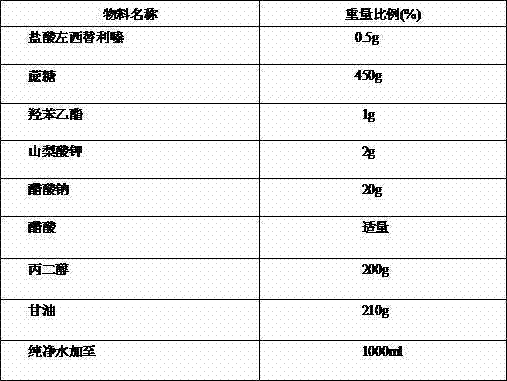
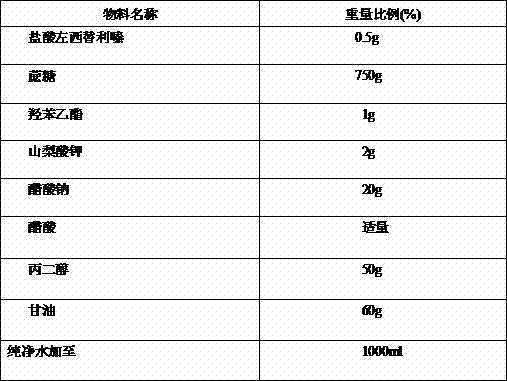
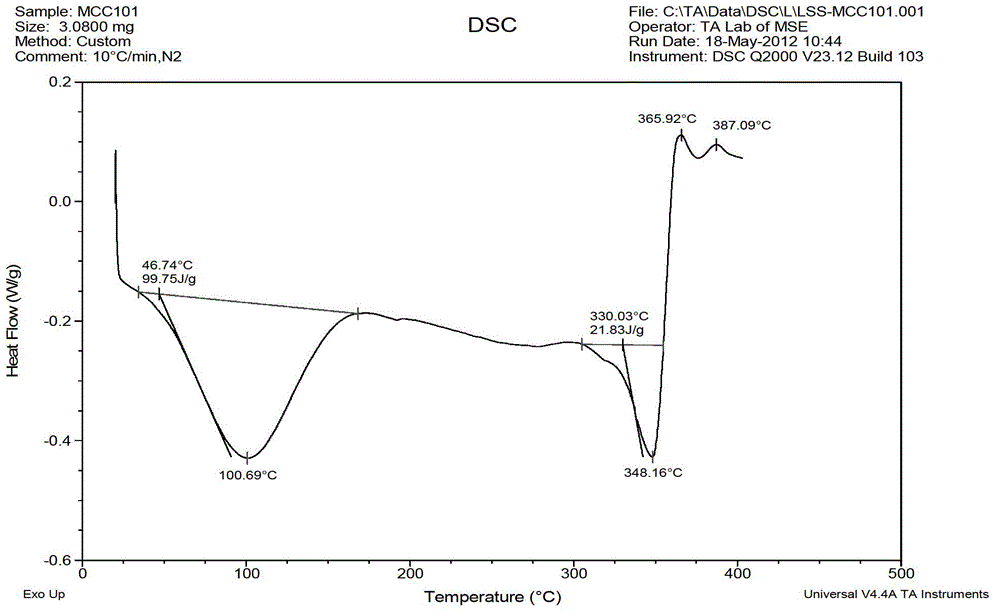
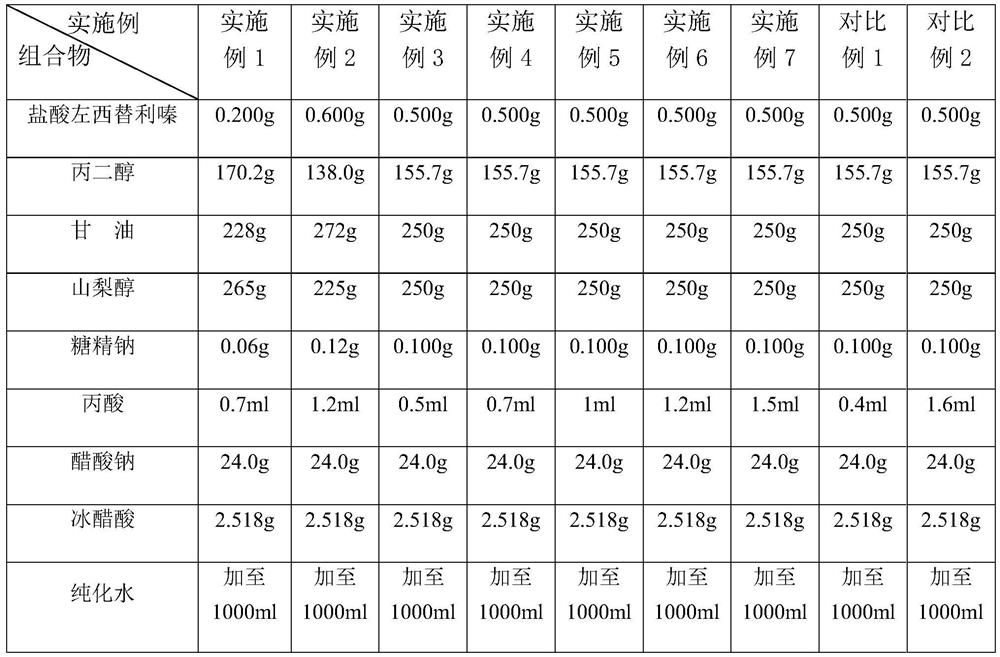
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
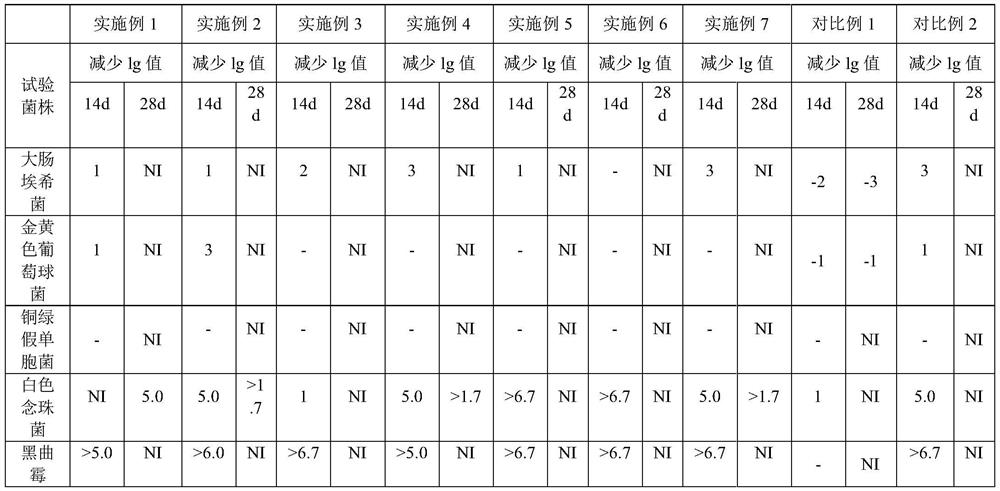
52 results about "Levocetirizine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Levocetirizine (as levocetirizine dihydrochloride) is a third-generation, non-sedating antihistamine, developed from the second-generation antihistamine cetirizine. Chemically, levocetirizine is simply the isolated levorotary enantiomer of cetirizine, which is sold as a racemic mixture.
Taste masking pharmaceutical composition containing levocetirizine
InactiveUS20060083786A1Increase surface areaOrganic active ingredientsPill deliveryAdditive ingredientLevocetirizine hydrochloride
A solid oral dosage composition is provided comprising a prophilactically or therapeutically effective amount of an active pharmaceutical ingredient comprising levocetirizine or a pharmaceutically acceptable salt thereof, the solid oral dosage composition having a coating thereon capable of providing taste masking of the levocetirizine or pharmaceutically acceptable salt thereof.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Levocetirizine dihydrochloride granule and preparation and detection methods thereof
ActiveCN101669913ALower control costsSimple processOrganic active ingredientsPharmaceutical non-active ingredientsCurative effectLactose
The invention discloses a levocetirizine dihydrochloride granule and preparation and detection methods thereof. The specification of the levocetirizine dihydrochloride particle is 2.5 mg, and milk sugar is used as filling agent. The levocetirizine dihydrochloride granule dispenses with the process of disintegration of tablets and capsules in a human body, the degree of dispersion in the human bodyis superior to the tablets and the capsules, and the absorption is faster than the tablets, the capsules and dispersible tablets; and the flowability, the dispersibility and the adhesiveness are better than the dispersible tablets, the granule is convenient to take, the mouthfeel is easier to adjust, and the curative effect of the medicine can be guaranteed to be better played.
Owner:HAINAN HONZ PHARMA
Levocetirizine and montelukast in the treatment of inflammation mediated conditions
The embodiments described herein include methods and formulations for treating viruses and diseases that are exacerbated by inflammatory responses in the body. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:IRR INC
Method for separating and determining levocetirizine hydrochloride and enantiomer thereof through HPLC method
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining levocetirizine hydrochloride and enantiomer thereof through an HPLC method. In the method, an adopted chromatographic column takes silica gel of which the surface is chemically bonded with beta-cyclodextrin as filler, elution is conducted through a mobile phase A and a mobile phase B, and detection is conducted by entering a detector; the mobile phase A is an inorganic salt buffer system, and the mobile phase B is organic solvent. The method is a reversed phase high performance liquid chromatography method, separation and determination of levocetirizine hydrochloride and the enantiomer thereof can be achieved simultaneously, excellent separation performance and durability are achieved, the method is simple, convenient, feasible, good in reproducibility, efficient and rapid, the analysis time is significantly shortened, the tailing factor and theoretical pedal number can both reach very good effects, the content of the enantiomer in levocetirizine hydrochloride can be effectively determined, and specificity is high.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Levo-cetirizine hydrochloride orally disintegrating tablets and preparation method thereof
InactiveCN101310711AFast disintegrationFast absorptionOrganic active ingredientsPill deliveryCetirizine HydrochlorideTreatment effect
The invention relates to a hydrochloric acid levocetirizine orally disintegrating tablet and a preparation method thereof. The hydrochloric acid levocetirizine orally disintegrating tablet is obtained by directly pressing principal medicine and accessories. The tablet of the invention can be dissolved fast in a mouth cavity and has no grit feeling and high biological availability, which takes effect fast and can have curative effect faster.
Owner:海南高升医药科技开发股份有限公司
Levocetirizine and montelukast in the treatment of inflammation mediated conditions
The embodiments described herein include methods and formulations for treating viruses and diseases that are exacerbated by inflammatory responses in the body. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:IRR INC
Method for separating and measuring levocetirizine dihydrochloride and related substances by using high performance liquid chromatography
ActiveCN110726788AEasy to separateStrong specificityComponent separationPolyethylene glycolHydrochloric acid
The invention relates to a method for separating and measuring levocetirizine dihydrochloride and 5 related substances by using high performance liquid chromatography. According to the method, a chromatographic column is a C18 chromatographic column, detection is performed after the elution of a mobile phase containing a mixed solution of sodium heptanesulfonate and acetonitrile, and alkali is added to the mobile phase. By adopting the method, separation and detection can be simultaneously performed any one or more of 5 related substances in a levocetirizine dihydrochloride tablet, that is, levocetirizine dihydrochloride, levocetirizine lactose ester thereof, levocetirizine polyethylene glycol ester, p-chlorobenzophenone, p-chlorobenzyl alcohol and p-chlorodiphenylmethylpiperazine. Compared with the prior art, the degree of separation of the levoctirizine hydrochloride and the related substances is good, the sensitivity is high and the separation and detection efficiency is improved.
Owner:HUNAN JIUDIAN PHARMA
H1-receptor-antagonist-containing inhalation preparation
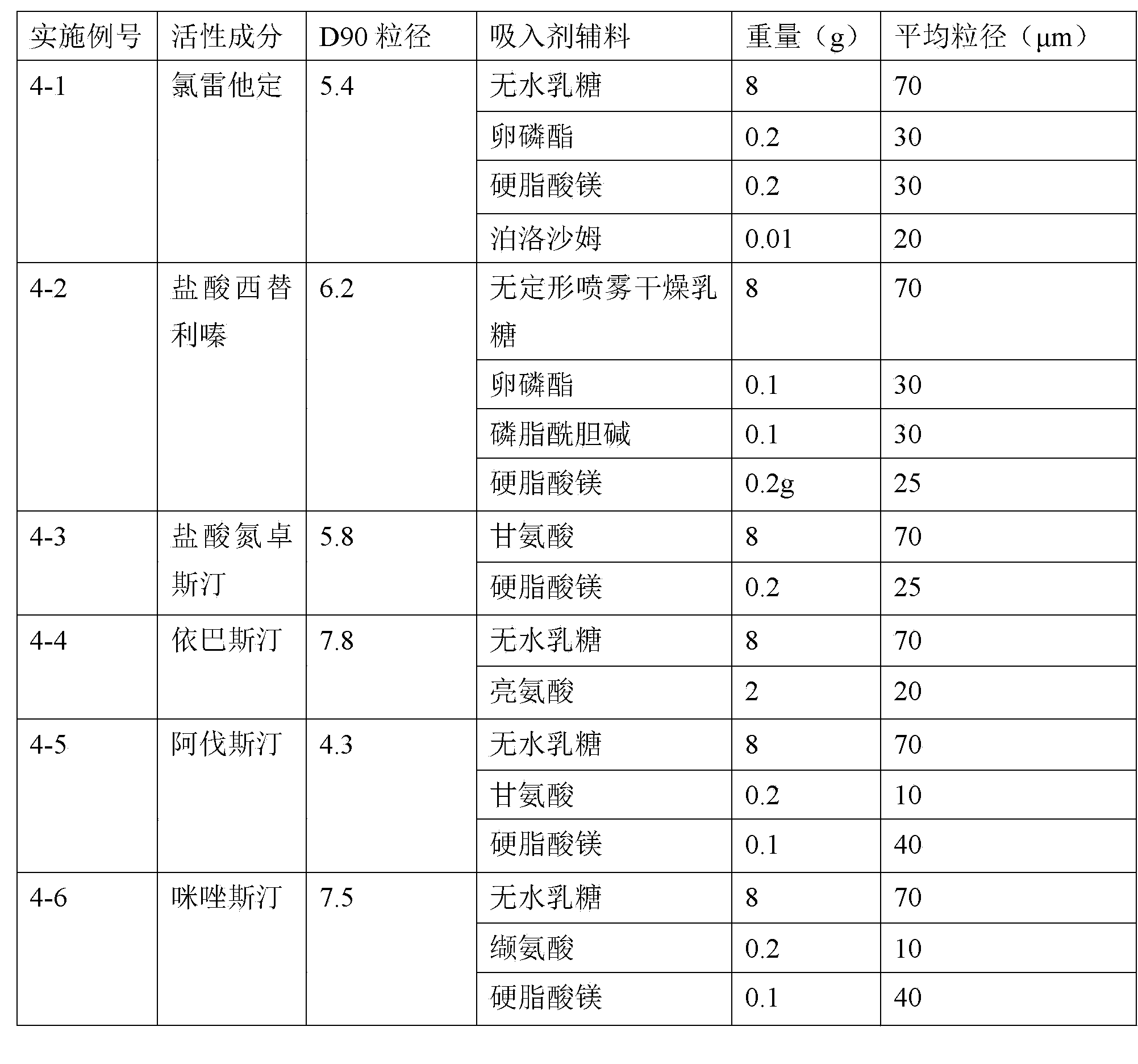
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Method for testing related substances of levocetirizine hydrochloride intermediate
The invention belongs to the field of analytical chemistry, and discloses a method for separately testing a levocetirizine hydrochloride intermediate and related substances of the levocetirizine hydrochloride intermediate by liquid chromatography. The method comprises the following steps: a chromatographic column taking octadecylsilane chemically bonded silica as a filler uses a certain proportion of buffer salt solution-organic phase as a mobile phase to quantificationally test the content of the levocetirizine hydrochloride intermediate and the content of the related substances of levocetirizine hydrochloride intermediate, so that the quality of the levocetirizine hydrochloride intermediate is effectively controlled, and the controllable quality of levocetirizine hydrochloride is ensured. The method is high in specificity and accuracy, and simple and convenient to operate.
Owner:AVENTIS PHARMA HAINAN
Levocetirizine hydrochloride syrup and preparation method
InactiveCN103860462AComfortable tasteEasy to acceptOrganic active ingredientsPharmaceutical delivery mechanismDrugs preparationsBitter taste
The invention belongs to the field of medicinal preparations and discloses levocetirizine hydrochloride syrup and a preparation method. The levocetirizine hydrochloride syrup and the preparation method have the advantages that the bitter taste of the levocetirizine hydrochloride is covered, the adaptability of clinical medicines is improved, the treating effect is more ideal, and the preparation process is simple and is high in reliability.
Owner:AVENTIS PHARMA HAINAN
Oral anti-allergy compound pharmaceutical composition
The invention relates to an oral anti-allergy compound pharmaceutical composition, which contains levocetirizine and mast cell membrane stabilizer. The dosage forms of the composition comprise tablet, capsule, suspending agent, dry suspending agent, oral solution, granule, and the like. The oral medicament is applied to the treatment of allergic diseases such as urticaria, eczema, dermatitis, allergic rhinitis, skin pruritus, and the like.
Owner:CHONGQING HUAPONT PHARMA
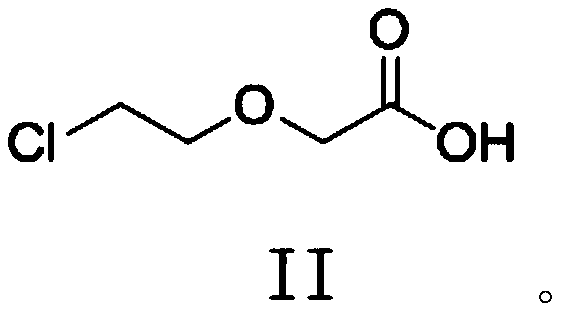
New procedure for preparation of levocetirizine and its intermediates
The present invention describes a novel process for the preparation of levocetirizine and pharmaceutically acceptable acid addition salts thereof using diglycolic acid or derivatives thereof and new intermediates used in that process.
Owner:克卡制药新梅斯托股份公司
External preparation of Levocetirizine hydrochloric acid
ActiveCN1957942AAntagonism of sensitizationPromote transdermal absorptionOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
An exterior-applied levocetirizine hydrochloride for preventing and treating the anaphylaxia and inflammation without untoward effect is disclosed.
Owner:LUNAN PHARMA GROUP CORPORATION
Levocetirizine hydrochloride chewable tablet and preparation method thereof
ActiveCN102716099AMask bitternessOrganic active ingredientsPill deliveryAllergy disordersBeta-Cyclodextrins
The invention relates to a levocetirizine hydrochloride chewable tablet for relieving hypersensitivity caused by allergy diseases and the preparation technology thereof. Levocetirizine hydrochloride has stronger bitterness, in the invention, by adopting the composite of Beta-cyclodextrin and microcrystalline cellulose, the medicine is prepared to be inclusion compound so as to cover up the bitterness of the medicine, the prepared medicine is better in mouth-feel and is convenient to take, the process operability in preparing the inclusion compound and the stability of the levocetirizine hydrochloride chewable tablet are enhanced at the same time, the levocetirizine hydrochloride chewable tablet is particularly suitable for old and child patients having difficulty in swallowing, enhances the compliance of the patients and has a better marketable value.
Owner:湖南千金湘江药业股份有限公司
Levocetirizine preparation method and levocetirizine dihydrochloride preparation method
ActiveCN103351361AImprove conversion rateHigh selectivityOrganic chemistryHydroxyzineCatalytic oxidation
The invention relates to a levocetirizine preparation method and a levocetirizine dihydrochloride preparation method. Levocetirizine is prepared through a synthesis route shown in the specification, and levocetirizine dihydrochloride is prepared through hydrochlorinating the prepared levocetirizine to form a salt, and recrystallizing the salt. The preparation method which adopts cheap and easily available Pd-M / C to realize the catalytic oxidation of L-hydroxyzine as a catalyst in order to prepare levocetirizine realizes the high conversion rate of the substrate L-hydroxyzine and the high selectivity and high optical purity of the target product levocetirizine, and is an environmentally-friendly technology.
Owner:HAISO TECH
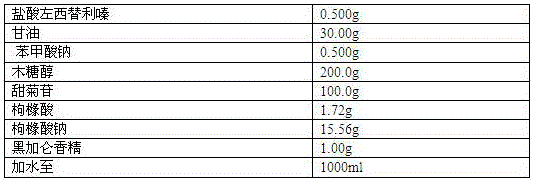
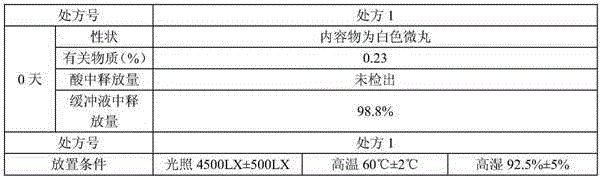
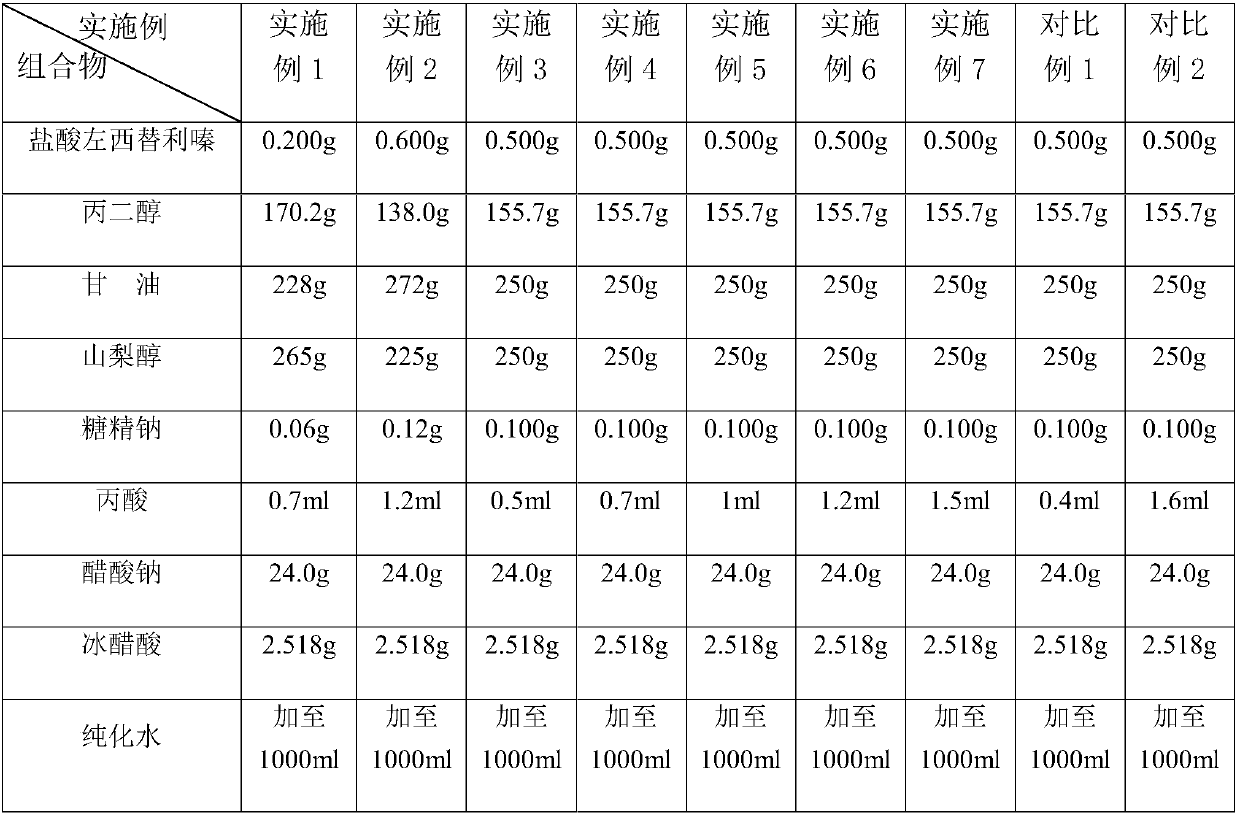
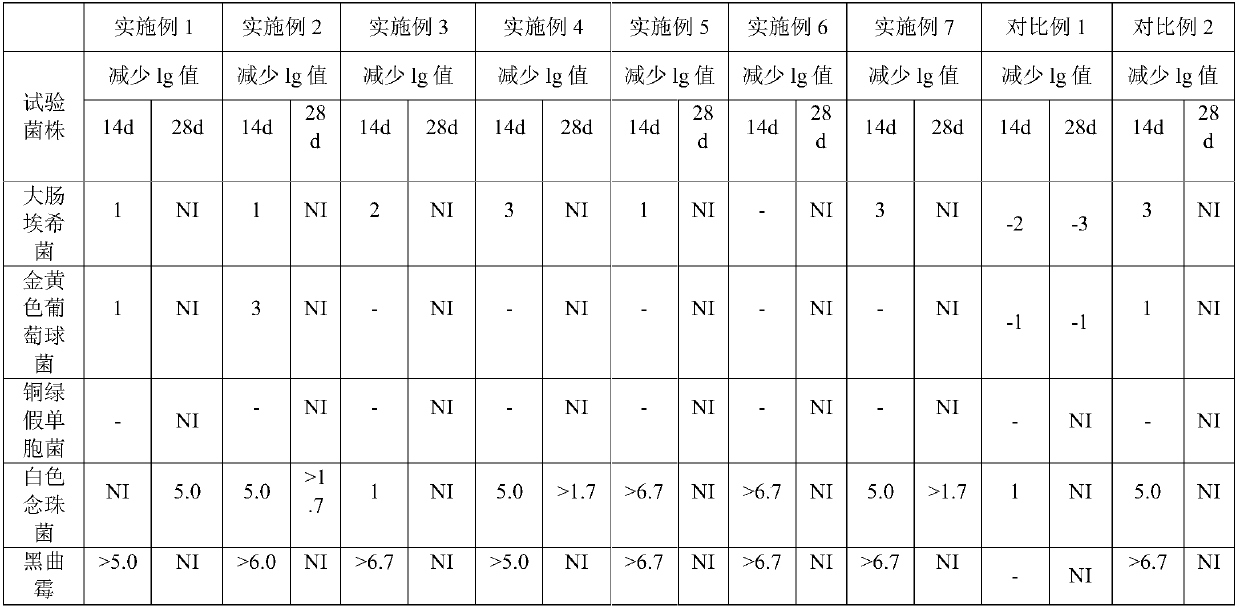
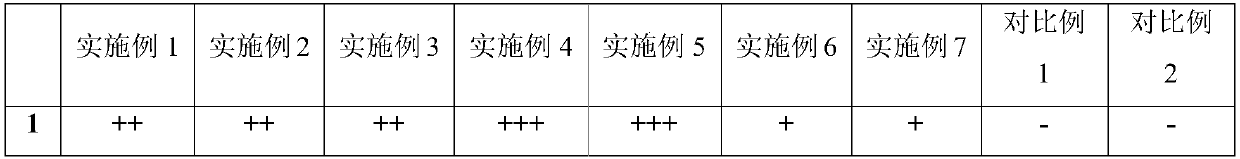
Stable levocetirizine hydrochloride oral solution and preparation method thereof
InactiveCN112006982ASolve poisoningSame antiseptic effectOrganic active ingredientsDispersion deliveryPropanoic acidPreservative
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a stable levocetirizine hydrochloride oral solution and a preparation method thereof. According to the levocetirizine hydrochloride oral solution, propionic acid is used as a preservative, and a volume percentage of propionic acid in the levocetirizine hydrochloride oral solution is 0.05%-0.15%. Propionic acid is added into the levocetirizine hydrochloride oral solution, and compared with a traditional preservative, propionic acid has the same preservative effect, and is smaller in toxicity and higher in safety. The levocetirizine hydrochloride oral solution prepared by the invention has the advantages of high stability, simple prescription, smaller preservative toxicity and higher safety, and the method for preparing the levocetirizine hydrochloride oral solution is simple to operate and easy for industrial production.
Owner:CHONGQING HUAPONT PHARMA
Levocetirizine hydrochloride oral drops and preparation method thereof
InactiveCN106491522ADefinite curative effectFully absorbedOrganic active ingredientsPharmaceutical delivery mechanismPreservativeOral Drops
The invention provides levocetirizine hydrochloride oral drops good in taste and a preparation method thereof and belongs to the field of drug preparation. The levocetirizine hydrochloride oral drops are prepared from levocetirizine hydrochloride material, a solvent, a corrective, a preservative, a stabilizer, and a colorant, and the liquid medicine is quantitatively pumped out through a mechanical snap valve by using a quantitative dropper pump; the levocetirizine hydrochloride material has a concentration of 0.1-5 mg / ml in the drops herein. The liquid quantity of quantitative drops per snap is 0.1-2 ml. The levocetirizine hydrochloride oral drops are taken by an adult two to three times a day, 1-2 ml for each time. The levocetirizine hydrochloride oral quantitative drops are of a solvent type preparation and have the advantages medicine is distributed in a molecular form in liquid, may be quickly absorbed after administration, and acts fast; that dosage of the quantitative drops given by using the quantitative dropper pump is accurate, scientific and reasonable; the quantitative drops are sprayed directly into the mouth through a spray tube such that administration is greatly facilitated; the levocetirizine hydrochloride oral drops are good in taste and easy for a patient to accept.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation for treating allergic rhinitis and preparation method thereof
InactiveCN102302497AFlat surfacePromote absorptionOrganic active ingredientsRespiratory disorderSide effectClinical efficacy
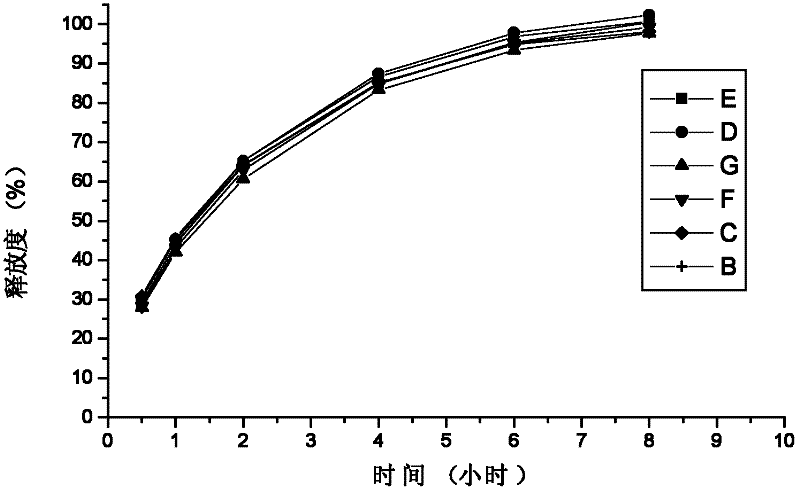
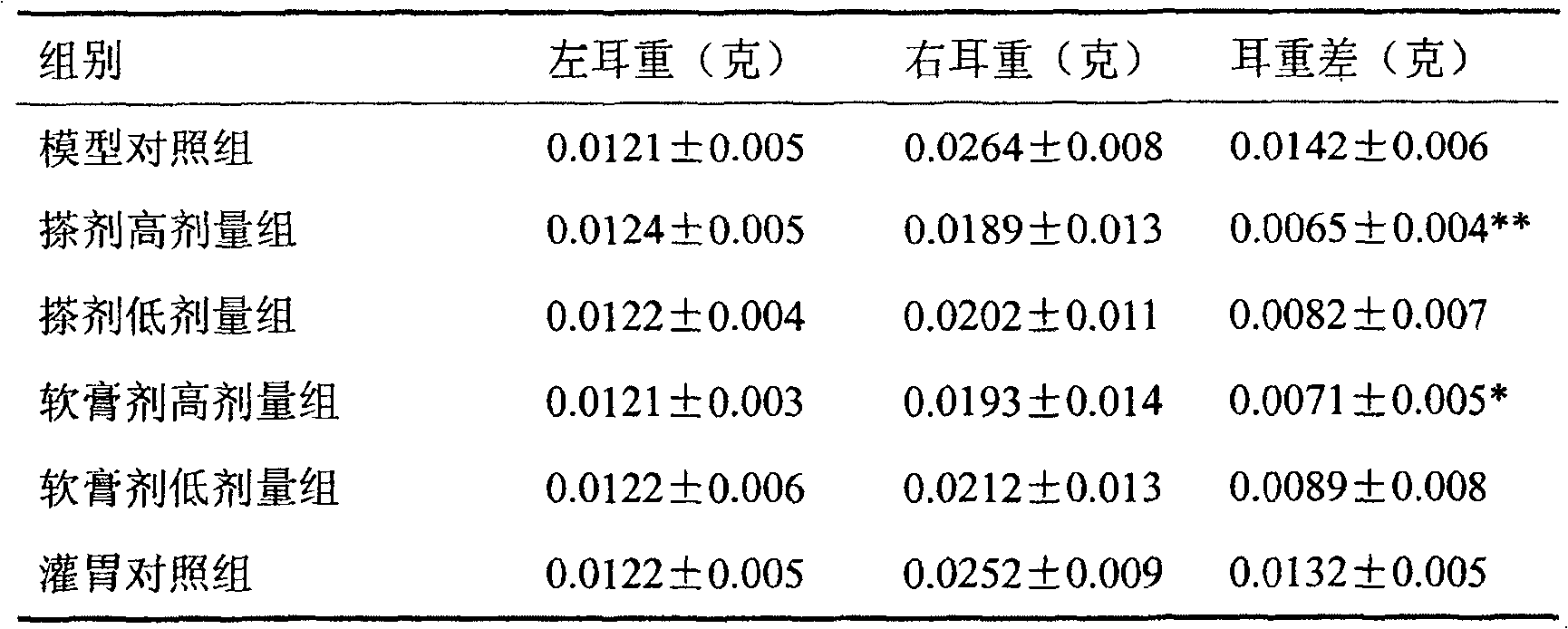
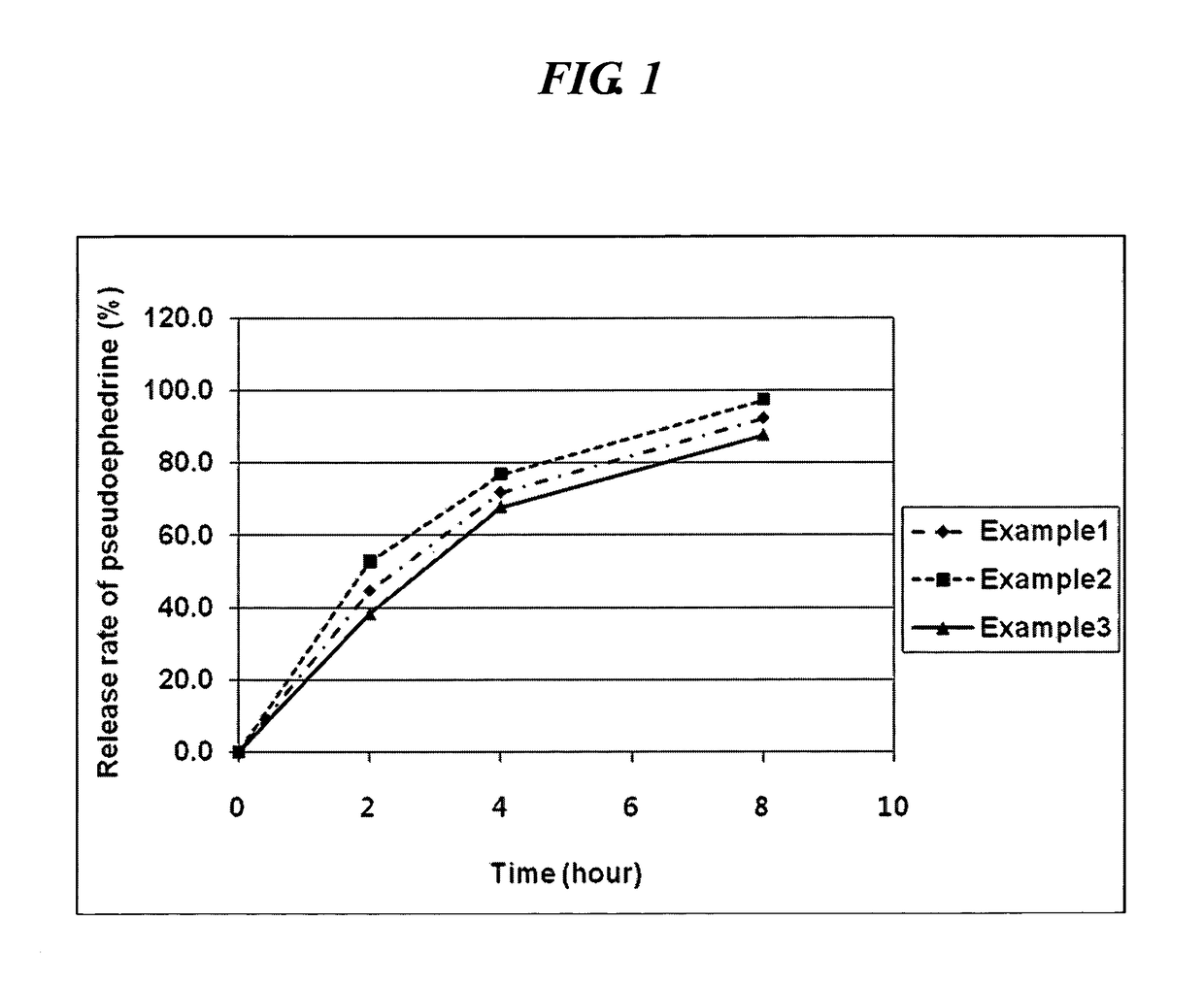
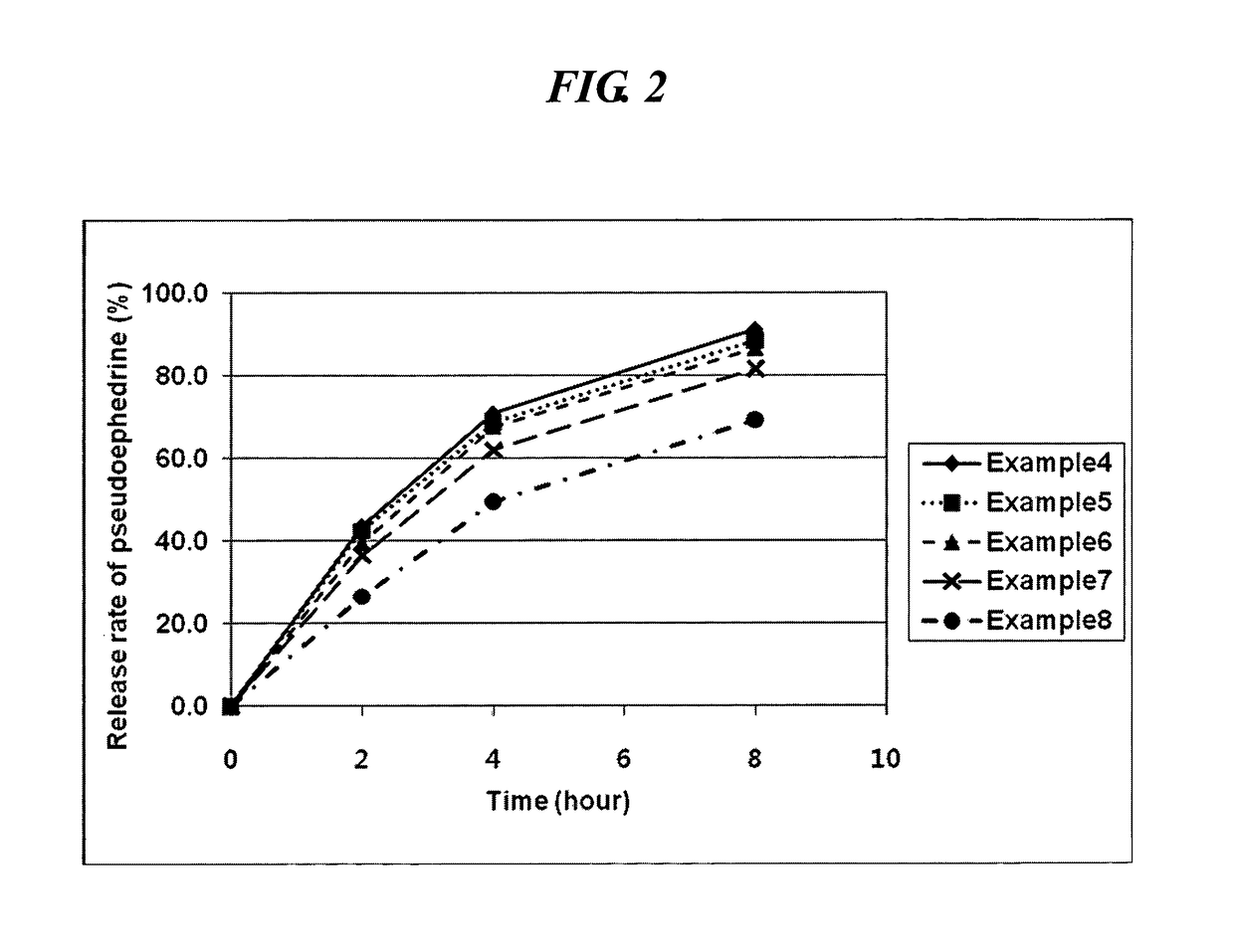
The invention relates to a preparation for treating allergic rhinitis. The preparation is prepared by pressing a quick release layer and a sustained-release layer into bilayer tablets and coating, wherein the quick release layer consists of the following components in percentage by mass: 1 to 10 percent of levocetirizine hydrochloride, 1 to 15 percent of disintegrating agent, 0.5 to 3 percent of lubricating agent, 0 to 8 percent of adhesive and the balance of filler; the sustained-release layer consists of the following components in percentage by mass: 20 to 30 percent of pseudoephedrine hydrochloride, 50 to 60 percent of sustained-release framework material, 0.5 to 3 percent of lubricating agent, 0 to 8 percent of adhesive and the balance of filler; and the mass ratio of the levocetirizine hydrochloride to the pseudoephedrine hydrochloride is 1:48. The sustained-release tablets for treating the allergic rhinitis have bright and clean surfaces, and the release behavior of the main medicine pseudoephedrine hydrochloride of the sustained-release tablets has high consistency with that of American marketed medicine cetirizine hydrochloride and pseudoephedrine hydrochloride sustained-release tablets through detection; and the dissolution rate of the main medicine levocetirizine hydrochloride of the sustained-release tablets in 30 minutes is over 90 percent, so that the sustained-release preparation for treating the allergic rhinitis has high bioavailability, a good absorption effect, comprehensive and effective clinical curative effects, small side effects and ideal safety.
Owner:TIANSHENG PHARMA GROUP
Capsule formulation comprising montelukast and levocetirizine
Disclosed is a capsule formulation for preventing or treating allergic rhinitis and asthma, which comprises two separate layers of: (1) a Montelukast layer comprising montelukast or a pharmaceutically acceptable salt thereof; and (2) a Levocetirizine layer comprising levocetirizine or a pharmaceutically acceptable salt thereof; and a method for the preparation thereof. The capsule formulation according to the present invention can completely separate two active ingredients, thereby minimizing the reactivity between them and improving product stability against aging effects, and thus, can optimize the therapeutic effects.
Owner:HANMI PHARMA
Oral compound levocetirizine pseudoephedrine formulation and its preparation
The present invention belongs to the field of pharmaceutical technology, and discloses one kind of orally taken compound levo-cetirizine pseudoephedrine preparation. The preparation has activity similar to that of compound cetirizine pseudoephedrine preparation and is single optical isomer with half reduced dosage and thus long effect and reduced side effect. The preparation may be taken by adult and children. The preparation process is also provided.
Owner:北京曙光药业有限责任公司
Composition and preparation method thereof as well as oral liquid and preparation method of oral liquid
ActiveCN104666302AImprove stabilityPromote growthInorganic non-active ingredientsPharmaceutical delivery mechanismActive agentOrganosolv
The invention relates to the field of medicinal preparations, in particular to a composition and a preparation method thereof as well as an oral liquid and a preparation method of the oral liquid. The preparation method of the composition comprises the following steps: mixing montelukast sodium with first water to obtain a montelukast sodium solution; mixing levocetirizine hydrochloride with second water and adjusting the pH value to 6.5-9 to obtain a levocetirizine hydrochloride solution; mixing the montelukast sodium solution with the levocetirizine hydrochloride solution to obtain the composition. The montelukast sodium in the oral liquid provided by the invention is high in stability; the composition does not contain an organic solvent or a surfactant; medication of children is facilitated; and the preparation technology is simple.
Owner:BEIJING HANMI PHARMA CO LTD
Pharmaceutical composition comprising naringin and levocetirizine hydrochloride, and preparations thereof
InactiveUS20160303156A1Good cough relievingGood sputum reducingOrganic active ingredientsPill deliveryNaringinSputum Production
A naringin composition and preparations thereof are provided. The pharmaceutical composition comprises naringin and levocetirizine hydrochloride, and preferably, 27.5-275 mg of naringin and 1.25-12.5 mg of levocetirizine hydrochloride, where the preferred weight ratio of naringin to levocetirizine hydrochloride is 20:1. The pharmaceutical composition has good curative effects on cough and sputum production originating from various causes and on cough variant asthma. The efficacy of the pharmaceutical composition is obviously superior to that of naringin or levocetirizine hydrochloride that is used alone.
Owner:SUN YAT SEN UNIV
Method for separating and determining levocetirizine hydrochloride and genotoxic impurity E thereof by HPLC method
ActiveCN110988163AEasy to separateIncreased durabilityComponent separationHplc methodMonopotassium phosphate
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining levocetirizine hydrochloride and a genotoxic impurity E thereof by an HPLC method. A chromatographic column adopted in the method takes octadecyl bonded silica gel as a filler, a mixed mobile phase of potassium dihydrogen phosphate and methanol and / or acetonitrile is adopted for elution, and a product enters a detector for detection. The method is a reversed-phase high performance liquid chromatography method. Separation and detection of levocetirizine hydrochloride and genotoxic impurities E thereof can be realized at the same time; the method has excellent separation performance and durability, is simple, convenient and feasible, has good reproducibility, is efficient and rapid, can achieve very good effects on tailing factors and theoretical pedal numbers, can effectively determine the content of genotoxic impurities E in levocetirizine hydrochloride, and has strong specificity.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Naringin and levocetirizine hydrochloride pharmaceutical composition and preparation thereof
The invention relates to naringin composition and a preparation thereof. The pharmaceutical composition is characterized by comprising naringin and levocetirizine hydrochloride, wherein preferably, the naringin is in a range of 27.5-275 mg, and the levocetirizine hydrochloride is in a range of 1.25-12.5 mg; the preferable mass ratio of the naringin to the levocetirizine hydrochloride is 20:1. Tests prove that the pharmaceutical composition has good curative effects on coughs and excessive phlegm caused by various reasons and the cough variant asthma, and the efficacy of the composition is obviously superior to the efficacy of the independently-used naringin or the independently-used levocetirizine hydrochloride.
Owner:SUN YAT SEN UNIV
Montelukast sodium and levocetirizine hydrochloride gastrointestinal capsule pharmaceutical composition
InactiveCN104434919AImprove securityOptimizing Prescription VolumeMetabolism disorderGranular deliveryMontelukast SodiumTherapeutic effect
The invention provides a montelukast sodium and levocetirizine hydrochloride gastrointestinal capsule pharmaceutical composition, and can overcome the deficiency of the existing technology and effectively solve the problems of stability and the safety of clinical medication of the montelukast sodium and levocetirizine hydrochloride compound. The pharmaceutical composition achieves the purpose of simultaneous absorption of the montelukast sodium and levocetirizine hydrochloride in different parts, and can play a better synergistic therapeutic effect; through prescription screening and optimization, the montelukast sodium and levocetirizine hydrochloride gastrointestinal capsule pharmaceutical composition is provided; and the preparation ensures the stability of the drug during production and storage. A preparation method of the pharmaceutical composition is provided, and the method is simple and feasible, and applicable to industrial scale production through pilot-plant test.
Owner:TIANJIN SONGRUI MEDICAL TECH
Stable levocetirizine hydrochloride oral solution and preparation method of same
InactiveCN107823647ASame antiseptic effectLow toxicityOrganic active ingredientsDispersion deliveryPropanoic acidPreservative
The invention belongs to the field of medicine preparations, and particularly relates to a stable levocetirizine hydrochloride oral solution and a preparation method of same. Propionic acid is used asa preservative in the oral solution, volume percentage of the propionic acid being 0.05-0.15% in the oral solution. In the levocetirizine hydrochloride oral solution, the propionic acid is added, sothat the preservative effect as same as that of conventional preservatives is achieved, while toxicity is reduced and safety is increased. The levocetirizine hydrochloride oral solution has high stability and simple formula, is lower in toxicity of the preservative and has better safety. The preparation method is simple in operations and is easy to carry out industrially.
Owner:CHONGQING HUAPONT PHARMA
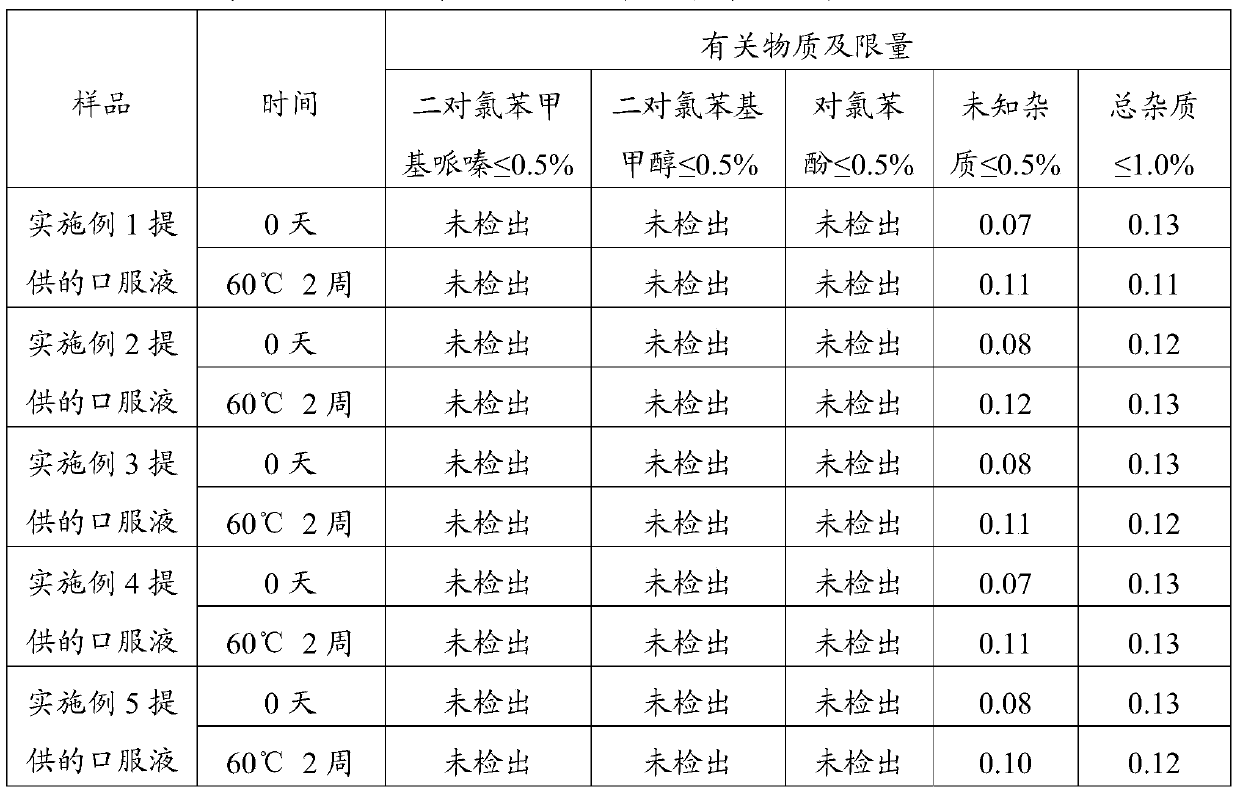
Method for detecting related substances in levocetirizine hydrochloride oral solution
The invention provides a method for detecting related substances in a levocetirizine hydrochloride oral solution, belongs to the technical field of pharmaceutical analysis, and the method comprises the following steps: adopting a high performance liquid chromatography to detect the related substances in the levocetirizine hydrochloride oral solution, and calculating the content of related substances in the levocetirizine hydrochloride oral solution by using an external standard method. According to the method, acetonitrile and a monopotassium phosphate buffer solution are used as mobile phases, gradient elution is performed on a high performance liquid chromatograph, and parachlorobenzhydrol, parachlorobenzophenone, parachlorobenzhydryl piperazine, levocetirizine amide and p-hydroxybenzoic acid can be accurately and efficiently detected at a time under a liquid phase condition.
Owner:浙江核力欣健药业有限公司
External preparation of Levocetirizine hydrochloric acid
ActiveCN100589805CAntagonism of sensitizationPromote transdermal absorptionOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
An exterior-applied levocetirizine hydrochloride for preventing and treating the anaphylaxia and inflammation without untoward effect is disclosed.
Owner:LUNAN PHARMA GROUP CORPORATION
Oral complex composition comprising pseudoephedrine and levocetirizine
InactiveUS20120301548A1Increase release rateOrganic active ingredientsPill deliveryPseudoephedrinePolyethylene glycol
An oral complex composition which comprises (i) a core comprising a swellable hydrogel-forming agent and pseudoephedrine, or a pharmaceutically acceptable salt thereof; (ii) a first coating layer encasing the core which comprises a water-soluble substance; and (iii) a second coating layer deposited on the first coating layer which comprises levocetirizine or a pharmaceutically acceptable salt thereof together with polyvinylalcohol, povidone, polyvinylalcohol-polyethyleneglycol graft copolymer or a mixture thereof, has an improved levocetirizine releasing rate and does not show a delayed release behavior even after a long storage period. Accordingly, the inventive oral complex composition is useful for treating perennial or seasonal allergic diseases including nasal obstruction, sneezing, and rhinorrhea.
Owner:HANMI SCI CO LTD
Oral complex composition comprising pseudoephedrine and levocetirizine
InactiveUS9694007B2Increase release rateOrganic active ingredientsGranular deliveryDiseasePseudoephedrine
An oral complex composition which comprises (i) a core comprising a swellable hydrogel-forming agent and pseudoephedrine, or a pharmaceutically acceptable salt thereof; (ii) a first coating layer encasing the core which comprises a water-soluble substance; and (iii) a second coating layer deposited on the first coating layer which comprises levocetirizine or a pharmaceutically acceptable salt thereof together with polyvinylalcohol, povidone, polyvinylalcohol-polyethyleneglycol graft copolymer or a mixture thereof, has an improved levocetirizine releasing rate and does not show a delayed release behavior even after a long storage period. Accordingly, the inventive oral complex composition is useful for treating perennial or seasonal allergic diseases including nasal obstruction, sneezing, and rhinorrhea.
Owner:HANMI SCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com