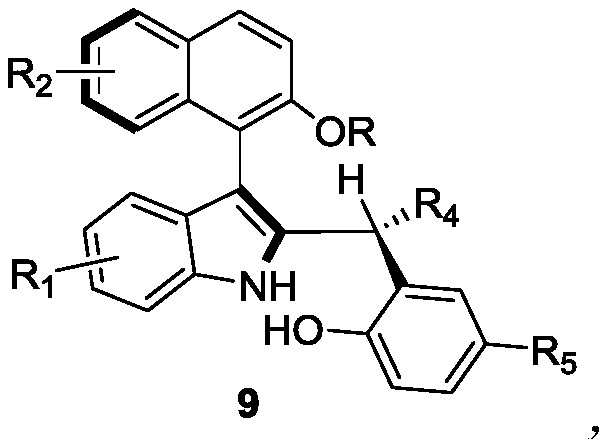
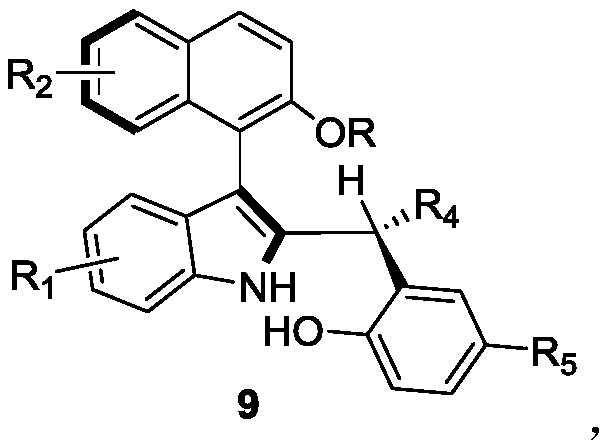
Axially-chiral indole-naphthalene compounds and preparation method thereof
A compound, axial chiral technology, applied in the field of organic synthetic chemistry, can solve limited problems, achieve mild reaction conditions, high yield, and broaden the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: in the mixed solvent (v / v=1:4) of 1 milliliter 1,1,2,2-tetrachloroethane and p-xylene, add 0.1 mmol of formula 7a compound and 0.12 mmol of formula Compound 8a as reactant, 100 mg Molecular sieves are used as additives, 0.01 mmol of chiral phosphoric acid (i.e. the compound of formula 6) is used as a catalyst, reacted at 25 ° C for 12 hours, TLC traced the reaction to the end, and filtered to remove Molecular sieves, wash the filter cake with ethyl acetate, and separate the obtained filtrate through silica gel column chromatography (the eluent is a mixed solution with a volume ratio of petroleum ether and ethyl acetate of 10:1) after concentration to obtain the axial chiral indole Indole-naphthalene 9aa, white solid.
[0030] The structural characterization data of product 9aa in Example 1 are as follows:
[0031] m.p.128-129°C; [α] D 20 =-14.5 (c 0.64, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ8.24(s,1H),7.78–7.70(m,2H),7.37(d,J=8.1Hz,1H),7.28(s,2H),7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com