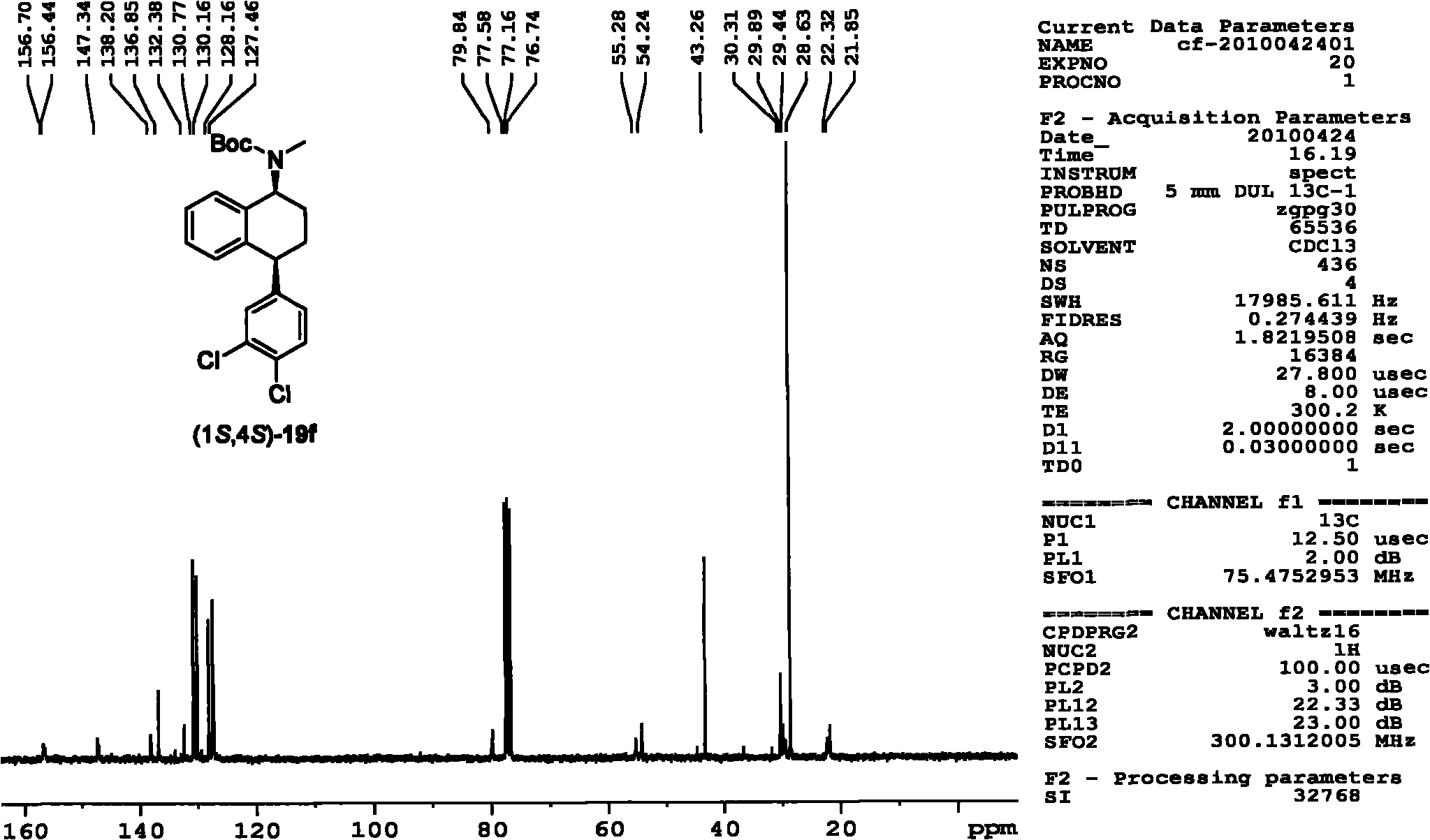
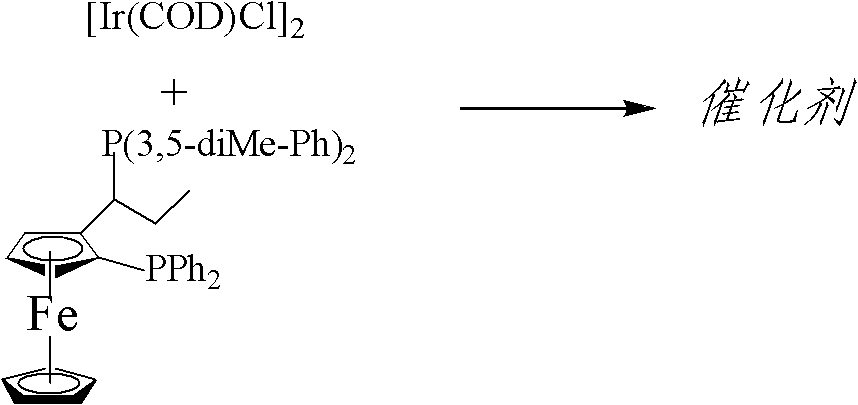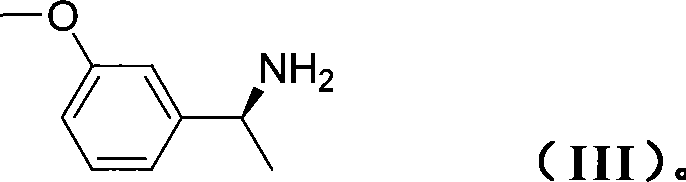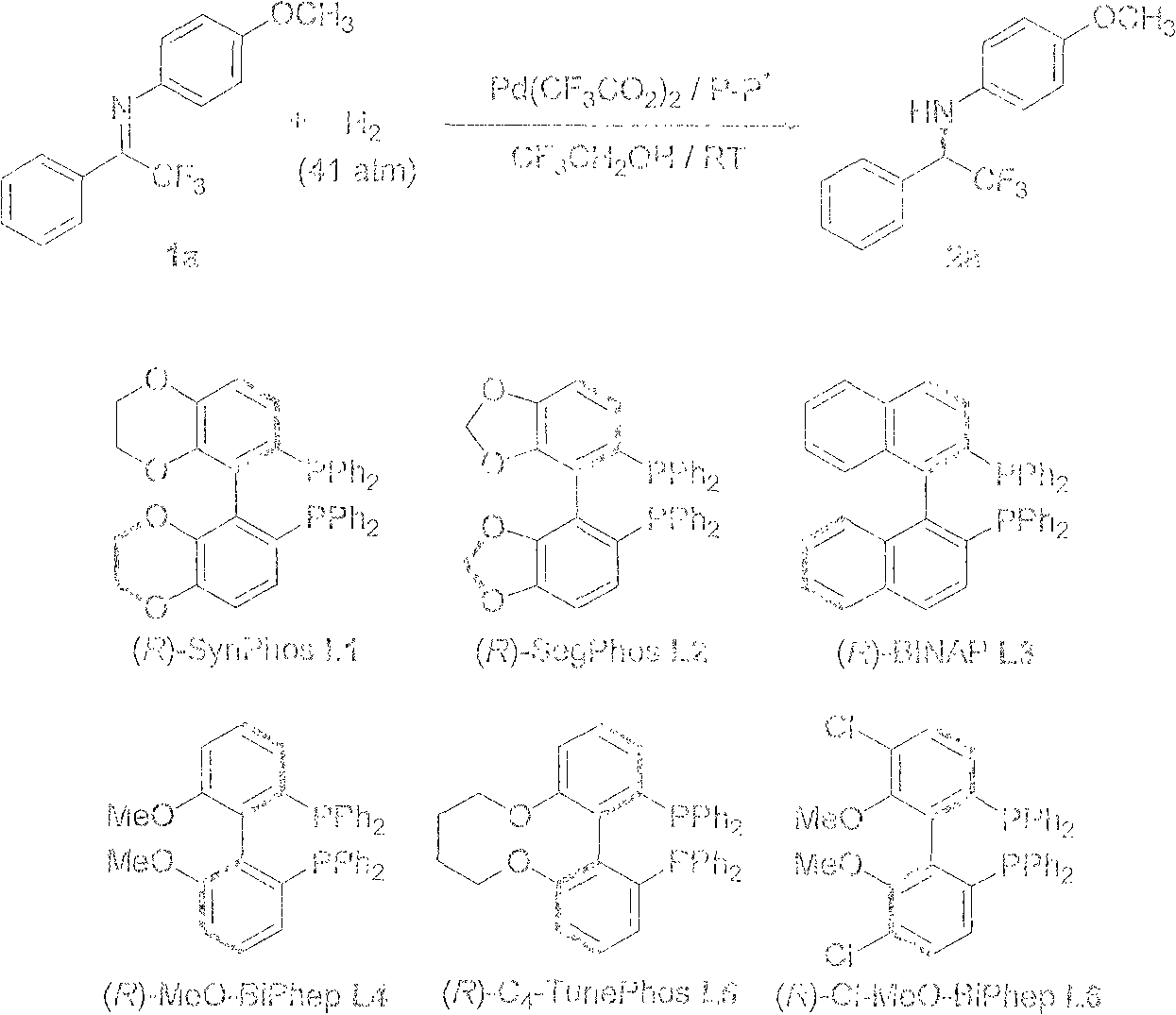Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
383 results about "Chiral amine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
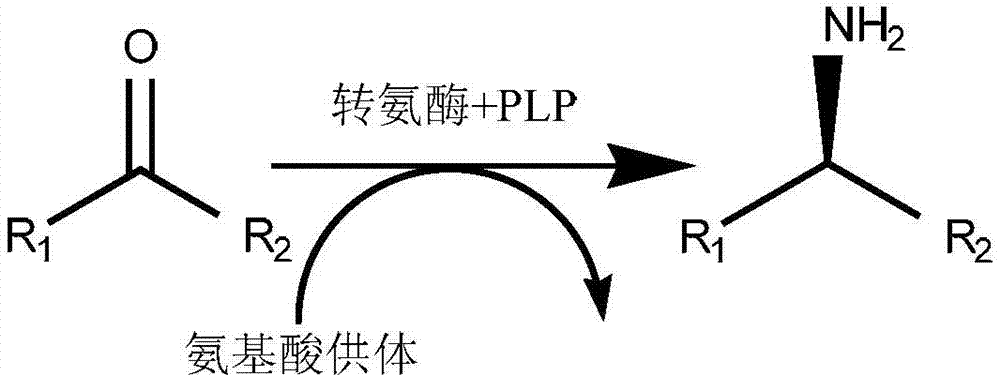
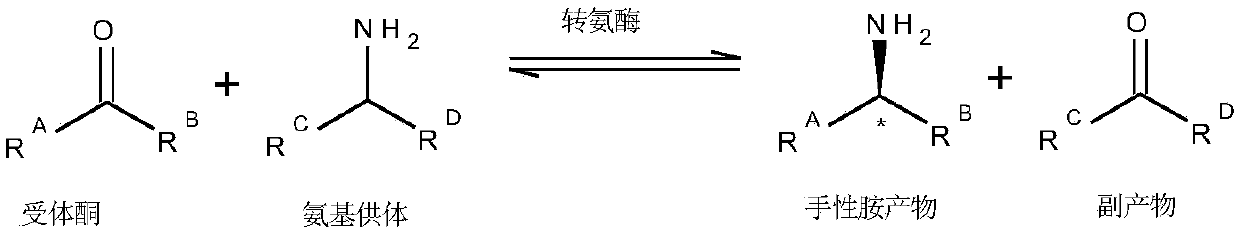
Α-Chiral amines, organic compounds in which the core amine moiety (-NH2) is connected to a chiral carbon atom, constitute the most widely used intermediates for the production of active pharmaceutical ingredients, fine chemicals and agrochemicals.
Preparation method for synthesizing apremilast intermediate
ActiveCN104447445AStable and cheapReaction is easy to controlOrganic chemistryOrganic compound preparationPhenyl groupSulfone
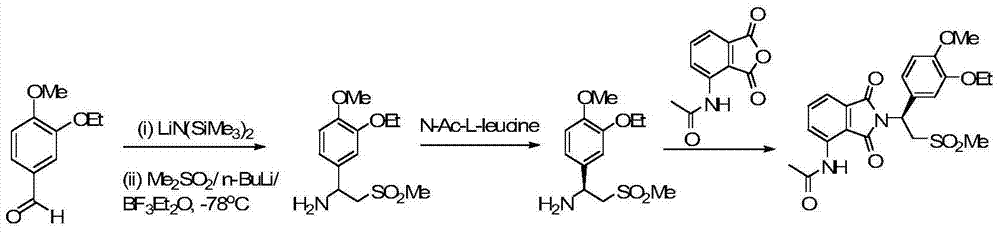
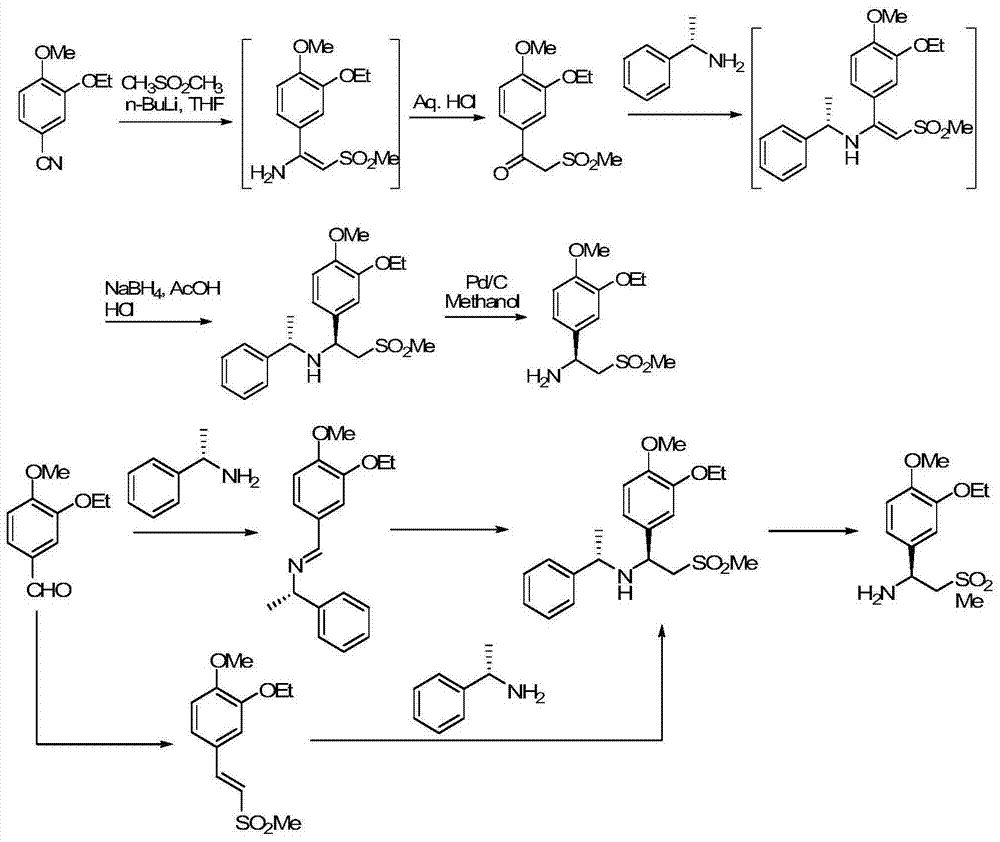
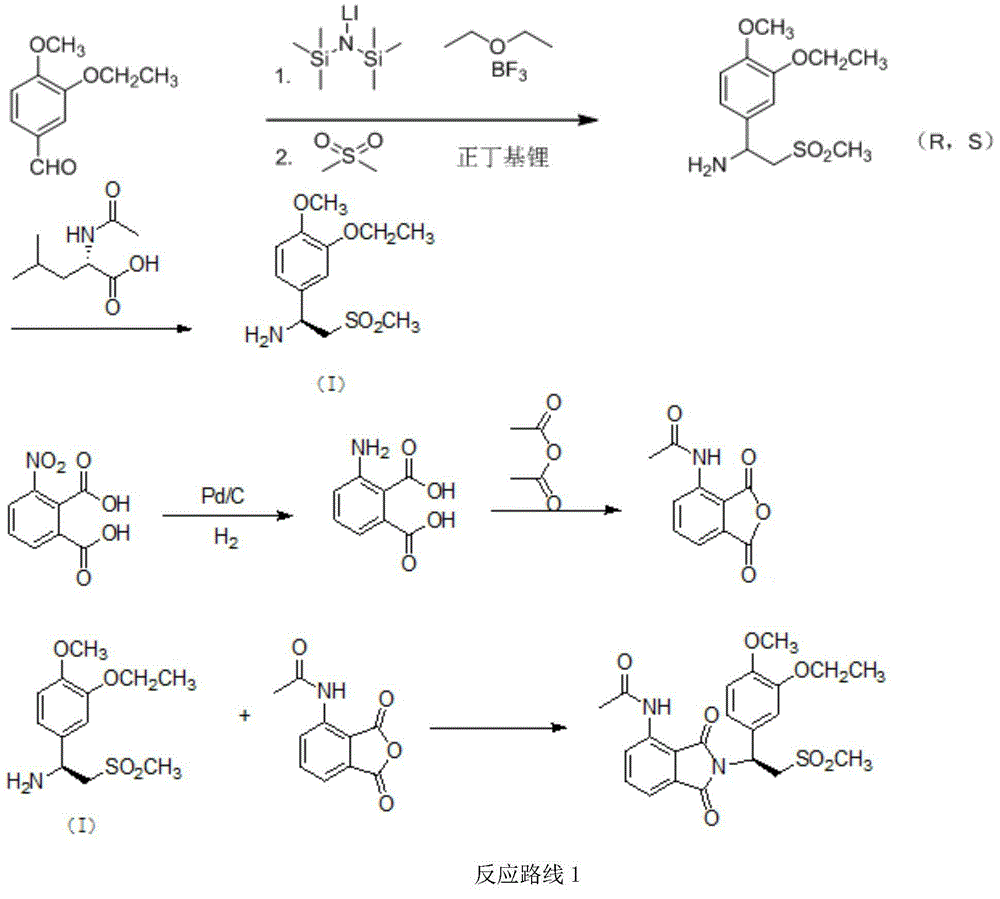
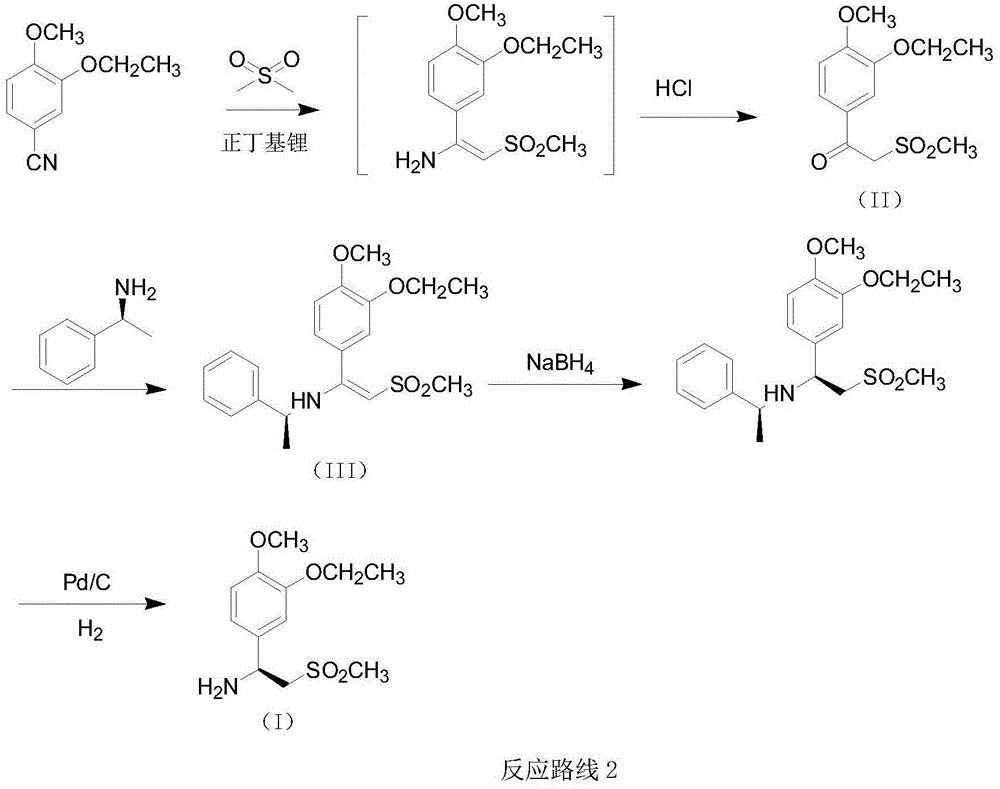
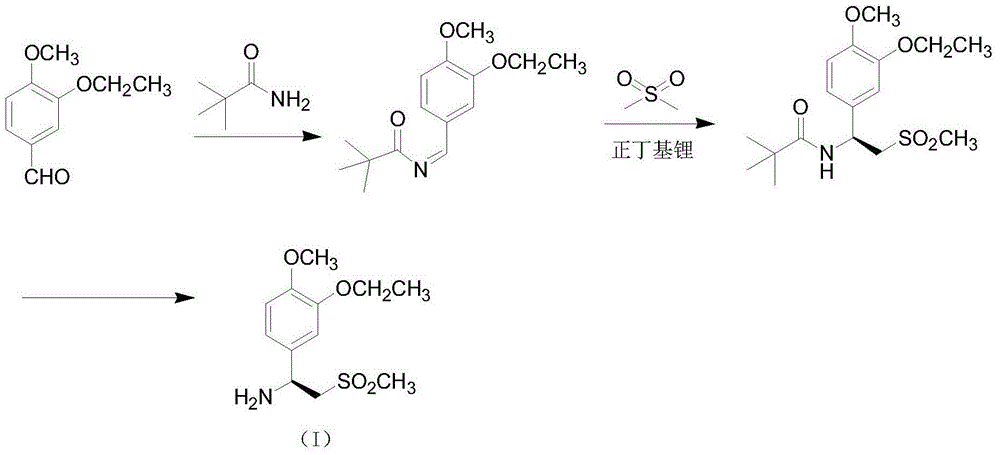
The invention relates to a preparation method for synthesizing an apremilast intermediate. The preparation method comprises the following steps of: carrying out condensation reaction on 3-ethoxyl-4-methoxyl-benzoate and dimethyl sulfone under an alkaline condition to generate 2-(3-ethoxy-4-methoxyphenyl)-1-methylsulfonyl acetone; reacting the compound II and chiral amine in the presence of an acidic catalyst to obtain 1-N-substituted amino-1-(3-ethoxyl-4-methoxyl) phenyl-2-methylsulfonyl ethylene (III), and directly hydrogenating the obtained compound III in the presence of a hydrogenation catalyst without separating the compound III to obtain a product (S)-1-(3-ethoxyl-4-methoxyl) phenyl-2-methanesulfonyl ethylamine (I), namely the apremilast intermediate, wherein the apremilast intermediate can be further prepared into N-acetyl L-leucinate. The invention also provides a preparation method of apremilast. The preparation method disclosed by the invention has the advantages of simple process flow, safety, environmental friendliness and low cost and is favorable to clean industrialized production.
Owner:XINFA PHARMA
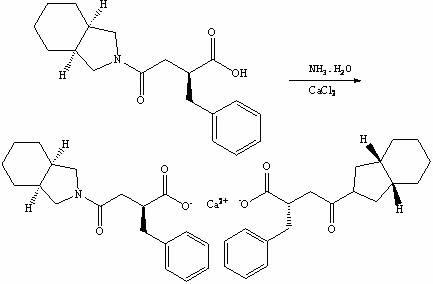
Preparation method of mitiglinide calcium
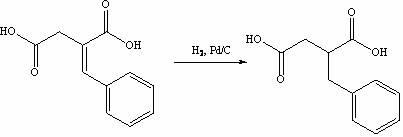
InactiveCN102101838ASave cis-hexahydroisoindoleHigh chiral separationOrganic chemistryAcetic anhydrideBenzaldehyde
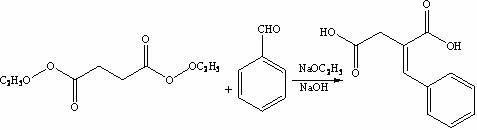
The invention discloses a preparation method of mitiglinide calcium. The method comprises the following steps of: performing Stobble condensation on diethyl succinate and benzaldehyde serving as raw materials in ethanol by using sodium alcoholate; hydrolyzing to obtain toluenyl butane diacid; performing catalytic hydrogenation on the toluenyl butane diacid to obtain DL-2-benzyl butane diacid; resolving the DL-2-benzyl butane diacid with (R)-chiral amine to obtain (S)-2-benzyl butane diacid; reacting the (S)-2-benzyl butane diacid under the action of acetic anhydride to obtain acid anhydride ; reacting the obtained acid anhydride with cis-hexahydroisoindole to obtain mitiglinide acid; and reacting the mitiglinide acid with calcium chloride and ammonia water to generate a mitiglinide calcium bihydrate. The preparation method of the mitiglinide calcium has the advantages of saving of cis-hexahydroisoindole serving as a raw material and high chiral separating degree.
Owner:周玉莲
Enantioselective resolution process for arylpropionic acid drugs from the racemic mixture
InactiveUS6093830AOrganic compound preparationOptically-active compound separationCelsius DegreeOrganic solvent
The invention relates to a novel non-catalytic enantioselective resolution process for the separation of enantiomer of arylpropionic acid drugs from the racemic mixture, which comprises dissolving the racemic mixture of the said drug an organic solvent, reacting this solution with an aqueous phase containing an ionic surfactant with or without a suitable co-surfactant, and an electrolyte in microemulsion / micellar / biphasic medium, reacting this mixture with an appropriate chiral amine at a temperature in the range of 0 to 70 degrees Celsius to obtain a diastereomeric salt, acid hydrolysing the diastereomeric salt to result in the pure enantiomer of the drug which is extracted by known methods.
Owner:COUNCIL OF SCI & IND RES
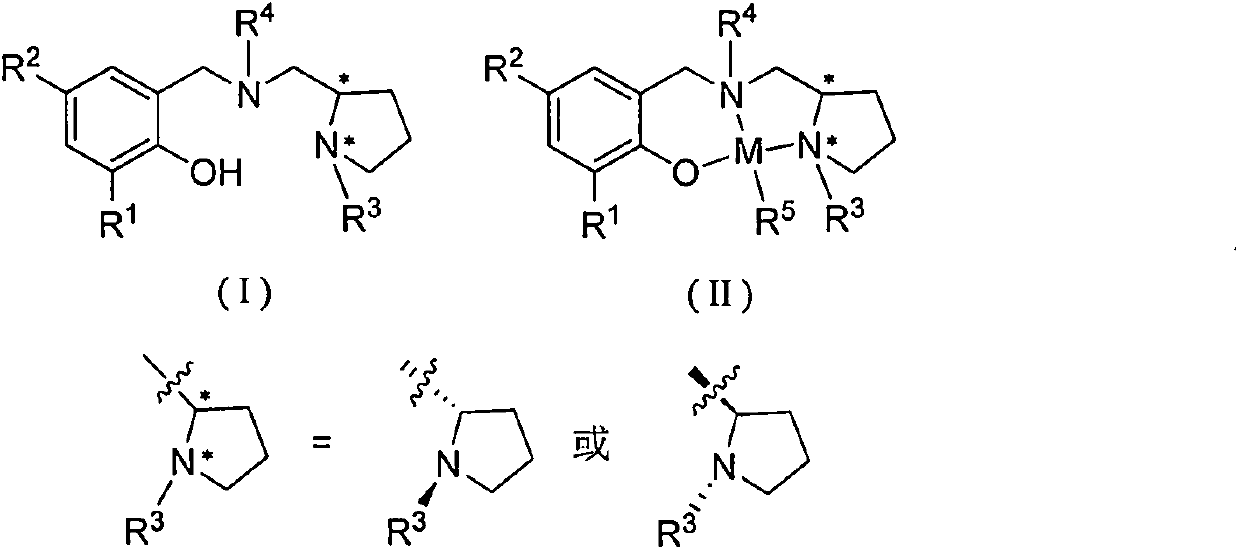
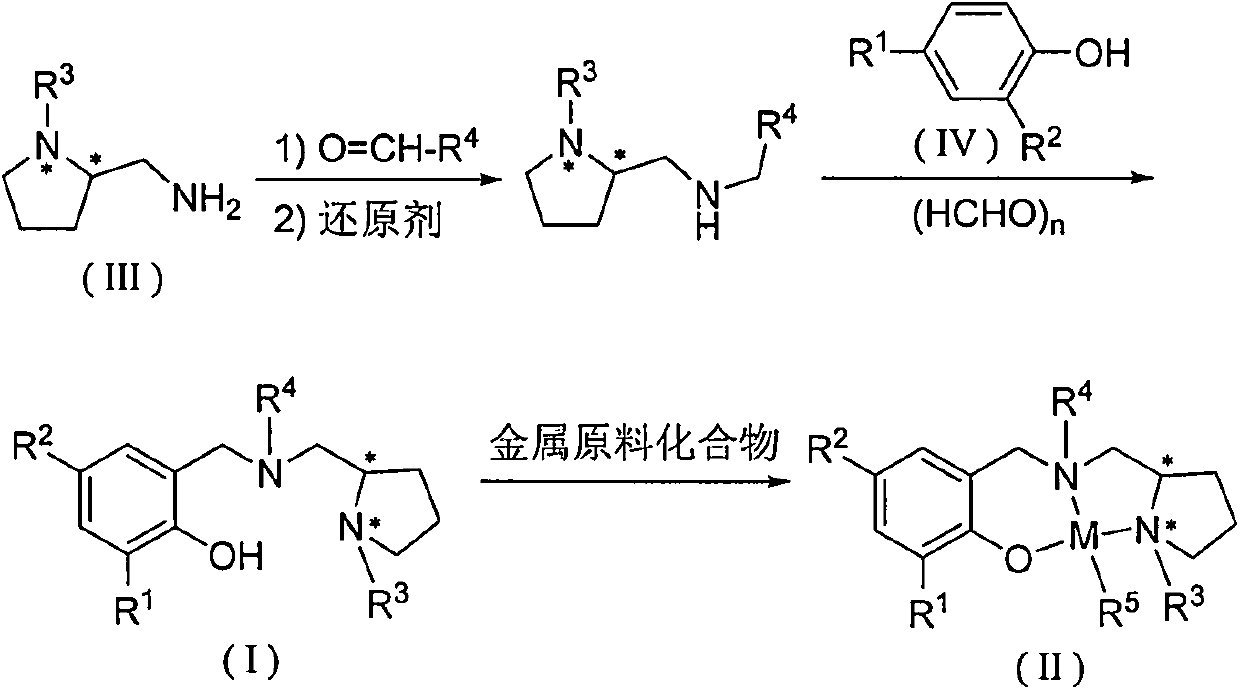
Chiral amino phenoxyl zinc and magnesium compound, and preparation method and application thereof
InactiveCN103787943ALigand raw materials are readily availableLigand raw materials are convenientGroup 4/14 element organic compoundsGroup 2/12 organic compounds without C-metal linkagesPolyesterLactide
The invention discloses a chiral amino phenoxyl zinc and magnesium compound, a preparation method of the chiral amino phenoxyl zinc and magnesium compound, and application of the chiral amino phenoxyl zinc and magnesium compound in ring opening polymerization of catalytic lactone with high activity and high selectivity. The preparation method comprises the following steps: directly reacting a neutral ligand with a metal raw material compound in an organic medium; performing filtration, concentration and re-crystallization to obtain a target compound. The chiral amino phenoxyl zinc and magnesium compound is an efficient lactone ring opening polymerization and can be applied to polymerization reaction of catalytic lactide and the like; particularly, high-isotacticity or high-heterotacticity polylactic acid can be obtained for racemization lactide. The chiral amino phenoxyl zinc and magnesium compound has the obvious advantages that raw materials are easily obtained; a synthetic route is simple; high product yield, high catalytic activity and high stereo selectivity are realized; a high-regularity and high-molecular-weight polymer material can be obtained; requirements of industrial departments can be met. A structural formula is shown as (img file='DSA00000897420700011.TIF' wi='860'he='608' / ).
Owner:EAST CHINA UNIV OF SCI & TECH
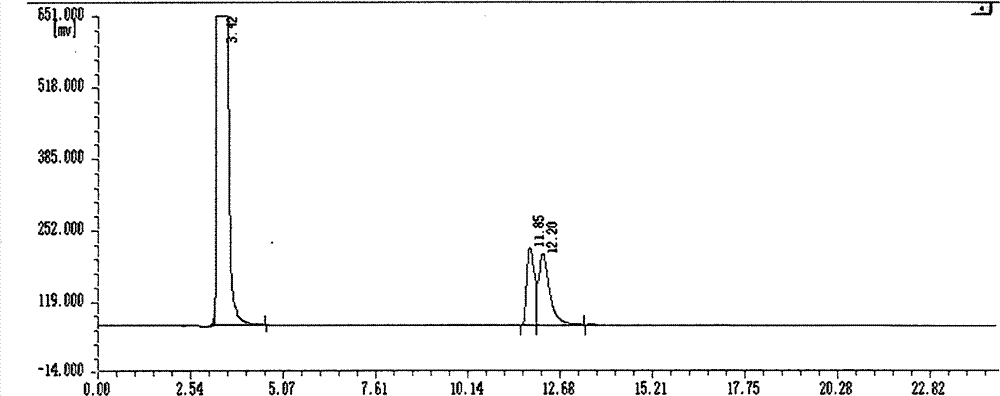
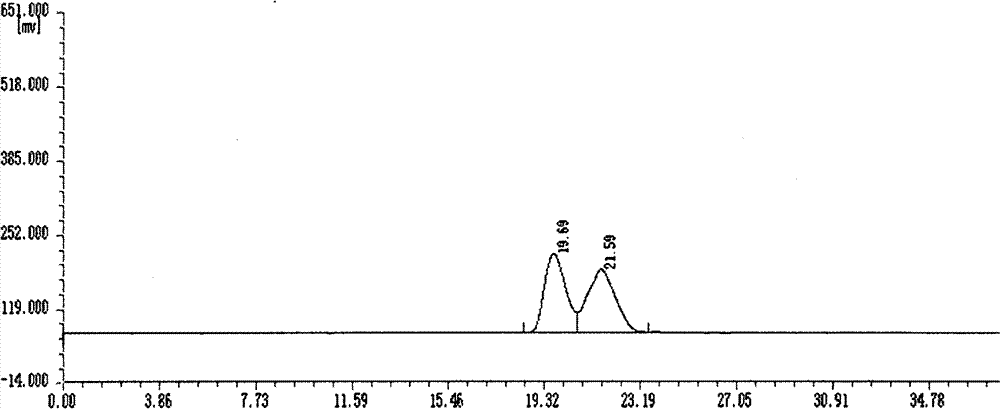
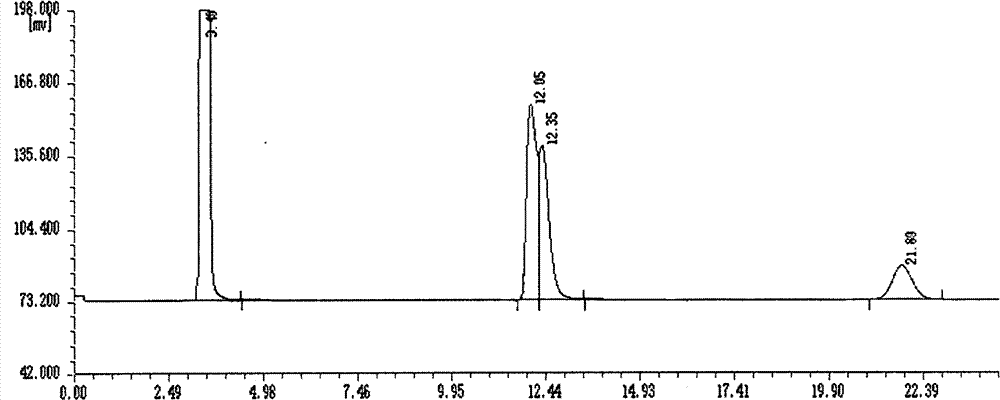
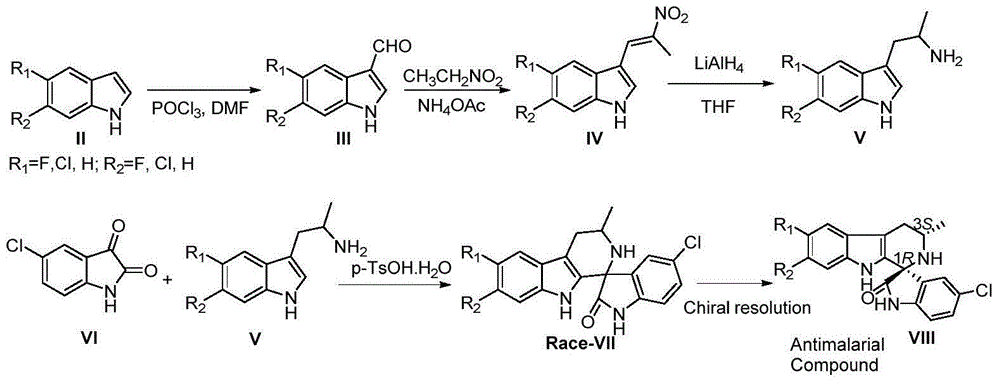
Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane
ActiveCN103044418AReduce processing costsShort processOrganic chemistryBulk chemical productionArylHydrogen
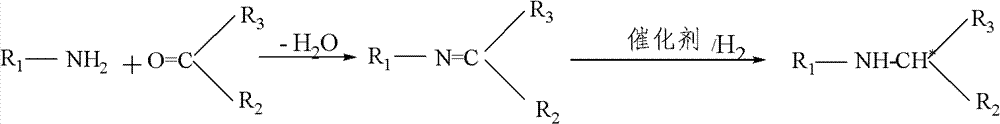
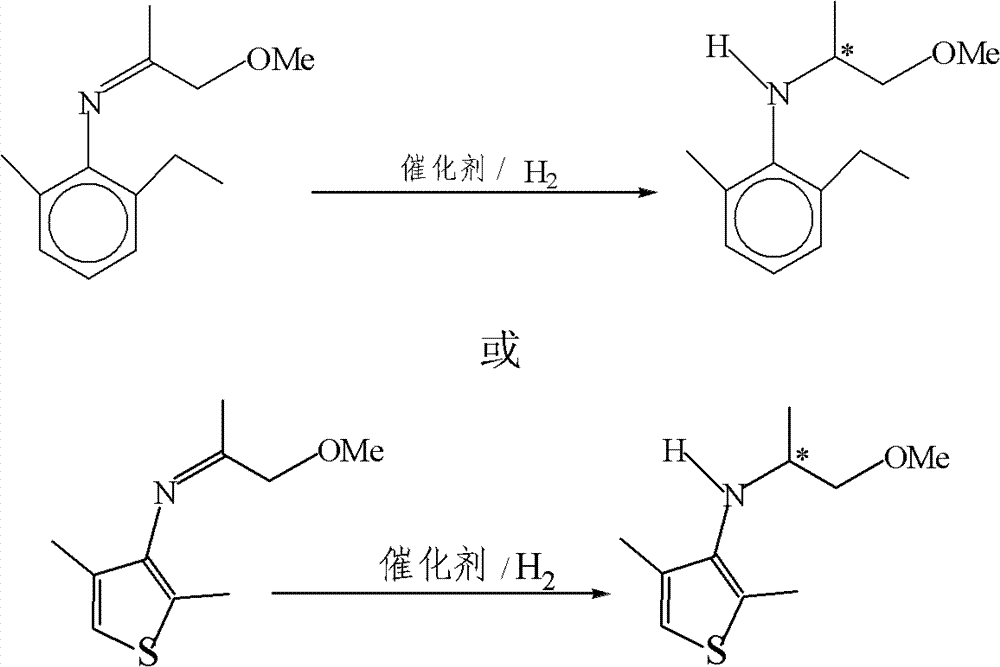
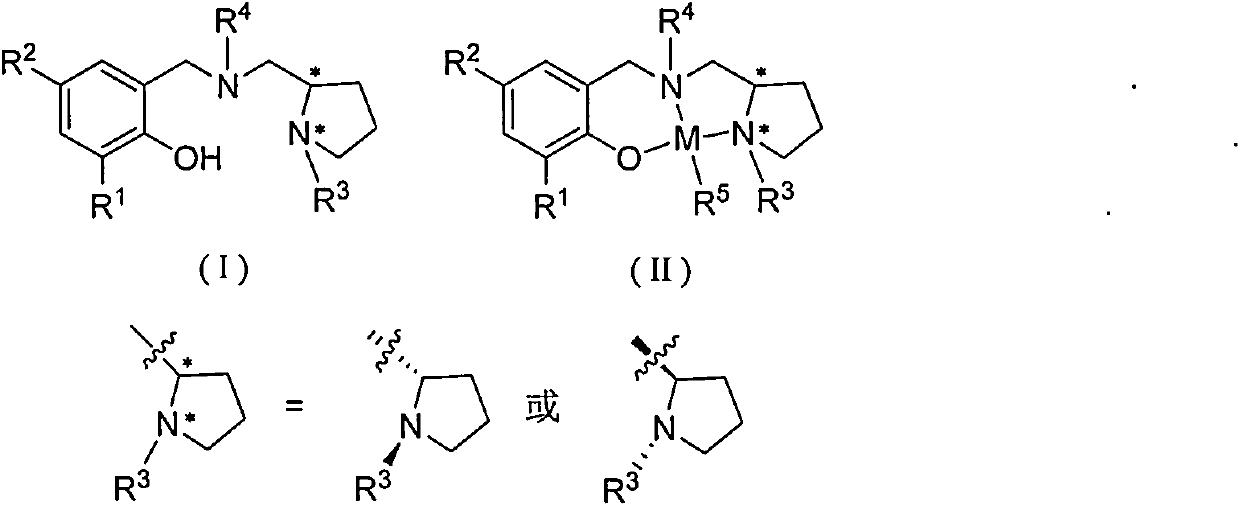
The invention relates to an asymmetric synthesis method of (S,S)-2,8-diazabicyclo[4,3,0] nonane (I) as a chiral intermediate of moxifloxacin. The asymmetric synthesis method comprises the steps of: firstly, carrying out a dewatering reaction on a 3-pyrrolidone compound (shown as formula III or IV) and chiral amine, reducing a dewatered product to obtain enantiopure compounds shown as a formula (II) under different reducing conditions, and carrying out intramolecularly cyclization and removal of chiral prothetic groups on the compounds shown as the formula (II) to obtain (S,S)-2,8-diazabicyclo[4, 3, 0] nonane (I). The invention also relates to 3-pyrrolidone shown as a formula (III) or (IV) and a preparation method thereof, wherein R is an amino protection group; in the formula (II), * is a chiral center mark of the chiral prothetic groups, R1 and R2 are respectively C1-4 alkyl, C1-4 hydroxyl or carboxyl substituted C1-4 alkyl, aryl, carboxyl, C1-4 carbalkoxy or carbamoyl, when Z is H2, Y is chlorine, bromine, iodine or hydroxyl sulphonate, when Z is O, Y is hydrogen, hydroxyl or C1-4 alkoxy; in the formula (III), R3 is hydrogen or C1-4 alkyl; and in the formula (IV), Y is chlorine, bromine, iodine or hydroxyl sulphonate.
Owner:ABA CHEM SHANGHAI
Preparation method of mono-chiral metallic organic frame material with function of splitting chiral amine
InactiveCN103113411AShort reaction timeEasy to operateIon-exchange process apparatusOxygen-containing compound preparationManganesePhenyl group
The invention provides a preparation method of a mono-chiral metallic organic frame material with a function of splitting chiral amine. The method comprises the following steps of: dissolving manganese salt and mono-chiral ligand (S)-3,3'-di-tert-butyl-5,5'-di(3,5-dicarboxylphenyl)-6,6'-dimethyl-2,2'-dihydroxyl-1,1'-bipheny into methanol and N,N-dimethyl formamide according to certain proportions, stirring for 10 minutes and then heating up to 80-85 DEG C, reacting for about 24 hours to generate colorless needle-like crystals, and then washing with aether to obtain the structurally stable mono-chiral metallic organic frame material with the function of splitting the chiral amine. The preparation method provided by the invention has the characteristics that the preparation condition is mild, the operation is simple, the separation ee value of the chiral amine is higher than 90%, and the chiral amine is reusable.
Owner:SHANGHAI JIAO TONG UNIV
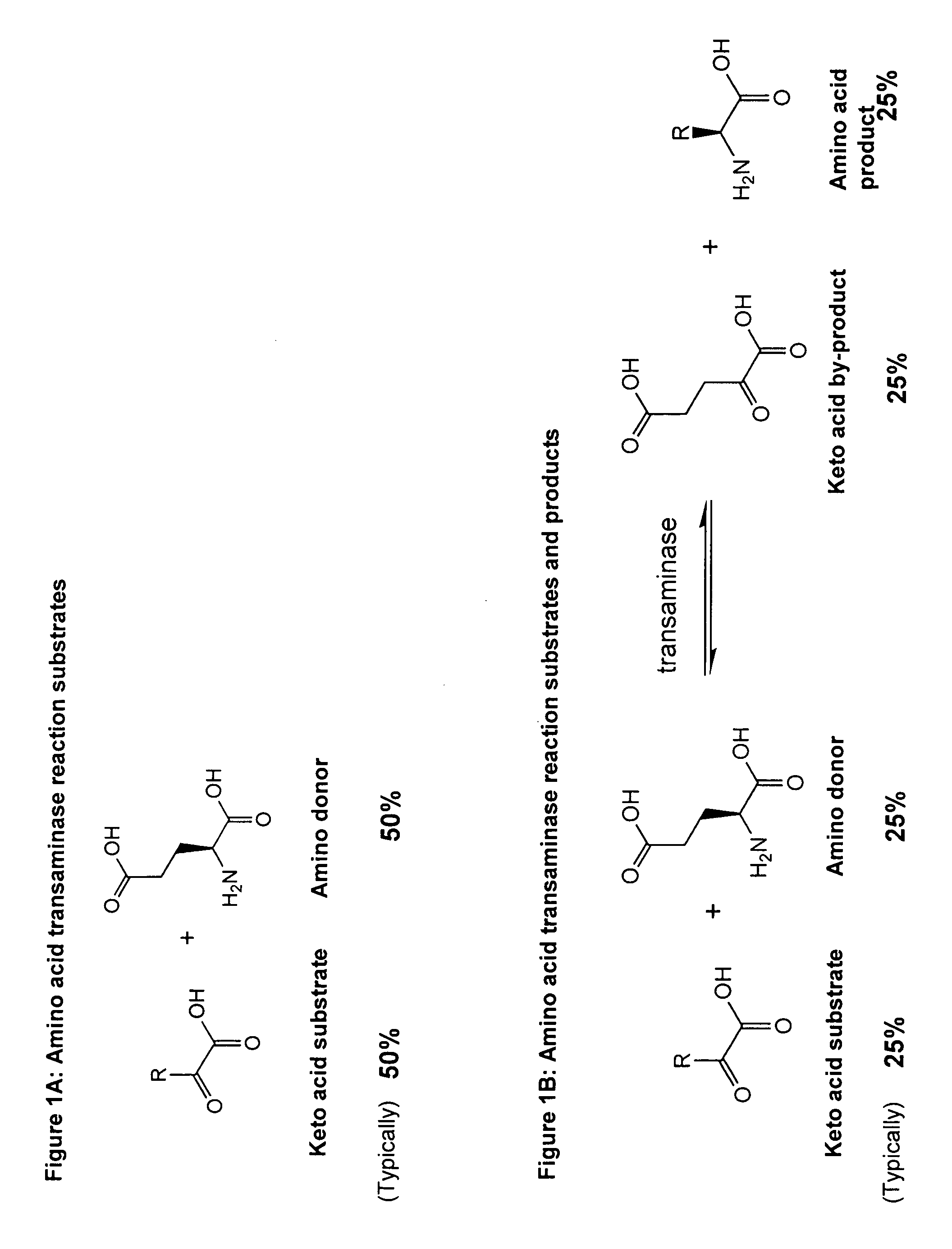
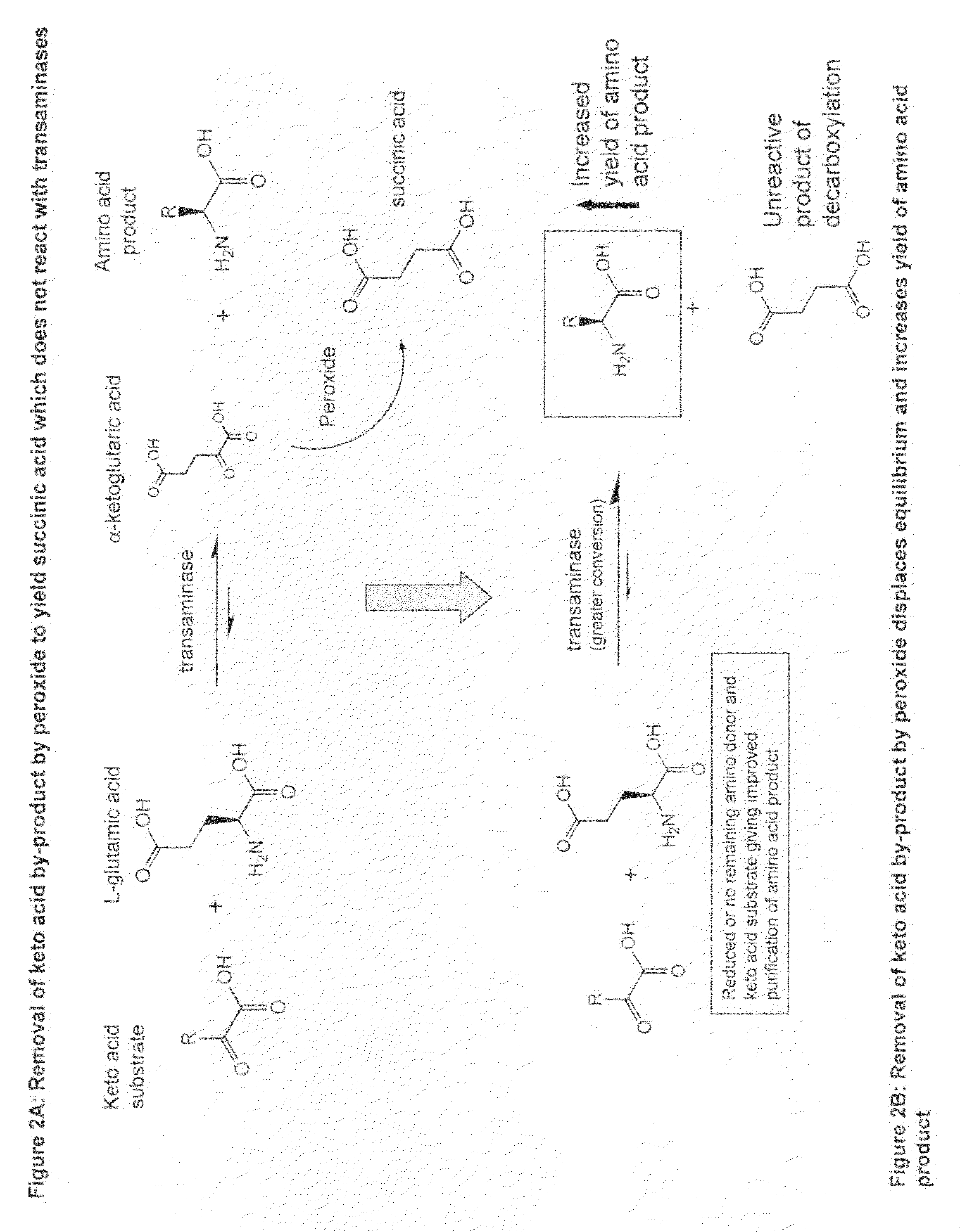
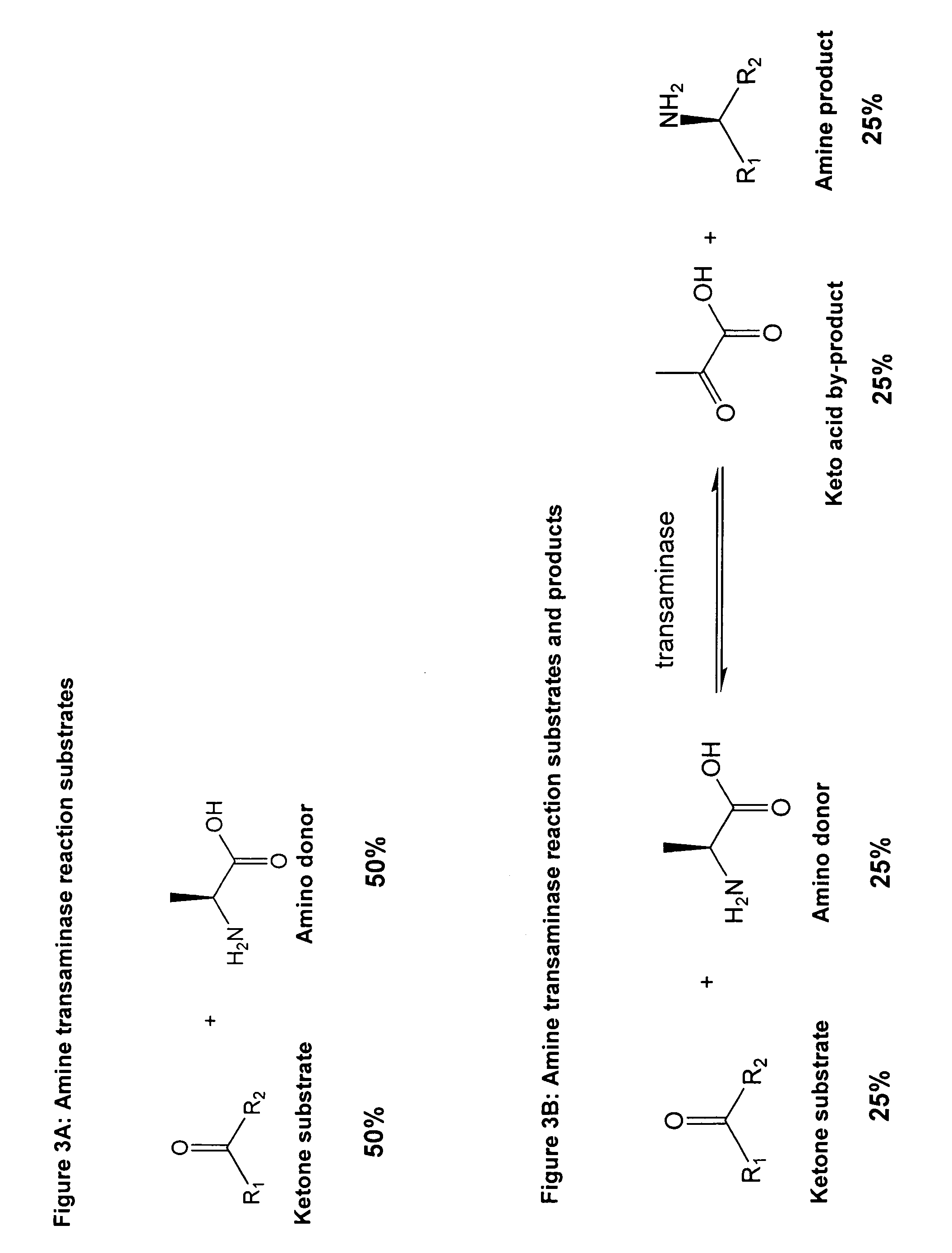
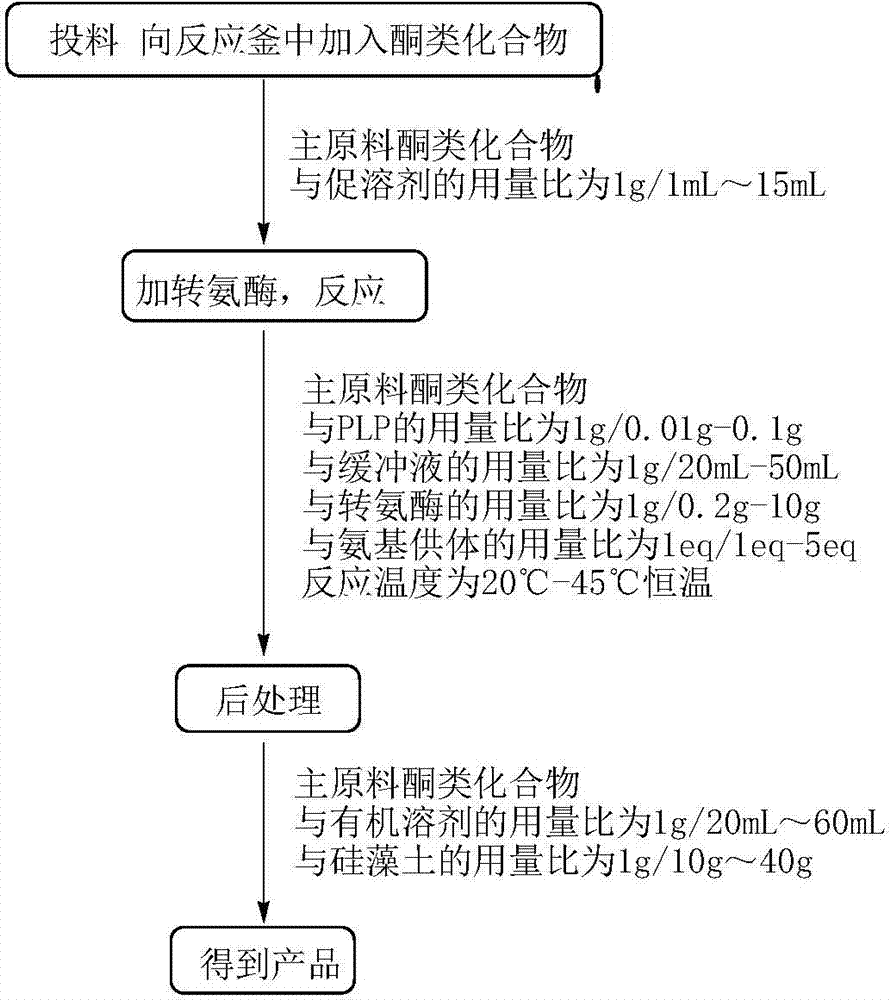
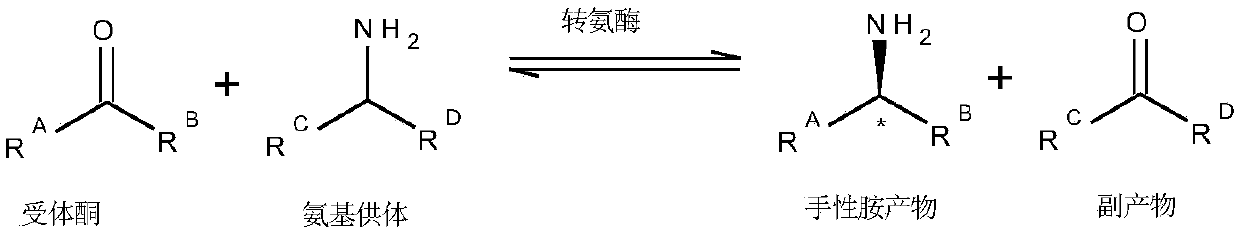
Method to increase the yield and improve purification of products from transaminase reactions
A process for producing high yields of enantioselective amino acids and chiral amines by reacting a keto acid or ketone and an amino acid donor in the presence of a transaminase biocatalyst to produce a keto acid by-product and an amino acid or amine product. Further reacting the keto acid by-product with a peroxide to increase the yield of additional amino acid or amine product.
Owner:RICHMOND CHEM CORP
Synthesis method of [3aS, 6aR]-1,3-dibenzyl-tetrahydro-4H-fruo [3,4-d]-imidazolyl-2,4 [1H]-diketone [I]
The present invention provides the synthesis process of (3aS, 6aR)-1, 3-dibenzyl-tetrahydro-4H-furo[3, 4-d]-imidazolyl-2, 4(1H)-dione. Compound 1, 3-dibenzyl imidazolidine-2-one-2H-furo[3, 4-d]-imidazolyl-2, 4, 6-trione is made to produce enantiotropic selective ring-opening reaction with fatty alcohol and arylkyl alcohol under the catalysis of chiral amine to produce (4S, 5R)-1, 3-dibenzyl-5-alkoxycarbonyl-2-oxyimidazolidine-4-carboxylic acid, which is then reduced with borohydride and ring-closed under Lewis acid catalysis inside organic solvent to produce (3aS, 6aR)-1, 3-dibenzyl-tetrahydro-4H-furo[3, 4-d]-imidazolyl-2, 4(1H)-dione in the total yield over 88 %. The said process uses easy-to-obtain material, has mild reaction condition and is suitable for industrial production.
Owner:FUDAN UNIV
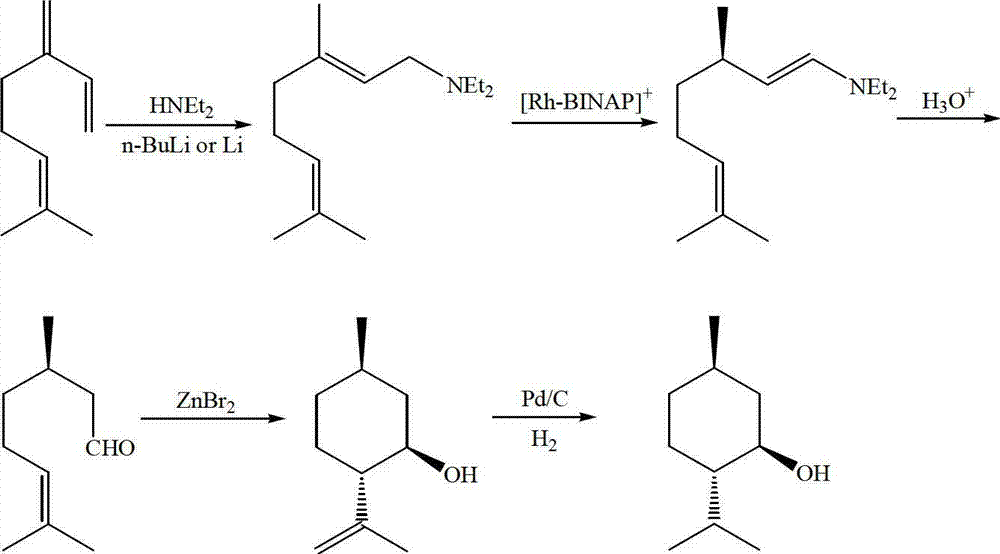
Method for asymmetric synthesis of levorotation menthol
InactiveCN103044204AEasy to makeMild reaction conditionsPreparation by hydrogenationLewis acid catalysisDihydropyridine derivatives
The invention discloses a method for asymmetric synthesis of levorotation menthol. The method comprises the following steps: citral is taken as an initial raw material, a dihydropyridine derivant is taken as a negative hydrogen source, chiral amine serves as a chiral auxiliary agent for catalytic and asymmetric hydrogenization synthesis of dextrorotation citronellal, the dextrorotation citronellal is catalyzed by Lewis acid for ring-closing synthesis of levorotation isopulegol, and the levorotation isopulegol is subject to catalytic hydrogenation to finally produce the levorotation menthol. The total yield of the levorotation menthol produced by adopting the method is larger than 60 percent, and the ee (enantiomeric excess) value is larger than 90 percent. The method has the characteristics of mild reaction conditions, simple synthetic process, simplicity in catalyst preparation, convenience in catalyst recovery and the like and is suitable for large-scale industrial production of levorotation menthol.
Owner:GUANGDONG FOOD IND INST +1
Kinetic resolution method of chiral amine
InactiveCN102766672AImprove performanceMeet various requirements of purityFermentationOrganic solventReaction temperature
The invention discloses a kinetic resolution method of chiral amine, comprising the following steps of: adding chiral amine and acyl donor at the mol ratio of 1:0.5-3 into an organic solvent reaction system, adding lipase which accounts for 10-80 wt% of the weight of chiral amine, and reacting at the reaction temperature of 30-70 DEG C for 12-48 hours to obtain amide with the conversion rate of 50% and e.e value being greater than 99%. According to the invention, resolution of chiral amine can be mildly realized under the condition of enzyme catalysis; and simultaneously, the product has good optical purity, high yield and great application values.
Owner:六安佳诺生化科技有限公司
Recombinant transaminase as well as preparation method and application of recombinant transaminase
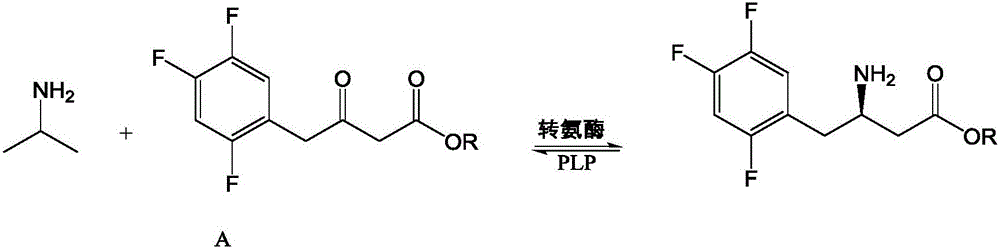
ActiveCN106801043AStrong specificityHigh catalytic activityOrganic chemistryBacteriaRegioselectivityMutant
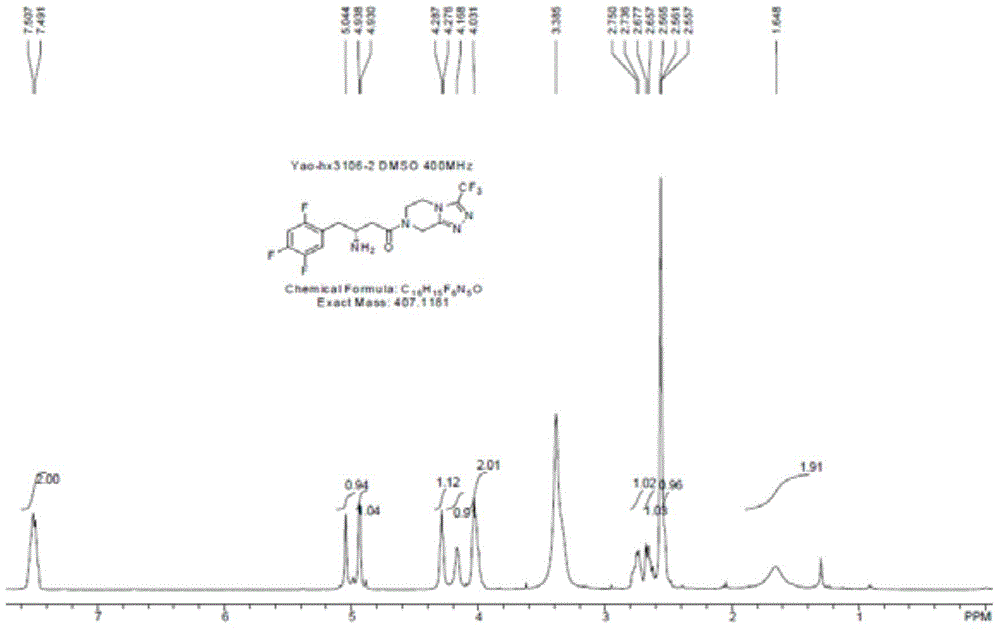
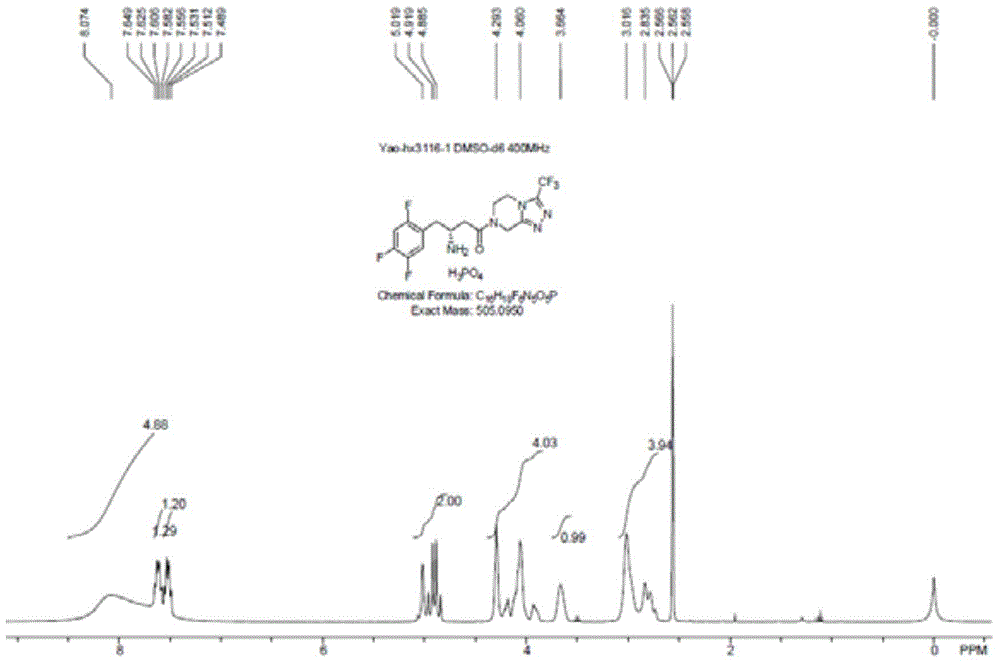
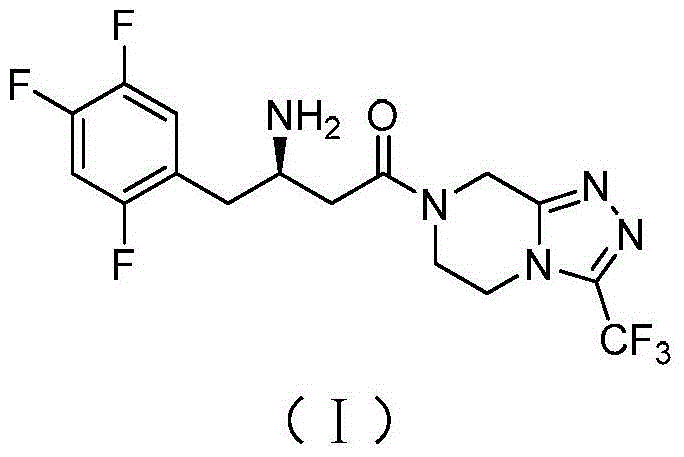
The invention discloses recombinant transaminase as well as a preparation method and application of the recombinant transaminase. An amino acid sequence of a high-activity recombinant transaminase mutant is as shown in SEQ ID NO.2 and a coding gene is as shown in SEQ ID NO.1. The method for preparing the recombinant transaminase compries the steps of fermenting and cultivating a gene engineering bacterium containing the coding gene, and collecting and preparing the recombinant transaminase. The recombinant transaminase is applied to asymmetric synthesis of a chiral amine compound, and particularly is applied to synthesis of a sitagliptin midbody (R)-3-amino-4-(2,4,5-trifluoromethyl phenyl)-methyl butyrate. The related transaminase has excellent stereoselectivity, regioselectivity and catalytic activity, the catalytic reaction is mild, the reaction conversion rate and the yield of a product are high, and the recombinant transaminase has relatively good application prospect.
Owner:JIANGSU ALPHA PHARM CO LTD
R-type omega-aminotransferase and application thereof
InactiveCN104328093AHigh chiral purityEfficient synthesisTransferasesFermentationNucleotideActive protein
The invention discloses an R-type aminotransferase and application thereof. The R-type omega-aminotransferase contains the amino acid sequence disclosed as SEQ ID NO:2, or the omega-aminotransferase-active protein amino acid sequence with high stereoselectivity-R configuration catalytic activity, which has at least 80% of homogeneity with the amino acid sequence disclosed as SEQ ID NO:2 or is subjected to substitution, deficiency or addition of one or more amino acids, wherein the high stereoselectivity means that the content of one stereomer is at least 1.1 times or so of the other stereomer. The R-type omega-aminotransferase has high stereoselectivity, can efficiently synthesize R configuration chiral amines with higher chiral purity, and is suitable for industrial production of chiral amines.
Owner:ASYMCHEM LAB TIANJIN +4
Omega-transaminase from bacillus pumilus and application in biological amination
ActiveCN109402188AIncrease productionCannot meet the requirements of industrial applicationTransferasesMicroorganism based processesEscherichia coliBacillus pumilus
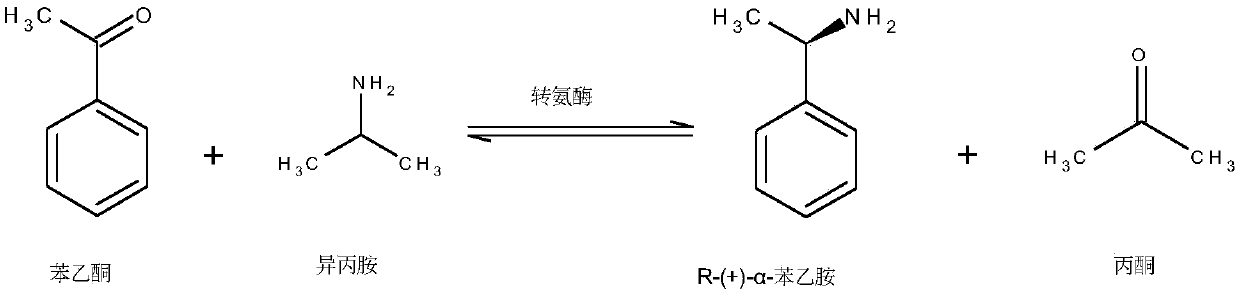
The invention discloses Omega-transaminase from bacillus pumilus and application in biological amination, and belongs to the technical field of bioengineering. In the invention, an Omega-transaminasegene is separated and identified from bacillus pumilus for the first time, and by adopting an escherichia coli recombination expression system to produce codon optimized Omega-transaminase, the yieldcan be remarkably increased, and the requirements of industrial application are met more easily. The invention discovers that the most proper action pH of the Omega-transaminase is 7.0 and the most proper action temperature is 45 DEG C when (R)-Alpha-phenethylamine serves as a substrate; meanwhile, the R-Omega-transaminase has excellent pH stability and temperature stability, and the catalytic activity on the experimental group (taking (R)-Alpha-phenethylamine as a substrate) is higher than that on the control group (taking (S)-Alpha-phenethylamine as a substrate), indicating that the recombinant Omega-transaminase has a function of synthesizing R-chiral amine with perfect selectivity and has relatively great application potential.
Owner:JIANGNAN UNIV
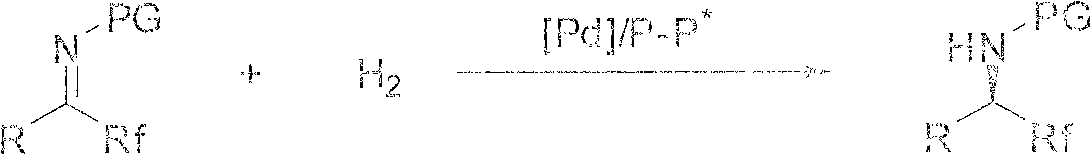
Asymmetric catalytic hydrogenation method for ketone-derived N-alkylimine
InactiveCN102050688AHigh enantioselectivityCarbamic acid derivatives preparationOrganic compound preparationAsymmetric hydrogenationKetone
The invention discloses a method for performing the asymmetric catalytic hydrogenation of ketone-derived N-alkylimine. In the method disclosed by the invention, the catalytic hydrogenation of the ketone-derived N-alkylimine is performed in the presence of a chiral catalyst formed by a chiral diamine ligand and a transitional metal to obtain a chiral amine product with high yield and high enantioselectivity. The enantiomeric excess of a chiral amine product formed by the hydrogenation of an acyclic N-alkylimine substrate may reach 98 percent ee; and the enantiomeric excess of a chiral amine product formed by the hydrogenation of an exocyclic N-alkylimine substrate may reach over 99 percent ee. The method disclosed by the invention realizes the asymmetric catalytic hydrogenation of ketone-derived N-alkylimine with high enantioselectivity, and the obtained chiral amine product is an important medicinal and material intermediate and has a bright actual application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for preparing optically active carbonyl compound
ActiveCN105541579AEasy to separate and purifyMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenDihydropyridine
The invention discloses a method for preparing an optically active carbonyl compound, which comprises the following step: under catalysis of a chiral amine salt and a transition metal catalyst, carrying out asymmetric catalytic hydrogenation reaction on alpha, beta-unsaturated aldehyde or alpha, beta-unsaturated ketone compound by taking hydrogen gas and a catalysis amount of dihydropyridine compound as a hydrogen source, so as to obtain the optically active carbonyl compound. The method has mild reaction conditions and simple operation, and the use amount of the dihydropyridine compound is the catalysis amount, so that a target product is easily separated and purified from a reaction system; and at the same time, the metal catalyst can be recycled, thereby meeting the economy requirement.
Owner:ZHEJIANG NHU CO LTD +2
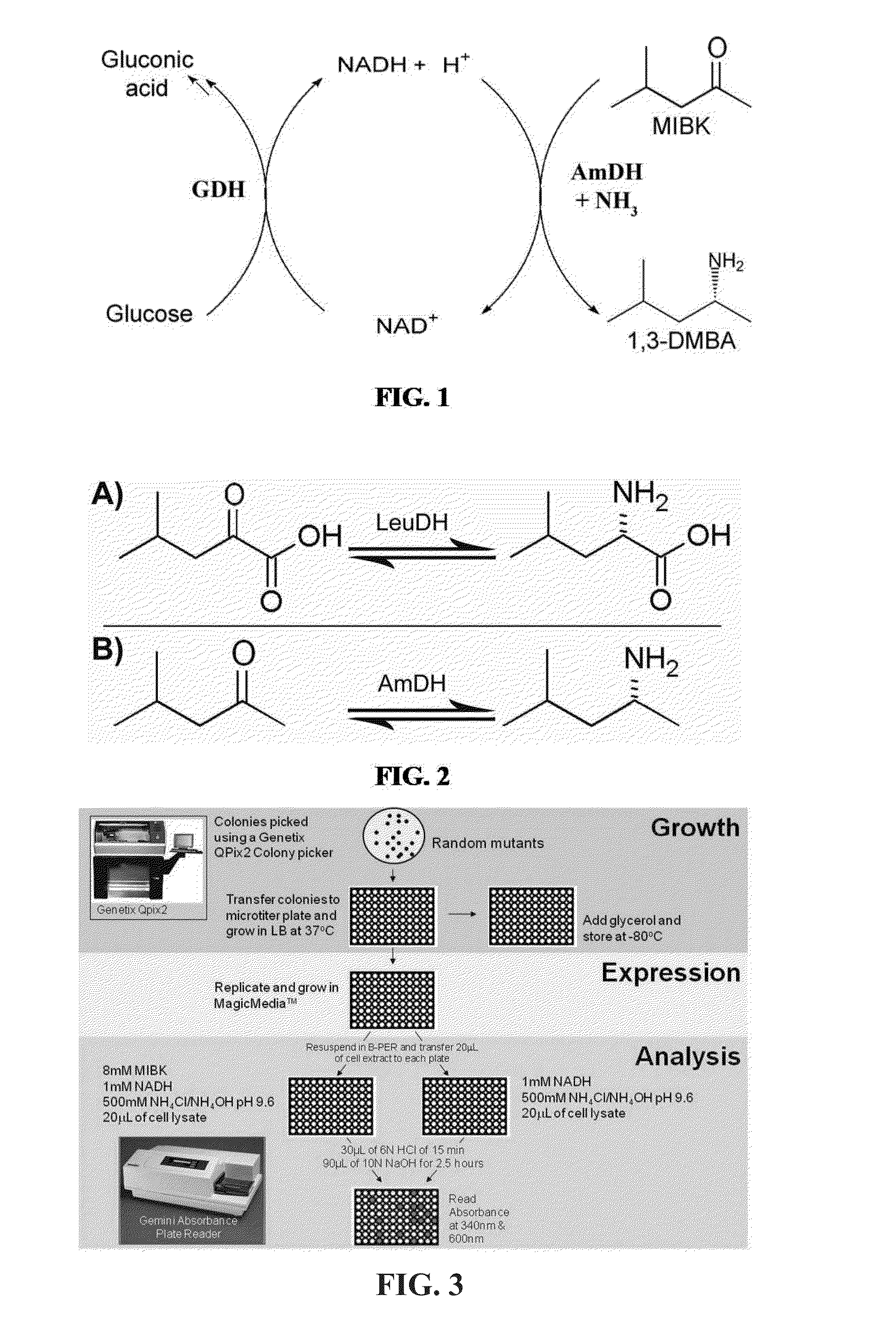
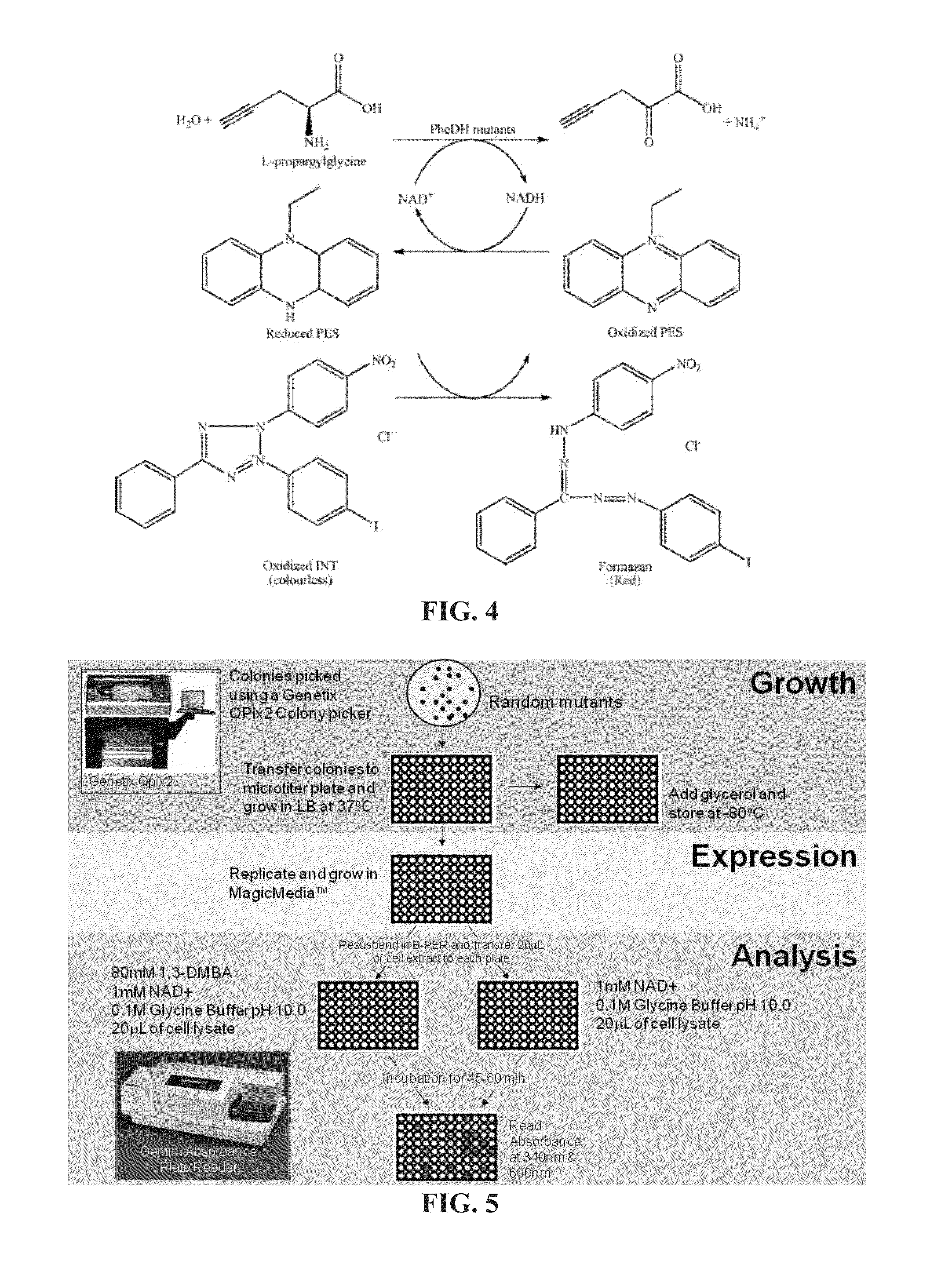
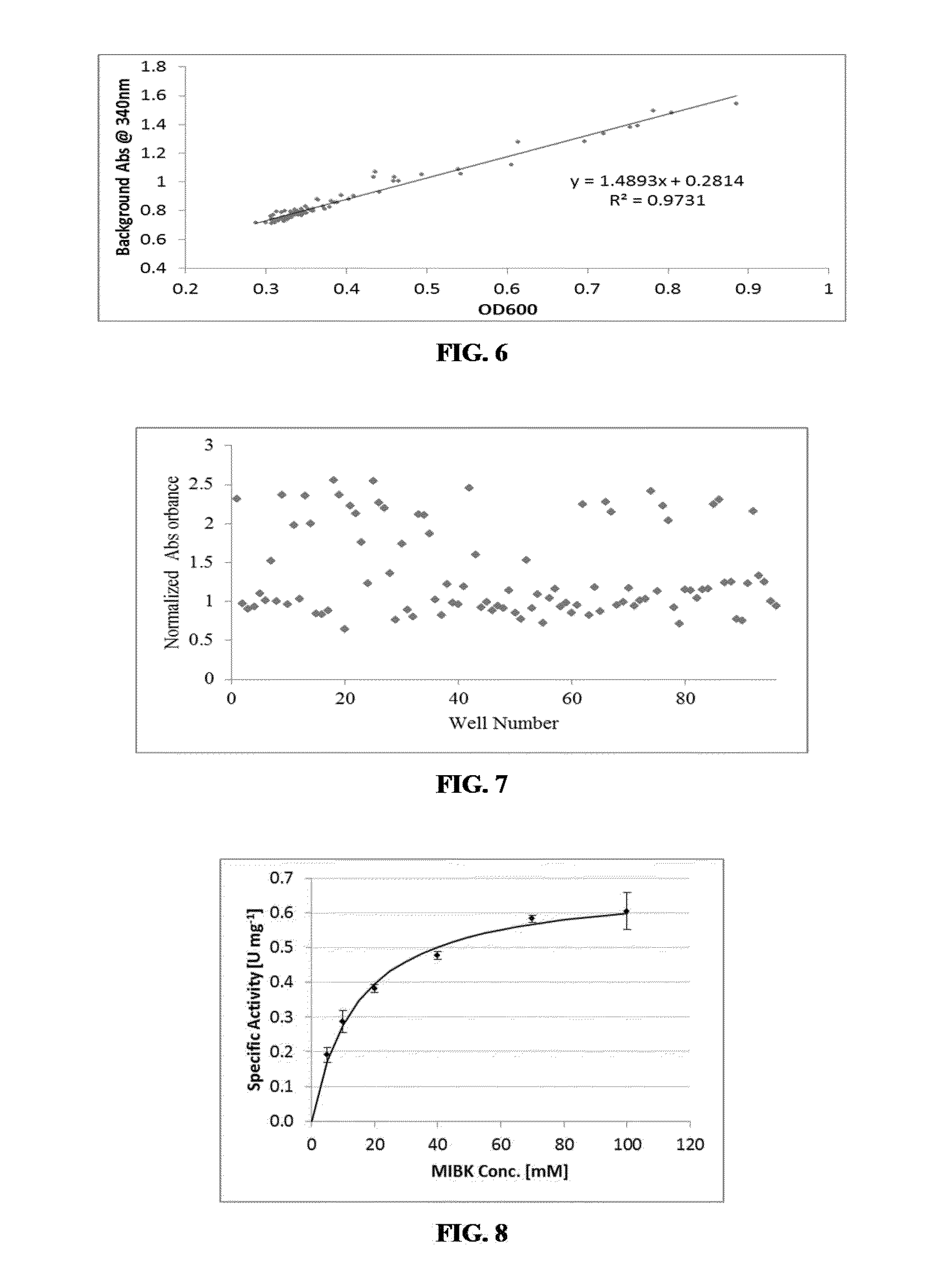
Engineered Amine Dehydrogenases and Methods of Use Thereof
Non-naturally occurring amine dehydrogenases (AmDH) and methods of use thereof the produce chiral amines are disclosed. The AmDH are variants of amino acid dehydrogenases. AmDH based on phenylalanine, leucine, and valine scaffolds are provided. The AmDH typically have one, two, three, four, or more amino acid alterations relative to the scaffold. The alterations to the scaffold result in an enzyme that accepts the analogous ketone, such as methyl isobutyl ketone (MIBK), instead of the wild-type α-keto acid. Chimeric AmDH are also disclosed. The chimeras are fusion proteins that include a substrate binding domain from a first AmDH and a cofactor binding domain from a second AmDH. In a preferred embodiment, one of the domains is from a PheDH-based AmDH and one of the domains is from a LeuDH-based AmDH.
Owner:GEORGIA TECH RES CORP
Synthetic method of apremilast chiral amine intermediate
ActiveCN104761474AChiral avoidanceReduce dosageOrganic chemistryOrganic compound preparationAlcoholAsymmetric hydrogenation
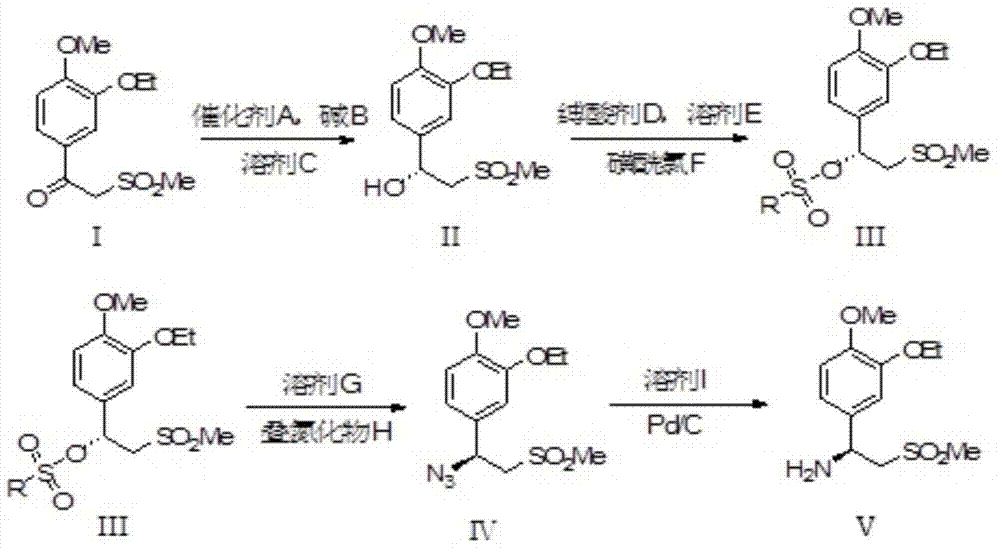
The invention relates to a synthetic method of an apremilast chiral amine intermediate (S)-2-[1-(3-ethoxyl-4-methoxylphenyl)]-1-methylsulfonyl-2-ethylamine (V). The synthetic method is characterized by including following steps: (1) with 1-(3-ethoxyl-4-methoxylphenyl)-2-(methylsulfonyl)ethyl ketone (I) as a raw material, performing asymmetric hydrogenation reduction to obtain methyl sulfonyl ethanol (II); (2) performing an esterification reaction to obtain methyl sulfonyl ester (III); (3) performing an azidation reaction to obtain an azide compound (IV); and (4) performing hydrogenation reduction to obtain the chiral amine intermediate (V) being high in chiral purity. The synthetic method is simple in process, is stable in reaction processes, and is environmental-protective and low-cost. In the synthetic method, the use amount of a catalyst during catalytic hydrogenation is less and the conversion rate reaches higher than 98%. The chiral amine can be prepared through chiral alcohol with very high yield and purity so that the synthetic method has a quite excellent commercial value and develops a new approach for synthesizing the apremilast chiral amine intermediate.
Owner:ENANTIOTECH CORP
Preparation method of high optical purity shikonin and alkannin, and derivatives thereof
InactiveCN102399139AHigh optical purityHigh yieldOrganic compound preparationCarboxylic acid esters preparationSide chainEnantiomer
The invention discloses a preparation method of high optical purity shikonin and alkannin, and derivatives thereof. According to the invention, an intermediate separation means is used to prepare the high optical purity shikonin and alkannin, and the derivatives thereof, wherein the intermediate separation is that an amide diastereomer is formed from a carboxyl-contained intermediate and chiral amine, and separation is carried out through column chromatography or a recrystallization process. The derivatives of shikonin and alkannin are tetramethoxylated derivatives with shikonin and alkannin as mother nuclei, dimethoxylated-2-sidechainisomer derivatives and dimethoxylated-6-sidechainisomer derivatives with shikonin and alkannin as mother nuclei, 1,4-diacetoxylated-5,8-dimethoxylated-2-sidechainisomer derivatives and 1,4-diacetoxylated-5,8-dimethoxylated-6-sidechainisomer derivatives with shikonin and alkannin as mother nuclei, and diacetoxylated-6-sidechainisomer derivatives and diacetoxylated-2-sidechainisomer derivatives with shikonin and alkannin as mother nuclei. The preparation method of the invention, which has the advantages of easily available raw material, low price and high yield of each step reaction, is suitable for the large scale preparation.
Owner:SHANGHAI JIAO TONG UNIV
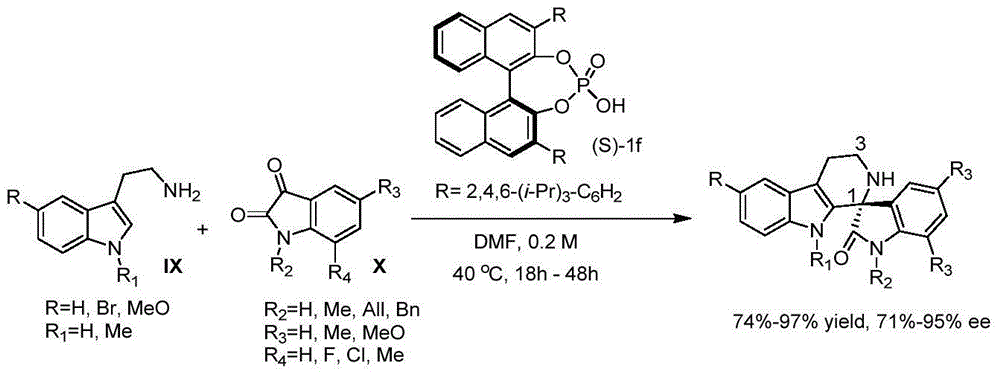
Axially-chiral indole-naphthalene compounds and preparation method thereof
ActiveCN110452150AHigh yieldControlling enantioselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsXylyleneChemical structure
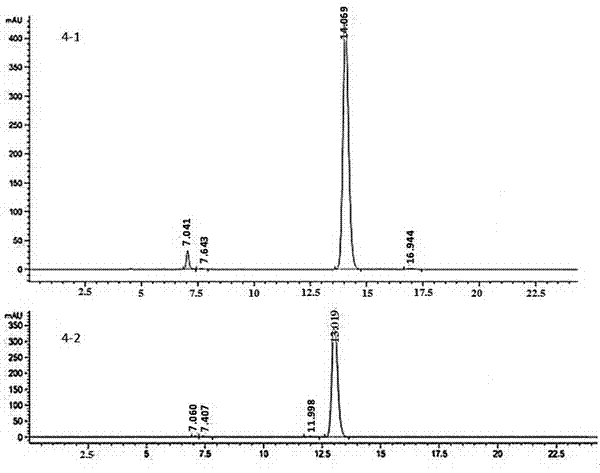
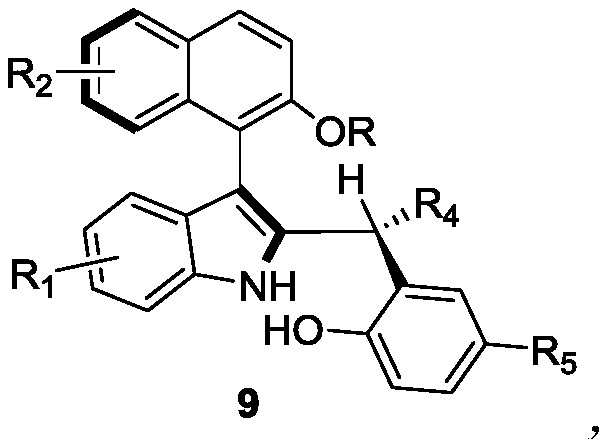
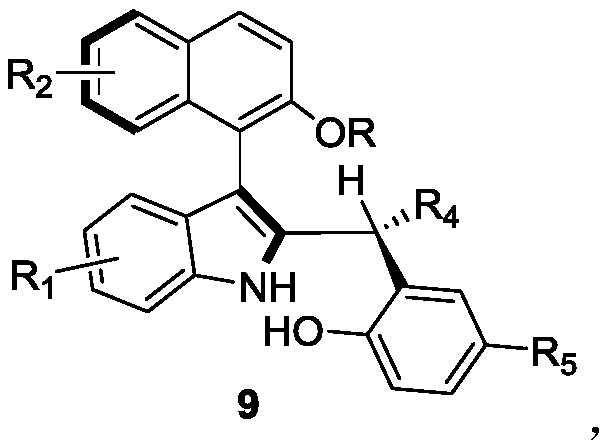
The invention discloses axially-chiral indole-naphthalene compounds and a preparation method thereof. The axially-chiral indole-naphthalene compounds have a chemical structure represented by a formula9 shown in the description; and the method comprises the following steps: a compound represented by a formula 7 and a compound represented by a formula 8 are used as reaction raw materials, a mixed solution of 1,1,2,2-tetrachloroethane and p-xylene is used as a solvent, a molecular sieve is added, a reaction is performed under stirring under the action of a chiral phosphoric acid catalyst, the reaction is tracked by TLC until the reaction is completed, filtration is performed, concentration is performed, purification is performed, and therefore one compound is obtained. The method for preparing the axially-chiral indole-naphthalene compounds provided by the invention is an asymmetric addition reaction under catalysis of organic small molecules, starts from the racemic raw material to construct the axially-chiral indole-naphthalene structure in one step, and has the advantages of simple and convenient operation, mild reaction conditions and economical easily-available raw materials, and the prepared axially-chiral indole-naphthalene compounds have high optical purity; and the axially-chiral indole-naphthalene compounds prepared by the method are expected to be widely used in the field of asymmetric catalysis.
Owner:XUZHOU NORMAL UNIVERSITY
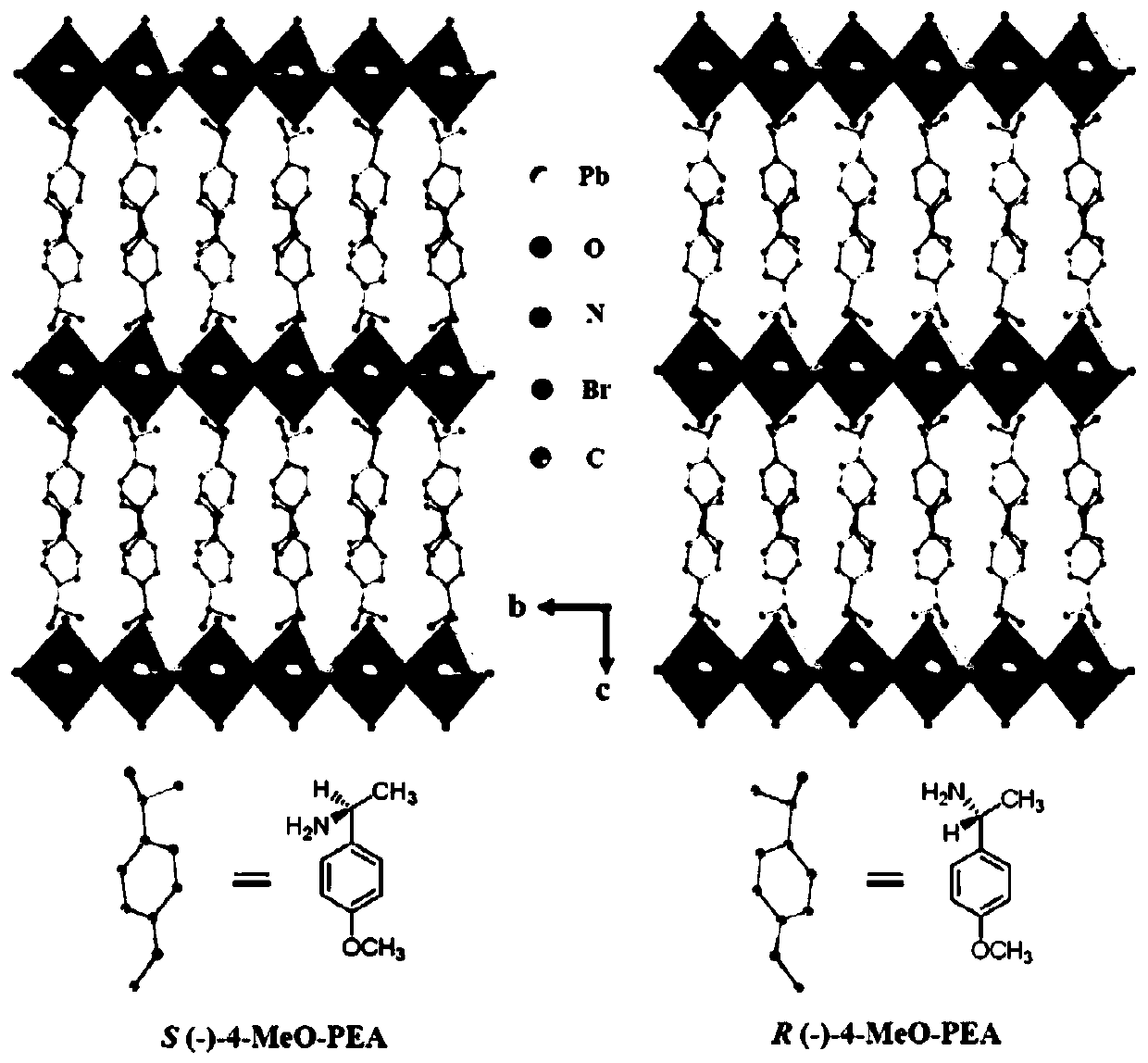
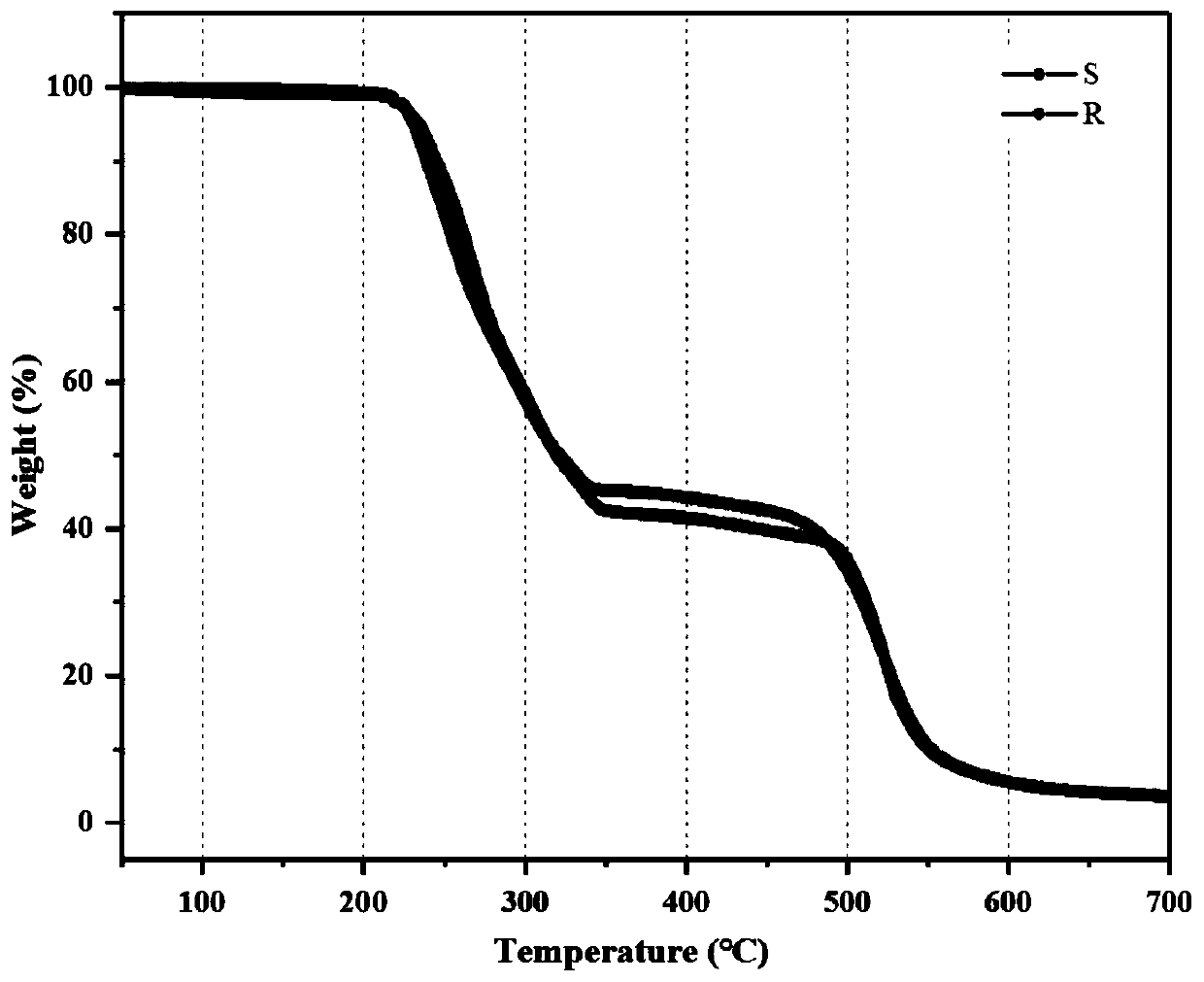
Hybrid organic-inorganic chiral perovskite single crystal and synthesis method thereof
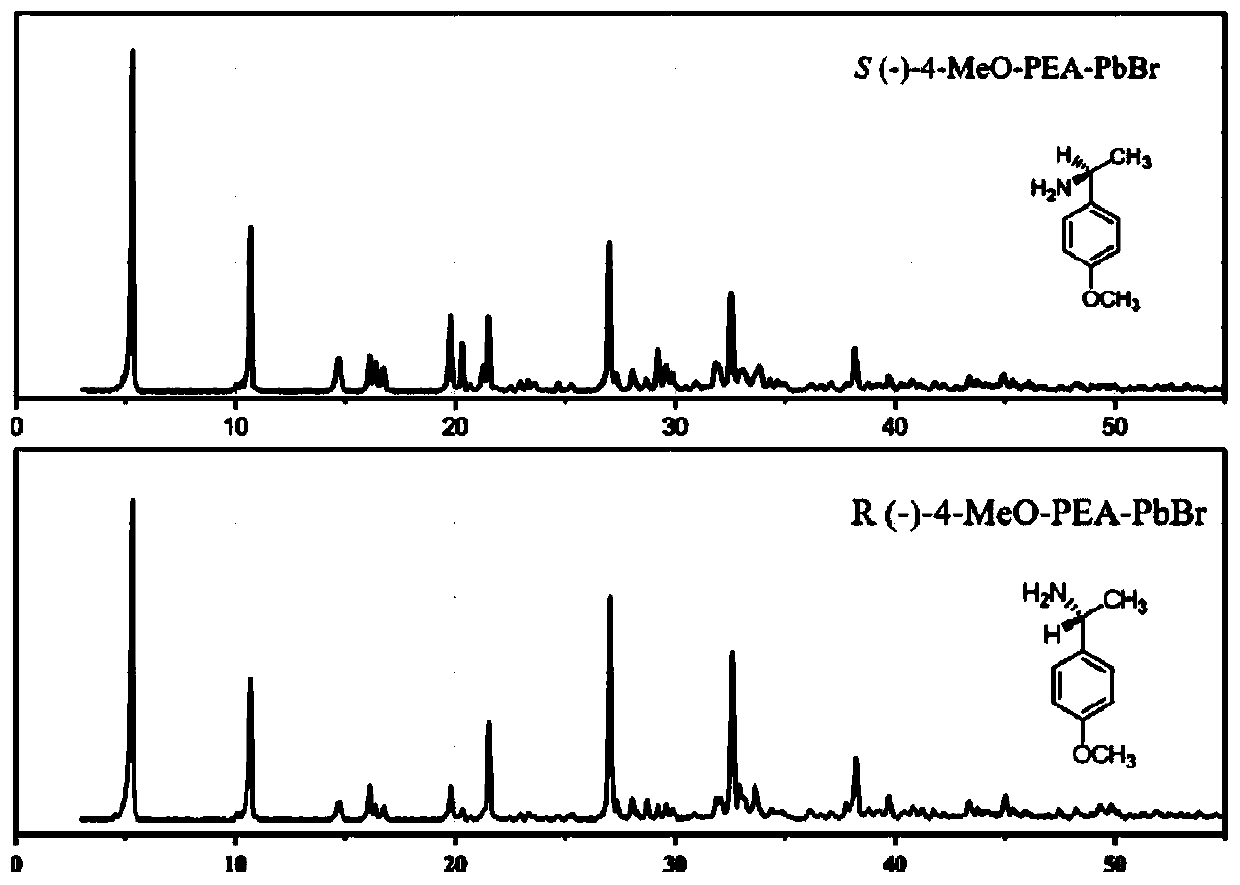
InactiveCN110863246AReduce pollutionImprove stabilityPolycrystalline material growthFrom normal temperature solutionsPhysical chemistrySingle crystal
The invention relates to a hybrid organic-inorganic chiral perovskite single crystal and a synthesis method thereof. According to the invention, a chiral organic amine ligand is used as a raw materialto prepare a novel non-centrosymmetric hybrid organic-inorganic chiral perovskite single crystal. The preparation method comprises the following steps: dissolving a mixture of PbBr2 and chiral aminein a molar ratio of 2: 1 in HBr and N, N-dimethylformamide, stirring for 20 minutes at room temperature, gradually clarifying the solution, standing in a toluene atmosphere for several days to obtaincolorless bulk monocrystals, and drying in a drying oven to obtain the hybrid organic-inorganic chiral perovskite monocrystals. The hybrid organic-inorganic chiral perovskite single crystal synthesized by the method disclosed by the invention has relatively strong second-order nonlinear optical properties. The preparation method provided by the invention is simple and efficient, the product stability is high, the environmental pollution is small, and a foundation is laid for the development and application of the novel hybrid organic-inorganic perovskite material in the photoelectric field.
Owner:NANKAI UNIV
Engineered transaminase polypeptide and application thereof
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
Synthetic method of sitagliptin and salt thereof
The invention discloses a synthetic method of sitagliptin and a salt thereof. The synthetic method comprises the steps: carrying out an esterification reaction, a reduction reaction, an oxidizing reaction and a witting reaction on 2,4,5-trifluorophenylacetic acid as a starting raw material to obtain 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate; then carrying out a hydroamination reaction on 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate and chiral amine in the presence of butyl lithium or hexamethyldisilazane sodium to form a chiral hydroamination product; carrying out an esterolysis reaction, a condensation reaction and a hydrogenation reaction to obtain sitagliptin. The raw materials used in the synthetic method of sitagliptin are low in price and easy to obtain; the synthetic method of sitagliptin is less in step, easy to operate and capable of effectively reducing cost. By the use of the method, high-purity sitagliptin can be obtained, a sitgliptin phosphate obtained through salifying has an HPLC (High Performance Liquid Chromatography) and an ee (enantiomeric excess) value of more than 99% and can be applied to the field of medicine.
Owner:ZHEJIANG NHU CO LTD +1
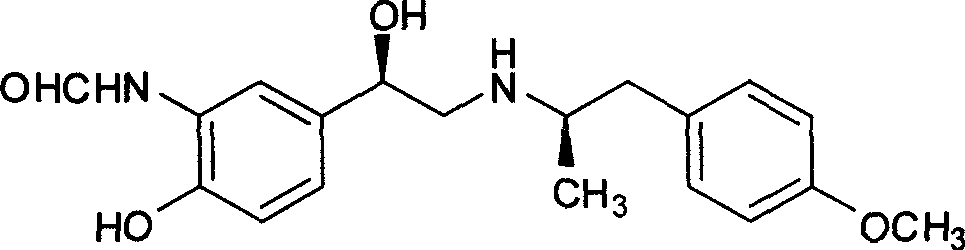
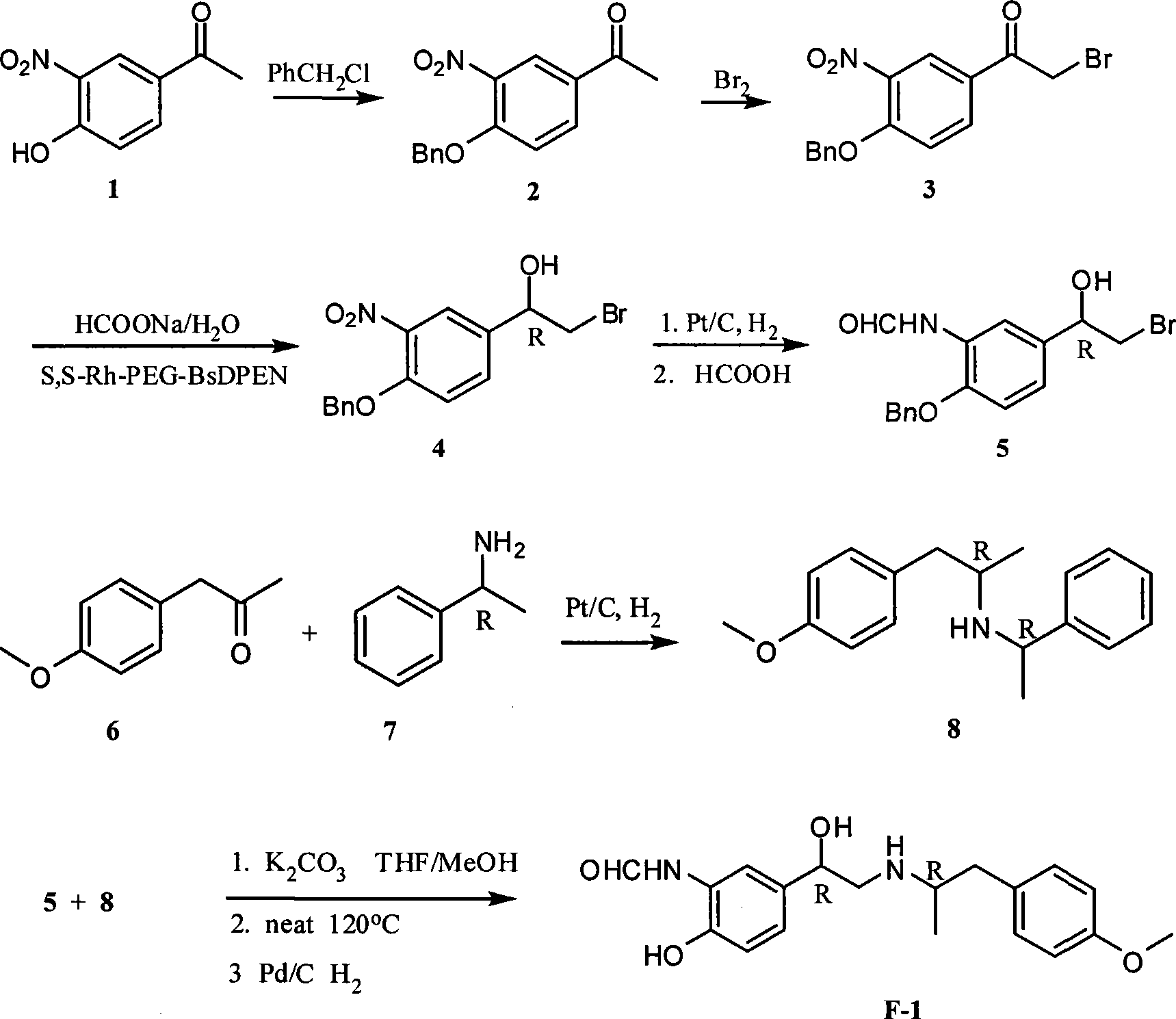
Unsymmetrical hydrogen migration synthesizing method for (R, R)-formoterol
InactiveCN101468954AIn line with the concept of green chemistryLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
The invention relates to an asymmetric hydrogen transfer synthesis method for (R,R)-formoterol, and relates to a novel method for synthesizing an optical pure beta 2-adrenoreceptor excitant, namely formoterol. The method comprises: firstly, taking 4-hydroxyl-3 nitroacetophenone as a raw material, using benzyl groups to protect phenolic hydroxyl groups, and obtaining alpha-bromo keto after bromination; secondly, taking (S,S)-Rh-PEG-BsDPEN as a catalyst and formic acid and derivatives of the formic acid as hydrogen sources, and synthesizing chiral alcohol intermediate by an asymmetric hydrogen transfer method; thirdly, using (R)-alpha-methyl phenylethylamine and methoxyl phenylacetone to generate imine compounds, and obtaining chiral amine intermediate through hydrogenation reduction under the catalysis of Pt / C; and fourthly, reacting and coupling the chiral alcohol intermediate and the chiral amine intermediate, removing protective groups, and obtaining the (R,R)-formoterol. The invention uses the asymmetric hydrogen transfer method and a chiral auxiliary reagent to synthesize the (R,R)-formoterol, and has high yield and good ee value. Compared with a method for synthesizing chiral formoterol through chemical splitting, the method has the advantages of high total yield, mild reaction conditions, low cost and so on, and is favorable for industrial production.
Owner:SUN YAT SEN UNIV
Asymmetric catalytic hydrogenation method of imine
InactiveCN102951980ASuitable for large-scale preparationLigands are readily availableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumIodised salt
The invention discloses an asymmetric catalytic hydrogenation method of imine. Chiral amine is prepared through asymmetric hydrogenation of the imine through using a complex as a catalyst in the presence of an iodate or iodine under the co-action of an acid, wherein the complex is prepared through an in-situ reaction of {(R)-1-[(S)-2-di-phenyl-phosphino-ferrocene]}-propyl-di-(3,5-dimethyl-phenyl)phosphine and an iridium complex; and the proper molar ratio (s / c) of the raw material imine to the catalyst is 100-500000. The method has the advantages of easily available ligand, convenience of large-scale preparation, simple operation, and realization of continuous operation, is suitable for the large-scale preparation of the chiral amine, enables the e.e value of the chiral amine to be above 70%, completely satisfies requirements of a pesticide intermediate, is especially suitable for preparing chiral intermediates of a pesticide S-metolachlor, and has a very good industrial practicality.
Owner:中国中化股份有限公司 +1
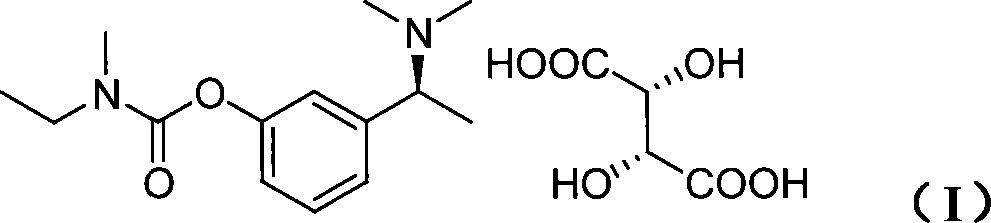
Technique for producing rivastigmine hydrogen tartrate
InactiveCN101239934AAvoid huge lossesLow production costCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsRivastigmine
The invention discloses a production process of rivastigmine, comprising using m-methoxy hypnone as raw material, adding chiral auxiliary, reducing and aminating through an asymmetry synthesis method to obtain chiral amine, then demethylatig and acylating to prepare rivastigmine, and finally reacting rivastigmine with tartaric acid to obtain rivastigmine. The invention has advantages of simple operation, low cost, high yield, small pollution, and is suitable for industrialisation production.
Owner:苏州凯达生物医药技术有限公司
Method for synthesizing dihydrogen phenanthridine
InactiveCN102952073ARaw materials are easy to getFew reaction stepsOrganic chemistryMetallolePhenanthridine
The invention relates to a method for synthesizing dihydrogen phenanthridine. According to the invention, transition metals [Ru (II), Rh (I), and Ir (I)] are adopted as catalyst for realizing phenanthridine catalytic hydrogenation. Also, according to the invention, 9,10-dihydrogen phenanthridine in-situ regeneration is applied in asymmetric transfer hydrogenation of unsaturated imine. Only a catalytic amount of phenanthridine is needed to be added, and chiral amine can be synthesized with high enantioselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
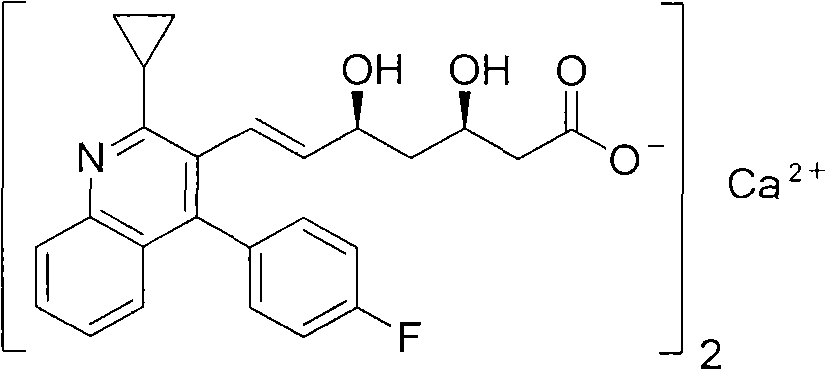
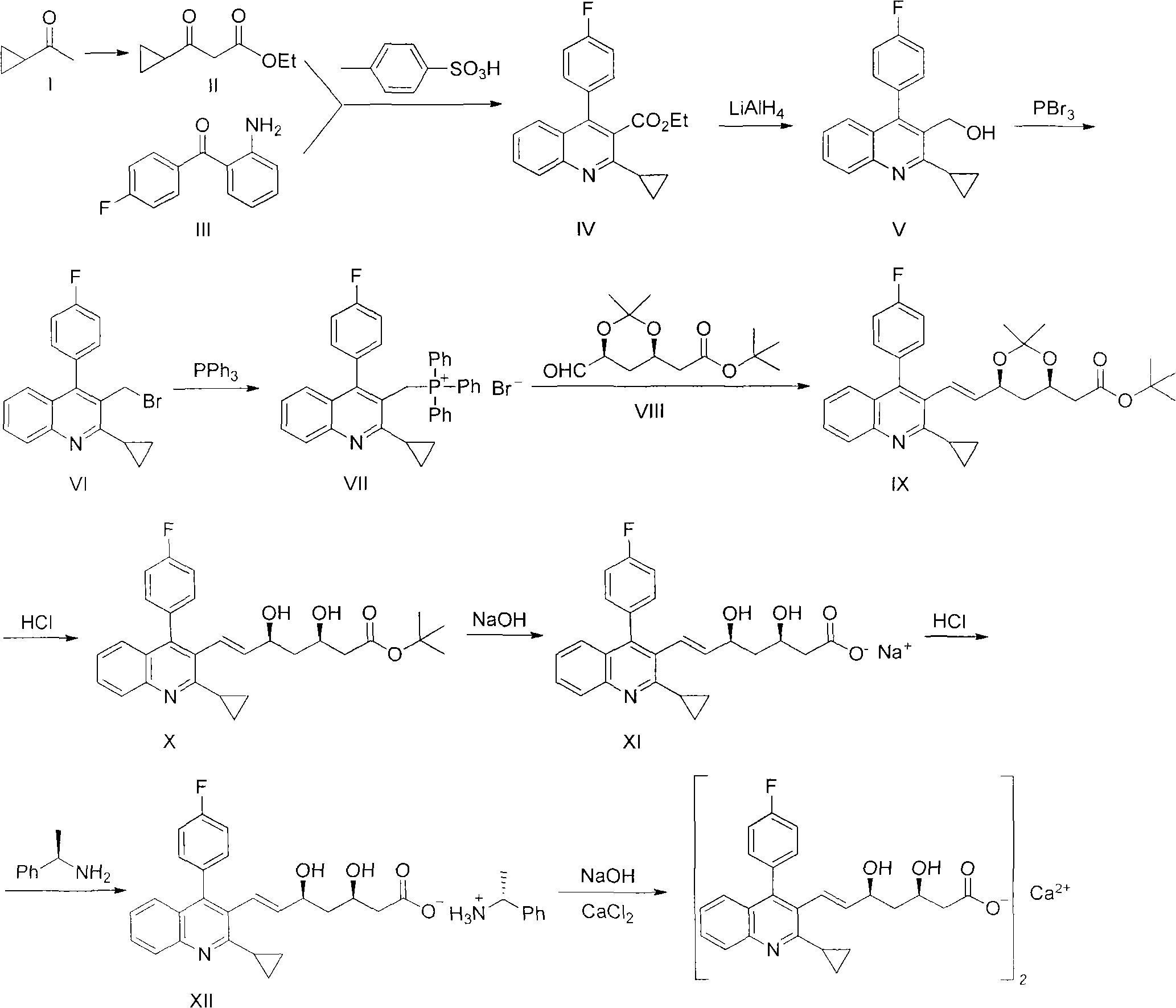
Preparation method of pitavastatin calcium
InactiveCN103508947AReasonable designSimple post-processingOrganic chemistryPhosphonium saltPhosphonium
The invention relates to a preparation method of pitavastatin calcium for treating hyperlipidemia. The preparation method comprises the following steps: performing cyclization on 2-amino-4'-fluorobenzophenone (III) and ethyl 3-cyclopropyl-3-oxo-propanoate (II), and then reducing with LiAlH4 to obtain 2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline methanol (V); bromizing the V to obtain 2-cyclopropyl-3-bromomethyl-4-(4-fluorophenyl) quinoline (VI); reacting the VI with triphenylphosphine to obtain (2-cyclopropyl-4-(4-fluorophenyl)-quinoline-3-yl) methyltriphenylphosphonium bromide (VII), performing alkali treatment on a phosphonium salt, then forming phosphonium ylide, performing condensation with (3R, 5S)-6-oxo-3, 5-isopropylidene-dioxo-6-heptenoic acid tert-butyl ester (VIII) to obtain a compound IX; and acidifying the compound IX with hydrochloric acid, performing hydrolysis deprotection with sodium hydroxide, performing salt formation and purification with chiral amine and forming a calcium slat to obtain a final product. The whole route has reasonable design, the process flow is simple, starting raw materials and reagents used by the preparation method can be purchased from the market, the reagents with severe toxicity and serious pollution are not used in a reaction process, the post-treatment of an intermediate is simple, and the preparation method has the advantages of high yield and is easy to purify.
Owner:WEIHAI WEITAI PHARMA TECH DEV
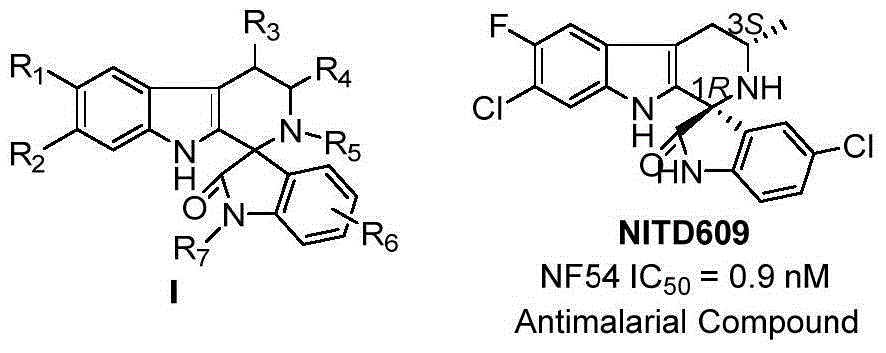
Method for asymmetric catalytic synthesis of spirocyclic tetrahydrocarbazoline compound
The invention discloses a method for asymmetric catalytic synthesis of a spirocyclic tetrahydrocarbazoline compound, comprising the steps: by taking 3-alkenyl indole and isatin derived ketimine as raw materials, a complex formed by chiral amine oxide and nickel trifluoromethane sulfonate as a catalyst and dichloromethane as a solvent, performing reaction on the raw materials at (-30) DEG C to (-10) DEG C at the normal pressure for 96-192h; then adding 6.0M HCl(solution) and performing reaction at 30 DEG C for 4-48h to obtain the chiral spirocyclic tetrahydro carbazoline compound, wherein the yield of the chiral spirocyclic tetrahydro carbazoline compound can reach up to 95%, and the enantioselectivity can reach up to 99%. According to the method provided by the invention, the catalytic reaction conforms with the green chemistry atom economy and has a good prospect in industrial application, thereby providing a new path for asymmetric synthesis of compounds with high anti-malarial activity (NITD609).
Owner:SICHUAN UNIV
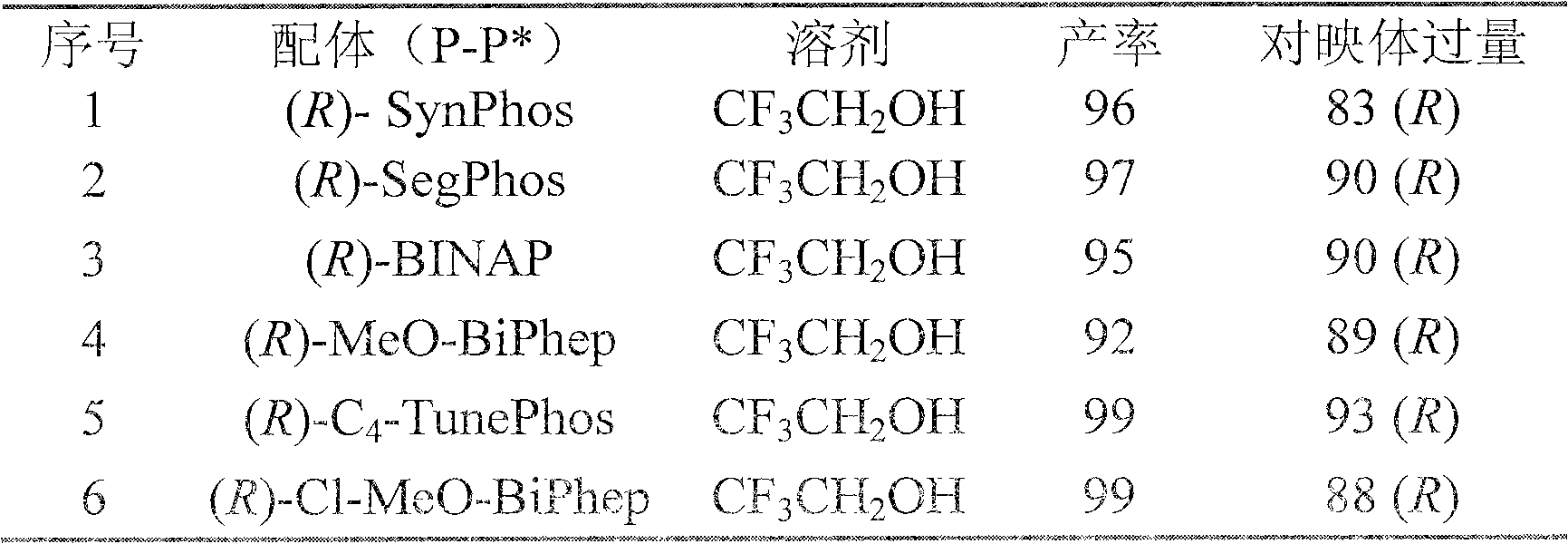
Method for synthesizing chiral fluoroamine by palladium catalytic asymmetric hydrogenation
ActiveCN102336621AHigh reactivityHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolventFluoroamine
The invention discloses a method for synthesizing chiral fluoroamine by palladium catalytic asymmetric hydrogenation, wherein, the used catalysis system comprises a palladium chiral diphosphite complex. The reaction is carried out under the following conditions that: the temperature is 0-50 DEG C, the solvent is a 2,2,2-trifluoroethyl alcohol, the pressure is 1-42 atm, the ratio of the substrate to the catalyst is 50 : 1, the used metal precursor is palladium trifluoroacetate, the used chiral ligand is a chiral diphosphite ligand. The catalyst is prepared by stirring the palladium metal precursor and the chiral diphosphite ligand in acetone at room temperature, and then carrying out vacuum condensation. According to the invention, by the hydrogenation of imine containing trifluoromethyl, corresponding chiral amine containing trifluoromethyl is obtained, the enantiomeric excess can reach to 94 %; by the hydrogenation of imine containing perfluoroalkyl, corresponding chiral amine containing perfluoroalkyl is obtained, and the enantiomeric excess can reach to 86 %. The present invention has the advantages of simple and practical operation, high enantioselectivitiy, good yield, green atom economy of the reaction, and no environmental pollution.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Asymmetric synthesis method of dextral citronellal
InactiveCN103724170AEasy to makeMild reaction conditionsOrganic compound preparationCarbonyl compound preparationHydrogenDihydropyridine
The invention discloses an asymmetric synthesis method of dextral citronellal. The method takes citral as a starting material, dihydropyridine derivative as a negative hydrogen source, and chiral amine salt as a chiral auxiliary agent, and asymmetric hydrogenation reaction is generated in a bi-catalytic system to synthesize dextral citronellal. By adopting the synthesis method disclosed by the invention for the synthesis of dextral citronellal, the total yield is more than 85%, and the ee (enantiomeric excess) value is greater than 80%; the synthesis method has the characteristics of mild reaction conditions, simple synthesis process, simple catalyst preparation, convenience in recycling and the like, and is suitable for industrial production of dextral citronellal.
Owner:GUANGDONG FOOD IND INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28fd47c2-a1f4-4277-a641-21397b12afd8/BDA0000098780580000011.PNG)
![Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28fd47c2-a1f4-4277-a641-21397b12afd8/BDA0000098780580000012.PNG)
![Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane Asymmetric synthesis method, relevant raw materials and preparation method of (S,S)-2,8-diazabicyclo[4,3,0] nonane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28fd47c2-a1f4-4277-a641-21397b12afd8/BDA0000098780580000021.PNG)