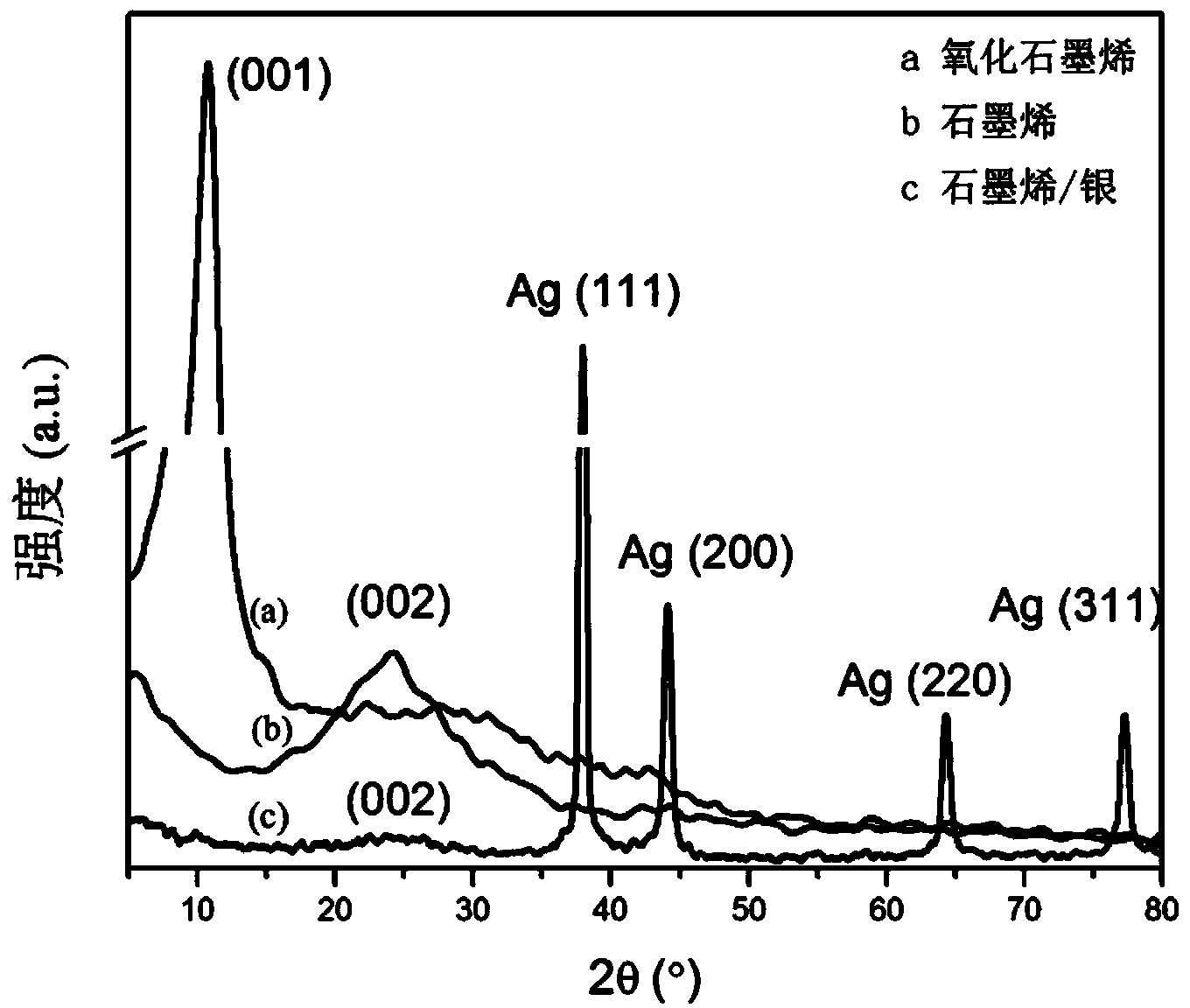
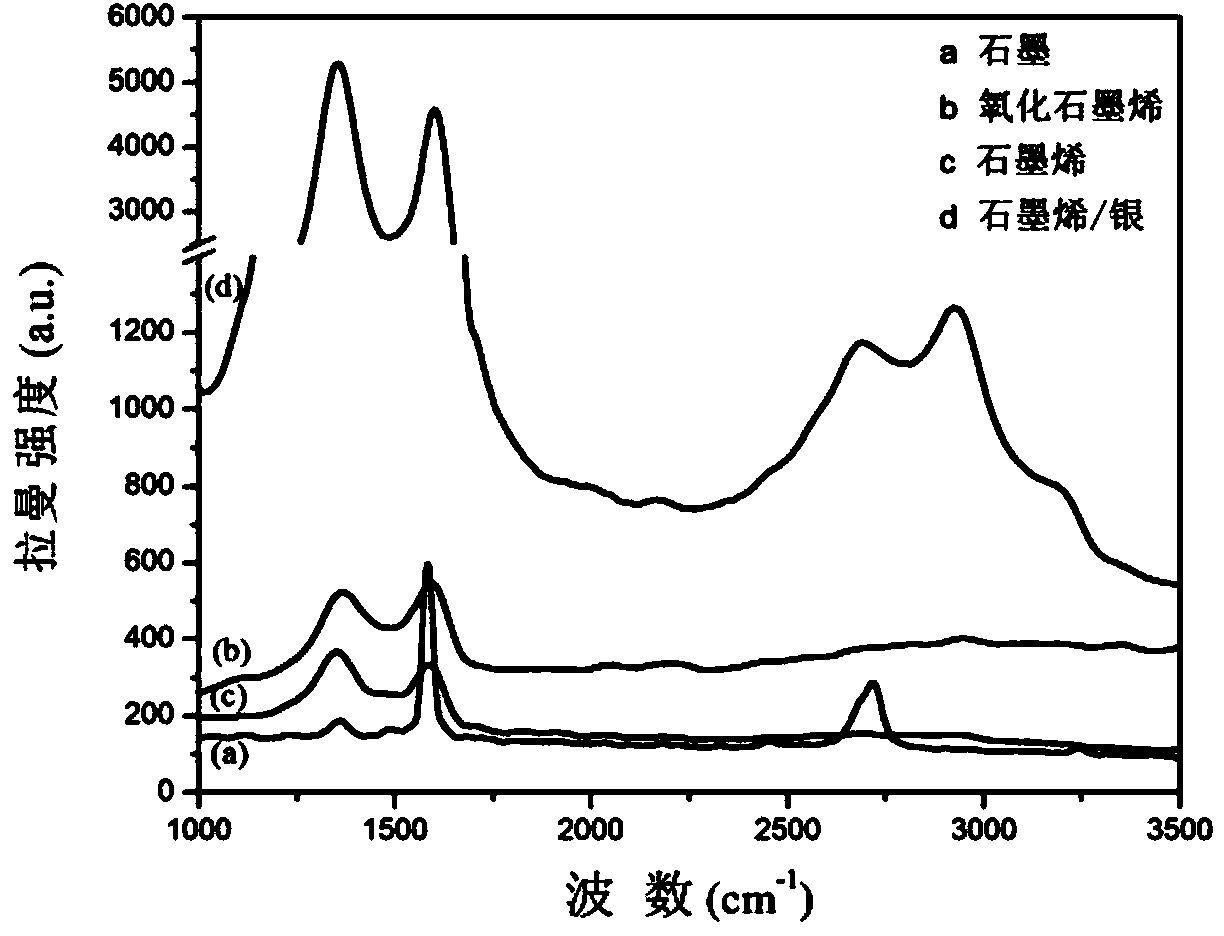
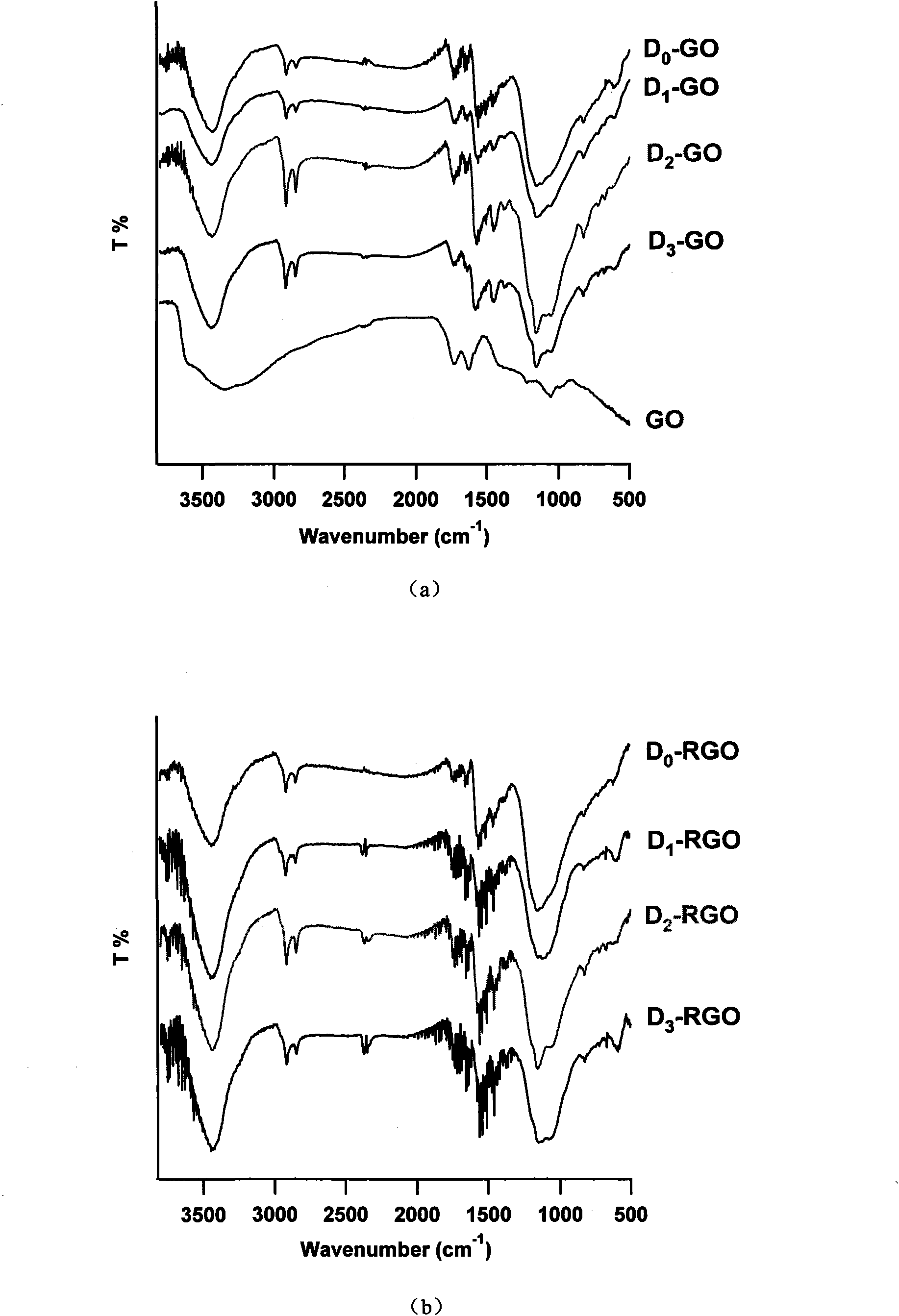
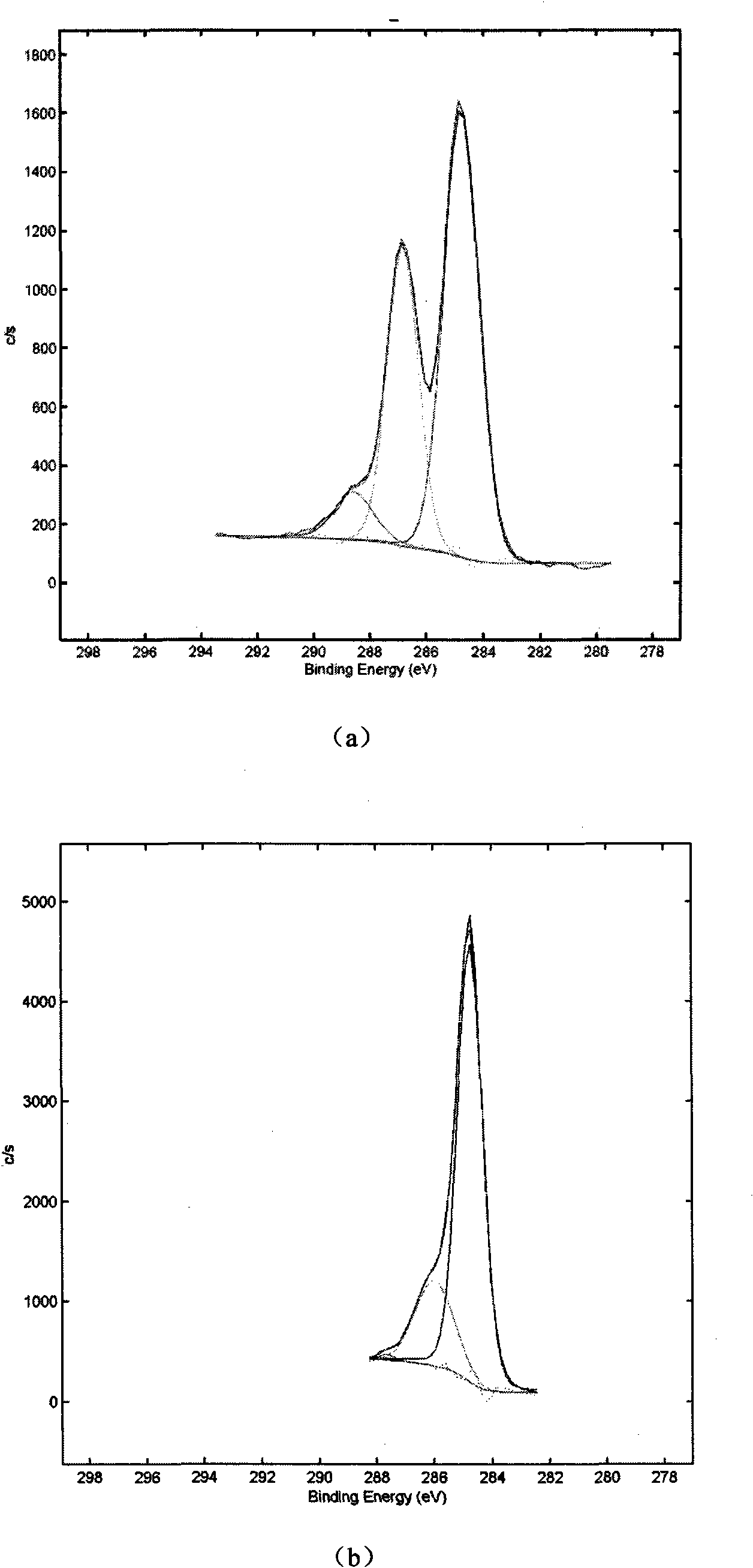
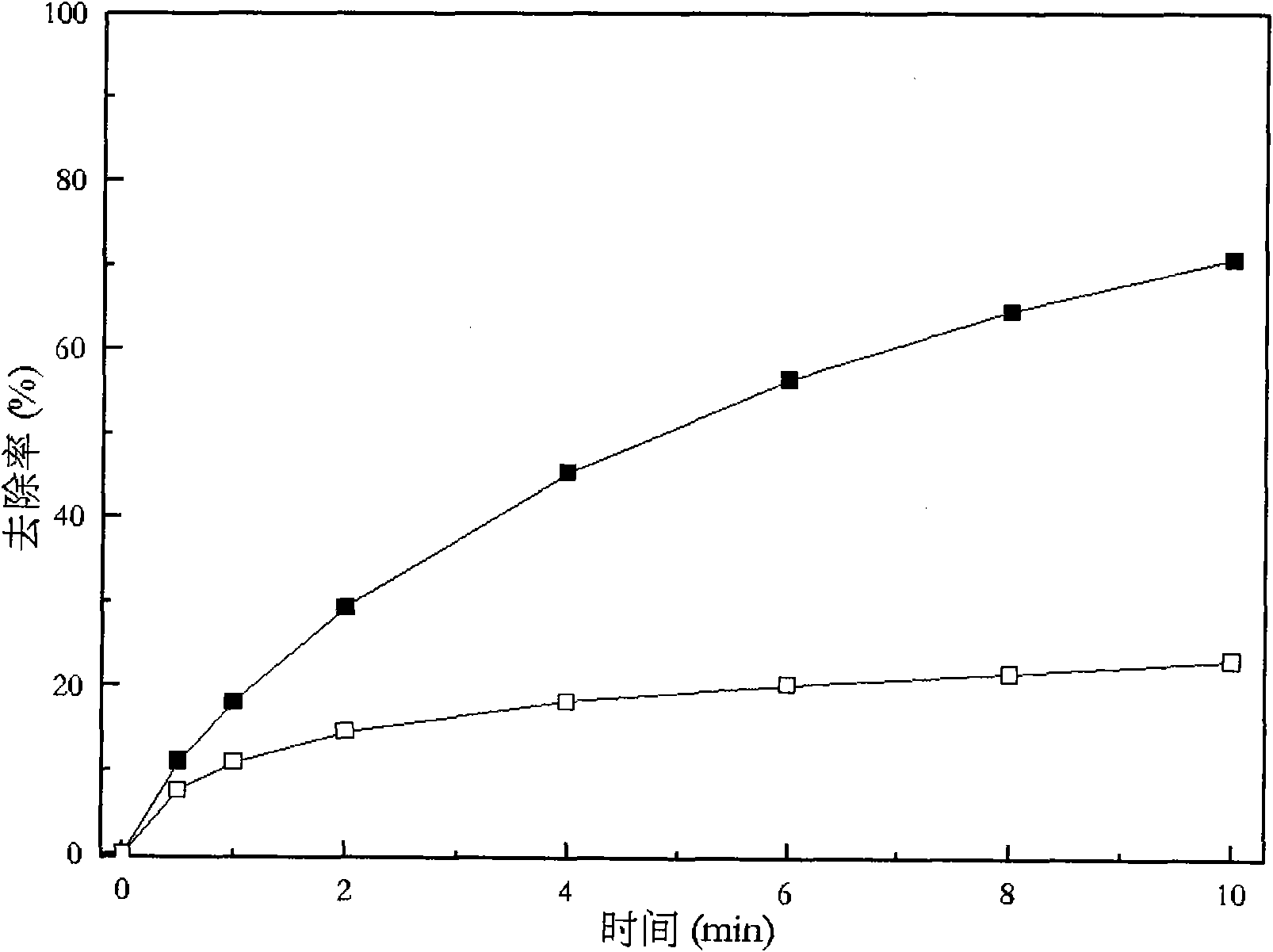
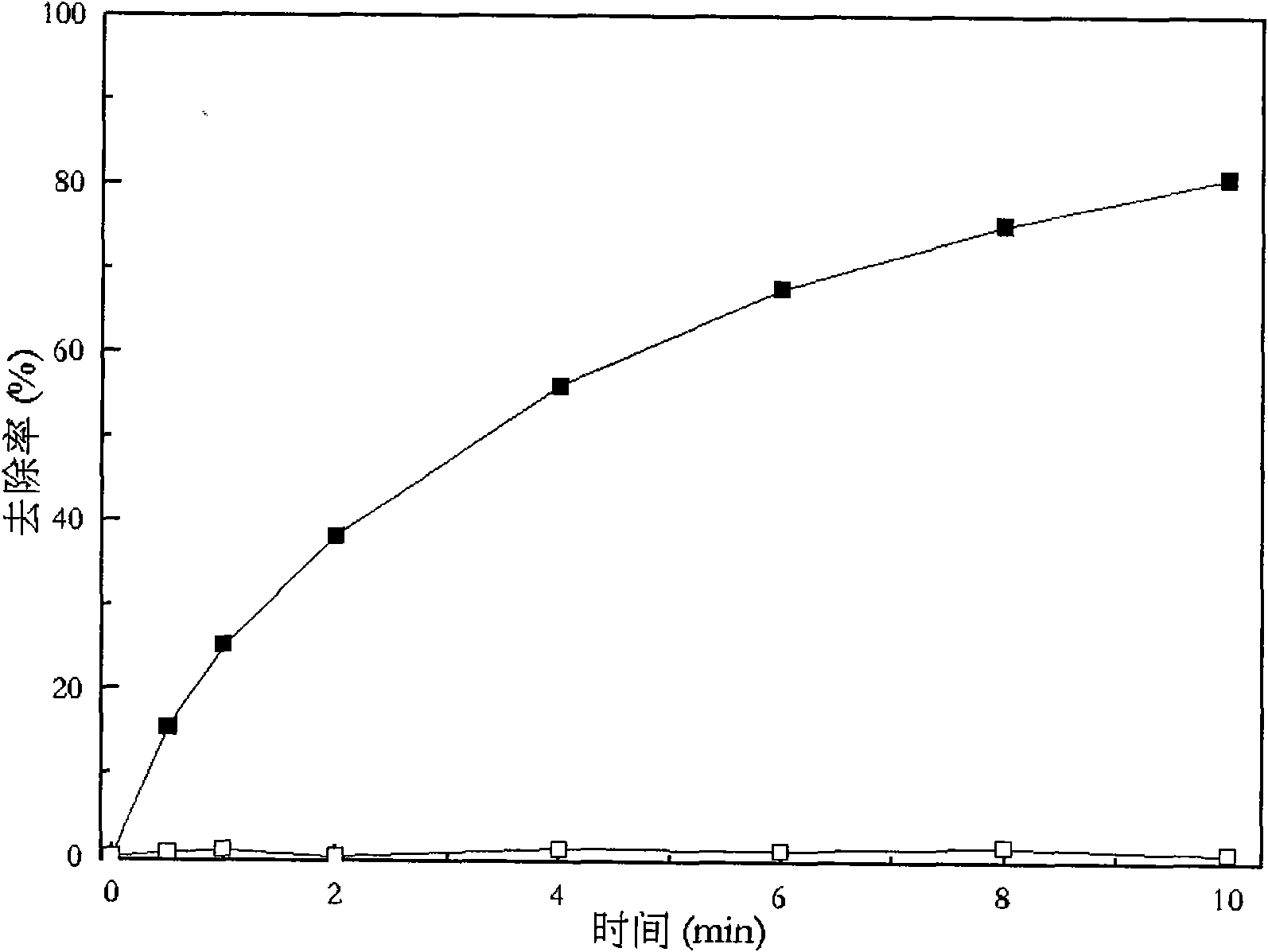
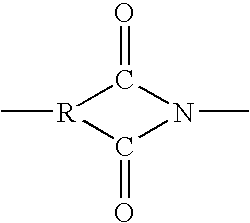
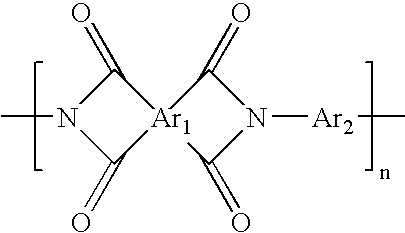
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2578 results about "Hydrazine compound" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrazine, also known as diazine, is a chemical compound. It is composed of nitrogen and hydrogen ions. Its chemical formula is N2H4. It contains hydrogen in its +1 and nitrogen in its -2 oxidation state.
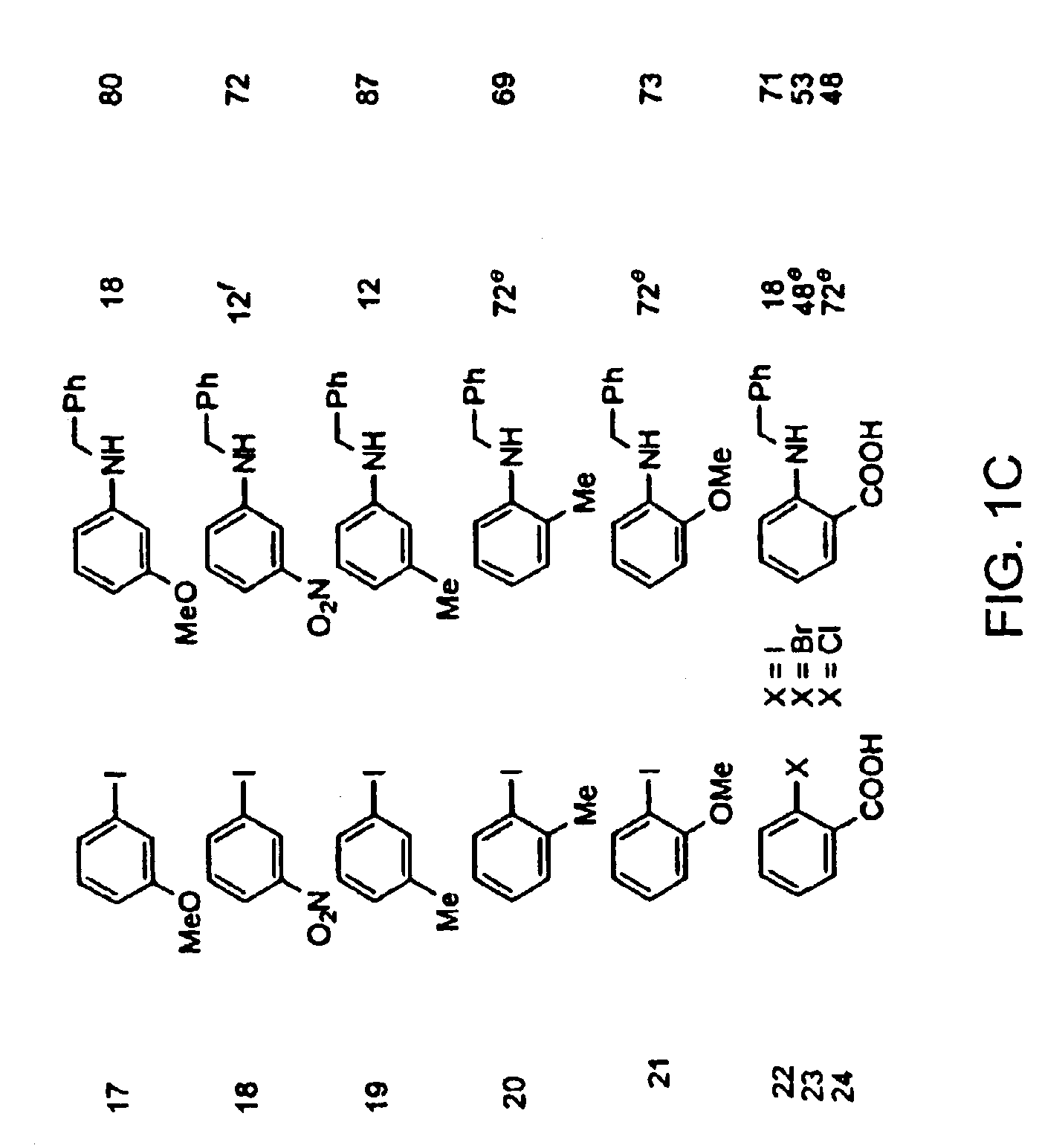
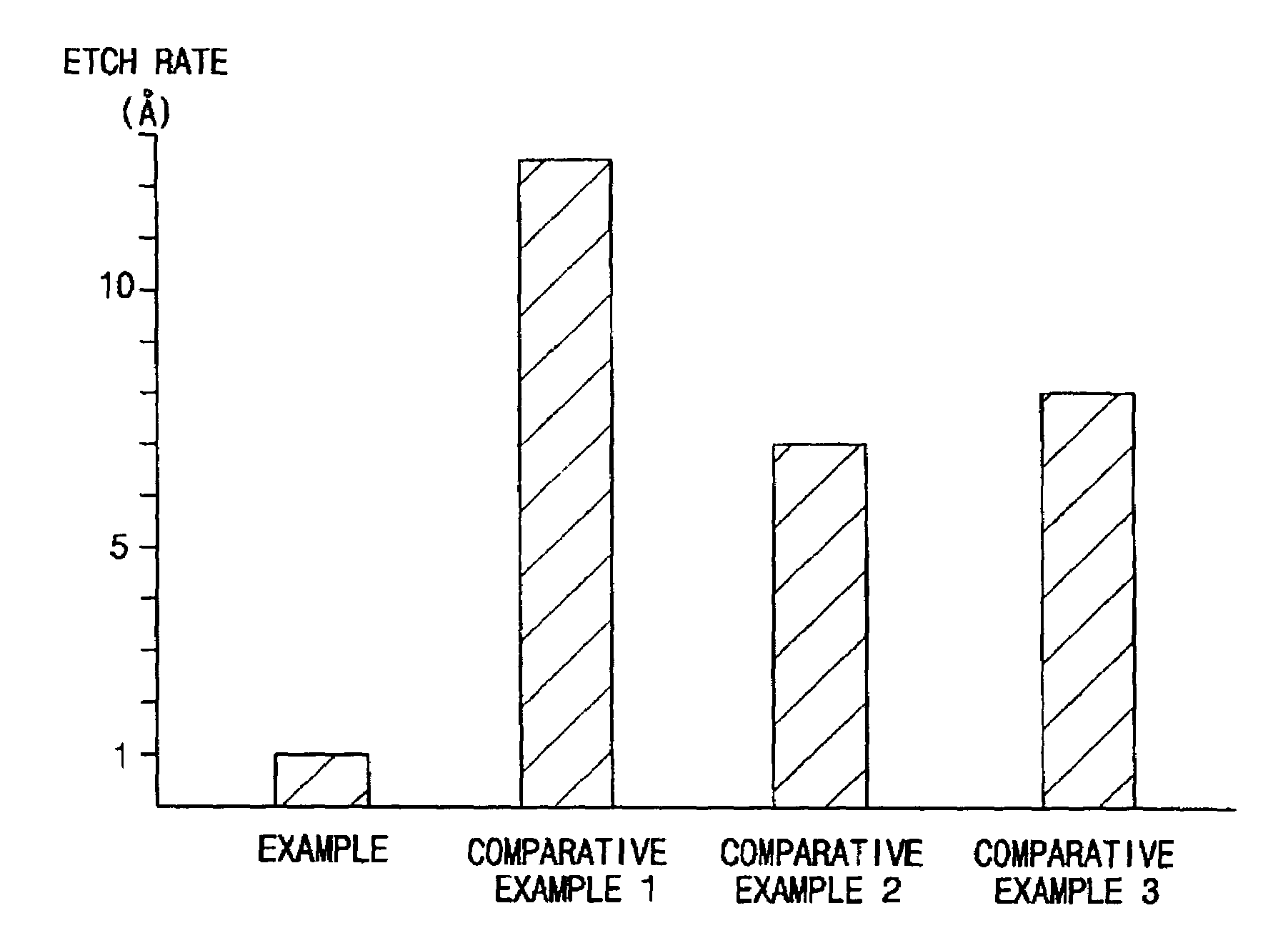
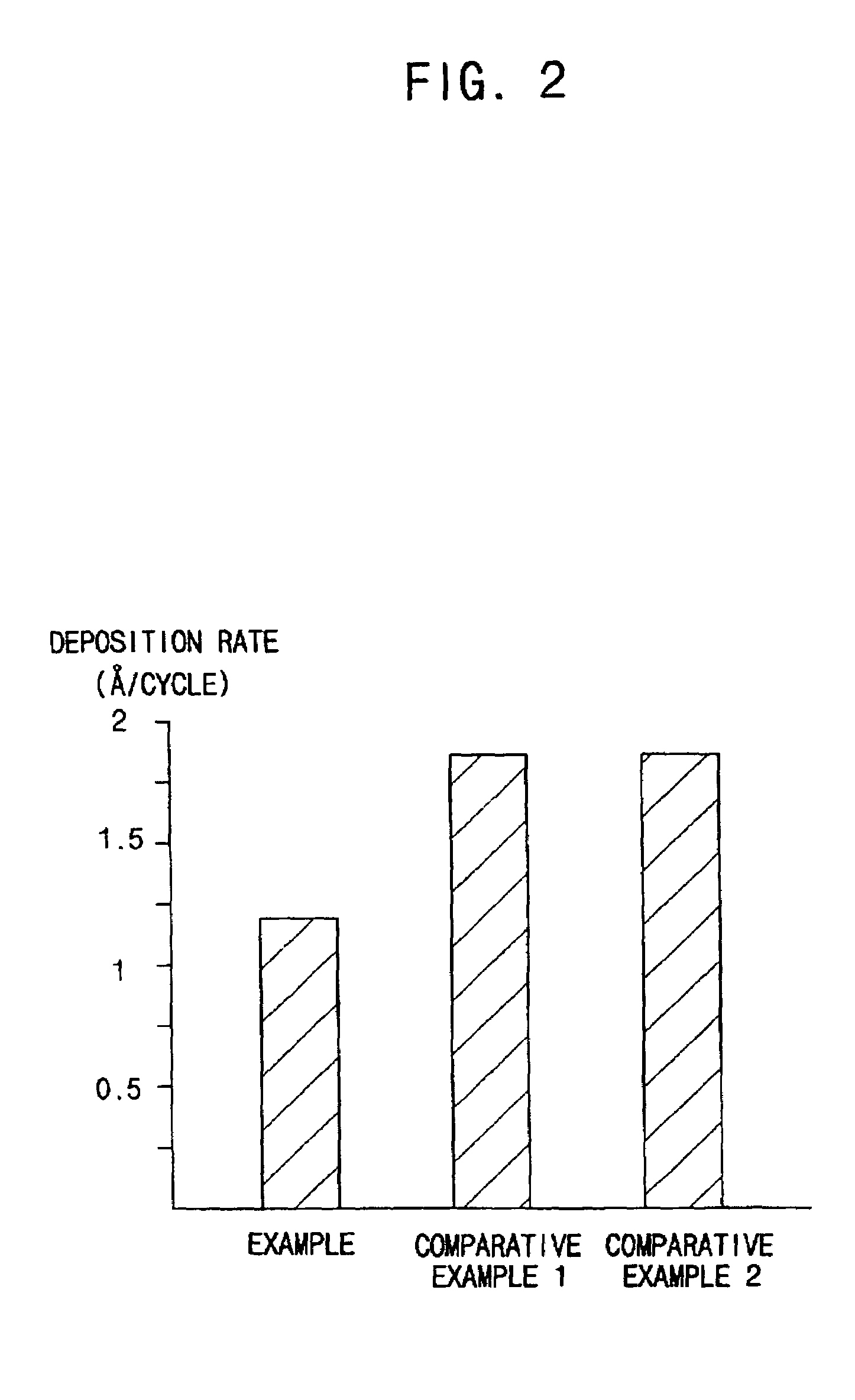
Methods for forming a metallic film on a substrate by a cyclical deposition and related semiconductor device structures
ActiveUS20190252196A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingHydrazine compoundOptoelectronics
Methods for forming a metallic film on a substrate by cyclical deposition are provided. In some embodiments methods may include contacting the substrate with a first reactant comprising a non-halogen containing metal precursor comprising at least one of copper, nickel or cobalt and contacting the substrate with a second reactant comprising a hydrocarbon substituted hydrazine. In some embodiments related semiconductor device structures may include at least a portion of a metallic interconnect formed by cyclical deposition processes.
Owner:ASM IP HLDG BV
Stabilized silver nanoparticles and their use
A process comprising: reacting a silver compound with a reducing agent comprising a hydrazine compound in the presence of a thermally removable stabilizer in a reaction mixture comprising the silver compound, the reducing agent, the stabilizer, and an optional solvent, to form a plurality of silver-containing nanoparticles with molecules of the stabilizer on the surface of the silver-containing nanoparticles.
Owner:XEROX CORP
Stabilized silver nanoparticles and their use
A process comprising: reacting a silver compound with a reducing agent comprising a hydrazine compound in the presence of a thermally removable stabilizer in a reaction mixture comprising the silver compound, the reducing agent, the stabilizer, and an optional solvent, to form a plurality of silver-containing nanoparticles with molecules of the stabilizer on the surface of the silver-containing nanoparticles.
Owner:XEROX CORP
Post clean treatment
InactiveUS6546939B1Inorganic/elemental detergent compounding agentsOrganic detergent compounding agentsHydroxylamineHydrazine compound
A composition for removal of chemical residues from metal or dielectric surfaces or for chemical mechanical polishing of a copper or aluminum surface is an aqueous solution with a pH between about 3.5 and about 7. The composition contains a monofunctional, difunctional or trifunctional organic acid and a buffering amount of a quaternary amine, ammonium hydroxide, hydroxylamine, hydroxylamine salt, hydrazine or hydrazine salt base. A method in accordance with the invention for removal of chemical residues from a metal or dielectric surface comprises contacting the metal or dielectric surface with the above composition for a time sufficient to remove the chemical residues. A method in accordance with the invention for chemical mechanical polishing of a copper or aluminum surface comprises applying the above composition to the copper or aluminum surface, and polishing the surface in the presence of the composition.
Owner:DUPONT AIR PRODS NANOMATERIALS
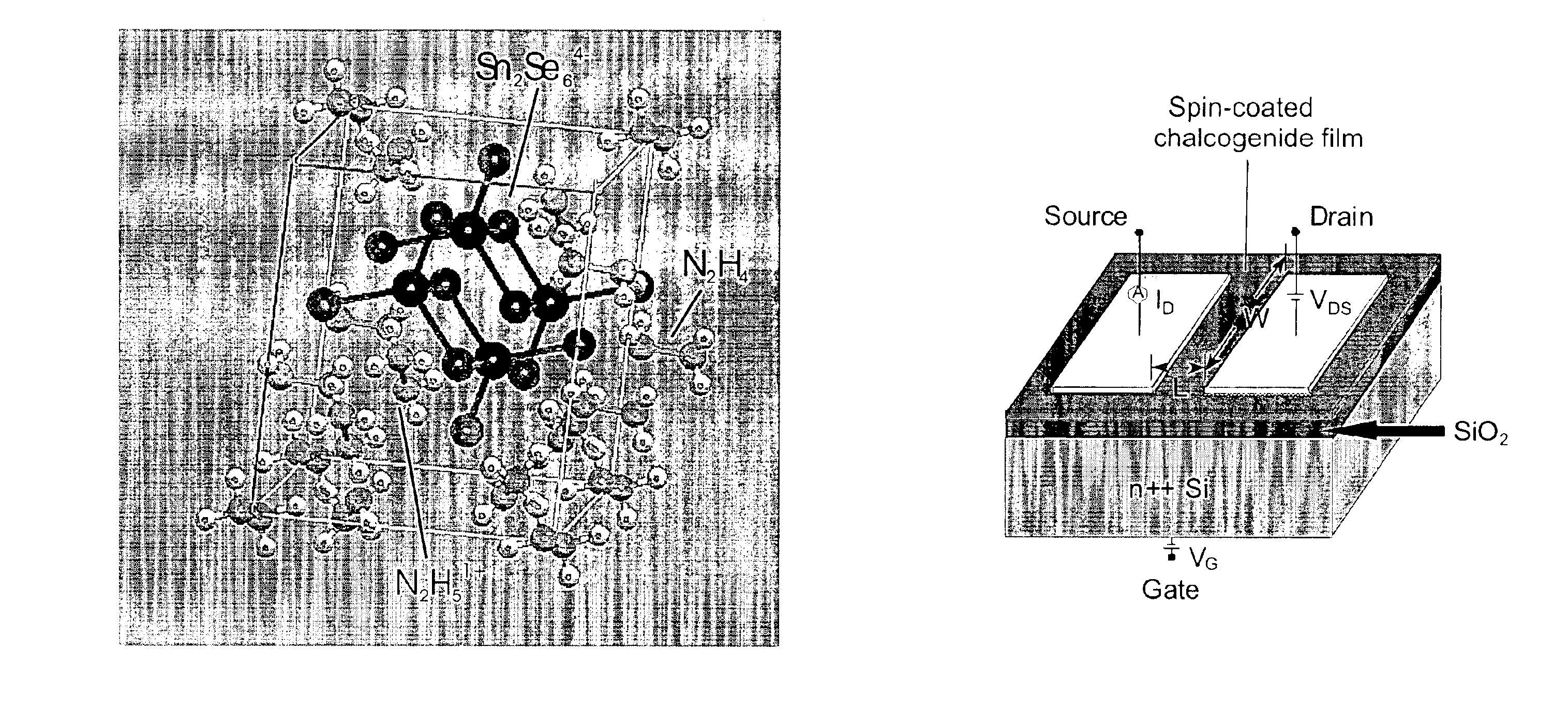
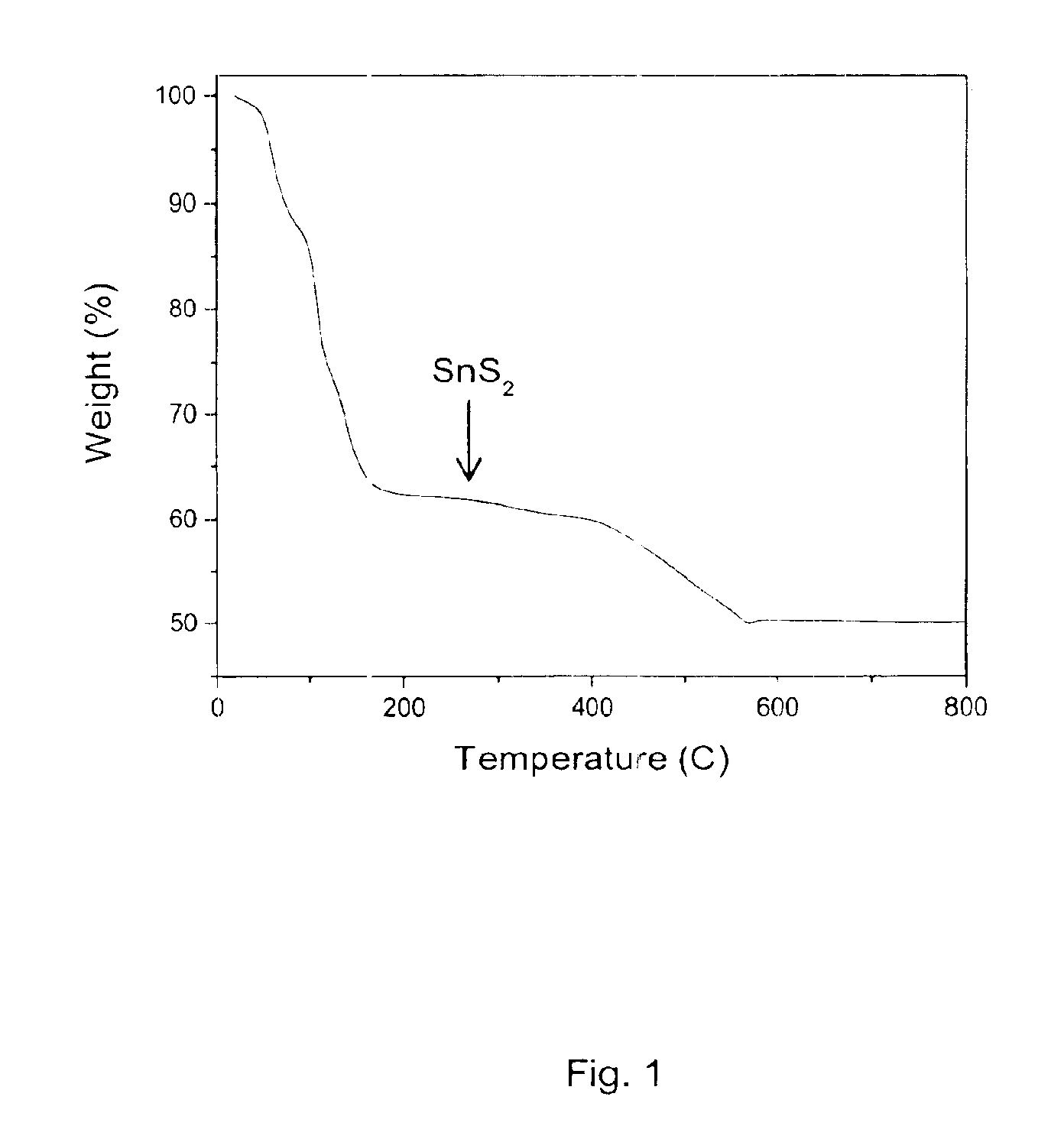
Solution deposition of chalcogenide films
A method of depositing a film of a metal chalcogenide. The first of these methods includes the steps of: contacting at least one metal chalcogenide, a hydrazine compound and optionally, an elemental chalcogen, to produce a solution of a hydrazinium-based precursor of the metal chalcogenide; applying the solution of the hydrazinium-based precursor of the metal chalcogenide onto a substrate to produce a film of the precursor; and thereafter annealing the film of the precursor to remove excess hydrazine and hydrazinium chalcogenide salts to produce a metal chalcogenide film on the substrate. The second of these methods includes the steps of: contacting: at least one metal chalcogenide and a salt of an amine compound to produce an ammonium-based precursor of the metal chalcogenide; contacting the ammonium-based precursor of the metal chalcogenide and a hydrazine compound, and optionally, an elemental chalcogen, to produce a solution of a hydrazinium-based precursor of the metal chalcogenide in the hydrazine compound; applying the solution of the hydrazinium-based precursor onto a substrate to produce a film; and thereafter, annealing to produce a metal chalcogenide film. Also provided is a thin-film field-effect transistor device using the metal chalcogenides as the channel layer.
Owner:GLOBALFOUNDRIES INC
Methods of chemically derivatizing single-wall carbon nanotubes
InactiveUS6841139B2Increase resistanceHigh yieldMaterial nanotechnologyPigmenting treatmentFiberLithium
This invention is directed to making chemical derivatives of carbon nanotubes and to uses for the derivatized nanotubes, including making arrays as a basis for synthesis of carbon fibers. In one embodiment, this invention also provides a method for preparing single wall carbon nanotubes having substituents attached to the side wall of the nanotube by reacting single wall carbon nanotubes with fluorine gas and recovering fluorine derivatized carbon nanotubes, then reacting fluorine derivatized carbon nanotubes with a nucleophile. Some of the fluorine substituents are replaced by nucleophilic substitution. If desired, the remaining fluorine can be completely or partially eliminated to produce single wall carbon nanotubes having substituents attached to the side wall of the nanotube. The substituents will, of course, be dependent on the nucleophile, and preferred nucleophiles include alkyl lithium species such as methyl lithium. Alternatively, fluorine may be fully or partially removed from fluorine derivatized carbon nanotubes by reacting the fluorine derivatized carbon nanotubes with various amounts of hydrazine, substituted hydrazine or alkyl amine. The present invention also provides seed materials for growth of single wall carbon nanotubes comprising a plurality of single wall carbon nanotubes or short tubular molecules having a catalyst precursor moiety covalently bound or physisorbed on the outer surface of the sidewall to provide the optimum metal cluster size under conditions that result in migration of the metal moiety to the tube end.
Owner:RICE UNIV
Chemically modifying single wall carbon nanotubes to facilitate dispersal in solvents
InactiveUS6875412B2High yieldIncrease resistanceMaterial nanotechnologyIndividual molecule manipulationFiberCarbon fibers
Owner:RICE UNIV
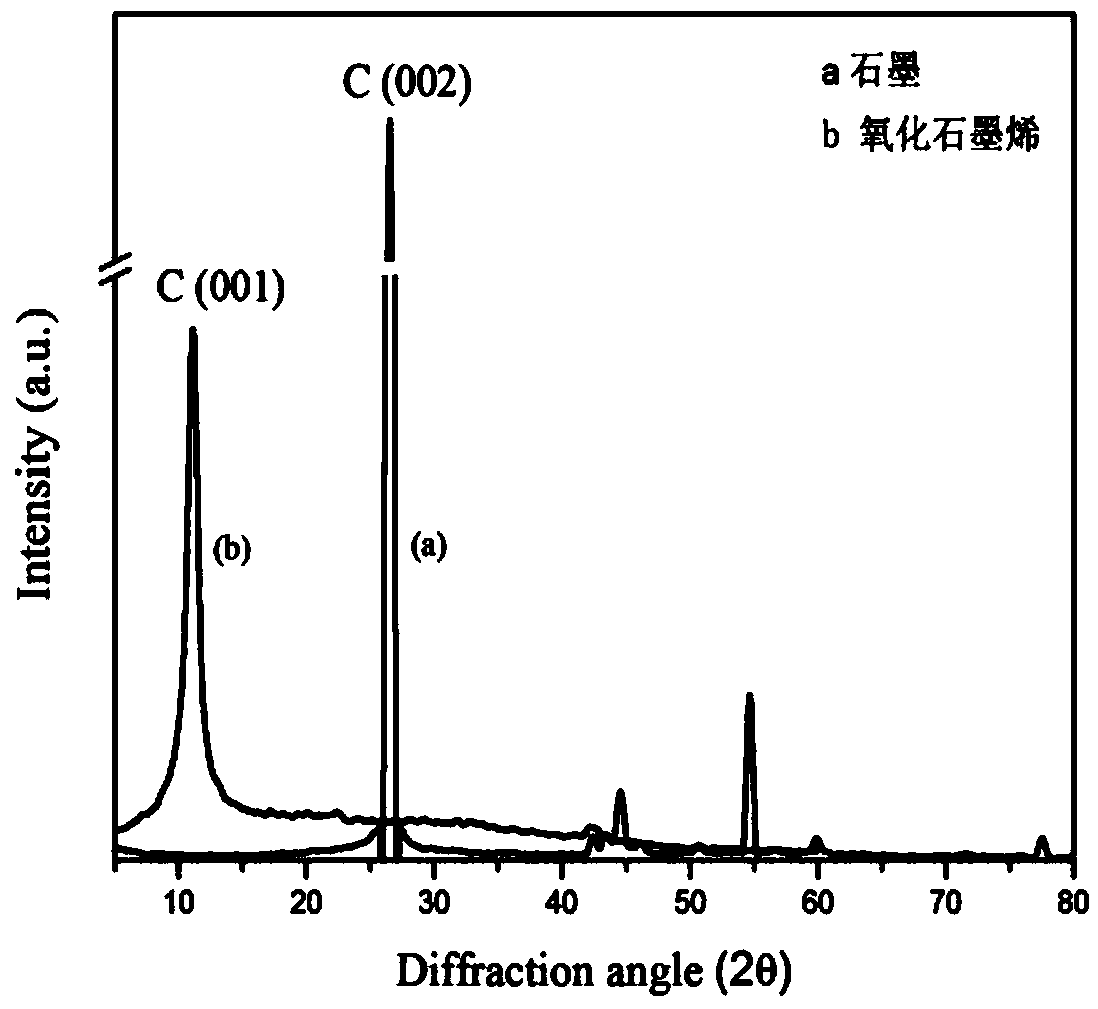
Method for preparing graphene loaded ferroferric oxide magnetic nanometer particle composite material
InactiveCN101941842AGood dispersionPromote crystallizationMaterial electrochemical variablesElectrodesHydrazine compoundNitrogen gas
The invention relates to a method for preparing a graphene loaded ferroferric oxide magnetic nanometer particle composite material. The method comprises the following steps of: (1) dispersing graphite oxide into deionized water, pouring into a vessel after ultrasonic and centrifuging, putting the vessel into an oil-bath pan, adding hydrazine hydrate and an anionic surfactant for heating, condensing and refluxing, and cooling and drying to obtain modified graphene; and (2) dispersing the graphene into aqueous solution, and ultrasonically stirring and centrifuging to obtain modified graphene dispersion liquid; and weighing a soluble trivalent ferric salt and divalent ferric salt, dissolving the salt into the deionized water, introducing nitrogen, pouring into the modified graphene dispersion liquid, dripping ammonia after uniform ultrasonic stirring, heating for reaction, washing and collecting a product, and drying the product. The method is simple and easy for industrial production; and the prepared composite material has the advantages of pure crystal phase, uniform distribution and excellent electrocatalytic activity.
Owner:DONGHUA UNIV
Aqueous-based method of forming semiconductor film and photovoltaic device including the film
InactiveUS20110097496A1Easy to controlOptimal rheologicalFinal product manufactureSemiconductor/solid-state device manufacturingHydrazine compoundThin layer
A method with enhanced safety characteristics of depositing a kesterite film, which includes a compound of the formula: Cu2−xZn1+ySn(S1−zSez)4+q, wherein 0≦x≦1; 0≦y≦1; 0≦z≦1; −1≦q≦1. The method includes contacting an aqueous solvent, ammonia, a source of hydrazine, a source of Cu, a source of Sn, a source of Zn, a source of at least one of S and Se, under conditions sufficient to form an aqueous dispersion which includes solid particles; applying the dispersion onto a substrate to form a thin layer of the dispersion on the substrate; and annealing at a temperature, pressure, and length of time sufficient to form the kesterite film. An annealing composition and a photovoltaic device including the kesterite film formed by the above method are also provided.
Owner:ELPIS TECH INC
Gas generants comprising transition metal nitrite complexes
InactiveUS6077371AMetal azide explosive compositionsAlkali metal salt explosive compositionsNitriteHydrazine compound
High nitrogen gas generant compositions, useful for inflating passenger restraint gas inflator bags, comprise a nitrogen rich coordination compound selected from coordination complexes comprised of anionic nitro and nitrito ligands coordinated with a transitional metal template, and nonmetallic or nonmetallic / metallic cations. The gas generant compositions generate relatively more gas and less solids, and are safer than known gas generant compositions. Certain gas generant compositions ignite at lower autoignition temperatures thereby facilitating the use of an aluminum or light weight metal pressure vessel. Other gas generants self-deflagrate eliminating the need for other constituents in the composition. Novel methods for the synthesis of nonmetal derivative coordination complexes, guanidine and hydrazine for example, are also presented.
Owner:AUTOMOTIVE SYST LAB +1
Hydrazine-free solution deposition of chalcogenide films
InactiveUS20050009225A1Efficient preparationLow costTransistorFrom solid stateHydrazine compoundSulfur
A method of depositing a film of a metal chalcogenide including the steps of: contacting an isolated hydrazinium-based precursor of a metal chalcogenide and a solvent having therein a solubilizing additive to form a solution of a complex thereof; applying the solution of the complex onto a substrate to produce a coating of the solution on the substrate; removing the solvent from the coating to produce a film of the complex on the substrate; and thereafter annealing the film of the complex to produce a metal chalcogenide film on the substrate. Also provided is a process for preparing an isolated hydrazinium-based precursor of a metal chalcogenide as well as a thin-film field-effect transistor device using the metal chalcogenides as the channel layer.
Owner:GLOBALFOUNDRIES US INC
Preparation of 2,2-bi-[4-(4-aminophenoxy)phenyl]propane
InactiveCN1472193AEasy to operateImprove working environmentOrganic compound preparationAmino-hyroxy compound preparationActivated carbonAlcohol
A process for preparing 2,2-bis-[4-(4-aminophenyloxy)phenyl] propane includes such steps as reflux reacting between 4,4'-dihydroxydiphenyl propane (BPA), 1-chloro-4-nitrobenzene (CNB) and salting agent in the mixture of non-protonic transferring polar solvent and dewatering agent at 110-150 deg.C to obtain 2,2-bis-[4-(4-nitrophenyloxy)phenyl] propane (BNPP), and reducing in alcohol as solvent under action of hexahydrated iron chloride, activated carbon and hydrazine hydrate. Its advantages are simple process, low cost and high quality and output rate of product.
Owner:TANSUO SCI & TECH NANTONG CITY
Process for microwave decomposition of hazardous matter
InactiveUS6187988B1Promote oxidationNitrogen compoundsLiquid separation by electricityActivated carbonHydrazine compound
This process occurs in the presence of activated carbon or its equivalent by decomposing adsorbed hazardous materials, such as hydrazine and microorganisms, on the carbon surface by radiofrequency energy in the microwave range at near ambient conditions of temperature and pressure. Further microwave oxidation to nonhazardous gases occurs in the presence of a microwaves enhanced oxidation catalyst.
Owner:CHA CHANG YUL
Synthesis of several metal selenides and tellurides as semiconductor material
InactiveCN1384047AOvercome the problems of high temperature, highly toxic raw materials, complicated process, etc.Low reaction temperatureSemiconductor/solid-state device manufacturingBinary selenium/tellurium compoundsSemiconductor materialsHydroxylamine
By using the soluble salt of transition metalz Zn, Cd, Pb, Mn, Co, Ni, Cu, Ag, Sb and Bi, selenious acid or its solutl salt, or antimonous acid or its soluble salt as raw material, and hydrazine hydrate, sodium borohydride, potassium borohydride, hydroxylamine or hydrazine sulfate as reductant, and through hydrothermal reduction reaction at 100-200 deg.c in a sealed container for 2 hr to 5 days, selenides or tellurides of the said metals as semiconductor material may be synthesized. Unlike available synthesis process, which needs high temperature, toxic feedstock and complex technological course, the present invention has the advantages of low-cost material, simple apparatus, easy control, good technological reproducibility, stable product quality, etc.
Owner:TSINGHUA UNIV
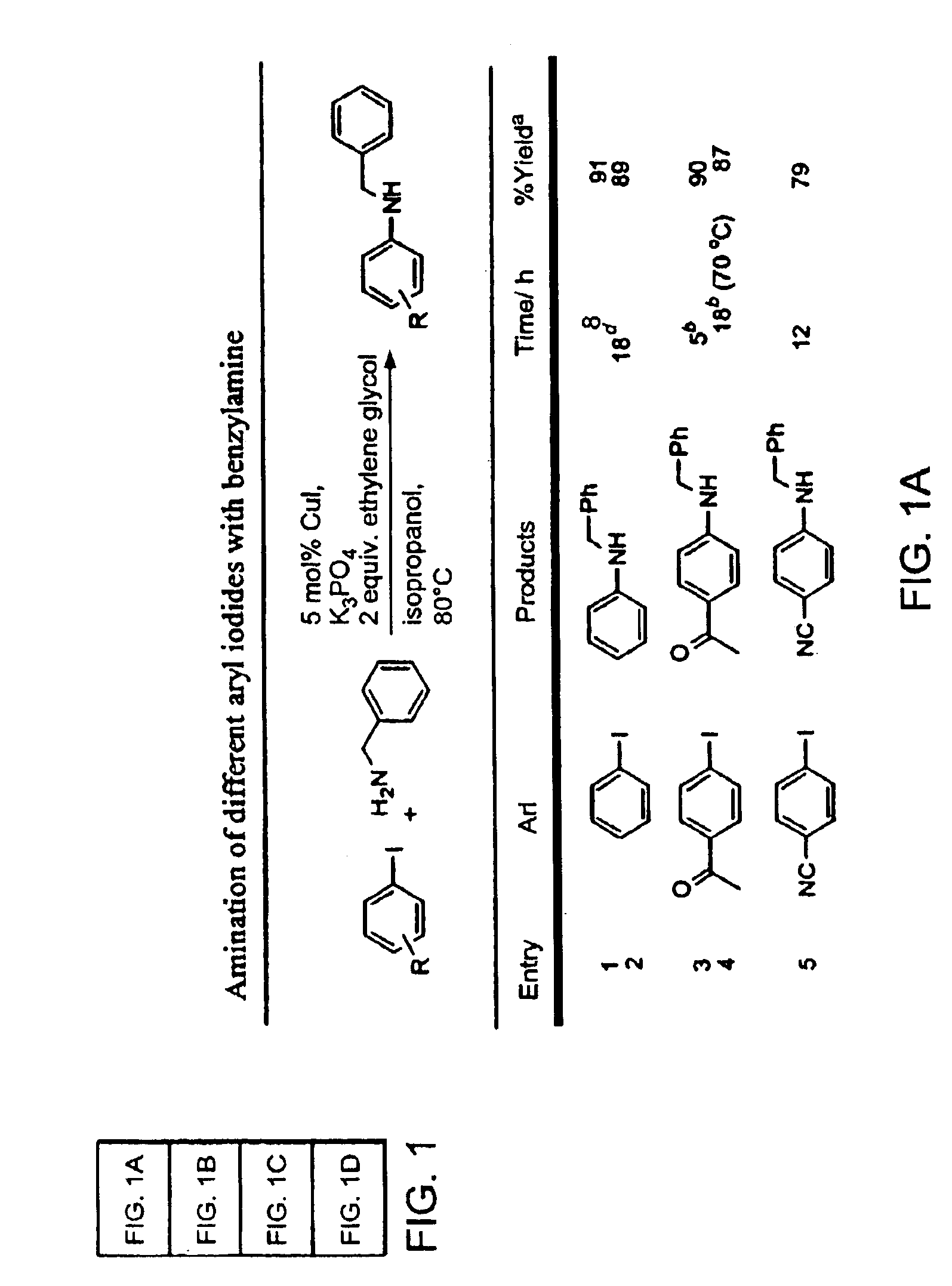
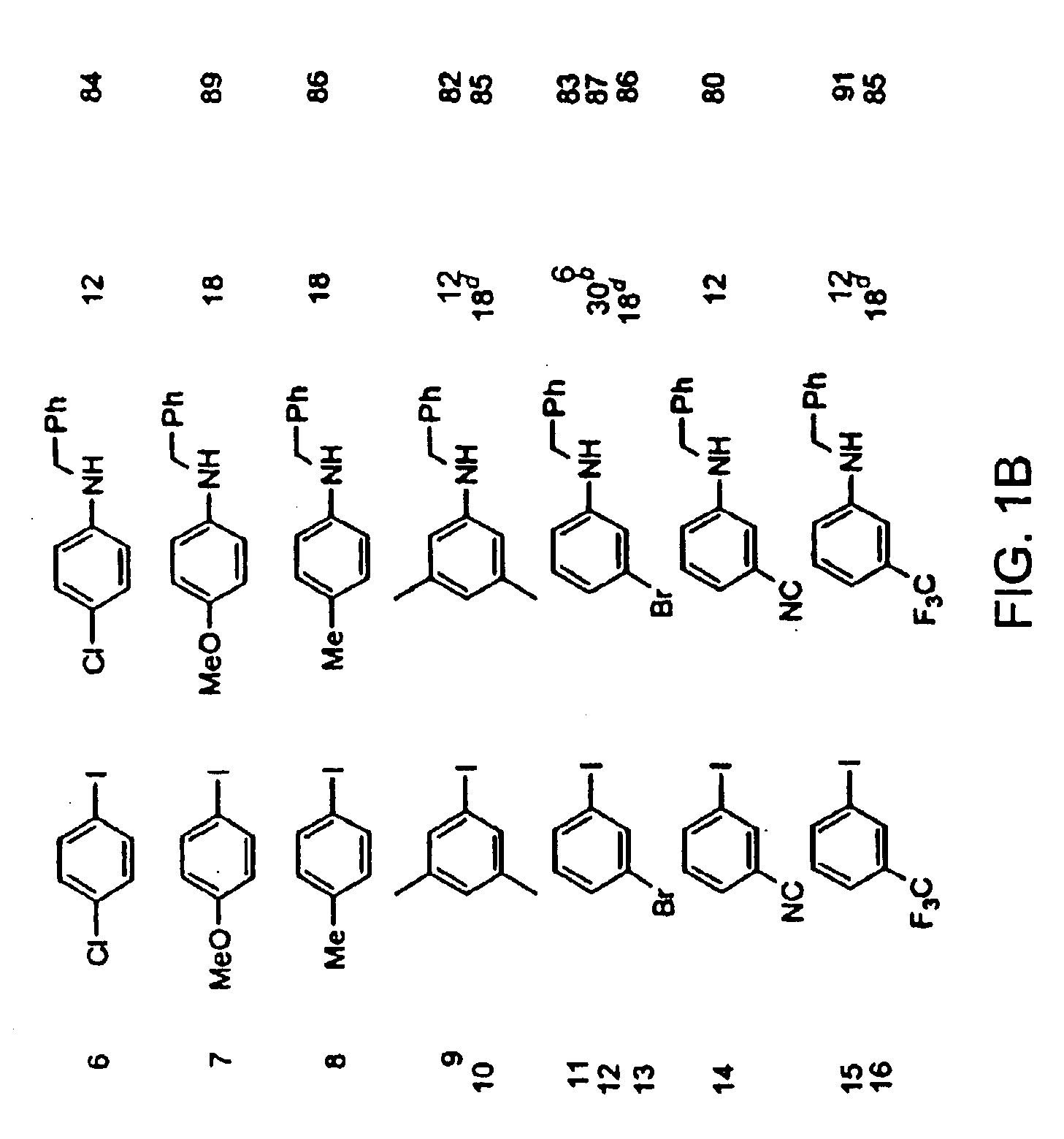
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
InactiveUS6867298B2Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
Methods of forming silicon nitride layers using nitrogenous compositions
The present invention provides nitrogenous compositions for forming a silicon nitride layer, wherein the nitrogenous composition comprises a hydrazine compound, an amine compound or a mixture thereof. The present invention further provides source compositions for forming a silicon nitride layer, wherein the source composition comprises a nitrogenous composition comprising a hydrazine compound, an amine compound or a mixture thereof, and a silicon source comprising hexachlorodisilane. Methods for forming silicon nitride layers are further provided. The silicon nitride layers provided herein may be formed on a substrate at a low temperature and may further exhibit improved breakdown voltage and an enhanced etch resistance.
Owner:SAMSUNG ELECTRONICS CO LTD
Preparation method for high-conductivity graphene and silver nanoparticle composite materials
ActiveCN103639421AEnhanced surface contactGuaranteed electrical conductivityMaterial nanotechnologyFreeze-dryingHydrazine compound
The invention relates to a preparation method for high-conductivity graphene and silver nanoparticle composite materials. The method comprises the steps that graphene oxide is prepared at first; a graphene oxide water solution is prepared; silver nitrate is added into the obtained graphene oxide water solution, the temperature is risen to be 90+ / -10 DEG C, sodium citrate is added in the solution, and stirring and reaction are conducted; ammonium hydroxide and hydrazine hydrate are added into the obtained solution, and stirring and reaction are conducted at the temperature of 90+ / -10 DEG C; deionized water and ethyl alcohol are adopted to clean the reaction product, vacuum freeze drying is conducted on the cleaned reaction product, and then the conductive graphene and silver nanoparticle composite materials are obtained. The conductivity of the composite materials is 3.71-18.32S / cm, the graphene and silver nanoparticle composite materials prepared based on the method can greatly improve the conductivity of graphene, and can be further applied in the field of printed electronics.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
A kind of preparation method of graphene and manganese dioxide nanocomposite material
InactiveCN102275903AReduce typesLow costMaterial nanotechnologyManganese oxides/hydroxidesHydrazine compoundManganese
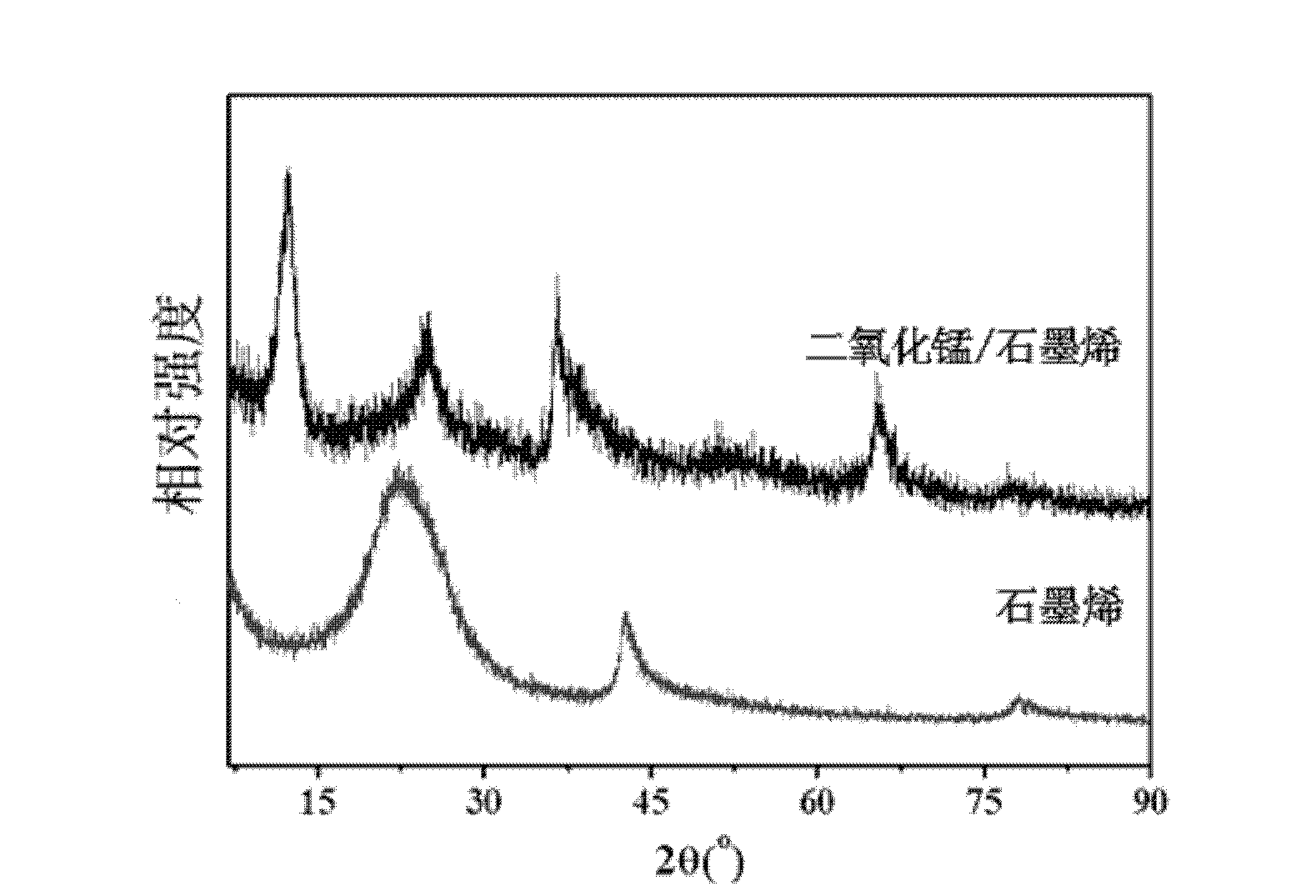
The invention relates to a preparation method of a graphene and manganese dioxide nanocomposite material, comprising: (1) stirring and mixing graphite, potassium nitrate and concentrated sulfur evenly, adding potassium permanganate, and reacting at 30-40° C. for 20-40 minutes , add deionized water at room temperature, add hydrogen peroxide after reacting for 15 to 30 minutes to obtain graphite oxide; (2) disperse the above graphite oxide in water, add hydrazine hydrate, and react at 95°C for 1 to 24 hours to obtain graphene (3) ultrasonically disperse the graphene in a saturated potassium permanganate solution, add acid, and react at 60-80° C. for 1-5 hours to obtain graphene and manganese dioxide nanocomposite material. The invention has the advantages of simple reaction, easy control, convenient operation and simple process; the obtained composite material has broad application prospects and can be used for catalysts, biosensing materials, electrode materials of lithium ion batteries and supercapacitor electrode materials, and the like.
Owner:DONGHUA UNIV
Bi-functional oxygen catalyst for graphene/nickel iron type hydrotalcite as well as preparation method and application thereof
InactiveCN105618060AImprove conductivityIncrease the areaCell electrodesMetal/metal-oxides/metal-hydroxide catalystsPorous grapheneHydrazine compound
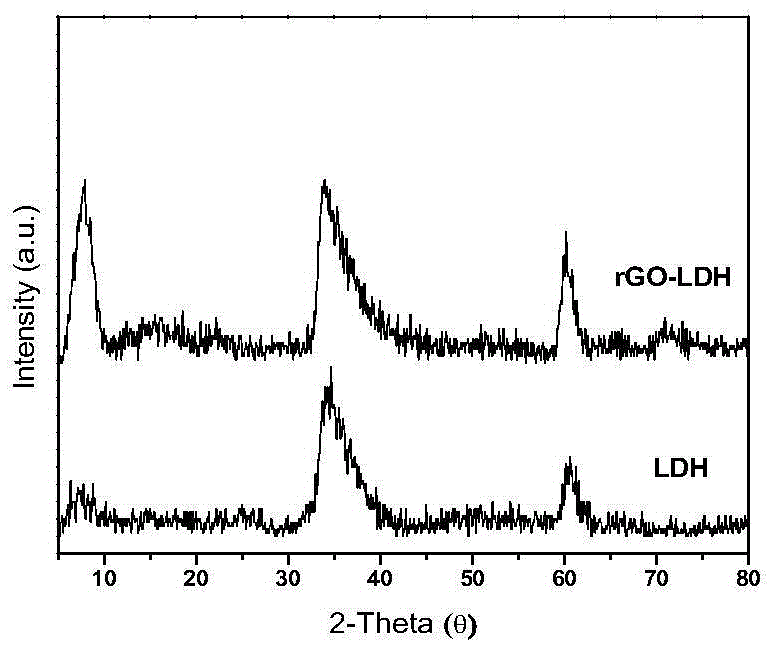
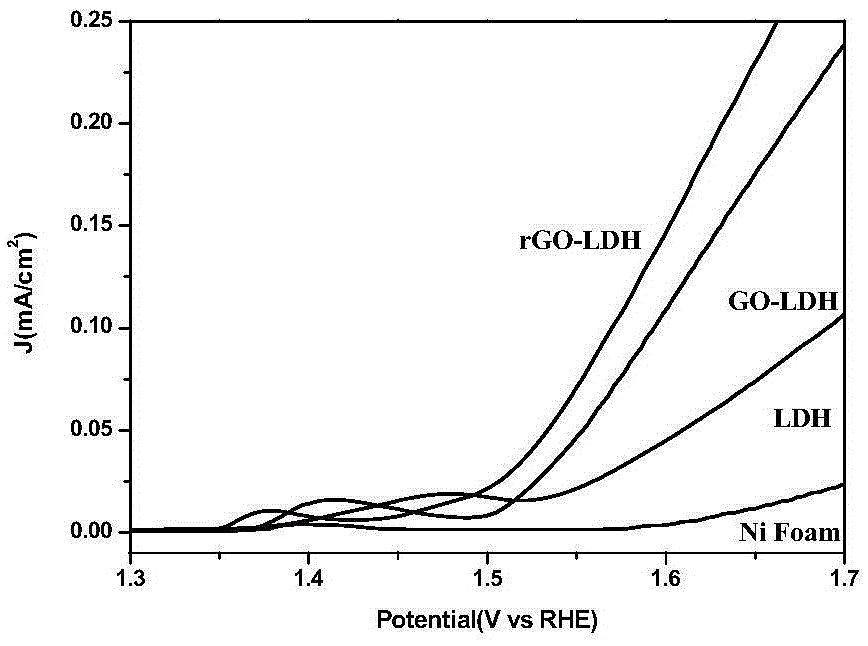
The invention relates to a non-metallic bi-functional oxygen catalyst for graphene / nickel iron type hydrotalcite as well as a preparation method and electric catalytic application thereof to oxygen evolution reaction and oxygen reduction reaction in an alkaline medium. The catalyst takes a micelle as a template, and under the hydrothermal and reducing conditions, the nickel iron type hydrotalcite is assembled onto graphene in sequence to form a spherical porous graphene oxide / nickel iron type hydrotalcite compound. The method comprises the following steps: dispersing graphene oxide and metal salt in the micelle, introducing an alkali source, synthesizing the graphene oxide / nickel iron type hydrotalcite compound under the hydrothermal conditions, and performing hydrazine hydrate reduction on an obtained product to obtain the catalyst. The catalyst prepared by the method has high oxygen evolution and oxygen reduction catalytic activity, good stability and excellent methanol tolerance under the alkaline conditions and is low in cost of raw materials used, simple in preparation method, easy to operate and convenient for large-scale production.
Owner:杭州新灵峰润滑油有限公司
Preparation method of grapheme capable of dispersing in organic solvent
ActiveCN101863465AExcellent solvent dispersion performanceEasy to synthesizeOrganic solventHydrazine compound
The invention relates to a preparation method of grapheme capable of dispersing in an organic solvent, comprising chemical reduction of a graphene oxide stem grafting arborization substituent group and graphene oxide. In the invention, the arborization substituent group with huge volume is utilized to functionalize graphene oxide, thereby the obtained graphene oxide can be dispersed in a majority of organic solvents, and after the graphene oxide is reduced by hydrazine hydrate, the obtained graphene still keeps excellent organic solvent dispersibility. In the invention, excess reactants, impurities and solvent in the preparation process of a solution method are removed by utilizing means of filtering and washing so that a purified graphene product is obtained, and the graphene product is powdery, is convenient to store and transport, and meets the requirement of preparation in macroscopic quantity. The graphene prepared by utilizing the invention has excellent solvent dispersibility, therefore, a powdery graphene sample can be dispersed in a specific organic solvent again as required to obtain required graphene sol for large-scale application.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Fenton and Fenton-like system fortifier and using method thereof
ActiveCN101792205AReduce dosageEasy to useWater treatment parameter controlWater treatment compoundsSulfite saltHydrazine compound
The invention provides a Fenton and Fenton-like system fortifier and a using method thereof, which relate to a water treating fortifier and a using method thereof and overcomes the defects that the pH value of the Fenton and Fenton-like system reaction water body is limited, the adding amount of Fe<2+> is overhigh in the Fenton reaction, and the reaction speed is low in Fenton-like reaction. The fortifier is selected from ascorbic acid, sodium sulfite, lithium sulfite, potassium sulfite, magnesium sulfite, calcium sulfite, hydroxylamine, hydroxyl-ammonium perchlorate, hydroxylamine sulfate, hydrazine, N,N-diethylhydroxylamine, carbohydrazide, ethanolamine, a hydroxylamine solution or N,N,N',N'-tetra substituted phenylenediamines. The using method has the following steps of: adding the Fenton and Fenton-like system fortifier, fortificated chemicals and peroxide into the treated water body; and then, uniformly stirring to react. The fortifier can accelerate the reaction of water treatment and decrease the using amount of the fortificated chemicals.
Owner:HARBIN INST OF TECH
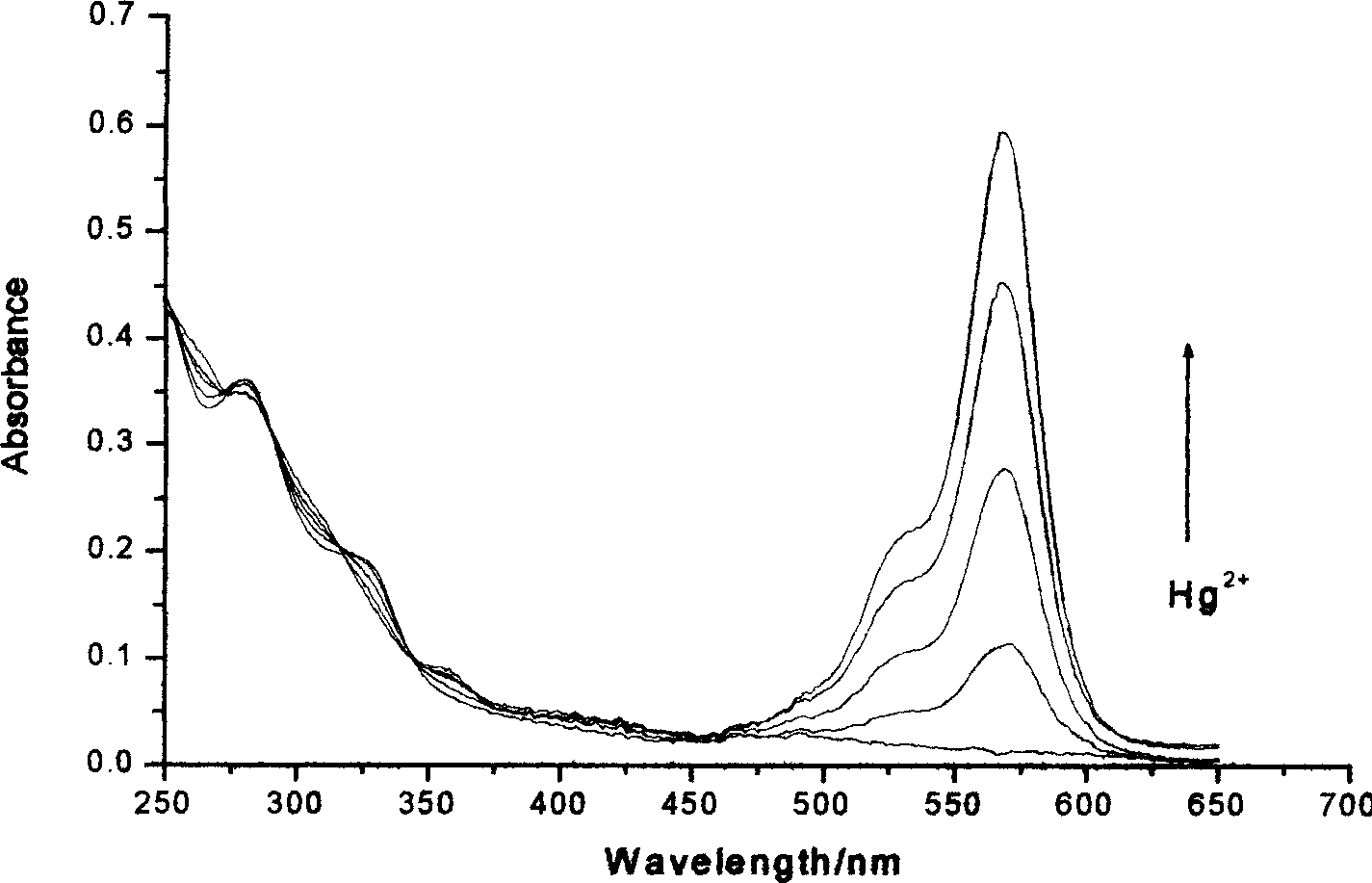
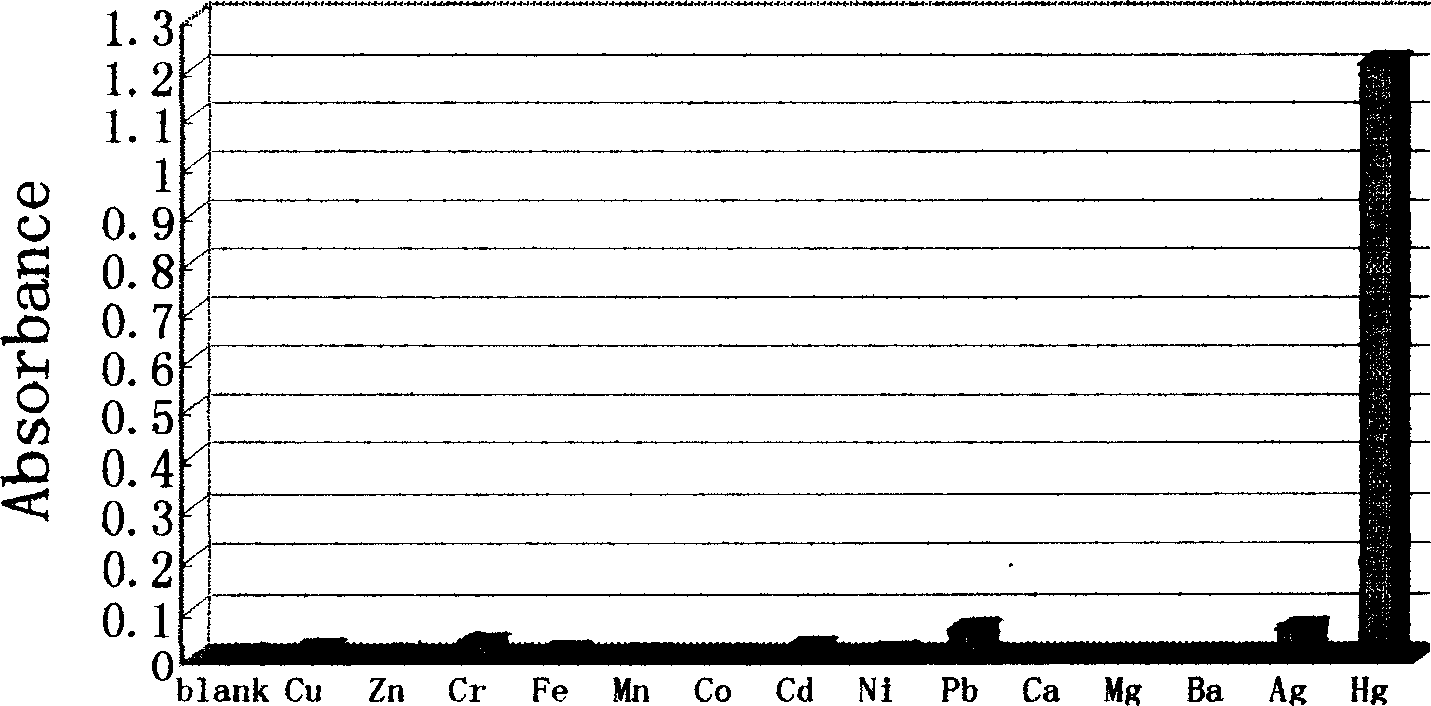
Reagent for detecting mercury ion in water and its preparation method
InactiveCN1752750ADetection fieldQuick checkComponent separationChromatographic separationSynthesis methods
Owner:XIAMEN UNIV
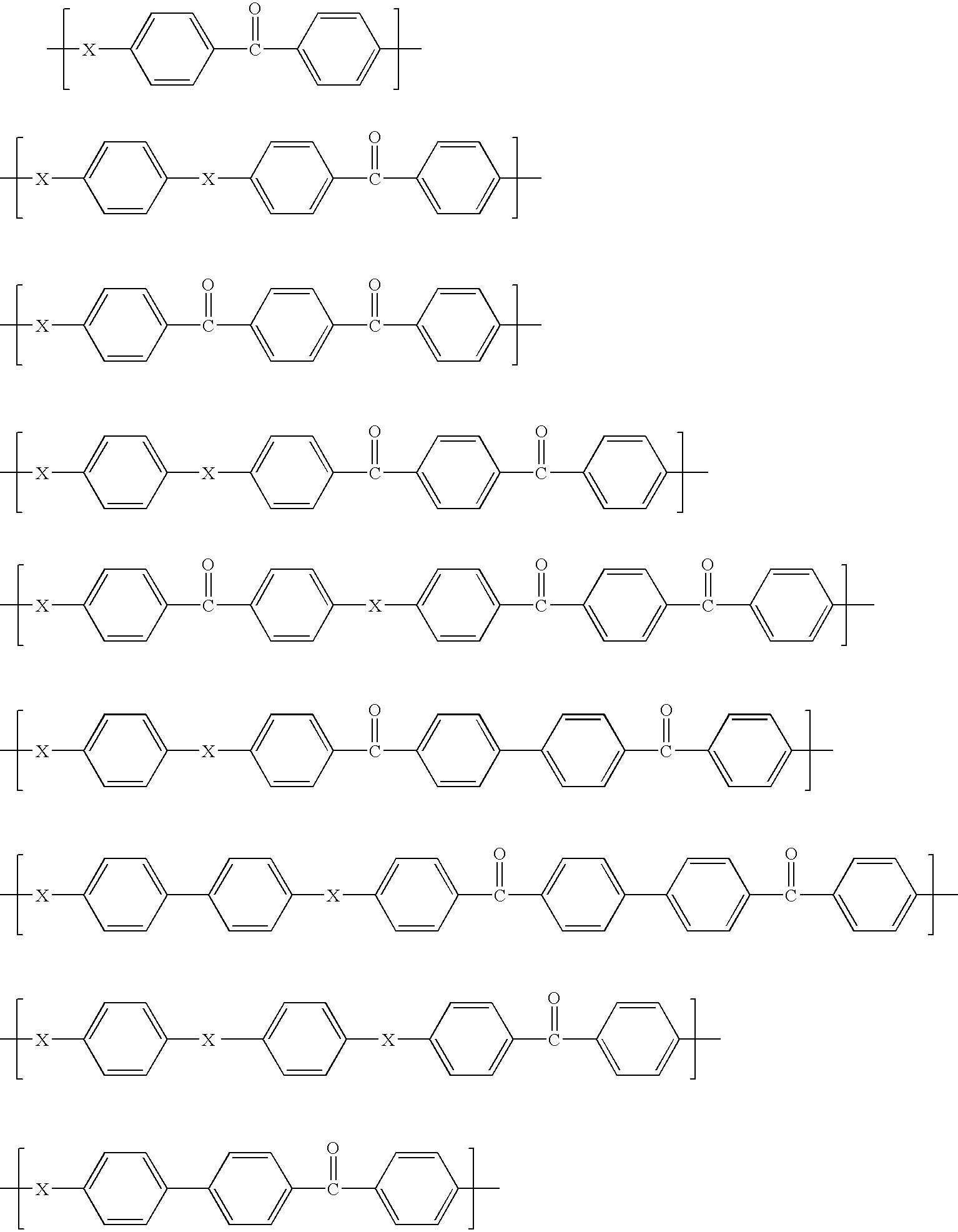
Functionalized porous poly(aryl ether ketone) materials and their use
ActiveUS20060094852A1Good chemical resistanceSimple and cost-effective and industrially feasibleMembranesSemi-permeable membranesPolymer scienceHydrazine compound
Functionalized porous poly(aryl ether ketone) articles are prepared by reacting ketone groups in the backbone of poly(aryl ether ketone) polymer with a primary amine reagent. Preferred functional primary amines are primary aliphatic amines or substituted hydrazines containing one or more target functional groups including polar groups, such as hydroxyl groups, ˜OH, amino groups, ˜NH2, ˜NHR, ˜NRR′, and ethylene oxide groups, ˜OCH2CH2—, negatively or positively charged ionic groups, such as ˜SO3−, ˜COO−, and ˜NH4+ groups, hydrophobic groups such as siloxane or perfluorcarbone groups, and non-polar groups, such as linear or branched hydrocarbon groups. The functionalized porous poly(aryl ether ketone) article can be prepared by reacting primary amine with a pre-formed, shaped porous poly(aryl ether ketone) article or by functionalizing the surface of a non-porous precursor article that is subsequently converted into a porous article.
Owner:MASSACHUSETTS DEV FINANCE AGENCY
Composite article of aluminum alloy with resin and method for production thereof
ActiveUS20060127684A1Effective combinationExhibit some effectSynthetic resin layered productsPaper/cardboard layered productsHydrazine compoundElectrical devices
The present invention allows both the advantages of a metallic housing and those of a synthetic resin structure to be exhibited in electronic devices, home electrical devices, etc., and achieves high productivity and mass productivity and further enables a desired configuration and structure to be designed freely. As a pretreatment, a shaped aluminum alloy material is dipped in an aqueous solution of at least one selected from the group consisting of ammonia, hydrazine, and a water-soluble amine compound. A thermoplastic resin composition containing polyphenylene sulfide as a component is integrally bonded to the surface of the treated shaped aluminum alloy material by injection molding or other method. The molded article is a product made of the shaped aluminum alloy material and the thermoplastic resin composition containing PPS. Thus, the characteristic features of metal can be utilized in terms of mechanical strength and external appearance design. Moreover, a complicated configuration and structure can be formed inside the housing.
Owner:TAISEI PLAS CO LTD
Method for preparing nano-Pd or Pd platinum alloy electrocatalyst for fuel cell
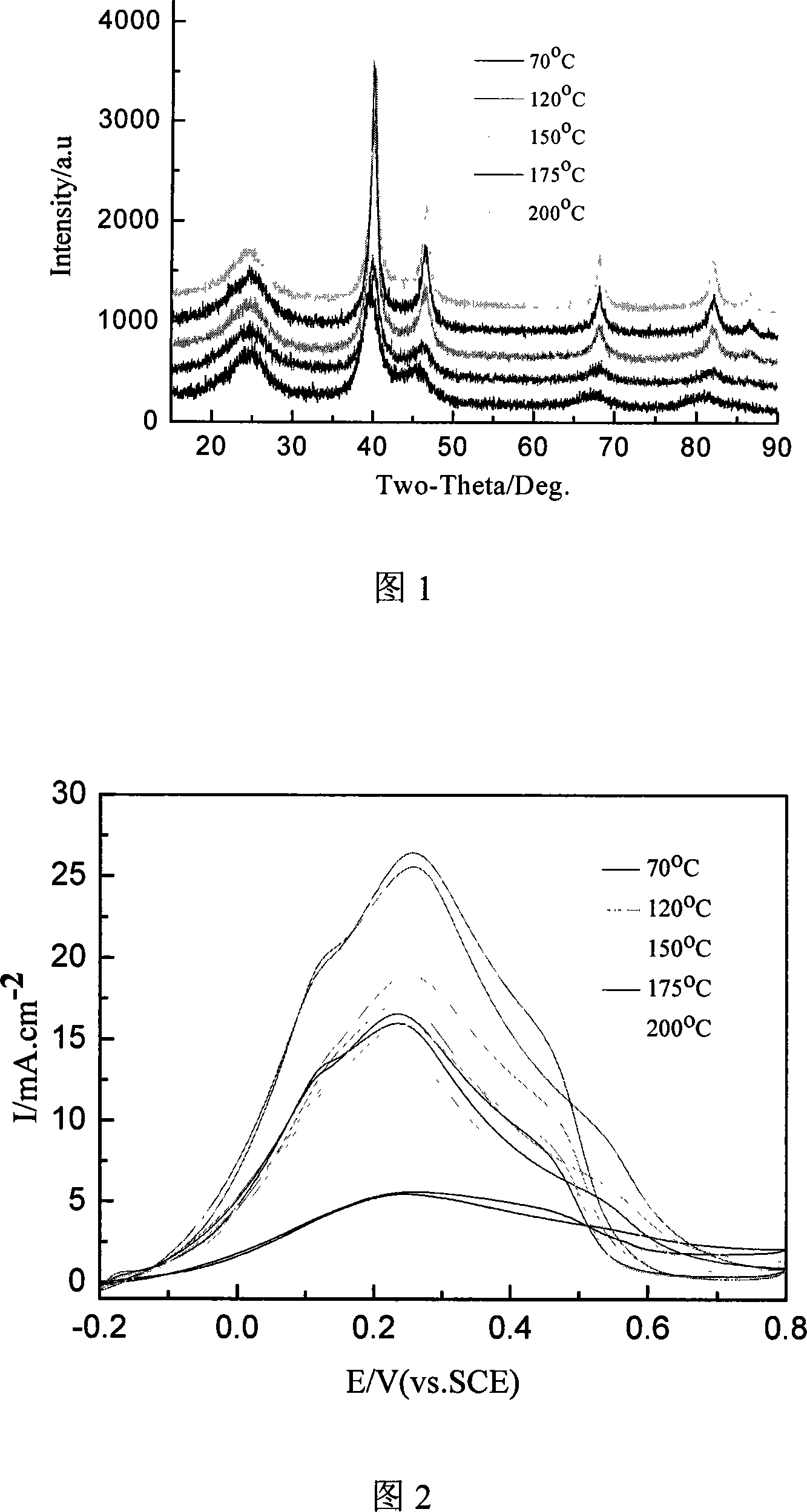
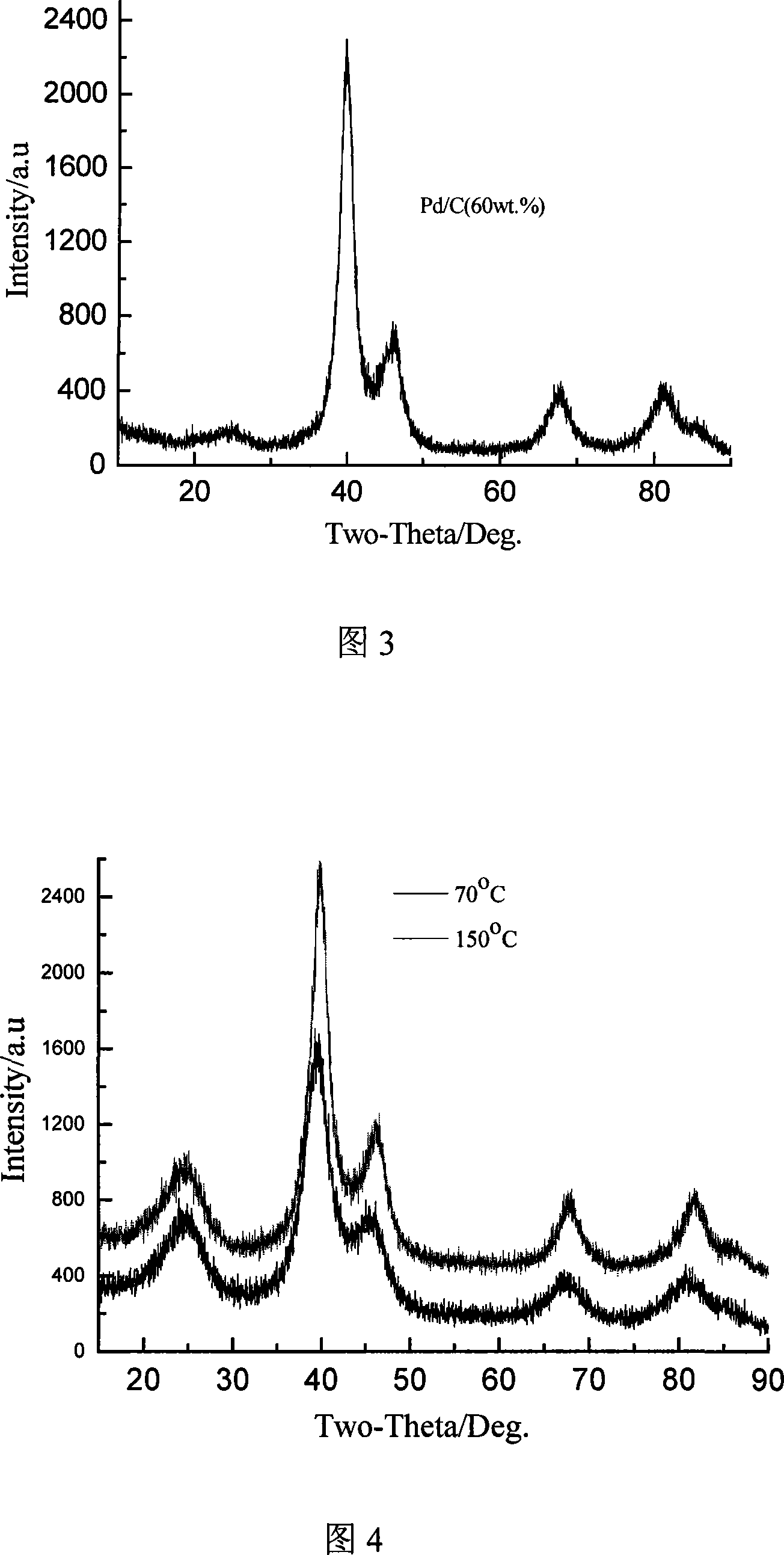
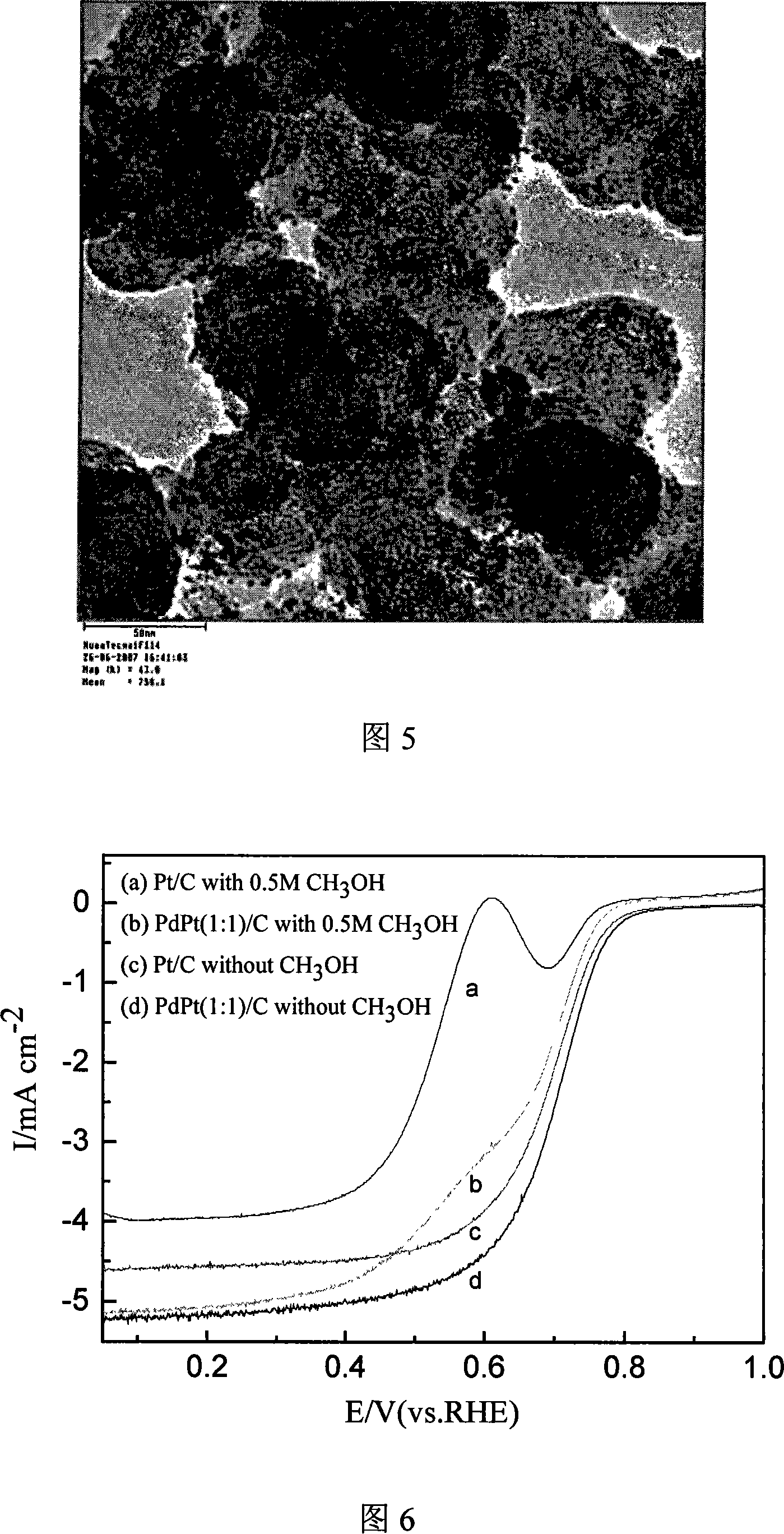
InactiveCN101083325ANarrow particle size distributionSimple methodCell electrodesCatalyst activation/preparationPlatinum saltsHydrazine compound
The invention relates to a preparation method of a nanometer Pd or the Pd platinum gold electrocatalysis catalyzer which is used in a kind of fuel cell, whose characteristic lies in that dissolve the mixture of the ration Pd salt or the Pd salt and the platinum salt (in which the Pd atomic ratio accounts for metal quantity 10-100%) in the water, after joining the right amount complexing agent solution, elevates temperature to 0-80deg.C and keeps the temperature 5 minutes to 8 hours, then cooling to the room temperature, adjusts the pH value to 5 to 12 and adds the carbon carrier, then adds the solution of hydroboration sodium, hydrazine or formic acid and so on reducing agent under the 0 to 80deg.C , and maintains 10 minutes to 10 hours, then filtrating, laundering, dry, finally in inert atmosphere or reducing atmosphere through 100 to 300deg.C heat treatment in 0.5 to 10h, namely carries the carbon Pd or the Pd platinum electrocatalysis. The particle size of catalyst is controllable, adjustable, the composition is controllable, regards the heat treatment temperature to be different, the particle size which obtains is relatively 1.8nm to 20nm above, and the granule distribution is narrow, is suitable to serve as the direct formic acid fuel cell anode catalyst as well as the direct methanol fuel cell anti-methyl alcohol negative pole catalyst.
Owner:上海新微科技集团有限公司 +1
Preparation of benzotrizole light stabilizer
InactiveCN101029032AEasy to cleanReduce manufacturing costOrganic chemistryIce waterHydrazine compound
A process for preparing the benzotriazole as photostabilizer includes such steps as dissolving 2-nitro-2'-hydroxy-5'-methyl azobenzene and 2-nitro-2'-hydroxy-3'- tert-butyl-5'-methyl propionate azobenzene in the mixture of alcohol and alkali solution, adding hydrazine hydrate, heating, reflux reaction, using hydrochloric acid to neutralize the reaction liquid, depositing, filtering, water washing, drying to obtain an intermediate, dissolving it in the mixture of alcohol and alkali, adding sodium dithionate, stirring while reacting, adding ice water, neutralizing, filtering, drying, and recrystallizing in alcohol or isopropanol.
Owner:TIANJIN UNIV
Organic metal framework supported palladium, preparation method and application thereof
InactiveCN101733162AImprove stabilityHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical industryN dimethylformamide
The invention discloses an organic metal framework supported palladium in the technical field of chemical industry, a preparation method and application thereof. The organic metal framework supported palladium is prepared by the method comprising the steps of: dissolving terephthalic acid and zinc nitrate hexahydrate into N, N-dimethylformamide, dripping triethylamine with the stirring, continuing stirring the obtained solution, filtering the obtained solution, and washing a precipitate to obtain a solid, namely MOF-5; and dissolving Na2PdC14 into the N, N-dimethylformamide to obtain solution, adding the MOF-5 into the solution, and then dripping hydrazine hydrate to obtain Pd@MOF-5 through stirring, filtration, washing and vacuum drying. The invention also relates to the method for preparing the organic metal framework supported palladium, application thereof taken as a catalyst in a Sonogashira reaction, and the method for preparing diaryl acetylene. The organic metal framework supported palladium in the invention has good stability, can catalyze the reaction with high selectivity and high efficiency under rather low loading of the catalyst, and can be repeatedly used.
Owner:SHANGHAI JIAO TONG UNIV
Silver nanoparticle process
ActiveUS20100034693A1Easy to processShort reaction timeMaterial nanotechnologyNanostructure manufactureHydrazine compoundSolvent
A process comprising: (a) preparing a reaction mixture comprising a silver salt, the reducing agent comprising a hydrazine compound, a thermally removable stabilizer, and an optional solvent, to form a plurality of silver-containing nanoparticles with molecules of the stabilizer on the surface of the silver-containing nanoparticles, wherein the reaction mixture generates an acid; and (b) removing the acid to produce the silver-containing nanoparticles substantially free of acid.
Owner:XEROX CORP
Method for recovery of copper, indium, gallium, and selenium
InactiveUS20100329970A1Simple processShorten operation timePhotography auxillary processesGallium/indium/thallium compoundsProduction lineIndium
A method for the recovery of copper, indium, gallium, and selenium is provided. The method includes steps of using a mixed solution containing a hydrochloric acid and hydrogen peroxide to dissolve the copper, indium, gallium, and selenium. After using the hydrazine to separate the selenium out, the copper is reduced by indium metal. Later, a combination of a supported liquid membrane (SLM) and a strip dispersion solution separates the gallium from the indium. The acid performed in all the steps of the method is hydrochloric acid. Therefore, the copper, indium, gallium, and selenium can be separated one by one in a single production line without changing the solution during the operation process, thereby simplifying the process, shortening the operation time and lowering the manufacture cost.
Owner:SOLAR APPLIED MATERIALS TECHNOLOGY CORPORATION
Method for recycling and preparing superfine nickel powder from nickel-hydrogen cell
ActiveCN101383440ARich sourcesLow priceWaste accumulators reclaimingBattery recyclingHydrazine compoundManganese
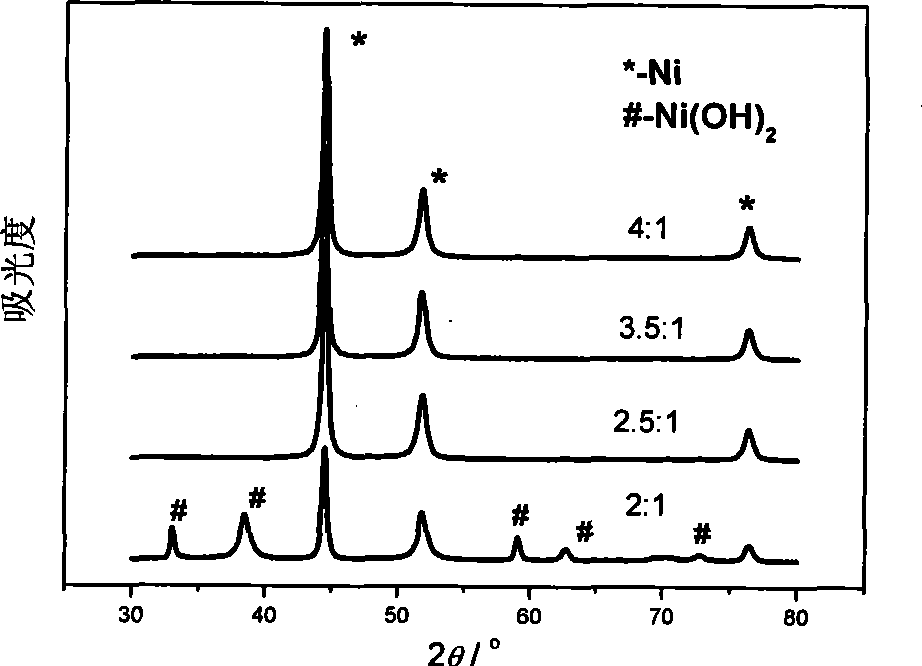
The invention discloses a method for directly reclaiming and preparing superfine nickel powder in positive material of nickel-hydrogen batteries. The method is characterized in that firstly, a professional disassembling machine is adopted to disassemble waste nickel-hydrogen batteries to obtain positive waste material which is crushed; secondly, lixiviating is performed by adopting a sulphuric acid and oxyful system; iron is removed and then impurities are extracted using P204 from lixivium to cause impurities such as calcium, copper, manganese, zincum and the like to transfer to an organic phase and cause nickel and cobalt to be left in a water phase; thirdly, solution containing nickel and cobalt is separated and extracted by using P507 so cause the cobalt to transfer to the organic phase the nickel to be left in the water phase; at last, faffinate containing nickel is deoxidized using hydrazine hydrate to obtain the superfine nickel powder. Through the application of the method, the coefficient of recovery of nickel in the positive waste material can reach more than 98.5 percent, and the purity of the obtained nickel reaches more than 99.7 percent; and the superfine nickel powder with the average grain diameter of 400 nm and face centered cubic crystal type sphericity is obtained by applying the method.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com