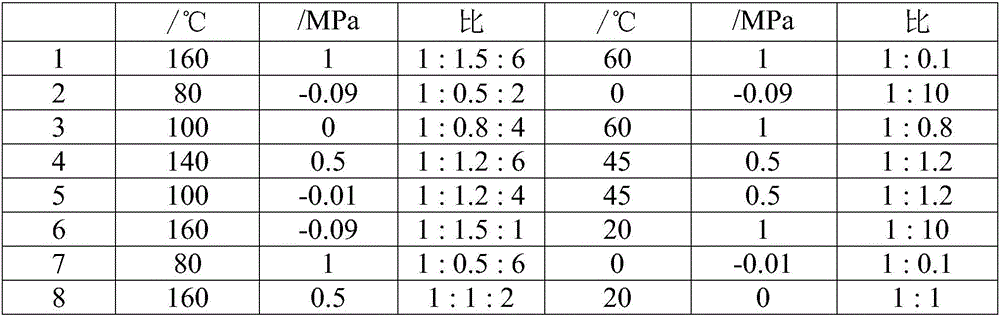
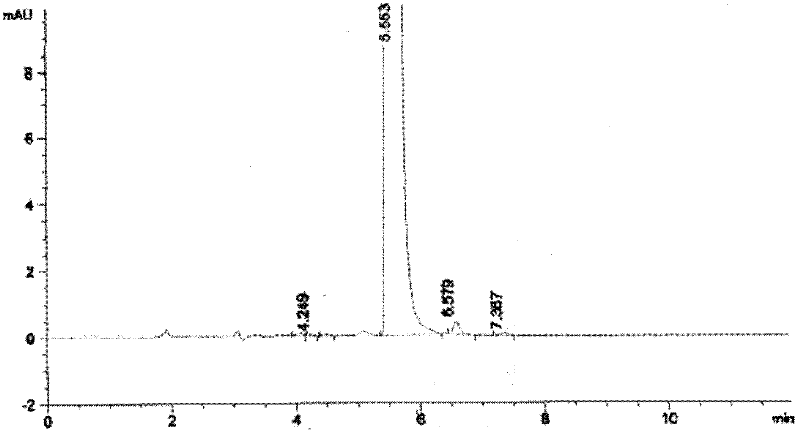
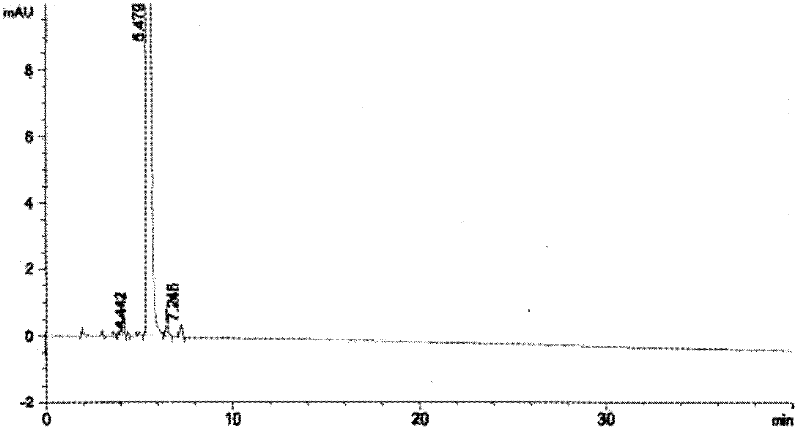
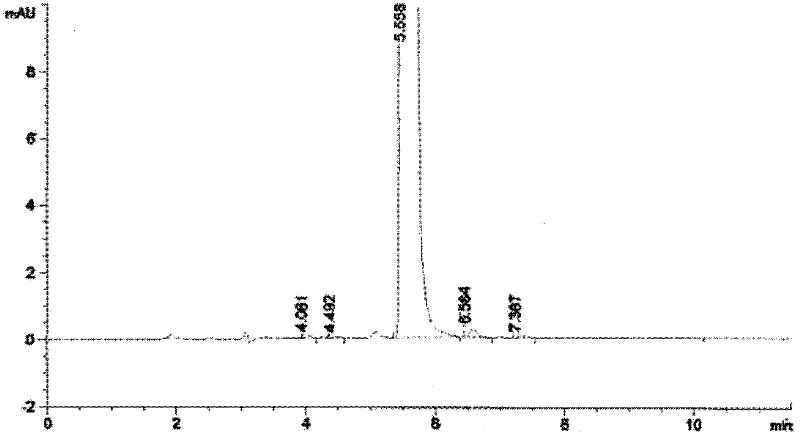
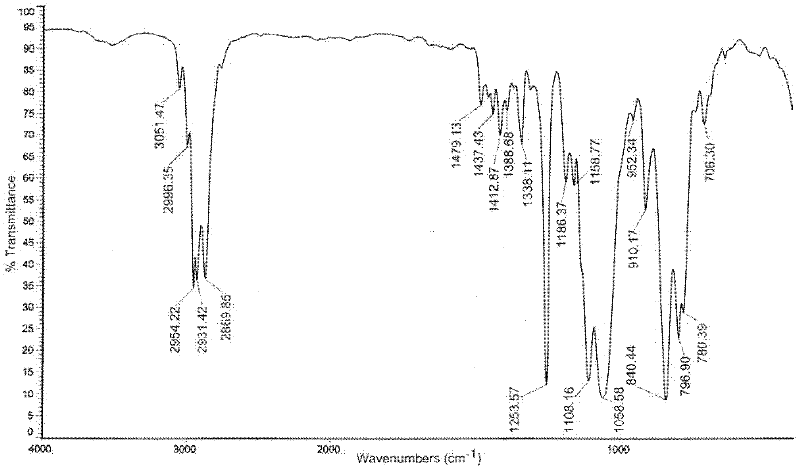
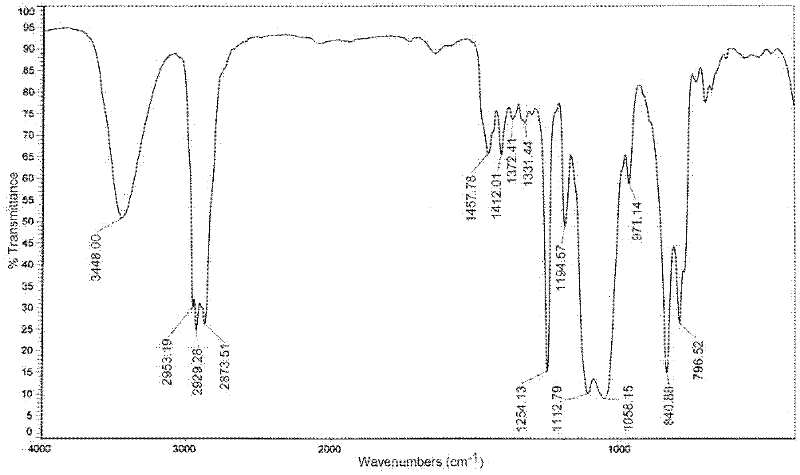
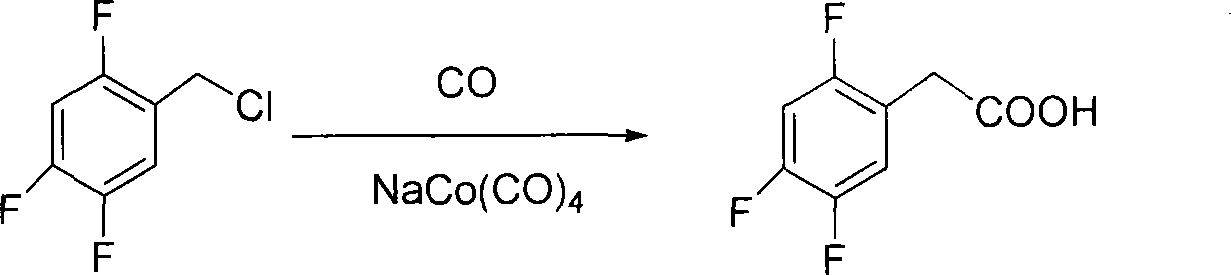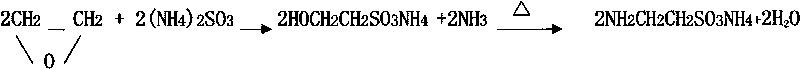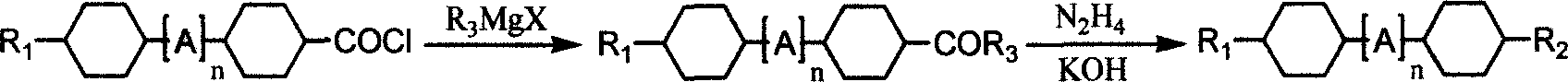Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2071results about How to "Few reaction steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of difluoro-sulfimide and lithium difluoro-sulfimide
InactiveCN106365132AFew reaction stepsSimple processNitrosyl chlorideAmidosulfonic acidChemical synthesisDistillation
The invention relates to a preparation method of difluoro-sulfimide and lithium difluoro-sulfimide, and belongs to the field of fluorine chemical synthesis. The preparation method comprises the steps that difluoro-sulfimide is obtained by adding fluoro-sulfoxide into a mixture of sulfamic acid or amino sulfonyl fluoride and fluorosulfuric acid for reacting and then subjected to recrystallization and filtration which are conducted at the temperature of minus 100 DEG C to 16 DEG C and / or reduced / normal-pressure distillation and purification which are conducted at the temperature of 60 DEG C to 169 DEG C; difluoro-sulfimide and a lithium-containing substance react in a solvent to obtain lithium difluoro-sulfimide, and then recrystallization, filtration and purification are conducted. According to the preparation method, difluoro-sulfimide can be prepared through one step, lithium difluoro-sulfimide is obtained through lithiation, and the prepared products can be purified; few steps are needed, the technology is simple, the process is easy to control, the requirement on production devices is low, the production efficiency, the product yield and the purity are high, and large-scale production and application can be achieved.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Polysaccharide conjugate of carboxylic acid drug, preparation method thereof and application thereof
InactiveCN101745119AMild conditionsFew reaction stepsPharmaceutical non-active ingredientsPharmaceutical active ingredientsCarboxylic acidPolysaccharide
The invention discloses a polysaccharide conjugate of carboxylic acid drug, a preparation thereof and application thereof. In the technical scheme, sub-alkyl diamine with 2-12 carbon atoms is used as a connecting arm, and a carboxylic acid drug and polysaccharide carboxyl are connected with each other through an amido link. Compared with the original carboxylic acid drug, the conjugate enhances the pharmacological effect, reduces the adverse effect and improve the safety. In addition, the conjugate can have the amphipathic performance through using the hydrophobic carboxylic acid drug, so as to be used as a carrier of a slightly soluble or sparingly soluble drug. The preparation method of the invention is simple, the process is mature, the yield is high, and the preparation method is applicable to industrial production.
Owner:CHINA PHARM UNIV
Method for preparing difluoromethoxy bridge type liquid crystal
InactiveCN102675062AFew reaction stepsHigh reaction yieldLiquid crystal compositionsOrganic chemistryReaction intermediateBoric acid
The invention discloses a method for preparing a difluoromethoxy bridge type liquid crystal compound, which comprises the following steps: uniformly mixing a compound shown in a formula II and a compound shown in a formula III with alkali under the condition that a catalyst exists for reaction and obtaining a compound shown in a formula I after reaction. The method has the beneficial effects that a reaction substrate is changed into the compound shown in the formula II and a phenylo boric acid derivative shown in the formula III, the reaction steps are reduced, a reaction intermediate is simplified, the total reaction yield is greatly improved, and different group compounds can use the same intermediate formula I. The method is particularly suitable for preparing the difluoromethoxy bridge type liquid crystal compound. Formula II, formula III and formula I are shown in the instruction.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Method for preparing 2,3,3,3-tetrafluoropropene
ActiveCN102001910AFew reaction stepsPreparation by hydrogen halide split-offHydrogen fluorideDistillation
The invention discloses a method for preparing 2,3,3,3-tetrafluoropropene, comprising the following steps: a. in the presence of a fluorinated catalyst, hydrogen fluoride and 1,1,2,3-tetrafluoropropene enter into a first reactor to react; b. in the presence of the fluorinated catalyst, 2-chloro-3,3,3 trifluoropropene, 2-chloro-1,1,1,2-tetrafluoropropane, 1,1,1,2,2-pentafluoropropane and hydrogen fluoride enter into a second reactor to react; c. the product flows obtained in the steps a and b enter into a first distillation tower to be separated; the tower top components are hydrogen chloride and 2,3,3,3-tetrafluoropropene which break away from the system and others are column reactor components; and d. the column reactor components in the step c enter into a second distillation tower to be separated; the column reactor components include 2-chloro-3,3,3 trifluoropropene and 2-chloro-1,1,1,2-tetrafluoropropane which are recycled to the second reactor; and the tower top components, including hydrogen fluoride and 1,1,1,2,2-pentafluoropropane, are recycled to the first reactor or / and the second reactor to be used.
Owner:山东华安近代环保科技有限公司
Method for preparing cabazitaxel
ActiveCN102336726AFew reaction stepsShort reaction cycleOrganic chemistryBulk chemical productionCabazitaxelChemical synthesis
The invention relates to the field of chemical synthesis, in particular to a method for preparing cabazitaxel. Reaction steps are reduced, a protective group is removed in a mild mode, the reaction period is shortened, and the high-purity cabazitaxel is obtained. The whole preparation method has the advantages of a few reaction steps, light pollution and suitability for industrial production.
Owner:重庆兴泰濠制药有限公司
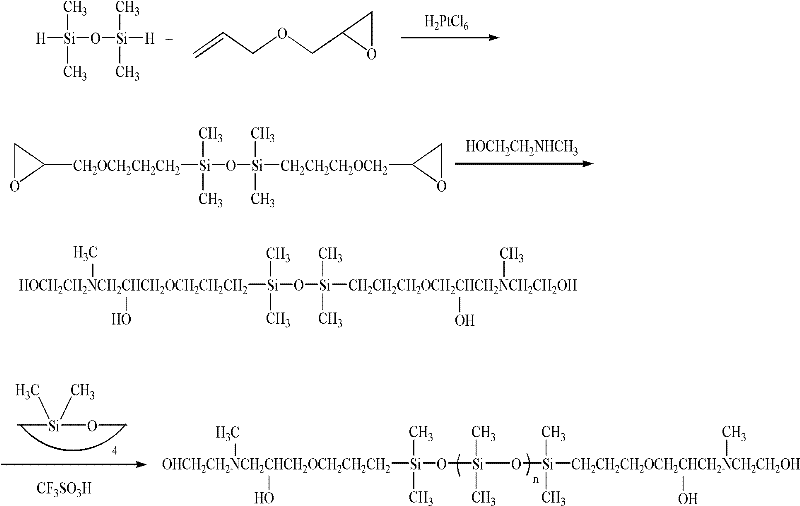
Method for synthesizing dihydroxyl-terminated polysiloxane
InactiveCN102504260AReduce manufacturing costShort overall reaction timeEpoxyTrimethylsilyl chloride
The invention discloses a method for synthesizing dihydroxyl-terminated polysiloxane. The method synthesizes the target dihydroxyl-terminated polysiloxane compound by using unsaturated epoxy compound, tetramethyldisiloxane, methylaminoethanol and octamethylcyclotetrasiloxane as starting materials and by hydrosilylation, epoxy-opening and equilibrium polymerization. The hexamethyldisilazane or trimethylchlorosilane are not needed as a hydroxyl protector, so the production cost is reduced; and hydroxyl protection and deprotection are avoided in a preparation process, so reaction steps are reduced, the total reaction time for synthesizing the final product is short and the production efficiency is increased.
Owner:邬元娟
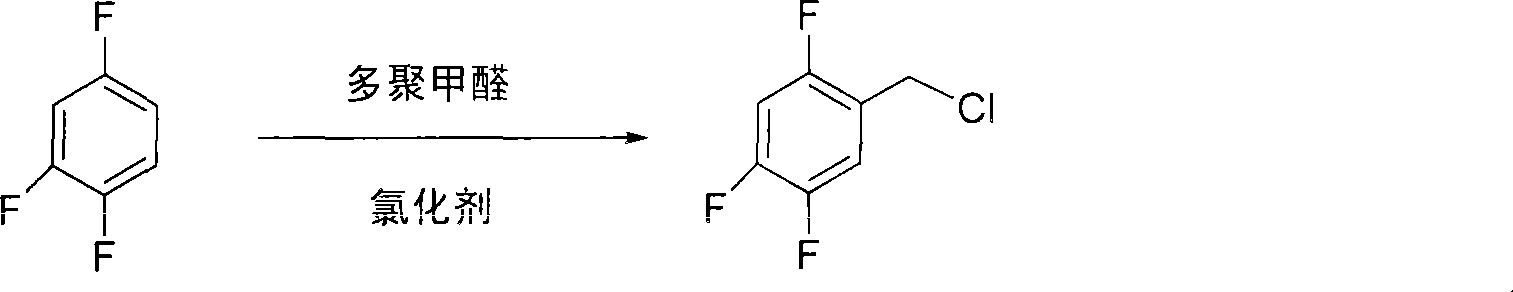
Method for preparing 2,4,5 trifluorobenzene acetic acid
ActiveCN101092345ALow priceFew reaction stepsCarboxylic preparation from carbon monoxide reactionAcetic acidPtru catalyst
This invention provides a method for preparing 2, 4, 5-trifluorophenylacetic acid. The method comprises: (1) reacting 1,2,4-trifluorobenzene and chlorinating agent in paraformaldehyde to obtain 2,4,5-trifluorobenzyl chloride; (2) performing carbonylation reaction with CO in the presence of catalyst to obtain 2,4,5-trifluorophenylacetic acid. The catalyst is alkali cobalt tetracarbonyl. The method has such advantages as few reaction procedures, and mild reaction conditions, and is suitable for industrial production of 2, 4, 5-trifluorophenylacetic acid.
Owner:ZHEJIANG YONGTAI TECH CO LTD
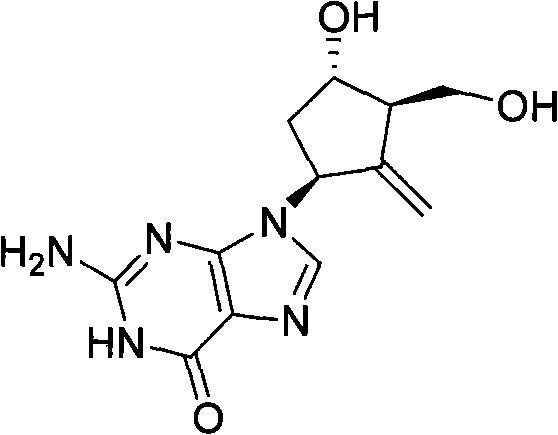
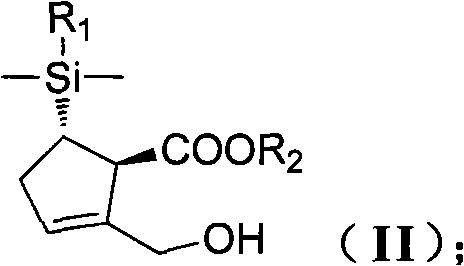
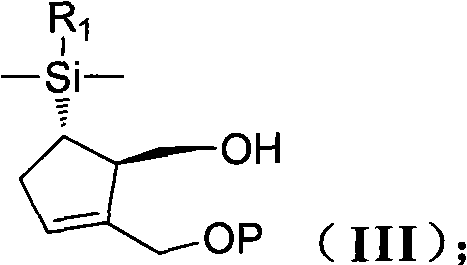
New synthesis process of antiviral drug entecavir
ActiveCN101891741AHigh purityEliminate the purification processOrganic chemistryBulk chemical productionAntiviral drugCombinatorial chemistry
The invention discloses a method for preparing a compound shown as the formula (I), which is characterized by comprising the following steps of: (1) carrying out hydroxyl protection on a compound shown as the formula (II) by using a first hydroxyl protecting group, and then, reacting with hydride to generate a compound shown as the formula (III); (2) carrying out hydroxyl protection on the compound shown as the formula (III) by using a second hydroxyl protecting group, and then, removing the first hydroxyl protecting group to obtain a compound shown as the formula (IV); (3) enabling the compound shown as the formula (IV) to carry out non-corresponding selective epoxidation reaction to generate a compound shown as the formula (V); (4) reacting the compound shown as the formula (V) with thecompound shown as the formula (VI) in a polar aprotic solvent to obtain a compound shown as the formula (VII); and (5) carrying out condensation, desilylation and oxidization on the compound shown asthe formula (VII) to generate the compound shown as the formula (I). In the general formulas of the compounds in each step, R1 is selected from naphthyl or any substituted naphthyl; R2 is selected from alkyl or benzyl of C1-C4; P is selected from hydroxyl protecting groups, such as 2-methoxyl propyl or p-methoxyl benzyl and the like; P' is a hydroxyl protecting group capable of resisting and removing P, such as benzyl; and X is selected from Cl, Br, I or benzyloxyl.
Owner:聊城高新生物技术有限公司
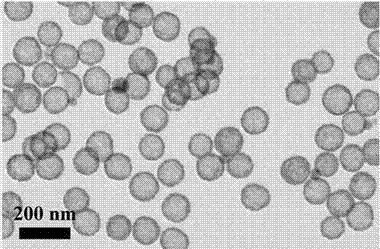
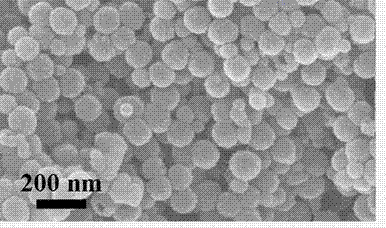
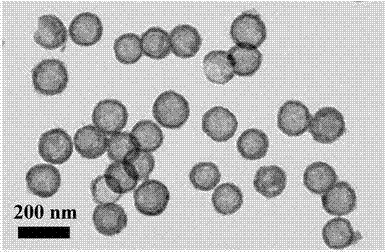
Preparation method and application of hollow SnO2@C nanosphere in lithium ion battery
InactiveCN103193263AThe synthesis method is simpleUniform particle sizeMaterial nanotechnologyCell electrodesCarbon layerElectrical battery
The invention belongs to the technical field of nanocomposites, and in particular relates to a preparation method of a hollow SnO2@C nanosphere with a core-shell structure and an application of the hollow SnO2@C nanosphere in a lithium ion battery. The preparation method comprises the following steps: synthesizing a hollow SnO2 nanosphere with high dispersibility and uniform particle diameter by taking silica as a template via one-step process; and wrapping a carbon layer on the hollow SnO2 nanosphere by taking PAA (Phenolic Aldehyde Amine) as a carbon source, thereby preparing the hollow SnO2@C nanosphere with the core-shell structure. According to the invention, the purpose of integrating the regulating effects of the nanoscale dispersion of nanoparticles to the volume expansion of SnO2, the absorbing effects of the hollow structure to the internal stress generated by volume changes and the restricting and buffering effects of the carbon wrapping layer to stannic oxide particles is achieved; and when the hollow SnO2@C nanosphere is applied to the lithium ion battery, the lithium ion battery can have a larger volume, better cycling stability and longer service life.
Owner:NORTHEAST NORMAL UNIVERSITY
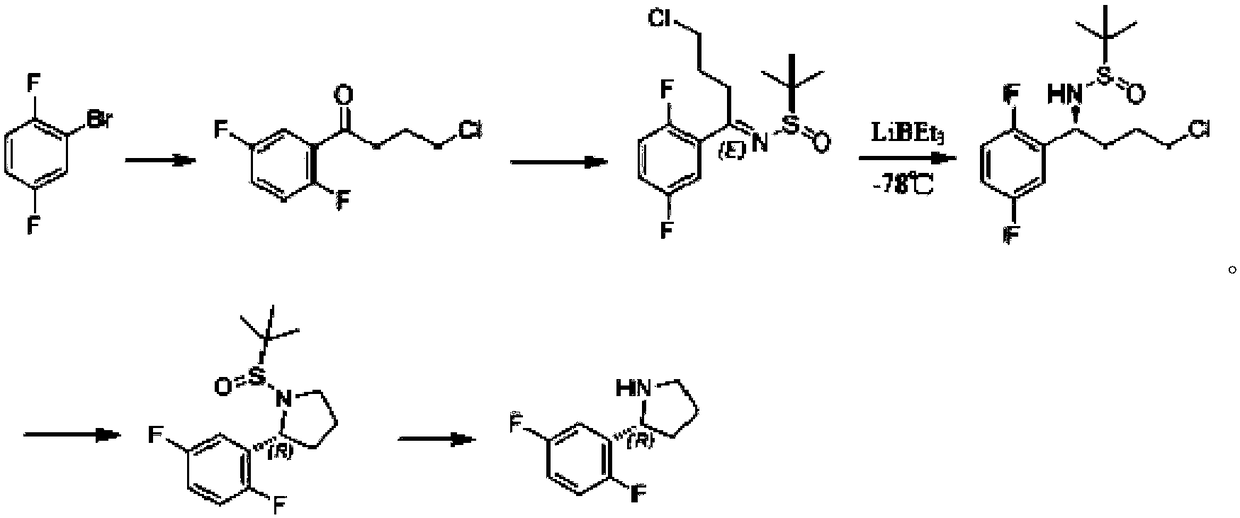
Synthesis process of chiral pyrrolidine and intermediates
The invention discloses a synthesis process of chiral pyrrolidine and intermediates. The synthesis process adopts 2, 5-difluorohalobenze or 5-fluoro-3 halogenated pyridine as the raw material substrate, and employs a chiral catalyst to induce chiral reaction so as to reduce a ketone compound into a corresponding chiral alcohol compound, and then carries out substitution reaction to introduce an easily leavable group to an alcoholic hydroxyl group so as to facilitate ring formation reaction (or carry out ring formation reaction directly). The process provided by the invention has the advantagesof significant increase of product yield and reduction of cost, also the reaction temperature is mild, and the process is easy to control and industrialize.
Owner:上海鑫凯化学科技有限公司
Synthesis method of taurine
InactiveCN101717353AFew reaction stepsSimple craftSulfonic acid preparationChemical synthesisSynthesis methods
The invention relates to a synthesis method of taurine, belonging to the technical field of organic chemical synthesis. The synthesis method comprises the following steps of: reacting the oxirane with ammonium sulfite to obtain taurine ammonium salt, and acidating and refining to obtain the taurine. The reaction steps of the technical scheme are less, the taurine ammonium salt can be obtained just by one-step reaction between the oxirane and the ammonium sulfite, the crude taurine can be obtained by acidating, and the taurine is obtained by refining, therefore, the synthesis method has simple process and can embody the economical efficiency and the cleaning production.
Owner:JIANGSU YUANYANG PHARMA
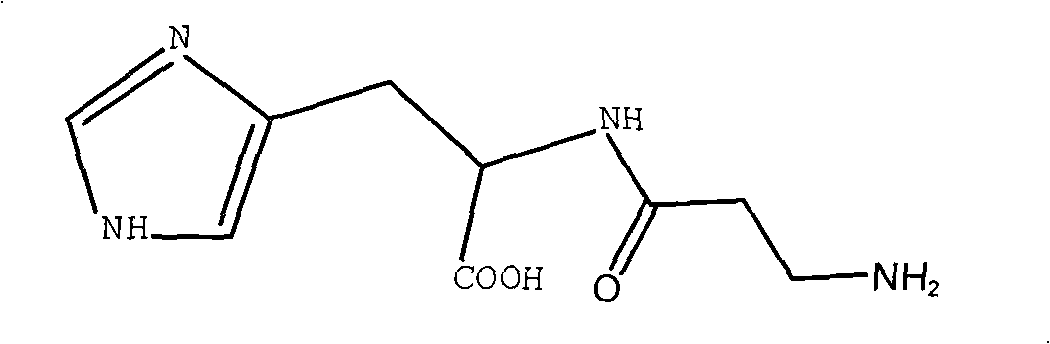
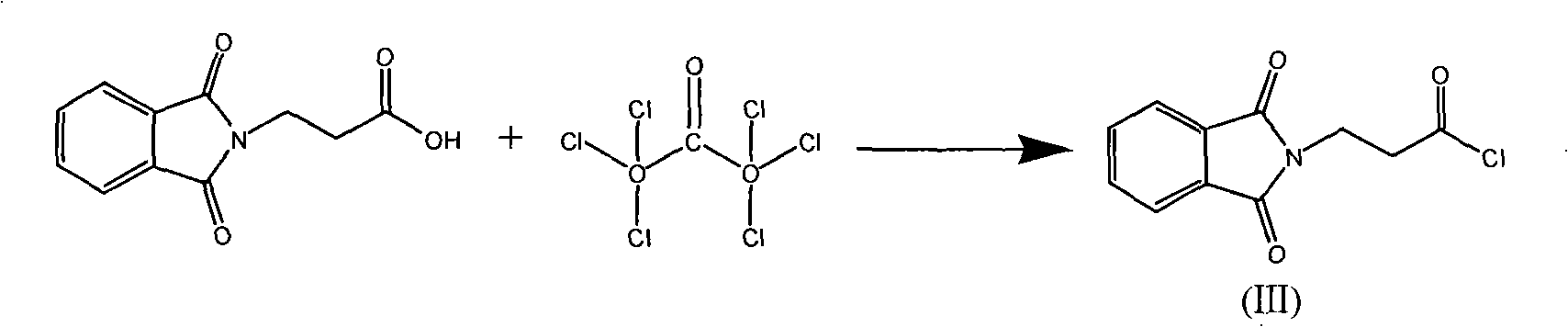
Synthetic method of L-carnosine
ActiveCN101284862AAvoid formingReduce consumptionPeptide preparation methodsBulk chemical productionHydrazine compoundOrganic synthesis
A method for synthesizing L-carnosine belongs to the organic synthesis technical field. The method is as follows: beta-alanine is dissolved in non-polar solvent to be reacted with phthalic anhydride under the catalysis of organic amine, and then phthaloyl-beta-alanine is obtained through water recrystallization; the phthaloyl-beta-alanine is dissolved in solvent to synthesize phthaloyl-beta-alanyl chloride through the chlorinated reagent of acyl chloride phthaloyl-beta-alanine; L-histidine is reacted with hexamethyl disilazane or trimethylchlorosilane to obtain L-histidine trimethylsilane protector; the protector is reacted with the phthaloyl-beta-alanyl chloride to obtain hydrochloride product with the protecting group divested by water, and then neutralization product is obtained by the hydrochloride product obtained through the neutralization condensation reaction of alkaline reagent, thereby obtaining L-carnosine crude product through the hydrazinolysis of the neutralization product by hydrazine hydrate; and the crude product is purified to obtain L-carnosine finished product. The method has low raw material consumption, short reaction procedure and high yield; moreover, the quality of synthesized L-carnosine can meet the requirements of industrial production.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Synthetic method of kyprolis
ActiveCN103641890AHigh reaction yieldHigh yieldPeptidesBulk chemical productionAcetic acidMorpholine
The invention relates to the technical field of drug synthesis, and particularly relates to a synthetic method of kyprolis. The synthetic method comprises the following steps: carrying out condensation reaction on morpholine-4-base acetic acid, L-homophenylalanine ester and salt, and then carrying out decarboxylation protection to generate a compound V; carrying out the condensation reaction on the decarboxylation, the salt and N-Boc-L-leucine, and then carrying out deamination protection to generate a compound VI; carrying out the condensation reaction on the compound V and the compound VI, and then carrying out decarboxylation protection to generate a compound VII; carrying out the condensation reaction on the compound VII and a compound VIII to obtain the kyprolis. The method disclosed by the invention can be used for enhancing the reaction yield by adopting a converging synthetic method; the used reagent is easy, convenient, easy to obtain and less in pollution. The process disclosed by the invention only relates to the condensation and deprotection between amino acids and is simple and controllable in reaction and suitable for industrial production.
Owner:重庆兴泰濠制药有限公司
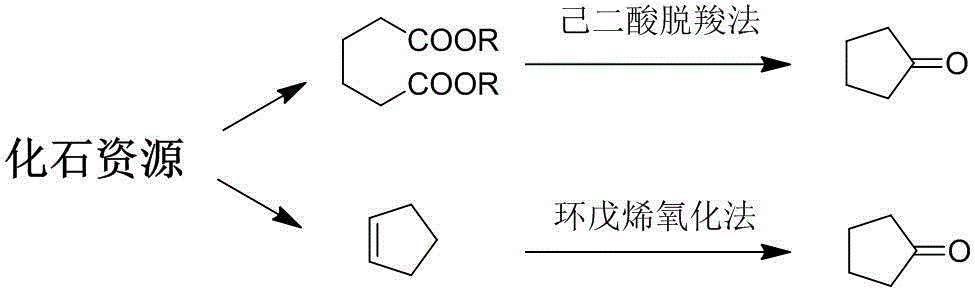
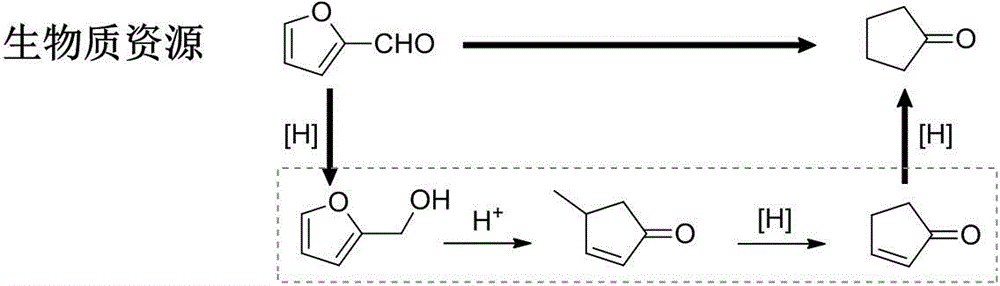
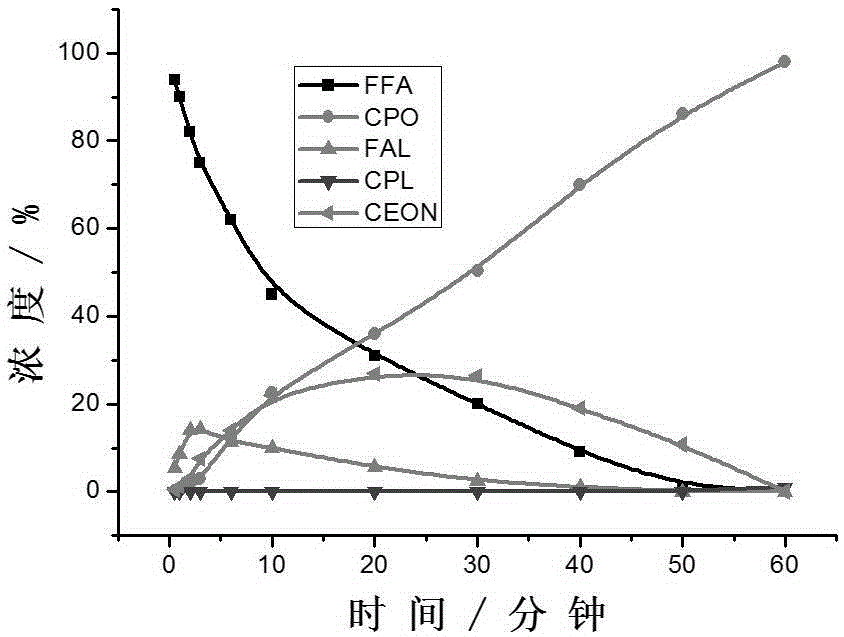
Method for preparing cyclopentanone by taking biomass resource as raw material
InactiveCN105330523AReduce yieldFew stepsMolecular sieve catalystsChemical industryFixed bedFurfural
The invention belongs to the technical field of biomass conversion utilization, and concretely relates to a method for preparing cyclopentanone by taking biomass resource as a raw material. The method comprises taking furfural coming from biomass as the raw material, taking a hydrogen-containing gas as a reducing agent, and in the presence of a metal supported type catalyst, performing hydrogenation rearrangement reaction in a high-pressure reaction kettle or a fixed bed reactor, so as to obtain cyclopentanone in one step, wherein the carrier of the metal supported type catalyst is selected from Nb2O5, H-ZSM-5 molecular sieve, HY molecular sieve, Fe2O3, ZrO2, Al2O3, SiO2, CeO2, MgO, active carbon, and TiO2 in various crystal forms, the active composition is selected from Au, Pt, Ru, Rh, Pd, Ir, Ni and Cu, and the active composition load capacity is 0.1-5% of the catalyst. The method is mild in reaction technological conditions, and cheap and easily-available in raw materials, is capable of realizing quantitative conversion of furfural to cyclopentanone, and belongs to an environment-friendly green chemical technology.
Owner:FUDAN UNIV
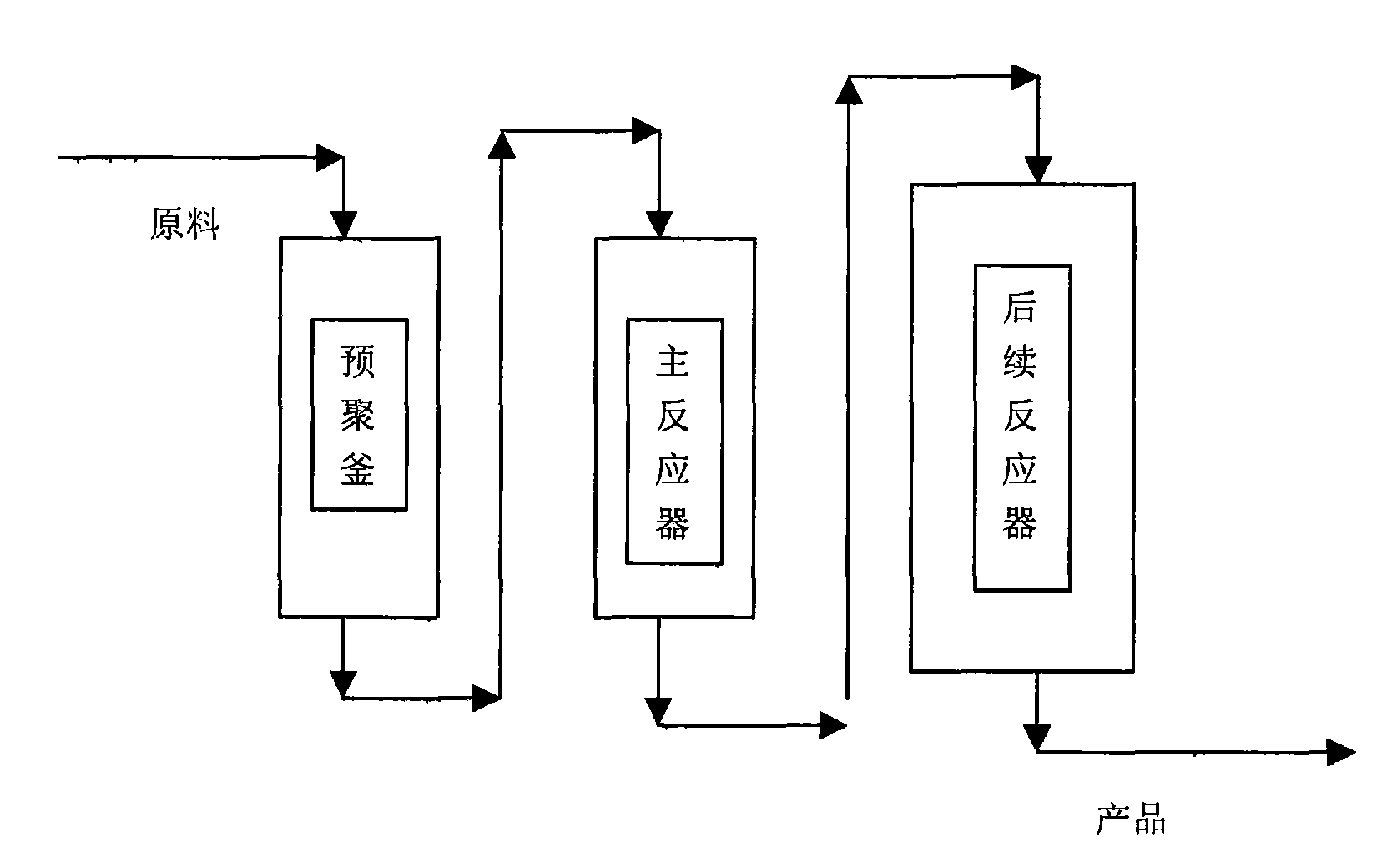
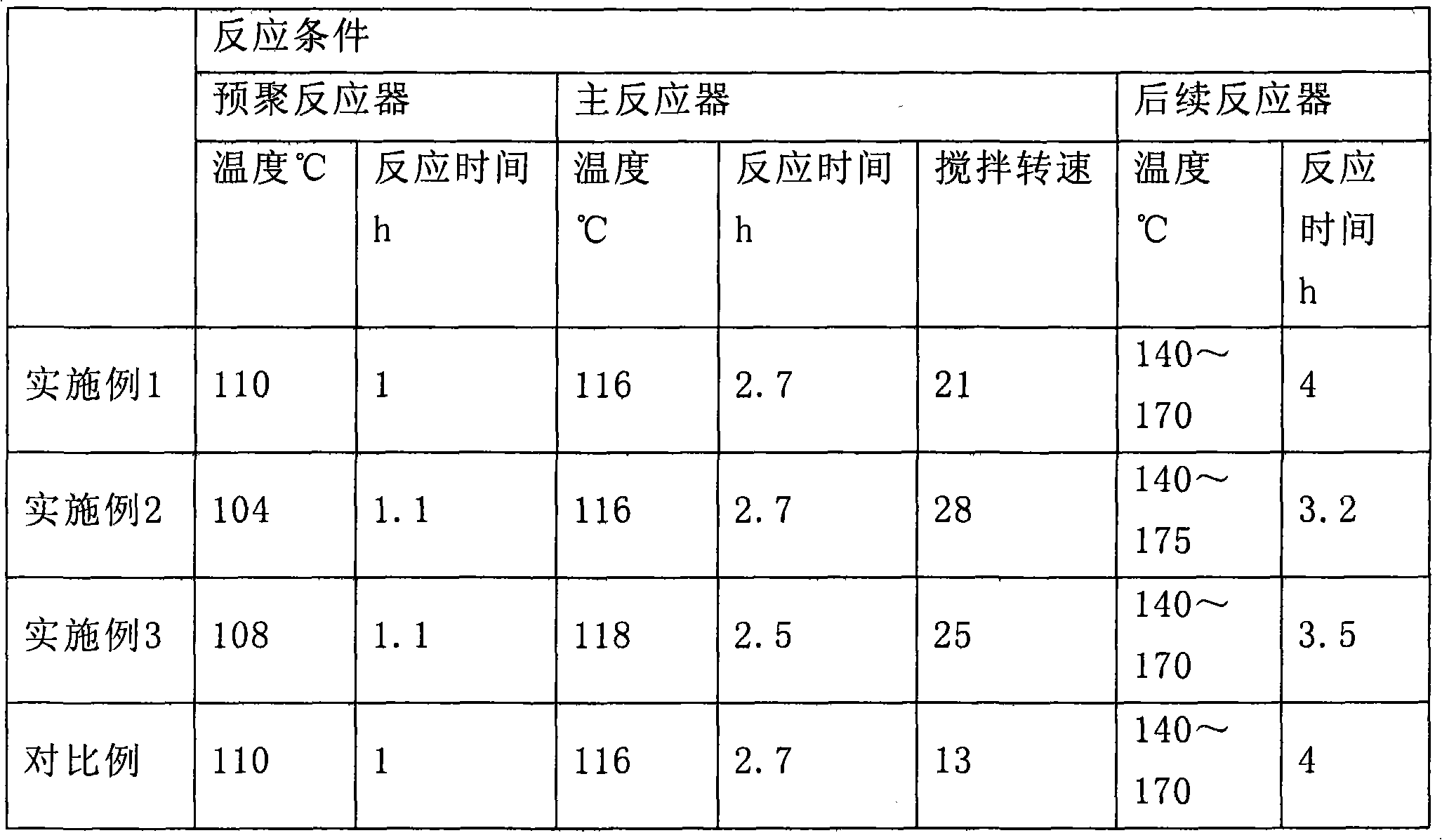
High impact high gloss bimodal polystyrene material and preparation method thereof
The invention provides a high impact high gloss bimodal polystyrene material and preparation method thereof, comprising the following steps: (a) the solution of polybutadiene rubber component mixed with polystyrene monomers is provided; wherein, the polybutadiene rubber component is selected from high cis-polybutadiene and / or low cis-polybutadiene, the cis-content of high cis-polybutadiene is not less than 94% by mole and the cis-content of low cis-polybutadiene is between 33% to 40% by mole ; (b) the solution obtained from the step (a) is used to perform prepolymerization reaction of styrene monomers to obtain a prepolymerization solution system which does not generate phase transition; (c) the prepolymerization solution system obtained from the step (b) is used to perform further prepolymerization reaction of styrene monomers in shearing force field until the system generates phase transition to obtain rubbery state material of which the particle size presents bimodal distribution; (d) the conversion rate of styrene monomers in the material obtained from the step (c) is further increased to obtain the high impact high gloss bimodal polystyrene material. The invention also provides a preparation method of the high impact high gloss bimodal polystyrene material.
Owner:SHANGHAI SECCO PETROCHEM
Semi-synthesis method for high-purity and high-stability gastrodin
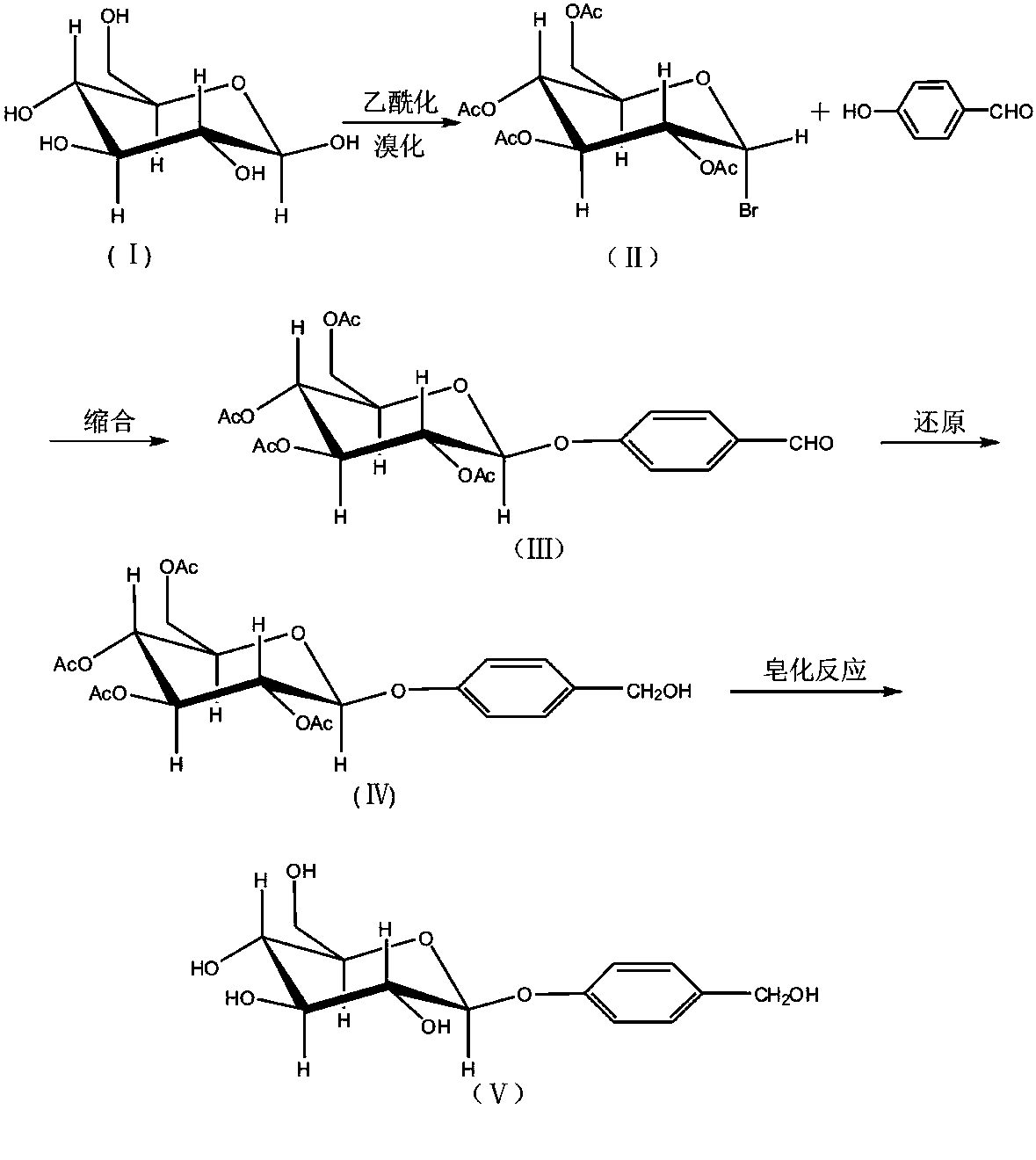
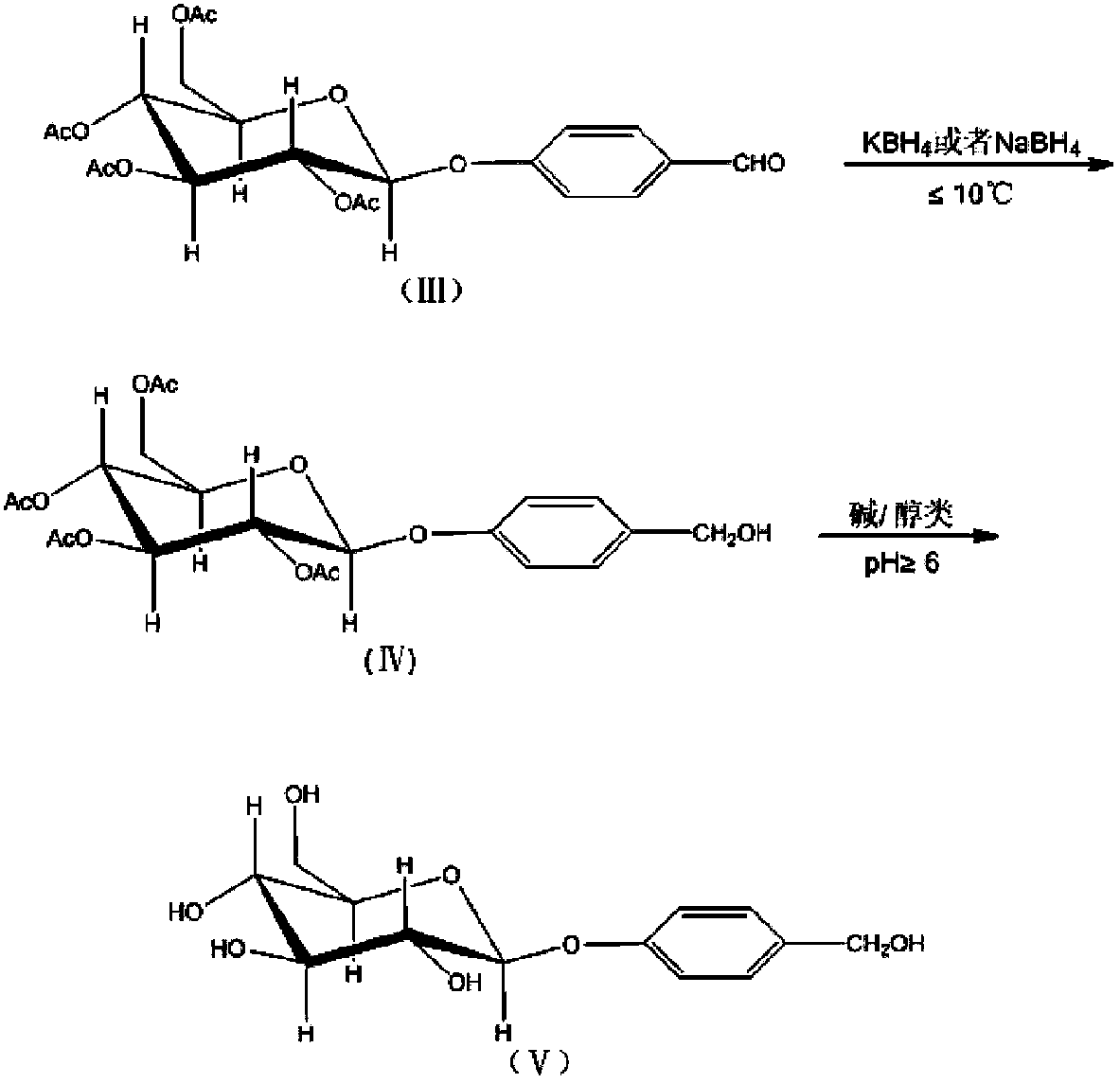
ActiveCN103804438AHigh purityFew reaction stepsSugar derivativesSugar derivatives preparationSolventChemistry
The invention discloses a semi-synthesis method for high-purity and high-stability gastrodin. The semi-synthesis method comprises the following steps: carrying out a reduction reaction by taking tetraacethyl as a raw material; concentrating and adding a suitable amount of water into concentrated liquid; filtering and drying to obtain acethyl gastrodin; recycling a filtering solution which is used as a mother solution a; adding an alcohol solvent into the acethyl gastrodin to carry out a reflowing reaction, and concentrating; repeatedly reflowing and concentrating to obtain a concentrated solution; adding a non-polar solvent and filtering; drying solids to obtain a gastrodin rough product; recycling a filtering solution which is used as a mother solution b; adding alcohol and / or an ester solvent into the rough product of gastrodin; heating and completely dissolving; filtering and separating out crystals to obtain a refined product of gastrodin; recycling a filtering solution which is used as a mother solution c; mixing the mother solution b and the mother solution c; filtering and drying to obtain the rough product of gastrodin; and refining to obtain the refined product of gastrodin. The acethyl gastrodin prepared by the semi-synthesis method disclosed by the invention is stable, can be directly used as a crude drug, and can also be used as an intermediate to synthesize the high-purity gastrodin; the semi-synthesis method for the high-purity and high-stability gastrodin is very good for meeting the clinical demands on gastrodin and acethyl gastrodin.
Owner:KPC PHARM INC
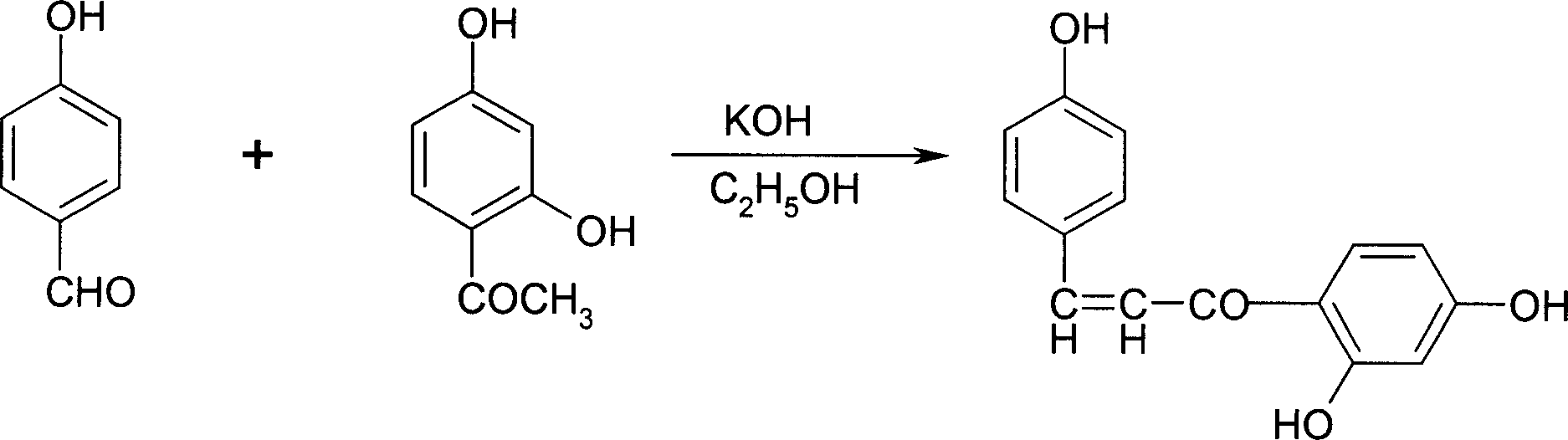
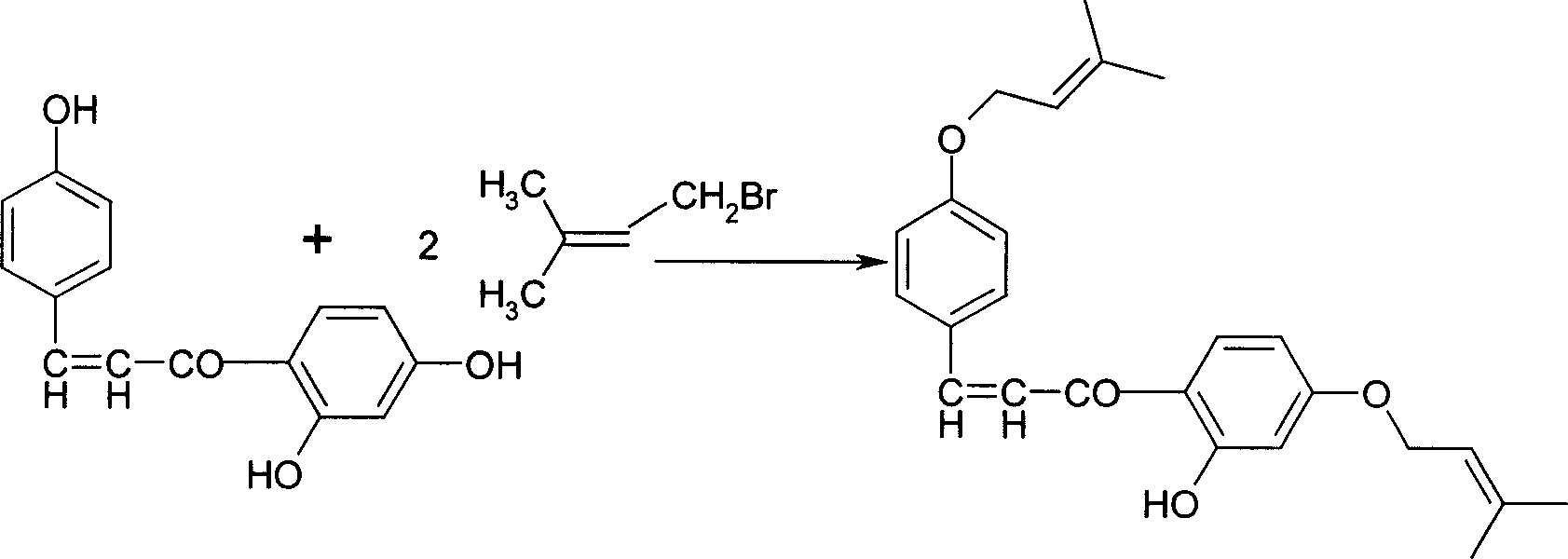
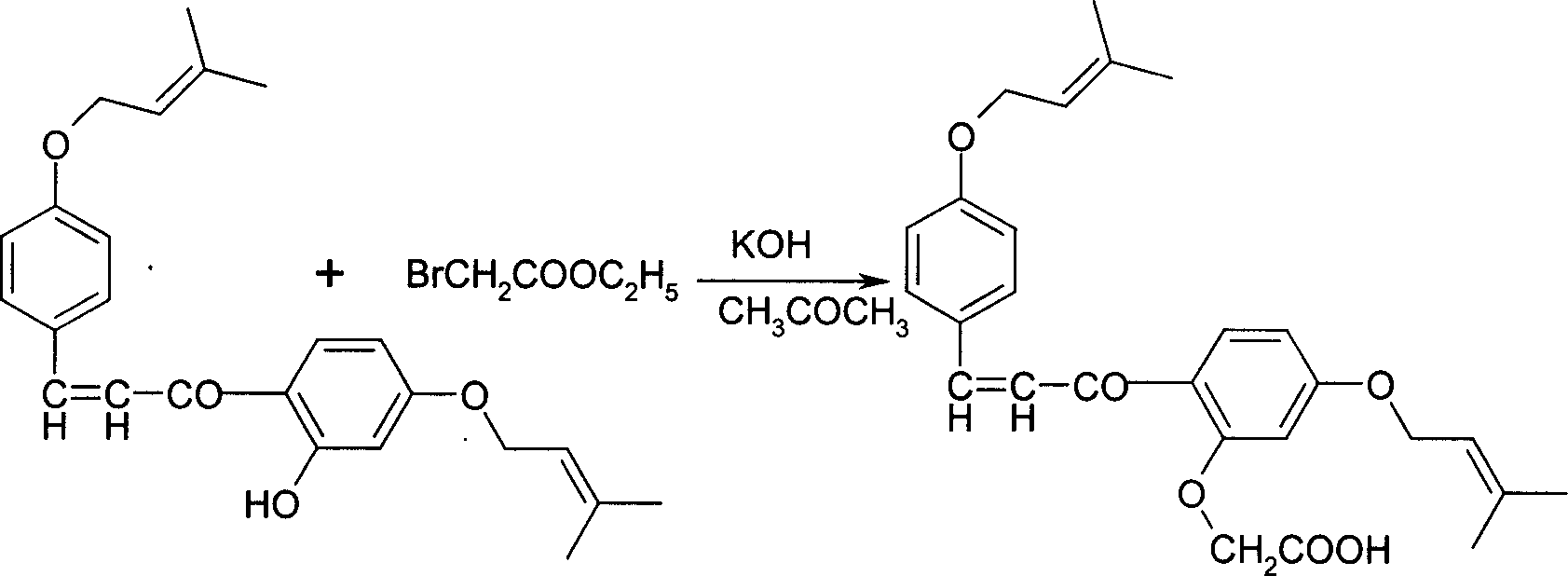
Sofalcone preparation method
InactiveCN1733682AFew synthetic stepsFew reaction stepsOrganic compound preparationCarboxylic compound preparationKetone2-Butene
Disclosed is a Sofalcone preparation method which mainly comprises, (1) subjecting p-hydroxybenzaldehyde and 2,4-ihydroxyacetophenone to condensation reaction under alkaline condition, obtaining 2,4,4-Trihydro-xychalcone, (2) subjecting 2,4,4-trihydro-xychalcone and 1-bromo-3-methyl-2-butene to condensation reaction, obtaining 2-hydroxyl-4,4-bis (3-methyl-2-butenyloxy) chalcone, (3) subjecting the 2-hydroxyl-4,4-bis (3-methyl-2-butenyloxy) chalcone and ethyl bromoacetate to condensation under the action of potasium carbonate, thus obtaining sofalcone. The mol ratio of p-hydroxybenzaldehyde, 2.4-dihydroxyacetophenone, 1-bromo-3-methyl-2-butene, and ethyl bromoacetate can be 1 : (0.90-1.50) : (1.60-2.55) : (0.60-1.25).
Owner:阮华君
Rust-resistant agent for concrete reinforcing bar and preparation method thereof
InactiveCN101948264AExcellent chelating antirust abilityGood migration and diffusion propertiesRebar corrosionAlkali soil
The invention discloses a rust-resistant agent for a concrete reinforcing bar and a preparation method thereof. The rust-resistant agent for the concrete reinforcing bar is prepared based on a gas-phase corrosion inhibitor prepared by reacting a dicarboxylic acid with organic amine. The rust-resistant agent for the concrete reinforcing bar comprises the following raw materials in percentage by mass: 5 to 10 percent of sodium monofluorophosphate, 2 to 5 percent of calcium gluconate, 20 to 40 percent of the gas-phase corrosion inhibitor and the balance of water. The rust-resistant agent for theconcrete reinforcing bar of the invention does not comprise nitrite, comprises the gas-phase corrosion inhibitor and has a function of migrating and diffusing in the concrete. Compared with the traditional rust-resistant agent for the reinforcing bar, the rust-resistant agent for the concrete reinforcing bar has the advantages of excellent corrosion inhibition and rust-resistant properties, high environmentally-friendly property and the like, can be widely applied to fresh reinforced concrete construction of harbour engineering, coastal buildings, buildings in saline and alkaline lands and other buildings with the requirements of preventing the concrete reinforcing bar from rust and corrosion, and can also be applied to the repair of the reinforced concrete structures in major construction projects and routine maintenance.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
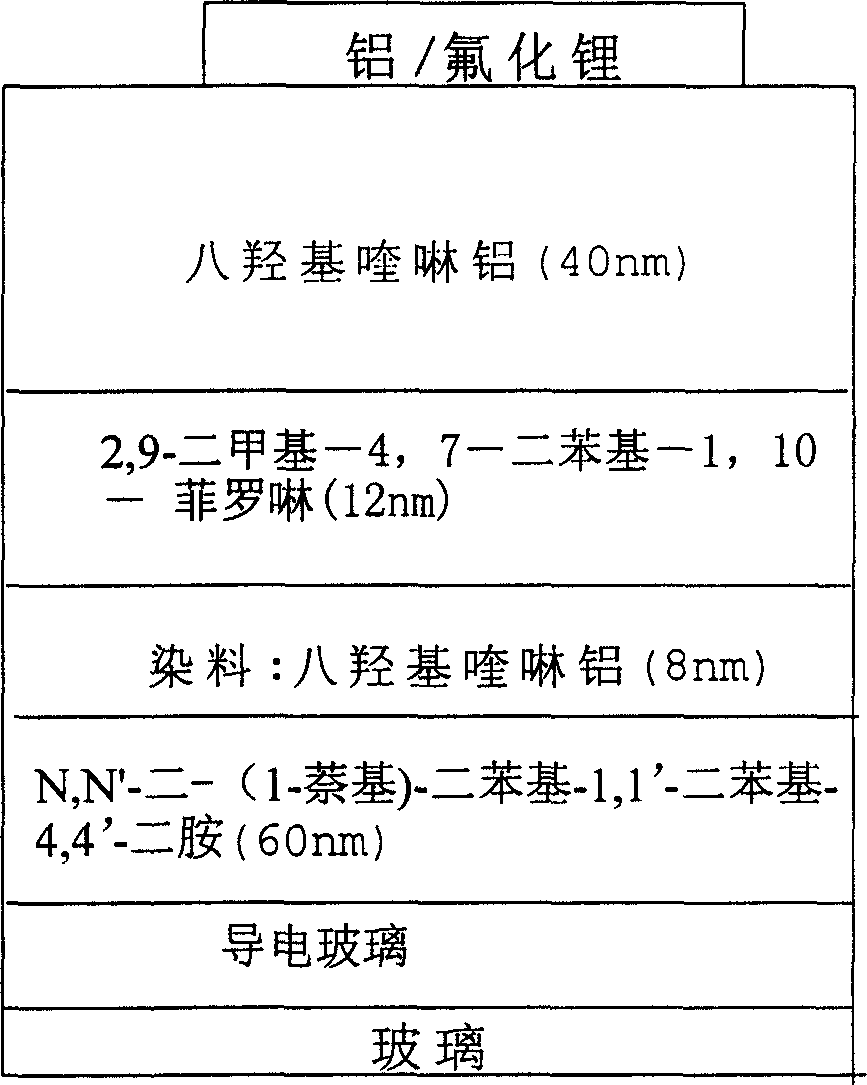
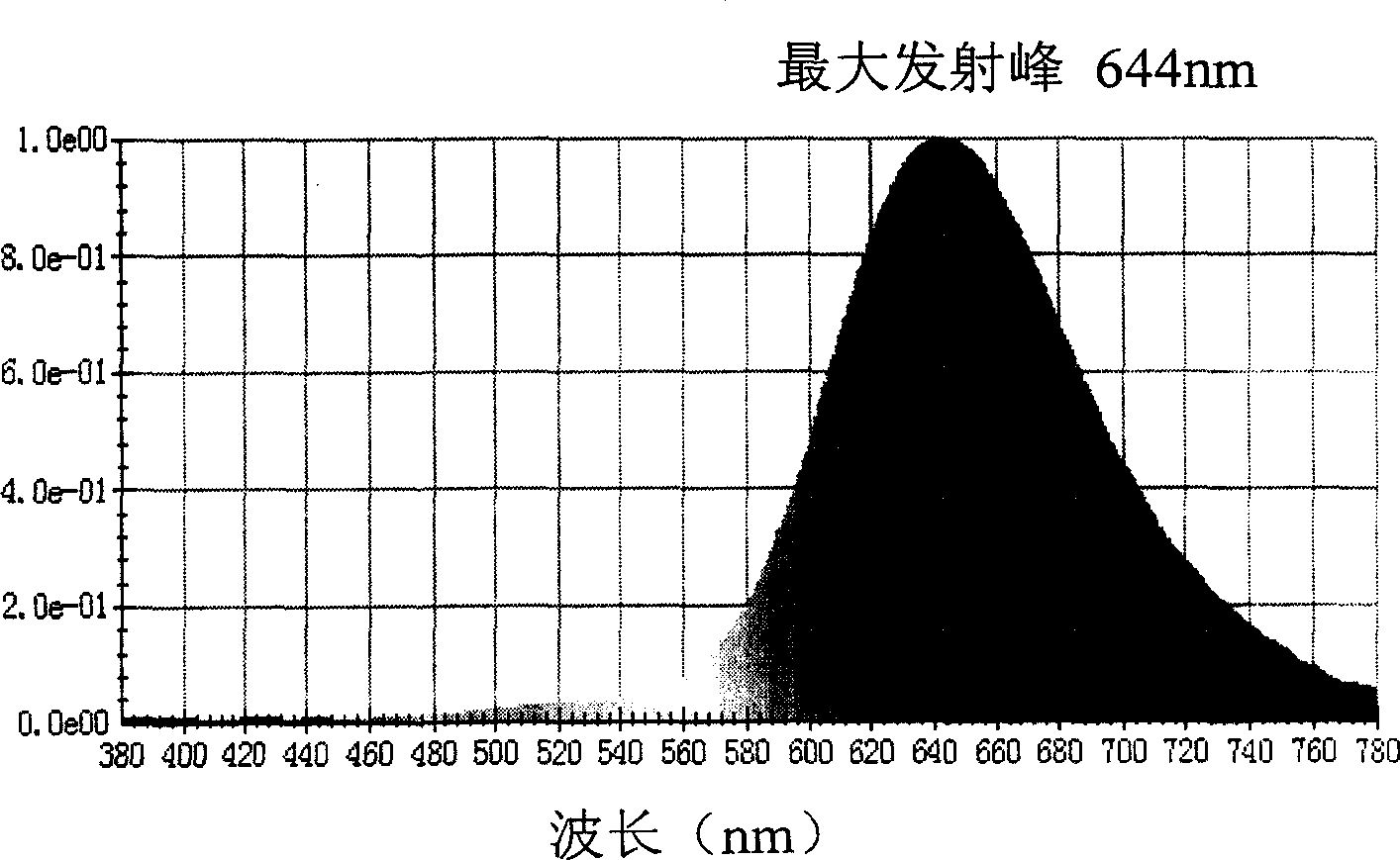
Red light emitting fluorescent dye, synthesizing process and use thereof
InactiveCN1861740ARealize separation and purificationRaw materials are easy to obtainElectrical apparatusElectroluminescent light sourcesPyroneRed light
The invention relates to the red beam luminescent dye which the receptor group is the 1, 3-indandione and the derivant 4H-pyrones. So the quenching effect of the new dyestuff has decreased efficiently and it has the saturated colour-purity. It can keep high efficient and the brightness in the high doping content. It is used for the organic photoconductor, the organic nonlinear optical material and the luminescent material of the electroluminescent cell. The emission band spectrum is in the red region of the visible spectrum and it has the high fluorescence quantum efficiency.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of trichlorosucrose-6-acetate
InactiveCN102070678AReduce dosageLess side effectsEsterified saccharide compoundsSugar derivativesFood sweetenersSide reaction
The invention relates to a preparation method of an intermediate of the food sweetening agent trichlorosucrose, belonging to the technical field of fine chemicals. The theory of the invention is as follows: chloroethane is used as reaction solvent, thus replacing dimethyl formamide (DMF) which is generally used as solvent; and DMF is used as catalyst, thereby greatly reducing the dosage of DMF and saving the cost; and the generated hydrogen chloride and sulfur dioxide can fast leave the reaction system, thereby reducing side reactions. The preparation method has the following beneficial effects; the method has low cost and less reaction steps, the yield is obviously improved by about 20% compared with the method using DMF as solvent, and the preparation method has obvious advantages.
Owner:CHANGZHOU NIUTANG CHEM PLANT CO LTD +1
Preparation method of conjugated linoleic acid
InactiveCN101565367AHigh yieldReduce manufacturing costFatty acid isomerisationOrganic compound preparationIsomerizationVegetable oil
The invention relates to a preparation method of conjugated linoleic acid, comprising the following steps: placing the vegetable oil containing linoleic acid glyceride or mixed fatty acid containing linoleic acid or extracted and separated linoleic acid product as raw material, low boiling point alcohols solvent and base catalyst into a reaction kettle and performing the isomerization reaction at a certain temperature. The reaction product is subjected to evaporating, acidifying, extracting and vacuum drying to obtain the conjugated linoleic acid. The conversion of the linoleic acid is above 97.5% and the total yield of the conjugated linoleic acid is above 85%, the proportions of the cis-9, trans-11 isomer and trans-10, cis-12 isomer in the total conjugated linoleic acid are above 96%. The preparation method has features of less reaction steps, easy recovery and repeated use of solvent, simple follow-up separation, low production cost, and easy industrialization.
Owner:ZHEJIANG UNIV
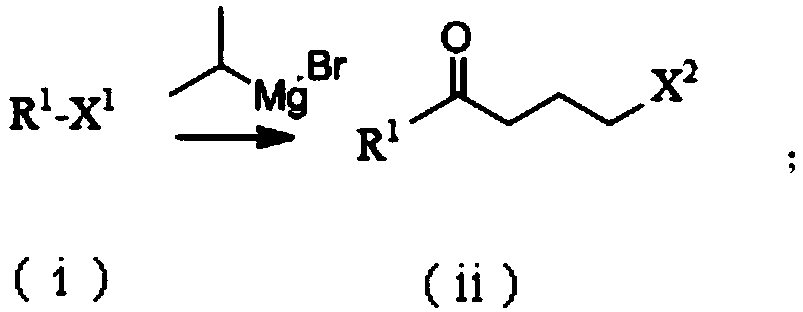
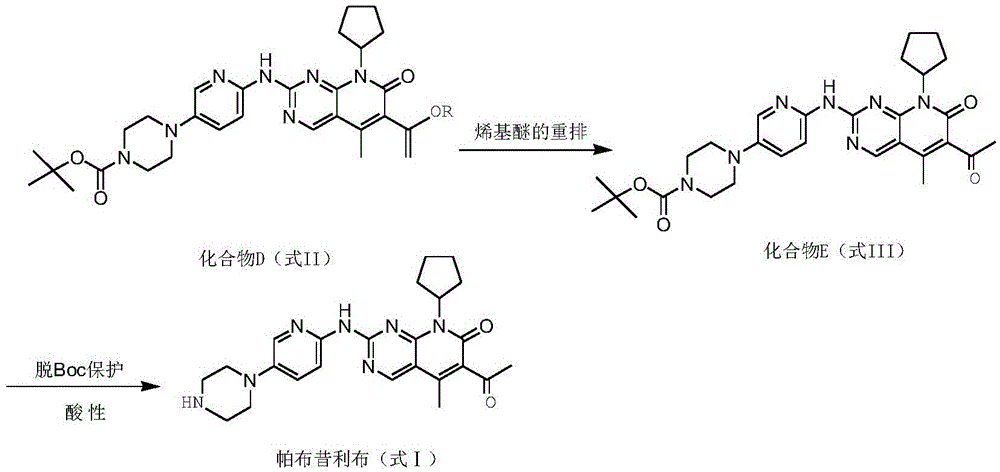
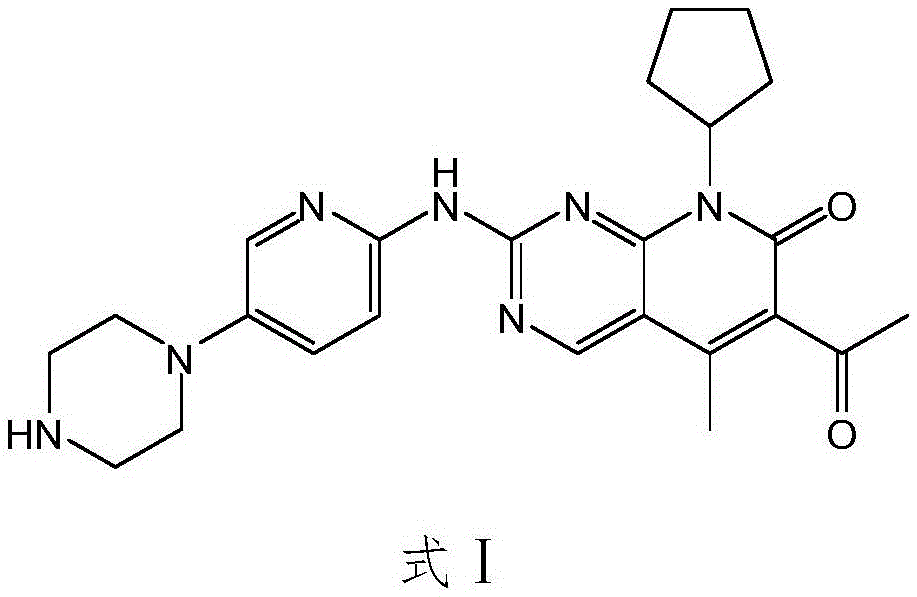
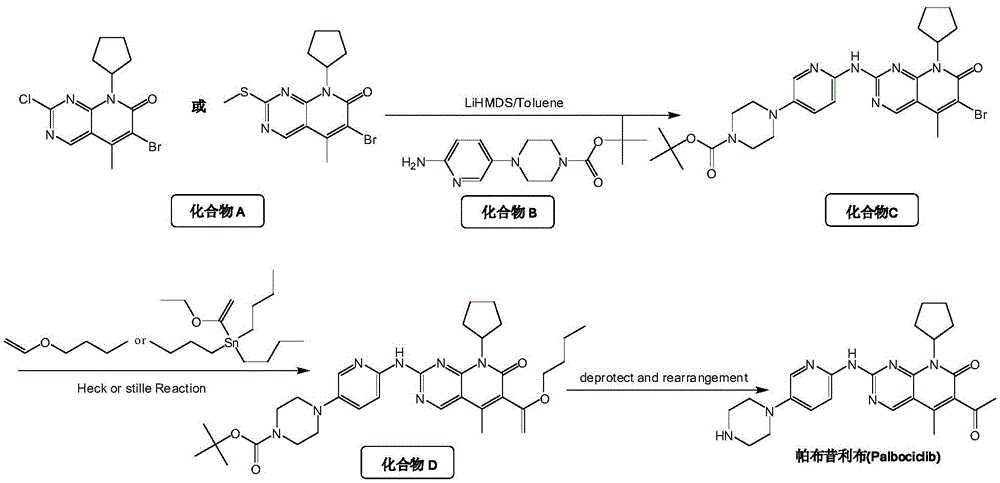
Method for preparing high-purity palbociclib and reaction intermediate of palbociclib
InactiveCN105418603AImprove solubilityReduce the difficulty of purificationOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupReaction intermediate
The invention relates to a method for preparing compound palbociclib (shown as the formula I). A compound D (shown as the formula II) serves as a raw material, and a compound E (shown as the formula III) is obtained through a rearrangement reaction of alkenyl ether; then the compound E is subjected to t-butyloxycarboryl protection group removal under the acidic condition, and target product palbociclib is obtained; an R group in a compound D is selected from any one of a C1-C6 alkyl group, a C1-C6 halogenate alkyl group, C1-C6 hydroxyalkyl and a C1-C6 naphthenic base. The process procedure and operation steps are easy and convenient, prepared palbociclib is high in purity, and good popularization prospects are achieved.
Owner:重庆莱美隆宇药业有限公司
Nitrogen-doped porous carbon-supported bimetallic catalyst and preparation method and application thereof
ActiveCN109999880ALarge specific surface areaRich porosityOrganic compound preparationHydroxy compound preparationCellulosePorous carbon
The invention discloses a thermometal-nitrogen-doped carbon-based bifunctional catalyst synthesized through a one-pot method by using nitrogen-doped bio-based porous carbon as a carrier, and a preparation method and application thereof. The catalyst can be used for catalyzing efficient selective hydrogenation of biomasses such as bio-based sorbitol, xylitol, cellulose, lignocellulose, etc. for thepreparation of low-carbon alcohol such as diol, etc. since cheap and renewable biomasses are used as raw materials for preparation of a porous nitrogen-dope carbon material without adding nitrogenousorganic compounds as the nitrogen-doped element, the catalyst of the invention is green and environmentally-friendly and the cost is low. When being used for selective hydrogenation of biomasses in awater-phase system, the prepared metal supported catalyst has excellent of catalytic activity, stability and selectivity. In addition, the separation of products and the catalyst is simple; yield ofglycol and propylene glycol in the products can reach up to 85% and above; the reaction steps are less, the condition is mild, and the operation is simple; and the catalyst has a broad application prospect.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Preparation method of erlotinib hydrochloride
The invention provides a preparation method of erlotinib hydrochloride. The method comprises the following steps: taking 6,7-dimethoxy-3,4-dihydro-quinazoline as an initial raw material, chlorinating directly, then reacting with 3-aminophenylacetylene, afterwards removing methyl on the positions of 6 and 7 and finally introducing a methoxyethyl side chain to generate the erlotinib hydrochloride. The invention has the technological lines of mild reaction condition, no need of high temperature, deep cooling, energy saving and environment friendliness; the whole line has better yield and quality; and the reaction steps are little, the reaction is stable and controllable, the postprocessing is very simple and convenient, and the industrialized production is easy.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
Preparation method of low-cost ZSM-5 type zeolite molecular sieve, and application thereof
InactiveCN103787366ALower synthesis costHigh activityOther chemical processesWater contaminantsWater bathsMolecular sieve
The invention discloses a preparation method of low-cost ZSM-5 type zeolite molecular sieve, and application of the low-cost ZSM-5 type zeolite molecular sieve, belonging to the field of a molecular sieve preparation technology. The method comprises the steps of a. drying pulverized fuel ash raw powder to constant weight, mixing HCl solution with the dried pulverized fuel ash, stirring, centrifuging, washing and drying for later use; b. grinding the pulverized fuel ash treated by the step a, mixing the ground pulverized fuel ash with NaOH for melting, cooling, grinding and screening by a molecular sieve; feeding the ground melt into deionized water, mixing, stirring under the condition of water bath, and carrying out centrifugal separation to obtain liquid supernatant; c. taking the liquid supernatant obtained in the step b, taking tetrapropylammonium hydroxide as a template agent and NH4F as a mineralizer, mixing and carrying out crystallization reaction; after that, taking out the product, quenching to the room temperature, washing, drying and roasting to obtain the zeolite molecular sieve. According to the method, the pulverized fuel ash discharged by a thermal power plant is fully utilized as a silicon-alumininm source and is used for replacing pure chemical reagents Si and Al for synthesizing the molecular sieve, so that the synthesis cost of the molecular sieve is lowered, and the utilization value is high.
Owner:HUAINAN NORMAL UNIV
N-amino-1,2-cyclopentanediformylimine and preparation method thereof
InactiveCN101235011AFew reaction stepsReduce manufacturing costOrganic chemistryImideCombinatorial chemistry
The invention discloses an N-amino-1, 2-cyclopentane dicarboximide whose formula is represented as right. The invention also discloses a corresponding preparation method which comprises using cyclopentane ortho-anhydride as raw material, carrying out hydrazine hydrate in solvent for refluxing reaction for 0.5-12h while the mol ratio of cyclopentane ortho-anhydride and hydrazine hydrate is 1:1-2.5, removing solvent after reaction, and drying to obtain N-amino-1, 2-cyclopentane dicarboximide. The N-amino-1, 2-cyclopentane dicarboximide can be used as the intermediate of gliclazide, thereby reducing the reaction route of gliclazide to reduce waste.
Owner:ZHEJIANG UNIV
Synthetic process of chiral 2-amido-1-(6-fluorine-3,4-dihydrobenzopyranyl) alCohol
This invention is attributed to the field of organic chemistry and specifically relates to the method to synthesize drug intermediates (R)-2-amino-1-((R)-6-fluoro-3, 4-dihydrochromeno) ethanol and (R)-2-amino-1-((S)-6-fluoro-3, 4-dihydrochromeno) ethanol. The method is that, drug intermediates (2R)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone and (2S)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone are adopted as raw materials and reduced by Clemmensens method with the products reacting with p-toluenesulfonyl chloride in pyridine. The consequent products are dissolved in solvent and dry ammonia is introduced for heating reflux. The total yield can be as much as 32%.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
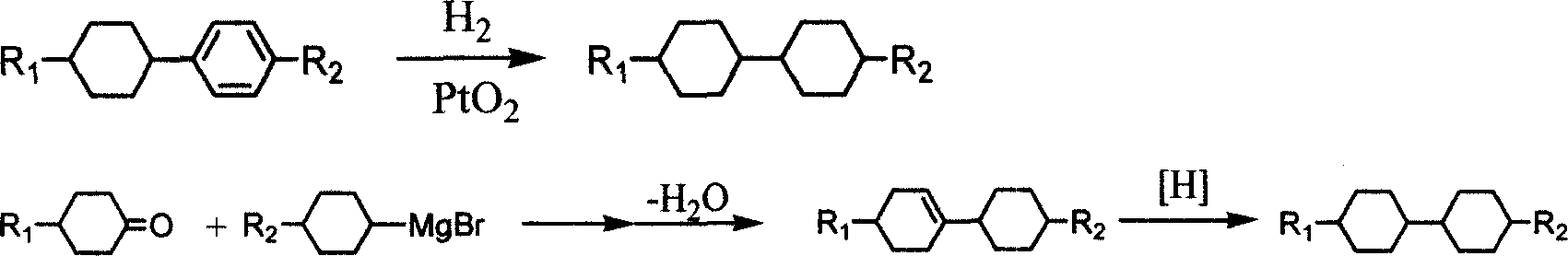
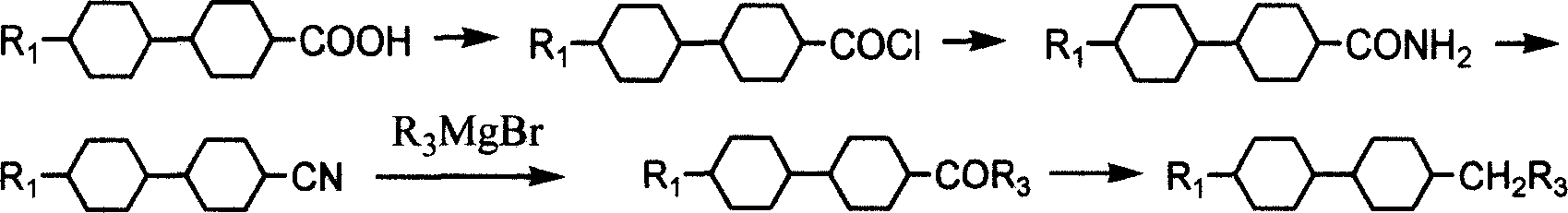
Method for preparing bis cyclohexane monomer liquid crystal using grignard reaction
InactiveCN1962580AAvoid drop in yieldLow costLiquid crystal compositionsHydrocarbon from oxygen organic compoundsGrignard reactionStructural formula
The invention discloses a preparing method of bicyclohexane monomer LCD in the monomer LCD preparing technical domain, which utilizes Grignard reaction to prepare the product with structural formula as graph I and synthetic feature route as graph II, wherein R1 and R2 is C2-C10; R3 is C1-C9 straight-chain alkyl; X is Cl or Br or I; A is C2H4 or cyclohexane; n is 0 or 1.
Owner:VALIANT CO LTD
Hyper-branched chitosan or hyper-branched glycol chitosan and preparation method thereof
The invention discloses a process of preparing of an over-expenditure structure chitose or an over-expenditure ethylene alcohol chitose, which comprises the following steps: degrading the chitose or the ethylene alcohol chitose with nitrous acid or nitrite; getting fractionally more narrow molecular weight distribution high molecular chain a end-groupof which is aldehyde group chitose or ethylene alcohol chitose with method of precipitation or volumetric exclusive chromatography; getting the Schiff's base dissolving in the weakly-acidic or neutral solution; getting the over-expenditure structure chitose or the over-expenditure ethylene alcohol chitose by deacidizing SCHIFF'S base( when R is H in the structural formula, the structure is the over-expenditure structure chitose; when R is CH2CH2OH, the structure is the over-expenditure ethylene alcohol chitose; wherein RI is the same structure branched chain to the broken line part in the above structural formula; the molecular weight range of the over-expenditure structure chitose or the over-expenditure ethylene alcohol chitose is >= 5000Da).
Owner:INST OF CHEM CHINESE ACAD OF SCI
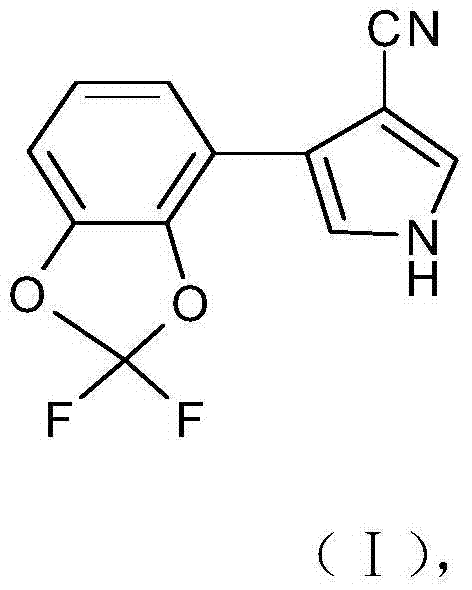
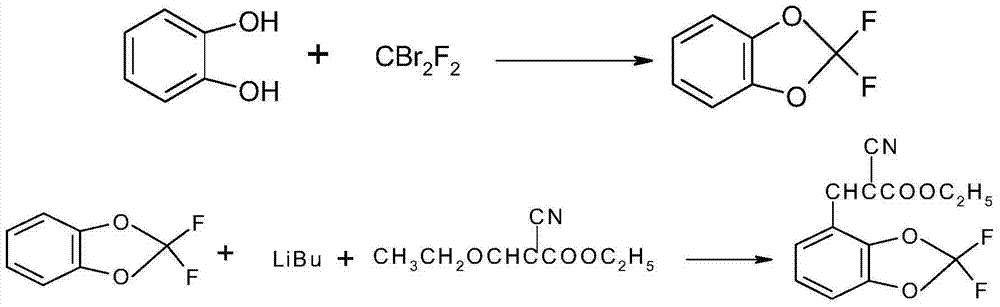
Synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile
The invention discloses a synthetic method of 4-(2,2-difluoro-1,3-benzodioxole-4-yl)pyrrole-3-nitrile. The method comprises the following steps of: preparing an intermediate 2,2-difluorobenz-1,3-dioxole through reacting catechol with dibromodifluoromethane, and preparing an intermediate 2-cyano-3-(2,2-difluorobenz-1,3-dioxole-4-yl)-2-acrylate. The fludioxonil prepared by the synthetic method provided by the invention has the purity of more than 99.0% and the total yield of more than 45.0%; the synthetic method has the advantages of cheap and easily available raw materials, simple process, high product yield in each step, good purity, low production cost, applicability to batch industrial production and the like.
Owner:XIAN MODERN CHEM RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com