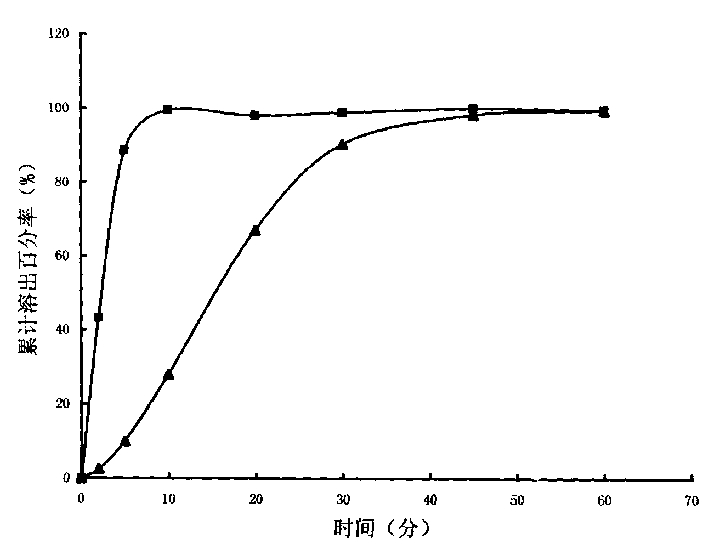
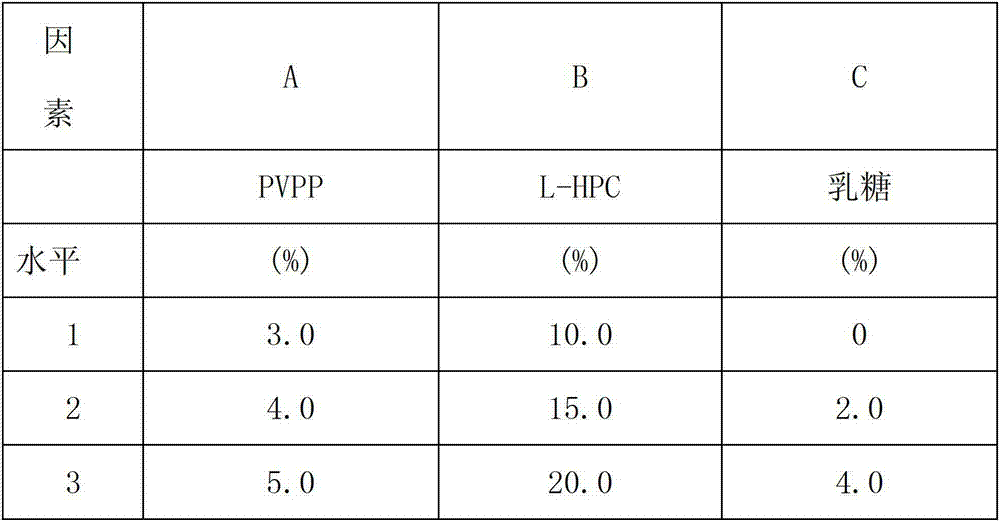
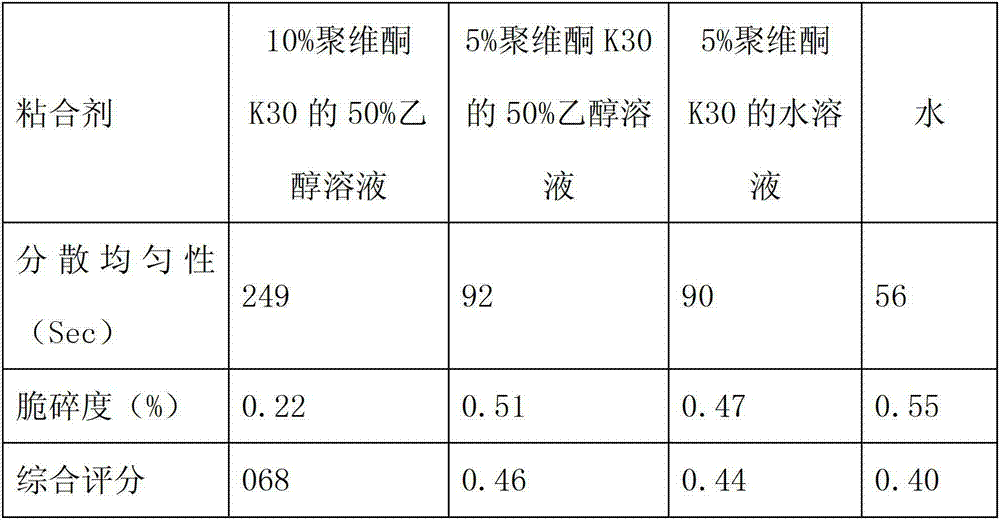
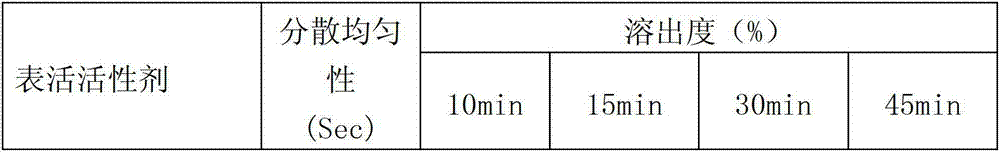
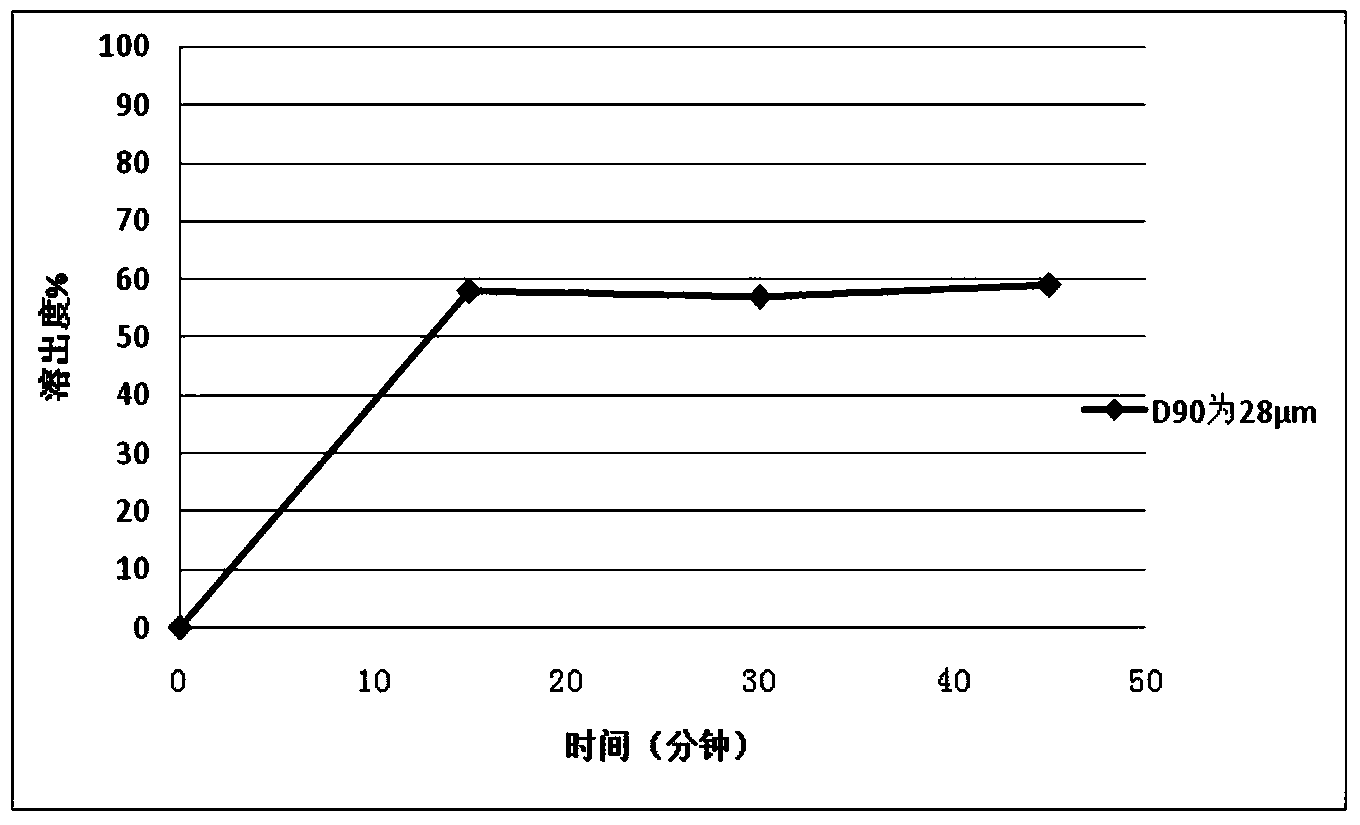
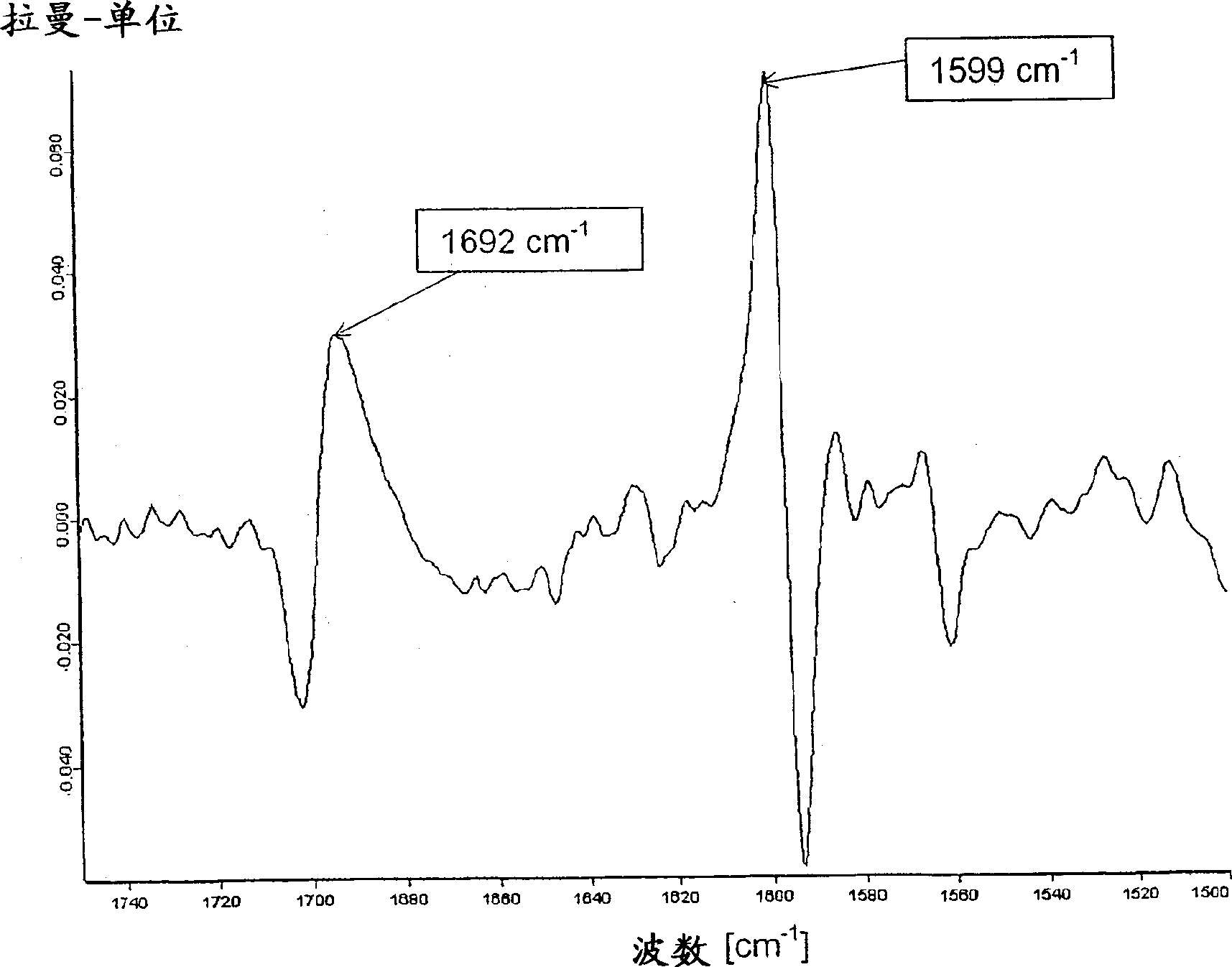
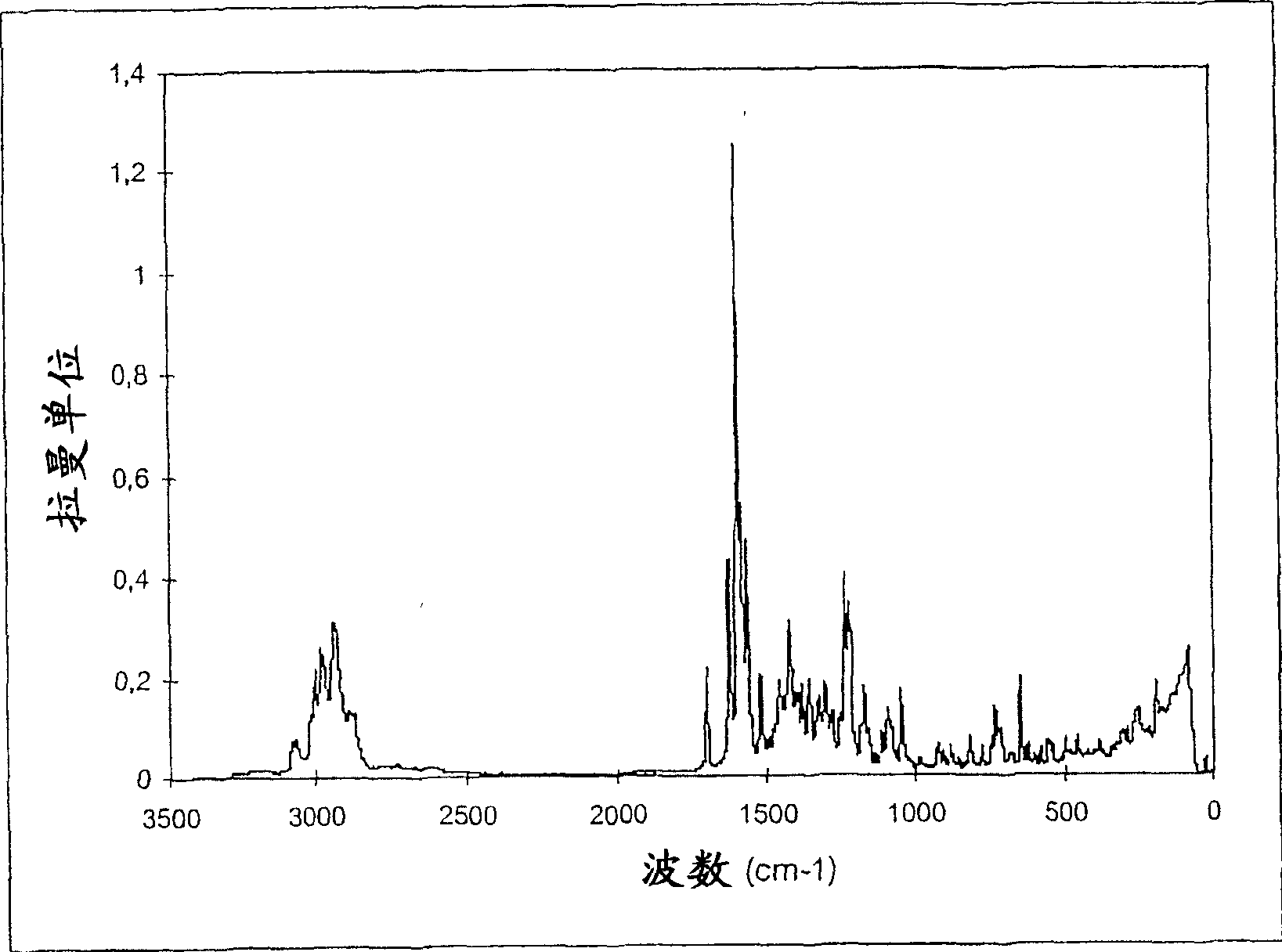
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
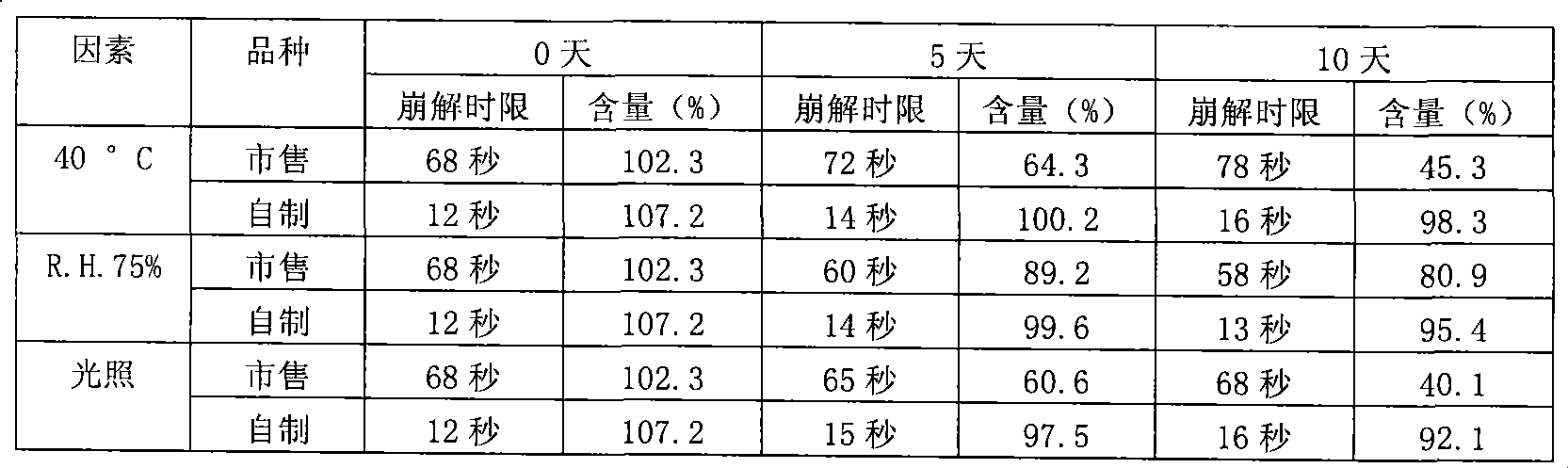
530results about How to "Short disintegration time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Direct compression metformin hydrochloride tablets
InactiveUS6117451AGood compressibilityImproved flowabilityPowder deliveryBiocideMetformin HydrochlorideHigh doses
Metformin Hydrochloride (herein referred to as metformin HCl) that may be 98.5%-100% pure is a high dose drug capable of being directly compressed with specific excipients into tablets having desired, hardness, disintegrating ability, and acceptable dissolution characteristics. Metformin HCl is not inherently compressible and thus presents formulation problems. Excipients used in the formulation enhance the flow and compaction properties of the drug and tableting mix. Optimal flow contributes to uniform die fill and weight control. The binder used ensures sufficient cohesive properties that allow metformin HCl to be compressed using the direct compression method. The tablets produced provide an acceptable in-vitro dissolution profile.
Owner:PHARMALOGIX
Nicotine containing stimulant unit
InactiveUS6110495AIncrease stimulationLarge deformationTobacco treatmentConfectioneryStimulantAdditive ingredient
A saliva-soluble stimulant unit comprising an active ingredient and optional ingredients comprising flavor and aroma additives incorporated in a gel prepared by gelling a water-binding gelling agent, in which the active ingredient comprises nicotine or other alkaloids with the same direction of activity, said unit having i) a texture profile, determined by texture profile analysis, with parameter values of firmness, hardness, brittleness, adhesiveness, elasticity, and cohesiveness within given ranges; (ii) a disintegration time within the range 5-60 minutes; and (iii) a nicotine content from 0.5 to 10 mg or a corresponding content of said alkaloids.
Owner:DAM ANDERS
Oral quick-dissolving film preparation and preparation method thereof
InactiveCN102824333AImprove disintegration time limitSolve the shortcomings of taking waterPharmaceutical non-active ingredientsSexual disorderVardenafilTadalafil
The invention discloses an oral quick-dissolving film preparation and a preparation method thereof. The oral quick-dissolving film comprises the following components in percentage by weight: 20 to 40 percent of medicinal active component, 40 to 75 percent of water-soluble film forming material, 10 to 25 percent of plasticizer, 0 to 25 percent of disintegrating agent, and 0.1 to 8 percent of water, wherein the medicinal active component is one of sildenafil, tadalafil, vardenafil or salts thereof. According to the oral quick-dissolving film preparation, the disintegration time limited of the film preparation can be remarkably accelerated, the problem that most oral solid preparations should be taken with water can be solved, medicine taking time cannot be delayed under a condition without water, and the taking compliance of a patient can be improved.
Owner:SUZHOU UNIV
Cefixime dispersing tablet and preparation methods thereof
ActiveCN101606913AIncrease contentEasy to takeAntibacterial agentsOrganic active ingredientsMagnesium stearatePharmaceutical preservatives
The invention discloses a cefixime dispersing tablet and preparation methods thereof, belonging to the technical field of pharmaceutical preparation. The cefixime dispersing tablet comprises 40-420 mg of cefixime, 0-100 mg of starch, 0-250 mg of amylum pregelatinisatum, 10-80 mg of mannite, 0-150 mg of microcrystalline cellulose, 10-60 mg of carboxyrnethyl starch sodium, 2-20 mg of polyvidone K30, 0.4-10 mg of magnesium stearate, 0-10 mg of Steviosin and 0-10 mg of orange compound perfume. The preparation method comprises the following steps: evenly mixing basic remedies and excipients; adding adhesive to prepare granulates; drying; sorting and then adding with other excipients; and tabletting. The other preparation method is described as follows: the basic remedies are added after granulating. The invention aims to solve the technical problem that cefixime preparation has shorter disintegration time, better leachability, higher content of the basic remedies, production cost reduction, quality detection time reduction and production environment pollution reduction.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Atorvastatin calcium tablet and preparation method thereof
ActiveCN102920675AHigh dissolution rateImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsFiller ExcipientHardness
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet consists of the following components in parts by mass: 7.22 parts of main medicine atorvastatin calcium, 84.55 parts of filler, 6 parts of disintegrating agent croscarmellose sodium, 1.33 parts of adhesive hydroxy propyl cellulose, 800.4 parts of cosolvent polysorbate and 0.5 part of lubricating agent magnesium stearate, wherein the filler comprises the following raw materials in parts by mass: 22.01 parts of calcium carbonate, 21.87 parts of milk sugar and 40.67 parts of microcrystalline cellulose. The atorvastatin calcium tablet has the characteristics of short disintegrating time, fast dissolving-out speed, high bioavailability and small particle diameter, and is convenient to take. Furthermore, the hardness of the tablet can reach 60-70N, so that the tablet is hardly broken, and therefore, the packing and transporting costs are reduced, and the industrialized popularization of the tablet is easily realized.
Owner:HENAN RUNHONG PHARMA
Starch soft capsules
InactiveCN104721167AHigh transparencyImprove toughnessPharmaceutical non-active ingredientsCapsule deliveryPlasticizerBreakage rate
The invention relates to capsules widely used in the industries of medicines, health products and foods, and particularly relates to starch soft capsules. The starch soft capsules contain starch and a plasticizer which are used as a matrix, and vegetable gelatine used as a gel, a raw material for preparing the starch soft capsules is obtained after mixing the starch, the plasticizer and the vegetable gelatine with deionized water, and then the starch soft capsules are prepared by a conventional process for preparing soft capsules. The plasticizer in the starch soft capsules disclosed by the invention is used for improving the movability of the molecular chains of starch, reducing the crystallinity of the molecular chains of the starch, and further playing a role of enhancing the elongation and flexibility of the starch. In addition, the starch soft capsules prepared from the abovementioned raw materials have a low breakage rate and a good disintegration performance.
Owner:HUNAN ER KANG PHARMA
Nicardipine hydrochloride dispersion piece and method for making same
InactiveCN101156851AShort disintegration timeGood dispersionOrganic active ingredientsPill deliveryCross-linked polyethyleneStevioside
The invention discloses a nicardipine hydrochloride dispersible tablet which is characterized in that the nicardipine hydrochloride dispersible tablet is composed of components with the following weight percentage: 8 to 12 percent of nicardipine hydrochloride, 35 to 55 percent of starch, one or more than one of microcrystalline cellulose and lactose, 35 to 50 percent of sodium carboxymethyl starch, one or more than one of hydroxypropyl cellulose, hydroxypropyl methylcellulose, crosslinked polyvinyl pyrrolidone, and crosslinked sodium carboxymethyl cellulose, 5 to 2.5 percent of saccharin sodium or stevioside, and 0.25 to 1 percent of magnesium stearate. The disintegration time of the nicardipine hydrochloride dispersible tablet is short, the dispersed state is good, the drug is dissolved rapidly, the administration is convenient and flexible, the tablet can not only be sucked and swallowed, but also can be taken after being dispersed by adding water. The invention also discloses a preparation method of the nicardipine hydrochloride dispersible tablet.
Owner:刘全胜
Ambroxol hydrochloride oral cavity disintegrating tablet and method of producing the same
The invention discloses an oral disintegration tablet of alcaine aminobromisuo, which contains alcaine aminobromisuo with effective dosage and pharmacological findings, wherein the alcaine aminobromisuo is processed by metarchon with rate of aminobromisuo and metarchon at 1: 0. 1-1. 5, which is blended with pharmacologically acceptable findings; the metarchon can be medically inorganic oxide or / and medicinal organic acid and salt; the acceptable findings is filler, corrigent, disintegration agent, adhesive and lubricant; the oral disintegration tablet of alcaine aminobromisuo hasn't bitterness and numb sense to disintegrate within one minute with stripping degree not less than 90%. The invention is convenient and simple to control with high yield and low energy consumption, which widens the resource of metarchon to save cost.
Owner:CHONGQING CONQUER PHARML
Oral disintegrant tablet and its preparation method
InactiveCN1476826AGreat tasteShort disintegration timePill deliveryPharmaceutical non-active ingredientsCross-linkOrally disintegrating tablet
The present invention relates to an oral disintegration tablet and its preparation method. It includes the medicinal active component and auxiliary material. Auxiliary material includes diluting agent and disintegrant, in which the diluting agent is mannite and lactose and the disintegrant is microcrystal cellulose, low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone, the weight of disintegrant is less than 25% of total weight of the auxiliary material, the weight ratio of medicinal active component and auxiliary material is 1:2-75. Said disintegration tablet has enough hardness (strength).
Owner:北京中西经纬释药技术有限公司
Orodispersible pharmaceutical composition for oromucosal or sublingual administration of agomelatine
InactiveCN1981752AShort disintegration timeSpeed up entryOrganic active ingredientsNervous disorderSublingual administrationOral cavity
The invention relates to a coated solid orodispersible pharmaceutical composition for oral, oromucosal or sublingual administration of agomelatine.
Owner:LES LAB SERVIER
Melatonin orally disintegrating tablet and preparation method thereof
InactiveCN101143135AMeet technical requirementsQuick effectOrganic active ingredientsNervous disorderMedicineOrally disintegrating tablet
The invention discloses an oral disintegrating tablet of melatonin which is made by selecting the melatonin, a disintegrator, an effervescing agent, a filling agent, an odor corrective and a lubricating agent for smashing, drying, mixing and tablet forming. The drug can be promptly disintegrated inside the mouth without water and is especially applicable to the patients with the deglutition difficulty or under the special environment, such as the elder, the children, the narcose patient etc. The drug also has good effect when used in the environment, which lacks the water, such as the outdoors, the battle field etc. The invention has simple technology and cheap cost and is provided with the advantages of short production period, simple production equipment etc. The oral disintegrating tablet which is prepared by the method of the invention has the enough rigidity to meet the requirements of the production, the packaging, the storage and the transportation, and at the same time the oral disintegrating tablet has good taste and short disintegrating time, and the inside-body disintegrating time is less than thirty seconds; the dissolved quantity inside the 37 DEG C artificial gastric juice and intestinal juice within one minute is 40 percent, and the dissolved quantity within six minutes is as high as 90 percent.
Owner:徐贵丽 +1
Compound rhodiola rosea chewing tablet and preparing method thereof
InactiveCN103977056APromote dissolutionIncrease surface areaOrganic active ingredientsAnthropod material medical ingredientsAdjuvantRHODIOLA ROSEA ROOT
The invention provides a compound rhodiola rosea chewing tablet and a preparing method thereof. The tablet comprises following raw materials by weight: 1-2% of a rhodiola rosea clathrate compound that is the main medicine, 3.6-4.6% of adjuvants, 76-86% of a filling agent, 7.4-17.5% of a corrigent, 0.7-1% of a lubricant and a proper amount of a wetting agent. The tablet has characteristics of good dispersion state, short disintegration time, rapid medicine digestion, rapid absorption, high bioavailability and taking convenience.
Owner:凌春生
Zhongshengmycin wettable powder and preparation method thereof
InactiveCN101171931AReduce pollutionGood dispersionBiocideAnimal repellantsAdditive ingredientDisinfectant
The invention discloses mesophilous bacterial chlorin water dispersion granule agent in disinfectant, and belongs to the disinfectant technology in the plant protection field. The invention comprises the components that the raw material medicine mesophilous bacterial chlorin occupies 100 to 600 shares, auxiliary agent occupies 180 to 360 shares, carrier packing material occupies 250 to 600 shares, and binding agent occupies 10 to 150 shares. The ingredients are mixed proportionally, after being evenly mixed, the mixture is crushed to obtain the target powder, the target powder and the binding agent are evenly mixed, and the mesophilous bacterial chlorin water dispersion granule can be obtained through granulation, drying and screening. The invention overcomes the disadvantage of the wettable powder of the mesophilous bacterial chlorin, both the us-cost of the farmer and the environment pollution are reduced. The invention has the advantages that the content is high, the disintegration is quick, the dispersivity is good, the invention is advantageous for storage and transportation and the stability is good, the activeness is high, the efficiency is good, and the invention is secure.
Owner:SHAANXI MEIBANG PHARMA GRP CO LTD
Dispersion tablet of vasilowy hydrochlaride and its prepn. method
ActiveCN1513454AShort disintegration timeGood dispersionAerosol deliveryAntiviralsCross-linked polyethyleneHydroxypropylmethyl cellulose
A dispersing tablet of Faxiluowei hydrochloride contains Faxiluowei hydrochloride, the starch, microcrystalline cellulose and / or calcium hydrogen phosphate, the carboxymethyl starch sodium, hydroxypropyl cellulose, polyvinyl pyrrolidone and / or carboxymethyl cellulose sodium, the polyvinyl pyrrolidone or hydroxypropylmethyl cellulose, the sodium saccharin, aspartame, or stevioside, the menthol, the magnesium stearate, and the talc powder or micropowder silica gel. Its advantages are short disintegration time, and high biologic utilization rate.
Owner:湖北科益药业股份有限公司
Albendazol dispersible tablets and preparation method thereof
InactiveCN102805736AImprove bioavailabilityGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsPatient complianceCurative effect
The invention relates to albendazol dispersible tablets and a preparation method thereof. The albendazol dispersible tablets comprise, by weight ratio, 200g of albendazol, 50-160g of disintegrant, 18-118g of diluent, 2-12g of surfactant, 6-16g of sweetener, 3-12g of glidant, and 1-4g of lubricant. Bioavailability of the albendazol dispersible tablets made from the proper accessories is improved evidently, and curative effect of the albendazol dispersible tablets is improved. The albendazol dispersible tablets are available to take in multiple ways, and different patients can take the albendazol dispersible tablets in different ways, so that patient compliance is improved. Practice shows that preparation technology of the albendazol dispersible tablets is high in production mechanization level, simple to operate, high in production efficiency and low in cost. The dispersible tablets produced by the preparation technology have the advantages of high dispersing uniformity, short disintegrating time, and fast dissolution, and the product quality completely meets the requirements.
Owner:宁夏启元国药有限公司
A medication for treating headache, and preparation method
A Chinese medicine in the form of dispersing tablet for treating headache is prepared from 11 Chinese-medicinal materials including Chinese angelica root, Chuan-xiong rhizome, white peony root, prunella spike, etc and proper auxiliaries including disintegrant, filler and lubricant. Its preparing process is also disclosed.
Owner:天士力东北现代中药资源有限公司
Healthcare product combination for clearing and nourishing throat and preparation method thereof
The invention relates to a healthcare product combination for clearing and nourishing the throat and a preparation method thereof. The combination comprises the following raw and auxiliary materials in parts by weight: 25-75 parts of rosa roxbughii tratt powder, 100-200 parts of phyllanthus emblica powder, 40-250 parts of sorbitol, 200-900 parts of Isomalt, 1-10 parts of stevioside and 2-18 partsof magnesium stearate. The healthcare product combination for clearing and nourishing the throat, provided by the invention, has slightly astringent taste and sweet aftertaste.
Owner:NEW ERA HEALTH IND GRP +1
Orodispersible tablets
InactiveUS20120077888A1Short disintegration timeHighly robustBiocideAntipyreticMANNITOL/SORBITOLActive agent
A directly compressed orodispersible tablet comprises 0.1 to 50% of a ungranulated active agent (w / w), 10 to 80% of a sugar-based direct compression base, and 10 to 80% of a microcrystalline cellulose (MCC) direct compression base, and has a hardness of at least 60N, and a disintegration time of less than 40 seconds. The sugar-based direct compression base is a DC sugar alcohol, especially direct compression mannitol, and the MCC base is a silicified MCC, especially a Prosolv. The active is a hydrophobic active, typically a high-dose active. Also disclosed is a method of producing an orodispersible tablet comprising the steps of directly compressing a mixture of components at a compression force of at least 5 k N to form the tablet, wherein the mixture of components comprises 0.1 to 50% of an active agent (w / w), 10 to 80% of a sugar-based direct compression base (w / w); and 10 to 80% of a microcrystalline cellulose (MCC) direct compression base (w / w).
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Oral rehydration salt effervescent tablet and application thereof
ActiveCN101732342AReduce joinComplete acid-base reactionOrganic active ingredientsMetabolism disorderSodium bicarbonateNuclear chemistry
The invention discloses an oral rehydration salt effervescent tablet which mainly contains sodium chloride, postassium chloride, anhydrous dextrose, citric acid, sodium bicarbonate and / or sodium carbonate and water-soluble lubricant. The invention also discloses the application of the oral rehydration salt effervescent tablet. The oral rehydration salt effervescent tablet is convenient to carry and use. The dosages of an acid source and an alkali source in the oral rehydration salt effervescent tablet can reflect the dosage of sodium citrate needed by the prescription for producing oral rehydration salt (ORSII), thereby reducing the addition of the raw material sodium citrate and lowering the production cost effectively.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Vonoprazan oral quick-dissolving film agent and method for preparing same
ActiveCN105663096AAvoid difficultiesImprove complianceOrganic active ingredientsDigestive systemVonoprazanPharmaceutical formulation
The invention discloses a Vonoprazan oral quick-dissolving film agent and a method for preparing the same, and belongs to the field of medicine preparations.The Vonoprazan oral quick-dissolving film agent particularly comprises, by weight, 10-15% of Vonoprazan active monomers, 50-55% of hydroxypropyl methylcellulose, 25-30% of maltodextrin, 0.8-1.0% of hyaluronic acid and 5-10% of plasticizers.The Vonoprazan oral quick-dissolving film agent and the method have the advantages that the water-soluble hydroxypropyl methylcellulose, the water-soluble maltodextrin and the water-soluble hyaluronic acid are preferably used as film-forming materials, the appropriate plasticizers with the appropriate weight ratio are screened, and accordingly the film agent with excellent disintegration time and excellent mechanical performance can be prepared; the disintegration time limit of the Vonoprazan oral quick-dissolving film agent can be obviously shortened, accordingly, the shortcoming that water is required when existing most oral solid preparations are about to be administered can be overcome, the medicine administration time cannot be delayed even under the condition of deficiency of water resources, and the medication compliance of patients can be improved.
Owner:NANJING GRITPHARMA CO LTD
Laminated laundry detergent tablet and preparation method thereof
InactiveCN106190626AEasy to operateWide operating rangeInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAdhesiveRoom temperature
The invention discloses a laminated laundry detergent tablet and a preparation method thereof. The laminated laundry detergent tablet is prepared from, by weight, 5%-10% of a non-ionic surfactant, 15%-35% of an anionic surfactant, 3%-10% of an auxiliary washing agent, 0.1%-5% of an adhesive, 40%-60% of a disintegrating agent, 0.1%-5% of a laminating agent and 1%-5% of a solvent. The laminated laundry detergent tablet which is made from biodegradable materials is highly concentrated, low in consumption and high in detergence; by combination of an effervescence system and a polymer swelling system, high disintegration speed is realized; due to water-soluble laminates on surfaces of material particles, interaction or dampness of a disintegration agent can be avoided, requirements on a storage environment are lowered, and the shelf life is prolonged. The preparation method is completed under a normal-temperature normal-pressure condition, convenient, quick and simple in operation, free of special equipment, wide in application range and low in cost.
Owner:南京尚易环保科技有限公司
Prepared Chinese medicine capsule and its preparation and quality control method
ActiveCN101239110ADisintegrates quicklyShort disintegration timeComponent separationDigestive systemDiseaseSpleen
The invention discloses a Chinese medicine capsule for curing weak spleen and stomach, cold coagulation indigestion, pain in stomach, diet indigestion, chronic gastritis and peptic ulcer, which is characterized in that the raw materials of the capsule such as south gypsum rubrum, piper longum, elecampane, pomegranate rind, savoury rhododendron, hawthorn, pawpaw and elecampane are crushed into powder and mixed uniform, right amount of starch is added into the mixed powders and loaded in capsules. The capsule provided by the invention has the advantages of easy to collapse, easy to take and good effect and can be used to cure the diseases such as weak spleen and stomach, cold coagulation indigestion, pain in stomach, diet indigestion, chronic gastritis and peptic ulcer, etc.
Owner:杨文龙
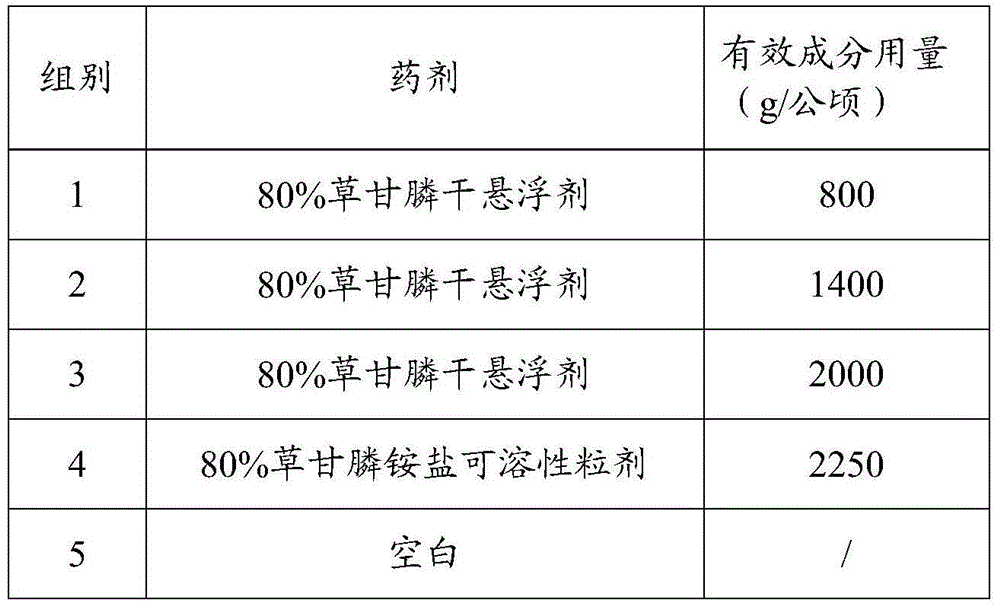
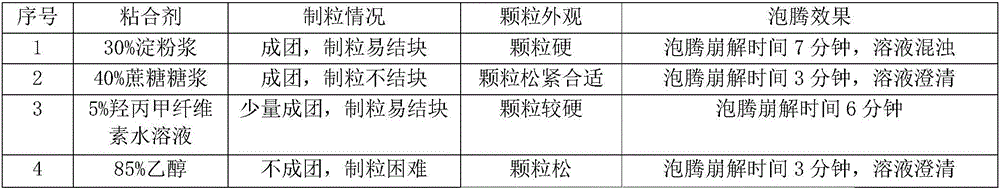
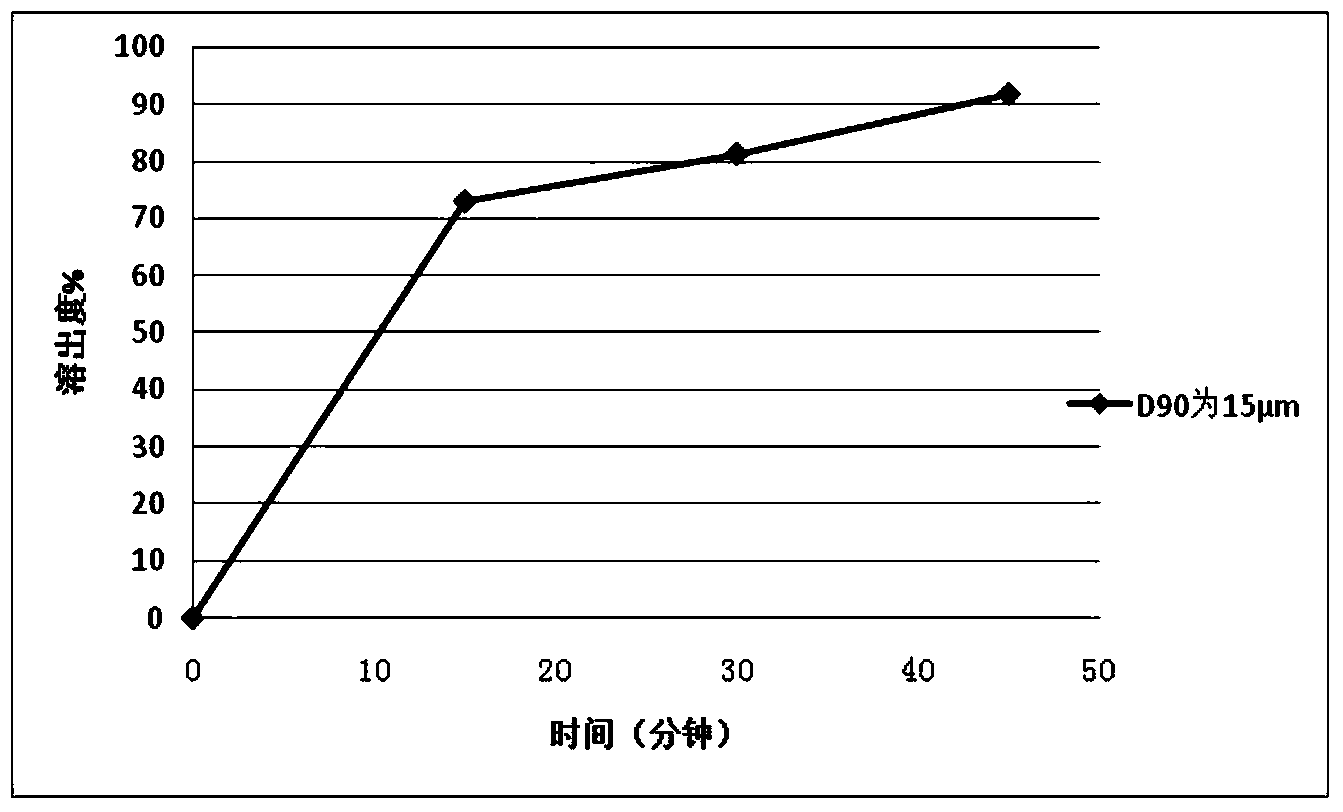
Glyphosate dry suspension and preparation method and application thereof
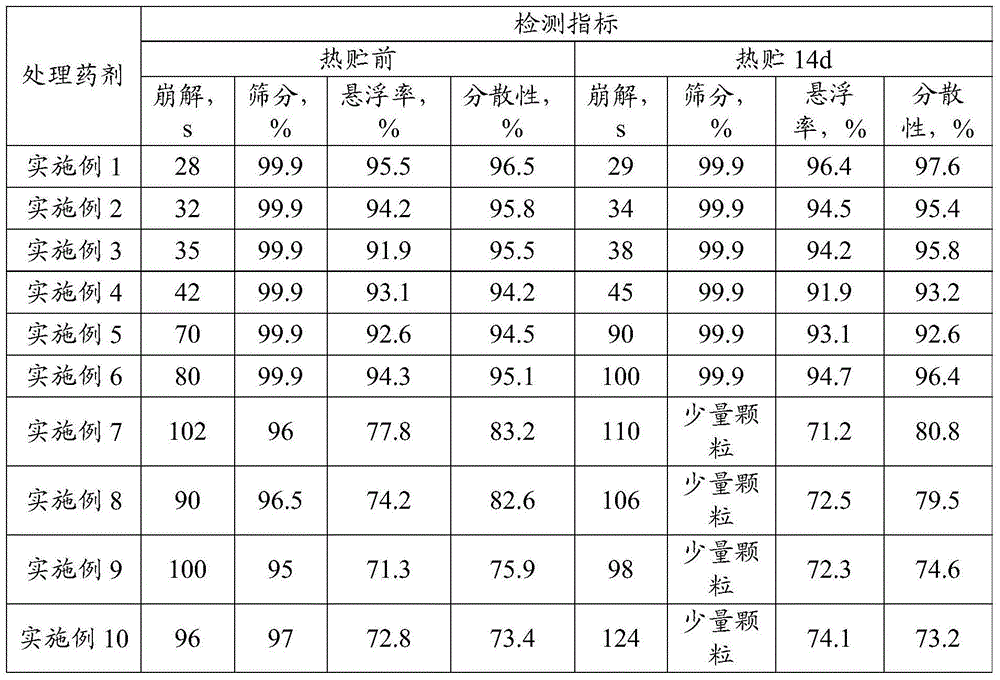
The invention provides a glyphosate dry suspension and a preparation method and application thereof. The glyphosate dry suspension is prepared by using the following components in percentage by mass: 30-85 percent of glyphosate, 2-20 percent of wetting agent, 1-20 percent of dispersing agent, 0.1-5 percent of defoaming agent, 1-5 percent of bonding agent, 1-10 percent of disintegrating agent and balance of carrier, wherein the disintegrating agent comprises one or a plurality of urea, sodium chloride, sulfate, carbonate and sodium carboxymethylcellulose. The glyphosate dry suspension provided by the invention has relatively excellent stability and disintegrating property through the proportioning of the components with the contents; the content of effective components is higher; the control efficiency is higher; according to experimental results, after 14-day heated storage, no particles occur in more than 60 percent of the glyphosate dry suspension after screening; the suspension rate highly reaches 96.4 percent; the disintegrating time is 29-124s; the control efficiency is 88-99.05 percent after 14 days after application; the control efficiency is 88-99.72 percent after 28 days after application.
Owner:SHANDONG WEIFANG RAINBOW CHEM
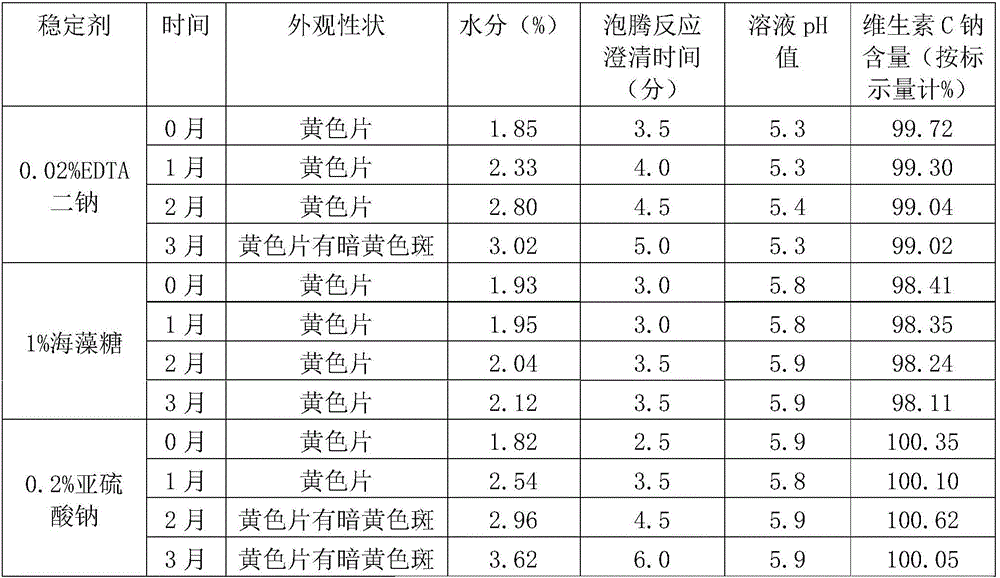
Effervescent tablet containing vitamin C sodium and preparation method of effervescent tablet
InactiveCN106420655ASuitable for long-term useTake applicableOrganic active ingredientsNervous disorderSodium bicarbonateEffervescent tablet
The invention discloses an effervescent tablet containing vitamin C sodium. The effervescent tablet is prepared from the following components in parts by weight: 200-1120 parts of the vitamin C sodium, 560-1300 parts of tartaric acid, 600-1500 parts of sodium bicarbonate and 200-600 parts of sucrose. The effervescent tablet containing the vitamin C sodium provided by the invention aims at solving the problems of an existing vitamin C effervescent tablet which is high in acidity after being dissolved, relatively serious in irritation to oral cavities, throats and esophagi as well as to gastric mucosae and not applicable to long-term taking, the problems that in a storage process, vitamin C can become oxidized and invalid easily and the effervescent tablet is easily affected with damp and easily absorb moisture, and the like; and the preparation (the effervescent tablet) is simple and convenient and is stable in quality.
Owner:GUANGXI SANPOTEL HEALTH IND CO LTD
Repaglinide troche and preparation method thereof
ActiveCN103610677AAvoid stickingGood dispersionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention relates to an oral troche which contains repaglinide or pharmaceutically acceptable derivatives of repaglinide as well as a preparation method of the oral troche. According to the preparation method, powder of repaglinide or pharmaceutically acceptable derivatives of repaglinide is directly pressed into troche, so that the production cost is remarkably lowered, and the disintegration and the dissolution rate are greatly improved. The bioavailability and the stability of the medicine can be improved, and the problem of low content uniformity of existing small-dose medicines formed by the direct pressing method is overcome, so that the quality of the troche is better guaranteed.
Owner:华益泰康药业股份有限公司
Legemiddel inneholdende vardenafilhydrokloridtrihydrat
ActiveCN1681481AWell definedRapid disintegrationOrganic chemistryCoatingsWater contentSource material
Production of solid medicaments (A) containing vardenafil hydrochoride (I) in the form of the trihydrate involves using (I) of any water content in the production of (A) and converting (I) into the trihydrate form at an intermediate processing stage or in the final product. An independent claim is included for the medicaments (A) (specifically in coated tablet form) obtained by the process. - ACTIVITY : Vasotropic. - MECHANISM OF ACTION : None given in the source material.
Owner:BAYER IP GMBH
Prepn process and formula of compound chondroitin sulfate tablet
InactiveCN1531932AHas the function of health foodShort disintegration timeOrganic active ingredientsSkeletal disorderHigh absorptionSulfate
The present invention relates to health food with the function of preventing and auxiliarily treating osteoarthropathy, and is especially preparation process and formula of compound chondroitin sulfate tablet. The compound chondroitin sulfate tablet formula contains chodroitin sulfate 30-50 wt%, glycosamine chlorate 40-60 wt%, soybean isoflvone 2-3 wt%except supplementary material. The compound chondroitin sulfate tablet has both the treating function of chondroitin sulfate and the preventing function of soybean isoflvone, and is one kind of good health food with fast disintegrating, high leaching rate, easy taking and high absorption and other features.
Owner:唐山市福乐药业有限公司
Macitentan oral disintegrating tablet for treating PAH (pulmonary arterial hypertension) and preparation method of macitentan oral disintegrating tablet
InactiveCN107913256ADisintegrates quicklyGreat tasteOrganic active ingredientsRespiratory disorderDiseaseSolubility
The invention discloses a macitentan orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is a drug mixture comprising macitentan, flavoring agent, filler, disintegrating agent, lubricant, wetting agent and binder, wherein the physiologically active ingredient macitentan is The weight percentage of disintegrating tablets is 5-10%. The solid pharmaceutical preparation provided by the invention adopts solid dispersion technology. By mixing and grinding the main drug and the filler, the solubility of the main drug is improved, the dissolution effect is improved, and the dissolution rate of the preparation is ensured. High content uniformity and high bioavailability. In addition, the macitentan orally disintegrating tablet with convenient administration, rapid onset of effect and high bioavailability of the present invention can improve the drug compliance of patients and is beneficial to the treatment of diseases.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Nitroglycerin tablets and preparation method thereof
InactiveCN101485634AGood value for moneyNon hygroscopicPill deliveryEster active ingredientsLactoseMagnesium stearate
The invention relates to a glonoin tablet which is a drug for treating angina and a method for preparing the same. The glonoin tablet contains 0.5 milligram of glonoin and a proper amount of pharmaceutic adjuvants which are selected from a bulking agent, a stabilizing agent, a bonding agent, a disintegrating agent and a lubricating agent. In the prescription, the valuing ranges of all components for preparing one thousand tablets are that: 5.0 milliliter of 10 percent glonoin solution, 1 to 15 grams of microcrystalline cellulose, 0.1 to 1.0 gram of polyvinyl pyrrolidon, 10 to 30 grams of lactose, 1.0 to 4.0 grams of cross-linking polyvidone, 0.1 to 0.4 gram of magnesium stearate, and 10 to 20 gram of 65 percent ethanol; and the raw materials and the pharmaceutic adjuvants are screened, mixed, granulated and tableted to prepare the glonoin tablet. In the glonoin tablet, the stabilizing agent is added in the prescription, thereby lowering the vapor pressure for volatilizing the glonoin, shortening disintegration time, improving stabilization and prolonging period of validity.
Owner:BEIJING YIMIN PHARMA
Traditional Chinese medicine for treating acute and chronic gastroenteritis and bacterial dysentery and its processing technology
InactiveCN101007071AWeight increaseReduce weightAntibacterial agentsDigestive systemGastritisChinese knotweed
The invention relates to a process for preparing Chinese medicinal composition for the treatment of chronic enteritis, gastritis and bacillary dysentery, which is prepared from Chinese herbal materials including hairy euphorbia, Chinese knotweed and iron holly bark through steps of grinding, grilling, merging grilling liquid, concentrating into electuary, drying and granulating.
Owner:刘晓
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com