Azepine argireline or pharmaceutically acceptable salt thereof and preparation method and application thereof
A nitrogen-heterocyclic and pharmaceutical technology, applied in the field of azacyclohexyl peptide or its pharmaceutically acceptable salt and its preparation, can solve the problems that the compound has no significant stereoselectivity, cannot be applied to industrial production, and the yield is not high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
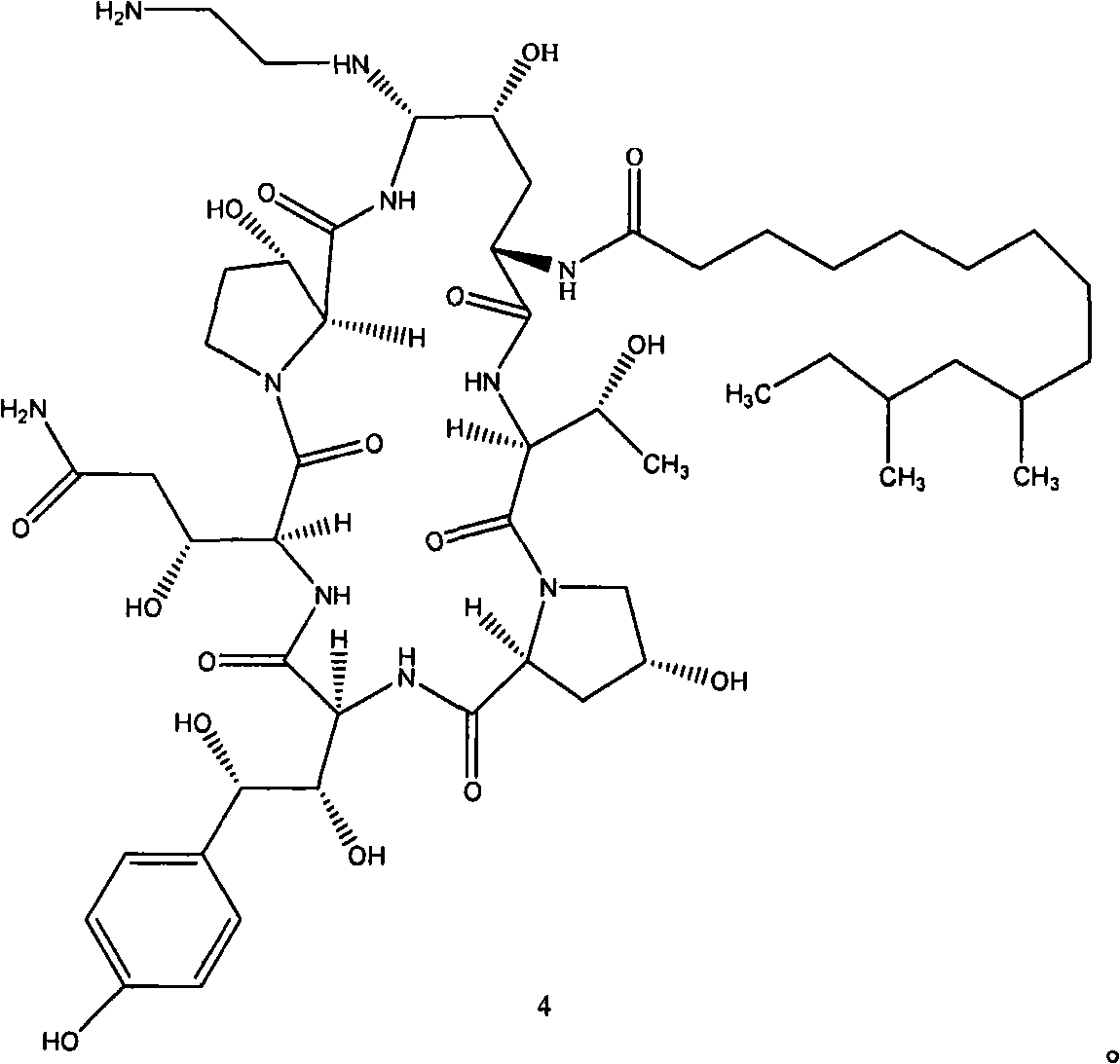
[0044] The present invention provides a kind of preparation method of the compound shown in formula 4, described method comprises the following steps:
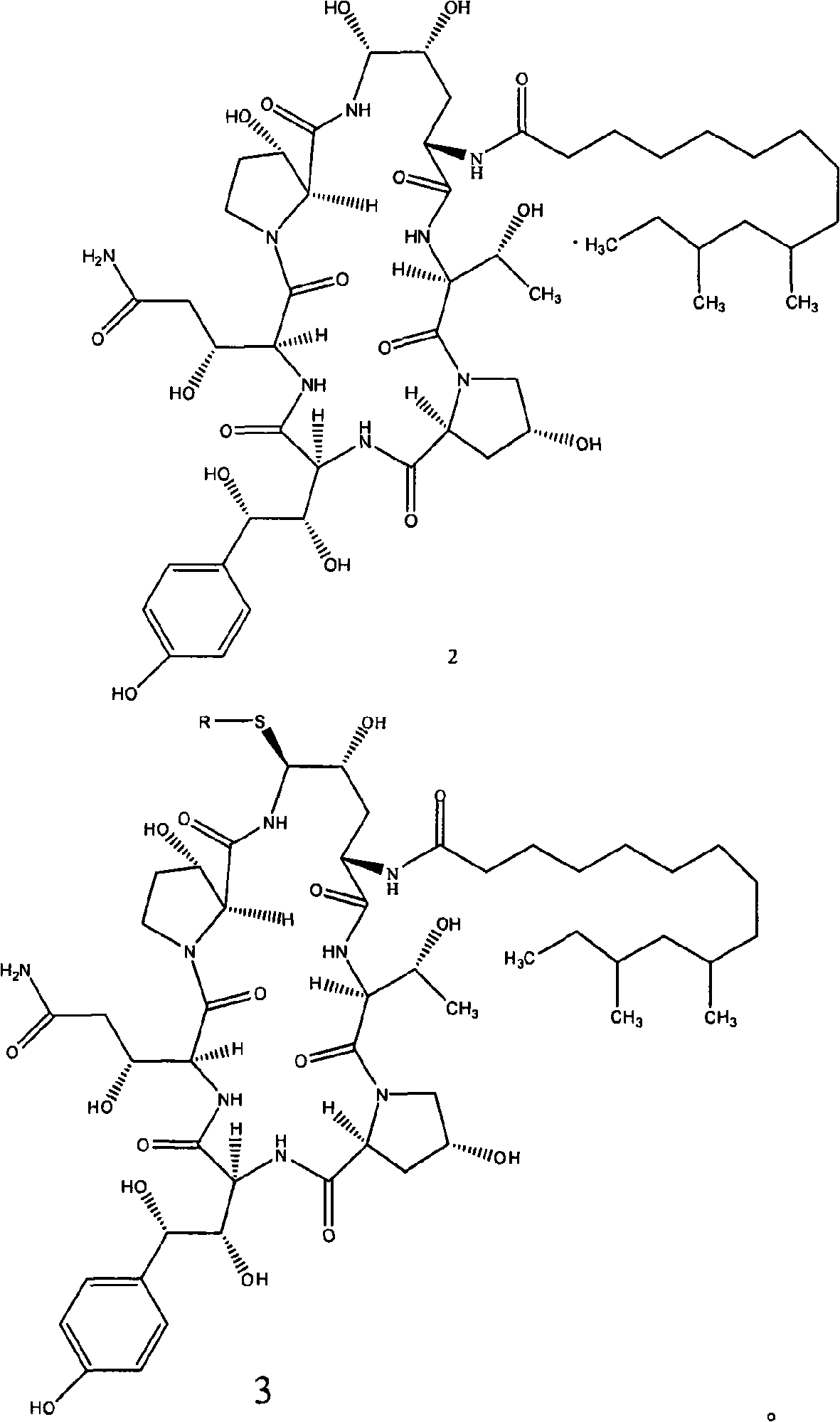
[0045] In the first step, the compound of formula 2 is mixed with a strong leaving group, and reacted to obtain the compound shown in formula 3;
[0046] In the second step, the compound of formula 3 and ethylenediamine are mixed and reacted to obtain the compound shown in formula 4.
[0047] Further, the obtained compound of formula 4 can be mixed with a reducing agent to react to obtain the compound shown in formula 1.
[0048]
[0049] The starting compound of formula 2 in the preparation method provided by the present invention can be prepared by methods well known in the art, such as but not limited to, described in US Patent No. 5,021,341 published on June 4, 1991: in the rich Zalerionarboricola ATCC 20868 was grown in a nutrient medium with mannitol as the main carbon source.
[0050] The strong leaving group in th...
Embodiment 1
[0082] Compound of formula 2 prepares compound of formula 7
[0083]
[0084] Under nitrogen protection, compound 2 (100g, 94.0mmol, dehydrated), phenylboronic acid (22.9g, 188mmol) and thiophenol (29.0ml, 282mmol) were added to 3L acetonitrile, the suspension was cooled to -15°C, and added Trifluoromethanesulfonic acid (24.9ml, 282mmol) was maintained at -15°C for 2.5h, and the reaction was completed. An aqueous solution of sodium acetate (333ml, 282mol) was added to produce a large amount of precipitate. The suspension was heated to 17°C, stirred for 2 hours, then cooled to 0°C, filtered, and washed with 1:9 (v / v) water / acetonitrile Post-drying gave compound 7 (93.4 g, 93.4%).
[0085] MS(ESI)1157.6(M+H + ), 1179.6 (M+Na + );
[0086] 1H NMR (500MHz, CD3OD) δ7.56-7.55 (om, 2H), 7.28-7.22 (om, 3H), 7.13 (m, 2H), 6.76-6.74 (m, 2H), 5.58 (d, 1H), 5.05(d, 1H), 4.94(d, 1H), 4.57(dd, 1H), 4.42-4.26(om, 9H), 3.88(om, 3H), 3.70(om, 2H), 2.76(dd, 1H) , 2.45(dd, 1H), 2.40(om,...
Embodiment 2
[0089] Compound of formula 2 prepares compound of formula 8
[0090]
[0091] Under nitrogen protection, compound 2 (10.0g, 9.4mmol), phenylboronic acid (2.3g, 18.8mmol) and p-methylthiophenol (3.56g, 28.6mmol) were added to 3L acetonitrile, and the suspension Cool to -15°C, add trifluoromethanesulfonic acid (2.49ml, 28.2mmol), maintain -15°C for 2.5h, and the reaction ends. An aqueous solution of sodium acetate (33.3ml, 28.2mol) was added to produce a large amount of precipitation. The suspension was warmed up to 17°C, stirred for 2 hours, then cooled to 0°C, filtered, and washed with 1:9 (v / v) water / After washing with acetonitrile, it was dried to obtain compound 5 (9.0 g, 90%).
[0092]Under the protection of nitrogen, compound 5 (9.0g, 7.7mmol) was dissolved in 36ml of methanol, cooled to -10°C, and ethylenediamine (36ml, 537.8mmol) was slowly added dropwise, and the dropping temperature was controlled not to exceed 2°C. , reacted at 30° C., reacted overnight, added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com