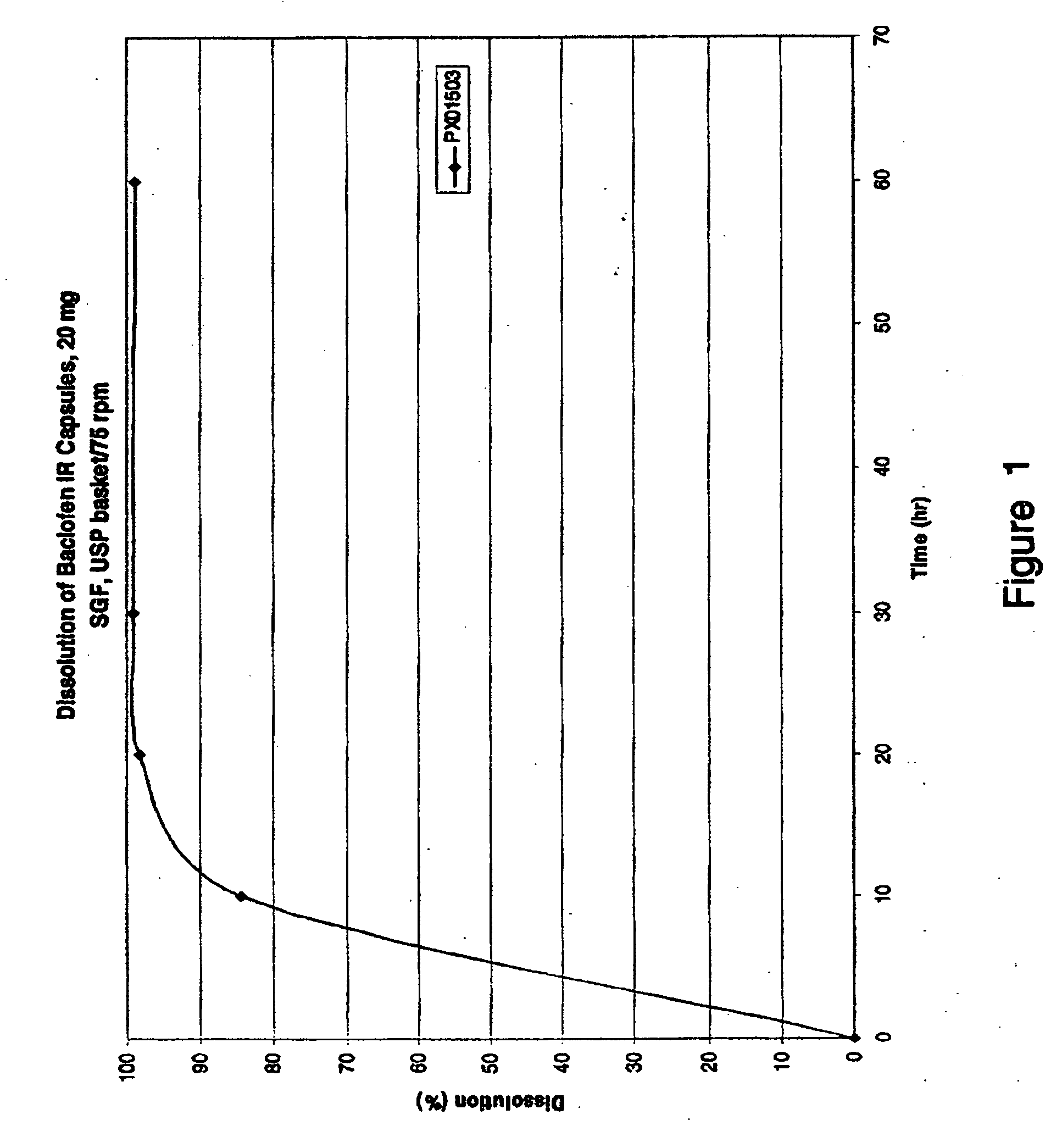
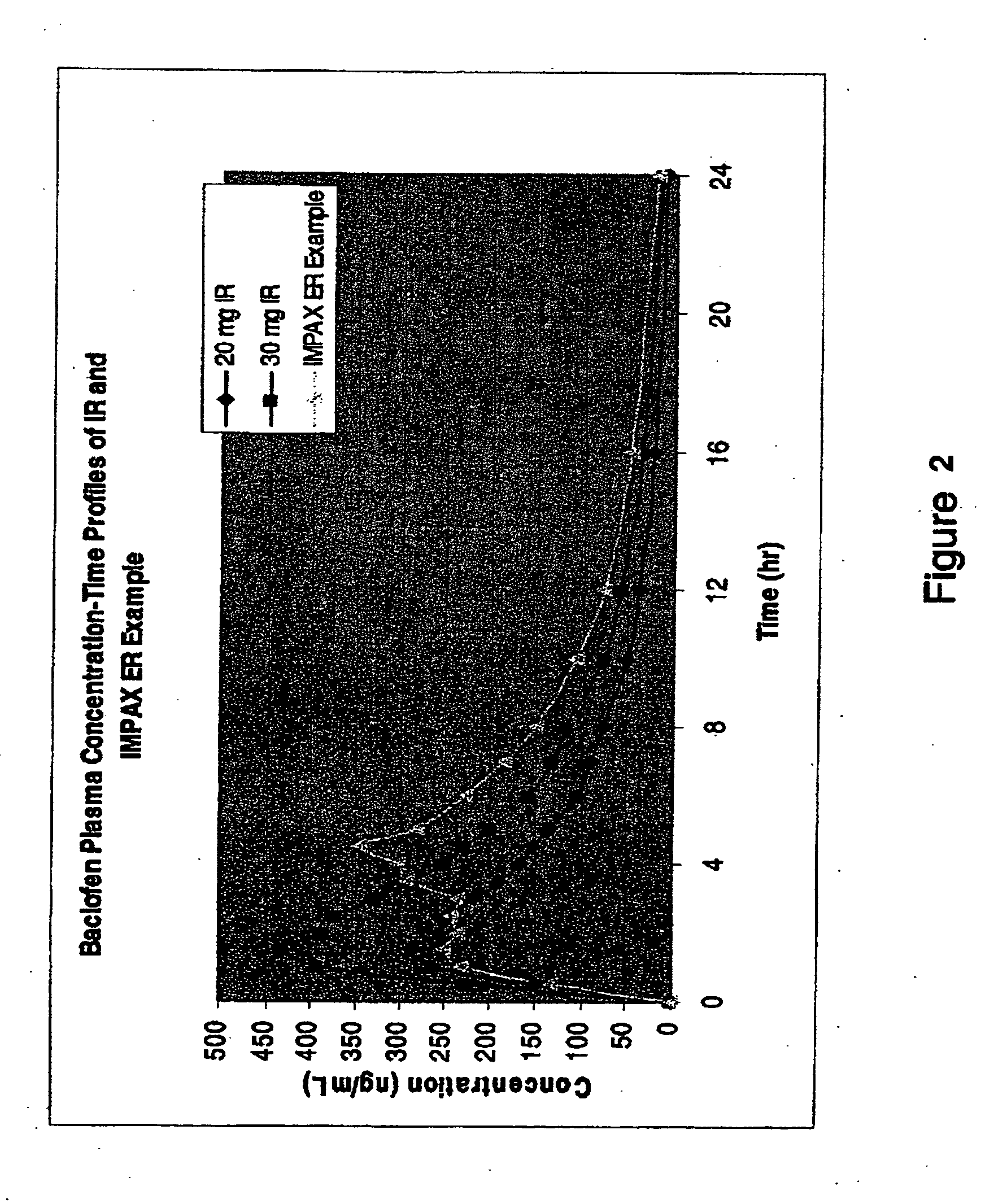
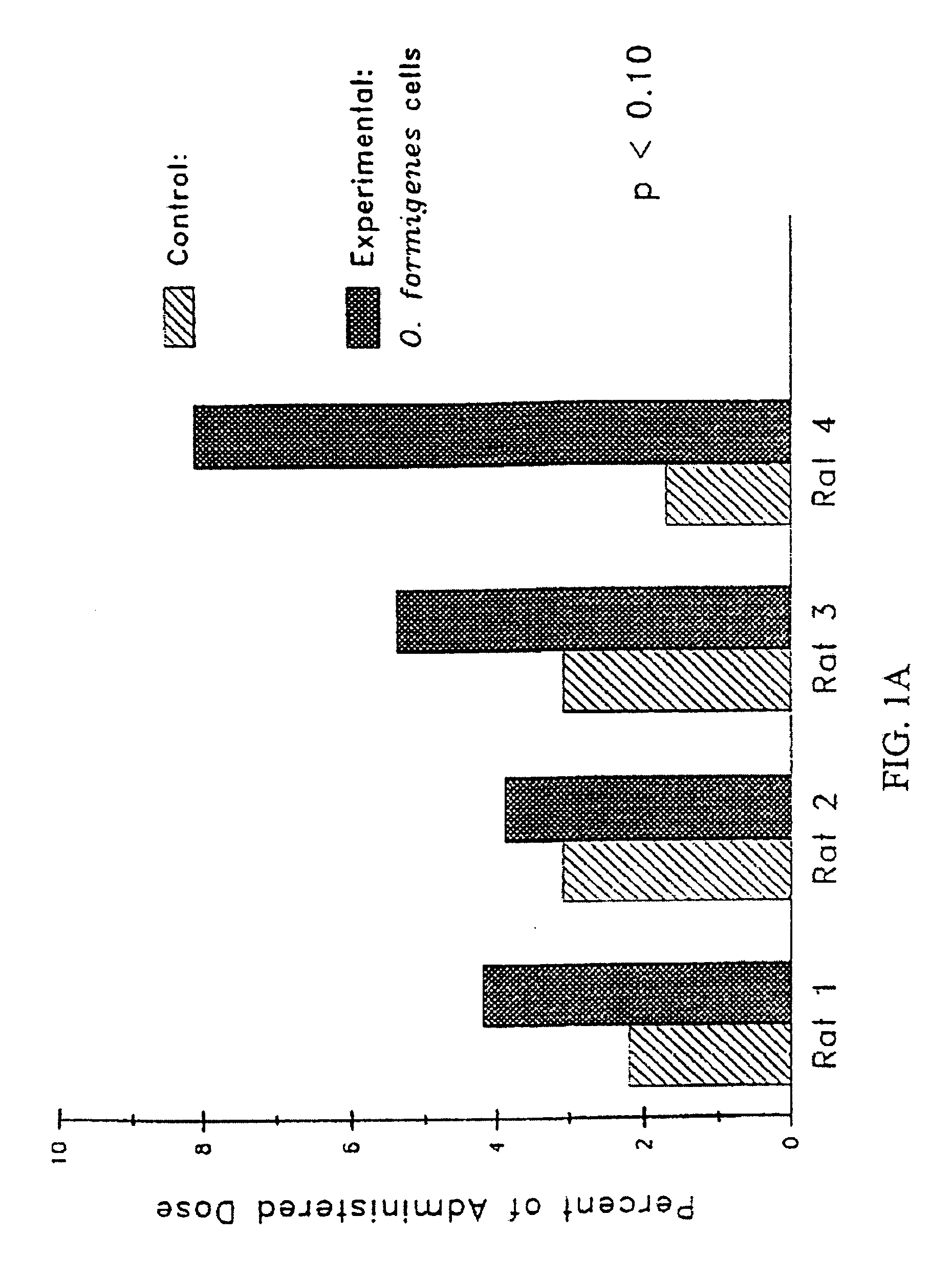
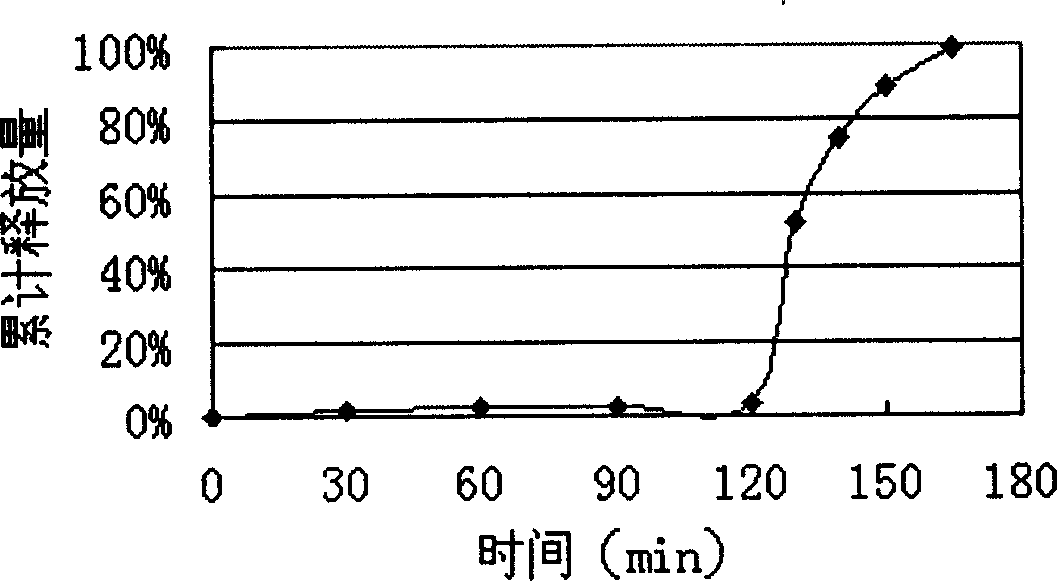
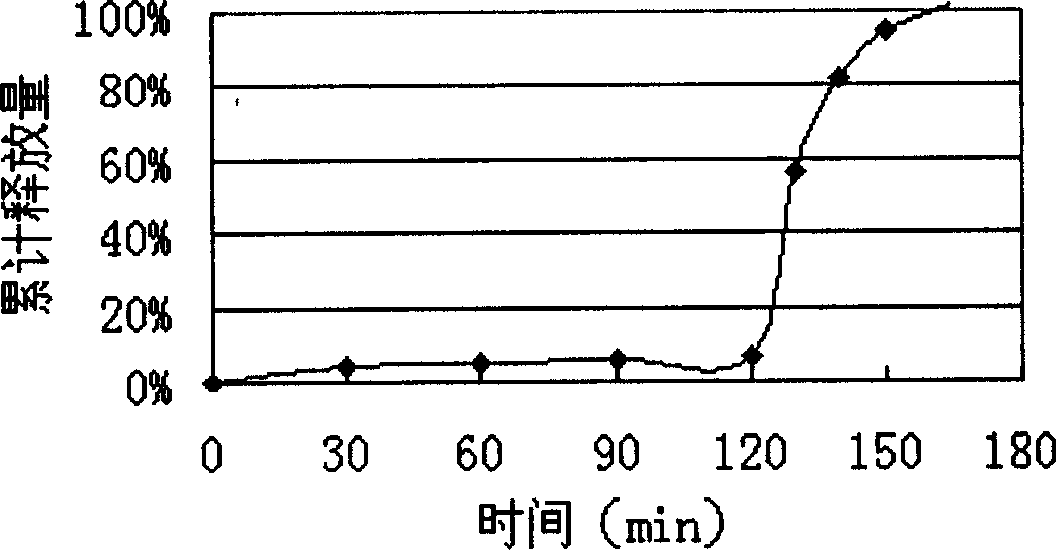
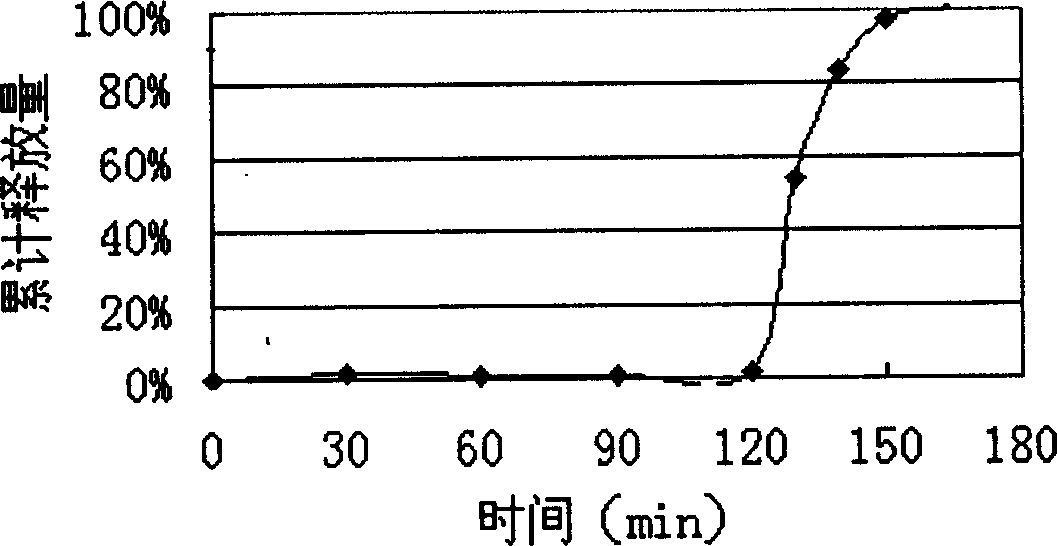
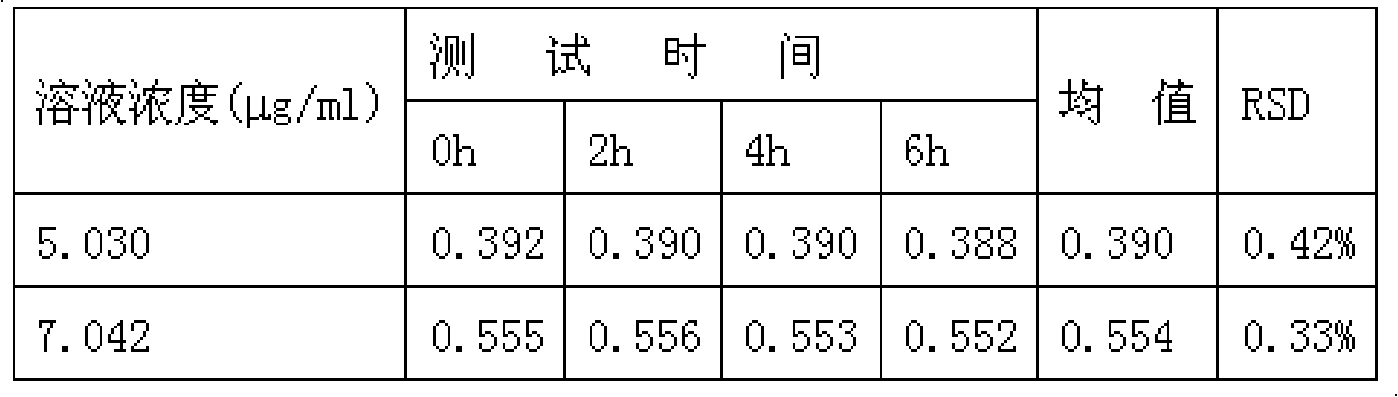
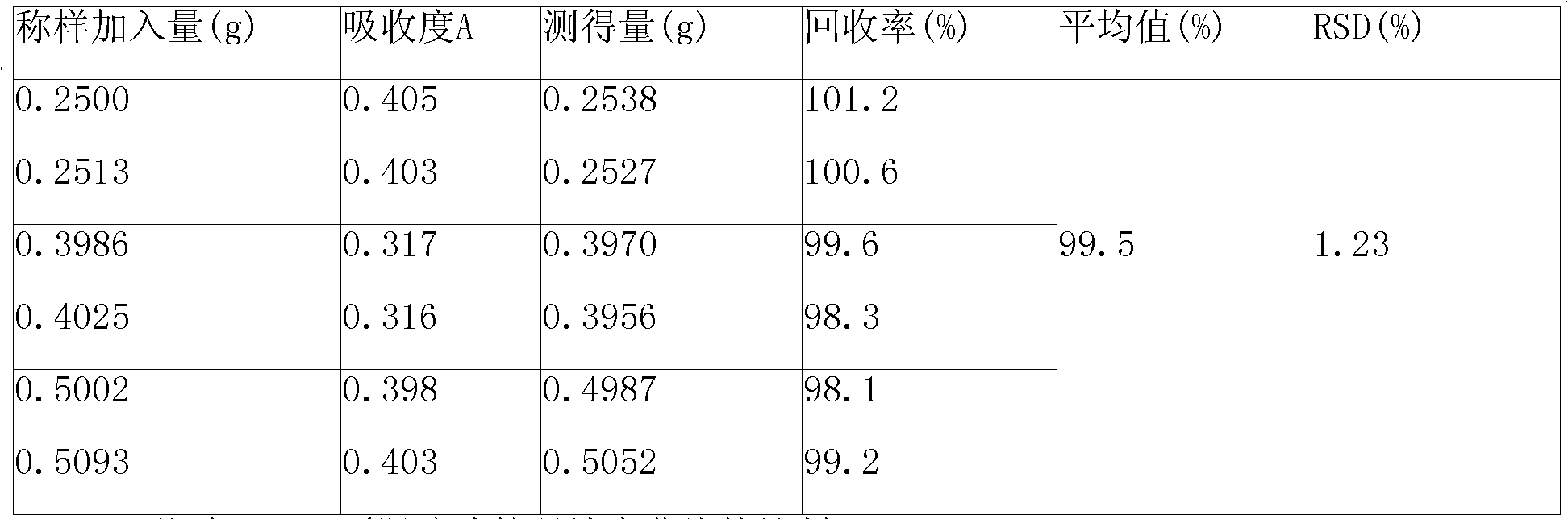
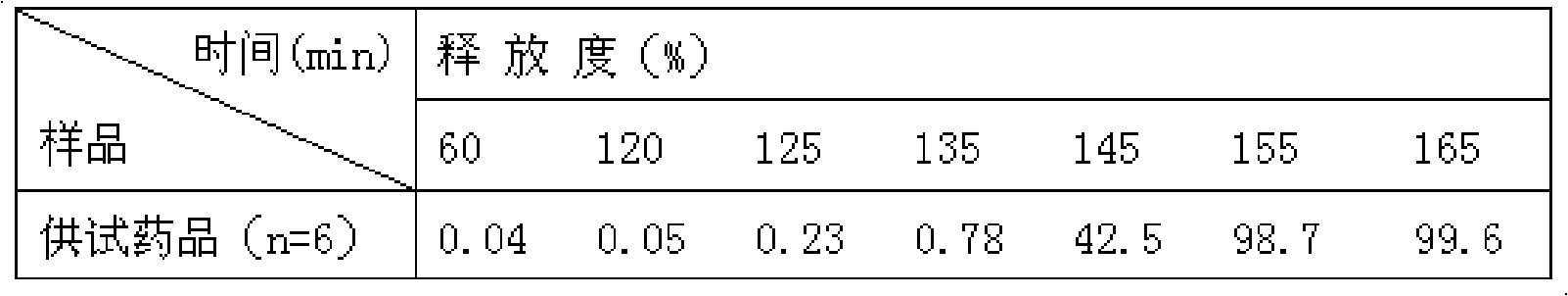
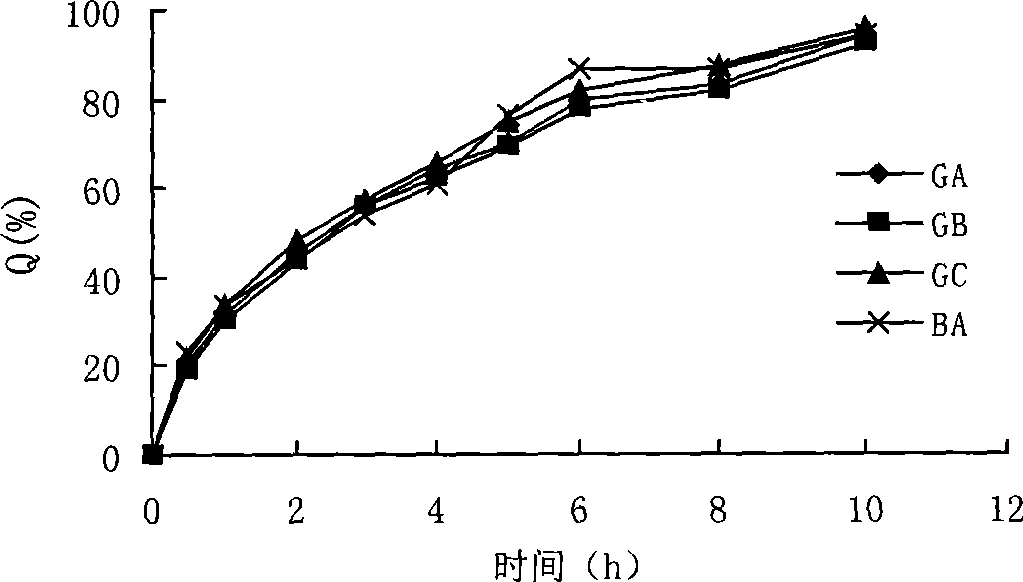
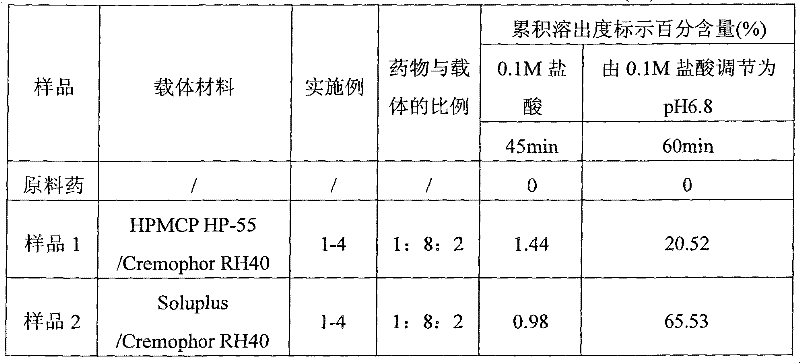
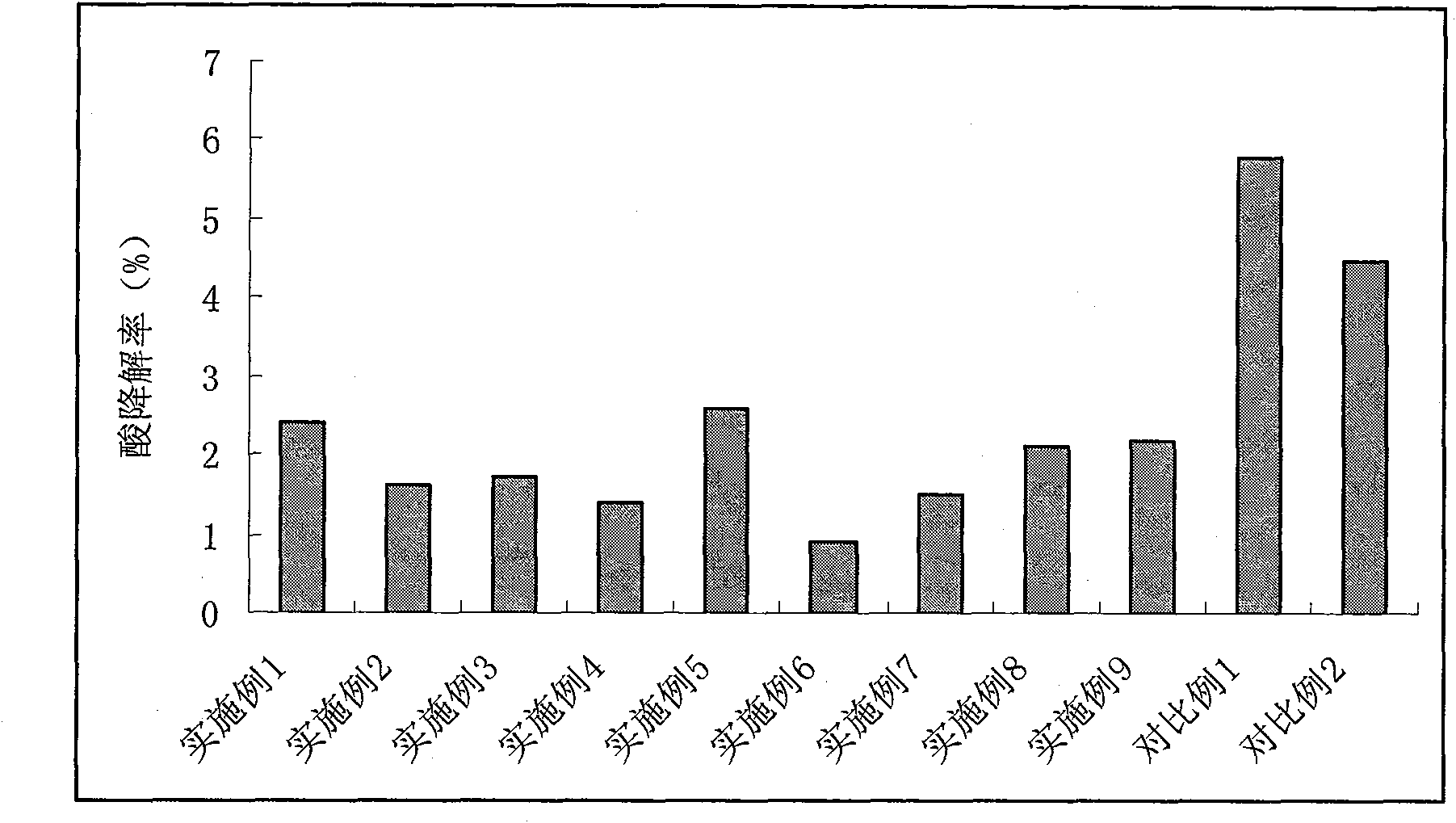
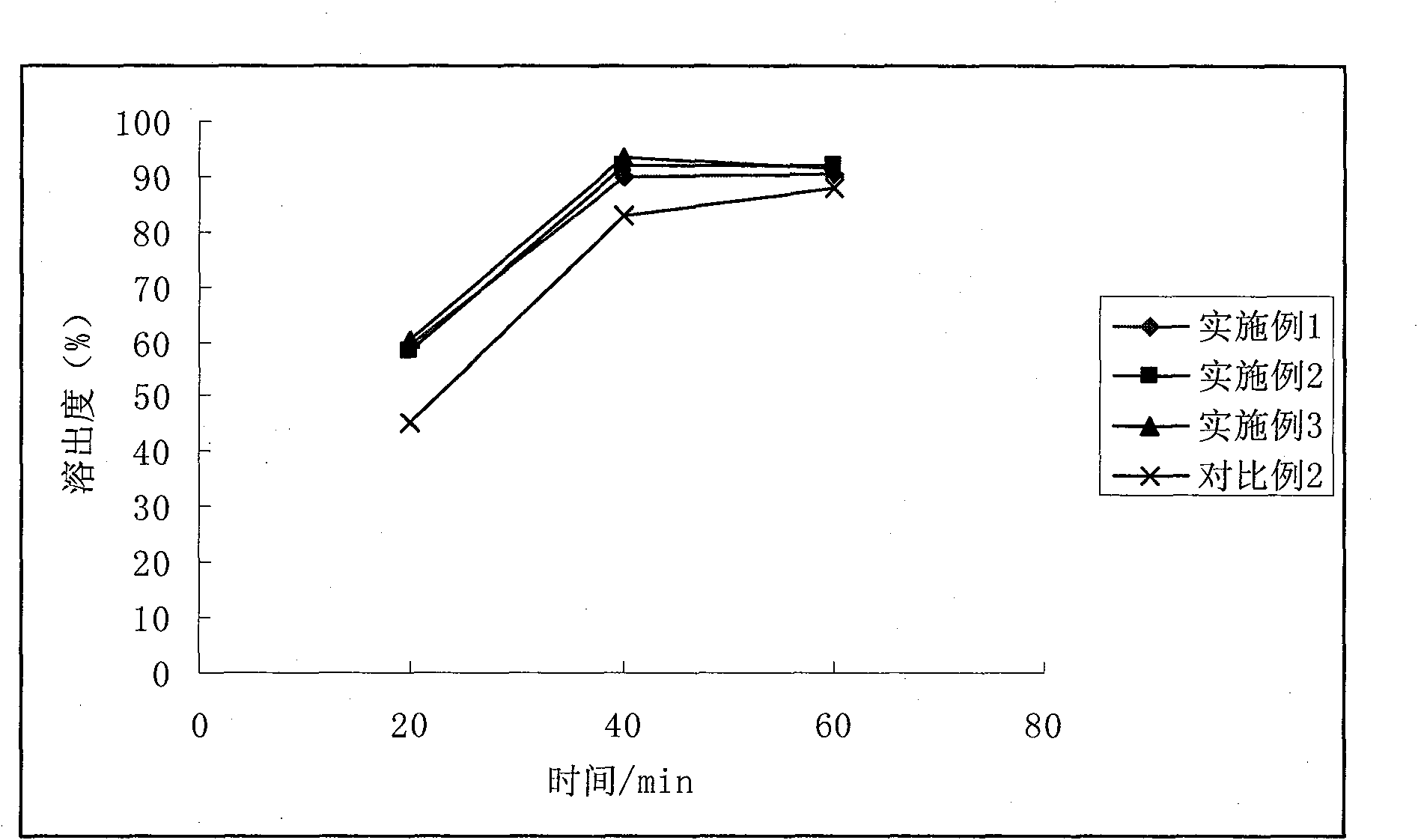
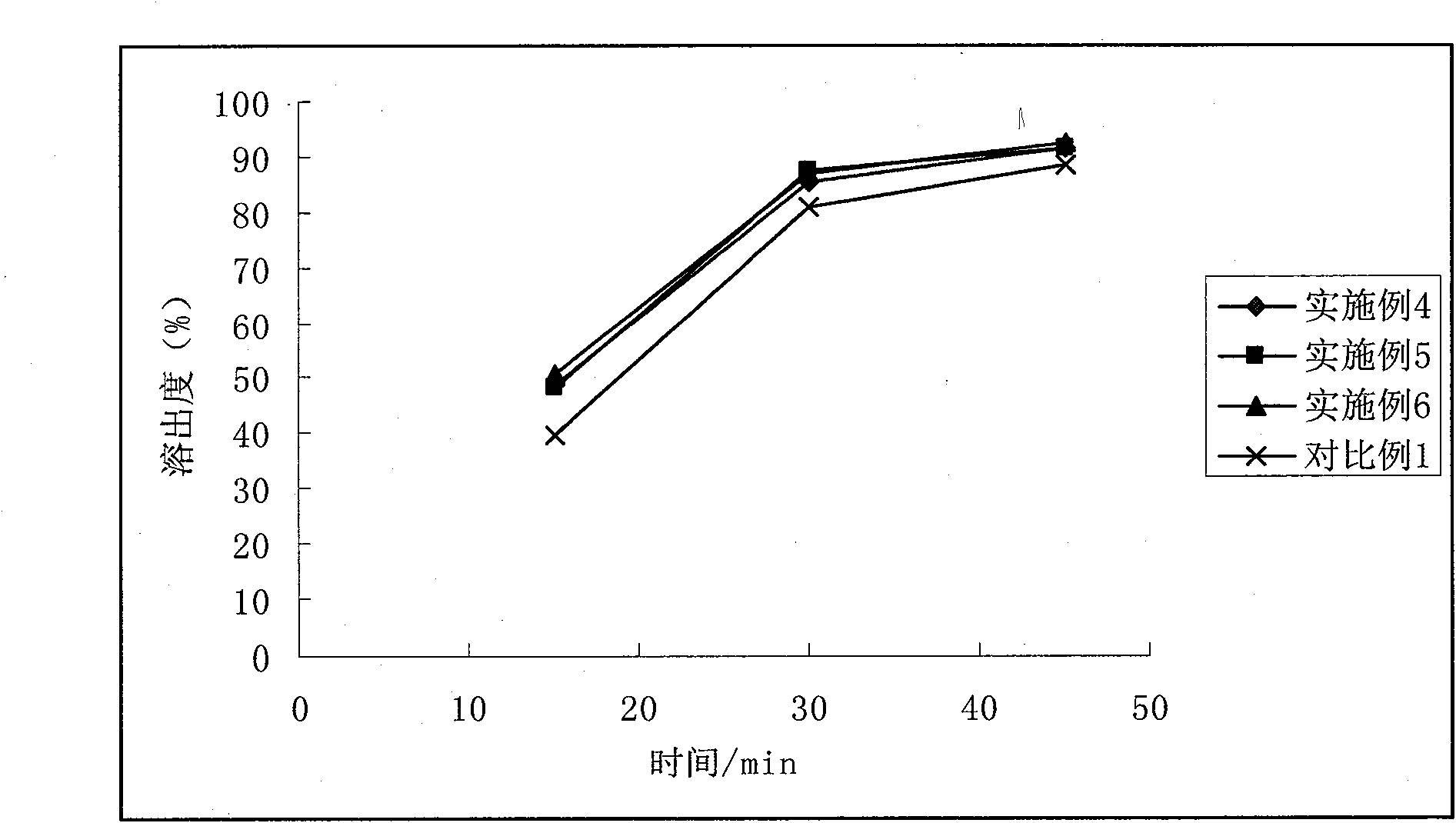
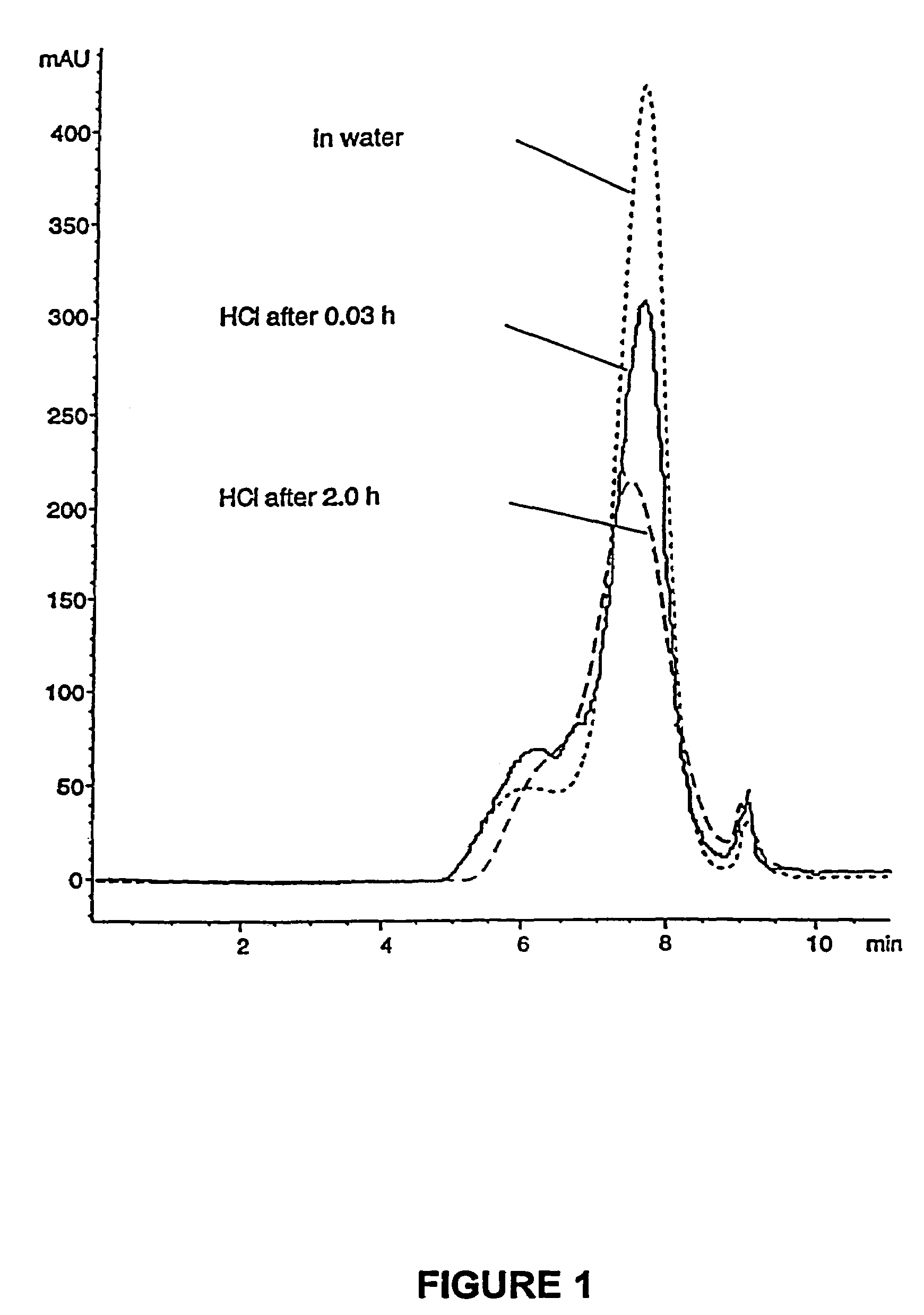
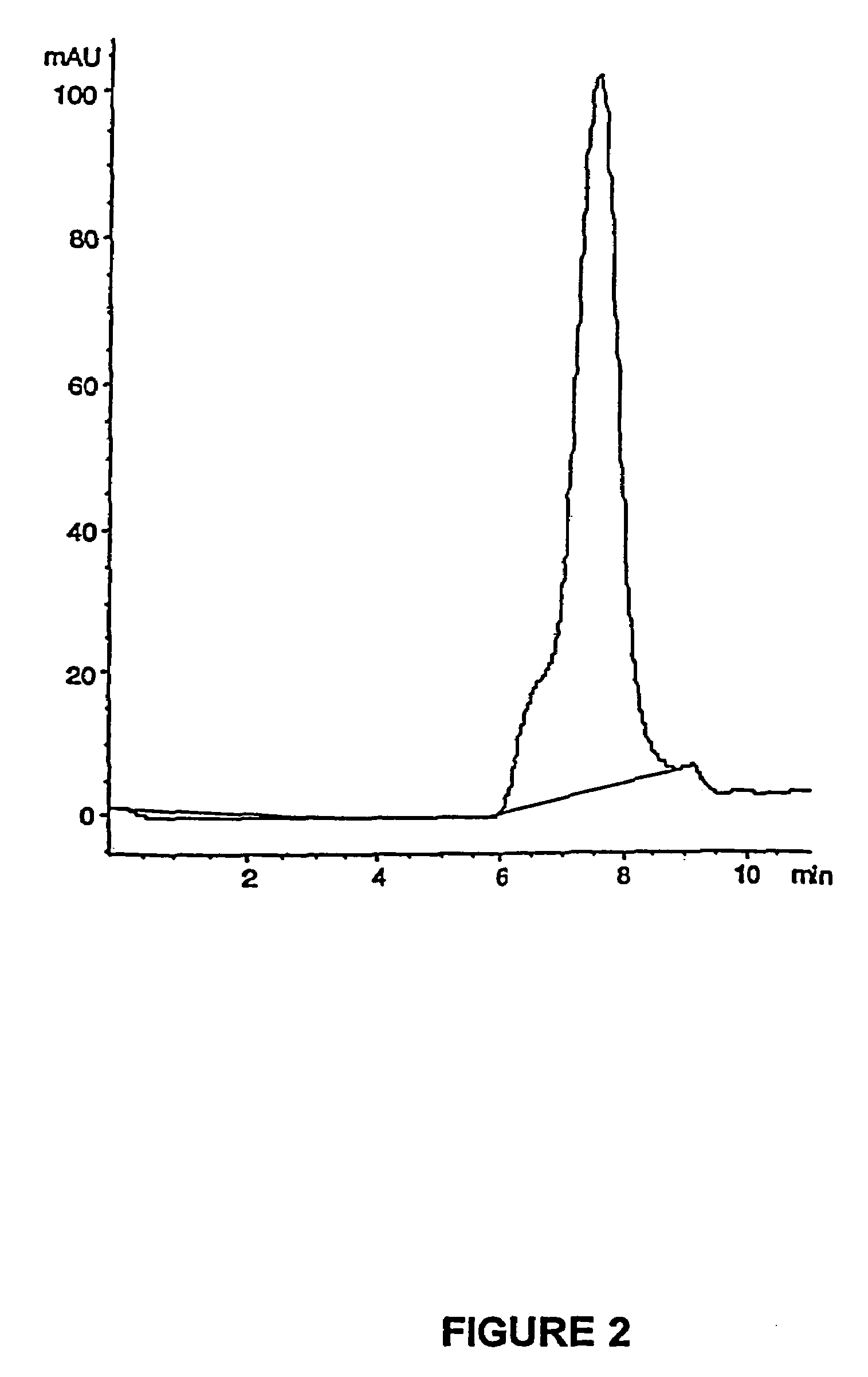
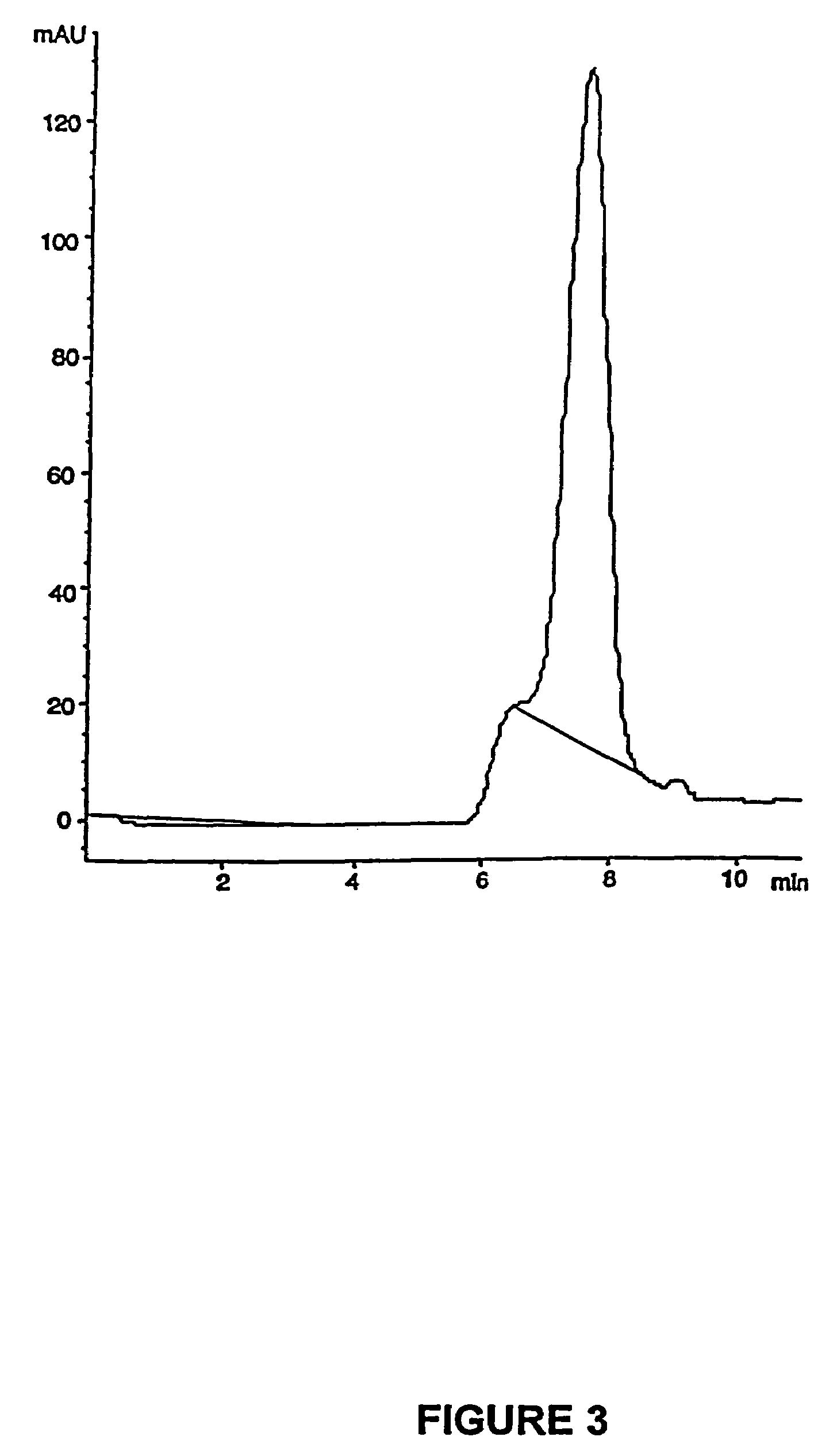
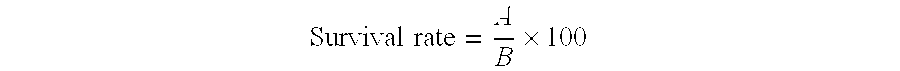Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
906 results about "Enteric coated" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosage forms using drug-loaded ion exchange resins
InactiveUS20050181050A1Avoid breakingPharmaceutical non-active ingredientsPill deliveryOral medicationImmediate release
A multiparticulate, modified release composition for oral administration has been developed. The formulation is made by complexing a drug with an ion-exchange resin in the form of small particles, typically less than 150 microns. The present invention provides novel extended release coated ion exchange particles comprising drug-resin complexes, produced by binding the salt form of the drug, that do not require impregnating agents to insure the integrity of the extended release coat. To prepare a modified release formulation, one or more of the following types of particles are formulated into a final dosage form: (a) Immediate release particles, (b) Enteric coated particles, (c) Extended release particles, (d) Enteric coated-extended release particles; and (e) Delayed release particles. The various drug-containing particles described above can be further formulated into a number of different easy-to-swallow final dosage forms including, but not limited to, a liquid suspension, gel, chewable tablet, crushable tablet, rapidly dissolving tablet, or unit of use sachet or capsule for reconstitution
Owner:COLLEGIUM PHARMA INC
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
Enteric coated stable oral pharmaceutical composition of acid unstable drug and process for preparing the same
InactiveUS20040028737A1Long storage periodImprove solubilityBiocideGranular deliveryNeutral phMedicine
Enteric coated stable oral pharmaceutical composition of acid unstable drug. The enteric coating is a bilayer with a pH gradient across its thickness comprising an inner layer of neutral or near neutral pH 7-7.5 and an outer layer of acidic pH 2-6. Also process for preparng the enteric coated stable oral pharmaceutical composition of acid unstable drug. The enteric coating is first carried out at neutral or near neutral pH of 7-7.5 to form an inner layer of neutral or near neutral pH and then at acidic pH of 2-6 to form an outer layer of acidic pH.
Owner:KOPRAN RES LAB LTD
Oral pharmaceutical formulations containing non-steroidal anti-inflammatory drugs and acid inhibitors
InactiveUS20070154542A1AntipyreticDigestive systemSide effectNonsteroidal Antiinflammatory Drugs/NSAIDs
The present disclosure provides enteric coated capsules and orally dissolving films comprising non-steroidal anti-inflammatory drugs and acid inhibitors, as well as methods of treating treatment humans for pain and / or inflammation while reducing gastrointestinal side effects.
Owner:HORIZON PHARMA USA
Enteric-coated multilayer encapsulated probiotic microcapsule and preparation method thereof
InactiveCN1969889AGrowth inhibitionPromote formationMetabolism disorderBacteria material medical ingredientsAcid-fastSolubility
The invention discloses an enteric-solubility multilayer encysted bacterium microcapsule and making method in the biological agent technical domain, which is characterized by the following: adopting sodium alginate, calcium chloride and chitose as clad material of microcapsule; making lactic acid bacteria or bifidobacteria as core-clad material; proceeding ionic exchange for sodium alginate and calcium chloride; forming second clad on the surface of calcium alginate through different isoelectric points of calcium alginate and chitose; freezing at low temperature to obtain the product.
Owner:JINAN SYNBIOTICS BIOENG
Enzyme composition for improving food digestion
InactiveUS20080199448A1Improving food absorptionPromote digestionOrganic active ingredientsPeptide/protein ingredientsProteinase activityAdditive ingredient
An orally administered composition for improving food absorption and digestion contains therapeutically effective dosages of digestive enzymes and L-glutamine as active ingredients. The digestive enzymes include at least one each of a lipase, a protease, and an amylase, and at least a portion of each of these enzymes is enteric coated.
Owner:NUTRI-HEALTH SUPPLEMENTS LLC
Controlled release formulations using intelligent polymers
InactiveUS6893661B1Promote absorptionMaintenance of therapeutically effective blood levelPowder deliveryOrganic active ingredientsSmart polymerWater contact
An extended release dosage composition of pharmaceutically active substances that have a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848 presented as a matrix tablet containing the said pharmaceutically active substances, with / without suitable pharmaceutical excipients in intimate mixture with two groups of intelligent polymers having opposing wettability characteristics, one demonstrating a stronger tendency towards hydrophobicity and the other a stronger tendency towards hydrophilicity, the polymer combination being between the ratios of 1:50 and 50:1 amounts effective to control the release of said pharmaceutically active substances in a mathematically predictable manner, wherein the polymer demonstrating a stronger tendency towards hydrophobicity is not less than 5% wt / wt and preferably between 5-70% wt / wt of the final formulation composition. The intelligent polymers being ethylcellulose (EC) as a more strongly hydrophobic and hydroxyethylcellulose (HEC) and / or hydroxypropyl methylcellulose (HPMC) as more strongly hydrophilic (the ratio of HEC to HPMC being between 1:100 and 100:1). The matrix tablet is optionally coated with an enteric coat, 0-5%-15% wt / wt to prevent the initial burst effect seen in such systems and to impart gastrointestinal tract (GIT) “stealth” characteristics especially in the presence of food.
Owner:VALEANT INT BERMUDA
Drug Delivery Formulations For Targeted Delivery
The size and location of microsphere uptake / delivery are important determinants of the final biodistribution of oral microsphere systems. Formulations, kits, methods of administering the formulations, and using the kits are described herein. The formulations are oral dosage formulations. In one embodiment, the formulations contain microparticles and / or nanoparticles having a homogenous size range selected to optimize uptake in a specific region of the GI tract and target drug delivery to specific organs. In some embodiments, the dosage formulation contains an enteric coating and / or a magnetic material. In a preferred embodiment, the formulation contains a magnetic material and an active agent to be delivered, optionally the active agent is in the form of micro- or nano-particles. In some embodiments metallomucoadhesive materials and / or magnetic materials are employed as magnetic and / or mucoadhesive sources. Formulations containing magnetic materials can be localized using the kits and methods disclosed herein. In one embodiment, the method includes orally administering the formulation and applying an extracorporeal magnet to a site on the outside surface of the patient's body in an area that closely apposes the location in the gastrointestinal tract to which delivery of the formulation is desired. The extracorporeal magnet is applied for a suitable time period to allow for the drug to be released from the formulation and / or to allow for the formulation to adhere to the site. Both magnetic and mucoadhesive forces may be utilized to site-direct and retain the dosage form in the region of the gastrointestinal (GI) tract most suitable for the desired delivery.
Owner:PEROSPHERE INC
Enteric coated microgranules for stabilizing lactic acid bacteria
InactiveUS6365148B1Improve stabilityIncreased proliferationBiocideMilk preparationEnteric-coated granulesLactobacillus
The present invention relates to an enteric coated granule prepared by coating lactic acid bacteria-containing seed with a water-miscilble coating material and then, if desired, subjecting the first coated product to the second coating with a controlled-release coating material.
Owner:IL YANG PHARMA CO LTD
Orally disintegrable tablets
InactiveUS20020142034A1High strengthGood disintegrationBiocidePowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Pharmaceutical dosage forms having immediate release and/or controlled release properties
ActiveUS20060057197A1Prolonged in vivo absorptionPromote absorptionPowder deliveryPharmaceutical non-active ingredientsControl releaseMedicine
The present invention relates generally to pharmaceutical dosage forms comprising: an absorption window active agent; a controlled release component comprising enteric-coated controlled release beads, wherein the enteric-coated release beads comprise at least two pH-sensitive polymer layers. The controlled-release dosage forms provide good bioavailability of absorption window active agents.
Owner:IMPAX LAB LLC
Oral pharmaceutical extended release dosage form
An enteric coated pharmaceutical extended release dosage form of a H+, K+-ATPase inhibitor giving an extended plasma concentration profile of a H+, K+-ATPase inhibitor. The extended plasma profile is obtained by a pharmaceutical composition which comprises a core material of a hydrophilic or hydrophobic matrix, and the H+, K+-ATPase inhibitor and optionally pharmaceutically acceptable excipients. The dosage form may be administered once daily.
Owner:ASTRAZENECA AB
Enteric coated particles containing an active ingredient
InactiveUS20070292510A1Sufficient taste-masking propertyEfficient releaseBiocideSalicyclic acid active ingredientsMedicineImmediate release
Enterically coated particles and chewable tablets made therefrom are disclosed. The enterically coated particles are comprised of a core containing an active ingredient, a first coating layer comprised of polymeric composition having a Tg less than about 40° C. that substantially covers the core; and a second coating layer, which substantially covers the first coating layer, comprised of a high temperature film forming polymer. The particles may be produced into a tablet form, such as a chewable tablet form, that provides for the immediate release of the active ingredient.
Owner:MCNEIL PPC INC
Enteric coated omeprazole pellets capsule and the preparing method thereof
The invention relates to an omeprazole enteric micropill capsule and a process for preparing the same. The content of the capsule is omeprazole enteric micropill including a blank pill core; an activity medicine layer having an alkaline component; a separator containing magnesia and titanium pigment; and an enteric coat layer. According to synergistic reaction, stability of the medicine is distinctly improved. The omeprazole enteric micropill capsule of the invention has period of validity of the preparation to three years or more.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Enteric formulations of proanthocyanidin polymer antidiarrheal compositions
InactiveUS20050019389A1Reduce secretionReduce acidityAntibacterial agentsBiocideSecretory diarrheaPharmaceutical formulation
Pharmaceutical compositions containing a proanthocyanidin polymer composition which are useful for the treatment and prevention of secretory diarrhea are provided. The invention specifically relates to pharmaceutical formulations of a proanthocyanidin polymer composition which has been isolated from a Croton spp. or a Calophyllum spp. In particular, the invention relates to a formulation of a proanthocyanidin polymer composition which protects the composition from the effects of stomach acid after oral administration, particularly to those formulations which are enteric coated. The invention also relates to methods of producing a directly compressible proanthocyanidin polymer composition, as well as compositions containing the directly compressible proanthocyanidin polymer composition.
Owner:NAPO PHARMA INC
Pantoprazole multiparticulate formulations
ActiveUS20050129761A1Low variabilityProlong the action timeBiocideDispersion deliveryMedicineEnantiomer
Pantoprazole sodium multiparticulates are described which avoid sticking to nasogastric and gastronomy tubes. The pantoprazole multiparticulates have a spheroid core of pantoprazole or an enantiomer thereof, or a salt thereof, a surfactant, and a distintegrant; a sub coat which is comprised of hydroxypropyl methylcellulose (hypromellose) and water, an enteric coat on the sub-coat, and a final seal coat over the enteric coat, which is composed of hydroxypropyl methylcellulose (hypromellose) and water.
Owner:WYETH LLC
Novel soft-gelatin capsule comprising S-adenosylmethionine and a method for producing the same
InactiveUS20020164369A1Easy to handleReadily availableBiocideSugar derivativesS-Adenosyl methionineGelatin film
The invention provides a novel soft gelatin capsule comprising a fill material consisting essentially of S-adenosylmethionine (SAMe) salt disposed within an enteric coated soft gelatin film.
Owner:ORCHID CHEM & PHARM LTD
Pharmaceutical formulation of omeprazole
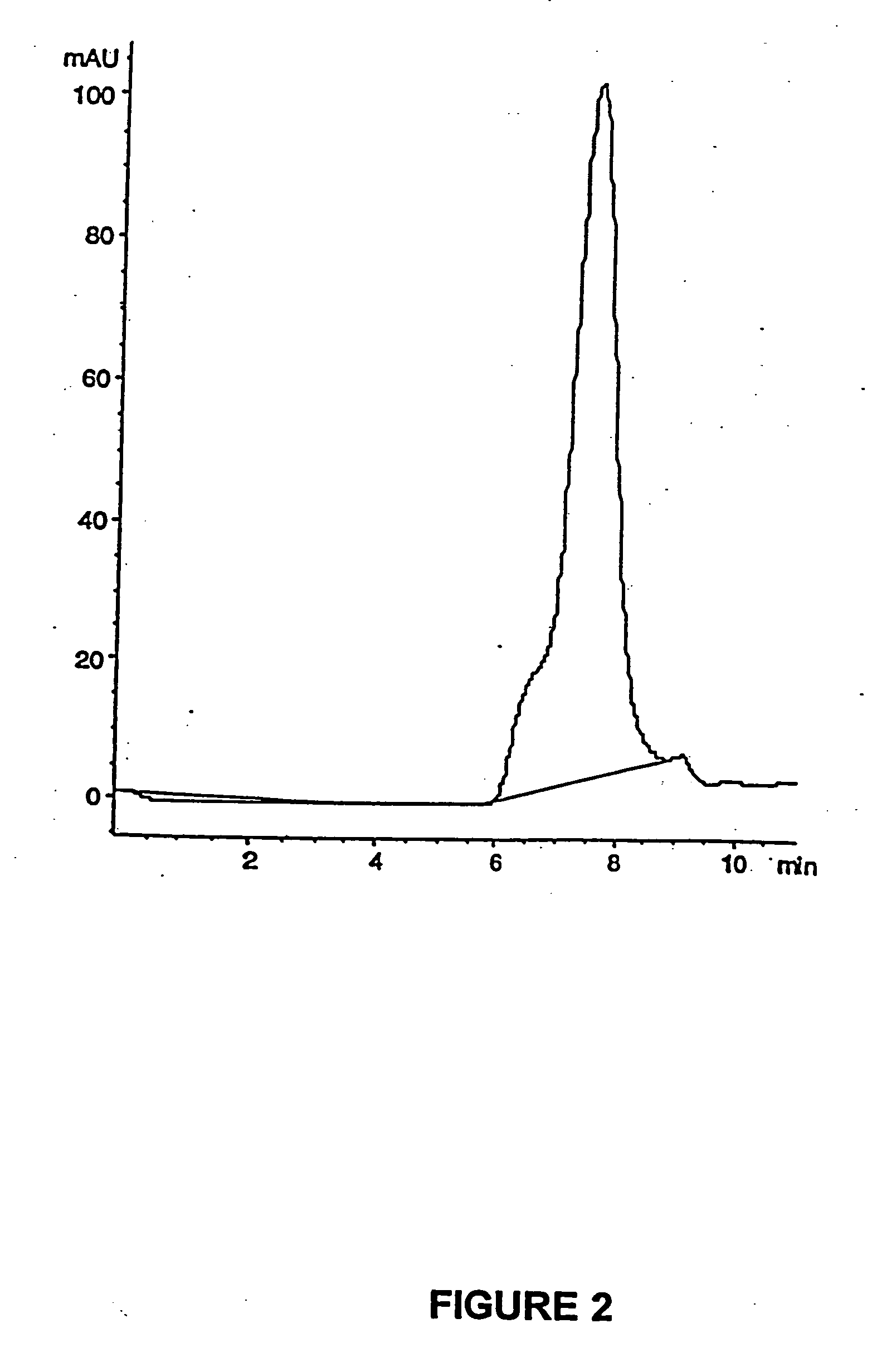
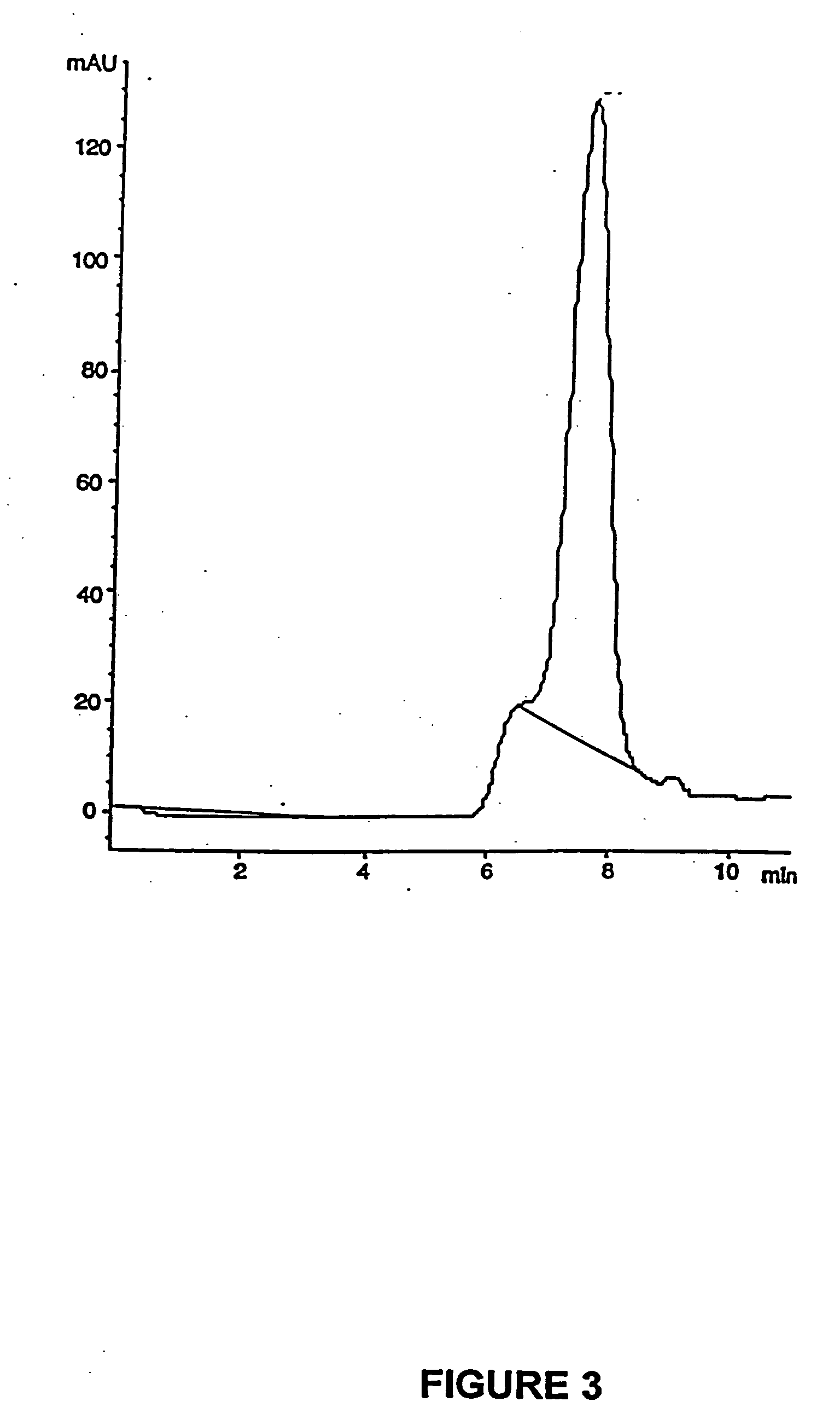
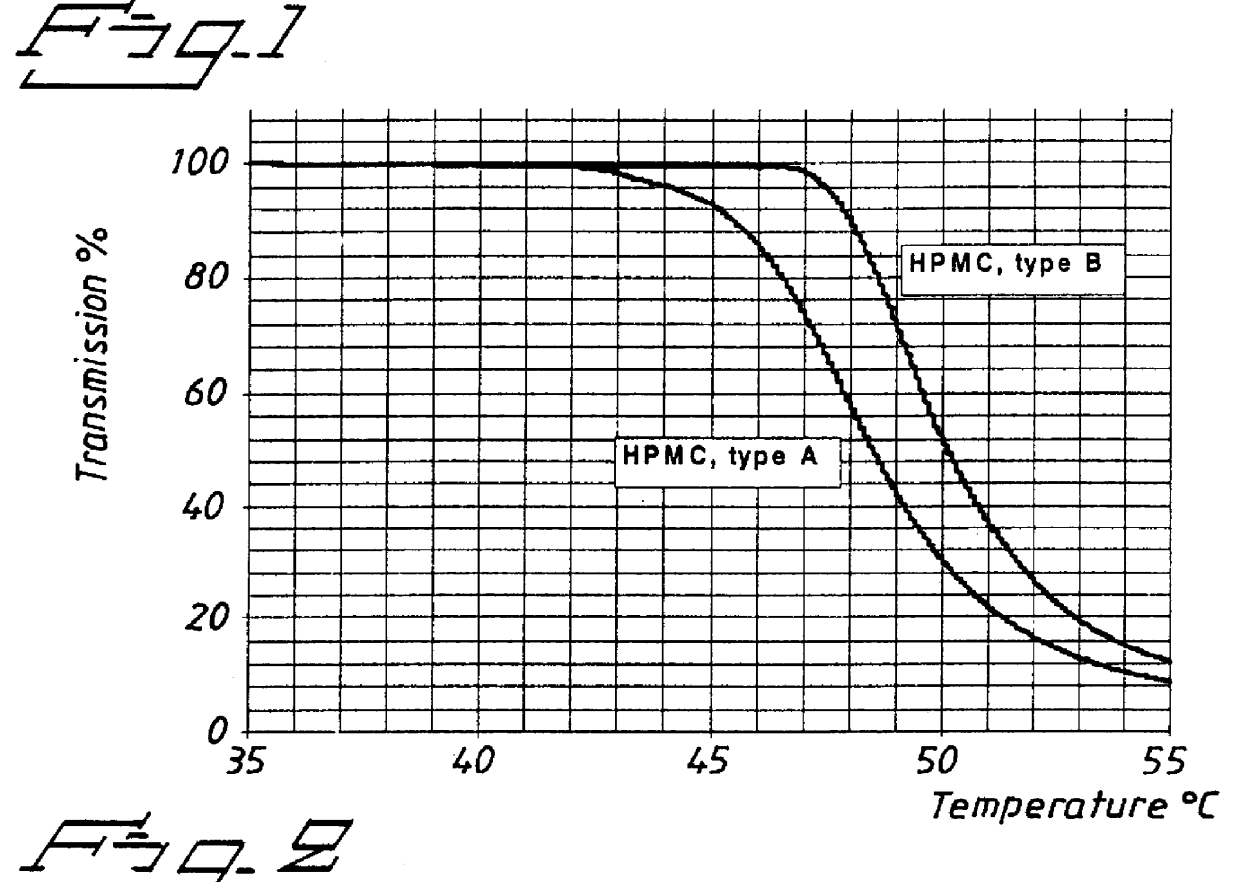
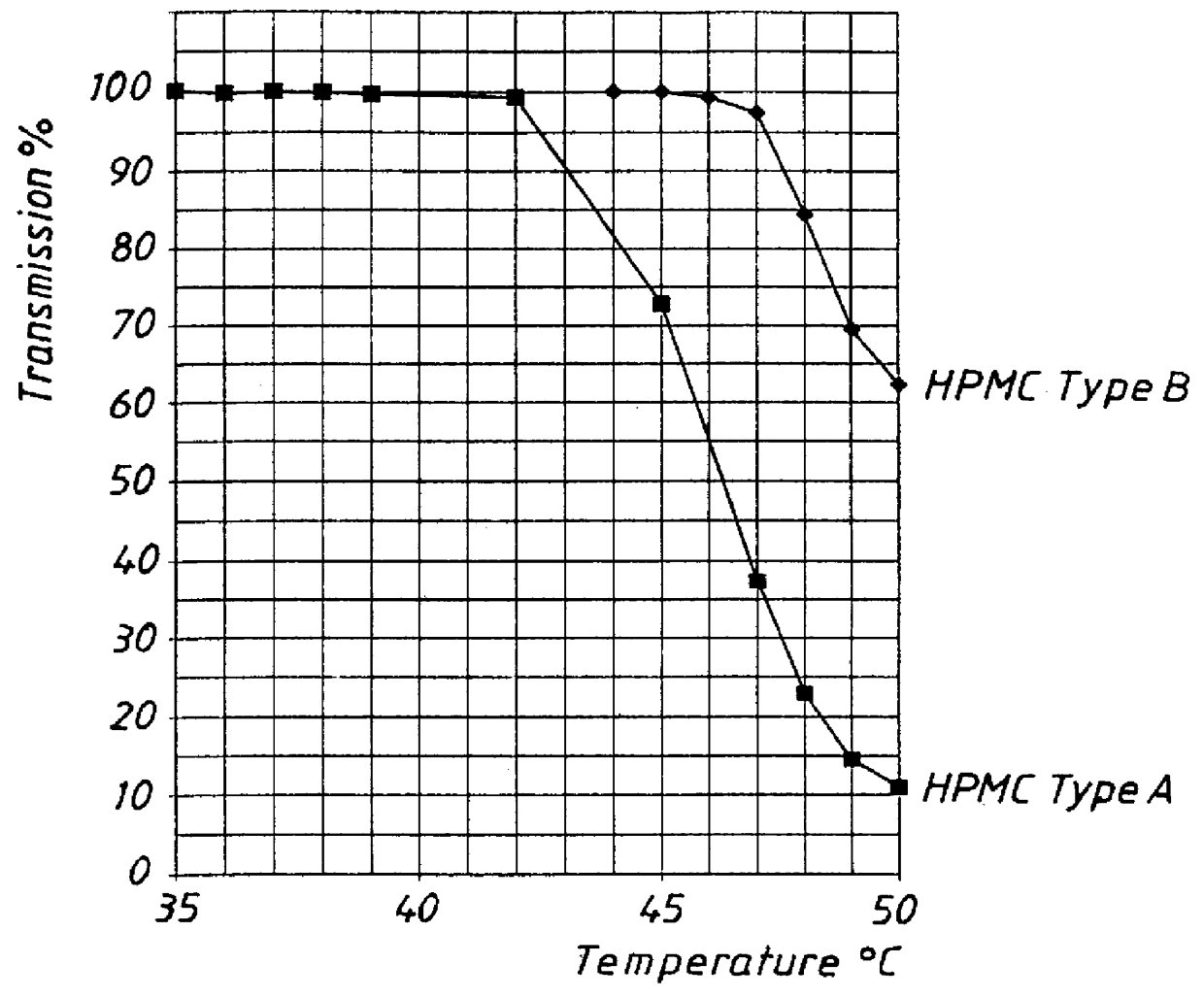
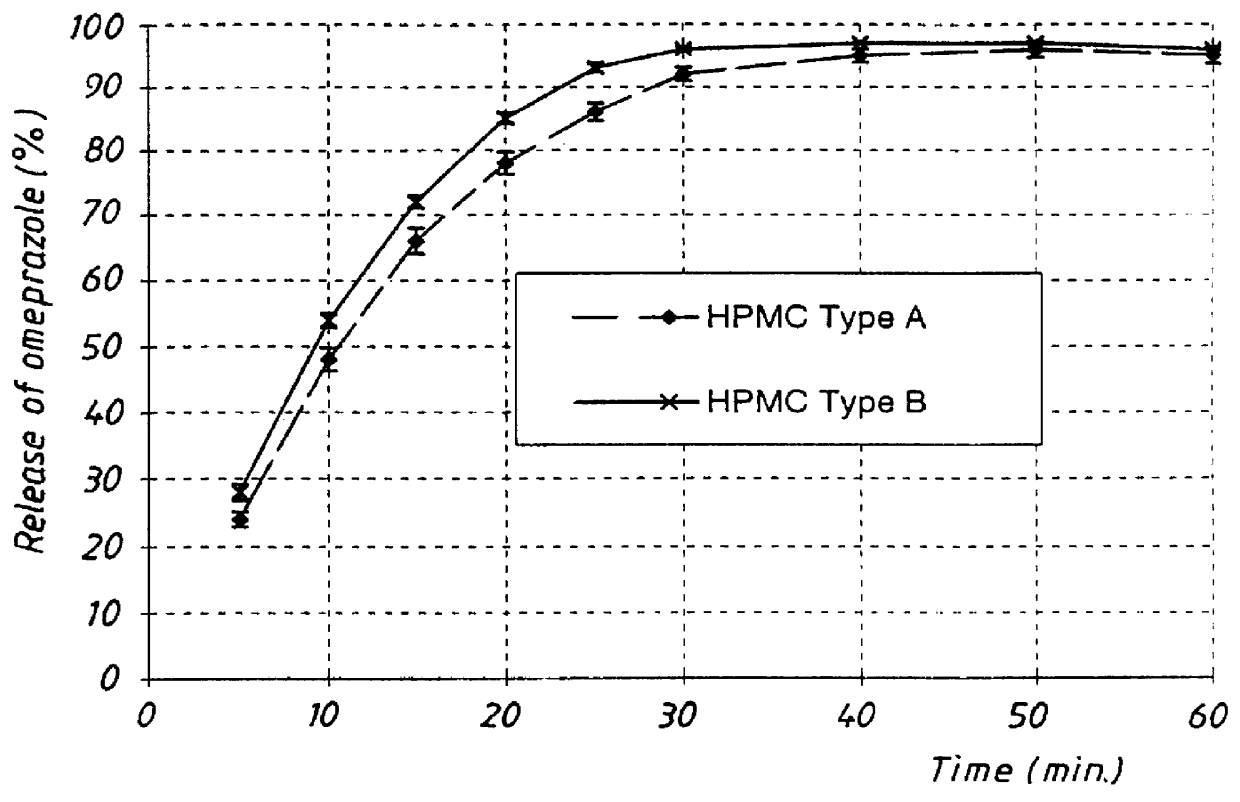
PCT No. PCT / SE98 / 00922 Sec. 371 Date Jun. 8, 1998 Sec. 102(e) Date Jun. 8, 1998 PCT Filed May 18, 1998 PCT Pub. No. WO98 / 53803 PCT Pub. Date Dec. 3, 1998An enteric coated oral pharmaceutical formulation comprising as active ingredient a compound selected from the group of omeprazole, an alkaline salt of omeprazole, the (-)-enantiomer of omeprazole and an alkaline salt of the (-)-enantiomer of omeprazole, wherein the formulation comprises a core material of the active ingredient and optionally an alkaline reacting compound, the active ingredient is in admixture with a pharmaceutically acceptable excipient, such as for instance a binding agent, and on said core material a separating layer and an enteric coating layer. A hydroxypropyl methylcellulose (HPMC) of low viscosity with a specific cloud point is used in the manufacture of pharmaceutical formulations. Furthermore, the application describes the processes for their preparation and the use of the claimed formualtions in medicine.
Owner:ASTRAZENECA AB
Controlled release dosage forms combining immediate release and sustainted release of low-solubility drug
InactiveUS20080299188A1Short biological half-lifePromote absorptionPowder deliveryOrganic active ingredientsSolubilityImmediate release
A controlled release dosage form comprises an immediate release portion and an enteric coated sustained release core.
Owner:PFIZER INC
Enveloping acidifier for feed and preparation method thereof
ActiveCN102106461AHigh total acid contentImprove protectionAnimal feeding stuffAccessory food factorsMaterial consumptionMethyl cellulose
The invention relates to enveloping acidifier and the preparation method thereof. The enveloping acidifier comprises acidifier solid microparticles and an enveloping layer on the surface of the acidifier solid microparticles, wherein the enveloping layer comprises an ethyecellulose (EC) and hydroxypropyl methyl cellulose (HPMC) composite layer and an enteric-coated polyacrylic resin layer. In theinvention, sustained-release coating material with good film forming property and the coating process are adopted, so that excessive enveloping material consumption is avoided, the weight of the acidifier solid microparticles is increased slightly, the large total effective acid of the acidifier is kept, and the acidifier has good sustained-release effect.
Owner:安徽泰格生物技术股份有限公司
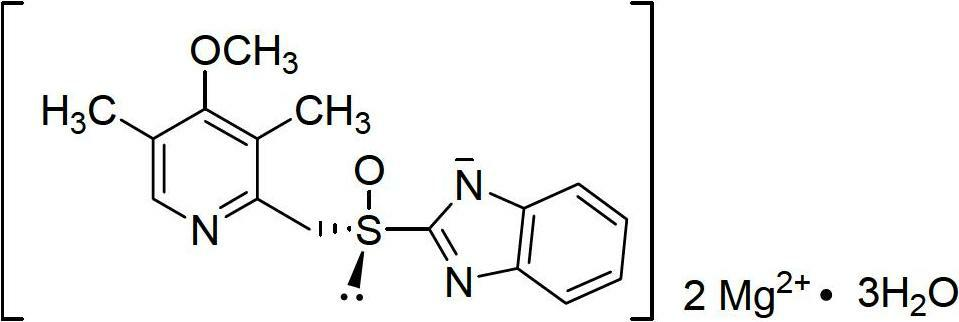
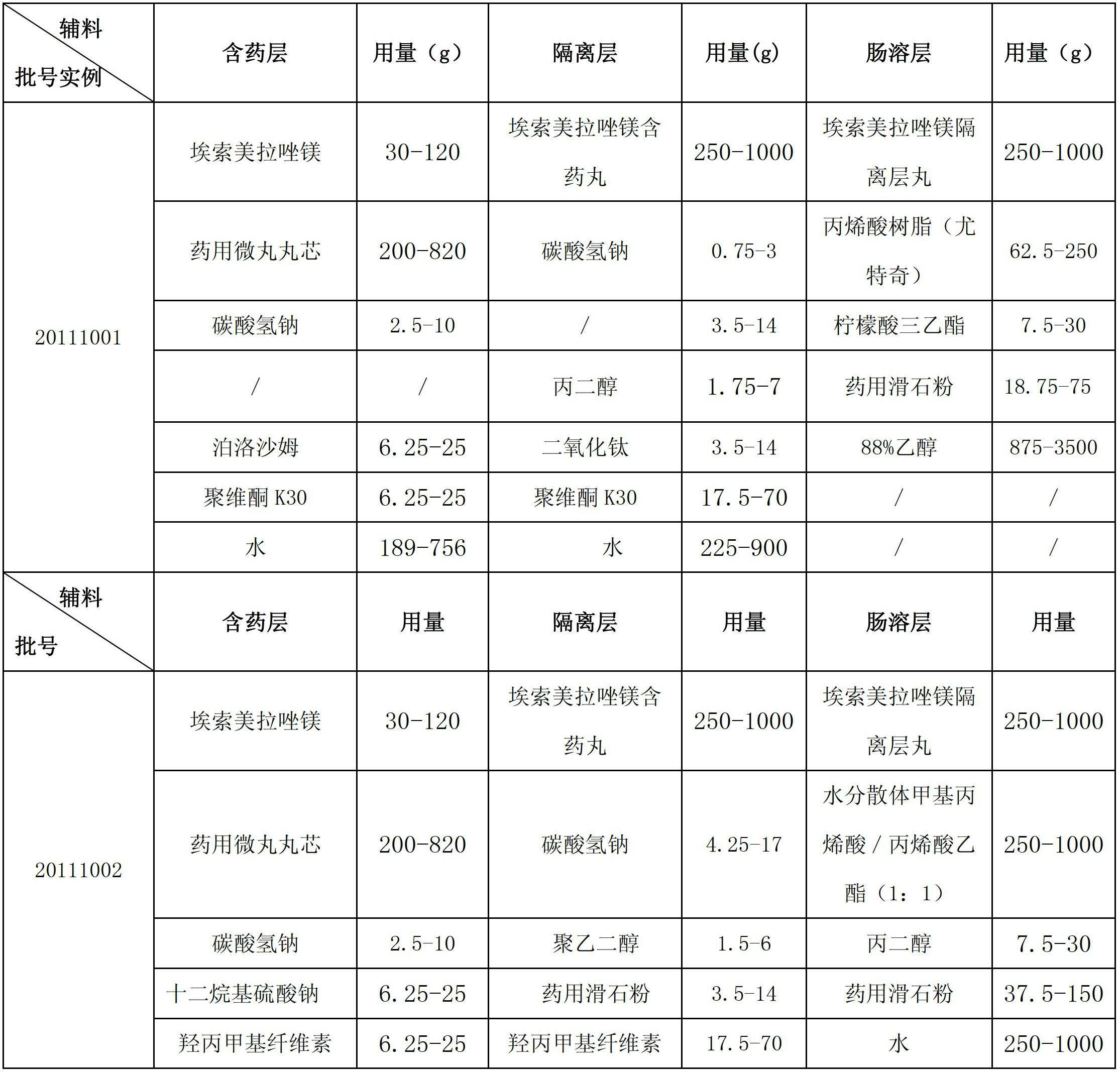
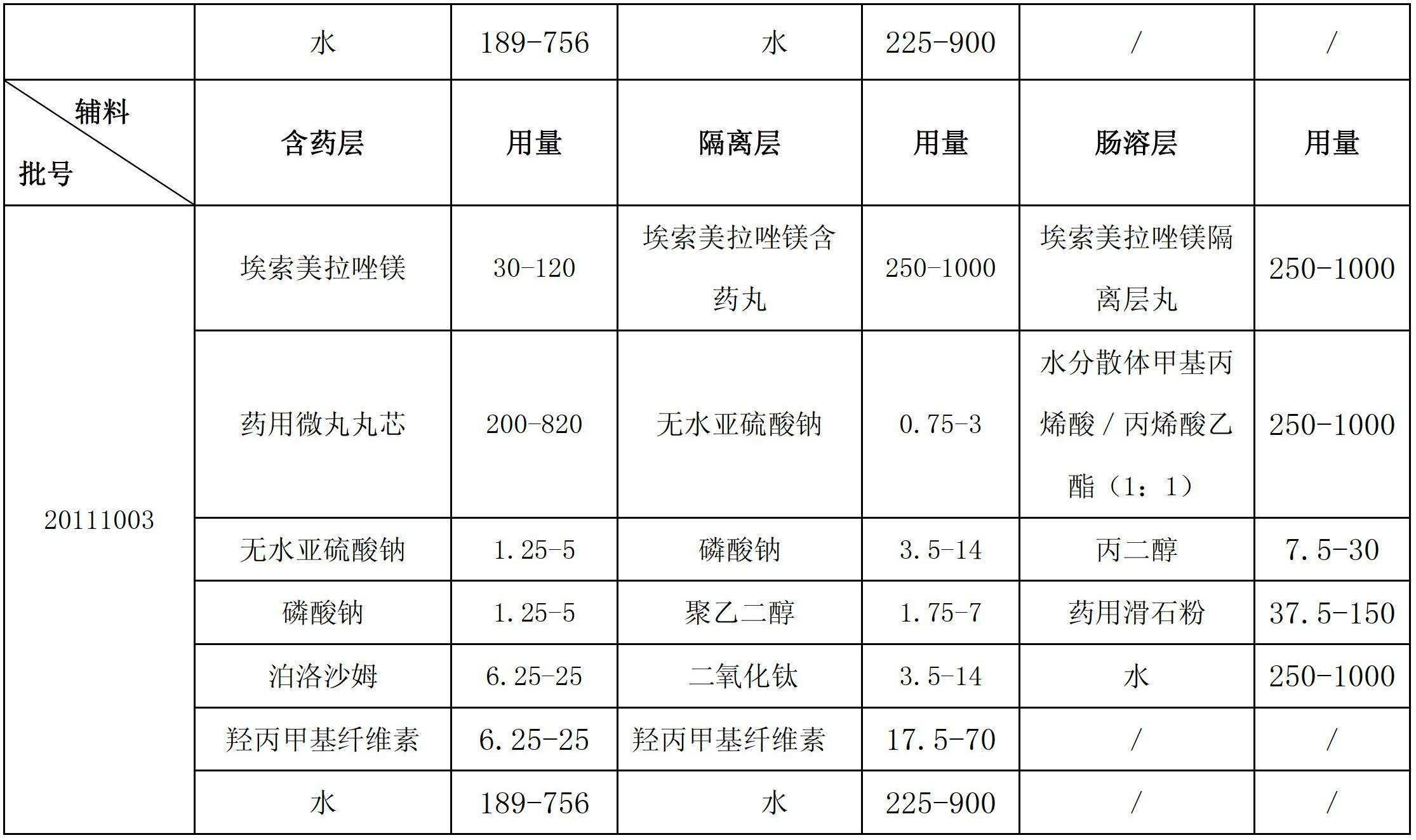
Esomeprazole magnesium enteric-coated pellet and preparation method thereof
ActiveCN102670521AProtect active ingredientsFix stability issuesOrganic active ingredientsDigestive systemMedicineIsolation layer
The invention belongs to the field of pharmacy and relates to a medicinal preparation taking esomeprazole magnesium as an active ingredient, in particular to an esomeprazole magnesium enteric-coated pellet and a preparation method thereof. According to the esomeprazole magnesium enteric-coated pellet, medicines can be rapidly released in intestinal tracts. The esomeprazole magnesium enteric-coated pellet comprises the following structural layers in sequence from inside to outside: a medicine-contained layer, an isolation layer and an enteric-coated layer.
Owner:珠海润都制药股份有限公司
Pharmaceutical compositions and methods for treating or preventing oxalate-related disease
ActiveUS20070178070A1Suitable storage shelf-lifeAvoid any substantial degradationBiocideNervous disorderOral medicationDelivery vehicle
The present invention comprises methods and compositions for the reduction of oxalate in humans, animals and plants. For example, the invention provides methods and compositions for the delivery of one or more oxalate-reducing pharmaceutical compositions to the intestinal tracts of persons and animals. The methods and compositions can be used in treating and preventing oxalate-related conditions. A composition of the invention comprises an oral delivery vehicle comprising an oxalate degrading bacteria, one or more cryopreserving agents and one or more excipients. A composition of the invention is enteric coated and has a suitable shelf-life and acceptable properties to avoid negative impact from gastric fluid when it is orally administered.
Owner:OXTHERA INTPROP
Duloxetine enteric coated tiny pill capsule, and preparation method
ActiveCN1759829AImprove solubilityReduced bioavailabilityNervous disorderGranular deliveryDuloxetineMedicine
An enteric micropill (microsoftgel) of Duluoxiting and its preparing process are disclosed. Said micropill is composed of an empty core pill and a coated external layer consisting of a principal medicine layer, an isolating layer and an enteric layer. It features that its principal medicine can be quickly released only in small intestine.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and composition and formulation thereof
ActiveCN101537020APromote growth and reproductionStrong antagonistic effectOrganic active ingredientsBacteria material medical ingredientsBacillus licheniformisSynbiotics
The invention relates to a synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and a composite and a formulation thereof, being characterized in that the synbiotics comprises bacillus licheniformis medical live bacterial powder and the oligosaccharide class prebiotics, wherein the bacillus licheniformis medical live bacterial powder contains live bacteria about 30 billion / gram; the weight ratio of the bacillus licheniformis medical live bacterial powder to the oligosaccharide class prebiotics is 1:1 to 76; the weight ratio of the medical live bacterial powder of the composite, the oligosaccharide class prebiotics and auxiliary materials is as follows: 1.25-5 percent of the bacillus licheniformis medical live bacterial powder, 5-95 percent of the oligosaccharide class prebiotics, 20-50 percent of preferable oligosaccharide class prebiotics, 30-65 percent of diluting agents, 1-20 percent of bonding agents, 1-15 percent of disintegrating agents, 0.1-3 percent of lubricating agents, 1-10 percent of coating agents, 0.01-0.1 percent of flavoring agents, 0.0001-0.001 percent of coloring agents, and 0.1-15 percent of suspending agents. The synbiotics composite is prepared into oral common tablets, oral cavity disintegration tablets, dispersing tablets, enteric-coated tablets, granular formulation, enteric-coated granular formulation, capsules, enteric-coated capsules, dry supensoid agents and external tablets according to a conventional process; and an in vitro test shows that each formulation can promote the growth and the reproduction of the synbiotics of bacillus licheniformis, restrain the growth of harmful bacteria, enhance the immunizing power of parasitifers, reduce diarrhea and enhance the health care.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
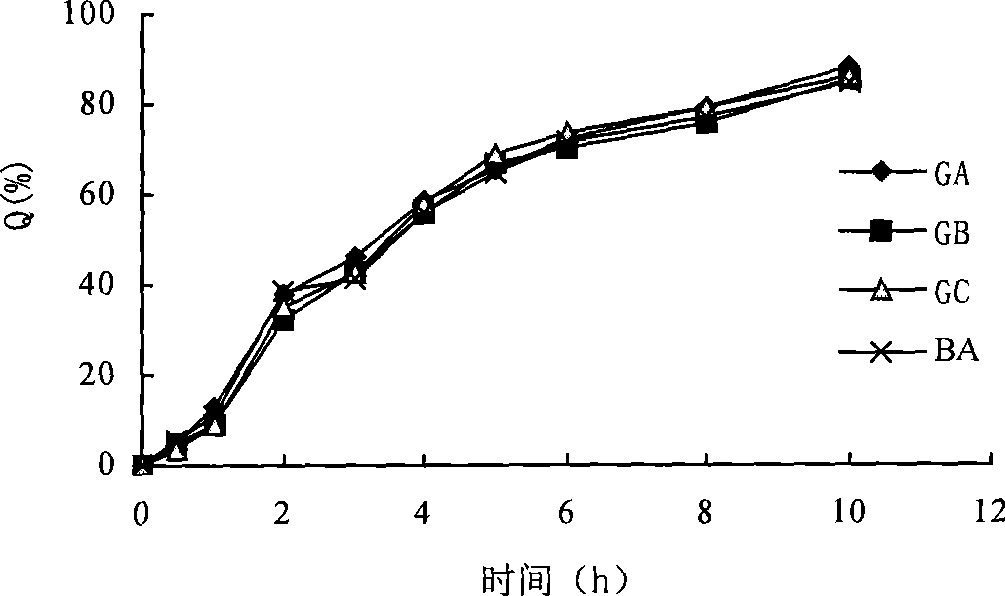
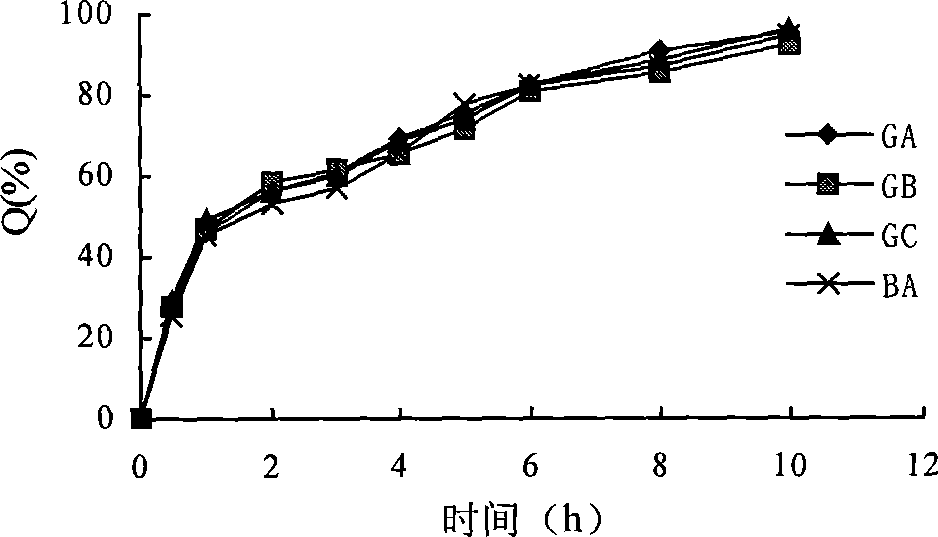
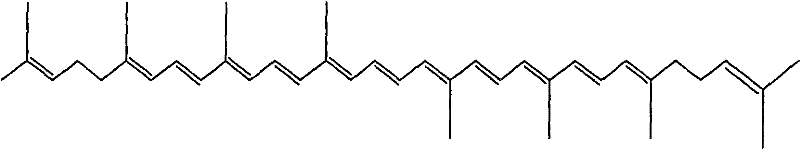
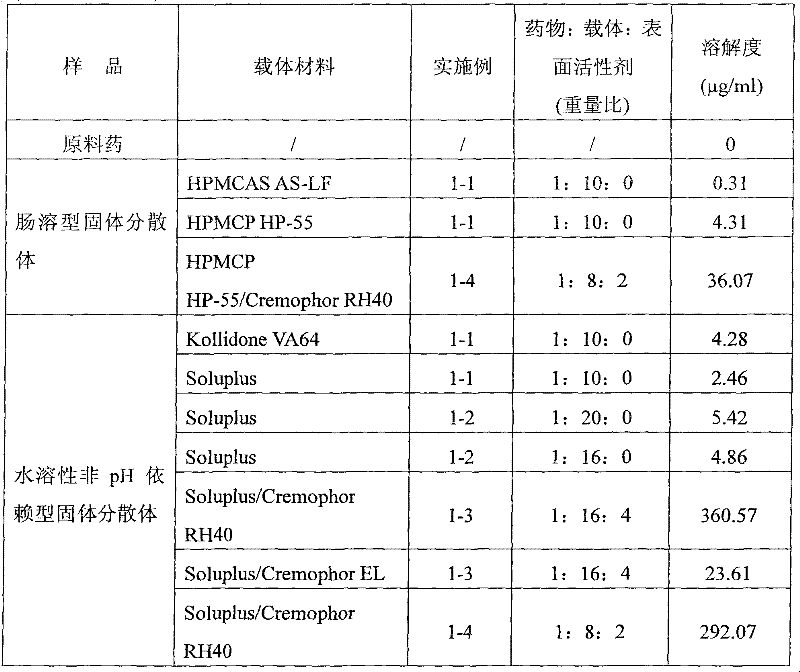
Enteric solid preparation containing lycopene, resveratrol or melatonin and preparation method of enteric solid preparation
The invention relates to the field of medical preparations, in particular to an enteric solid preparation containing lycopene, resveratrol or melatonin and a preparation method of the enteric solid preparation. The enteric solid preparation comprises one or more of lycopene, resveratrol and melatonin as an active ingredient, water-soluble and / or enteric carrier adjuvants or other pharmaceutic adjuvants. The water-soluble carrier adjuvants can be used as water-soluble solid dispersoid carriers; and the enteric carrier adjuvants are enteric polymers and can be used as enteric solid dispersoid carriers or enteric coating film materials. The lycopene, the resveratrol and the melatonin of the enteric solid preparation have favorable dissolubility in the intestinal tract, so that the medicament, namely, the enteric solid preparation, can be rapidly dissolved and released in the intestinal tract, and thus absorption and bioavailability of the lycopene, the resveratrol and the melatonin are increased. The enteric solid preparation containing the lycopene, the resveratrol and the melatonin can be suitable for application and industrial production of oral preparations, such as tablets, particles, pellets, capsules, enteric capsules, enteric coating tablets, enteric coating pellets, enteric coating particles and the like.
Owner:SINOTHERAPEUTICS
Lansoprazole orally disintegrating tablets
InactiveUS20070141151A1Dissolve fastPrevent degradationBiocidePill deliveryLansoprazoleOrally disintegrating tablet
The invention provides orally disintegrating tablets that readily disintegrates in the mouth, releasing enteric coated drug sub-tablets.
Owner:TEVA PHARM USA INC
Enteric-coated pellet preparation of proton pump inhibitor and preparation method thereof
ActiveCN102119927AEvenly dispersedAvoid degradationOrganic active ingredientsDigestive systemWater solubleBioavailability
The invention relate to an enteric-coated pellet preparation of proton pump inhibitor and a preparation method thereof. The enteric-coated pellet preparation of proton pump inhibitor provided by the invention is composed of a blank pellet core, a drug-loaded layer, isolating layers (I) and (II) and an enteric-coated layer, wherein both the drug-loaded layer and the isolating layer (I) contain water soluble inorganic bases, and the base used in the drug-loaded layer contains sodium hydroxide and another water soluble inorganic base which can form buffer with sodium hydroxide in a water solution and the pH of which is in an alkaline environment of 11-12 (excluding 11). The enteric-coated pellet of proton pump inhibitor provided by the invention successfully solves the technical problems of the existing enteric-coated pellet preparation of proton pump inhibitor, and is a superior preparation with high drug loading efficiency, good anti-acid effect, high release rate, good repeatability, high bioavailability and good stability.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Enteric formulations of proanthocyanidin polymer antidiarrheal compositions
InactiveUS7323195B2Improve compression performanceReduce weightAntibacterial agentsBiocideSecretory diarrheaPharmaceutical formulation
Pharmaceutical compositions containing a proanthocyanidin polymer composition which are useful for the treatment and prevention of secretory diarrhea are provided. The invention specifically relates to pharmaceutical formulations of a proanthocyanidin polymer composition which has been isolated from a Croton spp. or a Calophyllum spp. In particular, the invention relates to a formulation of a proanthocyanidin polymer composition which protects the composition from the effects of stomach acid after oral administration, particularly to those formulations which are enteric coated. The invention also relates to methods of producing a directly compressible proanthocyanidin polymer composition, as well as compositions containing the directly compressible proanthocyanidin polymer composition.
Owner:NAPO PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com