Caspofungin preparation with low impurity content and preparation method and application thereof
一种杂质、含量的技术,应用在治疗和/或预防真菌感染的药用组合物领域,能够解决患者安全性隐患、降解杂质未进行有效的严格控制、不现实等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
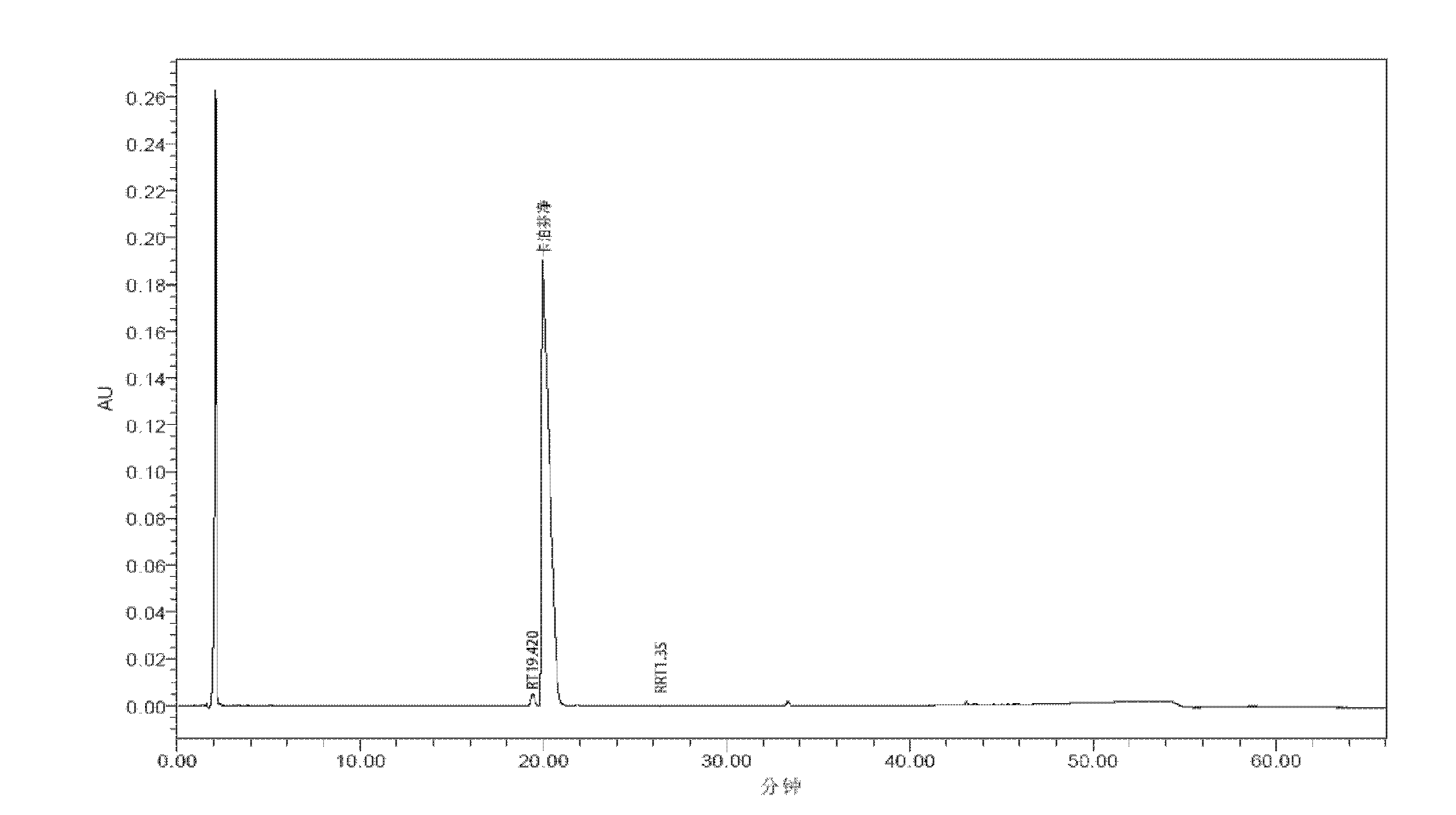
[0136] Caspofungin HPLC analysis method:
[0137] High performance liquid chromatography: WATERS 2695-2998
[0138] Analytical column: YMC-Pack ODS-A column, specification: 250×4.6mm, S-5um, 1.2nm
[0139] Column temperature: 35°C
[0140] Detection wavelength: 220nm
[0141] Mobile phase: A: 0.1% perchloric acid (analytical pure, Shanghai Jinlu Chemical Co., Ltd.) and 0.075% sodium chloride (analytical pure, Sinopharm Chemical Reagent Co., Ltd.) solution (get perchloric acid 1.0ml and chlorine Sodium chloride 0.75g, add water to dissolve and dilute to 1000ml);
[0142] B: Acetonitrile (HPLC grade, TEDIA).
[0143] The gradient conditions are shown in the table below:
[0144] time (minutes)
A%
B%
initial
65.5
34.5
14.5
65.5
34.5
[0145] 35
50
50
45
35
65
50
20
80
52
20
80
53
65.5
34.5
66 ...
Embodiment 1
[0184] Preparation of caspofungin pharmaceutical composition
[0185] The preparation process is as follows: first dissolve the carbohydrate protecting agent and glycine (or other amino acids) in water or in a solution containing an optional pH regulator, then add the compound of formula I or its pharmaceutically acceptable salt to dissolve it, Dilute to a certain volume, and then lyophilize the solution obtained above.
[0186] Different formulations can be obtained by changing the concentration of caspofungin and / or the concentration of carbohydrate protecting agent and / or glycine (or other amino acids), and the pH or concentration of pH regulator. The composition of each formulation of the composition before lyophilization is as follows:
[0187]
[0188]
[0189]
[0190] Each freeze-drying procedure is as follows:
[0191] Freeze-drying procedure A:
[0192] a. The shelf temperature drops to -40°C at a rate of 1°C / min;
[0193] b. The shelf temperature is mai...
Embodiment 2
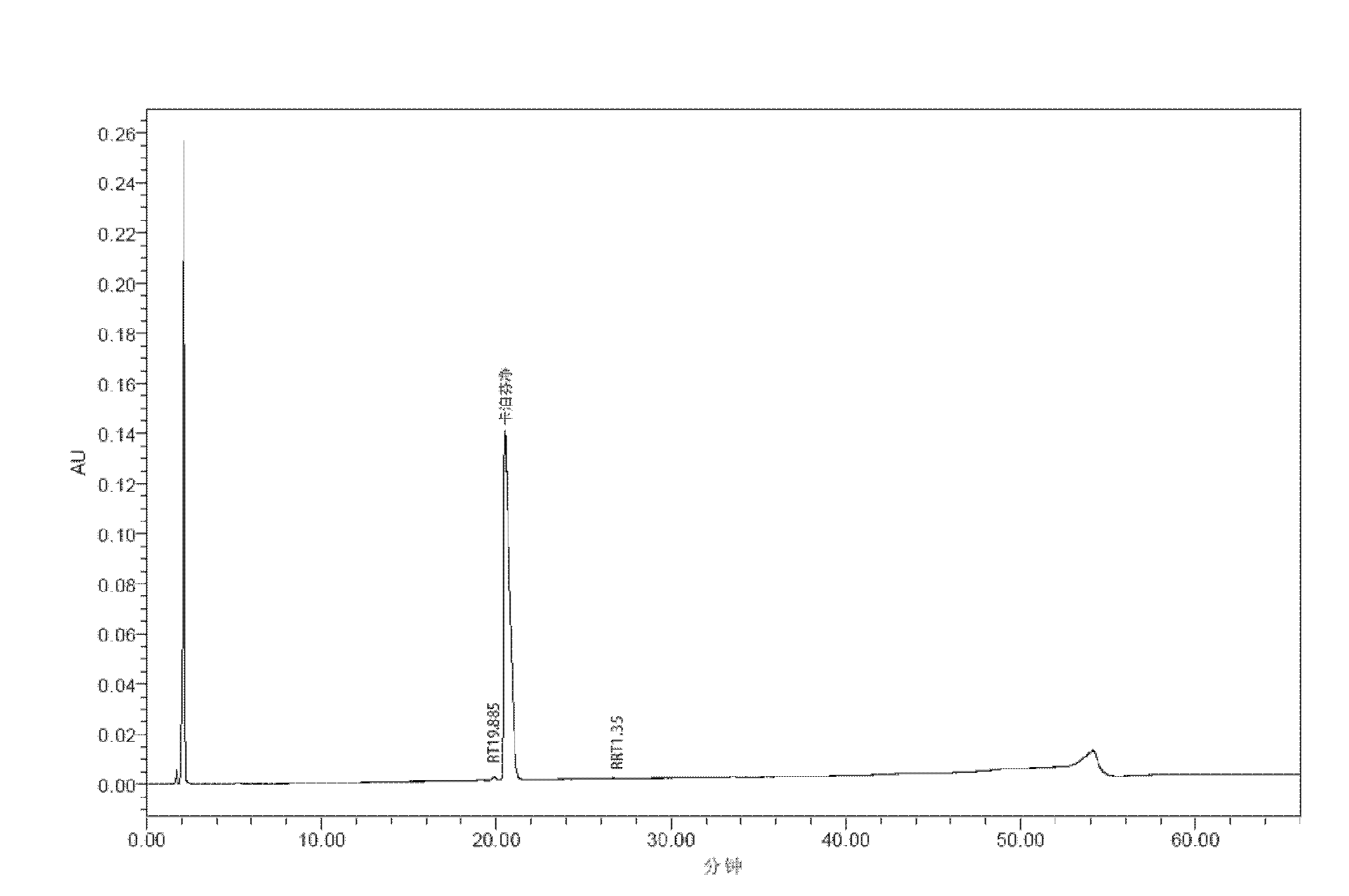
[0255] Get 0.75g sorbitol, 0.5g mannitol, dissolve in 20ml of water, then add 1.05g of caspofungin base, then add sodium dihydrogen phosphate, until its final constant volume concentration is 20mM, and adjust the pH to 6.0, add water to make up to 25mL final volume. Then through a 0.22 μm membrane filter, the composition of the composition (formulation 22) before freeze-drying is as follows:
[0256]
[0257] Fill vials at 1.25ml / bottle and lyophilize.
[0258] The freeze-dried products were placed at 40°C for stability inspection, and samples were taken for HPLC analysis after 8 weeks and 24 weeks, respectively, and stability inspections were carried out at 25°C, 65% RH and 2-8°C, and respectively Samples were taken after 24 weeks for HPLC analysis (including data at time 0).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com