Levocetirizine hydrochloride oral drops and preparation method thereof
A technology of levocetirizine hydrochloride and drops, which is applied in the direction of feeding oral medicine utensils, pharmaceutical formulas, and medical preparations with non-effective ingredients, etc., can solve the problems of inability to improve patients' medication compliance, difficulty in accurately controlling the dosage, Inconvenient to take and other problems, to achieve the effect of accurate, scientific and reasonable dosage, solving dosage and hygiene problems, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of oral quantitative drops of levocetirizine hydrochloride
[0026] Prescription composition:
[0027]
[0028] Preparation Process:
[0029] 1) Weigh the prescribed amount of glycerin and 40% purified water, add sodium acetate and the main ingredient in a water bath at 50°C, stir to dissolve, then add sucrose, stir to dissolve, let cool to room temperature, and obtain solution 1;
[0030] 2) Preheat the prescribed amount of propylene glycol in a water bath at 50°C, then add ethylparaben, stir to dissolve to obtain solution 2;
[0031] 3) Mix solution 1 and solution 2 evenly, add glacial acetic acid and orange essence, and then set the volume to 1000ml, and stir evenly;
[0032] 4) Filtrate, after passing the quality inspection, fill in drop bottles, screw on the bottle caps with quantitative valves, and fill each bottle with 100ml of liquid medicine.
Embodiment 2
[0033] Embodiment 2: the preparation of oral quantitative drops of levocetirizine hydrochloride
[0034] Prescription composition:
[0035]
[0036] Preparation Process:
[0037] 1) Weigh the prescribed amount of glycerin and 40% purified water, add sodium citrate and the main ingredient in a water bath at 50°C, stir to dissolve, then add sucrose and fructose, stir to dissolve, let cool to room temperature, and obtain a solution 1;
[0038] 2) Preheat the prescribed amount of propylene glycol in a water bath at 50°C, then add methylparaben, and stir to dissolve it to obtain solution 2;
[0039] 3) Mix solution 1 and solution 2 evenly, add citric acid and peach essence, make the volume to 1000ml, and stir evenly;
[0040] 4) Filtrate, after passing the quality inspection, fill in drop bottles, screw on the bottle caps with quantitative valves, and fill each bottle with 100ml of liquid medicine.
Embodiment 3
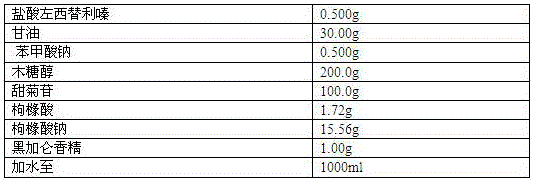
[0041] Embodiment 3: the preparation of oral quantitative drops of levocetirizine hydrochloride
[0042] Prescription composition:
[0043]
[0044] Preparation Process:
[0045] 1) Weigh the prescribed amount of glycerin and 40% purified water, add sodium citrate and the main ingredient in a water bath at 50°C, stir to dissolve, then add xylitol and stevioside, stir to dissolve, let cool to room temperature , to obtain solution 1;
[0046] 2) Weigh 20% purified water, add sodium benzoate, stir to dissolve to obtain solution 2;
[0047] 3) Mix solution 1 and solution 2 evenly, add citric acid and blackcurrant essence, and then set the volume to 1000ml, and stir evenly;
[0048] 4) Filtrate, after passing the quality inspection, fill in drop bottles, screw on the bottle caps with quantitative valves, and fill each bottle with 100ml of liquid medicine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com