Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
252 results about "Agomelatine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Agomelatine is an atypical antidepressant used to treat major depressive disorder. One review found that it does not appear to be better than other antidepressants. Another review found it was similarly effective to many other antidepressants.
New crystalline form v of agomelatine, a process for its preparation and pharmaceutical compositions containing it
The crystalline form V of a compound of formula (I) is characterised by the X diffraction diagram thereof on a powder.
Owner:LES LAB SERVIER
Crystalline form iv of agomelatine, a process for its preparation and pharmaceutical compositions containing it
The crystalline form IV of a compound of formula (I) is characterised by the X diffraction diagram thereof on a powder.
Owner:SERVIER LAB
New crystalline form iii of agomelatine, a process for its preparation and pharmaceutical compositions containing it
The crystalline form III of a compound of formula (I) is characterised by the X diffraction diagram thereof on a powder, and the drugs contains it.
Owner:LES LAB SERVIER
Process for the synthesis and crystalline form of agomelatine
ActiveUS20050182276A1Nervous disorderCarboxylic acid nitrile preparationPharmacy medicineCompound (substance)
A process for the industrial synthesis and a new crystalline form of the compound of formula (I): Medicinal products containing the same which are useful in treating disorders of the melatoninergic system.
Owner:LES LAB SERVIER
Preparation method of agomelatine I type crystal
The invention discloses a preparation method of an agomelatine I type crystal which is an anti-depressant. The preparation method comprises the following steps of: dissolving crude agomelatine in a hydrophily organic solvent, filtering, dropping the filtrate into water under stirring, separating out solids and drying; wherein the weight part ratio of the hydrophily organic solvent to the water is 1:2-50. The agomelatine I type crystal prepared by the invention has excellent quality, good reproducibility, and high purity of HPLC normalization method to be above 99 percent, and is more suitable for large-scale industrialization production.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
Agomelatine and medicine composition thereof
ActiveCN101781226AQuality improvementImprove solubilityOrganic active ingredientsNervous disorderK-alphaPowder diffraction
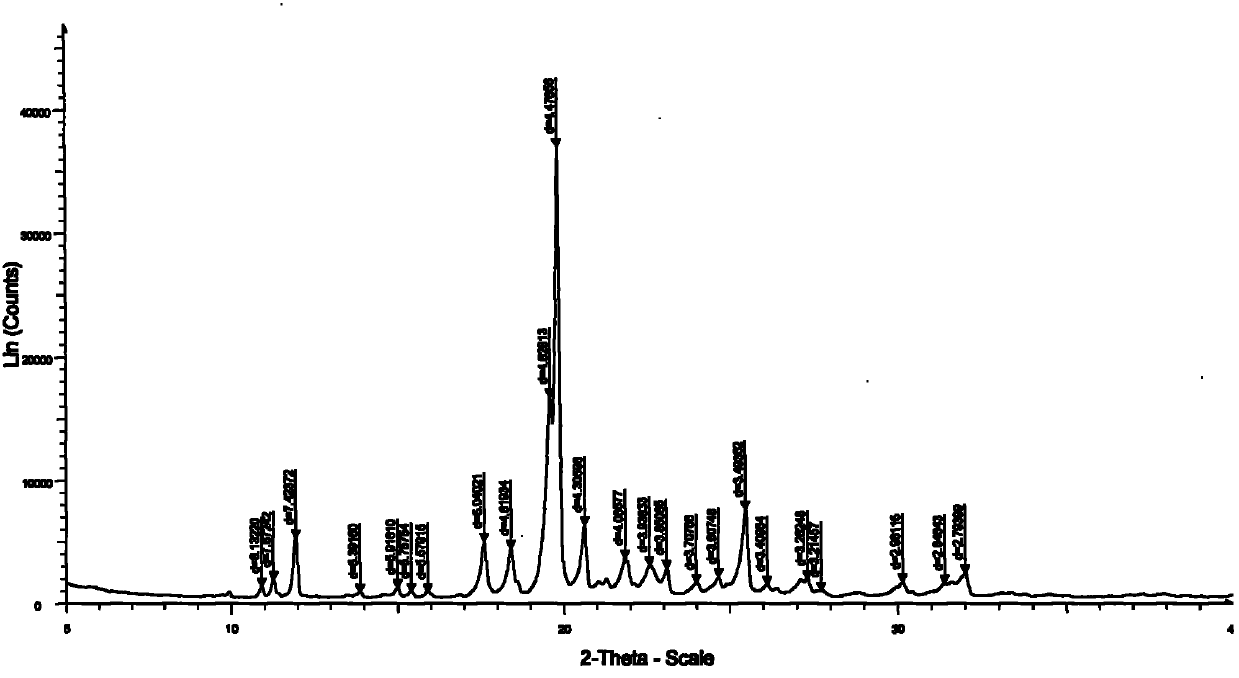
The invention discloses a medicine of Agomelatine for treating depression, in particular an Agomelatine crystal and a medicine composition thereof. The Agomelatine crystal is irradiated by using Cu-K alpha; and an X-ray powder diffraction spectrum expressed by the degree of 2theta of the Agomelatine crystal has characteristic diffraction peaks at positions of 12.84, 13.84, 16.14, 14.18, 18.56, 19.12, 20.86, 21.20 and 23.84, and an infrared absorption spectrum of the Agomelatine crystal has characteristic diffraction peaks approximately at position of 3234, 3060, 2940, 1638, 1511, 1436, 1249, 1215, 1184, 1032, 908, 828, 755 and 588cm-1. The DSP heat absorption transition is 97.6 DEG C. The invention further discloses application of the medicine composition containing the Agomelatine crystal as an active effective component to the preparation of medicines for treating the depression.
Owner:TIPR PHARM CO LTD
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
Pharmaceutical composition for treating depression
The invention belongs to the technical field of medicines, and discloses a pharmaceutical composition for treating depression. The pharmaceutical composition is composed of a raw material agomelatine and auxiliary materials, wherein the raw material agomelatine is micronized agomelatine. Based on a deep research of the physical and chemical properties of agomelatine, the scientific researchers of the company accidentally find that agomelatine can be micronized for obtaining agomelatine with a certain particle size to produce a preparation, the invention has the advantages of uniform quality and good dissolution rate.
Owner:北京美迪康信医药科技有限公司
Preparation method of agomelatine I crystal form
ActiveCN101921205AHigh purityEasy to operateCarboxylic acid amide separation/purificationSingle crystalSolvent
The invention discloses a preparation method of an agomelatine I crystal form, which comprises the steps of: adding agomelatine into a mixed solvent of amide and water, heating for dissolving, then reducing the temperature and separating out crystals, and drying to obtain a solid. The agomelatine is dissolved in the heated mixed solvent of the amide and water, and separated out slowly in a cooled mixed solvent of the amide and water, and the high-purity I crystal form can be stably obtained. The method has the advantages of high purity of reaching above 99 percent of the single crystal form, simple operation and good repeatability, and the like and is suitable for industrialized production.
Owner:NHWA PHARMA CORPORATION
Agomelatine benzenesulfonic acid compound and preparation method thereof
The invention relates to agomelatine benzenesulfonic acid compound of a formula I and a preparation method thereof; the agomelatine benzenesulfonic acid compound obtained by the invention is good in product stability, high in purity and is suitable for application demand when finished drug is prepared; preparation technology is also very simple; and a high-purity product can be obtained without special operation, wherein in the formula (I), R=CH3, H.
Owner:SHANGHAI RIGHTHAND PHARMTECH
Copolymer containing amorphous agomelatine, and preparation method, pharmaceutical composition and application thereof
ActiveCN102716493AHigh dissolution rateFast absorptionOrganic active ingredientsNervous disorderX-raySolvent
The invention provides a copolymer containing amorphous agomelatine. The detection of a sample of the copolymer by Differential Scanning Calorimetry (DSC) and X-ray powder diffraction shows that the sample has stable properties. The copolymer mainly comprises agomelatine raw material, carrier and solvent. The invention also provides a preparation method of the copolymer containing amorphous agomelatine, and the method has good reproducibility. The invention further provides a pharmaceutical composition containing the copolymer containing amorphous agomelatine and an application of the copolymer containing amorphous agomelatine in preparing medicines for treating depression.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Agomelatine-containing orally disintegrating tablet
InactiveCN101836966AOrganic active ingredientsNervous disorderOrally disintegrating tabletDissolution
The invention provides an agomelatine-containing orally disintegrating tablet, which is characterized in that the inclusion technology is adopted to prepare the agomelatine-containing orally disintegrating tablet. The invention effectively covers up the tingling of agomelatine, improves the dissolution of the agomelatine, and is suitable for industrial production.
Owner:万全万特制药江苏有限公司
Crystalline Agomelatine solvate and preparation method thereof
InactiveCN101870662ALess solventReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationAcetic acidSolvent
The invention discloses a crystalline Agomelatine ethylene glycol, acetic acid solvate and a preparation method thereof. The preparation method of the crystalline Agomelatine ethylene glycol solvate comprises the steps of: mixing Agomelatine with ethylene glycol according to the proportion of 1:2-5, heating the mixture to 100 to 130 DEG C, and melting and cooling the heated mixture to result in solids. The preparation method of the crystalline Agomelatine ethylene glycol solvate further comprises the steps of: dissolving Agomelatine in acetic acid to result in solution, and adding the solution into antisolvent, followed by standing still or constantly stirring or evaporating the solvent below 50 DEG C to result in crystal. The crystalline Agomelatine ethylene glycol, acetic acid solvate prepared according to the method has the advantages of: obviously reduced melting point of the solvate, high crystallization purity, good reproducibility, less amount of the solvent used, no need of protection from inert gas, low production cost, simple operation, mild reaction condition and easy control.
Owner:SUN YAT SEN UNIV
Agomelatine crystal form B, preparation method thereof and medicinal composition containing same
InactiveCN101723844AReduce difficultyLow costOrganic active ingredientsNervous disorderMedicineAgomelatine
The invention relates to an agomelatine crystal form B, a preparation method thereof and a medicinal composition containing the same.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Preparation method of agomelatine crystal form A
InactiveCN101781225AReduce difficultyLow costOrganic compound preparationCarboxylic acid amides preparationMedicinal chemistryDifferential scanning calorimetry
The invention provides a preparation method of agomelatine crystal form A which has a single endothermic peak at about 100 DEG C under the differential scanning calorimetry.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Agomelatine solid preparation
ActiveCN102670514AQuality improvementGood in vitro dissolution performanceOrganic active ingredientsPowder deliveryHot meltOrganic chemistry
The invention provides an agomelatine solid preparation. Agomelatine and a carrier are subjected to hot melt extrusion to form solid dispersoid of agomelatine, and the solid dispersoid of agomelatine can be further prepared into the solid preparation. A product can be dissolved out well and is controllable in quality.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
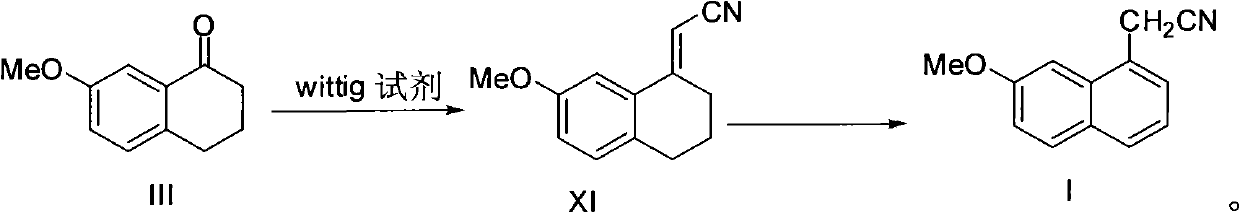
Preparation method of 2-(7-methoxy-1-naphthyl) acetonitrile
InactiveCN102336686ACarboxylic acid nitrile preparationOrganic compound preparationWittig reactionAromatization
The invention provides a new preparation method of an important intermediate of the antidepressant agomelatine, i.e. 2-(7-methoxy-1-naphthyl) acetonitrile. In the method, a compound of formula III is subjected to a Wittig reaction so as to obtain a compound of formula XI, which then undergoes an aromatization reaction, so that 2-(7-methoxy-1-naphthyl) acetonitrile can be obtained. The preparation method of the invention has a short reaction route, the Wittig reaction and the aromatization reaction both have high yield. With mild reaction conditions and without anything to do with reagents and solvents etc. of high toxicity, the preparation method provided in the invention is suitable for industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
New process for the synthesis of agomelatine
InactiveCN101643434AAvoid structure reactionHas crystalline propertiesNervous disorderMetabolism disorderMedicinal chemistryAgomelatine
The invention relates to new process for the synthesis of agomelatine. More particularly, the invention relates to a process for the industrial synthesis of the compound of formula (I).
Owner:LES LAB SERVIER
Process for the synthesis and crystalline form of agomelatine
A process for the industrial synthesis and a new crystalline form of the compound of formula (I):Medicinal products containing the same which are useful in treating disorders of the melatoninergic system.
Owner:LES LAB SERVIER
Crystalline form V of agomelatine, a process for its preparation and pharmaceutical compositions containing it
Crystalline form V of the compound of formula (I): characterised by its powder X-ray diffraction diagram. Medicinal products containing the same which are useful in the treatment of melatoninergic disorders.
Owner:LES LAB SERVIER
Orodispersible pharmaceutical composition for oromucosal or sublingual administration of agomelatine
InactiveCN1981752AShort disintegration timeSpeed up entryOrganic active ingredientsNervous disorderSublingual administrationOral cavity
The invention relates to a coated solid orodispersible pharmaceutical composition for oral, oromucosal or sublingual administration of agomelatine.
Owner:LES LAB SERVIER
Preparation of agomelatine midbody, 2-(7-anisyl-1-naphthyl) ethylamine
ActiveCN101709036AReduce one step reactionHigh yieldOrganic compound preparationAmino-hyroxy compound preparationReaction stepAcetamide
The invention aims at providing a preparation method of 2-(7-anisyl-1- naphthyl) ethylamine (II) which is an important midbody of agomelatine. The preparation method uses 2- (7-anisyl-1-naphthyl) acetamide (VII) as an initiative raw material, only needs one reaction step, has higher yield, abolishes high-voltage hydrogenation, has mild conditions and does not need special devices.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Stable Agomelatine capsule medicine composition
InactiveCN101991559AMask the smellMask bitternessOrganic active ingredientsNervous disorderMannitolBioavailability
The invention discloses a stable Agomelatine capsule medicine composition which is characterized in that 1000 capsules comprise the following components of 15-30g of Agomelatine, 70-140g of mannitol, 0.5-1g of superfine silica powder and a proper amount of 10 percent pre-gelatinized starch. The invention also relates to a preparation method of an Agomelatine capsule. The Agomelatine capsule prepared by adopting the formula and the preparation method provided in the invention has the advantages of good flowability, good dissolution rate, small content uniformity, high bioavailability and good treatment effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
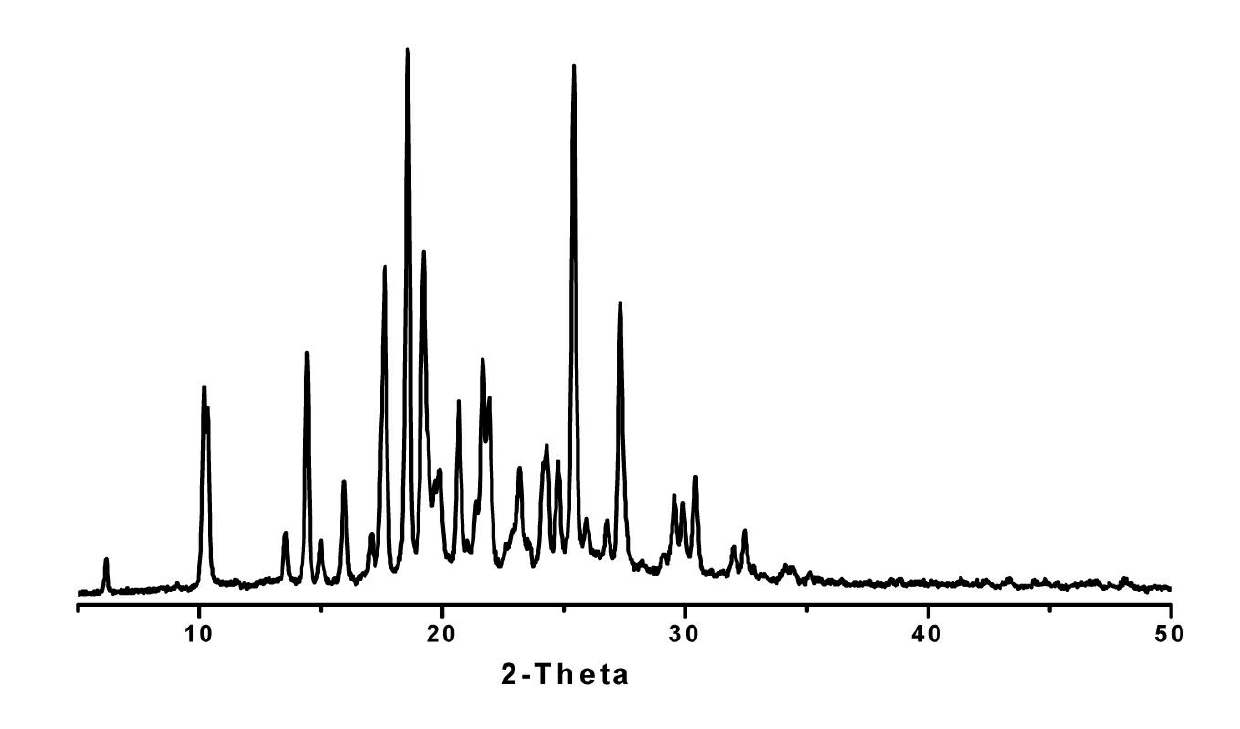
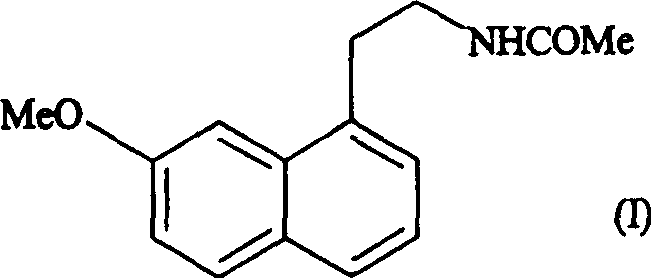
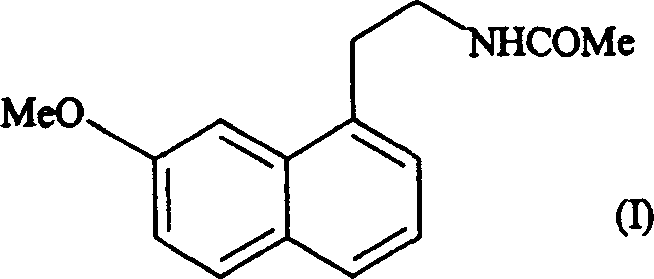
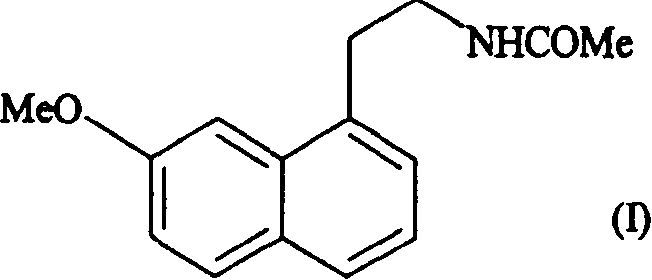
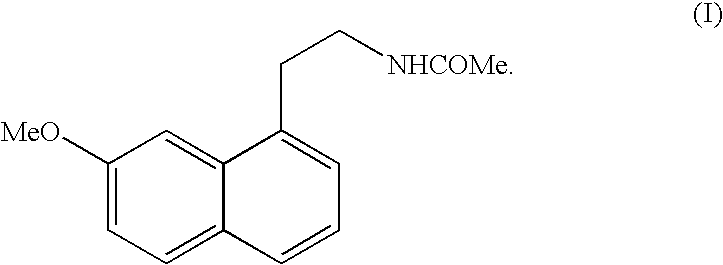
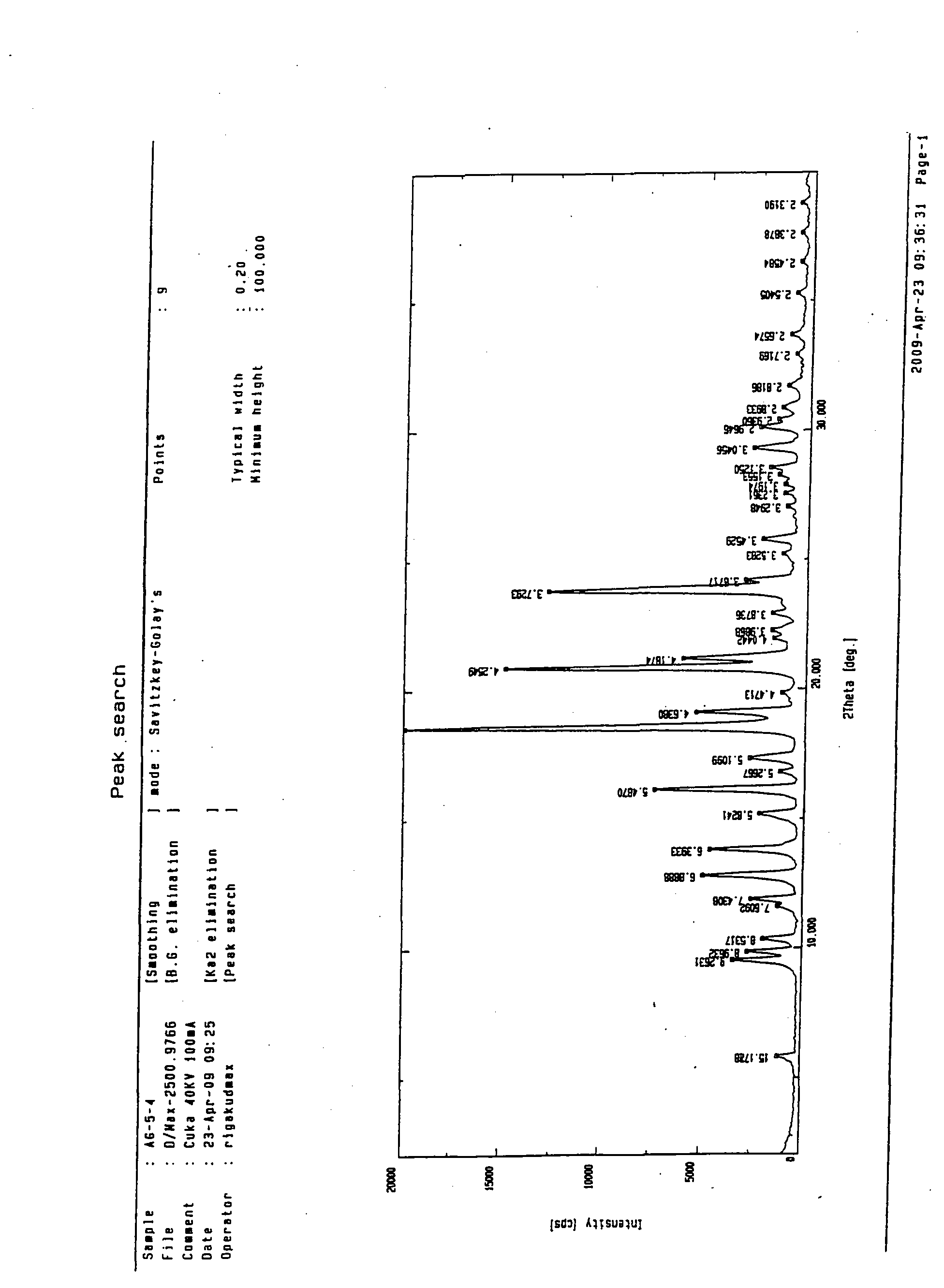
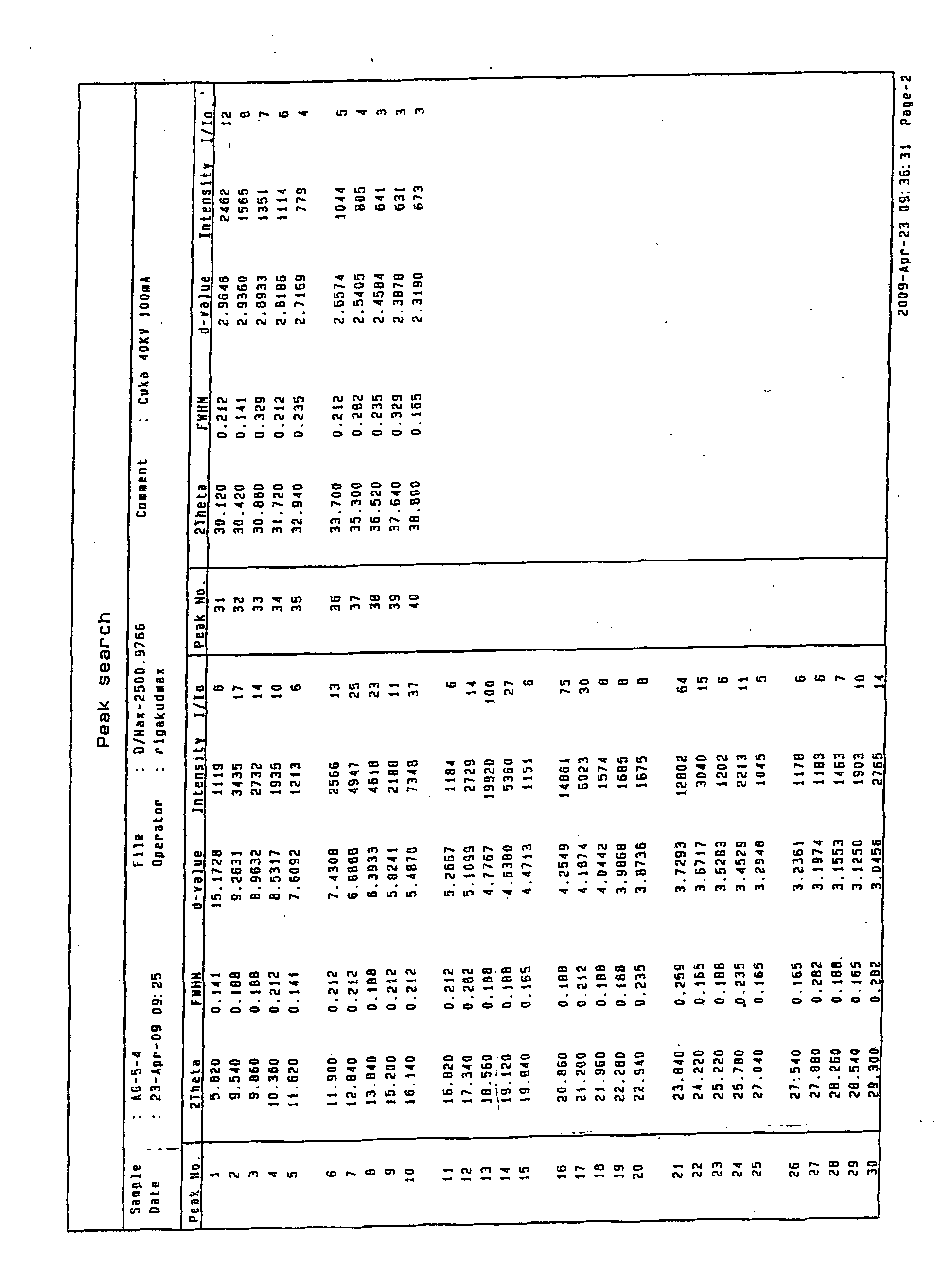
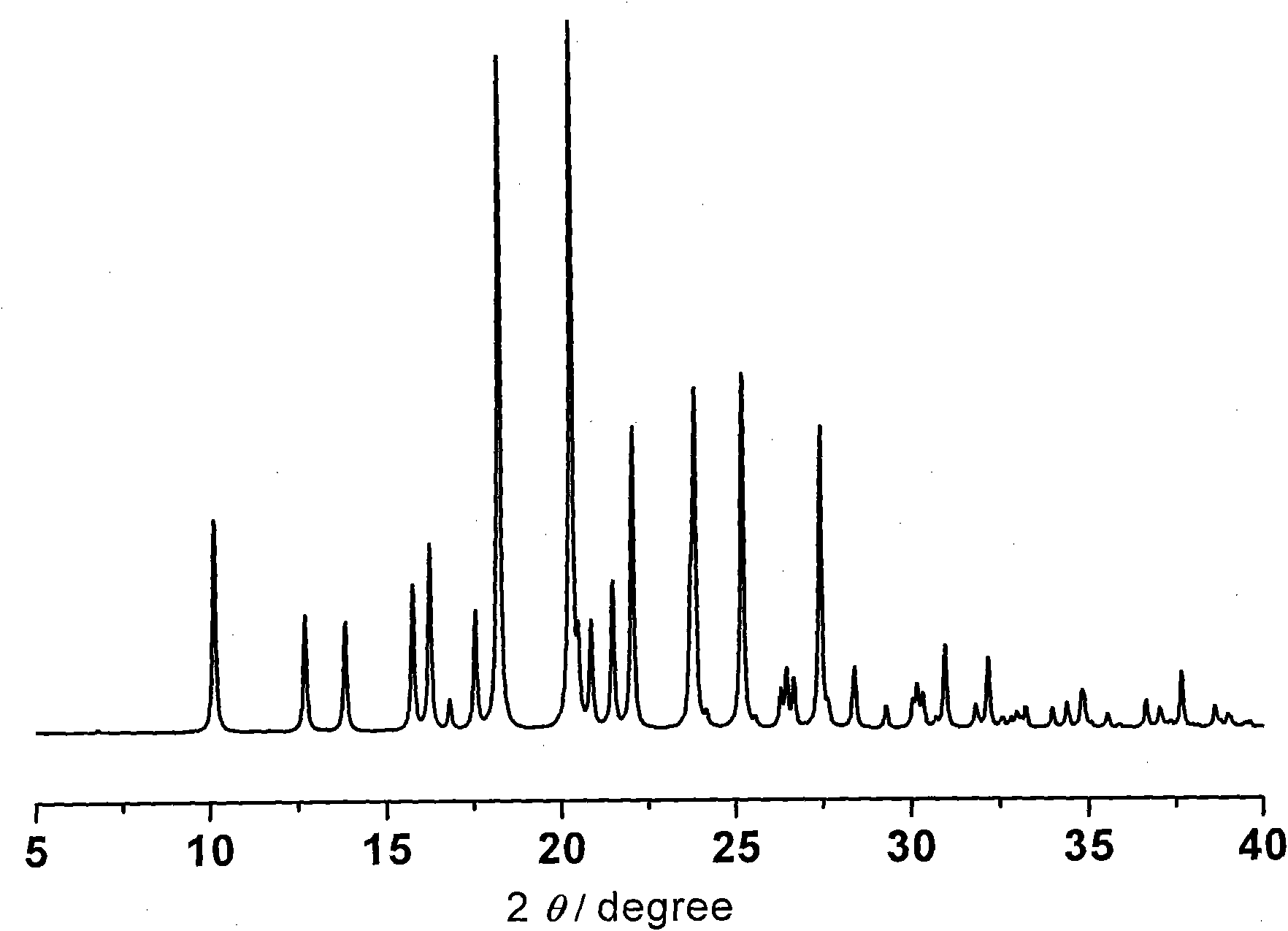
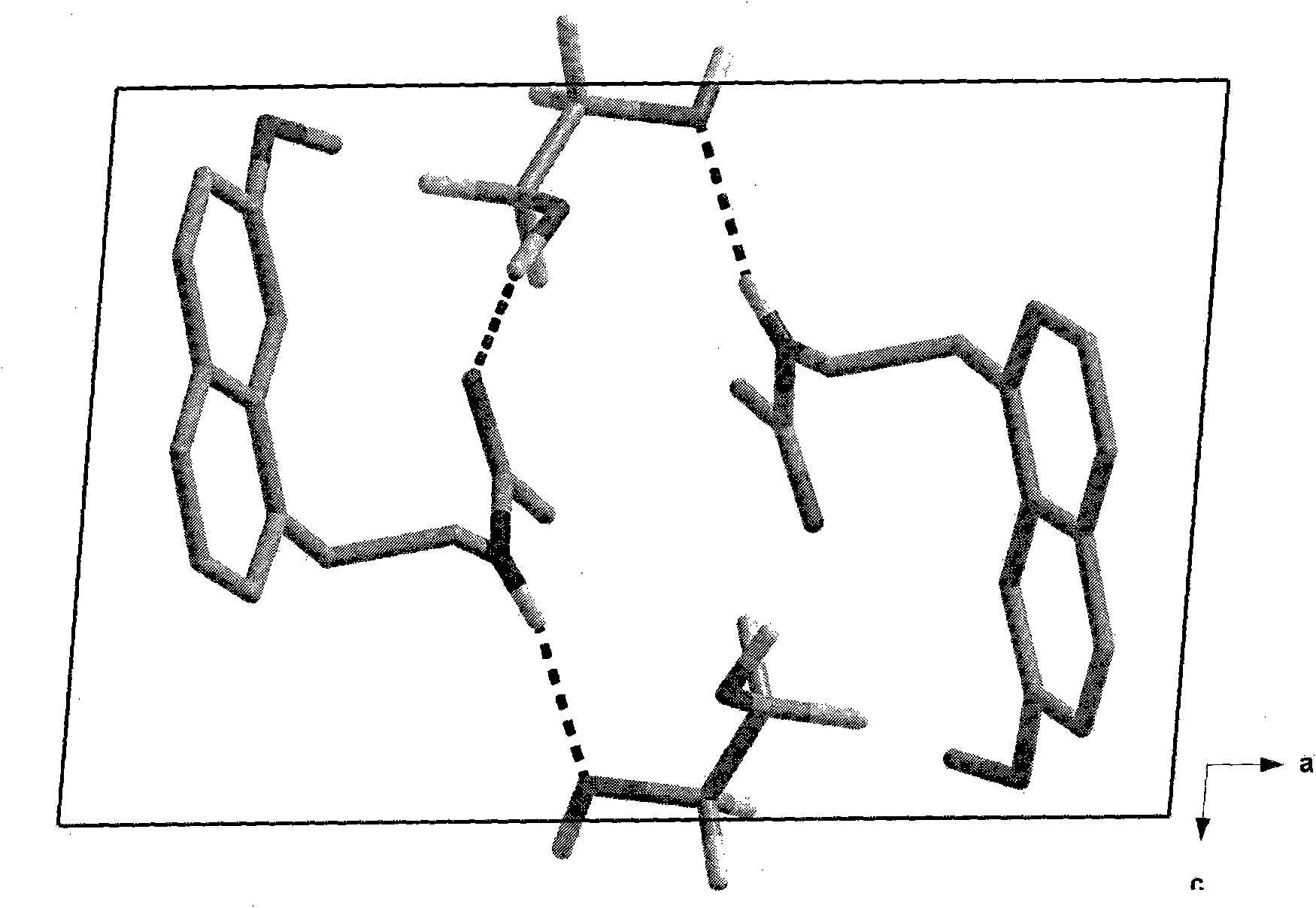
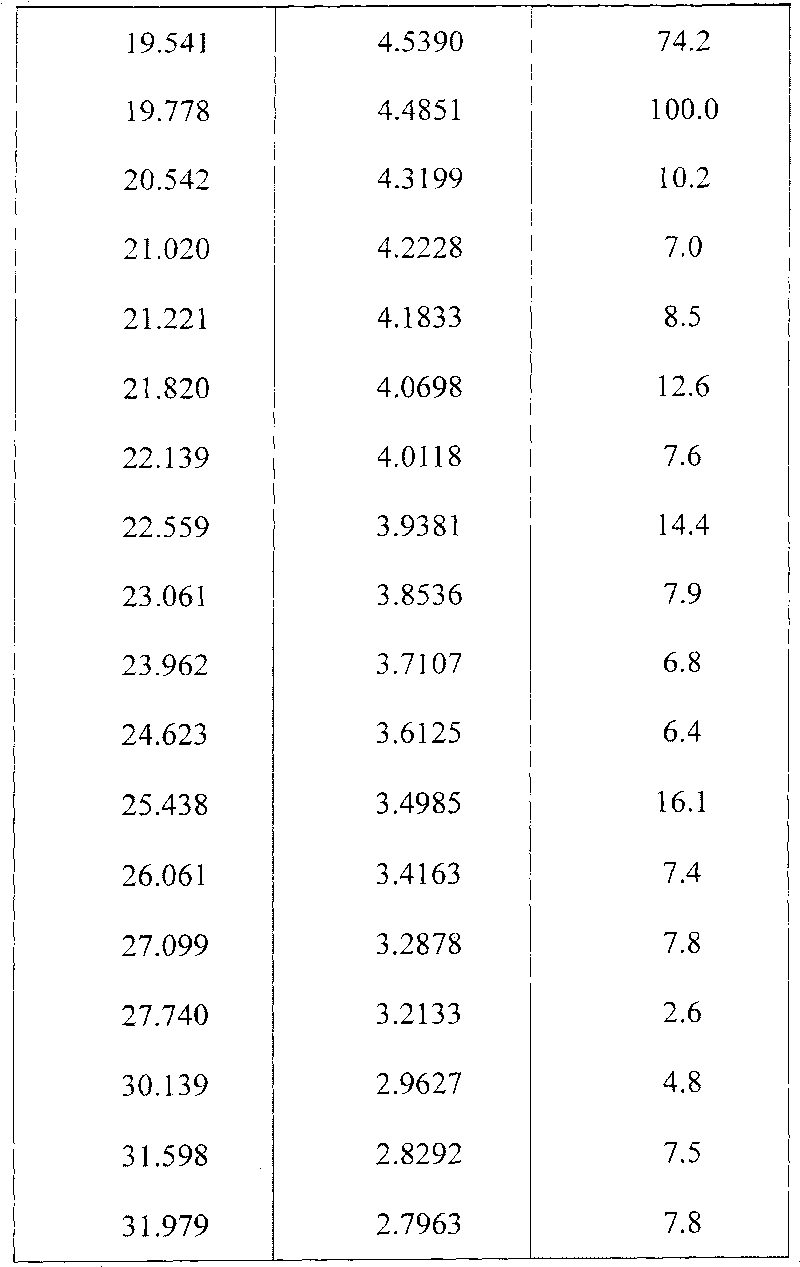
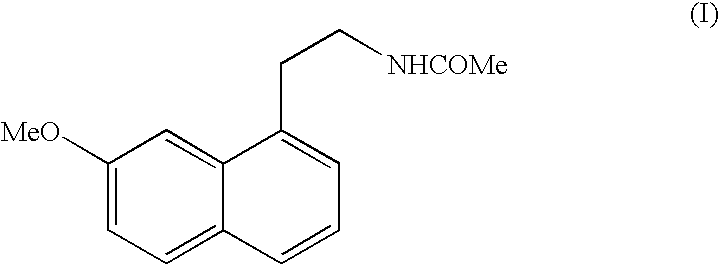
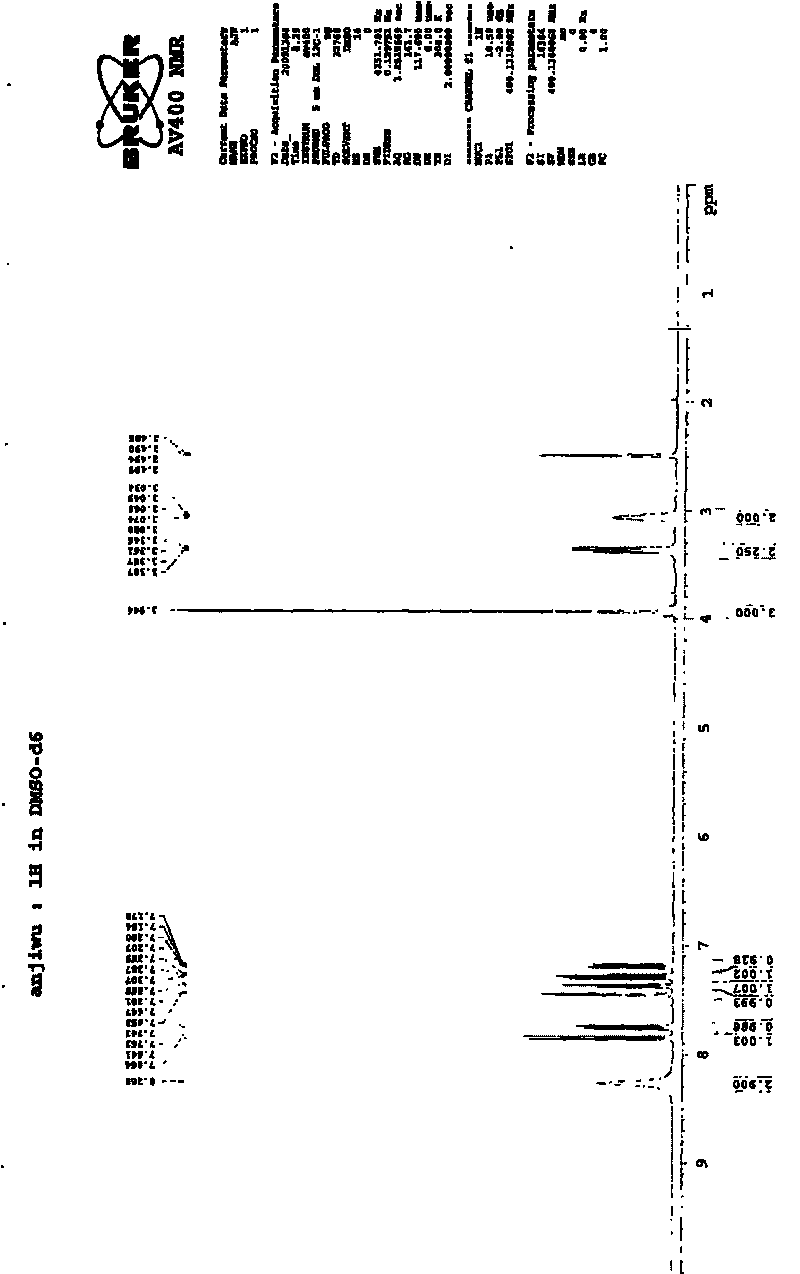
Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide
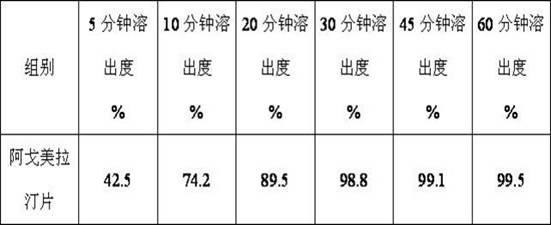
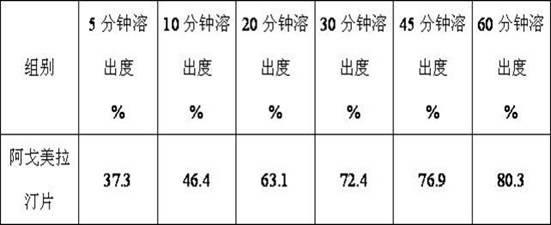
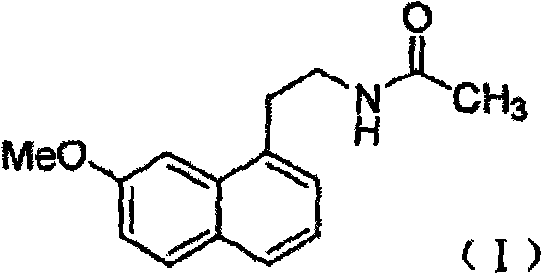
InactiveCN101759591AMeet the requirements of medicinal valueComply with purityOrganic active ingredientsNervous disorderDehydrogenationAntidepressants drugs
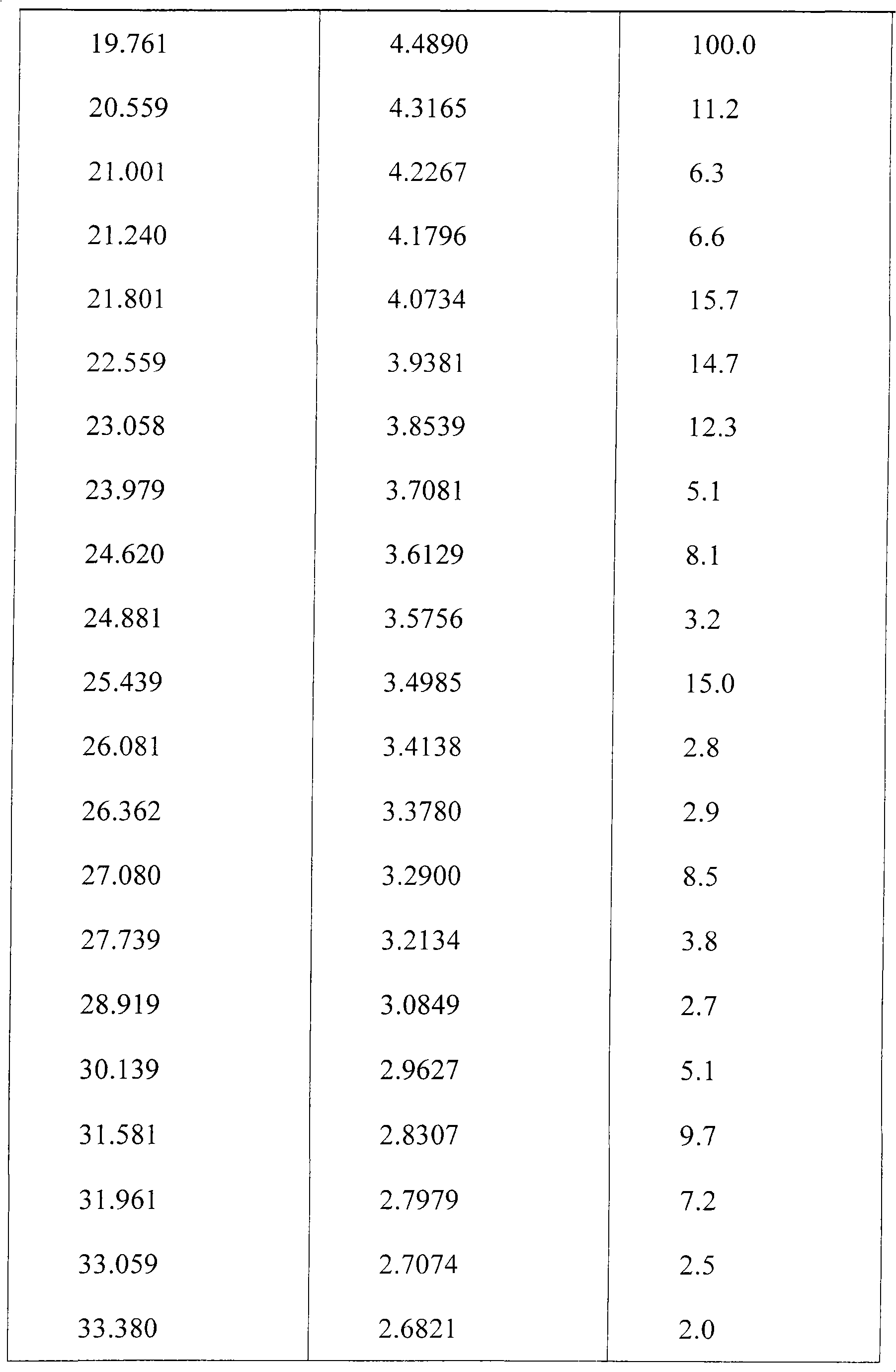
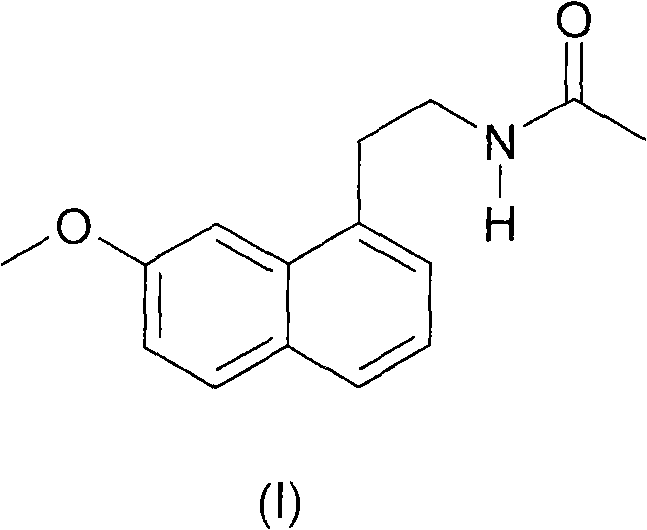
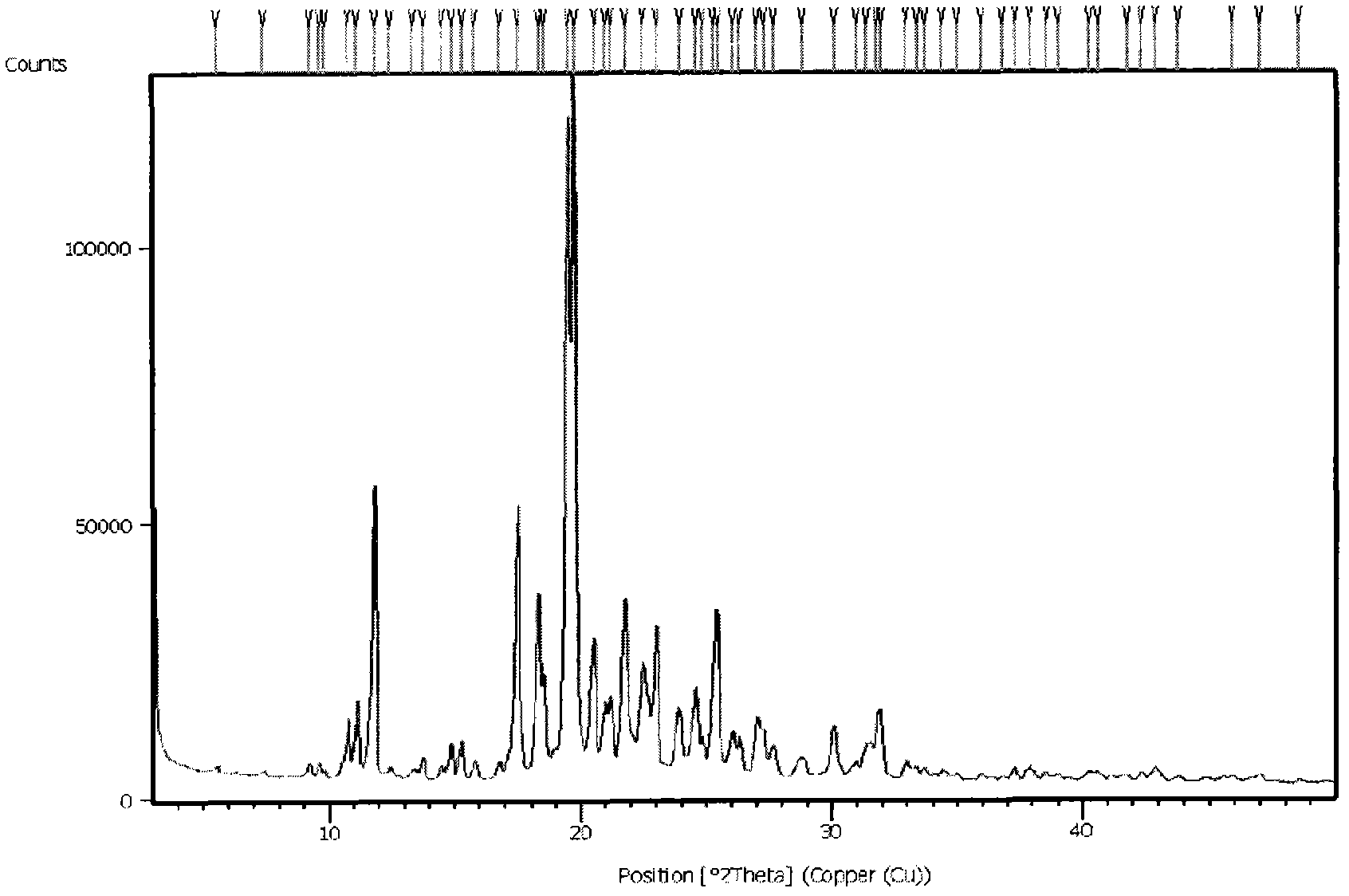
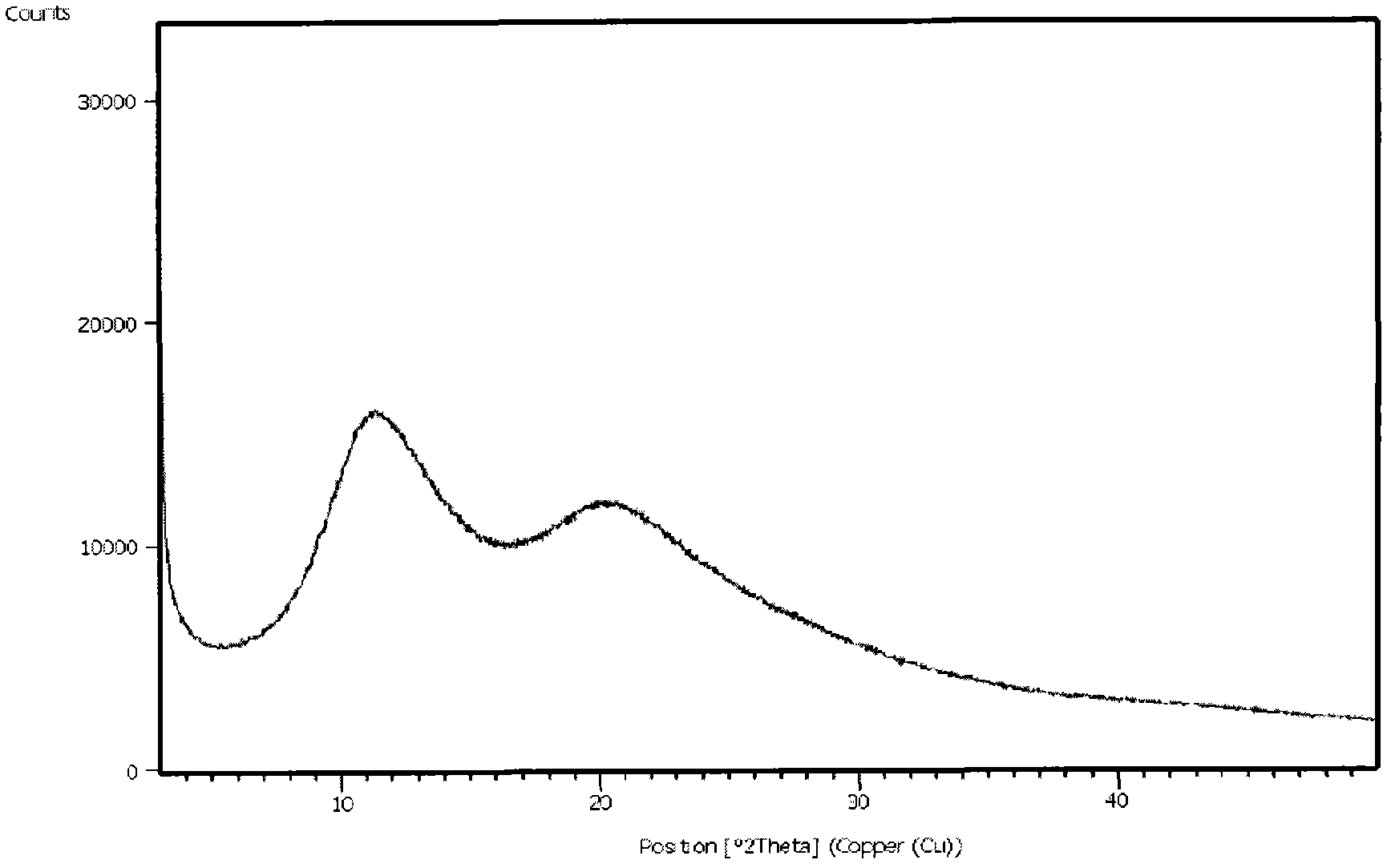
The present invention relates to a preparing method of agomelatine (N-[2-(7- anisyl-1- naphthyl) ethide] acetamide) of melatonin antidepressant drugs of commercial production. Firstly, 7- anisyl-1-tetralone is used as raw material, and is prepared into 2-(1,2,3,4- tetrahydrochysene-1- oxhydryl-7- methoxynaphthalene-1-base) acetonitrile in a cyanophoric way; then, 2-(1,2- dihydro-1-oxhydryl-7- methoxynaphthalene-1-base) acetonitrile is generated in an aromatization dehydrogenation way, and (7-- anisyl-1- naphthyl) acetonitrile is generated in a backflow reaction dehydrogenation way; finally, the agomelatine (N-[2-(7- anisyl-1- naphthyl) ethide] acetamide) is generated by reducing and acetylizing in a one-kettle way. The present invention has the advantages of high product purity, friendly environment, convenient operation and low cost, and is suitable for the commercial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Agomelatine sulfuric acid composition and preparation method thereof
The invention relates to an agomelatine sulfuric acid composition shown in the formula I and a preparation method of the agomelatine sulfuric acid composition. The agomelatine sulfuric acid composition obtained by using the preparation method is good in stability and high in purity, and is suitable for application in finished-product medicinal preparations. The preparation process is also quite simple, so that the product with high purity can be obtained without special operations.
Owner:SHANGHAI RIGHTHAND PHARMTECH
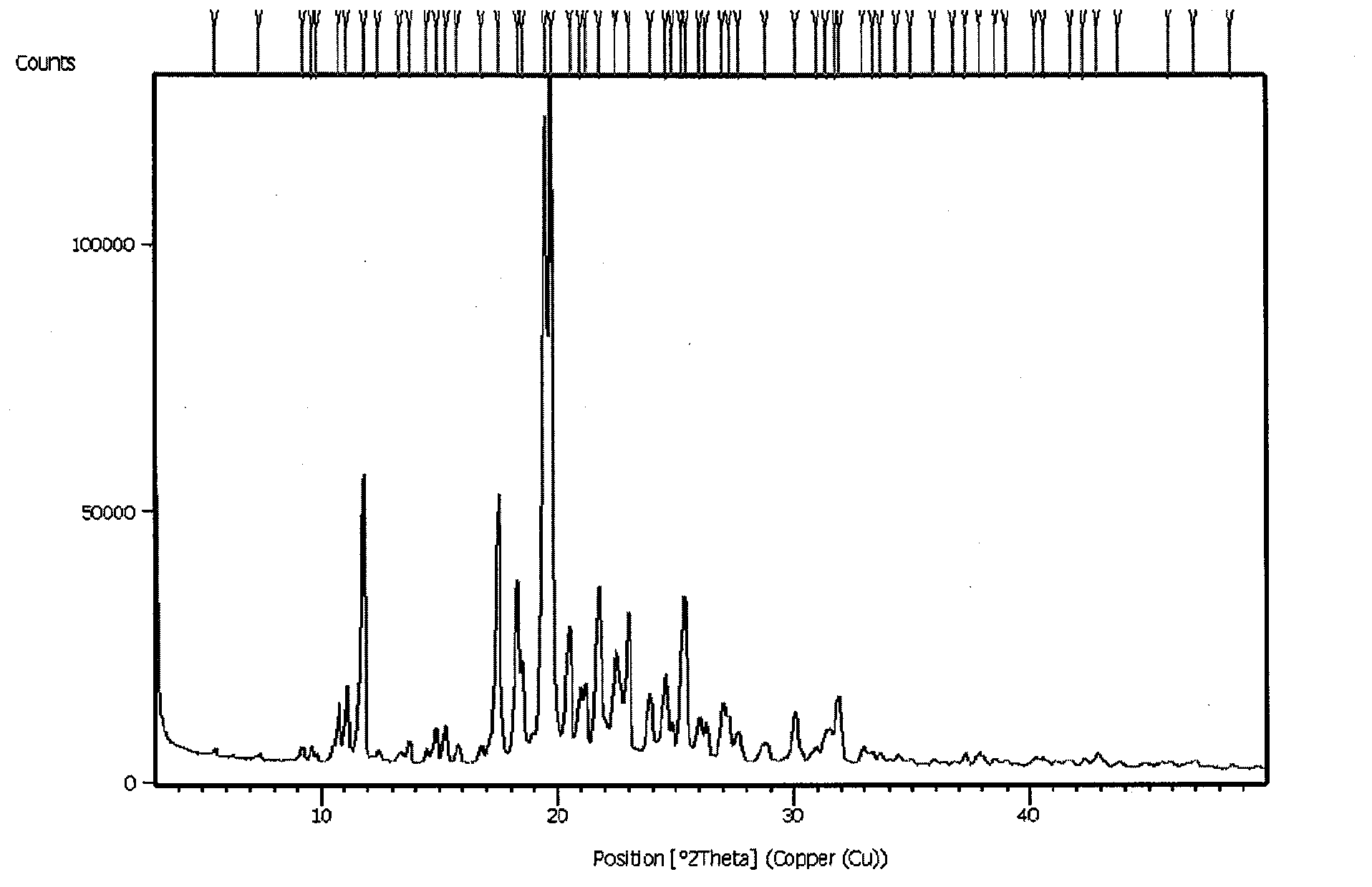
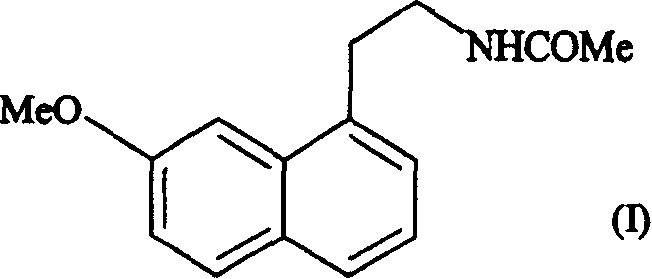
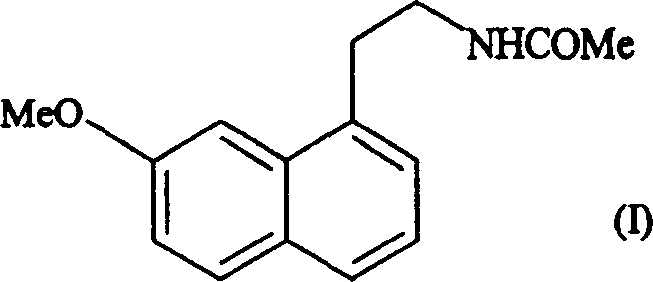
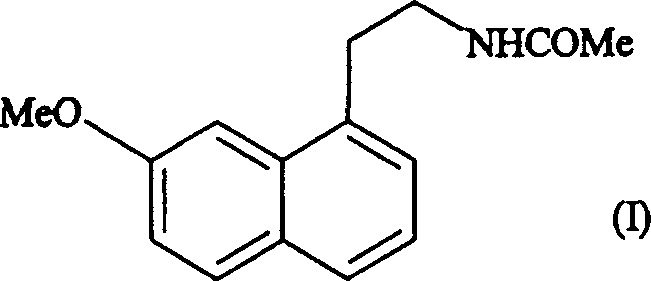
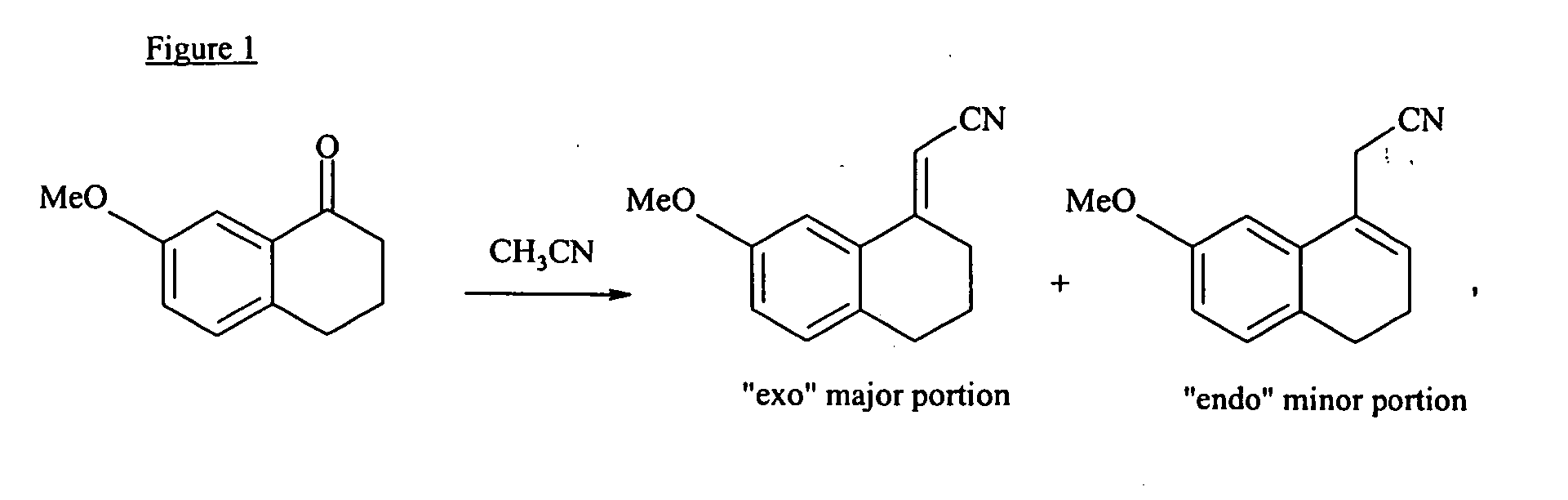
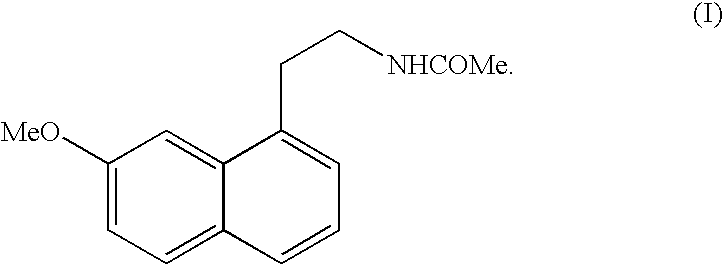
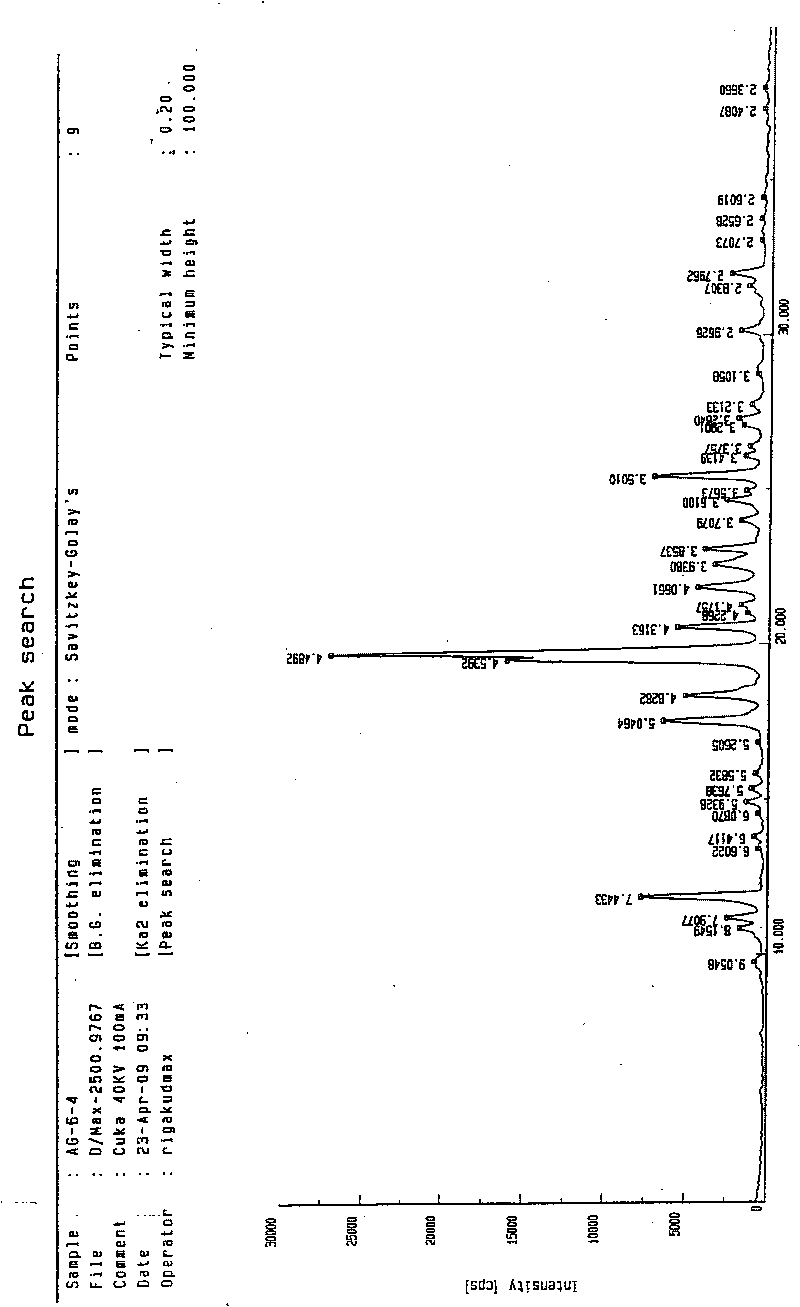
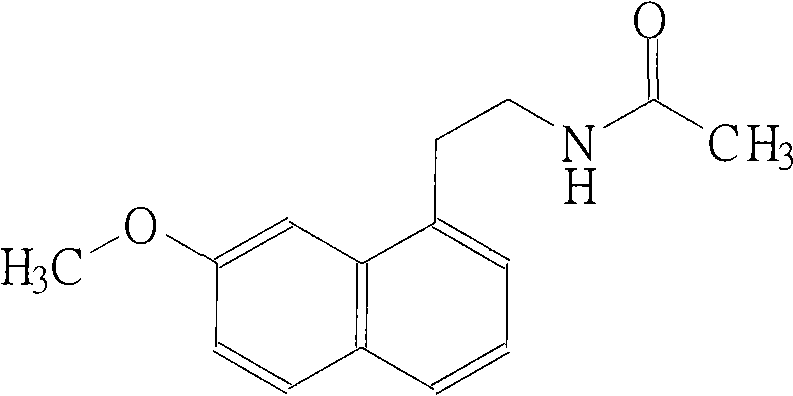
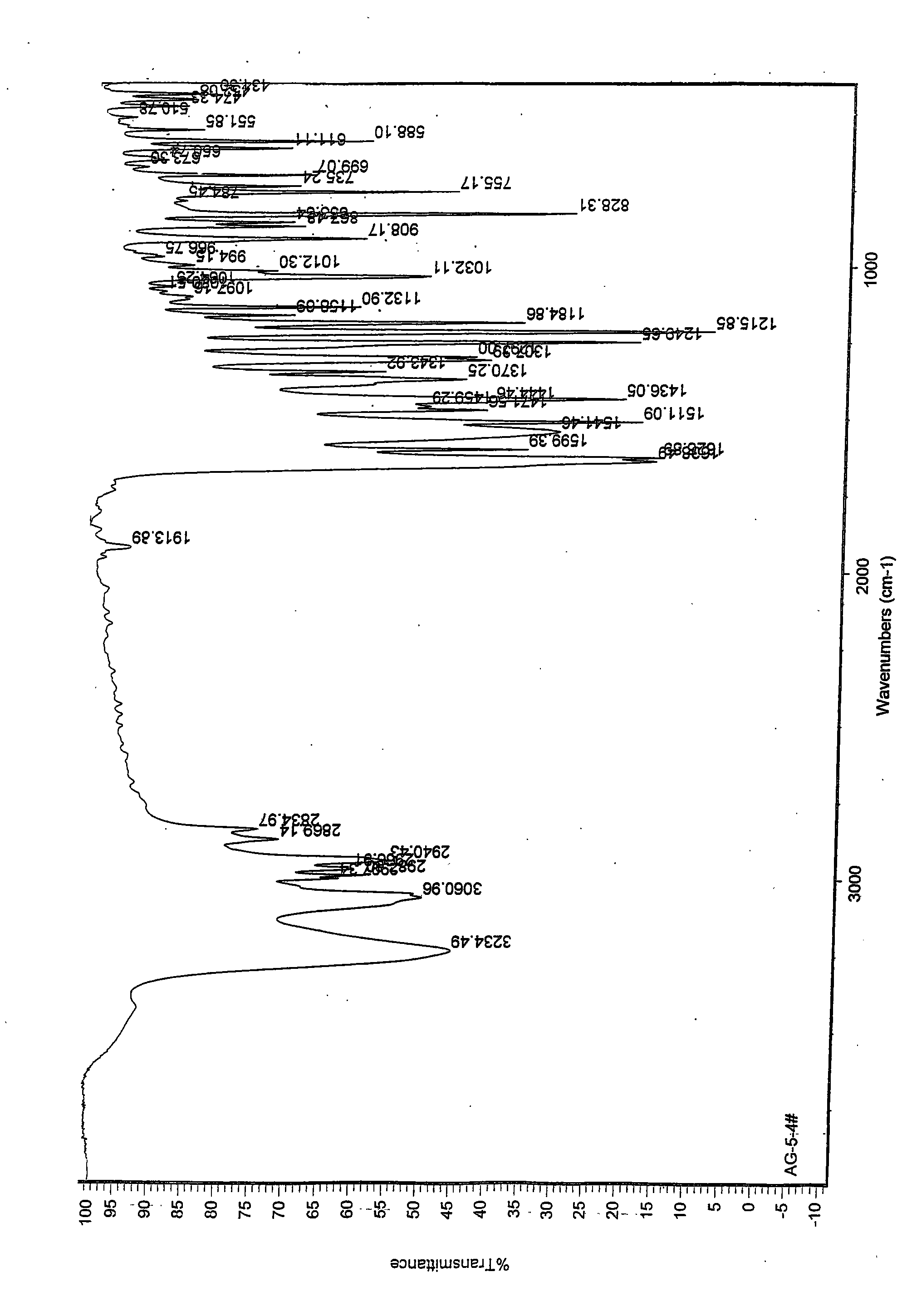
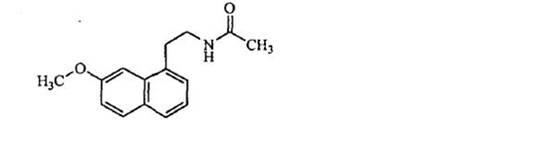
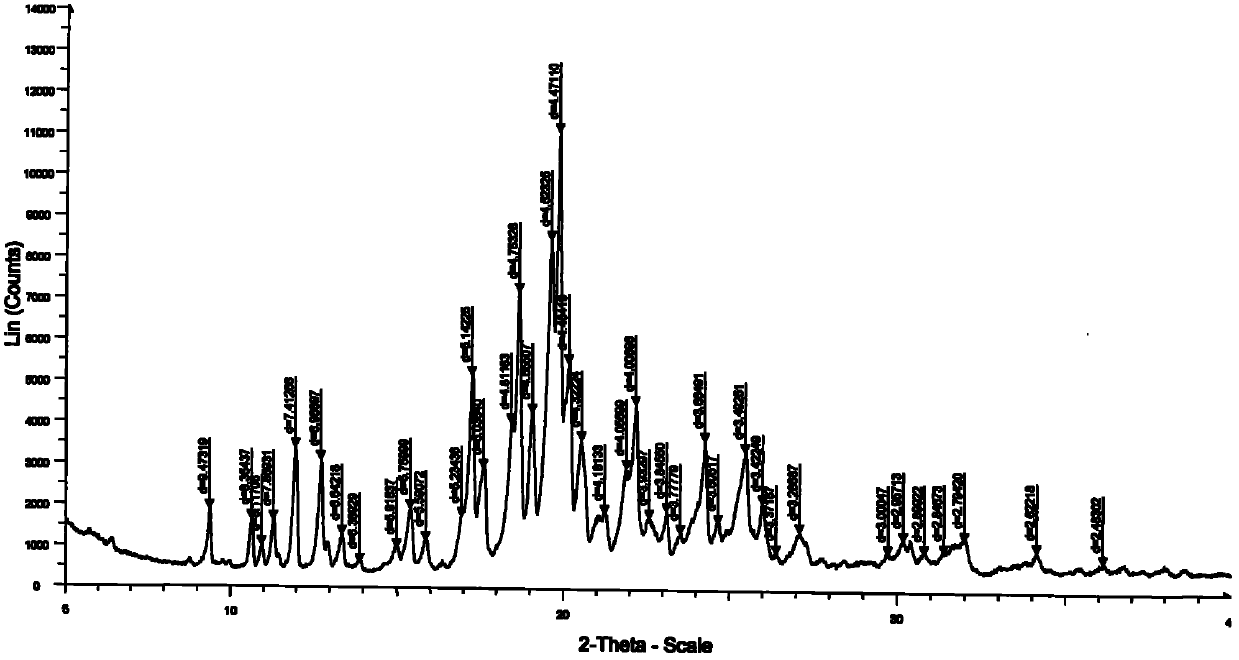
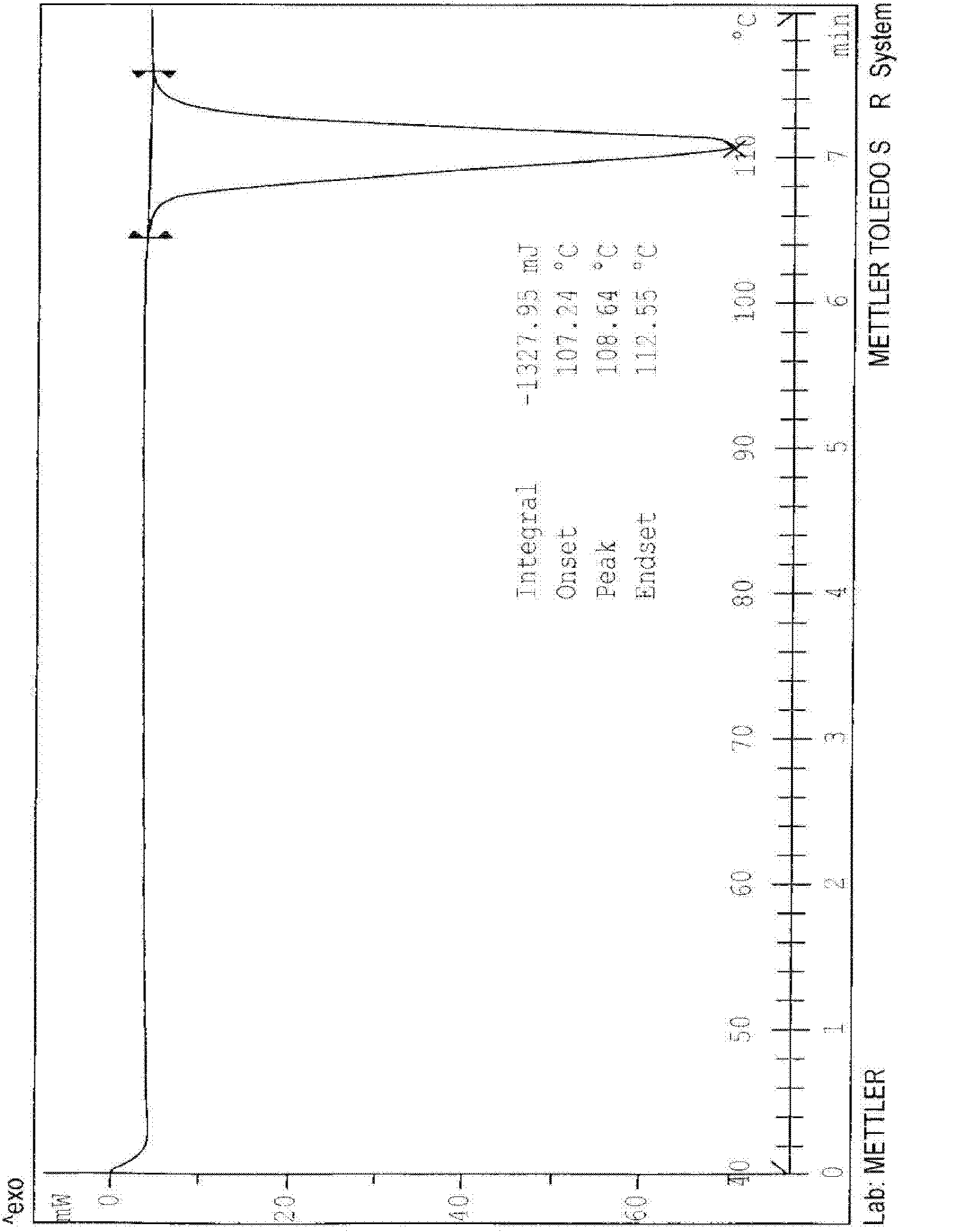
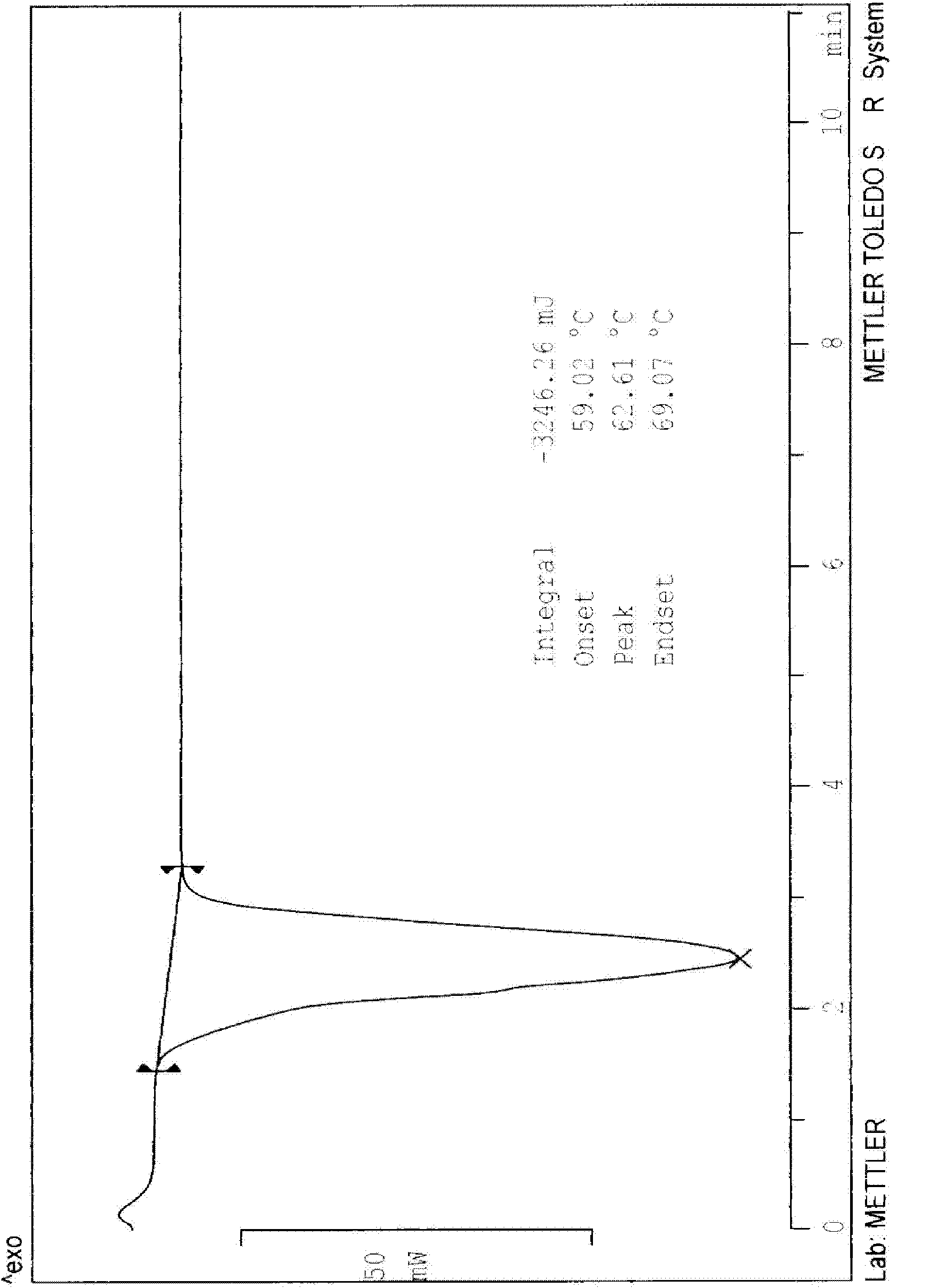
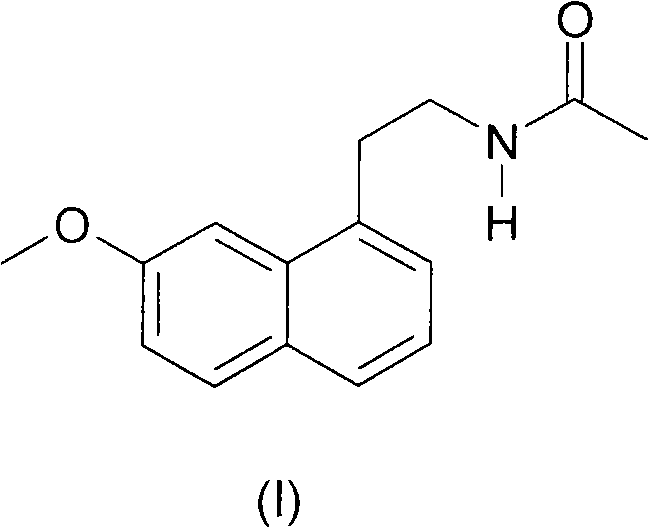
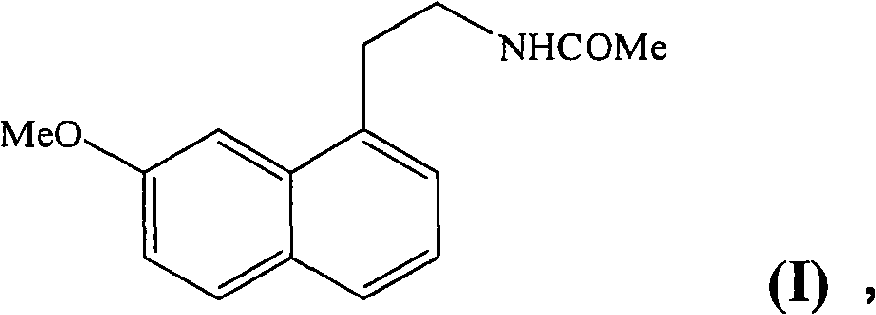
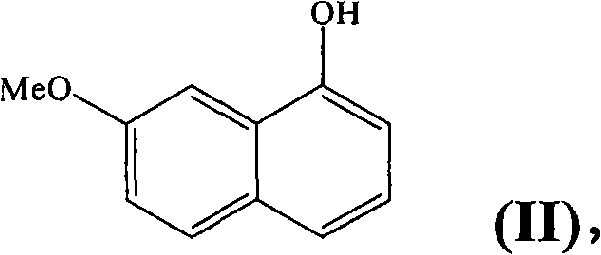
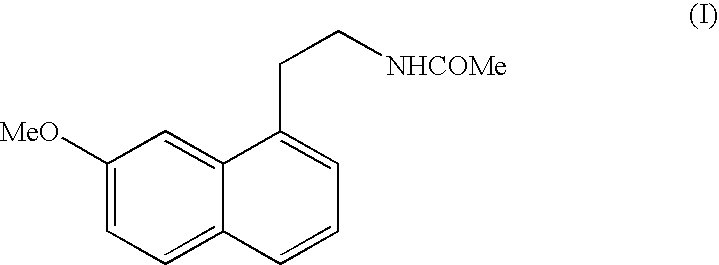
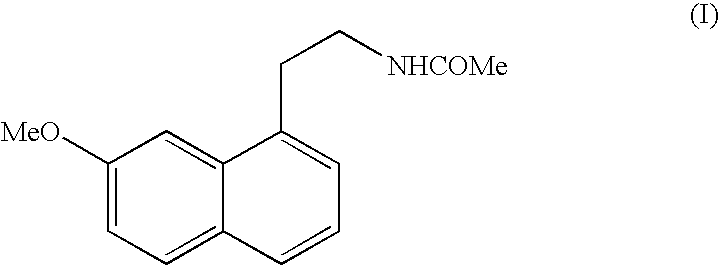
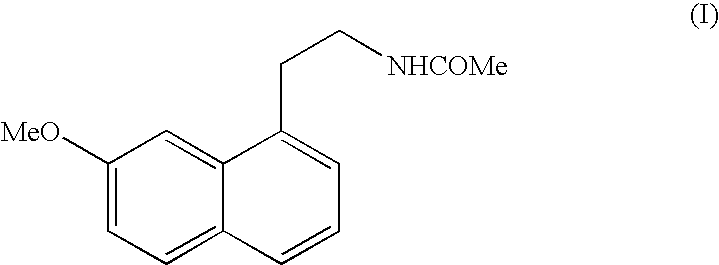
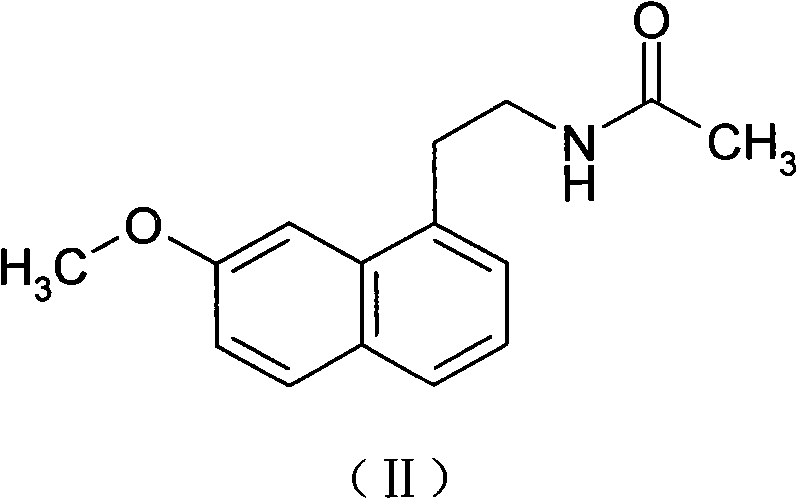
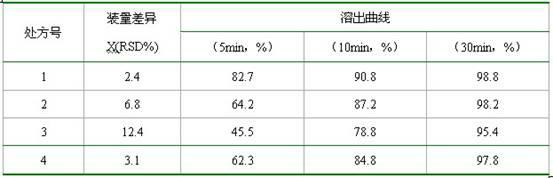
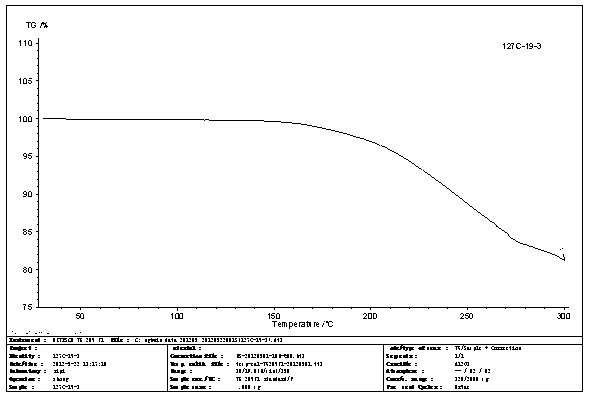
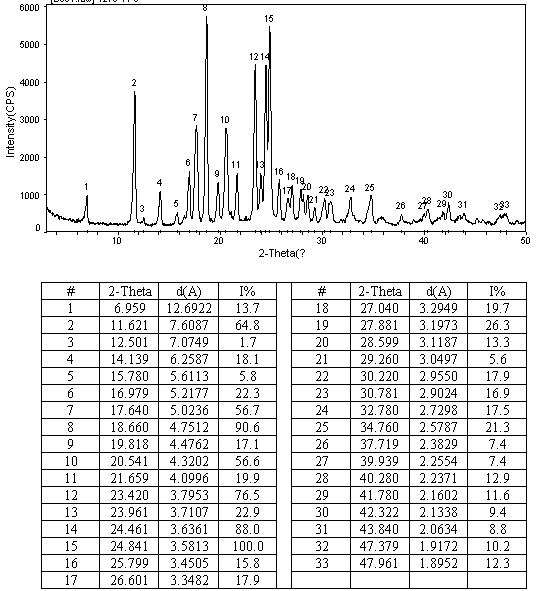
N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof
Owner:TIANJIN HANKANG PHARMA BIOTECH
Agomelatine-isonicotine eutectic crystal and compound and preparation method thereof
InactiveCN102503886AGood water solubilityImprove playbackOrganic active ingredientsNervous disorderDiffractionAgomelatine
The invention discloses agomelatine-isonicotine eutectic crystal which has the chemical formula (I). The diffraction angles 2theta can be 10.20 DEG, 14.43 DEG, 15.97 DEG, 17.64 DEG, 18.57 DEG, 19.22 DEG, 19.91 DEG, 20.68 DEG, 21.39 DEG, 21.66 DEG, 21.94 DEG, 23.16 DEG, 24.28 DEG, 24.75 DEG, 25.42 DEG, 29.54 DEG, 29.90 DEG and 30.39 DEG. The agomelatine-isonicotine eutectic crystal is prepared by melting agomelatine and isonicotine and cooling. The agomelatine-isonicotine eutectic crystal is easy to prepare and has low preparation cost.
Owner:SUN YAT SEN UNIV
Preparation of agomelatine intermediate 2-(7-methoxy-1-naphthyl) acetamide
InactiveCN101486665ALow costIncrease costOrganic compound preparationCarboxylic acid amides preparationAcetic acidEthyl ester
The invention relates to a preparation method for an intermediate compound for preparing agomelatine. The agomelatine intermediate 2-(7-methoxyl-1-naphthyl) acetamide is directly obtained by the ammonolysis of (7-methoxyl-1-naphthyl) ethyl acetate. The method has high reaction yield; reagents used have low cost; the method also has relatively mild reaction condition, safe operation and good controllability. Besides, the whole reaction process is not added with highly toxic reagent or solvent, thus meeting the requirement of environmental protection and being very suitable for industrialized production application.
Owner:SHANGHAI INST OF PHARMA IND
Preparation method of agomelatine solid preparation
ActiveCN102988315AQuality improvementSimple methodOrganic active ingredientsNervous disorderDissolutionHot melt
The invention provides a preparation method of an agomelatine solid preparation. The preparation method comprises the steps of mixing agomelatine or pharmaceutically acceptable salts with polyvinylpolypyrrolidone; hot-melting the mixture to prepare particles; and mixing the particles with other pharmaceutically acceptable carriers to be tabletted or filled to form capsules. The agomelatine solid preparation prepared by the method is stable and controllable in quality and high in dissolution.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Medicine composition containing agomelatine
InactiveCN102342924AImprove bioavailabilityOrganic active ingredientsNervous disorderDissolutionLactose
The invention belongs to the technical field of medicine, and discloses a medicine composition containing agomelatine prepared form components of, by weight: 11% to 25% of agomelatine, 30% to 65% of lactose, 11% to 40% of starch, 8% to 15% of carboxymethylstarch sodium, 0.5 to 5% of stearic acid, 0.6% to 2% of magnesium stearate, and 0.5% to 5% of silicon dioxide. As a result of experiments, preparations prepared with the medicine composition provided by the invention provide a better dissolution rate.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com













![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000012.PNG)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000031.PNG)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000051.PNG)

![N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4c39bf25-1d51-46ea-8f9e-d0ffe13c8b50/HSA00000027767200011.PNG)
![N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4c39bf25-1d51-46ea-8f9e-d0ffe13c8b50/HSA00000027767200021.PNG)
![N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof N-[2-(7-methoxyl-1-naphthyl)ethyl]acetamide and compound thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4c39bf25-1d51-46ea-8f9e-d0ffe13c8b50/HSA00000027767200031.PNG)