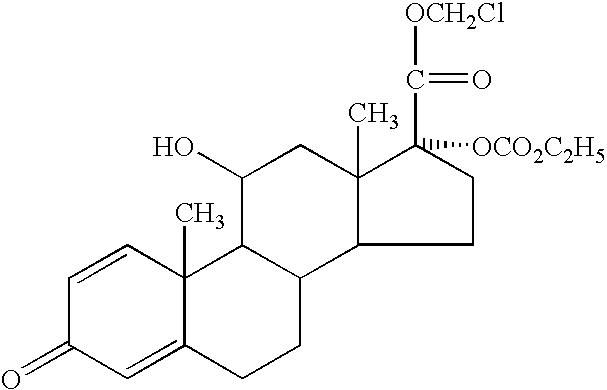
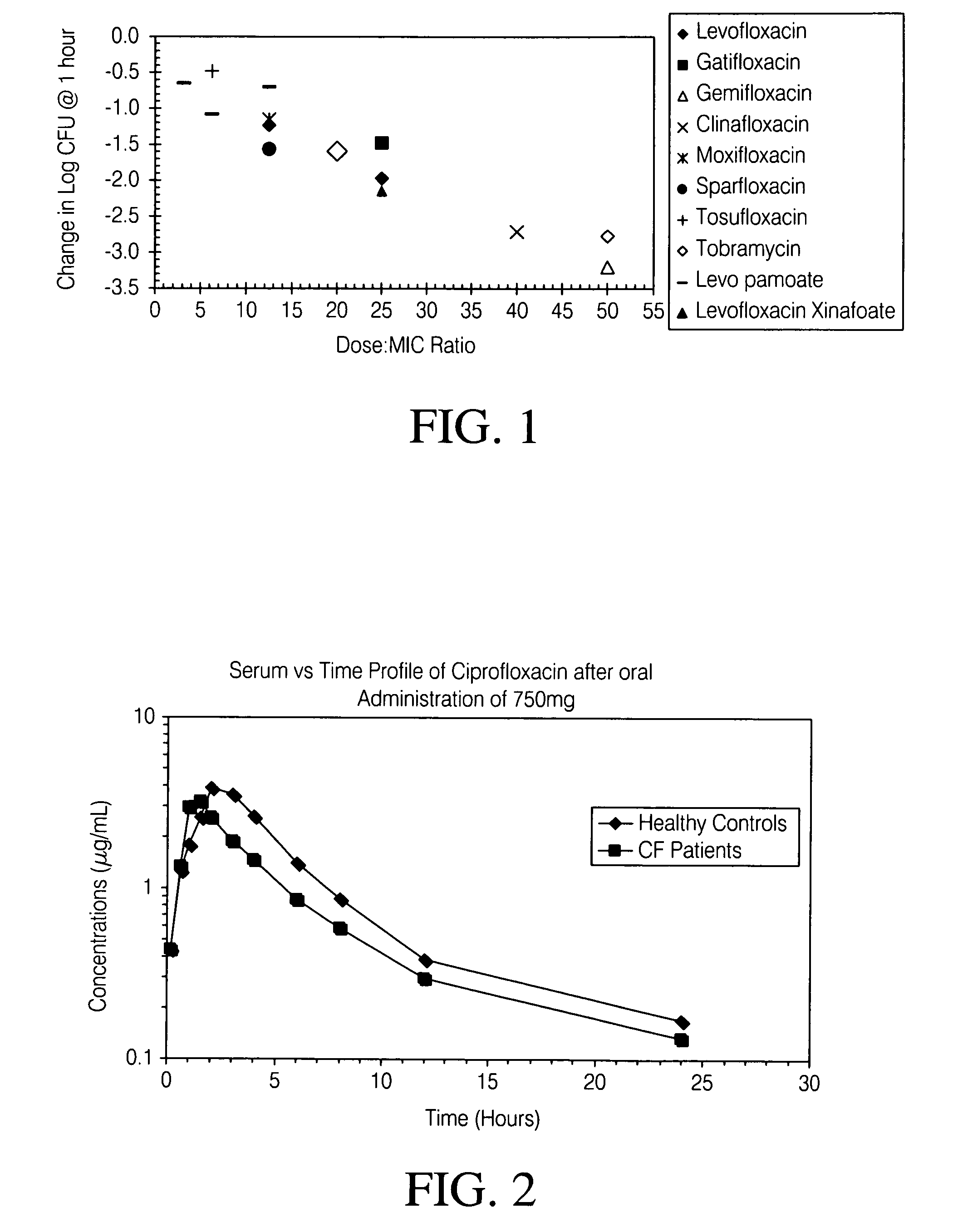
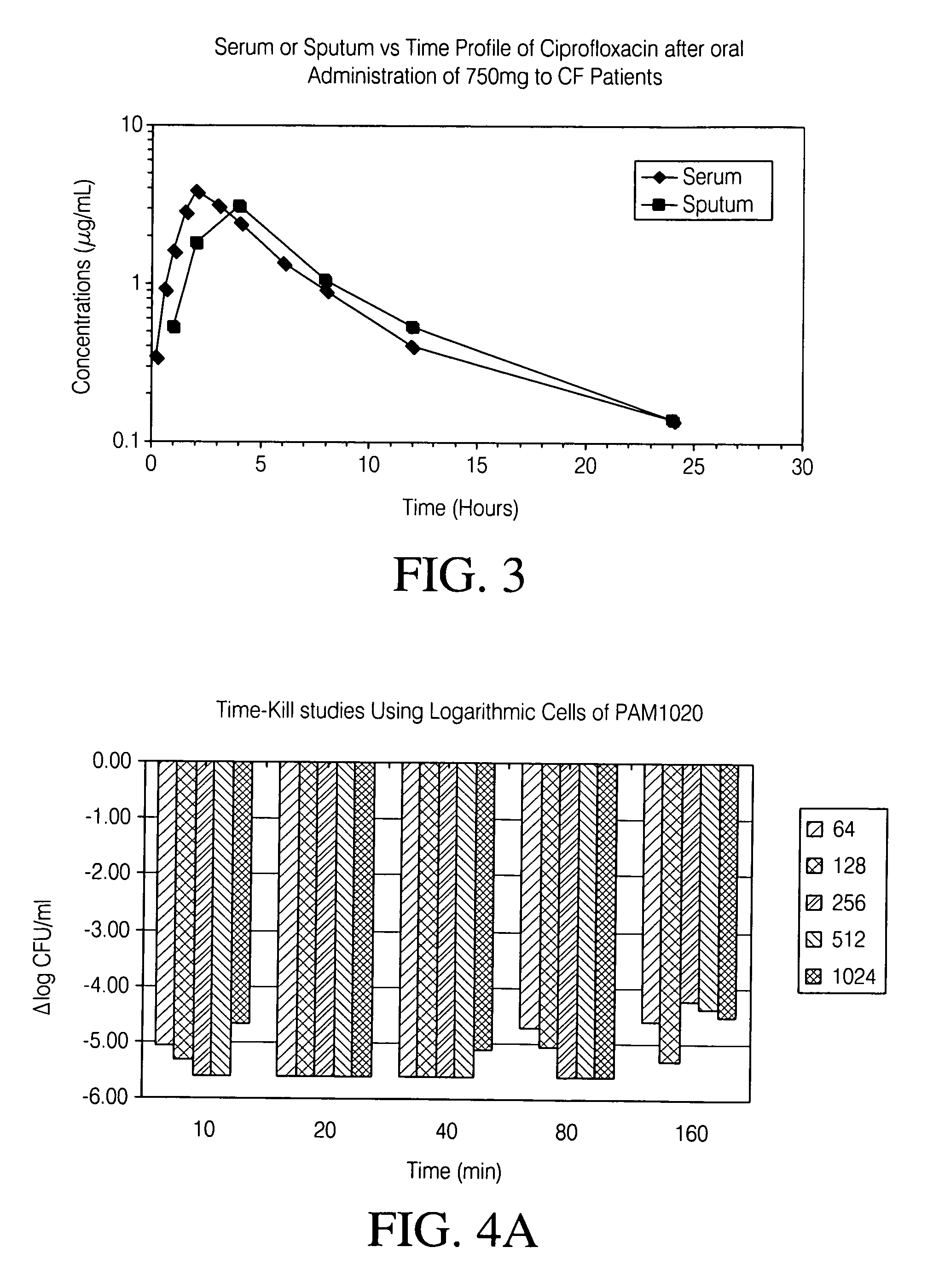
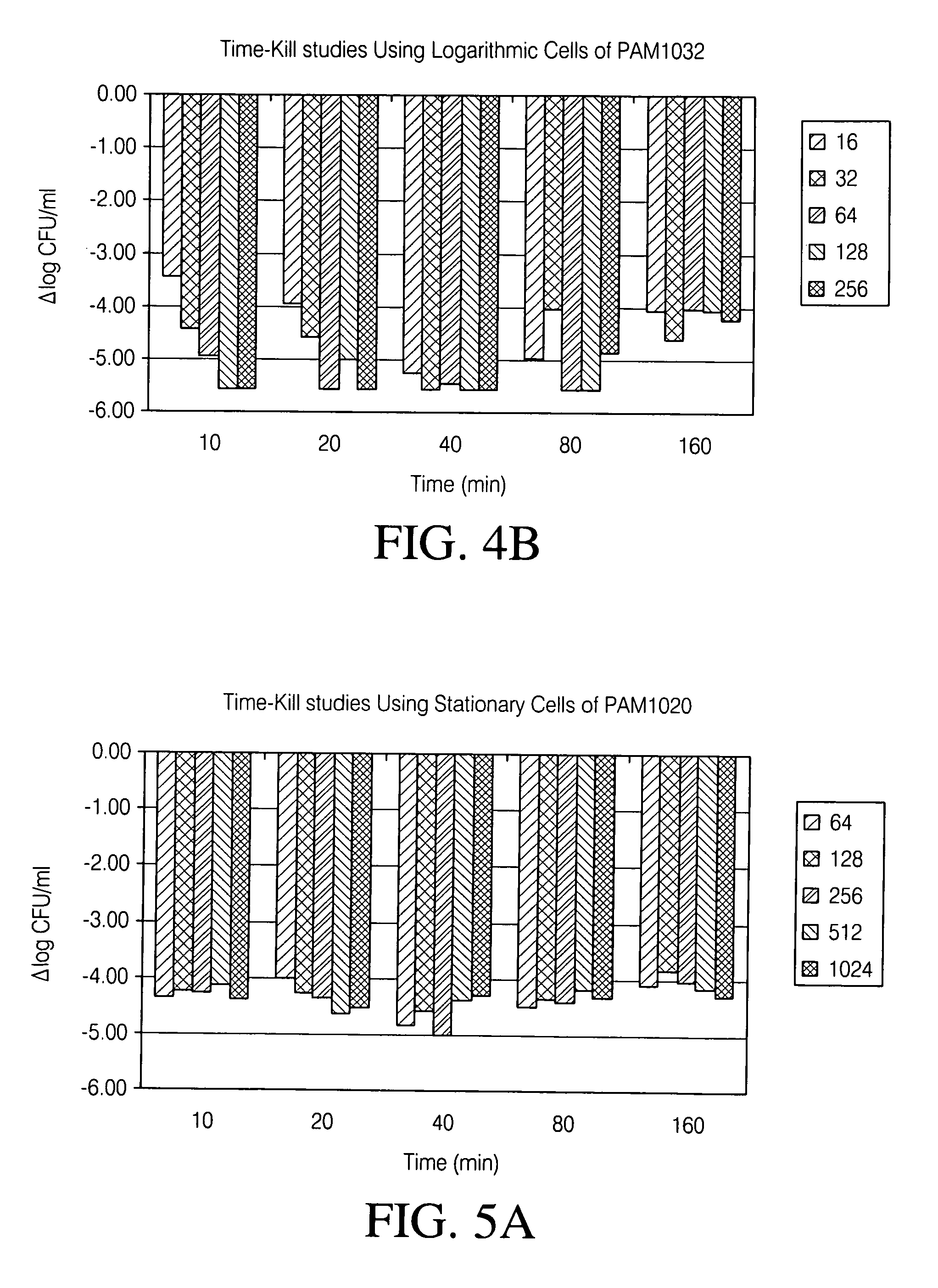
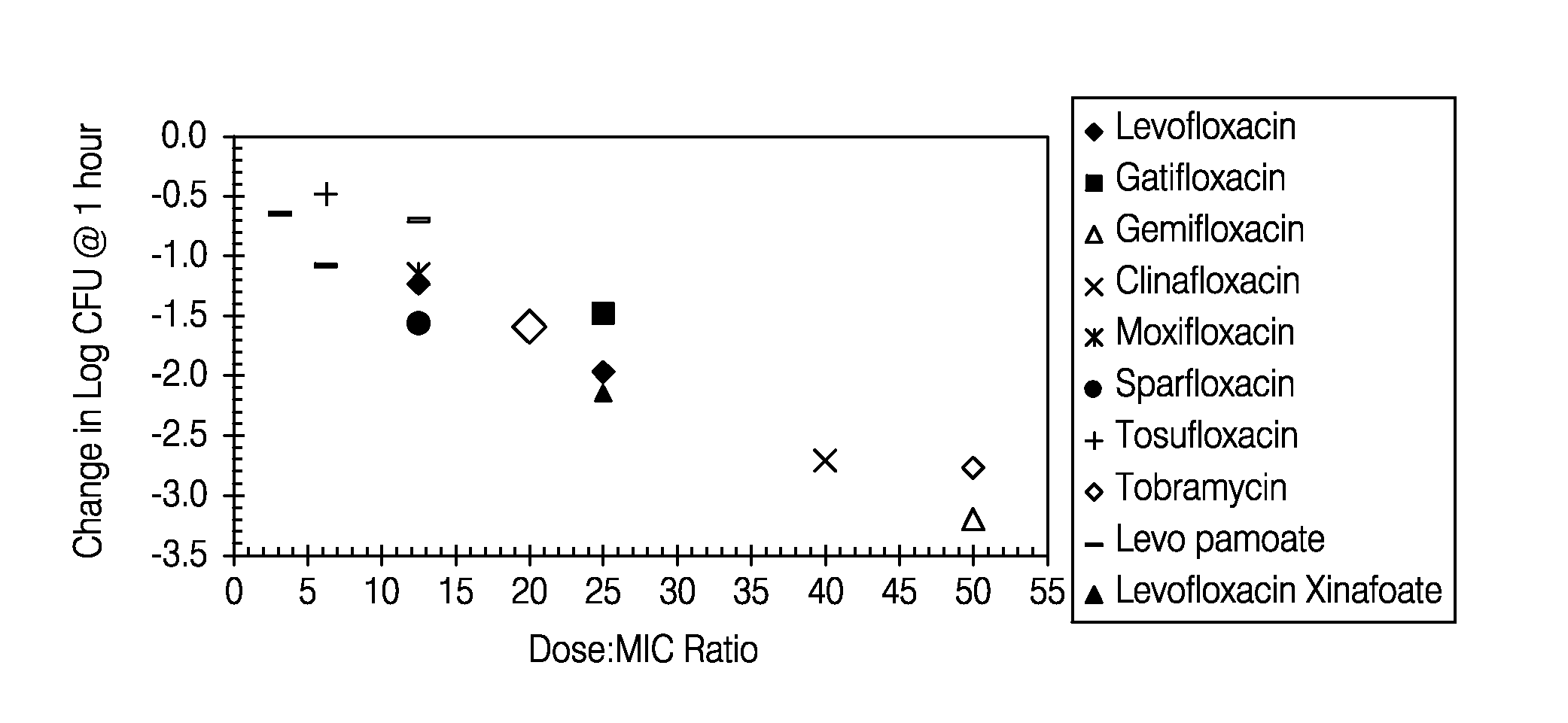
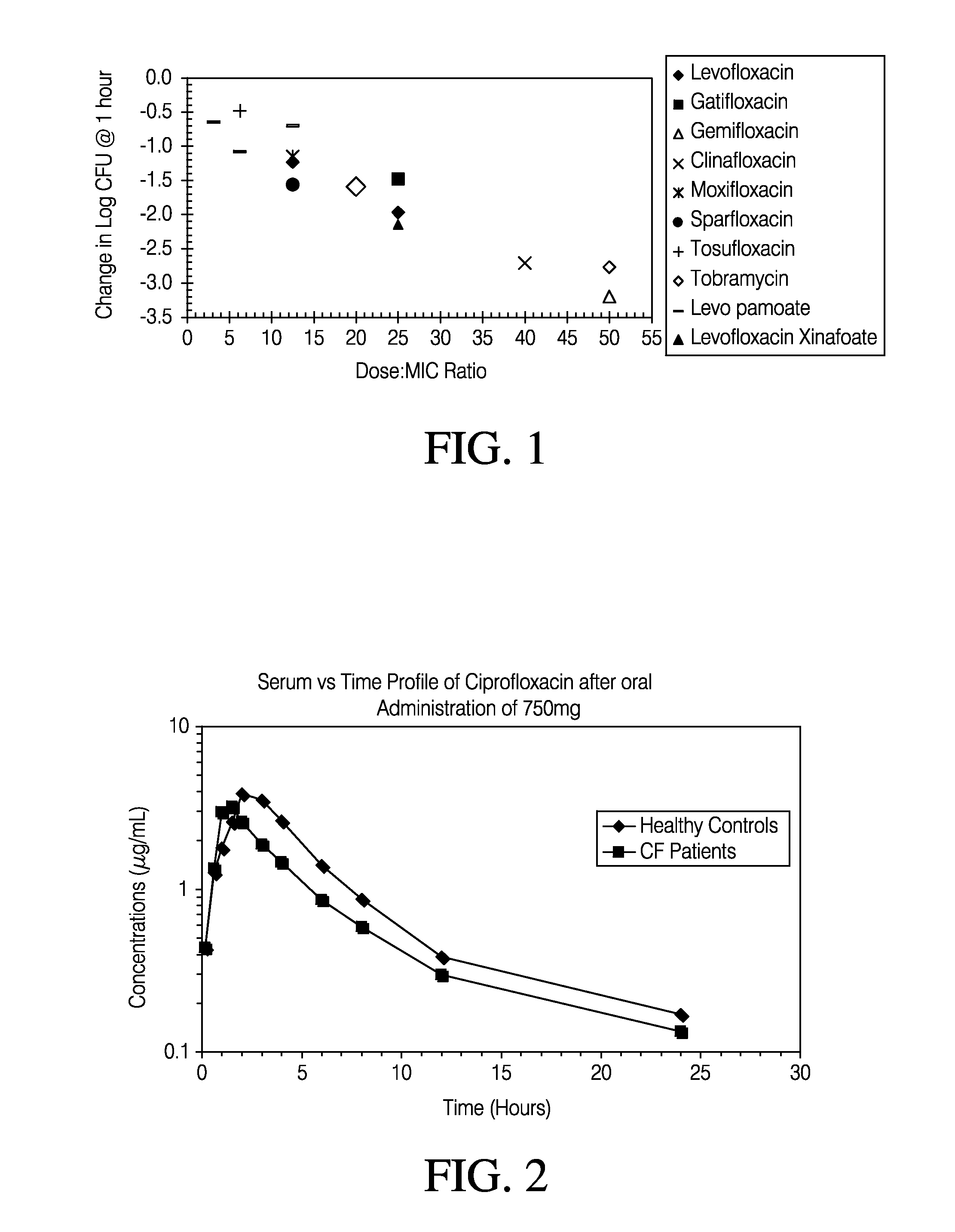
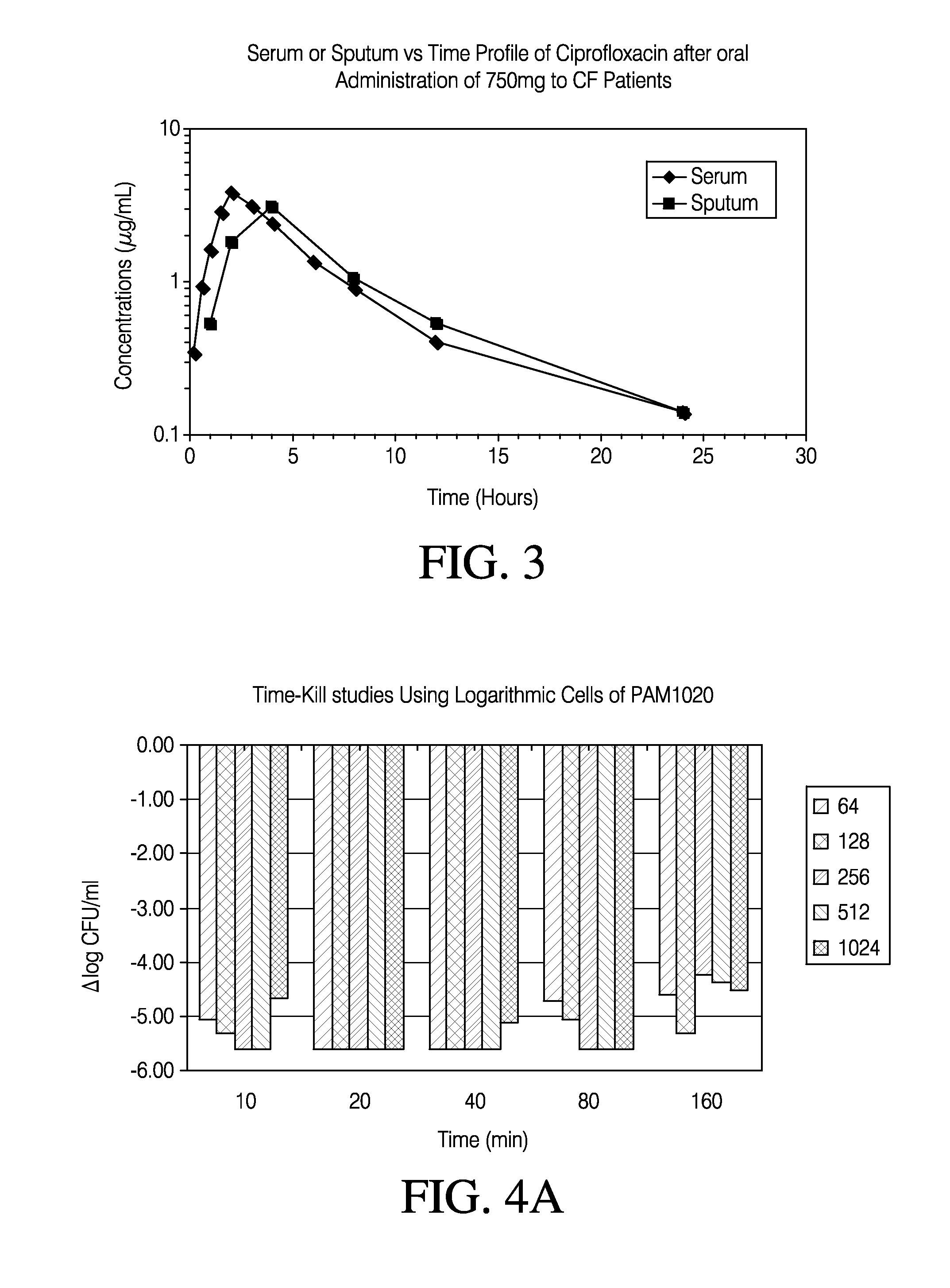
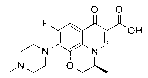
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
411 results about "Levofloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
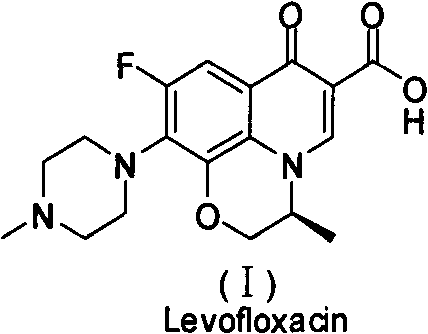
Levofloxacin is used to treat a variety of bacterial infections.
Ophthalmic, otic or nasal pharmaceutical composition and the use thereof
InactiveUS20100222308A1Effective treatmentPreventing increase of bacterial infection riskAntibacterial agentsBiocideInfective rhinitisNose
The invention provides an ophthalmic, otic or nasal pharmaceutical composition, comprising levofloxacin or the pharmaceutical acceptable salts thereof and loteprednol etabonate, wherein the weight ratio of loteprednol etabonate to levofloxacin is 1:0.2-5. The use of ophthalmic, otic or nasal pharmaceutical composition of the invention in preparation of the medication for treatment of conjunctivitis, keratitis, blepharitis, dacrycystitis, hordeolum, corneal ulcer and ocular infection accompanied with ophthalmitis and even inflammation of the surrounding tissues, to prevent increase of bacterial infection risks and the tissue inflammation of the infected area after the ophthalmic surgeries or ocular injuries, to treat or alleviate the bacterial infection in combination with the tissue inflammation of the infected area, or to treat tympanitis, otitis externa and infective rhinitis.
Owner:SHENZHEN REGOO LAB
Aerosolized fluoroquinolones and uses thereof
ActiveUS7838532B2Reduce riskHigh levelPowder deliveryHeavy metal active ingredientsAerosol drugsLevofloxacin
Owner:HORIZON ORPHAN LLC
Medicament composition for eyes or nose, and uses thereof
The present invention provides a combination of medicines for eyes or ears and nose, which comprises levofloxacin and loteprednol carbon ester; wherein, the weight ratio of the loteprednol carbon ester to the levofloxacin is between 1 to 0.2 and 1 to 5. The combination of medicine for eyes or ears and nose of the present invention is used for curing conjunctivitis, keratitis, blepharitis, dacryocystitis, hordeolum, corneal ulcer and eye infection with inflammation of eyes or even inflammation of tissue around eyes. The combination is also used for preventing bacterial infection risk after ophthalmology operation or eye injury and inflammation of the infected region, or the combination is used for curing or alleviating bacterial infection and tissue inflammation of the infected region after ophthalmology operation or eye injury, or cure tympanitis, otitis externa and infectious rhinitis.
Owner:SHENZHEN REGOO LAB
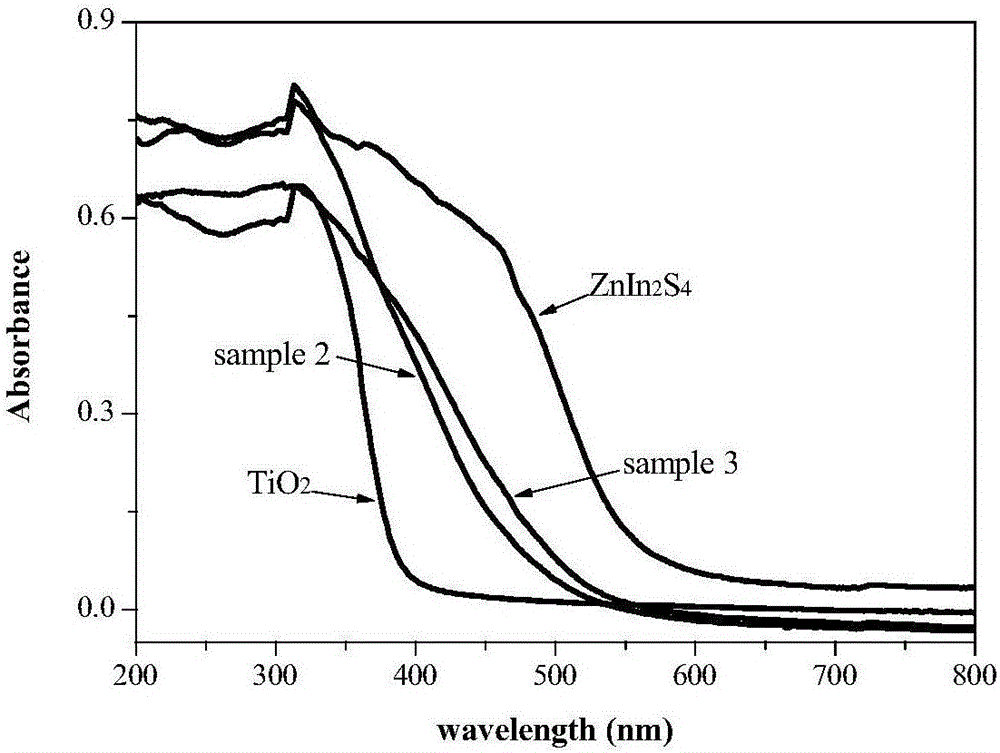
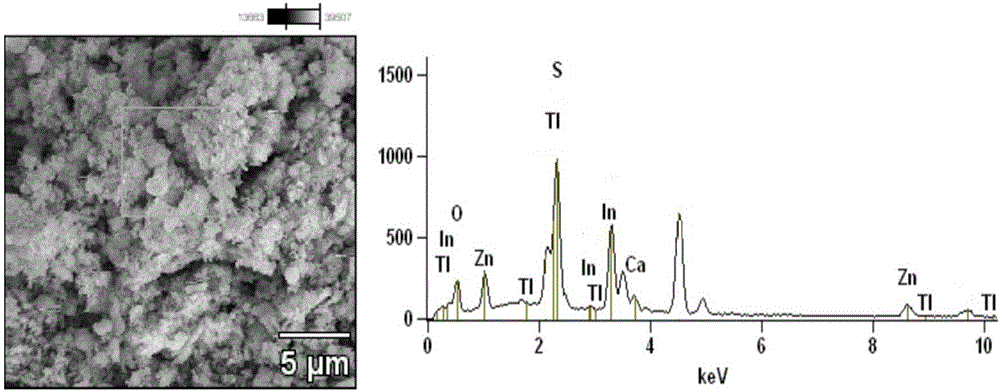
Preparation method and application of compound photocatalyst for growth of ZnIn2S4 on surfaces of TiO2 hollow spheres
ActiveCN106268868AEasy to prepareEasy to operatePhysical/chemical process catalystsWater/sewage treatment by irradiationLevofloxacinMethyl orange
The invention discloses a preparation method and application of a novel visible light response compound photocatalyst for growth of ZnIn2S4 on the surfaces of TiO2 hollow spheres. The method can be applied to treatment of water body pollution. The method includes the following steps of ultrasonically dispersing the obtained TiO2 hollow spheres in water, and then preparing a ZnIn2S4 / TiO2 nanometer compound hollow sphere photocatalyst through an in-situ hydrothermal method. The prepared ZnIn2S4 / TiO2 nanometer compound hollow sphere photocatalyst can be used for catalyzing and degrading tetracycline hydrochloride, levofloxacin and methyl orange under visible light. The obtained ZnIn2S4 / TiO2 nanometer compound hollow sphere photocatalyst can capture more visible light and has a matched band gap structure, photoelectron and hole separation efficiency can be improved, good interface contact promotes photoelectron transmission, and the visible light catalytic activity of the catalyst is remarkably improved.
Owner:JIANGSU UNIV
Pithecellobium clypearia extracts and application of extract in preparation of medicines for treating methicillin-resistant staphylococcus aureus
ActiveCN103385912AHas a sensitizing effectRealize comprehensive utilizationAntibacterial agentsPlant ingredientsEthyl acetatePharmaceutical Substances
The invention discloses water, ethanol and ethanol aqueous extracts of traditional Chinese medicine pithecellobium clypearia and application of corresponding petroleum ether, ethyl acetate, normal butanol and a water extractants in preparation of medicines for treating methicillin-resistant staphylococcus aureus (MRSA) and antibiotics anti-MRSA sensitization medicines. Meanwhile, the invention further discloses a preparation method of the extracts or extractants. Experimental results show that water, 10% ethanol, 30% ethanol, 60% ethanol, 95% ethanol extracts of pithecellobium clypearia and corresponding ethyl acetate, normal butanol and water extractants have stronger anti-MRSA effect, wherein the activity of the ethyl acetate extracting part of the 60% ethanol extract of pithecellobium clypearia is the strongest, and the ethyl acetate extracting part of the 60% ethanol extract of pithecellobium clypearia has sensitization effect on erythrocin, ceftriaxone sodium, levofloxacin for treating MRSA.
Owner:HUACHENG PHARMA FACTORY GAUNGZHOU
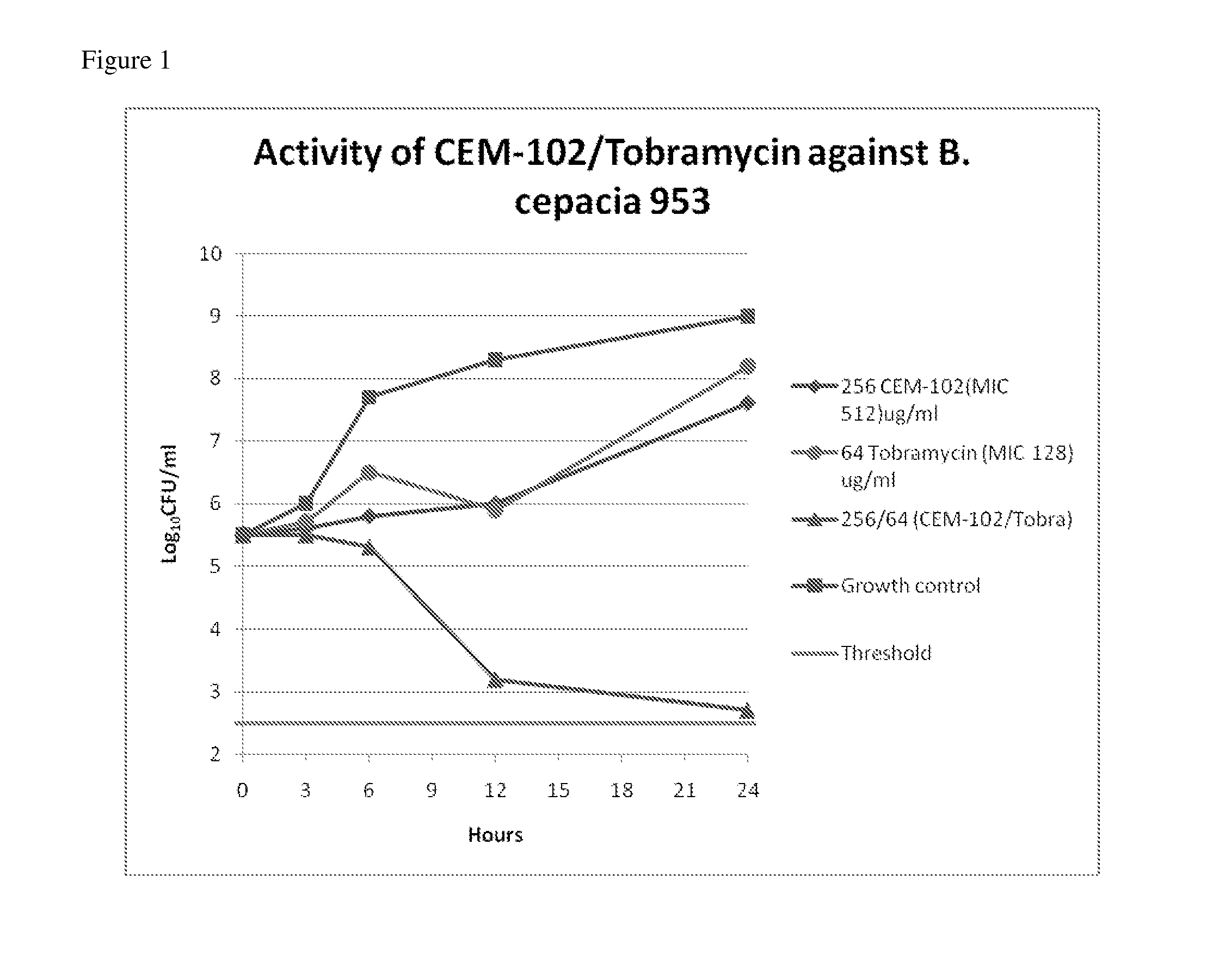
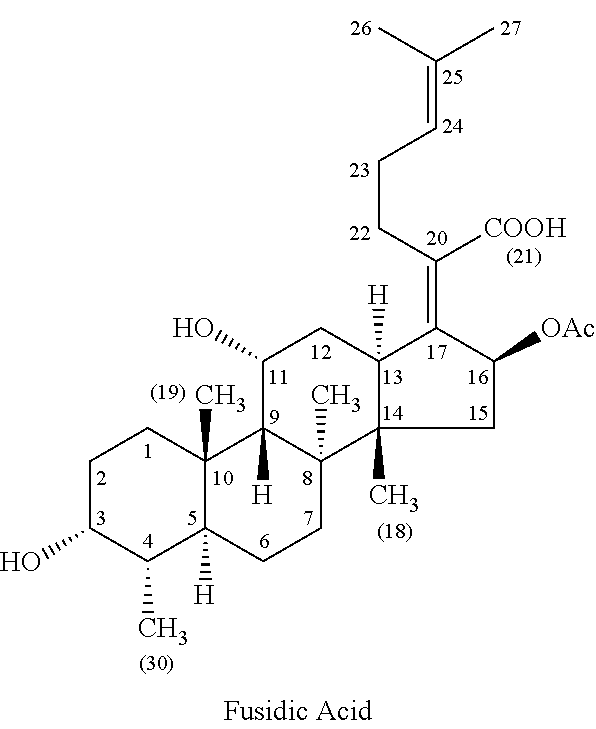
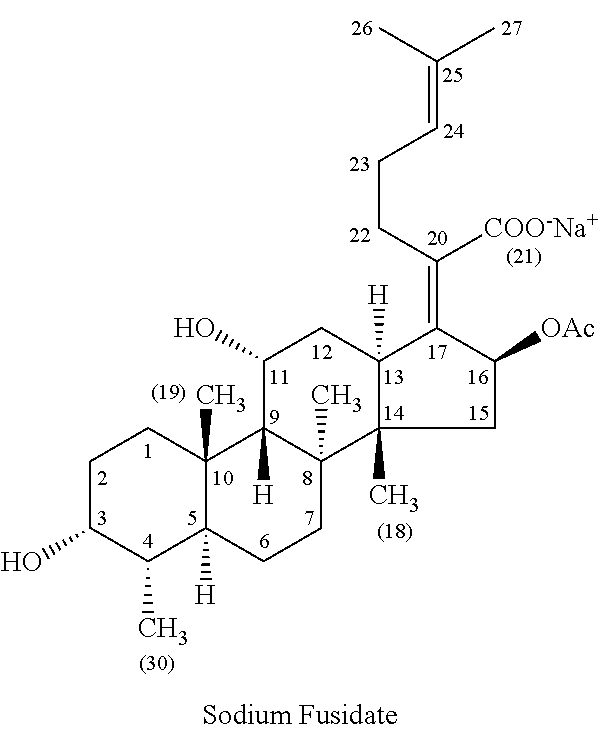
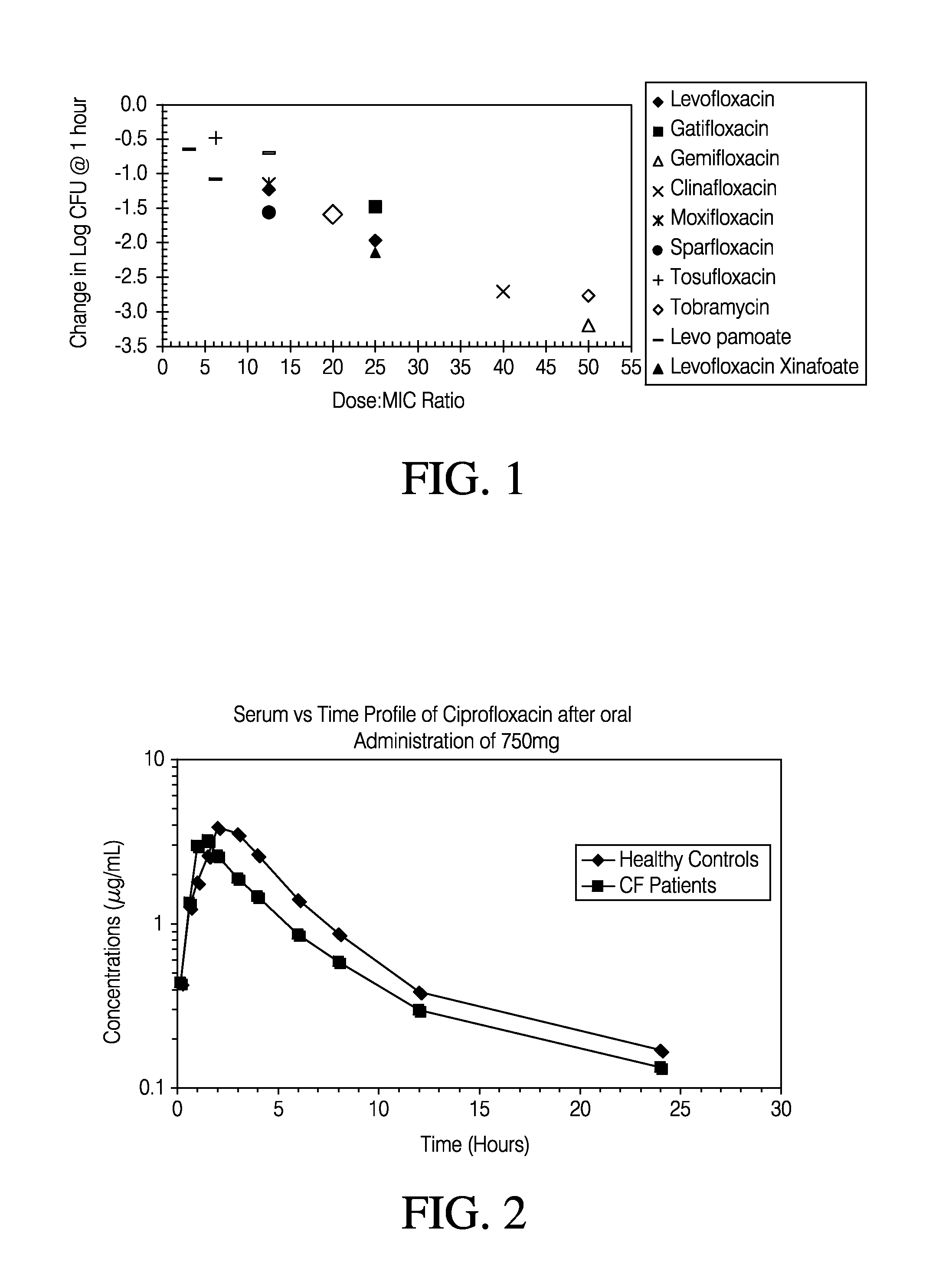
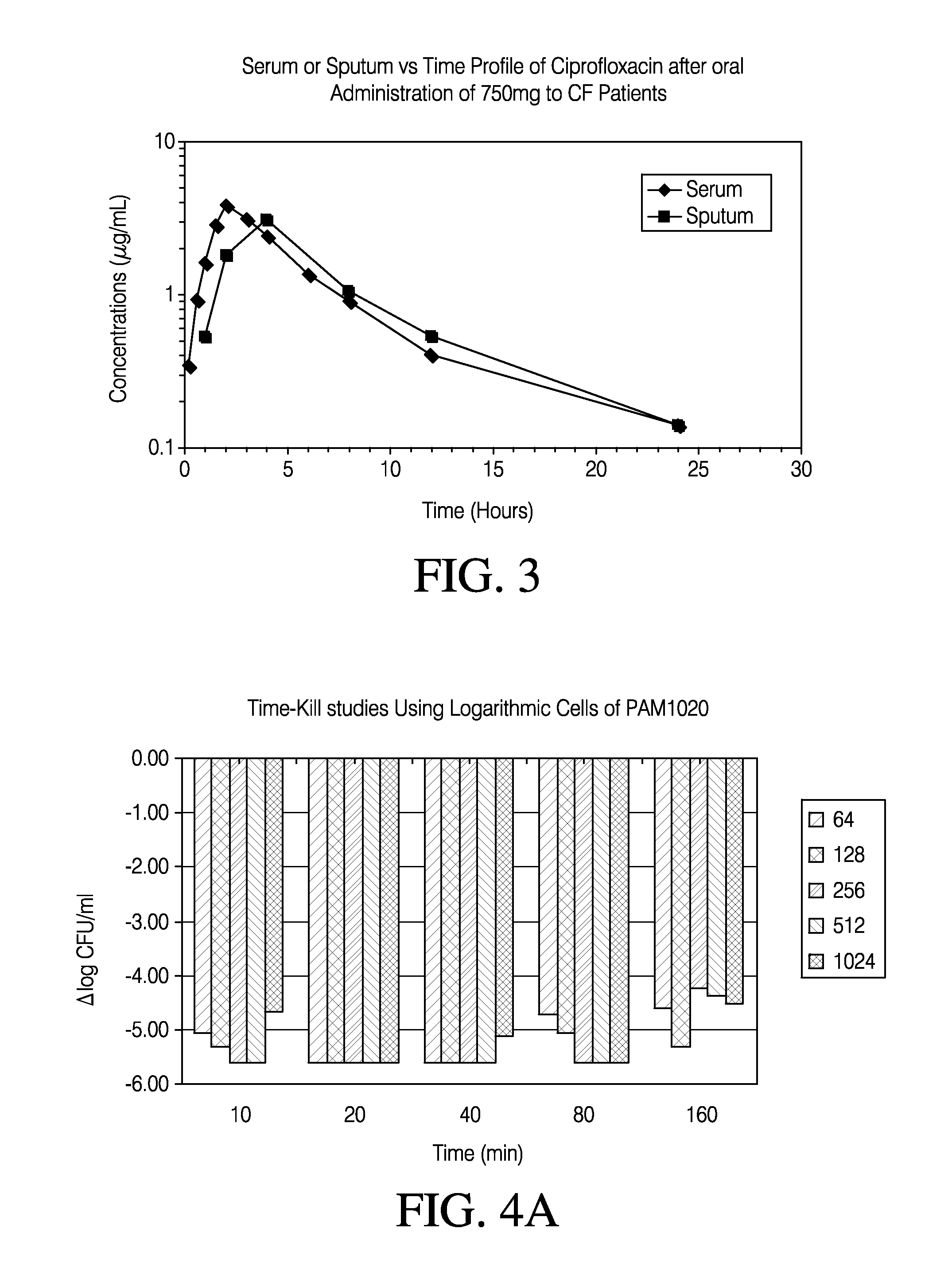
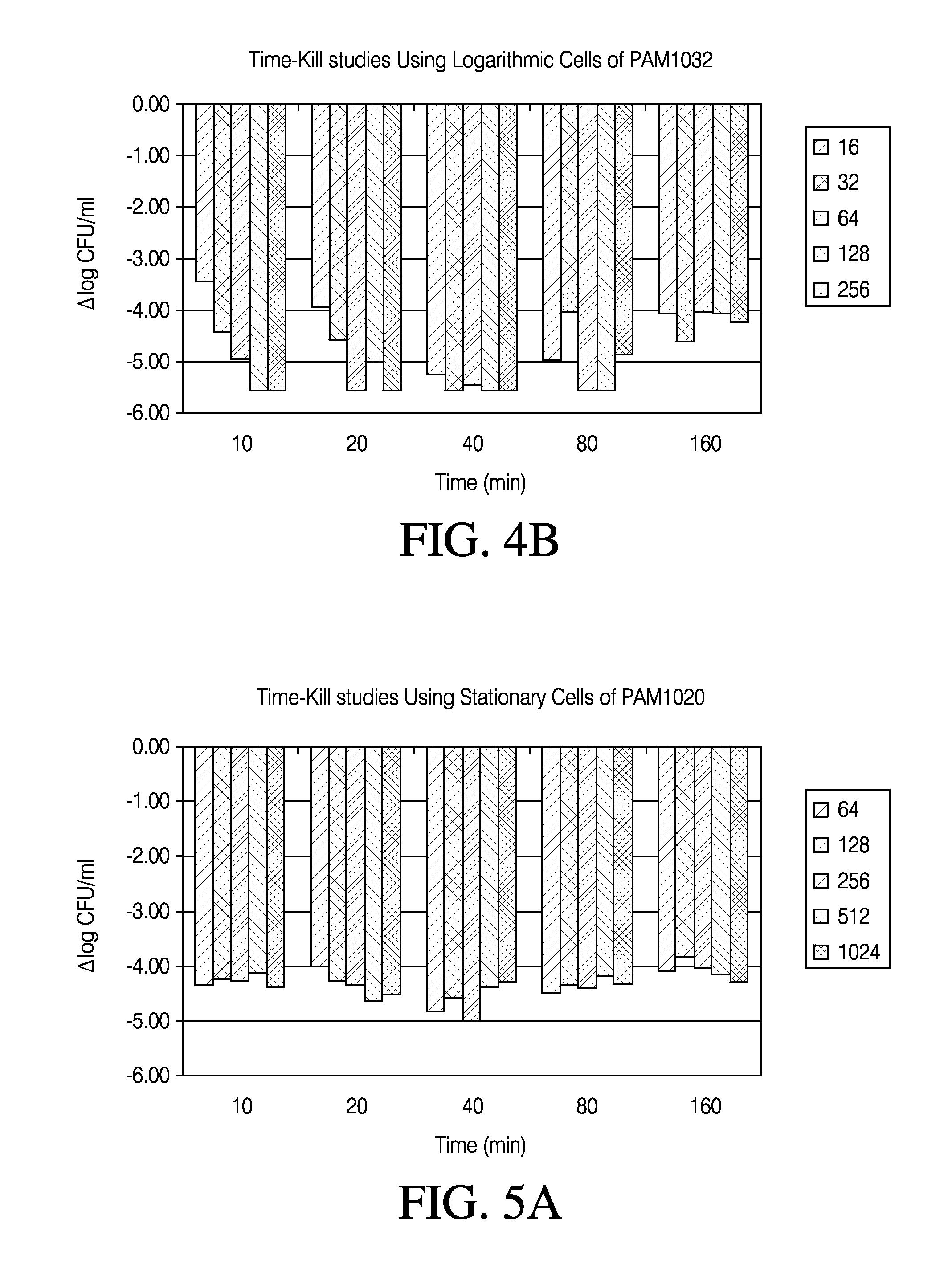
Methods of treating bacterial infections through pulmonary delivery of fusidic acid
Methods for the treatment of bacterial infections in the respiratory system of a subject, such as the lungs of a subject, using fusidic acid alone or in combination with a second bacterial agent such as tobramycin, amikacin, fosfomycin or levofloxacin are described.
Owner:CEMPRA PHARMA INC
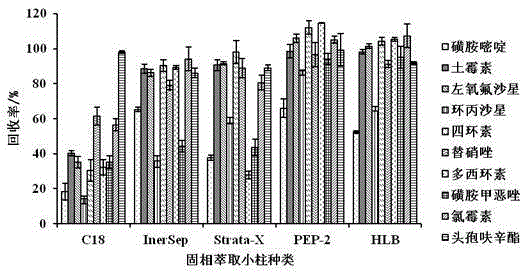
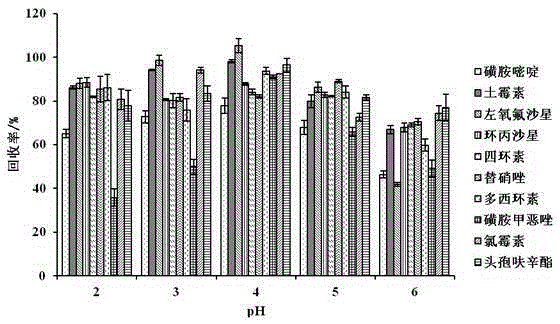
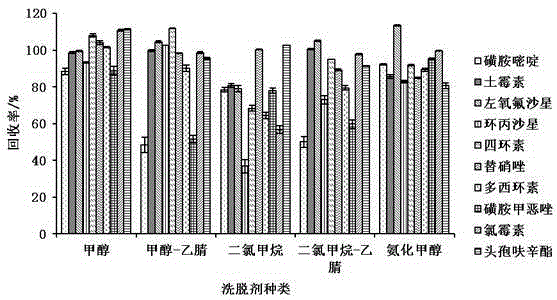
Method for determining 10 kinds of antibiotics in water environment through combination of sample pre-treatment technology and HPLC-MS
The present invention relates to a method for determining 10 kinds of antibiotics in a water environment through combination of a sample pre-treatment technology and HPLC-MS, and belongs to the field of detection of safety of trace organic contaminant residue in the water environment. The method is characterized in that a water sample is separated and enriched through combination of solid phase extraction and dispersive liquid-liquid microextraction (SPE-DLLME), and then an ultra-high performance liquid chromatography-mass spectrometry instrument (UPLC-MS / MS) is adopted as a detection tool to directly determine the contents of 10 kinds of common antibiotics in the water environment (drinking water, tap water, river water, sewage treatment plant influent and effluent), wherein the 10 kinds of the common antibiotics respectively are sulfadiazine, sulfamethoxazole, oxytetracycline, tetracycline, doxycycline, ciprofloxacin, levofloxacin, chloramphenicol, cefuroxime axetil and tinidazole. According to the present invention, the water sample pre-treatment method and the instrument detection conditions are investigated and optimized, and the optimal SPE-DLLME-UPLC-MS / MS method is established and is successfully applied for the real sample determination; and compared with the traditional method, the method of the present invention has advantages of high sensitivity, high extraction recovery rate, wide application objects, environmental protection, and the like.
Owner:SHENYANG PHARMA UNIVERSITY
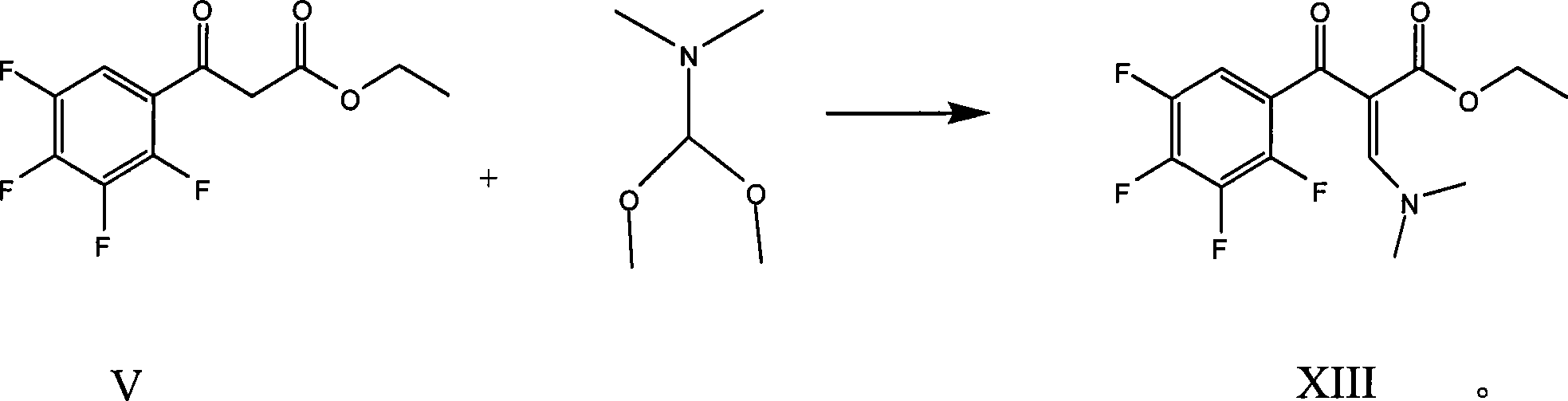
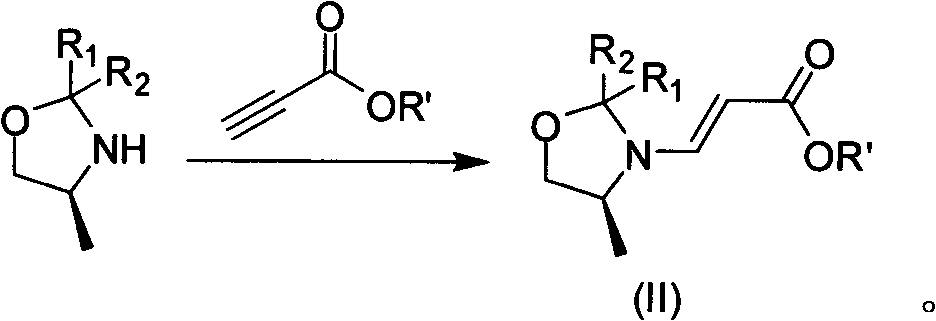
Preparation process of lavo-ofloxacin and ofloxacin
InactiveCN101519361AReduce generationIncrease production levelsOrganic compound preparationAntiinfectivesPotassium fluorideReaction temperature
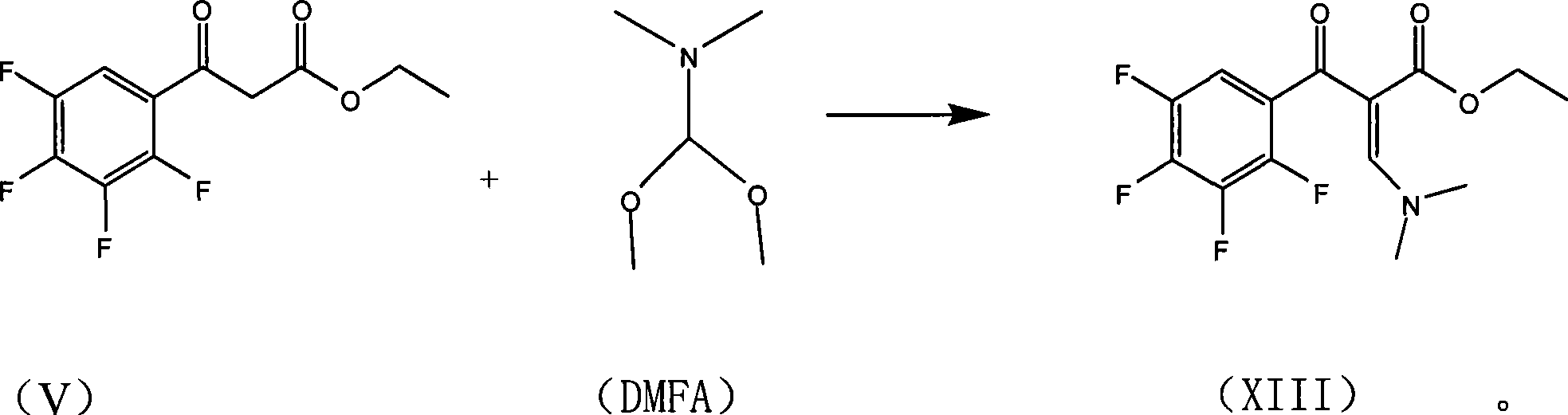
The invention relates to a preparation process of lavo-ofloxacin and ofloxacin which are anti-infectious medicaments, belonging to the synthetic process with tetrafluorobenzoic aid as raw material. The preparation method is characterized in that (2, 3, 4, 5-phenyl tetrafluoride formyl) ethyl acetate and DMFA react for 1.0-1.5h in toluene at 50-55 DEG C with the existence of acylating catalyst; the reaction product is washed by water, and an aqueous layer is separated; at 30-35 DEG C, L-amino propanol is dripped in an oil layer to carry out replacement reaction for 1.5-2.0h; toluene is decompressed, recovered and dried proper quantity of DMF is added to the oil layer for diluting; the diluted oil layer is dripped into back-flow DMF with the existence of anhydrous potassium fluoride to carry out back-flow reaction for 6h; DMF is recovered, water is added for centrifugation, acid is added to the obtained solid to be hydrolyzed to prepare lavo-perfluorocarboxylic acid, the lavo-perfluorocarboxylic acid reacts with N-methyl piperazine in DMSO at 90-110 DEG C by taking triethylamine as an acid-binding agent, and the lavo-ofloxacin is obtained after the fine purification of the product of reaction. The process improves the reaction condition of (2, 3, 4, 5-phenyl tetrafluoride formyl) ethyl acetate and DMFA, lowers the reaction temperature, shortens the reaction time and improves the reaction yield of lavo-fluoro ester serving as a reaction intermediate by 20 percent.
Owner:HENAN TOPFOND PHARMA
Polyvinyl alcohol/sodium alginate medicine-loading hydrogel dressing having light-responsive bacteria resistance and preparation method of polyvinyl alcohol/sodium alginate medicine-loading hydrogel dressing
ActiveCN107496975AWith mechanical propertiesGood biodescriptiveAntibacterial agentsOrganic active ingredientsBiocompatibility TestingUltraviolet lights
The invention relates to polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing having light-responsive bacteria resistance and a preparation method of the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing, wherein a macromolecular medicine realizing fracturing under ultraviolet light is distributed inside the hydrogel dressing. The preparation method of the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing comprises the following steps: grafting micromolecule antibiotic, namely, levofloxacin to polyethylene glycol macromolecular chains with amino groups at two ends through molecules with ultraviolet light response property, thus obtaining the macromolecular medicine realizing fracturing under ultraviolet light; and carrying out repeated freezing and thawing on a mixed solution of polyvinyl alcohol solution, sodium alginate solution and the macromolecular medicine realizing fracturing under ultraviolet light, thus obtaining the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing having light-responsive bacteria resistance. The adopted raw materials have good biocompatibility, the obtained hydrogel dressing has certain mechanical property and has the ultraviolet-light-responsive antibacterial property, namely, the hydrogel dressing can realize controlled release of antibiotics under irradiation of 365nm ultraviolet light, and thus the polyvinyl alcohol / sodium alginate medicine-loading hydrogel dressing is suitable for protecting and treating the wound surface.
Owner:ZHEJIANG UNIV
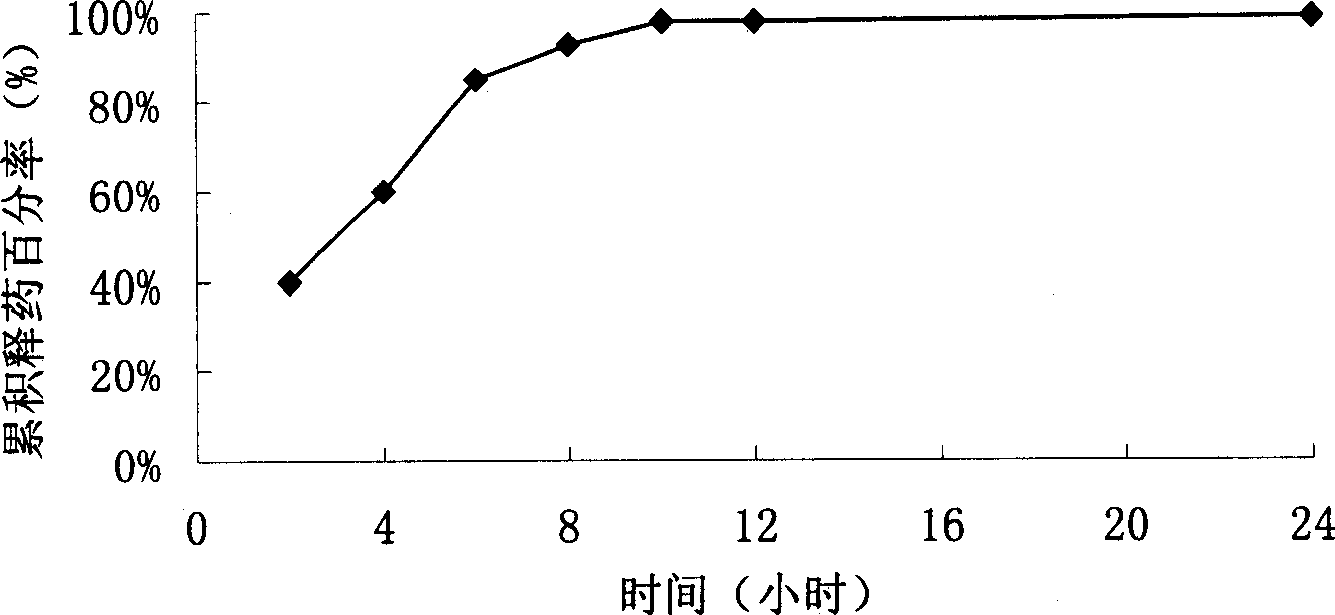
Long-acting sustained-release wound dressing containing levofloxacin sustained-release microspheres, and preparation method thereof
InactiveCN102302458ASmall particle size distributionFlat surfaceAntibacterial agentsOrganic active ingredientsWound dressingMicrosphere
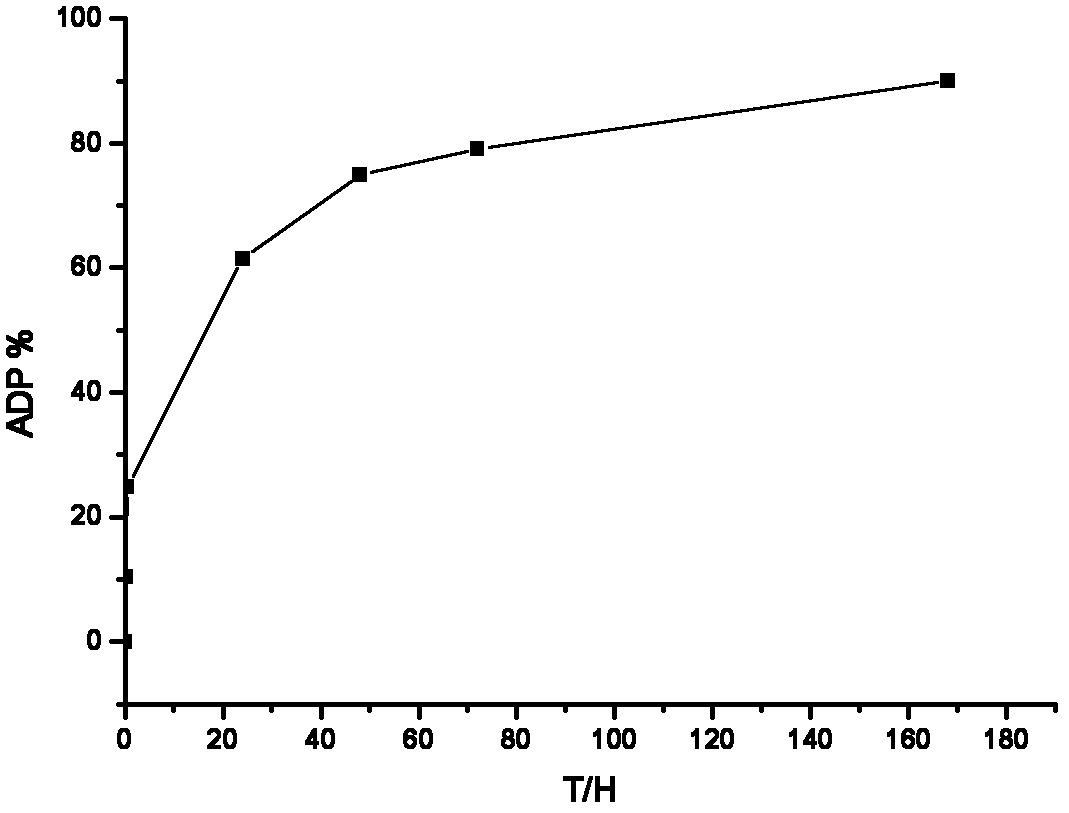
The invention discloses a long-acting sustained-release wound dressing containing levofloxacin sustained-release microspheres. According to the preparation method of the wound dressing, levofloxacin sustained-release microspheres with grain diameter between 10 and 20mu m are fixed in a composite medical non-woven fabric which mainly comprises chitosan fiber, alginate fiber, viscose fiber, and hydrophobic ethylene propylene fiber; and the long-acting sustained-release wound dressing is prepared by taking the levofloxacin sustained-release microspheres as a medicinal component. In a use process, the medicament quickly release levofloxacin after contacting the blood, tissues and surrounding skin of the wound, and can continuously release levofloxacin for a long time, and the effective release of levofloxacin accumulatively reaches 168h. The long-acting sustained-release wound dressing is applied to treatment of bacterium infection of burnt and scalded skin, and the microsphere state medicament can remarkably improve the bioavailability of effective components of the medicament; and the long-acting sustained-release wound dressing has excellent moisture penetrability, hygroscopicity, mechanical tensile property and biocompatibility, the frequency of dressing change can be reduced, and the curative effect and administration safety cam be enhanced.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100158957A1Reduce riskHigh levelAntibacterial agentsPowder deliveryAerosol drugsLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
One-step synthesizing method of levofloxacin and ofloxacin
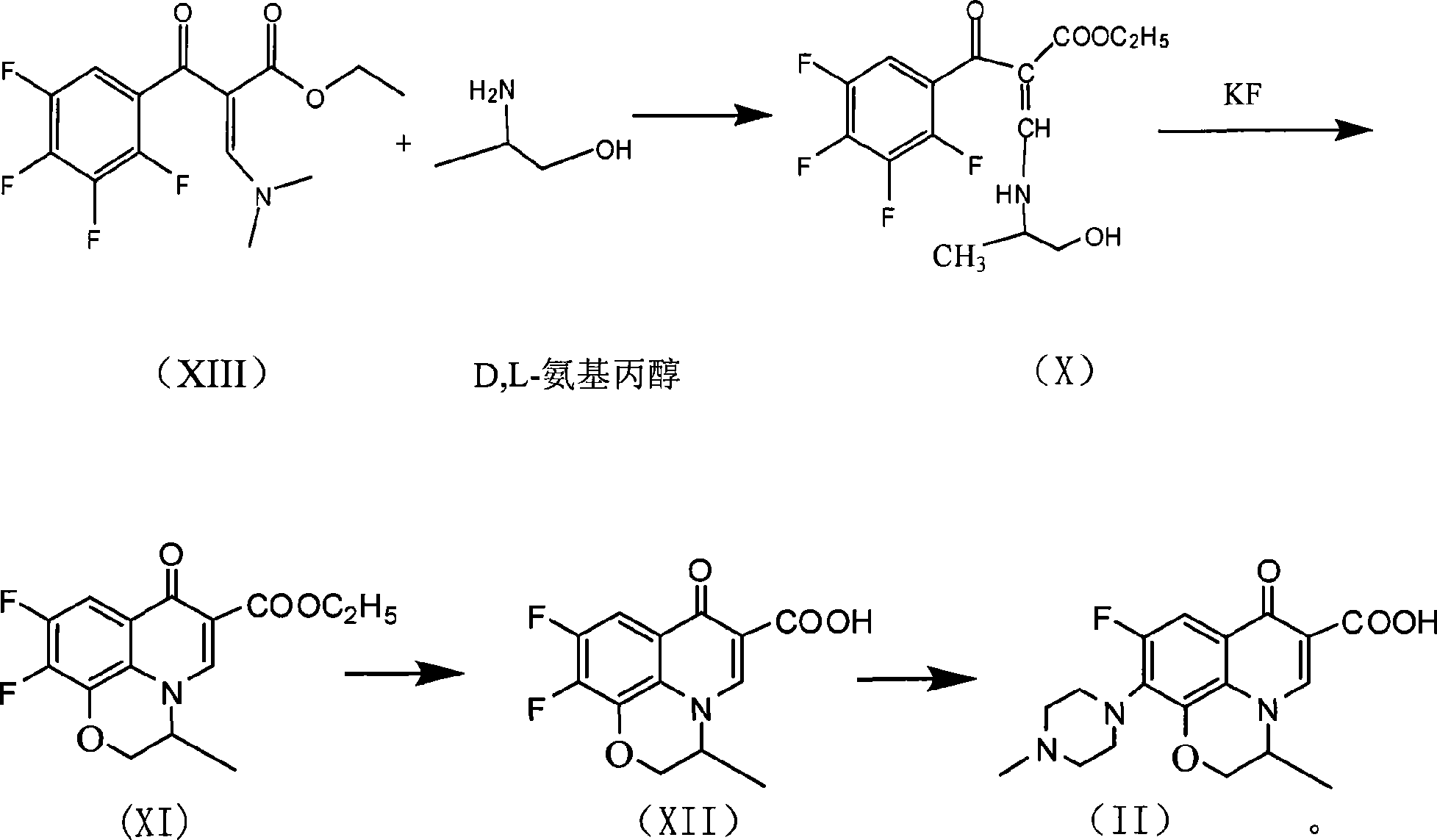
The invention provides a one-step synthesizing method of levofloxacin and ofloxacin. According to the invention, S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxygen-7H-pyrido[1,2,3-delta]-[1,4]-benzoxazine-6-carboxylic acid ester or 9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-delta]-[1,4]-benzoxazine-6-carboxylic acid ester is adopted as a raw material; the raw material is subjected to a reaction with alkali in an organic solvent or water or a mixed solvent of an organic solvent and water, such that a corresponding carboxylic acid salt is formed; the solvent is directly removed by evaporation after a hydrolysis process; The product is directly added into N-methylpiperazine in a form of a carboxylic acid salt; and a piperazine concentration reaction is carried out, such that levofloxacin or ofloxacin is obtained. The method provided by the invention is simple to operate. With the method, a hydrolysis and then acid adjusting process is not needed, reaction cost is reduced, production period is short, pollution is low, raw material utilization rate is high, the method is economical and simple, and the yield and purity of obtained levofloxacin and ofloxacin are high.
Owner:ZHEJIANG UNIV +1
Preparation method of magnetic microspheres-based levofloxacin surface imprinted material
InactiveCN104788612AImprove adsorption capacityGood magnetic responseOther chemical processesAlkali metal oxides/hydroxidesSynthesis methodsMicrosphere
The invention relates to a preparation method of a magnetic microspheres-based levofloxacin surface imprinted material. The preparation process mainly comprises three steps: preparation of magnetic microspheres; coating of the magnetic microspheres with a molecularly imprinted material; and elution of template molecules. A synthetic method provided by the invention is simple. The prepared magnetic microspheres-based surface molecularly imprinted material has specific recognition function, high adsorption capacity and rapid adsorption ability of levofloxacin and has chiral resolution function of ofloxacin, has good magnetic response and high mechanical strength, can be used as an absorption filler or a coating material, also can be used in preparation of a molecular imprinting sensor and a chip, and is of great significance for researches on specific recognition, chiral resolution and highly sensitive detection of floxacin drugs.
Owner:CHINA PHARM UNIV
Ophthalmic preparation of levofloxacin and prednisolone acetate and preparation method thereof
ActiveCN102085203ALess irritatingReduce secretionAntibacterial agentsOrganic active ingredientsOphthalmologyLevofloxacin
The invention relates to an ophthalmic preparation of levofloxacin and prednisolone acetate and a preparation method thereof. Particularly, the invention relates to an ophthalmic preparation containing levofloxacin and prednisolone acetate with an effective amount for treatment and / or prevention, a high polymer material, a surfactant, a complexing agent and water. The invention also relates to anophthalmic preparation containing the ophthalmic preparation provided by the invention and a medicinal excipient mixed with the ophthalmic preparation provided by the invention before application, and a preparation method of the ophthalmic preparation. The ophthalmic preparation provided by the invention not only has a favorable effect of treating eye diseases, but also has very low stimulation to the eyes.
Owner:SHENYANG XINGQI PHARM CO LTD
Magnesium modified biomass and application thereof
InactiveCN106423064AWide variety of sourcesLow priceOther chemical processesWater contaminantsTime rangeFiltration
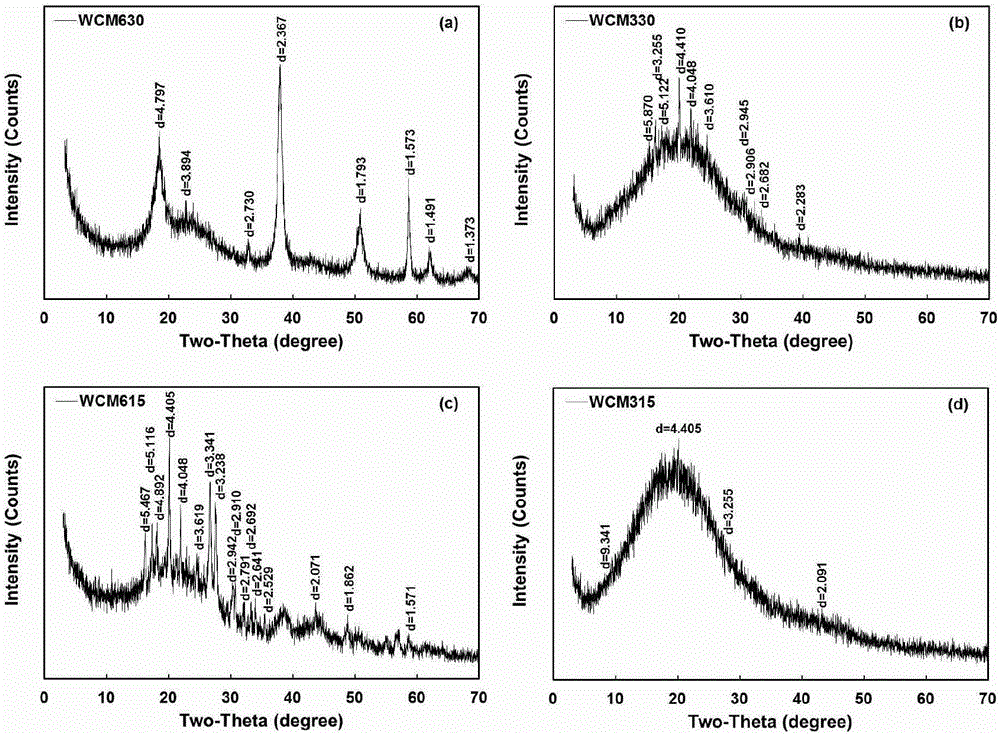
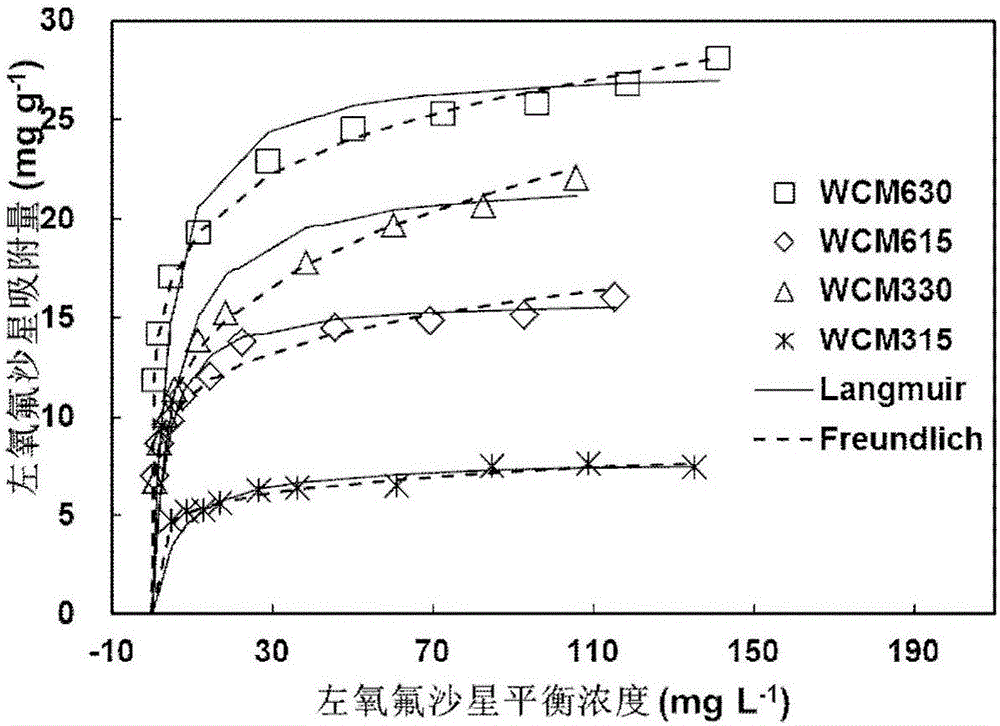
The invention discloses magnesium modified biomass, which is obtained by mixing biomass powder with a MgSO4 solution and then performing vacuum suction filtration, drying and high-temperature pyrolysis. The invention further provides application of the magnesium modified biomass to removal of antibiotics from underground water. The method has the advantages that the removal efficiency on levofloxacin in the underground water is high; the adsorption capability is high; the maximum adsorption quantity on the levofloxacin in the underground water reaches 7.5mg g<-1> to 27mg g<-1>; the levofloxacin in the underground water can be continuously adsorbed in a long time range; in an implantation process, new waste or pollutants cannot be generated; the environment risk is low; meanwhile, the preparation method is simple; the production period is short; special or expensive chemical engineering equipment is not needed; the industrial production can be easily realized.
Owner:NANJING UNIV
Disposable levofloxacin hydrochloride eye drops without bacteriostatic agent and preparation method thereof
InactiveCN101461777AAvoid side effectsAvoid potential dangerAntibacterial agentsOrganic active ingredientsLevofloxacinEye drop
The invention discloses disposable levofloxacin hydrochloride eye drops and a preparation method thereof. The disposable levofloxacin hydrochloride eye drops comprise levofloxacin hydrochloride, a pH regulator, an isotonic agent, a stabilizing agent, a thickening agent, and the like; and the preparation method adopts a bacteria-free bottling process or an autoclaving process. In addition, the eye drops are independently packaged in single dose for disposable use, thereby the sterility performance of products is guaranteed and the products are more safe, reliable, simple, convenient, and sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100040560A1Overcome resistancePrevent further resistanceAntibacterial agentsBiocideInhalationLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Levofloxacin slow release micropill, its preparation method and uses
InactiveCN1839846AFacilitated releaseReach plasma concentrationAntibacterial agentsOrganic active ingredientsLevofloxacinPharmaceutical formulation
The invention relates to a slow release micro-pellet preparation containing Levofloxacin, its preparation process and therapeutic use, wherein the micro-pellet preparation contains 0-40% of conventional medicinal particles and 60-100% of slow release medicinal micro-pellet, the micro-pellet contains medicinal core including Levofloxacin, and slow release coating layer whose content being 4-100% of the pellet core.
Owner:CHINA PHARM UNIV +2
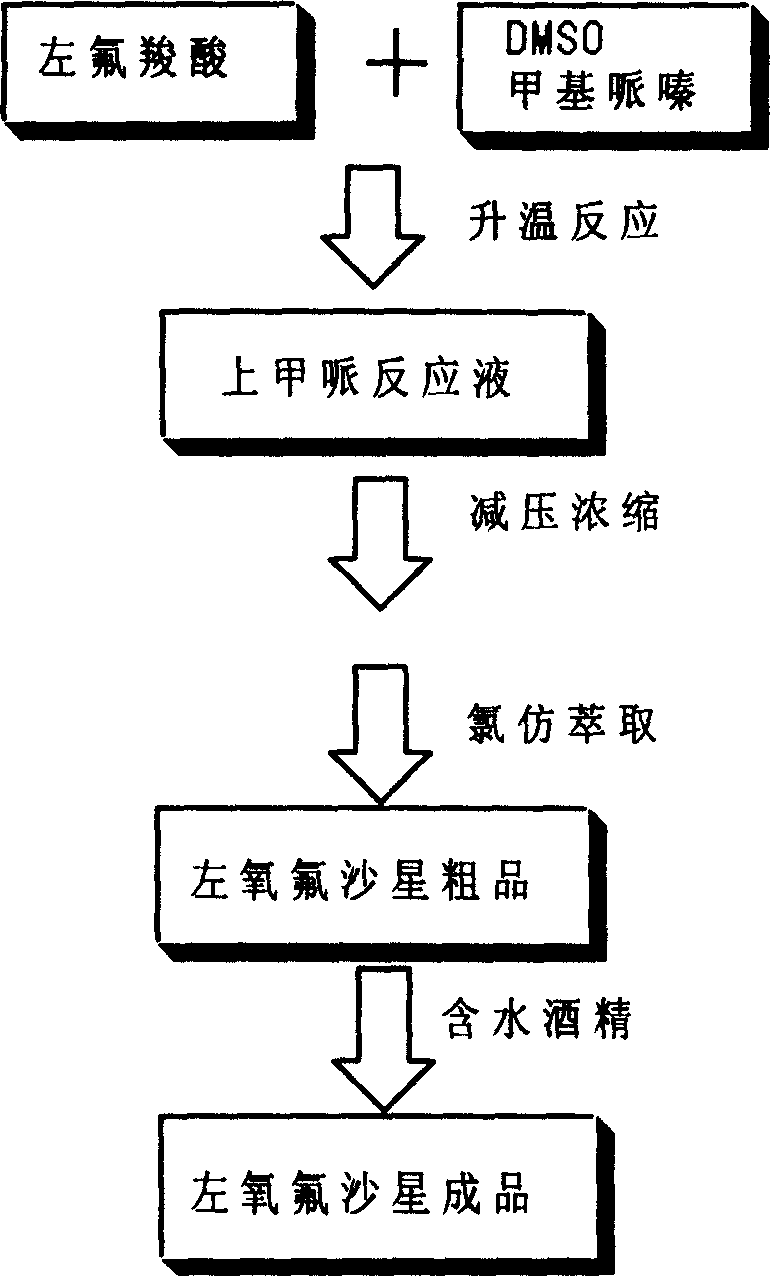
Post processing method for preparing levo-ofloxacin
The present invention provides an after-treatment method for preparation of levofloxacin. Said method is characterized by that after reaction with methylpipie it can directly recover solvent, and adopts the method of firstly salt-forming and then using alkali to make neutralization so as to effectively obtain levofloxacin with high purity.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Disposable levofloxacin lactate eye drops without bacteria inhibitor and preparation method thereof
InactiveCN101455633AAvoid side effectsAvoid potential dangerAntibacterial agentsOrganic active ingredientsLevofloxacinBiomedical engineering
The present invention discloses a disposable levofloxacin lactate eye drop and a preparing method thereof, wherein the disposable levofloxacin lactate eye drop comprises levofloxacin lactate, pH modifying agent, isoosmotic agent, stabilizing agent, thickening agent, etc. The preparing method adopts an aseptic manipulation filling technique or hot pressing sterilizing technique. Furthermore the eye drop according to the invention adopts single-dose independent packaging and disposable using. The sterilized performance of product is guaranteed. The product is safer, more reliable, easier and more sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Eye drop capable of significantly increasing medicament effect
ActiveCN101278908AGood antibacterial effectImprove antibacterial propertiesOrganic active ingredientsSenses disorderEye dropStabilizing Agents
The invention provides a levofloxacin eyedrops and a preparation method thereof. The eyedrops contains levofloxacin, sodium chloride, sorbic acid and water; refrigerant and thickening agent also can be added; and PH value is regulated. The sorbic acid is taken as antibacterial synergist, stabilizing agent of formulation, comforting agent of a product and bacterial inhibitor to be capable of remarkably strengthening bacteriostatic effect of the levofloxacin, lengthening residence time of drug in eye region, stabilizing active component, keeping drug effect, reducing production cost and being suitable for various men to use, thereby providing a simpler and more convenient dosage form composition for the levofloxacin eyedrops.
Owner:江苏广承药业有限公司
Eye in-situ gel of chiral anti-glaucoma medicine L-3alpha alkyla acyloxy-6belta alkyla acyloxy tropane and preparation method thereof
InactiveCN101396333AReduce eliminateAccurate doseSenses disorderPharmaceutical delivery mechanismAdjuvantIrritation
The invention relates to an ophthalmic in-situ jelly of chiral anti-glaucoma medicine levofloxacin tropane. The ophthalmic jellies concretely comprises 0.03 percent to 0.3 percent of levofloxacin tropane, 10 percent to 20 percent of hydrophilic polymer material, 0 percent to 20 percent of humectant, 0.1 percent to 10 percent of permeation pressure regulator, 0.01 percent to 0.3 percent of antiseptic / bacteriostat, pH value regulator and water. In a preparation method, the materials are weighted according to the percentage; the levofloxacin tropane is solved in injection water, the hydrophilic polymer material is added under the stirring state, and the solution is stood over a night; the adjuvant such as the humectant and the like is added, and the pH value is adjusted as 4 to 9; the solution is filtered, the injection water is added to the total quantity, and the ophthalmic in-situ jelly is obtained. The ophthalmic in-situ jelly has the advantages of uniform and smooth quality, proper viscosity, convenient use, exact dosage, and long-term uniform distribution on the surface of eye cornea; besides, the jelly causes no hypersusceptibility and irritation, no blurred vision, can effectively prolong the acting time of the medicine on the eye cornea, and has wide application prospect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Ophthalmic or otic and nasal composition containing difluprednate and lavo-ofloxacin and application thereof
InactiveCN101564395AAntibacterial agentsOrganic active ingredientsInfective rhinitisInfectious Rhinitis
The invention provides an ophthalmic or otic and nasal pharmaceutical composition, which comprises lavo-ofloxacin and salts thereof and difluprednate, wherein the weight ratio of the difluprednate to the lavo-ofloxacin is 1:1-1:10. The ophthalmic or otic and nasal medicament composition is applied to treating conjunctivitis, keratitis, eyelid inflammation, dacryocystitis, hordeolum, corneal ulcer and eye infections with inflammations of eyes or surrounding tissues, or preventing increased risk of bacterial infections and tissue inflammations of infected parts after ophthalmic surgeries or eye injuries, or treating or relieving the bacterial infections combined with tissue inflammations of the infected parts after the ophthalmic surgeries, or treating otitis media, otitis externa and infectious rhinitis.
Owner:SHANDONG INST OF PHARMA IND
Levofloxacin hydrochloride sustained-release eye drops
InactiveCN106236706AAvoid pollutionReduce risk of damageAntibacterial agentsOrganic active ingredientsSodium hyaluronateEye drop
The invention discloses levofloxacin hydrochloride sustained-release eye drops which are ophthalmic preparations prepared by using levofloxacin hydrochloride as a pharmacodynamic raw material, matching with sodium hyaluronate to play a role of a thickener, and then matching with a metal ion complexing agent pharmaceutically acceptable to local parts of eyes, an osmotic pressure regulator and a pH regulator, wherein per 100 parts by weight of a finished product preparation contains 0.30-1.00 part by weight of levofloxacin hydrochloride and 0.1-1.00 part by weight of sodium hyaluronate. The medicine liquid provided by the invention has high viscosity, prolongs the time of medicine staying in the eyes, improves the absorption of the medicine, and improves the bioavailability of the eye drops; no preservative is added to the eye drops provided by the invention, so as to improve the biological safety of the eye drops.
Owner:GUANGDONG WHOLEWIN TECH
Levofloxacin hydrochloride tablet and preparation method thereof
ActiveCN103520124AReduce alkalinityGuaranteed not to precipitateAntibacterial agentsOrganic active ingredientsAlkalinityAlcohol
The invention discloses a levofloxacin hydrochloride tablet and a preparation method of the levofloxacin hydrochloride tablet. The preparation is prepared by blending and tabletting medicine-containing particles, citric acidenteric particles and a lubricant, wherein the citric acidenteric particles is prepared by dissolving the citric acid and the polyvinyl acetatephthalic acid ester in ethyl alcohol. According to the invention, the citric acid is wrapped in an enteric material and is not dissolved in the stomach, the citric acid is dissolved and released in the intestines, so that the alkalinity of the intestinal juice is reduced, the levofloxacin hydrochloride is enabled not to be separated out, and therefore, the bioavailability of the medicine is improved.
Owner:NANJING REDWOOD FINE CHEM CO LTD
Insoluble magnetic cobalt/defective g-C3N4 composite catalyst and application thereof to catalytic Oxone wastewater degradation
InactiveCN107983391AImprove visible light absorptionImprove catalytic performanceWater/sewage treatment by irradiationWater contaminantsMaterial synthesisWastewater
The invention discloses an insoluble magnetic cobalt / defective g-C3N4 composite catalyst and application thereof to catalytic Oxone wastewater degradation, and belongs to the technical field of composite catalytic material synthesis and organic wastewater catalytic degradation. The composite catalyst is characterized in that a preparation method of the insoluble magnetic cobalt / defective g-C3N4 composite catalyst concretely comprises the steps of preparation of magnetic cobalt catalysts with the cobalt mass percentage content being 11 percent, preparation of insoluble magnetic cobalt / defectiveg-C3N4 composite catalysts with the cobalt mass percentage content being 1.2 percent and the like. The insoluble magnetic cobalt / defective g-C3N4 composite catalyst can be used for efficiently catalyzing Oxone to degrade levofloxacin wastewater. The prepared insoluble magnetic cobalt / defective g-C3N4 composite catalyst has better infusibility in neutral environment; meanwhile, better catalytic performance is realized; certain photocatalytic performance is also realized; the insoluble magnetic cobalt / defective g-C3N4 composite catalyst can be applied to the degradation of nonbiodegradable organic pollutants.
Owner:HENAN NORMAL UNIV
Preparation method of magnetic cobalt-loaded ordered mesoporous carbon material, and applications of magnetic cobalt-loaded ordered mesoporous carbon material in catalytic degradation of levofloxacin waste water with Oxone
InactiveCN107890869AIncrease the areaLarge pore volumeWater contaminantsCatalyst activation/preparationSodium hydroxideWater treatment
The invention discloses a magnetic cobalt-loaded ordered mesoporous carbon material, and applications of magnetic cobalt-loaded ordered mesoporous carbon material in catalytic degradation of levofloxacin waste water with Oxone, and belongs to the technical field of mesoporous carbon material synthesis and organic waste water treatment. According to the preparation method, cobaltous sulfate heptahydrate, sodium hydroxide, and ammoniacal liquor are taken as raw materials to prepare Co3O4 solid, and the obtained Co3O4 solid, melamine, and F127 are subjected to hydrothermal reaction so as to obtain the magnetic cobalt-loaded ordered mesoporous carbon material. The magnetic cobalt-loaded ordered mesoporous carbon material is relatively high in specific surface area and pore volume; an oxidationsystem composed of the magnetic cobalt-loaded ordered mesoporous carbon material and Oxone possesses high degradation efficiency on levofloxacin waste water, no toxic or harmful side product is generated in degradation process, and recycling is realized.
Owner:HENAN NORMAL UNIV
Method for preparing levofloxacin
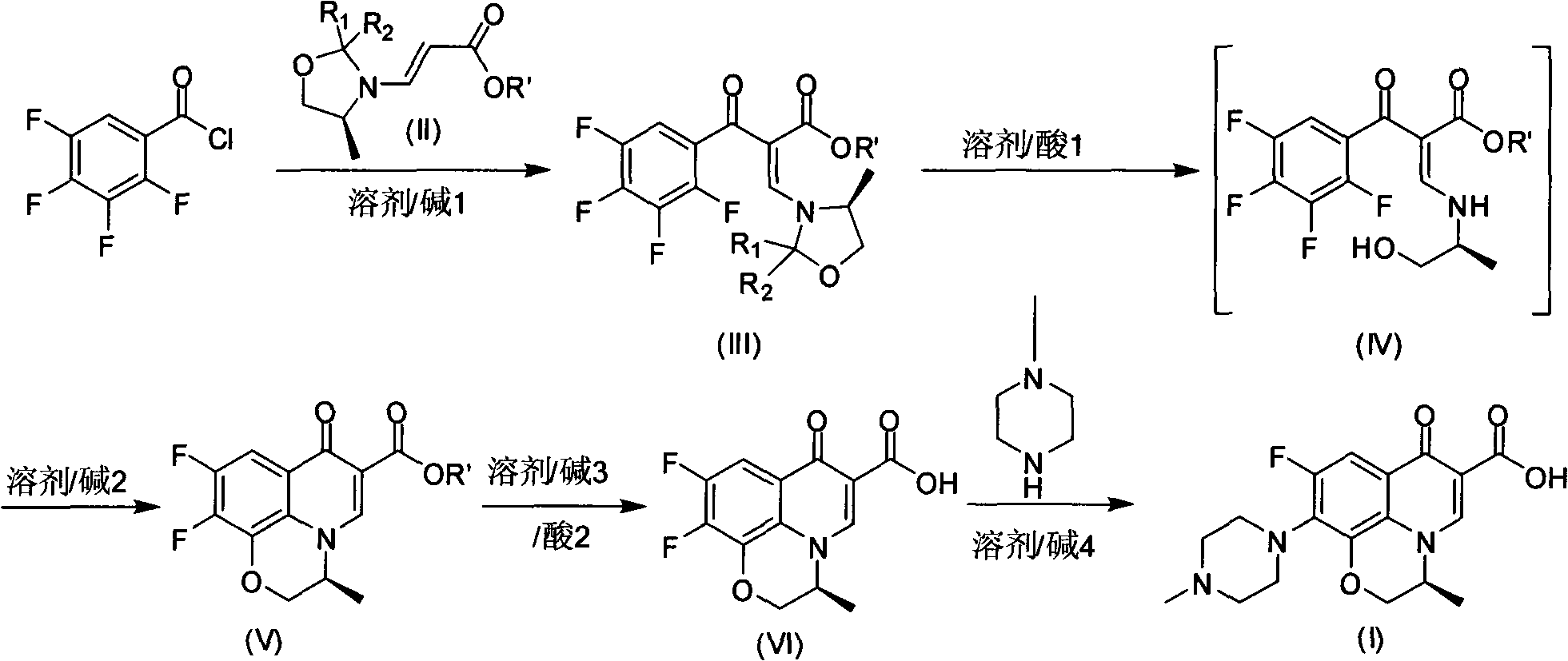
ActiveCN101659669AFew reaction stepsShort reaction timeOrganic chemistryOrganic solventSynthesis methods
The invention discloses a method for preparing levofloxacin, aiming at providing a method for preparing levofloxacin, with short production period, small pollution, high raw material utilization ratio, yield and purity and simple and convenient operation. The method comprises the following steps of: taking tetrafluorobenzoyl chloride as raw material to prepare S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxygen-7H-pyridino[1,2,3-de]-[1,4]- benzoxazine-6-carboxylic acid by a synthesis method, then reacting with alkali in organic solvent, and obtaining the levofloxacin. The method has the advantages that the tetrafluorobenzoyl chloride is utilized to react with S-3-(2-R1-2-R2-4-methyl imidazolidinyl) acrylic ester, and hydrolization is carried out directly while in post-treatment, and finally loopis closed, thus reducing reaction steps, shortening the reaction time and improving the reaction yield up to 85-90%. In preparing, organic or inorganic alkali is added, thus reducing the inventory amount of methyl piperazine and lowering the reaction cost and having good recovery rate.
Owner:CHENGDA PHARM CO LTD
Preparation method of titanium alloy material with good biocompatibility and antibacterial function coating
InactiveCN110115777AGood biocompatibilityImprove bindingMetallic material coating processesTissue regenerationApatiteBiocompatibility Testing
The invention discloses a preparation method of a titanium alloy material with good biocompatibility and an antibacterial function coating. The method comprises the steps that firstly, surface treatment is conducted, then the titanium alloy material after surface treatment and a calcium source solution are placed in a closed reaction container, and through a hydrothermal reaction, a hydroxyapatitecoating is formed on the surface of the titanium alloy material; a levofloxacin-ethyl alcohol antibiotics solution is used for bacterium inhibition treatment; washing and drying are conducted for obtaining the titanium alloy material with the good biocompatibility and the antibacterial function coating. The hydroxyapatite coating is good in biocompatibility and has a bacterium inhibition functionafter being treated through levofloxacin-ethyl alcohol, hydroxyapatite is firmly bonded to titanium alloy, no residual internal stress exists on the bonding face, the coating is uniform, high in purity, good in crystallization performance and easy to operate, and the prepared titanium alloy material with the function coating can be used as an orthopedic instrument implanted into an animal body.
Owner:YANGZHOU UNIV
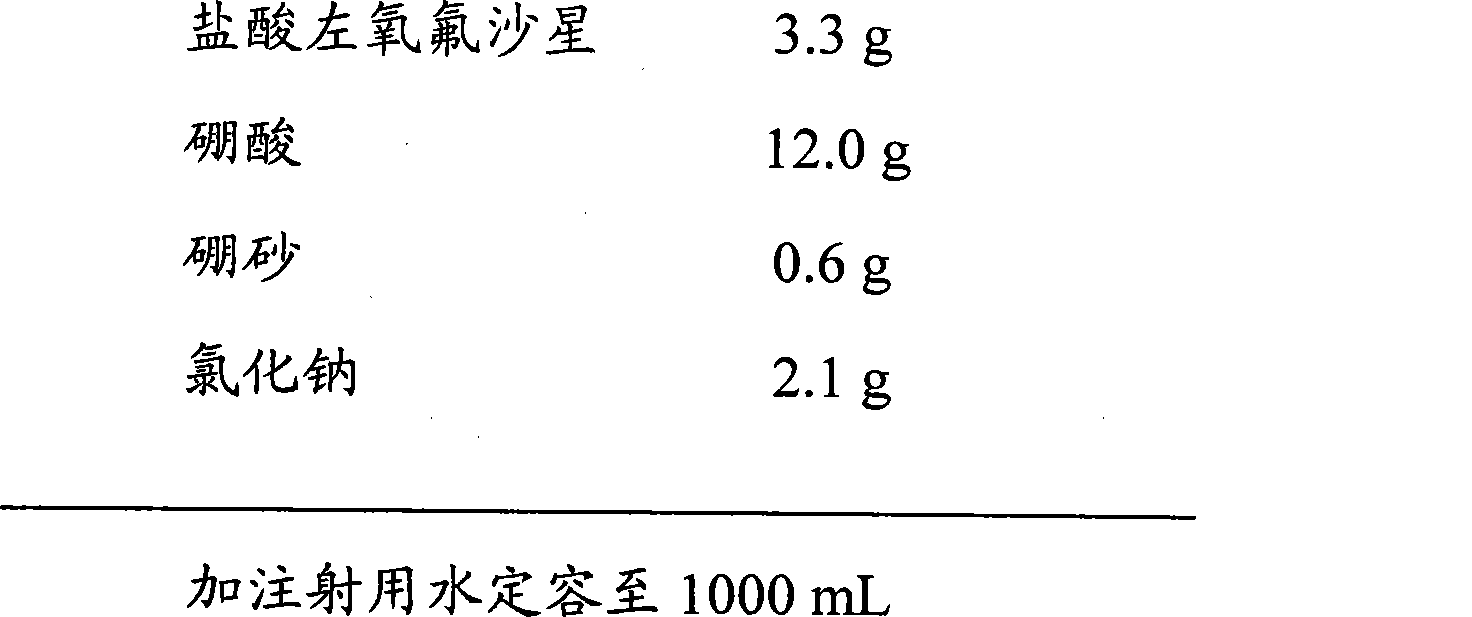
Method for preparing levofloxacin hydrochloride sodium chloride injection
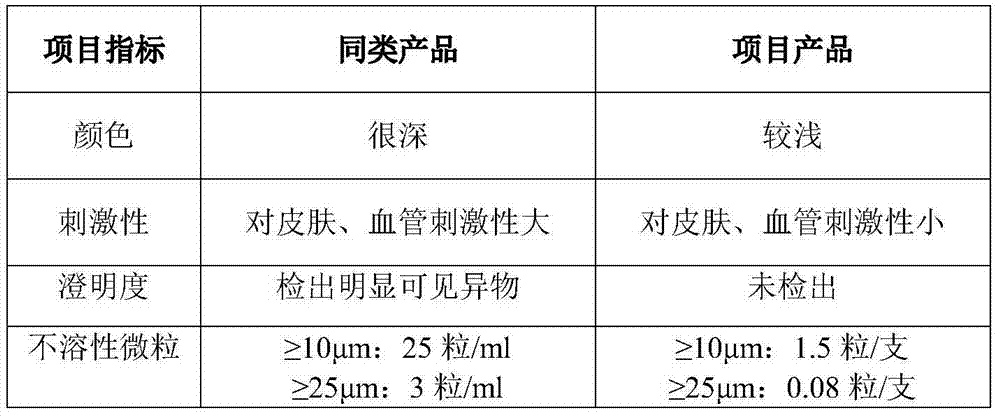
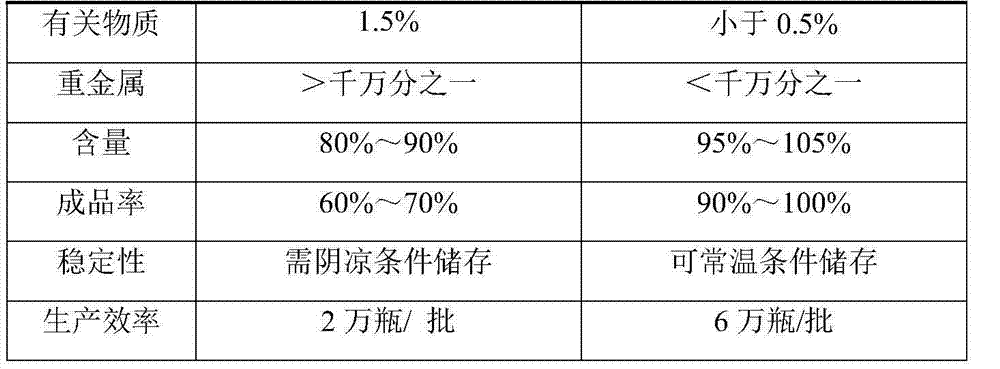
InactiveCN103479522ASubstance reductionHigh clarityAntibacterial agentsOrganic active ingredientsWater bathsSodium Chloride Injection
The invention provides a method for preparing levofloxacin hydrochloride sodium chloride injection. By researching a liquid medicine preparation method, medicinal charcoal pretreatment and a terminal sterilization process, product quality is ensured by the aid of technologies of a concentrated solution and diluted solution method, charcoal slurry preparation from medicinal charcoal, water bath sterilization and the like, production is facilitated, obtained finished products have higher stability, so that the storage life of the finished products can be prolonged, and clinical use effects are better.
Owner:HAINAN HOTMED TIANYA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com