Dispersible tablet containing antipsychotic medicines and application thereof
An antipsychotic and dispersible tablet technology, which can be used in medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., and can solve the problem that the pathogenesis of depression is not completely clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
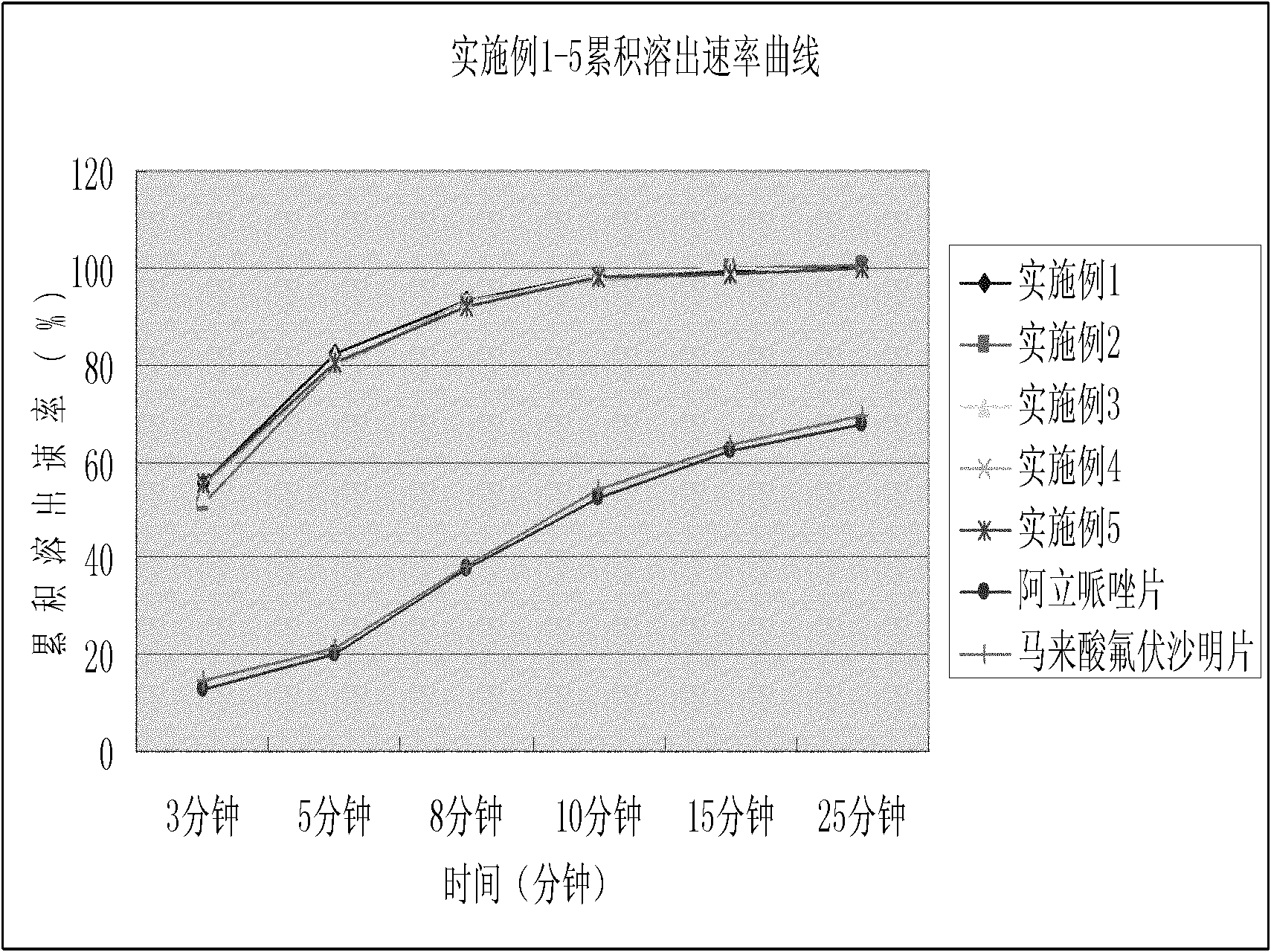
Image
Examples
Embodiment 1
[0124] Embodiment 1: Aripiprazole Dispersible Tablets
[0125] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
Dose 4(mg)
5.00
10.00
15.00
20.00
15.00
30.00
45.00
60.00
19.87
39.74
79.48
158.96
0.25
0.50
0.75
1.00
Povidone K30
5.00
10.00
15.00
20.00
0.50
1.00
1.50
2.00
2.50
5.00
7.50
10.00
1.50
3.00
4.50
6.00
Micropowder silica gel
0.33
0.66
0.99
1.32
0.05
0.10
0.15
0.20
Sheet weight
50.00
100.00
150.00
200.00
[0126] Preparation:
[0127] (A) Micronize the main ingredient after drying, and control...
Embodiment 2
[0132] Embodiment 2: fluvoxamine maleate dispersible tablet
[0133] Name of raw material
Embodiment 3
[0134] Embodiment 3: Escitalopram Oxalate Dispersible Tablets
[0135] Name of raw material
[0136] sodium starch glycolate
[0137] ※6.39mg of escitalopram oxalate is equivalent to 5.00mg of escitalopram.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com