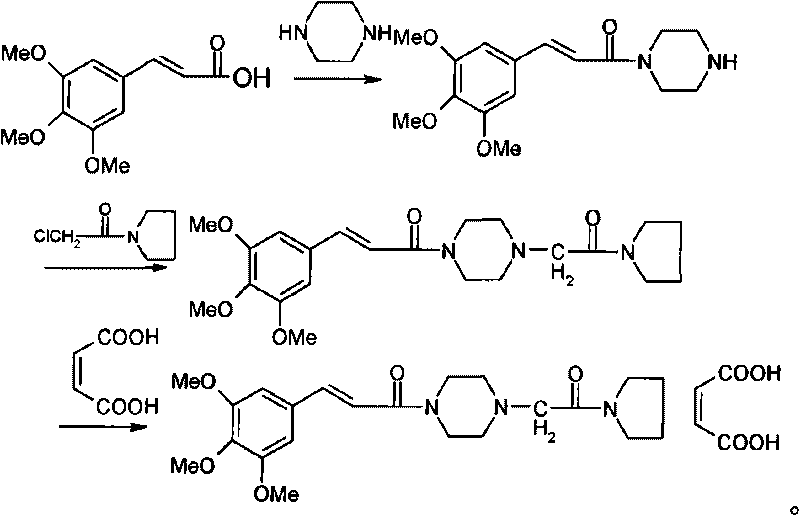
Method for synthesizing cinepazide
A synthesis method and technology of cinepazide are applied in the synthesis field of cinepazide, namely trans-1-[methyl]-4-piperazine, and can solve the problem of increasing reaction steps, harsh deprotection conditions and restricting application. etc., to achieve the effect of few reaction steps, high melting point and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] A synthetic method of cinepazide, comprising the steps of:
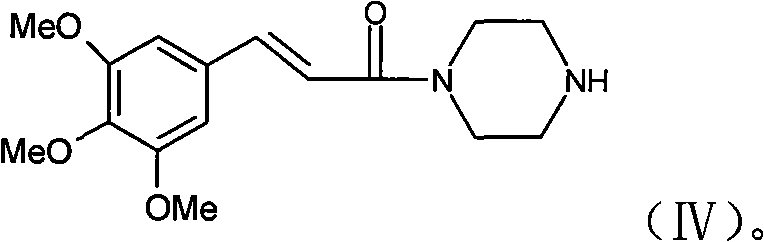
[0015] a) 3,4,5-trimethoxycinnamic acid reacts with piperazine to obtain 3,4,5-trimethoxycinnamoylpiperazine;
[0016] b) reacting 3,4,5-trimethoxycinnamoylpiperazine with chloroacetylpyrrolidine to obtain cinepazide;
[0017] Optionally, cinepazide maleate is further reacted with maleic acid to form a salt to obtain cinepazide maleate.
[0018] The synthetic route is as follows:
[0019]
[0020] The piperazine is anhydrous piperazine, piperazine containing crystal water or piperazine containing free water, wherein anhydrous piperazine is preferred.
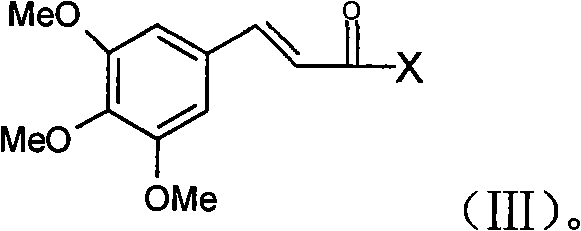
[0021] Wherein, in step a), 3,4,5-trimethoxycinnamic acid can be activated and reacted with piperazine to obtain 3,4,5-trimethoxycinnamoylpiperazine; 3,4,5-trimethoxycinnamic acid can also be The direct dehydration reaction of oxycinnamic acid and piperazine gives 3,4,5-trimethoxycinnamoylpiperazine.
[0022] More specifically, the preparation of step a) ...
Embodiment 1
[0045] Embodiment 1: the synthesis of cinepazide maleate:
[0046] 1) Synthesis of 3,4,5-trimethoxycinnamoylpiperazine
[0047] Dissolve 3,4,5-trimethoxycinnamic acid (50.0g, 0.210mol) in dichloromethane (250mL), add 2,4-dimethoxy-6-chloro-1,3,5-s Oxyzine (36.9 g, 0.210 mol), after complete dissolution, N-methylmorpholine (23.3 mL, 0.210 mol) was added. The stirring reaction was continued for 120 min. Dissolve piperazine (27.1g, 0.315mol) in dichloromethane (250mL), cool down to 0-10°C, add 3,4,5-trimethoxycinnamic acid active ester dropwise to piperazine, dropwise Afterwards, continue stirring reaction 30min. Stop responding. Extract with 2N hydrochloric acid (3×150 mL). The aqueous phase was adjusted to pH=10 with 6N sodium hydroxide solution. Extracted with dichloromethane (3×150 mL), combined organic phases, washed with saturated sodium chloride solution (1×150 mL), dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 54.9 g of yellow oil, yield 8...
Embodiment 2
[0053] Embodiment 2: the synthesis of cinepazide
[0054] 1) Synthesis of 3,4,5-trimethoxycinnamoylpiperazine
[0055] Dissolve 3,4,5-trimethoxycinnamic acid (50.0 g, 0.210 mol) in dichloromethane (250 mL), add thionyl chloride (75 g, 0.630 mol) dropwise, heat to reflux for 2 h, and set aside. Dissolve piperazine hexahydrate (122g, 0.630mol) in methanol (150mL), add hydrobromic acid (67mL, 0.630mol), cool down to 0-10°C, add 3,4,5-trimethoxycinnamoyl chloride dropwise Add it to the piperazine solution, drop it, and stir at 50°C for 120 minutes. Stop responding. The reaction solution was extracted with water (2×150 mL), and adjusted to pH=10 with 6N sodium hydroxide solution. Extracted with dichloromethane (3×150 mL), combined organic phases, washed with saturated sodium chloride solution (1×150 mL), dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 37.5 g of yellow oil, yield 58%. Leave to cure at room temperature.
[0056] 2) Synthesis of Cinepazid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com