Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
318 results about "Cholanic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
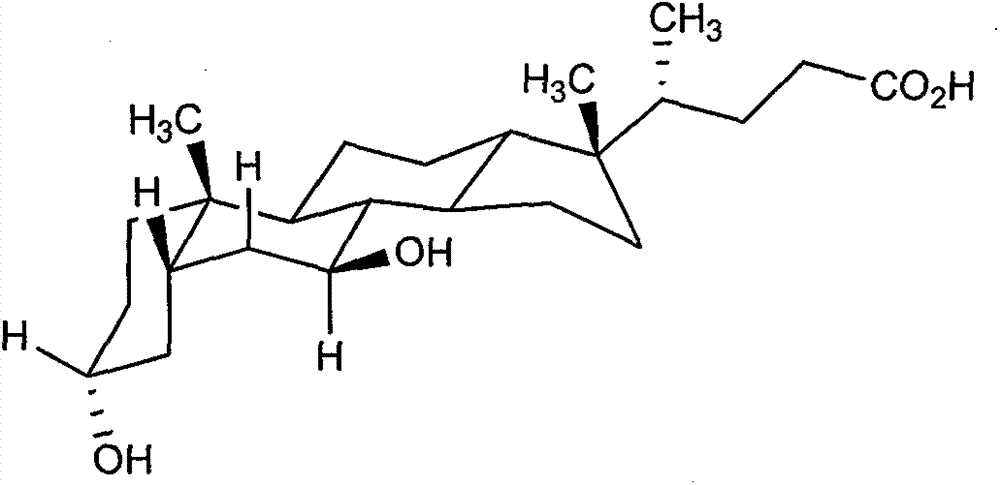
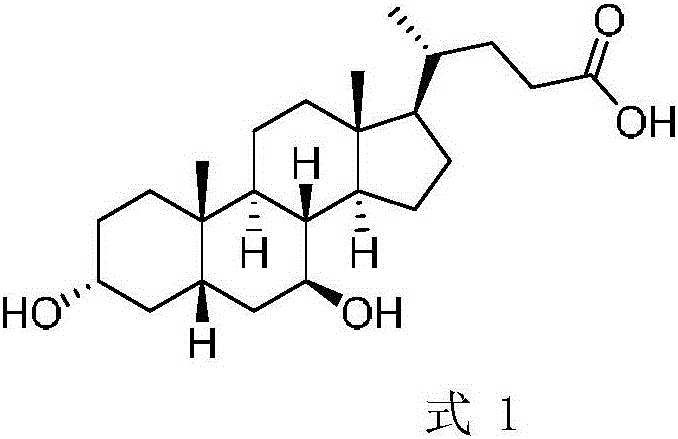
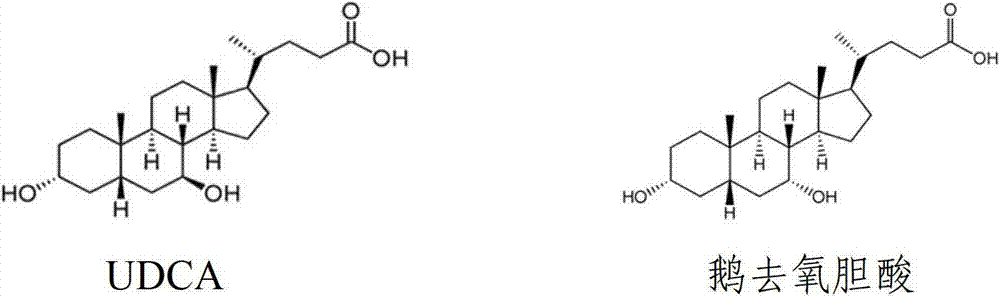
Chenodeoxycholic acid (also known as chenodesoxycholic acid, chenocholic acid and 3α,7α-dihydroxy-5β-cholan-24-oic acid) is a bile acid. It occurs as a white crystalline substance insoluble in water but soluble in alcohol and acetic acid, with melting point at 165–167 °C.
Oligonucleotides comprising a C5-modified pyrimidine
ActiveUS20050288244A1Improved pharmacokinetic propertiesAntibacterial agentsNervous disorderBenzoic acidPhosphate
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one ligand. In certain embodiments, a ligand is bound to only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, both of the oligonucleotide strands of the double-stranded oligonucleotide independently comprise a bound ligand. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. In certain embodiments, a phosphate linkage in one or both of the strands of the oligonucleotide has been replaced with a phosphorothioate or phosphorodithioate linkage. In a preferred embodiment, the ligand is cholesterol or 5β-cholanic acid. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one ligand. In certain embodiments, the oligonucleotide comprises at least one modified sugar moiety. In certain embodiments, a phosphate linkage of the oligonucleotide has been replaced with a phosphorothioate or phosphorodithioate linkage. In a preferred embodiment, the ligand is cholesterol or 5β-cholanic acid. The ligand improves the pharmacokinetic properties of the oligonucleotide.
Owner:ALNYLAM PHARM INC
Method for preparing binding-form ursodesoxycholic acid by two-step enzymatic method
ActiveCN102994604ASimple preparation processImprove conversion rateFermentationChenodeoxycholic acidSolution state
The invention relates to a method for preparing binding-form ursodesoxycholic acid by a two-step enzymatic method, belonging to the field of biotechnology. Under the water solution state, a substrate, namely binding-form chenodeoxycholic acid, is converted into the binding-form ursodesoxycholic acid in the presence of 7alpha-HSDH and 7beta-HSDH, the separation is not needed in the intermediate step, thus the method disclosed by the invention is very simple, and furthermore, the conversion efficiency is very high. Especially chicken bile, duck bile or goose bile in a mixture form can be subjected to reaction without separating and purifying the binding-form chenodeoxycholic acid. The preparation process is simple and easy to implement, and the new, simple and convenient preparation method is provided for the binding-form ursodesoxycholic acid.
Owner:SHANGHAI KAIBAO PHARMA
Method for catalyzing chenodeoxycholic acids to compound ursodesoxycholic acids through efficient whole-cells
ActiveCN105368828AFermentation methods are cheap and readily availableSuitable for industrial productionBacteriaMicroorganism based processesChemical synthesisLactate dehydrogenase
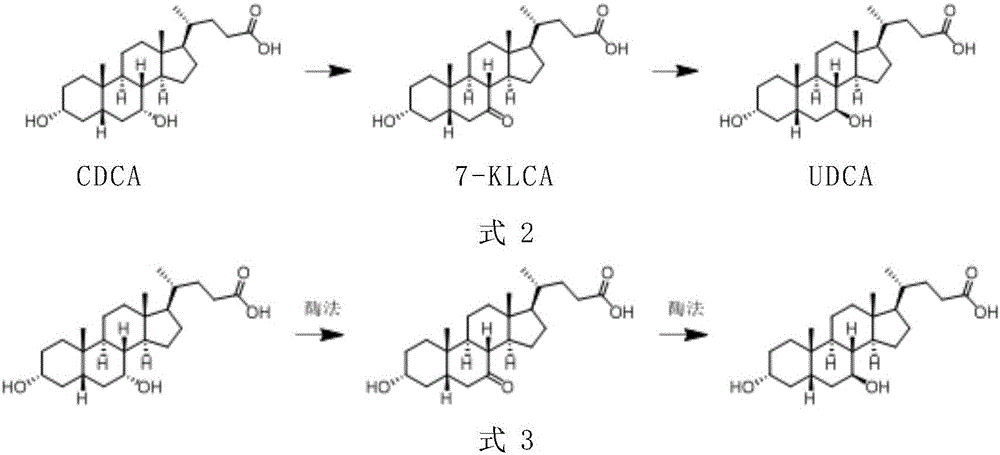
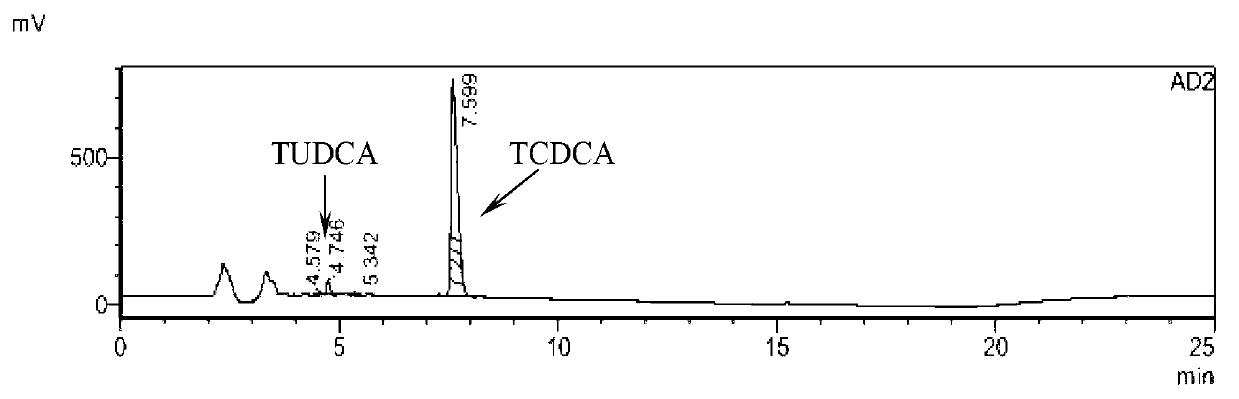
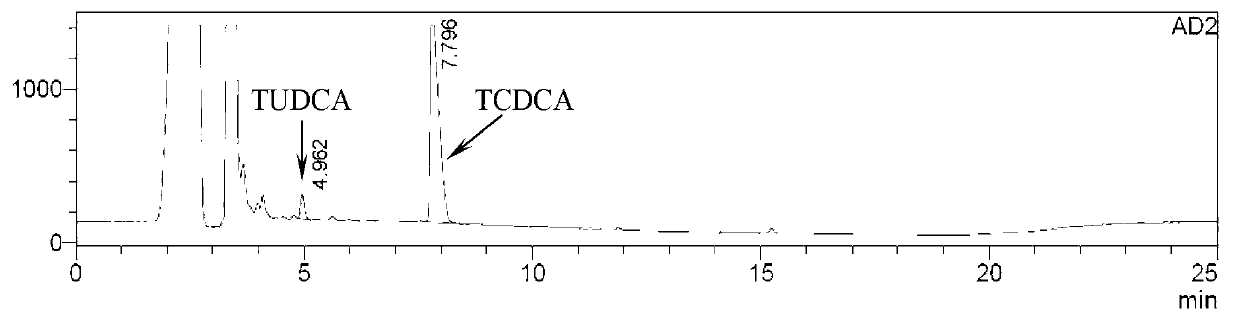
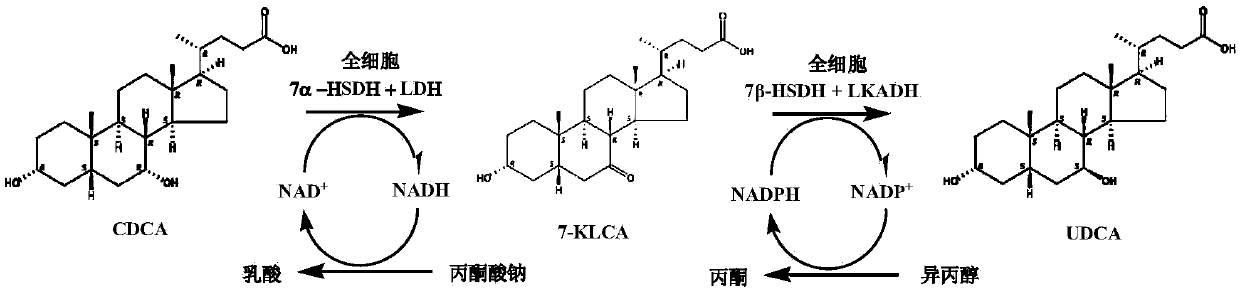
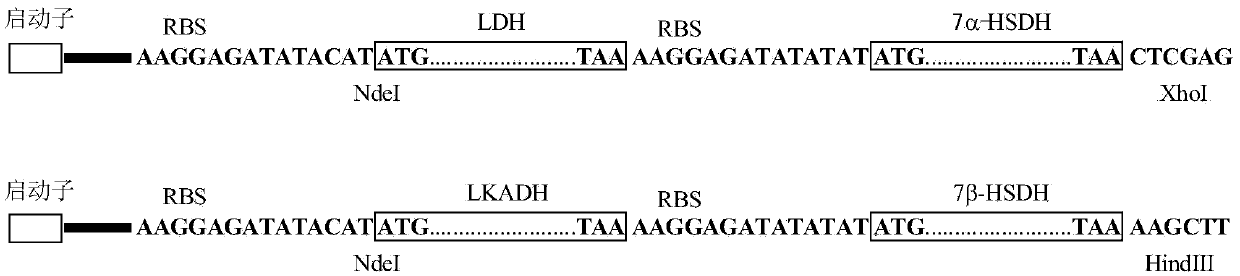
The invention provides a method for catalyzing chenodeoxycholic acids to compound ursodesoxycholic acids through efficient whole-cells. A 7a-hydroxysteroid dehydrogenase (7a-HSDH) and a lactic dehydrogenase (LDH) for regeneration of coenzyme nicotinamide adenine dinucleotide (NAD) are efficiently co-expressed in escherichia coli, escherichia coli whole cells are used to catalyze chenodeoxycholic acids (CDCA) to generate 3 alpha (Alpha)-hydroxyl-7-oxo-5bata (Beta)- cholanic acids (7-KLCA), and a reaction liquid which is obtained by catalyzing the chenodeoxycholic acids through whole cells is adjusted to be 7-KLCA crude products. Reconstitution cells can be easily obtained in low cost through a fermentation process, are better than a chemical synthesis method in production cost and product quality, and are suitable for commercial process.
Owner:苏州天绿生物制药有限公司
Process for preparing 3a(.beta.)-7a(.beta.)-dihydroxy-6a(.beta.)-alkyl-5.beta.-cholanic acid
ActiveUS7994352B2Highly toxic hexamethylenphosphonamideMetabolism disorderSteroidsKetoneMedicinal chemistry
Process for preparing 3α-7α(β)-dihydroxy-6α(β)-alkyl-5β-cholanic acid (I) in which R is a linear or branched C1-C5 alkyl and the relative intermediates 3α-hydroxy-6β-alkyl-7-keto-5β-cholanic (VIII) and 3α-hydroxy-6α-alkyl-7-keto-5β-cholanic (IX).
Owner:INTERCEPT PHARMA INC
Preparation method for 5 beta-3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-cholanic acid
The invention provides a preparation method for 5 beta-3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-cholanic acid. The method comprises the following steps: carrying out silanization on 5 beta-3 alpha-ethoxy carbonyloxy-7-carbonyl-methyl cholanate by silicon haloganide in an aprotic solvent in the presence of strong alkali, producing a mukaiyama aldol reaction, reducing 5 beta-3 alpha-ethoxy carbonyloxy-6-ethylidene-7-carbonyl-methyl cholanate by palladium carbon and hydrogen, and hydrolyzing to obtain 5 beta-3 alpha-hydroxy-6 alpha-ethyl-7-carbonyl-cholanic acid; finally, reducing 5 beta-3 alpha-hydroxy-6 alpha-ethyl-7-carbonyl-cholanic acid by sodium borohydride to obtain 6-ECDCA (6 alpha-ethyl-chenodeoxycholic acid). The raw materials adopted in the method are wide in source; only one-step siloxane protection is required, so that a process is simple; the product yield is high.
Owner:康美(北京)药物研究院有限公司 +2
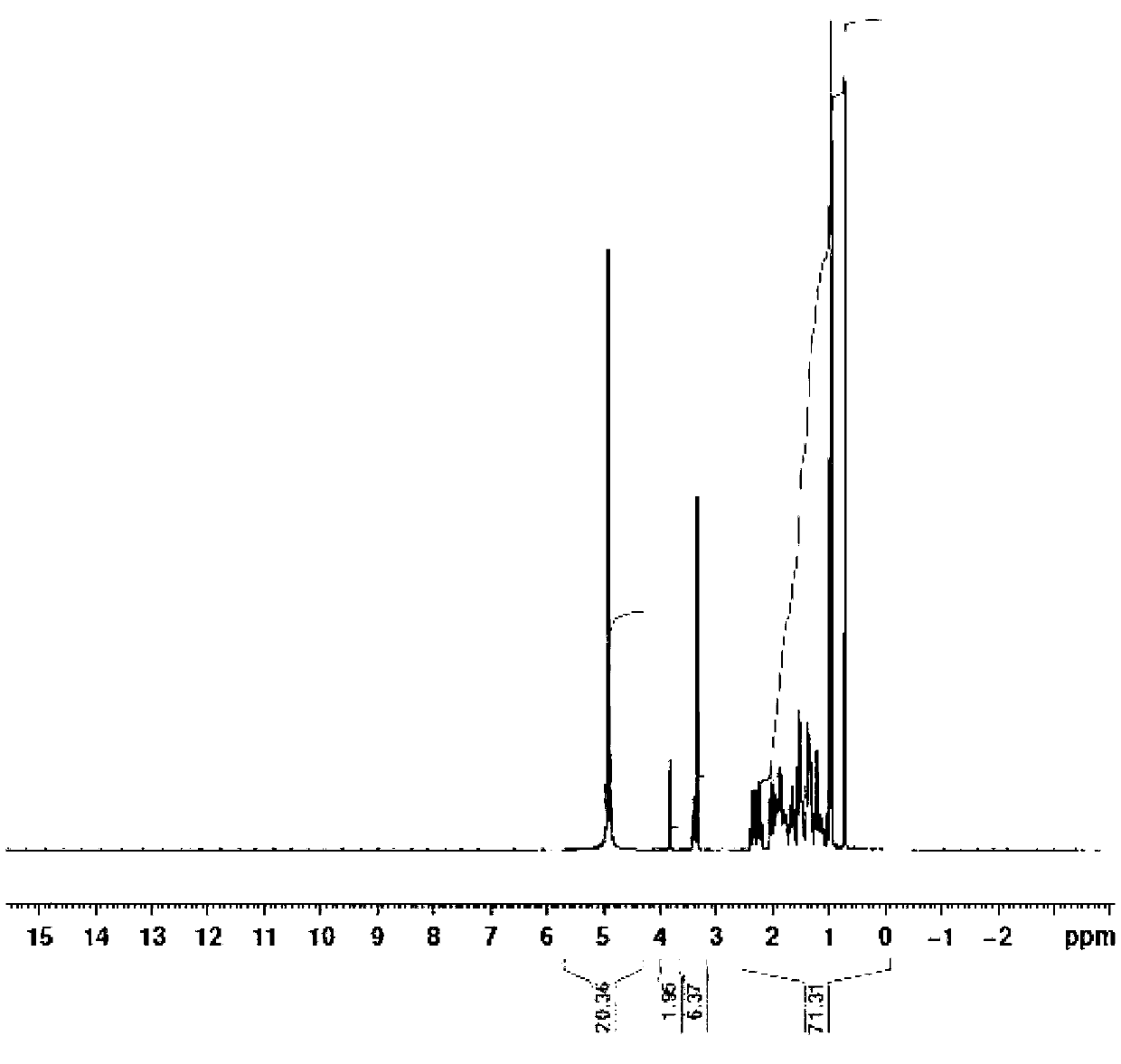
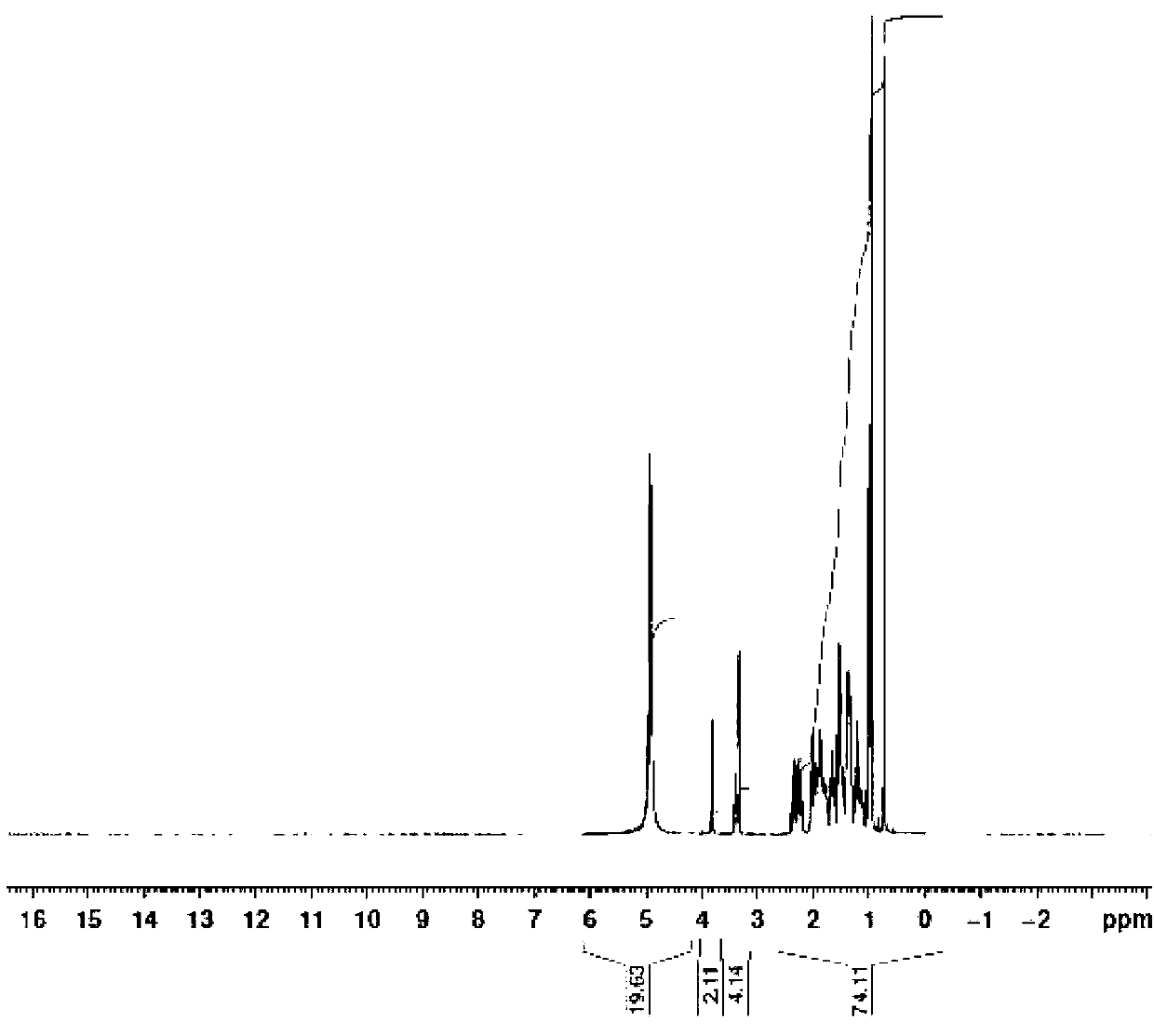
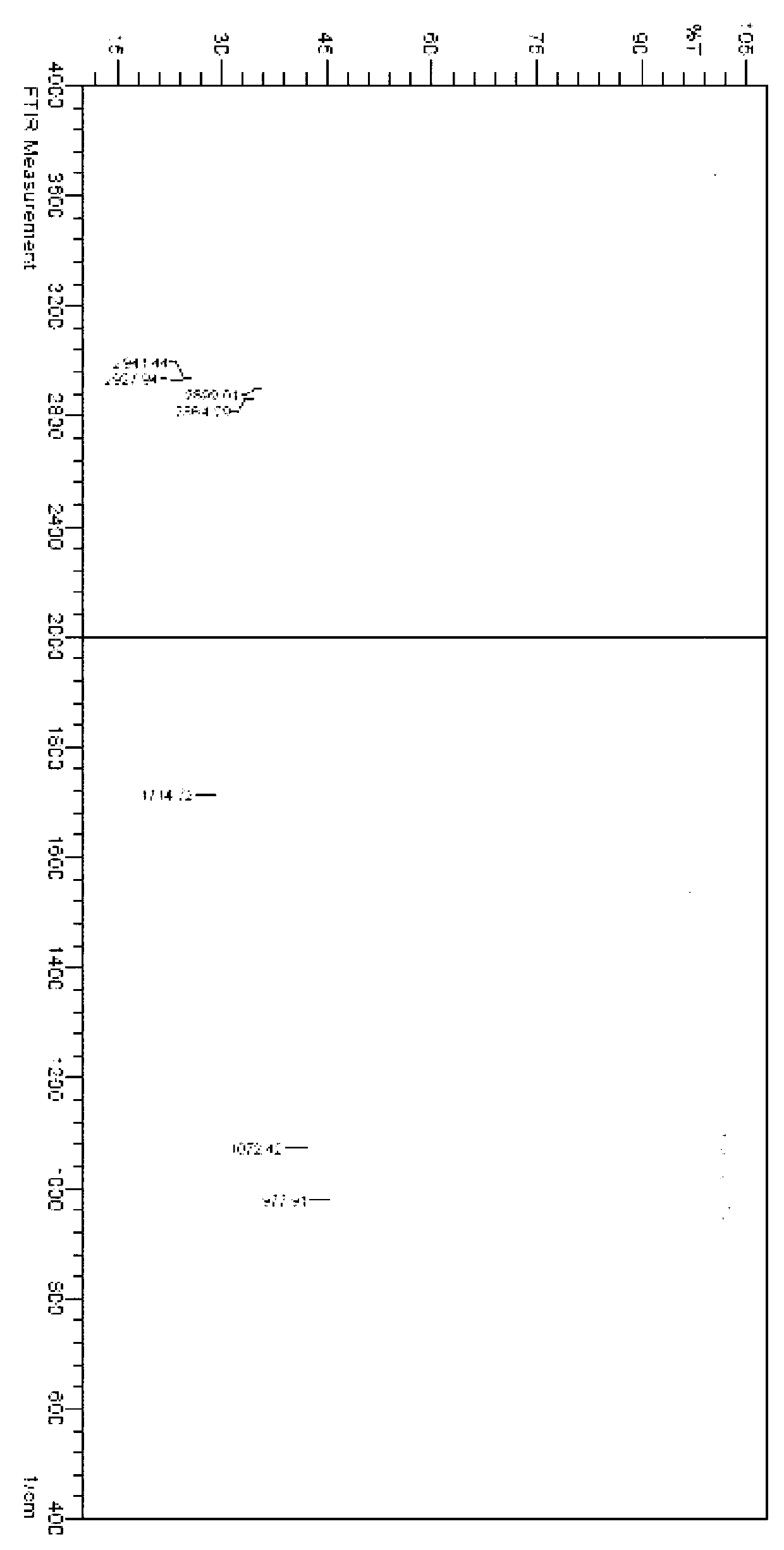
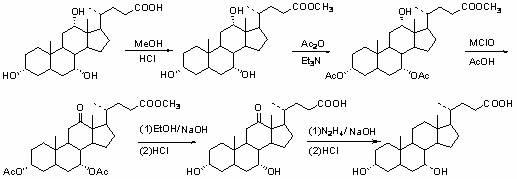
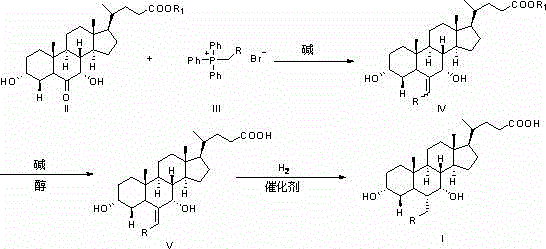
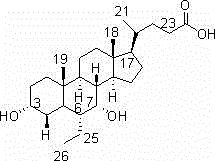
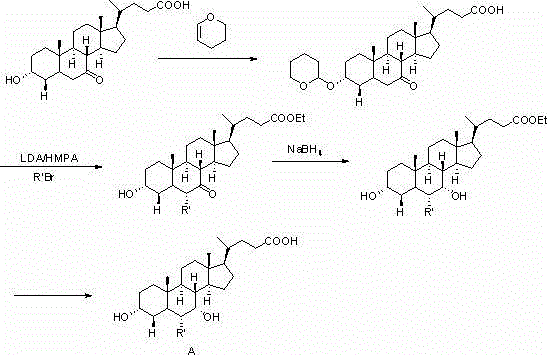
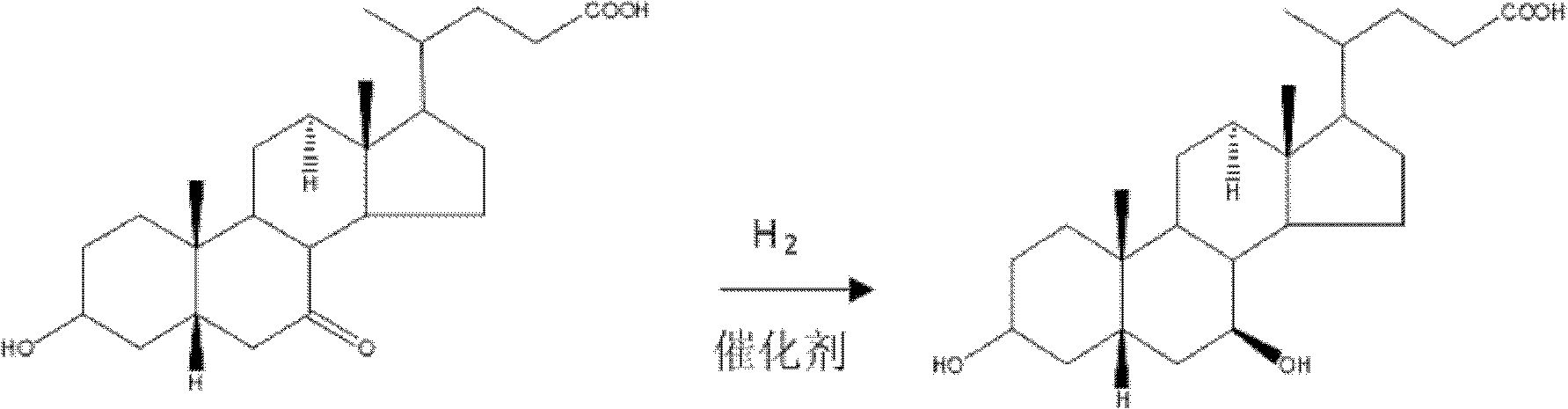
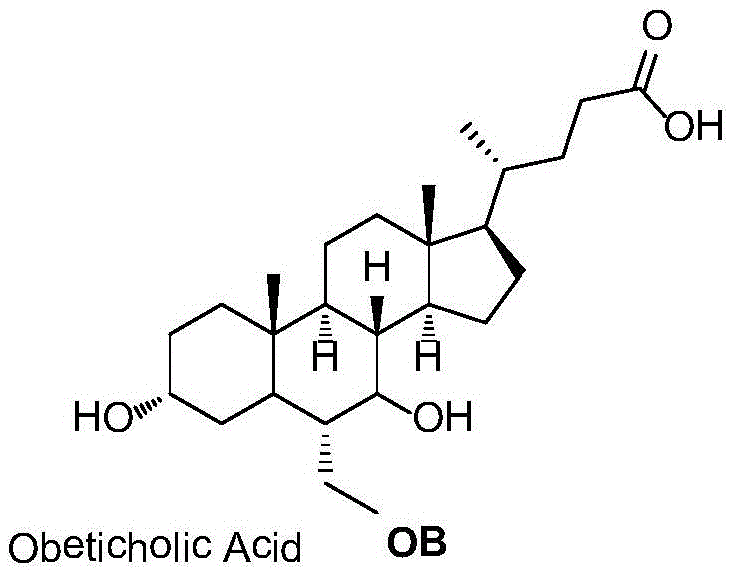
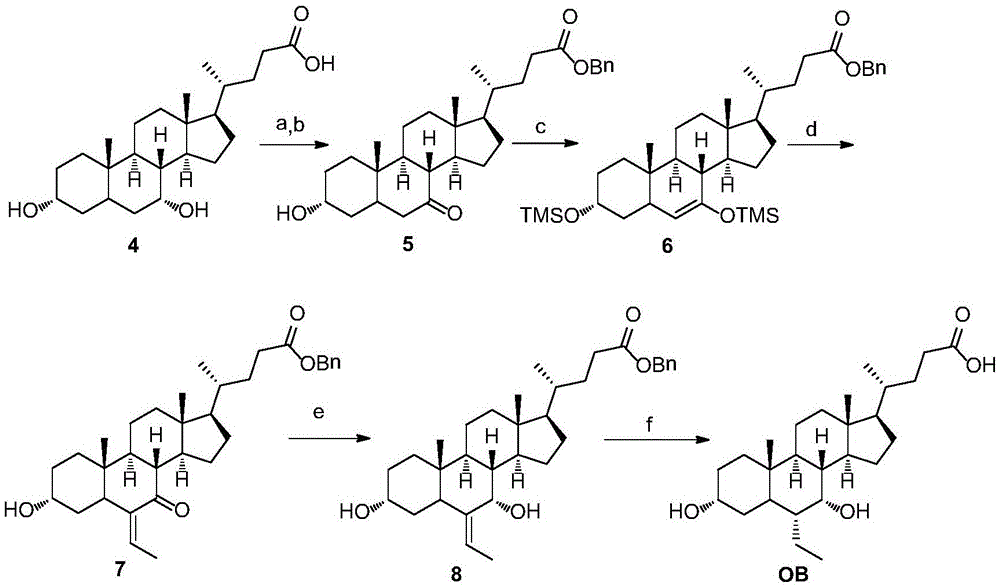
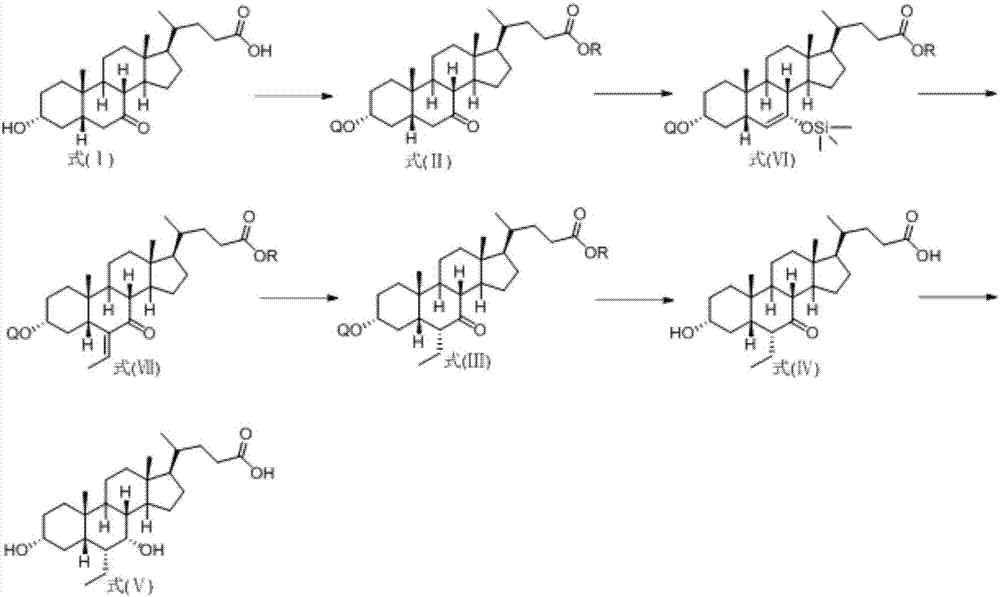
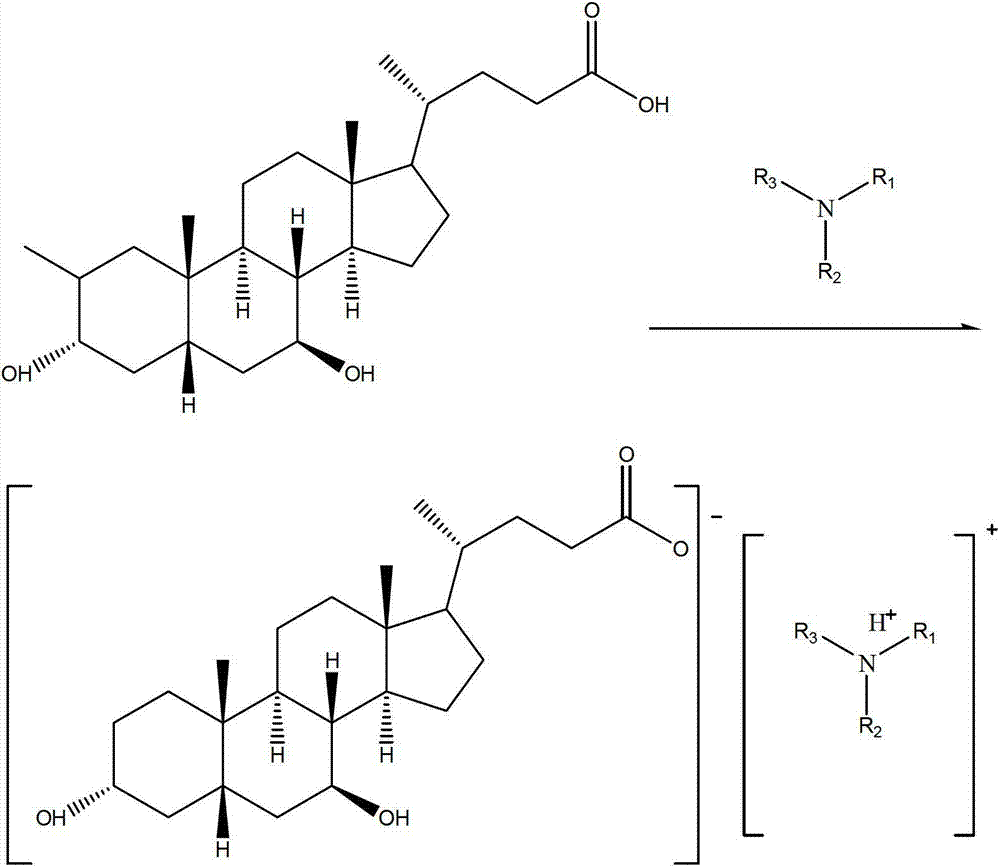
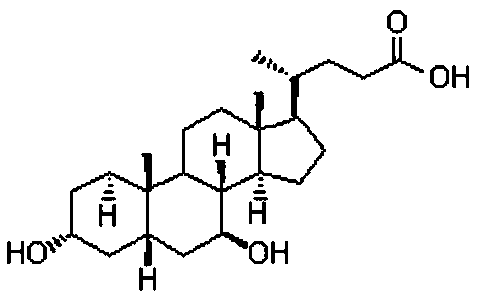
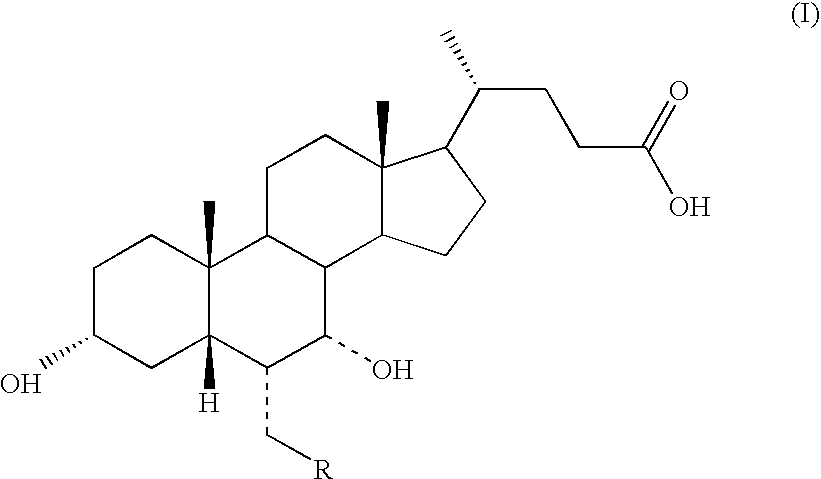
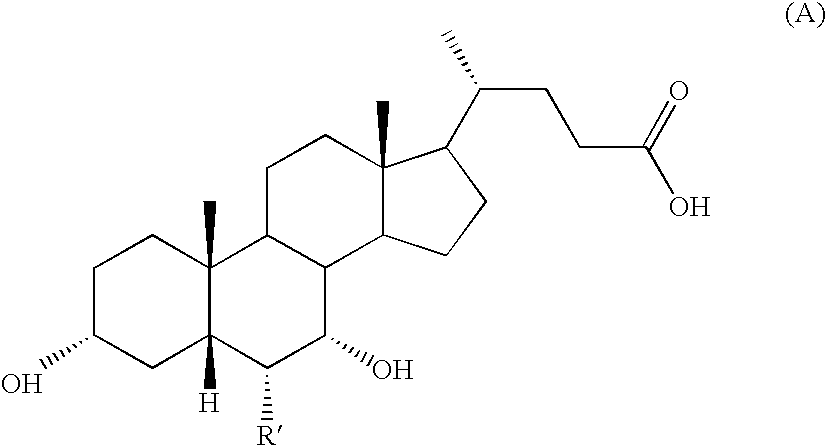
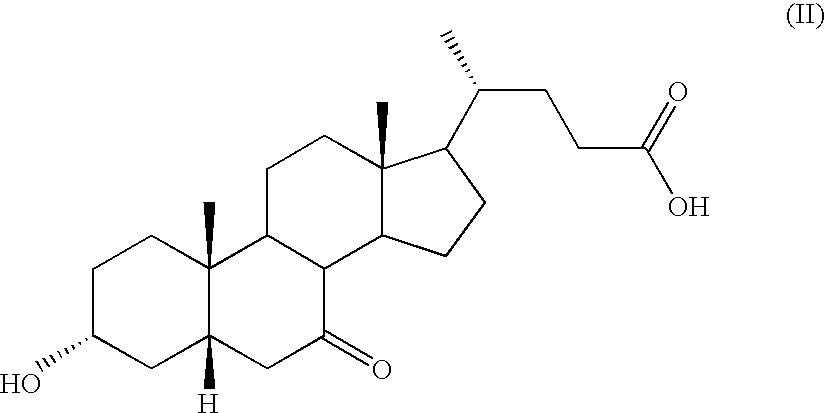
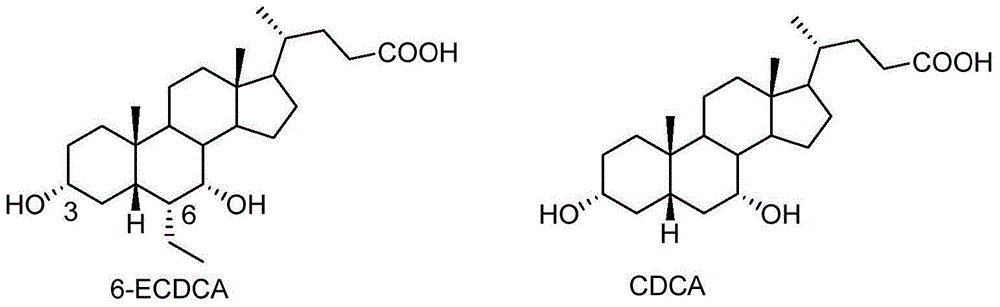
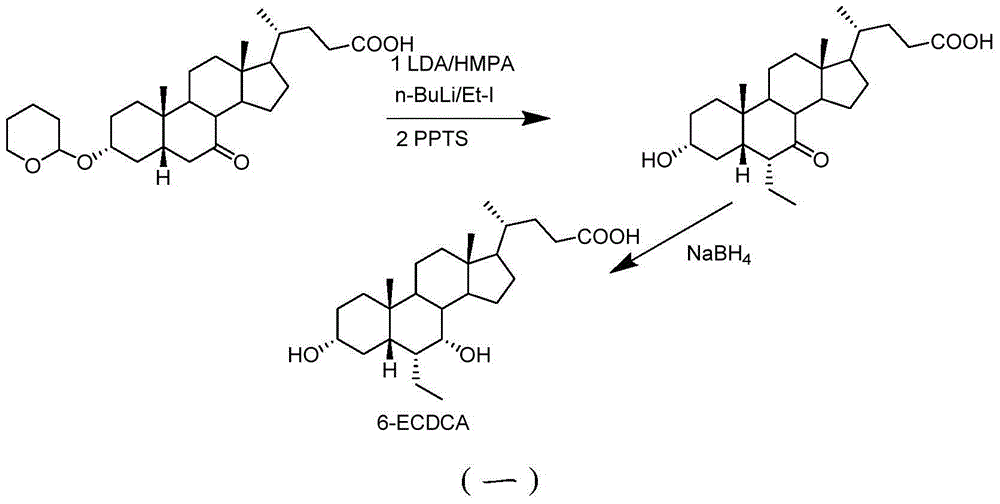
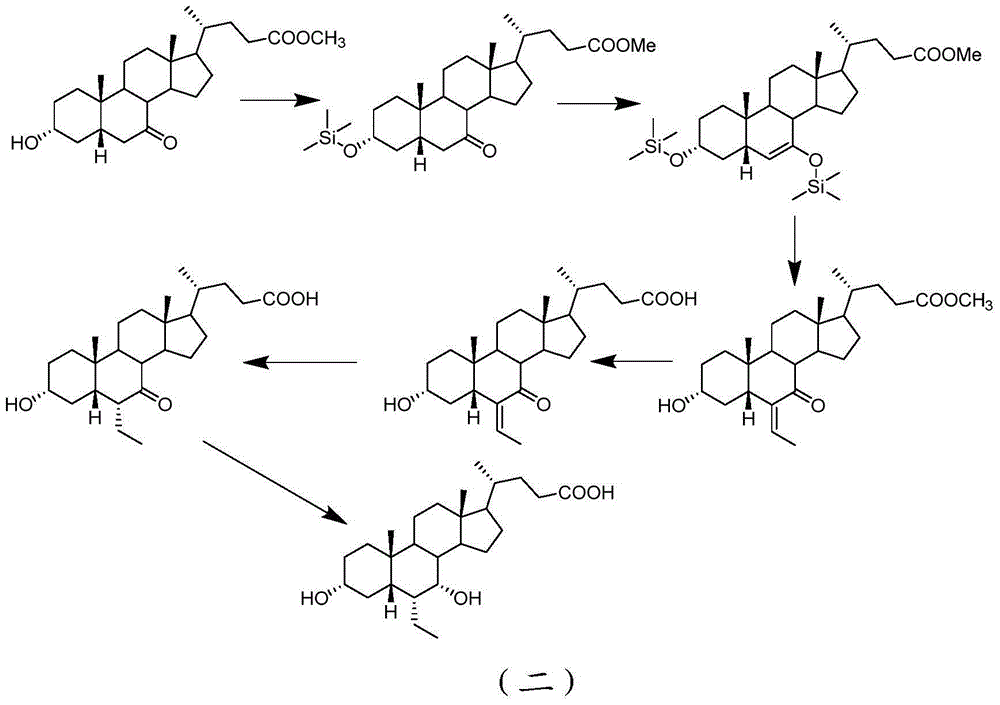
Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine
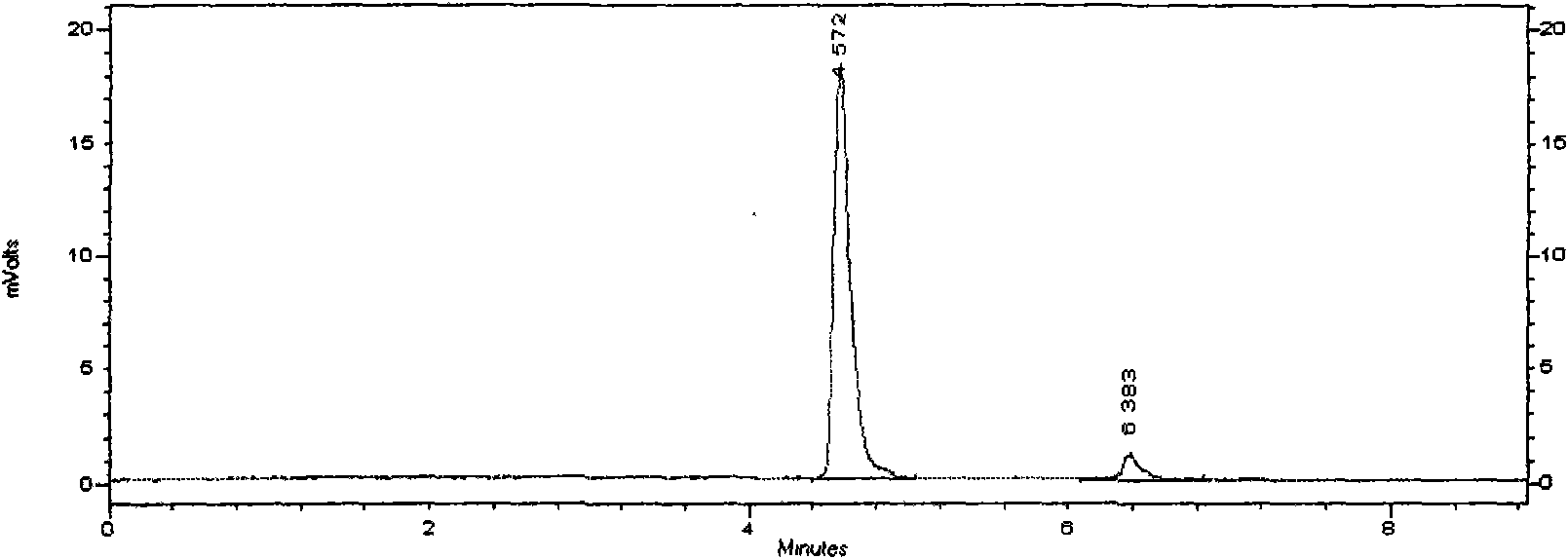
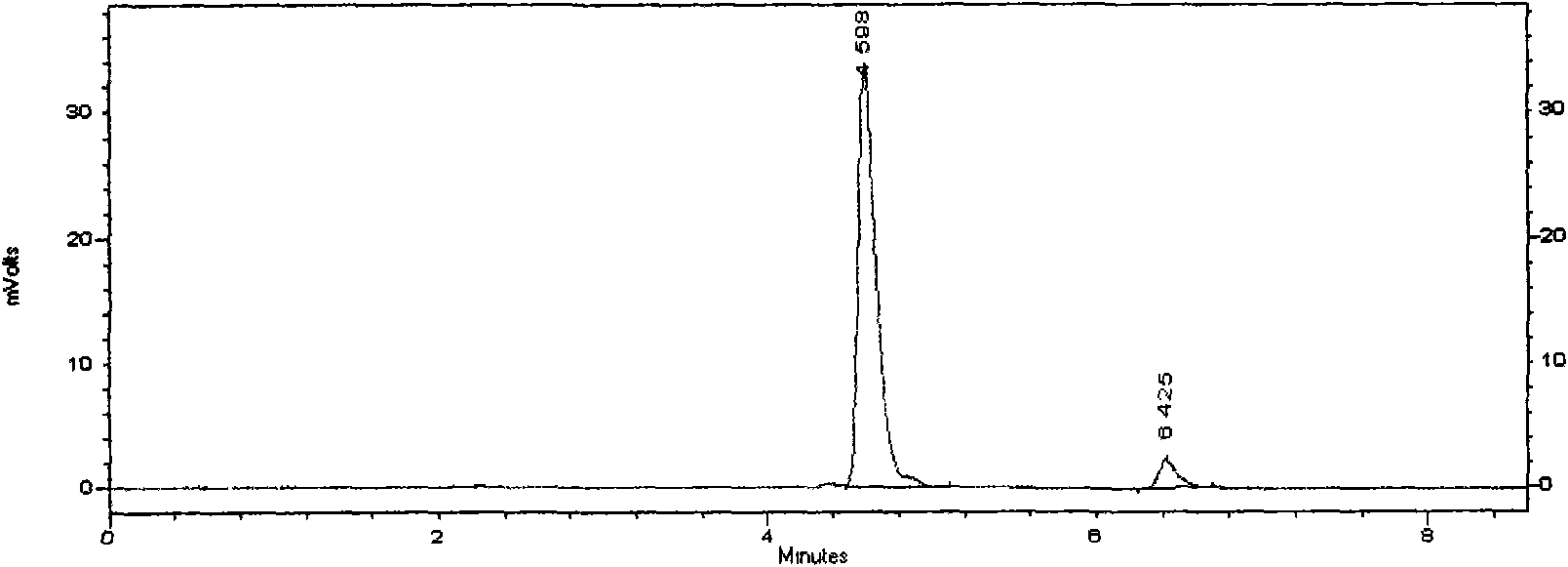
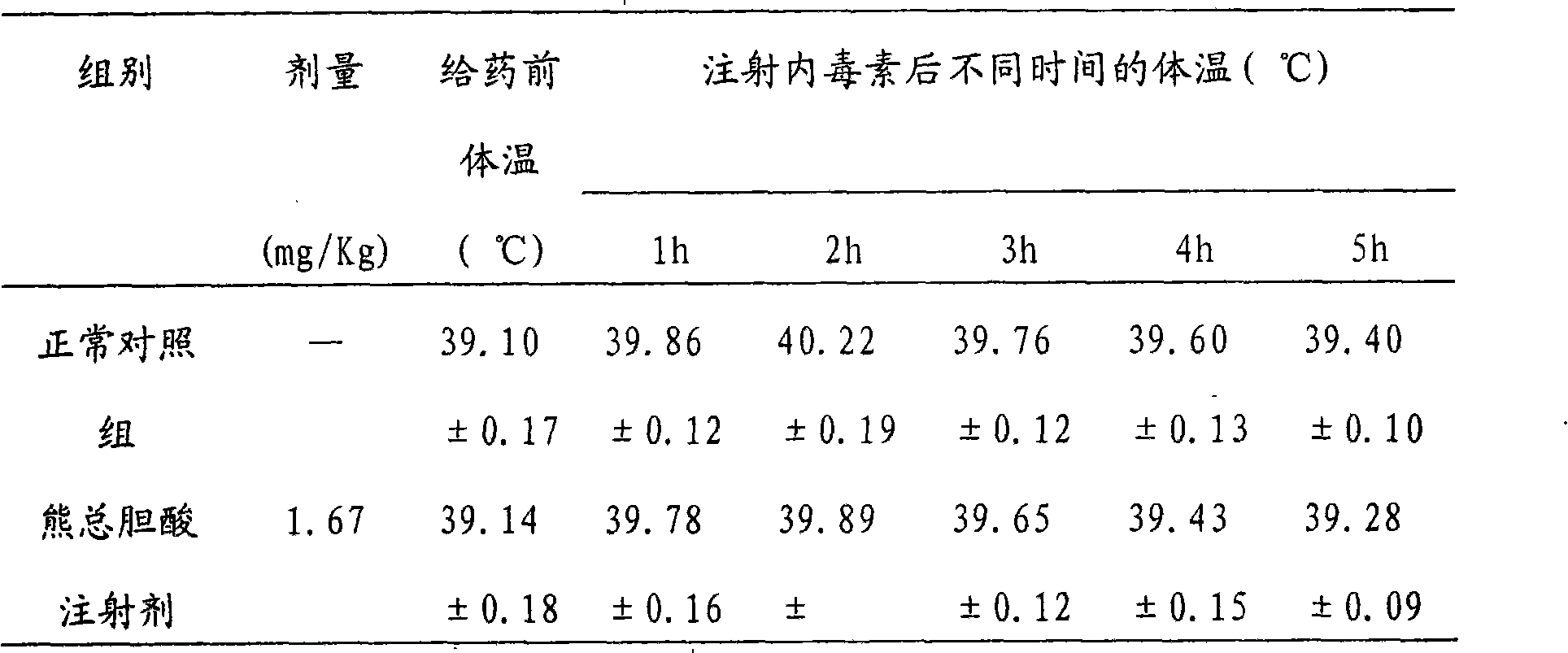
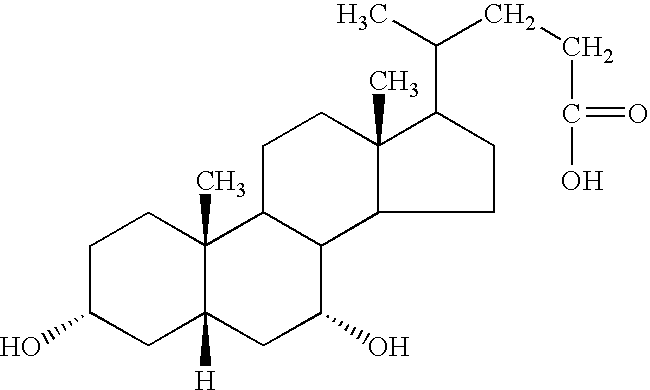
ActiveCN105669811ADifficult to purifyHigh purityMetabolism disorderSilicon compound active ingredientsKetoneMedicinal chemistry
The invention provides a preparation method of a 7-keto-6[alpha]-alkyl cholanic acid derivative. According to the preparation method provided by the invention, a 7-keto-6[beta]-alkyl cholanic acid derivative, as shown by a formula II, is used as a raw material, and the 7-keto-6[alpha]-alkyl cholanic acid derivative is prepared by converting a 6[beta] configuration into a 6[alpha] configuration under an acid or alkali condition. The invention also provides a 7-keto-6[beta]-alkyl cholanic acid derivative and an application thereof in preparation of 3[alpha],7[alpha]-dihydroxy-6[alpha]-alkyl-5[beta]-cholanic acid. The preparation method provided by the invention is simple and convenient, and is high in configuration conversion rate, and the product, the 7-keto-6[alpha]-alkyl cholanic acid derivative, is easy to purify, so that the purification difficulty for preparing the 3[alpha],7[alpha]-dihydroxy-6[alpha]-alkyl-5[beta]-cholanic acid is reduced.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method of ursodeoxycholic acid
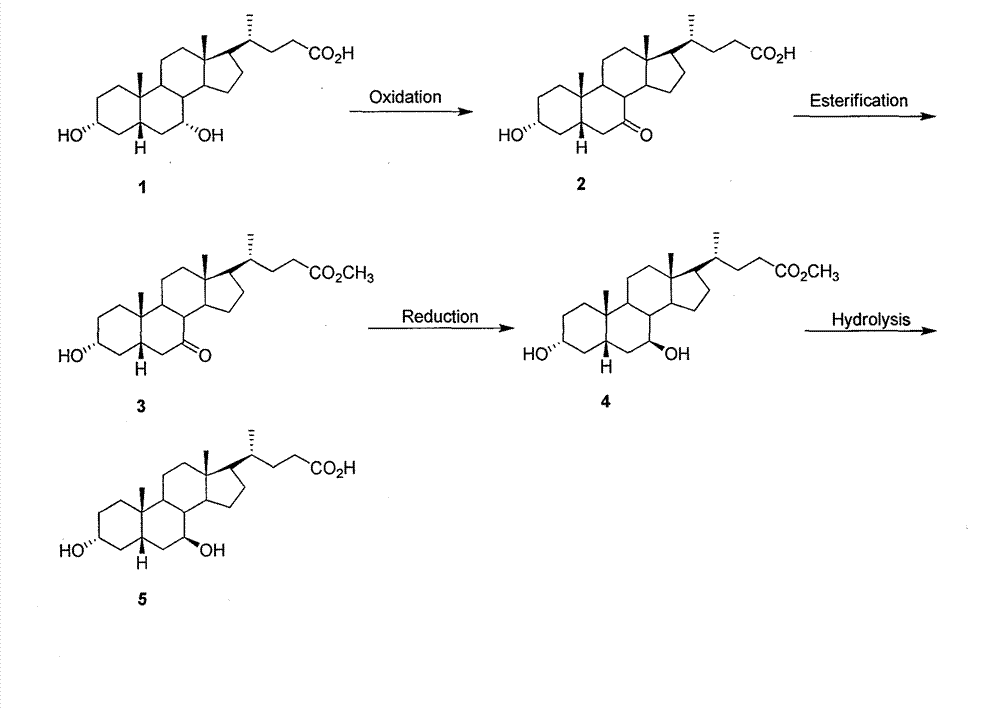
The invention provides a preparation method of ursodeoxycholic acid. Commercial available chenodeoxycholic acid is taken as raw materials, the ursodeoxycholic acid is obtained by four steps including selective oxidation, esterification, deoxidation and hydrolyzation, and the total yield is 85.7%. In a mixture of acetone and water, NBS is used for selective oxidation of hydroxy at C- 7 bit of the chenodeoxycholic acid, and the selective oxidation possesses excellent selectivity and high yield. NaBH14 / CeC13 may be used to deoxidize carbonyl at C-7 bit into hydroxy, and the ratio of alpha / beta is as high as 5 / 95.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing cheodexycholic acid
The invention relates to an industrialized preparing method of extracting and purifying chenodeoxycholic acid from fowl and animal biles. Its process includes the steps of saponifying bile by lye, preparing calcium salt deposit of total bile acid, decolorizing by oxydol and active carbon, converting calcium salt deposit of chenodeoxycholic acid by sodium carbonate and HCl, purifying by the marcoporous absorbing resin, and so on. It has advantages of friend environment, safety and innocuity, low cost and be easy for large-scale operation.
Owner:EAST CHINA UNIV OF SCI & TECH
A chemical-enzyme method of preparing ursodeoxycholic acid
ActiveCN106086149AHigh ee valueEliminate enzyme inactivationSteroidsFermentationChenodeoxycholic acidOxidizing agent
A chemical-enzyme method of preparing ursodeoxycholic acid is disclosed. The method adopts chenodeoxycholic acid as an initial substrate, and prepares the ursodeoxycholic acid through a chemical process and a bio-enzyme process in order, wherein 7-KLCA reductase is adopted as a biological catalyst. A situation that an oxidant residual in a process of preparing 7-ketolithocholic acid through a chemical manner causes later enzyme inactivation in the prior art does not occur. A product prepared by the method is high in ee value and low in comprehensive cost.
Owner:ENZYMEWORKS
Method for preparing high-purity hyodeoxycholic acid by pig bile
InactiveCN101037463AReduce the difficulty of purificationLow impurity contentDigestive systemUnknown materialsBenzeneHyodeoxycholic acid
The invention relates to a method for extracting the hyodeoxycholic acid from the leftovers of pig bile or extracted bilirubin, including saponify the leftovers with alkali, adjusting pH value, acetic ester extraction, decoloring with active carbon; concentrating the mother liquid to a proper volume, precipitating deposit, separating the deposit and drying to get coarse hyodeoxycholic acid; esterifying the coarse product, then doing an addition reaction with benzene, separating the methylhyodeoxycholanate-benzene addition compound; decomposing the addition compound with alkali, adjust pH value, getting the purified deoxycholic acid. The obtained mother liquid can be further extracted to get precious chenodeoxycholic acid. The craft has a lot of material, a low pollution, safety and no poison, a low cost, a high product purity and is suitable for industry production in a large scale.
Owner:JIANGSU UNIV
Separation purification preparation method of chenodeoxycholic acid in pig's bile
InactiveCN1869044ASimple and fast operationLow costUnknown materialsSteroidsCholic acidChenodeoxycholic acid
A process for separating the chenodeoxycholic acid from pig's gall and purifying it includes such steps as preparing general cholic acid from the mother liquid generated by extracting the cholerythrin from pig's gall, saponifying, regulating pH value to obtain crude chenodeoxycholic acid, decoloring, defatting, preparing the deposit of barium chenodeoxycholate, reacting on potassium carbonate to remove Ba, regulating pH value, and purifying by silicon gel column.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
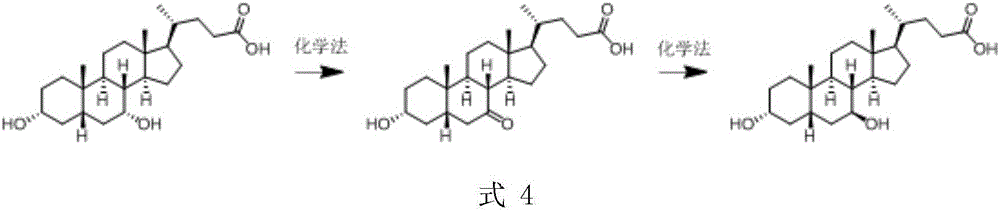
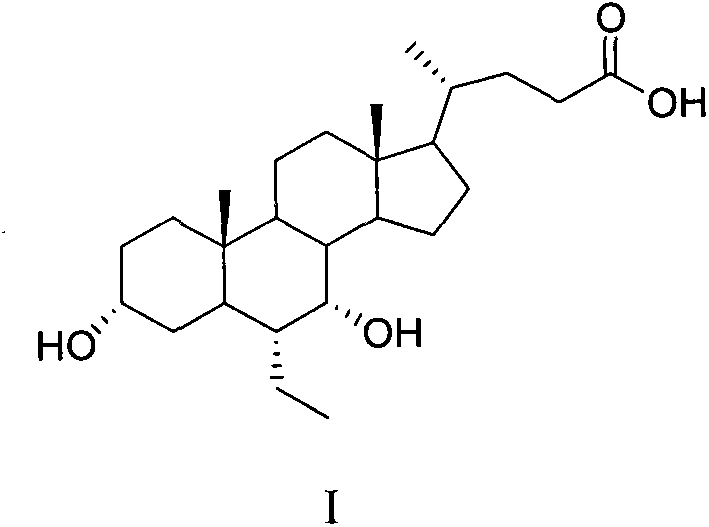
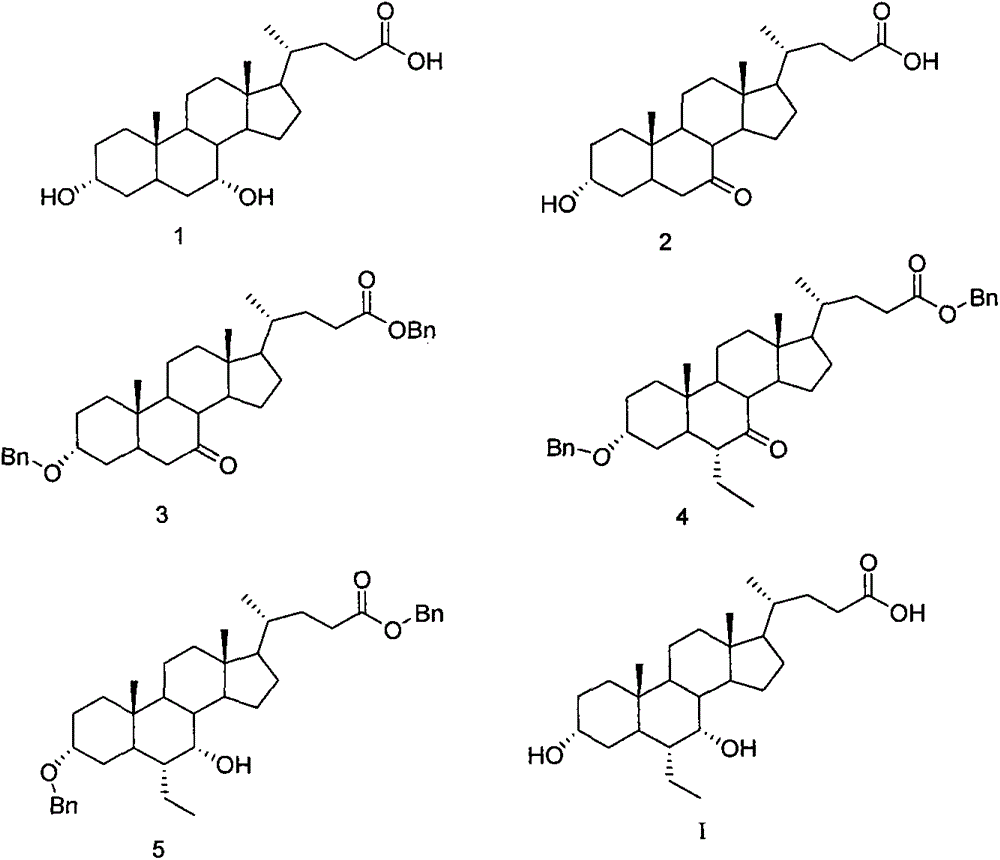
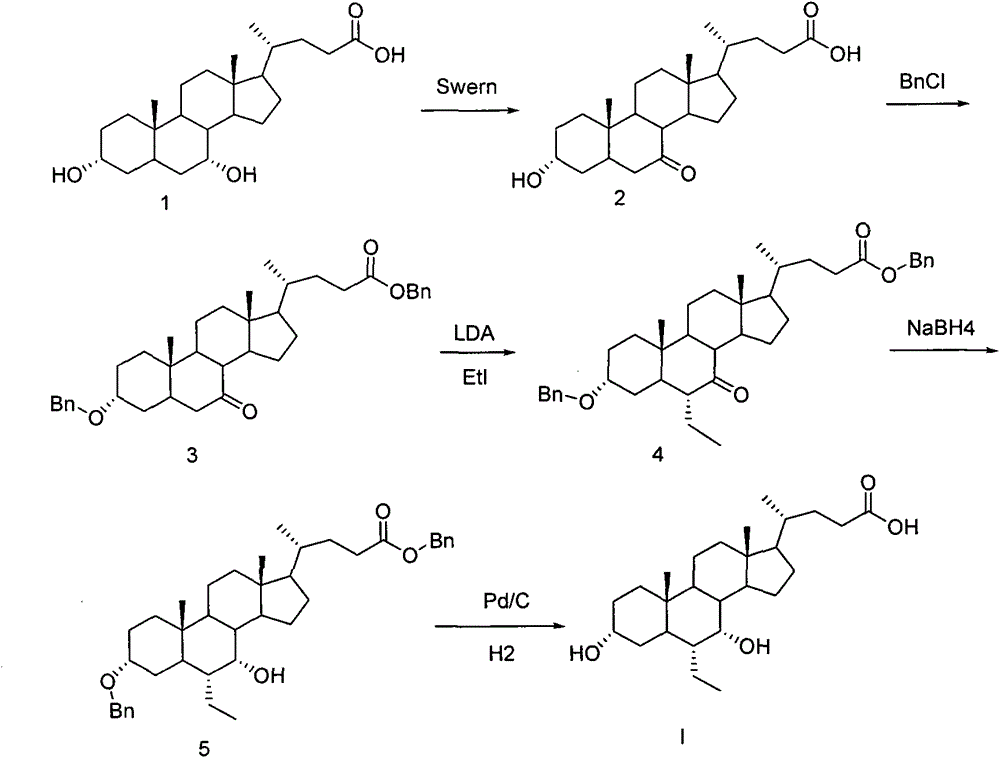
A preparing method of a chenodeoxycholic acid derivative
The invention relates to a preparing method of a chenodeoxycholic acid derivative, and particularly relates to a preparing method of a compound shown as a formula I. The method includes (1) subjecting a compound shown as a formula 1 to Swern oxidation to obtain a compound shown as a formula 2; (2) subjecting the compound shown as the formula 2 to hydroxy protection to obtain a compound shown as a formula 3; (3) bringing the compound shown as the formula 3 into contact with iodoethane to obtain a compound shown as a formula 4; (4) subjecting the compound shown as the formula 4 to a reduction reaction to obtain a compound shown as a formula 5; and (5) bringing the compound shown as the formula 5 into contact with hydrogen under the existence of palladium / carbon that is a catalyst to obtain the compound shown as the formula I. The method is short in steps, simple in operation, and suitable for industrial production, and raw materials are easily available.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
Total bile acid extract of bear bile powder and preparation method and application of injection thereof
ActiveCN101890048AAdd extraction stepEfficient removalAntibacterial agentsDigestive systemChenodeoxycholic acidAlkaline hydrolysis
The invention relates to a total bile acid extract of bear bile powder and a preparation method and application of injection thereof. The total bile acid extract of the bear bile powder is prepared by the following steps of: performing reflux extraction on the bear bile powder by using ethanol; recovering the ethanol from extracting solution, and concentrating the extracting solution; and performing alkaline hydrolysis, neutralization, acidification, ethyl acetate extraction, ethyl acetate crystallization and ethanol crystallization. The invention also provides a method for preparing the injection from the total bile acid extract of the bear bile powder. The prepared total bile acid extract of the bear bile powder and the injection thereof can be used for preparing medicaments for resisting bacteria and viruses, relieving cough and reducing sputum and protecting liver and gallbladder. The invention can improve the extraction yield and purity of ursodesoxycholic acid in the total bile acid extract of the bear bile powder and reduce the chenodeoxycholic acid content simultaneously, so the ursodesoxycholic acid content in the total bile acid extract of the bear bile powder is no less than 70 percent and the chenodeoxycholic acid content is no more than 20 percent. The ursodesoxycholic acid content in the total bile acid extract of the bear bile powder is higher, and the ursodesoxycholic acid yield is high and is over 18 percent generally.
Owner:SHANGHAI KAIBAO PHARMA
Use of chenodeoxycholic acid for reducing adipose tissue
Use of chenodeoxycholic acid is disclosed to reduce adipose tissue and thereby reduce weight in mammals. The chenodeoxycholic acid can be administered orally through the use of a tablet, pill, capsule or liquid suspension.
Owner:DOX
Method for preparing chenodeoxycholic acid in pig bile by esterification method
InactiveCN102372757AEasy to prepareHigh extraction ratioSteroidsAcetic anhydrideChenodeoxycholic acid
The invention discloses a method for preparing chenodeoxycholic acid in pig bile by an esterification method, which comprises the following steps that: methanol, concentrated sulfuric acid and sodium hydroxide are used for esterification; acetic anhydride is used for acidylation; sherwood oil is used for extraction; the primary crystallization and the secondary crystallization are realized through ethanol and the sherwood oil; the sodium hydroxide is used for hydrolytic acidification; ethyl acetate and active carbon are used for crystallization; and the chenodeoxycholic acid is obtained through drying. The chenodeoxycholic acid is prepared by the method disclosed by the invention, the preparation method is simple, safety and environment protection are realized, the method can be used for large-scale industrial production, the content of the extracted chenodeoxycholic acid is higher or equal to 98 percent, the extraction rate is high, and the purity requirement on products in markets can be met.
Owner:ANHUI KEBAO BIOLOGICAL ENG CO LTD
Method for separating and purifying chenodeoxycholic acid from duck gall
The invention provides a method for separating and purifying chenodeoxycholic acid from duck gall, and concretely comprises to the following steps: dissolving and extracting a duck gall paste crude product, extracting ethyl acetate, and ultrafiltering and removing impurities. The method has the following advantages that 1) the raw material source of chenodeoxycholic acid can be enlarged, the method for separating and purifying chenodeoxycholic acid from the duck gall is provided; and 2) the content of chenodeoxycholic acid extracted from the duck gall by a traditional method is low with about 60%. The HPLC content of chenodeoxycholic acid obtained by using the method of the invention can reach more than 96%, and the extraction efficiency of chenodeoxycholic acid is enhanced.
Owner:苏州天绿生物制药有限公司 +1
Chenodeoxycholic acid synthesis method
The invention relates to a chenodeoxycholic acid (3 alpha, 7 alpha-dihydroxyl-5 belta-cholestane-24-acid) chemical synthesis method, belonging to the field of organic chemical synthesis. Chenodeoxycholic acid is prepared by the following steps of: (1) preparing cholate; (2) preparing 3 alpha, 7 alpha-acetyl-12 alpha-hydroxy cholate; (3) preparing 3 alpha, 7 alpha-acetyl-12-oxo-chenodeoxycholic acid ester; (4) preparing 12-oxo-chenodeoxycholic acid; and (5) preparing chenodeoxycholic acid. The application of the method for synthesizing chenodeoxycholic acid has the advantages of high yield rate, low cost and no pollution and is particularly convenient for industrial production.
Owner:ZHENGZHOU UNIV
Method for extracting chenodeoxycholic acid from bile of fowl
InactiveCN101948496AQuality is not affectedImprove versatilitySteroidsCompound aChenodeoxycholic acid
A method for extracting chenodeoxycholic acid from bile of fowl comprises the following steps of: acquiring rough bile acid or rough bile acid calcium salt from fresh or frozen bile of fowl through saponification reaction, heating the bile acid resin solution together with the aqueous solution of organic nitrogenous compound A, removing the majority of hydrophilic impurities from the rough bile acid by using the organic nitrogenous compound A, reacting the acquired bile acid resin solution with organic nitrogenous compound B, dissolving the sediment, filtering, decolorizing and refining, so as to acquire chenodeoxycholic acid with a purity about 95%. The method of the invention has simple operation, the generality of the production equipment is high, and the chemic matter is harmless, therefore the invention is fit for industrialization of chenodeoxycholic acid.
Owner:HUNAN CREDIT CHEM
Method for preparing chenodeoxycholic acid
The invention relates to a method of distilling the chenodeoxychoilc acid from the offcuts which be gained by distilling the bilirubin from the bile of the pig. With the offcuts of the alkali saponification, the deposit is treated with the oxidation treatment by means of the hydrogen peroxide, the filtrate is treated by the dilute sulfuric acid and decolored by means of the active carbon and solved, crystaled using the ethyl acetate again and again; the coordinate production of the hyodeoxycholic acid is purified, the mother liquor is concentrated to the cream and melted using the alkali and have the salifying treatment with the chloride of barium, so the cholate is formed, the cholate is treated to take off barium when the cholate suspending the aqueous solution containing the sodium carbonate, then it is acidificated with the dilute sulfuric acid and is separated ,deposited and dried to gain the crude chenodeoxycholic acid which is decolored using the active carbon and is melted, rimed and dried using the ethyl acetate. Then the finished production of the chenodeoxycholic acid form.The whole technics has some merits of the abundant material, the amity of the environment, the safety and innocuity, producing many outputs from the single stuff and easy to the mass production. The invention is used for distilling the chenodeoxycholic acid.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Method for preparing chenodeoxycholic acid analogue
ActiveCN105777835AEasy to operateAvoid ultra-low temperature reactionsSteroidsChenodeoxycholic acidPurification methods
The invention discloses a new method for preparing 3 alpha, 7 alpha-dihydroxy-6 alpha-alkyl-5 beta-cholanic acid I and an intermediate thereof. The method requires no low-temperature reaction; reaction conditions are mild; a purification method is simple; and the method of the invention is suitable for industrial production.
Owner:YAOPHARMA CO LTD +1
Method for preparing ursodesoxycholic acid by chiral catalytic hydrogenation of 7-ketodesoxycholic acid
InactiveCN102070693AReduce generationReduced purification stepsSteroidsChenodeoxycholic acidDistillation
The invention discloses a method for preparing an ursodesoxycholic acid by the chiral catalytic hydrogenation of a 7-ketodesoxycholic acid, which is characterized by comprising the following steps of: performing oxidation to prepare the 7-ketodesoxycholic acid from a chenodeoxycholic acid serving as an initiative raw material by using a common method; dissolving the 7-ketodesoxycholic acid into a solvent, adding a chiral catalyst, maintaining the pressure of 0 to 20 MPa under alkali condition, introducing nitrogen to perform hydrogenation reduction reaction at 10 to 80 DEG C, and performing distillation after the reaction is finished to remove the solvent; adding purified water in a volume which is 10 to 100 times that of a hydrogenation reduction reaction product, and adding acid liquor to crystallize the hydrogenation reduction reaction product; and separating solids from liquid, and performing washing and drying to obtain solid powder which is the ursodesoxycholic acid. The method for preparing the ursodesoxycholic acid by the chiral catalytic hydrogenation of the 7-ketodesoxycholic acid aims to overcome the shortcomings of the prior art, and ensures a short production flow, high yield and high quality.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Preparation method for obeticholic acid and intermediate thereof
The invention relates to a preparation method for obeticholic acid and an intermediate thereof. The intermediate 3alpha-hydroxy-6alpha-ethyl-7-keto-5beta-cholanic acid is obtained by subjecting 3alpha-hydroxy-6-ethylidene-7-keto-5beta-cholanic acid benzyl ester compounds to a reaction under the action of a catalyst and a hydrogen donor. The catalyst is selected from Pd / C or PtO2 or Raney Ni. The hydrogen donor is selected from cyclohexene or cyclohexadiene or tetrahydronaphthalene. According to the method, the yield is high, the stereoselectivity is high, safety is good, the reaction condition is mild, and the method is applicable to industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
Method for preparing obeticholic acid from new derivative of 3alpha-hydroxy-7-oxo-5beta-cholanic acid
The invention discloses a method for preparing obeticholic acid from a new derivative of 3alpha-hydroxy-7-oxo-5beta-cholanic acid. The synthesis method comprises the following steps: firstly, carrying out hydroxyl and carboxyl protection on the 3alpha-hydroxy-7-oxo-5beta-cholanic acid to prepare the corresponding new derivative; secondly, obtaining the obeticholic acid respectively according to two synthesis routes. According to the method disclosed by the invention, a safer protecting group reagent is utilized, and the problem that ultraviolet absorption of an intermediate is not strong is solved; the intermediate is easier to purify, the yield is improved, and the cost is reduced, so that the method is more suitable for industrialized amplification, and has remarkable creativity and actual application value.
Owner:HANGZHOU HEZE PHARMA TECH
Method for respectively recovering ursodesoxycholic acid and chenodeoxycholic acid from ursodesoxycholic acid waste mother liquor
ActiveCN102766185AEasy to operateMild reaction conditionsSteroidsOrganic solventChenodeoxycholic acid
The invention relates to a method for respectively recovering ursodesoxycholic acid and chenodeoxycholic acid from ursodesoxycholic acid waste mother liquor. Firstly waste mother liquor is dissolved in an inorganic base solution, and inorganic acid is added to form mixed sediment containing the ursodesoxycholic acid and the chenodeoxycholic acid; secondly, an organic solvent and an organic amine are added to separate ursodesoxycholic acid ammonium salt; and thirdly the ursodesoxycholic acid ammonium salt is acidized to recover the ursodesoxycholic acid, chenodeoxycholic acid ammonium salt left in the organic solvent is acidized and filtered to recover the chenodeoxycholic acid. The recovering method can effectively utilize the ursodesoxycholic acid waste mother liquor, recovers the ursodesoxycholic acid and the chenodeoxycholic acid contained in the ursodesoxycholic acid waste mother liquor, and is high in recover efficiency and product purity. The recovering method is simple to operate and mild in reaction conditions, used reagent is low in cost and wide in source, and the method is suitable for large-scale industrialized production.
Owner:苏州天绿生物制药有限公司 +1
Preparation method of ursodesoxycholic acid and enzyme for preparation
ActiveCN106636285AImprove conversion rateEasy to operateOxidoreductasesFermentationPhotochemistryUrsodesoxycholic acid
The invention relates to a method for preparing an ursodesoxycholic acid by using a biological enzyme catalysis technology and 7beta-hydroxysteroid dehydrogenase for preparation of the ursodesoxycholic acid. The method comprises the following steps: adopting a 3alpha-hydroxyl -7-oxo-5beta-cholanic acid as a substrate and catalyzing the 3alpha-hydroxyl -7-oxo-5beta-cholanic acid by using the 7beta-hydroxysteroid dehydrogenase to prepare the ursodesoxycholic acid in the presence of an NADP, glucose, glucose dehydrogenase and a buffer solution, wherein the 7beta-hydroxysteroid dehydrogenase is from Turneriella parva. The method is simple in operation, short in reaction time, the reaction conditions are mild and easy to control, the conversion rate of the substrate can reach 99.8% and the content of the obtained product is greater than 98.5%.
Owner:眉山市新功生物科技有限公司 +1
7beta-hydroxysterol dehydrogenase mutant and application of 7beta-hydroxysterol dehydrogenase mutant in ursodeoxycholic acid synthesis
ActiveCN107099516AEasy to separate and extractOvercoming the problem of inactivation processingOxidoreductasesGenetic engineeringChenodeoxycholic acidSubstrate concentration
The invention discloses a 7beta-hydroxysterol dehydrogenase mutant with increased activity and stability which is obtained through molecular evolution, recombinant expression plasmid containing the 7beta-hydroxysterol dehydrogenase mutant gene and a recombinant expression transformant and a preparation method of a recombinant mutant enzyme preparation, and the invention also provides an application of the recombinant mutant enzyme preparation in ursodeoxycholic acid synthesis. The 7beta-hydroxysterol dehydrogenase has excellent activity and heat stability, can efficiently catalyze asymmetric reduction of 7-carbonyl lithocholic acid to prepare the ursodeoxycholic acid; the 7beta-hydroxysterol dehydrogenase is subjected to immobilization and then is subjected to couple by an enzyme method with the immobilized 7beta-hydroxysterol dehydrogenase, epimerization of a substrate chenodeoxycholic acid with low cost can be directly catalyzed, ursodeoxycholic acid can be prepared through continuous conversion, and the operation is simple. Compared with the prior art reported currently, ursodeoxycholic acid prepared by hydroxysterol dehydrogenase through catalysis has the advantages of high substrate concentration, short reaction time, complete reaction, and high product purity, and has strong industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH +1
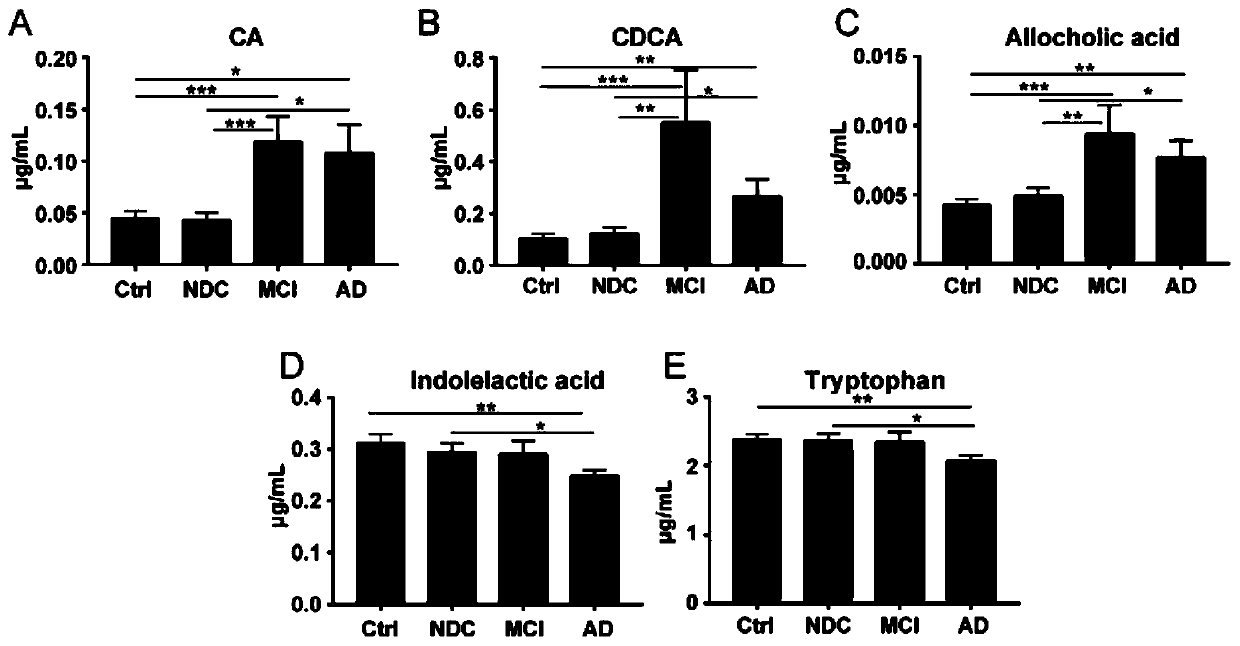
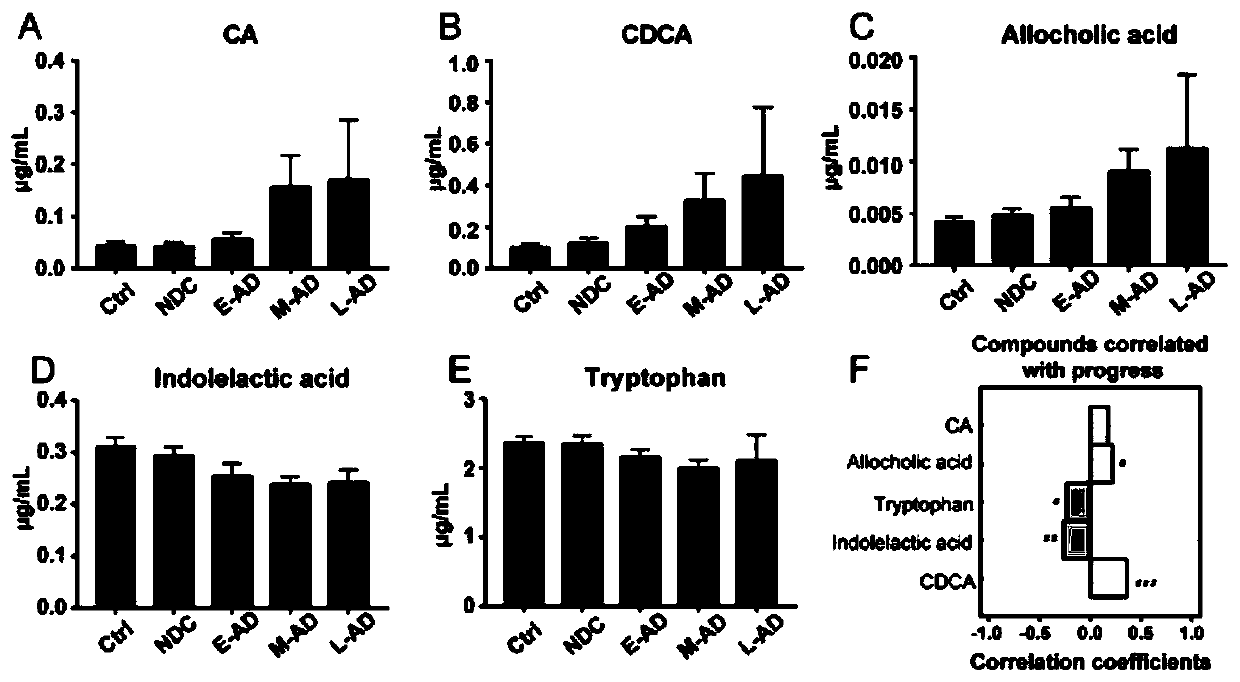
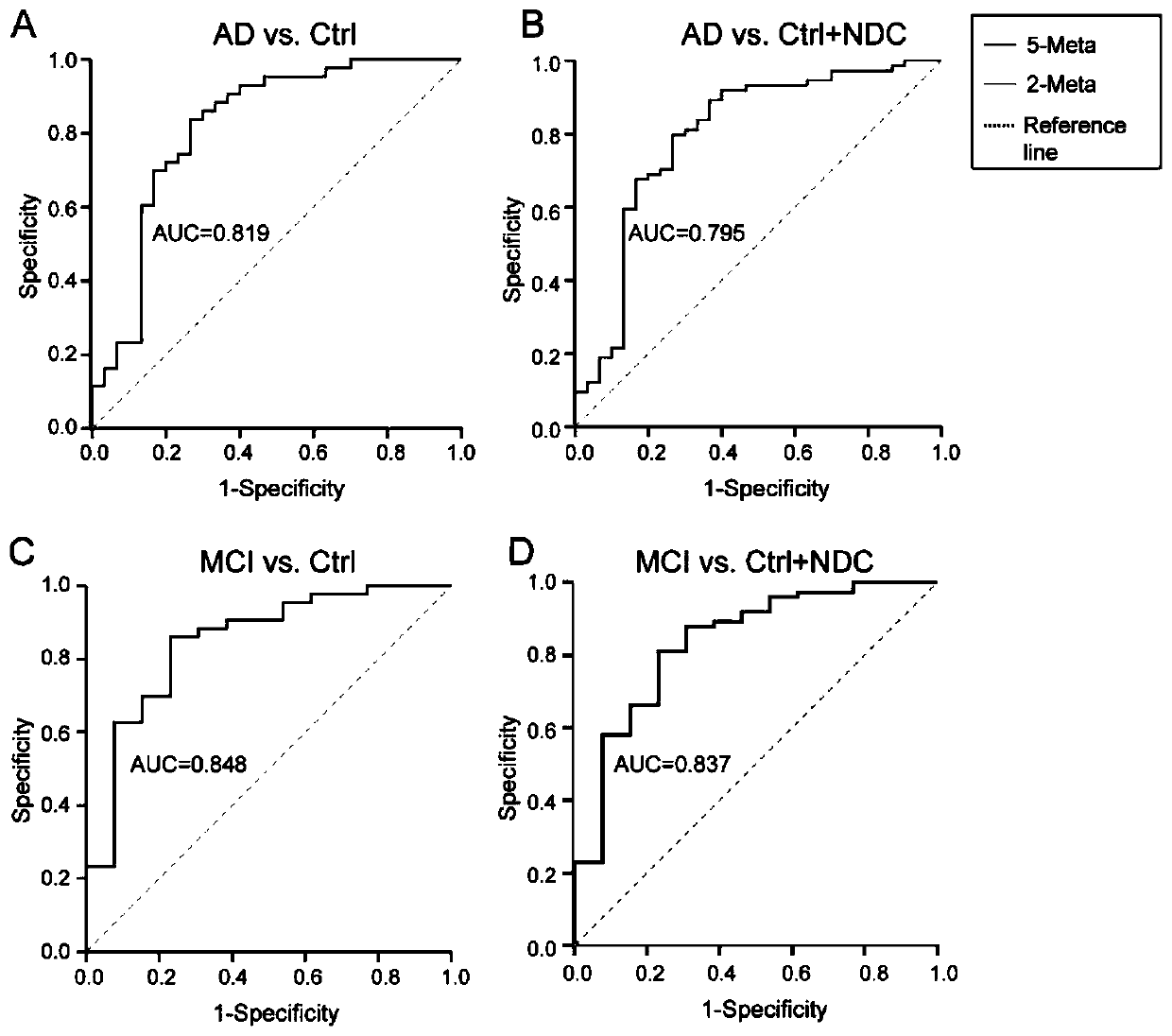
A set of biomarkers for diagnosing AD in a subject or determining a risk of the AD in the subject and an application thereof
The invention relates to a set of biomarkers for diagnosing AD in a subject or determining a risk of the AD in the subject. Diagnostic markers are a cholic acid, a chenodeoxycholic acid, an allocholicacid, a benzpyrole-3-lactic acid and tryptophan. The invention also provides the application of the above diagnostic markers in preparation of a differential diagnostic reagent for an Alzheimer's disease and a kit. Plasma fingerprint spectrum analysis of AD patients, MCI patients, Ctrl and NDC populations is performed through a plasma sample collection-plasma sample pretreatment-ultra-high performance liquid chromatography and mass spectrometry analysis method, and contents of the above five diagnostic markers are detected so that the markers are applied to preparation of products related todiagnosis of the Alzheimer's disease, and therapeutic evaluation. The markers can evaluate an early phase of the Alzheimer's disease, accuracy is high, a detection speed is fast, cost is low, traumasare small, and patients can easily accept. A scientific and effective treatment plan is provided for the Alzheimer's disease and a good application prospect is possessed.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
Ursodesoxycholic acid preparation method
The invention discloses an ursodesoxycholic acid preparation method. The method comprises the following steps: 1, adding chenodeoxycholic acid and a solvent A to a reaction container, stirring for dissolving, adding 7-alphaHSDH, 7-betaHSDH and a coenzyme II, and carrying out a reaction at a controlled temperature at a controlled pH value to convert chenodeoxycholic acid into ursodesoxycholic acid in order to obtain a conversion liquid; 2, heating the conversion liquid obtained in step 1 to denaturalize the 7-alphaHSDH and the 7-betaHSDH, centrifuging through a high speed centrifuge, removing proteins, adding a sodium hydroxide solution to the above obtained solution, distilling to remove the solvent A, adding water to dissolve obtained distillation residues, adding an acid, and crystallizing to obtain crude ursodesoxycholic acid; and 3, adding the crude ursodesoxycholic acid obtained in step 2 and a solvent B to the reaction container, heating and refluxing the crude ursodesoxycholic acid and the solvent B for 1h, cooling the obtained reaction product to normal temperature, and filtering the cooled product to obtain ursodesoxycholic acid with the purity being greater than 99%. The ursodesoxycholic acid preparation method has the advantages of simple technology, short synthesis route, high conversion rate, easy post-treatment and environmental protection.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method for synthesizing ursodeoxycholic acid through enzymatic method
The invention discloses a method for synthesizing ursodeoxycholic acid through an enzymatic method and a method for synthesizing the ursodeoxycholic acid by taking chenodeoxycholic acid as a raw material. The method takes the chenodeoxycholic acid as a base material and comprises the following steps: dissolving the chenodeoxycholic acid into a 50mM phosphate buffer solution; firstly, catalyticallyoxidizing the chenodeoxycholic acid by utilizing 7-alpha hydroxysteroid dehydrogenase in the presence of NAD, NOX2 and under the condition that oxygen is introduced, so as to obtain 7-ketolithocholicacid; then under the condition that the NAD, L-malic acid and malic dehydrogenase exist, catalytically reducing the 7-ketolithocholic acid by utilizing the 7-alpha hydroxysteroid dehydrogenase to obtain the ursodeoxycholic acid. According to the method disclosed by the invention, an organic solvent is not used and operation is simple; reaction conditions are moderate and easy to control and the utilization rate of the raw materials is high; the conversion rate reaches 99 percent or more.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method for purifying chenodeoxycholic acid
The invention discloses a method for purifying chenodeoxycholic acid. The method comprises the following steps: pretreatments such as degreasing, decoloration and impurity removal are carried out on commercially available crude bile paste or crude bile powder, so as to obtain a component to undergo chromatographic separation; the component to undergo chromatographic separation undergoes purification and separation by the use of a chromatographic column with a hydrophilic resin filler as a stationary phase; and separation products undergo concentration, acidification, washing and drying, so as to obtain chenodeoxycholic acid. In comparison with the prior art, the invention has the following beneficial effects: 1, content of the main component is high; 2, reappearance is high and operability is good; 3, short cycle: it only takes two days to prepare the high-purity chenodeoxycholic acid product from processing of the bile paste raw material; and 4, low content of organic residues: as processes such as solvent extraction, column chromatography on silica gel and the like in the traditional techniques are abandoned, an industrial chromatographic process with low dosage of an organic solvent is adopted and the final product undergoes drying process, the content of the residual organic solvent in the final product is especially low.
Owner:SHANGHAI FENPU NEW MATERIAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
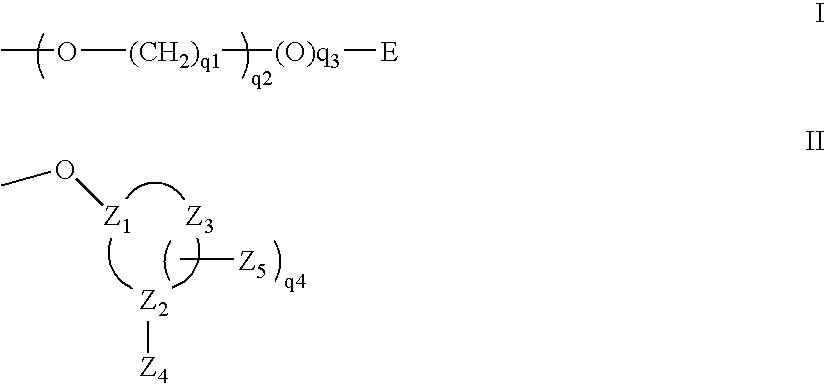

![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000011.PNG)
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000021.PNG)
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000022.PNG)