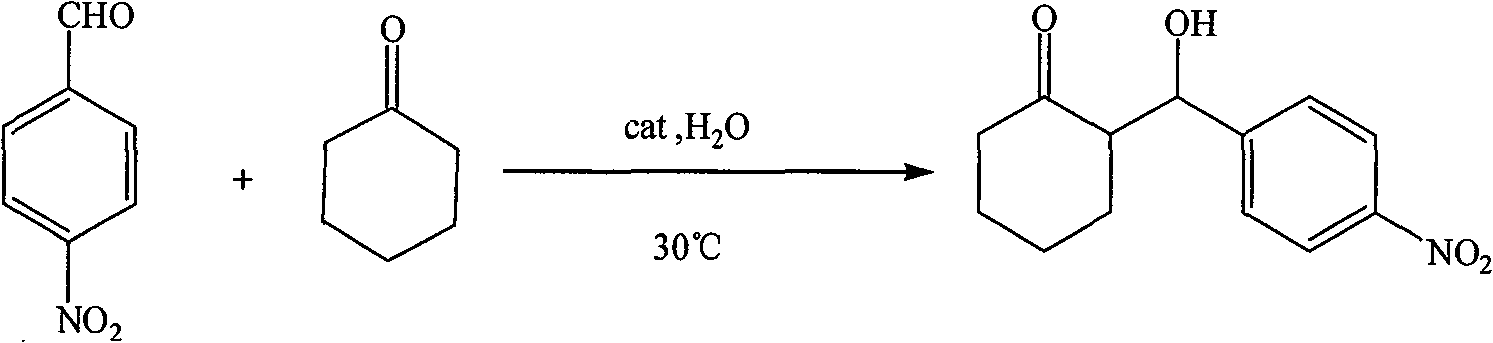
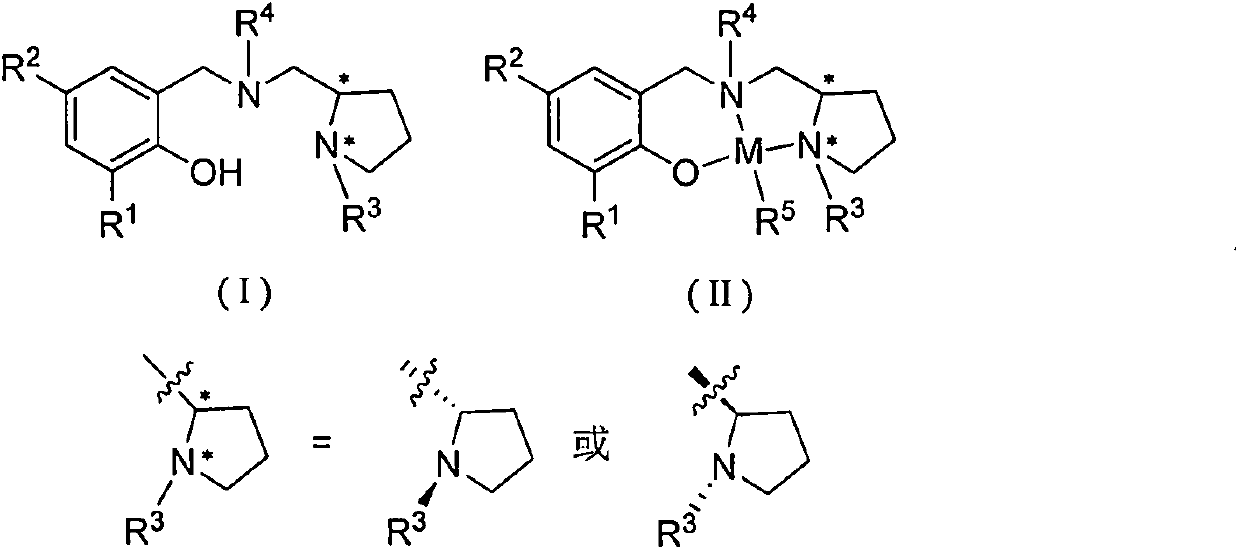
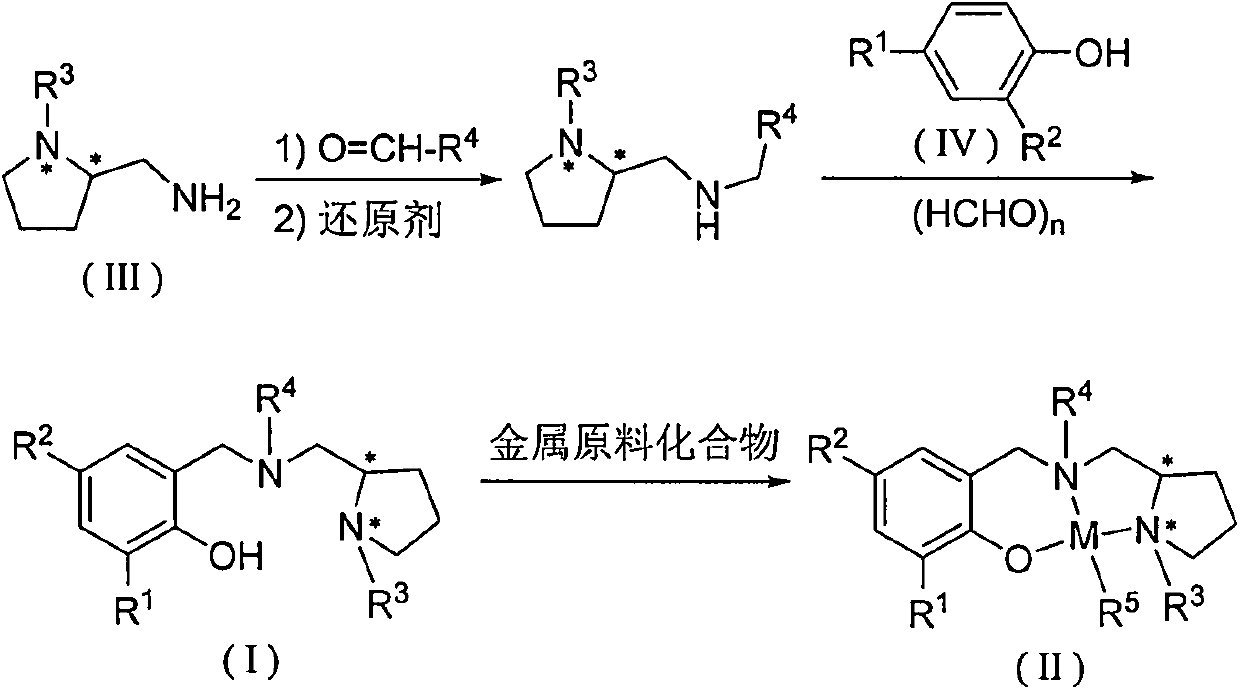
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1353results about How to "High stereoselectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
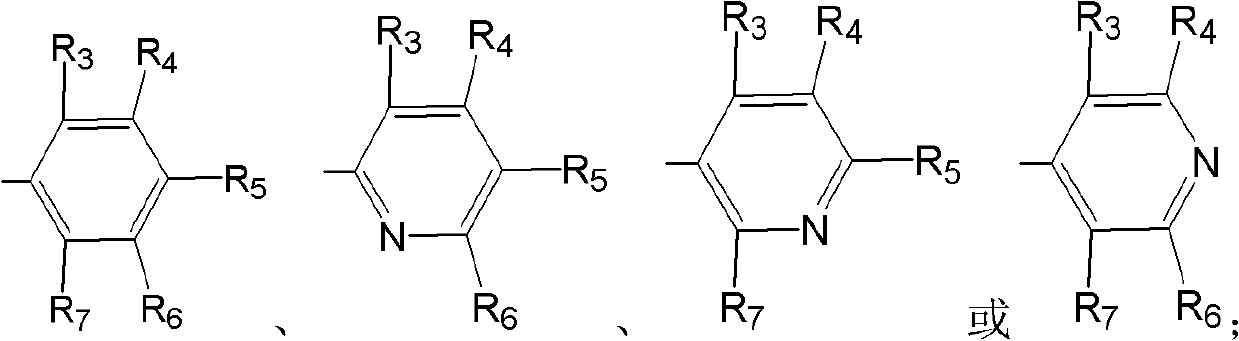
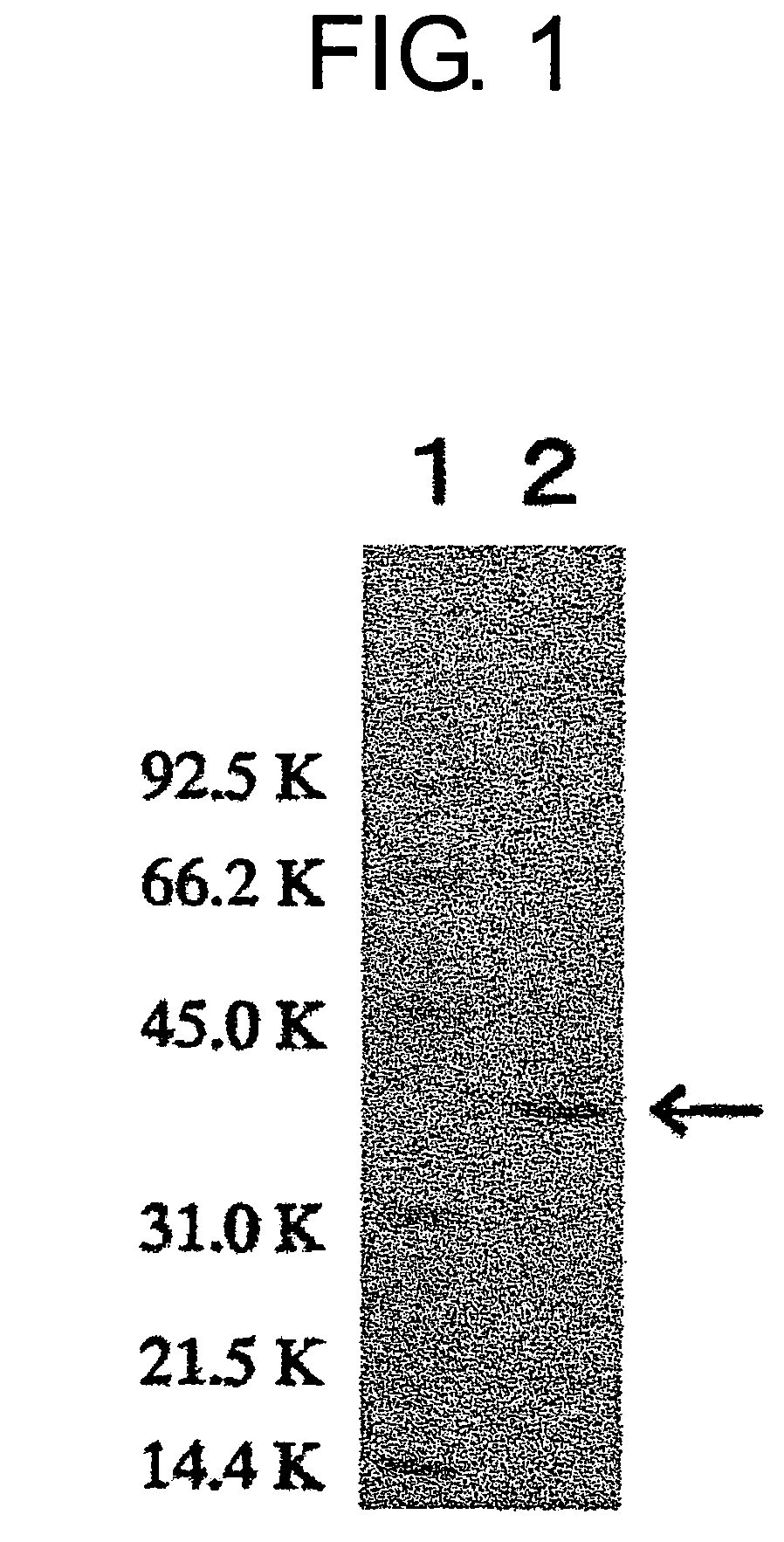
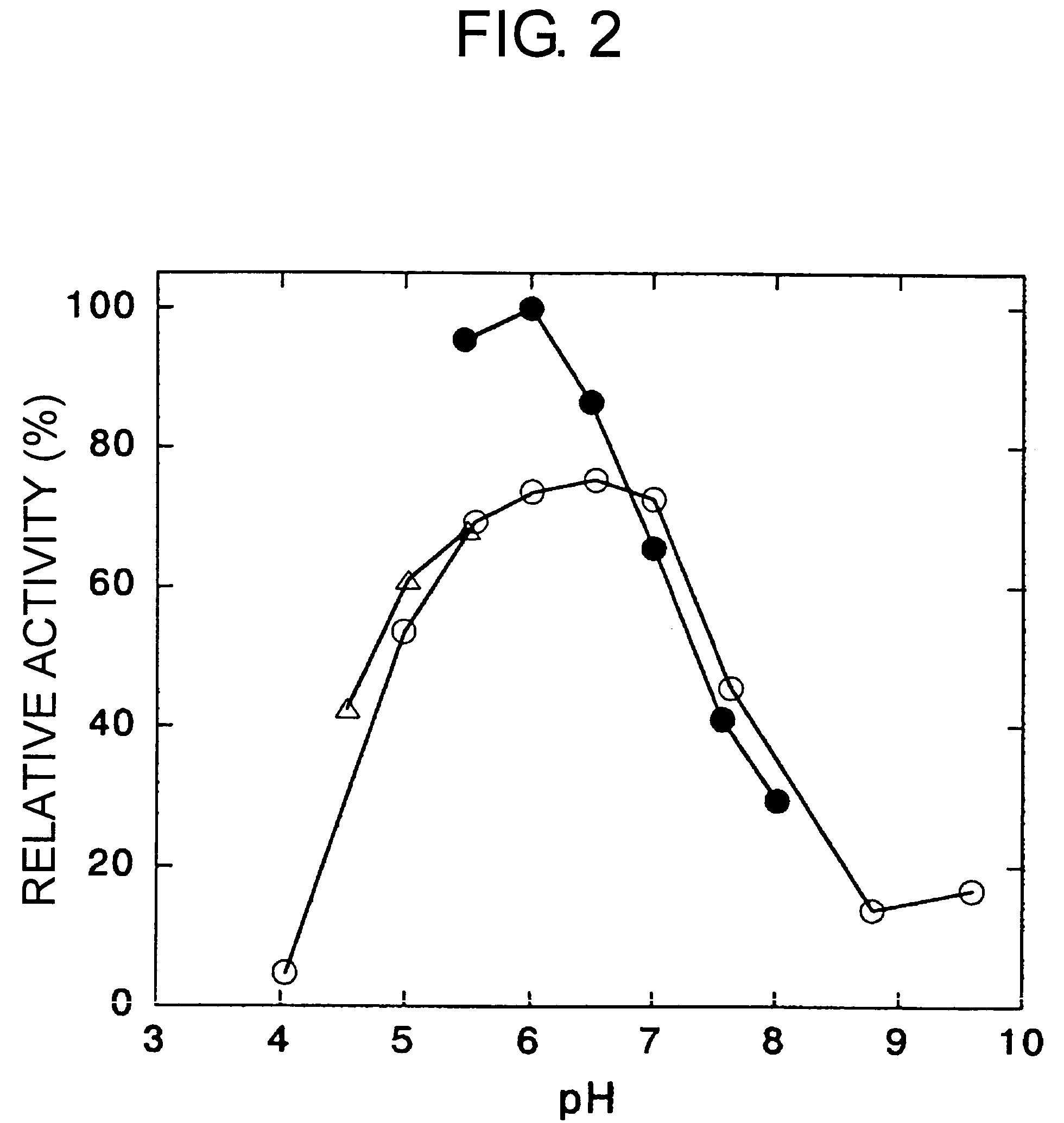
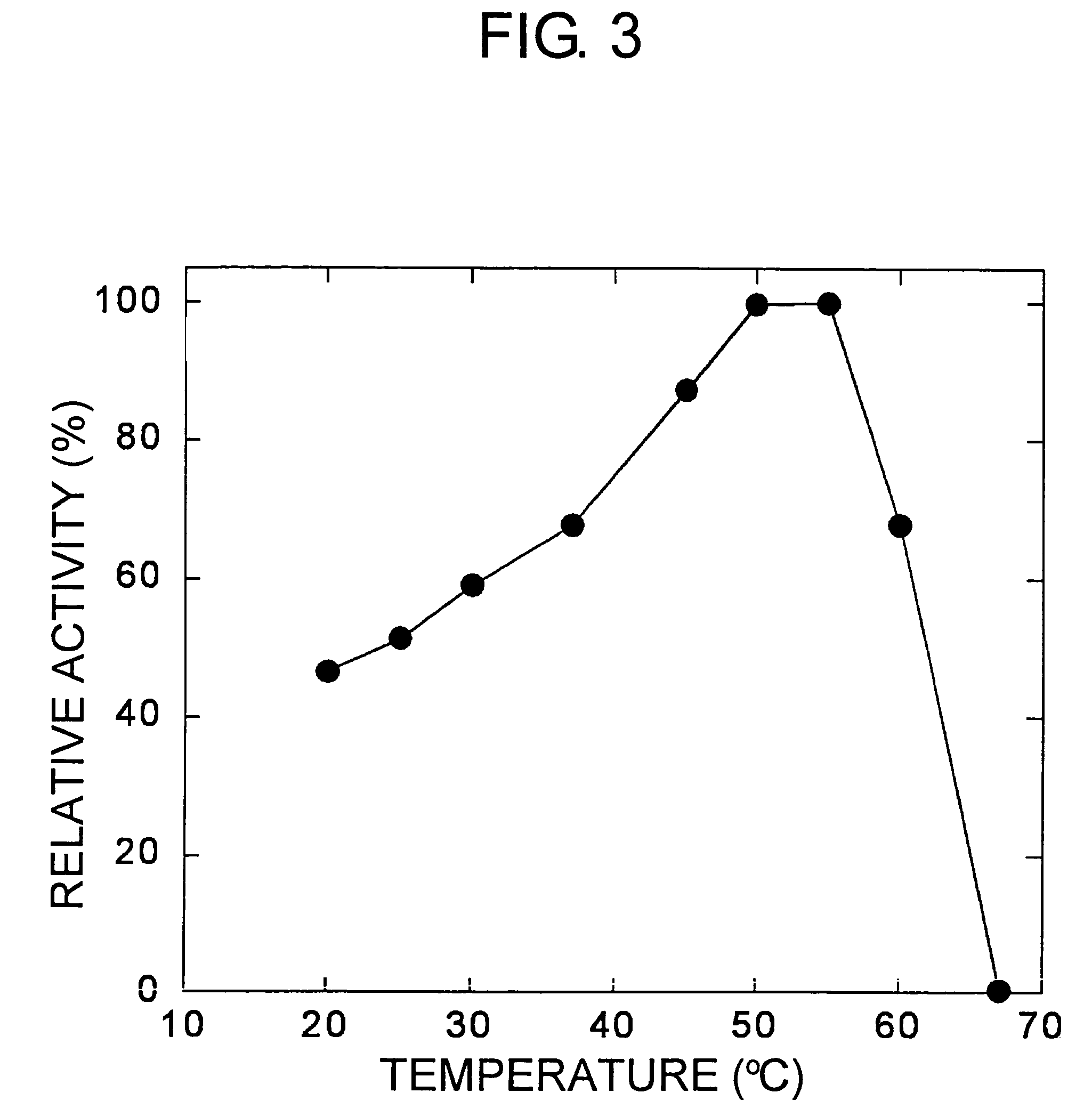
Carbonyl reductases, polynucleotides comprising DNA encoding the same, methods for producing the same, and methods for producing optically active alcohol utilizing the same
Owner:DAICEL CHEM IND LTD
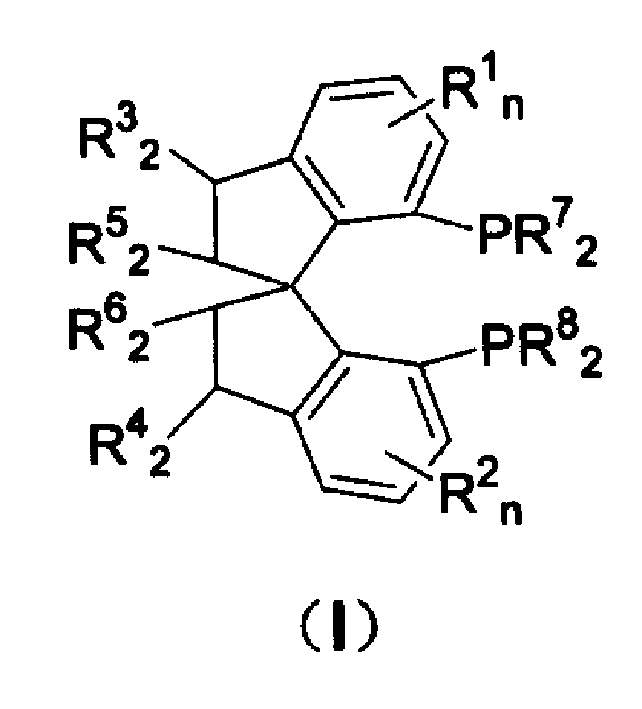
Spiro-diphosphine ligand
InactiveCN1439643AHigh stereoselectivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideDiphosphines
A spirocyclo-biphosphine ligand is prepared from spirocyclo-biphenol through esterifying by trifluoro methylsulfonic acid anhydride, coupling with diaryloxyphosphine under catalysis of Pd, and reduction reacting on trichlorosilane. It includes d-and levo-spricyclo-biphosphine ligands, whose mixture is dl-spirocyclo-biphosphine ligand. It can be used for asymmetrical catalytic hydrogenation reaction of latent chiral ketone with high stereo selectivity and e.e. value up to 99.5%.
Owner:NANKAI UNIV
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS7754902B2High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
Method for preparing aliphatic polycarbonates by catalyzing by metal cyanide coordination catalyst
The invention discloses a method for preparing aliphatic polycarbonates by catalyzing by the metal cyanide coordination catalyst. In a high pressure reactor, the metal cyanide coordination catalyst is utilized to catalyze epoxide and carbon dioxide body or solution copolymerization, wherein, the copolymerization temperature is 20-150 DEG C; the carbon dioxide pressure is 0.5-10MPa; the reaction time is 1-48h; the catalyst concentration is 1-100kg epoxide / g catalyst; the polymerization activity is more than 1.0kg of polymer / g catalyst; the weight-average molecular weight of the copolymer is more than 80 thousand; the molecular weight distribution is 1.5-4; the alternation degree of the copolymer is more than 90%; the cyclic carbonate by-product is less than 5wt%; the CO2 fixed rate of CO2 / epoxypropane copolymer is more than 40wt%; and the CO2 fixed rate of CO2 / cyclohexene oxide copolymer is more than 30wt%.
Owner:ZHEJIANG UNIV
Method for preparing ribonucleoside phosphorothioate
ActiveUS20130184450A1Improve efficiencyEfficient synthesisSugar derivativesBulk chemical productionRibonucleosideCombinatorial chemistry
Owner:WAVE LIFE SCI LTD
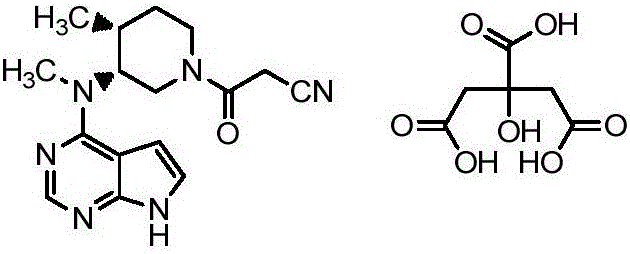
Preparation method of tofacitinib citrate
ActiveCN105884781AEasy to purifyGood stereoselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsCompound (substance)Benzyl group
The invention belongs to the field of medicine and chemical engineering and particularly relates to a preparation method of tofacitinib citrate. The method comprises steps as follows: 1-benzyl-4-methyl-2,6-dihydro-3-piperidone taken as a starting material is subjected to an asymmetric reduction reaction, 1-benzyl-4-methyl-3-piperidone is obtained, and (3R,4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride is produced under the action of a chiral catalyst; (3R,4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochlorid and a paratoluensulfonyl chloride protection product 4-chloro-7-(methyl-4-benzenesulfonyl) pyrrolo[2,3-d]pyrimidine of 4-chloropyrrolo[2,3-d]pyrimidine are subjected to a condensation reaction, [(3R,4R)]-1-benzyl-4-methyl-piperidine-3-yl]-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)-amine is obtained through deprotection, and tofacitinib citrate is obtained through debenzylation protection, an acylation reaction and citric acid salifying. The process route is short, the process cycle is short, chiral synthesis is performed by means of a catalyst, the product purity is improved, the cost is reduced, the yield is high, and the operation is simple and convenient.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
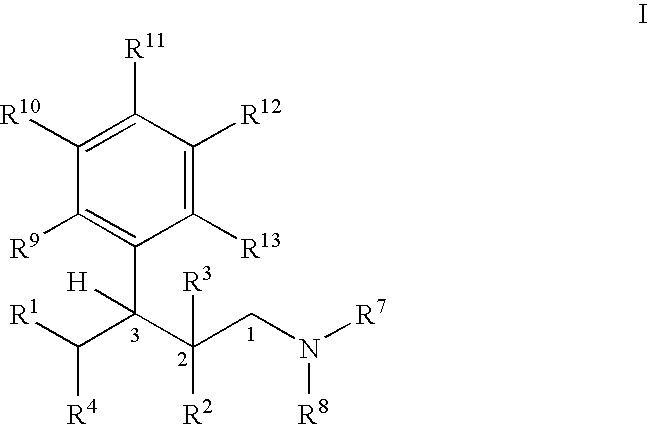
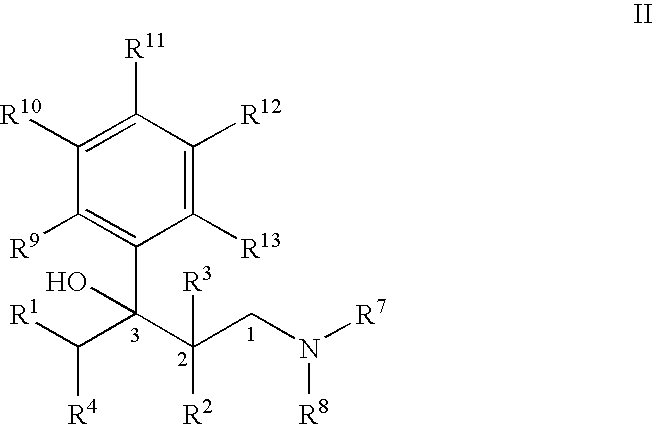
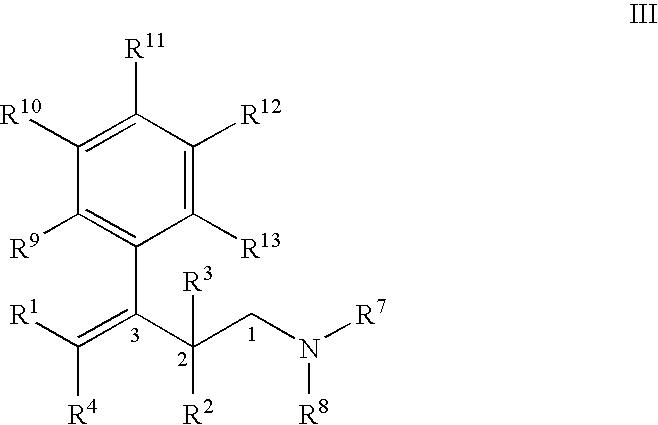
Process for the preparation of substituted 3-aryl-butylamine compounds
ActiveUS7417170B2High yieldImprove environmental friendlinessOrganic compound preparationAmino compound preparationArylAlcohol
Methods for the dehydration of substituted 1-amino-3-aryl-butan-3-ol compounds for the preparation of substituted 3-aryl-butyl-amine compounds.
Owner:GRUNENTHAL GMBH
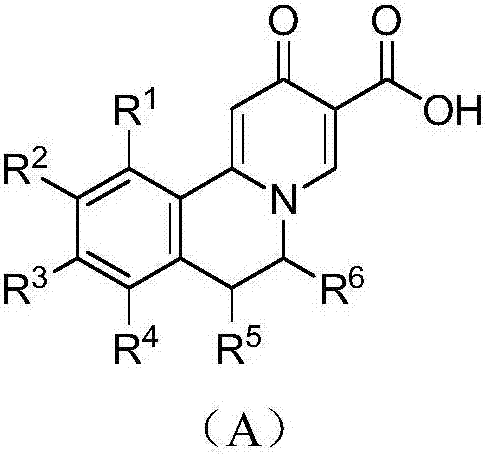
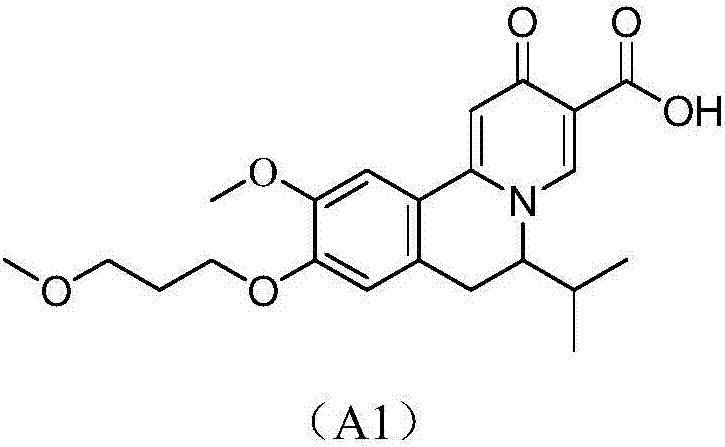
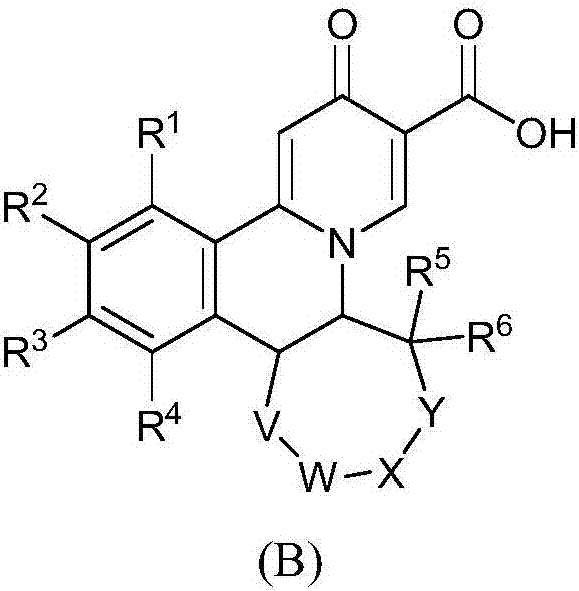
Quinolizinones compound and preparation method and application thereof
ActiveCN106928245ALower levelInhibition of replicationOrganic chemistry methodsDigestive systemToxicityHepatitis B Surface Antigens
The invention discloses a quinolizinones compound which comprises an optical isomer, a raceme and a cis-trans-isomer and a random combination or pharmaceutical salt thereof. A structure of the quinolizinones compound is as shown in a formula I: (with reference to the specification), wherein one of V and Y is O, and the other one of V and Y is CR8R9; R8 and R9 respectively independently are hydrogen or C1-6 alkyl. The invention further discloses a preparation method and application of the compound in the formula I. The compound in the formula I can obviously reduce a HBsAg (Hepatitis B surface antigen) level in vivo and meanwhile, inhibit replication of a HBV (Hepatitis B Virus), wherein when V or Y is O, solubility of the compound is obviously improved, the compound has excellent medicinal attributes and meanwhile, is also low in toxicity, pharmacokinetics and pharmacodynamics functions are improved, efficiency of combining with the HBV can be greatly improved, and a clearance ratio of the HBV in vivo can also be further improved.
Owner:河南春风医药科技有限公司
Di-carbonyl reduction enzyme, its gene and uses thereof
InactiveCN101429514AReduction efficiently catalyzesHigh stereoselectivityOxidoreductasesGenetic engineeringBiotechnologyDICARBONYL REDUCTASE
The invention discloses a gene of dicarbonyl reductase. The nucleotide sequence of the gene has over 80 percent of homology with SEQ ID NO.1. The dicarbonyl reductase consists of an amino acid sequence having over 80 percent of homology with SEQ ID NO.2. The obtained dicarbonyl reductase can be used for a reaction of catalyzing a dicarbonyl compound to be reduced into a dicarbonyl product, and has high stereo-selectivity.
Owner:常州金隆生物医药有限公司
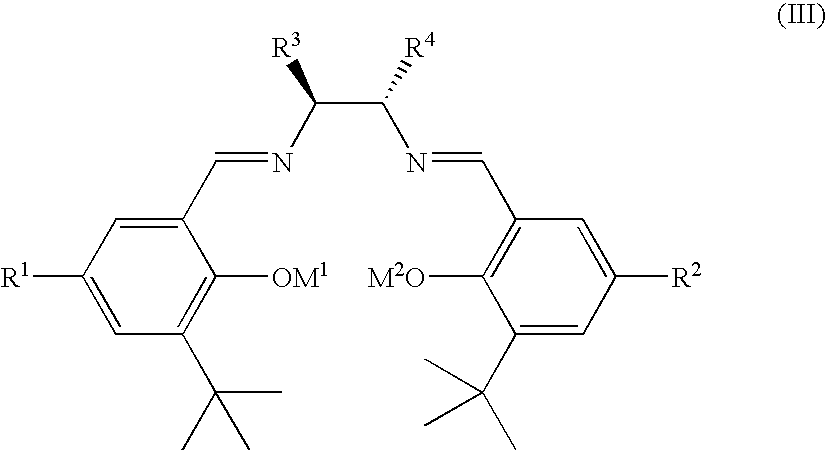
Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives
InactiveUS7560550B2High chemical yieldHigh stereoselectivitySilicon organic compoundsAntiviralsSilyleneSilanes
Glycosylation of 2,6-diaminopurines or silyl derivatives thereof with a compound of the structureis facilitated by the presence of an optionally silylated 1,3-dicarbonyl compound during glycosylation. Byproducts are minimized, while stereoselectivity may be altered by choice of Lewis acid and other reaction condictions.
Owner:RFS PHARMA
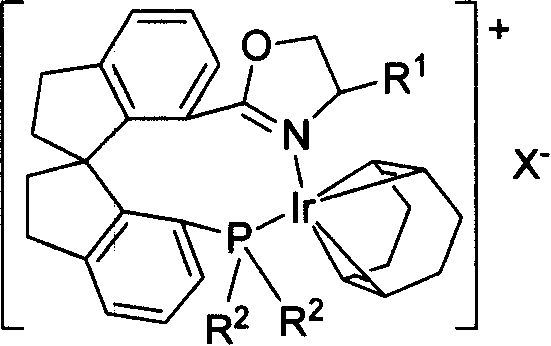
Phosphorus-oxazoline ligand with spiro backbone and its uses in asymmetrical catalytic hydrogenation
ActiveCN1884290AHigh stereoselectivityHigh reactivityGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsOrganic chemistryMedicinal chemistry
The invention relates to a new chiral toroid phosphine oxazoline ligand and its ionic iridium complex compound, and the application of said iridium complex compound in asymmetric catalytic hydrogenization for imines. The toroid phosphine oxazoline ligand is a widely used compound, for example, used as chiral ligand for asymmetric catalytic hydrogenization; especially said iridium complex compound is characterized by high stereoselectivity for asymmetric catalytic hydrogenization, the ee value reaches 97% and high reactive activity.
Owner:NANKAI UNIV
Directed evolution of recombinant monooxygenase nucleic acids and related polypeptides and methods of use
InactiveUS20060051782A1Improve abilitiesIncrease rangeBacteriaLibrary screeningNitrobenzeneMonooxygenase
The present invention relates to novel monooxygenase nucleic acids and polypeptides created using mutagenesis, DNA shuffling, or both, in a single iteration or multiple iterations, and methods for their creation and use. The monooxygenase enzymes of the present disclosure have particular utility as biocatalysts in industrial chemical redox reactions, such as the oxidation of aromatic hydrocarbons, for example, toluene, benzene, or nitrobenzene, into industrially desirable products. The systems and processes of the present invention are especially useful for the coupled synthesis and recovery of catechols, methylcatechols, resorcinols, methylresorcinols, hydroquinones, methylhydroquinones, hydroxybenzenes, cresols, nitrobenzenes, and nitrohydroxyquinones.
Owner:UNIV OF CONNECTICUT
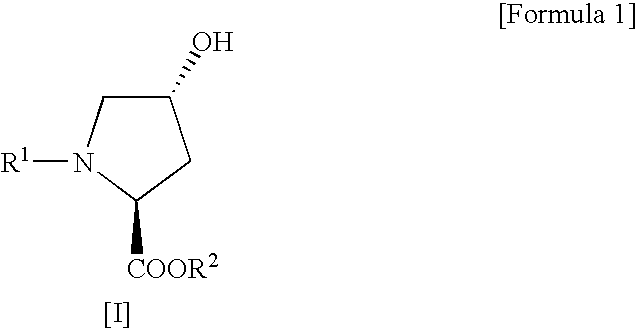
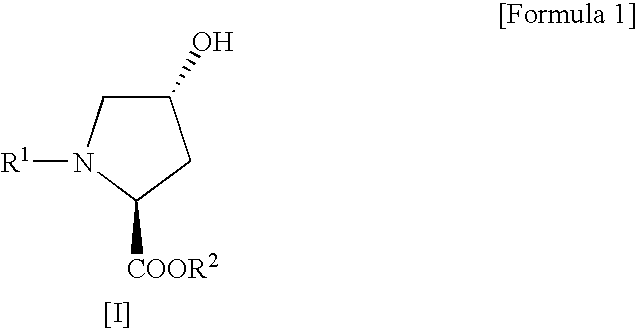
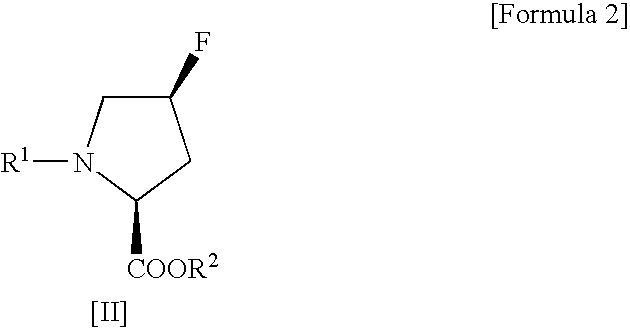
Process for production of cis-4-fluoro-l-proline derivatives
InactiveUS20060281927A1Enables industrial production of a target compound in high yieldInhibit side effectsOrganic chemistryBulk chemical productionHydrogen fluorideScavenger
The present invention provides a safer method for production of a cis-4-fluoro-L-proline derivative under milder conditions and in good yield to give a product of high purity on an industrial scale at low cost. Namely, the present invention provides a method for producing a cis-4-fluoro-L-proline derivative, which comprises reacting a trans-4-hydroxy-L-proline derivative of the following Formula [I]: (wherein R1 represents a protecting group for an α-amino group, and R2 represents a protecting group for a carboxyl group) with N,N-diethyl-N-(1,1,2,3,3,3-hexafluoropropyl)amine in the presence of a hydrogen fluoride-scavenger.
Owner:TAISHO PHARMACEUTICAL CO LTD
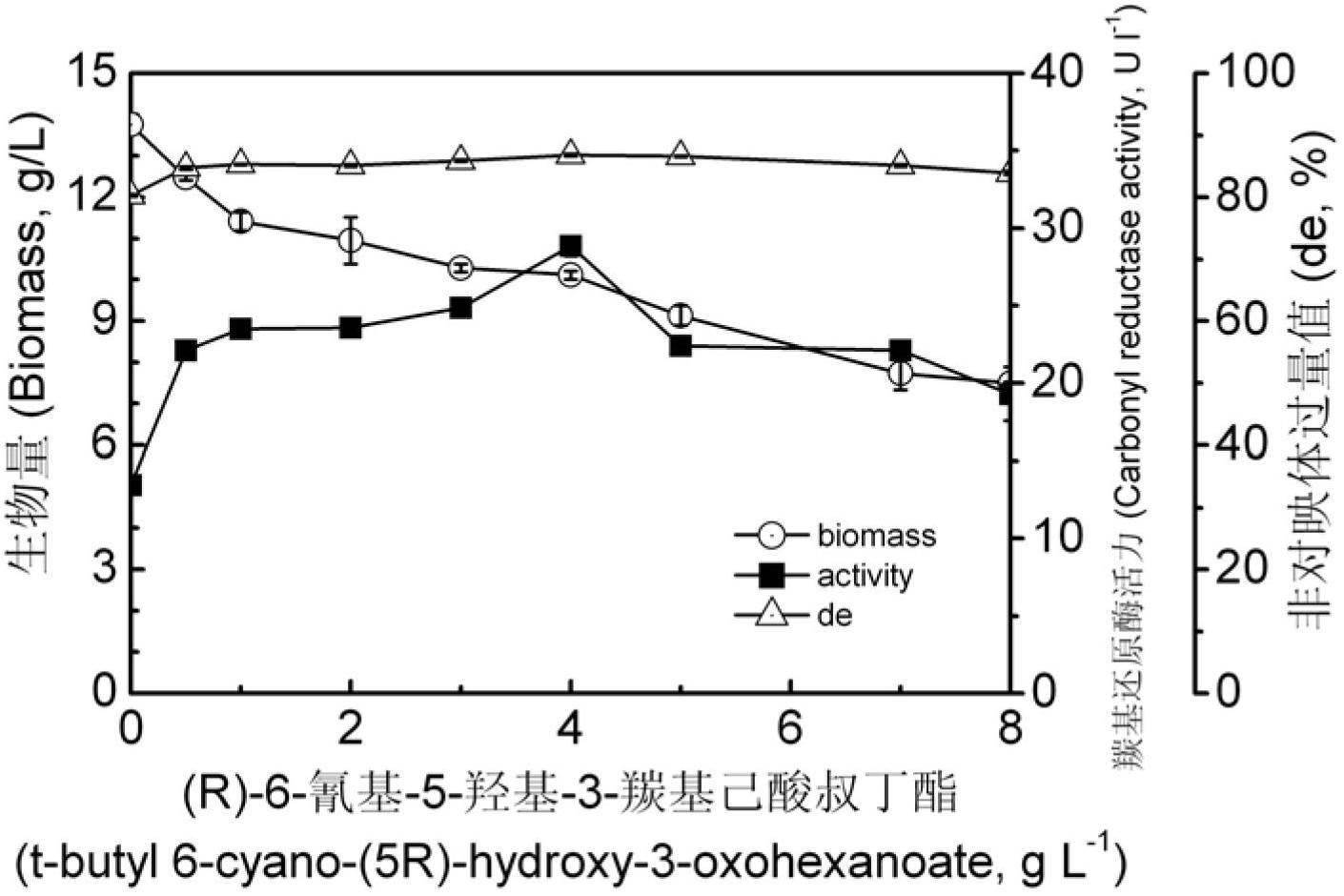
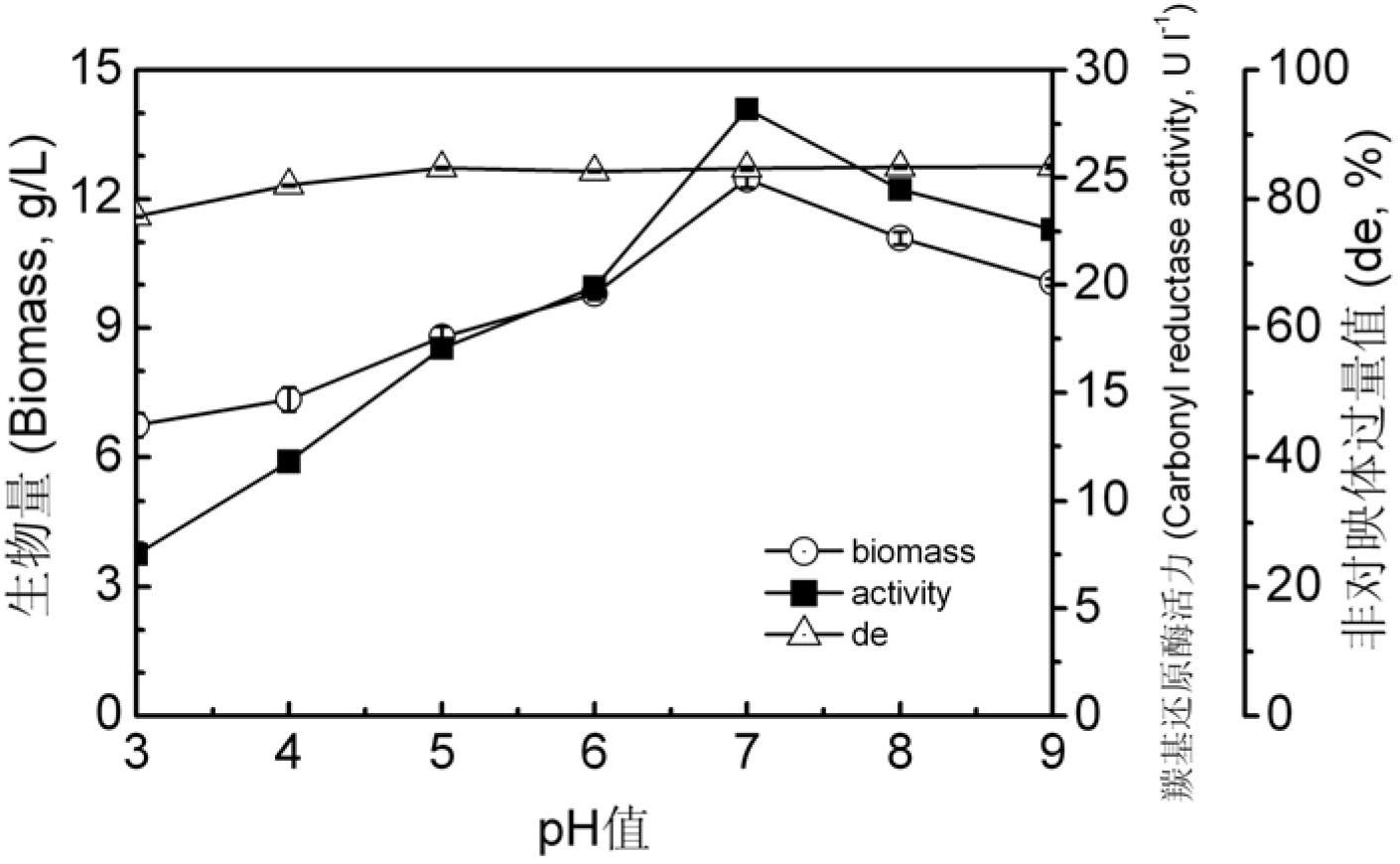
6- cyano-(3r, 5r)-dyhydroxyl hexanoic acid tert-butyl ester prepared by biological catalysis, and bacterial strain thereof
ActiveCN102643757AHigh stereoselectivityMild reaction conditionsFungiMicroorganism based processesCarbonyl ReductaseBacterial strain
The invention provides a Pichia guilliermondii X25 which is enriched and screened from soil, has high diastereoselectivity and is a carbonyl reductase active microbial new strain, and application of the Pichia guilliermondii X25 in preparation of 6-cyano-(3R, 5R)-dyhydroxyl hexanoic acid tert-butyl ester by biological asymmetric reduction (R)-6-cyano-5-hydroxyl-3-carbonyl hexanoic acid tert-butylester. The strain is preserved in China Center for Type Culture Collection (CCTCC), the address is Wuhan university, Wuhan, China, the post code is 430072, the CCTCC No. is M 2011386, and the preservation date is November11th,2011. The optical pure 6-cyano-(3R, 5R)-dyhydroxyl hexanoic acid tert-butyl ester prepared by using carbonyl reductase generated by the Pichia guilliermondii X25 to convert (R)-6-cyano-5-hydroxyl-3-carbonyl hexanoic acid tert-butyl ester is high in stereoselectivity, mild in reaction condition and environment-friendly.
Owner:ZHEJIANG UNIV OF TECH
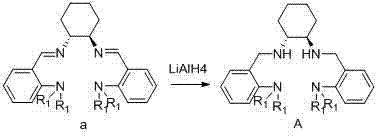
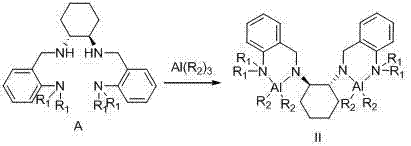
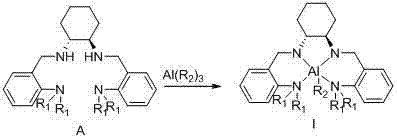
Chiral tetra-amino aniline ligand, aluminum compound thereof, preparation method and application
InactiveCN102924292AHigh catalytic activityWide molecular weight distributionOrganic compound preparationGroup 3/13 element organic compoundsLactideCombinatorial chemistry
The invention discloses a chiral tetra-amino aniline ligand, and a preparation method and application of an aluminum compound thereof. The structural formula of the ligand is represented as the formula (A), wherein R1 is alkyl in a C1-C4 straight chain branched chain structure. The aluminum coordination compound of the chiral tetra-amino aniline ligand can efficiently catalyze ring-opening polymerization reactions of lactide. The ligand and the aluminum compound of the ligand are simple to synthesize, high in product yield coefficient and stable in property, simultaneously catalyst activity is high, selectivity is good, the performance of an obtained polymer is good, and molecular weight and stereoselectivity of the polymer can be regulated by control of polymerization reaction conditions.
Owner:UNIV OF JINAN
Novel preparation method of netaglinide oxazolone
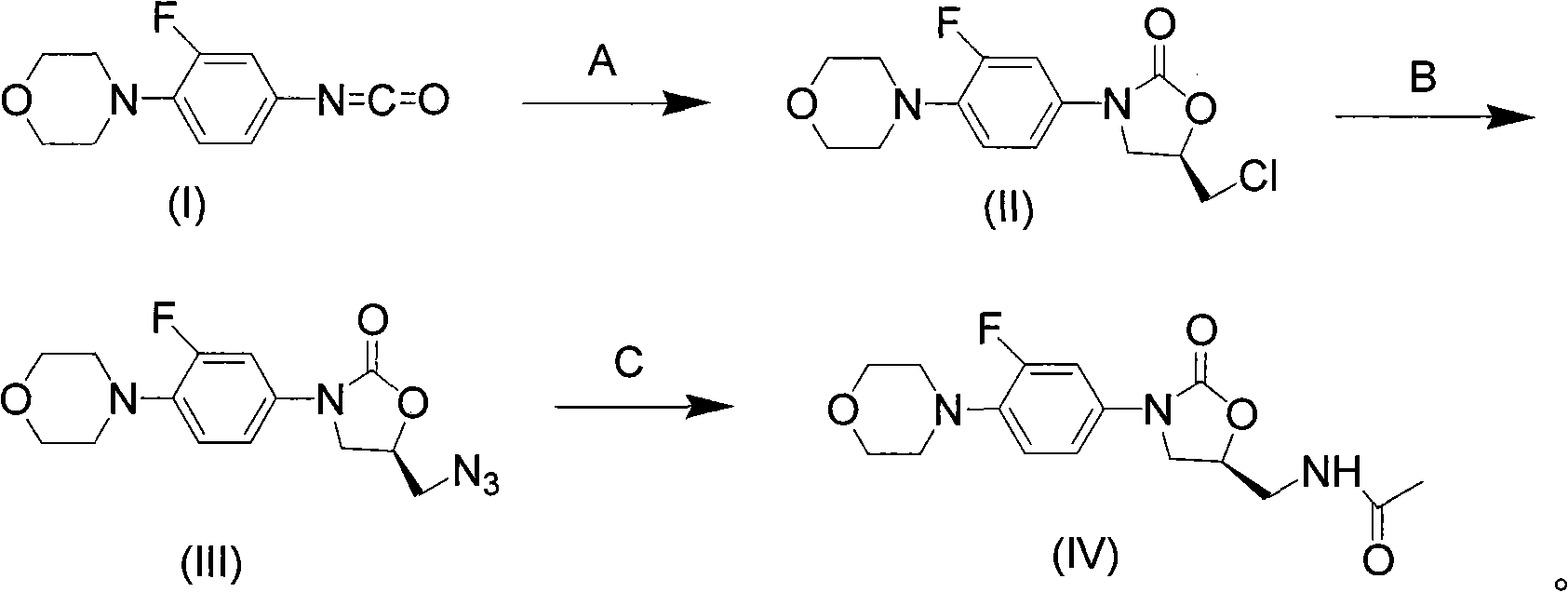
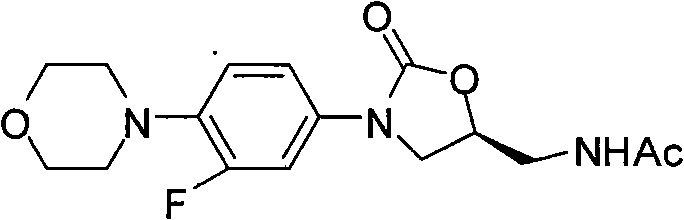
InactiveCN101638392AAtom utilization is highHigh stereoselectivityAntibacterial agentsOrganic chemistryEpoxyMorpholine
The invention discloses a novel preparation method of netaglinide oxazolone shown in the formula (IV), which comprises the following steps: under the action of a catalyst A, enabling 3-fluorine-4-morpholine phenyl isocyanate shown in the formula (I) to react with (R)-epoxy chloropropane to obtain a compound (II), wherein the catalyst A is magnesium diiodide, magnesium dibromide, magnesium dichloride, magnesium perchlorate or magnesium trifluoromethanesulfonic acid; enabling the compound (II) to react with sodium azide to obtain a compound (III); reducing the compound (III) by hydrogenation, and then, acetylating the reduced compound (III) to obtain the compound (IV). In the invention, the low-cost and environment-friendly catalyst (Lewis acid magnesium) is used for catalyzing the cycloaddition reaction of the (R)-epoxy compound and the isocyanate to establish a mother nucleus structure of the netaglinide oxazolone by one step, thus the prepared netaglinide oxazolone has high stereoselectivity, does not need rigorous operation conditions, such as low temperature, no water, no oxygen and the like, has the advantages of moderate reaction conditions, simple and convenient operation, high utilization ratio of atoms, environment protection, low production cost and the like, and is suitable for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
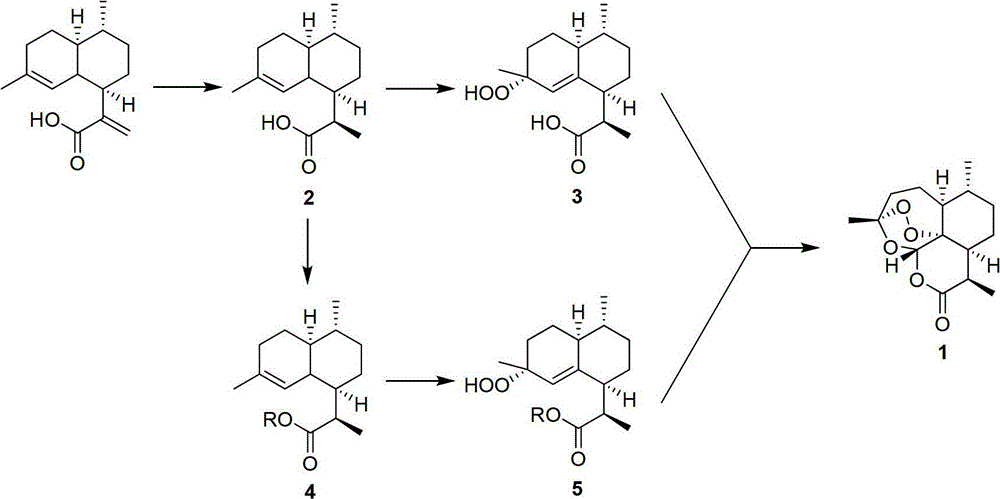
Method for preparing artemisinin through arteannuic acid
The invention discloses a method for preparing artemisinin through arteannuic acid. The method comprises the steps that first the arteannuic acid is processed to obtain a dihydroartemisinic acid under the effect of a reducing agent such as sodium borohydride / nickel chloride or a hydrogen / metal catalyst, and then the dihydroartemisinic acid is oxidized into a peroxided dihydroartemisinic acid through peroxide in the presence of the catalyst, and finally the target product artemisinin can be obtained with high yield under the catalyzing of the acid and the effect of oxygen; or a dihydroartemisinic acid derivative can be obtained from the dihydroartemisinic acid based on the protection on carboxyl, and the dihydroartemisinic acid derivative is oxidized into a relevant peroxided dihydroartemisinic acid derivative through the peroxide in the presence of the catalyst, and then the target product artemisinin can be obtained with high yield under the catalyzing of the acid and the effect of the oxygen. Compared with the prior art, the method for preparing the artemisinin through the arteannuic acid has the advantages as follows: the used agent has low cost, and is easy to obtain; the synthetic route is short; the reaction selectivity is high; the preparation process is environmental-friendly; the operation and post-processing are simple; the total yield is high; and the method for preparing artemisinin through the arteannuic acid is applied to industrial production.
Owner:SHANGHAI JIAO TONG UNIV
Novel polymer chiral catalyst, preparation method, and applications thereof
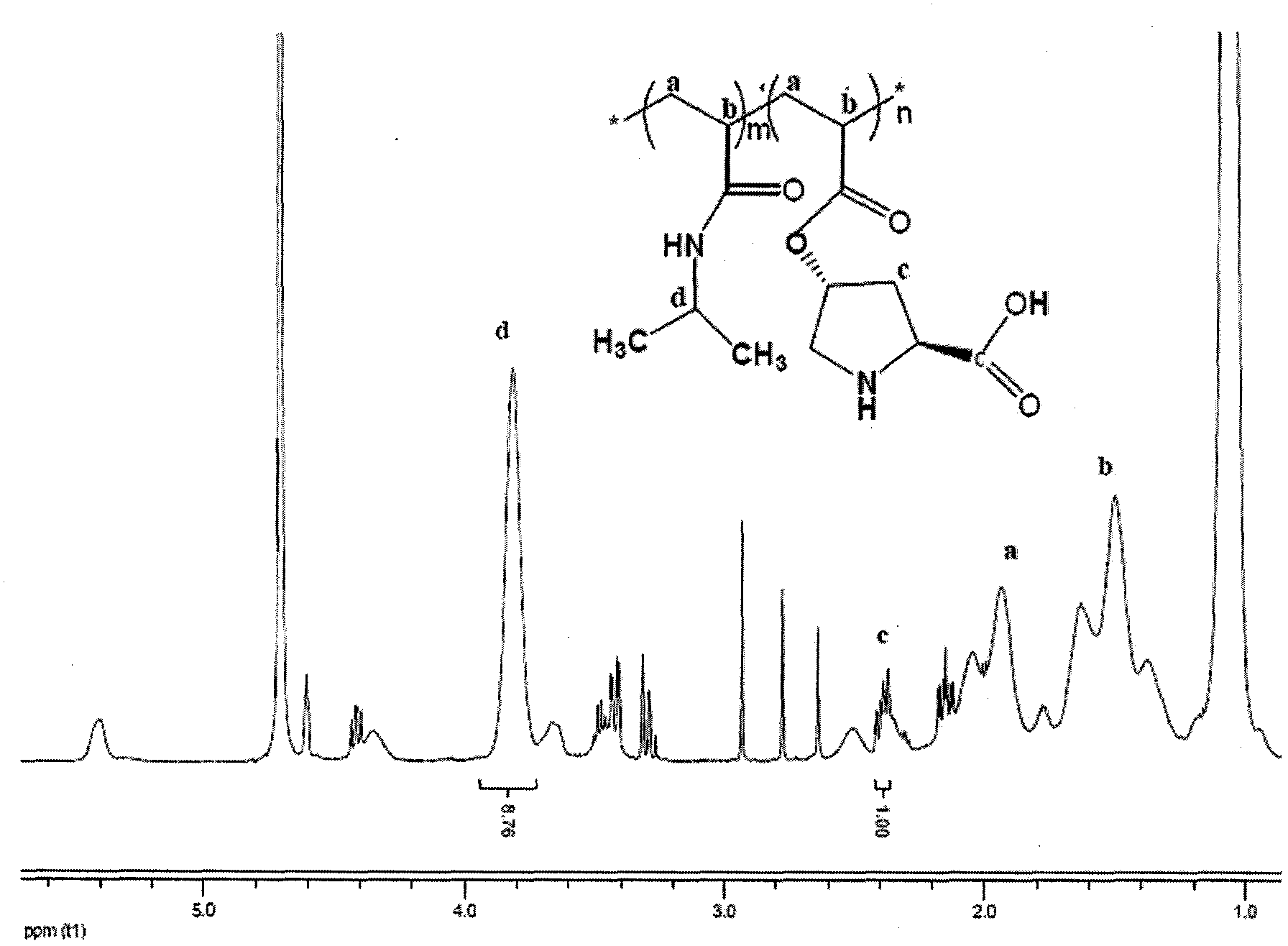
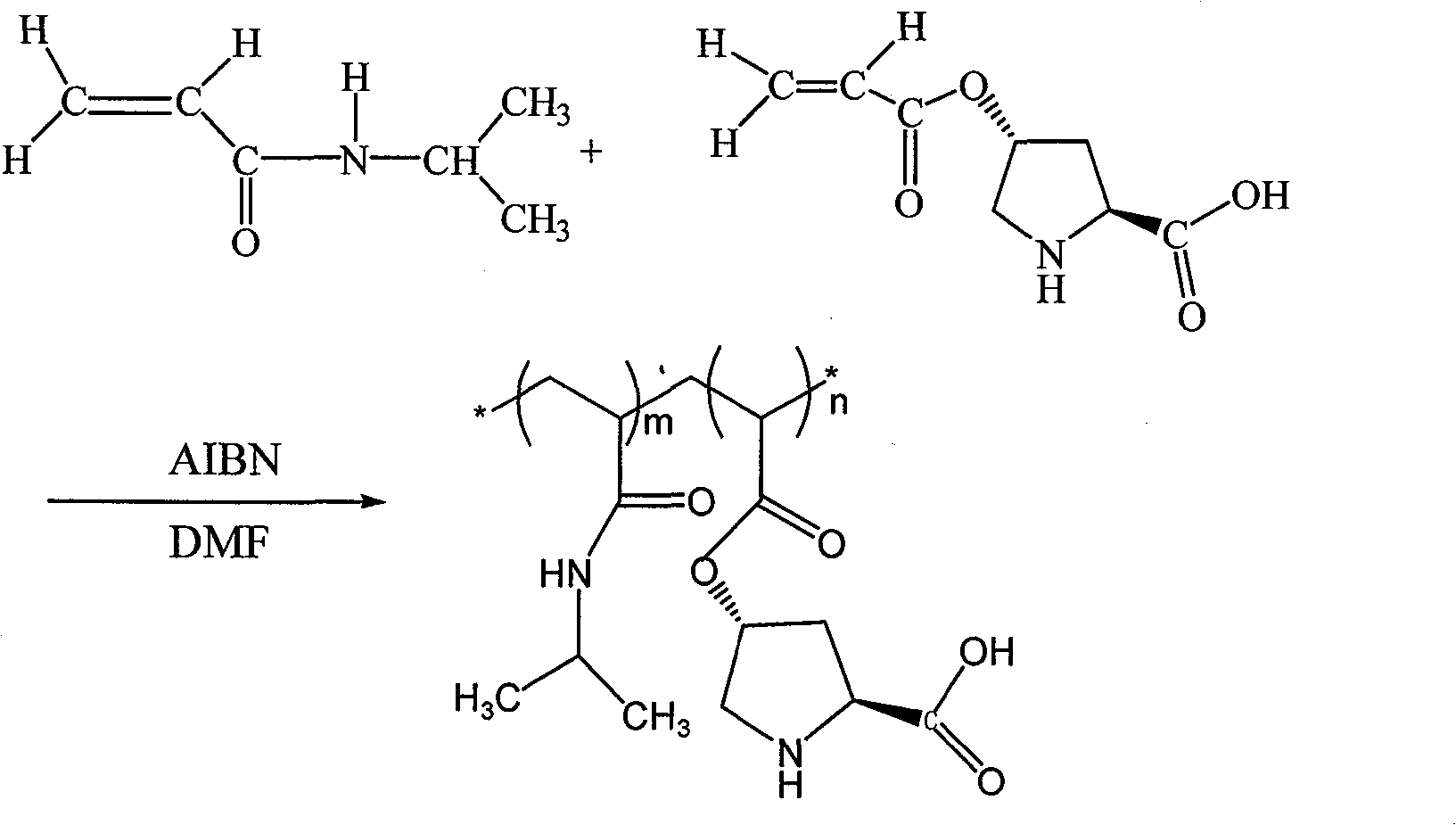
InactiveCN103447087AExtend your lifeImprove conversion rateOrganic-compounds/hydrides/coordination-complexes catalystsDimethyl formamideOrganic synthesis
The invention provides a soluble temperature sensitive polymer chiral catalyst, namely a polymer material copolymerized by L-proline derivatives and N-isopropylacrylamide. The catalyst is prepared by following steps: taking azodiisobutyronitrile (AIBN) as the initiator, and N,N-dimethylformamide (DMF) as the solvent, and then carrying out copolymerization reactions between N-isopropylacrylamide and free radicals of (s)-O-acryloyl-4-hydroxy-L-proline. The catalyst is capable of being used to catalyze asymmetric aldol condensation reactions, and has the advantages of high catalytic activity and good stereo-selectivity. The catalyst is capable of being used in an ordinary organic solvent system; furthermore, because the catalyst has a temperature sensitive effect, the catalyst is easy to be separated from the water system and recycled, the catalyst is an important way to achieve green organic synthesis, and has a vast application prospect.
Owner:NANJING UNIV +1
Alcohol dehydrogenase mutant and application thereof to synthesis of chiral biaryl alcohols
InactiveCN108359649AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesAlcoholMutant
The invention discloses an alcohol dehydrogenase mutant and an application thereof to synthesis of chiral biaryl alcohols, and belongs to the technical field of bioengineering. The alcohol dehydrogenase mutant has excellent catalytic activity and stereoselectivity and can be used for efficient catalysis for preparation of a series of R-configuration and S-configuration chiral biaryl alcohols. Alcohol dehydrogenase can be applied to synthesis of various chiral biaryl alcohol intermediates of antihistamine drugs by being coupled with glucose dehydrogenase or formate dehydrogenase. Compared withthe existing reports, a method for preparing the chiral biaryl alcohols from alcohol dehydrogenase through asymmetric catalytic reduction has the advantages of simple operation, high substrate concentration, complete reaction and high product purity, and has broad industrial application prospect.
Owner:JIANGNAN UNIV
Chiral amino phenoxyl zinc and magnesium compound, and preparation method and application thereof
InactiveCN103787943ALigand raw materials are readily availableLigand raw materials are convenientGroup 4/14 element organic compoundsGroup 2/12 organic compounds without C-metal linkagesPolyesterLactide
The invention discloses a chiral amino phenoxyl zinc and magnesium compound, a preparation method of the chiral amino phenoxyl zinc and magnesium compound, and application of the chiral amino phenoxyl zinc and magnesium compound in ring opening polymerization of catalytic lactone with high activity and high selectivity. The preparation method comprises the following steps: directly reacting a neutral ligand with a metal raw material compound in an organic medium; performing filtration, concentration and re-crystallization to obtain a target compound. The chiral amino phenoxyl zinc and magnesium compound is an efficient lactone ring opening polymerization and can be applied to polymerization reaction of catalytic lactide and the like; particularly, high-isotacticity or high-heterotacticity polylactic acid can be obtained for racemization lactide. The chiral amino phenoxyl zinc and magnesium compound has the obvious advantages that raw materials are easily obtained; a synthetic route is simple; high product yield, high catalytic activity and high stereo selectivity are realized; a high-regularity and high-molecular-weight polymer material can be obtained; requirements of industrial departments can be met. A structural formula is shown as (img file='DSA00000897420700011.TIF' wi='860'he='608' / ).
Owner:EAST CHINA UNIV OF SCI & TECH
Chiral diphosphine ligand and chiral catalyst, and preparation and application method thereof
ActiveCN103059064AHigh affinityReduced activityOrganic compound preparationGroup 5/15 element organic compoundsCyclic processDiphosphines
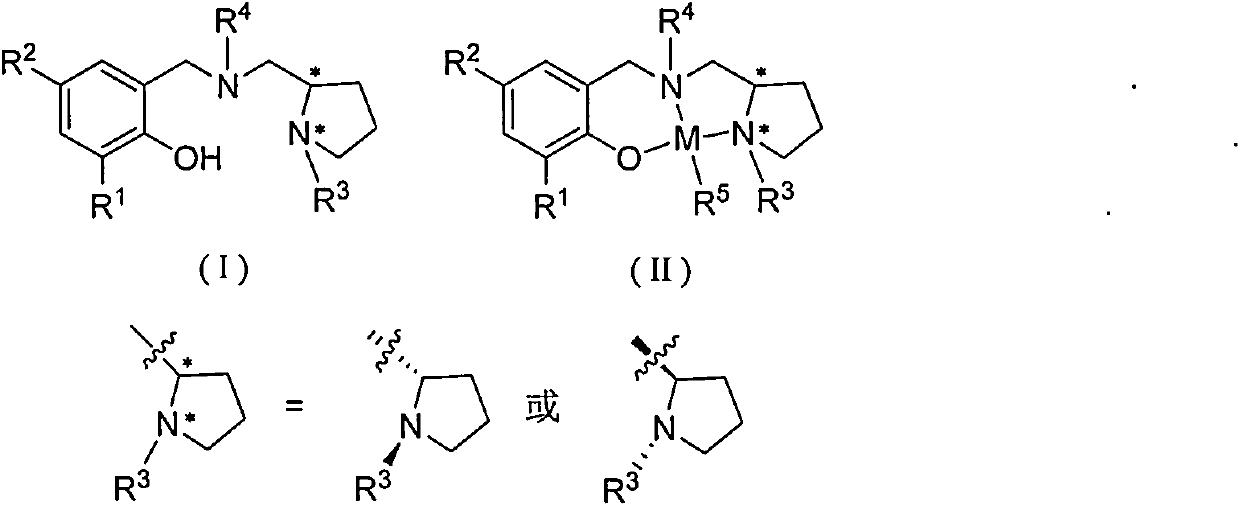
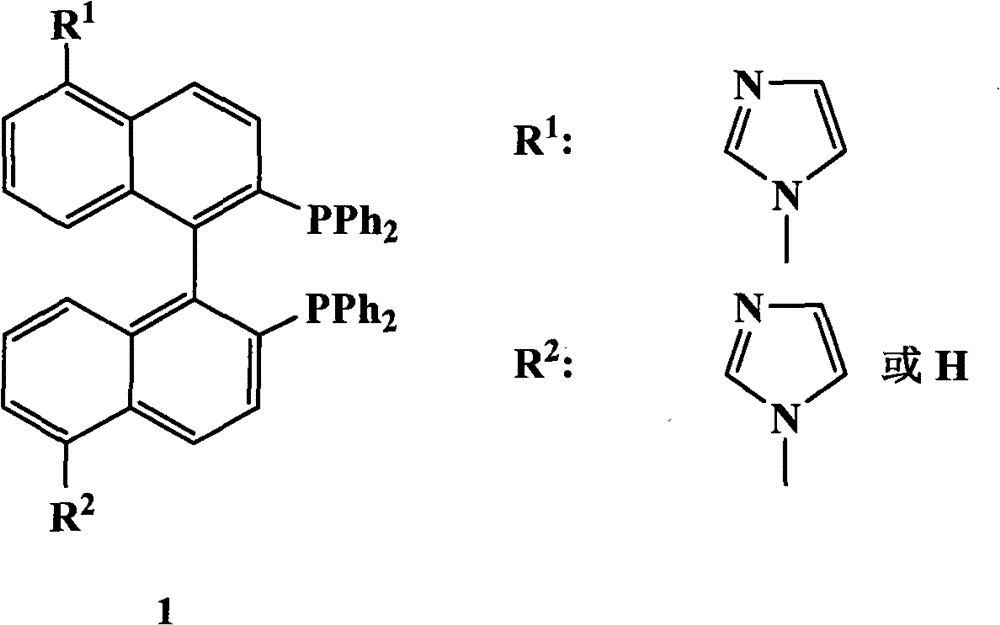
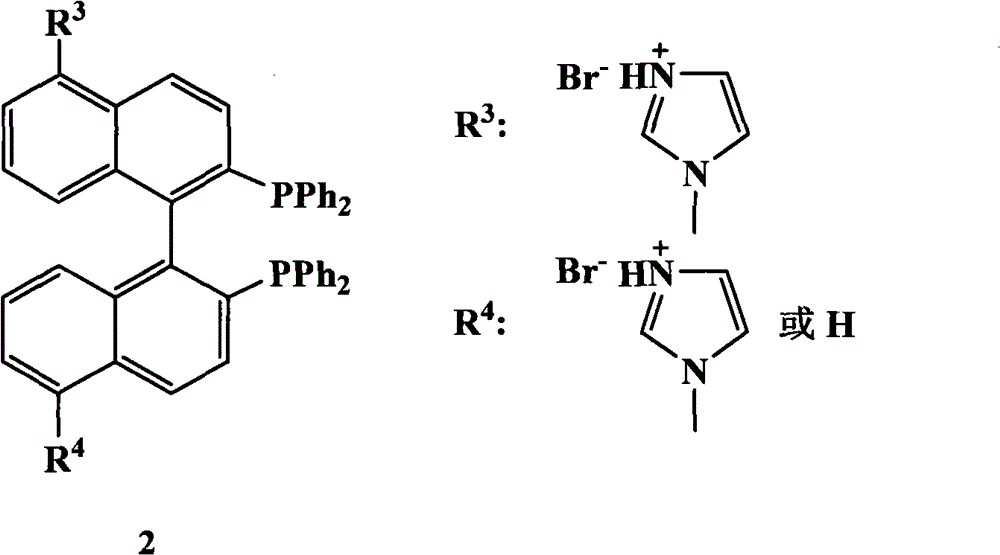
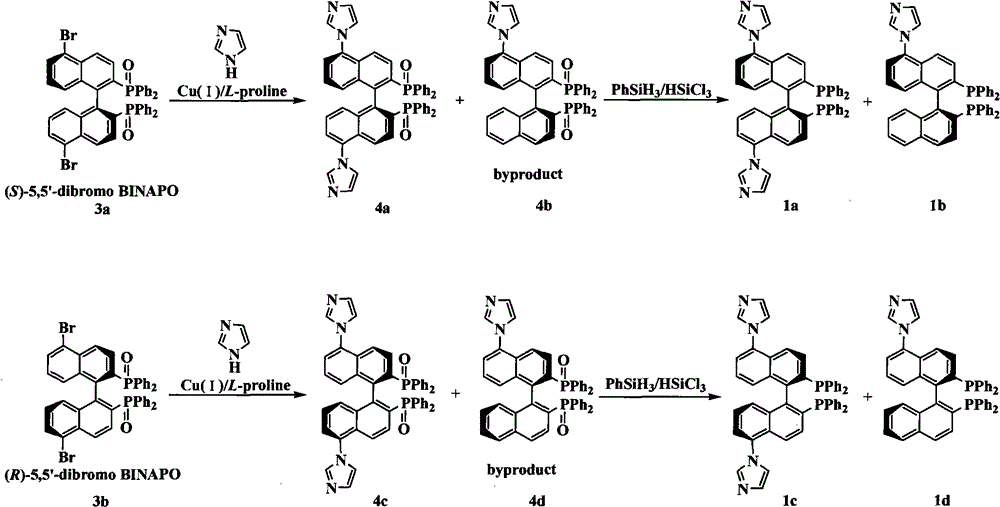
The invention relates to a chiral diphosphine ligand and a chiral catalyst, and a preparation and application method thereof. The preparation method of a BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) chiral diphosphine ligand based on novel imidazole and imidazole cation modification. Imidazole and imidazole cation are introduced to the 5,5'- position of the BINAP molecule framework to synthesize the chiral diphosphine ligand. The assembly of the imidazole and imidazole cation into the chiral diphosphine ligand molecule can effectively enhance the stability of the chiral catalyst in an ionic liquid and the affinity with the imidazole ionic liquid, and avoids loss of the catalyst in the cyclic process. The imidazole-modified chiral diphosphine ligand has the following chemical structural formula, wherein the spatial configuration of the imidazole-modified chiral diphosphine ligand is S type or R type.
Owner:山东聚强绿洲生物科技有限公司
Rhizopus microsporus root-shaped variant ZJPH1308 and application thereof in preparation of sitagliptin intermediate
ActiveCN104893989AHigh stereoselectivityHigh optical purityFungiMicroorganism based processesPyrazineButanone
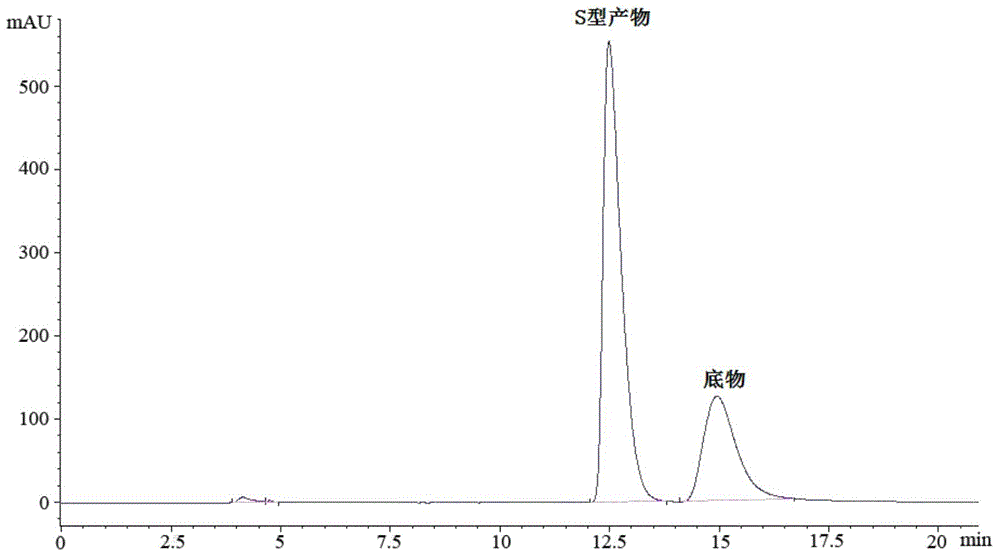
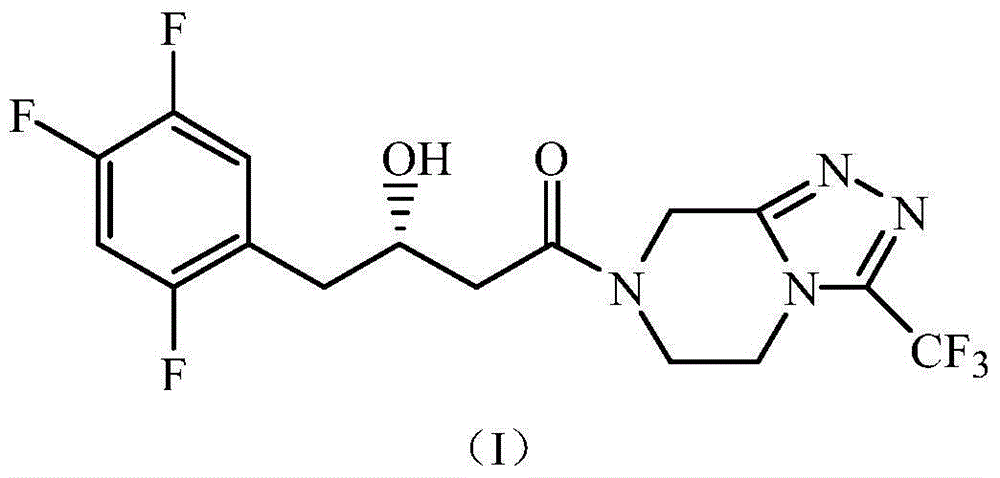
The invention discloses a rhizopus microsporus root-shaped variant ZJPH1308 and application thereof in preparation of a sitagliptin intermediate. A strain of the rhizopus microsporus root-shaped variant ZJPH1308 can be used for biologically and asymmetrically reducing 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazol[4,3-a]pyrazine-7-(8H)-yl]-1-(2,4,5-trifluorophenyl)butanone so as to obtain (S)-3-hydroxyl-1-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazol[4,3-a]pyrazine-7-(8H)-yl]-4-(2,4,5-trifluorophenyl)butanone. The strain has the advantages of good three-dimensional selectivity, high product optical purity and the like. The rhizopus microsporus root-shaped variant ZJPH1308 is fermented and cultured to obtain a wet thallus which serves as chiral biological catalyst; when the substrate concentration is 10mmol / L, the reduction product yield is as high as 95%, and the e.e. value of the product is greater than 99.9%.
Owner:JIANGSU TIANHE PHARMA CO LTD
Recombinant transaminase as well as preparation method and application of recombinant transaminase
ActiveCN106801043AStrong specificityHigh catalytic activityOrganic chemistryBacteriaRegioselectivityMutant
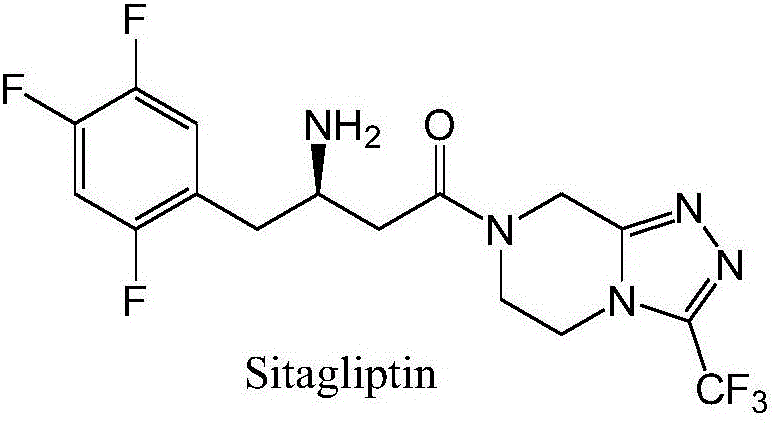
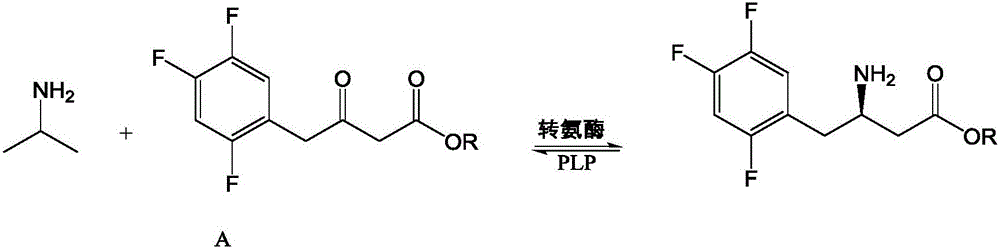
The invention discloses recombinant transaminase as well as a preparation method and application of the recombinant transaminase. An amino acid sequence of a high-activity recombinant transaminase mutant is as shown in SEQ ID NO.2 and a coding gene is as shown in SEQ ID NO.1. The method for preparing the recombinant transaminase compries the steps of fermenting and cultivating a gene engineering bacterium containing the coding gene, and collecting and preparing the recombinant transaminase. The recombinant transaminase is applied to asymmetric synthesis of a chiral amine compound, and particularly is applied to synthesis of a sitagliptin midbody (R)-3-amino-4-(2,4,5-trifluoromethyl phenyl)-methyl butyrate. The related transaminase has excellent stereoselectivity, regioselectivity and catalytic activity, the catalytic reaction is mild, the reaction conversion rate and the yield of a product are high, and the recombinant transaminase has relatively good application prospect.
Owner:JIANGSU ALPHA PHARM CO LTD
Method for asymmetrically synthesizing chiral beta-acetenyl ketone from beta-ketonic acid
InactiveCN104513146AHigh reactivityHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetonic acidsKetone
The invention relates to a method for synthesizing chiral beta-acetenyl ketone by catalytic intermolecular decarboxylation from beta-ketonic acid and a propargyl compound. A chiral copper catalyst adopted in the invention is synthesized in stiu from a copper salt and a chiral P,N,N-tridentate ligand in various polar solvents and nonpolar solvents. According to the invention, various chiral beta-acetenyl ketone compounds with substituent groups can be synthesized conveniently, and can obtain a percent enantiomeric excess as high as 95%. The method in the invention is advantaged by operational simplicity, available raw materials, wide substrate application range, high enantioselectivity, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Candida parapsilosis ZJPH1305 and application thereof in preparation of chiral alcohol
ActiveCN103849574AHigh optical purityHigh substrate concentrationFungiMicroorganism based processesMicroorganismAlcohol
The invention discloses a novel strain, namely candida parapsilosis ZJPH1305 and application thereof in preparation of high optical purity (5S)-(4-fluobenzene)-5-hydroxyvalerate by virtue of microbial catalytic asymmetric reduction of a 5-(4-fluobenzene)-5-keto pentanoic acid. The e.e value of a target product (5S)-(4-fluobenzene)-5-hydroxyvalerate achieves 99.9% and the yield achieves 95.37% when the concentration of a substrate is 47.62mmol / L and the final concentration of cosubstrate glucose is 100g / L by virtue of chiral biocatalysis of adopting the candida parapsilosis ZJPH1305 cell as a catalyst.
Owner:ZHONGRONG TECH CORP LTD
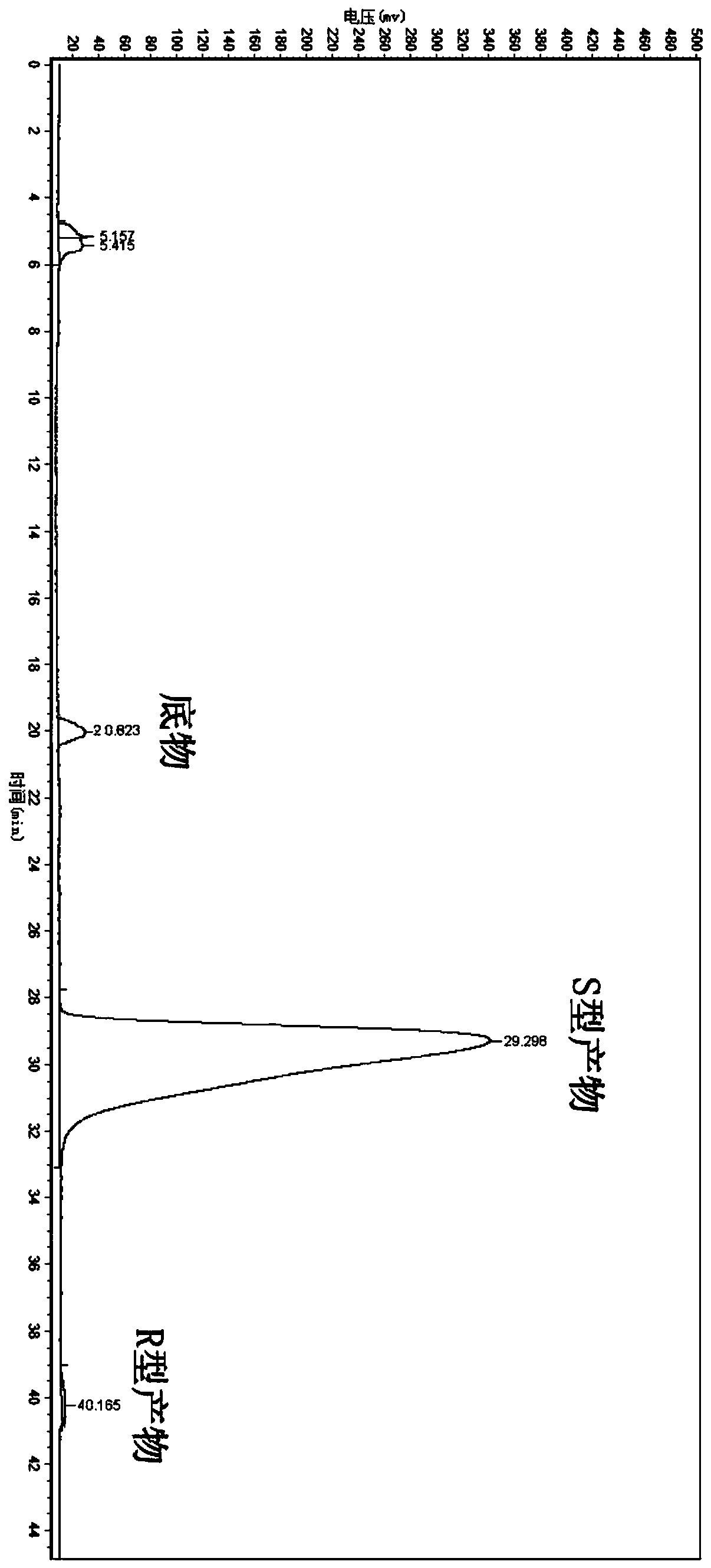
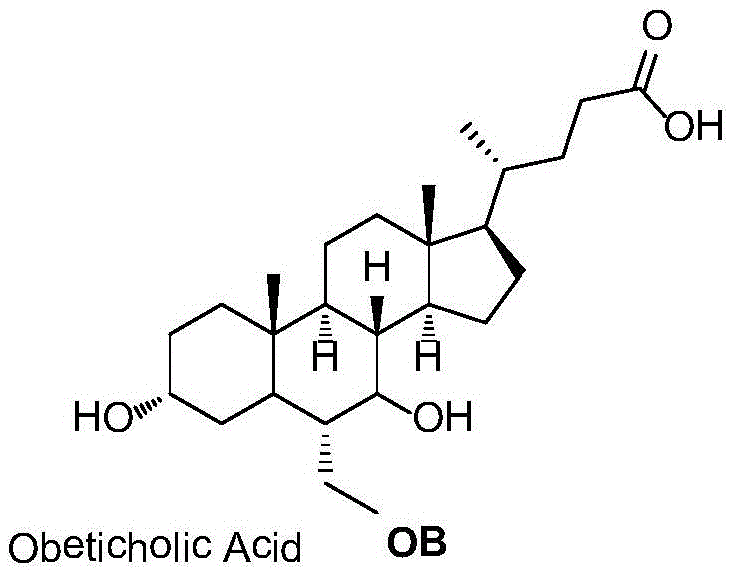
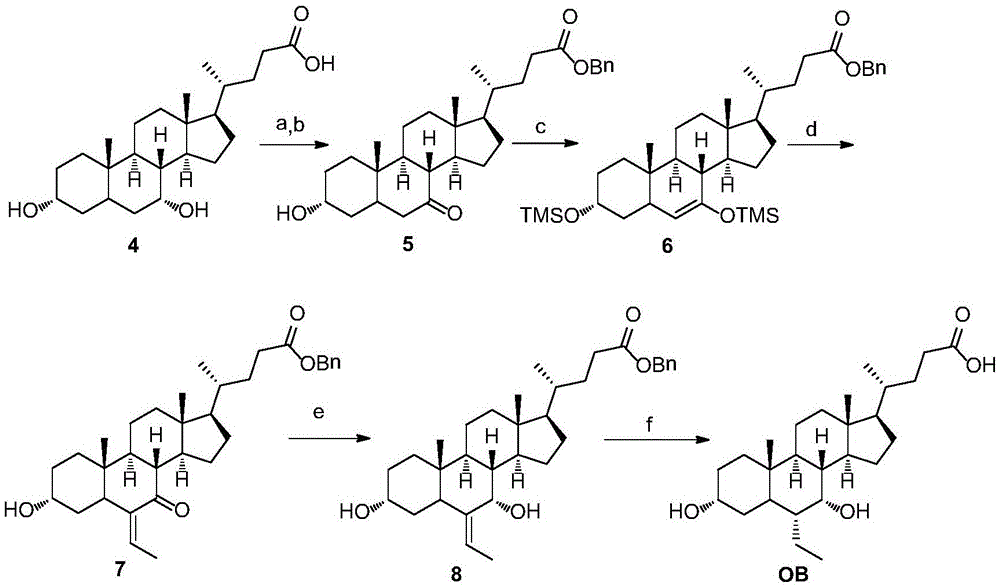
Preparation method for obeticholic acid and intermediate thereof
The invention relates to a preparation method for obeticholic acid and an intermediate thereof. The intermediate 3alpha-hydroxy-6alpha-ethyl-7-keto-5beta-cholanic acid is obtained by subjecting 3alpha-hydroxy-6-ethylidene-7-keto-5beta-cholanic acid benzyl ester compounds to a reaction under the action of a catalyst and a hydrogen donor. The catalyst is selected from Pd / C or PtO2 or Raney Ni. The hydrogen donor is selected from cyclohexene or cyclohexadiene or tetrahydronaphthalene. According to the method, the yield is high, the stereoselectivity is high, safety is good, the reaction condition is mild, and the method is applicable to industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
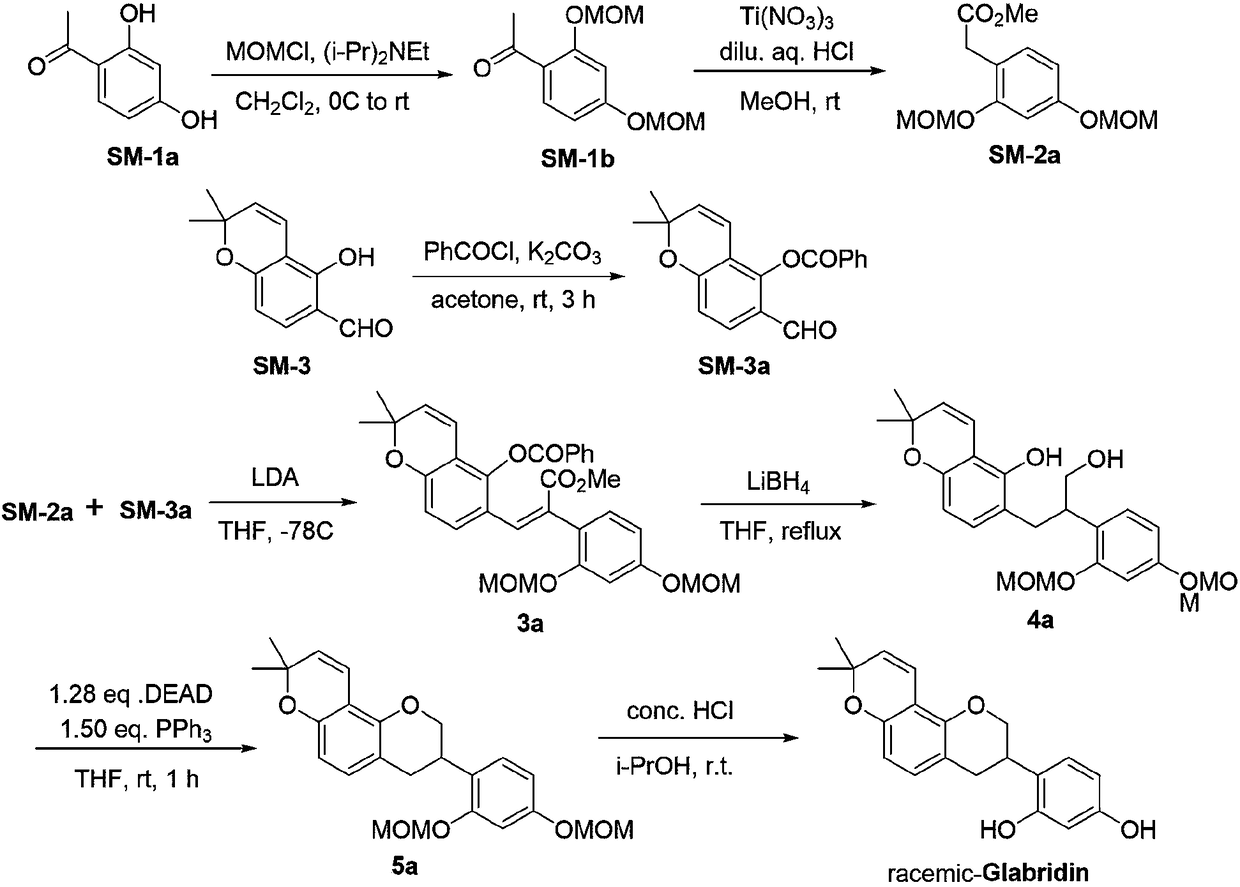
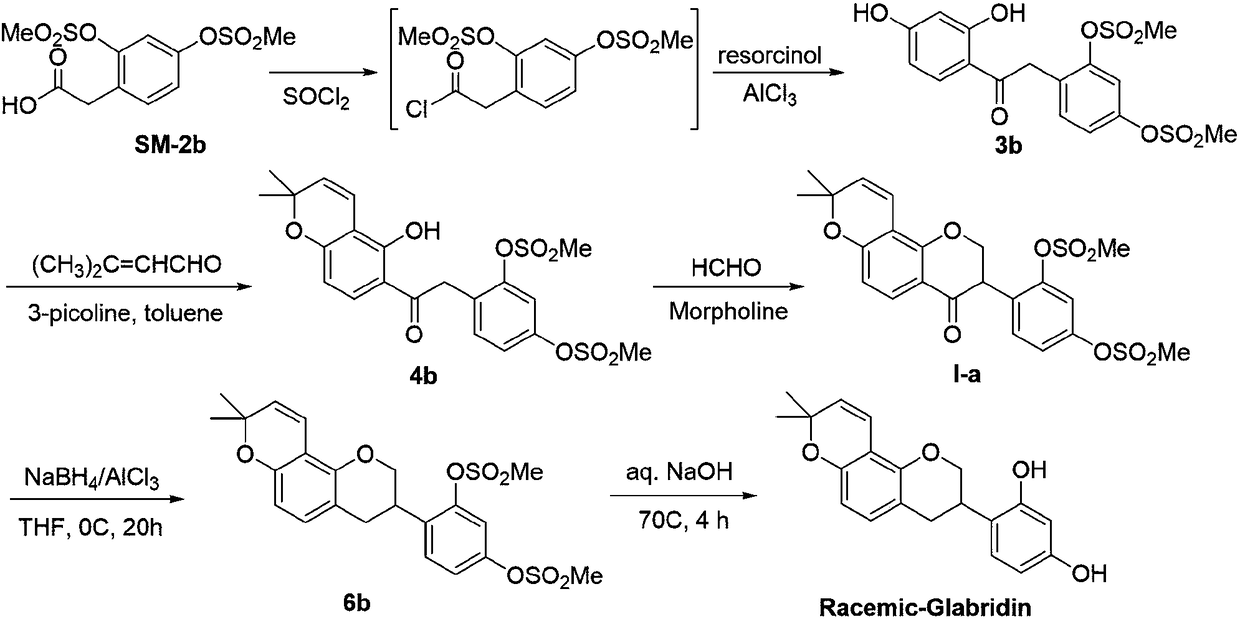
Method for asymmetrically synthesizing glabridin with optical purity under catalysis of ruthenium compound
ActiveCN108440553AHigh yieldHigh stereoselectivityOrganic chemistry methodsBulk chemical productionAbsolute configurationTrifluoroacetic acid
The invention relates to a method for asymmetrically synthesizing glabridin with optical purity under catalysis of a ruthenium compound. The method comprises the following steps: 1) taking isoflavoneprotected by a protection group as a raw material and carrying out dynamic kinetic asymmetric hydrogen transfer reaction under the catalysis effect of a ruthenium trichloride compound and the action of an acid-alkali buffering system to obtain chiral isoflavol with an absolute configuration being (3R, 4R); 2) removing hydroxyl of the chiral isoflavol under the action of triethylsilane and trifluoroacetic acid to obtain a product with an absolute configuration being (R); 3) removing a protection group of the product with the configuration being (R) in step 2) under an acidic or alkaline condition to obtain the glabridin with the configuration (R) and the optical purity. The method provided by the invention can be used for synthesizing the glabridin with the optical purity in a high-yield and high-stereoselectivity manner; the obtained product is completely the same as that of the glabridin extracted from glycyrrhiza glabra and can be used for replacing the glabridin derived from naturalplants to be industrially applied.
Owner:烟台六谛医药科技有限公司 +2
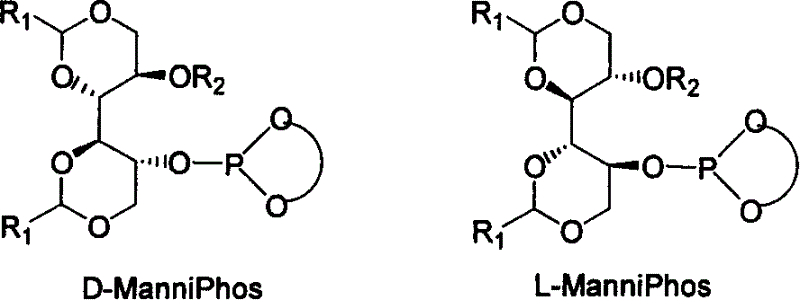
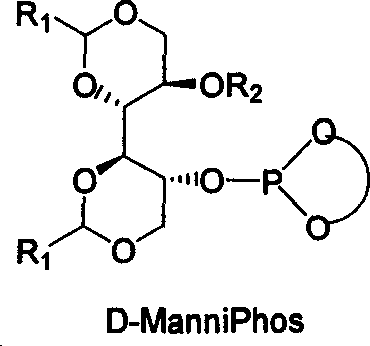
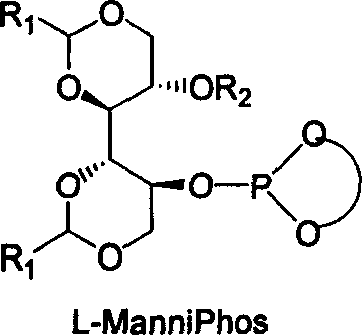
Chiral ligand metal complex catalyst system, and its preparation method and use
InactiveCN1579627ALower synthesis costHigh stereoselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPhosphateHigh pressure
The invention relates to chirality phosphine ligand metal complex catalyse system, which is composed by complex formed by ligand and metal Rh, Ru, Ir, Pt or Pd or ligand and metal precursor in of 1-2 mol ratio. The phosphine ligand is a kind of effective chirality phosphate ligand composed by D-mannitol or L-mannitol after reaction. The catalyst composed by the chirality ligand and rhodium metallic compound in asymmetry hydrogenation reaction can work in room temperature. With a wide applied range, catalyst's activation and stereoselectivity is not influenced from normal pressure to high pressure. Reaction time is 1-24 hours. Mol ratio of ligand and metal rhodium compound is 1:1-2:1. Ratio of reactant and catalyst is 100-10,000.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol
InactiveCN108384765AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesFormate dehydrogenase HGlycol synthesis
The invention discloses an alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol and belongs to the technical field of biological engineering. The alcohol dehydrogenase mutant disclosed by the invention has good catalytic activity and stereoselectivity, and a series of chiral diaryl alcohol with R- and S- configurations can be prepared through efficient catalysis. According to the alcohol dehydrogenase mutant, alcohol dehydrogenase is coupled with glucose dehydrogenase or formate dehydrogenase, and can be used for synthesizing various antihistamine drugs, i.e., a chiral diaryl alcohol intermediate. Compared with an existing report, a method for preparing the diaryl chiral alcohol through asymmetric catalysis of the alcohol dehydrogenase has the advantages of simplicity in operation, high substrate concentration, complete reaction and high product purity and has a very good industrial application prospect.
Owner:JIANGNAN UNIV
Optical pure 1,3-alkamine compound as well as preparation method and application thereof in preparing Dapoxetine and analogues thereof
ActiveCN101875666ANo wasteHigh stereoselectivityGroup 4/14 element organic compoundsCarbamic acid derivatives preparationStereochemistryDapoxetine-N-oxide
The invention specifically relates to an optical pure 1,3-alkamine compound as well as a preparation method and application thereof in preparing corresponding optical pure 1,3-alkamine and further preparing Dapoxetine and analogues thereof, belonging to the technical field of organic chemistry. The 1,3-alkamine compound is as shown in a formula I.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d059af67-daa6-4b9f-8b4a-5e15884fccc5/US07560550-20090714-C00001.png)
![Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d059af67-daa6-4b9f-8b4a-5e15884fccc5/US07560550-20090714-C00002.png)
![Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d059af67-daa6-4b9f-8b4a-5e15884fccc5/US07560550-20090714-C00003.png)