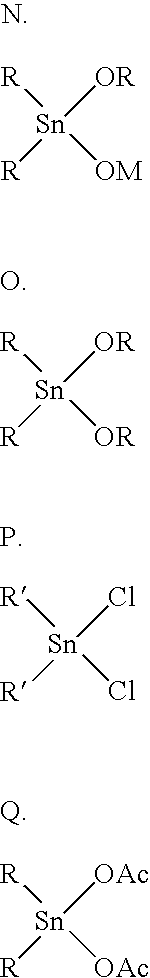
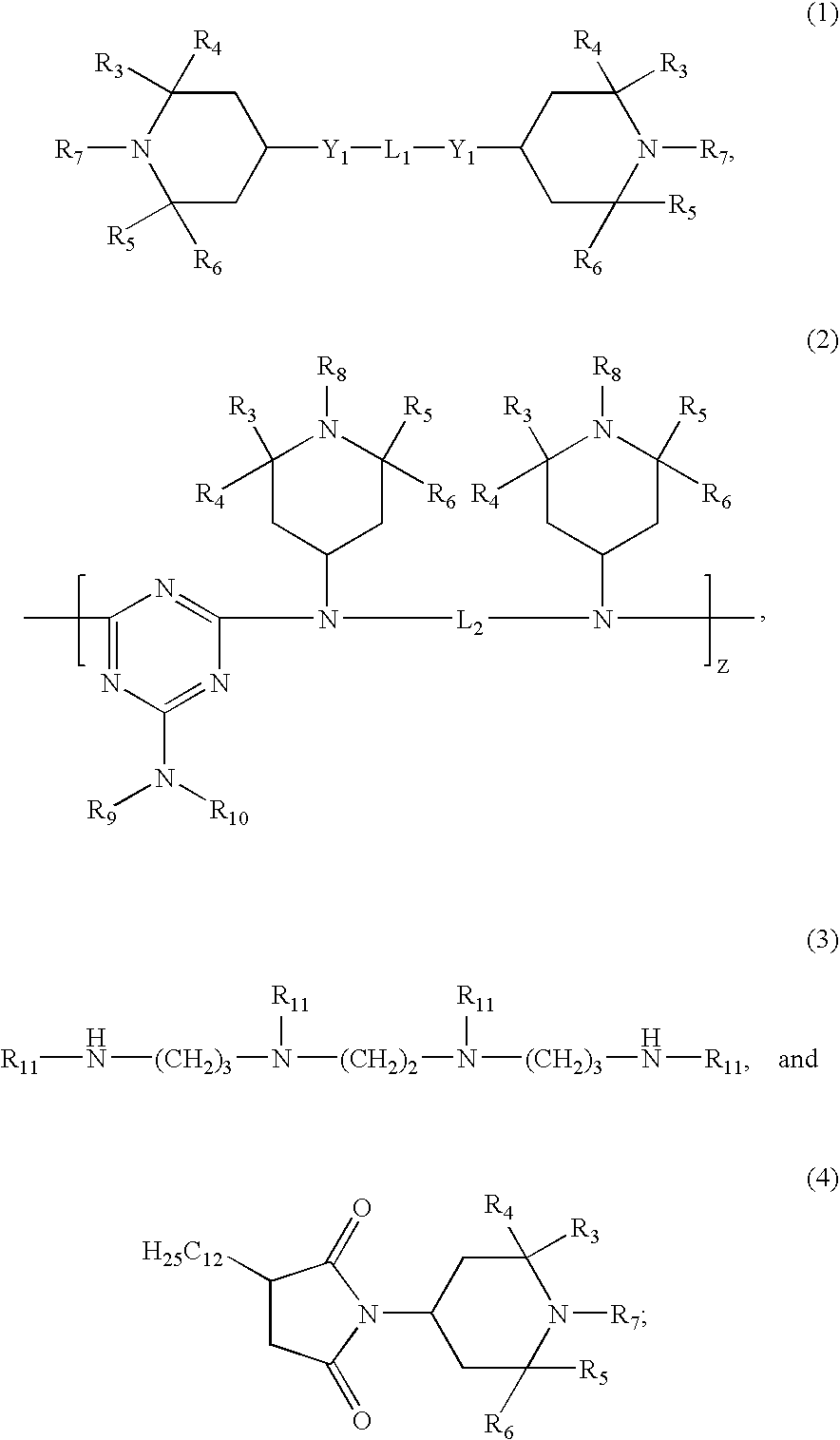
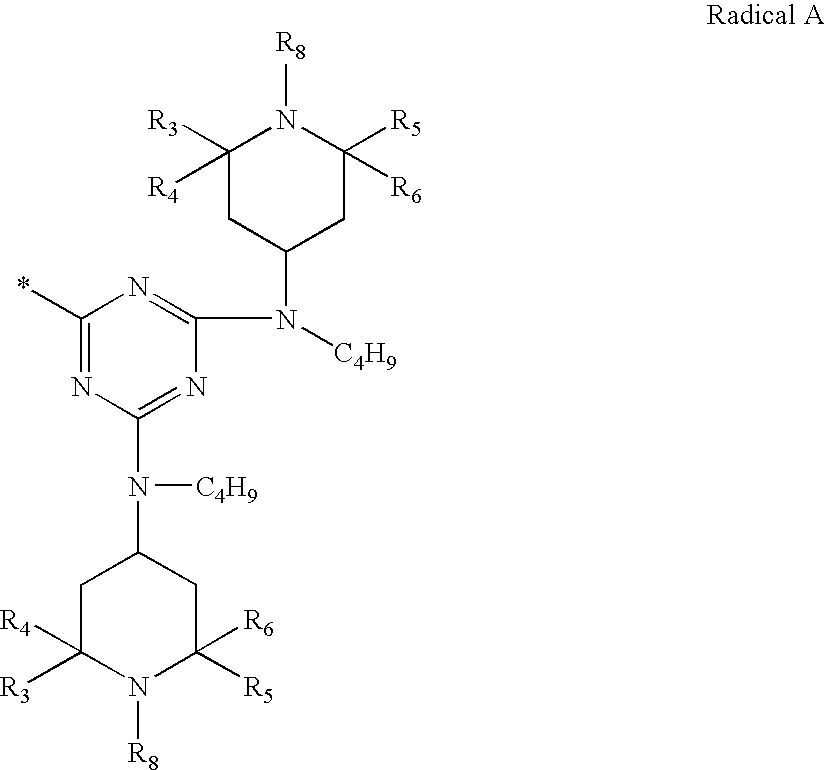
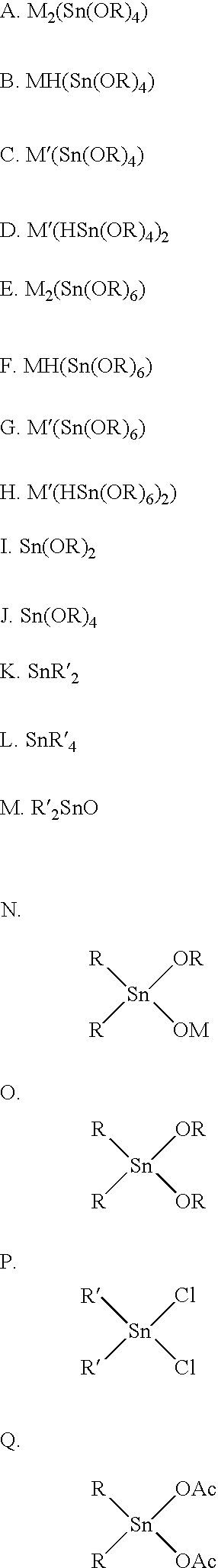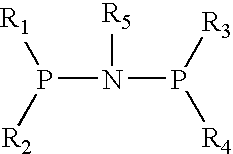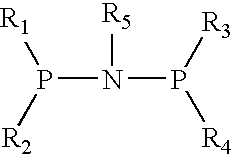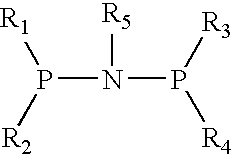Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
181 results about "Tetrachloroethane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetrachloroethane may refer to either of two isomeric chemical compounds: 1,1,1,2-Tetrachloroethane 1,1,2,2-Tetrachloroethane
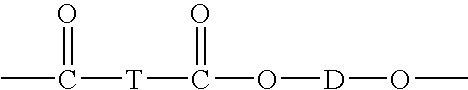
Certain polyester compositions which comprise cyclohexanedimethanol, moderate cyclobutanediol, cyclohexanedimethanol, and high trans cyclohexanedicarboxylic acid
InactiveUS20070232779A1Good chemical resistanceLess reactiveSynthetic resin layered productsOptical articlesPolyesterCyclohexanedimethanol
Described as one aspect of the invention are polyester compositions A polyester composition comprising at least one polyester which comprises:(A) a dicarboxylic acid component comprising:i) 70 to 100 mole % of cyclohexanedicarboxylic acid residues or an ester thereof comprising:(a) 80 to 99 mole % trans-cyclohexanedicarboxylic acid residues or an ester thereof; and(b) 1 to 20 mole % cis-cyclohexanedicarboxylic acid residues or an ester thereof;ii) 0 to 30 mole % of aliphatic dicarboxylic acid residues, other than cyclohexanedicarboxylic acid residues, having up to 16 carbon atoms or esters thereof, other than cyclohexanedicarboxylic acid residues; andiii) 0 to 10 mole % of aromatic dicarboxylic acid residues having up to 20 carbon atoms; and(B) a glycol component comprising:i) 5 to 35 mole % of 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues; andii) 65 to 95 mole % of 1,4-cyclohexanedimethanol residues, 1,3-cyclohexanedimethanol residues, 1,2-cyclohexanedimethanol residues or esters thereof or mixtures thereof,wherein the total mole % of said dicarboxylic acid component is equal to 100 mole %;the total mole % of said glycol component is equal to 100 mole %;wherein the inherent viscosity of said polyester is from 0.35 to 1.2 dL / g as determined in 60 / 40 (wt / wt) phenol / tetrachloroethane at a concentration of 0.5 g / 100 ml at 25° C.; and wherein said polyester has a Tg of from 66 to 120° C. The polyesters may be manufactured into articles.
Owner:EASTMAN CHEM CO
Polyester compositions which comprise cyclobutanediol ethylene glycol, titanium, and phosphorus with improved color and manufacturing processes therefor
Described as one aspect of the invention are polyester compositions comprising at least one polyester which comprises: (a) a dicarboxylic acid component comprising: (i) about 90 to about 100 mole % of terephthalic acid residues; (ii) about 0 to about 10 mole % of aromatic and / or aliphatic dicarboxylic acid residues having up to 20 carbon atoms; and (b) a glycol component comprising: (i) about 1 to less than 90 mole % 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues; and (ii) about 0 to about 89 mole % cyclohexanedimethanol residues; (iii) greater than 10 mole % ethylene glycol residues, and (iv) less than about 2 mole % of a modifying glycol having from 3 to 16 carbon atoms; (c) titanium atoms and phosphorus atoms, wherein the total mole % of the dicarboxylic acid component is 100 mole %, and wherein the total mole % of the glycol component is 100 mole %; and wherein the inherent viscosity of the polyester is from 0.50 to 1.2 dL / g as determined in 60 / 40 (wt / wt) phenol / tetrachloroethane at a concentration of 0.25 g / 50 ml at 25° C. The polyesters may be manufactured into articles.
Owner:EASTMAN CHEM CO
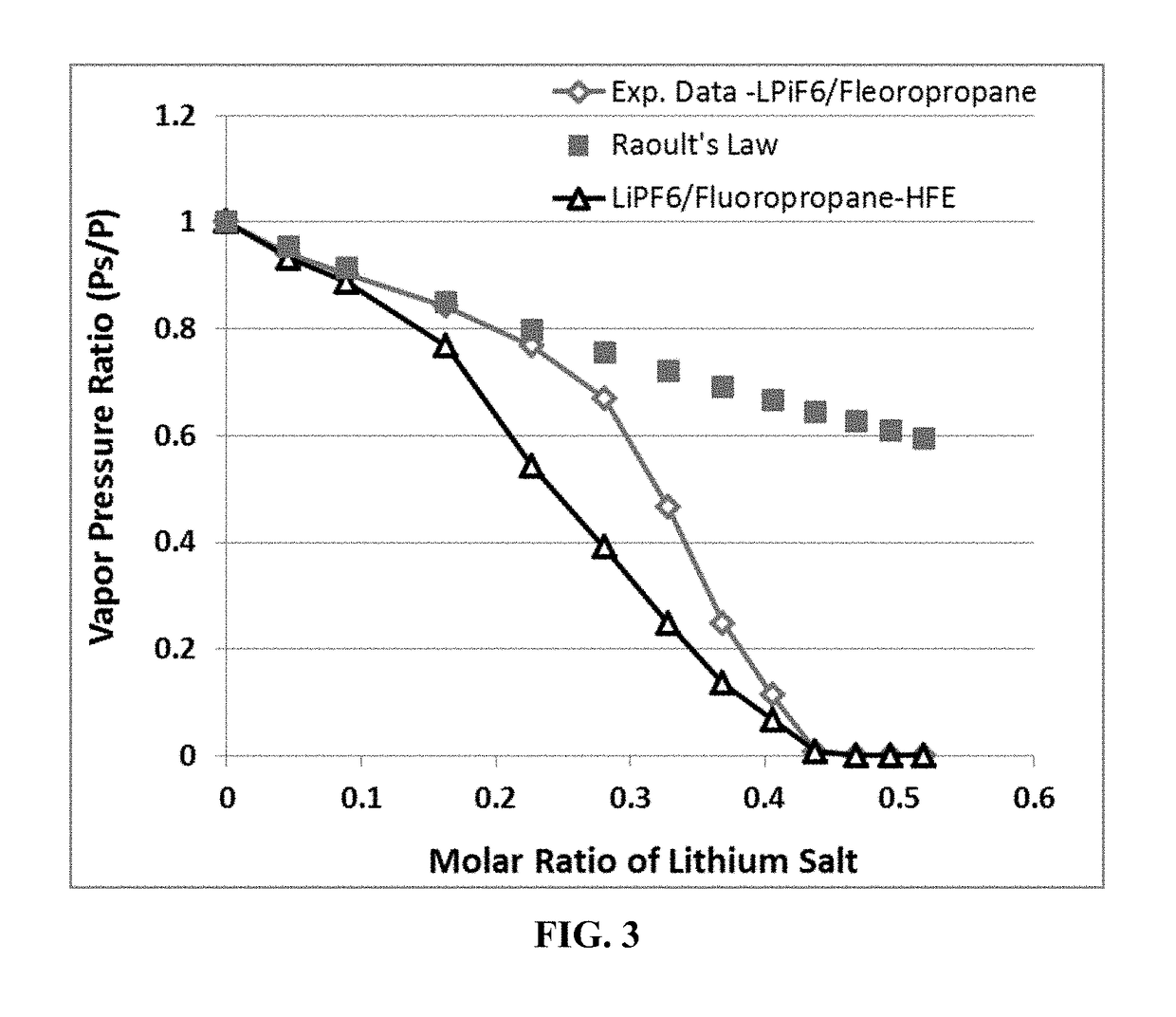
Non-flammable Electrolyte Containing Liquefied Gas and Lithium Secondary Batteries Containing Same
ActiveUS20180375156A1Flammability of any liquefied gas solvent can be effectively suppressedImprove solubilityOrganic chemistryCell electrodesElectrolytic agentDifluoroethyne
A rechargeable lithium cell comprising a cathode, an anode, an optional ion-permeable membrane disposed between the anode and the cathode, a non-flammable salt-retained liquefied gas electrolyte in contact with the cathode and the anode, wherein the electrolyte contains a lithium salt dissolved in or mixed with a liquefied gas solvent having a lithium salt concentration greater than 1.0 M so that the electrolyte exhibits a vapor pressure less than 1 kPa when measured at 20° C., a vapor pressure less than 60% of the vapor pressure of the liquefied gas solvent alone, a flash point at least 20 degrees Celsius higher than a flash point of the liquefied gas solvent alone, a flash point higher than 150° C., or no flash point, wherein the liquefied gas solvent is selected from methane, fluoromethane, difluoromethane, chloromethane, dichloromethane, ethane, fluoroethane, difluoroethane, tetrafluoroethane, chloroethane, dichloroethane, tetrachloroethane, propane, fluoropropane, chloropropane, ethylene, fluoroethylene, chloroethylene, or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
Certain polyester compositions which comprise cyclobutanediol, cyclohexanedimethanol, and high trans-cyclohexanedicarboxylic acid
InactiveUS20070232778A1Easily or more expensively stabilizedLess expensivePolyesterTetrachloroethane
Described as one aspect of the invention are polyester compositions comprising at least one polyester which comprises:(A) a dicarboxylic acid component comprising:i) 70 to 100 mole % of cyclohexanedicarboxylic acid residues or an ester thereof comprising:(a) 70 to 98 mole % trans-cyclohexanedicarboxylic acid residues or an ester thereof; and(b) 2 to 30 mole % cis-cyclohexanedicarboxylic acid residues or an ester thereof;ii) 0 to 30 mole % of aliphatic dicarboxylic acid residues, other than cyclohexanedicarboxylic acid residues, having up to 16 carbon atoms or esters thereof, other than cyclohexanedicarboxylic acid residues,; andiii) 0 to 10 mole % of aromatic dicarboxylic acid residues having up to 20 carbon atoms; and(B) a glycol component comprising:i) 1 to 99 mole % of 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues; andii) 1 to 99 mole % of 1,4-cyclohexanedimethanol residues, 1,3-cyclohexanedimethanol residues, 1,2-cyclohexanedimethanol residues or esters thereof or mixtures thereof,wherein the total mole % of said dicarboxylic acid component is equal to 100 mole %;the total mole % of said glycol component is equal to 100 mole %;wherein the inherent viscosity of said polyester is from 0.35 to 1.2 dL / g as determined in 60 / 40 (wt / wt) phenol / tetrachloroethane at a concentration of 0.5 g / 100 ml at 25° C.; and wherein said polyester has a Tg of from 60 to 155° C. The polyesters may be manufactured into articles.
Owner:EASTMAN CHEM CO
Polyester compositions which comprise cyclobutanediol, cyclohexanedimethanol, and ethylene glycol and manufacturing processes therefor
Described as one aspect of the invention are polyester compositions comprising at least one polyester which comprises: (a) a dicarboxylic acid component comprising: (i) about 90 to about 100 mole % of terephthalic acid residues; (ii) about 0 to about 10 mole % of aromatic and / or aliphatic dicarboxylic acid residues having up to 20 carbon atoms; and (b) a glycol component comprising: (i) about 20 to about 40 mole % 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues; and (ii) about 20 to about 40 mole % cyclohexanedimethanol residues; (iii) ethylene glycol residues, and (iv) less than about 2 mole % of a modifying glycol having from 3 to 16 carbon atoms; wherein the total mole % of the dicarboxylic acid component is 100 mole %, and wherein the total mole % of the glycol component is 100 mole %; and wherein the inherent viscosity of the polyester is from 0.50 to 1.2 dL / g as determined in 60 / 40 (wt / wt) phenol / tetrachloroethane at a concentration of 0.25 g / 50 ml at 25° C. The polyesters may be manufactured into articles.
Owner:EASTMAN CHEM CO
Crystalline polyester resins and processes for their preparation
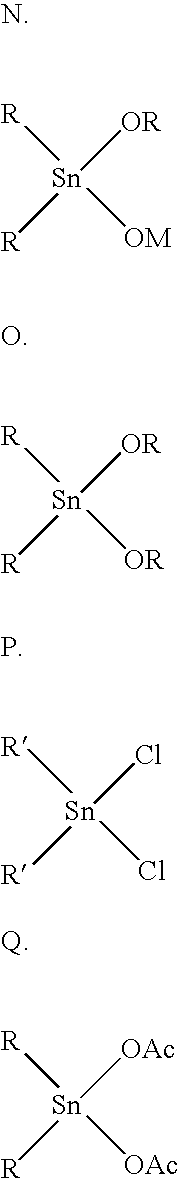
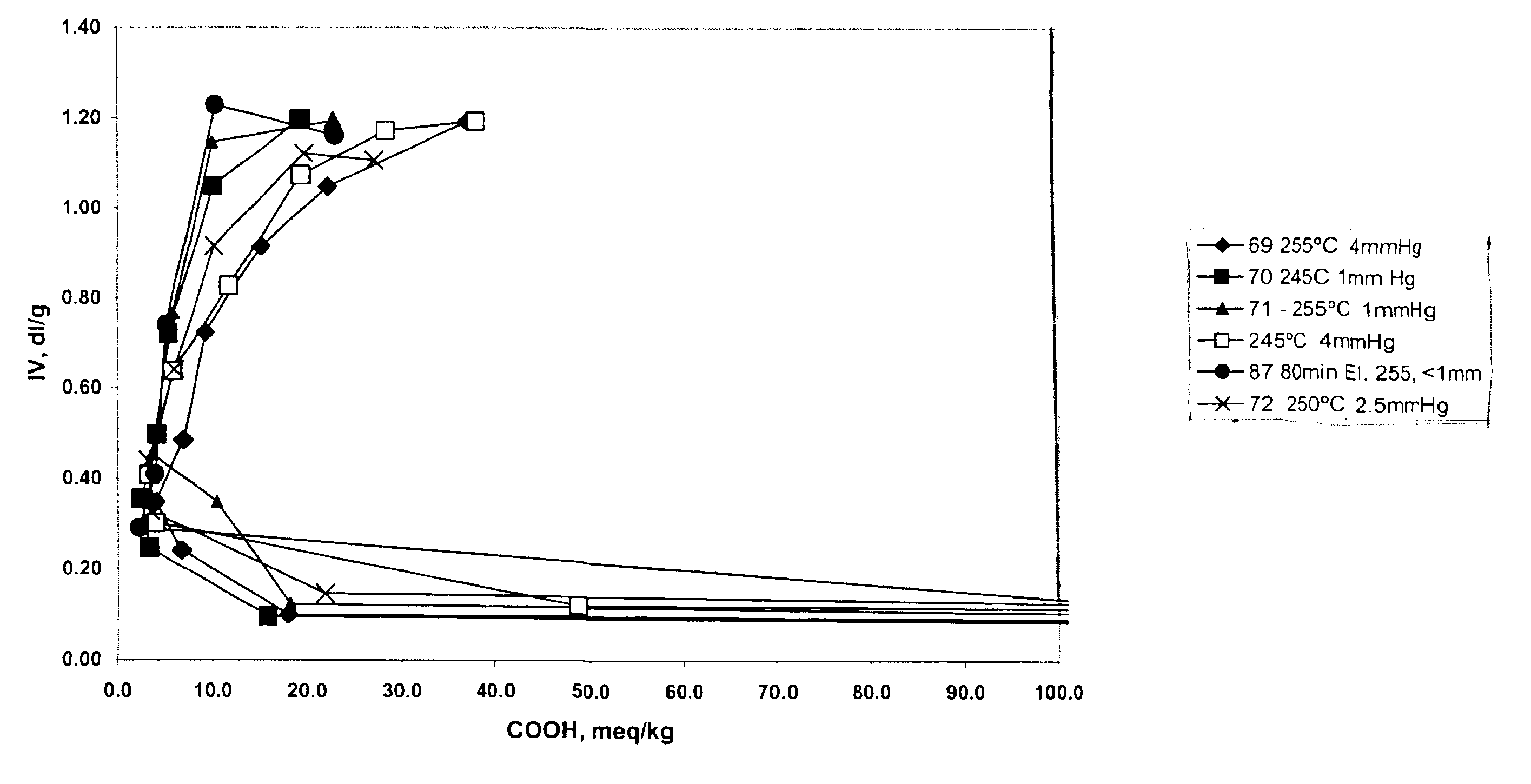
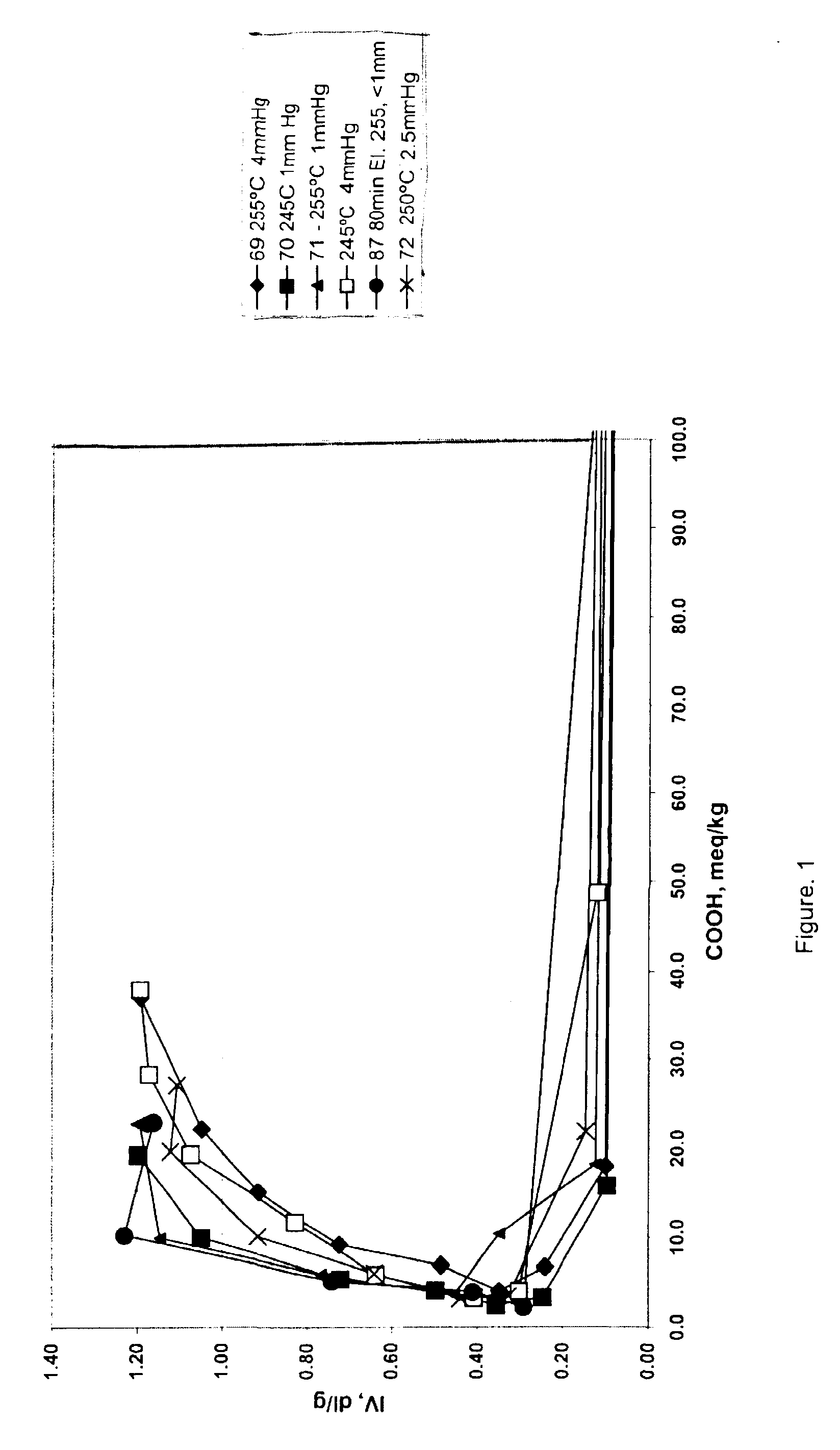
An improved process for the preparation of high molecular weight, linear polyester resins comprises reacting an aromatic dicarboxylic acid with an excess of an alkane diol under conditions effective to reach the clearing point of the reaction; pre-condensing the cleared reaction mixture under conditions effective to produce oligomers having an intrinsic viscosity measured in 60 / 40 phenol / 1,1,2,2-tetrachloroethane at 25° C. of less than about 0.70 deciliters / gram and a carboxylic acid end group level of less than or equal to about 100 milliequivalents per kilogram; and polycondensing the oligomer under conditions effective to produce a linear polyester resin having an intrinsic viscosity less than or equal to about 2.0 dl / g as measured in 60 / 40 phenol / 1,1,2,2-tetrachloroethane by weight at 25° C. and a carboxylic acid end group level of about 10 to about 40 milliequivalents per kilogram.
Owner:SABIC GLOBAL TECH BV
Catalyst composition for ethylene oligomerization and the use thereof
ActiveUS7786336B2High activityGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbons from unsaturated hydrocarbon addition1-OcteneAluminoxane
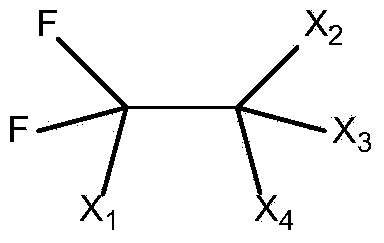
The present invention relates to a catalyst composition for ethylene oligomerization and the use thereof. Such catalyst composition includes chromium compound, ligand containing P and N, activator and accelerator; wherein the chromium compound is selected from the group consisting of acetyl acetone chromium, THF-chromium chloride and Cr(2-ethylhecanoate)3; general formula of the ligand containing P and N is shown as:in which R1, R2, R3 and R4 are phenyl, benzyl, or naphthyl. R5 is isopropyl, butyl, cyclopropyl, cyclopentyl, cyclohexyl or fluorenyl; the activatior is methyl aluminoxane, ethyl aluminoxane, propyl aluminoxane and / or butyl aluminoxane; the accelerator is selected from the group consisting of 1,1,2,2,-tetrachloroethane, 1,1,2,2-tetrabromoethane, 1,1,2,2-tetrafluoroethane, and compounds having a formula of X1R6X2, in which X1 and X2 are F, Cl, Br, I or alkoxyl, R6 is alkylene or arylene group; the molar ratio of chromium compound, ligand containing P and N, activator and accelerator is 1:0.5˜10:50˜3000:0.5˜10. After mixing the four components mentioned previously under nitrogen atmosphere for 10 minutes, they are incorporated to the reactor, or these four components are incorporated directly into the reactor. Then ethylene is introduced for oligomerization. Such catalyst can be used in producing 1-octene through ethylene oligomerization. It is advantageous in high catalysing activity, high 1-octene selectivity, etc. The catalytic activity is more than 1.0×106 g product·ma−1 Cr·h−1, the fraction of C8 linear α-olefin is more than 70% by mass.
Owner:PETROCHINA CO LTD
Cellulose ester blends
This invention relates to a blend comprising(a) about 2% to about 98% by weight of at least one ester of cellulose comprising an alkanoyl chain having from about 1 to about 10 carbon atoms, and having at a D.S. of about 2.3 to about 3.0, and an inherent viscosity of about 0.2 to about 3.0 deciliters / gram as measured at a temperature of 25° C. for a 0.5 g sample in 100 ml of a 60 / 40 parts by weight solution of phenol / tetrachloroethane, and(b) about 98% to about 2% by weight of at least one ester of cellulose comprising an alkanoyl chain having from about 1 to about 10 carbon atoms, and having a D.S. of about 1.5 to about 2.2, and an inherent viscosity of about 0.2 to about 3.0 deciliters / gram as measured at a temperature of 25° C. for a 0.5 g sample in 100 ml of a 60 / 40 parts by weight solution of phenol / tetrachloroethane, said percentages being based on the weight of component (a) plus component (b).
Owner:EASTMAN CHEM CO
Preparation method of trifluoroacetic acid
InactiveCN103524325ALow costPreparation from carboxylic acid halideHalogenated hydrocarbon preparationCatalytic oxidationTetrachloroethane
The invention discloses a preparation method of trifluoroacetic acid low in synthesis cost. The preparation method comprises the steps of (1) preparing 1,1-difluoro tetrachloroethane from 1,1-difluoroethane or chloride thereof through ultraviolet catalysis and a chlorine reaction; (2) implementing catalytic oxidation on the 1,1-difluoro tetrachloroethane to obtain 1,1-difluoro-1-chloroacetyl chloride, wherein in the catalytic oxidation, mol ratio of the 1,1-difluoro tetrachloroethane to an oxidizing agent is 1: (2-4), dosage of the catalyst is 0.5-5% of the total weight of an reactant, and reaction temperature is 50-70 DEG C; (3) carrying out hydrogen fluoride (HF) fluoridation on the 1,1-difluoro-1-chloroacetyl chloride under the catalyst to obtain trifluoroacetyl fluoride, wherein mol ratio of the 1,1-difluoro-1-chloroacetyl chloride to the HF is 1: (2-3), reaction temperature is 40-60 DEG C, and the catalyst is either antimony or antimony pentachloride; and (4) hydrolyzing the prepared trifluoroacetyl fluoride to obtain trifluoroacetic acid.
Owner:CHANGSHU 3F ZHENFU NEW MATERIALS CO LTD
Polyester compositions which comprise cyclobutanediol, cyclohexanedimethanol, and ethylene glycol and manufacturing processes therefor
Described as one aspect of the invention are polyester compositions comprising at least one polyester which comprises:(a) a dicarboxylic acid component comprising:(i) about 90 to about 100 mole % of terephthalic acid residues;(ii) about 0 to about 10 mole % of aromatic and / or aliphatic dicarboxylic acid residues having up to 20 carbon atoms; and(b) a glycol component comprising:(i) about 20 to about 40 mole % 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues; and(ii) about 20 to about 40 mole % cyclohexanedimethanol residues;(iii) ethylene glycol residues, and(iv) less than about 2 mole % of a modifying glycol having from 3 to 16 carbon atoms;wherein the total mole % of the dicarboxylic acid component is 100 mole %, andwherein the total mole % of the glycol component is 100 mole %; andwherein the inherent viscosity of the polyester is from 0.50 to 1.2 dL / g as determined in 60 / 40 (wt / wt) phenol / tetrachloroethane at a concentration of 0.25 g / 50 ml at 25° C. The polyesters may be manufactured into articles.
Owner:EASTMAN CHEM CO
Process for preparing brominated polystyrene
The invention provides a preparation method of bromized polystyrene. The method is characterized in that sulfur is taken as the scavenging agent of a small amount of water in the electrophilic bromination reaction of the polystyrene and as the quencher of free radical in the electrophilic bromination reaction of the polystyrene, and the mass of the sulfur is 0.1 to 12 percent of that of the polystyrene. In the organic solvent of dichloroethane, chloroform, bromochloromethane, carbon tetrachloride or tetrachloroethane, the anhydrous aluminium trichloride, the anhydrous ferric chloride, the titanium tetrachloride, the antimonous chloride, the aluminum powder, the zinc powder or the iron powder is taken as the catalyst, the sulfur is taken as the scavenging agent of the small amount of water and as the quencher of the free radical, and the bromized polystyrene is prepared by the electrophilic bromination reaction between the polystyrene and the bromine.
Owner:HUAIHAI INST OF TECH
A kind of method that takes dichloroethane as raw material to produce trichloroethylene and tetrachloroethylene
InactiveCN102267863ATo satisfy the market's needsSimple processPreparation by hydrogen halide split-offTetrachloroethaneEthylene Dichloride
The invention relates to a method for preparing trichloroethylene and perchloroethylene from dichloroethane as a raw material. The existing method has the defects of difficult realization and large amount of investment. The method of the invention comprises the following steps that dichloroethane undergoes chlorination to produce a mixture of dichloroethane, trichloroethane, tetrachloroethane, pentachloroethane and hexachloroethane, and hydrogen chloride gas under a dichloroethane gaseous or liquid phase; the mixture is transferred into a rectifying tower to be rectified; a tetrachloroethane / pentachloroethane mixture fraction with a top temperature of 147 to 162 DEG C is collected; the collected tetrachloroethane / pentachloroethane mixture fraction is vaporized and then is fed into a fixedbed reactor to form a trichloroethylene / perchloroethylene gas mixture; the trichloroethylene / perchloroethylene gas mixture is condensed into a liquid mixture; the liquid mixture and hydroquinone are fed into a rectifying tower to be rectified; a fraction with a top temperature of 86 to 87.5 DEG C is collected to obtain trichloroethylene finished products; and a fraction with a top temperature of 120 to 121.5 DEG C is collected to obtain perchloroethylene finished products. The method has the advantages of simple process, good controllability, easy acquirement of raw materials, low cost and little or no process wastewater or waste residue.
Owner:内蒙古磐迅科技有限责任公司
Nitrogen-doped activated carbon catalyst and application thereof
ActiveCN104475143AImprove catalytic performanceImprove conversion ratePreparation by hydrogen halide split-offPhysical/chemical process catalystsGas phaseTetrachloroethane
The invention discloses a nitrogen-doped activated carbon catalyst and application thereof. The preparation method of the nitrogen-doped activated carbon catalyst comprises the following steps: adding a nitrogen-containing compound into water to dissolve to obtain a nitride solution, wherein the nitrogen-containing compound is one or more of ammonia water, ammonia gas, pyridine, pyrrole, imidazole, acrylamide, polyacrylamide and polyvinylpyrrolidone; adding activated carbon into the nitride solution to soak; completely drying the soaked activated carbon, and calcining in nitrogen atmosphere, thereby preparing the nitrogen-doped activated carbon catalyst. The nitrogen-doped activated carbon catalyst disclosed by the invention has very good catalysis property in reaction of catalyzing tetrachloroethane and acetylene to generate trichloro ethylene and chloroethylene and catalyzing tetrachloroethane gas phase cracking to generate trichloro ethylene.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Inflaming retarding segmented copolymer containing phosphorus and silicon and preparation method thereof
ActiveCN104211880AMild reaction conditionsImprove flame retardant performanceGroup 5/15 element organic compoundsBenzoic acidFreeze thawing
An inflaming retarding segmented copolymer containing phosphorus and silicon and a preparation method thereof relate to the segmented copolymer. The preparation method includes mixing 9,10-dihydro-9-oxa-10- phosphaphenanthrene-10-oxide and carbon tetrachloride and adding the mixture into a solvent to obtain a mixture A; mixing methyl-hydroxyethyl acrylate and a triethylamine salt solution, adding the mixture into the mixture A, conducting filtering after reaction and conducting washing to obtain HEDOPO; dissolving the HEDOPO, dithio-benzoic acid-cumyl-ester and AIBN into the solvent, conducting freeze thawing degasification post polymerization reaction, stopping reaction through liquid nitrogen sudden cooling and conducting sedimentation to obtain a PHEDOPO macromolecule chain transfer agent; dissolving the PHEDOPO macromolecule chain transfer agent, 3-(isobutene-acyl-oxygen)-propyl-trimethoxy silane and the AIBN into tetrachloroethane, conducting continuous freeze thawing degasification post polymerization reaction, stopping the reaction through liquid nitrogen sudden cooling and conducting sedimentation through a precipitant to obtain the product the inflaming retarding segmented copolymer containing phosphorus and silicon.
Owner:XIAMEN UNIV
Biaxially oriented polyester film for sealing back surface of photovoltaics
ActiveUS20120178897A1Improve hydrolysis resistanceLow shrinkageDomestic articlesPhotovoltaic energy generationPolyesterPolymer science
The present invention provides a biaxially oriented polyester film for a backsheet of photovoltaics which exhibits an excellent hydrolysis resistance and a low shrinkage rate. The present invention relates to a biaxially oriented polyester film for a backsheet of photovoltaics, comprising a polyester having an intrinsic viscosity of 0.65 to 0.90 dL / g as measured in a mixed solvent comprising phenol and tetrachloroethane at a mass ratio of 50 / 50 at 23° C., a carboxyl end group content of 0 to 26 equivalents / t, and a phosphorus element content of 0 to 170 ppm, which biaxially oriented polyester film has a shrinkage rate of not more than 0.8% as measured in a longitudinal direction thereof after subjected to heat treatment at 150° C. for 30 min.
Owner:MITSUBISHI CHEM CORP
Biodegradable nanofiber nonwoven fabric capable of preventing postoperative adhesion and preparation thereof
InactiveCN1876926AGood flexibilityPromote absorptionFilament/thread formingNon-woven fabricsPolyesterTextile fiber
The invention provides a biodegradable textile fiber adhesive-bonded fabric to prevent adhesion after the operation and its preparing method, which belongs to the domain of medical article, and relates to the preparation of a nanometre-sized fiber adhesive-bonded fabric and the application as the anti-block film after the operation. The said adhesive-bonded fabric is prepared by adopting electrostatic spinning method, which includes preparing spin dope, dissolving the aliphatic polyester in the flux and mixing intimately to prepare the aliphatic polyester spin dope. The used flux of the spin dope can be one or compound system of two of carrene, chloroform, acetone, acetylene tetrachloride,acetic acid dimethylamide. Secondarily on the electrostatic spinning machine, by filling spin dope, electrostatic spinning and collecting superfine fibre, the aliphatic polyester superfine fibre is prepared, which carries out the adhesive-bonded fabric of electronic spinning nanometre-grade fiber and which can be used as blocking agent after the operation by the decontamination and biocidal treatment. The cloth has good flexibility and toughness, which can overcome the defect of anti-block film reported in the past. Because the degradable materials are selected, the adhesive-bonded fabric begins to degrade after about one month and completely degrade finally, which comes up to the anti-block function.
Owner:薛占强
Trichloroethylene gas phase catalysis production method
InactiveCN102775269ASimple processPreparation by hydrogen halide split-offGas phaseTetrachloroethane
The invention provides a trichloroethylene gas phase catalysis production method. Acetylene and chlorine are dried to be respectively sent to an acetylene chlorination tower, are subjected to chlorination reaction, then are gasified through a tetrachloroethane tower and a refined tetrachloroethane vaporizer, react through a splitting reactor, and are led in a desorber and a low boiling tower to reach a trichloroethylene tower to form trichloroethylene products, and matters produced after trichloroethylene is separated are processed through an inter-chamber tower to be led in another tetrachloroethylene tower to form tetrachloroethylene products. Mother liquor produced after the tetrachloroethylene is separated returns the back part of the tetrachloroethane tower to continuously participate in the reaction, materials passing through the desorber pass through an adsorption tower to adsorb hydrogen chloride, and residual materials are delivered to a soda plant for producing polyvinyl chloride raw materials. According to the trichloroethylene gas phase catalysis production method, the tetrachloroethylene products are obtained while trichloroethylene is produced, and by-products can be used for producing raw materials of polyvinyl chloride products. The trichloroethylene gas phase catalysis production method is applicable to application of production of the trichloroethylene products by using acetylene and chlorine.
Owner:HULUDAO DESIGN INST PETROCHINA NORTHEAST REFINING & CHEM ENG
Aromatic polyester and manufacturing process thereof
To provide an aromatic polyester which is formed into a film having excellent heat resistance, color, mechanical properties, dimensional stability and gas barrier properties and a manufacturing process thereof;the aromatic polyester comprises a dicarboxylic acid component and a diol component, wherein(i) the dicarboxylic acid component contains 50 to 100 mol % of a recurring unit represented by the following formula (A):wherein R is an alkylene group having 2 to 10 carbon atoms,(ii) the aromatic polyester has an intrinsic viscosity measured at 35° C. by using a mixed solvent of P-chlorophenol and 1,1,2,2-tetrachloroethane (weight ratio of 40 / 60) of 0.4 to 3.0;(iii) the aromatic polyester has a content of a recurring unit represented by the following formula (D) of less than 10 mol %:—O—CH2CH2—O—CH2CH2—O— (D)(iv) the aromatic polyester has a terminal carboxyl group concentration of 200 eq / ton or less; and(v) the aromatic polyester has an alkali metal content of 300 ppm or less.
Owner:TEIJIN LTD
Axially-chiral indole-naphthalene compounds and preparation method thereof
ActiveCN110452150AHigh yieldControlling enantioselectivityOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsXylyleneChemical structure
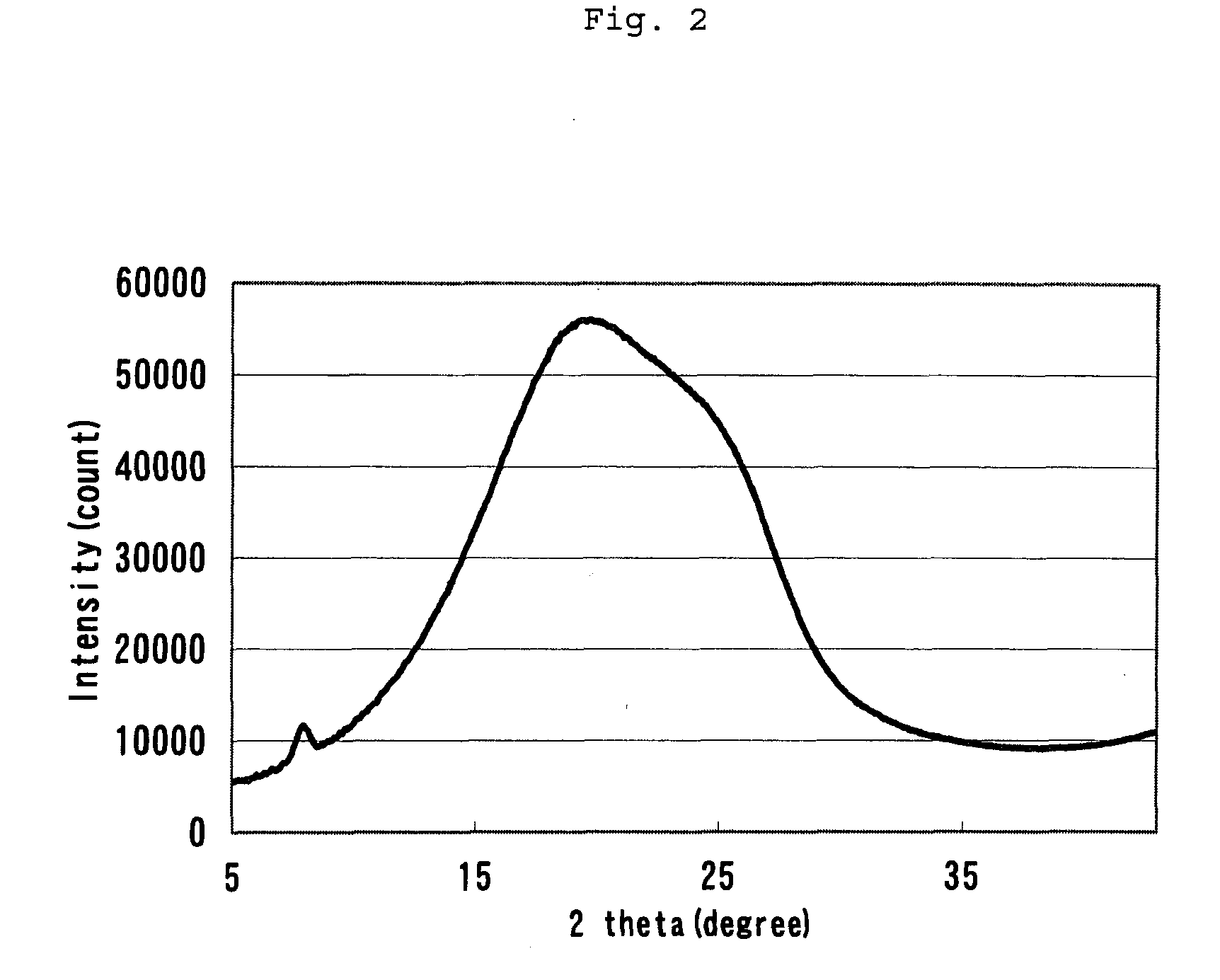
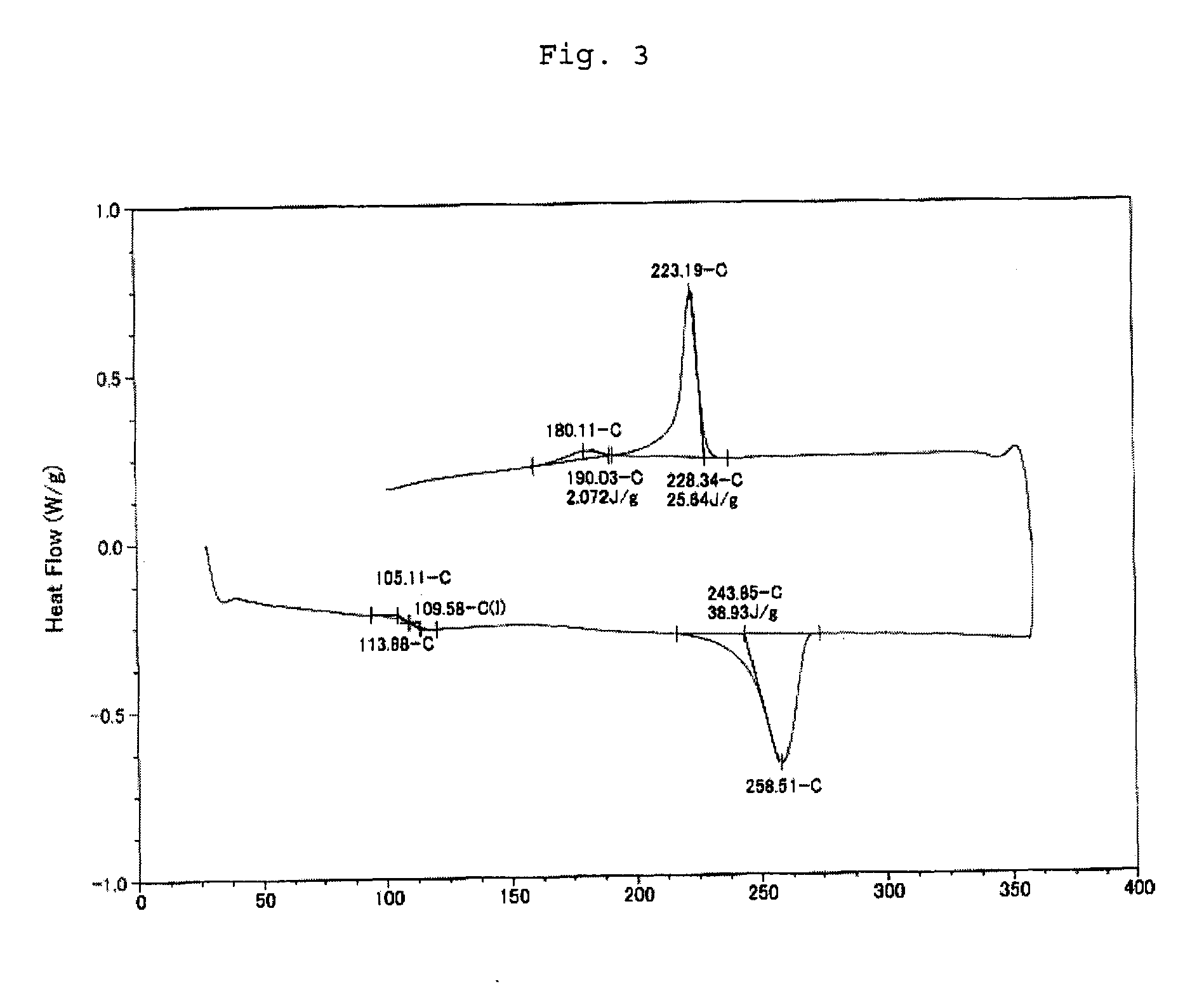
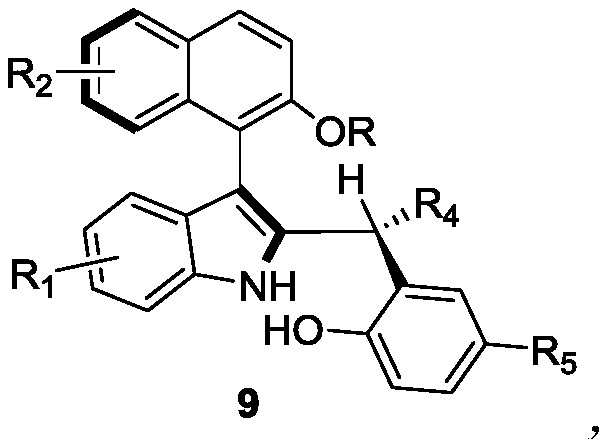
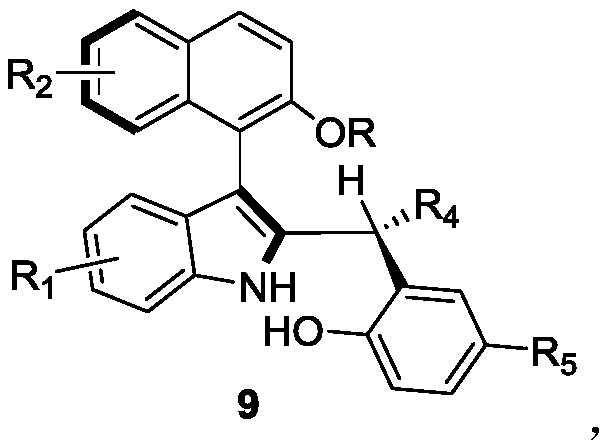
The invention discloses axially-chiral indole-naphthalene compounds and a preparation method thereof. The axially-chiral indole-naphthalene compounds have a chemical structure represented by a formula9 shown in the description; and the method comprises the following steps: a compound represented by a formula 7 and a compound represented by a formula 8 are used as reaction raw materials, a mixed solution of 1,1,2,2-tetrachloroethane and p-xylene is used as a solvent, a molecular sieve is added, a reaction is performed under stirring under the action of a chiral phosphoric acid catalyst, the reaction is tracked by TLC until the reaction is completed, filtration is performed, concentration is performed, purification is performed, and therefore one compound is obtained. The method for preparing the axially-chiral indole-naphthalene compounds provided by the invention is an asymmetric addition reaction under catalysis of organic small molecules, starts from the racemic raw material to construct the axially-chiral indole-naphthalene structure in one step, and has the advantages of simple and convenient operation, mild reaction conditions and economical easily-available raw materials, and the prepared axially-chiral indole-naphthalene compounds have high optical purity; and the axially-chiral indole-naphthalene compounds prepared by the method are expected to be widely used in the field of asymmetric catalysis.
Owner:XUZHOU NORMAL UNIVERSITY
Polybutylene Terephthalate Pellet, Compound Product and Molded Product Using the Same, and Processes for Producing the Compound Product and Molded Product
InactiveUS20070265382A1Excellent in color toneImprove hydrolysis resistanceConductive materialNon-conductive material with dispersed conductive materialPolytetramethylene terephthalateTetrachloroethane
An object of the present invention is to provide a polybutylene terephthalate pellet capable of producing a molded product which is excellent in color tone, hydrolysis resistance, transparency and molding stability, and has a less content of impurities. A polybutylene terephthalate pellet comprises polybutylene terephthalate containing titanium in an amount of not more than 90 ppm by weight, as calculated as titanium atom, and having an end methoxycarbonyl group concentration of not more than 0.5 μeq / g, wherein said pellet has an average intrinsic viscosity of 0.90 to 2.00 dL / g and a difference in intrinsic viscosity between a central portion and a surface layer portion of the pellet is not more than 0.10 dL / g. As preferred embodiments, there is exemplified a polybutylene terephthalate pellet having an end carboxyl concentration of 10 to 25 μeq / g, an end vinyl concentration of 0.1 to 10 μeq / g, and an solution haze of not more than 5%, when measured as a turbidity value of a solution prepared by dissolving 2.7 g of polybutylene terephthalate in 20 mL of a mixed solution containing phenol and tetrachloroethane at a weight ratio of 3:2.
Owner:MITSUBISHI CHEM CORP
Polybutylene terephthalate
InactiveUS20090264611A1Excellent color toneImprove heat stabilityPolytetramethylene terephthalateFiber
Polybutylene terephthalate has an intrinsic viscosity of 0.7 to 1.0 dL / g and an end carboxyl group concentration of 0.1 to 18 μeq / g, which is produced in a presence of a catalyst comprising a titanium compound and a metal compound containing a metal of Group 2A of the Periodic Table. In the preferable embodiment of the present invention, the polybutylene terephthalate has a crystallization temperature of 170 to 195° C. as measured at a temperature drop rate of 20° C. / min using a differential scanning calorimeter, an end vinyl group concentration of not more than 10 μeq / g, and not more than 10% of a solution haze of a solution prepared by dissolving 2.7 g of said polybutylene terephthalate in 20 mL of a mixed solvent containing phenol and tetrachloroethane at a weight ratio of 3:2.The polybutylene terephthalate of the present invention exhibits excellent color tone, hydrolysis resistance, heat stability, transparency and moldability as well as a less content of impurities, which is suitably applicable to films, monofilaments, fibers, electric and electronic parts, automobile parts, etc.
Owner:MITSUBISHI CHEM CORP
Method for preparing strong-hydrophilicity PET membrane
InactiveCN105435651AStrong anti-pollutionLow priceMembranesSemi-permeable membranesTetrachloroethaneCompanion animal
A disclosed method for preparing a strong-hydrophilicity PET membrane comprises the following steps: a, dissolving stannous chloride, cupric chloride or calcium chloride into an acetone solution, so as to obtain a doping solution; b, adding nanometer silicon dioxide into deionized water, performing ultrasonic dispersing, then adding a silane coupling agent under stirring condition, and adjusting pH value of the solution with a hydrochloric acid, so as to obtain a modified nanometer silicon dioxide solution; c, adding a PET particle into a mixed solvent of tetrachloroethane and phenol, heating to completely dissolve PET particle, and then adding the doping solution obtained in the steps a, so as to prepare a PET membrane forming solution, and dropwise adding the PET film forming solution to a glass slide, and then putting in a vacuum baker for drying, so as to obtain a blended membrane; and d, immersing the blended membrane obtained in the step c in the modified nanometer silicon dioxide solution obtained in the step b, and taking out and naturally drying in air after immersion is finished, so as to obtain the strong-hydrophilicity PET membrane. The prepared strong-hydrophilicity PET membrane possesses strong anti-pollution capability and possesses relatively good application prospect in the fields of water processing industry and the like.
Owner:SHANDONG UNIV OF SCI & TECH
Preparation method of high-molecular copolymer cationic polyacrylamide flocculant
ActiveCN104558399AApplicable treatmentHigh viscosity average molecular weightWater/sewage treatment by flocculation/precipitationPolymer scienceTetrachloroethane
The invention relates to a preparation method of a high-molecular copolymer cationic polyacrylamide flocculant. The product is prepared by copolymerization of acrylamide-methacrylic acid-2-oxylactone trimethylammonium chloride. Acrylamide (AM), trimethylamine hydrochloride, chloropropylene oxide, tetrachloroethane, methacrylic acid and deionized water, etc. used as raw materials undergo aqueous solution polymerization at normal pressure. During the reaction process, an additive, a polymerization inhibitor, an emulsifier and an initiator are added, and monomer concentration is controlled to 30-50%. Then, a cationic flocculant which meets usage requirements and has 2-5*106 of viscosity-average molecular weight can be prepared stably. Yield is greater than 95.0%. The whole polymerization only takes 6-7 hours to prepare the flocculant. Water quality index of sewage treated by the use of the prepared flocculant is more superior, and usability of the flocculant is approximately the same as that of a foreign similar product.
Owner:CHINA PETROLEUM & CHEM CORP +1
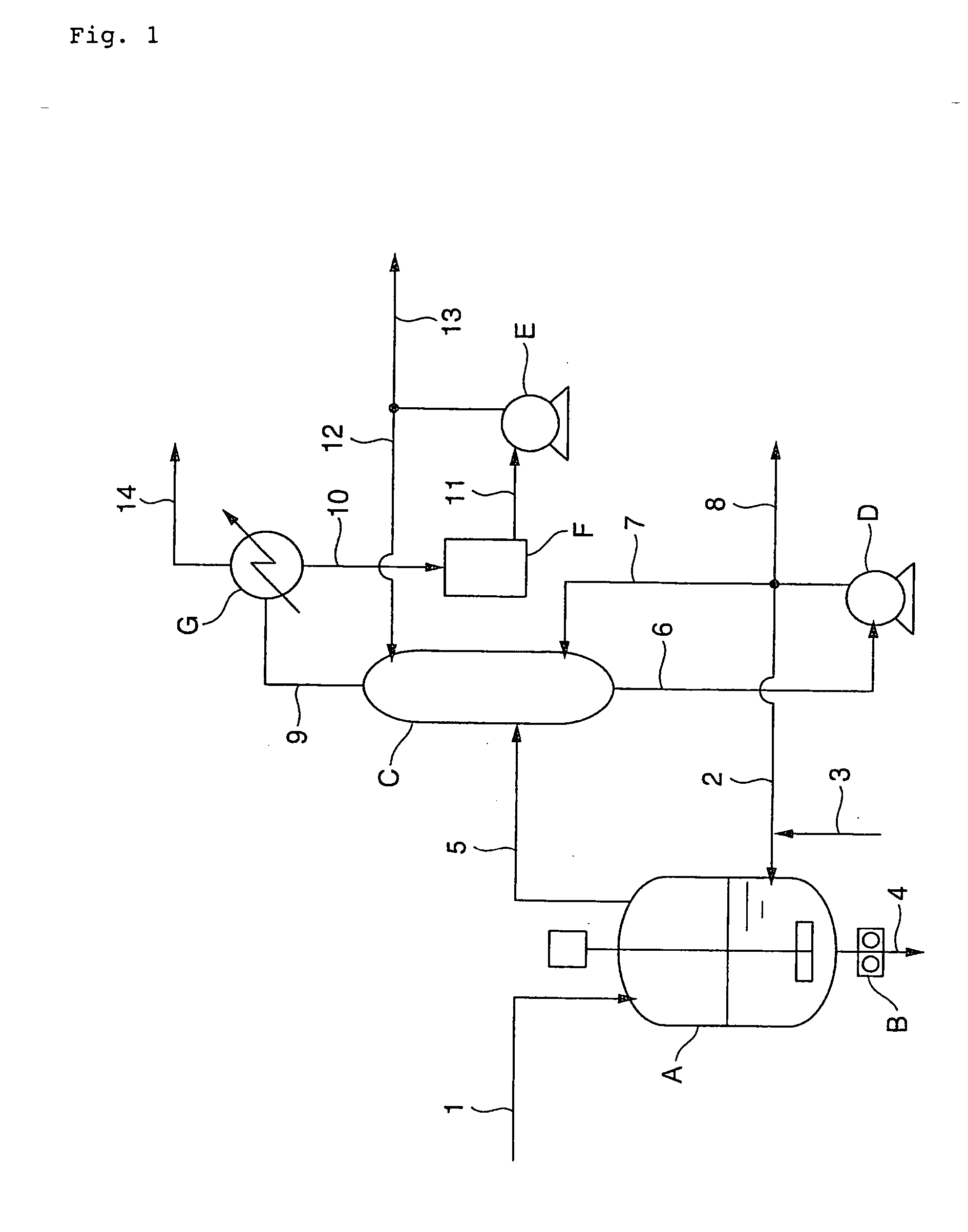
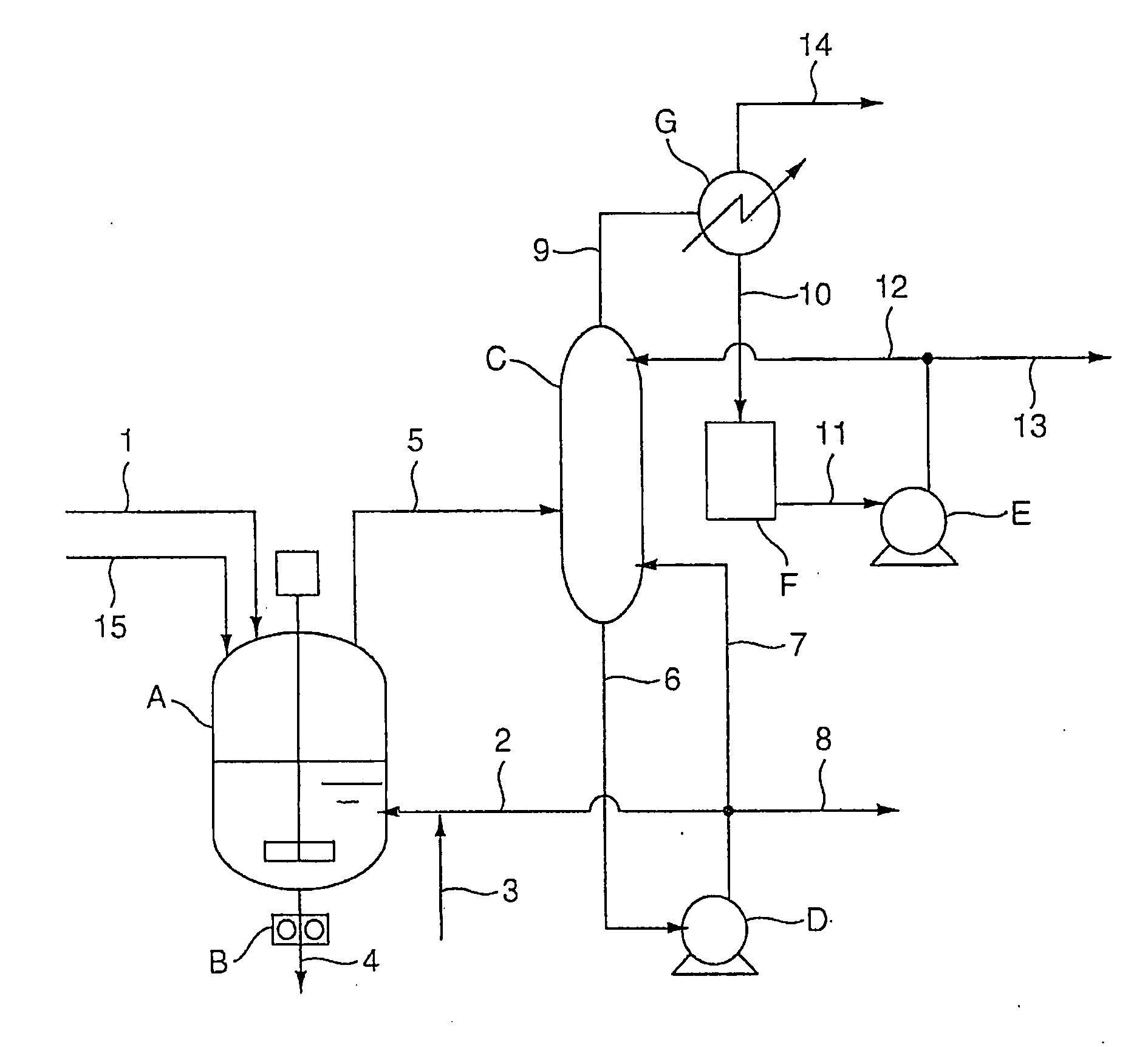
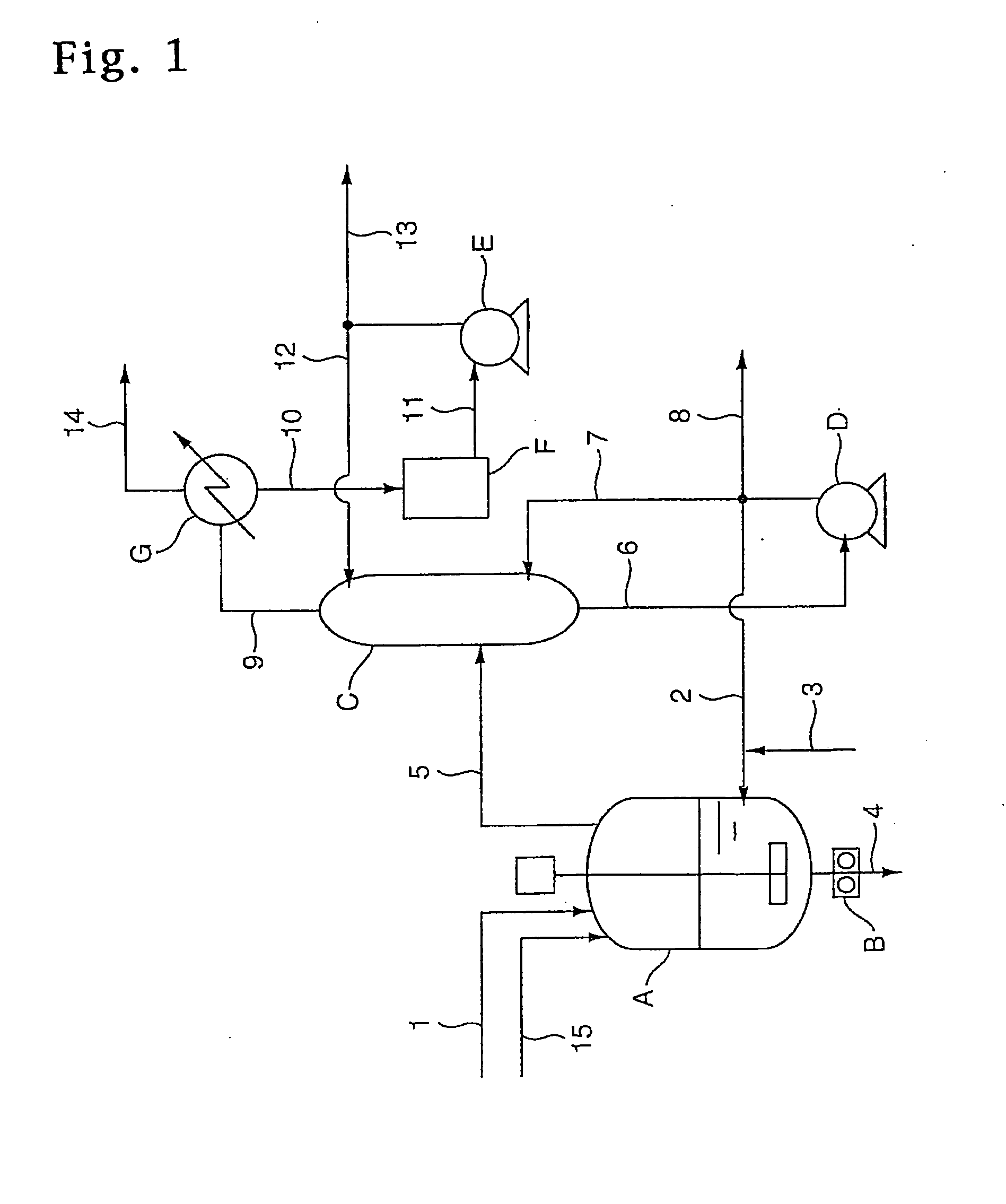
Trichlorethylene industrial production plant
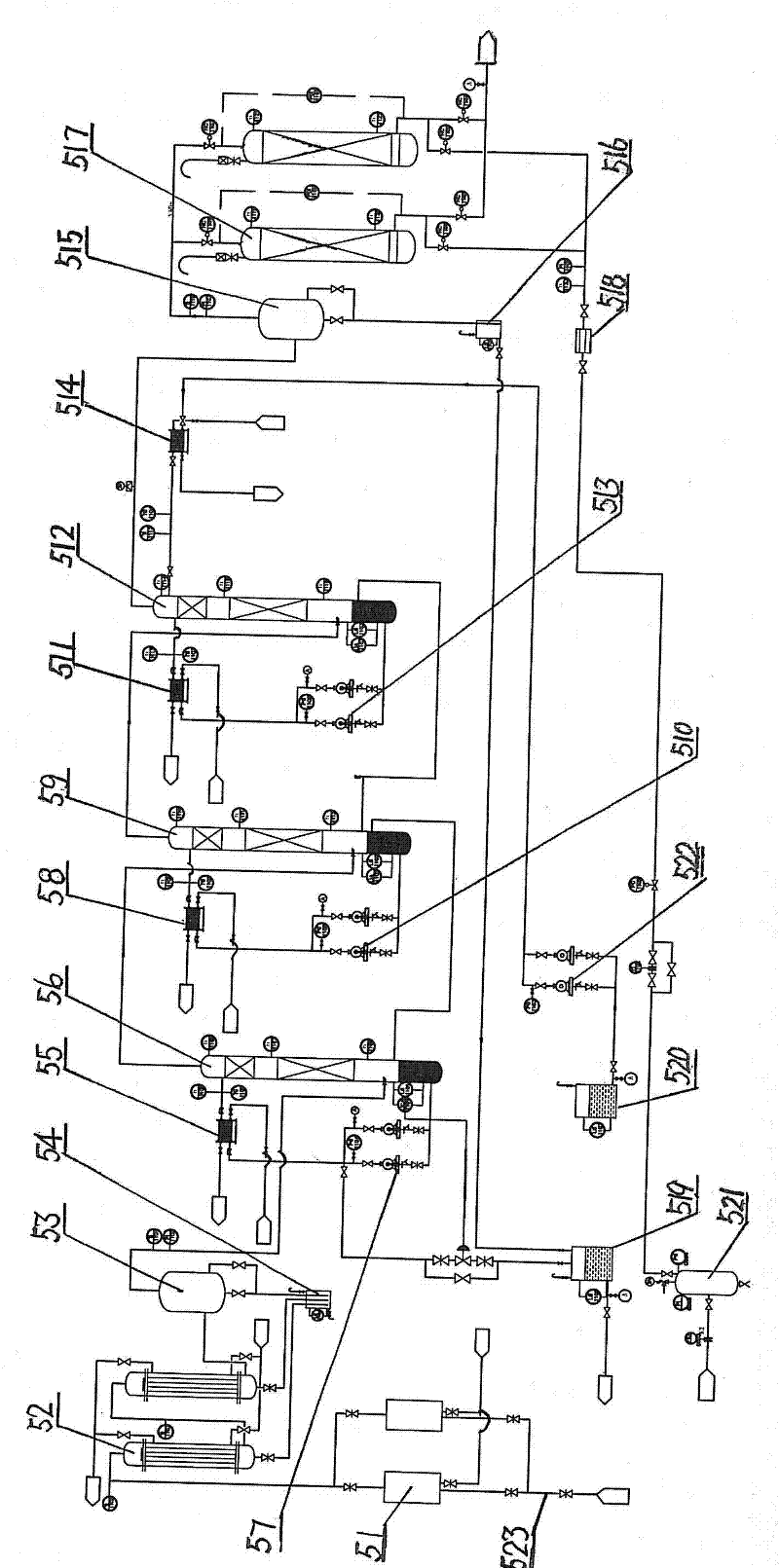
ActiveCN102267868AImprove product qualityReasonable structurePreparation by hydrogen halide split-offBiochemical engineeringDesorption
The invention provides an industrial production apparatus for trichloroethylene. The apparatus is characterized in that: an acetylene air inlet pipeline and a chlorine gas air inlet pipeline are respectively connected with an inlet end of a chlorination tower; an outlet end of a crude tetrachloroethane tank is connected with an inlet of a tetrachloroethane tower; an outlet end of a material feeding preheater of a dehydrochlorination reactor is connected with an inlet end of a desorber; an outlet end of a desorption residue tank is connected with an inlet end of a low-boiler tower; an outlet end arranged on the lower portion of the low-boiler tower is respectively connected with an inlet end of a reboiler of the low-boiler tower and an inlet end of a trichloroethylene tower; an outlet end of a trichloroethylene residue tank is connected with an inlet end of a middle distillate tower; an outlet end arranged on the lower portion of the middle distillate tower is respectively connected with an inlet end of a reboiler of the middle distillate tower and an inlet end of a tetrachloroethylene tower; heat exchanging devices of each complete condenser and each end cooler adopt graphite parts dipped in modified phenol formaldehyde resin. The industrial production apparatus for the trichloroethylene has characteristics of reasonable structure, large production capacity, safe, stable and long-period operation, and the prepared trichloroethylene has high quality.
Owner:BEFAR GROUP CO LTD
Grafted copolymer of cellulose and derivatives thereof and synthesizing method of grafted copolymer
The invention discloses a grafted copolymer of cellulose and derivatives thereof and a synthesizing method of the grafted copolymer. The method comprises the steps that 1, terephthalic acid and ethylene glycol are subjected to an esterification reaction according to the molar ratio of 1:(1.2-2) under the action of a catalyst at 200 DEG C to 240 DEG C, a condensation polymerization reaction is conducted for 10 min to 80 min on the condition that the temperature ranges from 250 DEG C to 285 DEG C and the vacuum degree ranges from 100 Pa to 6,000 Pa, and hydroxylation-terminated polyethylene glycol terephthalate with the molecular weight ranging from 2,000 to 15,000 is synthesized; 2, synthesized PET is dissolved in tetrachloroethane, diisocyanate and a catalyst are added, a reaction is conducted for 3 h to 6 h at 60 DEG C to 80 DEG C, and an isocyanate-terminated prepolymer is obtained; 3, the cellulose and derivatives thereof are ultrasonically dissolved into an acetone solution, the obtained product is added into the tetrachloroethane solution of the isocyanate-terminated prepolymer, a reaction is conducted for 3 days to 7 days, and the grafted copolymer is obtained after purification is conducted. The grafted copolymer is synthesized from the cellulose and derivatives thereof and small molecular weight PET, and the advantages of being simple in process and high in grafting ratio are achieved. The obtained grafted copolymer is used for cellulose and PET blending and can improve the compatibility of the cellulose and PET.
Owner:CHINA PETROLEUM & CHEM CORP +1
Polyester Compositions Which Comprise Cyclobutanediol and at Least One Phosphorus Compound
InactiveUS20100298523A1Improve thermal stabilityEasy to manufactureCyclohexanedimethanolPolymer science
The present invention relates to a process for making polyester compositions containing: (I) at least one polyester which comprises: (a) a dicarboxylic acid component comprising: (i) 70 to 100 mole % of terephthalic acid residues; (ii) 0 to 30 mole % of aromatic dicarboxylic acid residues having up to 20 carbon atoms; and (iii) 0 to 10 mole % of aliphatic dicarboxylic acid residues having up to 16 carbon atoms; and (b) a glycol component comprising: (i) 1 to 99 mole % of 2,2,4,4-tetramethyl-1,3-cyclobutanediol residues; and (ii) 1 to 99 mole % of cyclohexanedimethanol residues; and (II) at least one thermal stabilizer chosen from at least one phosphorus compound, reaction products thereof, and mixtures thereof; wherein the total mole % of the dicarboxylic acid component is 100 mole %, and wherein the total mole % of the glycol component is 100 mole %; wherein the inherent viscosity of the polyester is from 0.35 to 1.2 dL / g as determined in 60 / 40 (wt / wt) phenol / tetrachloroethane at a concentration of 0.25 g / 50 ml at 25° C., wherein the polyester has a Tg from 85 to 200 C.
Owner:EASTMAN CHEM CO
Polyester compositions, methods of manufacture, and uses thereof
A composition is disclosed, comprising, based on the total weight of the composition, a combination of from 44 to 80 weight percent of a thermoplastic poly(butylene terephthalate); from 20 to 50 weight percent of a thermoplastic poly(ethylene terephthalate) having an intrinsic viscosity of 0.5 to 0.8 deciliters per gram, measured in a 60:40 by weight phenol / 1,1,2,2-tetrachloroethane mixture at 23° C. using a relative viscometer operating with a closed loop system, which measures the solvent and sample viscosity simultaneously; from 5 to less than 20 weight percent of a talc filler having an average largest dimension of less than 0.9 micrometers, a median particle size of less than 0.9 micrometers, or both; and from 0.1 to 0.5 weight percent of a mold release agent. The compositions are useful in the manufacture of lighting articles, which can be directly metallized without the inclusion of a base coat.
Owner:SABIC GLOBAL TECH BV
Amorphous copolyesters
Disclosed are amorphous copolyesters having an inherent viscosity (IV) of at least about 0.5 dL / g measured at a temperature of 25° C. at 0.5 g / dL concentration in a solvent mixture of symmetric tetrachloroethane and phenol having a weight ratio of symmetric tetrachloroethane to phenol of 2:3 comprising (1) a diacid component comprising about 90 to 100 mole percent terephthalic acid residues and 0 to about 10 mole percent isophthalic acid residues; and (2) a diol component comprising about 10 to 70 mole percent 1,4-cyclohexanedimethanol residues and about 90 to 30 mole percent neopentyl glycol residues; wherein the amorphous copolyesters comprises 100 mole percent diacid component and 100 mole percent diol component. The amorphous copolyesters are useful in the manufacture or fabrication of medical devices which have improved resistance to degradation upon exposure to lipids, as a profile produced by profile extrusion and as an injection molded article. Also, a method of melt processing the amorphous copolyester is disclosed which allows for performing a minimal drying or no drying of the copolyester prior to melt processing.
Owner:EASTMAN CHEM CO
Preparation method of phosphonitrile chloride catalyst, and application of phosphonitrile chloride catalyst in preparation of alkoxy-terminated polysiloxane
ActiveCN110655049AHigh catalytic activityHigh activityNitrogen compoundsPtru catalystTetrachloroethane
The invention discloses a preparation method of a phosphonitrile chloride catalyst, and an application of the phosphonitrile chloride catalyst in the preparation of alkoxy-terminated polysiloxane. Thepreparation method comprises the following steps: purging a reaction device with nitrogen to achieve preheating treatment, opening the reaction device to remove condensate water, and sequentially infiltrating the reaction device with dichloromethane and tetrachloroethane; mixing and uniformly stirring tetrachloroethane and ammonium chloride to prepare a tetrachloroethane solution of ammonium chloride for later use; adding phosphorus pentachloride into the reaction device, slowly raising the temperature to 140-160 DEG C under the protection of nitrogen, then dropwise adding the prepared tetrachloroethane solution of ammonium chloride, carrying out a reflux reaction after the drop-by-drop addition is completed, then raising the temperature to 170-175 DEG C, continuously carrying out the reflux reaction, and cooling the reaction device to room temperature after the reaction is completed; and adding anisole into the reaction solution, and performing stirring under a vacuum condition to achieve uniform mixing in order to obtain target crystals which are the phosphonitrile chloride. The prepared phosphonitrile chloride has a high catalytic activity; and when the phosphonitrile chlorideis used for preparing alkoxy-terminated polysiloxane, a polymer with an excellent storage stability can be effectively prepared.
Owner:新纳奇材料科技江苏有限公司
Trichloro ethylene preparation method
ActiveCN102911008ASimplify the delivery processIncrease added valuePreparation by hydrogen halide split-offPreparation by halogen halide additionGas phaseTetrachloroethane
A trichloro ethylene preparation method relates to chlorinated alkene. The method comprises the following steps: 1), mixing vaporized tetrachloroethane with acetylene to form a raw material mixture gas, and controlling the molar ratio of tetrachloroethane to acetylene at 1:(0.5-2); 2), pumping the raw material mixture gas into a reactor filled with a catalyst for reaction at controlled temperature, pressure and gas flow rate, so as to allow the conversion rate of acetylene or tetrachloroethane to reach more than 70 percent; and 3), cooling the reaction product obtained in the step 2 to a room temperature by water, and separating out liquid-phase product and gas-phase product, wherein the liquid-phase product passes through a rectification column to produce a trichloro ethylene product, the gas-phase product, which is subjected to compression and freezing, is adopted for further liquification separation of vinyl chloride, and the uncooled gas after the completion of compression and freezing is used for circulating and recycling. According to the method provided by the invention, tetrachloroethane is adopted to eliminate coupling between the reaction and an acetylene addition reaction so as to form intermolecular energy direct conversion; and accordingly, the energy transfer process is simplified, the reaction energy consumption is reduced, the technological process is also simplified, and the equipment investment is lower. In addition, as for by-products, vinyl chloride is higher than hydrochloric acid in additional value.
Owner:ZHONGKE YIGONG SHANGHAI CHEM TECH CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com