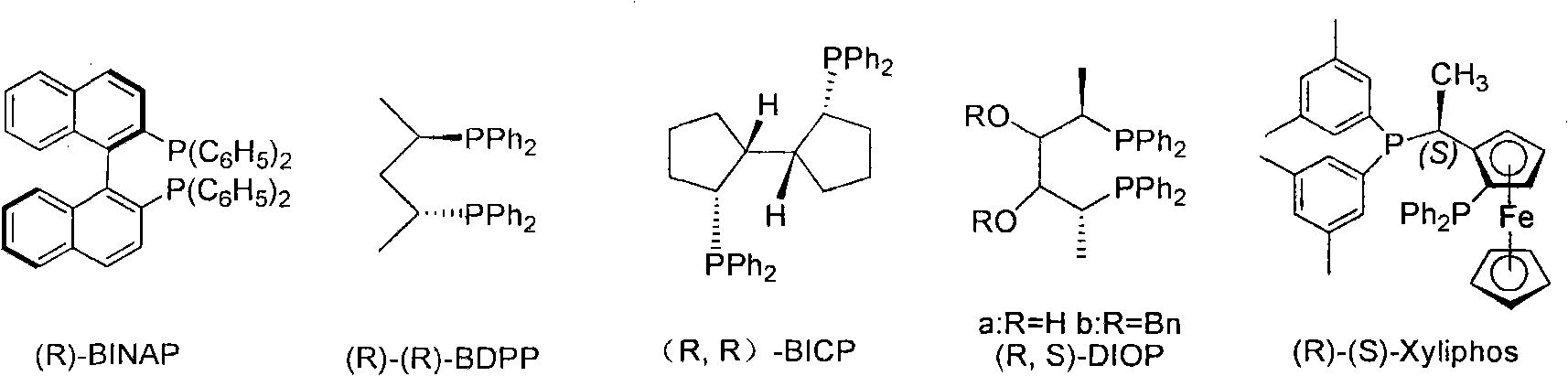
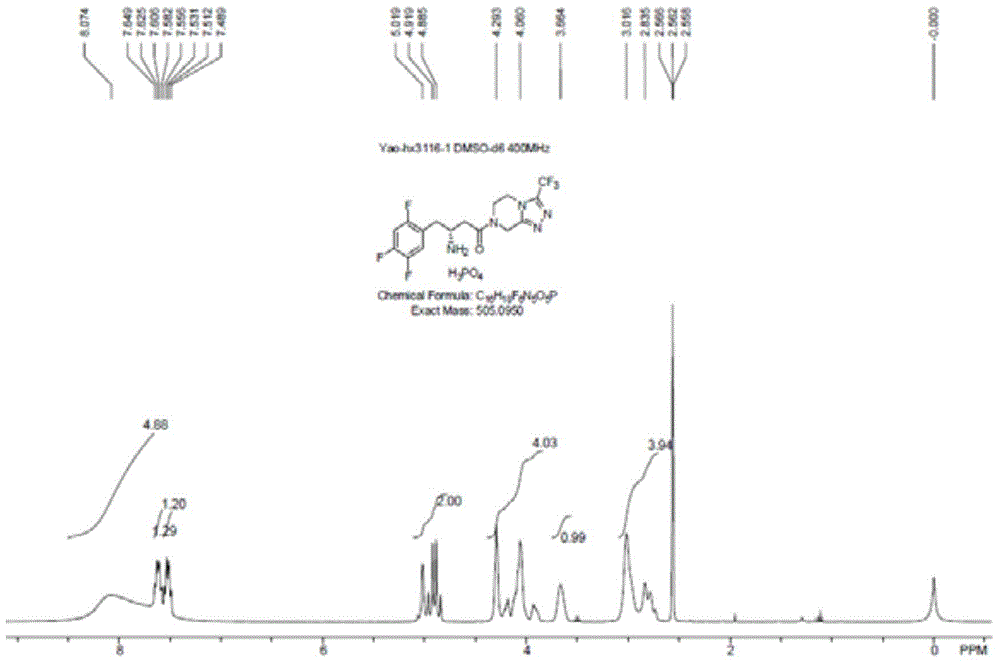
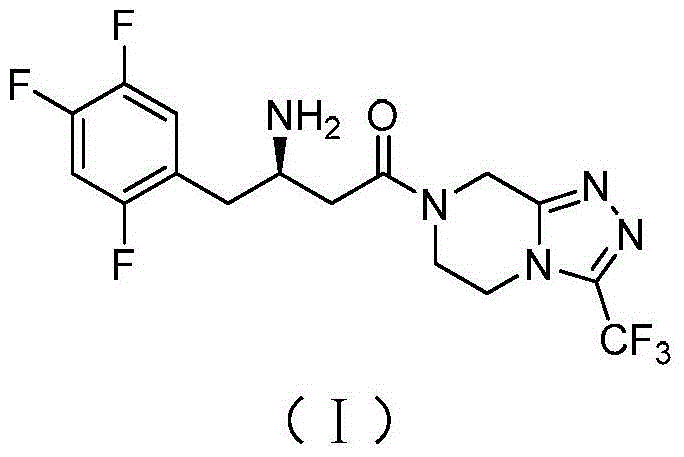
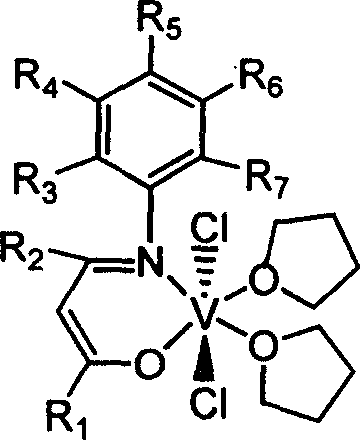
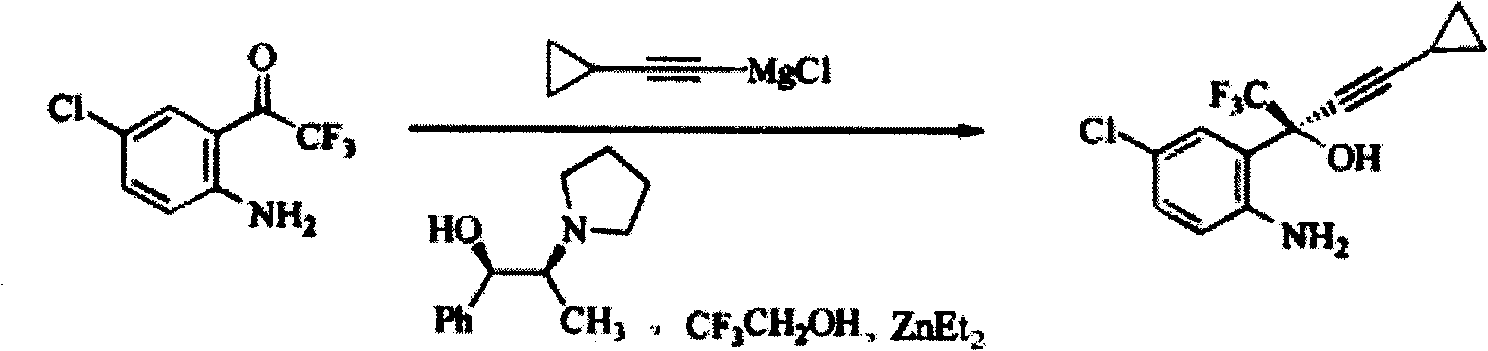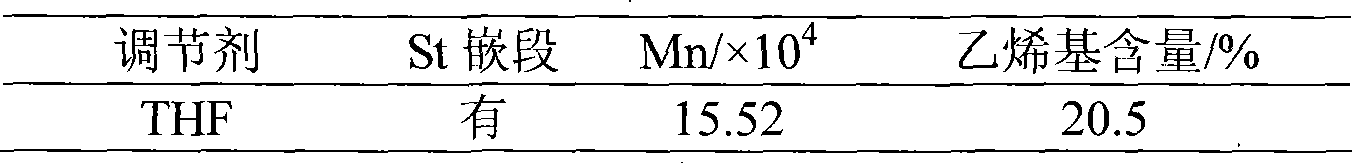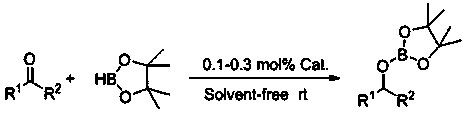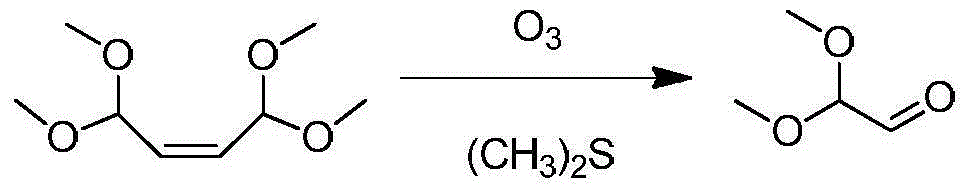Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
428 results about "Butyl lithium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyl lithium or butyillithium is a strong organic base, widely used in chemical synthesis. In organic reactions, butyl lithium is abbreviated as BuLi.
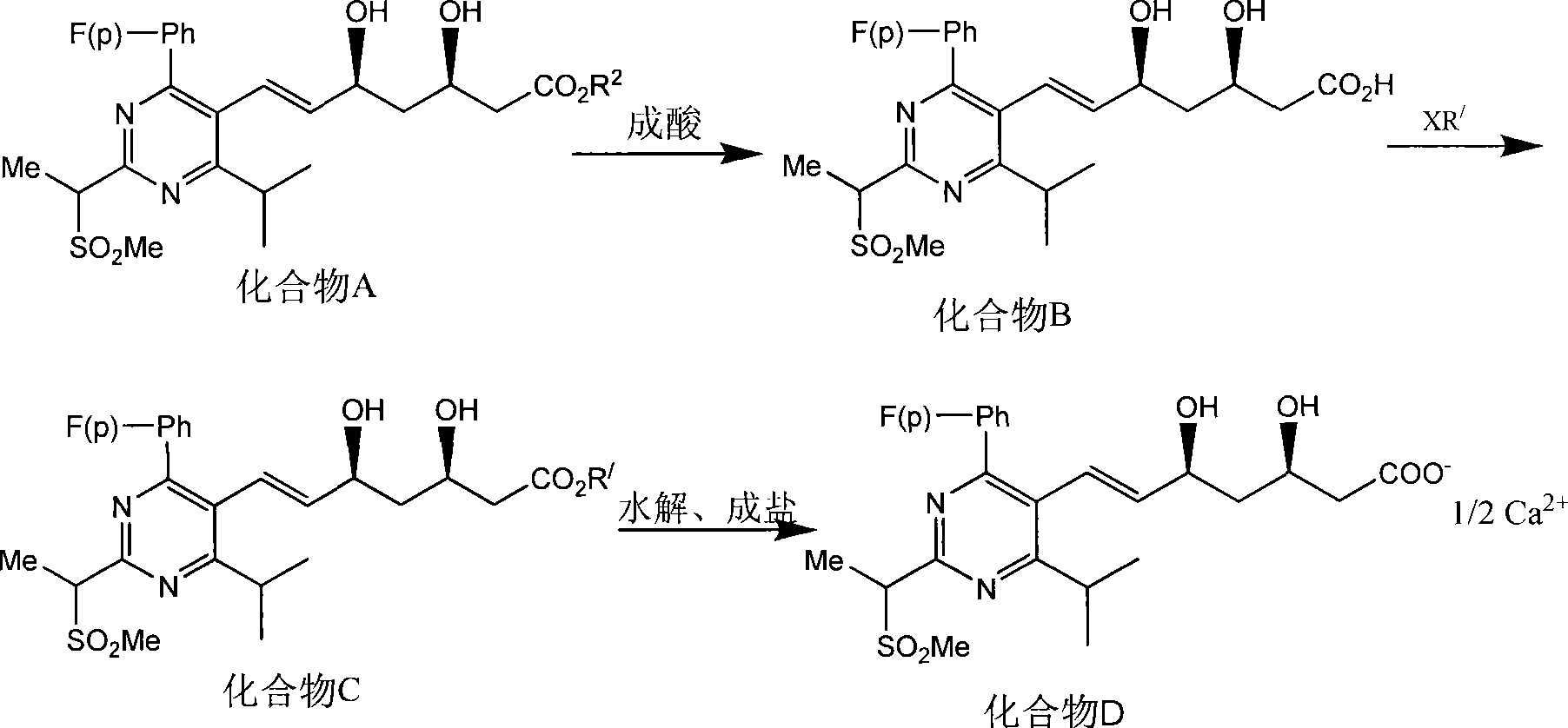
Method for synthesizing rosuvastatin intermediate and rosuvastatin
The invention provides a synthetic method of rosuvastatin calcium, which is characterized in that a compound 6 and branched chain react under the action of 2,2,6,6,-tetramethylpiperidine and n-butyl lithium to generate a compound 7, the reaction temperature is increased to minus 30 DEG C and the aspects such as reaction solvent and reaction reagent are improved so that the total reaction route has the advantages of mild condition, low device requirement and suitability for industrial production. Meanwhile, another improved synthetic route of rosuvastatin calcium comprises the following steps: converting esters into acids, synthesizing esters again, and synthesizing the rosuvastatin calcium. The rosuvastatin calcium purity is as high as 99.8%.
Owner:ENANTIOTECH CORP
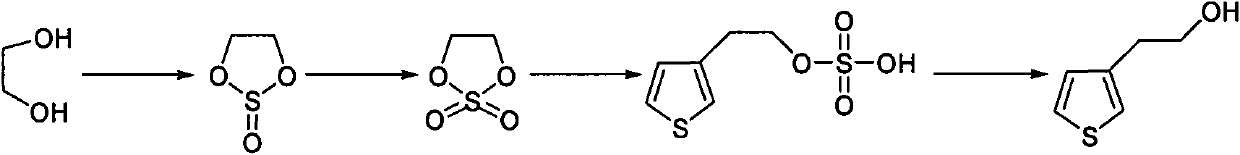
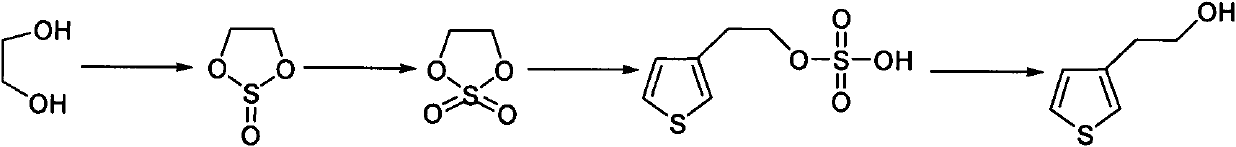
Synthetic method of thiophene-3-ethanol
The invention discloses a synthetic method of thiophene-3-ethanol. The method comprises the following steps of: adding a halogenated hydrocarbon solvent and ethylene glycol into a reaction kettle, dropwise adding thionyl chloride and preserving heat for reacting; separating liquid and extracting to obtain an organic phase containing a substance shown in the specifications; adding a ruthenium trichloride aqueous solution and a sodium bicarbonate aqueous solution in the presence of the halogenated hydrocarbon solvent and dropwise adding a sodium hypochlorite aqueous solution; after detecting that a system does not have oxidizing property, performing liquid separation, concentration, devitrification and drying to obtain a substance shown in the specifications, adding an ester solvent and butyl lithium into a reaction kettle, adding a prepared ester solution of tribromothiofuran and a prepared ester solution of the substance, separating the liquid and extracting to obtain a system containing a substance shown in the specifications; and adding a dilute sulfuric acid into the system containing the substance shown in the specifications, concentrating, neutralizing, extracting and concentrating to obtain an end product. The method has the advantages of high reaction purity and yield, stable process condition, easiness for operation and mass production capability; and the thiophene-3-ethanol is prepared from tribromothiofuran by performing low-temperature lithiation, so that the use of epoxy ethane serving as an explosive hazard is avoided, and mass production becomes possible.
Owner:ASYMCHEM LAB TIANJIN +4
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
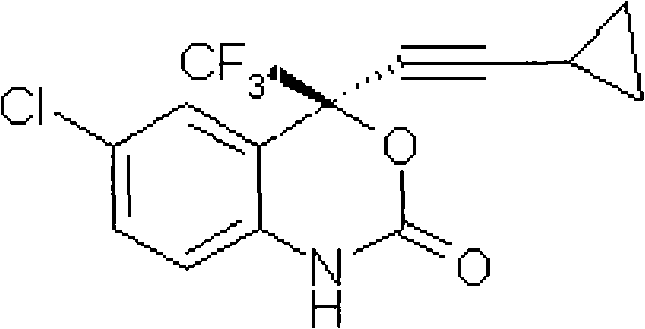
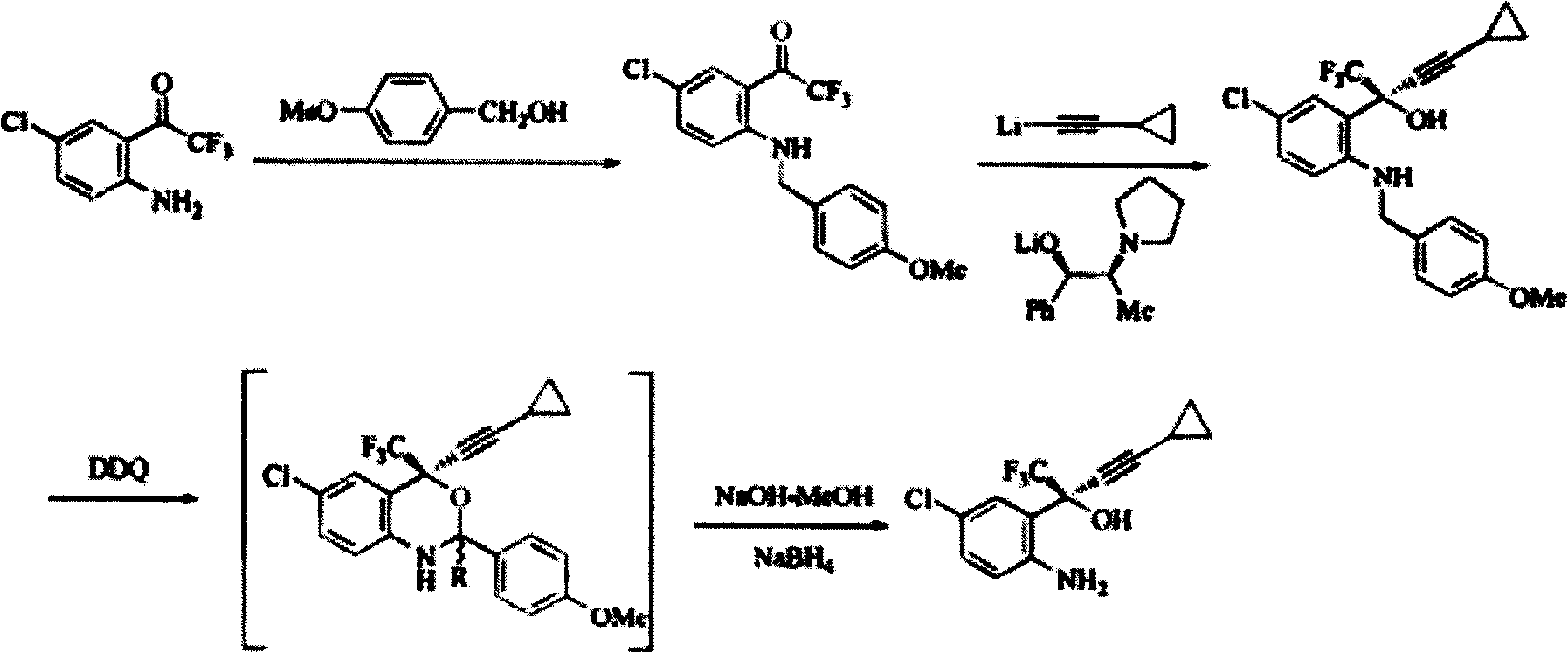
Method for preparing chiral cylopropyl acetenyl tertiary alcohol compound
InactiveCN101786959ALow costSuitable for industrial productionOrganic compound preparationAmino-hyroxy compound preparationTrifluorideButyl lithium
The invention discloses a method for preparing a chiral cylopropyl acetenyl tertiary alcohol compound.The steps of the method are as follows: (a) cylopropyl ethylnen or other derivatives react with a chiral induction reagent, a chirality auxiliary reagent and zinc halide in an organic solvent under the action of an alkaline reagent so as to obtain a zinc complex; (b) addition reaction is carried out between the obtained zinc complex and 5-chlorin-2-amino trifluoro-benzophenone; (c) (S)-2-amino-5-chlorin-alpha-cylopropyl ethylnen-alpha-benzyl alcohol trifluoride is collected from reaction liquid. Compared with butyl lithium and diethylzinc, the alkaline reagent adopted in the invention is safer; in addition, the cheap zinc halide is used as a ligand, which greatly reduces the cost. Therefore, the method is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP +1
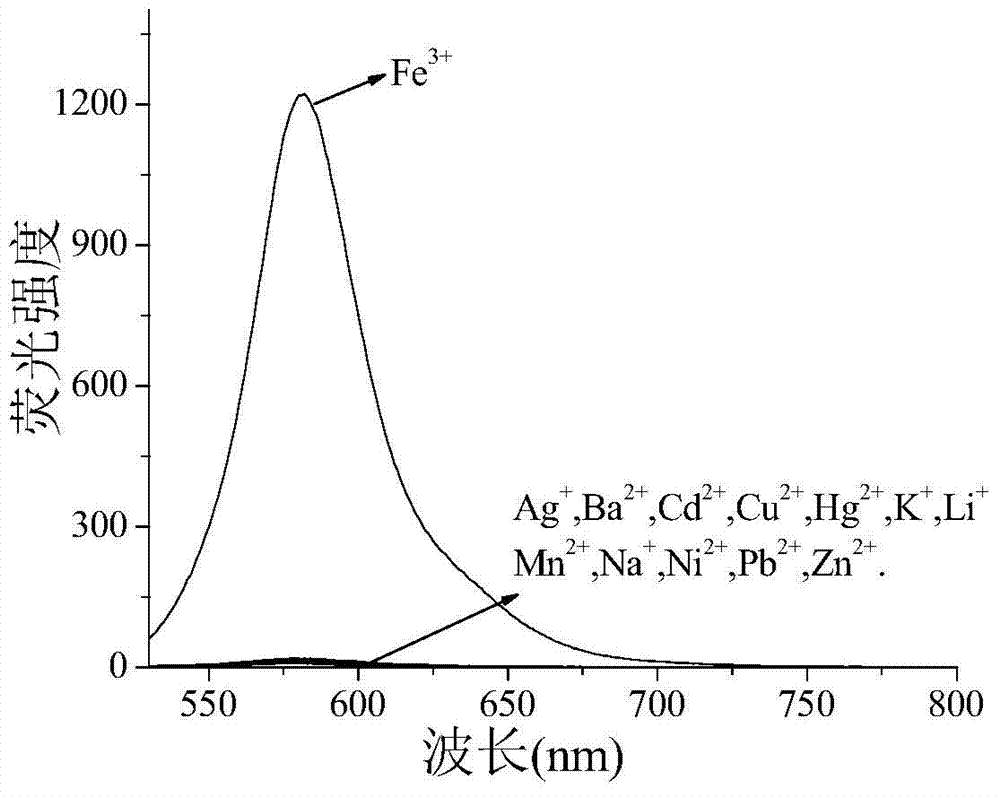
Ferric ion fluorescent probe compound as well as preparation method and application thereof
InactiveCN104496997AInnovative designGood choiceOrganic chemistryFluorescence/phosphorescenceN dimethylformamideButyl lithium
The invention relates to a ferric fluorescent probe compound as well as a preparation method and application thereof. The ferric fluorescent probe compound has the structure in formula I in the specification. The preparation method comprises the following steps: mixing rhodamine B with anhydrous ethanediamine and refluxing in ethyl alcohol to prepare amide of rhodamine; protecting hydroxyl of binaphthol by chloromethyl methyl ether, reacting with N,N-dimethylformamide under the action of butyl lithium, and removing methyl of methyl ether by hydrochloric acid to obtain binaphthol with aldehyde group; finally refluxing the amide of rhodamine and the binaphthol with aldehyde group in ethyl alcohol and washing to obtain the pure ferric fluorescent probe compound. The probe compound has favorable selectivity and sensitivity for the ferric ions, is simple to treat before sample detection and free of causing toxicity to cells, and is used for detecting the ferric ions in the water environment or the biological cells.
Owner:UNIV OF JINAN
(NH4+)XMOS2 intercalation compound and its preparing method
InactiveCN101024516ASolve storage stability issuesPrepared successfullyMolybdenum oxides/hydroxidesButyl lithiumMolybdenum disulfide
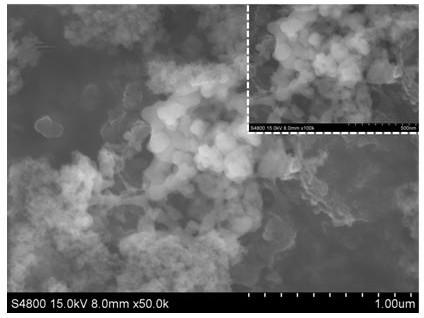
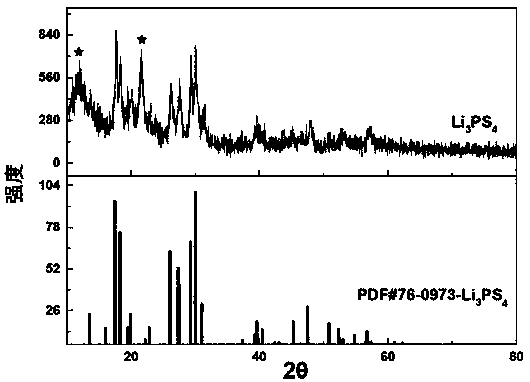
The invention discloses (NH4+)xMoS2 inserting compound and x is 0.2-3.0. The method includes the following steps: taking layer inserting to supramoly by using butyl lithium to gain layer inserting supramoly, making supramoly supernatant liquid, making (NH4+)xMoS2 layer inserting compound in ammonium chloride solution. The invention could be used as a good solid lubricant and could heating with alkali to gain single layer supramoly. It solves the storing problem of single layer of supramoly. It has crucial application value in mechanical, electric, aviation and spaceflight, chemical, etc.
Owner:HEFEI UNIV OF TECH
Preparation method of low-mould shrinkage ratio polypropylene alloy material
The invention discloses a preparation method of a low-mould shrinkage ratio polypropylene alloy material, which comprises the following two steps: (1) adopting an anionic polymerization method, taking n-butyl lithium as the initiator, tetrahydrofuran as the activator and styrene and isoprene as the monomer, preparing a PS-isoprene-styrene product; (2) putting 5 to 20 parts of PS-isoprene-styrene prepared in step (1), 60 to 80 parts of polypropylene resin, 2 to 10 parts of compatilizer, 200 to 1500ppm of nucleator and 500 to 2000ppm of antioxidant into a mixer to uniformly mix and prepare mixture; and then feeding the mixture into an extruder to extrude, cut into particles and dry, and obtain the polypropylene alloy material. The polypropylene alloy material has the mechanical property of ABS level, can be used for various plastic precision parts so as to replace ABS and realize universal plastic engineering.
Owner:CHINA PETROLEUM & CHEM CORP
Method for synthesizing lithium sulfide
InactiveCN111517288ASynthetic raw materials are cheap and easy to obtainThe process steps are simpleHybrid capacitor electrodesPhosphorus sulfur/selenium/tellurium compoundsMetallic lithiumN-Butyllithium
The invention mainly relates to the field of lithium battery materials, and provides a method for synthesizing lithium sulfide by taking a lithiation solution and elemental sulfur as raw materials through direct reaction, which comprises the following steps: preparing the lithiation solution, an organic ether solution containing a metal lithium-aromatic compound, a lithium iodide solution and an n-butyl lithium solution; adding elemental sulfur into the lithiation solution for mixing; and sequentially carrying out a room-temperature reaction, separation and precipitation, drying and heat treatment on the mixed solution to obtain the lithium sulfide. The synthesis method of lithium sulfide has the advantages of cheap and easily available raw materials, simple process, no harmful gas generation, and mild and controllable synthesis process.
Owner:TIANMU LAKE INST OF ADVANCED ENERGY STORAGE TECH CO LTD +2
Method for preparing high vinyl solution polymerized butylbenzene by high temperature continuous polymerization technique
The invention discloses a method for preparing high-vinyl solution styrene butadiene through a high-temperature continuous polymerization process. Butyl lithium is taken as an initiator of anionic polymerization, an alkyl tetrahydrofurfuryl ether is taken as a polarity regulator, and the continuous polymerization process is adopted to synthesize high-vinyl solution butadiene styrene at a polymerization temperature of between 100 and 130 DEG C. The vinyl content can reach more than 30 percent, thereby meeting the practical production requirements. The method has the advantages that the alkyl tetrahydrofurfuryl ether is taken as the anion regulator for the homopolymerization of alkadiene or the copolymerization of alkadiene and other olefins; under the condition of the high-temperature continuous polymerization, higher activity is still maintained so as to effectively regulate the vinyl content in the alkadiene.
Owner:DALIAN MARITIME UNIVERSITY
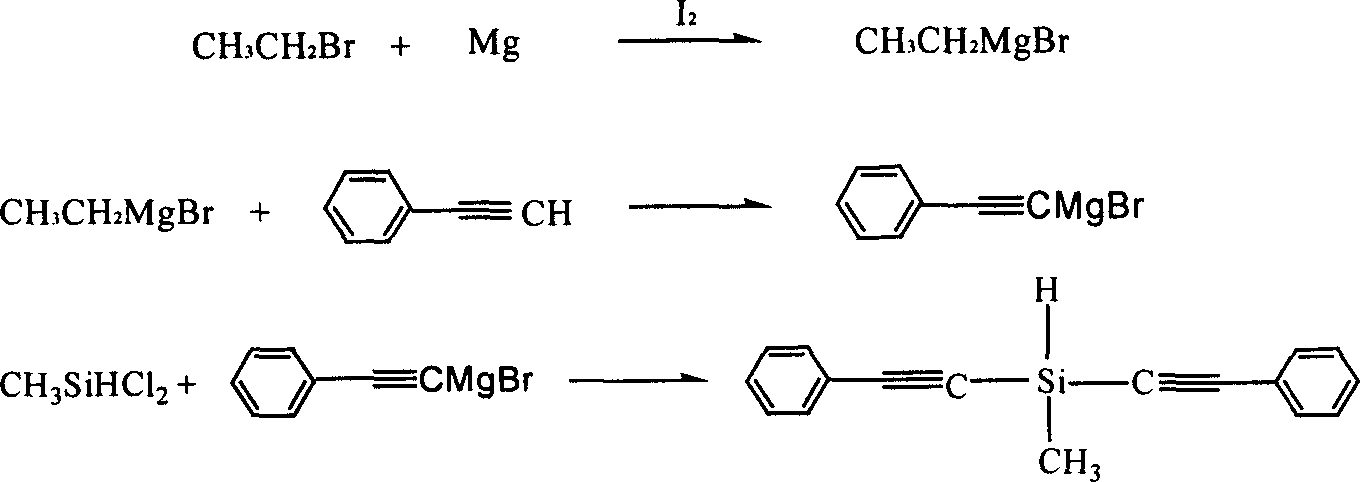
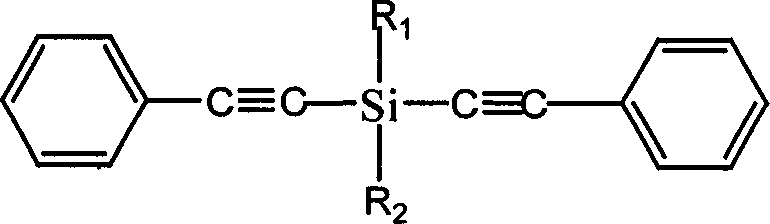
Diphenylacetylene silane novle synthesis method
InactiveCN1763053ASimple processSimple operation processSilicon organic compoundsSilanesSynthesis methods
The present invention is new type of diphenyl acetenyl silane synthesizing process. By using phenyl acetylene, organic lithium reagent and methyl dichlorosilane as material and anhydrous tetrahydrofuran as solvent, the present invention synthesizes methyl diphenyl acetenyl silane monomer through two-step reaction. Phenyl acetylene and butyl lithium first react to produce phenyl acetenyl lithium, and phenyl acetenyl lithium and hydrogen-containing dichlorosilane then react to produce phenyl acetenyl silane. The present invention has simple technological process, simple and feasible operation, short reaction time, controllable reaction condition, high product yield and purity and other advantages, and is suitable for industrial production. The prepared diphenyl acetenyl silane may be used in high performance composite material, ceramic precursor, heat resistant coating, etc.
Owner:EAST CHINA UNIV OF SCI & TECH
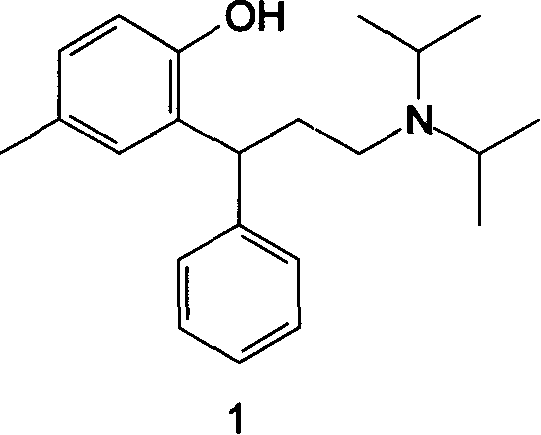
Method for preparing Tolterodine and tartrate
ActiveCN1626504AGood curative effectMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationAlcoholOrganic solvent
A process for preparing Tuoteluoding and its tartrate includes condensation reaction between anti-styryl alcohol ester and methylphenol, direct alkalizing, extracting in organic solvent, vacuum distilling to remove solvent, purifying to obtain Tuoteluoding, condensation reaction on diisopropylamine, reacting on L-(+) tartaric acid to become salt, splitting it into (L-(+)-tartrate-R-Tuoteluoding) and (L-(+)-tartrate-S-Tuoteluoding), reaction of L-(+)-tartrate-S-Tuoteluoding on alkali, butyl lithium, or magnesium isopropyl bromide, and reacting no tartaric acid to obtain L-(+)-tartrate-R-Tuoteluoding.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthesis method of oxetanone
ActiveCN103694201ASynthetic method safe scale-upReduce usageCarbonyl group formation/introductionMethyl azidePtru catalyst
The invention discloses a synthesis method of oxetanone. The method comprises the steps: in an organic solvent and in the presence of a catalyst, a halide and an alkali, utilizing an oxidant to oxidize oxetanol, and then separating and purifying to obtain oxetanone. The method adopts the organic oxidation system for oxidation production of oxetanone for the first time, is cheap in adopted materials and simple in operation, avoids use of methyl azide, butyl lithium, or 1,3-dichloroacetone and other dangerous chemicals, enables the synthesis method of oxetanone to be safely amplified, and can be produced in large scale; the method is friendly to the environment and avoids use of phosphorus pentoxide and other reagents, so that the process meets the requirements of environmental protection; and moreover, the method increases the reaction yield which is increased from 50% to 80% or more, thereby greatly reducing the production cost, and being in favor of further application and development of oxetanone in organic chemistry and biological medicines.
Owner:赣州康瑞泰药业有限公司
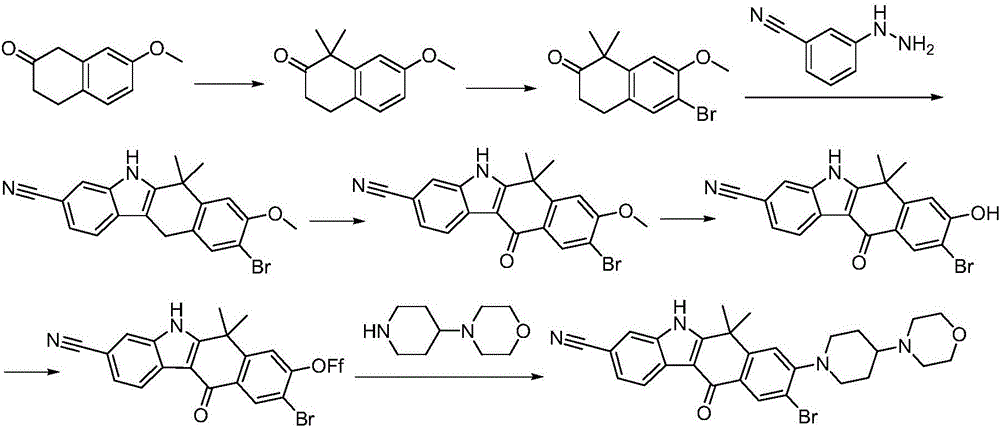
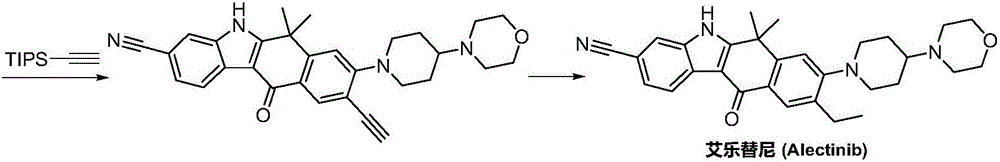
Synthesis method of Alectinib
ActiveCN105777710AMeet the needs of useApplicable generationOrganic chemistryButyl lithiumN-Butyllithium
The invention discloses a synthesis method of Alectinib. The method comprises the following steps: carrying out a borating reaction between 6-bromo-3,4-dihydro-2-naphthalenone and n-butyl lithium and then an organic boron reagent; carrying out a catalytic coupling reaction between the obtained 3,4,-dihydro-2-naphthalenone-6-boric acid and bromoethane; carrying out a dimethylation reaction between the obtained 6-ethyl-3,4-dihydro-2-naphthalenone and iodomethane; carrying out a bromination reaction between the obtained 1,1-dimethyl-6-ethyl-3,4-dihydro-2-naphthalenone and a bromination reagent; carrying out a substitution reaction between the obtained 1,1-dimethyl-6-ethyl-7-bromo-3,4-dihydro-2-naphthalenone and 4-(4-piperidyl)morpholine; carrying out a cyclization reaction between the obtained 1,1-dimethyl-6-ethyl-7-[4-(morpholine-4-yl)piperidine-1-yl]-3,4-dihydro-2-naphthalenone and 3-cyanophenylhydrazine; and carrying out an oxidation reaction between the obtained 9-ethyl-6,6-dimethyl-8-[4-(morpholine-4-yl)piperidine-1-yl]-6,11-dihydro-5H-benzo[b]carbazole-3-formonitrile and dichlorodicyanobenzoquinone to obtain a finished product of Alectinib. The synthesis method has the advantages of relatively short route, simplified operation and relatively low cost and is a green and environment-friendly method suitable for industrial production.
Owner:湖南欧亚药业有限公司
Application of n-butyllithium in catalytic hydroboration of ketone and borane
InactiveCN108654692AEfficient hydroboration reactionReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsN-ButyllithiumKetone
The invention discloses an application of n-butylllithium in catalytic hydroboration of ketone and borane. The n-butyllithium is a commercial n-butyl lithium reagent, and the method includes: adding borane to a reaction flask subjected to dehydration and deoxygenation treatment without oxygen and water at inert gas atmosphere; adding a catalyst n-butyllithium with even mixing prior to adding ketone for hydroboration. The catalyst has good universality to aromatic ketones with different substitution positions and different electron effects as well as heterocyclic ketone and aliphatic ketone, and more choices for borate compounds with different substituent structures are provided.
Owner:SUZHOU UNIV
Solution polymerized butadiene-styrene random copolymer, preparation method thereof, and applications of composite conditioning agent
The invention discloses a solution polymerized butadiene-styrene random copolymer, a preparation method thereof, and applications of a composite conditioning agent. The preparation method comprises following steps: under the protection of nitrogen, alkyl tetrahydrofurfuryl ether and an amine compound are taken as the composite conditioning agent, a premixed polymer monomer of butadiene and styrene and initiator butyl lithium are delivered into a reaction kettle uniformly continuously at the same time for random copolymerization reaction; after polymerization reaction, a coupling agent is added for coupling; after coupling reaction, an antioxidant is added, an obtained product is subjected to water vapor condensation, and is dried so as to obtain the solution polymerized butadiene-styrene random copolymer. The preparation method is simple, and is capable of inhibiting generation of gel in the polymerization kettle; maintenance and cleaning frequency is reduced; production efficiency is increased; and the preparation method is suitable for large-scale industrial production.
Owner:CHINA PETROLEUM & CHEM CORP
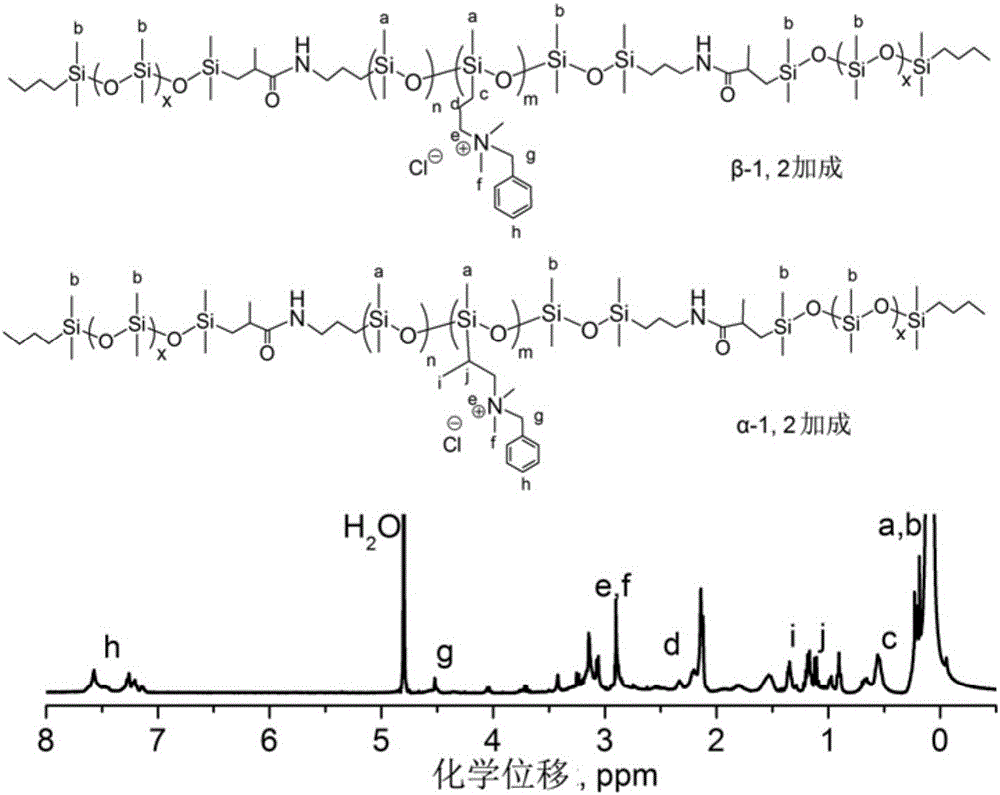
Quaternary ammonium salt group containing polysiloxane block copolymer and preparation method and application
ActiveCN105885054AGood water solubilityEnhanced inhibitory effectBiocideFungicidesQuaternary ammonium cationHalohydrocarbon
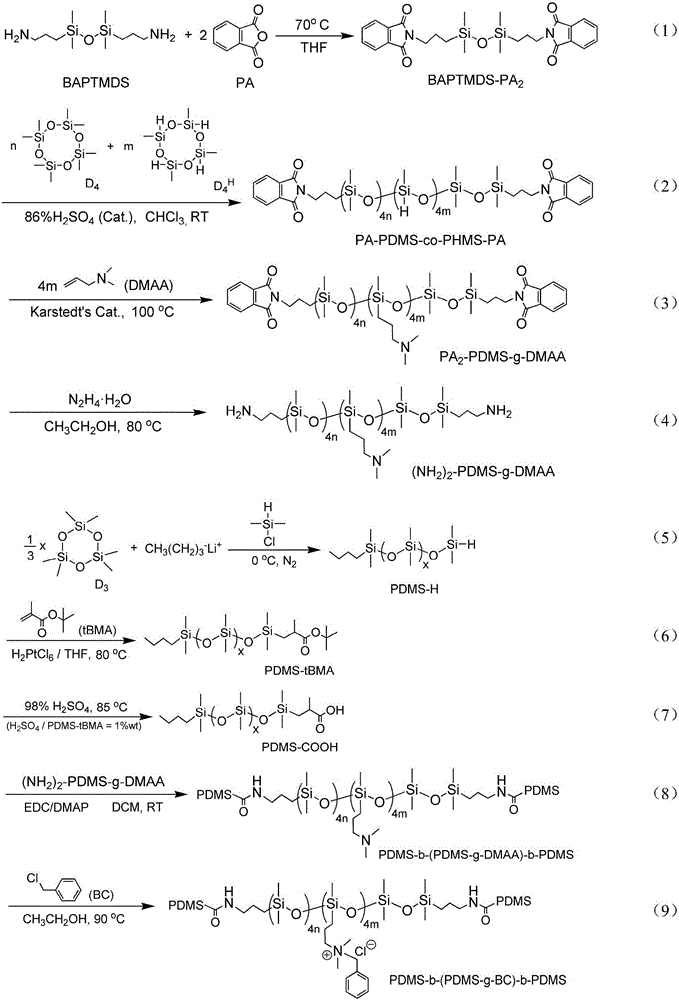
The invention discloses a quaternary ammonium salt group containing polysiloxane block copolymer and a preparation method and application. The primary amino in BAPTMDS is protected, and the obtained BAPTMDS-PA2 reacts with D4 and D4H to obtain hydrogen containing polysiloxane; the hydrogen containing polysiloxane reacts with DMAA to obtain tertiary amino polysiloxane; the tertiary amino polysiloxane reacts with hydrazine hydrate to obtain tertiary amino polysiloxane with terminated amino; D3 reacts with an n-butyl lithium solution to obtain single-ended hydrogen containing polysiloxane; the single-ended hydrogen containing polysiloxane reacts with tBMA to obtain single-ended ester-based polysiloxane; the single-ended ester-based polysiloxane reacts with sulfuric acid to obtain single-ended carboxyl polysiloxane; the single-ended carboxyl polysiloxane reacts with the tertiary amino polysiloxane with terminated amino and then reacts with halohydrocarbon to obtain the quaternary ammonium salt group containing polysiloxane block copolymer. The copolymer can effectively adhere to the hydrophobic surfaces of plant leaves and the like and plays the role of preventing plant fungi diseases.
Owner:SOUTH CHINA UNIV OF TECH
Polarized SEBS and its preparing mthod
The present invention provides a kind of polarized SEBS and its preparation process. The product may be expressed as SEBS-P, where S expresses polystyrene block, EB hydrogenated polybutadiene block, and P polarized block formed via polymerizing polar vinyl pyridine or methacrylate monomer. The preparation process adopts butyl lithium as initiator, tetrahydrofuran as activator and cyclohexane as solvent and includes the steps of the first preparing four block SBS-P polymer, and the subsequent selective hydrogenation to obtain polar SEBS. The present invention produces and polarizes SEBS simultaneously, and thus has high polar monomer converting rate, no need of single treatment of un-converted monomer, simple operation and low production cost. The prepared polar SEBS has obvious polarity, excellent performance, and expanded application field.
Owner:BALING PETRO CHEM CO LTD SINOPEC +1
Bridged diamidino group-IV metal catalyst and method for preparing same
The invention provides a bridged diamidino group-IV metal catalyst, which relates to an olefin polymerization catalyst, in particular to a compound taking metals of a group IV as central atoms and a seven-element skeleton with an N-C-N-Si-N-C-N characteristic as a ligand. A method for preparing the bridged diamidino group-IV metal catalyst comprises the following steps: in the protection of nitrogen, taking bridging diamine as an initial raw material and converting the bridging diamine into a dilithium salt by utilizing butyl lithium; adding cyanophenyl into the dilithium salt to produce an addition reaction, forming a bridged diamidino ligand after migrating a silicon base twice, and performing a complex reaction on the polydentate ligand and group-IV metal chloride of the group IV to prepare bridged diamidino group-IV metal chloride; and reacting lithium methide with the bridged diamidino group-IV metal chloride to displace helium atoms to produce a methyl substitute. The synthesis method has the advantages of universal applicability, moderate reaction condition, simple and easily-obtained materials, low cost, simple steps and relatively higher productivity. The compound has good catalytic effect on the polyreaction of olefin.
Owner:SHANXI UNIV
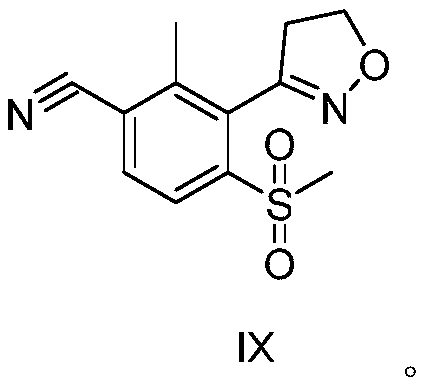
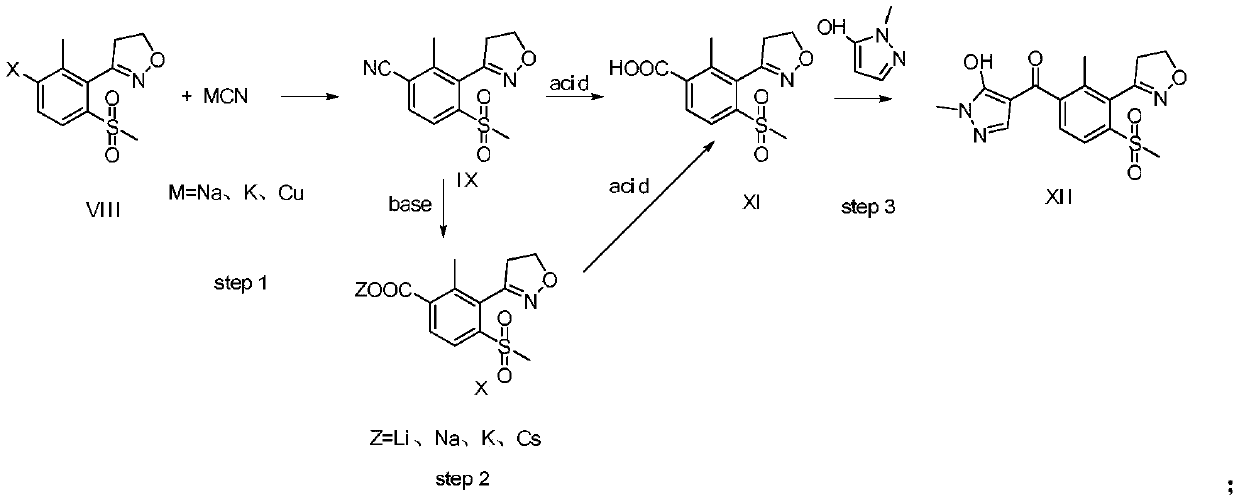
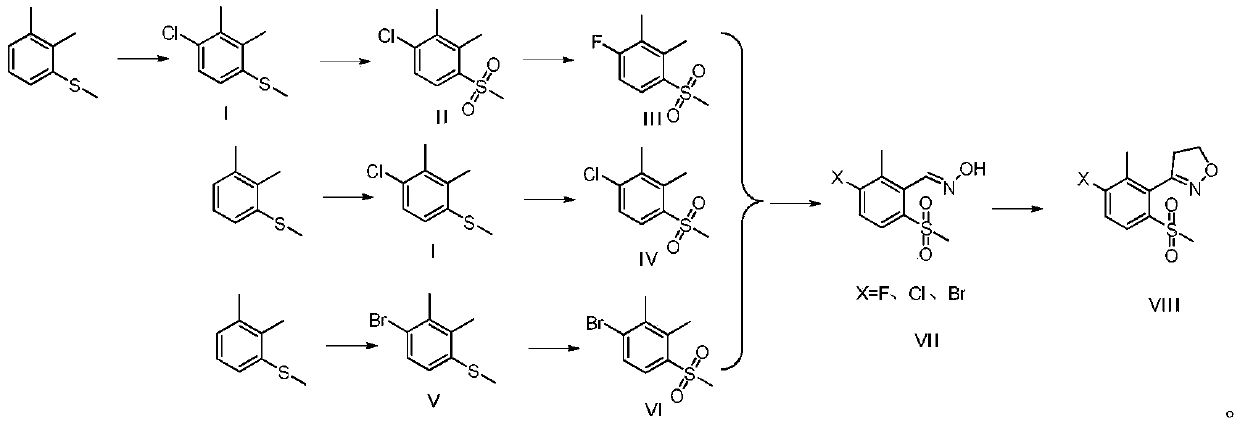
Topramezone intermediate and topramezone preparation method
The invention relates to the field of organic synthesis, in particular to a topramezone intermediate and a topramezone preparation method. The topramezone preparation method comprises the following steps: 3-[3-halogen-2-methyl-6-(methylsulfonyl)phenyl]-4, 5-dihydroisoxazole and cyanide are subjected to a substitution reaction to obtain 3-[3-cyano-2-methyl-6-(methylsulfonyl)phenyl]-4, 5-dihydroisoxazole; hydrolysis reaction is performed on 3-[3-cyano-2-methyl-6-(methylsulfonyl)phenyl]-4, 5-dihydroisoxazole under the action of acid or alkali to obtain 2-methyl-3-(4, 5-dihydroisoxazole-3-yl)-4-methylsulfonyl benzoic acid; and 2-methyl-3-(4, 5-dihydroisoxazole-3-yl)-4-methylsulfonyl benzoic acid and 1-methyl-5-hydroxypyrazole are condensed and rearranged to generate topramezone. According to the topramezone intermediate and the topramezone preparation method provided by the invention, the use of an expensive palladium catalyst and a dangerous butyl lithium reagent is avoided, the yield isrelatively high, the cost is reduced, the process is simplified, the defects in the prior art are overcome, and the industrial value is achieved.
Owner:JIANGSU FLAG CHEM IND
1, 1'-ferrocene perfluoroalkyl phosphine nitrogen ligand as well as preparation method and application thereof
ActiveCN103772445AEasy to synthesizeGood effectOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDiphenyl phosphiteDisplacement reactions
The invention relates to a 1, 1'-ferrocene perfluoroalkyl phosphine nitrogen ligand as well as a preparation method and an application thereof. The preparation method of the 1, 1'-ferrocene perfluoroalkyl phosphine nitrogen ligand comprises the following steps: (1) reacting oxazoline bromoferrocene with a butyl lithium compound in an organic solvent under the condition of existence of an additive; (2) mixing reaction liquid obtained in the step (1) with triphenyl phosphite; and (3) in the solvent, reacting an isodecyl diphenyl phosphite derivative obtained in the step (2) and cesium fluoride with TMSCnF2n+1. The invention also relates to the application of the ligand in an asymmetric allyl displacement reaction catalyzed by metallic palladium. The ligand provided by the invention can be used for catalyzing the allyl displacement reaction of single substituted allyl carbonic ester in a high domain selectivity and high enantioselectivity manner. The synthetic method is simple, mild in conditions, high in yield, convenient in purification and suitable for industrial production; starting materials are low in cost and easy to obtain.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Synthetic method of sitagliptin and salt thereof
The invention discloses a synthetic method of sitagliptin and a salt thereof. The synthetic method comprises the steps: carrying out an esterification reaction, a reduction reaction, an oxidizing reaction and a witting reaction on 2,4,5-trifluorophenylacetic acid as a starting raw material to obtain 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate; then carrying out a hydroamination reaction on 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate and chiral amine in the presence of butyl lithium or hexamethyldisilazane sodium to form a chiral hydroamination product; carrying out an esterolysis reaction, a condensation reaction and a hydrogenation reaction to obtain sitagliptin. The raw materials used in the synthetic method of sitagliptin are low in price and easy to obtain; the synthetic method of sitagliptin is less in step, easy to operate and capable of effectively reducing cost. By the use of the method, high-purity sitagliptin can be obtained, a sitgliptin phosphate obtained through salifying has an HPLC (High Performance Liquid Chromatography) and an ee (enantiomeric excess) value of more than 99% and can be applied to the field of medicine.
Owner:ZHEJIANG NHU CO LTD +1
Synthesis method for chiral intermediate of atorvastatin calcium
ActiveCN105153110AEasy to operateGood repeatabilityOrganic chemistry methodsChemical synthesisCyanide
The invention discloses a synthesis method for a chiral intermediate of atorvastatin calcium, and belongs to the technical field of medical intermediate synthesis. The synthesis method is characterized in that according to the process route, not only are dangerous, highly toxic and expensive chemicals such as butyl lithium, editpotassium cyanide and periodic acid in chemical synthesis prevented from being used, but also an ee value of the chiral intermediate is effectively improved due to usage of a mixed chiral catalysts of titanium iso-propylate and S-xenol. According to the synthesis method, the raw materials are low in cost and easy to obtain, the route operation is easy, the repeatability is good, the yield is very high, and the synthesis method is suitable for industrial production.
Owner:北京华素制药股份有限公司
Beta-diketo mono imine vanadium olefinic polymerization catalyst, and its preparing method and use
The invention discloses a beta-diketone mono-imide vanadium olefinic polymerization catalyst and the manufacture method and the application of ethylene polymerizing, ethylene and norborene polymerizing, ethylene and alpha-alkene or norborene copolymerization. Under the catalysis of formic acid, beta-diketone compound and aniline or the ramification of aniline taking condensation reaction in methanol solution to gain Schiff base; under the non water and non oxygen condition, the Schiff base taking reaction with butyl lithium to gain negative ion ligand; under the non-water, non oxygen condition, the negative ion ligand taking coordination reaction with VCl3, the beta-ketimine vanadium alkene polymerization catalyst could be gained. The invention could catalyze ethylene polymerization, and the copolymerizing of ethylene and alpha-alkene or norborene.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Cyclic esters compound polymerization catalyst, preparation method and application thereof
The invention belongs to a cyclic esters compound polymerization catalyst, a preparation method and the application thereof. The cyclic esters compound polymerization catalyst has the structure of dual (dual trimethyl silica-based amino) zinc (Zn (N (SiMe3)2)2. Hexamethyl bi-silica-based ammonia is taken as substrate, butyl lithium and anhydrous zinc chloride are taken as reactants, and the dual (dual trimethyl silica-based amino) zinc is synthesized by two-step reaction; the dual (dual trimethyl silica-based amino) zinc is uses as catalyst, an alcohols or phenolic compound is taken as initiator, l-lactide, caprolactone and cyclic carbonate are taken as monomers, polyesters homopolymer or copolymer can be prepared by adopting a way of solution polymerization, bulk polymerization or block copolymerization and being stirred for 5min-48h at the reaction temperature of 0-210 DEG C. The method has rapid speed of polymerization reaction and high monomer conversion rate; the obtained polyester has the number average molecular weight of 10-100 thousands and the molecular weight distribution of 1.1-1.7, is higher in three-dimensional regularity and melting point, and is suitable for the production and the application of high performance polyester materials with multiple specifications.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for preparing medium-high vinyl block-free solution-polymerized butylbenzene and product thereof
The invention discloses a method for preparing medium-high vinyl block-free solution-polymerized butylbenzene and a product thereof. The method mainly comprises the following steps of: A, undergoing a polymerization reaction on a vinyl aromatic hydrocarbon and an insufficient reacting dose of conjugated diene; and B, continually reacting the remaining reacting dose of conjugated diene. In a medium-high vinyl block-free solution-polymerized butylbenzene product, the content of a styrol block is zero, and the content of the vinyl is between 25 percent and 60 percent. In the method, n-butyl lithium is taken as an initiator for anionic polymerization, a compound product of alkyl tetrahydrofurfuryl ether and sodium alkyl benzene sulfonate is taken as a structure modifier, the alkyl tetrahydrofurfuryl ether can effectively adjust the content of vinyl in the product, the sodium alkyl benzene sulfonate can always keep high reaction activity, and further tail end functionalization is promoted; and high-temperature continuous polymerization is adopted, so that the produce efficiency of the product can be increased, and the product with wide molecular weight distribution can be produced; and the styrol block in the product is eliminated through stepwise reaction, so that practical requirements on production can be met.
Owner:周铁生
Preparation method of stable type compound nano anti-wearing agent
The invention discloses a preparation method of a stable type compound nano anti-wearing agent, and belongs to the technical field of preparation of lubricating oil additives. The preparation method comprises the following steps: firstly, enabling nano molybdenum disulfide to react with n-butyl lithium to obtain pretreated molybdenum disulfide; carrying out ultrasonic dispersion on the pretreated molybdenum disulfide and mercaptoethylamine; inserting the n-butyl lithium into a molybdenum disulfide sheet layer; stripping the molybdenum disulfide sheet layer in an ultrasonic treatment process, so as to expose an active group of the molybdenum disulfide; loading the mercaptoethylamine, and reacting with maleic anhydride and ethanol, so as to increase the lipophilic property of the molybdenum disulfide; coating the surface of the molybdenum disulfide with maleate, so that molybdenum disulfide powder is difficultly gathered, and the stability and uniformity are improved; finally, stirring with modified nano boron nitride, dispersing, and drying to obtain the anti-wearing agent. The anti-wearing agent prepared by the method has relatively good anti-wearing performance, and also has excellent stability and dispersity; the anti-wearing agent has no layered precipitation and no nano agglomeration phenomenon; after the anti-wearing agent is added into lubricating oil, the lubricating performance can be remarkably improved; the anti-wearing agent has relatively good application prospect.
Owner:唐林元
Synthesis of end amino phenylethylene/butadiene copolyer by tndcapping process and its method for preparing storage-stable modifie asphalt
InactiveCN1775823ASimple preparation processLow costBuilding insulationsPolymer modifiedPolymer science
The invention relates to an end capping method composing end amidogen cinnamene / butadiene copolymer and its preparation stockpile stable modified asphalt method. It belongs to polymer modified asphalt technique field. It uses butyl lithium as the anionic polymerization initiator, and 1, 5-dinirogen bicyclo caproylhydride as the anionic polymerization terminator. It makes omega-amidogen cinnamene / butadiene / cinnamene ternary block polymer. It adopts that end function SBS and the asphalt are mixed. This can effectively deduce the emanation degree of the SBS modified asphalt and obviously improve storage stability. It applies in modified asphalt manufacture field.
Owner:CHINA PETROLEUM & CHEM CORP
Non-metallocene catalyst and preparation method and application thereof
The invention discloses a non-metallocene catalyst and a preparation method and the application thereof. The general structural formula of the non-metallocene catalyst is as shown in formula I. The preparation method of the catalyst comprises the following steps: (1) pyridine or pyridone formaldehyde containing a substituent and amino phenol or amino thiophenol containing the other substituent are put into organic solvent to have reflux reaction and obtain a ligand; and (2) first the organic solution of the ligand obtained in step (1) reacts with butyl lithium, is added with metal halide to react and obtain the non-metallocene catalyst after reaction. When the non-metallocene catalyst is used for ethylene polymerization, not only dichloromethane with polarity but also non-polar toluene and heptane can be selected as the solvent during the ethylene polymerization process, and higher activity is also achieved during the process; and the polyethylene has high molecular weight, and the weight-average molecular weight is 800,000 to 1 million(Formula I is shown as the accompanying drawing.).
Owner:INST OF CHEM CHINESE ACAD OF SCI
Tetrakis(ethylmethylamino)hafnium synthesis method
InactiveCN103601750AEasy to operateSimple and fast operationGroup 4/14 element organic compoundsAlkaneN-Butyllithium
The invention relates to a tetrakis(ethylmethylamino)hafnium synthesis method, which comprises the following synthesis steps: (1) under an inert atmosphere, adding methylethylamine and an alkane solvent to a three-necked bottle, carrying out mechanical stirring, placing the reaction bottle into an environment with a temperature of -10 to -80 DEG C, adding a n-hexane solution of n-butyl lithium to the reaction bottle in a dropwise manner, and carrying out a stirring reaction after completing the addition; (2) adding hafnium tetrachloride to the reaction system, maintaining the temperature of the reaction system to not more than 60 DEG C, carrying out a stirring reaction on the reaction system under the protection of the inert gas after completing the addition; and (3) after completing the reaction, removing the reacting solvent under an atmospheric pressure, carrying out vacuum distillation after completely removing the solvent, and collecting the 110-115 DEG C / 4-5 mmHg fraction, wherein the fraction is the tetrakis(ethylmethylamino)hafnium compound. According to the present invention, the reaction adopts the simple and easily available materials such as methylethylamine, butyl lithium and hafnium tetrachloride as the raw materials, the operations are simple, and the cost is reduced.
Owner:NANJING UNIV
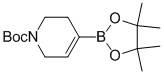
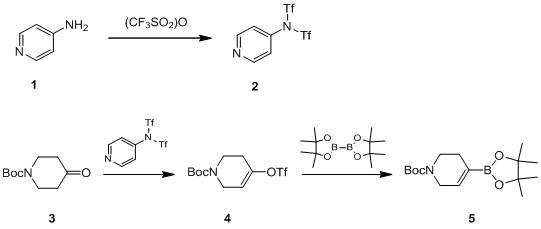
Method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester
InactiveCN102153579AShort process routeThe synthesis process is simpleGroup 3/13 element organic compoundsTrifluoromethanesulfonic anhydrideChemical synthesis
The invention provides a novel method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester, which belongs to the technical field of chemical synthesis. The method comprises the following steps of: obtaining a target product, namely the N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester through a three-step reaction by taking N-(tert-butoxycarbonyl)-4-piperidone, 4-aminopyridine, trifluoromethanesulfonic anhydride, n-butyl lithium, bis(pinacolato)diboron, triethylamine, diisopropylamine and potassium acetate as raw materials,dichloromethane, tetrahydrofuran and dioxane as solvents, and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) as a catalyst; and characterizing data through liquid chromatogram, nuclear magnetic spectrum and mass spectrum. By the method, the production period is short, the synthetic cost is low, a synthetic process is safe and reliable, and a post-treatment method is simple, convenient and quick; and the yield of a product is high (51 to 58 percent) and the purity of the product is high (98.2 to 99.6 percent).
Owner:LANZHOU MINUO BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com