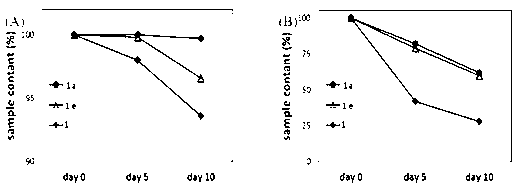
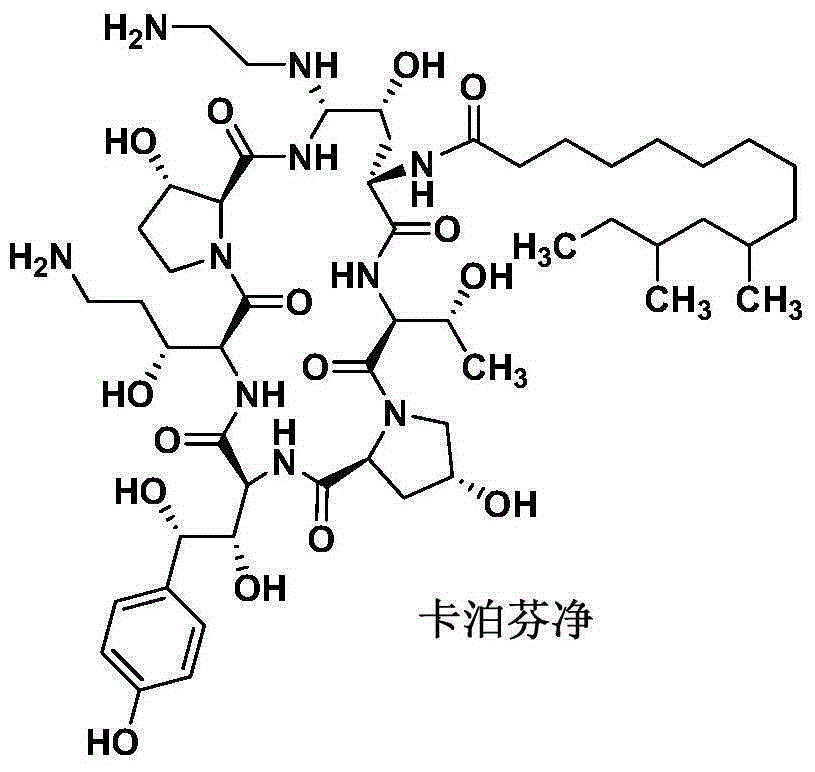
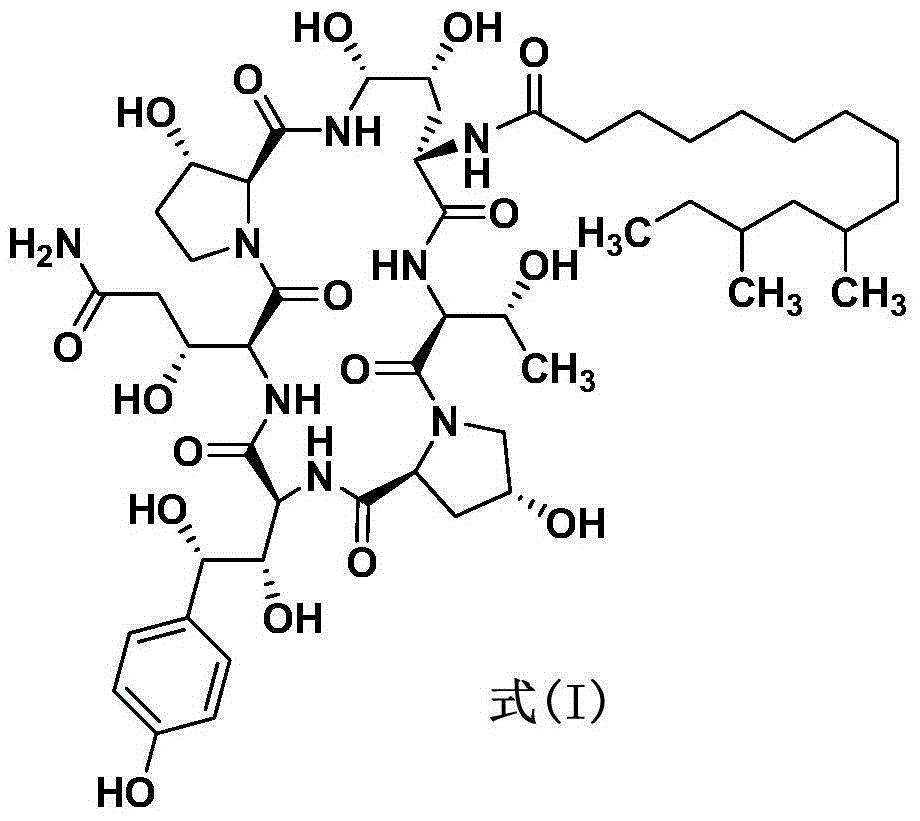
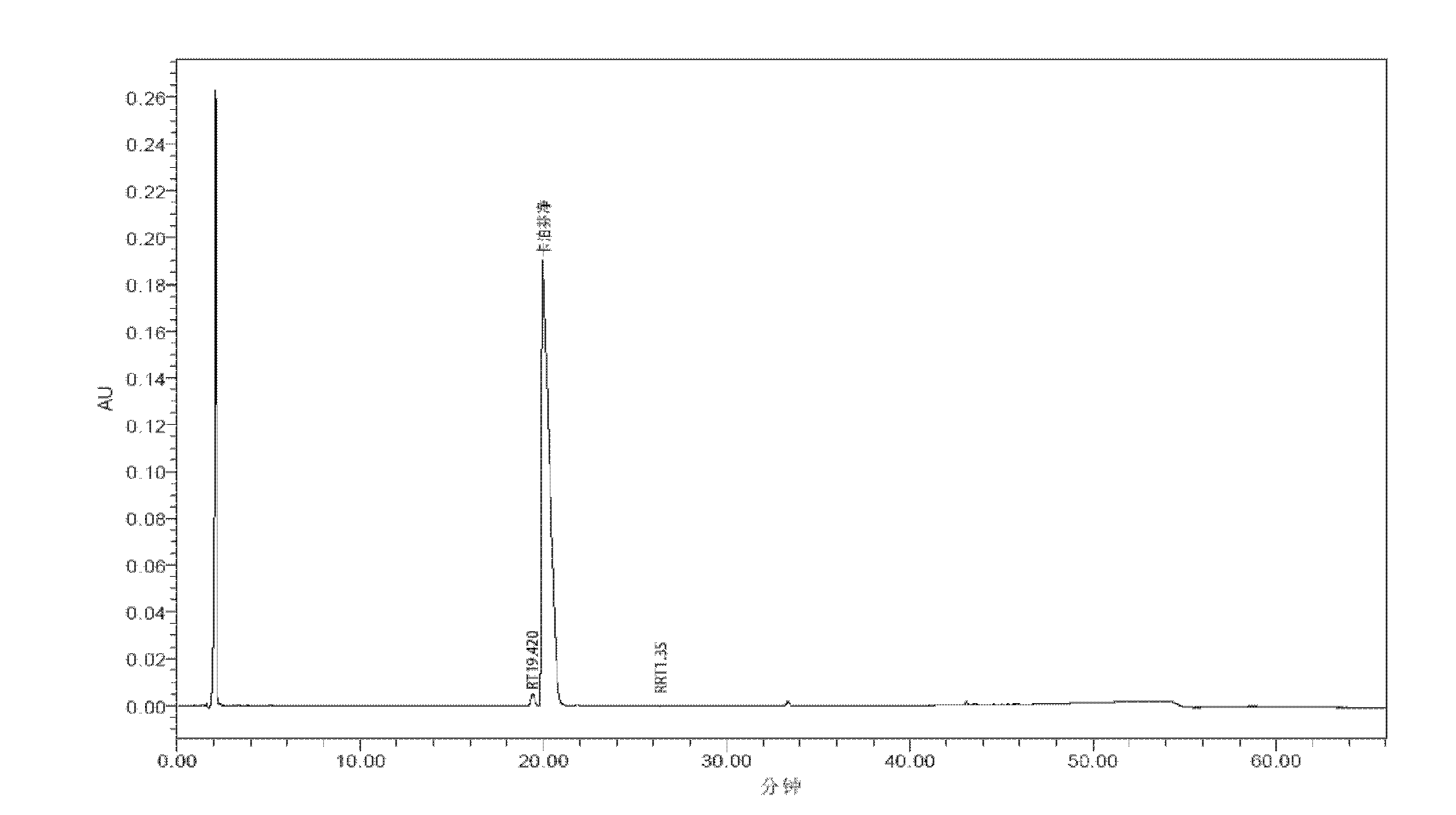
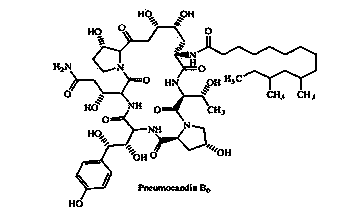
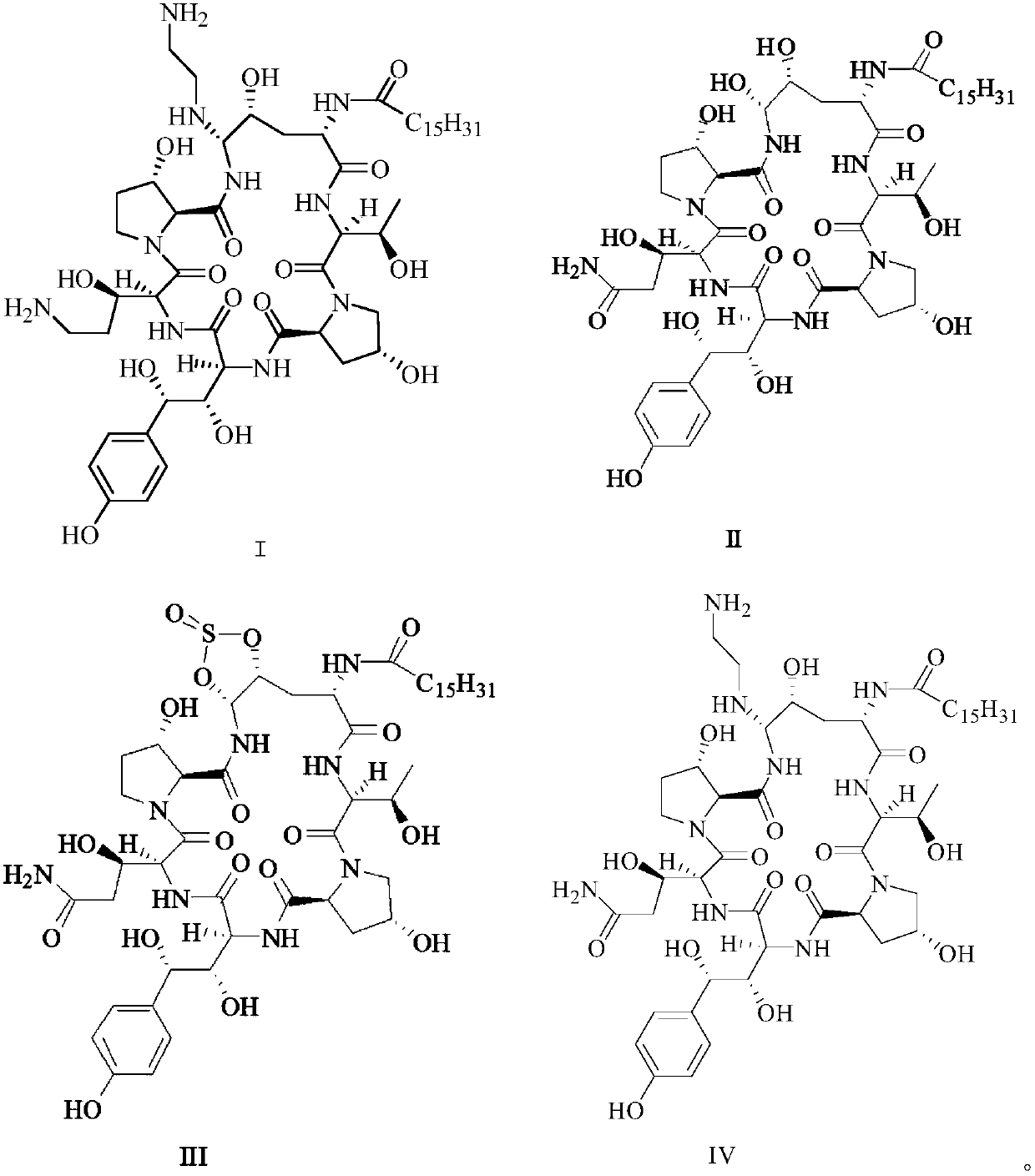
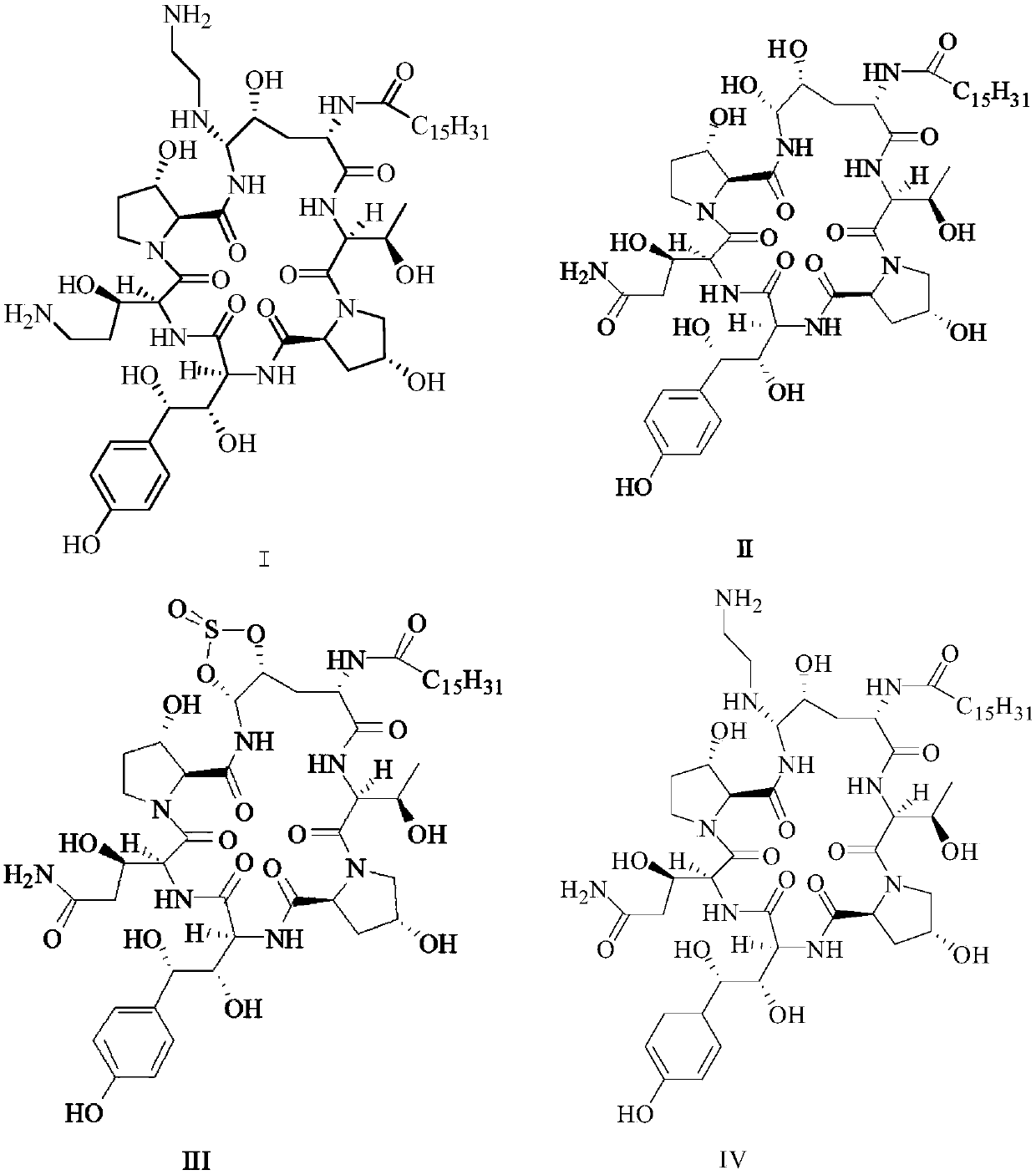
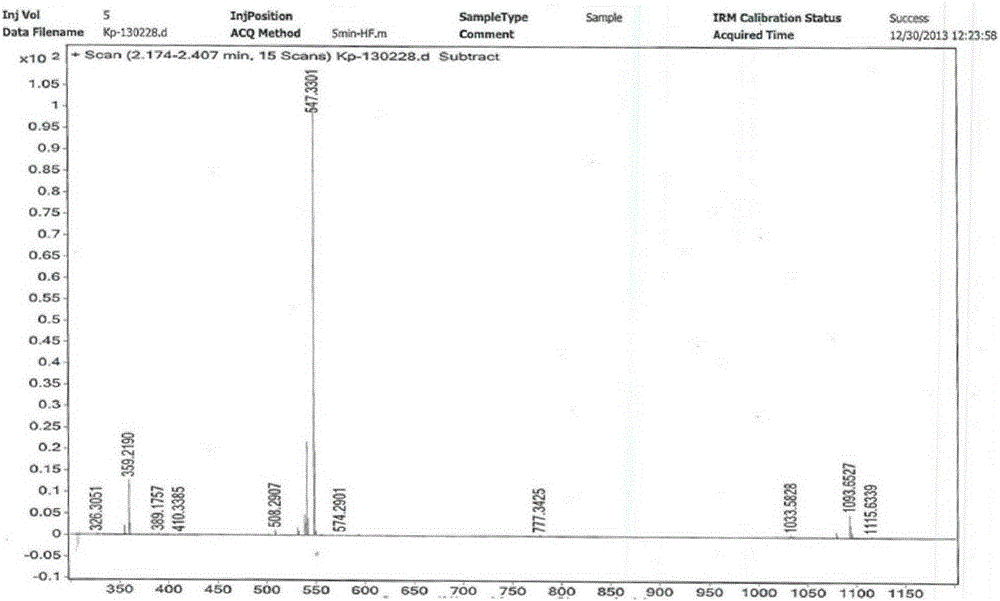
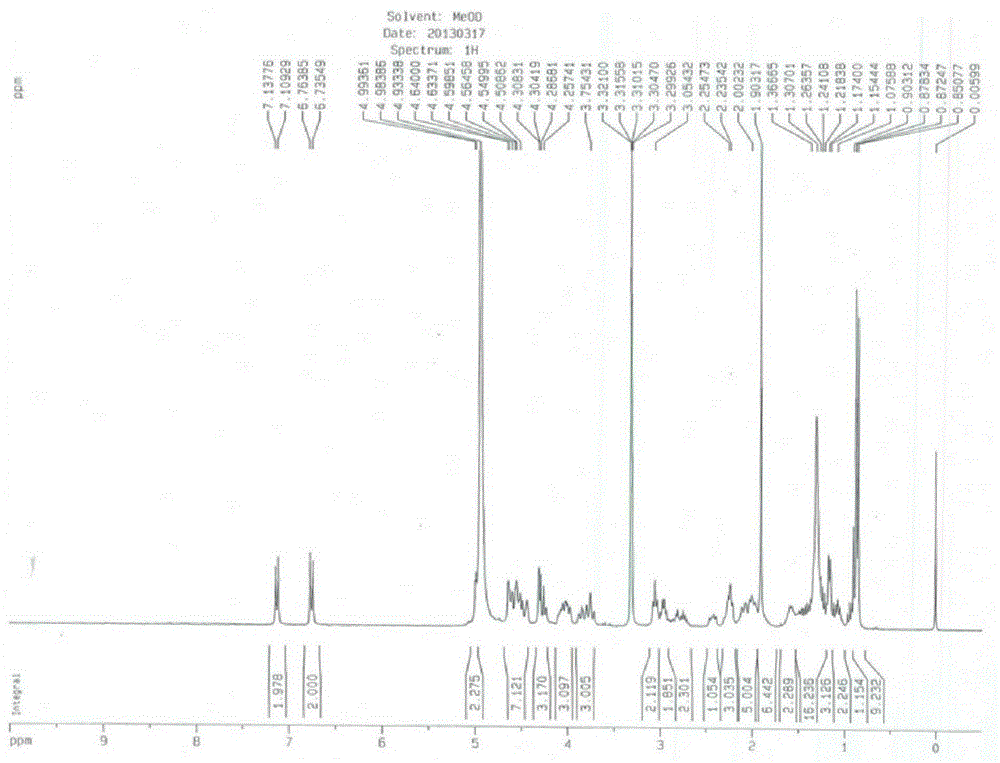
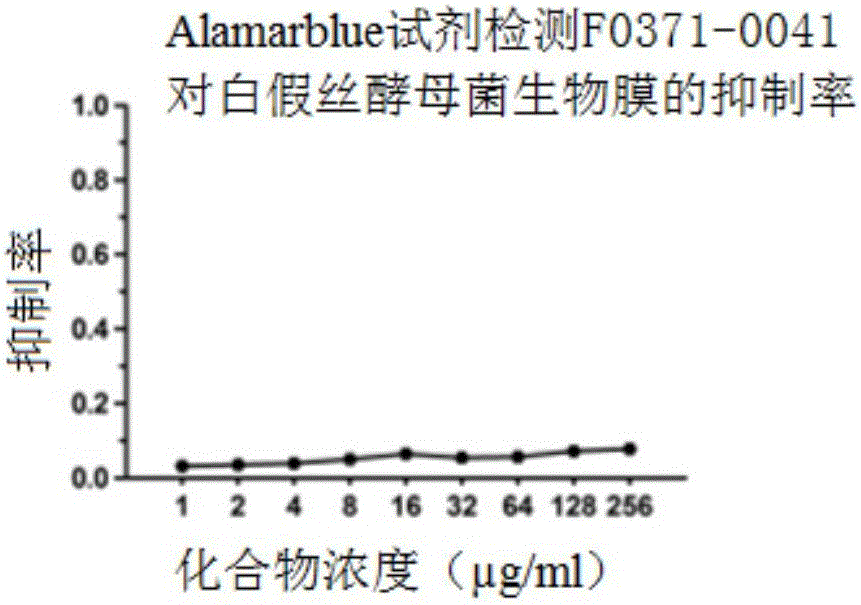
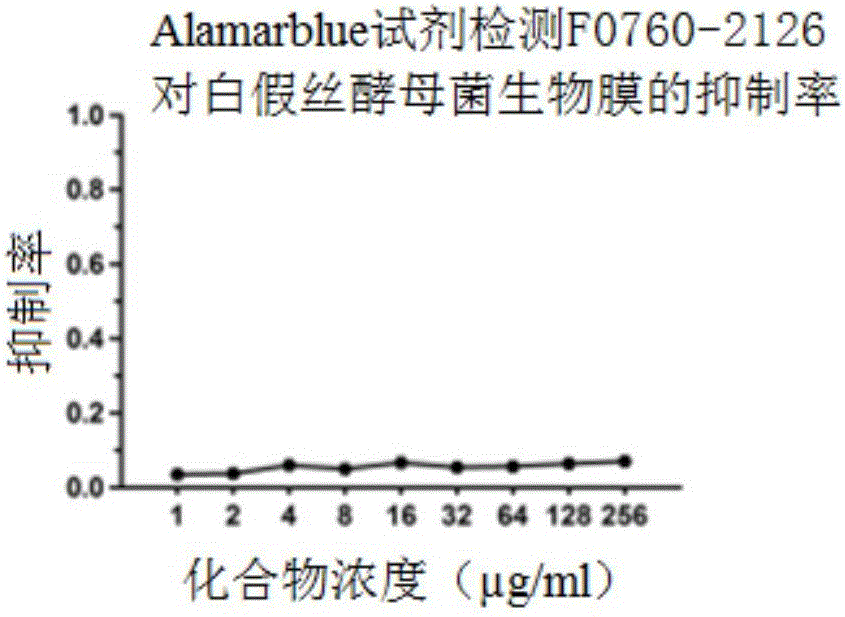
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Caspofungin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
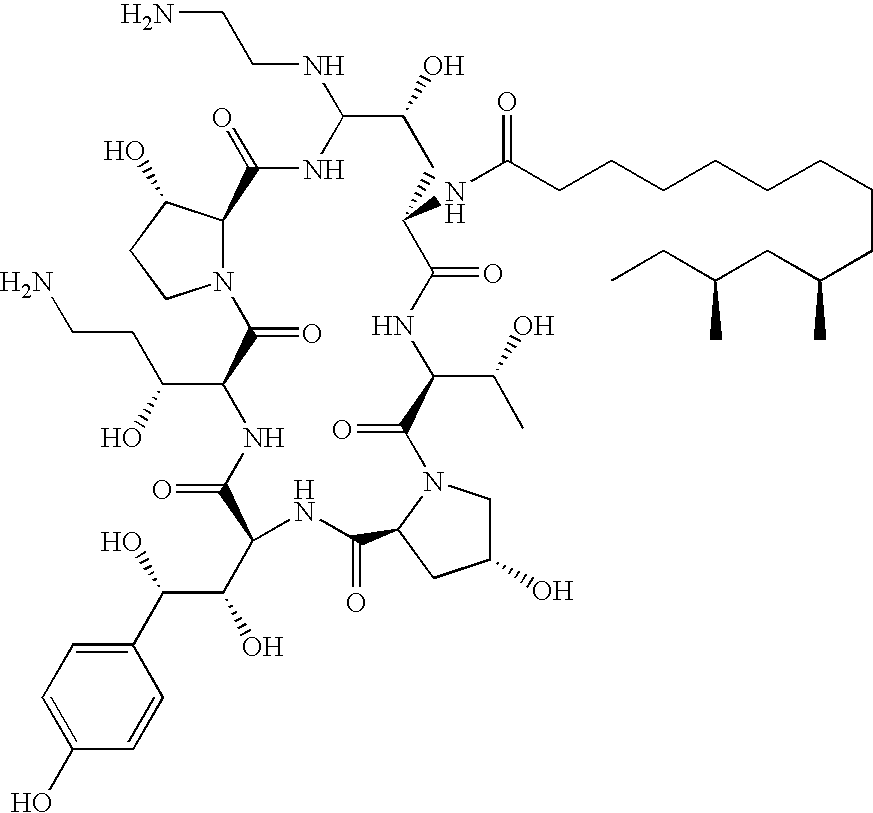
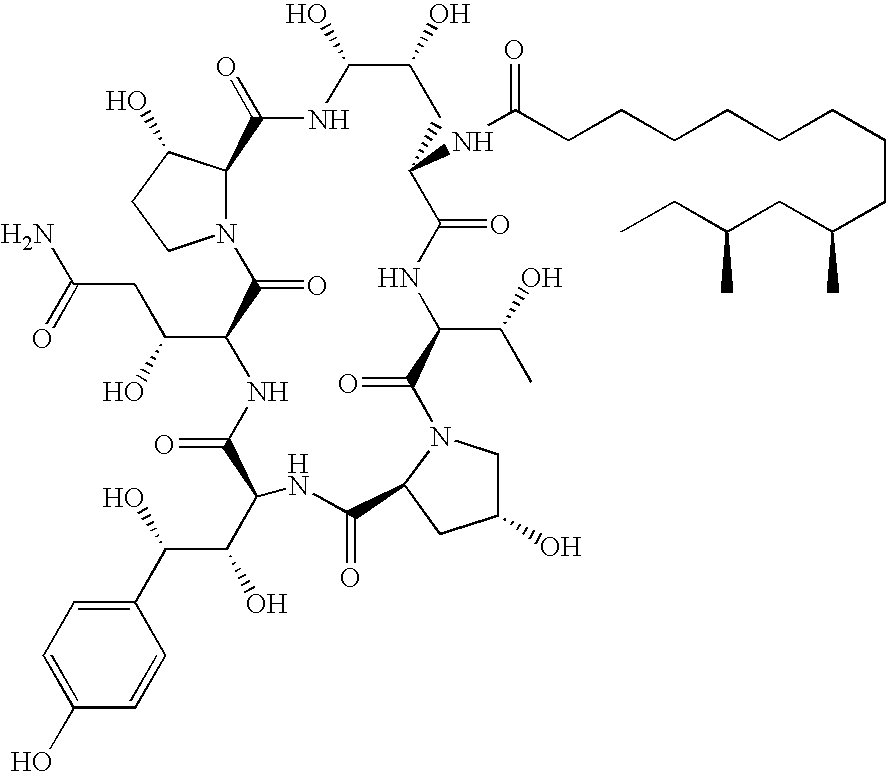
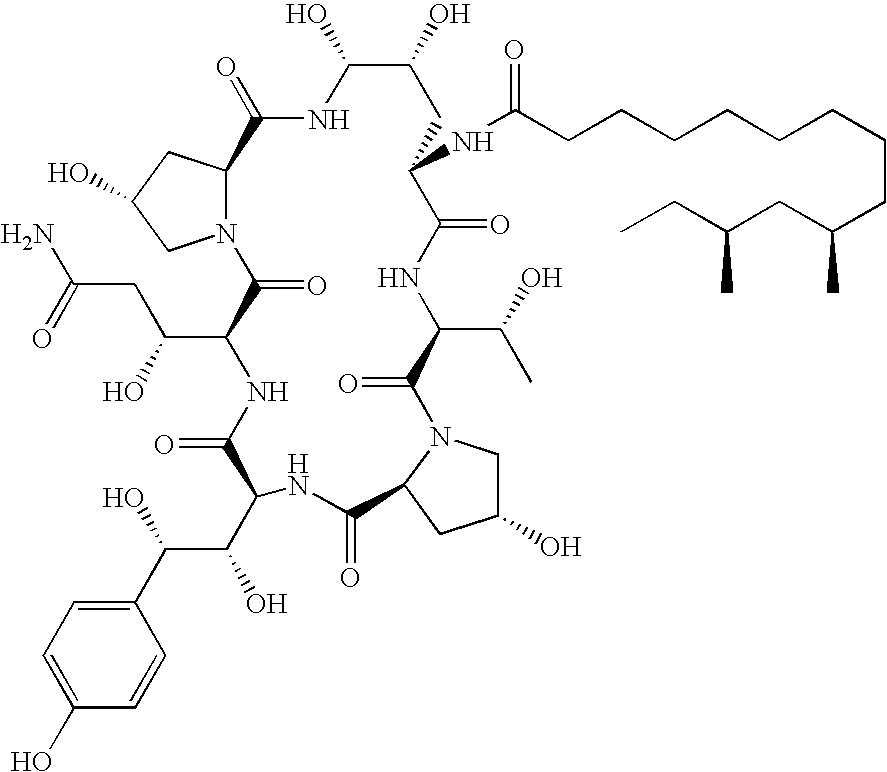
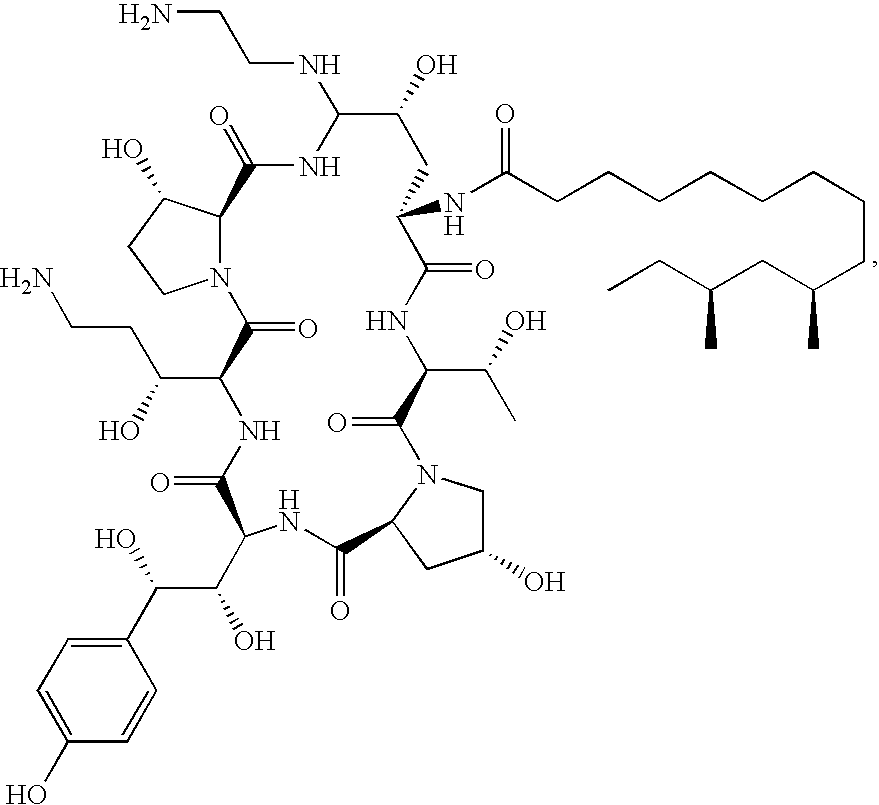
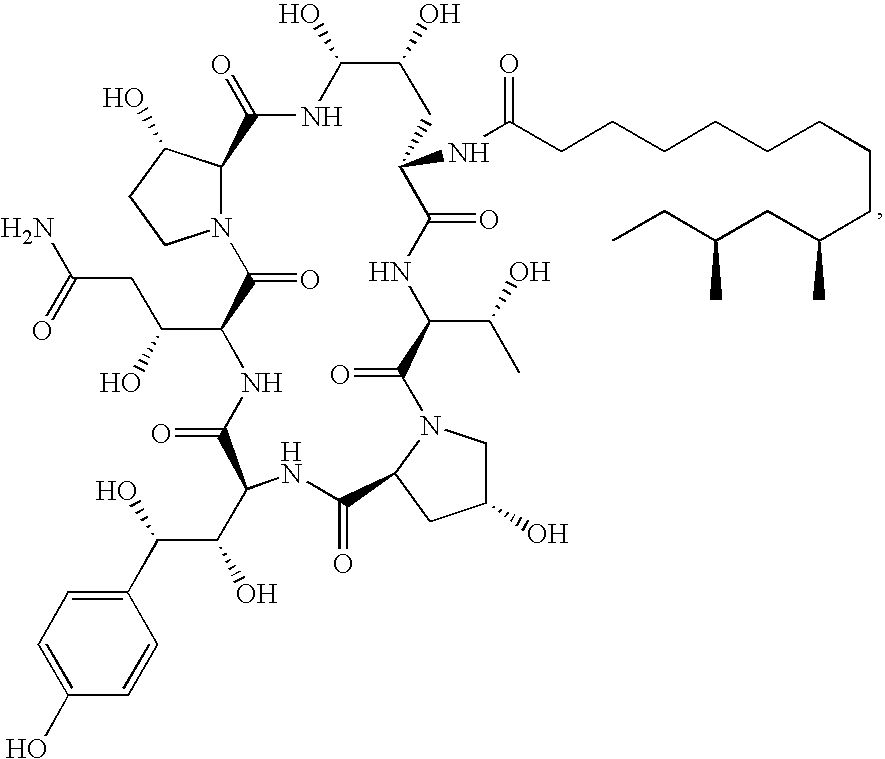
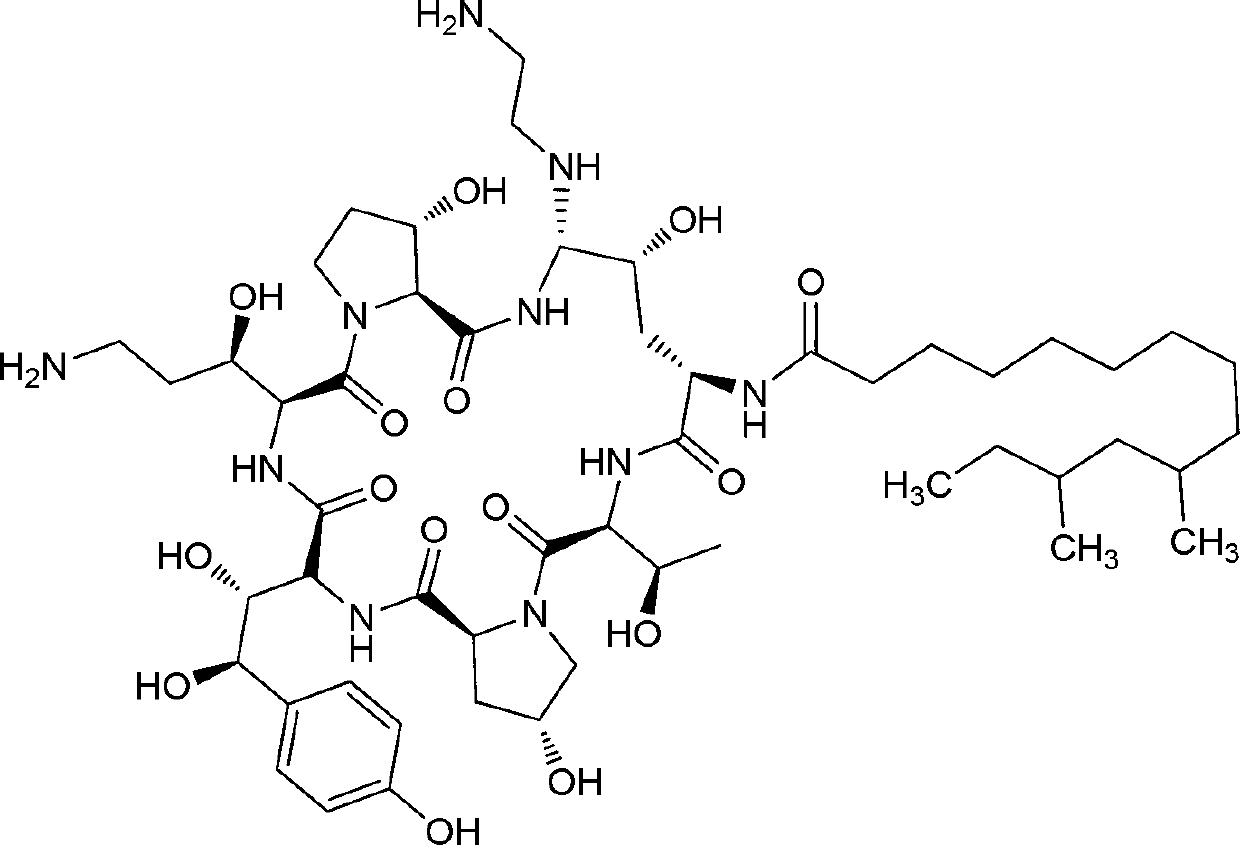
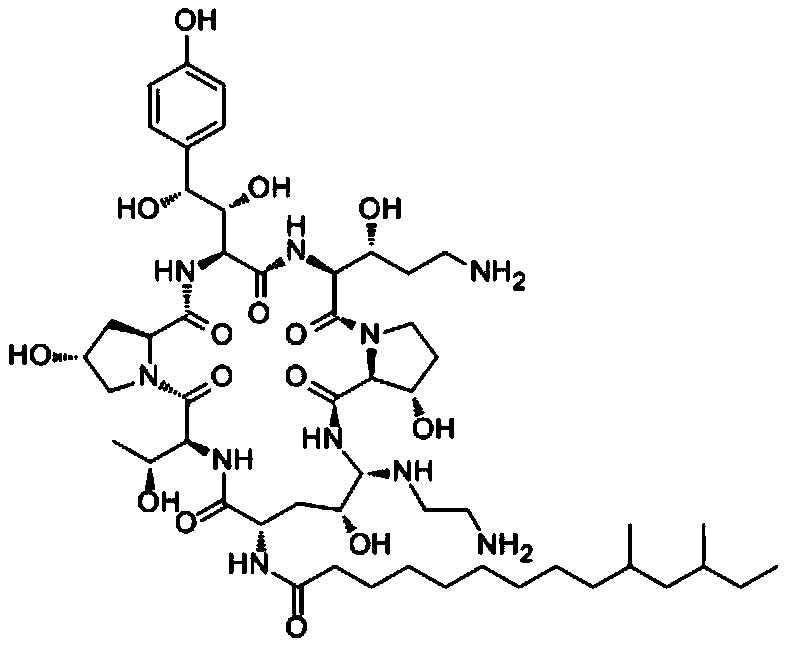
Caspofungin is used to treat a variety of fungal infections.
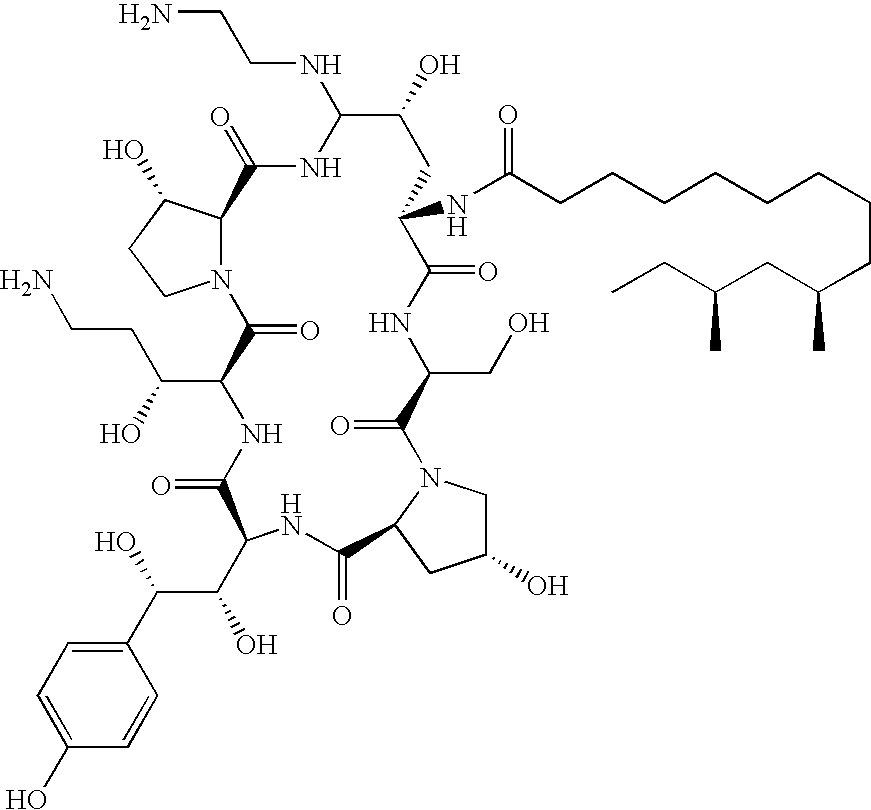
Capsofungin formulations
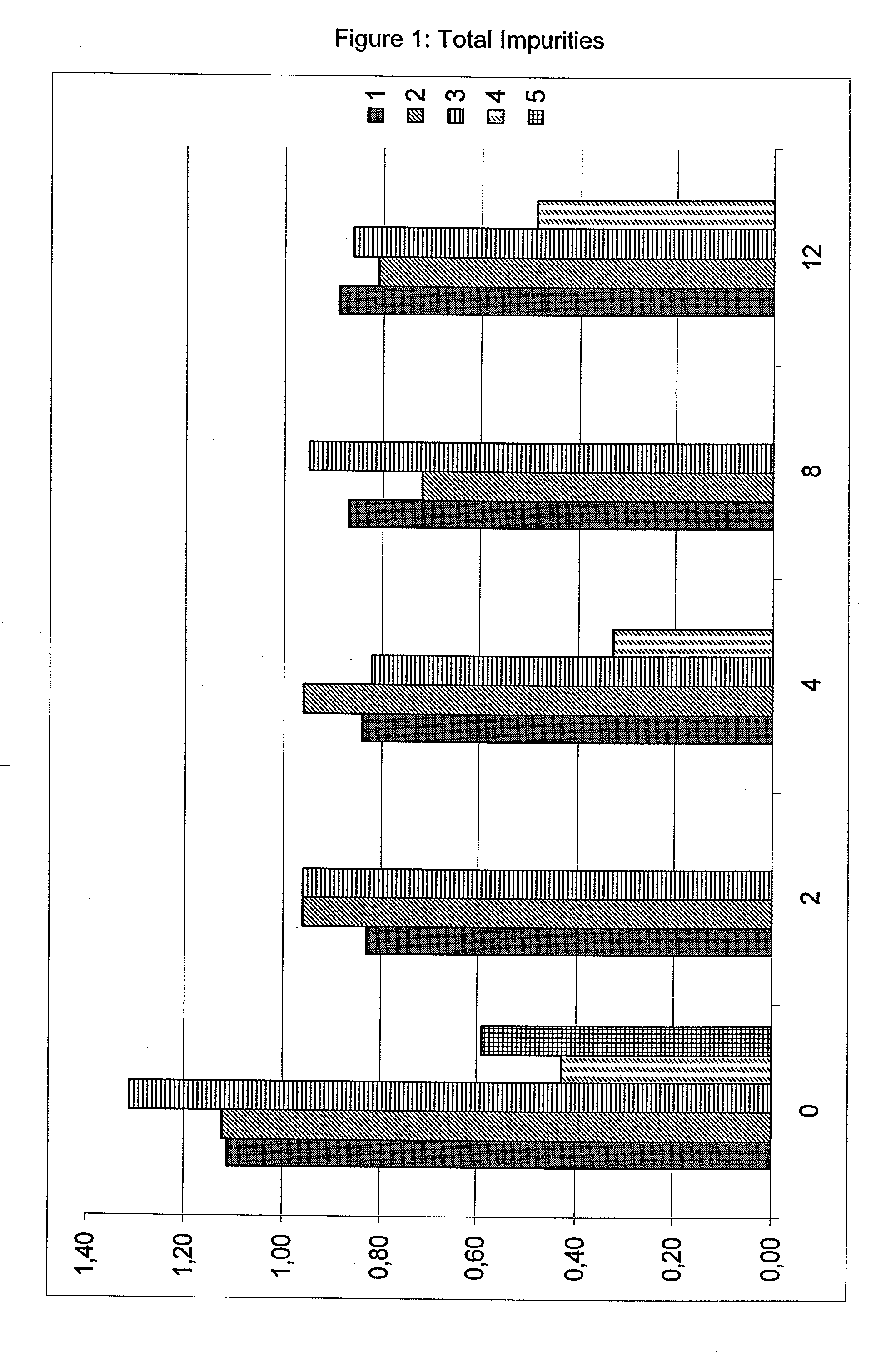
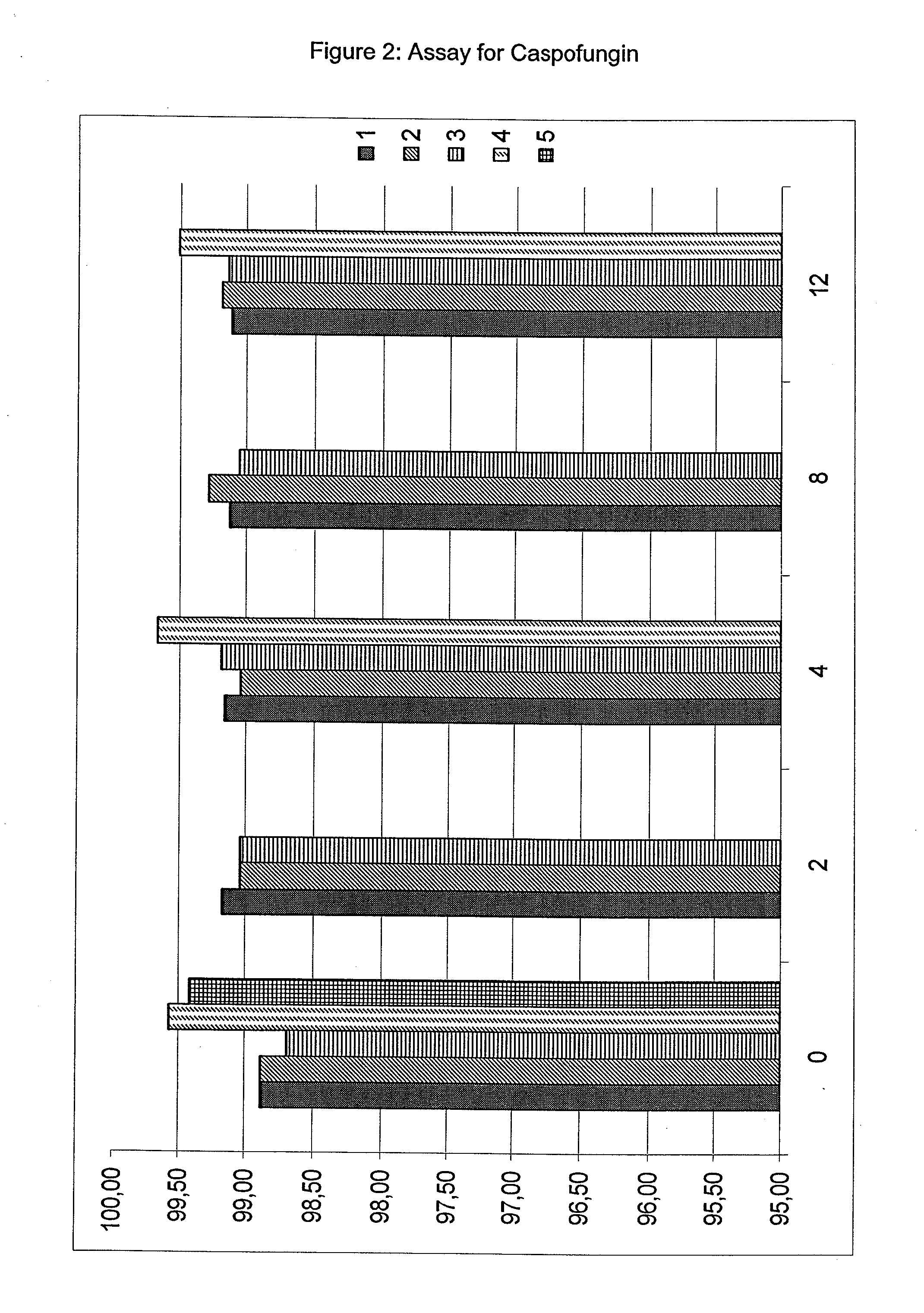
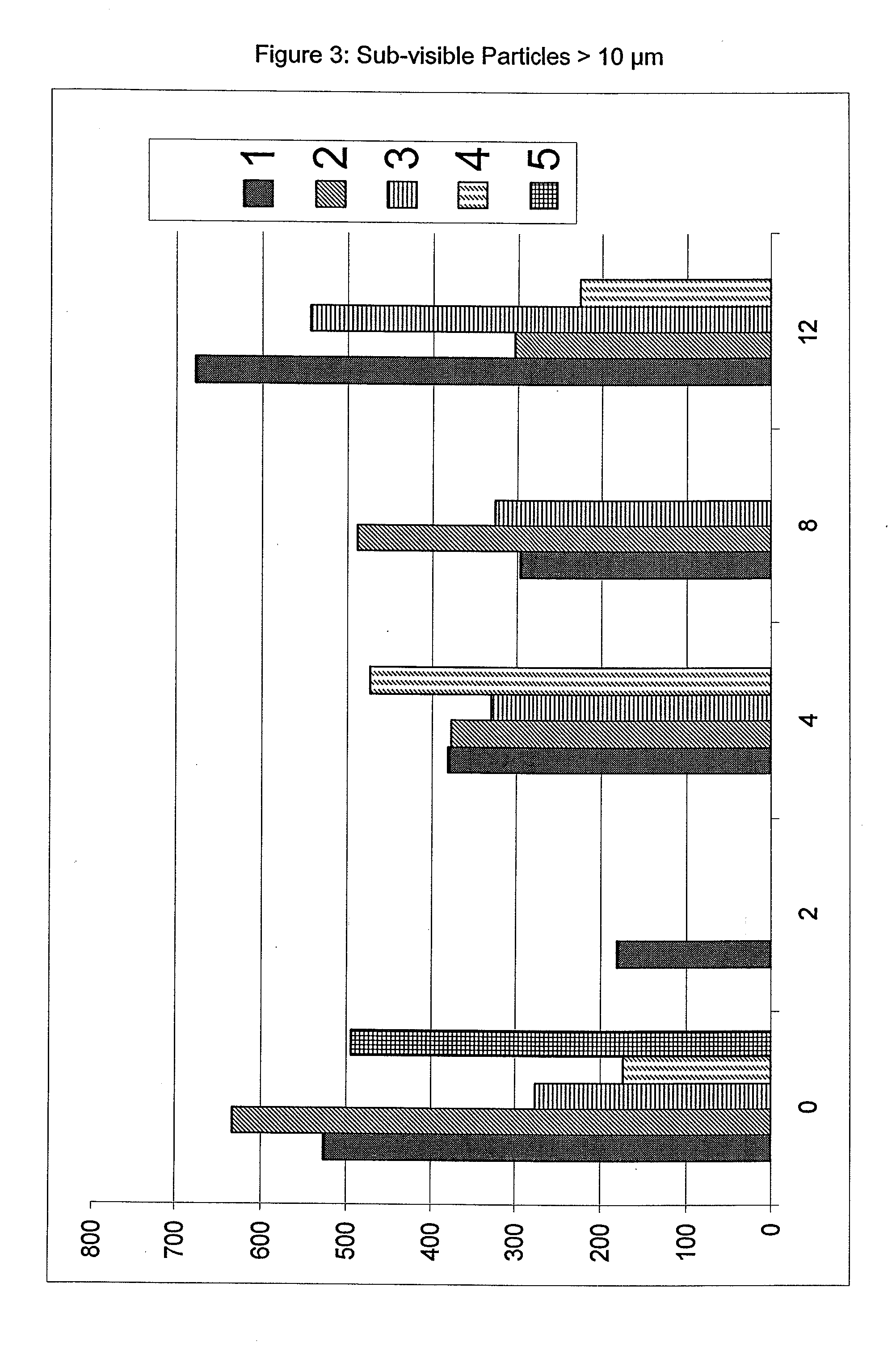
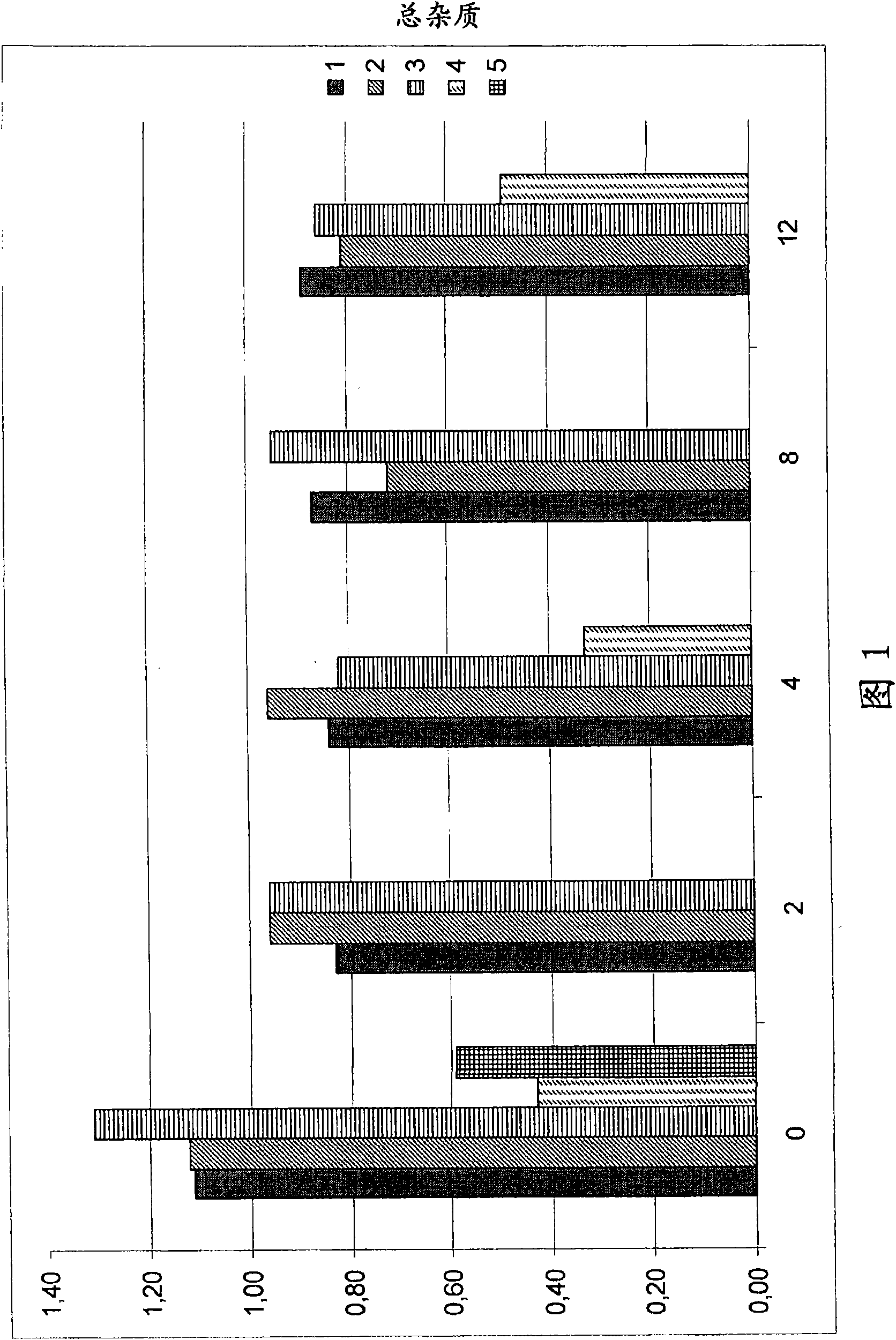
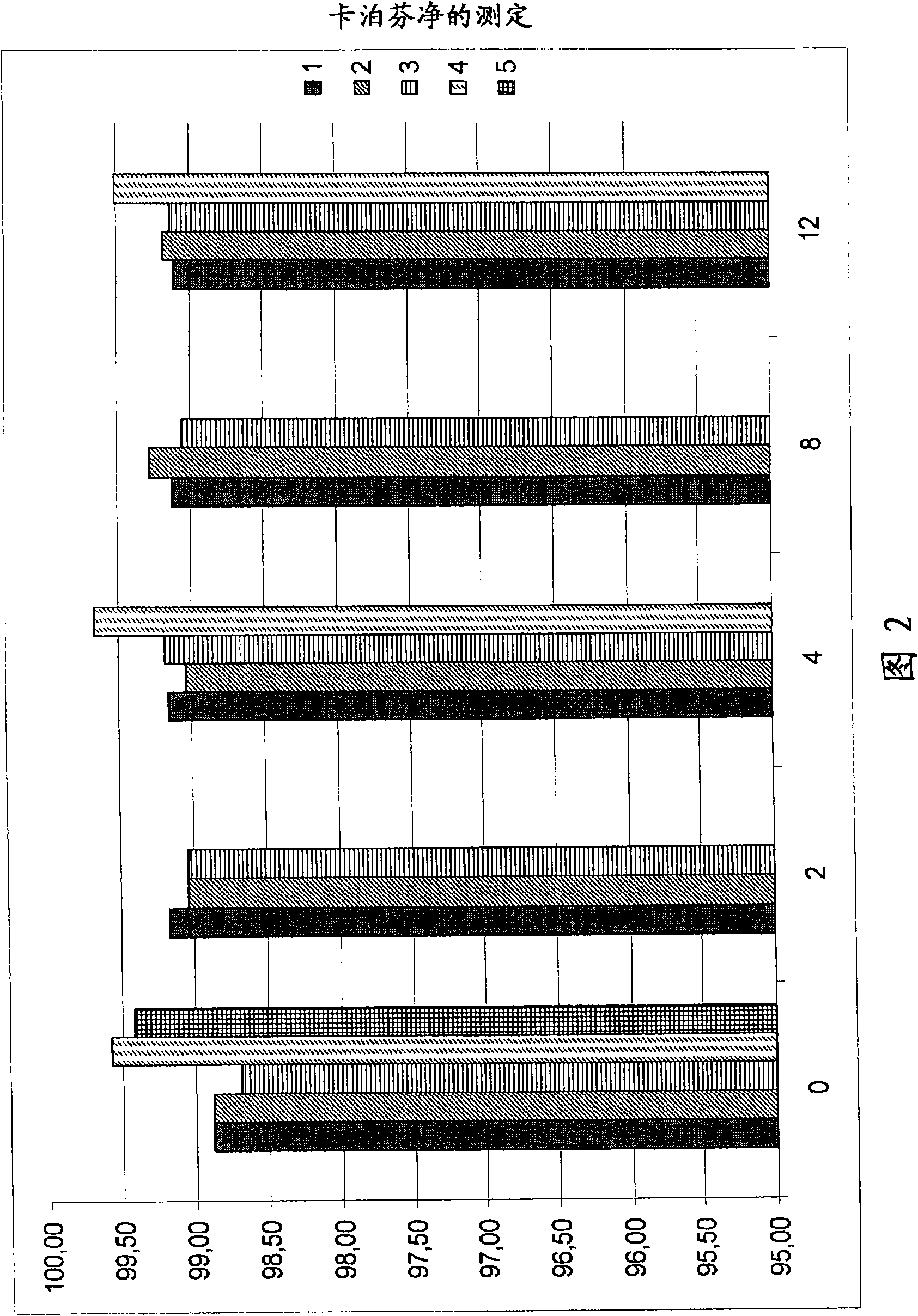
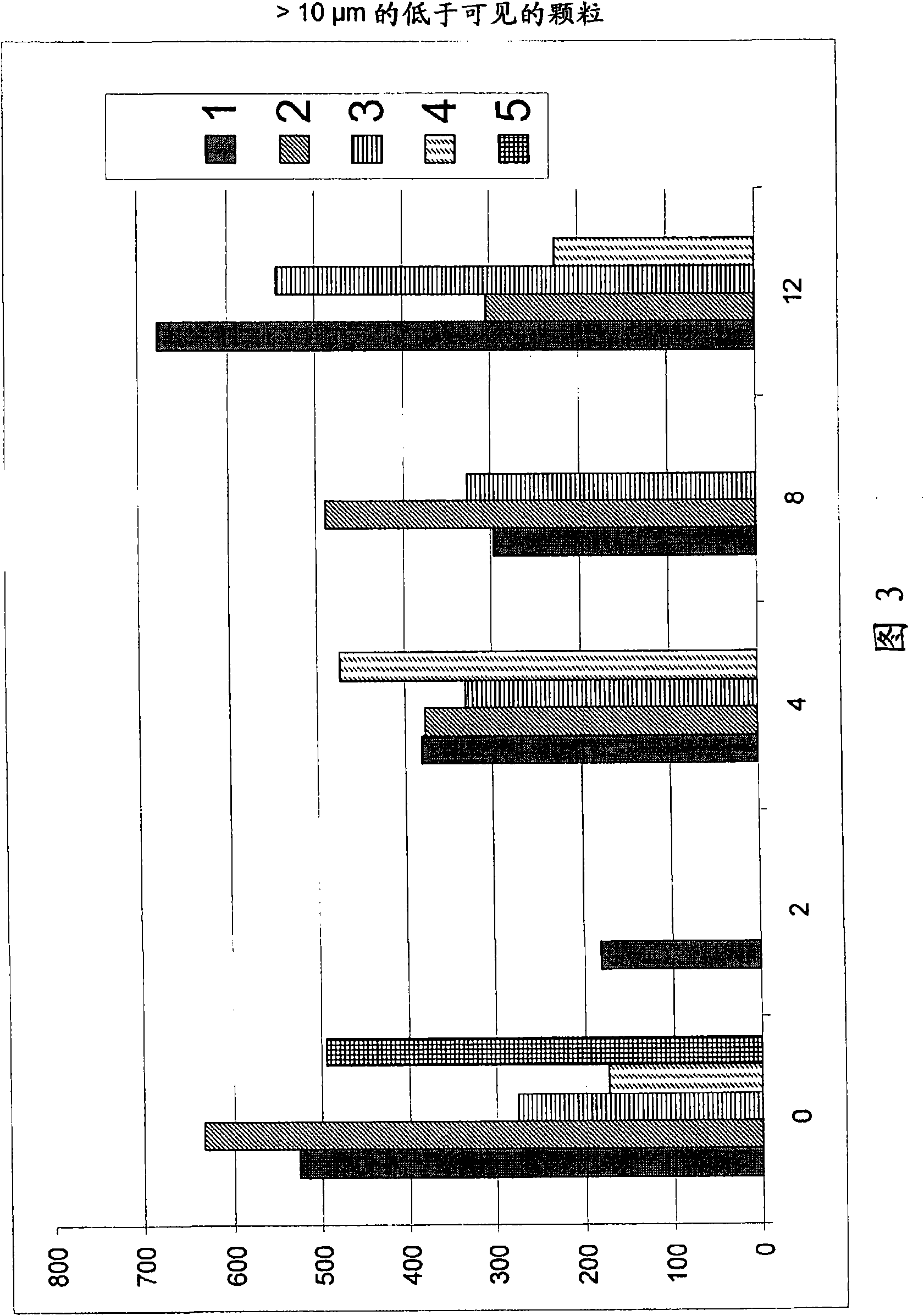
The present invention relates to pharmaceutical compositions comprising a pharmaceutically acceptable salt of caspofungin as active ingredient being useful for the prevention and / or treatment of fungal infections. Said compositions additionally comprise specific bulking agents and small amounts or no amounts of an additional pH modifier and may be in a liquid or solid form, e.g. may be lyophilized compositions. Said compositions show good stability and reduced amounts of sub-visible particulate matter formed in solutions which are reconstituted from the lyophilized product.
Owner:SANDOZ AG
Caspofungin free of caspofungin Co
The present invention provides Caspofungin and salts thereof substantially free of Caspofungin C0 and salts thereof. The present invention also provides processes for the preparation of said Caspofungin and salts thereof and processes for the determination of the amount of Caspofungin C0 and salts thereof present in Caspofungin and salts thereof. The present invention further provides pharmaceutical compositions comprising said Caspofungin and salts thereof.
Owner:TEVA PHARM USA INC
Caspofungin free of caspofungin impurity A
Provided is caspofungin free of caspofungin impurity A, methods for preparation thereof and isolation of caspofungin impurity A.
Owner:TEVA PHARM USA INC
Caspofungin formulations
The present invention relates to pharmaceutical compositions comprising a pharmaceutically acceptable salt of caspofungin as active ingredient being useful for the prevention and / or treatment of fungal infections. Said compositions additionally comprise specific bulking agents and small amounts or no amounts of an additional pH modifier and may be in a liquid or solid form, e.g. may be lyophilized compositions. Said compositions show good stability and reduced amounts of sub-visible particulate matter formed in solutions which are reconstituted from the lyophilized product.
Owner:SANDOZ AG
Method of treatment of otitis externa
This invention relates to a method of treating otitis externa, and in particular otitis externa of fungal etiology, using topical medication, including antifungal agents such as, for example fluconazole, voriconazole, itraconazole, clotrimazole, amphotericin B, caspofungin (Cancidas®), micafungin (Mycamine®), terbinafine, naftifine, butenafine, amorolfine, ravuconazole, posaconazole, flucytosine, econazole, enilaconazole, miconazole, oxiconazole, saperconazole, sulconazole, terconazole, tioconazole, nikkomycin Z, anidulafungin (LY303366), nystatin, pimaricin, griseofulvin, ciclopirox, haloprogin, tolnaftate, and undecylenate.
Owner:FAIRFIELD CLINICAL TRIALS
More stable nitrogen heterocyclic peptide preparation
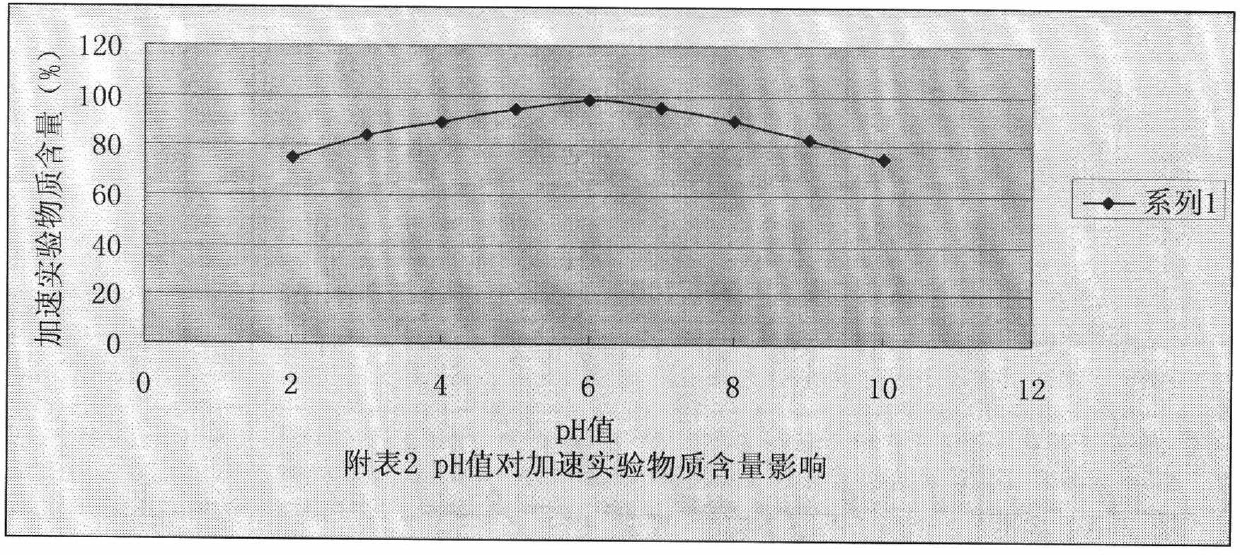
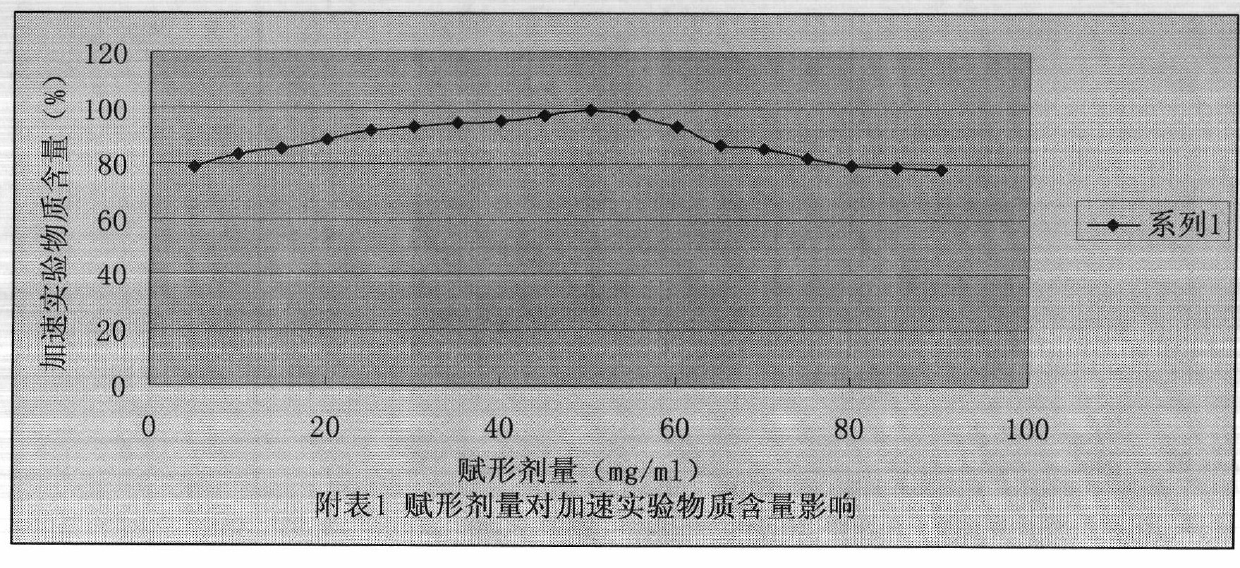
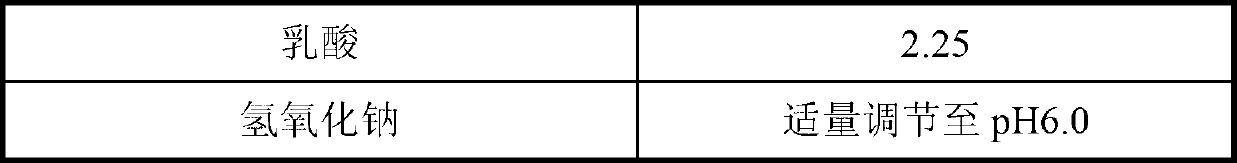
The invention belongs to the technical field of pharmaceutical preparations and provides a caspofungin injection preparation composition. The caspofungin injection preparation composition comprises 5 to 250 mg / ml of caspofungin or pharmaceutically available salt of the caspofungin, 5 to 150 mg / ml of excipient, 10 to 250 mM of buffer solution and an appropriate amount of sodium hydroxide or hydrochloric acid of which the pH value is regulated to be between 3.0 and 8.0, wherein the buffer solution is sulphate, citrate, phosphate, lactate or a mixture of the sulphate, the citrate, the phosphate and the lactate. The caspofungin injection preparation can be stored at room temperature, so that the stability is obviously improved, and the circulation and use of medicaments are facilitated.
Owner:SHENZHEN JYMED TECH
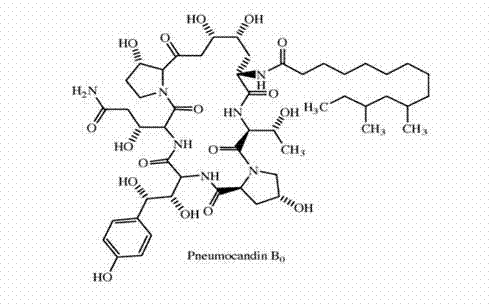
Method for purifying caspofungin precursor pneumocandin B0 component
ActiveCN102816207AHigh yieldSimplified purification stepsPeptide preparation methodsState of artPurification methods
The invention relates to a method for purifying a caspofungin precursor pneumocandin B0 component, and the method comprises the following steps of: carrying out reversed-phase column chromatography on a PB0 component crude product, collecting and carrying out reduced-pressure concentration to obtain a semi-pure product; carrying out normal phase column chromatography on the PB0 semi-pure product obtained in the step 1 to obtain high-purity PB0 component; and carrying out positive and negative phase liquid phase analysis, wherein the chromatographic purity is more than 95%. Compared with the prior art, the method has the beneficial effects that a normal phase column and a reversed-phase column are used for purifying the PB0 component, so that the purification steps are simple, the purification effect is good, the requirements for equipment are low, large-scale production is realized, and the production feasibility is high; and particularly, the PB0 component separated by the method is high in yield, but is lower in cost.
Owner:CHENGDU YATU BIOLOGICAL TECH
Fungus Glarea lozoyensis and application thereof for controlling microbial metabolite Pneumocandin Kangding B0
The invention discloses a fungus (Glarea lozoyensis) FIM 2006071. The strain is preserved in China Center for Type Culture Collection, the preservation number of the strain is CCTCC NO: M2012475, and the preservation date is 23rd, November, 2012. The invention further discloses a high-unit intermediate Pneumocandin Kangding B0 of caspofungin, which is obtained through controlling microbial metabolite. The fermentaition strain provided by the invention is good in performance, stable in production ability, high in fermentation unit and generates relatively less fermentation by-products, so that post-extraction difficulty is reduced, high-quality caspofungin is obtained favorably, and therefore, the strain is suitable for industrial mass production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
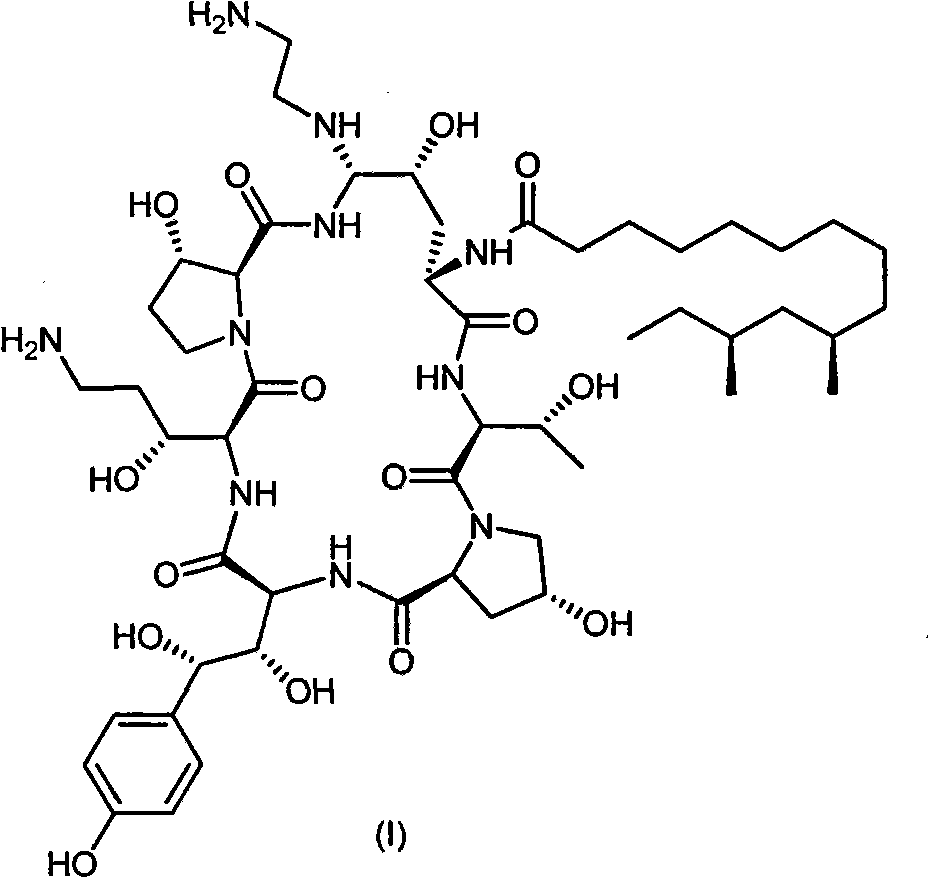
Process for preparing caspofungin and intermediates thereof
InactiveCN102112487ABatch process reductionAchieve preparationAntimycoticsPeptidesNitrogenMedicinal chemistry
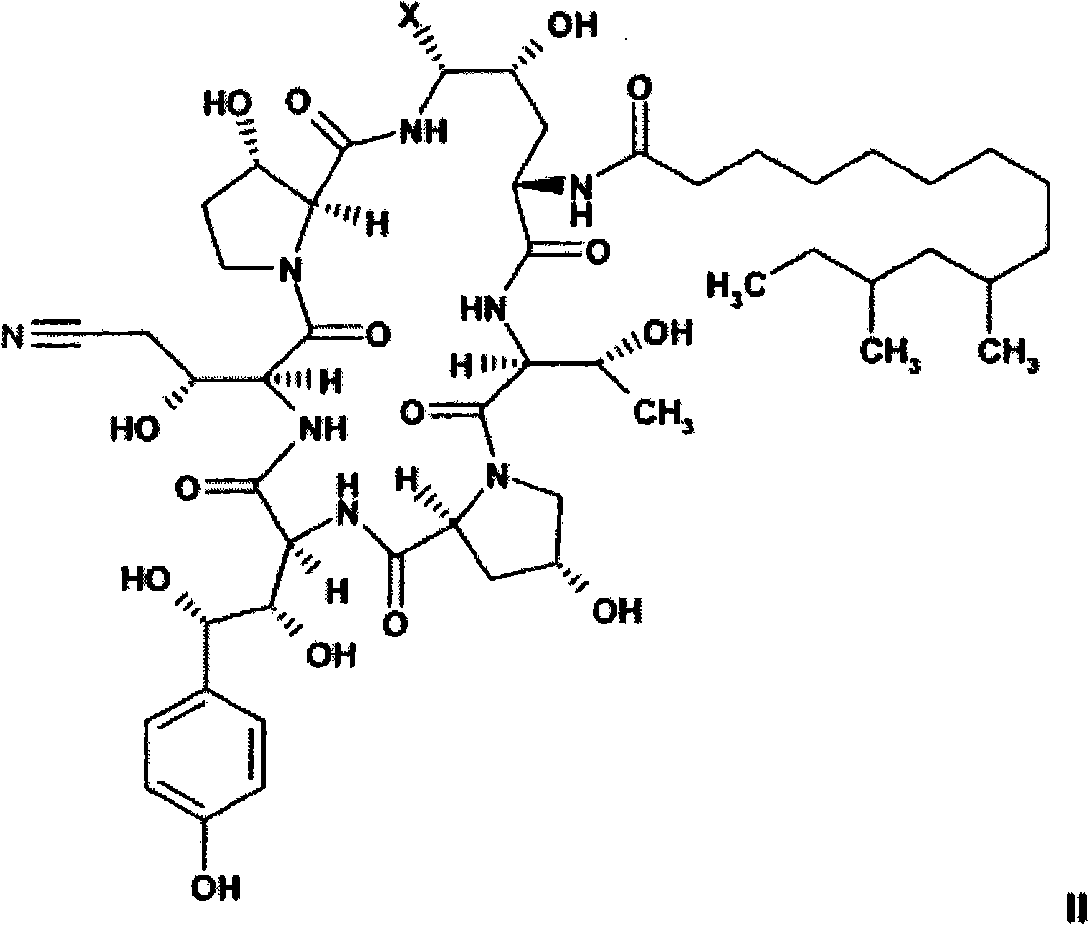
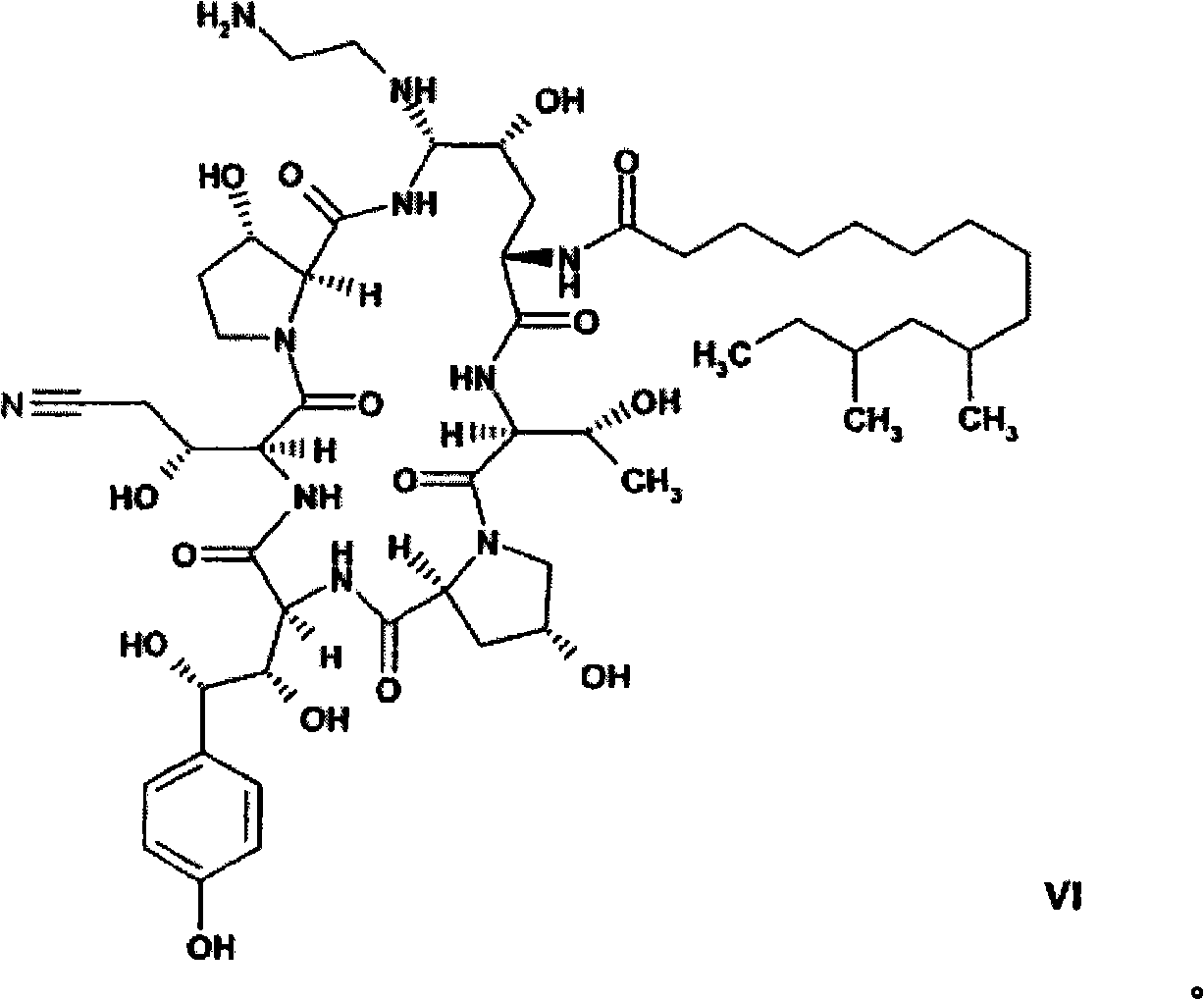
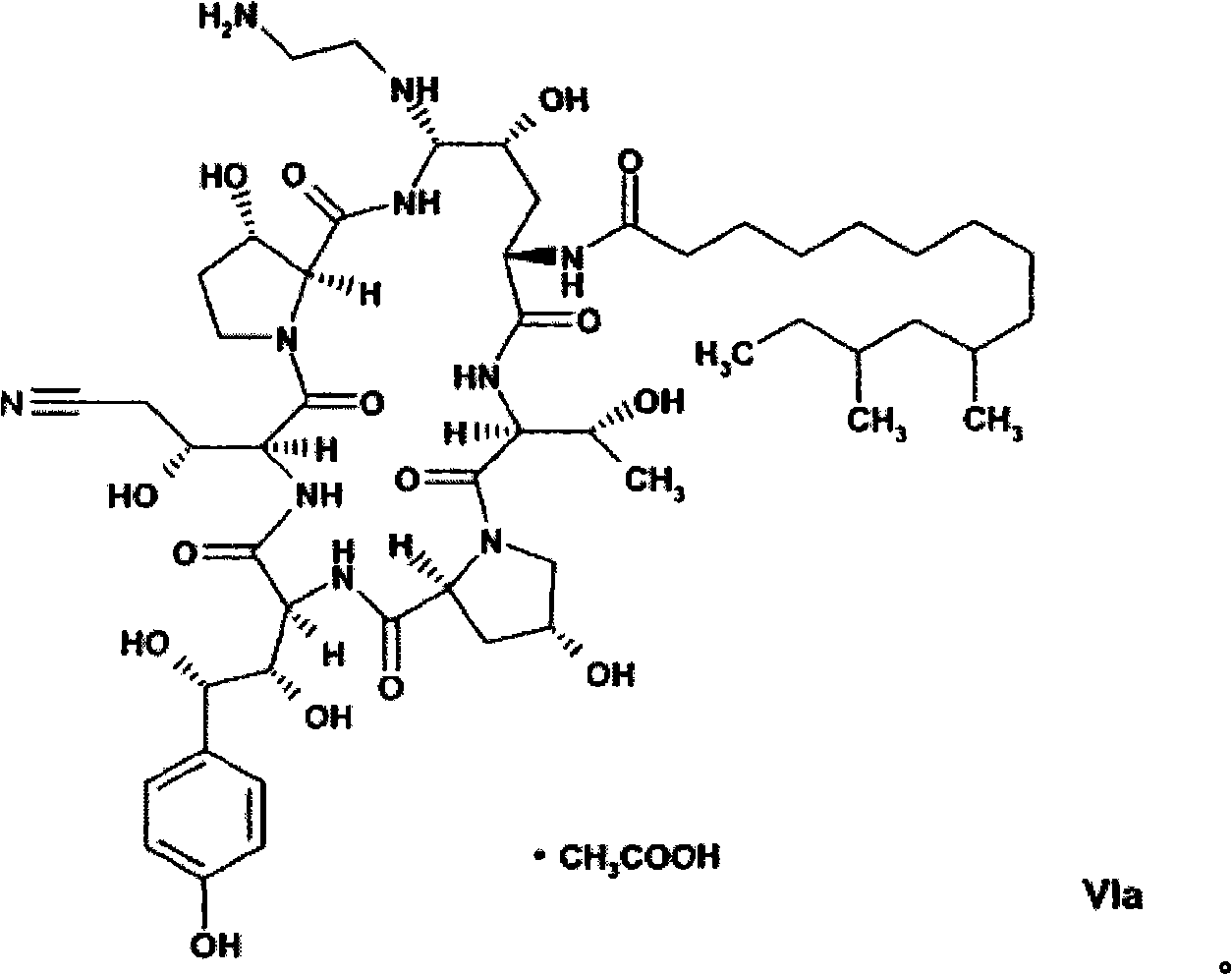
The present invention relates to novel intermediates of formula (VII), or an acid addition salt or a solvate thereof, wherein R1 is -(CO)NH2, -CH2NH2 or -CN; R2 = R3 = H or R2 and R3 together form a cyclic boronate or borate ester; X is a helping group selected from the group consisting of i) a five or six membered heterocyclic aromatic ring and derivatives thereof comprising at least one N-atom being a part of an imine-group, wherein said N-atom forms the point of connection to the cyclohexapeptide ring, and ii) tetrazolyl and derivatives thereof for which a nitrogen atom forms the point of connection to the cyclohexapeptide ring, and a process for the preparation of caspofungin utilizing said intermediates.
Owner:CELIA PHARM
Process and intermediates for the synthesis of caspofungin
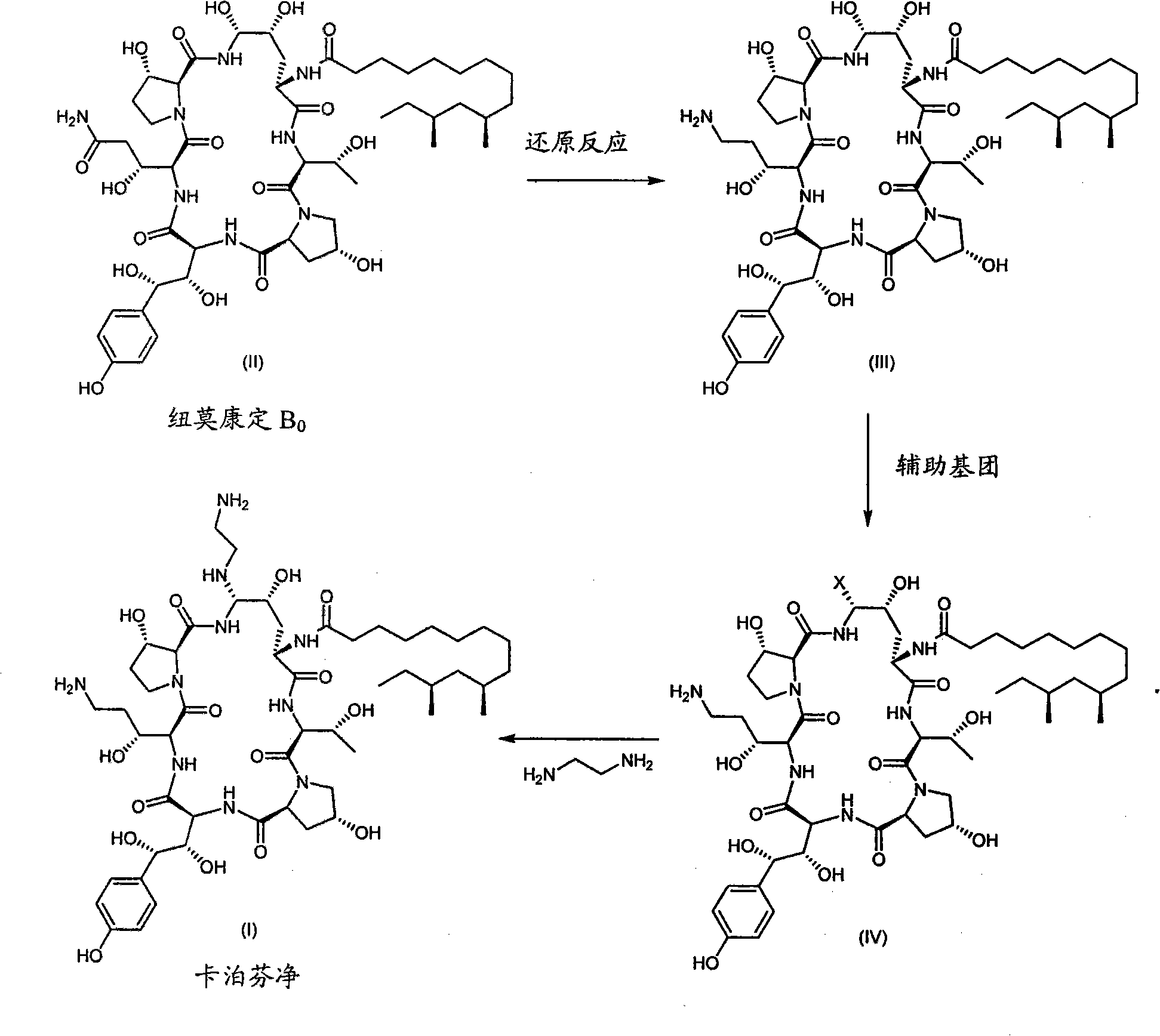
The present invention relates to novel processes for preparing certain aza cyclohexapeptide compounds, e.g. caspofungin, novel intermediates used in said processes and a process for preparing said intermediates. In particular, the intermediates have formula (II), wherein X is amino or substituted amino, and contains a cyano / nitrile functionality.
Owner:SANDOZ AG
Glycopeptide antifungal compound, and preparation method and application thereof
InactiveCN102766198AStrong inhibitory activityFix stability issuesAntimycoticsSaccharide peptide ingredientsAntifungal drugPharmaceutical medicine
The invention provides a glycopeptide antifungal compound and a pharmaceutically acceptable salt thereof, a preparation method and application thereof, and a pharmaceutical composition containing the active component. The glycopeptide antifungal compound has a general structural formula shown as below; n equals to 1, 2 or 3; and R represents monosaccharide or disaccharide, wherein the monosaccharide is glucose, galactose, xylose, rhamnose, mannose, ribose, glucosamine or acetyl glucosamine, and the disaccharide is maltose or lactose. Compared with existing clinical antifungal drugs, the compound provided by the invention has advantages of high efficiency, low toxicity and broad antibacterial spectrum, etc.; therefore, the compound can be applied to preparation of antifungal drugs. The preparation method provided by the invention successfully introduce different glycosyls onto primary amino group of Caspofungin, and has advantages of simple process and high yield.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method of caspofungin
The invention relates to a preparation method of caspofungin. The preparation method comprises the following steps: using p-thiocresol to replace hydroxide radicals of pneumocandins B0, then reducing acylamino groups by using a reducing agent, and finally using ethidene diamine to replace p-sulfonyl toluene, thus obtaining the caspofungin. According to the preparation method disclosed by the invention, an appropriate solvent is adopted for crystallizing and separating an intermediate, repeated separation and purification operation for chromatographic column preparation in the prior art is avoided, the reaction time is shortened, degradation of the intermediate is avoided, the operation is simplified, high-purity and high-yield caspofungin is obtained, and the preparation method is particularly suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Caspofungin preparation with low impurity content and preparation method and application thereof
ActiveCN102488889AReduce generationEasy to storeAntibacterial agentsAntimycoticsPhysical chemistryOrganic chemistry
The invention discloses a caspofungin medicinal composition with low impurity content, and discloses a method for preparing the caspofungin medicinal composition with low impurity content. The caspofungin medicinal composition provided by the invention has high stability.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
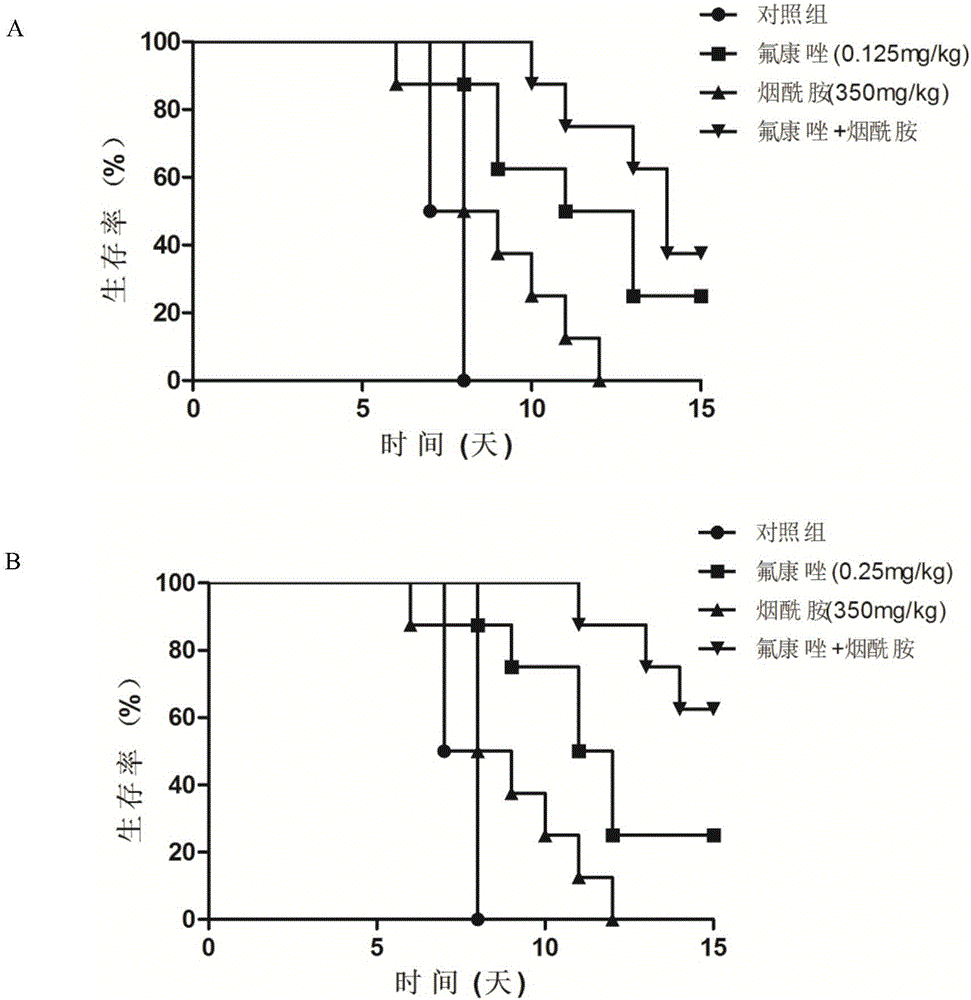
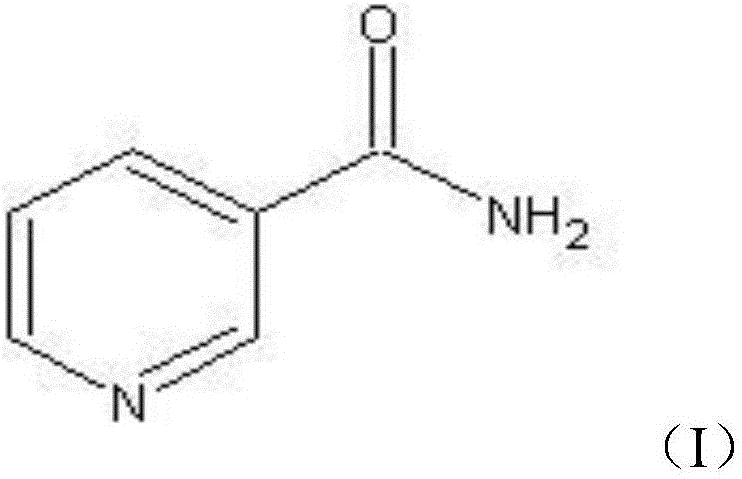
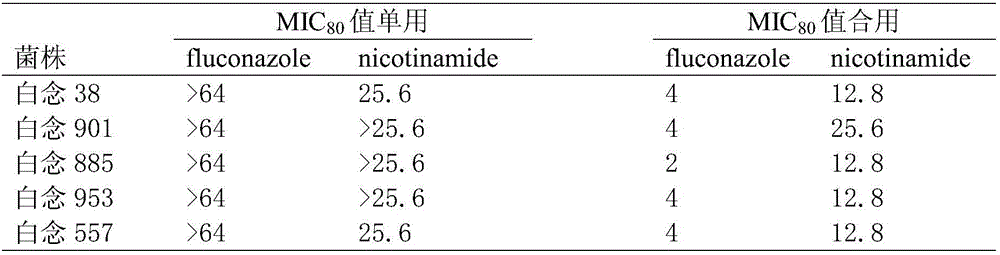
Applications of nicotinamide as antifungal drug synergist
ActiveCN106390130AEliminate side effectsLow toxicityAntibacterial agentsOrganic active ingredientsEchinocandinSide effect
The invention belongs to the technical field of medicine, and more specifically provides applications of nicotinamide in preparing an antifungal drug synergist. Novel applications of nicotinamide are provided; nicotinamide is capable of reducing dosages of antifungal drugs such as azoles, echinocandins, and polyenes as an antifungal drug synergist, and reducing toxic and side effect of drugs, especially fluconazole, voriconazole, caspofungin, and amphotericin B, accordingly. Nicotinamide is capable of recovering the effect of antifungal drugs on drug resistance funguses as a antifungal drug synergist, treating fungal infection especially drug resistance fungus infection effectively, reducing drug toxicity, and reducing economic burden of patients in drug therapy; and in addition, nicotinamide is capable of improving the effect of antifungal drugs on candida tropicalis and cryptococcus neoformans, and possesses high clinical application value.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Genetic recombination strain for producing pneumocandins B0, breeding method and application
ActiveCN104531535AShort fermentation cycleEasy to separate and extractFungiMicroorganism based processesLithium chlorideGlarea lozoyensis
The invention discloses a genetic recombination strain for producing pneumocandins B0, a breeding method and applications. The bacterial strain classification name of the genetic recombination strain is Glarea lozoyensis Q45; the preservation registration number is CCTCC NO: M2014416; and the preservation date is September 14, 2014. The method for breeding the strain includes the steps that an original strain is manufactured into a protoplast; a mutation library composed of a plurality of mutant strains is obtained through ion implantation and lithium chloride processing; the mutation library is manufactured into a protoplast again; random fusion is carried out on the protoplasts after ion implantation inactivation and heat inactivation are carried out; fermentation screening is carried out on high-yield genetic recombinant bacteria; and protoplast preparation and fusion and fusant screening are carried out on the screened genetic recombinant bacteria, and therefore the genetic recombination strain is obtained. The genetic recombination strain is excellent in performance and stable in production capacity; the yield of the pneumocandins B0 obtained through fermentation is 6 g / L; in addition, by-products are few; the post-extraction difficulty of the pneumocandins B0 is reduced; the application to high-quality caspofungin preparation is facilitated; and the genetic recombination strain has the important industrial value.
Owner:NANJING UNIV OF TECH
Echinocandin antifungal agent caspofungin containing medicinal composition, a preparation method and application thereof
The invention discloses an echinocandin antifungal agent caspofungin containing medicinal composition, which contains cane sugar used as an excipient. The medicinal composition provide by the invention has good stability.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Composition containing antifungal drug and lactate buffering agent
InactiveCN103212058AGood chemical stabilityImprove stabilityAntimycoticsPharmaceutical delivery mechanismAntifungal drugBuffering agent
The invention relates to a drug composition, containing caspofungin or pharmaceutically acceptable salts thereof, and a lactate buffering agent in a pharmaceutically acceptable amount. By addition of the lactate buffering agent, the composition greatly decreases generation of caspofungin degradation product, greatly raises stability of caspofungin, and is convenient for preservation and clinic usage, thereby raising usage security of caspofungin.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method for detecting antifungal drugs in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
InactiveCN111766312AHigh sensitivityStrong specificityComponent separationAntifungal drugPharmacology
Owner:南京品生医学检验实验室有限公司
Method for separating and purifying Pneumocandins B0
InactiveCN104250289ASolve problems that cannot be removedHigh purityPeptide preparation methodsAntifungal drugMedicine
The invention provides a method for separating and purifying Pneumocandins B0, which comprises the steps of acidifying and extracting a broth, separating a leaching solution by resin adsorption, concentrating, and then drying to obtain the Pneumocandins B0. The obtained product with high purity is a good raw material to prepare antifungal drug Pneumocandins B0. The prepared end product has the advantages of high yield, low production cost, and simple operation, and is especially suitable for industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Synthesis method of caspofungin
ActiveCN109721641AGood shape retentionFew reaction stepsPeptide preparation methodsEthylenediamineSynthesis methods
The invention belongs to the technical field of medicines, and particularly relates to a synthesis method of caspofungin. The synthesis method comprise the following steps: mixing a compound shown asformula II and thionyl chloride dissolved in a solvent A to form a compound shown as formula III, mixing the compound shown as the formula III without purification and ethidene diamine dissolved in asolvent B to form a compound shown as formula IV, mixing the compound shown as the formula IV and a borane complex to form a compound shown as formula I. The synthesis method replaces virulent and malodorous thiol or thiophenol compound with an irritating odor with thionyl chloride, and avoids using highly corrosive acid; formed cyclic sulphite facilitates configuration retention of a reactant; atthe same time, an ethidene diamine group is directly introduced into cyclic sulphite without the purification since cyclic sulphite has properties that cyclic sulphite is easy to remove and does notchange a chiral configuration of the reactant; a reaction procedure is shortened; the number of times of the purification for preparation is reduced; and the purity and yield are improved.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical composition of freeze-dried powder injection containing caspofungin
InactiveCN104116716AImprove stabilityShorten freeze-drying cyclePowder deliveryAntimycoticsSucroseFreeze-drying
The invention provides a pharmaceutical composition of a freeze-dried powder injection containing caspofungin or pharmaceutically acceptable salts of the caspofungin, and a preparation method of the pharmaceutical composition. The composition contains caspofungin or pharmaceutically acceptable salts of the caspofungin, a pH modifier and pharmaceutically acceptable auxiliary materials, wherein the pharmaceutically acceptable auxiliary materials are one or more of cane sugar, mannitol, newtol and sorbitol; and the pH modifier is one or more of appropriate lactic acid, sodium hydroxide and potassium hydroxide, so that the pH value of the pharmaceutical composition is kept within an acceptable range.
Owner:SICHUAN HAISCO PHARMA CO LTD
Formulations for improving the delivery of hydrophobic agents
PendingUS20210046019A1Reduce deliveryOrganic chemistryInorganic non-active ingredientsActive agentSurface-active agents
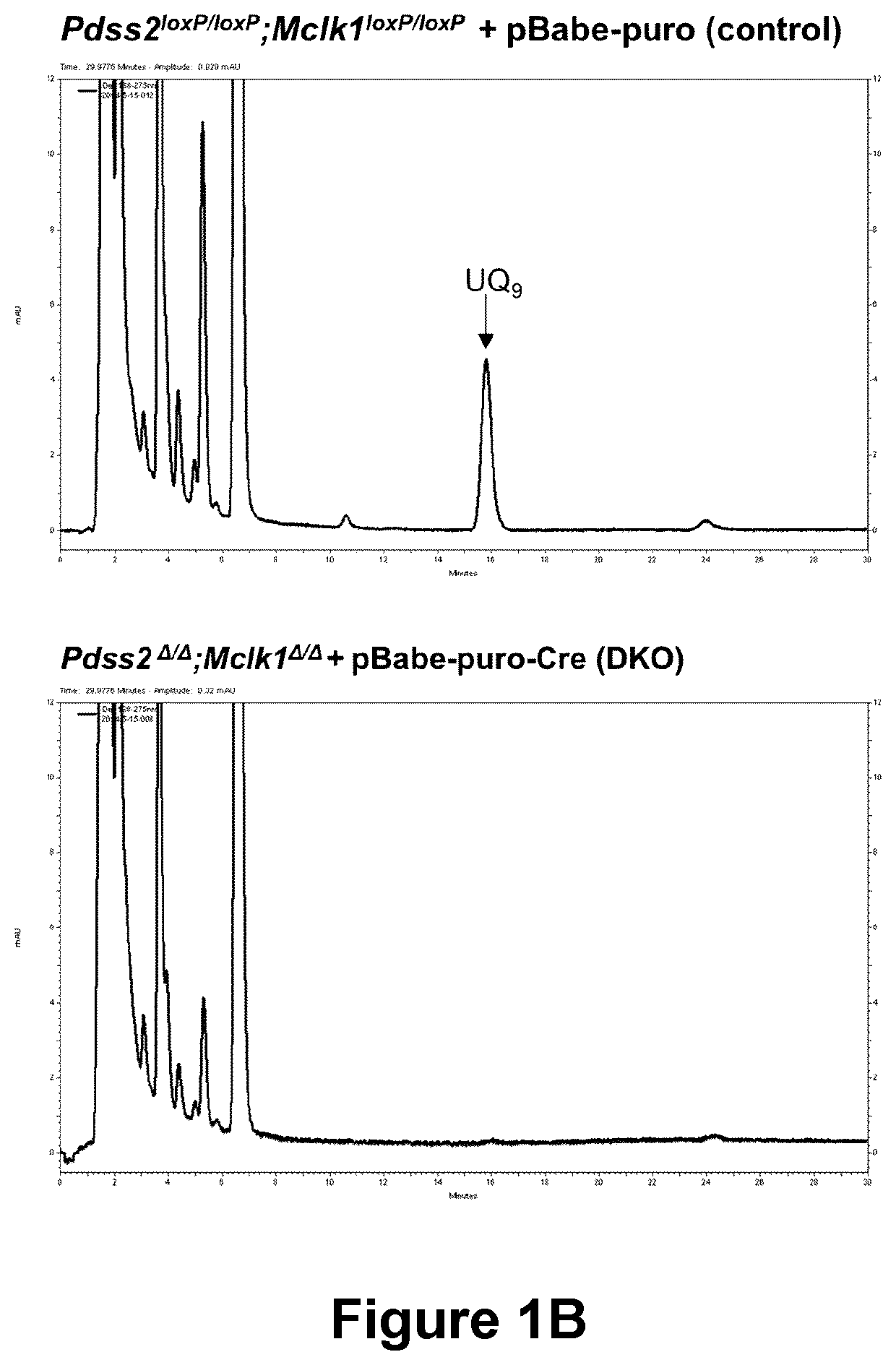
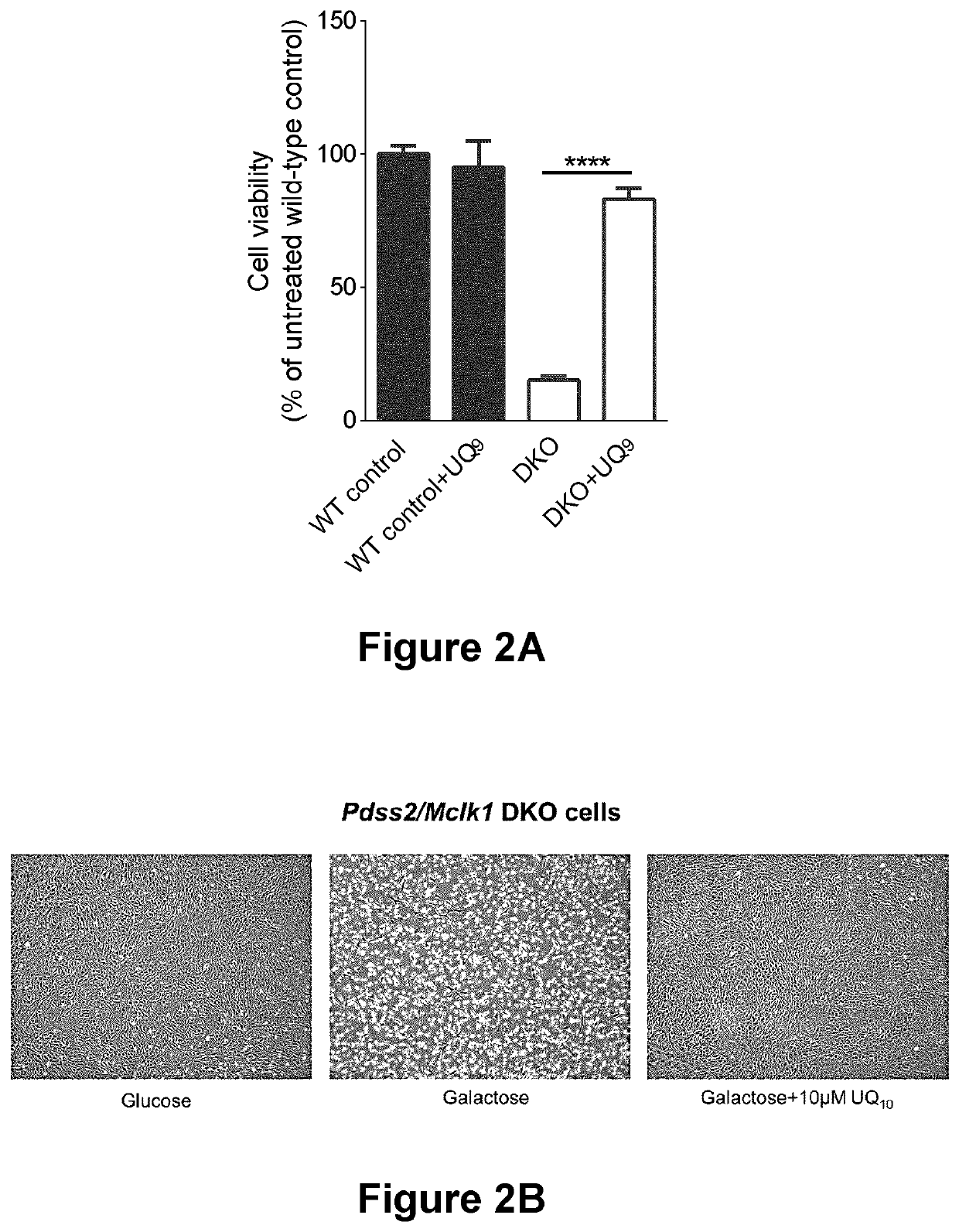
The present disclosure concerns methods and formulations for delivery of hydrophobic agents (such as ubiquinone or poorly soluble drugs) for therapeutic and bioanalytical use. It further concerns use of lipopeptides (e.g. caspofungin) or surfactants to solubilize hydrophobic agents and thus increase their bioavailability. Also described are therapeutic methods for the treatment of conditions that benefit from such hydrophobic agents. The present disclosure further relates to methods of identifying drug candidates for treatment of ubiquinone deficiency.
Owner:MCGILL UNIV
Liquid pharmaceutical composition containing echinocandin antifungal agent caspofungin
The invention discloses a liquid pharmaceutical composition containing echinocandin antifungal agent caspofungin, wherein the pharmaceutical composition containing carbohydrate as a stabilizer. The pharmaceutical composition provided by the invention has good stability.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
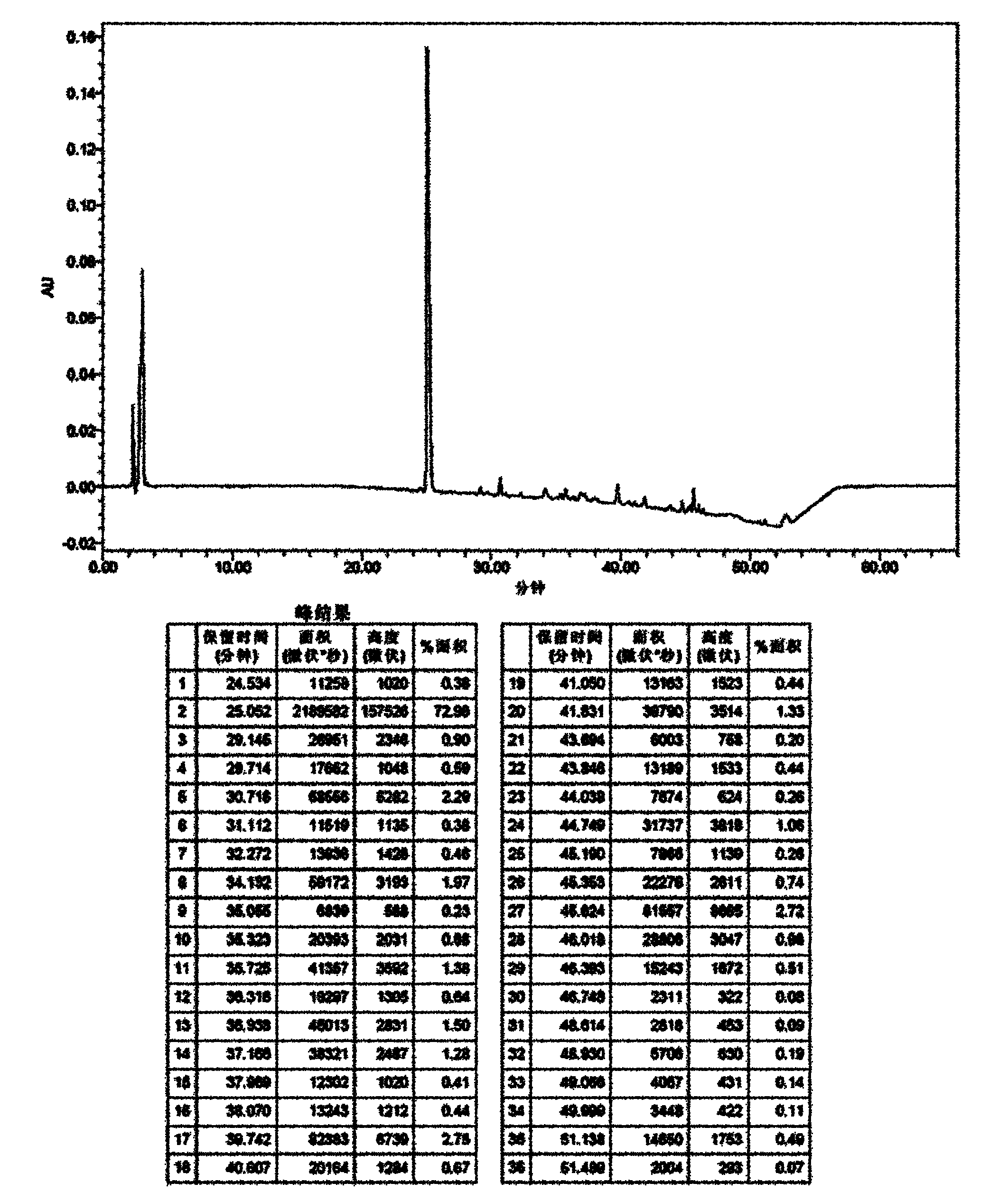
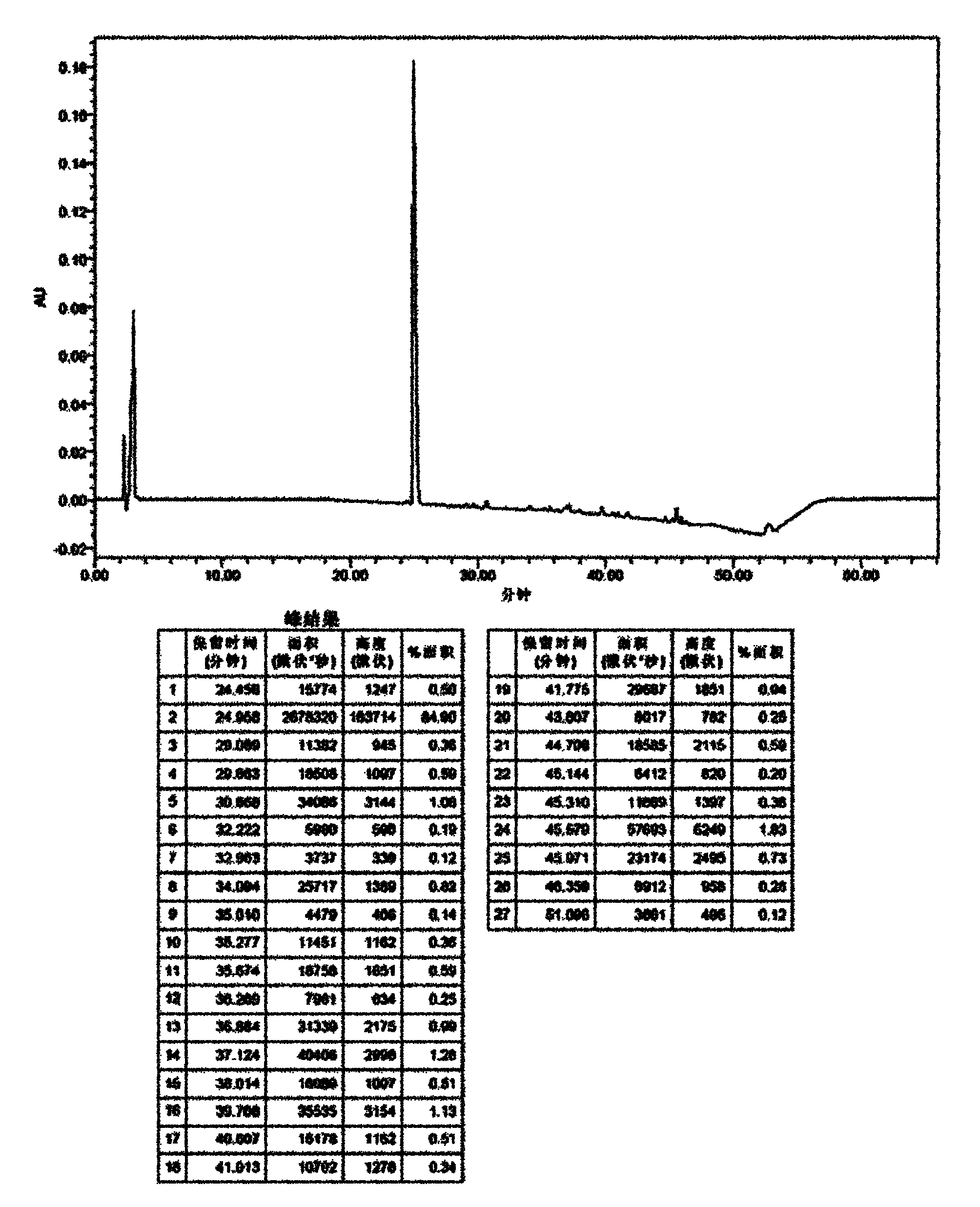
Method for separating and purifying caspofungin or its salt
InactiveCN104250290ASolve problems that cannot be removedQuality improvementPeptide preparation methodsEngineeringPharmaceutical medicine
The invention provides a method for separating and purifying caspofungin or its salt, which comprises the following steps: separating caspofungin or its pharmaceutically acceptable salt in a reversed column chromatography, then recrystallizing, and drying to obtain caspofungin or its pharmaceutically acceptable salt with high purity. The prepared end product has the advantages of high purity and low production cost, the medicine quality is increased, operation is simple, and the method is suitable for industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Caspofungin or salts thereof with high purity, as well as preparation method and use thereof
Disclosed are a high purity of caspofungin or salts thereof, and a preparation method thereof, and use thereof. Disclosed are a caspofungin or salts thereof with low solvent residue and hyposaline, and a preparation process comprising: dissolving a crude product of caspofungin or salts thereof into a system of water and acetic acid, then mixing with a first organic solvent ethyl alcohol, subsequently mixing with a second organic solvent ethyl acetate, then being subject to filtration and drying together with water, to obtain caspofungin or salts thereof with high stability, low solvent residue and hyposaline.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Preparation method of high-purity and high-yield caspofungin impurity CO
ActiveCN105218645ASimple processPeptide preparation methodsChromatographic separationPurification methods
The invention discloses a preparation method of a high-purity and high-yield caspofungin impurity CO. The preparation method comprises step1, an intermediate I is prepared from solids containing pneumocandins BO and CO; step 2, a crude product of an intermediate II is prepared from the intermediate I, chromatography purification and separation are performed with a reversed-phase chromatography method and a normal-phase chromatography method, and the intermediate II is obtained; step3, a crude product of the caspofungin impurity CO is prepared from the intermediate II, chromatographic separation is performed with a reversed phase chromatography method, and the high-purity caspofungin impurity CO is obtained. By means of a specific combination and purification method of raw materials, the caspofungin impurity CO with HPLC (high performance liquid chromatography) purity higher than 97% can be obtained, and a way is provided for preparation of the caspofungin impurity CO. The purity of the product obtained with the method can completely meet the requirements, and a process is relatively simple.
Owner:CHENGDU YATU BIOLOGICAL TECH
Application of small molecule compounds as adjuvant in preparation of antifungal drugs
ActiveCN105997965AImprove efficacyLower doseOrganic active ingredientsAntimycoticsAdjuvantAntifungal drug
The invention discloses an application of a kind of small molecule compounds as an adjuvant in the preparation of antifungal drugs. After 51,520 small molecule compounds in a national compound sample bank are subjected to high-throughput screening, the result shows that when small molecule compounds as shown in a formula I to a formula X independently act on a candida albicans biological membrane, the inhibition ratio is almost zero, but when the small molecule compounds are used with conventional antifungal drugs such as amphotericin B, fluconazole or caspofungin, the remarkable synergism is achieved, the efficacy of the conventional antifungal drugs is improved by 30% or above, the use dosage of the conventional antifungal drugs is reduced, and the inhibiting effect of the antifungal drugs to drug resistance fungi is improved.
Owner:SHANDONG UNIV
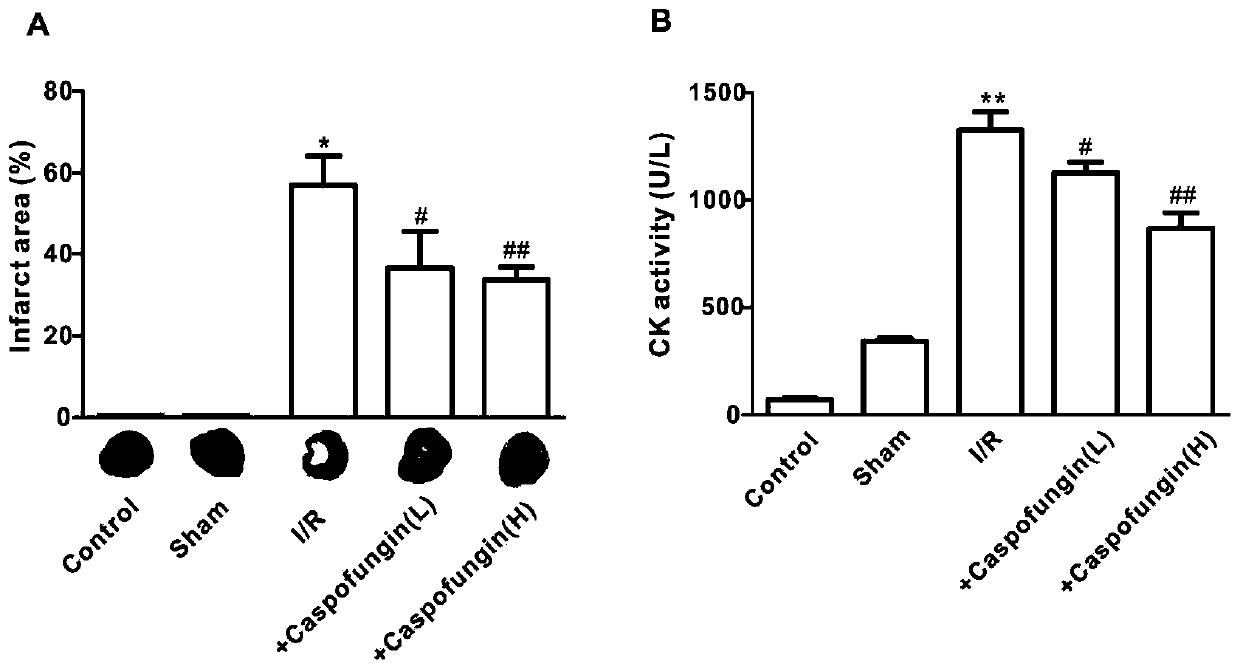
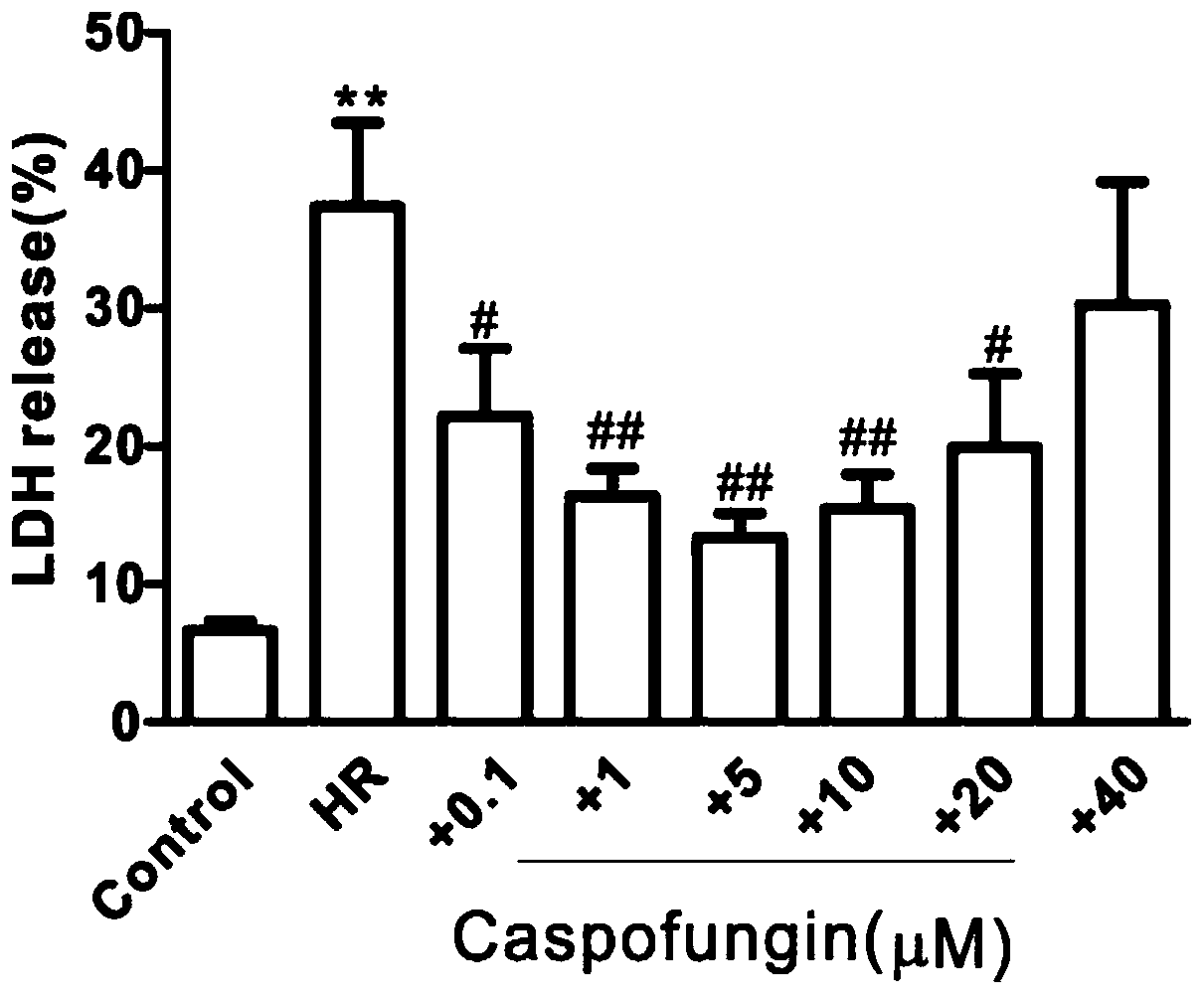
Application of caspofungin in preparation of medicine for treating ischemia/reperfusion injury
PendingCN110876798AReduce deathReduce infarct sizeCyclic peptide ingredientsCardiovascular disorderCardiac musclePharmaceutical drug
The invention relates to an application of caspofungin in preparation of a medicine for treating ischemia / reperfusion injury. The caspofungin is used for treating ischemia / reperfusion injury, particularly myocardial tissue ischemia, and more particularly has a protective effect on myocardial ischemia / reperfusion, so that the myocardial ischemia / reperfusion injury can be remarkably reduced.
Owner:CENT SOUTH UNIV
Applications of traditional Chinese medicine sanguisorba officinalis in preparing antifungal drug synergist
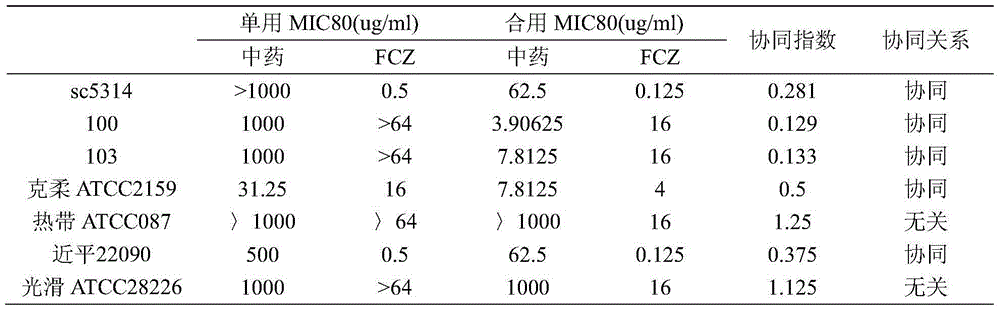
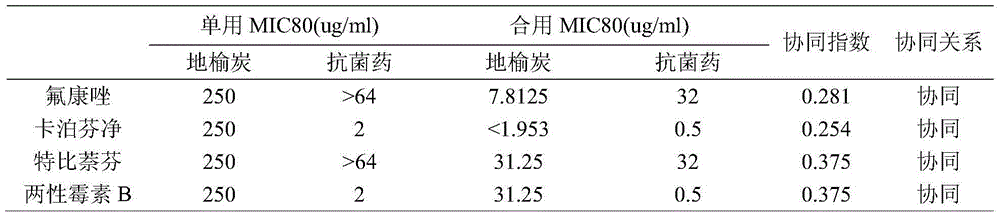
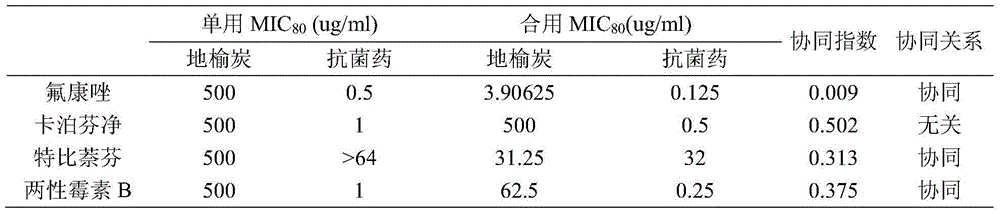
InactiveCN104857116AReduce drug doseEnhanced inhibitory effectAntimycoticsPlant ingredientsAntifungal drugsSide effect
The invention belongs to the technical field of medicine, and more specifically relates to applications of traditional Chinese medicine sanguisorba officinalis in preparing an antifungal drug synergist. It is confirmed by in vivo experiments that sanguisorba officinalis possesses synergistic effects with routine antifungal drugs such as fluconazole, miconazole, caspofungin, and amphotericin B; with regard to drug resistance funguses, when the antifungal drugs are combined with sanguisorba officinalis, drug dose of the routine antifungal drugs can be reduced obviously, inhibiting effects of the routine antifungal drugs on funguses can be improved to different degrees, effects of the antifungal drugs on the drug resistance funguses can be recovered, and inhibiting effects on the drug resistance funguses are improved. Novel applications of traditional Chinese medicine sanguisorba officinalis are provided; clinical fungus drug resistance can be widely observed, and drug resistant degree becomes higher, drug dose of the antifungal drugs can be reduced, medical cost is reduced for patients, and drug toxic and side effects are reduced.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Caspofungin formulations
InactiveUS8232245B2Reduce in quantityEasy to manufactureBiocideAntimycoticsMicrobiologyPharmaceutical medicine
The present invention relates to pharmaceutical compositions comprising a pharmaceutically acceptable salt of caspofungin as active ingredient being useful for the prevention and / or treatment of fungal infections. The compositions additionally comprise specific bulking agents and small amounts or no amounts of an additional pH modifier and may be in a liquid or solid form, e.g. may be lyophilized compositions. The compositions show good stability and reduced amounts of sub-visible particulate matter formed in solutions which are reconstituted from the lyophilized product.
Owner:SANDOZ AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com