Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
422results about How to "Short reaction steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
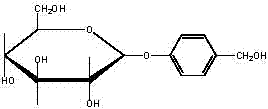
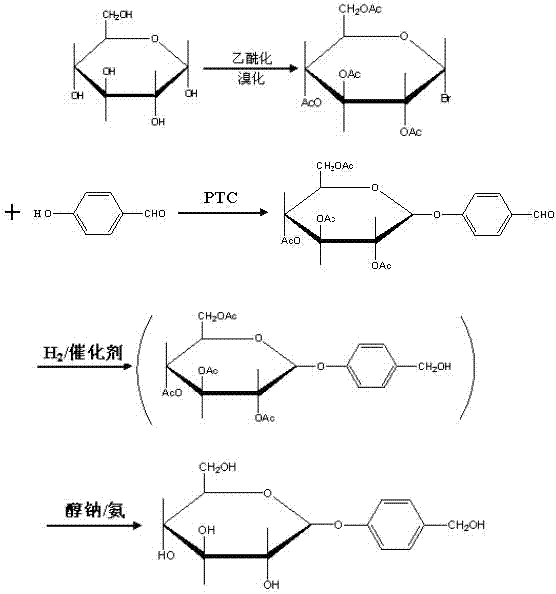
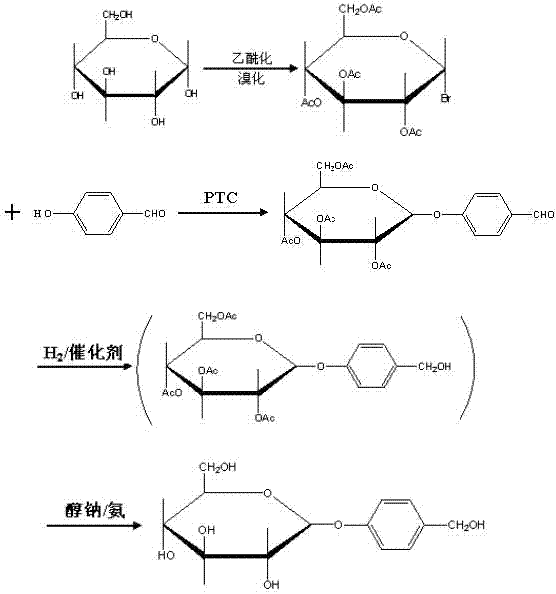
Gastrodin synthesizing method
InactiveCN102516329AReduce pollutionSuitable for industrial productionSugar derivativesSugar derivatives preparationKetone solventsPressure reduction
The invention relates to a gastrodin synthesizing method, which can effectively solve the preparation problem of gastrodin to meet the requirements of the gastrodin in pharmaceuticals. The method comprises the steps of adding catalyst perchloric acid, acetylating anhydrous dextrose by using acetic anhydride to produce per-acetyl dextrose, feeding hydrogen bromide to bromizing hemiacetal hydroxyl of the per-acetyl dextrose to produce bromo-tetraacethyl glucose, further and dropwise adding a bromo-tetraacethyl glucose solution into chloroform and tetrabutyl ammonium bromide, carbonate and para hydroxybenzene in water to obtain 4-formyl benzene-2', 3', 4', 6'- tetraacetyl-beta-D-glucopyranose, performing re-crystallization with ethanol, adding raney nickel or palladium and carbon, feeding hydrogen and pressurizing to hydrogenate, performing filtering, adding sodium alcoholate or ammonia in to filtrate to perform protecting group removal until the reaction is finished completely, performing pressure reduction and concentration to obtain crude gastrodin, and re-crystallizing the crude gastrodin by using alcohol or an alcohol and ester solvent or an alcohol and ketone solvent to obtain the gastrodin. The gastrodin synthesizing method is abundant and cheap in raw materials, simple in process, recycled in solvent, small in pollution and high in quality.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
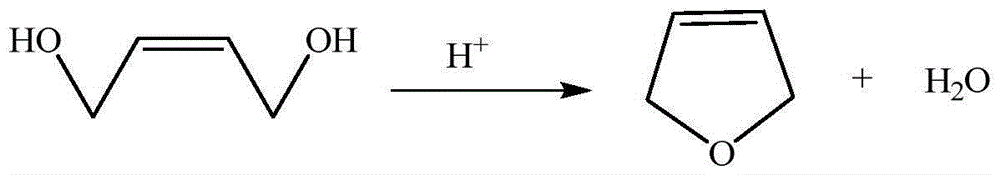
3-methylamine tetrahydrofuran preparation method
ActiveCN106397372ARaw materials are cheap and easy to getThe synthesis process is simpleOrganic chemistryCobalt acetateHydrogenation reaction
The invention provides a 3-methylamine tetrahydrofuran preparation method, wherein 1,4-butenediol is adopted as a raw material and is subjected to cyclization dehydration under the catalysis effect of a solid acid to produce 2,5-dihydrofuran, the 2,5-dihydrofuran reacts with water gas under the catalysis effect of cobalt acetate to produce 3-tetrahydrofuran formaldehyde, and the 3-tetrahydrofuran formaldehyde, ammonia gas and hydrogen gas are subjected to a hydrogenation reaction under the catalysis effect of 5% Pd / C to prepare the target product3-methylamine tetrahydrofuran, wherein the purity is 98.12%, and the total yield is 74.16%. According to the present invention, the preparation method has advantages of cheap and easily available raw materials, less steps, simple operation, high product purity, high yield, less three-waste pollution and production cost reducing, and is suitable for industrial production.
Owner:浙江捷达科技有限公司
Preparation method of Agomelatine
InactiveCN101735091AProcess stabilityEasy to operateOrganic compound preparationCarboxylic acid amides preparationAgomelatineStereochemistry
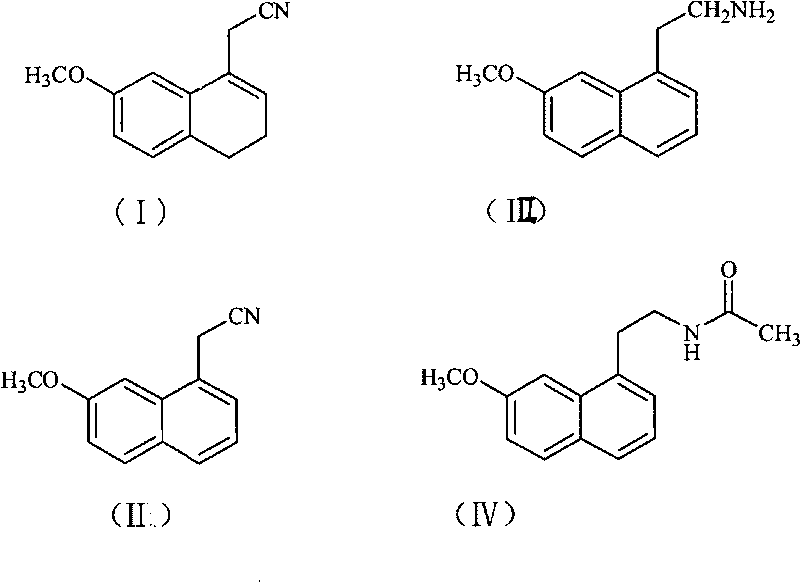
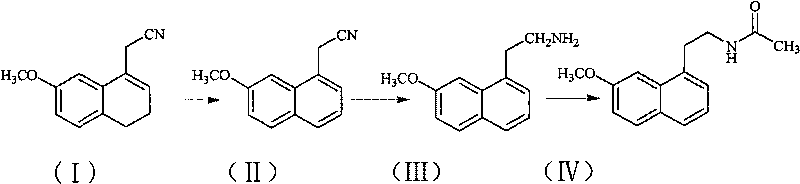
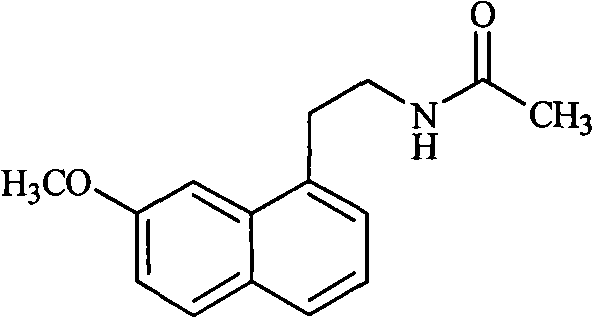
The invention relates to a preparation method of Agomelatine, which aims at economically preparing the Agomelatine on a large scale. The preparation method is characterized by comprising the following steps of: carrying out the dehydrogenation on a compound in a formula (I) under the action of DDQ to obtain a compound in a formula (II), reducing the compound in the formula (II) by sodium borohydride to obtain a compound in a formula (III), and reacting the compound in the formula (III) with acetylchloride to obtain a compound in a formula (IV), that is to say, the compound in the formula (IV) is the Agomelatine.
Owner:万全万特制药江苏有限公司
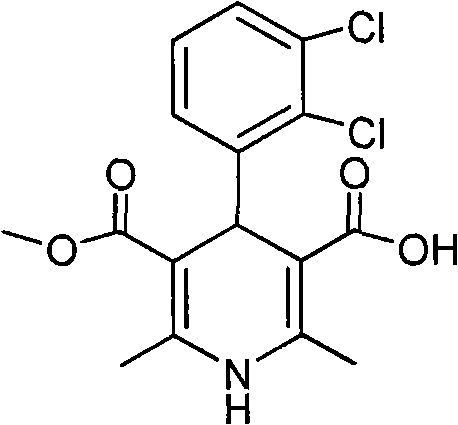
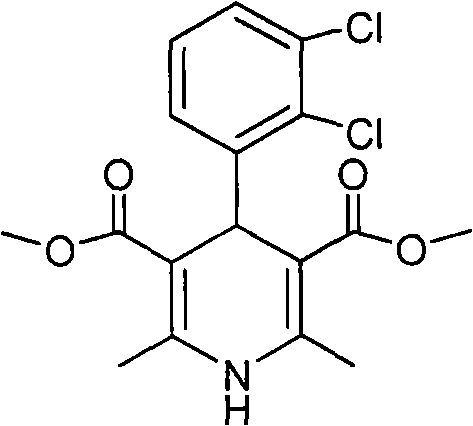
New preparation method of key intermediate of clevidipine butyrate
InactiveCN101602710AShort reaction stepsMild reaction conditionsOrganic chemistryAcetic acidClevidipine
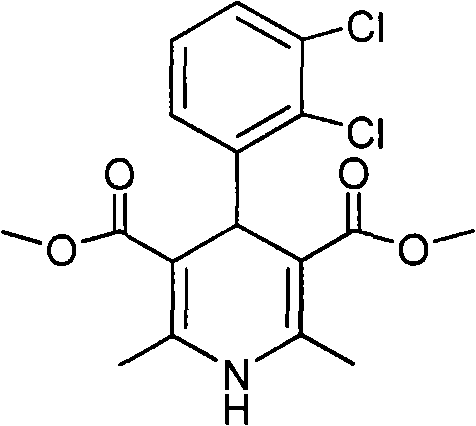
The invention relates to a preparation method of 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxylcarbonyl-3-pyridinecarboxylic acid (I). The method is characterized in that methyl acetoacetate, stronger ammonia water and 2, 3-dichlorobenzaldehyde are used as raw materials for direct cyclic condensation to obtain 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-3, 5-pyridinedicarboxylic acid methyl ester (II); part of the compound II is hydrolyzed under alkaline condition to obtain the product 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxylcarbonyl-3-pyridinecarboxylic acid (I). The method has the advantages that the starting materials are cheap and easy to obtain, the production cost is lowered, the reaction is easy to operate and industrialization is easy to realize.
Owner:CHINA PHARM UNIV
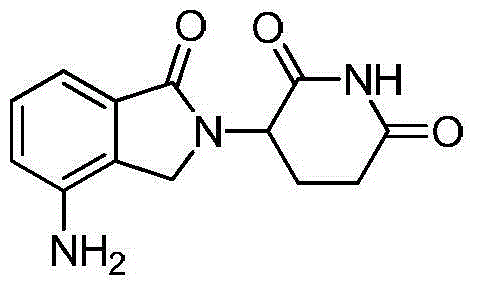
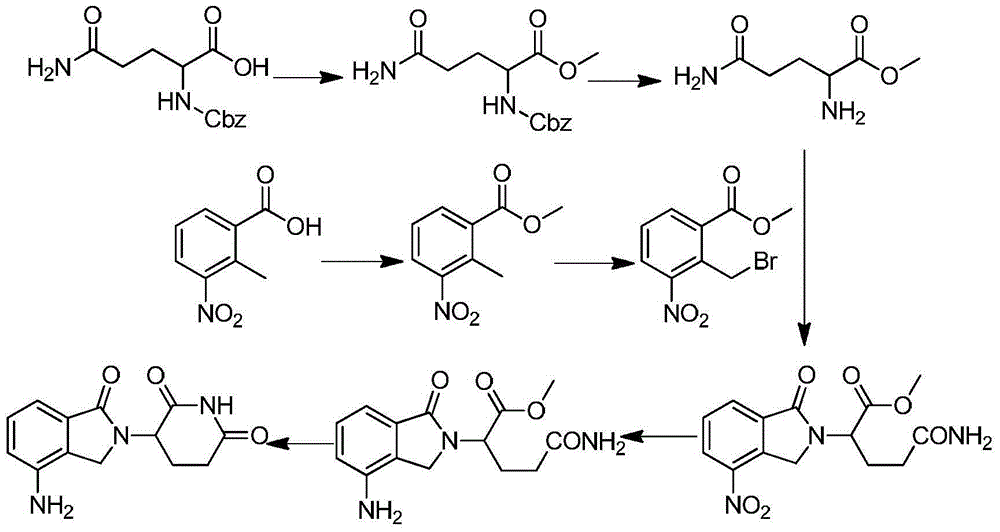
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
Synthetizing method of lacosamide
InactiveCN103113256AHighlight substantive featuresSignificant progressOrganic compound preparationCarboxylic acid amides preparationPtru catalystAmmonium chloride mixture
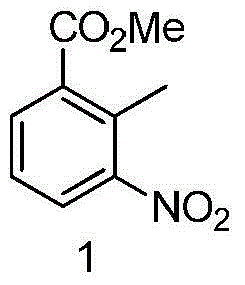
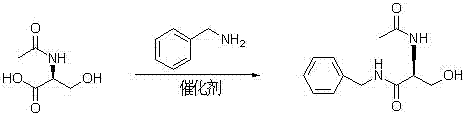
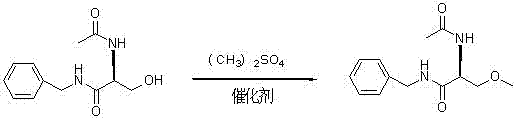
The invention provides a synthetizing method of lacosamide. The method comprises the steps of: based on D-serine as a raw material, performing an acylation reaction with acetic anhydride and then performing a condensation reaction with benzylamine; and finally, performing a methylation reaction with dimethyl sulfate, thereby obtaining lacosamide, wherein N,N' dicyclohexylcarbodiimide (DCC) or N,N' carbonyl diimidazole (CDI) is used as a catalyst in the condensation reaction; and a phase transfer catalyst including triethyl benzyl ammonium chloride (TEBA), tetrabutylammonium chloride (TBAC), tetrabutylammonium bromide (TBAB) or tetrabutylammonium hydrogen sulfate (TBAHS) is adopted in the methylation reaction. The method has the advantages of being simple in synthetizing process, moderate in reaction condition, simple in after-treatment, high in yield and high in product purity.
Owner:SUZHOU HONGRUI MEDICAL TECH
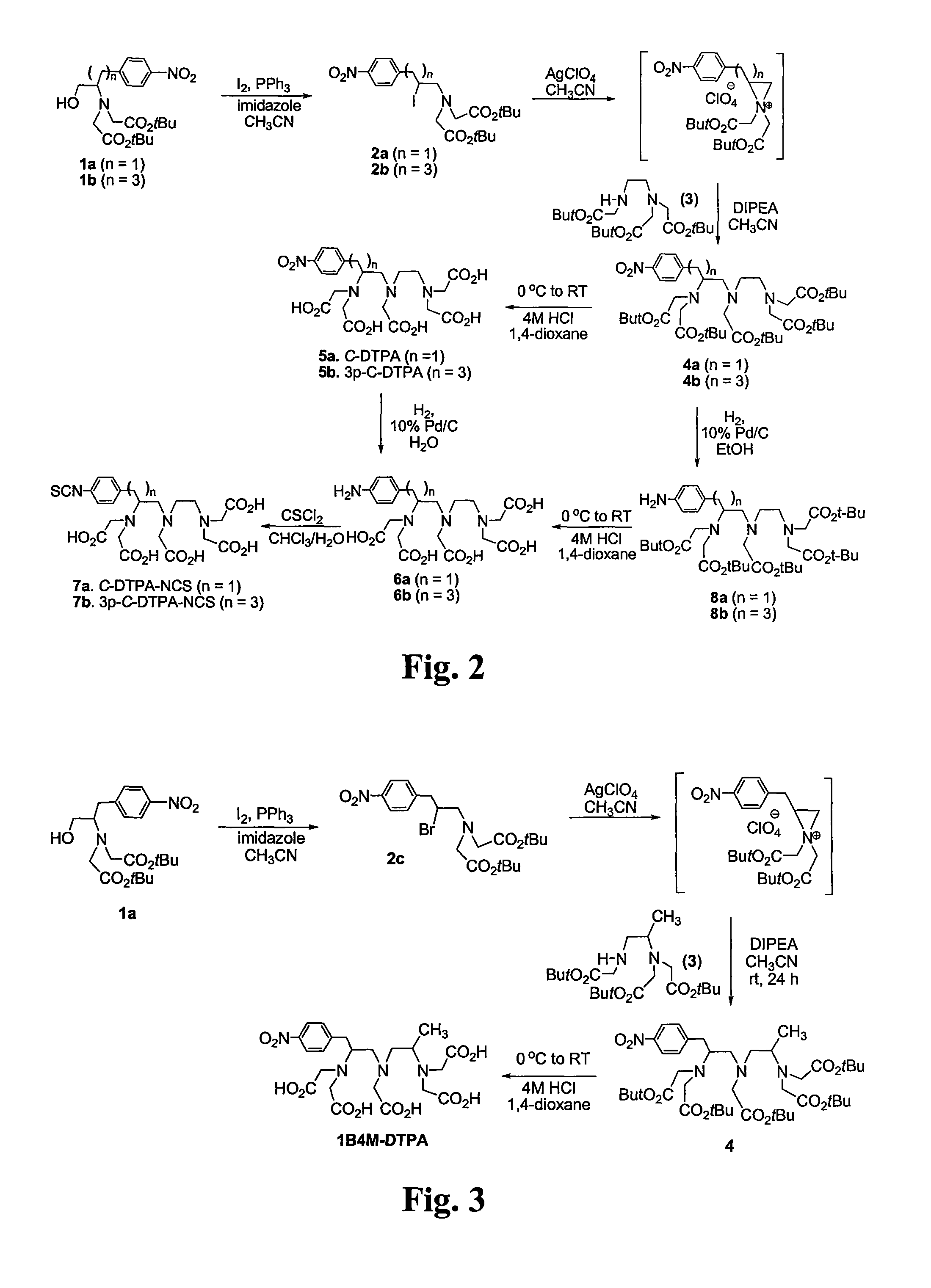
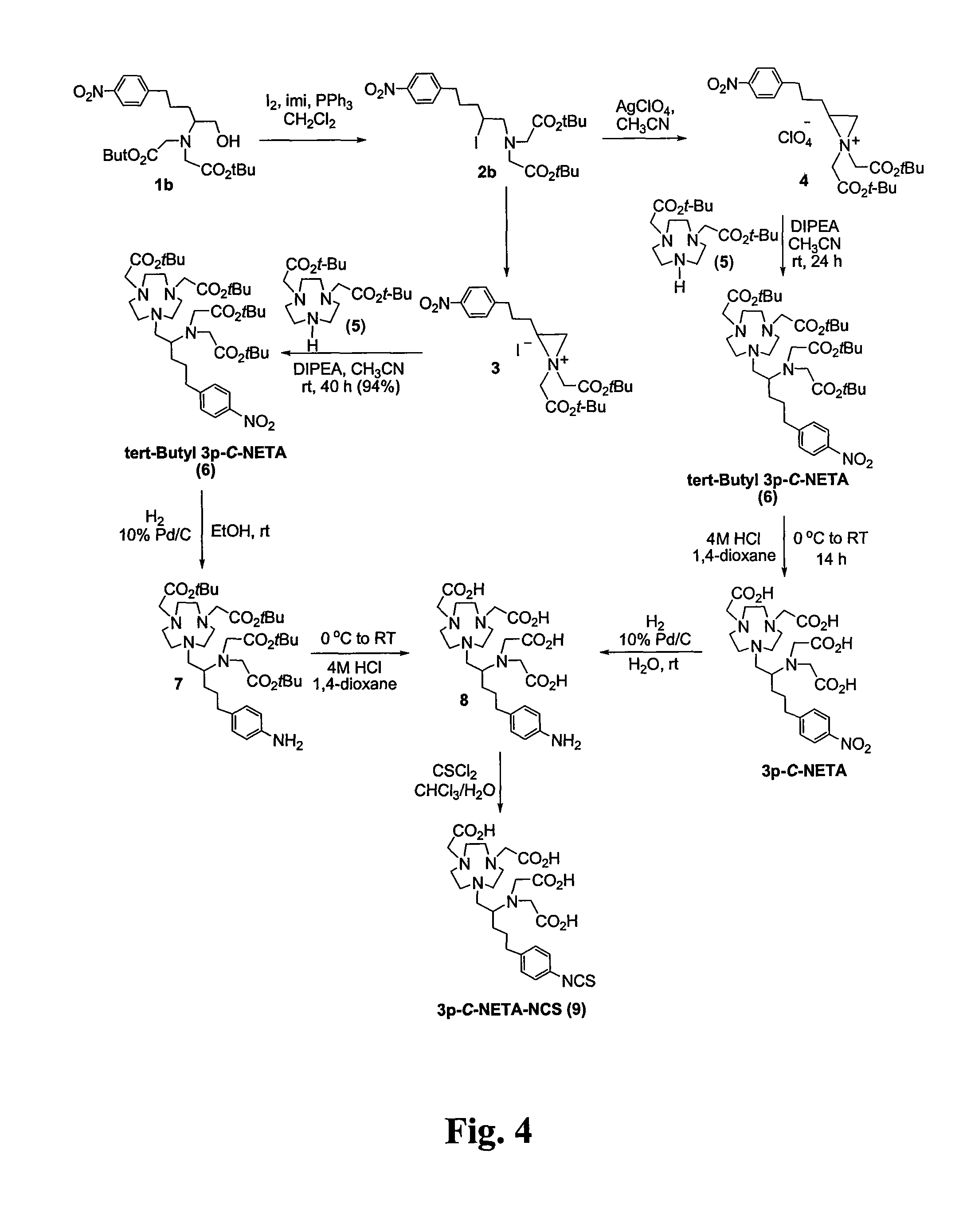
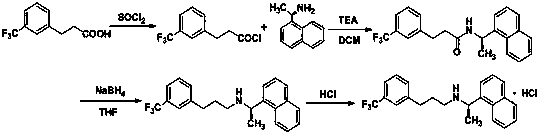
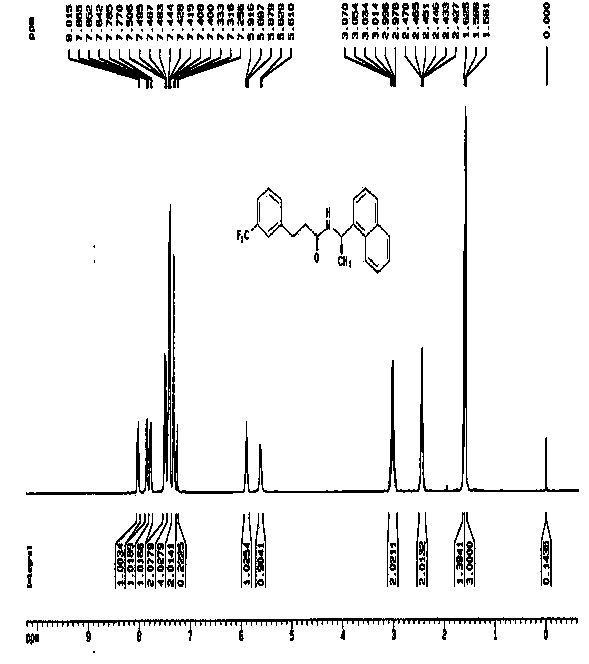
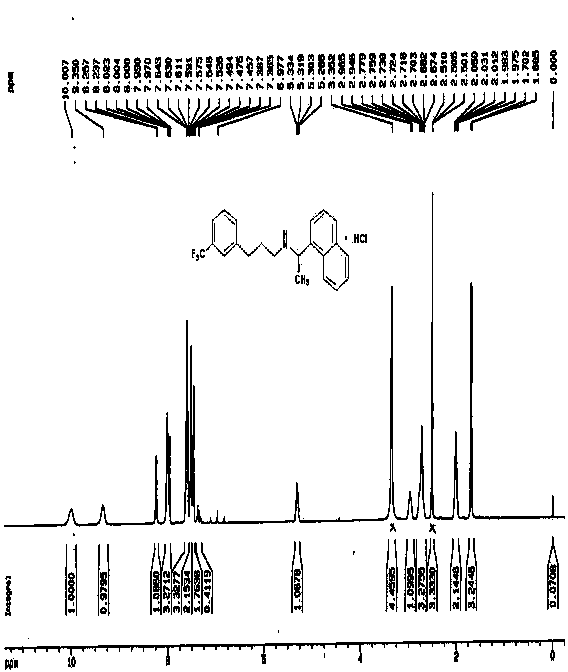
Synthesis of therapeutic and diagnostic drugs centered on regioselective and stereoselective ring opening of aziridinium ions
ActiveUS9446995B2Short reaction stepsHigh yieldThiol preparationOrganic compound preparationRegioselectivityStereoselectivity
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
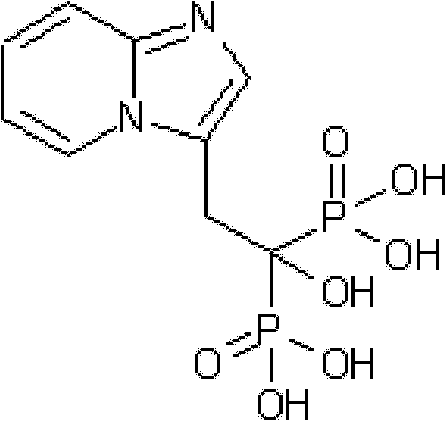
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585AAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
Preparation method of 3-mercaptopropyltriethoxysilane coupling agent
ActiveCN103408582AShort reaction stepsSimple production processGroup 4/14 element organic compoundsTriethoxysilaneCoupling
The invention relates to a preparation method of a 3-mercaptopropyltriethoxysilane coupling agent, belonging to the technical field of organic chemistry. The preparation method comprises the following steps of: with sodium hydrosulfide and 3-chloropropyltriethoxysilane as raw materials and water as a solvent, heating, stirring and reacting under the action of a phase transfer catalyst to obtain a crude product of silane; and drying the crude product, filtering and distilling to obtain the 3-mercaptopropyltriethoxysilane coupling agent product. The preparation method is simple in process, lower in production cost, high in product yield, little in odor, safe, environment-friendly and easy to realize large-scale industrial production.
Owner:JINGZHOU JIANGHAN FINE CHEM
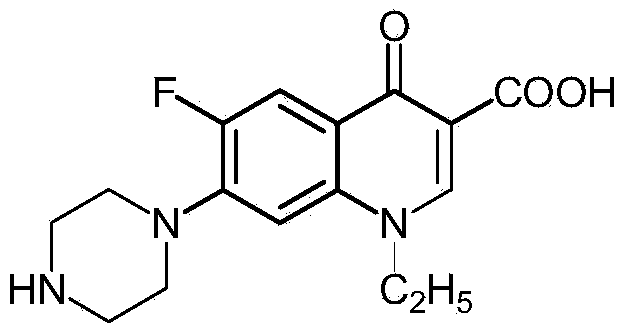
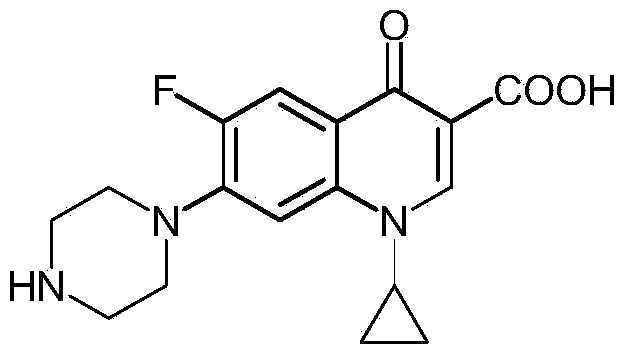
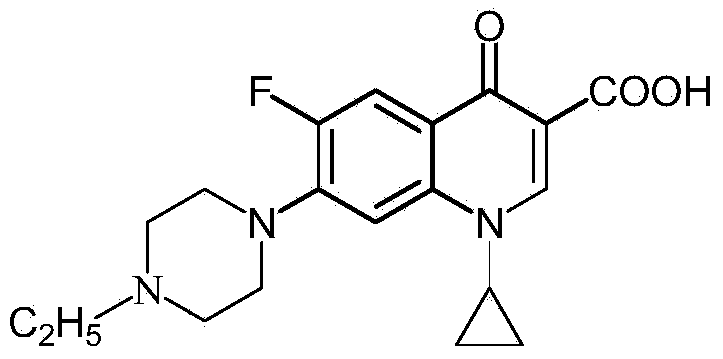
Preparation method of norfloxacin, ciprofloxacin and enrofloxacin
ActiveCN104292159AReduce consumptionEmission reductionOrganic chemistryCarboxylic saltReaction temperature
The invention discloses a preparation method of norfloxacin, ciprofloxacin and enrofloxacin. The preparation method comprises the following steps: directly reacting 1-ethyl-6-fluoro-7-chlo-4-oxo-1,4-dihydro-quinoline-3-carboxylate or 1-cyclopropyl-6-fluoro-7-chlo-4-oxo-1,4-dihydro-quinoline-3-carboxylate with piperazine (or N-ethyl piperazine); and then, performing after-treatment to prepare a corresponding target product norfloxacin (or ciprofloxacin or enrofloxacin). The preparation method disclosed by the invention is short in reaction step, convenient to operate, less investment and beneficial to industrial production; consumption of piperazine (or N-ethyl piperazine) can be reduced by more than half; under the catalytic action, the reaction temperature is low, the byproducts are less, the yield is high and the cost is low; heavy use of inorganic acid and inorganic alkaline is avoided, so that the pollution is reduced.
Owner:ZHEJIANG BENLI TECH CO LTD
Method for synthesizing and refining cinacalcet hydrochlorid
ActiveCN103739500AConvenient sourceLow costAmino compound purification/separationOrganic compound preparationEngineeringEnvironmental engineering
The invention explores a method for synthesizing and refining cinacalcet hydrochlorid, especially avoids toxic and expensive reaction reagents reported in literatures; the invention has the advantages of convenient sources of raw materials and reagents, low cost, little pollution to environment and simple operation, the invention is suitable for industrial production.
Owner:SINOPHARM A THINK PHARMA
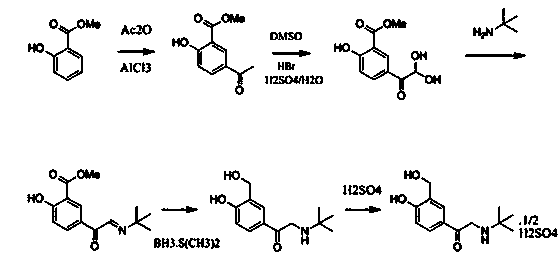
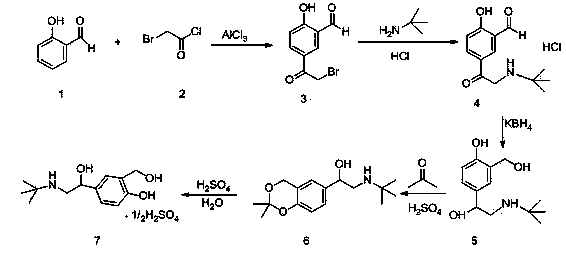
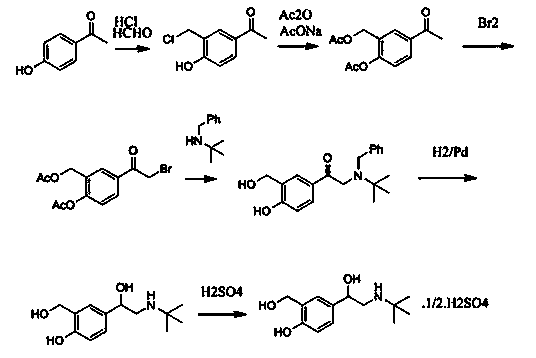
Production technology for synthetizing salbutamol sulphate
InactiveCN104356009AShort reaction stepsMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationChemical synthesisSalicylaldehyde
The invention discloses a production technology for synthetizing salbutamol sulphate, and relates to the field of chemical synthesis. The production technology comprises the following steps: firstly, performing an Friedel-crafts acylation reaction on salicylal to obtain a chemical compound 3, performing a substitution reaction of tert-butylamine, protecting dihydroxy by propylidene under the catalysis of concentrated sulfuric acid through the reduction of potassium borohydride or sodium borohydride, then performing extraction for desalting, and generating a hydrolysis reaction under acid conditions to obtain sulphuric acid salbutamol. The production technology disclosed by the invention is easy to control and operate, raw materials are simple and easy to obtain, the total mol yield is as high as 40%, the product purity is as high as 99.5%, and the product cost is low.
Owner:扬州市三药制药有限公司
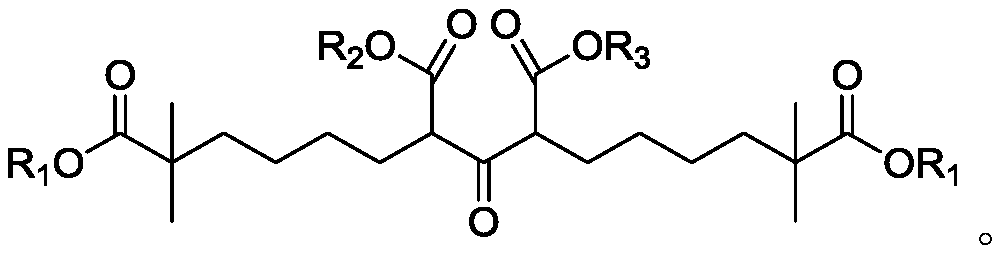
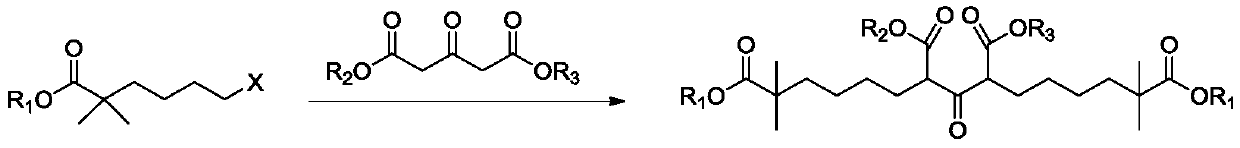
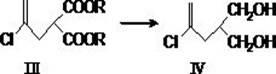
Compound and method for synthesizing 8-hydroxy-2,2,14,14-tetramethyl pentadecanedioic acid by adopting compound
ActiveCN111170855AReduce usageShort reaction stepsOrganic compound preparationCarboxylic acid esters preparationChemical synthesisBiochemical engineering
The invention belongs to the field of chemical synthesis, and relates to a compound with a structural formula as described in the specification. In the structural formula, R1, R2 and R3 are independently selected from C1-C6 alkyl groups, alkenyl groups and cycloalkyl groups respectively. The invention also provides a method for synthesizing 8-hydroxy-2,2,14,14-tetramethyl pentadecanedioic acid byusing the compound. The method has the advantages of short reaction steps, simplified operation and greatly reduced production cost; and the use of raw materials with high toxicity and danger is avoided, so the method is safer.
Owner:AURISCO PHARM(TIANJIN) INC +2
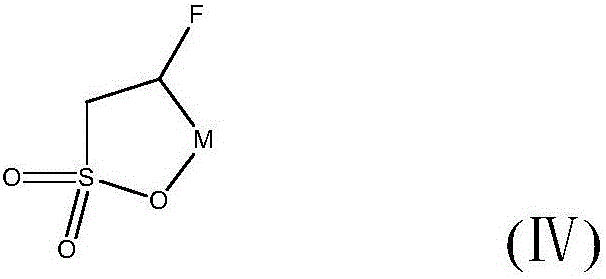
Preparation method of fluorosultone
The invention relates to generation of a sodium dihydroxyl sulfonate compound by a substitution reaction of a saturated aqueous solution of sodium sulfite and a chloro dihydroxy compound; a dehydration reaction is carried out for the sodium dihydroxyl sulfonate compound obtained by the step and excess dehydrating agents, after the reaction, excess dehydrating agents are removed; residual sodium dihydroxyl sulfonate is removed by washing and extracting the reaction products; drying and purification are carried out in order to obtain a hydroxy substituted sultone compound; a fluorination reaction is carried out for the obtained hydroxy substituted sultone compound and a fluorating agent, underpressure distillation and purification are carried out, and the fluorosultone compound is obtained. The method has the advantages of cheap raw material, easy purchase, simple operation steps, mild reaction conditions, short reaction steps, easy purification, and high purity of produced products.
Owner:宁德市凯欣电池材料有限公司
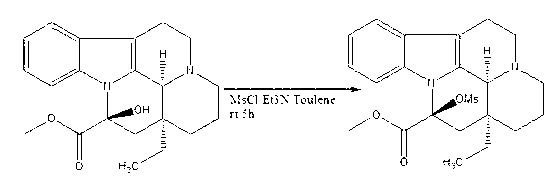
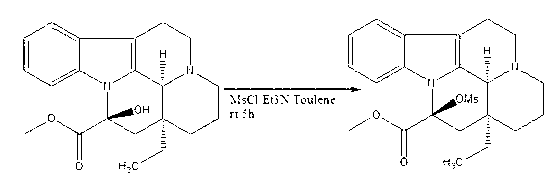
Synthesis method of vinpocetine
ActiveCN102702191AShort reaction stepsHigh product purity and yieldOrganic chemistryDistillationSodium ethoxide
The invention discloses a synthesis method of vinpocetine, comprising the following steps: adding vincamine into a three-opening bottle, subsequently, adding methylbenzene, stirring and dropwise adding triethylamine and methylsufonyl chloride in an ice-water bath, slowly heating to room temperature after stirring for 2 h, then continuously stirring for 5 h; stopping reaction, drying solvent by distillation, adding ethanol and water, regulating pH to 12 with saturated potassium carbonate solution, separating out solids, filtering, and drying in vacuum to obtain vinpocetine intermediate; adding absolute ethyl alcohol into the three-opening bottle, stirring in ice-water bath for 1 h, then slowly adding sodium ethoxide, stirring for 0.5 h, then adding the vinpocetine intermediate, subsequently, putting a reaction bottle into an oil bath, setting temperature as 80 degrees centigrade, evaporating most of solvent after reacting for 12 h, then pouring solution into hydrochloric acid, extracting with ethyl acetate, regulating pH to 12 with water phase, separating out solids, filtering and drying to obtain the vinpocetine. According to the synthesis method provided by the invention, reaction steps are short; purity and yield of products are high; energy consumption is low; and environmental pollution is less.
Owner:JIANGSU QINGJIANG PHARMA
Method for preparing N-dimethylamino propyl methyl acrylamide
InactiveCN101177403AShort reaction stepsEasy to operateOrganic compound preparationCarboxylic acid amides preparationReaction stepDiamine
The invention relates to a method for preparing N-dimethylamino propyl methacrylamide by catalyzing and cracking, which comprises the following technical steps: A. two incipient ingredients methyl methacrylate and N, N-dimethyl-1, 3-propane diamine are sealed in an autoclave for catalyzing and condensing, so as to generate the intermediate N-dimethylamino-Beta-dimethylamino methacrylamide; B. putting the intermediate N-dimethylamino-Beta-dimethylamino methacrylamide into a reactor with distilling apparatus and driving off the crude products of N-dimethylamino propyl methacrylamide through catalyzing and cracking; C. adding the crude products of N-dimethylamino propyl methacrylamide and polymerization inhibitor into a rectifying tower to generate the finished products. The invention has the advantages of less reaction steps, high conversion rate, small amount of byproducts, low cost for post treatment, and ability to meet application demand.
Owner:本溪万哈特化工有限公司
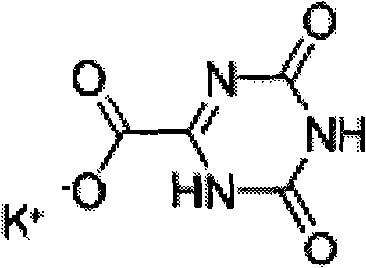
Preparation method suitable for industrially producing oteracil potassium
InactiveCN102250025AShort reaction stepsThe refining method is simpleOrganic chemistryQuality standardOxidizing agent
The invention provides a preparation method suitable for industrially producing oteracil potassium. The method provided by the invention has the advantages that: reaction steps are fewer, oxidants which generate gases polluting the environment are not used, a refining method is simple, the consumption of organic solvents which are harmful to the human body is decreased, the cost is saved, the total yield can reach more than 65%, and the method is suitable for the industrial production; the purity of a product reaches more than 99.8%, the impurity content of the single product is less than 0.1% and the purity and the impurity of the product both reach quality standards for the raw material medicine; and the obtained product is the oteracil potassium raw material medicine which is suitable for preparation of a medicinal preparation.
Owner:深圳万乐药业有限公司
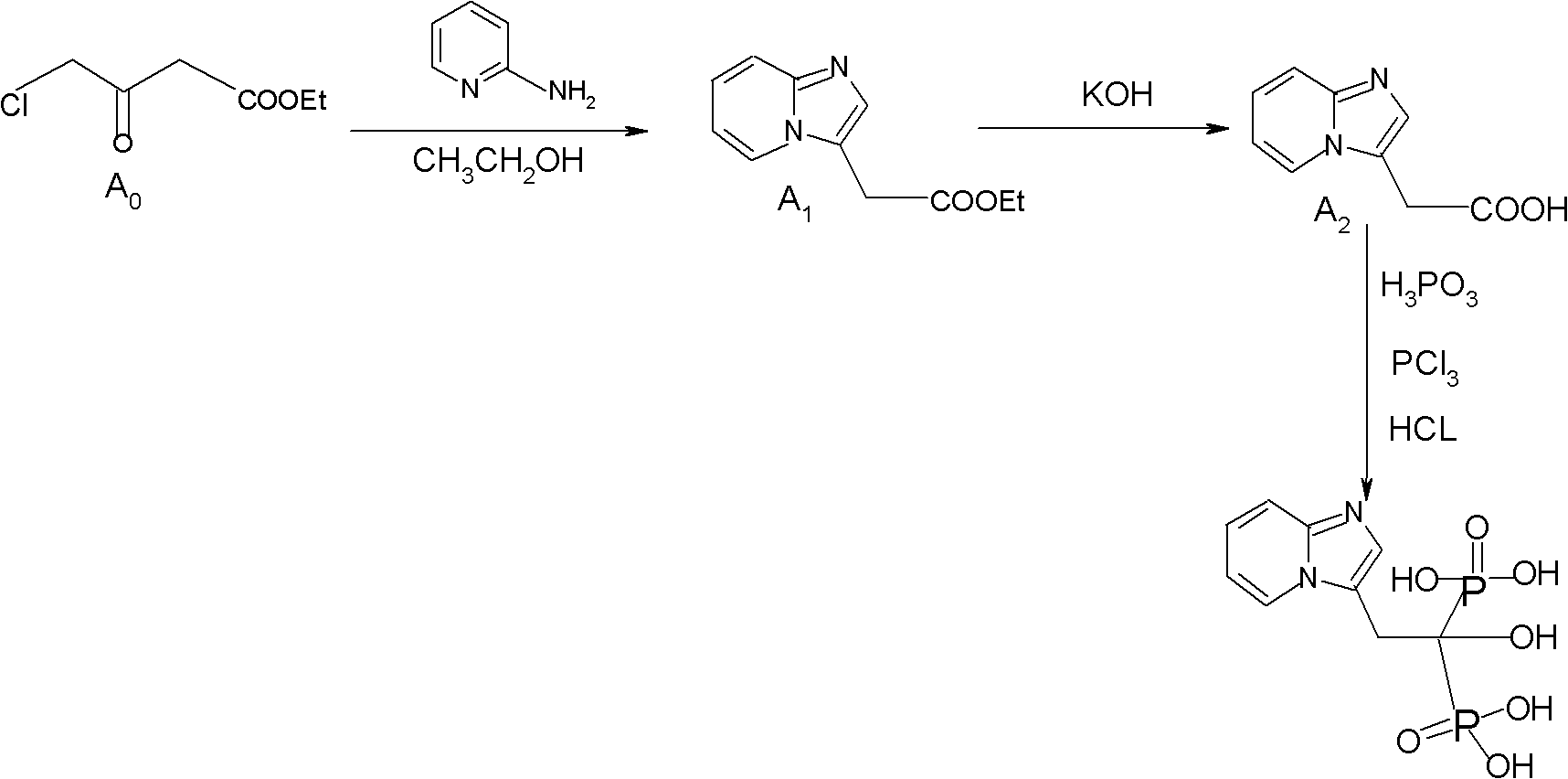
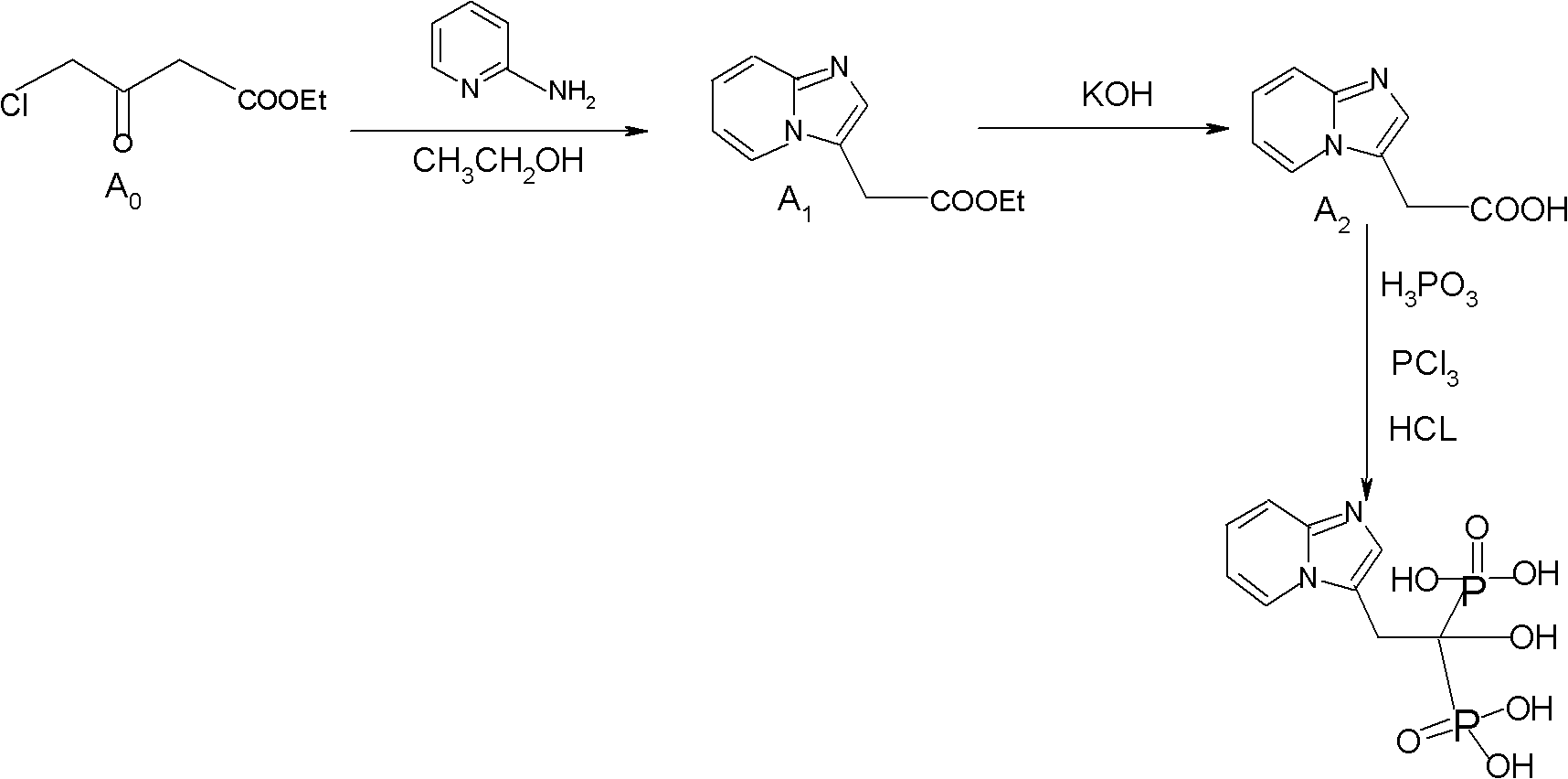
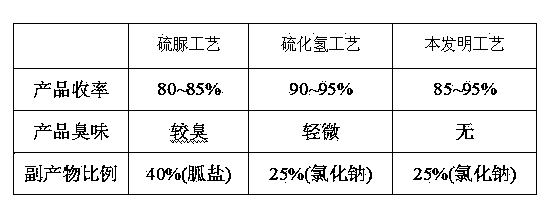
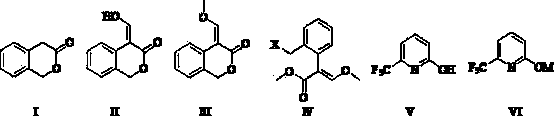
Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid
The invention provides a method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid, which comprises the following steps of: reacting 4-chloro-acetoacetic acid ester and 2-aminopyridine in an organic solvent in the presence of an acid binding agent to obtain 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid ester; and hydrolyzing the 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid under the acid or alkaline condition to obtain the 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid. In the method, raw materials have high stability and low price and are readily available; deadly toxic cyanides or high corrosion and unstable bromides are not used; the reaction step is short; the operation is simple; the method is safe and environment-friendly; special equipment is not needed; and the method is suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of carbazochrome sodium sulfonate
InactiveCN102757378AShort reaction stepsMild reaction conditionsOrganic chemistryHemostaticsSodium bisulfate
The invention relates to a preparation method of carbazochrome sodium sulfonate, belonging to the technical field of preparation of vascular hemostatics. The method comprises the following steps: raw materials dissolution and reaction: putting purified water, carbazochrome, sodium bisulfite and ascorbic acid into a reaction tank, heating while stirring until the solid substances are completely dissolved, and continuing keeping the temperature to react, thereby obtaining a reaction solution; decolorization and separation: adding a decolorant into the reaction solution, keeping the temperature while stirring for decolorization, after finishing decolorization, carrying out centrifugal filtration, washing residues with purified water, carrying out centrifugal drying, and merging the centrifugal filtrates to obtain a solution to be crystallized; crystallization: regulating the pH value of the solution to be crystallized with an alkaline matter, freezing to cooling, and standing to precipitate crystals; and refinement: carrying out centrifugal filtration on the crystals, washing, carrying out centrifugal drying, and drying in a vacuum drying oven to obtain the carbazochrome sodium sulfonate. The invention has the advantages of accessible reaction raw material, short reaction steps, mild reaction conditions, high reaction yield (up to more than 90%), better reaction product quality and high purity (up to more than 99%), and is convenient and simple to operate.
Owner:JIANGSU HI STONE PHARMA
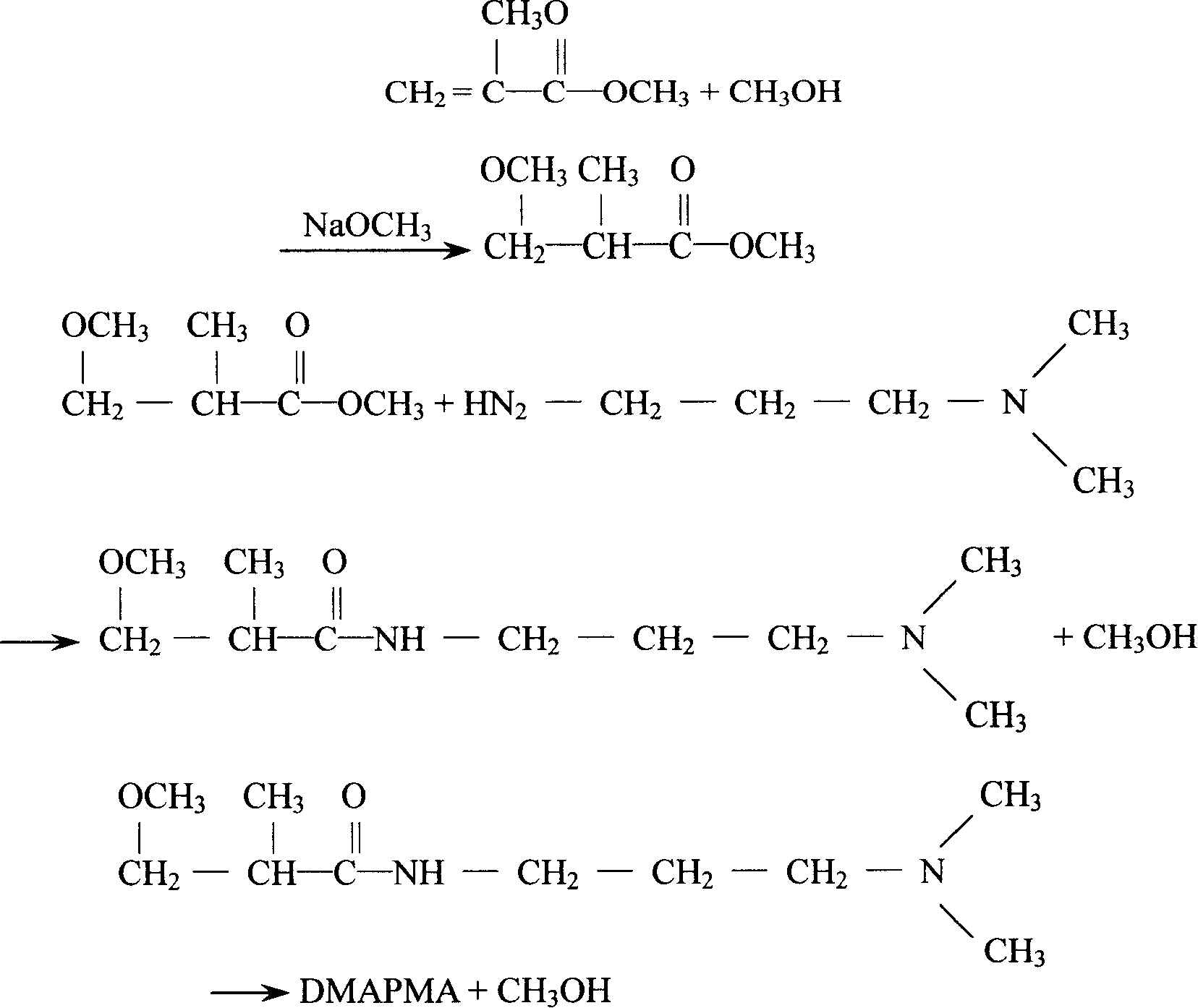
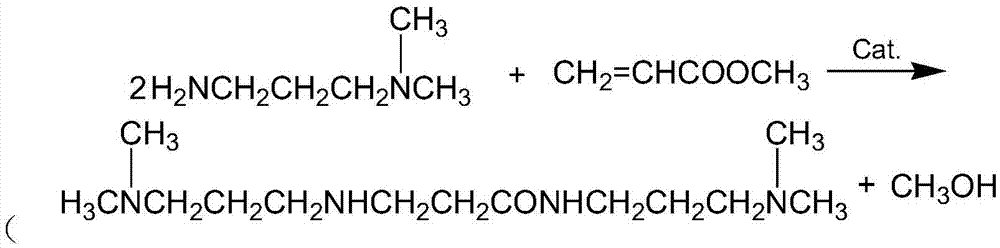
Method for preparing DMAPPA (N-(3-dimethyl aminopropyl) acrylamide) through catalytic amidation
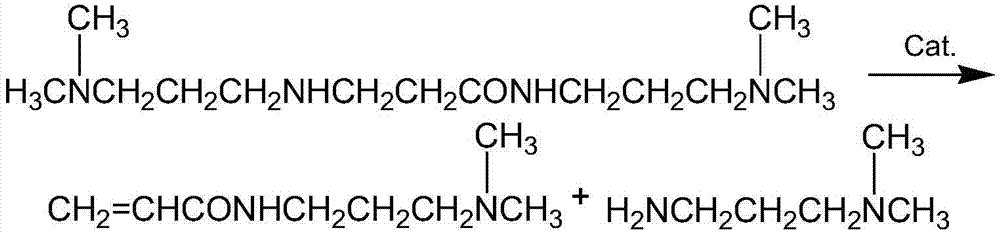
InactiveCN105439890AEasy to operateShort reaction stepsOrganic compound preparationCarboxylic acid amides preparationSolventOrganic synthesis
The invention belongs to the field of organic synthesis and particularly relates to a method for preparing DMAPPA (N-(3-dimethyl aminopropyl) acrylamide) through catalytic amidation. According to the method, methyl acrylate, N,N-dimethyl-1,3-propanediamine, a catalyst and an inhibitor are added to a reaction kettle, stirred, heated and cooled, excessive solvent is steamed out, an intermediate product is subjected to reduced pressure distillation treatment, then a distillate is added to a fractionating tower, reduced-pressure heating is performed for collection of a product distillate, and DMAPPA with the purity higher than or equal to 98% is obtained. The process developed with the method is simple and convenient to operate, a few reaction steps are included, and the raw material methyl acrylate is low in cost, easy to obtain and convenient to store and transport; methyl alcohol is generated in a reaction process, so that the reaction temperature is reduced, reaction conditions are mild, and side reactions are reduced; the novel catalyst is selected, accordingly, the reaction speed is high, the yield is high, the product quality is good, polymerization in a tower due to high temperature and high material activity in a distillation process is avoided, and three wastes are hardly generated.
Owner:SHENYANG RES INST OF CHEM IND
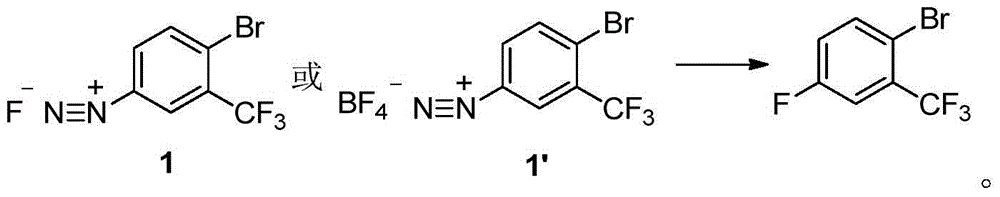
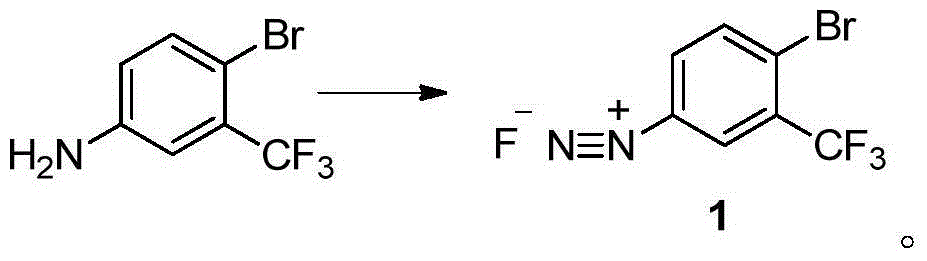
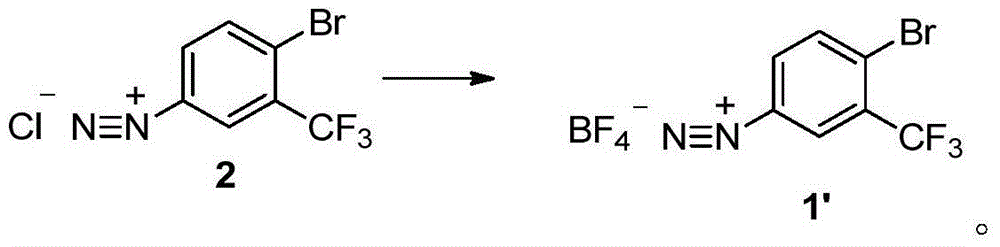
Method for preparing 2-bromine-5-trifluorotoluene chloride
ActiveCN104447183AIncrease profitHigh reaction conversion rateHalogenated hydrocarbon preparationBulk chemical productionOrganic solventBromine
The invention discloses a method for preparing 2-bromine-5-trifluorotoluene chloride. The method comprises the following step: under an anhydrous condition and in an organic solvent, performing cracking reaction on an anhydrous compound 1 or a compound 1', thereby preparing 2-bromine-5-trifluorotoluene chloride. According to the method, the reaction of an upper amino protecting group of m-trifluoromethyl phenylamine, the bromination reaction and the reaction of removing the amino protecting group can be all performed in one same reaction kettle without transferring or storing materials. The raw materials used in the method disclosed by the invention are cheap and easy to obtain, the reaction step is short, the reaction condition is gentle, the utilization rate of bromine is high, and the positioning selectivity of bromine feeding is high, so that a final product is low in isomeride impurity, high in reaction conversion rate, high in yield, high in product purity, low in production cost is low and applicable to industrialization production. The compound 1 and compound 1' are as shown in the specification.
Owner:JIANGSU LIANHE CHEM TECH +4
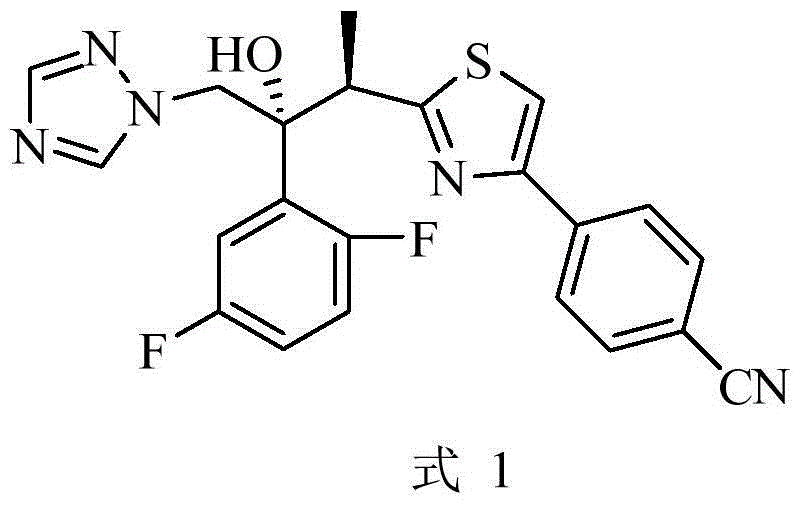
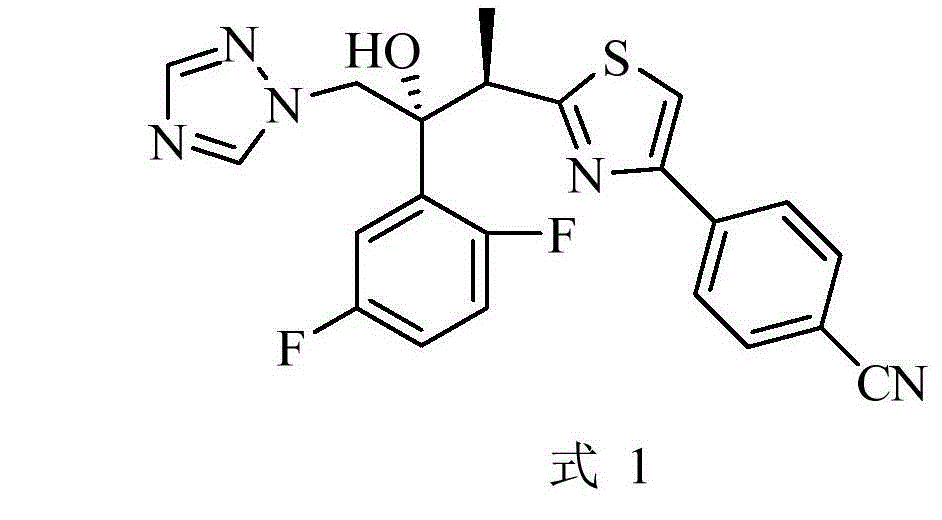
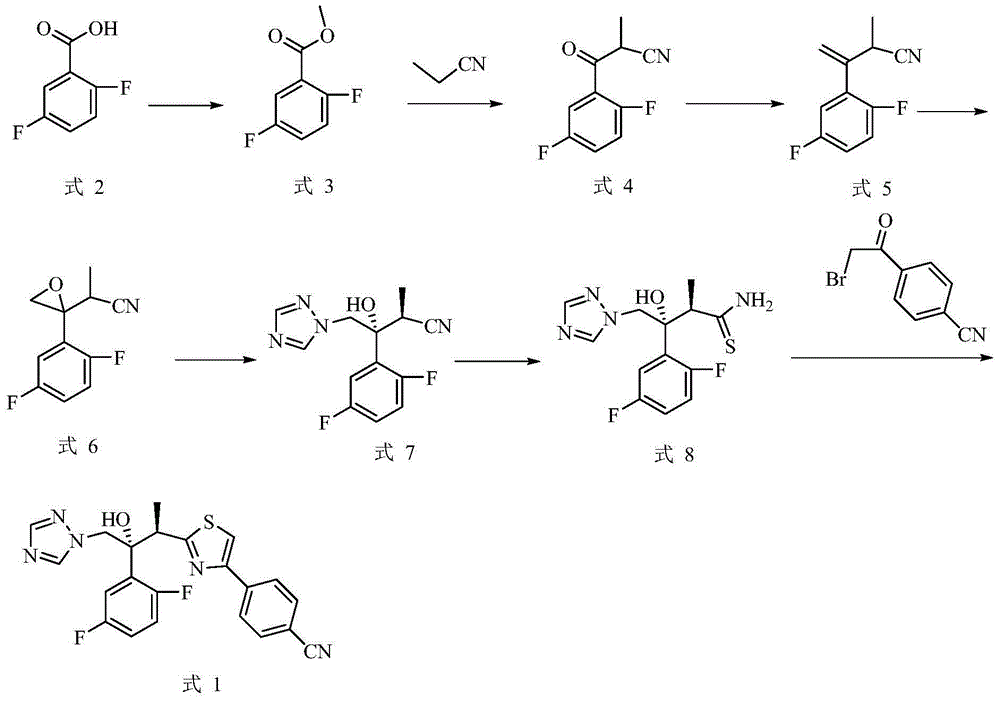
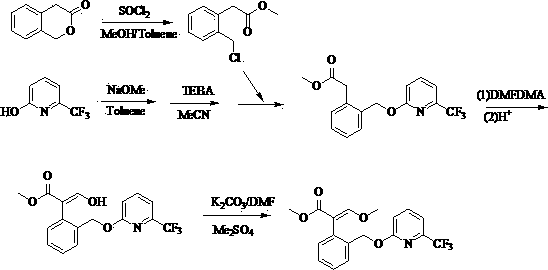
Preparation method of isavuconazole intermediate
InactiveCN105732605AShort reaction stepsMild reaction conditionsOrganic chemistryThiazoleCombinatorial chemistry
The invention belongs to the technical field of chemical drug intermediate preparation and relates to a preparation method of an isavuconazole intermediate. The preparation method is a 4-(2-((2R, 3R)-3-(2, 5-difluorophenyl)-3-hydroxy-4-(1H-1, 2, 4-triazole-1-yl)butane-2-yl)thiazole-4-yl)benzontrile preparation method. The 4-(2-((2R, 3R)-3-(2, 5-difluorophenyl)-3-hydroxy-4-(1H-1, 2, 4-triazole-1-yl)butane-2-yl)thiazole-4-yl)benzontrile has a structural formula (I) shown in the description.
Owner:KBP BIOSCIENCES CO LTD
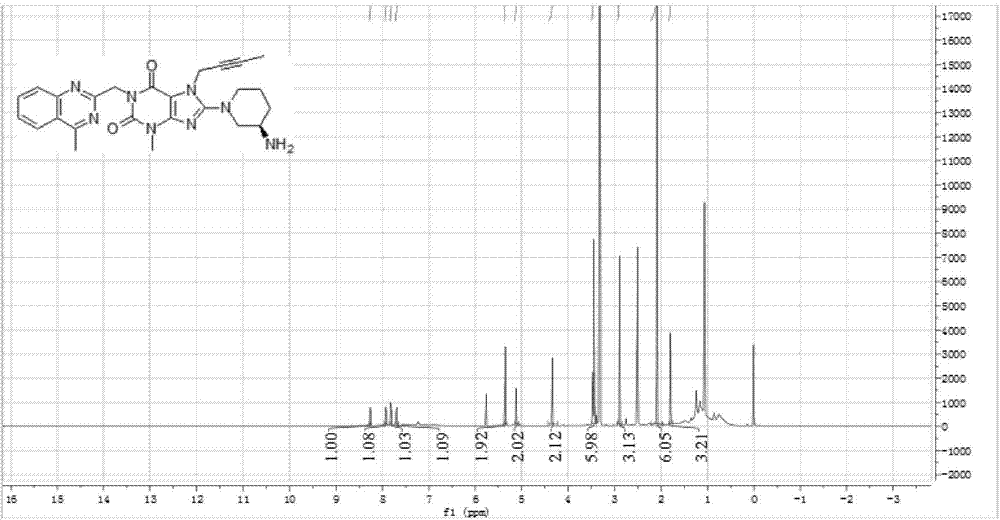
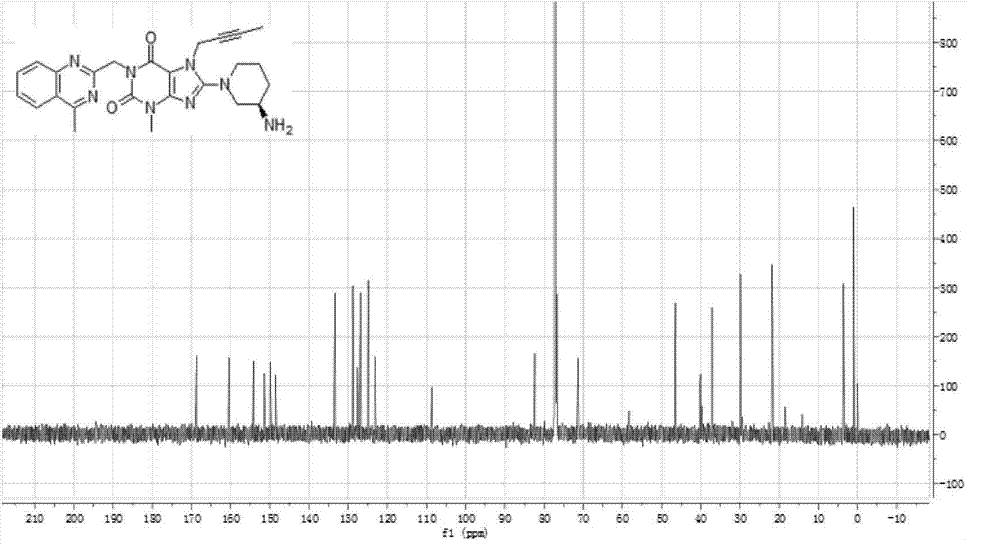
Simple preparation method of II-type antidiabetic drug linagliptin
InactiveCN104844603AEasy to operateShort reaction stepsOrganic chemistryMetabolism disorderFiltrationXanthine
The invention discloses a simple preparation method of II-type antidiabetic drug linagliptin which comprises the following steps: adding 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine into a DMSO (dimethylsulfoxide) solution of 2-chloromethyl-4-methyl-quinazoline and potassium carbonate A, and under the catalysis of potassium iodide, reacting for 7-8 hours at a temperature of 70-80 DEG C; adding potassium carbonate B and (R)-3-aminopiperidine, and reacting for 7-8 hours at a temperature of 70-80 DEG C; after the reaction is completed, adding saturated salt water, separating out solids, and carrying out suction filtration to obtain a crude product linagliptin; and recrystallizing the crude product linagliptin with methyl alcohol, carrying out suction filtration and drying, thus obtaining a target product. The method disclosed by the invention is implemented by using a one-pot method, so that the method is simple and easy to operate and short in reaction steps, and raw materials are cheap and easily available, therefore, the method is applicable to industrial mass production; and the method overcomes the problems in the conventional method that impurities are difficult to remove, line operations are complicated and time-consuming, and too many raw materials are required.
Owner:WUHAN UNIV OF TECH
Method for synthesizing high-efficiency green agriculture bactericide
Owner:ZHEJIANG TIDE CROP TECH
Preparation method of posaconazole intermediate
InactiveCN102643194ANovel process routeReasonable process conditionsOrganic compound preparationCarboxylic acid esters preparationBiochemical engineeringPharmaceutical drug
The invention belongs to the technical field of pharmacochemistry and in particular relates to a preparation method of a posaconazole intermediate. The method comprises four steps of alkylation, reduction, acylation and cross-coupling, so that a target compound (I) is obtained. Compared with the existing synthetic method, the synthetic method provided by the invention has the characteristics of low price and easiness in obtainment of raw materials, simple and convenient technology, high yield and the like.
Owner:FUZHOU UNIV
Synthetic method of bempedoic acid
PendingCN111825546AShort reaction stepsHigh yieldOxygen-containing compound preparationCarboxylic acid nitrile preparationBempedoic acidGrignard reagent
The invention discloses a synthetic method of bempedoic acid. The method comprises the following steps of: taking isobutyronitrile (ester) as a starting raw material, carrying out reaction with 2, 5-dibromopentane under the catalysis of alkali to generate 7-bromo-2, 2-dimethylheptonitrile (ester), then forming a Grignard reagent with magnesium, carrying out reaction with formate to generate 8-hydroxy-2, 2, 14, 14-tetramethylpentadecane dinitrile (ester), and finally carrying out alkaline hydrolysis and acidification to obtain bempedoic acid. The synthetic route is short, all the used raw materials are easy to obtain, the cost is low, the yield of each step of reaction is high, and the purity is high, therefore the method is suitable for industrial production.
Owner:合肥市梓熤科技贸易有限公司
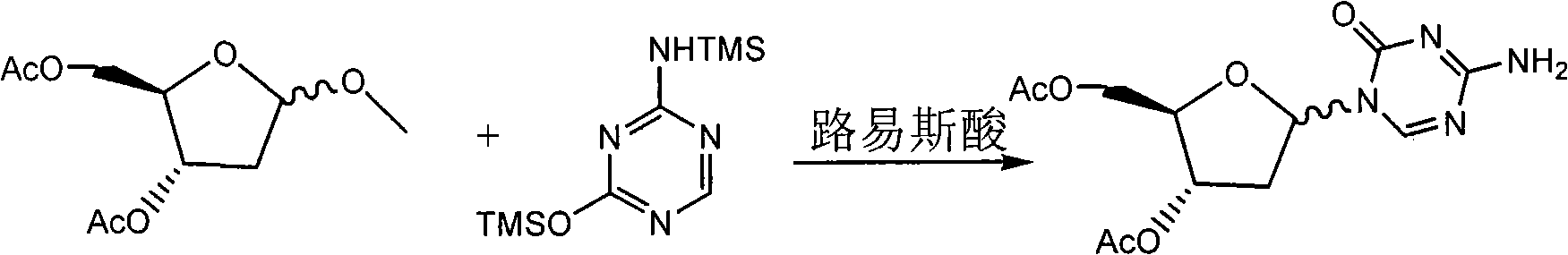
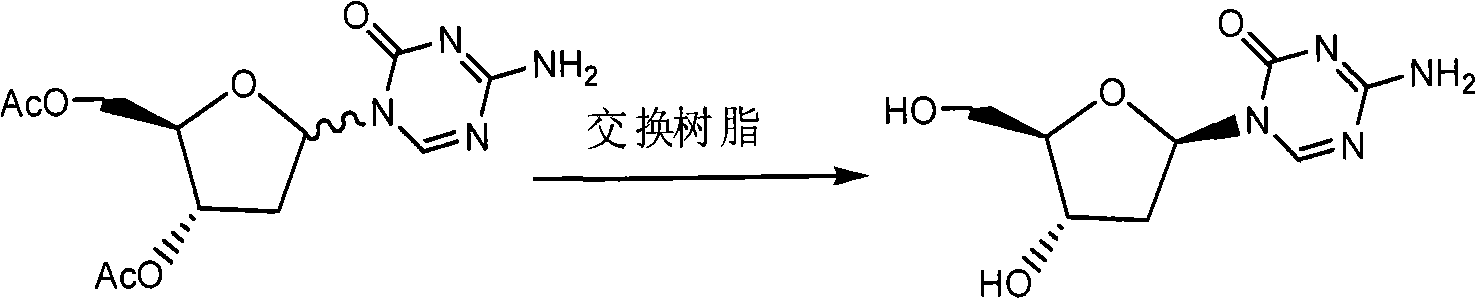
Preparation method of decitabine
ActiveCN101560233AShort reaction stepsLow costSugar derivativesSugar derivatives preparationAcetic anhydrideIon-exchange resin
Owner:SHANGHAI QINGSONG PHARMA
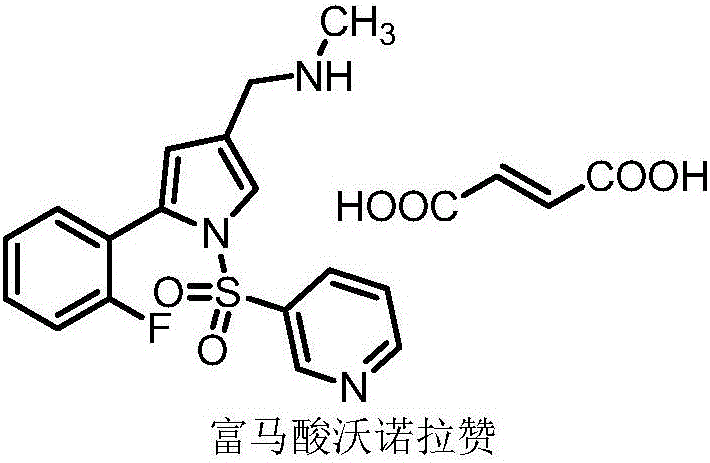
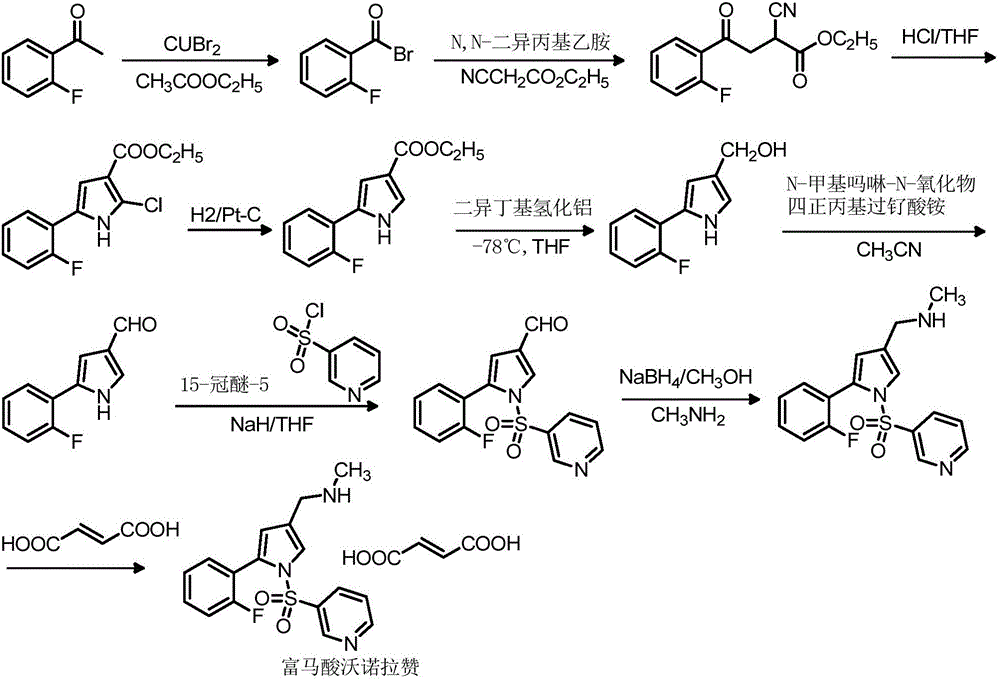
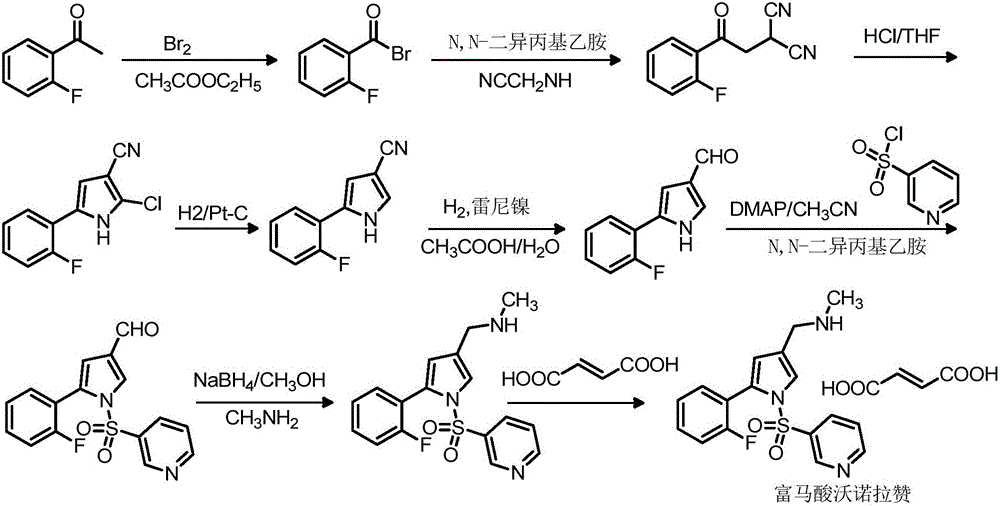
Vonoprazan fumarate midbody, preparation method thereof and method for preparing vonoprazan fumarate midbody
InactiveCN106146466AShort reaction stepsMild reaction conditionsOrganic chemistryState of artVonoprazan
The invention provides a vonoprazan fumarate midbody, a preparation method thereof and a method for preparing vonoprazan fumarate midbody. Compared with the prior art, vonoprazan fumarate is prepared through the vonoprazan fumarate midbody. The preparation method has the following advantages that a process route includes short reaction steps, raw materials are easy to obtain, reaction conditions are mild, operation is simple and easy, the method is economical and environmentally friendly, the total yield is obviously improved, and the vonoprazan fumarate midbody is suitable for large-scale production.
Owner:赛隆药业集团股份有限公司(长沙)医药研发中心
Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride
The invention relates to a novel method for preparing N- amino-3-DBU [3, 3, 0] octane bromide. The technical problem to be solved is to simplify the preparation process, reduce the pollution and avoid using a carcinogenic substance. The preparing method comprises the following steps that: 1) a substance shown as formula (II) is taken as a raw material and is added with water, the mixture reacts sufficiently with HXO hydrazine at a temperature of between 80 and 200 DEG C to produce the reaction fluid; 2) after the reaction finishes, the reaction fluid is concentrated, water-solubility ethanol is added to dissolve the reaction fluid and to separate out superfluous HXO hydrazine; 3) sediment is filtered; and the mother fluid is processed to produce the N- amino-3-DBU[3, 3, 0] octane bromide.
Owner:NINGBO CHEMGOO PHAMA TECH
Preparation method for aryl acetic acid derivative
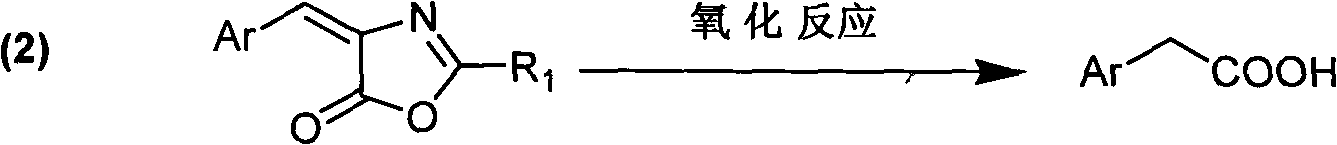
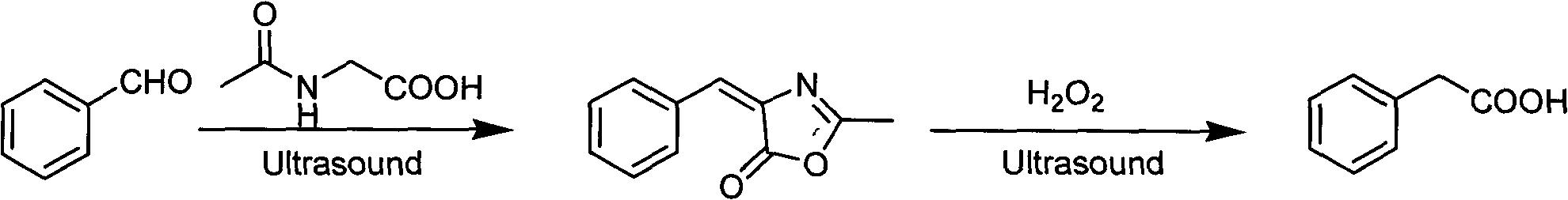
InactiveCN102070433AReduce pollutionMild reaction conditionsOrganic compound preparationCarboxylic compound preparationSodium acetateAcetic anhydride
The invention discloses a preparation method for an aryl acetic acid derivative. The preparation method comprises the following specific steps of: (1) adding sodium acetate, acetic anhydride and a cyclization agent into aryl methanal or alkyl original aliphatic aldehyde having more than four carbon atoms, and performing cyclization reaction to obtain an aryl oxazolone derivative, wherein the cyclization agent is N-acyl-glycine or an analogue thereof; and (2) uniformly mixing the aryl oxazolone derivative and aqueous alkali, adding an oxidizing agent and performing oxidization reaction to obtain the corresponding aryl acetic acid derivative, wherein the alkali is sodium hydroxide solution or potassium hydroxide, and the oxidizing agent is peroxy acid. Compared with the prior art, the method is mild in reaction condition, short in reaction steps, short in preparation cycle, low in cost, simple in posttreatment and low in environmental pollution, and is applicable for preparation of the aryl acetic acid derivative; by the method, the raw materials are readily available; moreover, the product prepared by the method is high in purity and stable in quality, and the method is suitable for large-scale industrial production.
Owner:GUILIN NORMAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
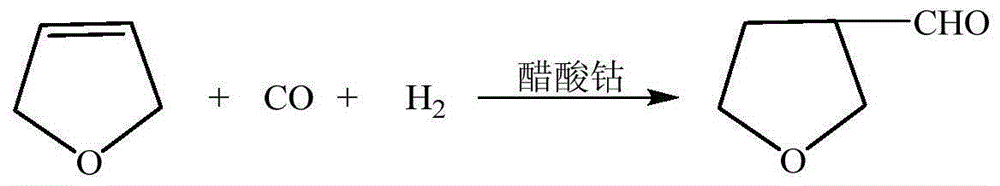
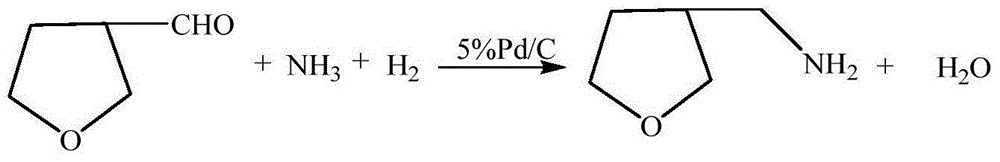

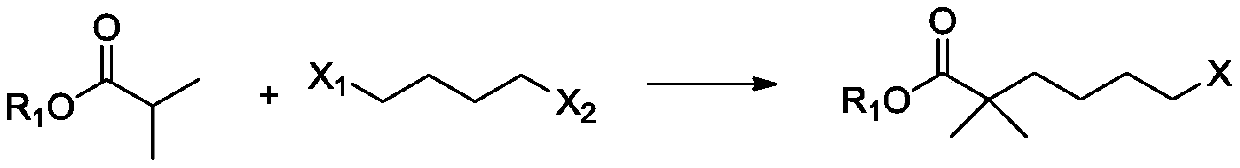

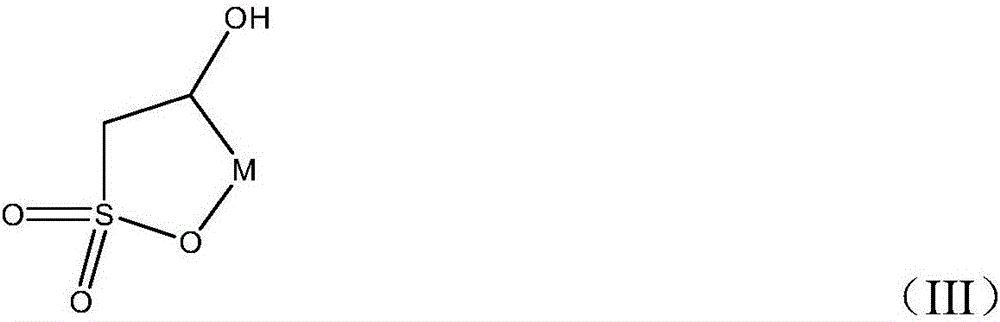
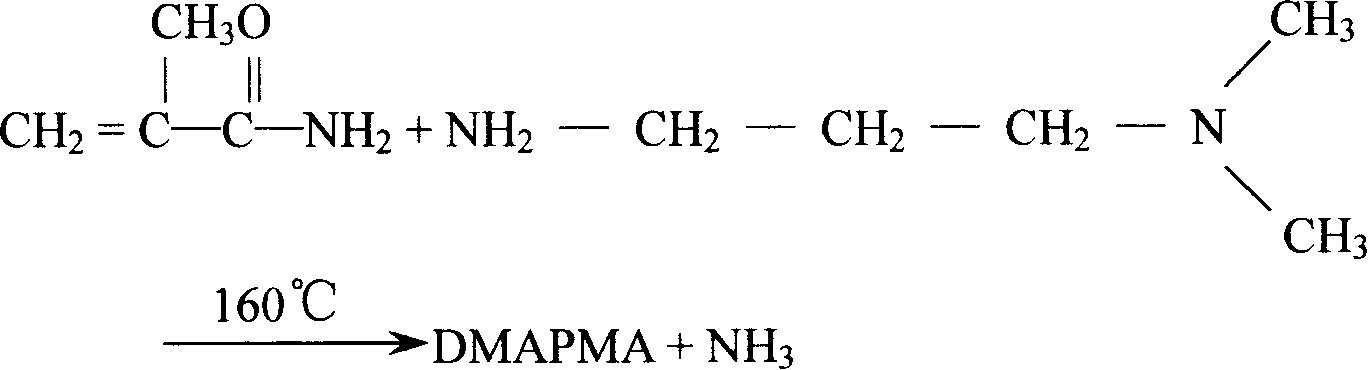
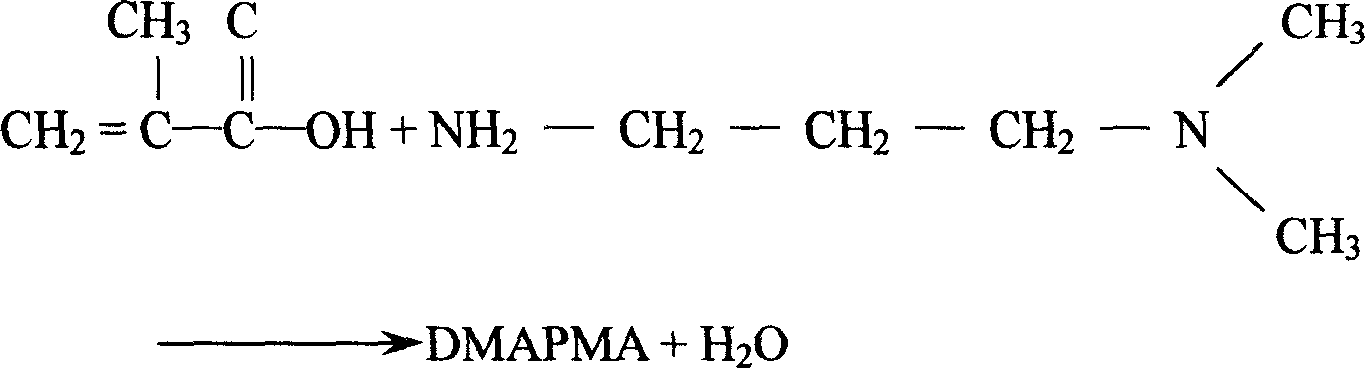
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700013.PNG)
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700021.PNG)
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700022.PNG)
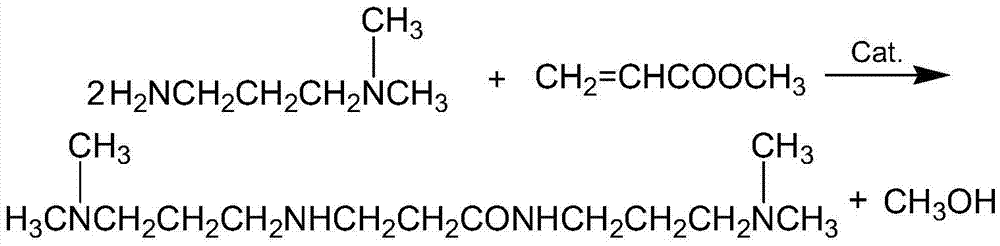
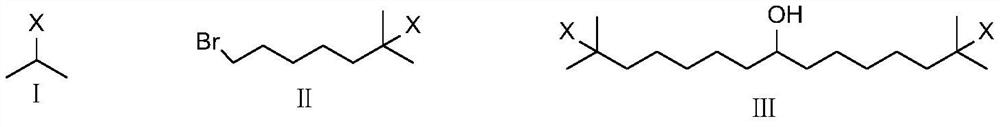


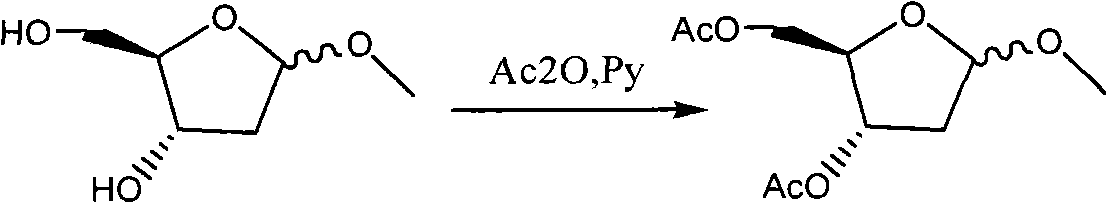
![Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/454b3bad-67dc-468d-b3b8-e6bb67152182/a2008100614210002c1.PNG)
![Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/454b3bad-67dc-468d-b3b8-e6bb67152182/a2008100614210002c2.PNG)
![Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride Method for preparing N-amino-3-azabicyclo[3,3,0]octane hydrochloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/454b3bad-67dc-468d-b3b8-e6bb67152182/a20081006142100031.PNG)