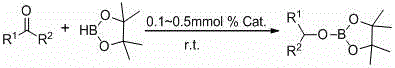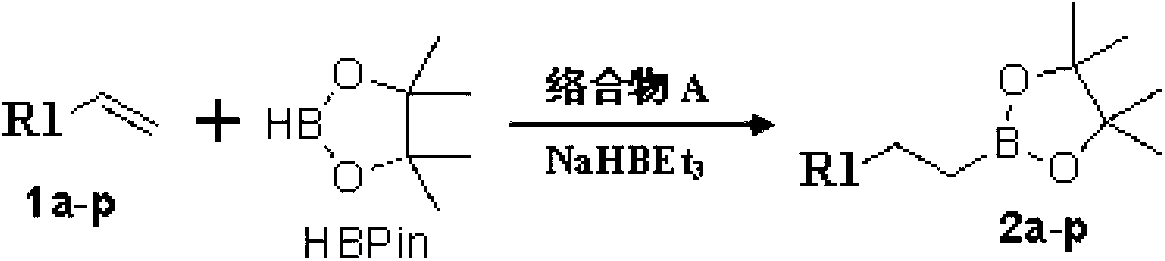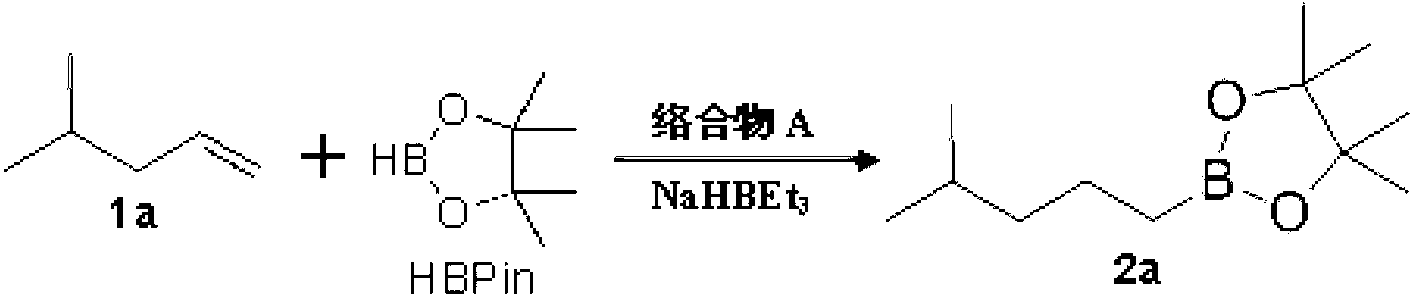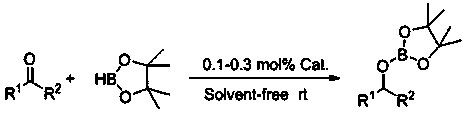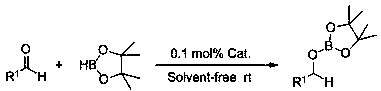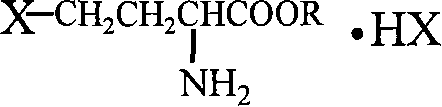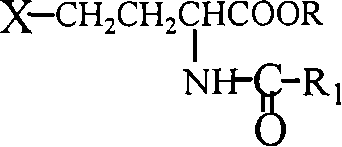Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
424 results about "Hydroboration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
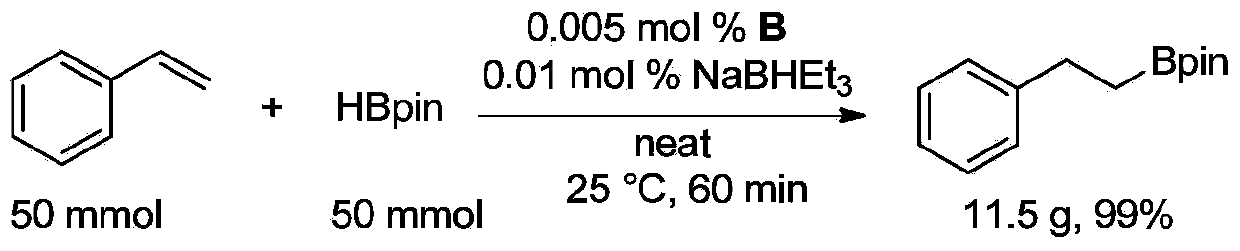
In chemistry, hydroboration refers to the addition of a hydrogen-boron bond to C-C, C-N, and C-O double bonds, as well as C-C triple bonds. This chemical reaction is useful in the organic synthesis of organic compounds. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the Nobel prize in chemistry with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates.
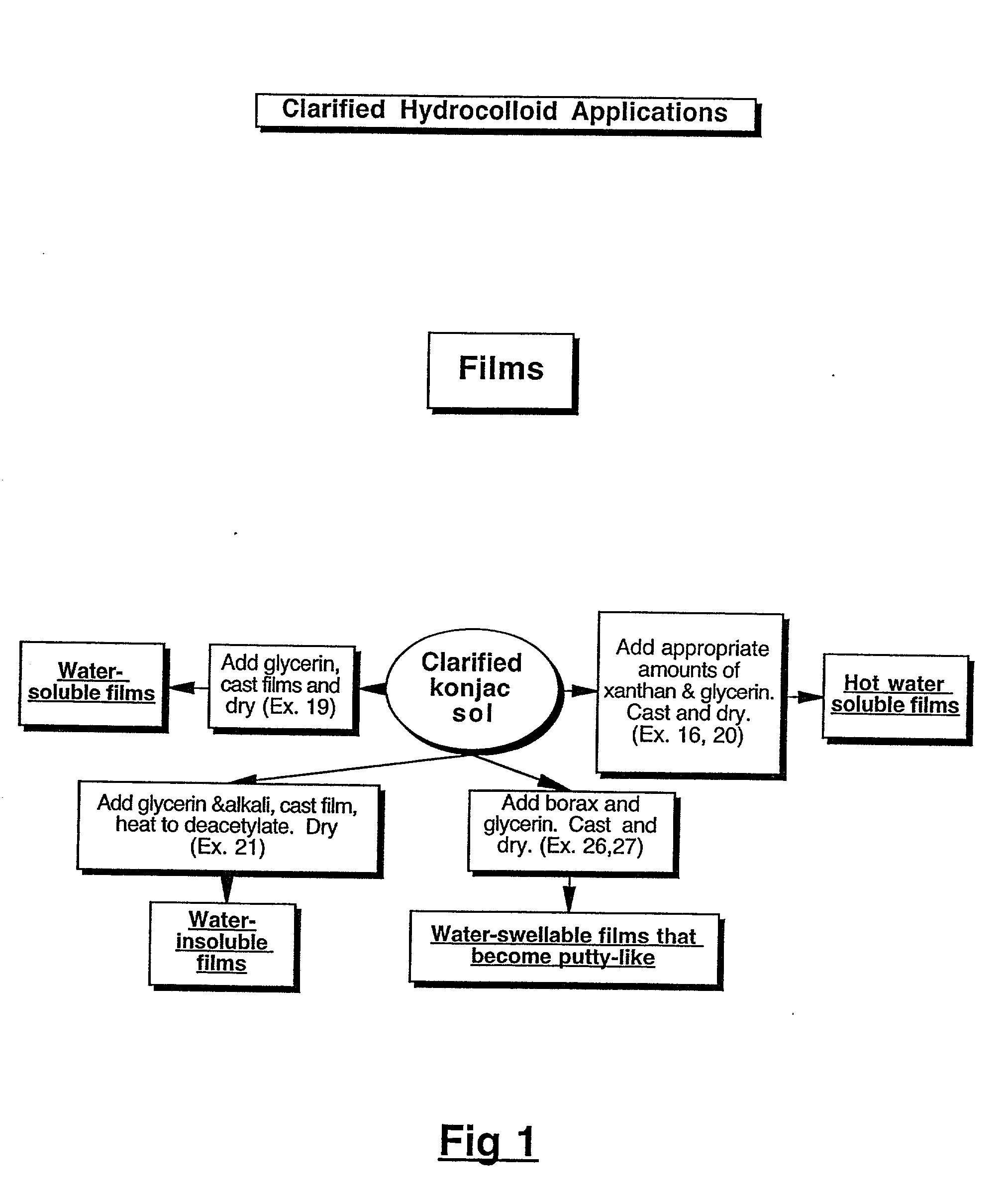
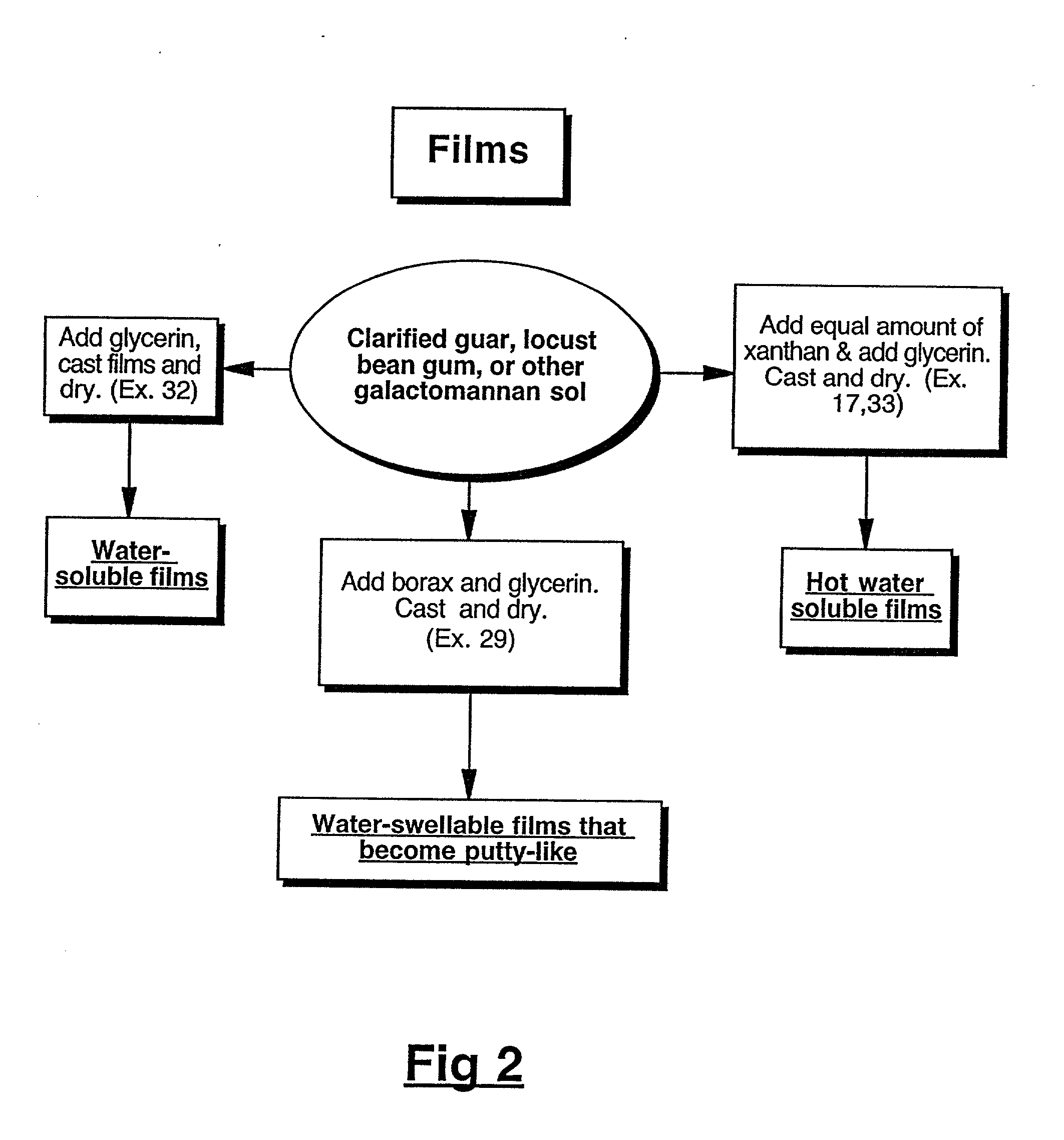
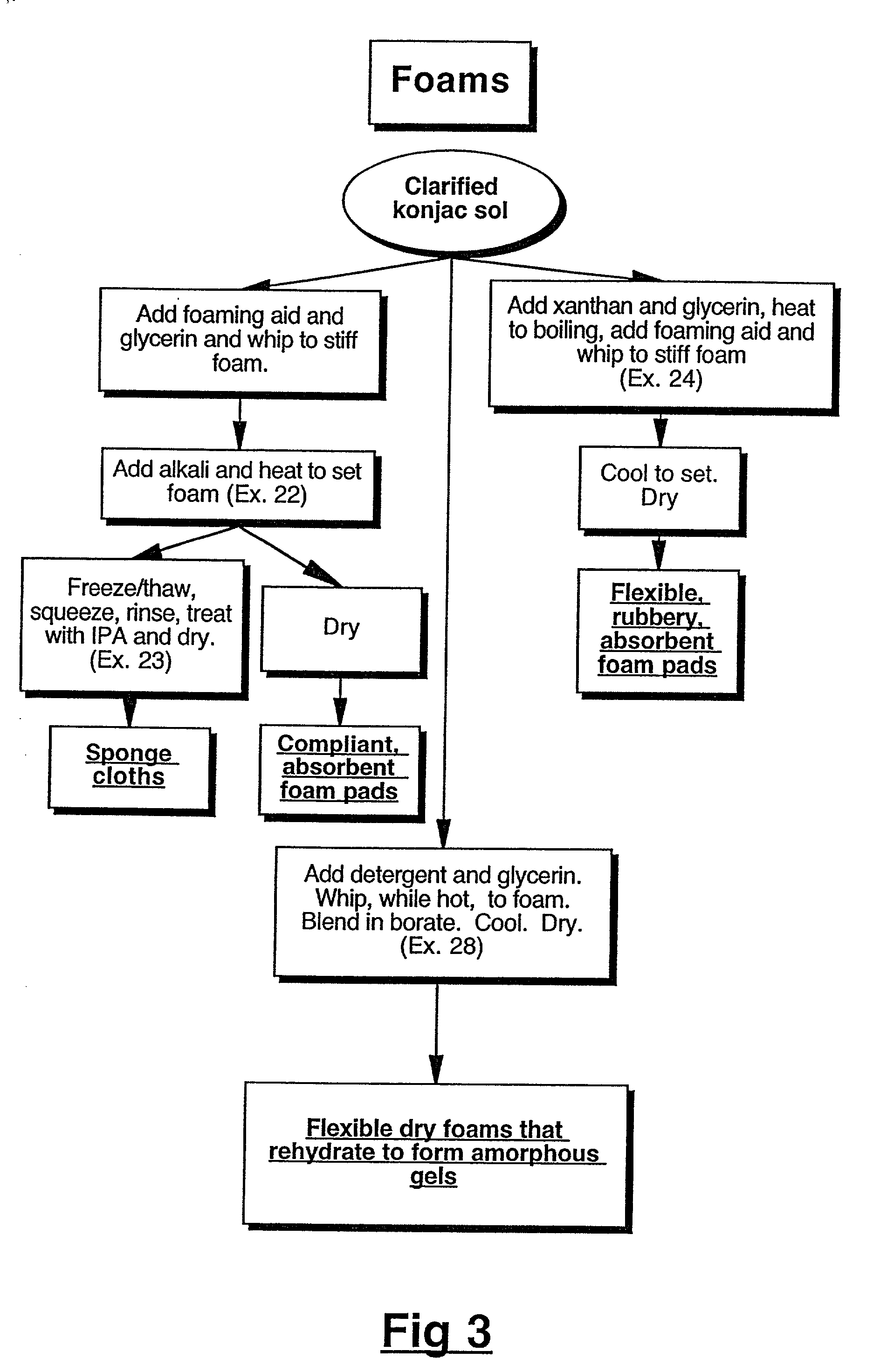
Physical forms of clarified hydrocolloids of undiminished properties and method of producing same
InactiveUS20020019447A1Good coagulationAssist dispersionOther chemical processesMixing methodsDiolPhysical form
This invention relates to novel forms of clarified hydrocolloids including gels, films, foams, capsules and sponges. The invention also pertains to novel processes for producing the various physical forms of the clarified hydrocolloids. The invention also includes clarified hydrocolloid composites; borated cis 1,2-diol containing hydrocolloids; and clarified hydrocolloids of low viscosity.
Owner:MARINE BIOPRODS INT
Preparation method for propellant fuel
InactiveCN108910843AShorter ignition delay timeHazard reductionOrganic chemistryHydrazineEvaporationIgnition delay
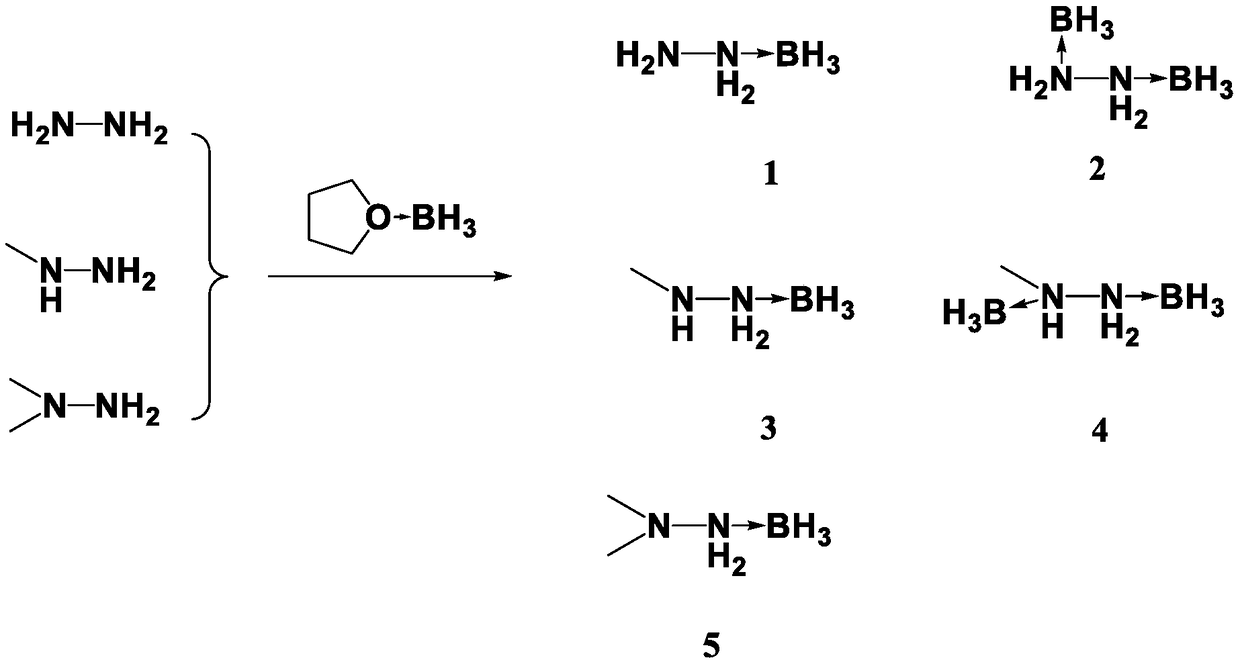
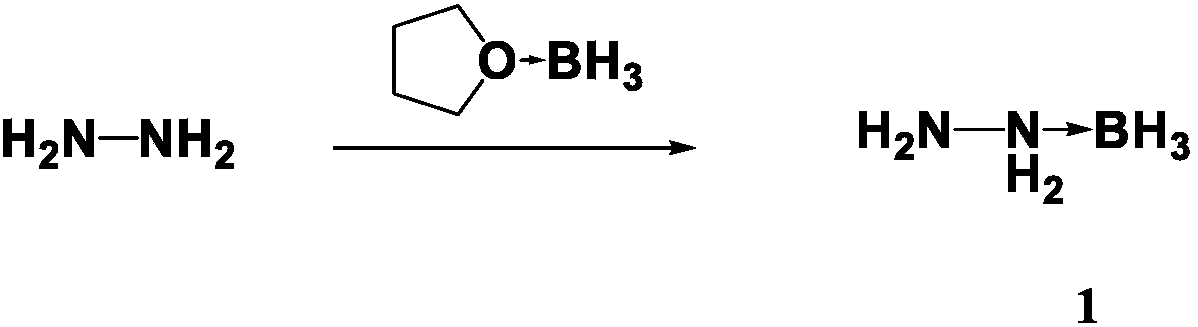
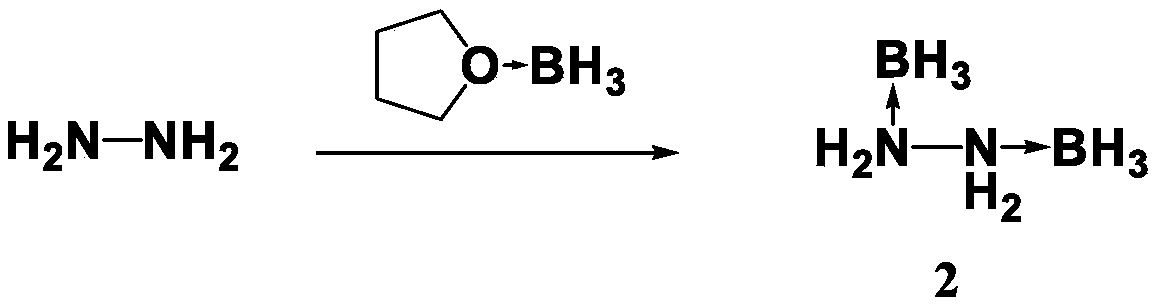
The invention discloses a preparation method for propellant fuel. The preparation method comprises the following steps: uniformly mixing hydrazine or a methyl derivative thereof with a tetrahydrofuransolution of borane to perform a reaction, and after the reaction, performing rotary evaporation on the tetrahydrofuran solution to obtain a first product; adding a first solvent into the first product, then performing washing, and then performing rotary evaporation to remove the first solvent, so as to obtain a hydrazinoborane derivative. According to the method, hydroboration is carried out on ahighly-volatile hydrazine fuel and a methylated derivative of hydrazine, the obtained borohydride of the hydrazine is a colorless non-volatile liquid or a white solid, has no volatility and greatly reduces hazard of the hydrazine fuel, and is a novel aerospace propellant fuel since the ignition delay time of the hydrazine borofluoride is extremely short.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
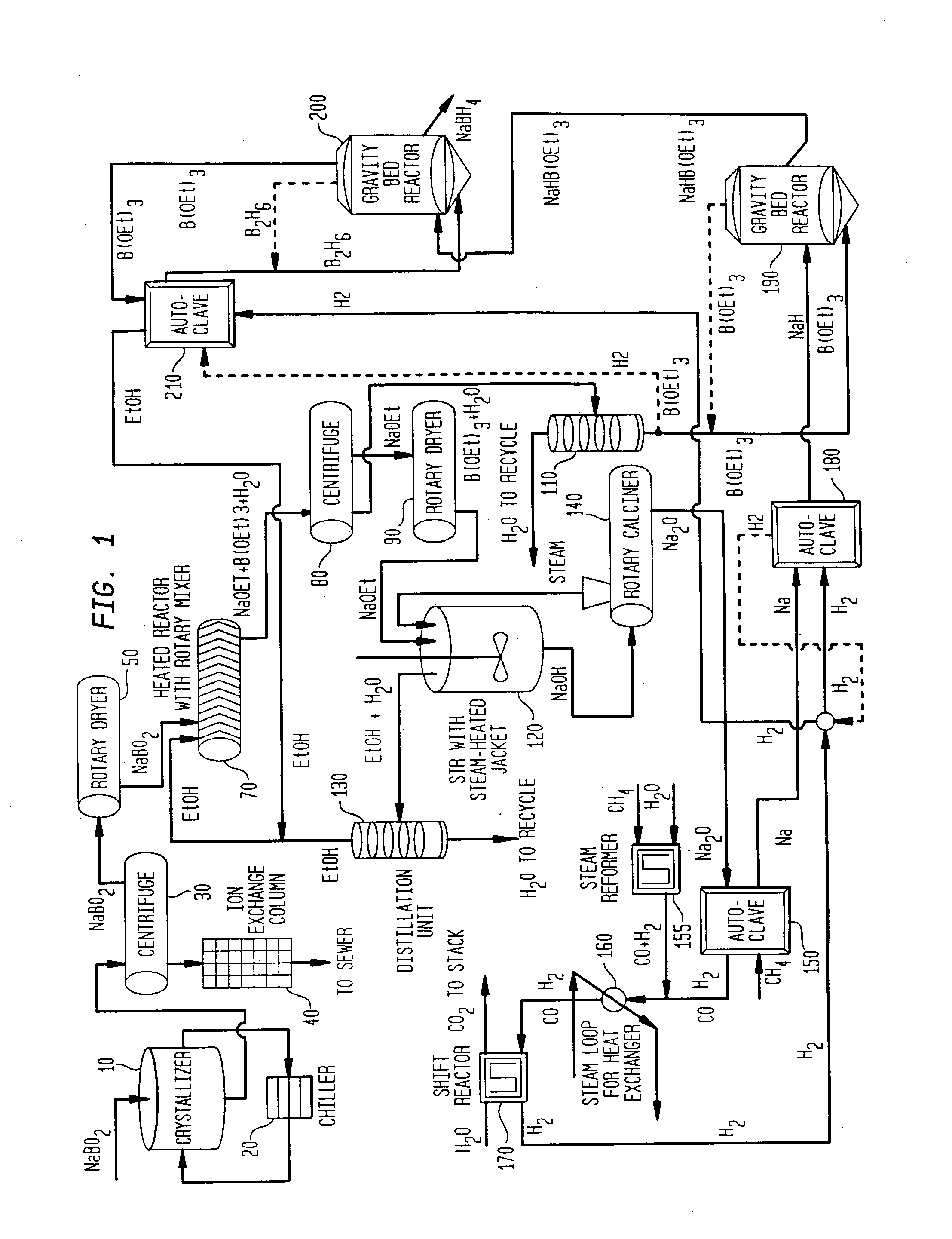
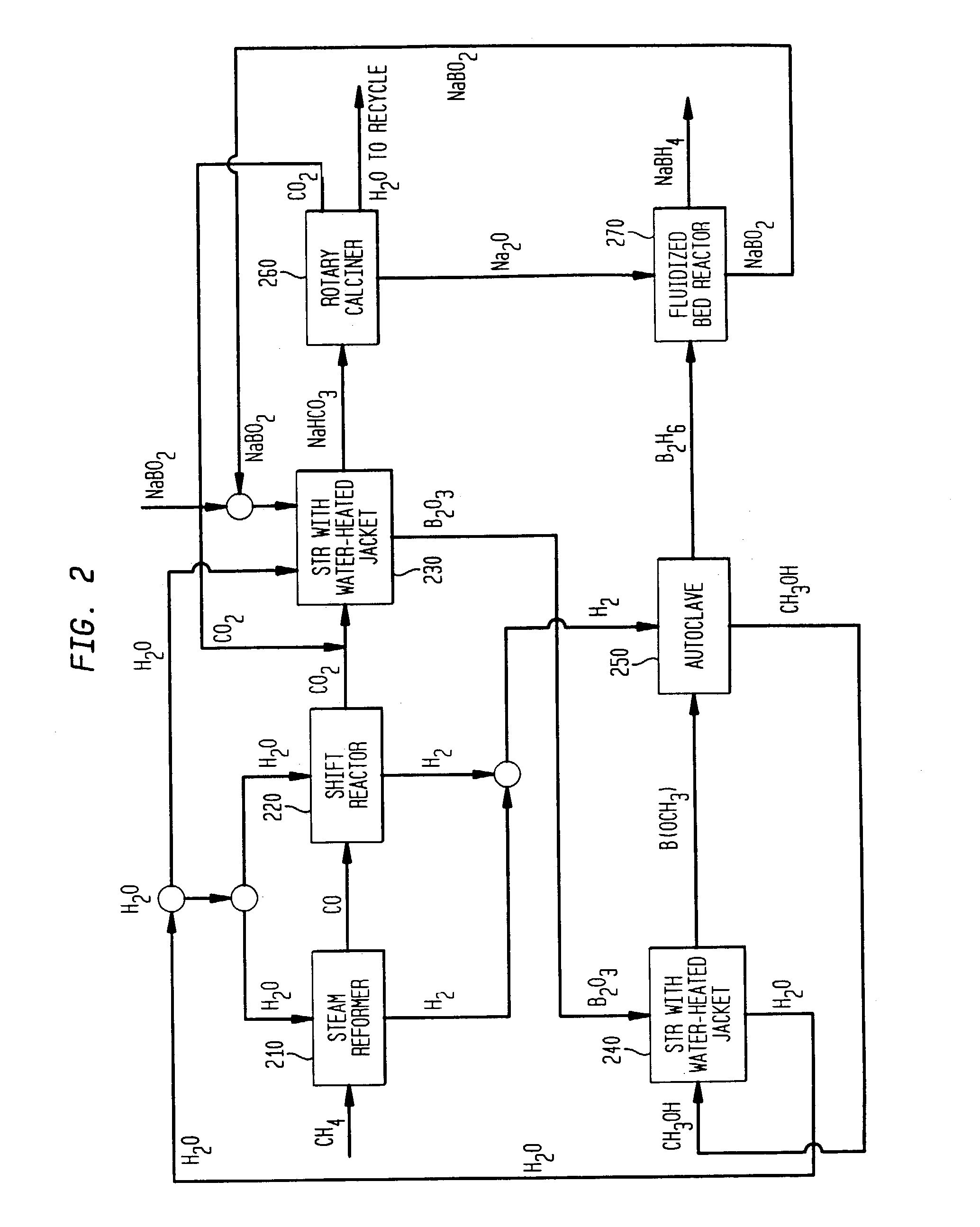
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Accelerated hydrogen generation through reactive mixing of two or more fluids
InactiveUS6818334B2Reactivity issueStability issueCell electrodesFuel cell auxillariesPtru catalystProton exchange membrane fuel cell
Owner:INTELLIGENT ENERGY LTD
Method for preparing nano-Pd or Pd platinum alloy electrocatalyst for fuel cell
InactiveCN101083325ANarrow particle size distributionSimple methodCell electrodesCatalyst activation/preparationPlatinum saltsHydrazine compound
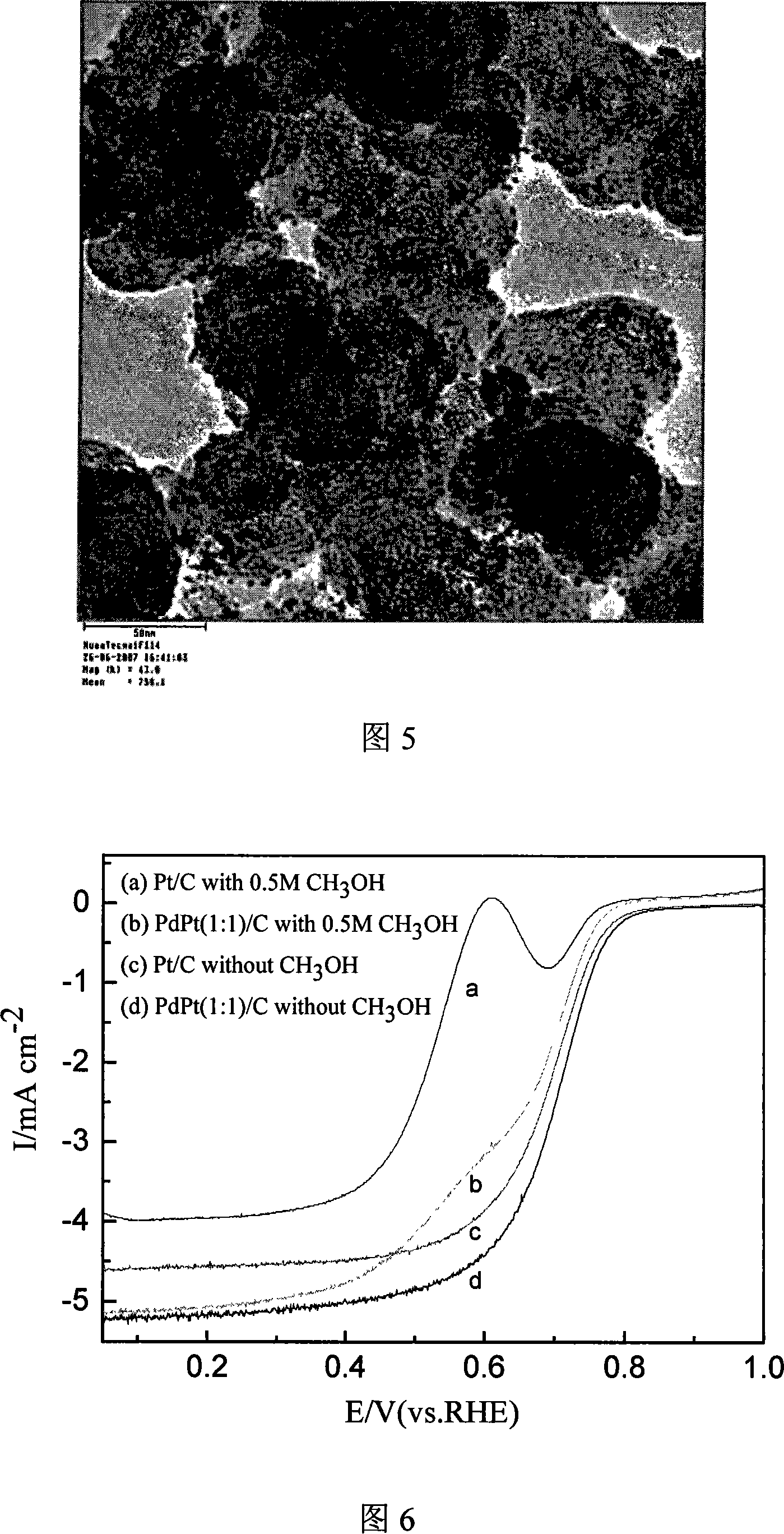
The invention relates to a preparation method of a nanometer Pd or the Pd platinum gold electrocatalysis catalyzer which is used in a kind of fuel cell, whose characteristic lies in that dissolve the mixture of the ration Pd salt or the Pd salt and the platinum salt (in which the Pd atomic ratio accounts for metal quantity 10-100%) in the water, after joining the right amount complexing agent solution, elevates temperature to 0-80deg.C and keeps the temperature 5 minutes to 8 hours, then cooling to the room temperature, adjusts the pH value to 5 to 12 and adds the carbon carrier, then adds the solution of hydroboration sodium, hydrazine or formic acid and so on reducing agent under the 0 to 80deg.C , and maintains 10 minutes to 10 hours, then filtrating, laundering, dry, finally in inert atmosphere or reducing atmosphere through 100 to 300deg.C heat treatment in 0.5 to 10h, namely carries the carbon Pd or the Pd platinum electrocatalysis. The particle size of catalyst is controllable, adjustable, the composition is controllable, regards the heat treatment temperature to be different, the particle size which obtains is relatively 1.8nm to 20nm above, and the granule distribution is narrow, is suitable to serve as the direct formic acid fuel cell anode catalyst as well as the direct methanol fuel cell anti-methyl alcohol negative pole catalyst.
Owner:上海新微科技集团有限公司 +1
Process for synthesizing d-tocotrienols from 2-vinylchromane compound
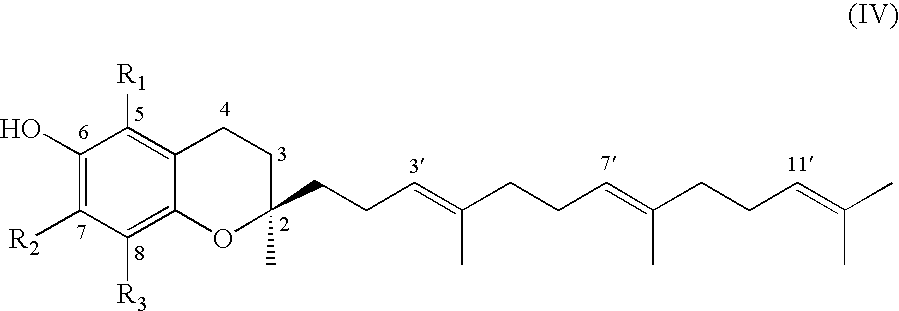
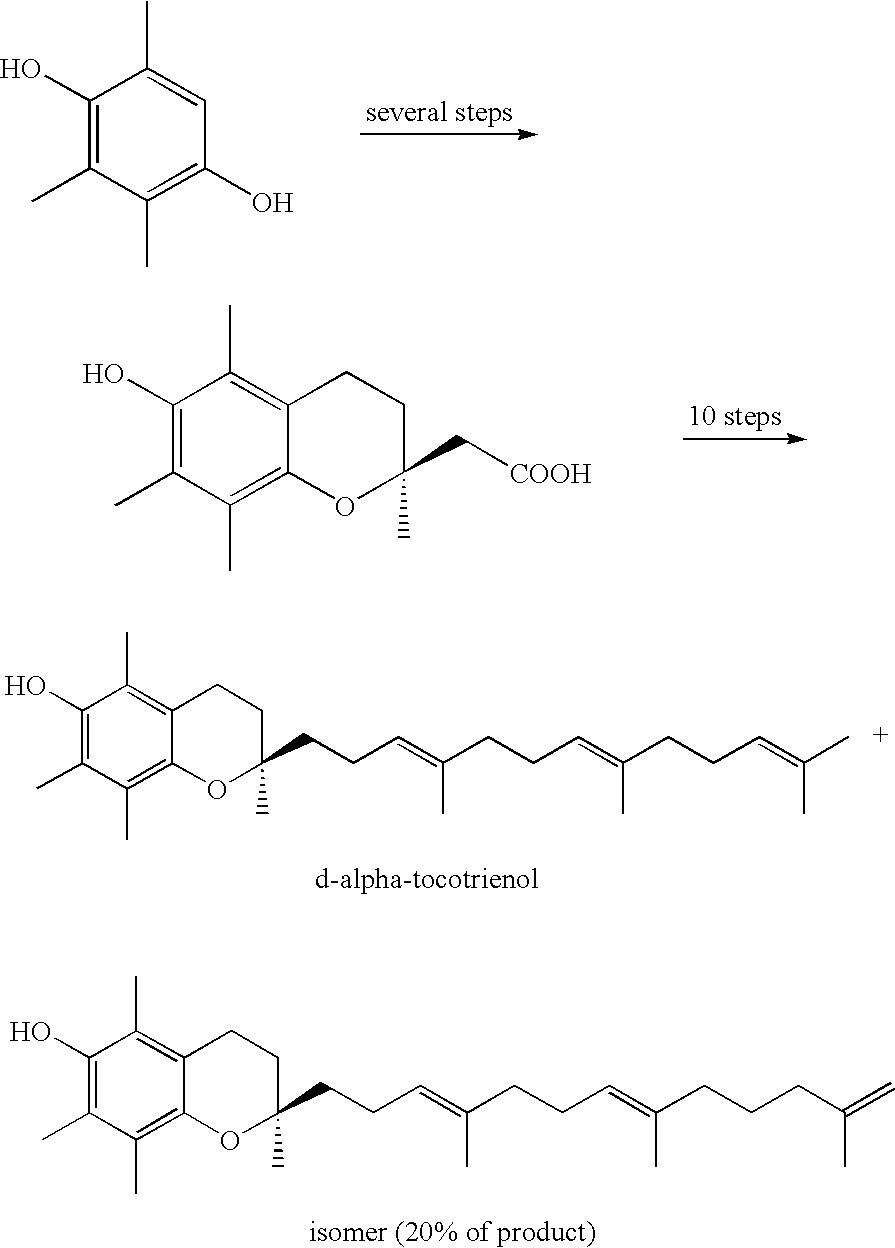
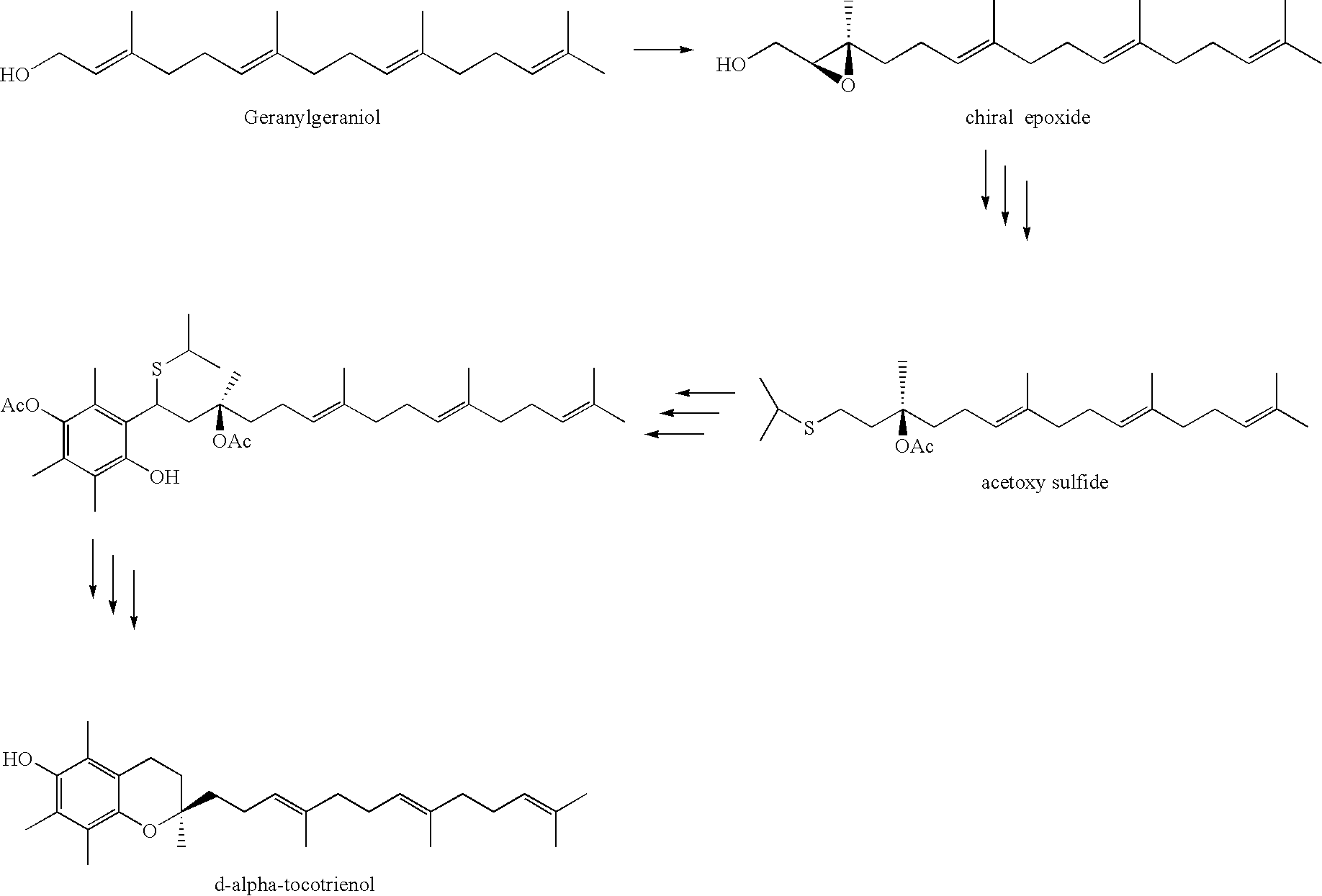
A process of forming a d-tocotrienol from a (2S)-vinylchromane compound, through hydroboration of the (2S)-vinylchromane to provide an organoborane, followed by coupling the organoborane with a halogenated C-14 sidechain compound under conditions of palladium-catalyzed cross-coupling is taught. Methods for providing the (2S)-vinylchromane compound and the halogenated C-14 compound are disclosed.
Owner:YASOO HEALTH
Carbon loaded type noble metal catalyst and preparation method thereof
ActiveCN101450308AHigh activityHigh selectivityCarboxylic preparation by ozone oxidationMetal/metal-oxides/metal-hydroxide catalystsPotassium borohydrideReduction treatment
The invention relates to a carbon loaded noble metal catalyst, which is characterized in that the catalyst consists of a carrier and palladium and ruthenium which are loaded on the carrier, wherein the carrier is powdery fruit shell active carbon and is between 85 and 99.7 percent by weight; the palladium is between 0.2 and 10 percent by weight; and the ruthenium is between 0.1 and 10 percent by weight. The catalyst is obtained by performing acid treatment, ash removal and oxidation treatment on the carrier, namely the active carbon for removing surface reduction functional groups first, using a solution of the palladium and the ruthenium to perform soakage treatment, and using one or more among hydrogen, potassium borohydride or hydrazine hydrate to perform reduction treatment. The catalyst has high activity and selectivity and good stability in hydrogenation reaction of organic compounds containing carbonyl.
Owner:CHINA PETROLEUM & CHEM CORP +1
Processes for synthesizing borohydride compounds
InactiveUS20030092877A1HydrogenMonoborane/diborane hydridesAlkaline earth metalCombinatorial chemistry
The present invention relates to processes for producing borohydride compounds. In particular, the present invention provides efficient processes and compositions for the large-scale production of borohydride compounds of the formula YBH.sub.4 by the reaction of a boron-containing compound represented by the formula BX.sub.3 with hydrogen or an aldehyde to obtain diborane and HX, and reacting the diborane with a Y-containing base selected from those represented by the formula Y.sub.2O, YOH and Y.sub.2CO.sub.3 to obtain YBH.sub.4 and YBO.sub.2. Y is selected from the group consisting of the alkali metals, pseudo-alkali metals, alkaline earth metals, an ammonium ion, and quaternary amines of the formula NR.sub.4.sup.+, wherein each R is independently selected from hydrogen and a straight- or branched-chain C.sub.1-4 alkyl group, and X is selected from the group consisting of halide ions, --OH, --R' and --OR' groups, chalcogens, and chalcogenides, wherein R' is a straight- or branched-chain C.sub.1-4 alkyl group.
Owner:MILLENNIUM CELL
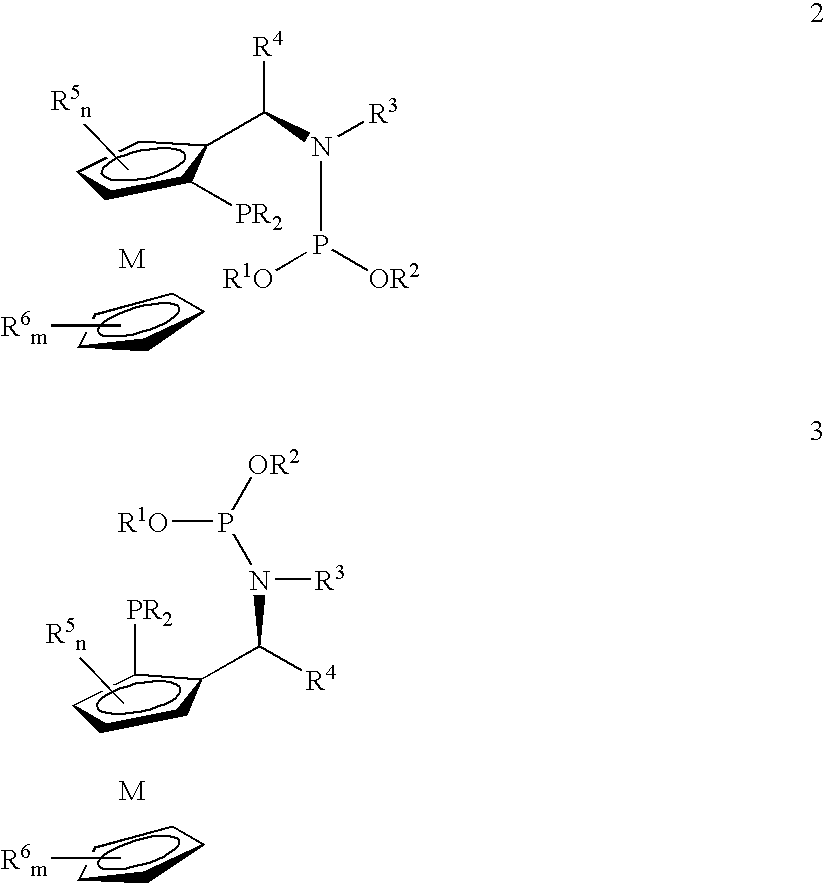
Phosphine-phosphoramidite compounds
ActiveUS6906212B1Hydrocarbon by hydrogenationGroup 8/9/10/18 element organic compoundsIsomerizationSilica hydride
Disclosed are novel phosphine-phosphoramidite compounds which may be employed in combination with a catalytically-active metal to effect a wide variety of reactions such as asymmetric hydrogenations, asymmetric reductions, asymmetric hydroborations, asymmetric olefin isomerizations, asymmetric hydrosilations, asymmetric allylations, asymmetric conjugate additions, and asymmetric organometallic additions. Also disclosed are a process for the preparation of the phosphine-phosphoramidite compounds, metal complex compounds comprising at least one of the phosphine-phosphoramidite compounds and a catalytically-active metal and hydrogenation processes utilizing the metal complex compounds.
Owner:JOHNSON MATTHEY PLC
Method for generation of hydrogen gas from borohydride
A method for generation of hydrogen by combining a solid composition containing a borohydride compound and a base with an aqueous solution of an acid.
Owner:YAMAMOTO JOHN HIROSHI
Preparation method for optical activity active 3-amino butanol and optical activity 3-amino butyric acid
ActiveCN104370755ANon-hazardousImprove conversion rateOrganic compound preparationAmino-carboxyl compound preparationSolventHydrolysis
The present invention discloses a preparation method for optical activity active 3-amino butanol and optical activity 3-amino butyric acid. The optical activity active 3-amino butanol preparation method comprises: in a solvent, under effects of a hydroboration reduction agent and a Lewis acid, carrying out a reduction reaction on a compound represented by a formlu 65 to produce a compound represented by a formlu 14. The optical activity active 3-amino butyric acid preparation method comprises: carrying out a hydrolysis reaction on a compound represented by a formlu 64 to produce a compound represented by a formlu 65. According to the present invention, the preparation method has characteristics of cheap and easily-available raw materials, simple operation, short process route, no hazard of raw materials, high yield, little waste production, environment protection, high raw material conversion rate, high product chemical purity and high product optical purity, and the industrialization is easily achieved. The formulas 64, 65 and 14 are defined in the instruction.
Owner:JIANGXI LONGLIFE BIO PHARM CO LTD +1
Accelerated hydrogen generation through reactive mixing of two or more fluids
InactiveUS20030228505A1Reactivity issueStability issueCell electrodesFuel cell auxillariesFuel cellsHydrogen
The rate of hydrogen generation in proton-exchange-membrane (PEM) fuel cells is greatly accelerated by mixing a first aqueous alkali borohydride solution with a second aqueous solution in the presence of one or more transition metal catalysts: wherein the first solution comprises (a) 5 to 50 wt % MBH4, where M is an alkali metal, (b) 5 to 40 wt % alkali hydroxide or alkaline metal hydroxide, and (c) the balance water, and wherein the second solution comprises (a) 51 to 100% water, and (b) the balance, if any, comprising at least one water-soluble additive.
Owner:INTELLIGENT ENERGY LTD
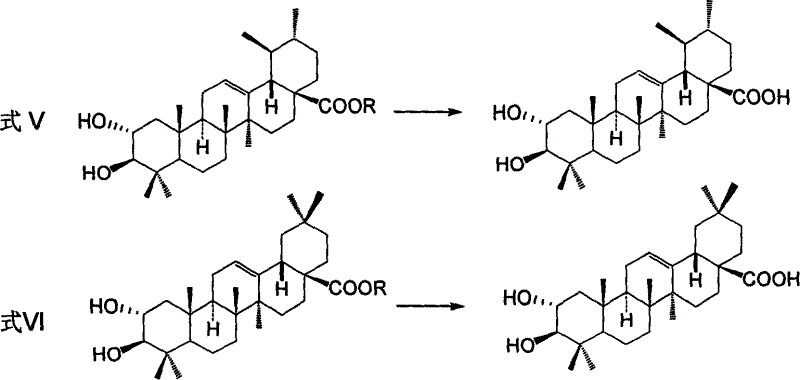
Process for preparing corosolic acid and crataegolic acid
InactiveCN1634971ASimple and efficient synthesisAvoid steric hindranceSteroidsAnti virusCorosolic acid
Disclosed are preparing processes for industrialized application of Corosolic acid with blood sugar lowering, weight reducing, anti-tumor and anti-inflammatory actions and maslinic acid with anti-tumor, anti-virus and cardiovascular diseases resistant actions. The processes include respectively preparing Corosolic acid and maslinic acid using 3-carbonyl ursolic acid ester and 3-carbonyl oleanolic acid ester by acid catalysed enolization esterification reaction, hydroboration oxidation reaction, catalytic hydrogenolysis reaction and haloidlysis reaction.
Owner:CHINA PHARM UNIV
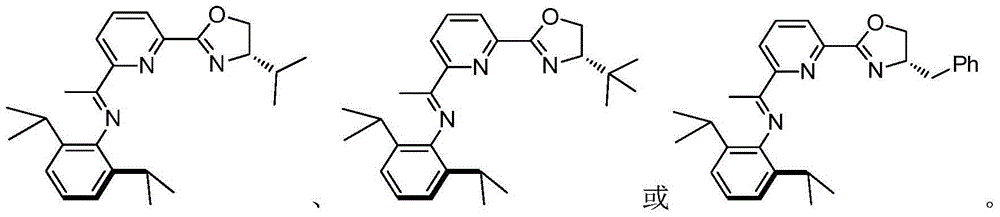
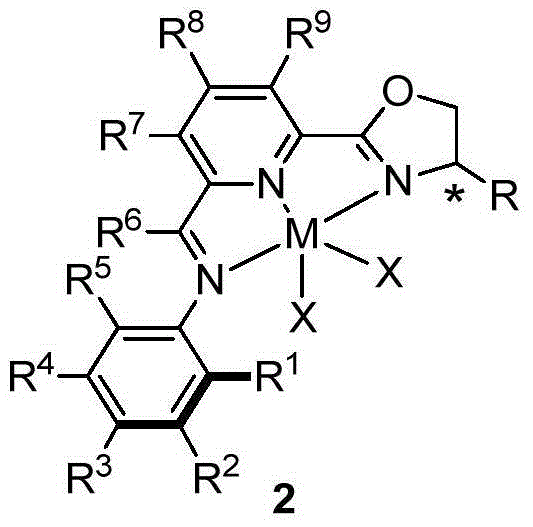
Chiral compound comprising iminopyridyl oxazoline and preparation method thereof
ActiveCN104447725AHigh chemical conversionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationDouble bond
The invention discloses a synthetic compound comprising iminopyridyl oxazoline, a preparation method thereof, a metal complex of the compound and application of a prochiral organic compound which contains at least one carbon / carbon or carbon / heteroatomic double bond in hydroboration. According to the compound, the total yield of two steps of a high-efficiency synthetic route can reach 50%, and the metal complex is an excellent catalyst or a catalyst precursor which is used for asymmetric synthesis (such as asymmetric hydrogenation of prochiral, unsaturated and organic compounds). The invention also provides application of the metal complex as a homogeneous catalyst. The catalyst is used for preparing a chiral organic compound by being subjected to the hydroboration and hydrosilation asymmetric addition on the carbon or carbon heteroatomic double bond of the prochiral organic compound, and an ee value can be more than 90%.
Owner:ZHEJIANG UNIV
Application of tri-silicon amine rare earth complex to catalyzing of hydroboration reaction of ketone and borane
ActiveCN106188118AMethods for Enriching Synthetic ReactionsSimple structureOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsRare earth2-acetylthiophene
The invention discloses application of tri-silicon amine rare earth complex to the catalyzing of hydroboration reaction of ketone and borane. The ketone is 2-acetylfuran, 2-acetylthiophene, 2-acetonaphthone or (substituted) phenyl ketone. The application has the advantages that the tri-silicon amine rare earth complex can catalyze the hydroboration reaction of the ketone and the borane under a mild condition (room temperature) in a high activity manner, and the use amount of catalyst is only 0.1-0.5% of the molar weight of the ketone; fast reaction is achieved, yield of above 90% can be reached after the reaction the performed for 10 minutes, catalyst use amount is lowered while yield is increased as compared with an existing catalyzing system, reaction time is short, the reaction condition is mild, and the economic atom synthesizing requirements are well satisfied.
Owner:SUZHOU UNIV
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6525210B1Carboxylic acid amides optical isomer preparationPreparation by carbon monoxide reactionIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
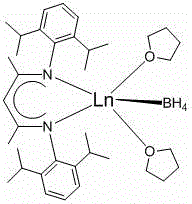
Application of beta-diimide bivalent rare earth boron hydrogen complex in catalysis of hydroboration reaction of ketone and boron hydride
ActiveCN106040303AMethods to Enrich Hydroboration ReactionsImprove reaction efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsRare earthKetone
The invention discloses application of a beta-diimide bivalent rare earth boron hydrogen complex in catalysis of hydroboration reaction of ketone and boron hydride. Beta-diimide rare earth dichloride and NaBH4 react in a tetrahydrofuran solvent, and in-situ Na / K reduction is carried out, so that the beta-diimide bivalent rare earth boron hydrogen complex, namely [2,6-ipr2-(C6H3)-NC(Me)CHC(Me)N-(C6H3)-2,6-ipr2]Ln-BH4.2THF, is obtained. The beta-diimide bivalent rare earth boron hydrogen complex disclosed by the invention can catalyze the hydroboration reaction of ketone and boron hydride with high activity under mild conditions and has the advantages of short reaction time and mild reaction conditions, and an aftertreatment method is simple and convenient.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV +1
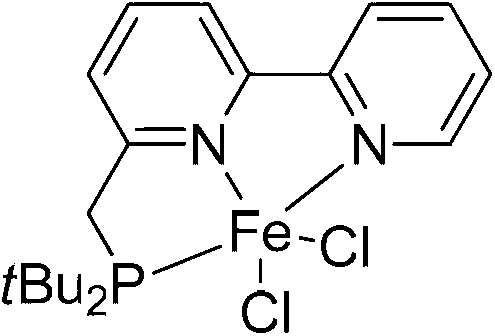
Pb(Ni1/3Nb2/3)O3 (PNN) ligand-iron complex catalyst and preparation method and application thereof
InactiveCN103071533A100% atomic availabilityGood functional group compatibilitySilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneHalogen
The invention discloses a Pb(Ni1 / 3Nb2 / 3)O3 (PNN) ligand-iron complex catalyst and a preparation method and application thereof. The catalyst is a compound with a general formula shown in the specification. In the general formula, R refers to an alkane group of C1-C30 or an alkane of C6-C30, and X refers to a halogen atom. The catalyst is prepared by performing a complexing reaction between a PNN ligand and FeX2. The PNN ligand-iron complex catalyst disclosed by the invention is good in catalytic activity on a hydroboration reaction of monoolefine and not only is high in selectivity and high in yield but also is mild in reaction condition. Moreover, the preparation method for the PNN ligand-iron complex catalyst is simple, is friendly to the environment, mild in reaction condition, higher in yield, simple in postprocessing and is easy for mass production, and raw materials are low in cost and can be easily got.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Ritodrine hydrochloride preparation method
InactiveCN102060716AShort cycleLow costOrganic compound preparationAmino-hyroxy compound preparationBromineRitodrine Hydrochloride
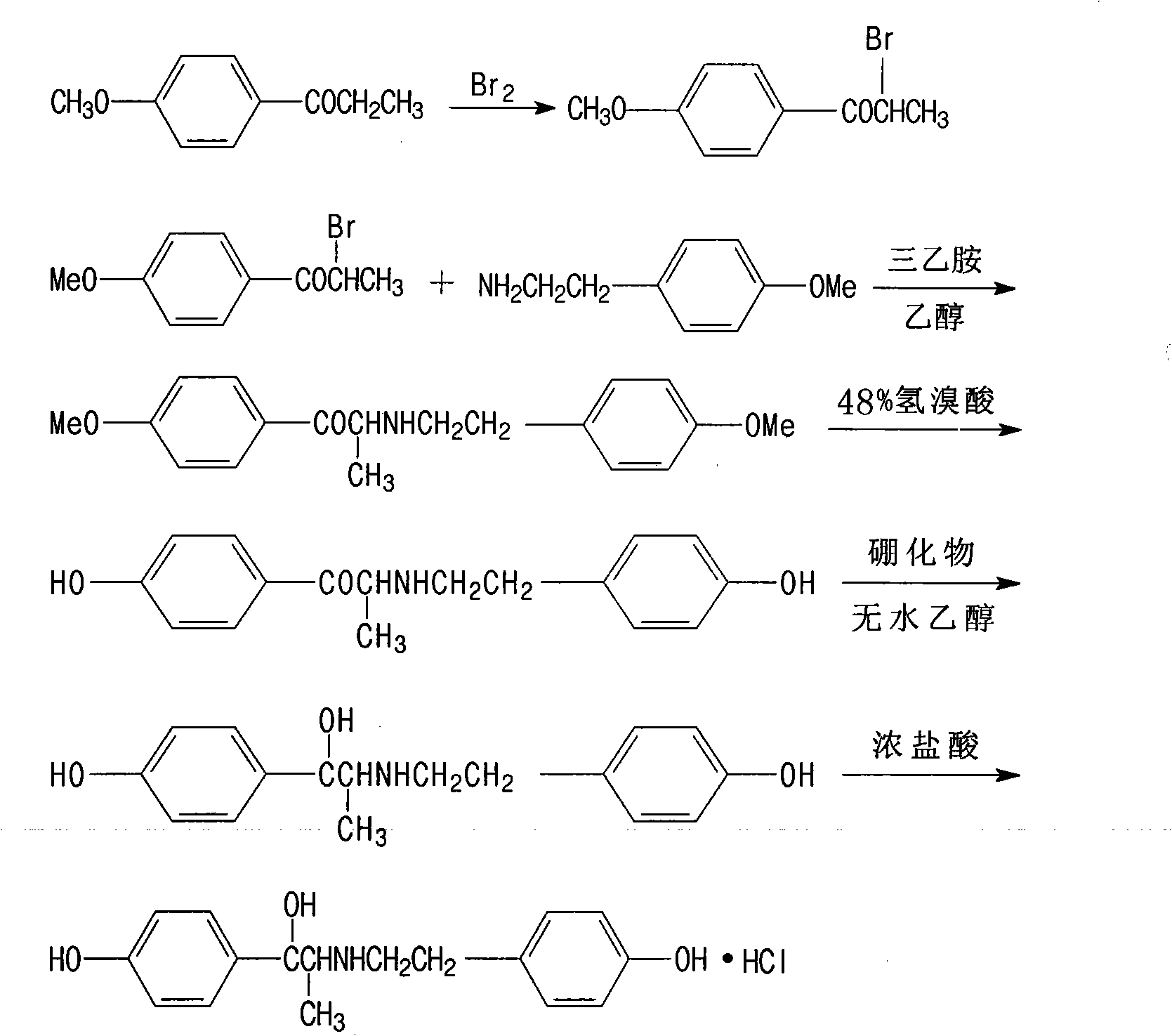
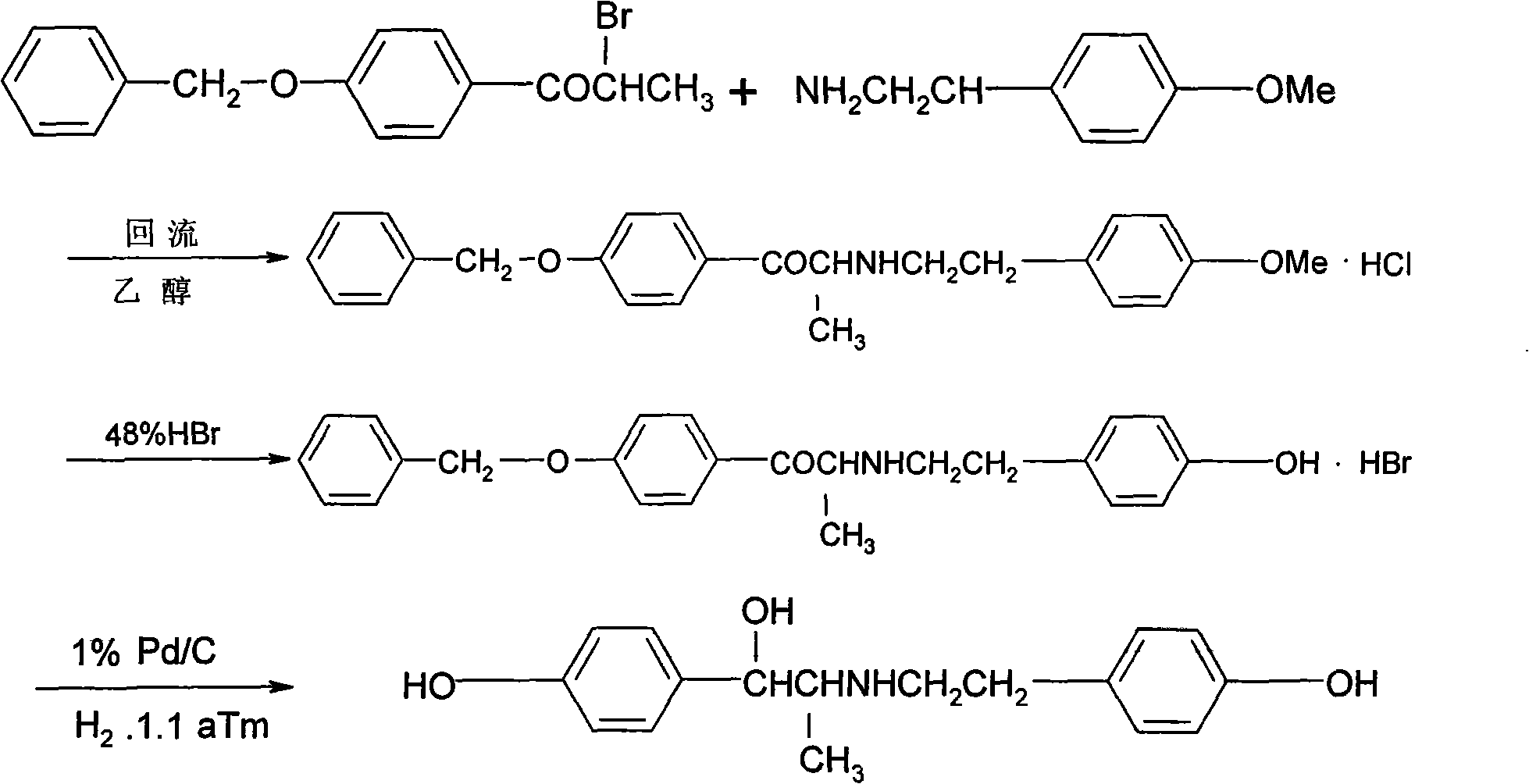
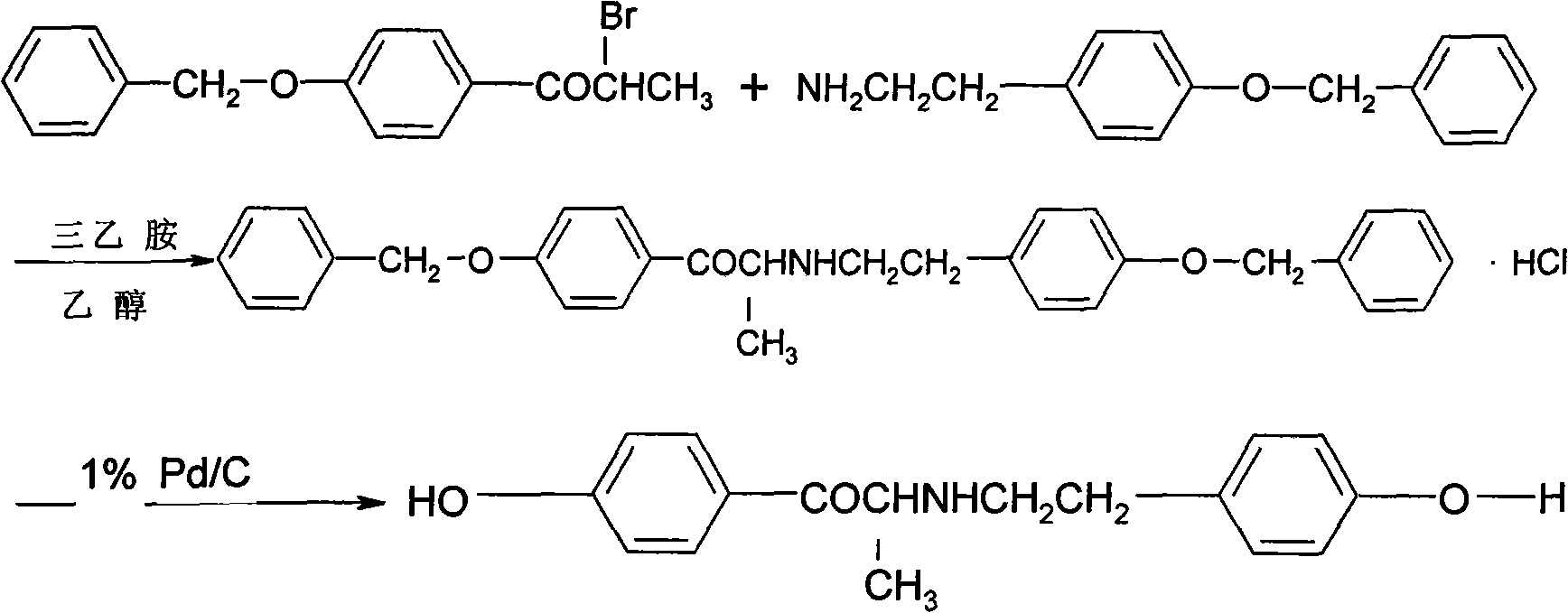
The invention relates to a ritodrine hydrochloride preparation method which comprises the following steps: using 4-methoxyphenylacetone as a raw material to react with liquid bromine so as to finish alpha-site bromization; then, reacting with methoxyphenylethylamine; and carrying out hydroxylation and hydroboration, and reducing to obtain the ritodrine hydrochloride product. By combining bromization, reaction with para-methoxyphenylethylamine and hydroxylation together, the invention realizes that intermediate steps do not need to be subjected to post treatment, thereby shortening processing steps to be adapted to industrial production. The ritodrine hydrochloride preparation method has the advantages of short period and low cost, and is convenient to operate, thereby greatly improving the production efficiency and yield.
Owner:双鹤药业(海南)有限责任公司
Application of n-butyllithium in catalytic hydroboration of ketone and borane
InactiveCN108654692AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsN-ButyllithiumKetone
The invention discloses an application of n-butylllithium in catalytic hydroboration of ketone and borane. The n-butyllithium is a commercial n-butyl lithium reagent, and the method includes: adding borane to a reaction flask subjected to dehydration and deoxygenation treatment without oxygen and water at inert gas atmosphere; adding a catalyst n-butyllithium with even mixing prior to adding ketone for hydroboration. The catalyst has good universality to aromatic ketones with different substitution positions and different electron effects as well as heterocyclic ketone and aliphatic ketone, and more choices for borate compounds with different substituent structures are provided.
Owner:SUZHOU UNIV
Chiral phosphines, transition metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6576772B1High enantioselectivityEnantioselectivity decreaseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include (R,S,S,R)-DIOP*. The ruthenium complex reduces enamide to the corresponding amine with up to 99% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation, hydride transfer, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, isomerization, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
Method of preparing borate ester based on aldehyde
InactiveCN108409772AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsLithiumBottle
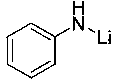
The invention relates to application of anilino lithium, in particular to a method of preparing borate ester based on hydroboration reaction of aldehyde and borane. The method comprises the followingsteps of under the conditions without water and oxygen, in an inert gas atmosphere, adding the borane in a reaction bottle subjected to dehydration and deoxygenation treatment, then adding the anilinolithium as a catalyst, performing uniform mixing, then adding the aldehyde for hydroboration reaction, and performing exposure in the air for reaction termination to obtain the borate ester as a product, wherein the aldehyde is selected from fatty aldehydes. By adopting the method provided by the invention, the condition that the anilino lithium can extremely efficiently catalyze cyclohexanecarboxaldehyde, propionaldehyde and heptanal to generate hydroboration reaction with the borane is discovered for the first time, and a new scheme is provided to prepare the borate ester by adopting a carbonyl compound and the borane to generate the hydroboration reaction.
Owner:NANTONG TEXTILE & SILK IND TECH RES INST +1
Application of p-methylanilinyl lithium to catalysis of aldehyde and borane to generate hydroboration reaction
ActiveCN108554446AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsSolvent freeOxygen
The invention relates to application of p-methylanilinyl lithium to catalysis of aldehyde and borane to generate hydroboration reaction. The application comprises the following processes: under the water-free and oxygen-free environment and in the inert gas atmosphere, adding borane to a reaction flask subjected to dehydration and deoxidization treatment, then adding p-methylanilinyl lithium as acatalyst, performing uniform mixing, then adding aldehyde to perform hydroboration reaction, and exposing to the air to stop the reaction so as to obtain a product borate, wherein aldehyde is selectedfrom aromatic aldehyde or heterocyclic aldehyde. The catalytic activity in catalysis of aldehyde and borane to generate hydroboration reaction by p-methylanilinyl lithium is high (the dosage of the catalyst is only 0.1%), the reaction conditions are mild (room temperature), the reaction time is short (10 min), the reaction yield is high, the reaction is simple and controllable, the after-treatment is simple, and the reaction adopts a solvent-free system so as to reduce the pollution to the environment.
Owner:SUZHOU UNIV
Application of o-methyl-anilino lithium in catalysis of aldehyde and borane hydroboration
InactiveCN108659027AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsLithiumBottle
The invention discloses application of o-methyl-anilino lithium in the catalysis of aldehyde and borane hydroboration. Borane is added into a reaction bottle that is dewatered and deoxidized in an inert gas atmosphere in an anhydrous oxygen-free environment; the catalyst, o-methyl-anilino lithium, is added; mixing is performed well before an aldehyde is added; hydroboration is carried out; the reaction is ended after exposure to air so as to obtain borates; the aldehyde is selected from aromatic aldehydes and heterocyclic aldehydes. The catalyst disclosed herein is well applicable to aromaticaldehydes and heterocyclic aldehydes having different substitution positions and electronic effects; more choices are provided to attain borates with different substituent structures.
Owner:SUZHOU UNIV
Preparation method for liquid polyborosilazane
The invention relates to a preparation method for liquid polyborosilazane and belongs to the field of inorganic nonmetallic materials. The preparation method comprises the following steps: liquid polyborosilazane and 9-borabicyclo [3,3,1]-nonane are added in a solvent in inert atmosphere to obtain mixed solution, then the obtained mixed solution is hydroborated under the stirring condition, and then the solvent is removed after completion of hydroboration to obtain the liquid polyborosilazane. The preparation method solves the problems in the traditional polymer route that excessive cross-linking is easily caused in the hydroboration addition reaction, and solid polyborosilazane has poorer fluidity. The prepared liquid polyborosilazane has good fluidity and high ceramic yield and can be directly used for the high polymer infiltration and pyrolysis method for preparing SiBCN ceramic matrix composites.
Owner:XIAMEN UNIV
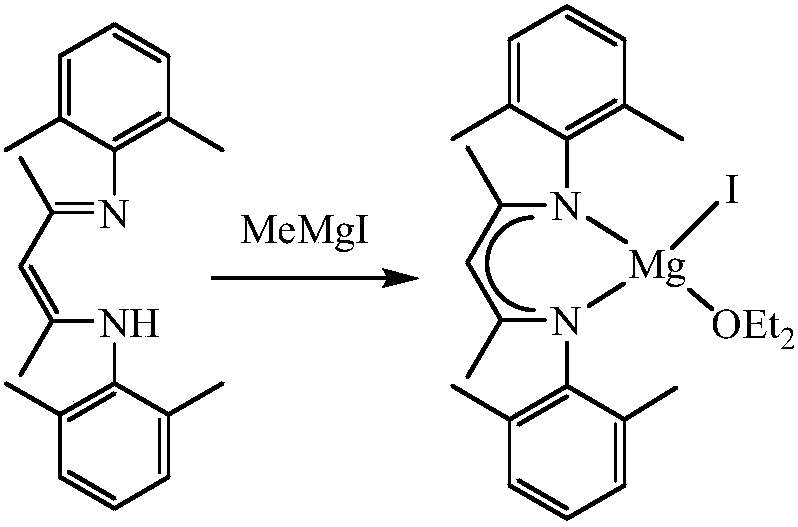
Beta-diimide monovalent magnesium compound, preparation method thereof and application of beta-diimide monovalent magnesium compound in hydroboration of aldehyde or ketone
ActiveCN107602595AEasy to purifyLow toxicityCarboxylic acid nitrile preparationOrganic compound preparationDiimineIodide
The invention discloses a beta-diimide monovalent magnesium compound, a preparation method thereof and an application of the beta-diimide monovalent magnesium compound in hydroboration of aldehyde orketone. The preparation method comprises the following steps: under anhydrous and anaerobic conditions, a beta-diimine ligand reacts with a Grignard reagent, iodide of magnesium is generated and reduced by sodium, and a yellow crystal is obtained and is the beta-diimide monovalent magnesium compound. The beta-diimide monovalent magnesium compound is simple to synthesize, convenient to separate andpurify, clear in structure and high in yield; activity of the compound as a catalyst in catalysis of a reaction of aldehyde or ketone with pinacolborane is high, and the substrate universality is broad.
Owner:厦门欧瑞捷生物科技有限公司
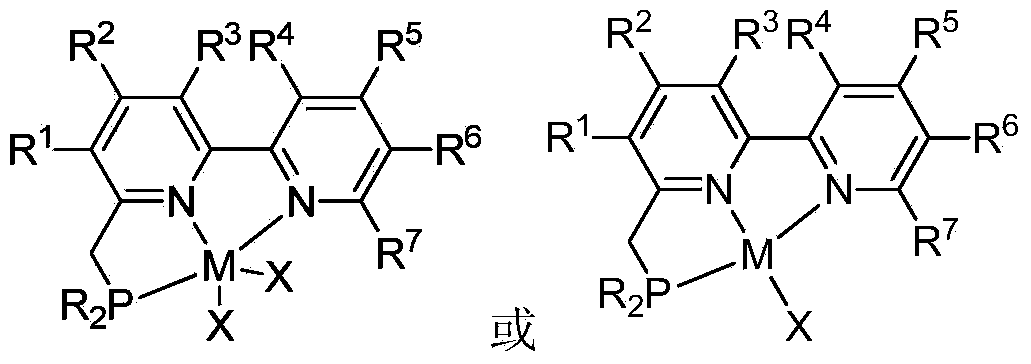
NNN ligand, metal complexes thereof, preparation methods and application
ActiveCN105294667AHigh yieldMild reaction conditionsCobalt organic compoundsIron organic compoundsRegioselectivityAlkene
The invention discloses an NNN ligand, metal complexes thereof, preparation methods and application, and particularly provides an NNN ligand 1, a metal complex 2 of the NNN ligand, a metal complex 3 of the NNN ligand and preparation methods of the NNN ligand 1 and the metal complexes, as well as application of the metal complex 3 of the NNN ligand to catalysis on hydroboration reaction of bis-substituted olefin, especially application of the metal complex 3 to catalysis on asymmetric hydroboration reaction of 1, 1-bis-substituted olefin. The metal complex 3 of the NNN ligand has good catalytic activity in the hydroboration reaction of the bis-substituted olefin, especially asymmetric hydroboration reaction of the 1, 1-bis-substituted olefin, has excellent regioselectivity and enantioselectivity, and is high in yield and mild in reaction conditions; besides, the preparation methods of the NNN ligand and the metal complexes thereof are simple, environment-friendly, mild in reaction conditions, relatively high in yield, and simple in post-processing, and the raw materials are low in cost and easy to obtain, so that the preparation methods are suitable for industrial production. (The formulas of the NNN ligand 1, metal complex 2 and metal complex 3 are shown in the description).
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
PNN ligand-metal complex catalyst as well as preparation method and application thereof
ActiveCN103962183AEasy to prepareRaw materials are cheap and easy to getSilicon organic compoundsOrganic compound preparationAlkaneOxygen
Owner:BIRDO (SHANGHAI) PHARMATECH CO LTD
Synthesis of animal nutrient additive amino-acid-selenomethionine
InactiveCN101073378AImprove securityMild reaction conditionsAnimal feeding stuffAccessory food factorsSelenium methionineOxygen
The invention is concerned with a kind of method to produce amino acid-selenium methionine as animal nutrition additive. Take dimethyl diselenide as material and deoxidize it in alkalescence solution to get methyl hydroselenide negative ion with borohydride metal or metal of borohydride. Then react with 4-halogen-alpha-amino acid or its ramification to get selenium methionine through hydrolyzing and adjusting pH valve. The reaction system is carrying in water solution without inert gases and avoids the operation without water and oxygen. The material is common with good safety and it is easy for operation and higher productivity with appropriate condition and it do not need special equipment.
Owner:广东新南都饲料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com