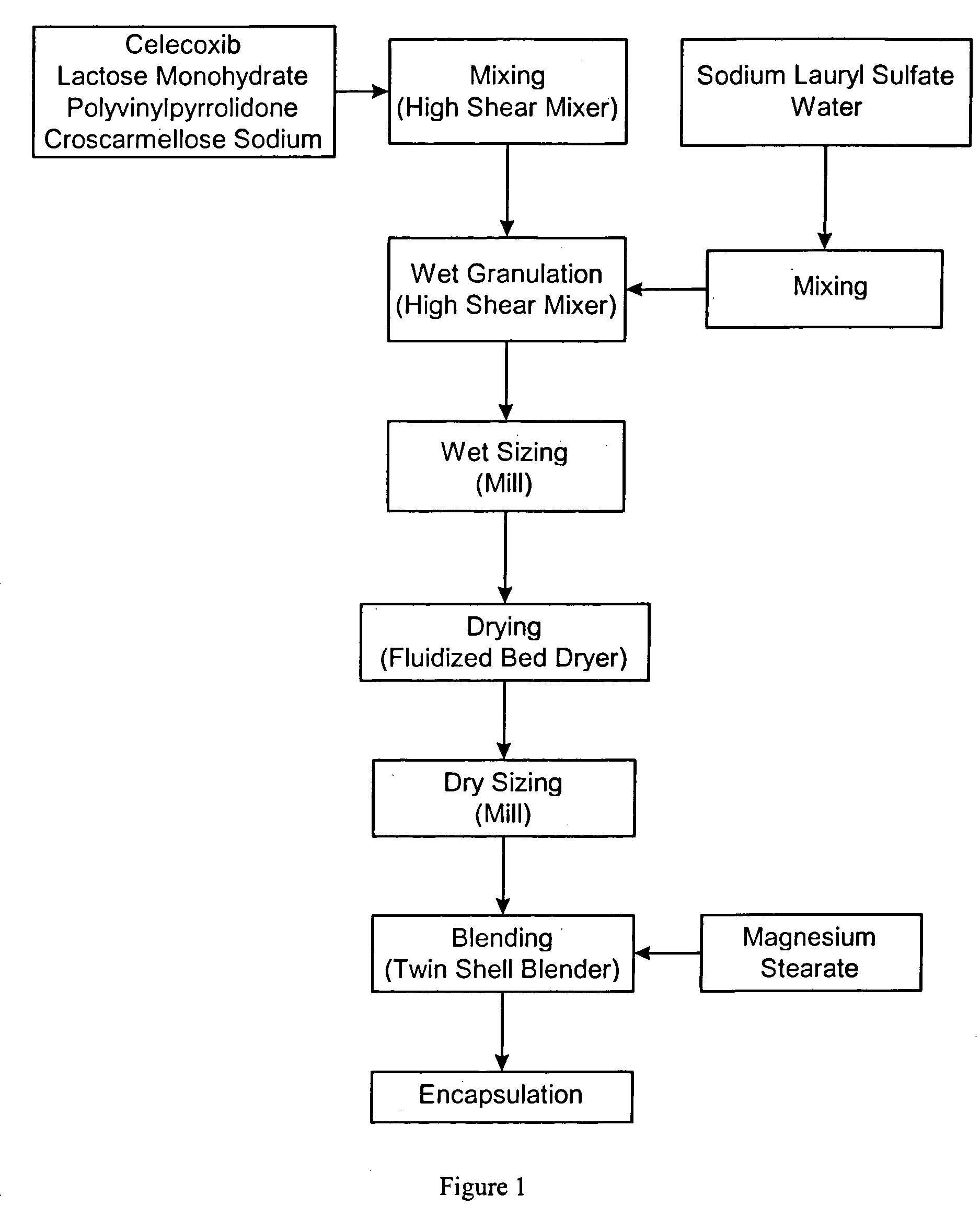
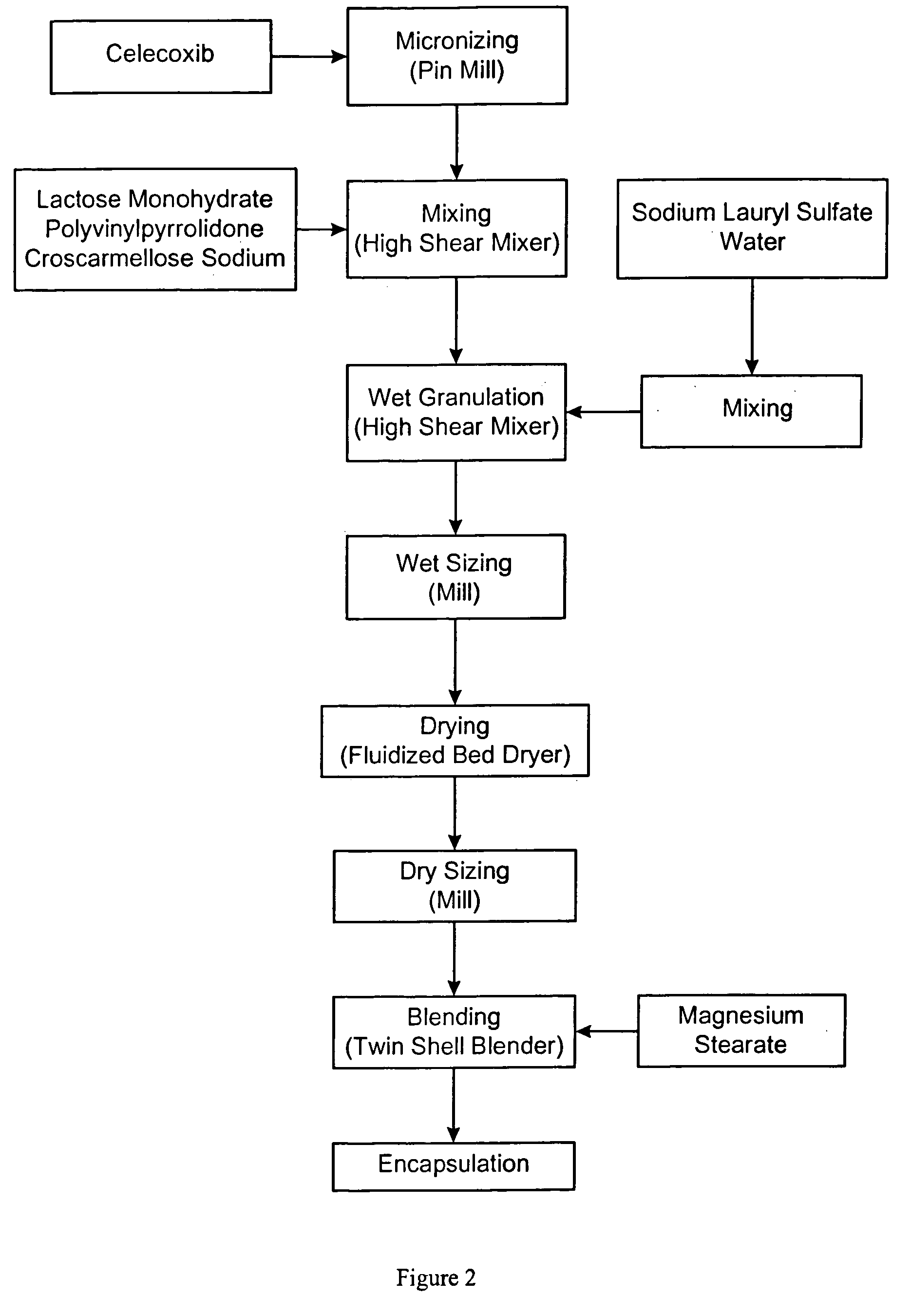
Celecoxib compositions
a composition and celecoxib technology, applied in the field of oral deliveryable pharmaceutical compositions, can solve the problems of undesirably large aggregates of celecoxib in the composition, difficult to prepare a pharmaceutical composition containing celecoxib that has the desired blend uniformity, and complicated formulation of celecoxib for effective oral administration to a subject. achieve the effect of relative bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0327] A capsule was prepared having the following composition:
TABLE 1WeightIngredientfraction (%)Amount (mg)Celecoxib37.04100Lactose monohydrate (NF, Ph Eur)55.46149.75Sodium lauryl sulfate (NF, Ph Eur)38.1Povidone (K29-32 USP)2.56.75Croscarmellose sodium (NF, Ph Eur)12.7Magnesium stearate (NF, Ph Eur)12.7Total capsule fill weight100270
[0328] The above unit dose composition was placed in a hard gelatin capsule (white opaque, size #2) comprising titanium dioxide (USP), gelatin (NF), and blue ink (SB-6018).
[0329] The lactose monohydrate used in each of the examples herein is commercially available from Formost Farms, Baraboo, Wis. The Ac-Di-Sol brand of croscarmellose sodium used in each of the examples herein is commercially available from FMC Corporation, Chicago, Ill. The sodium lauryl sulfate used in each of the examples herein is commercially available from Henkel Corporation, Cincinnati, Ohio. The povidone (polyvinylpyrrolidone) used in each of the exampl...
example 2
[0331] A capsule was prepared having the following composition:
TABLE 2IngredientWeight fraction (%)Amount (mg)Celecoxib74.07200Lactose monohydrate (NF, Ph Eur)18.4349.75Sodium lauryl sulfate(NF, Ph Eur)38.10Povidone (K29-32 USP)2.56.75Croscarmellose sodium12.7Magnesium stearate (NF, Ph Eur)12.7Total capsule fill weight100270
[0332] The above unit dose composition was placed in a hard gelatin capsule (white opaque, size #2) comprising titanium dioxide (USP), gelatin (NF), and blue ink (SB-6018).
example 3
100 mg Dose Tablet
[0333] Tablets were prepared having the following composition:
TABLE 3Amount / tabletWeightAmount / batchIngredient(mg)fraction (%)(kg)Celecoxib100406.40Lactose monohydrate (NF)101.8840.756.52Sodium lauryl sulfate (NF)7.530.48Povidone (K29 / 32, USP)6.252.50.40Croscarmellose sodium7.530.48(Type A, NF)Microcrystalline cellulose25101.60(Avicel PH-102, NF)Magnesium stearate (NF)1.880.750.12Total250.0110016Opadry White YS-1-18027A7.50
[0334] The tablets prepared were 0.210 inch×0.465 inch (5.0 mm×11.2 mm) modified oval shaped tablets.
[0335] The Avicel brand of microcrystalline cellulose was used in the preparation of the tablets of Examples 3 and 4 and is commercially available from FMC Corporation, Philadelphia, Pa.
[0336] Tablet dose strengths between 25 mg to 225 mg can be accomodated by increasing or decreasing the amounts of celecoxib and each of the carrier materials described above so as to maintain the same weight fractions exemplified above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle sizes | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com