Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Cinepazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
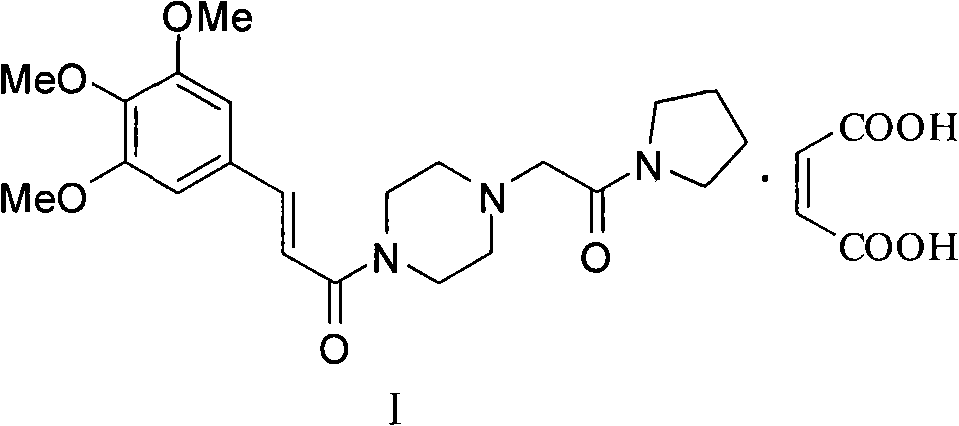
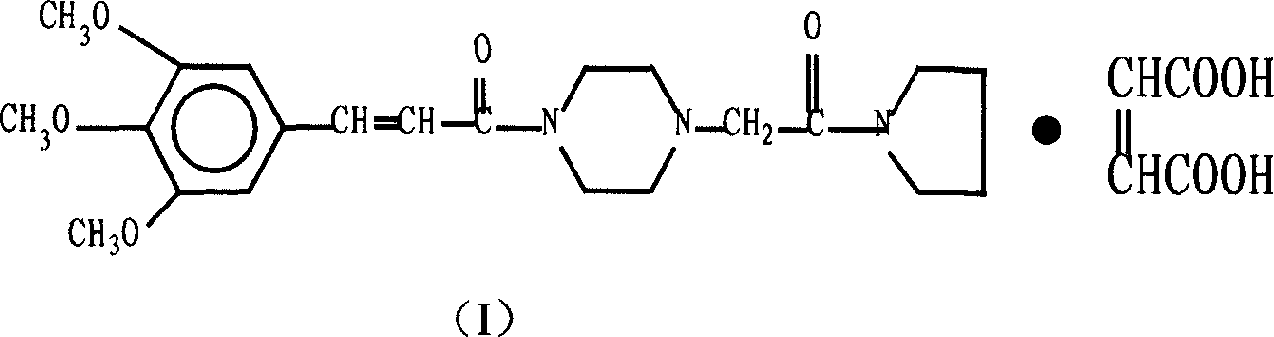
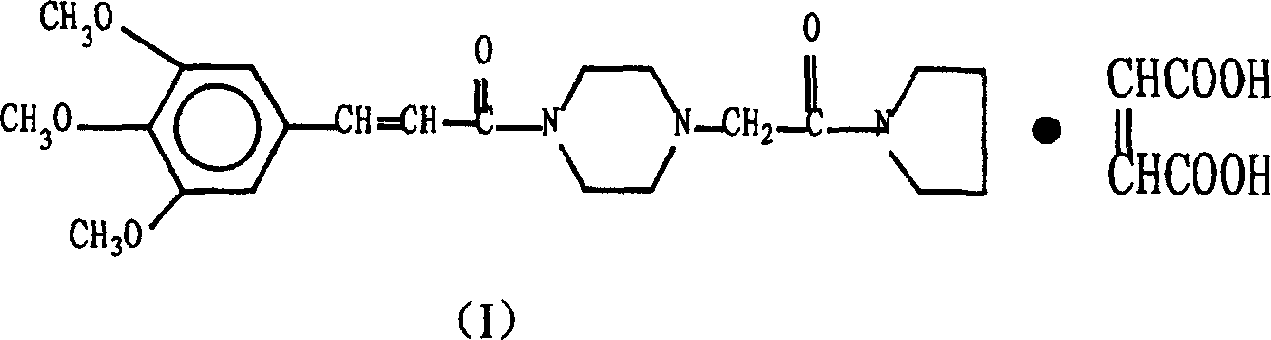
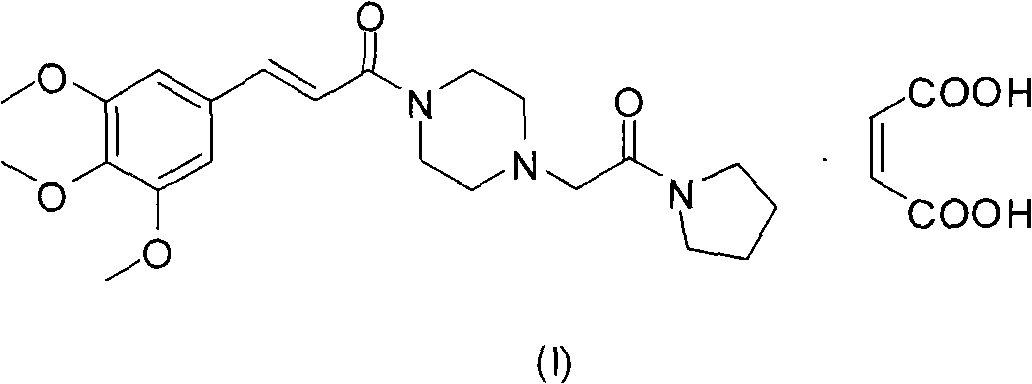
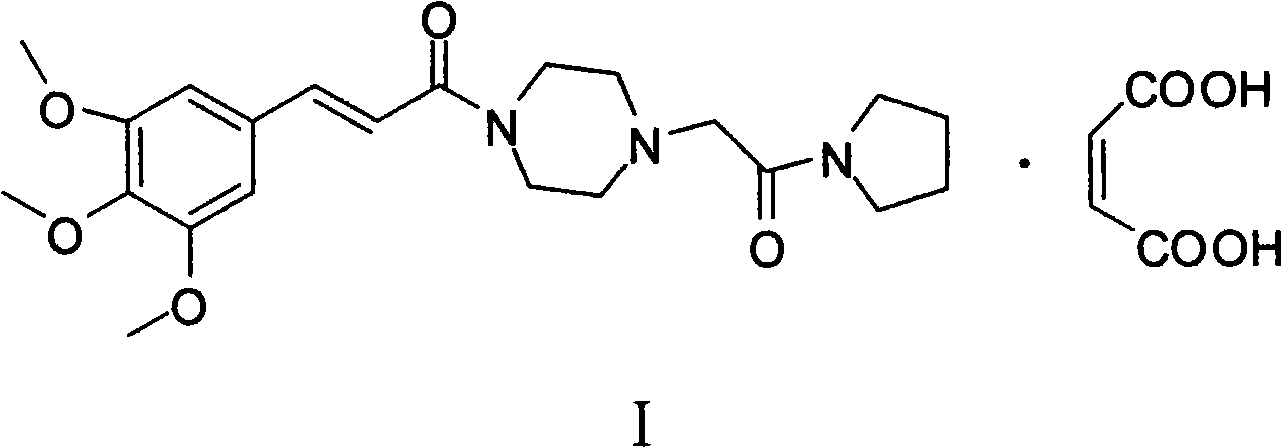
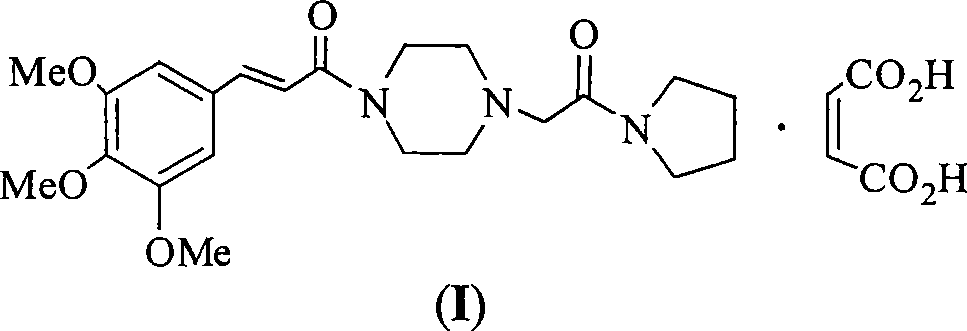
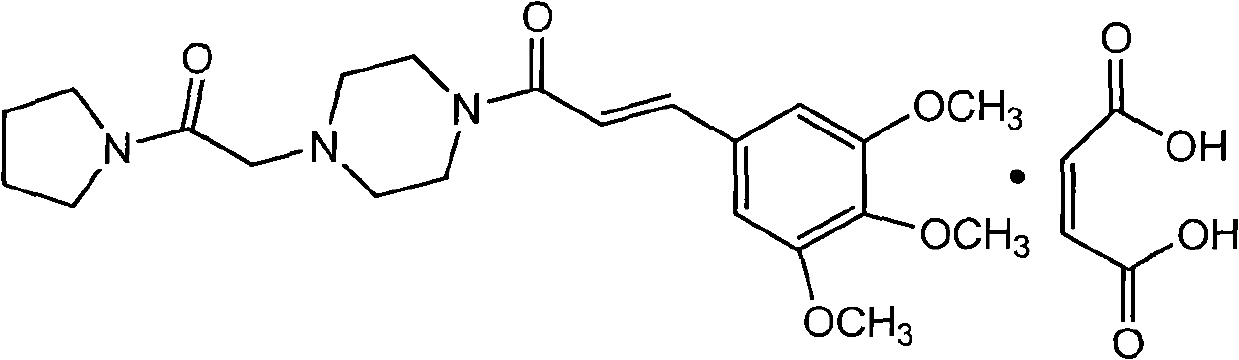
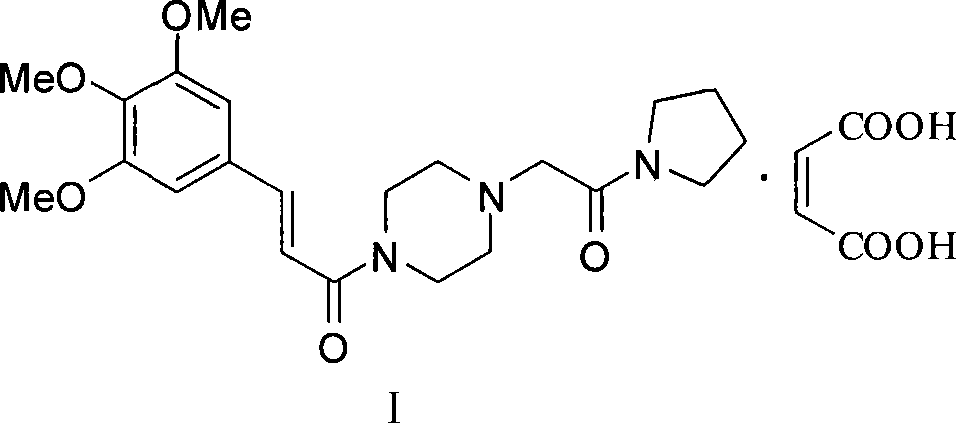
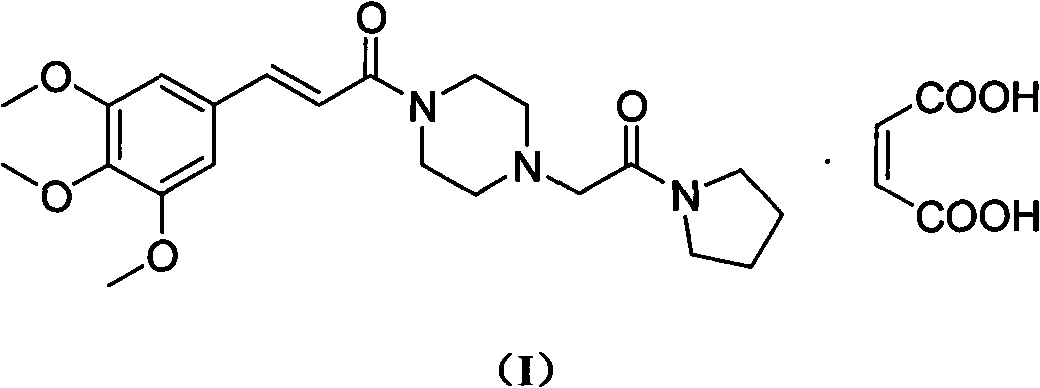
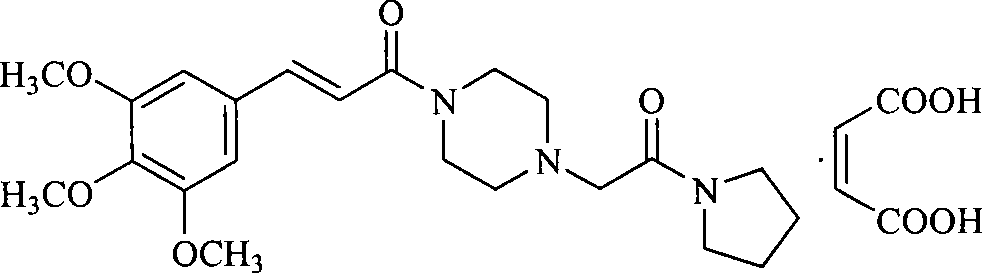
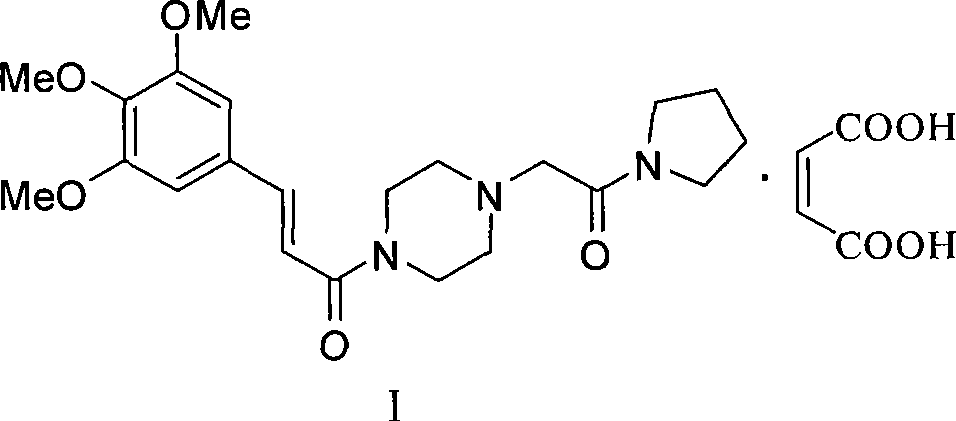
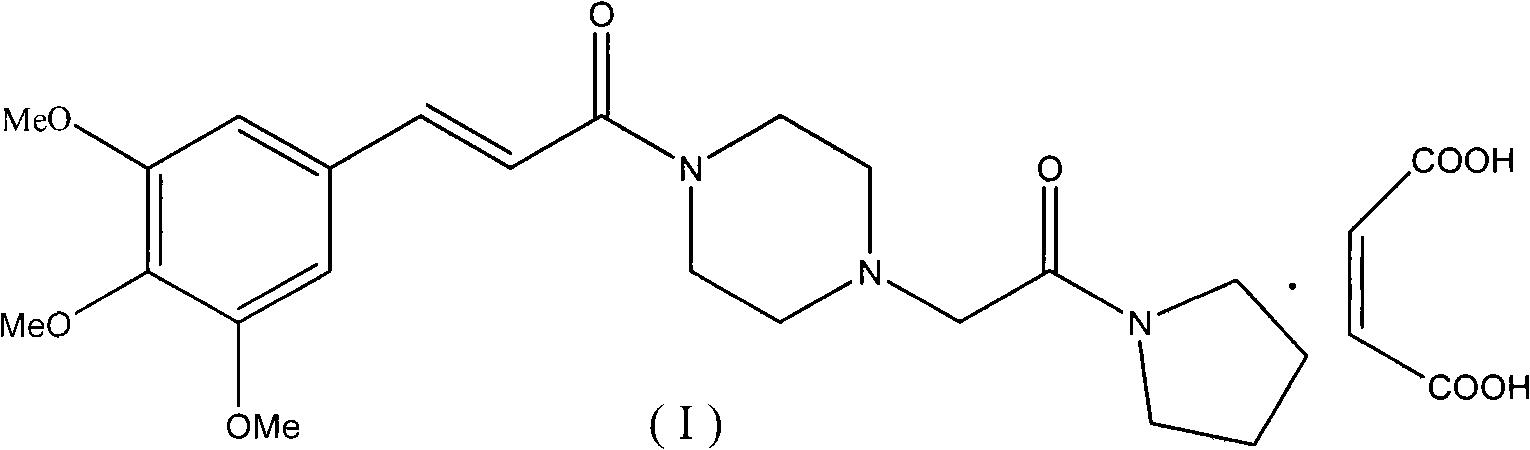
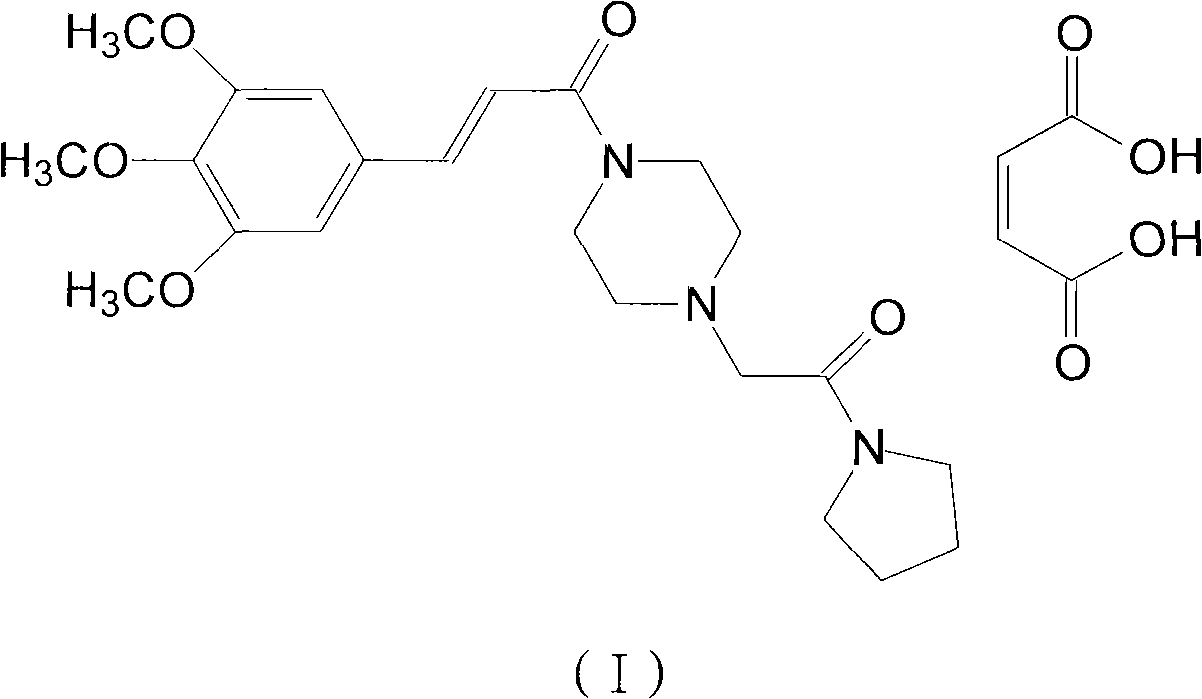
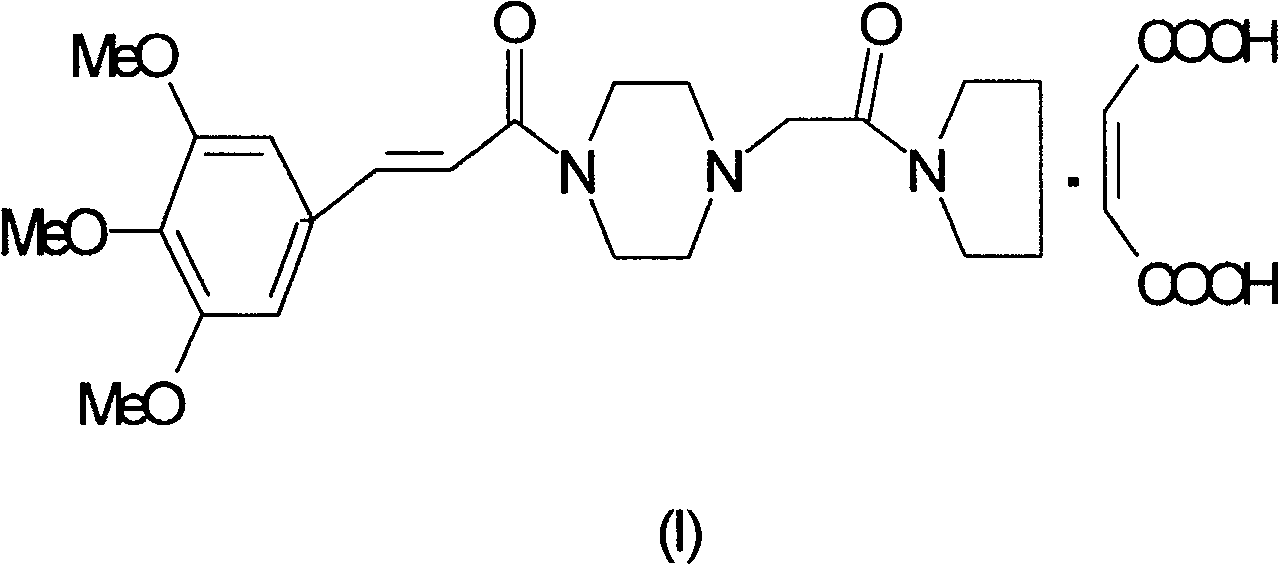
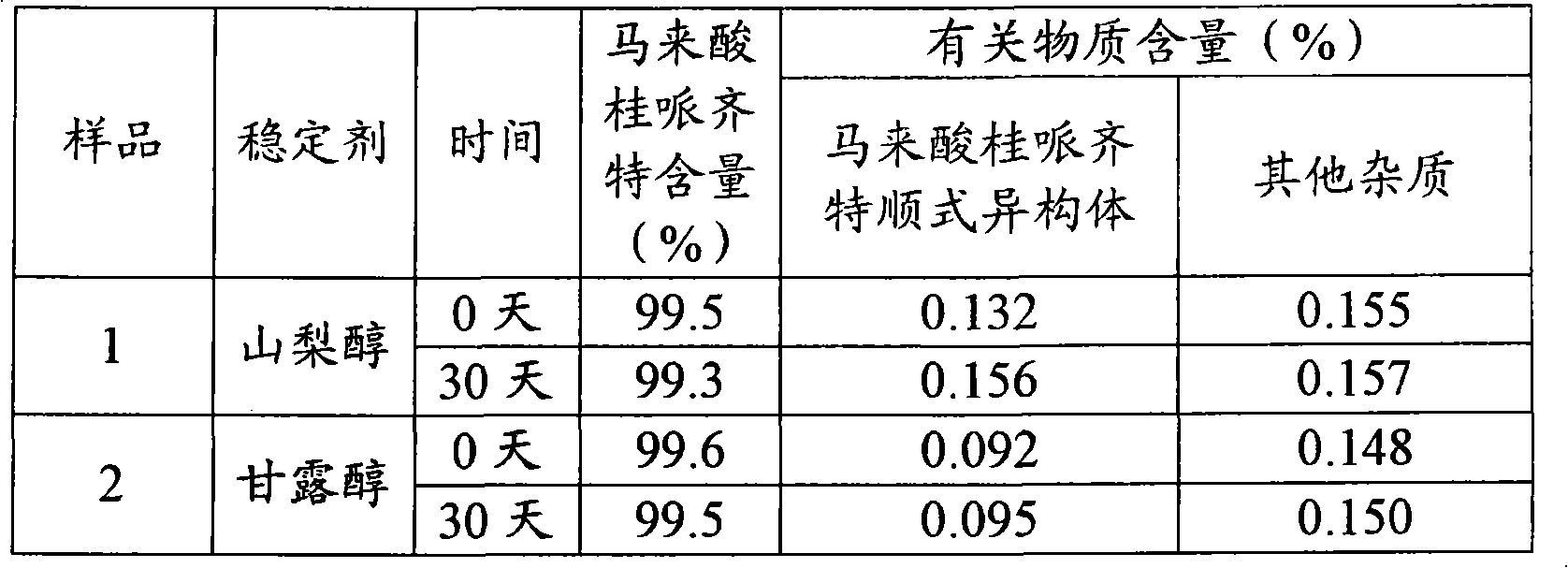
Cinepazide or cinepazide maleate (Kelinao or Anjieli in China) is a vasodilator used in China for the treatment of cardiovascular and cerebrovascular diseases, and peripheral vascular diseases. It appears to work by potentiating A2 adenosine receptors.
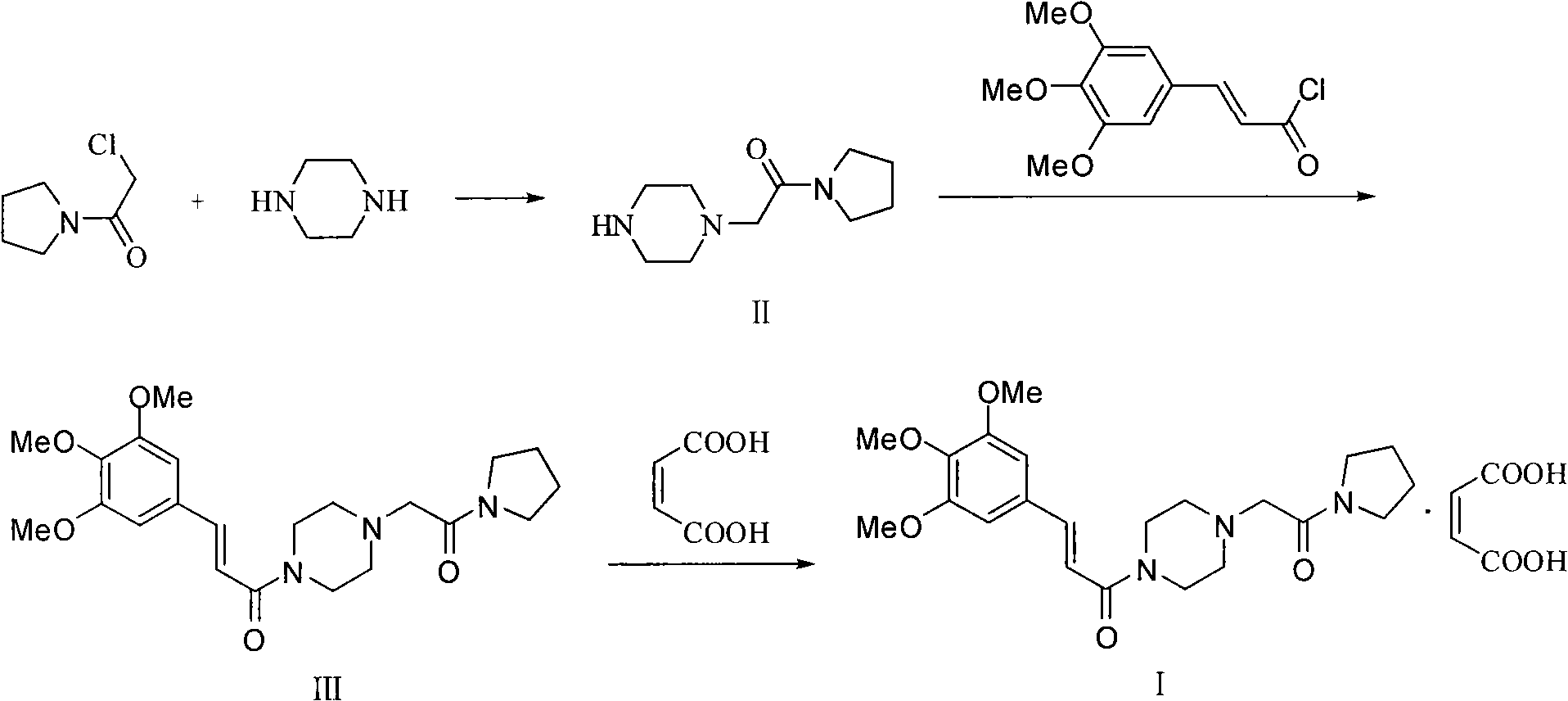
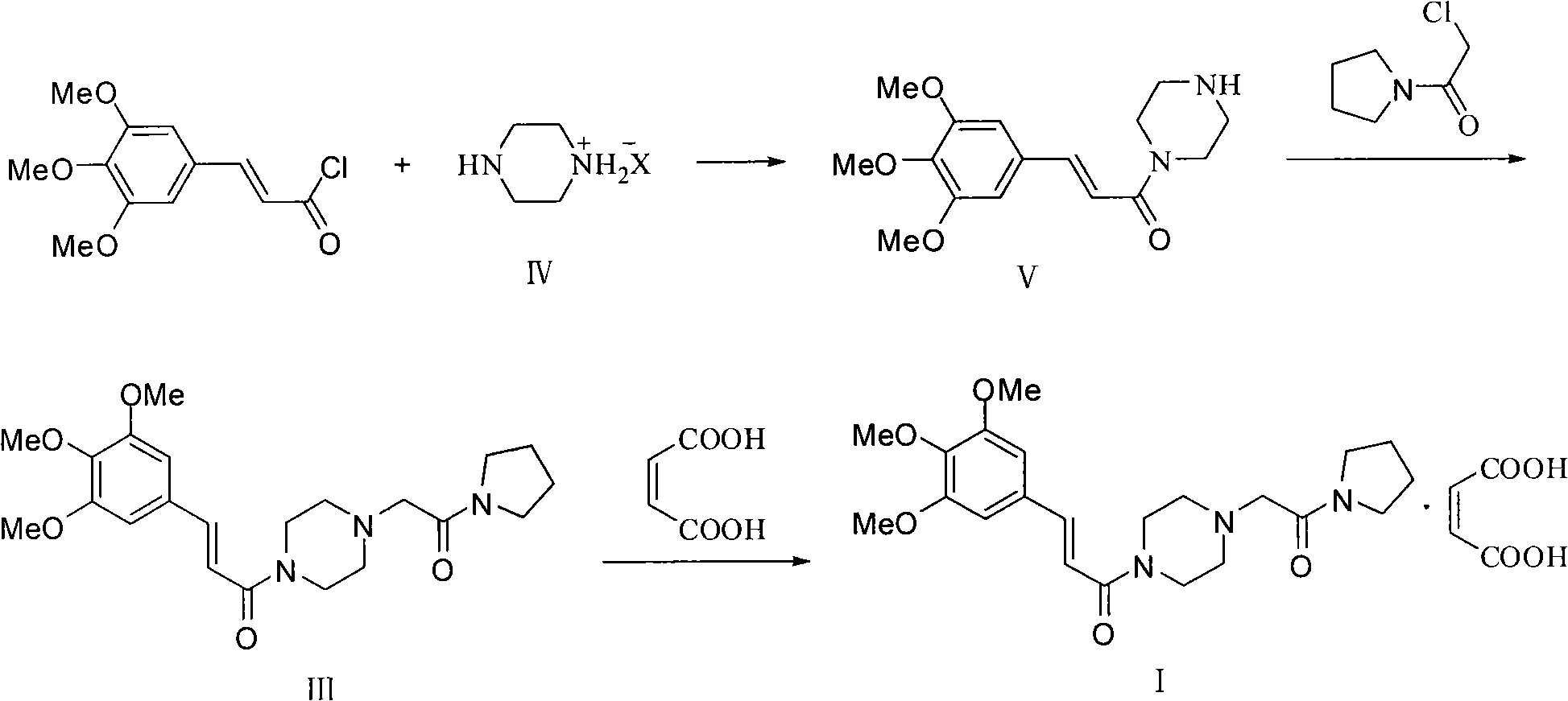
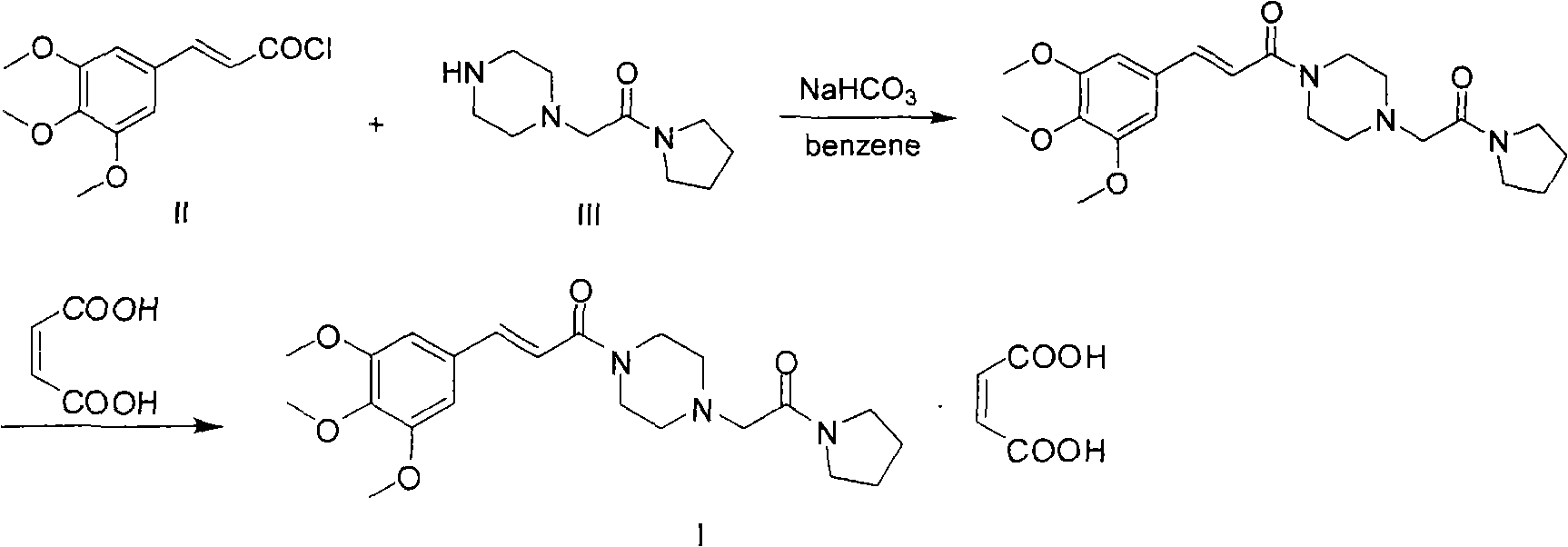
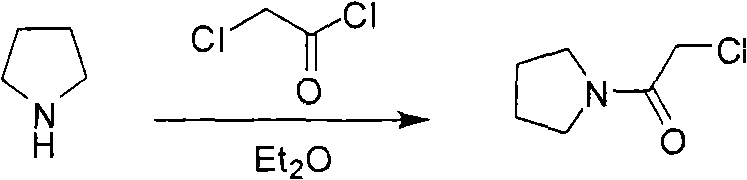
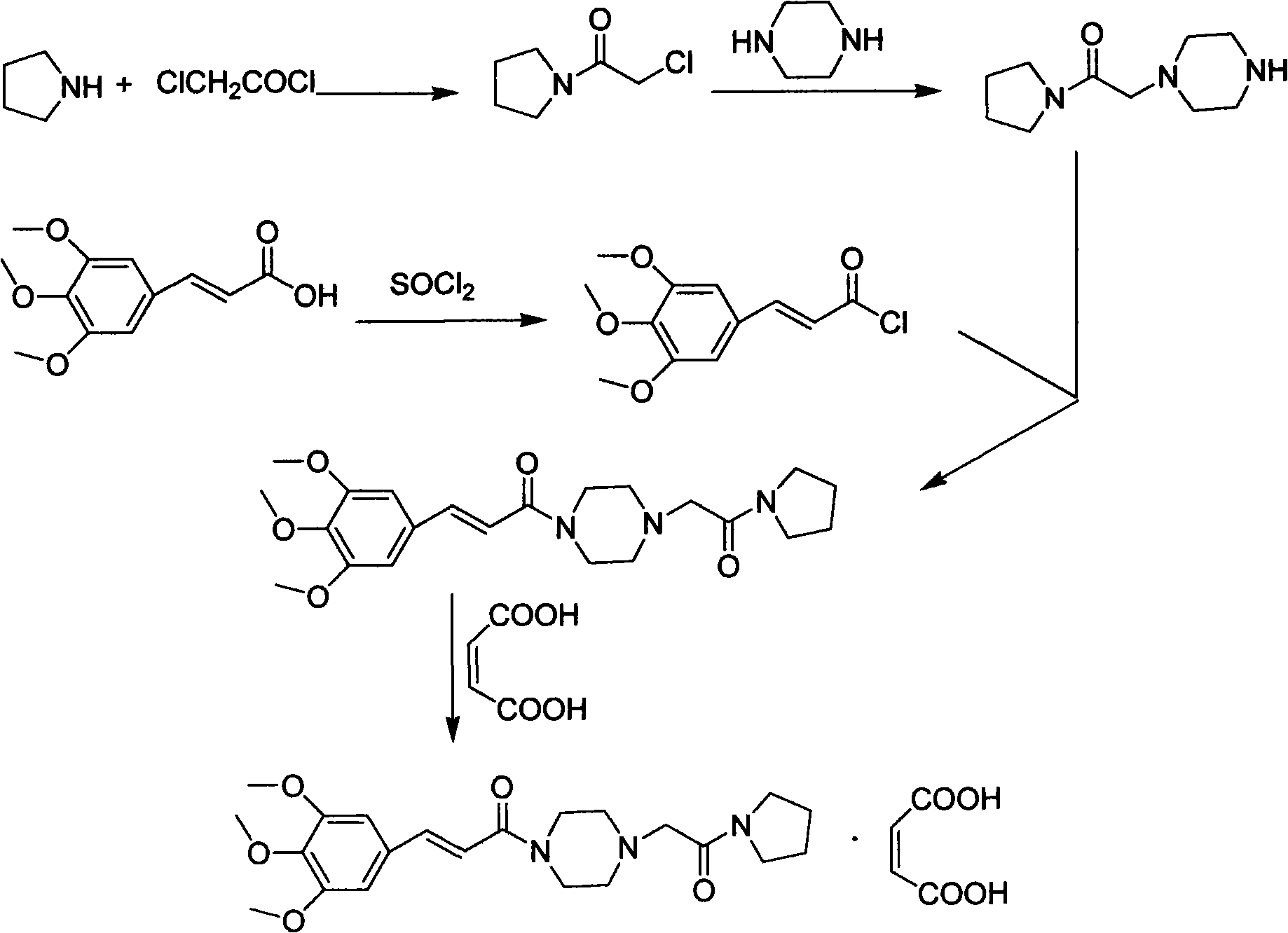
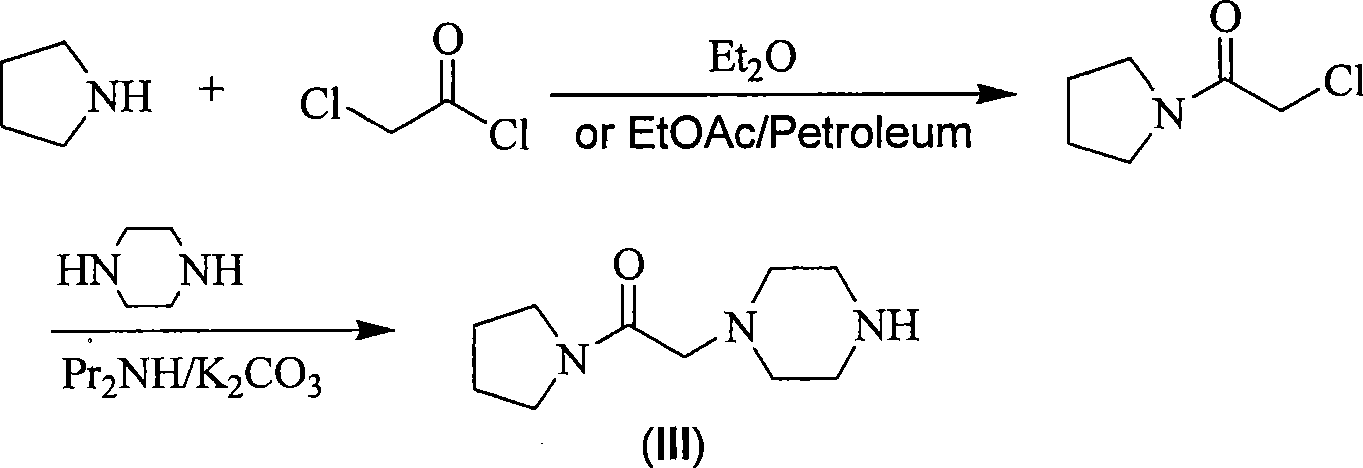
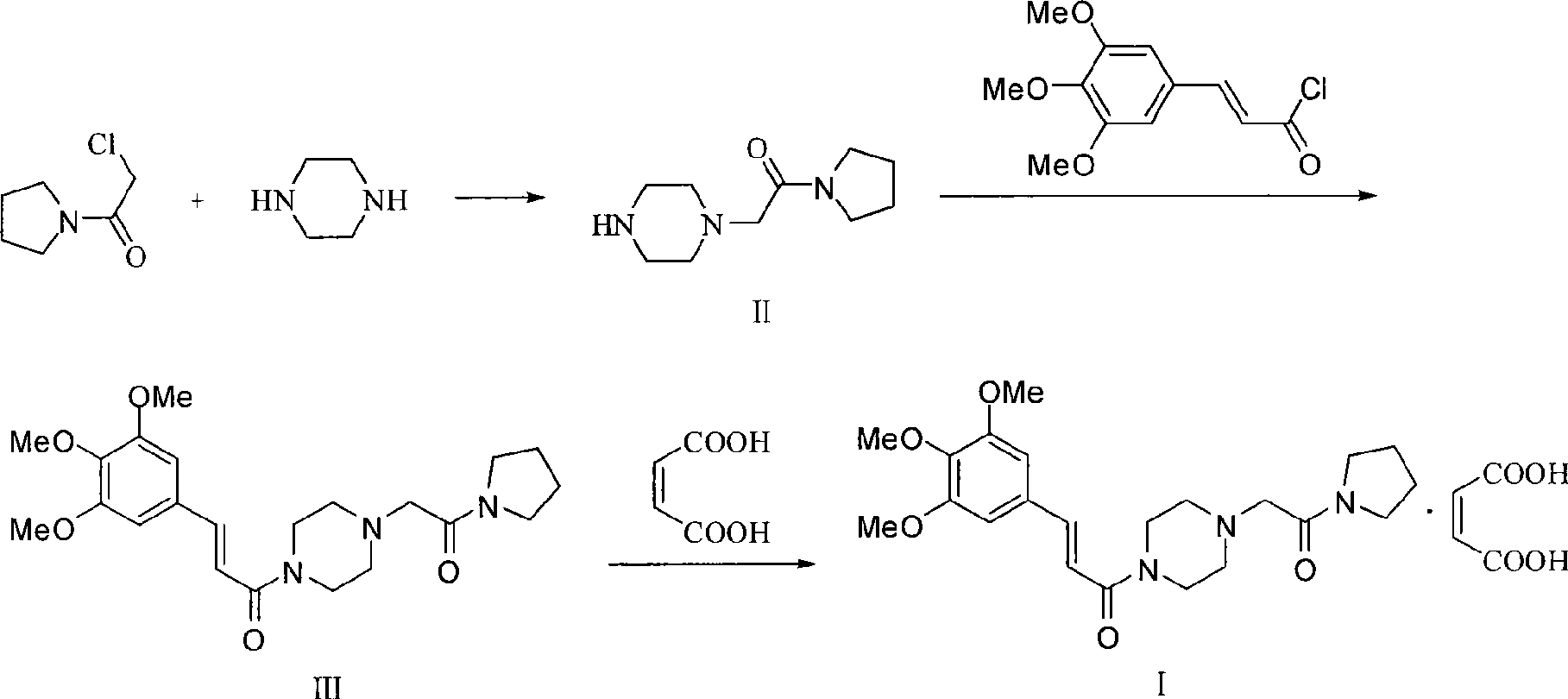
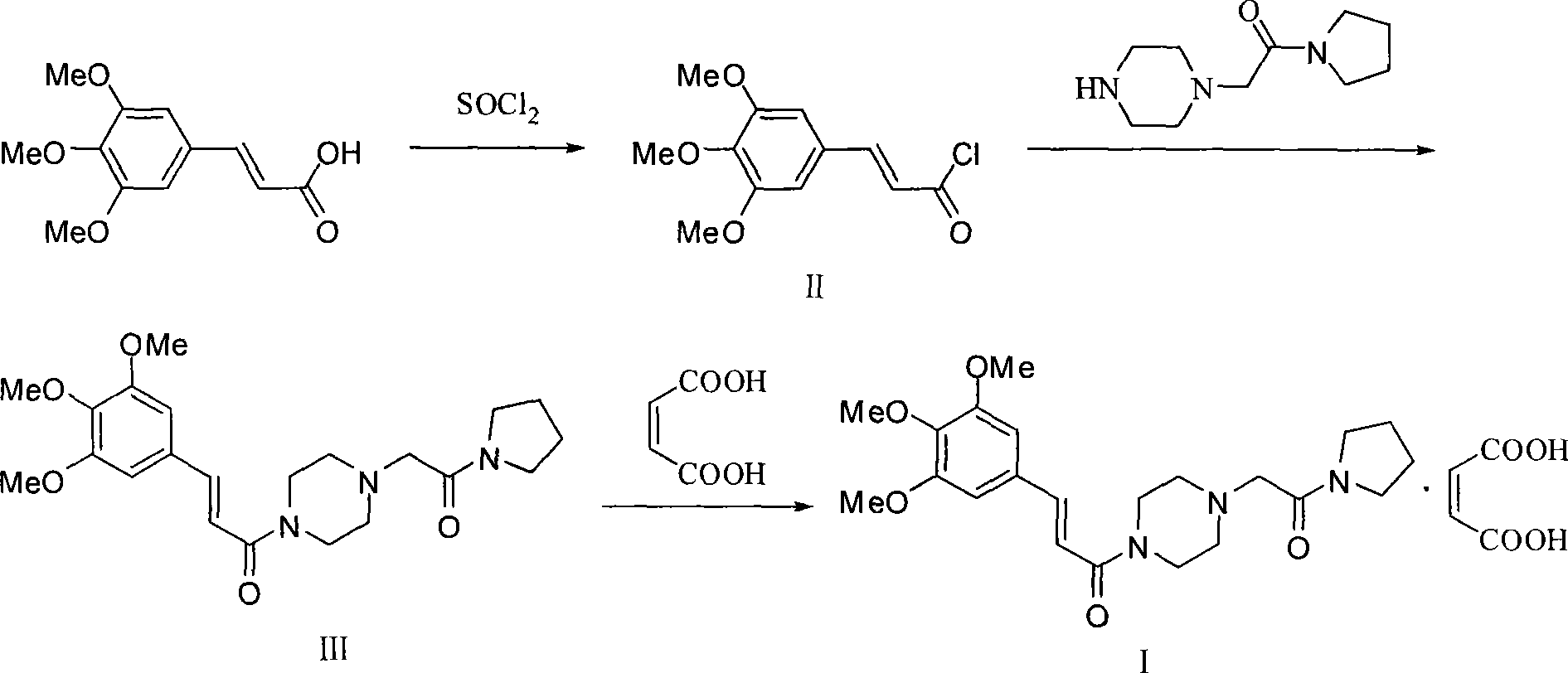
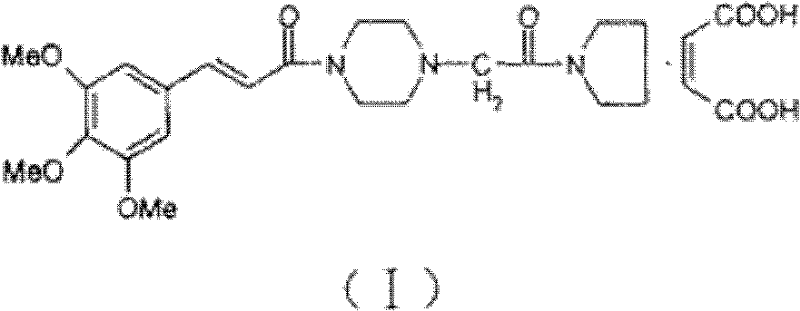
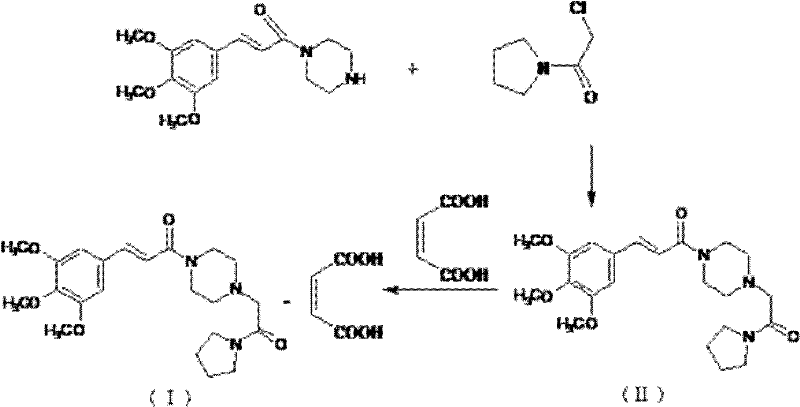
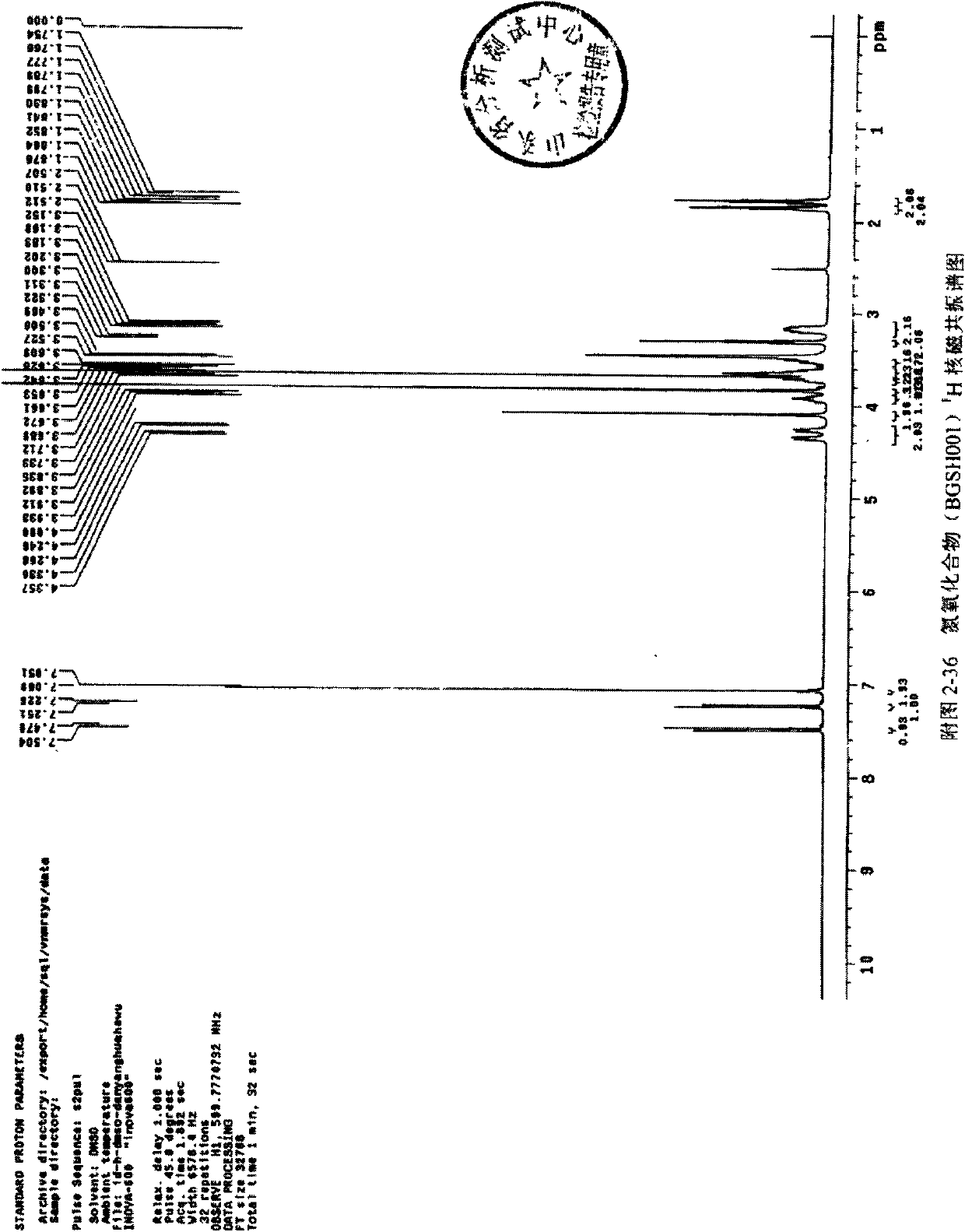
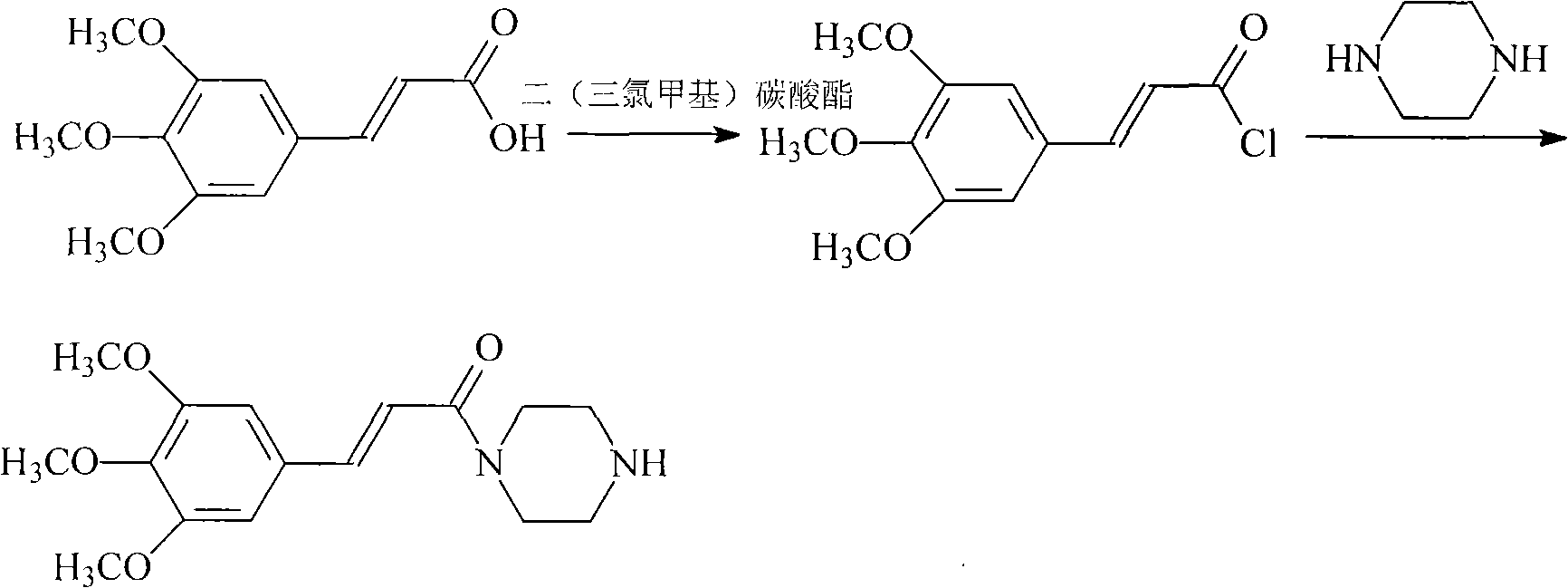
Synthesis method of cinepazide maleate
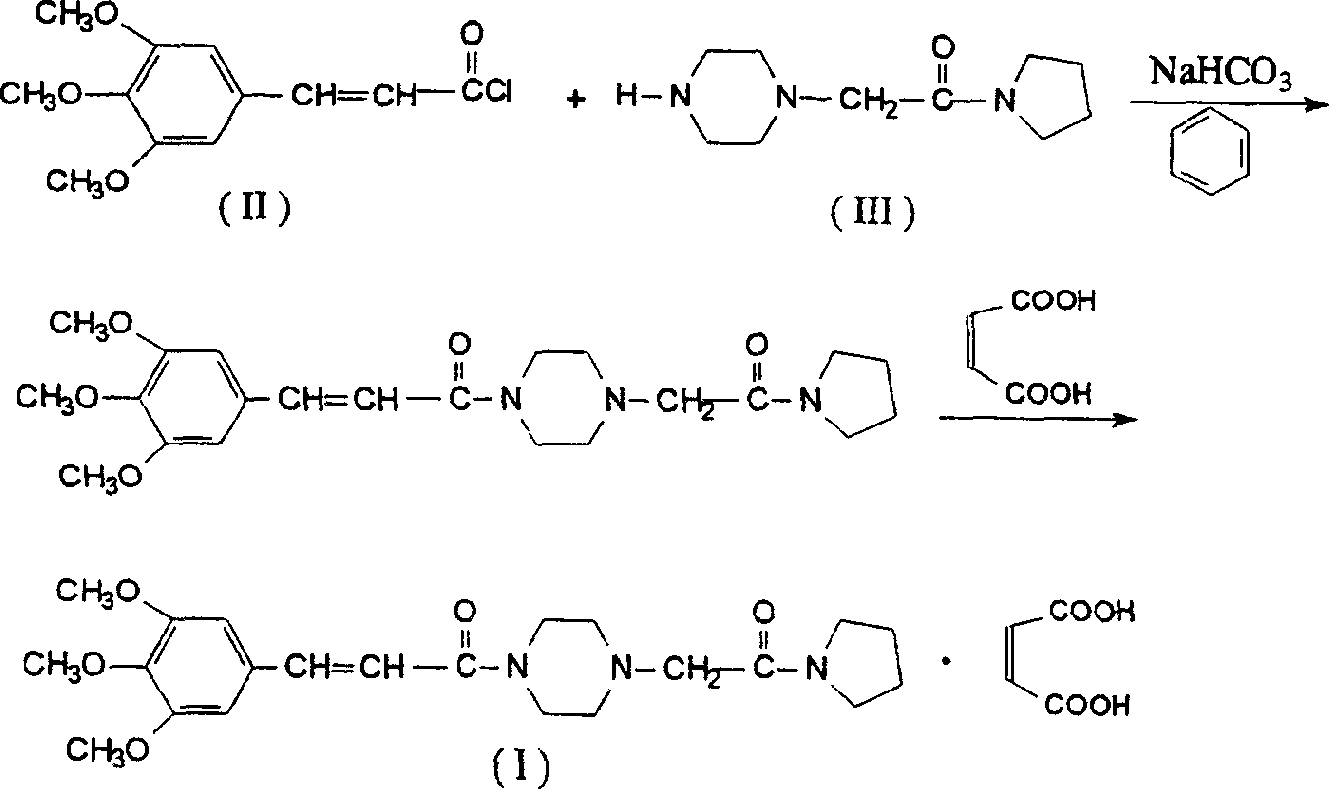
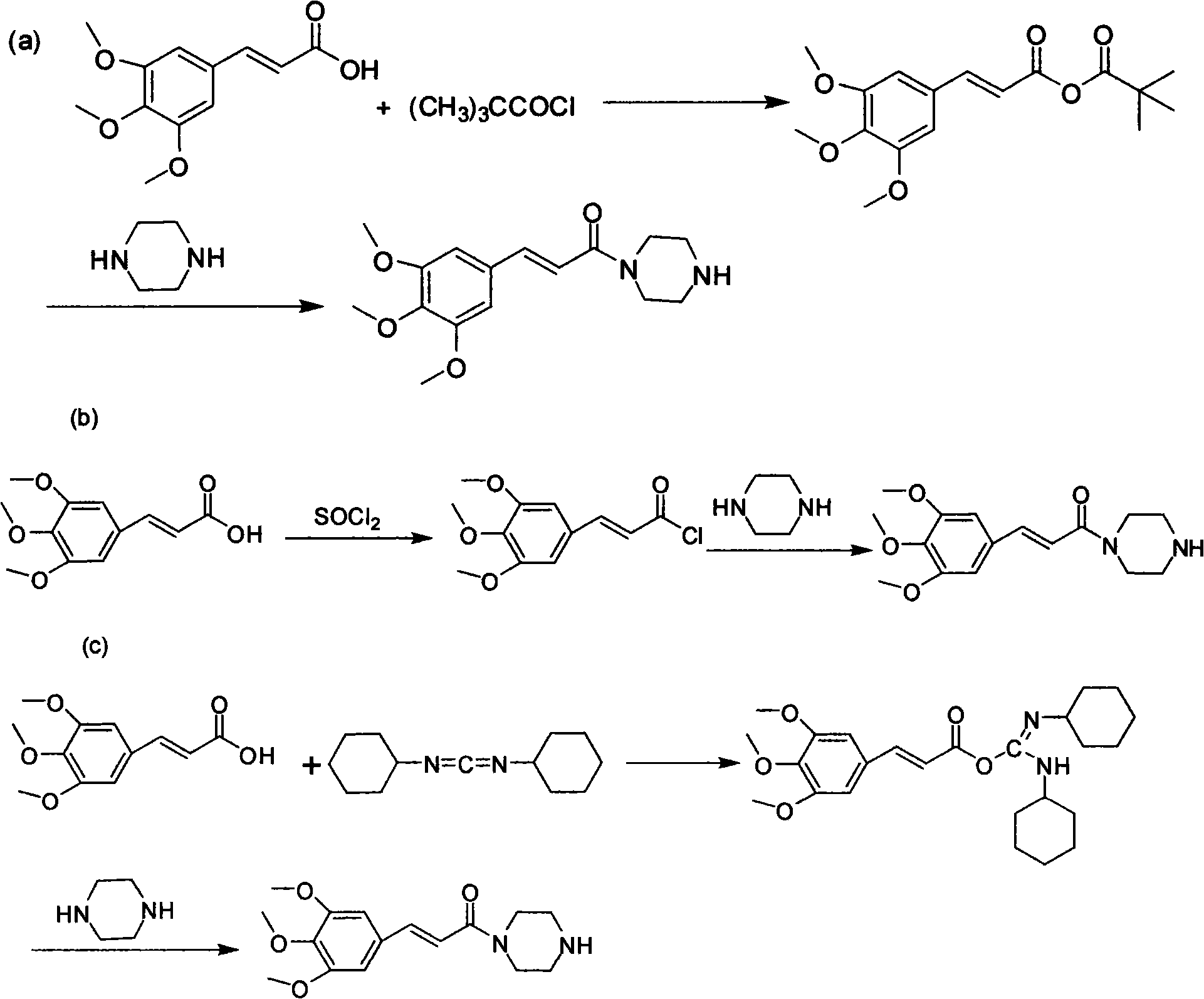
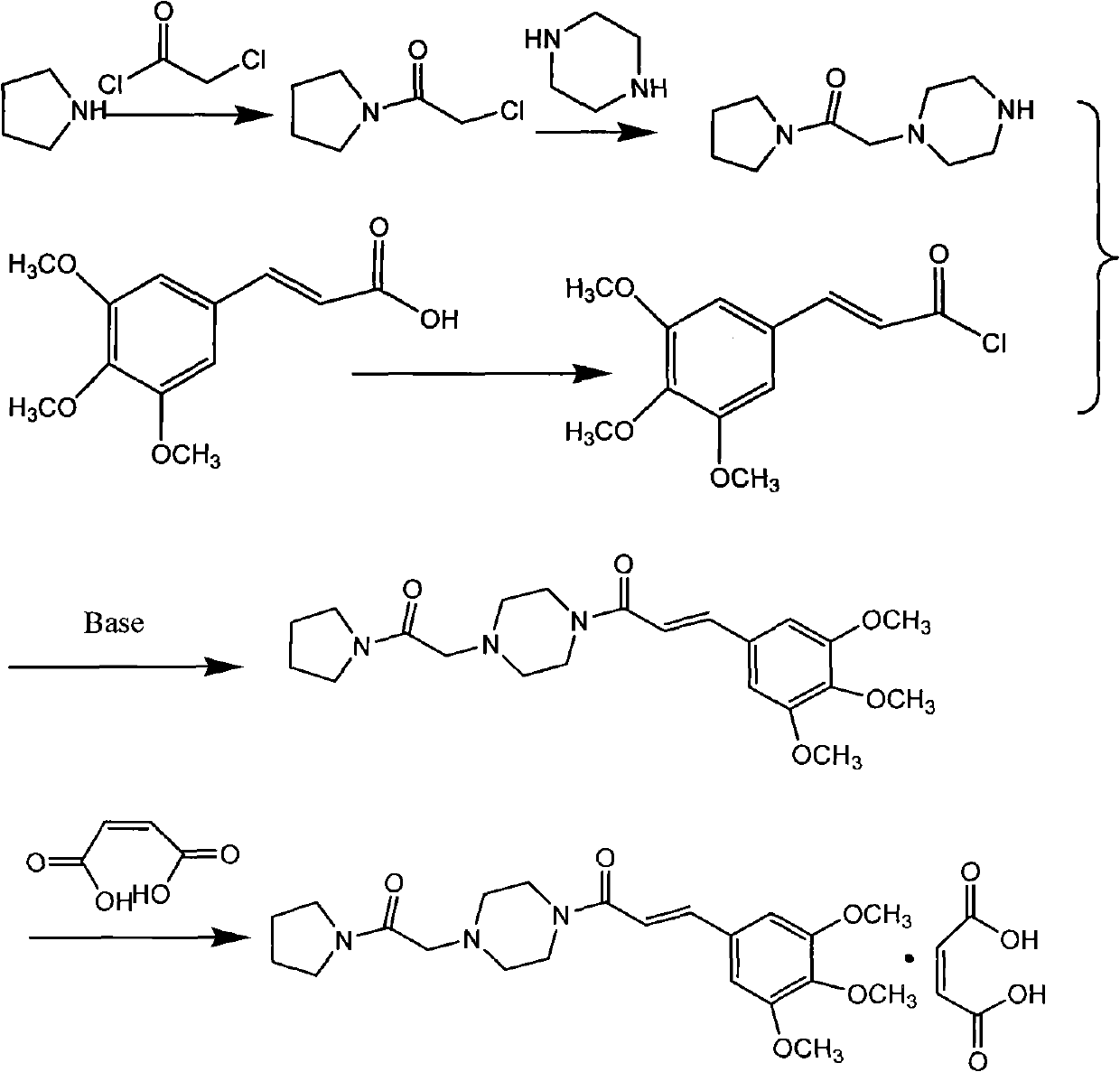
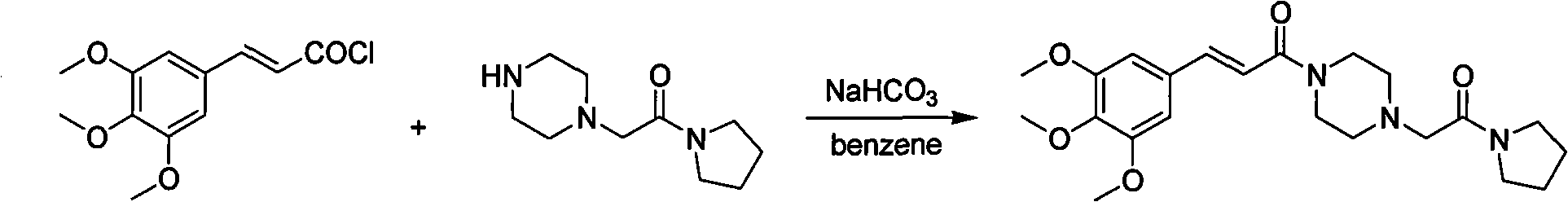
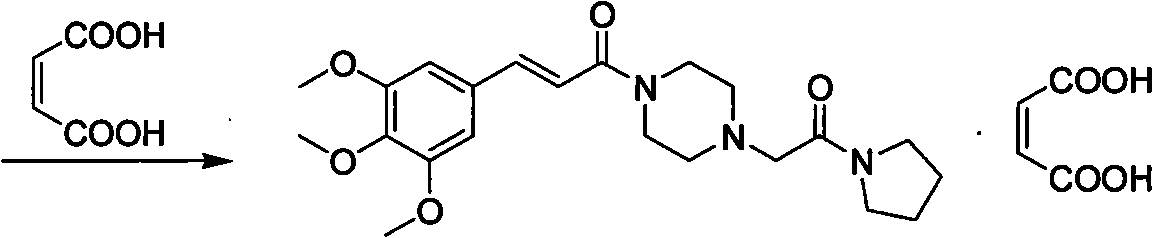
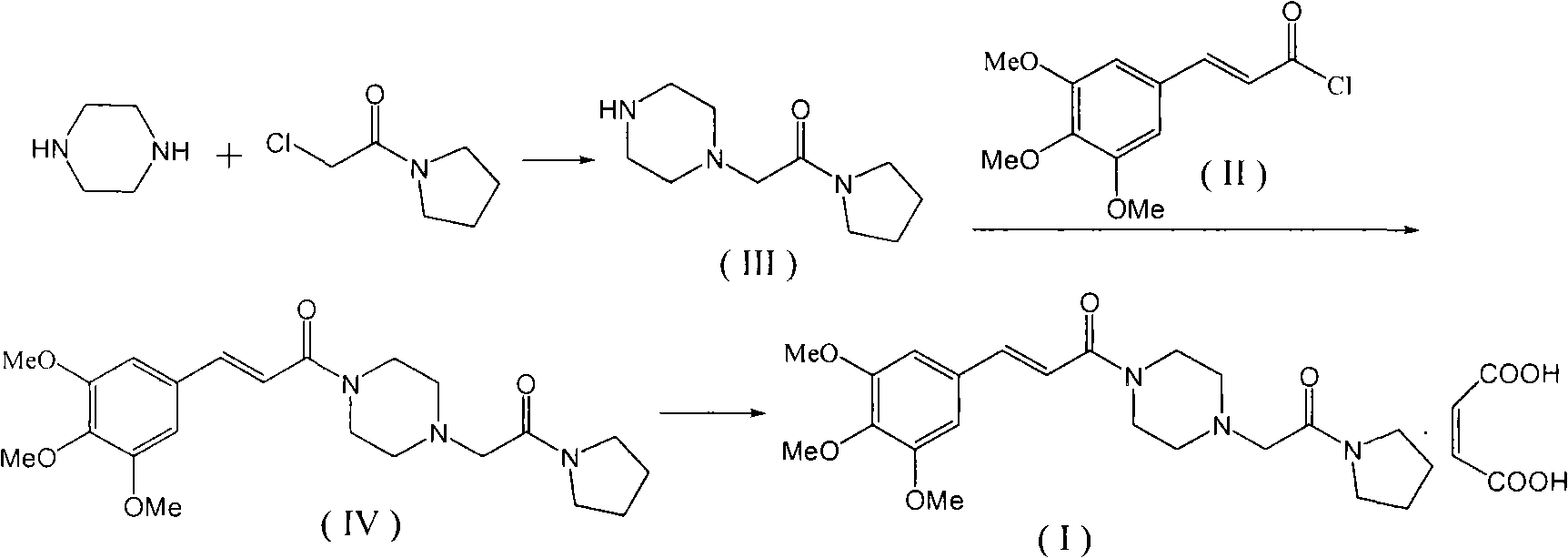
The invention relates to a method to synthesize maleic acid cinepazide. (2) reacts with 3,4,5- cinnamyl chloride, aquiring free alkali of cinepazide, the solvent is chosen from 5, add 6 and acquire the (7), the method for separating (1) is filtration. The invention uses safe solvent in the synthesis of (2), and the method of filtration and 8, making it suitable for industrialized production. The method uses the solvent with low toxicity and good safety, simplized productive condition and course, has high productivity and low cost.
Owner:BEIJING SIHUAN PHARMA +2
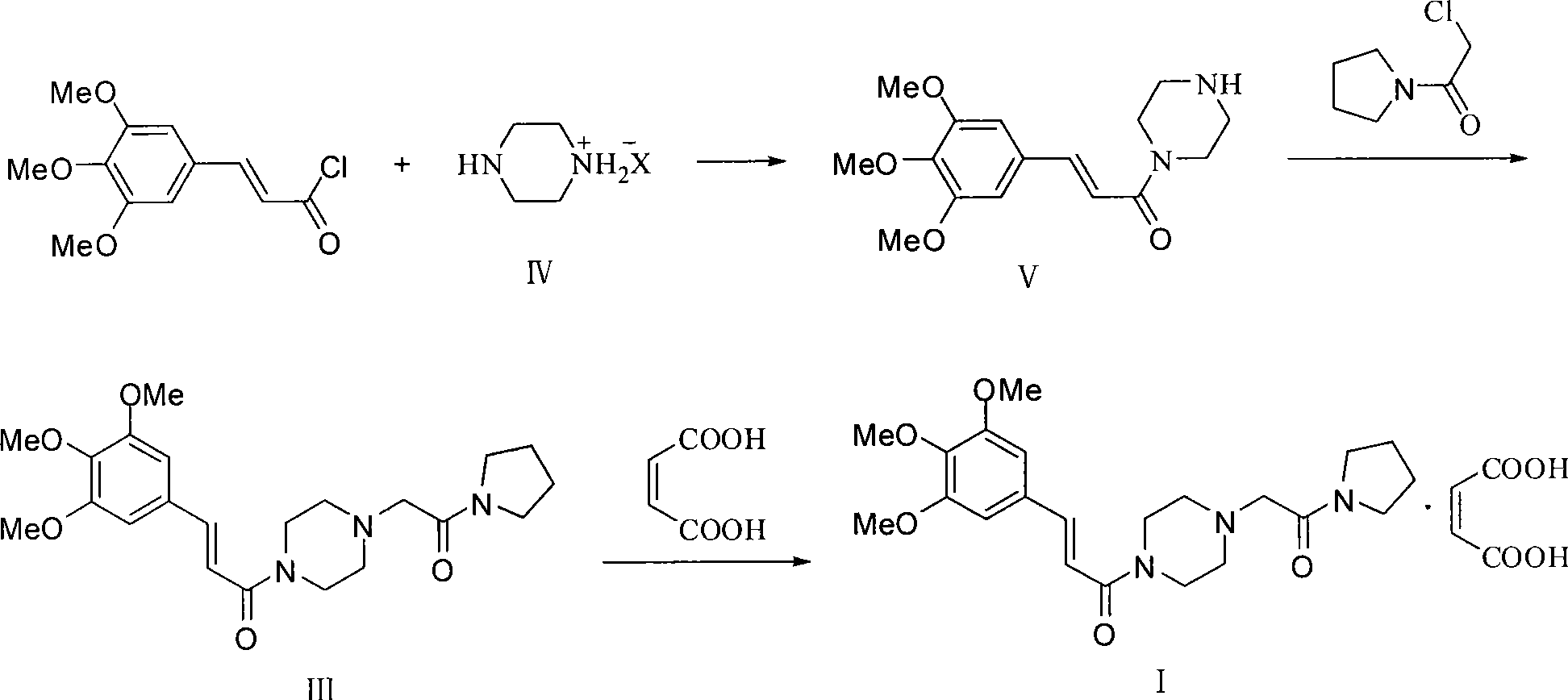
Synthesis method of cinepazide maleate
Owner:BEIJING SIHUAN PHARMA +2
Method for preparing cinepazide maleate
InactiveCN101260092AThree wastes less pollutionSave energyNervous disorderOrganic chemistrySolventPyrrole
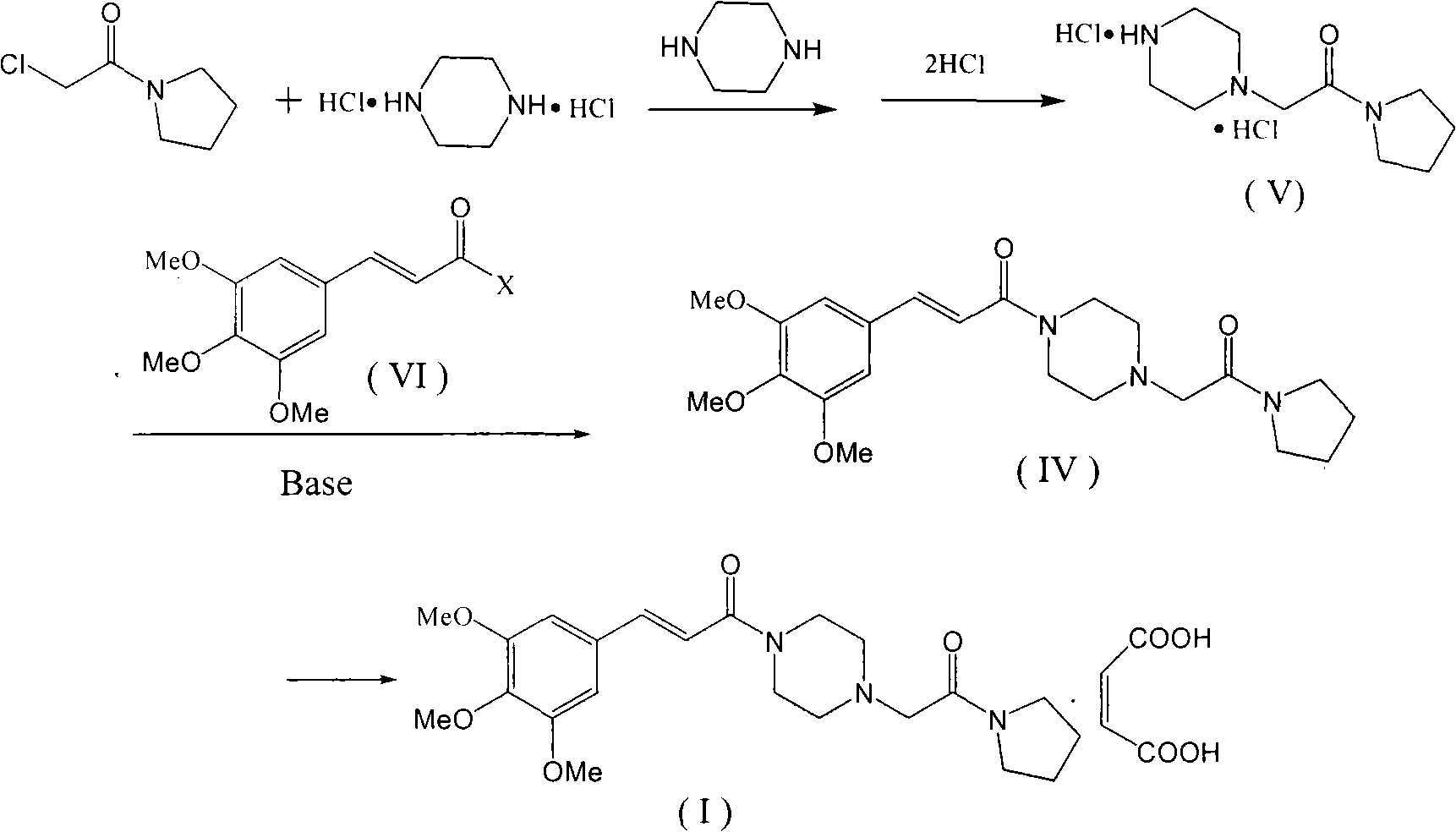
The invention provides a method for preparing cinepazide maleate, comprising the following steps of: allowing chloride acid pyrrole , piperazine and Hydrochloric acid to react in a water solvent, adding alkaline substances to adjust PH value, and then collecting 1-piperazine acetyl pyrrole, allowing product to react with 3, 4 and 5 - trimethoxy chloride cinnamon in Acetic ester solvent, collecting Cinepazide free base in the reaction product and then salifying the free base with maleic acid to obtain cinepazide maleate. The cinepazide maleate obtained is heated to reflow in mixed solvent of alcohols and acetone, and then collecting cinepazide maleate with stable crystal form from the system. The melting point of the cinepazide maleate obtained is at a temperature of between 173 and 174 DEG C. The cinepazide maleate prepared in the method has a low cost, the method is simple to operate, is high in the yield, the product quality is stable and is suitable for industrialized production.
Owner:罗军 +1
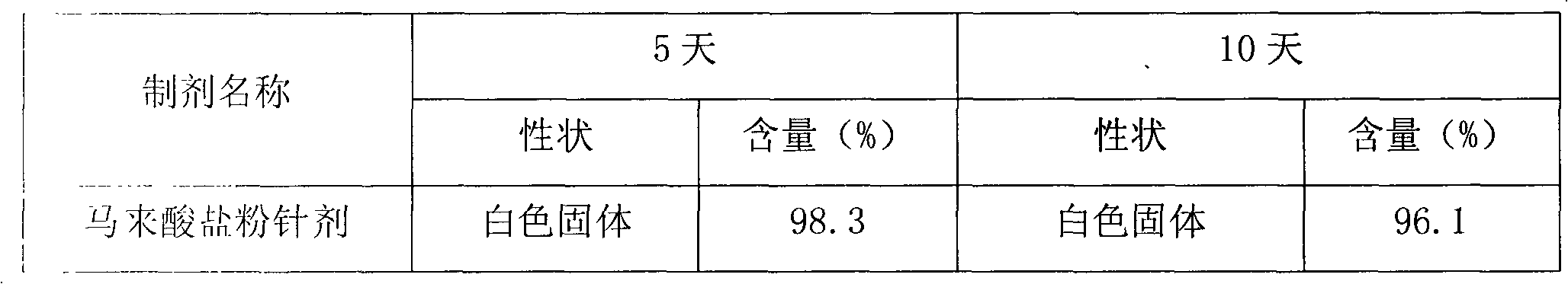
Cinepazide maleate injection and preparation method thereof
ActiveCN101204372AImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismCinepazideDisodium hydrogen phosphate
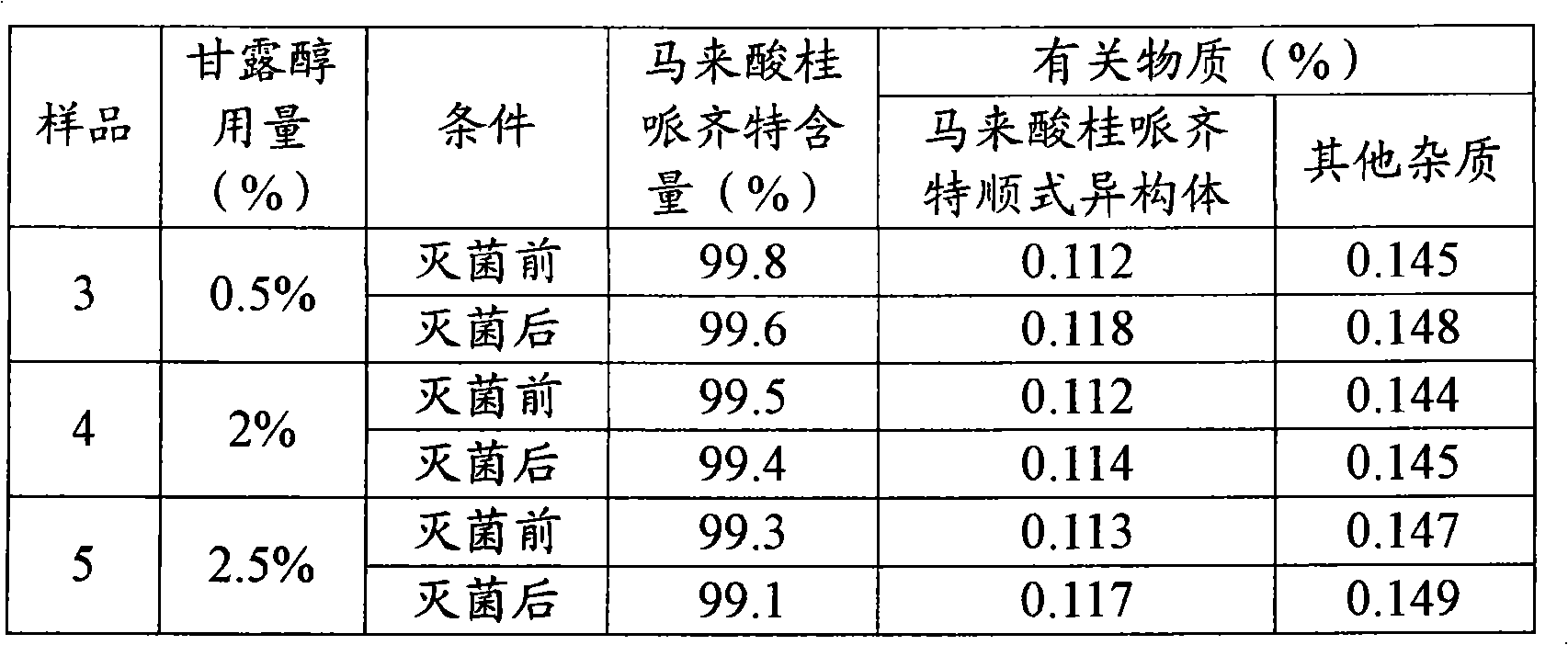
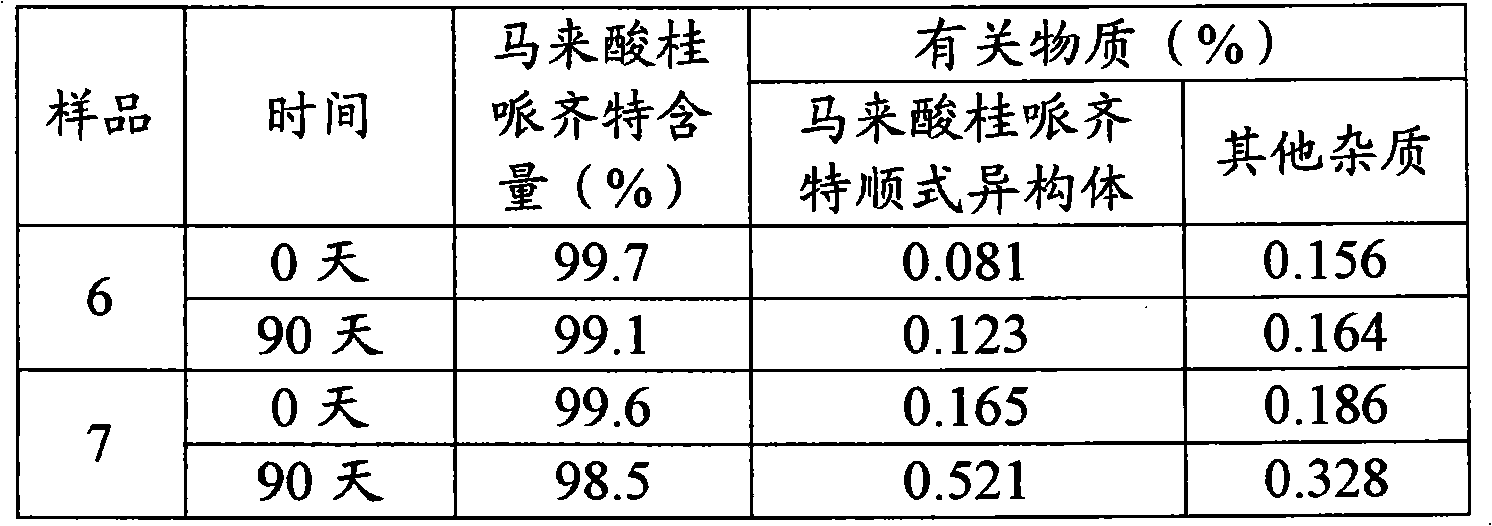
The invention belonging to the field of medicine technology discloses a cinepazide maleate injection and a manufacturing method thereof. In every 1000ml of injection, 35-45g of cinepazide maleate is contained, 20-30g of D-sorbierite, 2-3.5g of disodium hydrogen phosphate, and proper water for injection are contained. The manufacturing method includes the following steps: dissolving the D-sorbierite in water; adding the cinepazide maleate into the D-sorbierite solution, mingling in order to partially dissolve; adding part of disodium hydrogen phosphate solution in order to fully dissolve the cinepazide maleate; then slowly adding the rest disodium hydrogen phosphate solution to adjust the pH value; finally filtering and preparing into injection preparation. The cinepazide maleate injection of the invention effectively reduces the content of cinepazide maleate cis-isomer for the long-term storage.
Owner:HAINAN SIHUAN PHARMA +1
Method for preparing cinepazide maleate
InactiveCN101591310AEasy to removeSimple ingredientsOrganic chemistryCardiovascular disorderCarboxylic acid halidesPyrrole
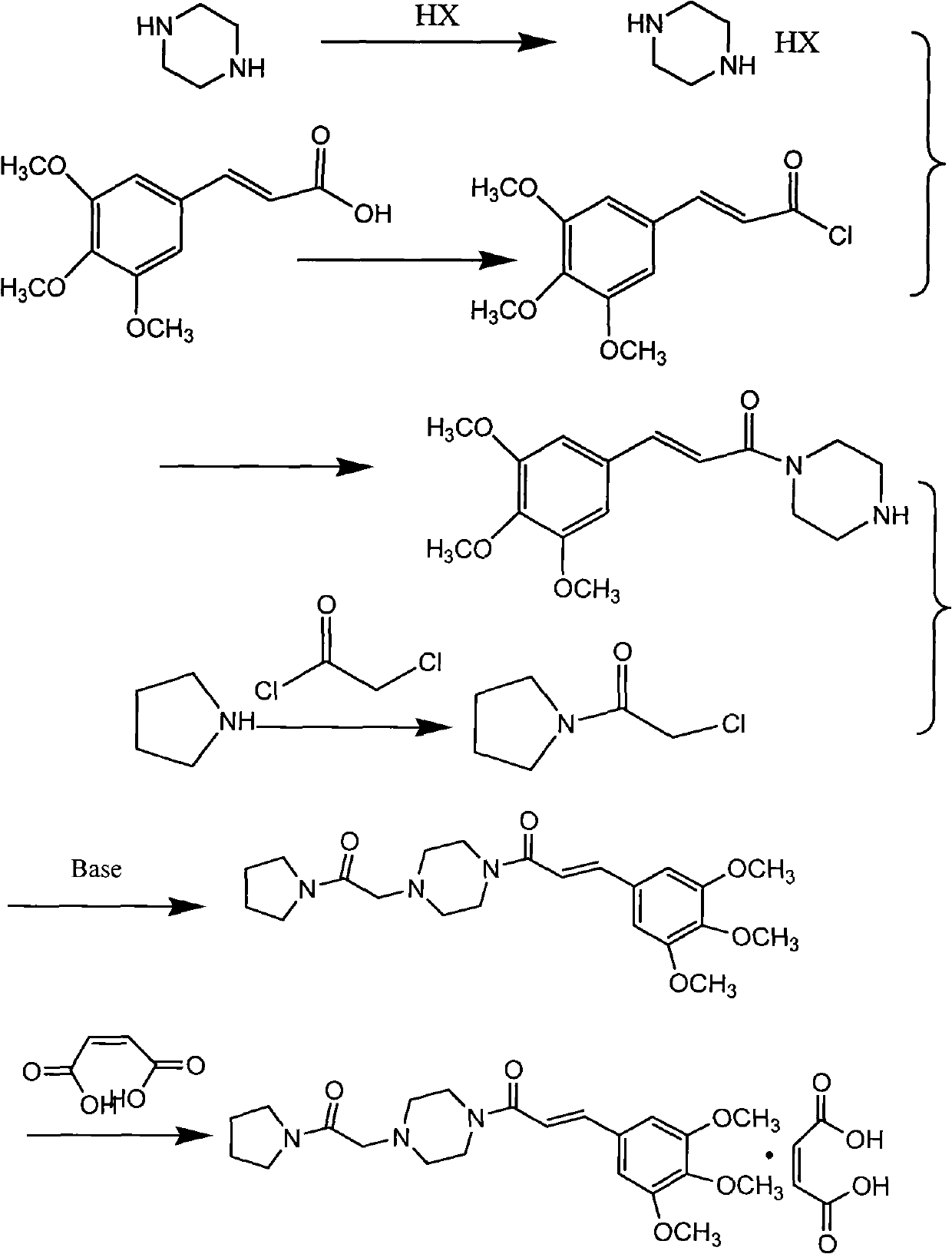
The invention provides a method for preparing cinepazide maleate. The method comprises the following steps: preparing mixed acid anhydride or acyl halide by taking 3,4,5-trimethoxycinnamic acid as a raw material; then, reacting the mixed acid anhydride or the acyl halide with absolute piperazidine to prepare 1-(3,4,5-trimethoxyphenyl)piperazidine, or dehydrating the 3,4,5-trimethoxycinnamic acid and the absolute piperazidine to generate 1-(3,4,5-trimethoxyphenyl)piperazidine under the action of dicyclohexylcarbodiimide; reacting chloracetyl chloride with nafoxidine to prepare N-(2-chloracetyl)nafoxidine; reacting the N-(2-chloracetyl)nafoxidine with 1-(3,4,5-trimethoxyphenyl)piperazidine to prepare cinepazide free alkali; and salifying the cinepazide free alkali and maleic acid to prepare the cinepazide maleate. The method has the advantages of accessible raw materials, mild reaction condition, simple and easy operation, short synthesis route, high yield, industrial production availability and the like.
Owner:北京兴德通医药科技股份有限公司
Method for preparing cinepazide maleate
InactiveCN101492431AGroup 5/15 element organic compoundsSulfonic acid preparationDiethyl phosphateMethyl group
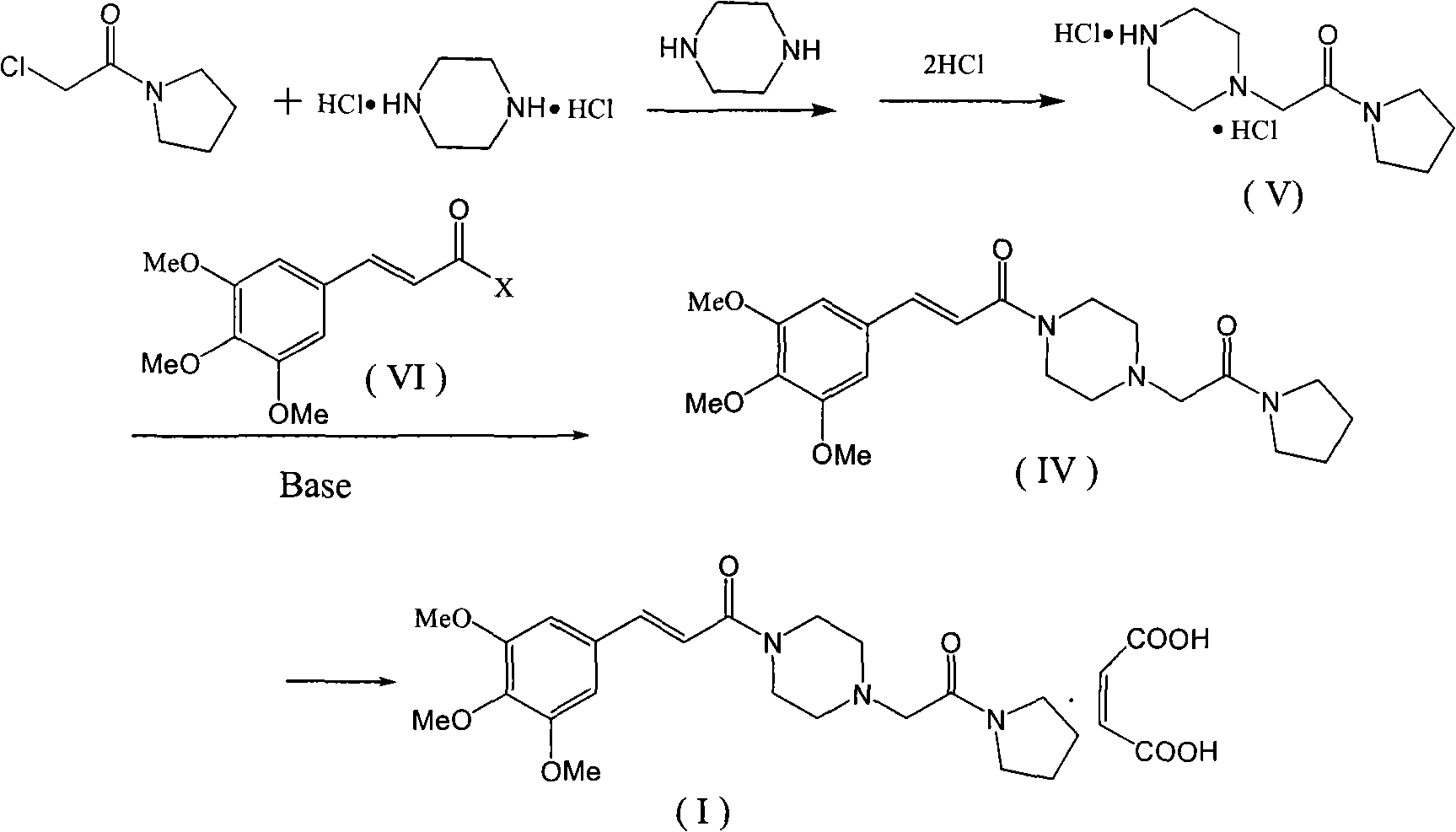
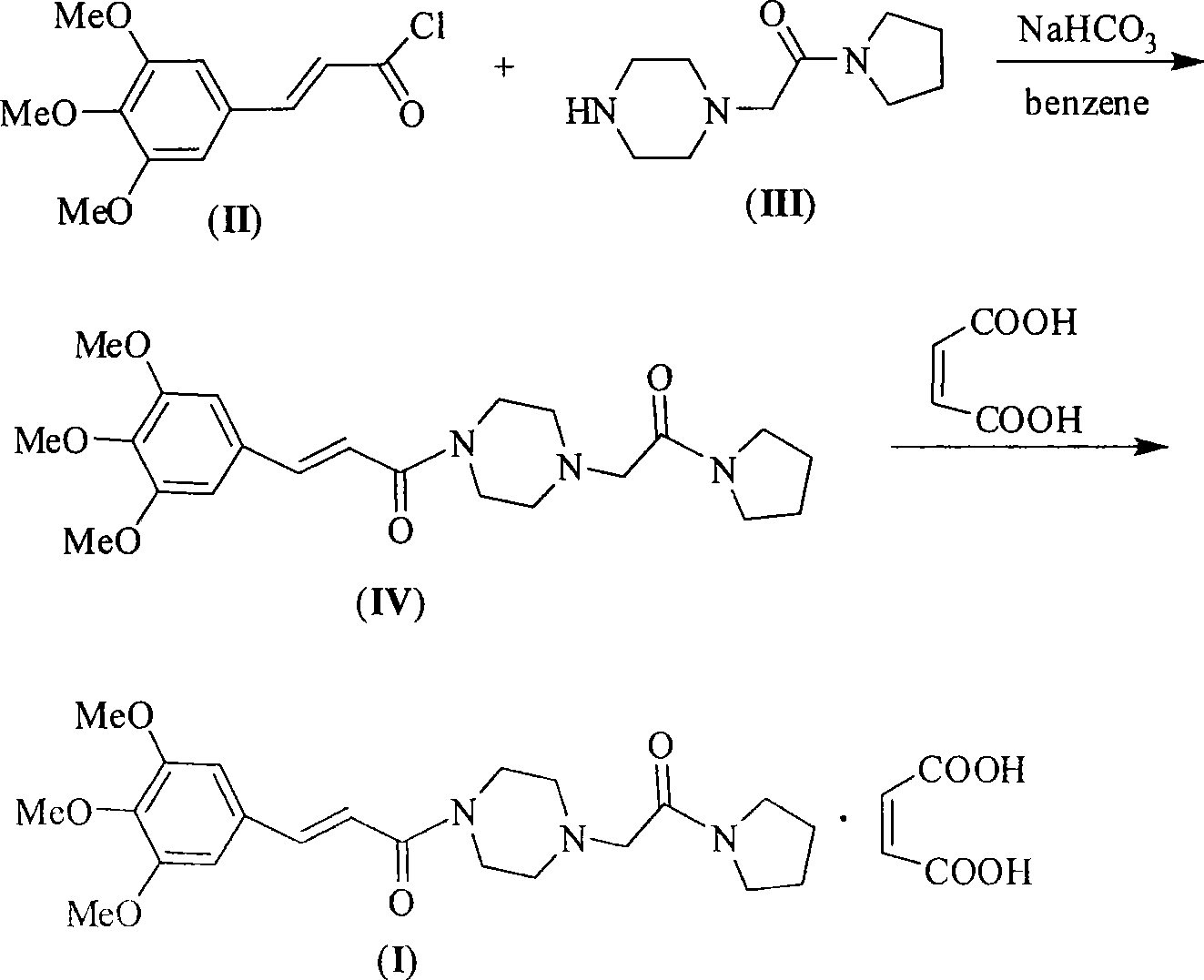
The invention relates to a preparation method of cinepazide maleate, comprising the following steps: 3, 4, 5-trimethoxycinnamylic acid reacts with chlorinating agent, chloro diethyl phosphate and benzenesulfonyl chloride for preparing corresponding acyl active matters of the 3, 4, 5-trimethoxycinnamylic acid. The acyl active matters react with 1-[(1-pyrrolidine carbonyl) methyl] piperazine double hydrochloride for preparing 1-[(1-pyrrolidine carbonyl) methyl]-4-(3, 4, 5-trimethoxycinnamylic acid acyl) piperazine which is separated and forms salt with maleic acid for preparing cinepazide maleate or can be directly used in ethanol or acetone solution for forming salt with maleic acid. And after the crystallization, the cinepazide maleate with high melting point and stable crystal form is prepared. Chloroacetyl pyrrole perimidine reacts with mixture of piperazine and double hydrochloride of the piperazine with the ratio of 1 to 1 in lower alcohol, and then the chlorine hydride is pumped in to obtain the 1-[(1-pyrrolidine carbonyl) methyl] piperazine double hydrochloride. The process has the advantages of simple operation and high yield.
Owner:SHIJIANGZHUANG ZHIHENG PHARMACY TECH CO LTD
Synthesis method for improved cinepazide maleate
InactiveCN101531643AReduce usageAvoid distillationOrganic chemistryCardiovascular disorderHydrogenTert-Butyloxycarbonyl protecting group
The invention discloses a synthesis method for improved cinepazide maleate. The synthesis method comprises the following steps: step one: preparing chloracetyl tetrohydropyrrole; step two: preparing 1-[(1-tetrahydropyrrylcarbonyl) methyl]-4-tertbutoxylcarboxypiperazidine; step three: preparing 1-[(1-tetrahydropyrrylcarbonyl) methyl]-piperazidine hydrochloride; step four: preparing 3, 4, 5-trimethoxylcinnamoyl chloride; step five: preparing 1-[(1-tetrahydropyrrylcarbonyl) methyl]-4-(3, 4, 5-trimethoxylcinnamoyl)-piperazidine; step six: preparing 1-[(1-tetrahydropyrrylcarbonyl) methyl]-4-(3, 4, 5-trimethoxylcinnamoyl)-piperazidine maleate. The synthesis route of the environment-friendly cinepazide maleate is economic and practical, green, simplifies operation, saves energy, and generates little waste.
Owner:SHANGHAI NEAO BIO PHARMA
Methanesulfonic acid cinepazide crystal form III and preparation method thereof
ActiveCN102351812AImprove solubilityGood chemical stabilityOrganic active ingredientsOrganic chemistrySolubilityCinepazide
The invention relates to a methanesulfonic acid cinepazide crystal form III and a preparation method thereof, and belongs to the field of chemical pharmaceutics. The water solubility of the prepared methanesulfonic acid cinepazide crystal form III is higher than that of an amorphous material, and the methanesulfonic acid cinepazide crystal form III is particularly suitable for injection, has higher chemical stability than the amorphous material, facilitates production of medicines, and storage and transportation of raw material medicines, improves the safety of the medicines and provides safe guarantee for clinical application of the medicines.
Owner:BEIJING SIHUAN PHARMA +1
Preparation method of cinepazide maleate
The invention relates to a preparation method of cinepazide maleate, comprising the following steps: taking (E)-3, 4, 5-trimethoxy cinnamate as a raw material, preparing and obtaining (E)-3, 4, 5-trimethoxy cinnamoyl piperazine by activating carboxyl with an activated agent and then reacting with piperazine anhydrous as a compound, and reacting the (E)-3, 4, 5-trimethoxy cinnamoyl piperazine with chloracetyl pyrrolidine to prepare cinepazide free alkali which becomes salt after adding maleic acid. The preparation of the chloracetyl pyrrolidine adopts a solventless method. Since the solventless method is adopted for preparing the chloracetyl pyrrolidine, the preparation method prevents from using an organic solvent with larger toxicity and low boiling point, has high safety, is environment-friendly, and simplifies the production condition and process. The cinepazide maleate crystal with the melting point of 170 DEG C to 175 DEG C is obtained by adopting an alcohol-butanone mixed solvent, has high melting point and stable crystal form and is suitable for industrial production on large scale.
Owner:安徽金太阳生化药业有限公司
Improved preparation method for cinepazide maleate
InactiveCN101508685ASimple methodLow equipment requirementsOrganic chemistryBlood disorderCyclohexanoneFiltration
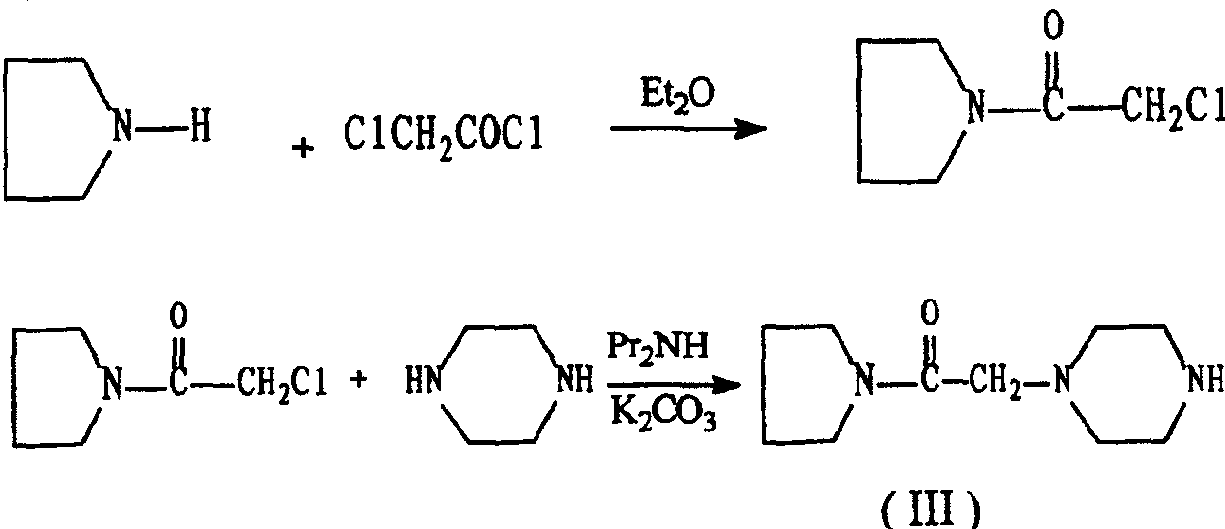
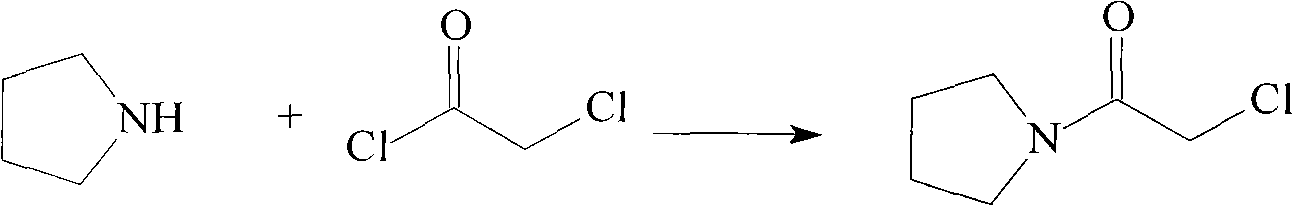
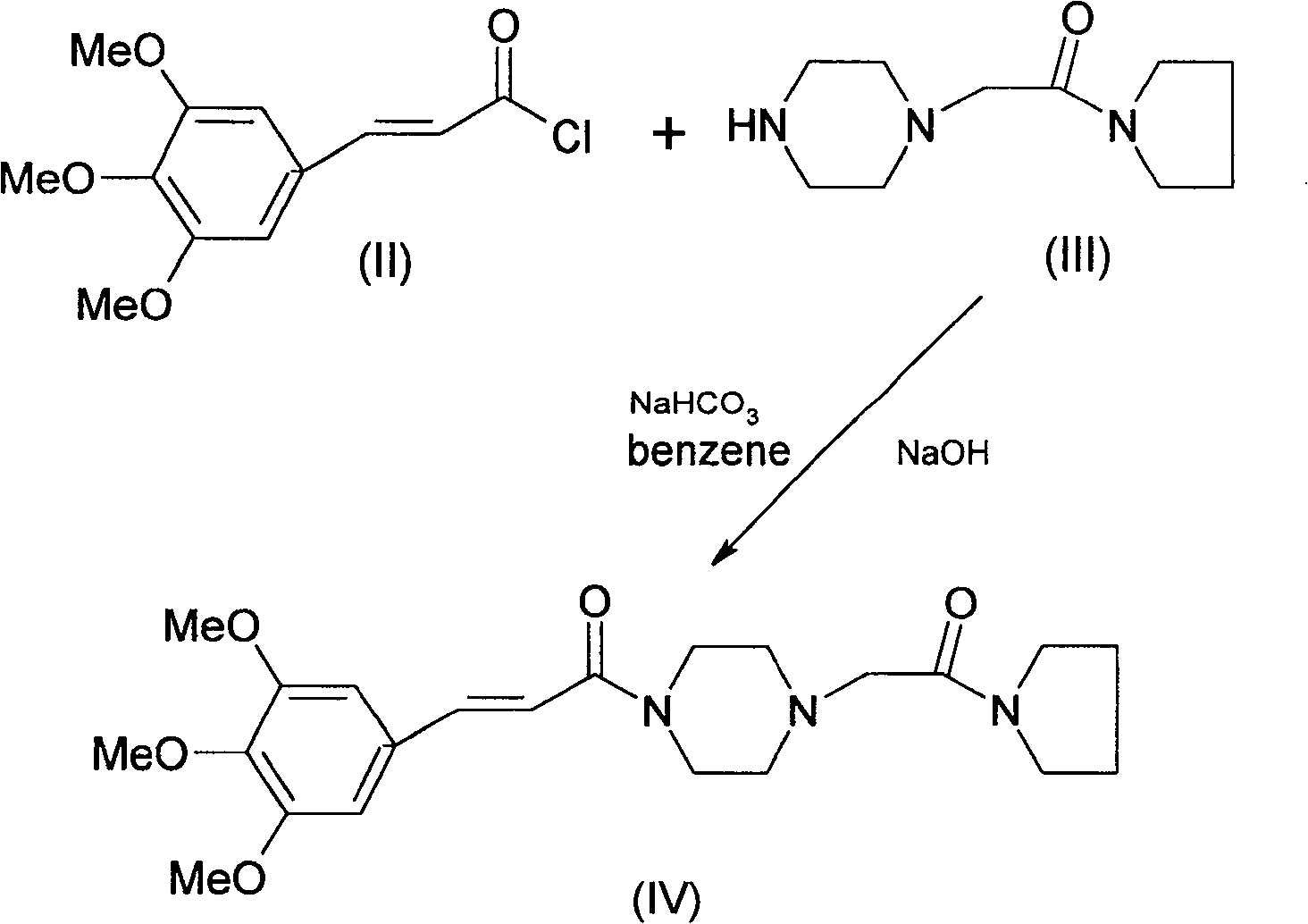
An improved preparation method of cinepazide maleate belongs to the pharmaceutical technical field. The improved preparation method is characterized in that anhydrous piperazine reacts with chloracetylpyrrolidine in absolute ethyl alcohol at room temperature to obtain 1-[(1-tetrahydropyrrolylcarbonyl)methyl]piperazine, post-treatment method is a acetone dissolution and filtration method; then the 1-[(1-tetrahydropyrrolylcarbonyl)methyl]piperazine reacts with 3,4,5-trimethoxycinnamoyl chloride at the room temperature to obtain cinepazide free alkali, an acid-binding agent used is triethylamine, and a solvent is selected from acetone or tetrahydrofuran, methyl ethyl ketone, methylbenzene, xylene, methyl isobutyl ketone, cyclohexanone and the like, and the acetone is preferred; finally, the cinepazide maleate is obtained after salifying with maleic acid. The improved preparation method has the effects and benefits of using a cheap and low-toxic solvent with good safety in the process of preparing the cinepazide maleate (I), mild reaction conditions, simple operation, fewer by-products, low energy consumption and equipment requirements, higher product yield and good product quality, and being suitable for industrial production.
Owner:DALIAN UNIV OF TECH +1
Powder injection of cinepazet maleate and its prepn. method
InactiveCN1903178ANot easy to decomposeAdvanced preparation technologyPowder deliveryOrganic active ingredientsActivated carbonCurative effect
A powder injection of cinepazide maleate is proportionally prepared from cinepazide maleate, solubilizer and filler through mixing for dissolving, decoloring by medical activated carbon, filtering with millipore membrane, dewatering and drying.
Owner:王义梅
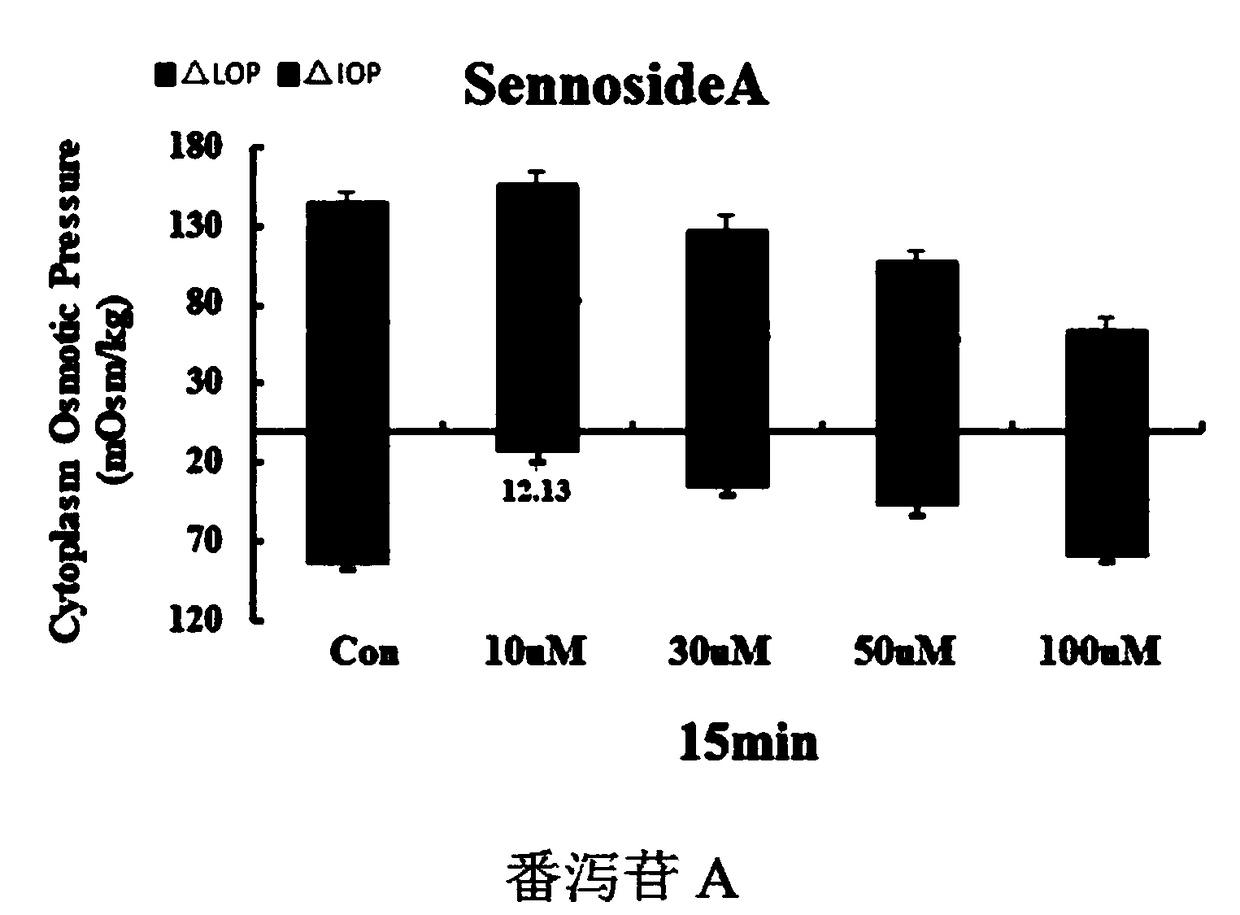
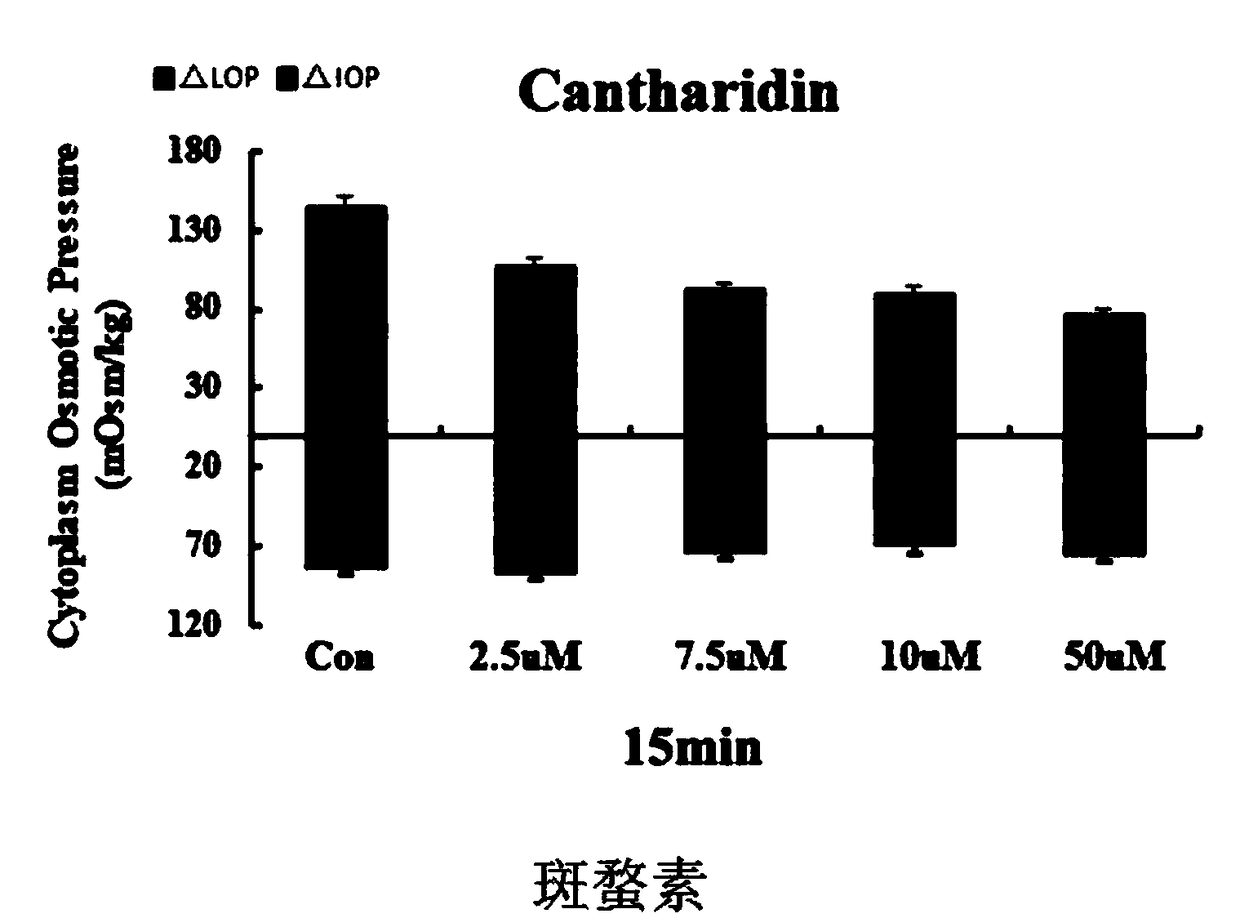
Drug composition for treating encephaledema by taking intracellular osmotic pressure as target
ActiveCN108653305AVolume stabilityConvenient treatmentNervous disorderBlood disorderNimodipineCantharidin
The invention relates to a drug composition for treating encephaledema and a preparation method of the drug composition. The compound drug composition comprises the following active ingredients: sennoside A, cantharidin or a derivative of cantharidin, nimodipine or nifedipine, cinepazide maleate and edaravone. An active drug and a drug combination capable of reducing colloid osmotic pressure and crystal osmotic pressure in brain cells so as to treat the encephaledema are looked for through a cell experiment, and a novel pharmaceutical application is provided clinically.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
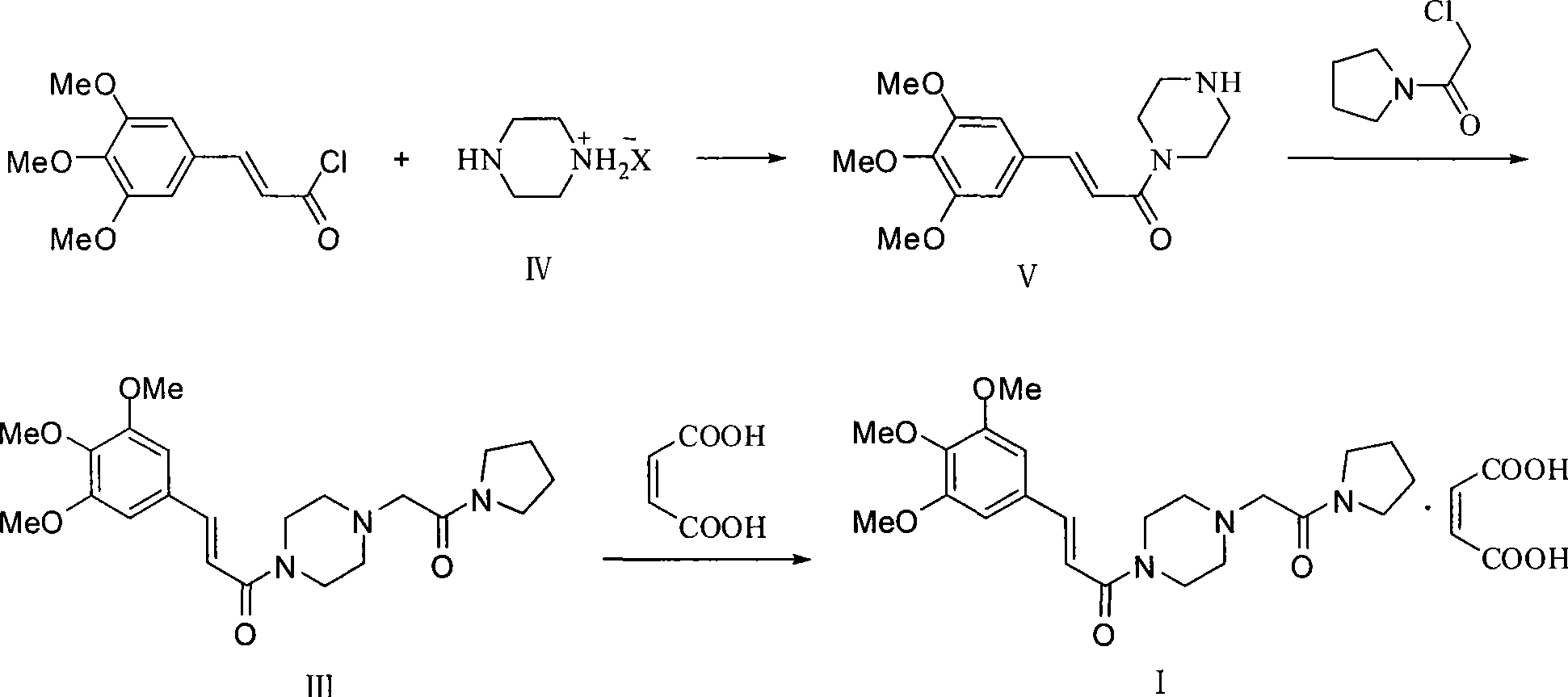
Preparation method of cinepazide maleate
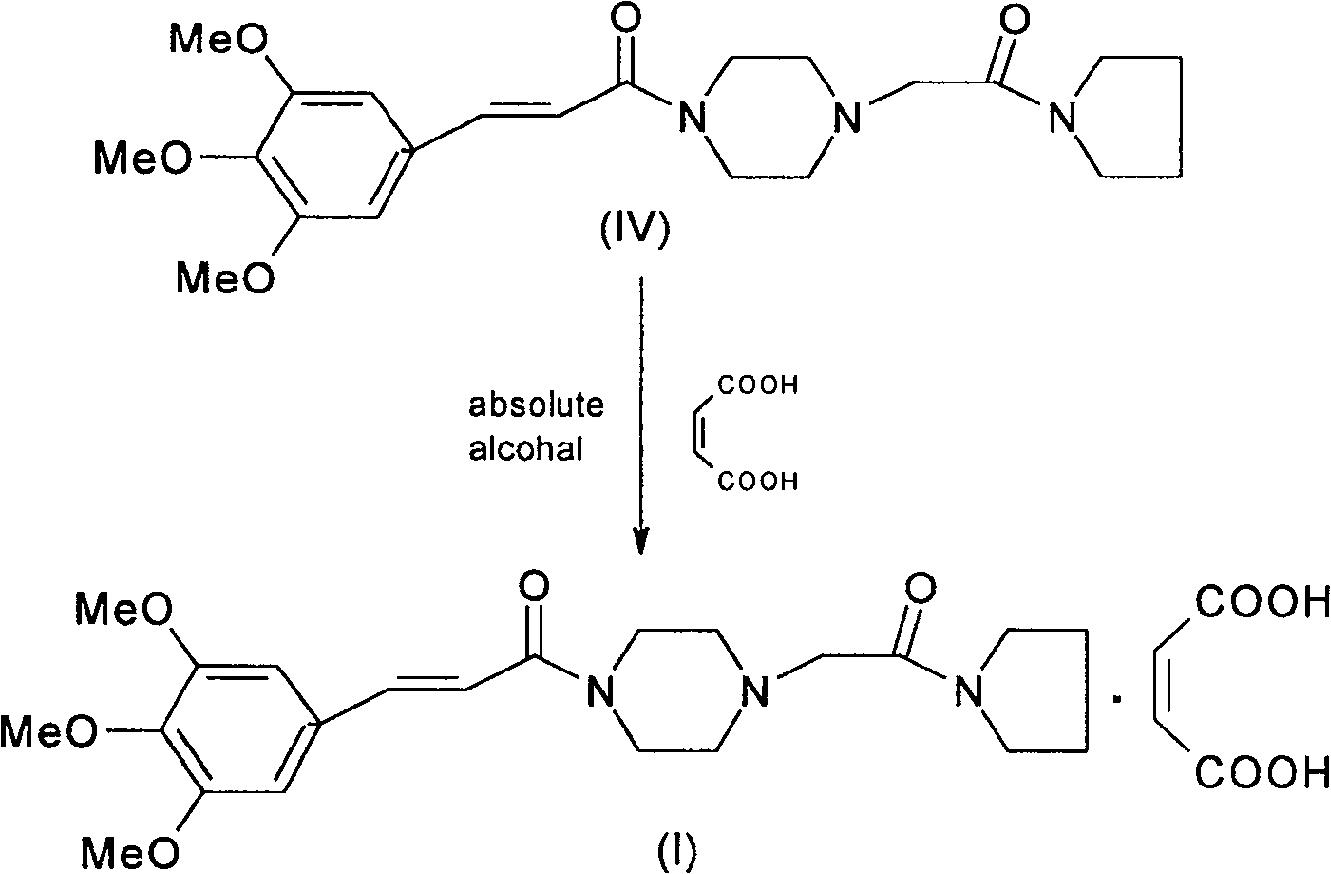
InactiveCN101602751AIncrease profitReduce processing difficultyOrganic chemistryCardiovascular disorderCinepazidePyrrolidine
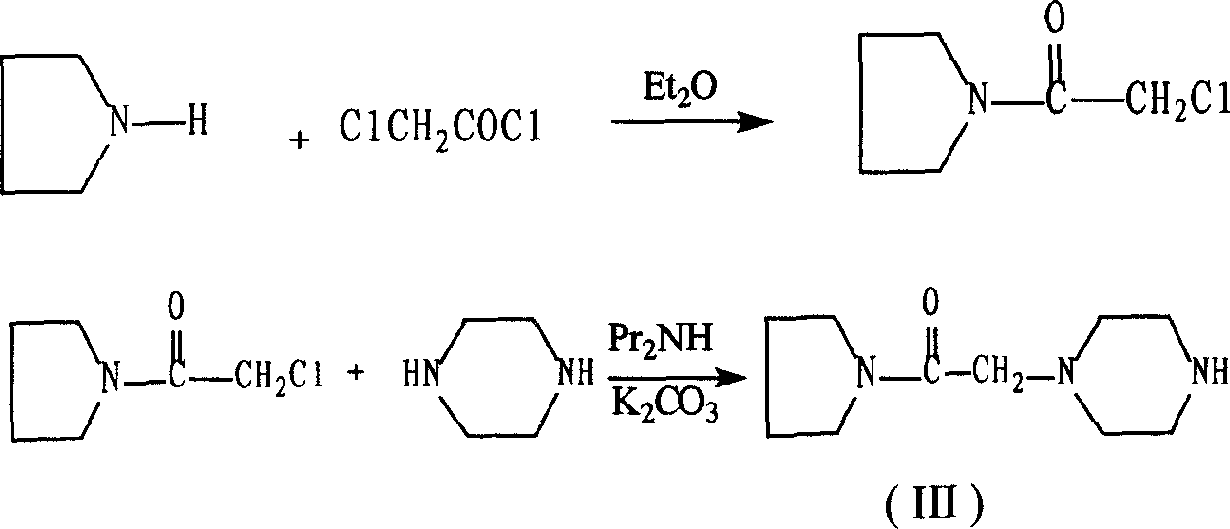
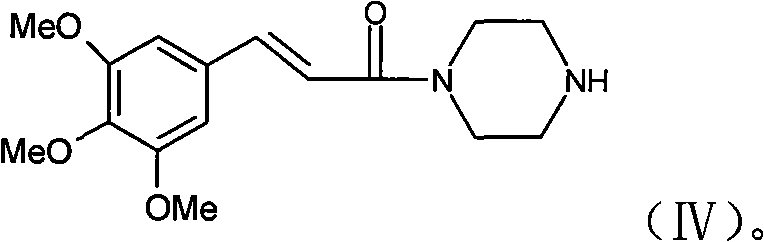
The invention discloses a preparation method of cinepazide maleate, comprising the steps of (1) preparing 1-piperazine acetyl pyrrolidine (IV) and (2) taking 1-piperazine acetyl pyrrolidine as material to prepare cinepazide maleate. The preparation method of 1-piperazine acetyl pyrrolidine comprises the following steps: placing piperazine dihydrochloride in water to react with alkaline and obtaining a reaction system containing piperazine monohydrochloride; (2) adding chloracetyl pyrrolidine into the reaction system for reaction, adjusting pH to 10-13 by using alkaline material, collecting 1-piperazine acetyl pyrrolidine from reaction products. The method for preparing the cinepazide maleate has low cost, high material utilization ratio, simple and convenient operation and higher yield and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for synthesizing cinepazide
InactiveCN101717382AFew reaction stepsEasy to operateOrganic chemistryCardiovascular disorderCinepazidePyrrolidine
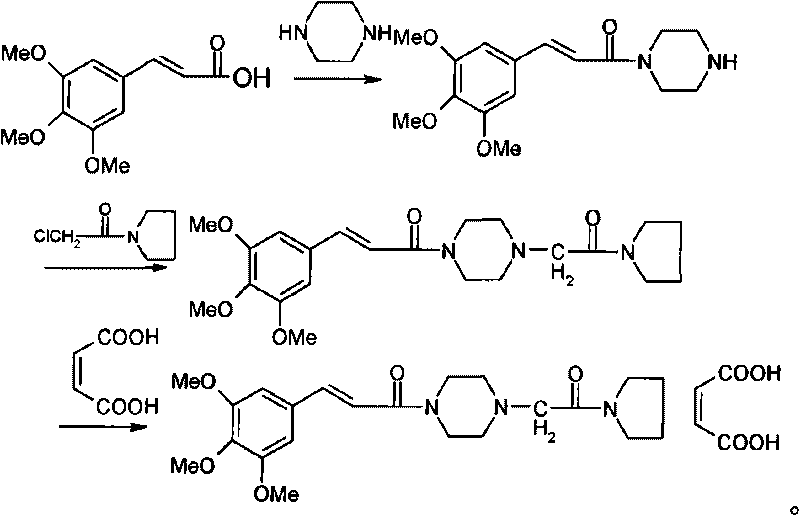
The invention relates to a method for synthesizing cinepazide. The method comprises: obtaining 3,4,5-trimethoxy cinnamoyl piperazine through the reaction of 3,4,5-trimethoxy cinnamic acid and piperazine; obtaining cinepazide through the reaction of 3,4,5-trimethoxy cinnamoyl piperazine and chloroacetyl pyrrolidine; and forming salts from cinepazide and maleic acid optionally. The method provided by the invention has the advantages of short route, simple operation, not high requirements for production equipment, environment-friendly reagents used, high reaction yield, high purity of obtained products and suitability for industrial scale production.
Owner:QILU PHARMA CO LTD
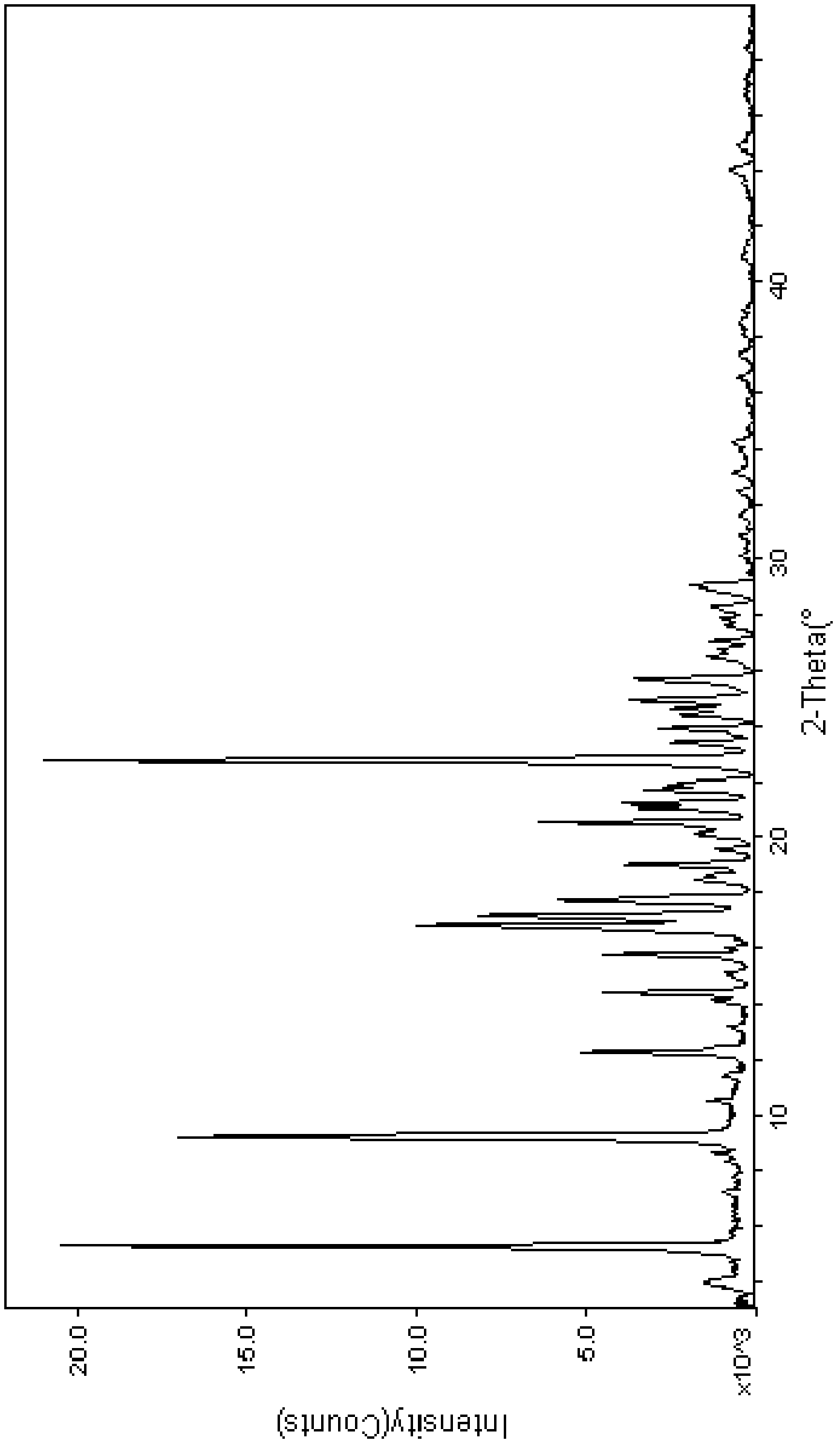
Cinepazide maleate crystal form and preparation thereof
InactiveCN101376648AImprove dissolution rateImprove solubilityOrganic chemistryCardiovascular disorderCinepazideCondensed matter physics
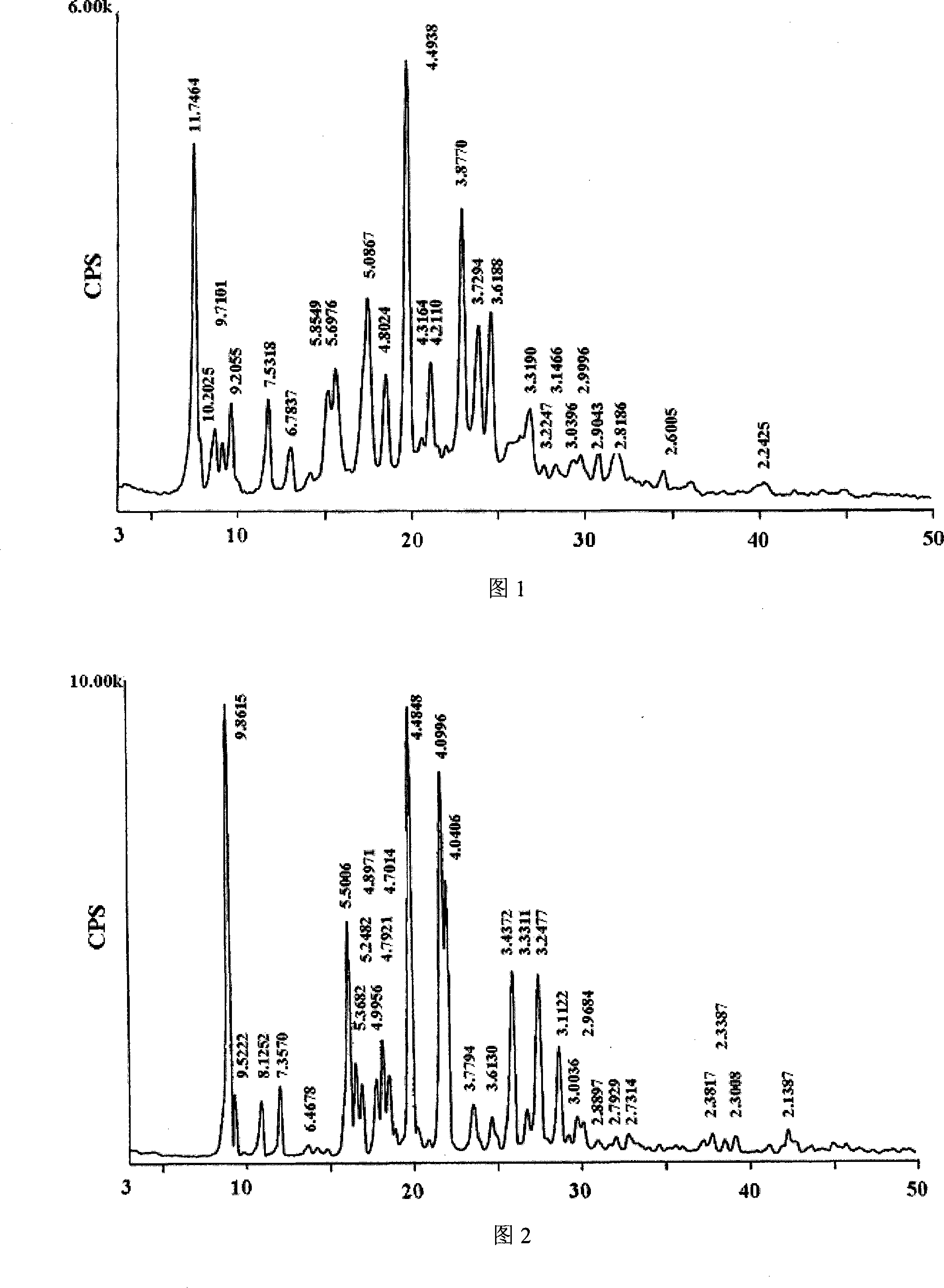
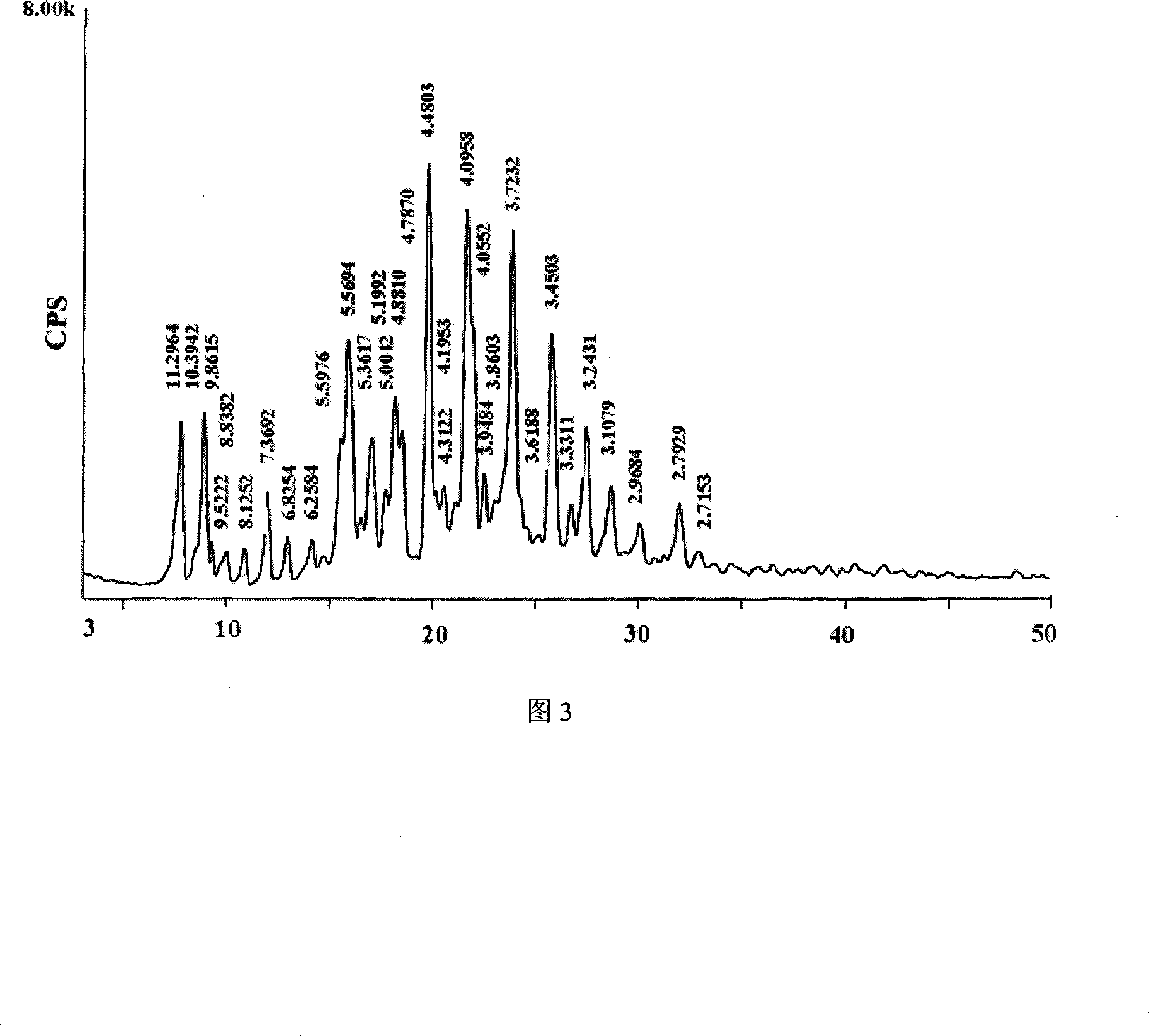
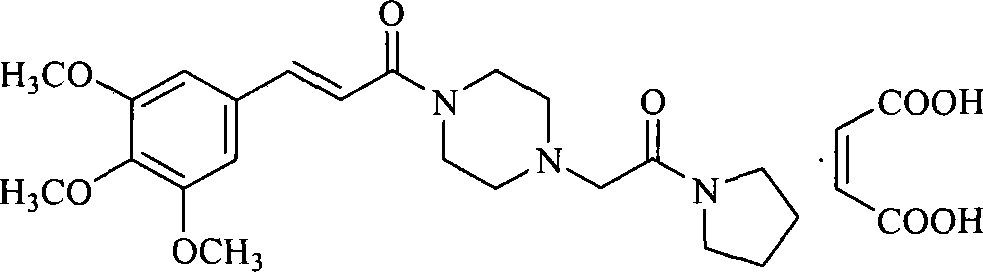
The invention belongs to the pharmaceutical technical field, particularly relates to maleate cinepazide crystal form (including maleate cinepazide A crystal form and maleate cinepazide B crystal form) and preparation method thereof. The maleate cinepazide crystal form has characteristic peak at 9.0 plus or minus 0.2, 16.1 plus or minus 0.2, 19.8 plus or minus 0.2 and 21.7 plus or minus 0.2 in X-ray powder diffraction expressed by 2 THETA using Cu-Ka radiation. The maleate cinepazide crystal form has characteristic peak at 7.6 plus or minus 0.2, 19.7 plus or minus 0.2, and 22.9 plus or minus 0.2 in X-ray powder diffraction expressed by 2 THETA using Cu-Ka radiation.
Owner:BEIJING SIHUAN PHARMA +1
Synthesis of cinepazide maleate
InactiveCN101508684AHarm reductionFew reaction stepsOrganic chemistryBlood disorderSulfonyl chlorideOrganic base
A synthetic method of cinepazide maleate belongs to the pharmaceutical technical field. The synthetic method is characterized in that trans-3,4,5-trimethoxycinnamic acid is taken as a raw material, and a carboxy activator is employed to synthesize by a 'one pot' method to obtain cinepazide free alkali in the presence of organic base. The used activator is any one of alkyl chloroformate, methylsulfonyl chloride, benzene sulfonyl chloride, p-methylsufonyl chloride, diphenylphosphinyl chloride, 2,4,6-trimethylbenzenesulfonyl chloride, N,N-dicyclohexylcarbodiimide(DCC) and carbonyldiimidazole (CDI); ethyl chlorocarbonate is preferred, the reaction temperature is minus 10 DEG C and a solvent is dichloromethane. The cinepazide maleate is obtained after salifying the free alkali. The synthetic method has the effects and benefits of simplifying production conditions and procedure, avoiding application of a reactive intermediate trans-3,4,5-trimethoxycinnamoyl chloride, reducing environmental hazards and reducing equipment requirements; and providing a new synthetic route which is simple and easily controlled, has mild reaction conditions, is environment-friendly and applicable to large-scale industrial production.
Owner:DALIAN UNIV OF TECH +1
Preparation method of cinepazide maleate
The invention relates to a preparation method of cinepazide maleate, comprising the steps of: reacting 3,4,5-trimethoxycinnamic acid with a chlorination agent, diethyl phosphorochloridate and benzene sulfonyl chloride to prepare a corresponding acyl active matter of the 3,4,5-trimethoxycinnamic acid; reacting the acyl active matter of the 3,4,5-trimethoxycinnamic acid with 1-[(1-pyrrolidine carbonyl) methyl] piperazine dihydrochloride to prepare 1-[(1-pyrrolidine carbonyl) methyl]-4-(3,4,5-trimethoxyphenyl) piperazine; and separating the 1-[(1-pyrrolidine carbonyl) methyl]-4-(3,4,5-trimethoxyphenyl) piperazine to salify with maleic acid so as to prepare cinepazide maleate, or directly salifying with the maleic acid in an ethanol or acetone solution and then crystallizing to prepare the cinepazide maleate with high melting point and stable crystal form, wherein the 1-[(1-pyrrolidine carbonyl) methyl] piperazine dihydrochloride can be prepared through reacting chloracetyl pyrrolidine with the dihydrochloride mixture of the piperazine and piperazine in proportion of 1:1 in lower alcohol, and introducing hydrogen chloride. The process has the advantages of simple operation and high yield.
Owner:SHIJIANGZHUANG ZHIHENG PHARMACY TECH CO LTD
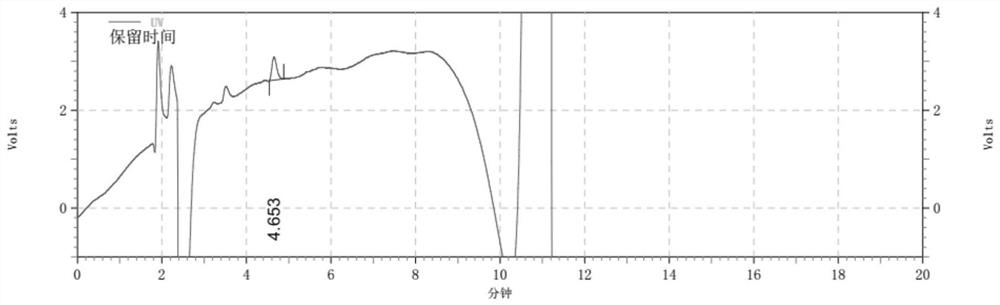
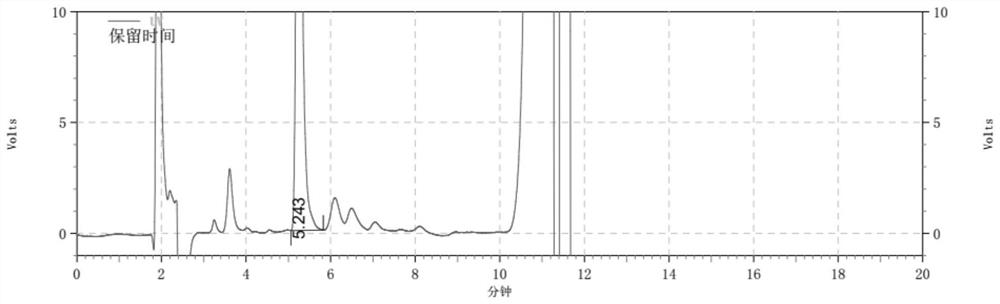
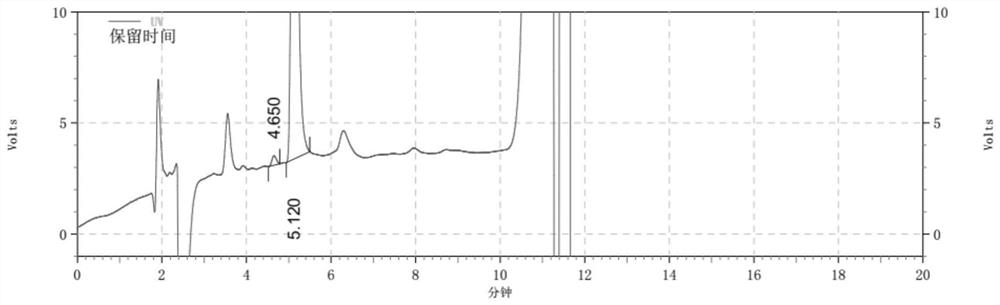
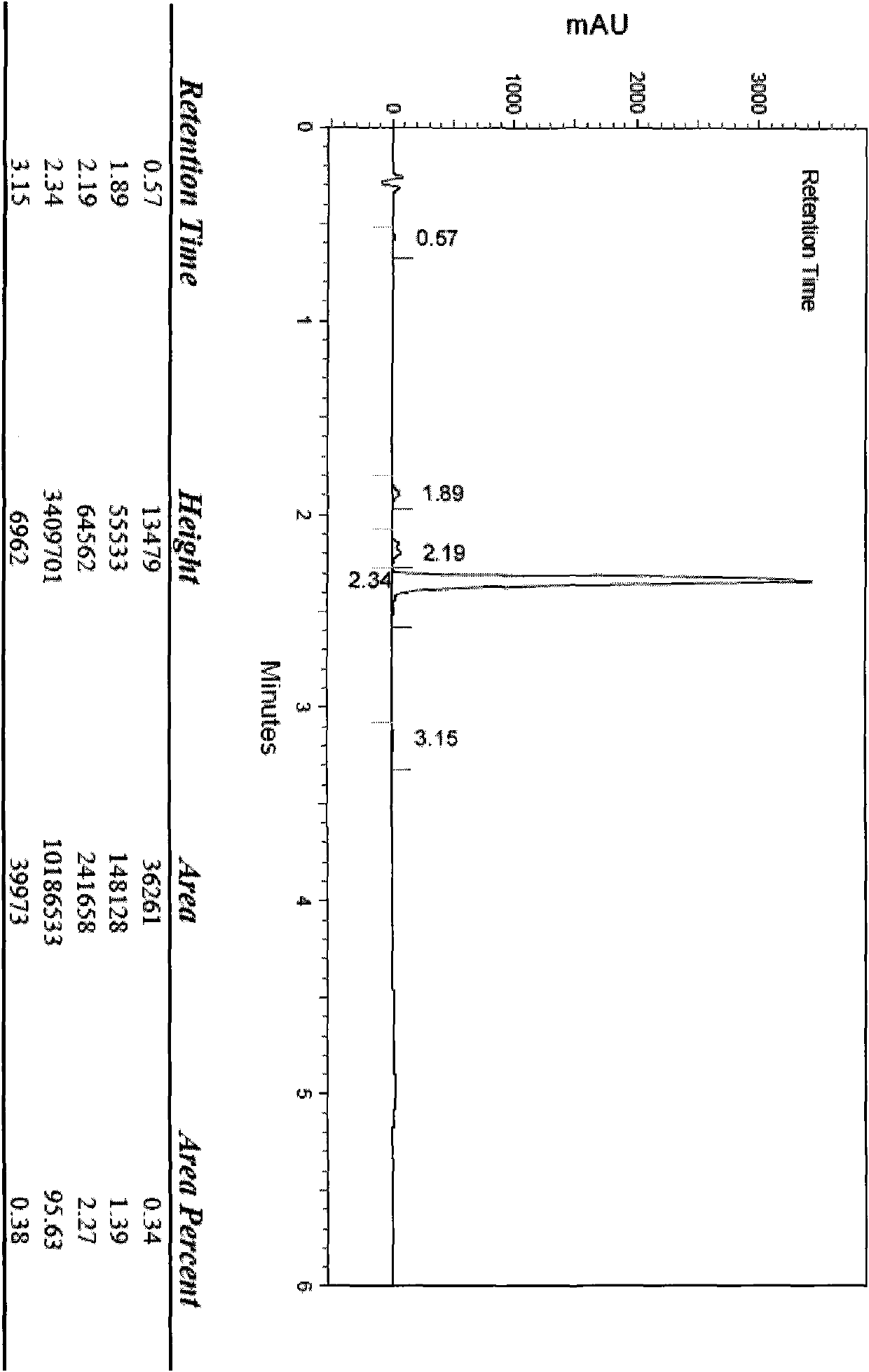
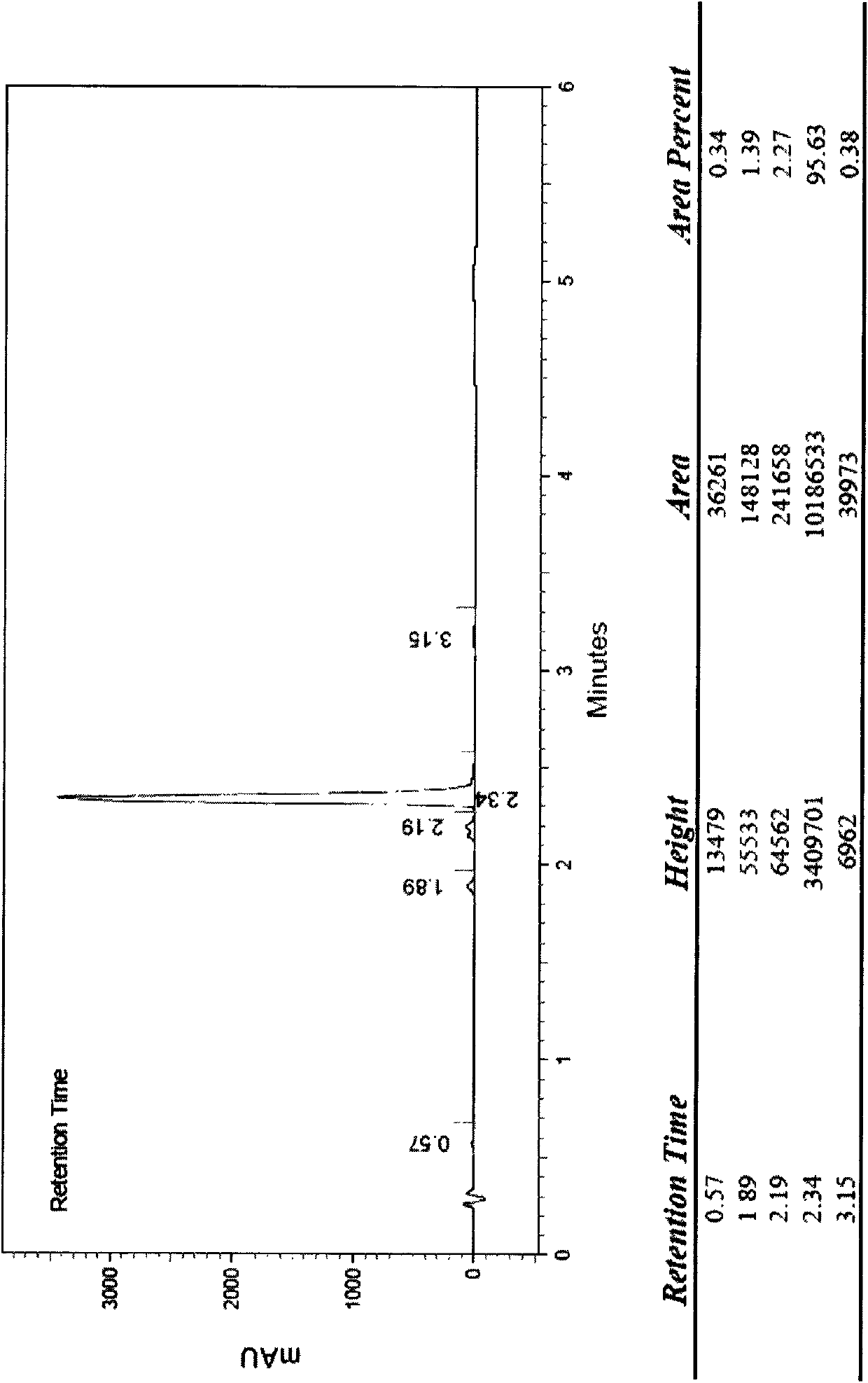
Detection method for determining chloroacetic acid residues in cinepazide maleate intermediate through HPLC method
The invention belongs to the technical field of chloroacetic acid detection, and particularly relates to a detection method for determining chloroacetic acid residues in a cinepazide maleate intermediate through an HPLC method. The method comprises the following steps: (1) preparing a reference solution; (2) preparing a sample solution; (3) performing sample injection detection in HPLC, wherein chromatographic detection conditions are as follows: chromatographic column: a silane bonding silica gel column is a high performance liquid chromatography column of a stationary phase, a mobile phase A: water-methanol-trifluoroacetic acid in a volume ratio of 96: 4: 0.1, a mobile phase B: methanol, the flow velocity of the mobile phase being 0.6-1ml / min, the column temperature of the chromatographic column is 33-37 DEG C, the sample size is 20mu l, and the detection wavelength is 212-216nm; and (4) calculating the content of chloroacetic acid: calculating the content of chloroacetic acid according to the peak area through an external standard method. The method has the beneficial effects that the chloroacetic acid is detected by using the HPLC method, the operation is simple, and the problems in the prior art are solved.
Owner:HARMONIA TESTING TECH TIANJIN LTD
Method for synthesizing cinepazide
ActiveCN102229583AIndustrial Production SafetyFew reaction stepsOrganic chemistryCinepazidePyrrolidine
The invention discloses a method for synthesizing cinepazide. The method for synthesizing the cinepazide comprises the step of reacting an activated substance of 3,4,5-trimethoxy cinnamic acid with 1-[1-(1-pyrrolidine carbonyl)methyl]piperazine or salt thereof to obtain the cinepazide. Benzene high-toxicity solvents are not used in the whole reaction process, so the method is environment-friendly and safe in industrial production; the reaction steps are few, so the method is easy to operate; the method is mild in condition, simple in aftertreatment and suitable for industrialized production; special or complex reaction equipment is not required; and the product has high purity. The invention provides a synthetic route of the cinepazide, with high reaction yield and low cost.
Owner:JIANGSU SHENLONG PHARMA
Cinepazide medicinal composition with high safety, preparation method and application thereof
ActiveCN101708179BDecreased blood countToxicOrganic active ingredientsPharmaceutical delivery mechanismPharmacyNitrogen oxides
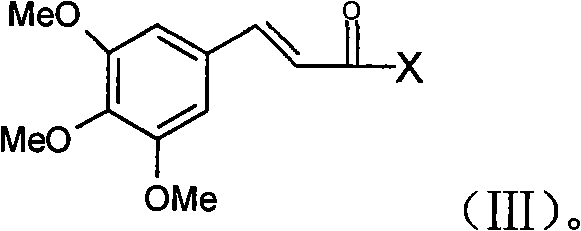
The invention relates to a medicine composition with high safety, a preparation method and an application thereof. The medicine composition contains cinepazide or salt thereof which is acceptable in pharmacy and a structural compound which has the content not higher than 0.5% and is shown in the formula (III). The cinepazide medicine composition and a preparation thereof have better safety, effectiveness and stability and can effectively lower or avoid the adverse reactions of hemogram lowering and the like of a patient caused by the cinepazide. Furthermore, the invention also separates, purifies and characterizes a novel cinepazide nitrogen oxide, researches the novel cinepazide nitrogen oxide fully and discovers that the toxicity of the compound is far greater than that of the cinepazide and the compound has an insecticidal activity which is different from that of the cinepazide. Meanwhile, the research discovers that the cinepazide nitrogen oxide can cause the leukopenia of animals, even influences the formation and the differentiation of granular cells and is possibly a main reason of the adverse reactions of the hemogram lowering and the like of the patient caused by the cinepazide.
Owner:BEIJING SIHUAN PHARMA
High-safety medicinal composition of cinepazide, and preparation method and application thereof
ActiveCN102100695ADecreased blood countToxicOrganic active ingredientsOrganic chemistryWhite blood cellCinepazide
The invention relates to a high-safety medicinal composition of cinepazide, and a preparation method and application thereof. The medicinal composition comprises cinepazide or pharmaceutically acceptable salt thereof and compounds, of which the content is less than 0.5 and which have the structure shown in the formula III. The medicinal composition of cinepazide and the preparations thereof have higher safety, effectiveness and stability and can effectively reduce or avoid adverse reactions, such as decreasing hemogram, of patients caused by the cinepazide. Moreover, the invention also separates, purifies and characterizes new nitrogen oxides of cinepazide. Complete research on the new nitrogen oxides of cinepazide shows that the toxicity of nitrogen oxides of cinepazide is far higher than that of the cinepazide, and the nitrogen oxides of cinepazide have the insecticidal activity different from that of the cinepazide; moreover, the research shows that the nitrogen oxides of cinepazide can cause the reduction of leukocytes of animals, even affect the formation and the differentiation of granulocytes, which are possibly the main reason for the cinepazide causing adverse reactions such as the decreasing hemogram of patients.
Owner:BEIJING SIHUAN PHARMA
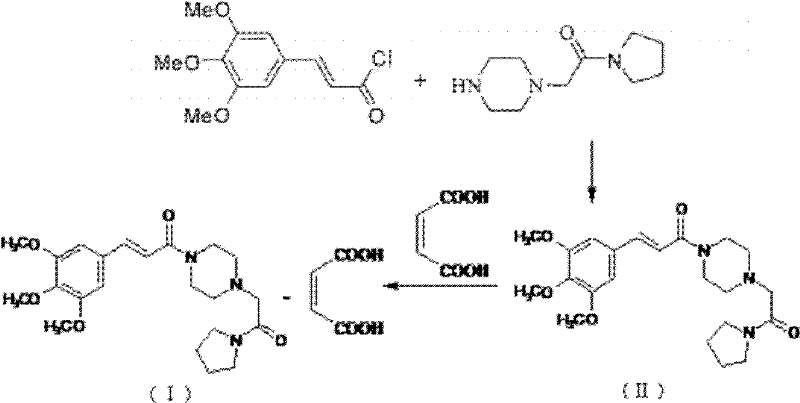
Cinepazide maleate compound with novel route
The invention provides a cinepazide maleate compound with a novel route. Concretely, the method comprises the step of making 3, 4, 5-trimethoxy cinnamic acid and piperazidine take reaction in the existence of dichloromethyl (trichloromethyl) carbonic ether for producing 3, 4, 5-trimethoxy cinnamoyl piperazine. Through using the dichloromethyl (trichloromethyl) carbonic ether as acid activated groups, the invention simplifies the reaction steps, and reduces the environment pollution, in addition, the superfluous piperazidine is separated through the reaction with the piperazidine at first, and the processing steps are simplified, so the subsequent purification becomes easy.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Improved cinepazide maleate preparation method
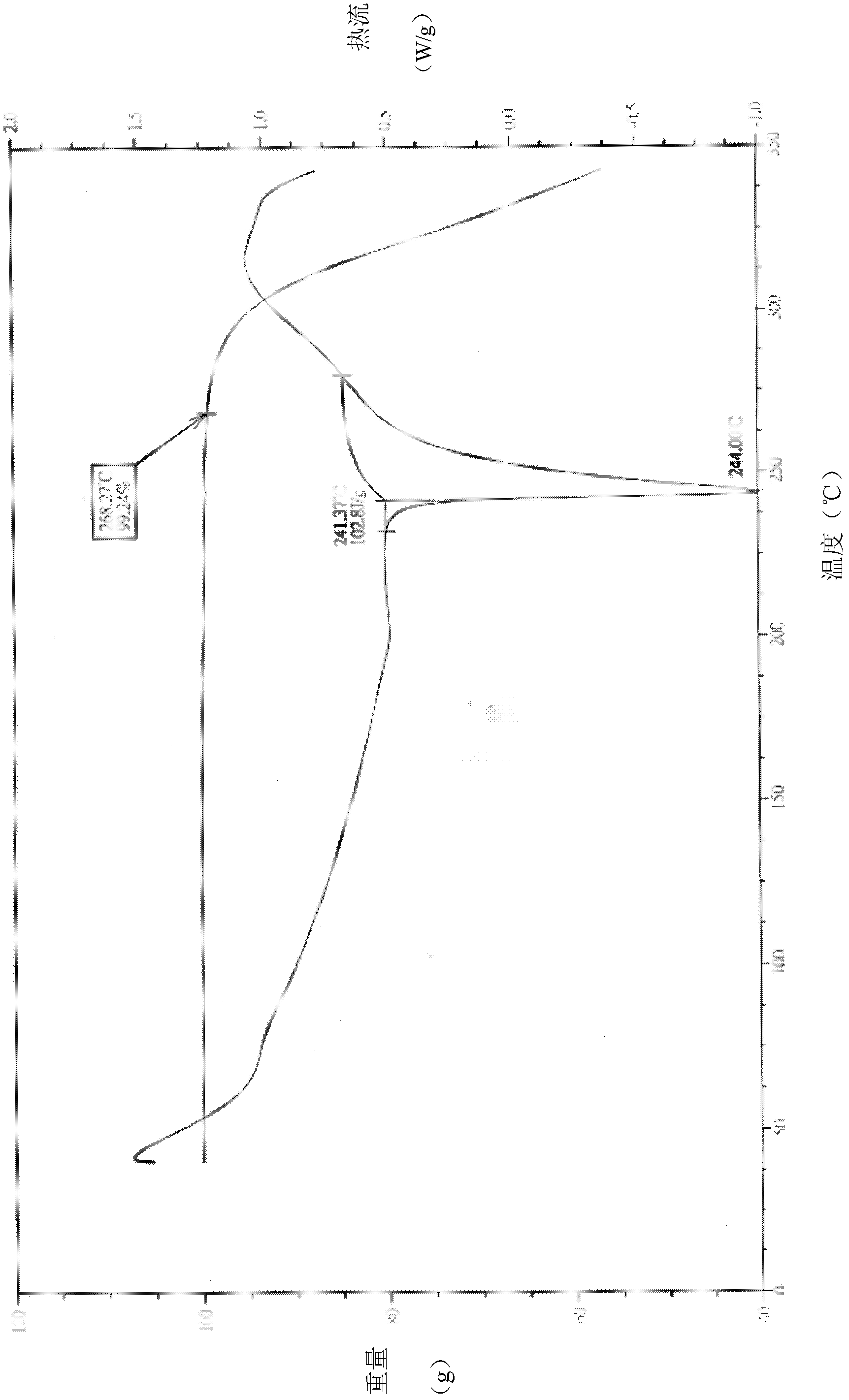
ActiveCN100572378CHigh melting pointCrystal stableOrganic active ingredientsOrganic chemistrySolventMethyl group
The invention relates to a preparation method of cinepazide maleate. Preparation of chloroacetylpyrrolidine; Preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine; Preparation of 3,4,5-trimethoxycinnamoyl chloride; 1-[(1-Pyrrolidinecarbonyl ) methyl]-4-(3,4,5-trimethoxycinnamoyl)piperazine; 1-[(1-pyrrolidinecarbonyl)methyl]-4-(3,4,5-trimethoxy Preparation of base cinnamoyl) piperazine maleate; purification of cinepazide maleate and preparation of stable crystal form; characterized in that: the solvent used in the preparation step of cinepazide maleate stable crystal form One or any combination of chloroform and acetone. The product prepared by the preparation method of cinepazide maleate has a melting point of 170-175°C. The product prepared by the method for preparing cinepazide maleate provided by the invention has high melting point and stable crystal form, and is suitable for industrial scale production.
Owner:BEIJING SIHUAN PHARMA
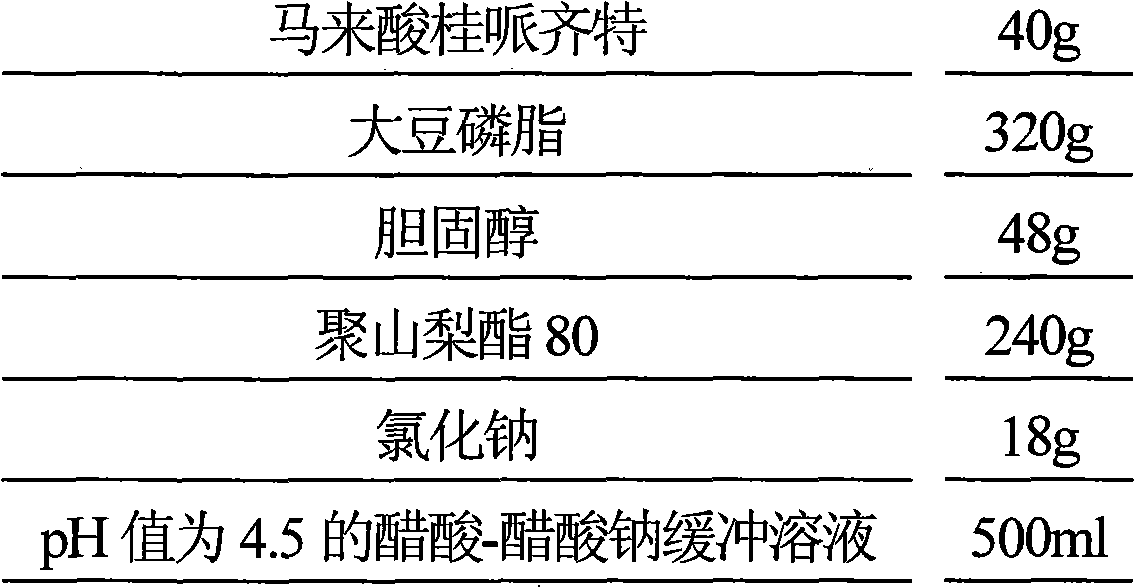
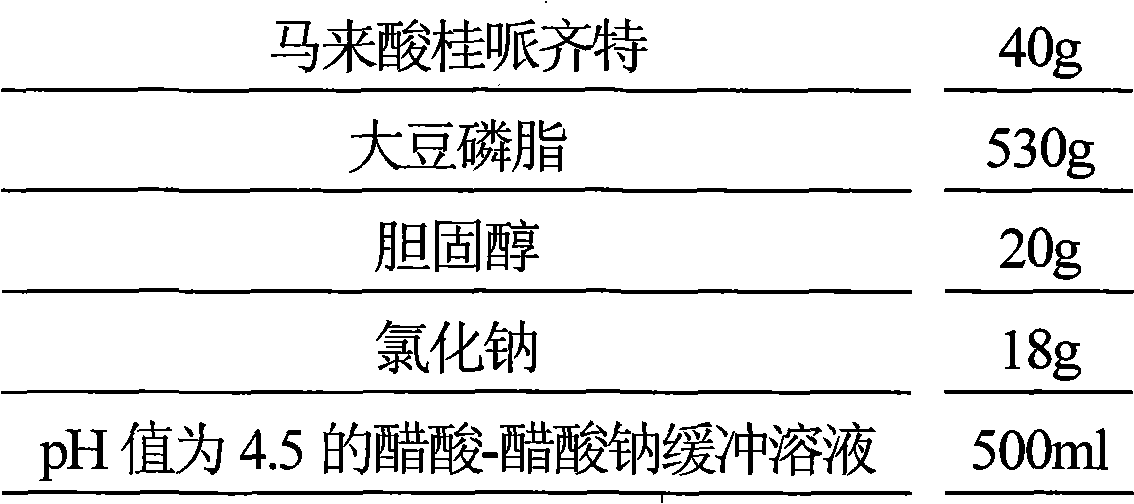
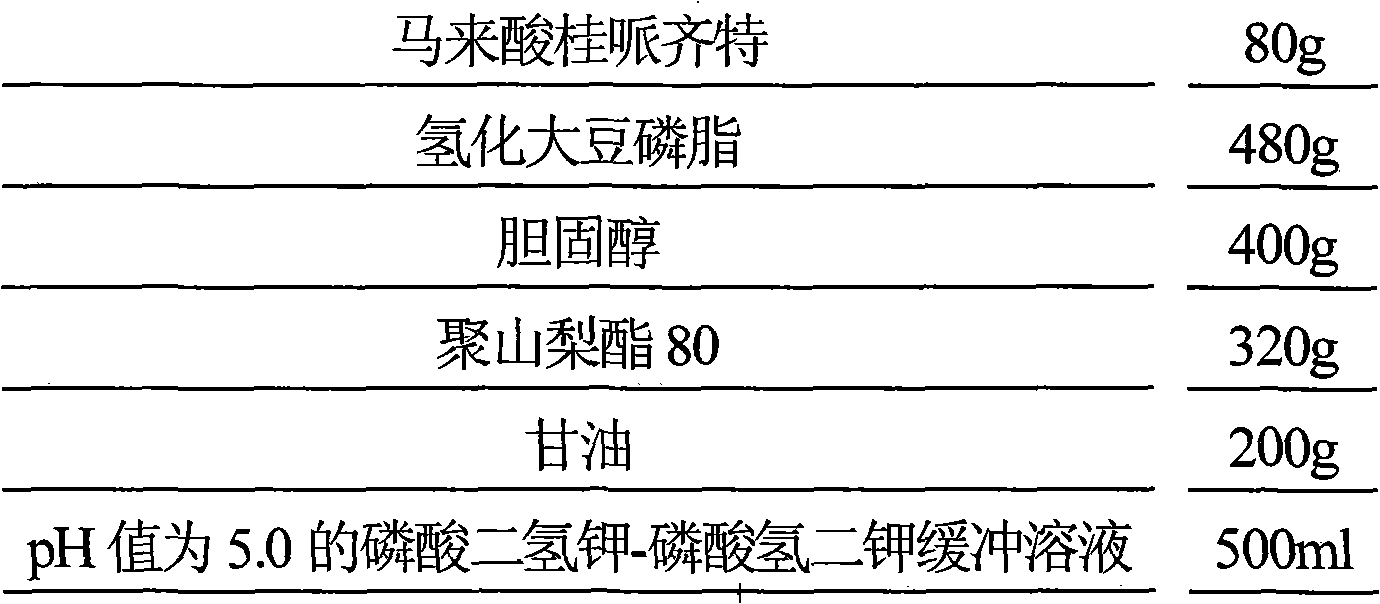
Maleic acid cinepazide liposome injection and new application thereof
InactiveCN101601655BImprove stabilityGuarantee product qualityOrganic active ingredientsNervous disorderMedicineCholesterol
The invention relates to maleic acid cinepazide liposome injection and new application thereof. The invention discloses the maleic acid cinepazide liposome injection and a preparation method thereof. The maleic acid cinepazide liposome injection mainly comprises the following components according to parts by weight: 1 part of maleic acid cinepazide, 1 to 13 parts of phospholipid, 0.6 to 6 parts of cholesterol and 2 to 10 parts of polysorbate 80. The preparation has favorable curative effect in treating blood vessel type migraine.
Owner:HAINAN YONGTIAN PHARMA INST
Cinepazide maleate crystal system and method for making same
The invention relates to maleate cinepazide crystal forms and preparation methods, belonging to the medicine technical field, which comprises maleate cinepazide crystal form A, crystal form B, polymorphism containing crystal form B, and the preparation methods of the crystal forms.
Owner:BEIJING SIHUAN PHARMA
Cinepazide maleate injection and preparation method thereof
InactiveCN101474147BReduce contentImprove securityOrganic active ingredientsInorganic non-active ingredientsWater useMANNITOL/SORBITOL
The invention relates to a cinepazide maleate injection and a preparation method thereof. Every 1000ml injection contains 30-45g of cinepazide maleate, 5-23g of mannite, 0.5g-1.5g of disodium hydrogen phosphate used for regulating the pH value, and the rest of water used for injection. The preparation method comprises: under the condition of keeping out of the sun, the mannite is added to and dissolved in the water which accounts for 80% of the total quantity, the cinepazide maleate is then added into the mannite aqueous solution to be stirred and dissolved, the pH value of the mixed solutionis regulated by the solution with 4% of the disodium hydrogen phosphate, and the water used for injection is added until the total quantity is achieved. The invention selects the mannite with high safety as stabilizing agent, and the operation which is kept out of the sun is adopted in the preparation process of the product, so that the content of the cis isomer of the cinepazide maleate is more effectively reduced, and the stability as well as the safety of clinical medication can be improved.
Owner:北京康瑞达彤科技有限公司
Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate
A liposome pharmaceutical formulation of the compound of 6,9-bis-[(2-aminoethyl)-amino]benzo[g]isoquinoline-5,10-dione dimaleate, the method for the preparation and the use thereof.
Owner:CELL THERAPEUTICS EURO SRL
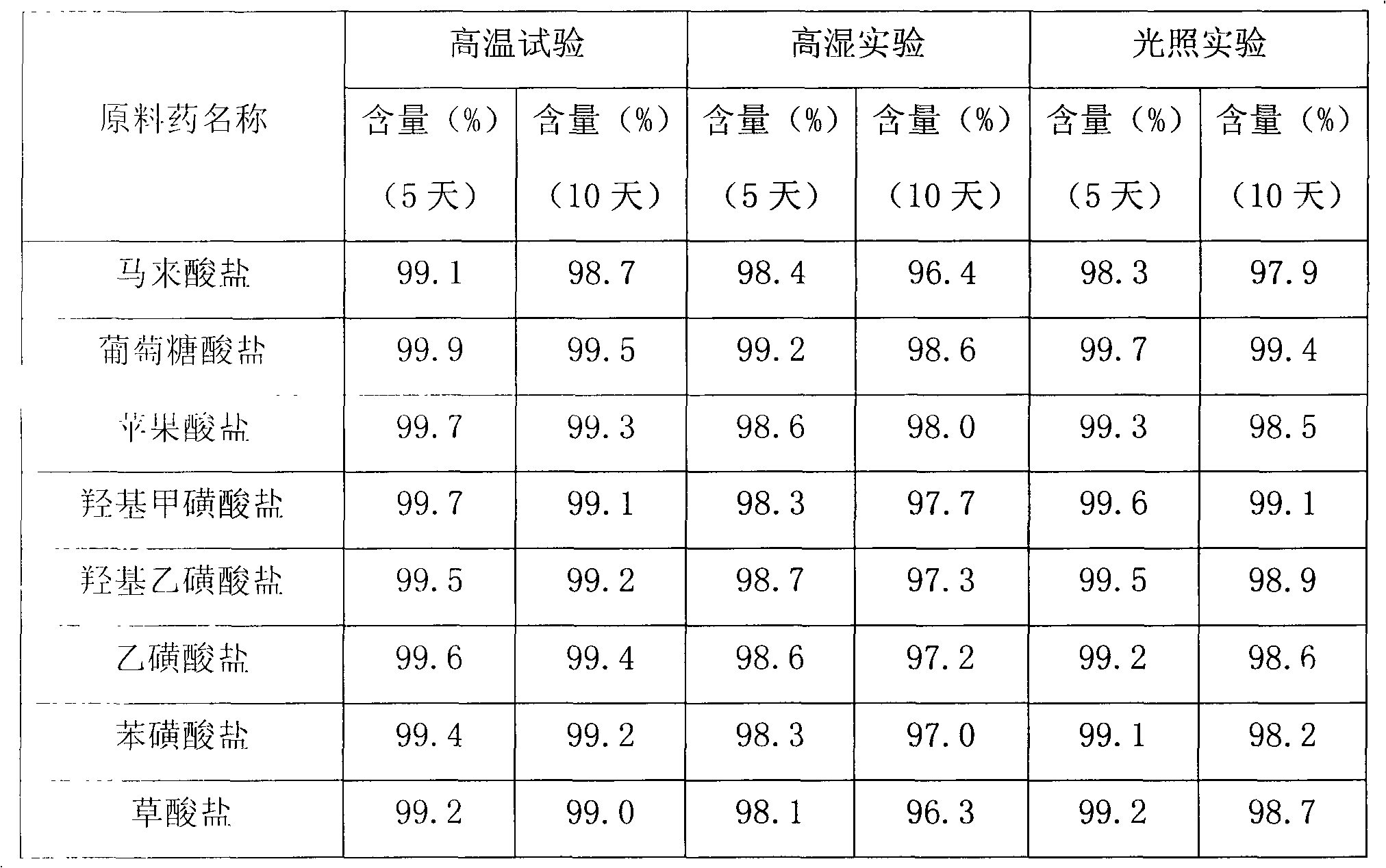
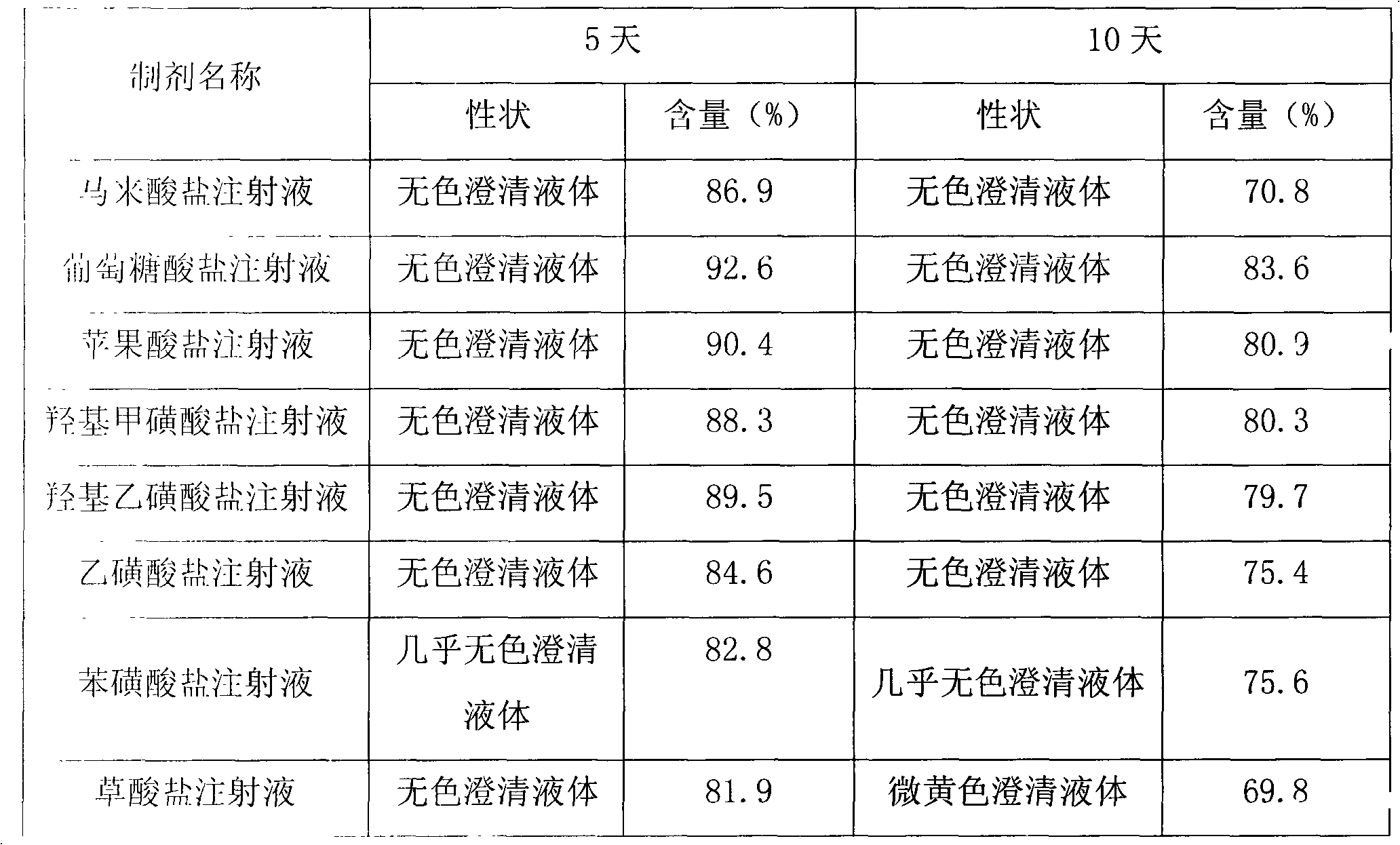
Cinepazide acid addition salt and preparation method thereof
ActiveCN102060808AImprove stabilityImprove securityOrganic active ingredientsOrganic chemistrySolubilityGluconic acid
The invention discloses a cinepazide organic acid medicinal salt and provides a preparation method and medicinal preparation of the cinepazide organic acid medicinal salt, werein,organic acid comprises gluconic acid, hydroxyl methanesulfonic acid, isethionic acid, ethyl sulfonic acid, benzene sulfonic acid, malic acid and oxalic acid,preferably gluconic acid. The cinepazide organic acid medicinalsalt is mainly used for treating and preventing cerebrovascular disease, cardiovascular disease and peripherial ascular disease. The cinepazide organic acid medicinal salt provided by the invention has the characteristics of good stability and good dissolvability, and is especially used for solving the defect of optical unstability of the maleic acid cinepazide.
Owner:北京亿灵医药科技发展有限公司
Cinepazide maleate injection and preparation method thereof
ActiveCN101204372BImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismCinepazideChemistry
The invention belonging to the field of medicine technology discloses a cinepazide maleate injection and a manufacturing method thereof. In every 1000ml of injection, 35-45g of cinepazide maleate is contained, 20-30g of D-sorbierite, 2-3.5g of disodium hydrogen phosphate, and proper water for injection are contained. The manufacturing method includes the following steps: dissolving the D-sorbierite in water; adding the cinepazide maleate into the D-sorbierite solution, mingling in order to partially dissolve; adding part of disodium hydrogen phosphate solution in order to fully dissolve the cinepazide maleate; then slowly adding the rest disodium hydrogen phosphate solution to adjust the pH value; finally filtering and preparing into injection preparation. The cinepazide maleate injectionof the invention effectively reduces the content of cinepazide maleate cis-isomer for the long-term storage.
Owner:HAINAN SIHUAN PHARMA +1
Improved preparation method for cinepazide maleate
InactiveCN101508685BSimple methodImprove securityOrganic chemistryBlood disorderCyclohexanoneFiltration
An improved preparation method of cinepazide maleate belongs to the pharmaceutical technical field. The improved preparation method is characterized in that anhydrous piperazine reacts with chloracetylpyrrolidine in absolute ethyl alcohol at room temperature to obtain 1-[(1-tetrahydropyrrolylcarbonyl)methyl]piperazine, post-treatment method is a acetone dissolution and filtration method; then the1-[(1-tetrahydropyrrolylcarbonyl)methyl]piperazine reacts with 3,4,5-trimethoxycinnamoyl chloride at the room temperature to obtain cinepazide free alkali, an acid-binding agent used is triethylamine, and a solvent is selected from acetone or tetrahydrofuran, methyl ethyl ketone, methylbenzene, xylene, methyl isobutyl ketone, cyclohexanone and the like, and the acetone is preferred; finally, the cinepazide maleate is obtained after salifying with maleic acid. The improved preparation method has the effects and benefits of using a cheap and low-toxic solvent with good safety in the process of preparing the cinepazide maleate (I), mild reaction conditions, simple operation, fewer by-products, low energy consumption and equipment requirements, higher product yield and good product quality, and being suitable for industrial production.
Owner:DALIAN UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8610d70-8ec5-4e19-bee3-cc14db2a2806/US20060078607A1-20060413-D00001.png)
![Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8610d70-8ec5-4e19-bee3-cc14db2a2806/US20060078607A1-20060413-D00002.png)
![Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate Liposome formulation of 6,9-bis[(2-aminoethyl)-amino]benzo[g]isoquinoline-5, 10-dione dimaleate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8610d70-8ec5-4e19-bee3-cc14db2a2806/US20060078607A1-20060413-D00003.png)