Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
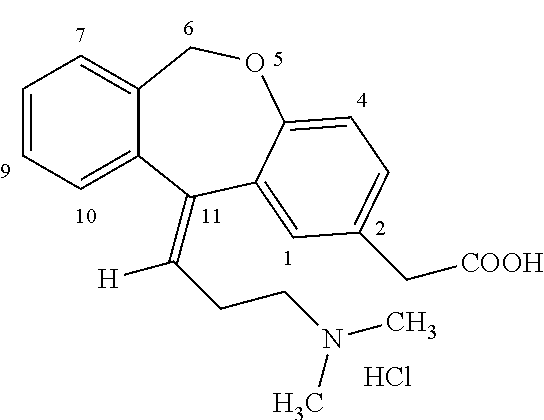
32 results about "Olopatadine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
OLOPATADINE (oh loe pa TA deen) drops are used in the eye to treat symptoms caused by allergies.
Use of connective tissue mast cell stabilizers to facilitate ocular surface re-epithelization and wound repair
InactiveUS20080139531A1Inhibition releaseOvercomes drawbackBiocideOrganic chemistryConjunctival woundDihydropyridine
Disclosed are methods of treating a wound in a subject that involve administering to the subject a pharmaceutically effective amount of a composition that includes one or more human connective tissue mast cell stabilizers, wherein administration of the composition results in treatment of the wound. In particular embodiments, the wound is an ophthalmic or dermal wound, such as a corneal epithelial defect, a conjunctival wound, or dermal abrasion. Administration, for example, may be by topical application of the composition to the ocular surface or skin. Exemplary mast cell stabilizers include olopatadine, variants of olopatadine, alcaftidine, derivatives of alcaftidine, dihydropyridines, and spleen tyrosine kinase inhibitors.
Owner:ALCON RES LTD
Olopatadine formulations for topical nasal administration
Owner:NOVARTIS AG
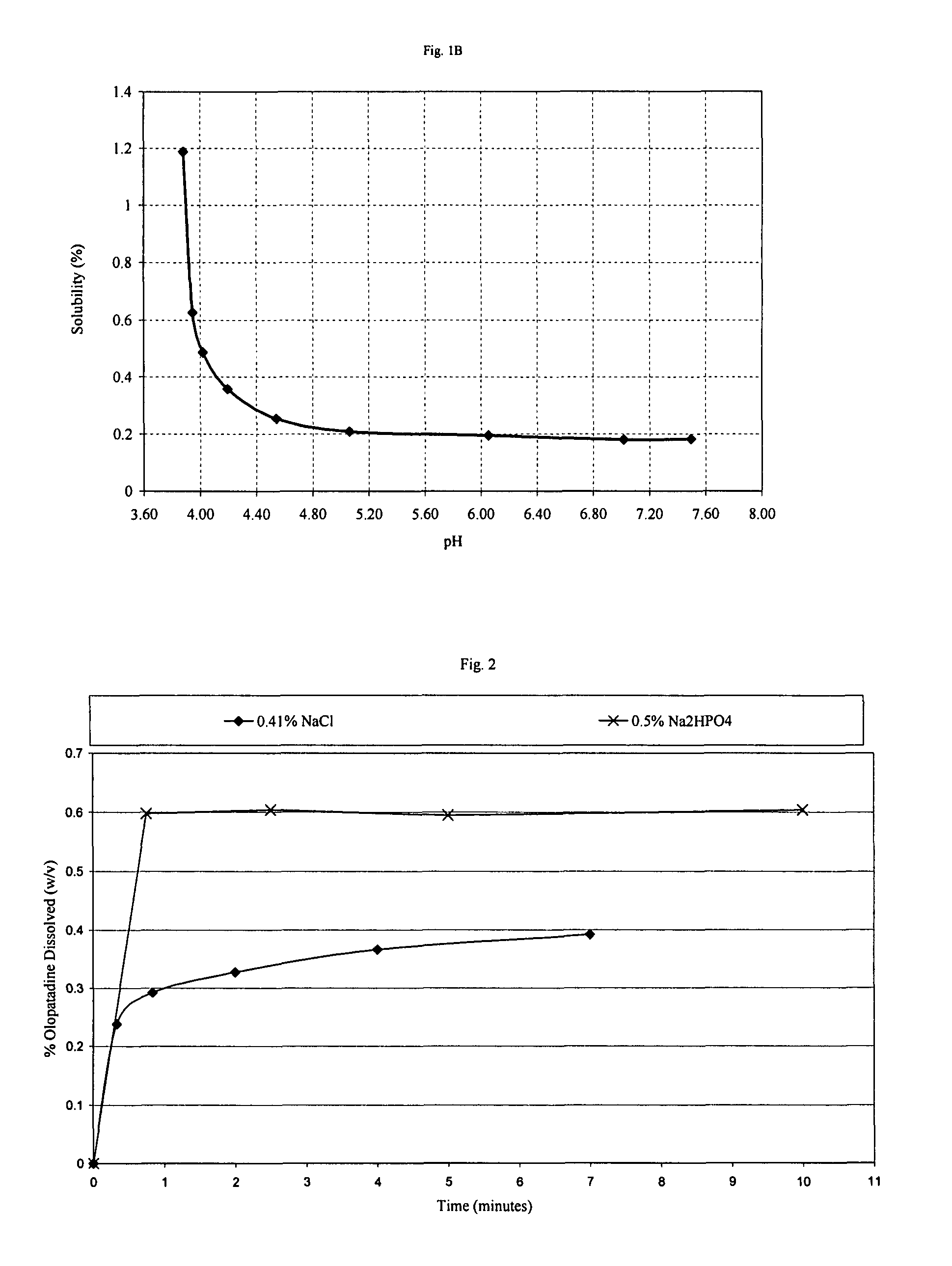
High concentration olopatadine ophthalmic composition
ActiveUS20120295967A1Improve solubilityOrganic active ingredientsBiocideHigh concentrationLate phase
The present invention is an ophthalmic composition containing a relatively high concentration of olopatadine. The composition is typically an ophthalmic aqueous solution containing relatively high concentrations of olopatadine solubilized within the solution. The composition is preferably capable of providing enhanced relief from symptoms of ocular allergic conjunctivitis, particularly late phase symptoms of ocular allergic conjunctivitis.
Owner:ALCON INC
High concentration olopatadine ophthalmic composition
The present invention is an ophthalmic composition containing a relatively high concentration of olopatadine. The composition is typically an ophthalmic aqueous solution containing relatively high concentrations of olopatadine solubilized within the solution. The composition is preferably capable of providing enhanced relief from symptoms of ocular allergic conjunctivitis, particularly late phase symptoms of ocular allergic conjunctivitis.
Owner:ALCON INC
Percutaneously Absorptive Ophthalmic Preparation Comprising Olopatadine
The present invention provides a percutaneously absorptive preparation for preventing or treating allergic eye disease, which comprises olopatadine or a salt thereof as an active ingredient. In addition, the present invention provides a method for preventing or treating allergic eye disease, which comprises applying a percutaneously absorptive preparation comprising olopatadine or a salt thereof to the skin surface including the skin surface of an eyelid, thereby casing transfer of a therapeutically effective amount of olopatadine or a salt thereof from the preparation to an anterior ocular segment through the skin of the eyelid rather than a systemic blood flow. The present preparation can exert a pharmacological effect over a prolonged period by a single application, as compared to conventional preparations such as eye drops.
Owner:SENJU PHARMA CO LTD
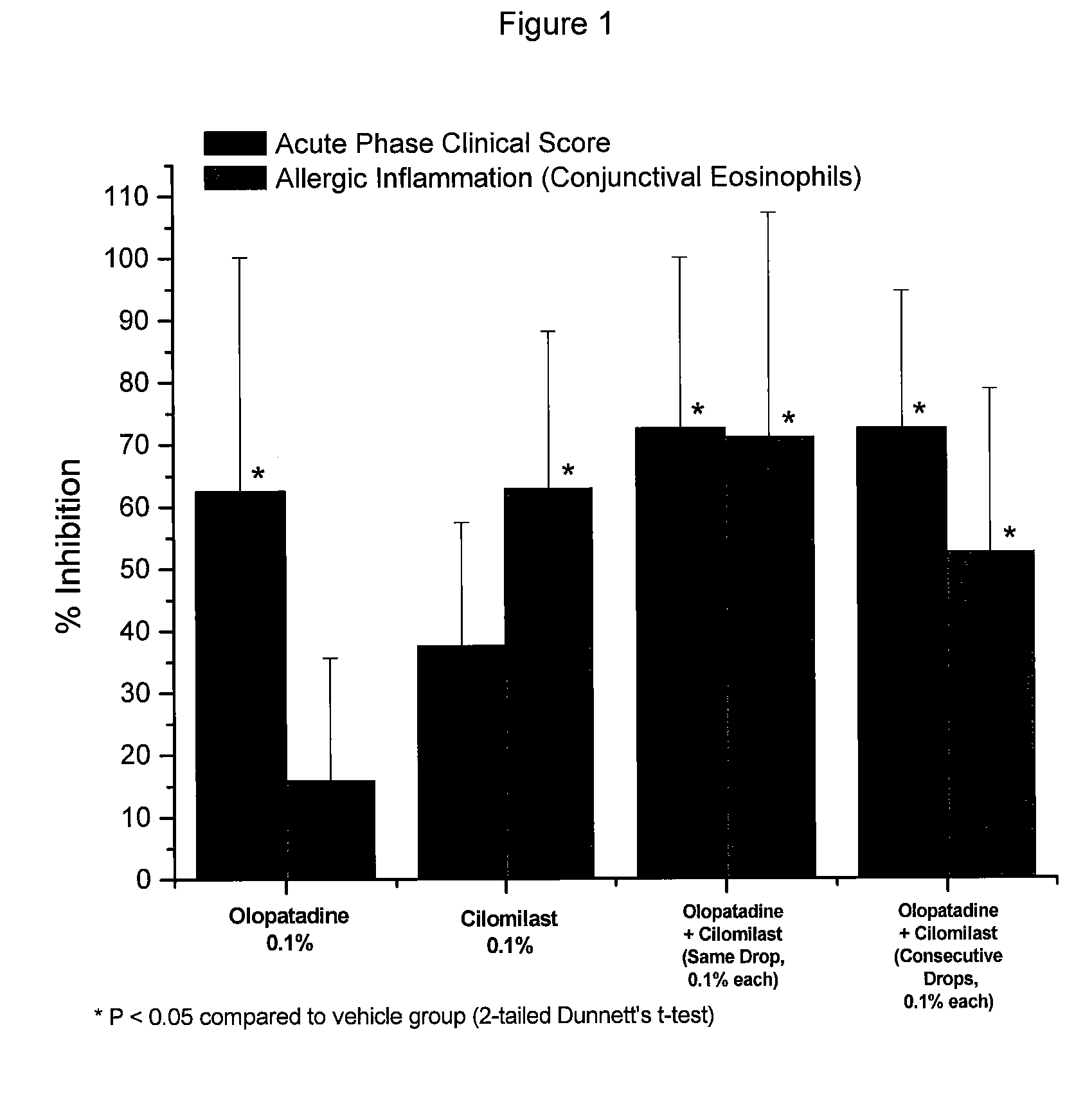
Use of a combination of olopatadine and cilomilast to treat non-infectious rhinitis and allergic conjunctivitis
InactiveUS20090182035A1Immediate and long-term reliefOvercomes drawbackBiocideSenses disorderGynecologyInfective rhinitis
Disclosed are methods of treating allergic conjunctivitis and non-infectious rhinitis in a subject that involve topically administering to the subject a composition comprising olopatadine and cilomilast.
Owner:ALCON RES LTD
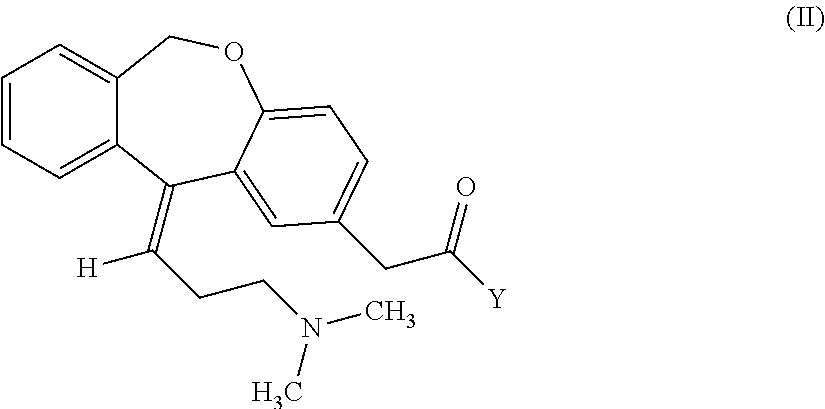
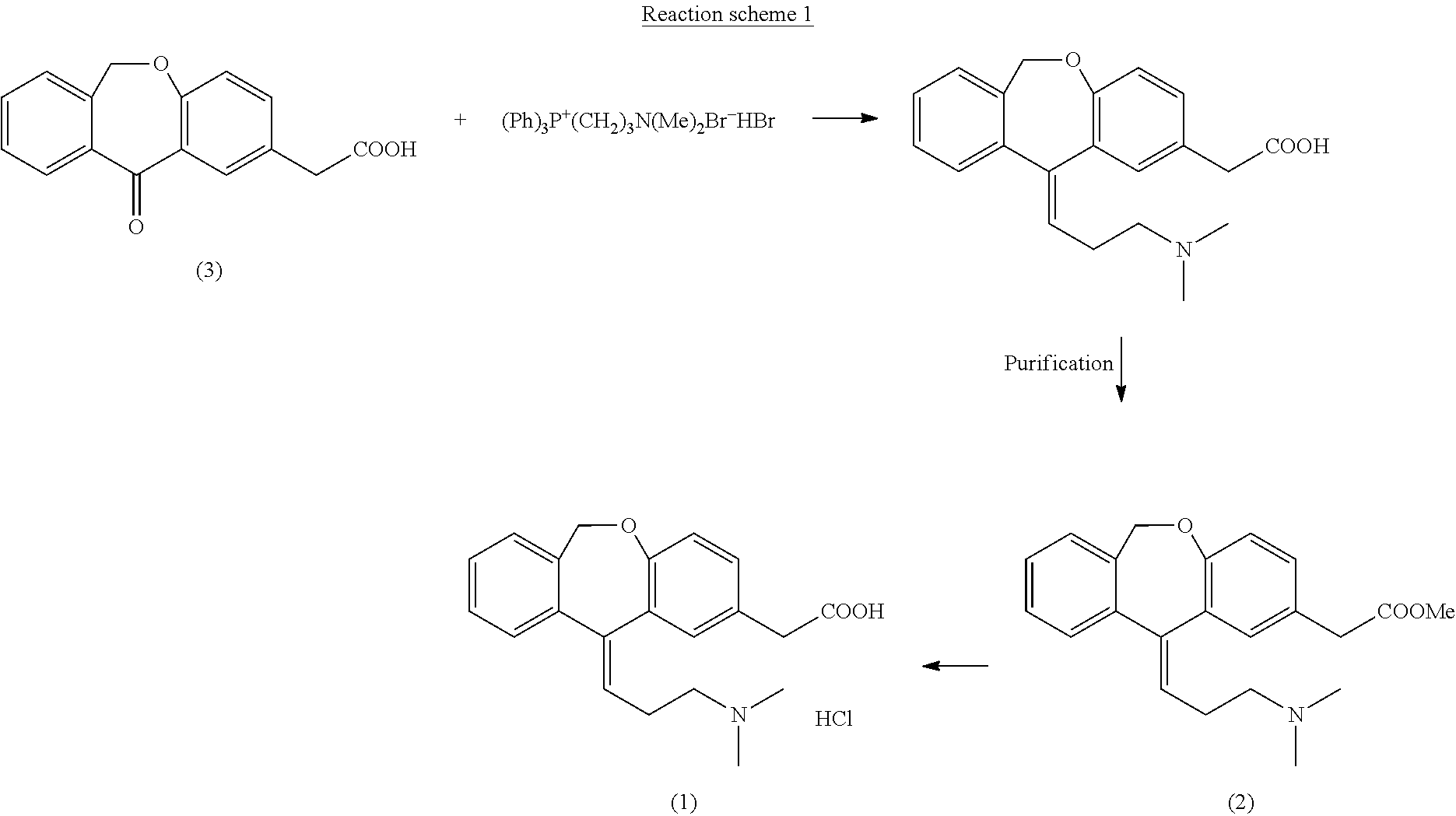
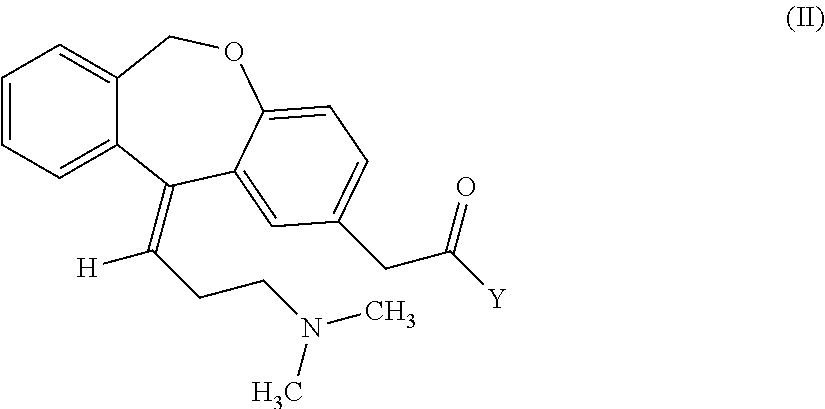
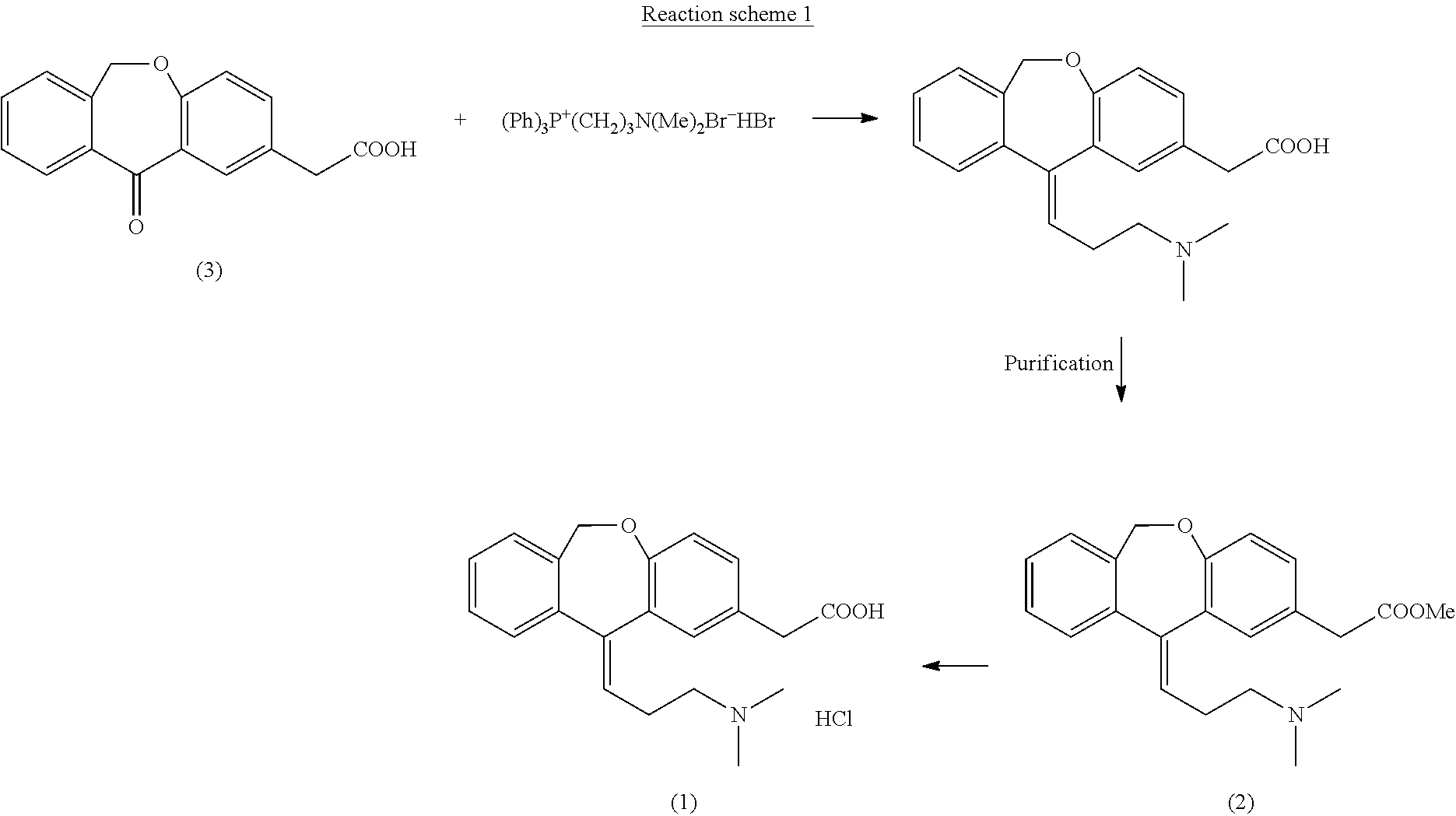
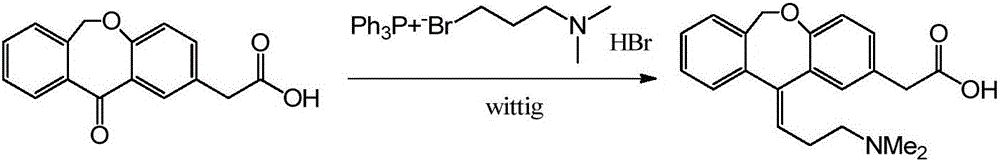
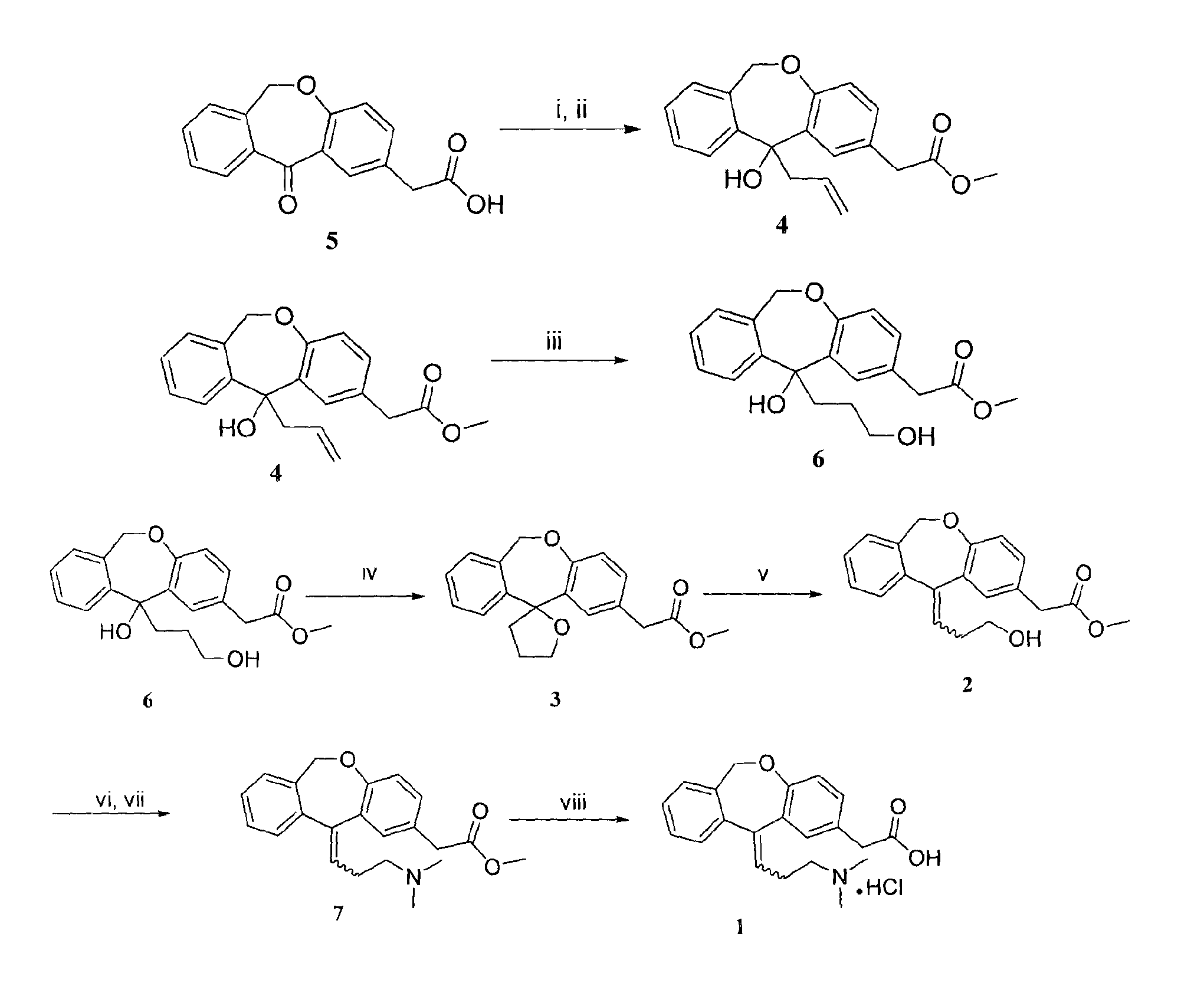
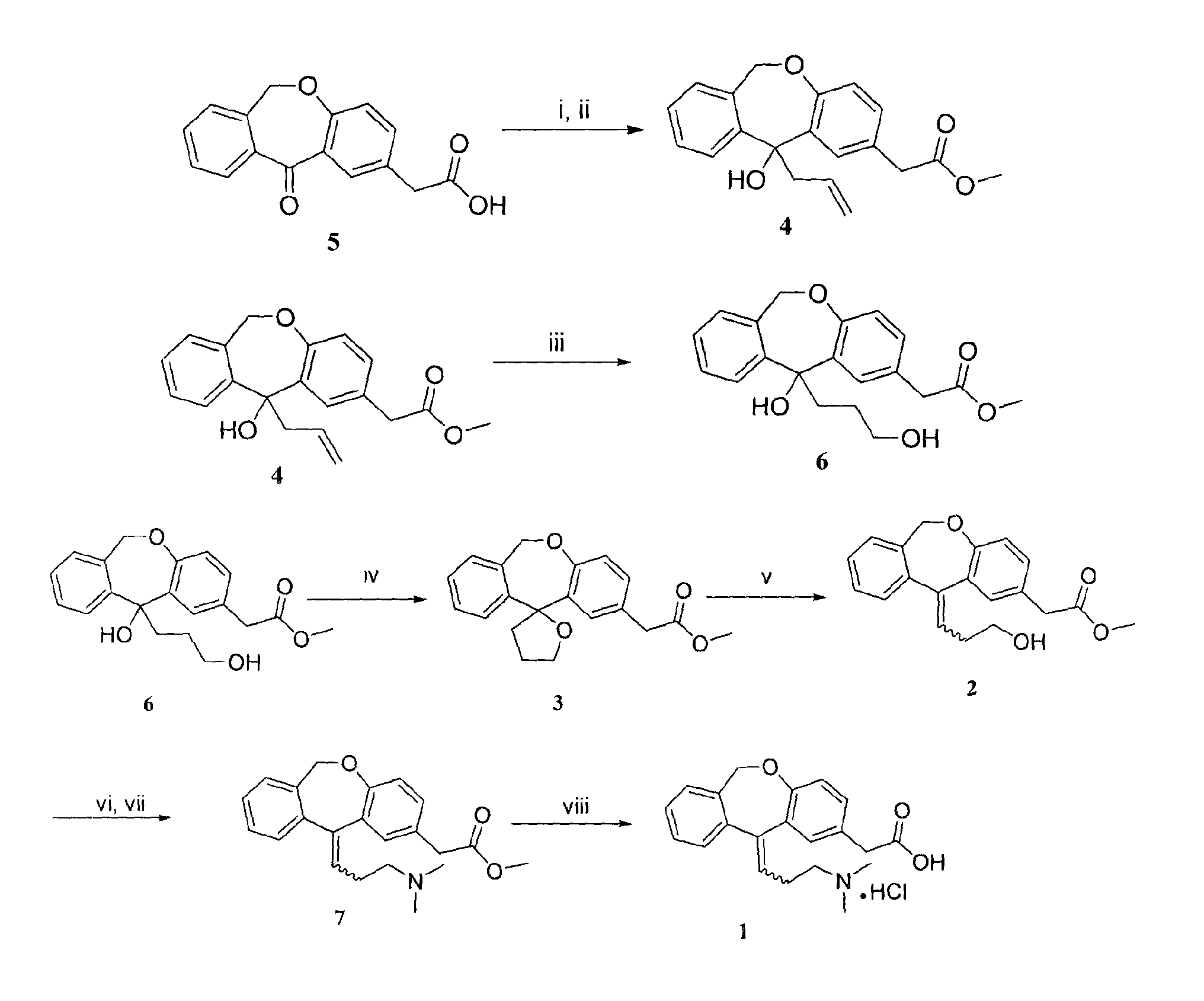
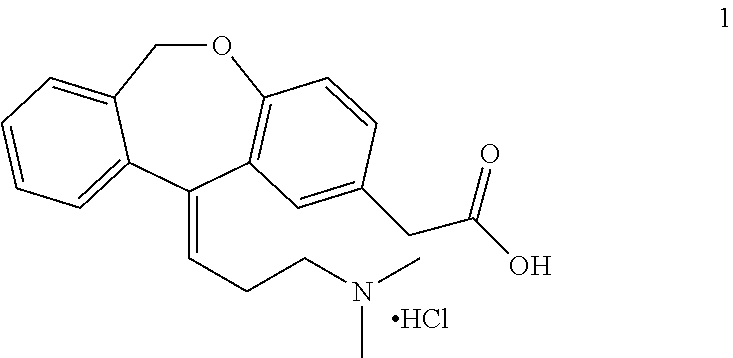
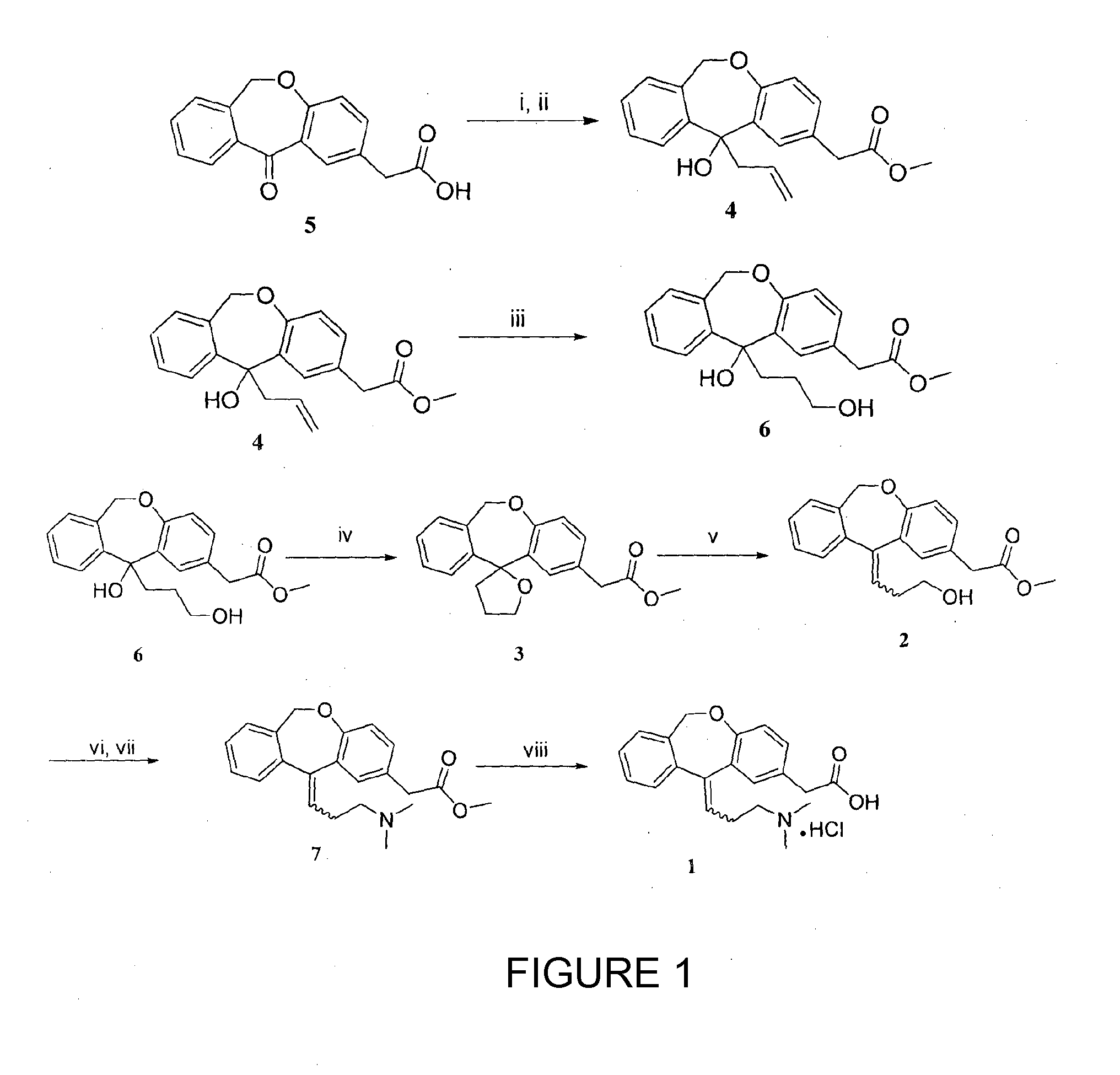
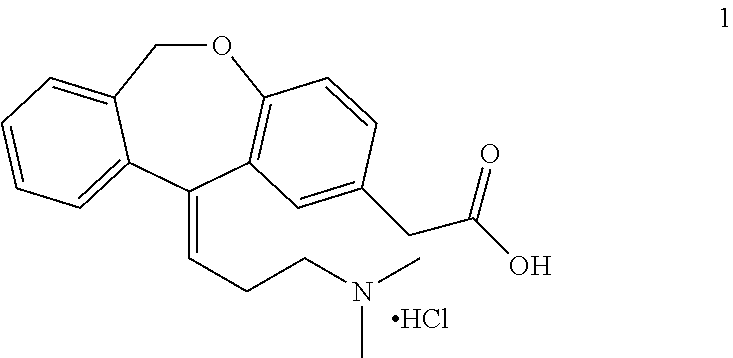
Process for obtaining olopatadine and intermediates
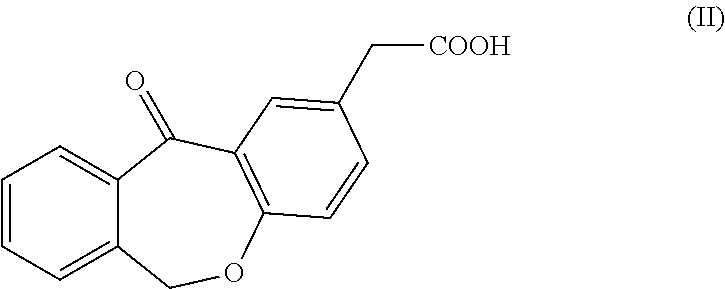
ActiveUS20120004426A1High purityHigh yieldSenses disorderOrganic chemistryAcetic acidOrganic solvent
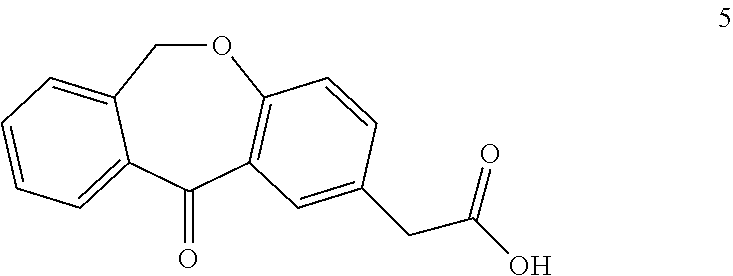
Olopatadine can be obtained by means of a process comprising hydrolysis of a compound of general formula (II), wherein Y is OR1, wherein R1 is C1-C7 alkyl, C3-C7 cycloalkyl, aryl, arylalkyl, or heterocycle; or NR2R3, wherein R2 and R3, independently from each other, are C1-C7 alkyl, aryl, arylalkyl, or R2 and R3 together with the nitrogen atom to which they are bound form a heterocycle of 3 to 7 members, obtained by means of a process comprising reacting the corresponding ester or amide of 6,11-dihydro-11-oxodibenz[b,e]oxepin-2-acetic acid with a suitable Wittig reagent, in the presence of a base in a reaction medium comprising an organic solvent.
Owner:CRYSTAL PHARMA SA
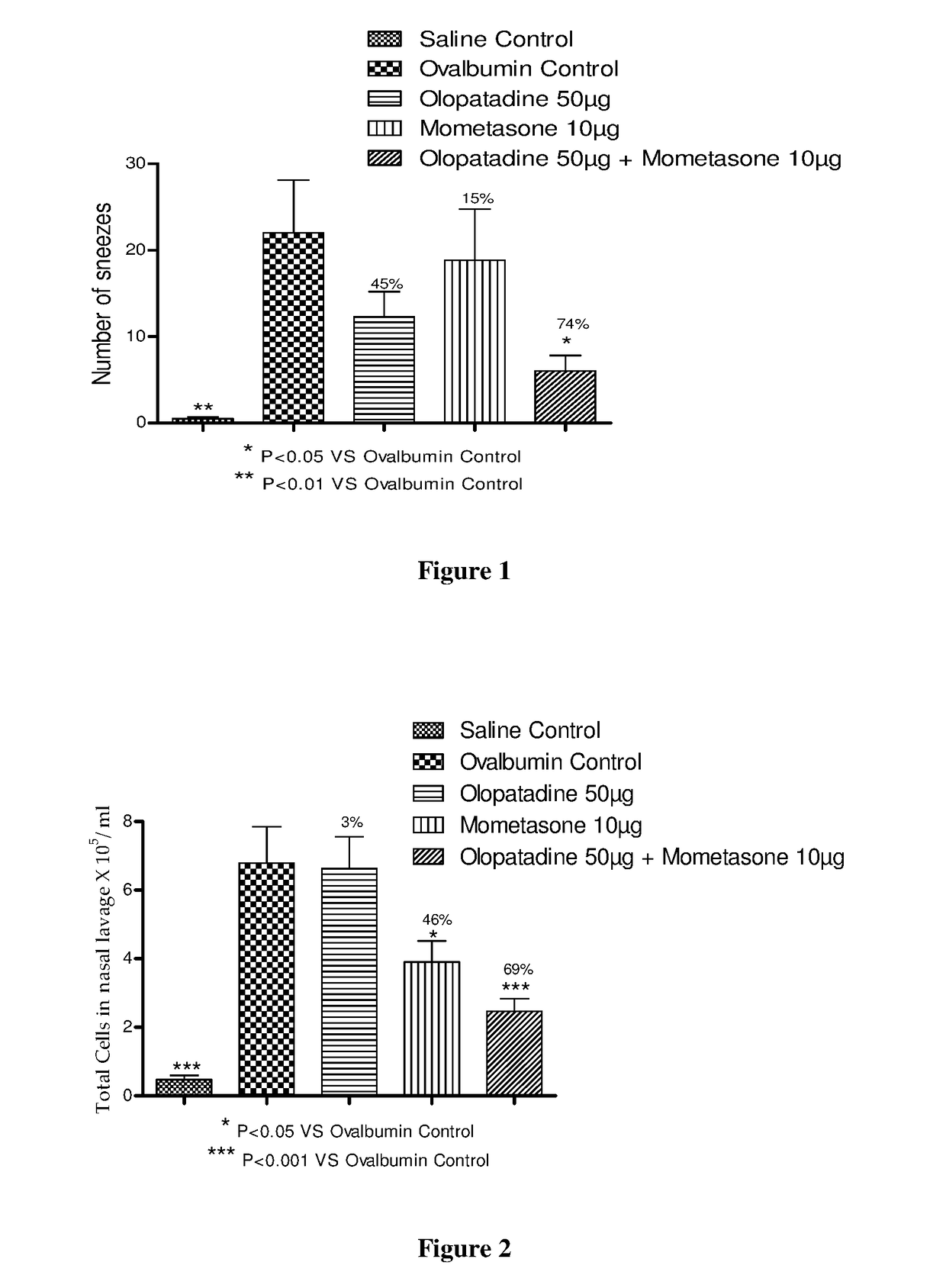
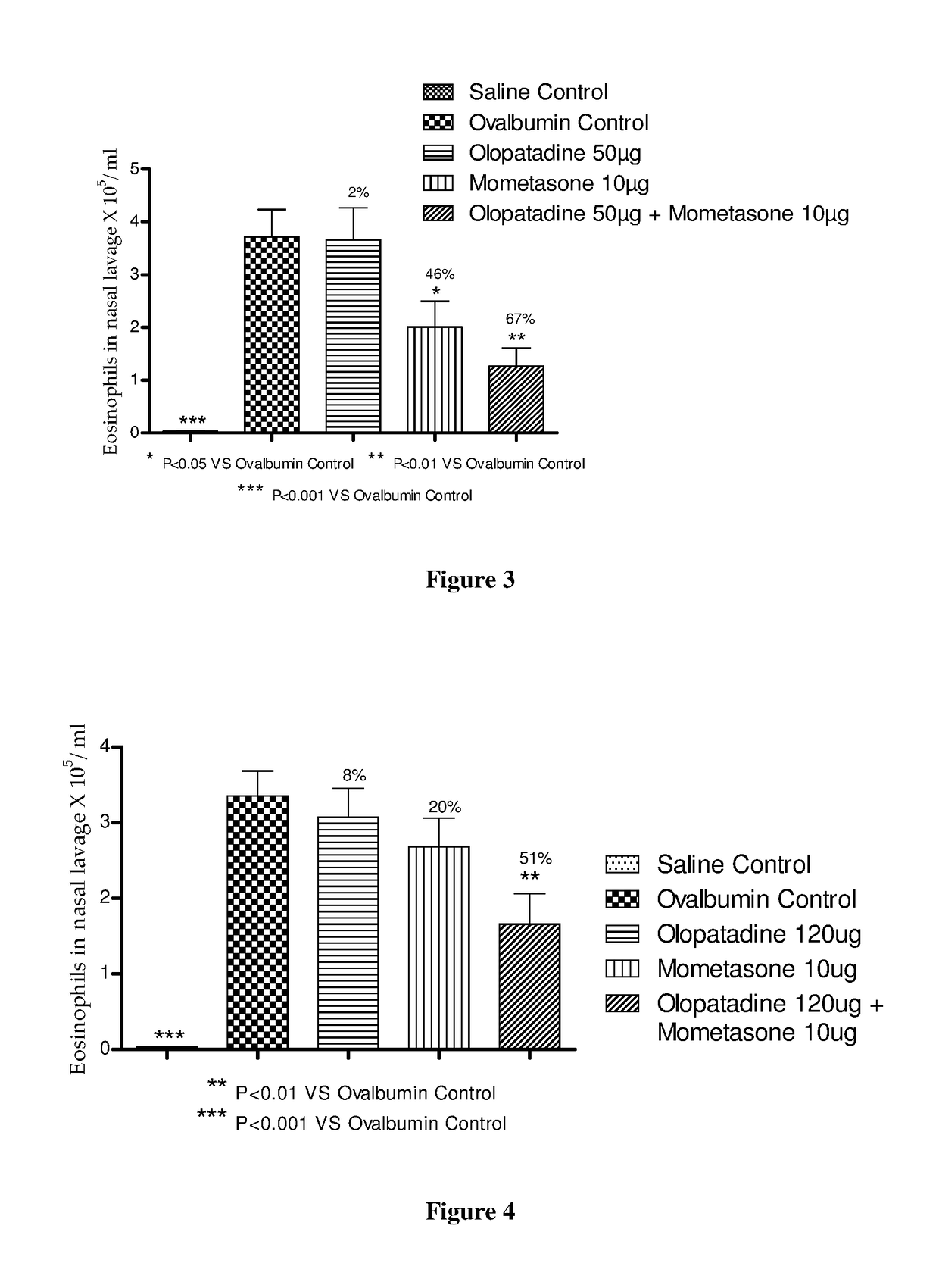
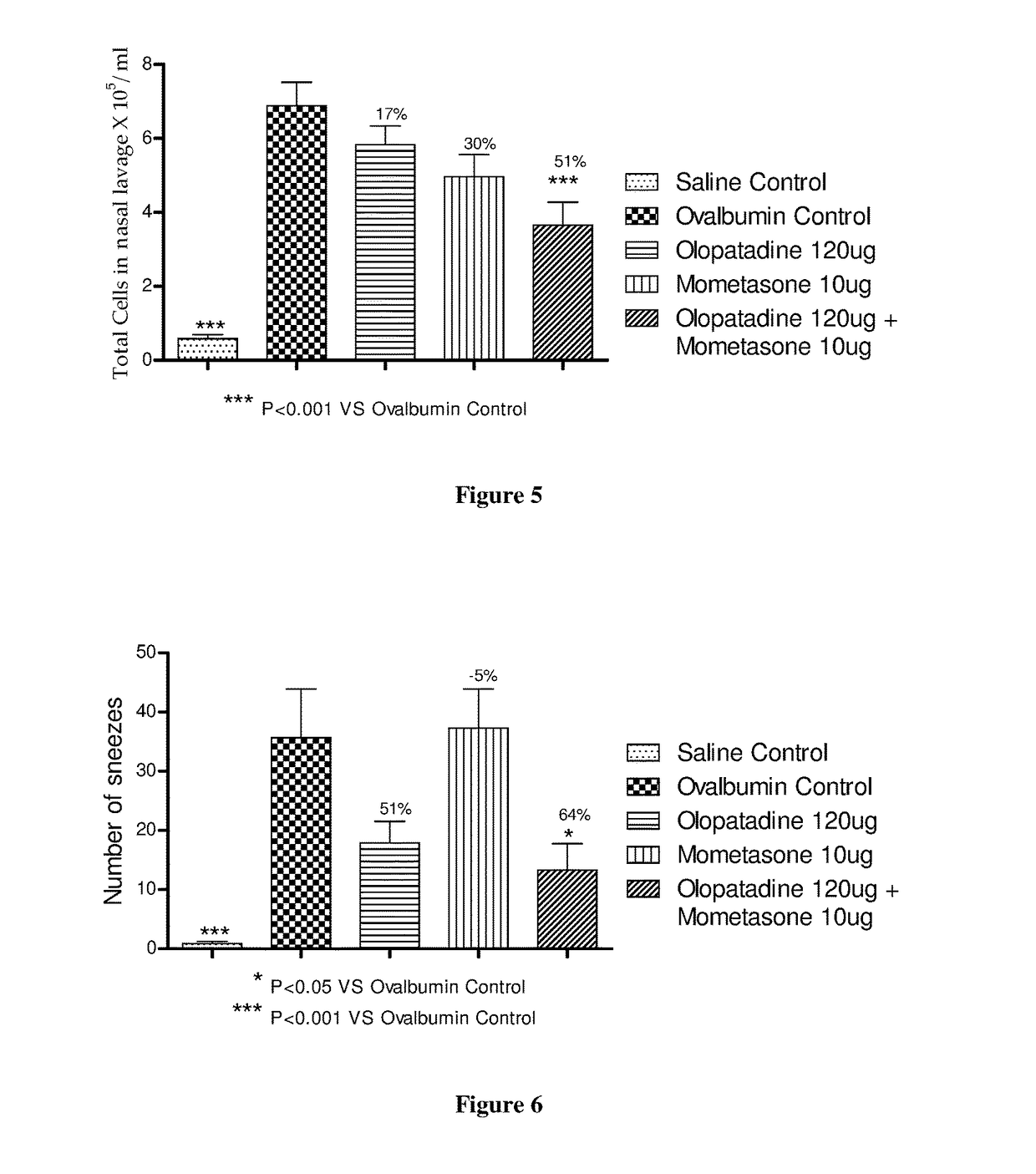
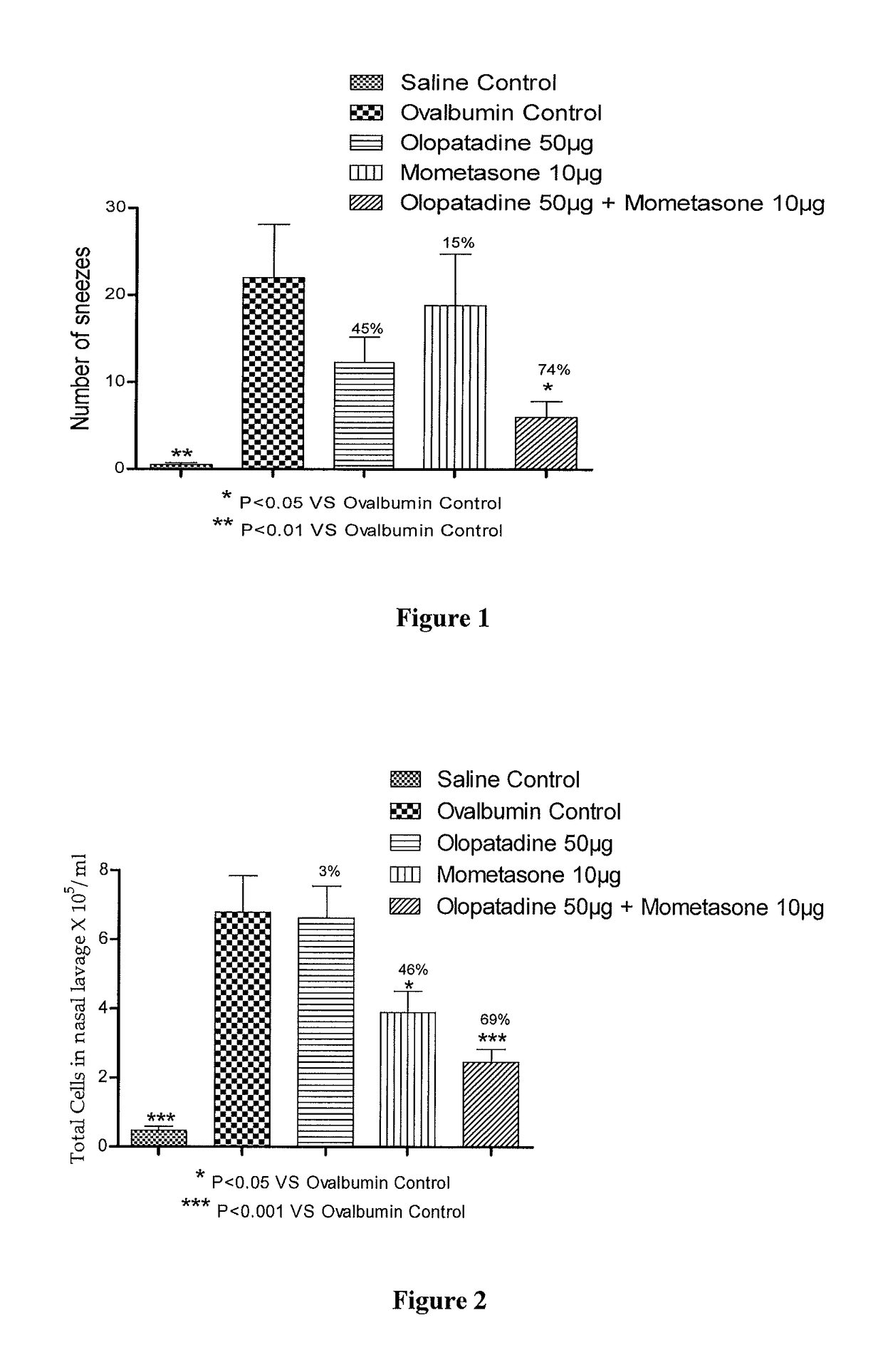
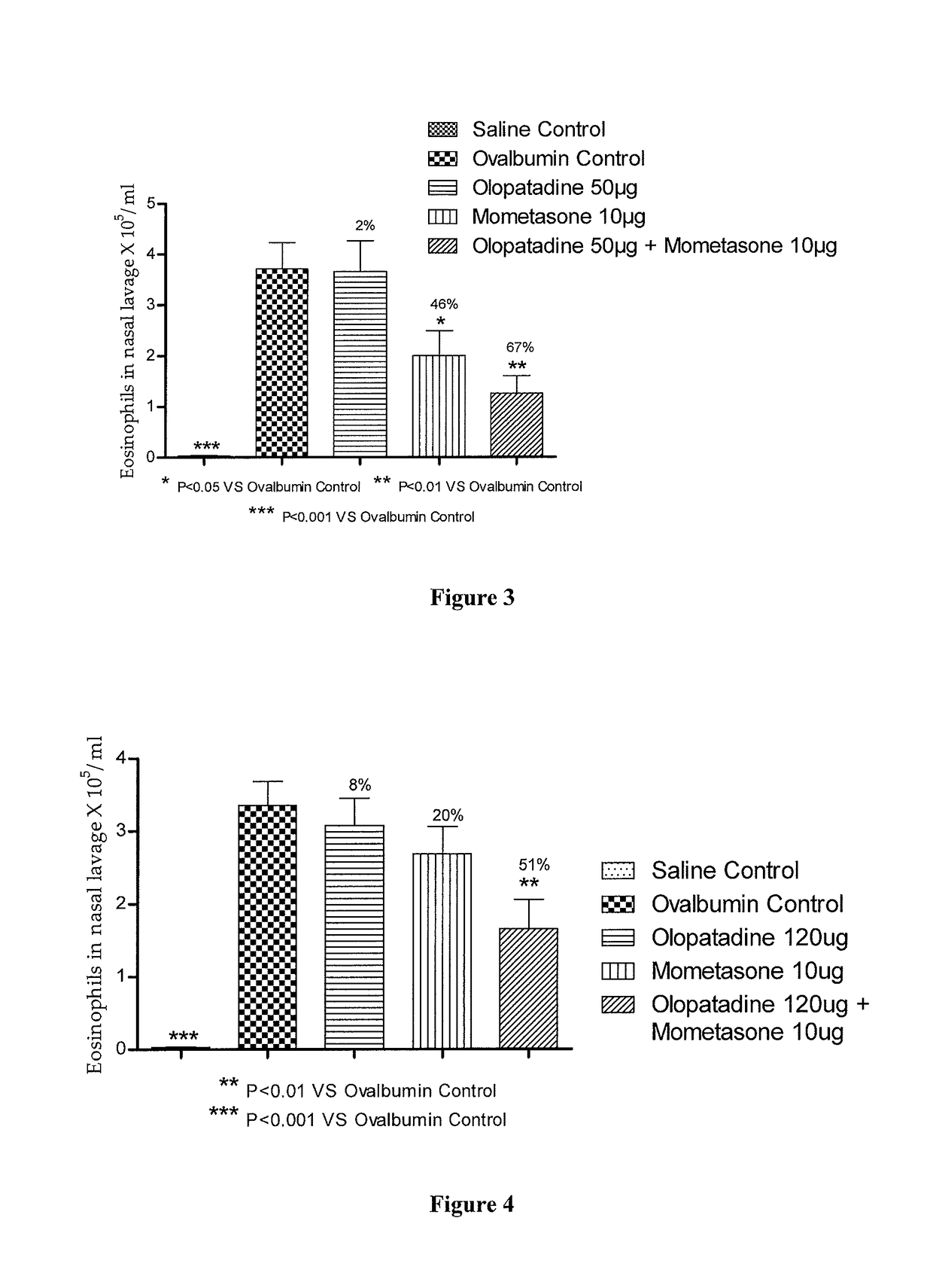
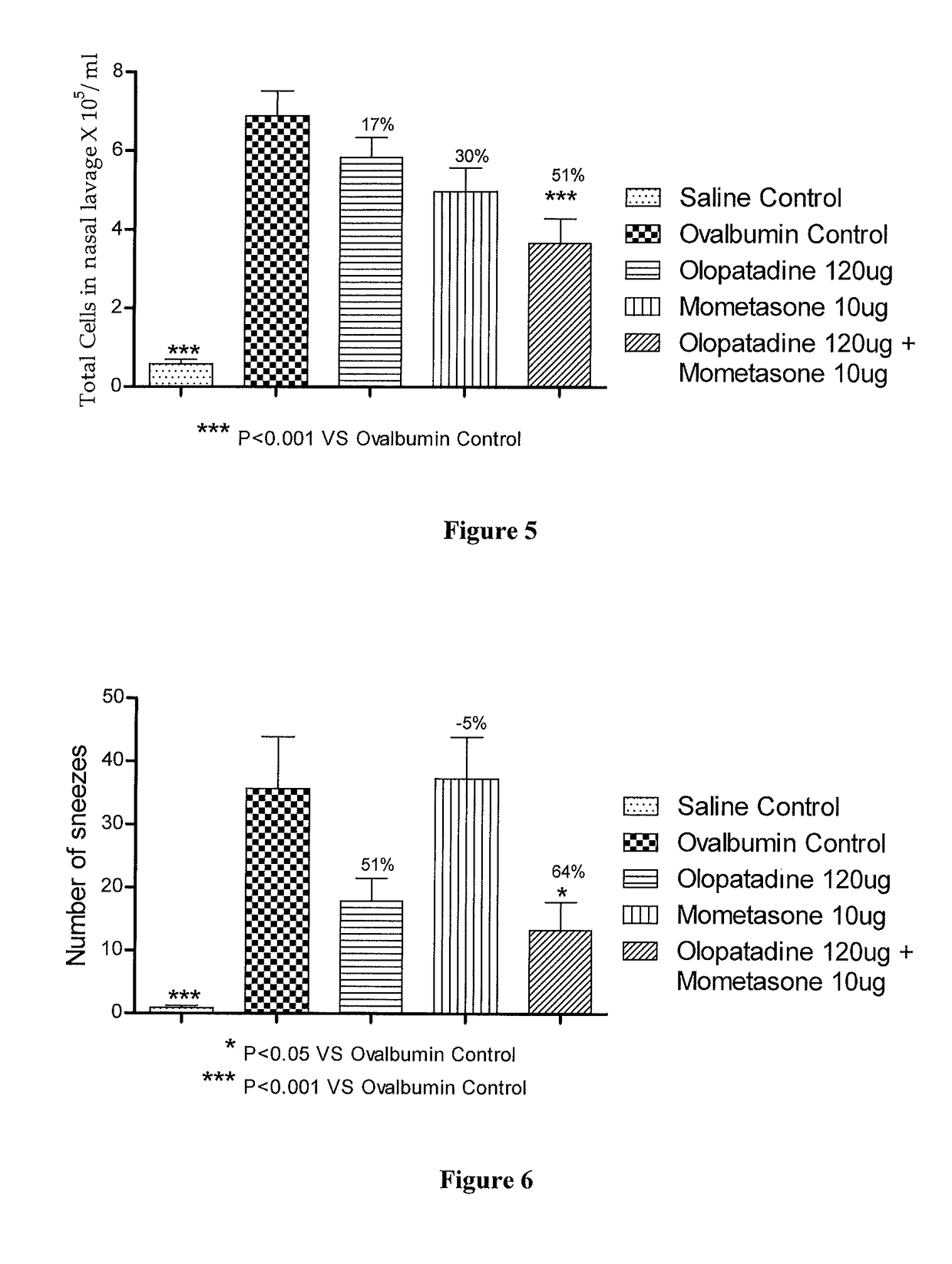
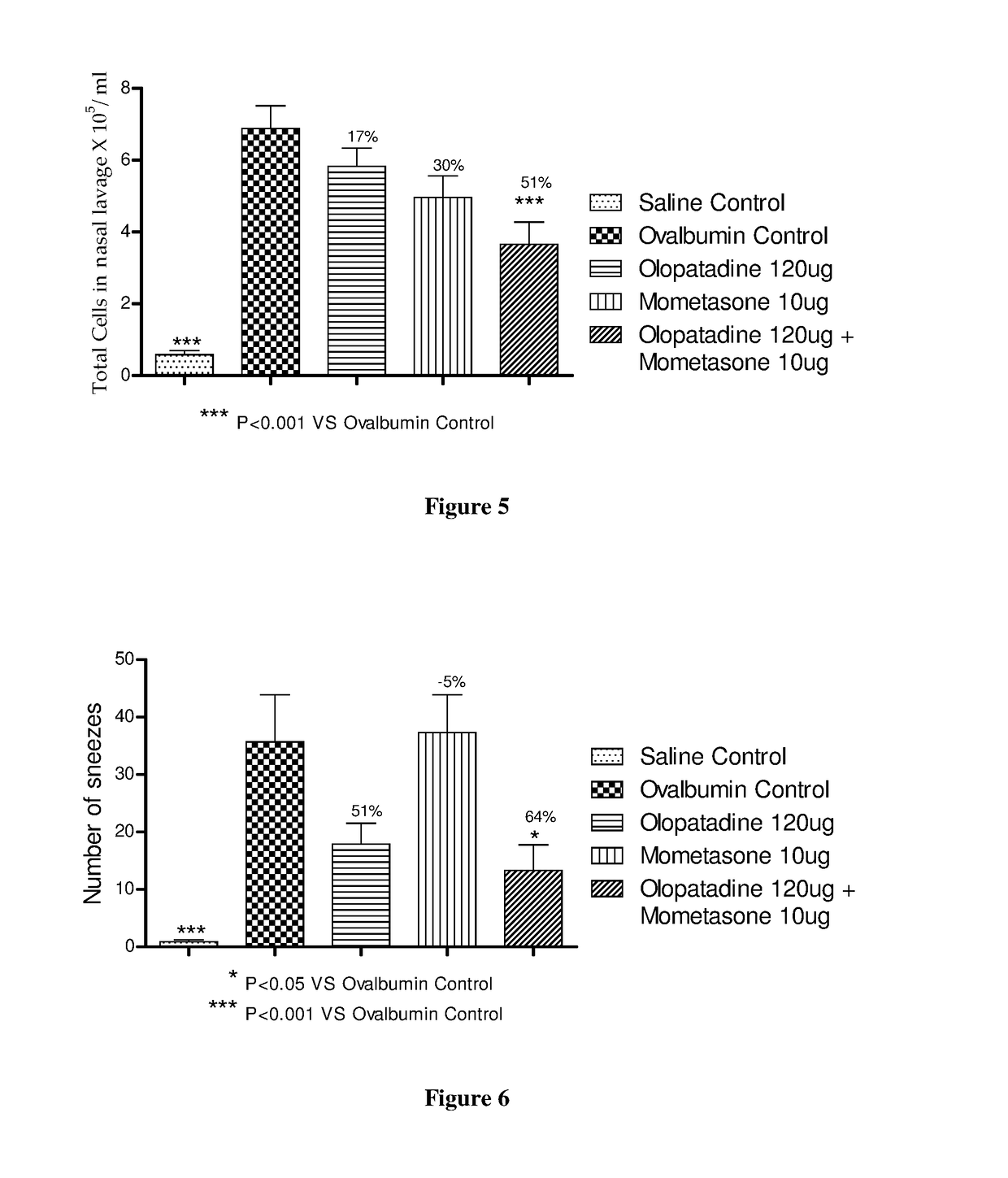
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS20190030047A1Better therapeutic valueIncrease valueOrganic active ingredientsSenses disorderMometasoneOlopatadine
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a pediatric human subject) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
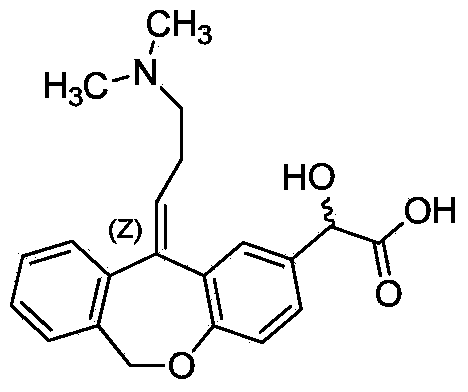
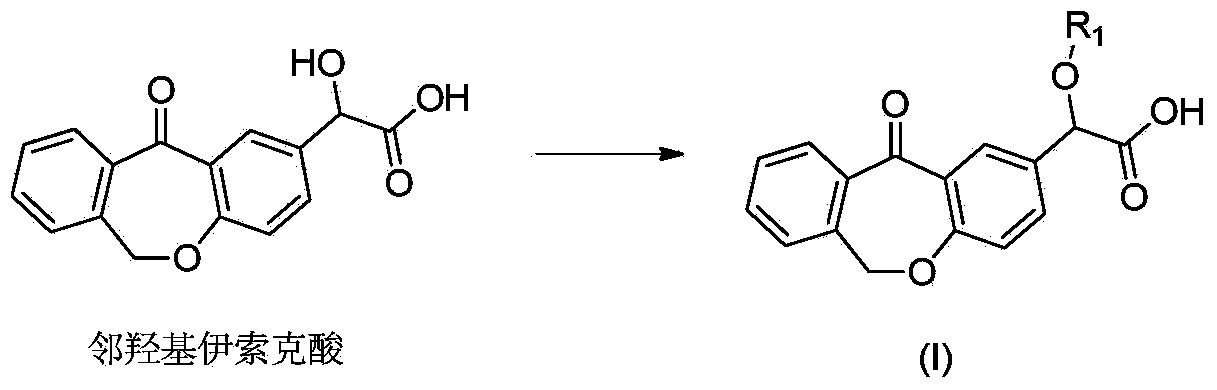
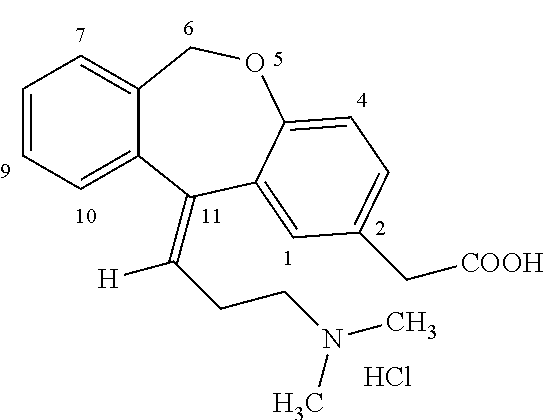
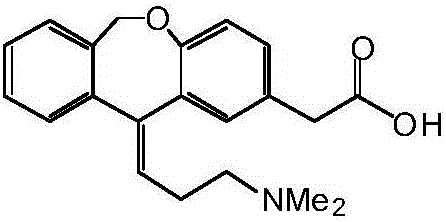
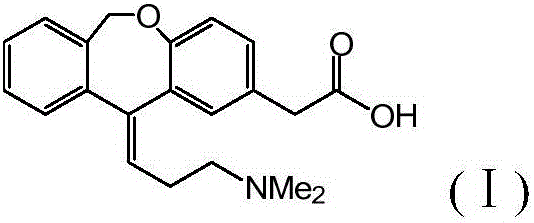
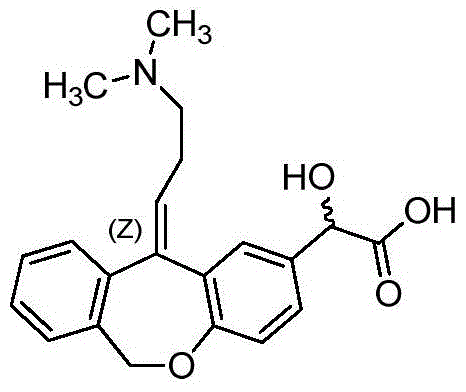
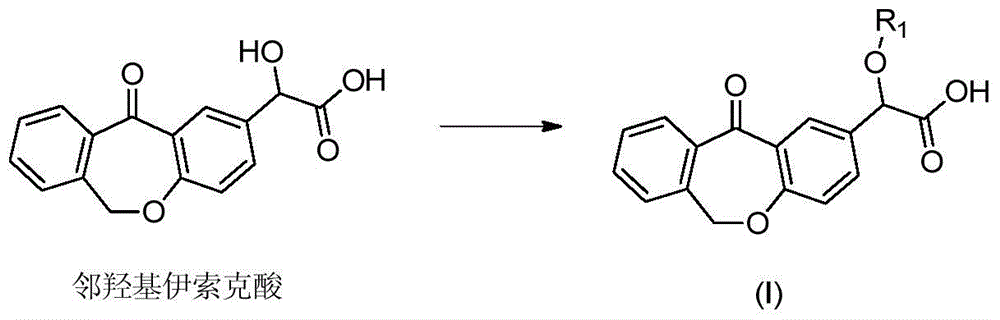
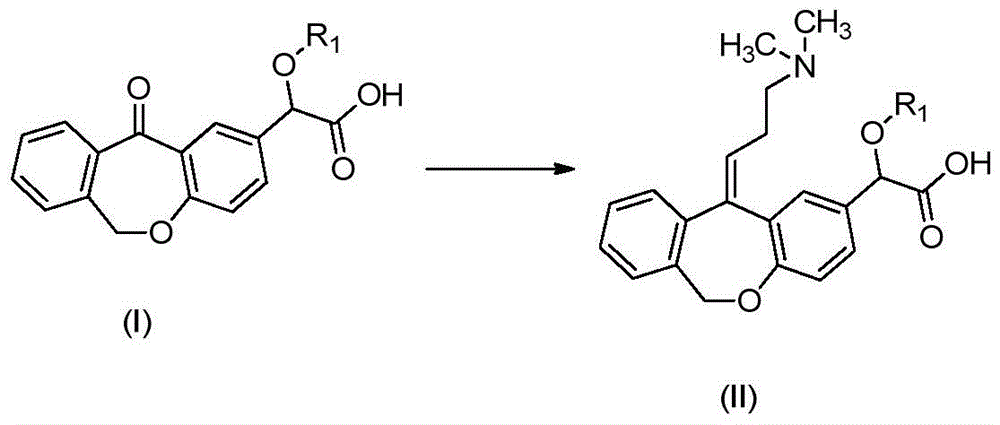
Preparation method of o-hydroxyl Olopatadine
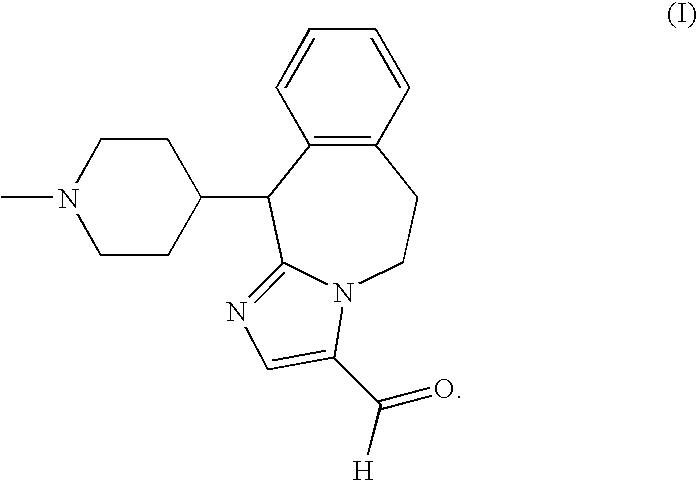
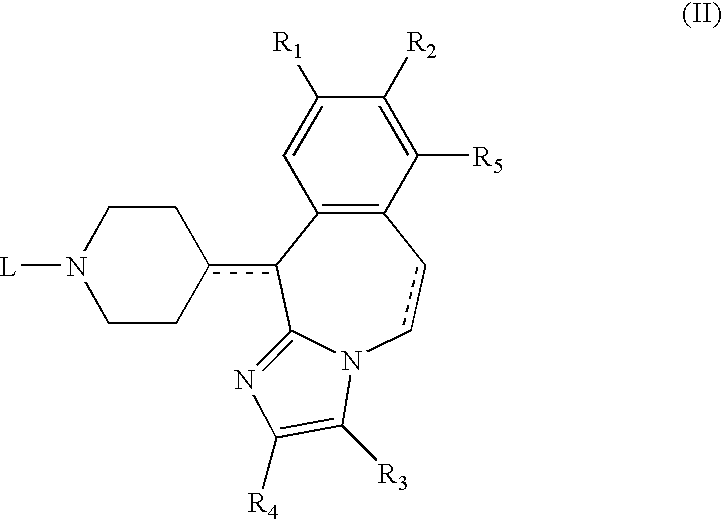
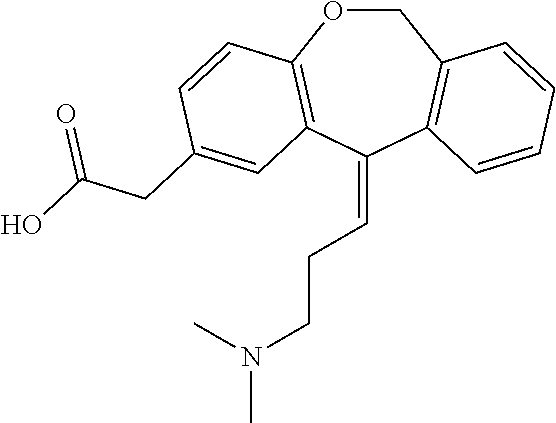
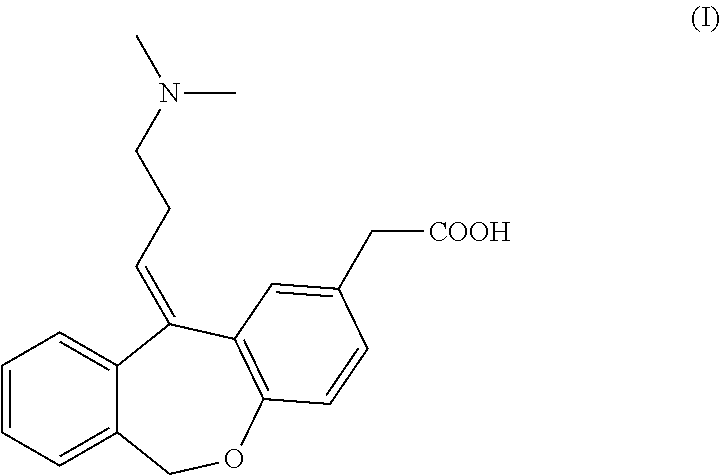
The invention discloses a preparation method of o-hydroxyl Olopatadine. The preparation method comprises the following steps: (1) performing a hydroxyl protection reaction on o-hydroxyl Isoxepac to obtain a compound shown in the general formula (I); (2) performing a Wittig reaction between the compound shown in the general formula (I) and [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide to obtain a compound shown in the general formula (II); and (3) performing a protecting group removal reaction on the compound shown in the general formula (II) to obtain the o-hydroxyl Olopatadine, wherein the definition of R1 is specified in the specification. Moreover, the invention also discloses an intermediate compound for preparing o-hydroxyl Olopatadine.
Owner:BEIJING JIALIN PHARM INC
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
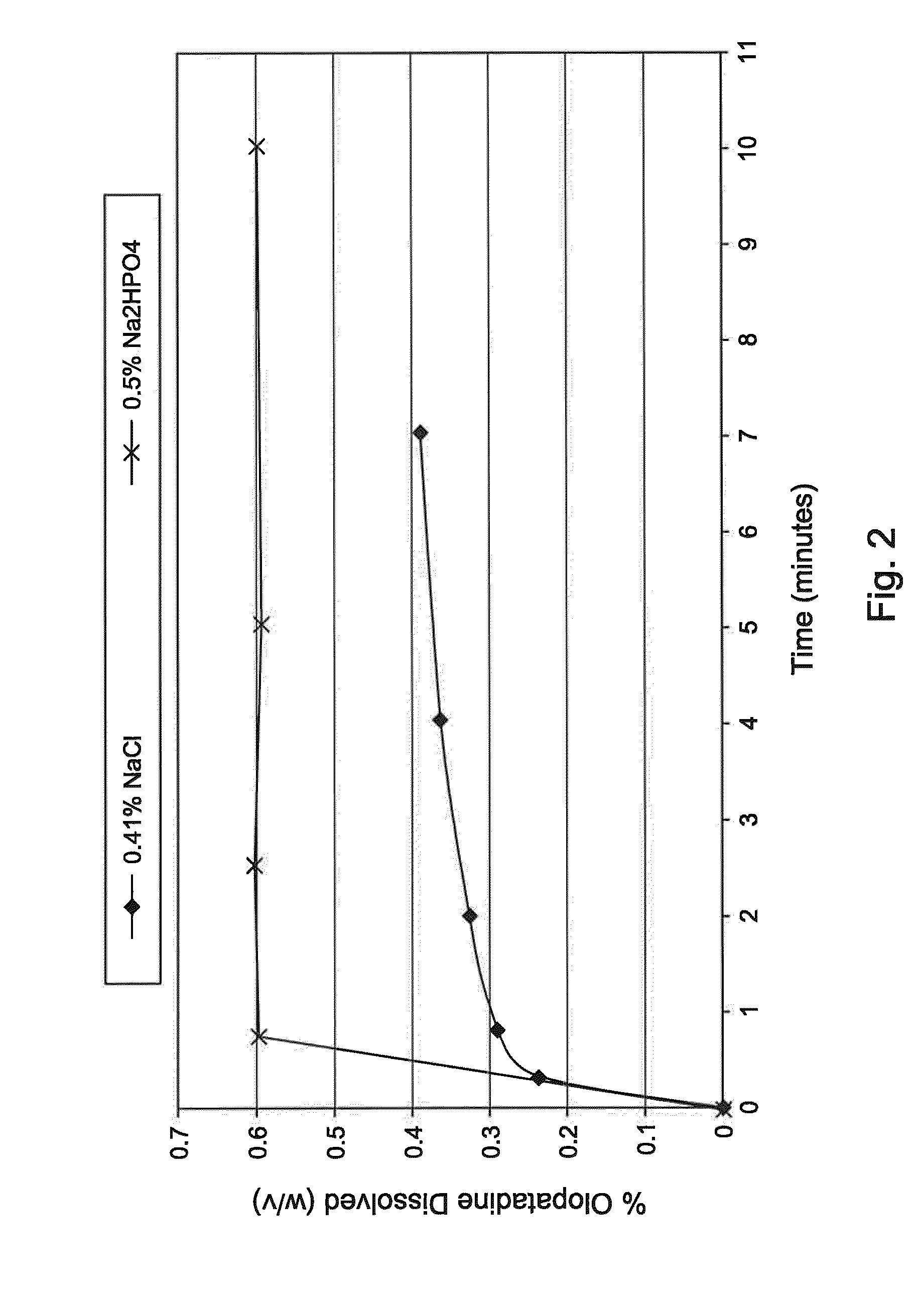
Process of preparing aqueous ophthalmic solution of olopatadine
InactiveUS20160338951A1Improve solubilityOrganic active ingredientsInorganic non-active ingredientsHigh concentrationMedicine
An aqueous ophthalmic solution containing relatively high concentration of olopatadine in solubilized form and process for making such solution is provided. The process for preparing the aqueous ophthalmic solution comprises a step of sizing olopatadine particles, preferably by using a microfluidizer, a ball mill or a colloidal mill. The solution is useful for providing enhanced relief from symptoms of ocular allergic disorders (e.g. conjunctivitis).
Owner:SOMERSET THERAPEUTICS LLC
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS10016443B2Increase valueAct quicklyOrganic active ingredientsPharmaceutical delivery mechanismMometasoneOlopatadine
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a human) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
Process for obtaining olopatadine and intermediates
Owner:CRYSTAL PHARMA SA
Percutaneously absorptive ophthalmic preparation comprising olopatadine
The present invention provides a percutaneously absorptive preparation for preventing or treating allergic eye disease, which comprises olopatadine or a salt thereof as an active ingredient. In addition, the present invention provides a method for preventing or treating allergic eye disease, which comprises applying a percutaneously absorptive preparation comprising olopatadine or a salt thereof to the skin surface including the skin surface of an eyelid, thereby casing transfer of a therapeutically effective amount of olopatadine or a salt thereof from the preparation to an anterior ocular segment through the skin of the eyelid rather than a systemic blood flow. The present preparation can exert a pharmacological effect over a prolonged period by a single application, as compared to conventional preparations such as eye drops.
Owner:SENJU PHARMA CO LTD
Preparation method of olopatadine
The invention provides an industrial preparation method of olopatadine. The method can accelerate the reaction speed and lower the environmental sensitivity, the process controllability is improved, the production cost is lowered, and safety is improved.
Owner:北京京卫燕康药物研究所有限公司
Combination of a non-steroidal Anti-inflammatory drug with an Anti-histaminic drug intended for ophthalmic use
InactiveUS20130281506A1Treating and preventing ocular surface allergyPreventing signBiocidePharmaceutical delivery mechanismDiseaseKetorolac
The present invention is directed to a stable formulation of a combination of ketorolac (non-steroidal anti-inflammatory drug) with olopatadine (anti-histaminic drug) intended for ophthalmic use. This pharmaceutical composition is used for treatment of ophthalmic diseases and conditions, particularly seasonal ocular surface allergy.
Owner:ALLERGAN INC
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS20180369187A1Better therapeutic valueIncrease valueOrganic active ingredientsSenses disorderMometasoneOlopatadine
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a human) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
Aqueous ophthalmic solution of olopatadine
ActiveUS9707174B2Organic active ingredientsInorganic non-active ingredientsHigh concentrationCyclodextrin
An aqueous ophthalmic solution containing a relatively high concentration of olopatadine in solubilized form is provided. The solution comprises a combination of at least two non-ionic surfactants and is essentially devoid of cyclodextrins. The solution is useful for providing enhanced relief from symptoms of ocular allergic disorders (e.g. conjunctivitis).
Owner:SOMERSET THERAPEUTICS LLC
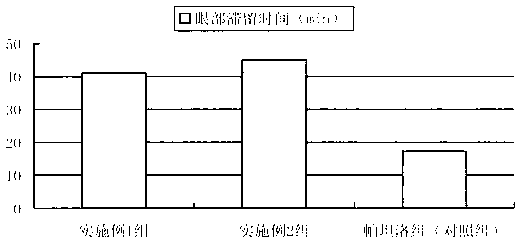
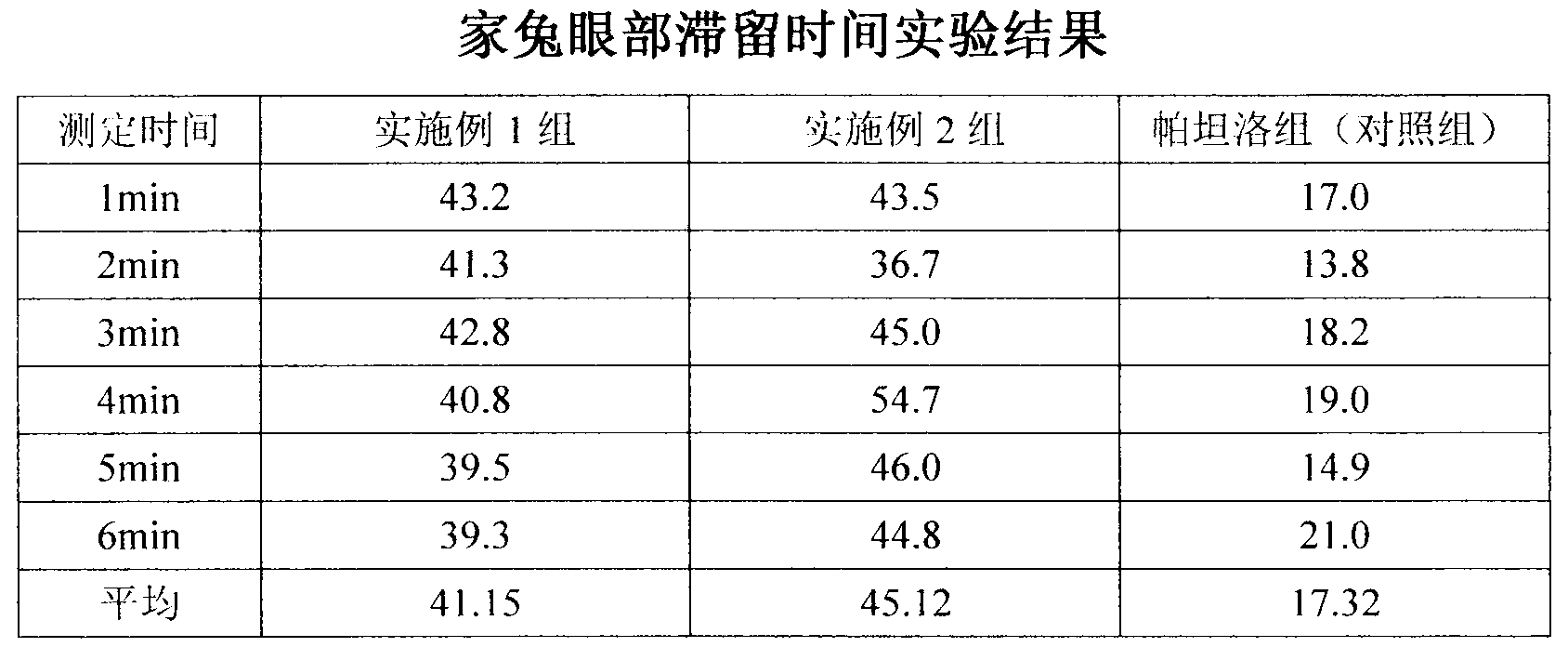
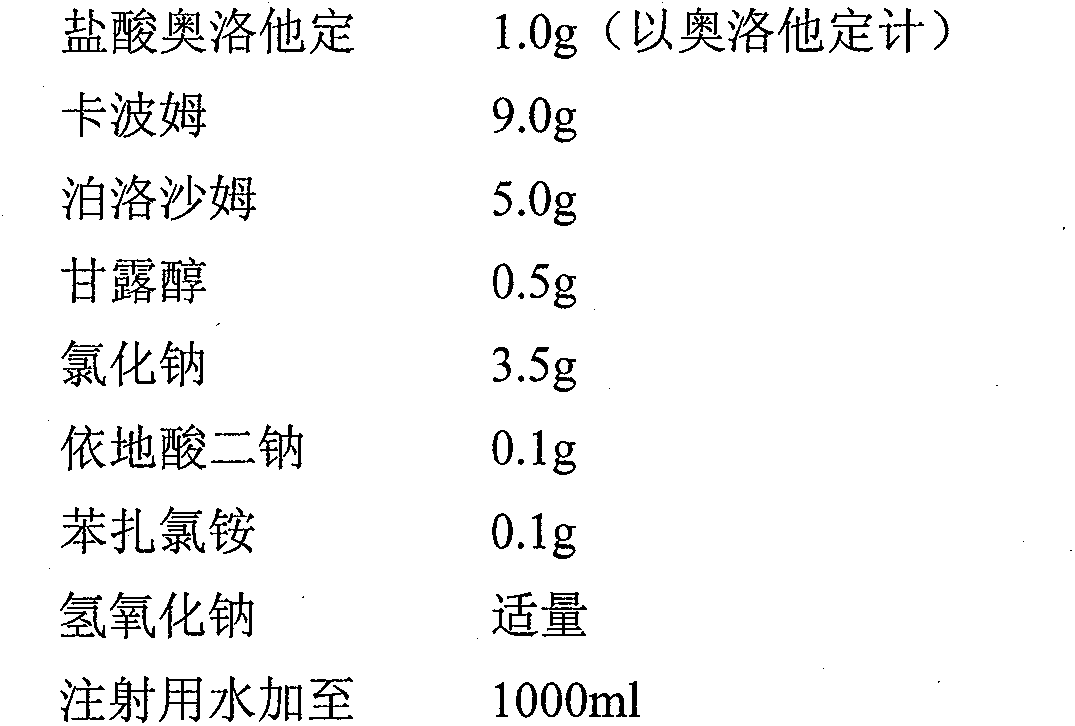
Pharmaceutical composition of olopatadine or salts of olopatadine, and preparation method thereof
InactiveCN103202833AExtended stayImprove retentionOrganic active ingredientsSenses disorderOlopatadineChemistry
The invention discloses a pharmaceutical composition of olopatadine or salts of olopatadine for treating allergic conjunctivitis, and a preparation method thereof. The composition mainly comprises the olopatadine or the salts of the olopatadine, and polycarbophil or polycarbophil salt or carbomer. The preparation method comprises firstly fully swelling the polycarbophil or the polycarbophil salt or the carbomer with a proper amount of pure water, and secondly adding the olopatadine or the salts of the olopatadine and other accessories.
Owner:JIANGSU YABANG AIPUSEN PHARMA
Process for the synthesis of olopatadine
The present invention provides a process for the synthesis of olopatadine. Further, the invention discloses a process that results in improved yield of the desired Z isomer.
Owner:COUNCIL OF SCI & IND RES
A kind of preparation method of o-hydroxy olopatadine
The invention discloses a preparation method of o-hydroxyl Olopatadine. The preparation method comprises the following steps: (1) performing a hydroxyl protection reaction on o-hydroxyl Isoxepac to obtain a compound shown in the general formula (I); (2) performing a Wittig reaction between the compound shown in the general formula (I) and [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide to obtain a compound shown in the general formula (II); and (3) performing a protecting group removal reaction on the compound shown in the general formula (II) to obtain the o-hydroxyl Olopatadine, wherein the definition of R1 is specified in the specification. Moreover, the invention also discloses an intermediate compound for preparing o-hydroxyl Olopatadine.
Owner:BEIJING JIALIN PHARM INC
A process for the synthesis of olopatadine
The present invention provides a process for the synthesis of olopatadine. Further, the invention discloses a process that results in improved yield of the desired Z isomer.
Owner:COUNCIL OF SCI & IND RES
Aqueous ophthalmic solution of olopatadine
ActiveUS20170105930A1Organic active ingredientsInorganic non-active ingredientsHigh concentrationCyclodextrin
An aqueous ophthalmic solution containing a relatively high concentration of olopatadine in solubilized form is provided. The solution comprises a combination of at least two non-ionic surfactants and is essentially devoid of cyclodextrins. The solution is useful for providing enhanced relief from symptoms of ocular allergic disorders (e.g. conjunctivitis).
Owner:SOMERSET THERAPEUTICS LLC
Formulation of olopatadine
InactiveUS20160271097A1Easy to manufactureAvoid the needOrganic active ingredientsSenses disorderMedicineOlopatadine
Stable formulations of Olopatadine, methods of making such formulations and methods of treatment using such formulations are provided.
Owner:NEPHRON PHARM CORP
Process for the preparation of olopatadine and sylil intermediates thereof
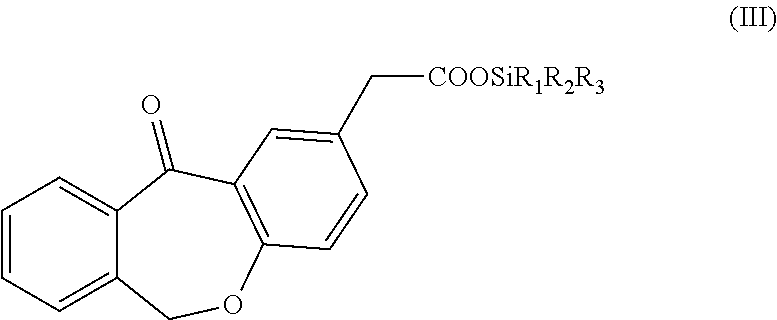
ActiveUS9708284B2Industrially and economically convenientGroup 4/14 element organic compoundsPhotochemistryOlopatadine
The present invention refers to a new “one-pot” process for the preparation of olopatadine via intermediates of formula (III).
Owner:LAB CHIM INTERNAZ
Olopatadine composition and preparation method thereof
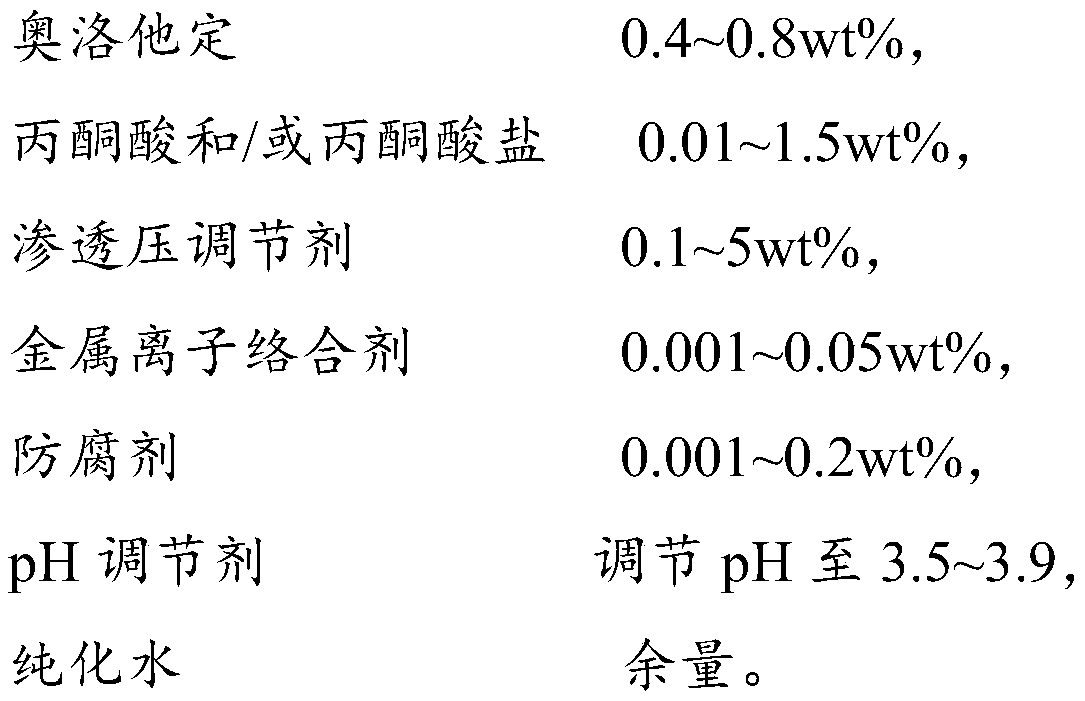
ActiveCN109806224APrevent spoilageGood function and effectOrganic active ingredientsPharmaceutical delivery mechanismNasal cavityMedicine
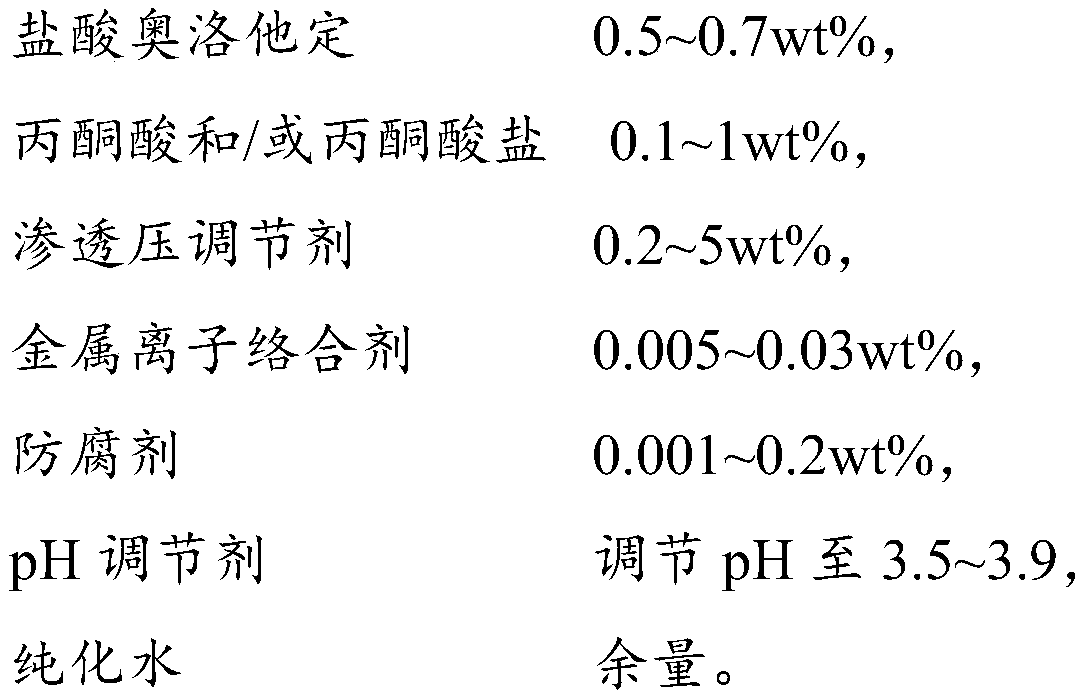
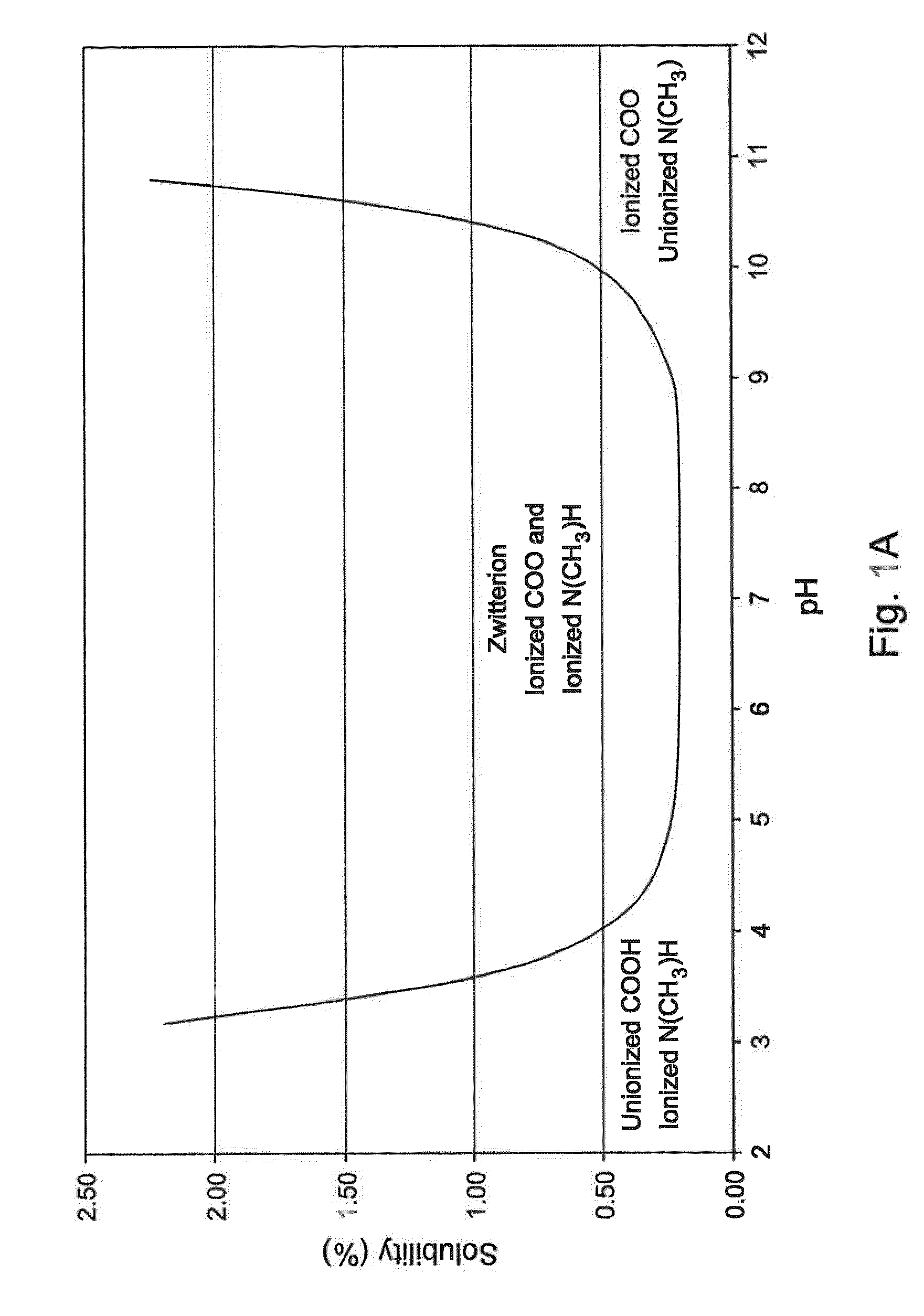
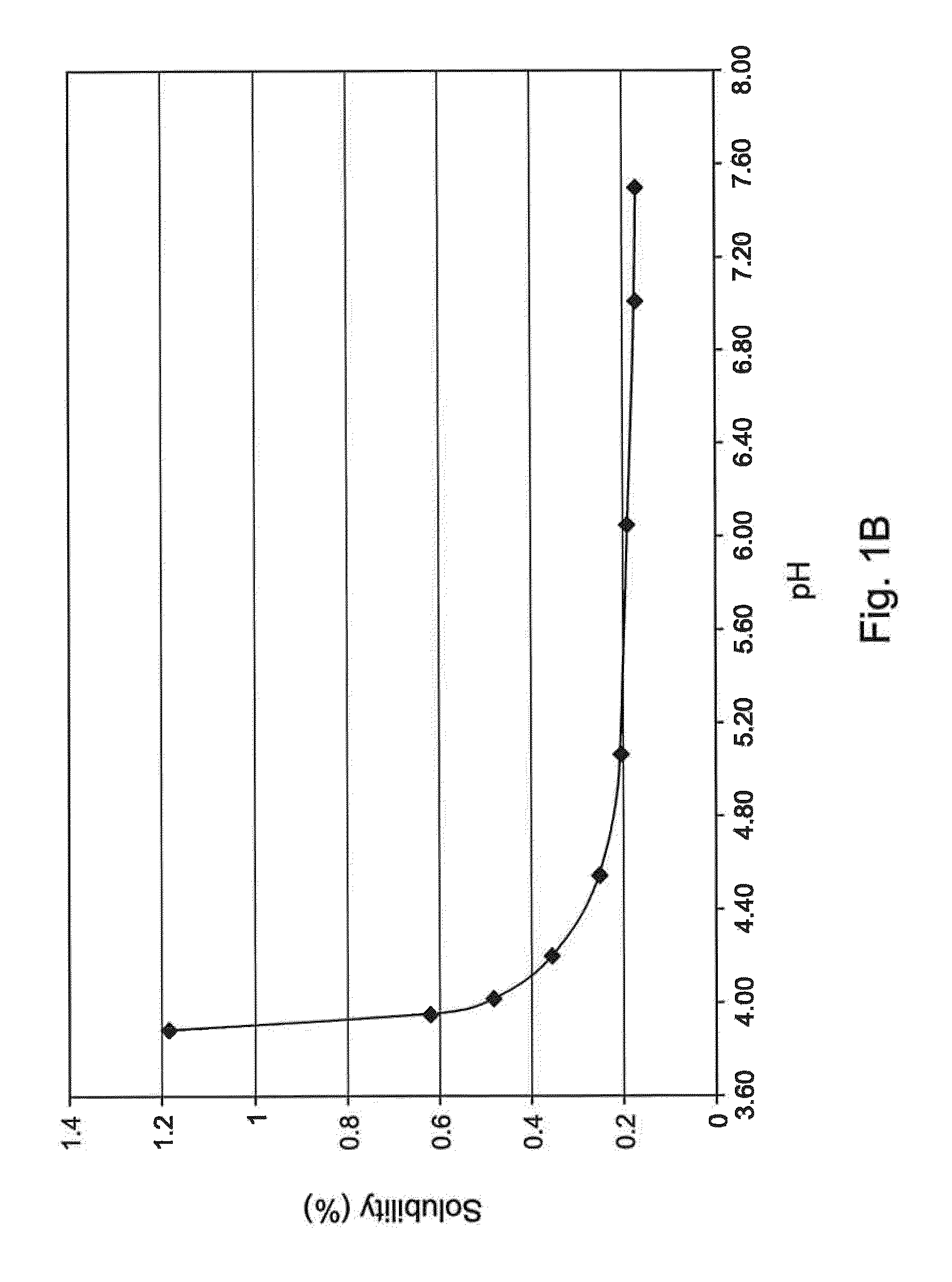
The invention provides an olopatadine composition. The composition consists of olopatadine, pyruvic acid and / or pyruvate, osmotic pressure regulator, metal ion complexing agent, preservative, pH regulator and purified water. The composition disclosed by the invention can increase the stability of olopatadine, decrease irritation to nasal mucous membranes, accelerate the recovery of nasal mucous membranes, alleviate the uncomfortable symptoms of the nose and increase the safety of olopatadine in use.
Owner:JIANG SU PHARMAMAXCORP
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
Owner:GLENMARK SPECIALTY
Olopatadine formulations for topical nasal administration
InactiveUS20110306659A1BiocideInorganic phosphorous active ingredientsMedicinal chemistryInflammatory disorder
Topical formulations of olopatadine for treatment of allergic or inflammatory disorders of the nose are disclosed. The aqueous formulations contain approximately 0.6% (w / v) of olopatadine.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com