Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
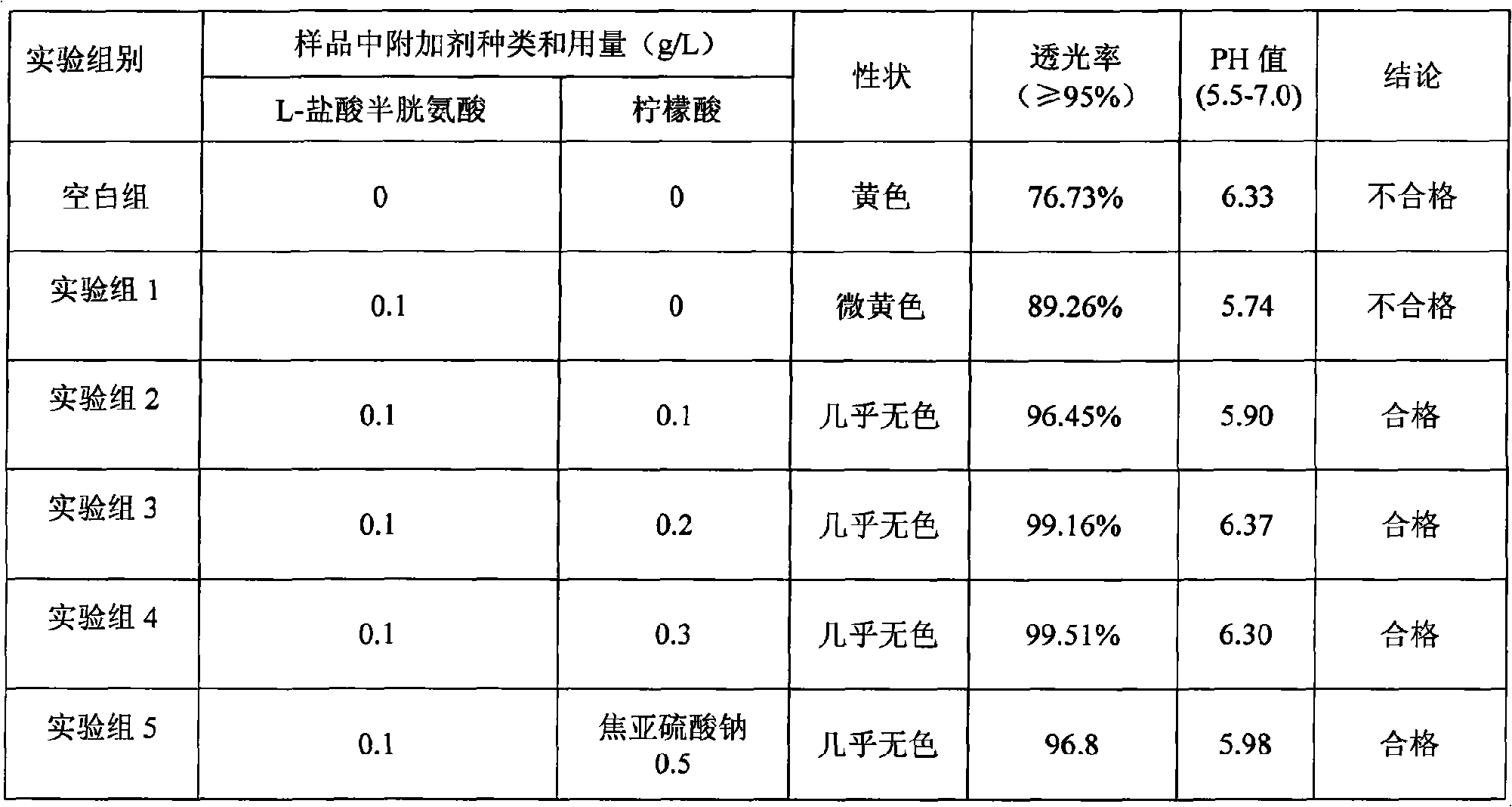
433 results about "Cysteine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
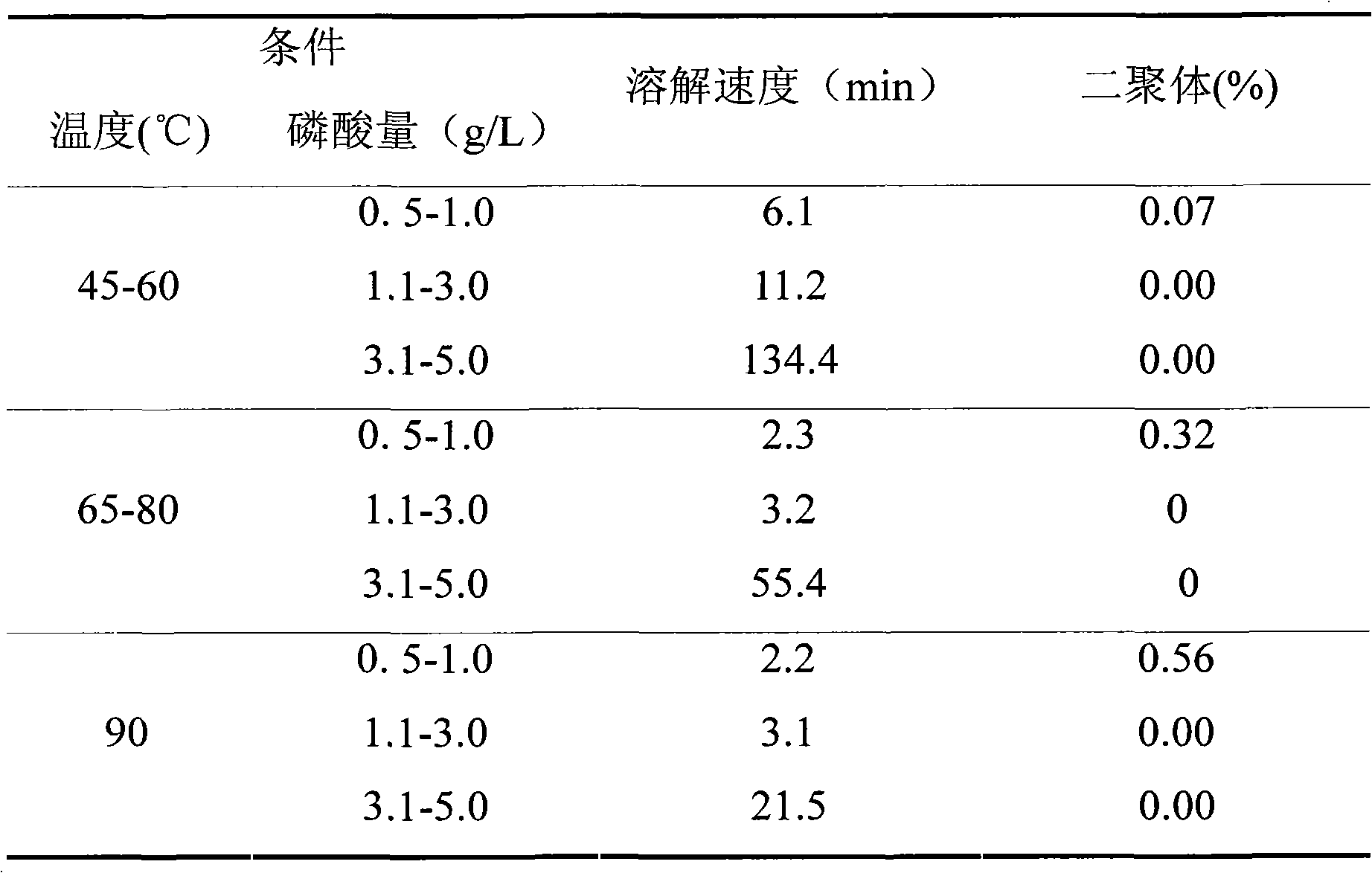
Cysteine Hydrochloride Injection, USP 0.5 gram is a sterile, nonpyrogenic solution containing 0.5 gram of Cysteine Hydrochloride, monohydrate in 10 mL of water for injection. The pH is 1.3 (1.0 to 2.5).
Method for preparing a freeze-drying crisp fruit and vegetable piece
The invention discloses a method for preparing a freeze-drying crisp fruit and vegetable piece, which includes the following steps: blanching raw materials for killing enzyme; preparing a color protection liquid using citric acid concentration, sodium isoascorbate concentration, or cysteine hydrochloride concentration, sodium phytate concentration and embedding medium, wherein, the ratio between the color protection liquid and the slice liquid material is 0.25-1:1; slowly freezing fruit paste with 4-7 mm thickness below a eutectic point at minus 30 DEG C. for one hour; placing the fruit paste on a separator plate of a vacuum lyophilizer after the machine is precooled to a cold trap minus 50 DEG C. with 30 Pa vacuum degree and 40 DEG C. separator plate temperature; heating and drying by programs, wherein, 3-5 DEG C. rises every hour when the temperature is below 10 DEG C.; keeping temperature at 10 DEG C. for 1 hour; heating continuously for rising 5-7 DEG C. every hour; keeping temperature for 2 hours at 40 DEG C.; obtaining the crisp fruit and vegetable pieces with water content below 7% after refrigeration; slicing and packing products in an independent drying room with air humidity 40-50%. The invention has a distinct fruit and vegetable original flavour with larger market prospect.
Owner:AGRI PROD PROCESSING INST GUANGXI ACADEMY OF AGRI SCI
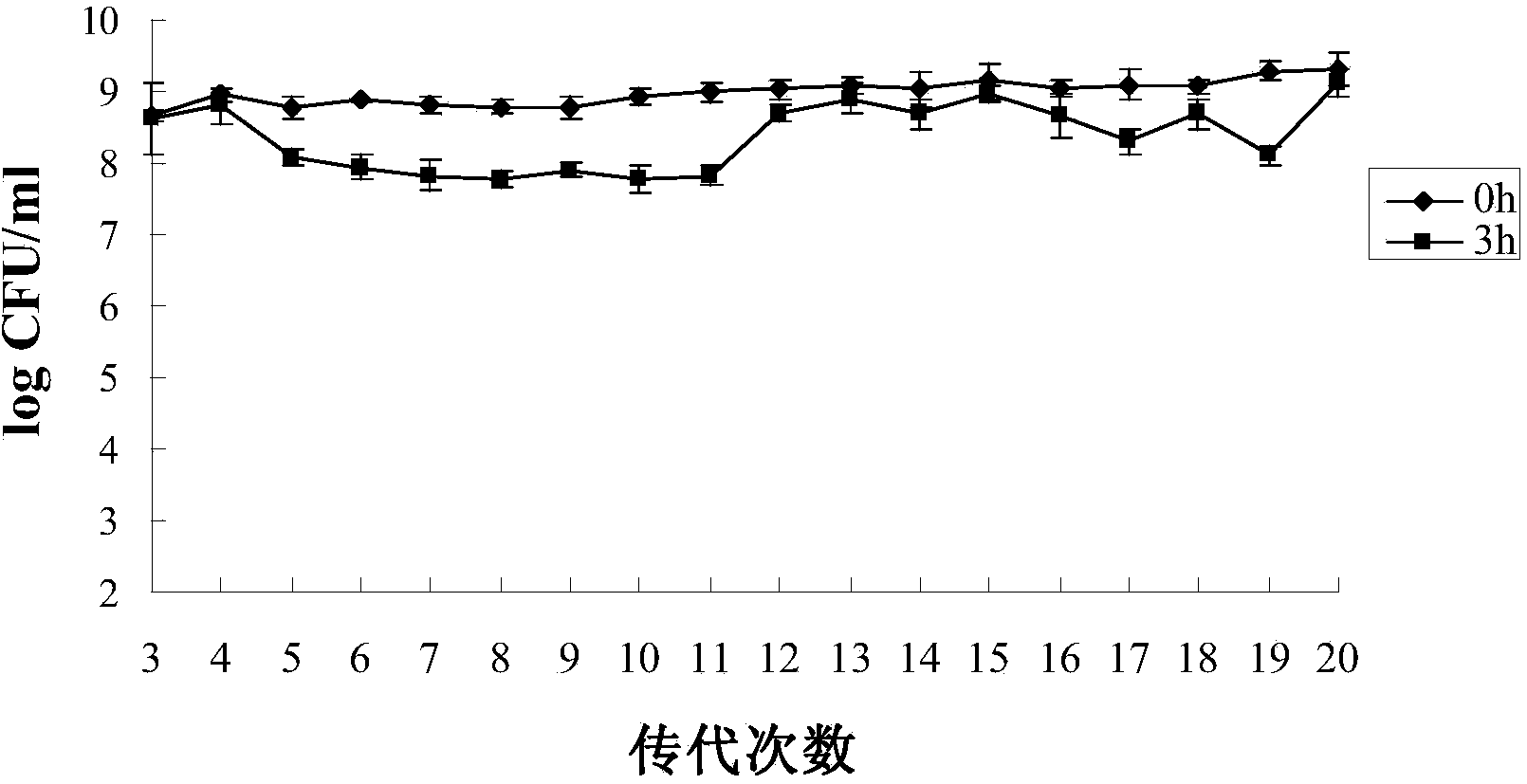
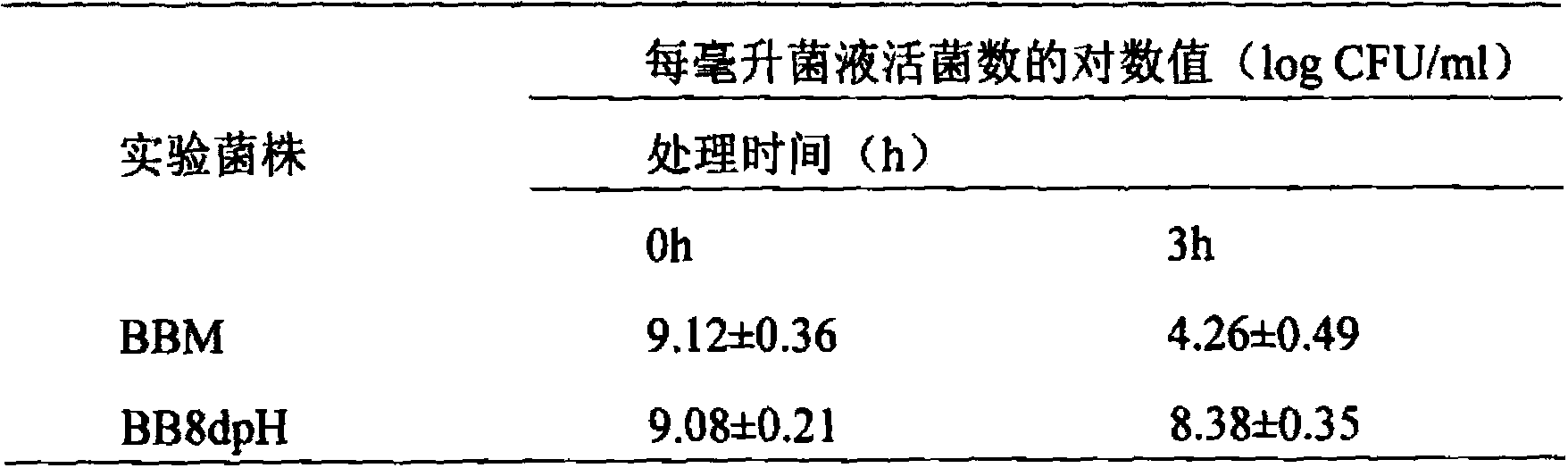
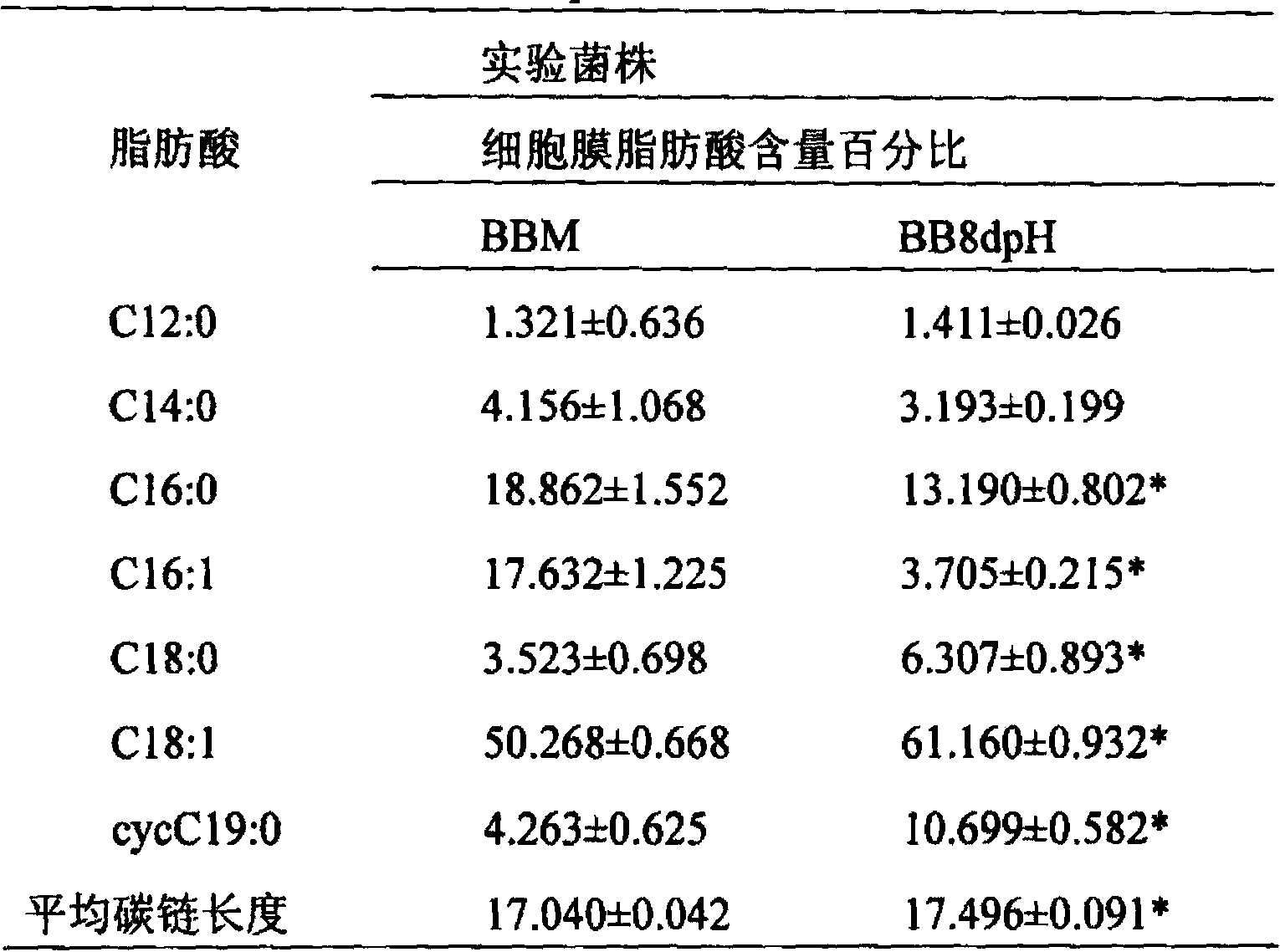
Acid-resistant bifidobacterium breve BB8dpH and application thereof
InactiveCN103849590AExcellent acid resistanceGenetically stableBacteriaMicroorganism based processesFecesCell membrane
The invention provides an acid-resistant bifidobacterium breve BB8dpH and an application thereof. The nucleotide sequence of the 16SrRNA gene part of the bifidobacterium breve BB8dpH is shown in SEQ ID NO.1, and is stored with the preservation number of CGMCCNo.8370. The bifidobacterium breve BB8dpH is a strain obtained by separating from the dejecta of healthy young people and further screening under the pH 3.2 acid condition. According to the fermentation and culturing process, anaerobic culture at 37 DEG C is carried out in a BL liquid substrate (containing 0.05% cysteine hydrochloride) for 24 hours. The bifidobacterium breve BB8dpH has remarkably better acid resistance than the common strains, and the acid resistance has genetic stability; the bifidobacterium breve BB8dpH has different percent contents of cell membrane fatty acids from the common strains, the average carbon chain length of the bifidobacterium breve BB8dpH is remarkably longer than that of the common strains, and the cell membrane fluidity of the bifidobacterium breve BB8dpH is remarkably lower than that of the common strains; and the bifidobacterium breve BB8dpH is used in the production field of daily fermented food, health-care food and medicines.
Owner:SHANGHAI JIAO TONG UNIV
Chicken essence and preparation method thereof
ActiveCN103750254ALong storage timeGreat tasteFood homogenisationFood dryingMaillard reactionMonosodium glutamate
The invention relates to a preparation method of chicken essence. The method comprises the steps of weighing chicken breast and chicken skeleton, adding water, boiling at high temperature, and then, passing through a colloid mill, so as to obtain colloidal chicken juice; hydrolyzing the colloidal chicken juice, then, carrying out enzyme deactivation and filtrating, so as to obtain enzymolysis chicken juice; uniformly mixing the enzymolysis chicken juice, L-cysteine hydrochloride, ribose, L-proline, xylose, dextrose monohydrate, L-glycine, water and chicken oil, and then, adding the mixture into a reaction kettle for Maillard reaction, so as to obtain a chicken juice reactant; uniformly mixing the chicken juice reactant, salt, white granulated sugar, monosodium glutamate, modified starch, maltodextrin, cyclodextrin, water, I+G, chicken oil and chicken flavoring base, then, passing through a colloid mill, filtrating to obtain colloidal chicken cream, emulsifying the colloidal chicken cream, and then, carrying out spray drying on the emulsified colloidal chicken cream through a homogenizer, thereby obtaining the chicken essence. The invention further relates to a product of the preparation method of the chicken essence. Compared with the existing products, the chicken essence prepared by the method has the advantages that the chicken fragrance is unique, the flavor is harmonious, the taste of chicken is strong and full, and the fragrance and the flavor can be of long-term coexistence.
Owner:GUANGZHOU TIANHUI FOOD
Method for producing chicken flavor essence base material by using chicken framework
The invention relates to a method for producing a chicken flavor essence base material by using a chicken framework, which comprises the following steps: firstly, crushing the cleaned chicken framework by a bone crusher, grinding the crushed chicken framework into chicken bone cement of which diameter is 10 + / - 5mu m by a colloid grinder, directly delivering the chicken bone cement into a reaction kettle without high-temperature boiling, adding water into the chicken bone cement to make the solid content reach 8 percent, and adjusting the pH to 7.0 by using 10 percent NaOH solution; adding 0.5 to 1.5 percent of composite proteinase by mass of the chicken bone cement into the mixture, and carrying out enzymolysis for 3 to 5 hours at a temperature of 55 DEG C; adding a thermal reaction mixture into the reaction kettle; heating the reaction kettle to make the mixture react at a temperature of between 100 and 125 DEG C; and after reacting for 30 to 180 minutes, obtaining the essence base material with intense chicken flavor, wherein the thermal reaction mixture comprises: 5 to 15 percent of glucose, 5 to 10 percent of xylose, 1 to 3 percent of cysteine hydrochloride, 1 to 3 percent of glycin, 30 to 50 percent of enzymolysis liquid, 1 to 5 percent of chicken fat, 5 to 20 percent of yeast powder, and 0.5 to 1.5 percent of vitamin B1.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
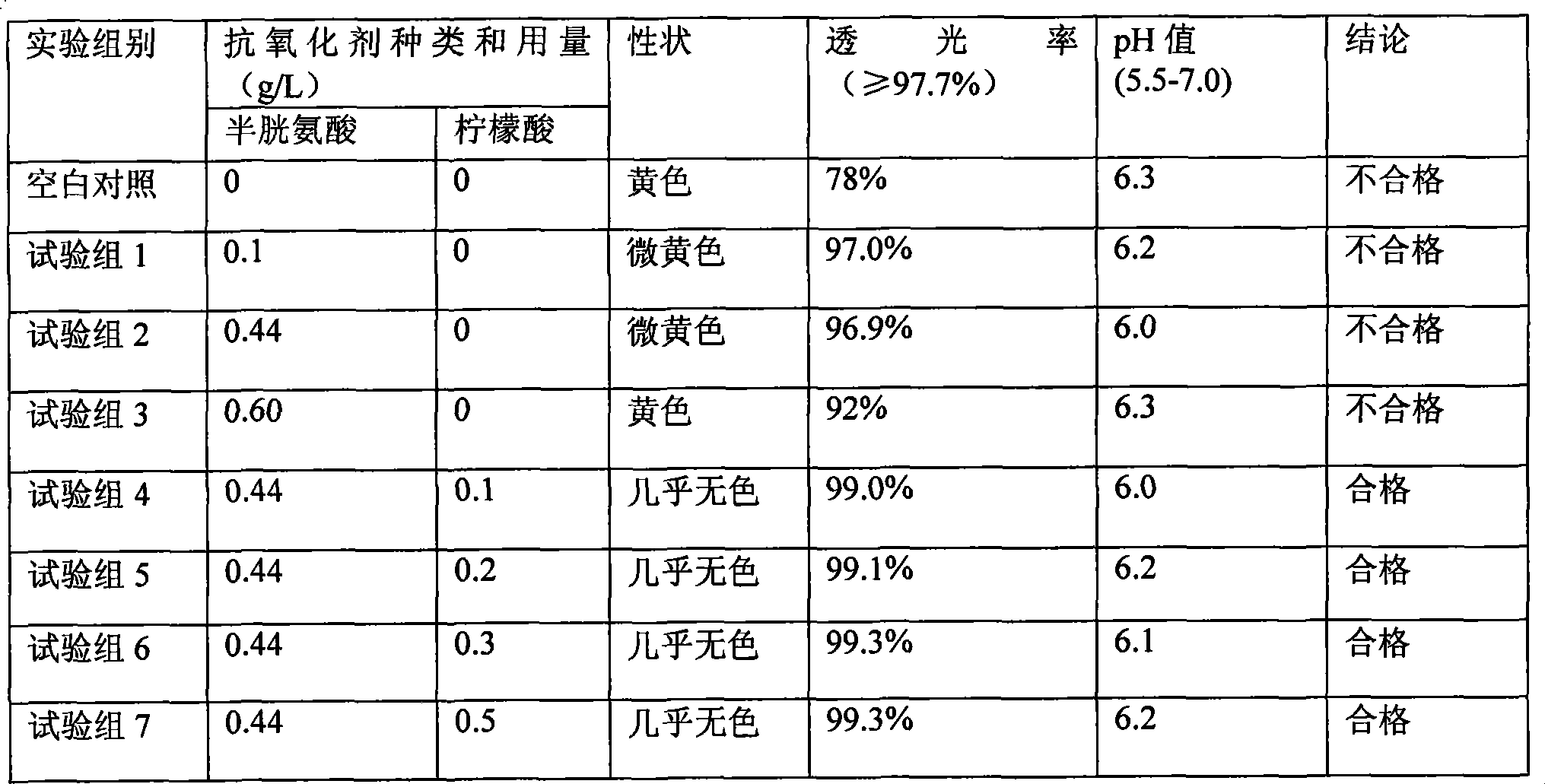
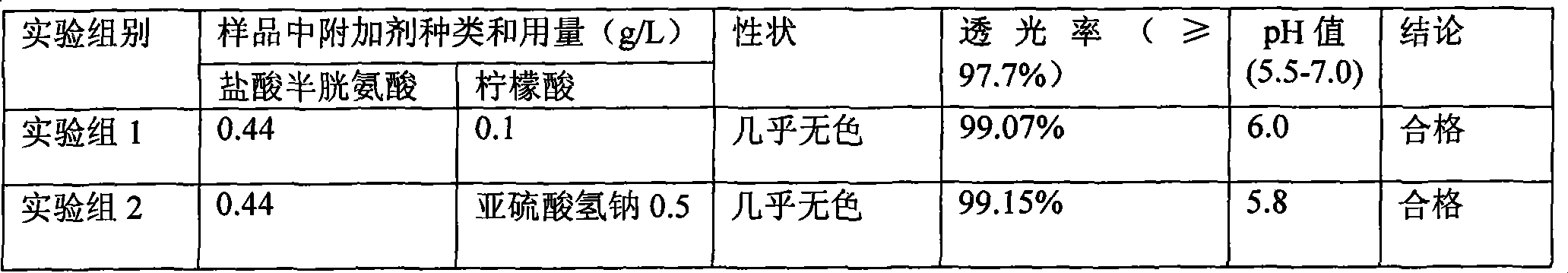
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
Water hardness on-line measurement device based on solution image technology and measurement method thereof
InactiveCN102183520AReduce pollutionReduce consumptionMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationChemical industryMeasurement device
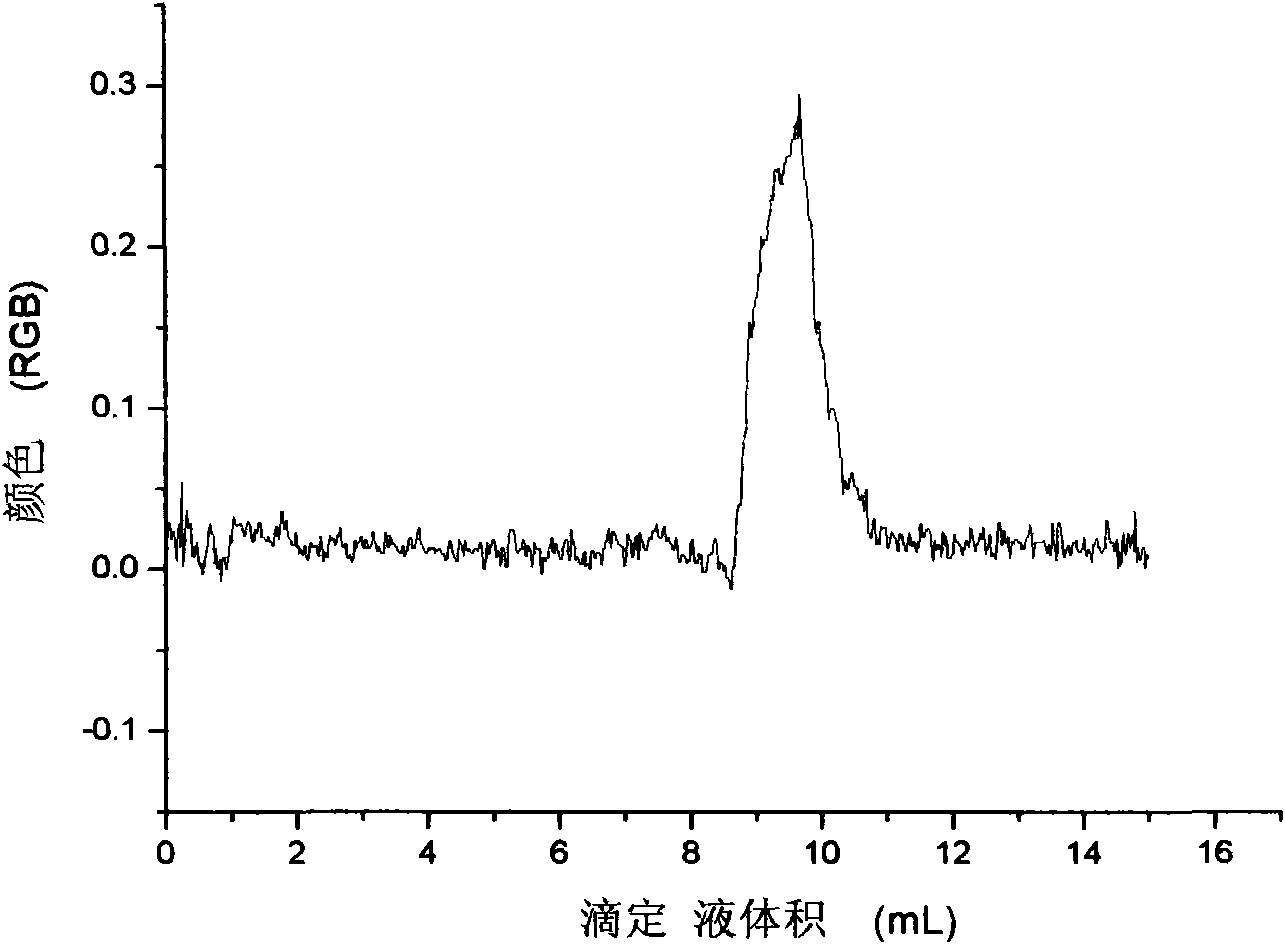
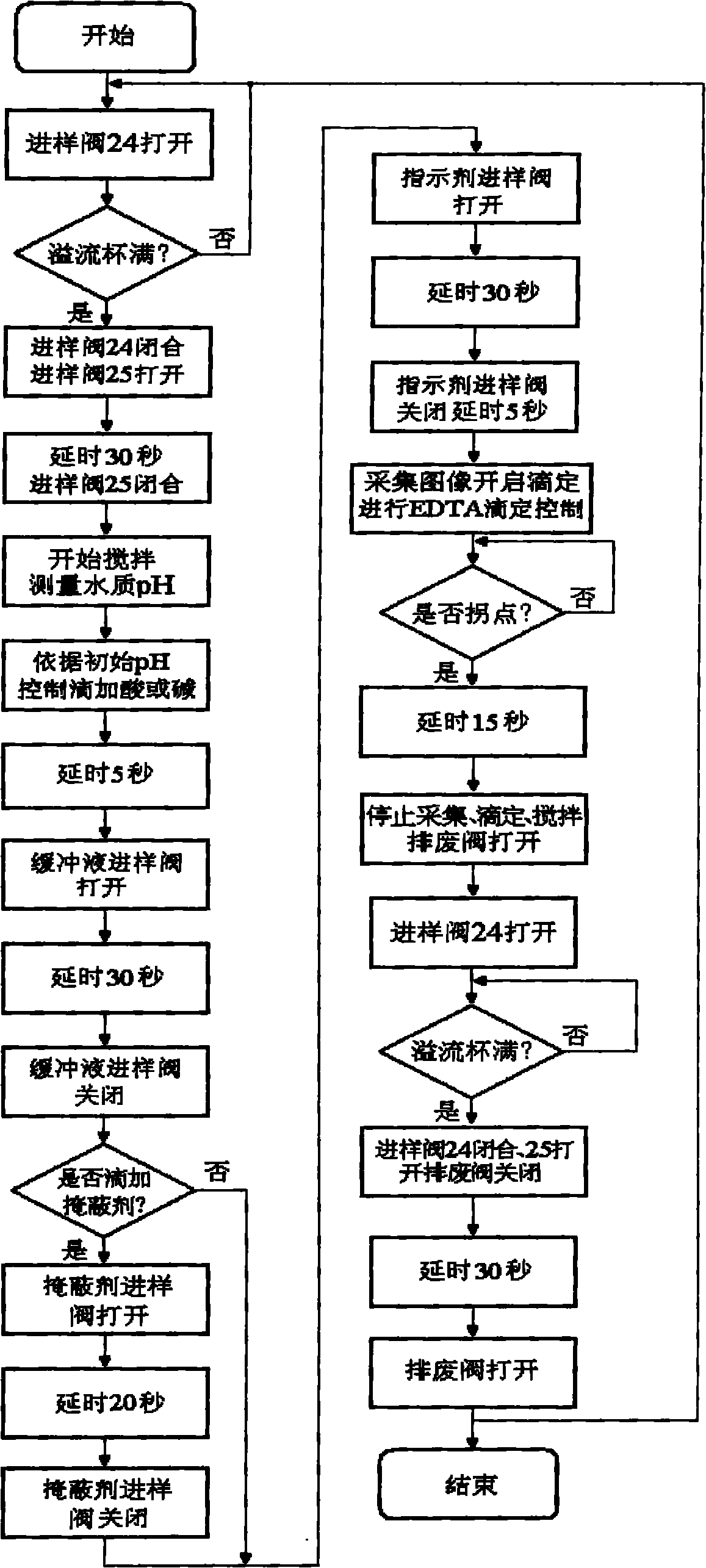
The invention relates to a water harness on-line measurement device. A measurement method of the water hardness on-line measurement device based on solution image technology is characterized by adding dropwise EDTA into a quantitative water solution with the pH of 10.0 plus or minus 0.1 by using a constant flow adding pump with chrome black T or acid chrome blue K as indicator, L-cysteine hydrochloride and triethanolaminesolution as screening agent and ethylenediaminetetraacetic disodiumsalt solution as titrant, recording the volume of added EDTA (Ethylene Diamine Tetraacetic Acid) solution by a computer, simultaneously collecting water solution image colors by using a CCD (Charged Coupled Device) image sensor, displaying image color RGB (Red, Green and Blue)-titrating solution volume real-time curve on a computer screen, and calculating the water hardness according to the titrating solution volume corresponding to the inflection point of the RGB-titrating solution volume curve, wherein the detection precision is improved by adopting a color constancy algorithm based on a neutral network to reduce the interference of factors such as a light source, sensor noise and the like. The invention have the choices of high hardness and low hardness on-line measurement, is simple and convenient in operation, and can be used in the industries of electric power, petroleum, chemical industry, metallurgy and the like.
Owner:NORTHEAST DIANLI UNIVERSITY
Clear liquid fermentation medium for clostridium butyricum and fermentation culture method thereof
InactiveCN102363754AHigh effective contentSimple post-processingBacteriaMicroorganism based processesBiotechnologySodium bicarbonate
The invention relates to a clear liquid fermentation medium for clostridium butyricum. The clear liquid fermentation medium, the pH value of which is 7-8, is made from glucose, tryptone, a yeast extract powder, ammonium sulfate, sodium bicarbonate, a maize liquid powder and the like. The clear liquid fermentation medium for clostridium butyricum contains metal salts for promoting the growth of gemma, wherein the metal salts are manganese sulfate, magnesium sulfate and ferrous sulphate. The enrichment medium of the clear liquid fermentation medium for clostridium butyricum contains yeast extract, beef extract, tryptone, glucose, soluble starch, sodium chloride, sodium acetate trihydrate, cysteine hydrochloride, methylene blue at the concentration of 0.5% and distilled water; and the pH is adjusted to 7.1. A fermentation culture method of the clear liquid fermentation medium for clostridium butyricum comprises the following steps of: glycerin tube refrigerated strain activation, heating and optimization, Erlenmeyer flask first-order seed culture, liquid fermentation and spray drying. The production of clostridium butyricum has advantages of simple operation, high amount of zymocyte, high gemma yield, simple post-treatment, less impurities in a bacterial powder sample and high amount of effective live bacteria, and saves cost at large.
Owner:HUAZHONG AGRI UNIV
Color fixative for yam slices and use method thereof
The invention discloses a color fixative for yam slices and a use method thereof. The invention aims to provide a sulfur-free color fixative for yam slices, which is free of sulfur and has favorable effect of color protection, and applications thereof. The invention has the technical proposal that the sulfur-free color fixative comprises phytic acid, citric acid, ascorbic acid and L-Cysteine hydrochloride, wherein the concentration of the phytic acid is arranged from 1.36 to1.69%, the concentration of the citric acid is ranged from 0.37 to 0.79%, the concentration of the ascorbic acid is 0.11-0.255, and the concentration of the L-Cysteine hydrochloride is ranged from 0.21 to 0.26%. Compared with the prior art, the color fixative had the obvious advantages of favorable effect of color protection, and high safety. The invention is used for sulfur-free protection of fresh slices during the processing of yam herbal pieces.
Owner:HENAN NORMAL UNIV
Natural yolk essence and preparation method thereof
The invention belongs to the technical field of essence for food, and in particular, relates to natural yolk essence and a preparation method thereof. The natural yolk essence is obtained from the following substances, such as dry yolk powder, protease, methionine, cysteine hydrochloride, a sweetening agent and a preservative by enzymolysis and Mailard thermal reaction. The natural yolk essence provided by the invention has intense natural yolk aroma, and has no egg fishy smell or other chemical odors; and the preparation method is simple.
Owner:许静
'Yanhuning' frozen-dried powder injection and its preparation method
ActiveCN101057841AStable pH environmentSlow oxidationPowder deliveryOrganic active ingredientsPhosphateAntioxidant
The invention discloses a freeze dried injection of potassium sodium dehydroandrographolide succinate which is prepared from potassium sodium dehydroandrographolide succinate, ionic cushion pairs and anti-oxidizing agents, the preferred ionic cushion pairs being phosphate cushion pairs, and the antioxidant can be L-cysteine hydrochloride.
Owner:HARBIN ZHENBAO PHARMA
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439031ASolve the problem of trace oxygenSolve the oxygen redissolution problemOrganic active ingredientsMetabolism disorderAntioxidantArginine
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-II) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 1.50 of aspartic acid, 2.50 of glutamic acid, 1.90 of serine, 3.00 of histidine, 3.50 of glycine, 2.50 of threonine, 7.20 of alanine, 4.90 of arginine, 0.20 of tyrosine, 0.20 of cystine, 3.20 of valine, 2.50 of methionine, 0.85 of tryptophan, 3.50 of phenylalanine, 2.50 of isoleucine, 3.40 of leucine, 5.50 of lysine acetate, 2.90 of proline, 0.10 of cysteine hydrochloride and 0.20 of lemon acid. The composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test, a test result shows that the pharmaceutical composition containing 18 amino acids is as stable as or more stable than like products which are sold in the markets and contain sulfites.
Owner:郑飞雄
Medicine composition containing 15 kinds of amino acid and preparation method thereof
ActiveCN101357118ASolve the problem of trace oxygenSolve the problem of oxygen incorporationOrganic active ingredientsPharmaceutical delivery mechanismArginineTryptophan
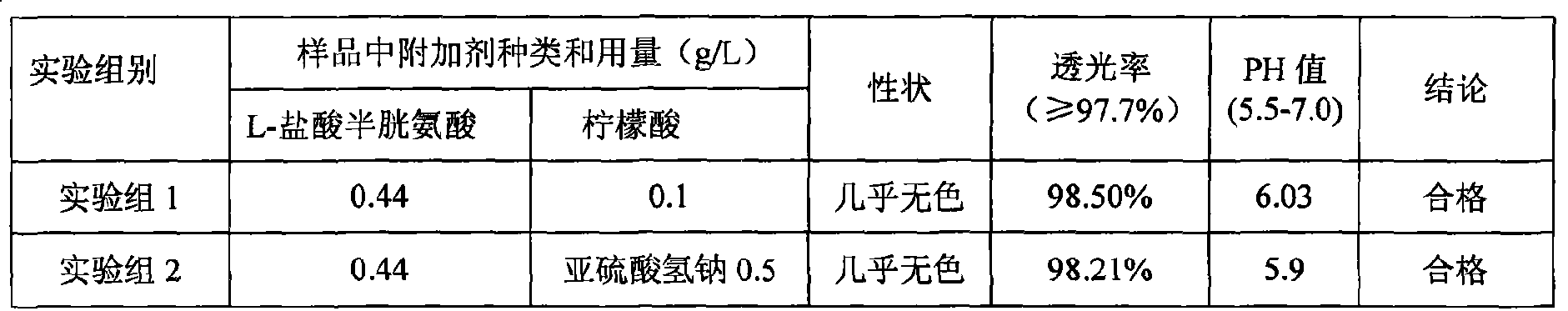
The invention discloses a medicine combination which contains 15 amino acids and the preparation method thereof; the medicine combination is characterized in that a compound amino acid injecta with different concentrations are prepared by the 15 amino acids which serve as raw materials and admixture according to the following parts by weight: 6.1-10.8 parts of L-Isoleucine, 8.8-16.6 parts of L-Leucine, 4.6-10.4 parts of L-Lysine Acetate, 0.8-3.0 parts of L-Methionine, 0.8-3.9 parts of L-Phenylalanine, 1.6-5.4 parts of L-threonine, 0.5-1.1 parts of L-Tryptophan, 6.7-10.7 parts of L-Valine, 3.2-9.3 parts of L-alanine, 4.6-7.2 parts of L-arginine, 1.2-2.9 parts of L-Histidine, 5.0-9.6 parts of L-proline, 2.6-6.0 parts of L-serine, 2.6-10.8 parts of glycin, 0.1-1.0 parts of L-Cysteine hydrochloride, 0.1-0.5 parts of citric acid and moderate water for injection. The injecta does not contain sulphite type chemical inhibitor, thoroughly solves the harm of sulphite type on human body, and ensures that the obtained products are safer. The PH value of the injecta is 5.5-7.0. By the accelerated test and quality test, the results show that the stability of the medicine combination which contains 15 amino acids is the same as or better than like products which contains sulphite type sold on market.
Owner:郑飞雄
Antibrowning agent for litchi and method for storing and preserving litchi
InactiveCN102302056AInhibition of decayGuaranteed qualityFruit and vegetables preservationLychee fruitCysteine Hydrochloride
The invention discloses an antibrowning agent for litchi and a method for storing and preserving litchi. The antibrowning agent for litchi comprises the following components by weight percent: 0.05-2% of cysteine hydrochloride, 0.5-5% of citric acid, 0.05-0.5% of EDTA (ethylenediaminetetraacetic acid) disodium salt and the balance of water, wherein the total weight of the components is 100%. The method for storing and preserving litchi comprises the following steps: harvesting 80-90% matured litchi without mechanical damage and plant diseases and insect pests at sunny days; performing sterilization treatment and antibrowning treatment on the litchi; and precooling, packaging and storing the litchi, wherein the antibrowning treatment is performed in the manner of soaking the litchi in the antibrowning agent for 1-3 minutes. According to the method provided by the invention, the litchi harvested at proper time is subjected to the sterilization treatment and the antibrowning treatment, is precooled, is packaged with a polyethylene film bag and then is stored at the temperature of 2-4 DEG C, thereby effectively inhibiting fruit rottenness and peel browning, maintaining the fruit quality, achieving the intact fruit ratio above 95% after storing the fruit for 30-40 days, excellently controlling browning and substantially being free from change in fruit quality.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
Method for preparing beef essence
InactiveCN101690579AFragrance image authenticity improvedEnhance the sense of cookingFood preparationHydrolysateDL-methionine
The invention relates to a method for preparing beef essence. The method comprises the following steps: taking 0 to 10 percent of glucose, 0 to 3 percent of D-xylose, 0 to 5 percent of glycine, 0 to 5 percent of DL-alanine, 0 to 6 percent of L-cysteine hydrochloride, 0 to 4 percent of L-cysteine, 0 to 4 percent of DL-methionine, 5 to 20 percent of refined butter, 0 to 4 percent of VC, 0 to 6 percent of VB1, 10 to 40 percent of plant protein hydrolysate, 10 to 40 percent of liquid yeast extract, 10 to 40 percent of beef hydrolysate, 0 to 2 percent of anise oleoresin, 0 to 2 percent of cinnamon oleoresin, 0 to 2 percent of wild pepper oleoresin, 0 to 2 percent of fennel oleoresin, and 10 to 30 percent of salt; sequentially adding the materials to a reaction kettle; stirring the materials for 20 minutes at room temperature; heating to raise temperature; performing reaction for 1 to 3 hours at a reaction temperature between 100 and 120 DEG C; naturally cooling to 50 DEG C; passing the obtained product through a 40-mesh vibrating screen; discharging the obtained product and obtaining the beef essence.
Owner:TIANJIN CHUNFA BIO TECH GRP
Method for enhancing washing resistance of nano-silver antibacterial cotton fabric by surface modification of cotton fabric
The invention discloses a method for enhancing washing resistance of nano-silver antibacterial cotton fabric by surface modification of cotton fabric. The method comprises the following steps: Step 1, impregnating cotton fabric into a cysteine hydrochloride solution, heating to carry out an esterification reaction between carboxyl group of cysteine hydrochloride and hydroxyl group of cellulose, cleaning, and drying; and Step 2, impregnating the above modified cotton fabric into nano-silver sol, fixing nano-silver by complexation of mercapto group, drying, and cleaning to finally obtain the antibacterial cotton fabric. Particle size of nano-silver particles obtained is 20-60 nm. Sterilizing rates of escherichia coli and staphylococcus aureus both reach 100%. After 20 times of standard washing, sterilizing rate of escherichia coli and staphylococcus aureus is still maintained at 100%. The method of the invention has high safety to human body and is pollution-free to the environment. By the method, excellent performance of the cotton fabric is maintained. The method has practical application potential in antibacterial modification of cotton fabric.
Owner:ZHEJIANG SCI-TECH UNIV
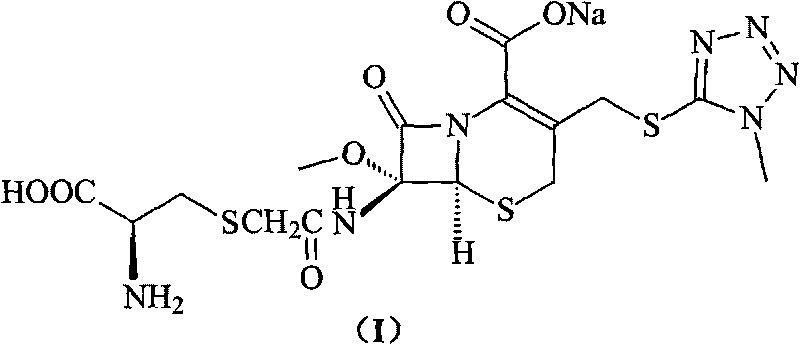
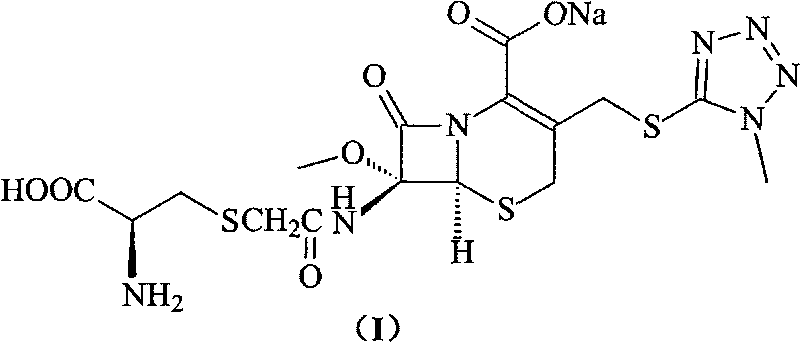
Cefminox sodium compound of new route
The invention provides a cefminox sodium compound of a new route, in particular a method for producing the cefminox sodium compound. The method comprises a step of generating cefminox sodium through the reaction between 7beta-bromoacetamido-7a-methoxyl-3-(1-methyl-1H-tetrazole-5-S-methyl)-3-cephem-4-carboxylic acid and D-cysteine hydrochloride, and is characterized in that: the reaction condition of the step is that the reaction is performed in aqueous solution, sodium iodide is used as a catalyst, the pH value of the reaction system is between 7.5 and 8.0, and the reaction temperature is kept at 30+ / -5 DEG C. Compared with the method for producing the cefminox sodium compound in the prior art, the method of the invention for producing the cefminox sodium compound greatly improves the product yield and the purity, and solves the problem that a cefminox sodium bulk drug always has a low purity in the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of meaty paste essence
The invention relates to a preparation method of meaty paste essence. The preparation method comprises the following steps of: 1). reacting glucose, xylose, pig bone stocks, pig bone oil, hydrolyzed vegetable protein, cysteine hydrochloride, glutamic acid, glycine, salt, cinnamon powder, ginger powder, shallot oil and water to prepare a Maillard reaction product; 2). evenly mixing ethyl maltol, 2-methyl-3-furan mercaptan, cassia oil, star anise oil, 4-methyl-5-(beta hydroxyethyl) thiazole, 2-methyl pyrazine, 2,5-dimethyl-3 (2H) furfuran ketone, difurfuryl sulfide and propylene glycol to obtain meat-flavor base; and 3). mixing the Maillard reaction product, the meat-flavor base, 10% furfuran ketone, the salt, monosodium glutamate, I+G, 10% sodium carboxymethyl cellulose, Tween-80 and water to be homogeneous to obtain the meaty paste essence. The meaty paste essence prepared by the preparation method is coordinative and natural in flavor, mellow in taste, strong in natural feeling and stable in state.
Owner:TIANJIN CHUNFA BIO TECH GRP
Chicken meal and preparation method thereof
The invention relates to chicken meal and a preparation method thereof. The chicken meal consists of the following raw materials in parts by weight: 70 to 90 parts of chicken skeleton, 0.03 to 0.2 part of papain, 10 to 30 parts of water, 0.2 to 2 parts of glucose, 0.1 to 2 parts of D-xylose, 0 to 1 part of glycine, 0 to 1 part of DL-alanine, 0.1 to 0.8 part of L-cysteine hydrochloride, 2 to 5 parts of modified starch, 0.1 to 0.3 part of citric acid, and 0.001 to 0.002 part of butylated hydroxyanisole (BHA). The chicken meal product prepared by the method is natural, the total nitrogen contentof the prepared chicken meal can reach over 7 percent, and the chicken meal has strong cooking feeling, lingering aftertaste and strong heat resistance.
Owner:TIANJIN CHUNFA BIO TECH GRP
Stable and safe edaravone injecta
ActiveCN102144964AImprove stabilityQuality improvementOrganic active ingredientsInorganic non-active ingredientsEdaravone InjectionPhosphate
The invention belongs to the field of pharmaceutical preparations, in particular to an edaravone injecta and a preparation process thereof. The edaravone injecta contains edaravone and pharmaceutically acceptable auxiliary materials and is characterized in that the injecta contains an antioxidant and a pH regulator, wherein the antioxidant contains phosphate, L-cysteine hydrochloride and sodium bisulfite. A whole-process nitrogen-filing and oxide-emitting method and a post-sterilization rapid cooling method are adopted in the preparing and filling process of the edaravone injecta to improve the stability of the edaravone injecta, remarkably reduce the content of a related substance, i.e., dimmer and provide the stable and safe edaravone injecta for clinical administration.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Preparation method of cilastatin sodium
InactiveCN101792410ASolve the pollution problemEasy to solveSulfide preparationSodium hydroxideChrominance
The invention discloses a preparation method of cilastatin sodium, comprising the following steps of: taking 7-chloro-oxo-ethyl oenanthate and (S)-(+)-2, 2-dimethyl cyclopropane formamide as raw materials, and carrying out condensation reaction and basic hydrolysis to obtain (Z)-7-chloro-2((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid; then, adding alkaline compound for reaction, and separating and purifying to obtain (Z)-7-chloro-2((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid metal salt crystal; leading the metal salt crystal and cysteine hydrochloride for condensation reaction, acidizing the reaction liquid by hydrochloric acid, washing by organic solvent and concentrating, then loading sample into macroporous absorption resin for purifying, directly using sodium hydroxide solution for elution, and obtaining cilastatin sodium solid after the treatment of eluent. The preparation method simplifies the preparation technique, and effectively improves the chrominance, the purity and the yield of the cilastatin sodium.
Owner:ZHEJIANG UNIV OF TECH +1
Soo guy chicken processing technique
The invention relates to a soo guy chicken processing technique, belonging to the technical field of deep chicken processing. L-cysteine hydrochloride, D-xylopyranose, thiamin hydrochloride and redistilled water are mixed to prepare an aroma enhancing liquid; 3 g of fructus amomi, 18 g of galanga, 18 g of radix angelicae, 1 g of clove, 6g of tsaoko amomum fruit, 18g of cinnamon, 6 g of dried tangerine peel and 3 g of cardamom are wrapped by gauze and then mixed with 250 g of edible salt, 10 g of sodium glutamate to prepare a curing liquid. The processing technique comprises the steps of thawing, cleaning, leaching, curing, shaping, three-sectional drying (drying I, drying II and drying III), vacuum packaging, sterilization, cooling, checking and product finishing. The processing technique can effectively improve the color and the taste of a chicken product, obviously reduce the generation of cancerogenic substances, i.e. naphtho-(alpha)pyrene, and improve the safety, sanitation and eating quality of the chicken product, and is beneficial to improving the economic benefit of relative enterprises and quickening the development of poulard raising industry in China.
Owner:NANJING AGRICULTURAL UNIVERSITY
Peptide-rich flavour development based material and preparation method
The invention discloses a seasoning material rich in peptide, the composition and mass percent of which are: water: 20-65; protein sources raw material: 15-50; reducing sugar: 1-10; L-cysteine Hydrochloride: 1-5; Disodium 5'-ribonucleotide: 0.1-2.5; sauce: 2-15; hydrolyzed vegetable protein: 2-15; 5-disodium inosinate: 0.6-3.8; cane sugar: 2-18; salt: 2-20; monosodium glutamate: 2-15; grease: 2-15; excipient: 5-20; spice:0.5-2; and edible spice: 0.2-1.5. The invention also discloses a method for preparing the seasoning material rich in peptide. The method consists of the steps of enzymolysis, filtering, dissolvation, pH adjusting, reaction, preparation, drying, crushing and the like. The seasoning material rich in peptide has long-lasting flavor and heavy aftertaste, is easy to use and can be applied to instant noodles, snack foods, complex condiment, meat products and the like.
Owner:广东汇香源生物科技股份有限公司
Formulation of l-cysteine hydrochloride amenable to terminal sterilization
InactiveUS20190247307A1Amenable to terminal sterilizationOrganic active ingredientsInorganic non-active ingredientsCysteine HydrochloridePharmacology
Stable formulation of L-cysteine hydrochloride amenable to terminal sterilization; a terminally sterilized stable formulation of L-cysteine hydrochloride; a process of manufacturing a stable formulation of L-cysteine hydrochloride amenable to terminal sterilization; and a container comprising the formulations of the invention.
Owner:AL PHARMA INC
Preparation of injecting soluble vitamines
InactiveCN1939333ARaise storage temperatureImprove antioxidant capacityOrganic active ingredientsMetabolism disorderGlycineVitamin C
A process for preparing the injection of water-soluble vitamin includes such steps as thermally dissolving glycine in the water for injection while filling inertial gas, adding 12 compounds including cysteine hydrochloride, endrate disodium, vitamin C sodium, folic acid, etc, stirring, regulating pH=5.6-6.1, adding the water for injection, stirring, aseptic filtering, pouring in containers, pre-freezing, vacuumizing, filling inertial gas, pre-freezing, heating to 30-35 deg.C, holding the temp for 6-10 hr, filling inertial gas, and sealing.
Owner:YAOPHARMA CO LTD
Cake powder quality improver and preparation method thereof
ActiveCN101485346AResistance useIncrease health functionDough treatmentBaking processesAmylaseCellulose
The invention relates to an agent for improving the quality of cake mix and a preparation method thereof. The agent for improving the quality of the cake mix comprises the following compositions in portion by weight: 0.6 to 5.5 portions of stearic propylene glycol ester, 1.5 to 6 portions of CSL / SSL, 0.02 to 0.6 portion of L-cysteine hydrochloride, 0.13 to 0.25 portion of lipase, 0.02 to 0.15 portion of alpha-amylase, 0.005 to 0.025 portion of neutral protease, 14.4 to 29 portions of mono / di-glyceride, 2.6 to 6 portions of gelatin, 6 to 30 portions of insoluble cellulose (CMC) and 37 to 56.3 portions of anticaking agent. The preparation method is to mix various raw materials in a cone-type mixer for 20 to 30 minutes according to proportion, and obtain finished products of the agent for improving the quality of the cake mix. The invention makes an emulsifier have better complementary relation with sensitive enzyme in the aspect of stabilizing the quality of flour, and fully reveals the synergistic reaction of the emulsifier and enzyme preparation.
Owner:中山市南方新元食品生物工程有限公司
High-intensity culture method for lactobacilli
InactiveCN101967454AProlong logarithmic phaseHigh activityBacteriaMicroorganism based processesHigh concentrationMetabolite
The invention relates to a high-intensity culture method for lactobacilli. The method comprises the following steps of: adjusting the initial pH value to be between 7.0 and 7.2, wherein lactobacillus acidophilus, lactobacillus fermentium and enterococcus faecalis are taken as strains, a special lactobacillus culture medium is used and a basic formula comprises the following component in percentage by weight: 1 percent of soy peptone, 1 percent of yeast leaching powder, 1 percent of glucose, 4 percent of inorganic salt solution and 0.05 percent of cysteine hydrochloride; and feeding Na2CO3, KOH, NaOH or ammonia water in a culturing process so as to stabilize the pH value of culture fluid between 6.0 and 6.8. Suppression of a self-metabolite on the growth of the lactobacilli is released, high-activity and high-concentration lactobacillus culture fluid is obtained and the viable count of the lactobacilli is up to 3.0 to 5.0*10<12> cfu / g by centrifuging and drying.
Owner:上海明博生物科技有限公司
Natural beef flavor
InactiveCN101669617AFragrance with high authenticityStrong sense of cookingFood preparationFennel OilFood flavor
The invention relates to natural beef flavor which is prepared by the following method: tanking 0-10% of glucose, 0-3% of D-wood sugar, 0-5% of glycocoll, 0-5% of DL- alanine, 0-6% of L- cysteine hydrochloride, 0-4% of sulfo-aminolactic acid, 0-4% of DL- methionine, 5-20% of refining beef tallow, 0-4% of VC, 0-6% of VB1, 10-40% of vegetable protein hydrolyzate, 10-40% of liquid state yeast extract, 10-40% of beef hydrolyzate, 0-2% of anise oil resin, 0-2% of cinnamon oil resin, 0-2% of pepper oil resin, 0-2% of fennel oil resin, and 10-30% of table salt which are added into a reactor in sequence and stirred in the room temperature for 20 minutes, heated up and keeps warm in the temperature of 100-120 DEG C for 1-3 hours, naturally cools down to 50 DEG C, is sieved with a vibrating screen with 40 meshes and discharged to obtain the beef flavor.
Owner:TIANJIN CHUNFA BIO TECH GRP
Powder injection of compound glycyrrhizic acid glycosides and preparation method thereof
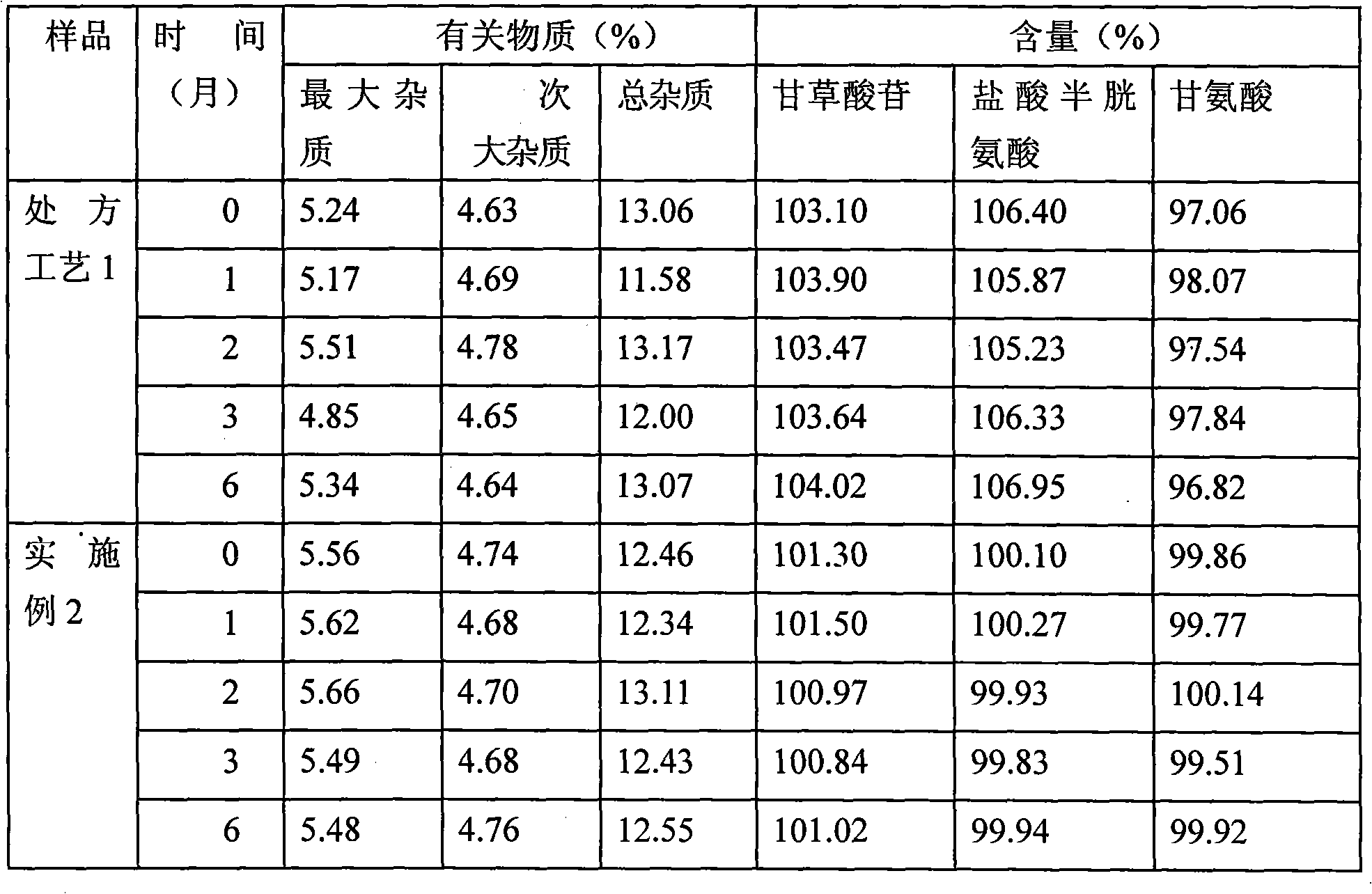
The present invention relates to compound glycyrrhizin type powder injection and the preparation method thereof, in particular to compound glycyrrhizin for injection, compound glycyrrhizic acid mono-ammonium S powder injection and the preparation method thereof, which are characterized in that the powder injection consisting of glycyrrhizin (or mono-ammonium glycyrrhizinate), glycine and cysteine hydrochloride that are taken as the active ingredient and the bearer acceptable in medicine and the preparation method are included in the prescription; wherein, the bearer acceptable in medicine contains dextran. The compound glycyrrhizin type powder injection of the present invention can be preserved at room temperature, thus remarkably improving the stability of the medicine, better guaranteeing safety and significance of the medicine and effectively decreasing the storage and transportation cost in production and transportation processes of the medicine.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
Compound monoammonium glycyrrhizinate S pharmaceutical composition and method for preparing high-capacity injection
The invention belongs to the technical field of pharmaceutical preparations, in particular to a compound monoammonium glycyrrhizinate S pharmaceutical composition and a method for preparing a high-capacity injection. The composition is a compound monoammonium glycyrrhizinate S sodium chloride injection. The injection is characterized by comprising the following components: 40-160g of monoammonium glycyrrhizinate S, 30-120g of cysteine hydrochloride, 400-1,600g of glycine, 40-160g of anhydrous sodium sulphite, 4-16g of sodium citrate, 900-1,800g of sodium chloride, a proper amount of sodium hydroxide and 100-200L of water for injection. The high-capacity injection is more stable and safer.
Owner:九瑞健康股份有限公司
Preparation method of cefminox sodium
The invention discloses a preparation method of cefminox sodium, which comprises the following steps: 7 beta-bromoacetamide-7 alpha-methoxy-3-(1-methyl-1H-5-tetrazyl)sulfur methyl-3- cephem-4-carboxylic acid and D-cysteine hydrochloride are dissolved in water, the pH value is regulated to 6.0-7.0 by sodium bicarbonate, condensation reaction is carried out, and reaction products are post-treated to obtain the cefminox sodium. In the method, cefminox sodium raw material can be prepared through low-temperature reaction, a nonpolar macroporous resin X5 chromatography column is used for purification, ethanol-aqueous solution or anhydrous alcohol recrystallization and other simple operations are adopted to obtain target products, the yield and the purity of the target products are high, the products have uniform crystal forms and good fluidity, no special equipment is needed for the production, and the method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com