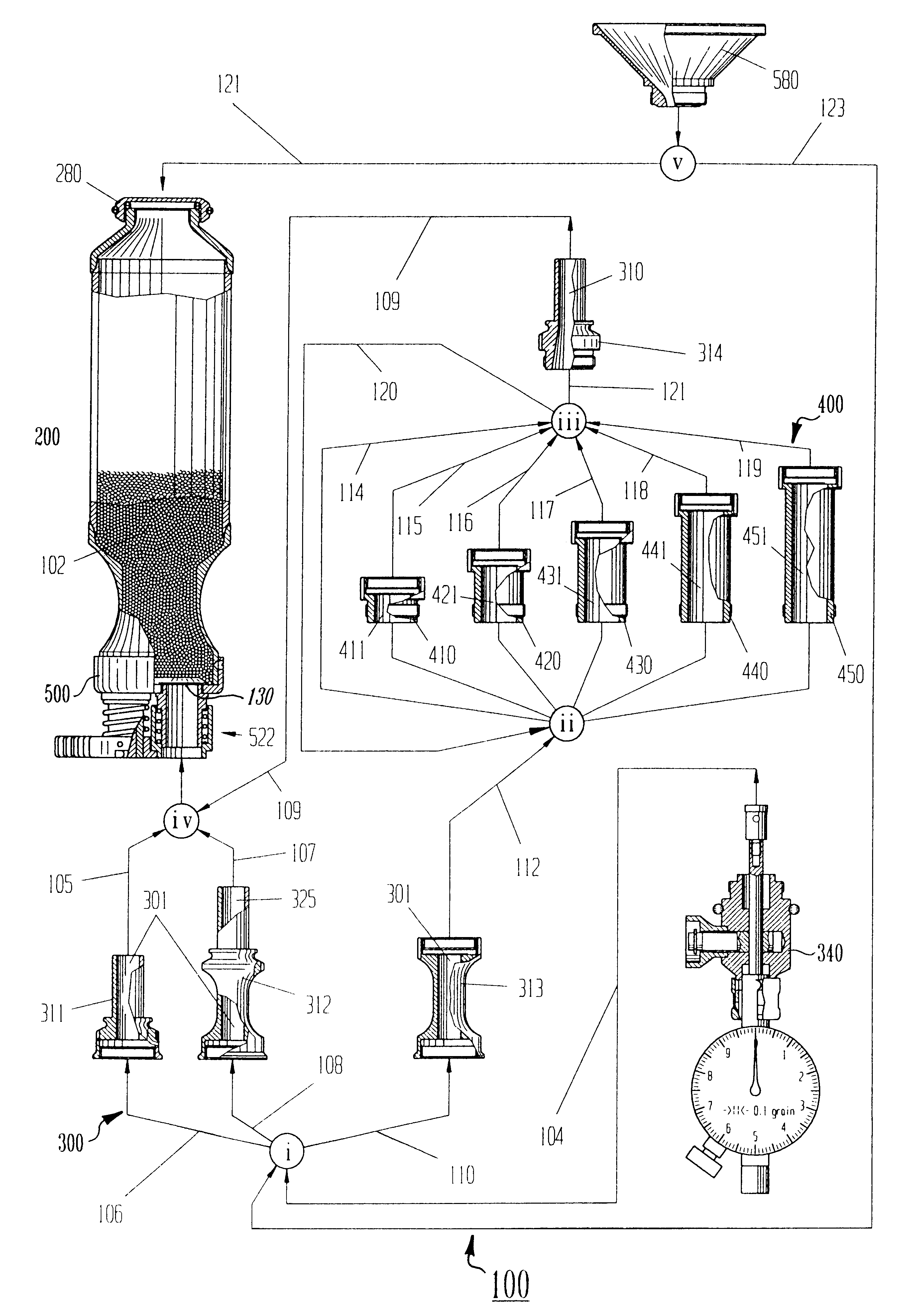
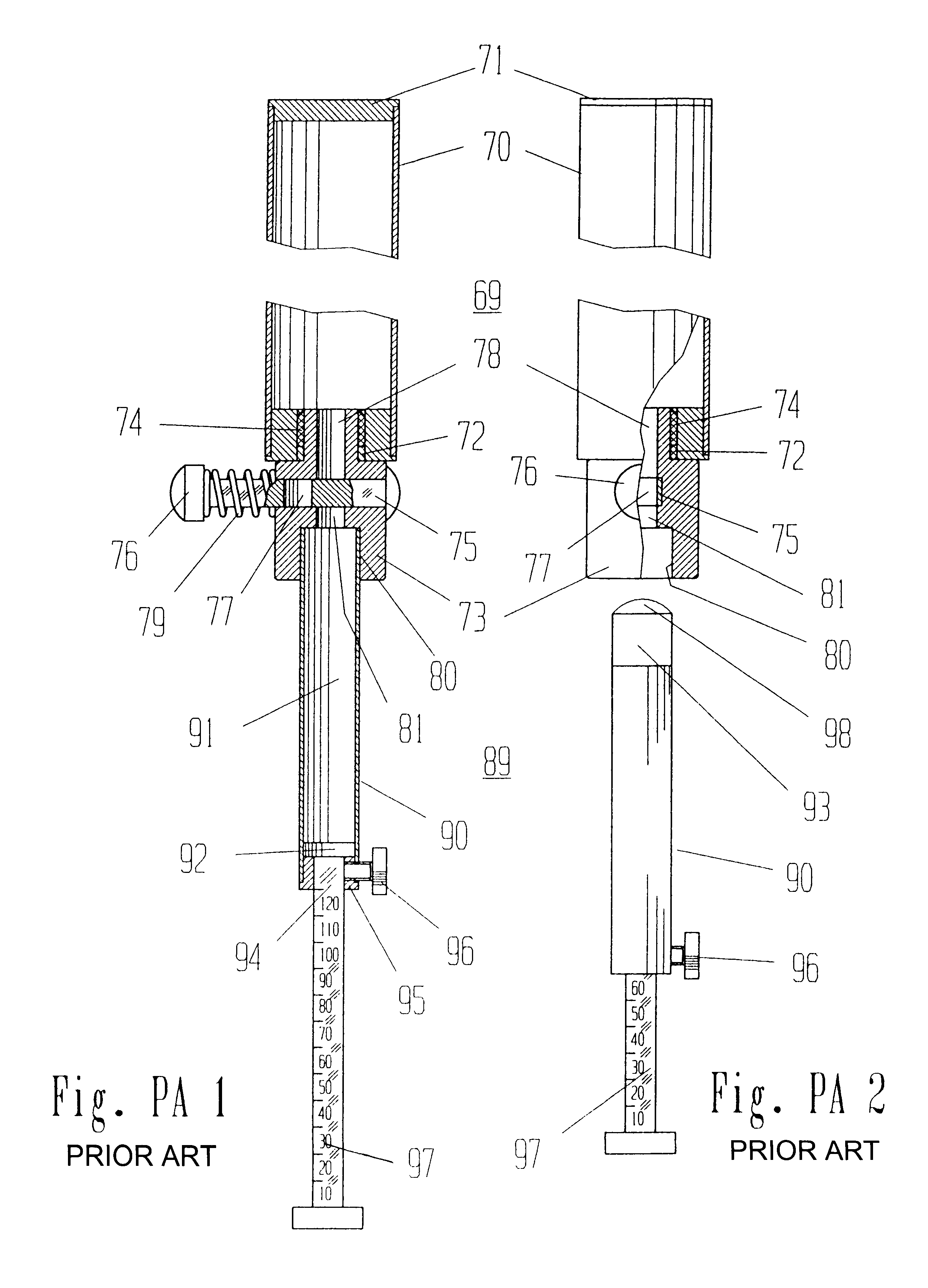
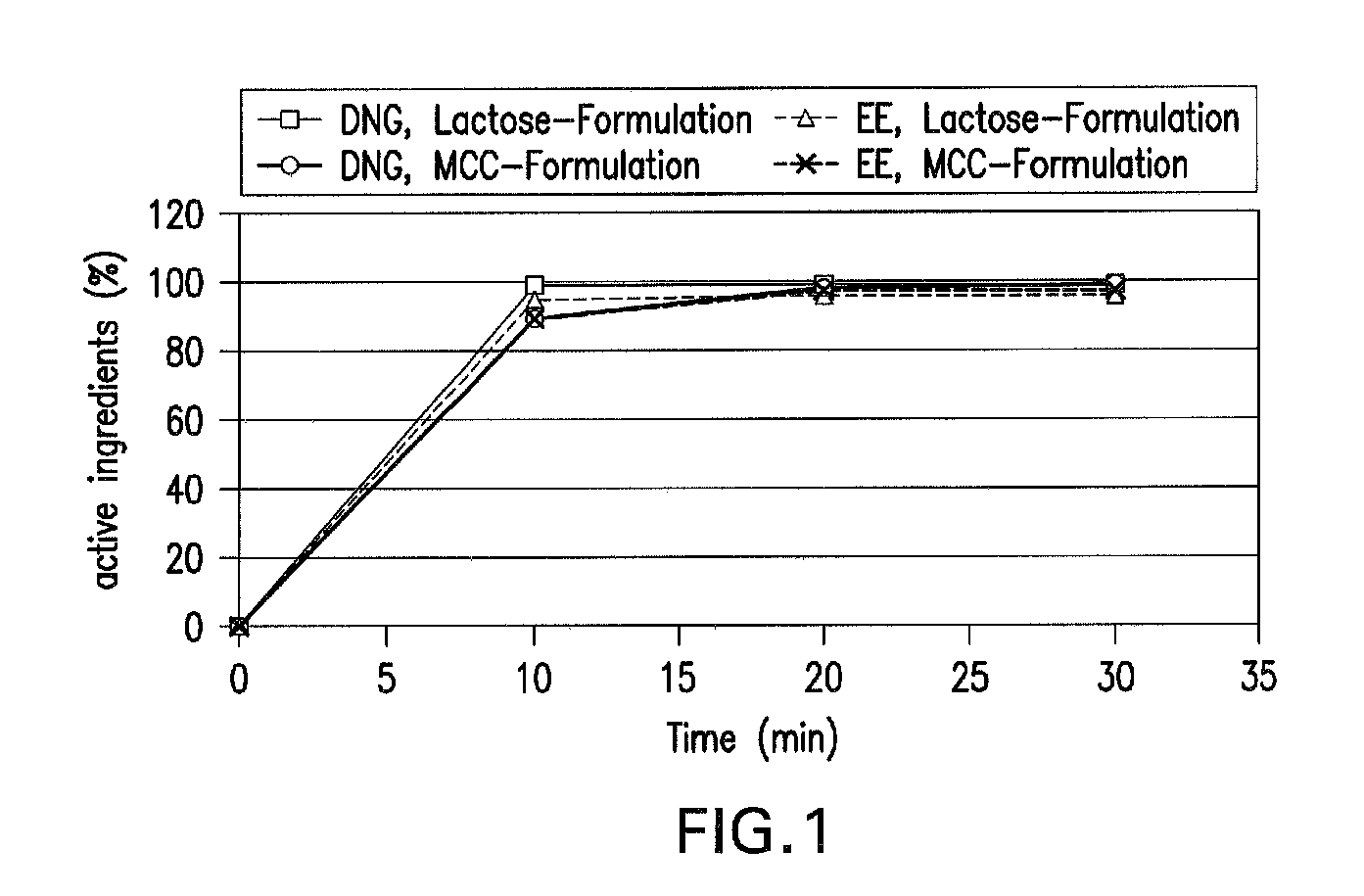
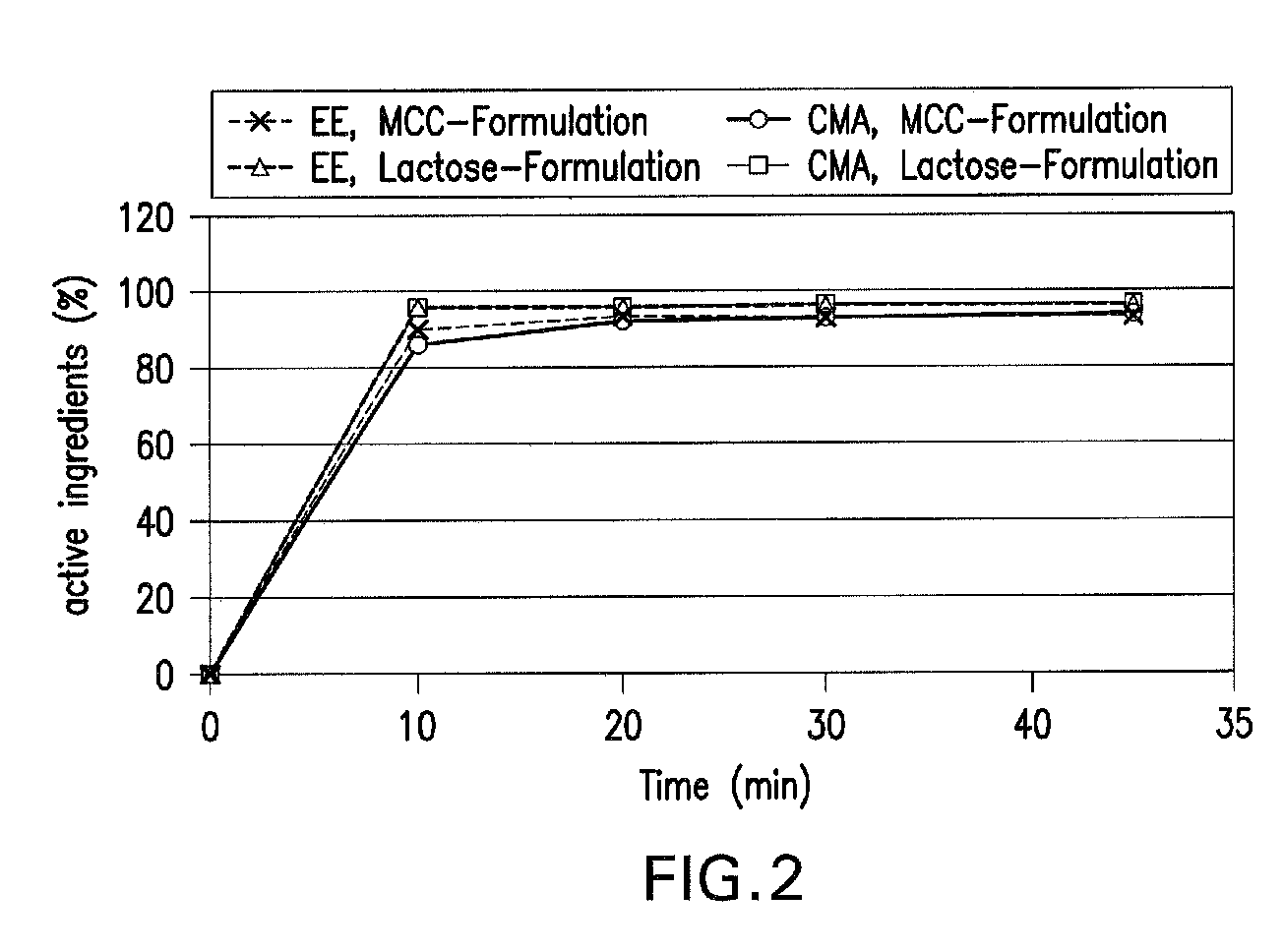
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Dose Units" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Unit dose. a method of preparing medications in which individual doses of patient medications are prepared by the pharmacy and delivered in individual labeled packets to the patient's unit to be administered by the nurses on an ordered schedule. One intent of unit dose is to decrease administration error.
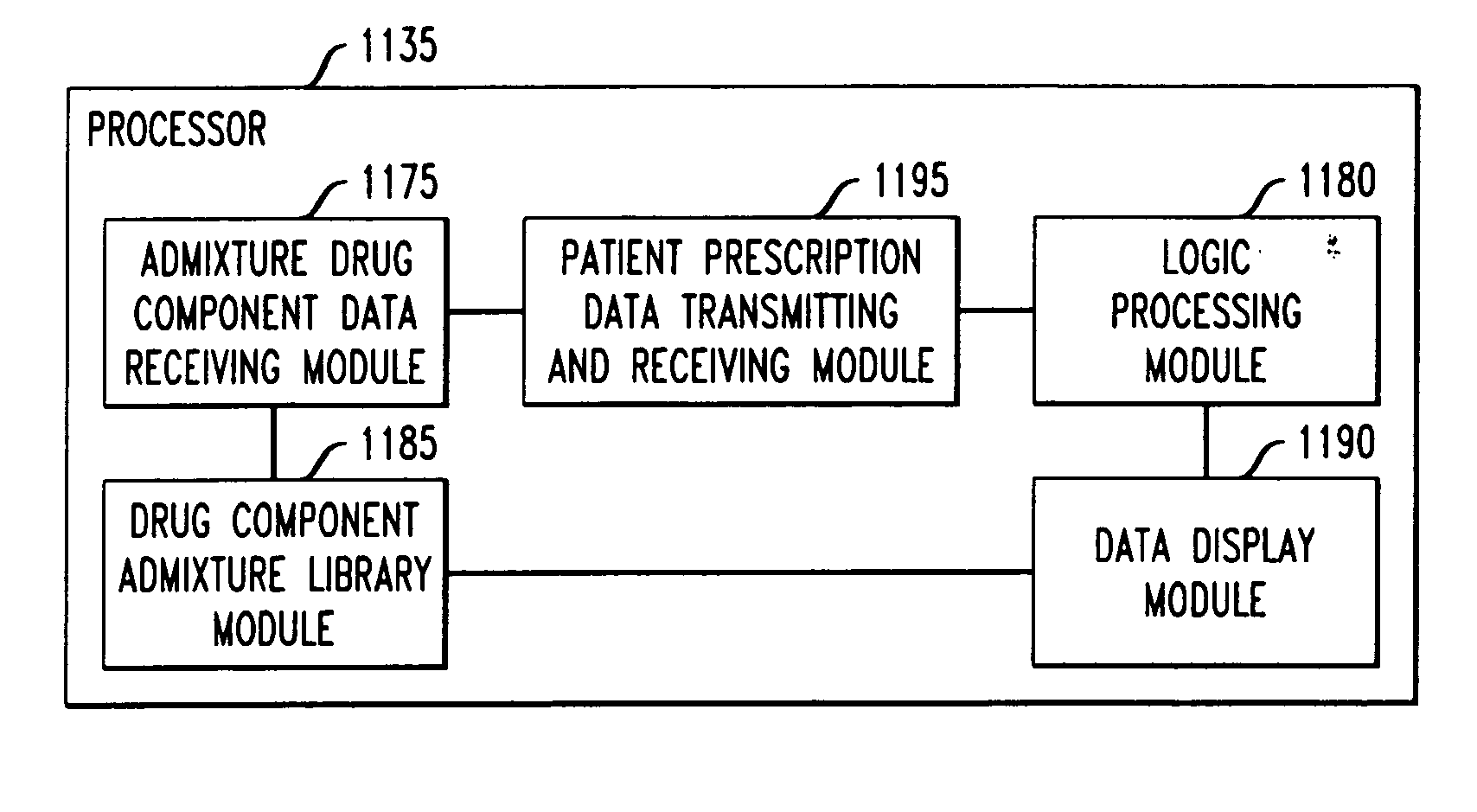
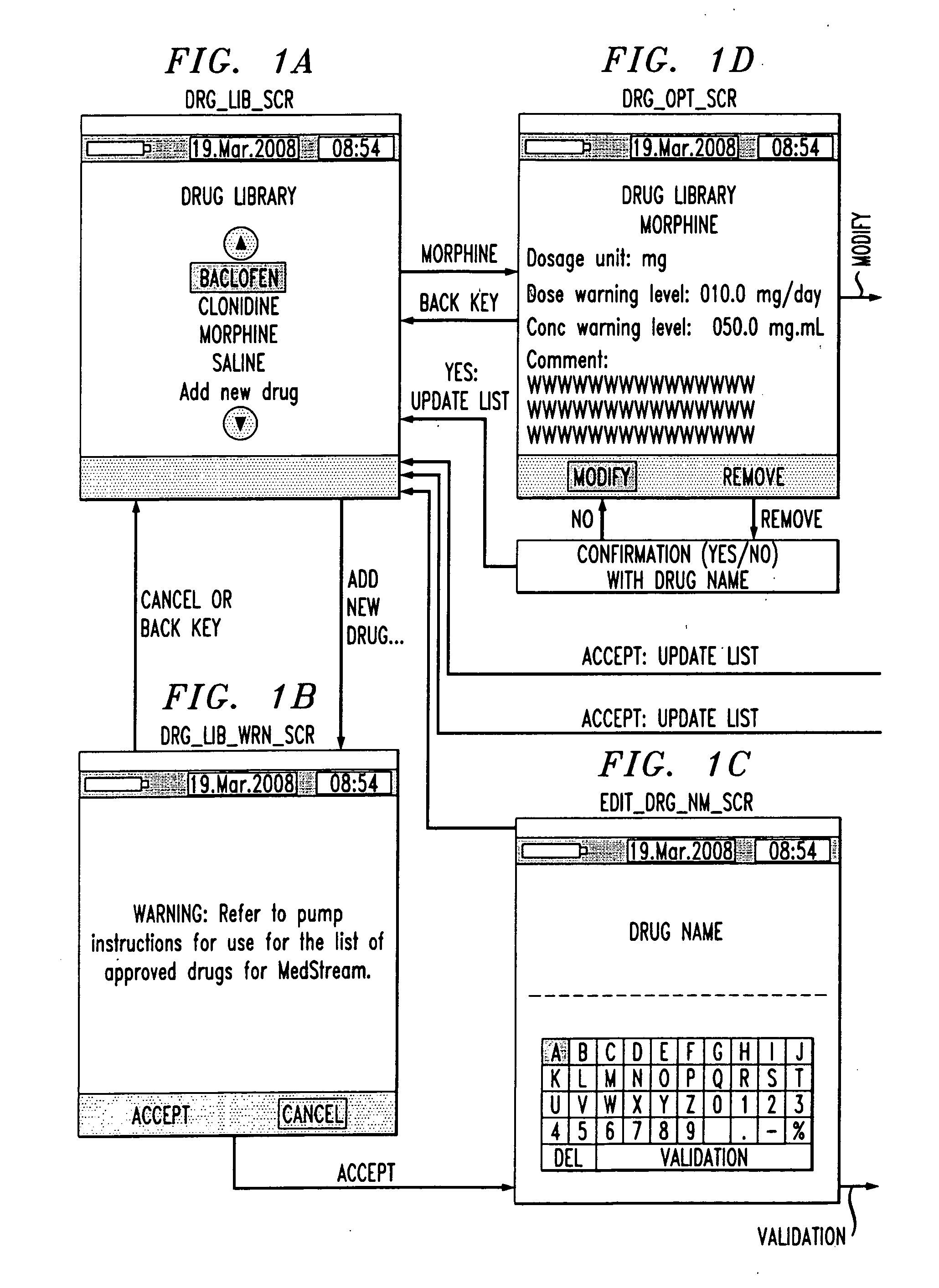
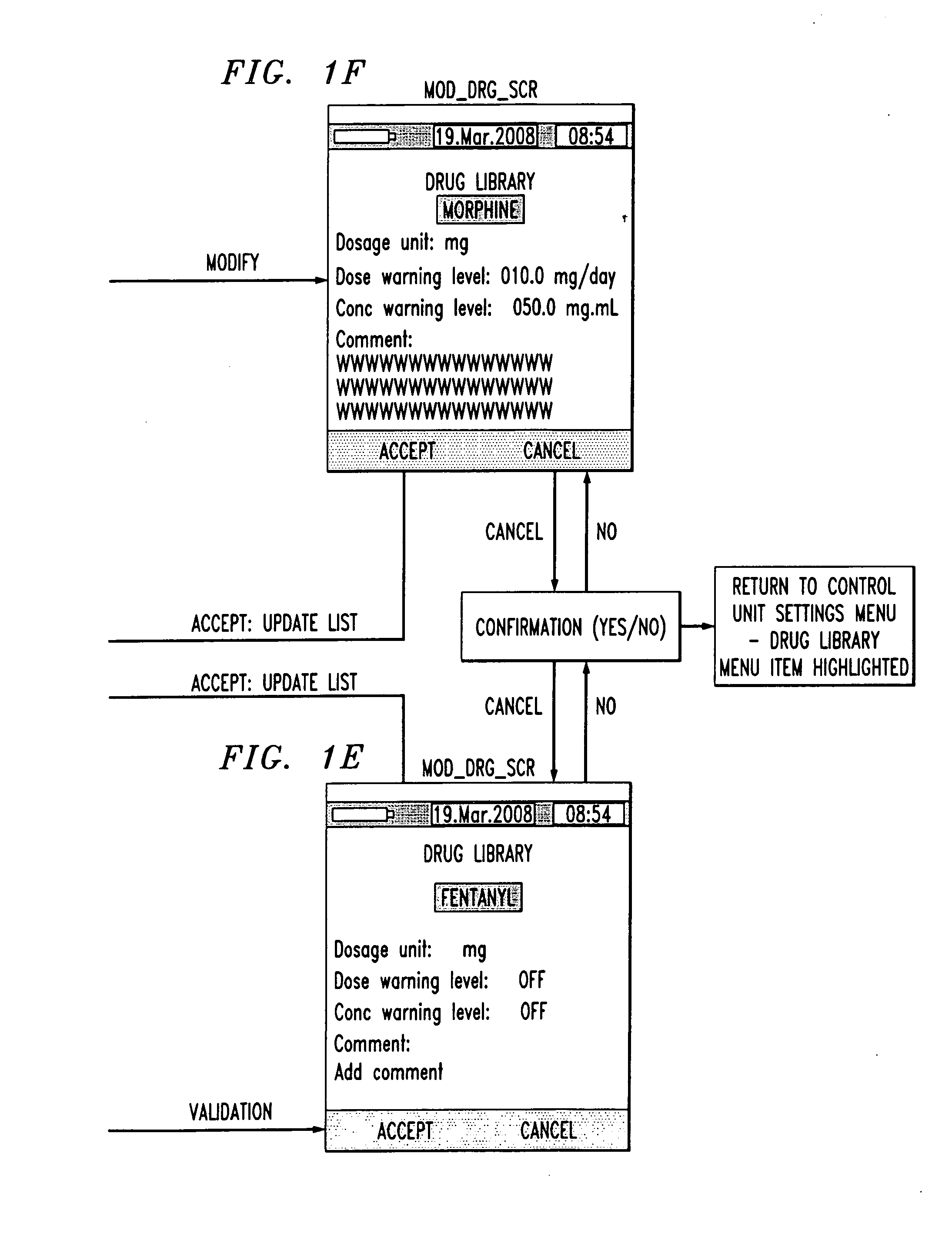
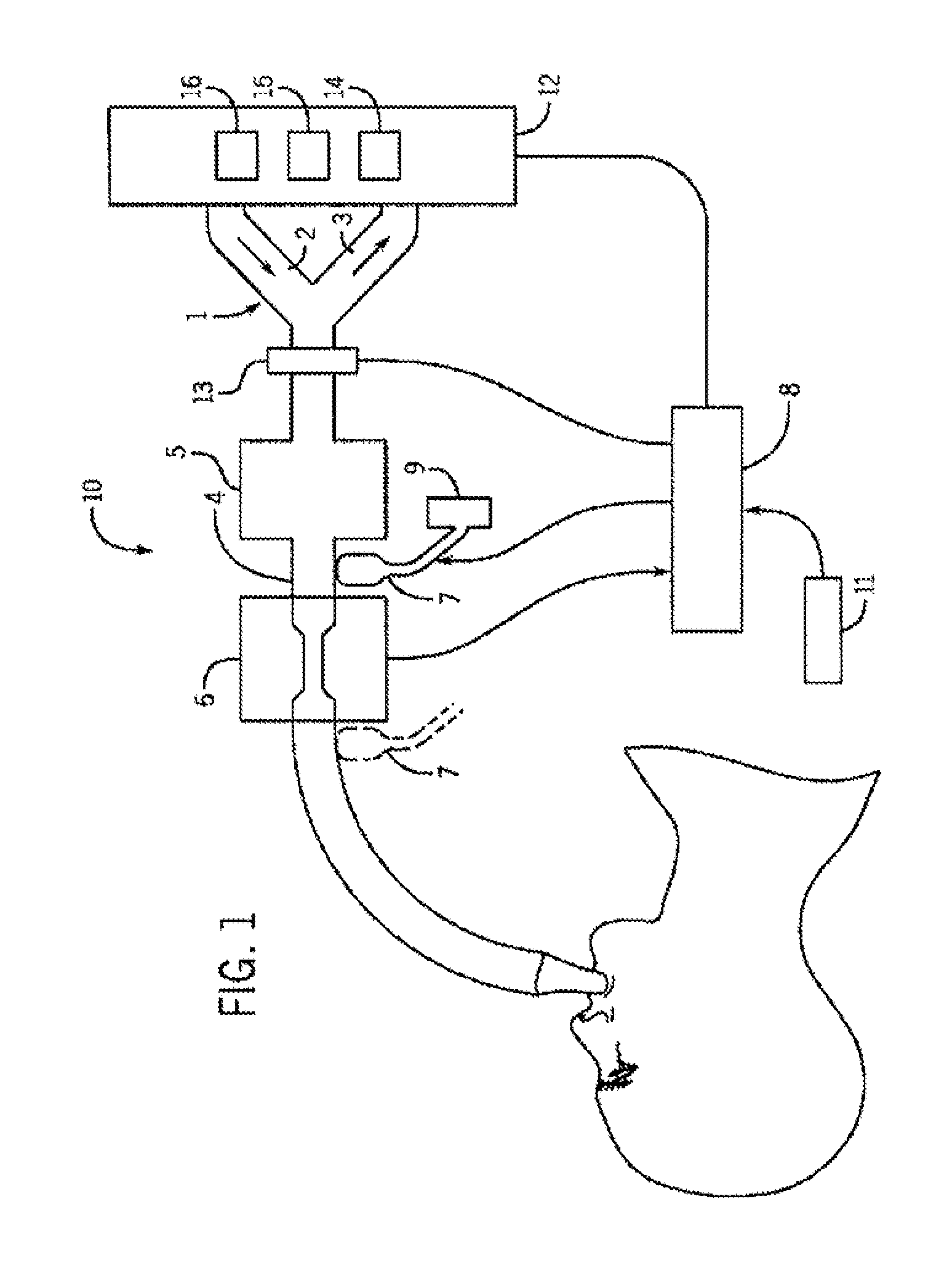
Drug component admixture library for a drug infusion delivery system
InactiveUS20110320049A1Shorten the timeReduce generationLevel controlControlling ratio of multiple fluid flowsDrugs infusionDelivery system
Minimizing improper dosage of a drug admixture (including a single primary drug component and at least one second drug component). For each drug component in the drug admixture, receiving a name of the drug component along with its dosage unit, a maximum dose warning level and a maximum concentration warning level. Receiving a concentration for each of the single primary drug component and the at least one secondary drug component; and a dose setting of only the primary drug component. Automatically calculating a dose of each of the at least one secondary drug component. Generating an alert when: (i) the received dose setting of the primary drug component or calculated dose setting of the at least one secondary drug component exceeds the dose warning level; or (ii) the received concentration of the primary drug component or the at least one secondary drug component exceeds the concentration warning level.
Owner:MEDOS INT SARL
Infusion device data set analyzer
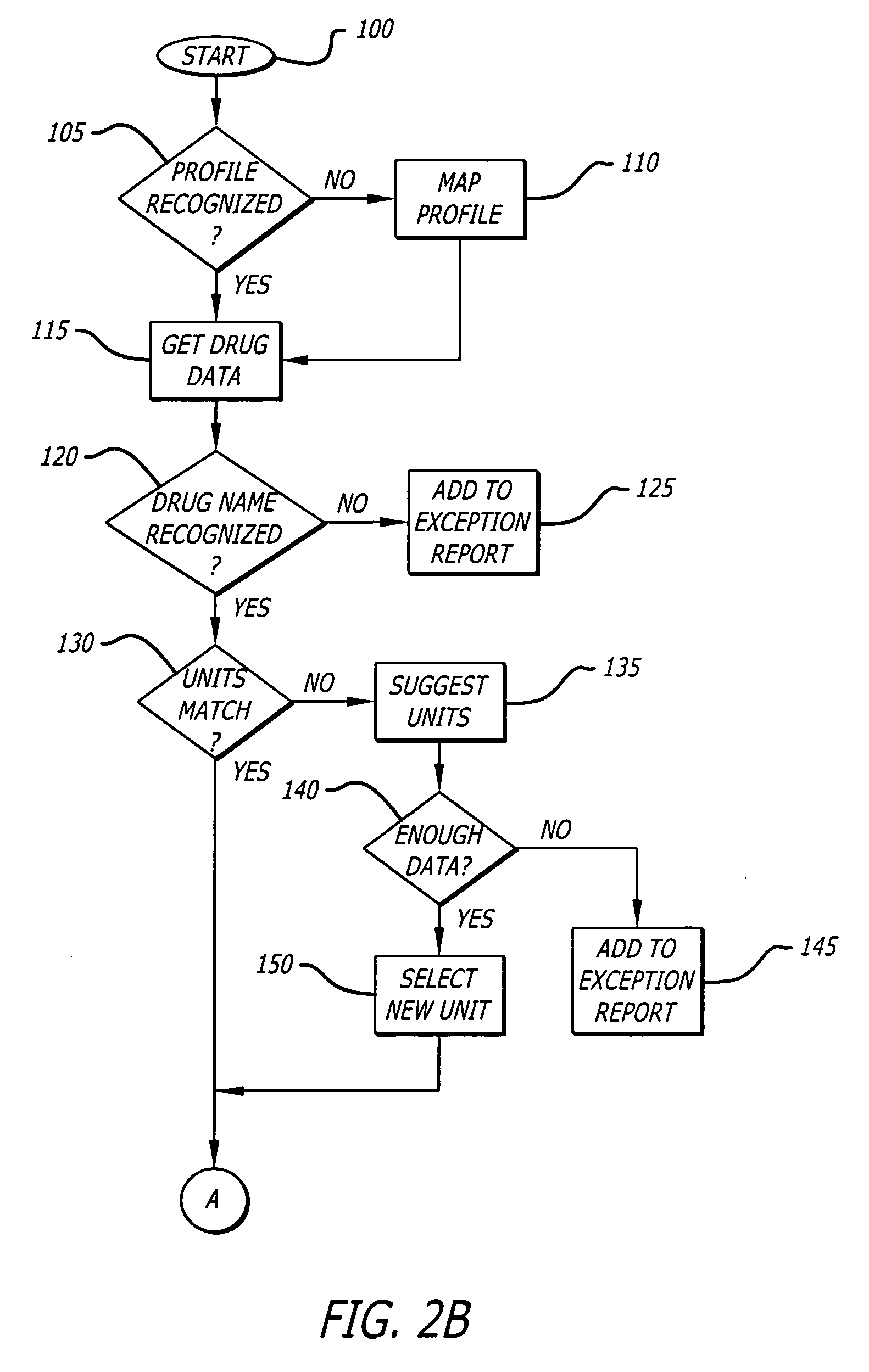
A system and method for evaluating drug data sets is provided. The system and method includes identifying when entries in a current data set differ from a data set comprising an aggregate of entries for a plurality of institutions, and prompting a user to determine if a change in the current data set should be changed. The system and method also identifies cases where different dose units are used for the same drug. A report is generated noting exceptions.
Owner:CAREFUSION 303 INC
Controlled-release drug delivery system
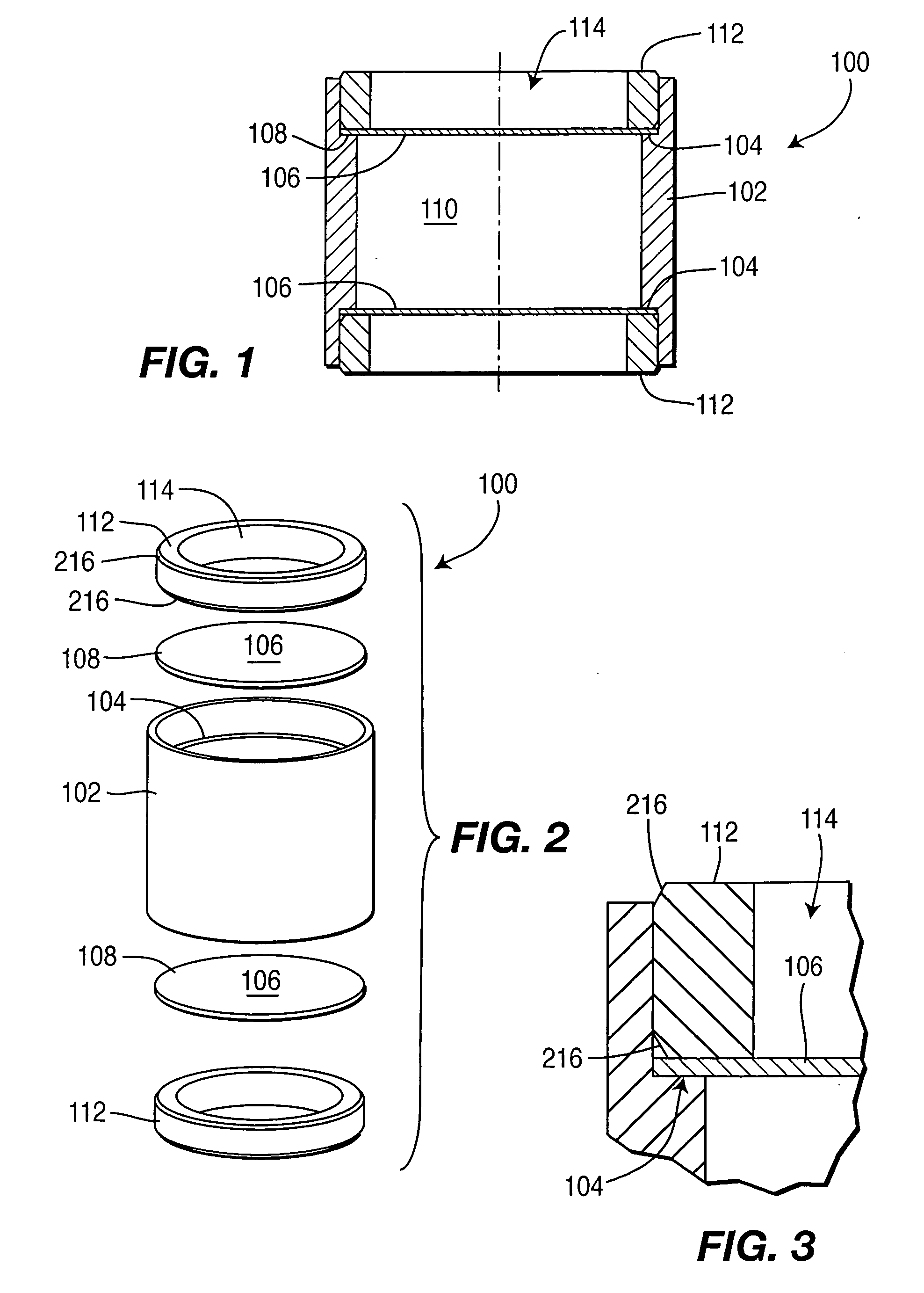
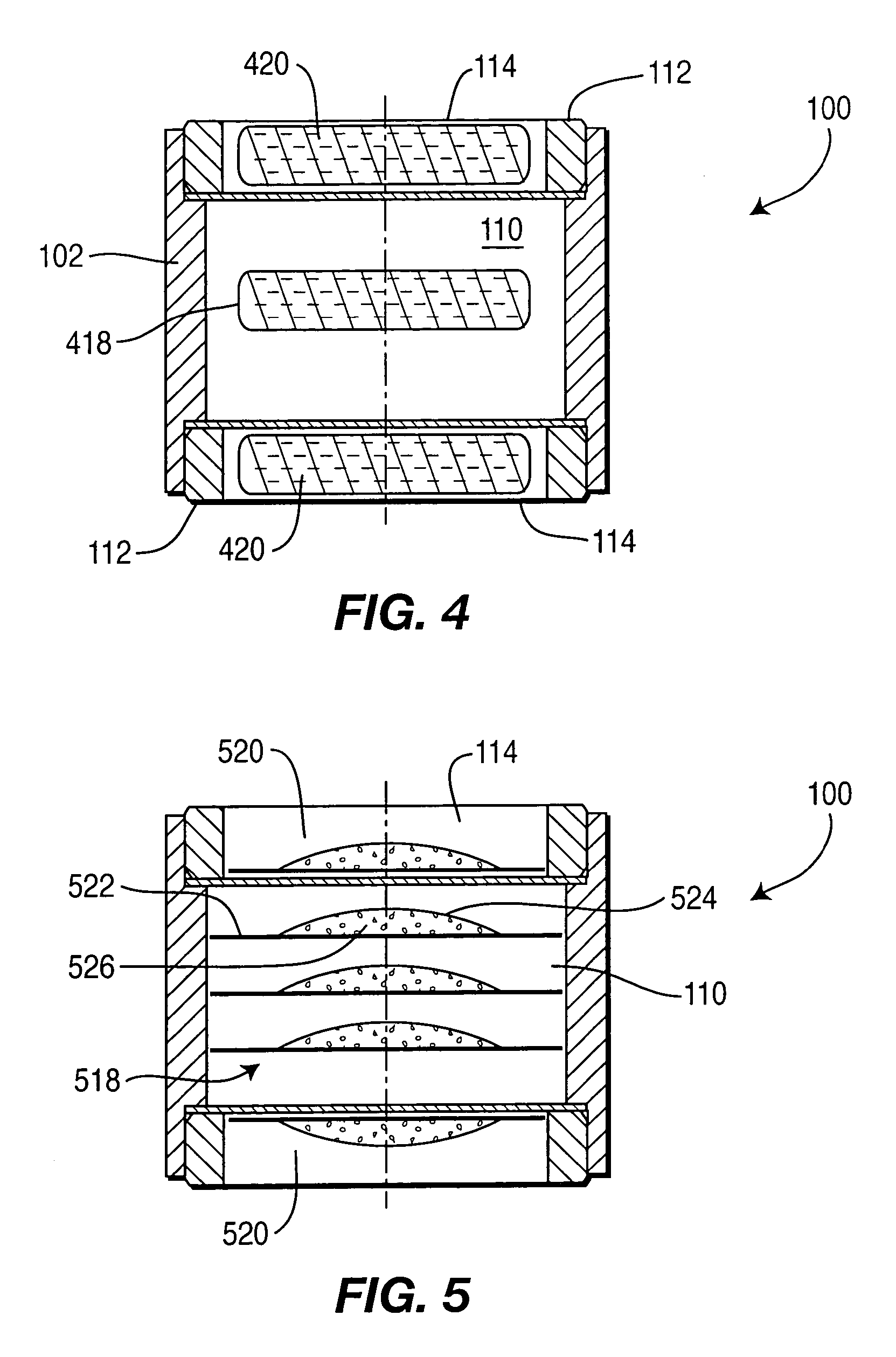
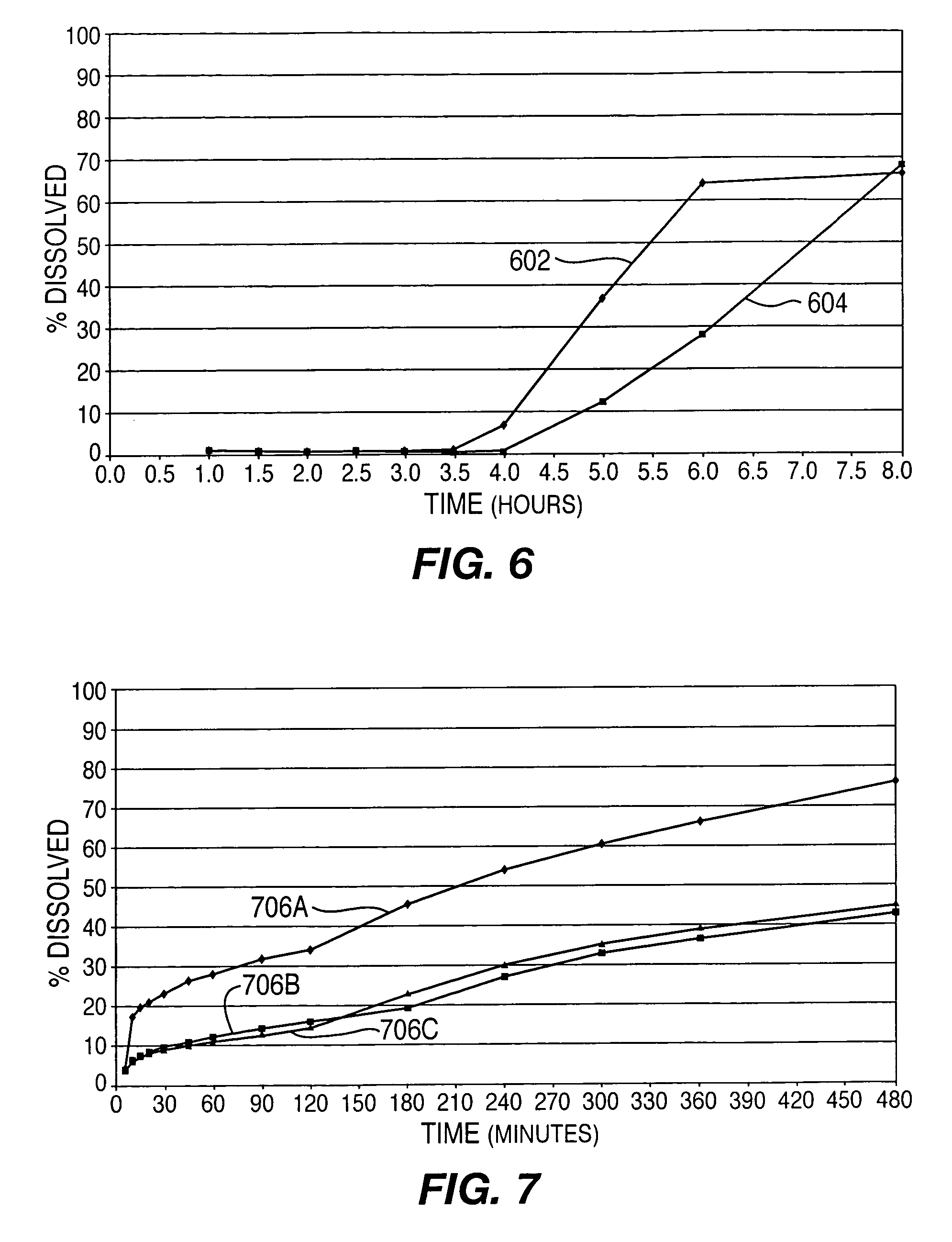
A controlled-release drug delivery system advantageously includes an open-ended, inflexible sleeve, at least two controlled-release layers and two open-center caps. Each controlled-release layer abuts a sealing surface that is located within and near each end of the sleeve. The caps seal each controlled-release layer against the abutting sealing surface. One or more dose units of drug are disposed in a region that is formed between the controlled-release layers. The controlled-release layers dissolve, at a predetermined rate, by the action of body fluids that are in contact with those layers through the center of the caps. Release of drug is delayed at least until the controlled-release layers dissolve. The dose unit itself, which is advantageously a core, can be tailored to provide an extended period of drug release. One or more dose units that provide an immediate release component can also be disposed near each end of the sleeve.
Owner:SARNOFF CORP
Pharmaceutical preparation containing a gestagen, and kit and method for treating endometriosis using the preparation
InactiveUS20080214512A1Significant positive effectEndometriosis can be reducedOrganic active ingredientsBiocideSide effectBone density
The pharmaceutical preparation for treating endometriosis contains at least 28, preferably 30, daily dose units, each of which contain dienogest, cyproterone acetate, or chlormadinone acetate at a daily dose that is at most twice that required to inhibit ovulation together with one or more pharmaceutical aids and / or carriers. The daily dose units are administered in a method of prophylaxis and / or therapy of endometriosis continuously during a time interval of at least 169 days or 25 weeks, preferably more than two years. The method effectively reduces endometriosis and associated pain, while undesirable side effects including bone density decrease are reduced or eliminated.
Owner:BAYER SCHERING PHARMA AG
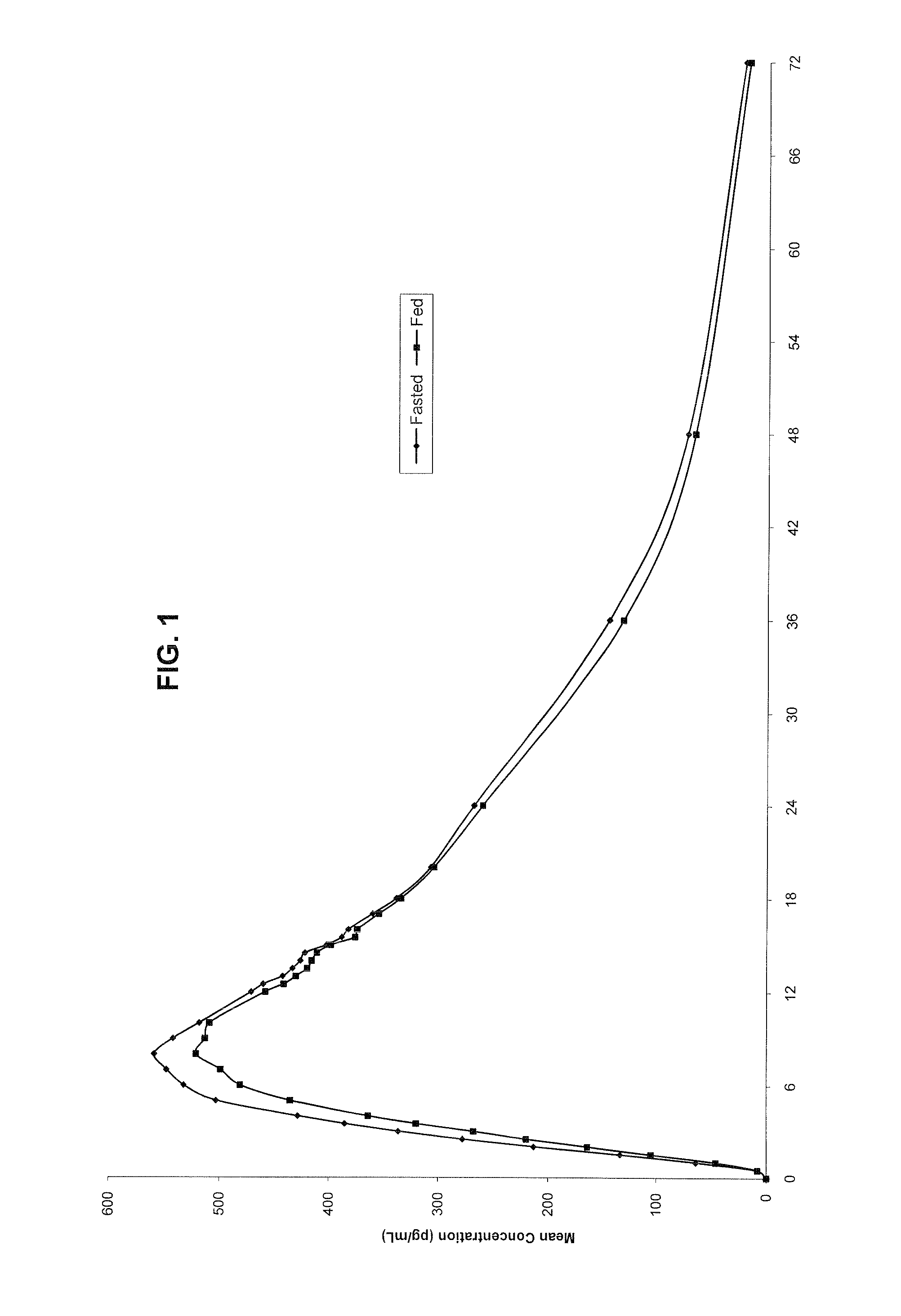
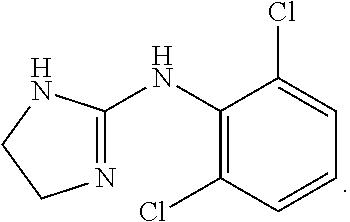
Novel clonidine formulation
Owner:TRIS PHARMA
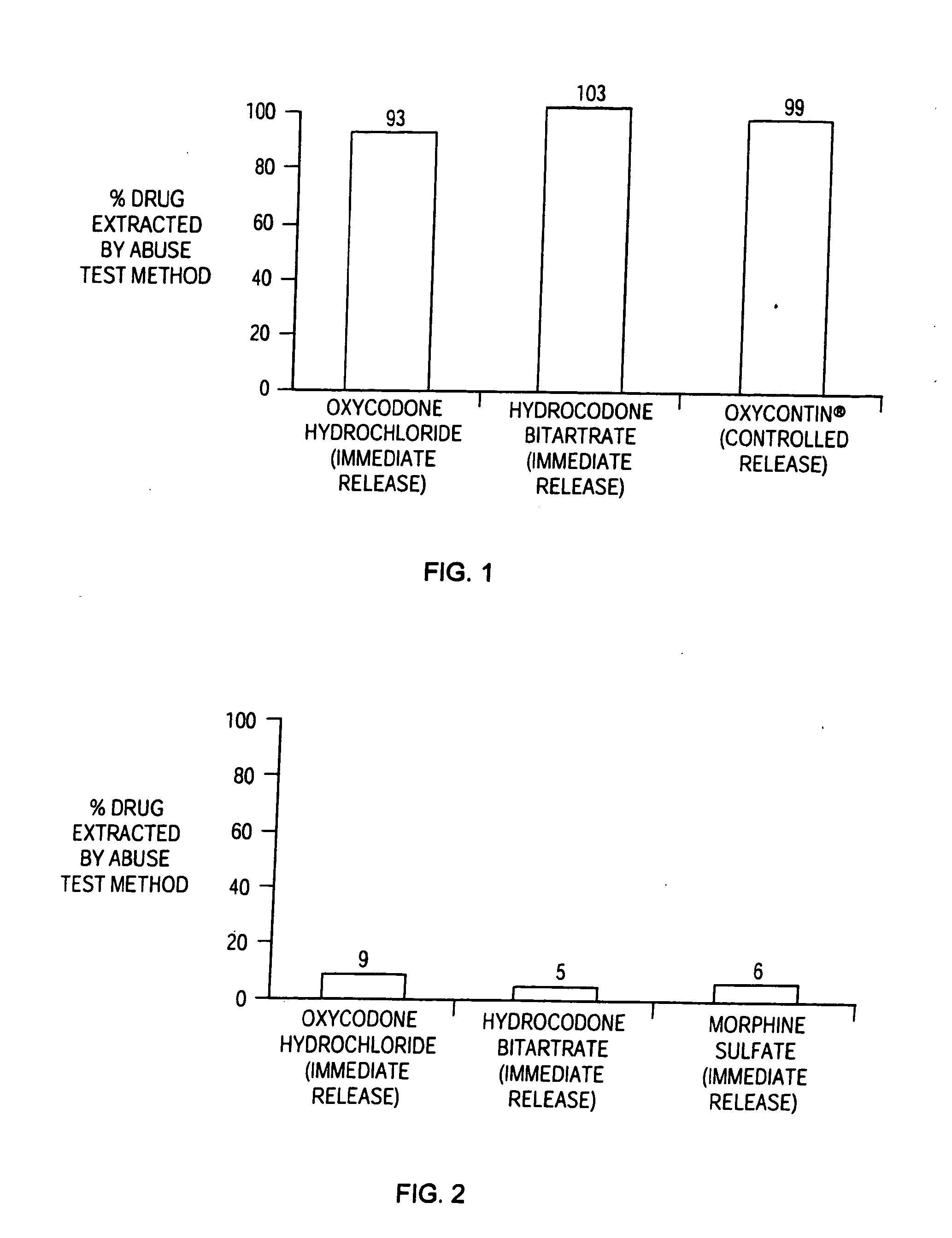
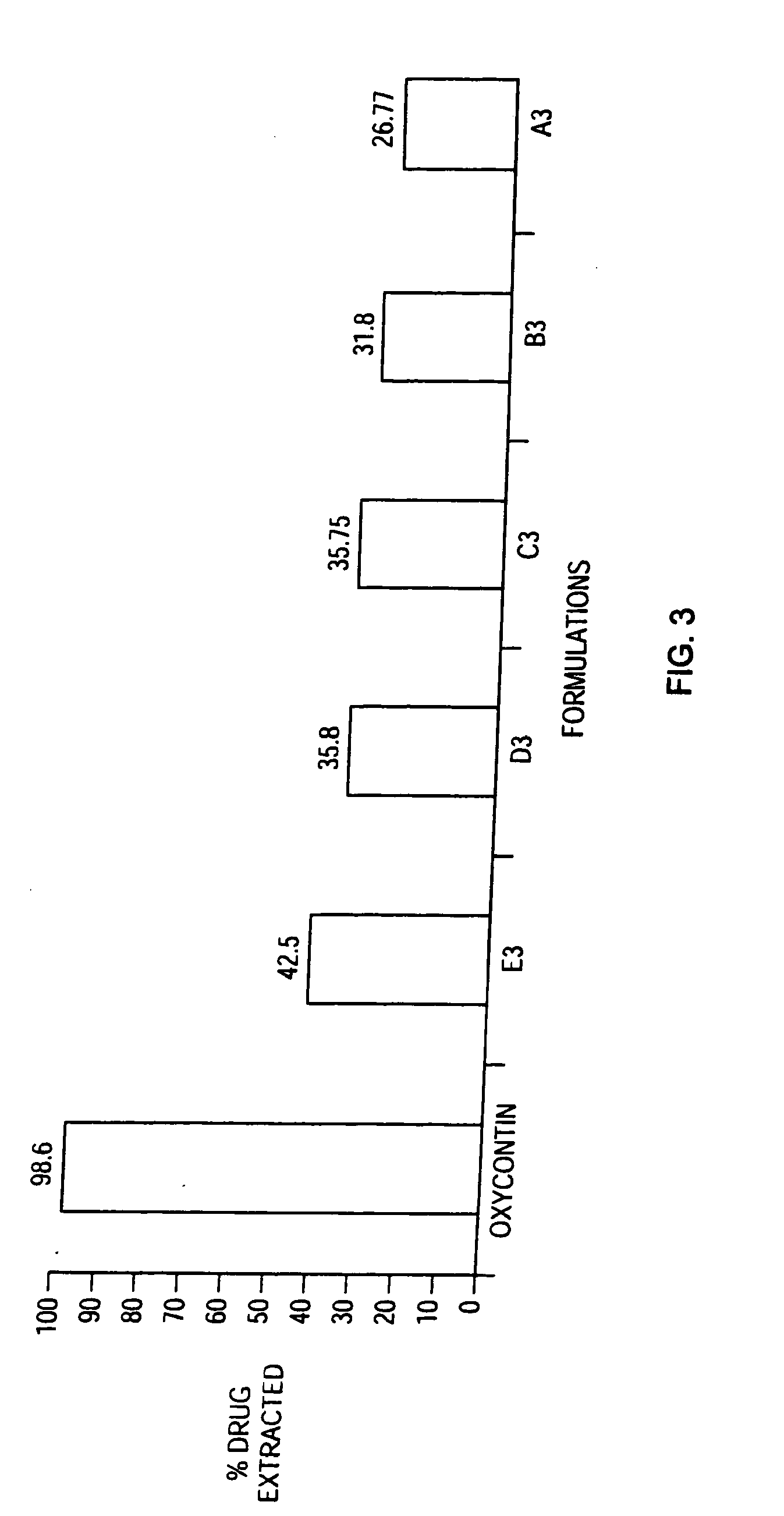
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Medication dosing system based upon patient classification codes
InactiveUS20090149815A1Reduce the possibilityData processing applicationsInfusion syringesMedication doseStandardization
Medication dosing system for one or more medications based on patient classification codes and standardized dosage units, where the patient classification codes are correlated with one or more characteristics of the patient, such as weight or age, and each medication is formulated so that the dosage unit for the classification code contains the recommended amount of medication for patients within that classification code.
Owner:CODADOSE
Method and device for vaporization and inhalation of isolated substances
A dose unit comprising at least one isolated bioactive agent applied on a carrier material in thermal contact with an electrically heating element configured to vaporize a pre-determined amount of the agent for pulmonary delivery thereof is provided herein, as well as devices for effecting vaporization and pulmonary delivery of the isolated agent, and methods for preparing the dose unit, controllably releasing the agent therefrom, methods for pulmonary delivery thereof and methods of treatment of medical conditions treatable by pulmonary delivery of the isolated bioactive agent.
Owner:SYQE MEDICAL
Antihistamine/decongestant regimens for treating rhinitis
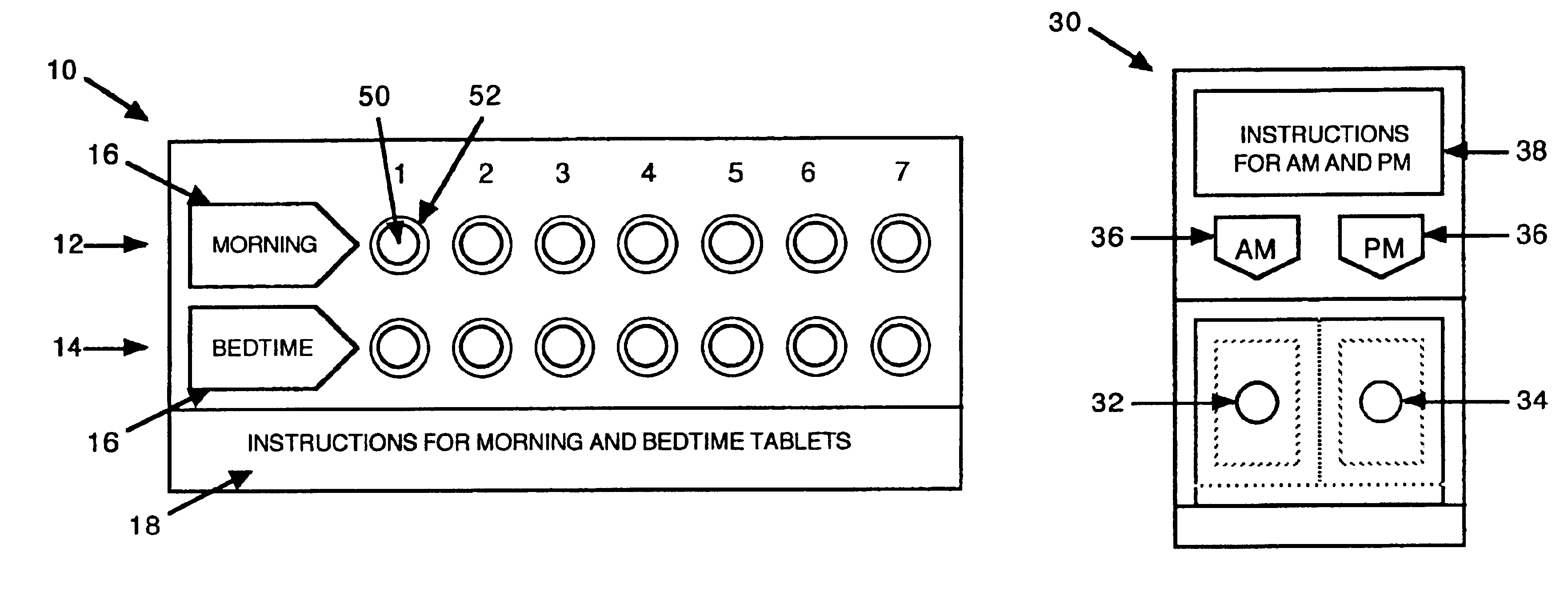
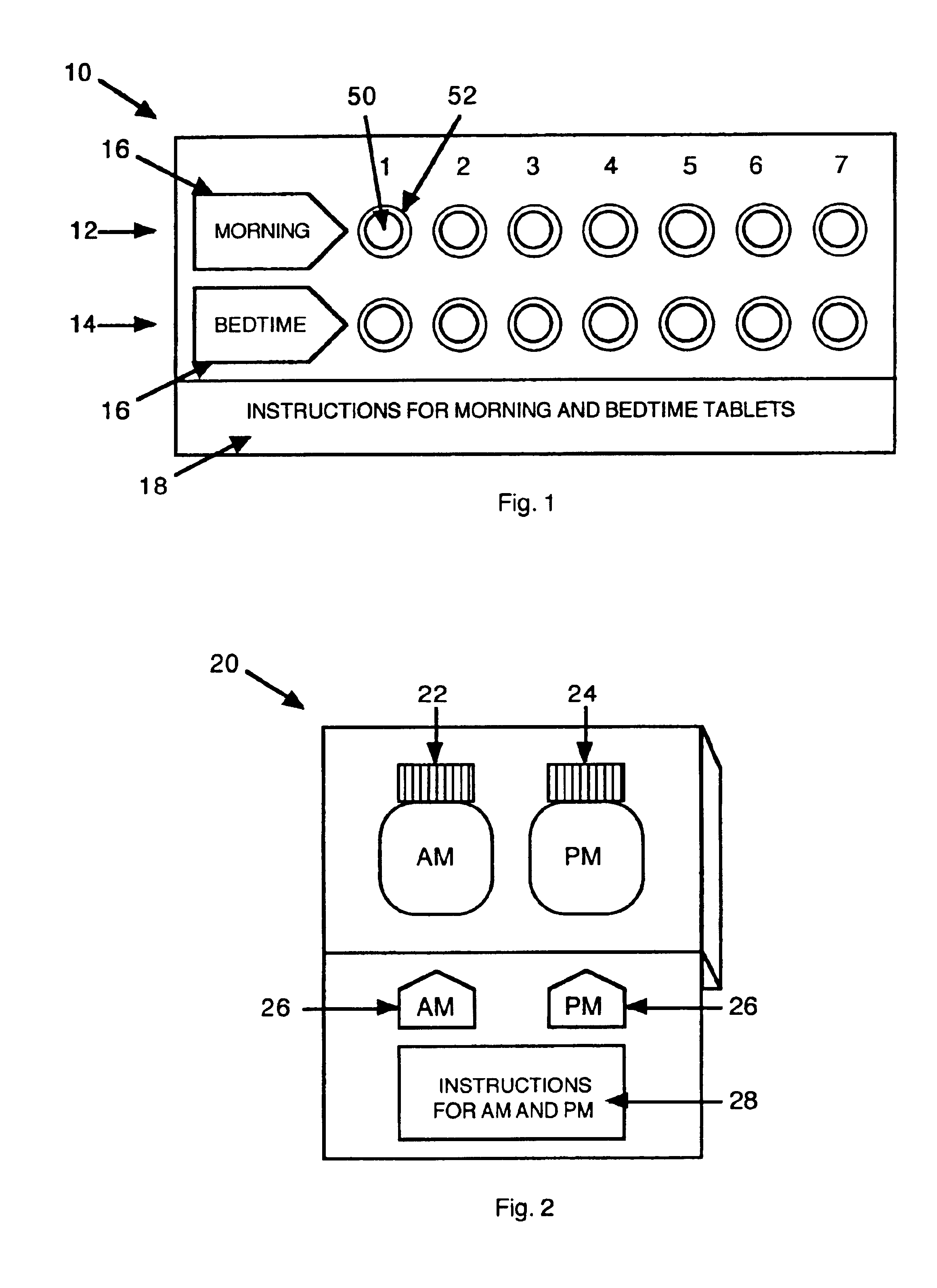
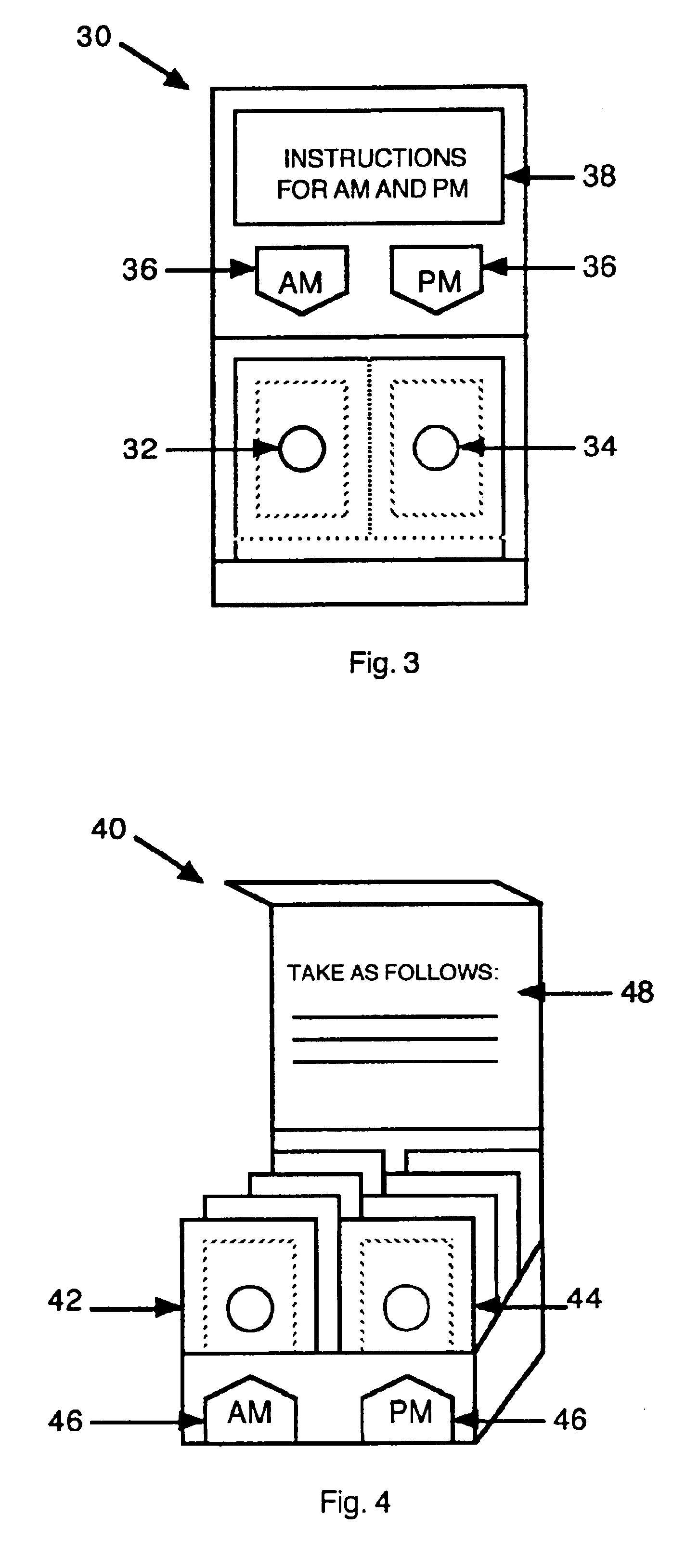
InactiveUS6843372B2Minimizing stimulationReduce errorsSmall article dispensingPharmaceutical containersDrugDose Units
A prepackaged, therapeutic regimen includes a non-sedating first dosage unit that includes a nasal decongestant, a second dosage unit that includes an antihistamine and an attenuated dosage of nasal decongestant, indicia for distinguishing between the first and second dosage units, administration instructions that teach the coordinated use of the first and second dosage units, and a pharmaceutical dispensing container containing the first and second dosage units and incorporating the indicia and coordinating instructions.
Owner:WEINSTEIN ALLAN M
Method and device for vaporization and inhalation of isolated substances
Owner:SYQE MEDICAL LTD
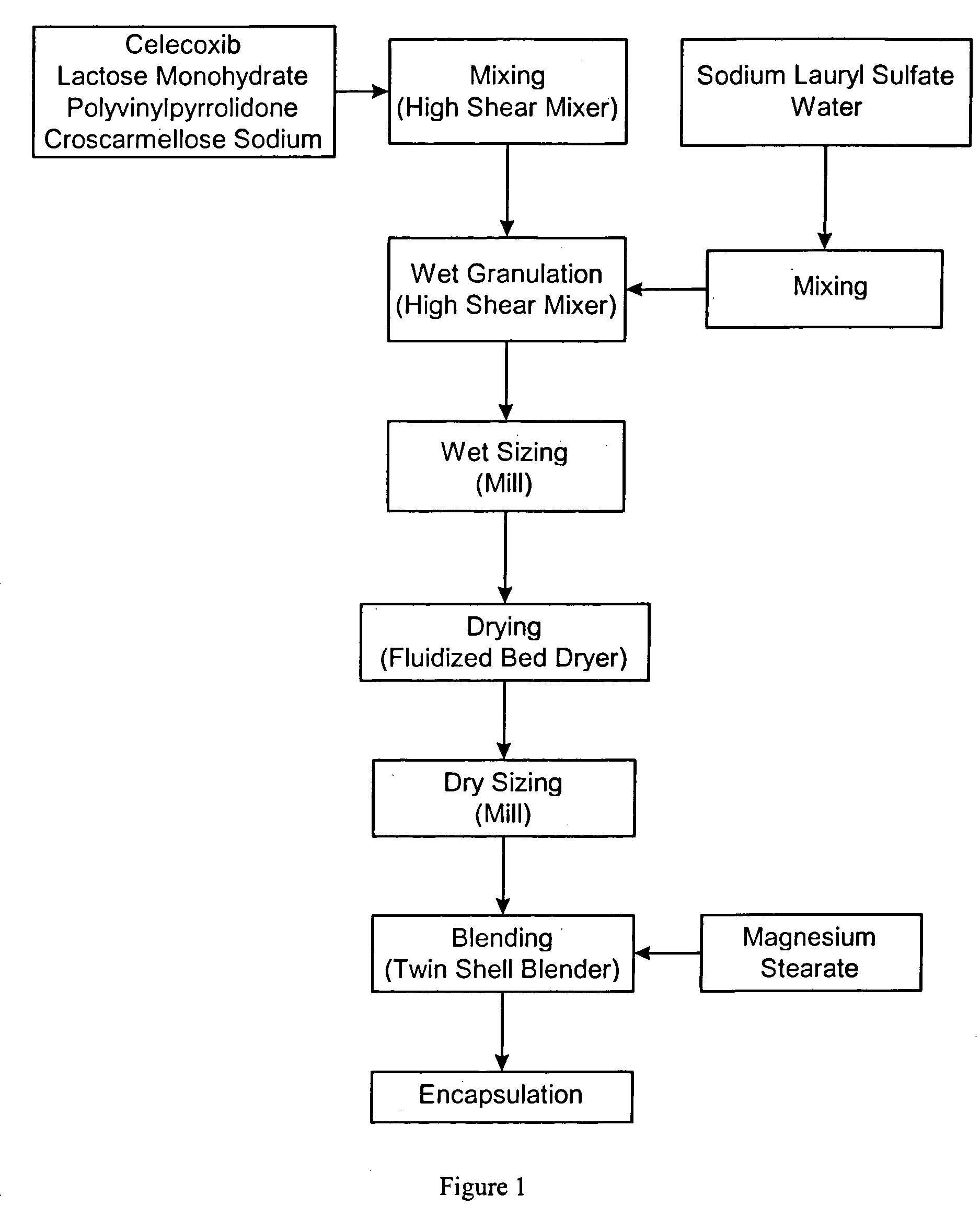
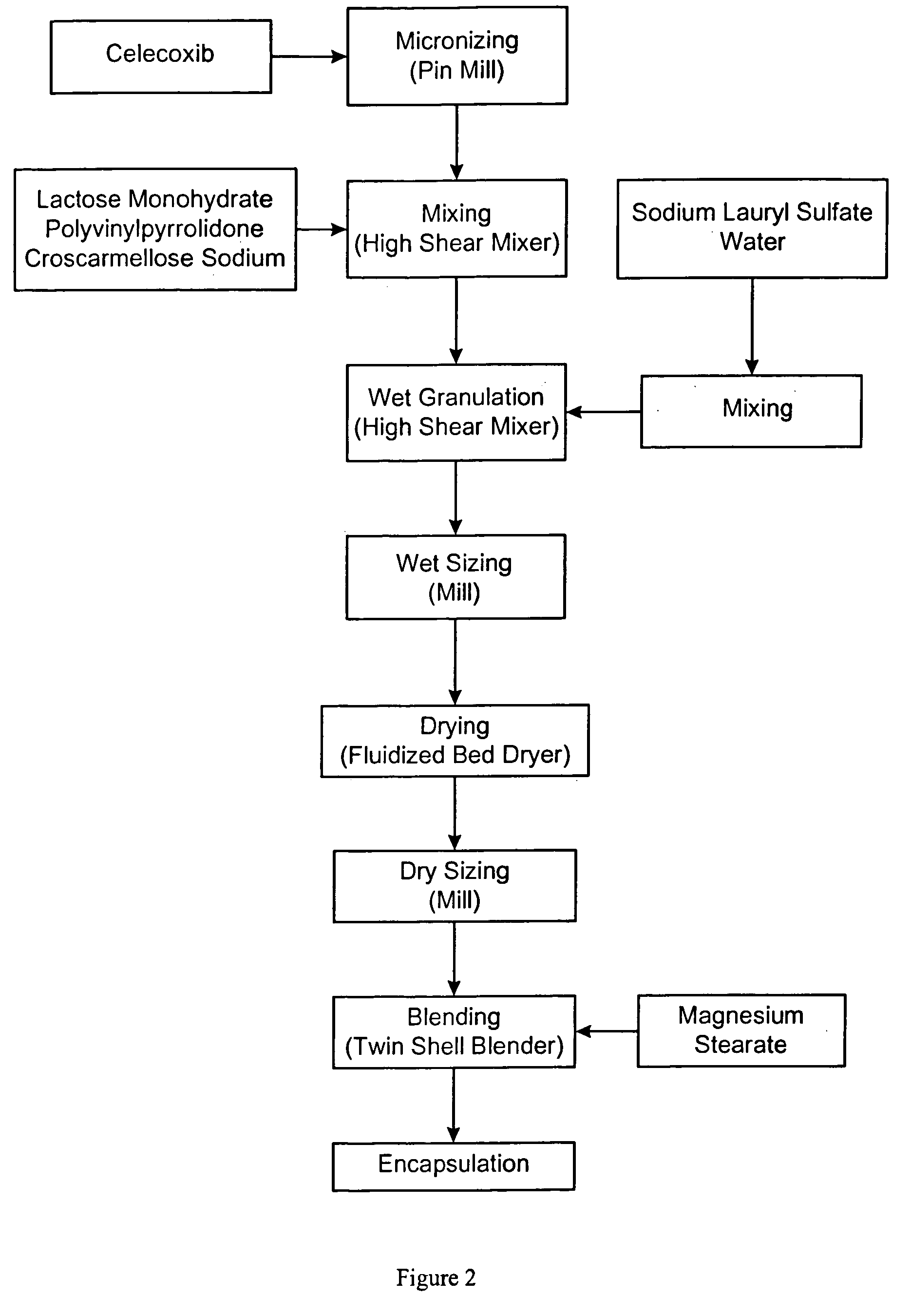
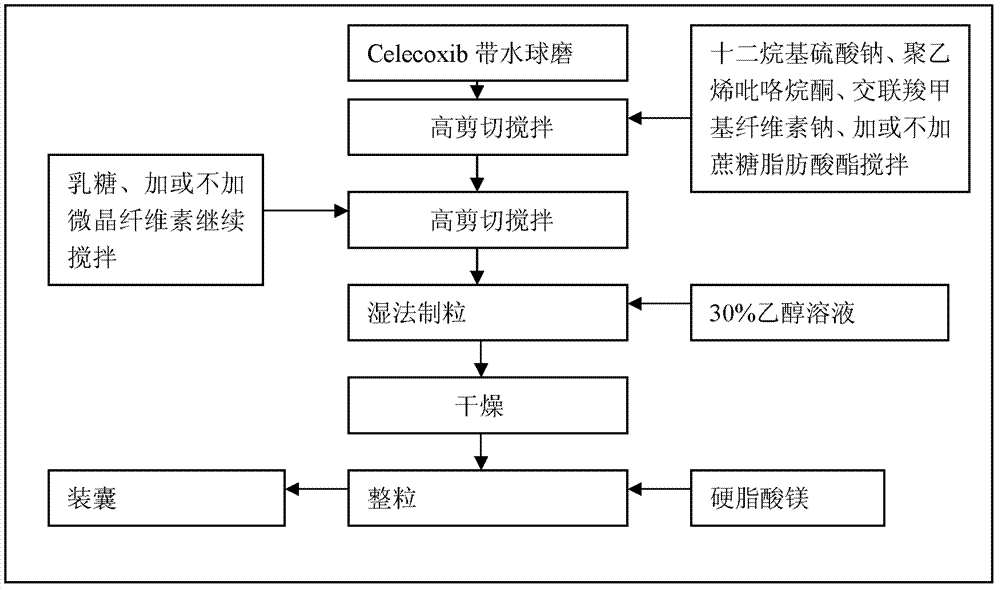
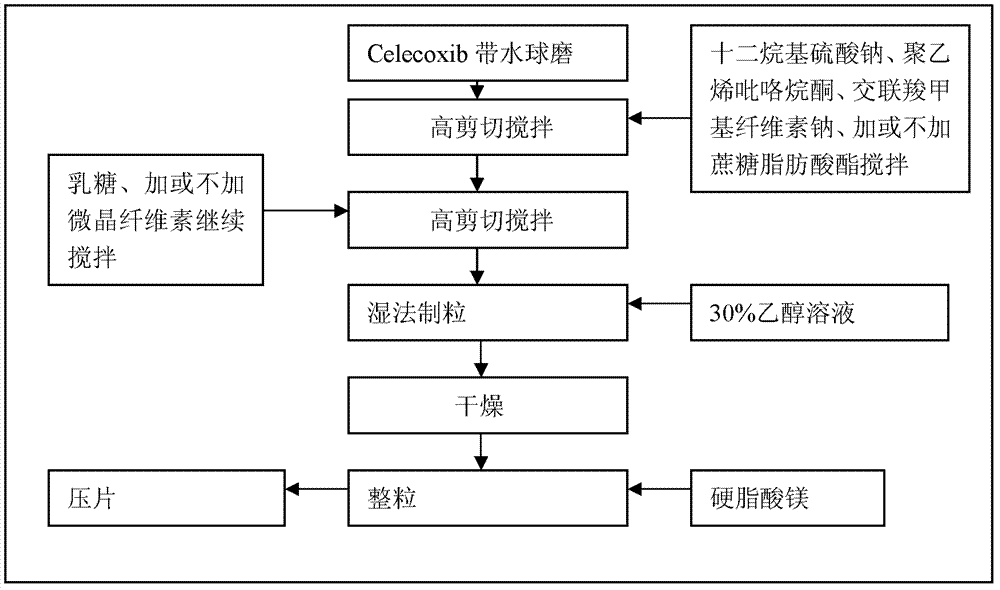
Celecoxib compositions
InactiveUS20050267189A1BioavailabilityLess-harmful side effectBiocideSenses disorderParticulatesCyclooxygenase
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising particulate celecoxib in an amount of about 10 mg to about 1000 mg in intimate mixture with one or more pharmaceutically acceptable excipients. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders.
Owner:GD SEARLE & CO
Method and system for dosing a pharmaceutical sample in a packaging machine
InactiveUS7536843B2Securing of qualityImprove accuracyLiquid fillingSolid materialBiomedical engineeringDosing units
The present invention relates to a method and system for dosing a pharmaceutical product in a packaging machine having at least one volumetric dosing unit with a dosing chamber. The system check-weighs the volumetrically dosed product with appropriate speed and is integrated into a filling or packaging machine. In accordance with the invention, a volume of a first pharmaceutical component is metered and weighed before being introduced into a package. The procedure is repeated with a second pharmaceutical component. The package containing the components is then weighed. Advantageously, accuracy and precision of the amount of the weighed components can be monitored with the present invention to secure the quality of the pharmaceutical product.
Owner:ASTRAZENECA AB
Formulations and use of a beta-blocker and an ACE-inhibitor for the treatment of cardiovascular diseases
InactiveUS20050032879A1Easy and rapid titrationReduce the burden onBiocideAnimal repellantsCongestive heart failure chfDose Units
The use of a beta-blocker and an ACE-inhibitor in combination for the treatment of cardiovascular disorders, including hypertension and congestive heart failure, is provided. In particular, pharmaceutical formulations which use metoprolol tartrate and enalapril maleate in a therapeutically effective combination in a single extended release dosing unit for the treatment of congestive heart failure are disclosed as well as methods of using those formulations, thereby resulting in a decrease in a patient's overall pill burden.
Owner:INNOVATIVE PHARMA PROD
Polymorphic crystalline forms of celecoxib
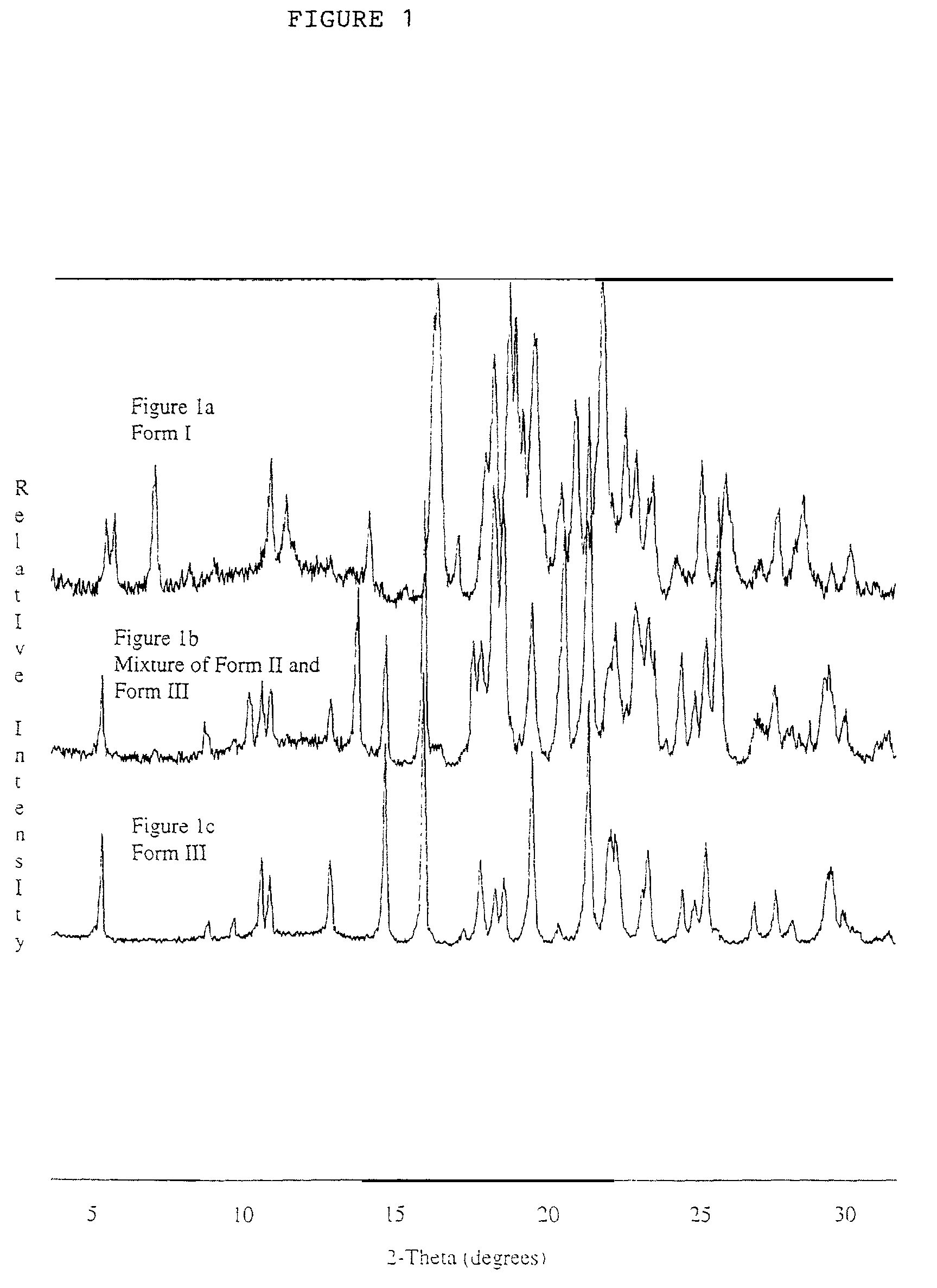
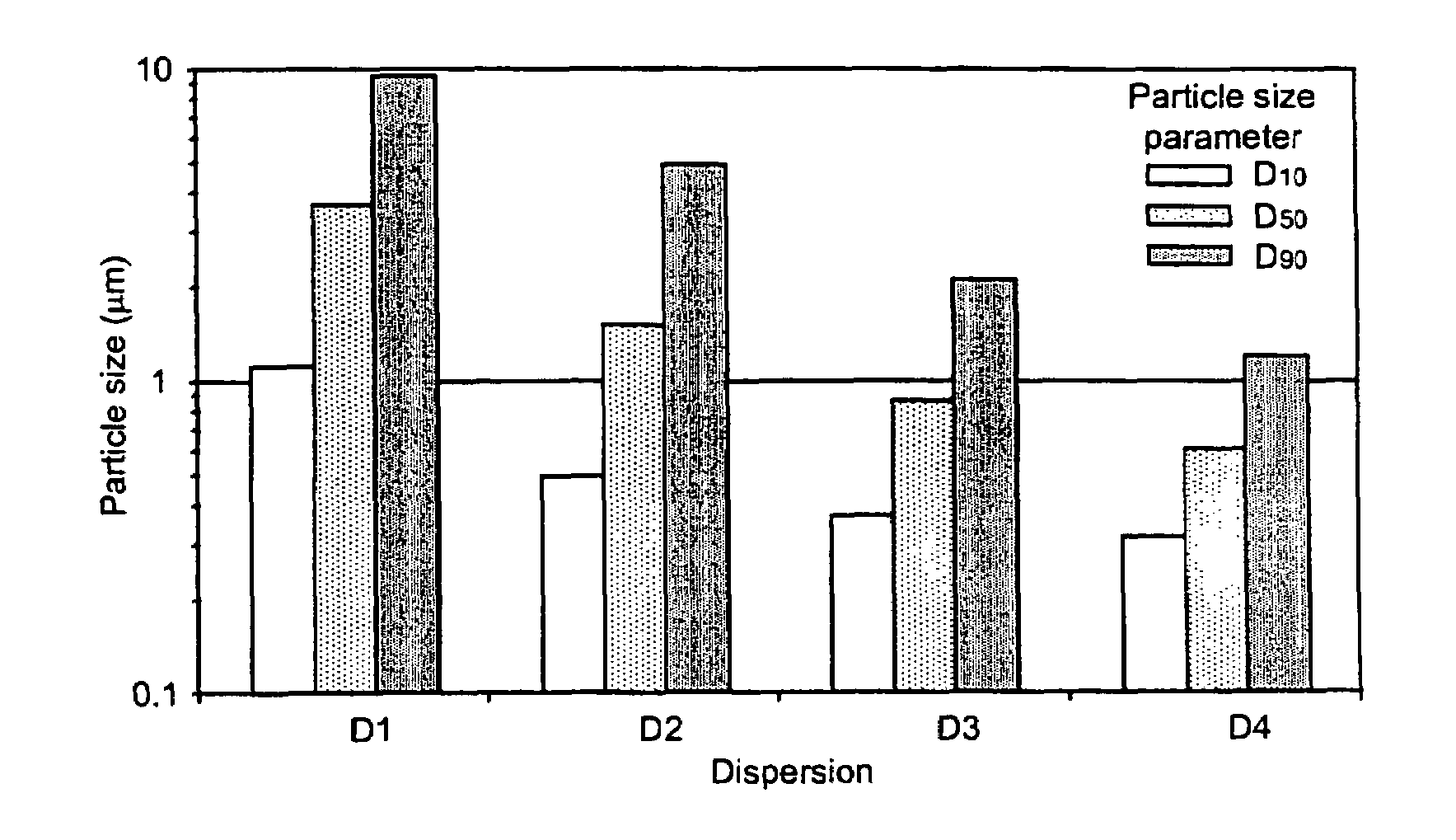
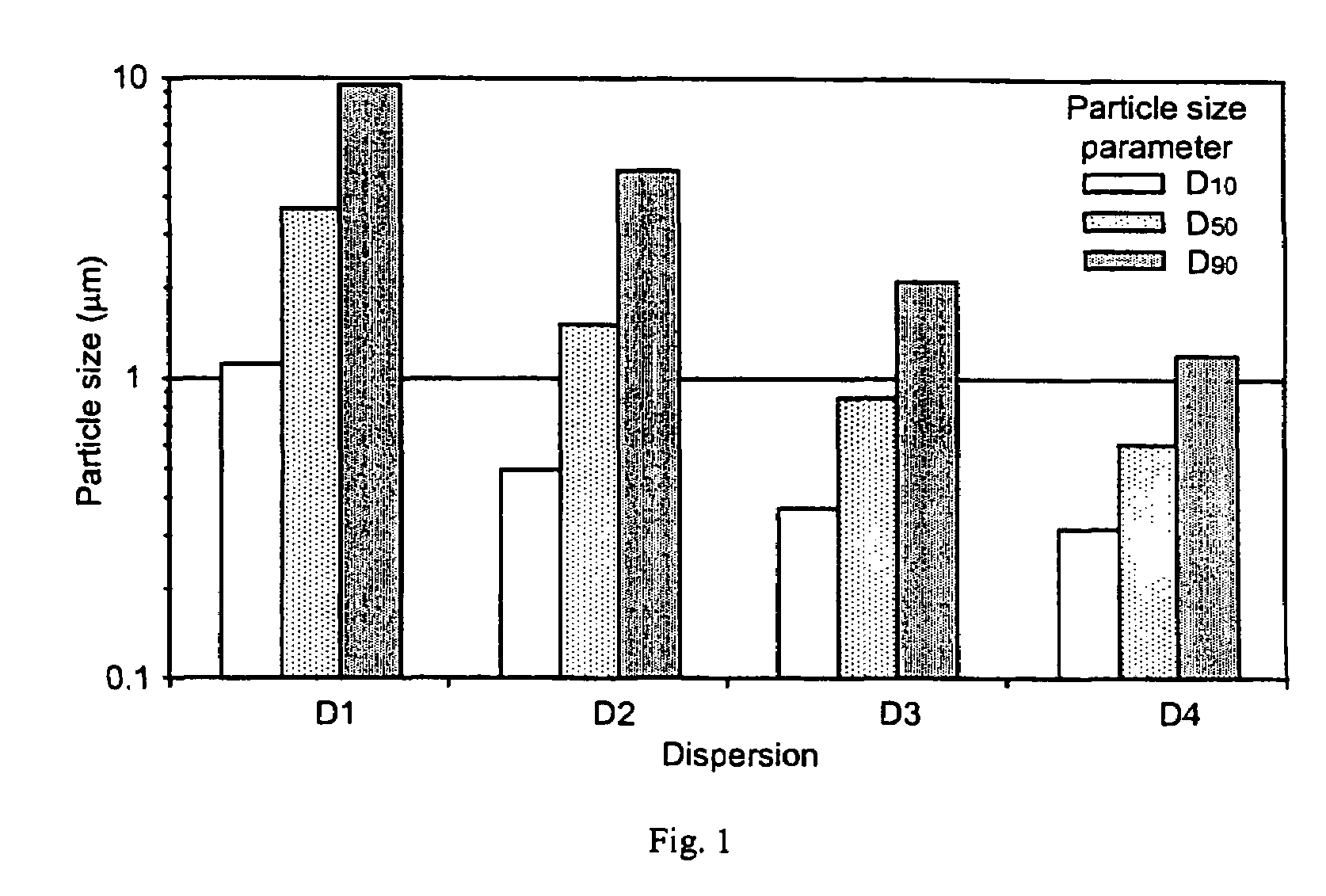
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising a selective cyclooxygenase-2 inhibitory compound of low water solubility in a therapeutically effective amount, wherein the compound is present in the form of solid particles, about 25% to 100% by weight of which are smaller than 1 micrometer. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders and have particular advantages where rapid onset of therapeutic effect is desired. The novel Form I and Form II crystalline forms of celecoxib are described. The crystalline forms have unique chemical and physical properties relative to other solid state forms of celecoxib and are characterized by their powder x-ray diffraction (PXRD) patterns, differential scanning calorimetric (DSC) thermograms, and other physical characterizations.
Owner:PHARMACIA CORP
Celecoxib composition, and preparation method and use thereof
The invention provides a celecoxib composition. The celecoxib composition contains one or more types of dose units which can be orally released, and each of the dose units contains 50-500mg of celecoxib D95 particles and one or more types of mixtures of pharmaceutical excipients. The composition can be used for treating or preventing diseases caused by COX-2.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
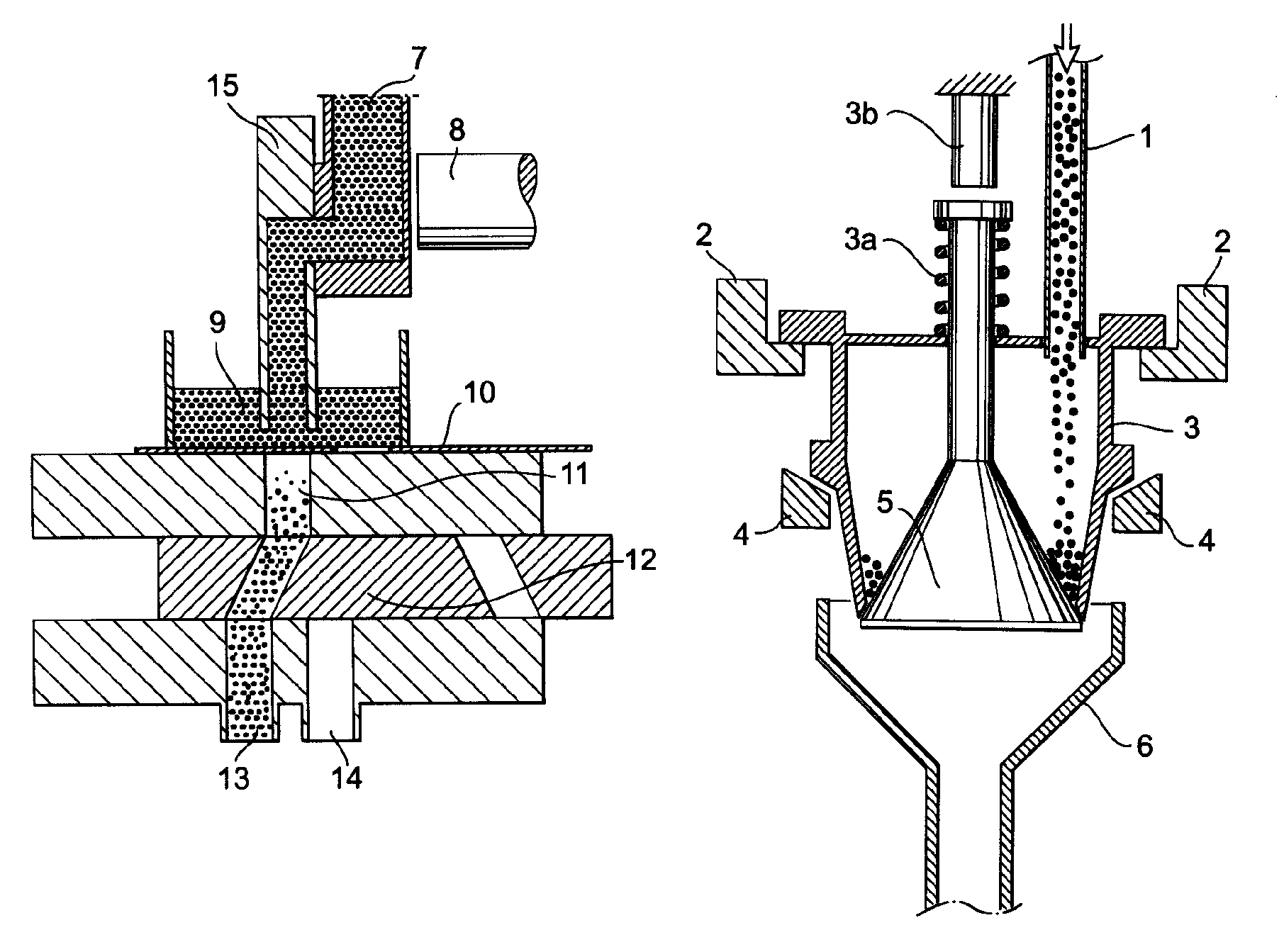
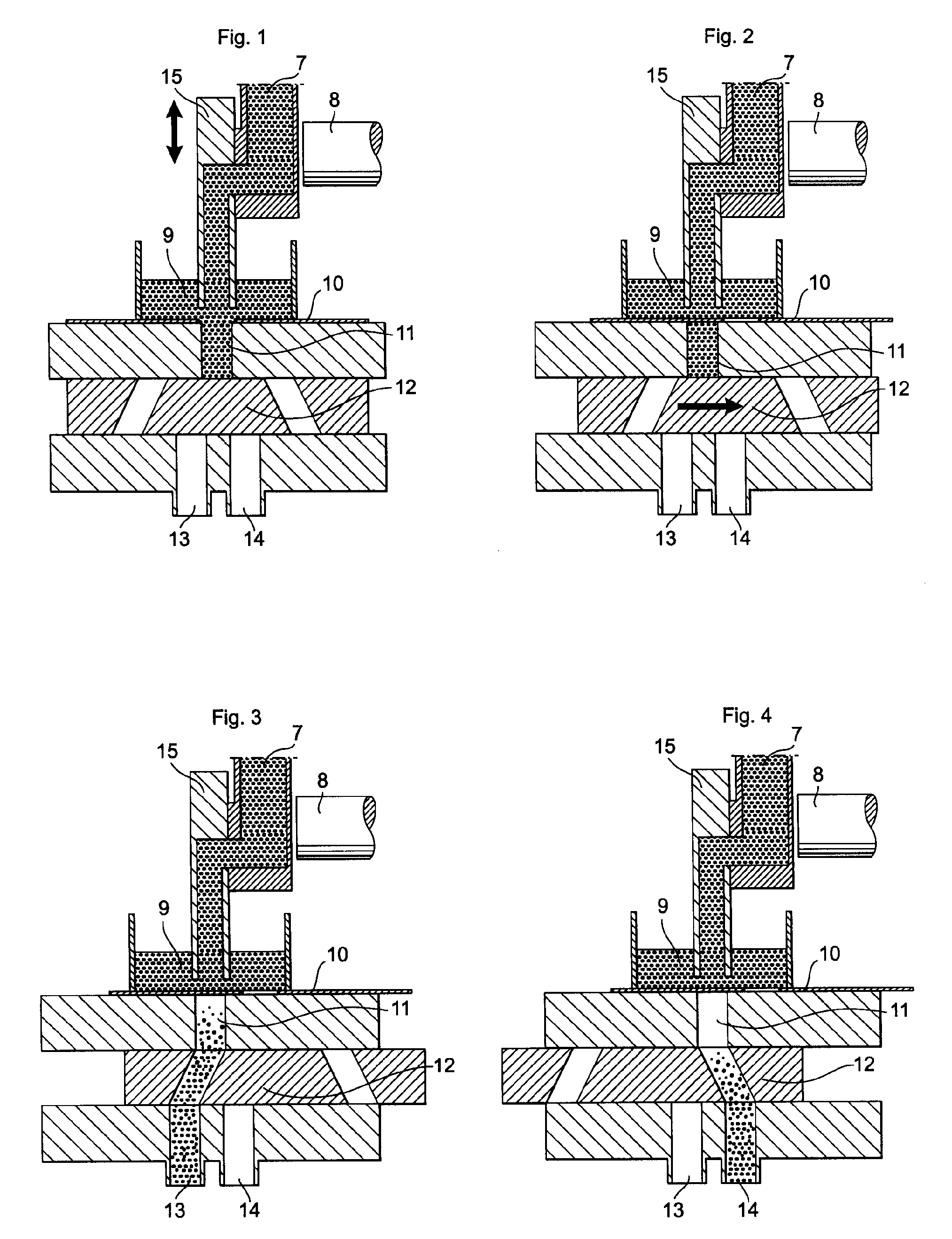
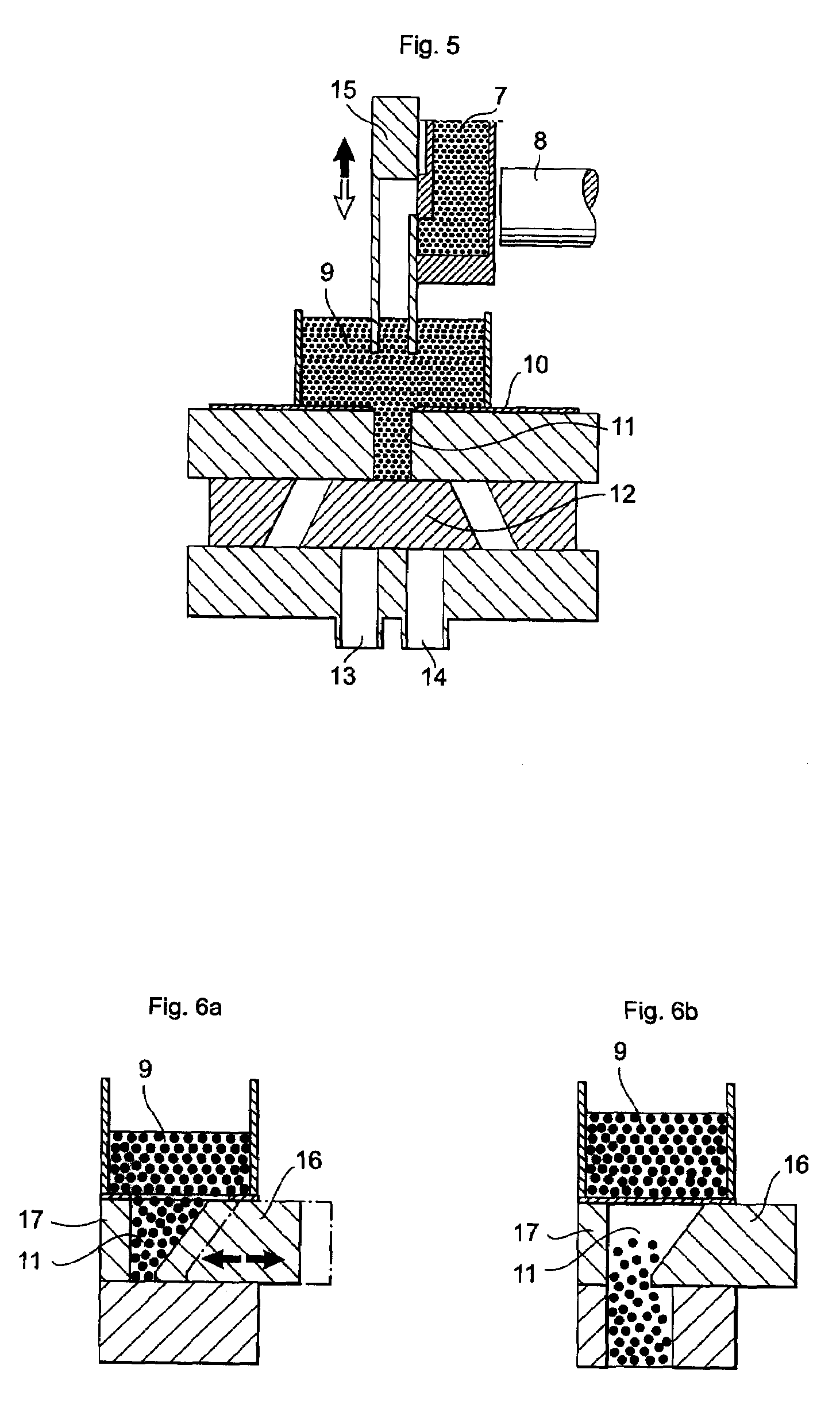
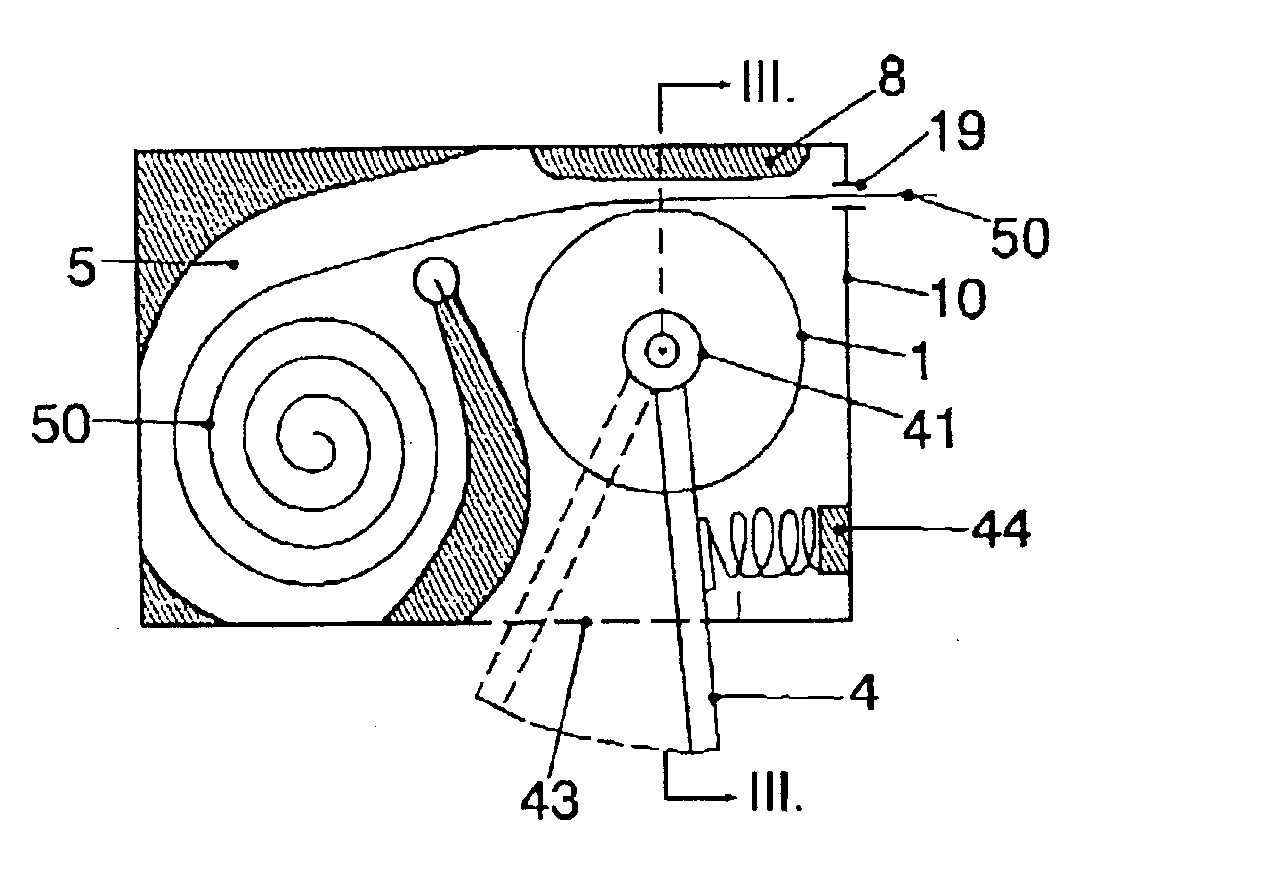
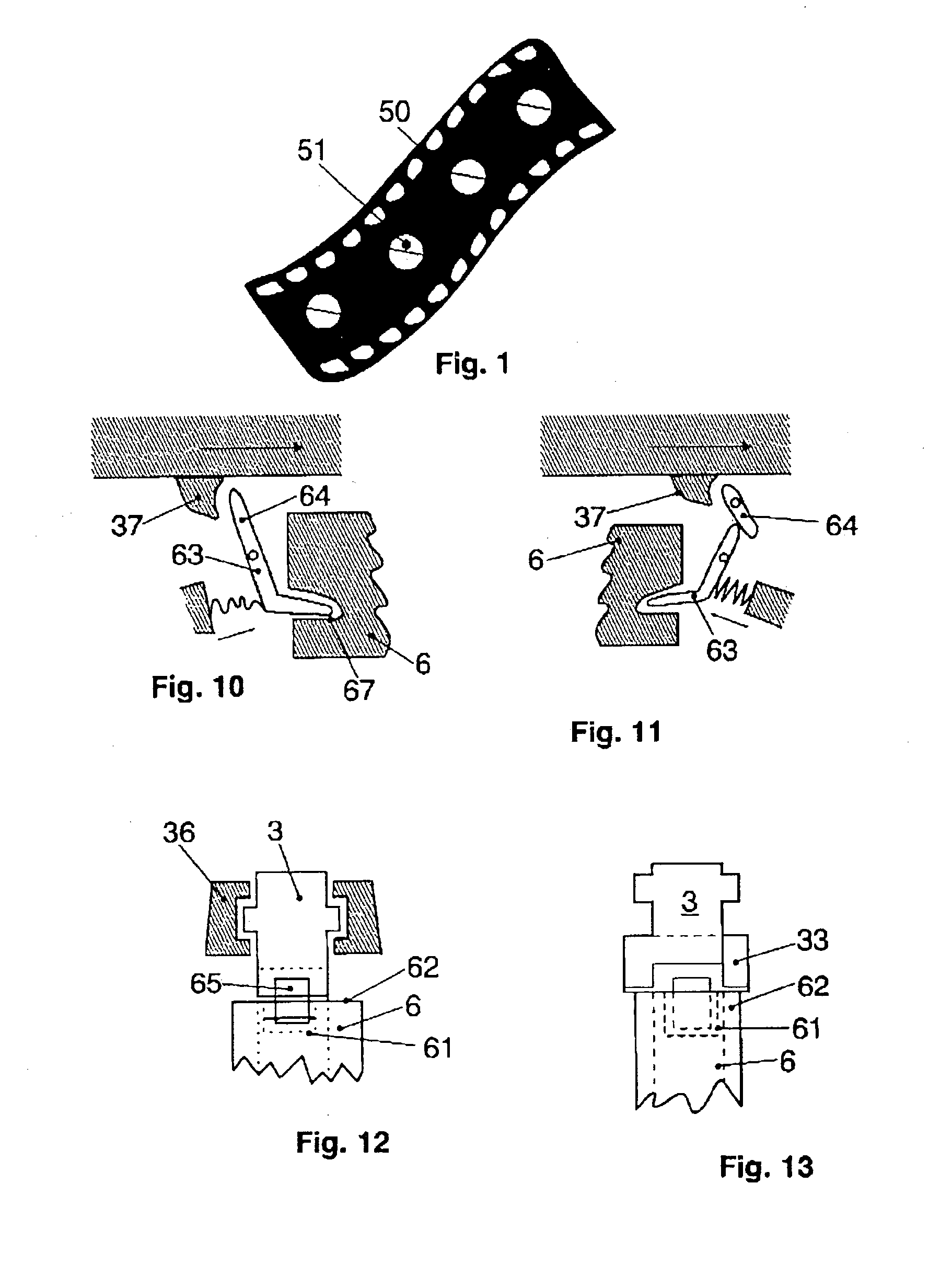
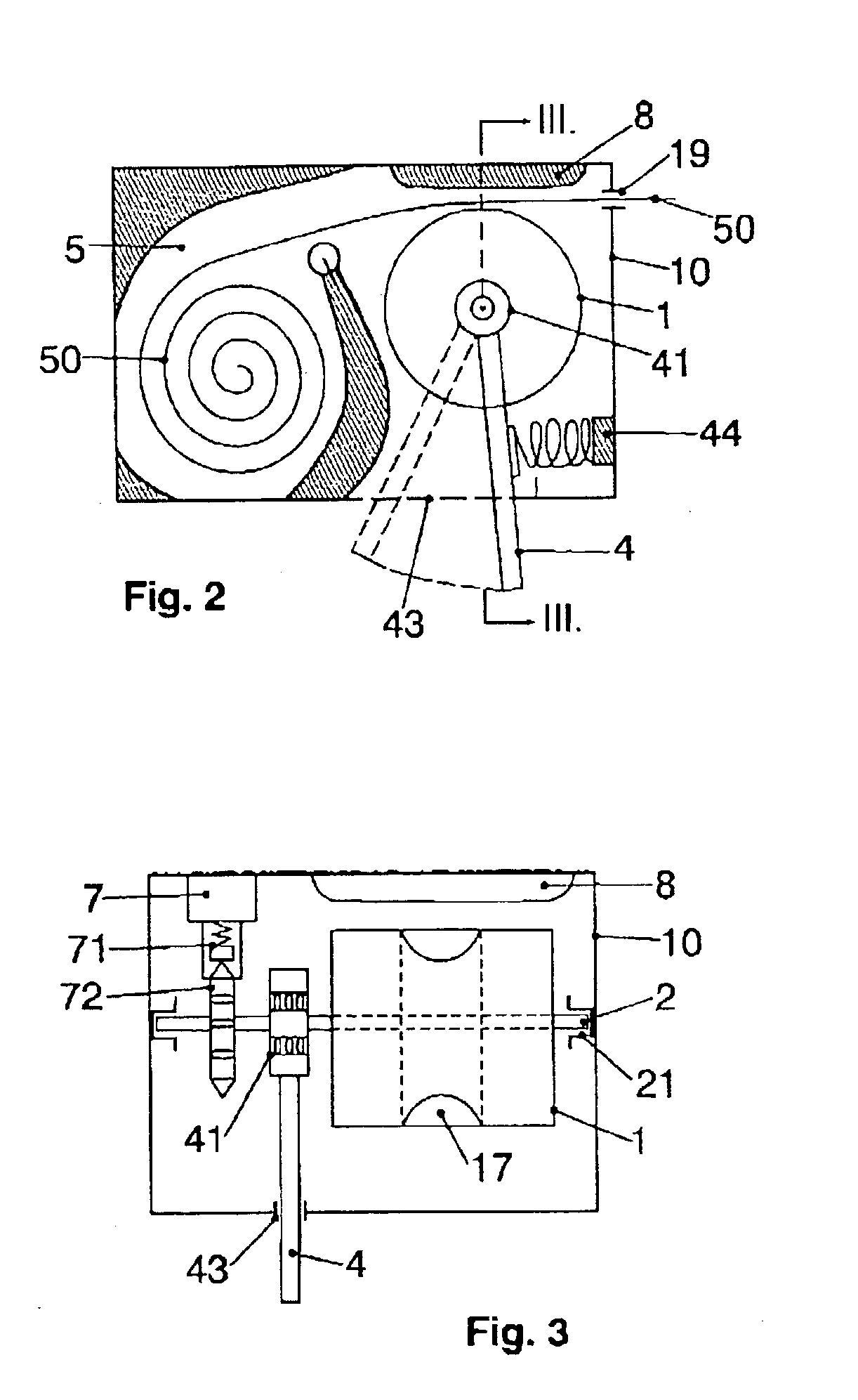
Apparatus for dosage of medicaments
InactiveUS20030102324A1Counter becomes reusableEasy assessment processCoin-freed apparatus detailsPharmaceutical containersSubject matterBiomedical engineering
The subject matter of the invention is an apparatus to dose medicaments and control taking in the medicaments, which apparatus consists of a storage unit (5) to store tablets or capsules, a dosing unit and a counter (7). The characteristic feature of the apparatus according to invention is that the medicaments (like tablets or capsules) (51) are packed in a medicament tape (50) and stored in the storage unit (5) and cylinders (1) furthering said medicament tape (50) are attached to the storage unit (5) and the said cylinders are mechanically coupled with a manual driving unit (4).
Owner:ILLYES MIKLOS
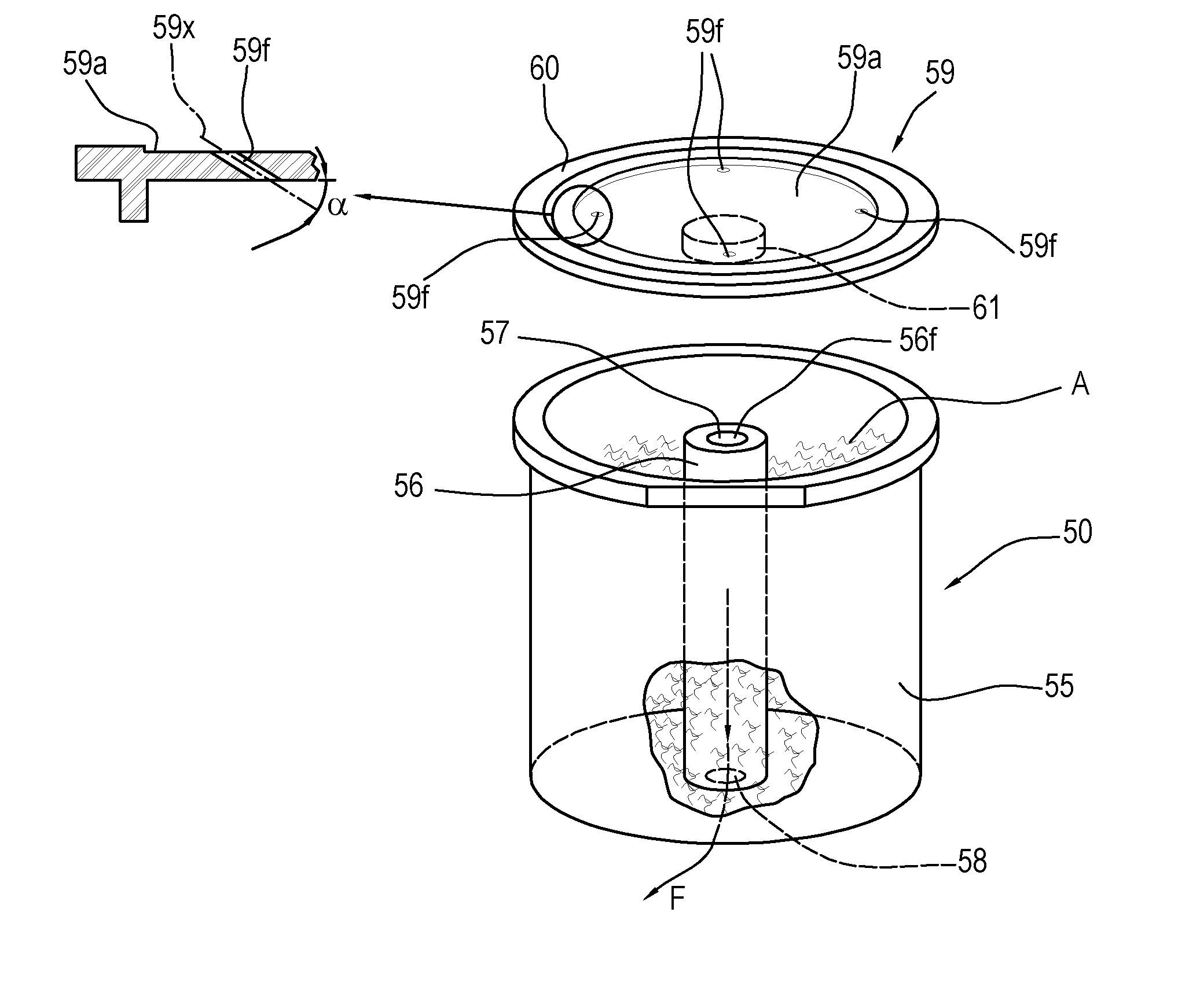
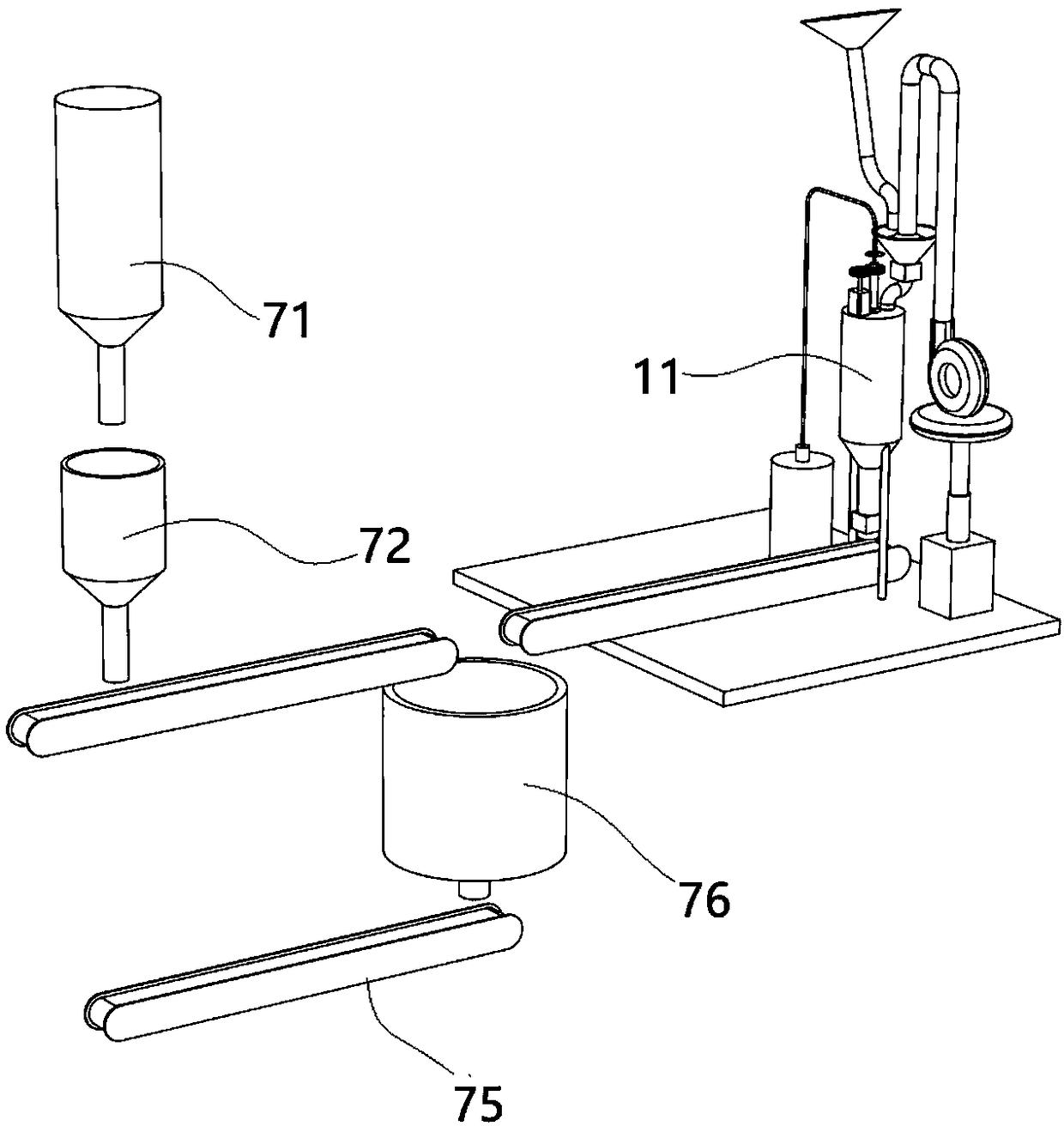
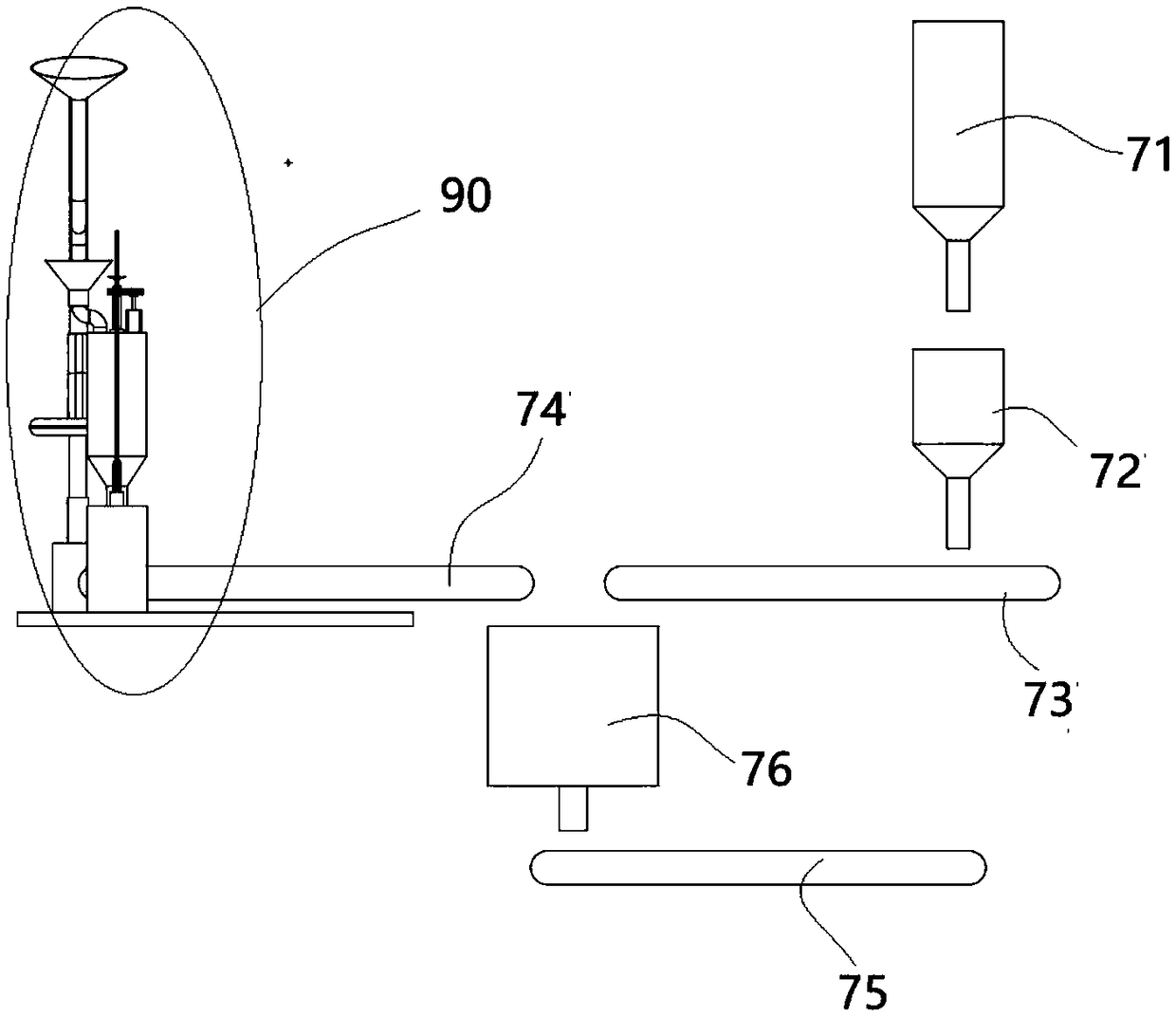
Apparatus and method for preparing and dispensing a single dose of a food product and a relative single-dose unit
ActiveUS20160316781A1Low production costHealth and safetyFrozen sweetsBeverage vesselsEngineeringSolvent
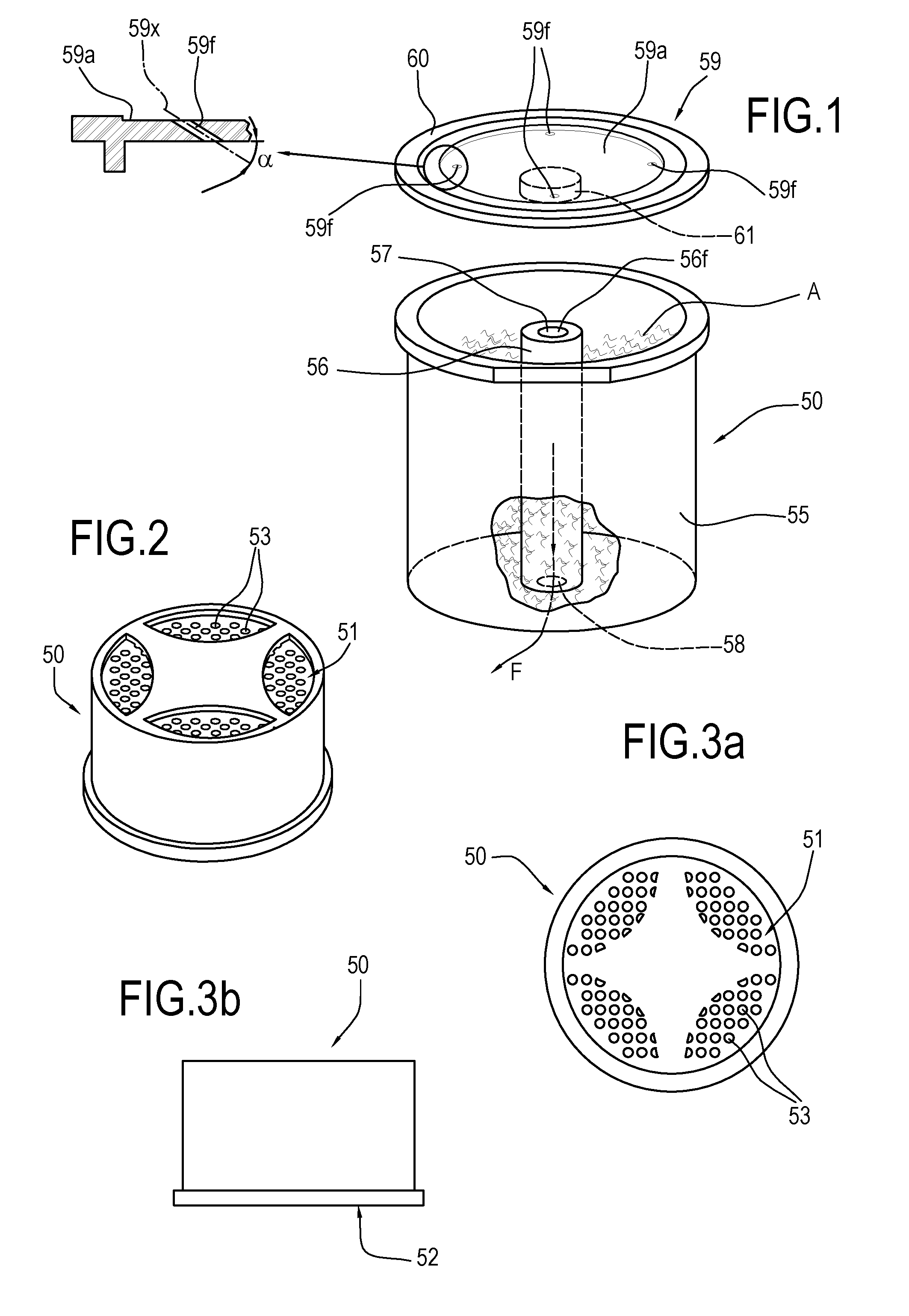
Described is an apparatus for preparing and dispensing a single dose of a food product comprising:—means (2) for feeding a solvent liquid having at least one duct (28) for dispensing the liquid;—a cavity (11) for housing a single-dose unit (50) of a food product comprising an inlet connected to the duct (28) for dispensing the liquid designed for feeding the liquid to the single-dose unit (50); the cavity having at least one duct (8) for transferring the product-liquid mixture;—a unit or chamber (9) for cooling the mixture fed by the transfer duct (8) inside the cooling unit;—a unit (14) for dispensing the product formed inside the cooling unit (9) connected to the same unit, for dispensing the product formed in a single dose.
Owner:RDL
Cyclooxygenase-2 inhibitor compositions having rapid onset of therapeutic effect
Pharmaceutical compositions are provided comprising one or more orally deliverable dose units, each comprising a selective cyclooxygenase-2 inhibitory drug of low water solubility in a therapeutically effective amount, wherein the drug is present in the form of solid particle, about 25% to 100% by weight of which are smaller than 1 μm. The compositions are useful in treatment or prophylaxis of cyclooxygenase-2 mediated conditions and disorders and have particular advantages where rapid onset of therapeutic effect is desired.
Owner:PHARMACIA CORP
Method and apparatus for monitoring, dosing and distribution of chemical solutions
InactiveUS20050051433A1Maintain qualityCellsSemiconductor/solid-state device manufacturingChemical treatmentChemical composition
It is an object of the present invention to provide a system and method for monitoring, dosing and distribution of a chemical composition in a material treatment process, the chemical composition containing at least one additive for maintaining quality of the chemical treatment process. The system and method include: at least one chemical containing unit configured to contain the chemical composition for the chemical treatment process; a dosing unit fluidly communicating with the at least one chemical containing unit configured to receive the chemical composition therefrom and to add a selected dose of the at least one additive to the chemical composition therein; an online monitor configured to monitor a property of the chemical composition at the dosing unit and to transmit a signal corresponding to the monitored property; and a controller programmed and configured to receive the signal from the online monitor and send a signal to the dosing system to add the selected dose to the chemical composition therein in response to the monitored property.
Owner:ZDUNEK ALAN D +2
Apparatus, system and method for administering an anesthetic agent for a subject breathing
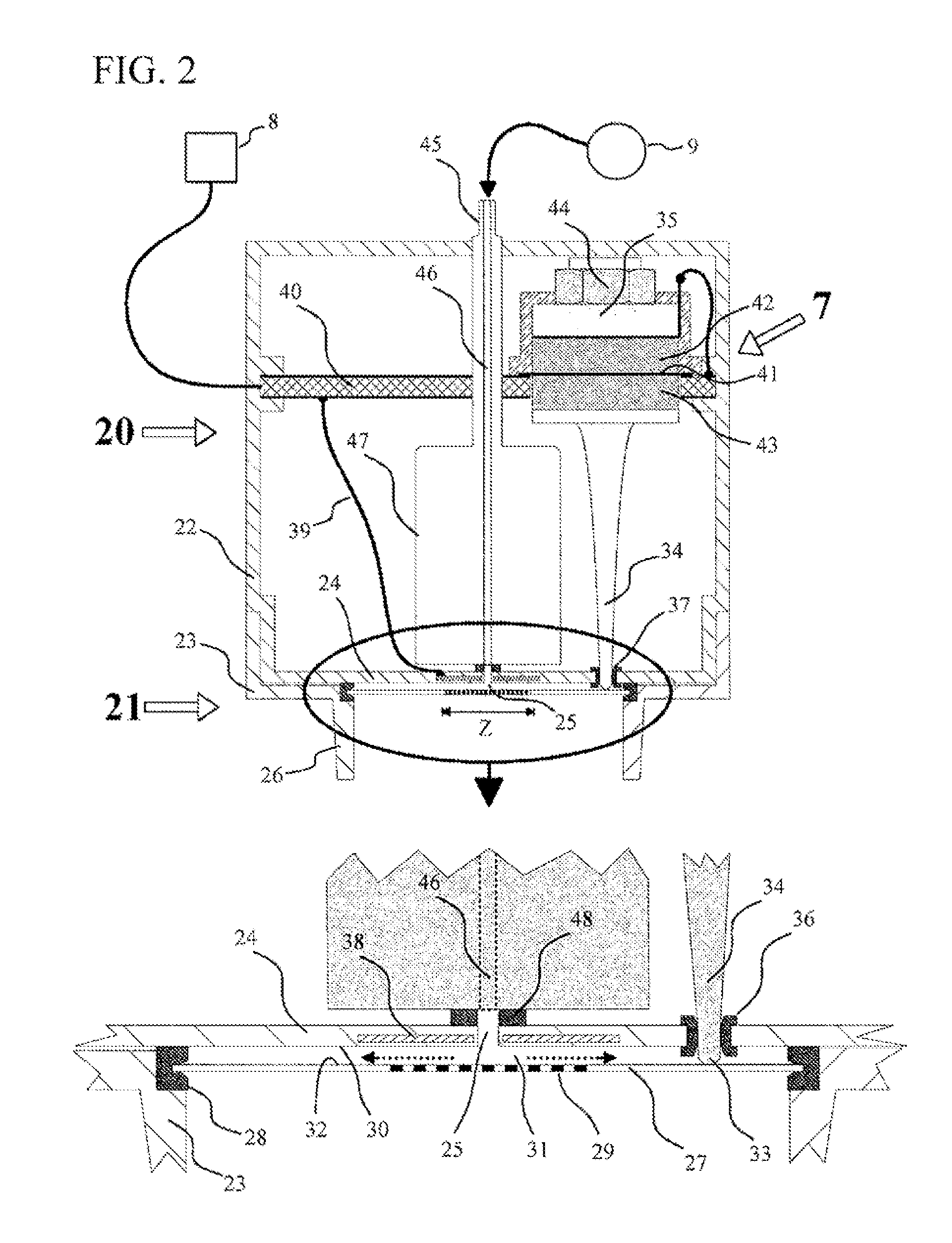
InactiveUS20090288659A1RespiratorsControlling ratio of multiple fluid flowsAnesthetic AgentBreathing gas
An apparatus for administering an anesthetic agent for a subject breathing. The apparatus includes a liquid reservoir for an anesthetic agent and an anesthetic agent dosing unit in fluid connection with the liquid reservoir and which anesthetic agent dosing unit is connectable to an airway tube for delivering breathing gases to a subject, said dosing unit including a first member to produce anesthetic agent droplets having a diameter less than 100 μm.
Owner:GENERAL ELECTRIC CO
Fertility-increasing type soil remediation system and remediation method thereof
InactiveCN108856268AEliminate the disadvantages of nutrient lossHeating evenlySoil lifting machinesClimate change adaptationSoil remediationEngineering
The invention discloses a fertility-increasing type soil remediation system. The system comprises a soil thermal desorption unit, an excrement fermentation unit, a stirring and dosing unit and a soil-excrement mixing unit; a soil source discharging end of the soil thermal desorption unit is connected with a soil source feeding end of the soil-excrement mixing unit through a first conveying device;a discharging end of the excrement fermentation unit is connected with a feeding end of the stirring and dosing unit, the discharging end of the stirring and dosing unit is connected with an excrement feeding end of the soil-excrement mixing unit through a second conveying device, and the discharging end of the soil-excrement mixing unit is connected with a third conveying device. The system hasthe advantages that the structure is simple, the soil after the heat desorption and the fermented excrement are mixed, and a disadvantage of the nutrient loss of partial soil after thermal desorptionis eliminated.
Owner:南通劲凌智能科技有限公司
NSAID Dose Unit Formulations with H2-Receptor Antagonists and Methods of Use
The present invention generally relates to pharmaceutical unit dosage forms of NSAIDs and H2-receptor antagonists, in which the H2-receptor antagonist is formulated so as to be released in a sustained manner over a predetermined period of time so as to maintain gastric pH above a desired level for a duration of time. The NSAID may then be formulated for immediate release. The pharmaceutical unit dosage forms may be administered to subjects susceptible to the development of NSAID induced gastric and / or duodenal ulcers, as the sustained release H2-receptor antagonist is formulated so as to maintain the gastric environment above the pH levels where NSAID-induced ulceration typically occurs.
Owner:HORIZON PHARMA USA
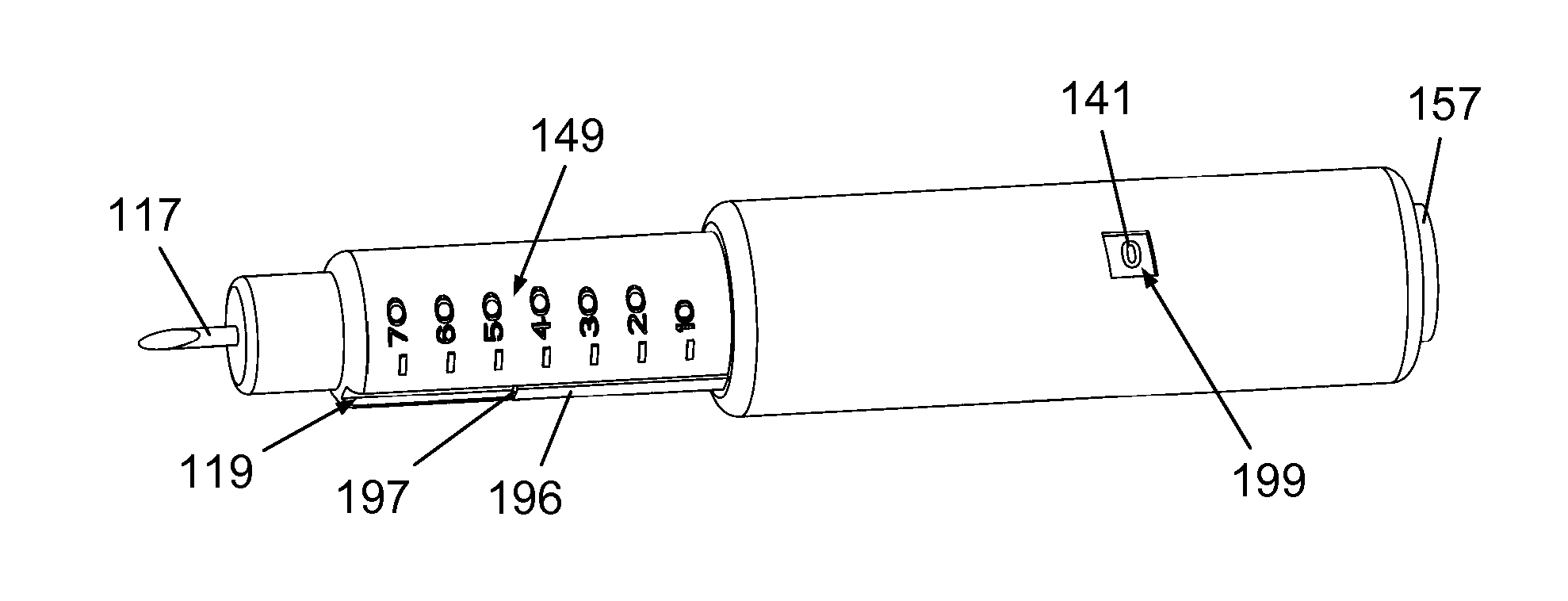
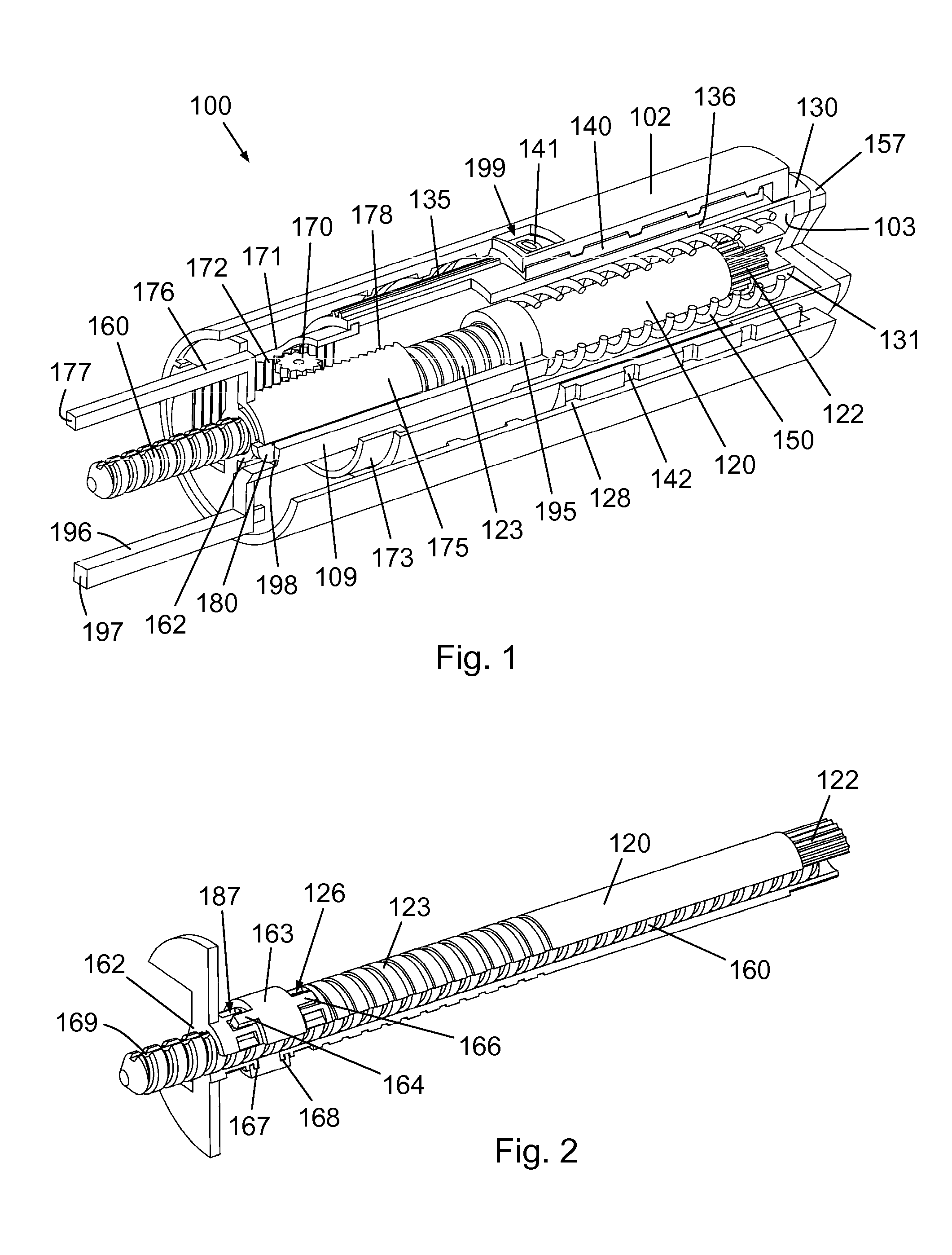
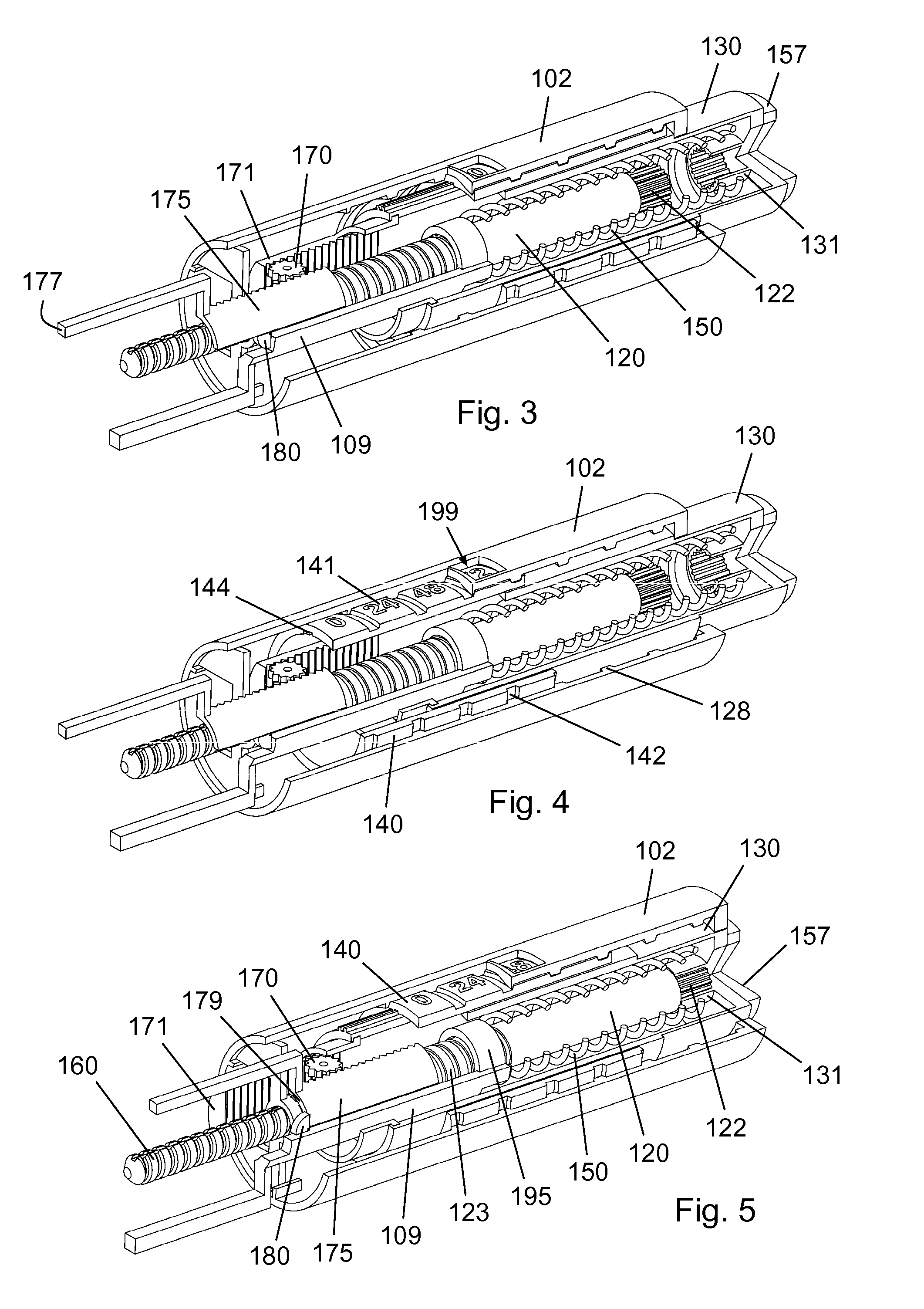
Mechanical Dose Expelled Indicator
InactiveUS20160317751A1Eliminate or reduce at least one drawback of the prior artAmpoule syringesAutomatic syringesDose deliveryMedicine
The present invention concerns a dosing unit for an injection device (100) and an injection device incorporating the dosing unit. The dosing unit comprises a housing (102) extending along a longitudinal axis from a proximal housing end to a distal housing end, the distal housing end being adapted for connection with a variable volume reservoir, an injection mechanism for expelling a dose of a substance held in a connected variable volume reservoir, the injection mechanism being arranged at least partially in the housing (102) and comprising an injection button (157), a dose defining structure (140) configured to move from a start position to an end-of-dose position in response to a dose expelling operation of the injection button (157), and a piston rod (160) configured to move in a dose delivery direction from a first position to a second position in response to a movement of the dose defining structure (140) from the start position to the end-of-dose position, and a dose delivered indicator (109) configured to move axially in a distal direction from a dose ready position to a dose delivered position in response to the movement of the dose defining structure (140) from the start position to the end-of-dose position, wherein during movement from the dose ready position to the dose de livered position a portion of the dose delivered indicator (109) emerges increasingly from the distal housing end.
Owner:NOVO NORDISK AS
Arrangement for dosing pourable substances and associated uses
Arrangement for dosing granular materials, propellants, explosives, gunpowder and other pourable substances comprising at least a container with a closure and a dosing unit comprising a measuring chamber for the take up of said substance, said dosing unit is defined connectable with the container via a means of association for the purpose of dosing, whereby said closure is held self-powered in a closed position and is transferable by a means of actuating into an open position for opening. At least one means of locking is provided securing the closure in the closed position in order to prevent unintentional release of substance, whereby the means of locking is releasable preferentially by the dosing unit while connecting with the container latest while attaining the filling location in order to rest the closure in the closed position as possible long and effect an constrained control. For additional security at least one means of holding my be provided in order to rest the association of dosing unit and container in the filling position. Said arrangement comprising a container for taking up of the substances, which is utilizing an active generation of a throttle effect by means of a field of lateral force. The container may be equipped with a funnel-shaped section being closable by means of a lid for the easier clearing of the container. Said arrangement further comprises a preferred clamping holder for taking up the container and a preferred holding device comprising a means of pressure equalization; furthermore preferred uses of the dosing unit and the arrangement and a preferred adhesive-joint for the container increasing the stability under load, particularly at swelling loads as well as three-dimensional stress condition. And finally a preferred tenter tool preventing the dosing unit from damages while assembling it.
Owner:HOERMANN KARL LUDWIG HARAL
Oral contraceptive containing a gestagen and an estrogen combined with pharmaceutically acceptable auxiliary agents and/or excipients, but not containing lactose, and method of making same
InactiveUS20090117183A1Easy to solveLow costBiocideOrganic active ingredientsPhysiologyAdditive ingredient
The method produces a lactose-free oral contraceptive composition containing a combination of a gestagen and an estrogen together with one or more pharmaceutically acceptable auxiliary agents and / or excipients. The contraceptive composition is a tablet, powder, or capsule that contains the gestagen and estrogen, filler material such as microcrystalline cellulose and a binder such as hydroxypropylcellulose, but no lactose. Preferably the gestagen is dienogest, chlormadinone acetate, or levonorgestrel and the estrogen is ethinylestradiol, 17β-estradiol, or estradiol valerate. A method is provided for improving the prophylaxis of lactose intolerance in women taking oral contraceptives. The oral contraceptive preparations for a standard 28-day cycle or for long-term use contain at least 21 daily dose units of the gestagen and the estrogen in a low-dosage but without lactose and at most 7 daily dose units containing no active ingredient or a placebo.
Owner:BAYER SCHERING PHARMA AG
Application of composition packet in preparing food, medicines, health care products and nutrition for improving and treating human Prader-Willi syndrome
InactiveCN106310006AIncrease the number of producing bacteriaIncrease the number ofMetabolism disorderPlant ingredientsPolygonum fagopyrumMedicine
The invention discloses an application of a composition packet in preparing food, medicines, health care products and nutrition for improving and treating human Prader-Willi syndrome. The composition packet includes the following compositions each of which is in a uniform dose unit form convenient for dose administration, and each dose unit form is a single-dose physical dispersing unit, wherein the first composition consists of coix seeds, oats, buckwheat, semen lablab album, yellow corn, semen phaseoli, soybeans, rhizoma dioscoreae, fructus ziziphi jujubae, peanuts, lotus seeds and fruits of Chinese wolfberry; the first composition can also consist of rye, wheat, quinoa and hulless barley; the second composition consists of fructus momordicae charantiae, soluble dietary fibers and oligosaccharide; and the third composition consists of soluble dietary fibers and oligosaccharide. By reasonably adjusting the diet nutrition of patients with the human Prader-Willi syndrome, the purposes of relieving, removing, remedying, preventing or improving the symptoms of the human Prader-Willi syndrome and recovering health can be achieved.
Owner:PERFECT CHINA
Celecoxib composition, and preparation method and application thereof
InactiveCN102949403AInhibit uterine contractionsReduce gastrointestinal toxicityOrganic active ingredientsAntipyreticDose UnitsOral medication
The invention provides a celecoxib composition which contains one or a plurality of dose units releasable through oral administration. Each dose unit contains 50 to 500 mg of a mixture of a celecoxib D97 particle and one or a plurality of medicinal excipients. The composition can be used for treating or preventing diseases caused by COX-2.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Pharmaceutical compositions
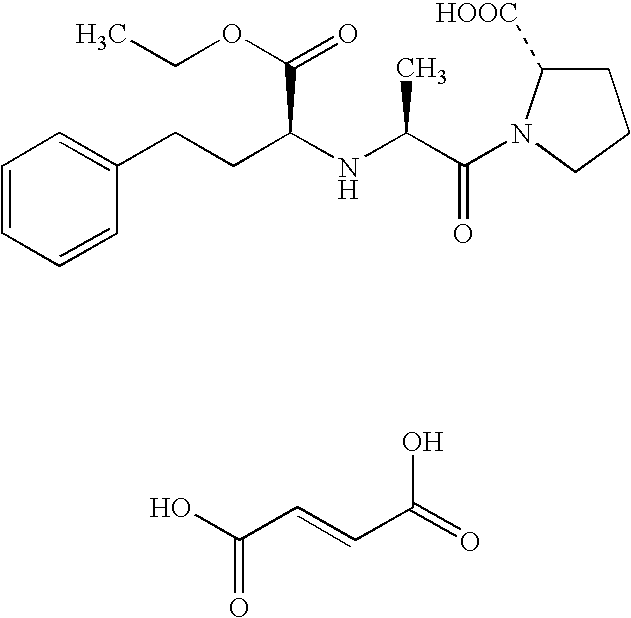
Provided herein are formulations and methods for treating pain in human beings. Also provide are optimal ratios at which an opioid and an opioid antagonist may be combined for administration to humans such that the opioid activity is inhibited. These ratios may also be used to formulate compositions containing both an opioid and an opioid antagonist within a single pharmaceutical dosing unit.
Owner:ALPHARMA PHARMA
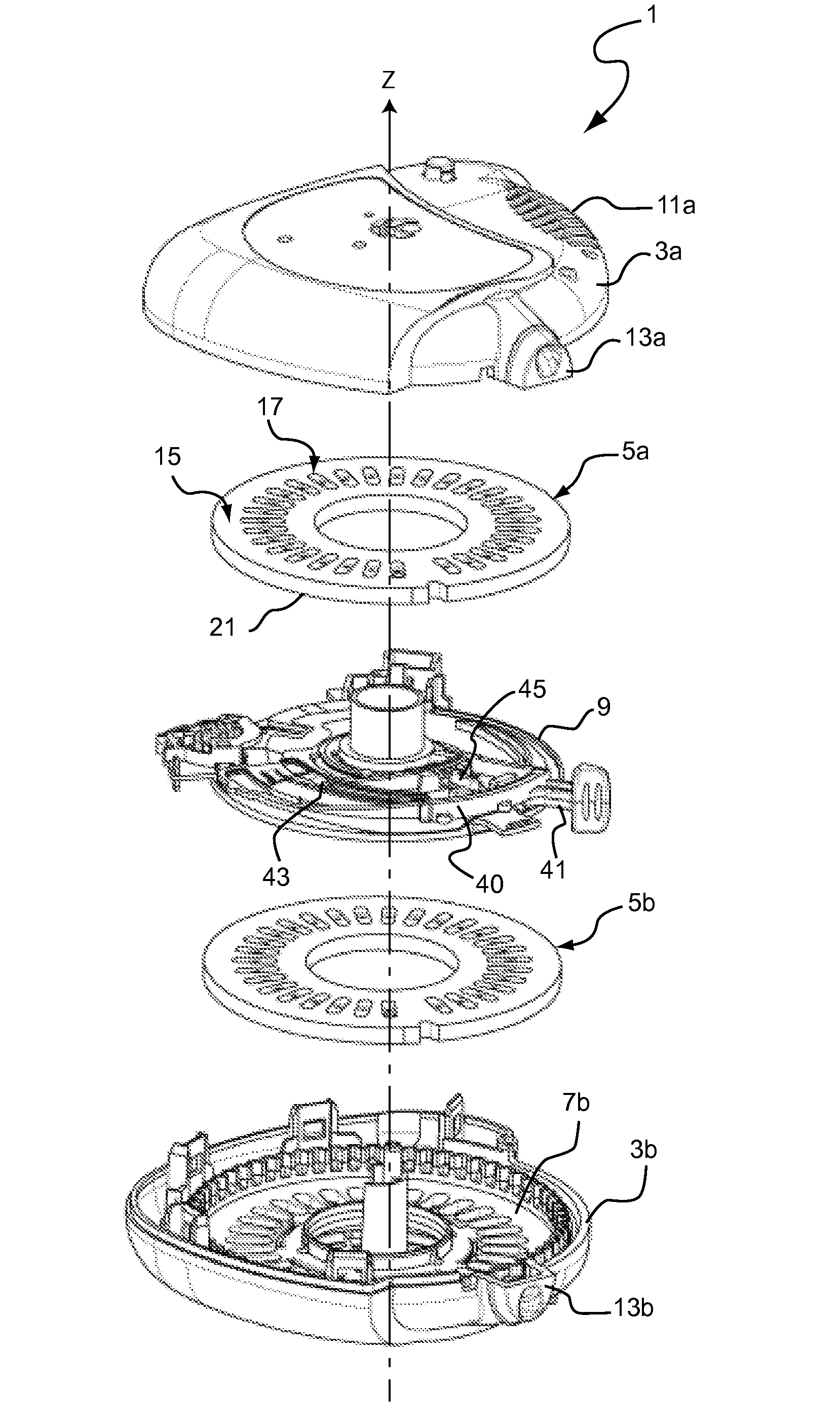
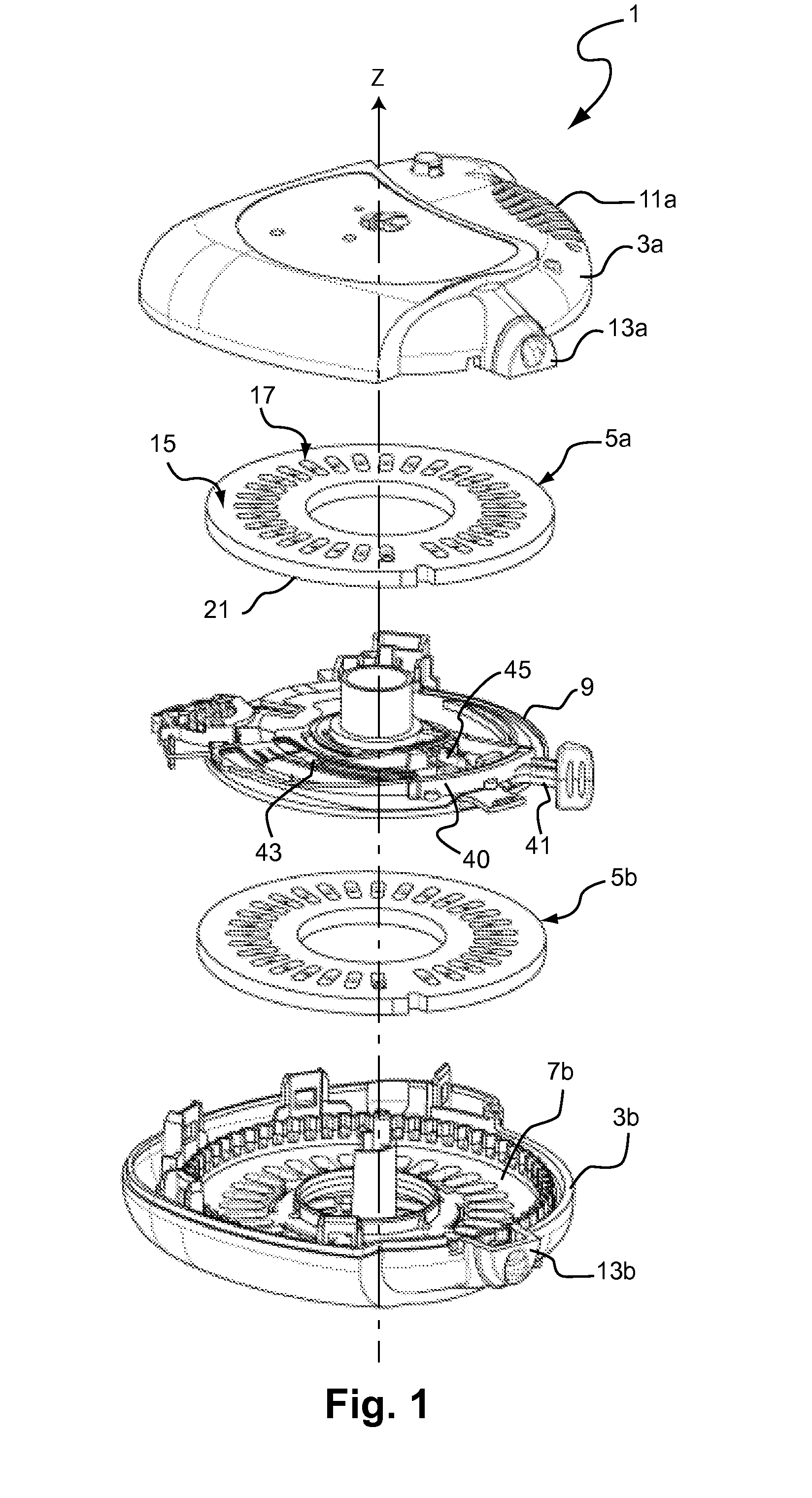
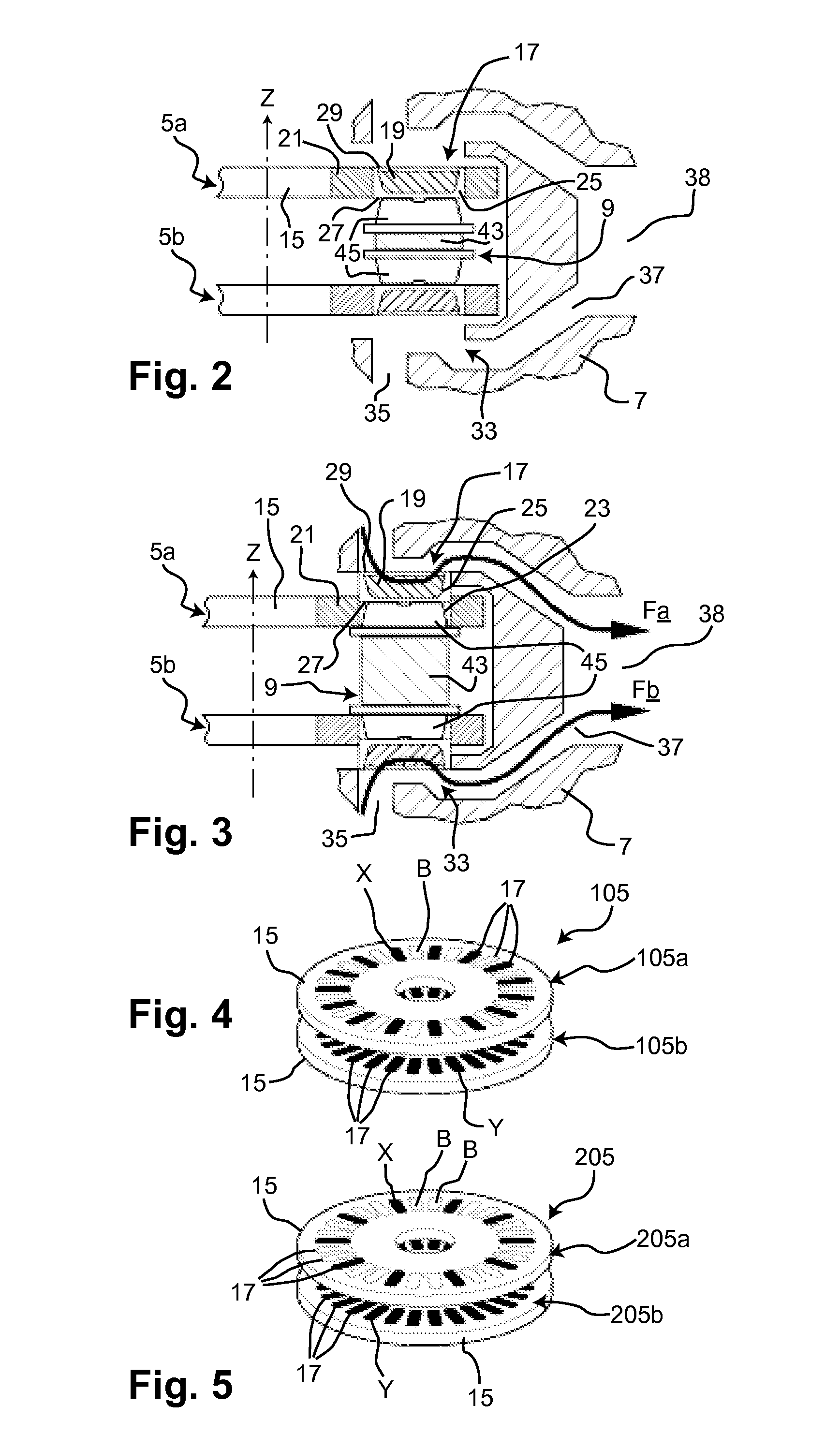
Does Unit, Pack of Dose Units and Inhaler for Inhalation of Combination of Drugs
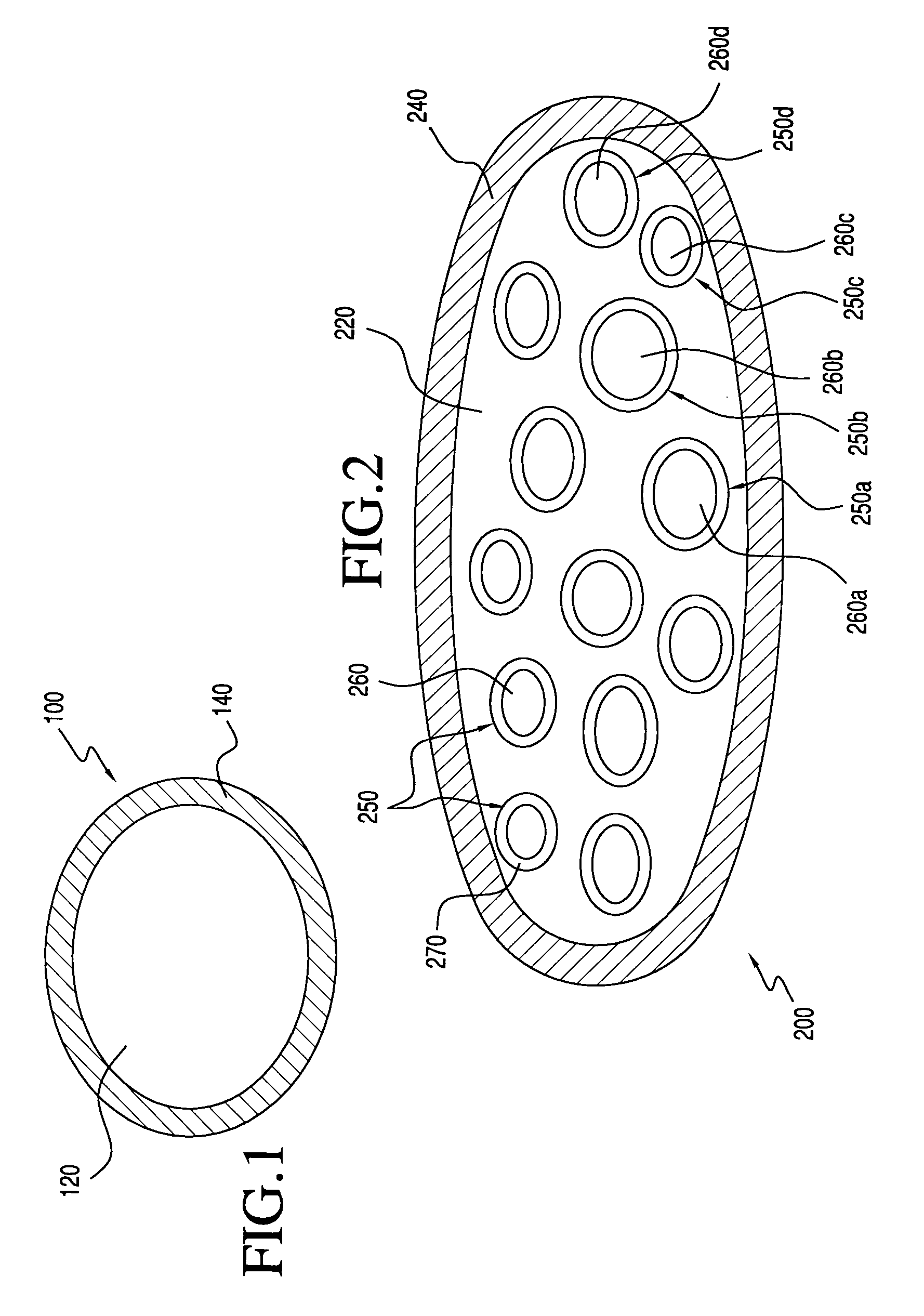
The invention relates to a dose unit for a dry powder inhaler comprising:—a dose carrier including a plurality of pockets (17) each adapted to contain a dose of medication powder suitable for inhalation, said pockets being sequentially arranged such that the content of the pockets (17) can be sequentially exposed to a flow of air for successive inhalations and—a plurality of medication powder doses (X) arranged in pockets (17) of the dose carrier (15). The doses are regularly distributed in the pockets according to a sequence of identical groups, each group including at least one blank pocket (B) and one pocket containing a dose of medication powder (X). The invention also relates to a pack (105) comprised of one such dose unit (105a) and one further dose unit (105b) with all pockets containing a medication powder (Y). The invention further relates to a dry powder inhaler including such a pack (105) of dose units.
Owner:PFIZER LTD
Pharmaceutical preparation containing a gestagen, and kit and method for treating endometriosis using the preparation
InactiveUS20110008409A1Endometriosis can be reducedOrganic active ingredientsPharmaceutical delivery mechanismBone densitySide effect
The pharmaceutical preparation for treating endometriosis contains at least 28, preferably 30, daily dose units, each of which contain dienogest, cyproterone acetate, or chlormadinone acetate at a daily dose that is at most twice that required to inhibit ovulation together with one or more pharmaceutical aids and / or carriers. The daily dose units are administered in a method of prophylaxis and / or therapy of endometriosis continuously during a time interval of at least 169 days or 25 weeks, preferably more than two years. The method effectively reduces endometriosis and associated pain, while undesirable side effects including bone density decrease are reduced or eliminated.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com