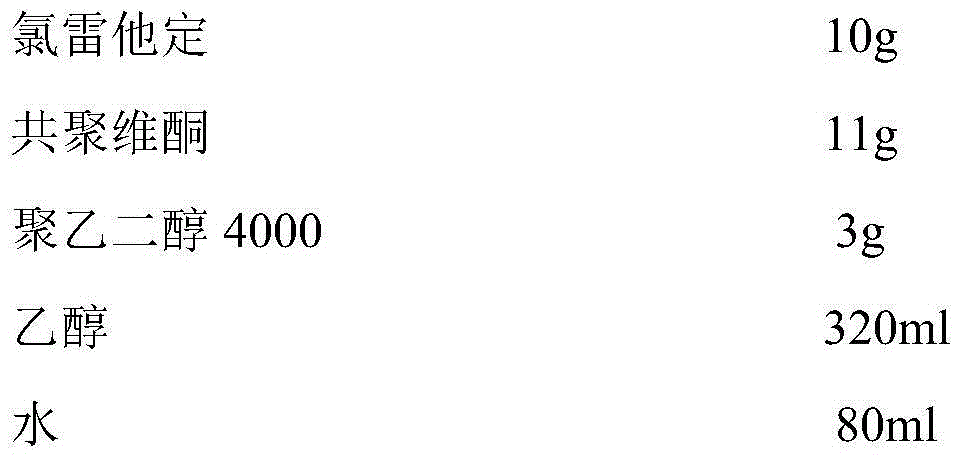Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
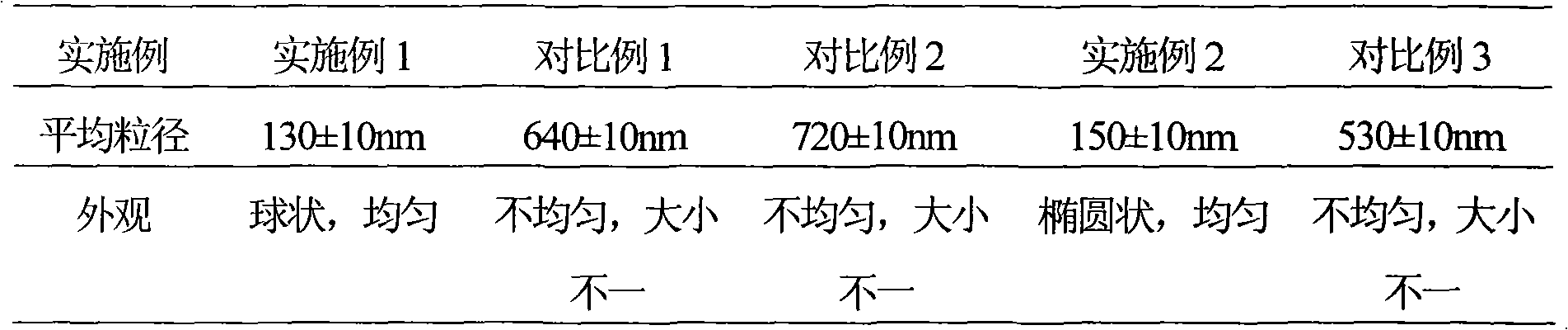
217 results about "Loratadine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is an antihistamine that treats symptoms such as itching, runny nose, watery eyes, and sneezing from "hay fever" and other allergies. It is also used to relieve itching from hives. Loratadine does not prevent hives or prevent/treat a serious allergic reaction (e.g., anaphylaxis).
[Instant dissolving tablet composition for loratidine and desloratidine]
Disclosed here is a tablet formulation of loratidine and desloratidine, non-sedating antihistaminic agents, that allows fast dissolution of tablets in the mouth allowing administration of these drugs without the aid of water. The formulation has pleasing taste and texture.
Owner:GULF PHARMA INDS
Lactose-free, non-hyproscopic and anhydrous pharmaceutical compositions of descarboethoxyloratadine
Stable pharmaceutical compositions of descarboethoxyloratadine (DCL), a metabolic derivative of loratadine, for the treatment of allergic rhinitis and other histamine-induced disorders are disclosed. The compositions are formulated to avoid the incompatibility between DCL and reactive excipients such as lactose and other mono- and di-saccharides. Disclosed compositions include lactose-free, non-hygroscopic and anhydrous stable pharmaceutical compositions of DCL.
Owner:SEPACOR INC
Oral liquid loratadine formulations and methods
InactiveUS20070286875A1Reduces headspaceGood impurity profileBiocideDispersion deliveryMetabolitePhenol
An oral liquid formulation including an effective amount of loratadine, or a pharmaceutically acceptable salt or metabolite thereof; and a pharmaceutically acceptable carrier including a mono- or poly-hydroxy phenol component, a solubilizing agent and a chelating agent. Methods of preparing and administering such formulations are also included.
Owner:WOCKHARDT EU OPERATIONS SWISS
Topical application of cetirizine and loratadine
The present invention relates to a drug composition containing the non-sedating H.sub.1 antihistamines cetirizine, loratadine, mixtures or pharmaceutically acceptable salts thereof for topical application in the form of a gel.
Owner:ORAMON ARZNEIMITTEL
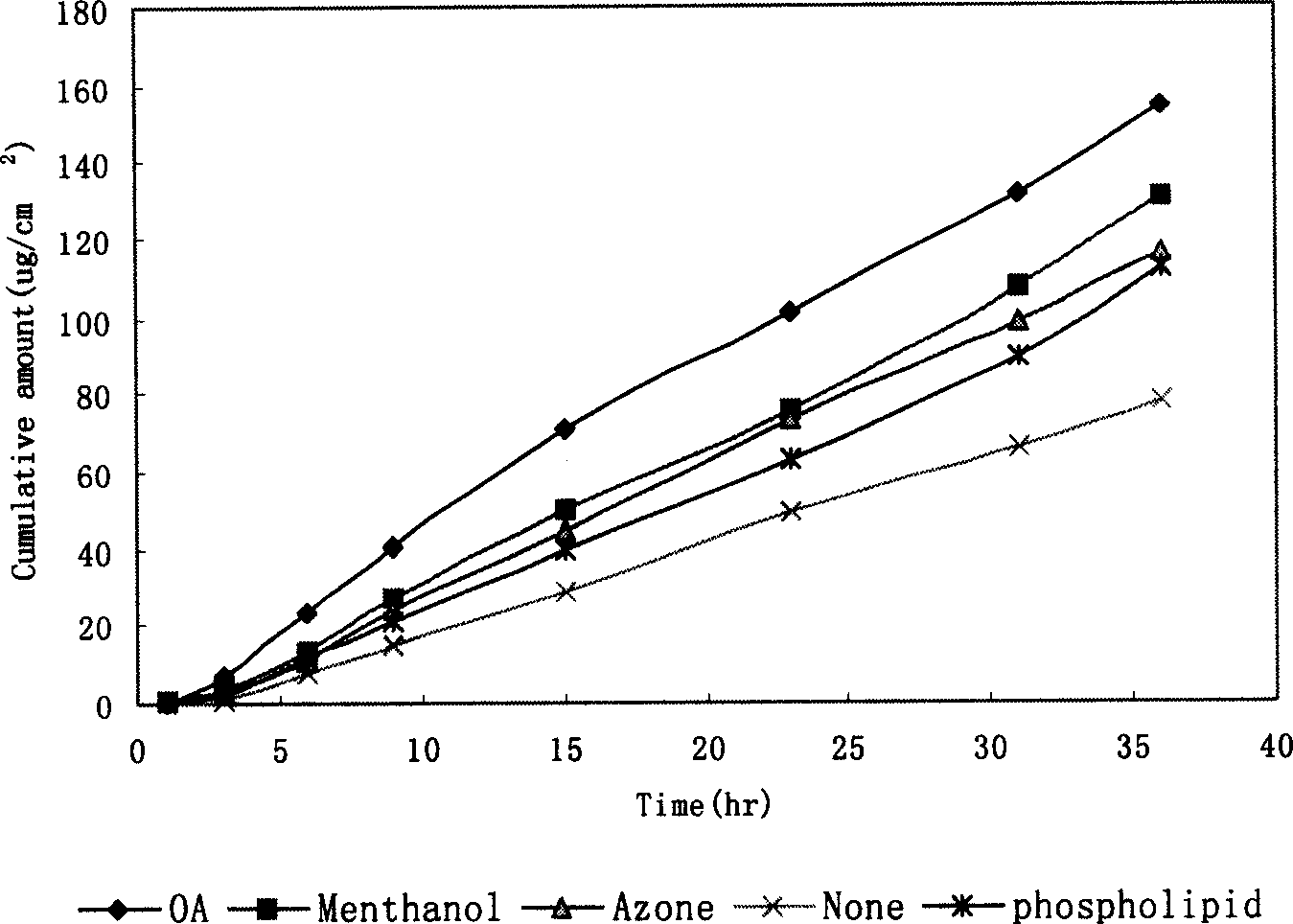
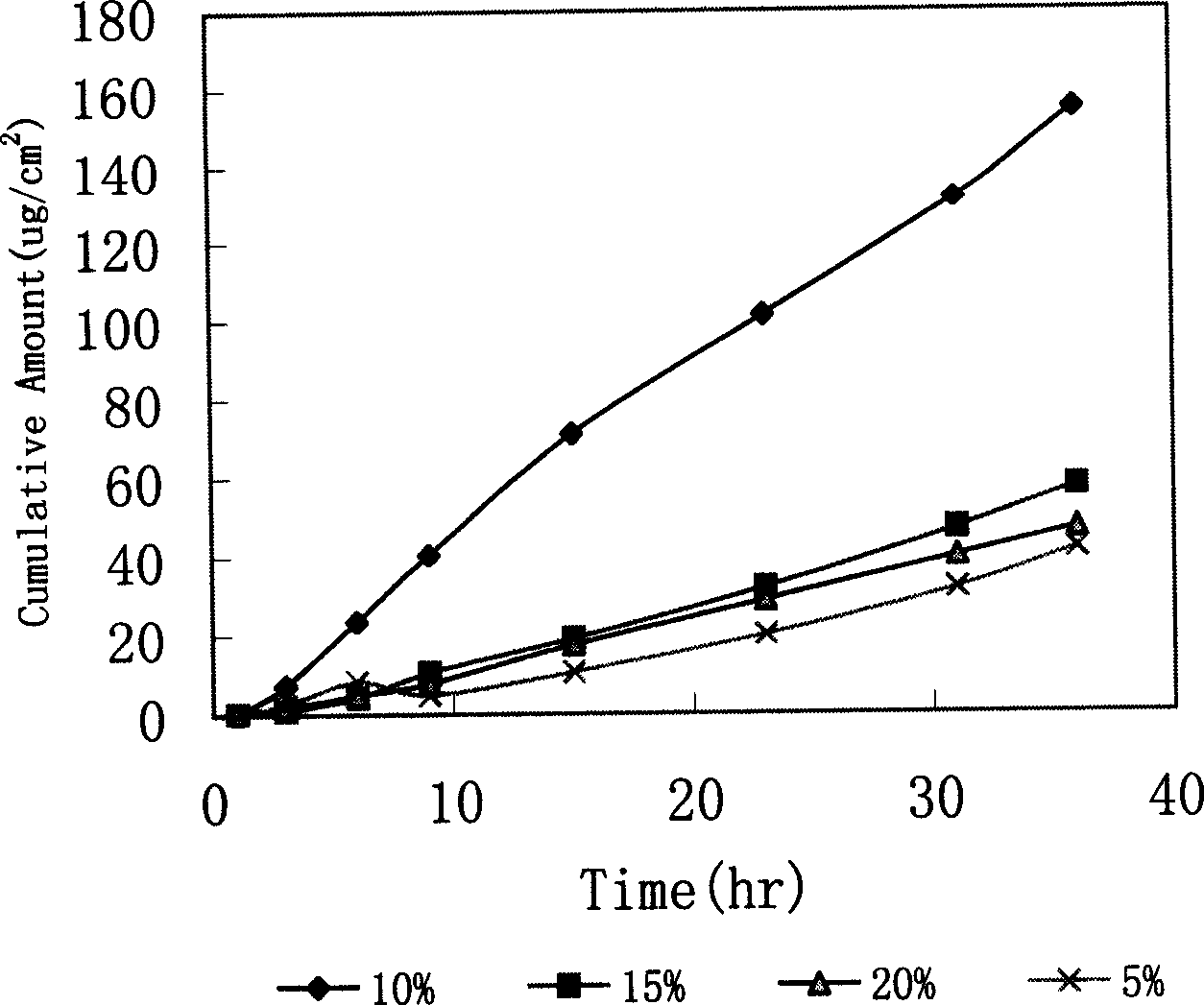
Loratadine paster of penetrating skin
InactiveCN1600297AEasy to takeLess irritatingOrganic active ingredientsMedical devicesSolventLoratadine
A transdermal disk of loratadine for treating allergic disease is prepared from loratadine, adhesive aggregate, cross-linking agent, plasticizer, solvent and smosis promoter through proportionally mixing.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Drug delivery device containing neuraminidase inhibitor and an H1 antagonist
The present invention provides a dual release solid dosage form containing a first composition that releases a neuraminidase inhibitor, such as oseltamivir, zanamivir, or peramivir, in a controlled manner and a second composition that releases an H1 antagonist in a rapid and / or immediate manner. A wide range of H1 antagonist antihistamines, especially fexofenadine and loratadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. The device is useful for the treatment of respiratory congestion and other viral infection associated symptoms.
Owner:ACELLA HLDG LLC +1
Liquid composition containing loratadine and ambroxol hydrochloride
InactiveCN101152181AOrganic active ingredientsPharmaceutical delivery mechanismMedicineActive component
The invention discloses a drug combination with loratadine and ambroxol hydrochloride as the active components and with the treating functions of allergy, relieving cough and expelling phlegm. The drug combination contains stabilizer, pH regulator, preservative, taste masking agent and metalion complexing agent, etc, and the existing forms of the product are syrups and oral liquid.
Owner:BEIJING D VENTUREPHARM TECH DEV
Treatment of behavioral disorders
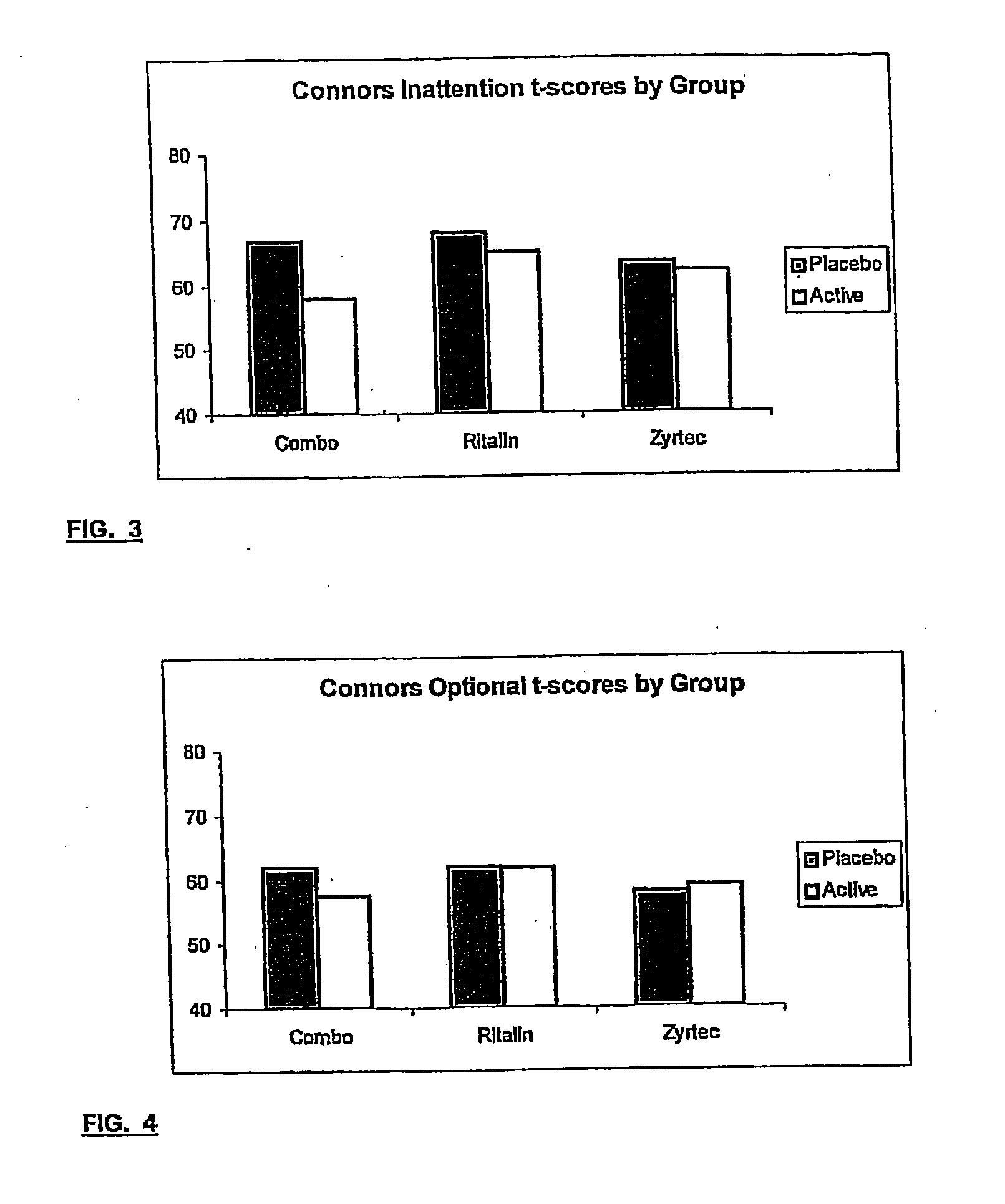
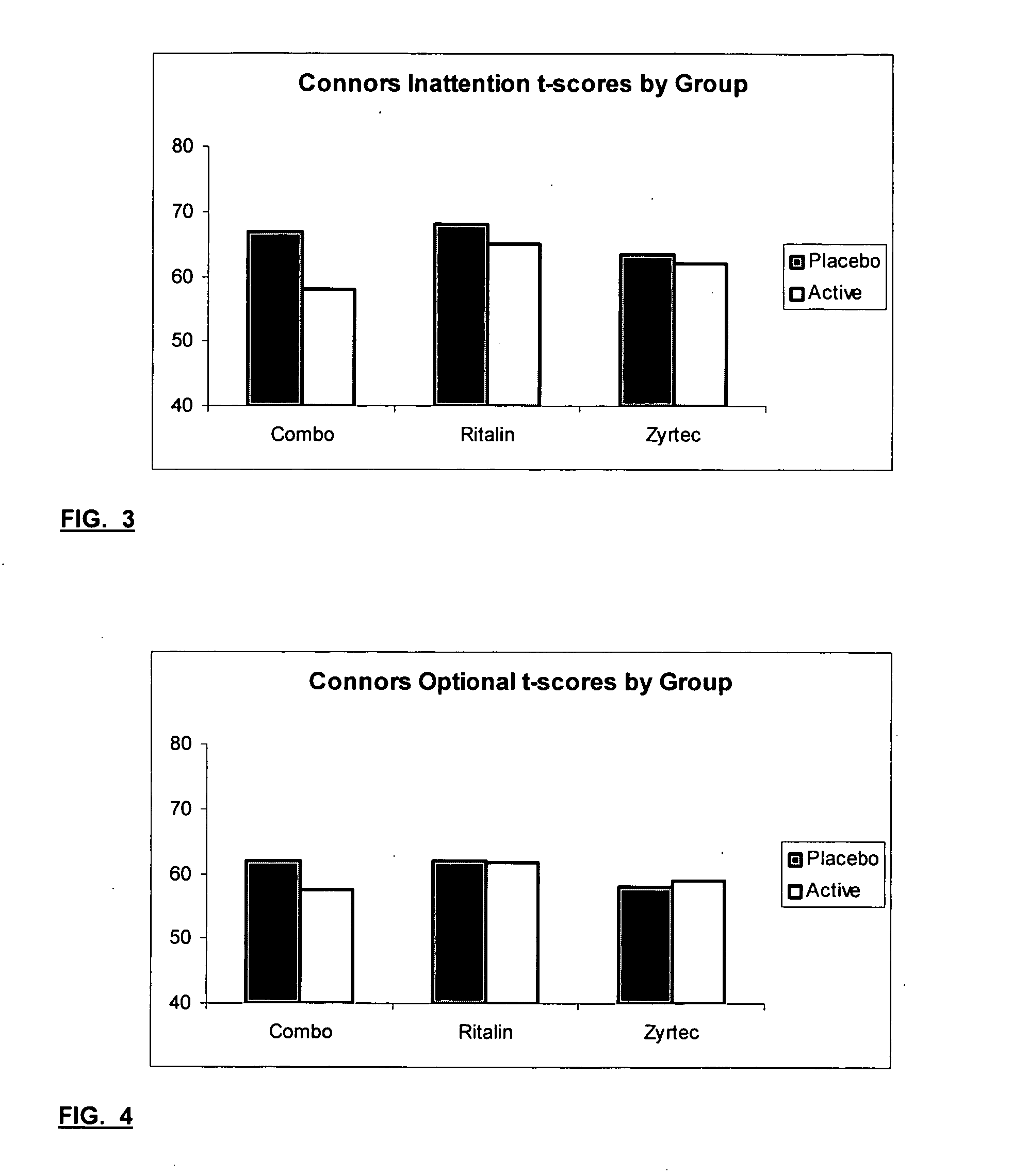
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Loratadine film preparation
ActiveCN103083283AReduce interactionImprove aestheticsOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMedicine
The invention discloses a loratadine film preparation. The preparation comprises a strip-shaped film tape and an effective amount of an active medicine loratadine loaded thereon; the strip-shaped film tape comprises an acidic strip-shaped film tape and an alkaline strip-shaped film tape, and the acidic strip-shaped film tape and the alkaline strip-shaped film tape are connected into one and are arranged in an interval mode; the acidic strip-shaped film tape is based on a high-water-solubility polymer film forming material, and contains 1-20wt% of an acidic agent; the alkaline strip-shaped film tape is based on the high-water-solubility polymer film forming material, and contains 1-20wt% of an alkaline agent; and loratadine is loaded on the acidic strip-shaped film tape, or on the alkaline strip-shaped film tape, or simultaneously on the acidic strip-shaped film tape and the alkaline strip-shaped film tape. The loratadine film preparation becomes an effervescent film after meeting with water, and can palsy olfaction to cover a smell, and the dissolution of the loratadine film preparation can be accelerated.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
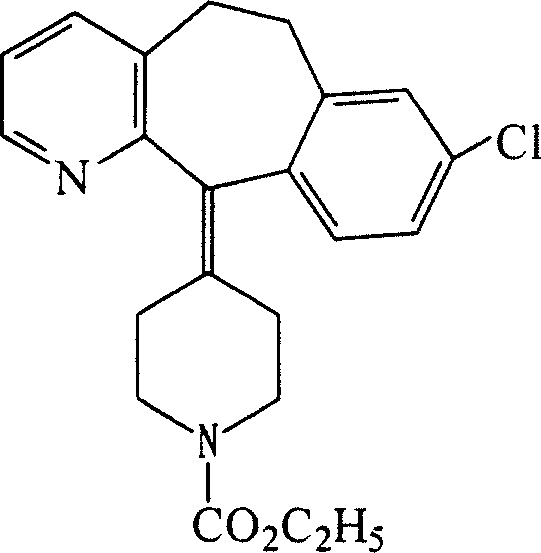
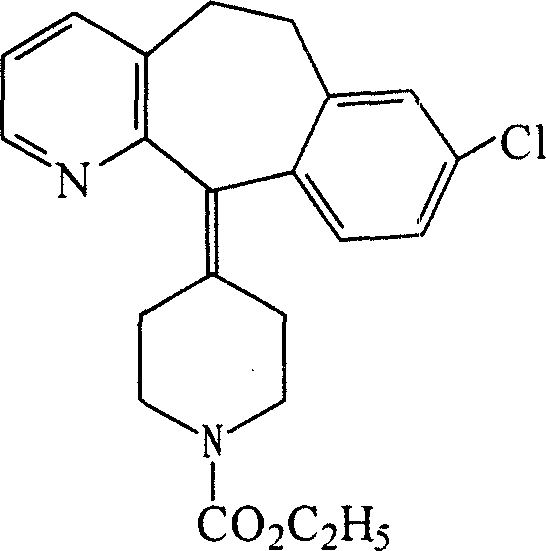
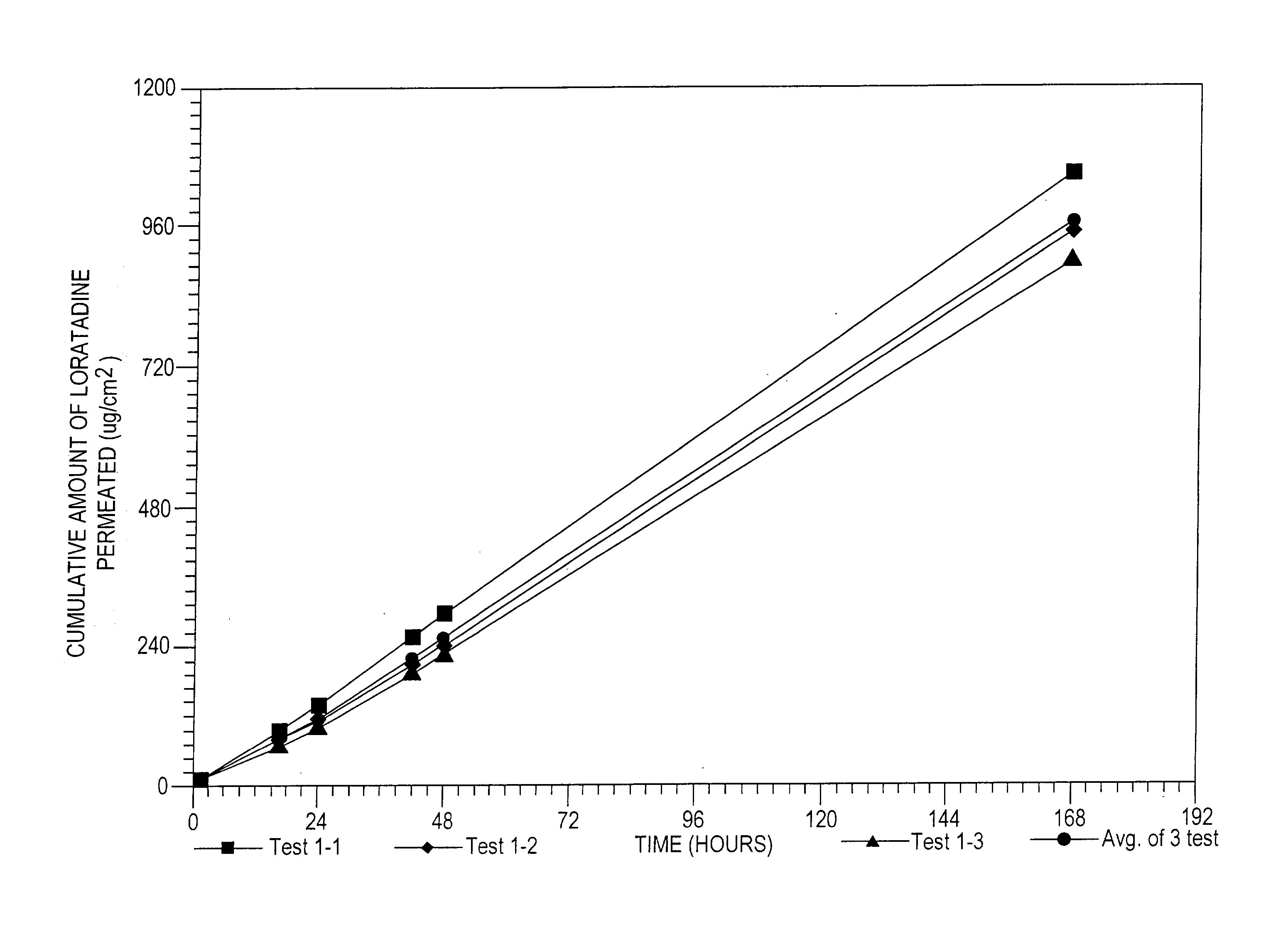
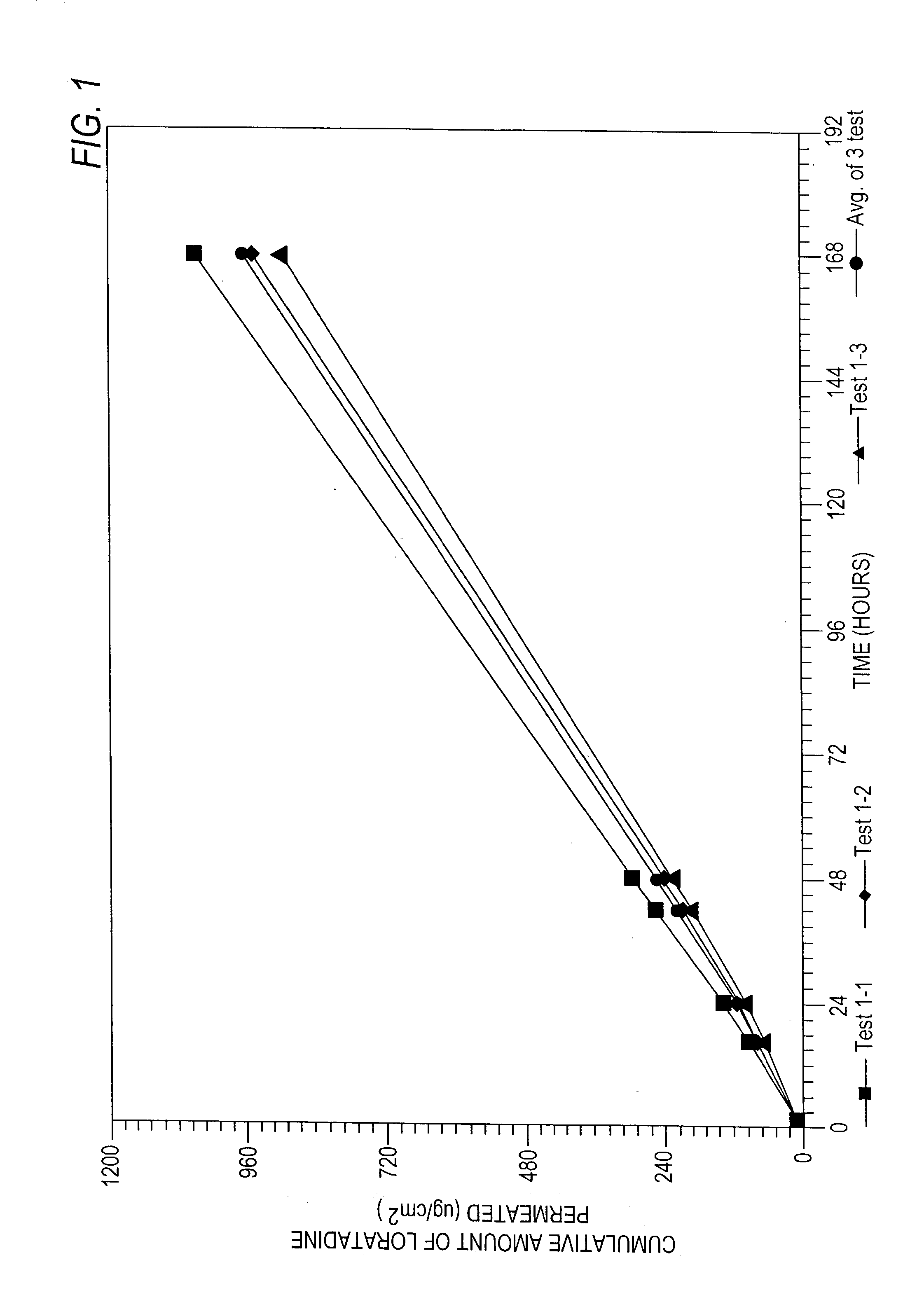
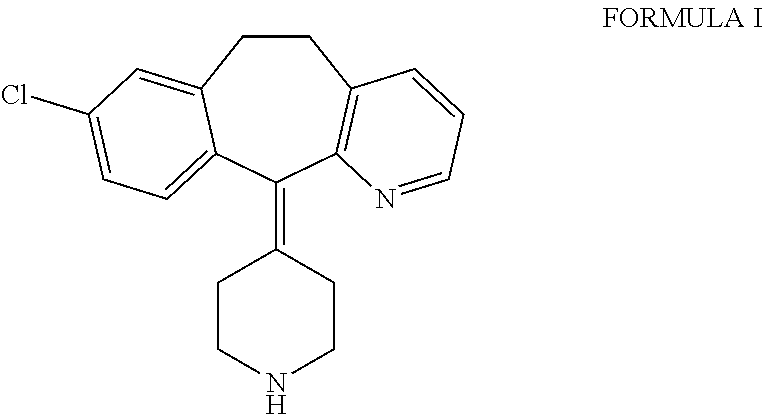
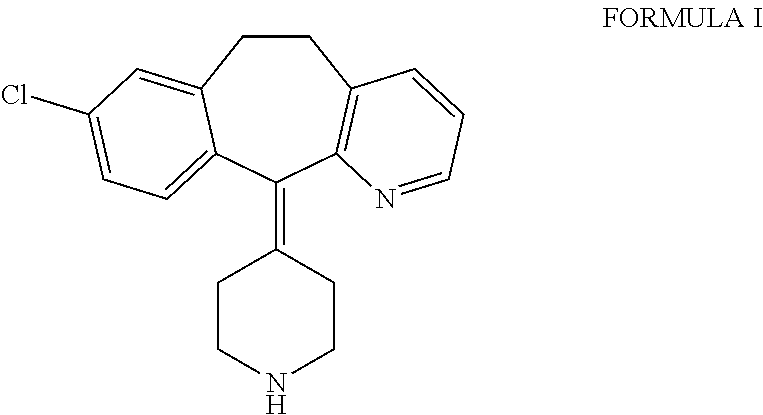
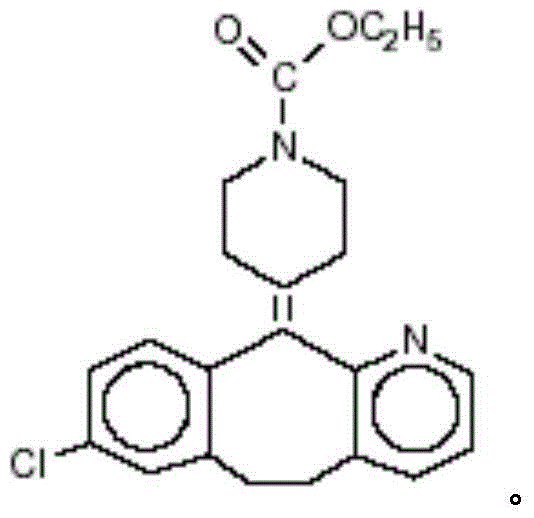
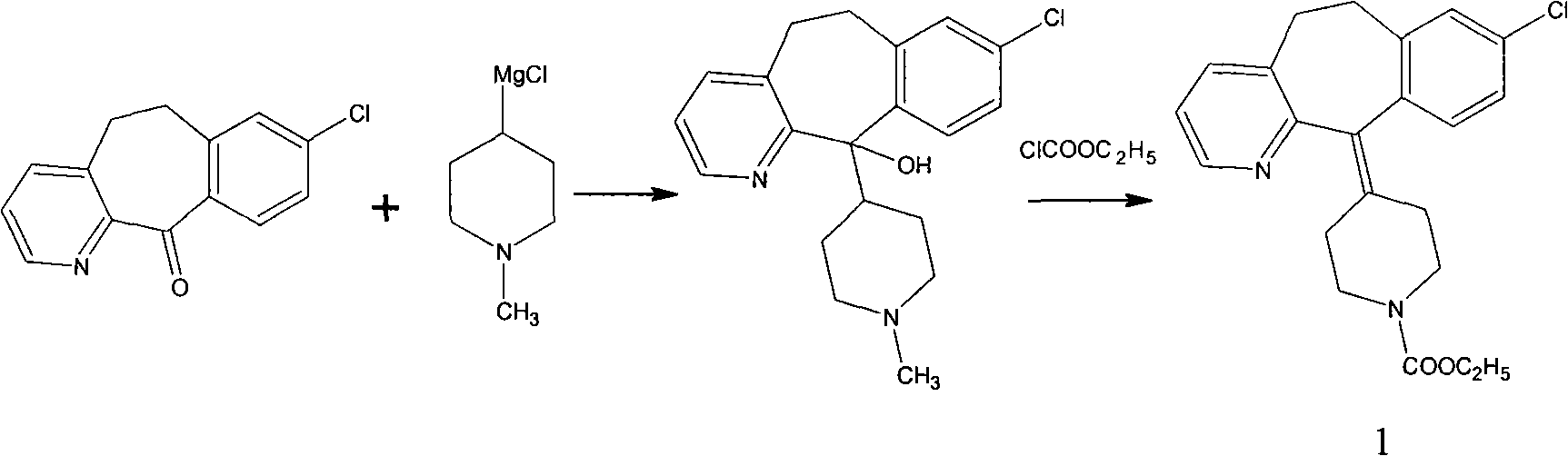
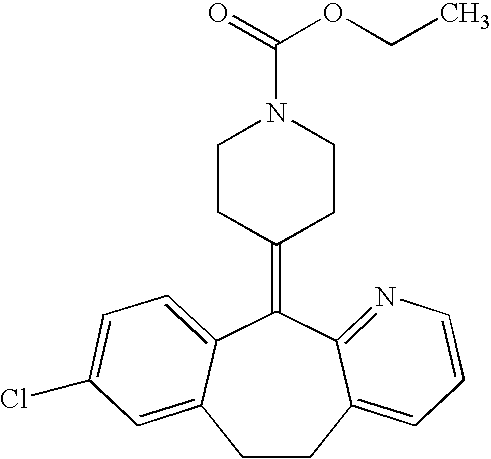
Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph
A polymorph form 2 of ethyl 4(8-chloro-5,6,-dihydro-11H- benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate (hereinafter "loratadine") represented by the formulaPharmaceutical composition containing the polymorph form 2, and methods of using the polymorph form 2 to treat allergic reactions in mammals such as man are disclosed.
Owner:SCHERING CORP
Oral cavity quick dissolved film containing civeran, and method for preparing the same
InactiveCN100998551AEasy to carryPleasant tasteOrganic active ingredientsPharmaceutical delivery mechanismChronic urticariaWater soluble
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Loratadine transdermal device and methods
InactiveUS20030035828A1Reduce morbidityEliminate side effectsBiocideCarbohydrate active ingredientsLoratadineChronic idiopathic urticaria
Owner:PURDUE PHARMA LP
Process for the preparation of desloratadine
The present invention provides a process for the preparation of desloratadine comprising contacting loratadine with a mixture of a weak inorganic base and sodium or potassium hydroxide in a ratio, ranging from 0.01 to 0.15 equivalents of sodium or potassium hydroxide per equivalent of weak inorganic base, in one or more suitable solvents) followed by isolation.
Owner:RANBAXY LAB LTD
Dry Syrup Containing Loratadine
Dry syrup preparations comprising loratadine as a hydrophobic medicinal drug are provided. The loratadine dry syrup preparations can be produced using a cellulose material or an argininic acid salt together with sugar.
Owner:SCHERING CORP
Granules of clarityne and their production
InactiveCN1679567AFragrant smellSweet tasteGranular deliveryRespiratory disorderLoratadineCitric acid
A particle of loratadine is prepared proportionally from loratadine, citric acid, and the additive chosen from edible essence, sweetening agent and edible pigment.
Owner:南京亿华药业有限公司
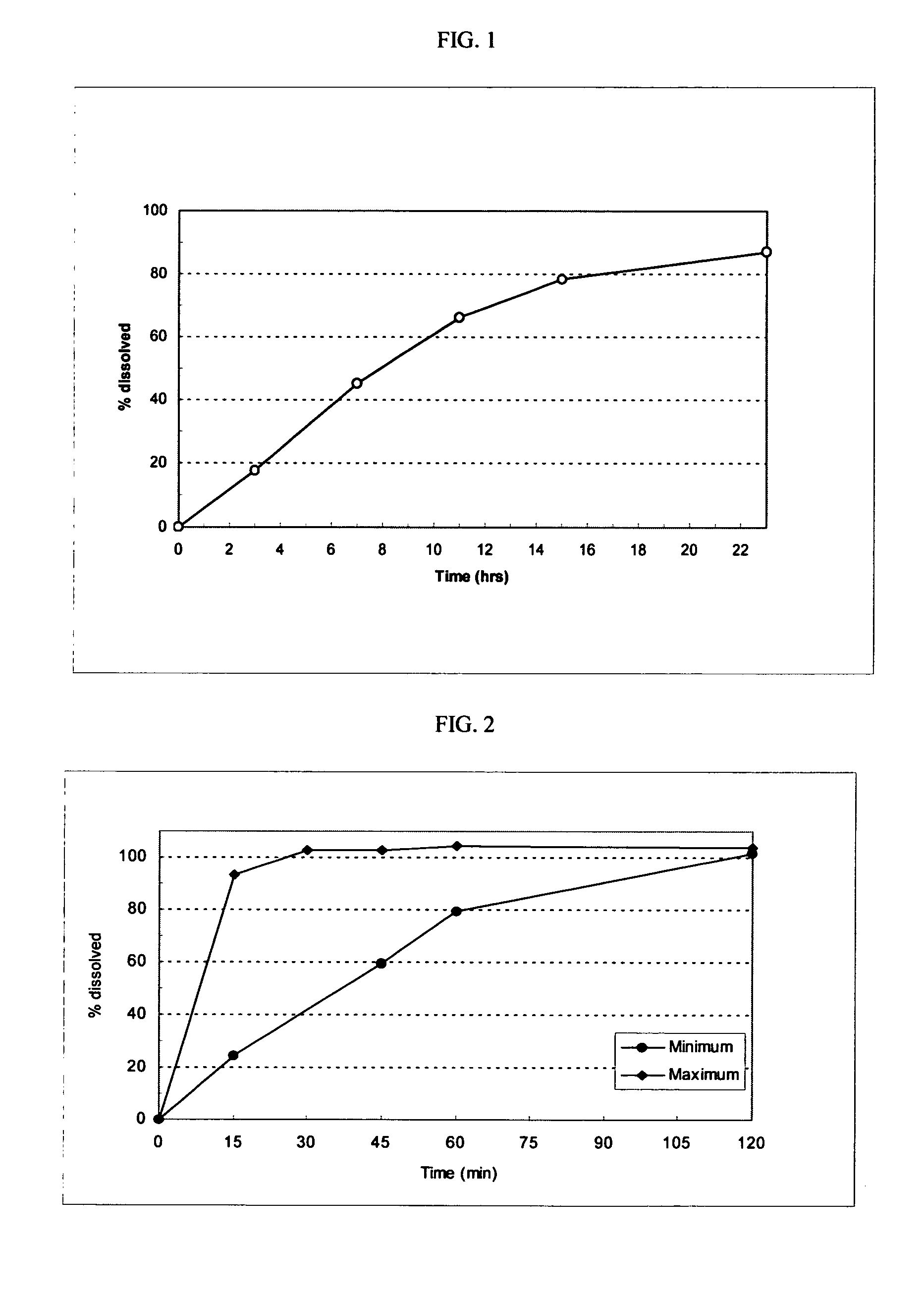
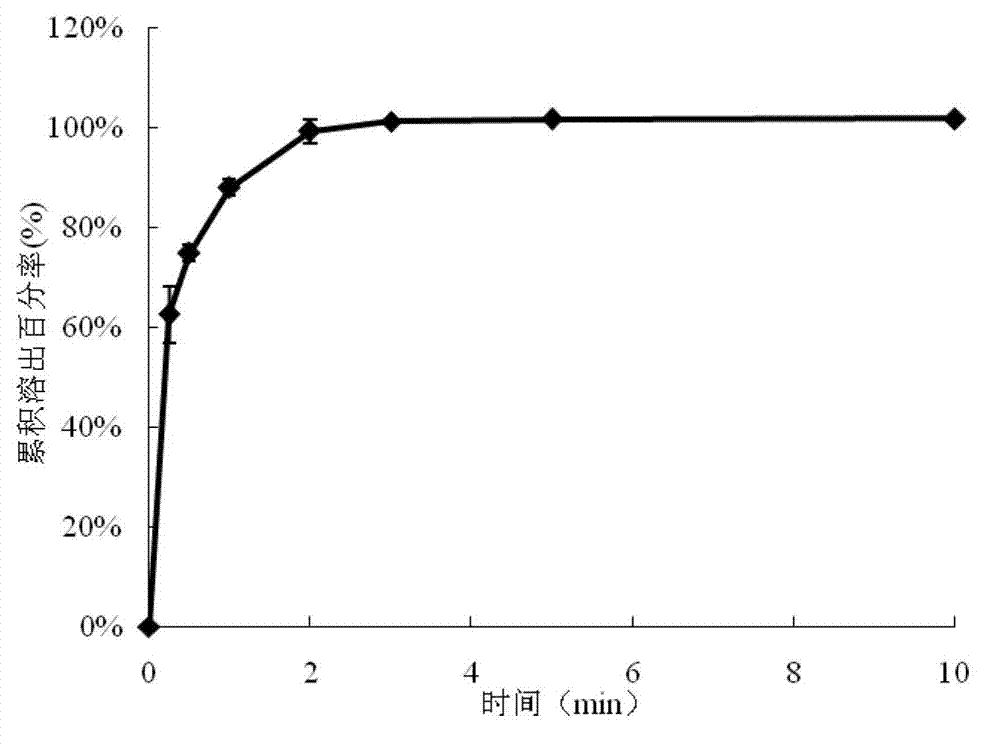
Rapidly-dissolved loratadine tablets and preparation process thereof
ActiveCN104523644APromote dissolutionSimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolDissolution
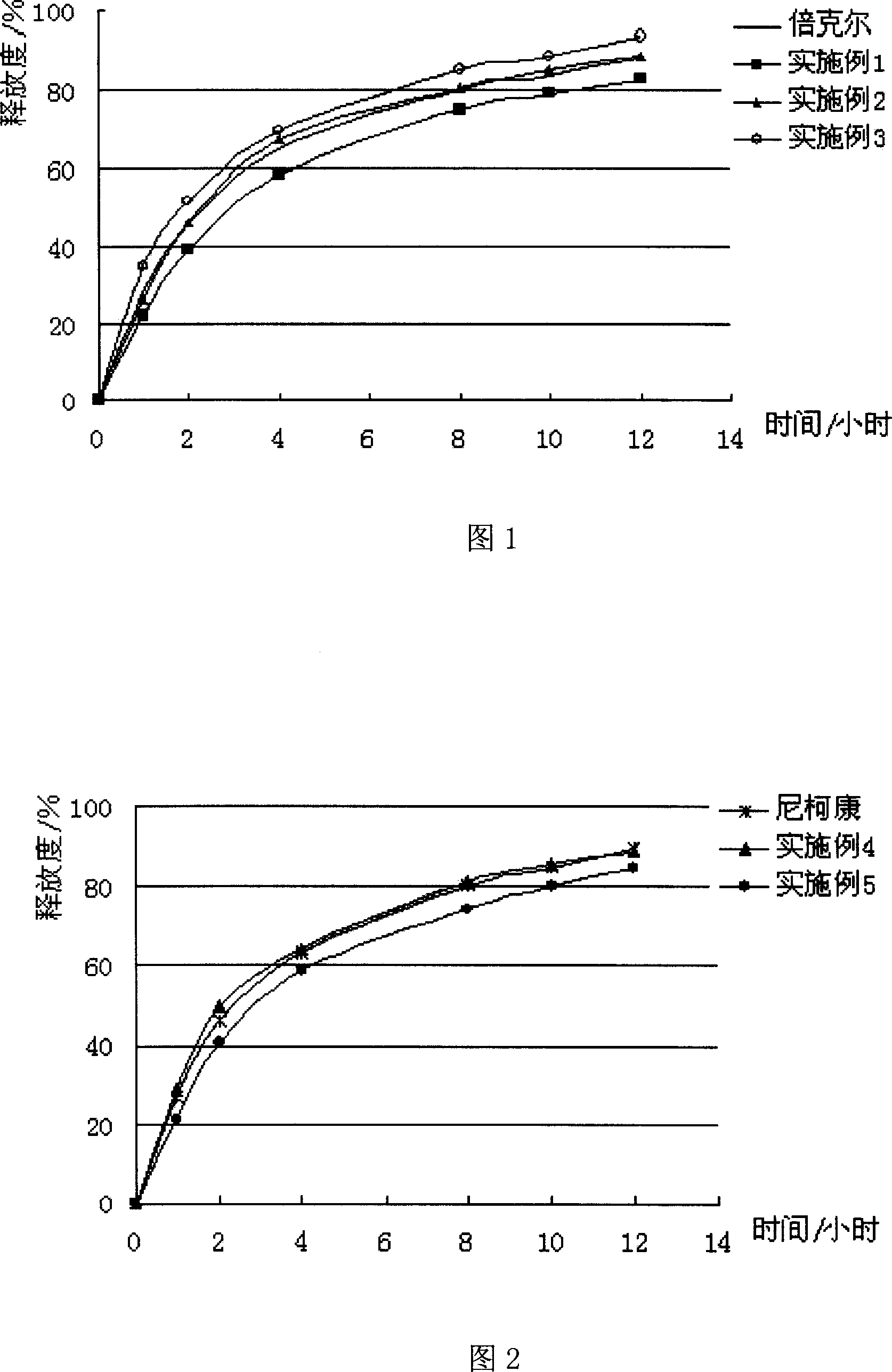
The invention discloses rapidly-dissolved loratadine tablets and a preparation process thereof. The tablets comprise blank tablet cores and medicine-containing coating layers, wherein each medicine-containing coating layer consists of loratadine, copovidone and polyethylene glycol; and the rapidly-dissolved loratadine tablets are prepared by coating the blank tablet cores by virtue of an efficient coating machine. The loratadine tablets disclosed by the invention are rapid in dissolution, and ensure that the dissolution rate can reach more than 95% in 5 minutes; and meanwhile, the loratadine tablets have the advantages of simple preparation process and easiness in operation.
Owner:JIANGSU LIANHUAN PHARMA
Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627998AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical product form changeYolkMedicine
The invention relates to a loratadine-ambroxol pharmaceutical composite and a liposome solid preparation thereof and a preparation method thereof; the liposome comprises the following components according to the parts by weight percent: 1 part of loratadine, 5 parts of ambroxol hydrochloride, 3-30 parts of yolk lecithin, 1-14 of cholesterol, 1.2-10 parts of sodium deoxycholate and 3-18 parts of poloxamer 188.
Owner:HAINAN YONGTIAN PHARMA INST
Novel loratadine oral quickly soluble film and preparation method thereof
InactiveCN106619577AImprove complianceQuick releaseOrganic active ingredientsPharmaceutical non-active ingredientsLoratadineEthanol
The invention relates to a novel loratadine oral quickly soluble film and a preparation method thereof. The oral quickly soluble film comprises the following components: loratadine, a surfactant, a film-forming material, a flavoring agent, a corrigent and a small amount of ethanol. The invention further provides a preparation method of the novel loratadine oral quickly soluble film. The oral quickly soluble film is small in size and convenient to carry; the dosage is convenient to control, a preparation process is simple and controllable, special production equipment is not required, the novel loratadine oral quickly soluble film is suitable for industrial massproduction, can be quickly dissolved in the oral cavity of a patient who does not need to drink water or chew, and tastes good; compliance of medication for children is greatly improved; the dissolution condition of the novel loratadine oral quickly soluble film in a citric acid-disodium hydrogen phosphate buffer solution with pH of 4.0 is remarkably better than the dissolution condition of commercially available tablets; and the novel loratadine oral quickly soluble film has significance on gerontal patients and other patients with achlorhydria.
Owner:CHINA PHARM UNIV
Preparation method of desloratadine
The invention relates to a preparation method of desloratadine. The preparation method comprises the following steps: (1) dissolving loratadine into an alcohol solvent with the volume concentration of 55-75% under the protection of nitrogen, adding potassium hydroxide, heating to 70-75 DEG C, beginning reflux reaction, controlling the temperature of the reflux reaction to keep a gradient change within 70-100 DEG C, and refluxing till complete reaction of the loratadine; (2) adding an ethyl acetate solvent into a completely reacted reaction solution for extracting, draining out a water layer, repeating the extracting step for 2-3 times, washing by using water, and crystallizing, decolorizing and recrystallizing to obtain desloratadine. By the preparation method, the technical problems that in the prior art, the product desloratadine cannot meet a requirement for purity, contains many impurities, and cannot meet requirements on clinical application are solved.
Owner:GUANGDONG JIUMING PHARMA
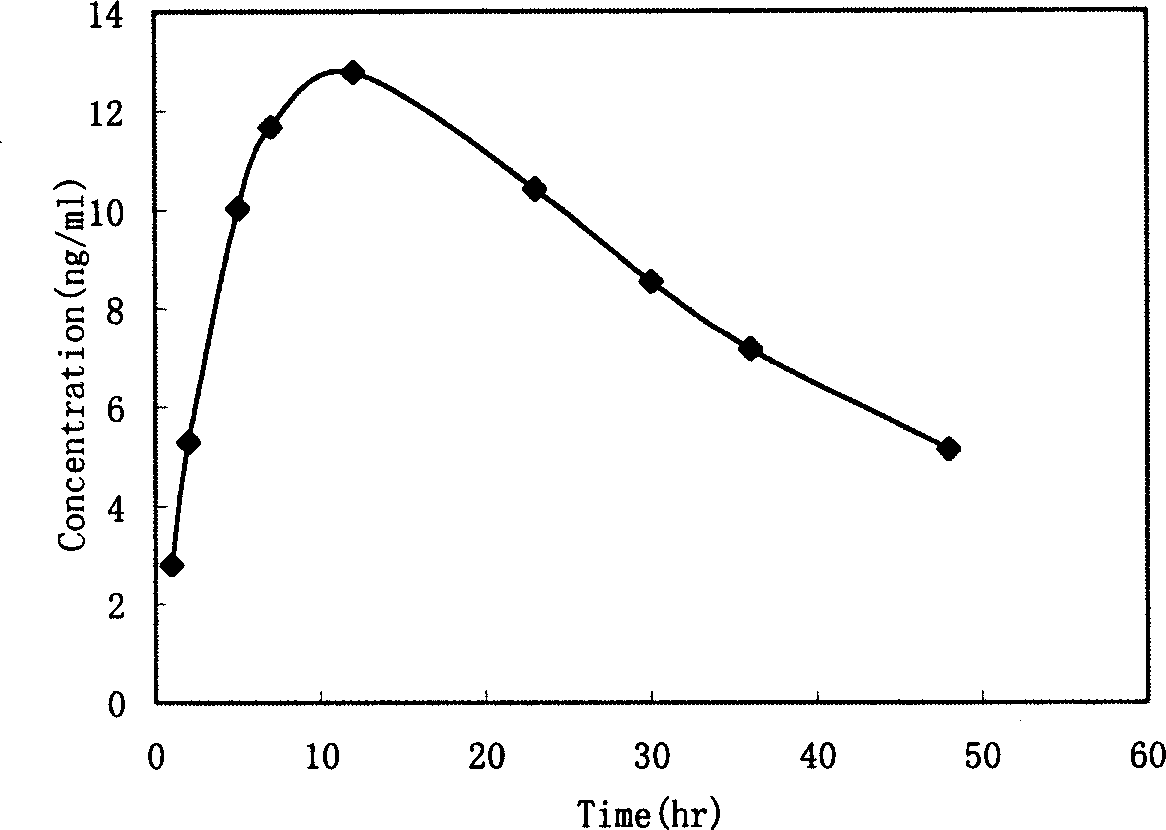
Desloratadine patch and preparation method thereof
InactiveCN101933914AImprove solubilityGood curative effectOrganic active ingredientsImmunological disordersCross-linkSolubility
The invention relates to a desloratadine patch and a preparation method thereof. The desloratadine patch comprises a patch film and medicament components on the patch film, wherein the medicament components comprise main component desloratadine and auxiliary materials; and the auxiliary materials comprise a solubilizing agent, a substrate, a cross-linking agent, an excipient and an osmosis reinforcing agent. The invention overcomes the bias that in the prior art, the desloratadine is considered to be difficult to externally penetrate through the skin cuticle and not to reach effective tissue concentration in the skin tissue so as not to exert the curative effects of allergic resistance and inflammation resistance, solves the problem of poor solubility of the desloratadine, improves the permeability of the desloratadine on the skin cuticle, ensures that the desloratadine rapidly arrives at the affected part and takes the effect rapidly and solves the problems of inconspicuous effect-taking and obvious first pass effect on gastrointestinal tract of the traditional patch. The desloratadine patch has the advantages of good curative effect, rapid action, less toxic or side effect and convenient use.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +1
Compound abiduoer preparation with wide spectrum in resisting influenza viral
The invention provides a compound Arbidol hydrochloride wide spectrum antiviral influenza medicament, which is prepared from Arbidol hydrochloride, acetaminopher and chlorpheniramine or Loratadine. The compound can be prepared into oral medication through the conventional preparation process.
Owner:吉林省吴太感康药业有限公司
Oral loratadine disintegrating tablet and its prepn
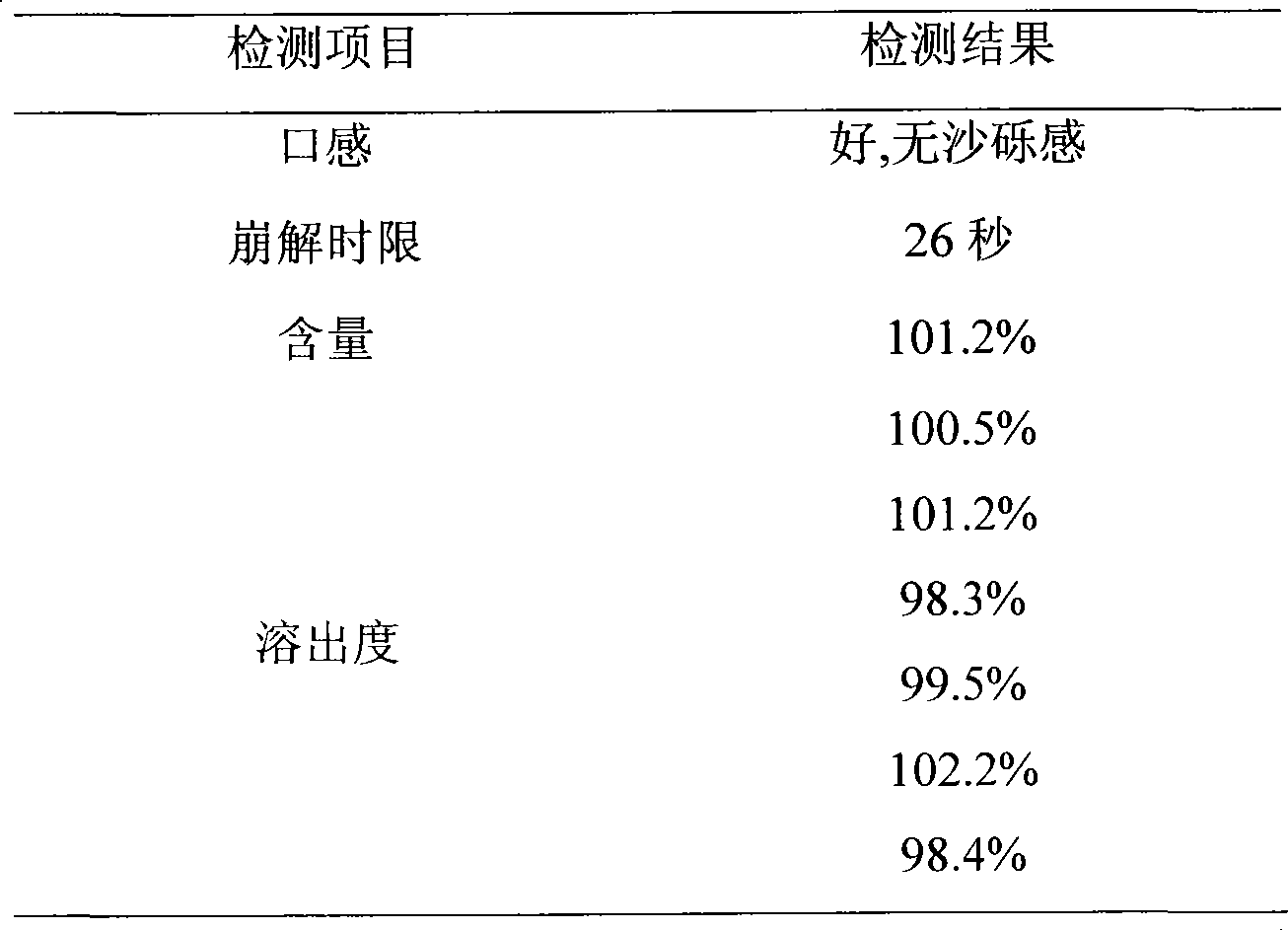
ActiveCN1739513AReduce stimulationQuick effectPill deliveryImmunological disordersDiseaseOrally disintegrating tablet
The present invention belongs to the field of medicine technology, and is especially oral loratadine disintegrating tablet for treating allergic diseases and its preparation process. The oral loratadine disintegrating tablet includes loratadine as effective medicine component, and excipient mixture comprising disintegrating agent, stuffing, soluble polyol and penetrant. The oral loratadine disintegrating tablet consists of loratadine 20-50 wt%, disintegrating agent 5-15 wt%, stuffing 10-30 wt%, soluble polyol 30-60 wt%, and penetrant 1-5 wt%. The oral loratadine disintegrating tablet, after being disintegrated fast, can cover gastrointestinal mucous membrane widely, and has fast acting, no first pass effect, high bioavailability, and convenient taking.
Owner:HAINAN PULIN PHARMA +1
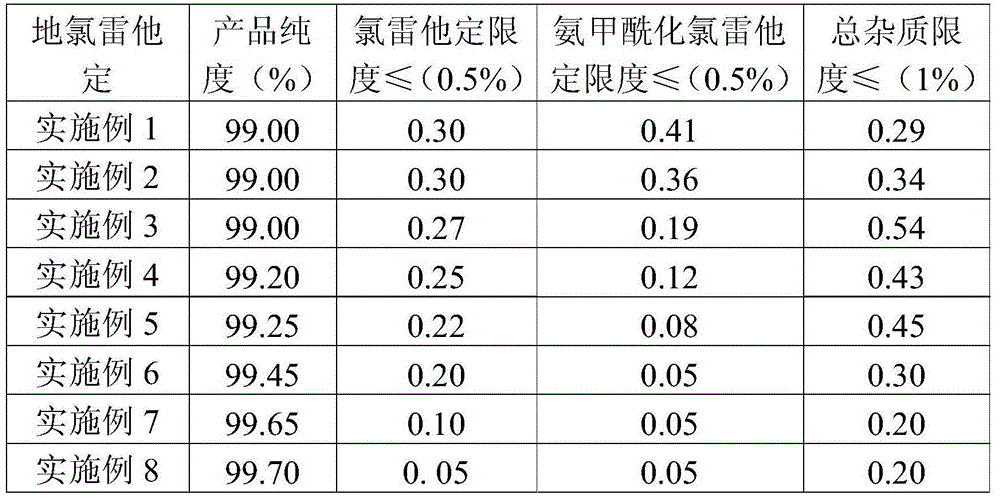
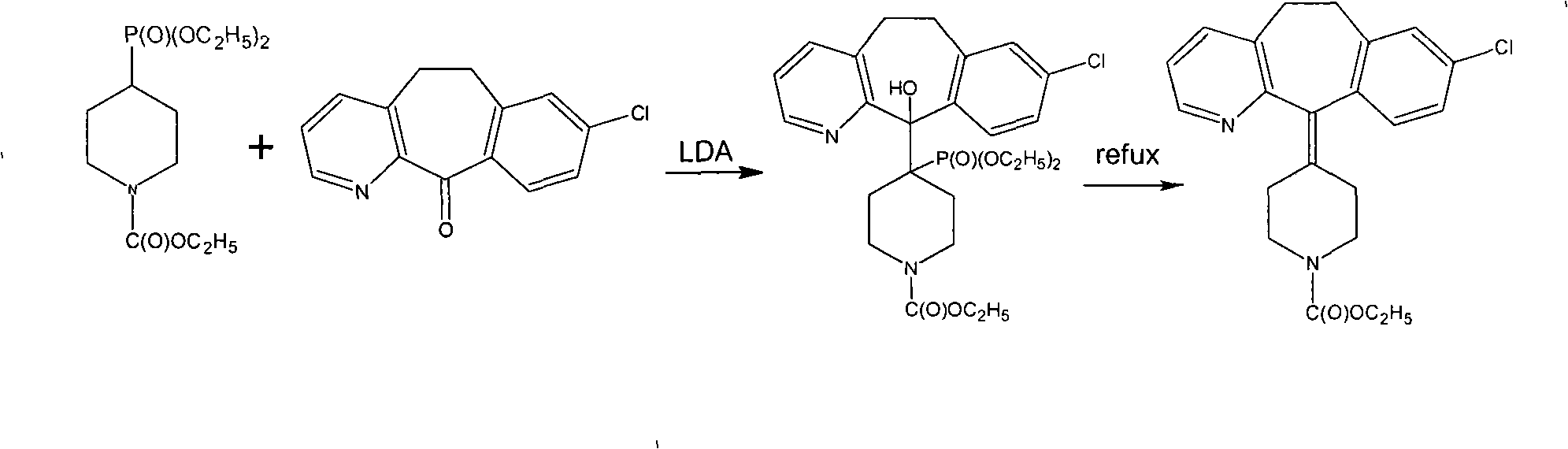
Novel synthetic method of loratadine
InactiveCN101348480ARaw materials are easy to getLow priceOrganic chemistryChemical recyclingKetoneOxygen
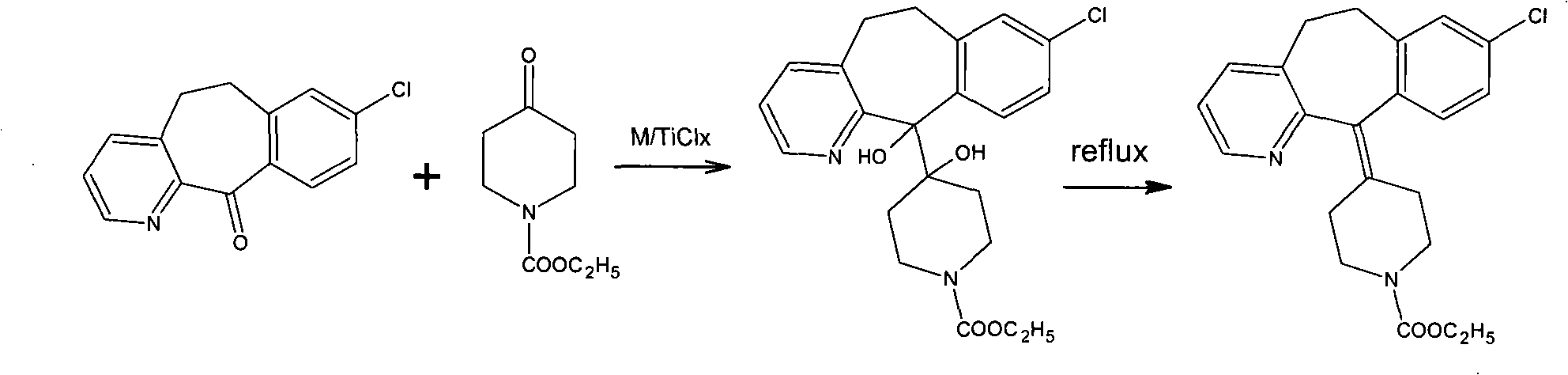
The invention discloses a method for preparing a drug of Loratadine. The prior art has stricter requirement on reaction conditions and is difficult to control, with higher price of raw materials and catalysts, high production cost; and high content of impurities in the final products and a great amount of produced pollutants. The invention comprises the following steps that tricyclic ketone and 4-Ethoxycarbon piperidone undergo condensation in an inertia solvent by a low valent titanium, followed by acid hydrolysis to obtain Loratadine. The invention lowers the cost on the basis of the ensured yield rate through using the low valent titanium, and has mild operation condition, easy control, generally no pollution, stable technology, and good repeatability.
Owner:ZHEJIANG JINGXIN PHARMA
Treatment of Behavioral Disorders
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as cetirizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxiety, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Loratadine oral compound medication composition
InactiveCN101015519AOrganic active ingredientsPharmaceutical delivery mechanismAntitussive drugsDrug effect
This invention relates to an orally adminstered compound Chinese medicinal composition of loratadine for treating cough. The composition comprises quick-release part containing loratadine and slow-release part containing centrum antitussive drug, wherein the centrum antitussive drug is preferably dextromethorphan. The invention maintains the quick and long lasting action of loratadine or desloratadine, overcomes the quick releasing and short action on relieving cough of dextromethorphan, slow releases dextromethorphan, and maintains the drug effect to arrive the purpose of relieving cough for a long time.
Owner:CHONGQING PHARMA RES INST
Oral desloratadine drops and preparation method thereof
InactiveCN106619504AEasy to acceptClear pharmacological propertiesOrganic active ingredientsSenses disorderNoseDisease patient
The invention belongs to the technical field of medicines and relates to a preparation method of oral desloratadine drops. The oral desloratadine drops are prepared from a loratadine raw material medicine, a preservative, a stabilizer, a coloring agent, a corrigent, a thickening agent, a cosolvent and the like. 10 mg of drops is orally taken every time for adults and adolescents of 12 years old or above, and infants are administrated according to kilogram and body weight. The oral desloratadine drops are syrup or a solution and has the following advantages in overall effect that the drug exists in a molecular state in liquid, can be rapidly absorbed after administration, plays a drug action and is high in initial effect time; the process is simple in operation, the cost is low, and the drops are convenient to take, stable in quality, reliable in treating effect and easy to absorb; the drops are good in taste, are conveniently accepted by patients, are suitable for patients difficult to take orally and infant and old diseased patients and has strong anti-inflammatory and antiallergic effects and rapid oral taking and absorbing effects, and the nose and eye symptoms and signs are relieved after the drug is orally taken.
Owner:BEIJING VENTUREPHARM BIOTECH
Compound composition of intal and Statins
InactiveCN101766617ASolve the irritatingOrganic active ingredientsSenses disorderDiseaseAdditive ingredient
The invention relates to a compound composition containing intal and statins claritin. The compound composition consists of the following components: a) a certain amount of intal; b) a certain amount of one of olopatadine hydrochloride, ioratadine, desloratadine, degreasing ioratadine, desloratadine, rupatadine and betahistine; c) other medicinal excipients. The compound composition can be made into external preparations such as eye drops, nose drops, aerosol, spray, inhalant, gelata, eye ointments, ointments or patch and the like. The compound composition can be used for curing the diseases such as anaphylactic eye diseases, anaphylactic rhinitis, skin urtication, urticaria, allergic asthma and the like, can significantly improve allergic symptoms, and has the characteristics of high efficiency, stability, safety, low adverse reaction rate, convenient use and the like.
Owner:北京华禧联合科技发展有限公司
Loratadine orally disintegrating tablet and technique for preparing the same
InactiveCN101461811AOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseOrally disintegrating tablet
The invention relates to a loratadine orally disintegrating tablet consisting of a special preparation and a preparation method thereof. The loratadine orally disintegrating tablet contains pharmaceutical excipients such as a disintegrant, a filling agent, an adhesive, a flavoring agent, a sweetening agent, a lubricating agent and so on, and can be used for the treatment of anaphylactic diseases.
Owner:AVENTIS PHARMA HAINAN
Loratadine dispersible tablets and its preparing method
InactiveCN101023949AOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseCarboxymethyl starch
The present invention relates to a loratadine dispersion tablet and its preparation method. Its dispersion tablet prescription contains 30-80 wt% of avicel or lactose, 2-10 wt% of pre-gelled starch, 1-20 wt% of carboxymethyl starch sodium or polyvinylpyrrolidone or their mixture, 1-10 wt% of aspartame, 0.5-2 wt% of sodium lauryl sulfate and 0.5-2 wt% of magnesium stearate. Said dispersion tablet can be used for curing the diseases of allergic rhinitis, chronic urticaraia, titillation dermatosis and other allergic dermatosis, etc.
Owner:BEIJING SL PHARMA
Loratadine oral quickly-soluble film and preparation method thereof
InactiveCN104958279AImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseDispersity
The invention discloses a loratadine oral quickly-soluble film and a preparation method thereof. By controlling parameters such as particle size of a loratadine drug, proportion of a film-forming material, content of water of a film product and the like, the product dissolution is improved, the bioavailability can be improved, the preparation can effectively prevent and cure such as allergic rhinitis, chronic idiopathic urticaria, allergic asthma, atopic dermatitis and associated symptoms caused by allergy such as skin diseases (eczema, dermatitis, pruritus cutanea), and the drug effect can be sufficiently realized. The loratadine oral quickly-soluble film does not need water, can be rapidly dissolved in the oral cavity and is convenient to take by patients and particularly suitable for the old people and children patients; moreover, the loratadine oral quickly-soluble film has characteristics of high dispersity, rapidness in absorption, fastness in effect, favorability for improving the bioavailability, and the like.
Owner:AVENTIS PHARMA HAINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/caa99178-f833-4c14-abd1-525060fb1a72/US06335347-20020101-C00001.png)
![Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/caa99178-f833-4c14-abd1-525060fb1a72/US06335347-20020101-C00002.png)
![Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph Ethyl 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidene carboxylate polymorph](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/caa99178-f833-4c14-abd1-525060fb1a72/US06335347-20020101-D00001.png)