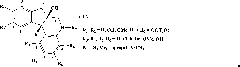Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
179 results about "Azepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Azepines are unsaturated heterocycles of seven atoms, with a nitrogen replacing a carbon at one position.
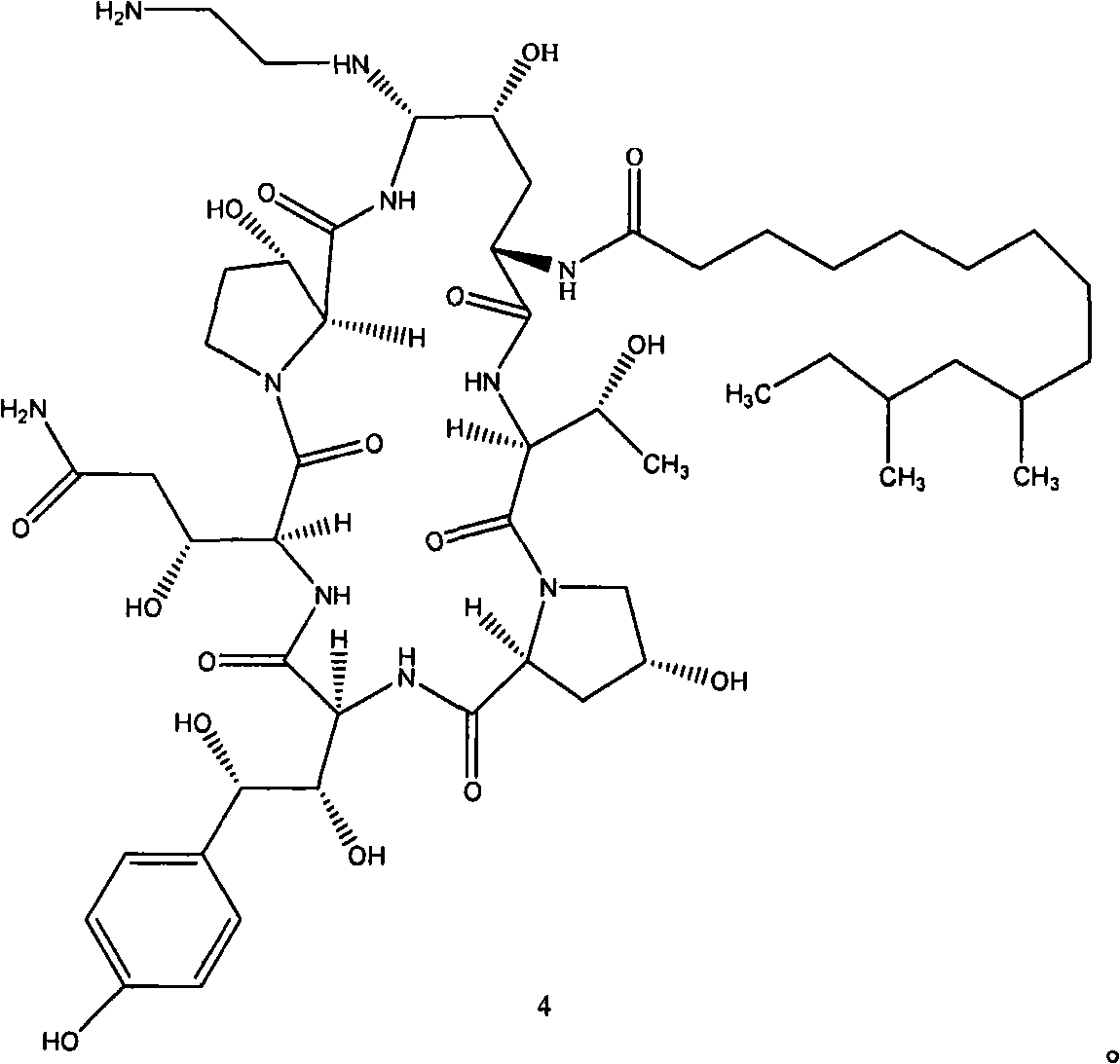
Azepine argireline or pharmaceutically acceptable salt thereof and preparation method and application thereof
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds
InactiveUS20070142635A1High yieldLow costAntiviralsPhosphorus organic compoundsCarboxylic saltHIV Integrase Inhibitors
Processes for preparing 10-amino-3-hydroxy-4-oxo-4,6,7,8,9,10-hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds are disclosed. The preparation of carboxamide derivatives from these carboxylates is also disclosed. The carboxamides are HIV integrase inhibitors and are useful for treating HIV infection and AIDS.
Owner:ASKIN DAVID +5
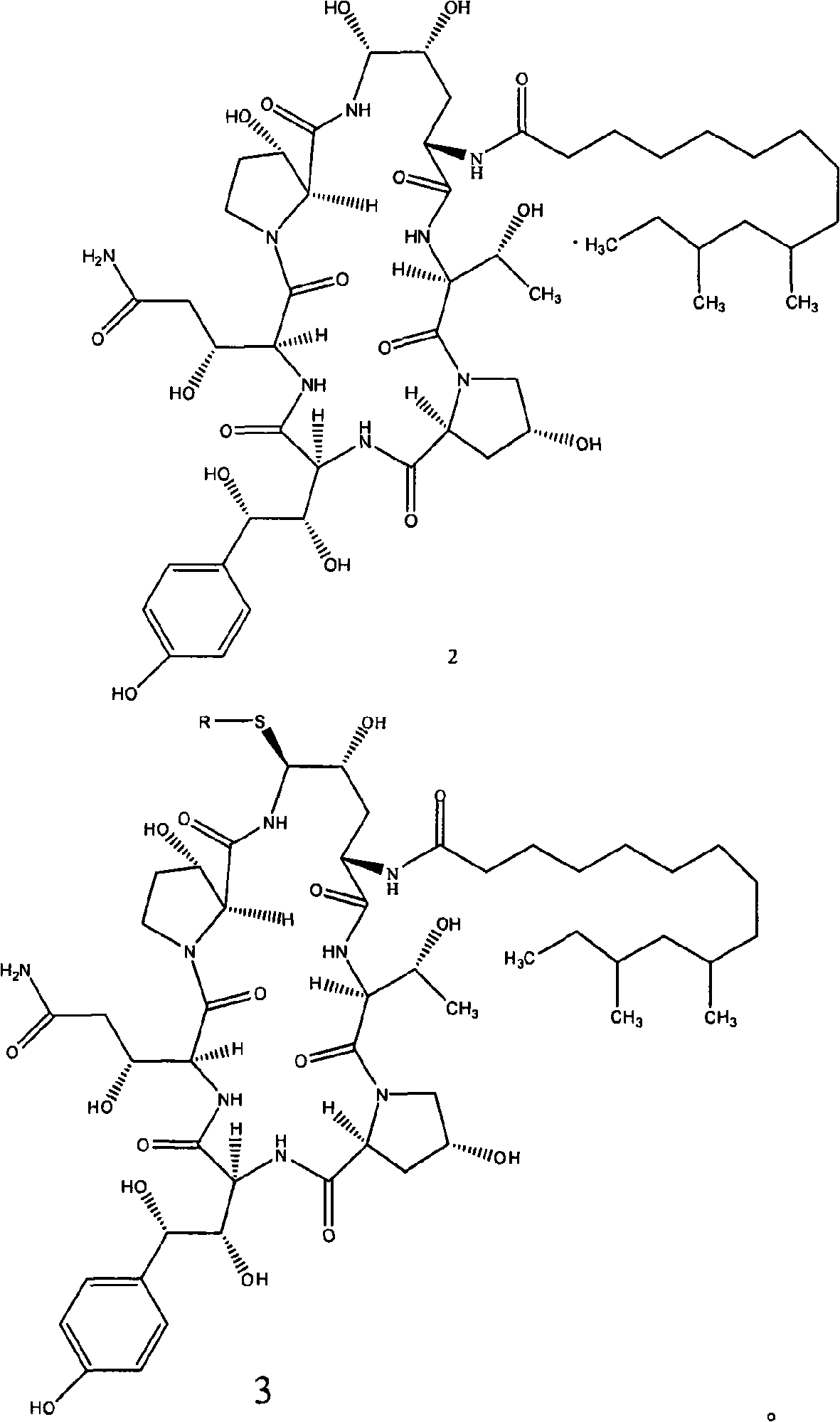
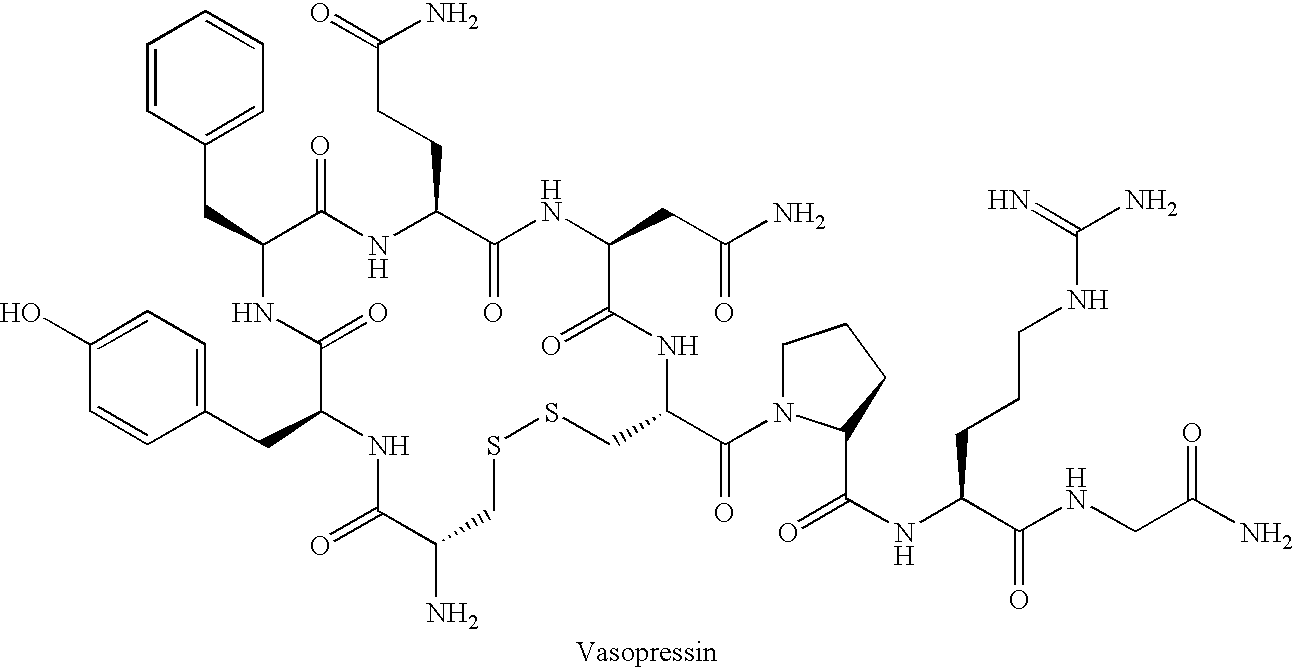
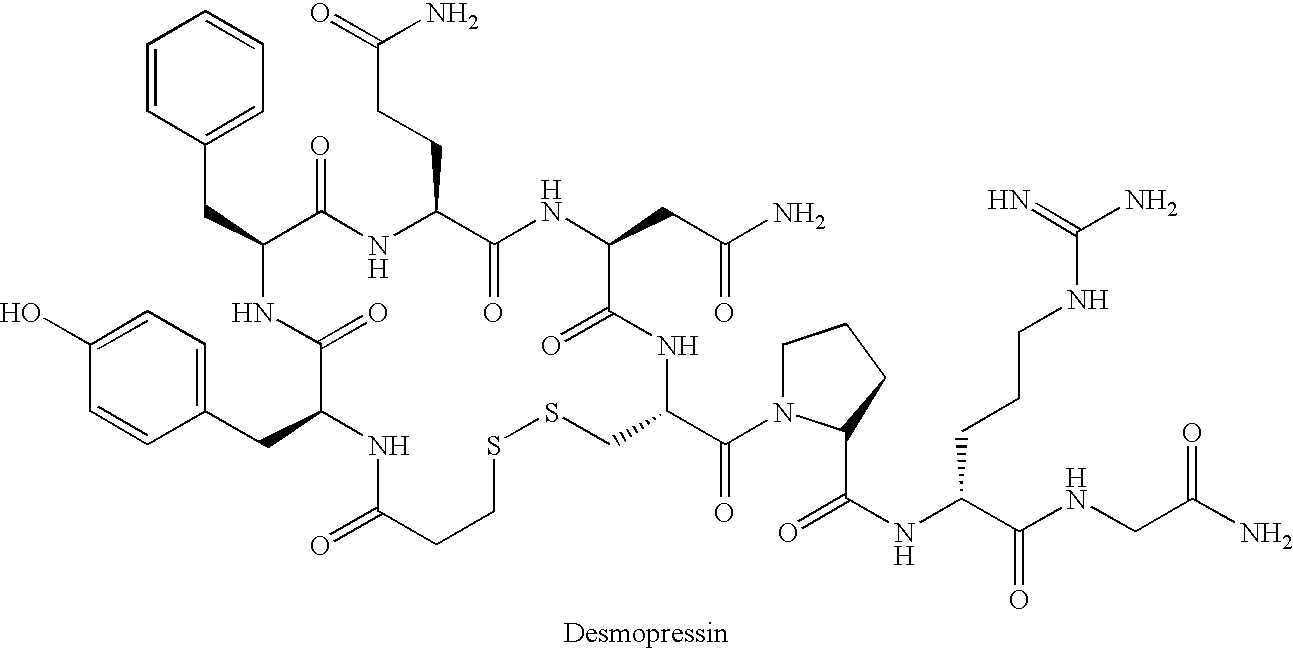
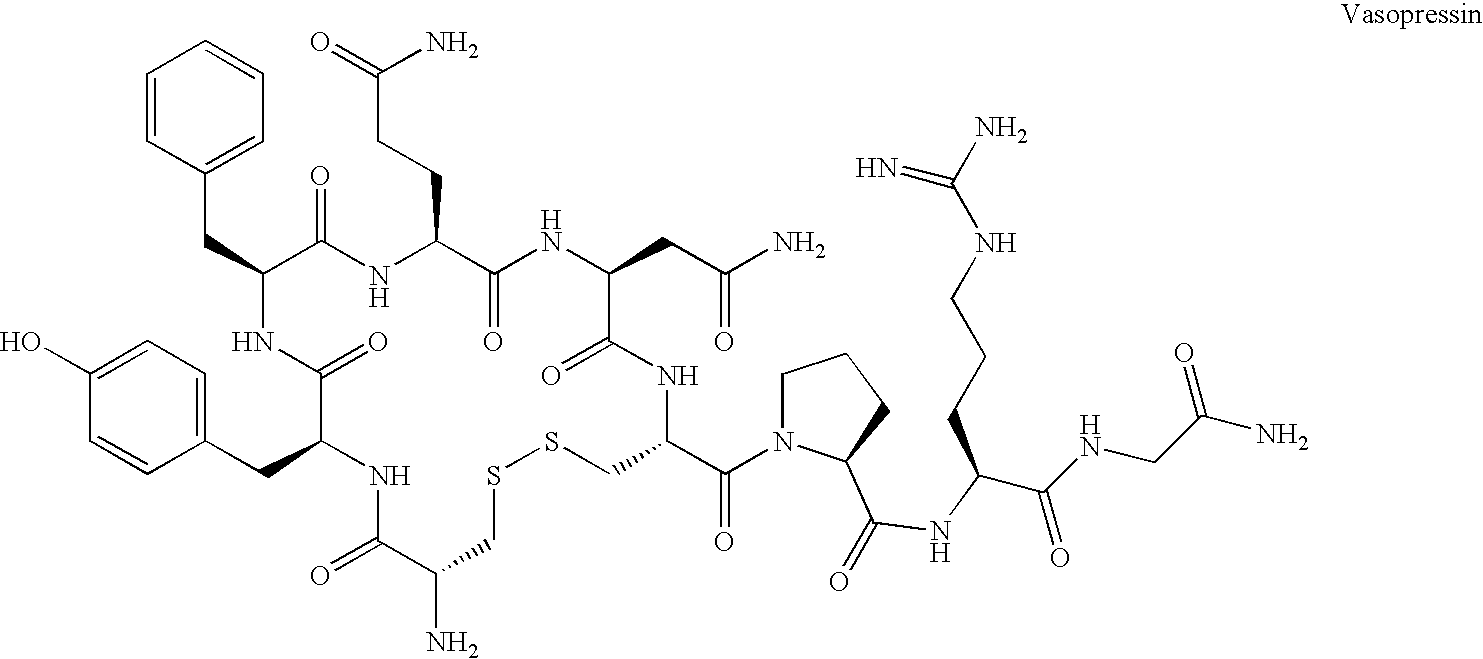
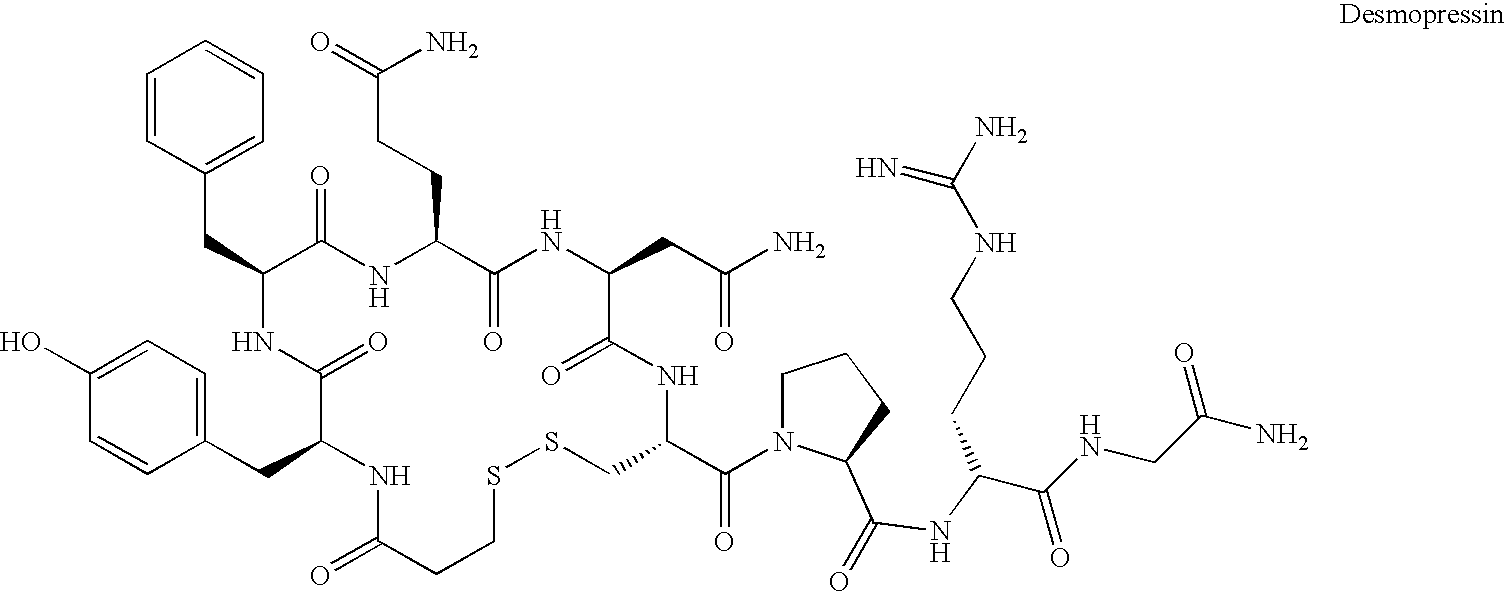
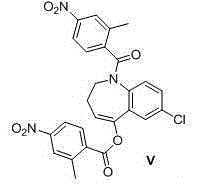
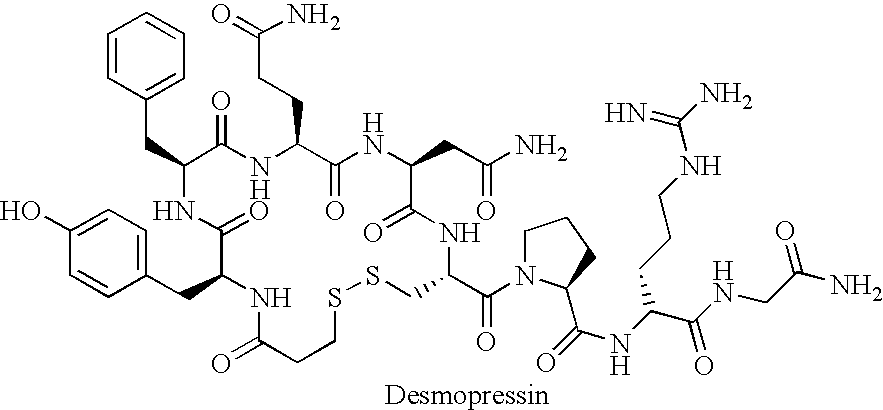
Fused azepine derivatives and their use as antidiuretic agents
Compounds according to general formulae (1 and 2), wherein G<1 >is an azepine derivative and G<2 >is a group according to general formulae (9-11) are new. Compounds according to the invention are vasopressin V2 receptor agonists. Pharmaceutical compositions of the compounds are useful as antidiuretic agents.
Owner:VANTIA
Aliphatic-aromatic copolyester, preparation method and application thereof
ActiveCN101717493AAvoid problems such as affecting performanceEvenly dispersedAdhesivesCarboxylic acid halidesMonomer
The invention provides aliphatic-aromatic copolyester, a preparation method and application thereof. Polymerization monomers comprise a compound selected from aliphatic dibasic acid, and naphthenic base dibasic acid or the ester, the anhydride and the acyl halide thereof, a compound selected from aromatic dibasic acid or the ester, the anhydride and the acyl halide thereof, a compound simultaneously with two functional groups selected from an amino-group, a mercapto group or a hydroxy or a compound of a derivative of the amino-group, the mercapto group or the hydroxyl with an epoxy group and an azepine ring, a compound selected from unsaturated acid with at least one C-C, C-O, C-N or C-S double bond and C-C or C-N triple bond or the ester, the anhydride and the acyl halide thereof and a compound of unsaturated alcohol with at least one C-C double bond or a C-C triple bond or an epoxide thereof. The aliphatic-aromatic copolyester is prepared by carrying out esterification and polycondensation after mixing the polymerization monomers, polymerizing the double bonds and / or the triple bonds on the polymerization monomers under the action of an initiator and then carrying out a graftingand / or coupling reaction.
Owner:HANGZHOU XINFU TECH CO LTD
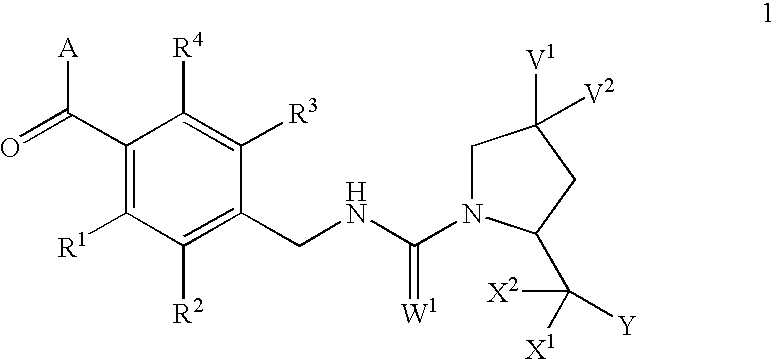
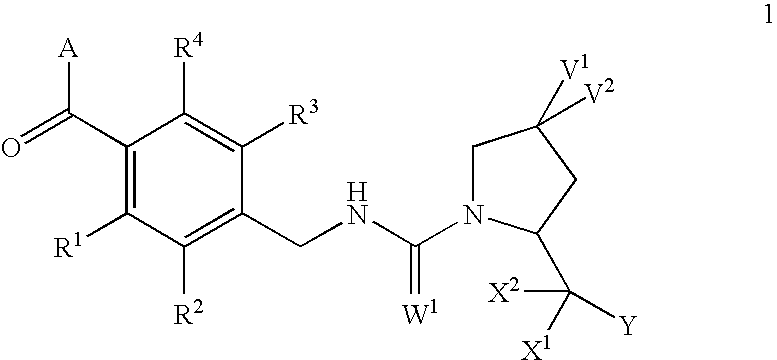
Condensed azepines as vasopressin agonists
This invention provides novel compounds according to general formula (1) wherein A is a bicyclic or tricyclic azepine derivative, V1 and V2 are both H, OMe or F, or one of V1 and V2 is Br, Cl, F, OH, OMe, OBn, OPh, O-acyl, N3, NH2, NHBn or NH-acyl and the other is H, or V1 and V2 together are =O, -O(CH2)pO- or -S(CH2)pS-; W1 is either O or S; X1 and X2 are both H, or together are =O or =S; Y is OR5 or NR6R7; R1, R2, R3 and R4 are independently selected from H, lower alkyl, lower alkyloxy, F, Cl and Br; R5 is selected from H and lower alkyl; R6 and R7 are independently selected from H and lower alkyl, or together are -(CH2)n; n=3, 4, 5, 6; and p is 2 or 3. The compounds are agonists at the vasopressin V2 receptor and are useful as antidiuretics and procoagulants. The invention further comprises pharmaceutical compositions incorporating these vasopressin agonists, which compositions are particularly useful in the treatment of central diabetes insipidus, nocturnal enuresis and nocturia.
Owner:VANTIA
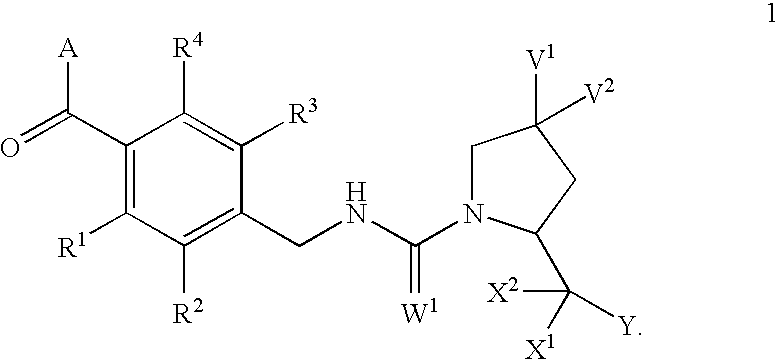
Condensed azepines as vasopressin agonists
This invention provides novel compounds according to general formula (1) wherein A is a bicyclic or tricyclic azepine derivative, V1 and V2 are both H, OMe or F, or one of V1 and V2 is Br, Cl, F, OH, OMe, OBn, OPh, O-acyl, N3, NH2, NHBn or NH-acyl and the other is H, or V1 and V2 together are ═O, —O(CH2)pO— or —S(CH2)pS—; W1 is either O or S; X1 and X2 are both H, or together are ═O or ═S; Y is OR5 or NR6R7; R1, R2, R3 and R4 are independently selected from H, lower alkyl, lower alkyloxy, F, Cl and Br; R5 is selected from H and lower alkyl; R6 and R7 are independently selected from H and lower alkyl, or together are —(CH2)n—; n=3, 4, 5, 6; and p is 2 or 3. The compounds are agonists at the vasopressin V2 receptor and are useful as antidiuretics and procoagulants. The invention further comprises pharmaceutical compositions incorporating these vasopressin agonists, which compositions are particularly useful in the treatment of central diabetes insipidus, nocturnal enuresis and nocturia.
Owner:VANTIA
Condensed azepines as vasopressin agonists
This invention provides novel compounds according to general formula (1) wherein A is a bicyclic or tricyclic azepine derivative, V1 and V2 are both H, OMe or F, or one of V1 and V2 is Br, Cl, F, OH, OMe, OBn, OPh, O-acyl, N3, NH2, NHBn or NH-acyl and the other is H, or V1 and V2 together are ═O, —O(CH2)pO— or —S(CH2)pS—; W1 is either O or S; X1 and X2 are both H, or together are ═O or ═S; Y is OR5 or NR6R7; R1, R2, R3 and R4 are independently selected from H, lower alkyl, lower alkyloxy, F, Cl and Br; R5 is selected from H and lower alkyl; R6 and R7 are independently selected from H and lower alkyl, or together are —(CH2)n; n=3, 4, 5, 6; and p is 2 or 3. The compounds are agonists at the vasopressin V2 receptor and are useful as antidiuretics and procoagulants. The invention further comprises pharmaceutical compositions incorporating these vasopressin agonists, which compositions are particularly useful in the treatment of central diabetes insipidus, nocturnal enuresis and nocturia.
Owner:VANTIA
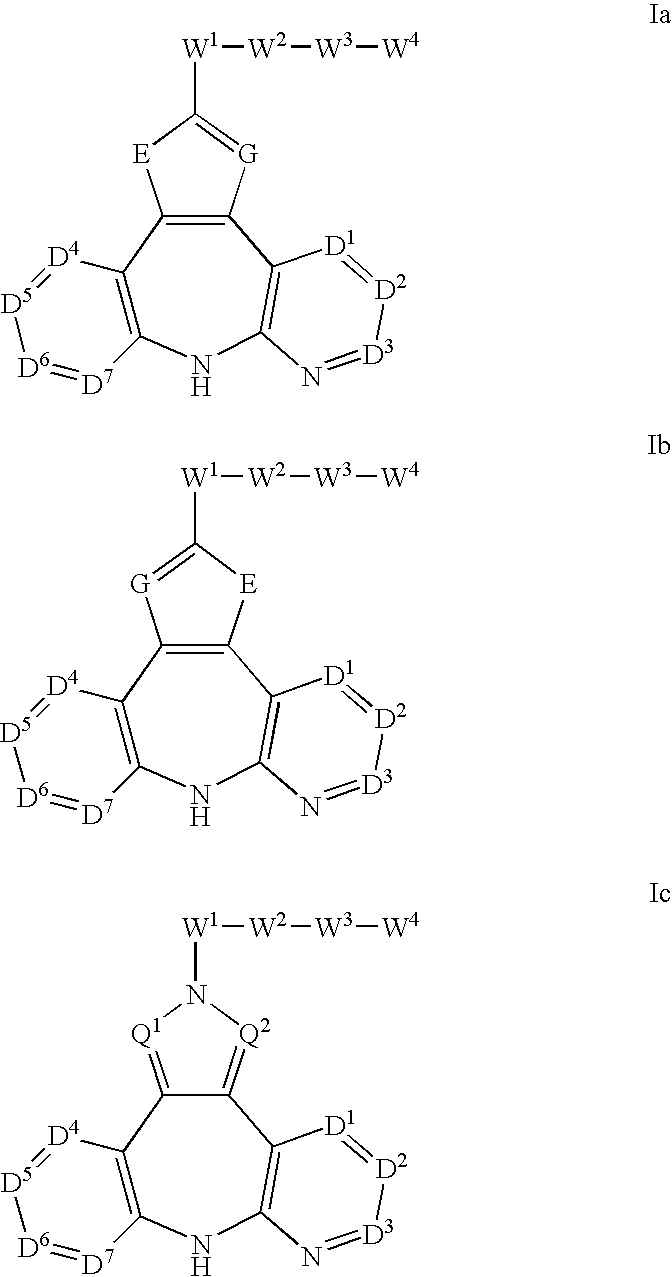
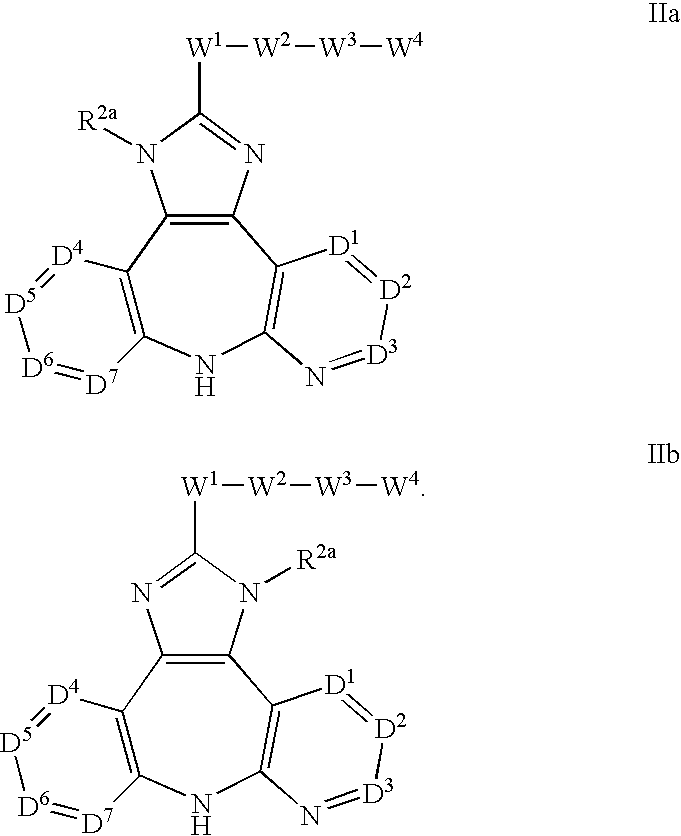
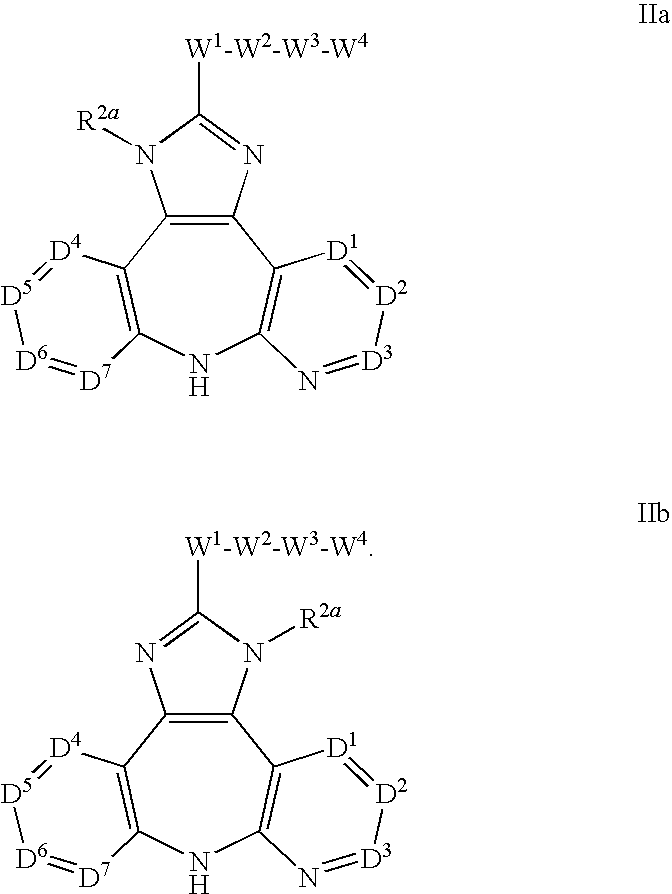
AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:HARMONY BIOSCIENCES LLC +1
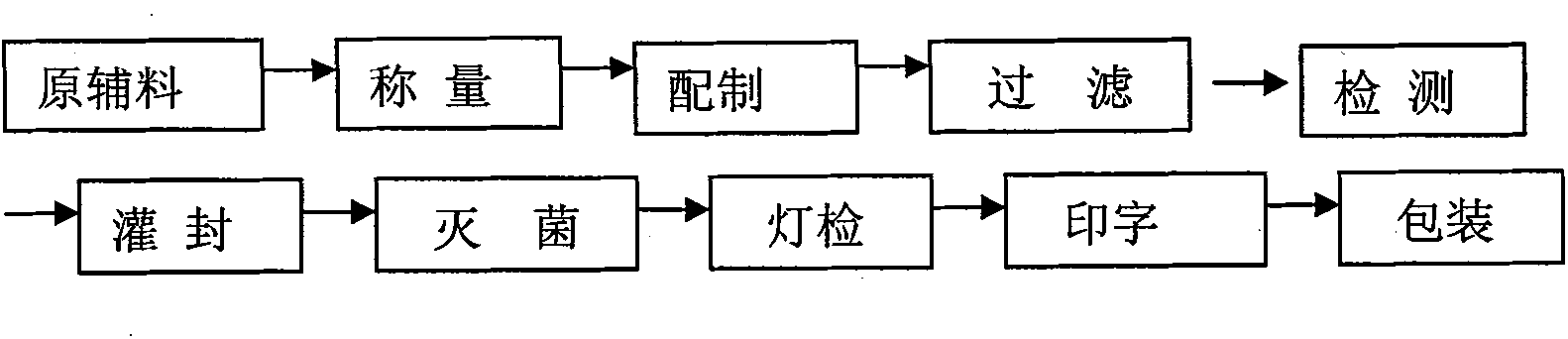
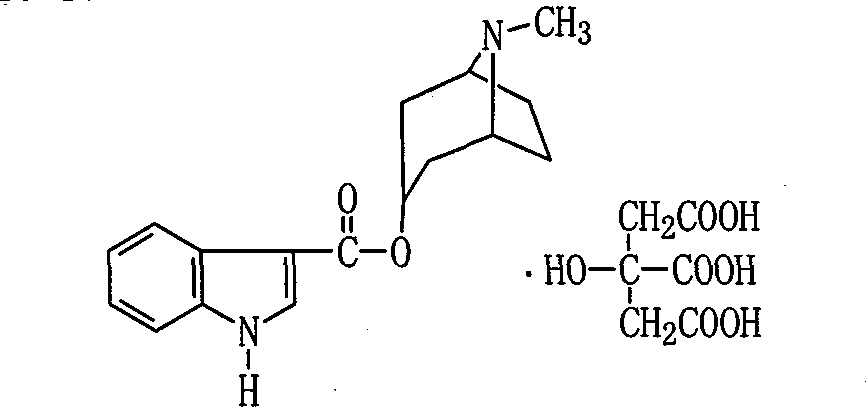
Citric acid tropisetron raw material medicine and preparation technology of raw material medicine and injection liquid
ActiveCN101838266AGood curative effectImprove securityOrganic chemistryDigestive systemTropaneFormate
The invention relates to a citric acid tropisetron raw material medicine and a preparation technology of the citric acid tropisetron raw material medicine and an injection liquid, and belongs to the field of chemical pharmacy; in the process of tropisetron salifying, the citric acid is used as acid radical; the chemical name of the citric acid tropane tropisetron is [(1alpha H, 5alpha H)-8-methyl-azepine bicyclo-(3, 2, 1) octyl group-3alpha-]-1H- benzpyrole-3- formic ether citrate; and the molecular formula is C23H28N209. In the process of tropisetron salifying, the tropisetron is dissolved in ethanol; the mixture is added with ethanol solution of the citric acid, evenly stirred and stands till the needed solid is separated out; the crude citric acid tropisetron is filtered, heated and dissolved in distilled water, is added with the activated carbon for backflowing and decoloring, cools and stands after being filtered so as to separate out white cystal; the mixture is filtered; the filter cake is dried and then distilled water is used for the secondary recrystallization to obtain the refined citric acid tropane tropisetron. The clinical research shows that compared with the imported muriatic acid tropisetron injection solution, the citric acid tropisetron injection solution has similar curative effect and safety.
Owner:JIANGXI DONGFU PHARMA CO LTD
Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines
Novel substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines, use of these compounds as pharmaceutical compositions, pharmaceutical compositions comprising the compounds, and a method of treatment employing these compounds and compositions. The compounds show a high and selective binding affinity to the histamine H3 receptor indicating histamine H3 receptor antagonistic, inverse agonistic or agonistic activity. As a result, the compounds are useful for the treatment of diseases and disorders related to the histamine H3 receptor.
Owner:VTV THERAPEUTICS LLC
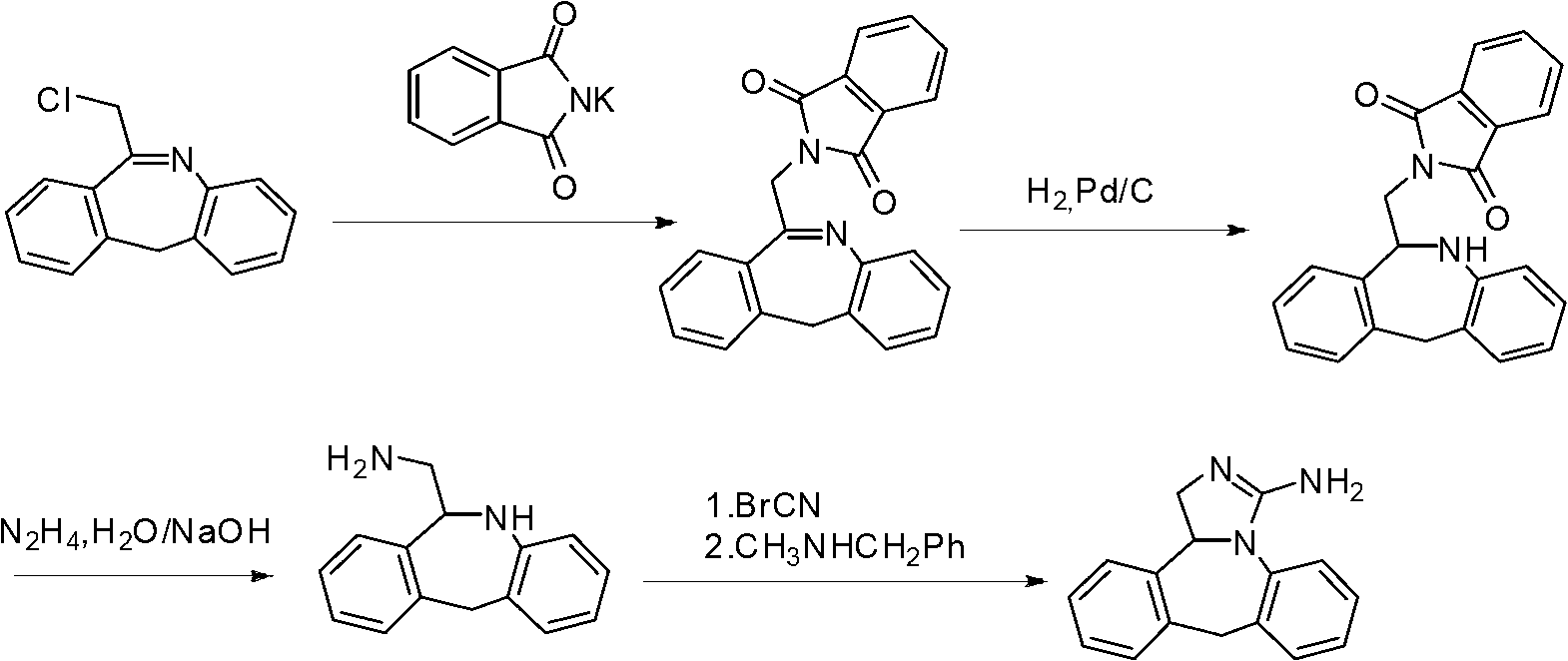
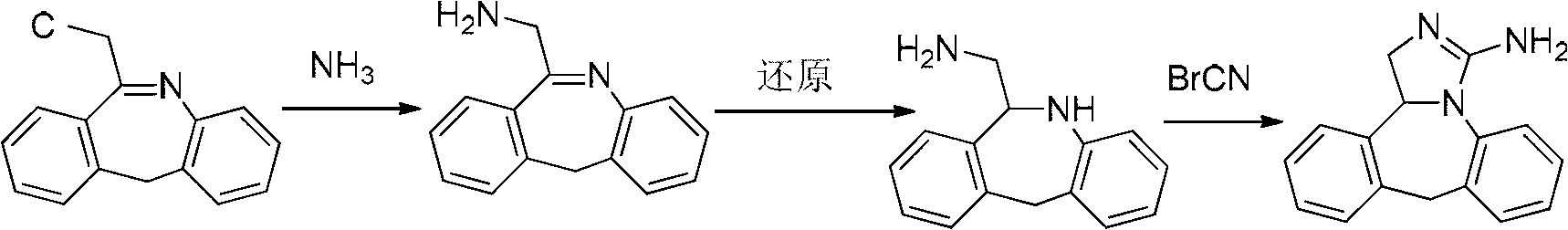
Synthesis method of epinastine
The invention discloses a synthesis method of epinastine. The synthesis method is implemented by taking 2-aminobenzophenone as a raw material and comprises the following steps of: reacting the 2-aminobenzophenone with a silane agent to obtain 2-benzylaniline; then, carrying out acylation reaction on the 2-benzylaniline and 2-chloroacetyl chloride to obtain N-(2-benzyl phenyl)-2-chloroacetamide; carrying out acidamide dehydration and cyclization on the N-(2-benzyl phenyl)-2-chloroacetamide under the action of a dehydrating agent to obtain 6-(chloromethyl)-11H-dibenzo[b,e] azepine; carrying out azidation reaction on the 6-(chloromethyl)-11H-dibenzo[b,e] azepine to obtain 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine; carrying out reduction on the 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine; and finally, carrying out cyclization on the 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine and cyanogen bromide to obtain the epinastine. The synthesis method disclosed by the invention avoids the application of expensive and flammable lithium aluminium hydride and aluminium hydride as well as hypertoxic sodium cyanide, so that the operation is safer in industrial production, and the cost is reduced. The method is simple in process and high in yield, requires mild conditions, and is suitable for industrialized production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
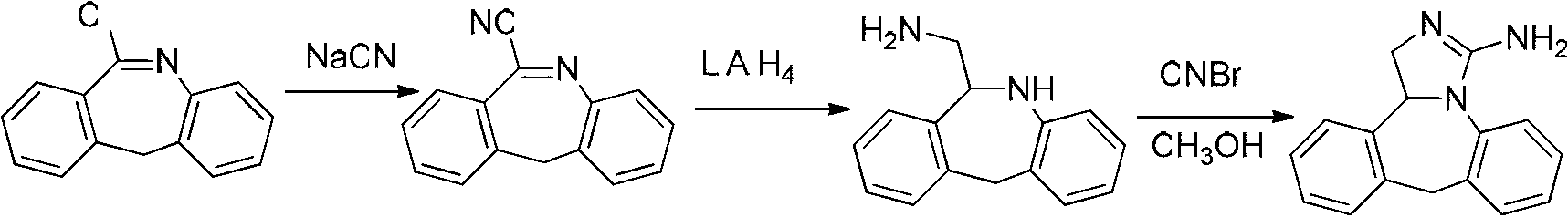
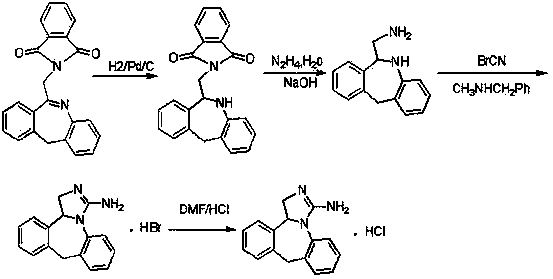
Preparation method of epinastine hydrochloride and intermediate thereof
The invention relates to a method preparing an epinastine hydrochloride intermediate and epinastine hydrochloride. The method comprises the following steps: reacting 6-chloromethyl-11-dihydro-dibenzo[b,e]azepine and an amino reagent under protection to generate an amino-protective compound; carrying out reduction reaction and deprotection reaction to generate 6-aminomethyl-6,11-dihydro-dibenzo[b,e]azepine; and finally, carrying out cyanogen bromide cyclization on the 6-aminomethyl-6,11-dihydro-dibenzo[b,e]azepine, neutralizing with alkali, and reacting with hydrochloric acid to generate the epinastine hydrochloride. The preparation method has the advantages of accessible reaction raw materials, fewer byproducts and high product purity, and is suitable for industrial production.
Owner:YAOPHARMA CO LTD +1
Heteroaryl pyridone and aza-pyridone amide compounds
Heteroaryl pyridone and aza-pyridone amide compounds of Formula I are provided, and various substituents including stereoisomers, tautomers, and pharmaceutically acceptable salts thereof, useful for inhibiting Btk, and for treating cancer and immune disorders such as inflammation mediated by Btk. Methods of using compounds of Formula I for in vitro, in situ, and in vivo diagnosis, and treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed.
Owner:GENENTECH INC
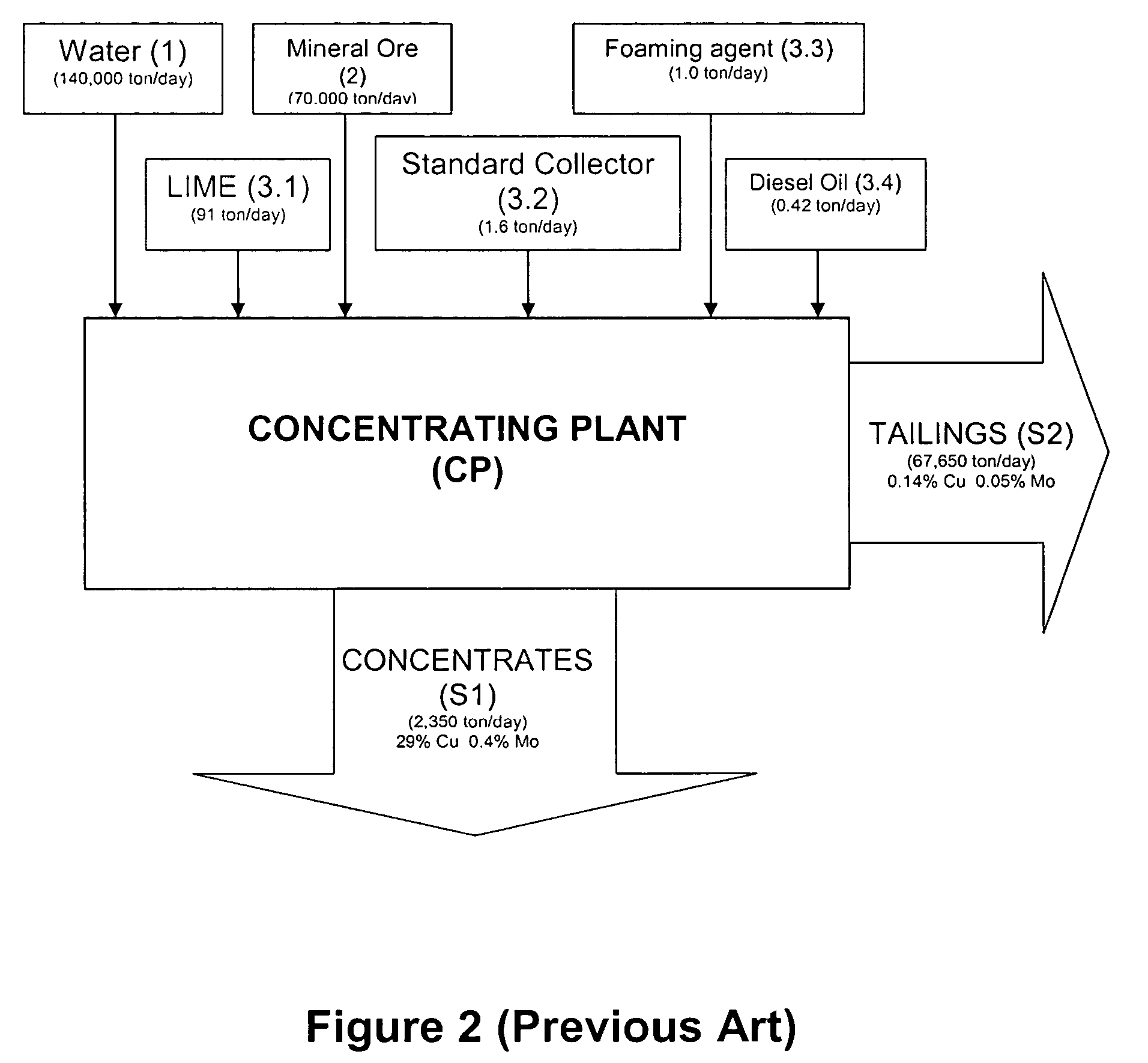
Collecting agent comprising ammoniated compounds (primary, secondary, tertiary amines), for use in the process of grinding and/or floating copper, molybdenum, zinc, and other contained mineral ores
Owner:PROCESOS MINEROS E IND CONOSUR
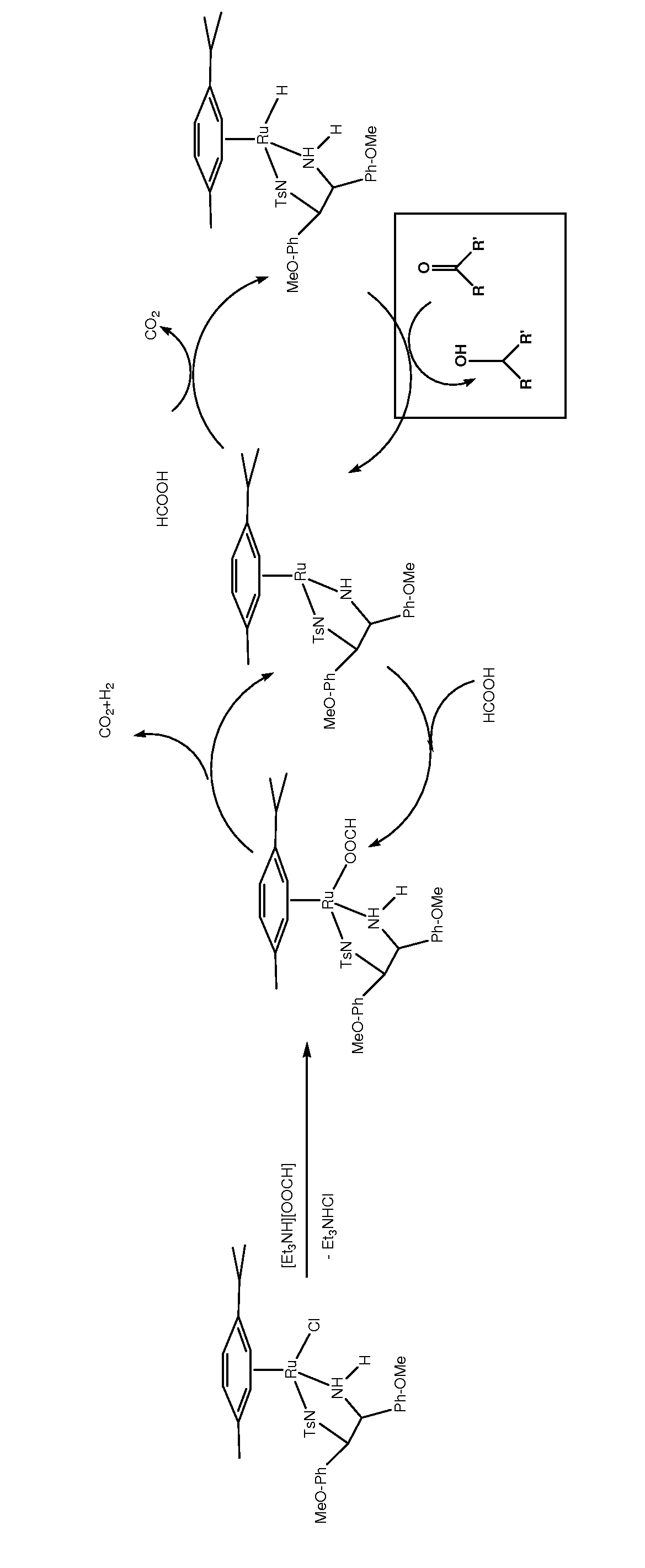
Asymmetric catalytic reduction of oxcarbazepine
ActiveUS8288532B2High substrate concentrationOptimize volumeAntibacterial agentsNervous disorderHydrogenAlkaline earth metal
A process for preparing (S)-(+)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide or (R)-(−)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide, by reduction of oxcarbazepine in the presence of a catalyst and a hydride source is disclosed. The catalyst is prepared from a combination of [RuX2(L)]2 wherein X is chlorine, bromine or iodine, and L is an aryl or aryl-aliphatic ligand, with a ligand of formula (A) or formula (B):wherein R1 is chosen from C1-6 alkoxy and C1-6 alkyl, n is a number from 0 to 5, and when n is a number from 2 to 5, R1 can be the same or different, and R2 is alkyl, substituted alkyl, aryl, substituted aryl, alkaryl or substituted alkaryl. The hydride source is either NR3R4R5 and formic acid, [R3R4R5NH][OOCH] and optionally formic acid, or [M][OOCH]x and formic acid, wherein R3, R4 and R5 are C1-6 alkyl, M is an alkali metal or alkaline earth metal and x is 1 or 2. A pH from 6.5 to 8 is maintained during the process.
Owner:BIAL PORTELA & CA SA
Preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine
The invention relates to a preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine. The 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine is an important intermediate for preparing arginine pitressin V2 receptor antagonist Tolvaptan. In the preparation method, the target product 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine is prepared by removing tosyl and carbalkoxy from 7-chlorine-5-oxo-4-carbalkoxy-1-tosyl-2,3,4,5-tetrahydro-1-benzoazepine serving as a raw material under the action of sulfuric acid and by a 'one-pot method'. The sulfuric acid is used for performing 'one-pot method' reaction, so reaction steps are reduced and reaction time is greatly shortened. The preparation method has the advantages of simpleness and convenience for operation, equipment and cost saving, simpleness and practicability in separation and purification, short reaction time, high yield and high product purity and is applicable to industrialized production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of tolvaptan
The invention discloses a preparation method of tolvaptan. According to the preparation method, 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one and 4-nitro-2-methyl bromobenzene are taken as the primary raw materials; high purity tolvaptan is obtained after steps of carbonyl inserting reactions, reduction reactions, and acylation reactions, and the yield is high. The preparation method has the advantages that no bromine or tin dichloride is used; the preparation method does not generate a large amount of industrial waste water, and the environment is protected. At the same time, the generation of impurities namely a compound V and a compound VIII is avoided, and the purification becomes easier. No explosive, flammable, and toxic solvent such as chloroform, ether, and the like, is used, the requirements on the protection of workers are lowered, and the safe production is guaranteed. Moreover, the route design is novel, the raw materials are easily available, the operation of the technology is simple and feasible, and a simple and feasible method is provided for the massive industrial production of tolvaptan.
Owner:天津泰普制药有限公司
Fused azepine derivatives and their use as antidiuretic agents
Compounds according to general formulae (1 and 2), wherein G1 is an azepine derivative and G2 is a group according to general formulae (9–11) are new. Compounds according to the invention are vasopressin V2 receptor agonists. Pharmaceutical compositions of the compounds are useful as antidiuretic agents
Owner:VANTIA
Novel Compounds
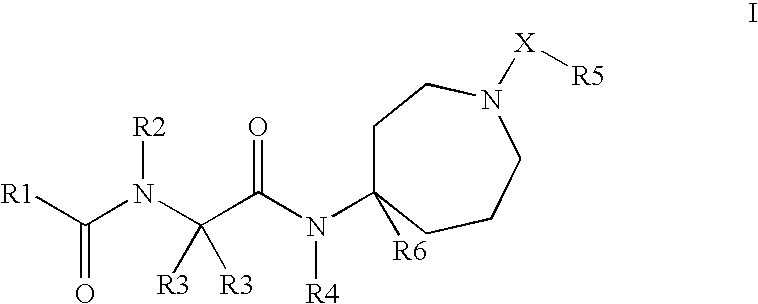
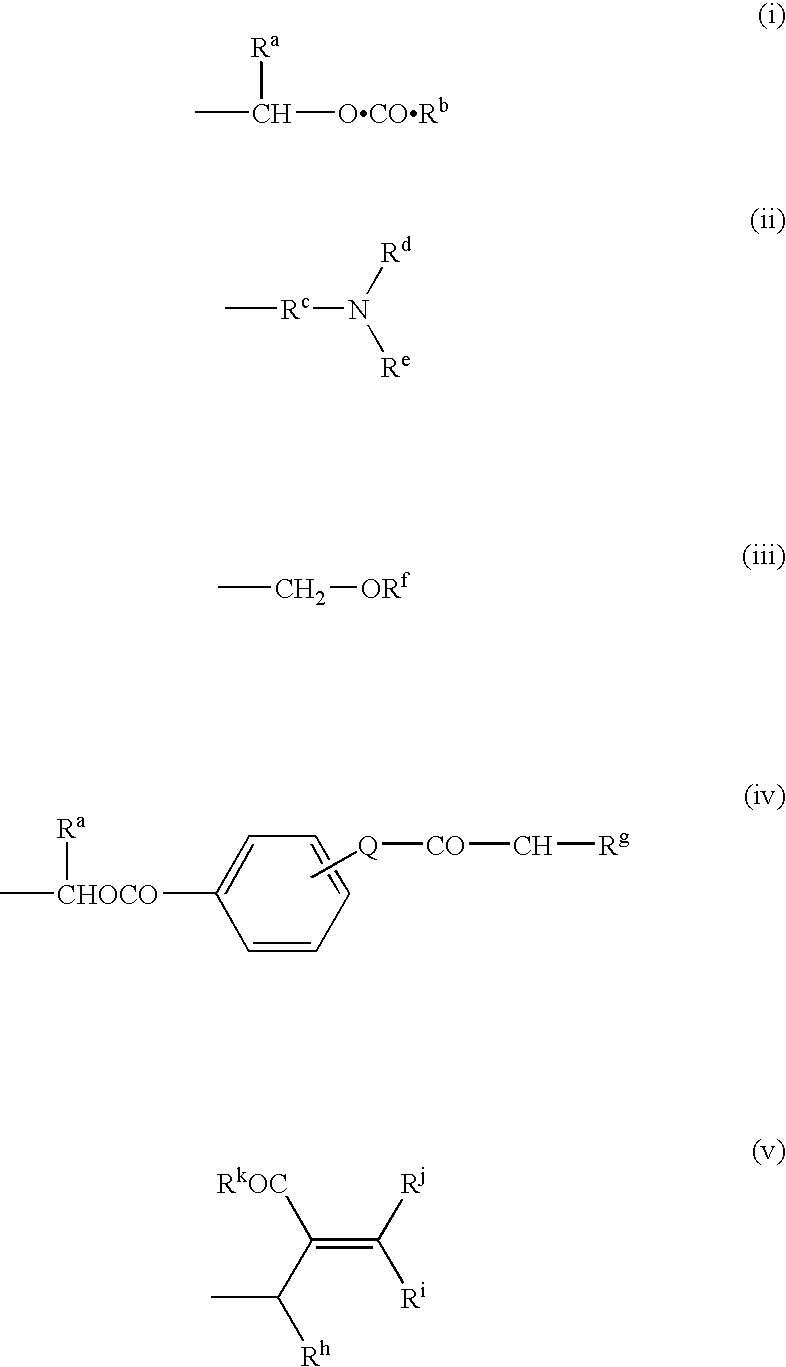
This invention relates to novel compounds useful in the treatment of diseases associated with TRPV4 channel receptor. More specifically, this invention relates to certain substituted amino-azepines, according to Formula I Specifically, the invention is directed to compounds according to Formula I wherein: R1 is optionally substituted C3-7cycloalkyl, optionally substituted C3-7cycloalkenyl, optionally substituted Het-C3-7alkyl, optionally substituted Het-C3-7alkenyl, optionally substituted aryl, optionally substituted heterocycloalkyl, optionally substituted heteroaryl, or optionally substituted indenyl; R2 is H, optionally substituted C1-6alkyl, C3-6cycloalkyl-C0-6alkyl, Ar—C0-6alkyl, or Het-C0-6alkyl; each R3 is independently H, optionally substituted C1-8alkyl, optionally substituted C2-8alkenyl, optionally substituted C2-8alkynyl, Het-C1-6 alkyl, optionally substituted C3-6cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, or optionally substituted C1-C6 alkoxy; R4 is H, or optionally substituted C1-C4 alkyl; R5 is H, optionally substituted C1-8alkyl, optionally substituted C2-8alkenyl, optionally substituted C2-8alkynyl, optionally substituted C3-6cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R6 is H or C1-6alkyl; and X is SO2, CO, CH2, or CONH, and pharmaceutically acceptable salts, hydrates, solvates and pro-drugs thereof.
Owner:SMITHKLINE BECKMAN CORP
Synthesis method of 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine
The invention relates to a synthesis method of 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine shown in the formula (I). The synthesis method comprises the following steps of: carrying out oxidizing reaction at 0-200 DEG C by taking 10,11-dihydro-5H-dibenzo(b,f) azepine shown in the formula (II) as a raw material, nitroxides shown in the formula (III) as a catalyst, acetate as a catalyst promoter, calcium hypochlorite as an oxidant and inorganic salt as a carrier, and after reaction, processing reaction liquid to obtain the 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine shown in the formula (I). The invention has the advantages of short reaction step, high conversion rate and yield, advanced process path, mild reaction conditions, small catalyst consumption, no use of brom-containing reagent like NBS (N-bromosuccinimide), liquid bromine and the like, simple after-treatment and less pollution to the environment.
Owner:ZHEJIANG UNIV OF TECH +1
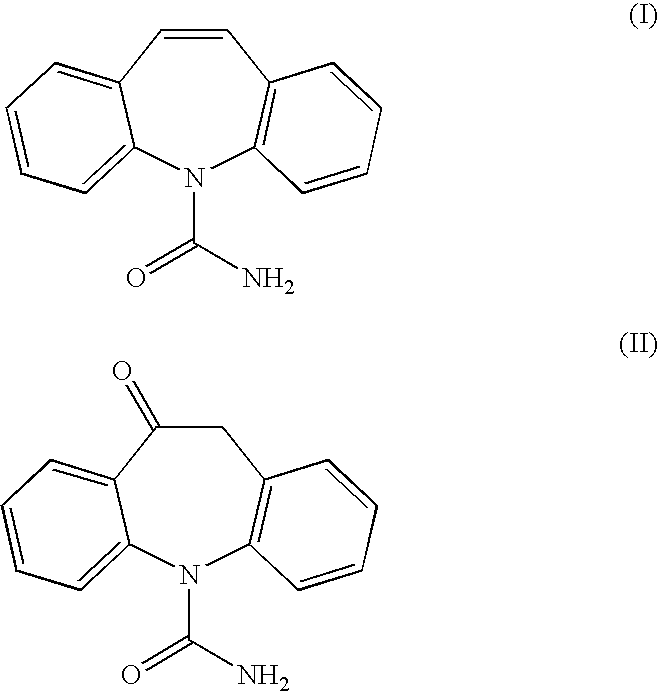
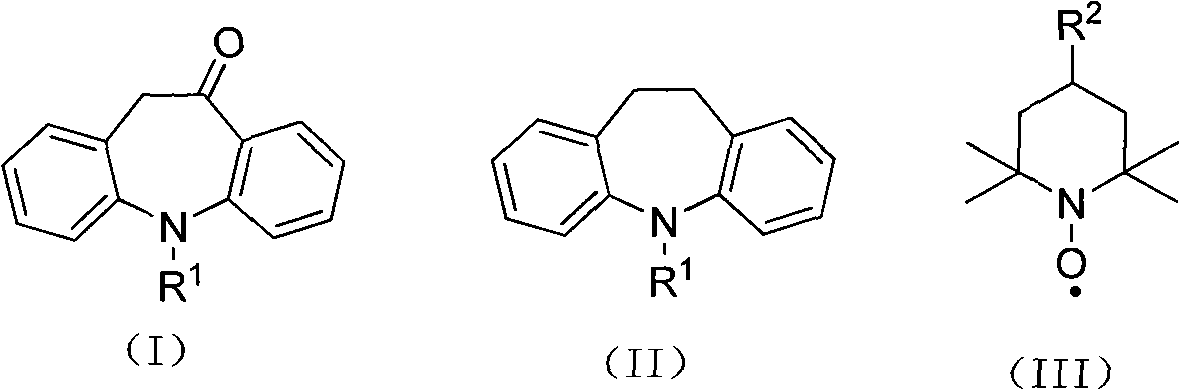
Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof
A high-yielding method and a useful intermediate, 10-chloro-10,11-dihydro-5H-dibenz / b,f / azepine-5-carboxamide (VII) is disclosed. Also disclosed are methods for the racemization of optically pure or optically enriched mixtures of (S)-(+)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide (I) and (R)-(−)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide (II) to racemic (±)-10,11-dihydro-10-hydroxy-5H-dibenz / b,f / azepine-5-carboxamide (III).
Owner:BIAL PORTELA & CA SA
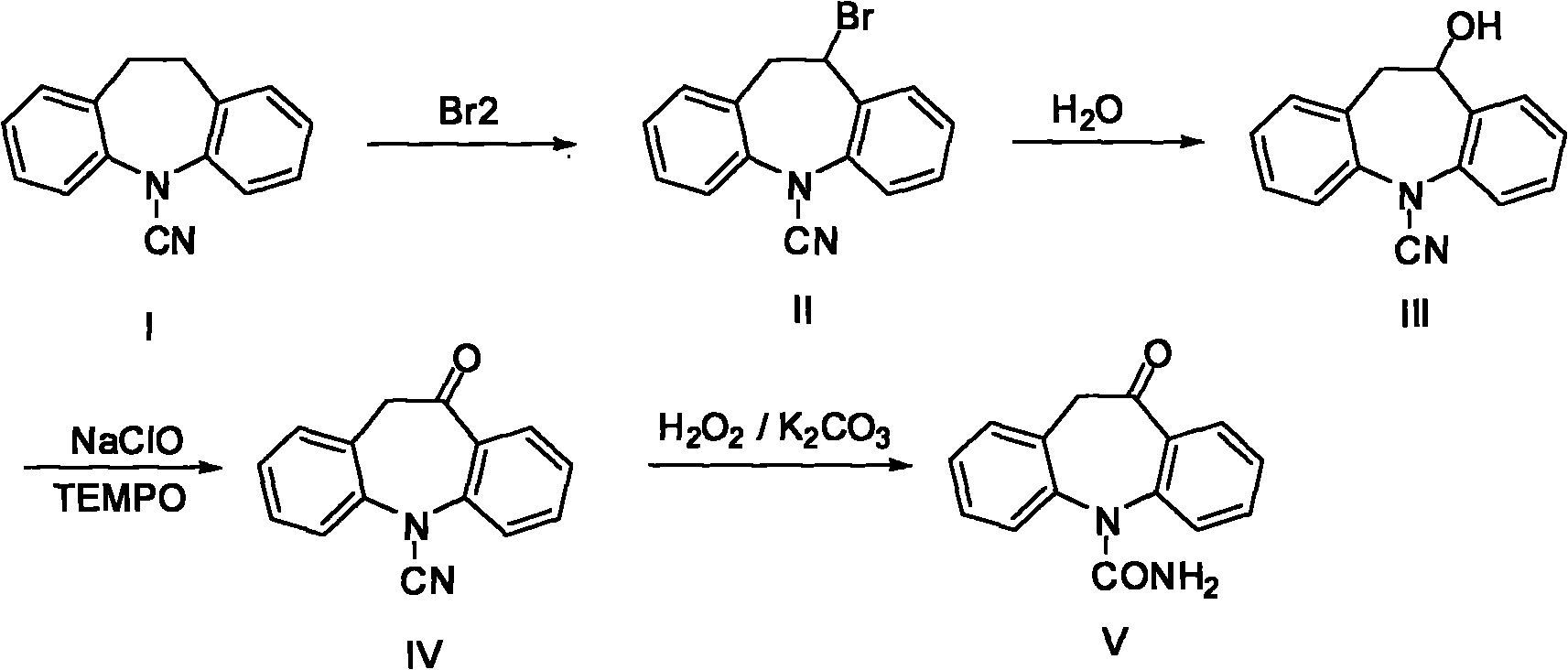
Chemical synthetic method of azepine derivate
ActiveCN101302198AHigh yieldReasonable process conditionsOrganic chemistryChemical synthesisHydrolysate
The invention discloses a synthesis method for an azepine derivative, in particular relating to a chemical synthesis method for 10-oxa-10,11-dihydro-5H-dibenzo[b,f]azepin-5-formylamino. The method of the invention is as follows: 10-cyano-10,11-dihydro-5H-dibenzo[b,f]azepin-5- formylamino undergoes bromination at the benzyl position to give a bromide. The bromide undergoes hydrolysis to give a hydrolysate which is oxidized under the condition of NaClO / TEMPO to give a carboxide. And finally a cyan in the carboxide is hydrolyzed by H2O2 in an alkaline condition to give the resultant. The method has the advantages of reasonable process condition, cheap materials, convenient and rapid operation, highly improved resultant yield, giving far more environment-friendly process due to the hydrolysis of cyan with hydrogen peroxide compared with that with concentrated sulphuric acid, having large advantages for industrial production. The method can be widely used in pharmaceutical industry.
Owner:浙江瑞博制药有限公司
Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride
InactiveUS20070032647A1Inhibition formationReduce the temperatureOrganic chemistryOrganic baseBis(trichloromethyl) carbonate
A process for preparation of 10-oxo-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide (oxcarbazepine) via intermediate 10-methoxy-5H-dibenz[b,f]azepine-5-carbonyl chloride, comprising the steps: a) Preparation of an intermediate 10-methoxy-5H-dibenz[b,f]azepine-5 carbonyl, chloride from 10-methoxyiminostillbene using bis (trichloromethyl) carbonate (BTC) with organic base such as aliphatic or aromatic tertiary amines in organic solvent; b) Conversion of the intermediate to 10-methoxy-5H-dibenz[b,f]azepine-5-carboxamide using ammonia in organic solvent; c) Formation of oxcarbazepine from step (b) using Bronsted acid in an organic solvent at a temperature between 25° C.-80° C., preferably at 50° C. to 70° C.; and d) Isolation of oxcarbazepine.
Owner:AMOLI ORGANICS LTD
Synthesis method of azepine anthraquinone
InactiveCN101712648AAchieve synthesisHas antibacterial activityOrganic chemistryPlatinum saltsIsomerization
The invention relates to a synthesis method of azepine anthraquinone. The azepine anthraquinone is obtained by carrying out intramolecular 6-endo-dig cycloisomerisation reaction shown as in formula (1) on N-propargyl quinine with a 1,5-eneyne structure under the action of a metal catalyst, and purifying, wherein the intramolecular 6-endo-dig cycloisomerisation reaction is homogeneous phase metal catalytic reaction, the metal catalyst is gold salt, platinum salt or univalent gold complex; and the use level of the metal catalyst is 0.01-0.5 equivalent weight of the N--propargyl quinine. The gold slat is auri chloridum (AuCl3) or aurous chloride (AuCl); the platinum salt is platinum tetrachloride, platinum bichloride or potassium chloroplatinate; and the univalent gold complex is PPh3AuOTf, PPh3AuSbF6, PPh3AuNTf2 or LAuNTf2, wherein L is nitrogen heterocyclic ring carbene ligand. The invention realizes the synthesis of the azepine anthraquinone by utilizing metal catalytic intramolecular eneyne cyclization reaction, and has the advantages of simple and easy-accessible raw materials and moderate reaction conditions.
Owner:NANJING UNIV
Azepine brazilin compound and synthesis method thereof
The invention relates to an azepine brazilin compound and a synthesis method thereof. By using the structure of a natural product brazilin as a simulation object, a series of brazilin structure compounds containing nitrogen atoms are designed; and substituted cinnamic acid is used as a raw material, and a series of azepine brazilin compounds is obtained through serial chemical conversion and synthesis. Partial azepine brazilin compounds have strong anti-cancer activity, partial compounds have anti-HIV (Human Immunodeficiency Virus) activity, and partial compounds have aldose reductase suppressive activity and obviously reduce the blood sugar level. Medical compositions prepared from the compounds and at least one pharmaceutically acceptable excipient or carrier can be used for treating cancer and resist virus infections.
Owner:YUNNAN UNIV
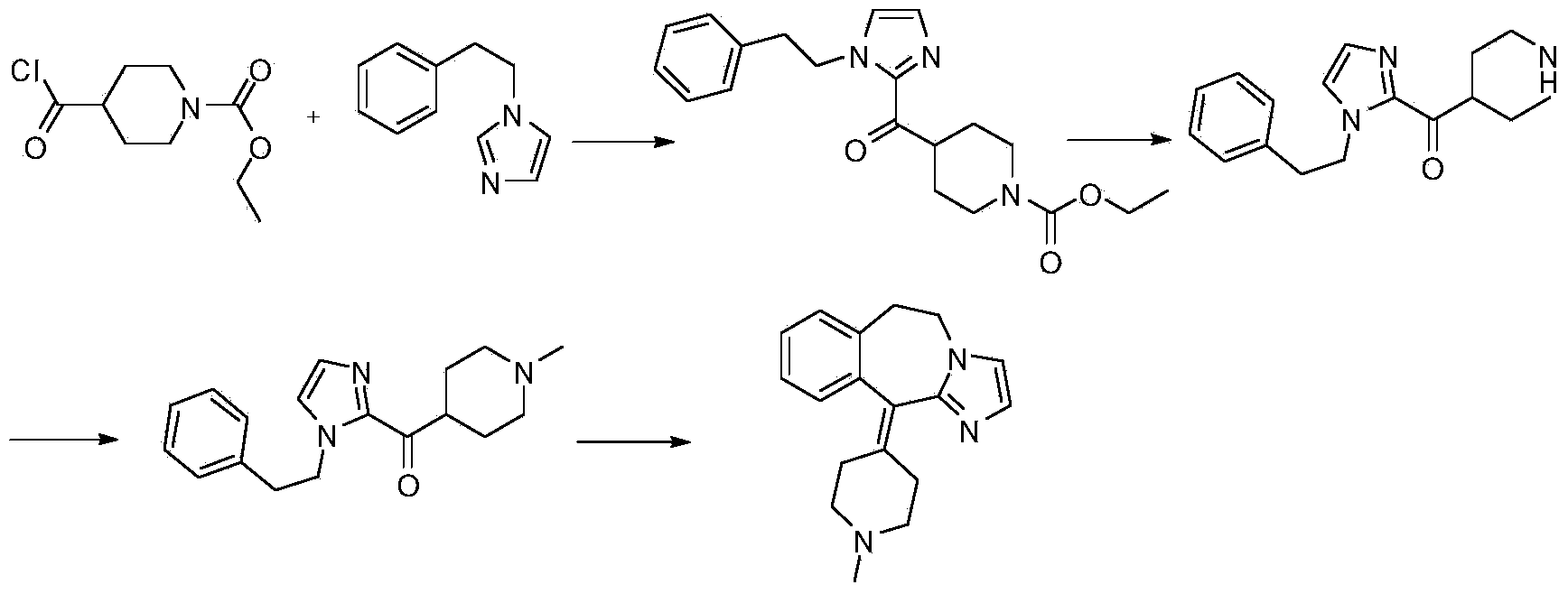
Preparation method of alcaftadine intermediate
The invention provides a preparation method of 6,11-dihydro-11-(1-methyl-4-subpiperidyl)-5H-imidazole[2,1-b][3] benzo azepine shown in a formula (I). The preparation method comprises the following steps of: with N-methyl-4-piperidine formyl chloride hydrochloride and 1-phenethyl-1H-imidazole as raw materials, carrying out an acylation reaction under the action of alkali to obtain [1-(2- phenethyl)-1H-imidazole-2-yl] (1-methyl-4-piperidyl) ketone, and then synthesizing 6,11-dihydro-11-(1-methyl-4-subpiperidyl)-5H-imidazole[2,1-b][3] benzo azepine. The invention provides a simple and efficient preparation method of 6,11-dihydro-11-(1-methyl-4-subpiperidyl)-5H-imidazole[2,1-b][3] benzo azepine, the preparation method is short in route and easy in operation, and can be used for avoiding a grignard reaction and introduction of precious metal, greatly shortening the existing reaction route and greatly increasing the reaction yield.
Owner:SHANGHAI PUKANG PHARMA
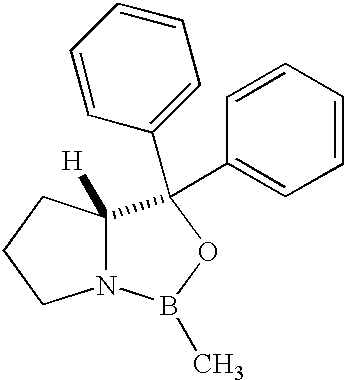
Methods for the stereoselective synthesis of substituted piperidines
One aspect of the present invention relates to methods of synthesizing substituted piperidines. A second aspect of the present invention relates to stereoselective methods of synthesizing substituted piperidines. The methods of the present invention will find use in the synthesis of compounds useful for treatment of numerous ailments, conditions and diseases that afflict mammals, including but not limited to addiction and pain. An additional aspect of the present invention relates to the synthesis of combinatorial libraries of the substituted piperidines using the methods of the present invention. An additional aspect of the present invention relates to enantiomerically substituted pyrrolidines, piperidines, and azepines.
Owner:SEPACOR INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/327ca0d5-2463-4ec2-9cc0-ebfc3042dd52/US20070142635A1-20070621-C00001.png)
![Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/327ca0d5-2463-4ec2-9cc0-ebfc3042dd52/US20070142635A1-20070621-C00002.png)
![Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds Process for preparing hexahydropyrimido[1,2-a]azepine-2-carboxylates and related compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/327ca0d5-2463-4ec2-9cc0-ebfc3042dd52/US20070142635A1-20070621-C00003.png)



![5,6-dihydro-4H-benzo[b]thieno-[2,3-d]azepine derivative 5,6-dihydro-4H-benzo[b]thieno-[2,3-d]azepine derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff7d0f02-9482-40d1-9999-959a72a70774/US09732098-20170815-D00001.png)
![5,6-dihydro-4H-benzo[b]thieno-[2,3-d]azepine derivative 5,6-dihydro-4H-benzo[b]thieno-[2,3-d]azepine derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff7d0f02-9482-40d1-9999-959a72a70774/US09732098-20170815-D00002.png)
![5,6-dihydro-4H-benzo[b]thieno-[2,3-d]azepine derivative 5,6-dihydro-4H-benzo[b]thieno-[2,3-d]azepine derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff7d0f02-9482-40d1-9999-959a72a70774/US09732098-20170815-C00001.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00001.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00002.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00003.png)
![Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/58fd7dae-39a4-4897-b809-98464c0347f7/US06906060-20050614-C00001.png)
![Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/58fd7dae-39a4-4897-b809-98464c0347f7/US06906060-20050614-C00002.png)
![Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines Substituted hexahydropyrrolo[1,2-a]pyrazines, octahydropyrido[1,2-a]-pyrazines and decahydropyrazino[1,2-a]azepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/58fd7dae-39a4-4897-b809-98464c0347f7/US06906060-20050614-C00003.png)















![Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7d8b9f0c-6a59-48dc-9f17-ce69ecddd93b/US07189846-20070313-C00001.png)
![Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7d8b9f0c-6a59-48dc-9f17-ce69ecddd93b/US07189846-20070313-C00002.png)
![Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof Method for racemization of (S)-(+)- and (R)-(-)-10,11-dihydro-10-hydroxy-5h-dibenz[B,F]azepine-5-carboxamide and optically enriched mixtures thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7d8b9f0c-6a59-48dc-9f17-ce69ecddd93b/US07189846-20070313-C00003.png)


![Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb90b6a6-a1f7-4a40-9209-d51f74866cc8/US20070032647A1-20070208-C00001.png)
![Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb90b6a6-a1f7-4a40-9209-d51f74866cc8/US20070032647A1-20070208-C00002.png)
![Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb90b6a6-a1f7-4a40-9209-d51f74866cc8/US20070032647A1-20070208-C00003.png)