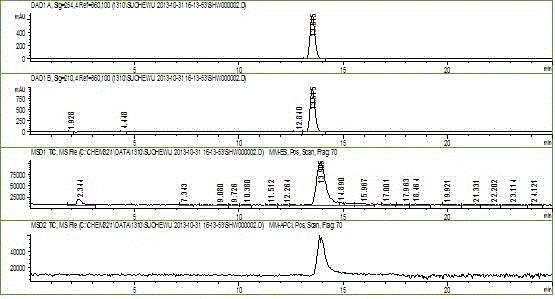
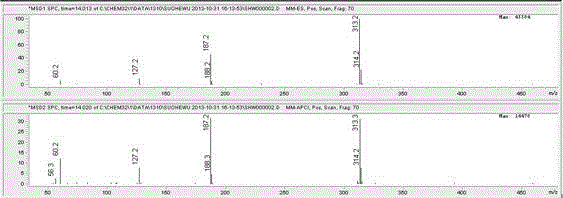
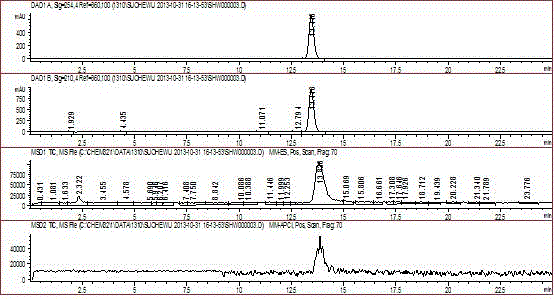
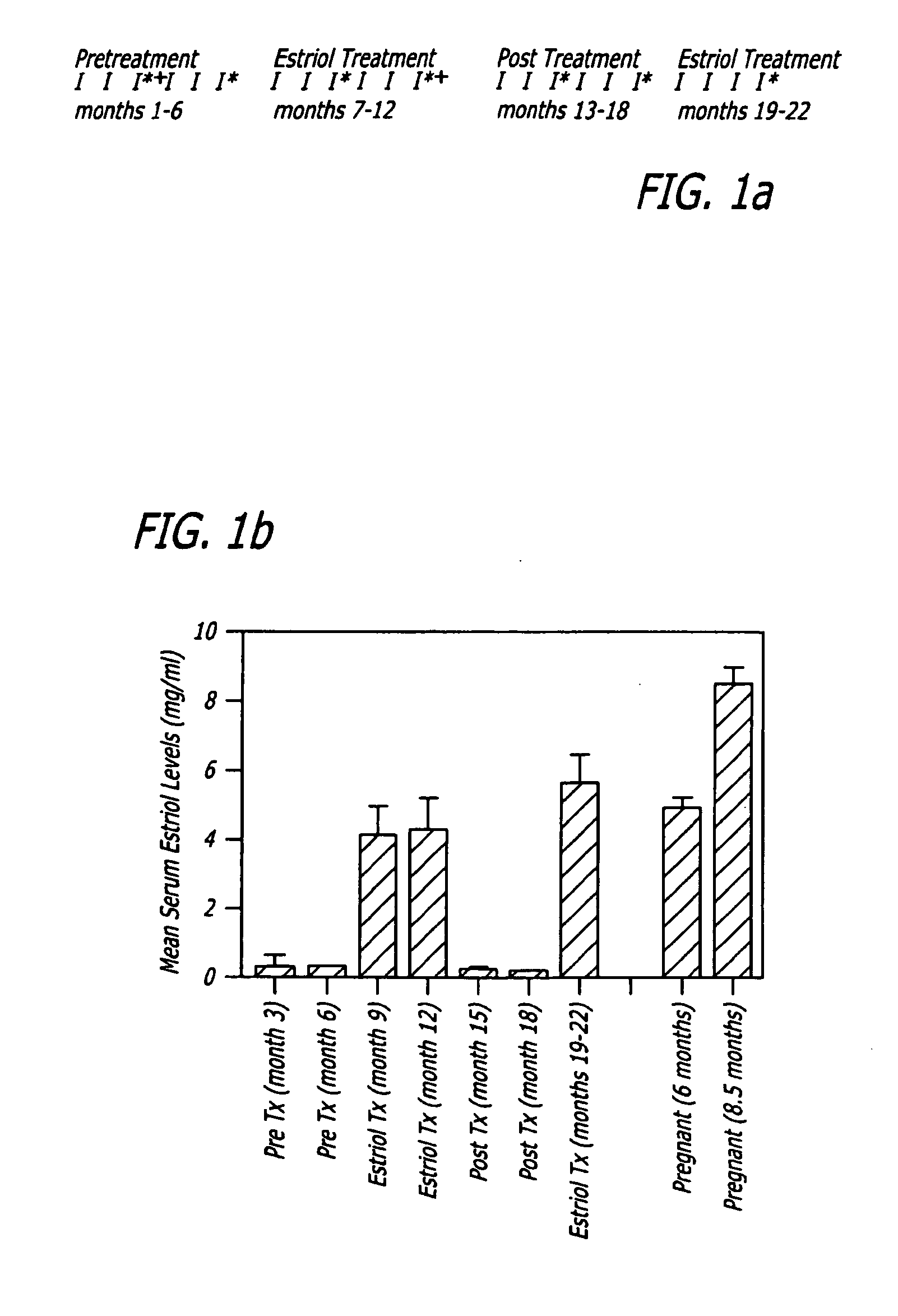
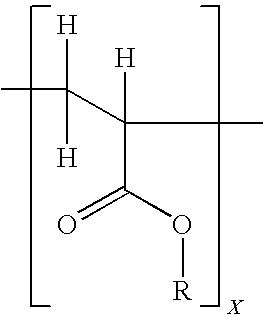
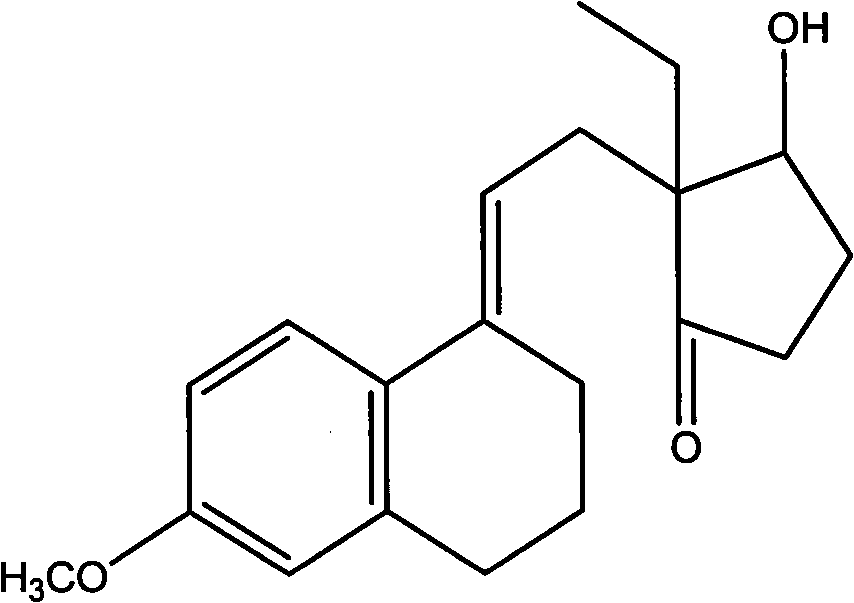
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Levonorgestrel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
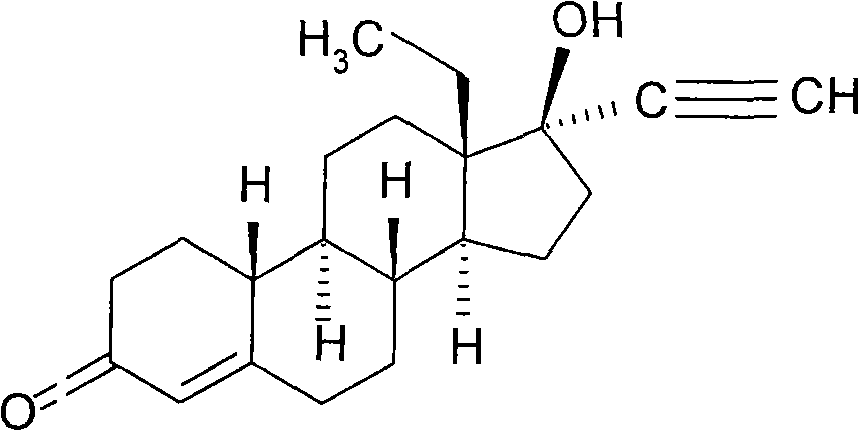
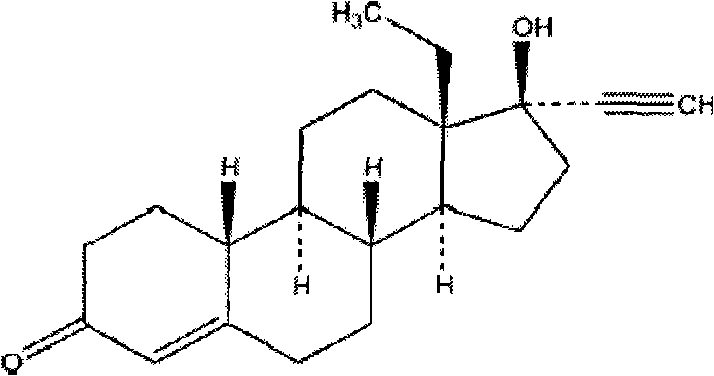
Levonorgestrel is a hormonal medication which is used in a number of birth control methods. As an emergency birth control, sold under the brand name Plan B among others, it is useful within 120 hours of unprotected sex. The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred. It decreases the chances of pregnancy by 57 to 93%. It is also combined with an estrogen to make combination birth control pills. In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy. An implantable form of levonorgestrel is also available in some countries.
Skin permeation enhancement composition for transdermal hormone delivery system
Owner:AGILE THERAPEUTICS
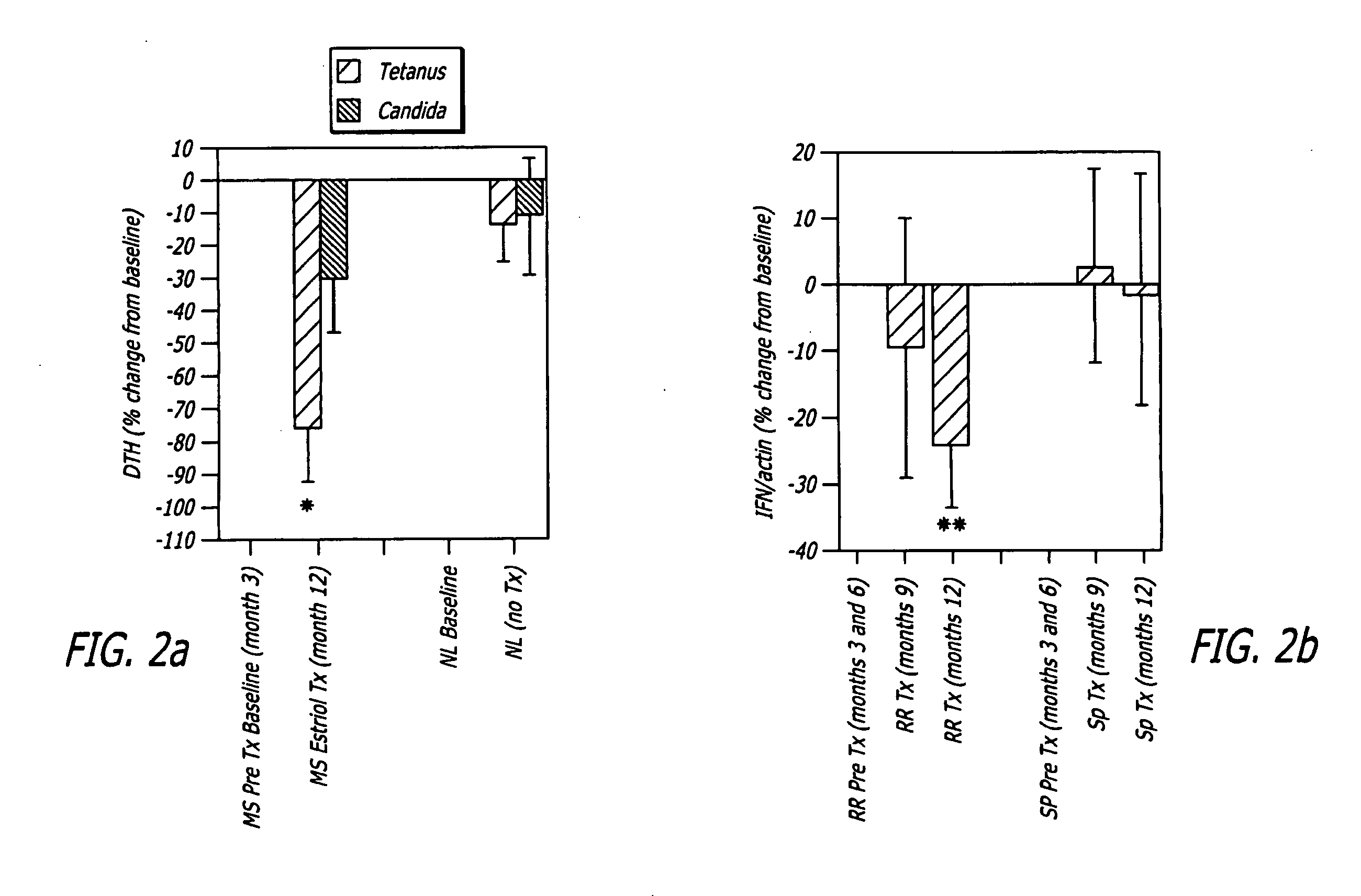
Pregnancy hormone combination for treatment of autoimmune diseases
InactiveUS8658627B2Slow onsetAvoid delayBiocideOrganic active ingredientsDiseaseImmunologic disorders
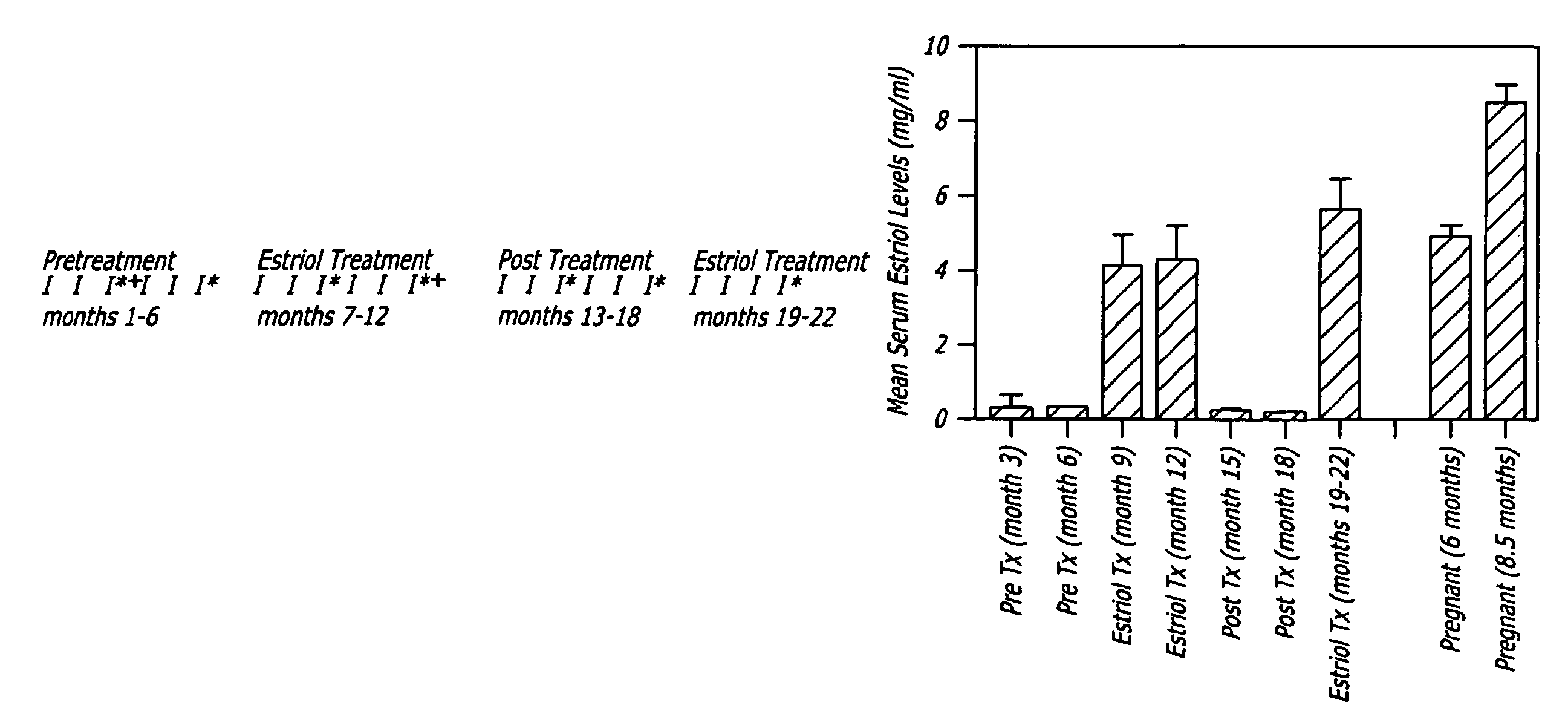
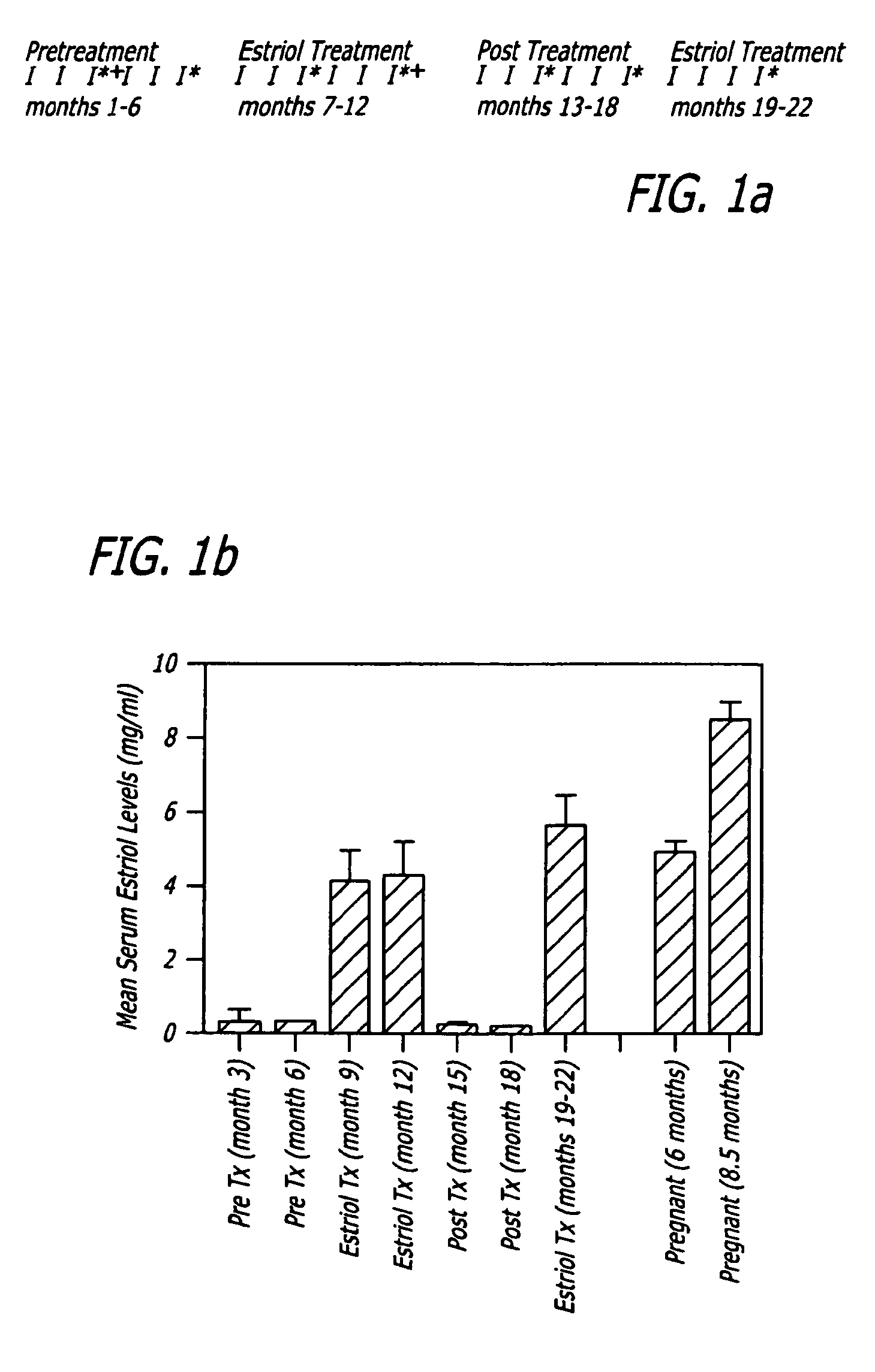
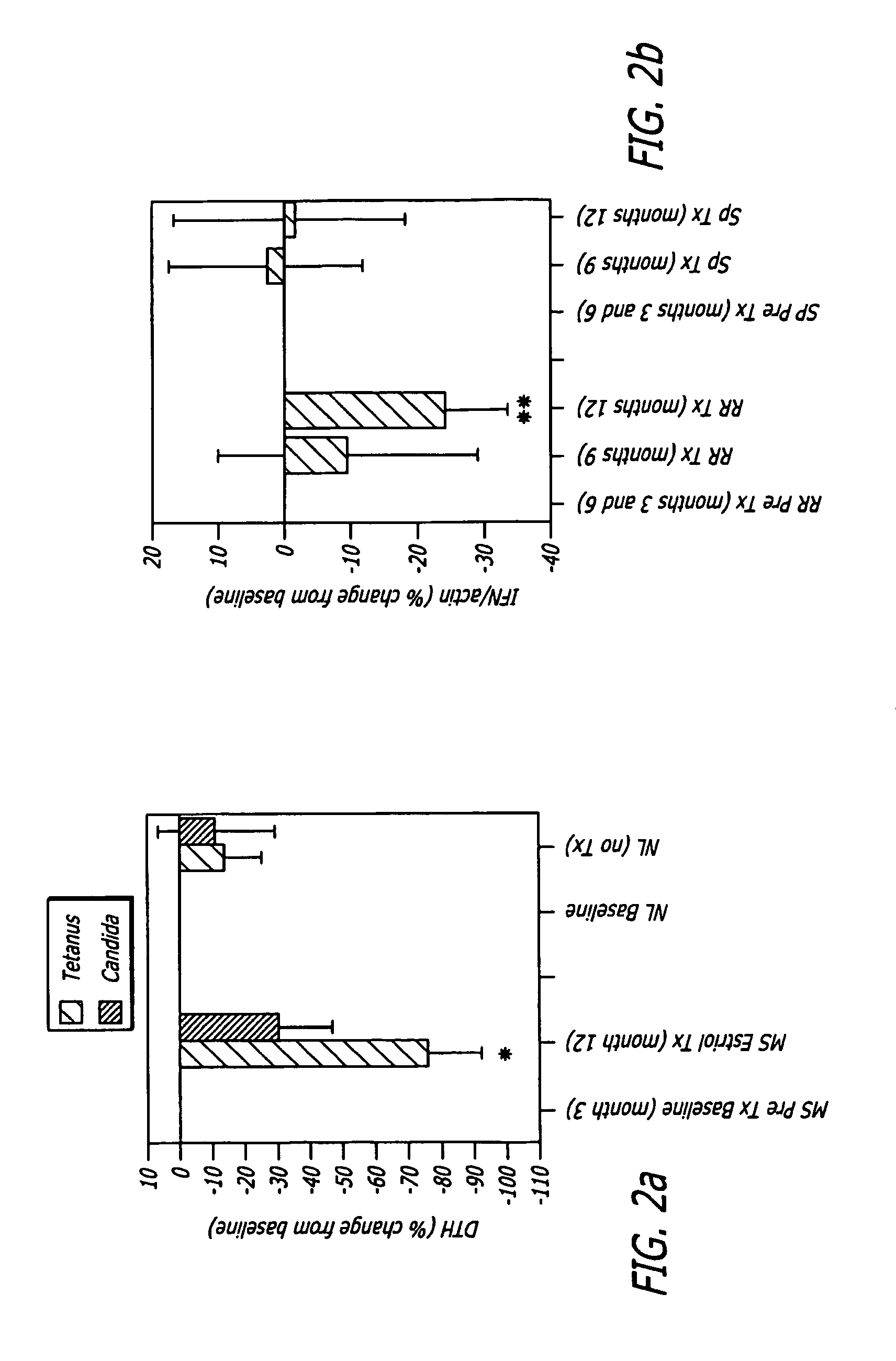
The present invention relates to pregnancy hormone combinations and methods of treatment for autoimmune diseases having at least two hormonal components, a pregnancy hormone (such as estriol), and a gestagen (such as levonorgestrel or norethindrone) thereby providing for the continuous, uninterrupted administration of pregnancy hormones for the treatment for autoimmune disorders, such as multiple sclerosis.
Owner:RGT UNIV OF CALIFORNIA
Method of preventing or treating benign gynaecological disorders
ActiveUS8071576B2Few side-effectsLow recurrence rateBiocideOrganic active ingredientsDiseaseGynecological disorders
The present invention relates to a method of preventing or treating benign estrogen sensitive gynecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
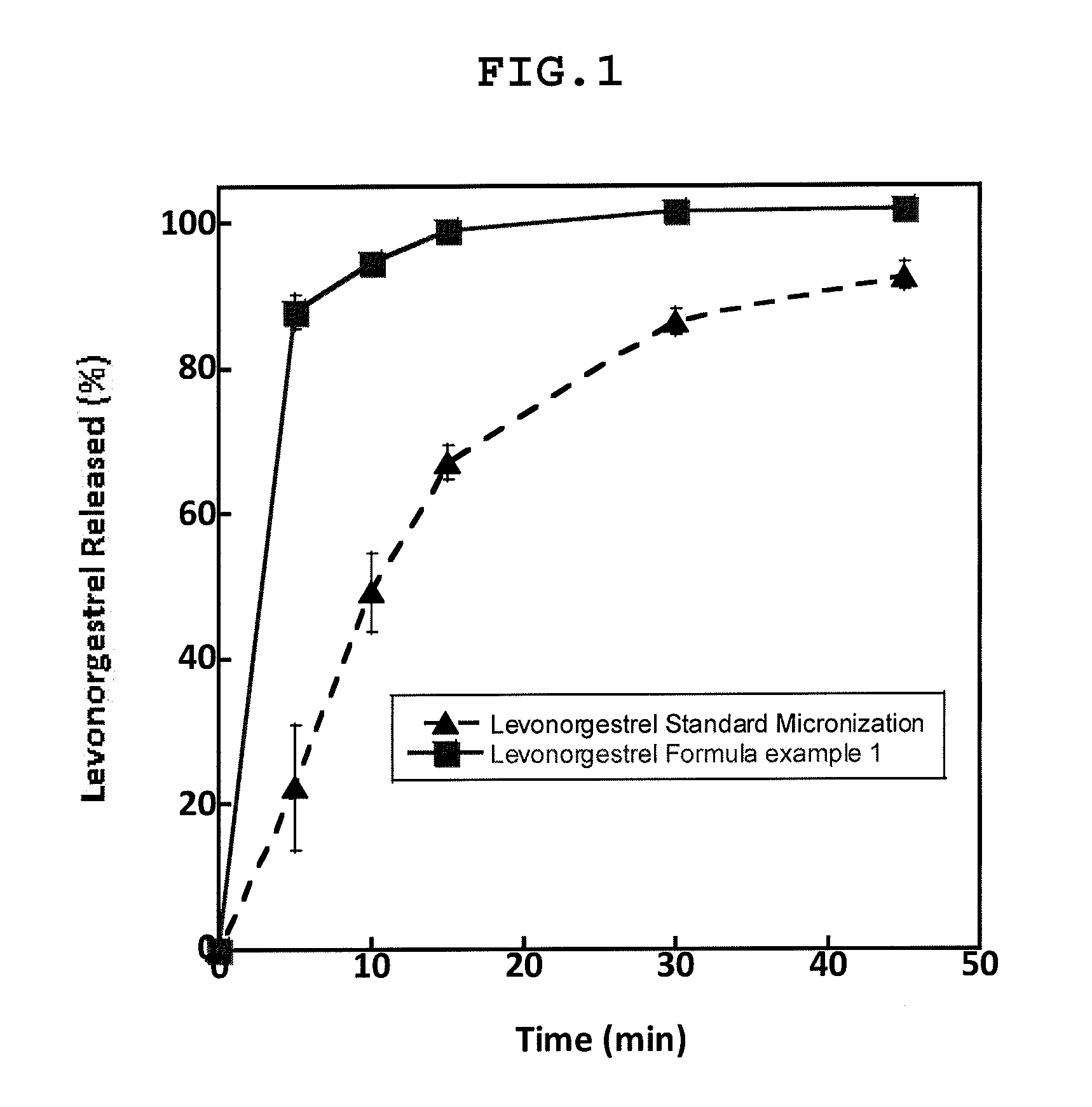
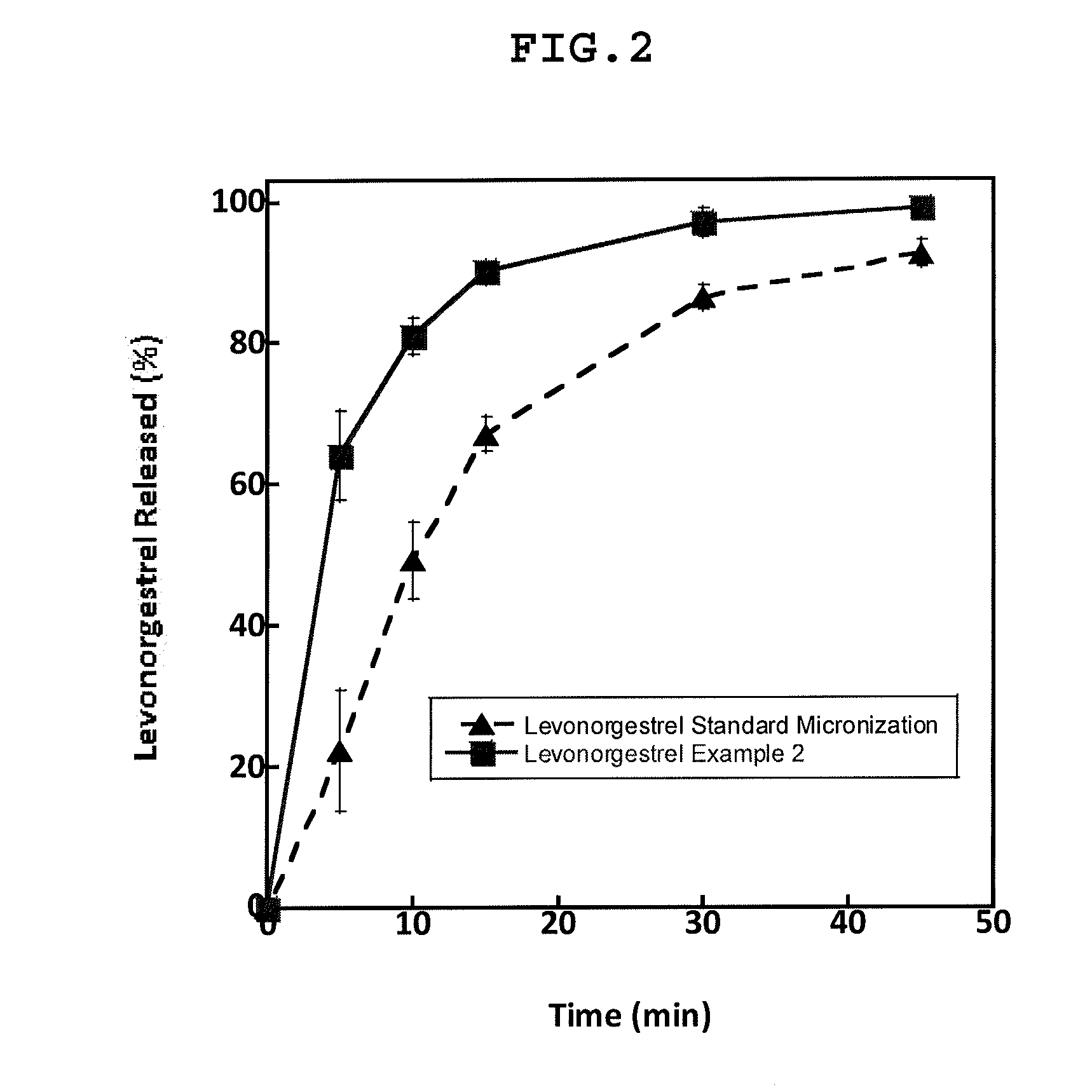
Micronized composition containing Levonorgestrel
ActiveCN101628002AQuick effectEmergency contraception goodOrganic active ingredientsPowder deliveryMedicineExcipient
The invention relates to a micronized composition containing Levonorgestrel, and aiming at the problem of low dissolution rate of the Levonorgestrel enteric coated preparation in the prior art, the invention provides the micronized composition containing Levonorgestrel. In the invention, 70 to 100 percent of Levonorgestrel is micronized to particles, and the grain diameter of each particle is less than or equal to 20 nanometers; the particles are combined with 60 to 97.9 percent of excipient to be prepared into a medicine core; and the medicine core is coated by a coating layer. The composition greatly improves the releasing degree and the peak time of the Levonorgestrel and Tmax.
Owner:CHINA RESOURCES ZIZHU PHARMA
Preparation method for solid dispersion levonorgestrel tablets
The invention provides a preparation method for solid dispersion levonorgestrel tablets. The preparation method employs a melting method to prepare a solid dispersion of the levonorgestrel; and mixing and tabletting with appropriate accessories. Compared with common levonorgestrel tablets obtained by directly tabletting, the levonorgestrel tablets of the invention are significantly increased in dissolution rate, so that the levonorgestrel tablets prepared by the method of the invention are convenient to take, fast in absorption, high in bioavailability, and the like, and can improve patient adaptability and increase choices of clinical medication for doctors and patients.
Owner:BEIJING YILING BIOENG
Levonorgestrel drop pills and preparation method thereof
ActiveCN101288660AImprove solubilityImprove wettabilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityBULK ACTIVE INGREDIENT
The invention provides a Levonorgestrel pill and a preparation method thereof. The Levonorgestrel pill consists of a pill core and a coating layer, wherein, the pill core comprises Levonorgestrel active ingredient, carrier used as dispersant and surface active agent with a weight ratio of 1: 10-60: 1-10; the coating material used by the coating layer consists of coating powders and correctives. The coating powders adopt gastric solubility film coating powders, the dosage of which is 2-6 percent (w / w) of the total amount of the Levonorgestrel pill; the dosage of the correctives is 0.1-2 percent (w / w) of the total amount of the Levonorgestrel pill. The preparation method of the Levonorgestrel pill is that the Levonorgestrel raw material is micronized to a grain diameter of less than 20 Mu m; in the preparation process, main medicine is evenly dispersed in the carrier to form a solid dispersoid, thus improving the drug water solubility, increasing the drug dissolution rate and bioavailability and more effectively playing a curative role.
Owner:SHANDONG RUIAN PHARMA CO LTD
Transdermal hormone delivery system: compositions and methods
InactiveUS20070065495A1Achieving Reliability RequirementsMinor side effectsBiocidePharmaceutical non-active ingredientsLevonorgestrelPhysiology
A transdermal hormone delivery system (THDS) is disclosed. The THDS is useful for control of fertility and as therapy for a variety of diseases and conditions treatable by robust delivery of progestin and estrogen hormones, particularly the progestin, levonorgestrel. The THDS comprises a backing layer, an adjoining adhesive polymer matrix comprising an effective amount of at least a progestin hormone, delivery of which is enhanced by one or more skin permeation enhancing agents present in pre-determined amounts. The THDS is capable of providing effective daily doses of progestin and estrogen hormones from a small surface area in contact with the skin, e.g., less than 20 square centimeters. Methods of fertility control and various types of hormone replacement therapy utilizing the THDS are also disclosed.
Owner:AGILE THERAPEUTICS
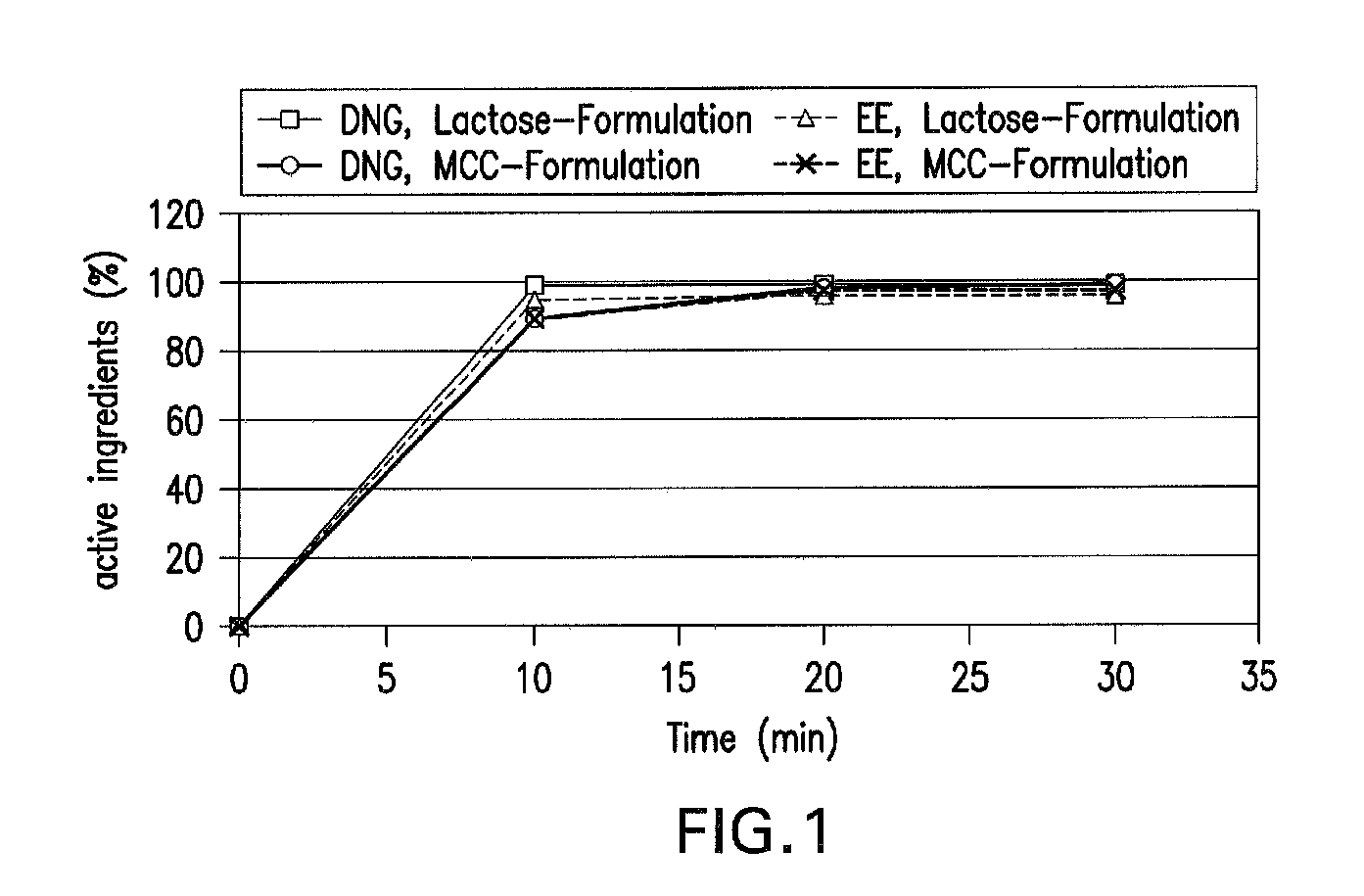
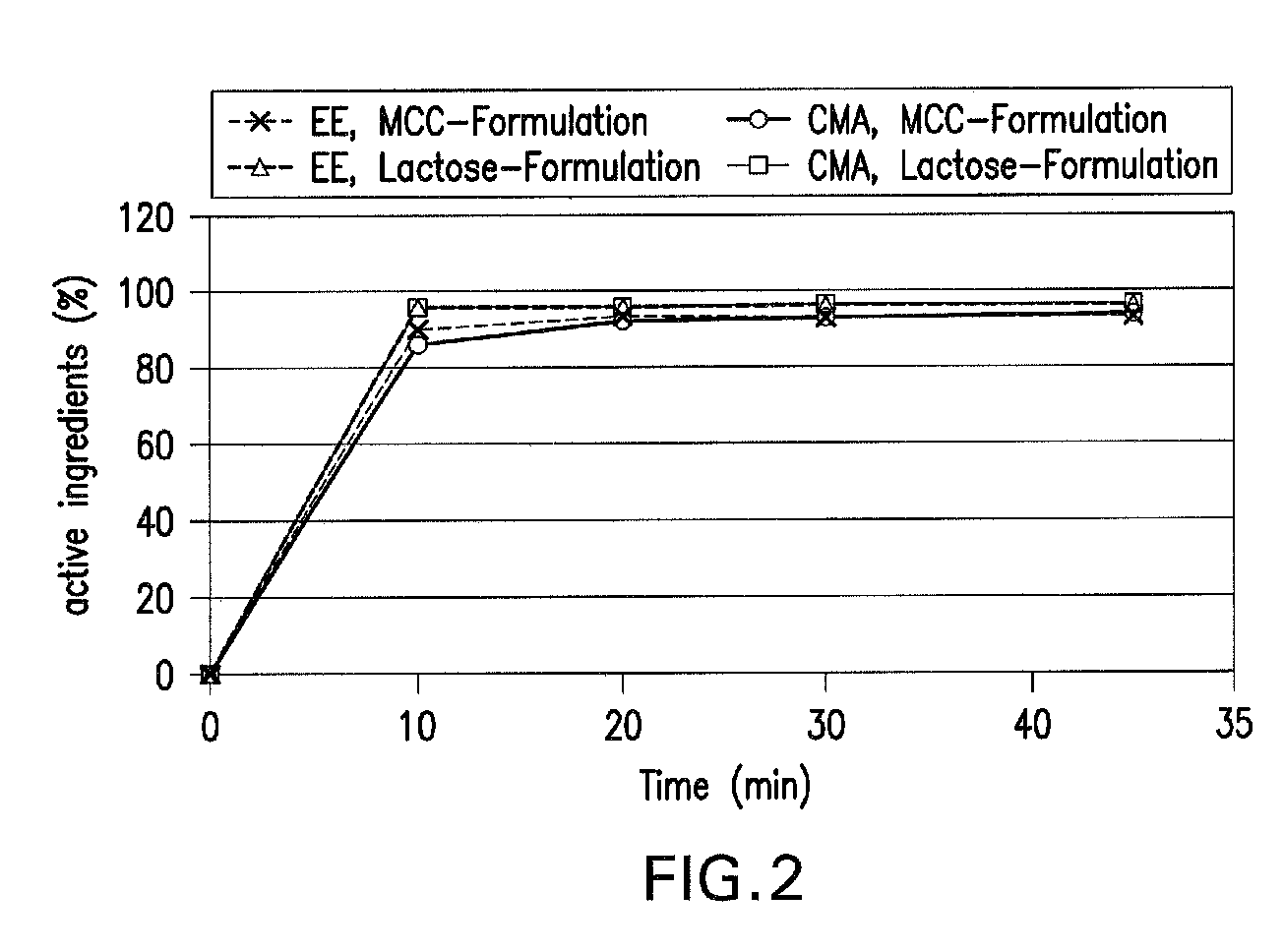
Oral contraceptive containing a gestagen and an estrogen combined with pharmaceutically acceptable auxiliary agents and/or excipients, but not containing lactose, and method of making same
InactiveUS20090117183A1Easy to solveLow costBiocideOrganic active ingredientsPhysiologyAdditive ingredient
The method produces a lactose-free oral contraceptive composition containing a combination of a gestagen and an estrogen together with one or more pharmaceutically acceptable auxiliary agents and / or excipients. The contraceptive composition is a tablet, powder, or capsule that contains the gestagen and estrogen, filler material such as microcrystalline cellulose and a binder such as hydroxypropylcellulose, but no lactose. Preferably the gestagen is dienogest, chlormadinone acetate, or levonorgestrel and the estrogen is ethinylestradiol, 17β-estradiol, or estradiol valerate. A method is provided for improving the prophylaxis of lactose intolerance in women taking oral contraceptives. The oral contraceptive preparations for a standard 28-day cycle or for long-term use contain at least 21 daily dose units of the gestagen and the estrogen in a low-dosage but without lactose and at most 7 daily dose units containing no active ingredient or a placebo.
Owner:BAYER SCHERING PHARMA AG
Levonorgestrel-containing emergency contraception medicament composition and preparation method thereof
ActiveCN101732324AHigh dissolution rateImprove bioavailabilityOrganic active ingredientsSexual disorderIntensive care medicineContraceptive Effect
The invention relates to an after emergency contraception medicament composition containing low-dose levonorgestrel without reducing contraceptive effect. In the medicament composition, the consumption of levonorgestrel contained by a medicament dosage unit with single-dosage medicament for emergency contraception is reduced from conventional 1.5 mg to 1.00 to 1.25 mg, and the consumption of levonorgestrel contained by the medicament dosage unit with two-dosage administration is reduced from conventional 0.75 mg to 0.50 to 0.625 mg. The composition reduces the dosage of the levonorgestrel used relative to conventional medicament dosage and reduces individual differences as well as adverse reaction, thereby having important clinical significance. The invention also provides a method for preparing the medicament composition, as well as the application of the medicament composition in the preparation of emergency contraception medicaments.
Owner:REGENEX PHARMA LTD
Preparation method for ultra-micro co-grinding levonorgestrel tablets
The invention provides a preparation method for ultra-micro co-grinding levonorgestrel tablets. The method employs an ultra-micro co-grinding technology, ultra-micro co-grinding levonorgestrel and appropriate fillers according to a certain proportion, and mixing and tabletting with appropriate accessories. Compared with common levonorgestrel tablets obtained by ultra-micro grinding bulk pharmaceutical chemicals and tabletting directly, the levonorgestrel tablets of the invention are significantly increased in dissolution rate, so that the levonorgestrel tablets prepared by the method of the invention are convenient to take, fast in absorption and high in bioavailability, and can improve patient adaptability and increase choices of clinical medication for doctors and patients.
Owner:BEIJING YILING BIOENG
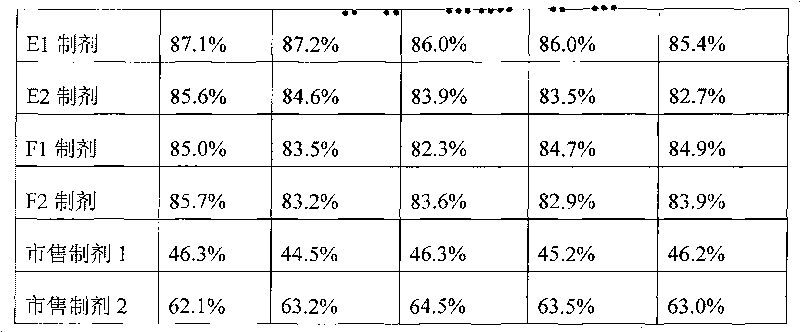
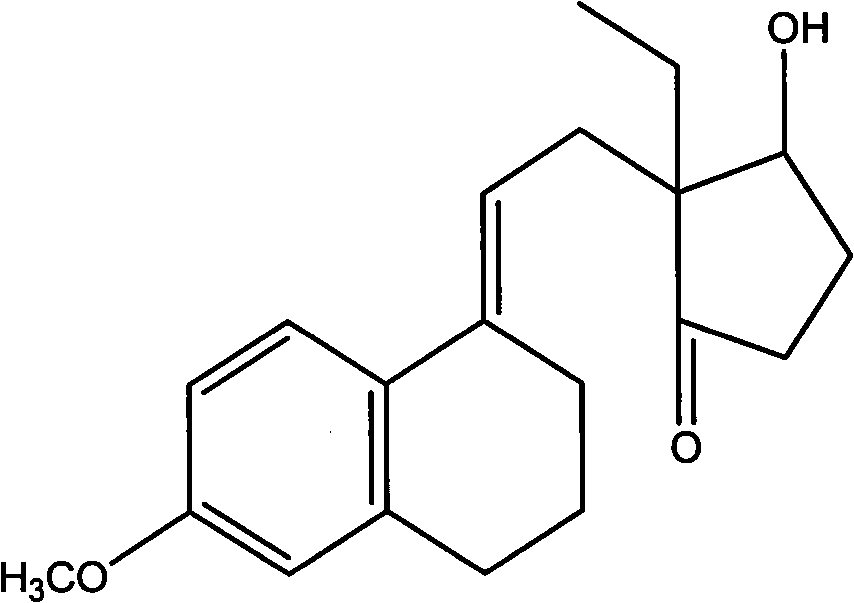
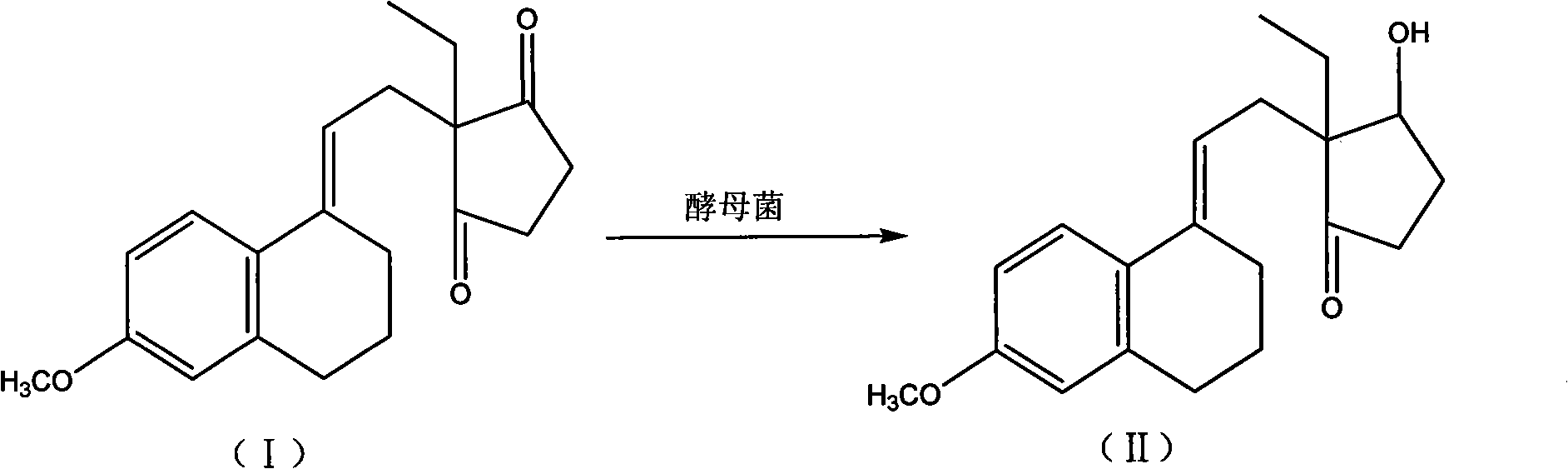
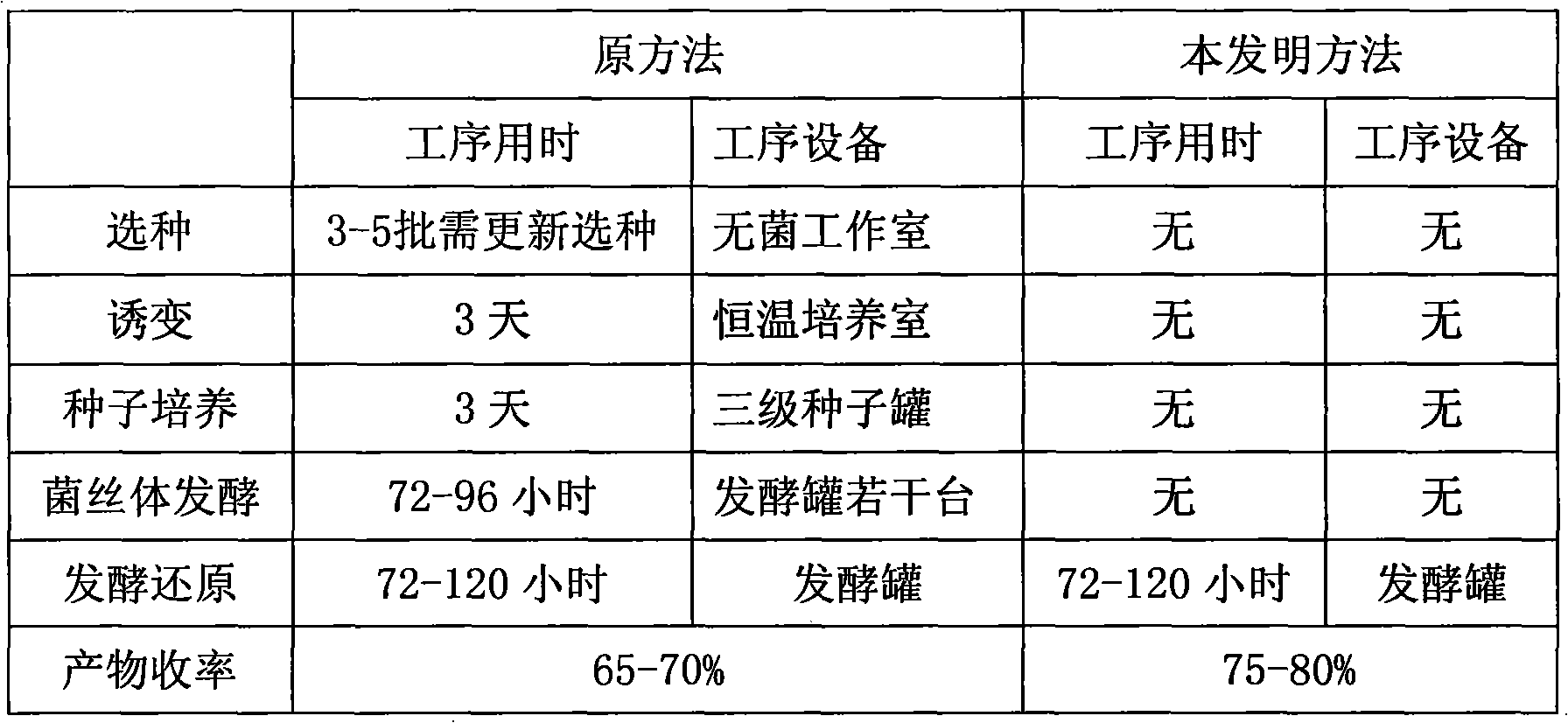
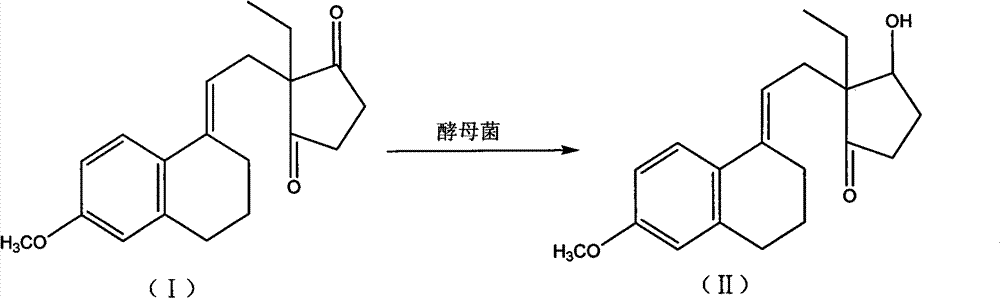
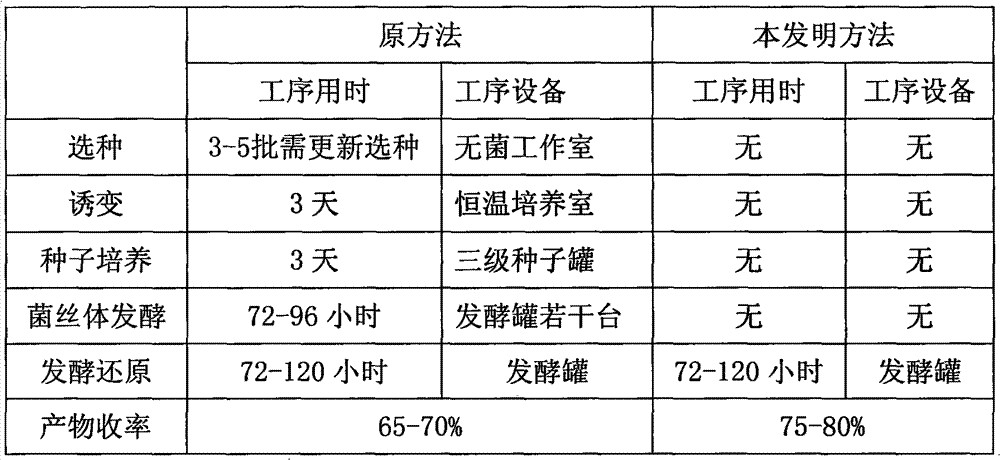
Novel fermentation reducing method for contraceptive midbody
InactiveCN102146421AExcellent reducing activitySimple methodMicroorganism based processesFermentationBiotechnologyAlcohol
The invention relates to a novel fermentation reducing method for midbody hydroxide D-18-methyl-3-methoxyl-8,14-open loop-delta1,3,5(10),9(11)-estratetraenol-17-beta-alcohol-14-ketone of emergency contraceptive levonorgestrel. The method is characterized by adopting dry brewing yeast as a strain to ferment and reduce a levonorgestrel midbody condensate to obtain the midbody hydroxide. The method is easy to control the fermenting process, is easy to maintain the activity of the strain, reduces the production cycle, has high product yield and stable quality and is suitable for mass production.
Owner:WUHAN TITON BIOTECH
Preparation method for cyclodextrin inclusion levonorgestral tablets
The invention provides a preparation method for cyclodextrin inclusion levonorgestral tablets. The preparation method comprises stirring beta-cyclodextrin and levonorgestrel in an appropriate solvent for some time; and mixing and tabletting with appropriate accessories. Compared with common levonorgestrel tablets obtained by directly tabletting, the levonorgestrel tablets of the invention are significantly increased in dissolution rate, so that the levonorgestrel tablets prepared by the method of the invention are convenient to take, fast in absorption, high in bioavailability, and the like, and can improve patient adaptability and increase choices of clinical medication for doctors and patients.
Owner:BEIJING YILING BIOENG
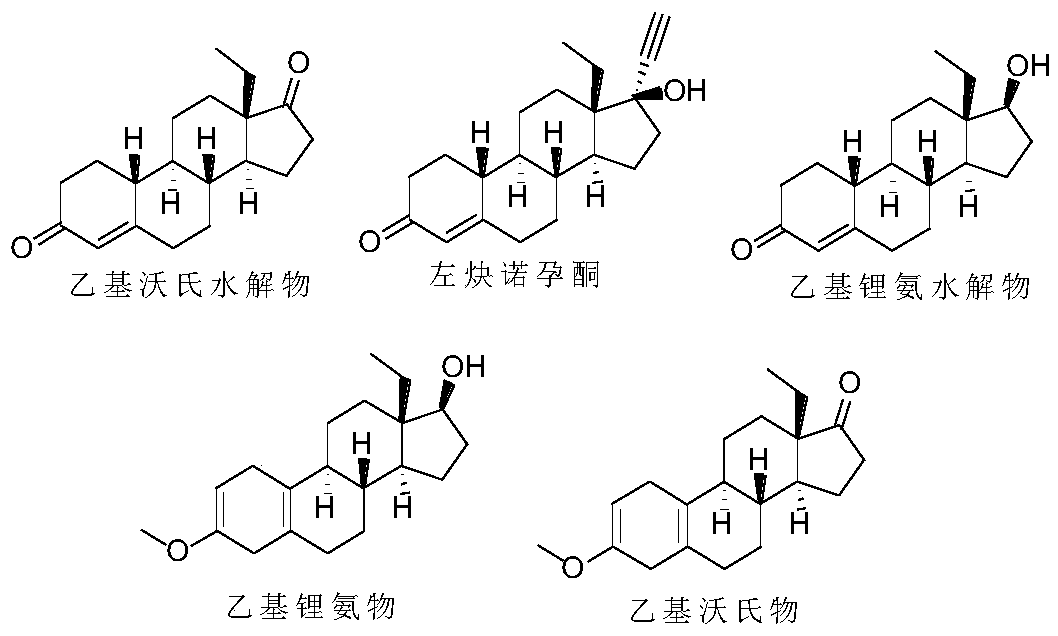
Method for preparing levonorgestrel intermediate condensation compound
ActiveCN103553891AQuality assuranceGuaranteed condensation reaction conversion rateEther separation/purificationOrganic compound preparationOrganic baseHydrolysate
The invention relates to the field of Grignard reaction of a levonorgestrel intermediate, and particularly relates to a preparation method for a levonorgestrel intermediate condensation compound. The preparation method mainly comprises step of adding a nitrogen-containing organic alkaline reagent as a stabilizer. The organic alkaline reagent plays a role in preventing tertiary hydroxyl from dehydroxylation, so that Grignard hydrolysate is stable, by-products are reduced, the conversion rate of condensation reaction is greatly improved, the weight yield is improved to be about 130% from 93% of the prior art, and the HPLC (high performance liquid chromatography) content of the product reaches 97%-99.8%.
Owner:ZHEJIANG XIANJU PHARMA
Pregnancy hormone combination for treatment of autoimmune diseases
InactiveUS20100203016A1Slow onsetAvoid delayBiocideOrganic active ingredientsImmunologic disordersObstetrics
The present invention relates to pregnancy hormone combinations and methods of treatment for autoimmune diseases having at least two hormonal components, a pregnancy hormone (such as estriol), and a gestagen (such as levonorgestrel or norethindrone) thereby providing for the continuous, uninterrupted administration of pregnancy hormones for the treatment for autoimmune disorders, such as multiple sclerosis.
Owner:RGT UNIV OF CALIFORNIA
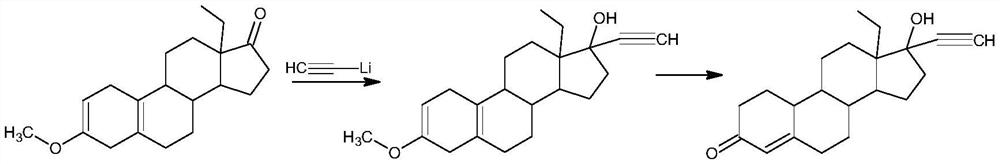
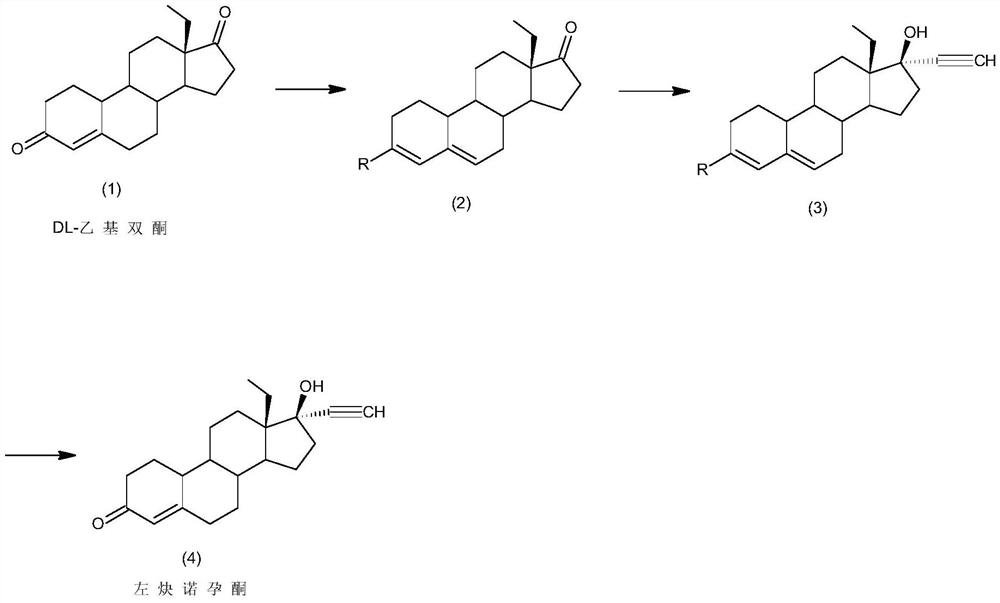
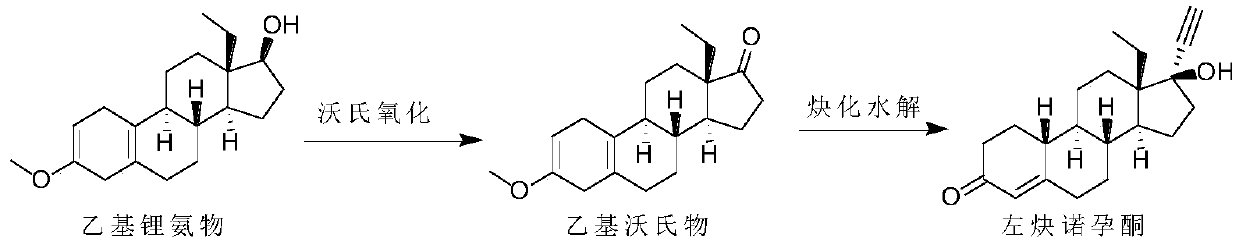
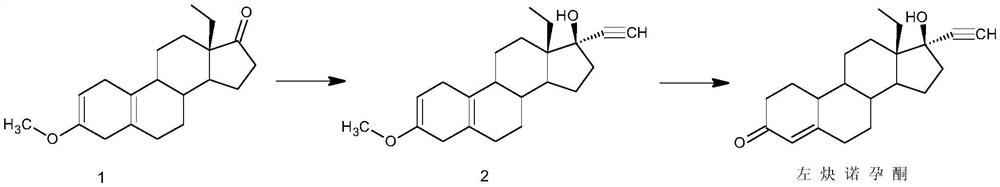
Synthesis method of levonorgestrel
The invention discloses a synthesis method of levonorgestrel, and belongs to the technical field of preparation and processing of medicines. According to the method, DL-ethyl diketone is used as an initial raw material, and the levonorgestrel disclosed by the invention is prepared through three steps of protection, ethynylation and hydrolysis. According to the preparation method of the levonorgestrel, the defects of a traditional process are overcome, a lithium ammonia reagent with large potential safety hazards is prevented from being used, reaction conditions are mild, operation is safe, andthe method is high in overall conversion rate, easy and convenient to operate, suitable for industrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Nano-gold cluster material radiotherapy sensitizer
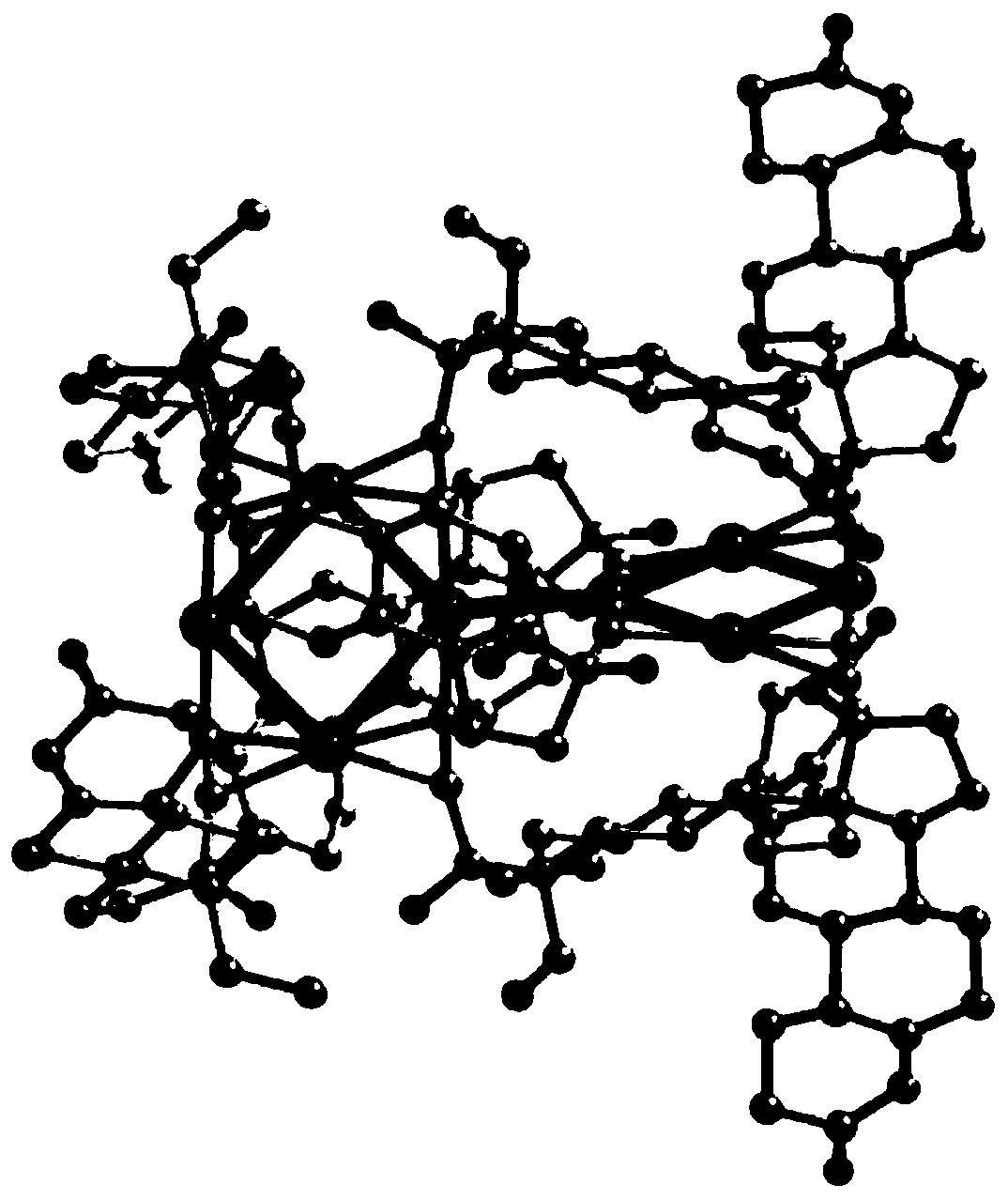
ActiveCN110204563ASmall sizeDimensionally precise and structurally preciseNanoopticsGold organic compoundsQuantum yieldGold cluster
The invention discloses an atom-accurate nano-gold cluster material radiotherapy sensitizer, and belongs to the crossing field of nano material chemistry and biochemistry. Levonorgestrel good in biocompatibility serves as a protective ligand, and a gold nano cluster which is high in yield, superior in fluorescence property and accurate in atom size is synthesized through a room-temperature volatilization one-pot method. The chemical formula of the nano-gold cluster is C168H216 Au8O16 (abbreviated as: Au8NC), and belongs to a triclinic system, and the space group is a chiral space group P1. Thegold nano cluster has strong yellow-green fluorescence at room temperature, the fluorescence quantum yield is 58.7%, the gold nano cluster has a small size (-2 nm) and good dispersibility and can beused for bright cell imaging. The nano-gold cluster material can serve as a radiotherapy sensitizer to enhance the radiotherapy sensitization effect.
Owner:ZHENGZHOU UNIV
Biological preparing method of key chiral intermediate of levonorgestrel
ActiveCN107881202AHigh substrate concentrationHigh stereoselectivityMicroorganism based processesFermentationSubstrate concentrationReaction system
The invention discloses a biological preparing method of a key chiral intermediate of levonorgestrel. The method includes the steps that wet cells obtained by fermenting cultivation of cryytococcus neoformans ZJPH1704 is used as an enzyme source, an ethyl condensation compound is used as a substrate, phosphate buffer with the pH of 5.8-8.0 is used as a reaction medium, a reaction system is formed,reaction is conducted at the temperature of 30-40 DEG C and the rotating speed of 150-250 rpm, and after the reaction is completed, reaction liquid is separated and purified to obtain the key chiralintermediate of levonorgestrel. The cryytococcus neoformans ZJPH1704 strain is used for catalytic reduction to prepare the intermediate, the key chiral intermediate has the advantages that the catalytic reduction substrate is high in concentration, and the stereoselectivity is high. When the substrate concentration is 7.0 g / L (22.4 mM), the yield reaches 73%, and the ee value is 100%.
Owner:ZHEJIANG UNIV OF TECH
Chewable tablets comprising levonorgestrel
The present invention relates to a chewable tablet comprising levonorgestrel and a pharmaceutically acceptable excipient for use in contraception, a method of use of same and a kit containing same.
Owner:EVERETT LABS
Emergency contraceptive
InactiveUS20120263784A1Enhance and maintain effective therapeutic concentrationSlow and prolonged absorptionPowder deliveryBiocideEmergency ContraceptivesEmergency medicine
The present invention discloses an emergency contraceptive formulation for nasal and / or pulmonary administration comprising of levonorgestrel optionally in combination with ethylestradiol. The said formulation can be in form of solution, suspension or carrier based systems selected from microemulsion and liposomes.
Owner:LYKA LABS LTD
Recovery treatment method and application of levonorgestrel mother liquor
The invention discloses a recovery treatment method and an application of a levonorgestrel mother liquor. The method comprises the following steps: adding concentrated hydrochloric acid into the levonorgestrel mother liquor, performing heating refluxing for acid hydrolysis, adding aluminum isopropoxide, carrying out Oppenauer oxidation to convert ethyl lithium ammonia hydrolysate, ethyl lithium ammonia and ethyl methoxydienone into ethyl Oppenauer hydrolysate, and obtaining the recovered product which is a mixture of the ethyl Oppenauer hydrolysate and levonorgestrel. The recovery method is simple to operate and high in recovery rate, and the recovered product can be used for preparing the levonorgestrel through return alkynylation, so that the utilization rate of each component of the mother liquor is improved.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
Transdermal Hormone Delivery System: Compositions And Methods
A transdermal hormone delivery system (THDS) is disclosed. The THDS is useful for control of fertility and as therapy for a variety of diseases and conditions treatable by robust delivery of progestin and estrogen hormones, particularly the progestin, levonorgestrel. The THDS comprises a backing layer, an adjoining adhesive polymer matrix comprising an effective amount of at least a progestin hormone, delivery of which is enhanced by one or more skin permeation enhancing agents present in pre-determined amounts. The THDS is capable of providing effective daily doses of progestin and estrogen hormones from a small surface area in contact with the skin, e.g., less than 20 square centimeters. Methods of fertility control and various types of hormone replacement therapy utilizing the THDS are also disclosed.
Owner:AGILE THERAPEUTICS
Novel fermentation reducing method for contraceptive intermediate
InactiveCN102146421BExcellent reducing activitySimple methodMicroorganism based processesFermentationBiotechnologyAlcohol
The invention relates to a novel fermentation reducing method for midbody hydroxide D-18-methyl-3-methoxyl-8,14-open loop-delta1,3,5(10),9(11)-estratetraenol-17-beta-alcohol-14-ketone of emergency contraceptive levonorgestrel. The method is characterized by adopting dry brewing yeast as a strain to ferment and reduce a levonorgestrel midbody condensate to obtain the midbody hydroxide. The method is easy to control the fermenting process, is easy to maintain the activity of the strain, reduces the production cycle, has high product yield and stable quality and is suitable for mass production.
Owner:WUHAN TITON BIOTECH
Hard Capsule Shell Compositions for the Oral Contraceptive Formulations
InactiveUS20190343770A1Organic active ingredientsInorganic non-active ingredientsDrospirenoneDietary supplement
A hard shell capsule includes a body and a cap cooperatively defining a hollow core hard shell capsule. Each of the body and the cap has a composition that includes a polymer forming a hard polymer structure of the body and of the cap and comprises a drug. The body further comprises a therapeutically effective amount of drug A loaded throughout the composition; the cap further comprises a composition comprising a therapeutically effective amount of drug B loaded throughout the composition. The body and cap compositions together containing a therapeutically effective amount of the drugs A and B; said drugs being oral contraceptive agents. The core of the capsule is filled with therapeutically effective amount of a second drug(s), a dietary supplement, minerals, a complexing agent and other excipients. With the help of FIG. 2, the invention can be very well understood easily. Drugs A and B are selected from the group of oral contraceptives, but are not limited to, Cyproterone acetate, Estradiol, Oestradiol, Norethindrone acetate, Ethinyl Estradiol, Levonorgestrel, Dienogest, Drospirenone, Desogestrel, Ethynodiol, Diacetate, Mestranol, Nomegestrol acetate, Norgestrel, Norgestimate, Dienogest, Norelgestromin, Norethisterone, Gestodene, Oestradiol valerate, and Ethynodiol diacetate.
Owner:JOSHI HEMANT N +1
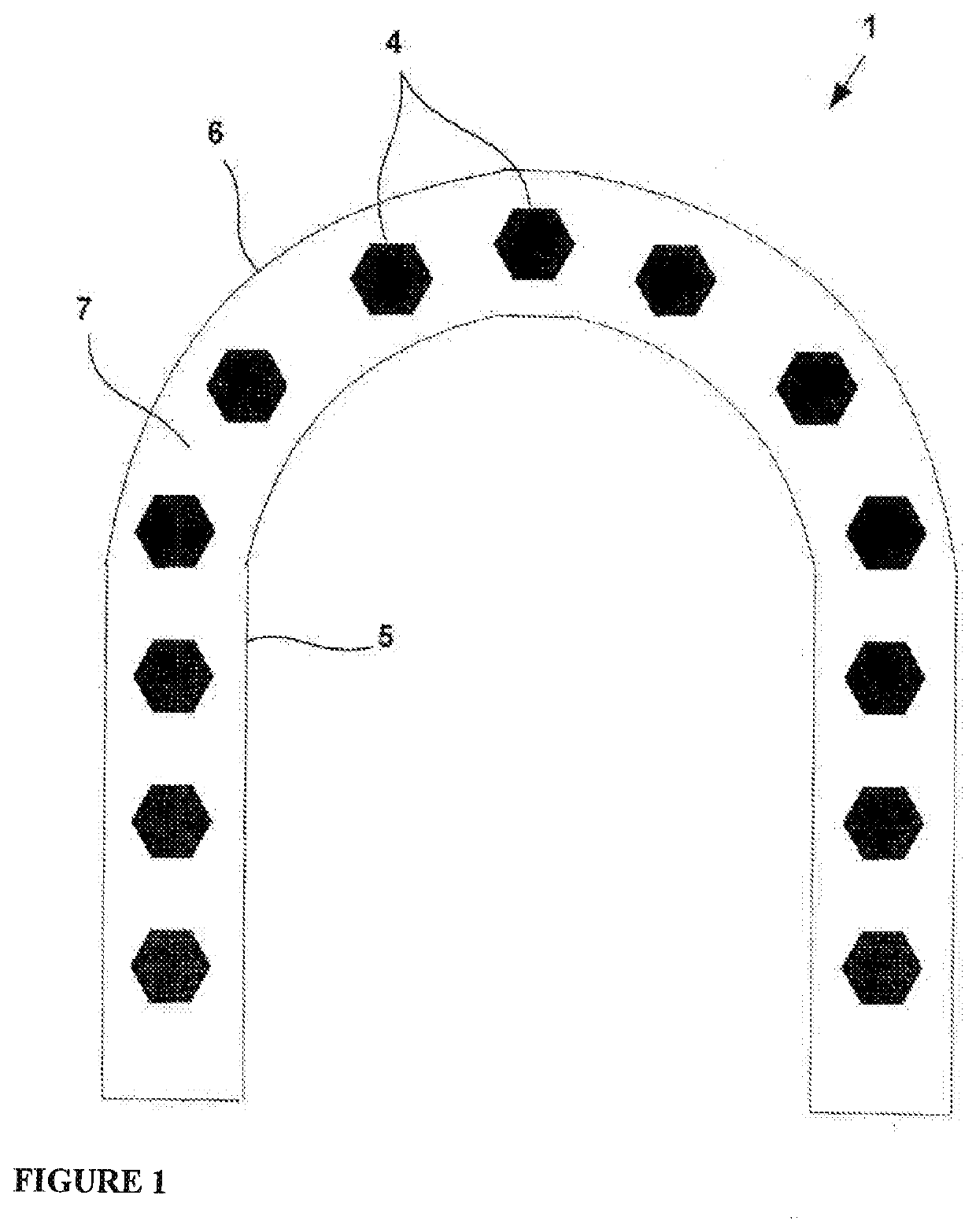
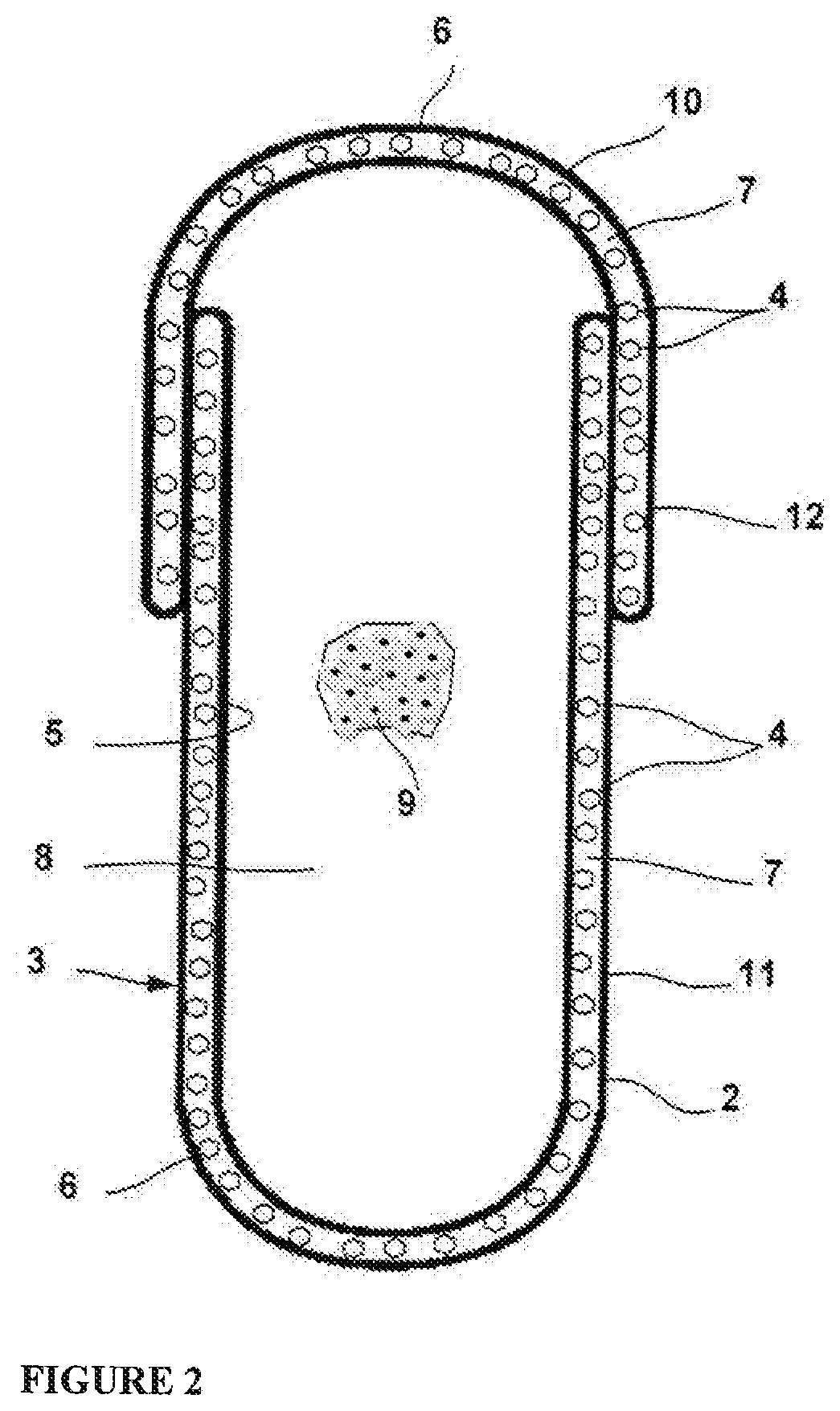
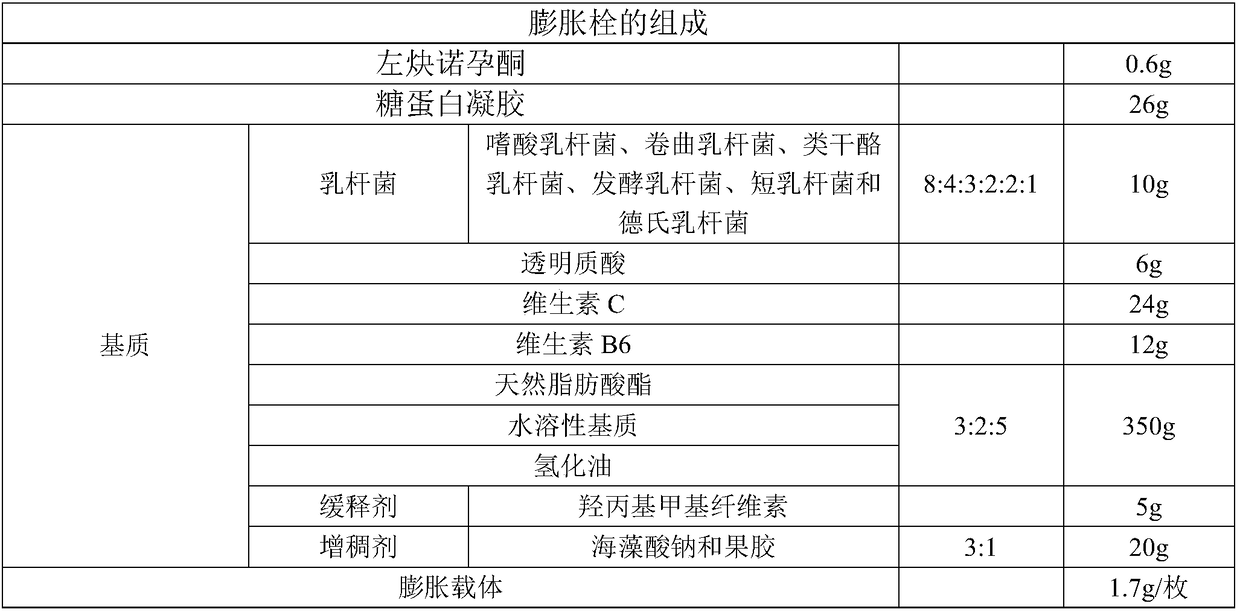
Levonorgestrel vagina expansion bolt and preparation method thereof
InactiveCN108451895ALess irritatingEliminate side effectsOrganic active ingredientsSuppositories deliverySide effectExpansion joint
The invention provides a vagina expansion bolt and a preparation method and application thereof. The expansion bolt comprises levonorgestrel, glycoprotein gel, a medium and an expandable expansion carrier. The expansion bolt further comprises lactobacillus, hyaluronic acid and vitamins. According to the vagina expansion bolt, the use amount of levonorgestrel is reduced, the side effect of levonorgestrel is reduced, the penetrating resistance of sperms is improved at the same time, the contraception efficiency can be effectively improved, the use comfort degree is high, and the vagina expansionbolt has the effect of nourishing the vagina.
Owner:霍尔果斯汉智医药科技有限公司
Synthesis method of levonorgestrel
ActiveCN114181272AAvoid water and fire problemsReduce separation and purification operationsSteroidsBulk chemical productionBiochemical engineeringProcess engineering
The invention relates to a method for synthesizing levonorgestrel, which is characterized in that a compound shown as a formula (I) is used as a raw material, the levonorgestrel is synthesized by adopting a one-pot method, the separation and purification operation of an intermediate is reduced, the production process is greatly simplified, the reaction speed is high, and the production period is short; the synthesis method does not need acetylene gas, and is good in safety, high in yield, low in production cost and suitable for large-scale industrial production.
Owner:湖南科益新生物医药有限公司
Method of contraception
InactiveUS20170312219A1High success rateStable amenorrheaOrganic active ingredientsPeptide/protein ingredientsObstetricsControl release
The invention is related to an improved method of contraception, for preventing or suppressing abnormal and / or irregular endometrial bleeding and achieving a rapid induction of amenorrhea by using an intrauterine delivery system comprising controlled release levonorgestrel over a prolonged period of time and at a therapeutic level required for contraception, and a sufficient amount of NSAID capable of suppressing abnormal and / or irregular endometrial bleeding
Owner:BAYER OY
Method of preventing or treating benign gynaecological disorders
ActiveUS20050148559A1Improve actionIncreased proliferationBiocideOrganic active ingredientsDiseasePresent method
The present invention relates to a method of preventing or treating benign estrogen sensitive gynaecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
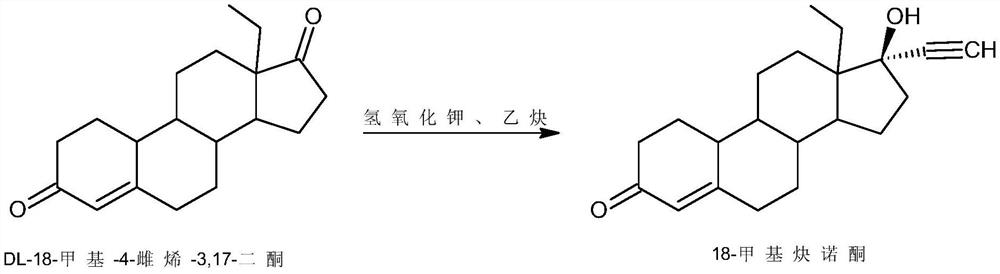
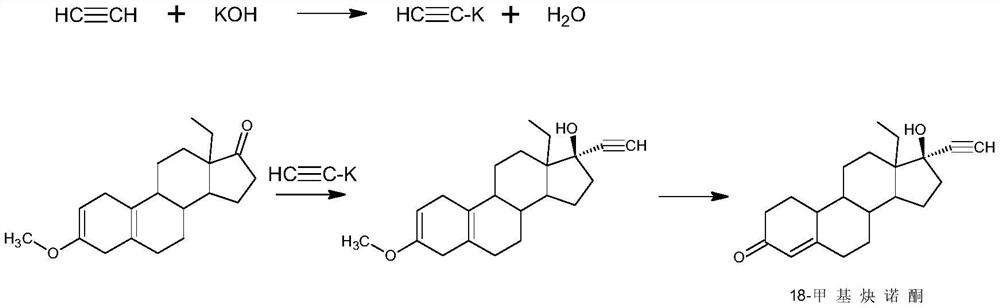
Preparation method of levonorgestrel
InactiveCN111647035AShort reaction pathLow running costSteroidsBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of levonorgestrel, and belongs to the technical field of medicine preparation and processing. According to the method, 18-methylestra-2,5(10)-diene-3-methoxy-17-ketone is used as an initial raw material, and the levonorgestrel disclosed by the invention is prepared by virtue of two steps of ethynylation and hydrolysis. According to the preparation method of the levonorgestrel, the defects of a traditional process are overcome and reaction conditions are mild; the method is high in overall conversion rate, easy and convenient to operate, suitable forindustrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Preparation method of levonorgestrel and ethinylestradiol tablets
ActiveCN105796573ASimple production processImprove solubilityOrganic active ingredientsPill deliveryFluidized bedSpray dried
The invention belongs to the field of pharmaceutic preparation technology, and concretely relates to a preparation method of levonorgestrel and ethinylestradiol tablets. The method comprises the following steps: two bulk drugs containing levonorgestrel and ethinylestradiol are dissolved into a solvent, and a binder is also dissolved into the solvent; in a fluidized bed device, spray drying, solid dispersion and one-step granulation are carried out at the same time for a filler, and the three processes are combined into one step for directly preparing granules; granules as well as a disintegrating agent and a lubricating agent of effective quantities are uniformly mixed, tabletting is carried out, and finally coating is carried out in order to obtain the levonorgestrel and ethinylestradiol tablets. The method is advantageous in that prior production processes are substantially simplified, dissolution rate of the finished product is improved, bioavailability of medicines is improved, and content uniformity is better controlled.
Owner:ZHEJIANG XIANJU PHARMA
Contraceptive
InactiveCN104546870ALittle side effectsInhibition of ovulationOrganic active ingredientsSexual disorderDrospirenoneNomegestrol
The invention relates to contraceptive. The contraceptive is characterized by comprising the following components by mass in single dosage: 10-50mu g of gestodene, 1.5-10mg of nomegestrol, 0.2-0.5mg of levonorgestrel, 1-20mg of drospirenone, 0.5-10mg of cyproterone, 10-20mu g of ethinyloestradiol, 40-80g of saccharose and 5-20mu g of mestranol. The sum of the content of the ethinyloestradiol and the content of the mestranol is not more than 30mu g; and the sum of the content of the cyproterone, the content of the ethinyloestradiol and the content of the saccharose is 2-20mg. The contraceptive adopts the means that multiple progestational hormones and estrogenic hormones are mixed for use so as to reduce the estrogenic hormones, so that the obtained contraceptive can guarantee less injury to a human body under the condition with better contraceptive effect.
Owner:江华瑶族自治县金牛开发建设有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com