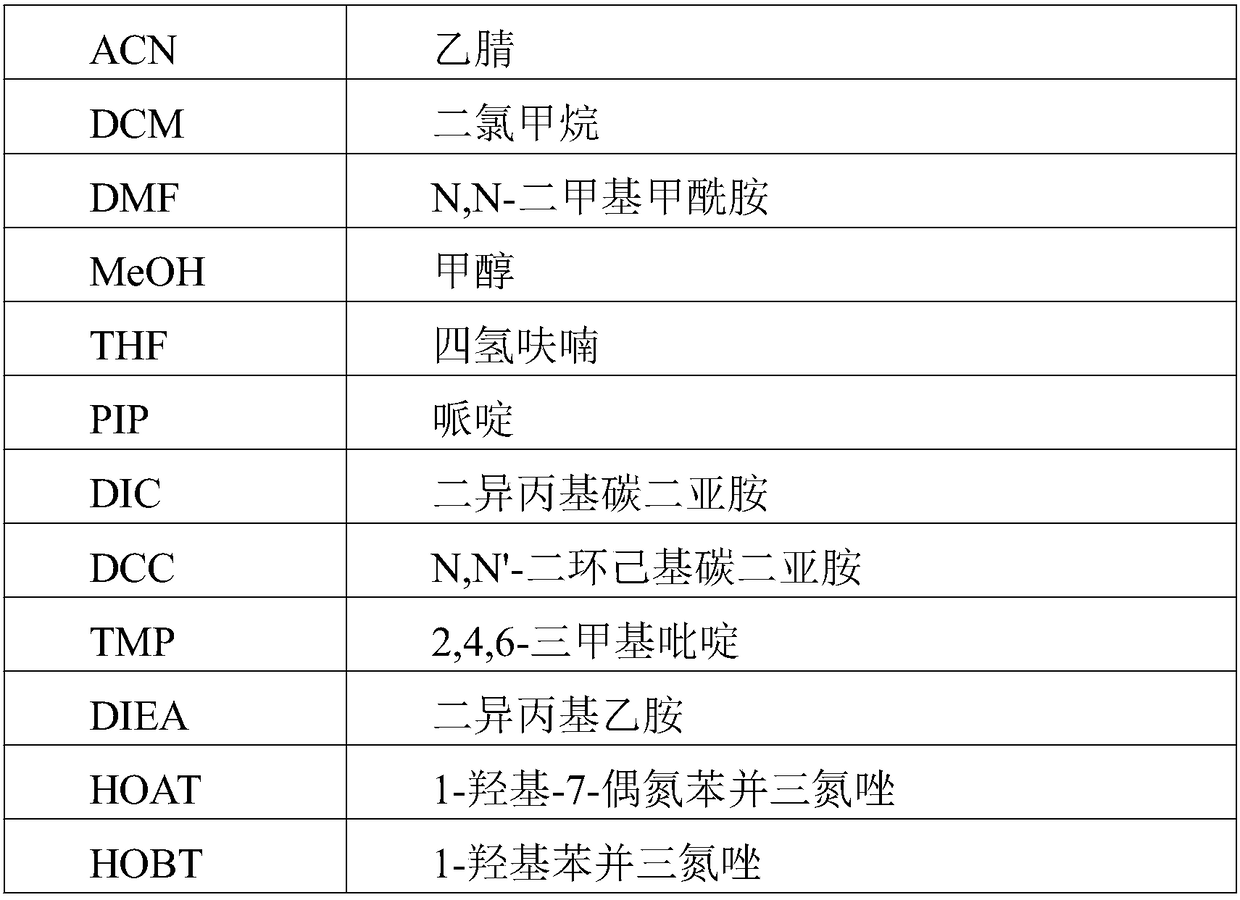
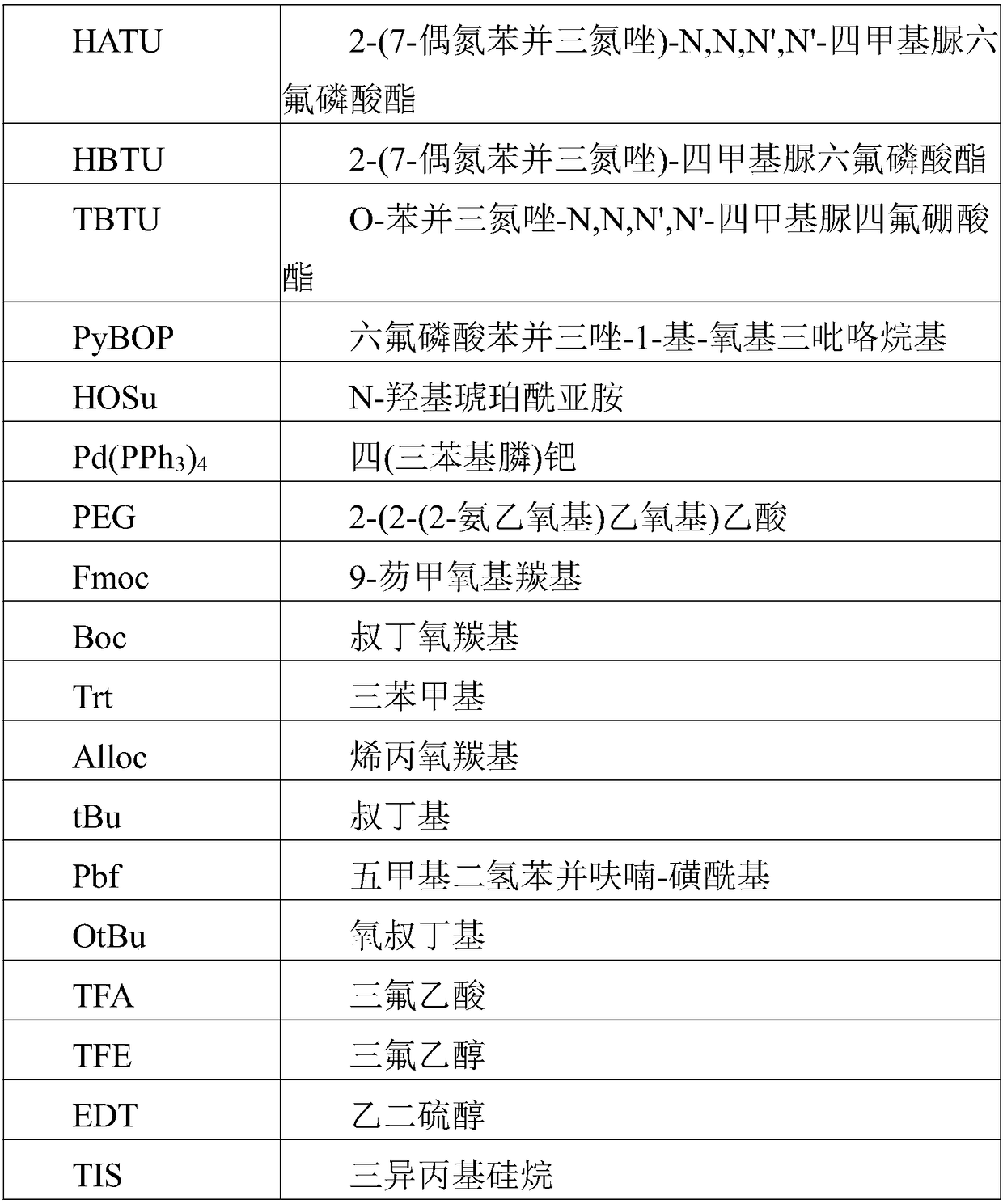
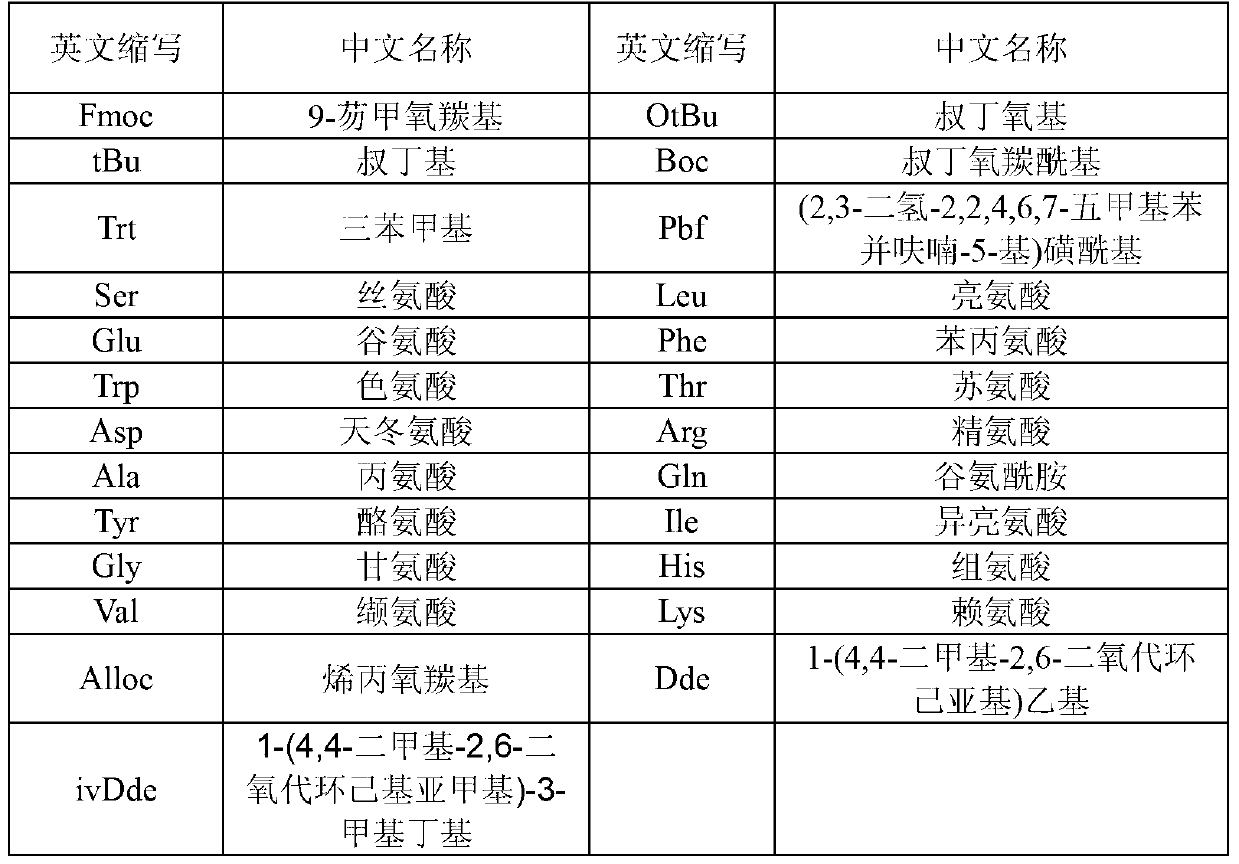
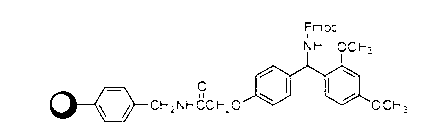
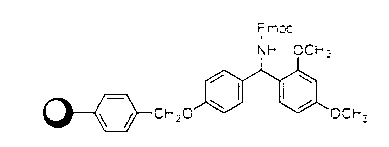
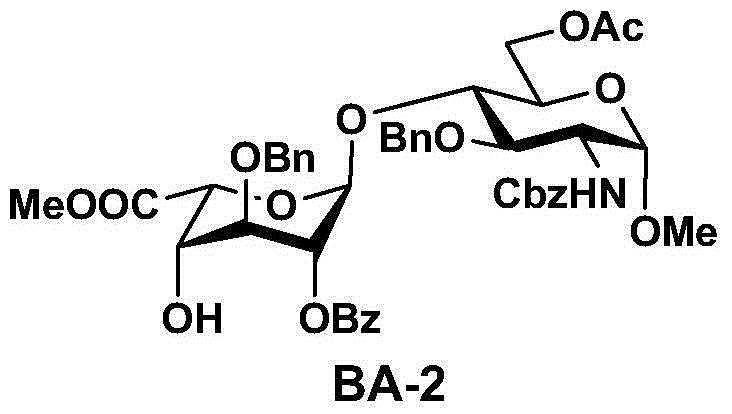
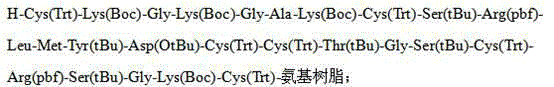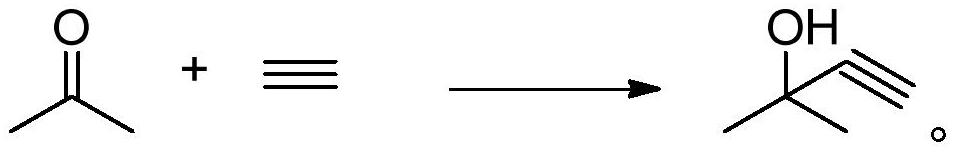Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
308results about How to "Reduce the difficulty of purification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
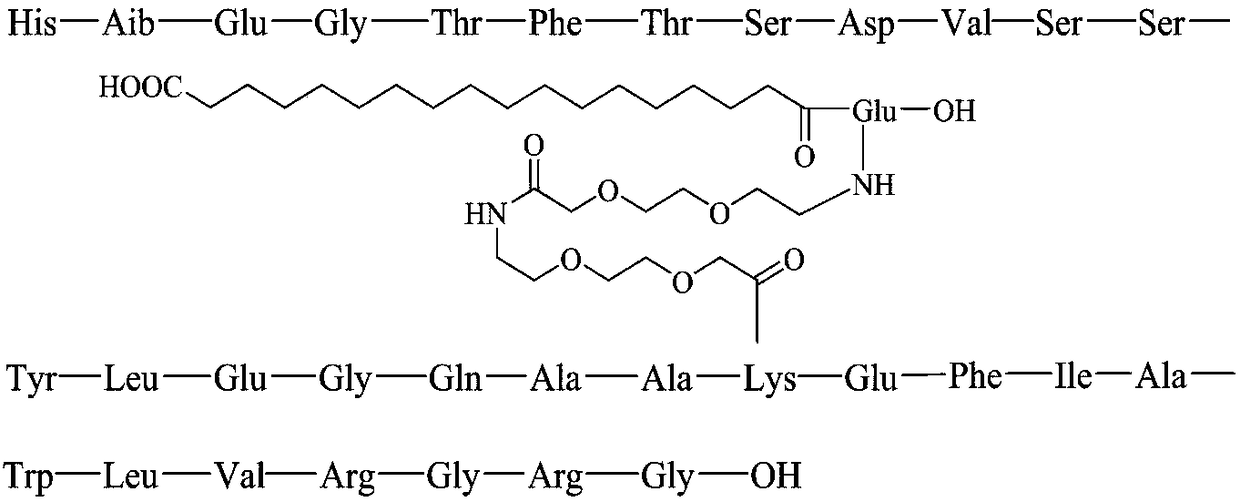
Method for preparing Semaglutide through solid and liquid combination
ActiveCN108059666AReduce generationAvoid it happening againPeptide preparation methodsBulk chemical productionDipeptideSide chain
The invention relates to a method for preparing Semaglutide through solid and liquid combination, and solves the technical problems that in the process for synthesizing long-sequence polypeptide by the existing technology, the synthesis period is long; the purification difficulty is high; the yield is low. The method for preparing Semaglutide through solid and liquid combination provided by the invention is characterized in that firstly, Lys and resin are condensed in an Alloc-Lys(Fmoc)-OH form by adopting a solid phase synthesis method; Fmoc protecting groups on epsilon-NH2 are removed; sidechain connection is performed; cracking is performed to obtain Alloc-Lys(PEG-PEG-gamma-Glu(OtBu)-Monobutyl octadecanate)-OH; meanwhile, 10 dipeptide or tripeptide or tetrapeptide fragments are simultaneously synthesized by a liquid phase synthesis method; then, the condensation reaction of the synthesized peptide fragments and single amino acid is performed by using the resin as a carrier; the 15-step solid phase condensation reaction is reduced in the process; the generation of lacked peptide impurities is reduced; the product purity and the yield are improved; meanwhile, the generation of the impurities of [+Gly]-Semaglutide and [+Ala]-Semaglutide is effectively avoided; the purification difficulty is greatly reduced. The method is widely applied to the technical field of polypeptide medicine preparation.
Owner:润辉生物技术(威海)有限公司
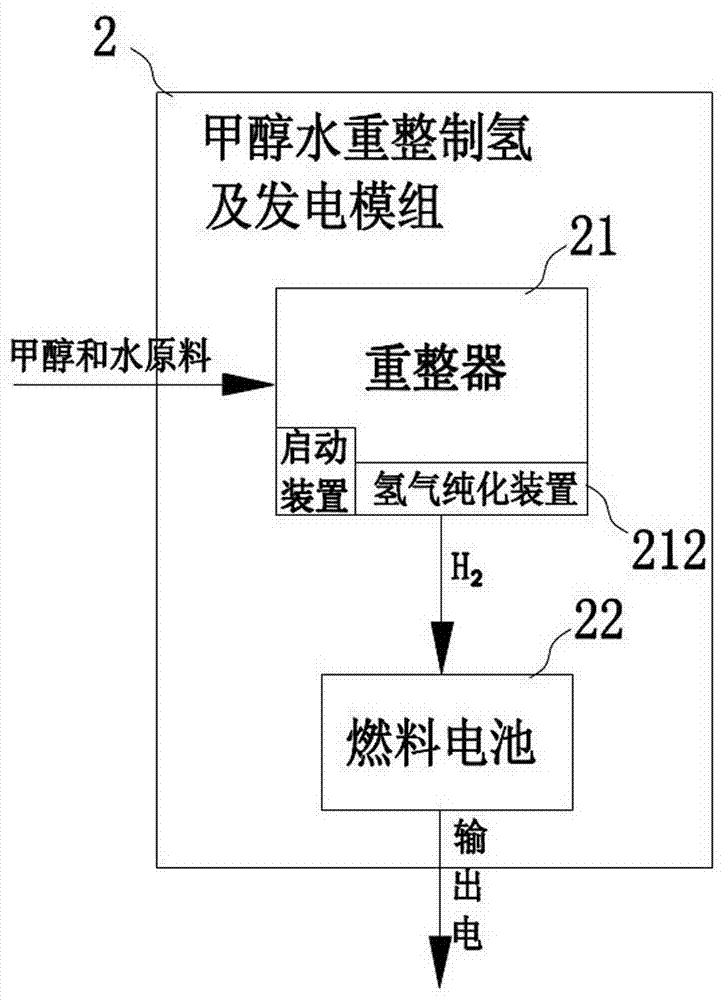
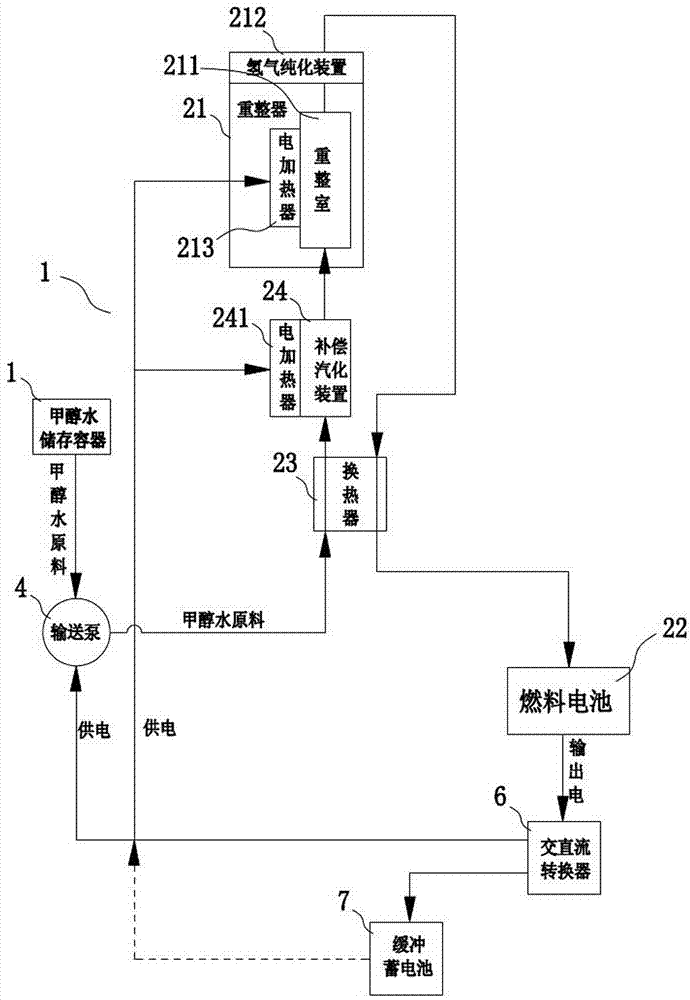
Fuel cell car
ActiveCN104555921AImprove power generation efficiencyIncrease output powerHydrogenVehicular energy storageElectricityElectrochemical response
The invention discloses a fuel cell car. The fuel cell car comprises a methyl alcohol hydrogen producer, a fuel cell and a car motor, wherein the methyl alcohol hydrogen producer comprises a methyl alcohol storage container, a raw material conveyor, a reformer and a hydrogen purifier; the reformer comprises a heat exchanger, a gasifying chamber, a reforming chamber and a separating chamber, and the hydrogen purifier is arranged in the separating chamber; the lower and middle parts of the reforming chamber are at the temperatures of 300-420 DEG C, and the upper part of the reforming chamber is at the temperature of 400-570 DEG C; the temperature in the separating chamber is set into 350-570 DEG C, hydrogen is obtained at the hydrogen producing end of the hydrogen purifier in the separating chamber, and the hydrogen is supplied to the fuel cell after heat exchange is carried out in the heat exchanger; the fuel cell comprises at least two fuel cell groups, and is used for producing electricity through the electrochemical reaction of the hydrogen and oxygen and supplying the car motor with electricity; the car motor is used for driving a car shaft to rotate and accordingly driving the car to run. The methyl alcohol raw material can be converted to the hydrogen at a high speed, the methanol conversion efficiency and utilizing rate are high, and the purification of hydrogen containing gas is easy.
Owner:GUANGDONG HYDROGEN ENERGY SCI & TECH
Preparation method for liraglutide
ActiveCN103275208AAvoid it happening againReduce the difficulty of purificationPeptide preparation methodsGlucagonsSolid phasesPAL
The invention belongs to the technical field of preparation methods for polypeptide medicines, and particularly relates to a preparation method for liraglutide, aiming at solving the technical problems of difficult separation and purification, and low total yield and purity of products, of the existing preparation method. The technical scheme for solving the technical problems, disclosed by the invention is providing a preparation method for liraglutide. The method comprises the following steps of: preparing liraglutide resin via a solid-phase polypeptide method; performing acidolysis to obtain a liraglutide crude product; and finally purifying to obtain a liraglutide pure product, wherein the solid-phase polypeptide method comprises the step of sequentially connecting amino resin to corresponding protected amino acids or protected amino acid segments in the following sequences via a solid-phase coupling synthesis method, so as to prepare the liraglutide resin: Boc-W(Trt)-X(OtBu)-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(OtBu)-Val-Ser(tBu)-Ser(tBu)-Tyr(tBu)-Leu-X(OtBu)-Gln(Trt) -Ala-Ala-Lys[Y(Alpha-OtBu)]-Glu(OtBu) -Phe-Ile-Ala-Trp(Boc)-Leu-Val-Z(Pbf)-Arg(Pbf)-Gly-resin, wherein W is His-Ala, X is Glu-Gly, Y is N alpha-PAL-Glu, and Z is Arg-Gly. The invention provides a novel method for shortening the production cycle, and improving the purity and the yield of the products.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for preparing bivalirudin
ActiveCN102532274AAvoid it happening againReduce the difficulty of purificationPeptide preparation methodsBulk chemical productionSynthesis methodsCombinatorial chemistry
The invention belongs to the technical field of preparation methods for polypeptide medicines, and particularly relates to a method for preparing bivalirudin. The method for preparing the bivalirudin comprises the following steps of: preparing a bivalirudin resin by a solid phase polypeptide synthesis method, performing acidolysis on the bivalirudin resin to obtain a bivalirudin crude product, and purifying the bivalirudin crude product to obtain a bivalirudin pure product, wherein the step of preparing the bivalirudin resin by the solid phase polypeptide synthesis method comprises the following substeps of: sequentially accessing the corresponding protection amino acid or fragments on an Fmoc-Leu-carrier resin by a solid phase coupling and synthesis method to obtain the bivalirudin resin, wherein the corresponding protection amino acid or fragments have the following sequences: R1-D-Phe-Pro-X-Y-Phe-Glu(OtBu)-Glu(OtBu)-Ile-Pro-Glu(OtBu)-Glu(OtBu)-Tyr(tBu)-Leu-resin, R1 is Fmoc, Boc or H; X is Arg(Pbf)-Pro; Y is Gly-Gly-Gly-Asn(R2)-Gly-Asp-(OtBu); and R2 is Trt or H. The purity of the product can reach above 99.5 percent.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for preparing bivalirudin through solid-liquid combination
ActiveCN104031127ASolve difficult coupling problemsReduce contentPeptide preparation methodsFluid phaseEngineering
The invention belongs to the field of polypeptide synthesis and relates to a method for preparing bivalirudin through solid-liquid combination. The bivalirudin is prepared by adopting a method for combining a liquid phase and a solid phase, impurity peptides can be well avoided, the purity of crude peptides is improved, and the production cost is reduced. The method comprises the following steps: synthesizing a fragmental hexapeptide Fmoc-Arg(pbf)-Pro-Gly-Gly-Gly-Gly-OH by adopting a liquid phase method, and connecting peptides onto the solid phase. By utilizing the method, impurity peptides Biv+ / -Gly and Biv+ / -2Gly can be avoided, and the problem that the peptides are difficultly completely coupled during solid phase Arg connection is avoided. By utilizing the synthesis process, the purity of the crude peptides can be over 90 percent, and the purification difficulty is reduced, so that the purity of the final product exceeds 99.5 percent, and the production cost is further reduced. Compared with the prior art, the method disclosed by the invention is simple in operation and low in synthesis cost, and is conductive to large-scale industrial production.
Owner:JINAN KANGHE MEDICAL TECH
Method for preparing high-purity hyodeoxycholic acid by pig bile
InactiveCN101037463AReduce the difficulty of purificationLow impurity contentDigestive systemUnknown materialsBenzeneHyodeoxycholic acid
The invention relates to a method for extracting the hyodeoxycholic acid from the leftovers of pig bile or extracted bilirubin, including saponify the leftovers with alkali, adjusting pH value, acetic ester extraction, decoloring with active carbon; concentrating the mother liquid to a proper volume, precipitating deposit, separating the deposit and drying to get coarse hyodeoxycholic acid; esterifying the coarse product, then doing an addition reaction with benzene, separating the methylhyodeoxycholanate-benzene addition compound; decomposing the addition compound with alkali, adjust pH value, getting the purified deoxycholic acid. The obtained mother liquid can be further extracted to get precious chenodeoxycholic acid. The craft has a lot of material, a low pollution, safety and no poison, a low cost, a high product purity and is suitable for industry production in a large scale.
Owner:JIANGSU UNIV
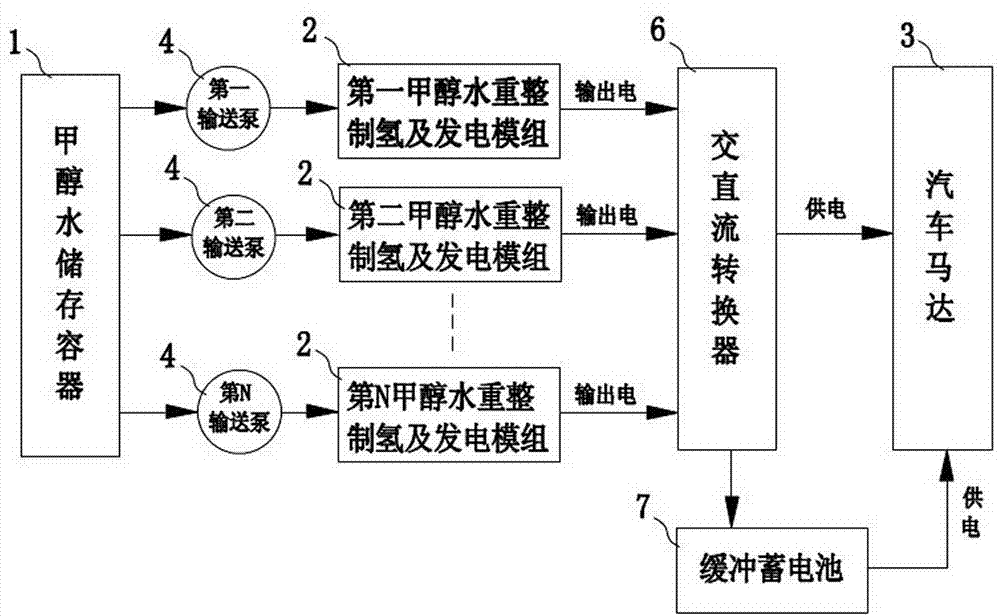
Multi-combination independent alcohol-water hydrogen generation fuel cell car
InactiveCN104752746AOverall small sizeSmall amount of hydrogen productionHydrogenFuel cell auxillariesMethanol waterAlcohol
The invention discloses a multi-combination independent alcohol-water hydrogen generation fuel cell car. The multi-combination independent alcohol-water hydrogen generation fuel cell car comprises a methanol water storage vessel, at least two groups of methanol water reforming hydrogen generation and power generation modules, a car motor and at least two conveying pumps, wherein the methanol water reforming hydrogen generation and power generation modules are integrated with a reforming device and a fuel cell, a reforming chamber and a hydrogen purifying device are arranged in the reforming device, the temperature in the reforming chamber is 300 to 570 DEG C, methanol and water have the reforming hydrogen generation reaction in the reforming chamber to prepare hydrogen-containing gas, the hydrogen-containing gas is purified by the hydrogen purifying device to obtain hydrogen, and obtained hydrogen is supplied to the fuel cell; the fuel cell is used for generating electric power which is used for powering the car motor; the car motor is used for driving a car axle to rotate so as to enable a car to run. According to the invention, the speed for converting methanol and water raw materials into hydrogen is high, the methanol conversion efficiency is high, the utilization rate of methanol is high, and the hydrogen-containing gas is easy to purify; the reforming device is easy to start, the stability is good, the vibration quantity is small, parameters such as the hydrogen generation temperature are sensitive to control, the safety is high, and the reliability is high.
Owner:GUANGDONG HYDROGEN ENERGY SCI & TECH
Method for preparing ziconotide
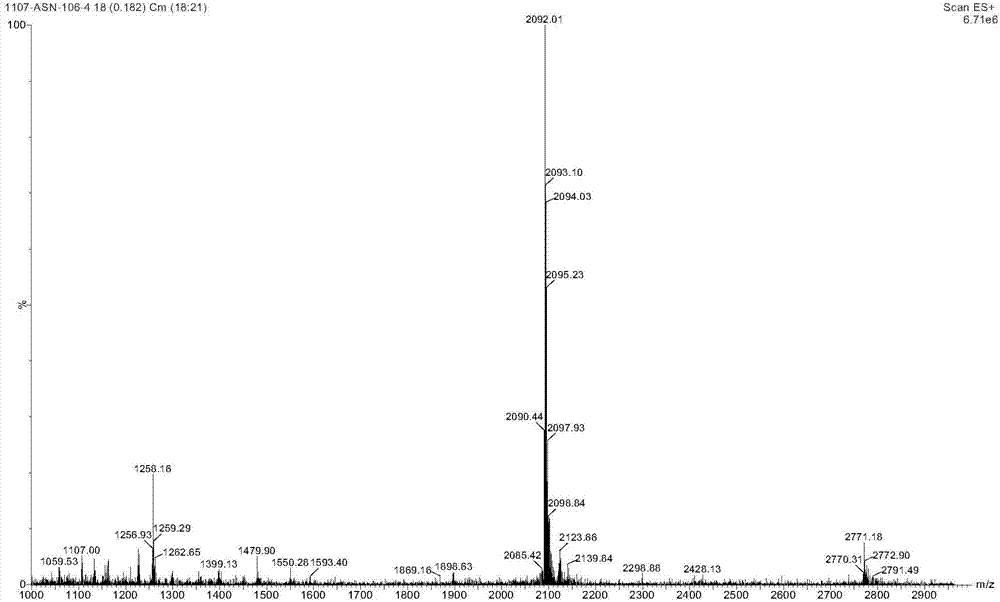
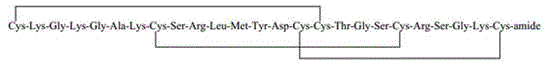
InactiveCN103304655AAvoid it happening againReduce the difficulty of purificationPeptide preparation methodsAnimals/human peptidesSynthesis methodsCombinatorial chemistry
The invention belongs to the technical field of polypeptide drugs, and particularly relates to a method for preparing ziconotide, and the method is used for solving the technical problems of difficult separation and purification and low total product yield and purity in the existing preparation methods. The method comprises the following steps of: preparation of ziconotide linear peptide resin based on a solid phase polytide method, acidolysis for obtaining a ziconotide linear peptide crude product, oxidation for obtaining a ziconotide crude product and purification for obtaining a ziconotide purified product, wherein the solid phase polytide method comprises the following steps of: preparing ziconotide linear peptide resin by sequentially connecting corresponding protected amino acid or protected amino acid fragment in the following sequence starting from amino resin through a solid phase coupling synthesis method: R-Cys(Trt)-X(Boc)-X(Boc)-Ala-Lys(Boc)-Cys(Trt)-Ser(tBu)-Arg(Pbf)-Leu-Met-Tyr(tBu)-Asp(OtBu)-Cys(Trt)-Cys(Trt)-Y(tBu)-Ser(tBu)-Cys(Trt)-Arg(Pbf)-Z(tBu)-Lys(Boc)-Cys(Trt)-amino resin, wherein R is Fmoc, Boc or H, X is Lys-Gly, Y is Thr-Gly, and Z is Ser-Gly. The invention provides a novel method for shortening the production period and improving the product purity and the product yield.
Owner:CHENGDU SHENGNUO BIOPHARM
Synthetic method for liraglutide
ActiveCN105732798AReduce synthesisReduce difficultyPeptide preparation methodsGlucagonsFreeze-dryingCombinatorial chemistry
The invention relates to products in the field of medicines, and provides a synthetic method for liraglutide, which includes the following steps: 1) condensing fragments to obtain various liraglutide intermediates; 2) deprotecting and cracking full-protected liraglutide; and 3) purifying and freeze-drying the liraglutide. The method employs a 4+5+7+6+9 synthetic method, wherein synthesis on five fragments is carried out at the same time, so that synthetic time of the product is greatly reduced. Meanwhile, through step-by-step analysis of synthesis factors, comprising His-Ala-Glu-Gly, Thr-Phe-Thr-Ser-Asp, Val-Ser-Ser-Tyr-Leu-Glu-Gly, Gln-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu]Lys-Glu-Phe, and Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH, synthesis difficulty of difficult peptide sequence in solid-phase synthesis is reduced, scale amplification in solid-phase synthesis is solved and synthetic efficiency is improved. Because fragment synthesis reduces purifying difficulty effectively, the production cost is greatly reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Boron-containing intermediate and application of same to medical industry
InactiveCN106496259AReduce the difficulty of purificationRaw materials are cheap and easy to getGroup 3/13 element organic compoundsCompound (substance)Boron containing
The invention discloses a preparation method for novel boron-containing intermediate I and a boron-containing drug MLN9708. The novel boron-containing intermediate I prepared by using a fluorination reagent is applied to preparation of the antitumor drug MLN9708; the intermediate has stable chemical properties in normal-temperature air, is easy to recrystallize and purify; and the intermediate and citric acid undergo a one-step reaction under the action of an alkaline reagent and a silicon reagent so as to prepare the antitumor drug MLN9708. The preparation method provided by the invention has no special requirements on production equipment, is mild in reaction conditions, uses commercially available raw materials and is suitable for industrial production.
Owner:CHENGDU BESTCHIRALBIO LIMITED LIABILITY
Preparation method of abaloparatide
InactiveCN108047329AEasy to purifyReduce consumptionPeptide preparation methodsParathyroid hormonesDipeptideImpurity
The invention relates to a preparation method of abaloparatide, aiming at solving the technical problems of a method in the prior art for synthesizing the abaloparatide that steps are complicated, theconsumption of raw materials is great and a synthesized product has low yield, low purity and more impurities and is difficult to purify. The preparation method of the abaloparatide, provided by theinvention, comprises the following steps: firstly, simultaneously synthesizing dipeptide segments Fmoc-Thr(OtBu)-Ala-OH, Fmoc-Lys(Boc)-Gly-OH and Fmoc-Ala-Val-OH and a tetrapeptide segment Fmoc-Arg-(Pbf)-Arg(Pbf)-Arg(Pbf)-Glu(OtBu)-OH; taking amino resin as a carrier of solid-phase synthesis; condensing completely protected amino acids containing alpha-NH2 with an Fmoc protection group and the synthesized dipeptide segments and tetrapeptide segment in sequence according to an amino acid sequence of the abaloparatide from a C end to an N end, so as to obtain completely protected abaloparatide resin, wherein 6 steps of solid-phase condensation reaction are reduced; cracking and purifying to obtain an abaloparatide pure product. The preparation method provided by the invention is widely applied to the technical field of preparation and synthesis of polypeptide drugs.
Owner:润辉生物技术(威海)有限公司
Preparation method of Tirzepatide
PendingCN110903355AHigh purityHigh yieldPeptide-nucleic acidsPeptide preparation methodsBiochemical engineeringCombinatorial chemistry
Owner:CHENGDU SHENGNUO BIOPHARM
Fondaparinux sodium, intermediates thereof and preparation methods
ActiveCN103601765AMild responseGood reaction selectivitySugar derivativesSugar derivatives preparationChemical compoundChemical preparation
The invention relates to fondaparinux sodium, intermediates thereof and preparation methods in the technical field of chemical preparation, particularly relates to the preparation methods of the fondaparinux sodium and the intermediates of the fondaparinux sodium, comprising the fondaparinux sodium, a disaccharide intermediate of the fondaparinux sodium, a tetrasccharide intermediate of the fondaparinux sodium, a pentasaccharide intermediate of the fondaparinux sodium and the corresponding preparation methods. The structure of the fondaparinux sodium is shown as the chemical compound (1). The invention also relates to the disaccharide intermediate, the tetrasccharide intermediate and the pentasaccharide intermediate which are used for preparing the fondaparinux sodium. The invention designs a novel synthetic route to prepare the fondaparinux sodium. The novel synthetic route has mild reactions, good reaction selectivity, strong controllability and low operation difficulty, and is prone to achievement of industrial production.
Owner:SHANGHAI ACANA PHARMTECH
Solid-phase fragment synthetic method of exenatide
ActiveCN103613656AReduce accumulationHigh synthesis efficiencyHormone peptidesPeptide preparation methodsCombinatorial chemistrySolid-phase synthesis
The invention discloses a solid-phase fragment synthetic method of exenatide. The solid-phase fragment synthetic method comprises the following steps: dividing 39 amino acids of the exenatide into 6 sections of full-protection fragments; firstly respectively synthesizing the 6 sections of the full-protection fragments; sequentially carrying out solid-phase fragment assembling and connection on the 6 sections of the full-protection fragments to obtain exenatide resin; then cutting to obtain an exenatide crude product; and purifying the crude product to obtain an exenatide product. According to the solid-phase fragment synthetic method of the exenatide, the problems that the existing solid-phase exenatide synthesizing process is complicated, is low in efficiency and difficult to purify and scale are solved; the synthesizing efficiency is improved and connection steps are simplified greatly; the accumulation of impurities is reduced; the invention provides a novel method for realizing the large-scale synthesis of the exenatide, improving the product purity and reducing the purification difficulty.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
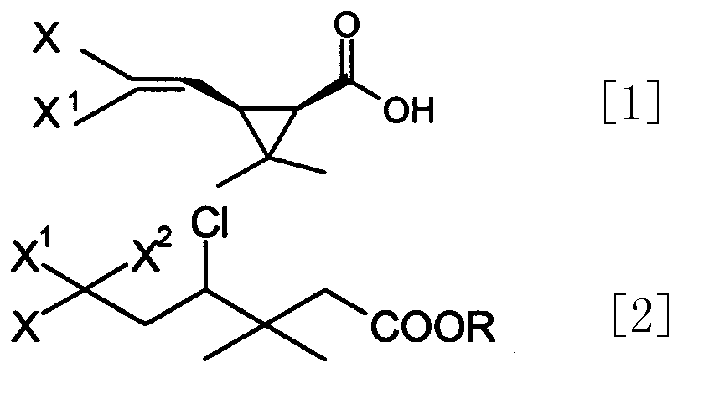
Process for preparing cis-halochrysanthemic acid
InactiveCN1390825AIncrease dosageReduce dosagePreparation from carboxylic acid esters/lactonesHexamethylphosphoramideTert butyl
A process for preparing cis-halochrysanthemic acid includes such steps as the addition reaction of raw material (sodium tert-butyl alkoxide), reaction for removing hydrogen chloride and hydrolysis, reflux with excess sodium hydroxide in the mixed solvent of methanol or ethanol and water to obtain cyclocompound, and acidation. Its advantages are simple process, high output rate, low cost and less pollution.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Method for synthesizing glucagon-like peptide (GLP)-1 analogue in solid-phase mode
InactiveCN102977204AReduce generationReduce the difficulty of purificationHormone peptidesPeptide preparation methodsSolid-phase synthesisGLP-1 Analogue
The invention relates to the field of polypeptide solid-phase synthesis and provides a method for synthesizing glucagon-like peptide (GLP)-1 analogue in a solid-phase mode. The method for synthesizing the GLP-1 analogue in the solid-phase mode solves the problems that in the process of synthesizing the GLP-1 analogue in the solid-phase mode, peptide deficiency products and by-products are generated because of difficult or incomplete amino acid sequence connection, and the by-products are resulted to be difficulty to separate in subsequent purification. The peptide deficiency products and by-products are quite same in properties. According to the method for synthesizing the GLP-1 analogue in the solid-phase mode, a segment convergent synthesis method and a substituent-introduced method are adopted in difficult-connection points and polypeptide synthesis yield coefficient is improved.
Owner:JILIN AOTENG BIOTECH
Solid-phase synthesis method of ziconotide by segment process
InactiveCN104974237AHigh yieldShort synthesis cyclePeptide preparation methodsAnimals/human peptidesDisulfide bondingDisulphide bond formation
The invention belongs to the field of polypeptide synthesis, and relates to a solid-phase synthesis method of ziconotide by a segment process. The method aims to solve the technical problems of long synthesis period and low crude peptide purity in the existing preparation method, and the technical problems of complex operation, low total yield and the like in the disulfide-bond formation process. The technical scheme is as follows: the method comprises the following steps: synthesizing four segment peptides [19-25]A, [12-18]B, [6-11]C and [1-5]D of ziconotide by a solid-phase process; sequentially connecting the all-protected peptide fragments B, C and D to the peptide resin A, and cracking to obtain a ziconotide linear peptide; and finally, carrying out liquid-phase one-step oxidization, which has the advantages of mild conditions, simplified operation, easy after-treatment and environment friendliness, to obtain the ziconotide. The technical scheme can greatly shorten the synthesis period, lower the synthesis and purification difficulty and the production cost, and enhance the purity of the linear peptide and the total yield of the product. The method is beneficial to industrial large-scale production.
Owner:JINAN KANGHE MEDICAL TECH
Method for preparation of moxidectin
ActiveCN104277050AReduce the difficulty of purificationReduce the number of analysisOrganic chemistryNemadectinTert butyl
The invention relates to a method for preparation of moxidectin. The method includes subjecting Nemadectin to protective reaction, oxidation reaction, oximation reaction and deprotection reaction in order, thus obtaining moxidectin. Specifically, the protective agent employed in the protective reaction is tert-butyl dimethylchlorosilane, and after the oximation reaction, the solid form crude product obtained by the oximation reaction is subjected to crystallization, and then the deprotection reaction is carried out on the obtained crystal. The method provided by the invention increases the product content, further reduces the difficulty for follow-up utilization of macroporous resin for purification, and improves the column loading amount, reduces the number of column chromatography, maintains or even improves the purity of the final product, and lowers the production cost.
Owner:NEW FOUNDER HLDG DEV LLC +2
Preparation method of liraglutide
ActiveCN107056927AShorten the production cycleHigh synthetic yieldPeptide preparation methodsBulk chemical productionSide chainCoupling
The invention discloses a preparation method of liraglutide. The preparation method comprises the following steps: using a deprotection reagent Fmoc-Gly-wang resin for deprotection, and removing Fmoc protective groups; coupling three protective amino acids at the 12th-14th sites by protective peptide fragments X; coupling three protective amino acids at 20-22 sites by protective peptide fragments Y; coupling protective amino acid at the 31th site by a Boc-His(trt)-OH protective form; removing alloc protection of lysine side chains for main-chain peptide resin of the liraglutide, coupling the side chains one by one and thus obtaining peptide resin of the liraglutide; cracking the peptide resin of the liraglutide in cracking liquid and thus obtaining a liraglutide crude product; purifying the liraglutide crude product and thus obtaining a liraglutide fine product. The preparation method disclosed by the invention has the advantages that segment coupling is carried out on amino sites which are easiest for folding in the process of synthesizing the main chain of the liraglutide, so that the problems of multiple times of coupling and low access rate for the amino acids due to folding in the synthesis process are avoided, the production cycle of the liraglutide is shortened, and the synthetic yield of the liraglutide is increased.
Owner:SICHUAN JISHENG BIOPHARM CO LTD
Monofunctional branched polyethyleneglycol
ActiveCN103044675ASmall hindranceEasy to convertSugar derivativesDepsipeptidesHydrogen atomPolyethylene glycol
The invention discloses a monofunctional branched polyethyleneglycol. The general formula of the monofunctional branched polyethyleneglycol is disclosed as Formula (1), wherein X1 and X2 are respectively an alkyl group with 1-20 carbon atoms; n1 and n2 are respectively a whole number ranging from 1 to 1000; n3 is a whole number ranging from 11 to 1000; L1 and L2 are respectively a linking group capable of existing stably under illumination, enzyme, acidic or alkaline conditions; p is 0 or 1; R1 is a hydrogen atom or alkyl group with 1-20 carbon atoms; and R is a functional group. The monofunctional branched polyethyleneglycol overcomes the defects of traditional multiarm polyethyleneglycol in drug modification application, can modify biologically relevant substances under mild conditions, and has the advantages of high modification rate, fewer byproducts, high activity retentivity and the like.
Owner:XIAMEN SINOPEG BIOTECH
Preparation process of glycyl-L-tyrosine
ActiveCN102993270AReduce the difficulty of purificationEasy to removePeptide preparation methodsChromatographic separationGlycyl-L-tyrosine
The invention discloses a preparation process of glycyl-L-tyrosine, and belongs to the field of pharmaceutical chemistry. The process comprises a condensation reaction and an ammonolysis reaction. In the ammonolysis reaction, N-chloroacetyl-L-tyrosine and concentrated aqueous ammonia are directly subjected to the ammonolysis reaction. When the reaction is finished, excessive aqueous ammonia is removed by reduced-pressure distillation under a temperature of 40-50 DEG C and a pressure of -0.08MPa to -0.10MPa. Residual substances are re-crystallized by using water, and the material is filtered, such that a glycyl-L-tyrosine crude product is obtained. In the ammonolysis step, N-chloroacetyl-L-tyrosine and concentrated aqueous ammonia are directly subjected to the reaction, and catalyzing of additional ammonium bicarbonate is not needed, such that glycyl-L-tyrosine purification difficulty is greatly reduced. Therefore, a problem of ammonium chloride removing difficulty caused by pH value regulation by using hydrochloric acid in post treatment can be avoided. Also, water re-crystallization is used for replacing WA-30 column chromatographic separation, such that product yield and purity are improved, and reaction cost is reduced. Obtained crude product can reach a medicinal grade through one-time refining, such that operation is greatly simplified, and industrialized production is facilitated.
Owner:SHANDONG QIDU PHARMA
Synthesis method of 4-methoxymethyl-2,3,5,6-tetrafluorobenzyl alcohol
ActiveCN102731269AReduce generationReduce the difficulty of purificationOrganic chemistryOrganic compound preparationLithiumHydrogen halide
The invention discloses a synthesis method of 4-methoxymethyl-2,3,5,6-tetrafluorobenzyl alcohol. The method comprises the following reaction steps of: 1, reacting 2,3,5,6-tetrafluorobenzyl alcohol with hydrogen halide in a solvent for 2-20h at 40-120 DEG C to obtain 3-halomethyl-1,2,4,5-tetrafluorobenzene; 2, reacting the 3-halomethyl-1,2,4,5-tetrafluorobenzene in methanol for 1-20h at 0-65 DEG C under the action of an inorganic alkali to obtain 3-methoxymethyl-1,2,4,5-tetrafluorobenzene; 3, dissolving the 3-methoxymethyl-1,2,4,5-tetrafluorobenzene into an inert solvent, dripping an organic lithium reagent at 0-78 DEG C to react for 0.5-5h, inputting formaldehyde gas into the reaction system to continuously react for 0.5-5h, and carrying out the quenching reaction by a protonic solvent to obtain the 4-methoxymethyl-2,3,5,6-tetrafluorobenzyl alcohol. The synthesis method provided by the invention has the advantages of good reaction selectivity, cheap and available reagents, and high reaction yield and high purity of the product.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Preparation method of Semaglutide
ActiveCN110078816AEnhanced couplingHigh purityPeptide preparation methodsBulk chemical productionSide chainImpurity
The invention provides a preparation method of Semaglutide. By using a short peptide chain Fmoc-Thr<13>-Ser<14>-Asp<15>-OH, impurity deletion caused by the a 14 locus Ser difficult reaction locus is directly avoided; by using a two-step method to couple side chains Fmoc-AEEA-AEEA-OH and Octa(OtBu)-Glu(alpha-OtBu)-gamma-COOH, the problems that the yield and purity are relatively low due to side chain fragment insolubility and low reaction activity are solved, the purification difficulty of the Semaglutide is greatly reduced, the yield and purity of a product are improved, the purity of the obtained high-quality product is greater than 99.0%, the cost of preparing the Semaglutide is also reduced, and the preparation method of the Semaglutide has broad practical value and application prospect.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
Separation method of 2-methyl-3-butyn-2-ol
ActiveCN111807929AHigh purityImprove conversion rateOrganic compound preparationHydroxy compound separation/purificationHexyneDistillation
The invention belongs to the technical field of acetylene alcohol production, and particularly relates to a separation method of 2-methyl-3-butyn-2-ol, which comprises the following steps: pretreatinga reaction solution containing 2-methyl-3-butyn-2-ol to remove unreacted acetone and contained salt from the reaction solution to obtain a 2-methyl-3-butyn-2-ol crude product, with the content of 2,5-dimethyl-3-hexyn-2,5-diol in the reaction solution containing 2-methyl-3-butyn-2-ol being not higher than 0.1 wt%; separating and purifying the 2-methyl-3-butyn-2-ol crude product by virtue of membrane separation treatment and reduced pressure distillation treatment, so as to obtain a 2-methyl-3-butyn-2-ol product. According to the method disclosed by the invention, water in the product can be removed and the content of the 2,5-dimethyl-3-hexyn-2,5-diol can be reduced under the condition that the investment and the energy consumption are reduced, so that the 2-methyl-3-butyn-2-ol with relatively high purity and conversion rate is obtained.
Owner:WANHUA CHEM GRP CO LTD
Iota-carrageenan degrading enzyme gene as well as preparation method and application thereof
ActiveCN103773785AIncrease temperatureImprove stabilityAntibacterial agentsOrganic active ingredientsCellulophaga sp.Enzyme Gene
The invention relates to an iota-carrageenan degrading enzyme gene as well as a preparation method and an application thereof and relates to iota-carrageenanase. An iota-carrageenanase gene cgiA2_Ce is a carrageenanase gene for coding Cellulophaga sp.KL-A. An iota-carrageenan recombinase CgiA2_Ce can be applied to iota-carrageenan degradation, degradation products are two iota-carrageenan oligosaccharides, and the degrees of polymerization of the two iota-carrageenan oligosaccharides are respectively 2-4 saccharide and 6-10 saccharide. The degradation products can be applied in preparation of antibacterial agents, antiviral agents, immunomodulators, antioxidants and the like, particularly antibacterial agents, antiviral agents, immunomodulators, antioxidants and the like for mariculture, so that the survival rate of prawns can be increased by 20%-30%; and a recombined protein of the iota-carrageenanase gene cgiA2_Ce can be obtained through a gene engineering technology.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Preparation method of industrial hemp full stalk pulp
ActiveCN102644212AReduce dustReduce alkali consumptionPulping with inorganic basesPulp beating/refining methodsFiberEcological environment
The invention provides a preparation method of industrial hemp full stalk pulp, comprising the following steps of: cutting air-dried hemp stalk into small sections, and retting to remove a part of pectin and non-fiber impurities; charging into a digester after dehydrating, digesting hemp stalk to obtain finished pulp by a sulfate method or a caustic soda-anthraquinone method; washing the finished pulp and beating, wherein the beating concentration is 1%-6%, the beating degree is less than or equal to 40oSR, and the wet weight of the fiber is within the range of 4.0g-10.0g; and screening, purifying, dehydrating, washing, and spirally extruding and concentrating to obtain the true color pulp of the industrial hemp full stalk. The method can be used for preparing the pulp by directly using the industrial hemp full stalk, so that the pulp is directly prepared from the hemp full stalk, and the ecological environment can be importantly improved; therefore, the preparation method is good in application prospect.
Owner:YUNNAN JUHENG TECH
A kind of solid-liquid combination prepares the method for semaglutide
ActiveCN108059666BReduce generationAvoid it happening againPeptide preparation methodsBulk chemical productionDipeptideSide chain
The invention relates to a method for preparing Semaglutide through solid and liquid combination, and solves the technical problems that in the process for synthesizing long-sequence polypeptide by the existing technology, the synthesis period is long; the purification difficulty is high; the yield is low. The method for preparing Semaglutide through solid and liquid combination provided by the invention is characterized in that firstly, Lys and resin are condensed in an Alloc-Lys(Fmoc)-OH form by adopting a solid phase synthesis method; Fmoc protecting groups on epsilon-NH2 are removed; sidechain connection is performed; cracking is performed to obtain Alloc-Lys(PEG-PEG-gamma-Glu(OtBu)-Monobutyl octadecanate)-OH; meanwhile, 10 dipeptide or tripeptide or tetrapeptide fragments are simultaneously synthesized by a liquid phase synthesis method; then, the condensation reaction of the synthesized peptide fragments and single amino acid is performed by using the resin as a carrier; the 15-step solid phase condensation reaction is reduced in the process; the generation of lacked peptide impurities is reduced; the product purity and the yield are improved; meanwhile, the generation of the impurities of [+Gly]-Semaglutide and [+Ala]-Semaglutide is effectively avoided; the purification difficulty is greatly reduced. The method is widely applied to the technical field of polypeptide medicine preparation.
Owner:润辉生物技术(威海)有限公司
Extracting technology of flavone of ageratum conyzoides by using response surface methodology and application thereof
ActiveCN107550964AReduce the difficulty of purificationLow costFood preservationAntinoxious agentsWater bathsDesign–Expert
The invention discloses an extracting technology of flavone of ageratum conyzoides by using response surface methodology and application thereof. According to the design principle of Box-Behnken test,the technology conditions of the flavone of the ageratum conyzoides are designed and optimized by Design-Expert software, such as concentration of ethyl alcohol, extracting time, material and liquidratio, and extracting frequency; the flavone of the ageratum conyzoides is extracted in an ultrasonic-assisted way. The extracting technology specifically comprises the following steps of drying the ageratum conyzoides, and crushing into powder; adding the ageratum conyzoides powder into an extracting tank, mixing with the ethyl alcohol solution, and performing ultrasonic extracting; placing an extracting solution into a rotary evaporator, relieving pressure and distilling, and ventilating air to volatize the solvent; evaporating by water bath, so as to obtain the finished product of the flavone of the ageratum conyzoides. The extracting technology has the advantages that the extracting rate is high; the anti-oxidizing ability of the extracted flavone of the ageratum conyzoides is strong;the stronger application effect is realized in anti-oxidizing medicines and food antioxidants.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Method for preparing exenatide and product thereof
ActiveCN106632655AReduce accumulationReduce the difficulty of purificationPeptide preparation methodsVasoactive intestinal peptideGly-Gly-ProImpurity
The invention discloses a method for preparing exenatide. The exenatide is prepared from a full protection fragment of Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-CONH2 and full protection fragments of following three fragments of Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-COOH, Thr-Ser-Asp-Leu-Ser-Lys-Gln-COOH and His-Gly-Glu-Gly-Thr-Phe-COOH. By means of the method, the problems that existing solid-phase-synthesis exenatide is difficult to purify and large-scale production is difficult are solved, the synthesis efficiency is improved, impurity accumulation is reduced, and the purifying difficulty is lowered.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
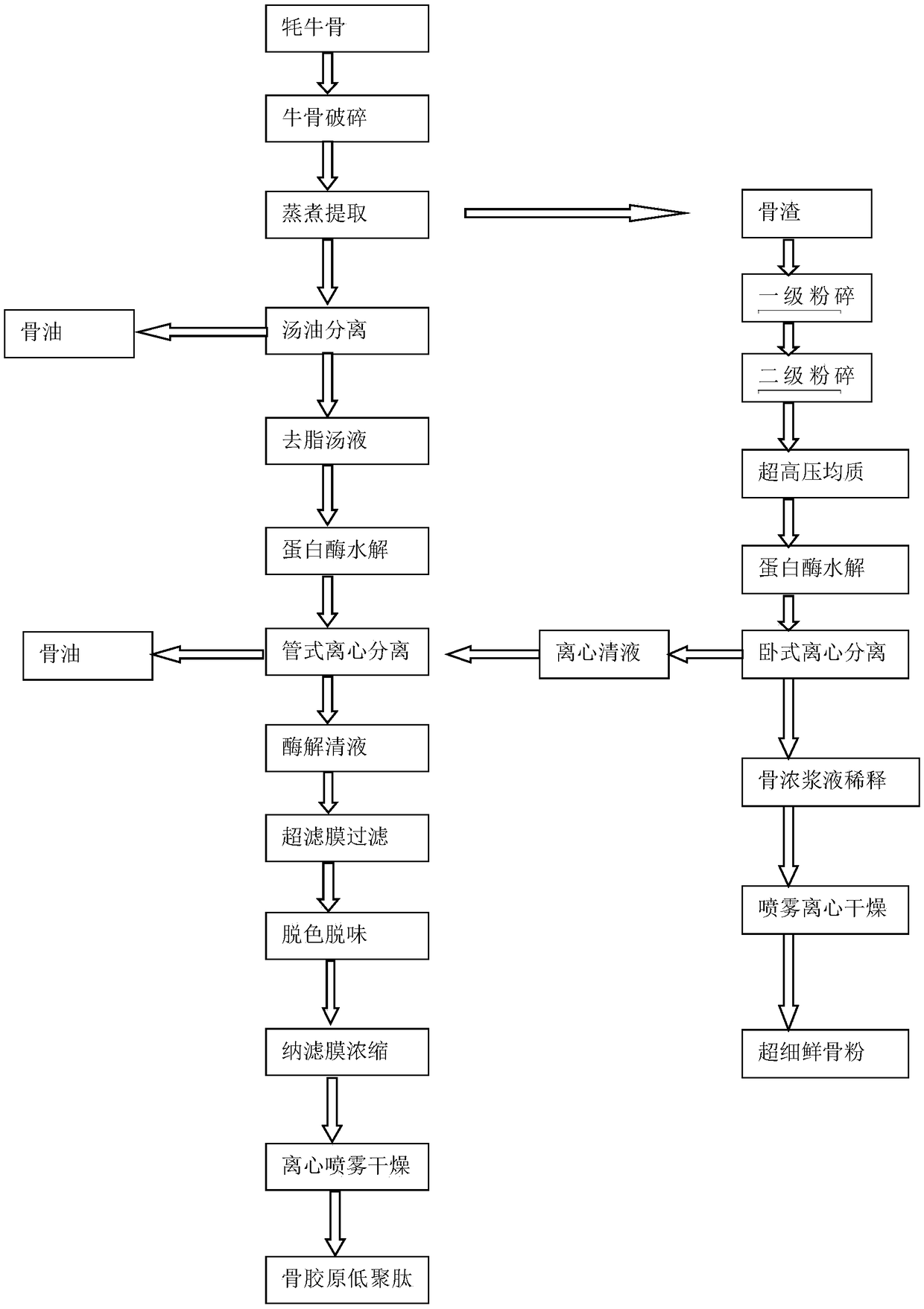
Method for processing yak bones by biotechnology
InactiveCN109287841AIncrease contactIncrease surface areaUltrafiltrationMulti-step food processesUltra high pressureFiltration
The invention relates to a method for processing yak bones by biotechnology. The method comprises the following steps: step 1, crushing yak bones to 3cm-6cm, putting the crushed yak bones into a cooking pot and performing high temperature and high pressure extraction to obtain oil-containing bone soup and oil-containing bone dregs; step 2, preparing the oil-containing bone soup obtained in the step 1 into ossein oligopeptide, and specifically, sequentially performing the following steps: soup-oil separation, protease hydrolysis, tubular centrifugal separation, filtration by an ultrafiltrationmembrane, discoloration and deodorization, concentration by a nanofiltration membrane and centrifugal spray drying; and step 3, preparing the oil-containing bone dregs obtained in the step 1 into superfine fresh bone meal, and specifically, sequentially performing the following steps: primary crushing, secondary crushing, ultra-high-pressure homogenization, protease hydrolysis, horizontal centrifugal separation, thick pulp dilution and centrifugal spray drying.
Owner:王书敏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com