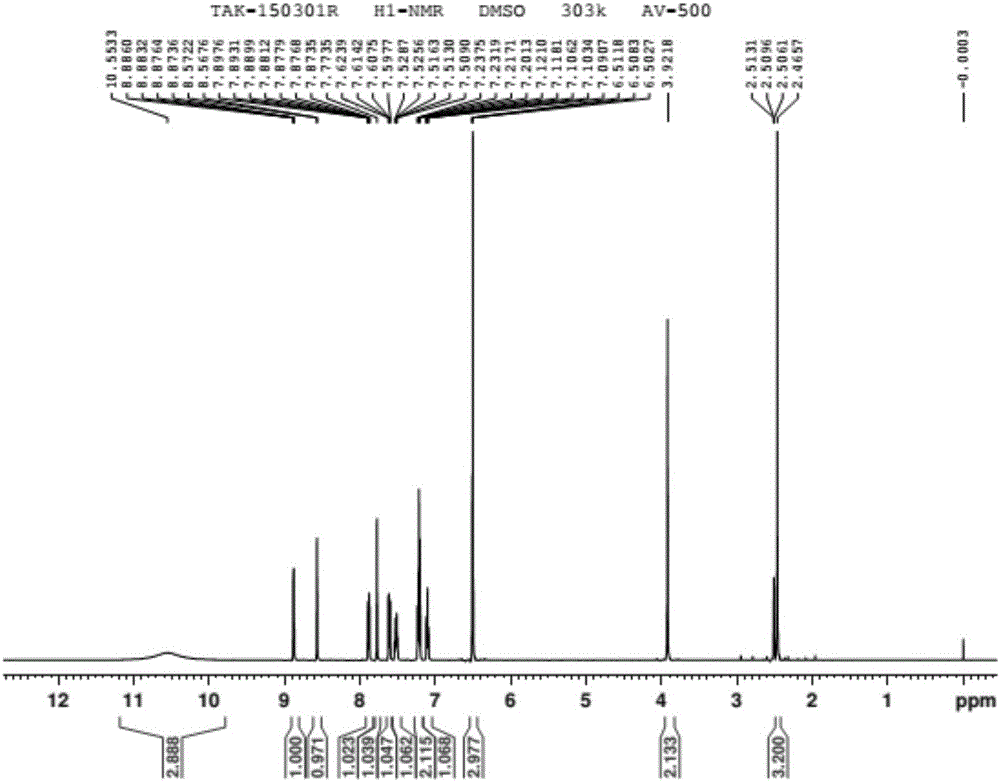
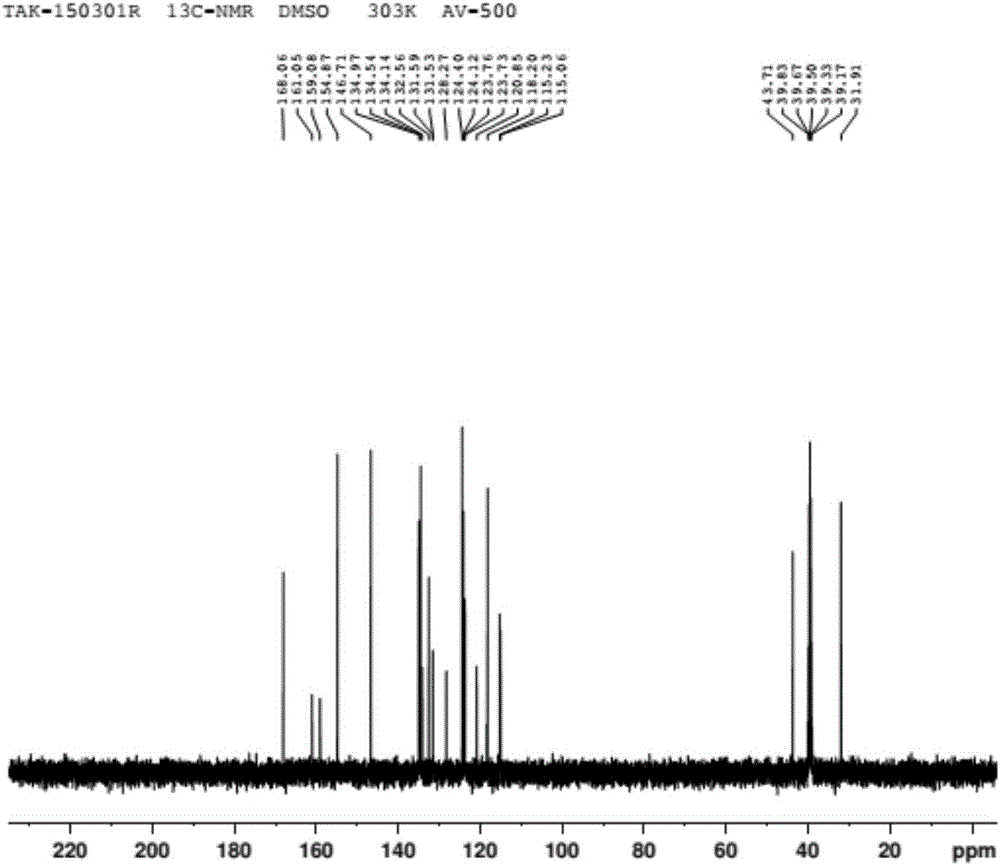
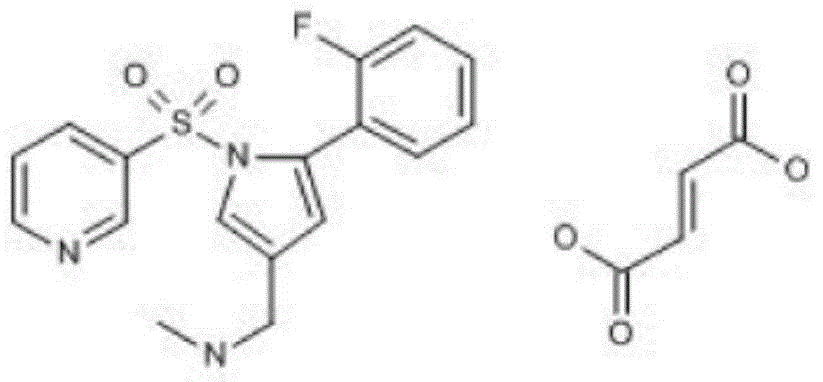
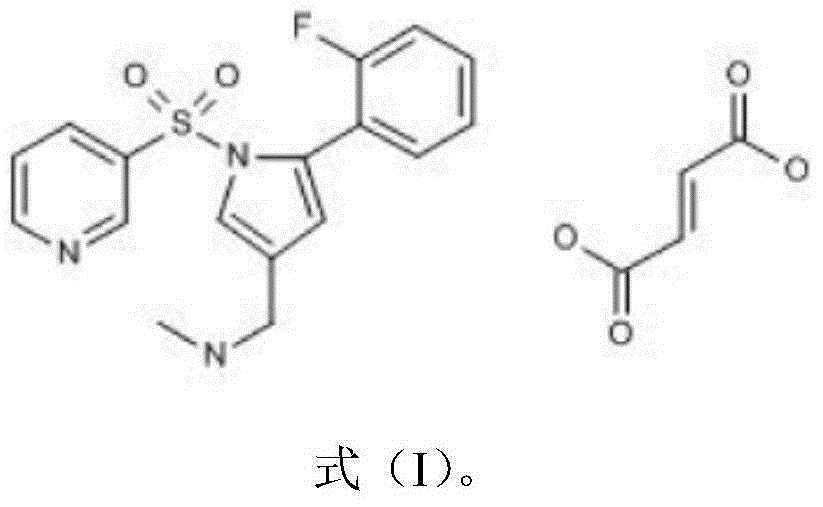
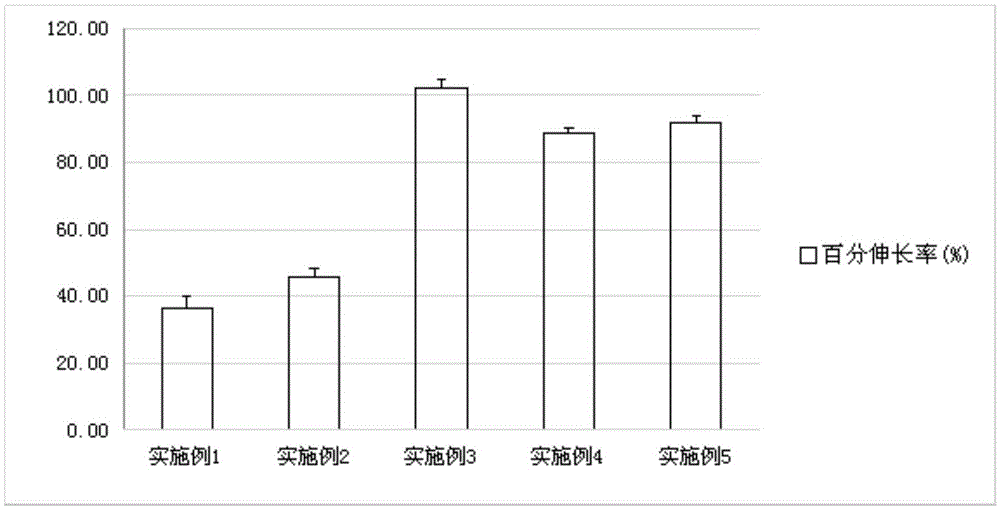
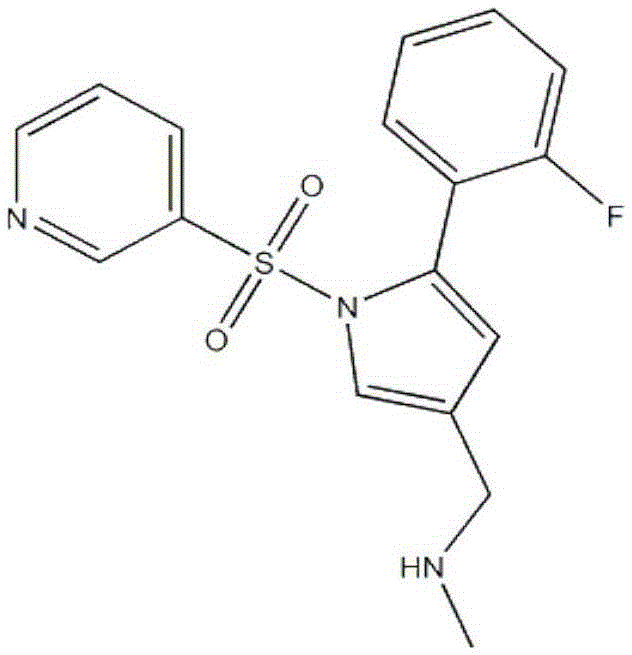
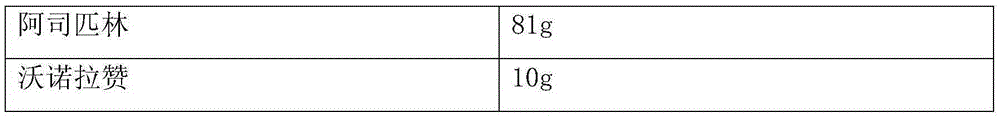
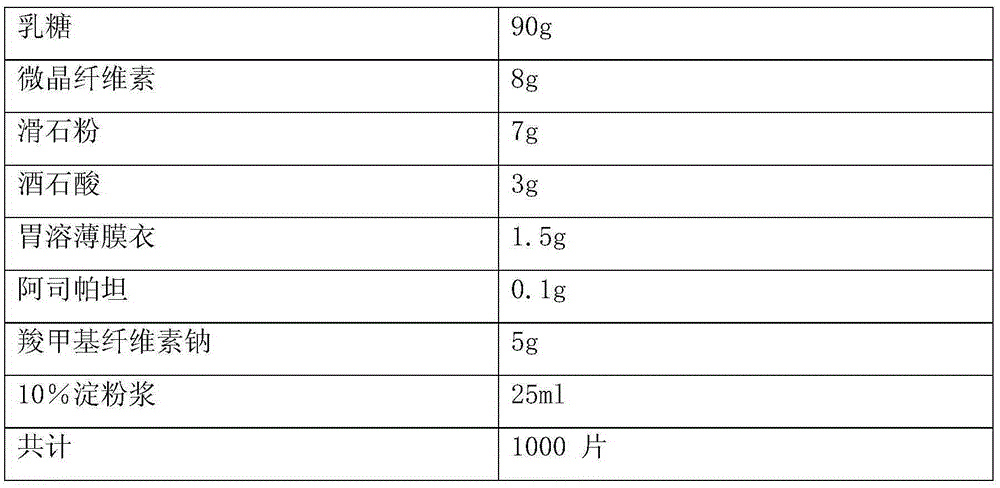
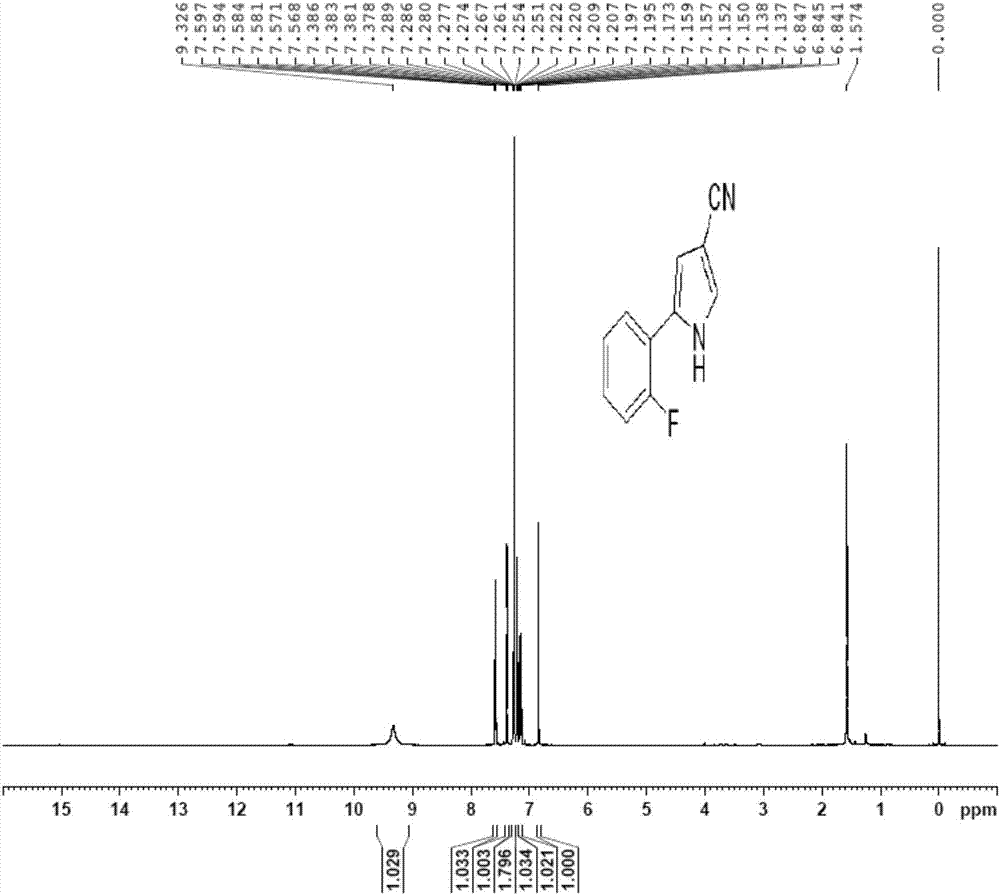
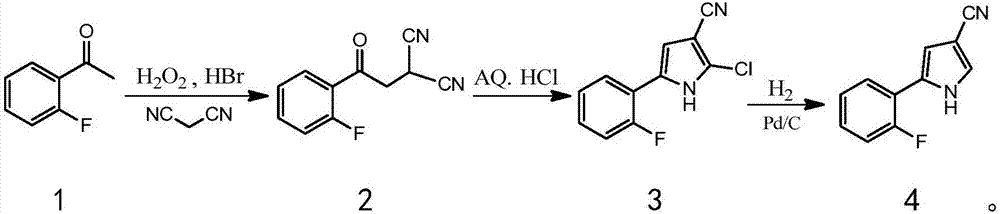
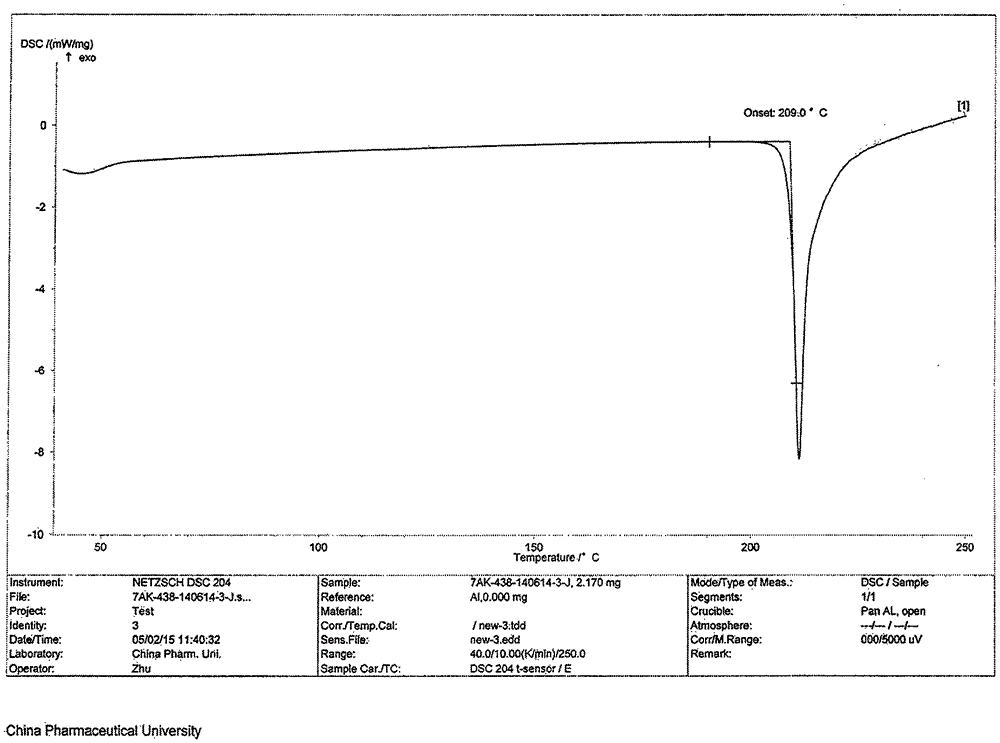
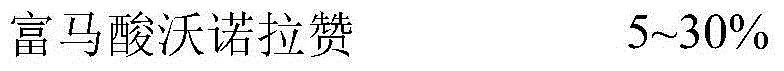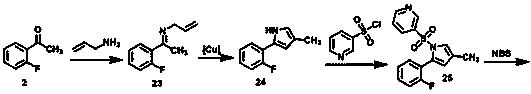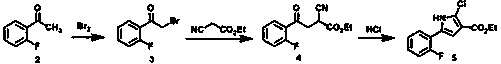Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Vonoprazan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
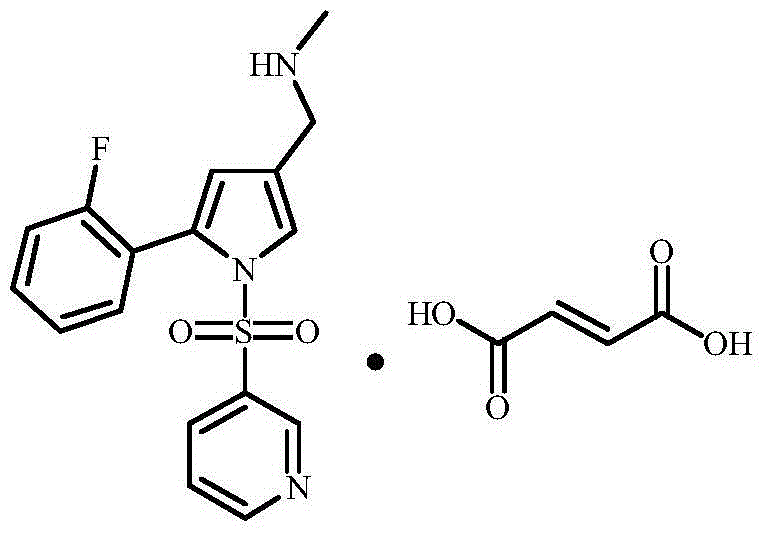
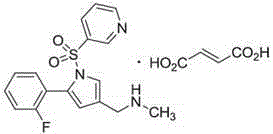
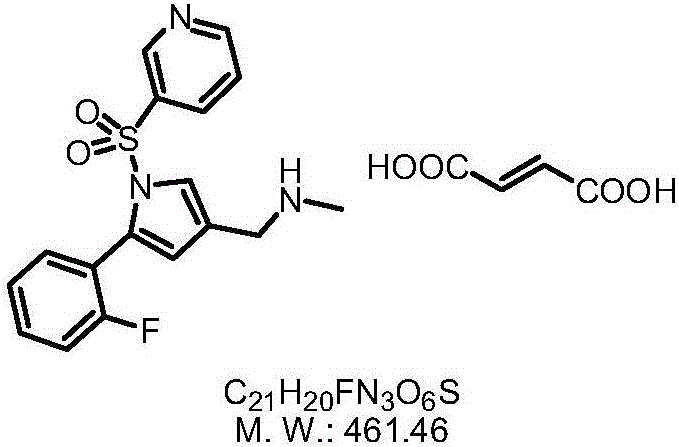
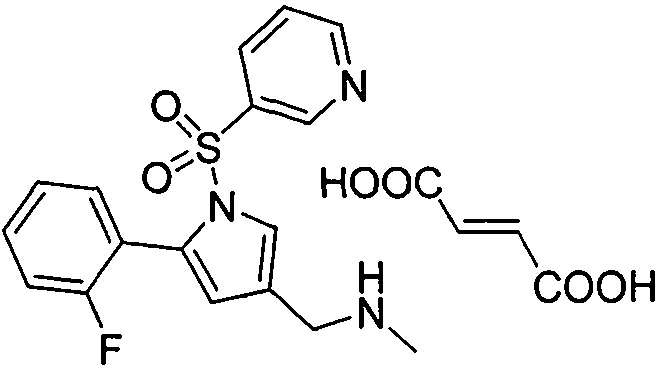
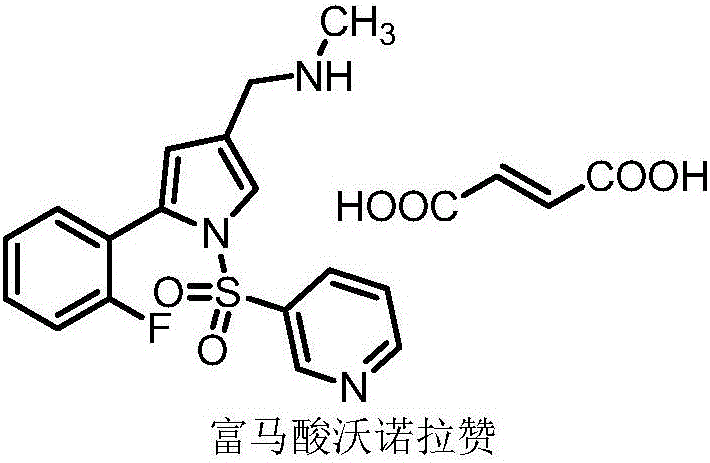
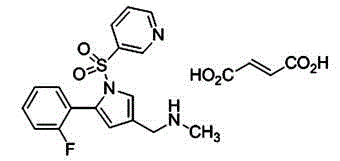
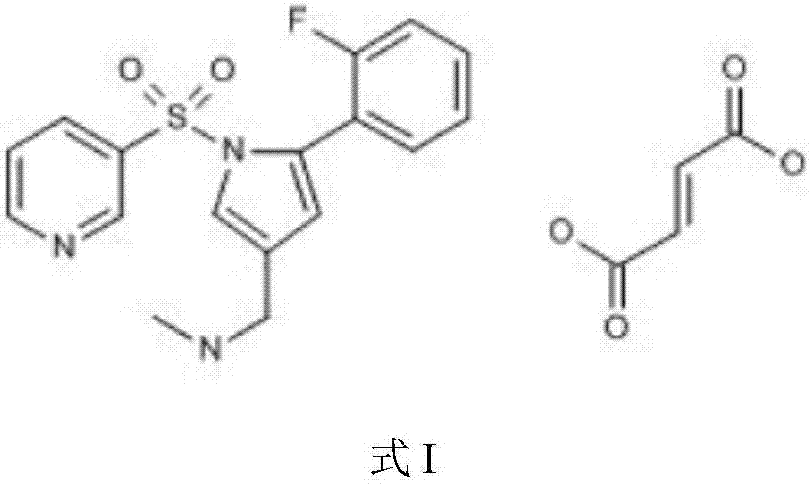
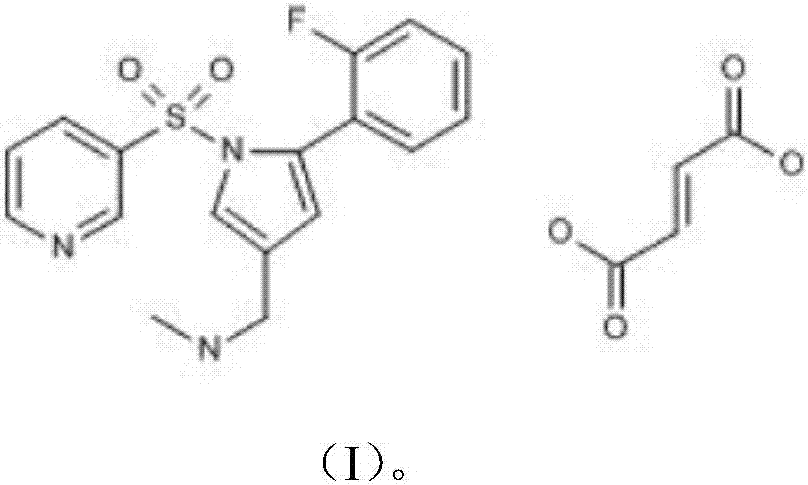
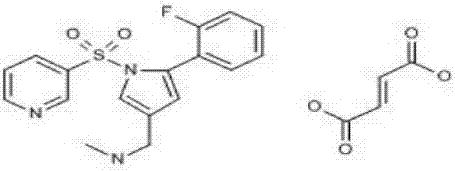
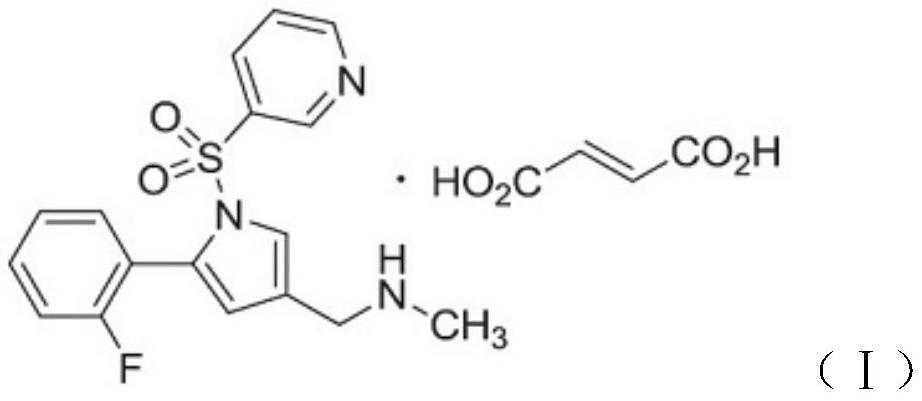
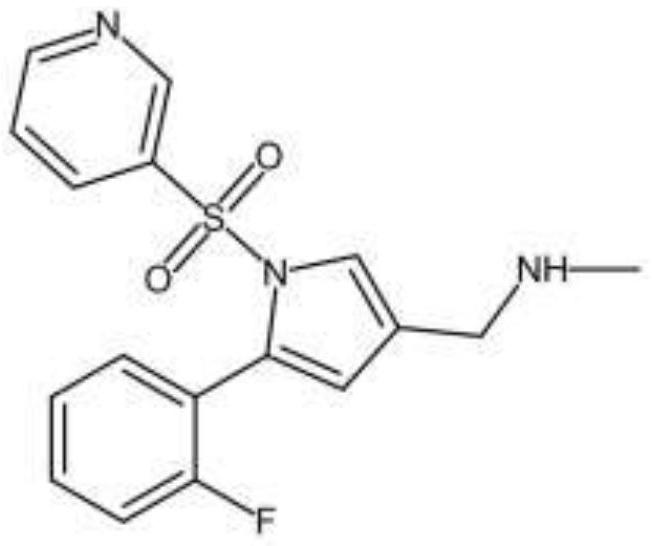
Vonoprazan fumarate is a first-in-class potassium-competitive acid blocker. It was approved in the Japanese market in February 2015. Vonoprazan can be used for the treatment of gastroduodenal ulcer (including some drug-induced peptic ulcers) and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori.
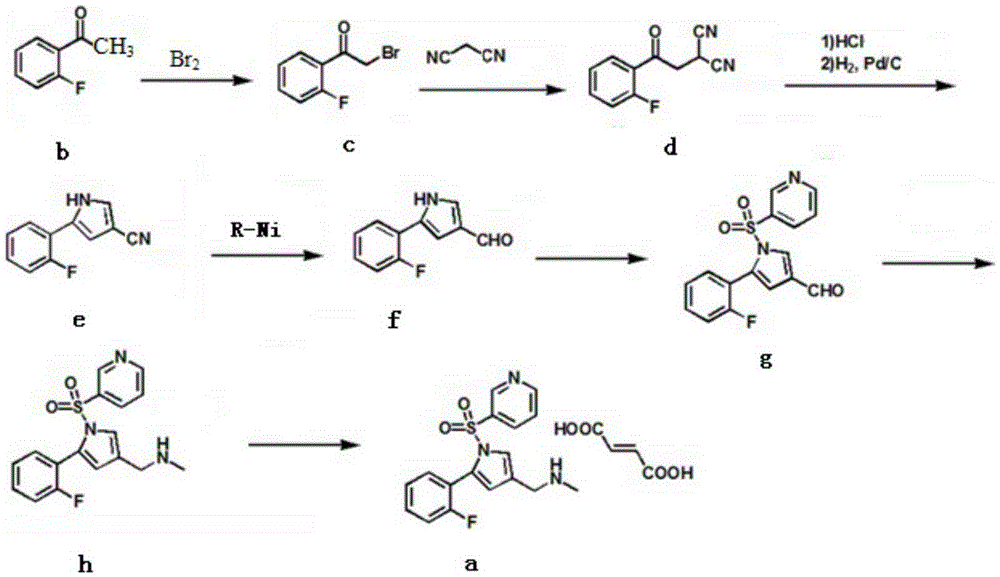
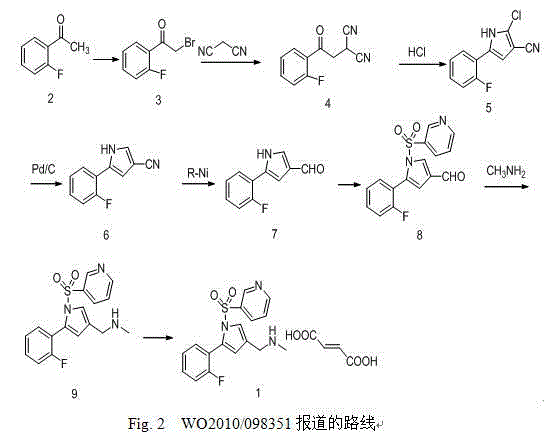
Preparation method of vonoprazan fumarate
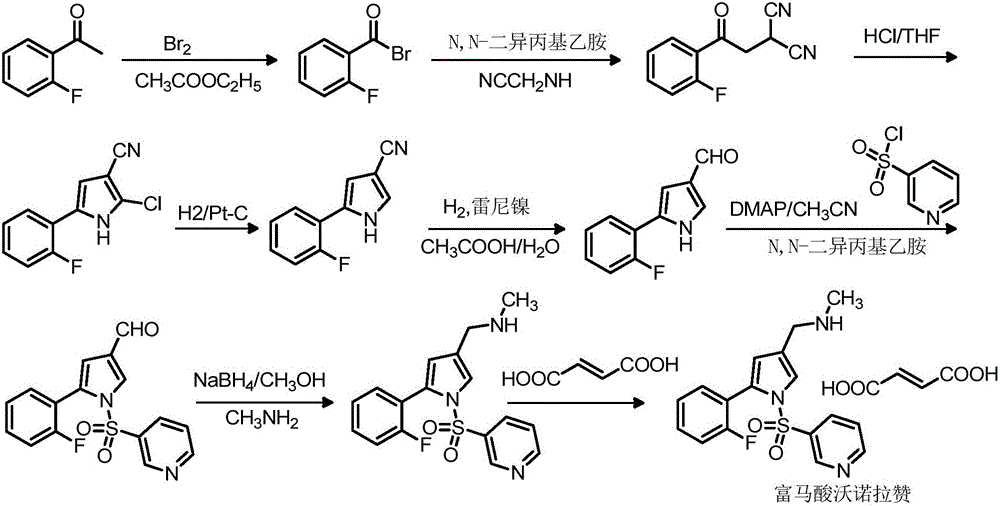
The invention relates to preparation of a medical compound, and in particular relates to a preparation method of a stomach-acid resistant medicine vonoprazan fumarate. A synthetic route of the compound is as shown in the specification.
Owner:ZHEJIANG CHENGYI PAHRMACEUTICAL
Preparation technology for vonoprazan fumarate
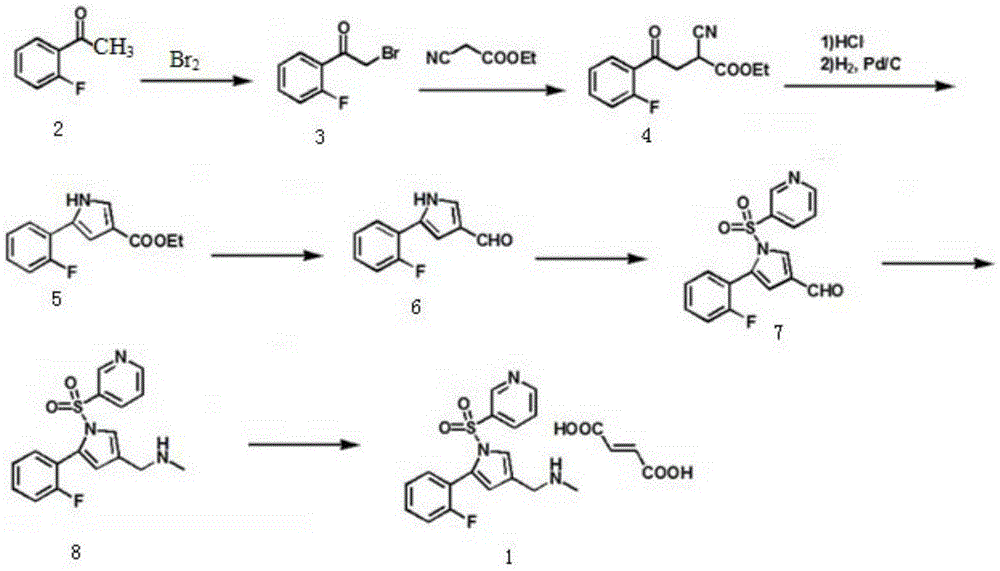
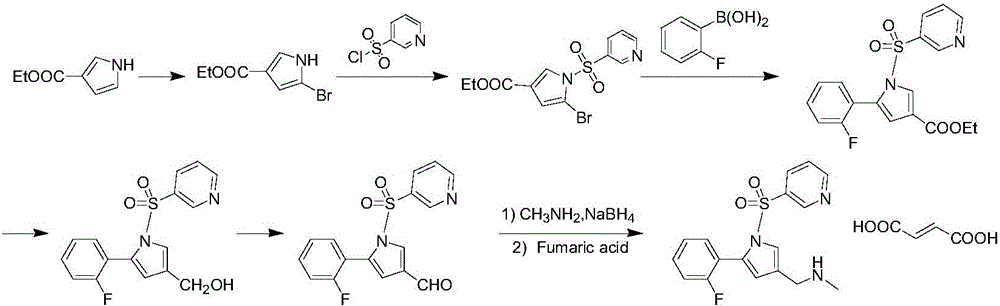
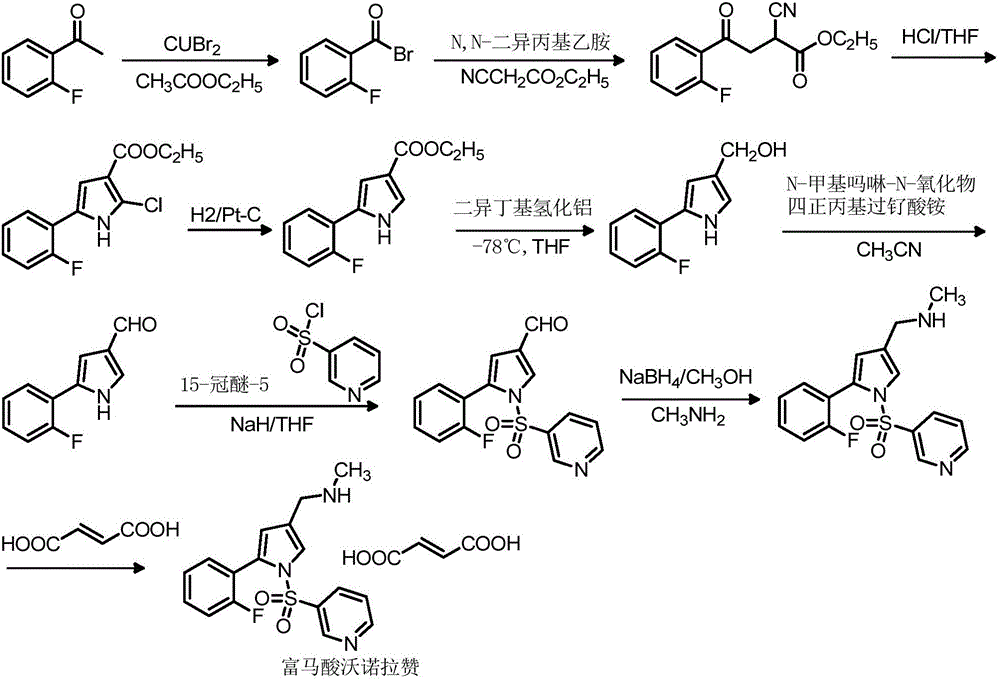
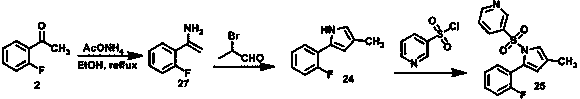
The invention relates to a preparation technology for vonoprazan fumarate. The preparation method comprises: reacting 2'-fluoroacetophenone (II) with ammonium acetate to generate 1-(2-fluorophenyl)ethen-1-amine (III), then reacting with 2-bromopropanal for cyclization for generating 2-(2-fluorophenyl)-4-methyl-1H-pyrrole (IV), reacting the compound IV with 3-pyridinesulfonyl chloride to generate 5-(2-fluorophenyl)-3-methyl-1-(pyridin-3-ylsulfonyl)-1H-pyrrole (V), then performing N-bromosuccinimide substitution to generate 5-(2-fluorophenyl)-3-bromomethyl-1-(pyridin-3-ylsulfonyl)-1H-pyrrole (VI), and finally performing methylamination reaction and salt forming, so as to obtain vonoprazan fumarate. The method avoids usage of a toxic reagent liquid bromine and hydrogen chloride gas capable of corroding equipment in the prior art, possesses the advantages of simple technological route, mild reaction conditions, controllable operation, environment friendliness and high product yield, and is suitable for large-scale industrialized production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
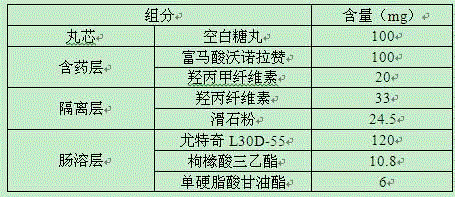
Vonoprazan fumarate enteric coated tablet and preparation method thereof
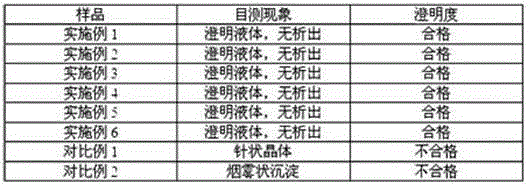
ActiveCN105030720ASolve the precipitation problemFix stability issuesOrganic active ingredientsDigestive systemAcrylic resinMannitol
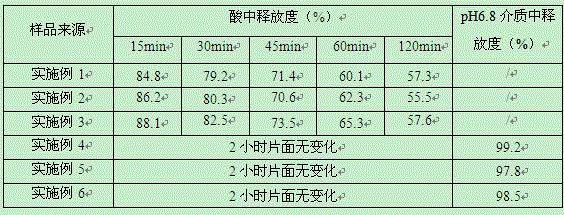
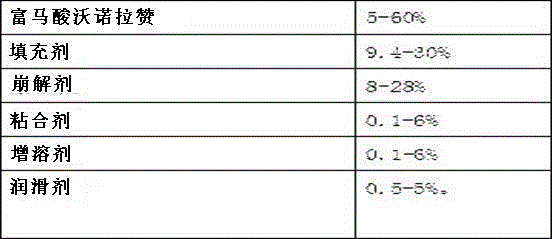
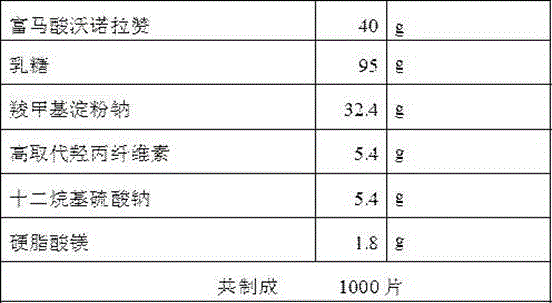
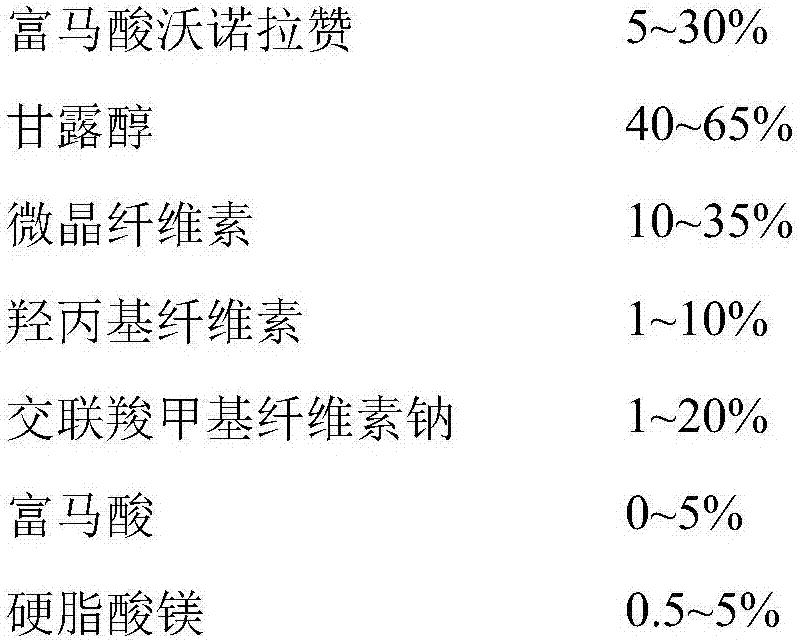
The invention belongs to the field of pharmaceutical preparations and particularly relates to a vonoprazan fumarate enteric coated tablet and a preparation method thereof. The invention adopts the following technical scheme: vonoprazan fumarate and pharmaceutical auxiliary materials are prepared into tablet cores, and then an enteric layer is coated; every 1000 tablet cores comprise 13.36g of vonoprazan fumarate, 40-50g of mannitol, 10-20g of microcrystalline cellulose, 3-8g of hydroxypropyl vonoprazan fumarate enteric coated tablet cellulose, 1-5g of fumaric acid, 8-30g of croscarmellose sodium and 1-3g of magnesium stearate; every 1000 enteric film-coated layers comprise 7.0g of acrylic resin, 0.1g of triethyl citrate and 3.9g of talcum powder. The technical scheme provided by the invention solves the problem that the release of common tablets in the stomach is unstable.
Owner:DISHA PHARMA GRP
Preparation method of vonoprazan fumarate
ActiveCN105085484AGet efficientlyLow costOrganic chemistryBulk chemical productionMethylene DichlorideVonoprazan
The invention discloses a preparation method of vonoprazan fumarate. The preparation method includes: S1, dissolving 5-(2-fluorophenyl)-1H-pyrrole-3-carboxaldehyde (I) in organic solvent, mixing with methylamine alcohol solution for 6-8h to generate imine, reducing with metal borohydride for 1-2h, and performing post-treatment to obtain a compound according to a formula II; S2, dissolving the compound prepared in the step S1 according to the formula II, in organic solvent, performing ice bathing and mixing with Boc anhydride to allow reaction for 1-2h, and performing post-treatment to obtain a compound according to a formula III; S3, dissolving the compound prepared in the step S2 according to the formula III, in organic solvent, adding sodium hydride and crown ether, adding 3-pyridine sulfuryl chloride, mixing for reaction for 1-2h, and performing post-treatment to obtain a compound according to a formula IV; S4, reacting the compound prepared in the step S3 according to the formula IV, in trifluoroacetic acid and methylene dichloride solution to obtain a compound according to a formula V; and S5, dissolving the compound prepared in the step S4 according to the formula V, in organic solvent to be salified with fumaric acid, thereby obtaining the vonoprazan fumarate (VI). The preparation method has few side reactions and high intermediate purity and allows simple post-treatment.
Owner:NANJING GRITPHARMA CO LTD
Pharmaceutical composition with vonoprazan fumarate and preparation method thereof
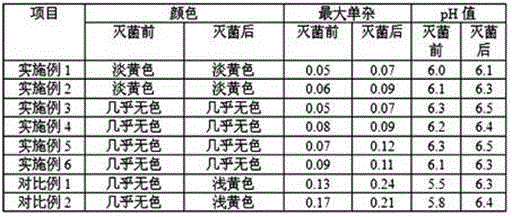
The invention discloses a pharmaceutical composition with vonoprazan fumarate and a preparation method thereof and particularly provides a pharmaceutical composition containing an active ingredient, vonoprazan fumarate, and a method of stabilizing the pharmaceutical composition. Specifically, the pharmaceutical composition containing the vonoprazan fumarate, plasticizer and excipient has good preparation stability and has good stability during illumination.
Owner:JIANGSU HANSOH PHARMA CO LTD
Vonoprazan fumarate preparation method
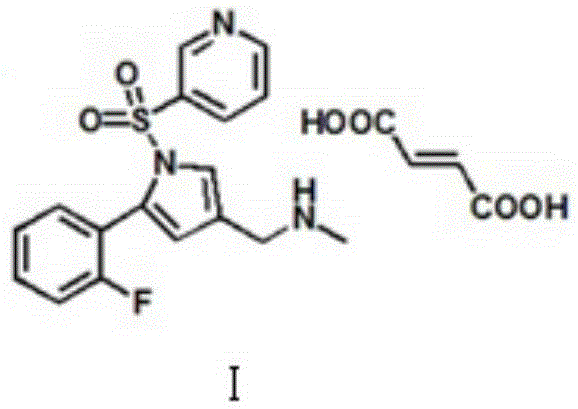
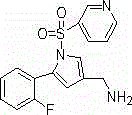
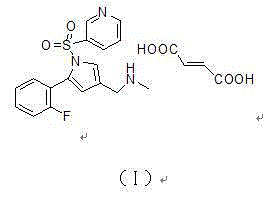
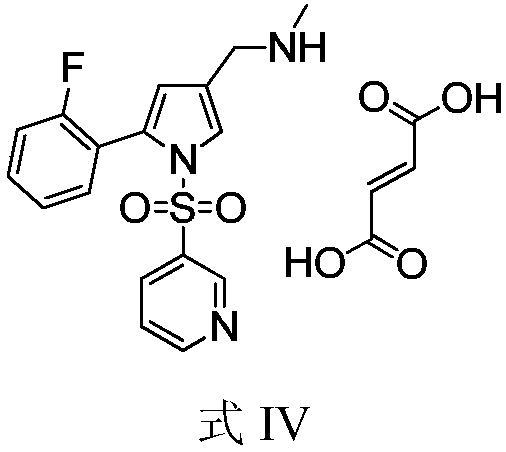
The invention relates to the field of drug preparation researches, and especially relates to a preparation method of 1-[5-(2-fluorophenyl)-1-(pyridyl-3-ylsulfonyl)-1H-pyrryl-3-yl]-N-methyl methylamine fumarate (represented by formula I) and an intermediate thereof. The method is characterized in that the above compound represented by formula (I) is prepared through nitrogen monomethylation and salt formation with a compound represented by formula (V) as an important intermediate. The structural formula of the compound represented by formula (V) is shown in the specification, and the structural formula of the compound represented by formula (I) is also shown in the specification.
Owner:山东康美乐医药科技有限公司
Vonoprazan fumarate preparation method
ActiveCN106366071AAccelerated corrosionHigh yieldCarboxylic acid salt preparationSulfonyl chlorideVonoprazan
The invention concretely relates to a vonoprazan fumarate preparation method, and belongs to the field of medicines and chemical engineering. The method comprises the following steps: 1, carrying out condensation on 2-fluoroacetophenone used as an initial raw material and allylamine to obtain a compound IV; 2, carrying out a ring closing reaction on the compound IV under the catalysis of a copper catalyst in the presence of a ligand in order to obtain a compound V; 3, carrying out a sulfoamidation reaction on the compound V and pyridine-3-sulfonyl chloride to generate a compound VII; 4, carrying out a bromination reaction on the compound VII by using N-bromosuccimide in order to generate a compound VIII; 5, carrying out an amination reaction on the compound VIII and methylamine hydrochloride under the action of a catalyst and an alkali in order to obtain vonoprazan alkali; and 6, carrying out salt formation on the vonoprazan alkali and fumaric acid in order to obtain vonoprazan fumarate. The preparation method has the advantages of simplicity in operation, mild reaction conditions, high yield and high purity of the product, and easiness in industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Preparation method of vonoprazan fumarate
ActiveCN108503621AReduce usageProcess environmental protectionOrganic chemistrySulfonyl chlorideVonoprazan
The invention relates to a preparation method of vonoprazan fumarate, belonging to the technical field of preparation of a raw material medicine. The preparation method of the vonoprazan fumarate comprises the following steps: 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile is used as a starting material to react with pyridine-3-sulfonyl chloride to form 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-nitrile; the 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-nitrile is reduced to prepare 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-formaldehyde; then vonoprazan is prepared; and then a salt formation step is used to prepare the vonoprazan fumarate. The preparation method is suitable for industrial production.
Owner:上海中拓医药科技有限公司 +1
Vonoprazan oral quick-dissolving film agent and method for preparing same
ActiveCN105663096AAvoid difficultiesImprove complianceOrganic active ingredientsDigestive systemVonoprazanPharmaceutical formulation
The invention discloses a Vonoprazan oral quick-dissolving film agent and a method for preparing the same, and belongs to the field of medicine preparations.The Vonoprazan oral quick-dissolving film agent particularly comprises, by weight, 10-15% of Vonoprazan active monomers, 50-55% of hydroxypropyl methylcellulose, 25-30% of maltodextrin, 0.8-1.0% of hyaluronic acid and 5-10% of plasticizers.The Vonoprazan oral quick-dissolving film agent and the method have the advantages that the water-soluble hydroxypropyl methylcellulose, the water-soluble maltodextrin and the water-soluble hyaluronic acid are preferably used as film-forming materials, the appropriate plasticizers with the appropriate weight ratio are screened, and accordingly the film agent with excellent disintegration time and excellent mechanical performance can be prepared; the disintegration time limit of the Vonoprazan oral quick-dissolving film agent can be obviously shortened, accordingly, the shortcoming that water is required when existing most oral solid preparations are about to be administered can be overcome, the medicine administration time cannot be delayed even under the condition of deficiency of water resources, and the medication compliance of patients can be improved.
Owner:NANJING GRITPHARMA CO LTD
Compound preparation containing vonoprazan and non-steroidal anti-inflammatory drugs
InactiveCN105412038AGuaranteed disintegration release speedPreparation technology is simple and easyOrganic active ingredientsBlood disorderAspirinVonoprazan
The invention provides a compound preparation containing vonoprazan and non-steroidal anti-inflammatory drugs. The compound preparation is characterized by being an inner-outer multi-layer preparation prepared from the vonoprazan and one or more than one non-steroidal anti-inflammatory drugs; an isolation layer is arranged between the inner-layer drug and the outer-layer drug to prevent contact of the vonoprazan and the non-steroidal anti-inflammatory drugs. The vonoprazan can inhibit gastric acid secretion, improve the clinical symptoms such as gastric acid increasing and epigastrium pain, reduce the pessimal stimulation of the non-steroidal anti-inflammatory drugs such as aspirin to the stomach and significantly reduce the occurrence rate of gastric mucosal erosion and anabrosis, and therefore the protective effect on the stomach is achieved.
Owner:FUKANGREN BIO PHARMA
Vonoprazan fumarate compound and pharmaceutical composition thereof
InactiveCN105566295AImprove stabilityHarm reductionOrganic active ingredientsOrganic chemistryVonoprazanStereochemistry
The invention provides a new crystal-form Vonoprazan fumarate compound and a preparation method and a pharmaceutical composition thereof. The Vonoprazan fumarate compound has the advantages of good stability and low hygroscopicity, and conforms to the medicinal requirements. The preparation process of the compound provided by the invention is stable and has good repeatability, conforms to the requirements of industrialized mass production, and has important application value in preparation of medicines.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Vonoprazan fumarate enteric-coated composition and preparation method thereof
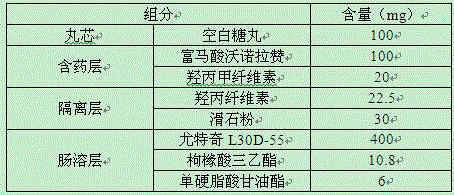
ActiveCN105030725AGood dispersionHigh dissolution rateAntibacterial agentsOrganic active ingredientsMedicineVonoprazan
The invention relates to a vonoprazan fumarate enteric-coated capsule and a preparation method thereof, particularly relates to a pellet type vonoprazan fumarate enteric-coated capsule and a preparation method thereof, and belongs to the technical field of medicines. The vonoprazan fumarate enteric-coated capsule sequentially comprises a hollow pill core, a main drug layer, an isolation layer, an enteric layer and a capsule layer from inside to outside, wherein the weight of the main drug layer is 80-130 percent of that of the hollow pill core, the weight of the isolation layer is 10-30 percent of the total weight of the hollow pill layer and the main drug layer, and the weight of the enteric layer is 30-80 percent of the total weight of the hollow pill layer, the main drug layer and the isolation layer. Each capsule contains 10-20mg of vonoprazan. By adopting the technical scheme, the dispersion of vonoprazan fumarate is improved, and the dissolution rate is increased.
Owner:DISHA PHARMA GRP
Vonoprazan fumarate single crystal, preparation method and uses thereof
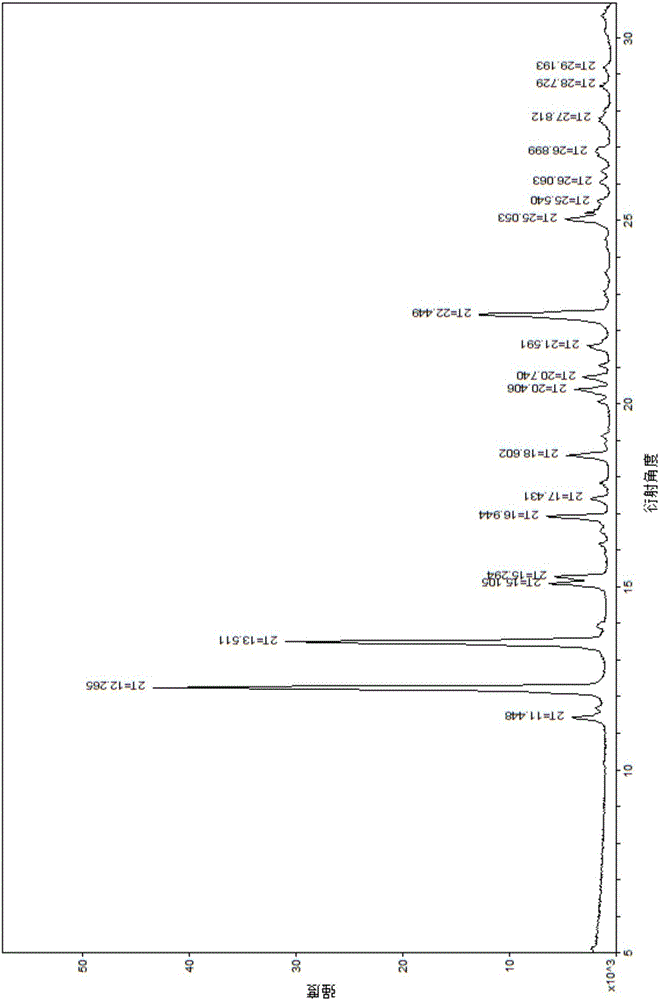
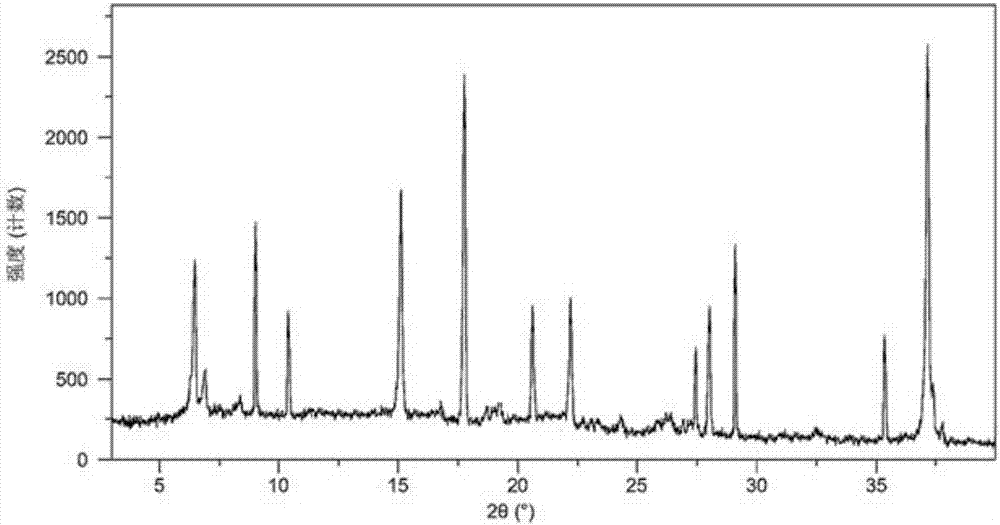
InactiveCN106478597AEasy to separateEasy to shapeAntibacterial agentsOrganic chemistry methodsSpace groupVonoprazan
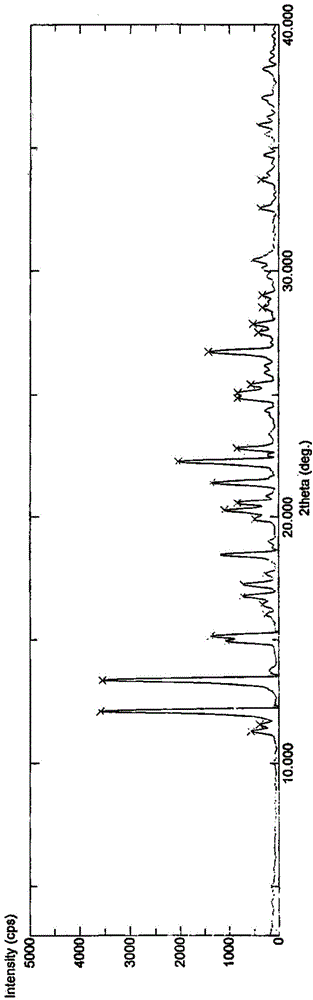
The present invention relates to a vonoprazan fumarate single crystal, a preparation method and uses thereof. According to the present invention, the X-ray powder diffraction results show that the characteristic peaks are showed at the 2[theta] of 11.4, 12.3, 13.5, 15.1, 15.3, 16.9, 18.6, 20.4, 20.7, 22.4 and 25.1, the obtained single crystal is not reported, and other determination results show that the crystal structure belongs to a monoclinic system, the space group is P2(1) / c, the intracellular molecular number Z is 4m the single crystal is a colorless orthorhombic crystal at a room temperature, the morphology is good, and the HPLC purity is up to more than 99.5%; and with the preparation method, the vonoprazan fumarate and other impurities are well separated, and the good reproducibility is good.
Owner:WATERSTONE PHARMA WUHAN
Preparation method of vonoprazan fumarate
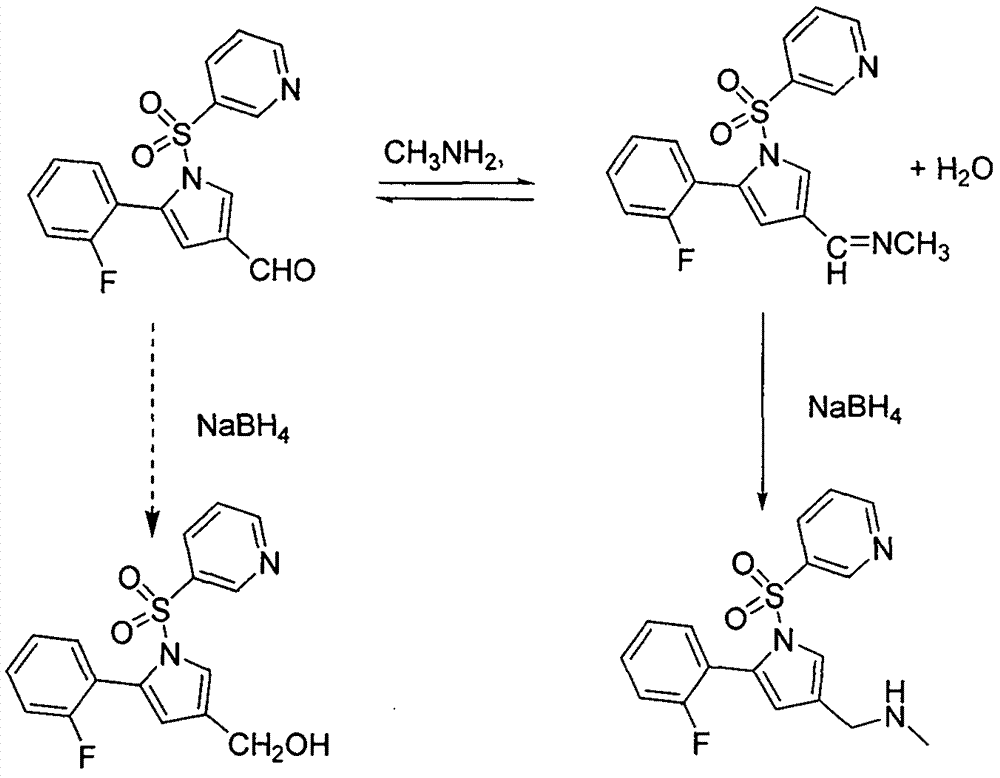
InactiveCN107011325AHigh yieldMild reaction conditionsCarboxylic acid salt preparationBorideCarbylamine reaction
The invention provides a preparation method of vonoprazan fumarate. The preparation method comprises the following steps of enabling 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1-H-pyrrole-3-formaldehyde and methylamine to react to generate Schiff base; using a mild boride reducing agent to reduce the Schiff base, so as to prepare vonoprazan. The preparation method has the advantages that the reaction treatment condition is mild, the yield rate is high, the product purity is high, and the like. The vonoprazan fumarate prepared by the preparation method has the advantages that the yield rate is increased to 95%, and the content of HPLC (high performance liquid chromatography) is 99%; when preparing by the sodium borohydride reduction method, the yield rate of 70%, and the content of HPLC of 96% are greatly improved.
Owner:GUANGDONG SCI FINDER PHARMA TECH CO LTD
Vonoprazan fumarate midbody, preparation method thereof and method for preparing vonoprazan fumarate midbody
InactiveCN106146466AShort reaction stepsMild reaction conditionsOrganic chemistryState of artVonoprazan
The invention provides a vonoprazan fumarate midbody, a preparation method thereof and a method for preparing vonoprazan fumarate midbody. Compared with the prior art, vonoprazan fumarate is prepared through the vonoprazan fumarate midbody. The preparation method has the following advantages that a process route includes short reaction steps, raw materials are easy to obtain, reaction conditions are mild, operation is simple and easy, the method is economical and environmentally friendly, the total yield is obviously improved, and the vonoprazan fumarate midbody is suitable for large-scale production.
Owner:赛隆药业集团股份有限公司(长沙)医药研发中心
Vonoprazan fumarate dispersible tablets and preparation method thereof
InactiveCN106074406ADisintegrates quicklyFast absorptionOrganic active ingredientsDigestive systemSide effectVonoprazan
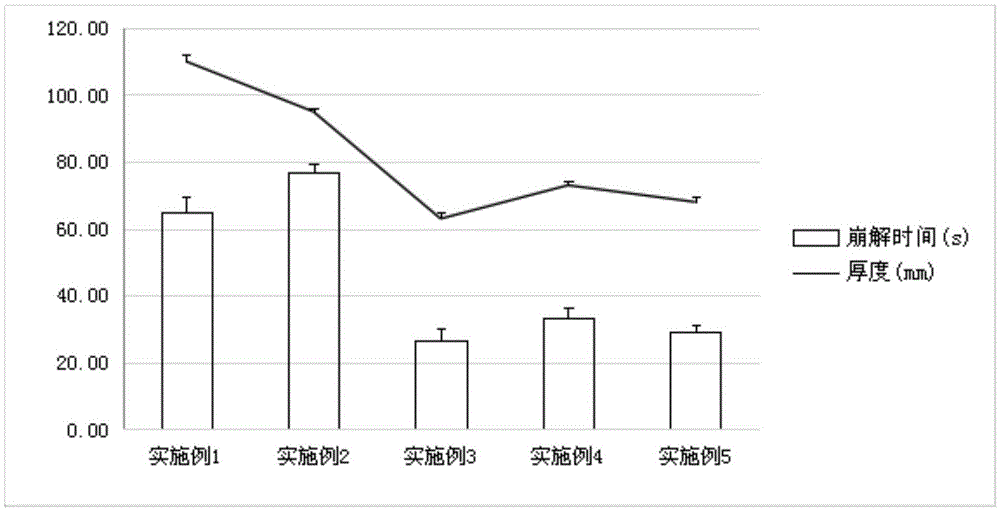
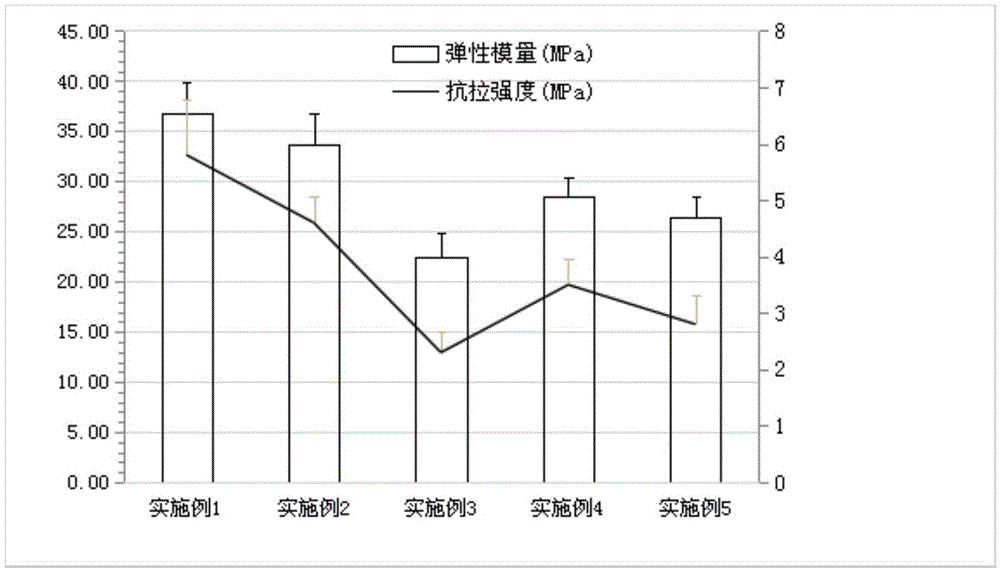
The invention discloses vonoprazan fumarate dispersible tablets, used for treating gastric and duodenal ulcers. The vonoprazan fumarate dispersible tablets are prepared from vonoprazan fumarate, with the addition of adjuvant materials. The vonoprazan fumarate dispersible tablets are rapid in disintegration and absorption, high in bioavailability, convenient to take, low in content of intestinal residues, light in side effects, sweet in taste and fragrant; and especially, the dispersible tablets are easy to improve the medication compliance of patients.
Preparation method of low-impurity Vonoprazan fumarate
PendingCN110590746ARemove won'tLong process cycleCarboxylic acid salt preparationCarboxylic compound separation/purificationHydrobromideVonoprazan
The invention provides a preparation method of low-impurity Vonoprazan fumarate. A method for removing impurities A-E during preparation of Vonoprazan comprises the steps: preparing Vonoprazan hydrobromide from Vonorazan, and removing the impurities A-E which are difficult to remove by using a recrystallization purifying method, wherein the method for removing the impurities A-E during preparationof Vonoprazan has good selectivity to the impurities A-E. The preparation method of low-impurity Vonoprazan fumarate comprises the steps: performing a reaction on 5-(2-fluorophenyl)-1-(pyridyl-3-ylsulfonyl)-1H-pyrrole-3-formaldehyde and methylamine or a salt of methylamine, then performing reduction so as to obtain Vonoprazan, then preparing Vonoprazan hydrobromide, then performing salt dissociation so as to obtain Vonoprazan free alkali, preparing Vonoprazan fumarate with a purity of 99.7% or above, and then performing recrystallization refining so as to obtain Vonoprazan fumarate with a purity of 99.9% or above. The invention provides an impurity D, a preparation method of the impurity D and application of the impurity D as an impurity reference substance in Vonoprazan fumarate.
Owner:JILIN HUIKANG PHARM CO LTD
New preparation method of Vonoprazan
ActiveCN107915720AReduce usageLow costDigestive systemCarboxylic acid salt preparationVonoprazanSolvent
The invention discloses an improved preparation method of Vonoprazan, and particularly discloses a preparation method of a compound Vonoprazan with a structure shown in the description. According to the method, a compound 5-(2-fluorophenyl)-1H-pyrrole-3-carboxylic ester is taken as a raw material and subjected to amination with water as a solvent, a product is reduced, and Vonoprazan is prepared.The technology adopts short steps, and is low in cost, environmentally friendly and suitable for large-scale industrial production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Vonoprazan fumarate key intermediate and preparation method thereof
The invention provides a vonoprazan fumarate key intermediate and a preparation method thereof. The preparation method of the vonoprazan fumarate key intermediate mainly comprises three procedures, and is simple in process and simple in aftertreatment; toxic reactants and unstable reactants are not required, difficulty and dangerousness of industrial operation are reduced, the use amount of a catalyst is small, the cost is low, the yield is high, and the vonoprazan fumarate key intermediate is suitable for large-scale production. The vonoprazan fumarate key intermediate is high in purity, and can be used for preparing vonoprazan fumarate.
Owner:BEIJING THTD PHARMA TECH JOINT CO LTD
Medicinal composition for resisting helicobacter pylori and preparation method as well as application thereof
ActiveCN104814964AAct quicklyReduce adverse reactionsAntibacterial agentsDigestive systemMedicineVonoprazan
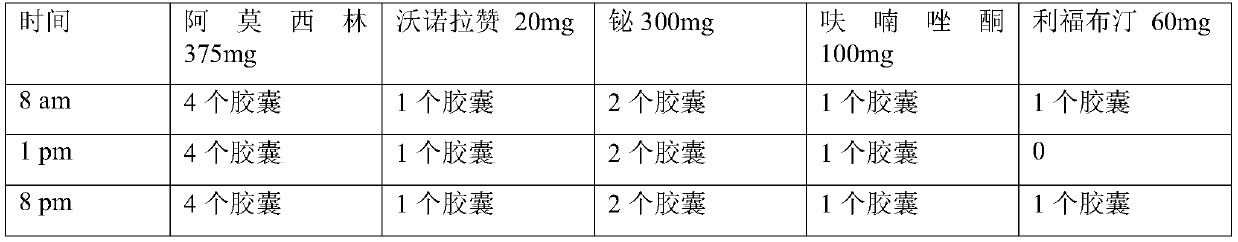
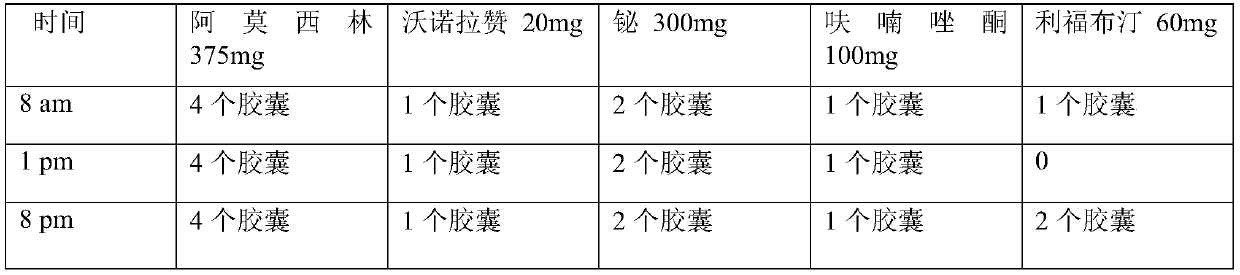
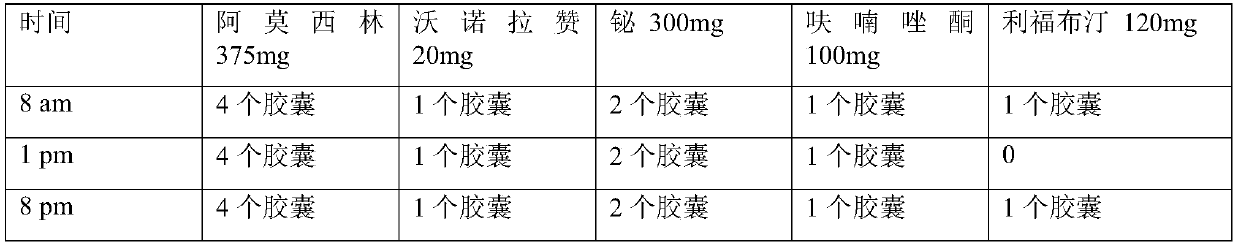
The invention relates to a medicinal composition for resisting helicobacter pylori. The medicinal composition comprises the following components in parts by weight: 50-500 parts of component A, 100-500 parts of component B and 5-50 parts of component C, wherein the component A is amoxicillin; the component B is selected from levofloxacin and pharmaceutically acceptable salts thereof, moxifloxacin and pharmaceutically acceptable salts thereof; the component C is selected from Vonoprazan and pharmaceutically acceptable salts thereof. The invention also provides a preparation method and application of the medicinal composition. The medicinal composition for resisting the helicobacter pylori has a strong effect of resisting the helicobacter pylori and has less adverse reaction and wide application, and the preparation method is simple and is easy to operate.
Owner:山东豪瑞恩制药有限公司
Vonoprazan fumarate polycrystalline forms and preparation method thereof
The invention discloses novel vonoprazan fumarate crystalline forms A and B and a preparation method suitable for industrial production. The prepared crystalline forms are simple in preparation technology, good in stability and capable of meeting the medicinal requirement.
Owner:NANJING HEALTHNICE MEDICAL TECH
Compositions and methods for treating, ameliorating and preventing h. pylori infections
PendingCN110366415AAntibacterial agentsSalicyclic acid active ingredientsVonoprazanPharmaceutical drug
Provided are methods for treating, ameliorating, reversing and / or preventing a Helicobacter pylori (H. pylori) infection in an individual in need thereof, comprising: administering to the individual in need thereof a therapeutic combination comprising: (a) a composition comprising or consisting of: vonoprazan or a vonoprazan fumarate, or a 5- (2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine monofumarate, or a 1-(5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)-N-methyl-methanamine fumarate), optionally TAKECABTM; and (b) an antimicrobial or antibiotic drug or composition.
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
Vonoprazan fumarate injection and preparation method thereof
ActiveCN106031710AExcellent long-term stabilityEasy to preparePowder deliveryOrganic active ingredientsDrugOral medication
The invention belongs to the technical field of pharmaceutical preparation, and in particular to a potassium ion competitive acid blocker Vonoprazan fumarate injection with the effect of treating gastric acid-related diseases, and a preparation method thereof. The invention overcomes the defect of poor solubility of the main drug Vonoprazan fumarate, uses an inclusion form of sulfobutylether-beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin for solution increase. The prepared injection has the advantages of good stability, high safety and no precipitation during long-term placement, and can meet the medication requirement for the patients who are not convenient for oral administration.
Owner:NANJING YOKO PHARMA +2
Vonoprazan fumarate pharmaceutical composition
ActiveCN107224438AImprove stabilityDoes not affect appearanceOrganic active ingredientsDigestive systemVonoprazanPlasticizer
The invention relates to a vonoprazan fumarate pharmaceutical composition; and the invention provides the pharmaceutical composition which contains vonoprazan fumarate as an active ingredient and a method for stabilizing the pharmaceutical composition. Specifically, the pharmaceutical composition containing the vonoprazan fumarate is free from a plasticizer; the pharmaceutical composition is excellent in preparation stability; and the pharmaceutical composition shows excellent stability in a high-temperature environment and during an illuminating phase.
Owner:JIANGSU HANSOH PHARMA CO LTD
Pharmaceutical compound for treating digestive system diseases, and preparation method thereof
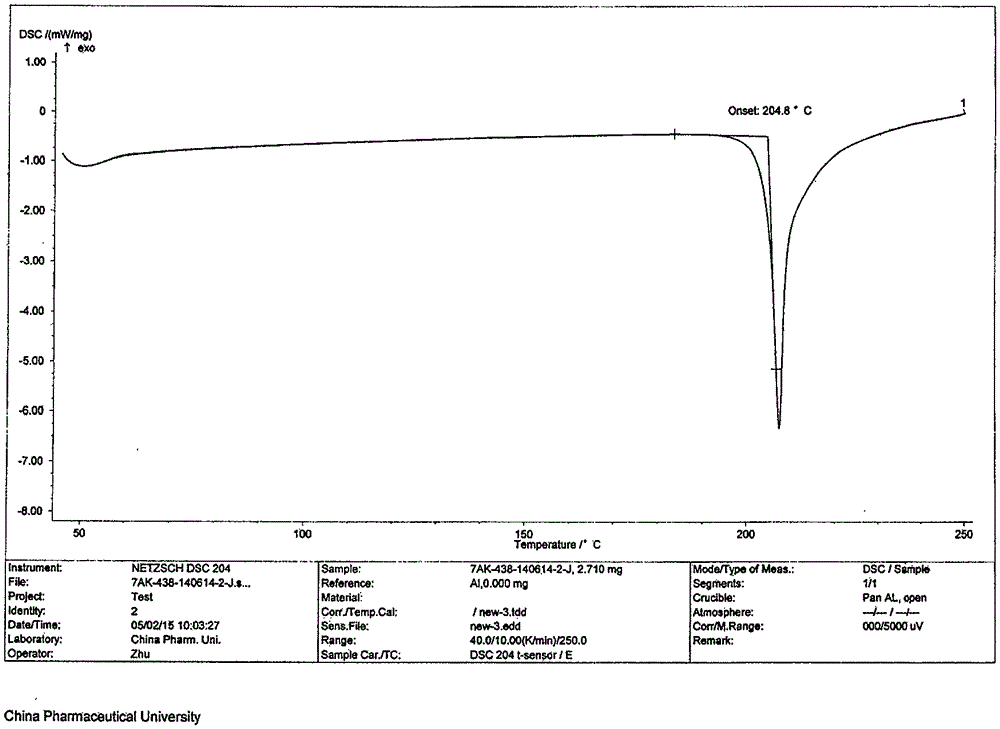
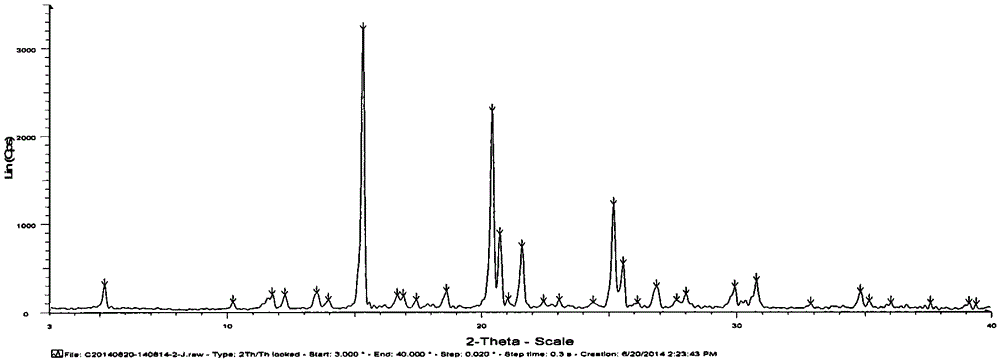
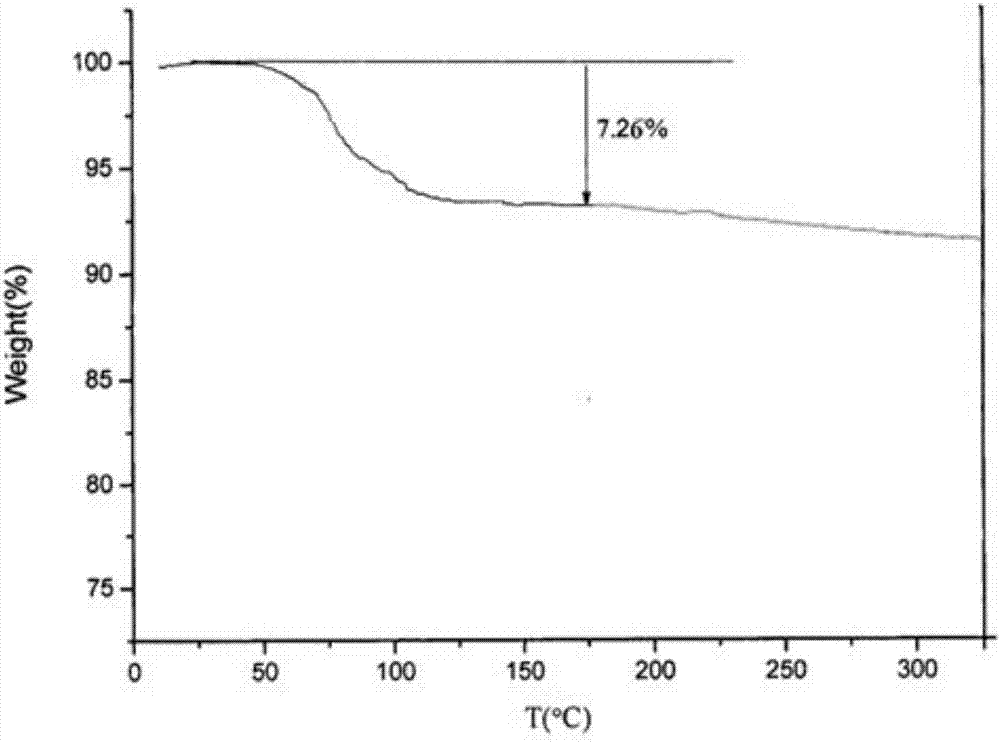
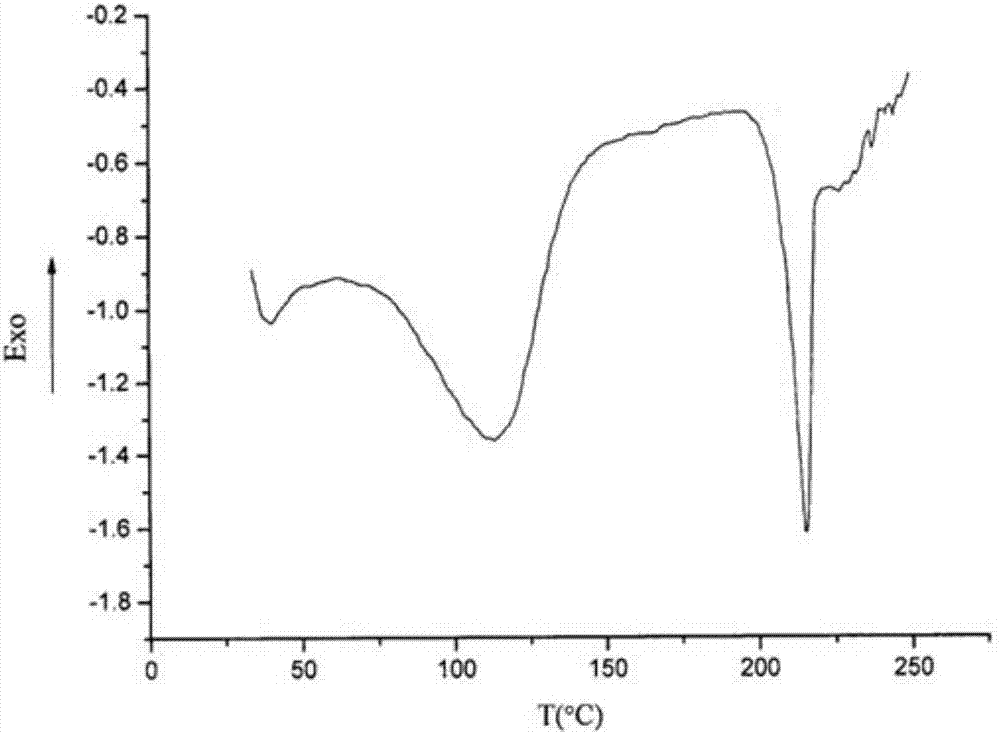
The invention belongs to the technical field of medicine and discloses a medicinal compound for treating digestive system diseases and a preparation method thereof. The provided vonoprazan fumaric acid dihydrate has high purity and good stability, and its diffraction angle is 2θ±0.2° The indicated X-ray powder diffraction pattern shows the Characteristic diffraction peak, the X-ray powder diffraction spectrogram that uses Cu-Kα ray measurement to obtain is shown in Figure 1, is completely different from the prior art, finds that the fumaric acid vonoprazan dihydrate obtained by the present invention is pleasantly surprised by experiments The solubility of the substance was significantly improved. The dissolution rate and stability of the composition tablet prepared from the vonoprazan fumarate dihydrate of the present invention are significantly improved, and are very suitable for clinical application.
Owner:HUNAN QIWEI TECH CO LTD
Vonoprazan fumarate solid dispersions and preparation method thereof
InactiveCN107496362AImprove in vitro dissolutionPromote absorptionOrganic active ingredientsPowder deliveryVonoprazanMass ratio
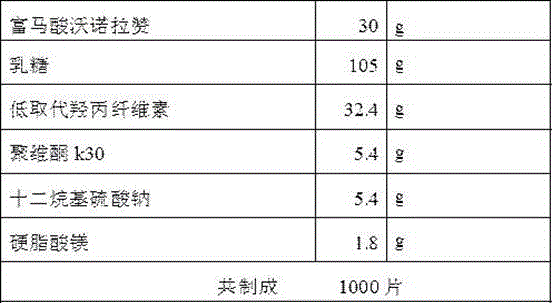
The invention discloses a preparation method of vonoprazan fumaric acid solid dispersion: dissolve vonoprazan fumaric acid and a carrier material in an organic solvent (acetone:methane=3:1), and reduce the temperature at 50 to 90°C. Pressure rotary evaporation, to obtain; the mass ratio of the fumaric acid vonoprazan to the carrier material is 1:1.5~1:10; the carrier material is selected from one of polyvinylpyrrolidone K30 and poloxamer or two. The present invention also provides a solid dispersion tablet of vonoprazan fumarate: made of solid dispersion of vonoprazan fumarate, filler, disintegrant, lubricant, wherein, the amount of filler accounts for 35%-65% of the total weight of the tablet; the dosage of the disintegrating agent is 1%-5% of the total weight of the tablet; the dosage of the lubricant is 0.5%-3% of the total weight of the tablet. The vonoprazan fumarate solid dispersion and tablet of the present invention improve the dissolution rate of vonoprazan fumarate in vitro, and improve the disadvantages of poor oral absorption and low bioavailability.
Owner:SUZHOU XINEN PHARMA
Vonoprazan fumarate-containing tablet and determination method for dissolution rate thereof
InactiveCN111973565ASimple processImprove stabilityOrganic active ingredientsComponent separationOrganic acidVonoprazan
The invention relates to a vonoprazan fumarate-containing tablet and a determination method for a dissolution rate thereof. The vonoprazan fumarate-containing tablet provided by the invention is composed of the following components in parts by weight: 10-15 parts of vonoprazan fumarate, 65-75 parts of mannitol, 2-5 parts of organic acid, 2-4 parts of hydroxy propyl cellulose, 8-12 parts of microcrystalline cellulose, 2-6 parts of croscarmellose sodium, 0.5-2 parts of magnesium stearate, 3-4 parts of coating powder and 0.2-1.0 part of polyethylene glycol. According to the tablet prepared by using the method, a damp-heat process is avoided; the granules of the tablet are good in flowability and compressibility, and the tablet is free of sticking, smooth in surface, qualified in friability and stable in tablet weight; after coating is completed, an aqueous polyethylene glycol solution is used for polishing treatment, so the appearance of the tablet is more glossy, a better moisture insulation effect is achieved on the tablet, and the dissolution behavior of the tablet is not influenced.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
Preparation method of high-purity Vonoprazan fumarate
PendingCN113861166AHigh purityRaw materials are easy to getOrganic compound preparationCarboxylic acid salt preparationVonoprazanAcyl group
The invention discloses a preparation method of high-purity Vonoprazan fumarate, which comprises the following steps: by taking 5-(2-fluorophenyl)-1[(pyridine-3-yl)sulfonyl]-1H-pyrrole-3-formaldehyde as an initial raw material, dissolving the initial raw material in a reaction solvent I, and reacting the solution with methylamine to generate Schiff base; reacting the solution with a reducing agent, salting out the product to obtain a free alkali Vonorazan crude product, and refining the product to obtain free alkali Vonorazan; and salifying the free alkali Vonorazan and fumaric acid in a reaction solvent II, and refining the product to obtain Vonorazan fumarate, wherein the HPLC purity of the product is greater than 99.9%.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
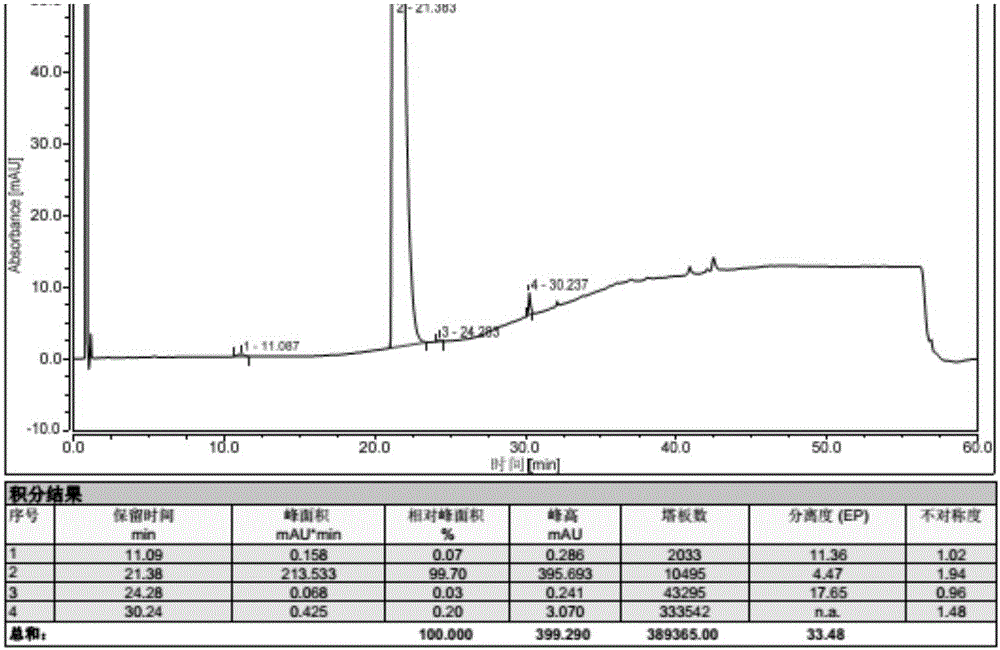
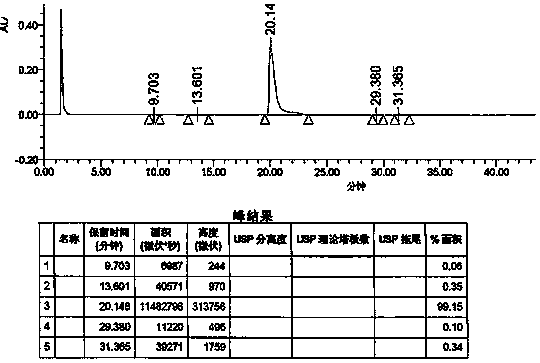
Detection method of Vonoprazan fumarate
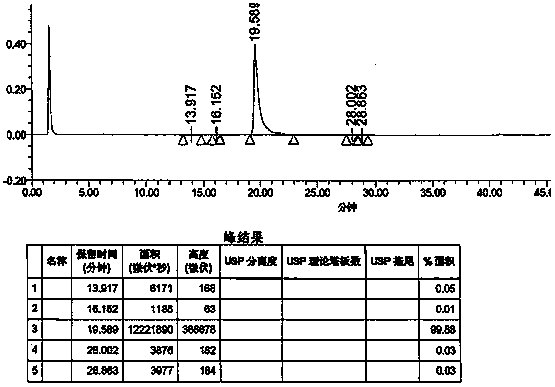
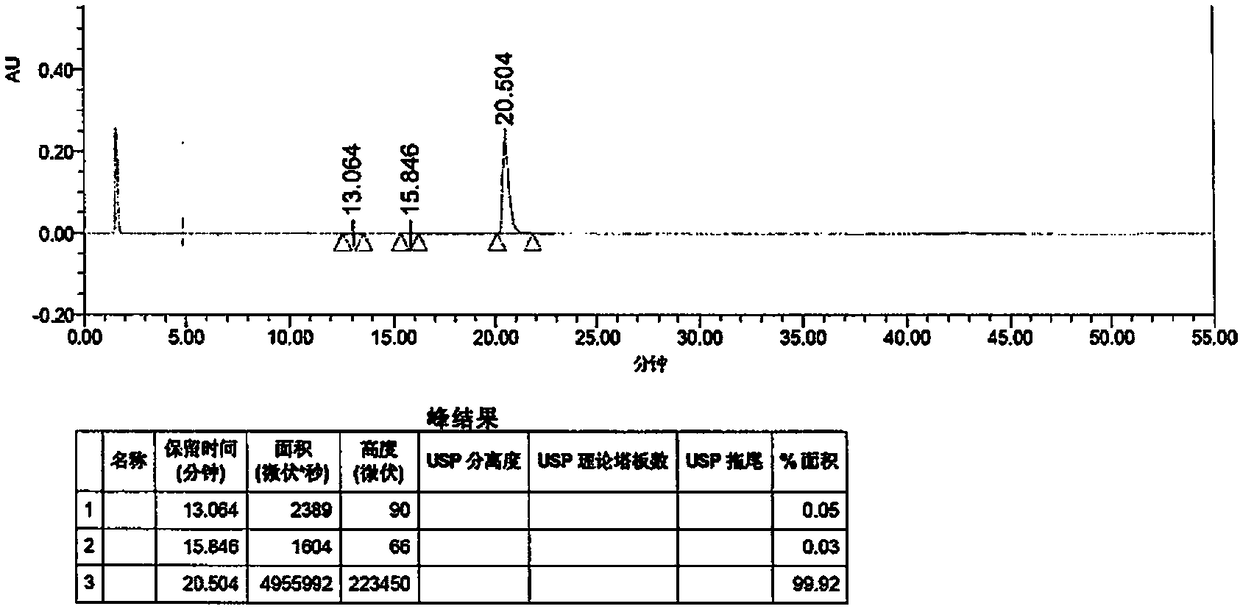
ActiveCN107941946AAccurate measurementHigh sensitivityComponent separationInjection volumeVonoprazan
The invention discloses a detection method of Vonoprazan fumarate. The detection method comprises the following steps: using a high performance liquid chromatography instrument to detect and an area normalization method to perform quantitative analysis, and controlling chromatographic conditions to be that the chromatographic column is Agilent ZORBAX SB-phenyl; the injection volume is 5 to 10 [mu]l; the flow velocity is 0.8 to 1.2 ml / min; the column temperature is 25 to 35 DEG C; the detection wavelength is 250 to 300 nm; the mobile phase comprises a mobile phase A which is a 0.1% formic acidaqueous solution and a mobile phase B which is acetonitrile in a volume ratio of (5-70): (30-95); the diluent comprises acetonitrile and water in a volume ratio of (40-60): 50; the detector is a UV detector; the detection method of Vonoprazan fumarate, disclosed by the invention, can simply, accurately, rapidly, efficiently and reliably detect the purity and impurities of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine fumarate, has very high sensitivity, is simple and convenient in operation, can realize complete separation, and further provides the basis for the research, development and quality detection of the compound.
Owner:ENANTIOTECH CORP
Preparation method of vonoprazan
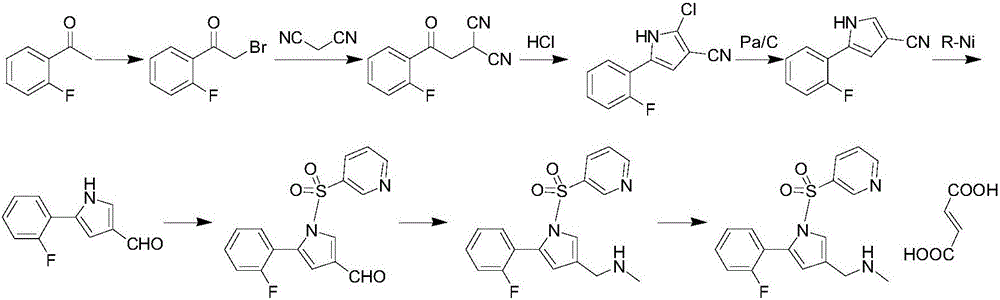
The invention relates to a preparation method of vonopraza. The preparation method of the vonoprazan, provided by the invention, comprises the following steps of taking 2-bromo-2'-fluoroacetophenone shown as a formula 3 as a starting raw material; reacting with 3-oxoethyl propionate to generate 4-(2-fluorophenyl)-2-formacyl-4-oxoethyl butyrate shown as a formula 39; then carrying out cyclization reaction on 4-(2-fluorophenyl)-2-formacyl-4-oxoethyl butyrate and pyridine-3-sulfonamide to generate 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-ethyl formate shown as a formula 40; then carrying out reduction and substation reaction to generate 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-yl]-N-methyl methylamine shown as a formula 1, i.e., the vonoprazan. The preparationmethod of the vonoprazan, provided by the invention, has the characteristics of few synthesis steps and high yield.
Owner:迪嘉药业集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com