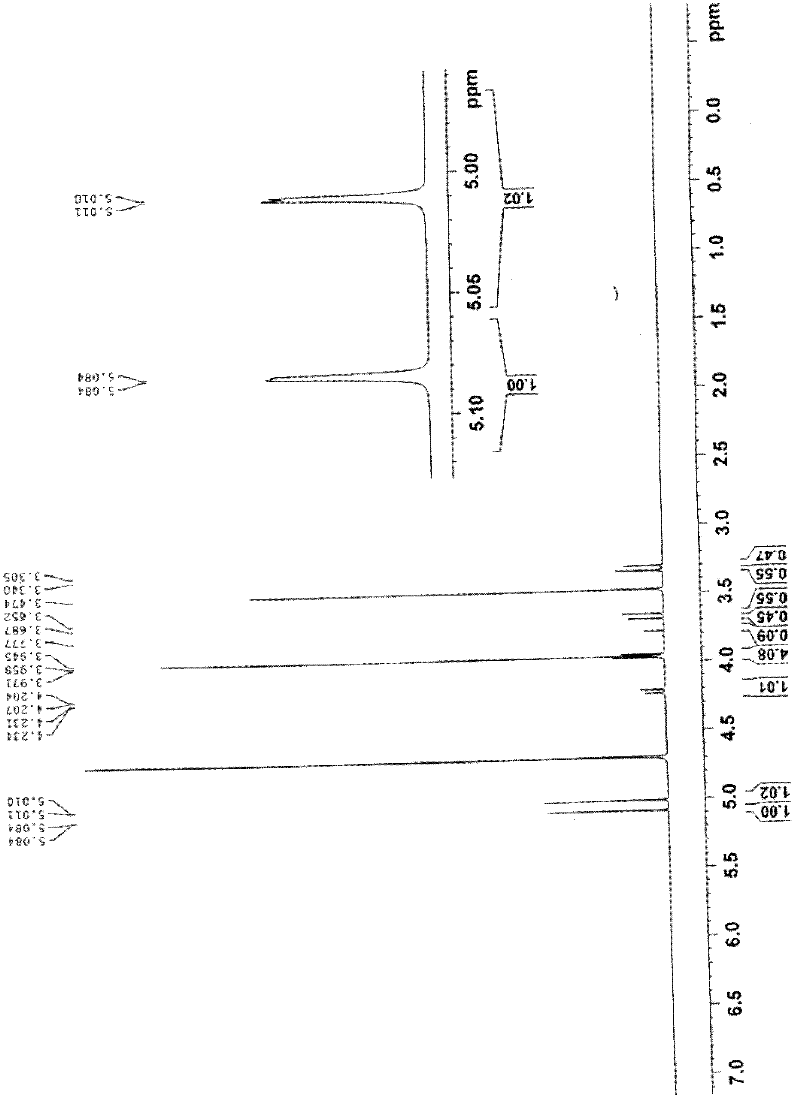
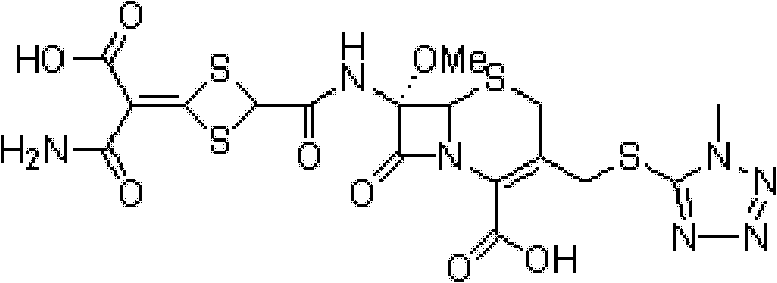
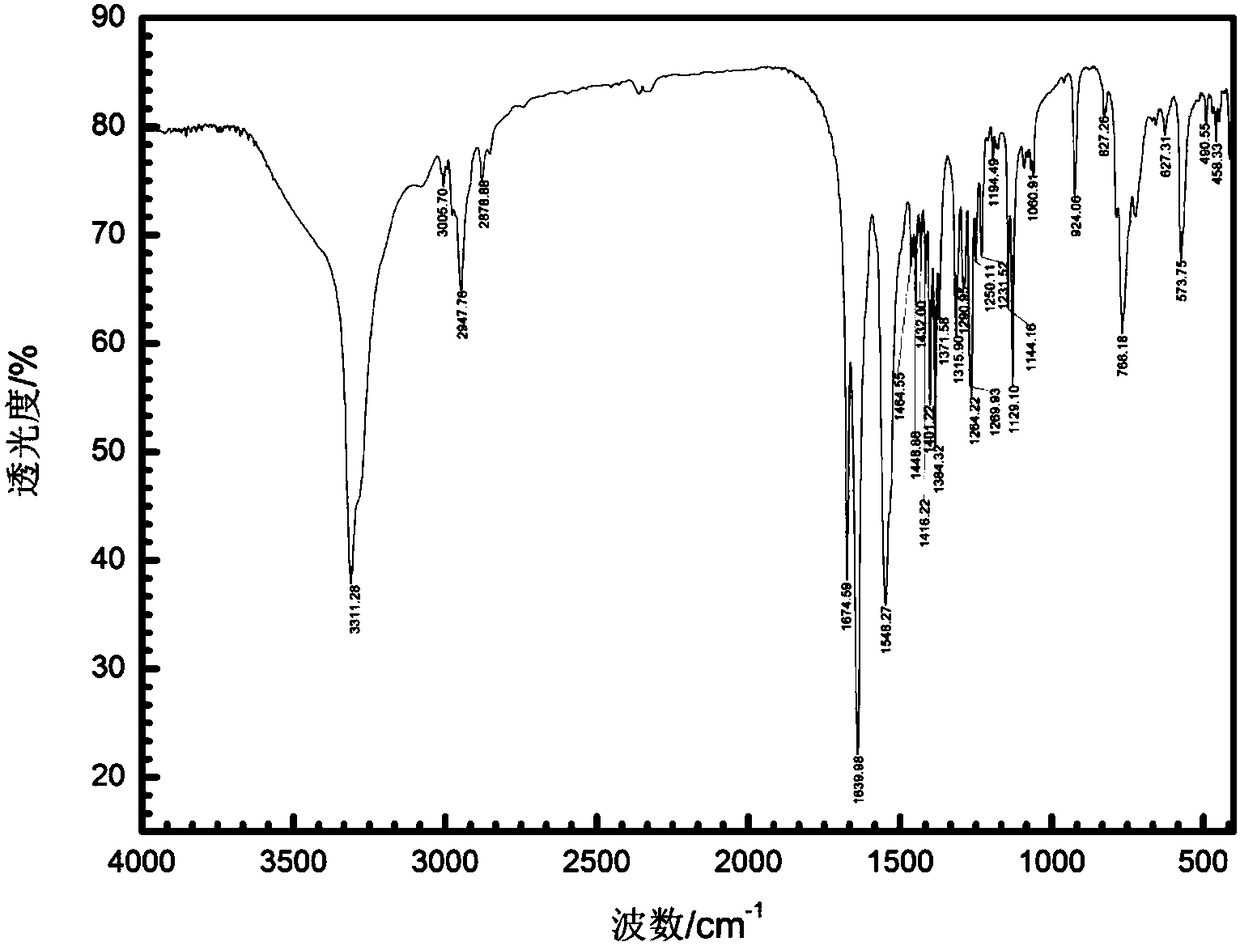
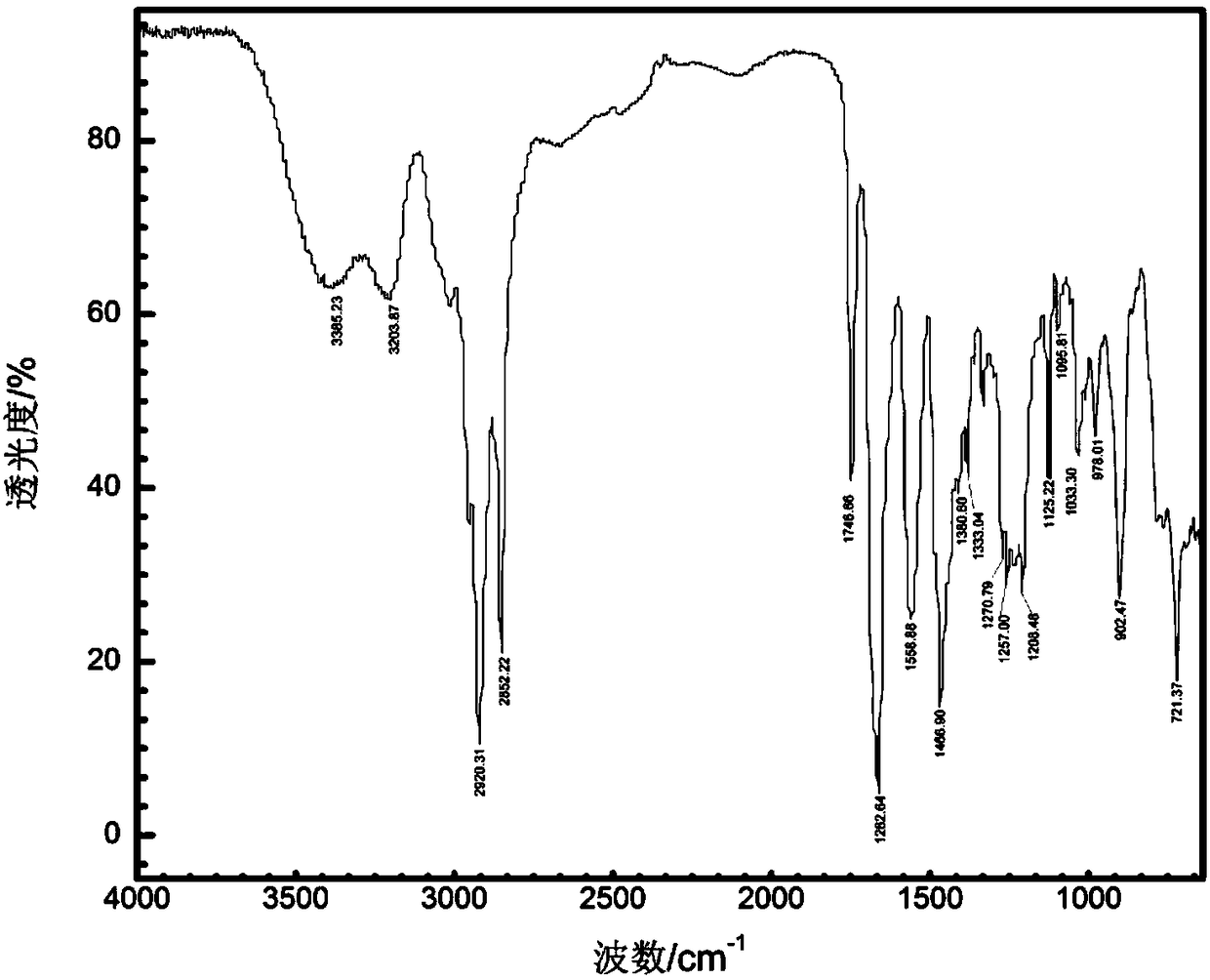
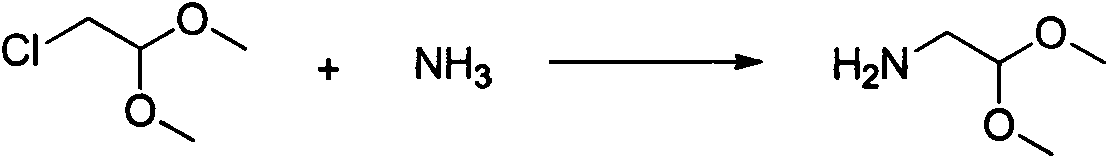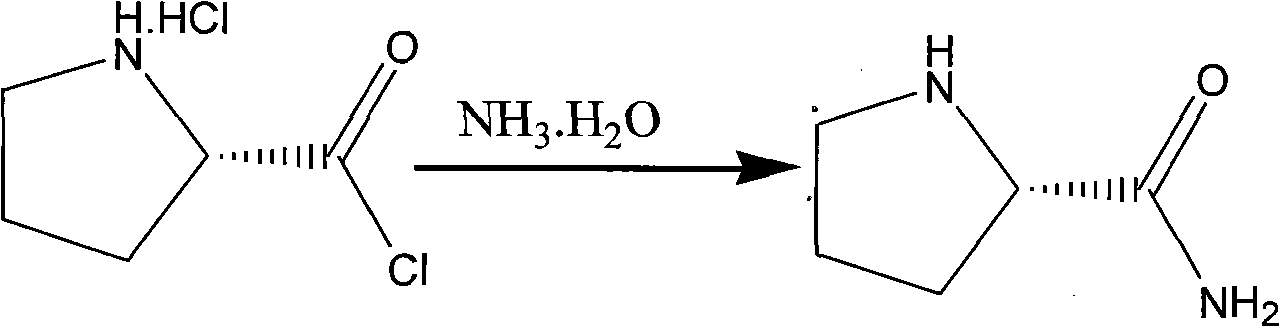Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
442 results about "Chloroacetyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
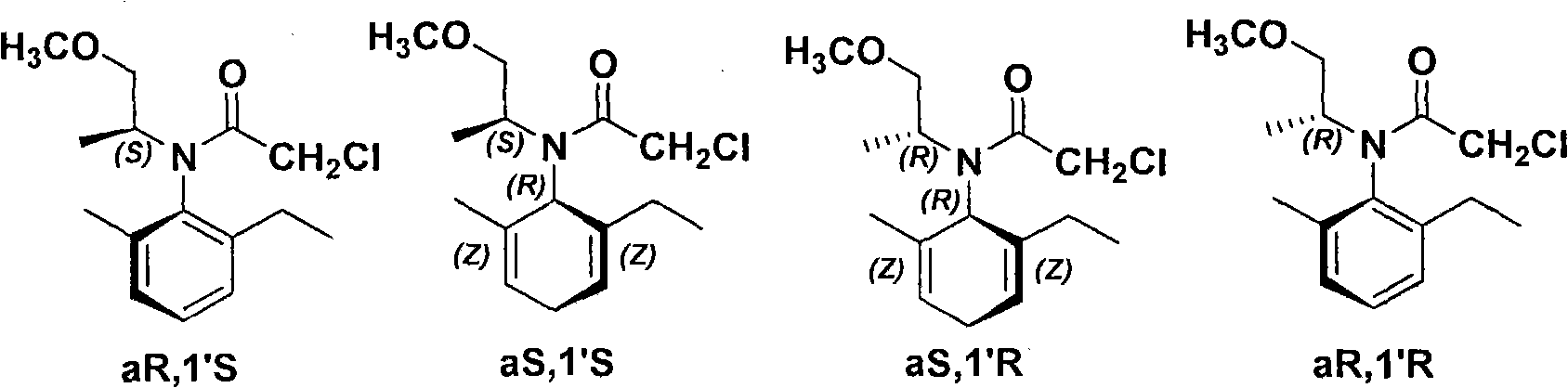
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
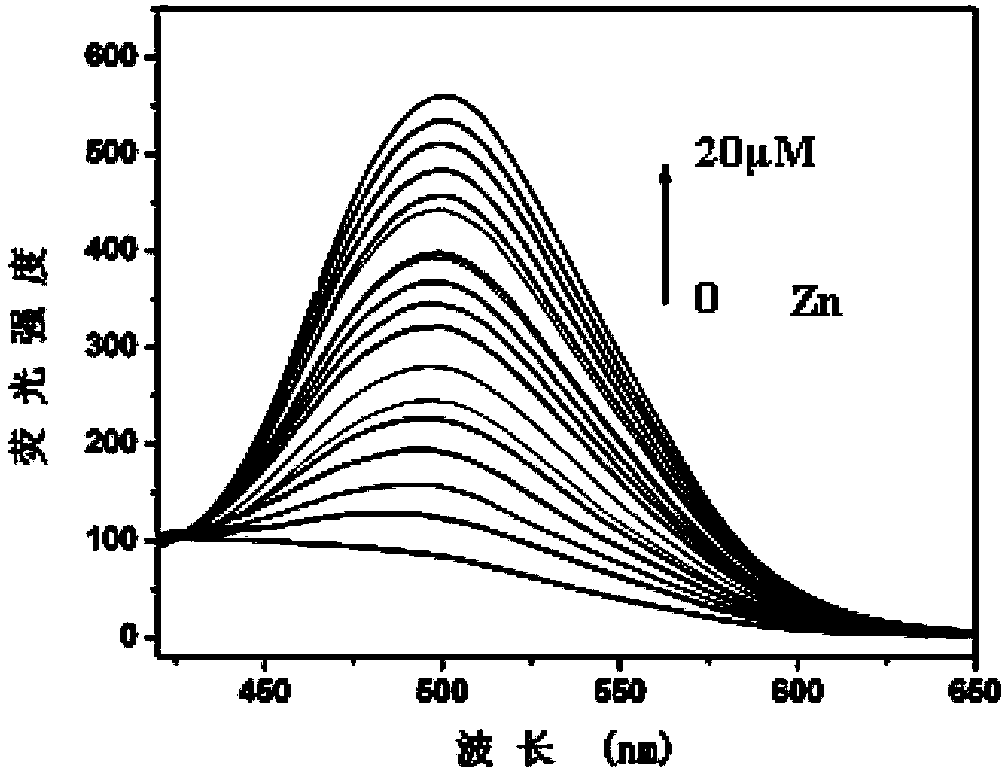
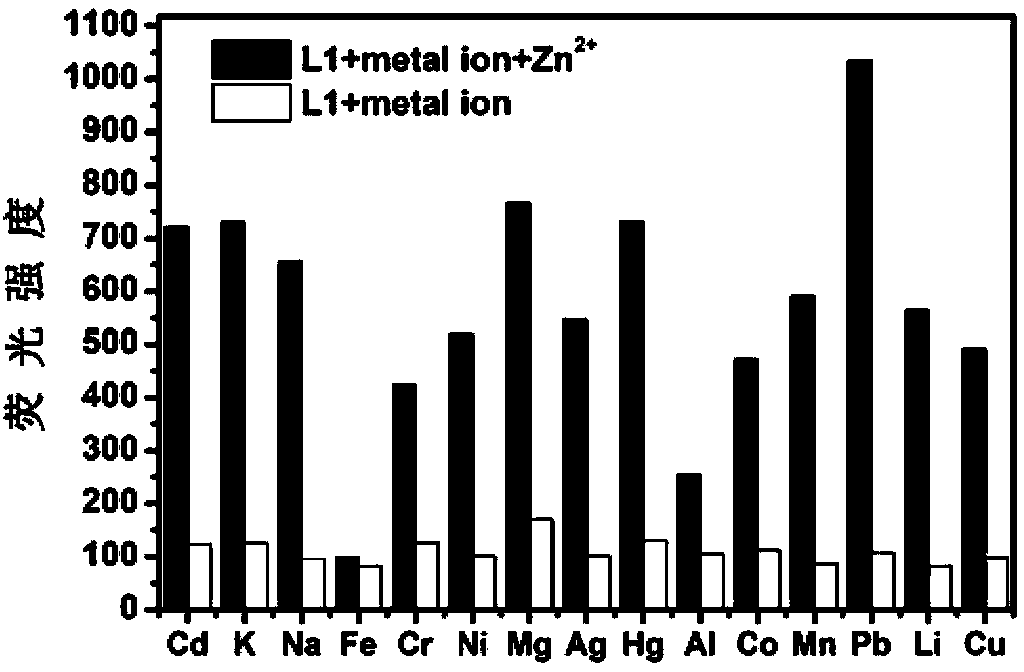
Fluorescence molecular probe for detecting zinc ions and preparation method thereof
The invention relates to a fluorescence molecular probe for detecting zinc ions. The fluorescence molecular probe has the following chemical structure. The fluorescence molecular probe is prepared by the following steps: carrying out a reaction on 8-amino quinoline and chloroacetyl chloride under the effect of pyridine to obtain an intermediate; and carrying out a reaction on the intermediate obtained under the effect of KOH and KI to obtain the fluorescence molecular probe. Compared with other probes for detecting zinc ions, the probe is easier to be dissolved in water, good in selectivity, free from interference quenching of copper, cobalt and nickel ions and strong in infiltration capacity, and can be used for quickly detecting zinc ions in organisms and plants.
Owner:LANZHOU UNIVERSITY
Preparation method of trifluoroacetic acid
InactiveCN103524325ALow costPreparation from carboxylic acid halideHalogenated hydrocarbon preparationCatalytic oxidationTetrachloroethane
The invention discloses a preparation method of trifluoroacetic acid low in synthesis cost. The preparation method comprises the steps of (1) preparing 1,1-difluoro tetrachloroethane from 1,1-difluoroethane or chloride thereof through ultraviolet catalysis and a chlorine reaction; (2) implementing catalytic oxidation on the 1,1-difluoro tetrachloroethane to obtain 1,1-difluoro-1-chloroacetyl chloride, wherein in the catalytic oxidation, mol ratio of the 1,1-difluoro tetrachloroethane to an oxidizing agent is 1: (2-4), dosage of the catalyst is 0.5-5% of the total weight of an reactant, and reaction temperature is 50-70 DEG C; (3) carrying out hydrogen fluoride (HF) fluoridation on the 1,1-difluoro-1-chloroacetyl chloride under the catalyst to obtain trifluoroacetyl fluoride, wherein mol ratio of the 1,1-difluoro-1-chloroacetyl chloride to the HF is 1: (2-3), reaction temperature is 40-60 DEG C, and the catalyst is either antimony or antimony pentachloride; and (4) hydrolyzing the prepared trifluoroacetyl fluoride to obtain trifluoroacetic acid.
Owner:CHANGSHU 3F ZHENFU NEW MATERIALS CO LTD
Preparation methods of anticoagulant and key intermediate of anticoagulant
The invention relates to a synthesis method of an anticoagulant and a key intermediate, namely 3-morpholinone and 4-[(4-nitrophenyl)]-3- morpholinone, of the anticoagulant. The synthesis of intermediate uses aminoethanol and chloroacetyl chloride as starting materials. And the intermediate is obtained through condensation, transesterification, cyclization, recondensation and reduction reaction. The operation is simple and suitable for industrial production. Dichloromethane or triethylamine is used as a solvent in the synthesis process of the product. The product is obtained after simple post-treatment. The purity of HPLC (high performance liquid chromatography) is more than or equal to 99.5%. The process is simple and easy to operate, and is suitable for industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Surface imprinted polymer for catalyzing degradation of organophosphorus pesticide and preparation method thereof
InactiveCN101942062AOvercome the disadvantages of poor mechanical properties and difficulty in repeated useOvercoming heterogeneous featuresOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkPolymer science
The invention discloses a surface imprinted polymer for catalyzing degradation of an organophosphorus pesticide and a preparation method thereof and relates to the field of surface imprinted polymers. The preparation method comprises the following steps of: preparing polystyrene seeds by a soap-free emulsion method; preparing polystyrene microspheres with particle diameter of 2 to 6 microns by a fractional swelling method; and bonding a chain transfer agent on the surfaces of the polystyrene microspheres. The preparation method comprises the main steps of: performing chloride acetylation on the microspheres with chloroacetic chloride, then aminating with ethydene diamine and then bonding the chain transfer agent with an amino; adding a template molecule under a proper solvent and performing graft polymerization on an amino acid derivative functional monomer and a cross-linking agent on the surfaces of the microspheres to form the needed polymer carrier surface template imprinted microspheres. The surface imprinted polymer with catalyzing, degrading and purifying functions on an organophosphorus compound is synthesized by taking an organophosphorus compound catalyzing and degradingreaction substrate as the template and organically combining the high-crosslinking degree insoluable porous polymeric microspheres with the template imprinting technology through a surface controllable graft hydrogel.
Owner:NANKAI UNIV
Method for preparing praziquantel
InactiveCN103739601AThe synthesis process is simpleImproved post-treatment processOrganic chemistryDimethyl acetalCyclohexanecarboxylic acid
The invention relates to a method for preparing praziquantel, which is a one-pot method and comprises the following steps: performing an ammonolysis reaction of chloroacetaldehyde dimethyl acetal and an ammonia aqueous solution to generate aminoacetaldehyde dimethyl acetal; performing a condensation reaction of beta-phenylethylamine and chloroacetyl chloride in an organic solvent in alkaline environment to generate an intermediate 1; performing a condensation reaction of the intermediate 1 and the aminoacetaldehyde dimethyl acetal in an organic solvent to generate an intermediate 2; performing cyclization of the intermediate 2 in the presence of an acidic catalyst to generate an intermediate 3; performing a reaction of the intermediate 3 and cyclohexanecarboxylic acid chloride in an organic solvent in alkaline environment, and performing solvent crystallization to obtain the target product of praziquantel.
Owner:JIANGSU CHENGXIN PHARMA
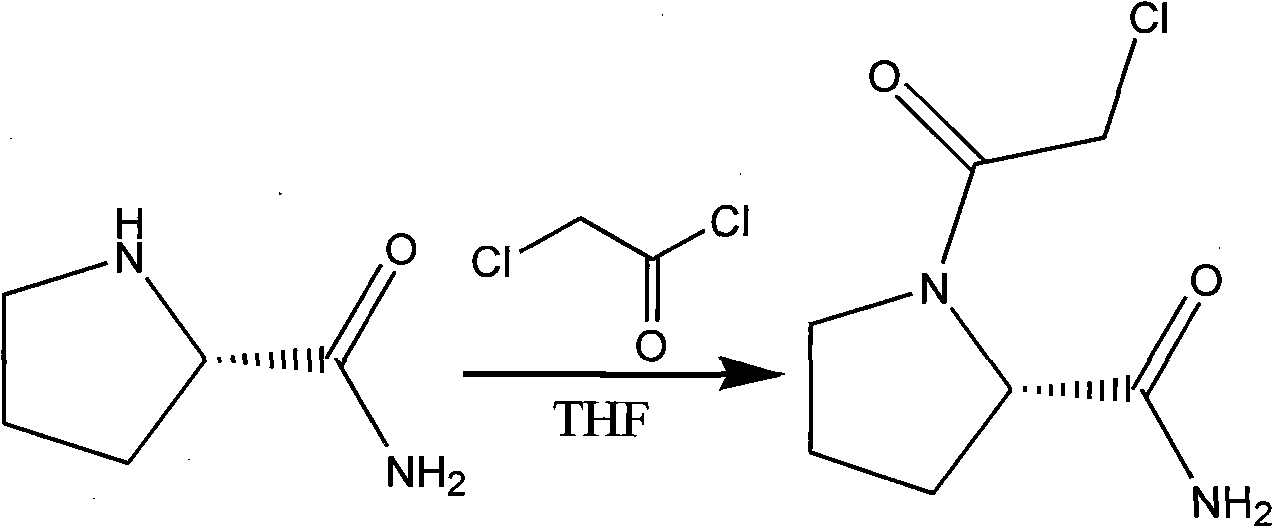
Efficient synthesis method of vildagliptin
ActiveCN104945299AReduce adverse effectsReduce solubilityOrganic chemistryOrganic baseSynthesis methods
The present invention relates to an efficient synthesis method of vildagliptin. According to the method, L-prolinamide is adopted as a raw material, N-chloro acetylation and amide dehydration are performed to generate an intermediate (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile, and the (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile and 3-amino adamantanol are subjected to condensation in acetonitrile in the presence of an organic base to obtain the target product vildagliptin. According to the present invention, the synthesis method operation only requires the separation of the one key intermediate, and the method has characteristics of simple and feasible operation, high efficiency, environmental protection, and easy industrial production achieving.
Owner:烟台万润药业有限公司
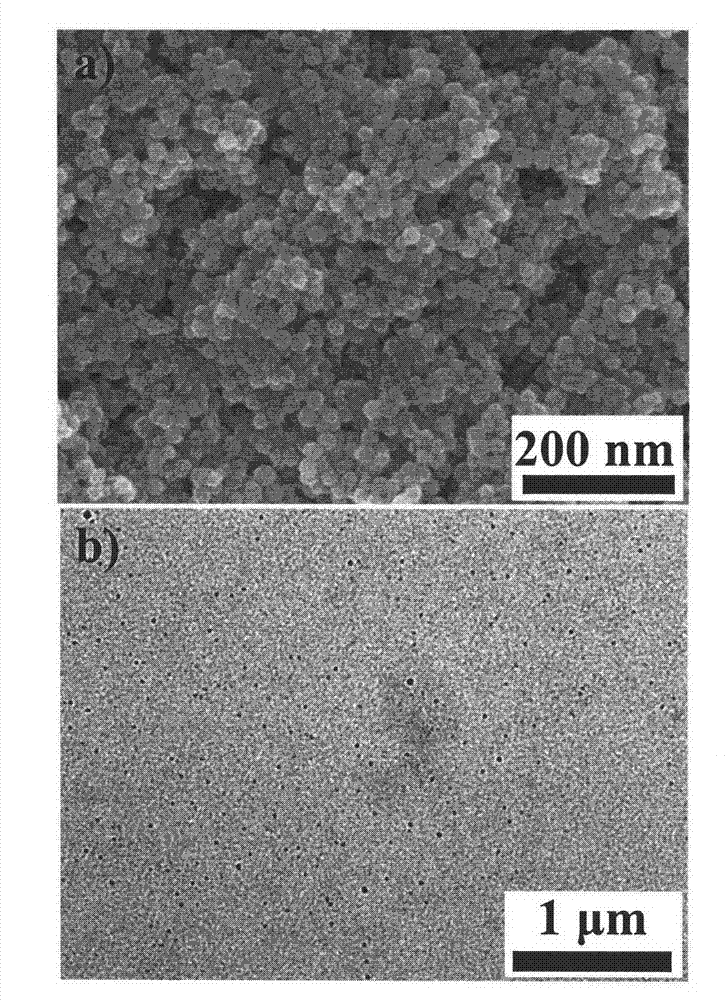
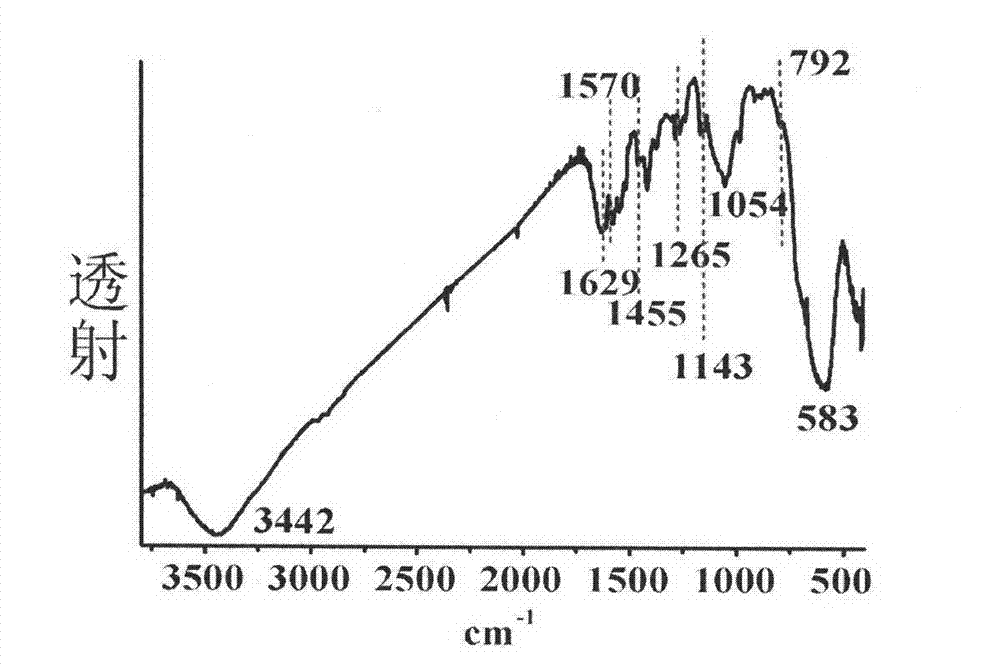
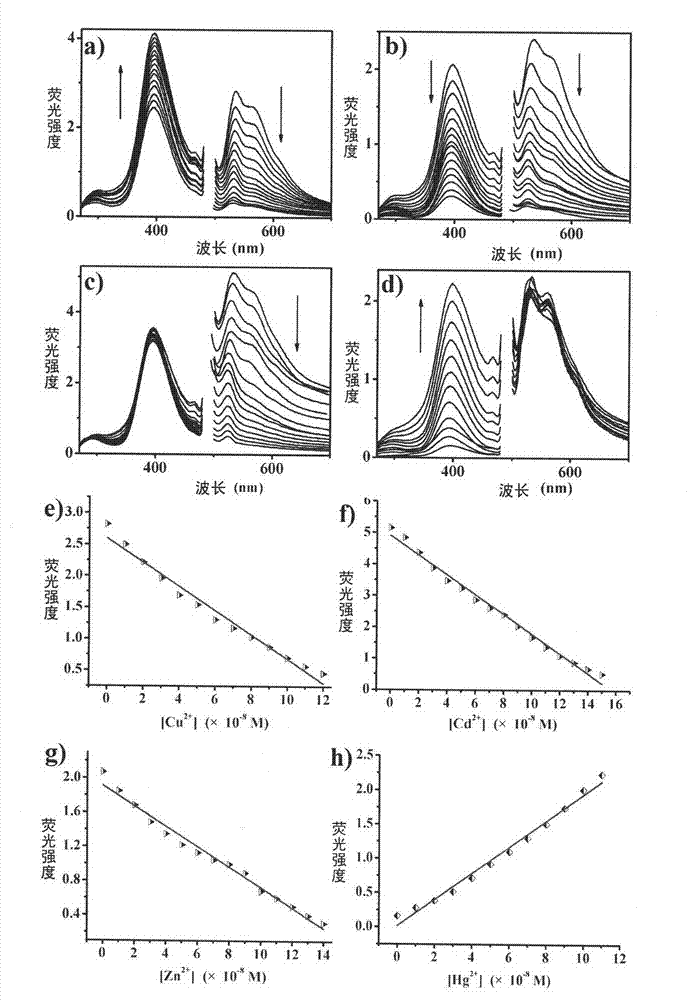
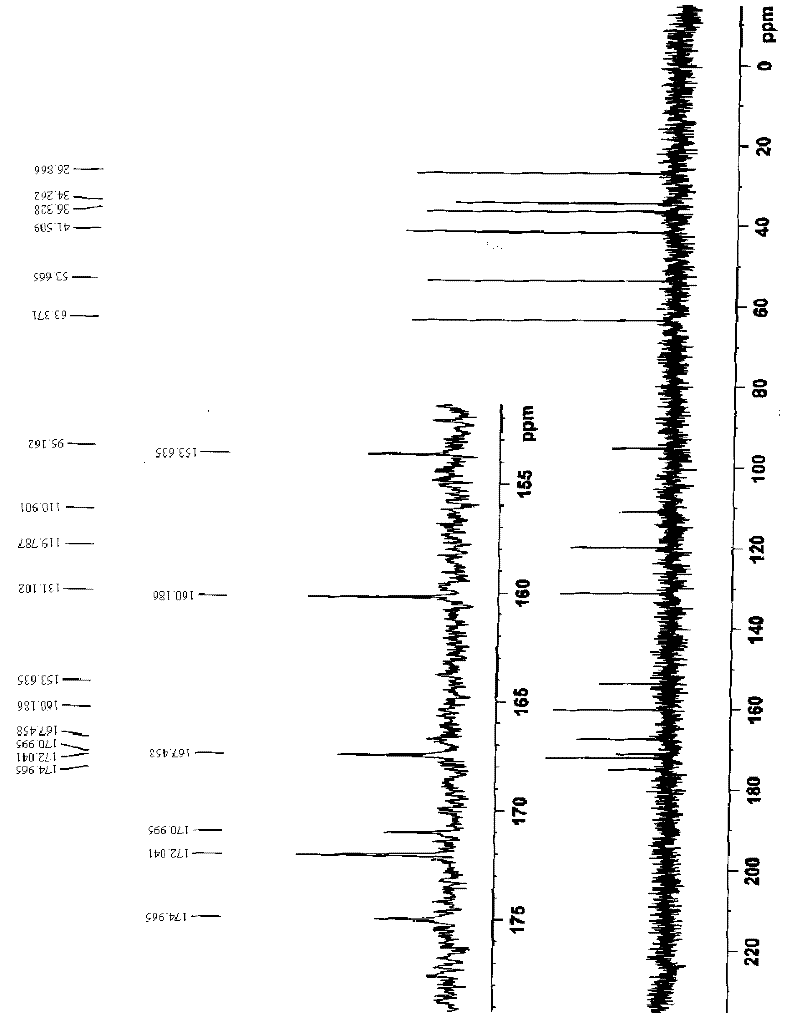
Ferroferric oxide nano-composite particle and preparation method and applications thereof
InactiveCN103241776AGood dispersionEfficient detectionFerroso-ferric oxidesNanotechnologySodium acetateN dimethylformamide
The invention discloses a ferroferric oxide nano-composite particle and a preparation method and applications thereof. The composite particle is a ferroferric oxide nano-composite particle with the particle size of 10-30nm, and 1,4-dihydroxy anthraquinone and fluorenylmethoxycarbonyl are modified on the surface of the ferroferric oxide nano-composite particle. The preparation method comprises the steps of: firstly, carrying out a hydrothermal method on ferric trichloride hexahydrate, sodium acetate, 1,6-hexanediamine and ethanediol to obtain the ferroferric oxide nano-composite particle, then producing 1,4-dihydroxy anthraquinone, chloroacetyl chloride, and N,N-dimethylformamide into dyes, then adding the ferroferric oxide nano-composite particle, sodium carbonate and the dyes into acetonitrile to carry out a reflux reaction, carrying out solid-liquid separation, washing and drying on the obtained reaction liquor to obtain an intermediate product, then dispersing the intermediate product into the N,N-dimethylformamide, adding dispersion liquid into the fluorenylmethoxycarbonyl, stirring for 2 hours, and carrying out solid-liquid separation, washing and drying to obtain a target product. The ferroferric oxide nano-composite particle can be used for fast detecting four heavy metal ions, namely copper, zinc, cadmium and mercury in an aqueous solution.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Method for preparing lidocaine
ActiveCN102070483AImprove responseHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideAcetyl chloride
The invention provides a method for preparing lidocaine. The method comprises the following steps: using 2,6-dimethylaniline and chloroacetic chloride as raw materials to prepare an intermediate, namely acetyl chloride-2,6-dimethylaniline, and using the prepared intermediate and diethylamine to react and obtain lidocaine, wherein acetone is used as solvent and carbonate is used as catalyst in thereaction process. The method of the invention has simple synthetic technology and does not require the complicated step that the intermediate is washed with acid firstly and washed with base secondlyin the post-treatment, thus avoiding unnecessary loss. Therefore, the yields of the intermediate and lidocaine prepared by the method are higher; and the prepared lidocaine has high purity which is more than 99%, and good industrial application prospect. In addition, the method of the invention uses acetone as solvent, thus the solvent is non-toxic basically and environmentally friendly, has no stimulation and can be recycled.
Owner:BENGBU BBCA MEDICINE SCI DEV
Preparation method of cefotetan
The invention relates to a preparation method of cefotetan. The method comprises the steps of: under an alkaline condition, reacting the starting raw material 7-MAC with chloroacetyl chloride in an organic solvent so as to obtain the intermediate CefoD-1; under nitrogen protection, adding aluminium trichloride into anisole, stirring for dissolving, and leaving the obtained solution for standby application; taking another organic solvent, into which the intermediate CefoD-1 is added under stirring, then adding the standby solution for reacting, thus obtaining the intermediate CefoD-2; reactingthe intermediate CefoD-2 with 3, 5-dithiol-4-isothiazole formic acid trisodium salt until that a sampled cefotetan tautomer is detected less than 4.0%, after the reaction conducting a post-treatment,thus obtaining cefotetan. With improved yield and reduced cost, the method of the invention, without final heating concentration, can generate products with a shallow color and almost invariant purity.
Owner:HAINAN HERUI PHARMA +1
Preparation method of polyamino phosphoric acid functional-born polyether sulfone functional separating membrane
ActiveCN106823824AEfficient removalEasy to operateMembranesSemi-permeable membranesPhosphorous acidPhosphorylation
The invention relates to a preparation method of polyamino phosphoric acid functional-born polyether sulfone functional separating membrane. The preparation method mainly comprises the following steps: by taking trichloromethane, chloroacetyl chloride and anhydrous aluminum chloride as main reagents, performing chloracetylation to the polyether sulfone at room temperature; then by taking N,N-dimethylacetylamide as a solvent and tetraethylenepentamine as an amination reagent, performing amination to the polyether sulfone, subjected to chloracetylation, at the temperature of 80-85DEG C, and preparing chloroacetyl chloride-tetraethylenepentamine- polyether sulfone anion-exchange membrane; then in methanol and triethylamine solution, by taking paraformaldehyde and phosphorous acid as reagents, performing phosphorylation graft modification to the chloroacetyl chloride-tetraethylenepentamine- polyether sulfone anion-exchange membrane, to prepare the polyamino phosphoric acid functional-born polyether sulfone functional separating membrane. The preparation method has good technological stability, the chelated functional groups of the prepared polyether sulfone functional separating membrane are difficult to lose, and the heavy metal pollutants in water environment can be effectively removed.
Owner:YANSHAN UNIV
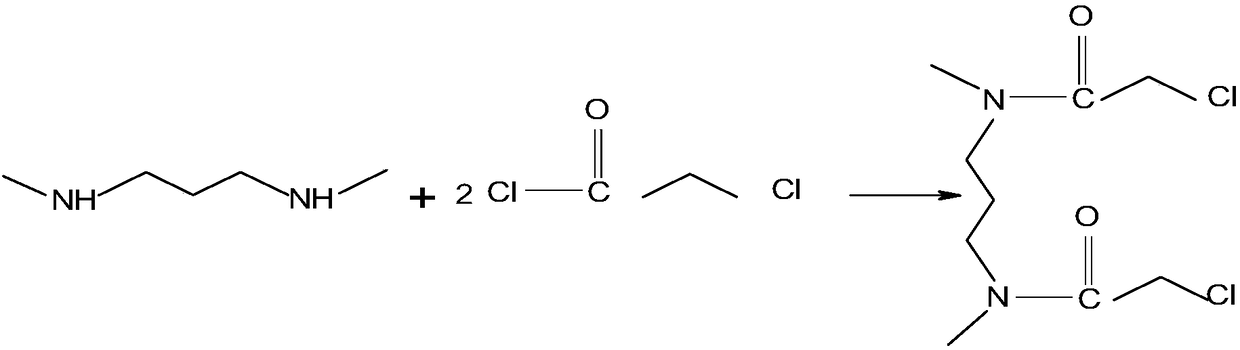
Method for synthesizing amide type gemini quaternary ammonium salt surfactant
InactiveCN108250095AImprove surface activityGood water solubilityOrganic compound preparationCarboxylic acid amides preparationSolubilityQuaternary ammonium cation
The invention provides a method for synthesizing an amide type gemini quaternary ammonium salt surfactant. The method comprises the following steps: under anhydrous conditions, firstly reacting 1,3-propanediamine and chloroacetyl chloride to synthesize an intermediate with bisamide groups and dichloro atoms; then reacting the intermediate and dodecyl dimethylamine, and synthesizing the final product belonging to quaternary ammonium salt Gemini surfactants. According to the method, the target product is synthesized by two steps, reactions are simple and feasible, and the product has the advantages of being high in surface activity, excellent in water solubility, small in irritation and degradable. The final product can be applied to an aluminum alloy corrosion inhibition system for aviation. Experiments prove that the corrosion inhibition property of the final product is better than that of the traditional single-chain quaternary ammonium salt corrosion inhibitor.
Owner:CIVIL AVIATION UNIV OF CHINA
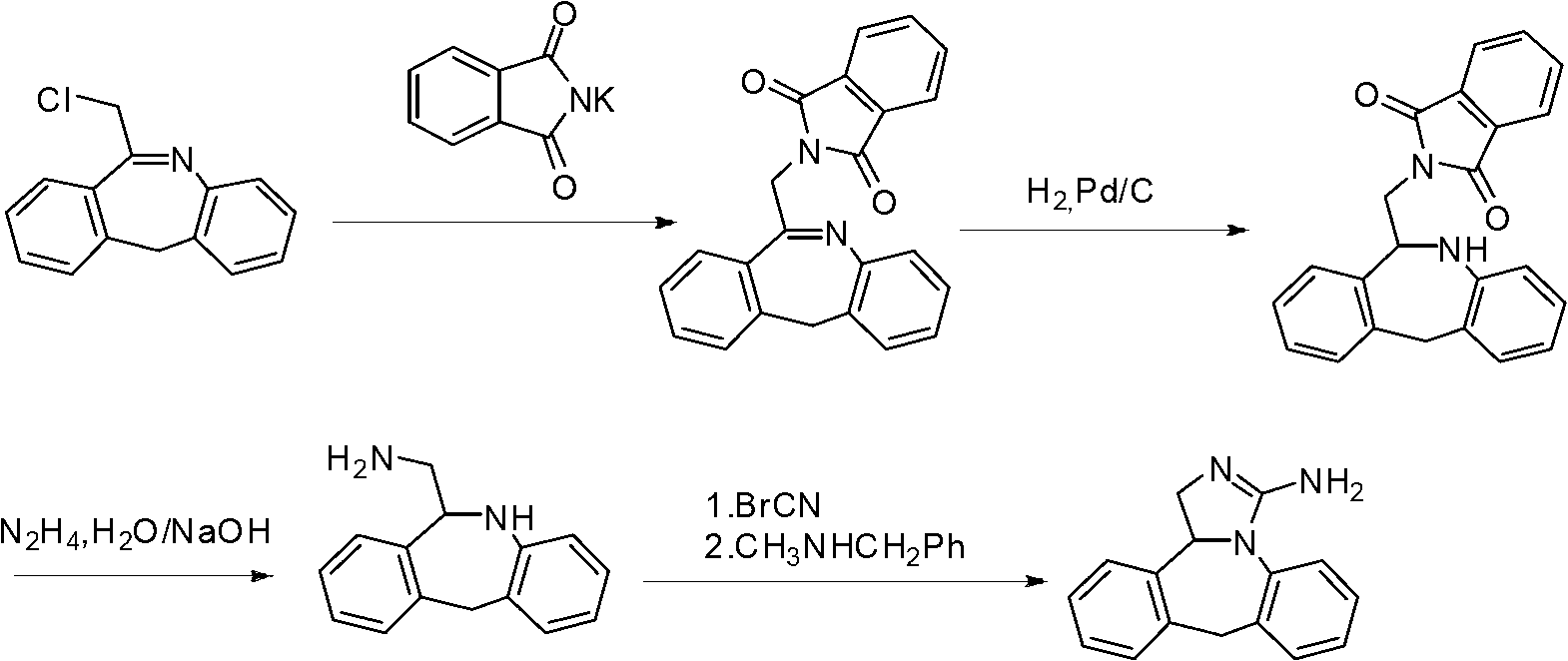
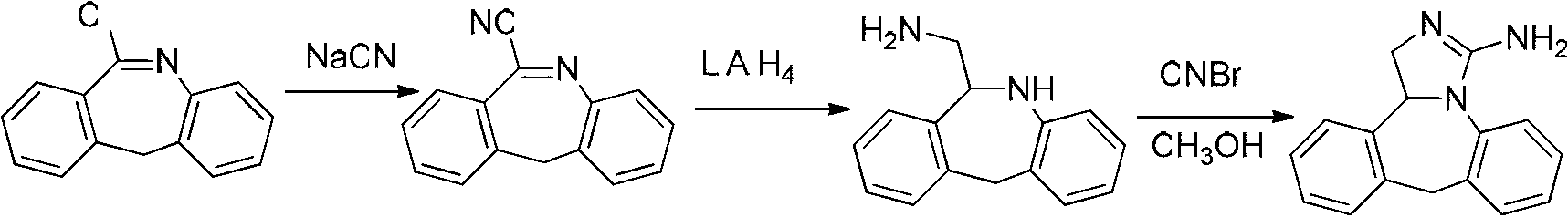
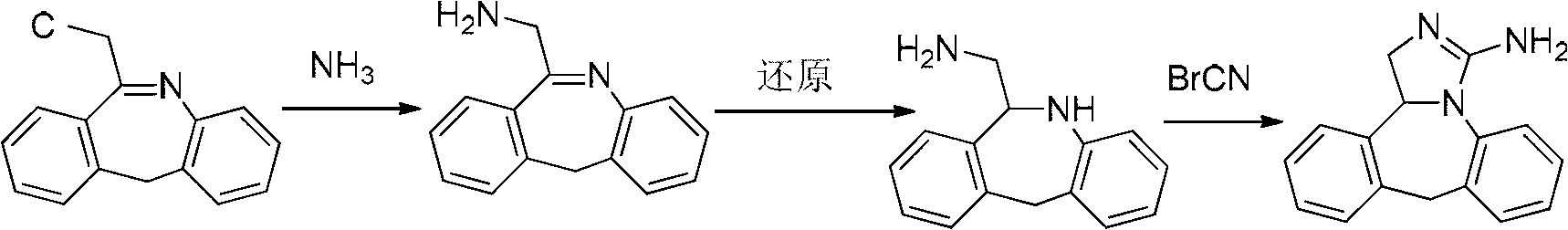
Synthesis method of epinastine
The invention discloses a synthesis method of epinastine. The synthesis method is implemented by taking 2-aminobenzophenone as a raw material and comprises the following steps of: reacting the 2-aminobenzophenone with a silane agent to obtain 2-benzylaniline; then, carrying out acylation reaction on the 2-benzylaniline and 2-chloroacetyl chloride to obtain N-(2-benzyl phenyl)-2-chloroacetamide; carrying out acidamide dehydration and cyclization on the N-(2-benzyl phenyl)-2-chloroacetamide under the action of a dehydrating agent to obtain 6-(chloromethyl)-11H-dibenzo[b,e] azepine; carrying out azidation reaction on the 6-(chloromethyl)-11H-dibenzo[b,e] azepine to obtain 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine; carrying out reduction on the 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine; and finally, carrying out cyclization on the 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine and cyanogen bromide to obtain the epinastine. The synthesis method disclosed by the invention avoids the application of expensive and flammable lithium aluminium hydride and aluminium hydride as well as hypertoxic sodium cyanide, so that the operation is safer in industrial production, and the cost is reduced. The method is simple in process and high in yield, requires mild conditions, and is suitable for industrialized production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
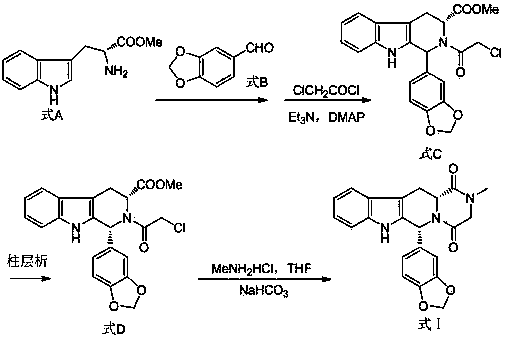
Improved tadalafil preparation method
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
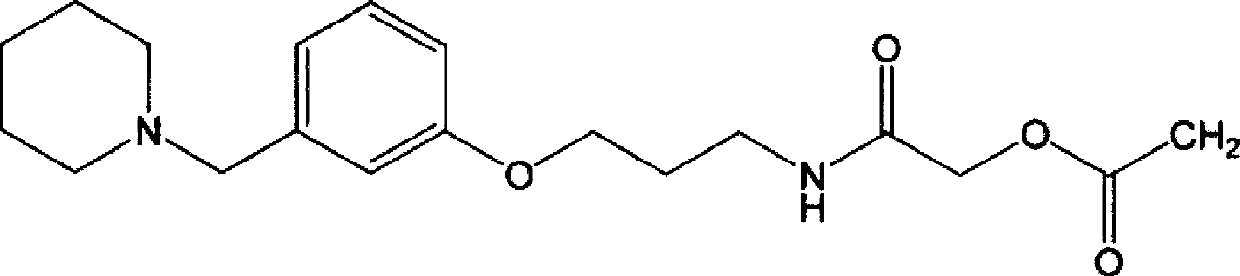
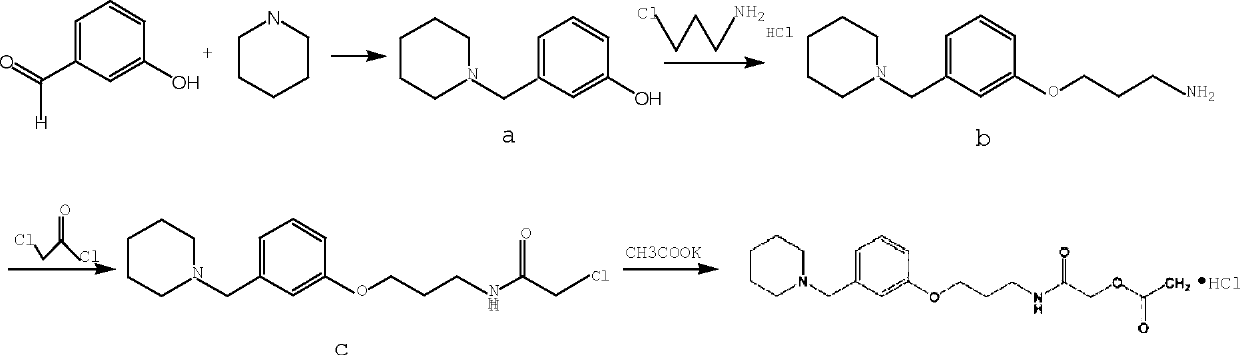
Synthetic method for preparing roxatidine acetate hydrochloride with high purity
InactiveCN102993121AHigh purityThe synthesis process is simple and safeOrganic chemistryPotassium acetatePhenol
The invention discloses a synthetic method for preparing roxatidine acetate hydrochloride with high purity. According to the invention, a starting material hydroxybenzaldehyde is subjected to condensation with piperidine to prepare 3-(1-piperidine methyl) phenol; an intermediate and 3-chloro amine hydrochloride are subjected to a backflow etherification reaction in a DMF at high temperature to obtain 3-(3-(1-piperidyl methyl) phenoxy) propylamine; the 3-(3-(1-piperidyl methyl) phenoxy) propylamine, chloroacetyl chloride and potassium acetate are subjected to a heating reflux reaction in a tetrahydrofuran solvent; and a HCl / THF solution is introduced to prepare a final product roxatidine acetate hydrochloride, with a total yield of 68%. The synthesis process has advantages of simpleness, low cost and high yield, can provide purity of the target product, and is in favor of industrial mass production of the product.
Owner:哈药集团人民同泰医药股份有限公司
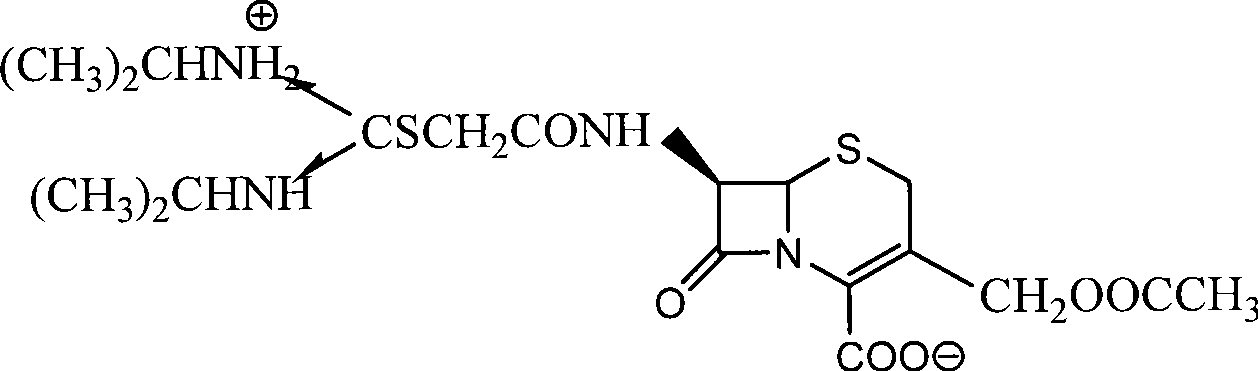
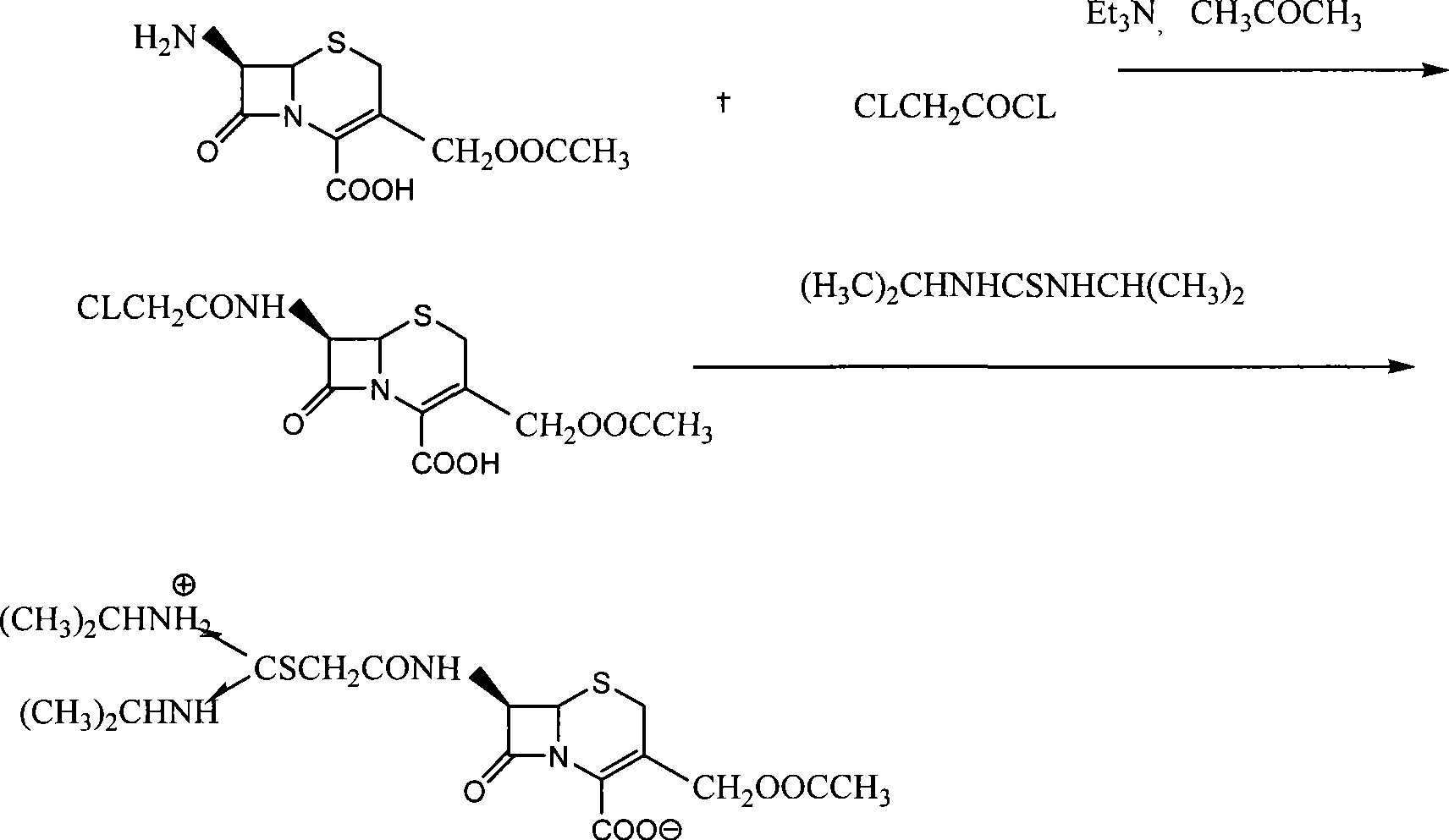
Process for preparing cefathiamidine
The invention relates to the field of the synthesis of chemical medicaments and discloses a preparation method of cefathiamidine; the method takes chloracetyl chloride as a raw material and comprises the following steps: (1) on the condition of the presence of a solvent, alkali is added so as to cause 7-ACA to be dissolved, and then the chloracetyl chloride is added for a condensation reaction; after the condensation reaction is finished, chloracetyl 7-ACA crystals are separated out with an acid; and (2) on the condition of the presence of both the solvent and a catalyst of a catalyzing amount, the chloracetyl 7-ACA reacts with N, N-di-isopropyl thiourea to produce the cefathiamidine. Besides the advantages of bromoacetyl-bromide preparation method of cefathiamidine, the technology of adopting chloracetyl chloride as the raw material to produce the cefathiamidine also has the advantages that: as no alkali is added into the reaction between the chloracetyl 7-ACA and the N, N-di-isopropyl thiourea, the produced cefathiamidine has lighter color, and better and more stable quality, is more beneficial to store and transport, improves the overall yield, lowers the cost and has broader prospects; and the price of the chloracetyl chloride is one sixth of that of the bromoacetyl bromide, which significantly reduce the cost.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
O-quaternary-N-thiourea chitosan, preparation method and application thereof
InactiveCN103980385AGood water solubilityGood antibacterial effectBiocideDisinfectantsBenzaldehydeThiourea
The present invention belongs to the field of polymer compound material. The invention discloses an O-quaternary-N-thiourea chitosan, a preparation method and application thereof, and the chitosan is a chemical modified functional derivative of chitosan. The preparation method of the O-quaternary-N-thiourea chitosan comprises the following steps: adopting chitosan as a raw material and carrying out reaction with benzaldehyde to obtain chitosan Schiff base, then carrying out reaction with 2,3-glycidyl trimethyl ammonium chloride to obtain O-quaternary-N-chitosan Schiff base; carrying out deprotection of an amino group positioned on the second carbon atom (C2) of the O-quaternary-N-chitosan Schiff base through a methanol-hydrochloric acid solution to obtain O-quaternary chitosan, adding the O-quaternary chitosan to the mixture of ammonium thiocyanate and chloroacetyl chloride to carry out reaction, and then obtaining the O-quaternary-N-thiourea chitosan. The O-quaternary-N-thiourea chitosan provided in the invention achieves a substantial increase in antibacterial property compared with chitosan. The invention can be used in a plurality of application fields of antibacterial material, daily chemical products, industrial wastewater treatment and the like.
Owner:SOUTH CHINA UNIV OF TECH
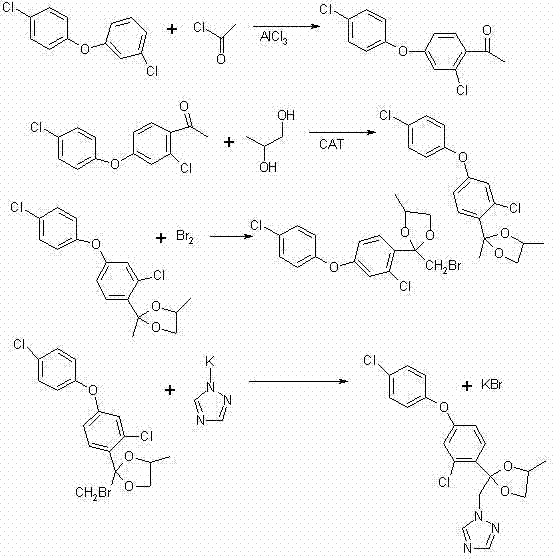
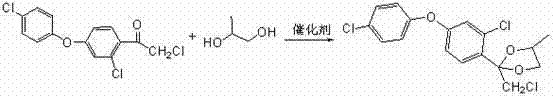
Preparation method of difenoconazole
InactiveCN102250072ASave bromination reaction stepHigh cost of solutionOrganic chemistrySulfolaneChlorobenzene
The invention relates to a preparation method of difenoconazole, comprising the steps of: (1) in the presence of aluminium trichloride, subjecting 3, 4'-dichloro diphenyl ether and chloroacetyl chloride to a Friedel-Crafts reaction so as to generate chlorobenzene ether ketone; (2) in the presence of a catalyst, conducting a cyclisation reaction to chlorobenzene ether ketone and 1, 2-propylene glycol, thus obtaining cis, trans-3-chlorine-4-(4-methyl-2-chloromethyl-1, 3-dioxolane-2-yl) phenyl-4'-chlorophenyl ether; (3) in the presence of sulfolane, carrying out a condensation reaction at a temperature of 190-230DEG C to cis, trans-3-chlorine-4-(4-methyl-2-chloromethyl-1, 3-dioxolane-2-yl) phenyl-4'-chlorophenyl ether and 1, 2, 4-triazole, thus obtaining cis, trans-3-chlorine-4-[4-methyl-2-1H-1, 2, 4-triazole-1-ylmethyl]-1, 3-dioxapentane-2-yl) phenyl 4-chlorophenyl ether, then performing filtration and exsolution when the reaction is over, thus obtaining a crude product of difenoconazole; (4) implementing rectification to the crude product, then carrying out crystallization with a solvent and performing centrifugation, thus obtaining the product of difenoconazole. The method provided in the invention has the advantages of short production period, low production cost and good production security.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Preparation of propacetamol hydrochloride
InactiveCN101353314ASimplify purification operationsHigh yieldOrganic compound preparationCarboxylic acid amides preparationAcetic acidPropacetamol
The invention provides a preparation method of propacetamol hydrochloride. The preparation method is characterized in that chloracetyl chloride and paracetamol carry out an acetylization chloride reaction in a polar aprotic solvent to obtain chloroactic acid-4-acetylamino phenyl ester which is directly aminated with diethylamine to obtain N, N'-diethylglycine 4-acetylamino phenyl ester, hydrochloric acid is used for adjusting the pH to be 4, and the propacetamol hydrochloride is obtained. The preparation method has the advantages of mild condition, convenient separation and purification, the total product yield is greatly increased, the use of the amount of the reaction solvent is low, and the preparation method reduces industrial pollution, and is applicable to industrialized production.
Owner:ANHUI PIOM PHARMA
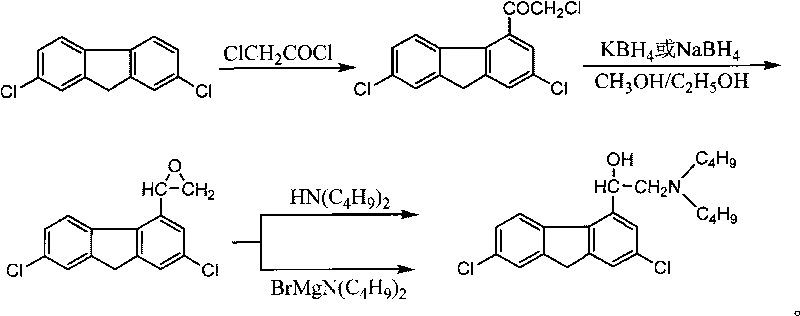
Method for preparing alpha-(di-n-butylaminomethyl)-2,7-dichloro-4-fluorenemethanol and the hydrochloride thereof
InactiveCN101747210AInexpensive and easy to use raw materialsMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationPotassium borohydrideEthyl acetate
The invention discloses a method for preparing benflumetol intermediate alpha-(di-n-butylaminomethyl)-2, 7- dichloro-4-fluorenemethanol and the hydrochloride thereof. The alpha-(di-n-butylaminomethyl)-2, 7- dichloro-4-fluorenemethanol is obtained by directly reacting 2, 7-Dichlorofluoren as raw material with the chloroacetyl chloride, then reducing the obtained mixture with potassium borohydride or sodium borohydride for 10 to 24 hours, washing the obtained solids with water, then adding the obtained solids into recycled ethanol mother solution concentrate, adding N-Dibutylamine into the mother solution concentrate and the target product is separated from the reaction mixture; the hydrochloride of the alpha-(di-n-butylaminomethyl)-2, 7- dichloro-4-fluorenemethanol is obtained by adding chlorhydric acid into the reaction mixture to salify the reaction mixture and then crystallizing the reaction mixture. The method has cheap raw materials, simple and convenient operations and high yield factor and in particular greatly reduces the production cost by reusing the ethanol mother solution.
Owner:GUILIN PHARMA
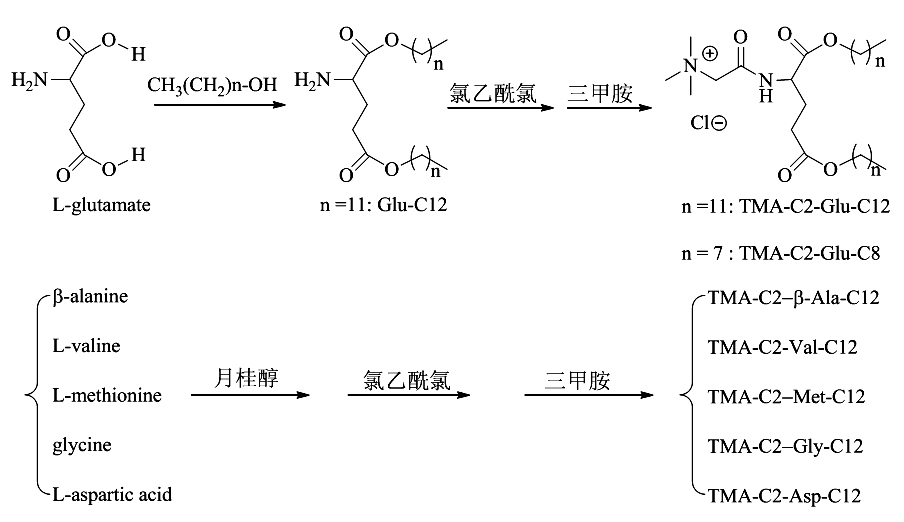
Preparation method of cationic liposome nanoparticles of amino acid
ActiveCN102060723AEfficient preparationLow costOrganic compound preparationGenetic material ingredientsChloroacetyl chlorideUltrasonic oscillation
The invention discloses a preparation method of amino acid cationic liposome nanoparticles. The method comprises the following steps: (1) adding amino acid which is used as a raw material into lauric acid or normal alcohol acid so as to be subjected to esterification reaction, separating and purifying to obtain white solid or paste; (2) after the esterification reaction in the step (1) is finished, dropping chloroacetyl chloride in the obtained solid slowly to be subjected to chloroacetylation reaction, concentrating the reactant, then adding trimethylamine to be subjected to quaternization reaction, and performing the corresponding separation and purification to the obtained white or colorless solid; and (3) performing ultrasonic oscillation water dispersion on the solid obtained in the step (2) to obtain the corresponding amino acid cationic liposome nanoparticles. The method provided by the invention has low cost, is easy to operate, is suitable for most of amino acids and can be used for efficiently preparing amino acid cationic liposome nanoparticles with different structures.
Owner:湖南远泰生物技术有限公司
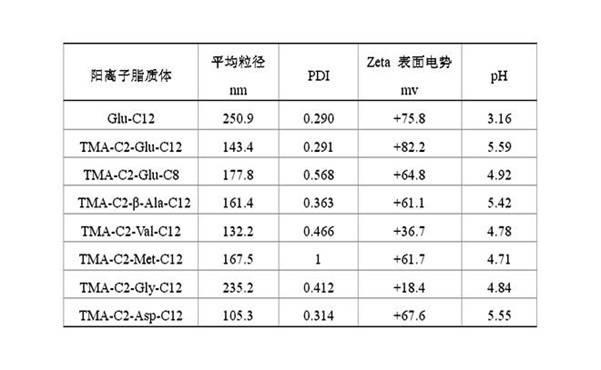
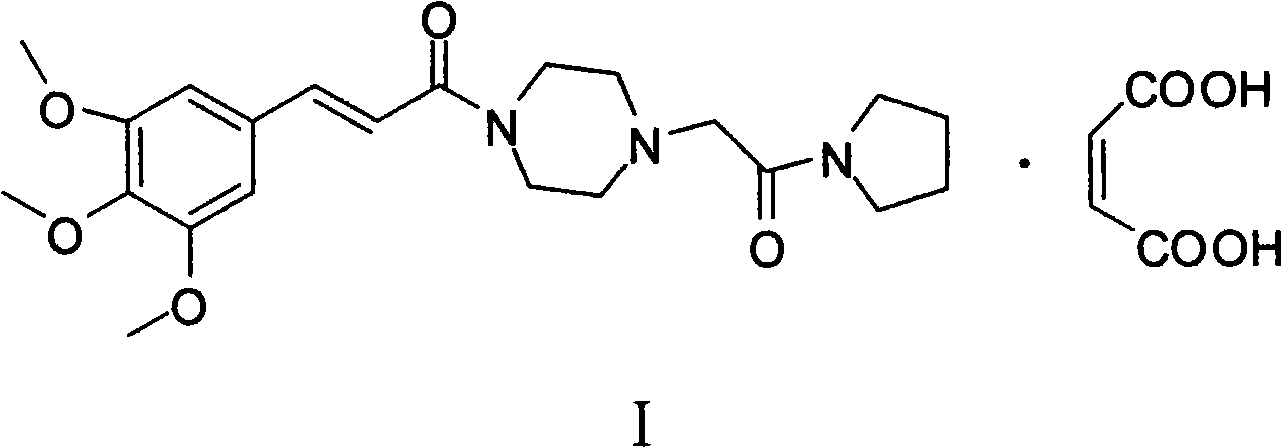
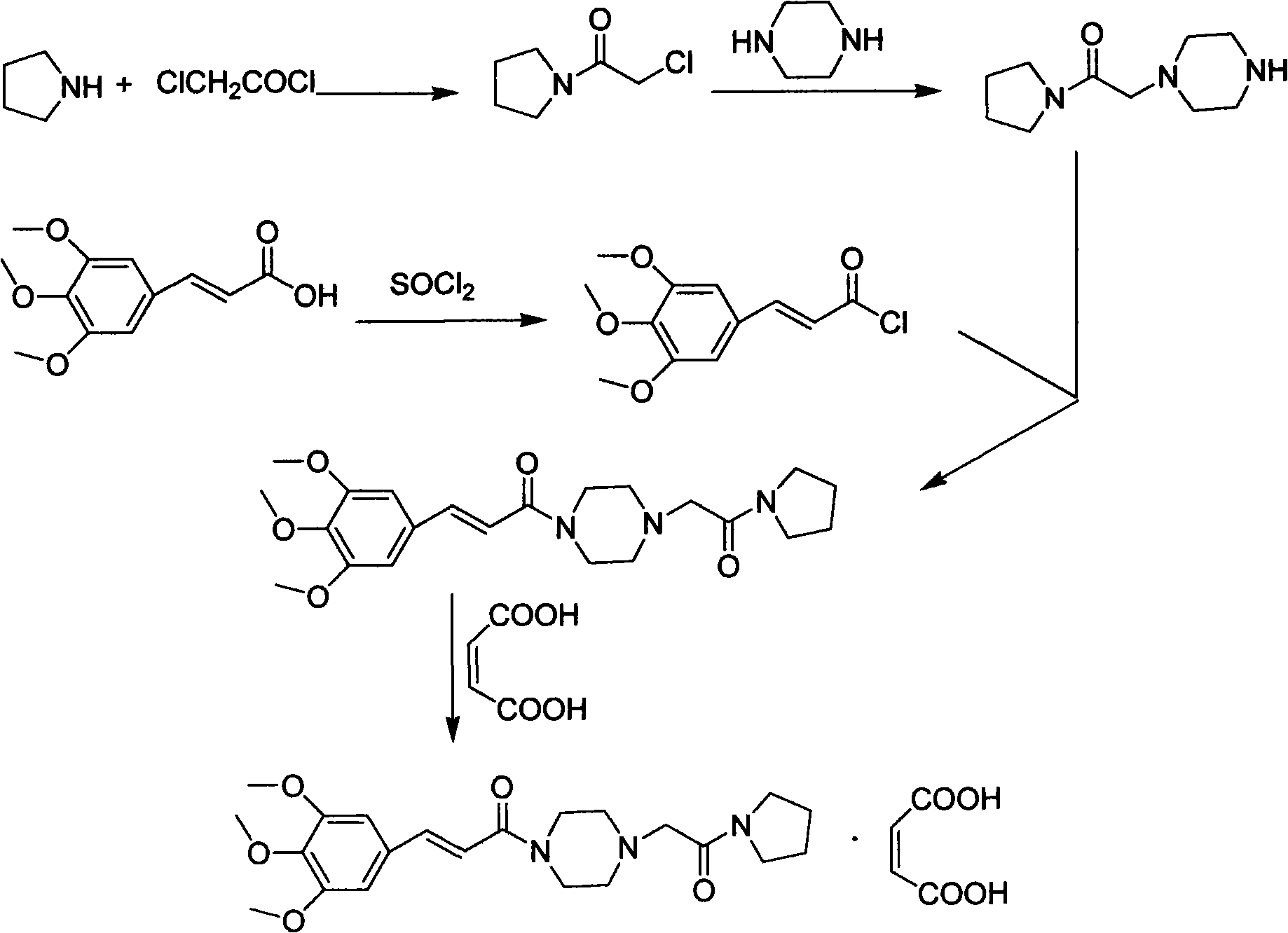
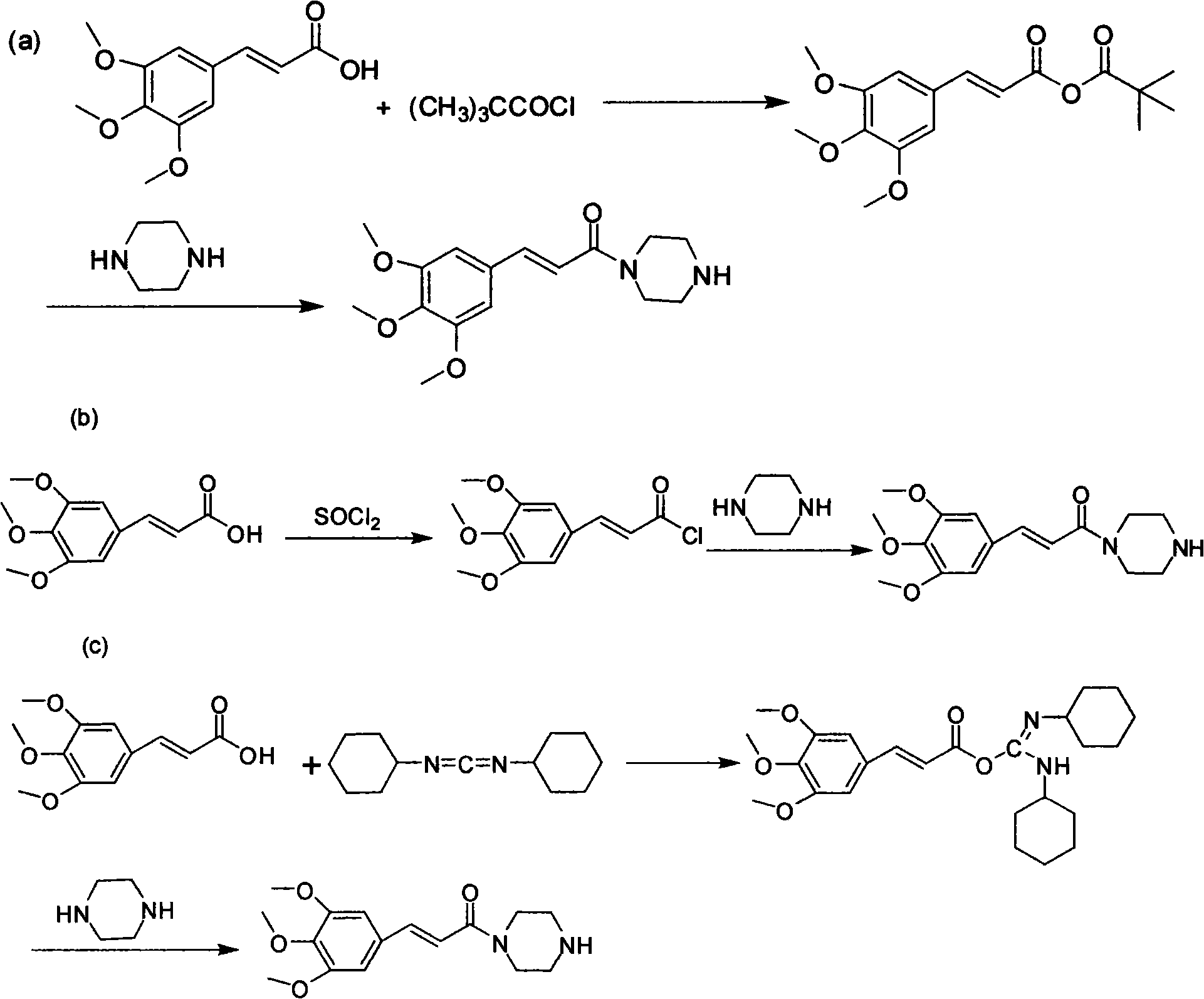
Method for preparing cinepazide maleate
InactiveCN101591310AEasy to removeSimple ingredientsOrganic chemistryCardiovascular disorderCarboxylic acid halidesPyrrole
The invention provides a method for preparing cinepazide maleate. The method comprises the following steps: preparing mixed acid anhydride or acyl halide by taking 3,4,5-trimethoxycinnamic acid as a raw material; then, reacting the mixed acid anhydride or the acyl halide with absolute piperazidine to prepare 1-(3,4,5-trimethoxyphenyl)piperazidine, or dehydrating the 3,4,5-trimethoxycinnamic acid and the absolute piperazidine to generate 1-(3,4,5-trimethoxyphenyl)piperazidine under the action of dicyclohexylcarbodiimide; reacting chloracetyl chloride with nafoxidine to prepare N-(2-chloracetyl)nafoxidine; reacting the N-(2-chloracetyl)nafoxidine with 1-(3,4,5-trimethoxyphenyl)piperazidine to prepare cinepazide free alkali; and salifying the cinepazide free alkali and maleic acid to prepare the cinepazide maleate. The method has the advantages of accessible raw materials, mild reaction condition, simple and easy operation, short synthesis route, high yield, industrial production availability and the like.
Owner:北京兴德通医药科技股份有限公司
Small-molecule fluorescent probe used for ratio recognition of human carbonic anhydrase, and synthetic method and application thereof
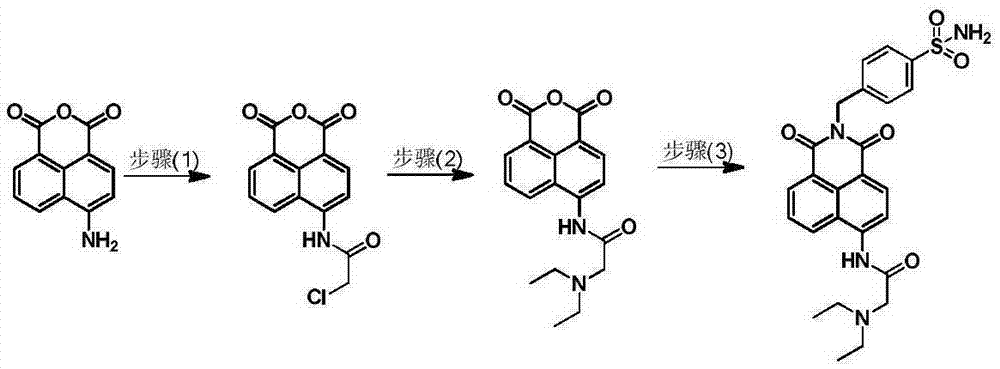
The invention relates to a small-molecule fluorescent probe used for ratio recognition of human carbonic anhydrase, and a synthetic method and application thereof. The probe is prepared by reacting 4-amino-1,8-naphthalic anhydride with chloroacetyl chloride, diethylamine, 4-aminomethylbenzenesulfonamide and like. The synthetic method is simple to operate and uses cheap raw materials. The probe emits blue fluorescent light with a wavelength of ~470 nm in an aqueous solution and emits green to yellow fluorescent light with a wavelength of 525 to 560 nm in polar solvents like methanol, dimethyl sulfoxide, N,N-dimethyl formamide and ethanol. In a 20-mM phosphoric acid buffer, when human carbonic anhydrase 1 with an equivalent of 0 to 0.7 is gradually added, a fluorescence peak at a wavelength of ~470 nm gradually attenuates and a fluorescence peak at a wavelength of ~530 nm gradually intensifies. Thus, the probe provided by the invention can realize ratio recognition of human carbonic anhydrase and eliminates influence of external factors like equipment and samples.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing (2S)-N-chloracetyl-2-cyano-group pyrrolidine
The invention provides a method for preparing (2S)-N-chloracetyl-2-cyano-group pyrrolidine, which belongs to the technical field of chemical medicine midbody preparation methods. The preparation method comprises the steps: (1) L-proline and thionyl chloride generate acylation reaction to obtain L-proline chloride; (2) the L-proline chloride and ammonia water generate reaction to produce L-proline amide; (3) the L-proline amide and chloroacetyl chloride generate reaction to obtain (2S)-N-chloracetyl-2-carbamoyl pyrrole; and (4) (2S)-N-chloracetyl-2-carbamoyl pyrrolidine and phosphorus oxychloride generate dehydration reaction under low temperature condition to obtain the (2S)-N-chloracetyl-2-cyano-group pyrrolidine. The method has the advantages of saving material cost, being simple in technological process, moderate in reaction condition, low in operation difficulty, convenient in aftertreatment, stable in quality and suitable for mass industrial production, having operability, and improving yield of prepared products by a large margin.
Owner:LINHAI TIANYU PHARMA
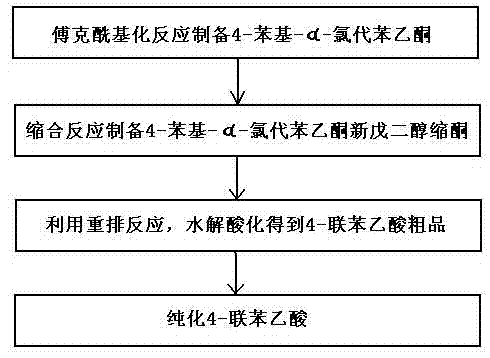
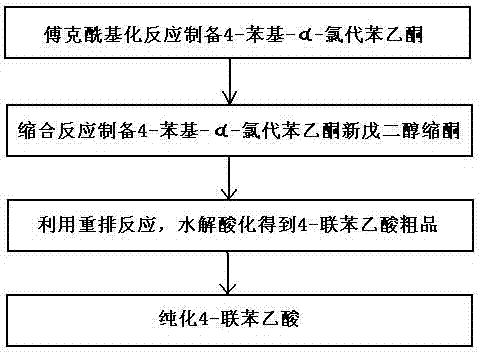
Method for preparing 4-felbinac through rearrangement reaction
InactiveCN102503805ALow toxicityToxicOxygen-containing compound preparationOrganic compound preparationChemical synthesisPtru catalyst
The invention relates to a medicinal chemistry synthesis technology, in particular to a method for preparing 4-felbinac through rearrangement reaction. The method comprises the following steps of: 1, performing Friedel-Crafts acylation on biphenyl and chloroacetyl chloride which serve as raw materials by using chloroform, dichloromethane or 1,2-dichloroethane as a solvent and alchlor as a catalyst to obtain 4-phenyl-alpha-chloroacetophenone; 2, performing condensation on the 4-phenyl-alpha-chloroacetophenon and neopentyl glycol under the catalysis of toluenesulfonic acid to prepare ketal; 3, performing hydrolysis and acidification through the rearrangement reaction to obtain coarse 4-felbinac; and 4, recrystallizing the coarse 4-felbinac by using ethanol, ethyl acetate or 40 to 70 percent acetic acid to obtain the 4-felbinac with the purity of 99.8 percent. The method has the advantages that: the raw materials are readily available and low in toxicity, and new compounds with high toxicity cannot be generated in production; and conventional equipment is used, the yield of the coarse product is 70 to 80 percent and the prepared 4-felbinac has the purity of 99.8 percent.
Owner:SHANGHAI TUPA MEDICAL TECH
Novel synthetic method of (S)-3-morpholinyl carboxylic acid
ActiveCN102617503AMild reaction conditionsSpecific responseOrganic chemistryCarboxylic acidTert butyl
The invention discloses a synthetic method of (S)-3-morpholinyl carboxylic acid, which comprises the following steps: (1) taking L-serine as a raw material to synthesize L-serine tert-butyl ester; (2) dissolving L-serine tert-butyl ester in dichloromethane, adding a dichloromethane solution of chloroacetyl chloride drop by drop to obtain N-chloroacetyl-L-serine tert-butyl ester; (3) dissolving N-chloroacetyl-L-serine tert-butyl ester in a toluene solution, adding the toluene solution of sodium ethoxide drop by drop to obtain (S)-5-oxo 3-morpholinyl carboxylic acid tert-butyl ester; (4) dissolving (S)-5-oxo 3-morpholinyl carboxylic acid tert-butyl ester in methanol, successively adding aluminum trichloride and sodium borohydride to carry out a reaction to obtain the (S)-3-morpholinyl carboxylic acid tert-butyl ester; (5) dissolving (S)-3-morpholinyl carboxylic acid tert-butyl ester in methanol, adding a methanol solution of hydrogen chloride for reacting to obtain the (S)-3-morpholinyl carboxylic acid. The method of the invention has the advantages of mild reaction condition, easily available raw material and less three waste, and is suitable for industrial production.
Owner:上海常丰生物医药科技有限公司
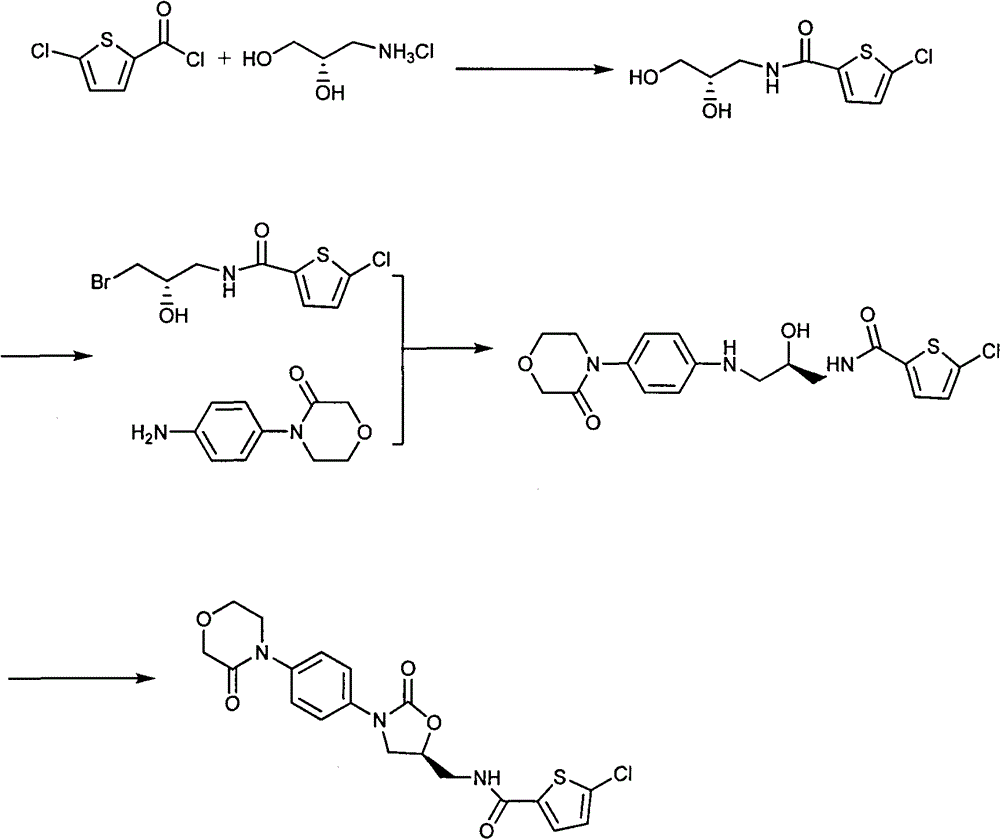
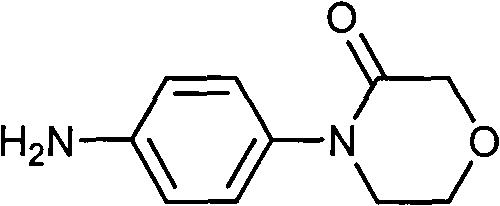
Preparation method of 4-(nitrobenzophenone)-3-morpholone and method for preparing rivaroxaban by using 4-(nitrobenzophenone)-3-morpholone
ActiveCN103980221ALow priceSimple operation processOrganic chemistryMorpholineTert-Butyloxycarbonyl protecting group
The invention relates to the technical field of preparation of rivaroxaban and an intermediate thereof and particularly relates to a preparation method of 4-(nitrobenzophenone)-3-morpholone which is prepared from halogenated nitrobenzene, ethanolamine and chloroacetyl chloride through a one-pot method. The method for preparing rivaroxaban comprises the steps of reducing 4-(nitrobenzophenone)-3-morpholone into 4-(aminophenyl)-3-morpholone; enabling 4-(aminophenyl)-3-morpholone to react with R-epichlorohydrin to obtain a product; enabling the product to react with N, N-carbonyldiimidazole to obtain a product; enabling the product to react with tert-butyl iminodicarboxylate; preparing hydrochloride; enabling hydrochloride to react with 5-penphene-2-carbonyl chloride. The preparation method of 4-(nitrobenzophenone)-3-morpholone is capable of realizing one-pot production and free of purifying intermediate products in the process, so that the operation process is simplified, the time is saved, and the labor cost is reduced; the preparation method of 4-(nitrobenzophenone)-3-morpholone is low in raw material price, high in obtained product yield and easy to realize large-scale industrial production; in addition, the method for preparing rivaroxaban is cheap, nontoxic and harmless in raw material, simple in process and high in product yield.
Owner:山东康美乐医药科技有限公司
Methylene technique for producing acetochlor
InactiveCN101270062AOrganic compound preparationCarboxylic acid amides preparationAnilineAromatic solvents
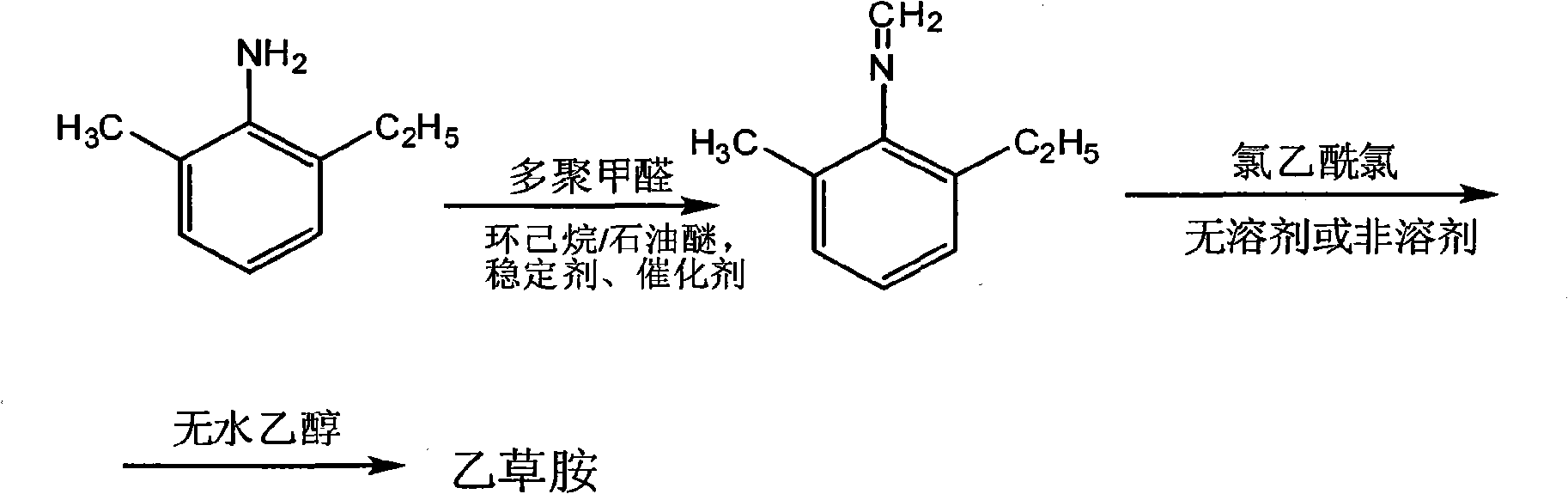
The present invention relates to a preparation process of acetochlor in a methylene method, and is characterized in that the production process comprises the following steps: (1) under the influence of a catalyst and a stabilizer, 2-methyl-6-diethylaniline and paraformaldehyde have dehydration reaction in non-aromatic solvent cyclohexane, petroleum ether or the mixture of the cyclohexane and the petroleum ether, to prepare 2-methyl-6-ethyl-N-methylene aniline that requires no purification for the reaction in the next step; (2) under the condition with non-aromatic solvents or without solvents, the prepared 2-methyl-6-ethyl-N-methylene aniline and chloroacetic chloride have acylation reaction; then the products prepared in the acylation reaction and anhydrous alcohol react to prepare the crude product; and the acetochlor with more than 93 percent of the acetochlor content can be separated and prepared.
Owner:INNER MONGOLIA HONGYU TECH
Diosgenin anti-tumor derivative and synthesizing method thereof
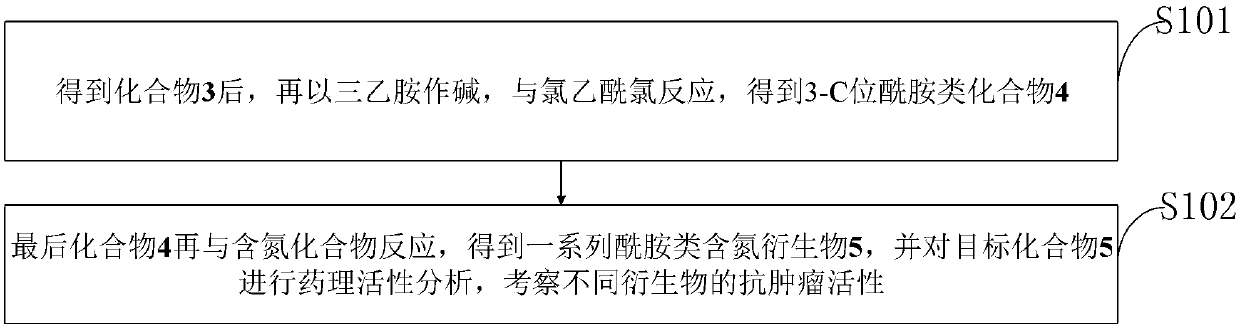
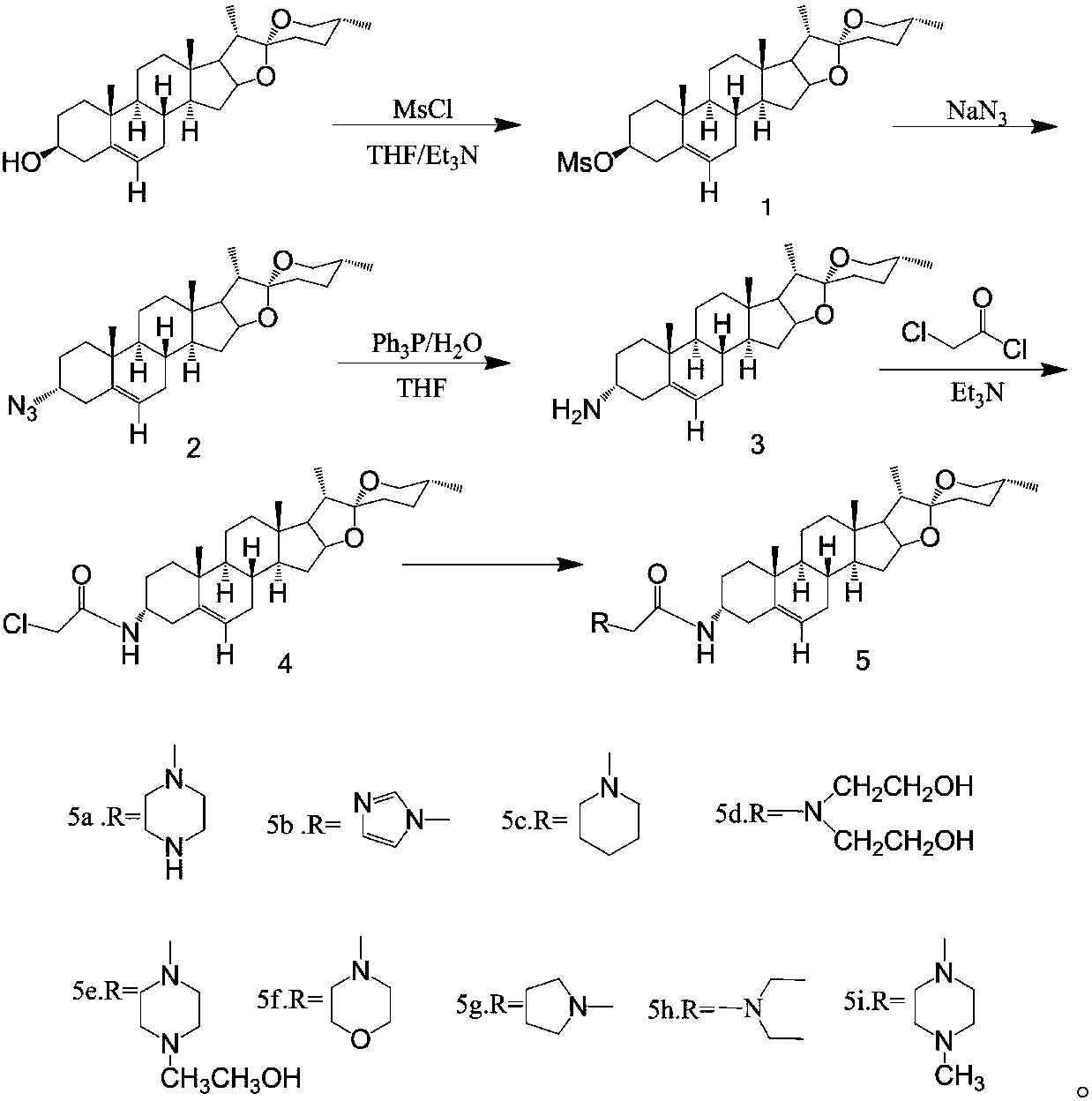
ActiveCN107827949AStrong inhibitory activityLow cytotoxicitySteroidsAntineoplastic agentsCytotoxicityNitrogen
The invention belongs to the technical field of medicine and discloses diosgenin anti-tumor derivative and a synthesizing method thereof. The synthesizing method comprises the steps: after compound 3is obtained, utilizing triethylamine as alkali to react with chloroacetyl chloride to obtain 3-Csite amide compound 4; finally, reacting the compound 4 with nitrogen-containing compound to obtain a series of amide nitrogen-containing compound 5. The derivative disclosed by the invention and intermediates of the derivative wholly show high inhibitory activity and low cytotoxicity, the derivative 5aand 5e containing piperazine and the intermediate 4 show stronger inhibitory activity, and the derivative 5c, 5e, 5f, 5g, 5h and 5i shows low cytotoxicity to HBE cells. The results of the derivativedisclosed by the invention can provide certain reference value for structural modification of the diosgenin and analysis of anti-tumor activity of different radicals.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Preparation method of imidazole aromatic alcohol analog derivative with optical activity
InactiveCN1765887AThe synthesis process is simpleLow costOrganic active ingredientsAntimycoticsSynthesis methodsKetone
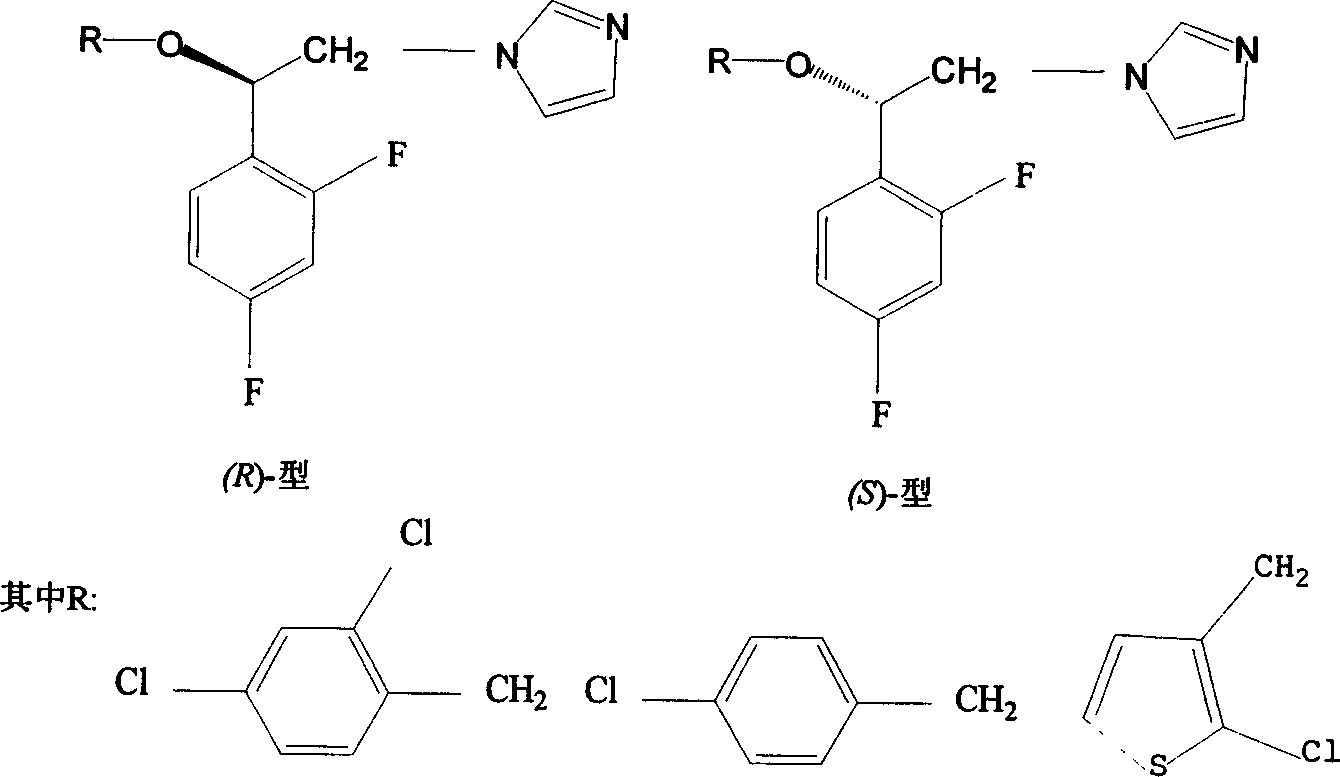
The invention discloses a preparation method for (R)- and (S)-alpha- alkoxy -beta-(1- imidazolyl)-2ú¼4-difluoroethylbenzenenitrate as optical-active imidazolyl aromatic derivant. Wherein, the synthesis method comprises: using chloracetyl chloride to acylate m-difluorobenzene into alpha- chloro-acetophenone; reducing ketone into alcohol; resolutioning racemic alcohol with organic-phase lipase catalyst to prepare (R)- and (S)- alcohol; on condition phase transferring, optical-active alcohol reacts with imidazole for N-alkylation reaction, then with benzylchlorine for O- alkylation reaction, using salt precipitation with nitric acid to obtain the objective product. The testings show: this product has antibacterial activity function, special the (R)- type, the technique has simple process and high yield.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com