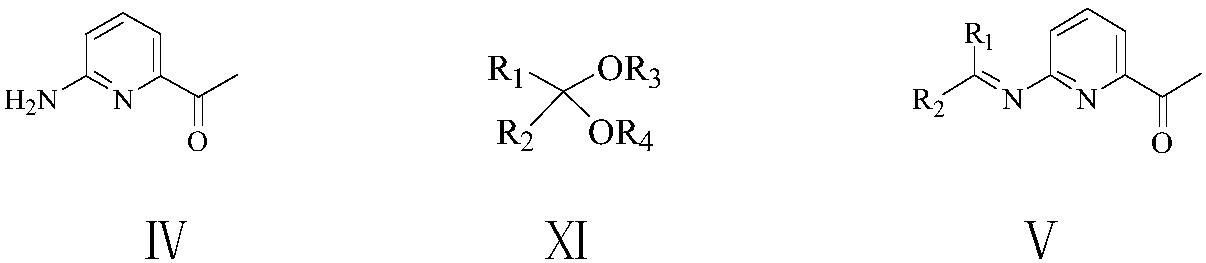
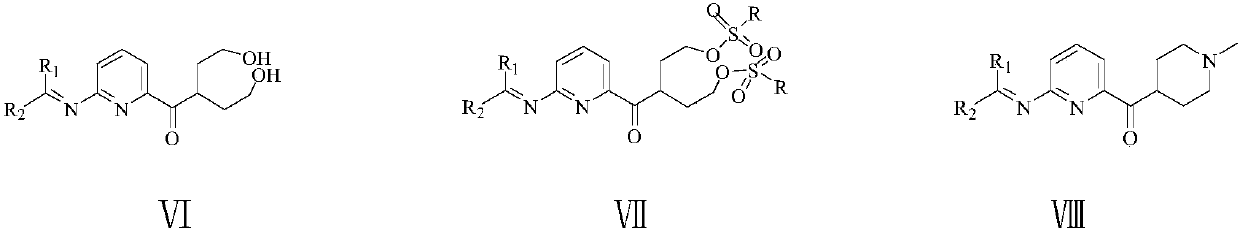
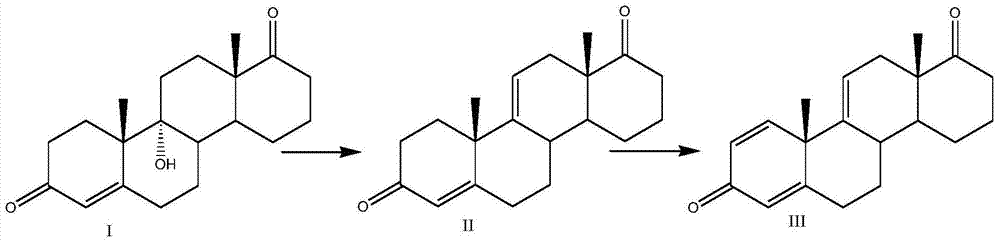
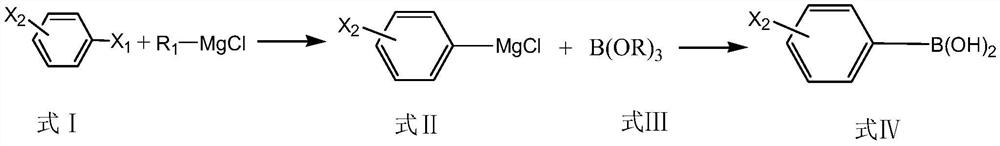
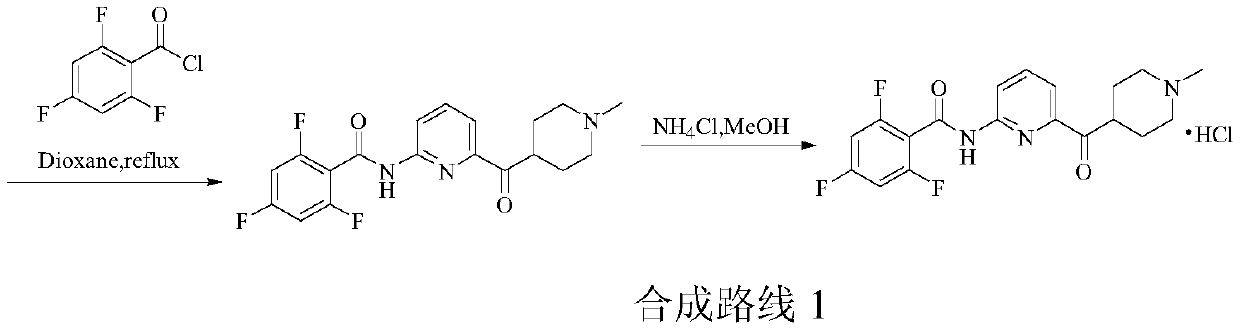
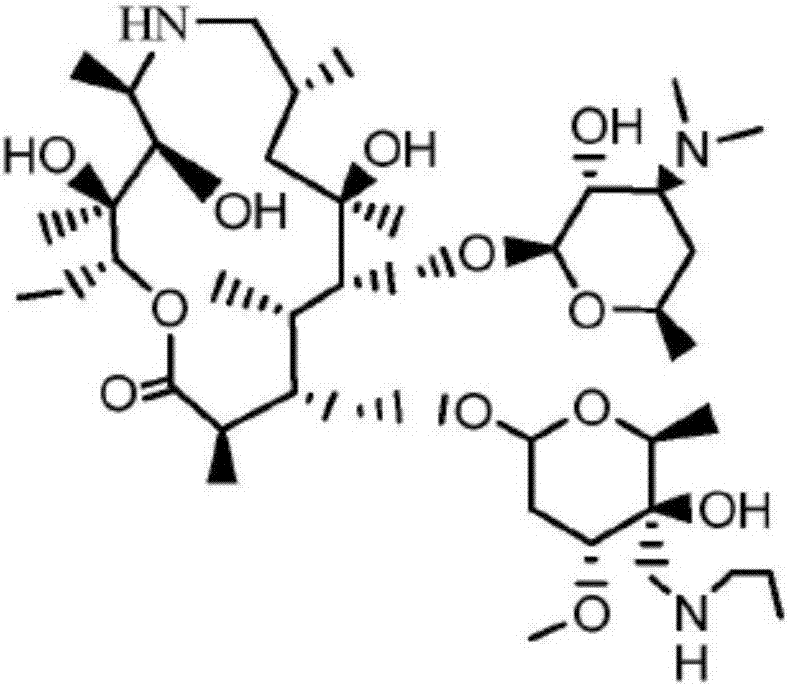
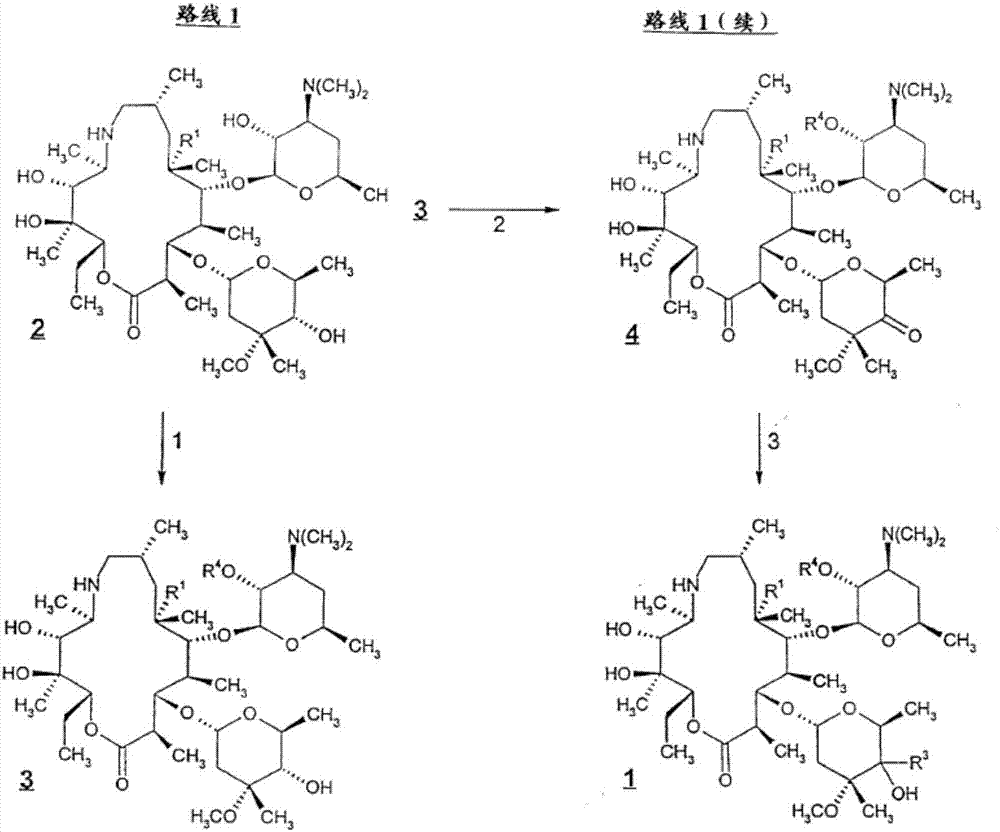
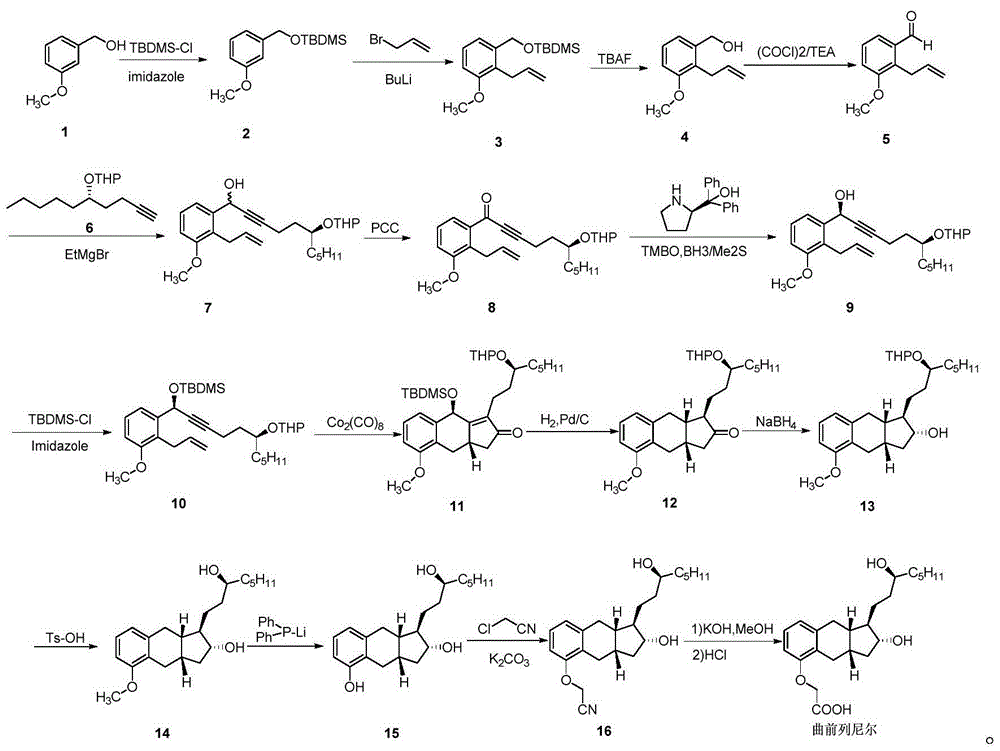
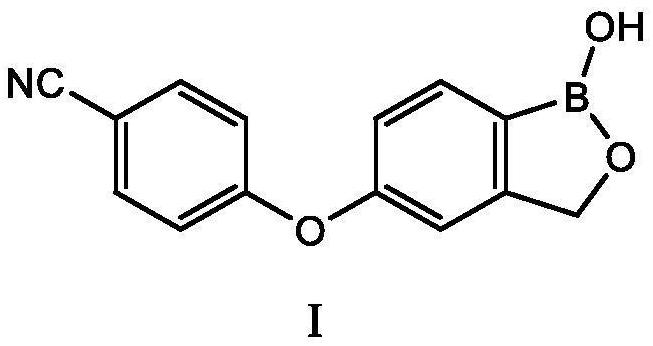
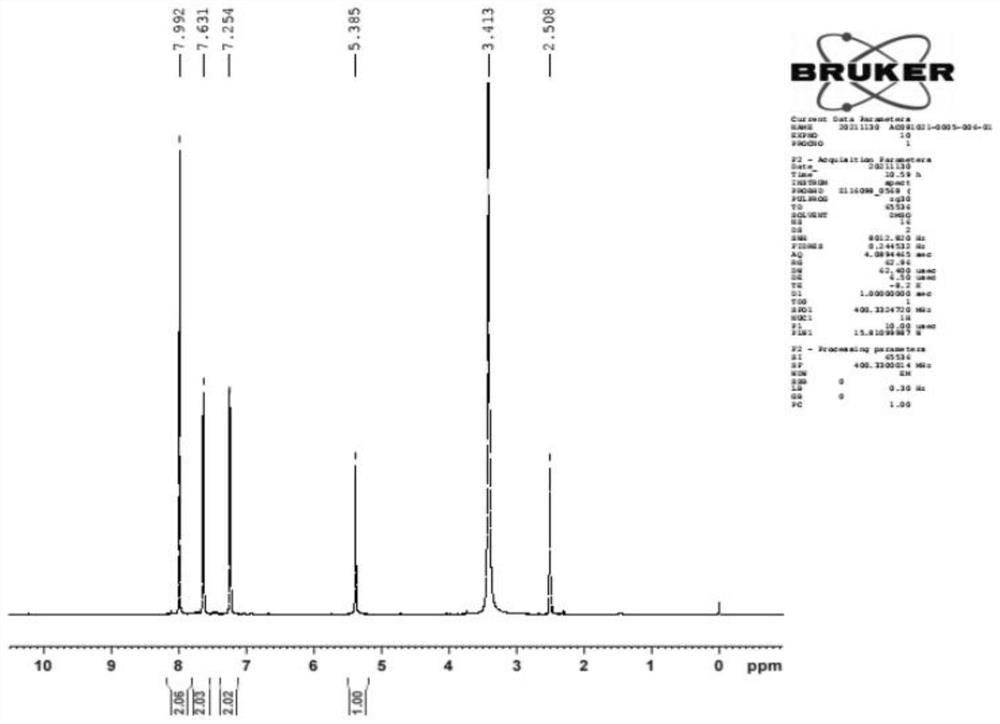
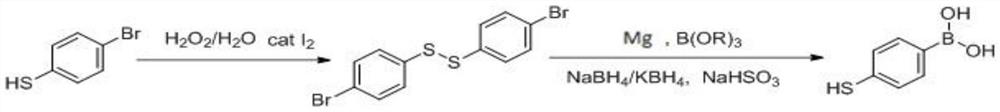
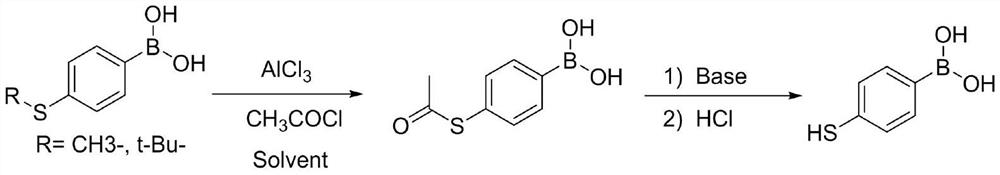
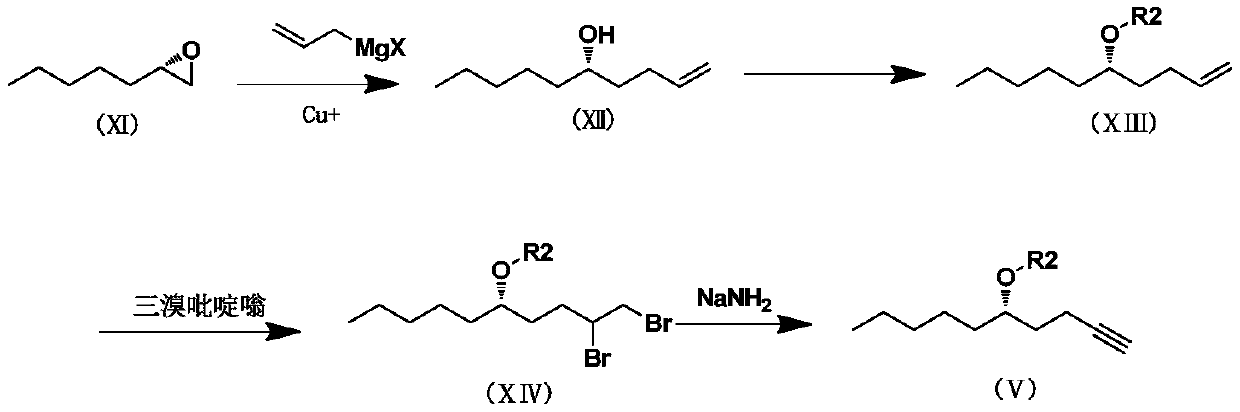
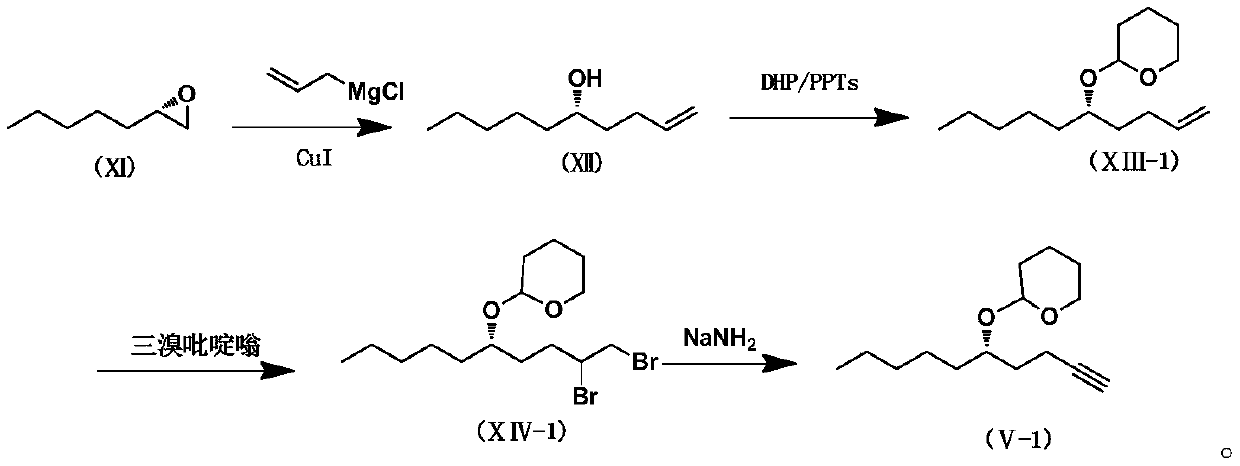
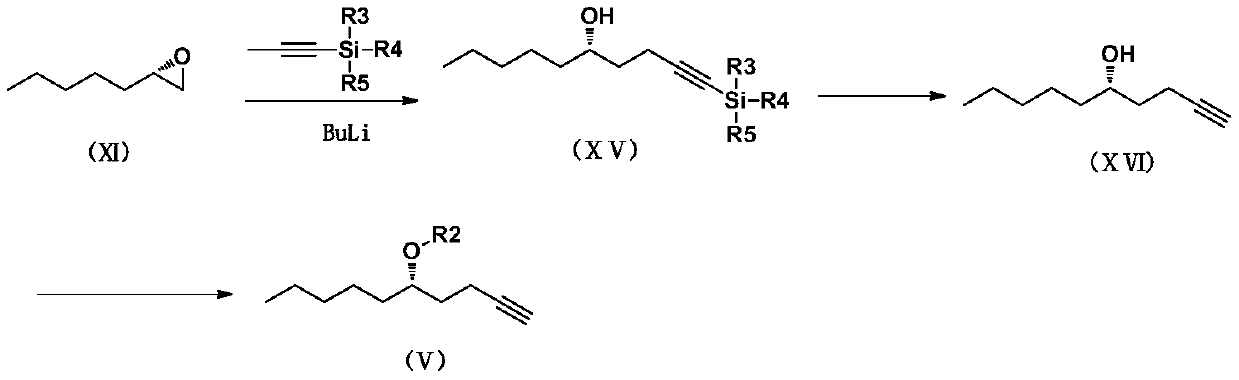
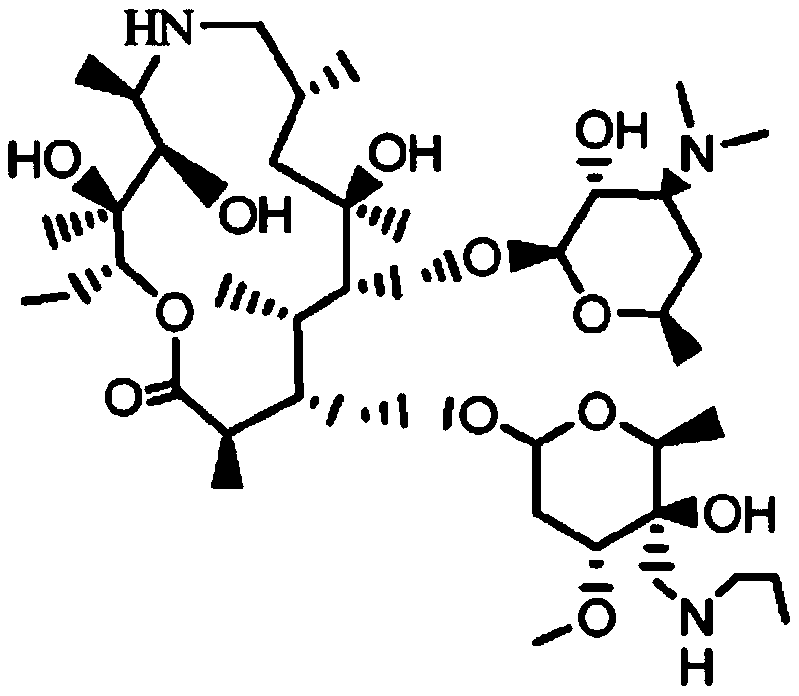
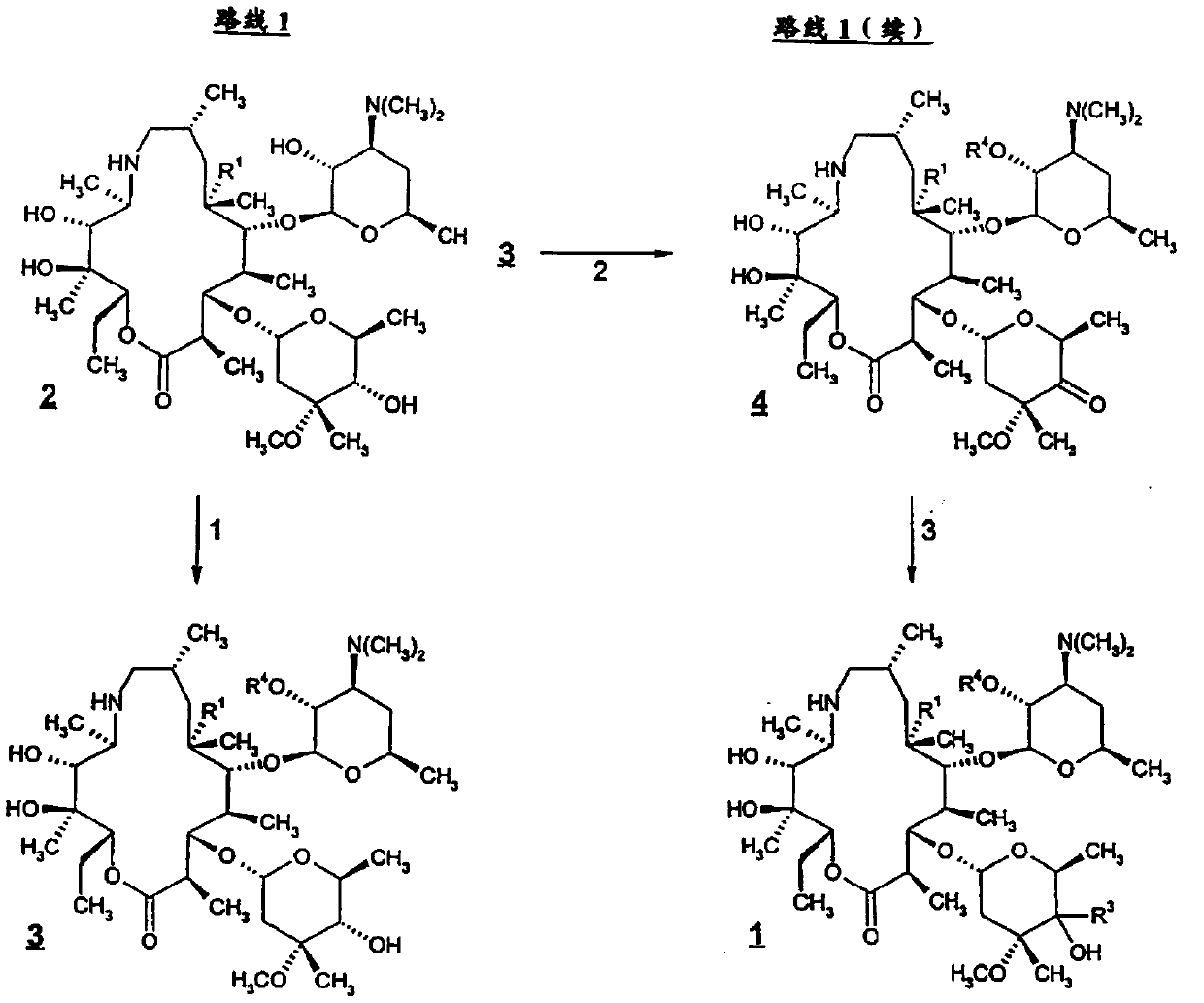
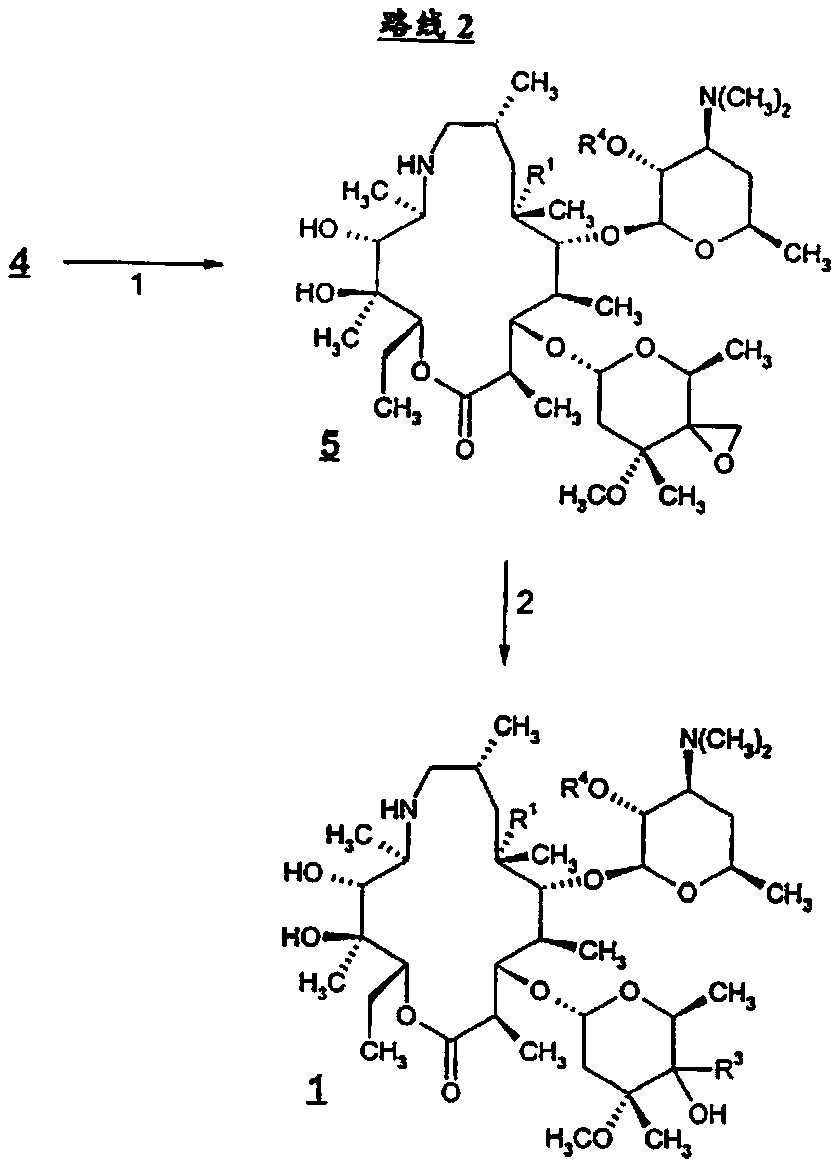
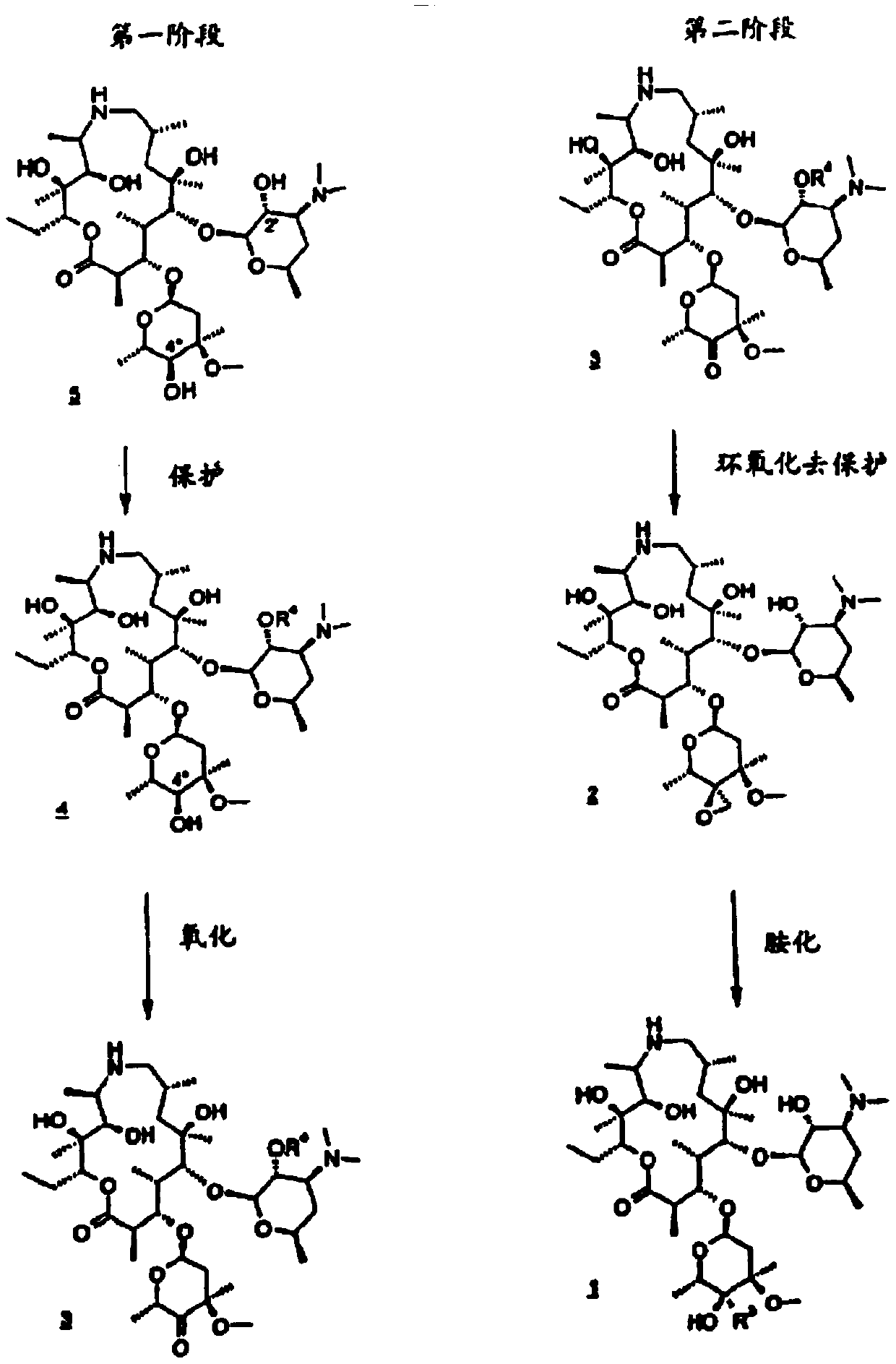
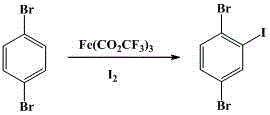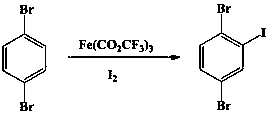Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32results about How to "Avoid ultra-low temperature reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
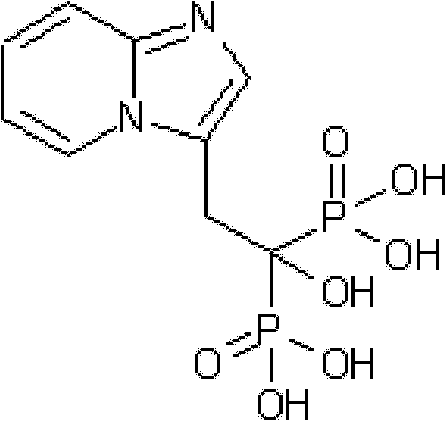
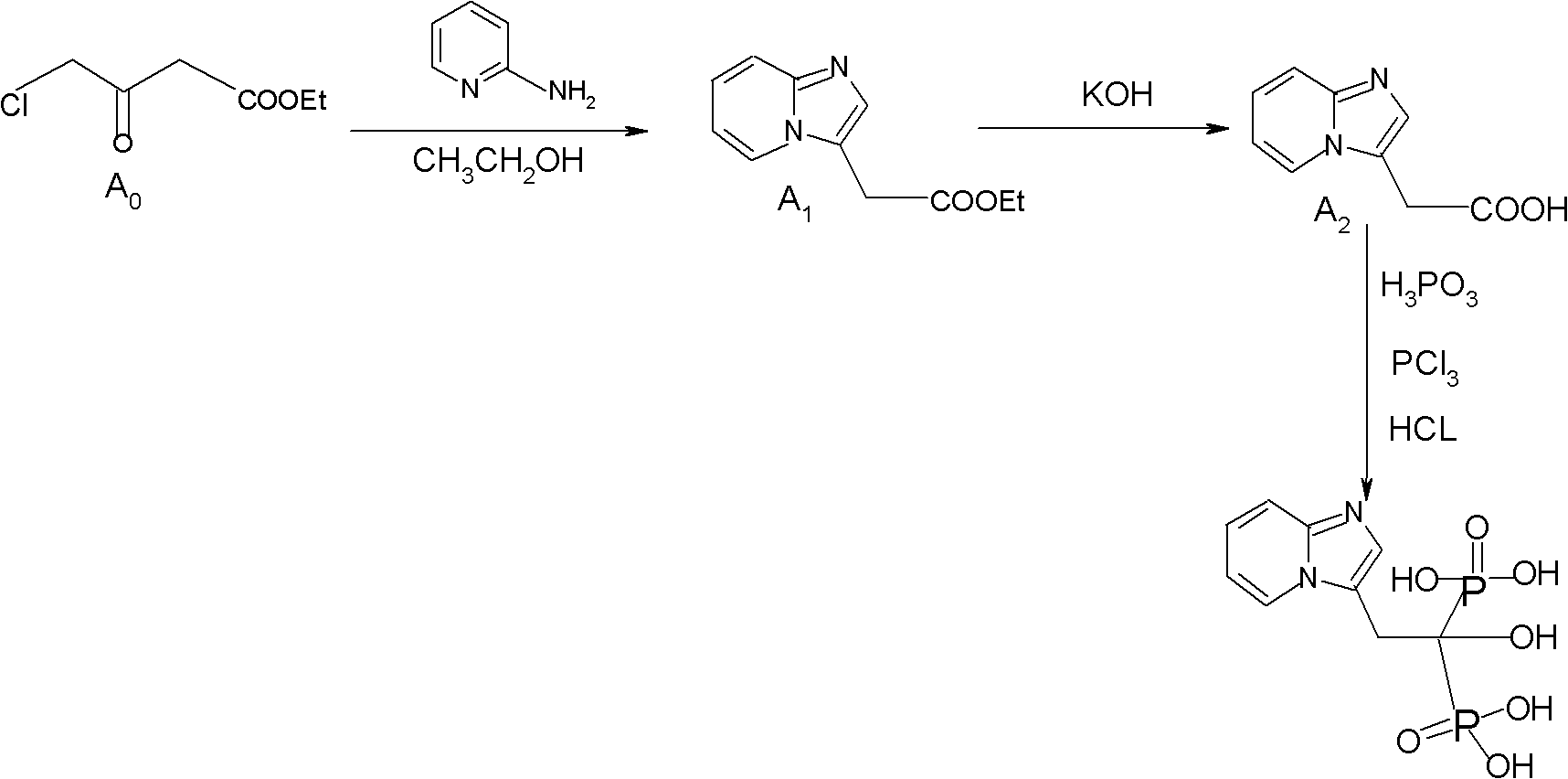
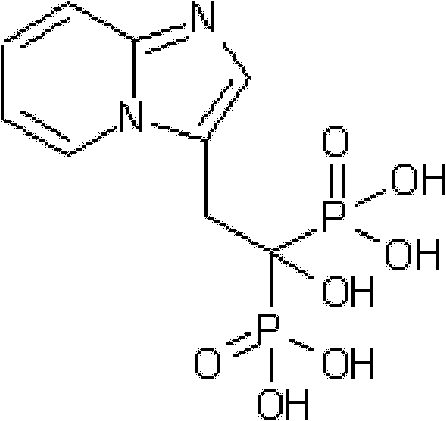
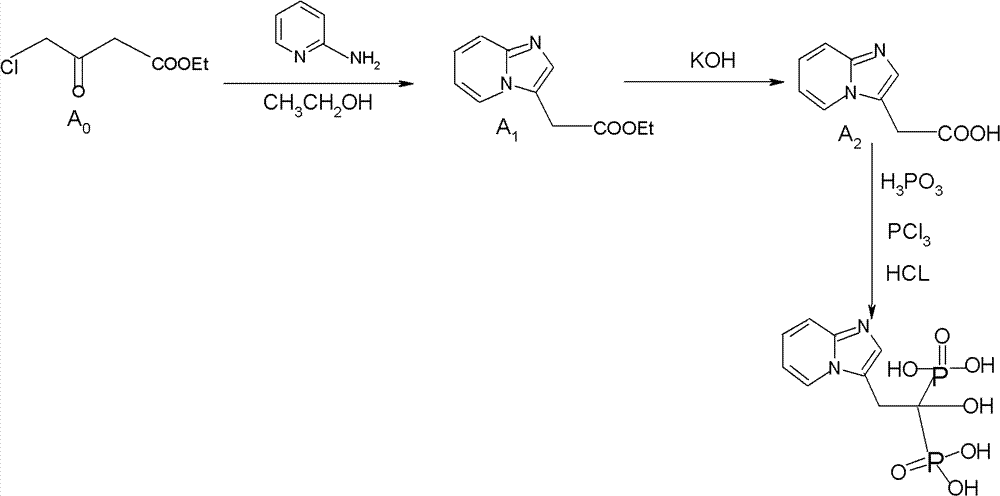
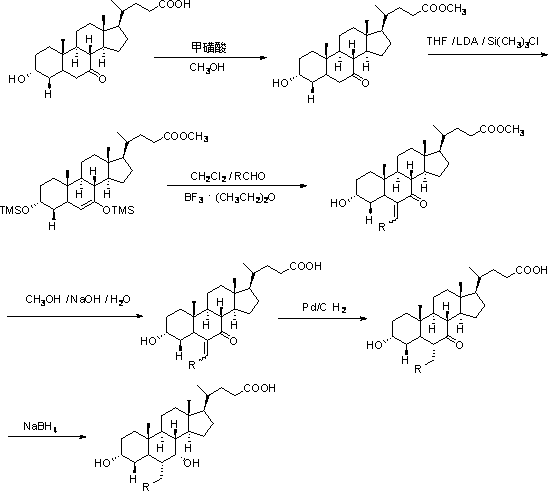
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585AAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
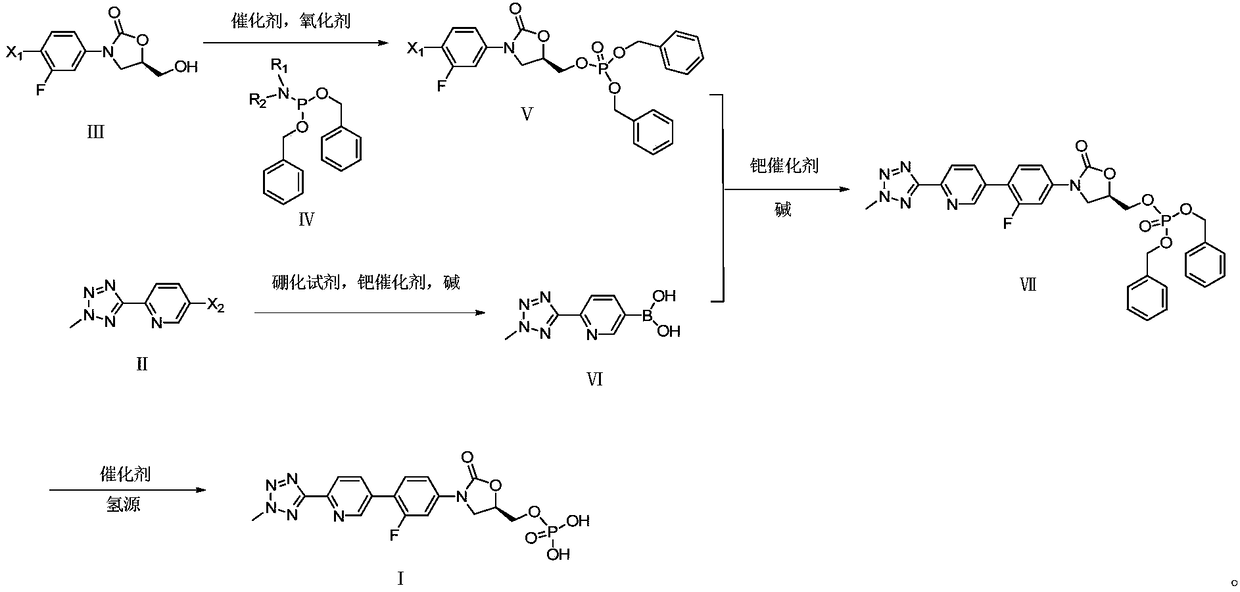
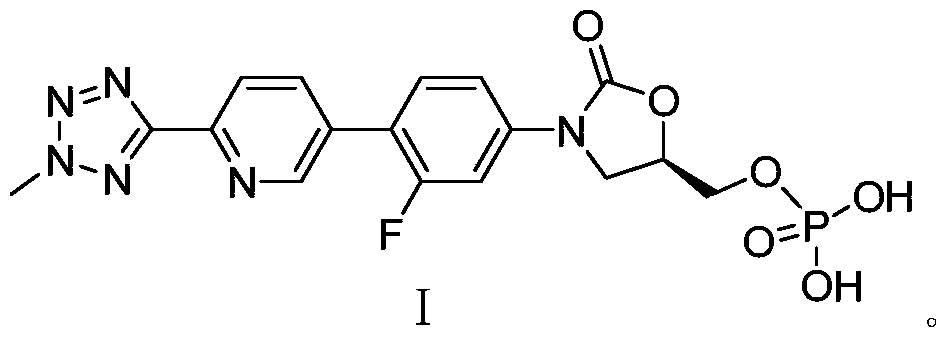
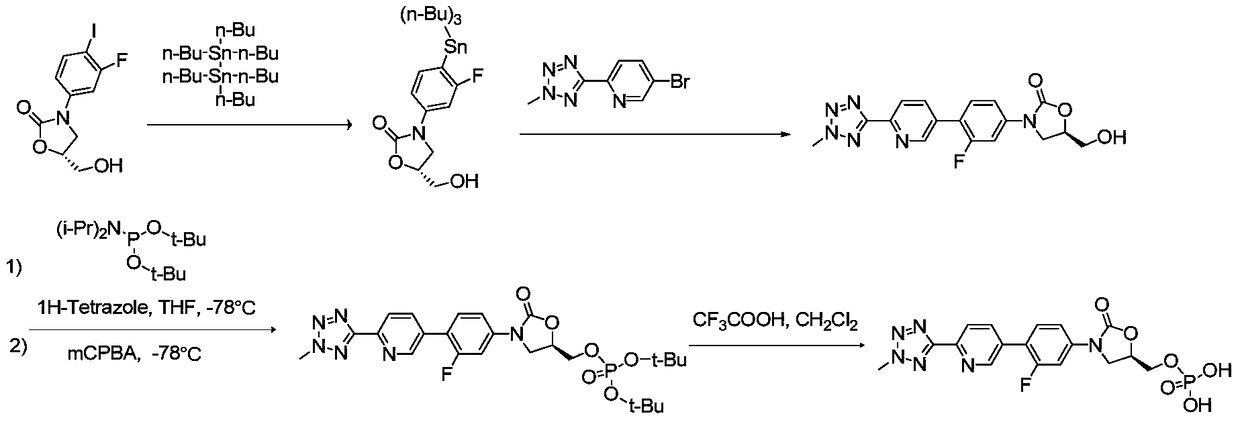
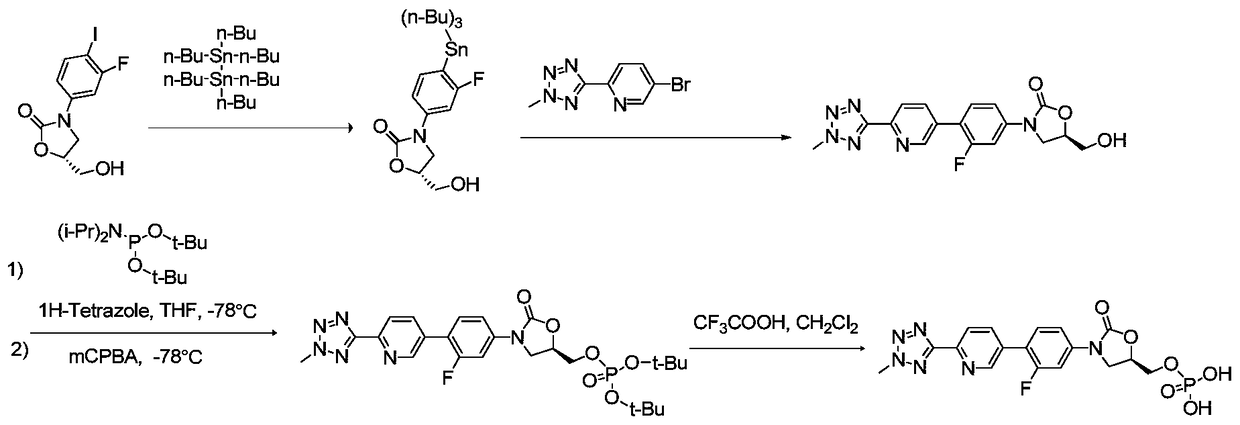
Preparation method for tedizolid phosphate
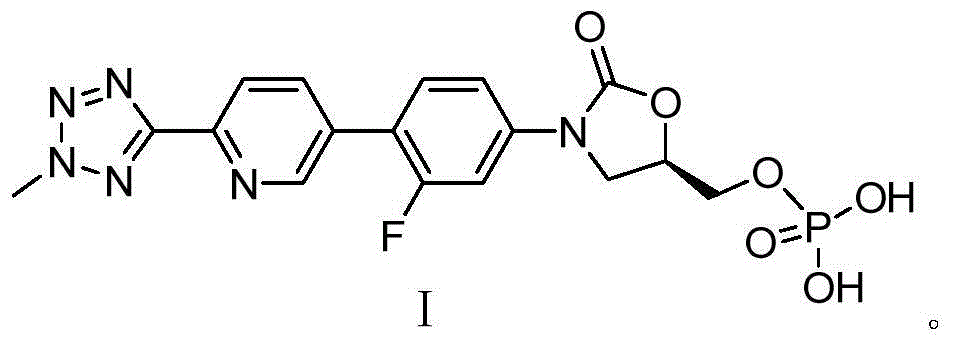
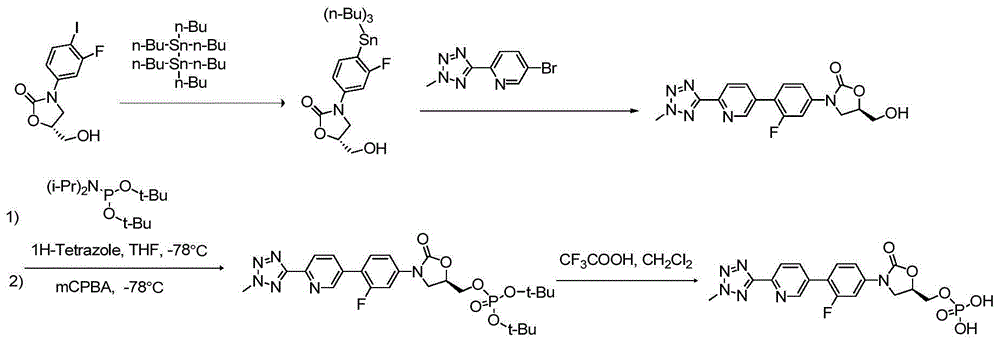
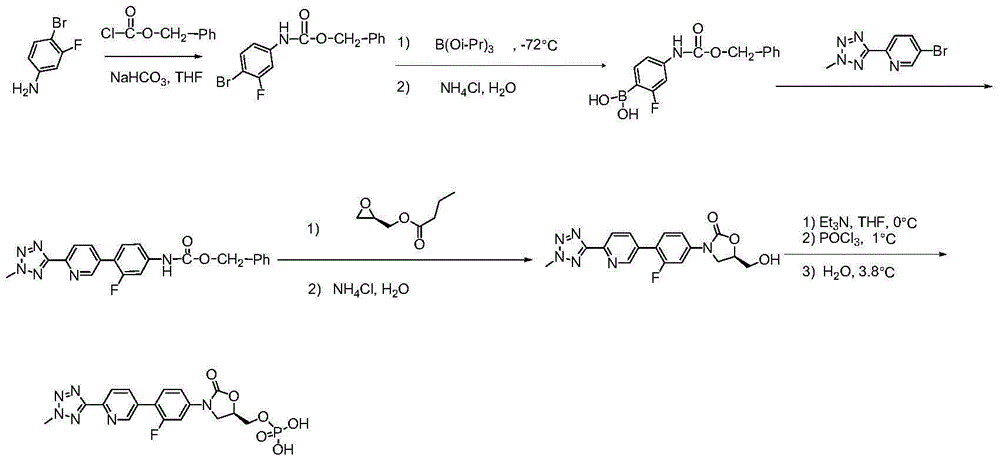
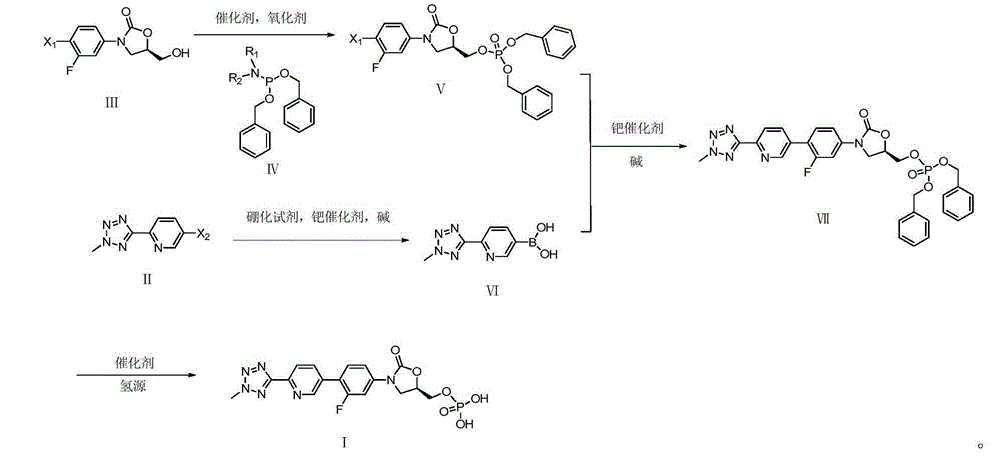
ActiveCN105418678AHigh purityAvoid it happening againGroup 5/15 element organic compoundsChemical industryPhosphate
The invention belongs to the field of medicine chemical industry and particularly relates to a preparation method for tedizolid phosphate. According to the preparation method provided by the invention, intermediates in the steps and the final product are high in purity. In addition, by using diiso-propylamido dibenzyl phosphite as a phosphorylation reagent, a dimerized product is further avoided, so that the preparation method provided by the invention is higher in yield. The preparation method provided by the invention is relatively short in route and mild in reaction condition, and further avoids use of toxic, irritant and strongly corrosive reagents, so that the preparation method is green and environmentally friendly. Meanwhile, ultralow temperature reaction is avoided, so that the preparation method is simple to operate, and the tedizolid phosphate is easy to prepare and high in production efficiency. Thereofe, the preparation method provided by the invention is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method of Draxxin
ActiveCN107556351AHigh yieldAvoid ultra-low temperature reactionsSugar derivativesSugar derivatives preparationPotassium tert-butoxideBenzyl chloroformate
The invention discloses a preparation method of Draxxin. The method comprises the following steps: enabling a reaction between an intermediate TA04 and benzyl chloroformate, so as to obtain TA05; oxidizing the TA05 with DMSO and IBX at the temperature of 20-30 DEG C, so as to obtain TA06; dissociating and performing aftertreatment after the TA06 becomes oxalate TA06-OX, so as to obtain purified TA06; oxidizing the TA06 with trimethylsulfonium bromide and potassium tert-butoxide, so as to obtain TA07; hydrogenating the TA07 to remove a protection group, so as to obtain TA08; enabling a reactionbetween the TA08 and n-propylamine, so as to obtain TA; dissociating and performing aftertreatment after the TA becomes oxalate TA-OX, so as to obtain purified end-product Draxxin. The total yield ofthe Draxxin prepared with the method is increased by above 20%.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Preparation method of 5-HT1F agonist compound
The invention relates to a preparation method of a 5-HT1F agonist compound. The preparation method of the 5-HT1F agonist compound comprises the steps that 2,3-butanedione and 2-halogenated acrylonitrile are subjected to 1,4-addition reaction to obtain 2-halogenated-5,6-dioxo n-heptanoic nitrile, then the 2-halogenated-5,6-dioxo n-heptanoic nitrile and ammonia are subjected to pyridine cyclizationand amino protection to obtain 2-disubstituted methylamino-6-acetyl pyridine, then 2-disubstituted methylamino-6-(1-methyl piperidine-4-base)formyl group piperidine is prepared through hydroxyethylation, sulfonic acid esterification and methylamine condensation reaction, and then the 5-HT1F agonist compound Lasmiditan is obtained through deprotection reaction and 2,4,6-trifluoro-benzoyl chloride amidation. The raw materials of the preparation method of the 5-HT1F agonist compound are easy to get, ultralow temperature reaction is avoided, the operation is simple and convenient, the cost is low,and industrial production of Lasmiditan is easy.
Owner:XINFA PHARMA
Preparation method of 1,4,9(11)-triene-androst-3,17-dione
InactiveCN104328159ACheap and easy to getAvoid ultra-low temperature reactionsMicroorganism based processesFermentationKetoneSolvent
The invention discloses a preparation method of 1,4,9(11)-triene-androst-3,17-dione, which uses a compound I 9alpha-OH-4AD as an initial raw material and comprises the following steps: A) elimination reaction: adding the compound I into an eliminating solvent to eliminate 9- alpha hydroxy group by using the eliminating reagent, and reacting to obtain a compound II; and B) fermentation conversion: preparing a fermentation culture medium by using the compound II as a raw material, inoculating a Nocard's bacillus strain into the fermentation culture medium, culturing for conversion, and extracting to obtain a compound III, namely 1,4,9(11)-triene-androst-3,17-dione. The new initial raw material adopted by the method is cheap and accessible; and the new elimination method avoids ultralow temperature reaction, is simple to operate, and has the advantages of low equipment investment, fewer side reactions and high yield.
Owner:江西赣亮医药原料有限公司
Preparation method of monohalogenated phenylboronic acid
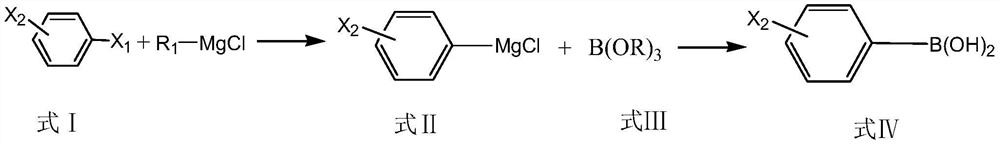
ActiveCN111647011ARealize industrial productionAvoid it happening againGroup 3/13 element organic compoundsBulk chemical productionChemical synthesisPtru catalyst
The invention relates to the technical field of chemical synthesis, and particularly discloses a preparation method of monohalogenated phenylboronic acid. The preparation method comprises the following steps of: by taking dihalogenated benzene as a raw material and a mixture of lithium salt and alkaline ionic liquid as a catalyst, carrying out Grignard exchange with R1MgCl to generate monohalogenated phenyl magnesium chloride, reacting with B (OR) 3 to generate monohalogenated phenyl borate, and hydrolyzing under acidic conditions to obtain monohalogenated phenylboronic acid. The HPLC (High Performance Liquid Chromatography) content of the monohalogenated phenylboronic acid prepared by the method is greater than 99.5%; the total yield of the product is greater than 80%, the contents of monohalogenated phenylboronic acid and phenyldiboronic acid impurities of another halogen are both less than 0.003%, the requirements of modern fine chemical synthesis are completely met, the raw materials are easily available, the operation is simple, the safety is high, and the industrial production of monohalogenated phenylboronic acid is realized.
Owner:宁夏中星显示材料有限公司
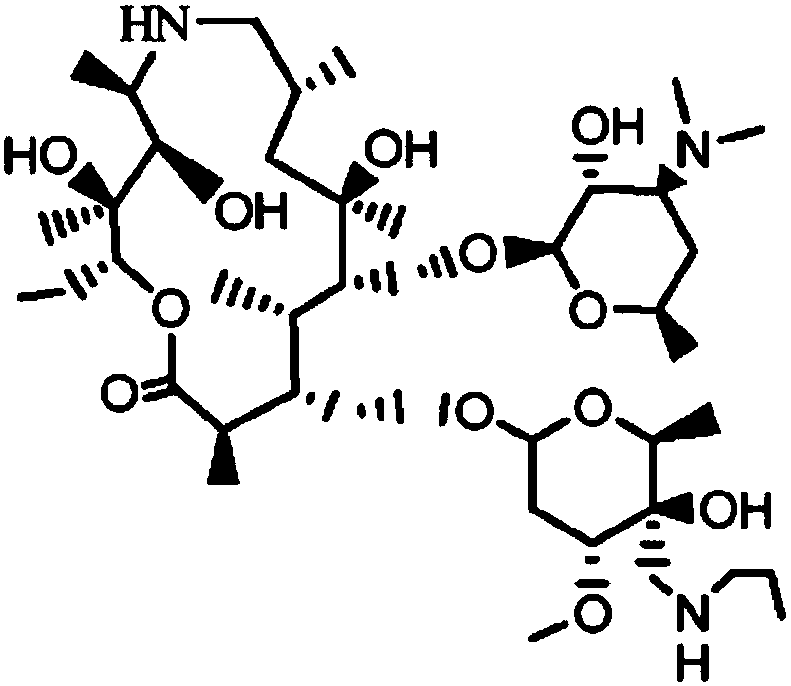
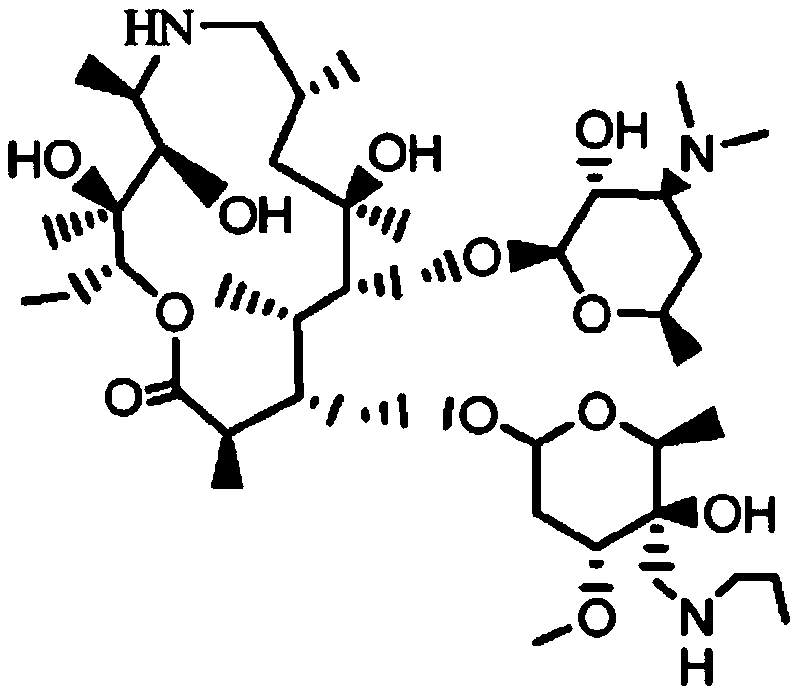
Tulathromycin oxalate
ActiveCN107400152AHigh yieldAvoid ultra-low temperature reactionsSugar derivativesSugar derivatives preparationTulathromycinInorganic chemistry
The invention discloses salt of tulathromycin, particularly oxalate, a preparation method of tulathromycin oxalate and application of tulathromycin oxalate to preparation of tulathromycin.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Preparation method of Dapagliflozin eutectic matter
InactiveCN107445932ALow costShort synthetic stepsMetabolism disorderOrganic chemistry methodsSolventNucleophilic substitution
The invention provides a preparation technology of a Dapagliflozin eutectic matter. The preparation technology comprises the following steps: (1) using 4-chlorin-3-(4-ethyoxyl benzyl) phenyl halide 6 as a raw material, carrying out an X / Li / Zn exchange reaction on the 6 with lithium alkylide and zinc salt in an appropriate solvent to prepare organic zinc reagent-di[4-chlorin-3-(4-ethyoxyl benzyl) phenyl] zinc, then carrying out a nucleophilic substitution reaction with 2,3,4,6-tetra-O-pivaloyl-alpha-D-bromo-glucopyranose 4 to prepare a compound 3; and (2) taking off a pivaloyl protecting group of a compound 3 to obtain Dapagliflozin 2, and directly reacting with (S)-1,2-propylene glycol and water in the appropriate solvent to prepare the Dapagliflozin eutectic matter 1. The synthetic route is as follows: the formula is as shown in the specification, wherein X in the 4-chlorin-3-(4-ethyoxyl benzyl) phenyl halide 6 structure is selected from bromine Br or iodine I. The reagent used in the route is a conventional reagent, and is low in cost and easily obtained; the route is simplified, the route cost is greatly reduced, the product yield and purity are relatively high, and a diastereoisomer is not contained in the product, so that the preparation technology is applicable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Bacterial protein synthesis inhibitor preparation method
ActiveCN105566392AHigh purityAvoid it happening againGroup 5/15 element organic compoundsGroup 3/13 element organic compoundsEnvironmental resistanceChemical industry
The present invention belongs to the field of medicine and chemical industry, relates to a bacterial protein synthesis inhibitor preparation method, and specifically relates to a tedizolid phosphate preparation method. According to the method, intermediates of every steps and a final product are high in purity. Further, by the use of diisopropylamine dibenzyl phosphite as a phosphorylating agent, a dimerization product can be avoided, and the preparation method has a higher yield. The preparation method is shorter in route and mild in reaction conditions, avoids the use of toxic, irritating and strongly-corrosive reagents, is green and environmentally-friendly, meanwhile avoids the use of ultra-low temperature reaction, and is simple and easy in preparation and high in production efficiency. Therefore, the preparation method is particularly adapted to industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Synthesis method for 2,5-dibromo-iodobenzene
ActiveCN105753643AControl generationImprove iodination reactivityHalogenated hydrocarbon preparationChemical synthesisBenzene
The invention discloses a novel synthesis method for 2,5-dibromo-iodobenzene and belongs to the field of organic chemistry synthesis.The synthesis method includes the steps that with 1,4-dibromo-benzene being a starting raw material, trifluoroacetic acid and iodine are subjected to an iodination reaction to synthesize the target product 2,5-dibromo-iodobenzene.The method avoids low-temperature reaction, is easy to implement, generates a small number of by-products, is suitable for industrialized production and has good application prospects.2,5- dibromo-iodobenzene is an important fine chemical intermediate and is widely applied to the fields of synthesis medicine, pesticide, dye, plastics, functional polymer materials and others.
Owner:郑州金上化成新材料有限公司
A kind of preparation method of 5-ht1f agonist compound
The invention relates to a preparation method of a 5-HT1F agonist compound. The preparation method of the 5-HT1F agonist compound comprises the steps that 2,3-butanedione and 2-halogenated acrylonitrile are subjected to 1,4-addition reaction to obtain 2-halogenated-5,6-dioxo n-heptanoic nitrile, then the 2-halogenated-5,6-dioxo n-heptanoic nitrile and ammonia are subjected to pyridine cyclizationand amino protection to obtain 2-disubstituted methylamino-6-acetyl pyridine, then 2-disubstituted methylamino-6-(1-methyl piperidine-4-base)formyl group piperidine is prepared through hydroxyethylation, sulfonic acid esterification and methylamine condensation reaction, and then the 5-HT1F agonist compound Lasmiditan is obtained through deprotection reaction and 2,4,6-trifluoro-benzoyl chloride amidation. The raw materials of the preparation method of the 5-HT1F agonist compound are easy to get, ultralow temperature reaction is avoided, the operation is simple and convenient, the cost is low,and industrial production of Lasmiditan is easy.
Owner:XINFA PHARMA
Salt for tulathromycin intermediate
ActiveCN107501364AHigh yieldAvoid ultra-low temperature reactionsSugar derivativesCarboxylic acid salt preparationOxalateTulathromycin
The invention discloses a salt for preparing a tulathromycin intermediate, and particularly relates to oxalate and a preparation method thereof as well as application of the oxalate in a method for preparing tulathromycin.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Preparation method for treprostinil intermediate
ActiveCN104892555AMild reaction conditionsEasy recrystallization purificationOrganic chemistryBulk chemical productionTreprostinilKetone
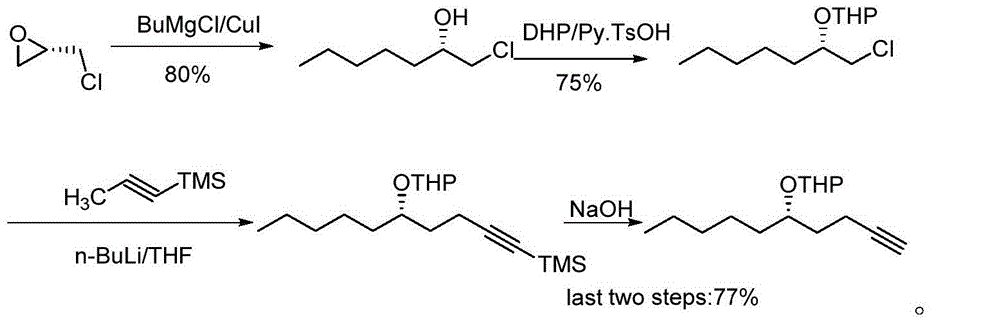
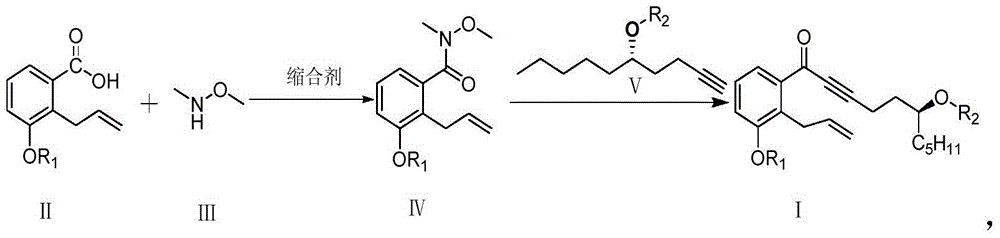
The invention relates to a preparation method for a treprostinil intermediate (I). The preparation method comprises the steps that: a compound of a formula (II) and a compound of a formula (III) or acidic salt thereof react in the presence of a condensing agent to obtain a compound of a formula (IV); the compound of the formula (IV) and a compound of a formula (V) react to obtain a compound of a formula (I). According to the preparation method for the treprostinil intermediate, weinreb amide and alkyne negative ions react to directly obtain a ketone compound (I), so that environment pollution caused by heavy metal (a PCC oxidant) is avoided, and the adoption of a butyl lithium low-temperature reaction method is also avoided. The preparation method for the treprostinil intermediate has the advantages that reaction conditions are mild, the yield is high, the purity of products is high, and the industrial application prospect is wide. (Formulae (I), (II), (III), (IV) and (V) are shown in the specification)
Owner:JIANGSU HANSOH PHARMA CO LTD +1
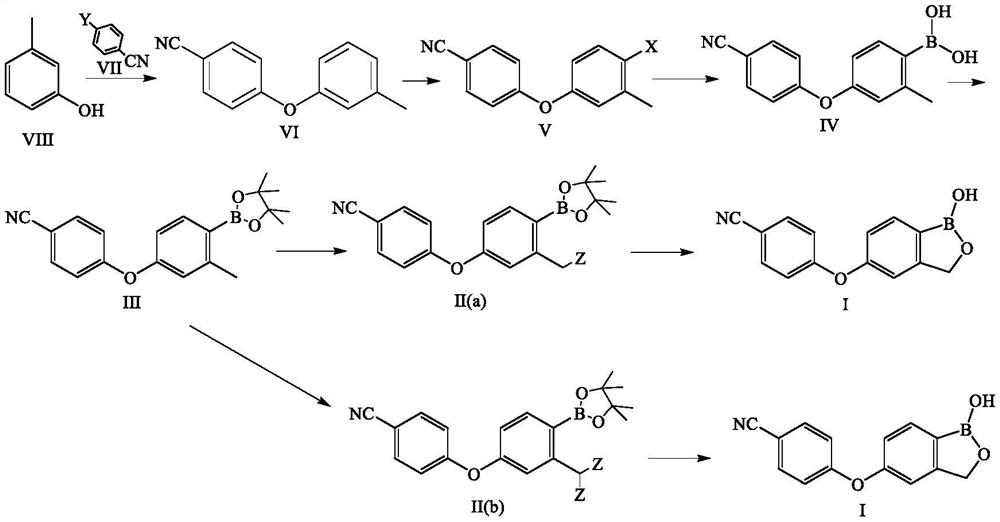
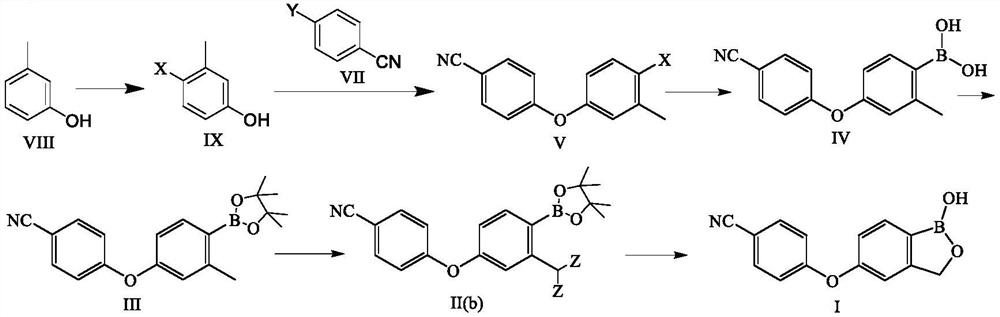
Preparation method of crisaborole
PendingCN113336781AStarting materials are cheap and readily availableMild responseGroup 3/13 element organic compoundsHalogenMethyl benzene
The invention discloses a preparation method of crisaborole. The preparation method comprises the following steps: by taking m-methylphenol as a raw material, firstly coupling the m-methylphenol with 4-halogenated-cyanophenyl, and then under the action of a halogenating reagent, increasing the yield of an intermediate product 4-(4-halogen-3-methylphenoxy) cyanophenyl; and carrying out halogen-metal exchange, halogenation, ring closing and other reactions to prepare the crisaborole. Compared with the prior art, the method has advantages that the raw materials are cheap and easy to obtain, and expensive reagents and raw materials in the prior art are not used; the reaction is mild, and ultralow-temperature reaction in the prior art can be avoided; the method is short in reaction time, free of column chromatography purification, simple in process, convenient to operate and suitable for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Process method for synthesizing 4-mercaptophenylboronic acid
PendingCN114057782AShort process stepsAtom economy is highGroup 3/13 element organic compoundsBiologyBorylation
The invention discloses a process method for synthesizing 4-mercaptophenylboronic acid, and belongs to the technical field of medical intermediates. The method comprises the following steps: taking p-bromothiophenol as a raw material, oxidizing the p-bromothiophenol with hydrogen peroxide to generate 4, 4 '-dibromodiphenyl disulfide; and then carrying out Grignard or n-butyllithium / boronation, carrying out one-pot reduction reaction in a sodium borohydride / alcohol solvent, quenching and acidifying to obtain the 4-mercaptophenylboronic acid. The method has the advantages of originality, short steps, high yield, mild conditions and simplicity in operation. The problems of long steps, low yield, ultralow temperature and difficulty in product purification of other literature methods are avoided. The method has potential technical advantages and is suitable for industrial large-scale production.
Owner:大连双硼医药化工有限公司
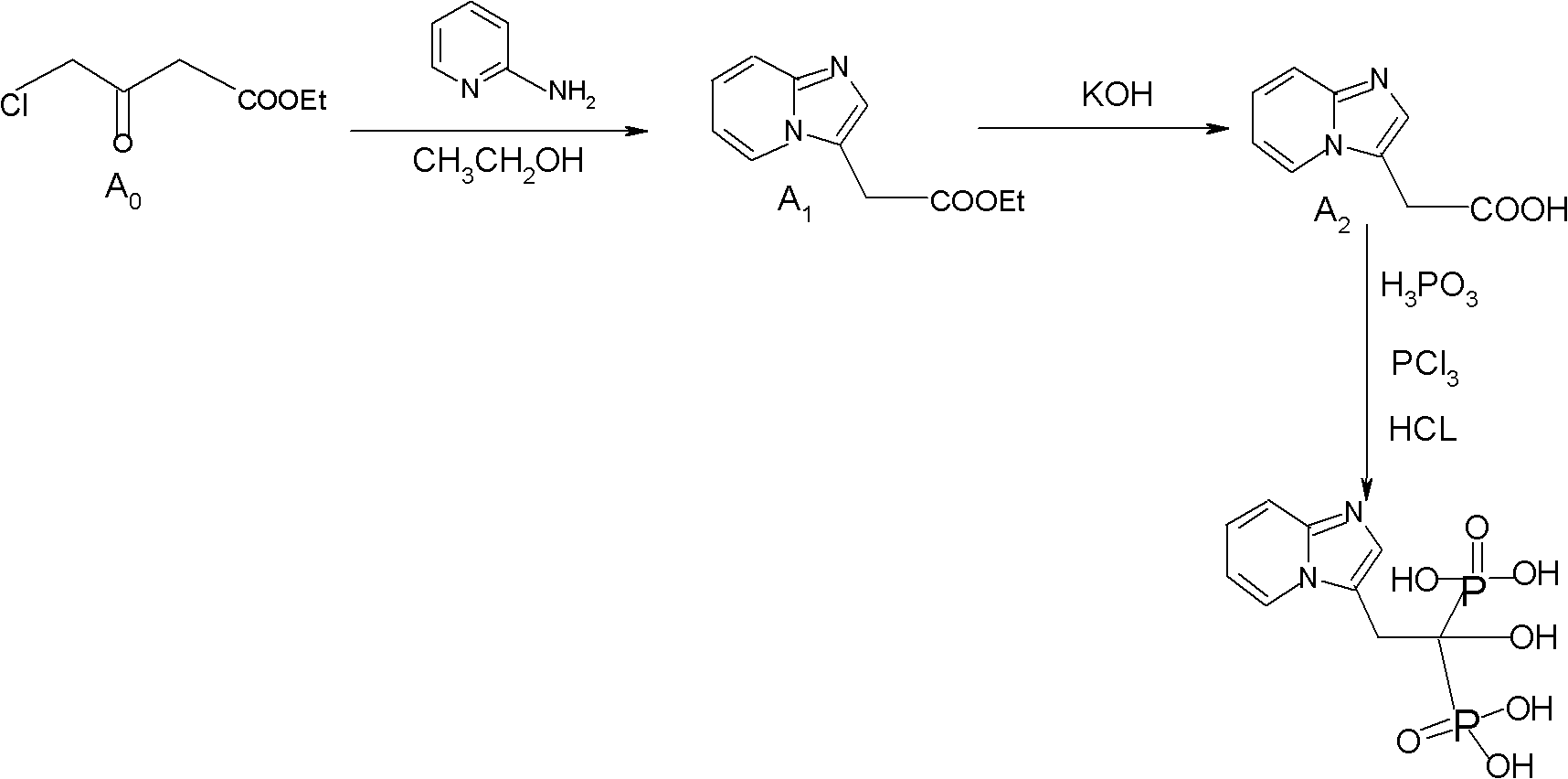
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585BAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
Preparation method of compound for preventing gram-positive bacteria
ActiveCN105503955AHigh purityAvoid it happening againGroup 5/15 element organic compoundsEnvironmental resistancePhosphorylation
The invention belongs to the field of pharmaceutical chemical engineering, relates to a preparation method of a compound for preventing gram-positive bacteria, and specifically relates to a preparation method of tedizolid. According to the preparation method, the purities of intermediates in each step and final product are very high. Moreover, diisopropylamino dibenzyl phosphite is taken as the phosphorylation reagent, dimerization is avoided, and thus the preparation method has a higher yield. The preparation method has the advantages that the route is short; the reaction conditions are mild; no toxic, irritant, or corrosive reagent is used; the preparation method is green and environment-friendly; at the same time, ultralow temperature reaction is avoided, the operation is simple and convenient, the production efficiency is high, and the preparation method is especially suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A kind of synthetic boron amine compound technology and product application
ActiveCN104926847BSolve the problem of easy self-couplingStrong boron nitrogen bond stabilityGroup 3/13 element organic compoundsGrignard reagentDistillation
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
A kind of synthetic method of 2,5-dibromoiodobenzene
ActiveCN105753643BControl generationImprove iodination reactivityHalogenated hydrocarbon preparationChemical synthesisBenzene
The invention discloses a novel synthesis method for 2,5-dibromo-iodobenzene and belongs to the field of organic chemistry synthesis.The synthesis method includes the steps that with 1,4-dibromo-benzene being a starting raw material, trifluoroacetic acid and iodine are subjected to an iodination reaction to synthesize the target product 2,5-dibromo-iodobenzene.The method avoids low-temperature reaction, is easy to implement, generates a small number of by-products, is suitable for industrialized production and has good application prospects.2,5- dibromo-iodobenzene is an important fine chemical intermediate and is widely applied to the fields of synthesis medicine, pesticide, dye, plastics, functional polymer materials and others.
Owner:郑州金上化成新材料有限公司
Preparation method of 2-methyl-1-tetralone
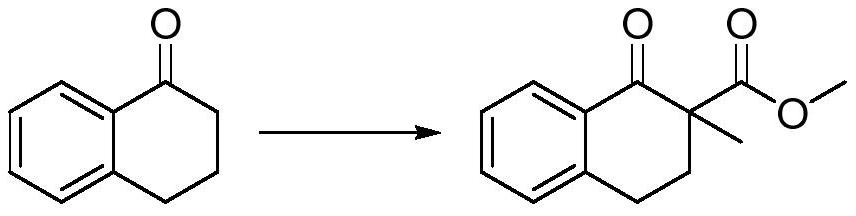
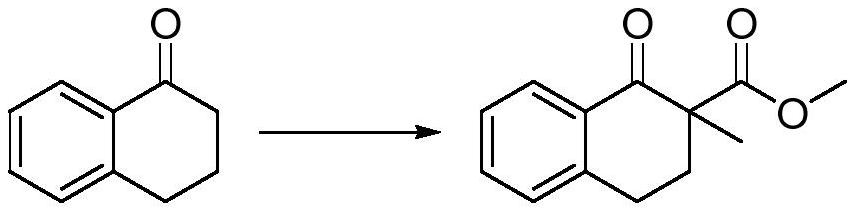
ActiveCN113563167AAvoid ultra-low temperature reactionsShort preparation routeOrganic compound preparationCarboxylic acid esters preparationTetraloneBenzenesulfinic acid
The invention relates to the field of synthesis of tetralone derivatives, and provides a preparation method of 2-methyl-1-tetralone. The preparation method comprises the steps of firstly, enabling 1-tetralone to be subjected to hydrogen extraction and methylation by using a one-pot method, replacing a conventional methylation reagent iodomethane with high toxicity with methyl trifluoroacetate, methyl p-benzenesulfonate or methyl bromide, and in the one-pot reaction, generating 1-tetralone-2-methyl-2-methyl formate; and reacting hydrobromic acid with 1-tetralone-2-methyl-2-methyl formate, and collecting a product in a reduced pressure distillation manner in a post-treatment step so as to finally obtain 2-methyl-1-tetralone. The preparation method of 2-methyl-1-tetralone provided by the embodiment of the invention has the advantages of short synthetic route, no need of ultralow-temperature reaction or column chromatography, simple purification and high yield (up to 84.3%).
Owner:BTC PHARMA TECH CO LTD
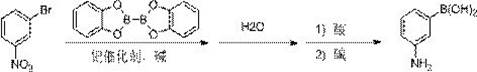
The synthetic method of 3-aminophenylboronic acid
ActiveCN109467569BReduce dosageEasy to operateGroup 3/13 element organic compoundsPtru catalystOrganic synthesis
The invention discloses a method for synthesizing 3-aminobenzeneboronic acid, and belongs to the field of organic synthesis. The method comprises the following steps: performing coupling reaction on 3-nitrobromobenzene and catechol diboron under action of a palladium catalyst; adding water for quenching; adding an acid for salifying a product and entering a water layer; adding alkali into the separated water layer to adjust to near neutral; and extracting and desolventizing to obtain the 3-aminobenzeneboronic acid. The method is simple in operation, high in purity of the obtained product and suitable for industrialized scale-up production.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Tyramectin oxalate
ActiveCN107400152BHigh yieldAvoid ultra-low temperature reactionsSugar derivativesSugar derivatives preparationTulathromycinInorganic chemistry
The invention discloses salt of tulathromycin, particularly oxalate, a preparation method of tulathromycin oxalate and application of tulathromycin oxalate to preparation of tulathromycin.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Treprostinil intermediate and preparation process thereof
ActiveCN111116419AMild reaction conditionsEasy recrystallization purificationOrganic chemistryBulk chemical productionTreprostinilCombinatorial chemistry
The invention relates to a treprostinil intermediate and a preparation process thereof. The preparation process comprises the following steps: reacting a compound as shown in a formula (II) with a compound as shown in a formula (III) or an acidic salt thereof in the presence of a condensing agent to obtain a compound as shown in a formula (IV); and reacting the compound as shown in the formula (IV) with a compound as shown in a formula (V) to obtain a compound as shown in a formula (I). According to the invention, the ketone compound (I) is directly obtained by a reaction of Weber amide and alkyne anions, environmental pollution caused by use of a heavy metal (a PCC oxidant) is avoided, and a low-temperature reaction method adopting butyl lithium is also avoided; and the method is mild inreaction conditions, high in yield, good in product purity and wide in industrial application prospects.
Owner:JIANGSU HANSOH PHARMA CO LTD +1
Salt of Tyramectin intermediate
ActiveCN107501364BHigh yieldAvoid ultra-low temperature reactionsSugar derivativesCarboxylic acid salt preparationOxalate
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
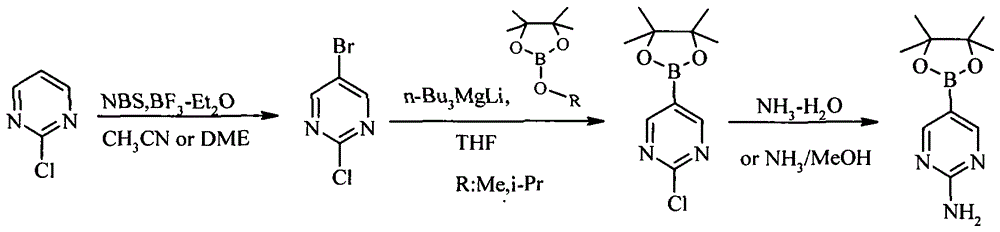
A method for preparing 2-aminopyrimidine-5-boronic acid pinacol ester
ActiveCN104788482BSynthetic process conditions are mildAvoid ultra-low temperature reactionsGroup 3/13 element organic compoundsBoronic acidCombinatorial chemistry
A method of preparing 2-aminopyrimidine-5-boronic acid pinacol ester is disclosed. The method includes subjecting 2-chloropyrimidine that is a raw material and NBS to bromization under catalysis of BF3-Et2O to obtain 2-chloro-5-bromopyrimidine; reacting the 2-chloro-5-bromopyrimidine with n-Bu3MgLi at a temperature ranging from -20 DEG C to -10 DEG C; adding methoxyboronic acid pinacol ester or isopropoxyboronic acid pinacol ester; performing boronization to obtain 2-chloropyrimidine-5-boronic acid pinacol ester; adding into ammonia water or a methanol-ammonia solution; and reacting at 80-100 DEG C in a sealed manner to obtain the 2-chloropyrimidine-5-boronic acid pinacol ester. Synthetic process conditions of the method are mild. An ultralow-temperature reaction is avoided. Reactions can be performed continuously. A pure product can be obtained only by simple recrystallization of the final product.
Owner:天津维智精细化工有限公司
A kind of preparation method of telamectin
ActiveCN107556351BHigh yieldAvoid ultra-low temperature reactionsSugar derivativesSugar derivatives preparationPotassium tert-butoxideBenzyl chloroformate
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
A kind of preparation method of bacterial protein synthesis inhibitor
ActiveCN105566392BHigh purityAvoid it happening againGroup 5/15 element organic compoundsGroup 3/13 element organic compoundsEnvironmental resistanceChemical industry
The present invention belongs to the field of medicine and chemical industry, relates to a bacterial protein synthesis inhibitor preparation method, and specifically relates to a tedizolid phosphate preparation method. According to the method, intermediates of every steps and a final product are high in purity. Further, by the use of diisopropylamine dibenzyl phosphite as a phosphorylating agent, a dimerization product can be avoided, and the preparation method has a higher yield. The preparation method is shorter in route and mild in reaction conditions, avoids the use of toxic, irritating and strongly-corrosive reagents, is green and environmentally-friendly, meanwhile avoids the use of ultra-low temperature reaction, and is simple and easy in preparation and high in production efficiency. Therefore, the preparation method is particularly adapted to industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A method of preparing 2-aminopyrimidine-5-boronic acid pinacol ester
ActiveCN104788482ASynthetic process conditions are mildAvoid ultra-low temperature reactionsGroup 3/13 element organic compoundsBoronic acidMethanol
A method of preparing 2-aminopyrimidine-5-boronic acid pinacol ester is disclosed. The method includes subjecting 2-chloropyrimidine that is a raw material and NBS to bromization under catalysis of BF3-Et2O to obtain 2-chloro-5-bromopyrimidine; reacting the 2-chloro-5-bromopyrimidine with n-Bu3MgLi at a temperature ranging from -20 DEG C to -10 DEG C; adding methoxyboronic acid pinacol ester or isopropoxyboronic acid pinacol ester; performing boronization to obtain 2-chloropyrimidine-5-boronic acid pinacol ester; adding into ammonia water or a methanol-ammonia solution; and reacting at 80-100 DEG C in a sealed manner to obtain the 2-chloropyrimidine-5-boronic acid pinacol ester. Synthetic process conditions of the method are mild. An ultralow-temperature reaction is avoided. Reactions can be performed continuously. A pure product can be obtained only by simple recrystallization of the final product.
Owner:天津维智精细化工有限公司
A kind of method for preparing chenodeoxycholic acid analog
ActiveCN105777835BEasy to operateAvoid ultra-low temperature reactionsSteroidsCholic acidChenodeoxycholic acid
The invention discloses a new method for preparing 3 alpha, 7 alpha-dihydroxy-6 alpha-alkyl-5 beta-cholanic acid I and an intermediate thereof. The method requires no low-temperature reaction; reaction conditions are mild; a purification method is simple; and the method of the invention is suitable for industrial production.
Owner:YAOPHARMA CO LTD +1
A kind of preparation method of tedizolid phosphate
ActiveCN105418678BHigh purityAvoid it happening againGroup 5/15 element organic compoundsChemical industryPhosphate
The invention belongs to the field of medicine chemical industry and particularly relates to a preparation method for tedizolid phosphate. According to the preparation method provided by the invention, intermediates in the steps and the final product are high in purity. In addition, by using diiso-propylamido dibenzyl phosphite as a phosphorylation reagent, a dimerized product is further avoided, so that the preparation method provided by the invention is higher in yield. The preparation method provided by the invention is relatively short in route and mild in reaction condition, and further avoids use of toxic, irritant and strongly corrosive reagents, so that the preparation method is green and environmentally friendly. Meanwhile, ultralow temperature reaction is avoided, so that the preparation method is simple to operate, and the tedizolid phosphate is easy to prepare and high in production efficiency. Thereofe, the preparation method provided by the invention is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com