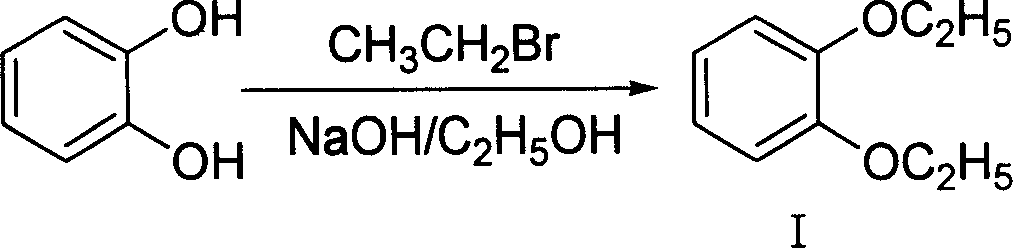Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
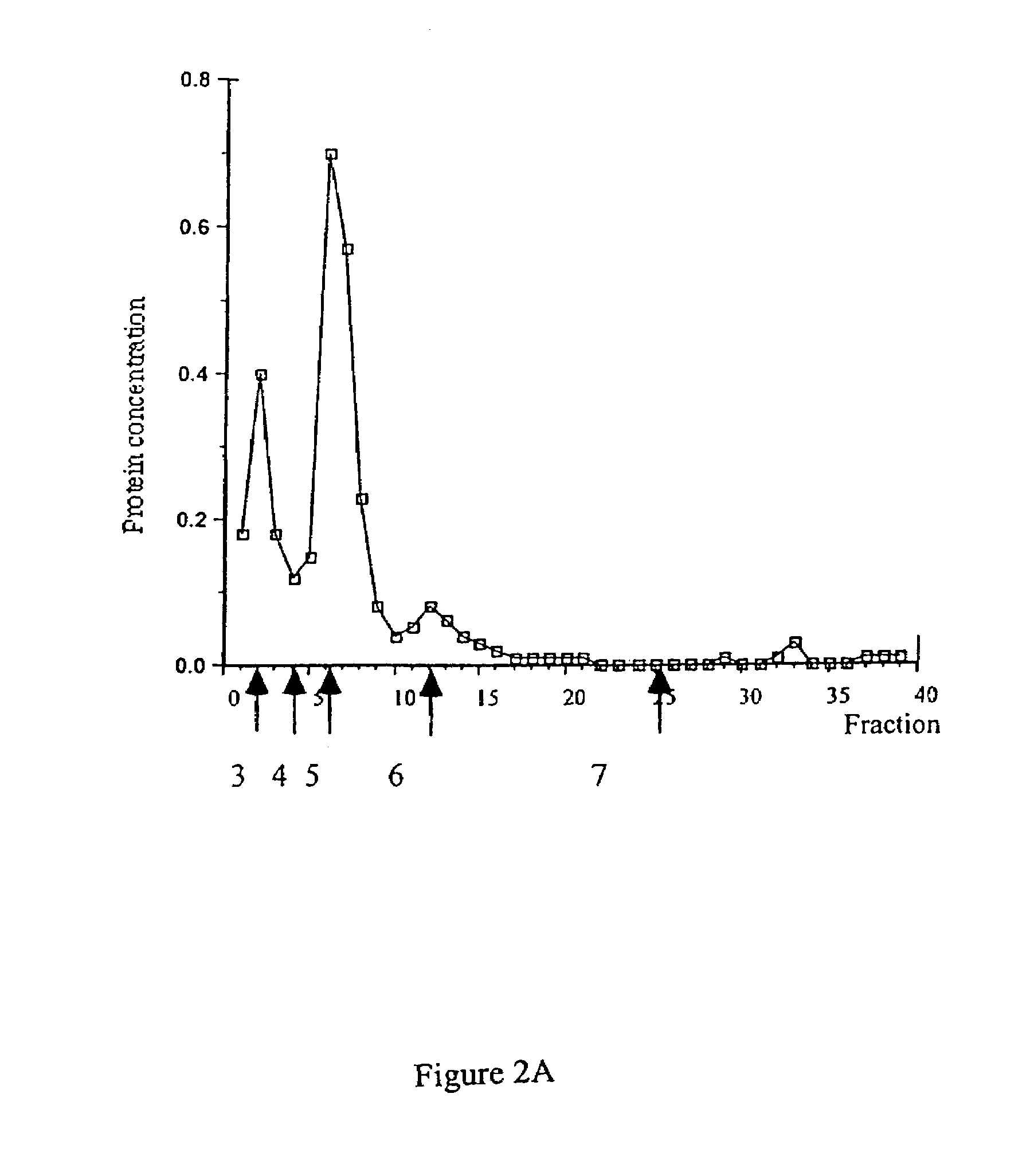
33 results about "Hyponitrite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, hyponitrite may refer to the anion N₂O²⁻₂ ([ON=NO]²⁻), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R¹−O−N=N−O−R², where R¹ and R² are organic groups. Such compounds can be viewed as salts and esters of respectively hyponitrous acid H₂N₂O₂ or HON=NOH.
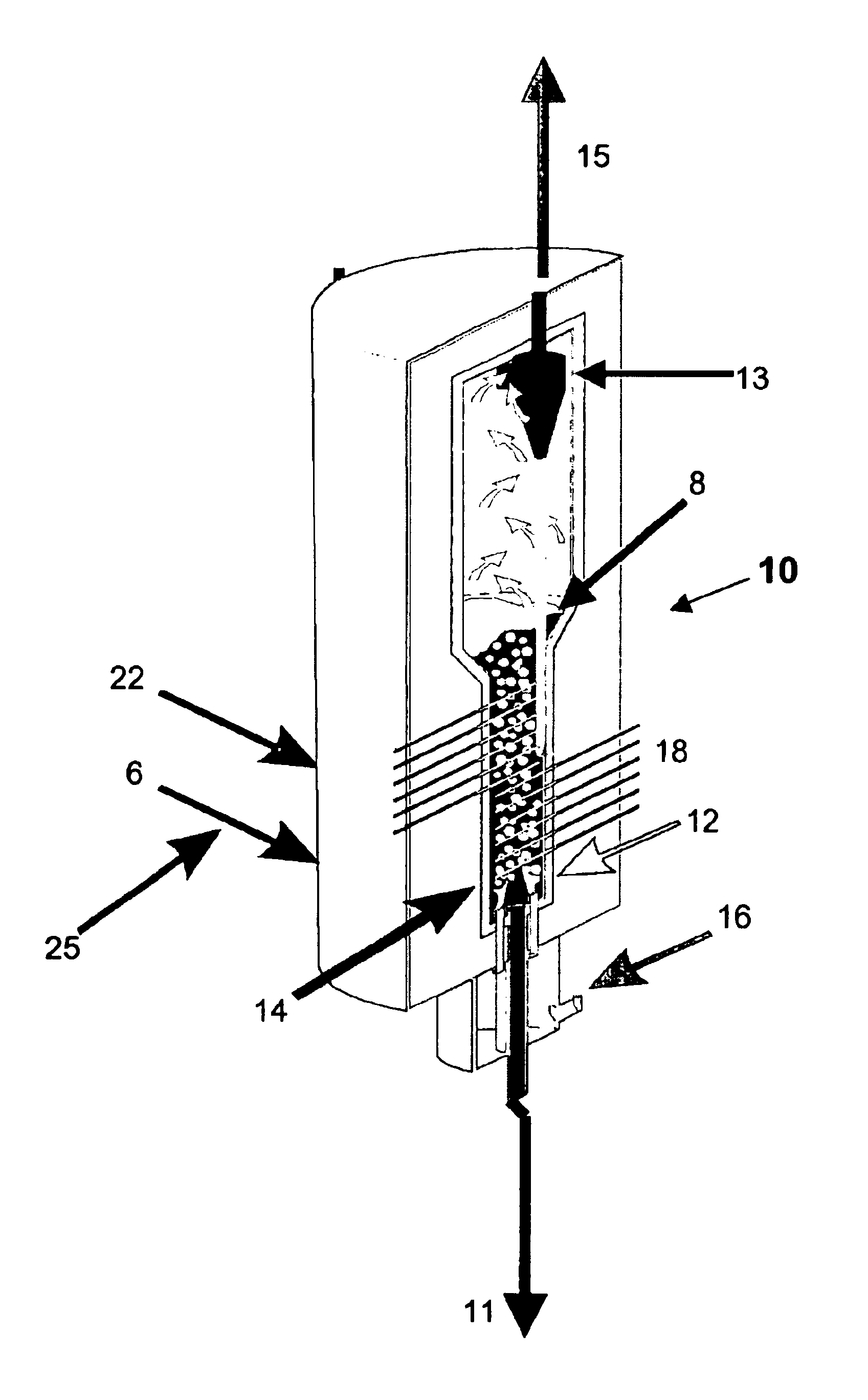
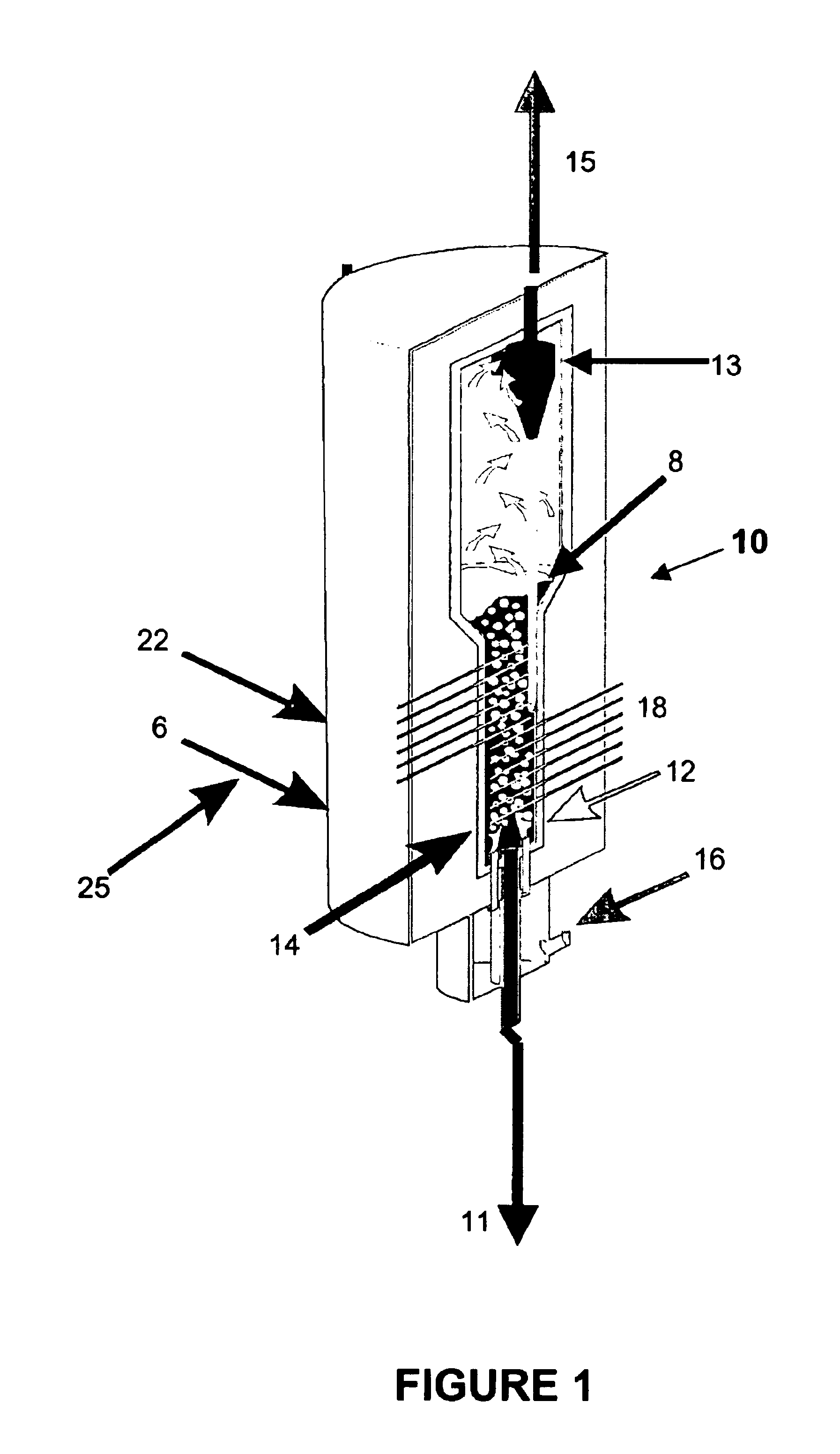
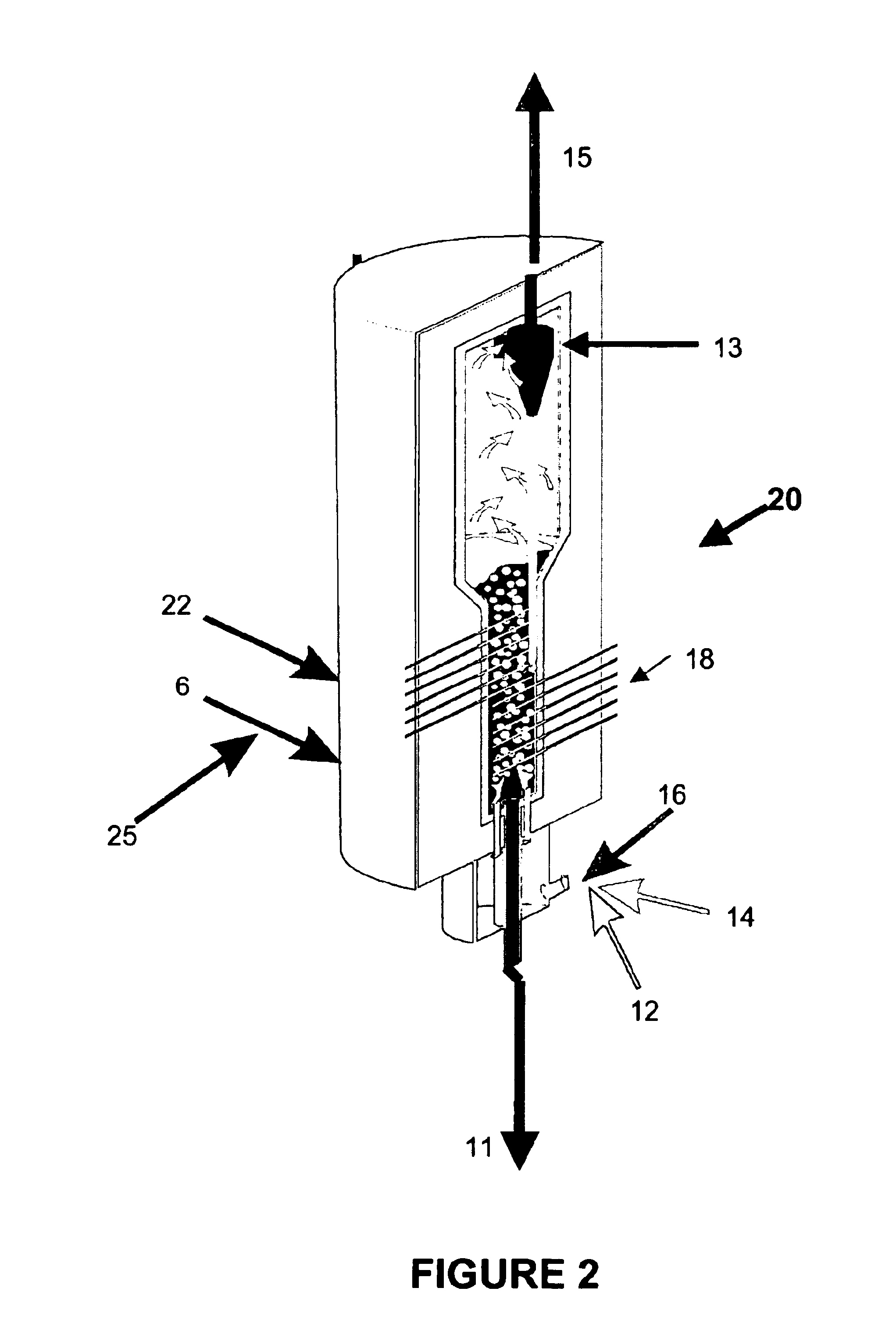
Process for the treatment of waste streams
The present invention is directed to a process for treating, reducing, and / or stabilizing various wastes or flue gases. In one embodiment, the process is directed to treatment of alkali bearing wastes that include nitrate and / or nitrite-rich wastes. Optionally, the disclosed method can be utilized for treatment of hazardous wastes, including radioactive hazardous waste compounds. In general, the present invention includes processing a waste or gaseous stream with the addition of suitable carbon-containing additives to treat and reduce nitrogen-containing compounds in the waste. Additives may be gaseous, liquid or solid reduction-promoting agents, catalysts, and the like. The reaction products obtained from the process of the invention include mainly alkali carbonate, nitrogen, hydrogen, carbon monoxide and carbon dioxide.
Owner:MFG & TECH CONVERSION INT
Iron rust conversion agent and preparation method thereof
InactiveCN103173757AStrong weather resistanceImprove mechanical propertiesMetallic material coating processesAtmospheric airSolvent
The invention relates to an iron rust conversion agent for removing and preventing rust on the surface of steel. The iron rust conversion agent can be coated on a rusty surface of the steel, the iron rust is conversed into a layer of a protective film, the complicated components of the rust layer are converted into stable products, and the stable products show no rust in the relatively long time in the atmosphere, therefore, the rusty surface of the steel can be directly coated and constructed without removing the rust. A synthetic resin emulsion is used as a main film-forming agent, pyrogallic acid and tannic acid are used as main conversion agents, citric acid is used as an auxiliary conversion agent, and a film-forming auxiliary agent is adopted to realize the curing film-forming at room temperature, thus the compactness and water resistance of the film are improved; and moreover, isopropyl alcohol is used as a solvent and penetrating agent, so as to stabilize the rusty layer. The iron rust conversion agent is sprayed to the rusty surface of the steel, thus a compact and uniform black film is formed on the surface of the steel in short time. In addition, the iron rust conversion agent has advantages of simple preparation process, low cost, stable performance, economy and practicality, safety and environmental friendliness, not containing of toxic and harmful ingredients such as formaldehyde, benzene and nitrite, and no simulation on operating personnel and the like.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Lithium secondary cell and nonaqueous electrolytic solution for use therein
ActiveCN102931434AExcellent low temperature discharge characteristicsElectrode thermal treatmentFinal product manufacturePropanoic acidHyponitrite
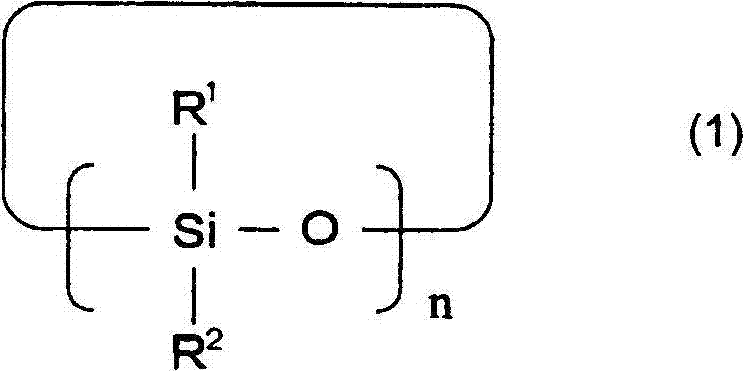
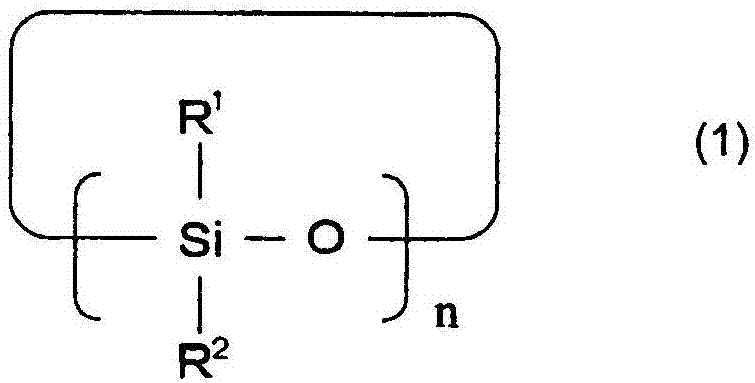
A lithium secondary cell which comprises a positive electrode and a negative electrode which have a specific composition and specific properties and a nonaqueous electrolytic solution containing a cyclic siloxane compound represented by the formula (1), a fluorosilane compound represented by the formula (2), a compound represented by the formula (3), a compound having an S-F bond, a nitric acid salt, a nitrous acid salt, a monofluorophosphoric acid salt, a difluorophosphoric acid salt, an acetic acid salt, or a propionic acid salt in an amount of at least 10 ppm of the whole nonaqueous electrolytic solution. The lithium secondary cell has a high capacity, long life, and high output. In the general formula (1), R<1> and R<2> each is a C1-12 organic group; and n is an integer of 3-10. In the general formula (2), R<3> to R<5> each is a C1-12 organic group; x is an integer of 1-3; and p, q, and r each is an integer of 0-3, provided that 1=p+q+r=3. In the general formula (3), R<6> to R<8> each is a C1-12 organic group; and A is a group constituted of H, C, N, O, F, S, Si, and / or P.
Owner:MITSUBISHI CHEM CORP +1
Vapor phase antirust blown polyethylene film and making method thereof
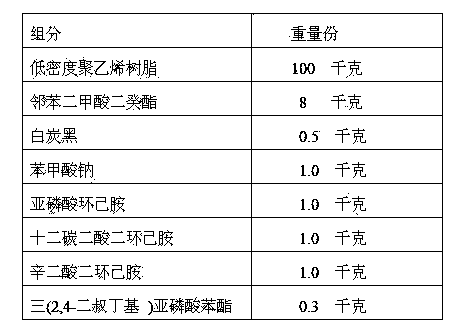
The invention discloses a vapor phase antirust blown polyethylene film and a making method thereof. The vapor phase antirust blown polyethylene film comprises low density polyethylene resin, a plasticizer, an auxiliary filler, sodium benzoate, cyclohexylamine phosphate, amine salt of aliphatic dibasic acid, and an antioxidant. Polyethylene materials comprising vapor phase antirust agents are utilized to make the vapor phase antirust blown polyethylene film through a blowing production process in order to realize convenient production and vapor phase antirust effects. The vapor phase antirust agents adopted in the invention are different from traditional nitrite vapor phase antirust agents, are in favor of realizing environmental protection and benefiting the health of human bodies. The film made in the invention has the advantages of composite structure, obstruction of the outward penetration of the vapor phase antirust agents, antirust effect increase and antirust time prolongation.
Owner:YANTAI HENGDIKE ENERGY TECH
Method of preparing 4-hydroxy-6-decyloxy-7-ethoxy-3-quinoline carboxylic acid ethyl ester
InactiveCN101012195AAvoid the disadvantages of greater pollutionWide variety of sourcesOrganic chemistryNitrobenzeneQuinoline
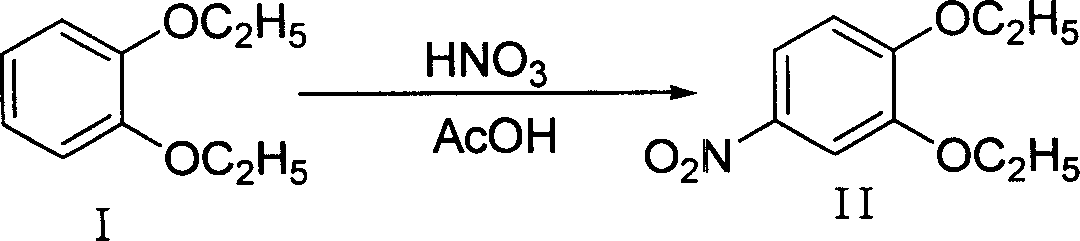
The invention discloses a making method of 4-hydroxy-6-oxy-7-ethoxy-3-quinoline carboxylic acid carbethoxy in the coccidiostat domain, which comprises the following steps: A. preparing catechol diethyl ether; B. making .3, 4-diethyloxy nitrobenzene; C. making 2-ethoxy-4-nitrophenol; D. making 3-ethoxy-4-oxynitrobenzene; E. making 3-ethoxy-4-oxyphenylamine; F. preparing N-methylene ethyl malonate-3-ethoxy-4-oxyphenylamine; G obtaining the product.
Owner:CHANGSHU OUYAJI BIOMEDICINE INST
Heteroatom-containing diamondoid transistors
InactiveUS7402835B2Solid-state devicesSemiconductor/solid-state device manufacturingField-effect transistorChemical vapor deposition
These heterodiamondoids are diamondoids that include heteroatoms in the diamond lattice structure. The heteroatoms may be either electron donating, such that an n-type heterodiamondoid is created, or electron withdrawing, such that a p-type heterodiamondoid is made. Bulk materials may be fabricated from these heterodiamondoids, and the techniques involved include chemical vapor deposition, polymerization, and crystal aggregation. Junctions may be made from the p-type and n-type heterodiamondoid based materials, and microelectronic devices may be made that utilize these junctions. The devices include diodes, bipolar junction transistors, and field effect transistors.
Owner:CHEVROU USA INC
Lithium secondary cell and nonaqueous electrolytic solution for use therein
InactiveCN107069091AExcellent low temperature discharge characteristicsFinal product manufactureActive material electrodesLithiumAcetic acid
A lithium secondary cell which comprises a positive electrode and a negative electrode which have a specific composition and specific properties and a nonaqueous electrolytic solution containing a cyclic siloxane compound represented by the formula (1), a fluorosilane compound represented by the formula (2), a compound represented by the formula (3), a compound having an S-F bond, a nitric acid salt, a nitrous acid salt, a monofluorophosphoric acid salt, a difluorophosphoric acid salt, an acetic acid salt, or a propionic acid salt in an amount of at least 10 ppm of the whole nonaqueous electrolytic solution. The lithium secondary cell has a high capacity, long life, and high output. In the general formula (1), R<1> and R<2> each is a C1-12 organic group; and n is an integer of 3-10. In the general formula (2), R<3> to R<5> each is a C1-12 organic group; x is an integer of 1-3; and p, q, and r each is an integer of 0-3, provided that 1=p+q+r=3. In the general formula (3), R<6> to R<8> each is a C1-12 organic group; and A is a group constituted of H, C, N, O, F, S, Si, and / or P.
Owner:MITSUBISHI CHEM CORP +1
Preparation of 2,3,4,5,6-pentafluorobenzyl alcohol
InactiveCN101412660ALow costReduce pollutionChemical recyclingPreparation by hydrolysisHydrogenOrganic solvent
The invention discloses a method for preparing 2, 3, 4, 5, 6-pentafluorophenylcarbinol. The preparation method comprises the following steps: using 2, 3, 4, 5, 6-pentafluorocyanobenzene as an initial raw material, introducing hydrogen in an organic solvent containing acid, and hydrogenating and reducing the raw material under the action of a catalyst at normal temperature of 70 DEG C and normal pressure of 30atm to obtain 2, 3, 4, 5, 6-pentafluorobenzylamine salt; and diazotizing the obtained 2, 3, 4, 5, 6-pentafluorobenzylamine salt and nitrite in an aqueous solution containing acid at a temperature of between 45 and 70 DEG C, then heating to a temperature of between 70 and 100 DEG C to carry out hydrolysis reaction, and after fully reacting, separating and drying the reaction liquid to obtain the 2, 3, 4, 5, 6-pentafluorophenylcarbinol. The method has the advantages that the product prepared by the method has high purity, and the total yield is more than 83 percent; the raw material cost is low, and the used solvent and catalyst can be recycled, so as to reduce pollution to environment; and diazotization and hydrolysis reaction are finished in one reactor in the whole reaction process, so the method has fewer steps, comparatively moderate conditions and simple operation, and is preferably applied to industrialized production.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD +1
Process for making amide acetals
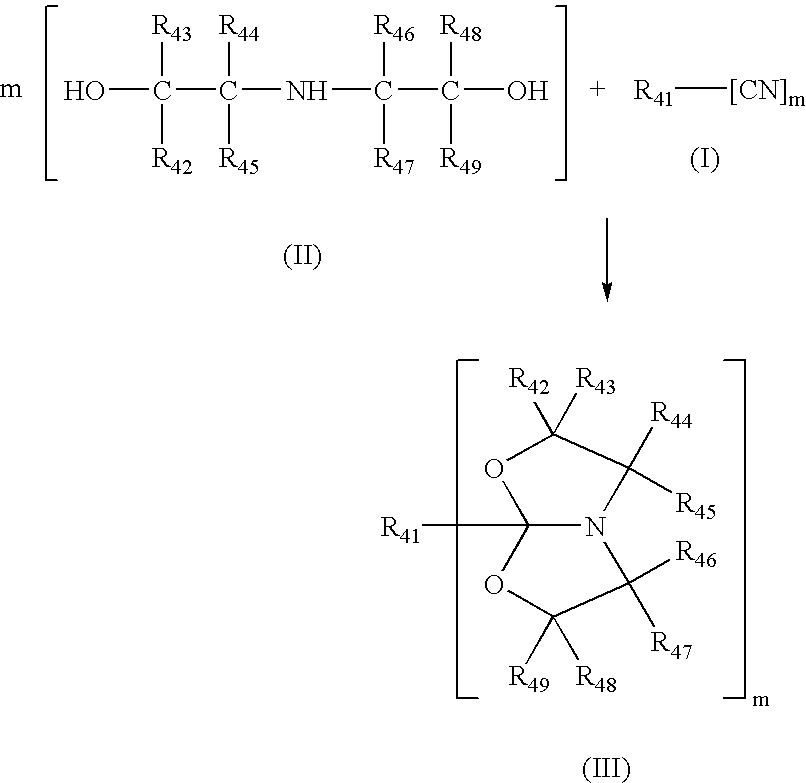
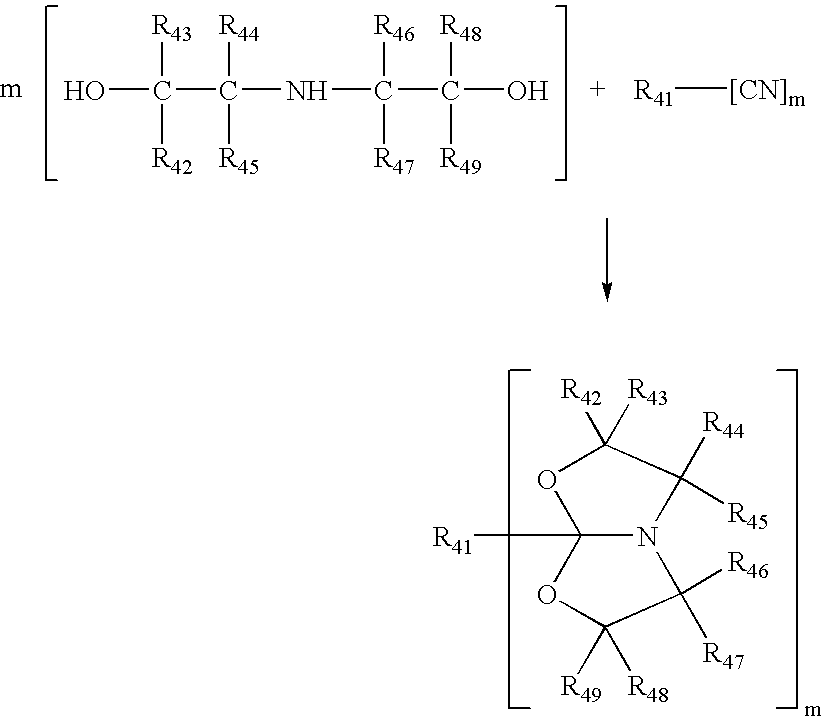
Described in this invention is a catalytic process for making amide acetals from nitrites and diethanolamines. Amide acetals can be further crosslinked by hydrolyzing the amide acetal groups, and subsequently reacting the hydroxyl groups and / or the amine functions that are formed, to crosslink the composition.
Owner:AXALTA COATING SYST IP CO LLC
Method of detecting peroxynitrite using a complex of a saccharide and an arylboronate-based fluorescent probe
InactiveUS20170059573A1Restores the PET quenching of the fluorescence of the fluorophore FpSimple fluorometricSugar derivativesGroup 3/13 element organic compoundsHyponitriteAryl radical
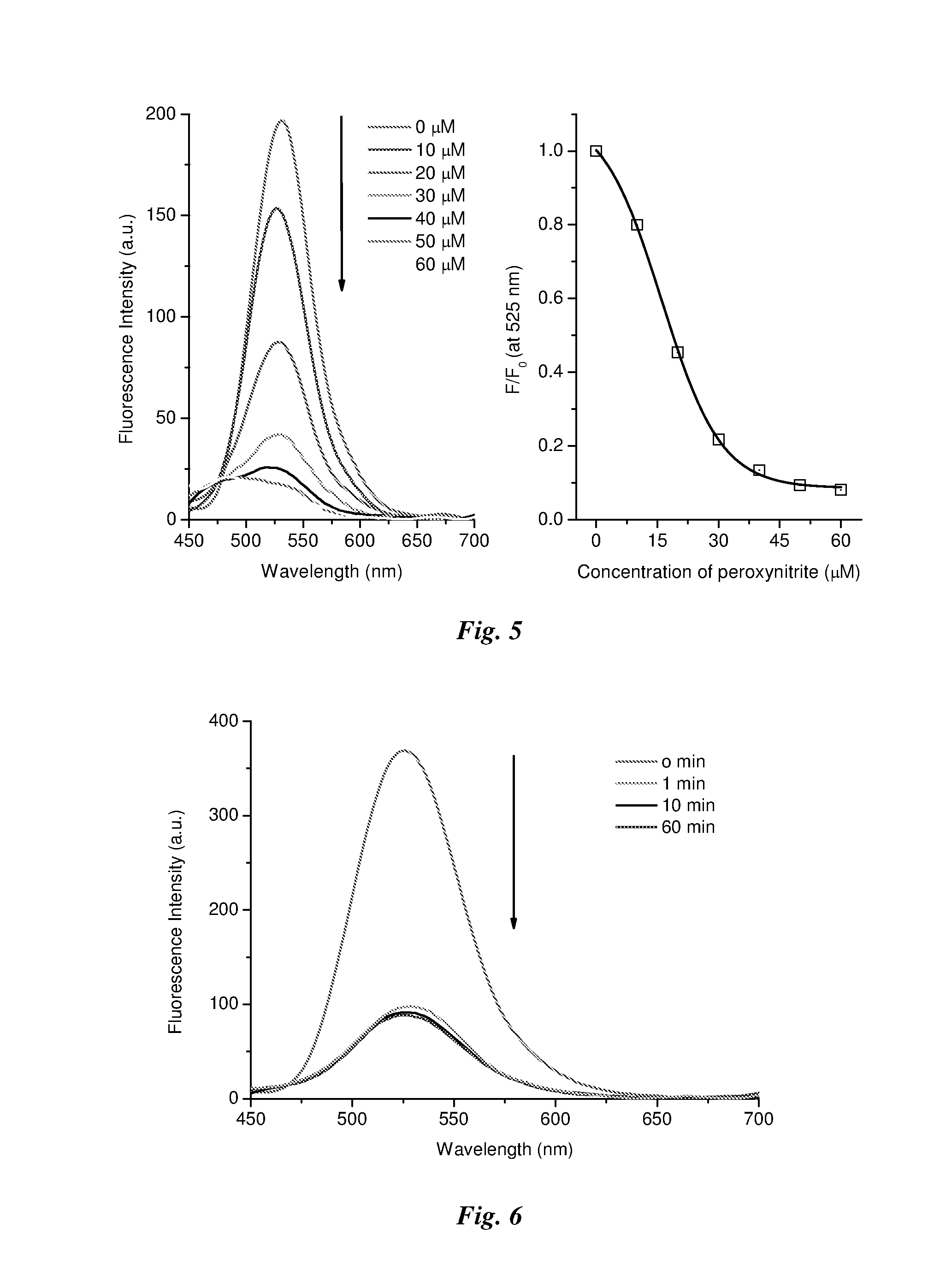
A method of detecting peroxynitrite in a sample is described comprising the steps of: (a) providing a complex of a saccharide with an aryl boronate compound of formula (I): Fp-L1-Z-L2-Ar—B(OH)2 (I) wherein: Fp comprises a fluorophore; L1 and L2 are linker groups; Z is a fluorescence switch; and Ar is optionally substituted aryl; (b) contacting said aryl boronate-saccharide complex with said sample, whereby peroxynitrite in said sample cleaves said aryl boronate-saccharide complex to produce a compound of formula (II): F-L1-Z-L2-Ar—OH (II); and (c) detecting a decrease in a fluorescence intensity of said fluorophore resulting from said cleavage reaction in step (b). Peroxynitrite reacts quantitatively, rapidly, and selectively in step (b) of the reaction, whereby medical conditions associated with elevated peroxynitrite can be diagnosed. Also provided are compounds of formula (I) for use in the methods.
Owner:UNIVERSITY OF BATH
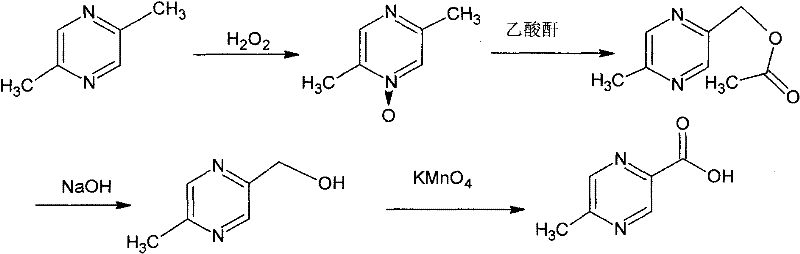
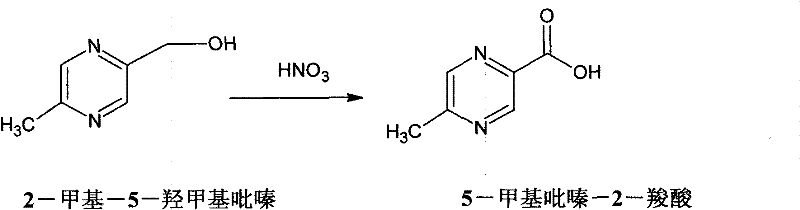
Method for synthesizing 5-methylpyrazine-2-carboxylic acid
InactiveCN102190630ALow priceGood choiceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsReaction rateReaction temperature
The invention relates to a method for synthesizing 5-methylpyrazine-2-carboxylic acid. According to the invention, 2-methyl-5-hydroxymethylpyrazine is used as a raw material, nitric acid is employed as an oxidant, and ammonium metavanadate or sodium metavanadate is employed as a catalyst. Hydroxymethyl is oxidized by the nitric acid to synthesize the 5-methylpyrazine-2-carboxylic acid, so that products have good selectivity and high yield. Moreover, the price of nitric acid is low, and tail gas that is produced during the process of synthesizing the 5-methylpyrazine-2-carboxylic acid by the method can be absorbed by alkali lye to synthesize nitrite or nitrate. Because the ammonium metavanadate or the sodium metavanadate is employed as the catalyst, the reaction temperature for oxidizing the 2-methyl-5-hydroxymethylpyrazine by the nitric acid directly can be reduced; meanwhile, the reaction rate can be enhanced, and side reaction can be reduced. Furthermore, tail gas NO2 produced by the technology in the invention can be absorbed by the alkali lye, so that environmental pollution can be reduced.
Owner:自贡市华气化学制品有限公司
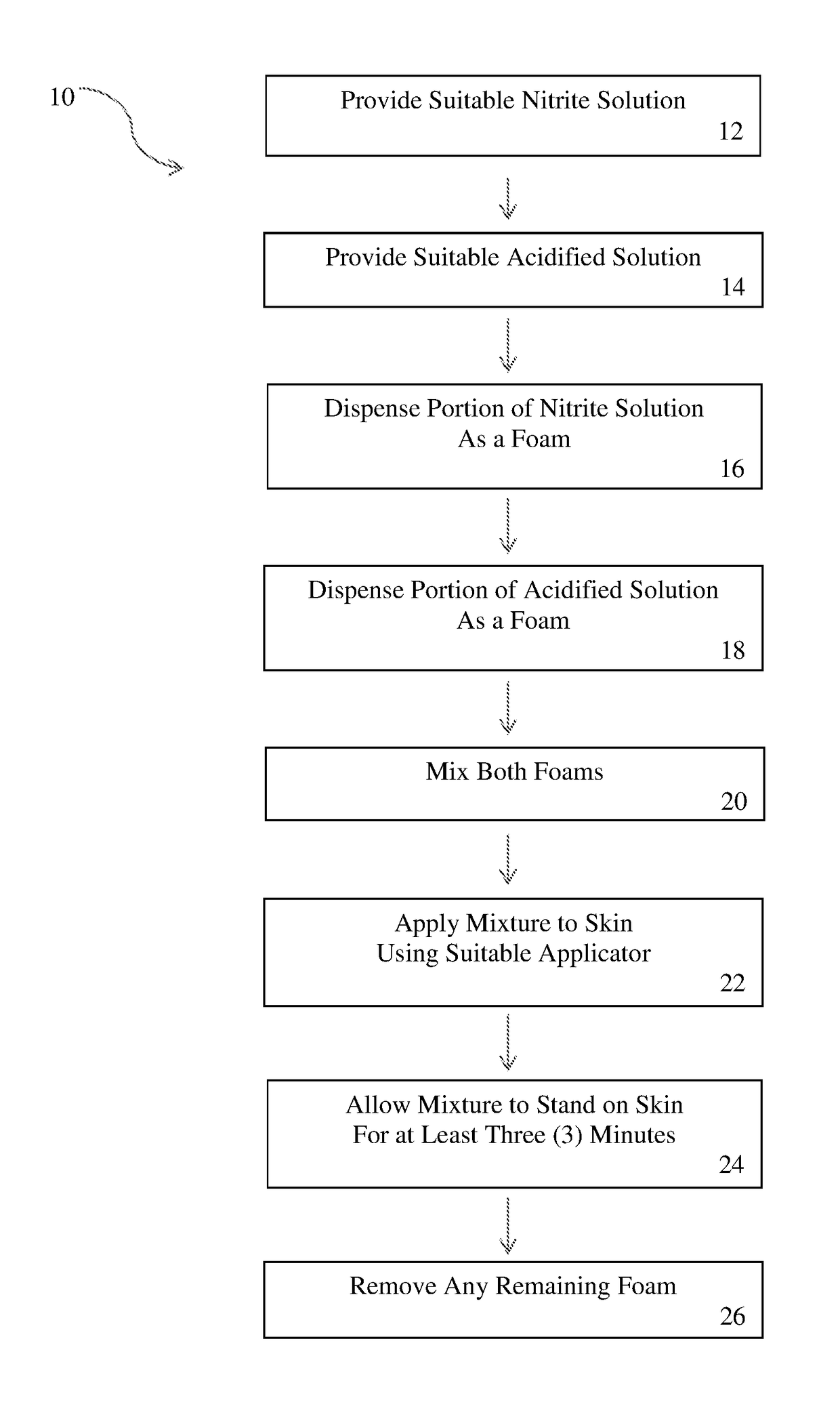
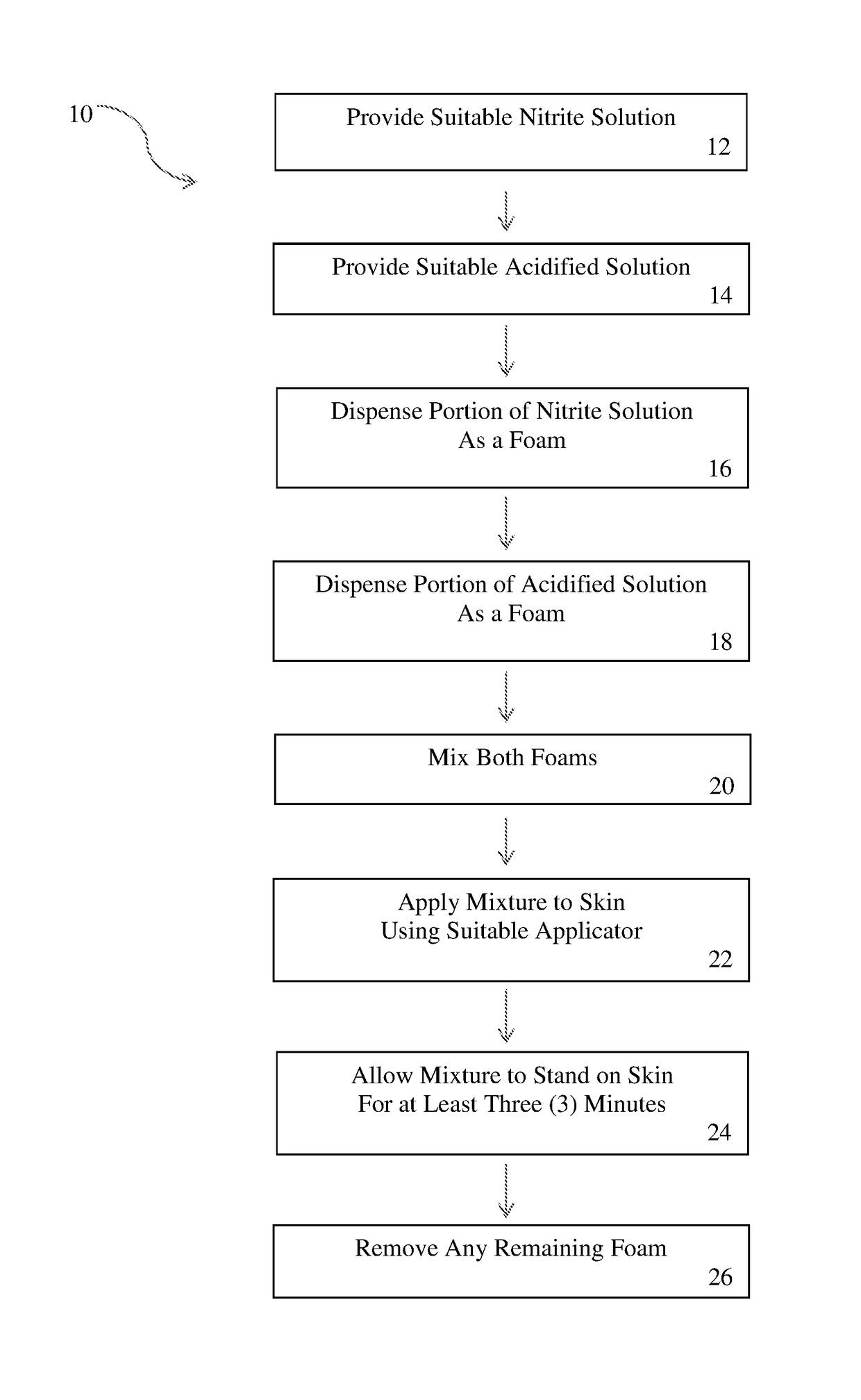
Nitric oxide topical application apparatus and methods
A topical application of nitric oxide may be provided by two separate containers, each containing an active component media to produce nitric oxide when combined. For example, a nitrite component media may be contained in one dispenser and an acidified component media may be contained in another dispenser. Each dispenser may dispense the respective component media as a foam. The resultant foams are combined to initiate the production of nitric oxide and the mixture of foams is applied topically to treat various skin disorders or wounds.
Owner:SYK TECH LLC
A kind of regeneration method of activated clay waste residue
InactiveCN102266759AAvoid pollutionSimple processOther chemical processesCombustible gas purificationSludgeSlag
The invention discloses a method for regenerating active white clay waste slag. The method comprises the following steps of: a) mixing the active white clay waste slag and sulfuric acid solution; b) making a solid matter by using the mixed sludge; c) burning the solid matter at temperature of 600 to 700 DEG C; and d) smashing the burned solid matter. By the method for regenerating the active white clay waste slag, the used active white clay waste slag is subjected to acidization and high-temperature roasting; the process is simple, the treatment time is short, the process is convenient to operate, and continuous industrial production can be realized; the regenerated active white clay can be reused, so that the fund is saved and pollution to the neighboring environment caused by peculiar smells due to long-time occupation stacking can be avoided; a using effect of the regenerated active white clay is basically the same as the using effect at the first time; and the active white clay waste slag can be regenerated for many times and applied to drinking water treatment so as to remove nitride, fluoride and the like from water.
Owner:QIDI ELECTRIC GROUP
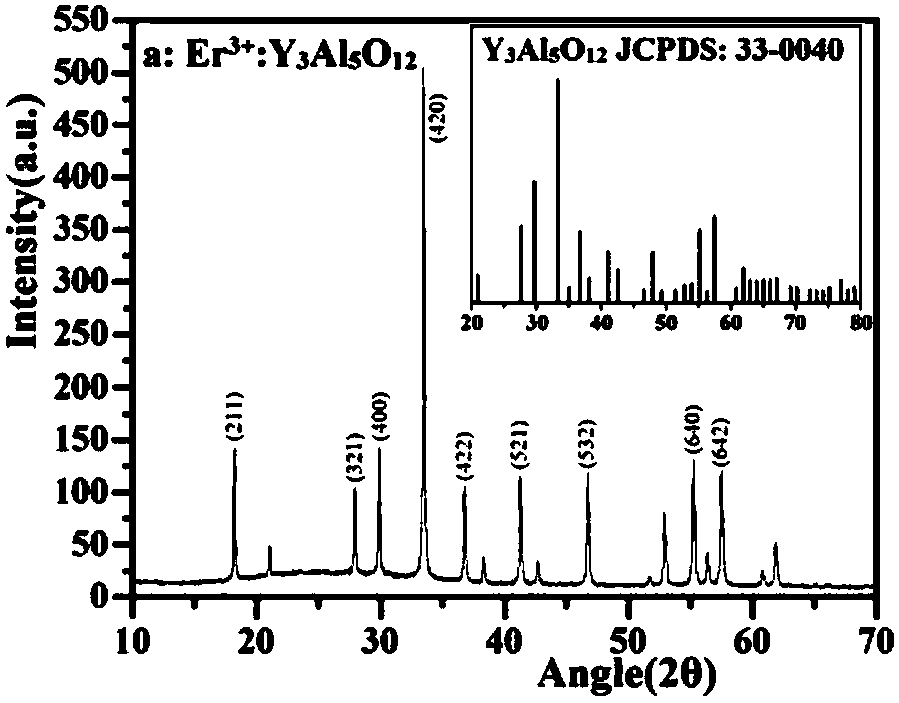
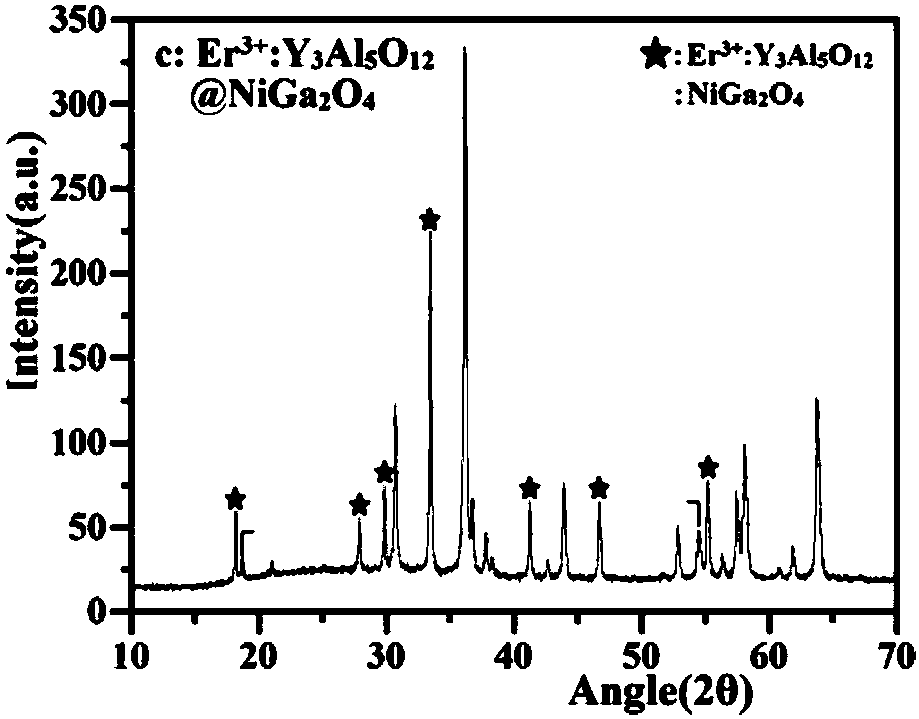
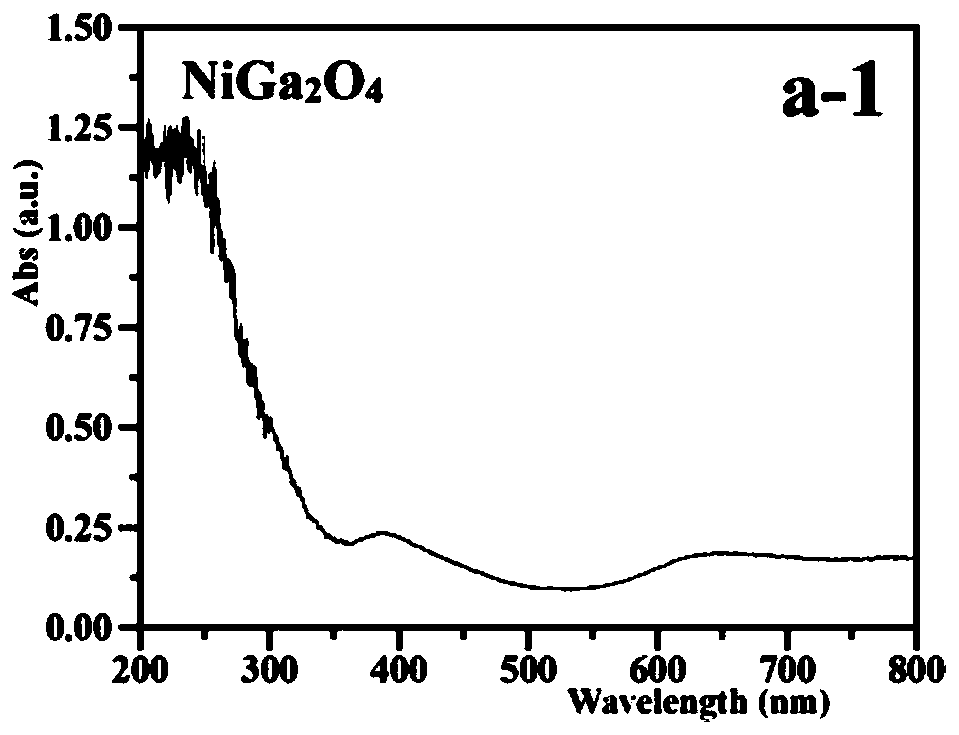
Z-shaped semiconductor photocatalyst with trapezoid structure as well as preparation method and application thereof
ActiveCN108273522AStable in natureImprove conversion of nitritePhysical/chemical process catalystsWater/sewage treatment by irradiationWastewaterSulfite
The invention relates to a Z-shaped semiconductor photocatalyst with a trapezoid structure as well as a preparation method and application thereof. The Z-shaped semiconductor photocatalyst is preparedthrough a sol-gel method, a hydrothermal method and a high-temperature calcination method; a narrow band gap semiconductor is embedded between Er<3+>:Y3Al5O12@NiGa2O4 and Bi2Sn2O7 and is used as a good conductor as a conductive ladder to form the Z-shaped semiconductor photocatalyst Er<3+>:Y3Al5O12@NiGa2O4 / NiS / Bi2Sn2O7 with the trapezoid structure; the composite material has highly-stable photocatalytic activity in nitrite and sulfite conversion processes; the conversion rates of nitrite and sulfite reach 86.23 percent to 94.44 percent respectively under the irradiation of simulated sunlight.The photocatalyst with the Z-shaped structure has stable and efficient photocatalytic activity and has a wide application prospect in nitrite and sulfite wastewater treatment.
Owner:LIAONING UNIVERSITY
Method for continuously preparing aniline diazonium salt and phenylhydrazine hydrochloride on basis of microreactor
InactiveCN109503417AHigh yieldShort reaction timeHydrazine preparationChemical/physical/physico-chemical microreactorsMicroreactorFiltration
The invention relates to a method for continuously preparing aniline diazonium salt and phenylhydrazine hydrochloride on the basis of a microreactor. An aniline salt and acid mixture and an nitrite aqueous solution which are prepared at a normal temperature are continuously injected into the microreactor to carry out diazotization reaction, and by the steps of reduction, acid precipitation and filtration, the phenylhydrazine hydrochloride is obtained. The method is easy to operate, the temperature of the diazotization reaction is high, the dosages of the reactants approximate the stoichiometric ratio, the time of the diazotization reaction is short, the yield is high, the aniline diazonium salt and the phenylhydrazine hydrochloride can be continuously produced, the energy consumption is reduced, the safety of the process is increased, and the method is applicable to industrial production.
Owner:SHANGHAI JIAO TONG UNIV
Copper oxide ultrafine particles
ActiveUS7767721B2Easy to operateGood dispersionNon-metal conductorsNanotechHydrazine compoundCopper oxide
Owner:ASAHI KASEI KK
Preparation method and application of modified electrode based on nanoporous platinum-cobalt oxide hybrid material
ActiveCN108956745AImprove electrocatalytic activityAvoid reunionMaterial nanotechnologyMetal/metal-oxides/metal-hydroxide catalystsPlatinumHybrid material
The invention relates to a food detection technology and particularly relates to a preparation method and application of a modified electrode based on nanoporous platinum-cobalt oxide hybrid material.The preparation method adopts a glassy carbon electrode as a base electrode. The surface of the base electrode is modified with the nanoporous platinum-cobalt oxide hybrid material, the nanoporous platinum-cobalt oxide hybrid material, carbon powder, ethanol and a Nafion solution are mixed under ultrasonic treatment to form a uniform ink-like suspension solution, and the ink-like suspension solution is added onto the polished surface of the glassy carbon electrode drop by drop and then is dried so that the modified electrode is obtained. The product detection process is fast, any sample labeling and probe modification are avoided, sensitivity is high and a detection limit is low. The hybrid material is a stable composition of nanoporous cobalt oxide and platinum nanoparticles, effectivelyprevents agglomeration of platinum nanoparticles, has a low platinum use amount and greatly reduces the cost of nitrite detection.
Owner:QILU UNIV OF TECH
Process for producing optically active carboxylic acid subtituted in 2-position
InactiveUS20030144546A1Easy to operateHigh yieldPreparation from carboxylic acid halideOrganic compound preparationCarboxylic acidAqueous solution
A nitrous acid salt is added at a temperature of 10 to 80° C. to an aqueous solution which contains an optically active 2-aminocarboxylic acid (4) and a protonic acid, the amount of the latter acid being 1 to 3 equivalents to the former, and which has a proton concentration of 0.5 to 2 mol / kg to conduct a reaction to thereby produce an optically active 2-hydroxycarboxylic acid (1). Thionyl chloride and a basic compound are caused to act on the compound (1) to chlorinate it and simultaneously invert the configuration in the 2-position. Thus, an optically active 2-chlorocarboxylic acid chloride (5) is induced. The compound (5) is hydrolyzed to induce an optically active 2-chlorocarboxylic acid (2). The compound (2) is reacted with a thioacetic acid salt to incorporate an acetylthio group thereinto and simultaneously invert the configuration in the 2-position to thereby produce an optically active 2-acetylthiocarboxylic acid (3).
Owner:KANEKA CORP
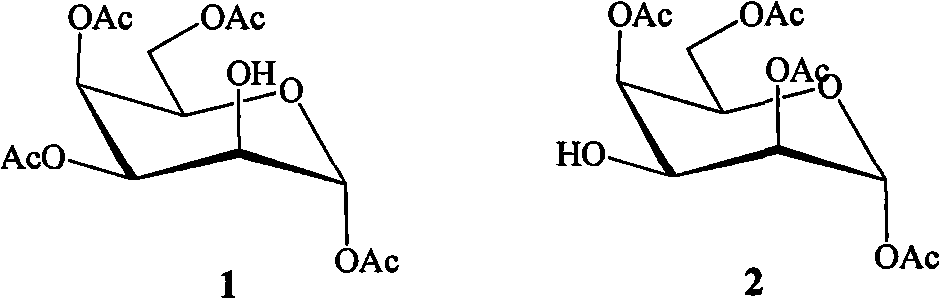
Tetraacetyl talose compound and preparation method thereof
InactiveCN102020681AEsterified saccharide compoundsSugar derivativesSodium acetateChemical synthesis
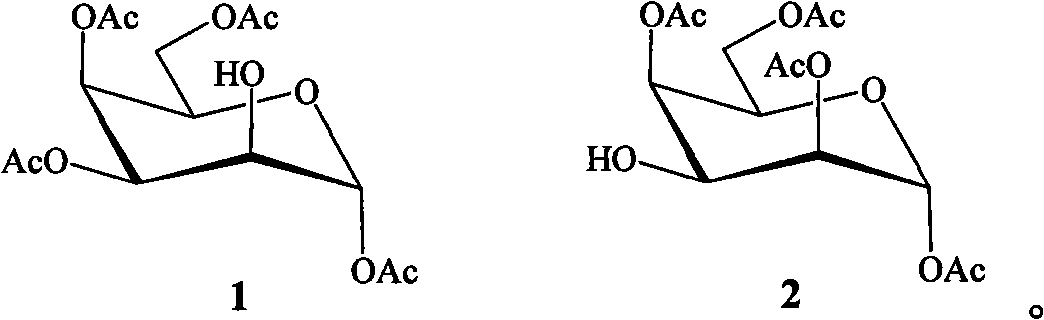
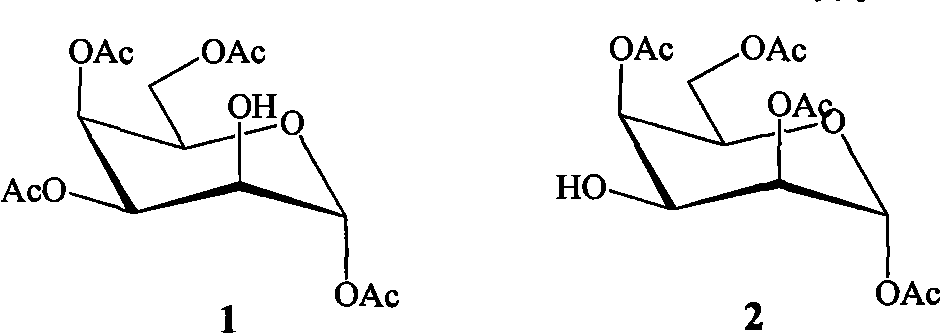
The invention belongs to the field of chemical synthesis, in particular relates to a preparation method for a tetraacetyl talose compound. The cheap and readily available per-O-acetyl galactose is taken as a raw material, and the method comprises the following steps of: reacting galactose in hot acetic anhydride in the presence of sodium acetate to obtain penta-O-acetyl-beta-D-galactopyranose; selectively removing acetyl of 2OH under the action of trifluoroacetic acid; protecting the 2OH by using sulfonyl; and finally, in a protonic solvent, performing simple and high-efficiency three-step reaction to obtain tetraacetyl talose with the structures 1 and 2 in high yield by taking water or nitrite as a reagent. The prepared tetraacetyl talose compound can be converted into talose or serve as a chiral raw material for synthesizing other compounds with excellent performance.
Owner:FUDAN UNIV
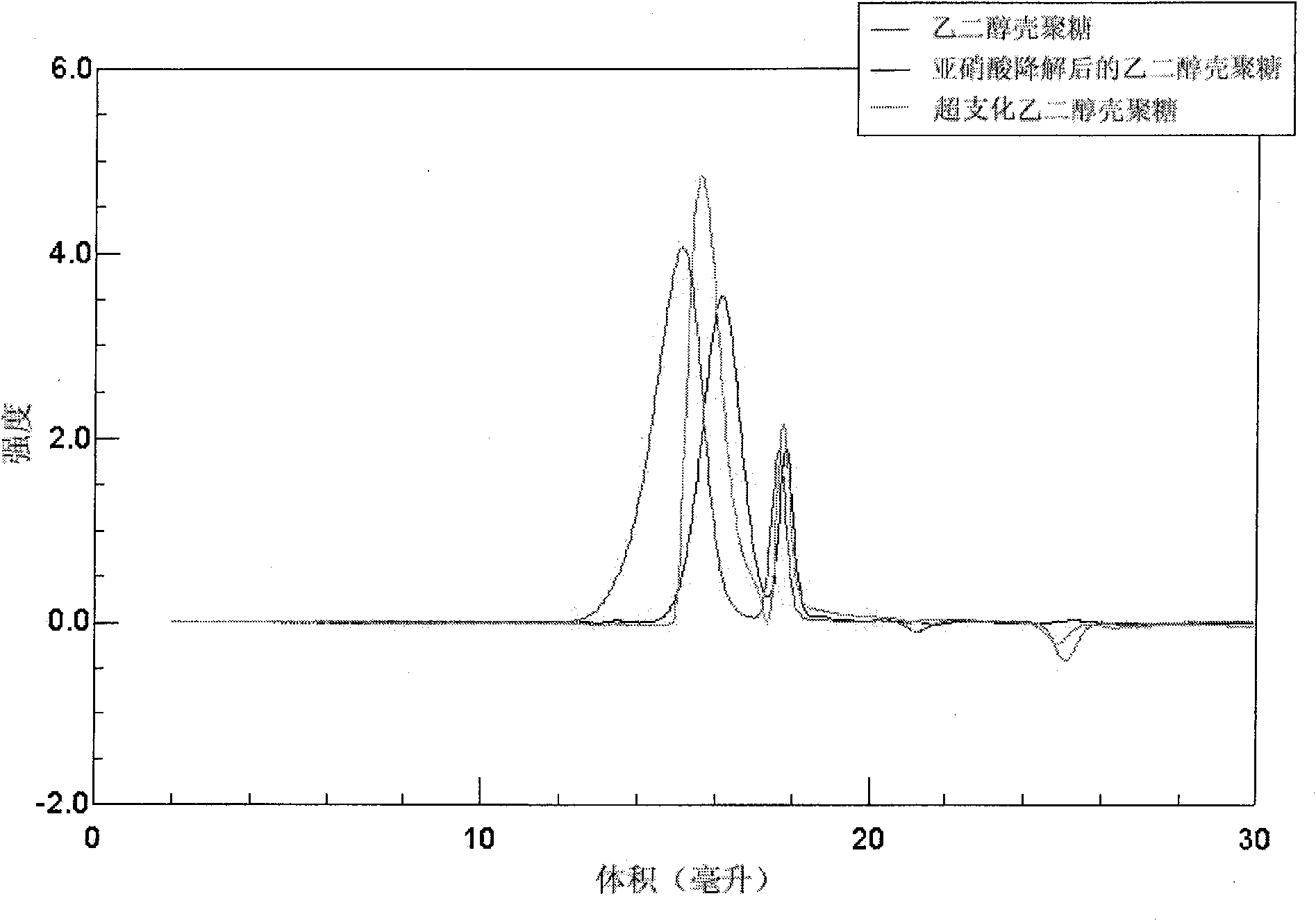
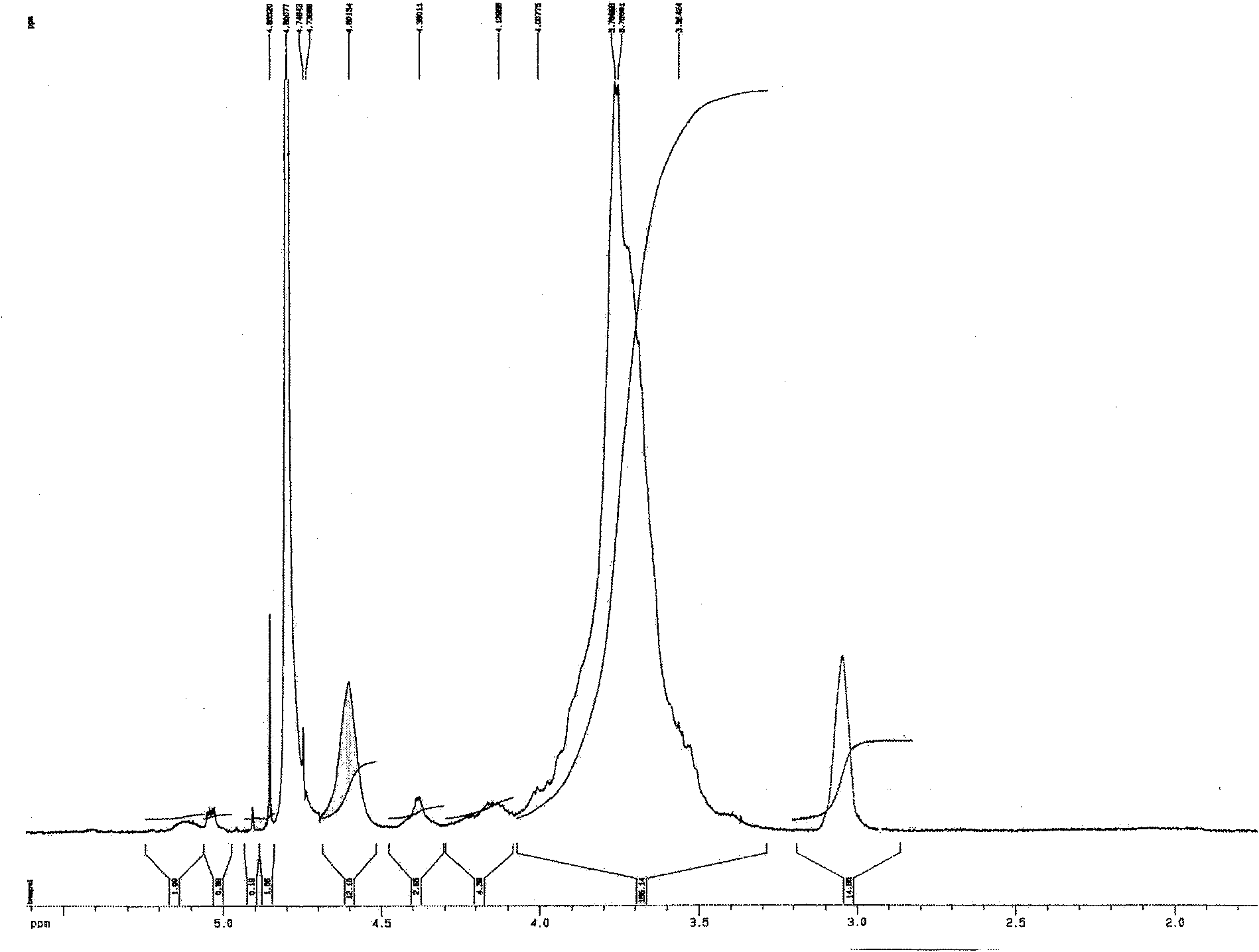
Hyper-branched chitosan or hyper-branched glycol chitosan and preparation method thereof
The invention discloses a process of preparing of an over-expenditure structure chitose or an over-expenditure ethylene alcohol chitose, which comprises the following steps: degrading the chitose or the ethylene alcohol chitose with nitrous acid or nitrite; getting fractionally more narrow molecular weight distribution high molecular chain a end-groupof which is aldehyde group chitose or ethylene alcohol chitose with method of precipitation or volumetric exclusive chromatography; getting the Schiff's base dissolving in the weakly-acidic or neutral solution; getting the over-expenditure structure chitose or the over-expenditure ethylene alcohol chitose by deacidizing SCHIFF'S base( when R is H in the structural formula, the structure is the over-expenditure structure chitose; when R is CH2CH2OH, the structure is the over-expenditure ethylene alcohol chitose; wherein RI is the same structure branched chain to the broken line part in the above structural formula; the molecular weight range of the over-expenditure structure chitose or the over-expenditure ethylene alcohol chitose is >= 5000Da).
Owner:INST OF CHEM CHINESE ACAD OF SCI
Nitric oxide releasing compositions
Nitric oxide (NO) generating compositions can include a nitrite component, an acidifying component, and a support material configured to carry one of the nitrite component and the acidifying agent. In some examples, the support material can minimize NO generation prior to addition of an activating amount of a suitable solvent.
Owner:SANOTIZE RES & DEV CORP
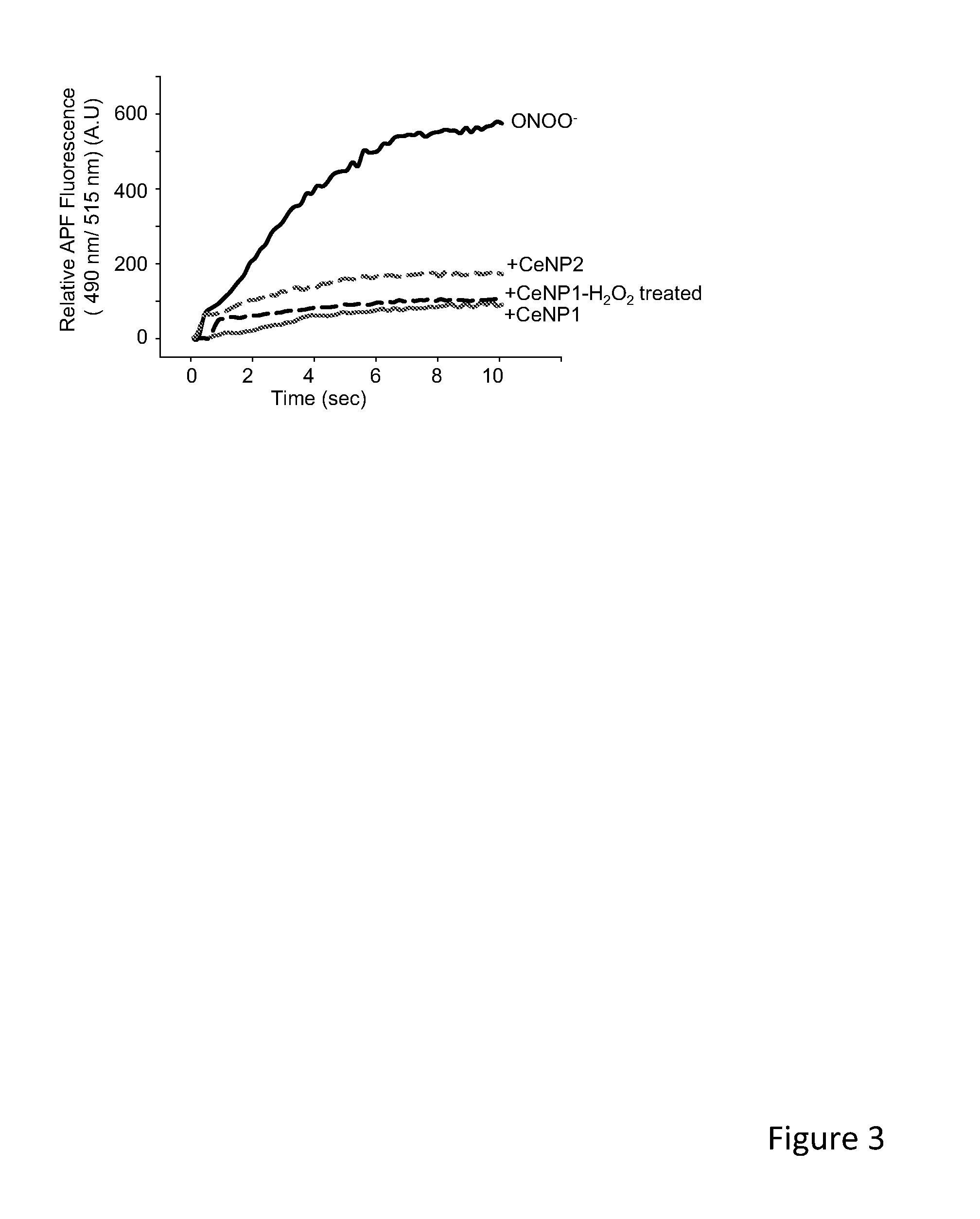
Cerium Oxide Nanoparticles Accelerate the Decay of Peroxynitrite (ONOO-)
Disclosed herein are methods and materials that may be implemented to scavenge a potentially destructive free radical, peroxynitrite. Specifically exemplified are cerium based nanoparticles that react with and reduce peroxynitrite. The discoveries disclosed herein reveal several therapeutic uses of such peroxynitrite reactive particles.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Preparation methods and application of phosphorescent iridium compound and organic and inorganic hybridized nano-silicon spheres
ActiveCN108250251ASolve the shortcomings of poor water solubilityLong launch lifeIndium organic compoundsFluorescence/phosphorescencePeroxynitriteSolubility
The invention discloses preparation methods and application of a phosphorescent iridium compound and organic and inorganic hybridized nano-silicon spheres (MSN-ONOO). A borate group with specific response on peroxynitrite is connected to an auxiliary ligand of an iridium compound through a covalent bond; the detection of a ratio method is realized through introducing a reference compound; the organic and inorganic hybridized nano-silicon spheres are prepared; the MSN-ONOO covers two iridium compound materials (Ir1 and Ir3*), wherein the Ir1 has the specific response on the peroxynitrite and the Ir3* is used as a ratio probe and has no response on the peroxynitrite. The Ir1 phosphorescent strength is weakened along the increasing of the concentration of the peroxynitrite; the phosphorescentservice life is reduced along the increasing of the concentration of the peroxynitrite; endogenous and exogenous peroxynitrite of cells is specifically detected through co-focusing imaging; mesoporous nanoparticles are prepared so that the disadvantage that the water solubility of a common fluorescent / phosphorescent probe is poor is successfully solved.
Owner:NANJING UNIV OF POSTS & TELECOMM
Carboxylic cationites and methods of manufacture
InactiveUS7071240B1Eliminate needCation exchanger materialsIon-exchanger regenerationSorbentIon exchange
A method for preparing synthetic polymerized resins for ion exchange, namely, carboxylic cationites, which are suitable as sorbents for preparative separation and purification of biologically active compounds by low pressure liquid chromatography (LPLC). The directional polymerization conditions enable such cationites to be prepared with polymeric structures which can be used for the separation of macromolecules, such as proteins, with high molecular mass from various microbiological raw materials and physiological liquids. Depending upon the embodiment of the method of manufacture according to the present invention, the resultant carboxylic cationites are obtained in one of three forms: a swollen block, if no pre-polymerization or dispersion stages are performed; and, if such stages are performed, depending upon the dispersion conditions, the resultant structures can be either irregular particles or spherical granules.
Owner:POLYGRAN
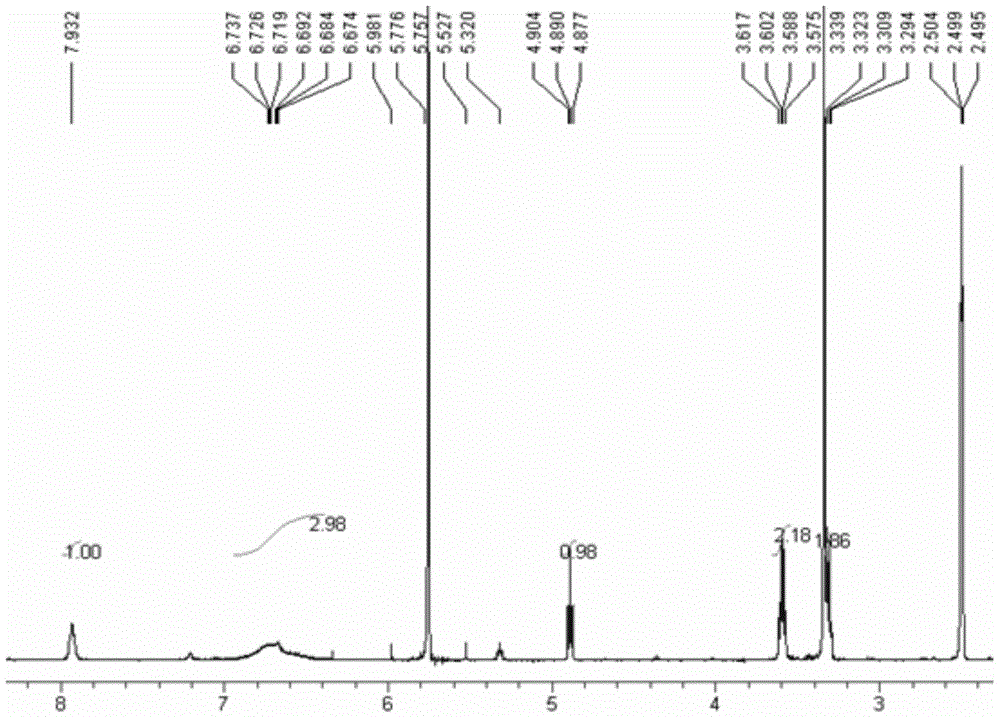
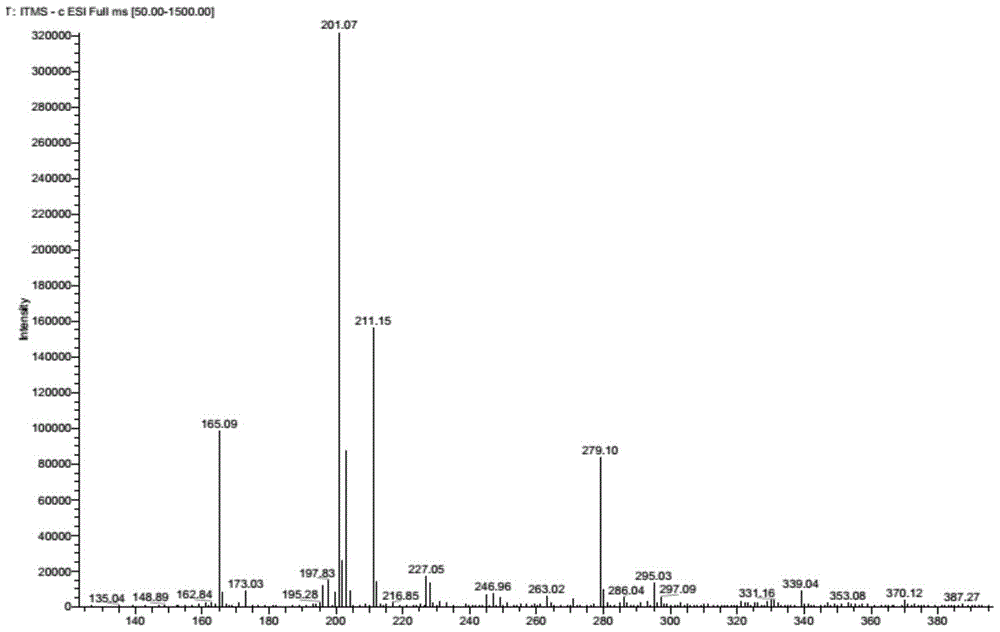
A kind of preparation method of rivaroxaban intermediate
The invention discloses a synthetic method of rivaroxabanintermediate4-(4-nitrosobenzene)-3-morpholine. The method comprises steps as follows: (1), halogenated benzene (I) and ethanolamine react under the condition of a catalyst and alkali to generate N-ethoxylaniline (II), N-ethoxylaniline (II) and nitrous acid or nitrite react to generate 4-nitroso-N-ethoxylaniline (III), and 4-nitroso-N-ethoxylaniline (III) and chloroacetyl chloride react to generate 4-(4-nitrosobenzene)-3-morpholine (IV). According to the synthetic method of 4-(4-nitrosobenzene)-3-morpholine, required raw materials and reagents are cheap and are easy to obtain, the yield is high, and the cost is low; the reaction condition is mild; fewer three wastes are produced; and the product quality is reliable and stable, and the whole process is very suitable for industrial production.
Owner:HANCHEM BIOPHARM TECH
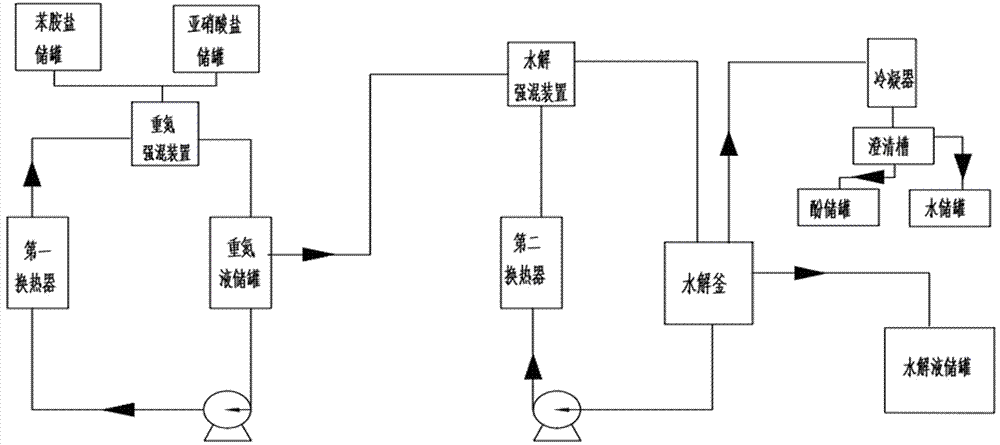
A kind of method and system for continuous production of 2,5-dichlorophenol
ActiveCN105384610BShort reaction timeLess side effectsOrganic chemistryOrganic compound preparationDichlorophenolAniline
The present invention provides a method and system for continuously producing 2,5-dichlorophenol. The method comprises an aniline salt diazotization process and a diazo solution hydrolysis process. The aniline salt diazotization process comprises the steps of continuously feeding aniline salt and nitrite into a diazo intensive mixing apparatus; performing a diazotization reaction under intensive mixing; feeding a diazo solution generated by the reaction into a diazo solution storing tank; back-mixing a part of the diazo solution in the diazo solution storing tank after cooling and feeding the part of the diazo solution into the diazo intensive mixing apparatus; removing all or a part of heat generated in the diazotization reaction by using the back-mixed cryogenic diazo solution; and directly performing continuous hydrolysis on the other part of the diazo solution. According to the method provided by the present invention, by adopting an intensive mixing process and a back-mixing process, mass and heat transfer during the diazotization reaction and the hydrolysis reaction can be quickly performed; heat can be taken away or given in time; a reaction time is shortened; the side reaction is reduced; the heat utilization rate is improved; the costs are lowered; and a safety factor and the device production capacity are improved.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Electrochemical sensor based on double metal porphyrin coordination polymer and its preparation method
InactiveCN104990972BUnique bimetallic activityUnique bimetallic electrocatalytic activityMaterial electrochemical variablesCarbon nanotubePorphyrin
An electrochemical sensor based on a double metal porphyrin coordination polymer, the double metal porphyrin coordination polymer or a double metal porphyrin coordination polymer / carbon nanotube composite is modified on the surface of the base electrode, the double The metal porphyrin coordination polymer is a coordination polymer formed by the self-assembly of Co, Cu and tetra-(p-carboxyphenyl) porphyrin, in which the metal Co coordinates with the four Ns of the porphyrin center, and each metal Cu Coordinate with the oxygens from the four carboxyl groups respectively, and each carboxyl group is bidentately coordinated with two metal Cu. The electrochemical sensor of the present invention is an amperometric sensor with unique bimetallic activity, the metal active site Cu has an electrocatalytic effect on the reduction process of hydrogen peroxide, and the metal active site Co has an electrocatalytic effect on the oxidation process of nitrite The incorporation of carbon nanotubes can significantly improve its electrical sensing performance.
Owner:NANJING NORMAL UNIVERSITY
Rapid response reversible photoisomerization perfluoroether chain-containing azobenzene and preparation method thereof
InactiveCN104926683BSolve the problem of green environmental protectionNon-bioaccumulativeOrganic chemistryTenebresent compositionsStructural formulaP-Phenylenediamine
The invention discloses quick-response reversible-photoisomerization perfluorinated-ether-chain azobenzene and a preparation method therefor. The perfluorinated-ether-chain azobenzene has a structural formula shown in the description. The preparation method comprises the steps: dissolving p-phenylene diamine and perfluoroether acyl fluoride, which serve as starting compounds, in an organic solvent so as to prepare (4-perfluoroether amide)phenylamine; diazotizing the compound by a hydrochloride / nitrite system, and then, enabling the diazotized compound to react with an alkali solution of phenol so as to prepare 4-perfluoroether amide-4'-hydroxyazobenzene; and in an anhydrous organic solvent, enabling 4-perfluoroether amide-4'-hydroxyazobenzene to react with methacryloyl chloride or acryloyl chloride so as to prepare 4-perfluoroether amide-4'-methacrylate azobenzene or 4-perfluoroether amide-4'-acrylate azobenzene. The compound disclosed by the invention has the advantages of reversible photoisomerization, quick response and high isomerization degree; a fluorinated ether chain is free of biological accumulation compared with a perfluoroalkyl hydrocarbon chain; and the compound contains an active functional group, i.e., propenyl which can participate in addition reaction or polymerization reaction, so that the field of application is broad.
Owner:DONGHUA UNIV
Preparation method for 4-phenyl-2,7-naphthyridine-1(2H)-ketone
The invention relates to a preparation method for 4-phenyl-2,7-naphthyridine-1(2H)-ketone, and belongs to the technical field of medicine synthesis. According to the preparation method for the 4-phenyl-2,7-naphthyridine-1(2H)-ketone, the problems that an existing reaction route is long and low in yield are solved. The preparation method comprises the steps that 3-amino-4-phenyl-1H-pyran-[3,4-c]pyridine-1-ketone reacts with ammonium water to obtain 3-amino-4-benzene-2,7-naphthyridine-1(2H)-ketone; on the acidic condition, a diazo-reaction takes place between the 3-amino-4-benzene-2,7-naphthyridine-1(2H)-ketone and nitrite to obtain a diazotized intermediate product, and after the reaction is completed, the temperature is increased to enable the diazotized intermediate product to be converted into the final product 4-phenyl-2,7-naphthyridine-1(2H)-ketone. The preparation method has the advantages that the raw materials are easy to obtain, the reaction requirements are low, operation is easy, only two steps of reactions are needed, the technology is simplified, and the total yield of the final obtained 4-phenyl-2,7-naphthyridine-1(2H)-ketone is higher.
Owner:台州复瑞生物科技有限公司
A self-assembled solar photocatalyst for efficiently transferring electrons and its preparation method and application
ActiveCN108057452BStable in natureImprove efficiencyWater/sewage treatment by irradiationWater treatment compoundsWastewaterSulfite
The invention relates to a self-assembled solar photocatalyst that efficiently transfers electrons and its preparation method and application. Through sol hydrothermal and calcination methods, in Er 3+ :Y 3 Al 5 O 12 @NiGa 2 O 4 and Bi 2 Sn 2 O 7 Insert MoS between 2 As a conductive channel, a new Z-shaped photocatalytic system is formed. The prepared Er 3+ :Y 3 Al 5 O 12 @NiGa 2 O 4 / MoS 2 / Bi 2 Sn 2 O 7 The composite material shows efficient and stable photocatalytic activity in the conversion process of nitrite and sulfite, and has broad application prospects in nitrite and sulfite wastewater treatment.
Owner:LIAONING UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com