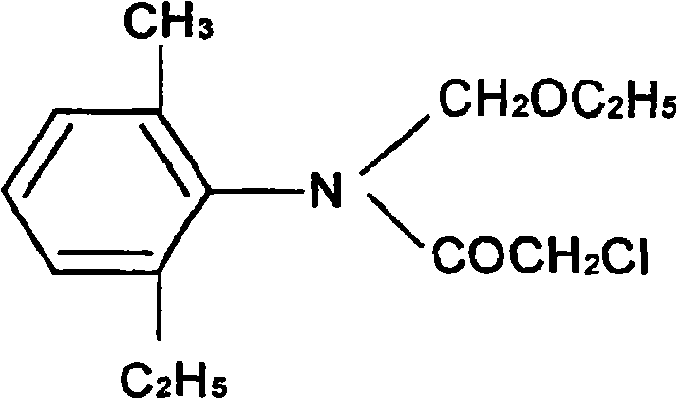
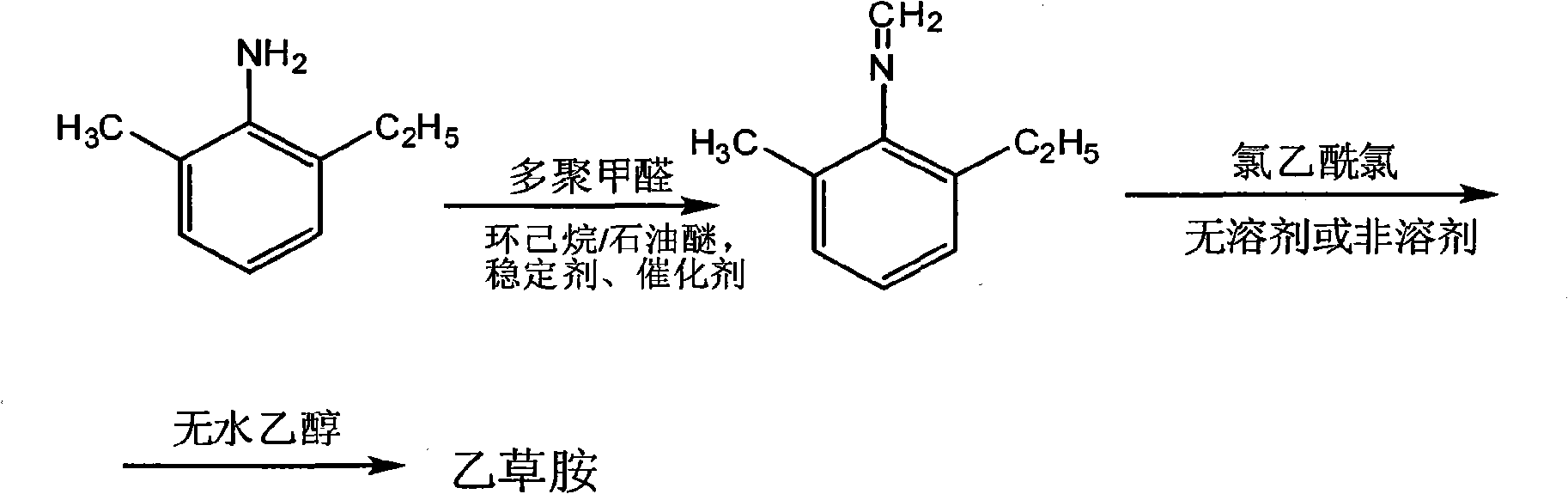
Methylene technique for producing acetochlor
A production process, the technology of acetochlor, applied in the field of pesticides, can solve the problems of excessive discharge of "three wastes", impact on product safety, and large solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 2-methyl-6-ethylaniline 62g, add cyclohexane 150g, 95% ethanol 16g, stabilizer NH 4 Add 0.5g of HS, 28g of paraformaldehyde, and 0.15g of catalyst sodium hydroxide into a 500ml four-neck flask, stir, raise the temperature to 36°C and react for 50 minutes, then start water separation. After the water separation is completed, distill out cyclohexane to obtain Methylidene 67.1g, content 99%, conversion rate 99.4%.
[0020] Take 60g of chloroacetyl chloride and 67.1g of methylene chloride, add them into a 500ml four-neck flask, stir, keep the temperature below 20°C, and react for 2 hours, add 100g of absolute ethanol to react for 2 hours, and pass through NH 3 When the pH was about 7-9, the solid ammonium chloride was filtered out, and the cyclohexane was distilled off from the filtrate to obtain 122.5 g of acetochlor with a content of 94.5% and a conversion rate of 94%.
Embodiment 2
[0022] Take 2-methyl-6-ethylaniline 62g, add cyclohexane and petroleum ether mixed solvent 110g (petroleum ether quality accounts for 80%), 95% ethanol 16g, the stabilizer NH that molar ratio is 1: 1 4 Add 0.3g of HS and Fe powder, 28g of paraformaldehyde, and 0.5g of tri-n-butylamine into a 500ml four-necked bottle and stir. After raising the temperature to 60°C and reacting for 40 minutes, water separation begins. After the water separation is completed, the solvent is evaporated. 67.3 g of methylene was obtained, the content was 99.1%, and the conversion rate was 98.8%.
[0023] Take 62g of chloroacetyl chloride and 67.3g of methylene chloride, add them into a 500ml four-neck flask, stir, keep below 20°C, react for 2 hours, add 200g of absolute ethanol to react for 2 hours, and pass through NH 3 When the pH was about 7-9, solid ammonium chloride was obtained by filtration, and cyclohexane and petroleum ether were distilled from the filtrate to obtain 121.9 g of acetochlor, ...
Embodiment 3
[0025] Get 2-methyl-6-ethylaniline 62g, add sherwood oil 60g respectively, 95% ethanol 16g, stabilizer (NH 4 ) 2 Add 0.35g of S, 28g of paraformaldehyde, and 0.5g of pyridine into a 500ml four-neck bottle, stir, heat up to 66°C and react for 40 minutes, then start water separation. After water separation, distill petroleum ether to obtain 66.8g of methylene , content 99.3%, conversion rate 98.3%.
[0026] Take 60g of chloroacetyl chloride and 67g of methylene chloride, add them into a 500ml four-neck flask, stir, keep the temperature below 20°C, and react for 2 hours, add 200g of absolute ethanol to react for 2 hours, and pass through NH 3 When the pH is about 7-9, solid ammonium chloride is obtained by filtration, and petroleum ether is evaporated from the filtrate to obtain 122.2 g of acetochlor with a content of 95% and a conversion rate of 94.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com