Preparation method of high-purity Vonoprazan fumarate
A technology for vonorasan fumarate and vonorasan maleate is applied in the field of preparation of high-purity vonorasan fumarate, can solve problems such as being unfavorable to industrialized production, and achieves simple operation and easy-to-obtain raw materials. , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of free alkaline Voro Late (Intermediate II)))
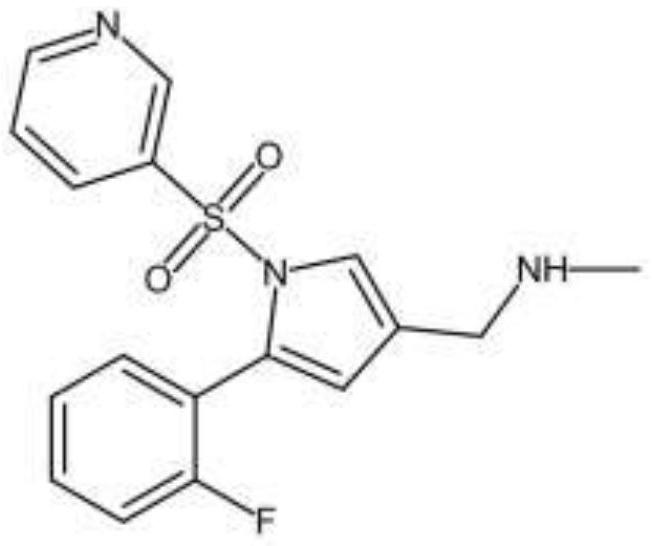
[0066] 100 g (0.3 mol) of 5- (2-fluorophenyl) -1 [(pyridin-3-yl) sulfonyl] -1H-pyrrole-3-formaldehyde was added to 800 g of methanol, and then 30% (quality) %) The methanol solution of the methal alcohol is about 34.54 g (methylamine 0.33 mol) 20 ° C for 4 h, cooling to 5 ° C, sodium borohydride 11.3 g (0.3 mol), 0-5 ° C insulation reaction 1 h; 2 mol / L hydrochloric acid was adjusted to be 5, and 200 g of saturated sodium chloride solution was added, and it was filtered to 0 ° C, and 83 g of free alkaline volfonora was obtained.
[0067] 83 g of free alkaline volfonola was added to 498 g of methanol 40 ° C dissolved, and the pH of 30% hydrochloric acid was 8.0, and 498 g of water was added dropwise, and the temperature was lowered to 0 ° C for 2 h, and the free alkaline volfonora boutique 61g (purity) ≥ 99.8%), yield 58.34% (with starting materials - 5- (2-fluorophenyl) -1 [(pyridin-3-yl) sulfonyl] -1...
Embodiment 2
[0068] Example 2: Preparation of free alkaline Vorora
[0069] 100 g (0.3 mol) of 5- (2-fluorophenyl) -1 [(pyridin-3-yl) sulfonyl] -1H-pyrrole-3-formaldehyde was added to 800 g of methanol, and 30% of the addition of 30% The amine methanol solution is about 62.8 g (0.60 mol) at 10 ° C for 2 h and 0.45 g (0.36 mol) of sodium borohydride, 1 h, and 2 mol / L hydrochloric acid is adjusted by 2 mol / L hydrochloride. 5. Add 200g of saturated sodium chloride solution, cool down to 0 ° C, resulting in free alkaline voltanolas. 76g.
[0070] 76 g of free alkaline volfonola was added to 304 g of methanol dissolved at 40 ° C, and the pH of 30% hydrochloric acid was 8.0, and 456 g of water was added dropwise, and the temperature was cooled to 0 ° C for 2 h, and filtered to obtain free alkaline voltanora boutique 67g ( Purity ≥99.8%), yield 64.07%.
Embodiment 3
[0071] Example 3: Preparation of high purity fumarate volcornsiii III
[0072] The free alkaline volfonola obtained in Example 1 was dissolved in 610 g (0.18 mol) in 610 g of ethanol, and 25.55 g (0.19 mol) was added to 73 ° C for 1 h, and the temperature was fired to 10 ° C for 1 h filtration, obtained Crude product 76g.
[0073] The above-mentioned crude product was added to 50% by volume of methanol solution to 50 ° C for 10 minutes filtration, and the resulting filtrate was lowered to 5 ° C for 2 h. Drying 72 g of rollandavila (yield 88.34%, with intermediate II).
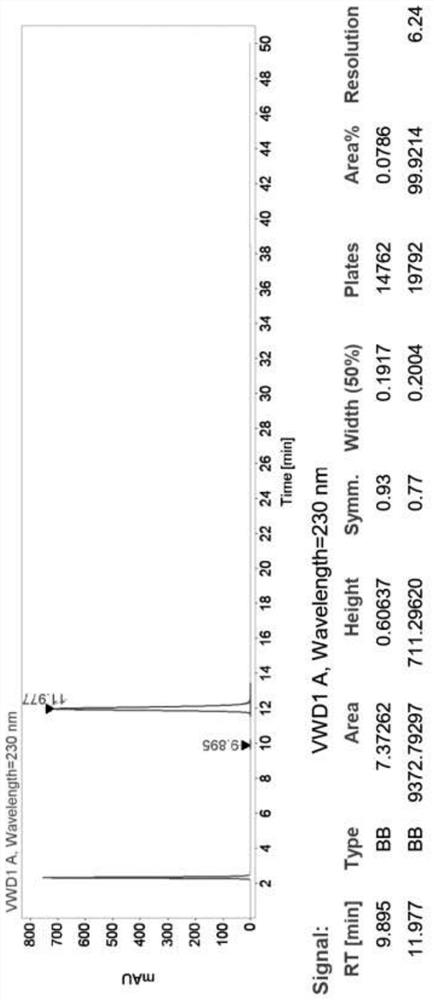
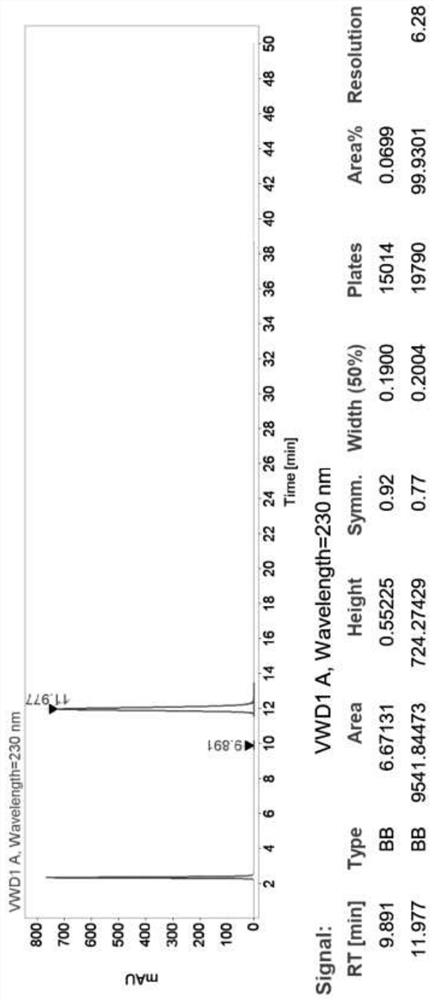
[0074] Detection by high efficiency chromatography, the chromatogram figure 1 , HPLC purity> 99.9%, and only one impurity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com