Method for preparing difluoromethoxy bridge type liquid crystal
A compound and selected technology, applied in liquid crystal materials, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low total yield, unfavorable, cost reduction, etc., and achieve the improvement of total reaction yield and reduction of reaction steps , Simplify the effect of reaction intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
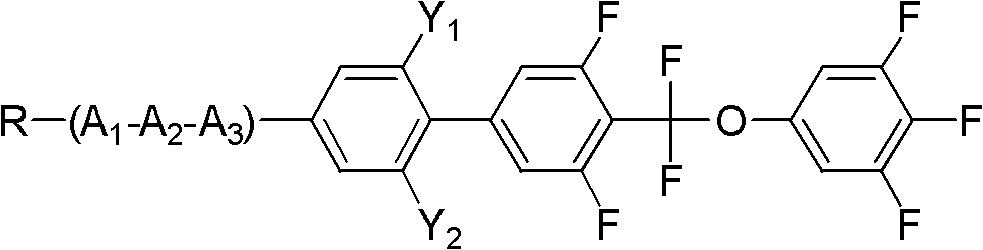
[0034] Embodiment 1, the preparation of 4-(difluoro(3,4,5-trifluorophenoxy)methyl)-3,5-difluoro-4'-propylbiphenyl
[0035]
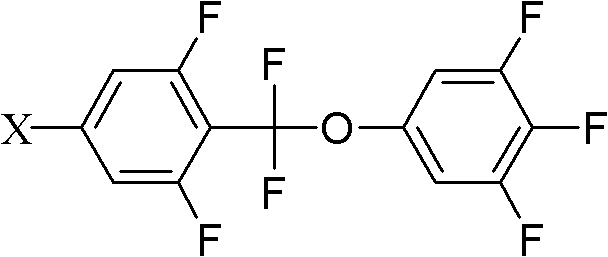
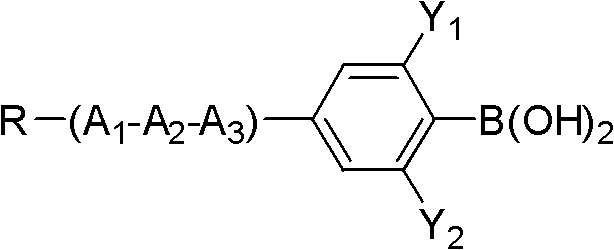
[0036] Add 38.9g (0.1mol) of compound 5-bromo-2-(difluoro(3,4,5-trifluorophenoxy)methyl)-1,3-difluorobenzene shown in formula II to a 250mL three-necked flask 18g (0.11mol) of compound propylphenylboronic acid shown in formula III, 32.1g (0.275mol) anhydrous sodium carbonate, 50ml toluene, 25ml ethanol, 25ml water, 1.15g (0.001mol) tetrakis triphenyl phosphide palladium, React at 90°C for 6 hours, add 100ml of toluene and 100ml of water, separate the lower aqueous phase and extract with 100ml×2 toluene, combine the organic phases, wash with 200ml×3 saturated saline, evaporate toluene to dryness, decolorize the product with silica gel column chromatography, and use 3 times of ethanol and 0.5 times of petroleum ether were recrystallized twice to obtain the target compound with a yield of 65%.
[0037] The experimental results are as follows:
[0038] ...
Embodiment 2
[0042] Example 2, 2-(4'-(difluoro(3,4,5-trifluorophenoxy)methyl)-3',5'-difluorobiphenyl)-5-propyltetrahydropyran preparation of
[0043]
[0044] Add 43.6g (0.1mol) compound 5-iodo-2-(difluoro(3,4,5-trifluorophenoxy)methyl)-1,3-difluorobenzene shown in formula II to a 1L three-necked flask 27.3g (0.11mol) of compound 4-(5-propyl tetrahydropyran-2-) phenylboronic acid shown in formula III, 32.1g (0.275mol) anhydrous sodium carbonate, 500ml toluene, 25ml ethanol, 25ml water, 1.25g (0.001mol) tetrakistriphenylphosphine palladium, react at 90°C for 6 hours, add 100ml toluene, 100ml water, separate the lower aqueous phase and extract it with 100ml×2 toluene, combine the organic phases, and use 200ml×3 saturated saline Wash with water, evaporate toluene to dryness, decolorize the product by silica gel column chromatography, and recrystallize twice with 3 times ethanol and 0.5 times petroleum ether to obtain the target compound with a yield of 70%.
[0045] The experimental resu...
Embodiment 3
[0050] Example 3, 2-(4'-(difluoro(3,4,5-trifluorophenoxy)methyl)-2,3',5',6-tetrafluorobiphenyl)-5-propyl Preparation of Tetrahydropyran
[0051] With reference to Example 1 in this embodiment, the reaction raw material propylphenylboronic acid in Example 1 was replaced by 2,6-difluoro-4-(5-propyltetrahydropyran 2-)phenylboronic acid to prepare the following compounds, The yield is 72%;
[0052]
[0053] (1) GC: 99.9%;
[0054] (2) GC-MS data analysis: 548 (M+0.4) 417 (100) 336 (27.8) 131 (14.4);
[0055] (3) mp: 83°C, Δε: 35.6, Δn: 0.1316, γ: 457.
[0056] It can be known from the above that the compound has a correct structure and is a compound shown in formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com