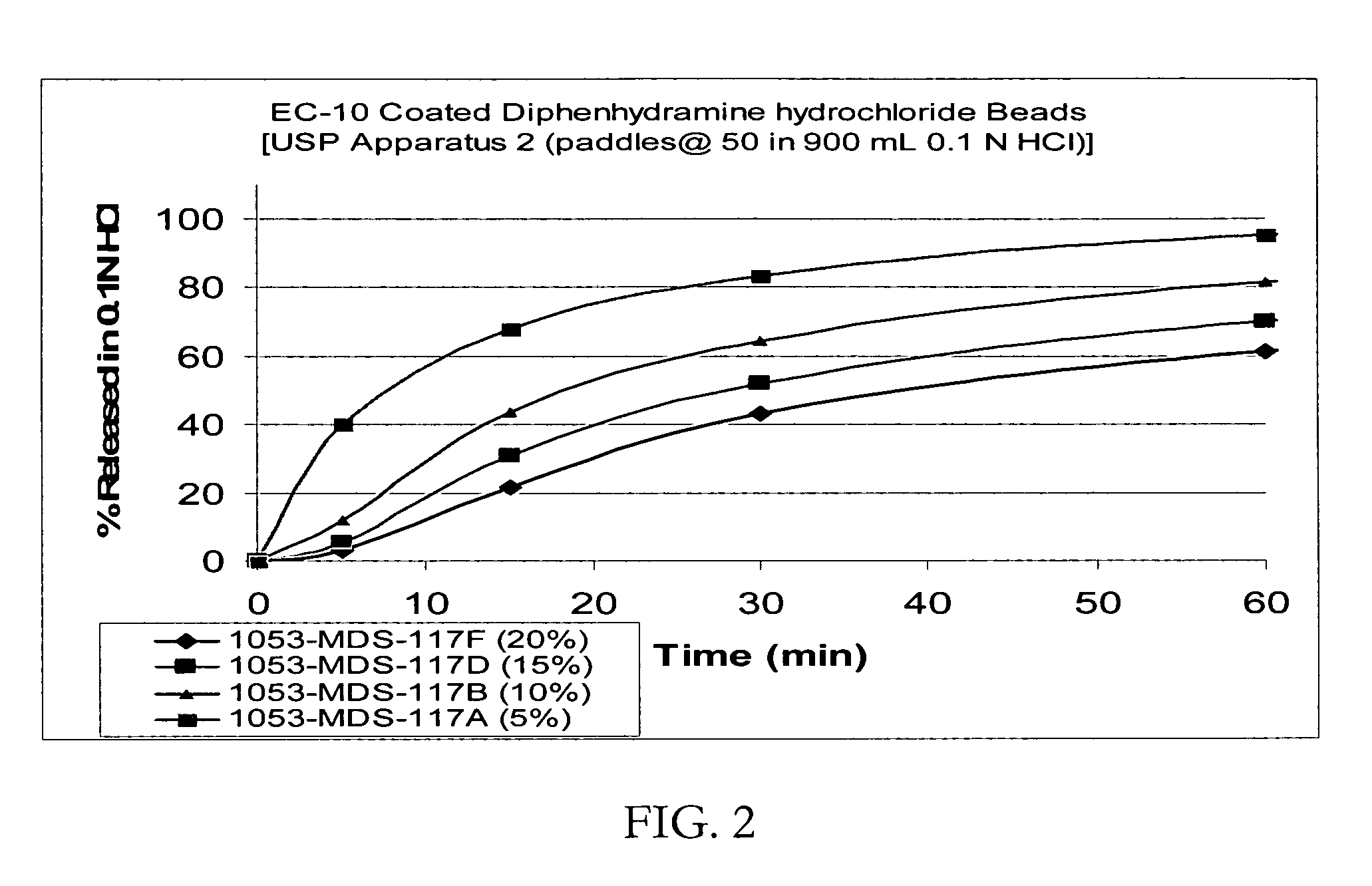
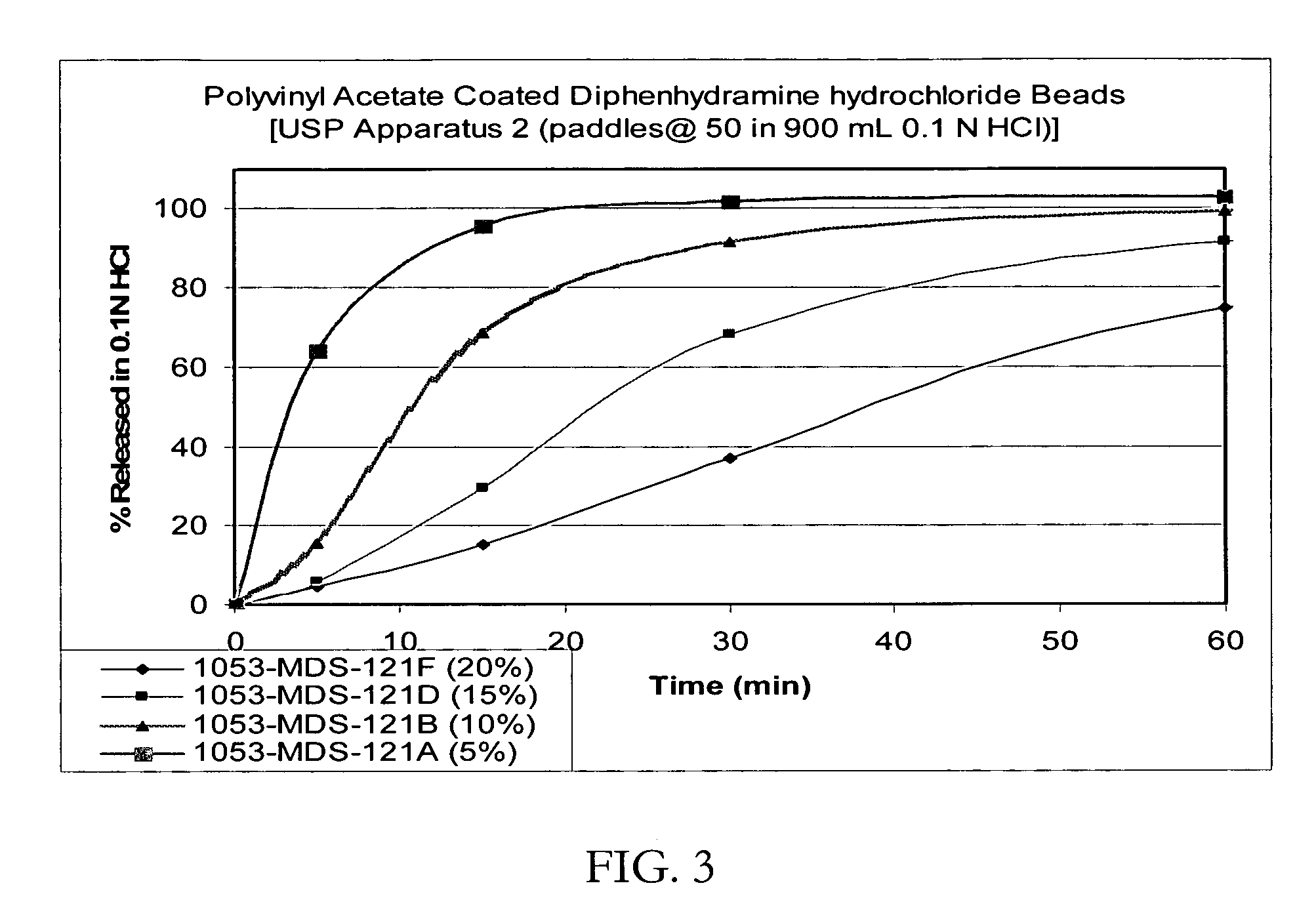
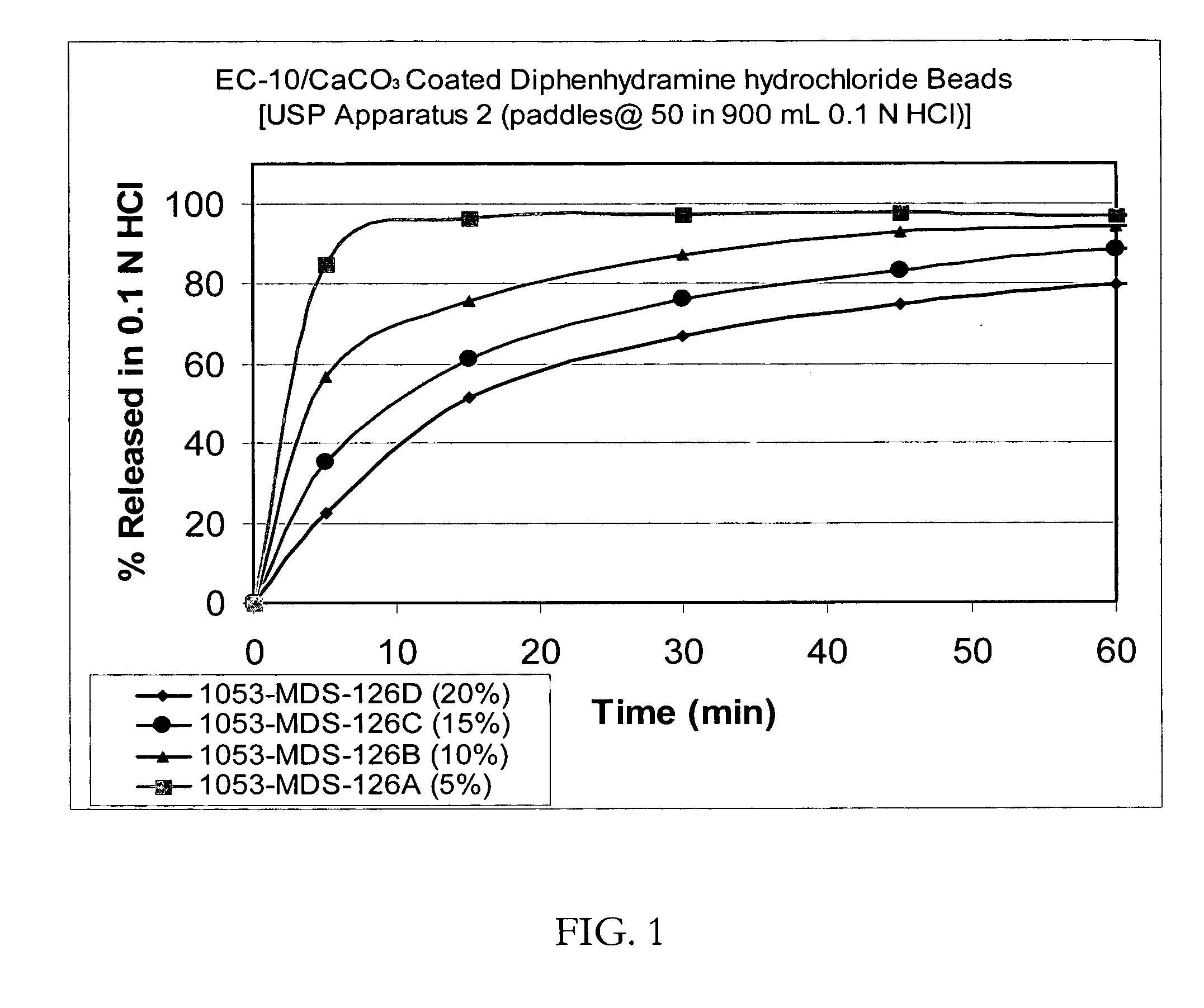
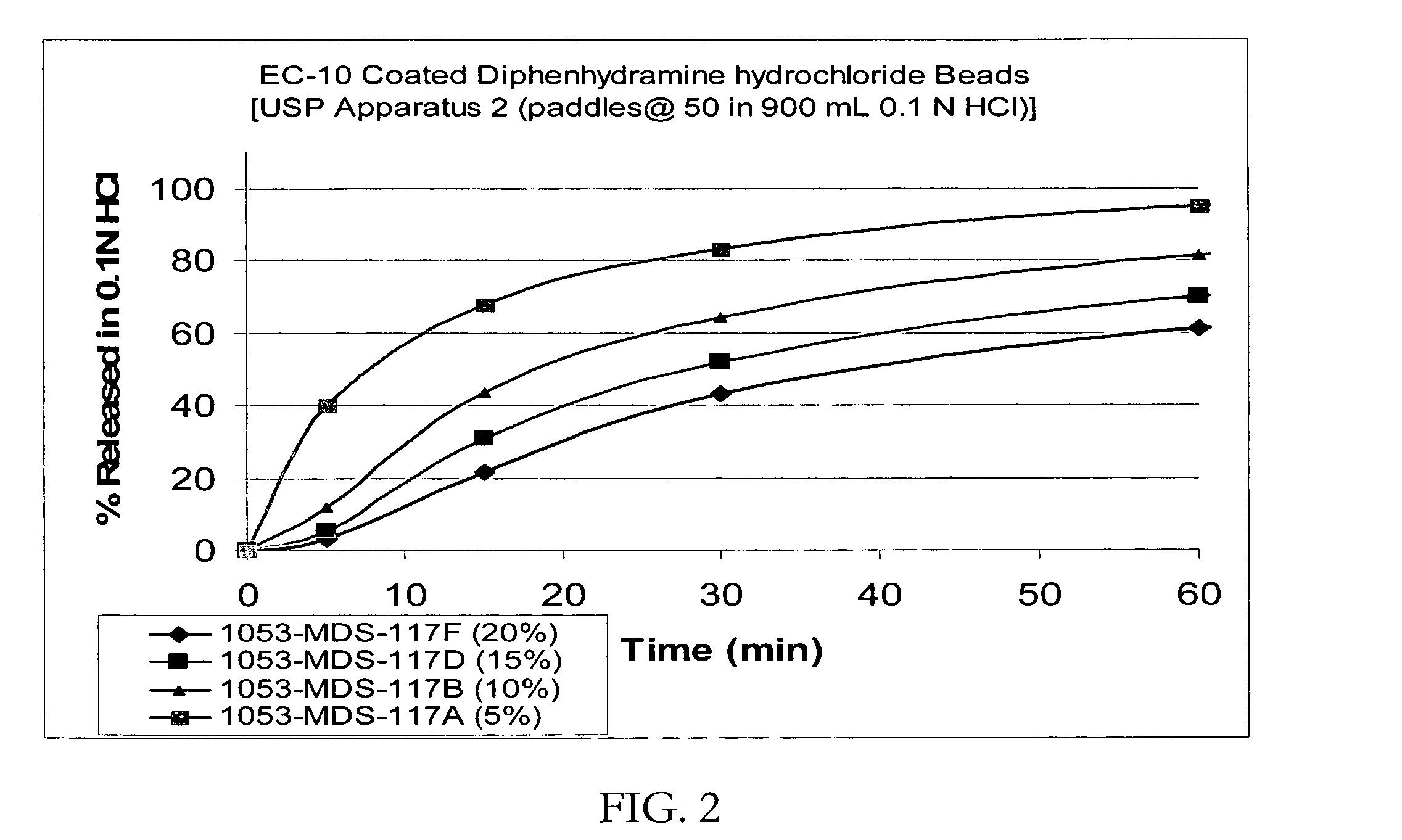
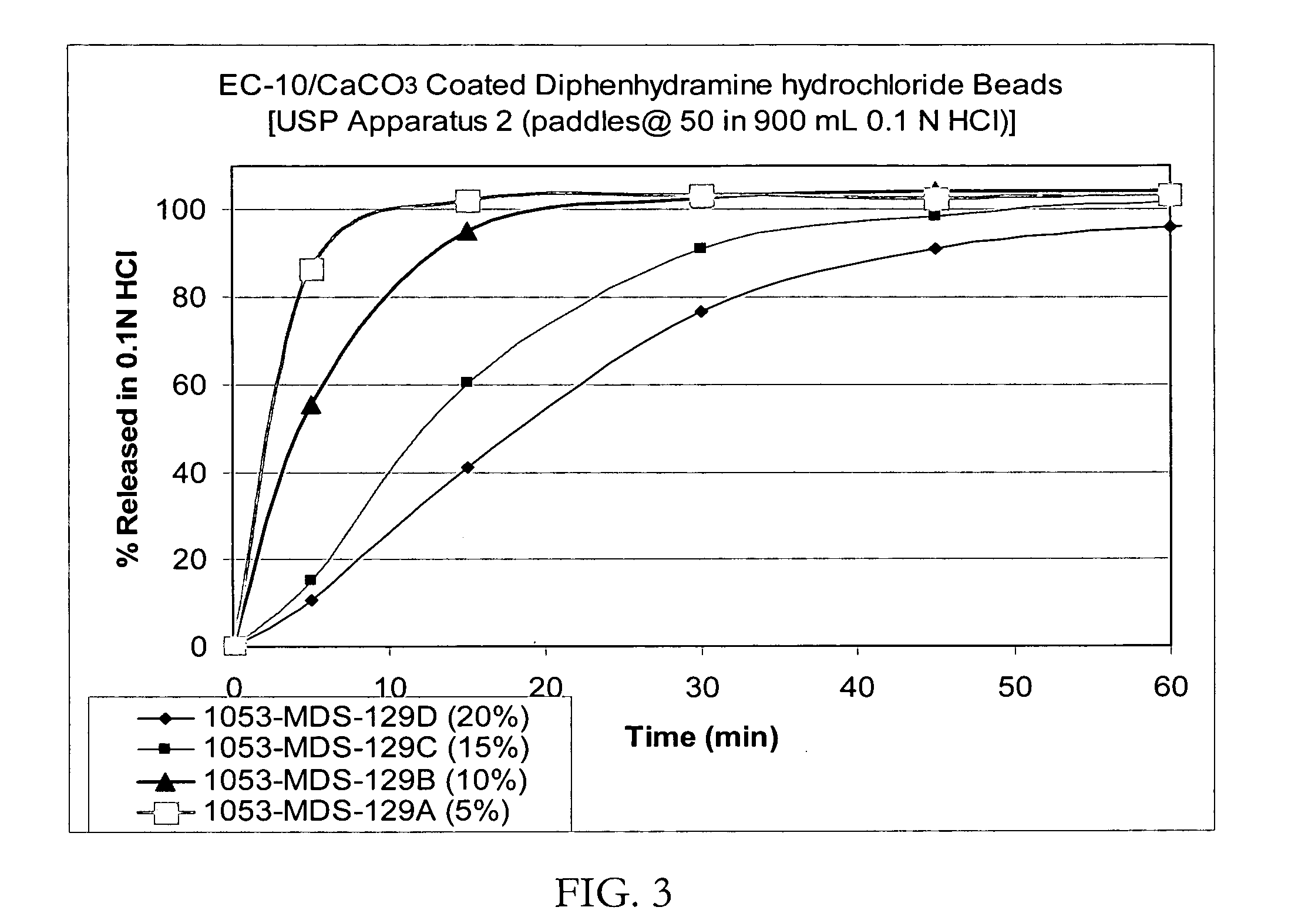
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
424 results about "Taste masking" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This is one of the simplest techniques used in taste masking. Excipients such as sweeteners and flavorants can be added to taste mask bitter API. Sweeteners, being highly water soluble, dissolve in saliva and coat the taste buds, thereby retarding the interaction of bitter API with taste buds.
Rapidly releasing and taste-masking pharmaceutical dosage form
InactiveUS6221402B1Inhibition releaseGood drug release profilePowder deliveryBiocideTaste maskingDosage form
A rapidly-releasing and taste-masking pharmaceutical dosage form and a process for preparing such oral dosage form are disclosed.
Owner:PFIZER PHAMACEUTICALS INC
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20060105038A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
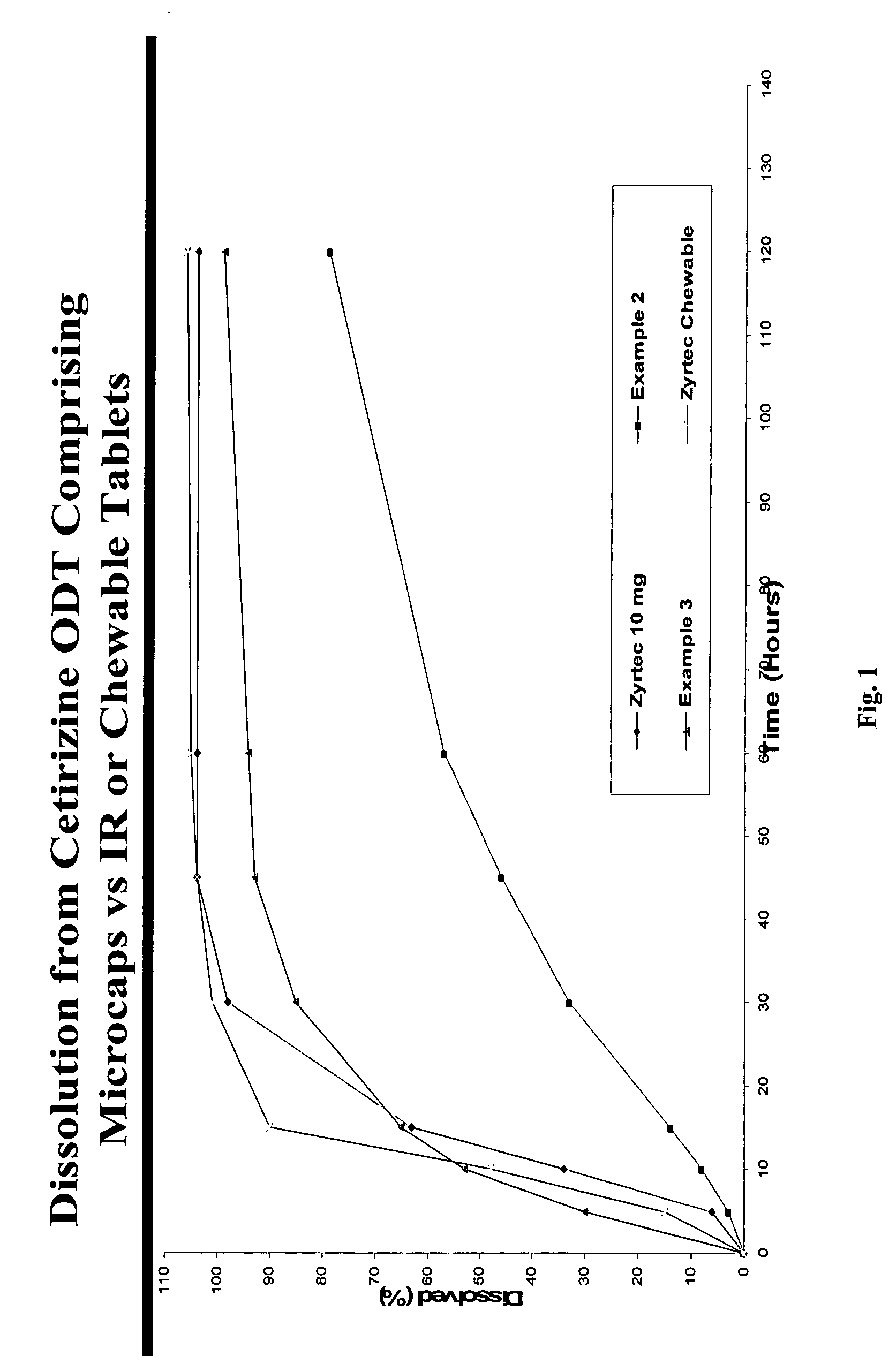
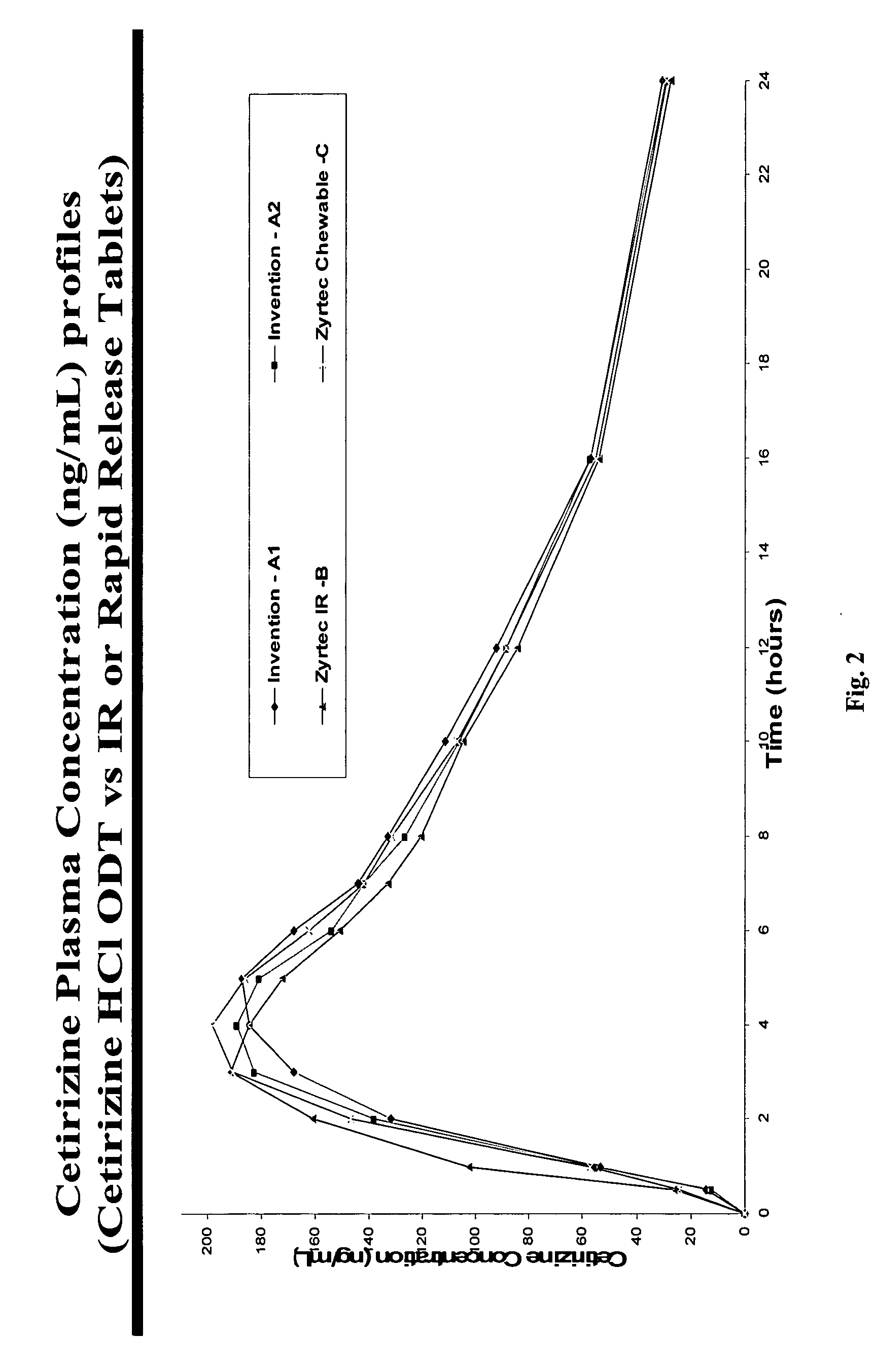
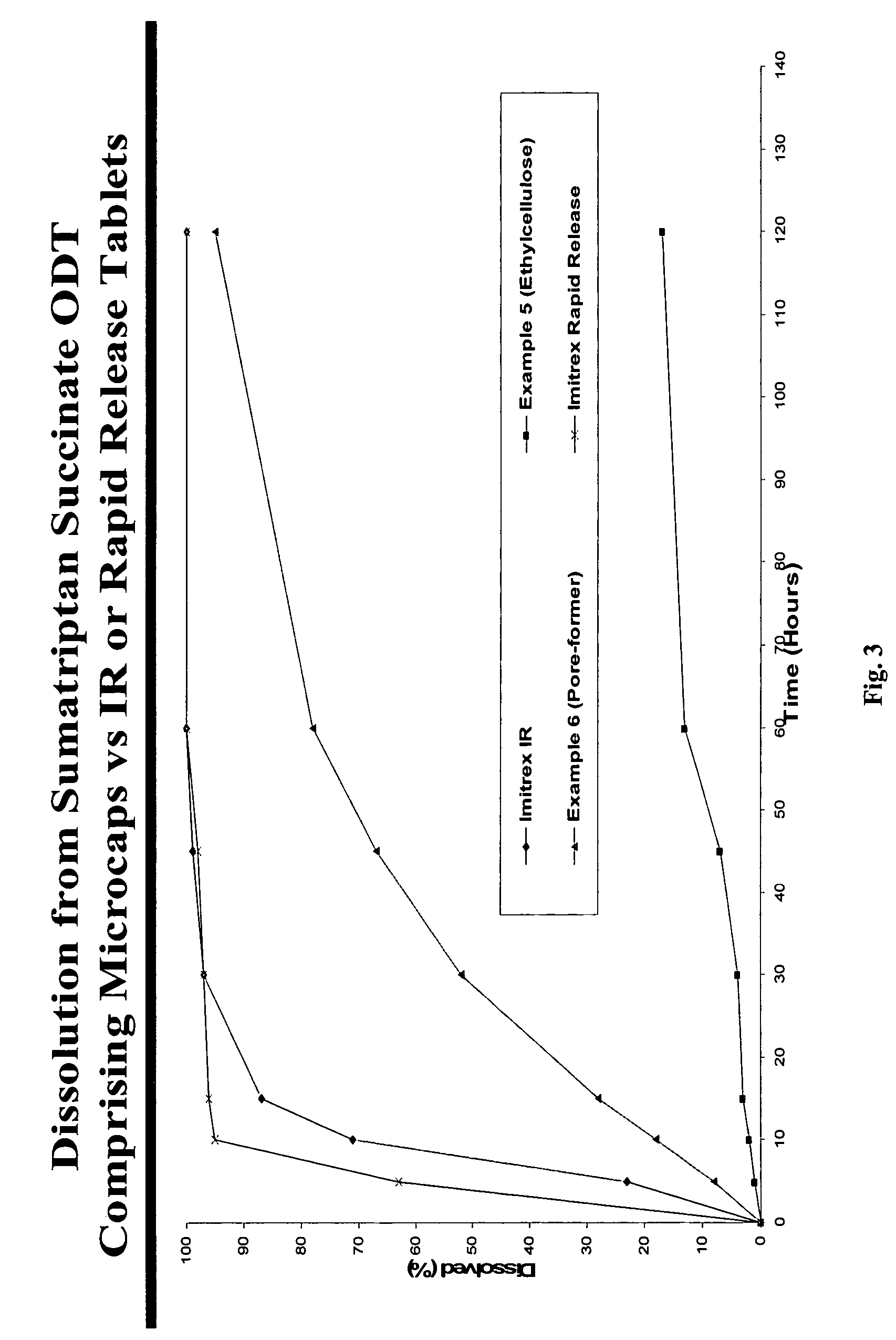
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Taste-masked pharmaceutical compositions with gastrosoluble pore-formers
InactiveUS20060105039A1Effective taste-maskingRapid/complete releaseOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active), coated with a taste-masking membrane comprising a water-insoluble polymer and one or more gastrosoluble inorganic or organic pore-formers (practically insoluble in water and saliva, but soluble in an acidic buffer), exhibit acceptable taste-masking when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:ADARE PHARM INC
Liquid compositions of calcium acetate
ActiveUS20080021104A1Patient compliance is goodLess side effectsBiocideOrganic chemistryAcetic acidAqueous solution
The invention relates to an aqueous liquid composition of calcium acetate, sweetener, and taste masking agent. Also provided is a method for binding phosphorus within the gastrointestinal tract of an individual by administering to the individual an aqueous solution of at least calcium acetate.
Owner:LYNE LAB
Uniform films for rapid dissolve dosage form incorporating taste-masking compositions
ActiveUS20080044454A1Evenly distributedMinimize incorporationPowder deliveryInorganic non-active ingredientsControl releaseOral medication
The present invention relates to rapid dissolve thin film drug delivery compositions for the oral administration of active components. The active components are provided as taste-masked or controlled-release coated particles uniformly distributed throughout the film composition. The compositions may be formed by wet casting methods, where the film is cast and controllably dried, or alternatively by an extrusion method.
Owner:AQUESTIVE THERAPEUTICS INC
Fast dissolving orally consumable films containing a sweetener
InactiveUS20030211136A1Fast deliverySufficient toleranceBiocideCosmetic preparationsActive agentSweetness
A consumable film adapted to adhere to and dissolve in the oral cavity of a warm-blooded animal including humans, comprising at least one water soluble polymer, a taste masking effective amount of a sweetener, and a pharmaceutically active agent having a sufficiently unpleasant taste that it is desirably masked by the sweetener.
Owner:MCNEIL PPC INC
Neramexane MR matrix tablet
InactiveUS20070141148A1Low peak concentrationReduce absorptionBiocideSenses disorderRegimenModified Release Dosage Form
The invention provides novel oral modified release dosage forms of neramexane which are useful for the continuous therapy of patients suffering from diseases and conditions such as Alzheimer's dementia and neuropathic pain. The compositions have drug release profiles that are suitable for achieving steady state plasma concentrations of neramexane which have relatively small fluctuation when administered on a twice-daily or even once-daily regimen. The dosage forms may be designed as modified release matrix tablets, which are optionally coated for taste masking. The invention further provides therapeutic methods of treating conditions such as Alzheimer's dementia and neuropathic pain which involve the administration of such dosage forms.
Owner:MERZ PHARMA GMBH & CO KGAA
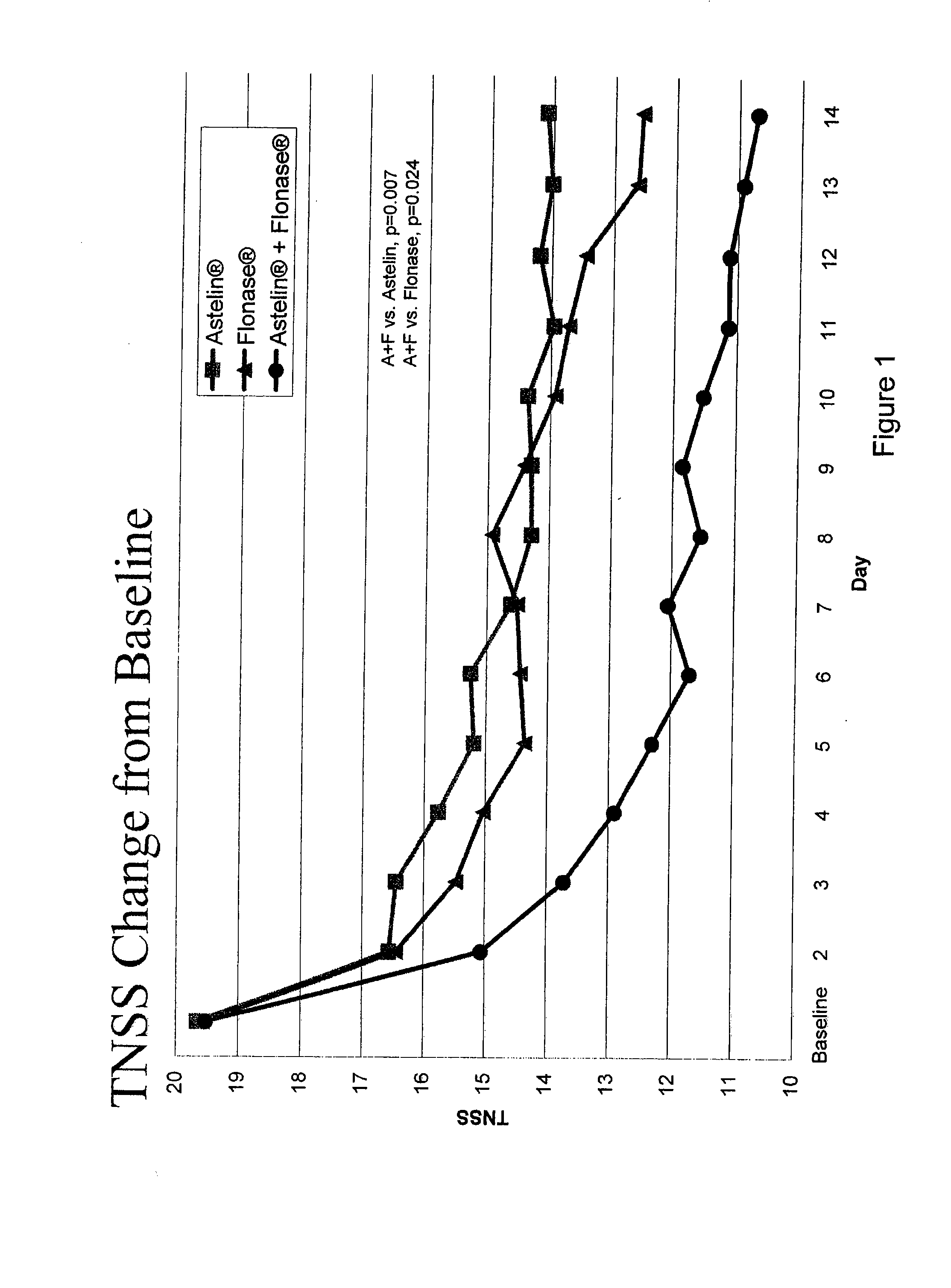
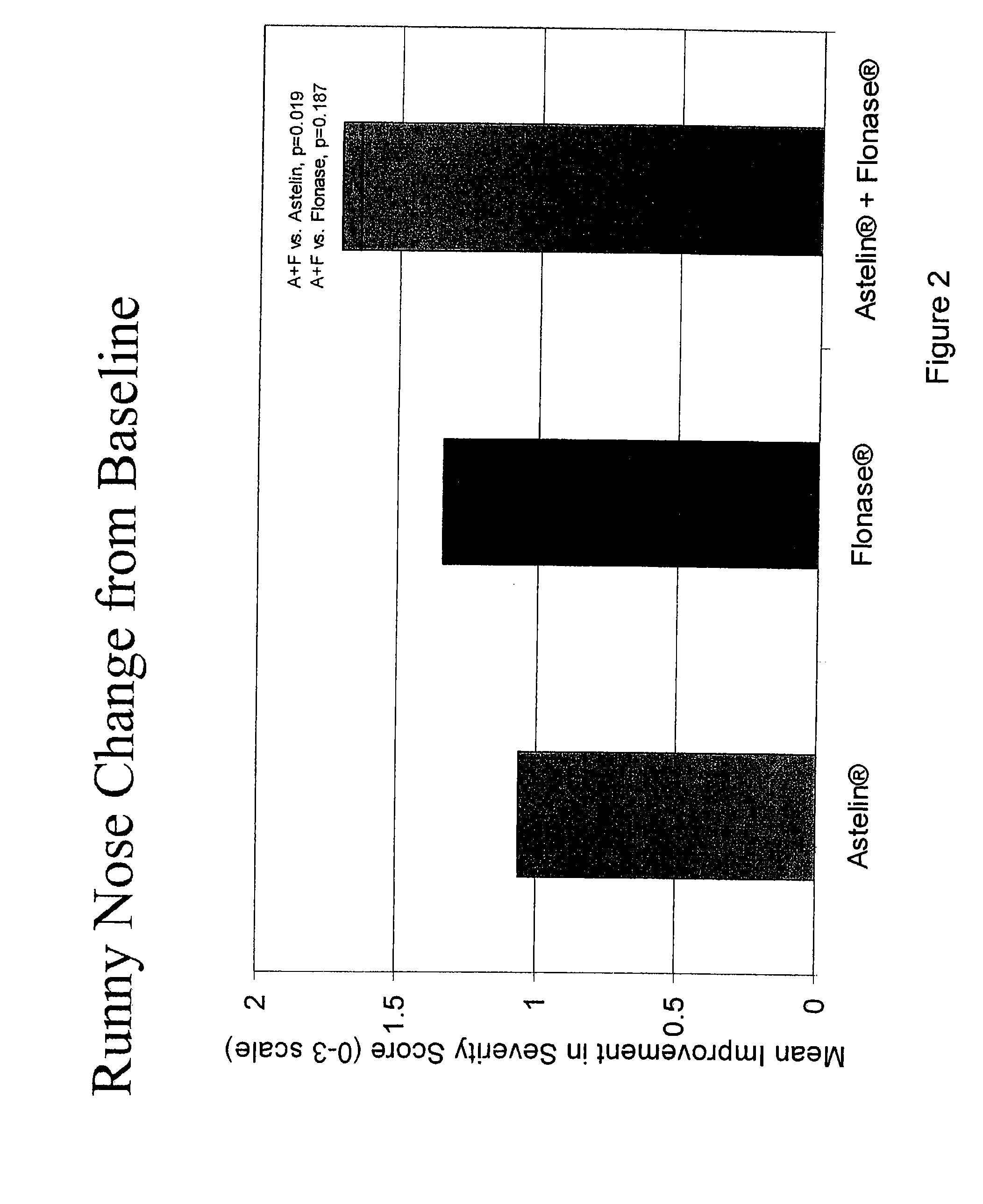
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Microencapsulation process and product
A composition comprising a core material, having a taste value and a polymeric coating. The polymeric coating substantially surrounds the core material and comprises a cationic polymer and optionally an anionic polymer. The polymeric coating has a uniform thickness ranging from 2 μm to 20 μm. The composition provides release of a portion of the core material which is taste masked over a time period ranging from 0.5 minute to 2 minutes in the oral cavity and provides a modified-release of the remaining core material in a gastrointestinal tract.
Owner:SPI PHARMA
Fast-melting tablets having taste-masking and sustained release properties
Fast-melting tablets contain particles of an active ingredient and ion-exchange resin complex to mask unpleasant taste associated with the active ingredient. The resin complex particles can be coated or uncoated to impart sustained release properties to the active ingredient. A fast-melting tablet also comprises a dry binder and bulk diluent to form highly plastic granules that are subsequently compressed into tablets.
Owner:AKINA INC
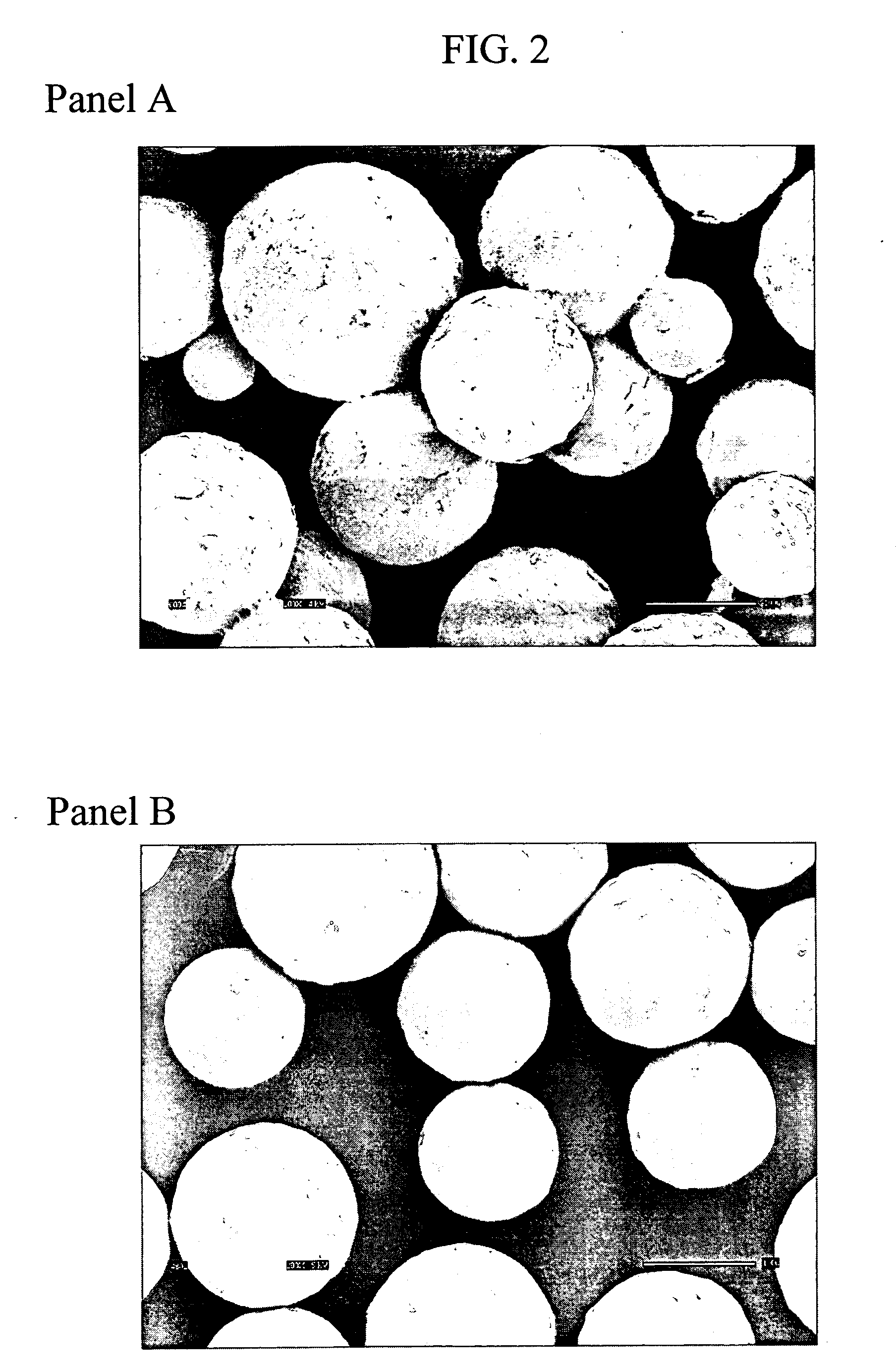
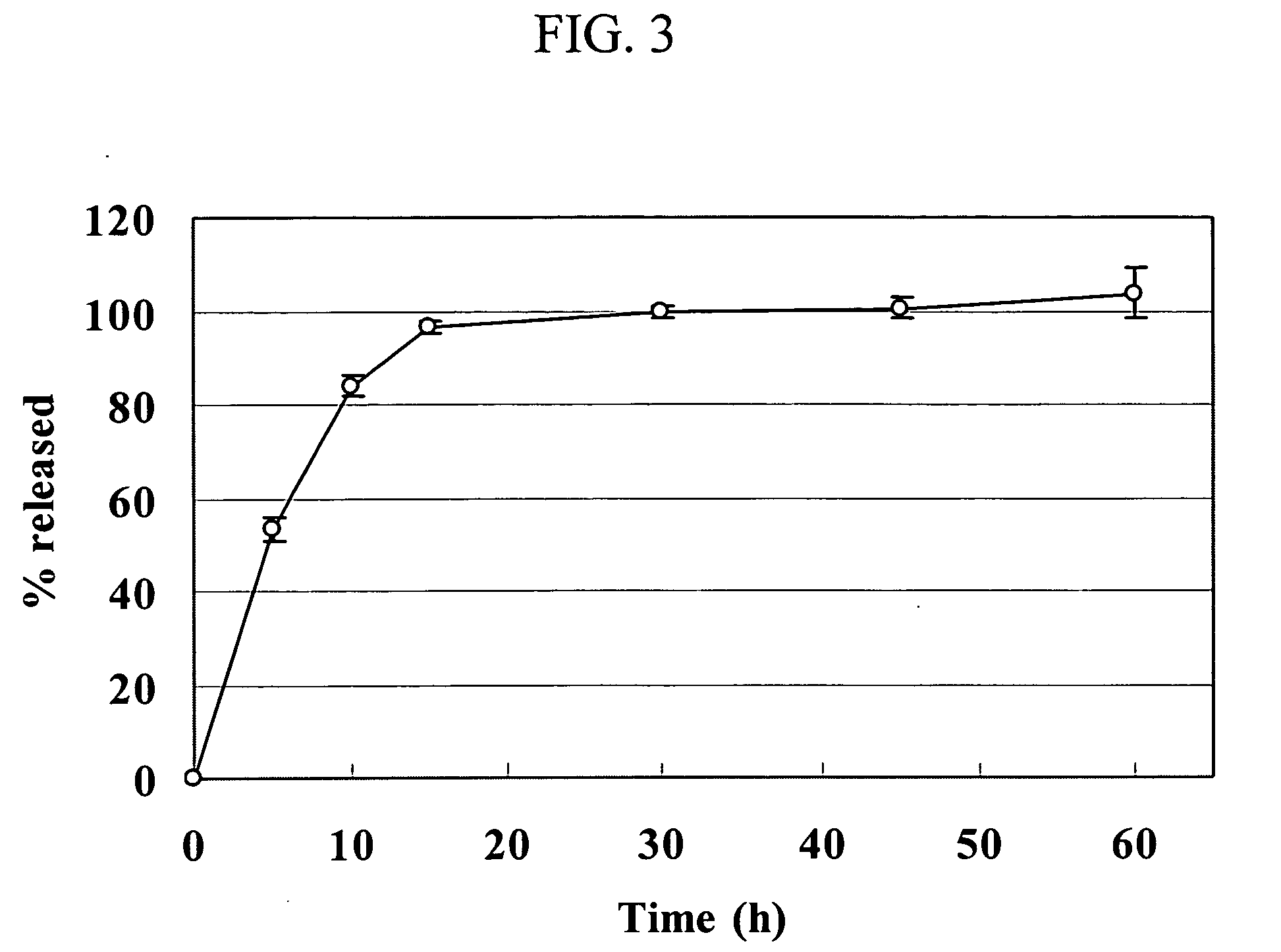
Food bars containing nutritional supplements
Owner:PBM PHARMA
Uniform films for rapid dissolve dosage form incorporating taste-masking compositions
ActiveUS8603514B2Minimize incorporationImprove uniformityPowder deliveryInorganic non-active ingredientsControlled releaseOral medication
The present invention relates to rapid dissolve thin film drug delivery compositions for the oral administration of active components. The active components are provided as taste-masked or controlled-release coated particles uniformly distributed throughout the film composition. The compositions may be formed by wet casting methods, where the film is cast and controllably dried, or alternatively by an extrusion method.
Owner:AQUESTIVE THERAPEUTICS INC
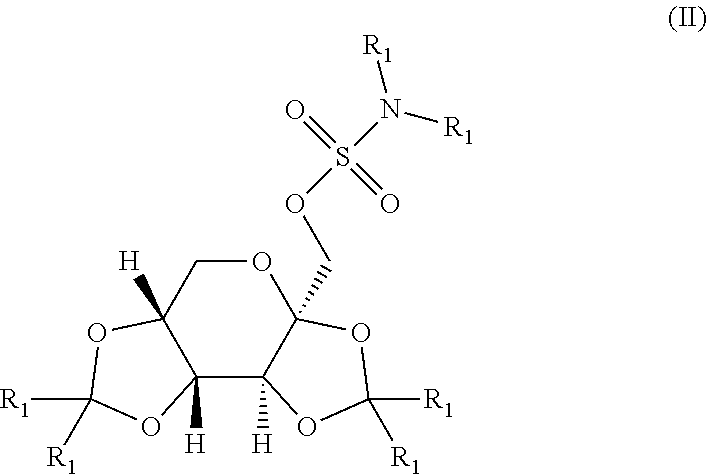
Taste masked topiramate composition and an orally disintegrating tablet comprising the same
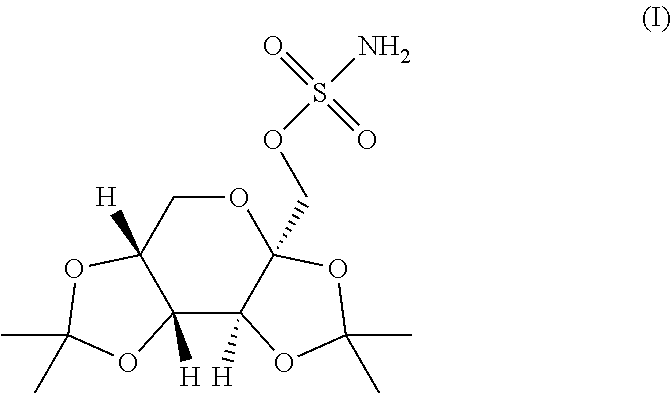
In various embodiments, the present invention is directed to a taste masked pharmaceutical composition comprising a therapeutically effective amount of taste masked sulfamate-substituted monosaccharide particles comprising a sulfamate-substituted monosaccharide or a pharmaceutically acceptable salt or derivative thereof that are coated with one or more taste-masking layers, and optionally one or more of taste-masked neltrexone, 5-HT3 receptor antagonist, phentermine, and vitamin B-12. The present invention relates to methods of making the taste masked and ODT compositions, and methods of using the compositions for treating a patient subject to an epileptic condition, migraines, dysphagia, achieving / maintaining weight loss, or alcoholism or drug addiction.
Owner:APTALIS PHARMATECH
Progestin-containing drug delivery system
The present invention relates to drug delivery compositions in the form of thin water-soluble films (wafers), which contain small particles that comprise at least one progestin and at least one protective agent. The protective agent provides effective taste-masking of the progestin due to limited release of the progestin in the mouth. The progestin is hence not absorbed via the buccal route, but rather via the enteral (per-oral) route.
Owner:BAYER SCHERING PHARMA AG
Multilayer tablets containing thiazolidinedione and biguanides and methods for producing them
InactiveUS20060057202A1Facilitated releaseEfficient compressionBiocideOrganic active ingredientsAcute hyperglycaemiaImmediate release
A novel patient-convenient, cost effective pharmaceutical composition, comprising of thiazolidinediones and biguanide for controlling hyperglycemia manufactured as multilayer tablet and its process of manufacturing, for immediate release of thiazolidinediones or thiazolidinediones and biguanide and prolonged release of the biguanide only, the tablet comprising of minimum two layers wherein one outer layer comprises of a mixture of excipients and thiazolidinediones or thiazolidinediones and biguanide allowing immediate release of thiazolidinediones or thiazolidinediones and biguanide respectively and the other layer arranged in contact with the immediate release layer which comprises of a novel composition of excipients and a minimum one or more non-biodegradable, inert polymer(s) and the biguanide allowing pH independent prolonged release of the biguanide up to a period of 8-12 hours. The tablets are for once a day dosing. The tablets may optionally be film coated or enrobed by soft gelatin ribbons for additional protection against oxidation, photodegradation, identification, ease of swallowing, taste masking and for aesthetic appeal without altering the dissolution profile.
Owner:INVENTIA HEALTHCARE LTD
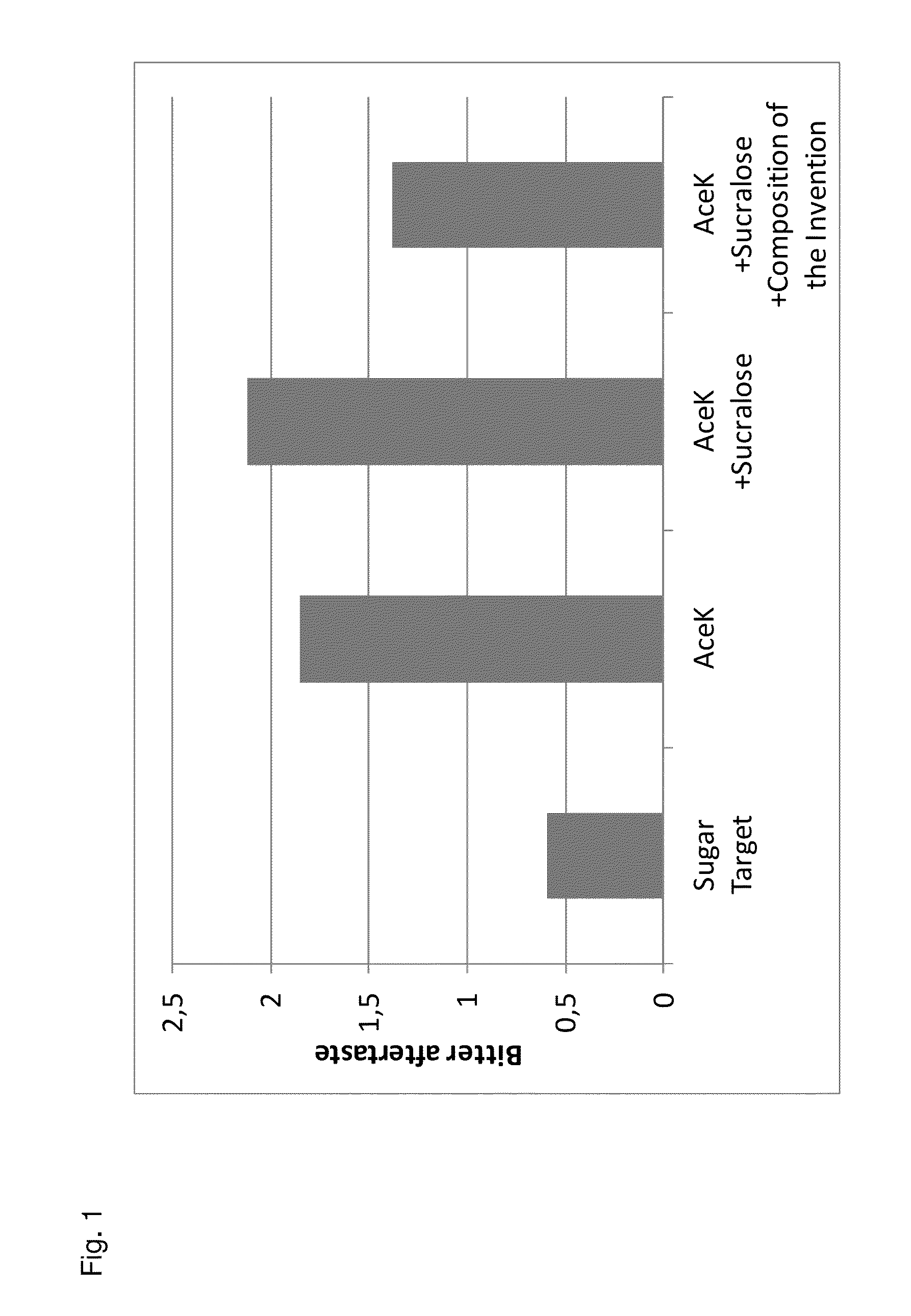
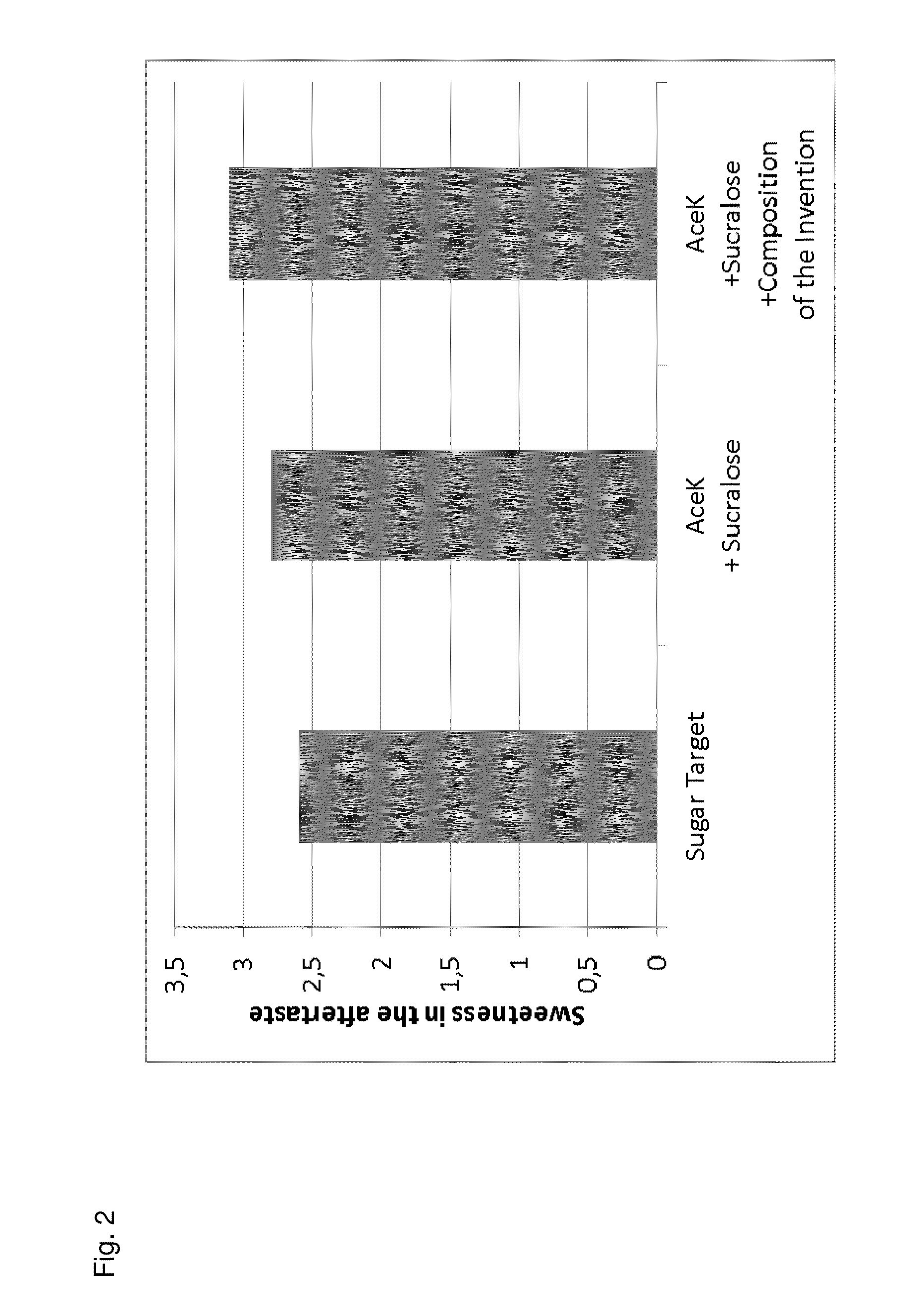
Sucralose formulations to mask unpleasant tastes
InactiveUS20060121066A1Unexpected synergy of bitter taste masking effectivenessBiocideOrganic active ingredientsSweetnessExcipient
The present invention is directed to a pharmaceutically acceptable taste masking liquid excipient base for administration of a relatively large amount of unpleasant tasting medicines. More particularly, the enhanced sweetness and taste masking effect are produced by the addition of sucralose to the excipient base with maintenance of a pH from about 2 to about 5. The invention is further directed to medicinal compositions comprising such a liquid excipient base and unpleasant tasting medicines. Still further, the invention is directed to a method for taste masking unpleasant tasting medicines through their incorporation into the claimed liquid excipient bases.
Owner:WYETH LLC
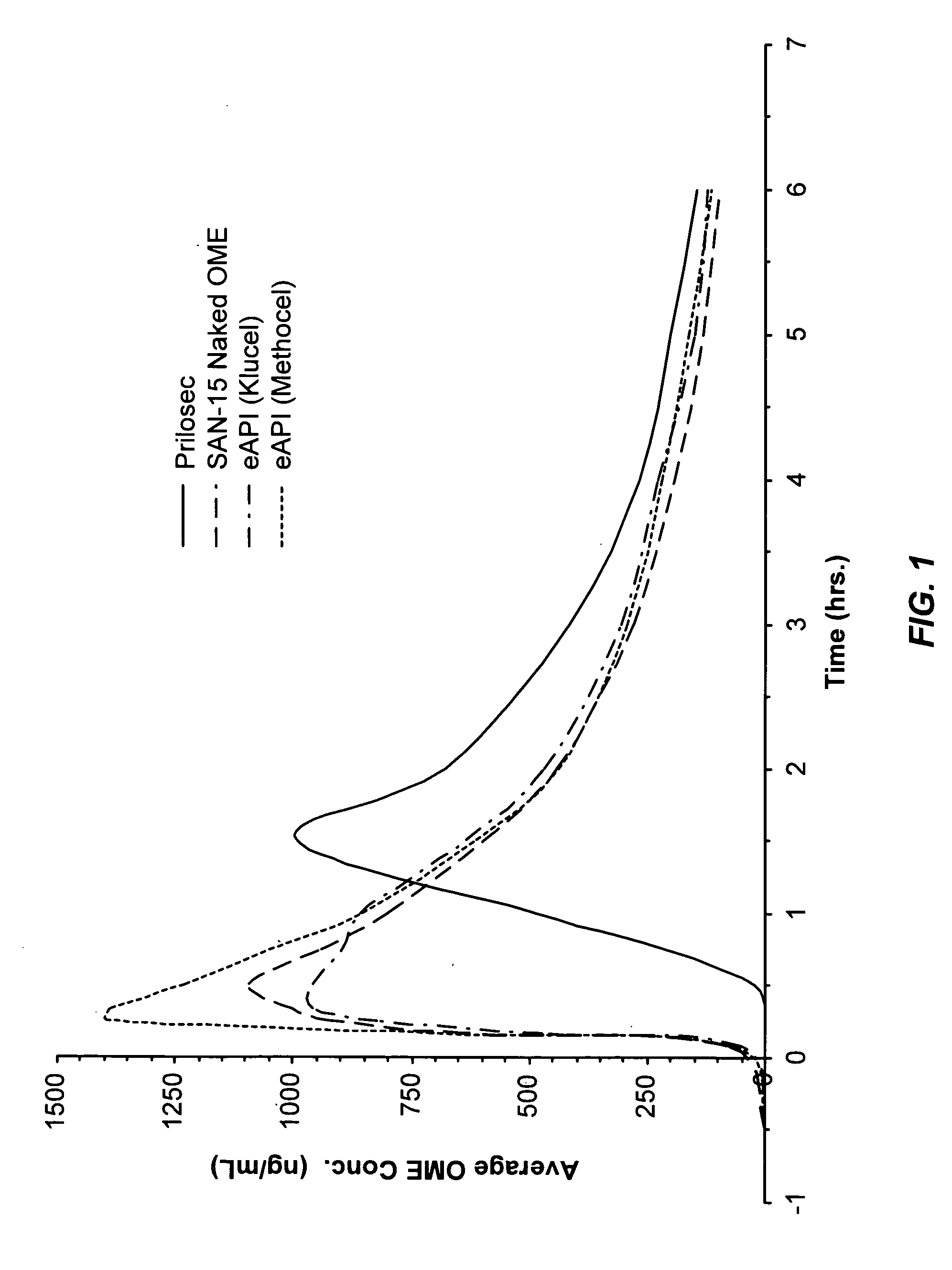
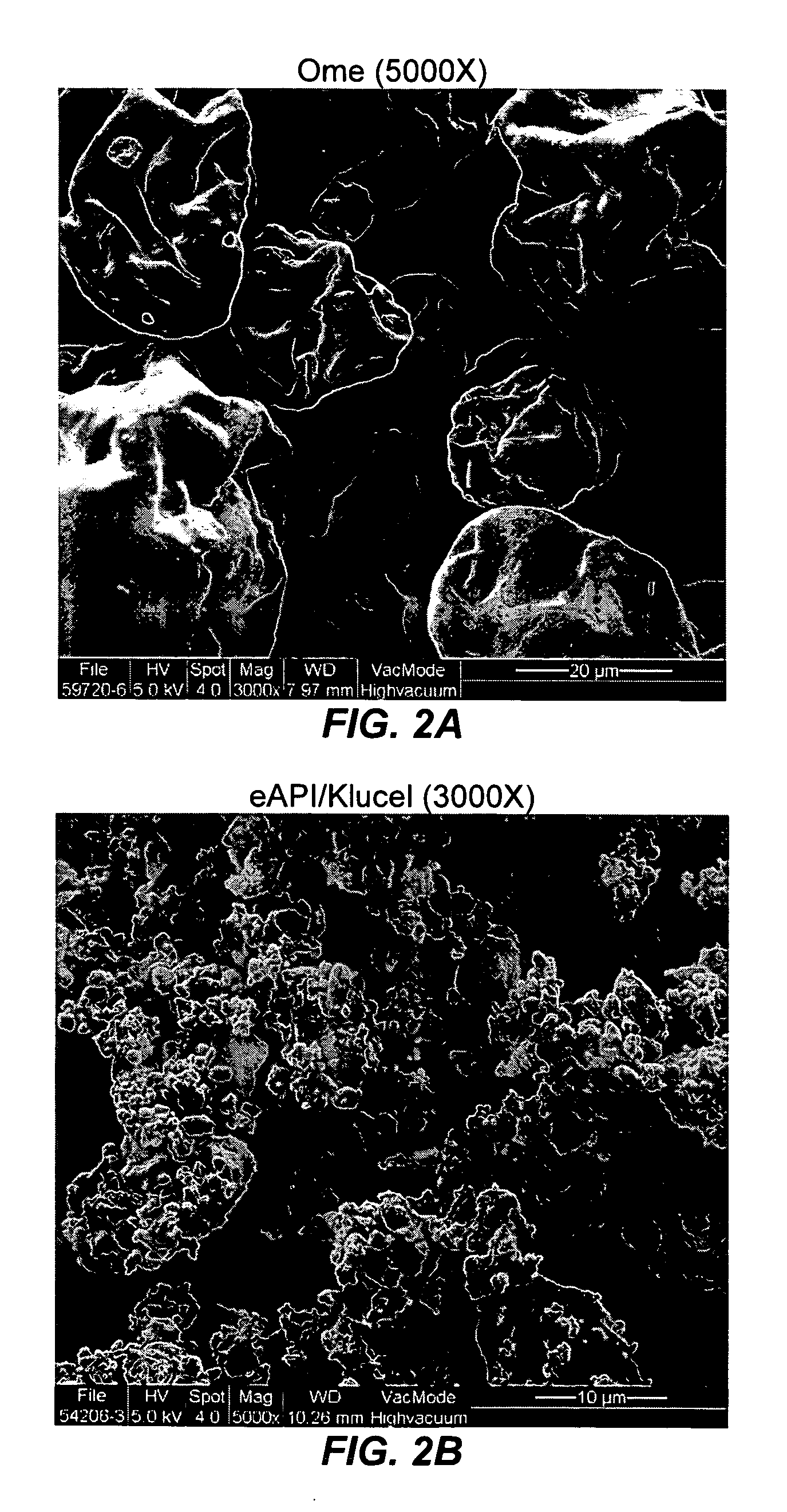
Pharmaceutical formulatins useful for inhibiting acid secretion and methods for making and using them
InactiveUS20050037070A1Extended shelf lifeEnhance processing and performancePowder deliveryBiocideSecretionSecreted substance
In one general aspect of the present invention, pharmaceutical formulations comprising both a proton pump inhibitor microencapsulated with a material that enhances the shelf-life of the pharmaceutical composition and one or more antacid are described. In another general aspect of the present invention, pharmaceutical formulations comprising both a proton pump inhibitor microencapsulated with a taste-masking material and one or more antacid are described.
Owner:SANTARUS
Taste-masked oral formulations of influenza antivirals
The present invention relates to taste-masked oral formulations of influenza antivirals. The taste-masked pharmaceutical formulations for oral administration comprise one or more influenza antivirals, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. Further, the taste-masked influenza antiviral formulations of the present invention are provided in the form of dispersible tablets, effervescent tablets, orally disintegrating tablets, chewable tablets, bite-dispersion tablets or the like, wherein the bitter taste of influenza antivirals is masked thereby providing palatable formulations.
Owner:RUBICON RES PTY LTD
Taste masked pharmaceutical compositions comprising bitter drug and pH sensitive polymer
InactiveUS20050136114A1Improve palatabilityA large amountPowder deliveryDispersion deliveryPH-sensitive polymersLiquid oral
The present invention discloses pharmaceutical compositions comprising of pH sensitive polymers used for taste masking highly bitter drugs. The pH sensitive polymer acts as a reverse enteric coating, which is soluble in the acidic pH range 1.0 to 3.0 normally found in the stomach but is insoluble in the pH range 3.5 to 7 thus inhibiting the release of the bitter drug at the pH of saliva and also at the pH of reconstitution medium in case of liquid orals.
Owner:COUNCIL OF SCI & IND RES
Taste masking pharmaceutical composition containing levocetirizine
InactiveUS20060083786A1Increase surface areaOrganic active ingredientsPill deliveryAdditive ingredientLevocetirizine hydrochloride
A solid oral dosage composition is provided comprising a prophilactically or therapeutically effective amount of an active pharmaceutical ingredient comprising levocetirizine or a pharmaceutically acceptable salt thereof, the solid oral dosage composition having a coating thereon capable of providing taste masking of the levocetirizine or pharmaceutically acceptable salt thereof.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Taste Making Formulation Comprising The Drug In A Dissolution-Retarded Form And/Or Cyclodextrin In A Dissolution-Enhanced Form
InactiveUS20080075784A1Great tasteBetter taste maskingBiocideOrganic active ingredientsCyclodextrinDissolution
Improved taste masking of pharmaceutical compositions of unpleasant-tasting drugs and cyclodextrin is achieved by forming a mixture of drug and cyclodextrin wherein the drug's dissolution rate has been retarded, or the the cyclodextrin's dissolution rate has been enhanced, or by both.
Owner:BEND RES
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Coating compositions for bitterness inhibition
InactiveUS7294347B2Mask bitternessImprove palatabilityPowder deliveryInksPH-sensitive polymersPh independent
The present invention discloses coating compositions with taste masking property, comprising a blend of pH sensitive polymers and optionally a pH independent polymer or a blend of the pH sensitive polymer and pH independent polymer used for taste masking of highly bitter drugs. The pH sensitive polymers used comprise the acid soluble polymers and the enteric polymers. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said coating compositions is disclosed. The concomitant use of the polymers inhibits the release of the bitter drug at the pH of saliva. The said coating compositions deliver substantial amount of the bitter drug immediately with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Controlled release and taste masking oral pharmaceutical composition
Controlled release and taste masking compositions containing one or more active principles inglobated in a three-component matrix structure, i.e. a structure formed by successive amphiphilic, lipophilic or inert matrices and finally inglobated or dispersed in hydrophilic matrices. The use of a plurality of systems for the control of the dissolution of the active ingredient modulates the dissolution rate of the active ingredient in aqueous and / or biological fluids, thereby controlling the release kinetics in the gastrointestinal tract.
Owner:COSMO TECH LTD
Controlled release and taste masking oral pharmaceutical composition
Controlled release and taste masking compositions containing one or more active principles inglobated in a three-component matrix structure, i.e. a structure formed by successive amphiphilic, lipophilic or inert matrices and finally inglobated or dispersed in hydrophilic matrices. The use of a plurality of systems for the control of the dissolution of the active ingredient modulates the dissolution rate of the active ingredient in aqueous and / or biological fluids, thereby controlling the release kinetics in the gastrointestinal tract.
Owner:COSMO TECH LTD
Taste-Masking Compositions, Sweetener Compositions and Consumable Product Compositions Containing the Same
In one embodiment, the present invention relates to a sweetener composition comprising: a sweetener and at least one flavoring. The at least one flavoring is suitable for modifying, masking, reducing and / or suppressing an unpleasant off-taste of the sweetener in a consumable product composition formed by adding the sweetener composition to a consumable product. A weight ratio of the at least one flavoring to the sweetener in the consumable product composition is such that the sweetness of the sweetener is detectable by taste in the consumable product composition and the flavor of the at least one flavoring is not detectable by taste in the consumable product composition.
Owner:CELANESE SALES GERMANY
Metered mixing technology for improved taste masking
InactiveUS20050232977A1Dissolve fastMinimized and eliminatedWood working apparatusGranular deliveryContact timeBitter taste
The present invention relates to metered mixing technology for producing a film type product having improved taste-masking properties of a drug substance. In one aspect, a film-based product and a process for making the product are provided wherein a drug substance coated with a taste-masking substance and a film-forming solution are suitably combined and mixed yet the contact time between the components is controlled such that a bitter taste quality is not sensed by a person ingesting the product.
Owner:MCNEIL PPC INC
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20090263480A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryWater insolubleAdditive ingredient
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
Chewy products and methods for making the same
InactiveUS20080008742A1Low viscosityPharmaceutical non-active ingredientsPill deliveryEmulsionActive agent
Methods for producing chewable emulsion compositions and compositions produced from such methods are disclosed. The emulsion base may be prepared by contacting an aqueous phase and a nonaqueous phase in the presence of an emulsifier. The aqueous phase is prepared under conditions that permit no substantial moisture loss and reduced viscosity. The methods permit preparing chewable compositions for delivering confectionary items, nutriceuticals, vitamins, minerals and therapeutically active agents. The compositions may be sugar-free or sugar-containing. The active agent may be added to the emulsion base at a lower temperature compared to the temperature at which either the aqueous or nonaqueous phase is formed. The compositions may be further coated with taste-masking materials, enteric coating materials, or slow-release materials. The compositions may be formed into compressible tablets, beads, granules, or otherwise generally known dosage forms.
Owner:CAPRICORN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com