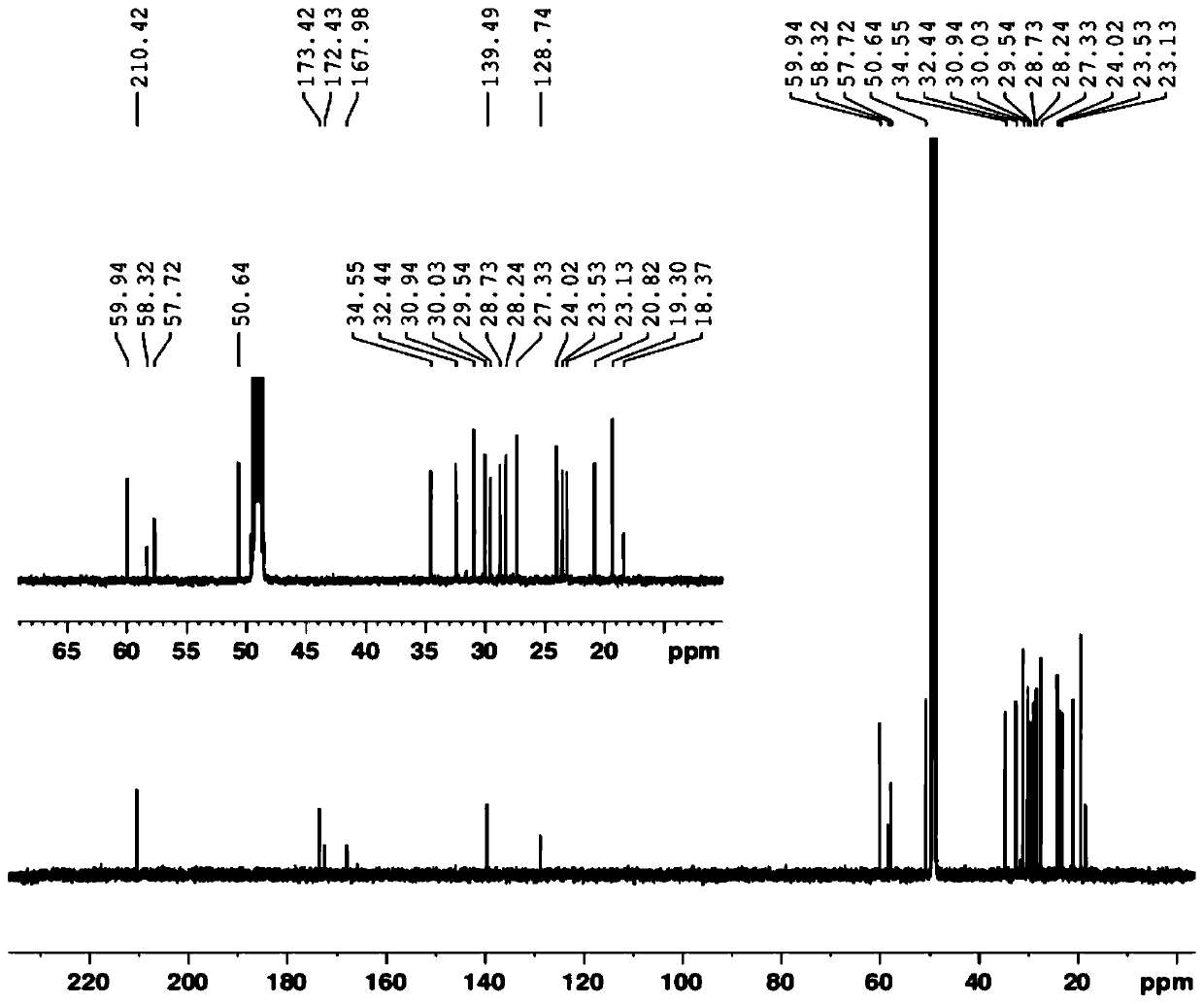
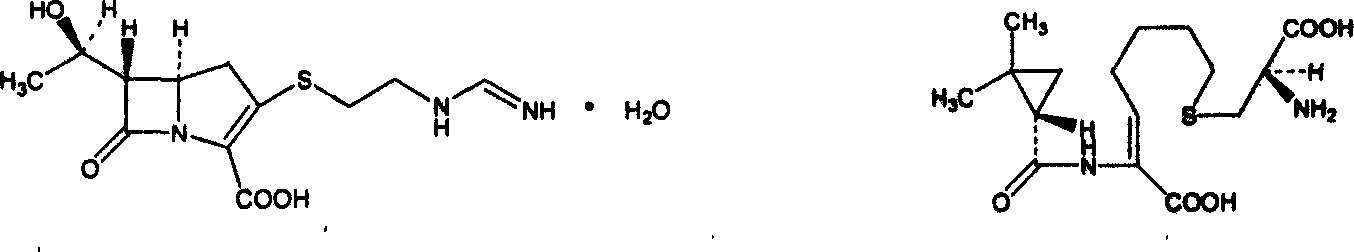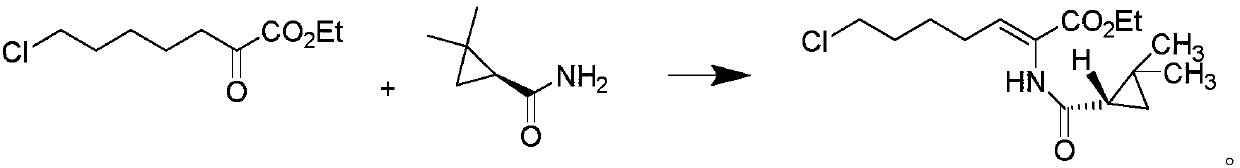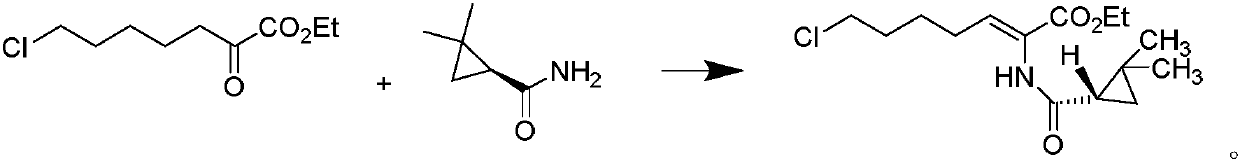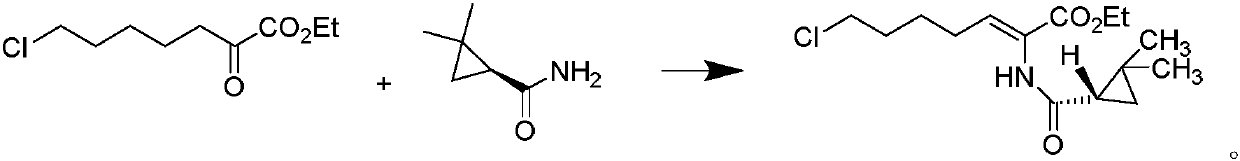Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
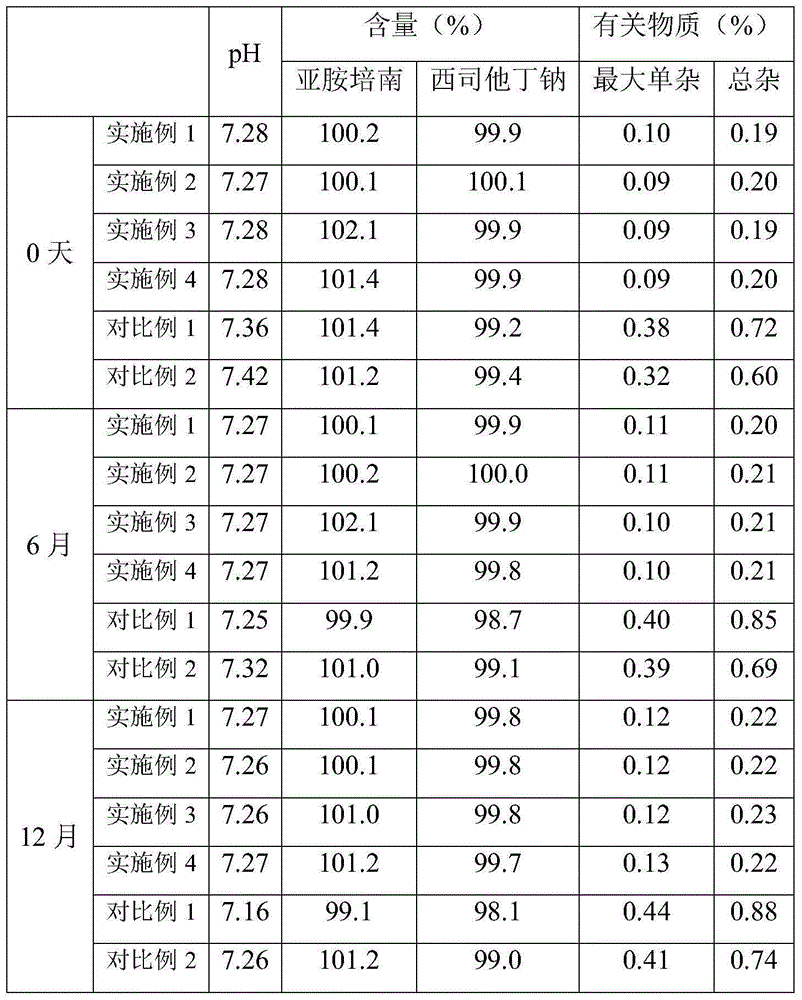
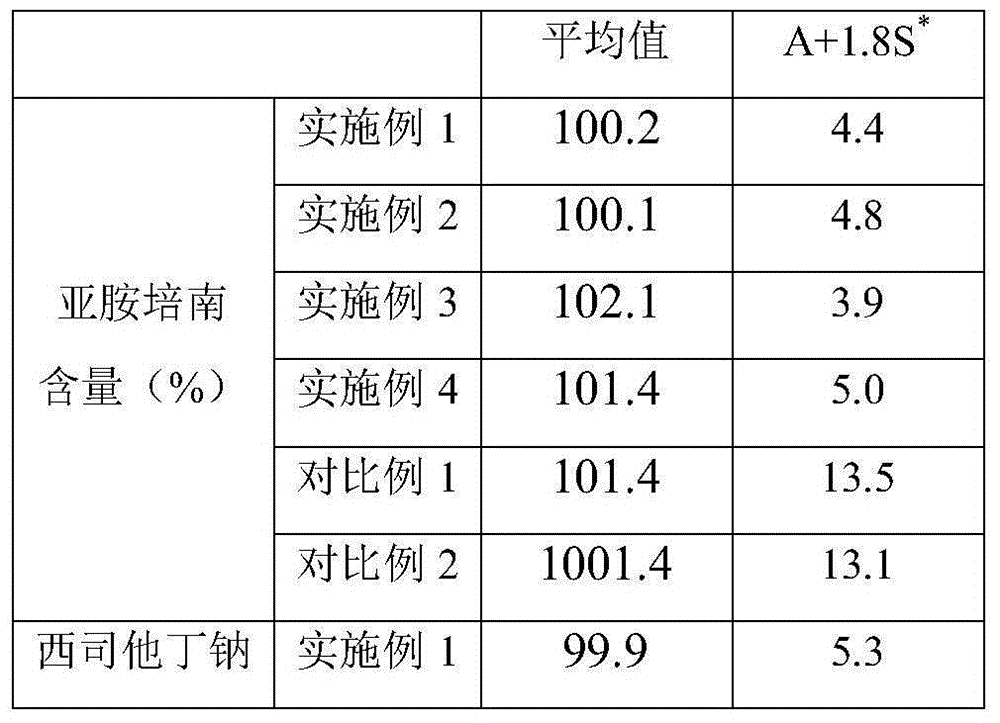
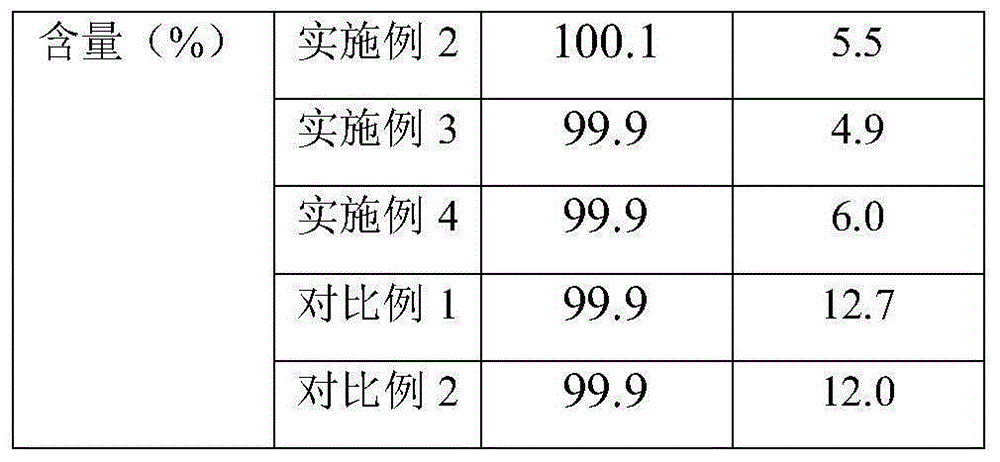
34 results about "Cilastatin sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
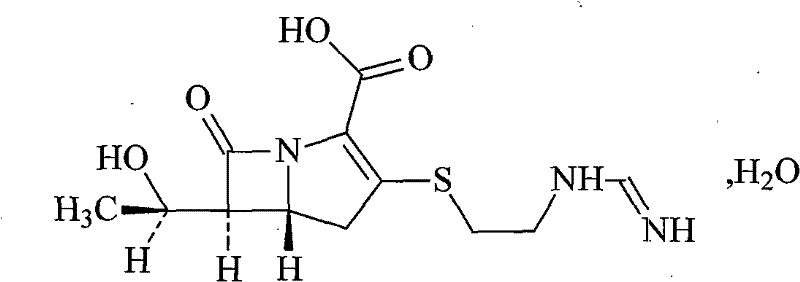
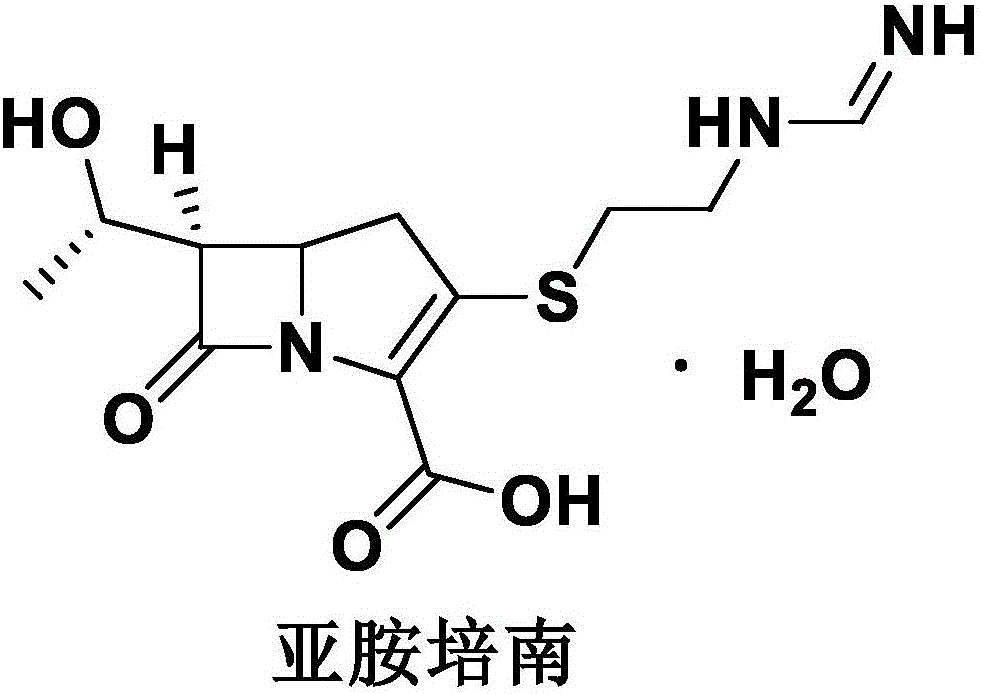
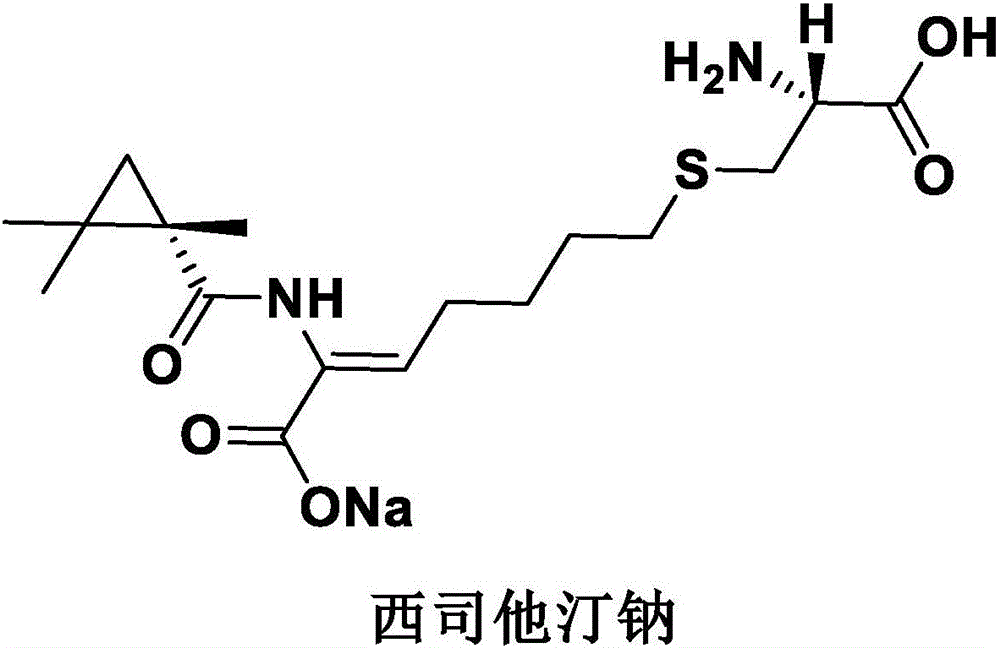
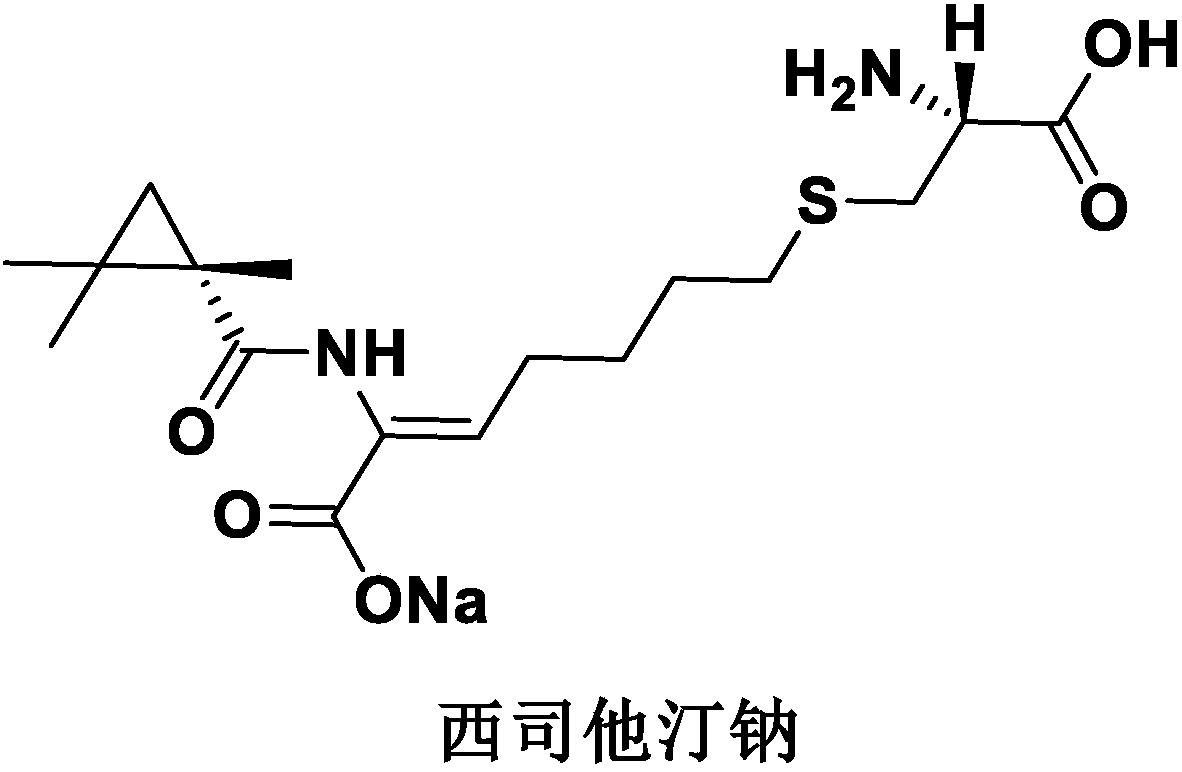
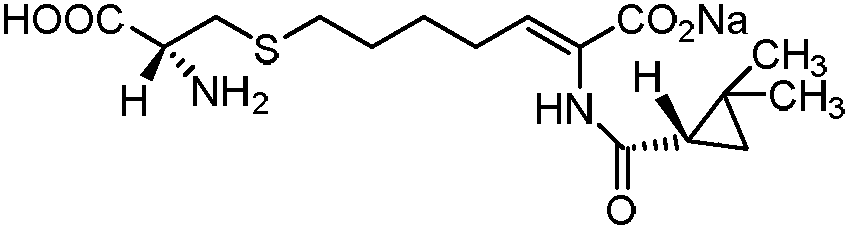
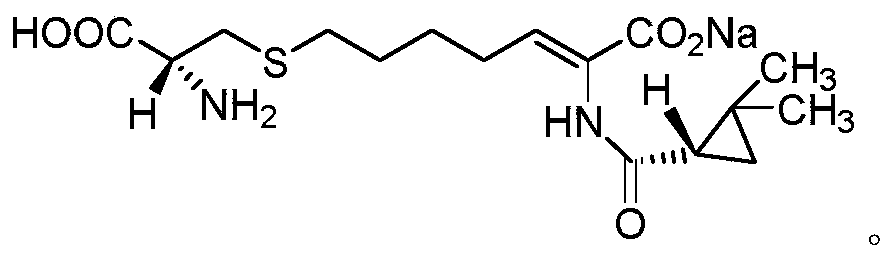
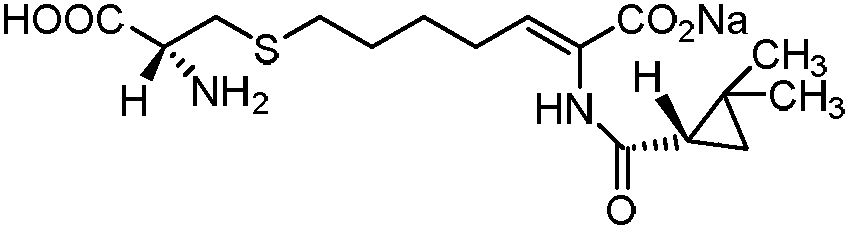
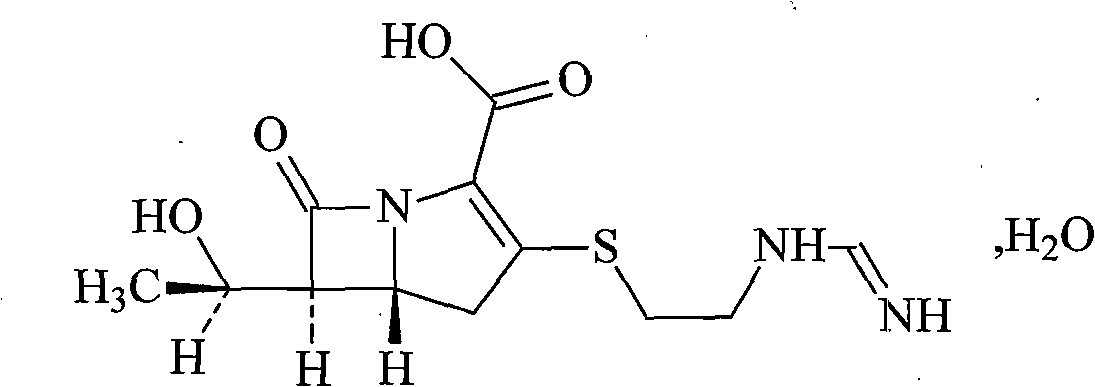
Cilastatin sodium is a renal dehydropeptidase-I and leukotriene D4 dipeptidase inhibitor. Since the antibiotic, IMIPENEM, is hydrolyzed by dehydropeptidase-I, which resides in the brush border of the renal tubule, cilastatin is administered with imipenem to increase its effectiveness.
Process for preparing cilastatin sodium
ActiveCN101307015AAvoid generatingReduce generationOrganic compound preparationSulfide preparationCilastatin sodiumPhotochemistry
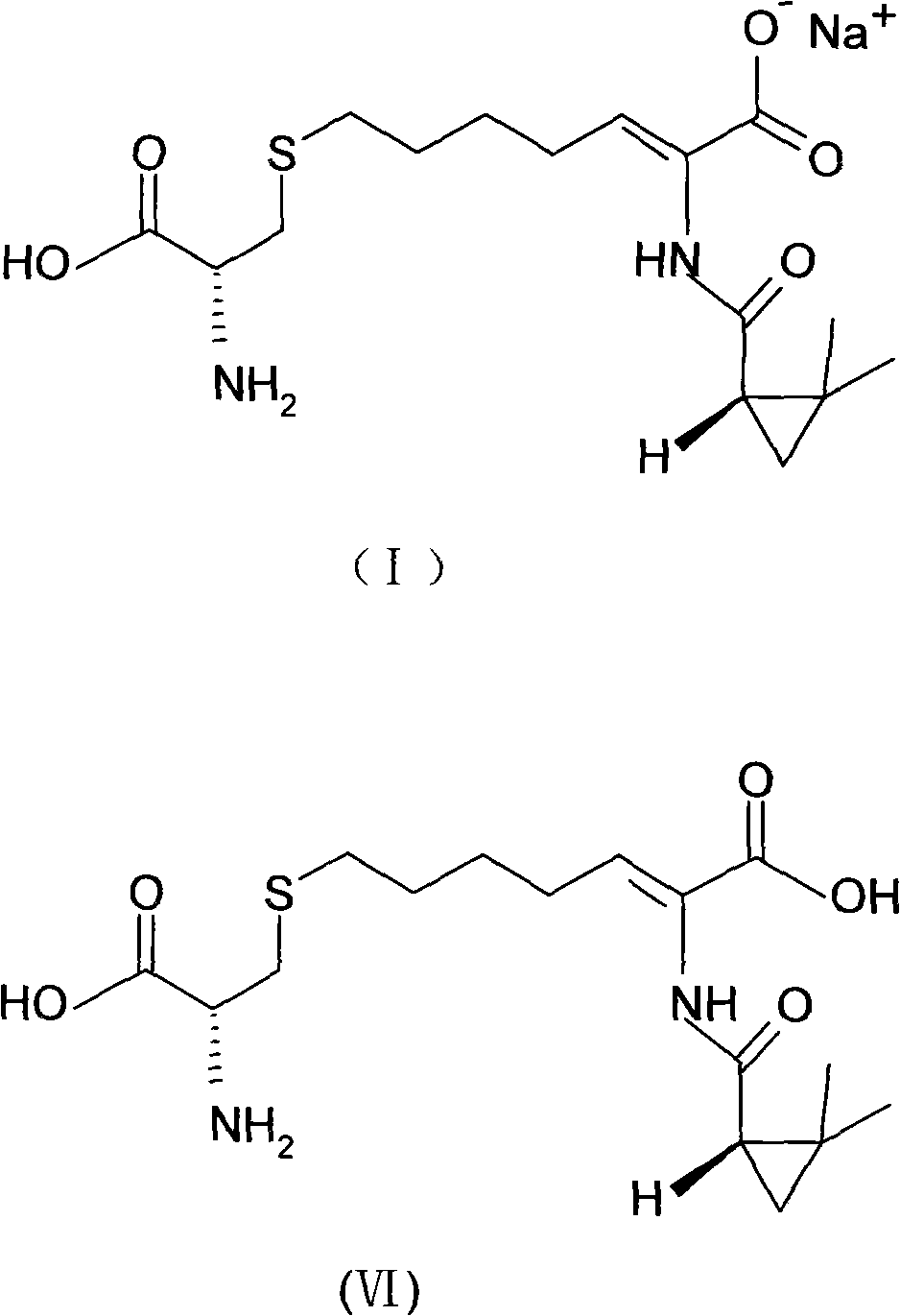
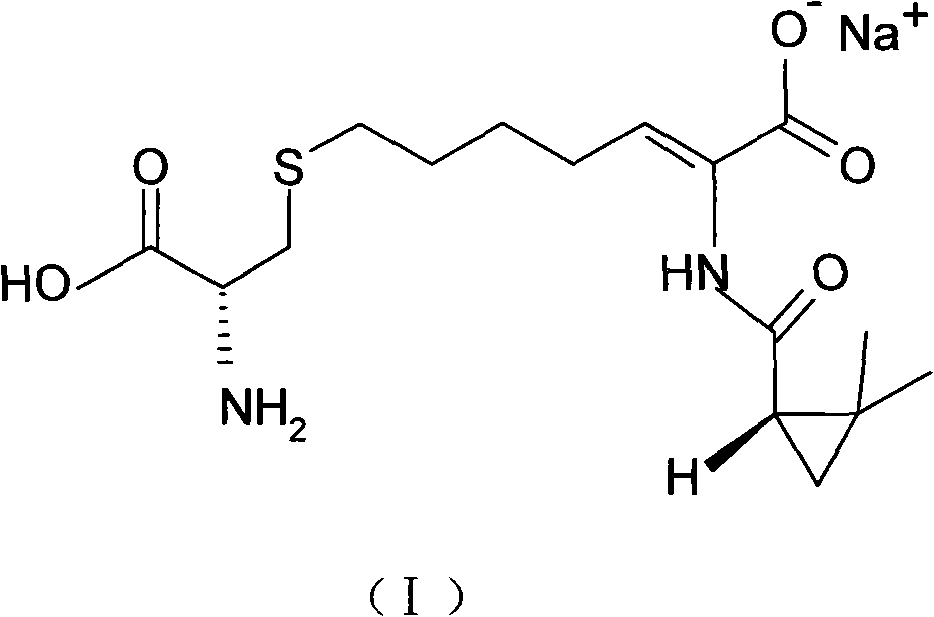
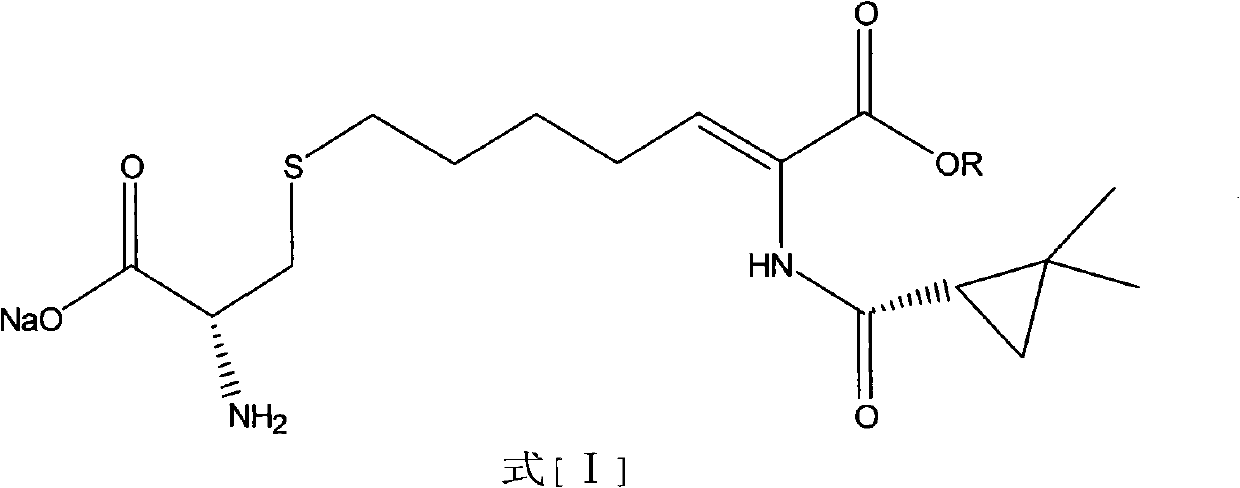
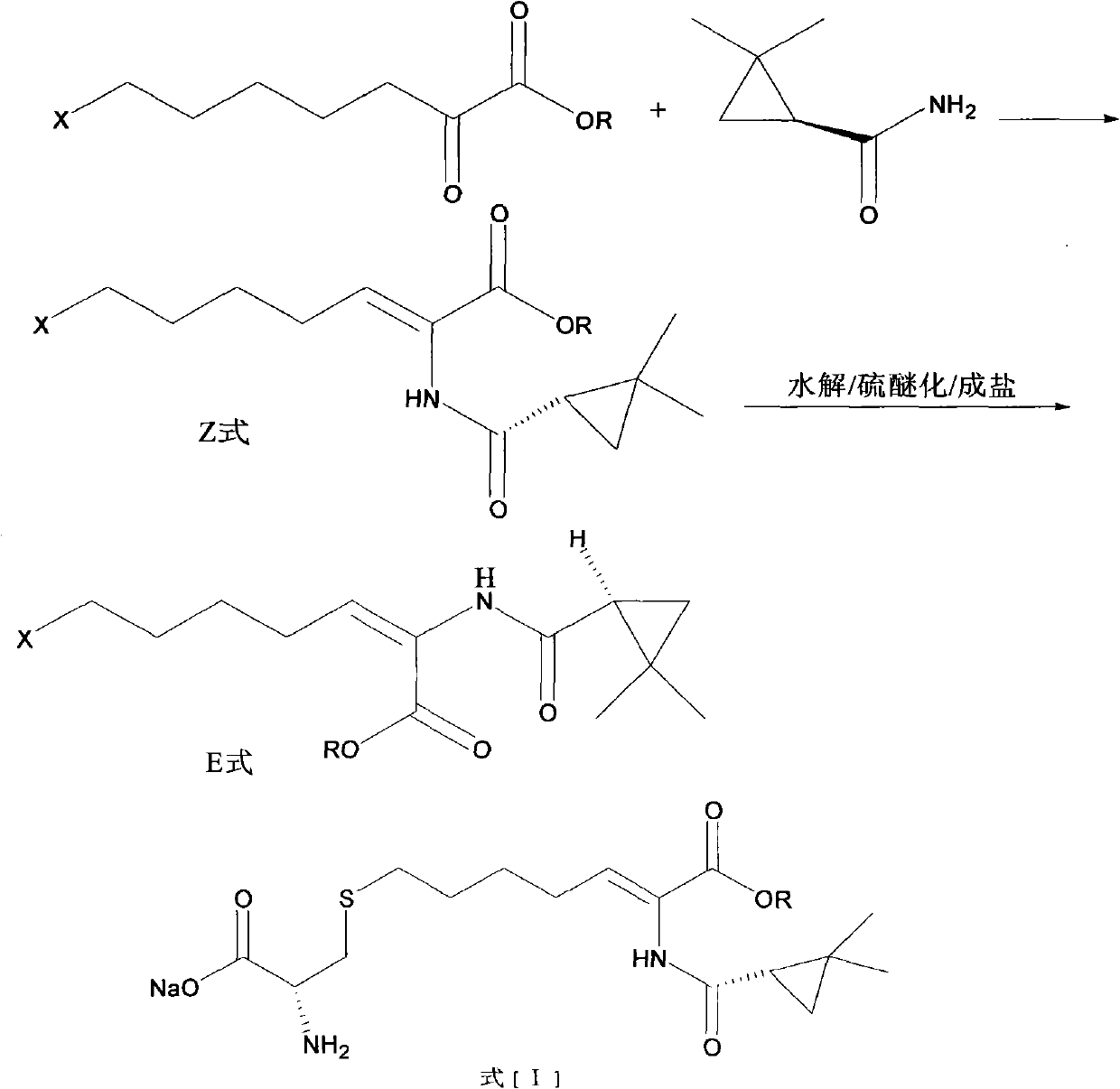
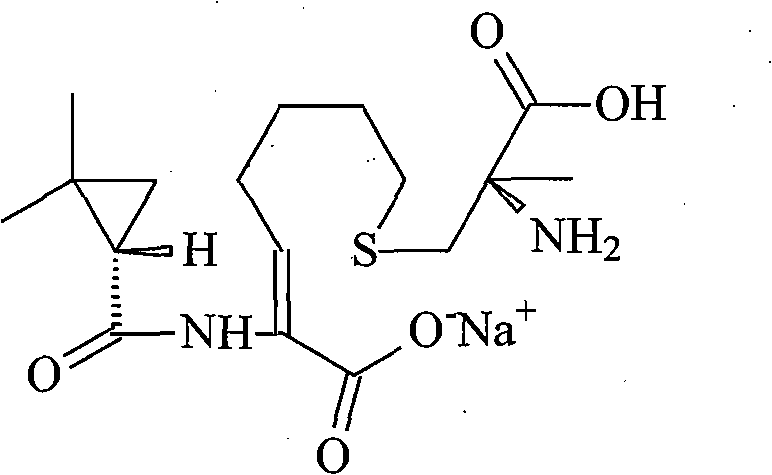
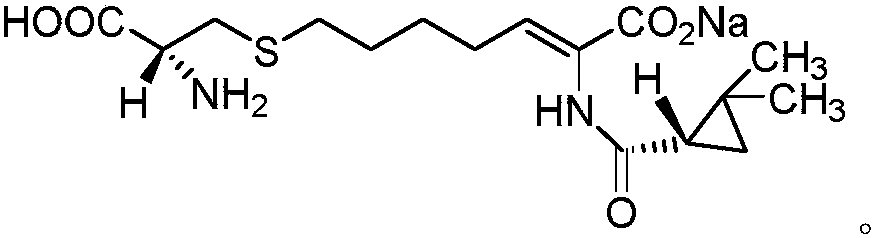
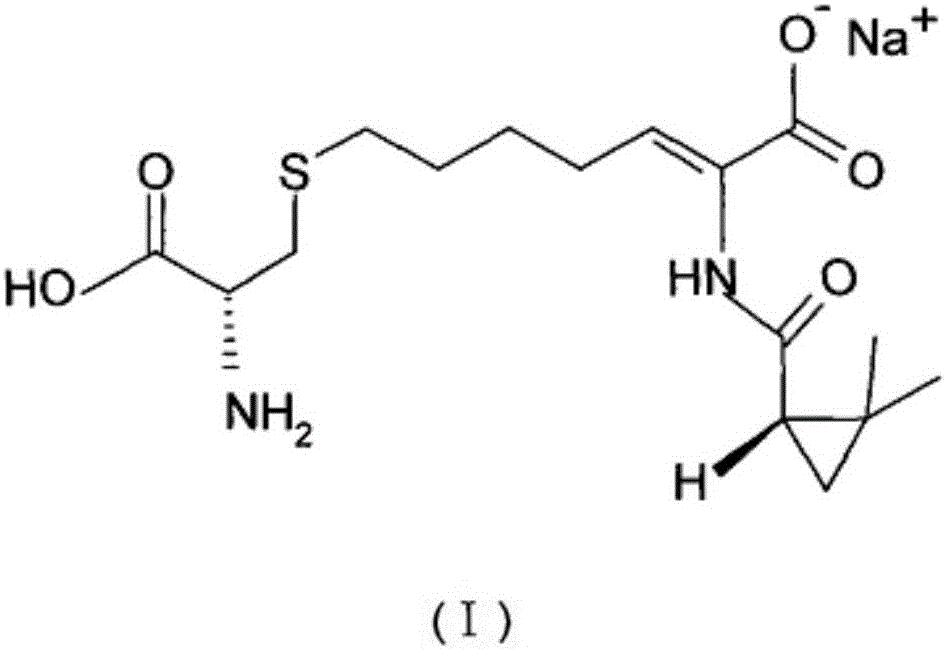
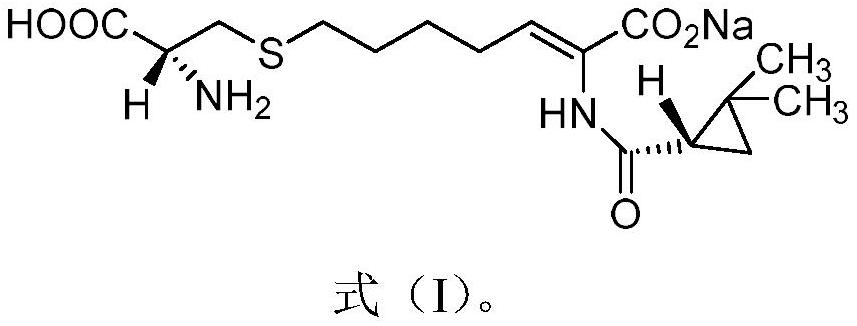
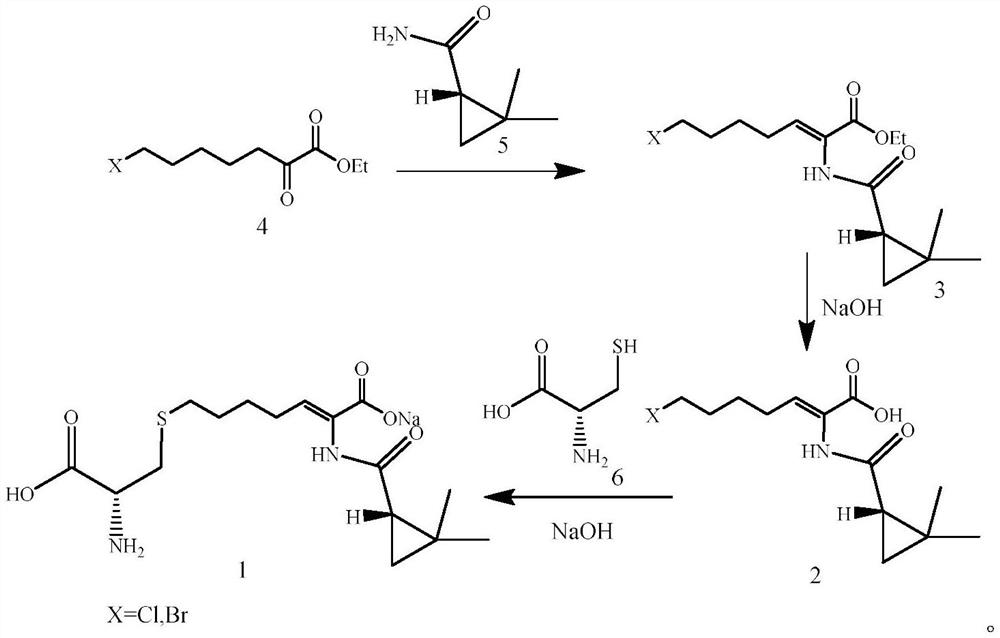
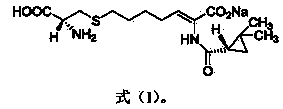
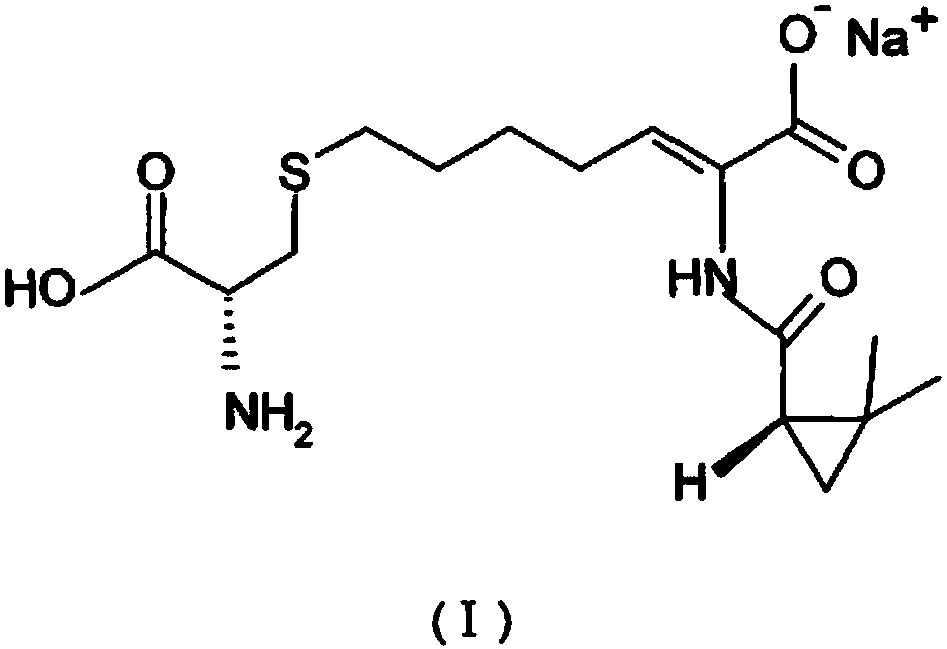
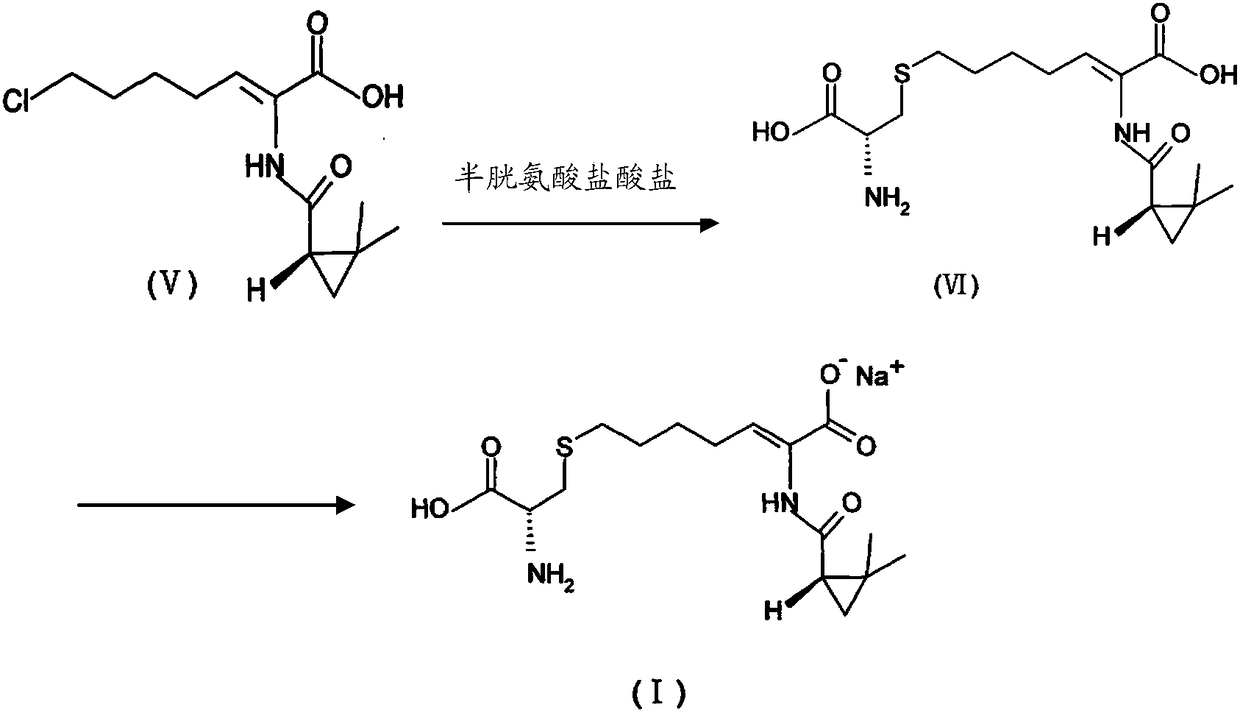
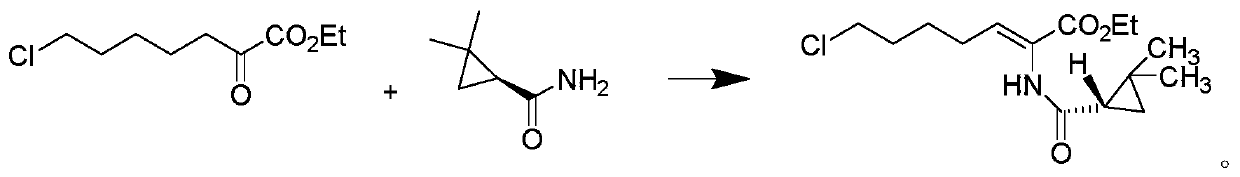
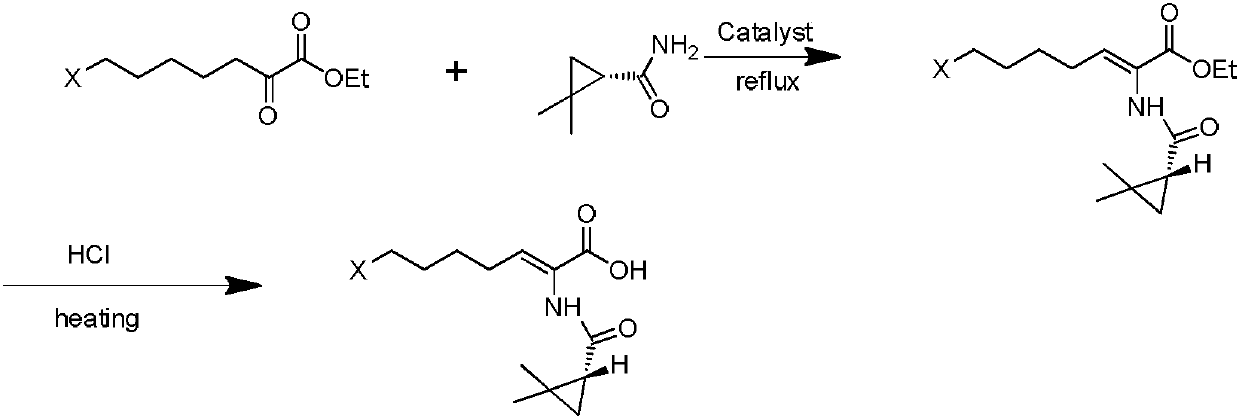
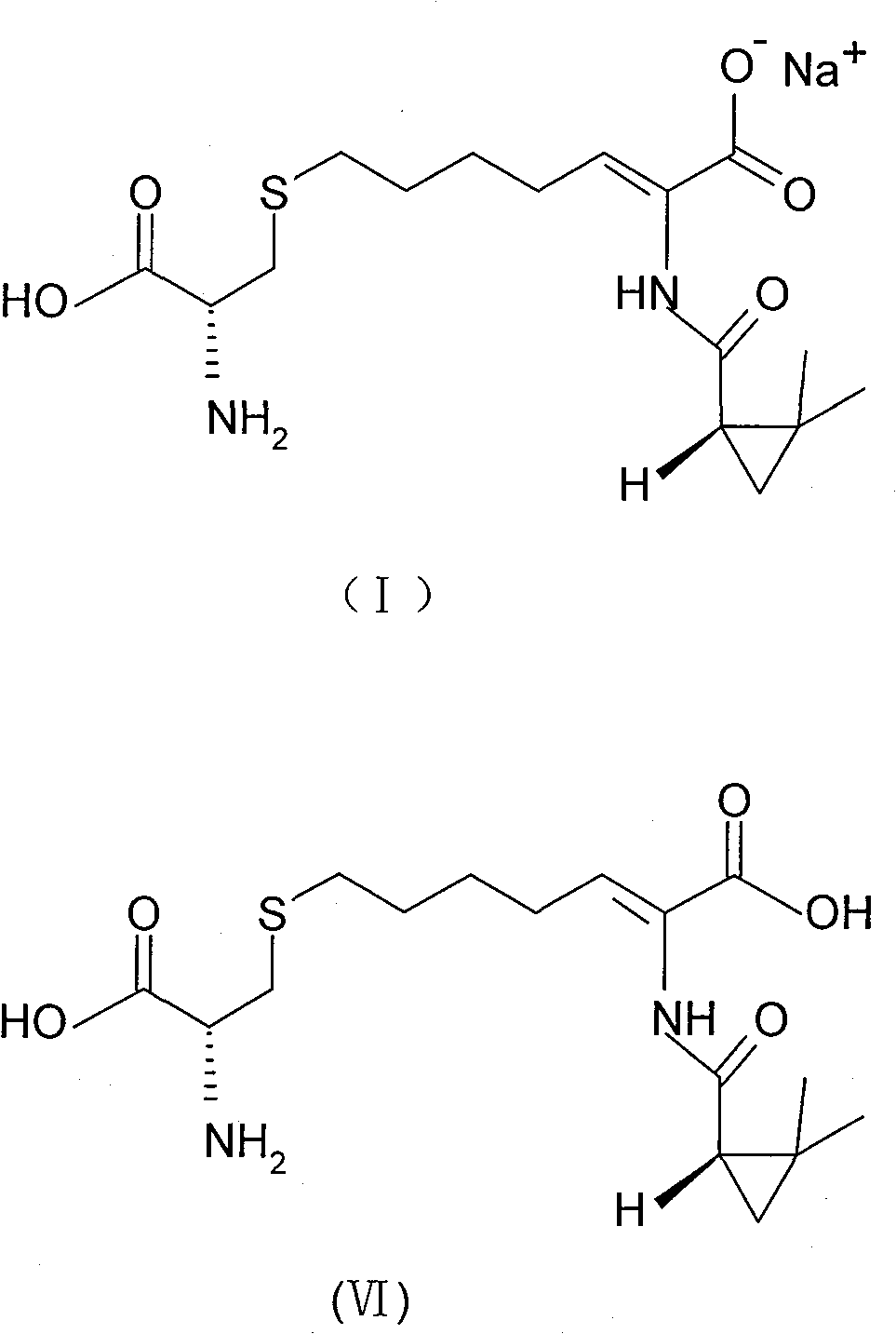
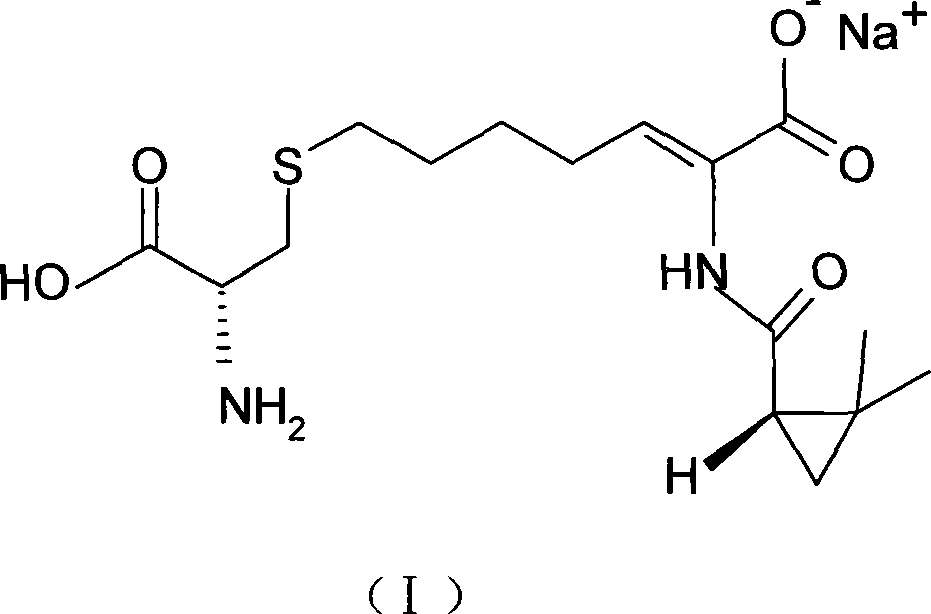
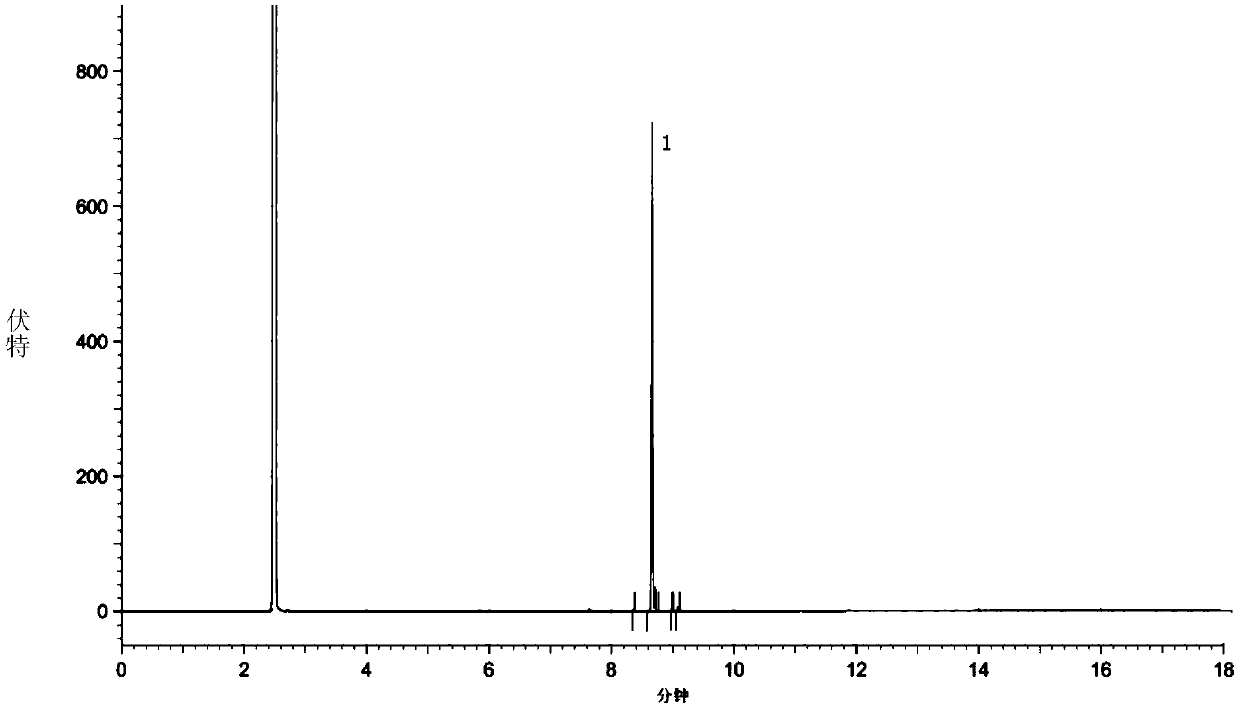
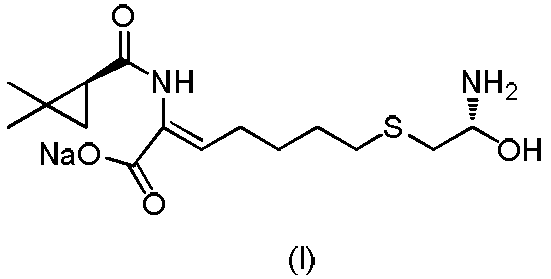
The invention relates to a method for preparing a compound called cilastatin sodium shown as a formula (I). Cilastatin is prepared by crystallizing and purifying (Z)-7-chlorine-2((S)-2, 2-dimethyl c-pr carbamoyl)-2-heptenoic acid and the cilastatin sodium is prepared by purifying the cilastatin through microporous resin, thereby improving the purity quotient and the yield coefficient of the cilastatin sodium.
Owner:SHENZHEN HAIBIN PHARMA
Preparation method of cilastatin sodium
InactiveCN101792410ASolve the pollution problemEasy to solveSulfide preparationSodium hydroxideChrominance
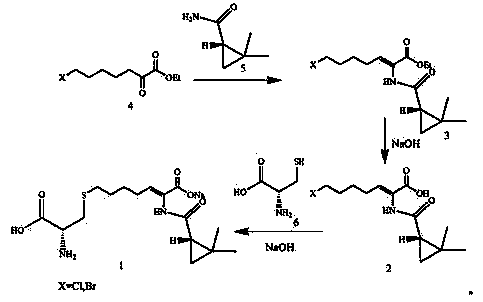
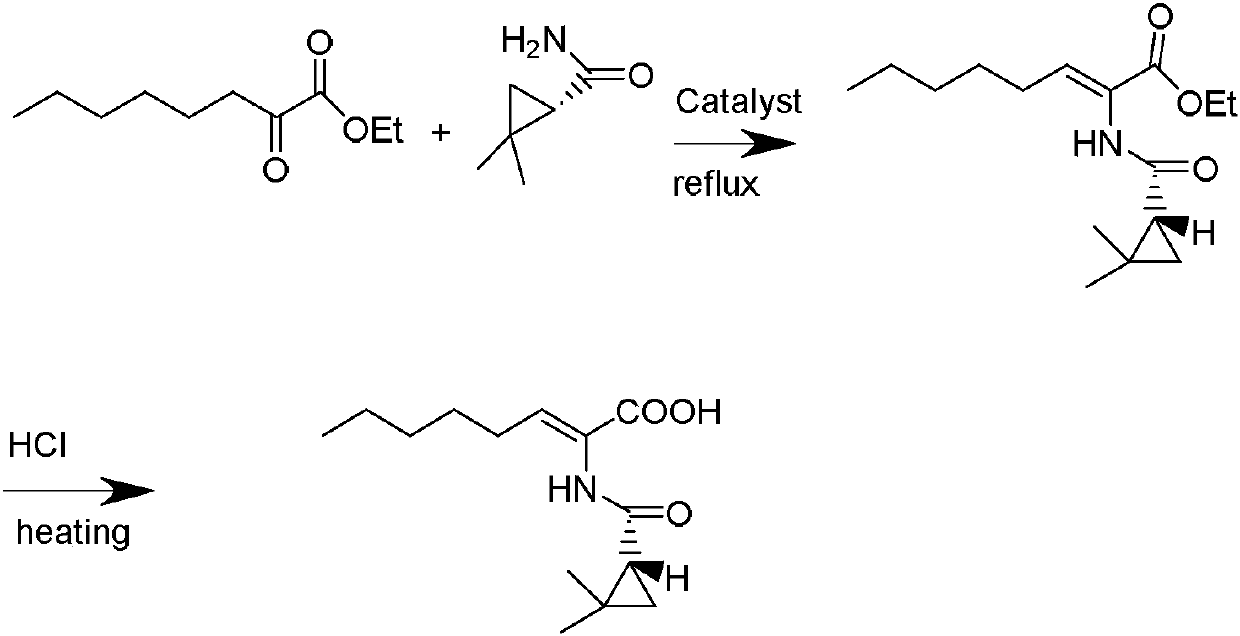
The invention discloses a preparation method of cilastatin sodium, comprising the following steps of: taking 7-chloro-oxo-ethyl oenanthate and (S)-(+)-2, 2-dimethyl cyclopropane formamide as raw materials, and carrying out condensation reaction and basic hydrolysis to obtain (Z)-7-chloro-2((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid; then, adding alkaline compound for reaction, and separating and purifying to obtain (Z)-7-chloro-2((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid metal salt crystal; leading the metal salt crystal and cysteine hydrochloride for condensation reaction, acidizing the reaction liquid by hydrochloric acid, washing by organic solvent and concentrating, then loading sample into macroporous absorption resin for purifying, directly using sodium hydroxide solution for elution, and obtaining cilastatin sodium solid after the treatment of eluent. The preparation method simplifies the preparation technique, and effectively improves the chrominance, the purity and the yield of the cilastatin sodium.
Owner:ZHEJIANG UNIV OF TECH +1
Preparation method of cilastatin sodium
ActiveCN102702051AReduce generationHigh yieldOrganic compound preparationSulfide preparationIsomerizationCilastatin sodium
The invention belongs to the field of pharmaceutical synthesis, and provides a preparation method of cilastatin sodium, and the method comprises the following steps: performing condensation, alkaline hydrolysis, and thioetherification of raw materials of 7-oxyhalogen alkyl heptylate and (s)-2,2-dimethylcyclopropane methanamide, adjusting the pH value of the thioetherified solution, washing by a nonpolar solvent, performing isomerization, purification by a neutral macroporous adsorption resin, and salt formation so as to obtain the cilastatin sodium solid. The invention reduces the generation of impurities, improves the isomerization efficiency, simplifies the preparation process, and effectively improves the yield and purity of cilastatin sodium.
Owner:SHANDONG NEWTIME PHARMA
Pharmaceutical composition containing imipenem cilastatin sodium and preparation thereof
ActiveCN104095847AHigh antibacterial activityImprove stabilityAntibacterial agentsOrganic active ingredientsCilastatin sodiumAdverse effect
The invention relates to a pharmaceutical composition containing imipenem cilastatin sodium and preparation thereof, belongs to the field of medicine and aims at overcoming the technical shortcoming that an imipenem cilastatin sodium powder injection in the prior art causes large adverse effect on the centre. The invention provides a powder injection containing the imipenem cilastatin sodium through reasonable compatibility. The powder injection can effectively reduce the adverse effect on the centre of an imipenem cilastatin sodium compound preparation when being used for clinic antibacterial treatment, the stability of the re-dissolved powder injection can be remarkably improved, and therefore, the pharmaceutical composition is suitable for development of clinic treatment medicine.
Owner:CHINA NAT MEDICINES GUORUI PHARMA
Preparing method of freeze-dried antibiotic formulation
InactiveCN1568975AEffective for severe infectionsNot easily oxidizedAntibacterial agentsOrganic active ingredientsImipenem/cilastatinFreeze-drying
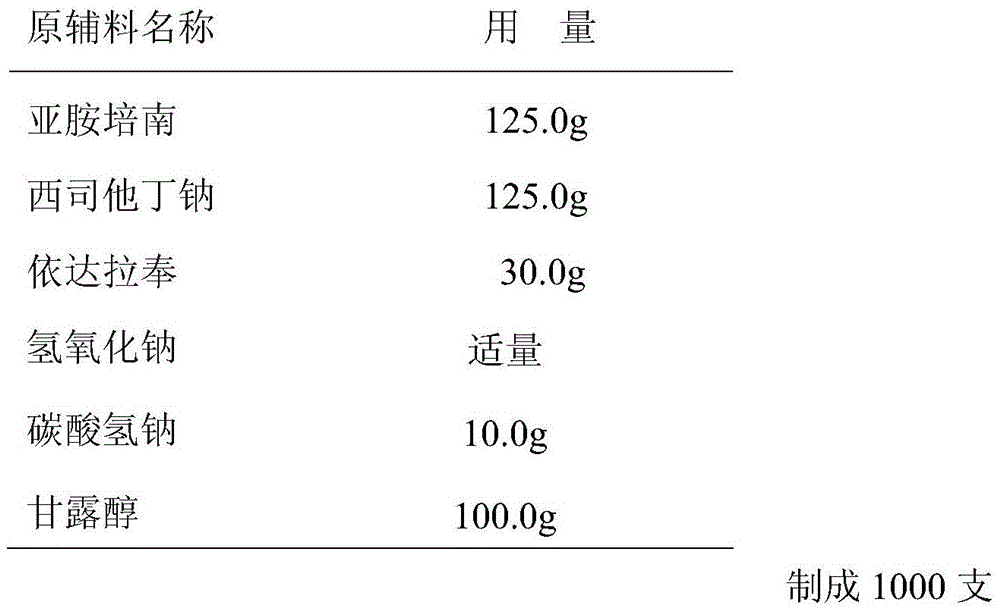
The invention dislcloses the process for preparing Imipenem / Cilastatin Sodiun cryodesiccation powder preparation, which is prepared from imipenem, Cilastatin Sodium or the mixture of imipenem and Cilastatin Sodium.
Owner:济南久创化学有限责任公司
Method for synthesizing cilastatin sodium
The invention discloses a method for synthesizing cilastatin sodium, comprising the following steps: preparing (Z)-7-iodine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester by reaction of (Z)-7-chlorine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester and sodium iodide; enabling (Z)-7-iodine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester, cysteine alkyl ester hydrochloride, alkali and a solvent to react to obtain cilastatin dialkyl ester; and enabling the cilastatin dialkyl ester and sodium hydroxide to react to obtain cilastatin sodium. The alkali used in the method can be one of or more of K2HPO4, CsCO3 and K3PO4, thereby avoiding the usage of strong alkali and an anhydrous reaction system and realizing mild reaction and easy operation. The method has the advantages of good economical efficiency, mild reaction conditions, high yield, little three wastes (waste gas, waste water and industrial residue) and no pollution and can be used for industrialization production. The product can be separated easily and has high purity.
Owner:ZHEJIANG NORMAL UNIVERSITY
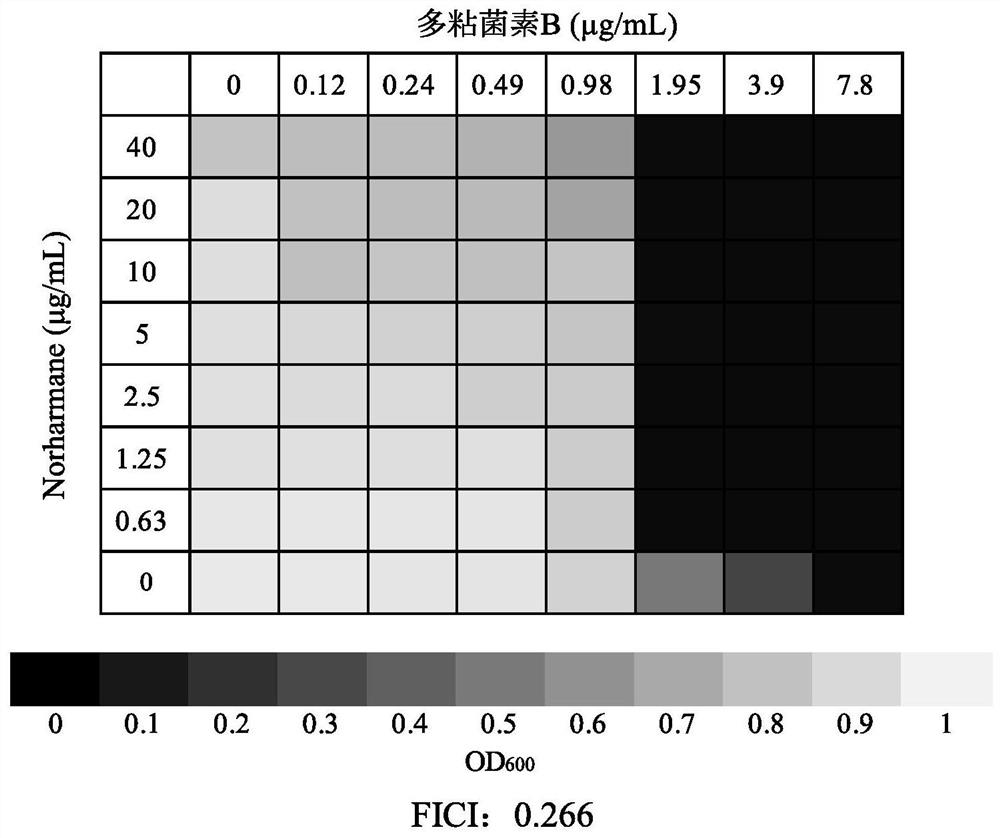
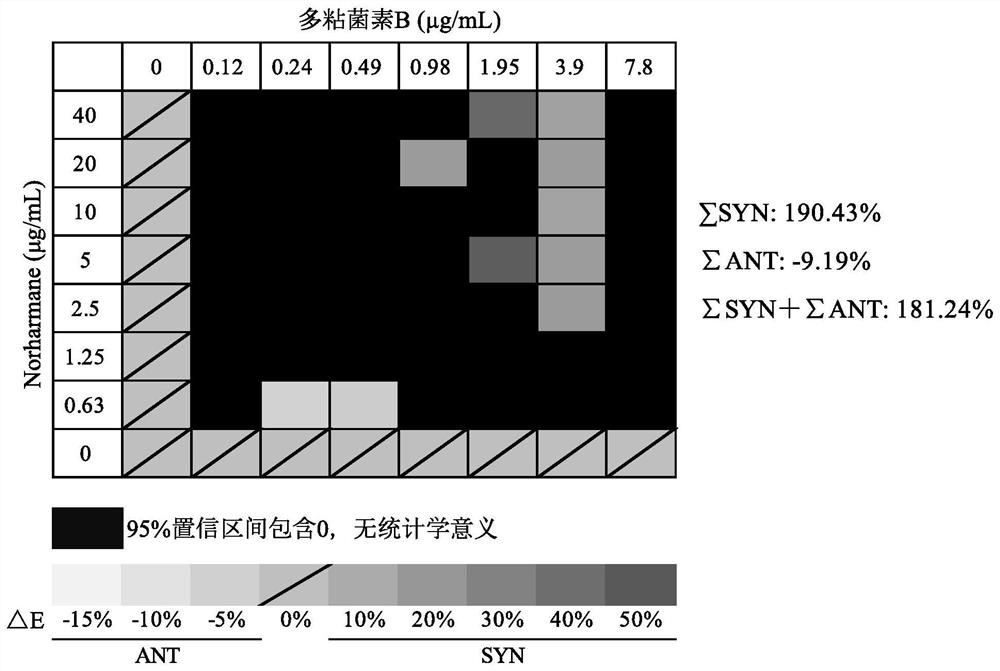
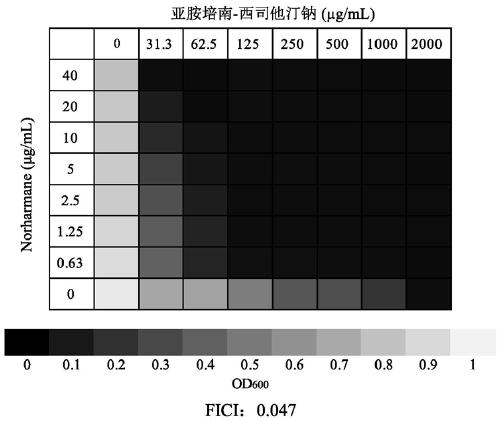
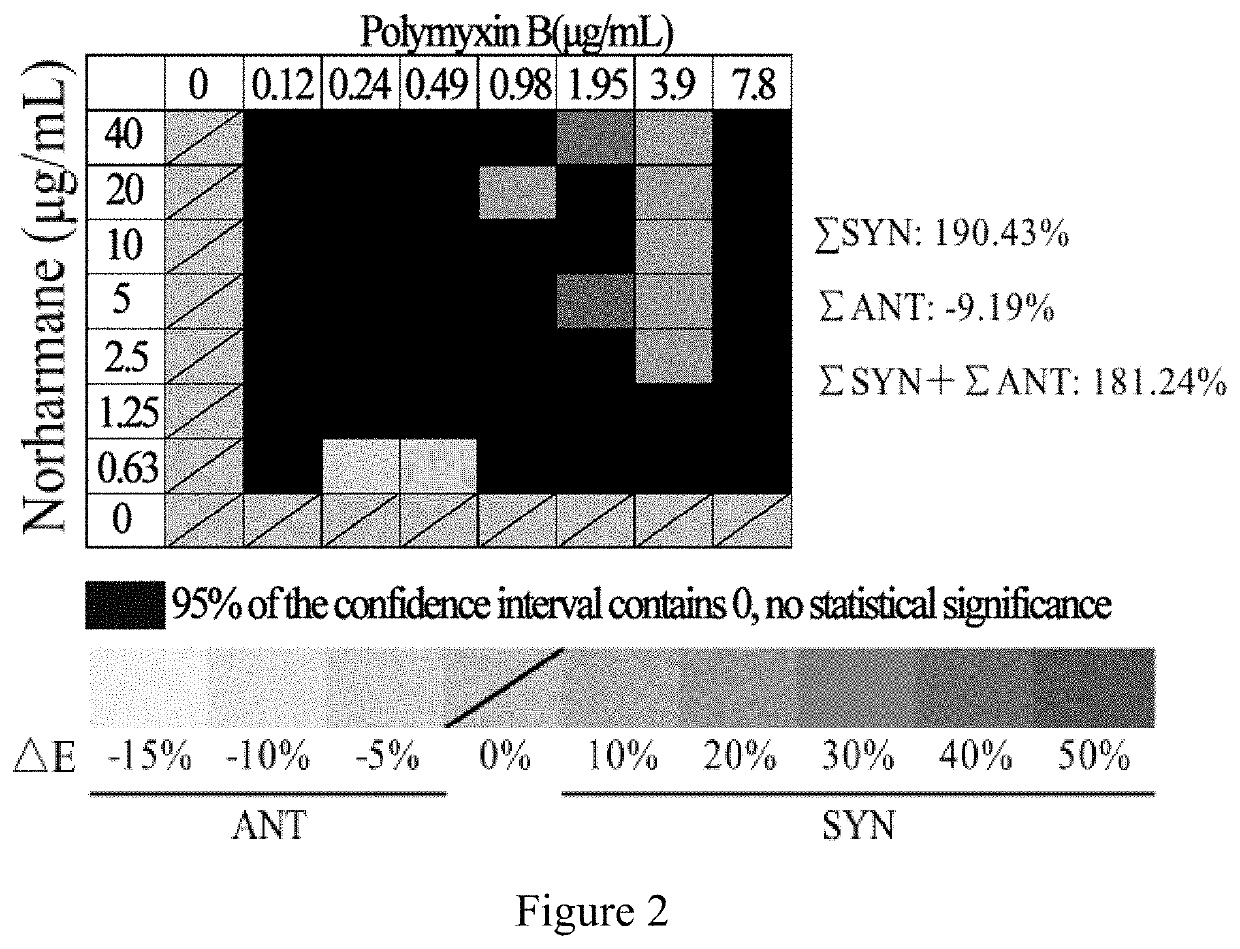
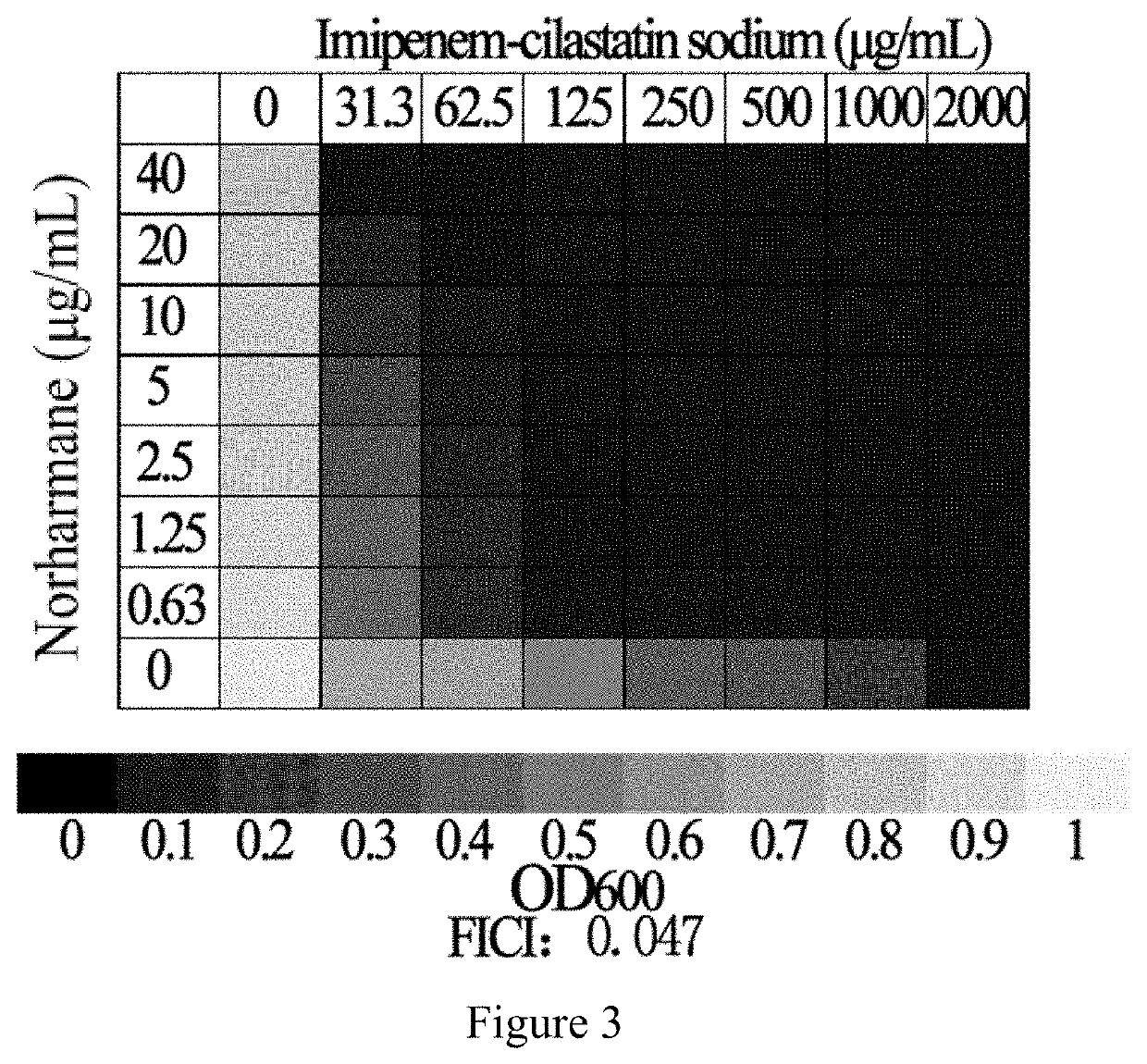
Application of norharmane in improvement of antibiotic antibacterial activity
ActiveCN111632051AReverse drug resistanceHigh antibacterial activityAntibacterial agentsOrganic active ingredientsAntibacterial activityPolymyxin B
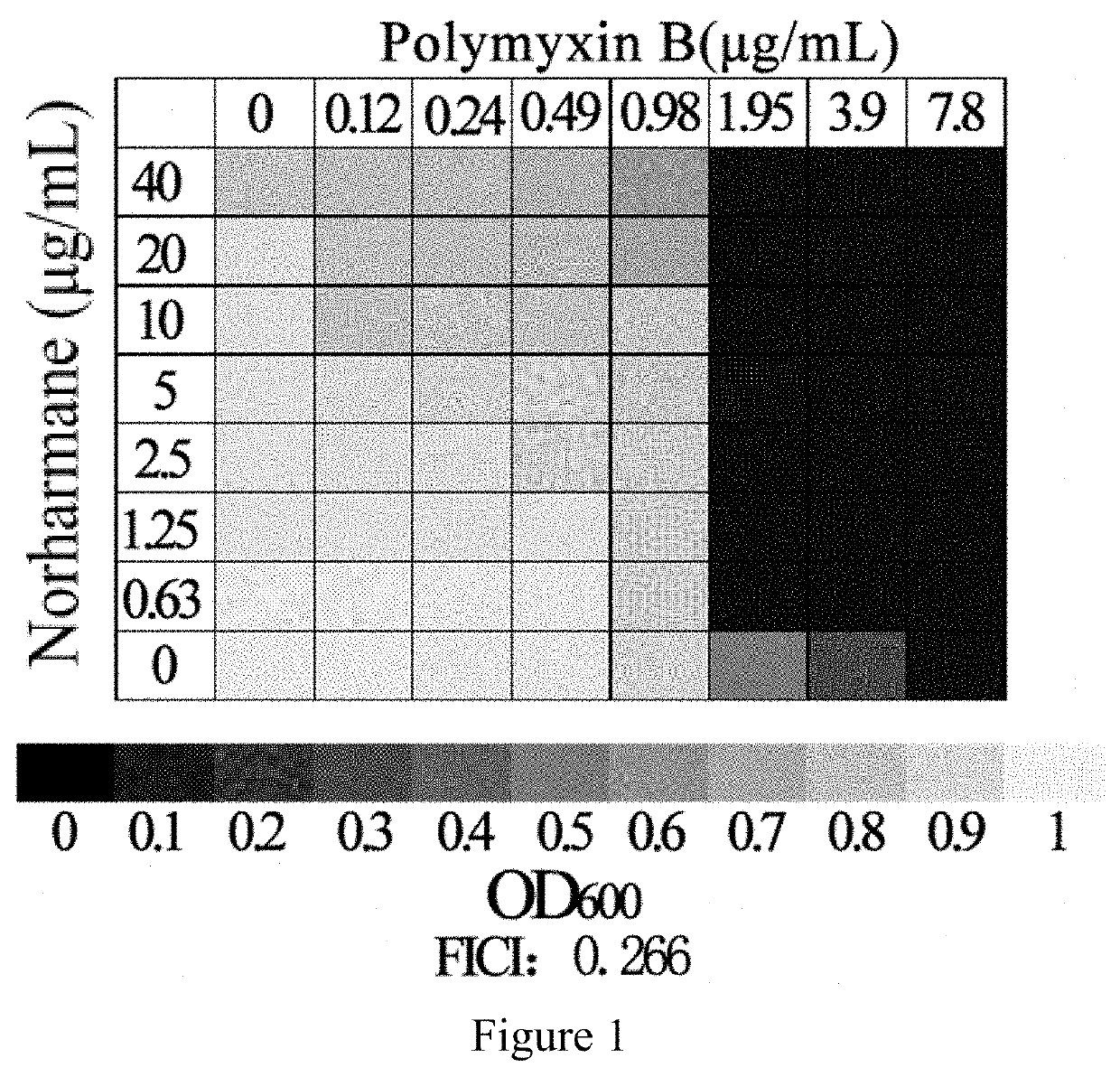
The invention discloses an application of norharmane in improvement of antibiotic antibacterial activity. When norharmane is combined with antibiotic polymyxin B, imipenem-cilastatin sodium or levofloxacin for application, the antibacterial activity on drug-resistant pseudomonas aeruginosa can be enhanced, and a synergistic antibacterial effect can be generated. According to the method for obviously improving the killing capacity of the antibiotics on the drug-resistant pseudomonas aeruginosa, the dosage of the antibiotics required for achieving the same treatment effect is greatly reduced, and a research direction is provided for the development of new drugs and the new use of old drugs.
Owner:ZHEJIANG UNIV OF TECH
A kind of imipenem cilastatin sodium pharmaceutical composition liposome injection
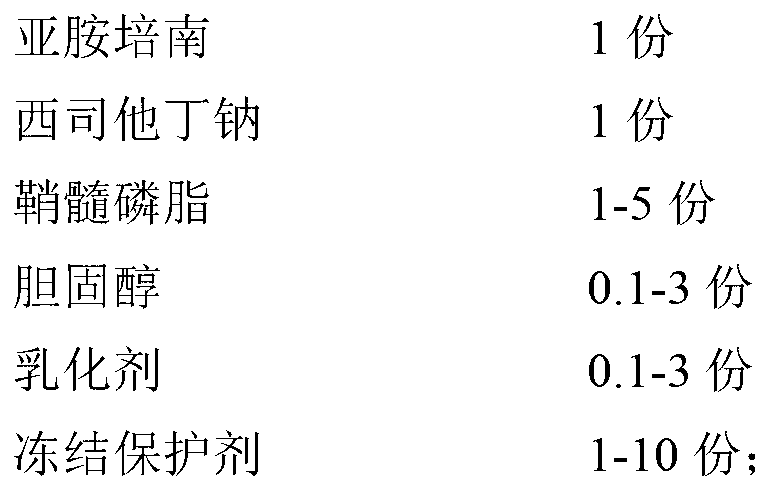
InactiveCN102266326AAvoid the dangers of toxicityQuality improvementAntibacterial agentsOrganic active ingredientsCilastatin sodiumBioavailability
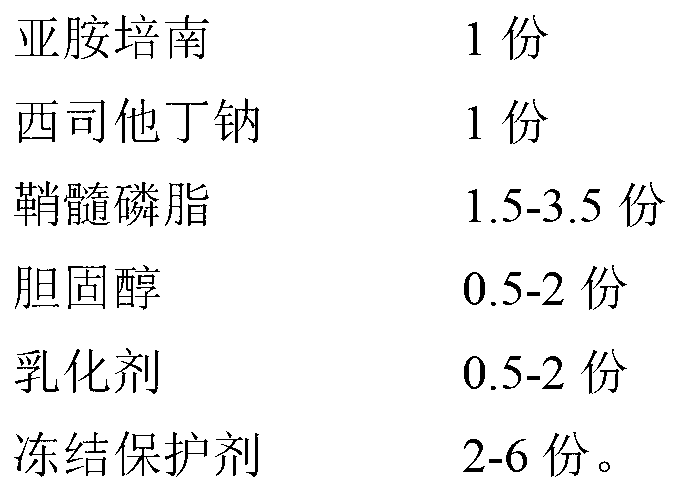
The invention provides an imipenem and cilastatin sodium pharmaceutical composition liposome injection, comprising imipenem, cilastatin sodium, sphingomyelin, cholesterol, an emulsifier and a cryoprotectant. The imipenem and cilastatin sodium injection prepared by using the method provided by the invention improves the quality of the preparation product, reduces toxic and adverse effects, increases bioavailability of medicinal components, and has good preparation stability. During the lyopilization process, liposomes do not break due to dehydration, fusion, ice crystals, and so on, and after hydration redissolution, liposomes also remain good encapsulation rate.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method of imipenem-cilastatin sodium sterile powder
ActiveCN105055169AUniform particle sizeImprove liquidityAntibacterial agentsPowder deliveryNitrogenCilastatin sodium
The invention provides a preparation method of imipenem-cilastatin sodium sterile powder. With the adoption of the preparation method provided by the present invention, the problem of difficulty in uniform mixing due to big grain diameter difference between imipenem and cilastatin sodium as well as the problem of proneness to oxidation due to sulfur contained in the components in existing preparation technology are solved through the measures of solvent adjusting, spray drying, nitrogen filling, etc.
Owner:LUNAN BETTER PHARMA
Imipenem and cilastatin sodium sterile powder preparation for injection and preparation method thereof
ActiveCN106176722AReduce adsorptionImprove solubilityAntibacterial agentsPowder deliverySolubilitySodium bicarbonate
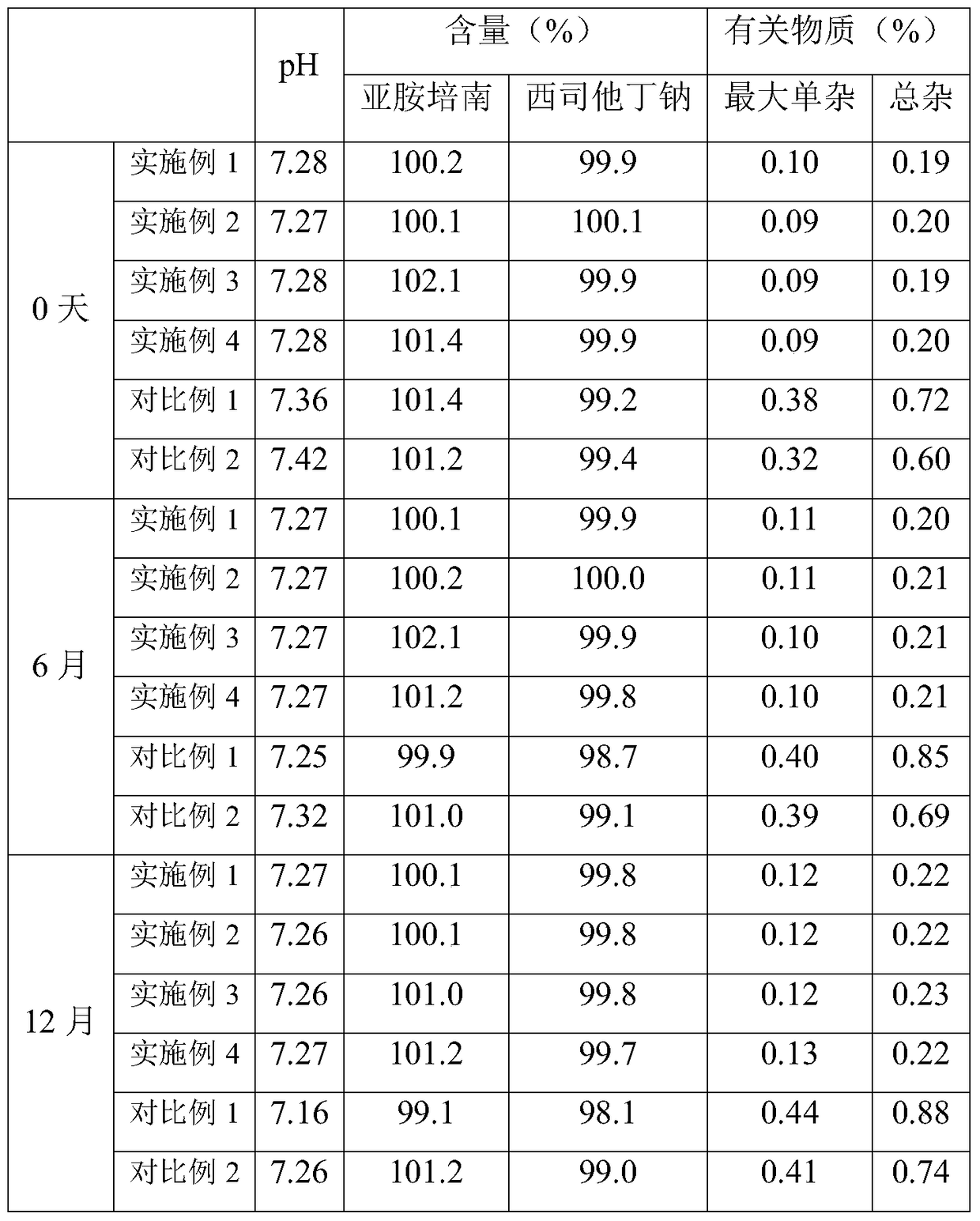
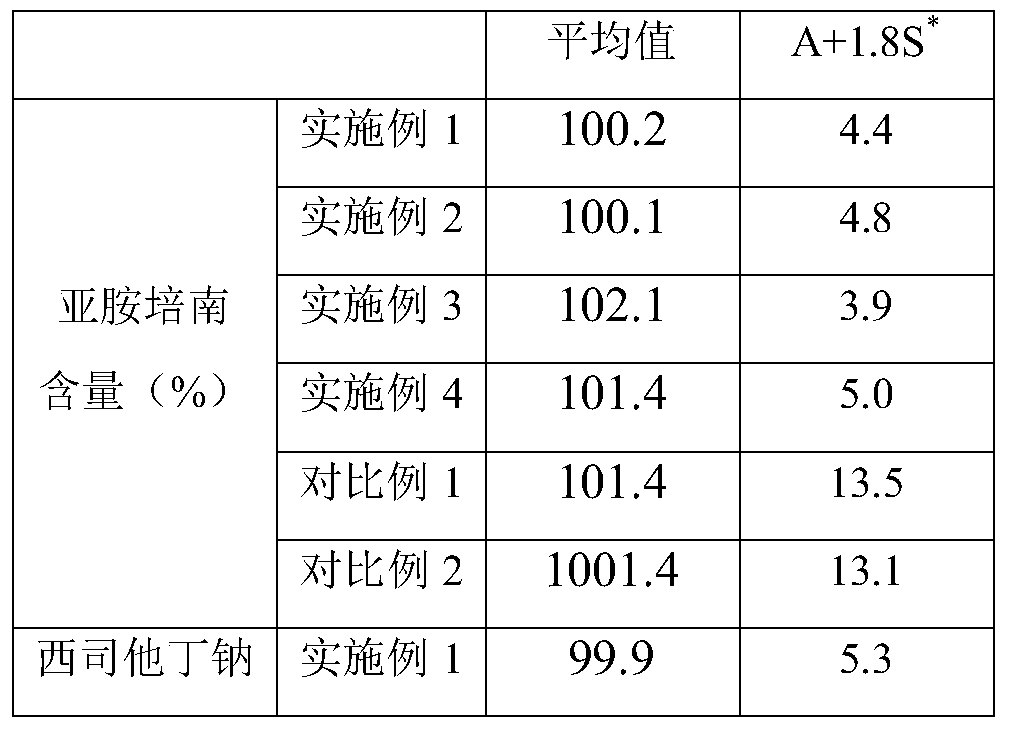
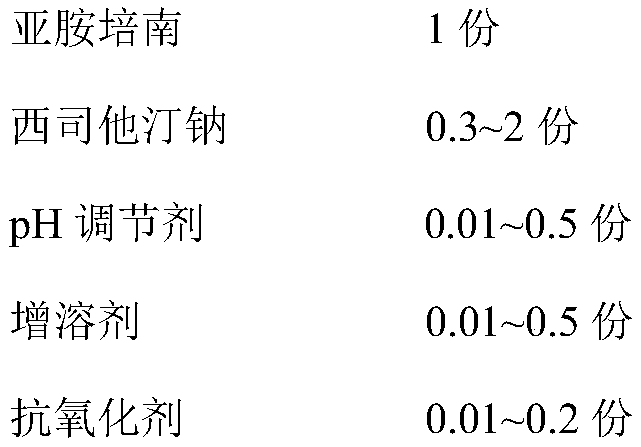
The invention provides an imipenem and cilastatin sodium sterile powder preparation for injection and a preparation method thereof. The solubilizing effect of a pH regulator of sodium bicarbonate on imipenem is utilized; the solubility of the imipenem in a water solution is increased; antioxidants are added, so that the oxidative deterioration of the imipenem and the cilastatin sodium sterile powder preparation in production and storage processes is reduced; the use of medical auxiliary materials is reduced; the manufacturing process is simplified; the cost is reduced; meanwhile, the contents of relevant substances are reduced; the production of the stable-quality imipenem and cilastatin sodium sterile powder preparation for injection can be ensured.
Owner:泊诺(天津)创新医药研究有限公司
Method for purifying intermediate of cilastatin sodium
ActiveCN110305033ASimple stepsReduce the possibilityOrganic chemistry methodsCarboxylic acid amide separation/purificationPurification methodsAlcohol
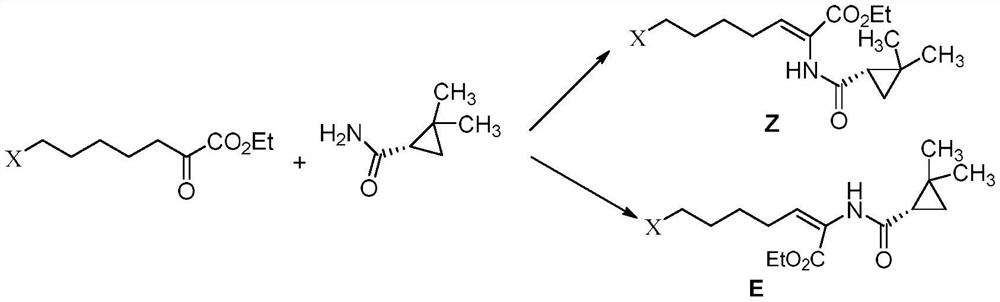
The invention belongs to the field of drug synthesis, and particularly discloses a method for purifying an intermediate of cilastatin sodium. The method includes adding (E-Z)-7-halo-2 ((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester into acetone, stirring, and adding a certain amount of organic alcohol; then dropping purified water, adjusting pH to 4-6 with acid after dropping, cooling to 0-10 DEG C, keeping the temperature and stirring for 3-4 hours; and filtering to obtain a solid, namely Z-7-halo-2 ((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester.Through the technical scheme, the HPLC purity of the product Z-7-halo-2 ((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester reaches more than 99.0%, and the impurity of the E configuration is reduced to less than 0.1%.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of omeprazole sodium pharmaceutical composition
ActiveCN105168211BGood curative effectAvoid injection irritationAntibacterial agentsPowder deliveryOmeprazole SodiumAntioxidant
The invention discloses an Omeprazole sodium pharmaceutical composition containing Omeprazole sodium, Imipenem and Cilastatin sodium. According to the composition, the treatment effect of Omeprazole can be improved, and Omeprazole sodium is unusually stable in the absence of antioxidants and metal chelating agents.
Owner:CHONGQING PHARSCIN PHARM CO LTD
Preparation method of cilastatin sodium active pharmaceutical ingredient
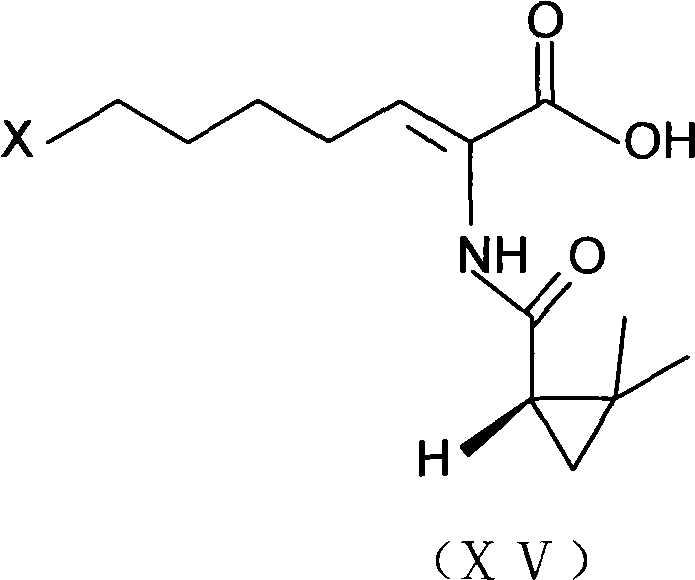
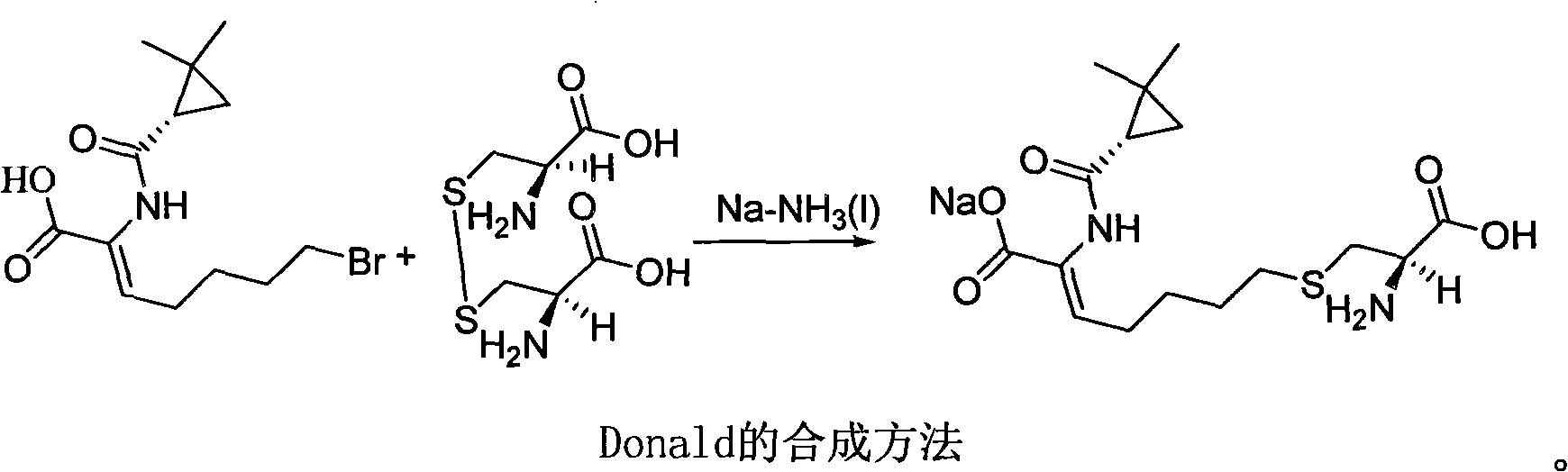
ActiveCN106518741AShort reaction timeHigh yieldAntibacterial agentsOrganic active ingredientsAdditive ingredientCysteine Hydrochloride
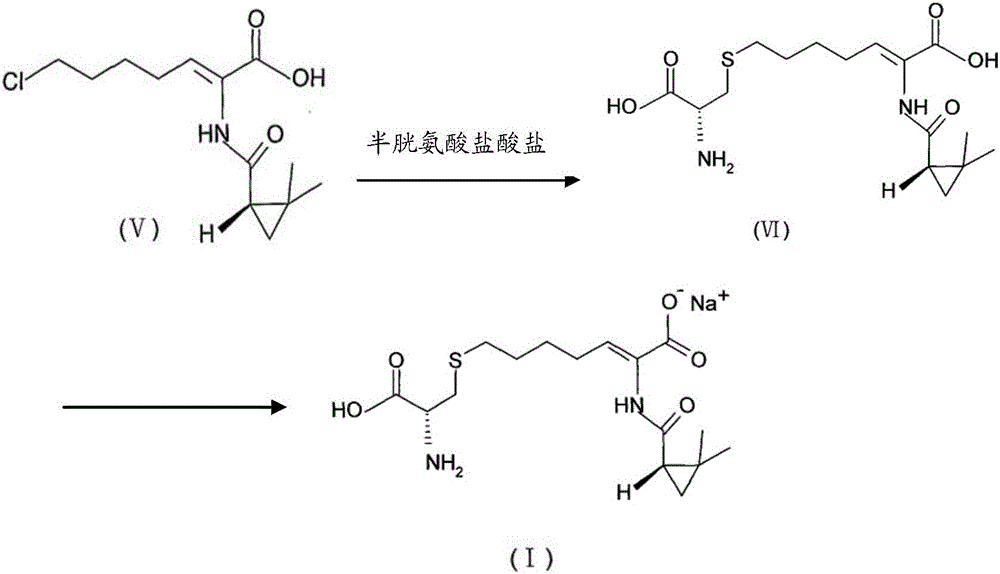
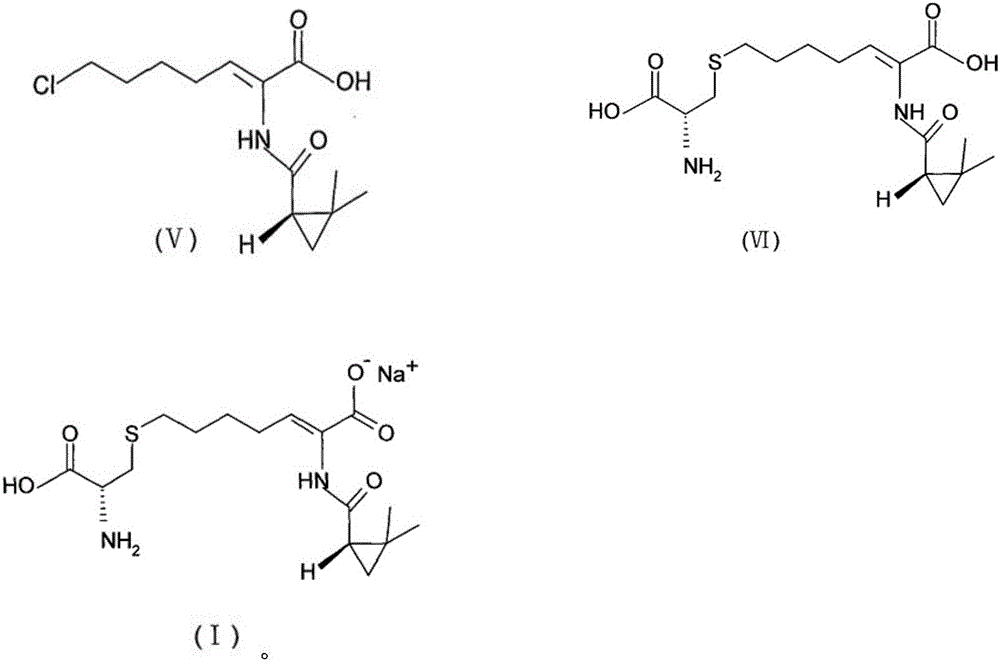
The invention provides a preparation method of a cilastatin sodium active pharmaceutical ingredient. The preparation method comprises the following steps: (1) mixing alkali and reaction solvent, adding a compound (Z)-7-chloro-2-((S)-2,2-dimethylcyclopropylformacyl)-2-heptenoic acid shown in Formula (V), then adding cysteine hydrochloride, reacting to obtain a reaction solution containing a compound cilastatin shown in Formula (VI); (2) purifying the reaction solution obtained in the step (1) through macroporous adsorption resin to obtain the compound cilastatin shown in Formula (VI); and (3) mixing sodium hydroxide and water, adding the cilastatin obtained in the step (2), regulating the pH value, and drying to obtain the compound cilastatin sodium shown in Formula (I). The preparation method is quick, efficient and suitable for industrial production; and the obtained cilastatin sodium active pharmaceutical ingredient is low in impurity content.
Owner:SHENZHEN HAIBIN PHARMA +1
A kind of preparation method of key intermediate of cilastatin sodium
ActiveCN111285781BReduce generationSimple and fast operationOrganic compound preparationOrganic chemistry methodsChemical synthesisIsomerization
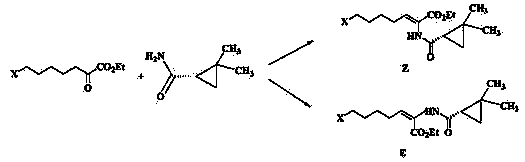
The invention belongs to the field of organic chemical synthesis and provides a method for preparing a key intermediate of cilastatin sodium, the method comprising: using 7-halo-2-ethyl oxoheptanoate and (S)-2, 2- Dimethylcyclopropanecarboxamide is a raw material, and through condensation reaction, Z / E type 7-halogen-2((S)-2,2-dimethylcyclopropanecarboxamide group)-2-heptenoic acid ethyl ester is obtained The reaction solution is placed under an ultraviolet lamp to irradiate to obtain a high-purity Z-type cilastatin sodium key intermediate. The invention reduces the generation of impurities, improves the efficiency of the isomerization process, simplifies the preparation process, and effectively improves the yield and purity of the key intermediate of cilastatin sodium.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of method for preparing imipenem cilastatin sodium sterile powder
ActiveCN105055169BUniform particle sizeImprove liquidityAntibacterial agentsOrganic active ingredientsNitrogenCilastatin sodium
The invention provides a preparation method of imipenem-cilastatin sodium sterile powder. With the adoption of the preparation method provided by the present invention, the problem of difficulty in uniform mixing due to big grain diameter difference between imipenem and cilastatin sodium as well as the problem of proneness to oxidation due to sulfur contained in the components in existing preparation technology are solved through the measures of solvent adjusting, spray drying, nitrogen filling, etc.
Owner:LUNAN BETTER PHARMA
Preparation method of cilastatin sodium key intermediate
ActiveCN111285781AHigh yieldHigh purityOrganic compound preparationOrganic chemistry methodsChemical synthesisIsomerization
The invention belongs to the field of organic chemical synthesis, and provides a method for preparing a cilastatin sodium key intermediate. The method comprises the following steps: by taking 7-halogen-2-oxoethyl heptanoate and (S)-2,2-dimethylcyclopropanecarboxamide as raw materials, carrying out a condensation reaction to obtain a reaction solution of Z / E type 7-halogen-2((S)-2,2-dimethylcyclopropanecarboxamide)-2-ethyl heptenoate, and irradiating the reaction solution under an ultraviolet lamp to obtain the high-purity Z type cilastatin sodium key intermediate. According to the method, thegeneration of impurities is reduced, the efficiency of the isomerization process is improved, the preparation process is simplified, and the yield and the purity of the cilastatin sodium key intermediate are effectively improved.
Owner:LUNAN PHARMA GROUP CORPORATION
Omeprazole sodium pharmaceutical composition
ActiveCN105168211AGood curative effectAvoid injection irritationAntibacterial agentsPowder deliveryOmeprazole SodiumAntioxidant
The invention discloses an Omeprazole sodium pharmaceutical composition containing Omeprazole sodium, Imipenem and Cilastatin sodium. According to the composition, the treatment effect of Omeprazole can be improved, and Omeprazole sodium is unusually stable in the absence of antioxidants and metal chelating agents.
Owner:CHONGQING PHARSCIN PHARM CO LTD
A kind of preparation method of cilastatin sodium crude drug
ActiveCN106518741BShort reaction timeHigh yieldAntibacterial agentsOrganic active ingredientsAdditive ingredientCysteine Hydrochloride
The invention provides a preparation method of a cilastatin sodium active pharmaceutical ingredient. The preparation method comprises the following steps: (1) mixing alkali and reaction solvent, adding a compound (Z)-7-chloro-2-((S)-2,2-dimethylcyclopropylformacyl)-2-heptenoic acid shown in Formula (V), then adding cysteine hydrochloride, reacting to obtain a reaction solution containing a compound cilastatin shown in Formula (VI); (2) purifying the reaction solution obtained in the step (1) through macroporous adsorption resin to obtain the compound cilastatin shown in Formula (VI); and (3) mixing sodium hydroxide and water, adding the cilastatin obtained in the step (2), regulating the pH value, and drying to obtain the compound cilastatin sodium shown in Formula (I). The preparation method is quick, efficient and suitable for industrial production; and the obtained cilastatin sodium active pharmaceutical ingredient is low in impurity content.
Owner:SHENZHEN HAIBIN PHARMA +1
A kind of imipenem cilastatin sodium sterile powder preparation for injection and preparation method thereof
ActiveCN106176722BReduce adsorptionImprove solubilityAntibacterial agentsPowder deliverySolubilitySodium bicarbonate
The invention provides an imipenem and cilastatin sodium sterile powder preparation for injection and a preparation method thereof. The solubilizing effect of a pH regulator of sodium bicarbonate on imipenem is utilized; the solubility of the imipenem in a water solution is increased; antioxidants are added, so that the oxidative deterioration of the imipenem and the cilastatin sodium sterile powder preparation in production and storage processes is reduced; the use of medical auxiliary materials is reduced; the manufacturing process is simplified; the cost is reduced; meanwhile, the contents of relevant substances are reduced; the production of the stable-quality imipenem and cilastatin sodium sterile powder preparation for injection can be ensured.
Owner:泊诺(天津)创新医药研究有限公司
A pharmaceutical composition comprising imipenem cilastatin sodium and its preparation
ActiveCN104095847BReduce central adverse reactionsStable pHAntibacterial agentsPowder deliveryImipenem/cilastatinMedicine
The invention relates to a pharmaceutical composition containing imipenem cilastatin sodium and a preparation thereof, belonging to the field of medicine. In order to overcome the technical deficiency of the large central adverse reaction of the powder injection of imipenem and cilastatin sodium in the prior art, the present invention provides a powder injection containing imipenem and cilastatin sodium through reasonable compatibility. When the powder injection is used for clinical antibacterial treatment, it can effectively reduce the central adverse reactions of imipenem cilastatin sodium compound preparation, and can significantly improve the stability of the powder injection after reconstitution, so it is suitable for development into clinical treatment drug.
Owner:CHINA NAT MEDICINES GUORUI PHARMA
A kind of preparation method of cilastatin sodium intermediate
ActiveCN110845354BHigh puritySolve the difficulty of recyclingOrganic compound preparationOrganic chemistry methodsPtru catalystEthyl ester
The invention belongs to the technical field of medicines, and particularly provides a cilastatin sodium intermediate preparation method, which comprises: simply preparing a (Z)-7-X-2((2s)-2,2-dimethylcyclopropaneformamido)-2-heptenoic acid crude product through a one-pot method by using 7-X-2-oxoheptanoic acid ethyl ester and (s)-2,2-dimethylcyclopropaneformamide as raw materials sequentially under the action of a catalyst, concentrated hydrochloric acid and hydrogen chloride gas, and re-crystallizing to prepare a (Z)-7-X-2((2s)-2,2-dimethylcyclopropaneformamido)-2-heptenoic acid refined product. According to the invention, the method has advantages of short synthetic route, simple operation and high product purity, and is suitableness for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of purification method of cilastatin sodium intermediate
ActiveCN110305033BSimple stepsReduce the possibilityOrganic chemistry methodsCarboxylic acid amide separation/purificationAmidogenPharmacology
Owner:LUNAN PHARMA GROUP CORPORATION
Imipenem and cilastatin sodium for injection and preparation process of imipenem and cilastatin sodium for injection
InactiveCN109381434AWell mixedReduce the impactAntibacterial agentsOrganic active ingredientsSodium bicarbonateCilastatin sodium
The invention relates to imipenem and cilastatin sodium for injection and a preparation process of the imipenem and cilastatin sodium for injection, and belongs to the field of medicines. The preparation process of the imipenem and cilastatin sodium for injection comprises the following steps: mixing imipenem, cilastatin sodium and sodium bicarbonate under a sterile condition, and separately packing the mixture in sterile glass bottles in a screw feeding mode. The problem that the imipenem and the cilastatin sodium are uniformly mixed difficultly is solved, meanwhile, drugs in the imipenem andcilastatin sodium for injection are uniform, and layering cannot be caused easily. The imipenem and cilastatin sodium prepared by the preparation process of the imipenem and cilastatin sodium for injection comprises the imipenem, the cilastatin sodium and the sodium bicarbonate according to the mass ratio of 1: 1: (0.01-0.1) successively, the quality of the imipenem and cilastatin sodium for injection is stable, the imipenem and the cilastatin sodium are mixed uniformly, and layering is not caused easily.
Owner:瀚晖制药有限公司
A kind of preparation method of cilastatin sodium
ActiveCN102702051BReduce generationHigh yieldOrganic compound preparationSulfide preparationIsomerizationCilastatin sodium
The invention belongs to the field of pharmaceutical synthesis, and provides a preparation method of cilastatin sodium, and the method comprises the following steps: performing condensation, alkaline hydrolysis, and thioetherification of raw materials of 7-oxyhalogen alkyl heptylate and (s)-2,2-dimethylcyclopropane methanamide, adjusting the pH value of the thioetherified solution, washing by a nonpolar solvent, performing isomerization, purification by a neutral macroporous adsorption resin, and salt formation so as to obtain the cilastatin sodium solid. The invention reduces the generation of impurities, improves the isomerization efficiency, simplifies the preparation process, and effectively improves the yield and purity of cilastatin sodium.
Owner:SHANDONG NEWTIME PHARMA
Cilastatin sodium intermediate preparation method
ActiveCN110845354AHigh puritySolve the difficulty of recyclingOrganic compound preparationOrganic chemistry methodsPtru catalystEthyl ester
The invention belongs to the technical field of medicines, and particularly provides a cilastatin sodium intermediate preparation method, which comprises: simply preparing a (Z)-7-X-2((2s)-2,2-dimethylcyclopropaneformamido)-2-heptenoic acid crude product through a one-pot method by using 7-X-2-oxoheptanoic acid ethyl ester and (s)-2,2-dimethylcyclopropaneformamide as raw materials sequentially under the action of a catalyst, concentrated hydrochloric acid and hydrogen chloride gas, and re-crystallizing to prepare a (Z)-7-X-2((2s)-2,2-dimethylcyclopropaneformamido)-2-heptenoic acid refined product. According to the invention, the method has advantages of short synthetic route, simple operation and high product purity, and is suitableness for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Imipenem and cilastatin sodium pharmaceutical composition liposome injection
InactiveCN102266326BAvoid the dangers of toxicityQuality improvementAntibacterial agentsOrganic active ingredientsBioavailabilityCilastatin sodium
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method of cilastatin sodium impurity C
InactiveCN111562333AHigh yieldThe preparation method has a short cycle timeComponent separationFluid phasePhysical chemistry
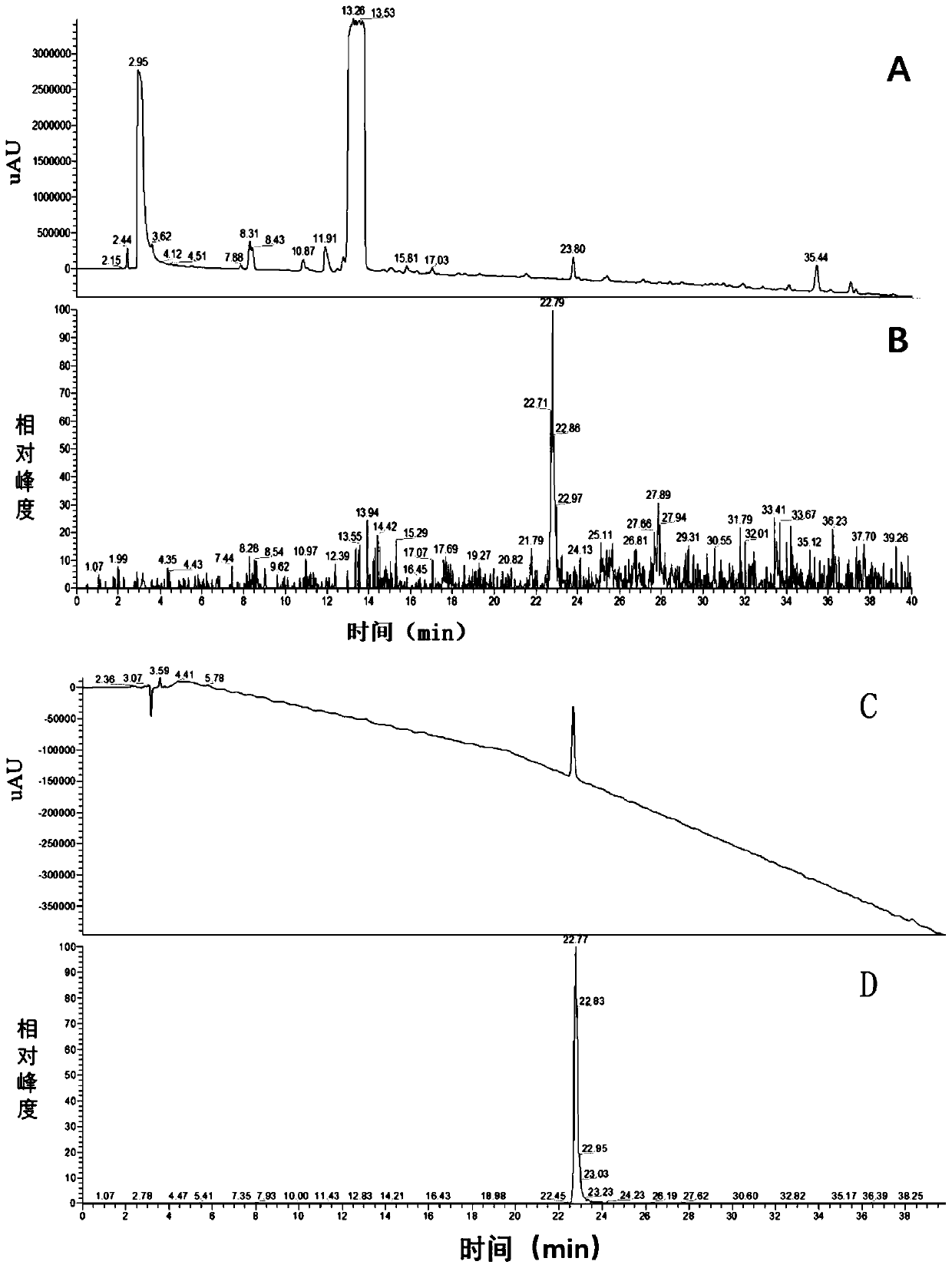
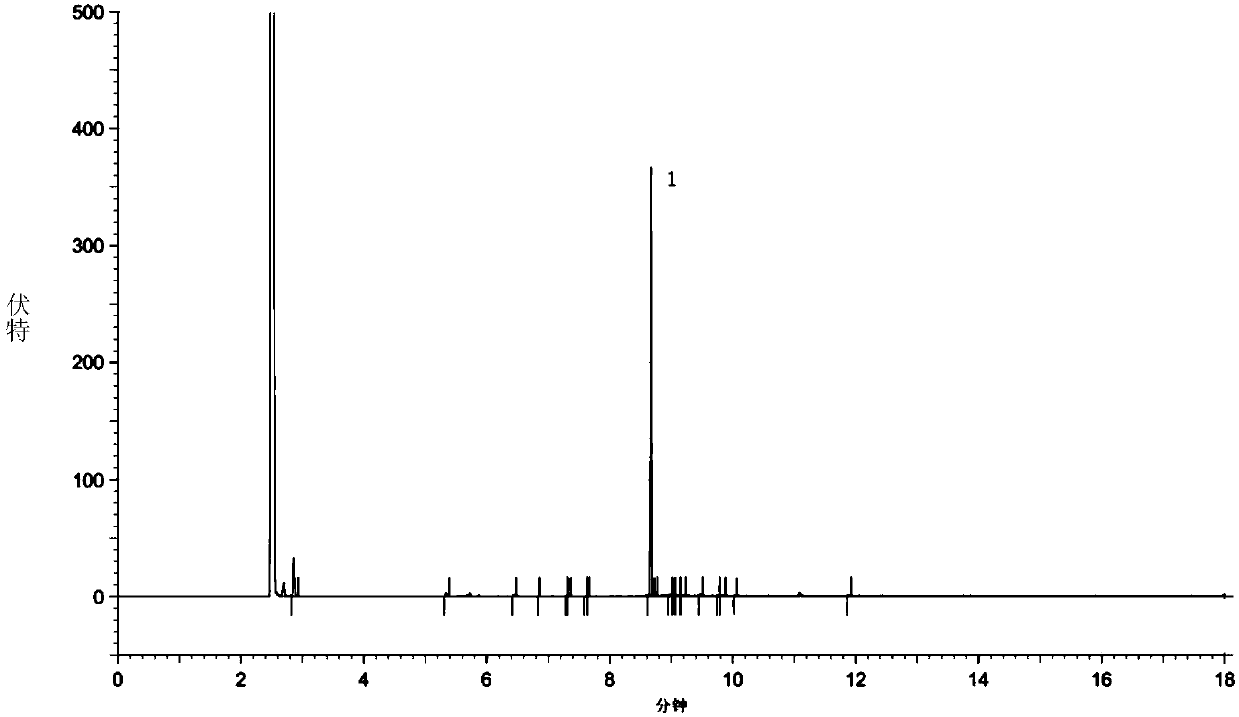
The invention discloses a preparation method of a cilastatin sodium impurity C. The preparation method comprises the steps of preparing a cilastatin sodium test solution, carrying out high-pressure liquid chromatography separation, concentrating, carrying out high-pressure liquid chromatography secondary separation, concentrating and drying to obtain the cilastatin sodium impurity C. Elution is carried out in a gradient manner, a mobile phase A is formic acid, and a mobile phase B is acetonitrile. The preparation method of the cilastatin sodium impurity C provided by the invention is short inperiod and high in detection sensitivity, has the advantages of being simple, rapid, high in purity, high in yield, low in cost and the like, and has important significance in quality control and pharmacology and toxicology research of cilastatin sodium.
Owner:LIVZON NEW NORTH RIVER PHARMA
Process for preparing cilastatin sodium
ActiveCN101307015BAvoid generatingReduce generationOrganic compound preparationSulfide preparationEthyl ChlorideCilastatin sodium
The invention relates to a method for preparing a compound called cilastatin sodium shown as a formula (I). Cilastatin is prepared by crystallizing and purifying (Z)-7-chlorine-2((S)-2, 2-dimethyl c-pr carbamoyl)-2-heptenoic acid and the cilastatin sodium is prepared by purifying the cilastatin through microporous resin, thereby improving the purity quotient and the yield coefficient of the cilastatin sodium.
Owner:SHENZHEN HAIBIN PHARMA
A kind of purification method of ethyl 7-chloro-2-oxoheptanoate
ActiveCN106117060BEnsure safetySimple and fast operationOrganic compound preparationCarboxylic acid esters preparationPurification methodsOrganic solvent
The invention provides a purification method of ethyl 7-chloro-2-oxoheptanoate, wherein the purification method comprises the steps: firstly, carrying out a reaction of ethyl 7-chloro-2-oxoheptanoate oil crude product and a hydrosulphite solution, and separating to obtain a sulfite solid of ethyl 7-chloro-2-oxoheptanoate; then, dissolving the sulfite solid of ethyl 7-chloro-2-oxoheptanoate in water, adding an acid or alkali at a certain temperature, and decomposing the sulfite of ethyl 7-chloro-2-oxoheptanoate into ethyl 7-chloro-2-oxoheptanoate; and finally, extracting with an organic solvent immiscible with water, to obtain ethyl 7-chloro-2-oxoheptanoate having the purity improved. The method is simple in operation and suitable for industrialized production, and ensures the purity of a subsequent product and final product cilastatin sodium.
Owner:SHENZHEN HAIBIN PHARMA +2
An application of norharmane in improving antibacterial activity of antibiotics
PendingUS20220304988A1Reduce the valueAvoid concentrationAntibacterial agentsOrganic active ingredientsAntibacterial activityPolymyxin B
The present invention discloses an application of norharmane in improving activity of an antibiotic in resisting bacteria, when norharmane of the present invention is combined with polymyxin B, imipenem-cilastatin sodium or levofloxacin, it can enhance antibacterial activity of the antibiotic against drug-resistant Pseudomonas aeruginosa, produce a synergistic antibacterial effect, and significantly improves the killing ability of antibiotics against drug-resistant Pseudomonas aeruginosa to reduce the amount of antibiotics needed for achieving the same therapeutic effect, thereby providing a research direction for the development of new drugs and new use of old drugs.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com