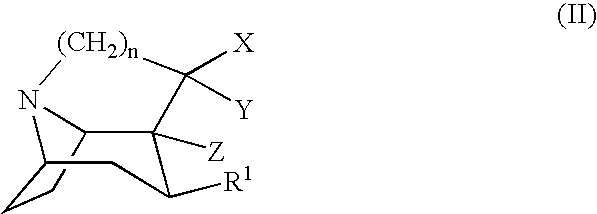Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Tropane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
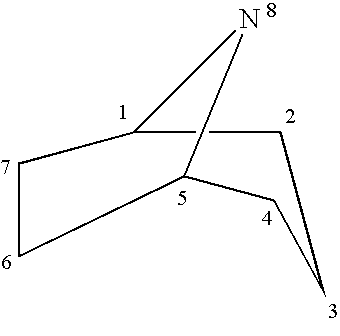
Tropane is a nitrogenous bicyclic organic compound. It is mainly known for a group of alkaloids derived from it (called tropane alkaloids), which include, among others, atropine and cocaine. Tropane alkaloids occur in plants of the families Erythroxylaceae (including coca) and Solanaceae (including mandrake, henbane, deadly nightshade, datura, potato, tomato).
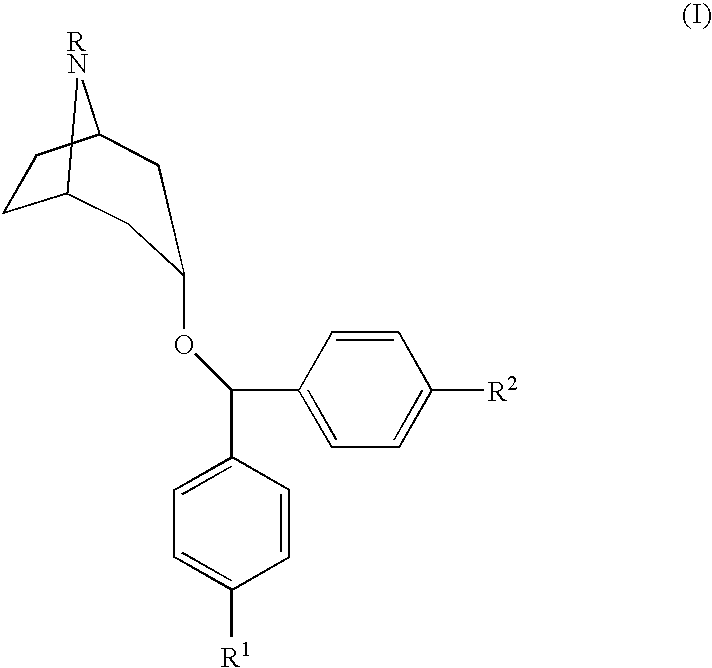
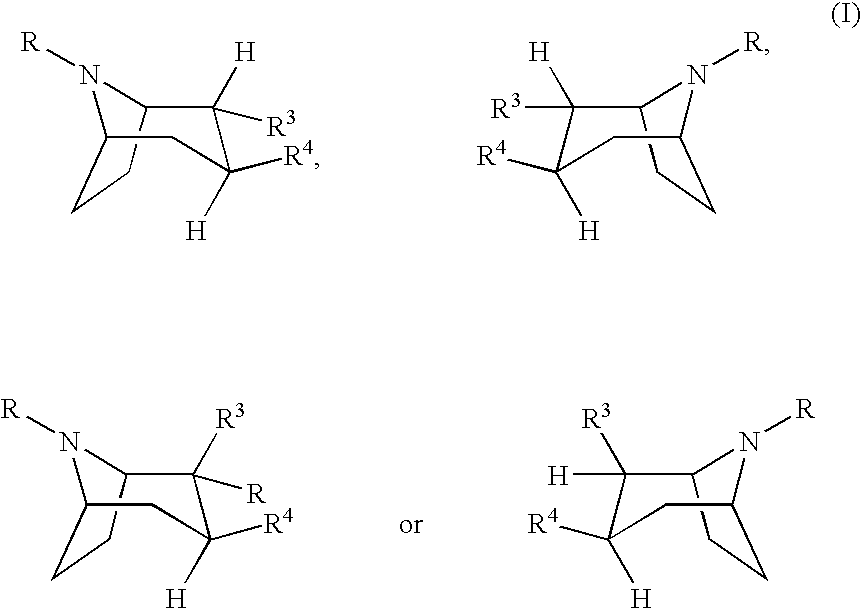
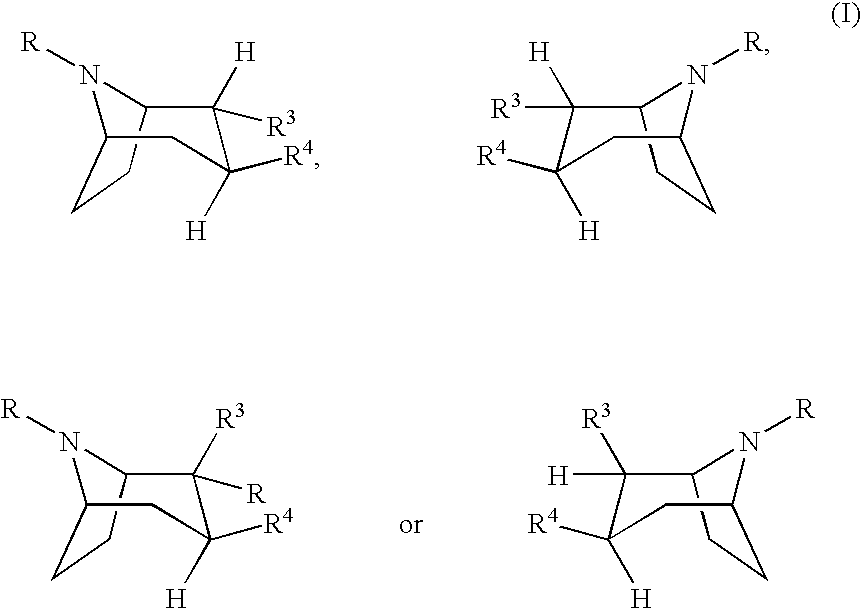
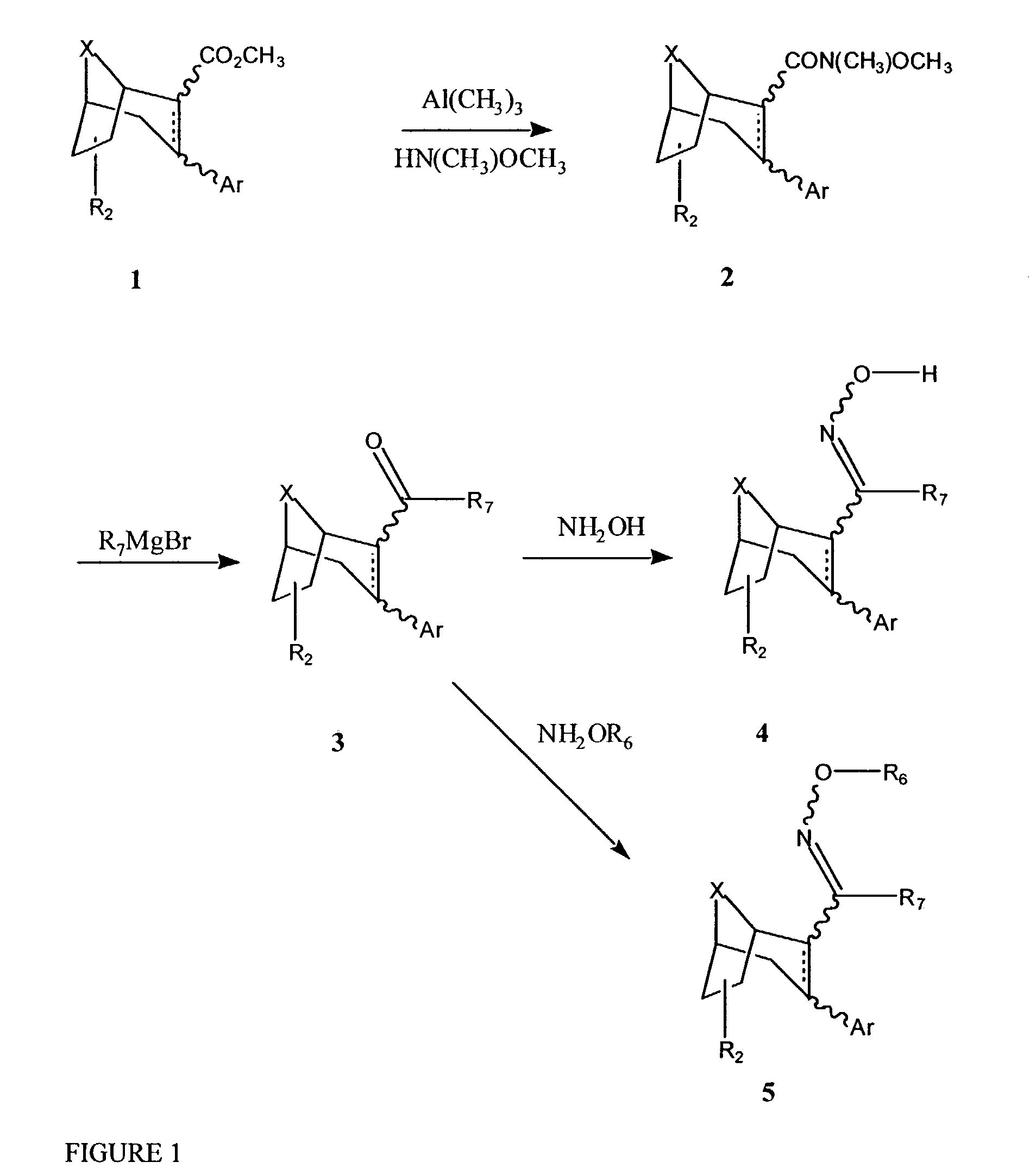
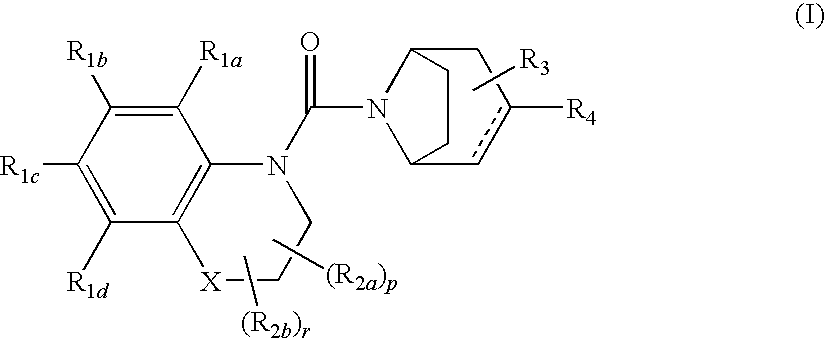
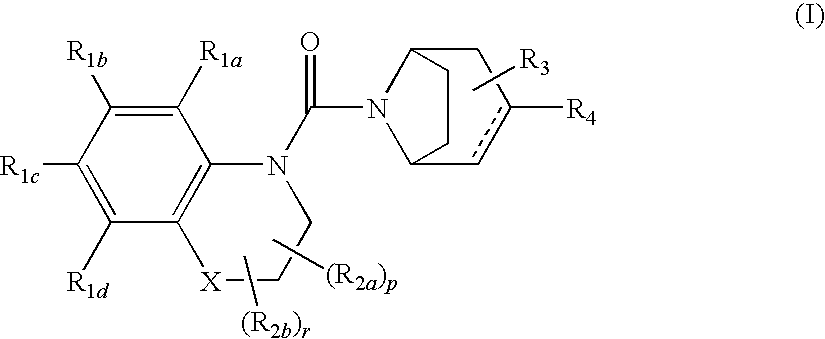
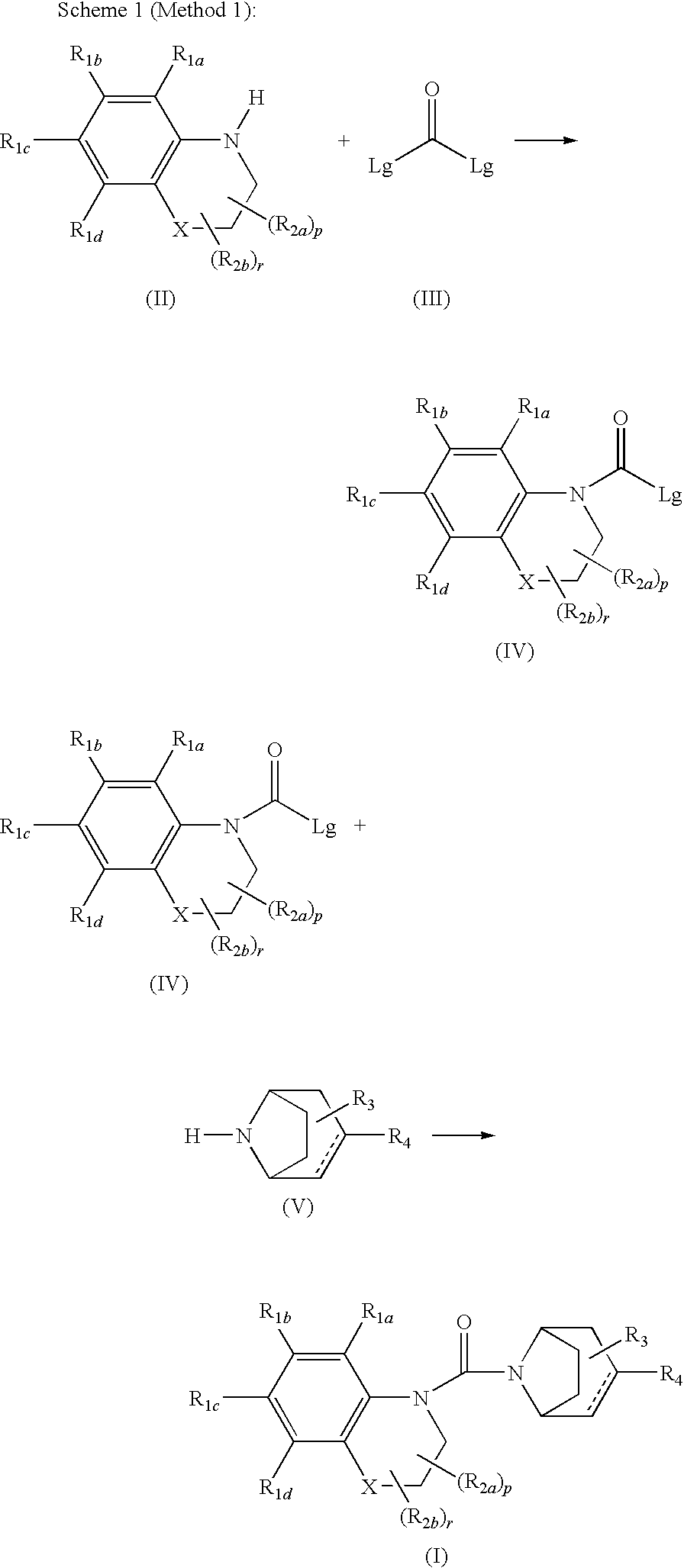
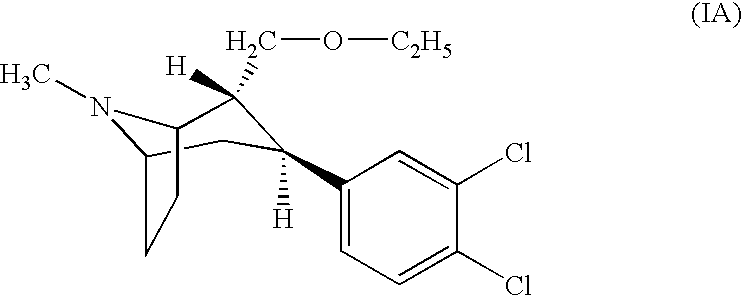
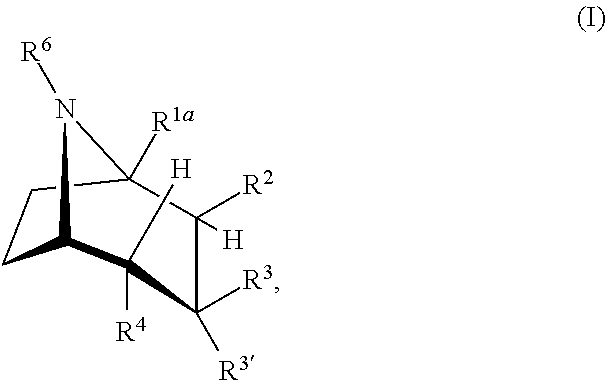
Urea derivatives of tropane, their preparation and their therapeutic application
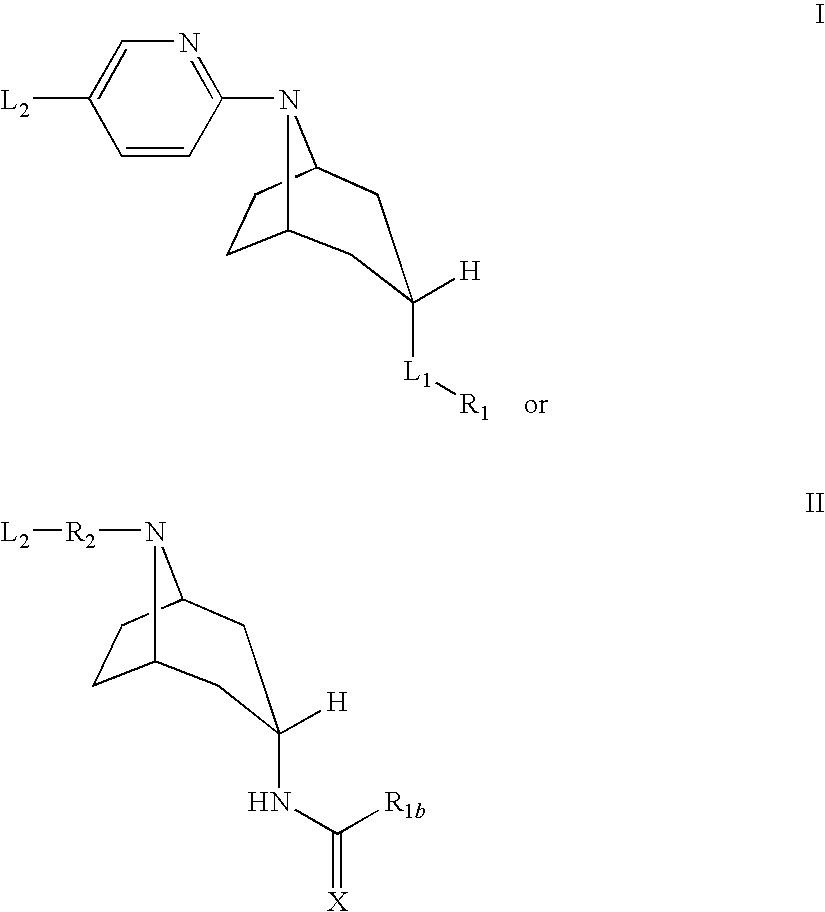
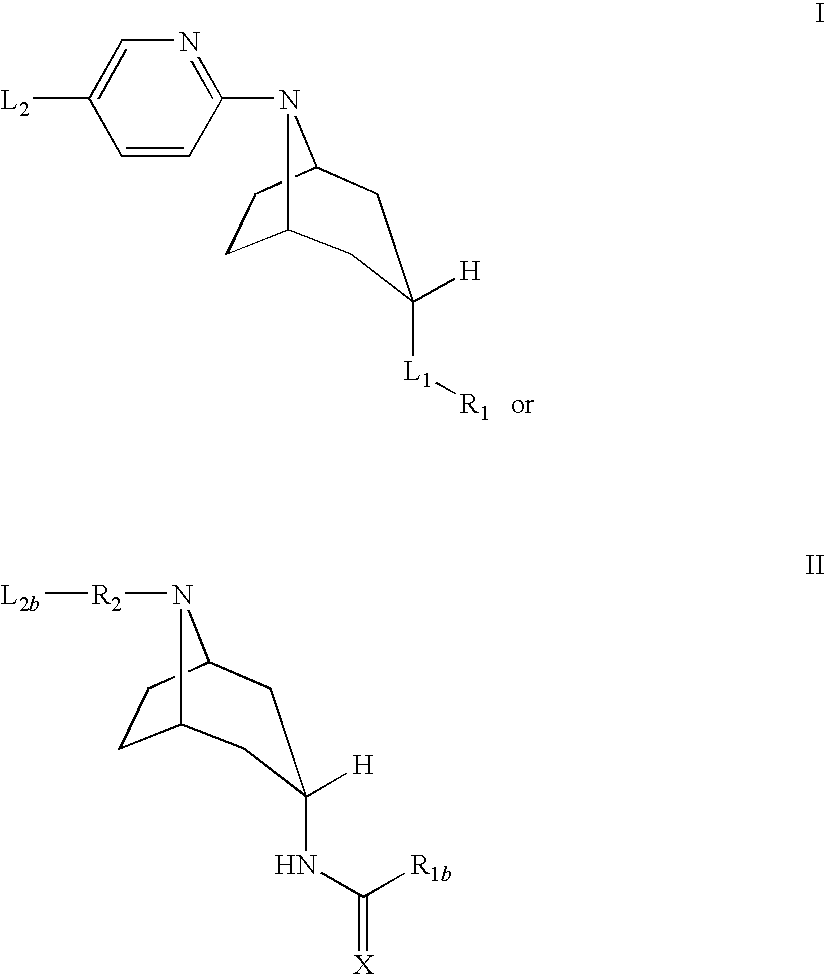
The present invention is related to a compound of formula (I)wherein R1a, R1b, R1c, R1d, R2a, R2b, R3, R4, p, r and are as defined herein, its preparation, pharmaceutical composition and use as a modulator of the activity of the 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1).
Owner:SANOFI SA
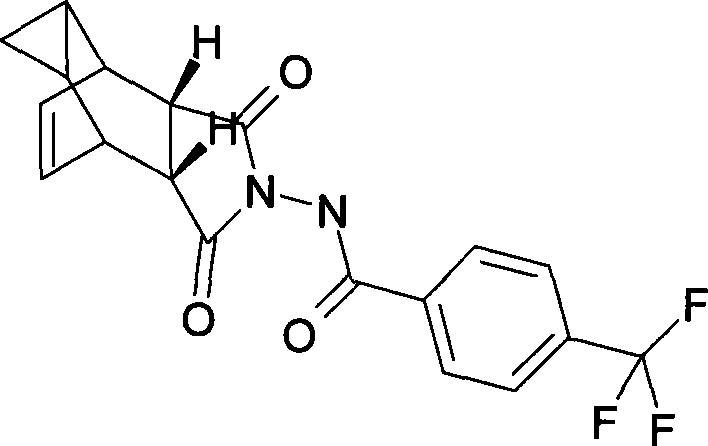
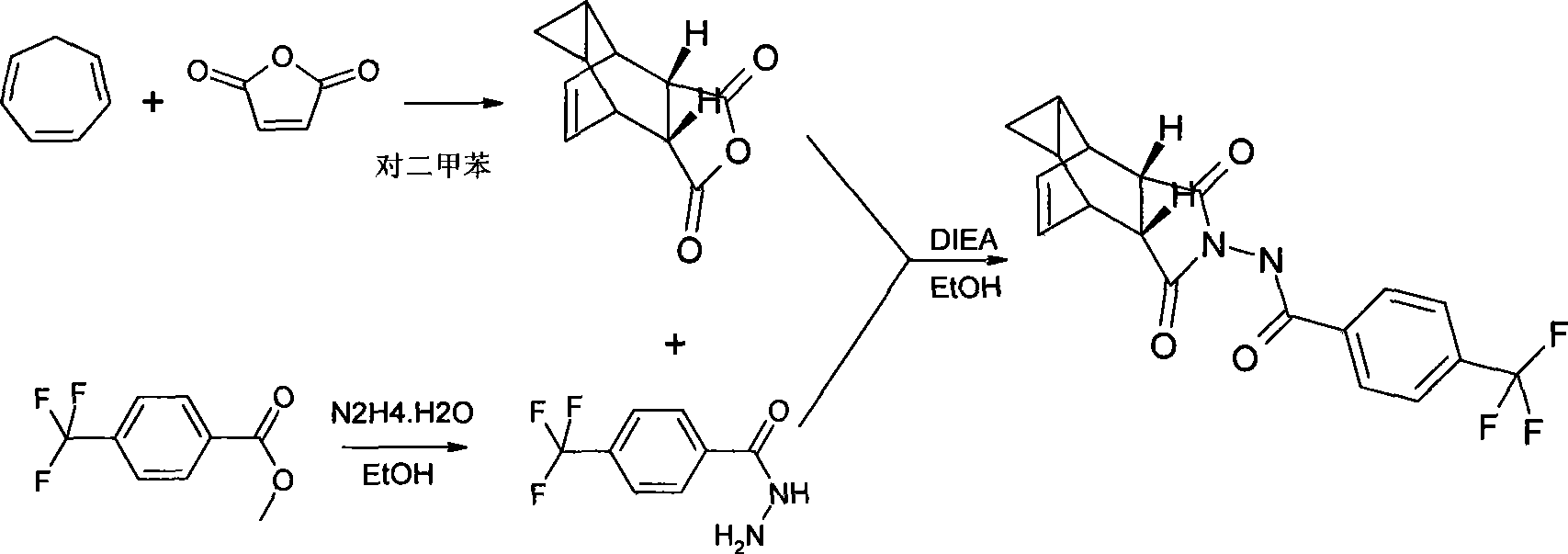
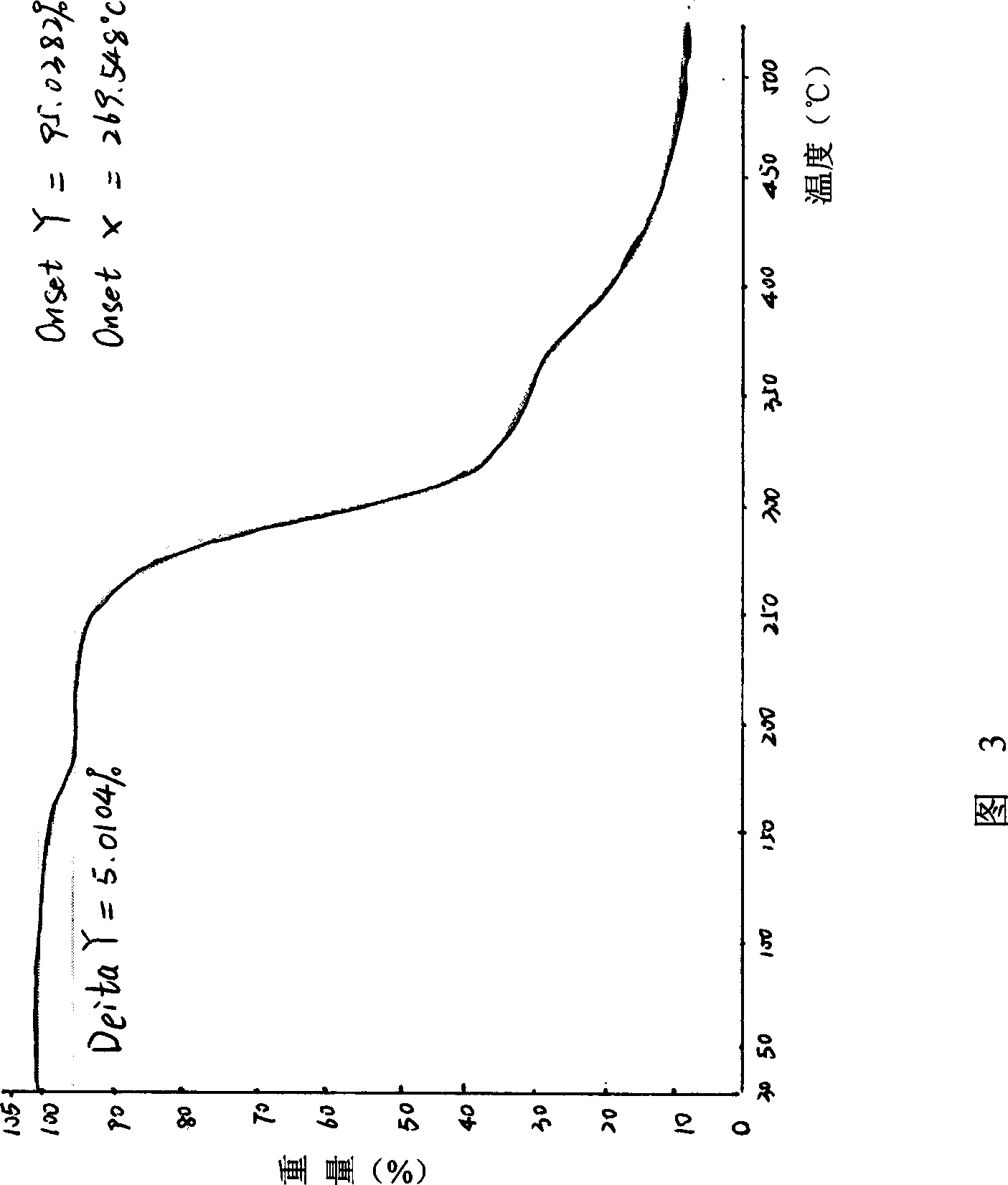
Compound ST-246 containing a crystal water, crystal thereof and preparation method thereof
ActiveCN101445478AStable structurePromote crystallizationOrganic active ingredientsOrganic chemistryOrganic baseTropane
The invention discloses a compound ST-246 containing a crystal water, known as ST-246.H2O. The ST-246.H2O is prepared according to the following method: in the presence of organic base and organic solvent and being under protection of nitrogen, tropane anhydride and p-trifluoromethyl benzoylhydrazine are heated and return flow and reaction solution is cooled and filtered, thereby obtaining the ST-246.H2O. The ST-246.H2O prepared by the method is steady at room temperature, is difficult to lose the crystal water or absorb moisture, is difficult to agglomerate after micronization and is beneficial for improving bioavailability. The compound can be used for preparing anti-poxvirus medicines.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
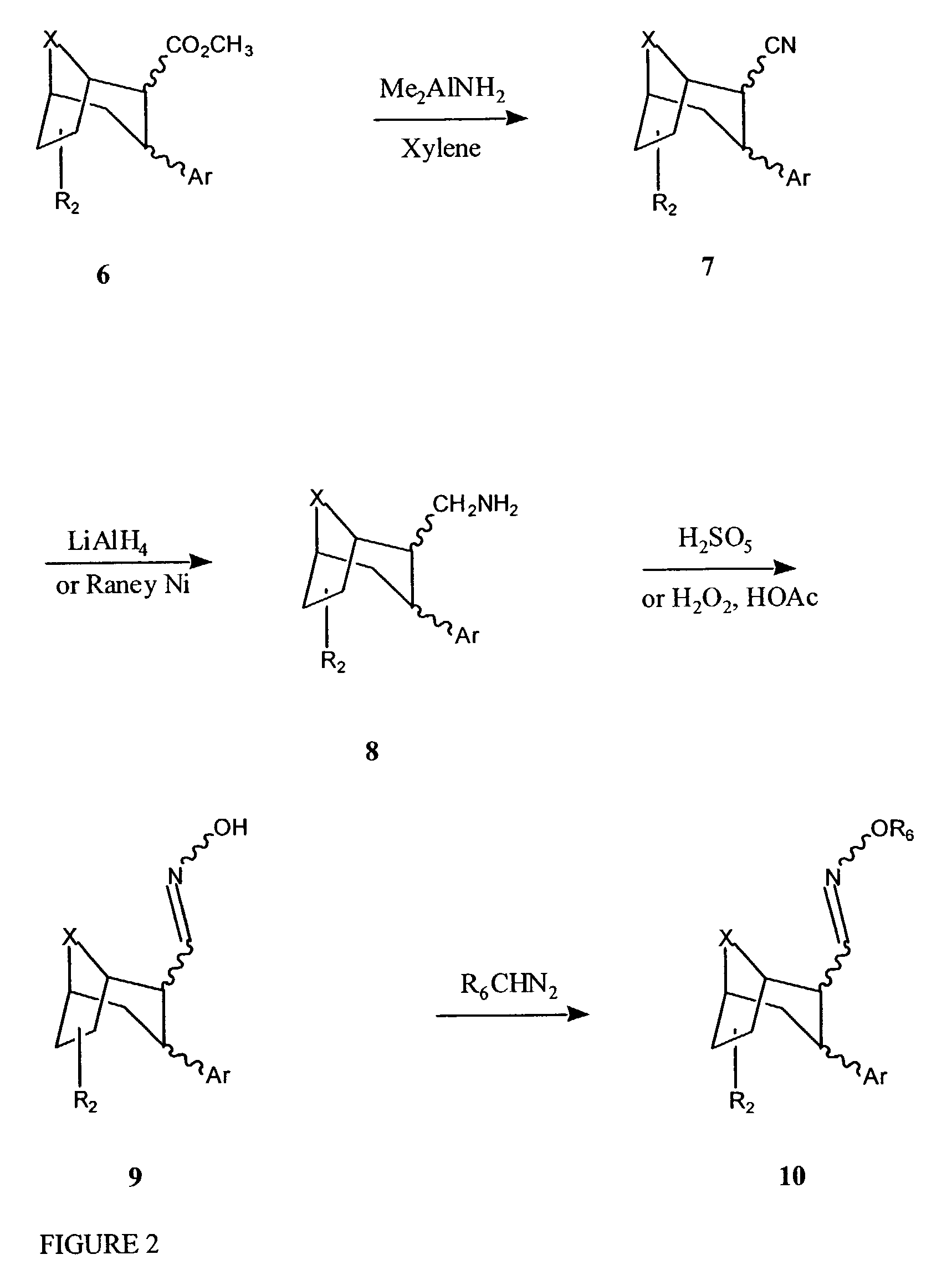
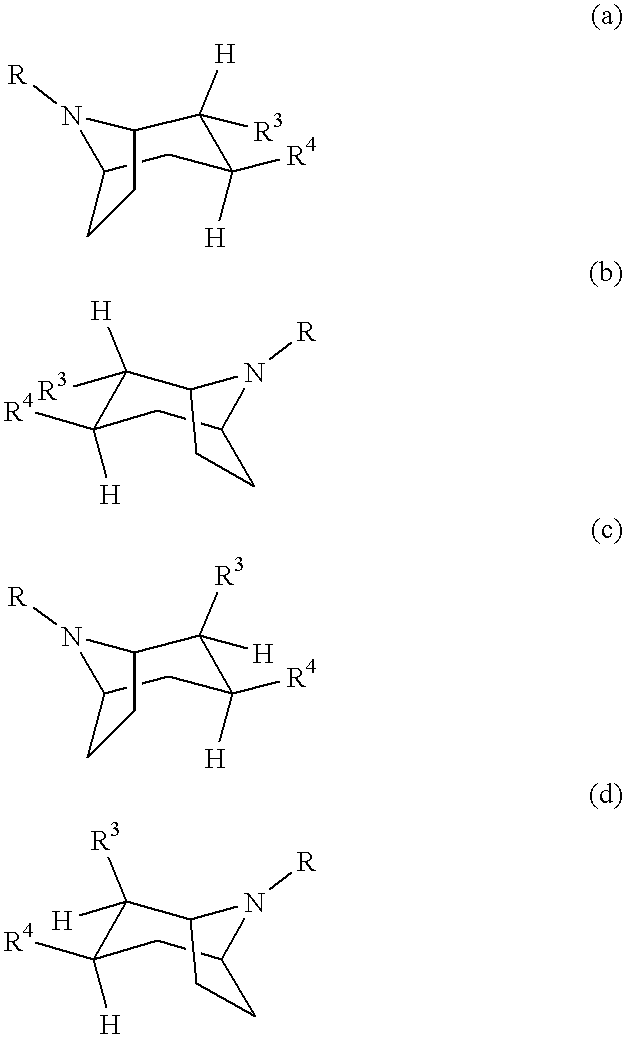
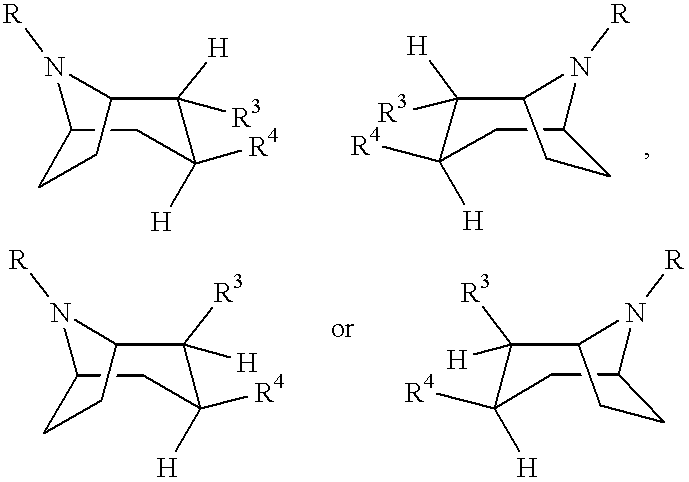
Tropane-derivatives, their preparation and use
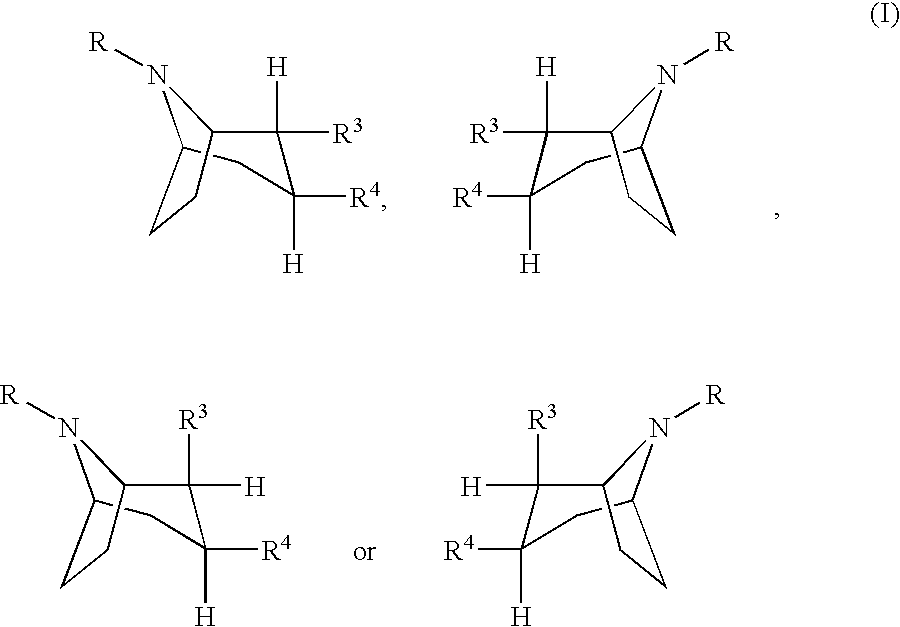
Compounds of formula (a), (b), (c), or (d), or any mixture thereof, or a pharmaceutically-acceptable salt thereof;wherein R is hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl or 2-hydroxyethyl; R3 is -CH2-X-R', wherein X is O, S or NR'', wherein R'' is hydrogen or alkyl and R' is alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, or -CO-alkyl; R4 is phenyl optionally substituted with halogen, CF3, CN, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl; 3,4-methylenedioxyphenyl; benzyl optionally substituted with halogen, CF3, CH, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino nitro, heteroaryl, or aryl; heteroaryl optionally substituted with halogen, CF3, CN, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl; or naphthyl optionally substituted with halogen, CF3, CN, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl. The compounds are monoamine neurotransmitter, i.e., dopamine, serotonin, noradrenaline, reuptake inhibitors.
Owner:NEUROSEARCH AS
Pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor and a dopamine agonist
InactiveUS20050182090A1Reduce pruningEliminate side effectsBiocideNervous disorderTropaneDopamine agonist agent
The invention relates to a pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor comprising a 2,3-disubstituted tropane moiety, or a tautomer, a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof, and at least one dopamine agonist or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
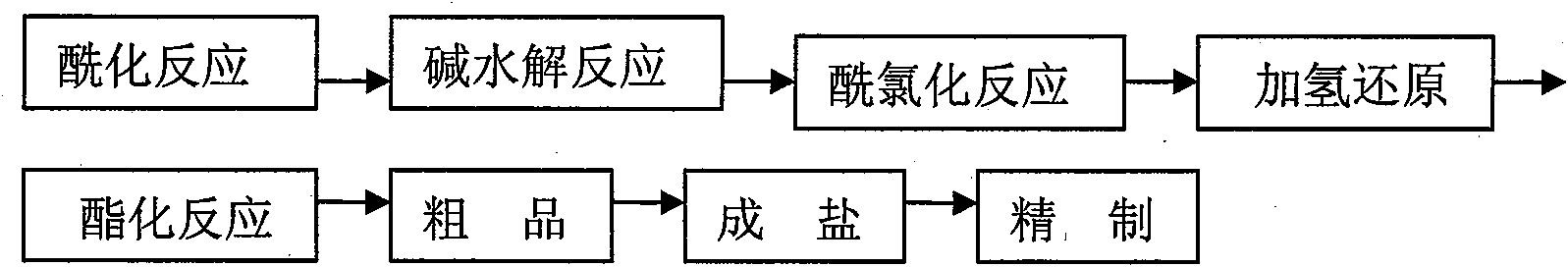
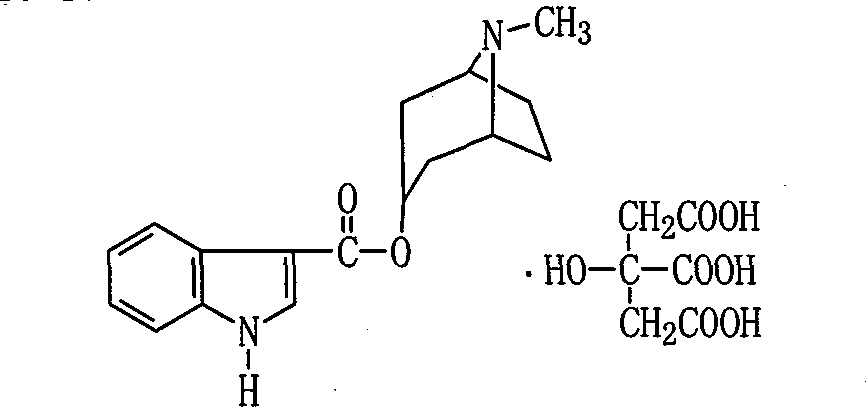
Citric acid tropisetron raw material medicine and preparation technology of raw material medicine and injection liquid
ActiveCN101838266AGood curative effectImprove securityOrganic chemistryDigestive systemTropaneFormate
The invention relates to a citric acid tropisetron raw material medicine and a preparation technology of the citric acid tropisetron raw material medicine and an injection liquid, and belongs to the field of chemical pharmacy; in the process of tropisetron salifying, the citric acid is used as acid radical; the chemical name of the citric acid tropane tropisetron is [(1alpha H, 5alpha H)-8-methyl-azepine bicyclo-(3, 2, 1) octyl group-3alpha-]-1H- benzpyrole-3- formic ether citrate; and the molecular formula is C23H28N209. In the process of tropisetron salifying, the tropisetron is dissolved in ethanol; the mixture is added with ethanol solution of the citric acid, evenly stirred and stands till the needed solid is separated out; the crude citric acid tropisetron is filtered, heated and dissolved in distilled water, is added with the activated carbon for backflowing and decoloring, cools and stands after being filtered so as to separate out white cystal; the mixture is filtered; the filter cake is dried and then distilled water is used for the secondary recrystallization to obtain the refined citric acid tropane tropisetron. The clinical research shows that compared with the imported muriatic acid tropisetron injection solution, the citric acid tropisetron injection solution has similar curative effect and safety.
Owner:JIANGXI DONGFU PHARMA CO LTD
Pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor and an N-methyl-D-aspartate (NMDA) receptors antagonist
InactiveUS20050182089A1Eliminate side effectsReduce pruningBiocideNervous disorderNR1 NMDA receptorTropane
Accordingly, the invention relates to a pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor comprising a 2,3-disubstituted tropane moiety, or a tautomer, a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof, and at least one NMDA receptor antagonists or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof.
Owner:NEUROSEARCH AS
Tropane Compounds and Pharmaceutical Compositions Comprising the Same as an Active Ingredient
InactiveUS20080027094A1Eliminate side effectsReduce adverse reactionsBiocideOrganic chemistrySide effectAnticholinergic Drugs
The conventional anticholinergic drugs for administration through inhalation have been considered to have the possibility of aggravating dysuria associated with prostatic hyperplasia mediated by blood, and it has been demanded that the conventional anticholinergic drugs for administration through inhalation will have to show reduced side effects or adverse reactions. The present invention relates to a compound represented by the general formula (I): (wherein A represents; and R1, R2, R3 and R1 each a hydrogen atom or a substituent; R5 is a substituent; X− is an anion; the symbol: denotes an exo-form or endo-form, or their mixture), its salt or solvation product thereof. They are useful as a prophylactic and / or therapeutic agent with reduced side effects or adverse reactions for the diseases mediated by the muscarinic receptor.
Owner:ONO PHARMA CO LTD
Pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor and an acetylcholinesterase inhibitor
InactiveUS20050154009A1Eliminate side effectsReduced effectivenessBiocideNervous disorderCholinesterase inhibitionAdditive ingredient
The invention relates to a pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor comprising a 2,3-disubstituted tropane moiety, or a tautomer, a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof, and at least one acetylcholinesterase inhibitor or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof, and a pharmaceutically acceptable carrier or excipient, and optionally one or more other therapeutic ingredients.
Owner:NEUROSEARCH AS
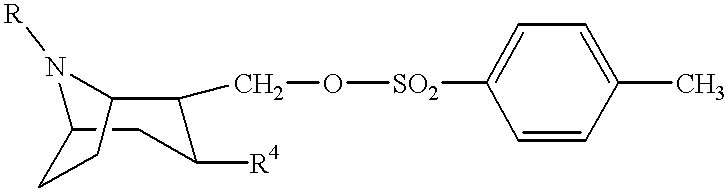
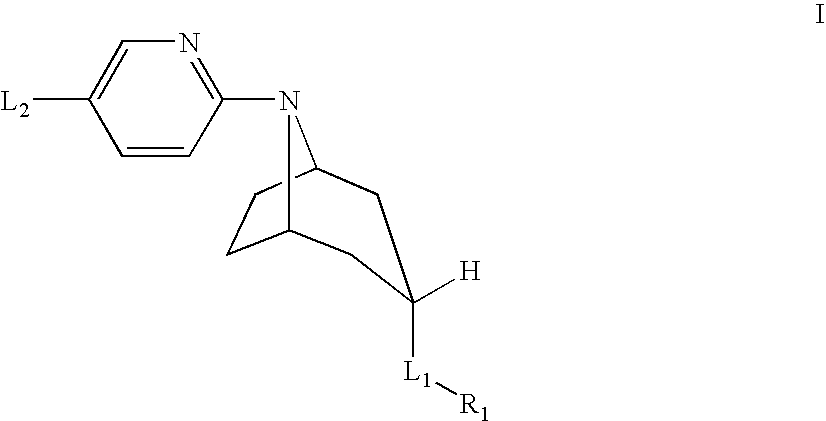
Tropane derivatives useable in particular for in vivo detection of dopamine transporters
The present invention relates to tropane derivatives having particular use for the in vivo detection of dopamine transporters.These derivatives meet the formulain which R1 is I or Sn (R3)3, R2 is for example the methyl group, and X and Y are various substituents.The derivatives with X=CH3 and Y=H show strong specificity for the dopamine transporter compared with the serotonin transporter (74% inhibition when the transporter is previously saturated with GBR 12909).
Owner:CIS BIO INT
Biological pesticide composition and its preparation method and use
A vegetative composite agricultural chemical features that it contains tropane meteloidine (0.5-6 Wt%), nicotine (0.5-20), veratrine (0.2-10) and carrier. It is prepared by mixing them proportionally. Its application is also disclosed. Its advantages are broad spectrum, high insecticiding and bactericide effect and quickly taking its effect.
Owner:SHANDONG ZEHE AGRI CHEM PROD
Eye in-situ gel of chiral anti-glaucoma medicine L-3alpha alkyla acyloxy-6belta alkyla acyloxy tropane and preparation method thereof
InactiveCN101396333AReduce eliminateAccurate doseSenses disorderPharmaceutical delivery mechanismAdjuvantIrritation
The invention relates to an ophthalmic in-situ jelly of chiral anti-glaucoma medicine levofloxacin tropane. The ophthalmic jellies concretely comprises 0.03 percent to 0.3 percent of levofloxacin tropane, 10 percent to 20 percent of hydrophilic polymer material, 0 percent to 20 percent of humectant, 0.1 percent to 10 percent of permeation pressure regulator, 0.01 percent to 0.3 percent of antiseptic / bacteriostat, pH value regulator and water. In a preparation method, the materials are weighted according to the percentage; the levofloxacin tropane is solved in injection water, the hydrophilic polymer material is added under the stirring state, and the solution is stood over a night; the adjuvant such as the humectant and the like is added, and the pH value is adjusted as 4 to 9; the solution is filtered, the injection water is added to the total quantity, and the ophthalmic in-situ jelly is obtained. The ophthalmic in-situ jelly has the advantages of uniform and smooth quality, proper viscosity, convenient use, exact dosage, and long-term uniform distribution on the surface of eye cornea; besides, the jelly causes no hypersusceptibility and irritation, no blurred vision, can effectively prolong the acting time of the medicine on the eye cornea, and has wide application prospect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
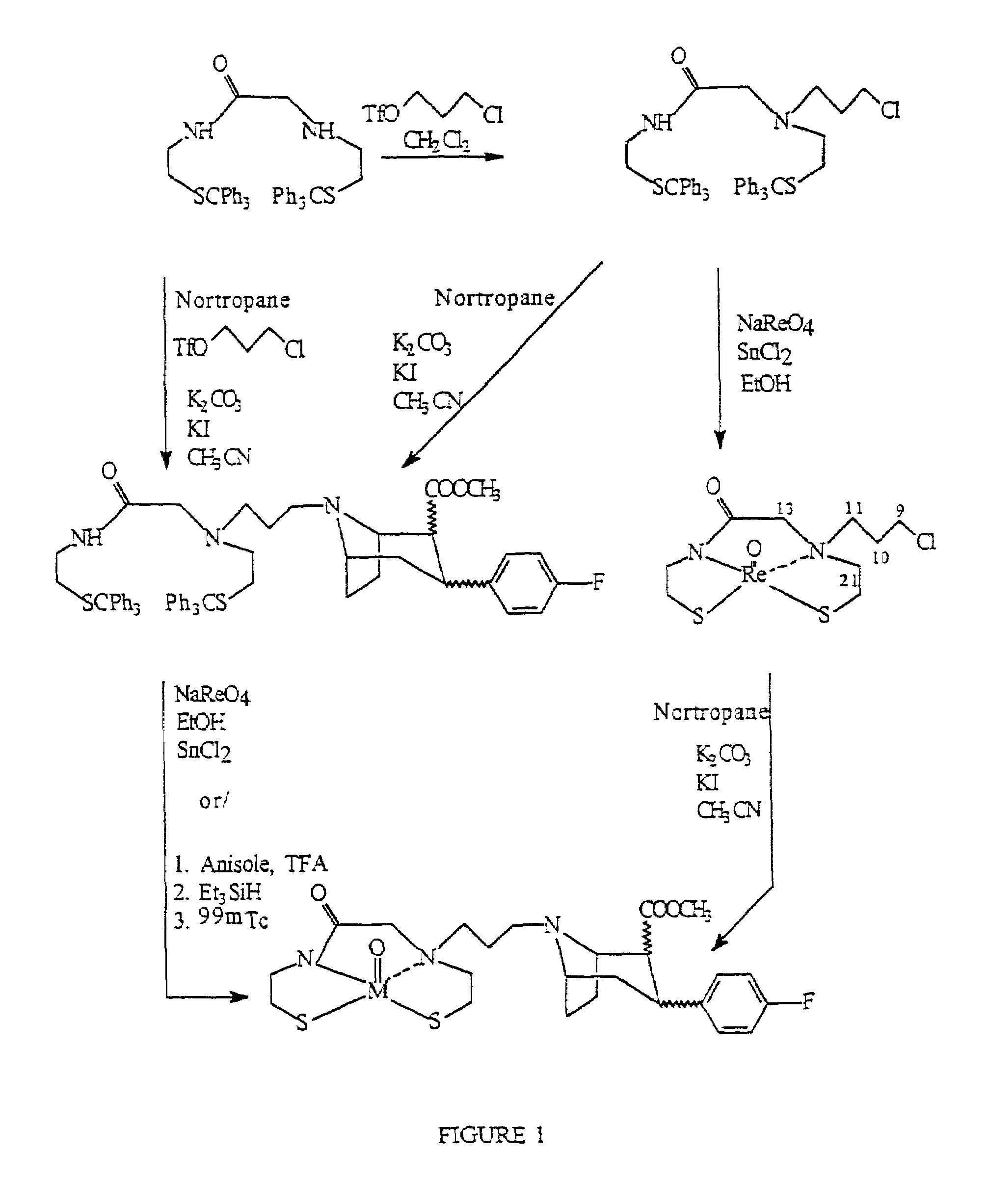
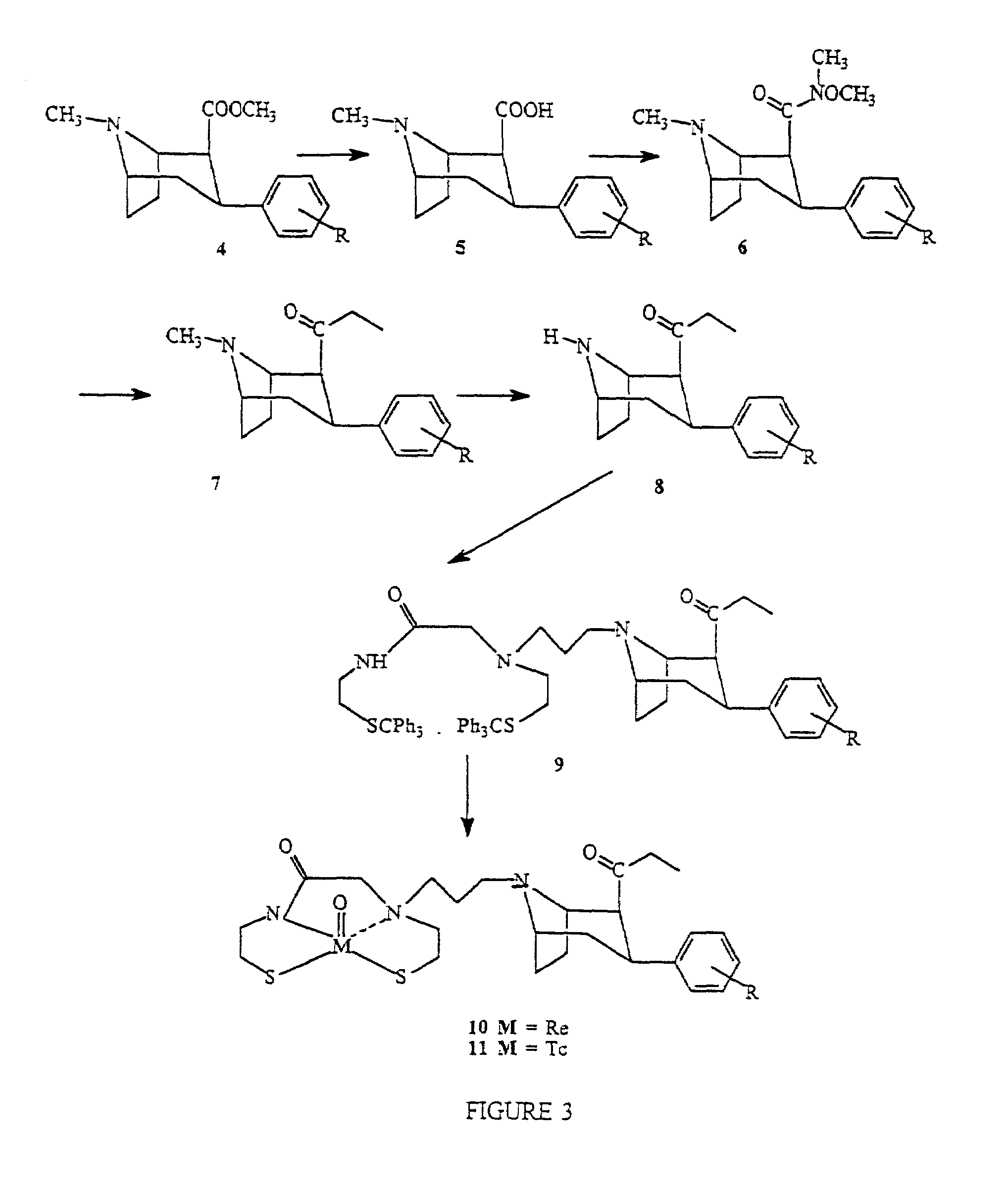
Boat tropanes
InactiveUS7105678B2Useful in therapyRadioactive preparation carriersRadiation therapyRheniumEnantiomer
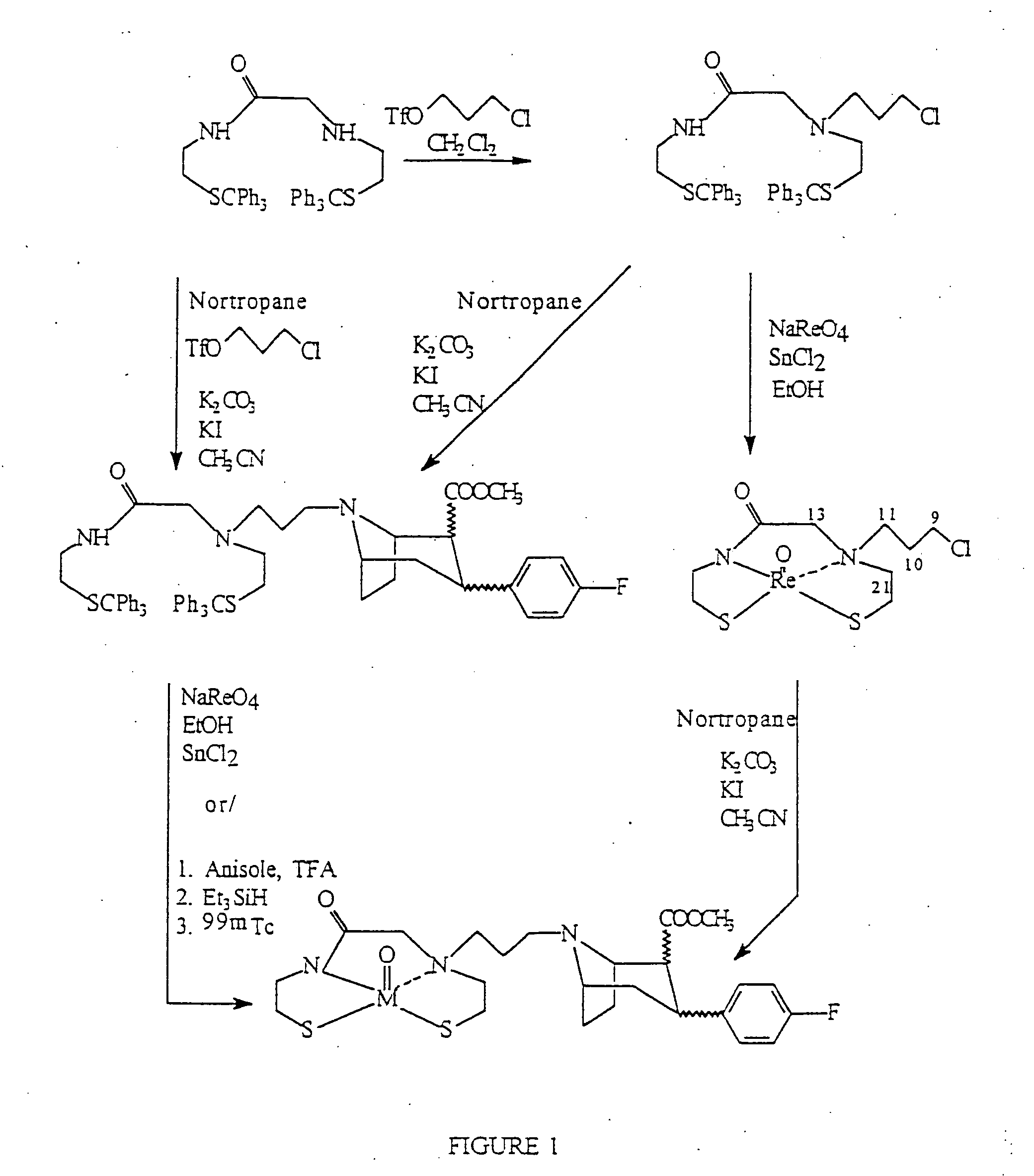
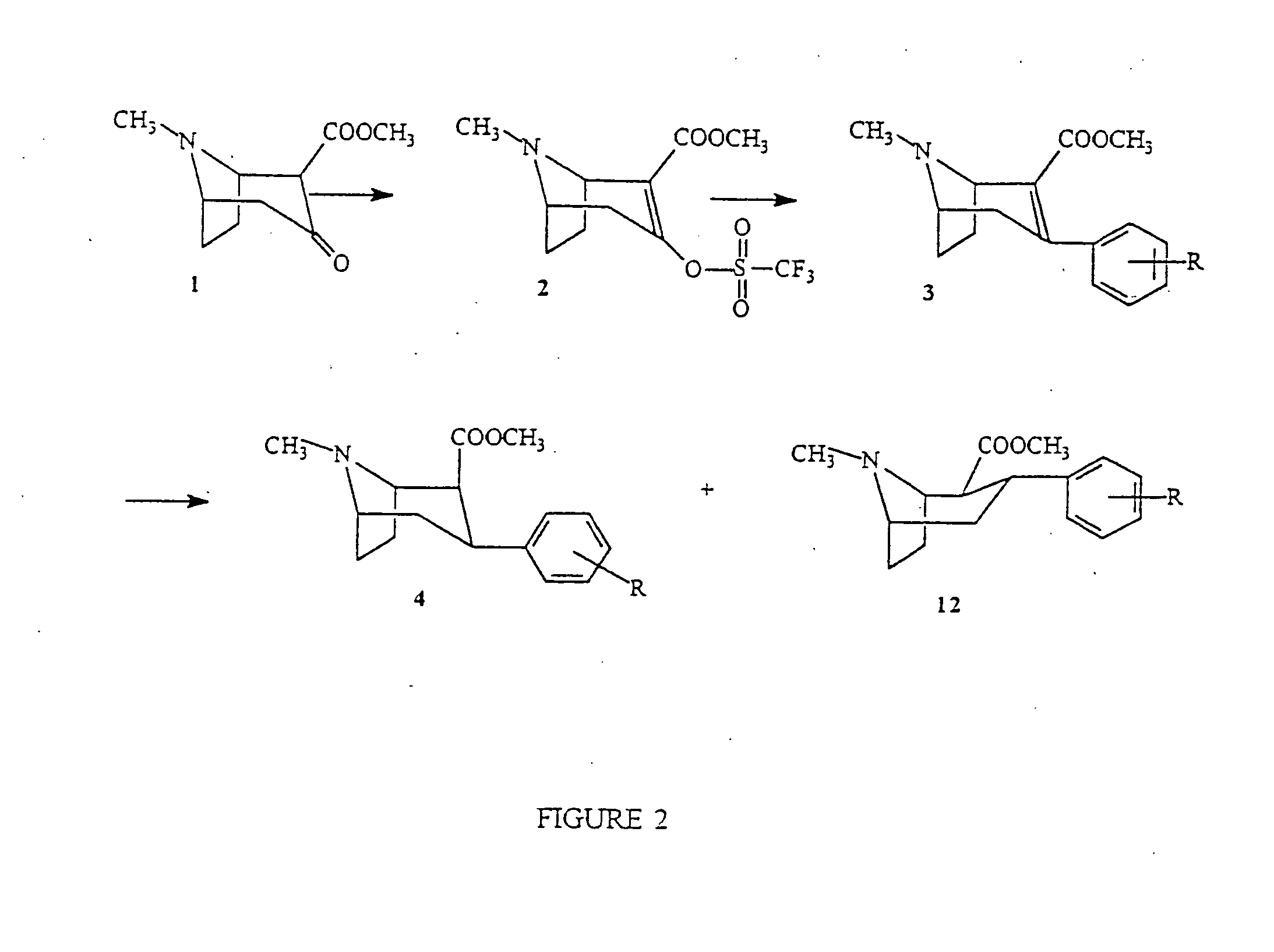
Radiopharmaceutical compounds are disclosed. A tropane compound is linked through the N atom at the 8-position to a chelating ligand capable of complexing technetium or rhenium to produce a neutral labeled complex that selectively binds to the dopamine transporter over the serotonin transporter with a ratio of 10 or more. These compounds can be prepared as separate diastereoisomers as well as a mixture of diastereoisomers. Also disclosed are radiopharmaceutical kits for preparing the labeled radiopharmaceutical compounds.
Owner:ORGANIX +2
Automatic synthesis method of methylation reagent [11C] CH3Br
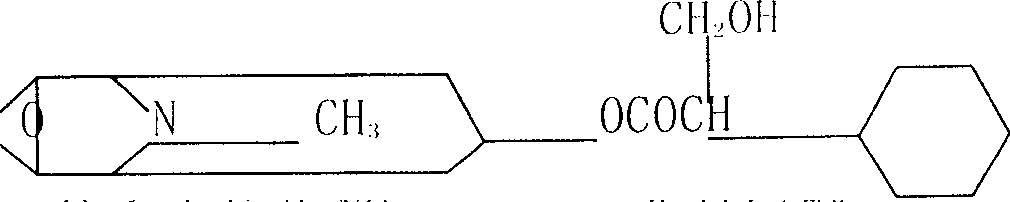
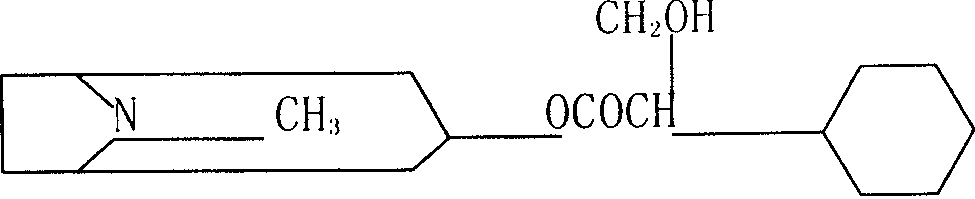
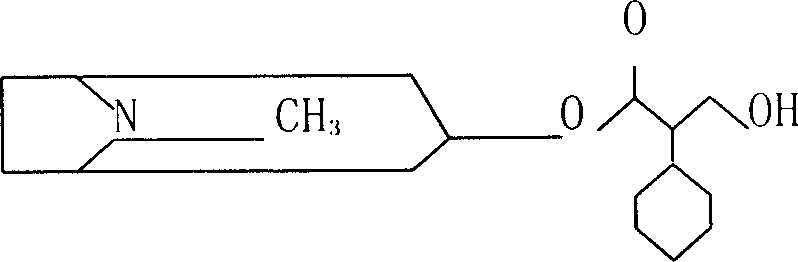
InactiveCN101798257AKeep tightKeep drySulfonic acid esters preparationIsotope introduction to organic compoundsTropaneSynthesis methods
The invention relates to an automatic synthesis method of [11C] CH3Br and an automatic synthesis system thereof. The mixed gas of 0.5% O2 and 99.5% N2 is used as a target gas to produce 11CO2 by a circular accelerator through 14N (p, a) 11C nuclear reaction. 11CO2 can generate 11CH3Br by LiAlH4 reduction, hydrolysis and brominating reaction. A PET-CS-II-IT-IllCH3I synthesis module or a similar synthesis module is adopted for synthesis. [11C] CH3Br is further transformed to [11CH3] Triflate in a heated CF3SO3Ag / graphite column, and can be further used in methylation reaction to prepare [11C] methyl-N-2beta-methyl ester-3beta-(4- fluorine- phenyl) tropane. [11C] CH3Br can be used in methylation reaction to prepare 11C labeled positron medicine containing N-11CH3, O-11CH3 and S-11CH3 bonds, such as S-[11CH3]-L-methionine, (S-[11CH3])-L-cysteine and (N-[11CH3]) sinkaline and [11C] methyl-N-2beta- methyl ester-3beta-(4-fluorine- phenyl) tropane. The invention can realize the automatic synthesis of 11 CH3Br, can realize automatic synthesis, short synthesis time, high radiochemical yield and high purity quotient of radiochemical products, and can realize the automated production of 11C labeled positron medicine by further using methylation reaction.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
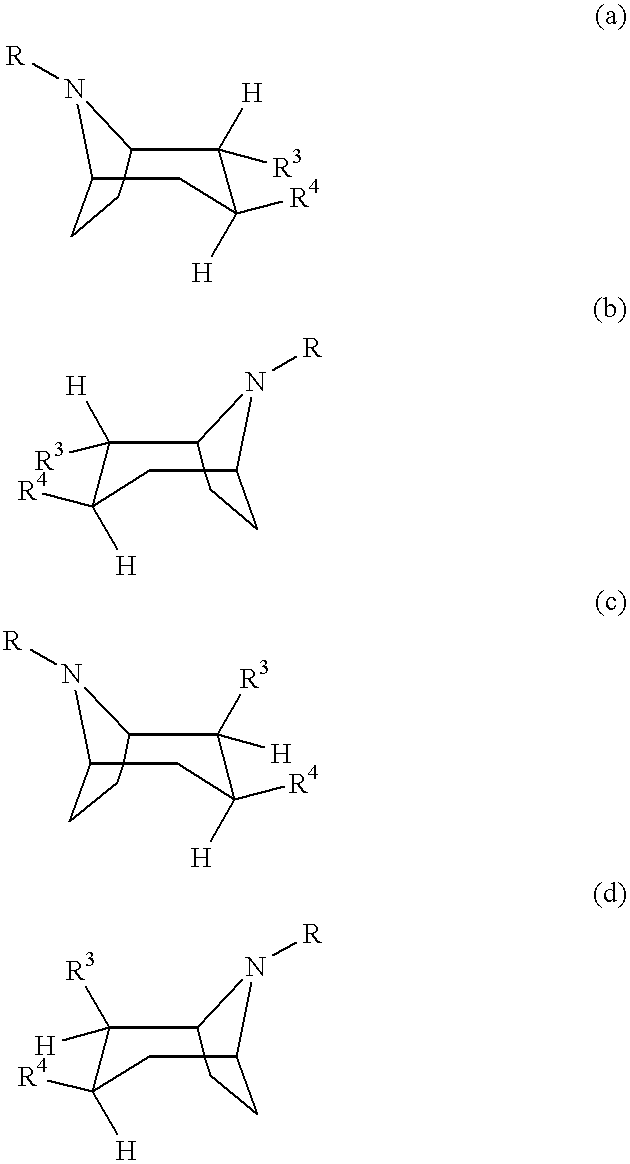
Tropane-derivatives, their preparation and use
Compounds of formula (a), (b), (c), or (d), or any mixture thereof, or a pharmaceutically-acceptable salt thereof; wherein R is hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl or 2-hydroxyethyl; R3 is -CH2-X-R', wherein X is O, S or NR'', wherein R'' is hydrogen or alkyl and R' is alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, or -CO-alkyl; R4 is phenyl optionally substituted with halogen, CF3, CN, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl; 3,4-methylenedioxyphenyl; benzyl optionally substituted with halogen, CF3, CH, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl; heteroaryl optionally substituted with halogen, CF3, CN, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl; or naphthyl optionally substituted with halogen, CF3, CN, alkoxy, cycloalkoxy, alkyl, cycloalkyl, alkenyl, alkynyl, amino, nitro, heteroaryl, or aryl. The compounds are monoamine neurotransmitter, i.e., dopamine, serotonin, noradrenaline, reuptake inhibitors.
Owner:NEUROSEARCH AS
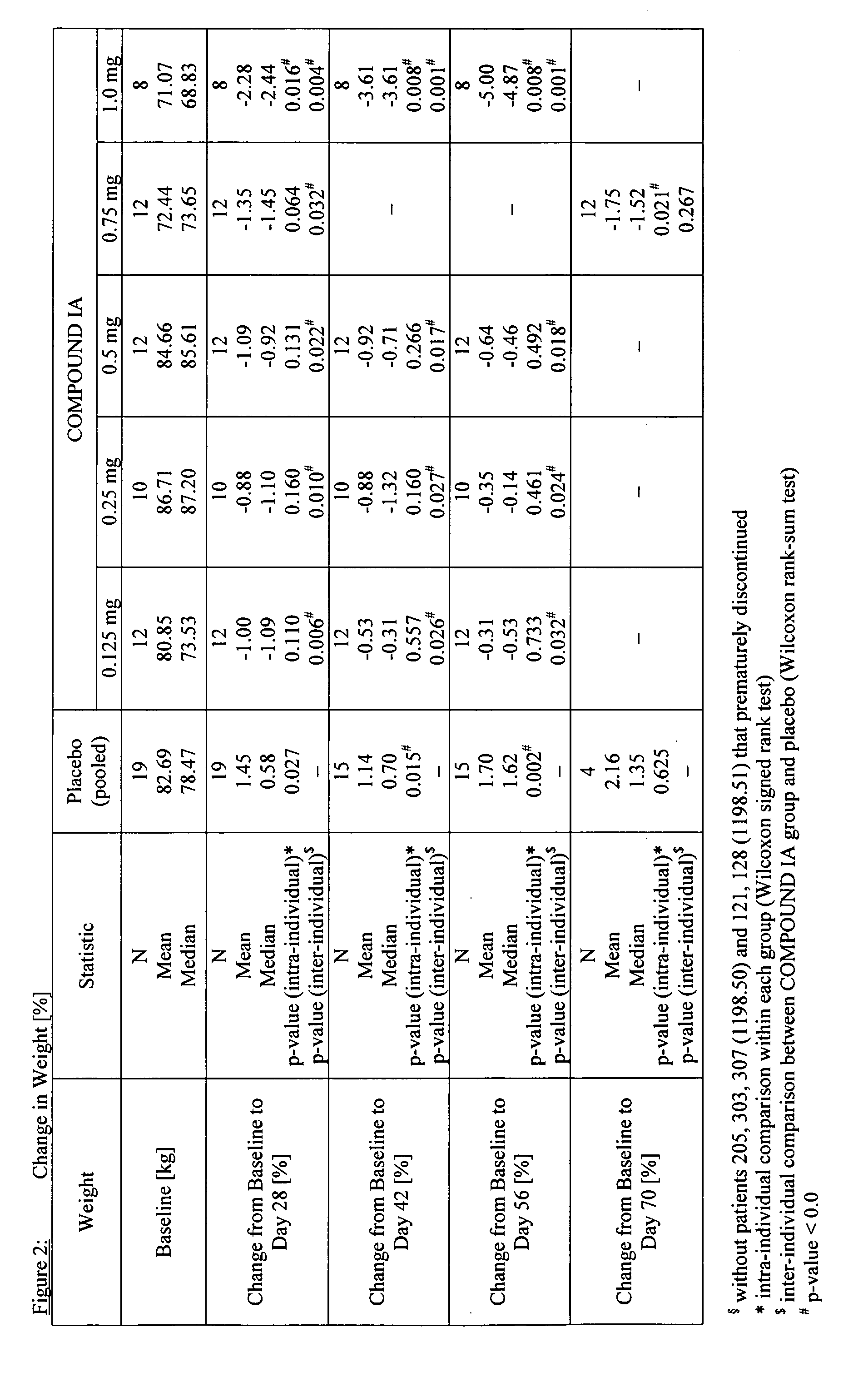
Compounds for the sustained reduction of body weight
InactiveUS20050203124A1Lose weightSustained reduction of body weightBiocideSugar derivativesTropaneBody weight
The invention relates to the use of a monoamine neurotransmitter re-uptake inhibitor comprising a 2,3-disubstituted tropane moiety, or a tautomer, a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof for a medicament for the for the sustained reduction of body weight.
Owner:NEUROSEARCH AS
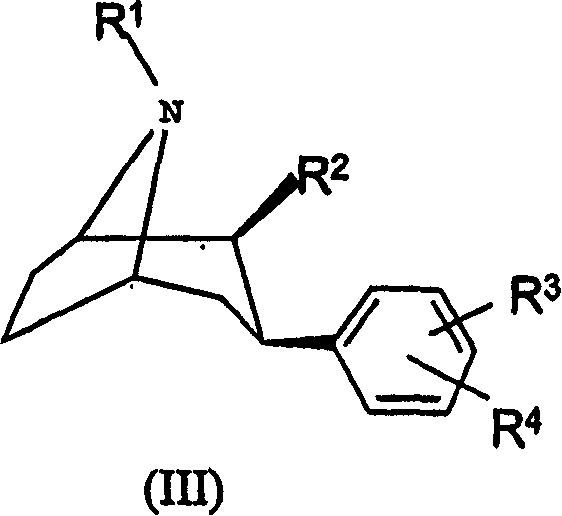
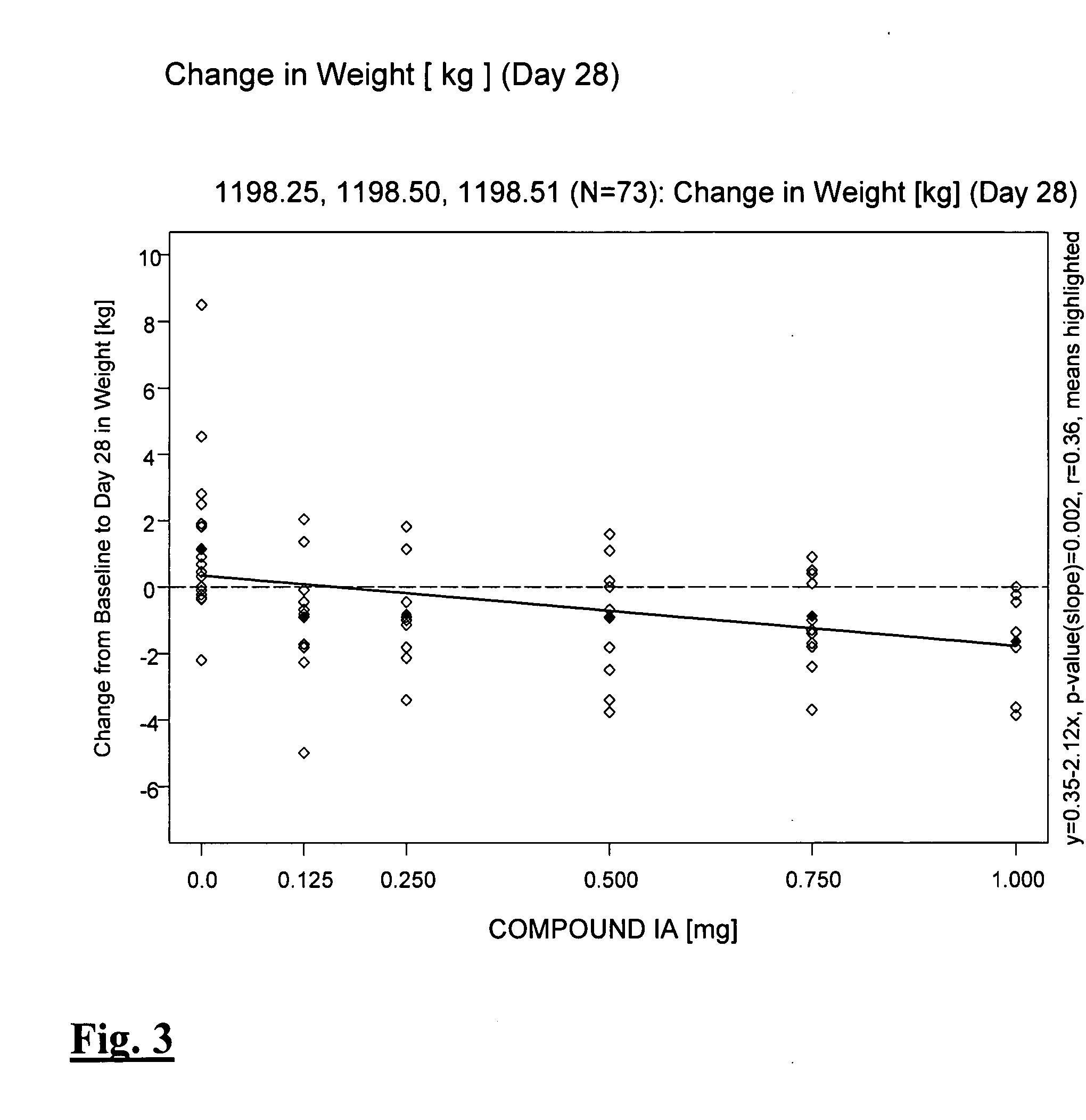
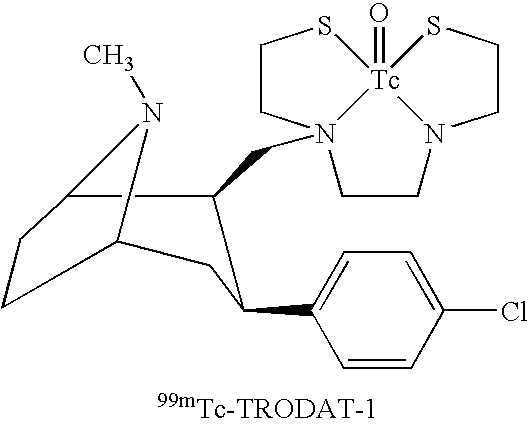
Method for measuring content of 2 beta-[N, Níõ-bis (2-mercaptoethyl) ethylenediamino] methyl, 3 beta-(4-chlorophenyl) tropane in kit
InactiveCN101042337AEasy to operateGood repeatabilityColor/spectral properties measurementsTropaneRepeatability
This invention relates to one method to test the content of 2beta-[N, N'-double(2-thioglycollic base) diethylenediamino] methyl -3beta-(4- chlorphenyl) tropane in the test technique field of TRODAT-1. This invention also provides one method to test TRODAT-1 content in drug case for the measurement of the object with easy operations and good repeat ability.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
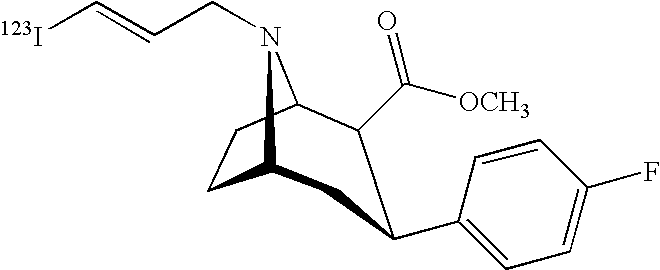
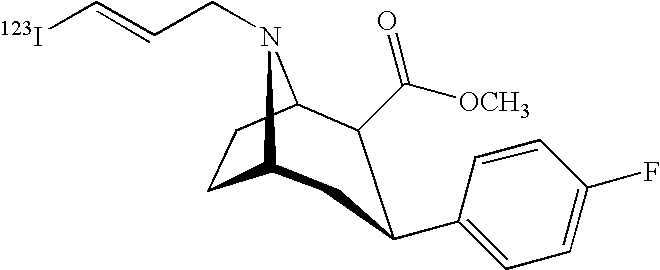
Labeled iodinated tropane formulation
A diagnostic formulation is provided comprising a tropane having a radioactive concentration of at least 1.6 mCi / mL at least about 51 hours post creation. The diagnostic formulation optionally comprises a radiolabeled dopamine transporter (DAT) ligand useful in the diagnosis of Parkinson's disease (PS). One example of a radiolabeled dopamine transporter (DAT) ligand example is [123I]-2β-carbomethoxy-3β-(4-flurophenyl)-N-(3-iodo-E-allyl) nortropane.
Owner:LIKEMINDS
Radiometal complex compositions
InactiveUS20070020177A1Increase concentrationSatisfactory RCPRadioactive preparation carriersRadioactive preparation formsRheniumTechnetium
The present invention relates to stabilised technetium and rhenium metal complex compositions comprising a radioprotectant and a radiometal complex of a tropane-tetradentate chelating agent conjugate, wherein the radiometal complex is neutral. Radiopharmaceuticals comprising the stabilised metal complex compositions, and kits for the preparation of the radiopharmaceuticals are also described.
Owner:MCGILL DAVID +1
Boat tropanes
InactiveUS20070009432A1Useful in therapyNervous disorderRadioactive preparation carriersRheniumTropane
Radiopharmaceutical compounds are disclosed. A tropane compound is linked through the N atom at the 8-position to a chelating ligand capable of complexing technetium or rhenium to produce a neutral labeled complex that selectively binds to the dopamine transporter over the serotonin transporter with a ratio of 10 or more. These compounds can be prepared as separate diastereoisomers as well as a mixture of diastereoisomers. Also disclosed are radiopharmaceutical kits for preparing the labeled radiopharmaceutical compounds.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Tropane compounds
Owner:EXELIXIS INC
Labeled iodinated tropane formulation
A diagnostic formulation is provided comprising a tropane having a radioactive concentration of at least 1.6 mCi / mL at least about 51 hours post creation. The diagnostic formulation optionally comprises a radiolabeled dopamine transporter (DAT) ligand useful in the diagnosis of Parkinson's disease (PS). One example of a radiolabeled dopamine transporter (DAT) ligand example is [123I]-2β-carbomethoxy-3β-(4-flurophenyl)-N-(3-iodo-E-allyl) nortropane.
Owner:LIKEMINDS
Biological pesticide composition and its preparation method and use
Owner:SHANDONG ZEHE AGRI CHEM PROD
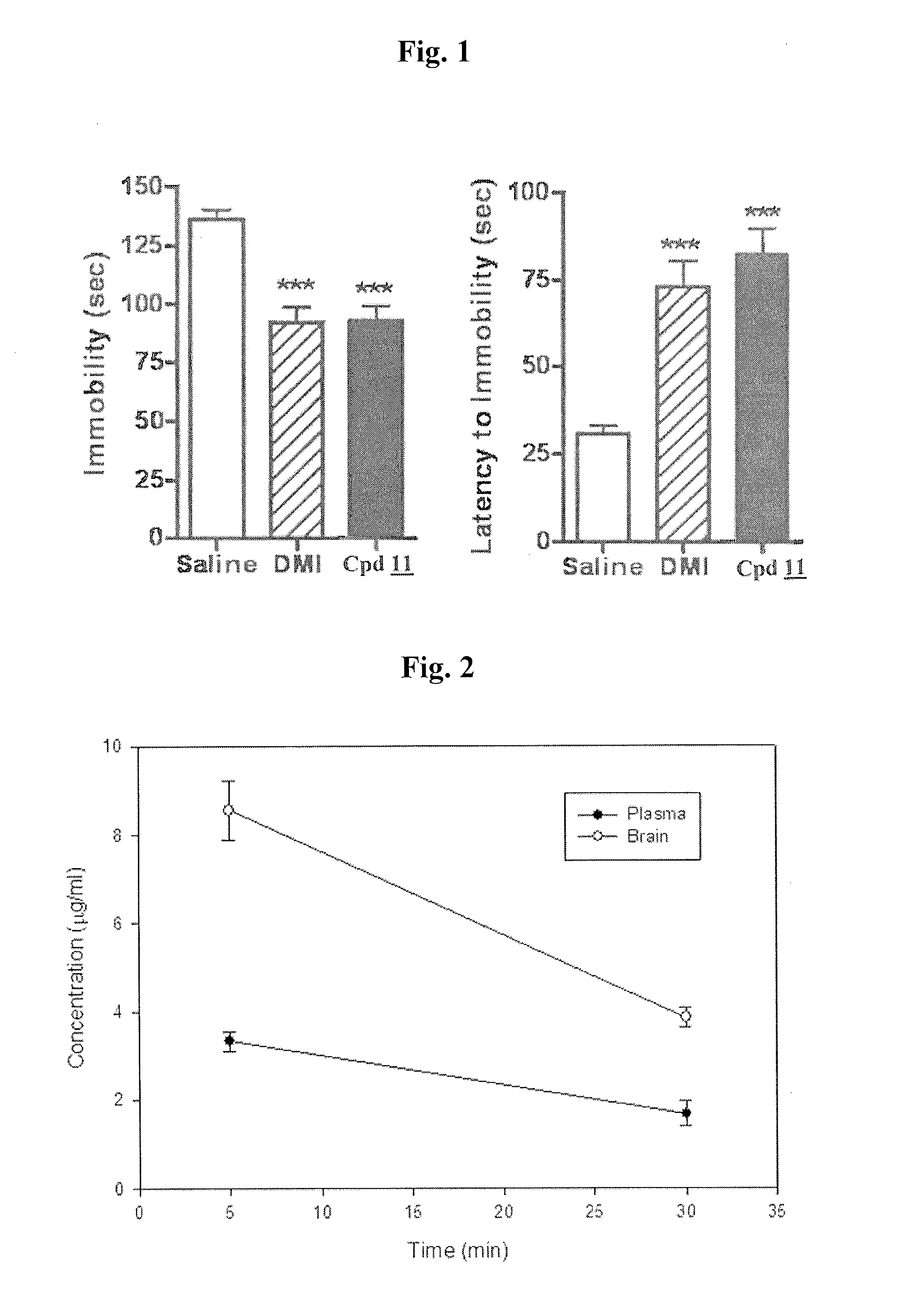
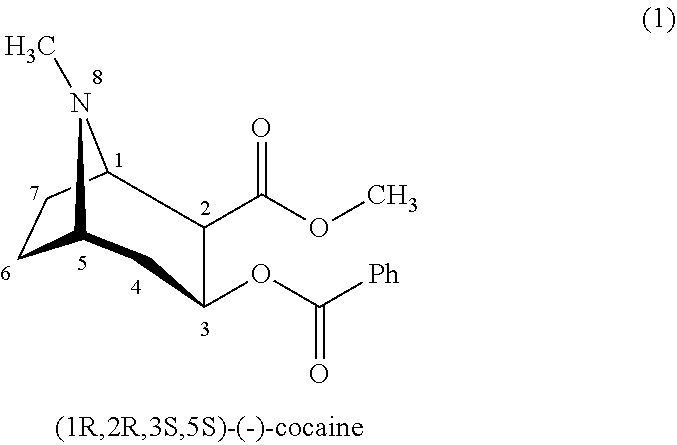
Cocaine Analogs and Methods of Preparation and Uses Thereof
ActiveUS20120329828A1Reduce development riskBiocideNervous disorderTropaneMonoamine neurotransmitter
The invention provides novel cocaine analogs. The invention also provides a method of preparing cocaine analogs with control over the substituents installed at the C-1, C-2, C-3, C-4 and N-8 positions of the tropane bicyclic scaffold. The invention further provides methods of providing anesthesia, blocking reuptake of a monoamine neurotransmitter, and treating depression, by administering to a subject in need of such treatment a pharmaceutical composition comprising a compound of the invention.
Owner:NEW YORK UNIV +1
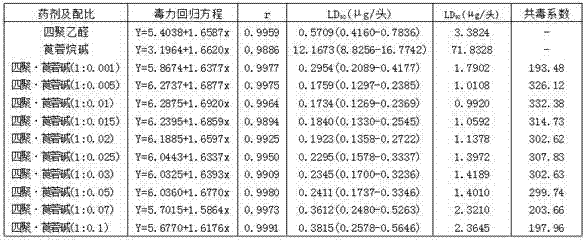
Agent composition containing metaldehyde and tropane alkali
InactiveCN107027783AGood synergyImprove efficiencyBiocideDead animal preservationTropaneAcetaldehyde
The invention belongs to the technical field of pesticides and particularly relates to an agent composition containing metaldehyde and tropane alkali and application thereof. The effective components of the agent composition are the metaldehyde and the tropane alkali, wherein the mass ratio of the metaldehyde to the tropane alkali is 1-30 to 0.001-3. In the composition, the tropane alkali is firstly applied to a snail killing pesticide, does not produce antagonism after being mutually mixed blended with the effective component metaldehyde, has a very good synergic effect and is obviously higher than a single agent in preventing effect, the snail killing efficiency is high, the usage dosage and usage times of agents are decreased, the economic cost is saved, too fast production of agent resistance is avoided, the lasting period of the agents is prolonged, agent residues in agricultural products and the environment are decreased, and environmental sustainable development is promoted. The composition has the special effect on snail killing.
Owner:SHANDONG ACADEMY OF PESTICIDE SCI
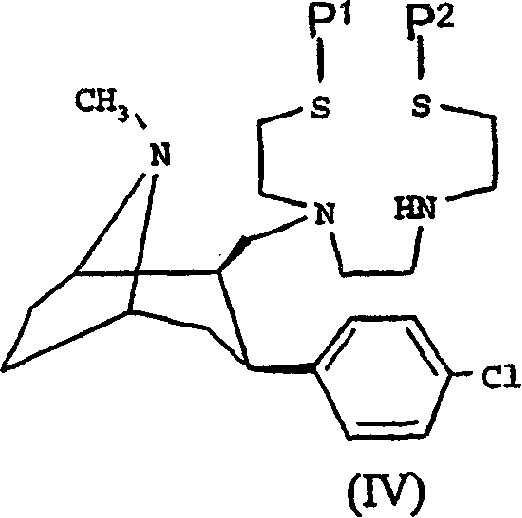
Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane
InactiveCN1762499AStable marking rateIncrease dosageIn-vivo radioactive preparationsEthylenediaminePh buffering
The technetium [99m Tc] cachet pharmaceutical case for the 2beta- [N, N'-(2-mercapto ethyl) ethylenediamine] methyl-3beta-(4- chlorphenyl) tropane (in the following, using TRODAT-1 as abbreviation), relating to technique field of single photon emission computer tomography (SPECT) imaging agent. The invention provides a medicine case formulation for the TRODAT-1, the operation is simple and the marking rate is stable and larger than 90% when the medicine case is used for the mark of [99mTc]. The medicine is prepared through a certain composition of TRODAT-1, stannous salt, gluceplate, edetate and pH buffering agent. There is no identical report of the medicine case raised in this invention in the international.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Solid pharmaceutical preparation
The invention relates to a solid pharmaceutical preparation containing one or more solid carriers and / or excipients and an active substance selected from among the Monoamine Neurotransmitter Re-uptake Inhibitors which have a 2,3-disubstituted tropane structure, the preparation thereof and use thereof for preparing a pharmaceutical composition for the treatment or prevention of central-nervous diseases or disorders.
Owner:BOEHRINGER INGELHEIM INT GMBH
4',4''-substituted 3alpha-(diphenylmethoxy) tropane analogs for treatment of mental disorders
The present invention describes a method for the treatment of attention deficit hyperactivity disorder (ADHD), conduct disorder, alcohol addiction, tobacco addiction, nicotine addiction, parkinsonism including Parkinson's disease, female and male orgasmic disorders, female and male sexual arousal disorders, hypoactive sexual desire disorder, and disorders characterized by anxiety and / or depression. In this method, a therapeutically effective, nontoxic dose of a 4′,4″-substituted 3α-(diphenylmethoxy) tropane analog or a pharmaceutically acceptable salt thereof is administered to the patient in need of such treatment.
Owner:GOLDSTEIN STEVEN J
Tropane derivatives having dopamine reuptake inhibitor activity for the treatment of ischemic diseases
The present invention relates to the use of tropane derivatives having dopamine reuptake inhibitor activity for the treatment of diseases associated with reduced blood flow to the brain or with instances of a temporary break in blood supply to the brain, such as ischemic diseases.
Owner:SANIONA AS
Improved radiometal complex compositions
The present invention relates to stabilised technetium and rhenium metal complex compositions comprising a radioprotectant and a radiometal complex of a tropane-tetradentate chelating agent conjugate, wherein the radiometal complex is neutral. Radiopharmaceuticals comprising the stabilised metal complex compositions, and kits for the preparation of the radiopharmaceuticals are also described.
Owner:GE HEALTHCARE LTD
Tropane compounds
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![Automatic synthesis method of methylation reagent [11C] CH3Br Automatic synthesis method of methylation reagent [11C] CH3Br](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0f39e24f-90cc-4de2-9642-332cbe06d9ce/HSA00000050772100011.PNG)
![Automatic synthesis method of methylation reagent [11C] CH3Br Automatic synthesis method of methylation reagent [11C] CH3Br](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0f39e24f-90cc-4de2-9642-332cbe06d9ce/HSA00000050772100012.PNG)
![Automatic synthesis method of methylation reagent [11C] CH3Br Automatic synthesis method of methylation reagent [11C] CH3Br](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0f39e24f-90cc-4de2-9642-332cbe06d9ce/HSA00000050772100021.PNG)


![Method for measuring content of 2 beta-[N, Níõ-bis (2-mercaptoethyl) ethylenediamino] methyl, 3 beta-(4-chlorophenyl) tropane in kit Method for measuring content of 2 beta-[N, Níõ-bis (2-mercaptoethyl) ethylenediamino] methyl, 3 beta-(4-chlorophenyl) tropane in kit](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5b58178b-5f08-49bf-95c4-3700f170d904/A20071002121100061.PNG)





![Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a30c9767-c2a8-46ba-b331-56a034ccc769/A20051009463500071.PNG)
![Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a30c9767-c2a8-46ba-b331-56a034ccc769/A20051009463500072.PNG)
![Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane Medicament box of 2beta-[N,N'-di(2-mercaptoethyl) ethylene diamine] methyl-3 beta-(4- chlorphenyl) tropane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a30c9767-c2a8-46ba-b331-56a034ccc769/A20051009463500073.PNG)