Itraconazole injection for dogs and preparation method thereof
An itraconazole and injection technology, applied in the field of veterinary medicines, can solve the problems of high price, restricted promotion and use, etc., and achieve the effects of high drug concentration, good therapeutic effect and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
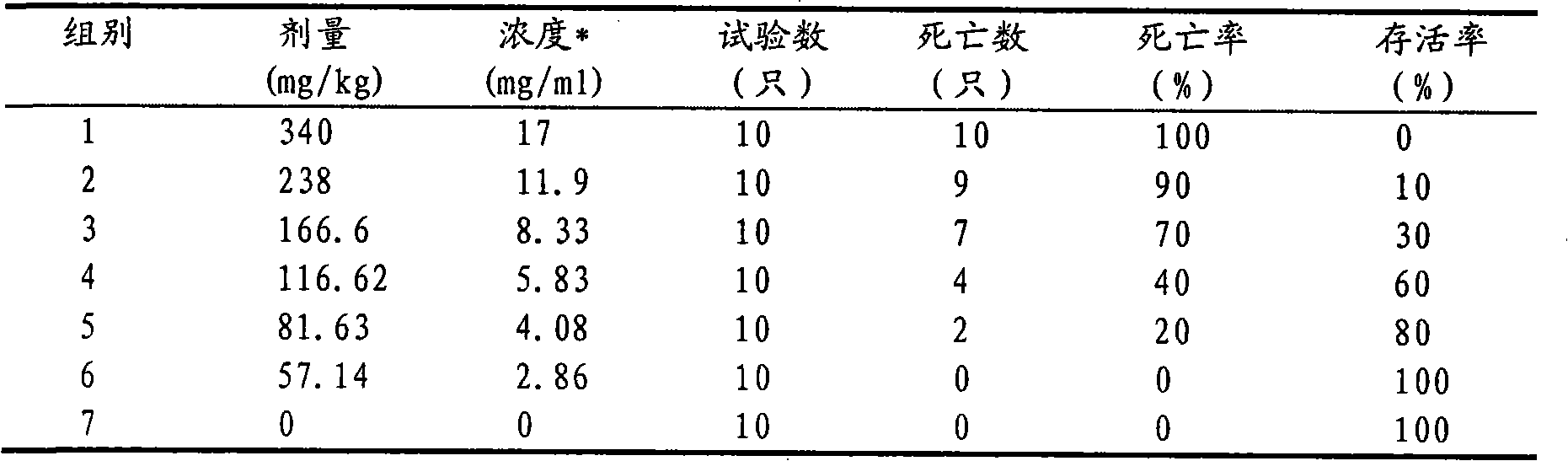
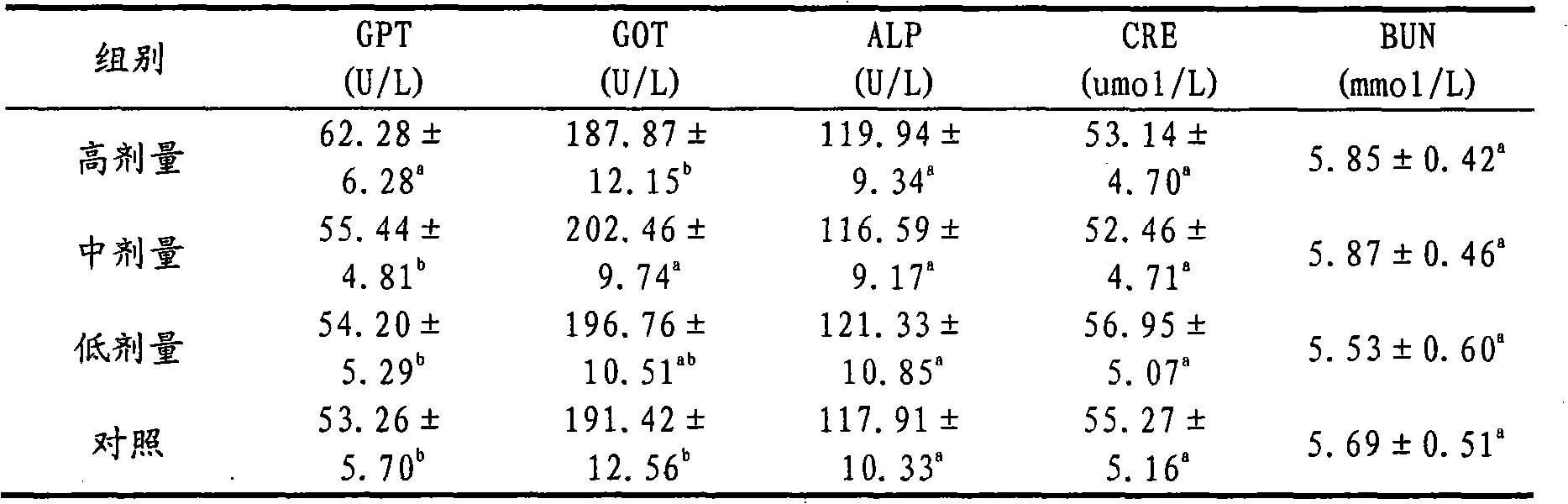
[0016] Weigh 1g of itraconazole and 25g of hydroxypropyl-β-cyclodextrin, and put them into Erlenmeyer flasks A and B respectively. Then add 5 mL of propylene glycol to bottle A, and slowly add 12 mol·L -1 Concentrated hydrochloric acid 370μL until itraconazole is completely dissolved; hydroxypropyl-β-cyclodextrin in B is dissolved in 30mL double distilled water. Then, under the condition of magnetic stirring, the itraconazole solution in A was slowly added dropwise to B, and the dropping time was 15 minutes. For inclusion, the inclusion time is 30h. After the inclusion is completed, the concentration of the inclusion solution is 0.8mol L -1 NaOH solution to adjust the pH to 5.5. Filter it with a 0.45 μm microporous membrane, and sterilize it by flowing steam at 100°C after bottling, that is, itraconazole injection for dogs.
[0017] In the injection prepared in this example, the inclusion rate of itraconazole can reach 80%. The prepared injection is clear and yellow. The...
Embodiment 2
[0019] Weigh 1g of itraconazole and 20g of hydroxypropyl-β-cyclodextrin, and put them into Erlenmeyer flasks A and B respectively. Then add 5mL of propylene glycol to bottle A, and then slowly add the concentration of 12mol L -1 Concentrated hydrochloric acid 370μL until itraconazole is completely dissolved; hydroxypropyl-β-cyclodextrin in B is dissolved in 24mL double distilled water. Then, under the condition of magnetic stirring, the itraconazole solution in A was slowly added dropwise to B, and the dropping time was 15 minutes. For inclusion, the inclusion time is 36h. After the inclusion is completed, the concentration of the inclusion solution is 0.8mol L -1 NaOH solution to adjust the pH to 5.5. Filter it with a 0.45 μm microporous membrane, and sterilize it by flowing steam at 100°C after bottling to obtain itraconazole injection for dogs.
[0020] The average particle size and encapsulation efficiency of the liposomes prepared in this example were 73%. The prepar...
Embodiment 3
[0022] Weigh 1g of itraconazole and 30g of hydroxypropyl-β-cyclodextrin, and put them into Erlenmeyer flasks A and B respectively. Then add 5mL of propylene glycol to bottle A, and then slowly add the concentration of 12mol L -1 Concentrated hydrochloric acid 370μL until itraconazole is completely dissolved; hydroxypropyl-β-cyclodextrin in B is dissolved in 36mL double distilled water. Then, under the condition of magnetic stirring, the itraconazole solution in A was slowly added dropwise to B, and the dropping time was 15 minutes. For inclusion, the inclusion time is 24h. After the inclusion is completed, the concentration of the inclusion solution is 0.8mol L -1 NaOH solution to adjust the pH to 5.5. Filter it with a 0.45 μm microporous membrane, and sterilize it by flowing steam at 100°C after bottling to obtain itraconazole injection for dogs.
[0023] The inclusion rate of itraconazole in the injection prepared in this example was 86%. The prepared injection solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ld50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com