External preparation of Levocetirizine hydrochloric acid
A technology of levocetirizine hydrochloride and ointment, which is applied in the field of pharmaceutical preparations, can solve the problems of unfavorable tissue distribution of levocetirizine hydrochloride, unsatisfactory administration methods, and less blood flow distribution, etc., and achieve good local anti-inflammatory effects. Allergic and anti-inflammatory effects, increased free drug concentration, good transdermal absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
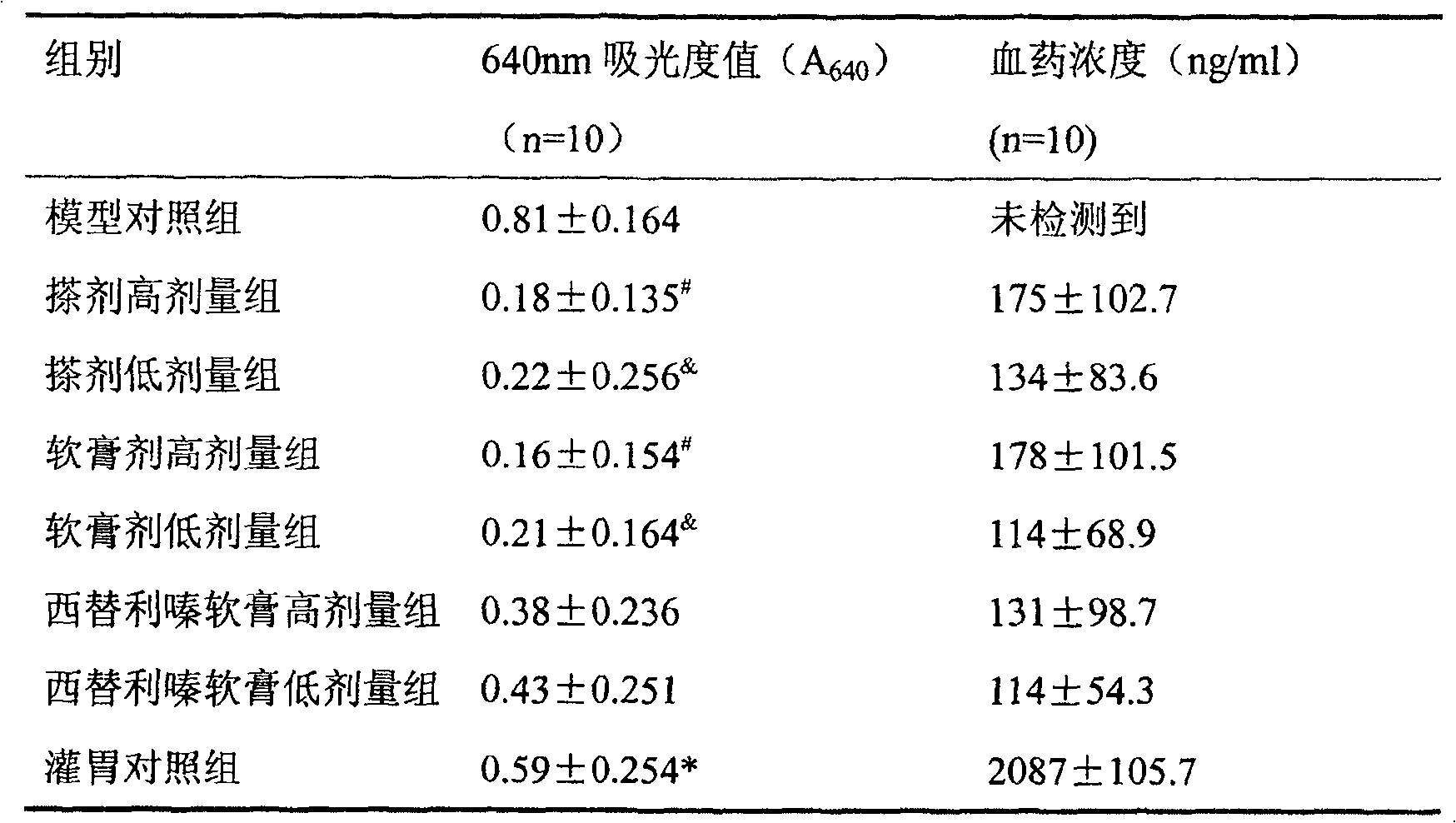
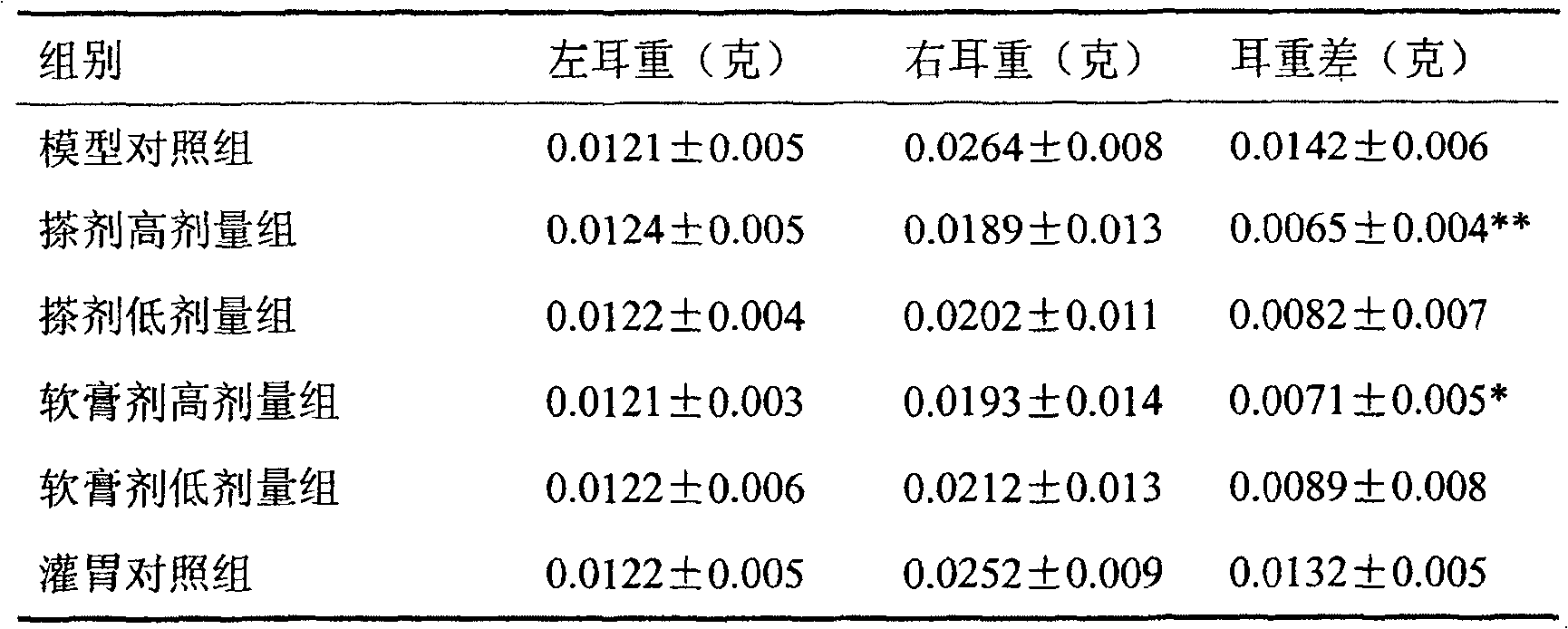
[0024] Preparation of antiserum: Take 8 healthy rats weighing 90-100g, and intramuscularly inject them with Na 2 SO 4 Ovalbumin (10mg / kg) recrystallized 3-5 times, and intraperitoneal injection of pertussis triple vaccine 2×10 10 / Only. 10-14 days after the sensitization, the animals were sacrificed to collect blood, and the serum was separated by low-speed centrifugation, and stored in a -40°C refrigerator for later use.
[0025] Operation method: Inject the serum of sensitized rats (containing rich IgE antibody) intradermally into the abdominal wall or back of normal rats, forming a mound at each point. IgE binds to the Fc receptors of local skin mast cells, making them passively sensitized. When the antigen is attacked, the local mast cells are caused to release allergic mediators, thereby increasing the permeability of the local blood vessels. Evans blue is injected, which can seep out of the pichu and form a coeruleus. According to the range or depth of coeruleus, the...
Embodiment 1
[0057] Levocetirizine Hydrochloride Liniment
[0058] Levocetirizine Hydrochloride 20g
[0059] Ethanol 35ml
[0060] Propylene glycol 100ml
[0061] Add phosphate buffered saline (PBS) to 1000ml
[0062] Preparation Process:
[0063] Dissolve levocetirizine hydrochloride in an appropriate amount of phosphate buffered saline (PBS), add the prescribed amount of ethanol and propylene glycol, and then add phosphate buffered saline (PBS) to 1000ml.
Embodiment 2
[0065] Levocetirizine Hydrochloride Liniment
[0066] Levocetirizine Hydrochloride 10g
[0067] Ethanol 200ml
[0068] Propylene glycol 300ml
[0069] Add distilled water to 1000ml
[0070] Preparation Process:
[0071] Dissolve levocetirizine hydrochloride in an appropriate amount of distilled water first, add the prescribed amount of ethanol and propylene glycol, and then add distilled water to 1000ml.
[0072] The liniment prepared in embodiment 1 and embodiment 2 is packed in a specific container (sprayer or atomizer), and can be used as a spray.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com