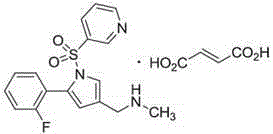
Vonoprazan fumarate enteric coated tablet and preparation method thereof
A technology for vonoprazan fumarate and enteric-coated tablets, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as unstable release of ordinary tablets, and achieve Good release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
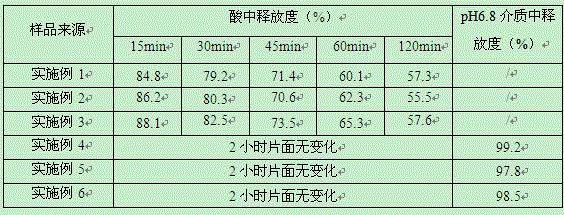
Embodiment 1
[0030] Embodiment 1, a kind of vonorazansu fumarate tablet (chip tablet), the composition of every 1000 tablets is as follows:
[0031] Chip:
[0032] Vonoprazan Fumarate 13.36g Mannitol 22g microcrystalline cellulose 58.14g Hydroxypropyl Cellulose 5.5g fumaric acid 3.3g Croscarmellose Sodium 6.6g Magnesium stearate 1.1g production 1000 pieces
[0033] The preparation process is as follows:
[0034] (1) Weigh the prescribed amount of mannitol, vonoprazan fumarate, microcrystalline cellulose, fumaric acid, and croscarmellose sodium and mix them in a wet granulator;
[0035] (2) Add hydroxypropyl cellulose aqueous solution to the wet granulator for wet mixing to make soft materials, and granulate with a 20-mesh screen;
[0036] (3) Dry the wet granules in an oven at 40°C, and granulate with a 20-mesh sieve;
[0037] (4) Add the prescribed amount of magnesium stearate to the dry granules, and mix them in a mixer;
[003...
Embodiment 2
[0039] Embodiment 2, a kind of vonorazansu fumarate tablet (tablet core tablet), the composition of every 1000 tablets is as follows:
[0040] Chip:
[0041] Vonoprazan Fumarate 13.36g Mannitol 44g microcrystalline cellulose 36.14g Hydroxypropyl Cellulose 5.5g fumaric acid 3.3g Croscarmellose Sodium 6.6g Magnesium stearate 1.1g production 1000 pieces
[0042] The preparation process is the same as in Example 1.
Embodiment 3
[0043] Embodiment 3, a kind of vonorazansu fumarate tablet (tablet core tablet), the composition of every 1000 tablets is as follows:
[0044] Chip:
[0045] Vonoprazan Fumarate 13.36g Mannitol 66g microcrystalline cellulose 14.14g Hydroxypropyl Cellulose 5.5g fumaric acid 3.3g Croscarmellose Sodium 6.6g Magnesium stearate 1.1g production 1000 pieces
[0046] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com