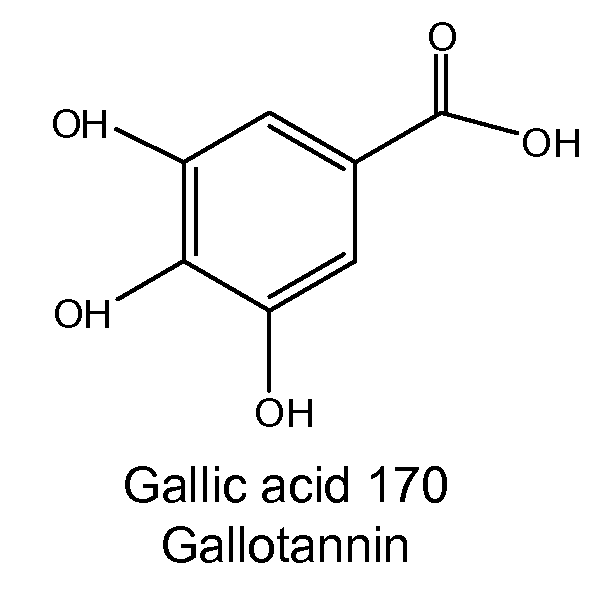
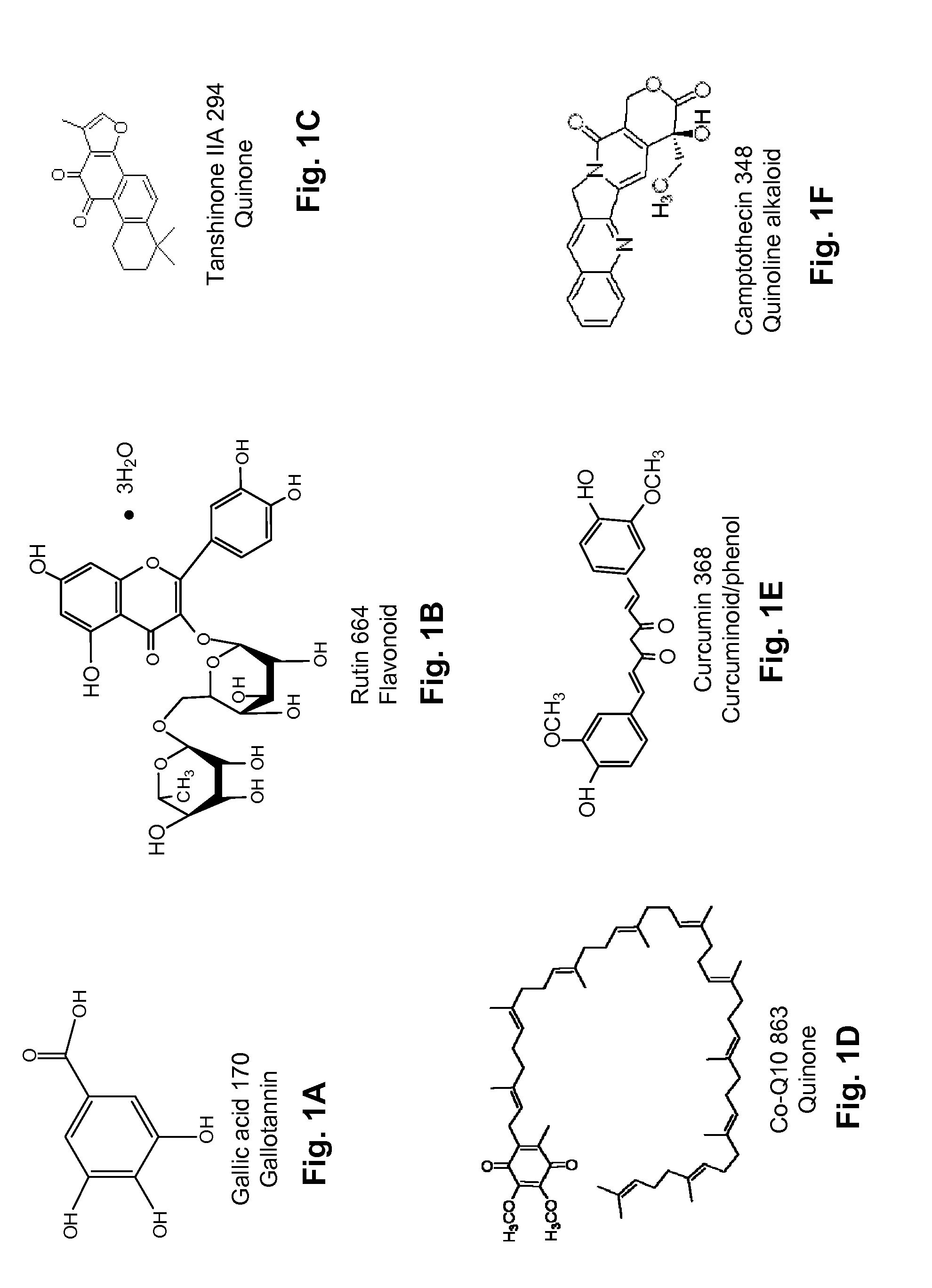
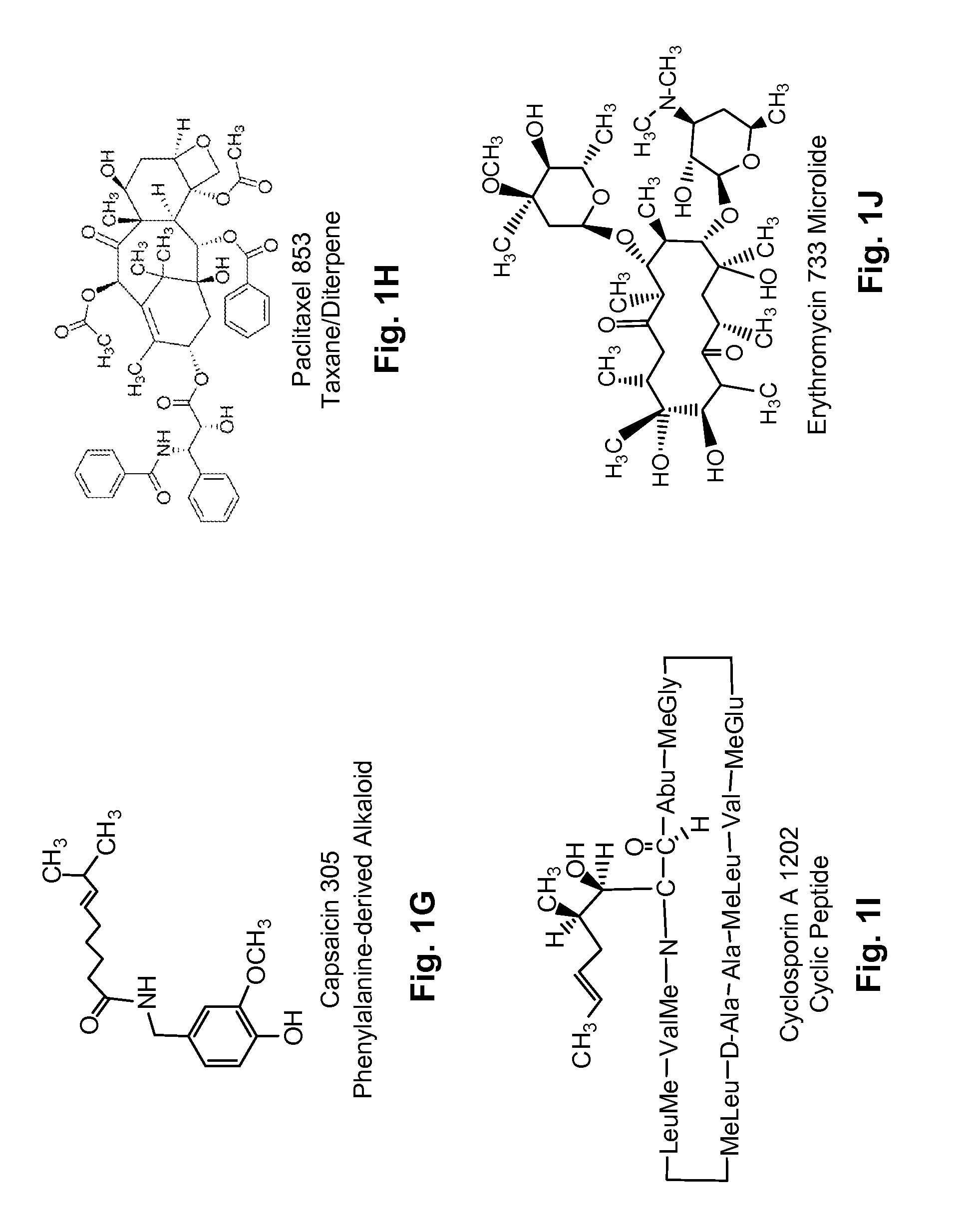
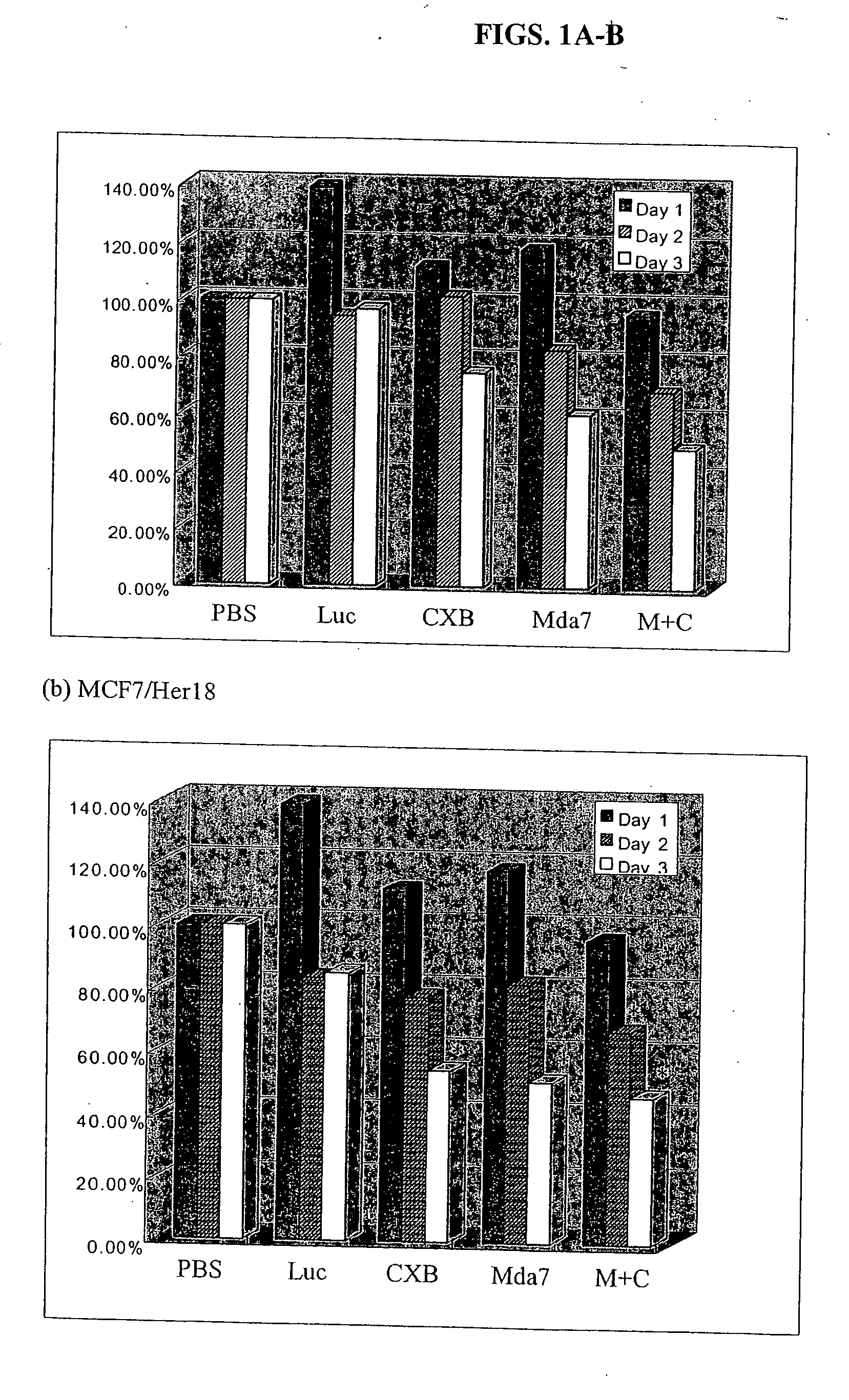
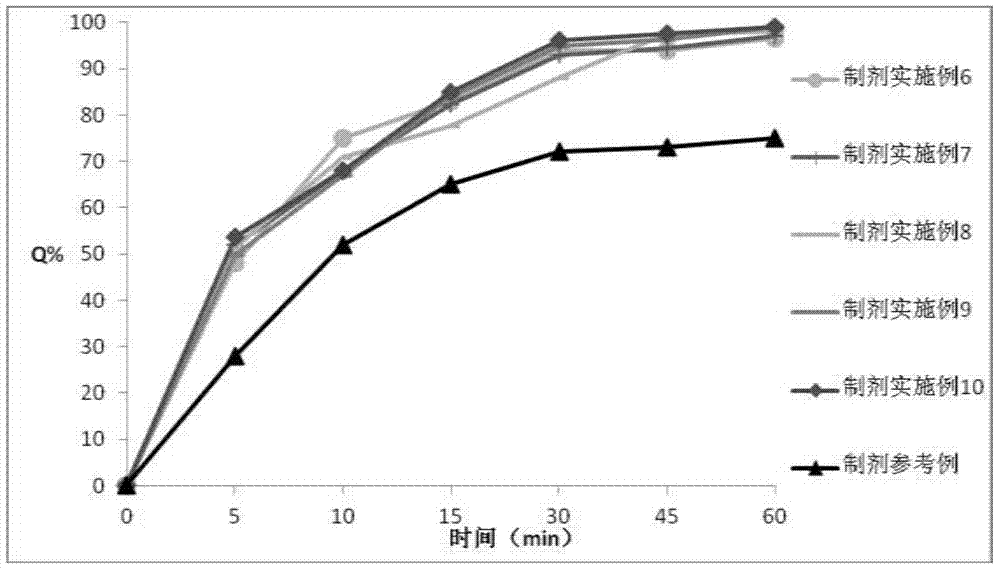
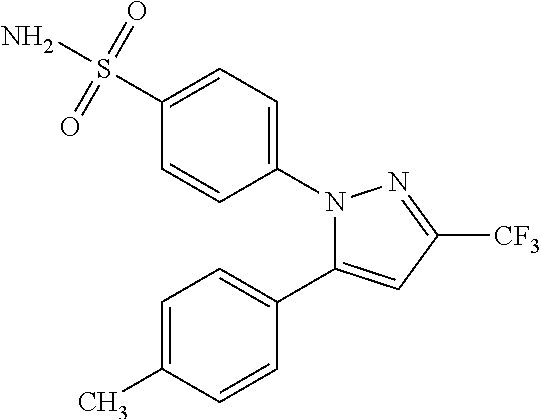
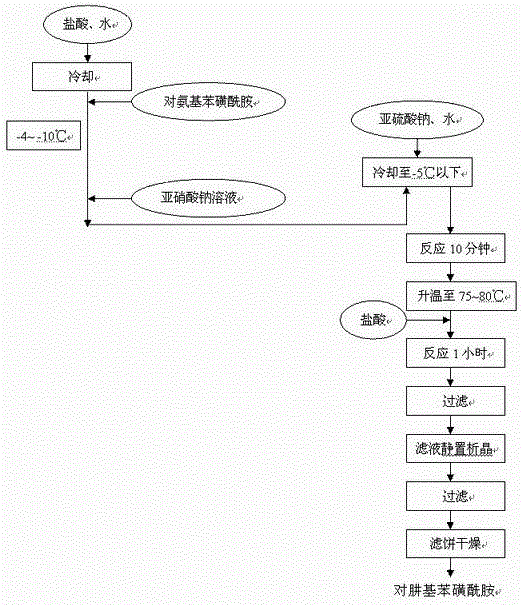
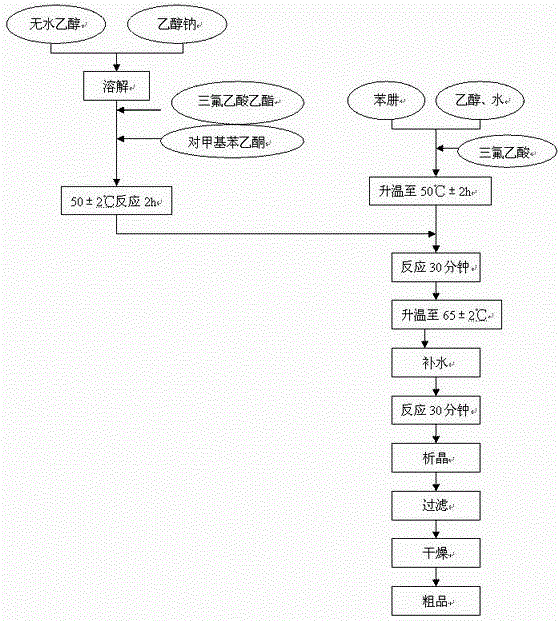
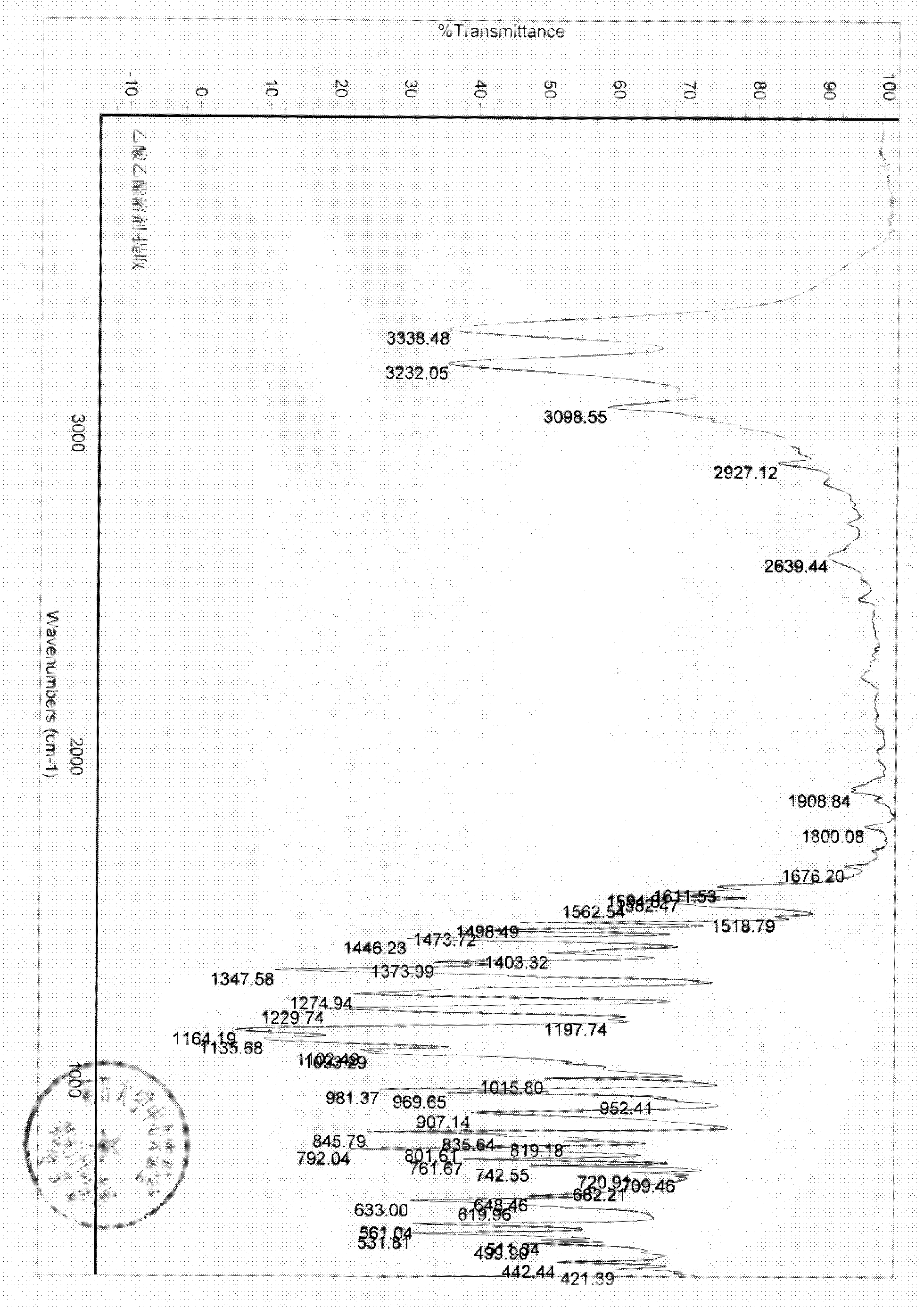
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
267 results about "Celecoxib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
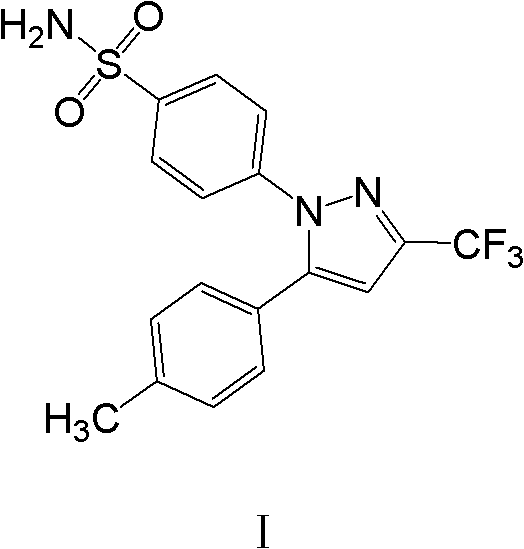
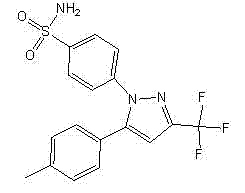
This medication is a nonsteroidal anti-inflammatory drug (NSAID), specifically a COX-2 inhibitor, which relieves pain and swelling (inflammation). It is used to treat arthritis, acute pain, and menstrual pain and discomfort. The pain and swelling relief provided by this medication helps you perform more of your normal daily activities.
Diterpene Glycosides as Natural Solubilizers
InactiveUS20110033525A1Improve solubilityRetain activityBiocideHydroxy compound active ingredientsItraconazoleCapsaicin
Several diterpene glycosides (e.g., rubusoside, rebaudioside, steviol monoside and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, and celecoxib. The use of the diterpene glycoside rubusoside increased solubility in all tested compounds. The diterpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries. Aqueous solutions by using rubusoside to increase the solubility of otherwise insoluble drugs will have several new routes of administration. In addition, aqueous solutions of therapeutic compounds with rubusoside were shown to retain the known pharmacological activity of the compounds.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Compositions and methods involving MDA-7 for the treatment of cancer
InactiveUS20070009484A1Promote apoptosisEnhanced inhibitory effectBiocidePeptide/protein ingredientsGeldanamycinHsp Inhibitor
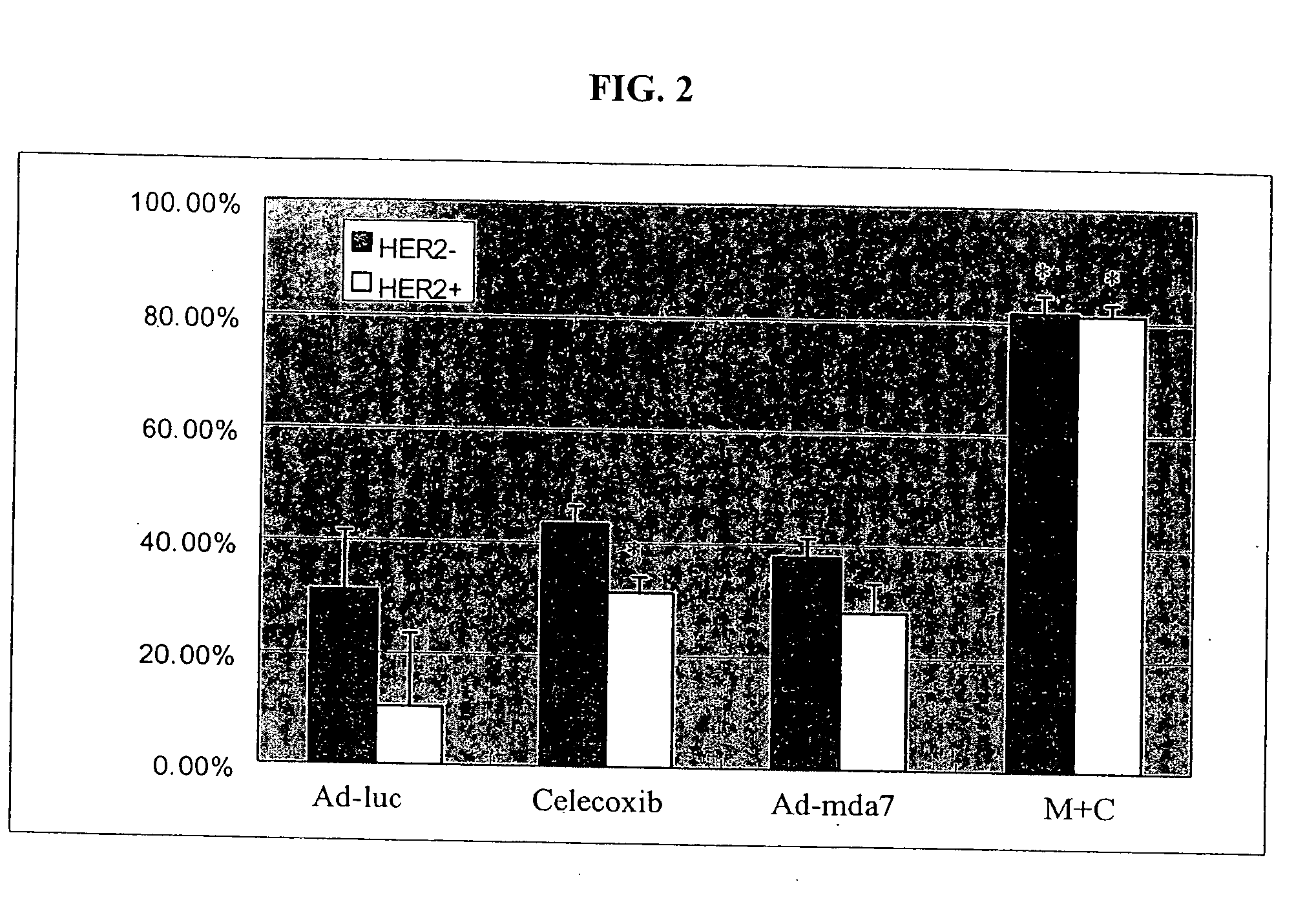
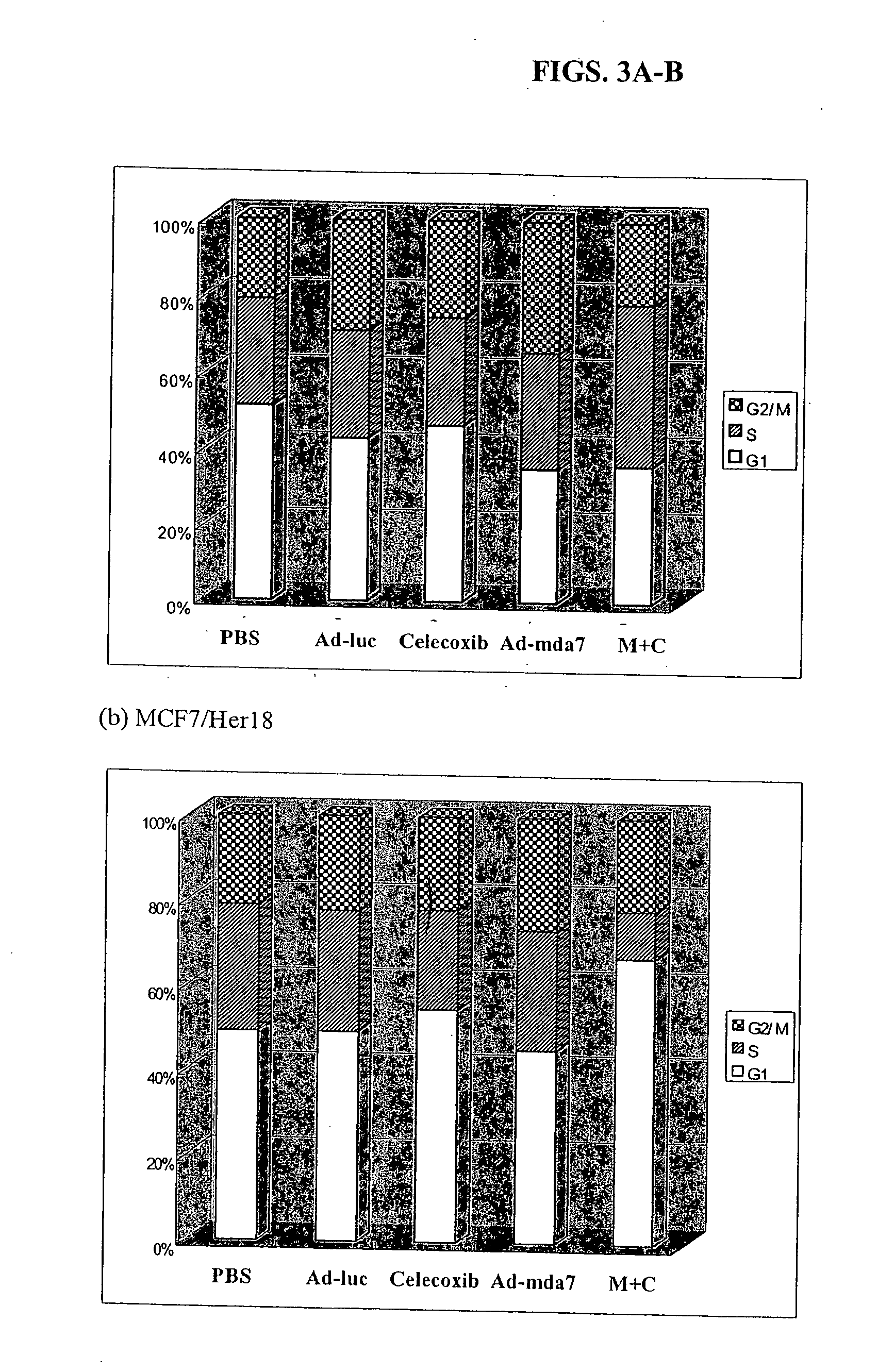
The present invention concerns methods and compositions involving MDA-7 protein or an MDA-7-encoding nucleic acid in combination with either 1) a COX-2 selective inhibitor, such as celecoxib, 2) an Hsp90 inhibitor, such as geldanamycin, or a geldanamycin derivative or analog, 3) a vitamin E compound, for the treatment of cancer, 4) a TNF, such as TNF-alpha, 5) a VEGF inhibitor, or 6) an inhibitor of IL-10. In certain examples, a treatment for breast cancer is provided. In other examples a treatment for lung cancer is provided. Such examples involve, in some cases, an adenovirus vector that expresses MDA-7 protein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Celecoxib compositions
InactiveUS20050267189A1BioavailabilityLess-harmful side effectBiocideSenses disorderParticulatesCyclooxygenase
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising particulate celecoxib in an amount of about 10 mg to about 1000 mg in intimate mixture with one or more pharmaceutically acceptable excipients. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders.
Owner:GD SEARLE & CO
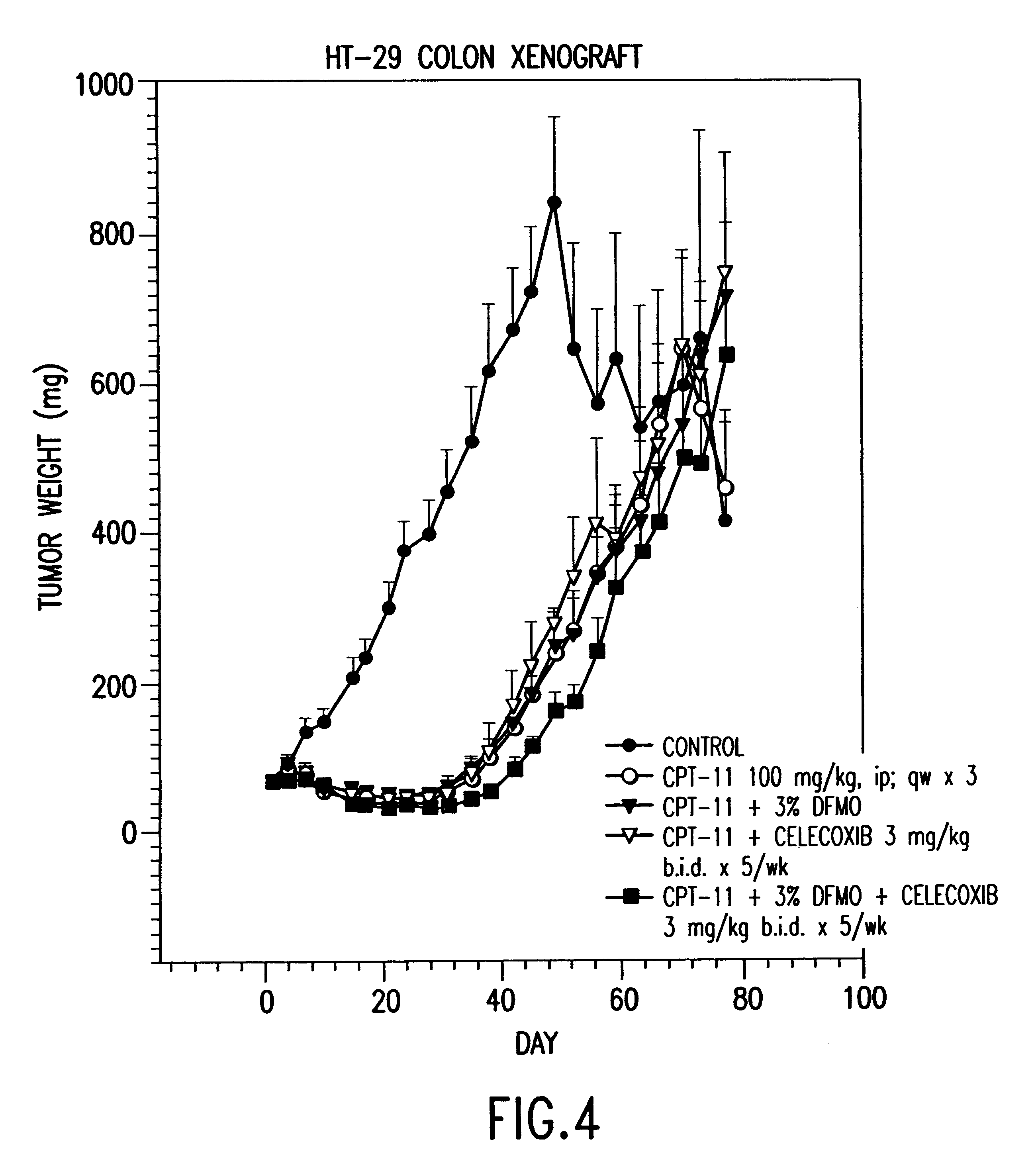
DFMO and celecoxib in combination for cancer chemoprevention and therapy
InactiveUS6573290B1Less effectEliminate side effectsBiocidePeptide/protein ingredientsNon steroidal anti inflammatoryCelecoxib
Celecoxib, a COX-2 specific non-steroidal anti-inflammatory agent, is provided in combination with DFMO for the prevention and / or treatment of cancers. Provided with the present invention are pharmaceutically acceptable compositions that include a non-steroidal anti-inflammatory agent, celecoxib, together with an effective amount of difluoromethylornithine.
Owner:ILEX ONCOLOGY
Solid dispersion containing celecoxib as well as preparation method and application thereof
InactiveCN102000018AImprove solubilityEasy to manufactureOrganic active ingredientsAntipyreticPolyethylene glycolCelecoxib
The invention relates to a solid dispersion containing celecoxib as well as a preparation method and application thereof. The invention needs to solve the technical problems of difficult pulverization of raw materials and difficult preparation of solid oral preparation of celecoxib. The solid dispersion is a solid dispersion containing the celecoxib and polyethylene glycol which are in the weight ratio of 1:0.5-10. The preparation method comprises the following steps of: melting solid polyethylene glycol by heating, adding the celecoxib and dissolving to obtain a clear solution; or mixing and heating the solid polyethylene glycol and the celecoxib, and melting to obtain a clear solution; and then rapidly cooling to obtain the solid dispersion. The solid dispersion can be prepared into various oral preparations after being pulverized.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Celecoxib-containing solid dispersion and preparation method thereof
InactiveCN103371976AImprove liquidityEasy to shapeOrganic active ingredientsPowder deliveryVitrificationPolyethylene glycol
The invention provides a celecoxib-containing solid dispersion and a preparation method thereof. The celecoxib-containing solid dispersion comprises celecoxib and copovidone. The celecoxib-containing solid dispersion provided by the invention has the advantages of high rigidity, good friability and moderate glass temperature, can be suitable for large-scale industrialized production and overcomes the defects of low glass temperature, soft material, easy melting and bonding, difficulty in crushing and the like of a celecoxib solid dispersion which takes polyethylene glycol as a carrier.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Celecoxib nanosuspension and preparation method thereof
InactiveCN105147607ASimple prescriptionSimple processOrganic active ingredientsAntipyreticSolubilityDissolution
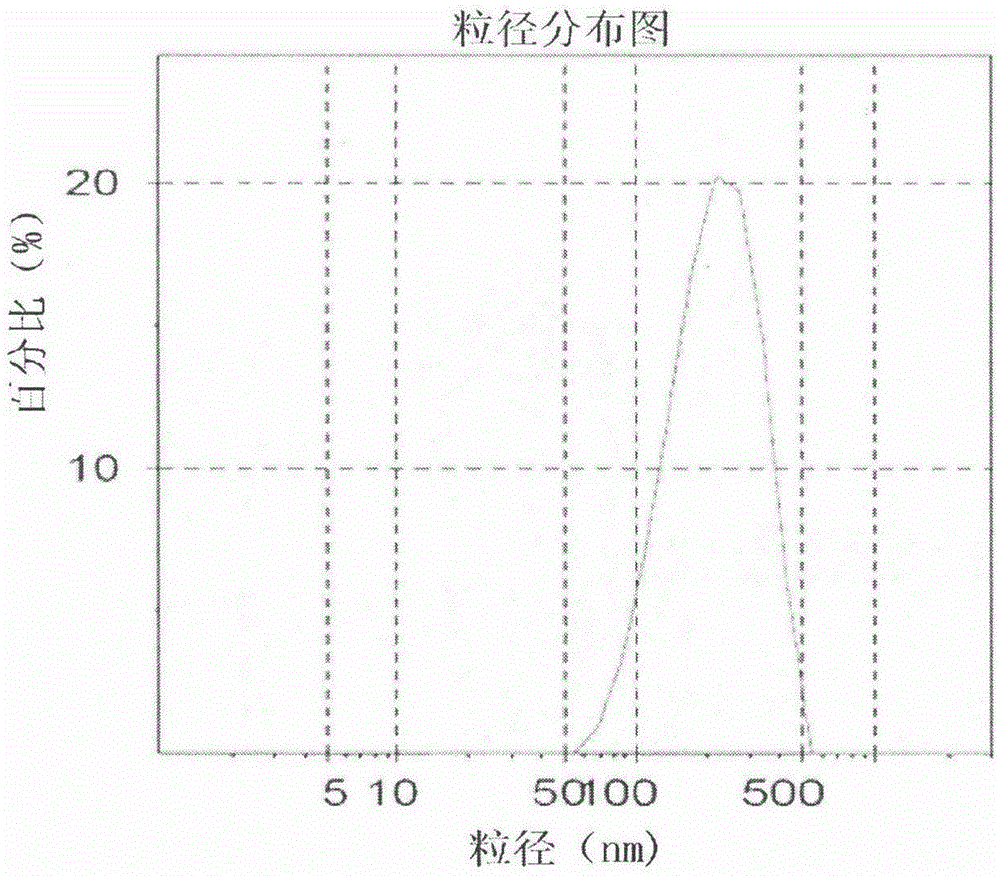
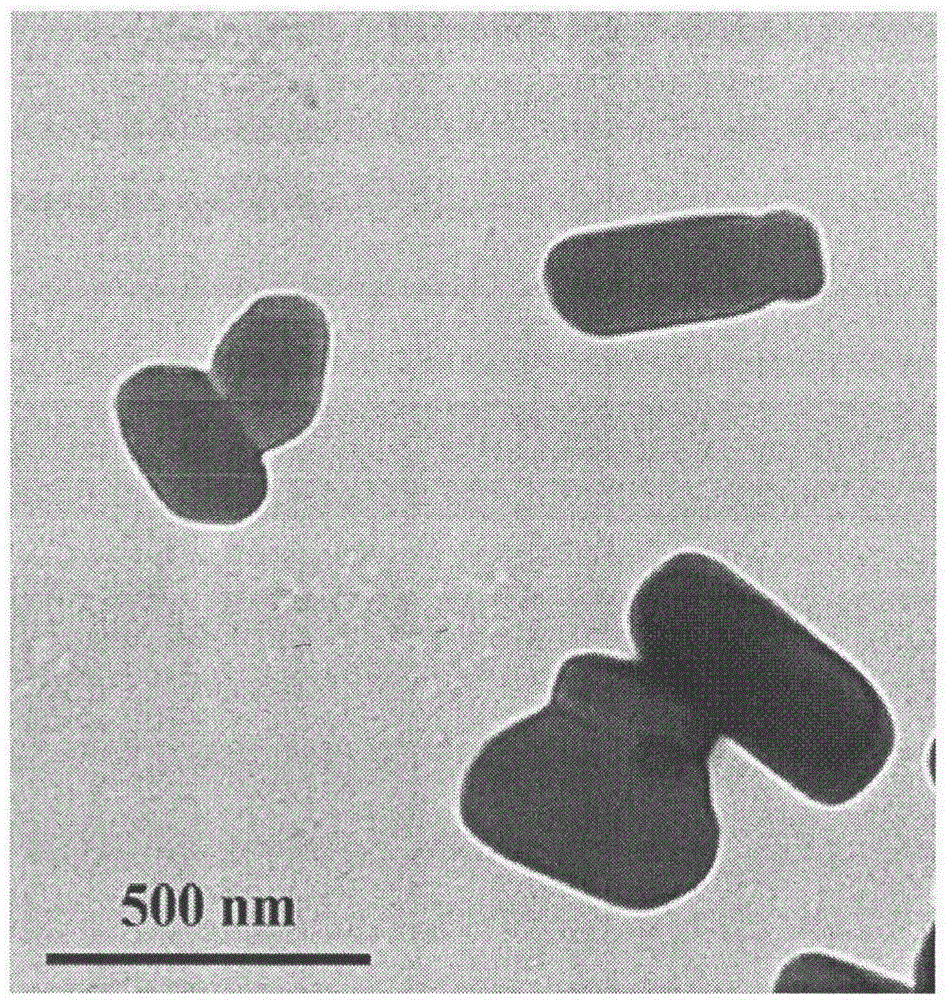
The invention belongs to the field of pharmaceutical preparations and provides a Celecoxib nanosuspension and a preparation method thereof. Celecoxib is a novel non-steroidal anti-inflammatory drug, inhibits cyclooxygenase -2(COX-2) is through specificity, has anti-inflammatory and pain-easing effects and is clinically used for treating osteoarthritis and rheumatoid arthritis. However, the Celecoxib has very low solubility and in-vivo bioavailability. In order to increase the dissolution and the bioavailability of the drug, a high-speed shearing combined high-pressure homogenizing method is adopted to prepare the Celecoxib nanosuspension, the particle size and the polydispersion index PI are used as indicators for formulation technology optimization, a laser particle analyzer and a transmission electron microscope are adopted to study the particle size and form of the drug, the dissolution of the drug is evaluated through in-vitro dissolution experiments, and rat in-vivo pharmacokinetic study is conducted. Measurement results prove that the drug particle size of the nanosuspension is 50-500 nm, and the dissolution and the in-vivo bioavailability of the drug are increased obviously.
Owner:CHINA PHARM UNIV
Celecoxib and preparing method thereof
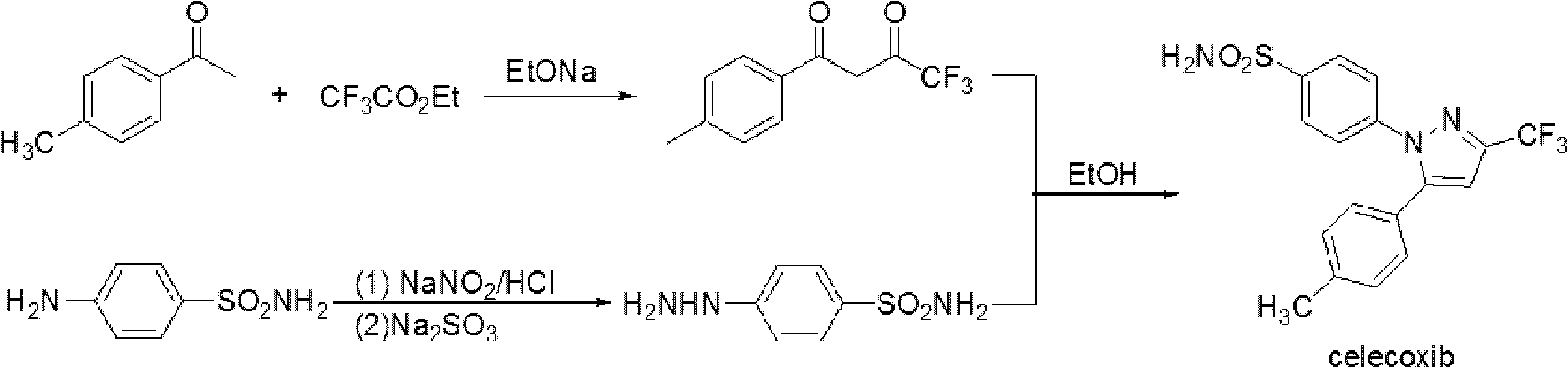
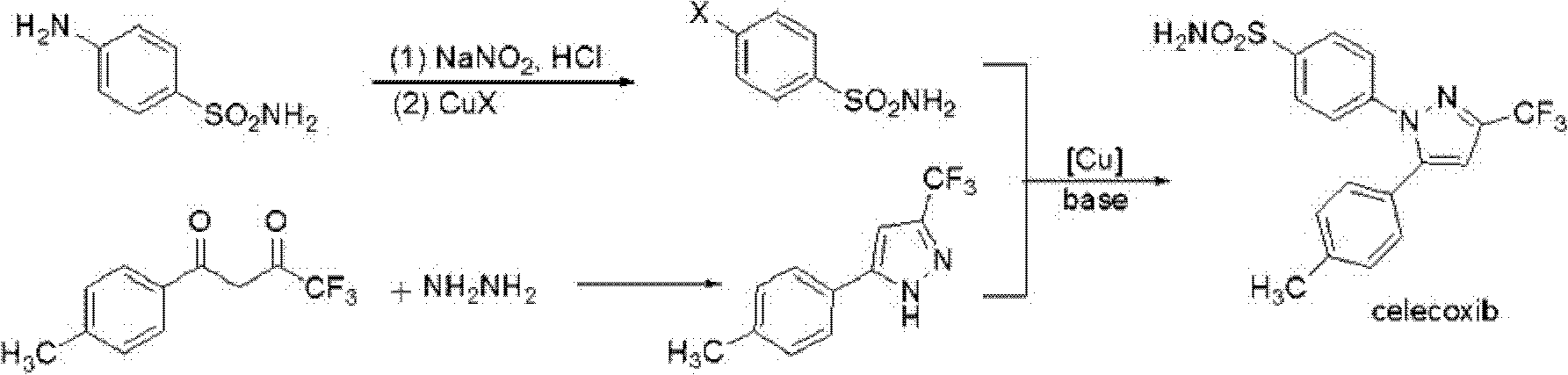
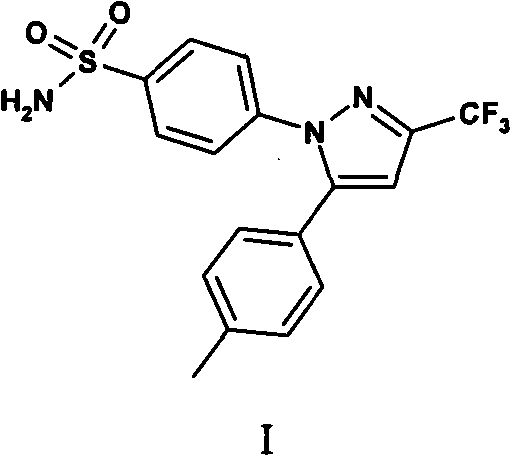
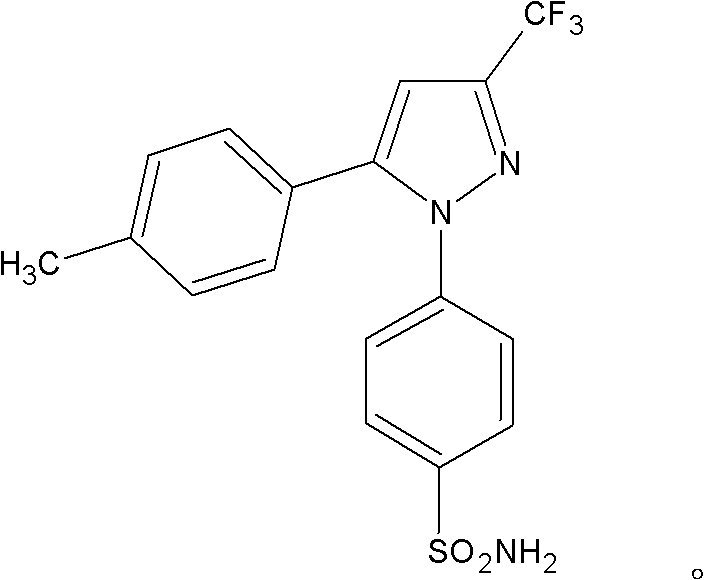
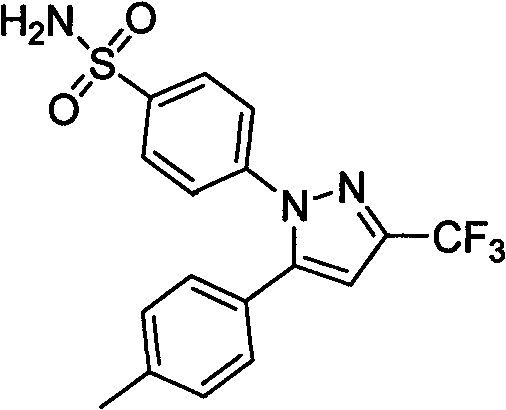
The invention provides celecoxib and a preparing method thereof. The preparing method of the celecoxib comprises the following steps: mixing 5- (4-methylphenyl) -3-trifluoromethyl-1H-pyrazole and 4-halogen benzene sulfonamide, then dissolving the mixture in the second organic solvent, and then adding alkali and catalyst to obtain the celecoxib after coupling reactions. The 5-(4-methylphenyl) -3-(trifluoromethyl)- 1H-pyrazole:(1) is prepared according to the following procedures: mixing dissolving 1-(4- methylphenyl) -4, 4, 4-trifluoro1, 3-butanedione and hydrazine hydrate in the second organic solvent, then obtaining 5-(4-methylphenyl)-3-(trifluoromethyl)-1H-crude pyrazole after heating and dehydration cyclization reaction; (2), obtaining 5-(4-methylphenyl) -3- (trifluoromethyl)-1H-pyrazole by purifying the crude pyrazole. The preparing method provide by the invention can effectively reduce the content of regional isomers in celecoxib, and dramatically improve the quality of products. The productivity can reach 81.3%, and the content of the regional isomer is 0.01%.
Owner:广东暨大基因药物工程研究中心有限公司
Celecoxib solid composition with high dissolution, preparation method and application
InactiveCN102764264AImprove solubilityDissolution rate is fastOrganic active ingredientsAntipyreticPharmaceutical medicineBioavailability
The invention provides a celecoxib solid composition with high dissolution, a preparation method and an application. The amorphous composition of celecoxib is obtained by dissolving the celecoxib into one or more pharmaceutically acceptable solvents and adsorbing and drying with pharmaceutically acceptable auxiliary materials. The composition can be further prepared into various solid preparation forms such as tablets, capsules and granules according to the actual requirement. Through the prepared celecoxib composition and preparations thereof, the dissolution of the celecoxib can be greatly improved, and the defects of low dissolution and low bioavailability of the celecoxib are overcome.
Owner:HANGZHOU HEZE PHARMA TECH
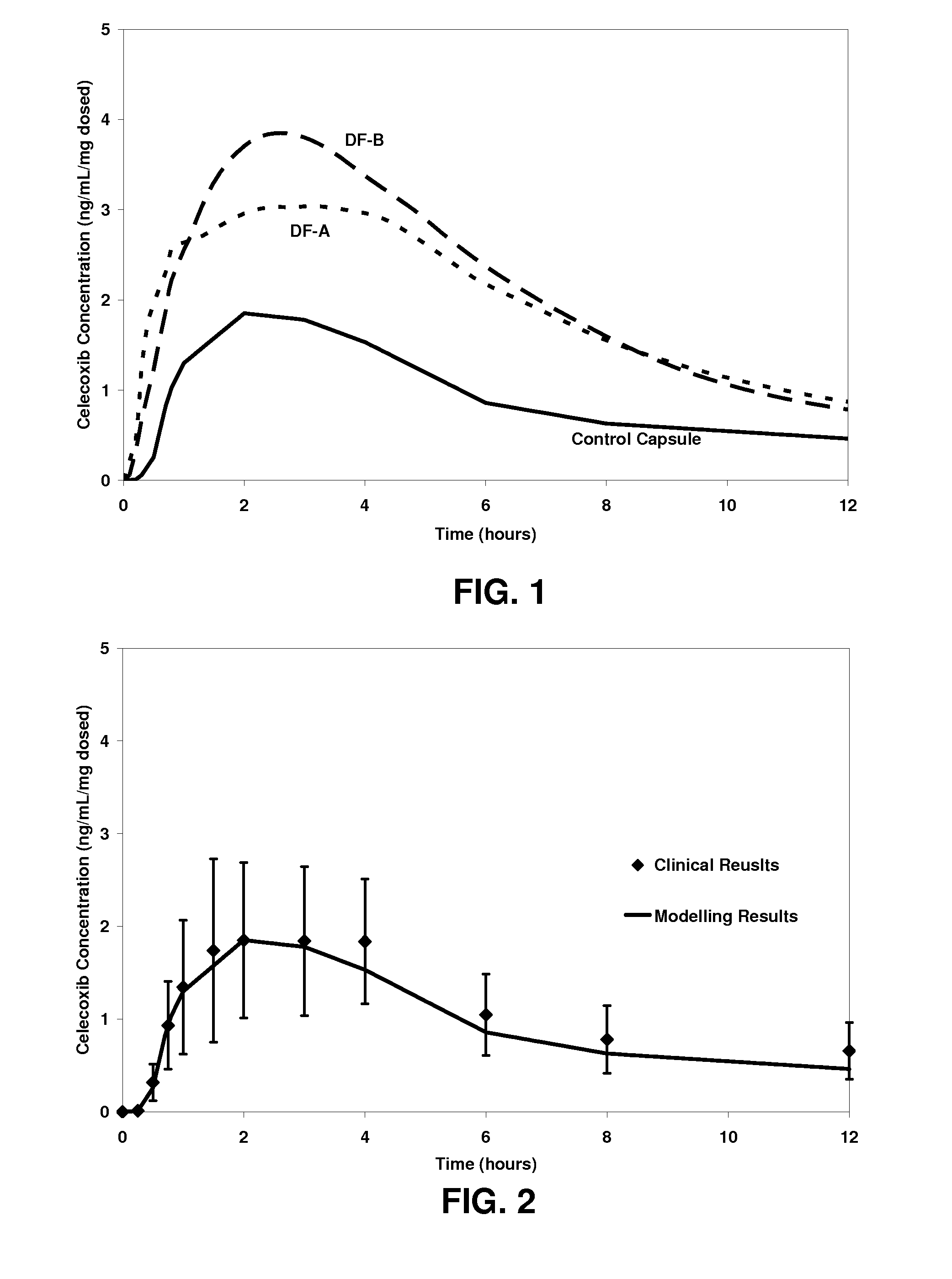
Dosage forms comprising celecoxib providing both rapid and sustained pain relief
InactiveUS20100233272A1Reduce the amount requiredReduce dosePowder deliveryOrganic active ingredientsMedicineHuman patient
A pharmaceutical dosage form comprising celecoxib and a pharmaceutically acceptable carrier, the dosage form when initially administered to at least 12 human patients in the fasted state in a crossover study providing: (a) a mean blood plasma concentration of celecoxib within 0.5 hour after administration (C0.5) of at least about 0.9 ng / ml per mg of celecoxib dosed; (b) a mean blood plasma concentration of celecoxib 12 hours after administration (Ci2) of at least about 0.6 ng / ml per mg of celecoxib dosed; (c) a mean area under the blood plasma concentration versus time curve for the 12 hour period following administration (AUC12) of at least 19 ng-hr / mL per mg of celecoxib dosed; and (d) a mean maximum blood plasma concentration (Cmax) of celecoxib of less than about 4.9 ng / ml per mg of celecoxib dosed.
Owner:APPEL LEAH ELIZABETH +4
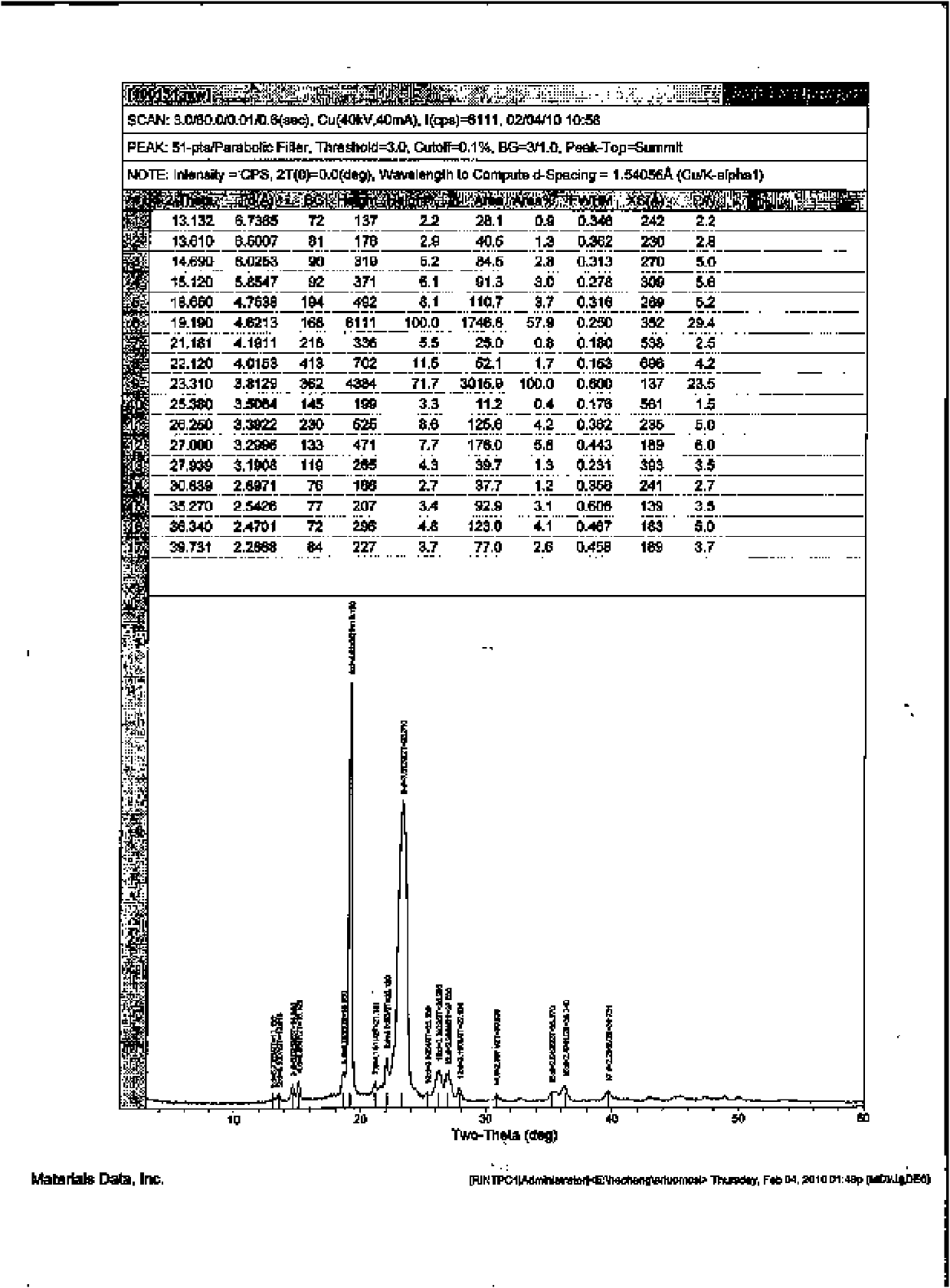
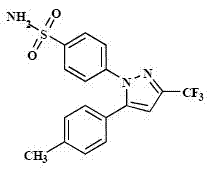
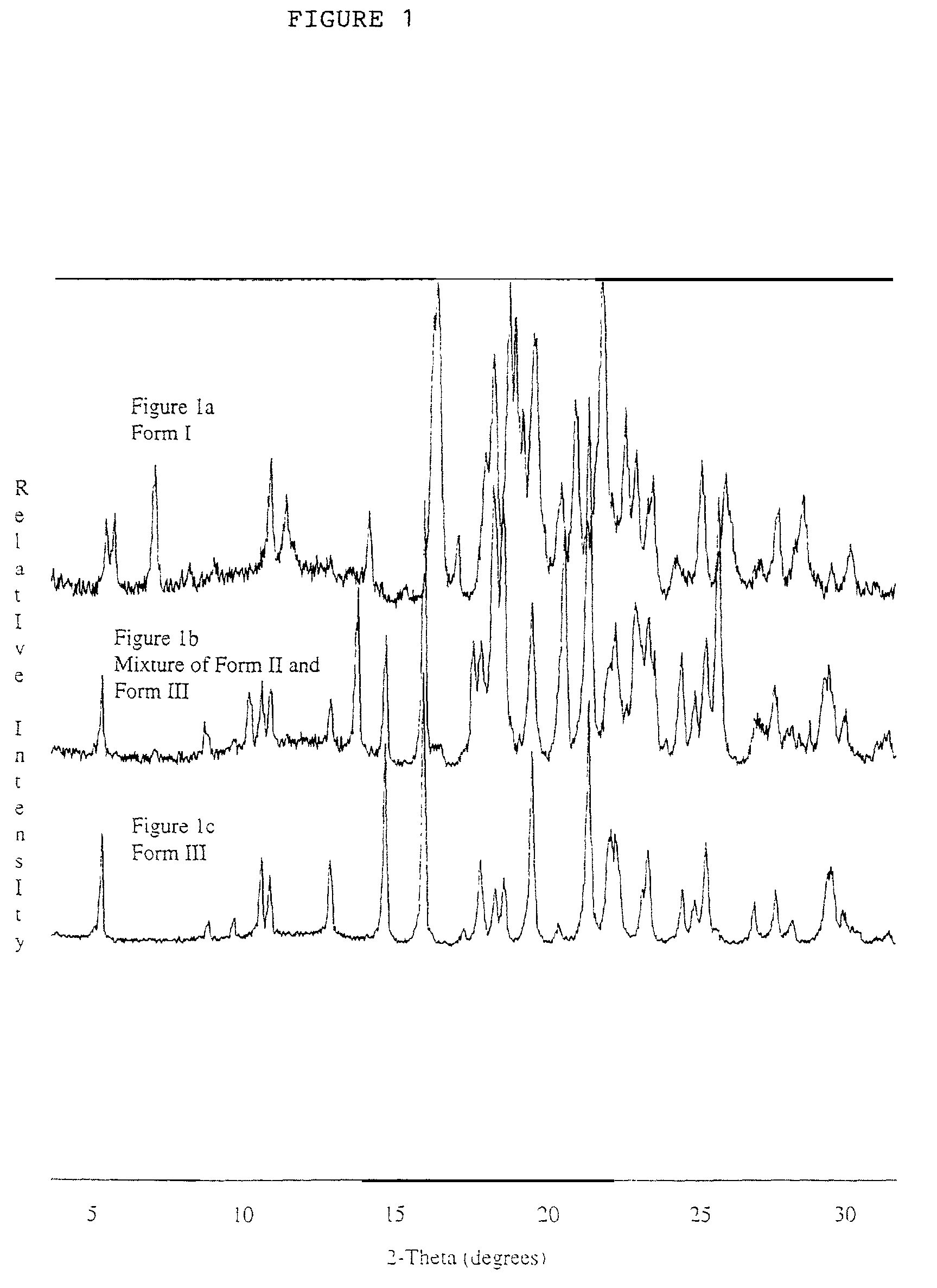
Polymorphic crystalline forms of celecoxib
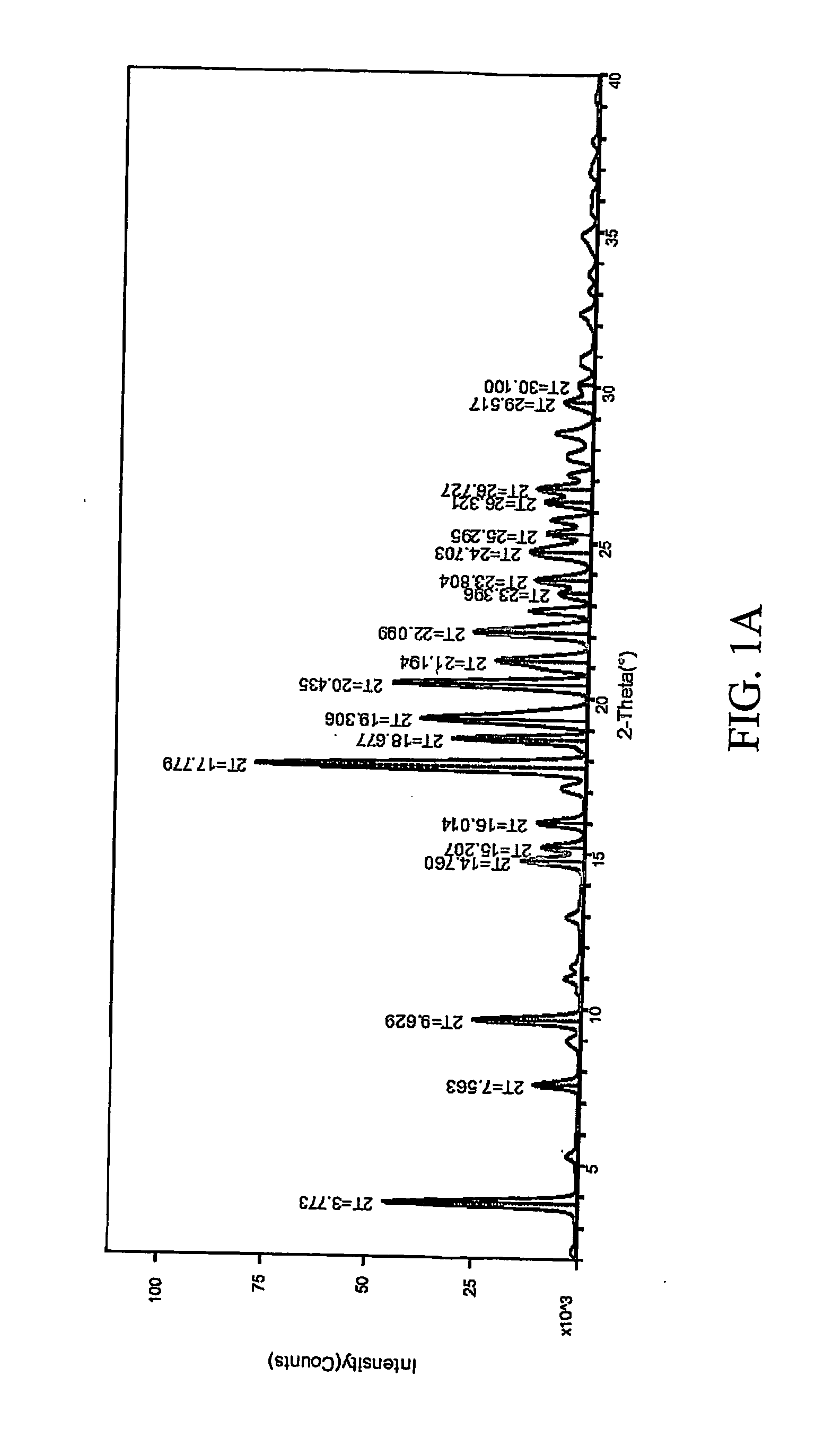
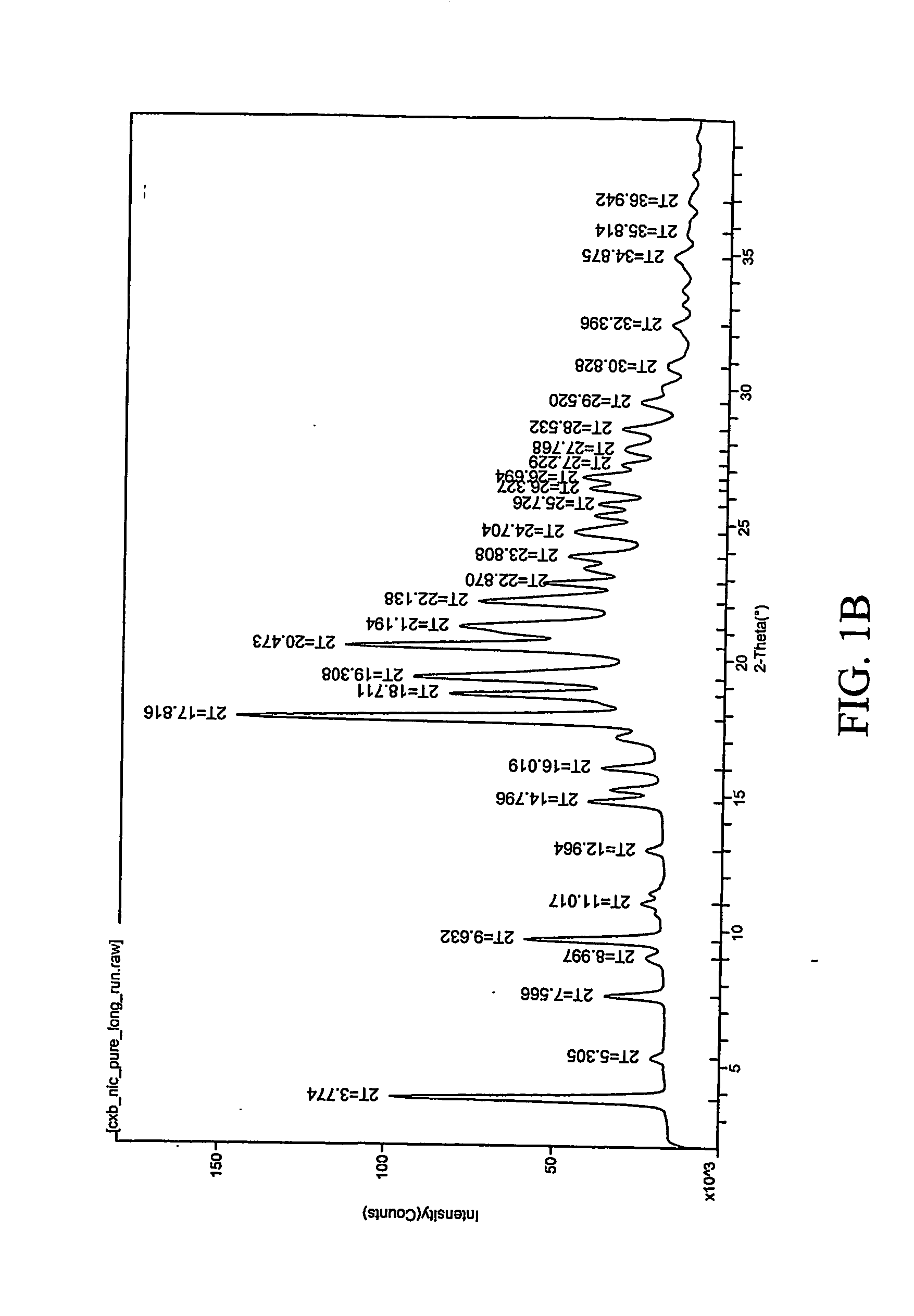
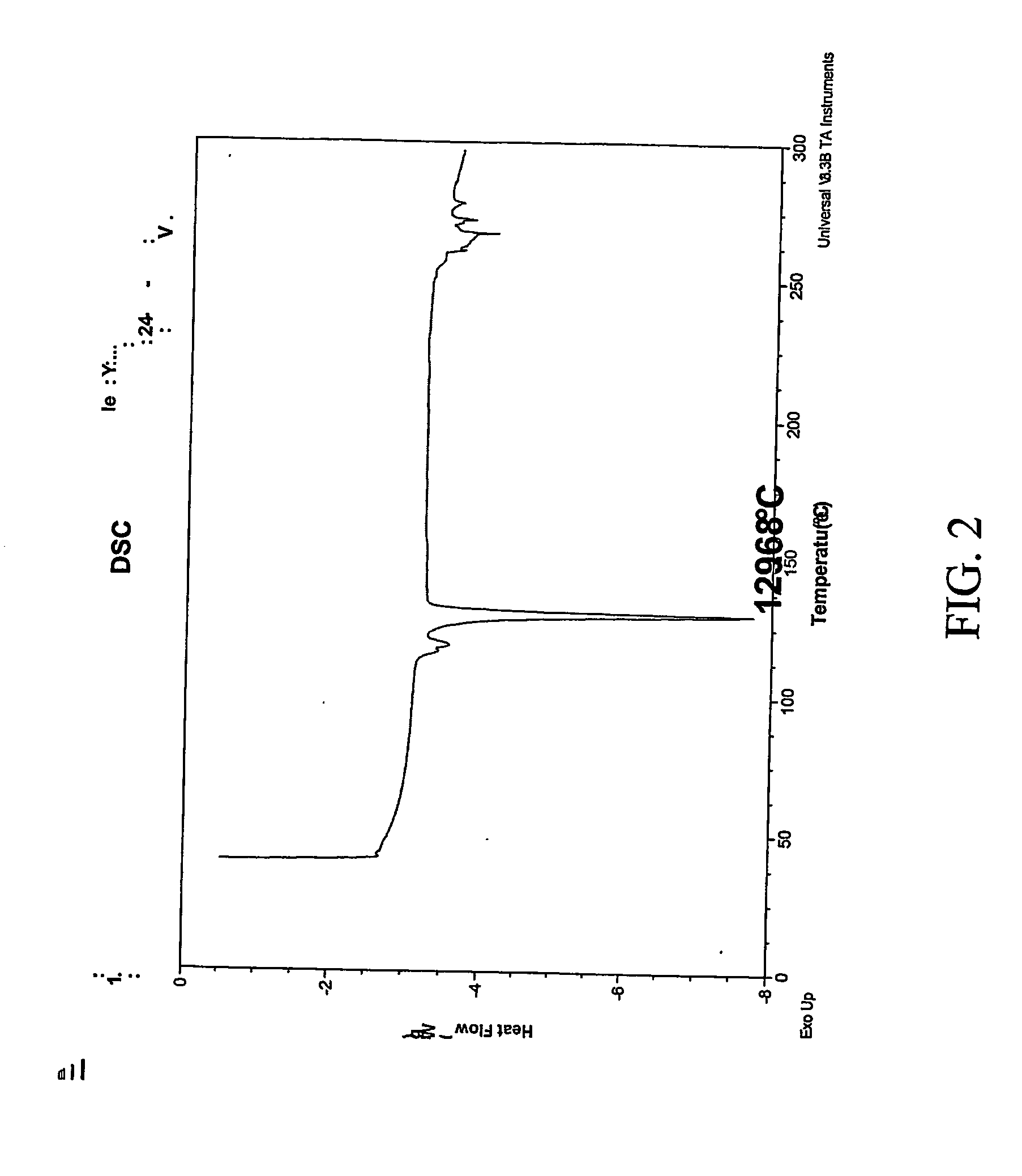
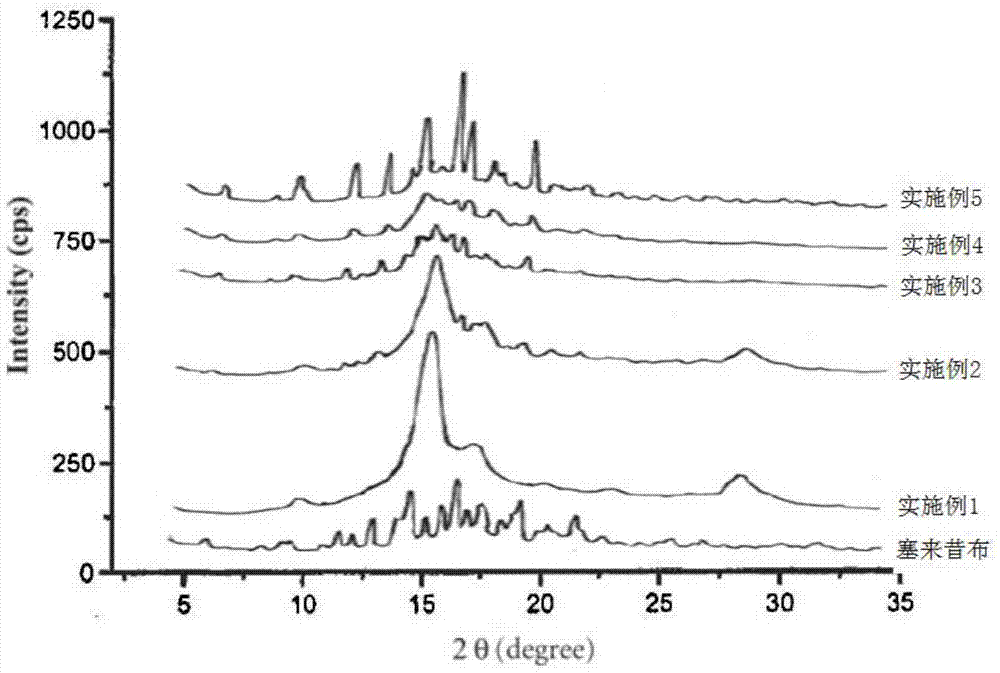
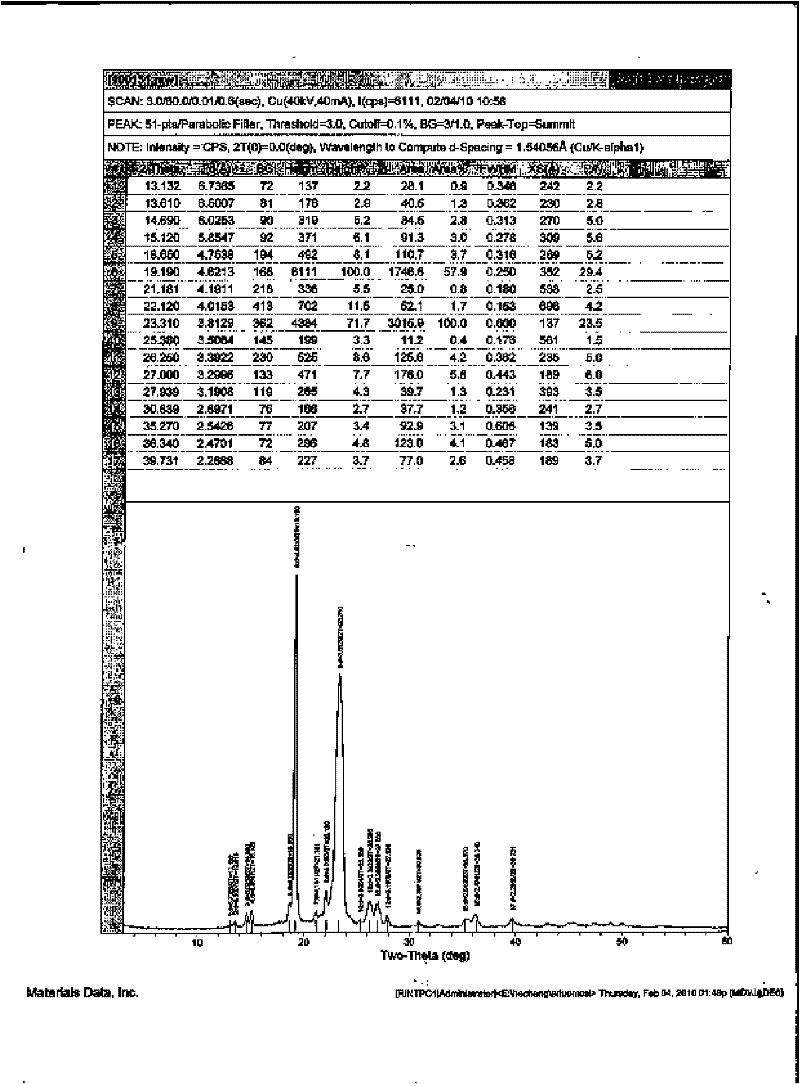
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising a selective cyclooxygenase-2 inhibitory compound of low water solubility in a therapeutically effective amount, wherein the compound is present in the form of solid particles, about 25% to 100% by weight of which are smaller than 1 micrometer. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders and have particular advantages where rapid onset of therapeutic effect is desired. The novel Form I and Form II crystalline forms of celecoxib are described. The crystalline forms have unique chemical and physical properties relative to other solid state forms of celecoxib and are characterized by their powder x-ray diffraction (PXRD) patterns, differential scanning calorimetric (DSC) thermograms, and other physical characterizations.
Owner:PHARMACIA CORP
Medicament microsphere and preparation method thereof
InactiveCN103610649AReduce solubilityHigh encapsulation efficiencyOrganic active ingredientsAntipyreticDipyridamoleMicrosphere
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a medicament microsphere and a preparation method thereof. The medicament microsphere comprises medicinal materials, a high polymer material and a surfactant, wherein the ratio of the medicament to the high polymer material is 1:(1-3); the medicament preferably is celecoxib, ketoprofen, dipyridamole and nimodipine. The medicament microsphere is prepared by adopting an O / W emulsified solvent diffusion-volatilization method. The O / W emulsified solvent diffusion-volatilization method comprises the following steps: respectively weighing the medicinal materials, the high polymer material and a release regulator according to the prescription amount, and then adding the weighted materials to an organic solvent; ultrasonically or mechanically stirring and dissolving as a disperse phase; taking a surfactant solution as a continuous phase; warming and stirring after low-temperature emulsification under an agitation state, so as to remove an organic solvent; and then carrying out solid separation, washing by distilled water, and drying, so as to obtain the medicament microsphere. Thus, the prepared microsphere is high in encapsulation efficiency and high in yield.
Owner:SHENYANG PHARMA UNIVERSITY
Celecoxib composition, and preparation method and use thereof
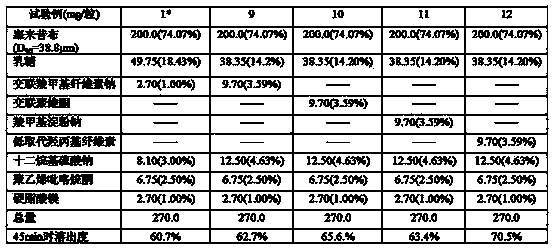
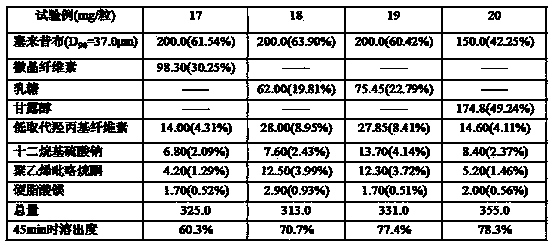
The invention provides a celecoxib composition. The celecoxib composition contains one or more types of dose units which can be orally released, and each of the dose units contains 50-500mg of celecoxib D95 particles and one or more types of mixtures of pharmaceutical excipients. The composition can be used for treating or preventing diseases caused by COX-2.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
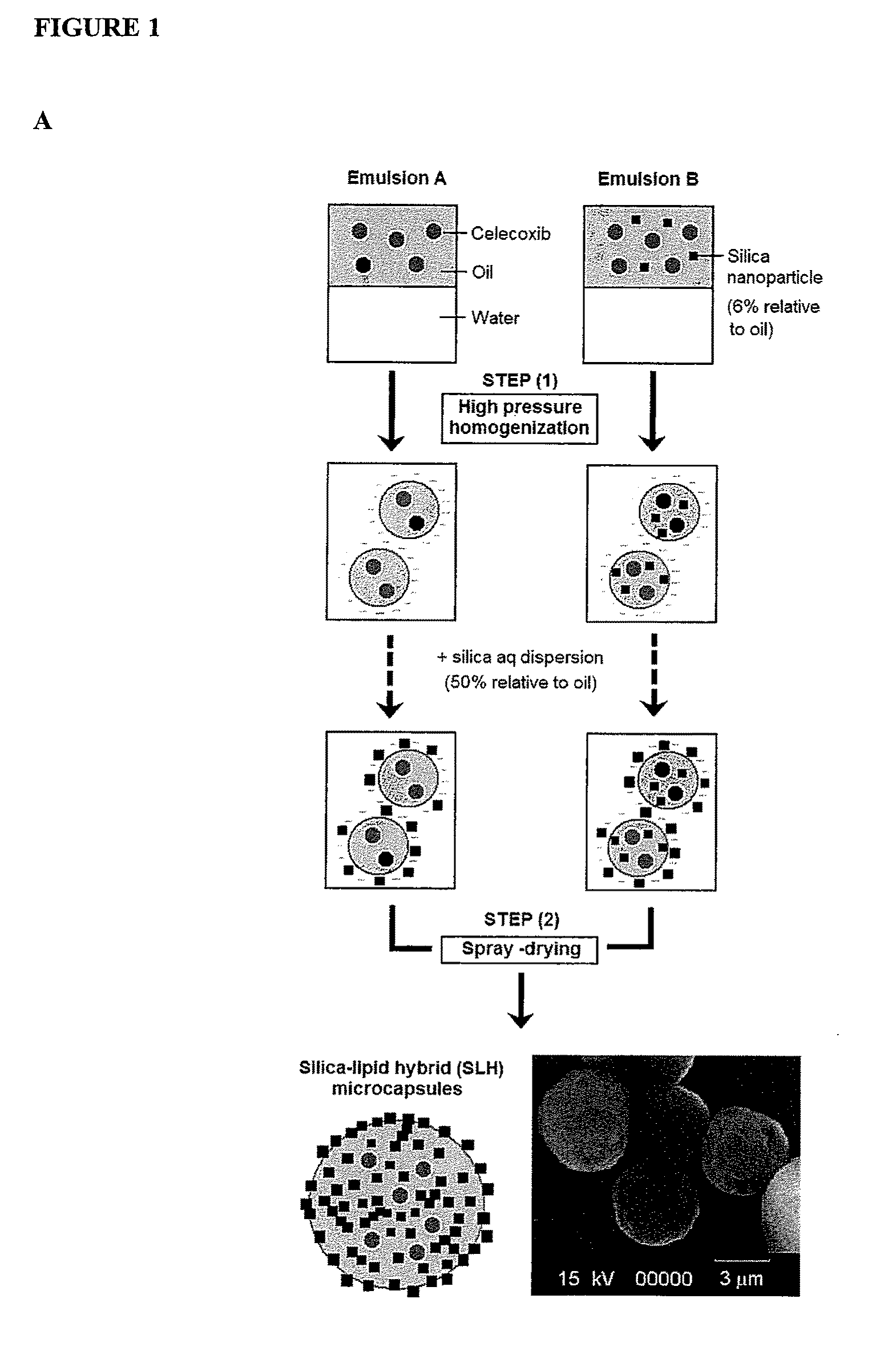
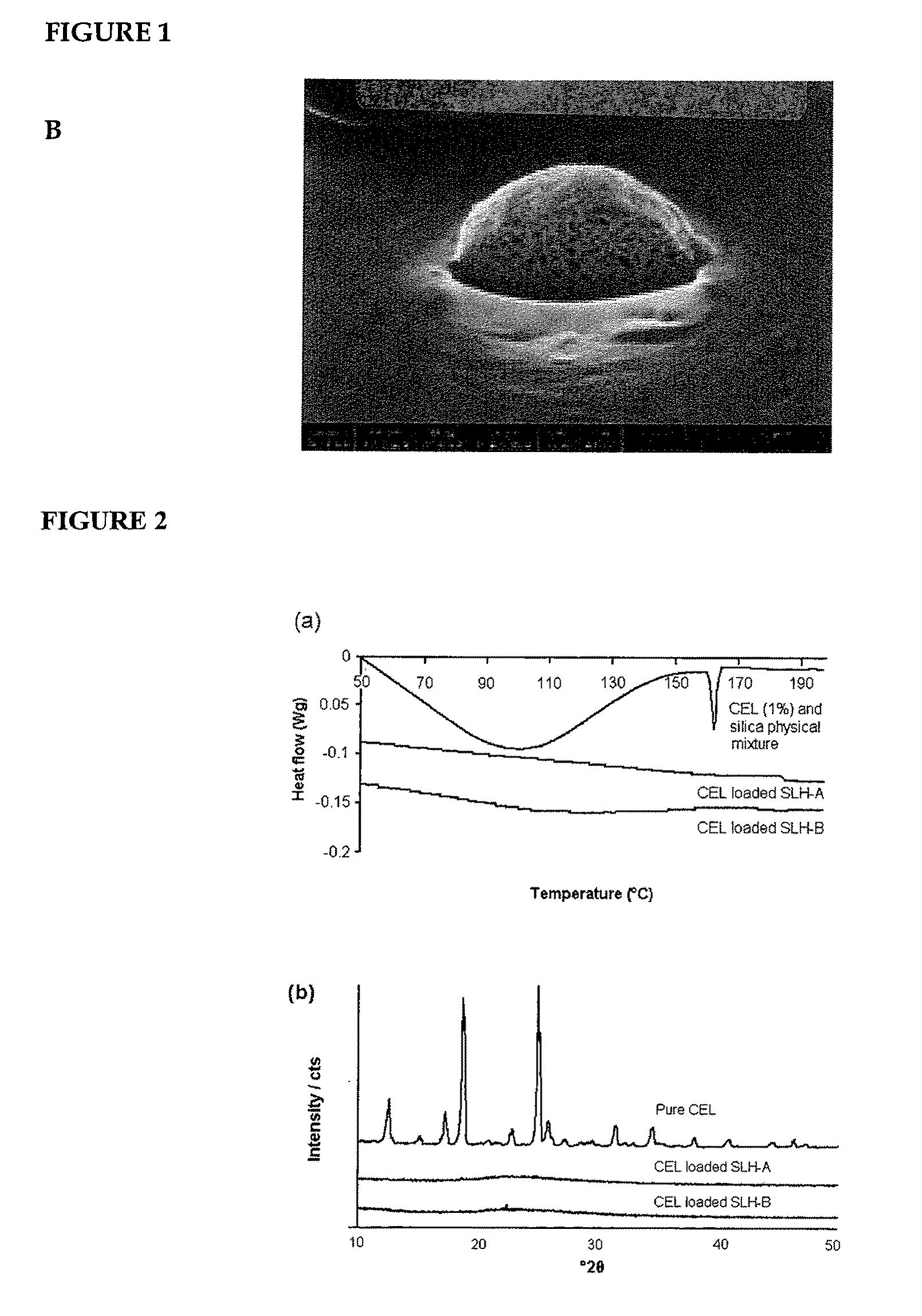
Nanoparticle-stabilized capsule formulation for treatment of inflammation
ActiveUS20090263486A1Quick releaseImprove bioavailabilityPowder deliveryBiocideAcetic acidAqueous droplet
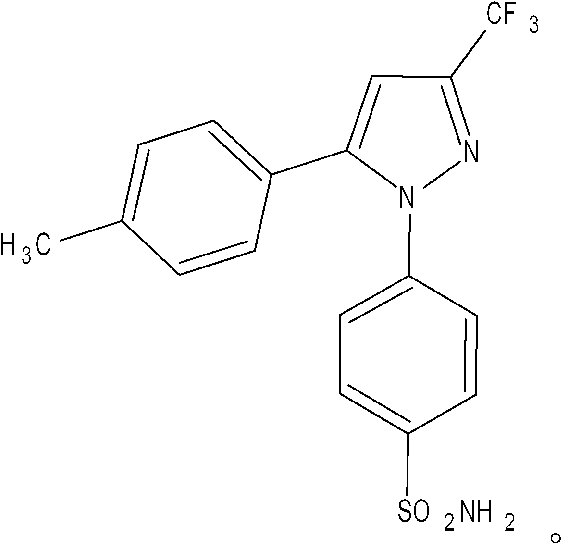
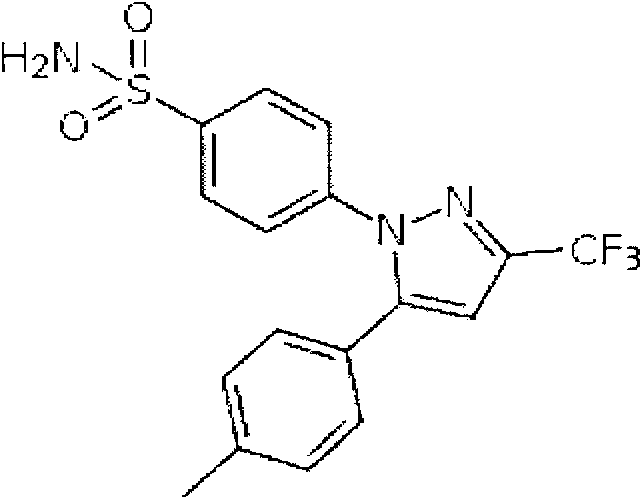
A formulation for the delivery of an anti-inflammatory agent to a subject is described. In one particular application of the invention, the formulation comprises oil-based or aqueous droplets comprising indomethacin (1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1-H-indole-3-acetic acid) or celecoxib (4-[5-(4-methylphenyl)-3-(trifluoromethyl) pyrazol-1-yl] benzenesulfonamide) stabilised by nanoparticles, particularly silica nanoparticles.
Owner:REFORMPHARM PTY LTD
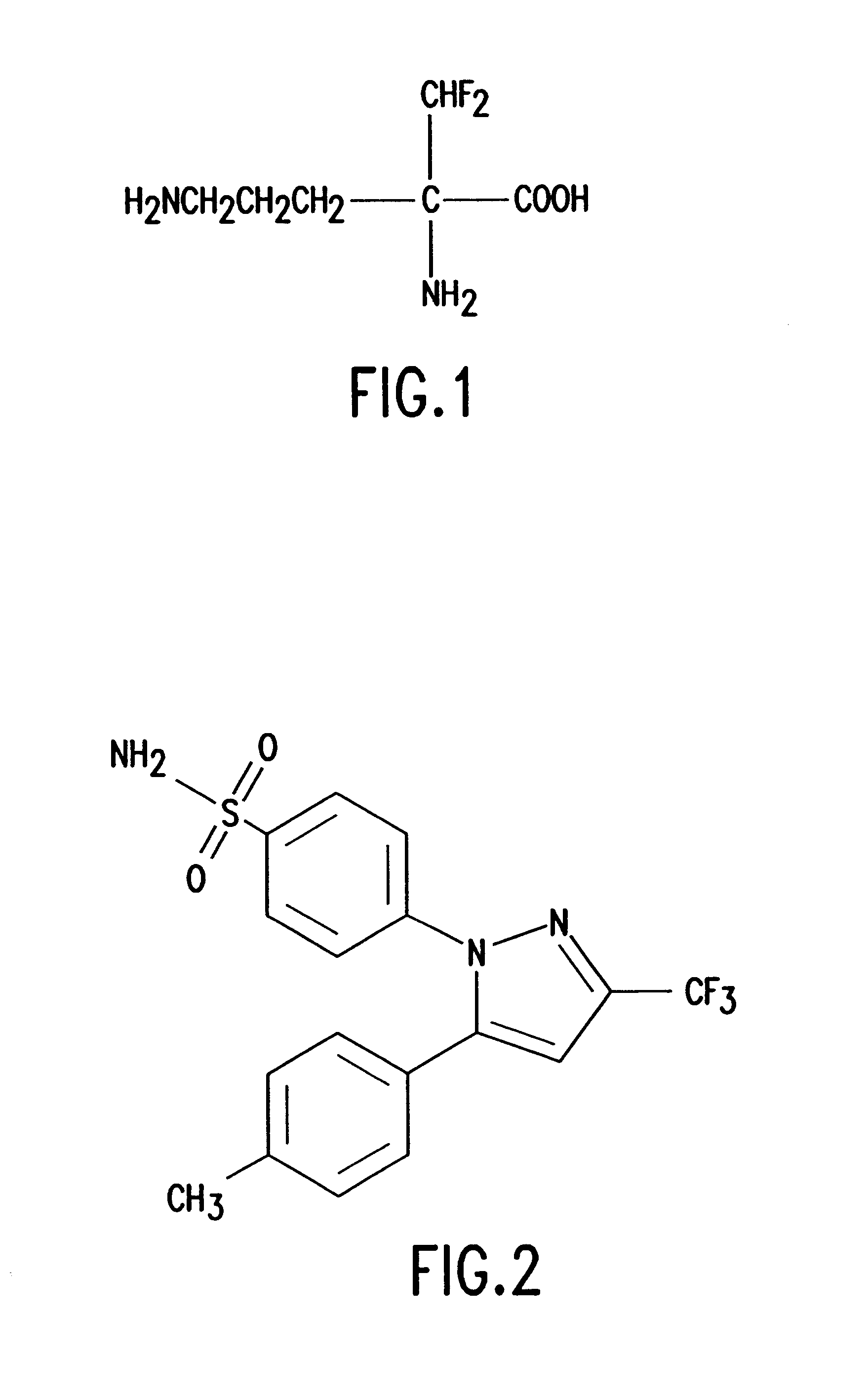
A kind of synthetic method of Erecoxib
ActiveCN104193664BHarm reductionThe reaction steps are simpleOrganic chemistry2-PyrrolidoneDrugs synthesis
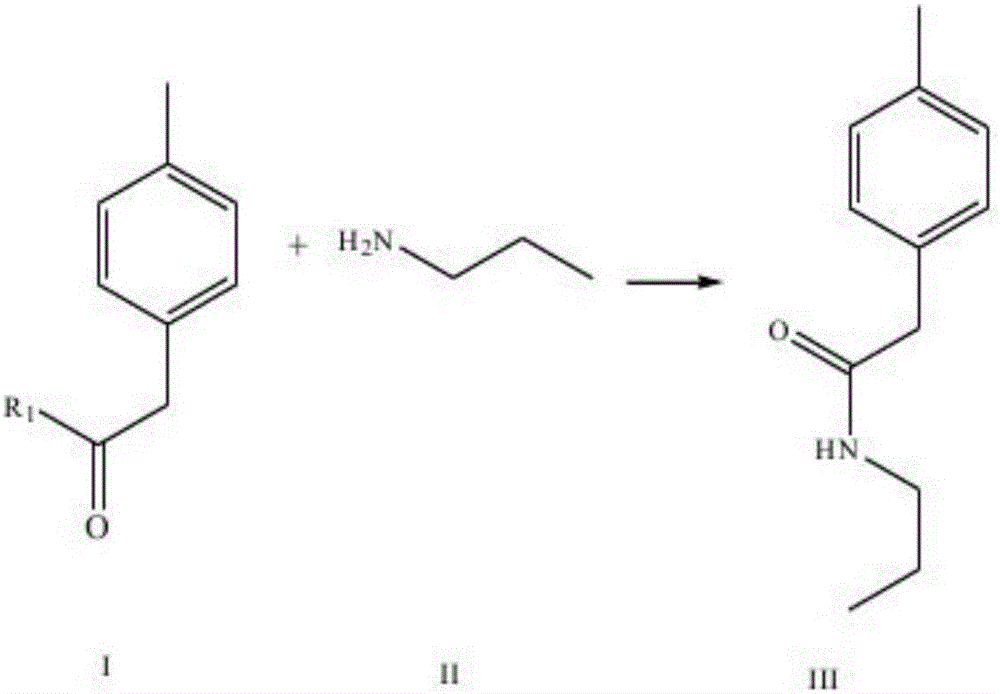
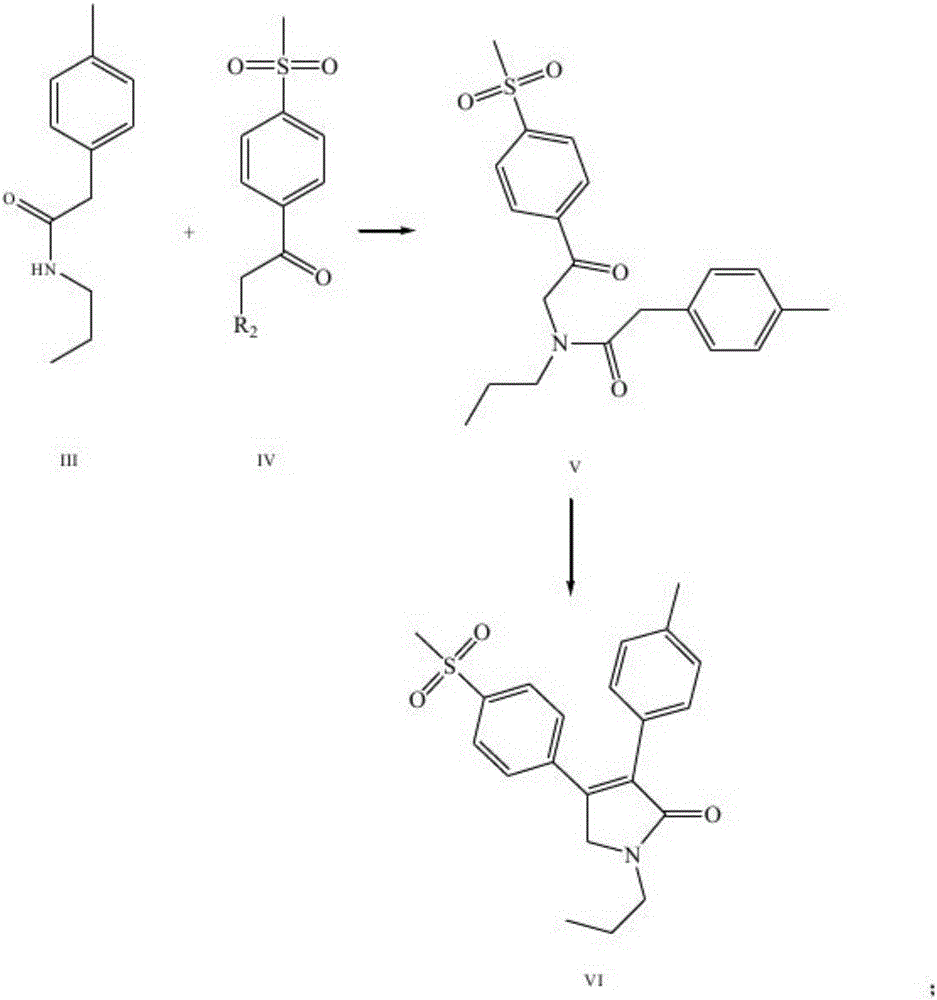
The invention discloses a synthesis method of imrecoxib and belongs to the field of drug synthesis. The method comprises the following steps: reacting p-toluene acetyl halide with propylamine to produce a compound III p-toluene levulinic amine; reacting the compound III with p-methylsulfonyl chloroacetophenone under an alkaline condition to produce a compound V; and then cyclizing to obtain a compound VII, namely n-propyl-3-(4-methyl phenyl)-4-(4-methylsulfonyl phenyl)-2,5-dihydro-1H-2-pyrrolidone. The method disclosed by the invention comprises simple reaction steps and can be put into industrial production easily.
Owner:SHANDONG BOYUAN PHARM CO LTD
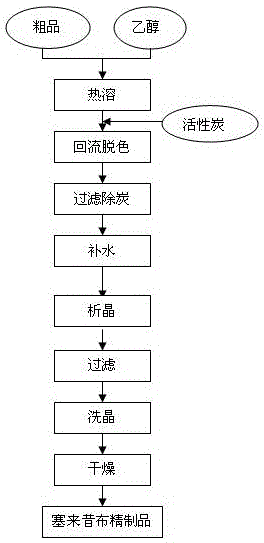
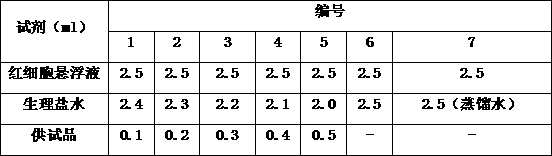
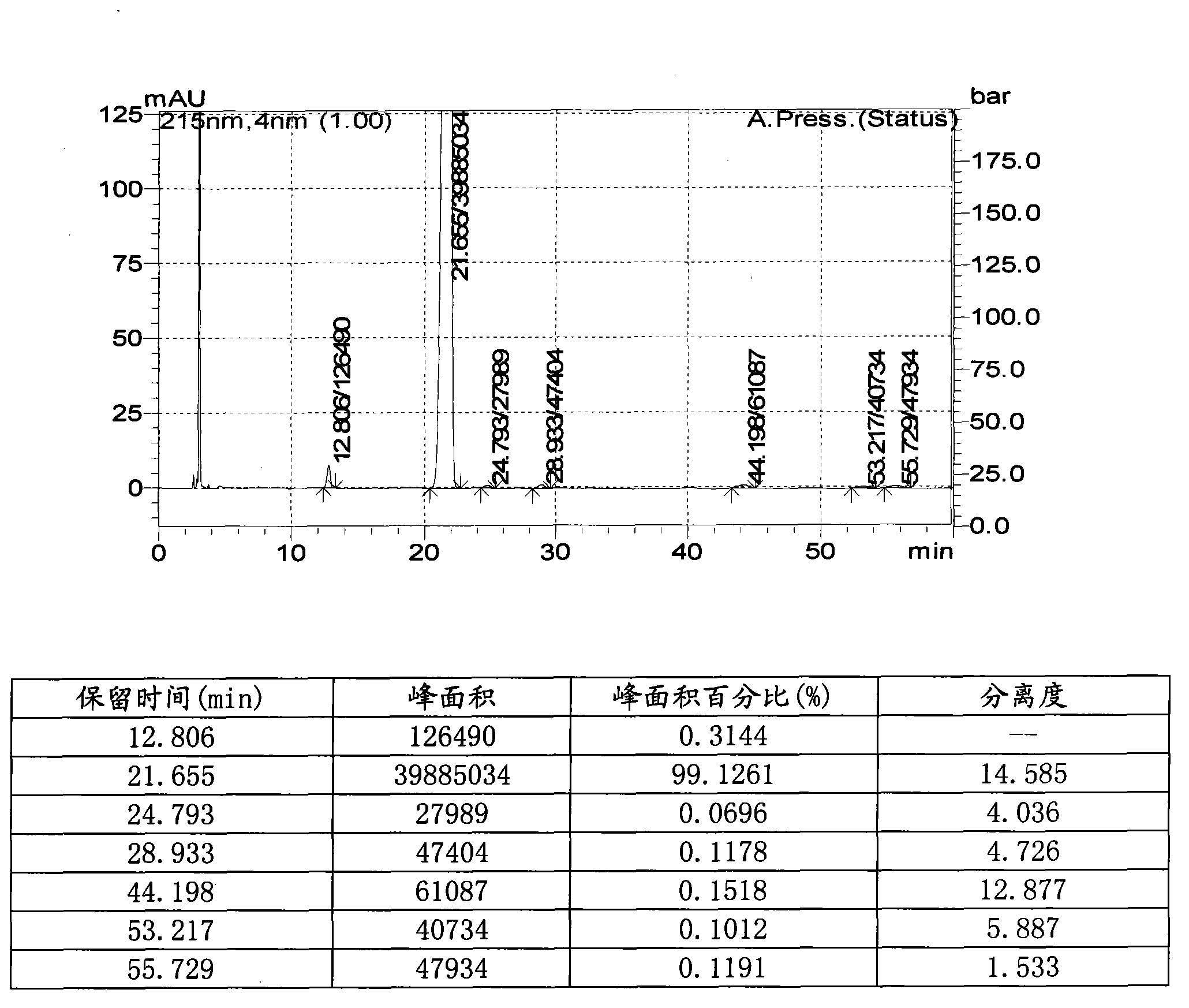
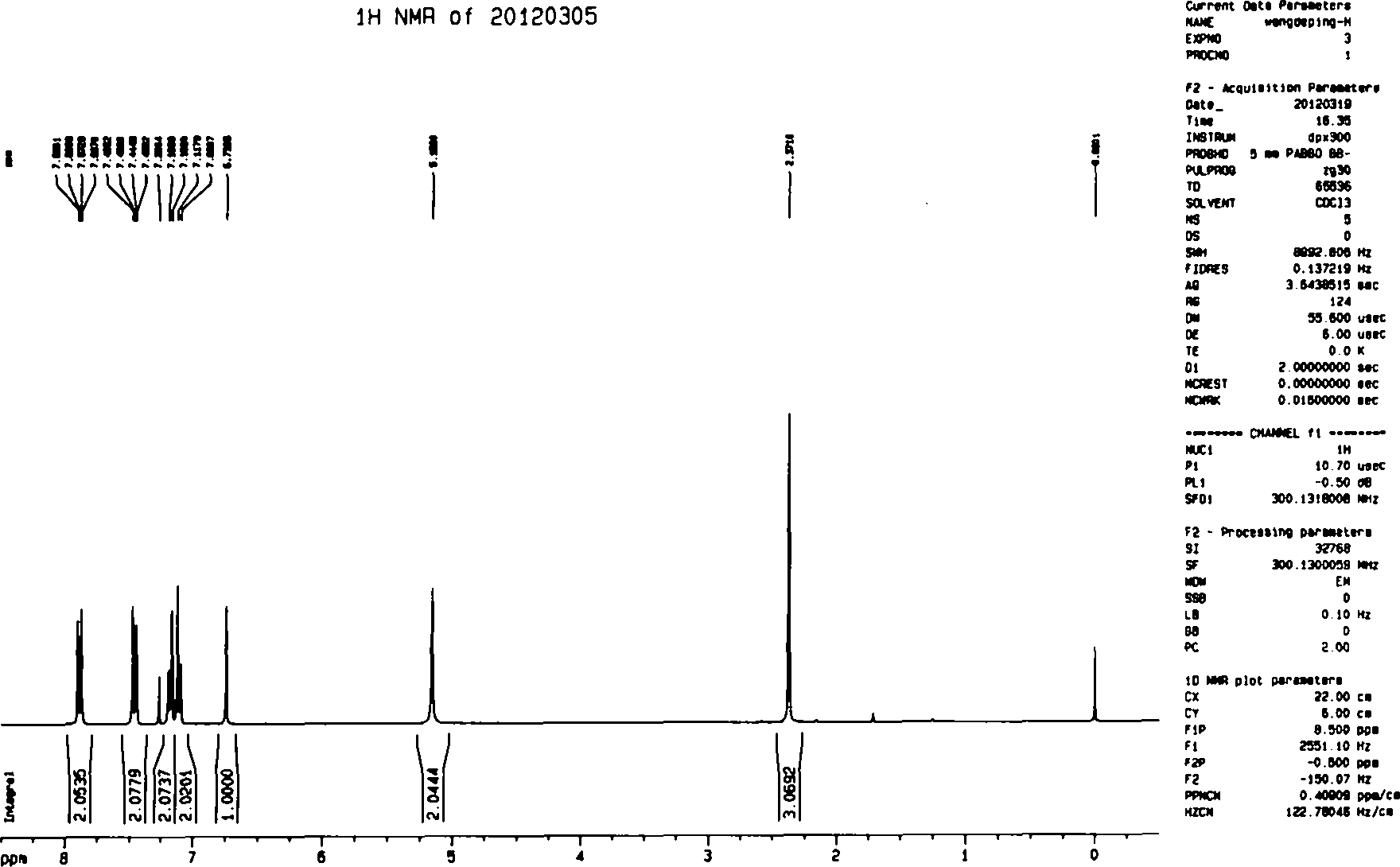
Preparation method of high-yield and high-purity celecoxib
The invention belongs to the technical field of preparation methods of drugs, and particularly relates to a preparation method of high-yield and high-purity celecoxib. The preparation method of the high-yield and high-purity celecoxib comprises the following steps: preparing a salt solution of 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione, preparing an alcohol water solution of hydrazinobenzene-1-sulfonamide hydrochloride, conducting reaction, drying, and refining. The preparation method has the benefits that compared with the traditional method, with the adoption of the preparation method of the celecoxib, the yield and purity are increased; the yield of the celecoxib reaches above 91%; the purity reaches above 99%; and the method is simple in technology, and can be applied to large-scale production easily.
Owner:山东诚汇双达药业有限公司
Celecoxib solid composition with increased dissolution rate, and preparation method and application thereof
ActiveCN103585164AImprove the defect of slow dissolutionPromote dissolutionOrganic active ingredientsAntipyreticPsychotherapeutic drugsDissolution
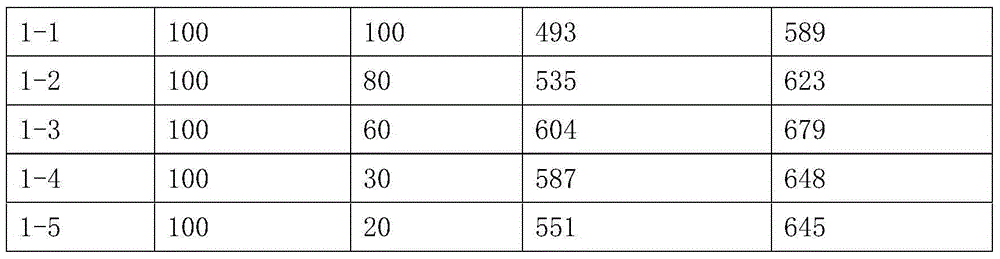
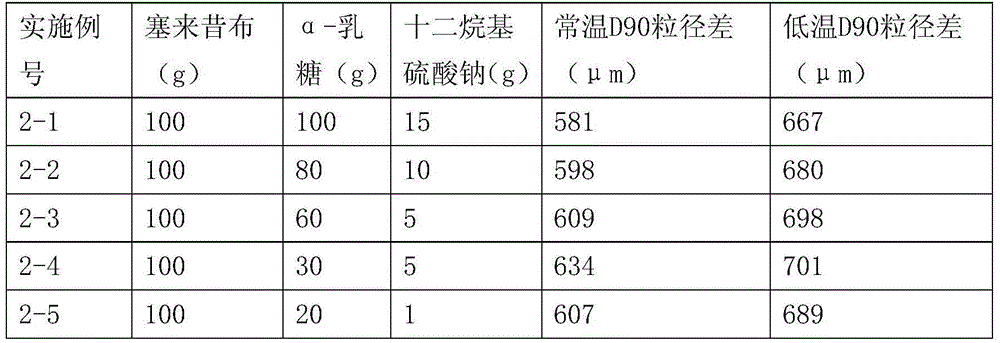
The invention relates to a psychotherapeutic drug for OA (Oarthritis) and RA (Rheumatoid Arthritis), in particular to a celecoxib solid composition with an increased dissolution rate, and the preparation method and application thereof. The celecoxib solid composition comprises celecoxib, a dispersion promoter and an alkali compound, wherein the mass ratio of the celecoxib, the dispersion promoter and the alkali compound is 100 : (5- 95) : (0.1- 1.5); the D90 of the celecoxib solid composition ranges from 5 to 20 micron. The celecoxib solid composition provided by the invention solves the technological difficulties of strong material static and low probability of powder mixing no matter under a condition that the celecoxib bulk drug is monocrystal or mixed polycrystal, so that the problem that the celecoxib is hydrophilic is solved, the physical property that the drug is difficult to dissolve is improved, and the dissolution rate of the drug is increased, thereby being beneficial to drug absorbing.
Owner:HAINAN HERUI PHARMA
Synthesis method of celecoxib
InactiveCN102391184AHigh purityHigh yieldOrganic chemistryPhenylhydrazine hydrochlorideClaisen condensation
The invention relates to a synthesis method of celecoxib, which comprises the following specific steps of: 1, carrying out claisen condensation on p-methylacetophenone and trifluoroacetic acid ethyl esters in an aprotic organic solvent under the catalysis of alkali to obtain 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione; and 2, reacting the obtained 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione with sulfonamide-phenylhydrazine hydrochloride to obtain celecoxib, wherein in the step 1, the alkali for catalysis is selected from one or more of sodium hydride, potassium hydride, lithium hydride and calcium hydride. The synthesis method of celecoxib provided by the invention is easy to operate, high in yield, high in product purity and easy for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Parenteral compositions of celecoxib
Parenteral (injectable) celecoxib emulsions and nanoemulsions are disclosed as are their use to treat pain in patients so afflicted. The emulsions are generally oil in water emulsions often comprised of an oil phase including an oil and a lecithin wherein the mean droplet size of the discontinuous oil phase is about 200 nanometers or less.
Owner:DR REDDYS LAB LTD
Celecoxib preparation method
InactiveCN105130901AFine granularitySuitable for productionOrganic chemistryClaisen condensationAcetophenone
The present invention relates to a celecoxib preparation method, wherein 4'-methylacetophenone and ethyl trifluoroacetate are adopted as raw materials, Claisen condensation is performed to obtain a beta-diketone intermediate, the beta-diketone intermediate and p-hydrazinobenzenesulfonamide hydrochloride are subjected to condensation cyclization in ethanol to obtain celecoxib, and refining and crystallization are performed to obtain the celecoxib crystal. According to the present invention, with the preparation method, the celecoxib can be obtained in the high yield manner, the purity of the obtained product is high, the single impurity can be controlled to be less than or equal to 0.5%, and the obtained celecoxib crystal has characteristics of fine particle size and uniform distribution, and is suitable for bulk drug production.
Owner:SUZHOU ERYE PHARMA CO LTD
Novel celecoxib composition and preparation process thereof
The invention relates to a novel composition of celecoxib and glucosyl-cyclodextrin or sulfobutyl-beta-cyclodextrin as well as a preparation method of the composition and application in medicine preparations. According to the invention, the prepared composition of celecoxib and glucosyl-cyclodextrin or sulfobutyl-beta-cyclodextrin has the advantages of improving the water solubility and the stability and has no adverse effect of hemolysis, so that the composition can serve as a starting material or a constituent for preparing intestinal-administration preparations or non-intestinal-administration preparations for treating tumors or rheumatoid arthritis; and the solid preparations have high bioavailability and the freeze-dried powder injections meet the requirements on the aspect of pH value and have no adverse effect of hemolysis.
Owner:北京博爱旺康医药科技有限公司
Inclusion compound containing celecoxib and preparation method thereof
InactiveCN103405782AImprove solubilityImprove in vitro dissolutionOrganic active ingredientsAntipyreticOrganic solventFreeze-drying
The invention relates to an inclusion compound containing celecoxib and a preparation method thereof. The inclusion compound containing celecoxib comprises celecoxib and an inclusion material, wherein the inclusion material is preferable one or two of beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin; the weight ratio of the inclusion material to the celecoxib is (0.5-10):1. The preparation method comprises the following steps: respectively dissolving the inclusion material and the celecoxib in water and an organic solvent; slowly dropwise adding the saturated aqueous solution of the inclusion material into the organic solution containing the celecoxib; continuously stirring to form a white suspension; preparing the inclusion compound containing the celecoxib by adopting a vacuum drying or freeze-drying mode. The inclusion compound can be crushed to be prepared into various solid preparations. According to the inclusion compound containing celecoxib, the problem of extremely poor dissolubility of celecoxib in water can be solved, a high-bioavailability solid preparation with stable property can be prepared by adopting a simple and practical process, and the problem that a celecoxib raw material medicine is difficult to crush can be solved.
Owner:JIANGSU QINGJIANG PHARMA
Celecoxib new formulation and preparation method thereof
InactiveCN103191065ASolve the problem of low dissolution rateAccelerate dispersal and absorptionOrganic active ingredientsAntipyreticAdenocarcinoma polypsAnkylosing spondylitis
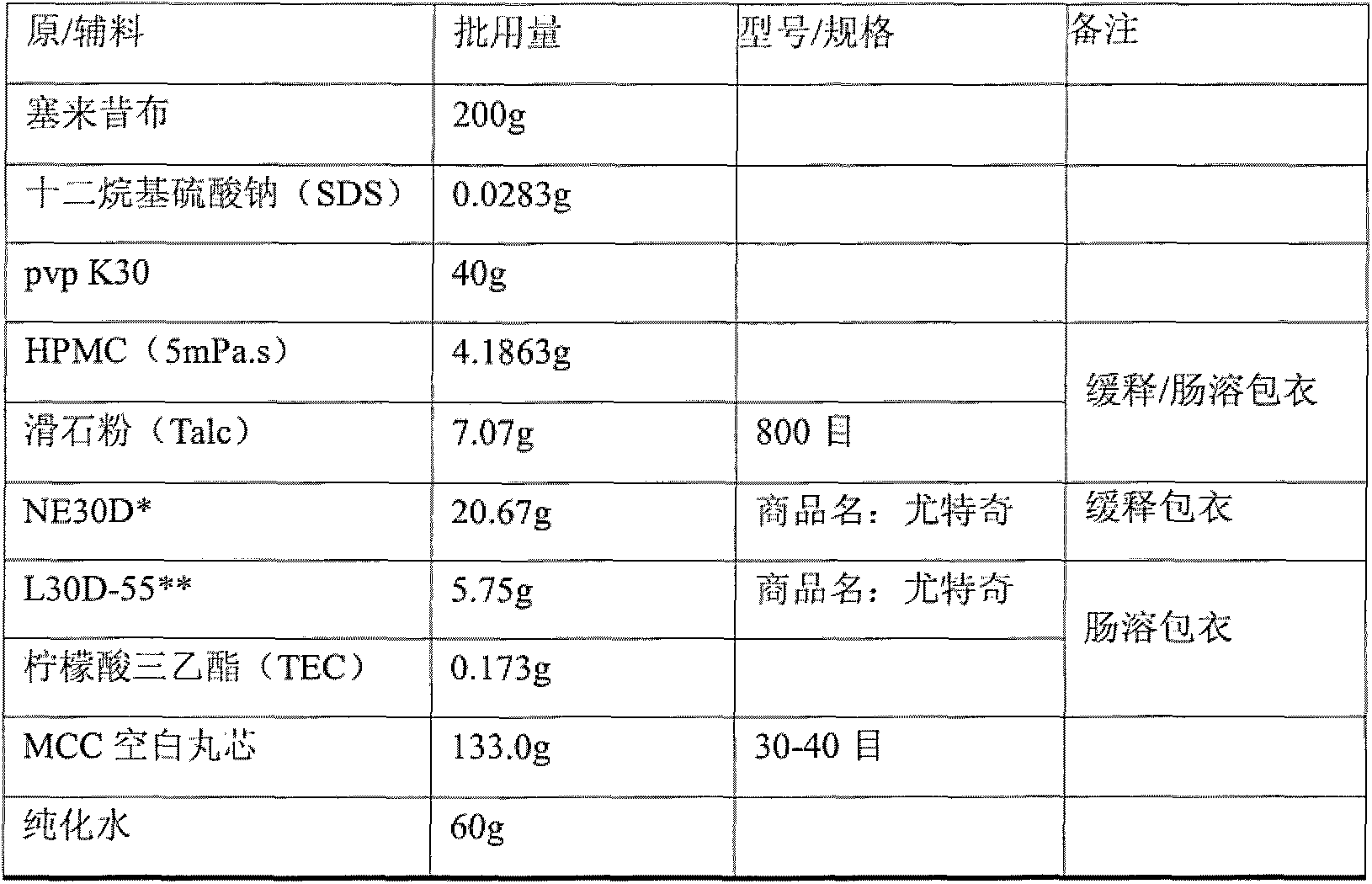
The invention discloses a celecoxib pellet preparation, which is used for treating rheumatic arthritis, osteoarthritis and ankylosing spondylitis, and also can be used for treating acute pain, dysmenorrheal, colorectal polyps, post-operation analgesia, low back pain, periarthritis of shoulder and tenosynovitis. According to the celecoxib new formulation and the preparation method thereof, the pellet preparation technology and the controlled-release pellet upper covering technology are adopted and microcrystalline cellulose pills are selected for dissolving the celecoxib material medicines in adhesive povidone solution, the medicine materials are uniformly sprayed on the surface of the pills and covered with isolating layers, so that the pellet is pressed and capsule. When the disintegration time limit of the celecoxib pellet preparation is remarkably shortened and the bioavailability is remarkably improved.
Owner:GUIZHOU LIANSHENG PHARMA
Solid dispersion containing celecoxib as well as preparation method and application thereof
InactiveCN102000018BImprove solubilityEasy to manufactureOrganic active ingredientsAntipyreticPolyethylene glycolCelecoxib
The invention relates to a solid dispersion containing celecoxib as well as a preparation method and application thereof. The invention needs to solve the technical problems of difficult pulverization of raw materials and difficult preparation of solid oral preparation of celecoxib. The solid dispersion is a solid dispersion containing the celecoxib and polyethylene glycol which are in the weightratio of 1:0.5-10. The preparation method comprises the following steps of: melting solid polyethylene glycol by heating, adding the celecoxib and dissolving to obtain a clear solution; or mixing andheating the solid polyethylene glycol and the celecoxib, and melting to obtain a clear solution; and then rapidly cooling to obtain the solid dispersion. The solid dispersion can be prepared into various oral preparations after being pulverized.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
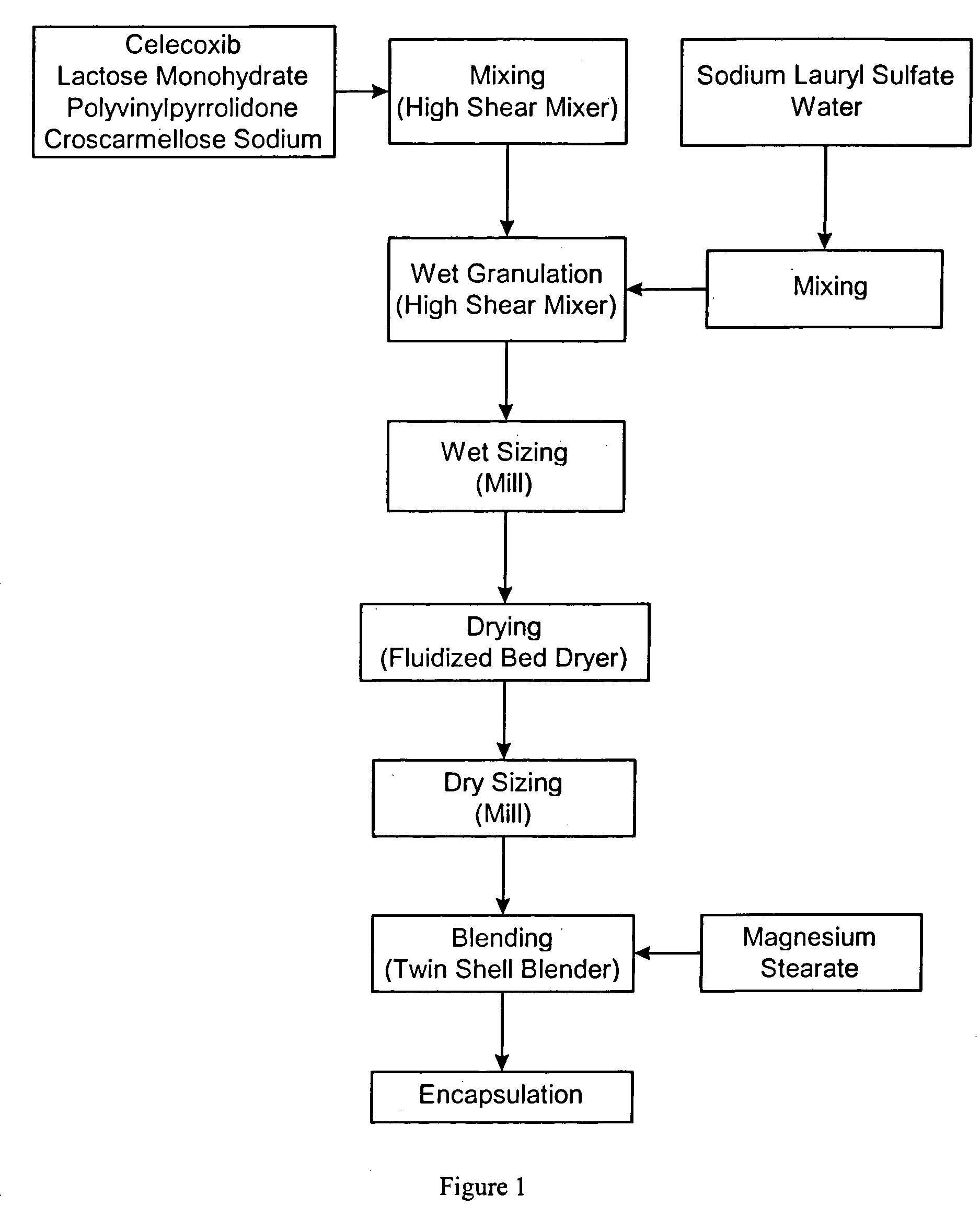
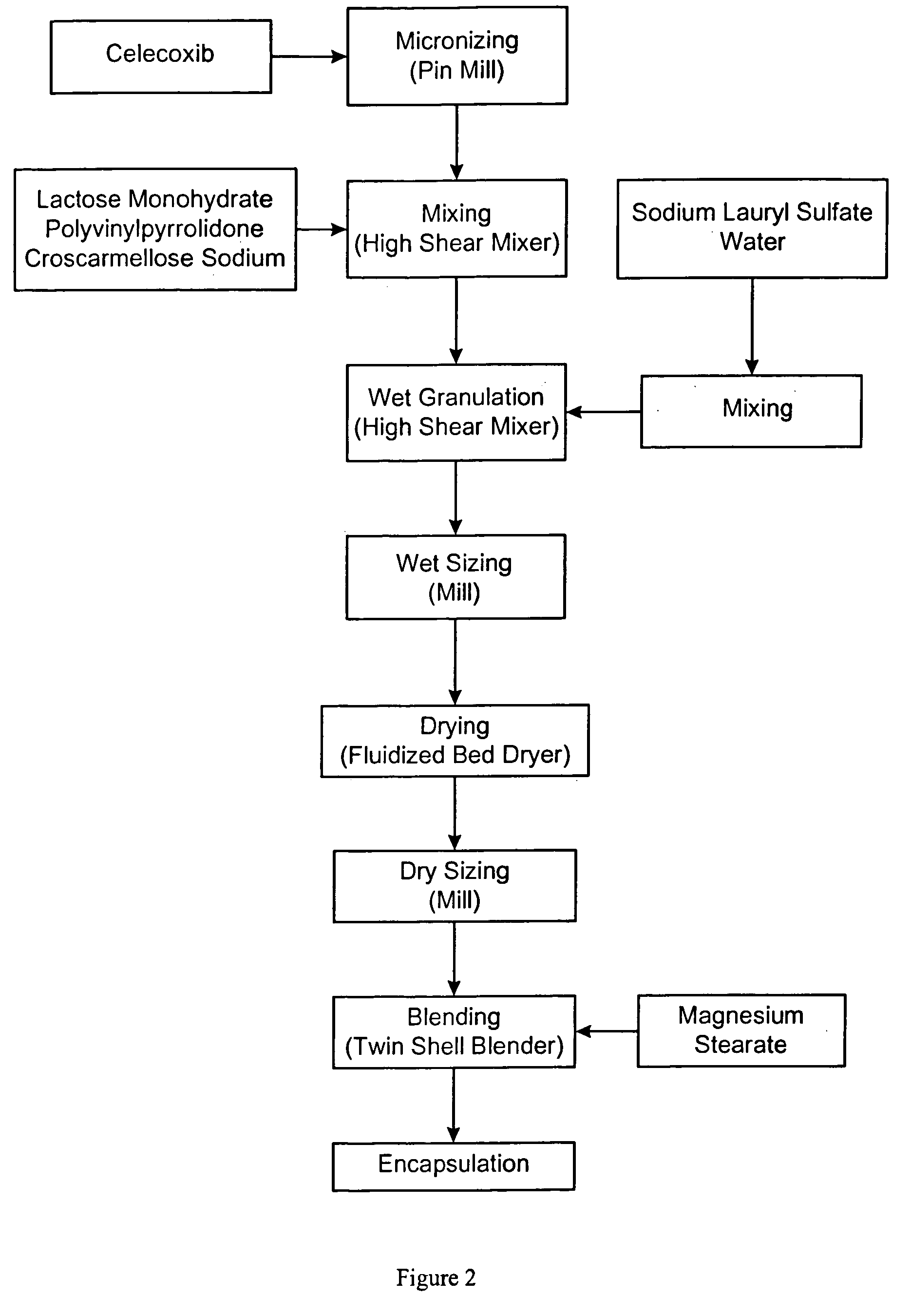
Celecoxib-containing capsule
ActiveCN103989657AOrganic active ingredientsAntipyreticLow-substituted hydroxypropylcelluloseDodecyl sulfate
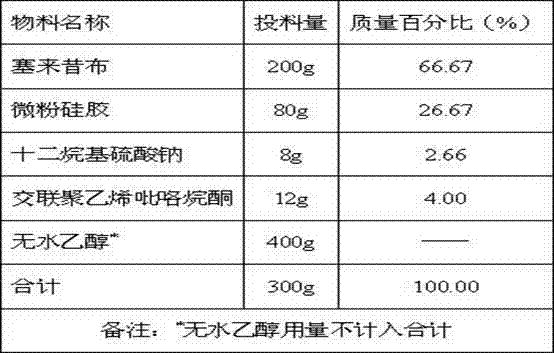
The invention relates to a celecoxib-containing capsule, which contains 40wt%-70wt% of celecoxib, 22wt%-50wt% of a water-soluble filler, 0.5wt%-25wt% of low substituted hydroxypropyl cellulose, and 0.5wt%-10wt% of sodium dodecyl sulfate. The celecoxib capsule with a prescription provided by the invention has no special requirement for the particle size of celecoxib, the celecoxib bulk drug directly synthesized by a conventional synthesis method can be taken as a preparation raw material, and no special treatment is needed. The preparation process is simple, the product quality is uniform and stable, and has effective bioavailability.
Owner:SICHUAN GOWELL PHARMA
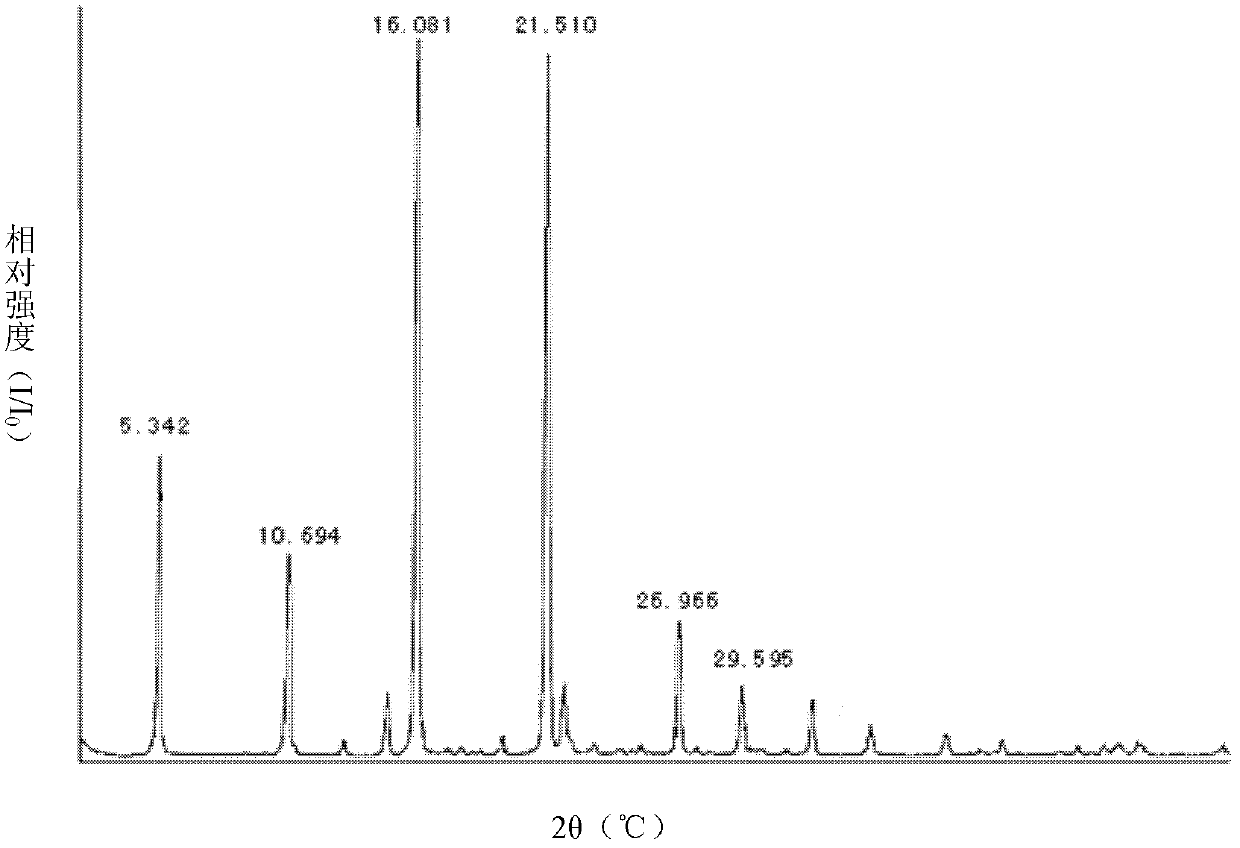
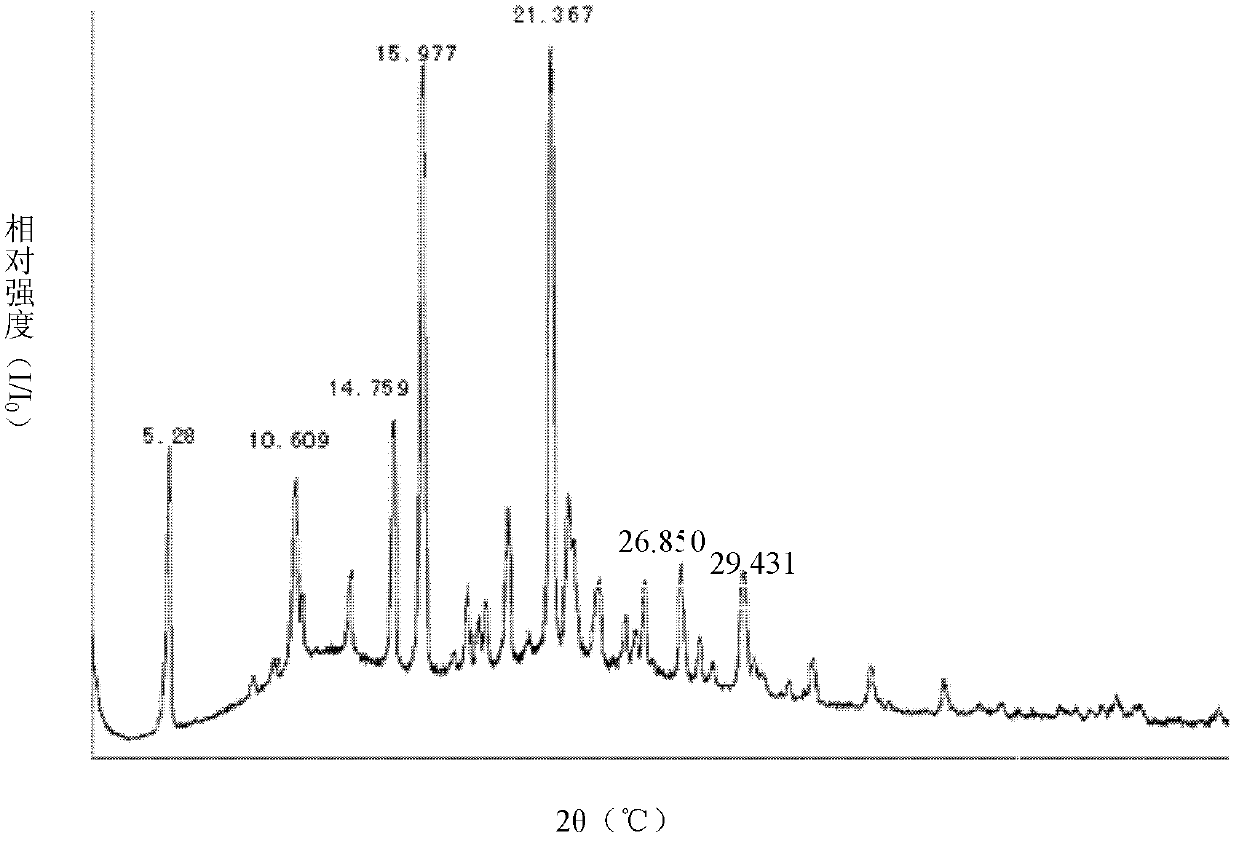
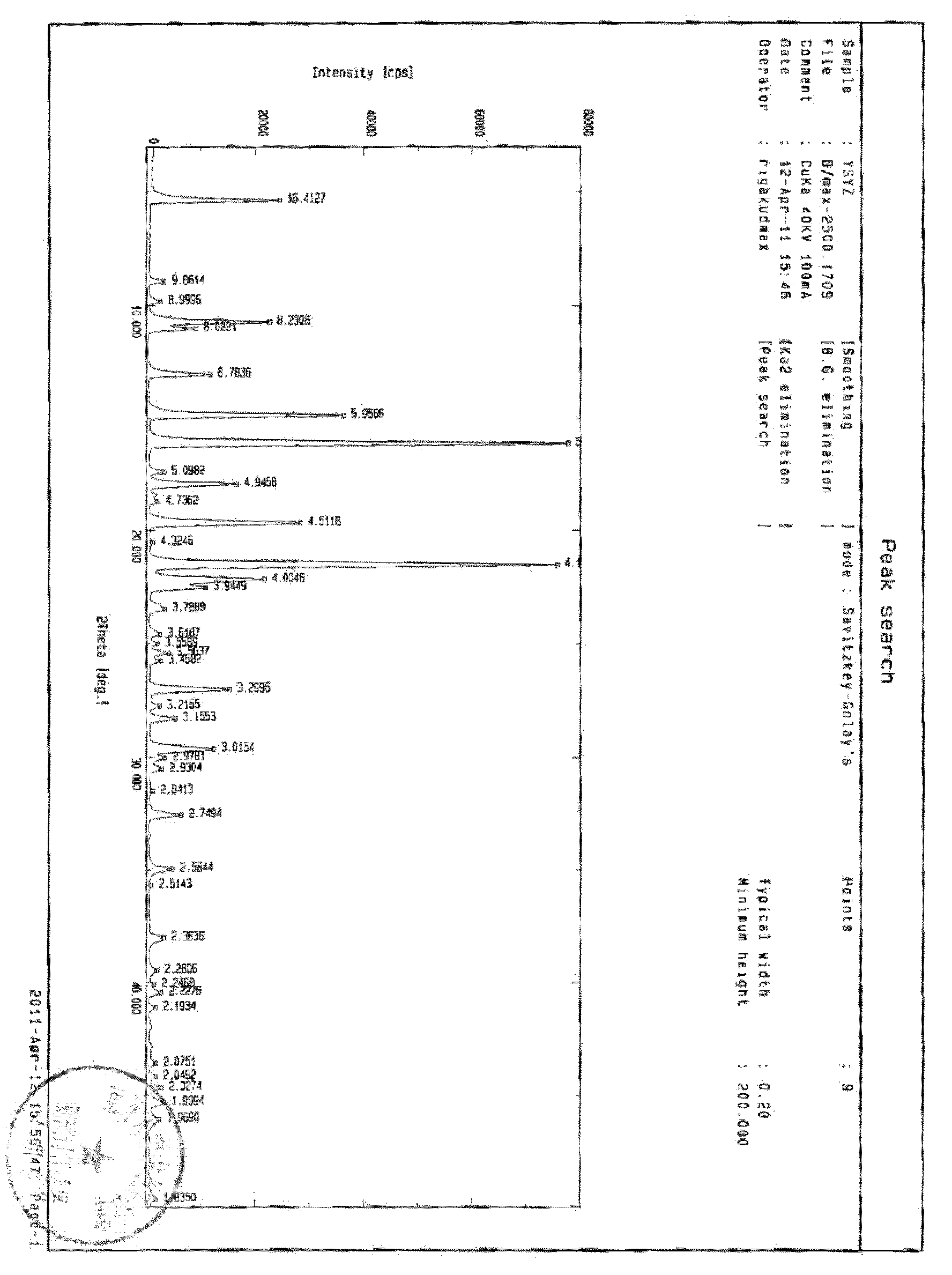
Crystalline form I of celecoxib, preparation method and purpose thereof
The invention relates to a crystalline form I of celecoxib and a preparation method thereof, and further relates to a pharmaceutical composition prepared by using the crystalline form I of celecoxib obtained by the invention, and an application of the pharmaceutical composition. The crystalline form I of celecoxib is characterized by an x-ray powder diffraction pattern spectrum, a differential thermal analysis spectrum and an infra-red spectrogram thereof.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Lactose celecoxib pharmaceutical composition
InactiveCN105168137AAvoid yellowingImprove solubilityOrganic active ingredientsPowder deliveryCoprecipitationLactose
Owner:TIANJIN JINYAO GRP
Celecoxib solid dispersion and preparation method and application thereof
InactiveCN103655478AGood dissolution effectGood disintegrationOrganic active ingredientsPowder deliverySolubilityDispersity
The invention specifically relates to a celecoxib solid dispersion and a preparation method and application thereof. The celecoxib solid dispersion is prepared from celecoxib and poloxamer 188 at a mass ratio of 1:(1-20) by a melting process and a solvent process. The dispersion provided by the invention has good dispersity and high stability; the solubility and dissolution rate of the medicine are increased; the solubility of the medicine in water is 5-200 times higher than that of the raw medicines; with good dissolution rate in water, the in-vivo absorption of the medicine can be enhanced so as to improve the bioavailability. The solid dispersion also can be made into multiple clinically acceptable dosage forms which are used as new dosage forms of celecoxib.
Owner:BEIJING SUNHO PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com