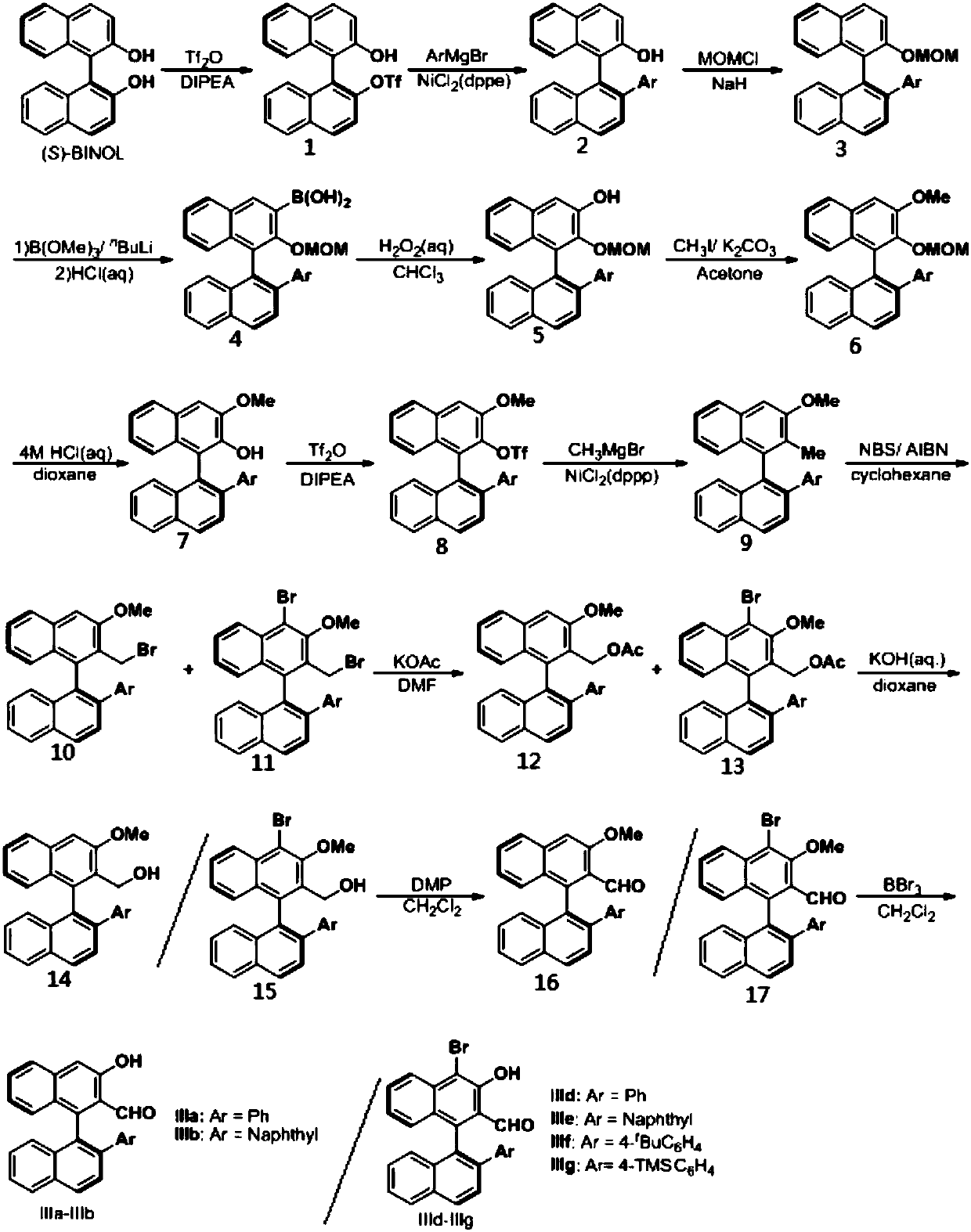
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
288 results about "Ester hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ion liquid of amino acid ester cation and its preparation method
InactiveCN1621152AEasy to getLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateAmino acid
The present invention is ion liquid of amino acid ester cation and its preparation process, and belongs to the field of new chemical material and its preparation technology. The ion liquid of amino acid ester cation is prepared with amino acid ester hydrochloride and through the substitution reaction with nitrate, tetrafluoroborate, hexafluorophosphate, bistrifluoromesyl imine salt or thiocyanate or the direct addition reaction with aluminum trichloride, ferric trichloride or zinc chloride, and the separation. The ion liquid thus prepared has the characteristic of chiral matter except the ion liquid characteristics, has low cost, simple preparation process and no pollution. The present invention is suitable for industrial production and is expected to become important green chemical material.
Owner:PEKING UNIV
Preparation method of ribofuranose phosphate derivative
ActiveCN104610404APurity is easy to controlHigh yield of docking reactionSugar derivativesSugar derivatives preparationPhosphateGrignard reagent
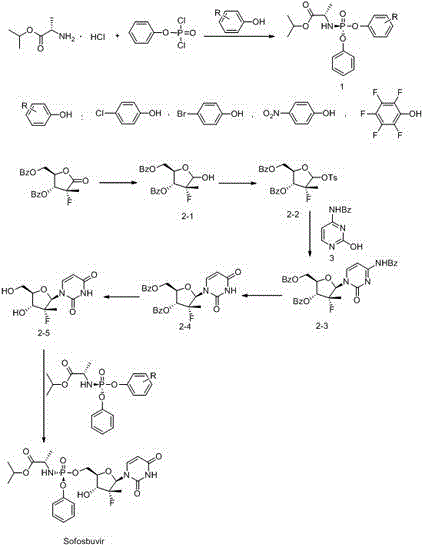
The invention discloses a preparation method of a ribofuranose phosphate derivative. The preparation method comprises preparation steps as follows: L-alanine isopropyl ester hydrochloride, phenol dichlorophosphate and substituted phenol are taken as starting materials and have a docking reaction under the action of alkali; (2R)-2-deoxy-2-difluoro-2-methyl-D-erythropentonic acid GAMMA-lactone and 3,5-dibenzoate reduce carbonyl in a dichloromethane or ether solvent into an alcoholic hydroxyl group under the action of a strong reducing agent; an intermediate with a formula 2-1 has a reaction with paratoluensulfonyl chloride under the action of alkali to obtain p-toluenesulfonates; an intermediate with a formula 2-2 and a benzoyl cytosine derivative have a docking reaction under the action of a condensing agent; an intermediate with a formula 2-3 converts cytosine into uracil under the action of organic acid; benzoyl protection for an intermediate with a formula 2-4 is released under the action of an alkaline agent; an intermediate with a formula 2-5 and an intermediate with a formula 1 are docked under the action of a Grignard reagent to obtain Sofosbuvir.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
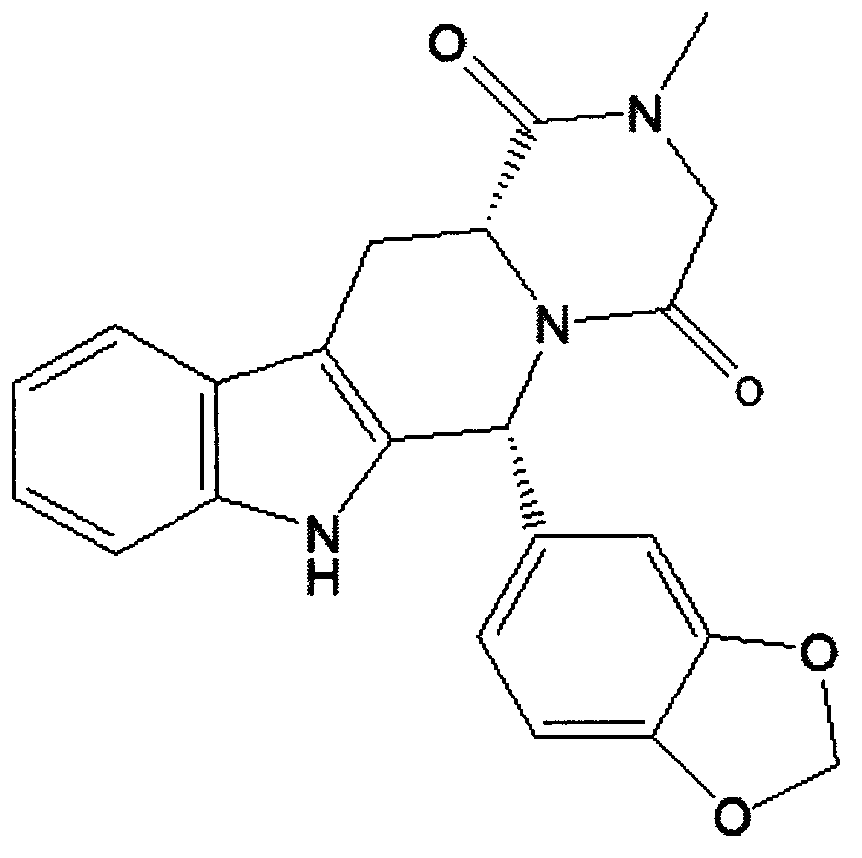
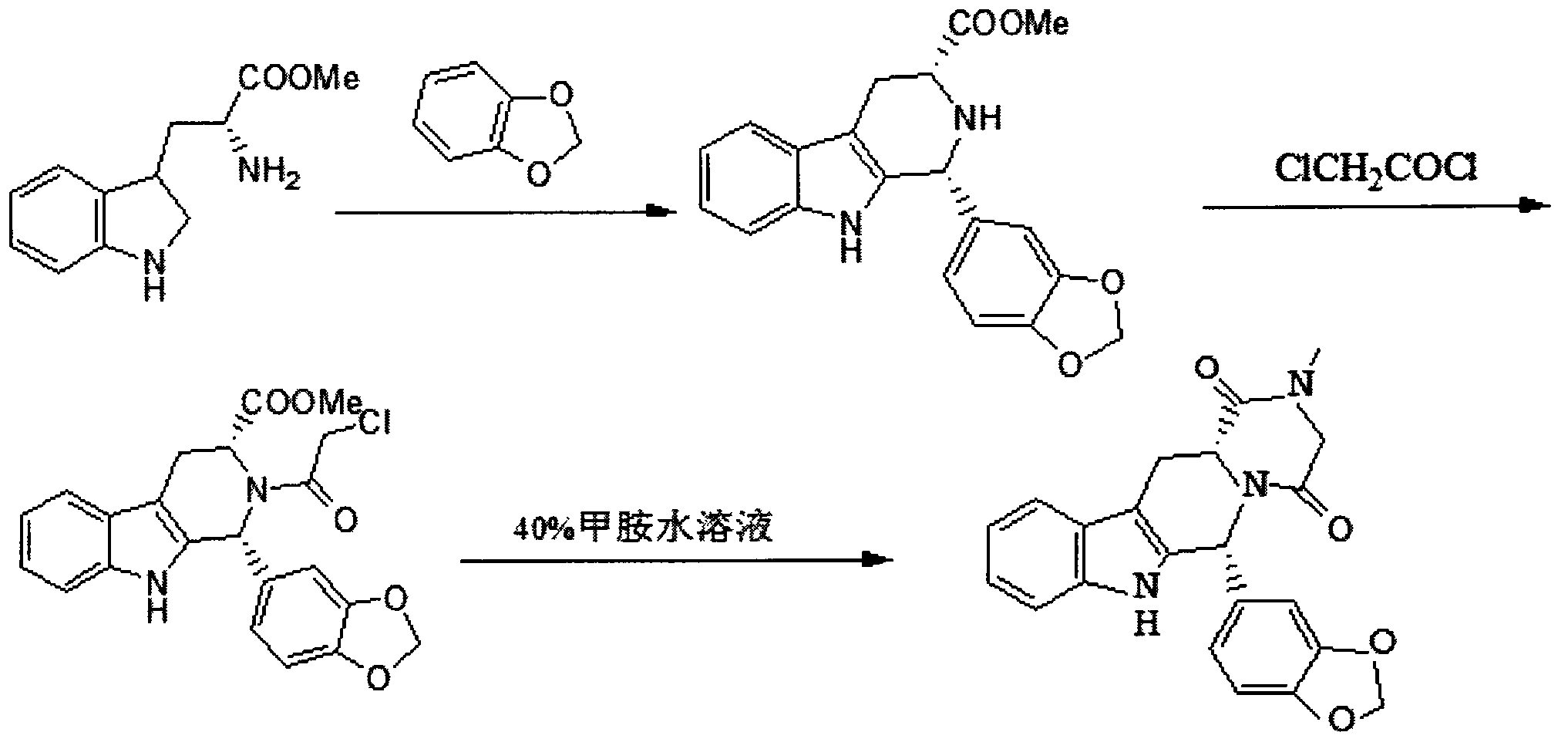
Simple preparation process of tadalafil
The invention relates to a preparation method of tadalafil. A product is obtained through condensation and cyclization, chloromethylation and aminolysis cyclization reaction based on D-tryptophan methyl ester hydrochloride and heliotropin as starting preparation materials. The simple preparation process is characterized in that condensation and cyclization are used for solving an isomer problem caused by Pictet-Spengler reaction by using isopropanol or nitromethane as a solvent; the yield of ethyl acetate in the aminolysis cyclization reaction is obviously improved; the aminolysis cyclization route includes three preparation steps, wherein the reaction yield of each step is high, the relevant impurities are easy to separate, the reaction conditions are simple, the production period is shorter, and toxic and highly corrosive reagents are not used, and therefore, the simple preparation process is safe and environment-friendly and easy to industrially produce, so that the high-purity qualified products are obtained.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Synthetic method of high-purity bortezomib and intermediate thereof
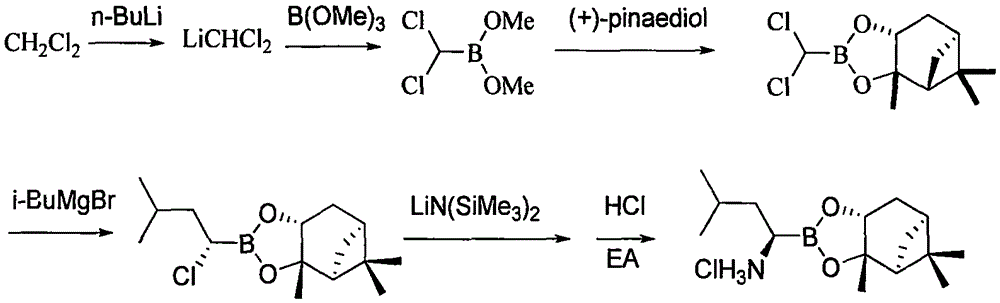
The invention belongs to the pharmaceutical and chemical fields and particularly relates to a synthetic method of high-purity bortezomib and an intermediate of the high-purity bortezomib. According to the invention, N-(2- pyrazine carbonyl)-L-phenylalanine benzyl ester is obtained from condensation reaction between 2-pyrazine carboxylic acid and L-phenylalanine benzyl ester; then the product is catalyzed and hydrogenated; and then the product is condensed and hydrolyzed with the hydrochloride of (aR,3aS,4S,6S,7aR)-hexahydro-3a,8,8-trimethyl-alpha-(2-methyl propyl)-4,6-methano-1,3,2- benzodioxoborane-2-methylamine or trifluoroacetate so that the bortezomib is obtained. The preparation process disclosed by the invention has the advantages of simplicity in operation, high purity and low cost. The bortezomib obtained through the method disclosed by the invention is in the form of white powder or crystals, the content is 99.8% or higher, and the total content of SS- and RR-isomers is not greater than 0.1%.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Synthetic method of bortezomib
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
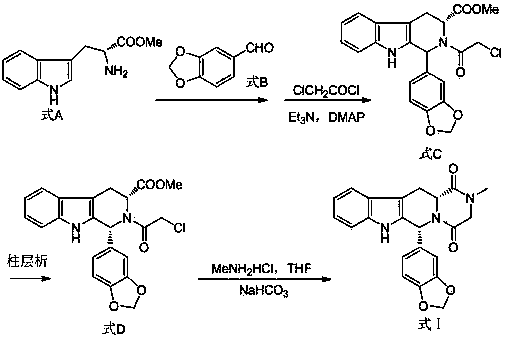
Improved tadalafil preparation method
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
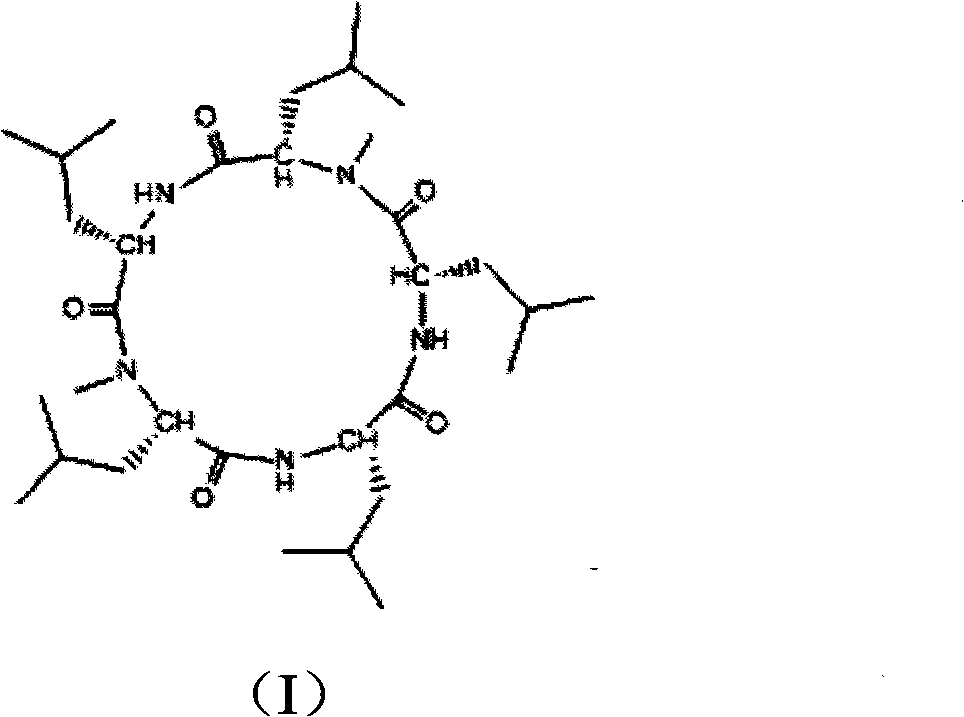
Cyclo-pentapeptide and synthesizing method
InactiveCN101270153AThe synthesis process is simpleMild reaction conditionsPeptide preparation methodsBulk chemical productionDipeptideTert-leucine
The present invention discloses a cyclic pentapeptide and a synthesization method, and the cyclic pentapeptide, the molecular formula of which is C32H59N5O5 and the constitutional formula of which is shown as the formula (I), is cyclic (leucyl-N-methylleucyl-leucyl-leucyl-N-methylleucyl). In the synthesization method of the present invention, butoxycarbonyl leucine and N-methylleucine benzyl ester hydrochloride are used as materials, which are condensed to produce protected dipeptide, the protected dipeptide is linked with a leucine to produce protected tripeptide, protected linear pentapeptide is produced by a ''2 plus 3'' synthesization strategy, products are respectively removed of the protecting groups of both ends, and a target product is produced after ring closure carried out by cyclizing reagent. In the synthesization method, ring closure is at the adjacent positions of two N-Mes, and ring closure yield reaches 62.7 percent. The synthesization process of the present invention has the advantages of simplicity, low material cost, bland reaction conditions and high purity, can be easily industrialized and can sufficiently satisfy the requirements of medical experiments and clinic applications.
Owner:JINAN UNIVERSITY
Lysine-modified benzofuroxan compound, synthetic method, application and recovery method of lysine-modified benzofuroxan compound as well as method of detecting concentration of copper ions
InactiveCN104478823AEasy to detectEasy to prepareOrganic chemistryFluorescence/phosphorescenceNitrobenzeneStructural formula
The invention discloses a lysine-modified benzofuroxan compound, a synthetic method, an application and a recovery method of the lysine-modified benzofuroxan compound as well as a method of detecting the concentration of copper ions. The structural formula of the lysine-modified benzofuroxan compound is shown in the specification. The synthetic method of the lysine-modified benzofuroxan compound comprises the following steps: 1) carrying out reaction on methanol, thionyl chloride and lysine to obtain lysine methyl ester hydrochloride; 2) in the presence of an acid binding agent, carrying out reaction on the lysine methyl ester hydrochloride and 4-chlorine-7-nitro-benzofuroxan, and carrying out column chromatography; and 3) dissolving the product obtained in the last step in alkali liquor, extracting for multiple times, regulating a pH value of a water phase, extracting for multiple times, collecting an extractant layer, and carrying out purification. The method of detecting the concentration of the copper ions comprises the following steps: 1) drawing a standard curve; 2) detecting and recording; and 3) carrying out calculation. The recovery method of the lysine-modified benzofuroxan compound comprises the following steps: adding a complexing agent aqueous solution into the compound solution containing the copper ions, uniformly mixing, extracting, collecting an organic phase, and removing a solvent. The lysine-modified benzofuroxan compound is applied to preparing a copper ion fluorescent probe. The synthesized lysine-modified benzofuroxan compound is capable of specifically detecting the copper ions, can be prepared into the probe and can also be recovered.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Synthetic method of boceprevir intermediate
ActiveCN103435532ARaw materials are cheap and easy to getReduce energy consumptionOrganic chemistryBulk chemical productionReaction conditions1-Naphthylamine
The invention relates to a synthetic method of a boceprevir intermediate, namely (1R, 2S, 5S)-6, 6-dimethyl-3-aza-bicyclo-[3. 1. 0] hexane-2-carboxylic acid methyl ester hydrochloride, belonging to the technical field of drug synthesis. The synthetic method solves the problems of high cost, complex reaction, low yield, and the like of the synthesis of the boceprevir intermediate in the prior art. The synthetic method comprises the following steps of carrying out amino protection on 6, 6-dimethyl-3-aza-bicyclo-[3. 1. 0] hexane hydrochloride which is taken as an original raw material; then reacting 6, 6-dimethyl-3-aza-bicyclo-[3. 1. 0] hexane hydrochloride with 1, 2, 3, 4-tetralin-1-naphthylamine for 3-4 hours at 30-35 DEG C under the action of a hydrogen drawing reagent by taking 4, 4'-difluoro benzophenone as a chiral inductive agent; finally removing an amino protecting group, and adding acid to form salt to directly obtain a final product. The synthetic method disclosed by the invention has the advantages of low cost, simple reaction condition, few reaction steps, short time and high purity and yield of the final product, namely the boceprevir intermediate.
Owner:SUZHOU UUGENE BIOPHARMA
Polymer for fluorescein angiogram and isotope angiogram
InactiveCN102070747AReduce distractionsHigh resistance to photobleachingRadioactive preparation carriersLuminescent compositionsTumour tissueSynthesis methods
The invention belongs to the field of polymer synthesis, and particularly relates to a polymer for fluorescein angiogram and isotope angiogram and a synthesis method of the polymer. The synthesis method mainly comprises the following steps: (1) reacting a hydroxyl-containing olefine acid ester substance with rhodamine, which contains a carboxyl group at the 3<-> position and is subjected to acyl chlorination to form a monomer containing a rhodamine group; (2) reacting tyrosine ester hydrochloride after deacidification with acrylic anhydride to obtain a monomer containing a tyrosine group; and (3) copolymerizing the two monomers and the hydroxyl-containing olefine acid ester substance to obtain a polymer contrast agent precursor. The polymer has strong red fluorescence of rhodamine substances and can lab radioisotopes; and the polymer can rapidly enter living cells, and can be enriched in not only cytoplasm but also in vivo tumour tissues.
Owner:SUZHOU UNIV
Method for synthesizing reduced glutathione
The invention relates to a method for synthesizing reduced glutathione. The method comprises the following steps of: (1), preparing R-glutamic acid; (2), preparing R-glutamic anhydride; (3), preparing R-glutamic-alpha-benzyl ester; (4), preparing R-glutamic-alpha-benzyl ester-gamma-acyl chloride; (5), preparing R-glutamic-alpha-benzyl ester-cystine; (6) preparing glycine-benzyl ester hydrochloride; (7) preparing bisR-S-S-glutathione-tetrabenzyl ester; and (8), preparing the reduced glutathione. The method has a simple and environmentally-friendly process step; the synthesizing steps are shortened; the yield is improved; and the used raw materials have relatively low cost. The method is the method for synthesizing the reduced glutathione, which has relatively high innovativeness.
Owner:天津市利发隆化工科技有限公司
Injectable parenteral medicinal preparation of temozolomide and preparation method thereof
ActiveCN102342931AImprove stabilityEasy to acceptOrganic active ingredientsPowder deliveryVitamin CPharmaceutical formulation
The invention relates to an injectable parenteral medicinal preparation of temozolomide and a preparation method thereof. The medicinal preparation comprises (1) temozolomide or pharmaceutically acceptable salt thereof, (2) at least one stabilizer, and (3) at least one aqueous diluent, wherein the stabilizer is selected from L-alanine, L-glycine, L-cysteine, L-cysteine hydrochloride anhydride, L-cysteine hydrochloride monohydrate, acetyl cysteine, S-carboxymethyl-L-cysteine, L-ethyl cysteine hydrochloride, L-methyl cysteine hydrochloride, vitamin C or a mixture thereof. The invention further relates to lyophilized power containing the medicinal preparation and products thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
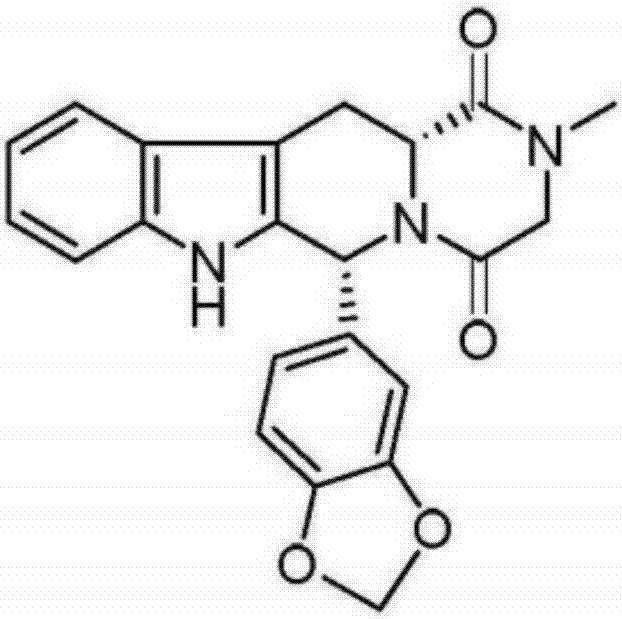
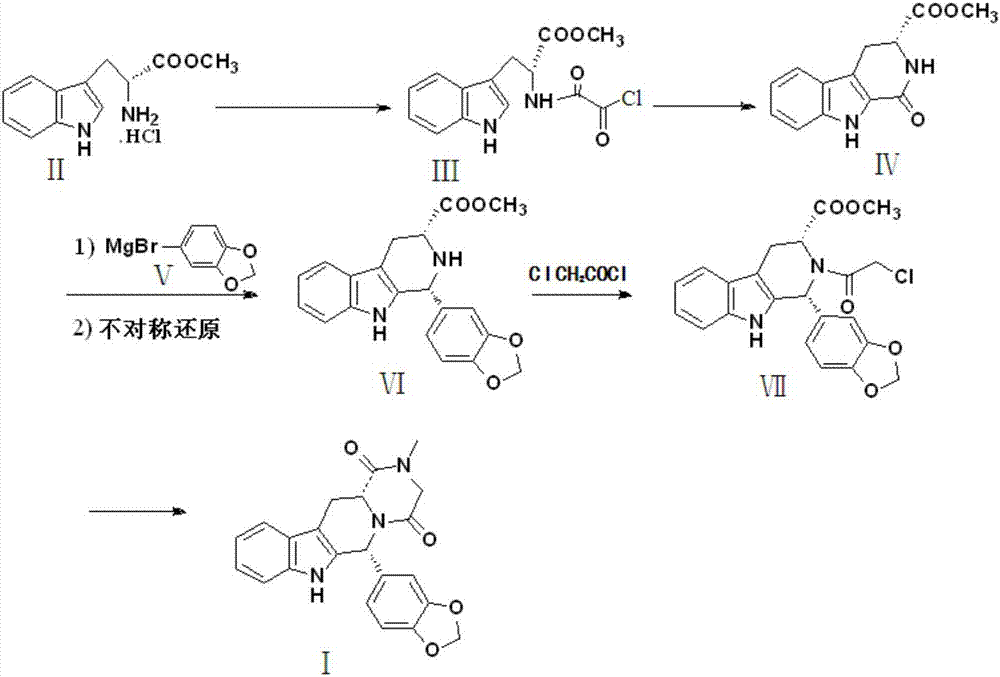
Preparation method of tadalafil
The invention discloses a preparation method of tadalafil, starting material D-Tryptophan methyl ester hydrochloride is reacted with oxalyl chloride to obtain an intermediate III, and the final product tadalafil (I) is obtained through cyclization, Grignard reaction, asymmetric reduction, substitution and condensation reaction. The use of national control chemical piperonal is avoided, an intermediate VI can be highly-selectively obtained by the asymmetric reduction, and the method has the advantages of simple postprocessing, short synthesis steps and high product total yield, and is suitable for industrialized production.
Owner:SHANDONG YUXIN PHARMA CO LTD
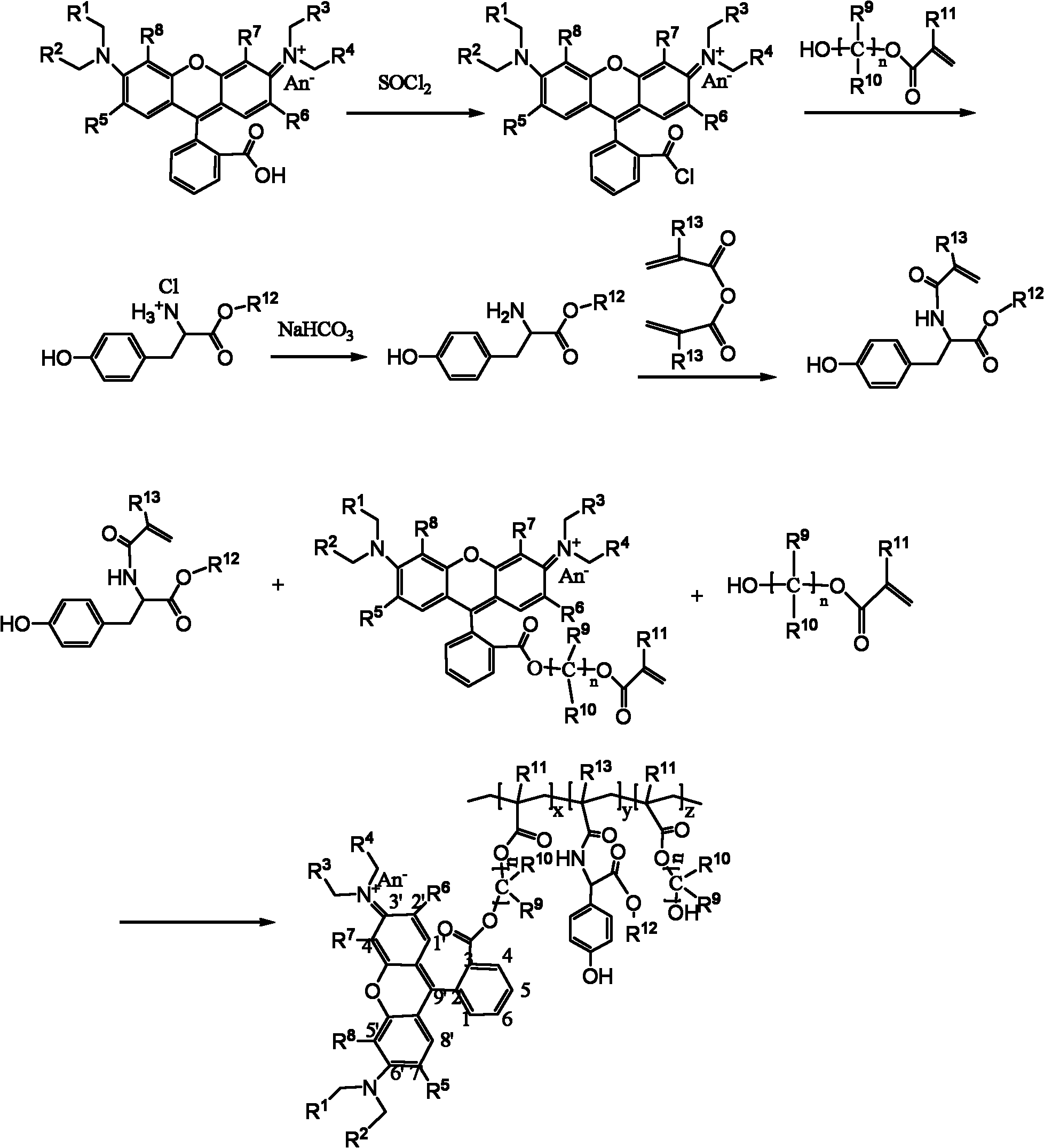
Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester
ActiveCN104761582ARealize large-scale productionSolution conditionsGroup 5/15 element organic compoundsOrganic solventPhenyl phosphate
The invention discloses a method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenyloxy)phenoxy phosphoryl]-L-alanine isopropyl ester; the method comprises the steps: first of all, carrying out a reflux reaction of dichloro phenyl phosphate and pentafluorophenol for 5-15 hours in an organic solvent and under an alkaline condition; followed by, cooling the reaction liquid down to room temperature, adding L-alanine isopropyl ester hydrochloride, and carrying out a stirring reaction for 2-8 hours at room temperature; and then carrying out suction filtration, concentrating the filtrate under reduced pressure, and carrying out recrystallization treatment on the concentrated residue. The method can effectively solve the defect problems that a conventional method has harsh reaction conditions, high requirements on equipment, relatively low yield and the like, prepares the high-purity N-[(S)-(2,3,4,5,6-pentafluorophenyloxy)phenoxy phosphoryl]-L-alanine isopropyl ester with simple operation, mild conditions and relatively high yield, and has an important value on achieving scale production of the compound.
Owner:SHANGHAI DESANO CHEM PHARMA +1
Synthetic method of proteasome inhibitor MLN9708
InactiveCN106608883AReduce usageEasy to operateGroup 3/13 element organic compoundsBulk chemical productionBenzoic acidProteasome Inhibition
The invention provides a synthetic method of a proteasome inhibitor MLN9708. The method comprises: taking 2,5-dichloro benzoic acid as an initial raw material, and performing condensation and saponification to prepare N-(2,5-dichlorobenzoyl) glycine; joining N-(2,5-dichlorobenzoyl) glycine to L-leucine boronic acid pinacol ester hydrochloride; purifying the obtained product through forming a complex with diethanolamine and performing hydrolysis for deprotection to obtain corresponding free boric acid; and reacting the obtained product with citric acid to obtain MLN9708. The method is cheap and available in raw materials, simple and convenient to operate, mild in reaction conditions and easy for industrial production.
Owner:PEKING UNIV
Method for preparing ceftizoxime alapivoxil hydrochloride
ActiveCN103059047AFew reaction stepsLow costOrganic chemistryBulk chemical productionBiotechnologyTert-Butyloxycarbonyl protecting group
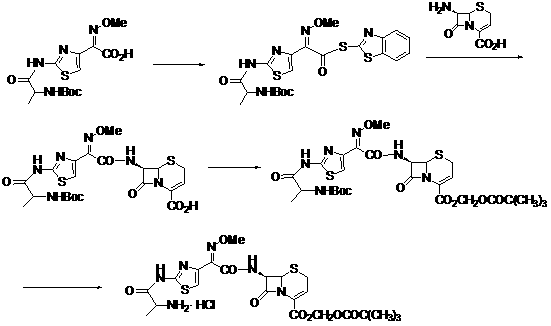
The invention discloses a new method for preparing ceftizoxime alapivoxil hydrochloride and belongs to the technical field of medicine synthesis. The method comprises the following steps: step (1), performing Boc protection on 7-amino of raw material 7-amino-3-cephem-4-carboxylic acid (7-ANCA) and then making the raw material have reaction with iodomethyl pivalate, then removing Boc protection group to obtain a midbody 7-amino-3-cephem-4-carboxylic pivaloyl oxymethyl ester (7-ANCA-POM); step (2), making N-t-butyloxycarboryl-L-alanine (Boc-L-Ala) and methoxyiminoacetic acid (ATMA) have condensation reaction to obtain a midbody 2-(2-N-t-butyloxycarboryl-amino-(S)-triacylamino-thiazole-4-yl)-2-(Z)-methoxyimino-acetic acid (Boc-L-Ala-ATMA); step (3), activating the midbody Boc-L-Ala-ATMA and making the midbody Boc-L-Ala-ATMA have condensation reaction with the midbody 7-ANCA-POM, and then removing the Boc protection group to prepare a target compound ceftizoxime alapivoxil hydrochloride (CZX-AP-HC1,I). The method adopts a collection type synthesis route, is simple and convenient to operate and mild in technical conditions, has high product quality and low cost, and avoids the problem about benzothiazole residue caused by the use of AE active ester in the present technique, so the collection type synthesis route is a good synthesis route suitable for industrial production.
Owner:江苏慈星药业有限公司
Application of fructus arctii aglycon methionine ester hydrochloride to preparation of antitumor medicine
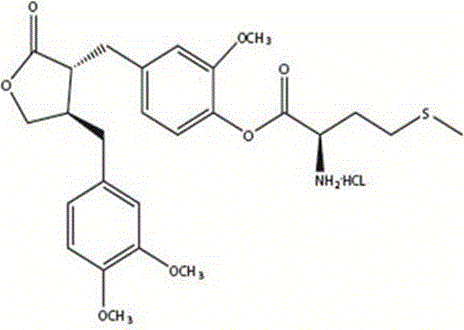
The invention provides a 4-(4-(3,4-dimethoxy phenmethyl)-2-carbonyl) tetrahydrofurfuryl-2-methoxyphenol-2-amidogen-4-methylthio butyrate hydrochloride shown as a structural formula I, which is used for tumor resistance and belongs to the field of antitumor medicine study. Experimental results show that the 4-(4-(3,4-dimethoxy phenmethyl)-2-carbonyl) tetrahydrofurfuryl-2-methoxyphenol-2-amidogen-4-methylthio butyrate hydrochloride has an obvious inhibition effect on H22 transplantation tumor mice; the immune organs of a body can be protected to a certain degree; the immune function of the body is enhanced. The formula I is shown as the accompanying drawing.
Owner:JILIN AGRICULTURAL UNIV
Prepn process of glycylglutamine
The preparation process of glycylglutamine includes preparing L-glutamine-gamma-methyl ester hydrochloride with L-glutamic acid, the subsequent preparing chloroacetyl glutamine-gamma-methyl ester hydrochloride with L-glutamine-gamma-methyl ester hydrochloride, and final aminolyzing chloroacetyl glutamine-gamma-methyl ester hydrochloride and purifying to obtain glycylglutamine. The preparation process utilizes chemical material chloroacetyl chloride directly, and has simple reaction, short period, high product yield and low production cost. The purified product has molting point higher than 204 deg.c, and content of 99-100 %.
Owner:崔焕兴
Application of arctigenin valine ester hydrochloride to preparation of anti-tumor drugs
The invention provides a 4-(4-(3, 4-dimethoxy benzene methyl)-2-carbonyl) tetrahydrofurfuryl-2-methoxyphenol 2-amino-3-methyl butyric acid ester hydrochloride for resisting tumors, and belongs to the field of research of anti-tumor drugs. Experimental results indicate that the 4-(4-(3, 4-dimethoxy benzene methyl)-2-carbonyl) tetrahydrofurfuryl-2-methoxyphenol 2-amino-3-methyl butyric acid ester hydrochloride has obvious inhibition effect on hepatocarcinoma 22 solid tumor-bearing mice, immune organs of organisms can be protected to some extent, and immune functions of the organisms are enhanced.
Owner:JILIN AGRICULTURAL UNIV
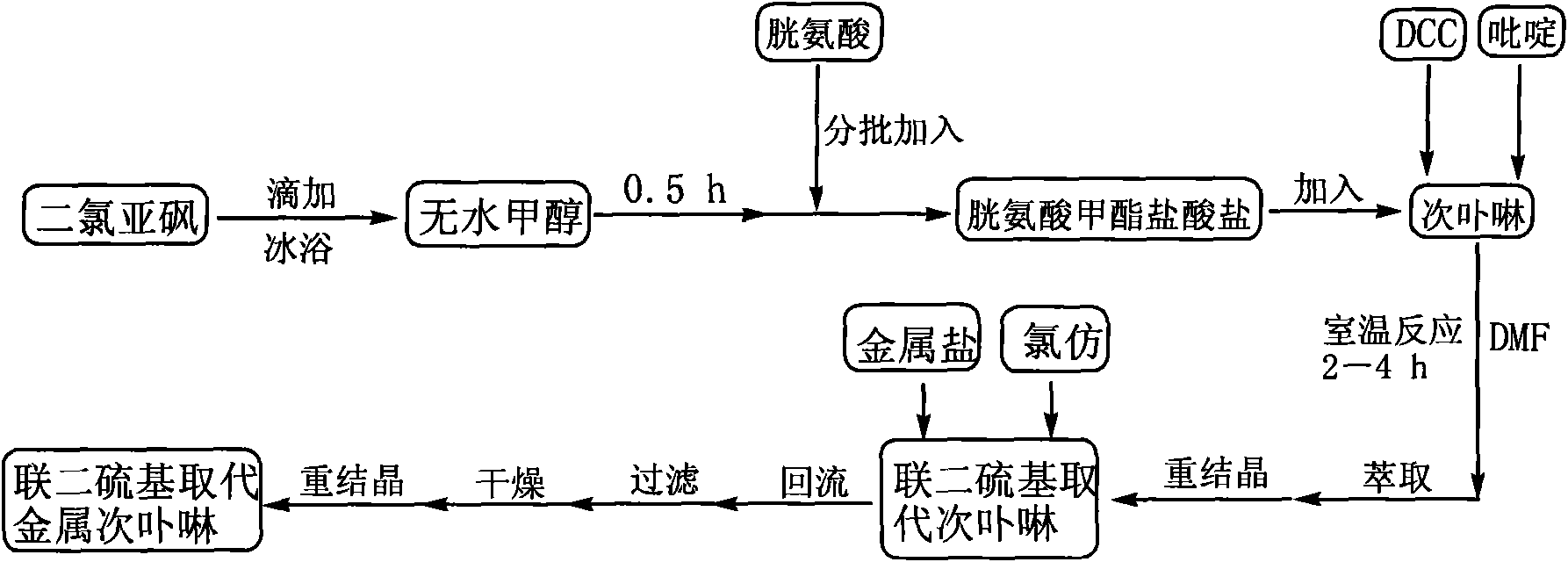
Linked disulfide group substituted deuteroporphyrin, metal complexes, preparation method and uses thereof
InactiveCN101585849AHigh activityHigh selectivityPreparation by oxidation reactionsOrganic compound preparationCyclohexanoneN dimethylformamide
The present invention discloses a linked disulfide group substituted deuteroporphyrin, metal complexes, preparation method and uses thereof, wherein linked disulfide group substituted deuteroporphyrin and metal complexes thereof, first using thionyl chloride and cystineto prepare cystine methyl ester hydrochloride solid; then using deuteroporphyrin, cystine methyl ester hydrochloride, dicyclohexylcarbodiimide DCC and N, N-dimethylformamide DMF to prepare linked disulfide group substituted deuteroporphyrin in the presence of catalyst pyridine. Then dissolving the linked disulfide group substituted deuteroporphyrin solid in chloroform, adding metal salts and proceeding refluxing reaction to get the linked disulfide group substituted deuteroporphyrin metal complexes. The invention provided linked disulfide group substituted deuteroporphyrin and metal complexes thereof are novel compounds capable of improving catalyst activity and selectivity, shorting reaction time, improving cyclohexane transformation ratio and selectivity of the cyclohexanone (cyclohexanol) in products.
Owner:NANJING UNIV OF SCI & TECH
Synthesis method of histidine and proline cyclodipeptide
ActiveCN106674230AAvoid defects that alter optical purityOrganic chemistryDipeptideMedicinal chemistry
The invention provides a synthesis method of histidine and proline cyclodipeptide. The method comprises the steps of preparing histidine and proline dipeptide methyl ester with protected amino from histidine and proline, wherein one of histidine and proline is in a form of ester hydrochloride and the other one is in a form of the protected amino; and carrying out deprotection on dipeptide methyl ester with the protected amino and forming histidine and proline cyclodipeptide through cyclization.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Method for preparing salts of polyene macrolide esters
InactiveUS6613889B2Promote conversionReducing methylationSugar derivativesGlycosidesCarbonateTetrahydrofuran
Owner:BIOSOURCE PHARM INC
Detection method for content of enantiomer in L-alanine isopropyl ester
InactiveCN110849980AQuality improvementEfficient separationComponent separationChromatography columnHplc mass spectrometry
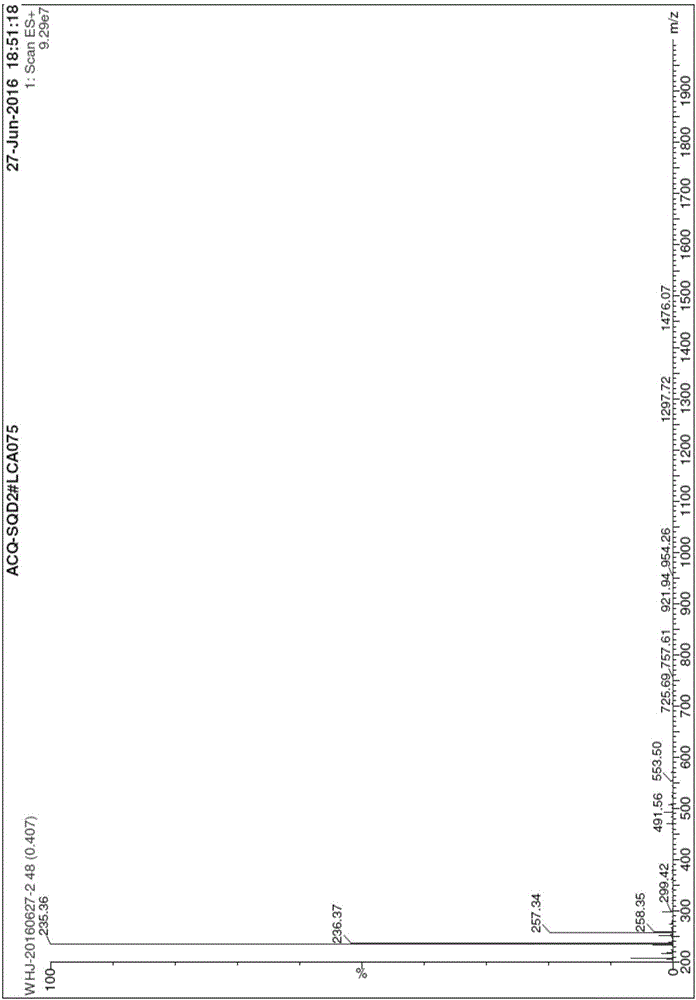
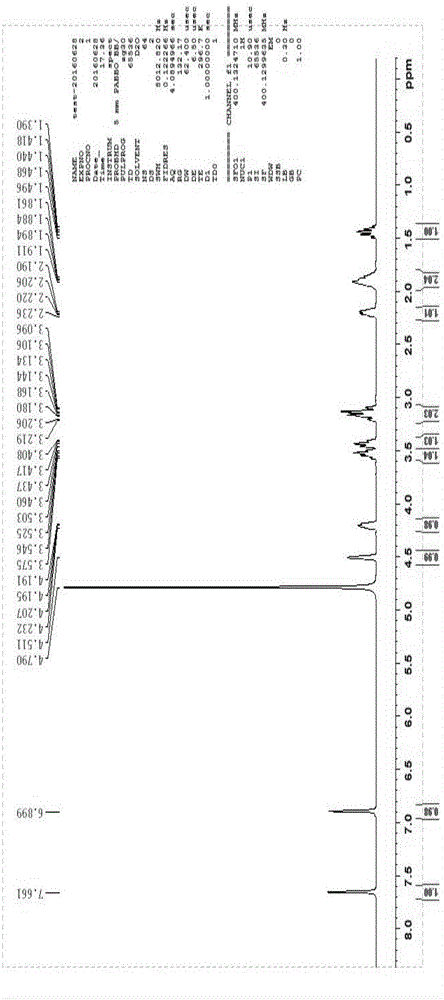
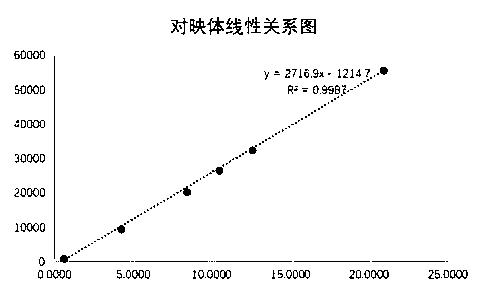
The invention belongs to the field of pharmaceutical analysis, and particularly relates to a high performance liquid chromatography detection method for the content of enantiomer in L-alanine isopropyl ester hydrochloride. According to the method, high performance liquid chromatography is used for determination, a crown ether chiral chromatographic column is used, and a perchloric acid aqueous solution with the pH value of 1.0 serves as the mobile phase. Thus, the L-alanine isopropyl ester hydrochloride can be effectively separated from the corresponding enantiomer, and the content of enantiomer can be detected more accurately. According to the method, a reversed-phase method and isocratic elution are used, operation is simple, peak shape is better, the separation degree is qualified, andvia methodological verification, the method is high in specificity, sensitivity, accuracy and durability.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
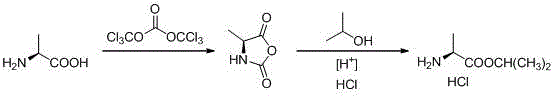
Novel preparation method of L-alanine isopropyl ester hydrochloride
InactiveCN106518694AStrong irritantHighly corrosiveOrganic compound preparationAmino-carboxyl compound preparationMedicinal chemistryPhosgene
The invention provides a novel preparation method of L-alanine isopropyl ester hydrochloride, and relates to a preparation method of an intermediate of a new drug sofosbuvir for treating chronic hepatitis. The method sequentially comprises the following steps that L-alanine is adopted as a raw material to react with triphosgene for ring closure, after ring opening is conducted through isopropanol under the acidic condition, salt formation is conducted, and the product L-alanine isopropyl ester hydrochloride is obtained. According to the novel preparation method, a brand-new synthetic route is provided, the adopted raw material is wide and sufficient in source, the cost is low, the reaction conditions are mild, the processes are simple, all the reactions are conventionally operated, the condition that a large quantity of high-irritation raw materials such as thionyl chloride are used is avoided, and a good industrial prospect is achieved.
Owner:ZHEJIANG GENEBEST PHARMA
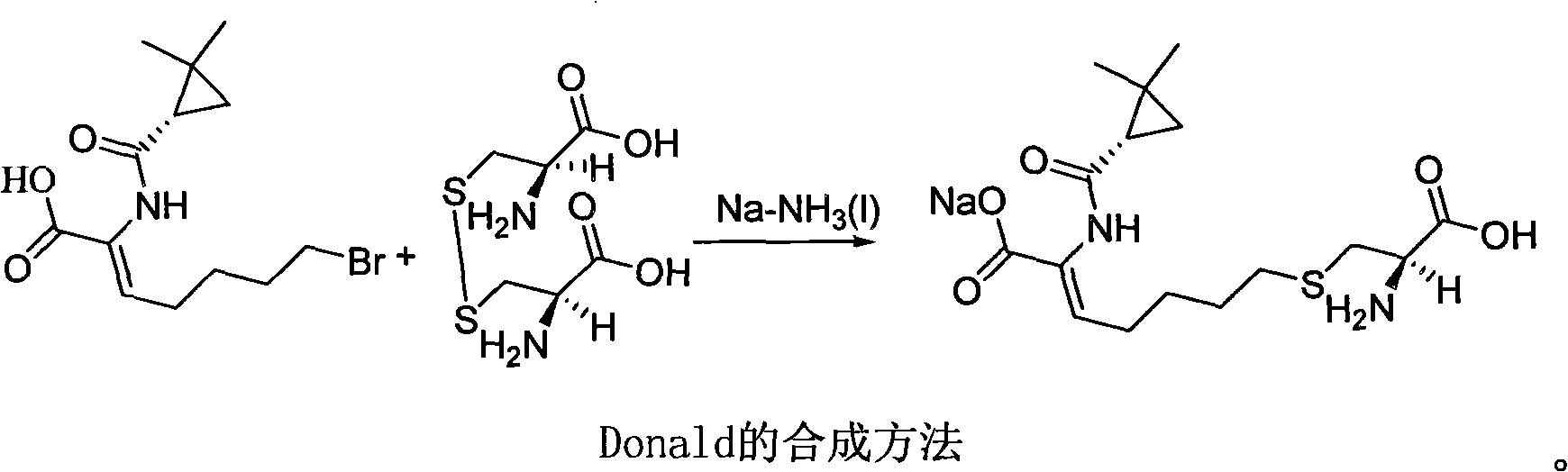
Method for synthesizing cilastatin sodium
The invention discloses a method for synthesizing cilastatin sodium, comprising the following steps: preparing (Z)-7-iodine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester by reaction of (Z)-7-chlorine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester and sodium iodide; enabling (Z)-7-iodine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester, cysteine alkyl ester hydrochloride, alkali and a solvent to react to obtain cilastatin dialkyl ester; and enabling the cilastatin dialkyl ester and sodium hydroxide to react to obtain cilastatin sodium. The alkali used in the method can be one of or more of K2HPO4, CsCO3 and K3PO4, thereby avoiding the usage of strong alkali and an anhydrous reaction system and realizing mild reaction and easy operation. The method has the advantages of good economical efficiency, mild reaction conditions, high yield, little three wastes (waste gas, waste water and industrial residue) and no pollution and can be used for industrialization production. The product can be separated easily and has high purity.
Owner:ZHEJIANG NORMAL UNIVERSITY
Chiral beta 2-amino acid derivative and preparing method thereof
ActiveCN106316871ASimple post-processingRaw materials are easy to obtainOrganic compound preparationCarboxylic acid esters preparationFuranCarbene
The invention belongs to the technical field of drug intermediate synthesis and particularly relates to a chiral beta 2-amino acid derivative and a preparing method thereof. The chiral beta2-amino acid derivative is represented with the following structural formula, wherein R is selected from C1-C16 linear chain or branched chain alkoxycarbonyl, and phenyl, oxole, thiophene, pyridine and naphthyl substituted with substitutes; the substituent is selected from any one of halogen, alkyl group, alkoxy, nitryl, ester group and amino group. In the method, mixed acid anhydride and protection benzylamine are regarded as starting materials; beta 2-amino acid methyl ester is prepared by catalyzing n-heterocyclic carbene and beta 2-amino acid methyl ester hydrochloride is prepared through hydrogenation and protection removal. The raw materials are easy to obtain and treat by the method, and the derivative has the high e.r value and overall yield and can provide important reference for industrial production.
Owner:中翌科技有限公司
Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
PendingCN106939327AIncreased molar yieldEasy to separateFermentationChemical synthesisCentrifugation
The invention relates to a method for preparing cefprozil in a pH responsive regenerative double aqueous phase system. The method comprises (1) preparing two double-aqueous phase systems, (2) orderly adding 7-APRA, D-p-hydroxyphenylglycine methyl ester hydrochloride into the systems, adjusting solution pH to 5.00-6.50 and controlling a solution temperature in a range of 10-30 DEG C, (3) adding immobilized penicillin acylase into the solution obtained by the step (2), and (4) standing the mixed solution, removing the immobilized penicillin acylase, adjusting the pH of the reaction solution, recovering P<ADBA> / <PMDB> and P<ADB> / P<MDB> polymers of the double-aqueous phase systems, feeding the supernatant to a crystallization section, carrying out crystallization, and carrying out centrifugation, washing and drying to obtain a product. The method reduces a chemical synthesis cost, improves a low product conversion rate of the monohydrolase catalytic reaction, effectively improves a yield, simplifies the operation, reduces a cost and realizes easy recovery of the double-aqueous phase systems.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of tadalafil intermediate
The invention relates to an improved method of preparing a compound (II). The improved method comprises the following steps: (the formula is shown in the description) 1, mixing D-tryptophan, methanol and toluene, adding thionyl chloride dropwise, and then carrying out reaction at the temperature of 70-85 DEG C to obtain D-tryptophan ester hydrochloride, wherein the structure is shown in a formula (III):(the formula is shown in the specification); and 2, mixing the D-tryptophan ester hydrochloride, a compound (IV) and a nitrile solvent, uniformly stirring, then raising the temperature to 75-85 DEG C, and carrying out reaction, (the formula is shown in the description). The improved preparation method of the compound shown by the D-tryptophan ester hydrochloride (II) has the following beneficial effects: 1, the dosage of the thionyl chloride in the first step is greatly reduced, so that the risk and the corrosion of the reaction are lowered, the post-processing is simple, and the yield is above 90 percent; 2, in the second step, the nitrile solvent is adopted to improve the stereoselectivity, and the enantiomer extra amount (ee %) is above 99 percent.
Owner:SUNSHINE LAKE PHARM CO LTD
Bearing-resisting compound and preparing method
InactiveCN1438217ASimple preparation processHigh product yieldOrganic active ingredientsSulfonic acids salts preparationMethane sulfonateEnzyme
The invention refers to anti-procreative compound 4-guanidino benzoic acid (4-carbomethoxy) phenyl ester methane sulfonate and the making method as well as anti-procreative preparation made from it. The compound has the activity to restrain the acrosome enzyme, its solubility small, the making method complex and the rate of preparation low.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Chiral aldehyde catalyst and preparation method thereof, and method for catalyzing asymmetric nucleophilic addition reaction
ActiveCN109908954AAvoid pollutionGood effectSilicon organic compoundsOrganic compound preparationBottleSolvent
The invention discloses a chiral aldehyde catalyst and a preparation method thereof, and a method for catalyzing an asymmetric nucleophilic addition reaction, wherein an amino acid ester or an amino acid ester hydrochloride and an alpha,beta-unsaturated carbonyl compound are added into a reaction bottle, the chiral aldehyde catalyst, 2,6-dicarboxypyridine, 1,5,7-triazacyclo[4.4.0]dec-5-ene, and anorganic solvent are sequentially added, and a complete stirring reaction is performed to perform the addition reaction. According to the present invention, the catalytic system uses the chiral aldehyde as the catalyst when the asymmetric nucleophilic addition reaction is catalyzed so as to avoid the pollution of metal ions to the product; and the asymmetric nucleophilic addition reaction method is widely used, and can achieve good effects on various alpha-beta-unsaturated ketones and various glycine esters, and the diversified synthesis of the products can easily achieve the further transformation and utilization.
Owner:SOUTHWEST UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
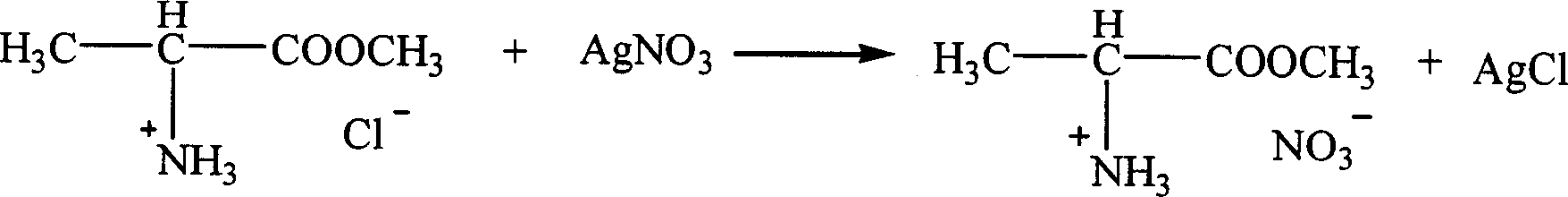
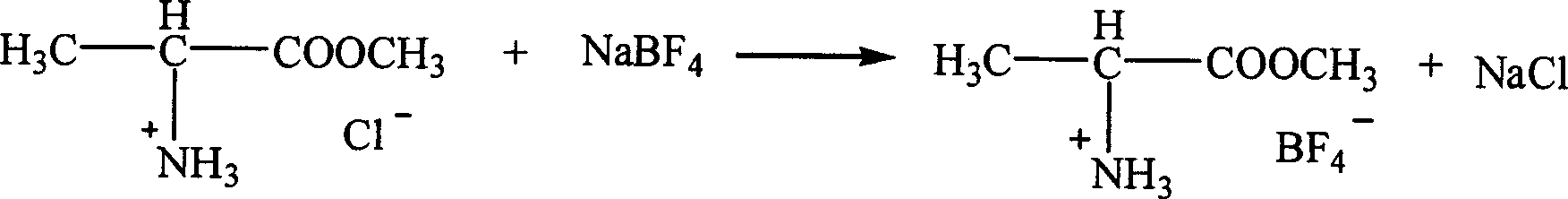
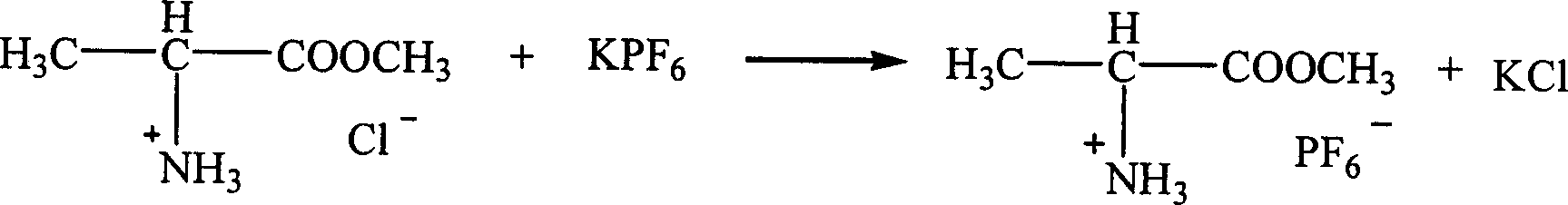



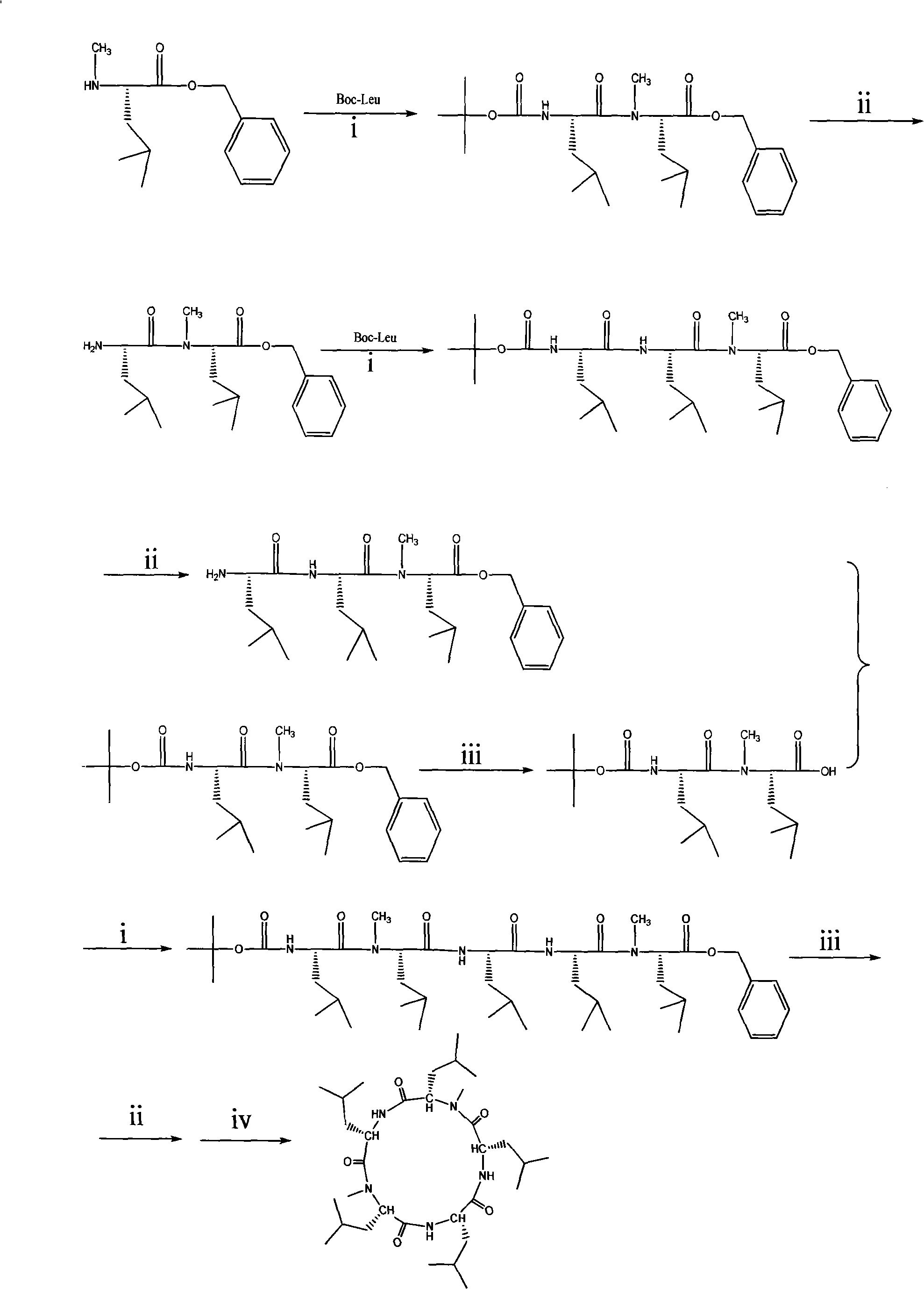








![Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2aa91e72-2b25-4b9d-aaae-9d4a77fc70db/BDA0000454418690000011.PNG)
![Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2aa91e72-2b25-4b9d-aaae-9d4a77fc70db/BDA0000454418690000012.PNG)
![Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester Method for preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxy phosphoryl]-L-alanine isopropyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2aa91e72-2b25-4b9d-aaae-9d4a77fc70db/BDA0000454418690000021.PNG)