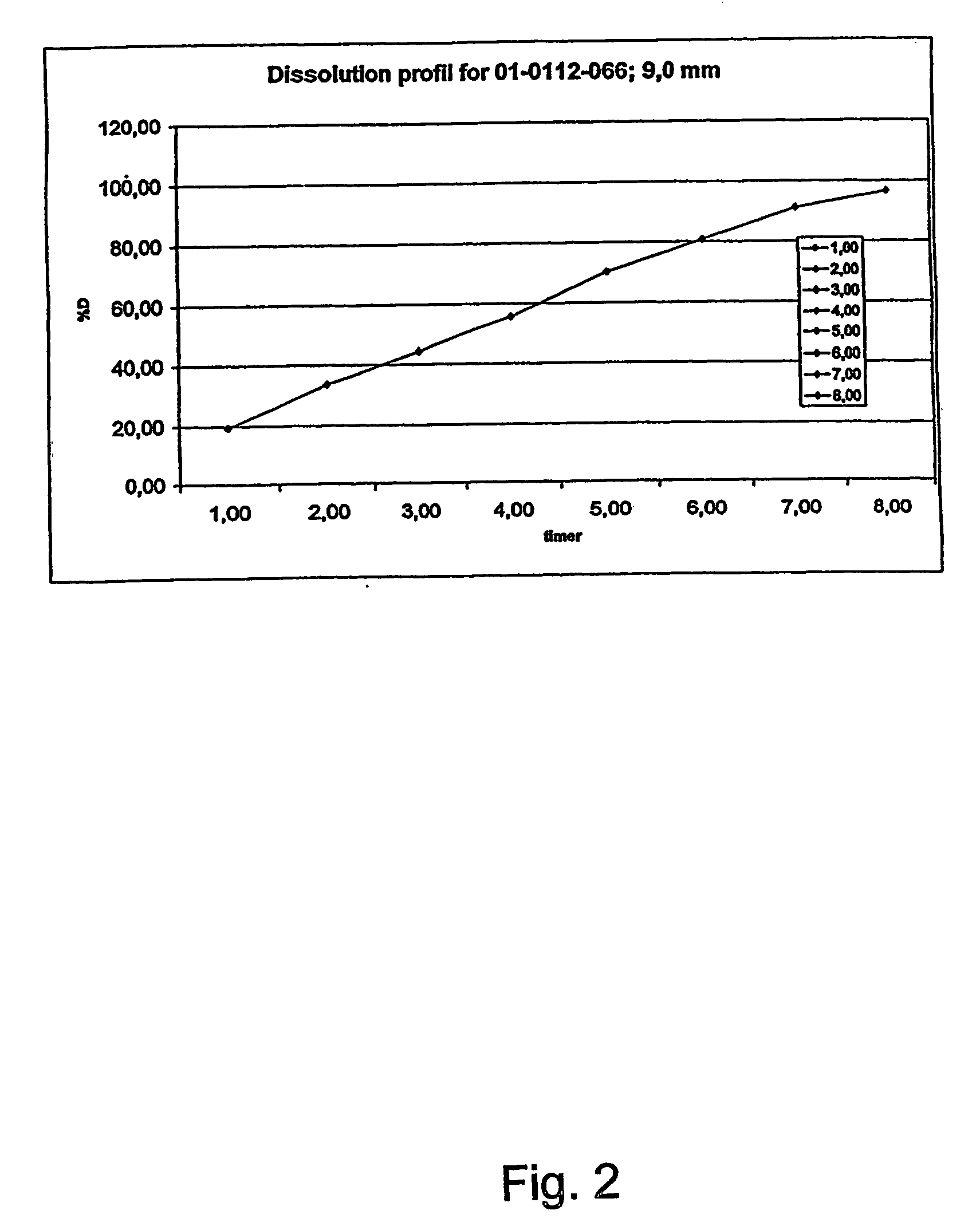
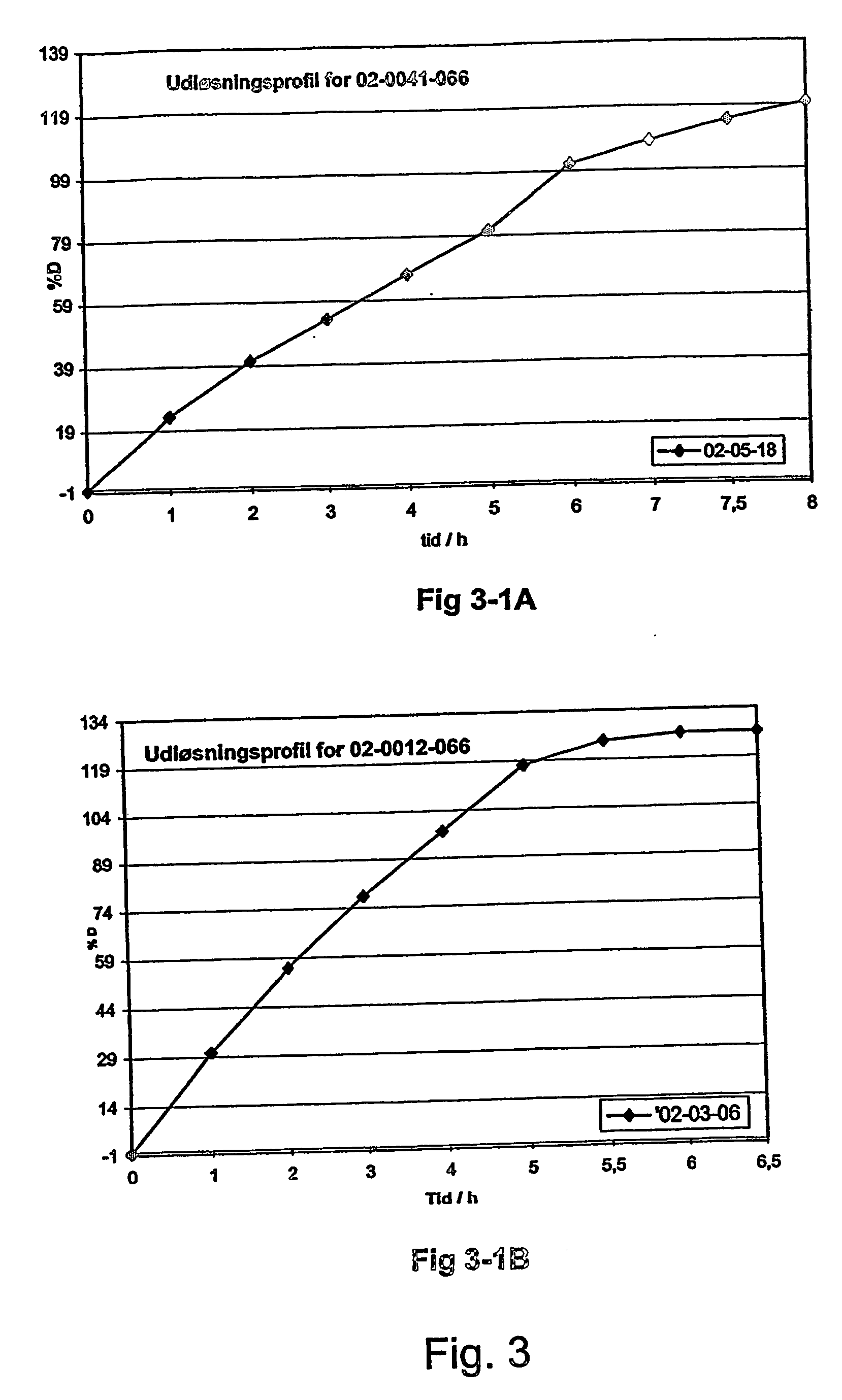
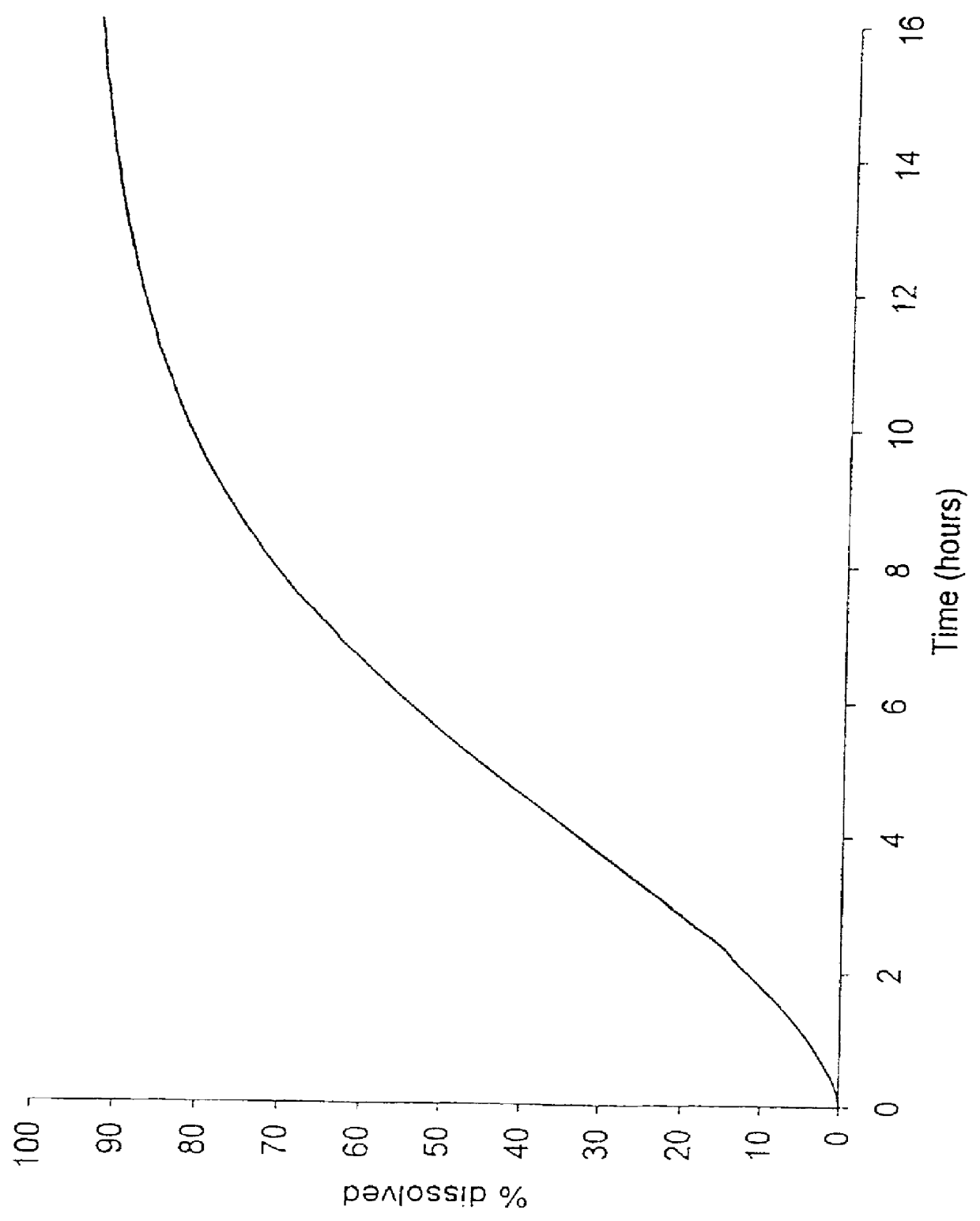
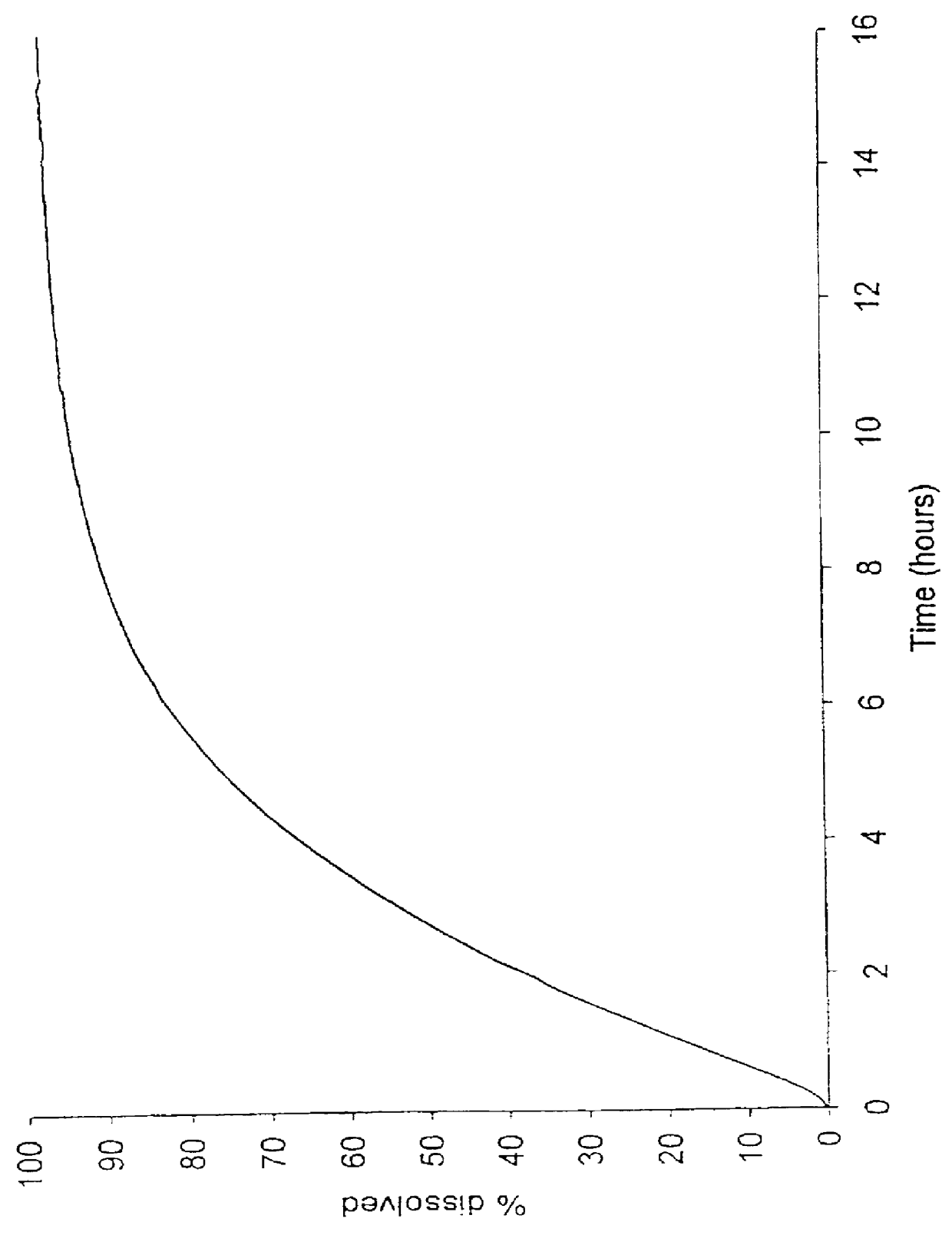
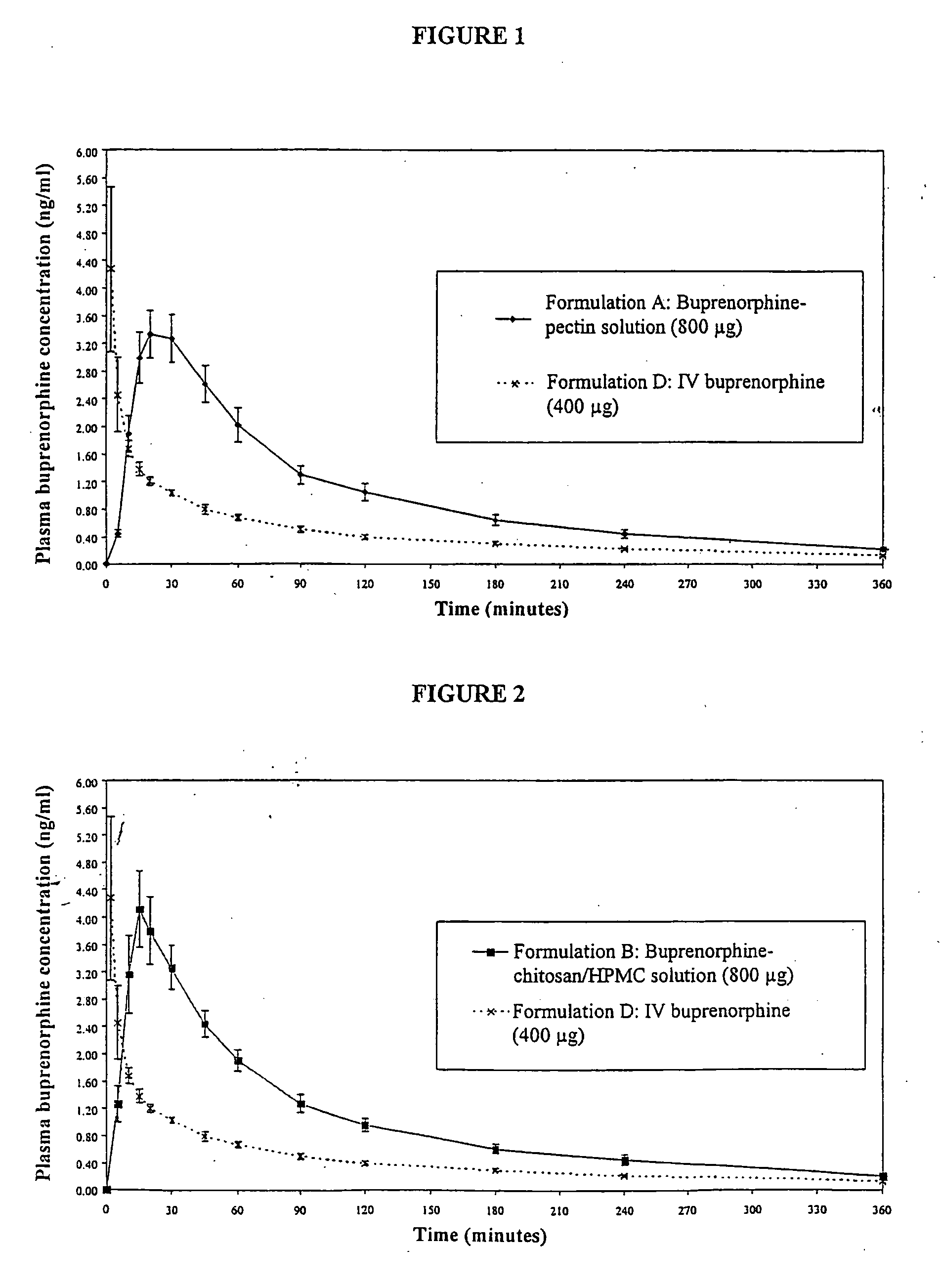
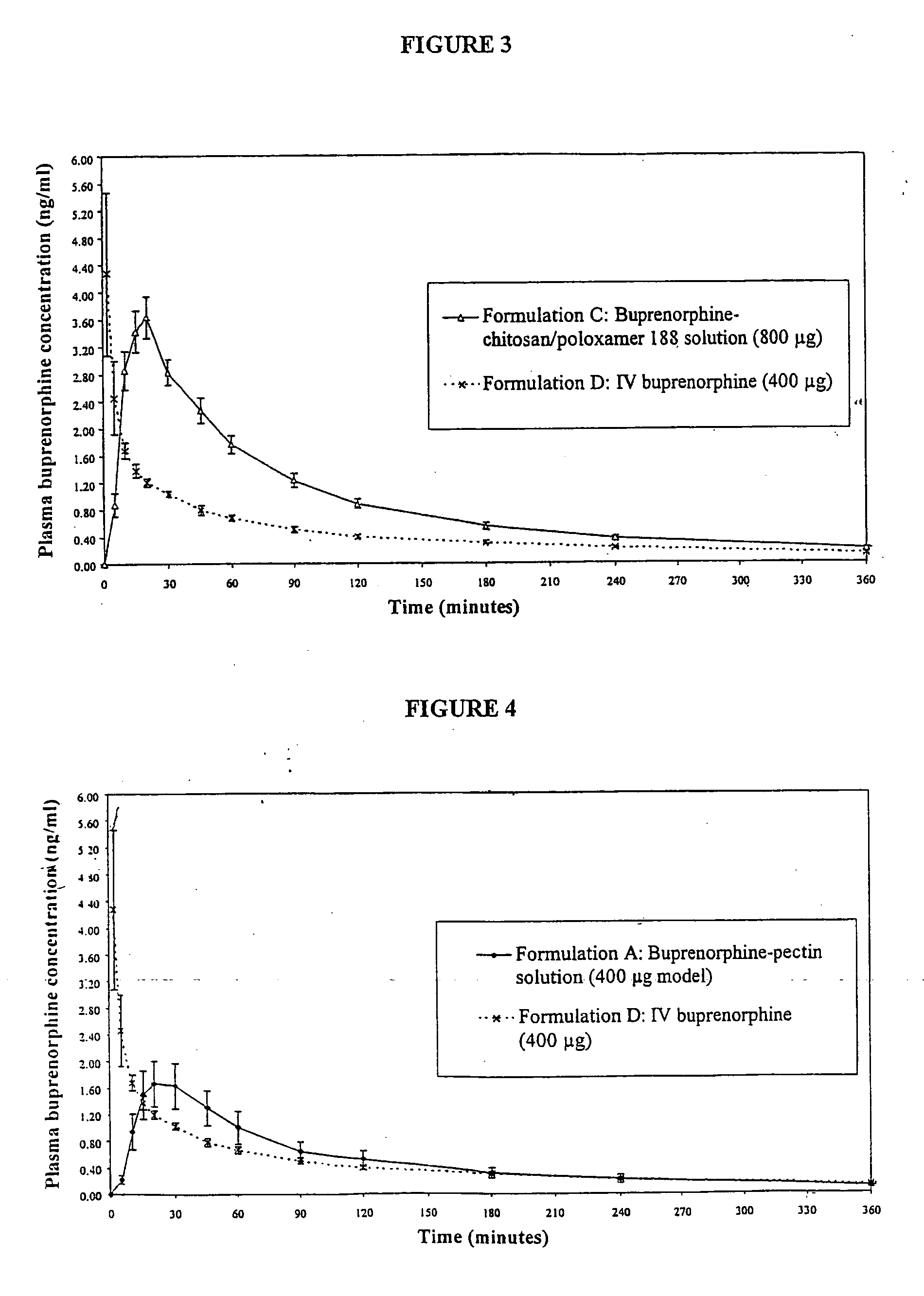
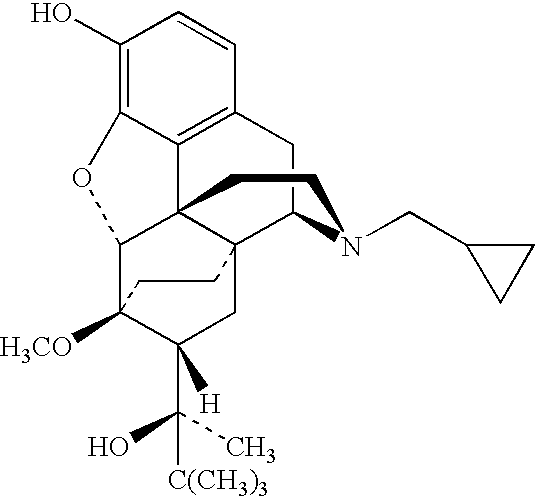
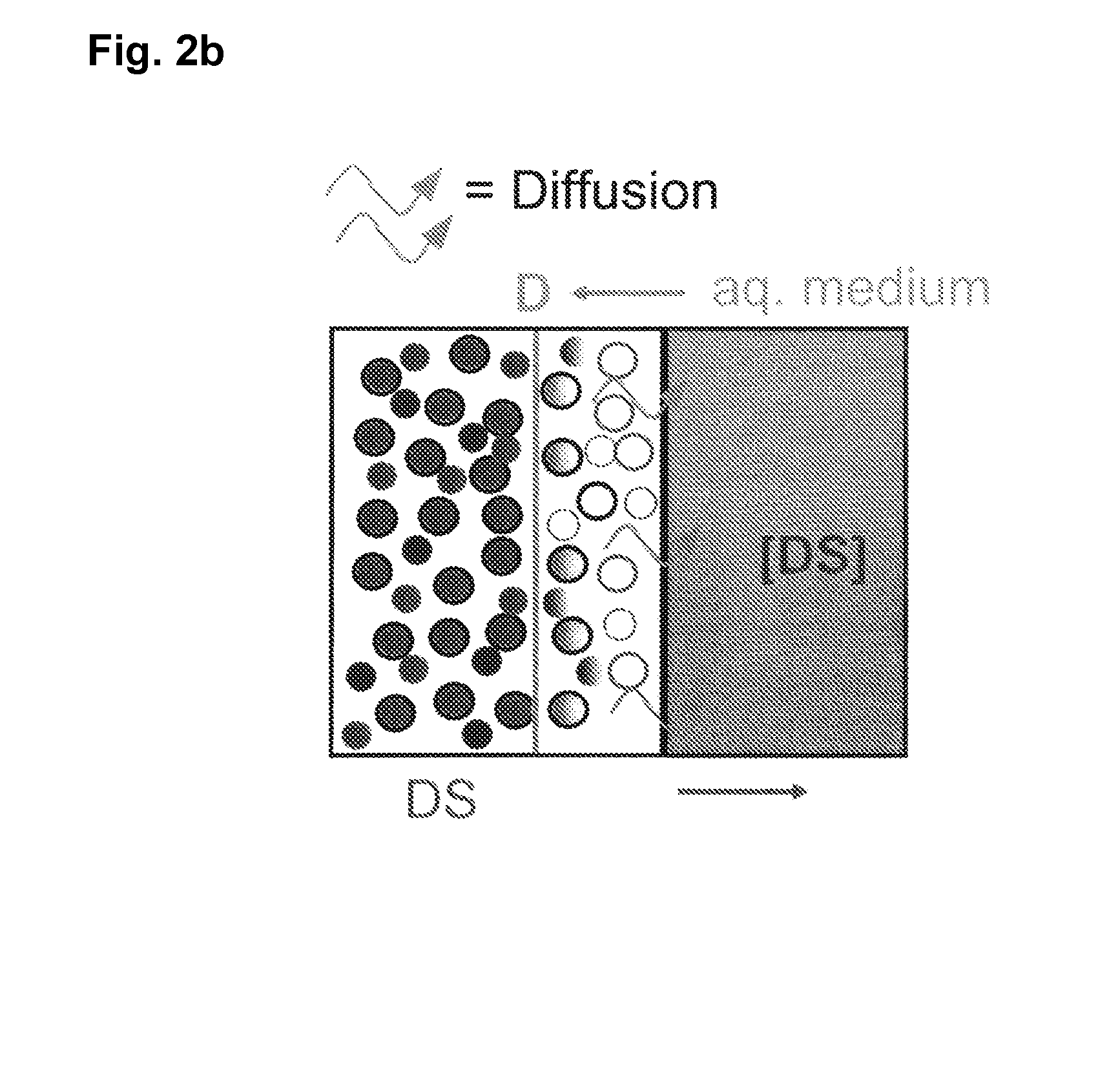
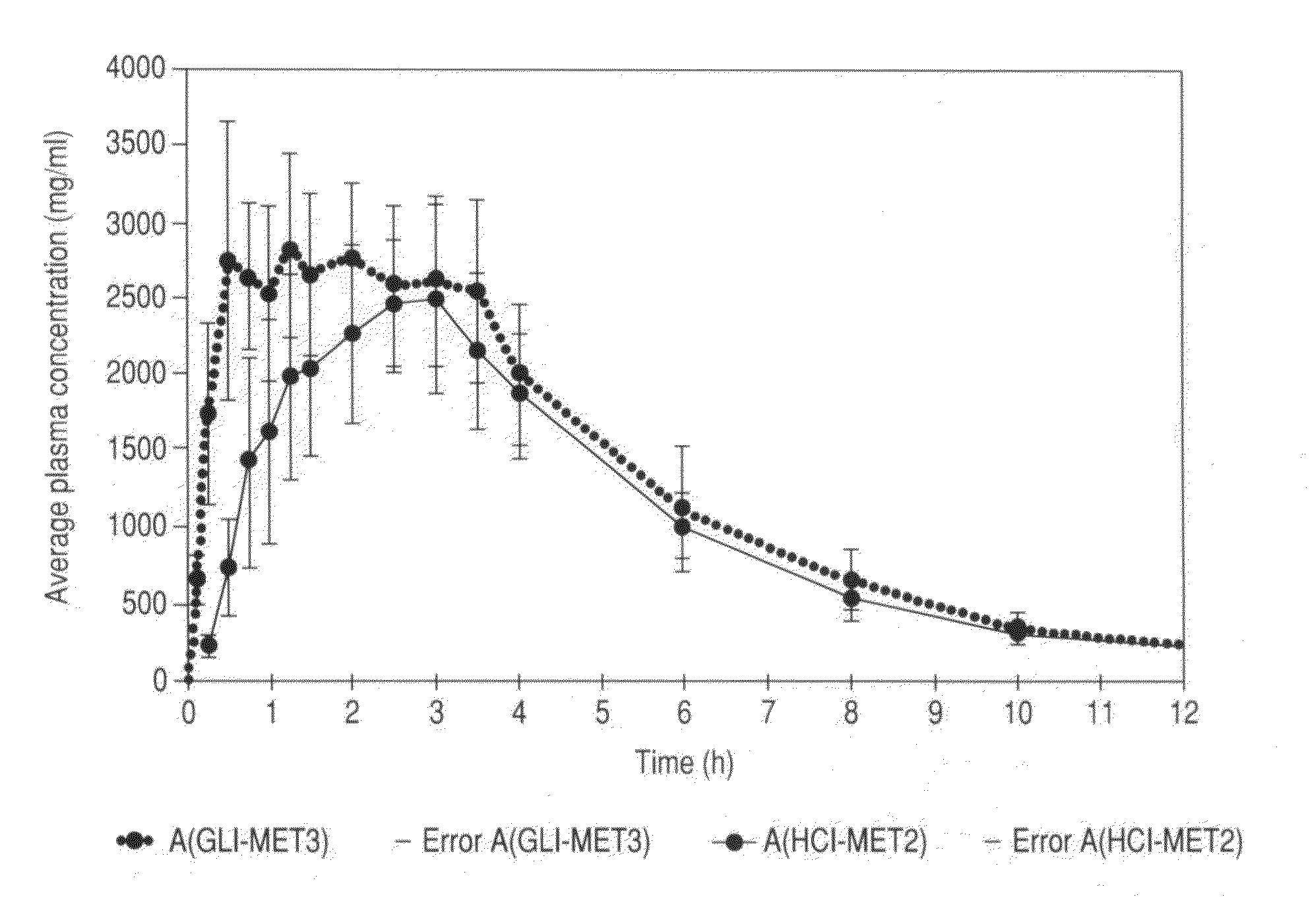
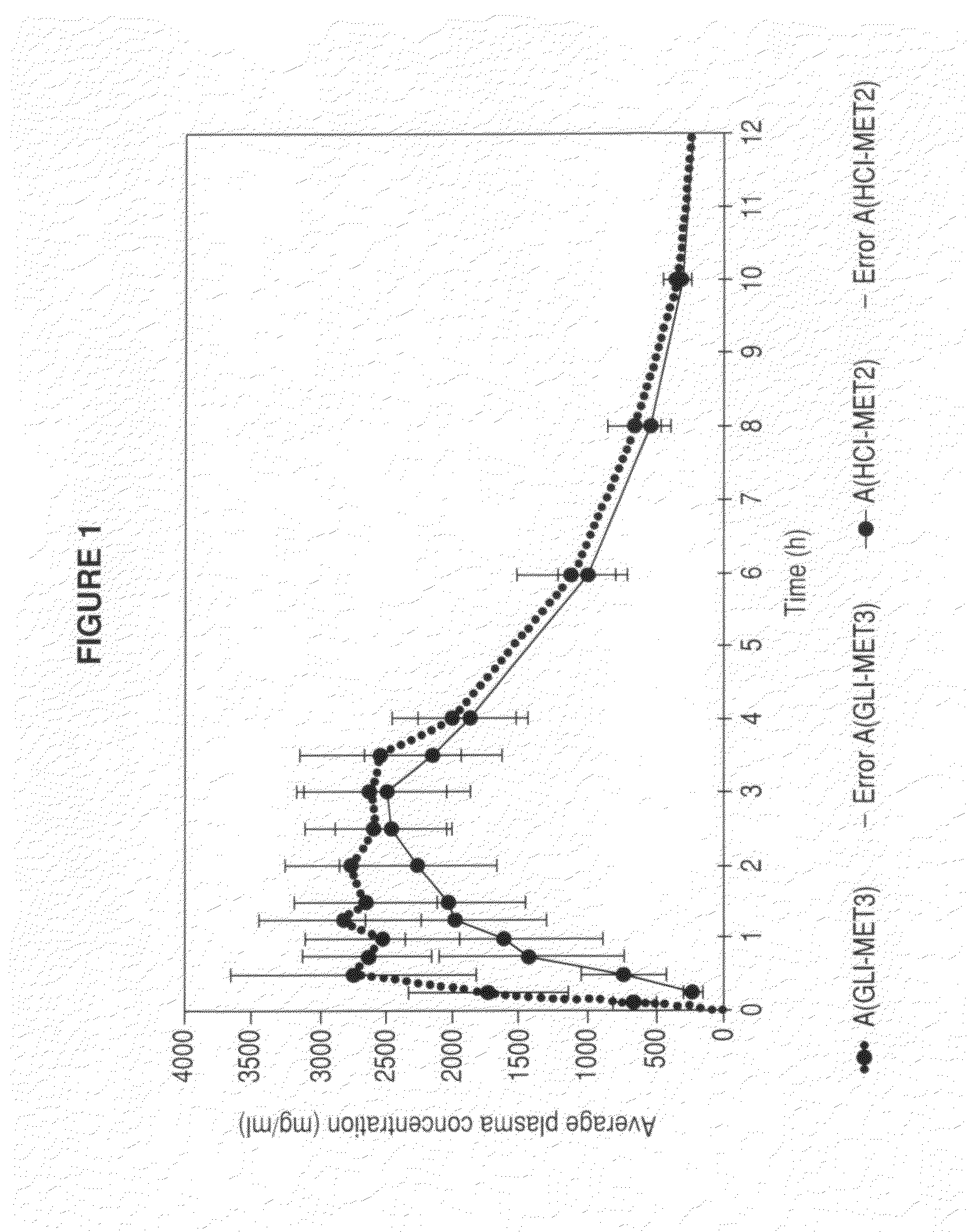
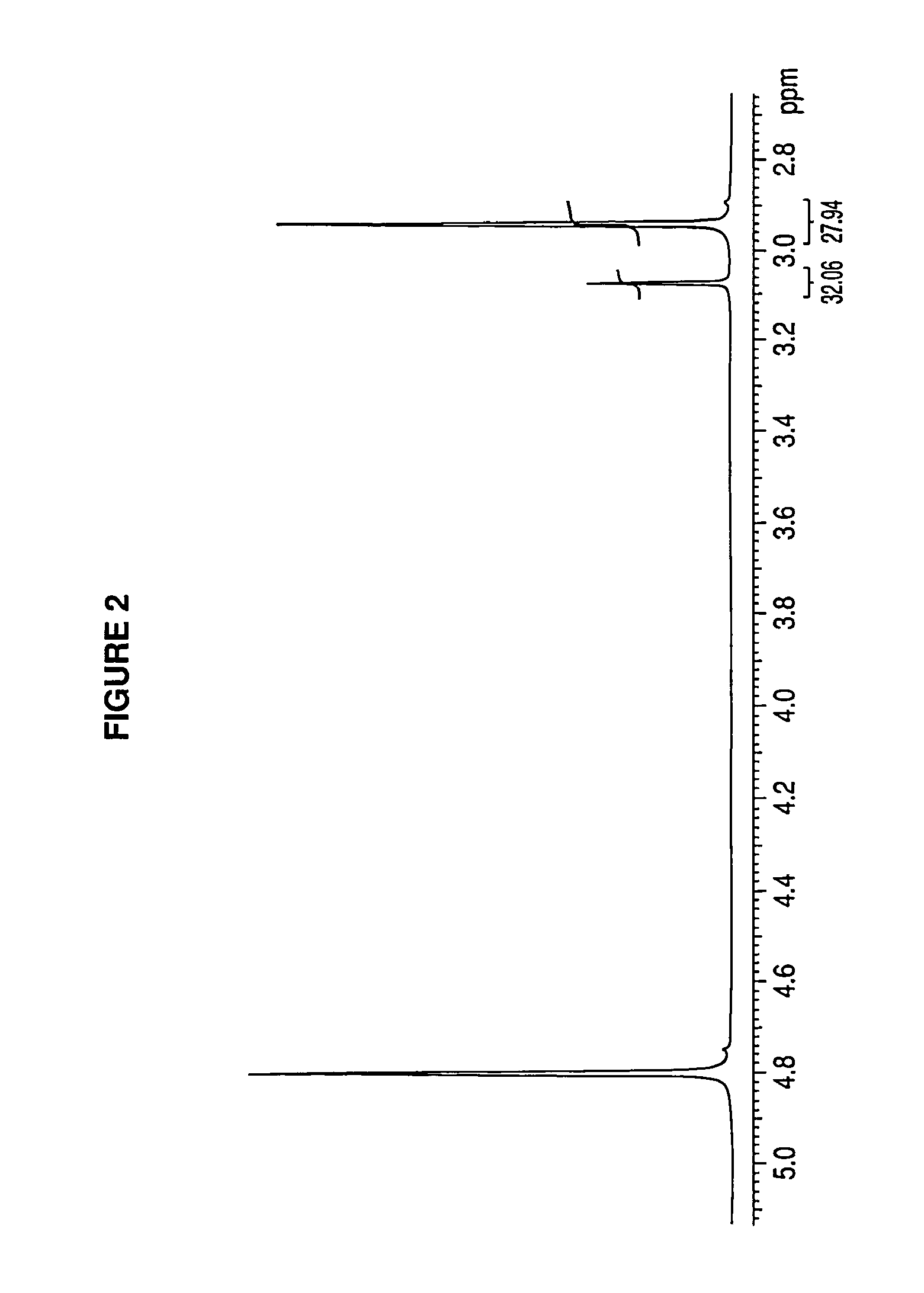
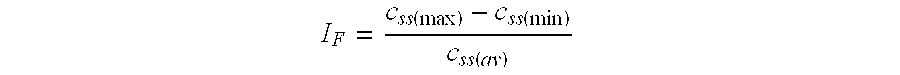Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
703 results about "Plasma concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
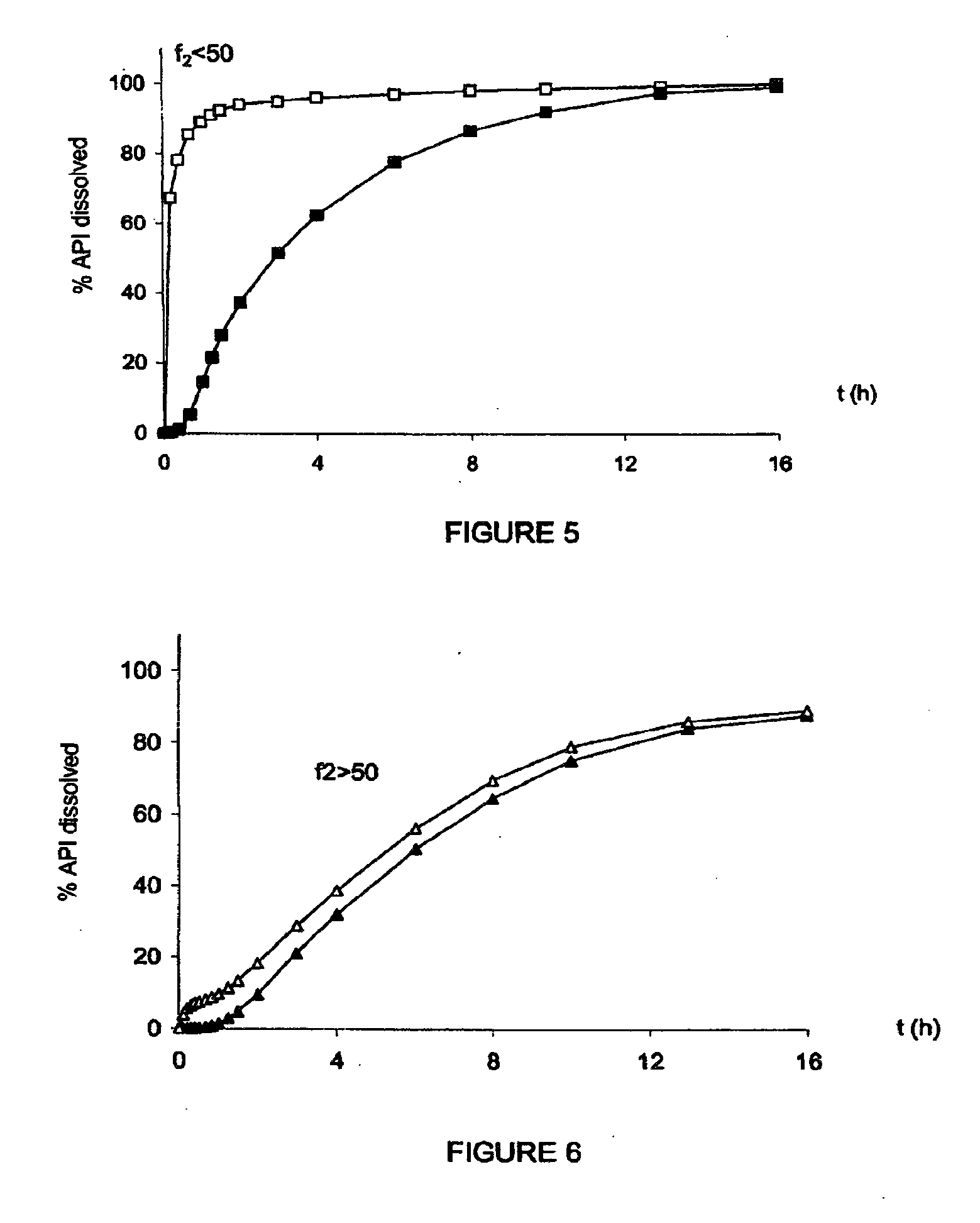
Plasma concentration is a measure of how much of a compound is present in a sample of plasma. This can be important information for the diagnosis, treatment, and management of disease. Lab testing is available at many facilities to provide quick plasma concentration data which may be needed in patient monitoring.
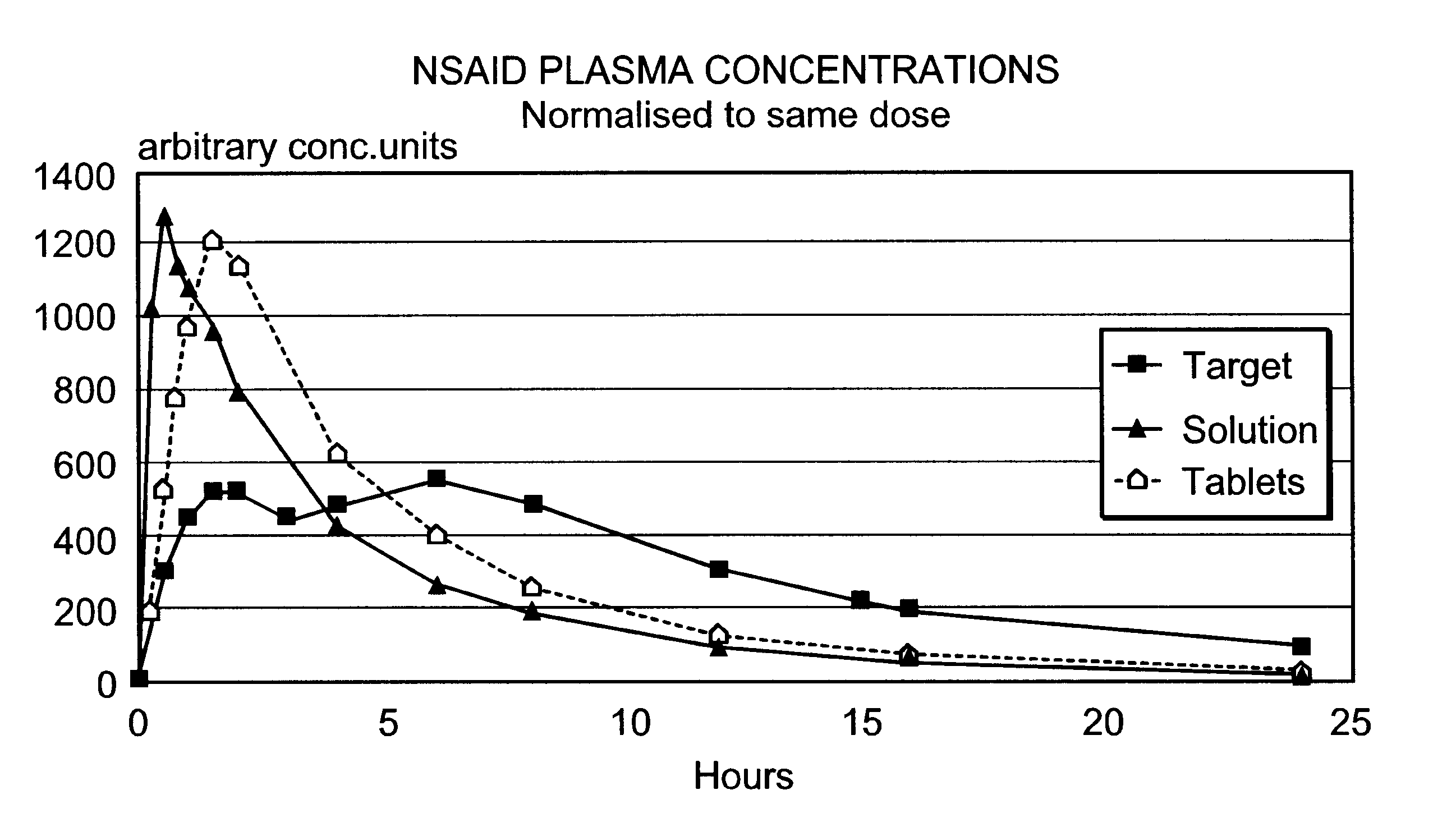
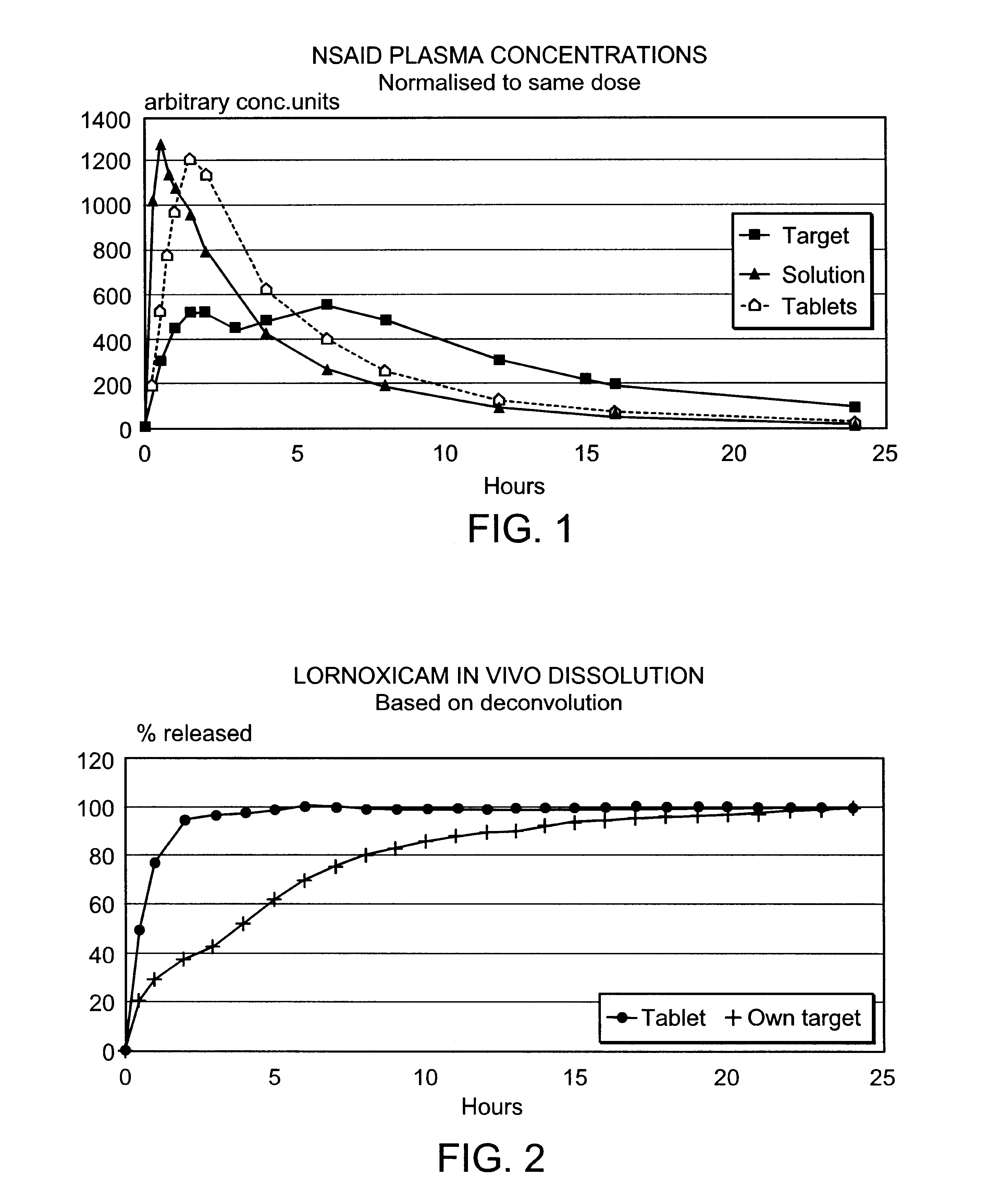
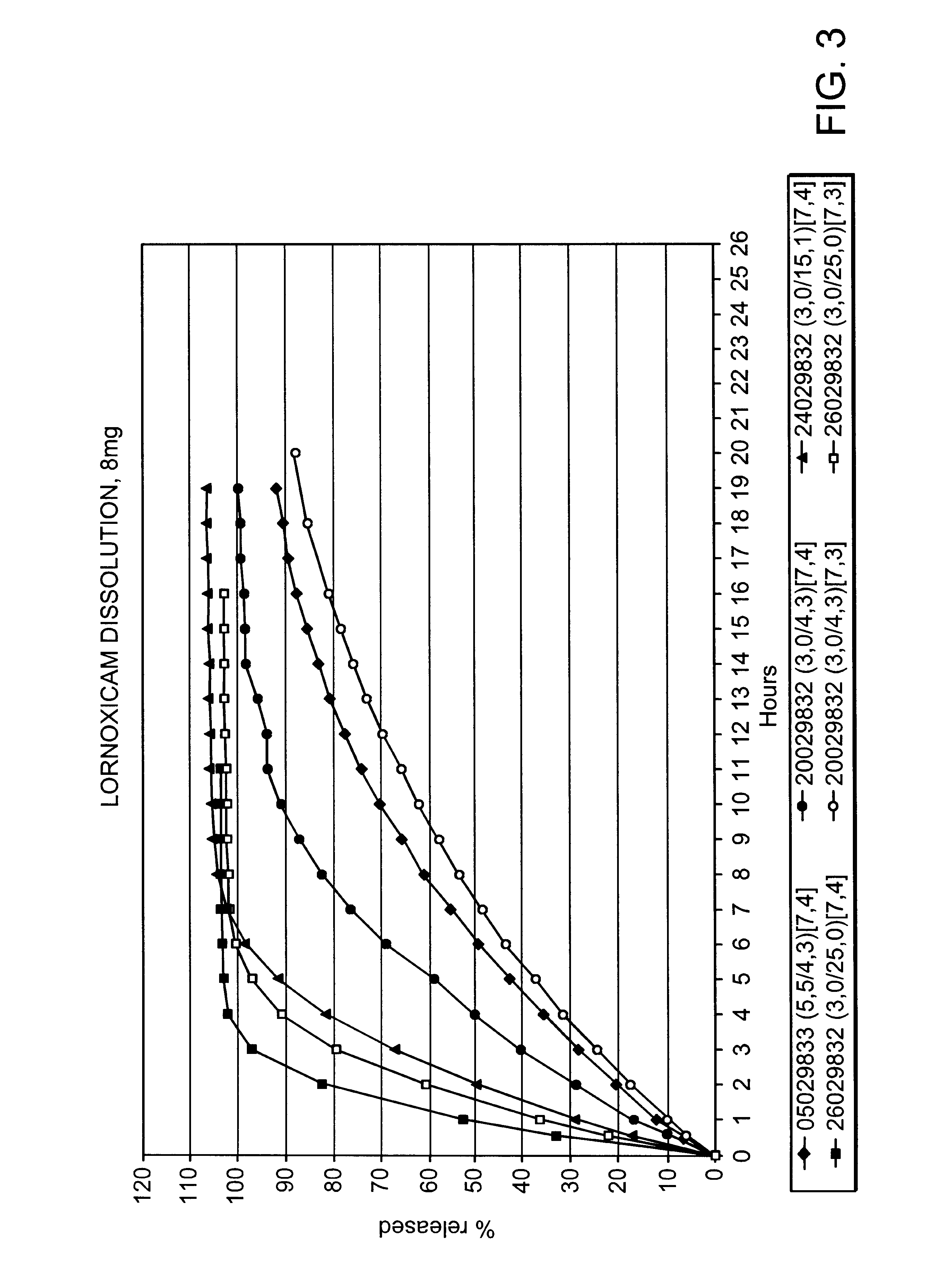
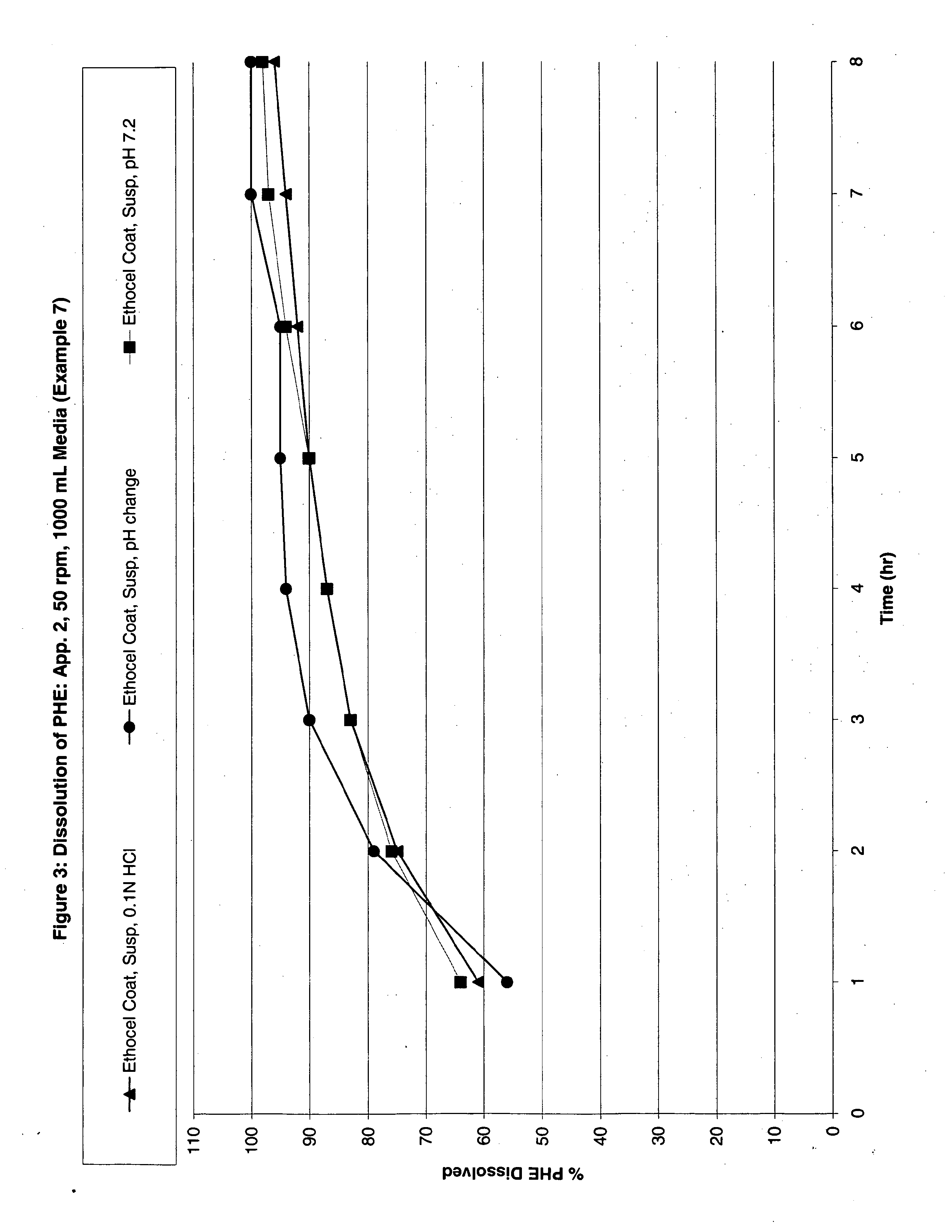
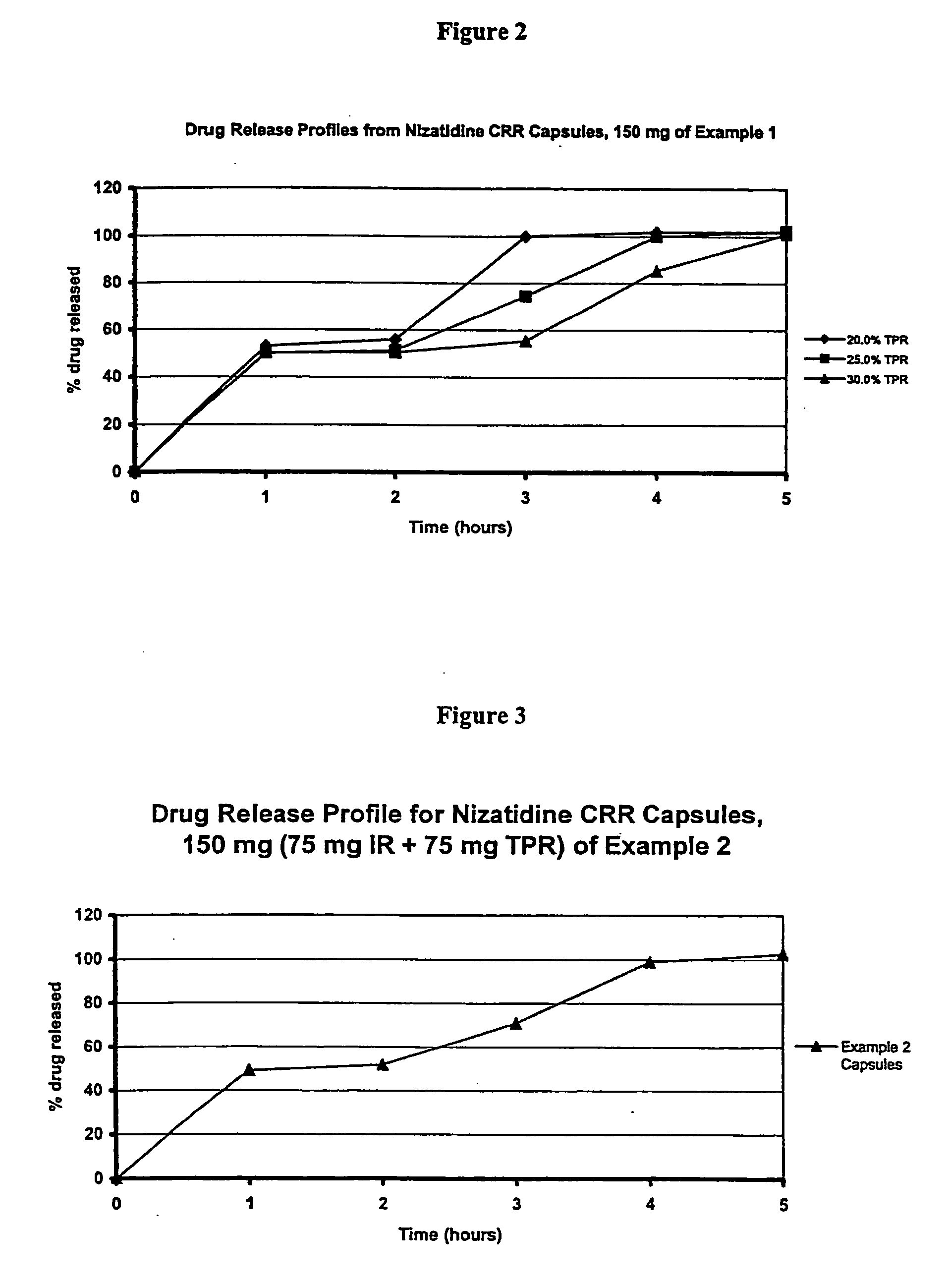
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
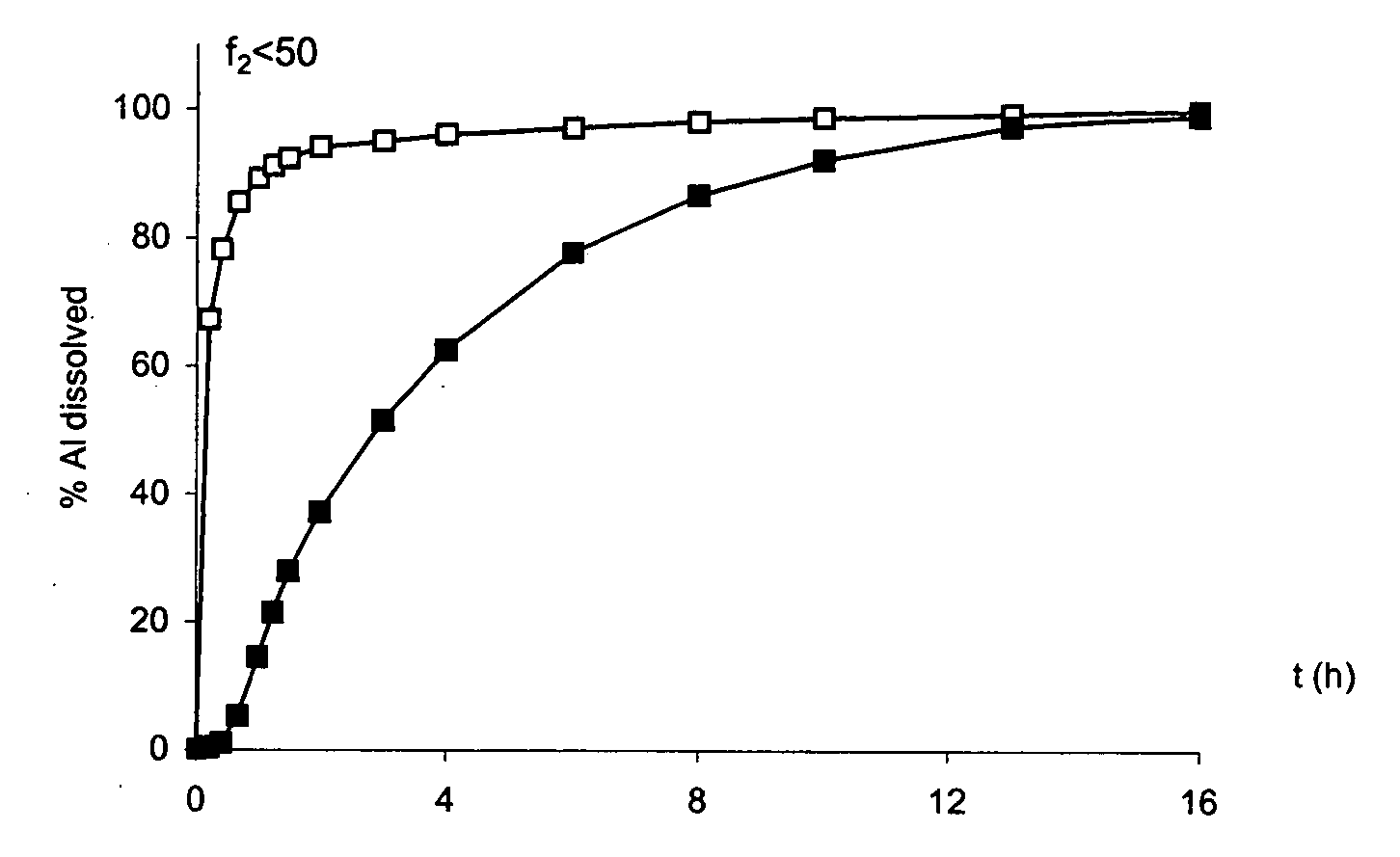
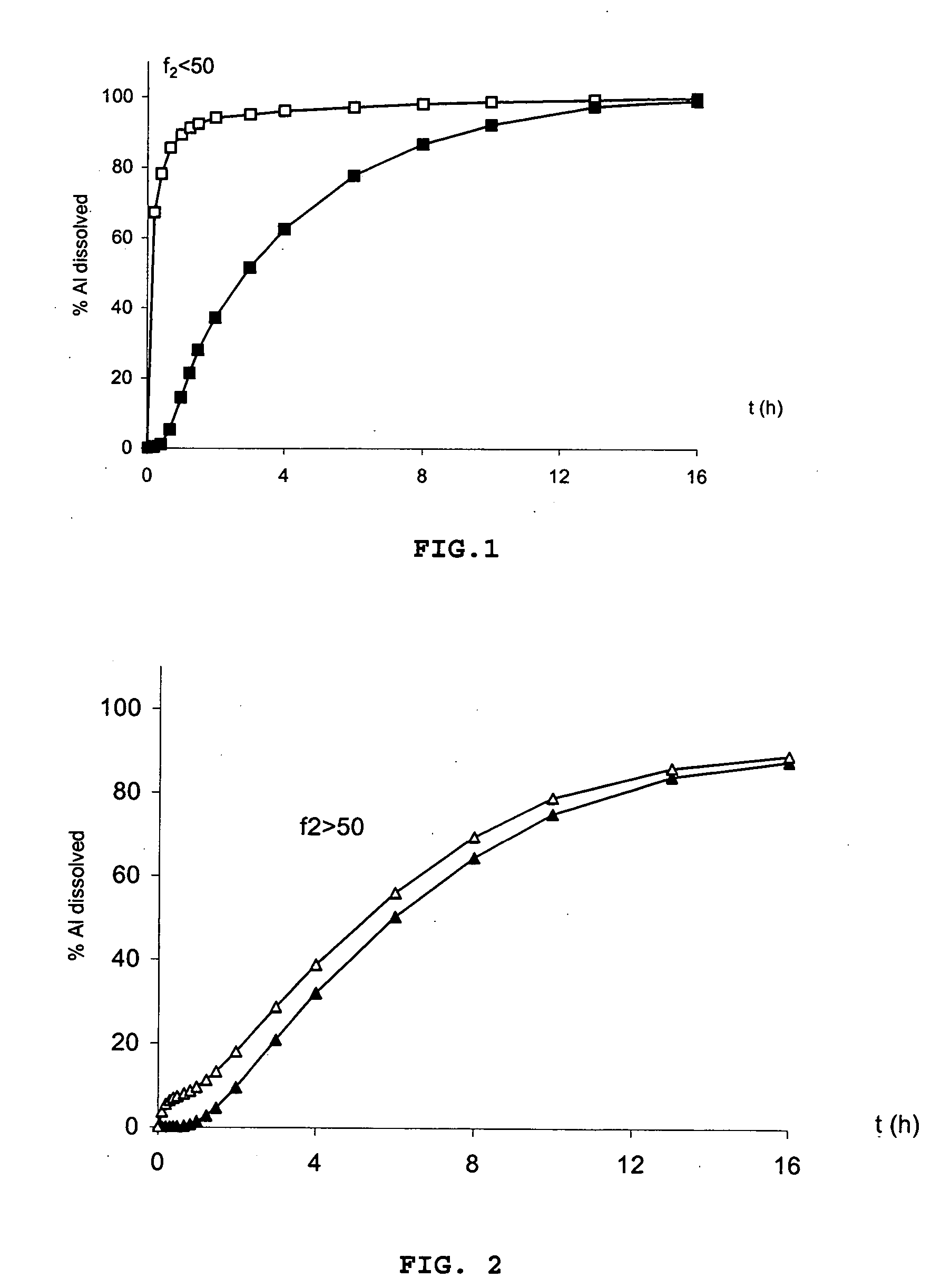
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
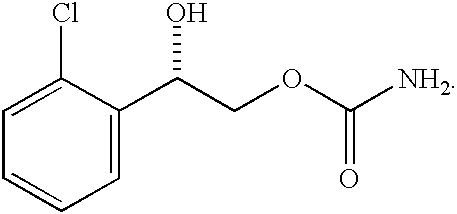
Phenylepherine containing dosage form
ActiveUS20060057205A1Good treatment effectRelieve symptomsBiocidePill deliveryNorphenylephrineBlood plasma
A pharmaceutical dosage form which comprises phenylepherine or a pharmaceutically acceptable salt thereof and a second drug. The dosage form provides a plasma concentration within the therapeutic range of the second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of phenylepherine. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:CAPELLON PHARMA LLC
Modified release multiple-units dosage composition for release of opioid compounds
InactiveUS6159501AQuick releaseShort timePowder deliveryOrganic active ingredientsFast releaseAnalgesics effects
PCT No. PCT / DK97 / 00101 Sec. 371 Date Jun. 22, 1998 Sec. 102(e) Date Jun. 22, 1998 PCT Filed Mar. 7, 1997 PCT Pub. No. WO97 / 32573 PCT Pub. Date Sep. 12, 1997An oral pharmaceutical modified release multiple-units composition for the administration of an analgesically effective amount of an opoid. The composition comprises at least two fractions wherein individual units containing an opoid are coated with a sustained release coating. A first fraction is adapted to relatively fast release while a second fraction is adapted to a delayed release. Such compositions make possible to obtain both a relatively fast onset of the analgesic effect and the maintenance of analgesically active plasma concentration for a relatively long period of time. The invention further relates to a process for the preparation of a composition according to the invention.
Owner:TAKEDA PHARMA AS
Anti-inflammatory and analgesic compositions and related methods
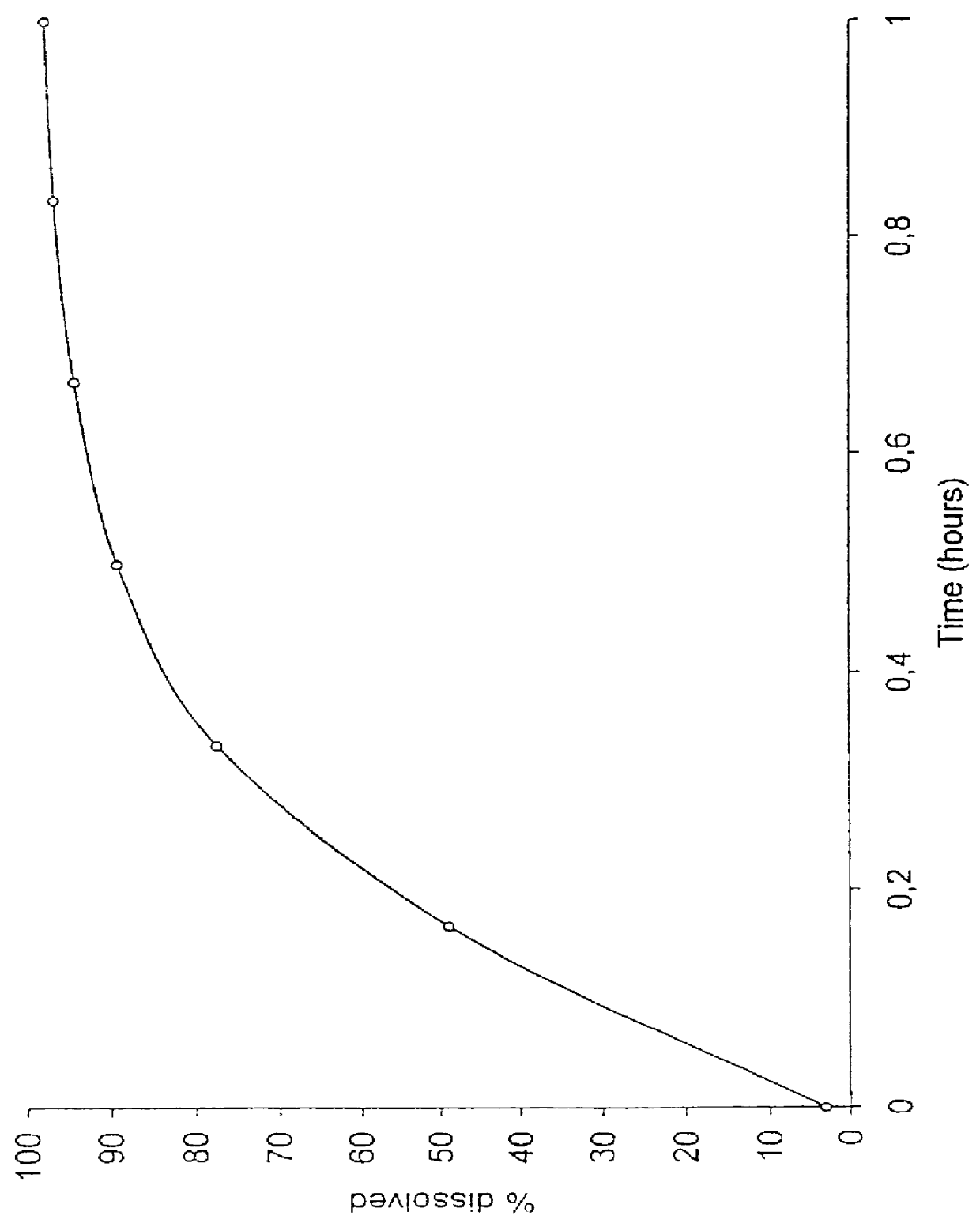
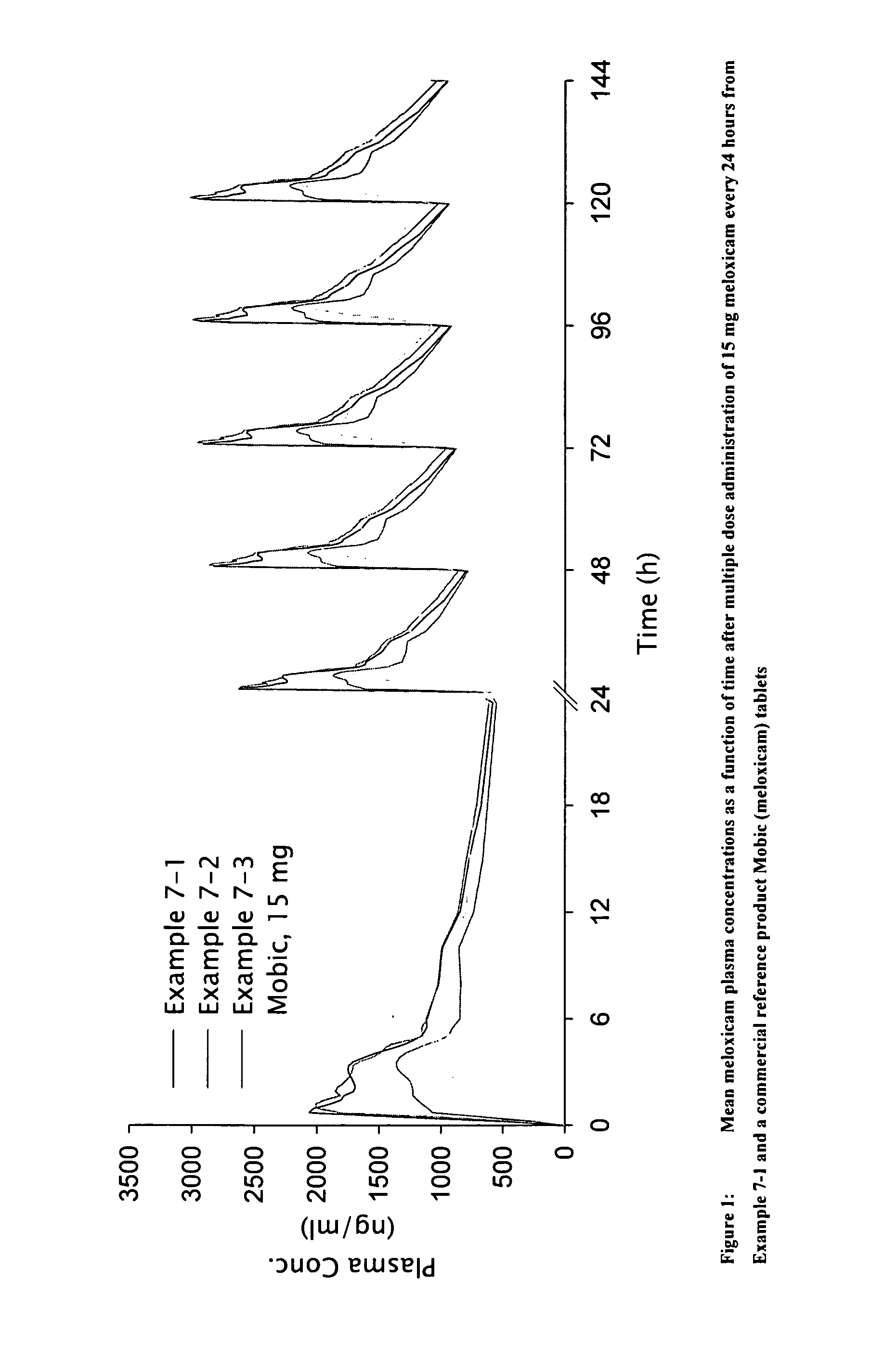
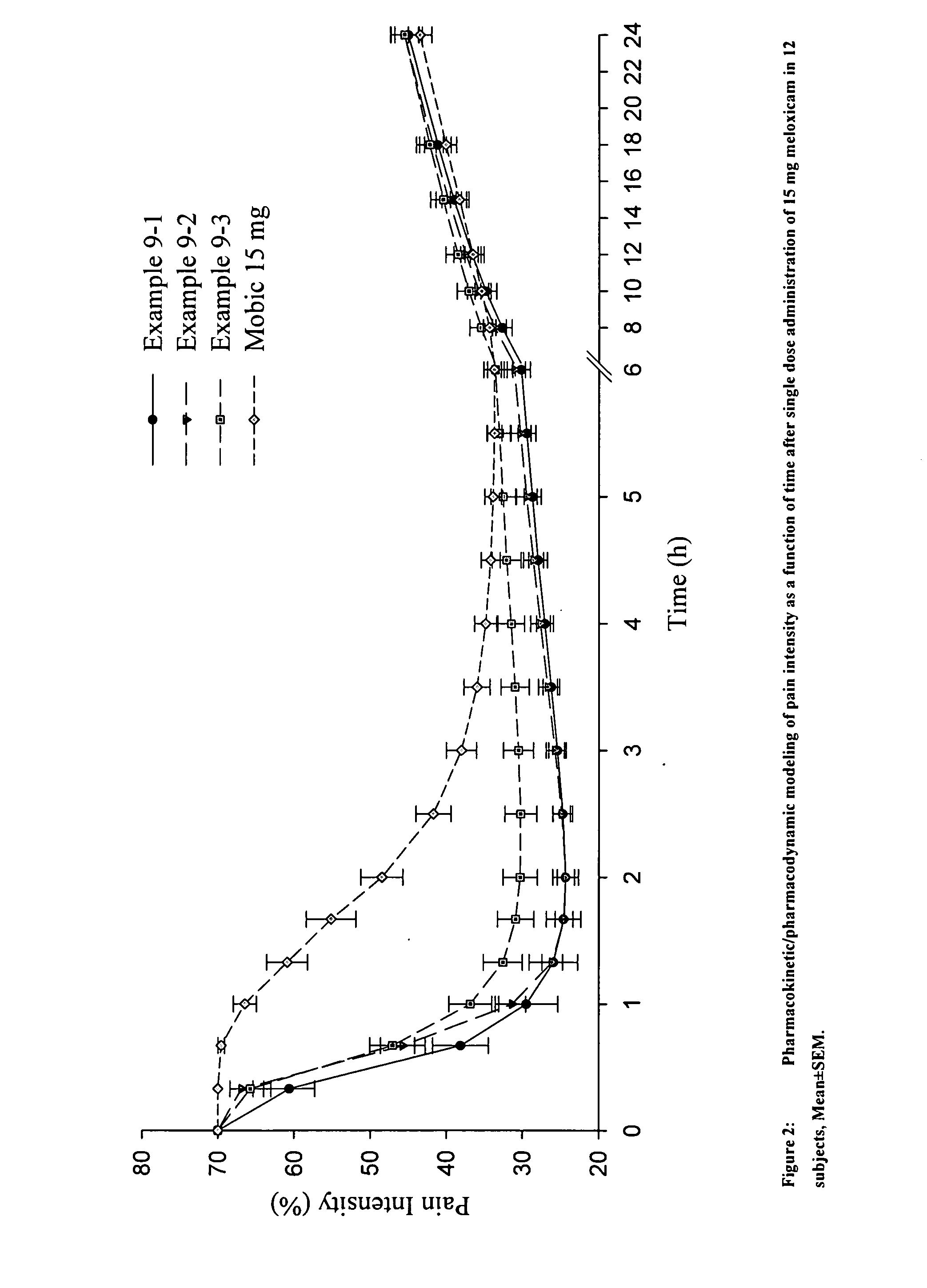
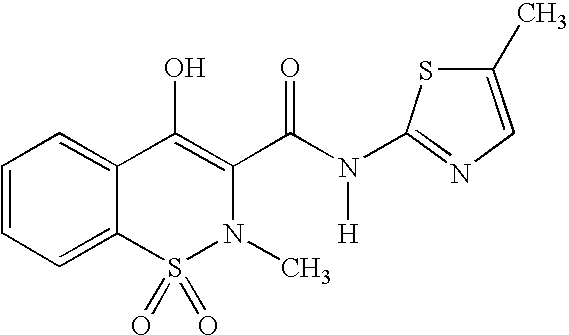
Methods and compositions for delivering a meloxicam compound are disclosed and described. In one aspect, a method may include perorally administering to a subject a therapeutically effective amount of a meloxicam compound that provides a meloxicam plasma concentration within 1 hour which is at least about 40% of the maximum plasma concentration attained by the formulation. In another aspect, a composition may include a therapeutically effective amount of a meloxicam compound in a pharmaceutically acceptable carrier including at least one of an alkalizer or a solubilizer, with the meloxicam compound having a solubility in the carrier that is greater than about 1.0 mg / gm.
Owner:LIPOCINE
Timed pulsatile drug delivery systems
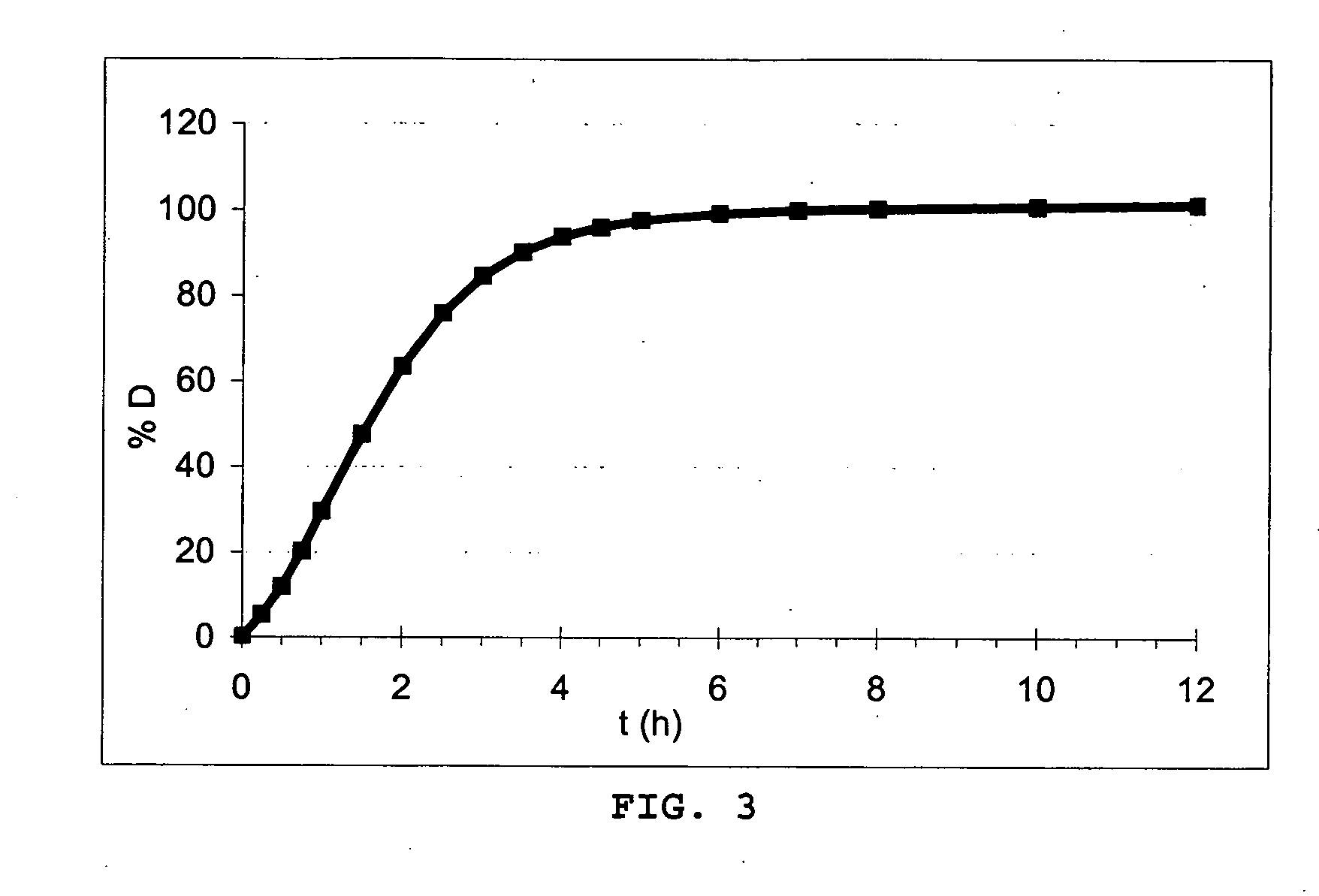
A pharmaceutical dosage form such as a capsule capable of delivering therapeutic agents into the body in a time-controlled or position-controlled pulsatile release fashion, is composed of a multitude of multicoated particulates (beads, pellets, granules, etc.) made of one or more populations of beads. Each of these beads except an immediate release bead has at least two coated membrane barriers. One of the membrane barriers is composed of an enteric polymer while the second membrane barrier is composed of a mixture of water insoluble polymer and an enteric polymer. The composition and the thickness of the polymeric membrane barriers determine the lag time and duration of drug release from each of the bead populations. Optionally, an organic acid containing intermediate membrane may be applied for further modifying the lag time and / or the duration of drug release. The pulsatile delivery may comprise one or more pulses to provide a plasma concentration-time profile for a therapeutic agent, predicted based on both its pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:ADARE PHARM INC
Dosage form containing promethazine and another drug
A pharmaceutical dosage form which comprises promethazine and / or a pharmaceutically acceptable salt thereof and at least one second drug. The dosage form provides a plasma concentration within the therapeutic range of the at least one second drug over a period which is coextensive with a substantial part of the period over which the dosage form provides a plasma concentration within the therapeutic range of promethazine or salt thereof. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Timed pulsatile drug delivery systems
A pharmaceutical dosage form such as a capsule capable of delivering therapeutic agents into the body in a time-controlled or position-controlled pulsatile release fashion, is composed of a multitude of multicoated particulates (beads, pellets, granules, etc.) made of one or more populations of beads. Each of these beads except an immediate release bead has at least two coated membrane barriers. One of the membrane barriers is composed of an enteric polymer while the second membrane barrier is composed of a mixture of water insoluble polymer and an enteric polymer. The composition and the thickness of the polymeric membrane barriers determine the lag time and duration of drug release from each of the bead populations. Optionally, an organic acid containing intermediate membrane may be applied for further modifying the lag time and / or the duration of drug release. The pulsatile delivery may comprise one or more pulses to provide a plasma concentration-time profile for a therapeutic agent, predicted based on both its pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:ADARE PHARM INC
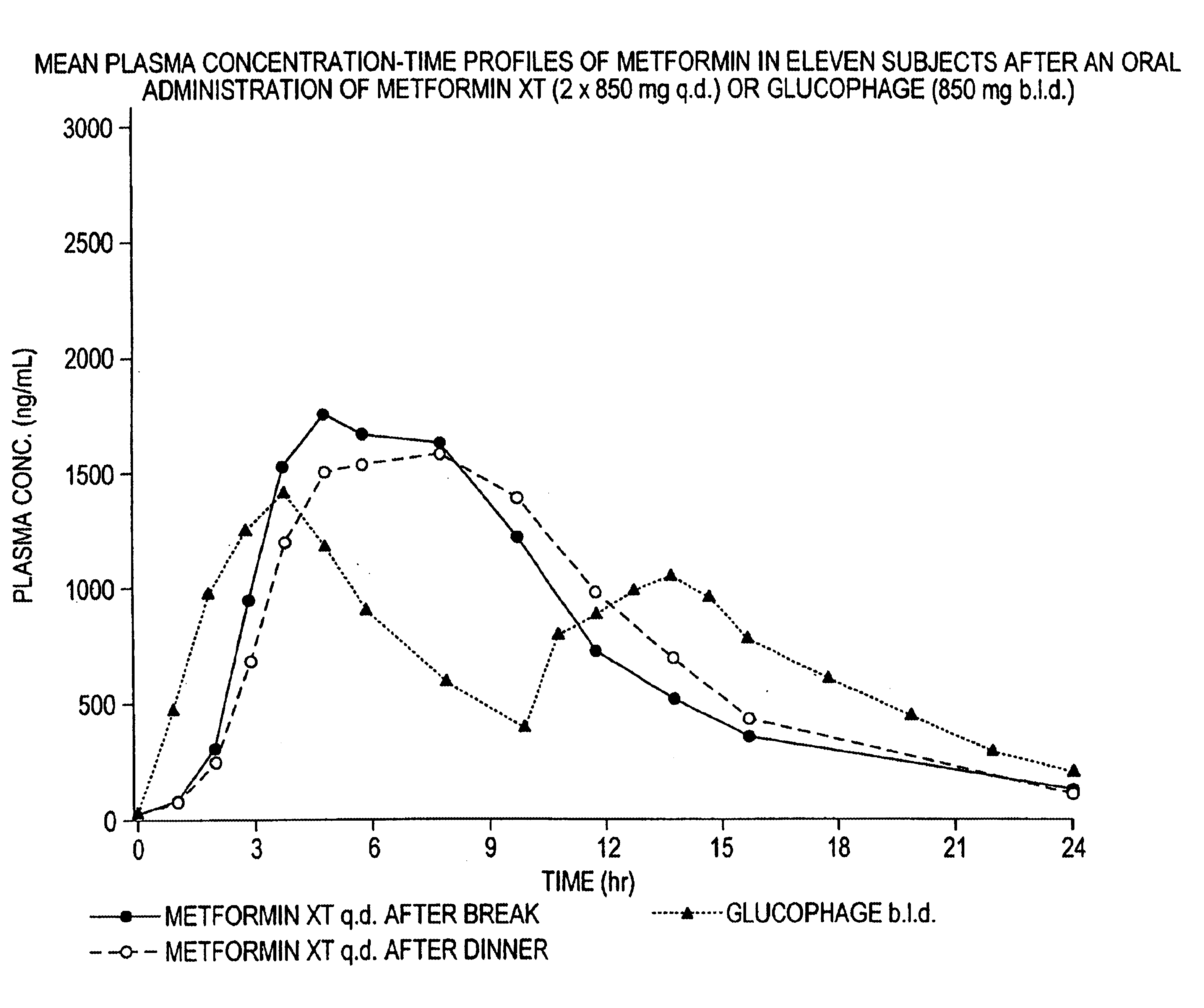
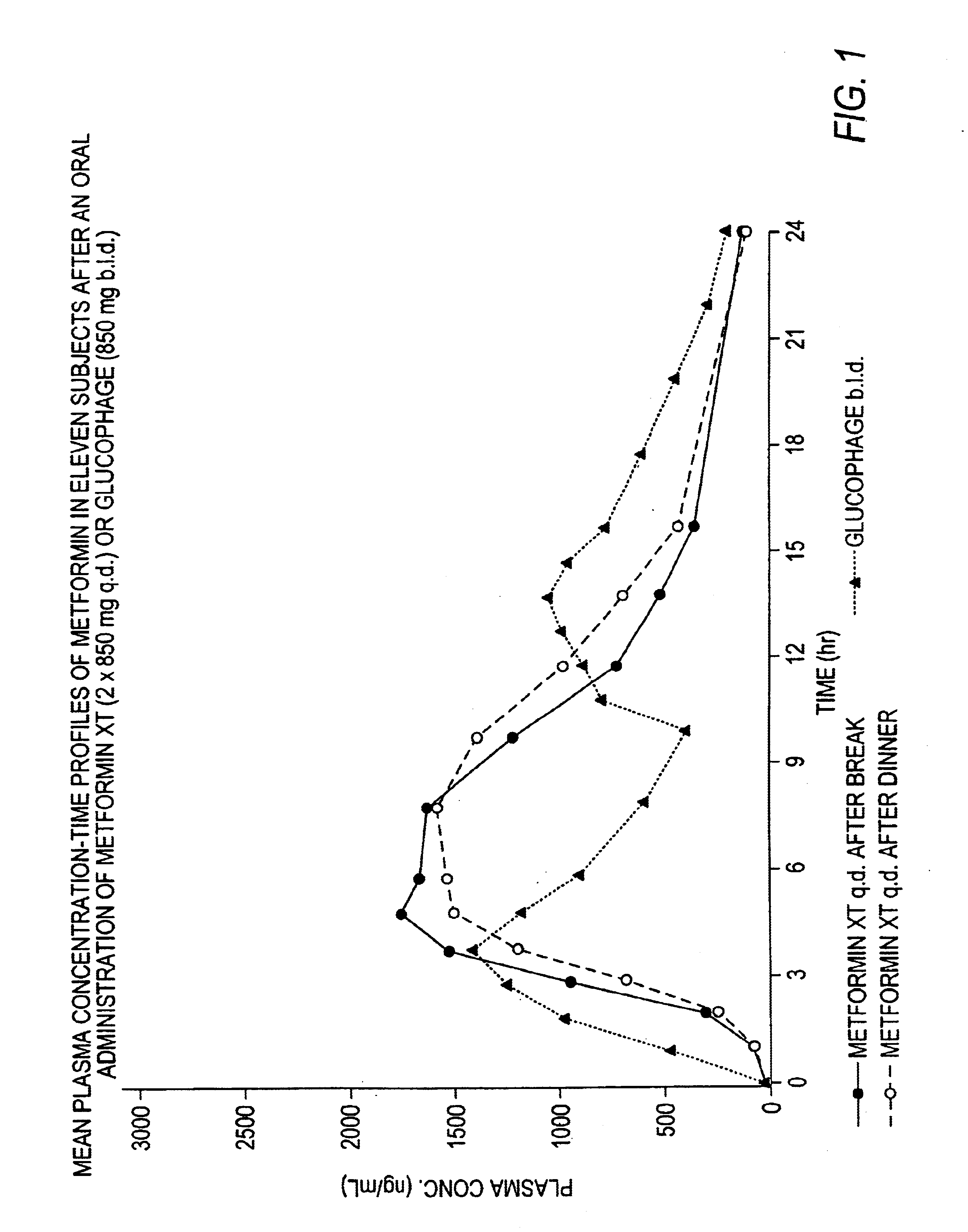
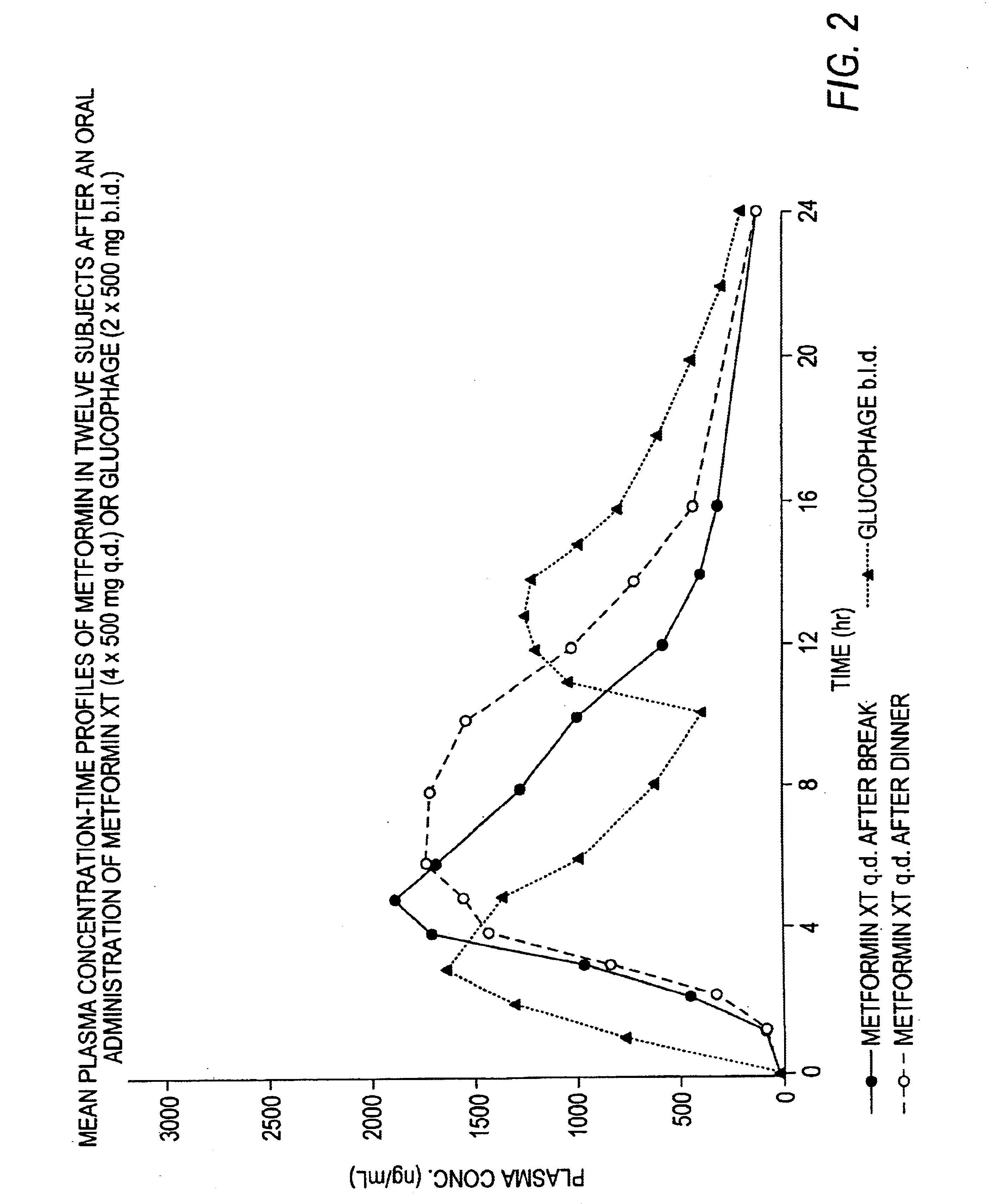
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Neramexane MR matrix tablet
InactiveUS20070141148A1Low peak concentrationReduce absorptionBiocideSenses disorderRegimenModified Release Dosage Form
The invention provides novel oral modified release dosage forms of neramexane which are useful for the continuous therapy of patients suffering from diseases and conditions such as Alzheimer's dementia and neuropathic pain. The compositions have drug release profiles that are suitable for achieving steady state plasma concentrations of neramexane which have relatively small fluctuation when administered on a twice-daily or even once-daily regimen. The dosage forms may be designed as modified release matrix tablets, which are optionally coated for taste masking. The invention further provides therapeutic methods of treating conditions such as Alzheimer's dementia and neuropathic pain which involve the administration of such dosage forms.
Owner:MERZ PHARMA GMBH & CO KGAA
Modified release dosage forms of skeletal muscle relaxants
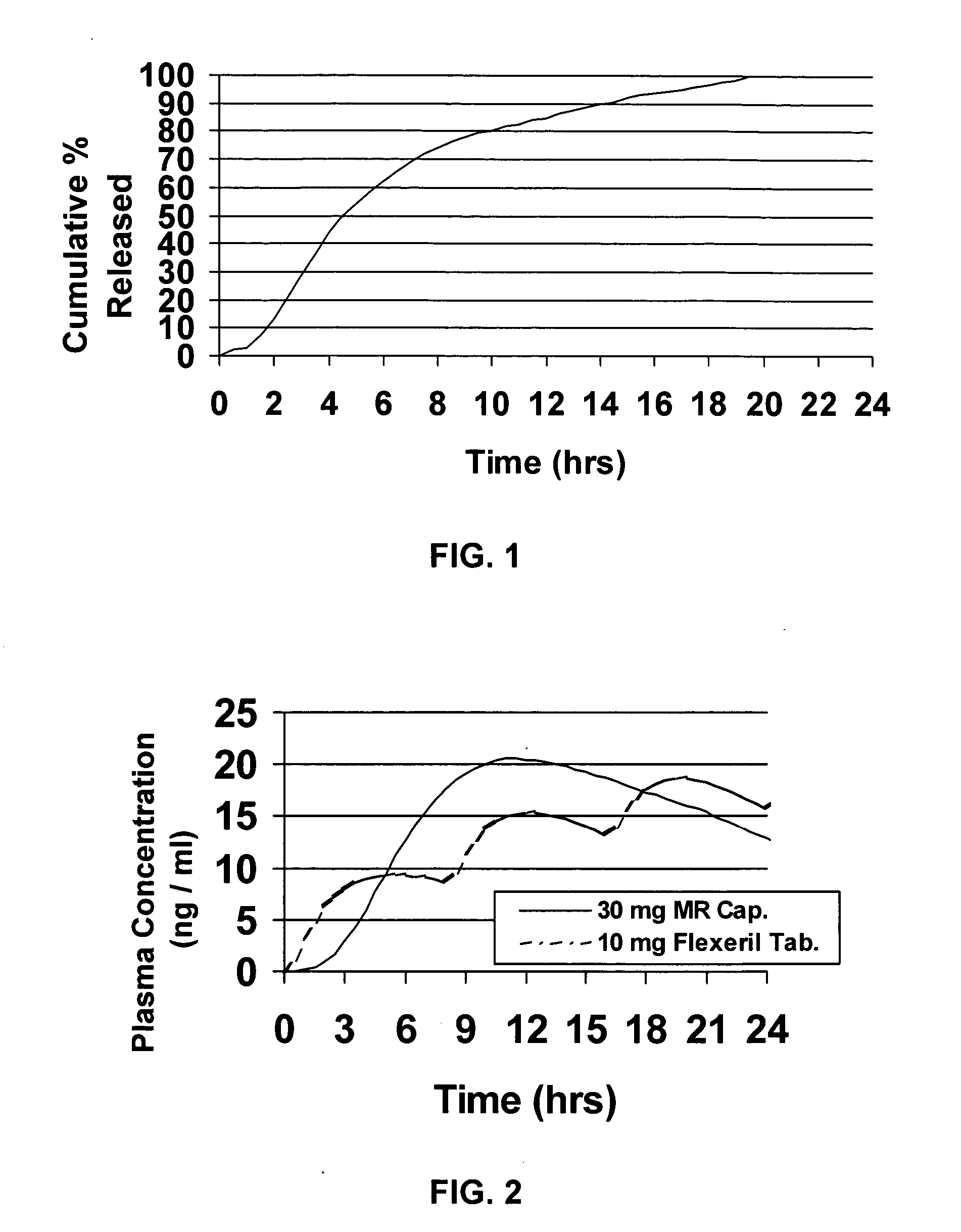
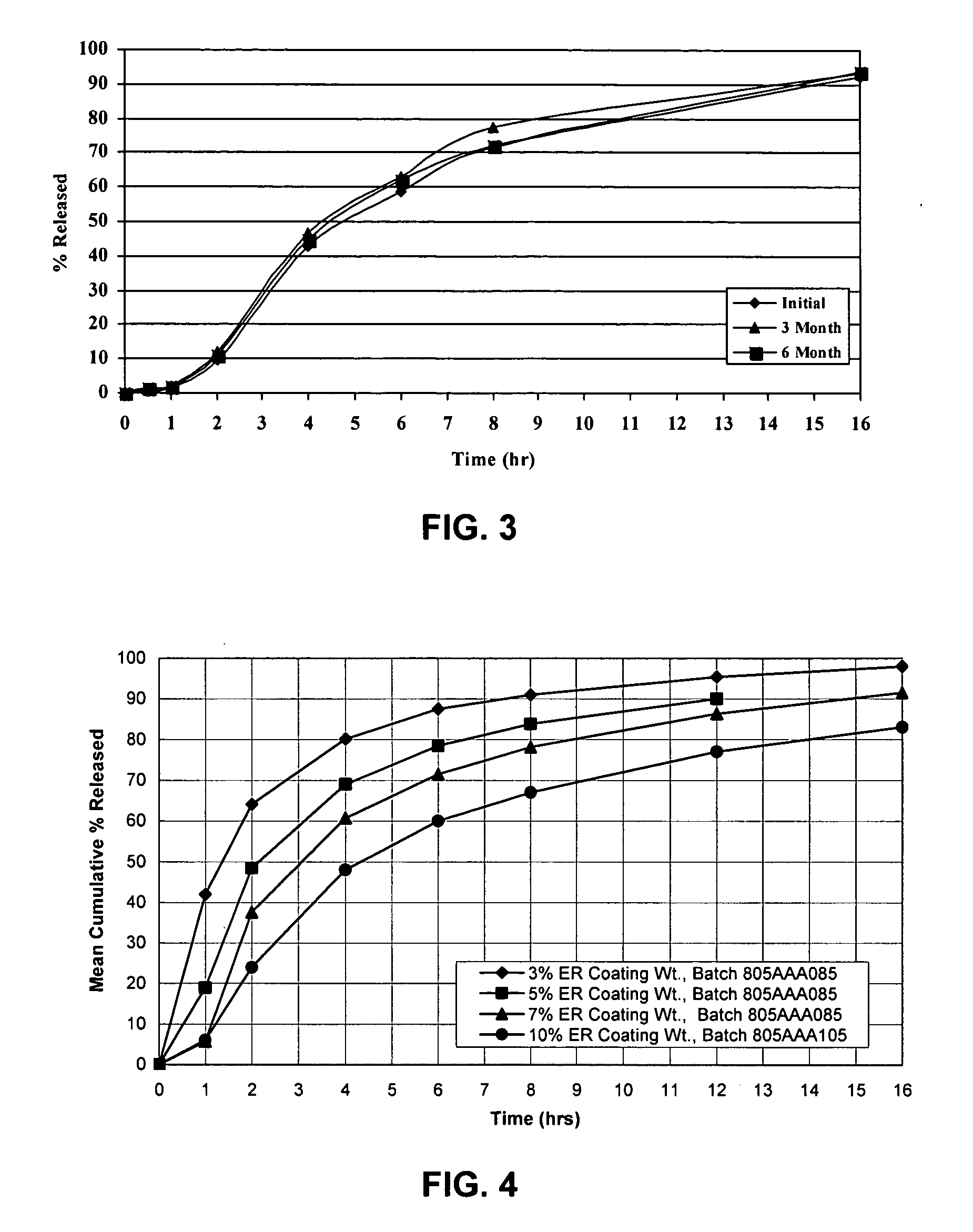
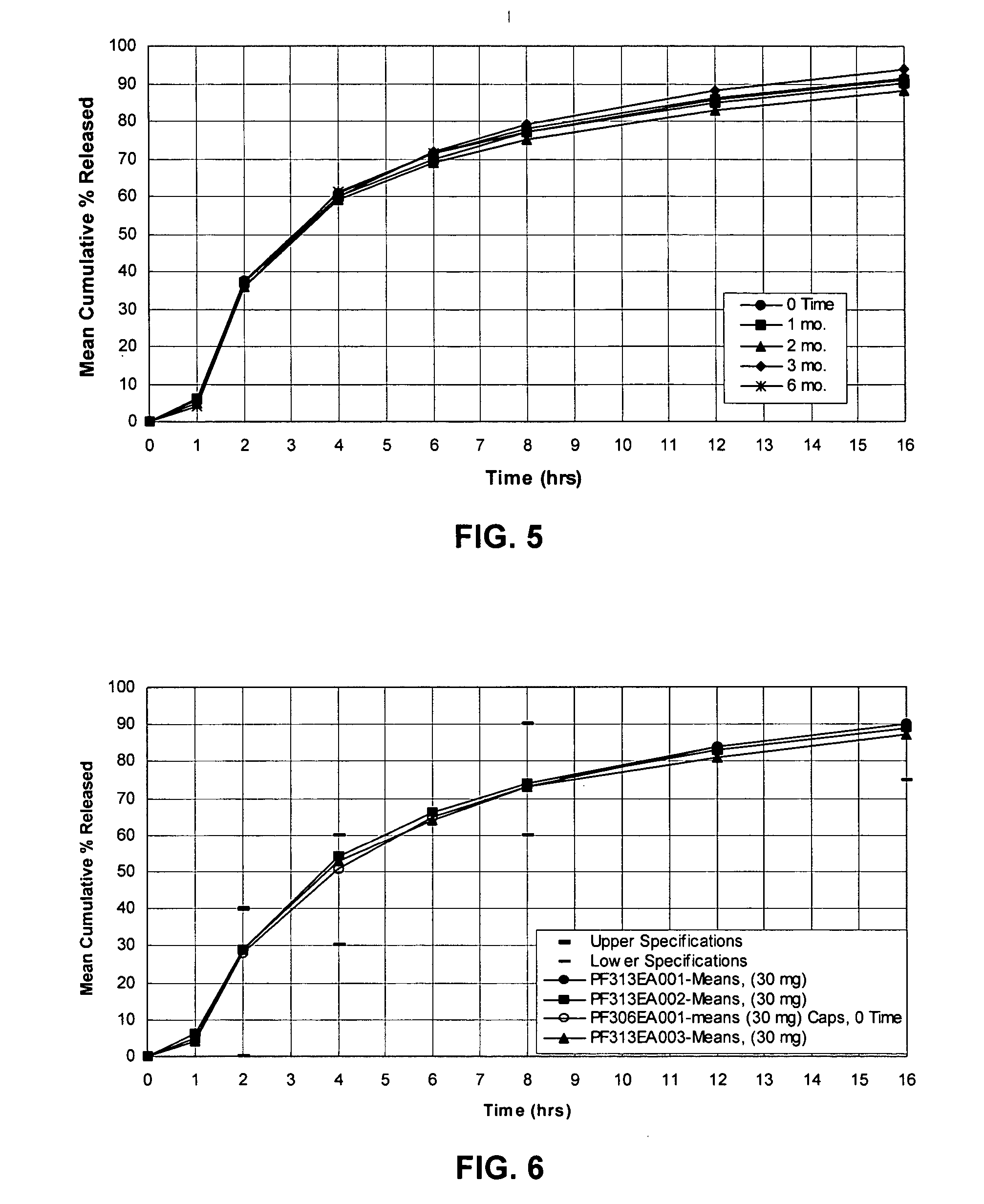
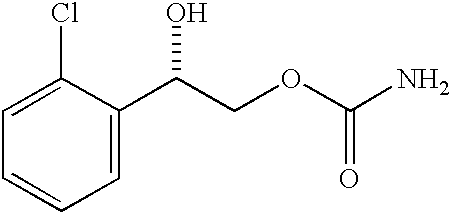
InactiveUS20050106247A1Patient compliance is goodEfficient ConcentrationBiocidePowder deliveryDiseaseModified Release Dosage Form
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration—time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Dosage form containing promethazine and another drug
InactiveUS20050232993A1Good treatment effectRelieve symptomsBiocideSalicyclic acid active ingredientsPromethazinePharmaceutical drug
A pharmaceutical dosage form which comprises promethazine and / or a pharmaceutically acceptable salt thereof and at least one second drug. The dosage form provides a plasma concentration within the therapeutic range of the at least one second drug over a period which is coextensive with a substantial part of the period over which the dosage form provides a plasma concentration within the therapeutic range of promethazine or salt thereof. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Modified release analgesic suspensions
A pharmaceutical dosage form comprising non-steroidal-anti-inflammatory drugs, in particular propionic acid derivatives such as ibuprofen, along with a second active ingredient having a shorter therapeutically effective plasma concentration duration, such as phenylephrine, and methods of administering the same are provided. This method provides improved therapeutic effect, in particular pain relief along with decongestant relief, over extended time periods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
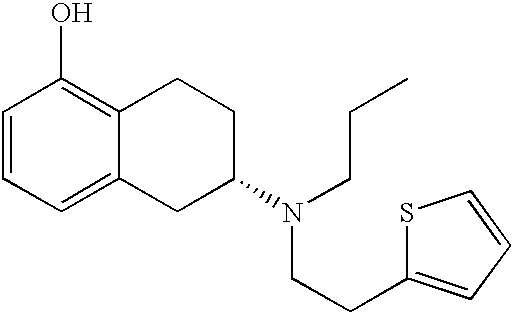
Transdermal therapeutic system for parkinson's disease inducing high plasma levels of rotigotine
This invention provides the use of a silicone-based transdermal therapeutic system having an area of 10 to 40 cm2 and containing 0.1 to 3.15 mg / cm2 of rotigotine as active ingredient, for the preparation of an anti-Parkinson medicament which induces a mean plasma concentration of rotigotine in the range of 0.4 to 2 ng / ml 24 h after administration.
Owner:UCB SA +1
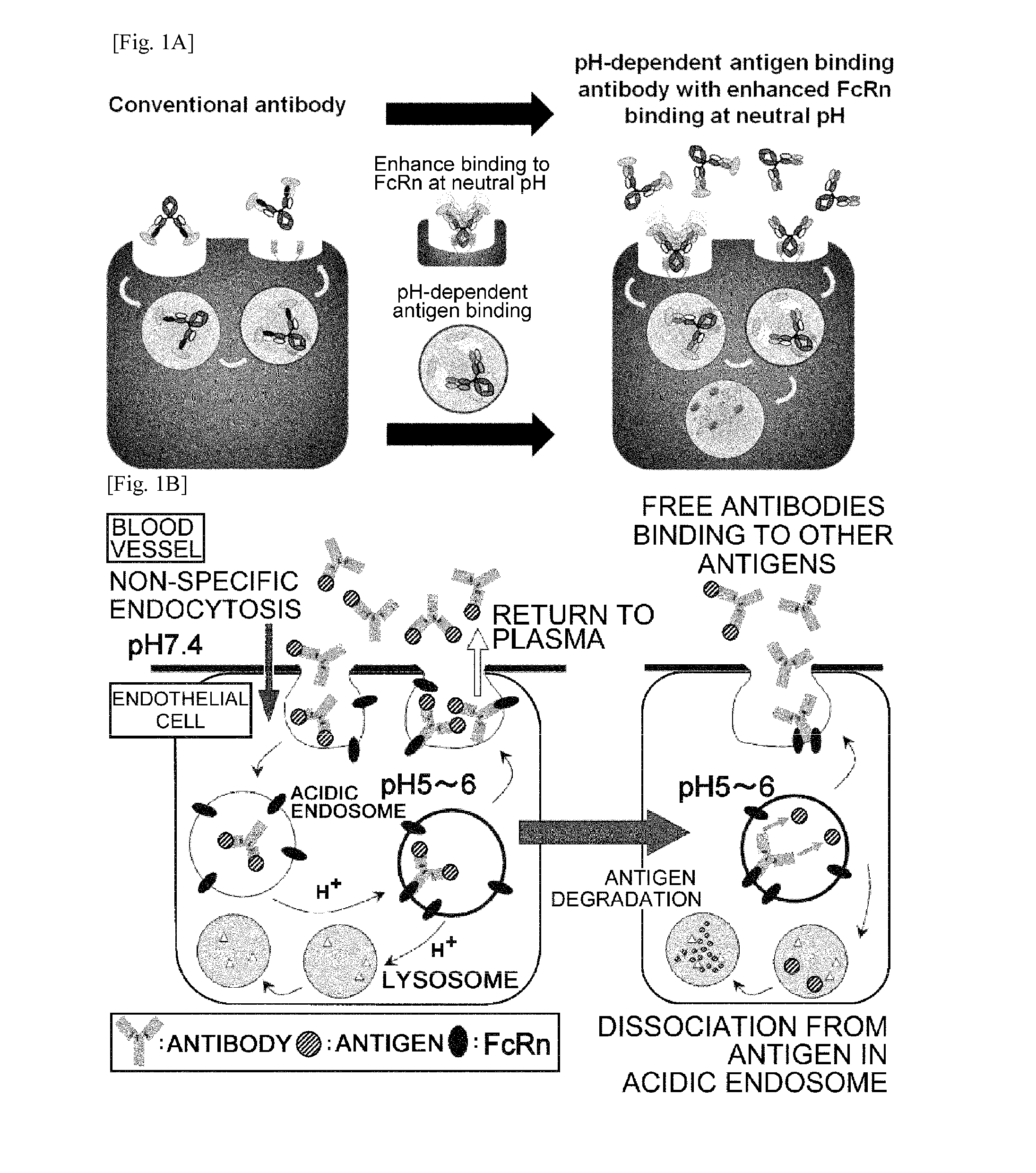
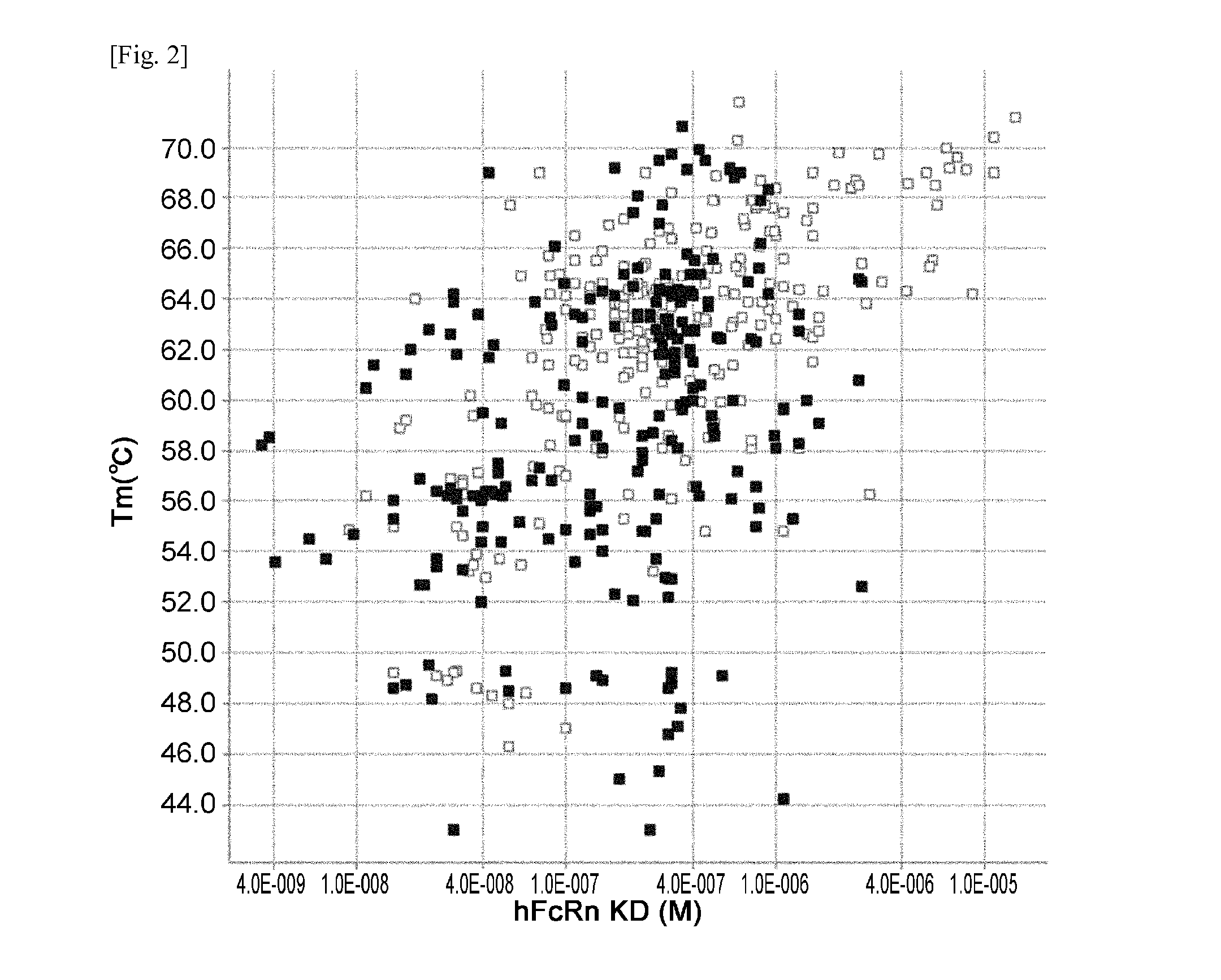
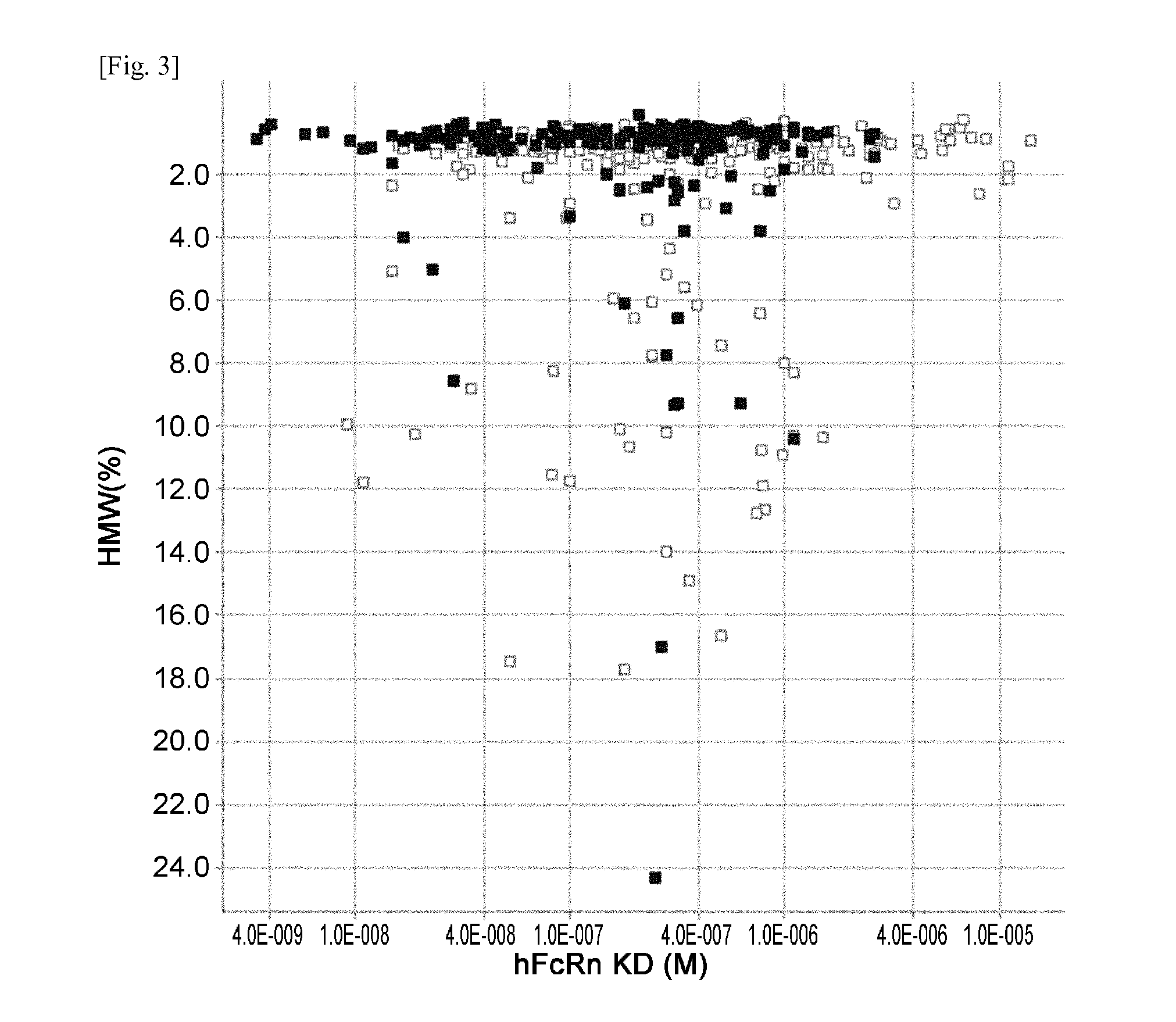
THERAPEUTIC ANTIGEN-BINDING MOLECULE WITH A FcRn-BINDING DOMAIN THAT PROMOTES ANTIGEN CLEARANCE
ActiveUS20140363428A1Low immunogenicityImprove stabilityAntipyreticAnalgesicsFc(alpha) receptorNeutral ph
The present invention provides: a modified FcRn-binding domain having an enhanced affinity for the Fc Receptor neonatal (FcRn) at neutral pH; an antigen-binding molecule comprising said FcRn-binding domain, which has low immunogenicity, high stability and form only a few aggregates; a modified antigen-binding molecule having an increased FcRn-binding activity at neutral or acidic pH without an increased binding activity at neutral pH for a pre-existing anti-drug antibody; use of the antigen-binding molecules for improving antigen-binding molecule-mediated antigen uptake into cells; use of the antigen-binding molecules for reducing the plasma concentration of a specific antigen; use of the modified FcRn-binding domain for increasing the total number of antigens to which a single antigen-binding molecule can bind before its degradation; use of the modified FcRn-binding domain for improving pharmacokinetics of an antigen-binding molecule; methods for decreasing the binding activity for a pre-existing anti-drug antibody; and methods for producing said antigen-binding molecules.
Owner:CHUGAI PHARMA CO LTD
Oral pharmaceutical extended release dosage form
An enteric coated pharmaceutical extended release dosage form of a H+, K+-ATPase inhibitor giving an extended plasma concentration profile of a H+, K+-ATPase inhibitor. The extended plasma profile is obtained by a pharmaceutical composition which comprises a core material of a hydrophilic or hydrophobic matrix, and the H+, K+-ATPase inhibitor and optionally pharmaceutically acceptable excipients. The dosage form may be administered once daily.
Owner:ASTRAZENECA AB
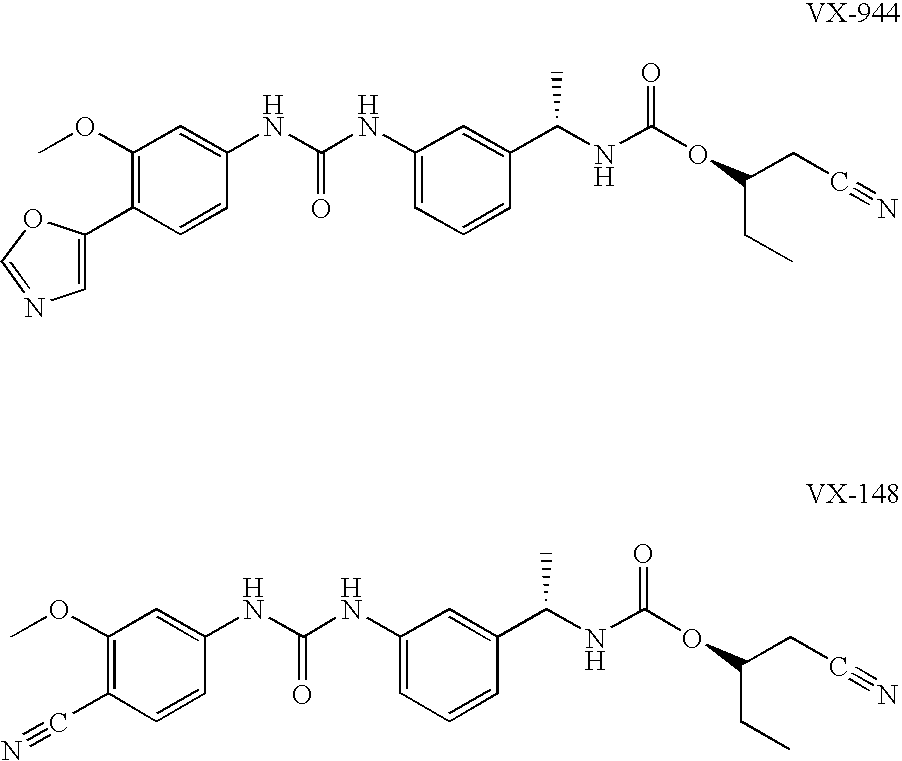
Compositions comprising IMPDH inhibitors and uses thereof for treating HCV infection
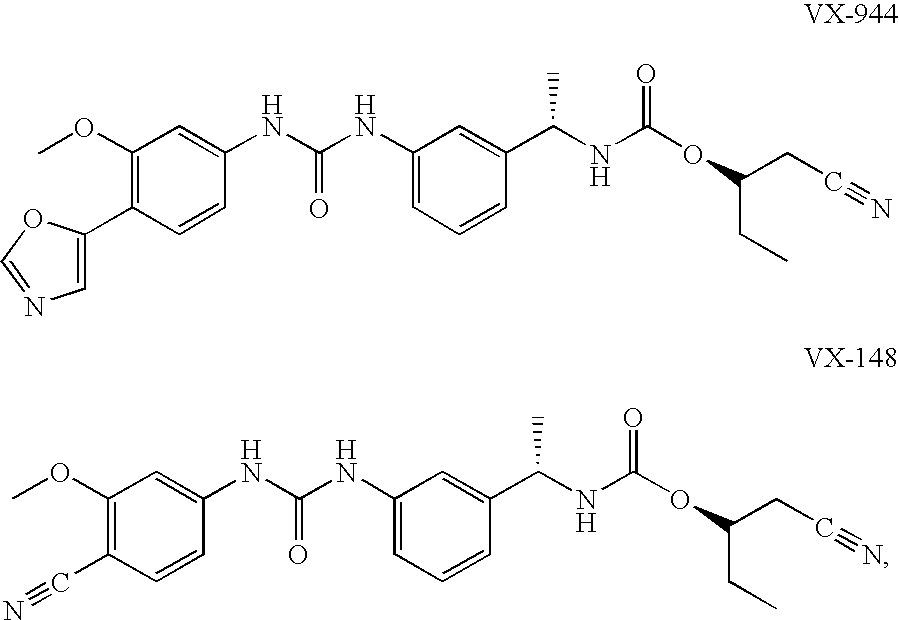
The present invention relates to optimal compositions useful in treating HCV infections in humans. These compositions comprise alpha-interferon or pegylated alpha-interferon and an IMPDH inhibitor selected from VX-148 or VX-944, wherein the IMPDH inhibitor is present in an amount such that a ratio of Cavg / Cmin is between 1 to 10, wherein:Cavg is average plasma concentration produced by said IMPDH inhibitor in said human; andCmin is estimated trough concentration produced by said IMPDH inhibitor in said human.The present invention also relates to methods of producing and using the optimal compositions to treat HCV infections in humans.
Owner:VERTEX PHARMA INC
Pulsatile release histamine H2 antagonist dosage form
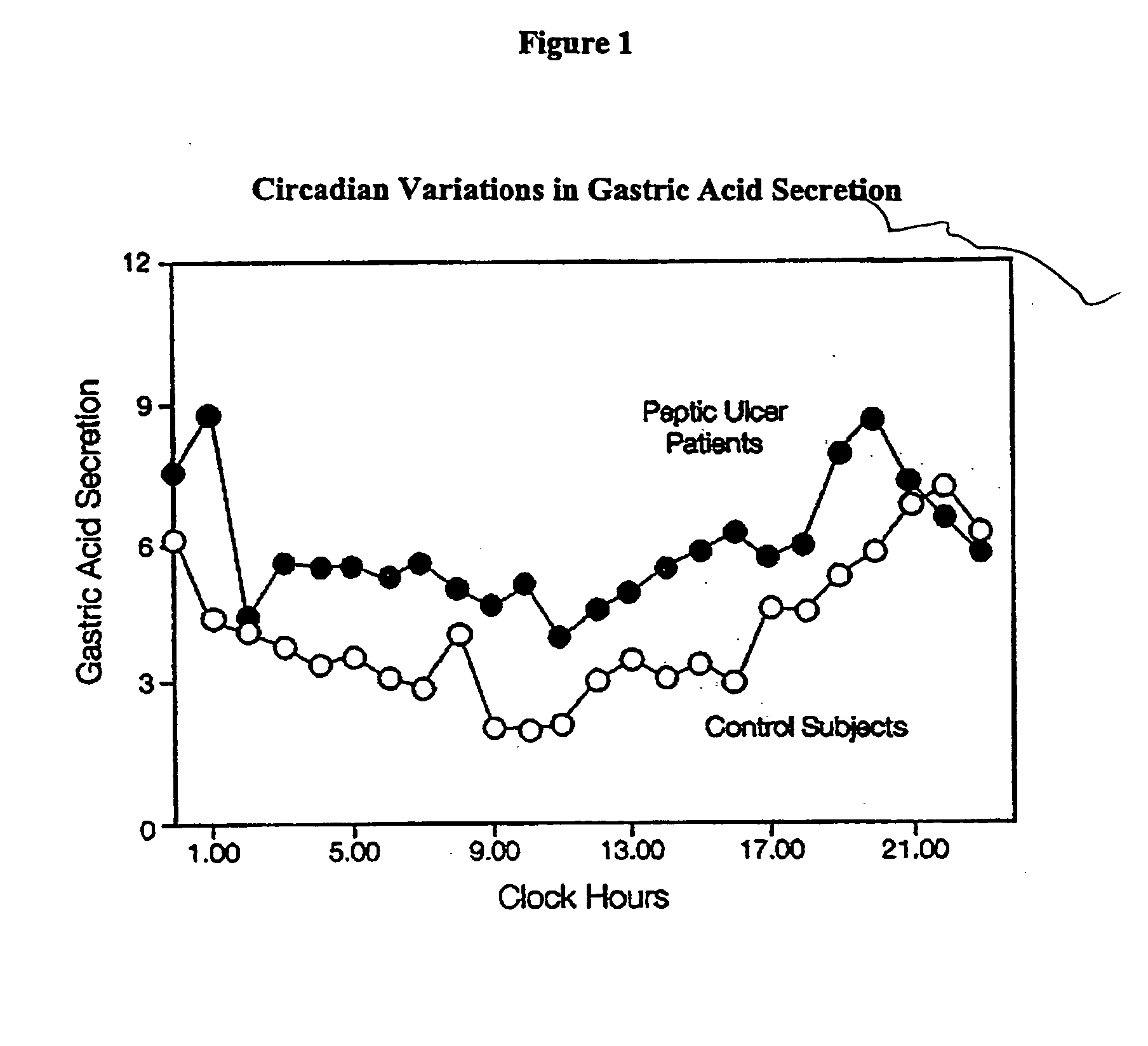
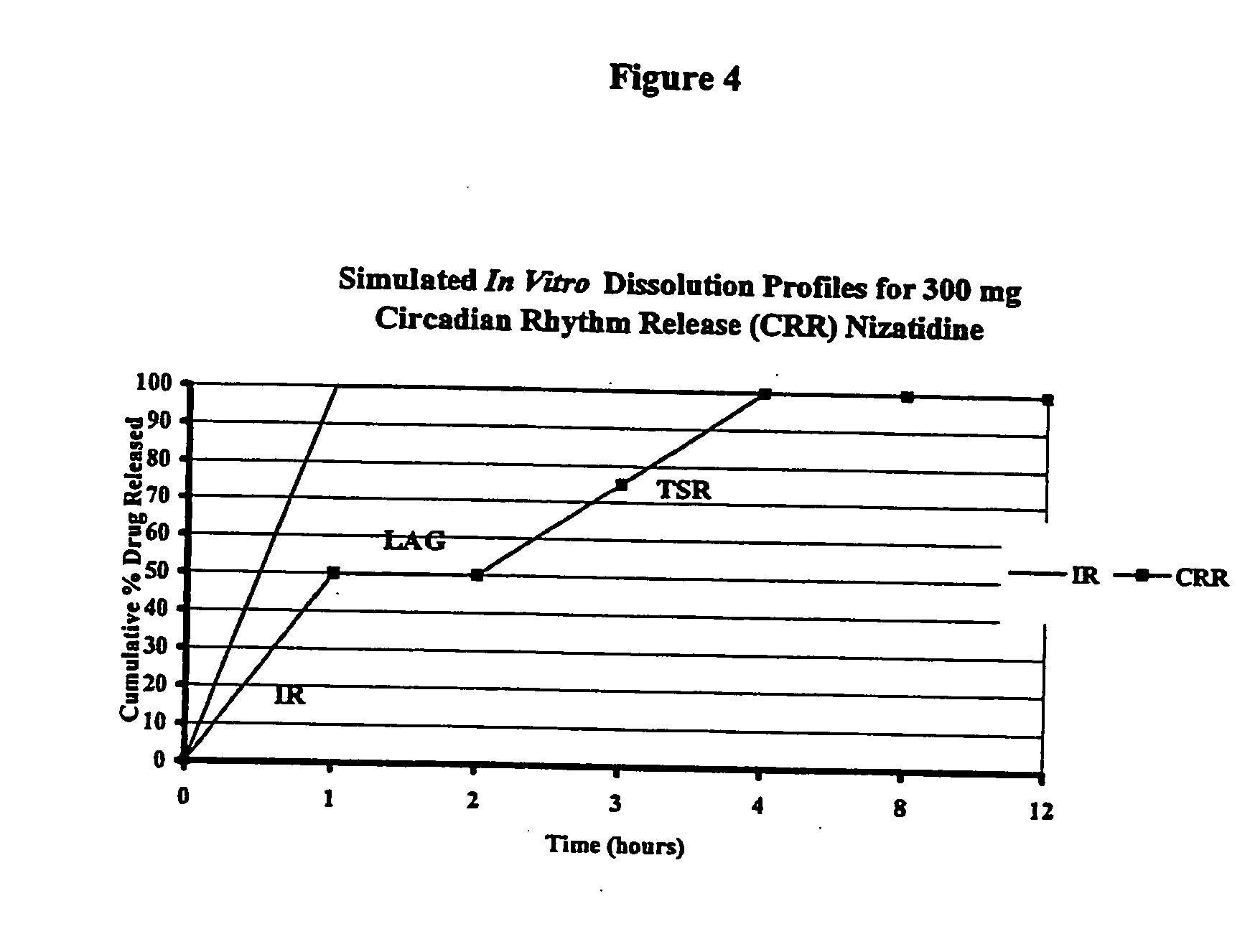
A unit dosage form, such as a capsule or the like, for delivering drugs into the body in a circadian release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. Each bead population exhibits a pre-designed rapid or sustained release profile with or without a predetermined lag time of 3 to 5 hours. Such a circadian rhythm release drug delivery system is designed to provide a plasma concentration-time profile, which varies according to physiological need at different times during the dosing period, i.e., mimicking the circadian rhythm and severity / manifestation of gastric acid secretion (and / or midnight gerd), predicted based on pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:APTALIS PHARMATECH
Dosage form containing carbetapentane and another drug
PendingUS20060029664A1Extended maintenance periodDispersion deliveryPill deliveryPharmacyBlood plasma
A pharmaceutical dosage form which comprises carbetapentane and / or a pharmaceutically acceptable salt thereof and an additional drug. The dosage form provides a plasma concentration within the therapeutic range of the additional drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of carbetapentane. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Anti-Misuse Microparticulate Oral Drug Form
InactiveUS20090041838A1Avoid misuseAvoid fraudulent abuseOrganic active ingredientsPowder deliveryAdditive ingredientMicroparticle
The invention relates to solid microparticulate oral dosage forms having a composition that prevents the misuse of the active pharmaceutical ingredient (API) contained therein. The aim of the invention is to prevent the improper use of solid oral drugs for any use other than the therapeutic use(s) officially approved by the appropriate public health authorities. Another aim of the invention is to provide novel analgesic drugs which can be used to: prevent the misuse of, and addiction to certain analgesics and / or to control plasma concentration variability and / or to facilitate oral; administration; and / or to combine analgesics with one another and / or with one or more active ingredients in the same oral form. More specifically, the invention relates to a solid oral drug form comprising anti-misuse means and at least one active ingredient, which is characterized in that: at least part of the active ingredient is contained in microparticles; and the anti-misuse means comprise anti-crushing means (a) which enable the microparticles of the active ingredient to resist crushing, such as to prevent the misuse thereof. According to the invention, the drug form can also comprise means (b) for preventing the misuse of the active ingredient following a possible liquid extraction process.
Owner:FLAMEL TECHNOLOGIES
Analgesics for nasal administration
InactiveUS20050142072A1Rapid uptakeRapid onsetPowder deliveryBiocideNasal Cavity EpitheliumBlood plasma
An analgesic and a delivery agent are combined in a pharmaceutical composition such that, on introduction into the nasal cavity of a patient to be treated, the analgesic may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours. The analgesic may be an opioid analgesic or a non-steroidal anti-inflammatory drug.
Owner:IONIX PHARMA +1
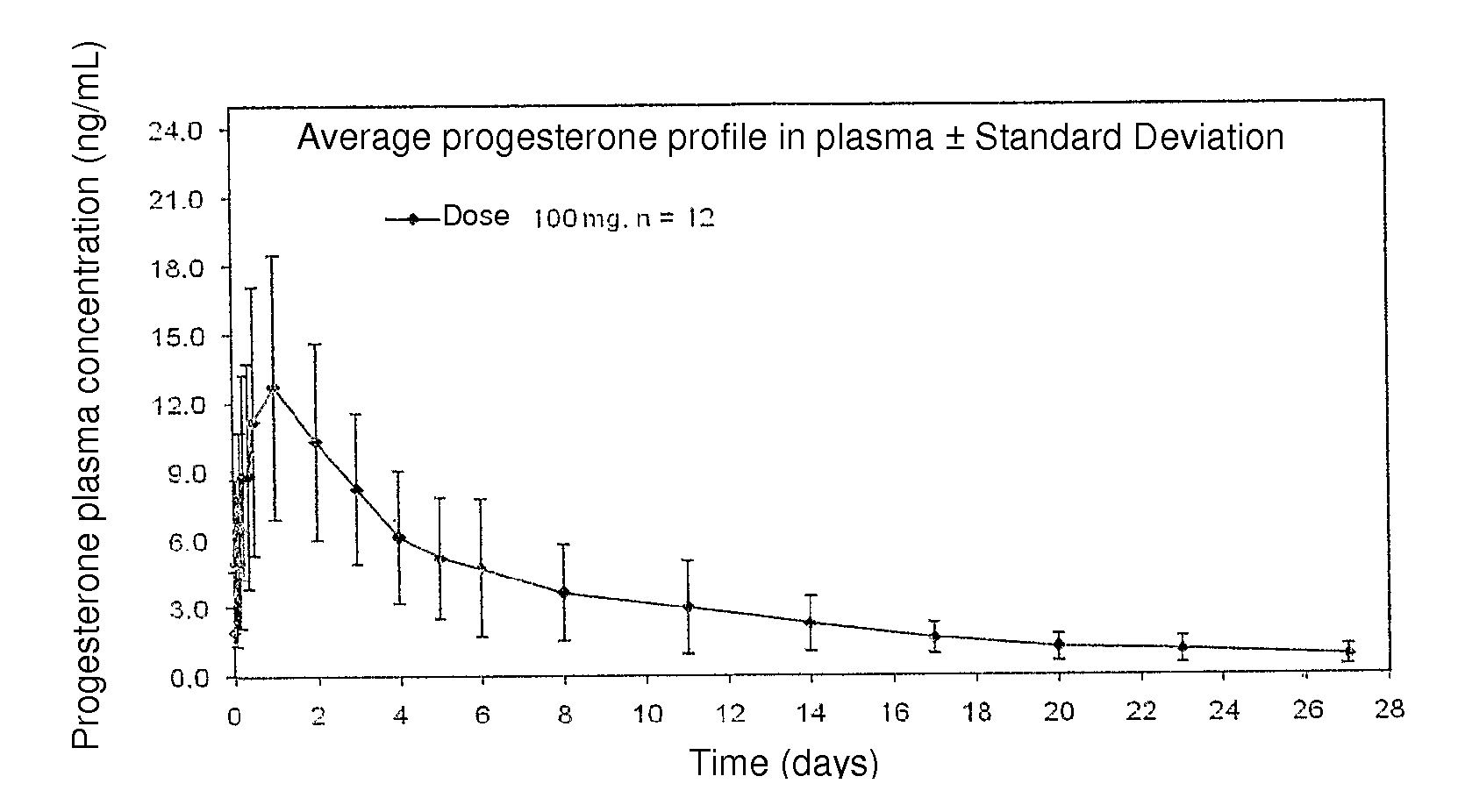
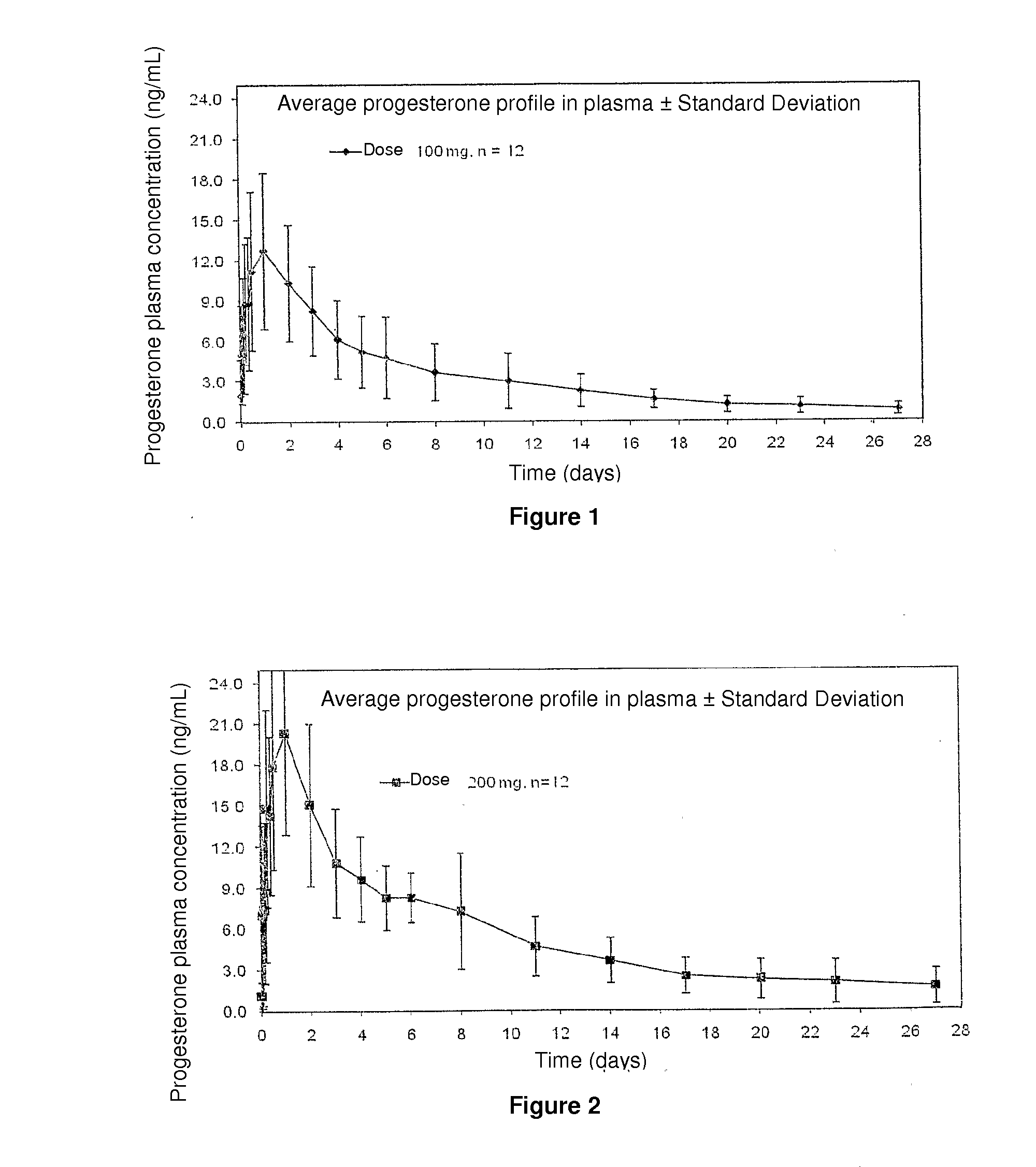
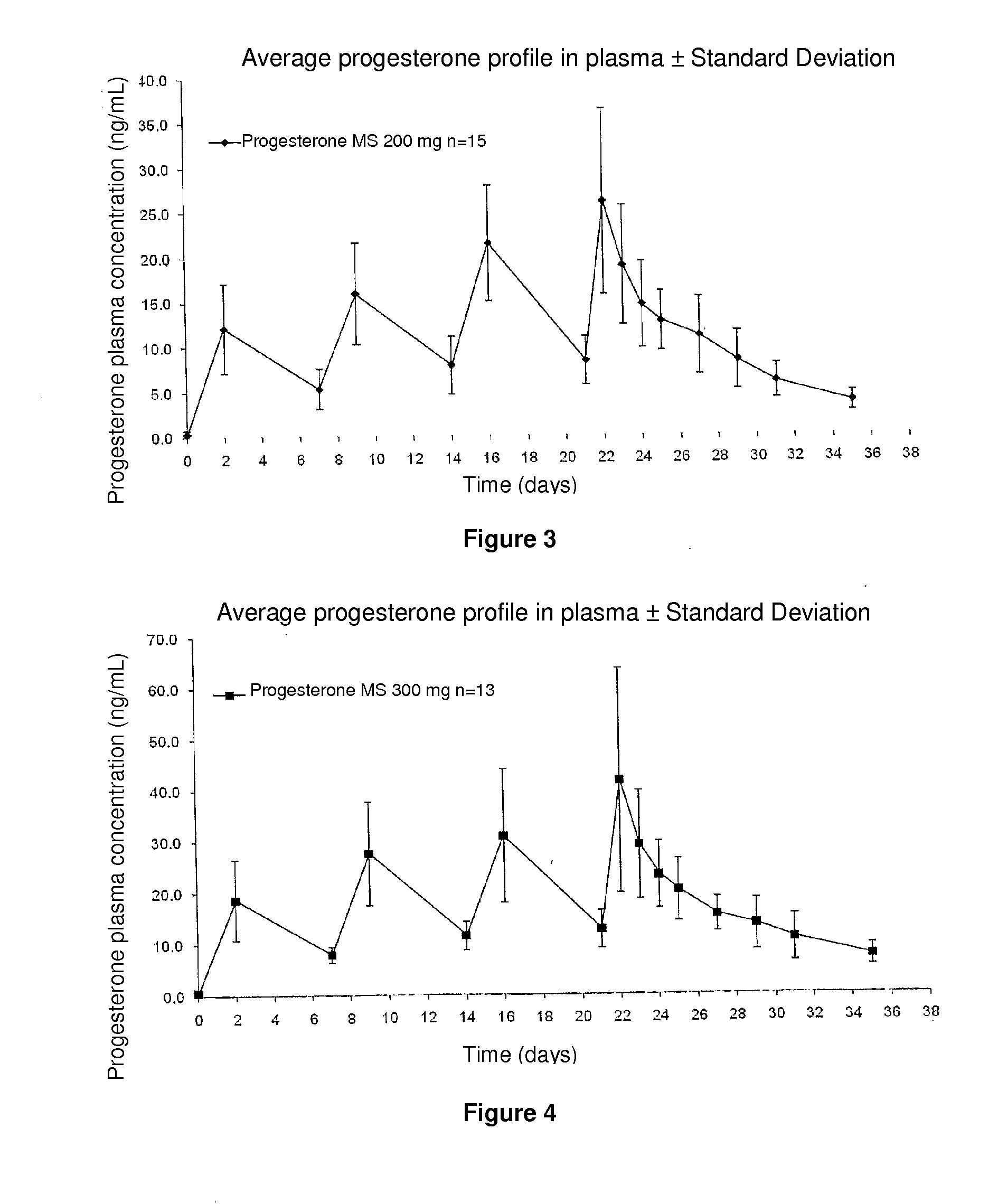
Method and pharmaceutical composition for obtaining the plasmatic progesterone levels required for different therapeutic indications
InactiveUS20110104289A1Decrease administration frequencyReduce the amount requiredOrganic active ingredientsPowder deliveryProgesterone levelProgesterones
Owner:POSI VISIONARY SOLUTIONS
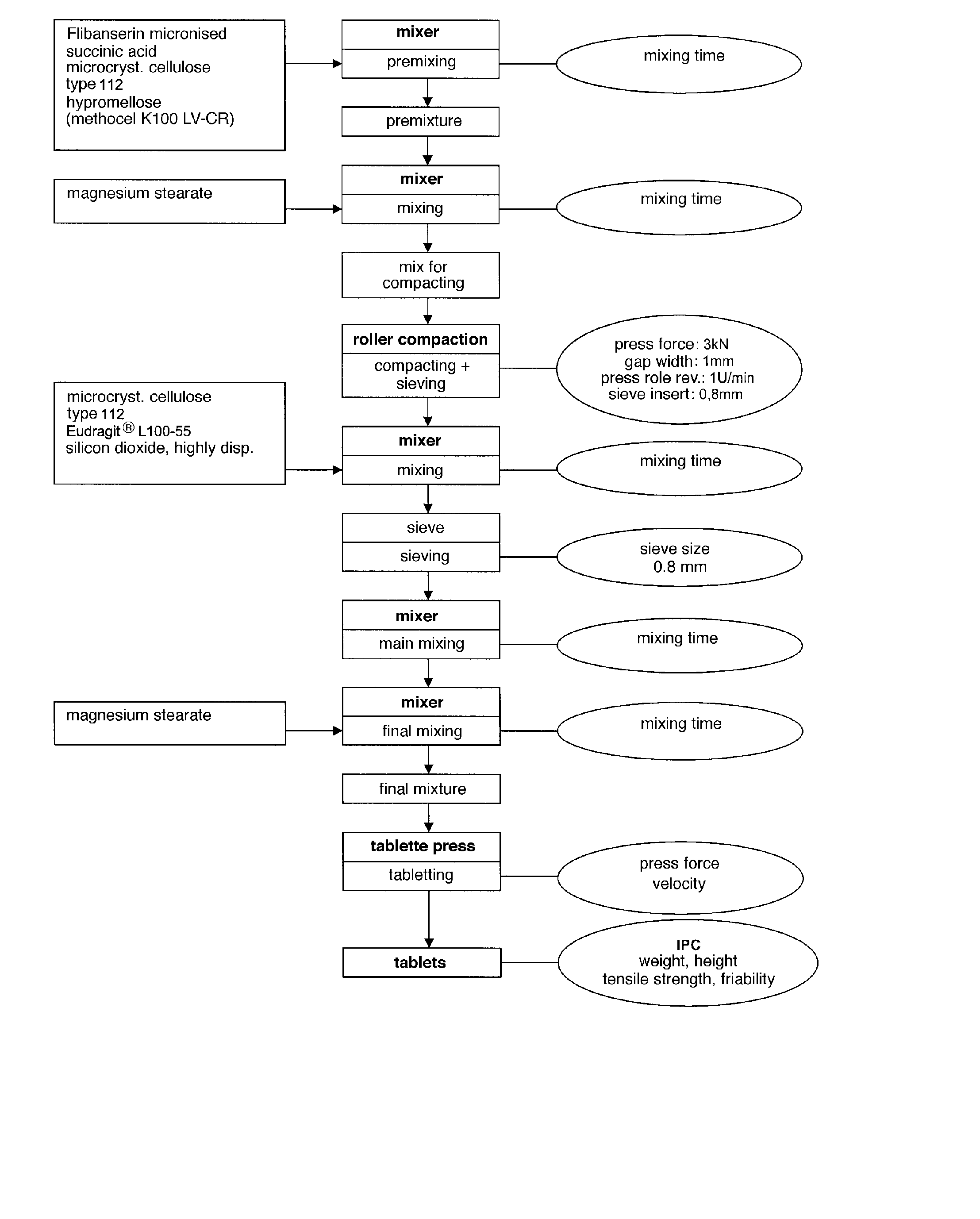
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038347A1Reduce wearExtended shelf lifeOrganic active ingredientsNervous disorderExtended release tabletsDrug release
The present invention provides pharmaceutical release systems comprising an therapeutically effective amount of flibanserin and at least one pharmaceutically acceptable excipient, characterized in that said pharmaceutical release systems exhibit a pharmacokinetic profile that is characterized by an average maximum flibanserin plasma concentration Cmax lower than 300 ng / mL, preferably lower than 200 ng / mL after administration of a single dose to healthy volunteers in fasted state or directly after a meal.
Owner:BOEHRINGER INGELHEIM INT GMBH
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
Anti-misuse oral microparticle medicinal formulation
InactiveUS20070264326A1Avoid fraudulent abuseReduce riskPowder deliveryDispersion deliveryOral medicineMean diameter
The field of the present invention is that of solid microparticulate analgesic oral medicines. The invention is that of providing novel analgesic medicines which allow at the same time the prevention of misuse and of addiction to certain analgesics, and / or the control of variability in the plasma concentration and / or the facilitation of oral administration; and / or the combination of analgesics with one another and / or with one or more active ingredients in the same oral form. The medicine according to the invention comprises (i) anti-misuse means and a plurality of microcapsules with modified release of analgesic(s), having a mean diameter of between 50 and 600 μm, (ii) at least 1000 microcapsules per dose; it being possible for this medicine to be administered once or twice a day for analgesic purposes.
Owner:FLAMEL TECHNOLOGIES
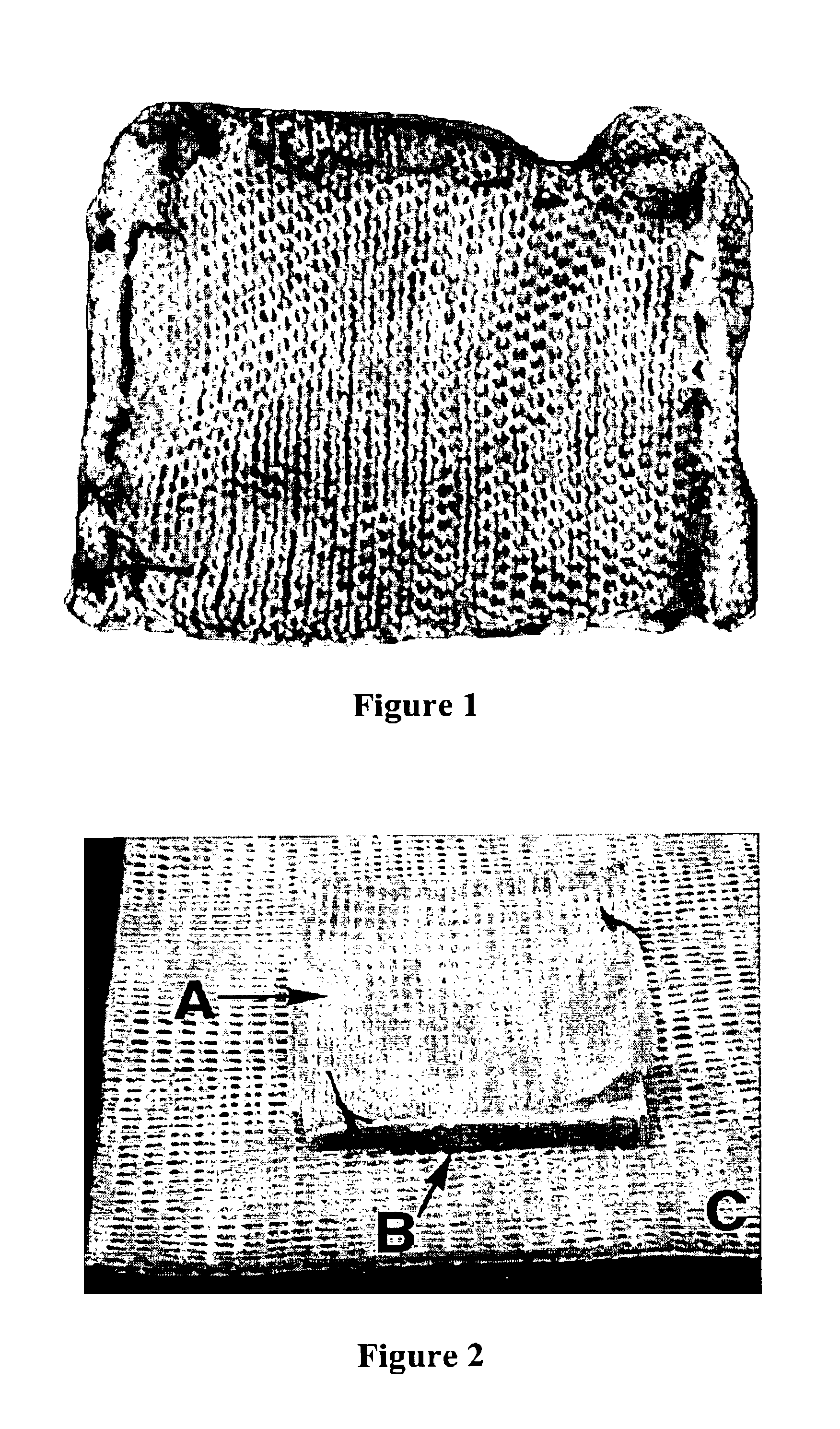
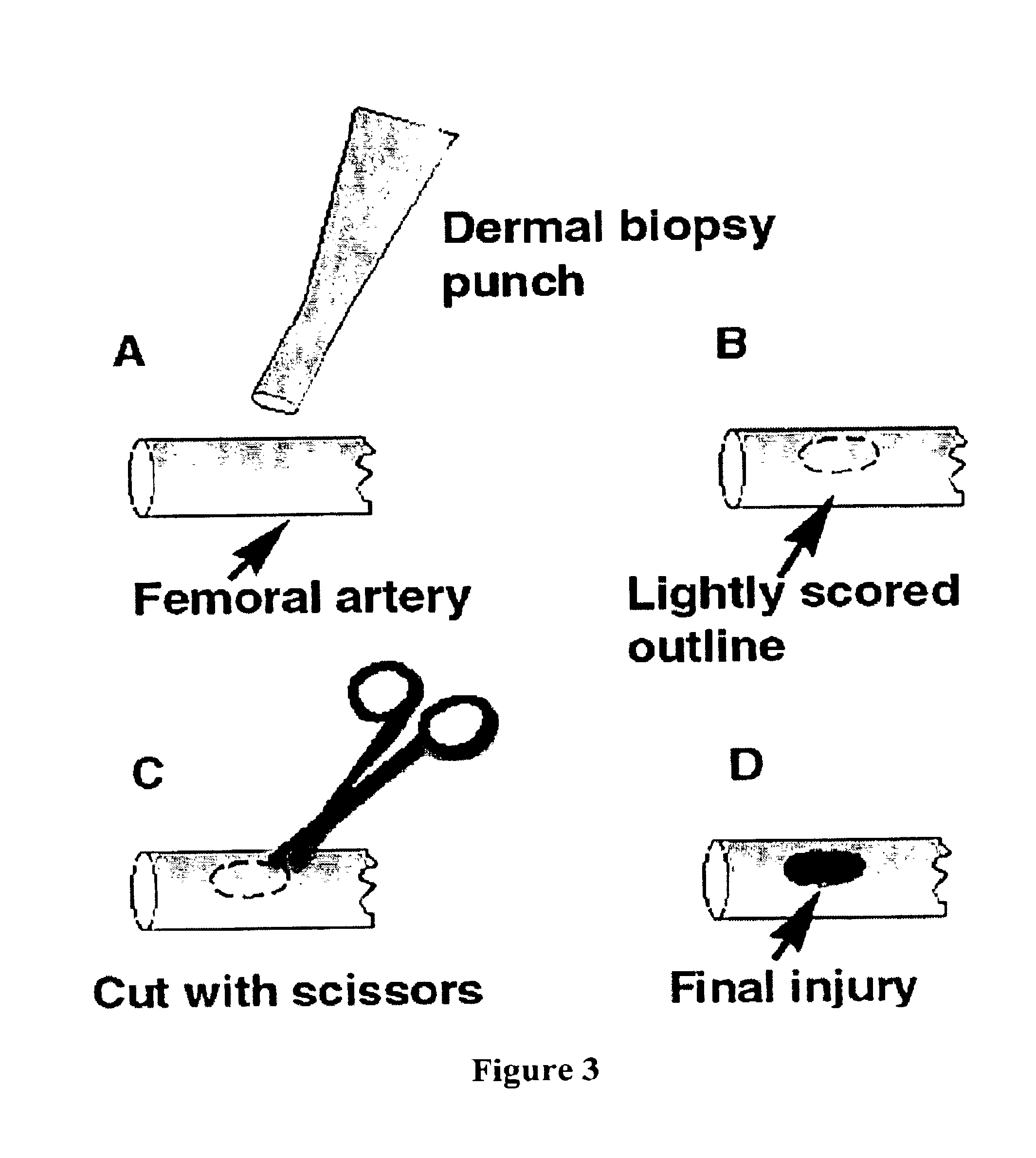
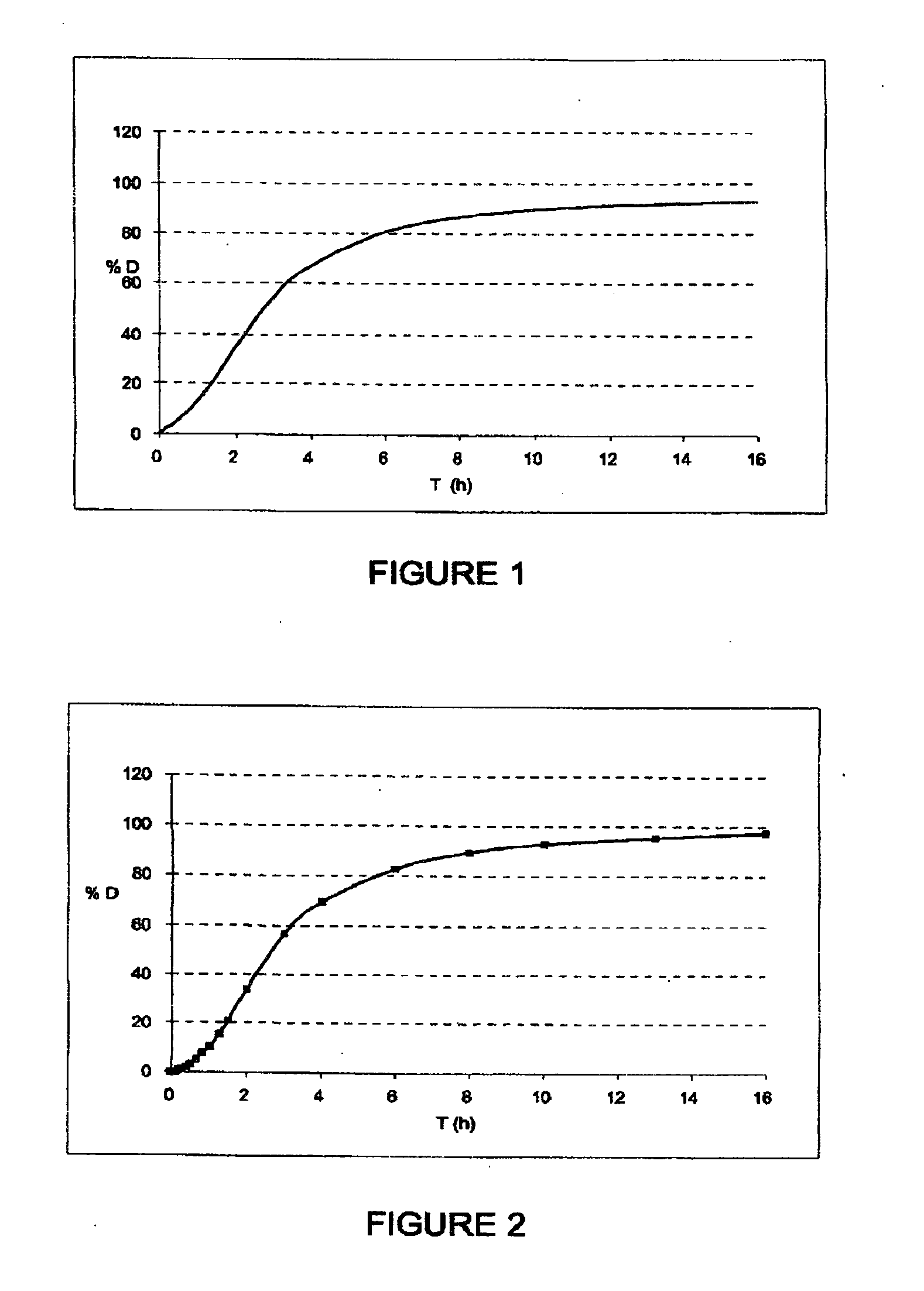
Fibrinogen bandages and arterial bleeding models and methods of making and using thereof
InactiveUS6891077B2Reduce the amount of solutionIncreasing of rate of clotSurgeryBaby linensBlood platelet countsClot formation
Disclosed herein are wound dressings comprising fibrinogen and at least one procoagulant such as propyl gallate in a therapeutic amount. Also disclosed are methods of treating wounds, increasing an amount of or rate of coagulation of blood from a wound, increasing an amount of or rate of clot formation over a wound, increasing blood platelet counts, activating a coagulation system, increasing the plasma concentration of fibrinogen, and decreasing the activated partial thromboplastin time. Also disclosed are an arterial bleeding model and methods of studying arterial bleeding.
Owner:UNITED STATES ARMY U S ARMY MEDICAL RES & MATERIEL COMMAND
Anti-misuse microparticulate oral drug form
InactiveUS20100266701A1Avoid fraudulent abuseReduce riskBiocidePowder deliveryAnalgesics drugsPublic health
The invention relates to solid microparticulate oral dosage forms having a composition that prevents the misuse of the active pharmaceutical ingredient (API) contained therein. The aim of the invention is to prevent the improper use of solid oral drugs for any use other than the therapeutic use(s) officially approved by the appropriate public health authorities. Another aim of the invention is to provide novel analgesic drugs which can be used to: prevent the misuse of, and addiction to certain analgesics and / or to control plasma concentration variability and / or to facilitate oral; administration; and / or to combine analgesics with one another and / or with one or more active ingredients in the same oral form. More specifically, the invention relates to a solid oral drug form comprising anti-misuse means and at least one active ingredient, which is characterized in that: at least part of the active ingredient is contained in microparticles; and the anti-misuse means comprise anti-crushing means (a) which enable the microparticles of the active ingredient to resist crushing, such as to prevent the misuse thereof. According to the invention, the drug form can also comprise means (b) for preventing the misuse of the active ingredient following a possible liquid extraction process.
Owner:FLAMEL TECHNOLOGIES
Rosiglitazone formulations
Rosiglitazone is a drug used to treat type 2 diabetes. Methods for the formation of amorphous rosiglitazone and formulations comprising the amorphous rosiglitazone are described. Other formulations include pulsed-release formulations and formulations for retention in the stomach and upper gastrointestinal tract. Controlled-release dosage form include those wherein the maximum plasma concentration of rosiglitazone occurs greater than one hour after administration to a human and / or wherein less than 75 percent by weight of the rosiglitazone is released at 1 hour after immersion in simulated gastric fluid.
Owner:ACTAVIS GRP PTC EHF
Device and Method for Treating Conditions of a Joint
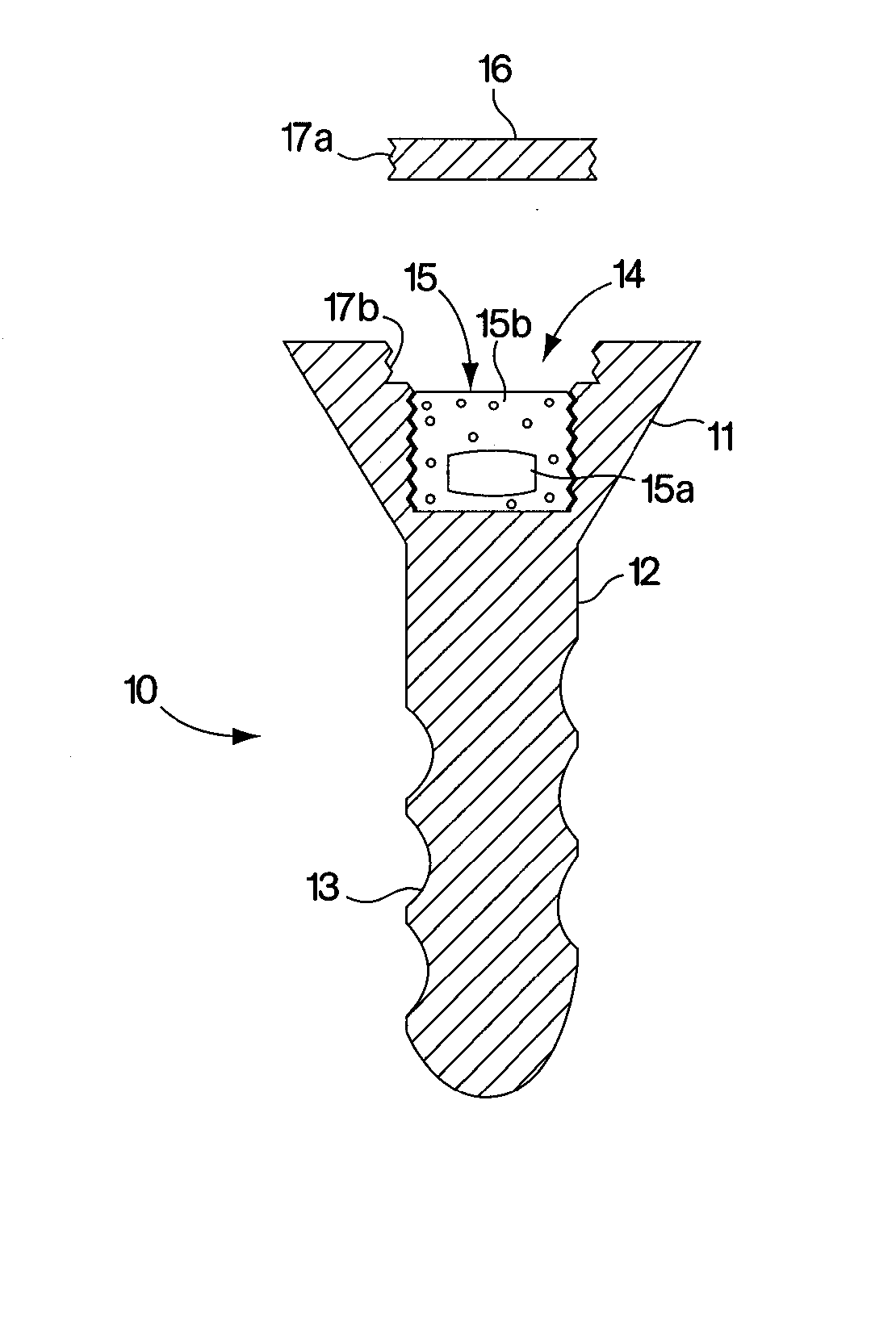
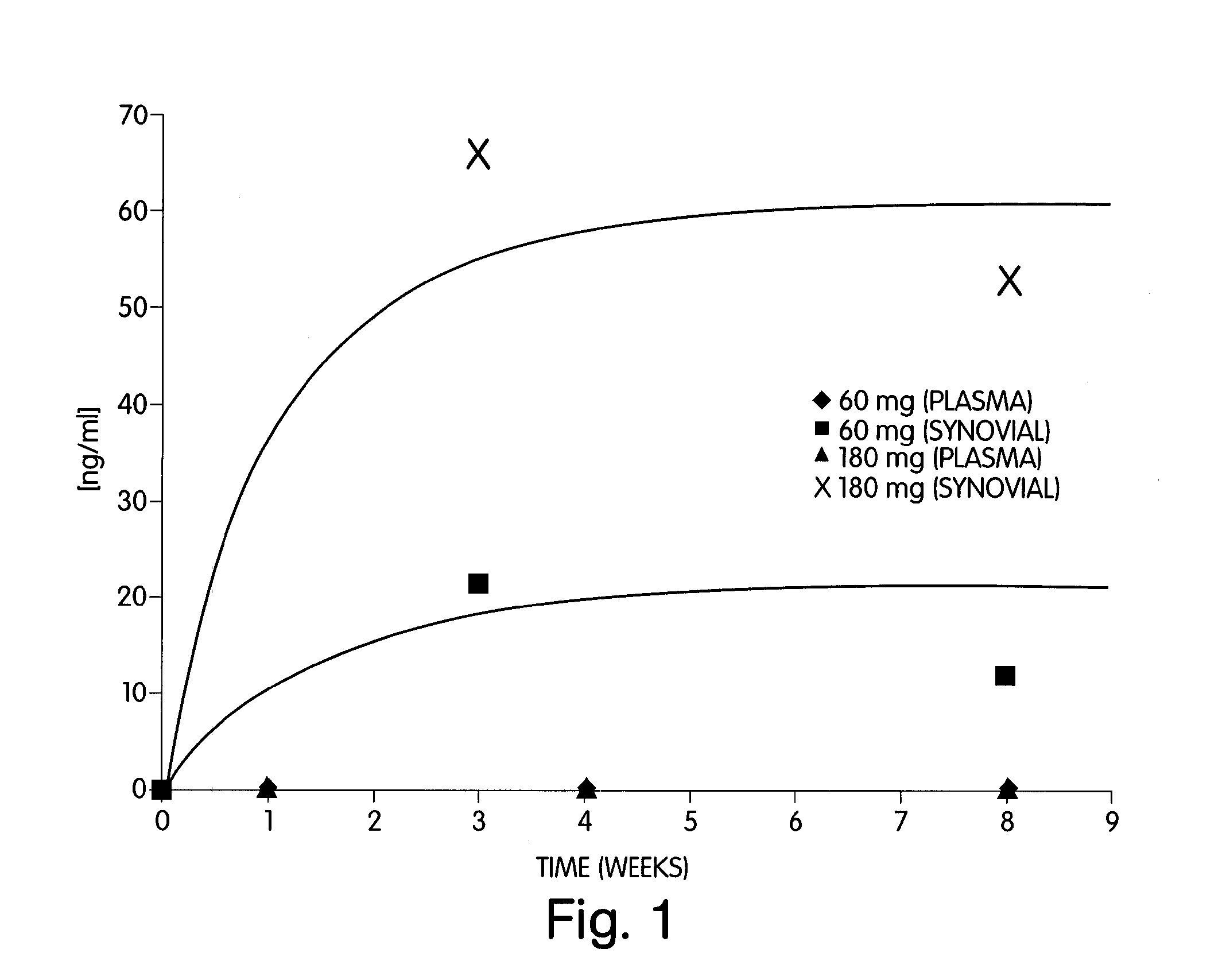
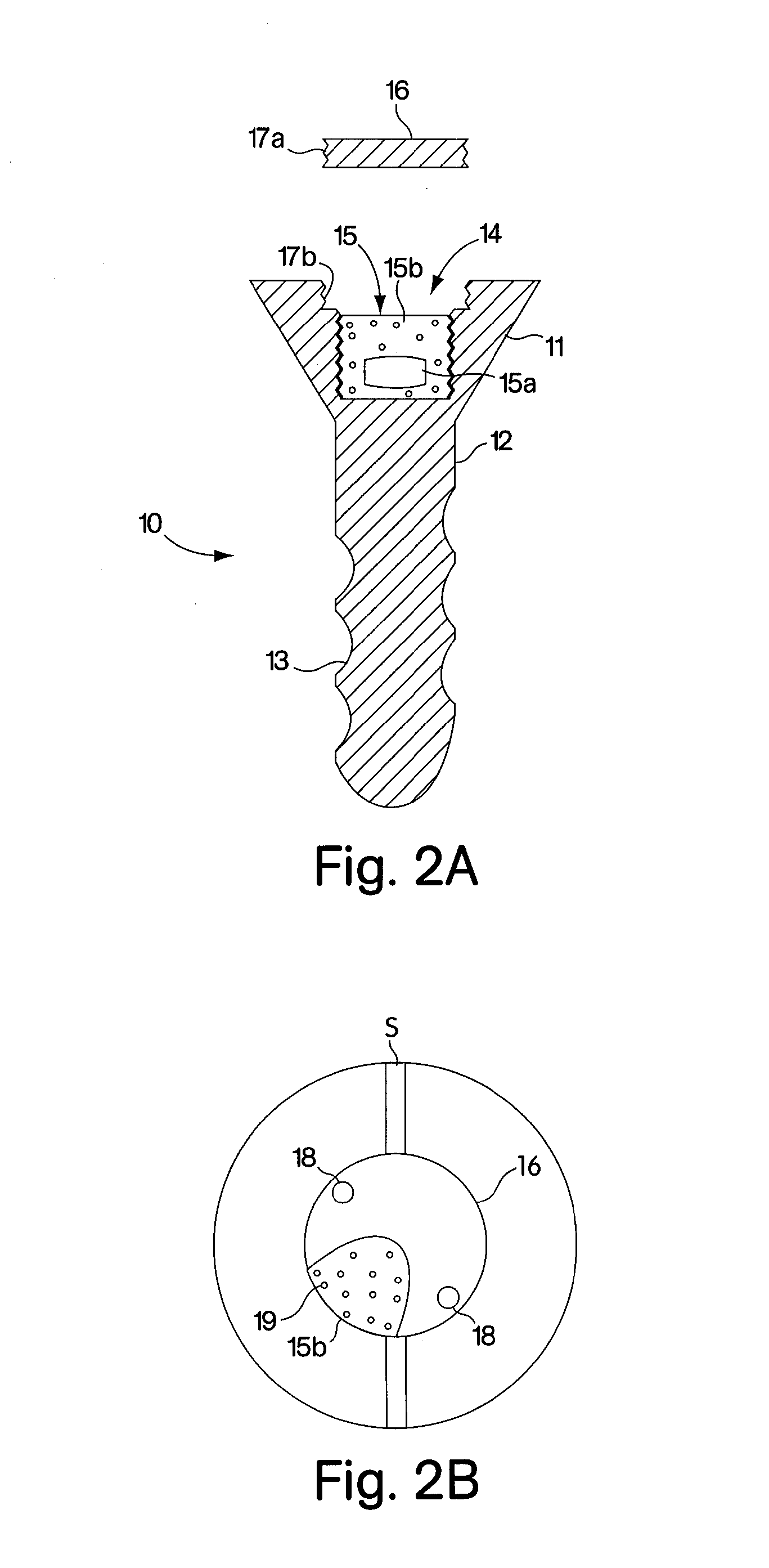
Abstract of Disclosure A therapeutically effective compound is locally administered by associating the compound with a piece of orthopedic hardware that is implanted at an appropriate site within a body. The compound is adapted, such as through a sustained release device, to administer an effective dosage continuously over an extended period of time. The compound may be administered, for example, to a joint of a mammal by intraarticularly implanting a sustained release device to deliver the therapeutically effective compound within a synovial capsule of the joint, such that synovial fluid concentration of the compound is greater than plasma concentration of the compound. A wide range of orthopedic hardware, such as bone screws and staples, may be adapted to use in the systems described herein to provide treatment for a variety of medical conditions.
Owner:EYEPOINT PHARMA US INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com