Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
613 results about "Dihydropyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dihydropyridine (DHP) is a molecule based upon pyridine, and the parent of a class of molecules that have been semi-saturated with two substituents replacing one double bond. They are particularly well known in pharmacology as L-type calcium channel blockers, used in the treatment of hypertension. Compared with certain other L-type calcium channel blockers (for example those of the phenylalkylamine class such as verapamil) that have significant action at the heart, they are relatively vascular selective in their mechanism of action in lowering blood pressure.
Use of connective tissue mast cell stabilizers to facilitate ocular surface re-epithelization and wound repair
InactiveUS20080139531A1Inhibition releaseOvercomes drawbackBiocideOrganic chemistryConjunctival woundDihydropyridine
Disclosed are methods of treating a wound in a subject that involve administering to the subject a pharmaceutically effective amount of a composition that includes one or more human connective tissue mast cell stabilizers, wherein administration of the composition results in treatment of the wound. In particular embodiments, the wound is an ophthalmic or dermal wound, such as a corneal epithelial defect, a conjunctival wound, or dermal abrasion. Administration, for example, may be by topical application of the composition to the ocular surface or skin. Exemplary mast cell stabilizers include olopatadine, variants of olopatadine, alcaftidine, derivatives of alcaftidine, dihydropyridines, and spleen tyrosine kinase inhibitors.
Owner:ALCON RES LTD
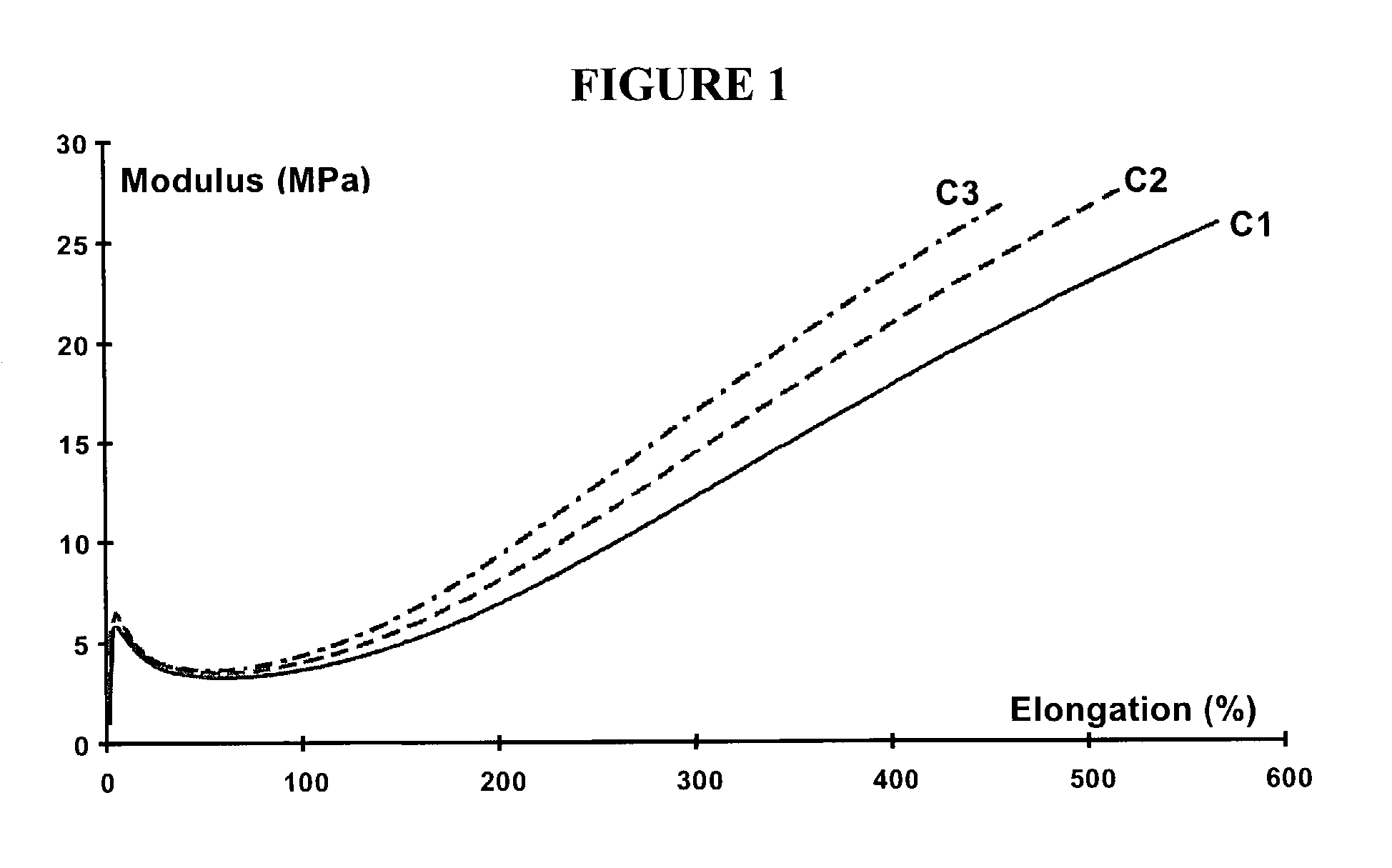
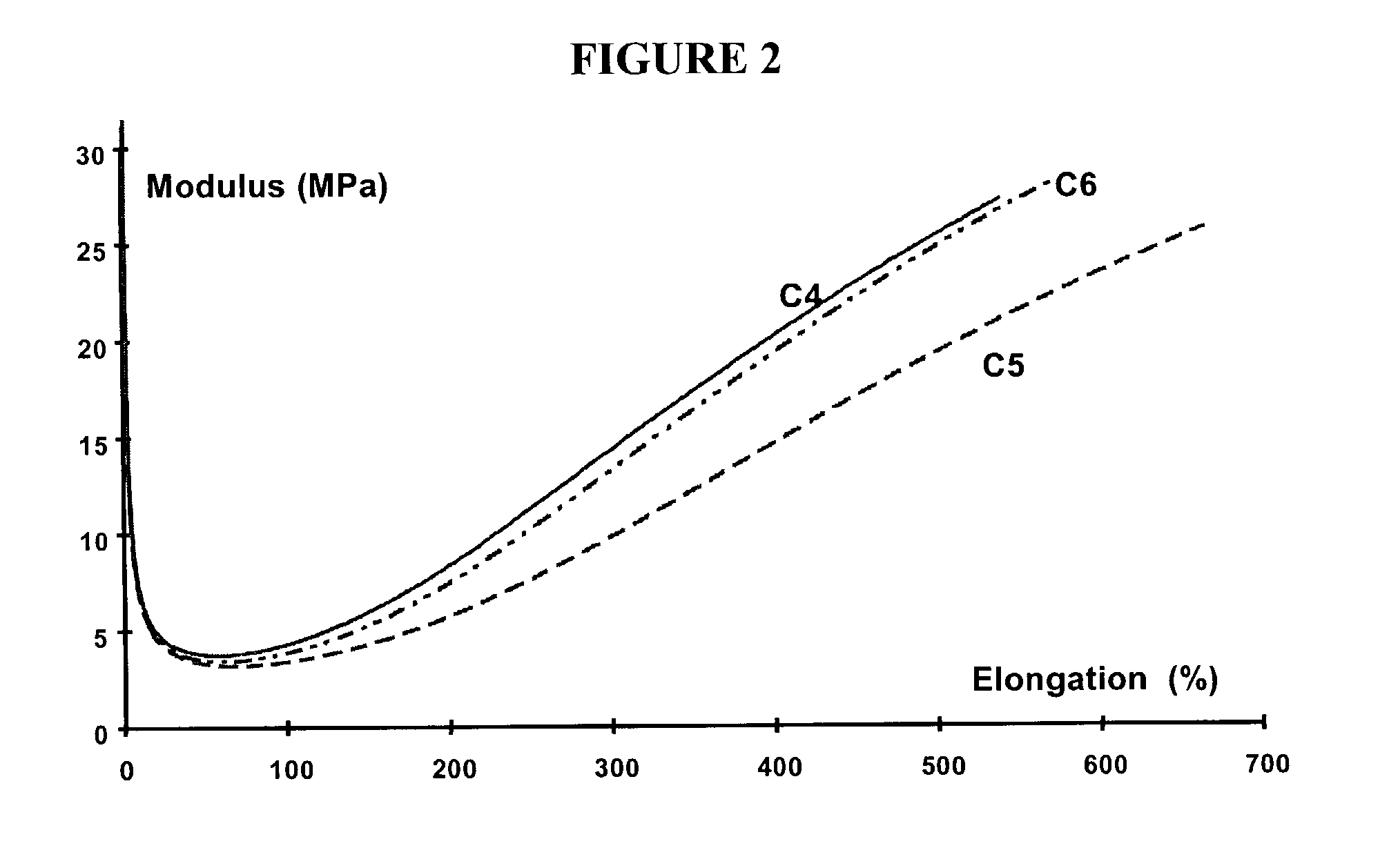
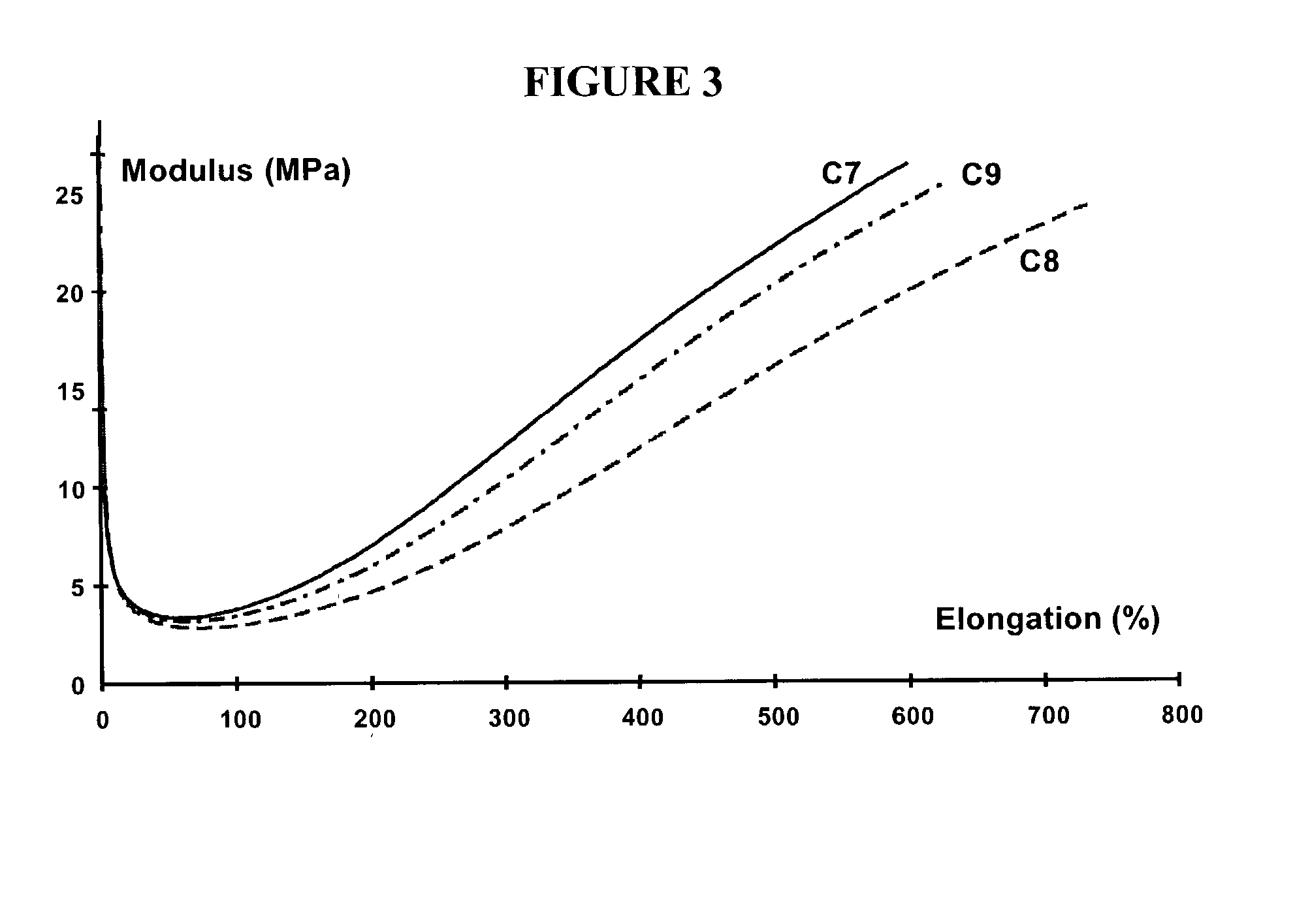
Rubber composition for a tire comprising a reinforcing inorganic filler and an (inorganic filler/elastomer) coupling system
InactiveUS6984689B2Improve efficiencyReduce in quantityOther chemical processesSpecial tyresGuanidine derivativesElastomer
The present invention is directed to a rubber composition that is useful for the manufacture of tires, where the composition is based on a diene elastomer, a reinforcing inorganic filler, and a coupling system. The coupling system comprises a polysulfurized alkoxysilane (“PSAS”) coupling agent (inorganic filler / diene elastomer) associated with a 1,2-dihydropyridine and a guanidine derivative. The present invention is further directed to tires and semi-finished products for tires comprising a rubber composition according to the invention. The invention is also directed to a coupling system (inorganic filler / diene elastomer) for a rubber composition based on a diene elastomer reinforced by an inorganic filler, where the coupling system comprises a polysulfurized alkoxysilane (PSAS) coupling agent in association with a 1,2-dihydropyridine and a guanidine derivative.
Owner:MICHELIN RECH & TECH SA
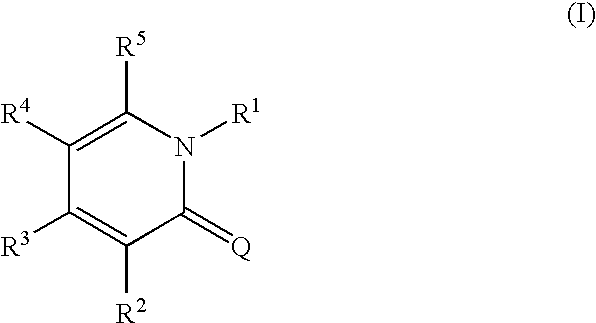
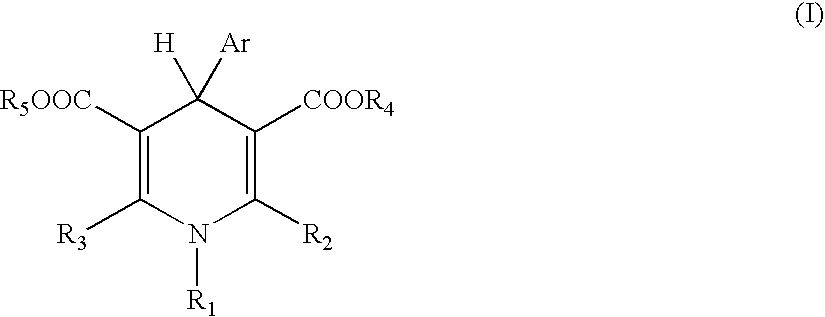
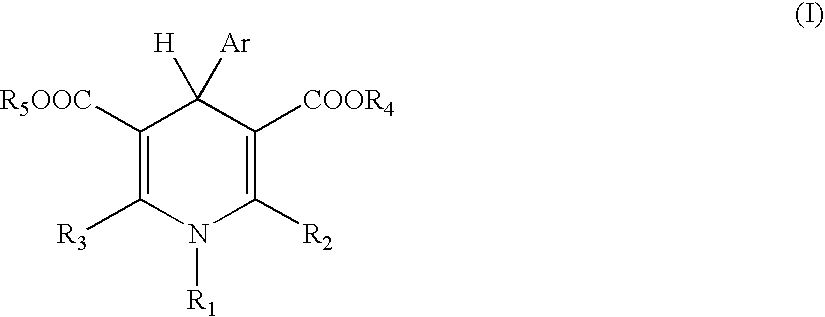
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
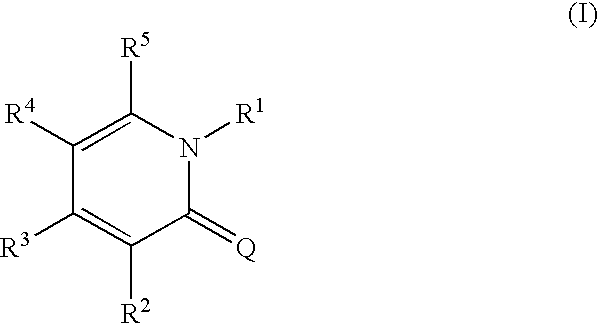
The present invention provides a novel compound having an excellent AMPA receptor inhibitory action and / or kainate inhibitory action. A compound represented by the following formula, a salt thereof or hydrates thereof. In the formula, Q indicates NH, O or S; and R1, R2, R3, R4 and R5 are the same as or different from each other and each indicates hydrogen atom, a halogen atom, a C1-6 alkyl group or a group represented by the formula —X-A (wherein X indicates a single bond, an optionally substituted C1-6 alkylene group etc.; and A indicates an optionally substituted C6-14 aromatic hydrocarbocyclic group or 5- to 14-membered aromatic heterocyclic group etc.).
Owner:CATALYST PHARM INC
Dihydropyridine and dihydropyridazine derivatives as inhibitors of MEK and methods of use thereof
Disclosed are compounds of the Formulas I and Vand pharmaceutically acceptable salts and prodrugs thereof, wherein R1, R2, R7, R8 and R9, W, X and Y are as defined in the specification. Such compounds are MEK inhibitors and useful in the treatment of hyperproliferative diseases, such as cancer and inflammation, in mammals, and inflammatory conditions. Also disclosed are methods of using such compounds in the treatment of hyperproliferative diseases in mammals and pharmaceutical compositions containing such compounds.
Owner:ARRAY BIOPHARMA
Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
InactiveUS20100047341A1Improve Medication AdherenceConstant controlBiocideAnimal repellantsCo administrationSide effect
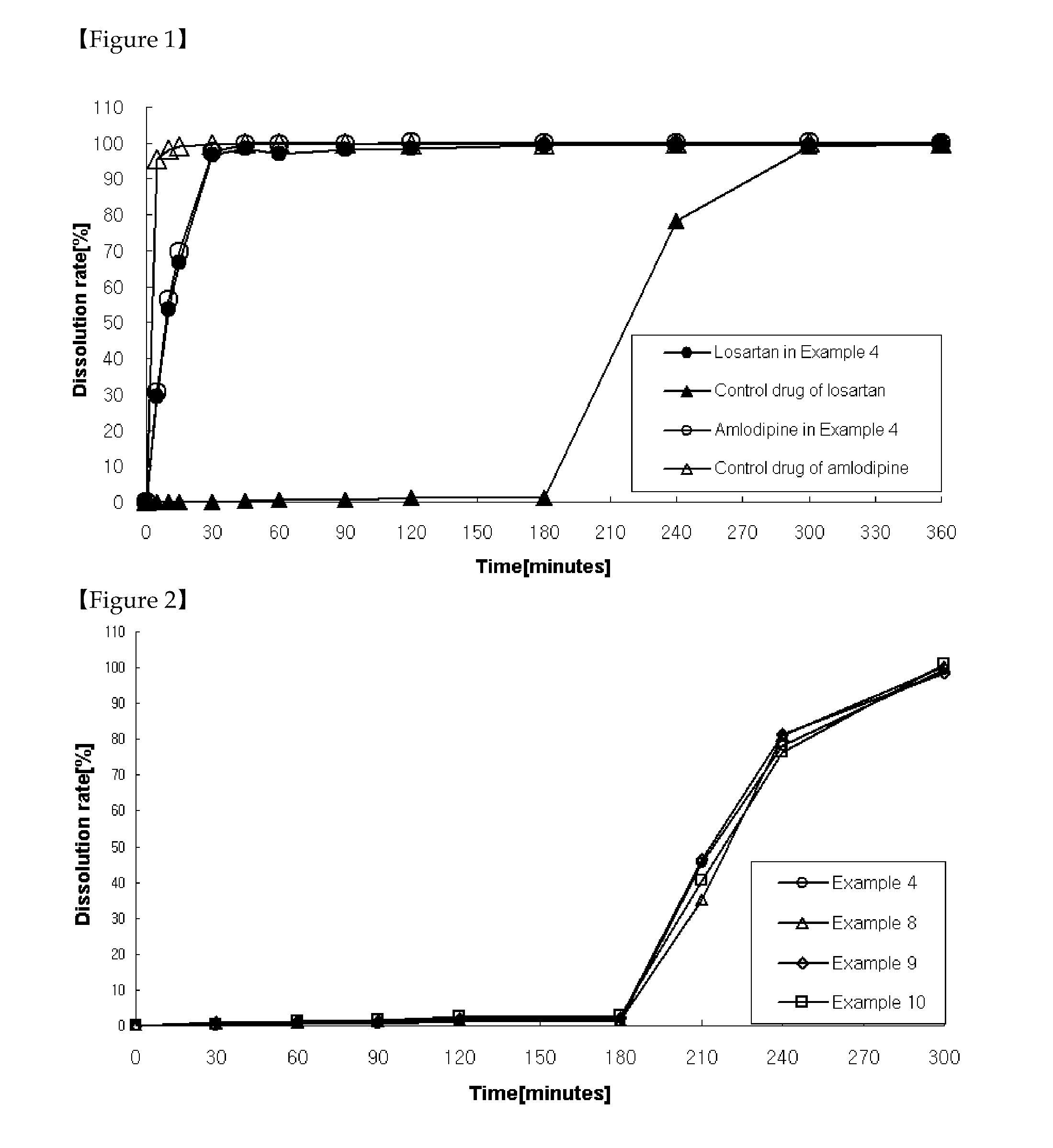
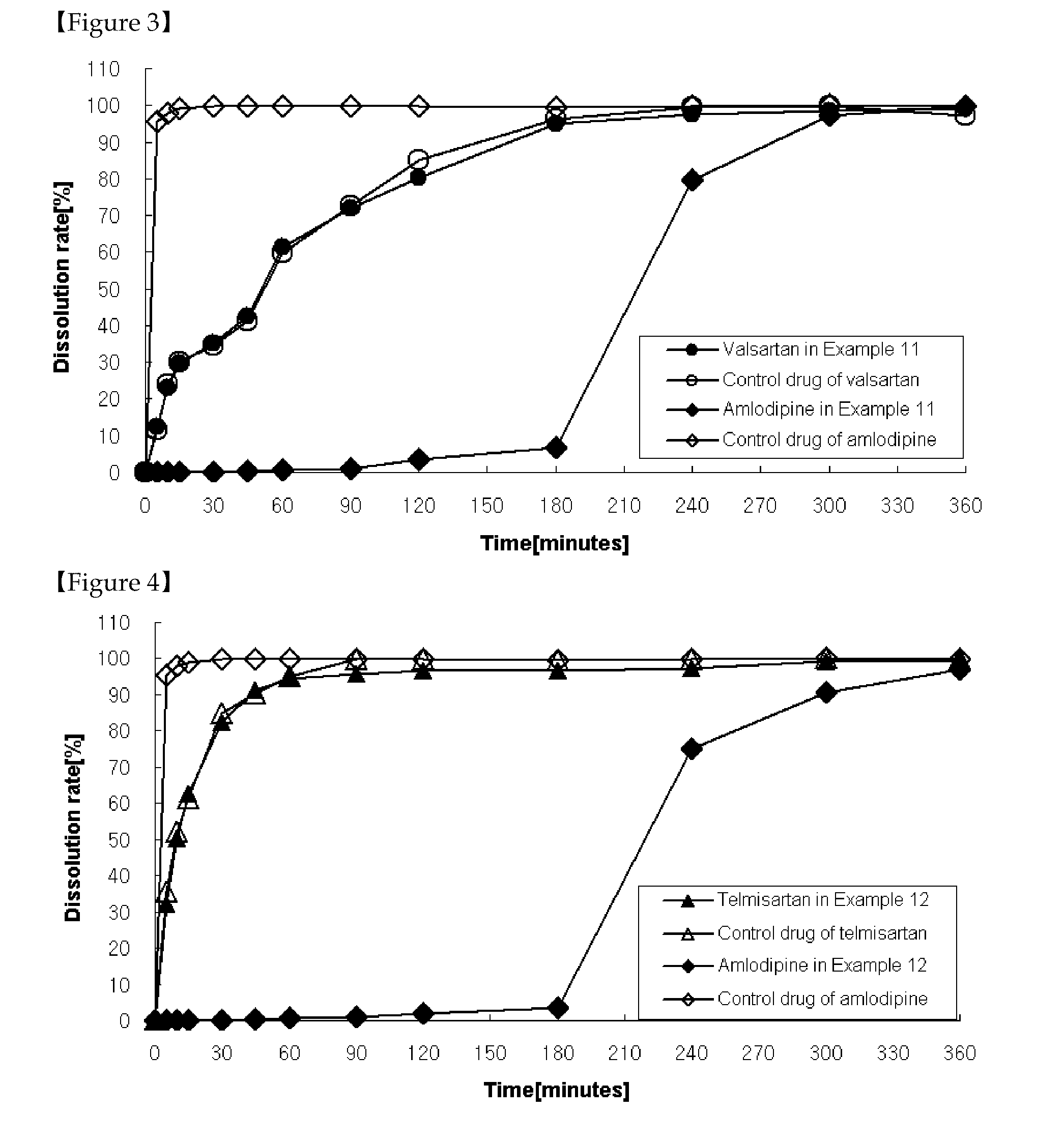
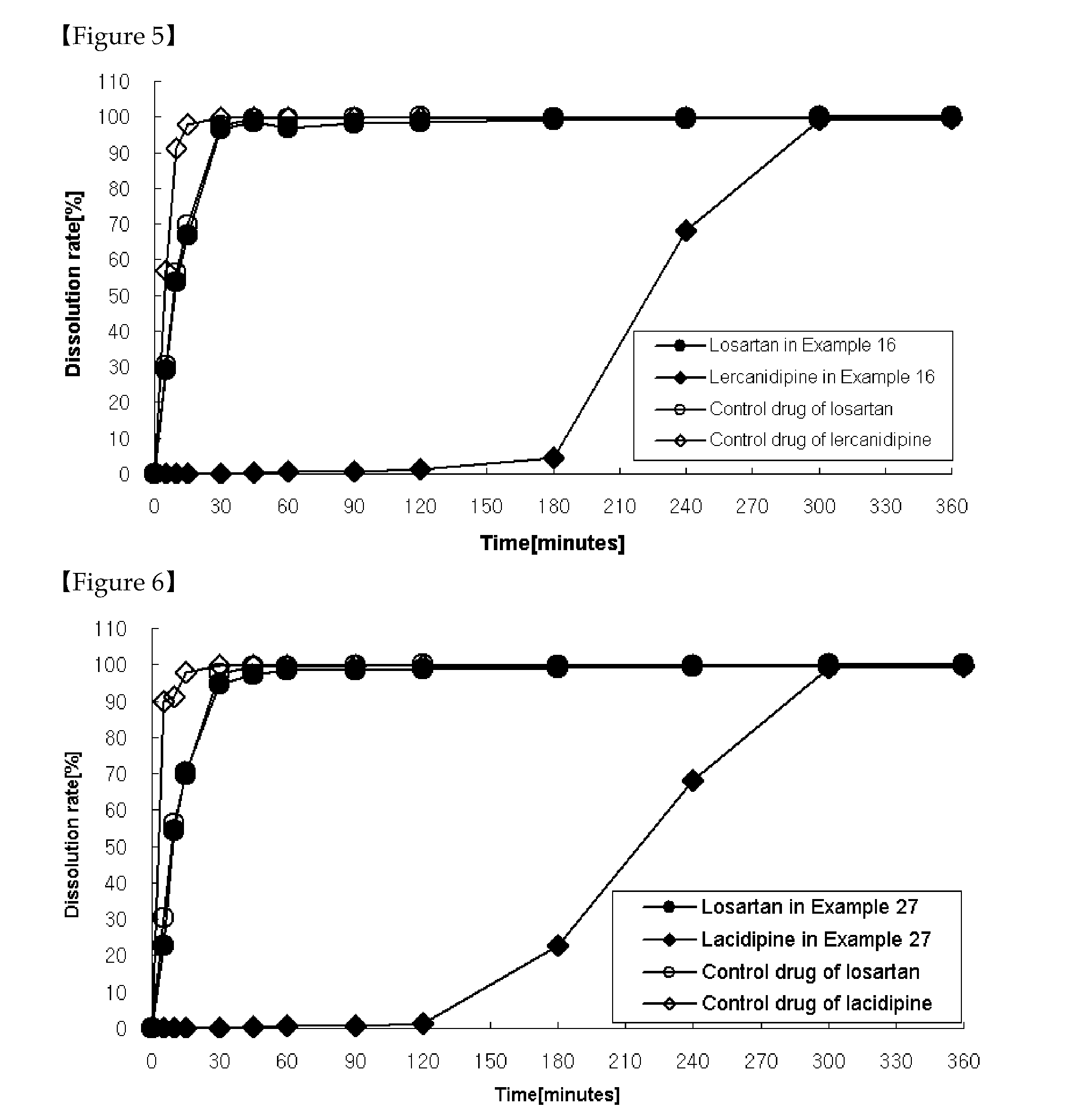
The present invention relates to a functional combination preparation comprising a dihydropyridine-based calcium channel blocker such as amlodipine and an ARB (Angiotensin-2 receptor blocker) such as losartan. In particular, the present invention relates to a chronotherapeutical combination pharmaceutical formulations with controlled-release for the prevention or treatment of cardiovascular disease, which is formulated in accordance with xenobiotics and chronotherapy for enabling the two drugs to be chronotherapeutically released, thereby improving the therapeutic activity as compared to the co-administration of each drug in the form of a single pill, while reducing side effects and maintaining the therapeutic activity as high as possible at the time of day when the risk of a complication of cardiovascular disease is highest.
Owner:HANALL PHARMA CO LTD
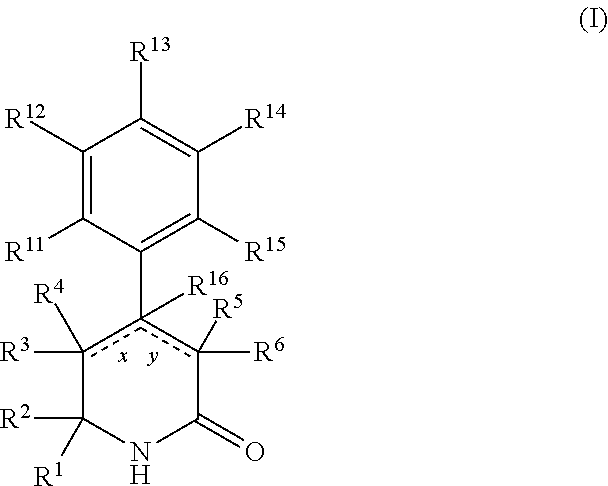
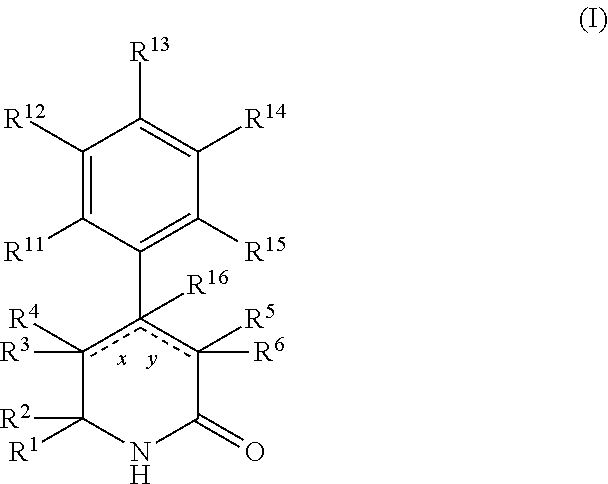
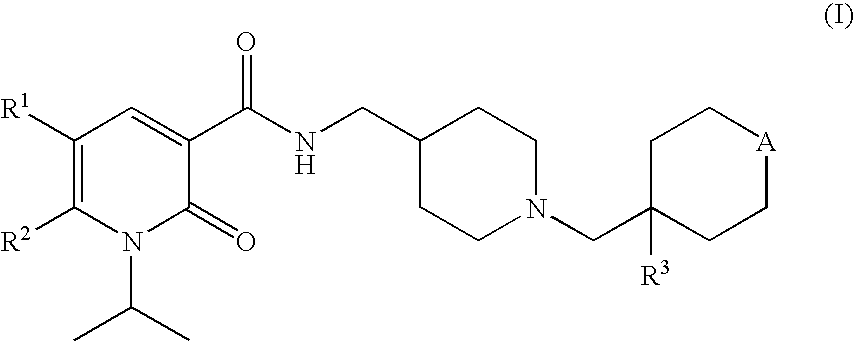
Aryl dihydropyridinone and piperidinone mgat2 inhibitors
The present invention provides compounds of Formula (I):or a stereoisomer, or a pharmaceutically acceptable salt thereof, wherein all of the variables are as defined herein. These compounds are monoacylglycerol acyltransferase type 2 (MGAT2) inhibitors which may be used as medicaments.
Owner:BRISTOL MYERS SQUIBB CO
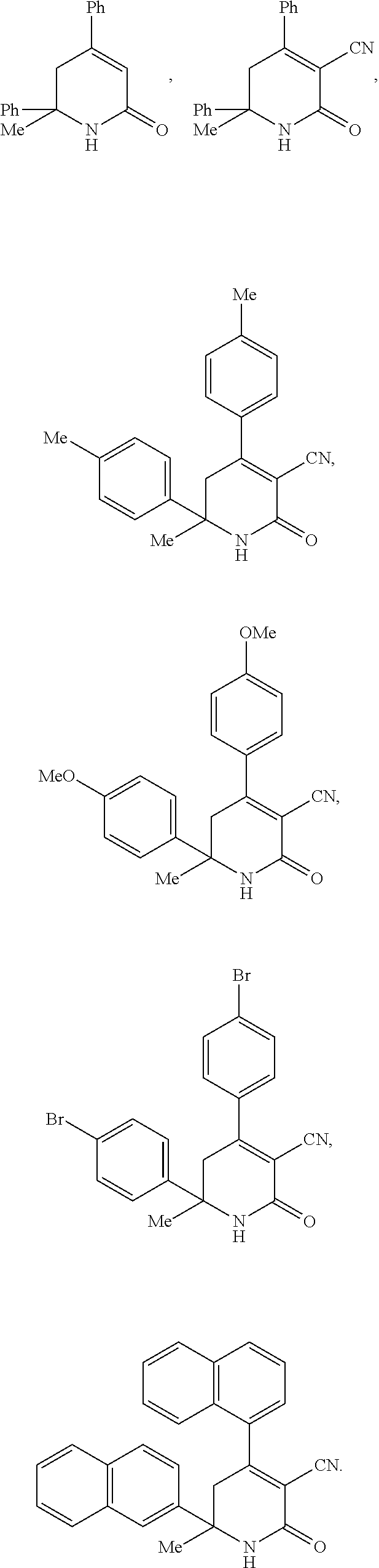
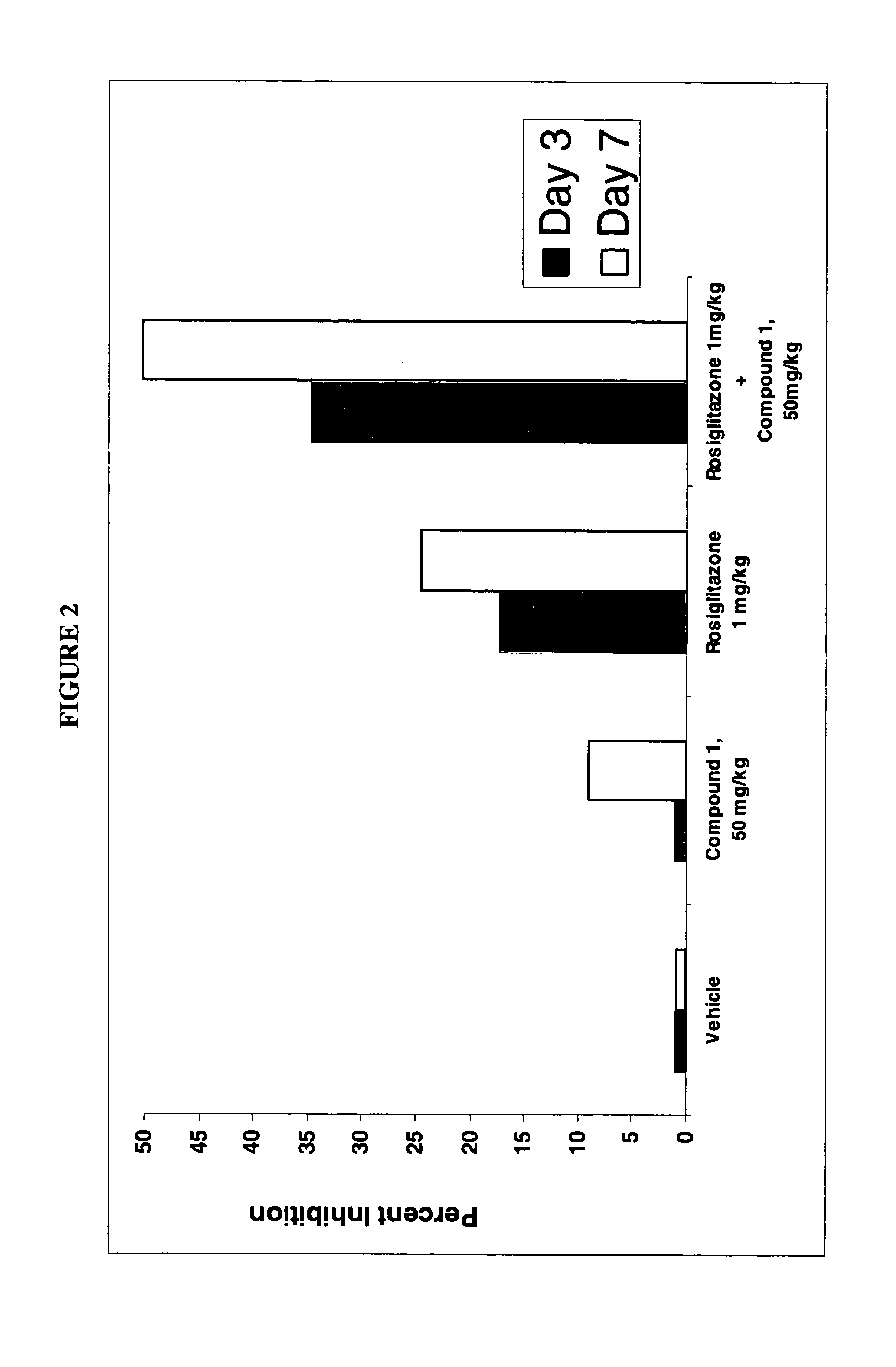
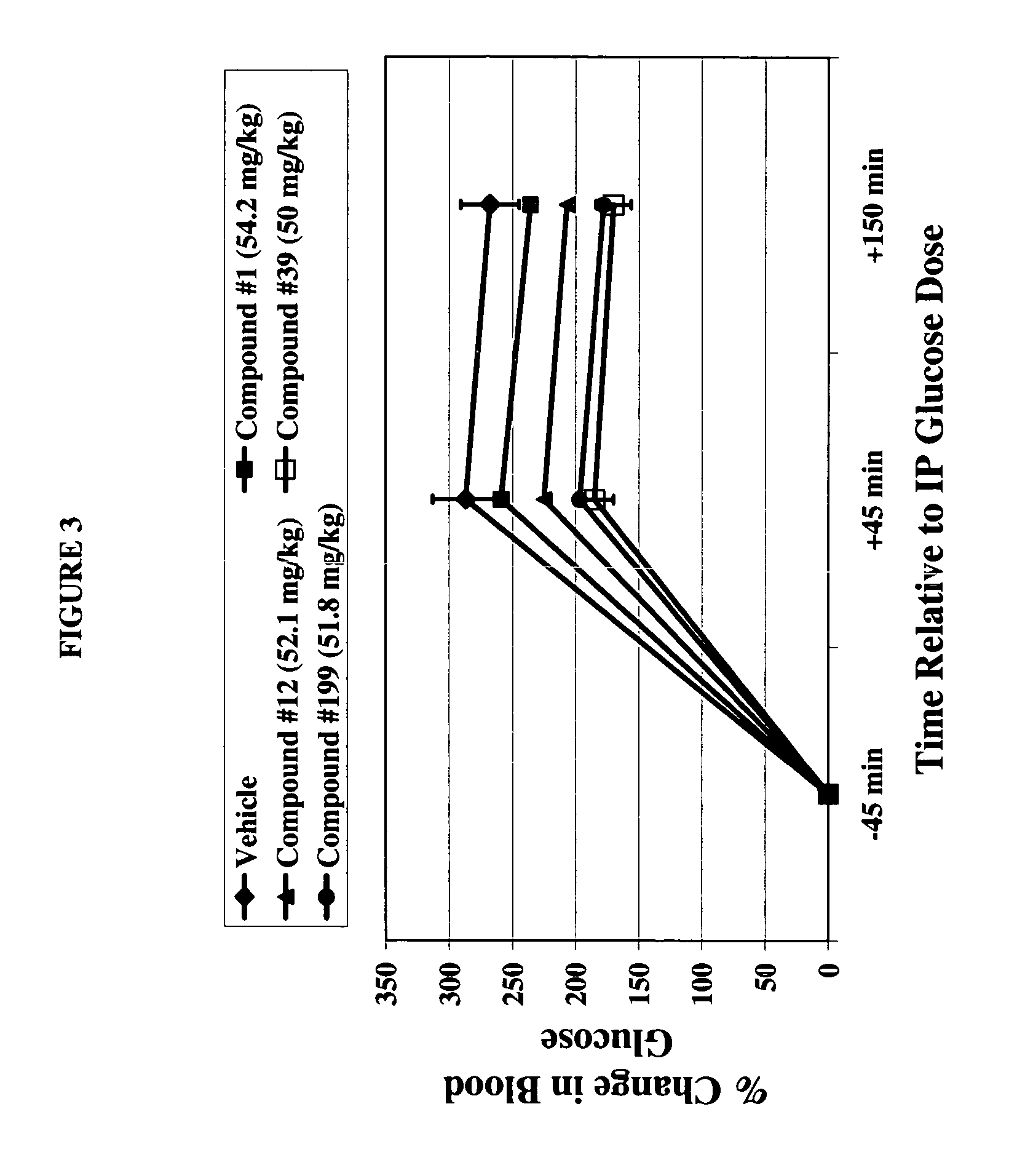
Dihydropyridine compounds for treating or preventing metabolic disorders
InactiveUS20050203119A1Lower blood sugar levelsImprove blood lipid levelsBiocideOrganic chemistryDiabetes mellitusDihydropyridine
This invention relates to dihydropyridine compounds of formula (I): or a pharmaceutically acceptable salt, solvate, clathrate, or prodrug thereof wherein A2, R12, R13, R14, R15, R16, R17, R18, R19, R20, R21, R22, and m are defined herein, and compositions comprising such compounds. The invention also relates to methods of preventing or treating metabolic disorders, such as diabetes mellitus, and conditions and complications associated with diabetes mellitus, comprising administering to a subject in need thereof a compound of formula (1) or a composition comprising such a compound. The invention further relates to kits comprising a compound of formula (I).
Owner:SYNTA PHARMA CORP
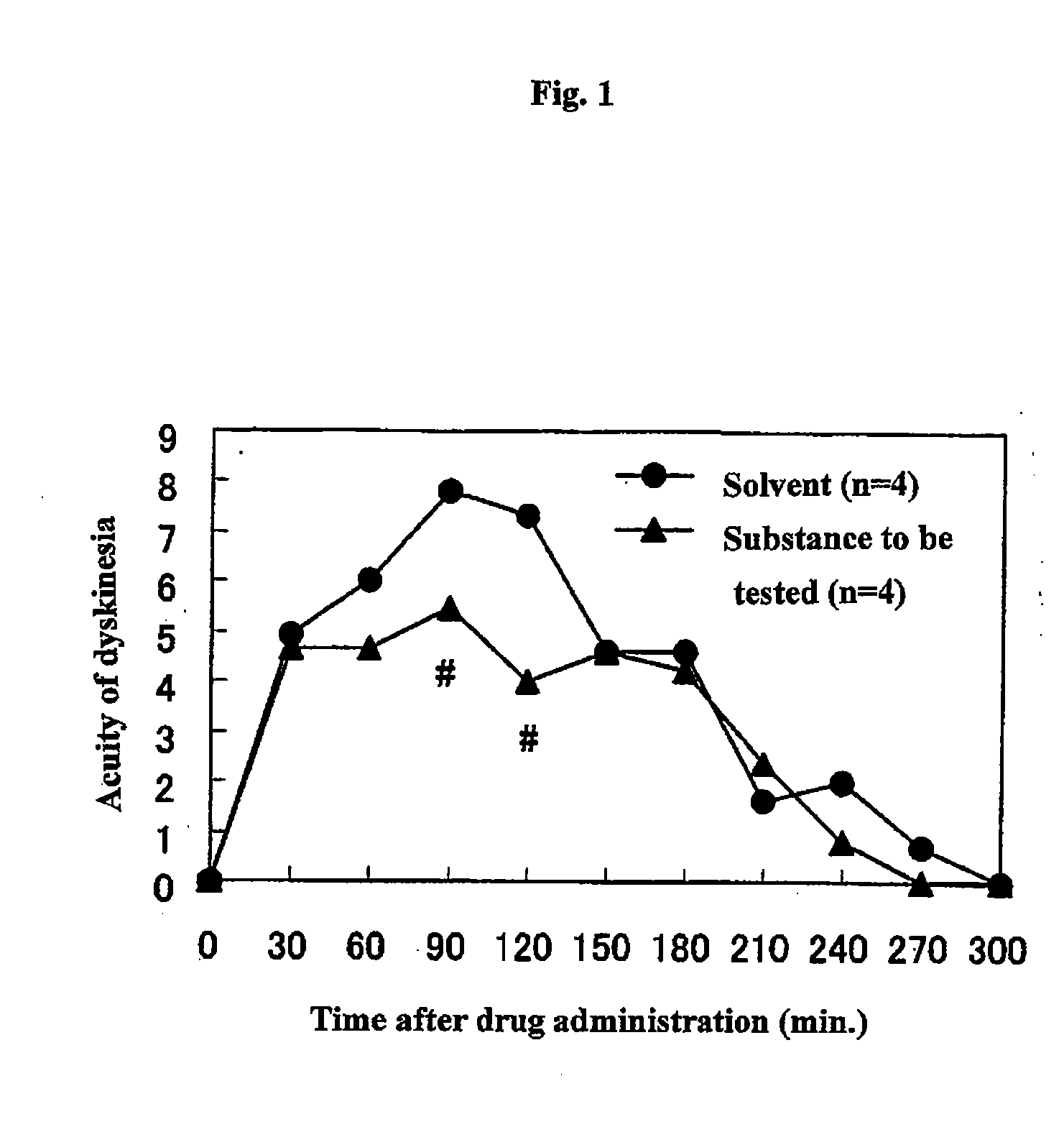
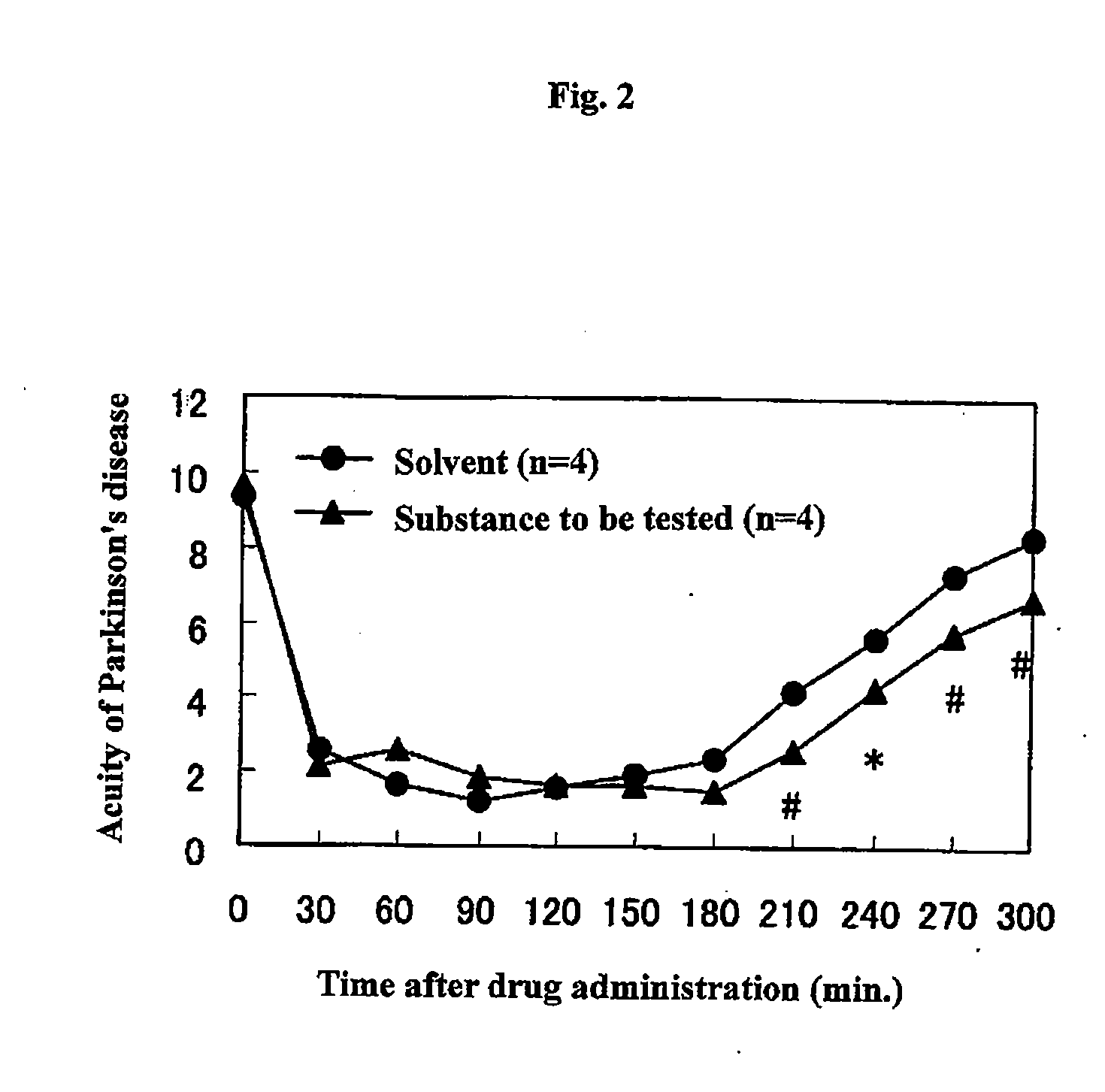
Therapeutic agent for dyskinesia
InactiveUS20090030017A1Symptoms improvedExcellent AMPA receptor antagonismBiocideNervous disorderDihydropyridineEXTREMITY TREMOR
Owner:EISIA R&D MANAGEMENT CO LTD
Feed additive for improving quality of fur of furry animal
ActiveCN102422993AImproves fur qualityIncrease hair lengthAnimal feeding stuffVitamin d 3Chelated zinc
The invention provides a feed additive for improving the quality of the fur of a furry animal. The additive is obtained by mixing a semi-finished vitamin product, a semi-finished trace element product, dihydropyridine, methionine, cystine and corn gluten meal according to a weight ratio of 1:1:0.3-1:0.2-0.9:0.1-0.6:2, wherein the semi-finished vitamin product is prepared by uniformly mixing the following components: 5-22 parts of vitamin A, 3-8 parts of vitamin D, 10-25 parts of vitamin E, 3-8 parts of vitamin B1, 8-15 parts of biotin, 1-3 parts of vitamin B6, and 120-160 parts of the corn gluten meal, and the semi-finished trace element product is prepared by uniformly mixing the following components: 10-20 parts of methionine chelated zinc, 2-5 parts of methionine chelated copper, and 70-90 parts of zeolite powder. The additive of the invention allows the quality of the fur of the furry animals (martens, foxes and raccoon dogs) to be substantially improved, the hair density to be increased, the hair length to be improved, the hide toughness to be increased and the culture benefit to be improved.
Owner:海宁市钱江兴业投资开发有限公司
Compounds and method for the prevention and treatment of diabetic retinopathy
InactiveUS6440933B1Reduce deliveryBlock deliverySenses disorderSomatostatinsDiabetic retinopathyDipeptide
The invention provides peptide derivatives designed to deliver peptides having growth factor inhibitory activity, especially somatostatin analogs, to the retina by sequential metabolism. The peptide derivatives, which comprise a dihydropyridine<CUSTOM-CHARACTER FILE="US06440933-20020827-P00900.TIF" ALT="custom character" HE="20" WI="20" ID="CUSTOM-CHARACTER-00001" / >pyridinium salt-type redox targetor moiety, a bulky lipophilic function and an amino acid / dipeptide / tripeptide spacer, are used in the prevention and treatment of diabetic retinopathy.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Premix for improving reproductive performance of fur animals
ActiveCN102150751AImprove reproductive performanceImprove weaning survival rateAnimal feeding stuffAccessory food factorsMinkManganese
The invention relates to a premix for improving the reproductive performance of fur animals, comprising trace element semi-finished product and a vitamin semi-finished product. The trace element semi-product is prepared from the following components: 10-40 parts of amino acid Fe-chelate, 10-50 parts of amino acid zinc chelate, 20-40 parts of amino acid manganese chelate, 5-20 parts of amino acid copper chelate, 1-8 parts of amino acid selenium chelate and 125-135 parts of zeolite powder; the vitamin semi-finished product is prepared from the following components: 2-8 parts of vitamin A, 1-4 parts of vitamin D, 15-35 parts of vitamin E, 30-70 parts of dihydropyridine, 10-20 parts of methionine and 100-125 parts of corn protein powder; and the two semi-products are uniformly mixed accordingto the ratio of 1: 1 to obtain the final product. The premix is applicable to a hybridization preparation period, a gestation period and a lactation period of foxes, minks and raccoons. By feeding the premix provided by the invention, reproductive performance of foxes, minks and raccoons, oestrus rate, conception rate, number born and survival rate of weaning cubs can be improved.
Owner:海宁市钱江兴业投资开发有限公司
Photosensitive resin composition and circuit board with metal support using the same
InactiveUS20120067626A1Curing shrinkage is smallSatisfactory photosensitivityPhotosensitive materialsPhotomechanical apparatusEpoxyDihydropyridine
A photosensitive resin composition contains a component (A) and at least one of a component (B) and a component (C). In addition, in the circuit board with metal support including: a metal support; a base insulating layer; a conductive layer formed of a wiring circuit pattern; and a cover insulating layer, at least one of the above-mentioned base insulating layer and cover insulating layer is made of the above-mentioned photosensitive resin composition.(A) a 1,4-dihydropyridine derivative represented by the following general formula (1)where R1 represents an alkyl group having 1 to 3 carbon atoms; and R2 and R3 each represent a hydrogen atom or an alkyl group having 1 or 2 carbon atoms and may be identical to or different from each other;(B) the following (x) and (y):(x) a carboxyl group-containing linear polymer; and(y) an epoxy resin(c) a linear polymer having a carboxyl group and an epoxy group
Owner:NITTO DENKO CORP
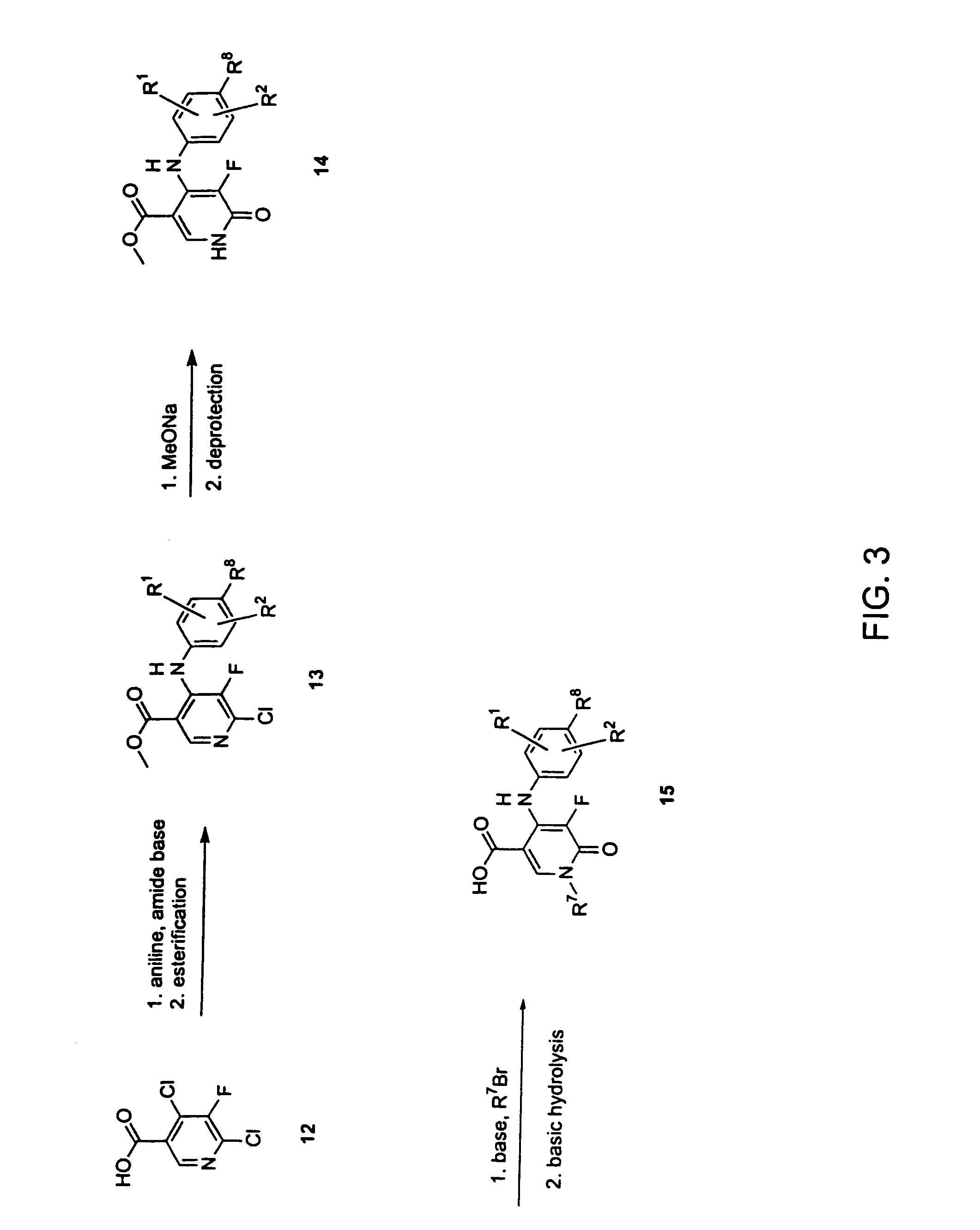
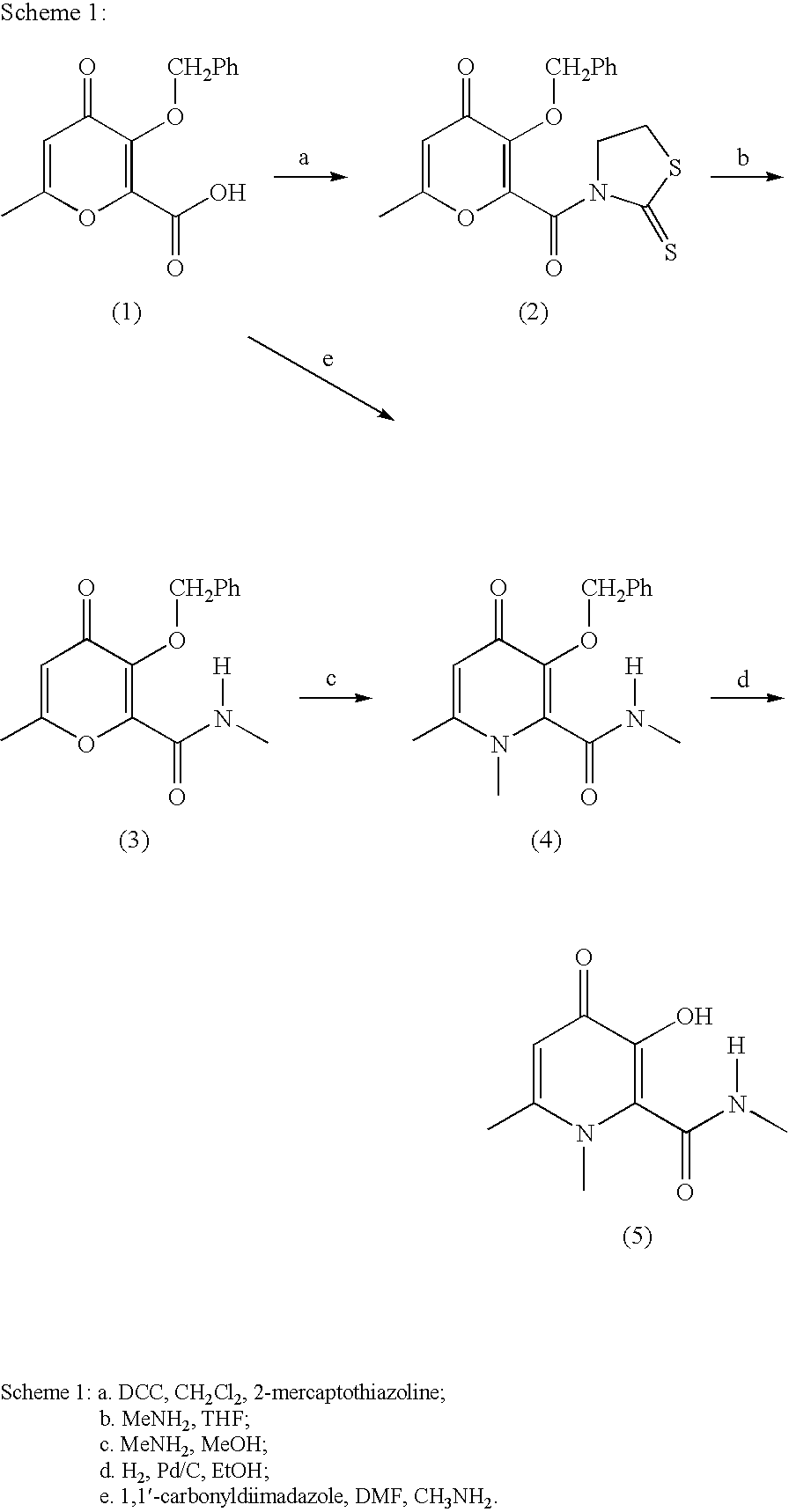
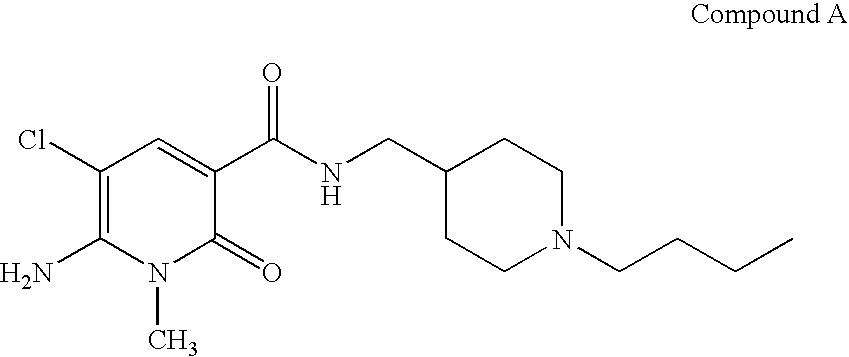
Process For The Manufacture Of 3-Hydroxy-N-Alkyl-1-Cycloalkyl-6-Alkyl-4-Oxo-1,4-Dihydropyridine-2-Carboxamide And Its Related Analogues
InactiveUS20080096886A1Removes costly aspectReduce the amount requiredBiocideOrganic chemistryOxalyl chlorideAmmonium chloride mixture
The present invention relates to a novel process for the preparation of 1-alkyl or 1-cycloalkyl derivatives of 3-hydroxy-4-oxo-1,4-dihydropyridine-2-carboxamide of formula I. The process includes reacting an amine R2NH2 with a compound of formula II in a solution of metal hydroxide in water to give a compound of formula III. Subsequent reaction of the compound of formula III with an acid chloride formation reagent in an inert solvent gives compounds of formula I. The acid chloride formation reagent is selected from oxalyl chloride and dimethylformamide, dimethylchloromethylene-ammonium chloride and thionyl chloride and dimethylformamide. If desired, a compound of formula I where R5 is hydrogen may be formed when an intermediate substituent is used wherein R5 is an alcohol protective group removable by catalytic hydrogenation.
Owner:APOTEX INC
5,6-dihydro-1H-pyridin-2-one compounds
Owner:ANDADYS PHARMA INC
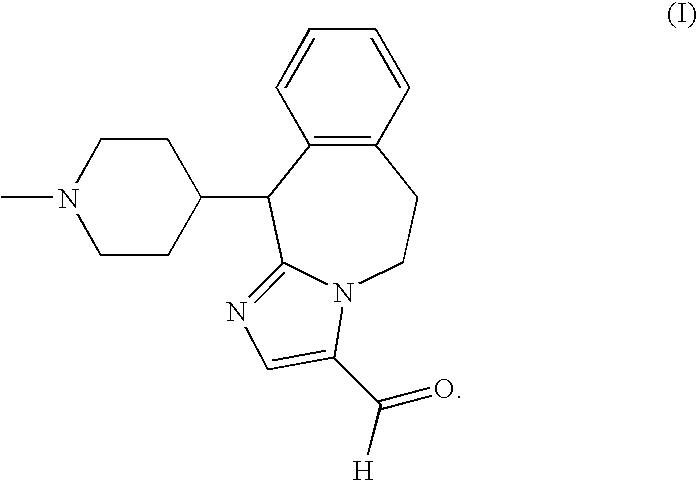
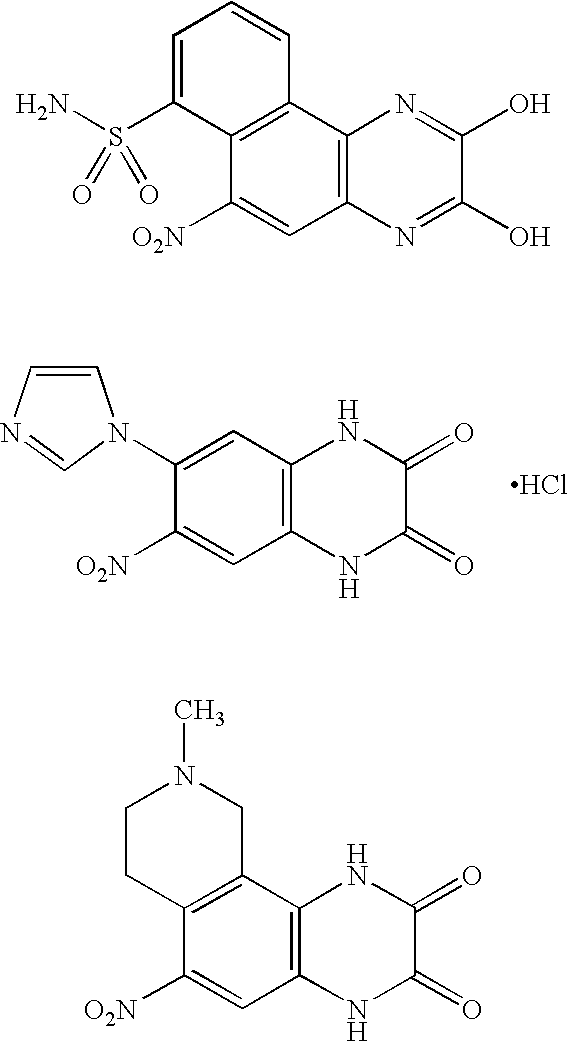
NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE)
The present invention is directed to novel 2-amino-3,4-dihydro-pyrido[3,4-d]pyrimidine derivatives, pharmaceutical compositions containing them and their use in the treatment of Alzheimer's disease (AD) and related disorders. The compounds of the invention are inhibitors of β-secretase, also known as β-site cleaving enzyme and BACE, BACE1, Asp2 and memapsin2.
Owner:JANSSEN PHARMA NV
Hydride reduction of alpha, beta-unsaturated carbonyl compounds using chiral organic catalysts
InactiveUS20060161024A1Lower Level RequirementsReduce compoundingOrganic compound preparationOrganic chemistry methodsGreek letter betaDihydropyridine
Nonmetallic, chiral organic catalysts are used to catalyze the 1,4-hydride reduction of an α,β-unsaturated carbonyl compound. The α,β-unsaturated carbonyl compound may be an aldehyde or cyclic ketone, and the hydride donor may be a dihydropyridine. The reaction is enantioselective, and proceeds with a variety of hydride donors, catalysts, and substrates. The invention also provides compositions effective in carrying out the 1,4-hydride addition of α,β-unsaturated carbonyl compounds.
Owner:CALIFORNIA INST OF TECH
Salt form of a human histone methyltransferase EZH2 inhibitor
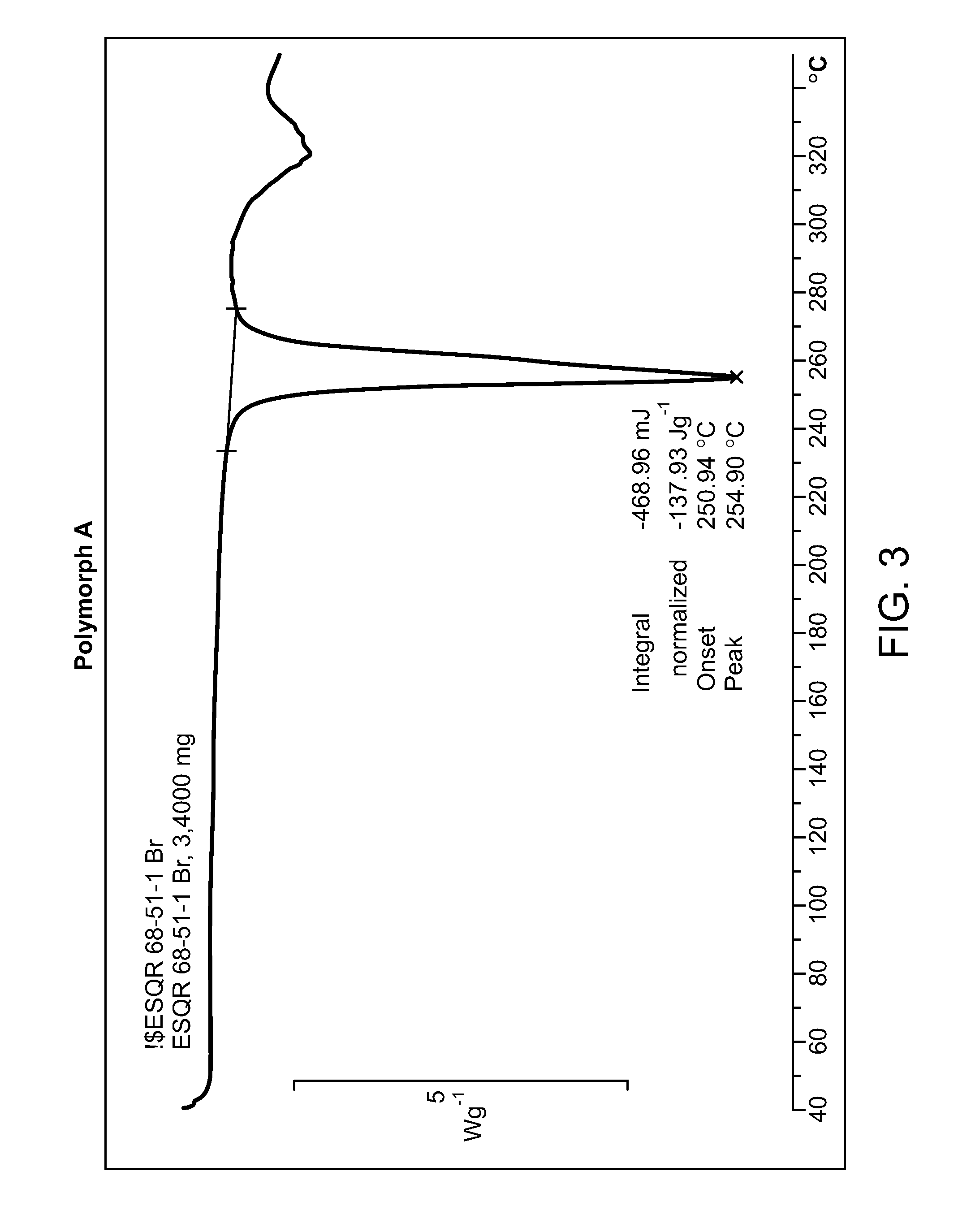
Provided herein is N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4′-(morpholinomethyl)-[1,1′-biphenyl]-3-carboxamide hydrobromide. Also provided herein is a particular polymorph form of this compound.
Owner:EISIA R&D MANAGEMENT CO LTD +1
Dihydropyridine compounds and compositions for headaches
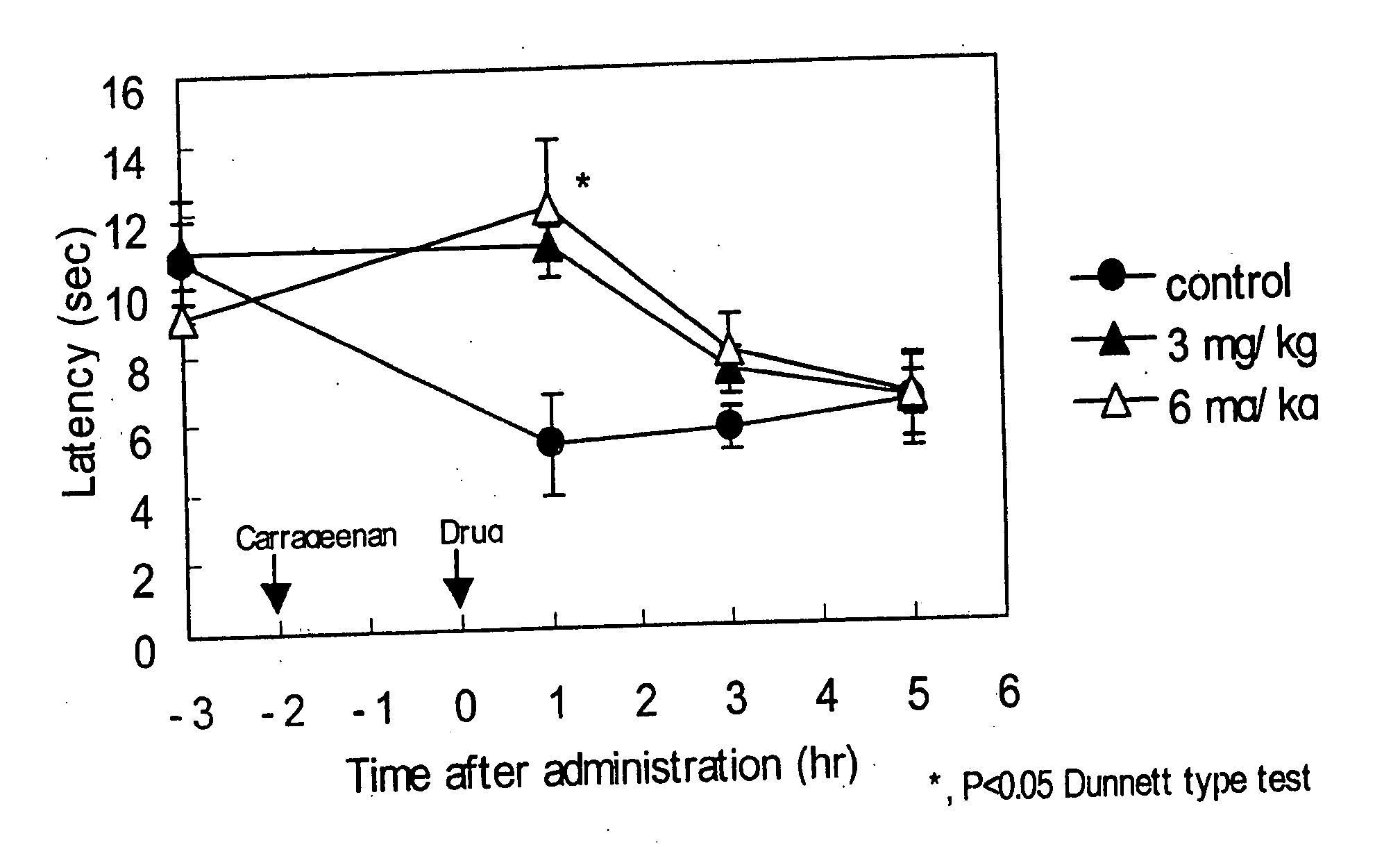
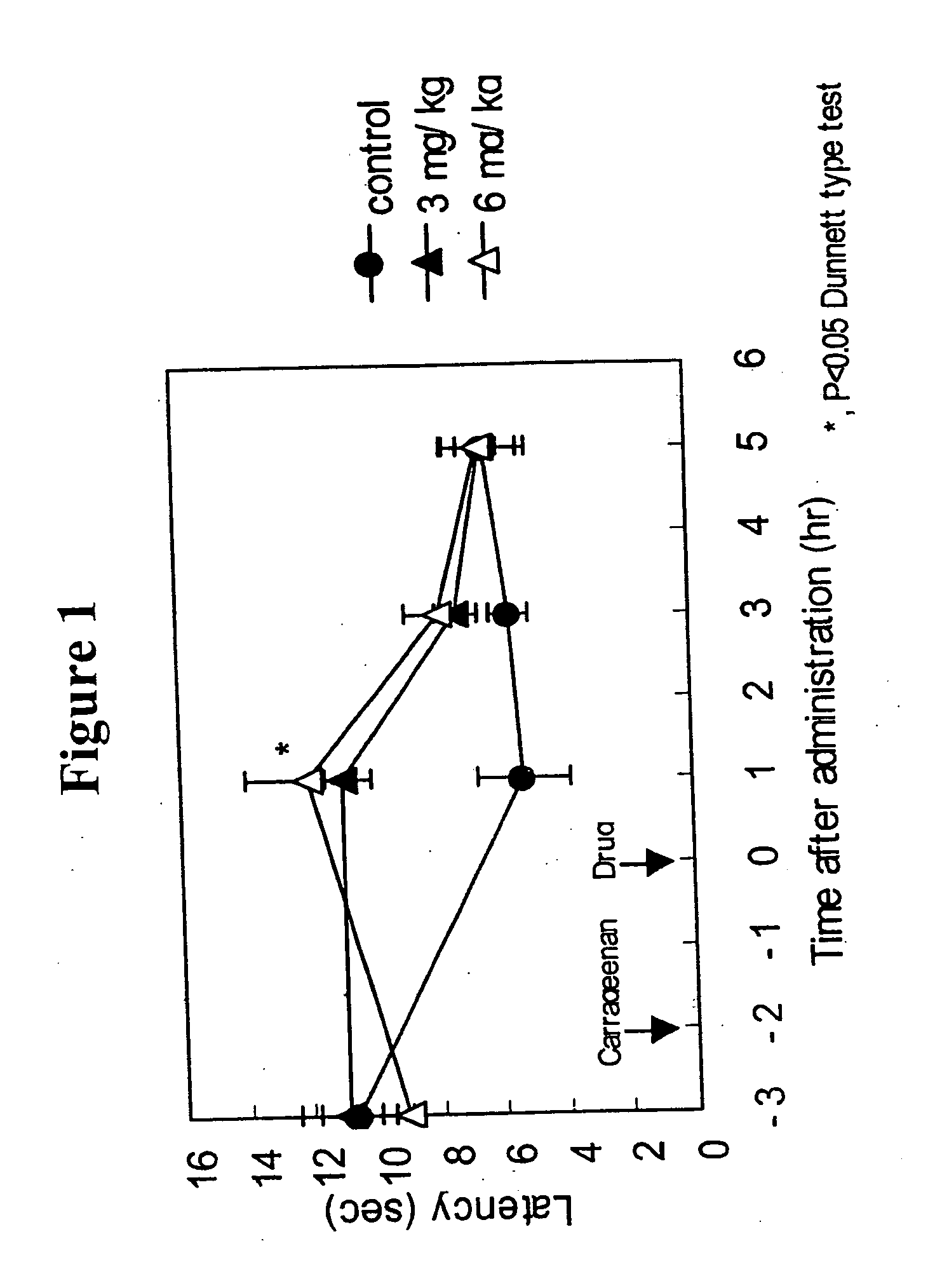
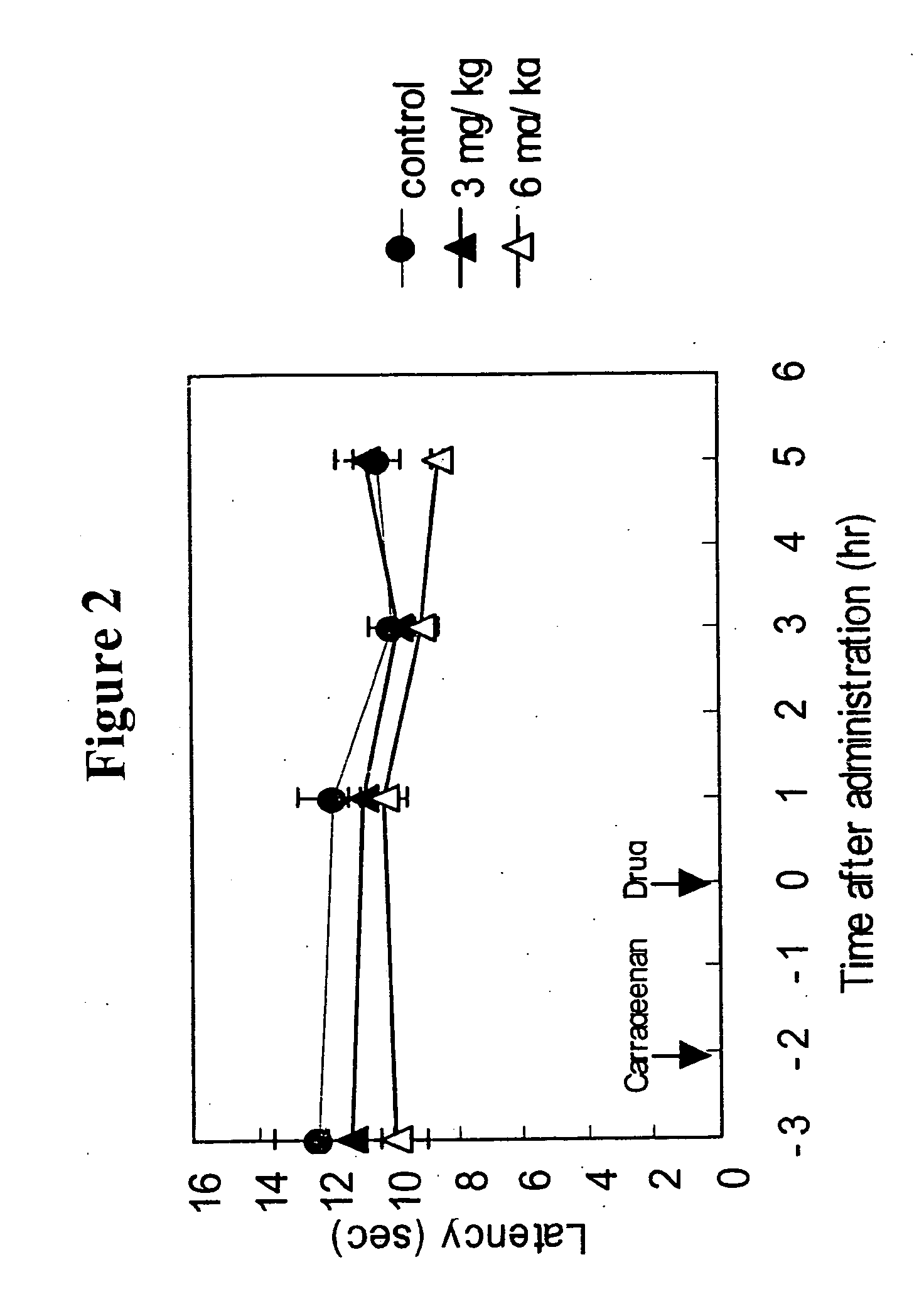
The invention provides methods for treating and / or preventing headaches by administering to patients therapeutically effective amounts of 1,2-dihydropyridine compounds, and, optionally, cholinesterase inhibitors and / or anti-migraine agents. The headaches may be primary headaches, such as migraines, or secondary headaches. The invention also provides combinations, commercial packages, and pharmaceutical compositions comprising therapeutically effective amounts of 1,2-dihydropyridine compounds and, optionally, cholinesterase inhibitors and / or anti-migraine agents. The 1,2-dihydropyridine compound may be, for example, 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one. The cholinesterase inhibitor may be, for example, 1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine.
Owner:EISIA R&D MANAGEMENT CO LTD
Treatment with dihydropyridine calcium channel blockers and omega-3 fatty acids and a combination product thereof
InactiveUS20070196465A1Good effectImprove efficiencyBiocideMetabolism disorderDihydropyridineFatty acid
Combinations of one or more dihydropyridine calcium channel blockers with mixtures of omega-3 fatty acids, methods of administering such combinations, and unit dosages of such combinations.
Owner:RELIANT PHARMACEUTICALS INC
A carbon quantum dot based on a copper complex and a preparing method thereof
ActiveCN104759283AGood catalyticMeet the requirements of green photochemistryOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSolubilityOxygen
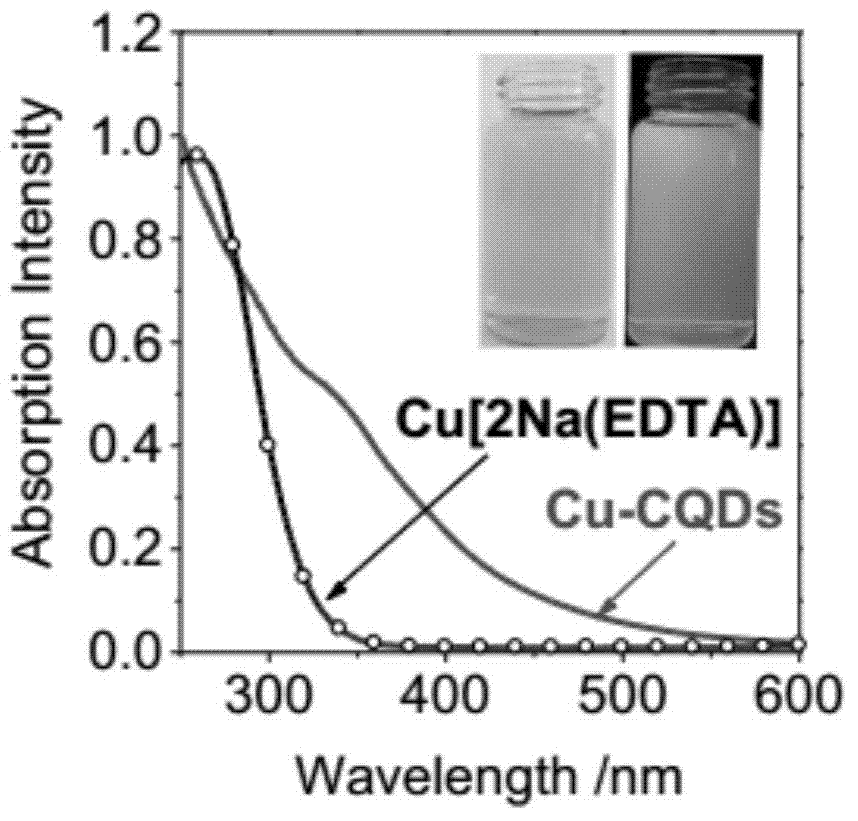
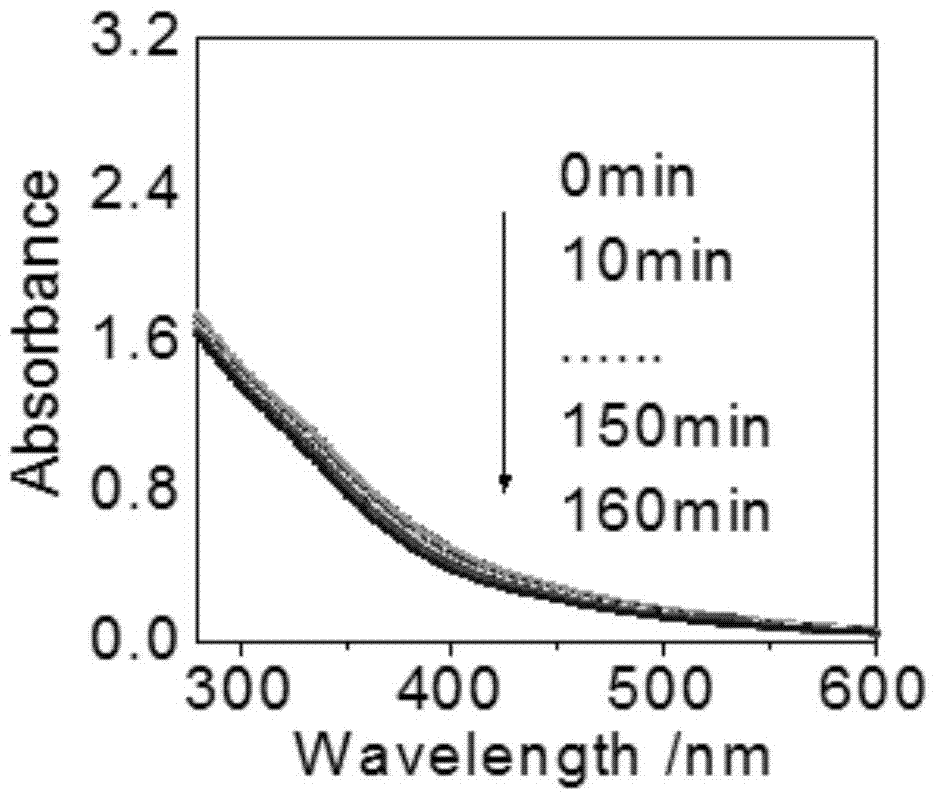
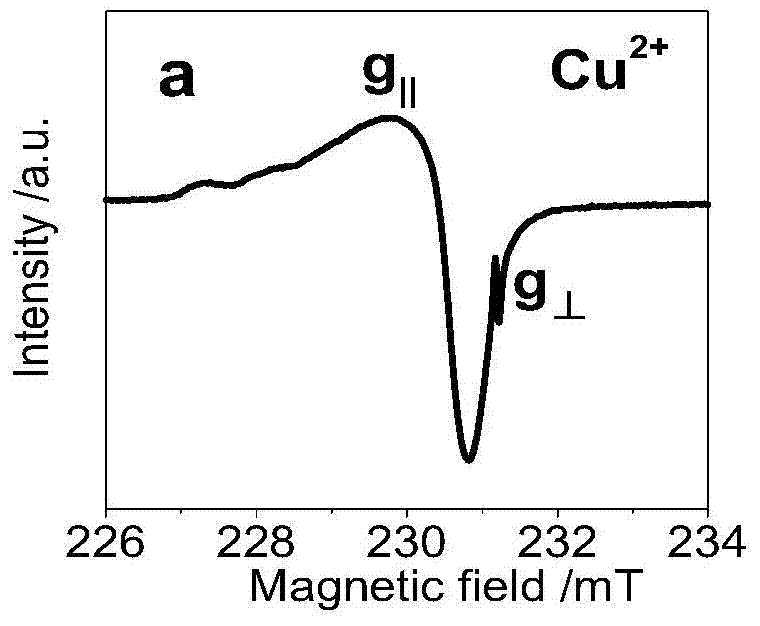
A carbon quantum dot based on a copper complex and a preparing method thereof are disclosed. Copper disodium ethylenediaminetetraacetate having a saturated Schiff base structure is adopted as a raw material, the copper complex and a carbon quantum dot having a structure similar to that of graphene are composited, and the carbon quantum dot based on the copper complex is prepared by a thermo-polymerization method so as to improve electron transfer capability of photosensitizers of this type. The prepared carbon quantum dot based on the copper complex is good in water solubility, electron receptivity, electron releasing capability and electrical conductivity and wide in visible-light response range, and is an excellent photosensitizer. By application of the photosensitizer into a photooxidation reaction of 1,4-dihydropyridine, efficient photo-catalytic oxidation of reactions of this type is achieved and byproducts only comprise water and oxygen.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Substituted dihydropyridines and methods of use
Compounds are provided that are modulators of the C5a receptor. The compounds are substituted dihydropyridines and are useful in pharmaceutical compositions, methods for the treatment of diseases and disorders involving the pathologic activtation of C5a receptors.
Owner:CHEMOCENTRYX INC
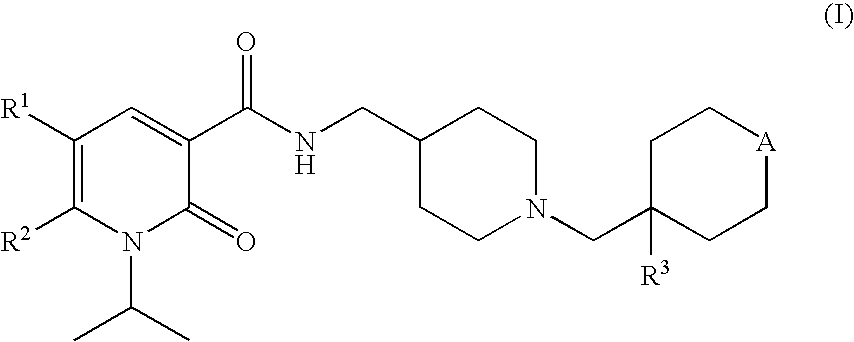
1-isopropyl-2-oxo-1,2-dihydropyridine-3-carboxamide derivatives having 5-HT4 receptor agonistic activity
InactiveUS7691881B2Improve breathabilityLess toxicityBiocideNervous disorder5-HT4 receptorIrritable bowel syndrome
This invention provides a compound of formula (I): wherein R1 represents an alkyl group having from 1 to 4 carbon atoms or a halogen atom, R2 represents an alkyl group having from 1 to 4 carbon atoms, R3 represents a hydrogen atom or a hydroxy group, and A represents an oxygen atom or a group of the formula —C(R4)(R5)— (in which R4 represents a hydrogen atom or an alkyl group having from 1 to 4 carbon atoms and R5 represents a hydroxy group or an alkoxy group having from 1 to 4 carbon atoms) or a pharmaceutically acceptable salts thereof. These compounds have 5-HT4 receptor agonistic activity, and thus are useful for the treatment of gastroesophageal reflux disease, non-ulcer dyspepsia, functional dyspepsia, irritable bowel syndrome or the like in mammalian, especially humans.
Owner:PFIZER INC
Photosensitive polyimide resin precursor composition, optical polyimide obtained from the composition, optical waveguide using the polyimide, and process for producing the optical waveguide
A photosensitive polyimide resin precursor composition capable of providing a polyimide resin that is not substantially colored, is transparent and has heat resistance, an optical polyimide resin obtained from the composition, and an optical waveguide using the polyimide resin. The photosensitive polyimide resin precursor composition contains (a) 100 parts by weight of a polyamic acid obtained from a tetracarboxylic acid dianhydride and a diamine, (b) 0.01 parts by weight or more and less than 5 parts by weight of a 1,4-dihydropyridine derivative, (c) 5-50 parts by weight of a glycol (ether). The optical polyimide resin is obtained by irradiating the photosensitive resin precursor composition with UV light, followed by exposure, heating, development, and then heating. The optical waveguide comprises a core layer comprising the optical polyimide resin, and a cladding layer thereof.
Owner:NITTO DENKO CORP
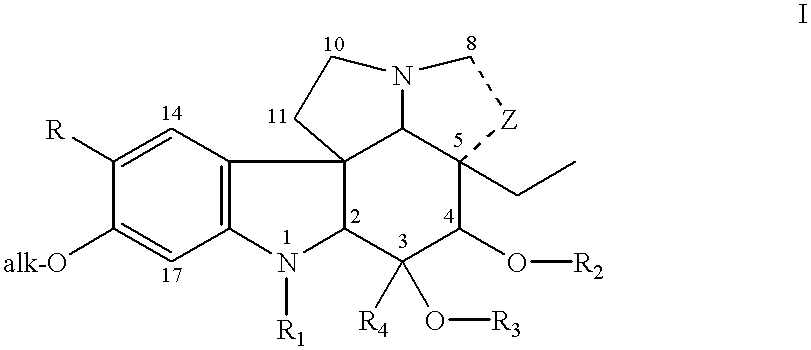
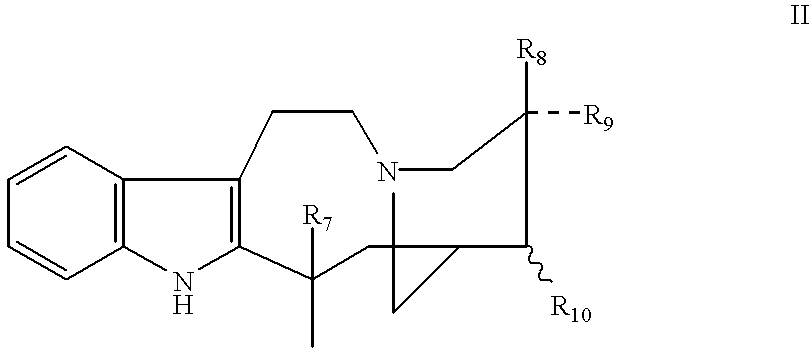
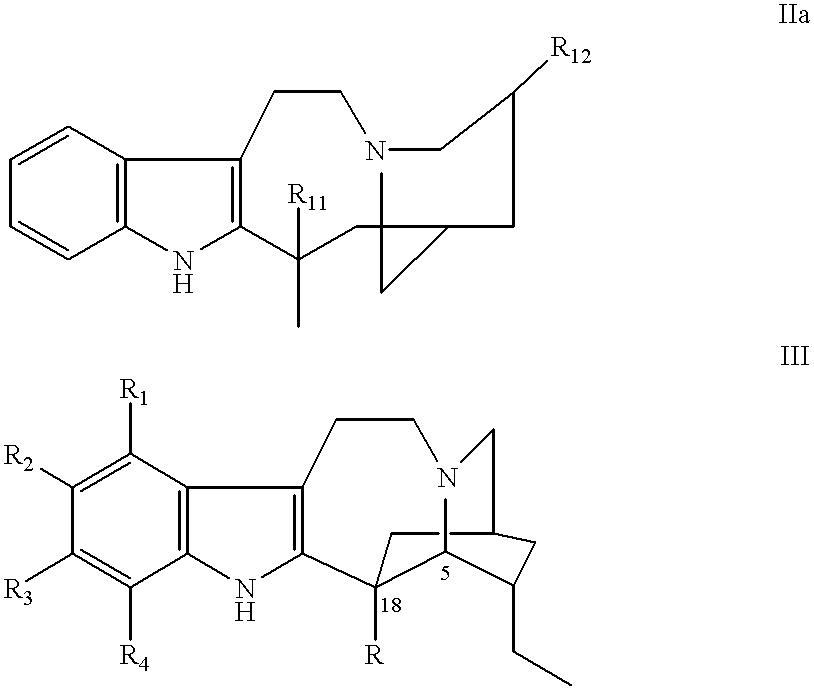
Process of synthesis of 3',4'-anhydrovinblastine, vinblastine and vincristine
The present invention relates to the synthesis of dimer alkaloid compounds, particularly those of the Catharantus (Vinca) family, from an indole unit, such as cantharanthine, and a dihydroindole unit, such as vindoline. A multi-step process is disclosed including the steps of (1) of 1,4-reduction of a first dimeric iminium intermediate to an enamine compound by reaction with a 1,4-dihydropyridine compound; (2) oxidative transformation of the resulting enamine to a second iminium intermediate under controlled aeration; (3) reduction of the second iminium intermediate to form the target dimer alkaloid compounds. The entire process can be conducted in a one-pot operation to obtain the target compounds without isolation of the intermediates.
Owner:THE UNIV OF BRITISH COLUMBIA
1, 2-Dihydropyridine compounds, manufacturing method thereof and use thereof
The present invention provides a novel compound having an excellent AMPA receptor inhibitory action and / or kainate inhibitory action. A compound represented by the following formula, a salt thereof or hydrates thereof. In the formula, Q indicates NH, O or S; and R1, R2, R3, R4 and R5 are the same as or different from each other and each indicates hydrogen atom, a halogen atom, a C1-6 alkyl group or a group represented by the formula —X-A (wherein X indicates a single bond, an optionally sbutituted C1-6 alkylene group etc.; and A indicates an optionally substituted C6-14 aromatic hydrocarbocyclic group or 5- to 14-membered aromatic heterocyclic group etc.).
Owner:EISIA R&D MANAGEMENT CO LTD
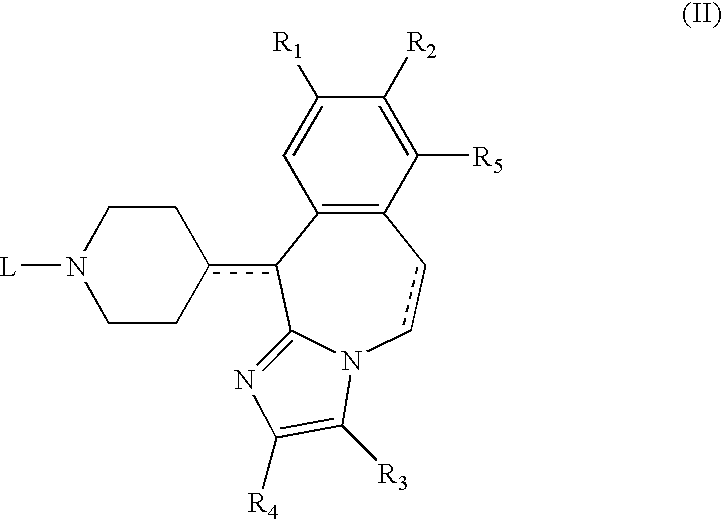
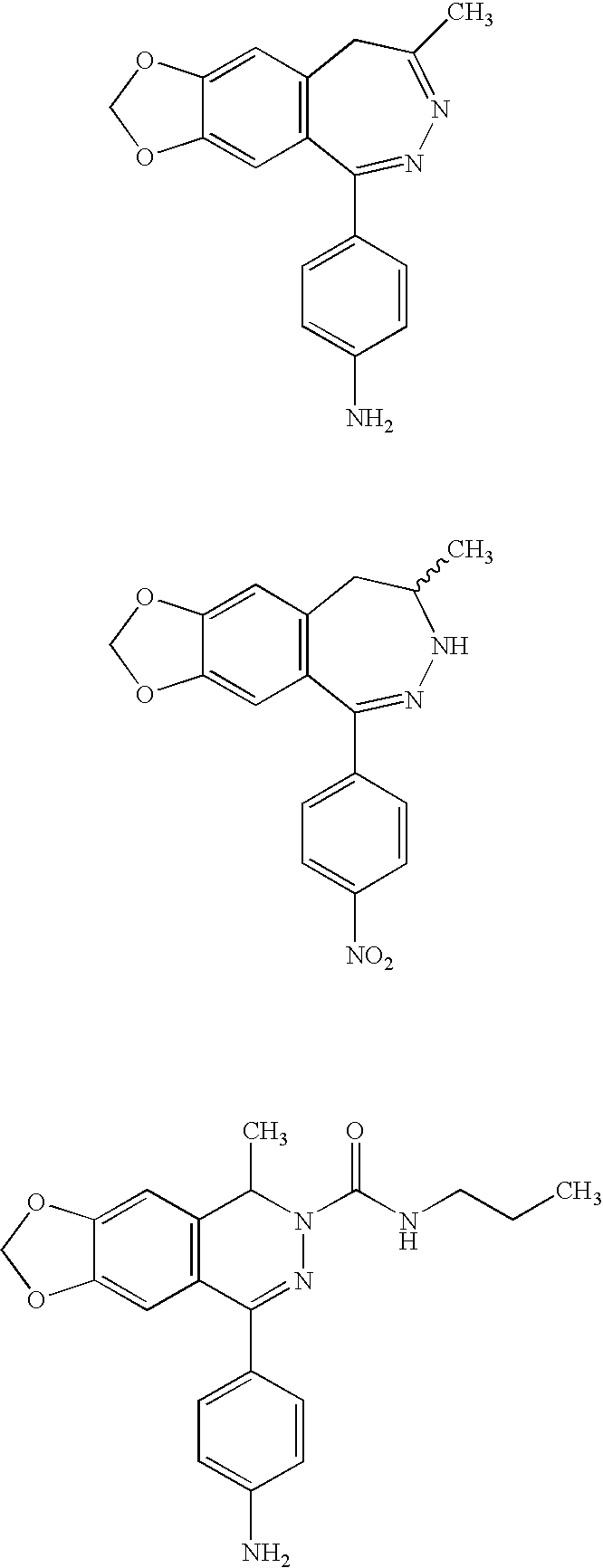
5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones
Owner:F HOFFMANN LA ROCHE & CO AG
Aryl dihydropyridinone and piperidinone MGAT2 inhibitors
Owner:BRISTOL MYERS SQUIBB CO
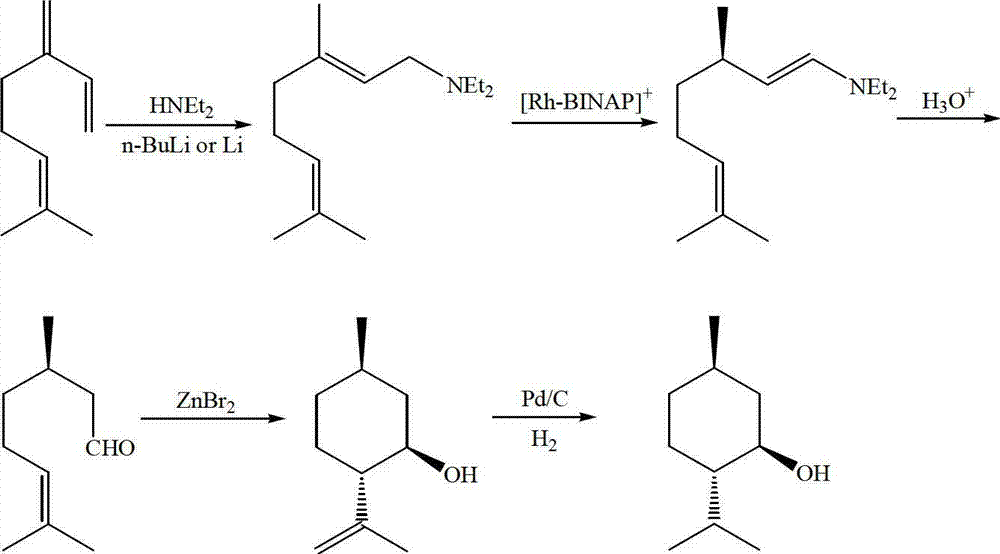
Method for asymmetric synthesis of levorotation menthol
InactiveCN103044204AEasy to makeMild reaction conditionsPreparation by hydrogenationLewis acid catalysisDihydropyridine derivatives
The invention discloses a method for asymmetric synthesis of levorotation menthol. The method comprises the following steps: citral is taken as an initial raw material, a dihydropyridine derivant is taken as a negative hydrogen source, chiral amine serves as a chiral auxiliary agent for catalytic and asymmetric hydrogenization synthesis of dextrorotation citronellal, the dextrorotation citronellal is catalyzed by Lewis acid for ring-closing synthesis of levorotation isopulegol, and the levorotation isopulegol is subject to catalytic hydrogenation to finally produce the levorotation menthol. The total yield of the levorotation menthol produced by adopting the method is larger than 60 percent, and the ee (enantiomeric excess) value is larger than 90 percent. The method has the characteristics of mild reaction conditions, simple synthetic process, simplicity in catalyst preparation, convenience in catalyst recovery and the like and is suitable for large-scale industrial production of levorotation menthol.
Owner:GUANGDONG FOOD IND INST +1
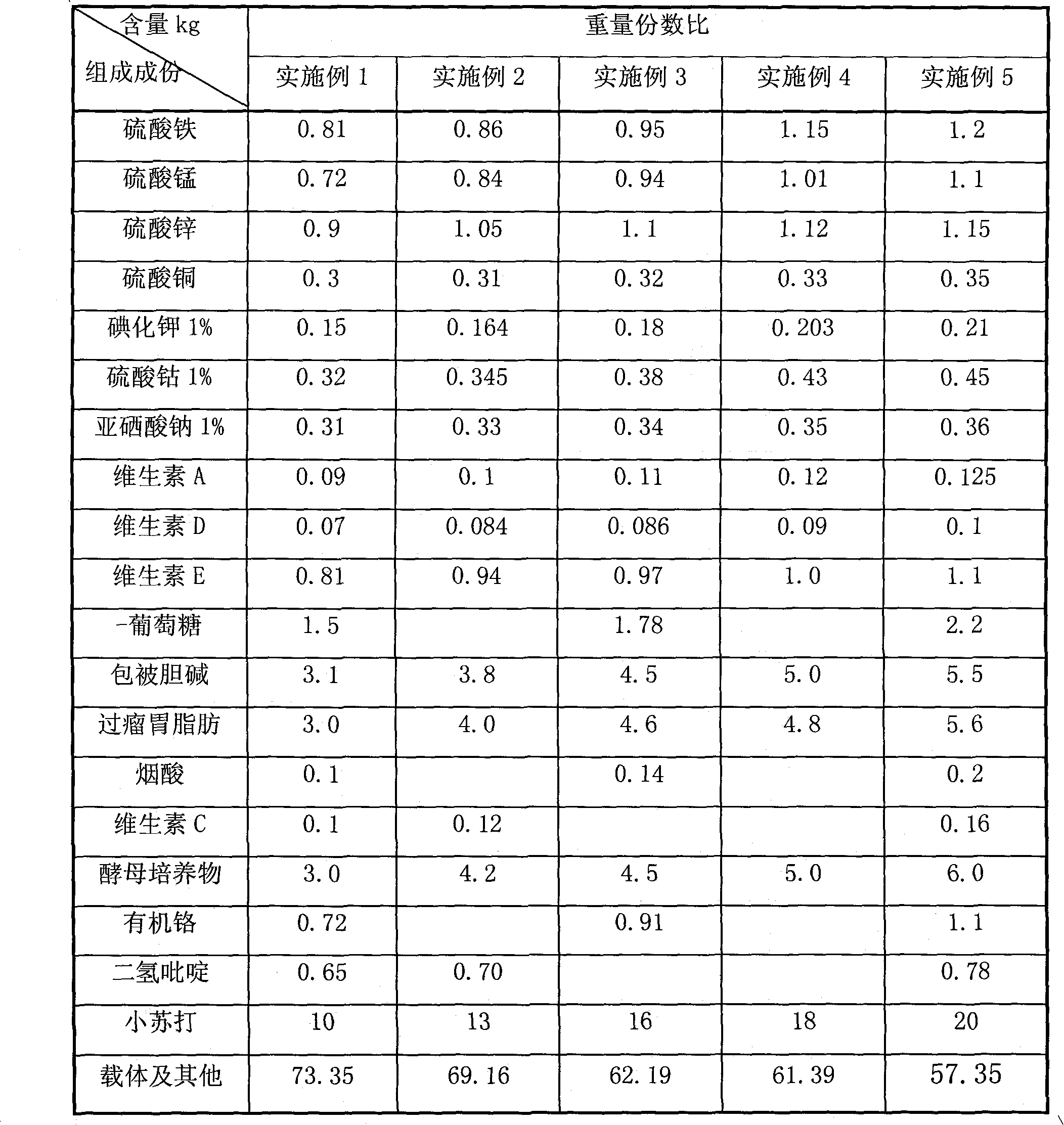
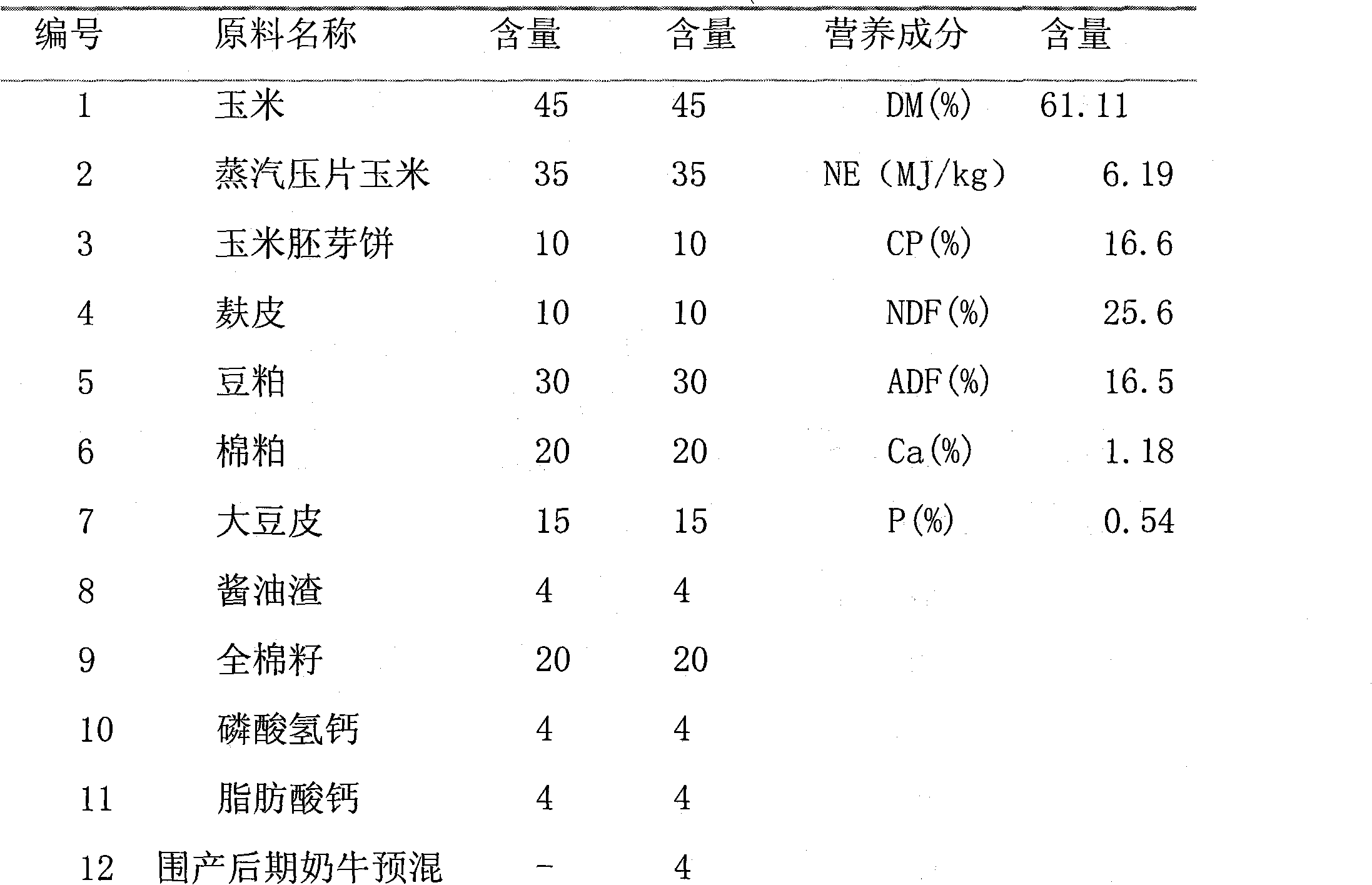
Compound pre-mixed material for dairy cows in post-perinatal period and application thereof
InactiveCN101785529AImprove effective conception rateIncrease estrus rateAnimal feeding stuffSodium bicarbonateIron sulfate
The invention relates to the technical field of animal feed, in particular to a compound pre-mixed material for dairy cows in the post-perinatal period and application thereof. The compound pre-mixed material consists of iron sulfate, manganese sulfate, zinc sulfate, copper sulfate, 1-percent potassium iodide, 1-percent cobalt sulfate, 1-percent sodium selenite, vitamin A, vitamin D, vitamin E, glucose, coated choline, rumen bypass fat, nicotinic acid, vitamin C, yeast cultures, organic chromium, dihydropyridine, sodium bicarbonate, and a carrier. The compound pre-mixed material can be safely and conveniently used, obviously improve an effective conception rate and oestrus rate of the dairy cows, reduce non-pregnant days of the dairy cows, shorten a claving interval of the dairy cows, enforce the body recovery of the dairy cows after delivery, lowers a culling ratio of the dairy cows, prolong a use period of the dairy cows, improve a feeding level of the dairy cows, and increase economic benefits of dairy cow farmers; and the compound pre-mixed material also has the advantages of easy source of raw materials, lower cost, obvious effect, easy popularization and wide application prospect, and is specially used for feeding the dairy cows in the post-perinatal period.
Owner:河北冀丰动物营养科技有限责任公司 +1
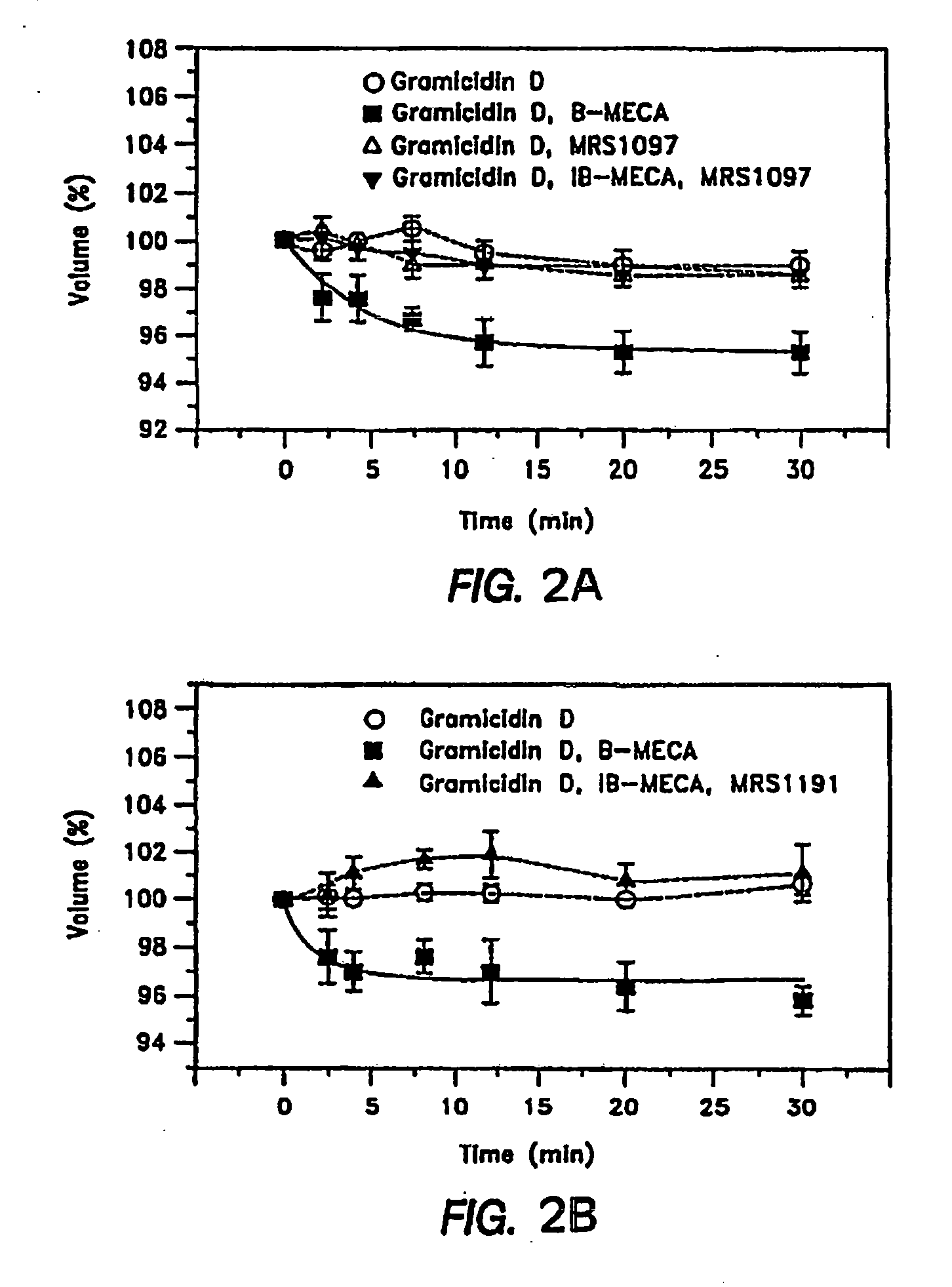
Effective delivery of cross-species a3 adenosine-receptor antagonists to reduce intraocular pressure
InactiveUS20090258836A1Good curative effectImprove actionBiocideSenses disorderPyridiniumNK1 receptor antagonist
Provided are methods for reducing intraocular pressure in an individual having an ocular disorder causing elevated intraocular pressure, such as glaucoma. The method comprises administering to the individual an effective intraocular pressure-reducing amount of a pharmaceutical composition comprising an A3 subtype adenosine receptor (A3AR) antagonist, including dihydropyridine, pyridine, pyridinium salt or triazoloquinazoline, and derivatives thereof expressly having A3AR antagonist activity, including, e.g., the nucleoside-based A3AR antagonist, MRS-3820. Further provided is a method for ensuring the delivery of a topically administered therapeutic composition for reducing intraocular pressure, wherein the method expressly requires physically opening a channel through the corneal barrier of the patient's eye by a microneedle or micropipette to permit transport of the topical composition to the anterior chamber of the eye.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE US SEC DEPT OF THE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











![NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE) NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/51459381-3e84-484d-8432-cae57fc7b999/US20070259898A1-20071108-C00001.png)
![NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE) NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/51459381-3e84-484d-8432-cae57fc7b999/US20070259898A1-20071108-C00002.png)
![NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE) NOVEL 2-AMINO-3,4-DIHYDRO-PYRIDO[3,4-D]PYRIMIDINE DERIVATIVES USEFUL AS INHIBITORS OF beta-SECRETASE (BACE)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/51459381-3e84-484d-8432-cae57fc7b999/US20070259898A1-20071108-C00003.png)






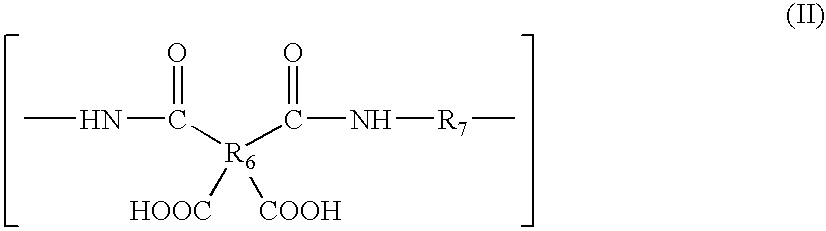

![5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones 5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/476e7a16-39ad-42c6-9bea-51a9750284a8/US07098332-20060829-C00001.png)
![5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones 5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/476e7a16-39ad-42c6-9bea-51a9750284a8/US07098332-20060829-C00002.png)
![5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones 5,8-Dihydro-6H-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/476e7a16-39ad-42c6-9bea-51a9750284a8/US07098332-20060829-C00003.png)