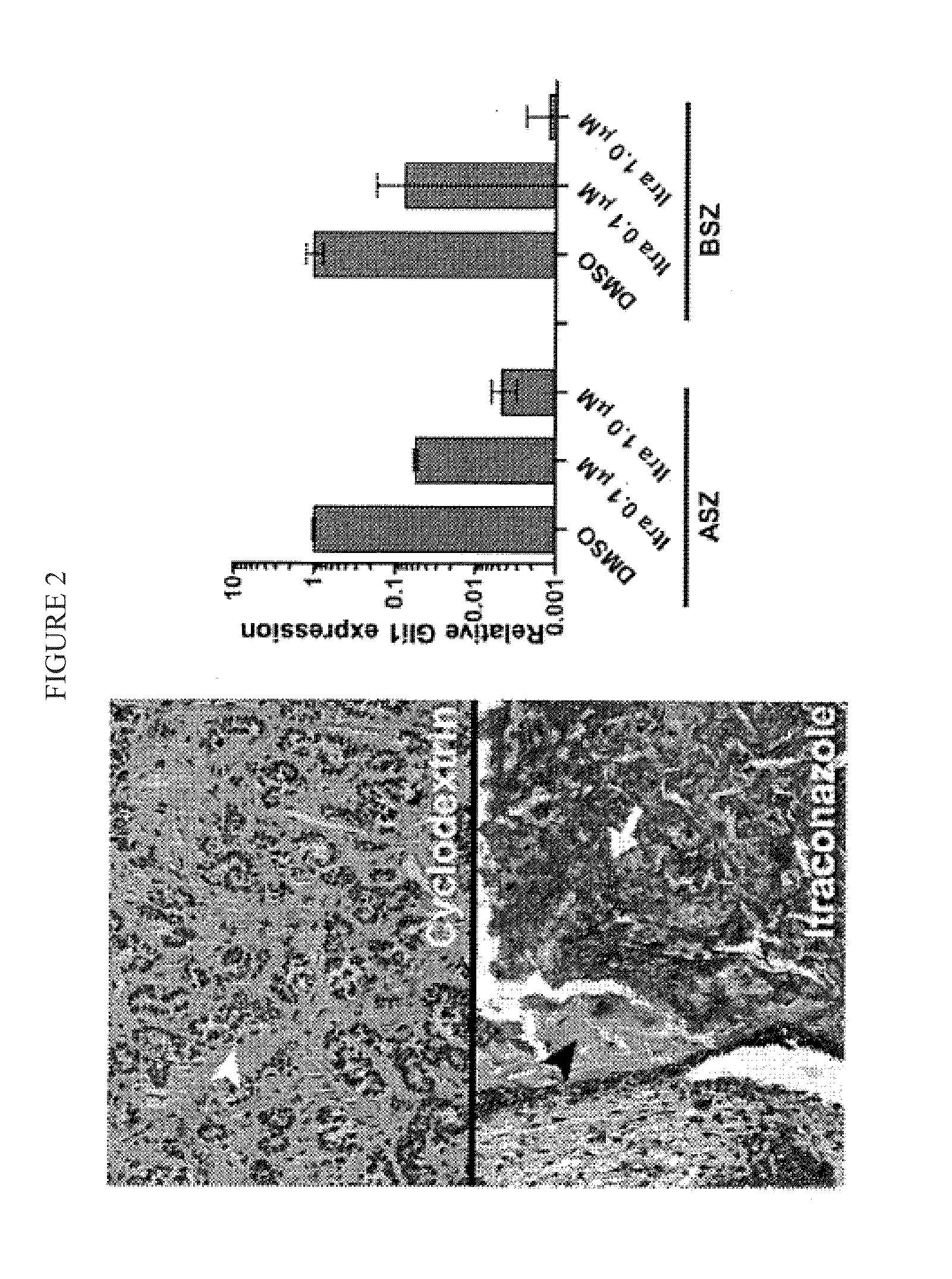
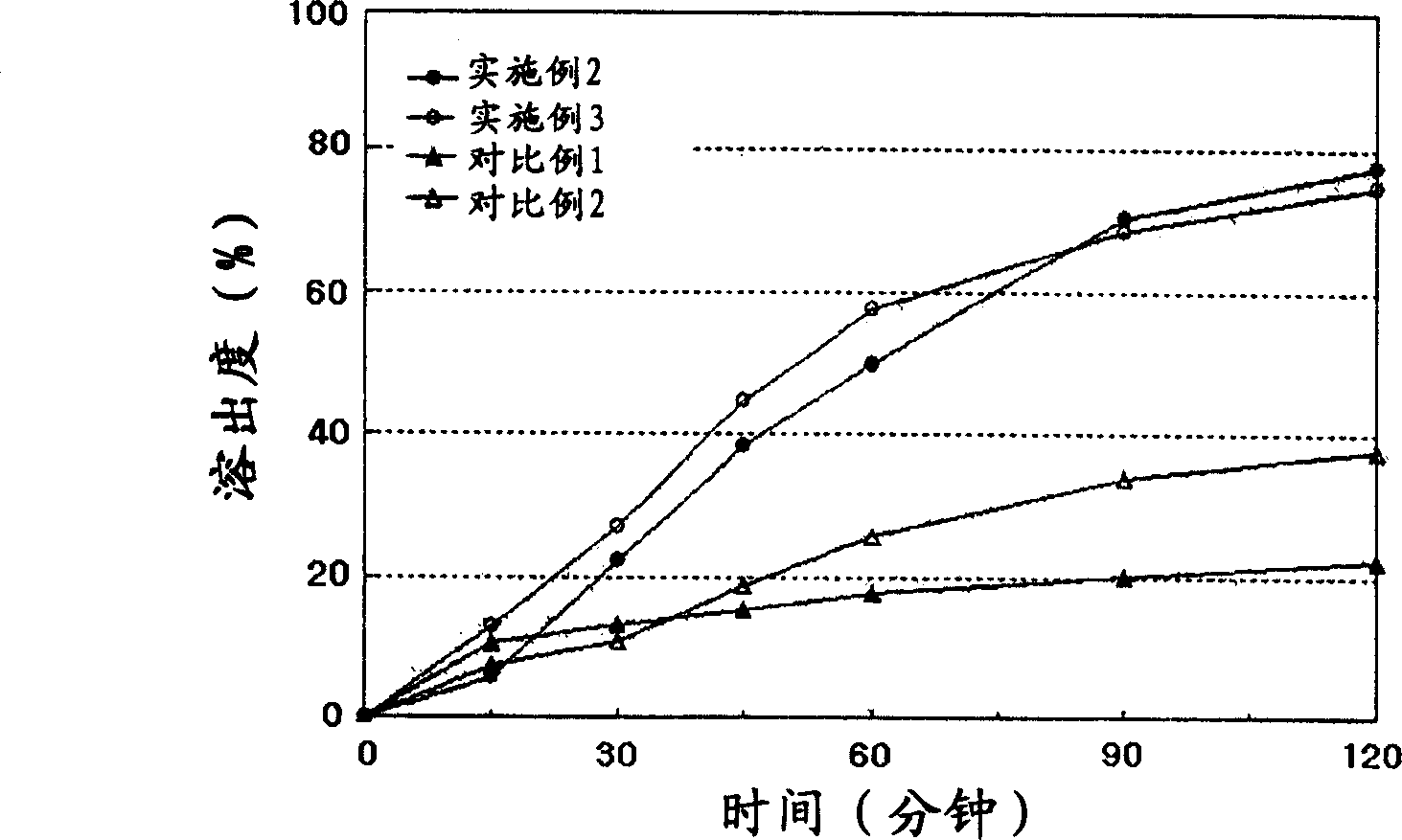
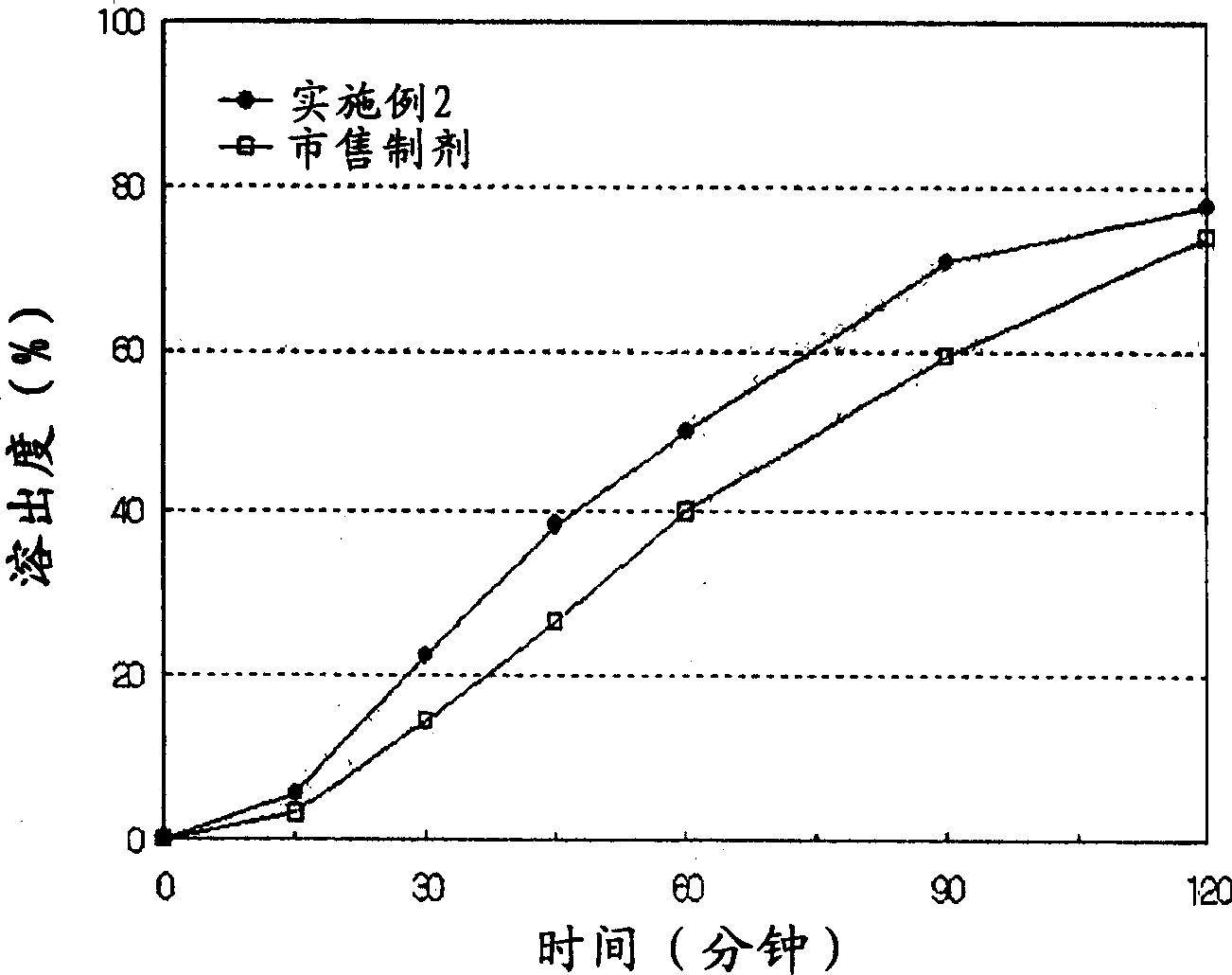
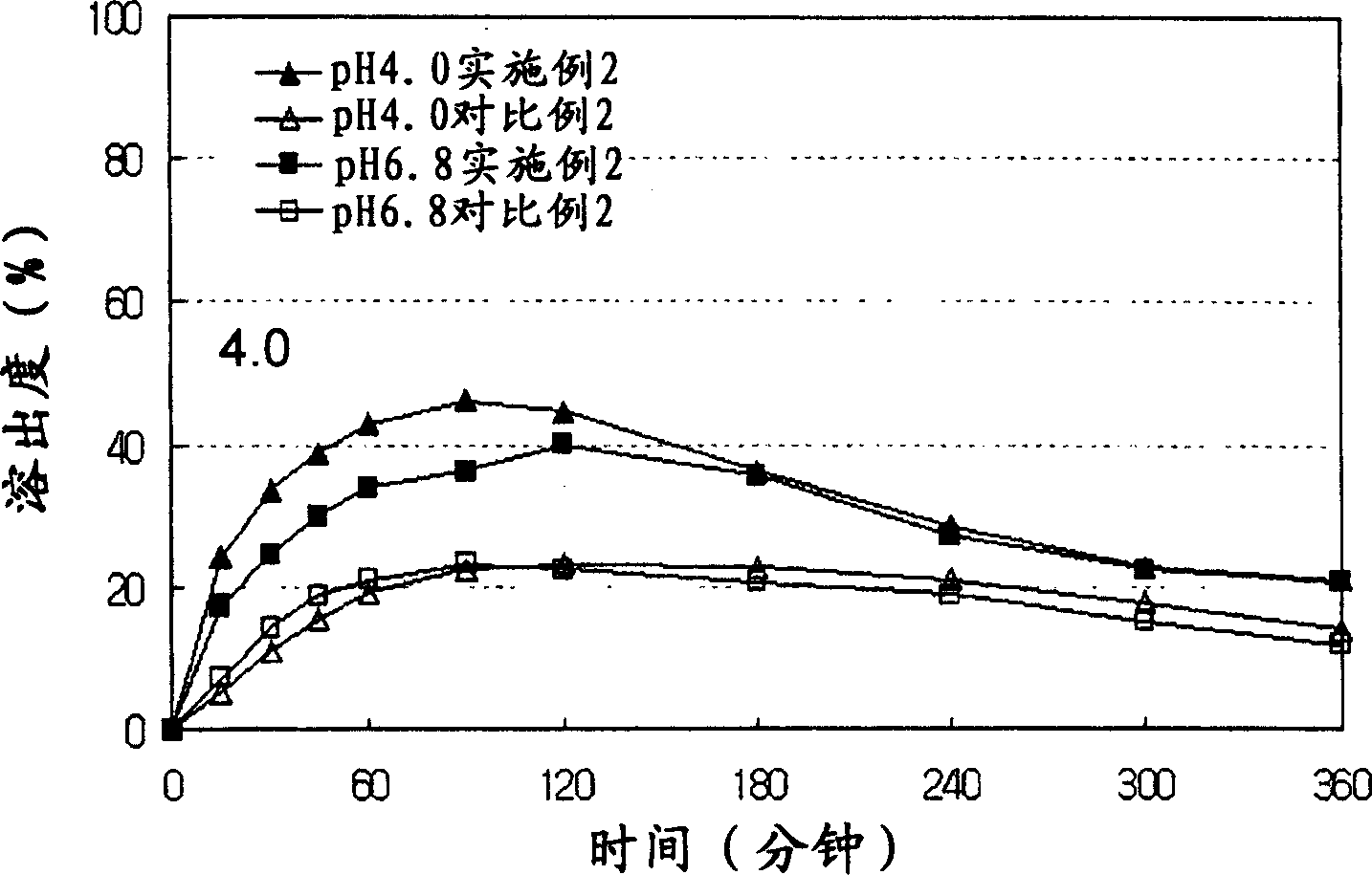
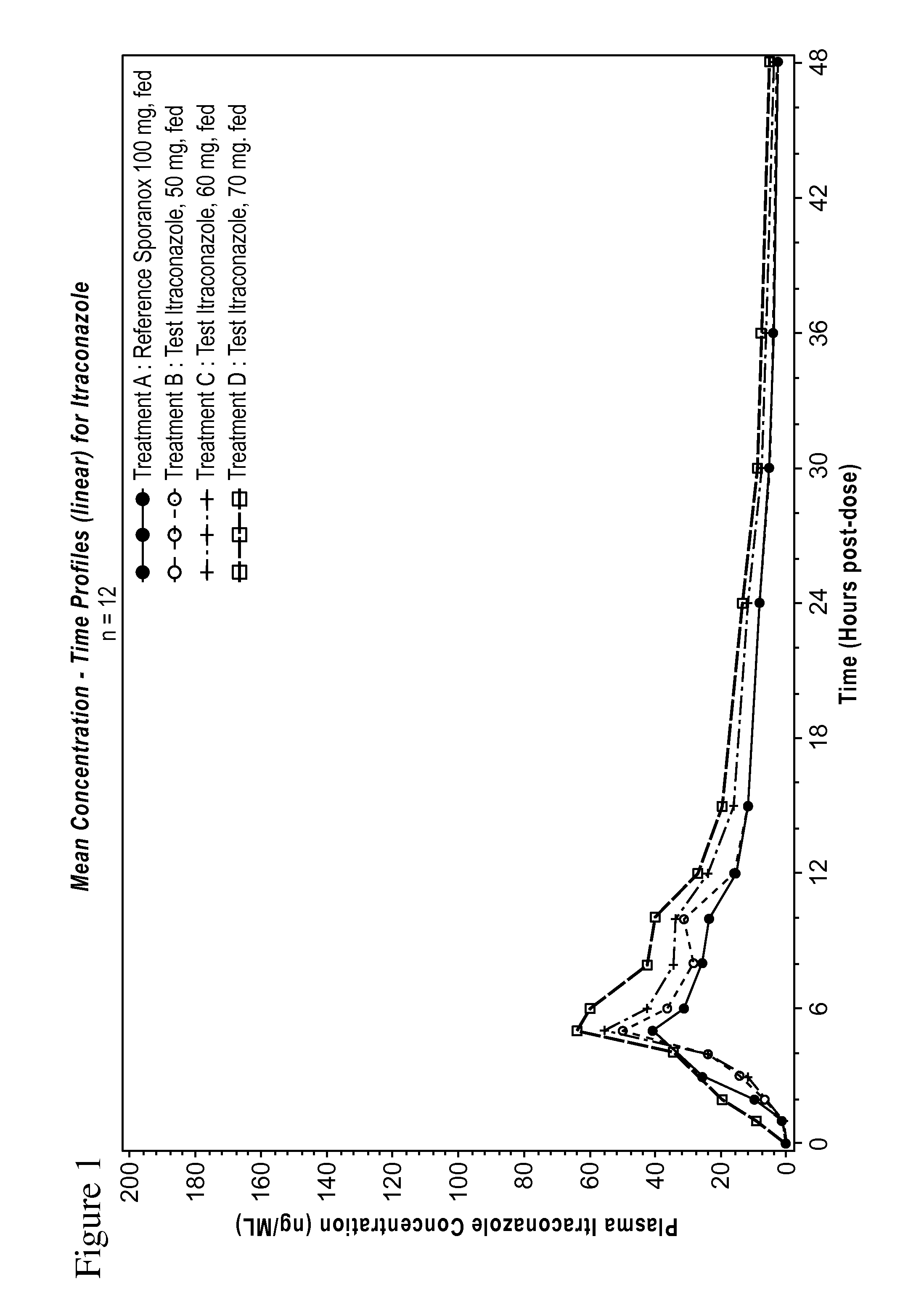
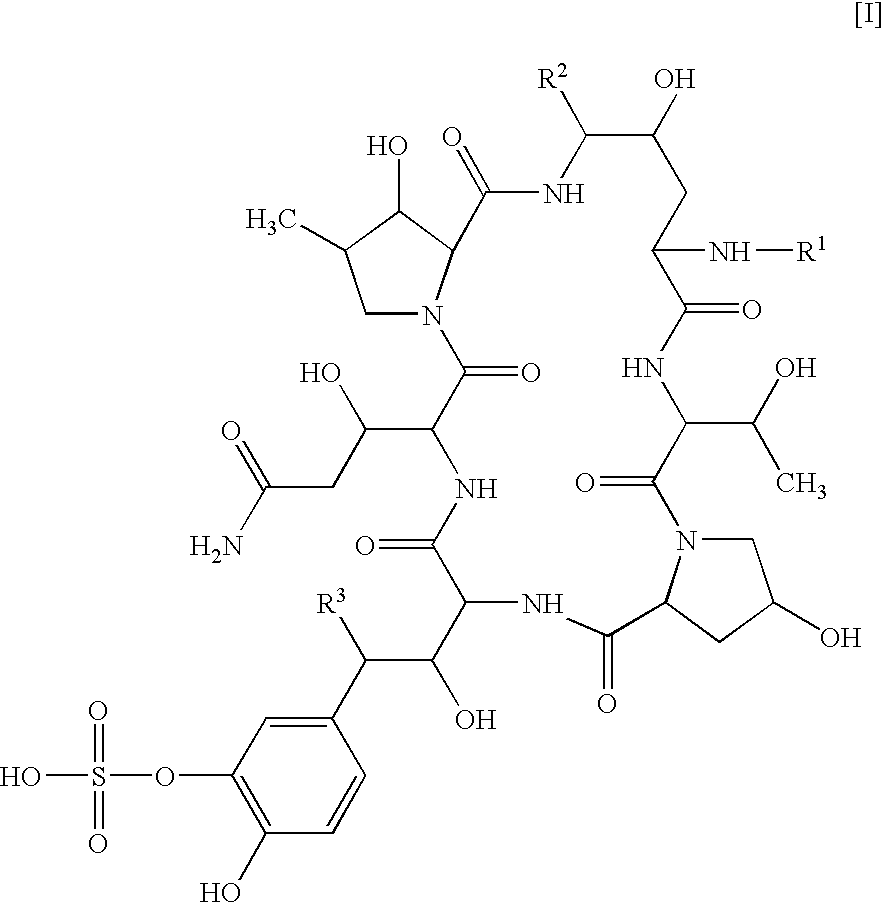
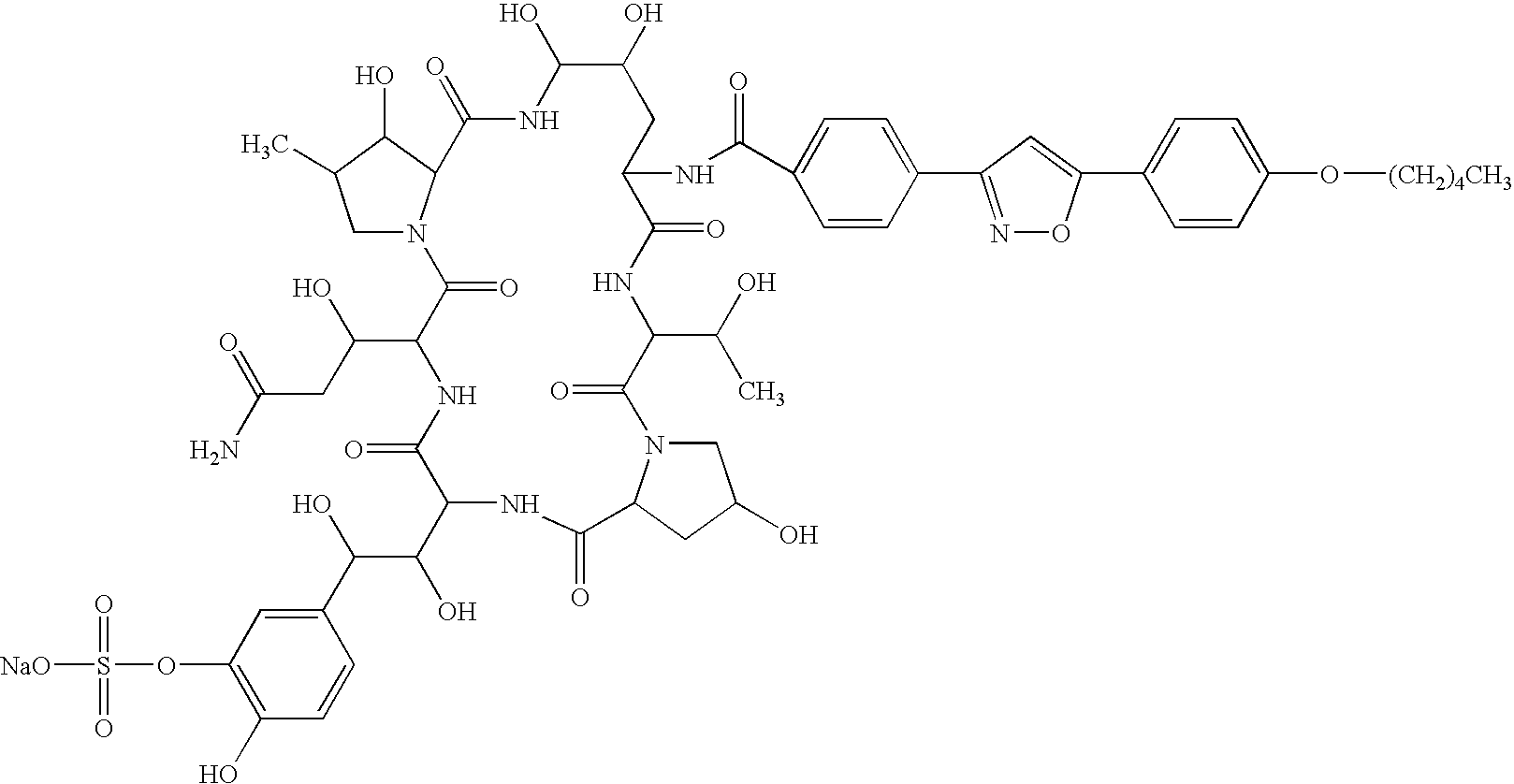
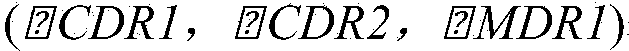Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
192 results about "Itraconazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Itraconazole is used to treat a variety of fungal infections.
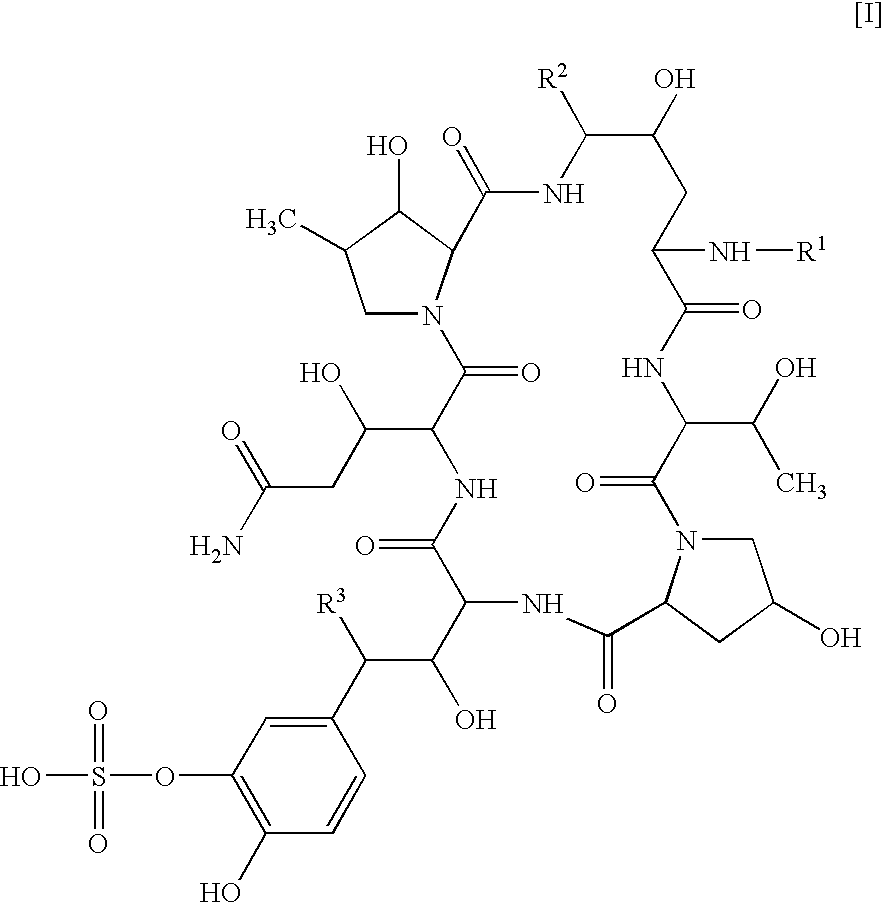
Diterpene Glycosides as Natural Solubilizers
InactiveUS20110033525A1Improve solubilityRetain activityBiocideHydroxy compound active ingredientsItraconazoleCapsaicin
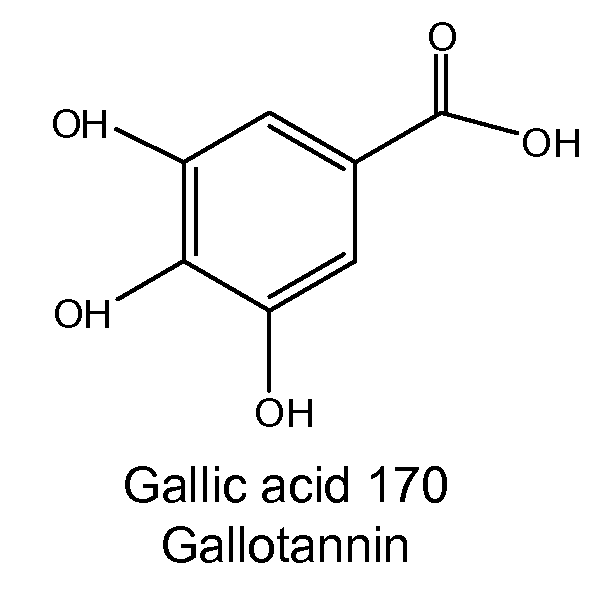
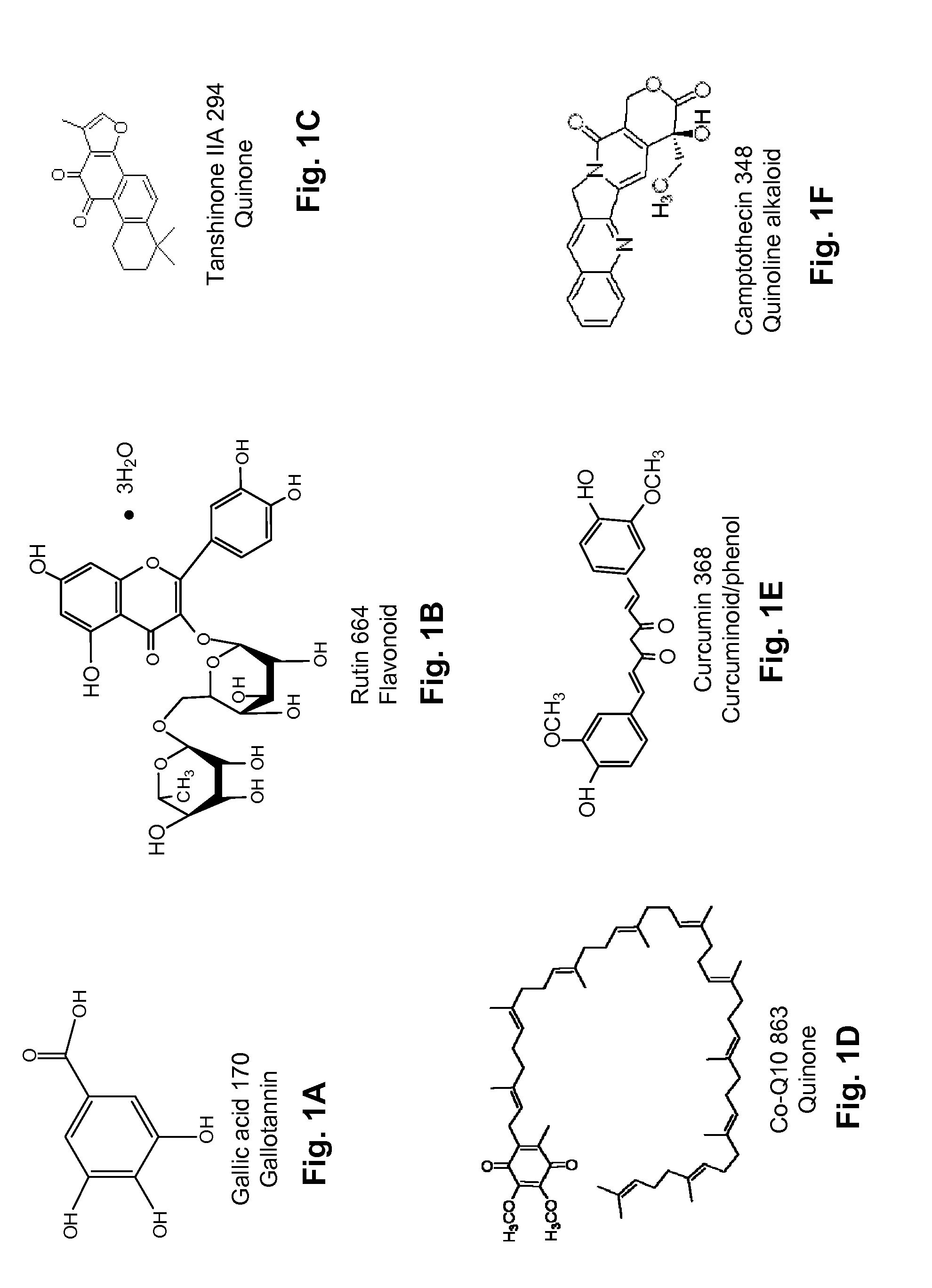
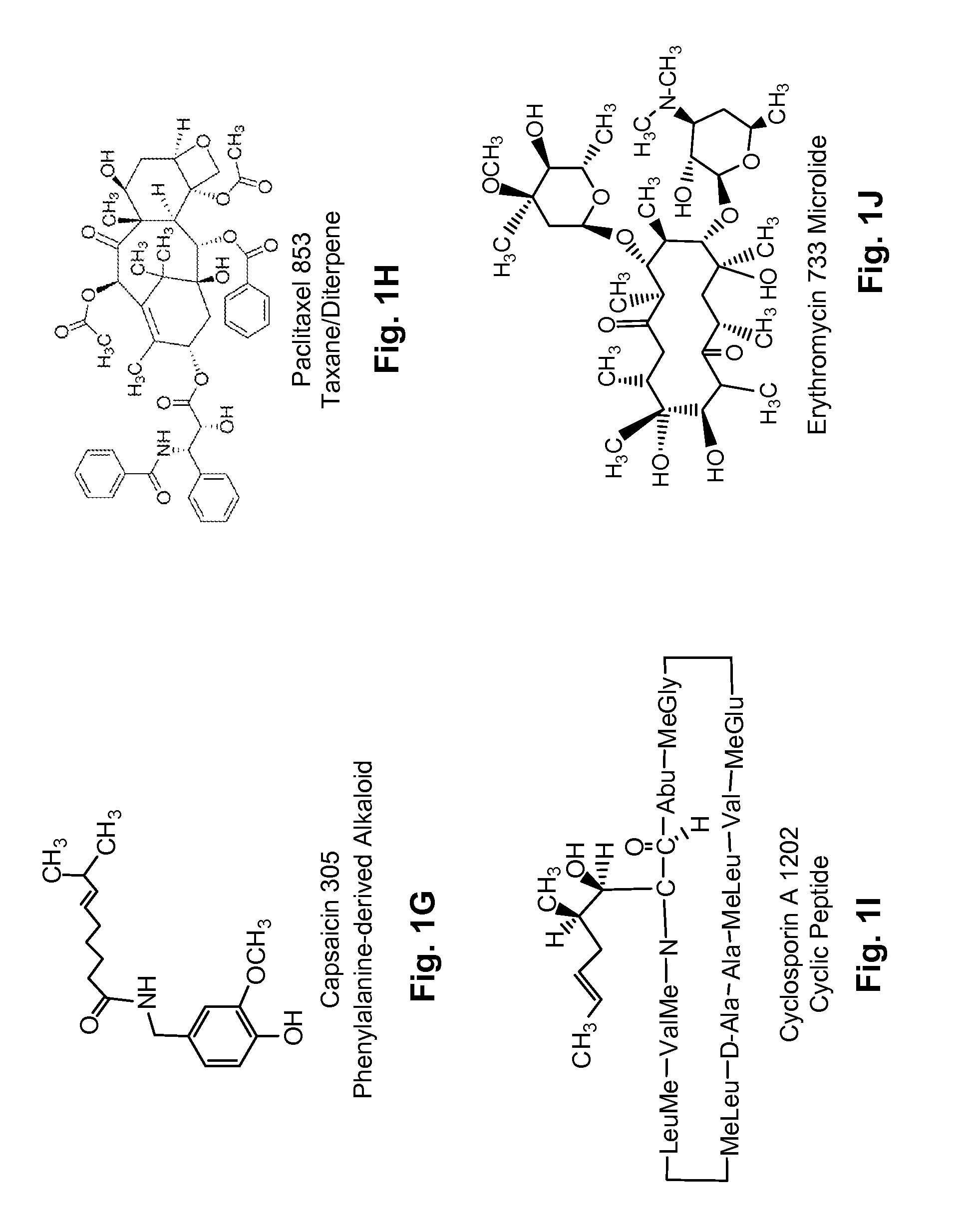
Several diterpene glycosides (e.g., rubusoside, rebaudioside, steviol monoside and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, and celecoxib. The use of the diterpene glycoside rubusoside increased solubility in all tested compounds. The diterpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries. Aqueous solutions by using rubusoside to increase the solubility of otherwise insoluble drugs will have several new routes of administration. In addition, aqueous solutions of therapeutic compounds with rubusoside were shown to retain the known pharmacological activity of the compounds.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Compositions containing itraconazole and their preparation methods
InactiveUS20040248901A1Improve bioavailabilityHigh dissolution ratePowder deliveryOrganic active ingredientsItraconazoleBioavailability
The present invention relates to compositions containing itraconazole with a greatly increased bioavailability and their preparation methods. More specifically, the present invention relates to compositions containing itraconazole which is a sparingly-soluble drug, fatty acid or fatty alcohol and surfactant and their preparation methods. Compositions according to the present invention act as Self-MicroEmulsifying Drug Delivery System(SMEDDS) wherein itraconazole which is a sparingly-soluble drug is dissolved and dispersed to form a mocoidal phase, and the mocoidal phase is dissolved in water to form microemulsion. And because of increased dissolution property and increased bioavailability, compositions according to the present invention show equal efficacy using less amount than commercial pharmaceutical preparations such as sporanox capsule and are cheaper rather than the commercial pharmaceutical preparations such as sporanox capsule.
Owner:WON JIN BIOPHARMA
Method of treatment of otitis externa
This invention relates to a method of treating otitis externa, and in particular otitis externa of fungal etiology, using topical medication, including antifungal agents such as itraconazole and miconazole, and optionally also including a second antifungal agent, which is formulated for topical application to the external ear canal, preferably as an ear drop in liquid form.
Owner:FERA DEV
Chirally pure isomers of itraconazole and inhibitors of lanosterol 14A-demethylase for use as angiogenesis inhibitors
Described herein are methods of inhibiting angiogenesis, and treating or preventing a disease or disorder (or symptoms thereof) associated with angiogenesis, wherein an anti-angiogenesis compound is administered to a subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Chirally pure isomers of itraconazole for use as angiogenesis inhibitors
ActiveUS20130102614A1Organic active ingredientsOrganic chemistryItraconazoleAngiogenesis growth factor
Described herein are methods of inhibiting angiogenesis, and treating and preventing disorders associated with angiogenesis by administering anti-angiogenesis compounds to a subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Itraconazole hydrochloride, oral solid combination and preparation method
InactiveCN1660841AImprove protectionSimple processOrganic active ingredientsAntimycoticsMethylene DichlorideItraconazole
An itraconazole hydrochloride and its orally taken solid composition are disclosed, which are prepared through reaction between itraconazole and hydrochloric acid to obtain itraconazole hydrochloride, and mixing it with cyclodextrin.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
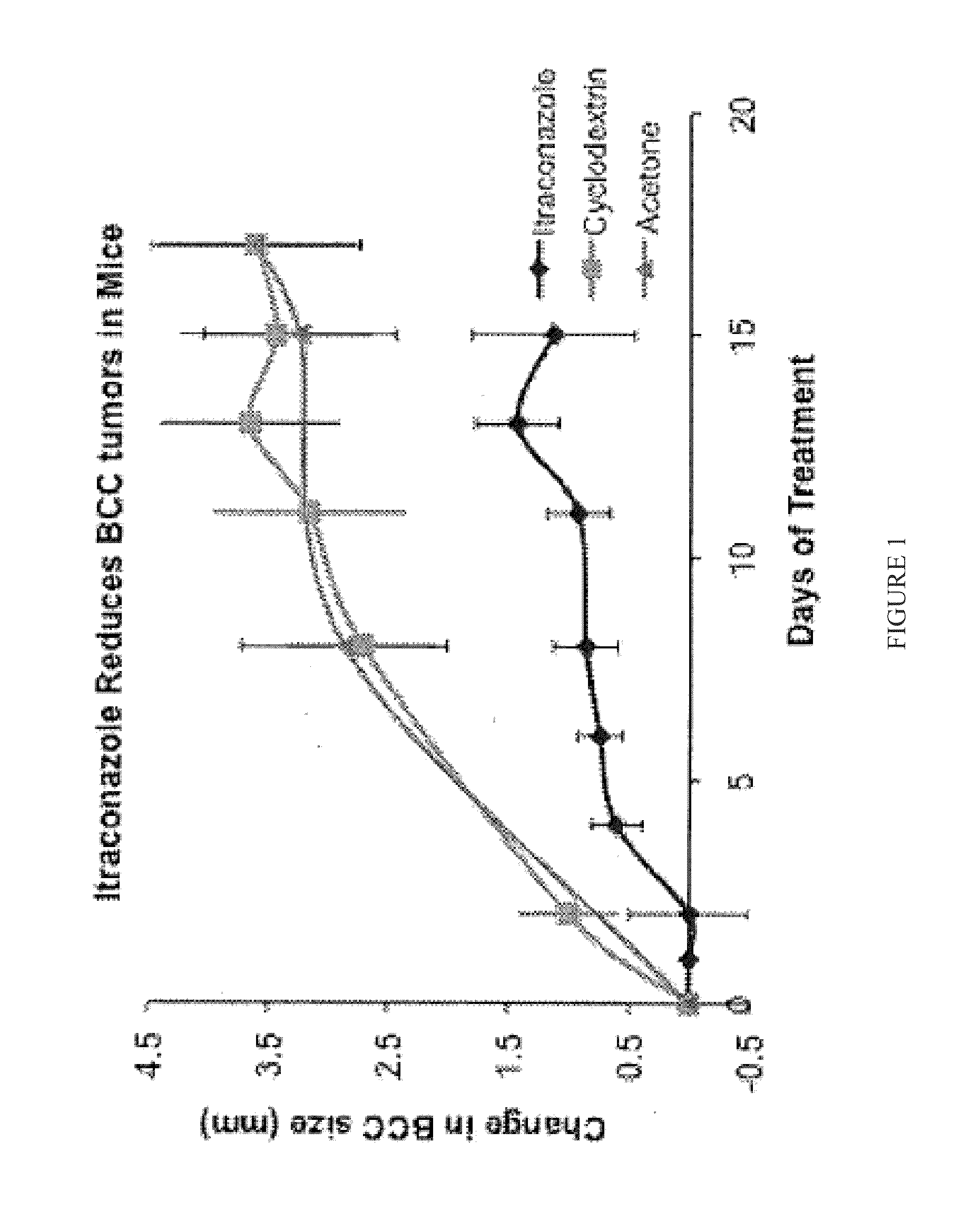
Topical Itraconazole Formulations and Uses Thereof
Methods of transdermally delivering a therapeutic amount of a triazole-triazolone compound are provided, e.g. for the prevention or treatment of basal cell carcinoma (BCC) in a subject. A therapeutic level of a triazole-triazolone compound such as itraconazole is delivered transdermally to a subject. Also provided are topical triazole-triazolone compositions that find use in practicing the subject methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Composition comprising itraconazole for oral administration
InactiveCN1853634AImprove bioavailabilityPowder deliveryOrganic active ingredientsOral medicationActive component
The present invention relates to an oral composition whose active component is itraconazole, and the composition contains 40-69.9wt% solid dispersion, 0.1-30wt% penetration inducer and 0.1-30wt% disintegrant , wherein the solid dispersion is prepared in an amorphous form by dispersing 1 part by weight of itraconazole in a mixture of 0.1 to 10 parts by weight of hydroxypropylmethylcellulose and 0.01 to 5 parts by weight of an absorption enhancer .
Owner:SAM CHUN DANG PHARM
Novel voriconazole broad-spectrum antifungal medicine compound, broad-spectrum antifungal medicine composition and application thereof
ActiveCN101575330ABroad and potent antifungal activityImprove mechanical propertiesOrganic active ingredientsAntimycoticsMonilinia laxaItraconazole
The invention relates to a novel broad-spectrum antifungal medicine compound, a broad-spectrum antifungal medicine composition, an application of the compound or the composition in the preparation of a broad-spectrum antifungal medicine, and an application of the compound or the composition in the preparation of a medicine used for treating severe invasive infection (comprising Candida krusei) caused by invasive aspergillosis and / or Fluconazole-resistant Monilia and / or severe infection caused by Scedosporium and fusarium. The novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition have extensive and strong antifungal activity and better dynamic property and safety. For deep mycotic infection, the novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition are better than the original voriconazole in the aspects of the antibiotic activity and the medicine resistance and is better than amphotericin B in the aspects of safety and effectiveness; and compared with the formerly applied voriconazole, Fluconazole, itraconazole and amphotericin B, the novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition have reinforced medicine effect, less adverse reaction and good safety.
Owner:LIVZON PHARM GRP INC
Itraconazole liposome and preparation process thereof
InactiveCN1969830AHighly toxicStrong side effectsOrganic active ingredientsAntimycoticsMANNITOL/SORBITOLPhosphate
The invention discloses an eosin liposome and making method, which comprises the following parts: 18-22mg eosin, 505-509mg lecithin, 90-95mg cholesterol, 73-77mg sodium deoxycholate, 505-509mg mannitol, 505-509mg lactose and 18-22mL phosphate buffer with pH value at 5.5-6.5. the making method comprises the following steps: (1) adding eosin, sodium deoxycholate, lecithin and cholesterol into chloroform; (2) decompressing; evaporating chloroform; (3) adding lactose and mannitol into phosphate buffer; (4) emulsifying phosphate buffer without fat film; (5) freezing; drying to obtain the product.
Owner:HARBIN MEDICAL UNIVERSITY
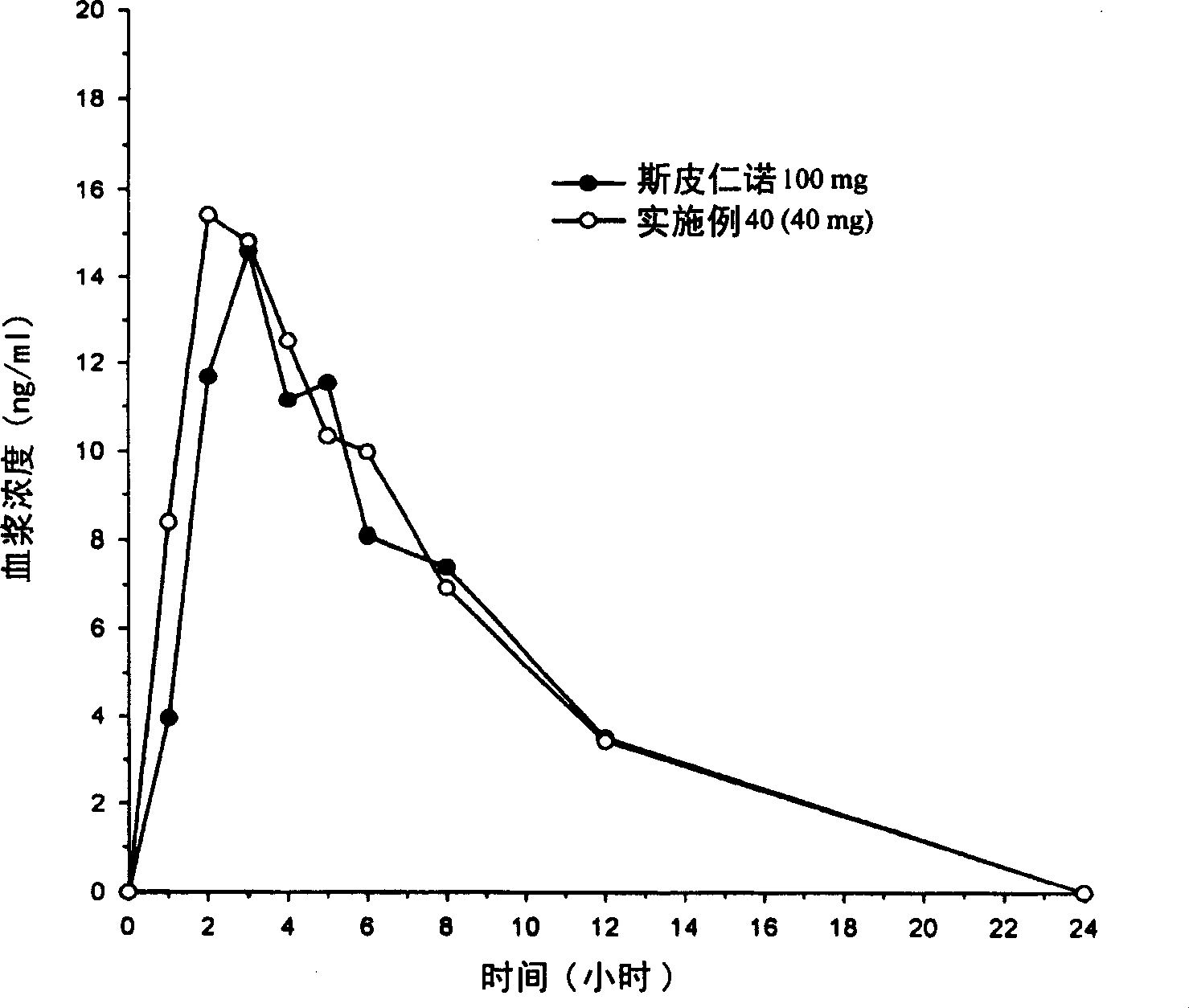
Itraconazole compositions and dosage forms, and methods of using the same
The disclosure relates to, among other things, pharmaceutical compositions, such as solid oral dosage forms, comprising itraconazole, methods of making the compositions, and methods of using the same for treating disorders including, but not limited to, fungal infections.
Owner:MAYNE PHARMA INT
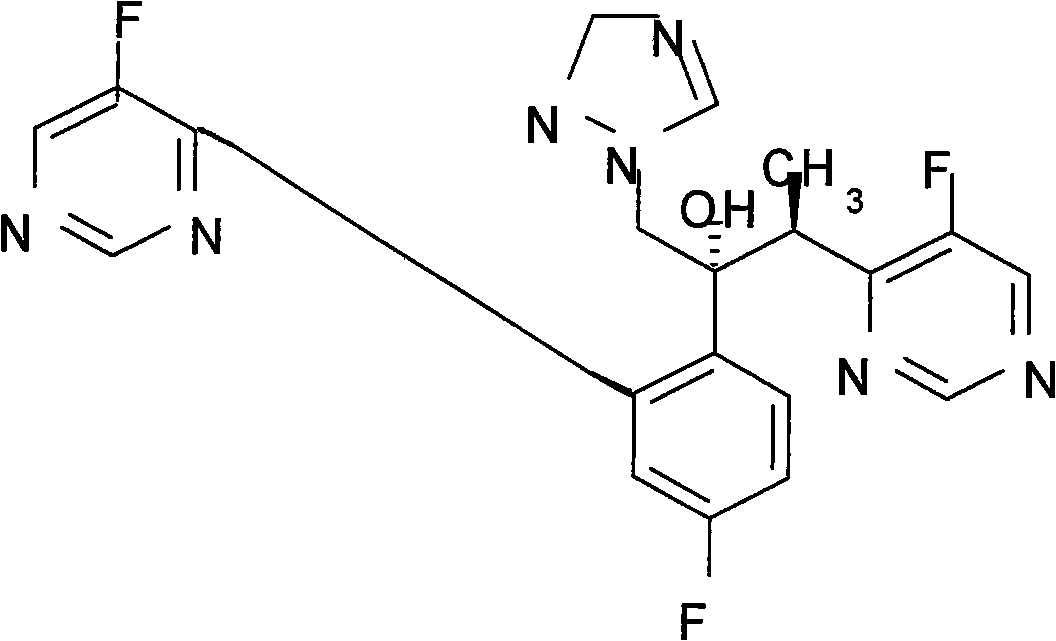
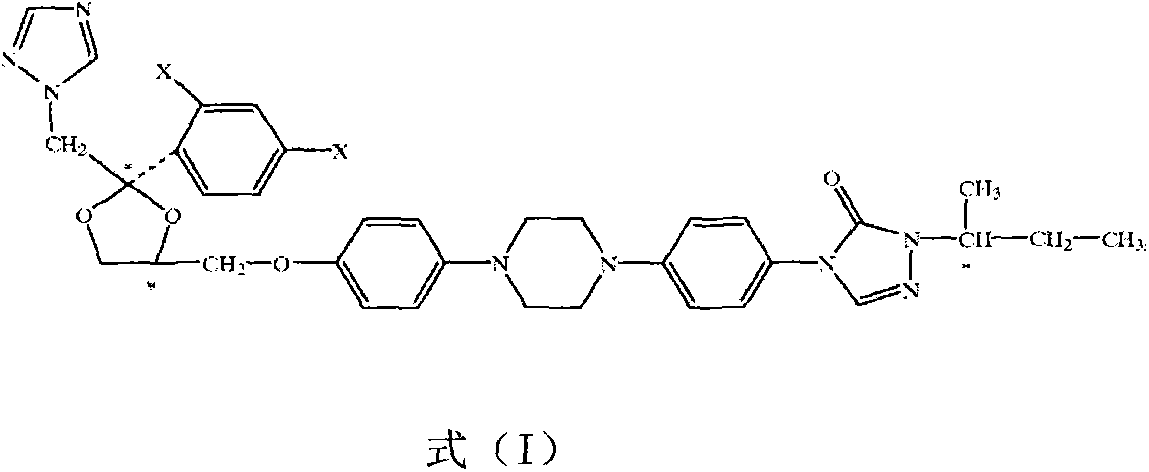
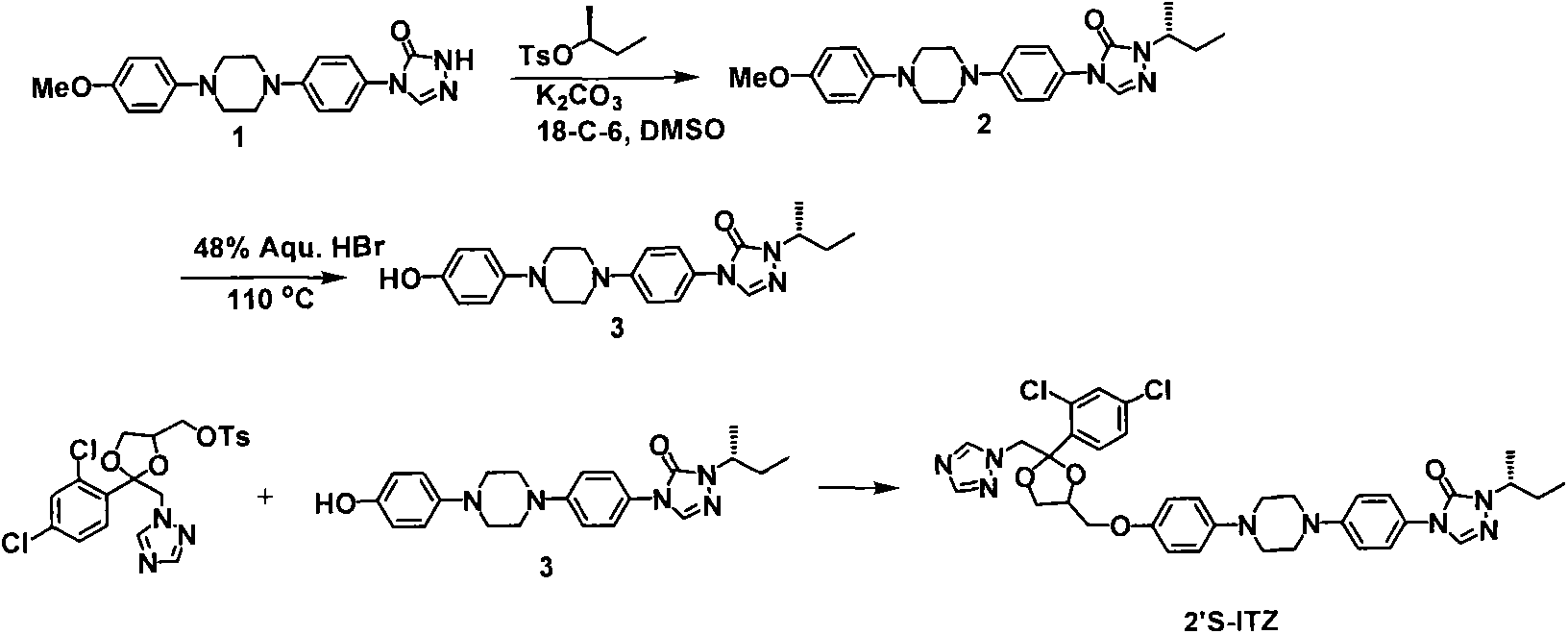
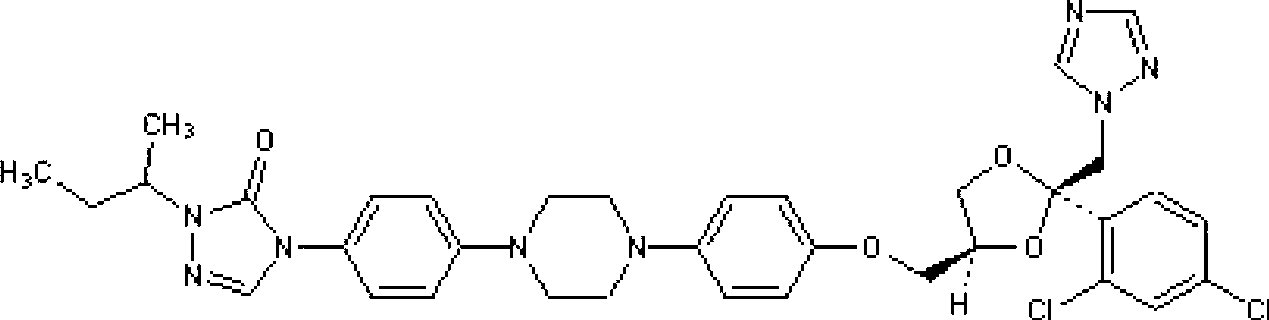
Optically pure itraconazole key intermediate, synthetic method thereof, and method for synthesizing optically pure itraconazole from the intermediate
The invention disclsoes an optically pure itraconazole key intermediate and synthetic method thereof, and a method for synthesizing the optically pure itraconazole from the intermediate. The method of the invention uses 1-(2,4-dichlorobenzene)-2-(1-methylene-1,2,4-triazole)-1-ketone for preparing the optically pure itraconazole key intermediate, and the optically pure itraconazole key intermediate is used for the preparation of optically pure itraconazole. The method uses easily available raw materials, not only reduces the production cost, but also obtains the product with high purity; through the control of the optical purity of the key intermediate compound VII, the optical purity of the target product itraconazole can be effectively controlled; therefore, the invention has with industrial value.
Owner:广州力鑫生物科技有限公司
Itraconazole gel for dogs and preparation method thereof
InactiveCN101889973AGood curative effectSmall side effectsOrganic active ingredientsAntimycoticsClinical efficacyItraconazole
The invention relates to an itraconazole gel for dogs and a preparation method thereof, belonging to animal medicines. The gel consists of itraconazole, hydroxypropyl-beta-cyclodextrin, carbomer 940, salicylic acid, polyethylene glycol-400, ethylparaben, absolute ethyl alcohol, concentrated hydrochloric acid, distilled water and triethanolamine. The preparation method comprises the following steps: preparing an itraconazole inclusion compound solution by a hydroxypropyl-beta-cyclodextrin inclusion technology; preparing the gel substrate from the carbomer 940, the salicylic acid, the polyethylene glycol-400, the ethylparaben and the distilled water; mixing and stirring the inclusion compound solution and the gel substrate evenly; regulating the pH value; and carrying out circulating steam sterilization to obtain the itraconazole gel. The finished product of the gel prepared by the invention is faint yellow, transparent and even, and has stable characters and good medicine penetrating effect, the content meets a criterion, the content of medicine in skin is higher, the bacteriostasis effect and the clinic curative effect are superior to that of general external antifungal agents, and the itraconazole gel has the characteristics of no stimulation, no toxicity, weak sensitization, safety and high efficiency.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Itraconazole isomer and medical application thereof
The invention provides a novel itraconazole isomer composition and a method for increasing the therapeutic index of the novel itraconazole isomer composition. The itraconazole isomer composition can be used for improving the selectivity to fungi and endothelial cells and also lowering the toxicity of liver cells. The itraconazole isomer composition provided by the invention is a drug with potential for treating fungi and angiogenesis related diseases, and meanwhile, the side effects caused by taking itraconazole are avoided.
Owner:广州安赛莱生物科技有限公司 +1
Itraconazole soft capsule, and its prepn. method
InactiveCN1559410ARapid dissolutionQuality improvementOrganic active ingredientsAntimycoticsVegetable oilPreservative
A softcapsule of itracnoazole is prepared through dissolving gelatin in water, adding glycerineand antiseptic, vacuum degrassing, adding the suspending aid to vegetable oil, mixing with itraconazole, adding antioxidizing agent, and die pressing.
Owner:SHENYANG PHARMA UNIVERSITY
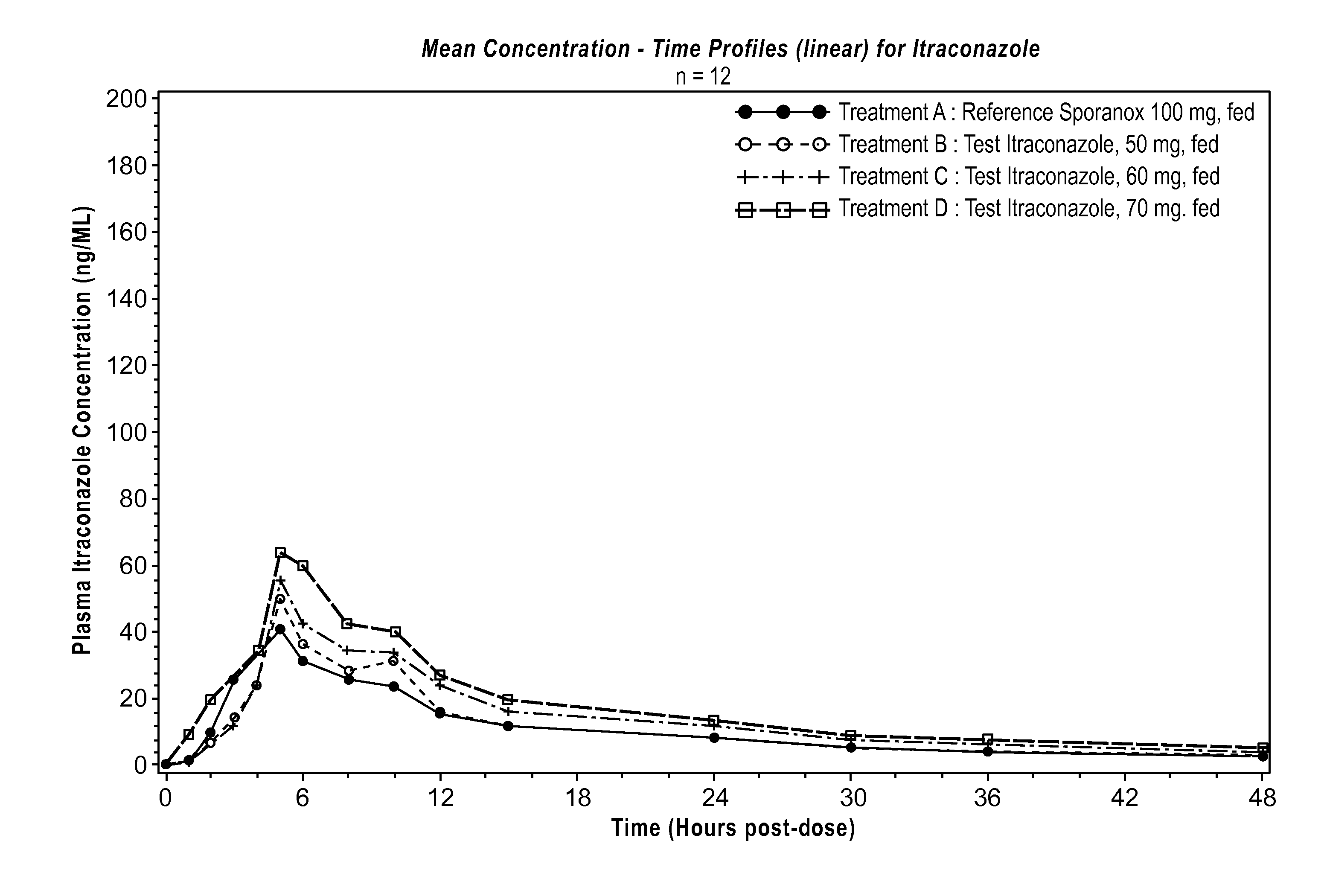
Compsns. contg. itraconazole with improved bioavailability and narrow intra-and inter-individual variation of its absorption
InactiveCN1398184ALarge specific surface areaNot hydrophobicPowder deliveryOrganic active ingredientsNormal peopleItraconazole
The present invention relates to agent containing itraconazole with both improved bioavailability, due to higher water-solubility and impressively reduced difference of pH-dependent solubility, and narrow intra- and inter-individual variation of its absorption- and a manufacturing method thereof. Agent containing itraconazole of the present invention consists of itraconazole, water-soluble macromolecule of 10-100 % w / w to itracotazole, solubilizing agent of 0.1-100 % w / w to itraconazole and pharmaceutically allowed additives. Agent containing itraconazole of the present invention minimizes absorption variation by dozing time after food intake as well as is available for adults with hypoacidity, AIDS patients and normal people. In addition, the manufacturing method introduces the elementary process, the spray drying, thereby control of physical properties of particles containing drug is easier and economy / production is improved.
Owner:DONG A PHARMA
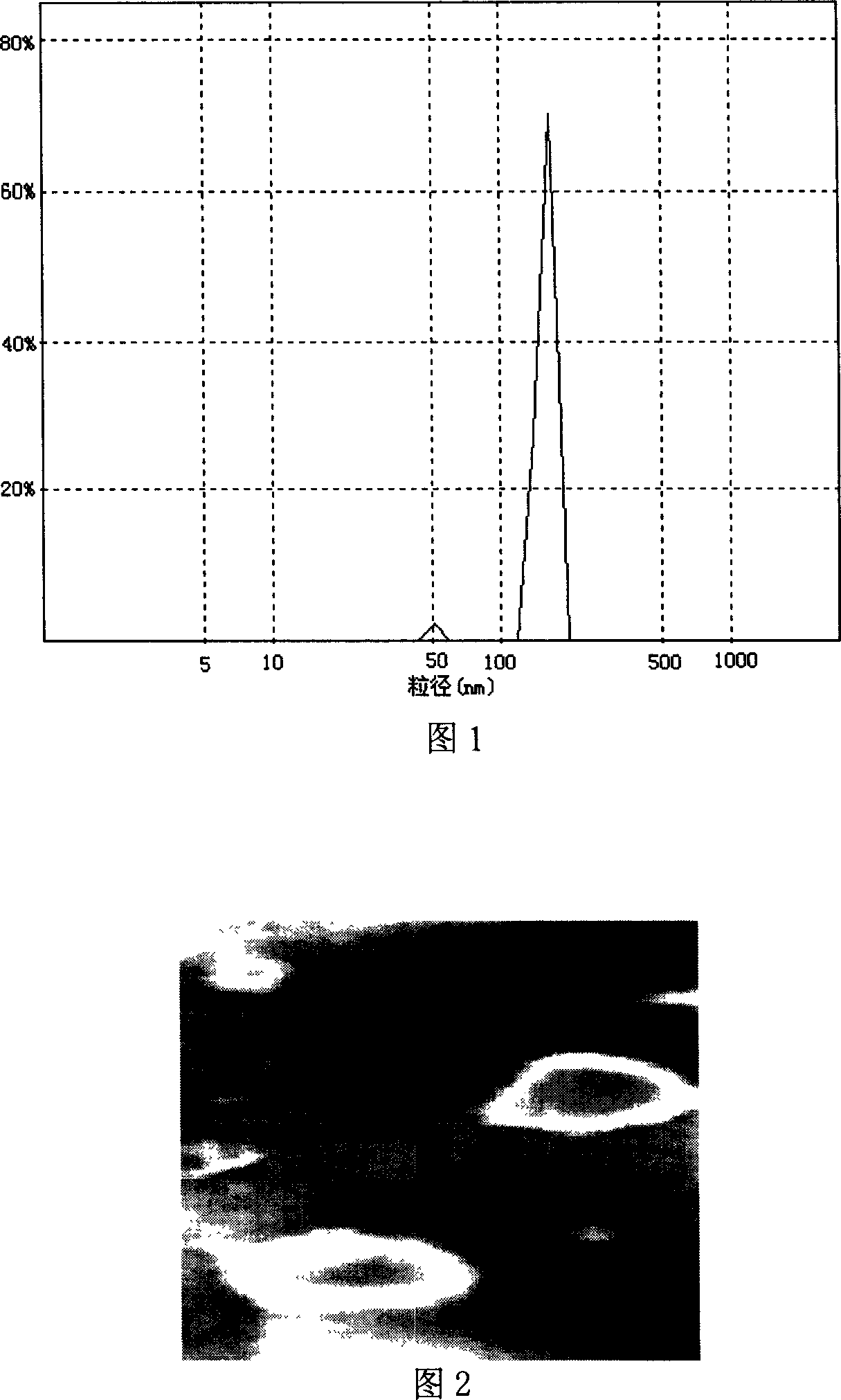
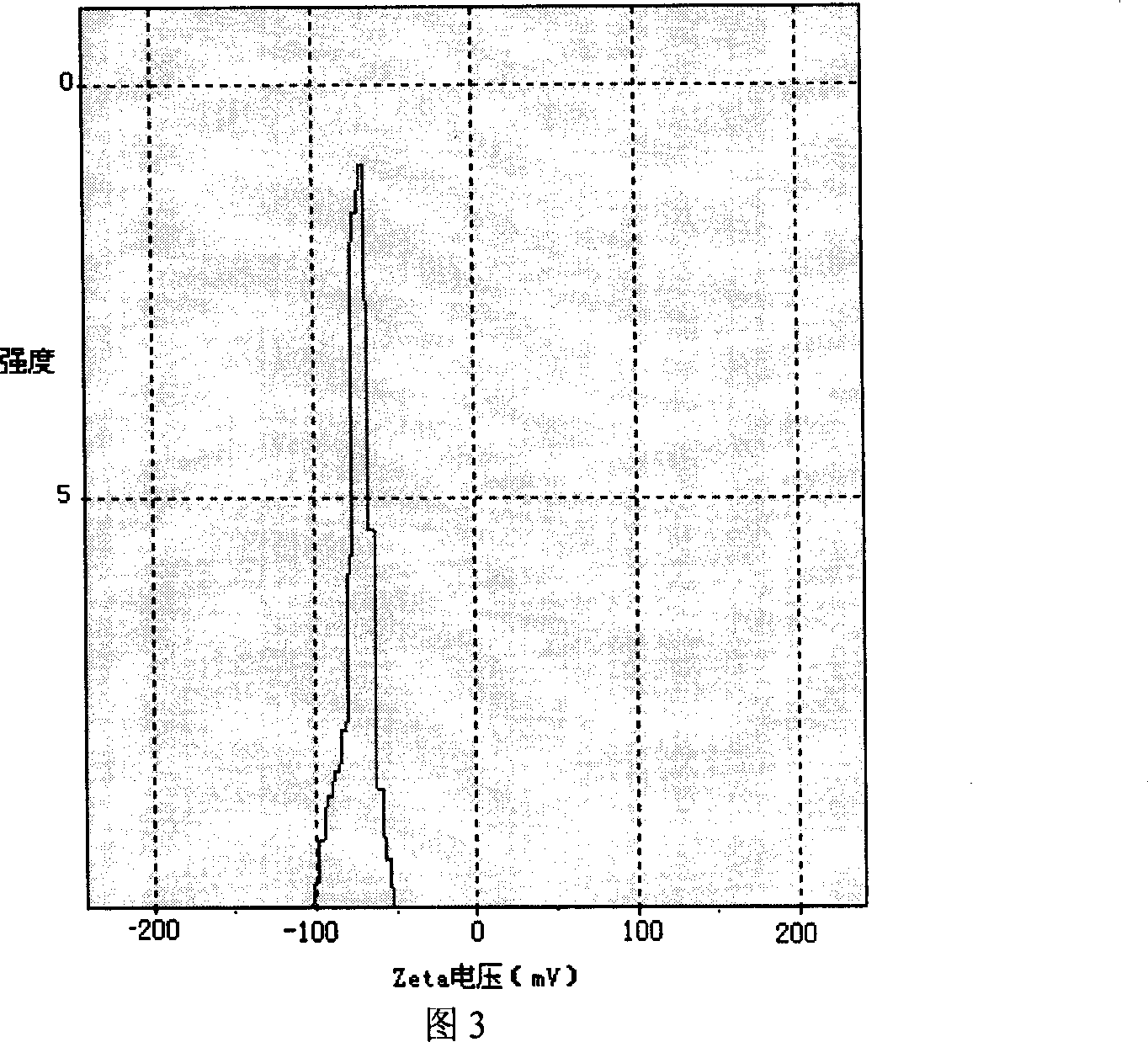
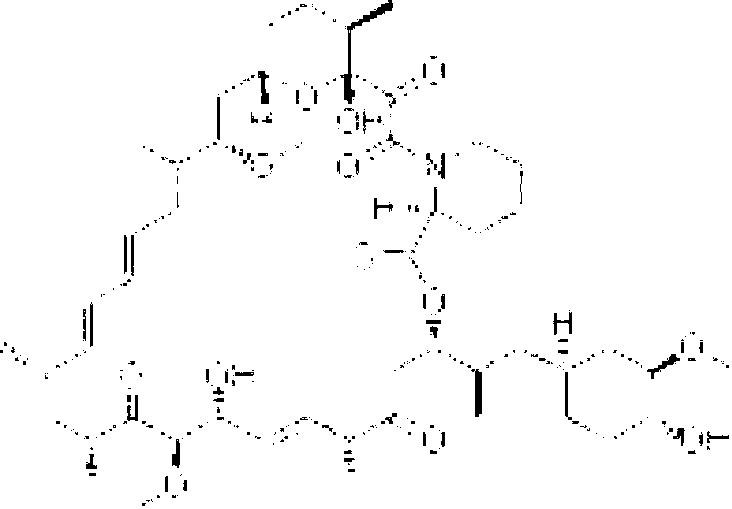
Itraconazole nanometer suspensions lyophilized compound and preparing , using method thereof
InactiveCN101199530ASmall particle sizeHigh drug loadingOrganic active ingredientsPowder deliveryHemolysisSerum protein albumin
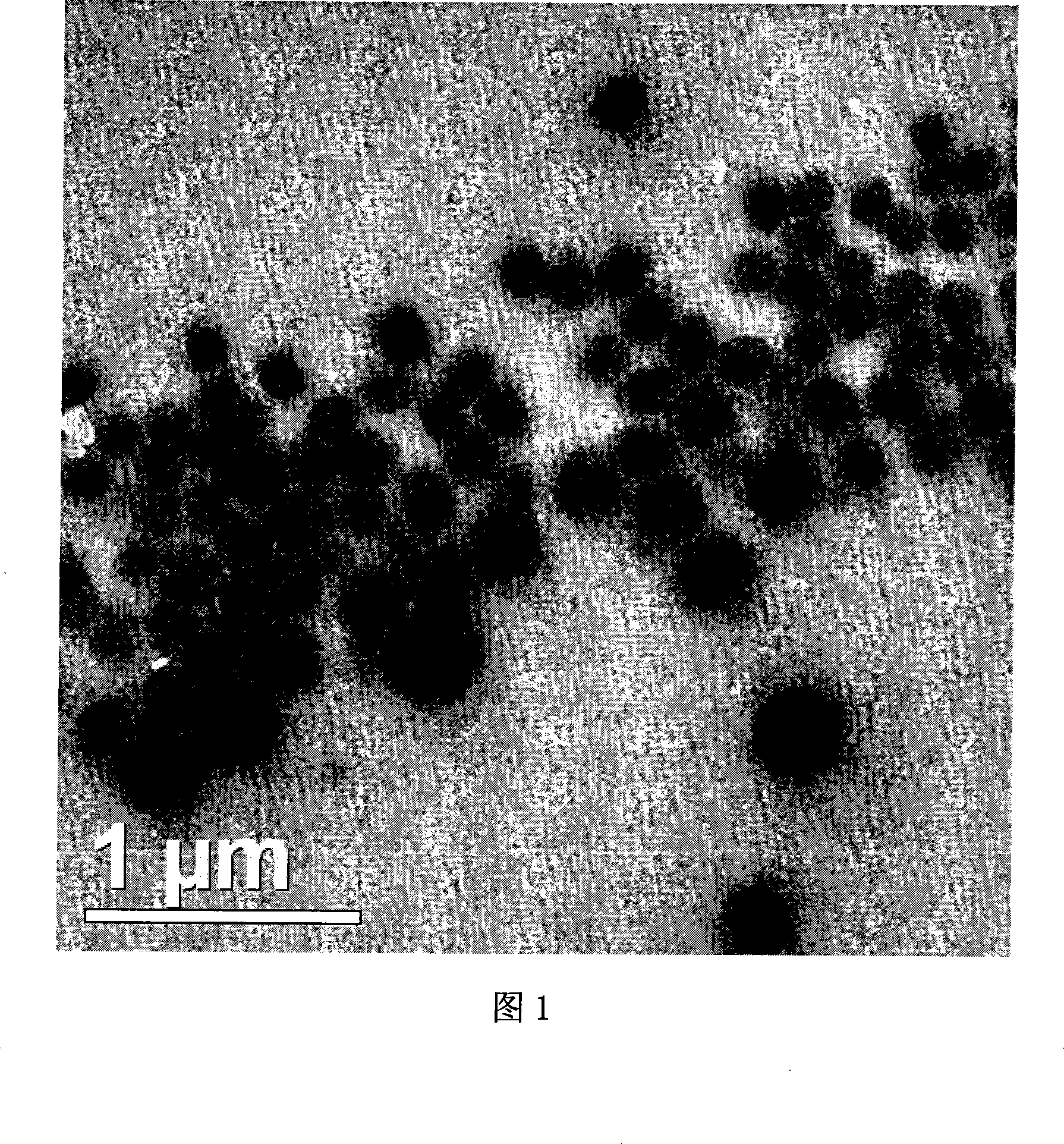
The invention discloses a freeze-drying combination of itraconazole nanosuspension for injection and preparation and using methods of the nanosuspension. The invention selects serum albumin of the human as main adjunct; itraconazole nano-sized granulation can be successfully realized by adopting the drying technology and freeze drying technology in combined liquid of high pressure homogenization nanometer emulsification technology; and the invention can be filtered to sterilize and can be stored in the form of freeze-dried powder. The itraconazole nanosuspension is prepared in acceptable waterborne diluent in pharmacy, and then the average particle size of the itraconazole nanosuspension is less than 0.3 microns after reconstruction, so the itraconazole nanosuspension can be made into nanosuspension for intravenous injection. The nanosuspension is composed of the following components : the ratio between the itraconazole and albumin is 1 : 200 to 10 : 1; and the nanosuspension also comprises surfactant, stent agent, osmo-regulator and other injection additives, etc. The nanosuspension has the advantages of drug loading, safety, low toxicity, high stability and good warming promotion without hemolysis or stimulation on vein vascular. And the preparation method is simple and easy, and thus the product forming can be realized easily.
Owner:CHINA PHARM UNIV
Itraconazole inhalation powder aerosol and its preparation method
ActiveCN104398497AImprove physical stabilityGood fluidity of powderOrganic active ingredientsAntimycoticsSide effectItraconazole
The invention discloses an itraconazole inhalation powder aerosol and its preparation method. The inhalation powder aerosol is prepared by using itraconazole and 20-80mass% of a carrier, and the carrier is at least one of lactose, mannitol, inulin and amino acids. The method comprises the following steps: mixing itraconazole with the carrier, adding the obtained mixture into a double screw hot melt extruder, setting the extrusion temperature at 150-170DEG C, starting screws after the preset temperature is reached, and carrying out strip extrusion under a screw rotating speed of 150-200r / min, cooling obtained strips in a dryer to room temperature, crushing by using a micro pulverization machine, and micronizing by using an airflow crusher to obtain the above powder. The inhalation powder aerosol has the advantages of good physical stability, good powder fluidity, high deposition amount at effective positions, high dissolution rate, fast dissolution speed, small drug dose, and low incidence rate of toxic side effects; and the method has the advantages of simple technology, no organic solvents, antiseptics or other impurities in the process, good reappearance and easy industrial production.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Compositions containing itraconazole and their preparation methods
InactiveCN1547467AImprove bioavailabilityPowder deliveryOrganic active ingredientsActive agentItraconazole
The present invention relates to compositions containing itraconazole with a greatly increased bioavailability and their preparation methods. More specifically, the present invention relates to compositions containing itraconazole which is a sparingly-soluble drug, fatty acid or fatty alcohol and surfactant and their preparation methods. Compositions according to the present invention act as Self-MicroEmulsifying Drug Delivery System(SMEDDS) wherein itraconazole which is a sparingly-soluble drug is dissolved and dispersed to form a mocoidal phase, and the mocoidal phase is dissolved in water to form microemulsion. And because of increased dissolution property and increased bioavailability, compositions according to the present invention show equal efficacy using less amount than commercial pharmaceutical preparations such as sporanox capsule and are cheaper rather than the commercial pharmaceutical preparations such as sporanox capsule.
Owner:WON JIN BIOPHARMA +1
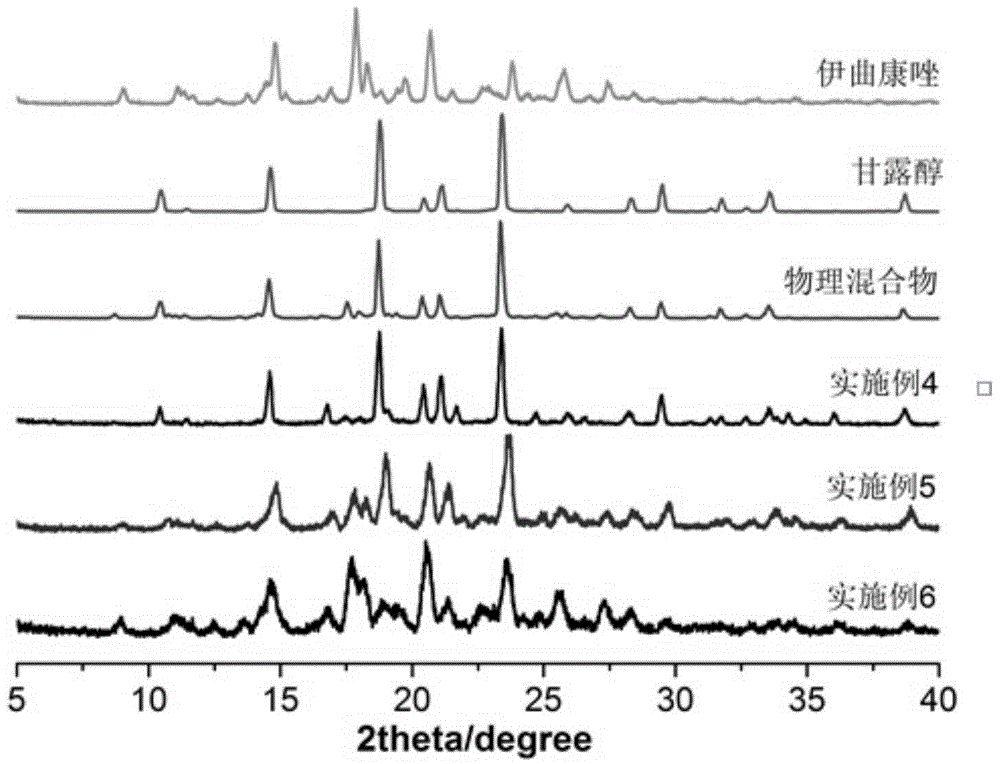
Preparation of submicron sized particles with polymorph control and new polymorph of itraconazole
The present invention provides a method of preparing particles with polymorph and size control of a pharmaceutical compound, the method including the steps of: (1) providing pharmaceutical compound in a first phase; (2) seeding the compound; (3) causing a phase change in the pharmaceutical compound to a second phase of a desired polymorphic form; and (4) wherein the mean particle size of the particles is less than 7 mum. The present invention further provides a polymorphic form of itraconazole.
Owner:BAXTER INT INC
Polymorphic form of itraconazole
Owner:BAXTER INT INC
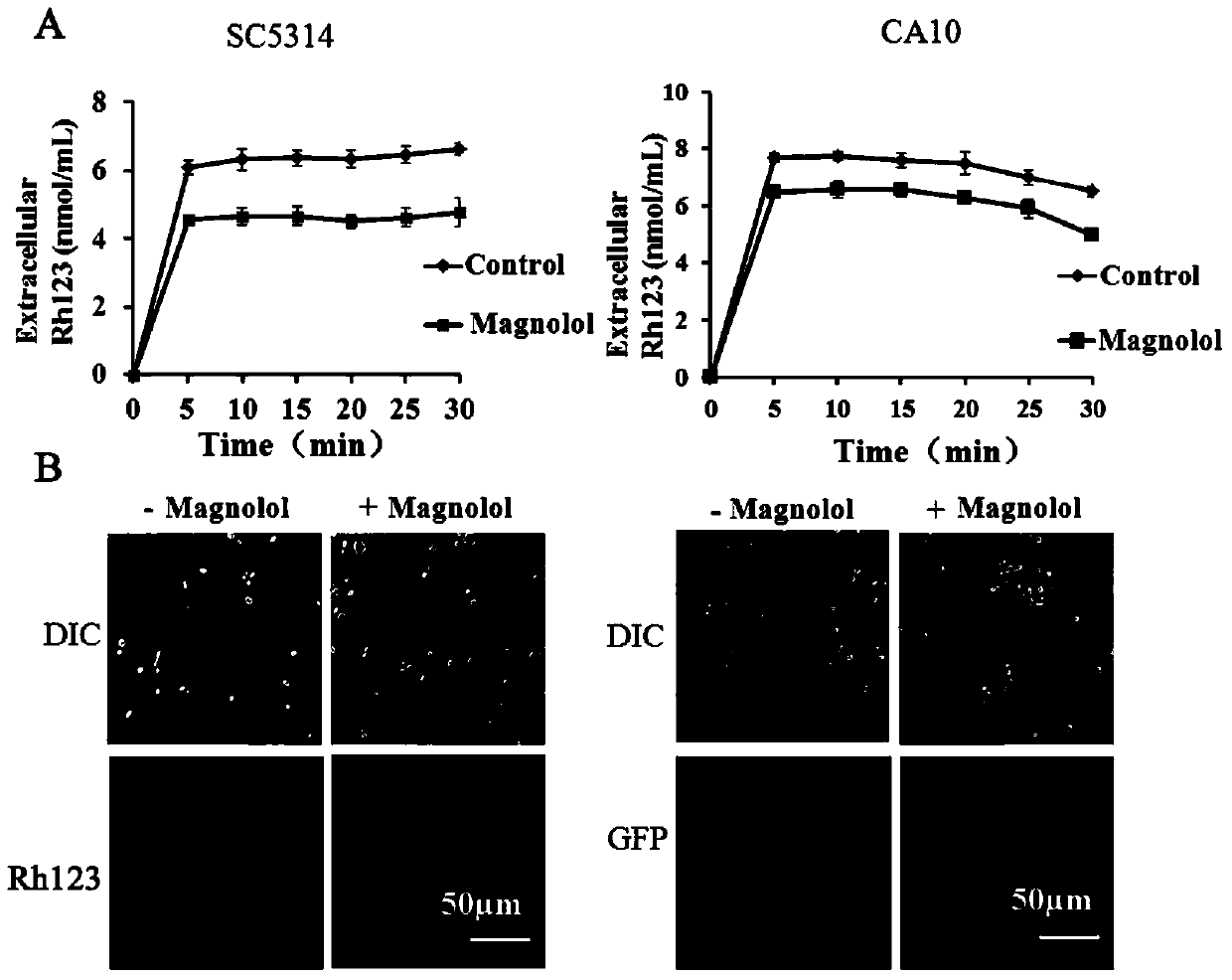
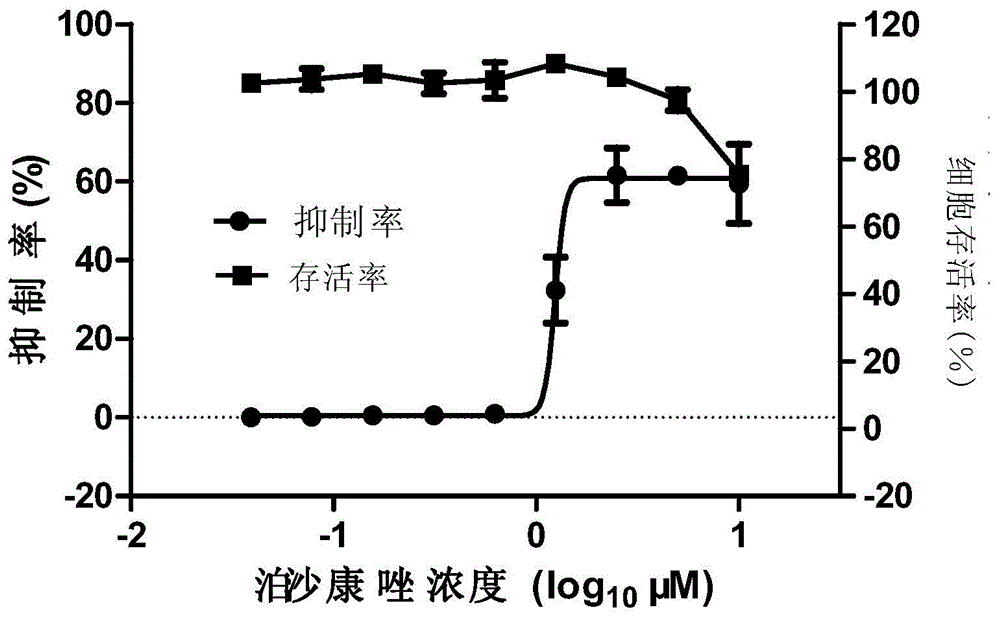
Application of magnolol and azole medicines to preparation of antifungal combined medicines
InactiveCN104188962AGood curative effectInhibit synthesisAntimycoticsHydroxy compound active ingredientsHonokiolMedicine
The invention provides application of magnolol and azole medicines to preparation of antifungal combined medicines. The application indicates that azole medicines such as fluconazole, ketoconazole, itraconazole, miconazole and voriconazole are used together with magnolol to generate a synergetic antifungal effect; the magnolol serving as an efflux pump Cdrlp substrate is utilized to compete with the azole medicines for an efflux system, so that the concentration of efflux medicines can be reduced, and the curative effect of medicines can be improved. Furthermore, magnolol acts on the synthetic pathway of ergosterol and is combined with azole medicines to form a double-edged sword to inhibit synthesis of ergosterol together.
Owner:SOUTHEAST UNIV
Itraconazole emulsion for injection and its prepn
InactiveCN1739519ADifficult to dispersePromote absorptionOrganic active ingredientsAntimycoticsSide effectEmulsion
The present invention relates to medicine technology, and is one kind of Itraconazole emulsion for injection and its preparation process. Each 1000 ml of the Itraconazole emulsion for injection contains solid Itraconazole compound 2-80 g, vegetable oil for injection 50-300 g, emulsifier 5-50 g, and isosmotic regulator 15-100 g, except pH regulator and water. By means of emulsifying technology and with oil-water interface film in carrying medicine, the present invention solves the problem of dispersing water and oil insoluble Itraconazole. Compared with intravenous injection and oral liquid of Itraconazole, the present invention has less negative effect, less toxic side effect, high bioavailability, low preparation cost and taking convenience.
Owner:SHENYANG PHARMA UNIVERSITY
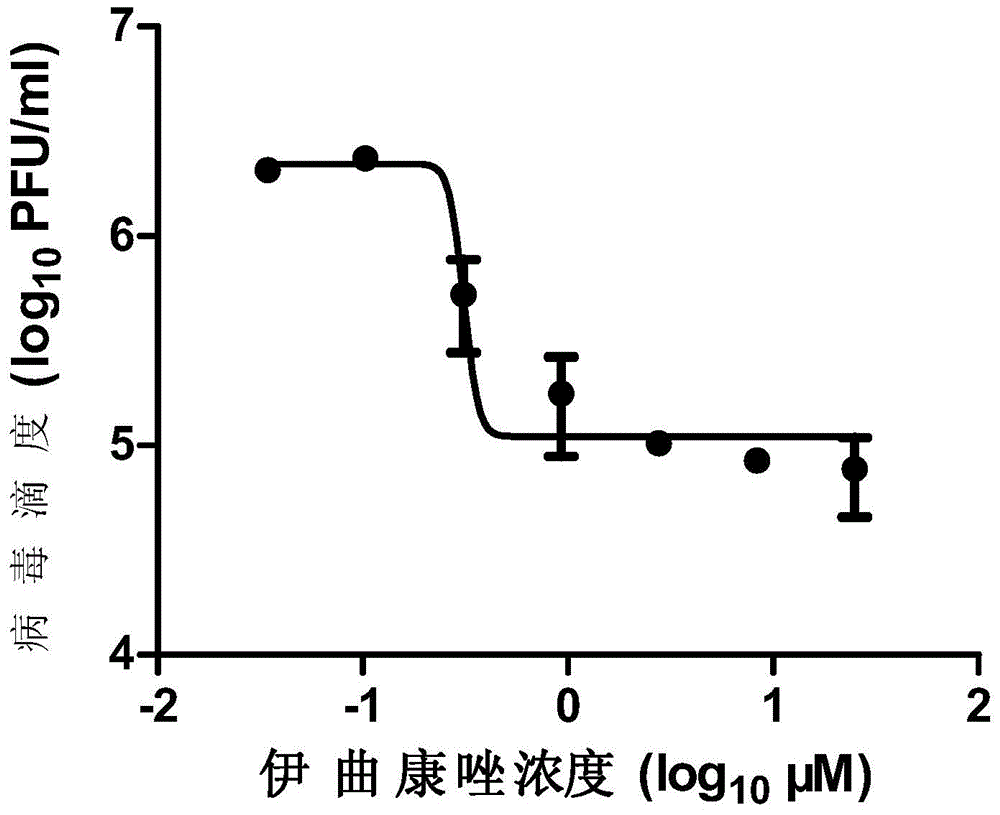
Micromolecule compound inhibitor for enterovirus and application of inhibitor
The invention provides a micromolecule compound inhibitor for enterovirus and an application of the inhibitor. Specifically, the invention provides an application of itraconazole, an itraconazole analogue, or pharmaceutically acceptable salt of itraconazole or the itraconazole analogue to preparation of a reagent, which is used for inhibiting growth or reproduction of enterovirus and / or for inhibiting synthesis of enterovirus RNA. The invention also provides an inhibitor and medicine composition containing itraconazole or the itraconazole analogue for enterovirus, and provides a method for in-vitro non-therapeutically inhibiting growth of enterovirus or killing enterovirus. Experimental results show that itraconazole and the itraconazole analogue has an excellent inhibition effect on multiple kinds of enterovirus.
Owner:中国科学院上海免疫与感染研究所
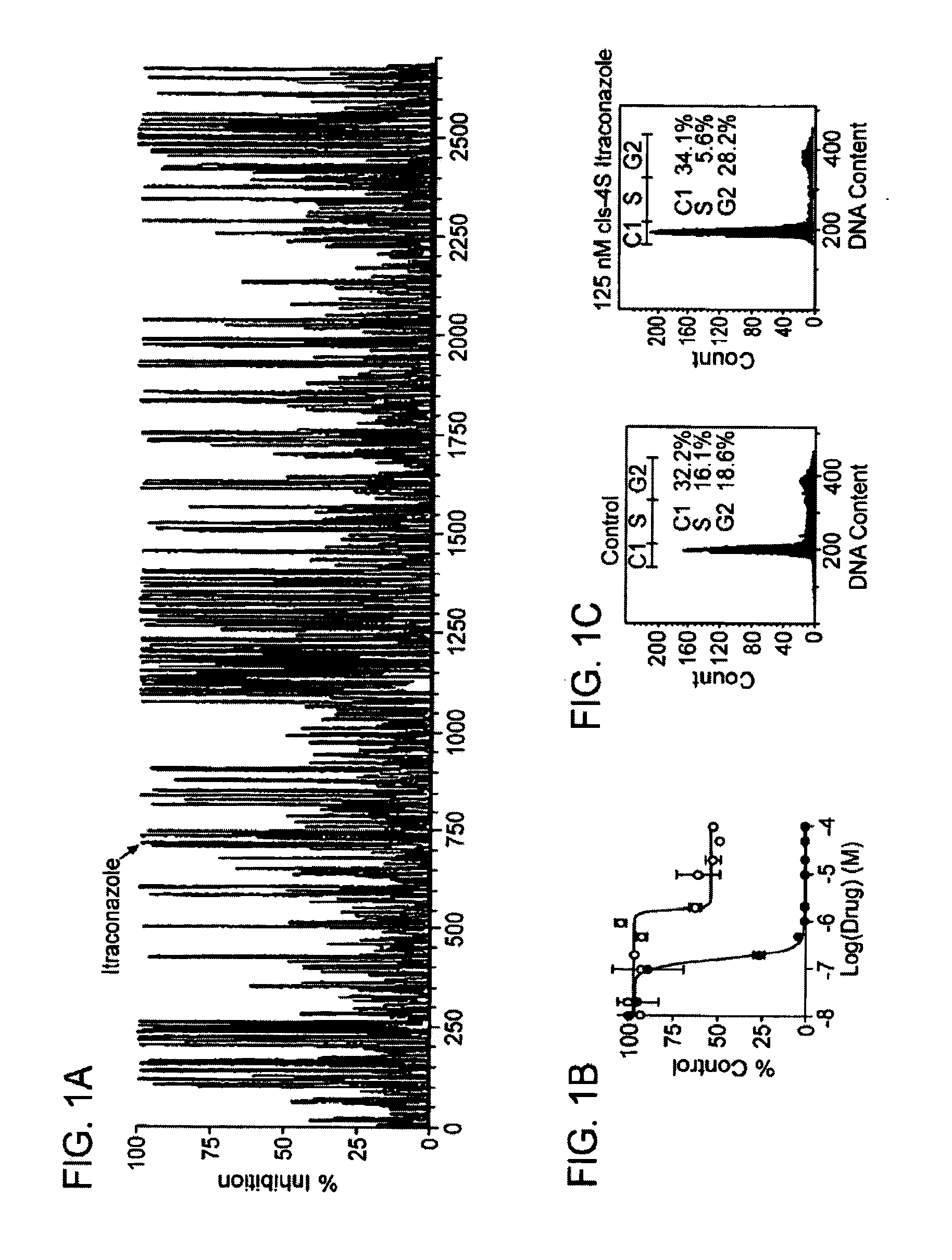
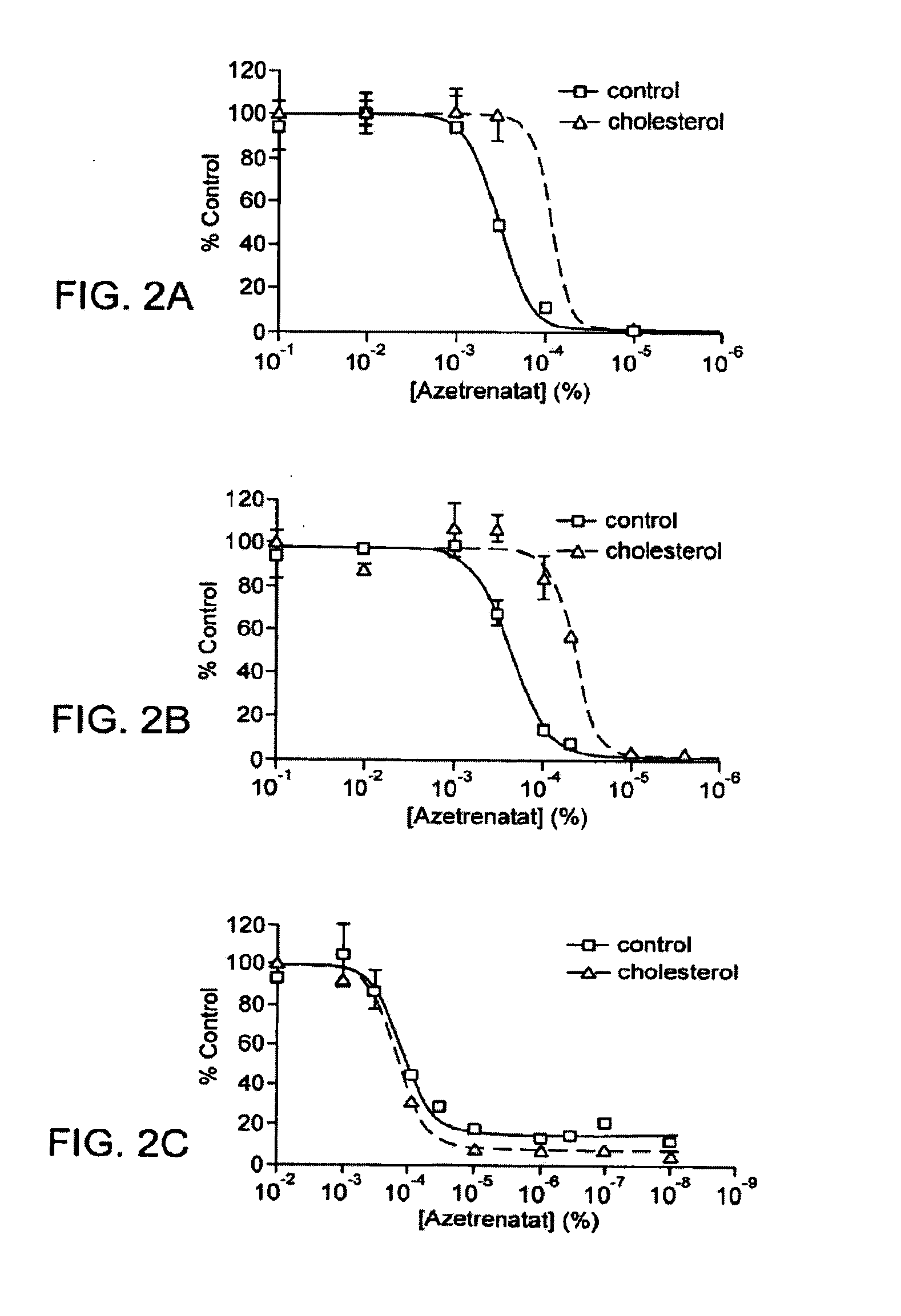
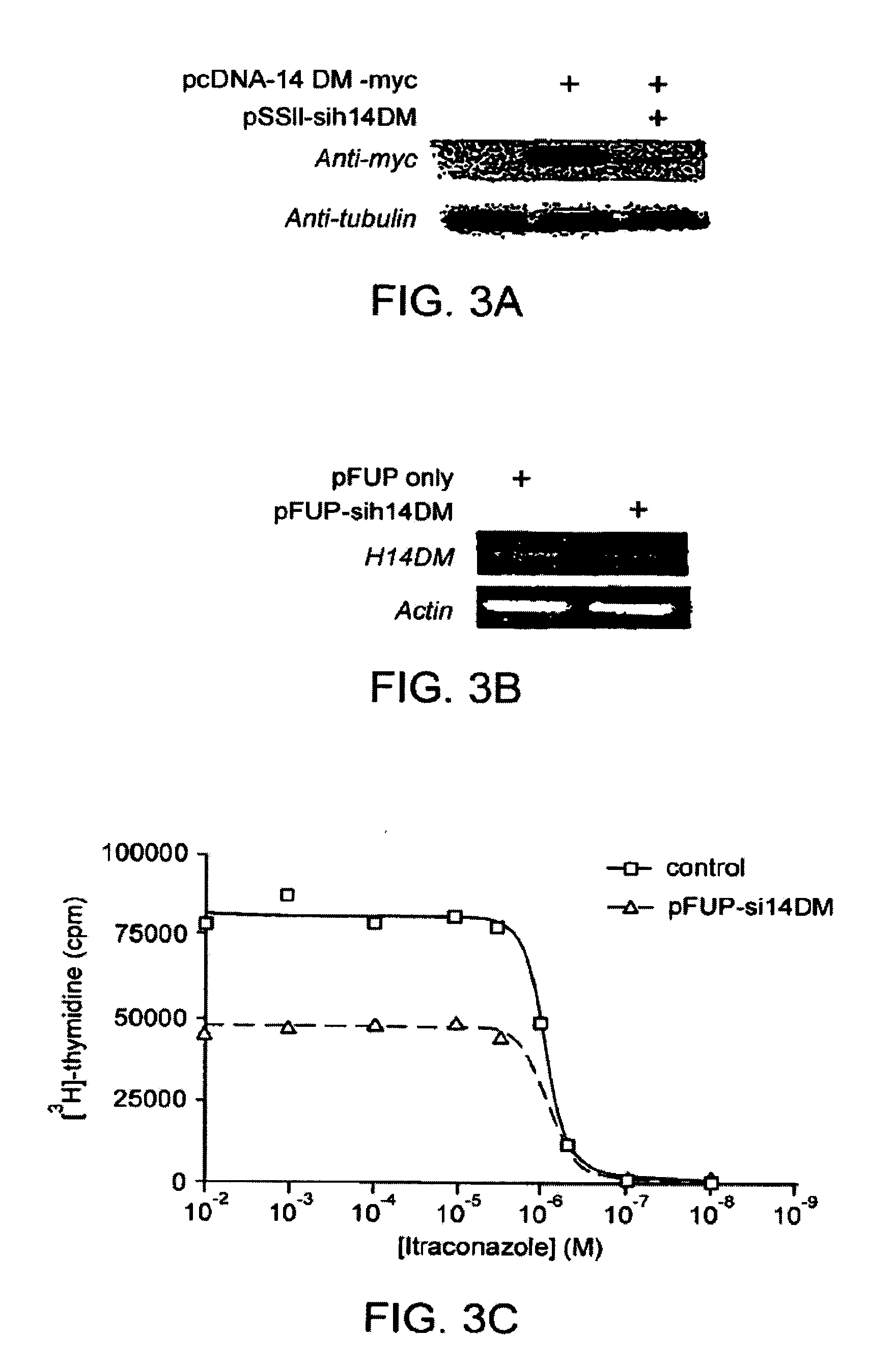
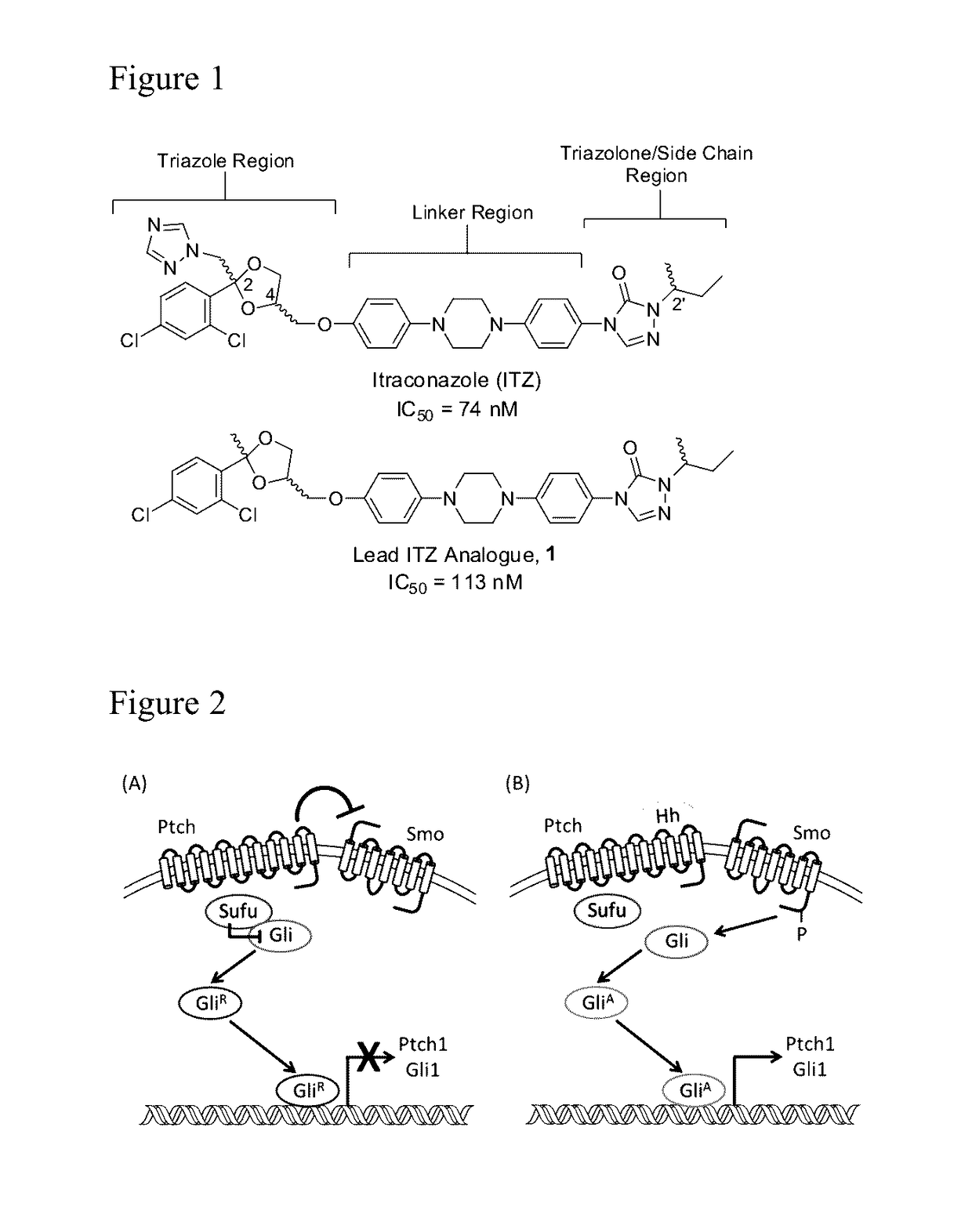
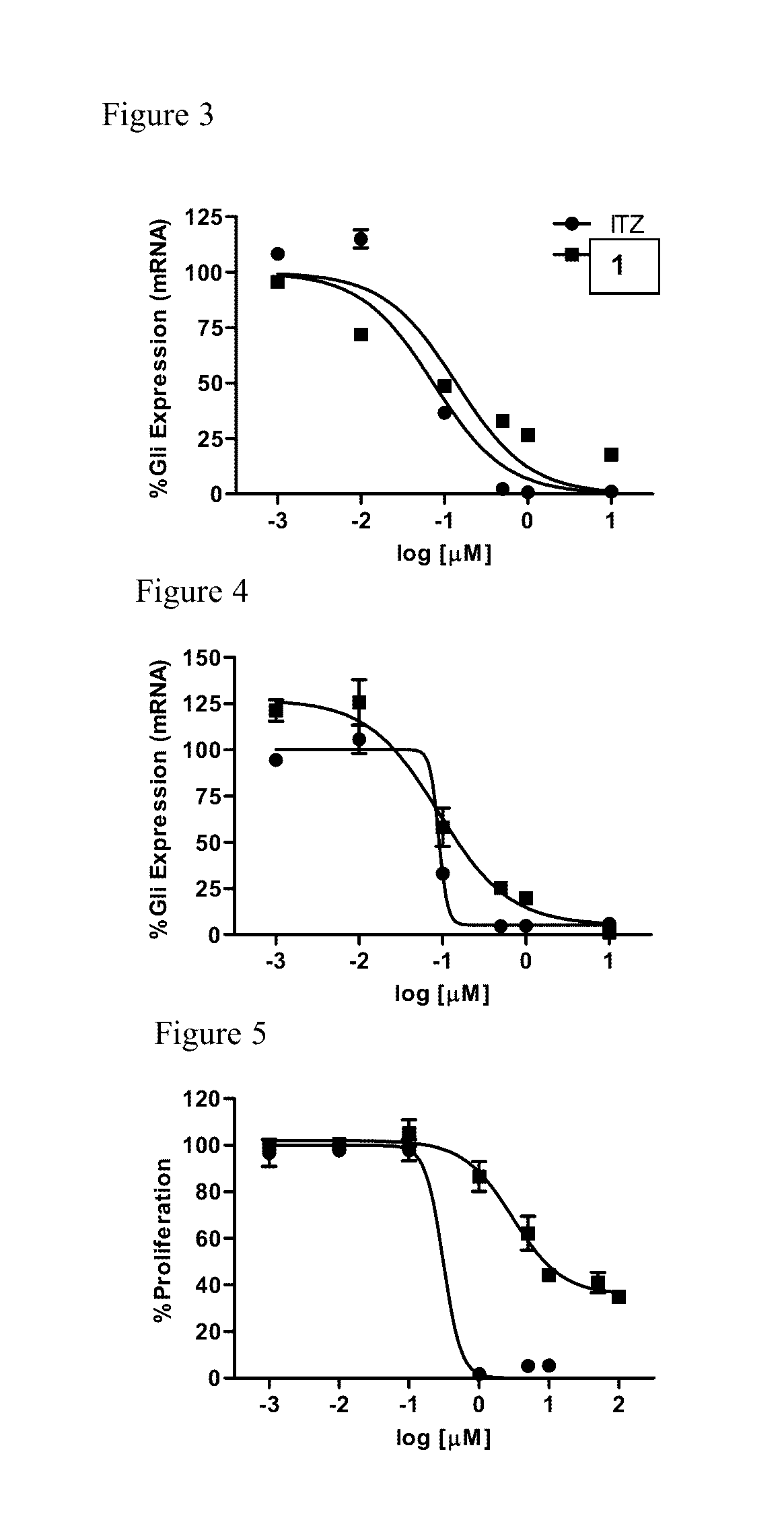
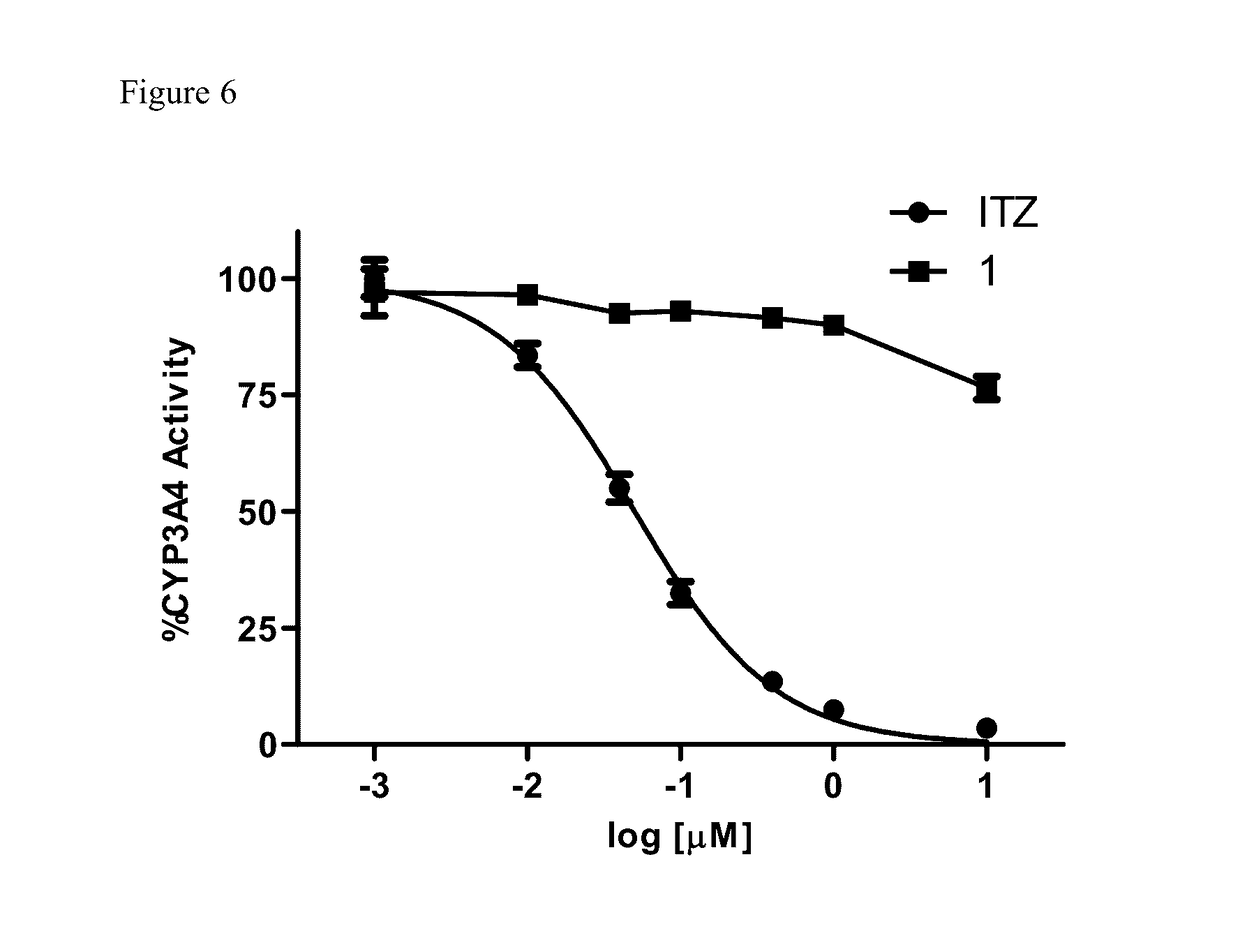
Itraconazole analogues and methods of use thereof
ActiveUS9650365B2Organic active ingredientsOrganic chemistryHedgehog signaling pathwayAngiogenesis growth factor
Disclosed herein are analogs of itraconazole that are both angiogenesis and hedgehog signaling pathway inhibitors. The compounds are expected to be useful in the treatment of cancer, particularly cancers that are dependent upon the hedgehog signaling pathway such as basal cell carcinoma and medulloblastoma.
Owner:UNIV OF CONNECTICUT
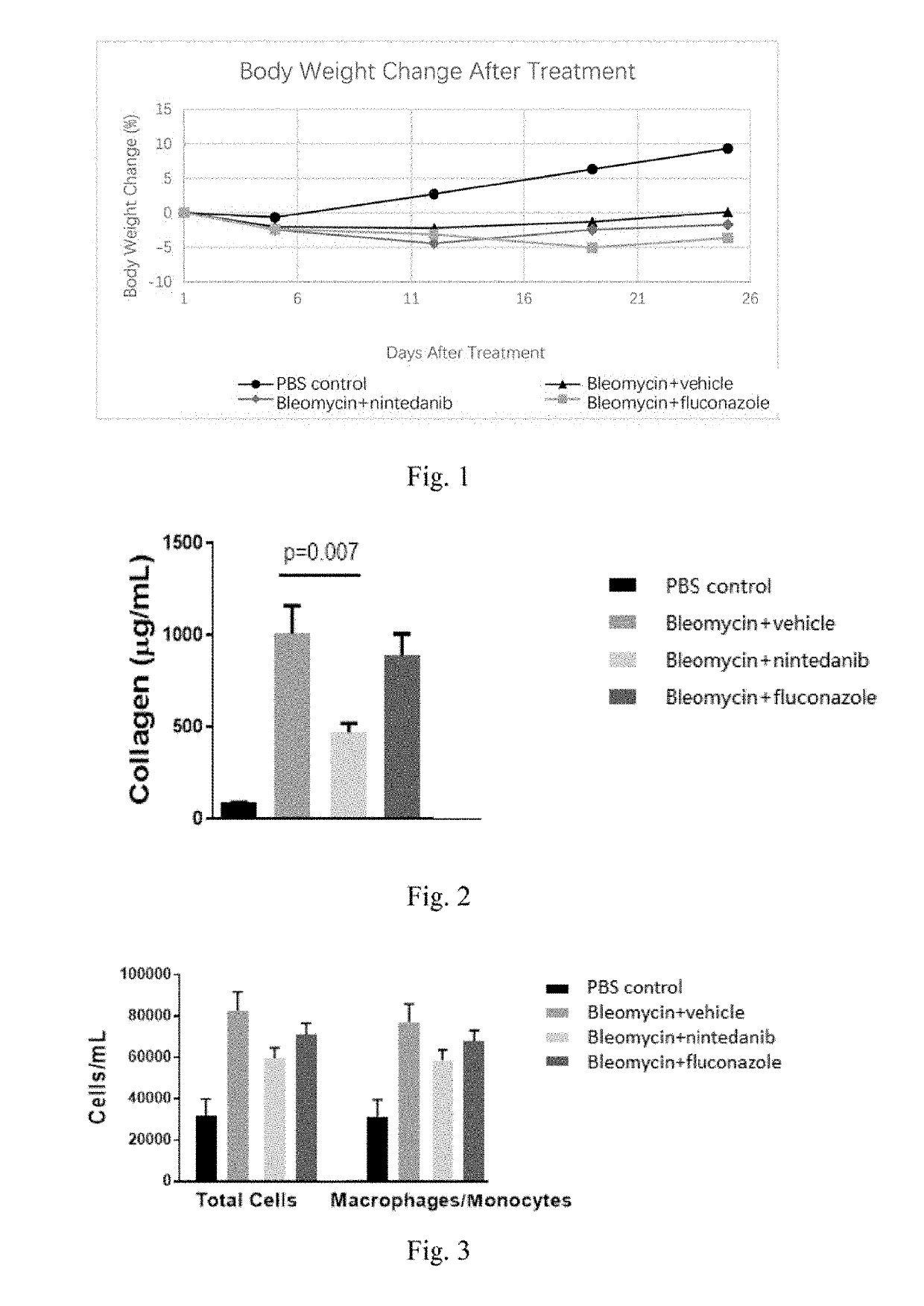
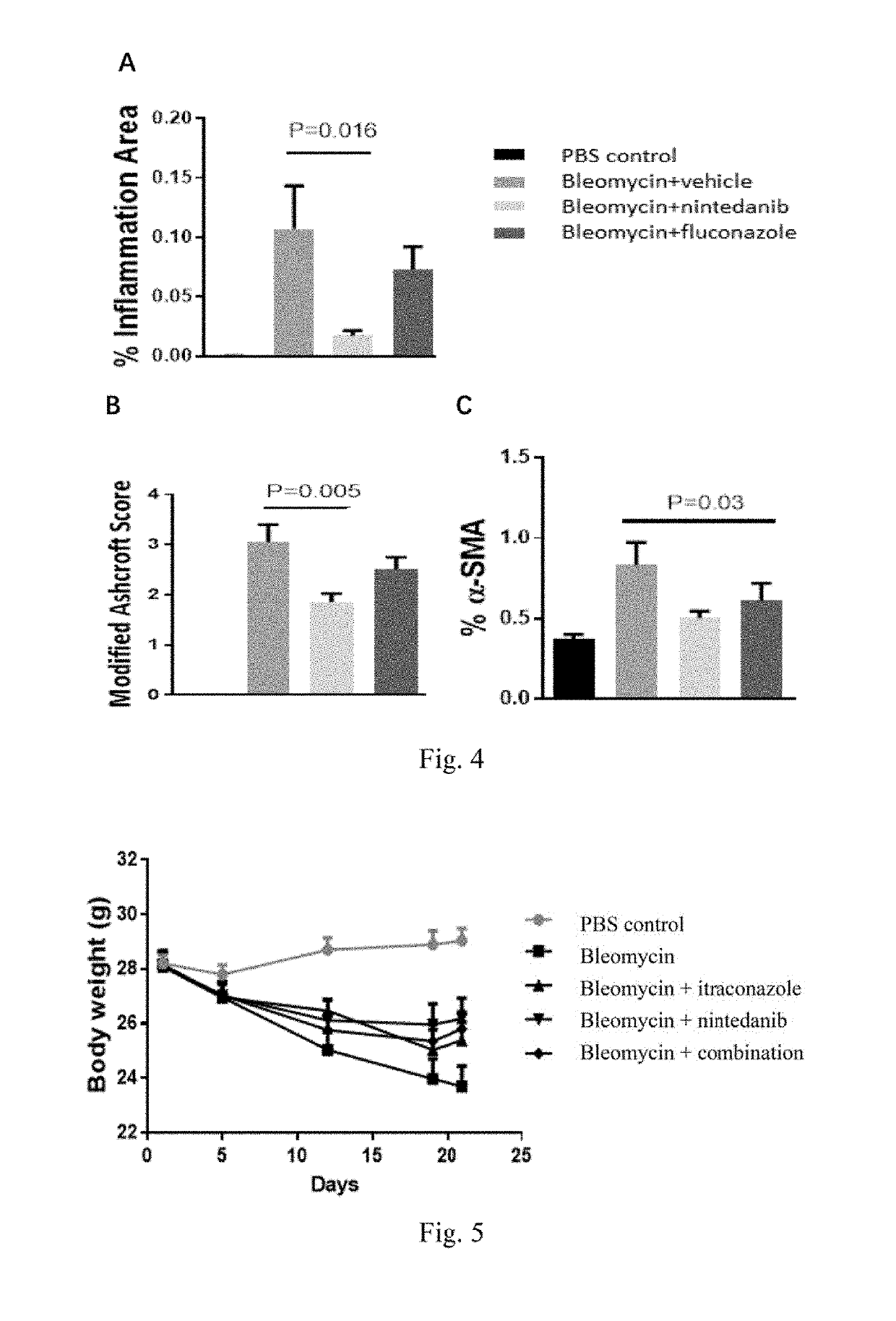
Methods and Compositions for Treating Idiopathic Pulmonary Fibrosis
InactiveUS20190282565A1Effective and safe mannerSafe and effectiveOrganic active ingredientsDispersion deliveryIdiopathic pulmonary fibrosisItraconazole
Provided is a pharmaceutical composition comprising an effective amount of itraconazole, and a pharmaceutically acceptable excipient. The use of the pharmaceutical composition for treatment of idiopathic pulmonary fibrosis is also provided.
Owner:LIANG GUI BAI
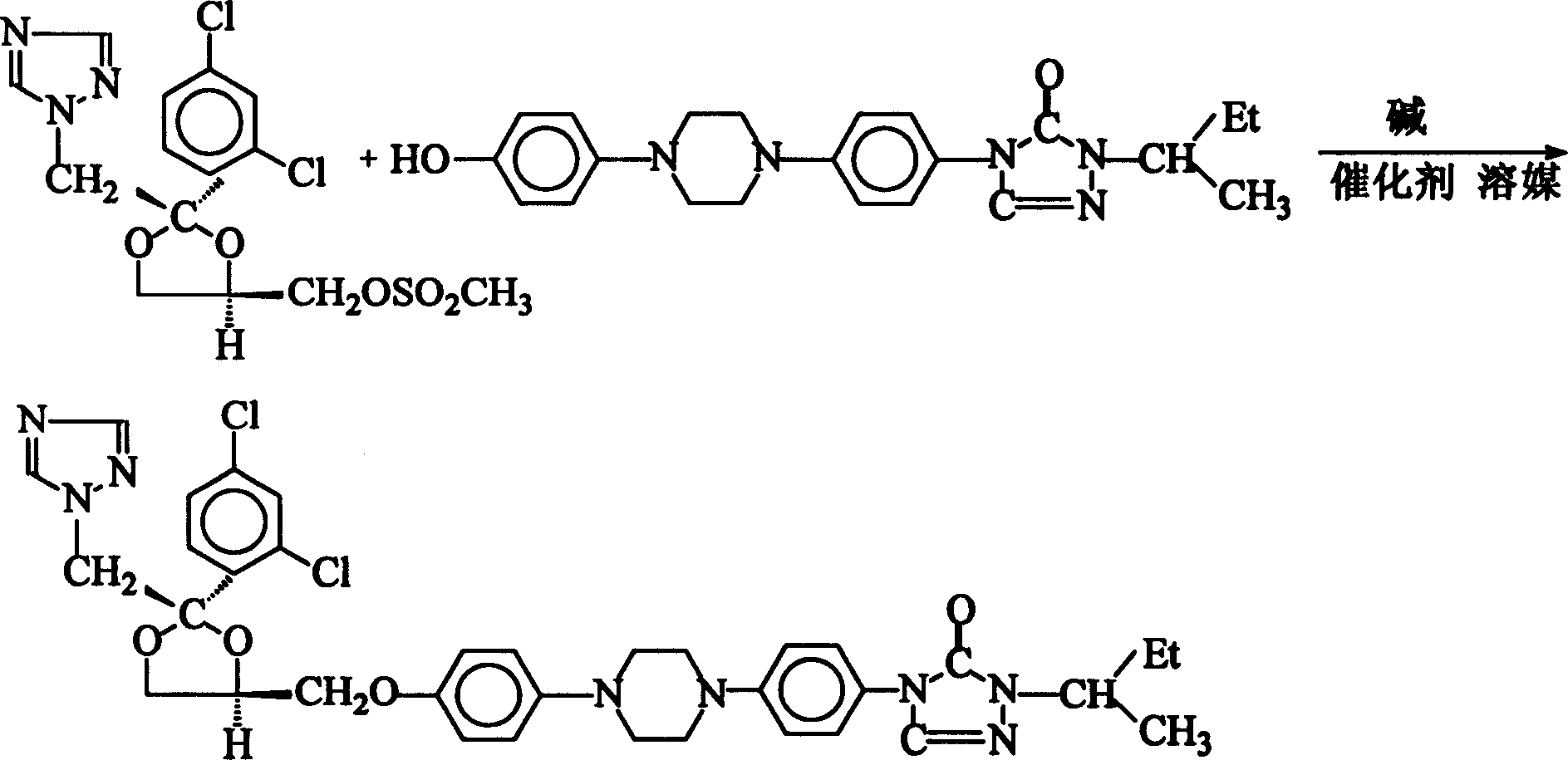
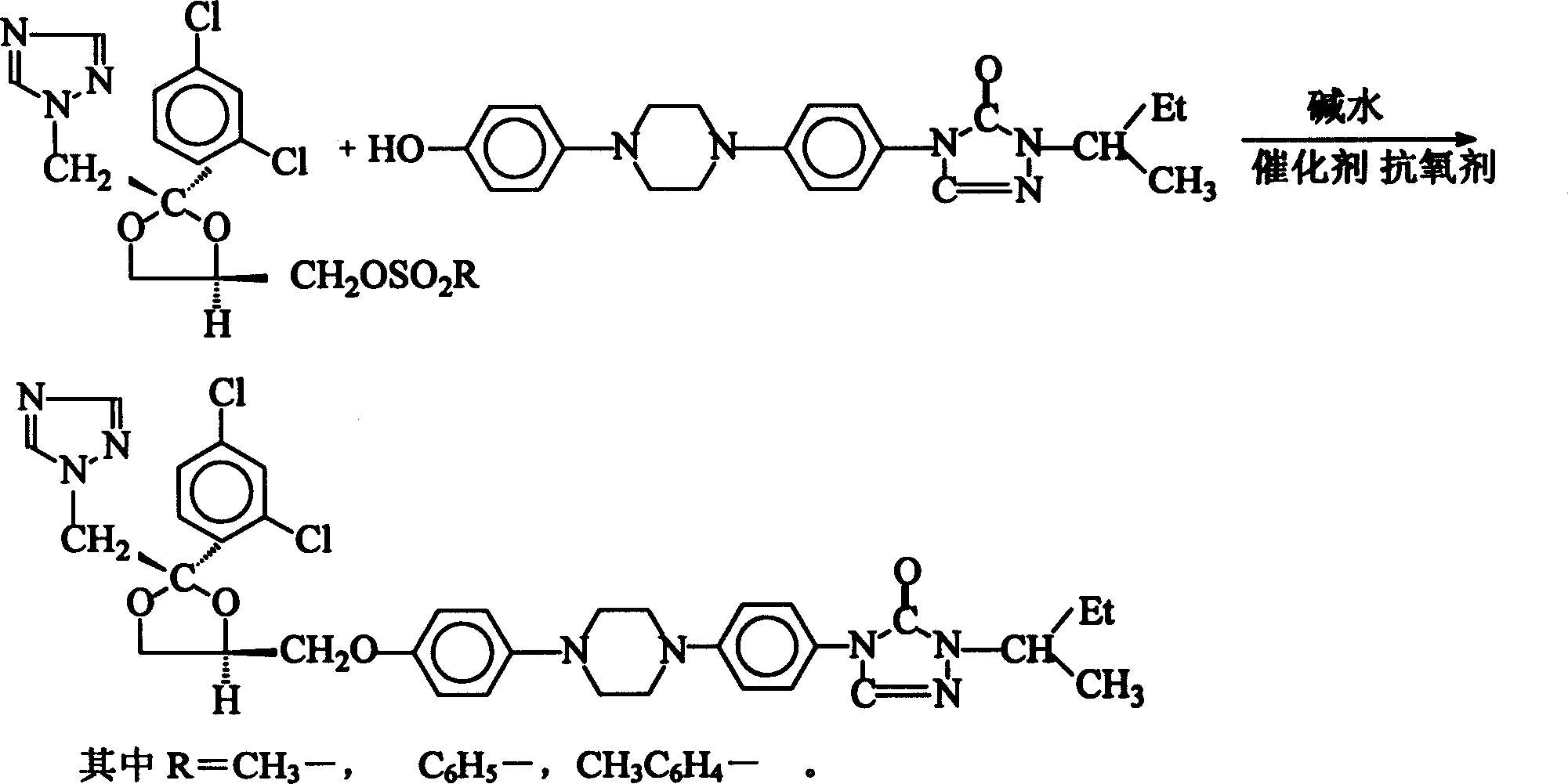
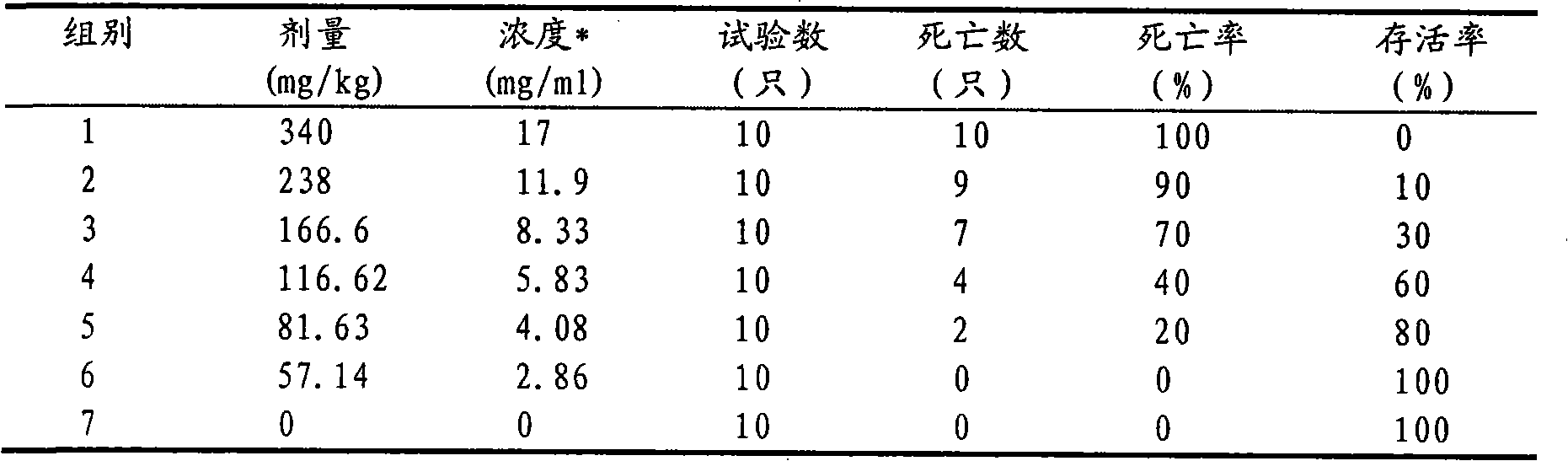
Method of synthesizing Itraconazole
The invention discloses a synthesizing method of yiqukangpyrazole, which comprises the following steps: dissolving sulfonate in the organic solvent; blending with inorganic alkaline solution, antioxidant and quaternary phase-transfering catalyst according to certain rate; adding hydroxy material; heating to 50-110 deg.c; refluxing 5-20 h; cooling to 40deg.c; removing water phase; cooling to 20-30 deg.c; obtaining rough product.
Owner:TIANJIN LISHENG PHARM CO LTD
Itraconazole injection for dogs and preparation method thereof
InactiveCN101889979AHigh inclusion rateGood curative effectOrganic active ingredientsAntimycoticsItraconazoleDistilled water
The invention relates to itraconazole injection for dogs and a preparation method thereof and belongs to an animal medicament. The injection consists of itraconazole, hydroxypropyl-beta-cyclodextrin, propylene glycol, 12 mol.L<-1> of concentrated hydrochloric acid and double distilled water. The preparation method comprises the following steps of: preparing itraconazole inclusion liquid by using hydroxypropyl-beta-cyclodextrin inclusion technology; adding sodium hydroxide solution into the inclusion liquid; adjusting the pH value; filtering the mixture; and circulating vapor for sterilization to obtain the itraconazole injection for the dogs. The injection finished product prepared by the method is yellow and transparent liquid and has the characteristics of high stability, sustained-release property and long-term effectiveness; the inclusion rate of the itraconazole can reach 80 percent; pharmacokinetic characteristic and pharmacodynamics are superior to those of the conventional formulations.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for solving problem of bacterial variation degradation of liquid-strain nutrition liquid
The invention relates to a method for solving a problem of bacterial variation degradation of liquid-strain nutrition liquid, in particular to a method for mushroom-liquid liquid strains. The method comprises a step of using an efficient mutagenic agent, wherein the efficient mutagenic agent comprises itraconazole and rapamycin, and the weight percentage of the itraconazole and the rapamycin in the nutrition liquid is respectively 0.5-5 percent and 1-7 percent. The method also comprises a step of low-temperature preservation strain, a step of adjusting culture conditions and a step of separation and purification. The method taking the itraconazole and the rapamycin as the efficient mutagenic agent as the core method, the mutagenesis is mild, and meanwhile, the strain preservation condition, the nutritional condition of the nutrition liquid, the passage number condition, the purification condition and the like are optimized. Experiments prove that the problem of bacterial variation degradation of various kinds of mushroom liquid-strain nutrition liquid can be basically solved, the method has an obvious economic value, and high-producing strain is obtained.
Owner:陕西众兴菌业科技有限公司
Antifungal combination use
Owner:ASTELLAS PHARMA INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com