Vonoprazan fumarate preparation method
A technology for methyl methylamine fumarate and compound, which is applied in the field of drug preparation, can solve the problems of complicated post-processing, long synthesis steps, unfriendly environment and the like, and achieves simple and easy post-processing, mild reaction conditions, and low cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
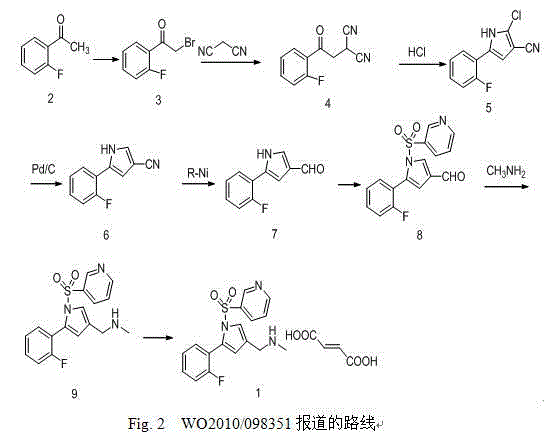
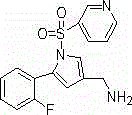
[0037] 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbonitrile
[0038]In a 500m reaction flask, add 200ml of acetonitrile, 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (50g, 0.27mol), N,N-diisopropylethylamine (41.6g, 0.32mol ) and 4-N, N-dimethylaminopyridine (4.69g, 38.4mmol), stirred at room temperature. Control the temperature of the reaction solution not higher than 30°C, and add dropwise a mixed solution of 3-pyridinesulfonyl chloride (52.5 g, 0.29 mol) in 100 ml of acetonitrile. After dropping, react at 25°C for 2 hours. After the reaction is complete, add 300ml of purified water to the reaction solution, control the temperature of the reaction solution not higher than 30°C, add concentrated hydrochloric acid dropwise to adjust the pH value to 4-5, and stir at 25°C for 2 hours. Filter, wash with acetonitrile and water mixture, collect the solid, and dry under reduced pressure at 50°C to obtain 79.5g of 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrro...
Embodiment 2
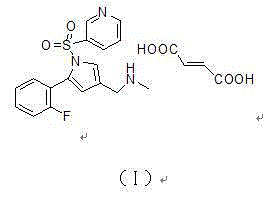
[0040] 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethylamine fumarate
[0041] Add 30g of 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbonitrile and 450ml of methanol into the reaction kettle, add Raney nickel, nitrogen replacement three times, hydrogen replacement Three times, keep 2 atmospheres, stir at 15-25°C for 5 hours, nitrogen replacement three times, filter out Raney nickel, add 4g paraformaldehyde to the filtrate, reflux at 65°C and stir for 8 hours, cool to 0°C, add in batches 10.4 g of sodium borohydride was stirred at room temperature for 4 hours. Add 450 g of purified water to terminate the reaction, concentrate, add 240 ml of dichloromethane for extraction. The organic phase was dried over anhydrous sodium sulfate. Filter, concentrate, add 300ml of isopropanol, heat to 50°C, stir to dissolve. Fumaric acid was added and stirred for 1 hour. Cool to room temperature, filter, wash with isopropanol, collect the solid, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com