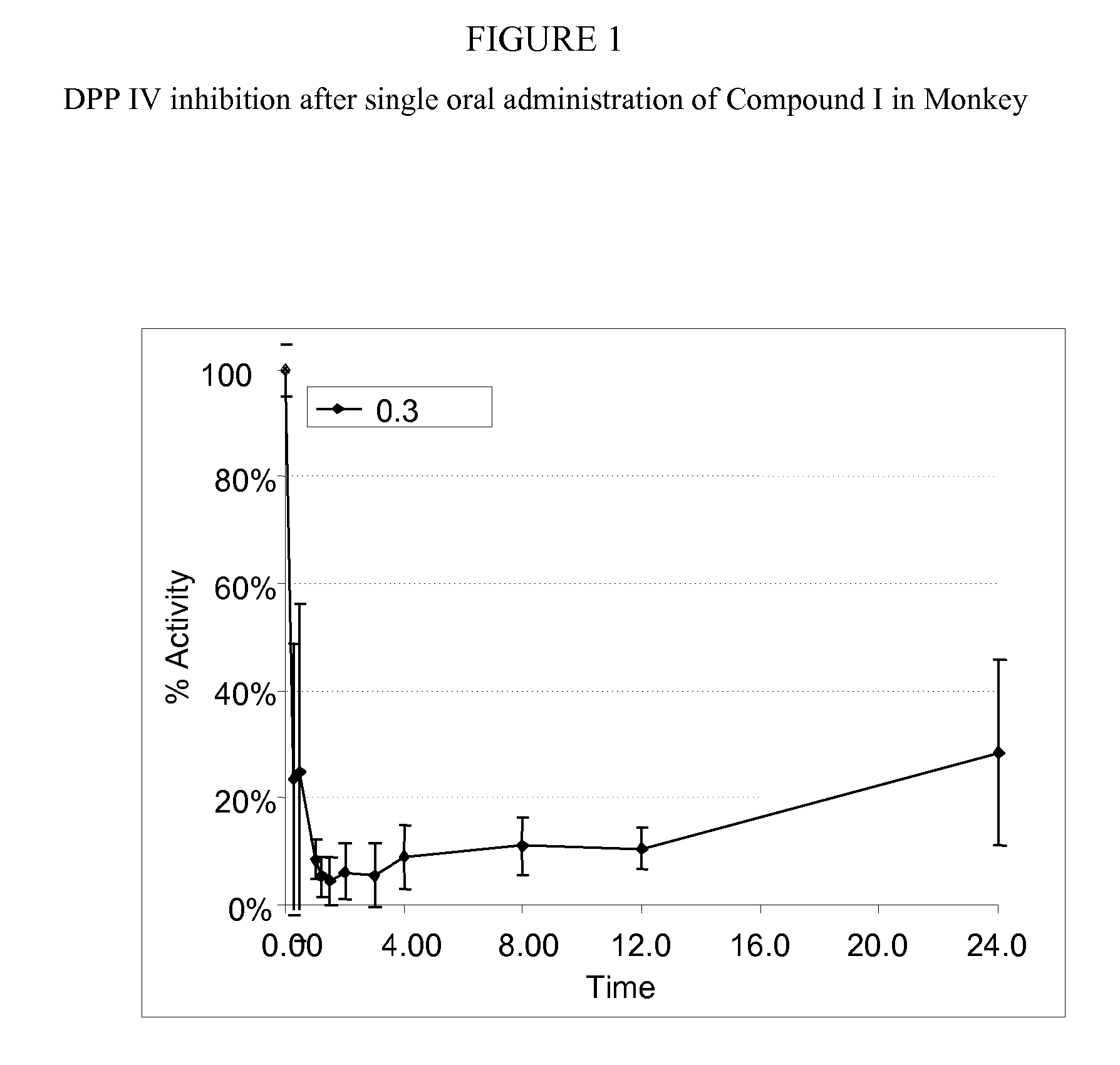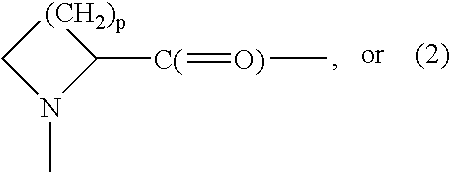Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1731results about "Glucagons" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
Peptide agonists of GLP-1 activity
InactiveUS6528486B1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsMetabolism disorderD-GlucoseGlycemic
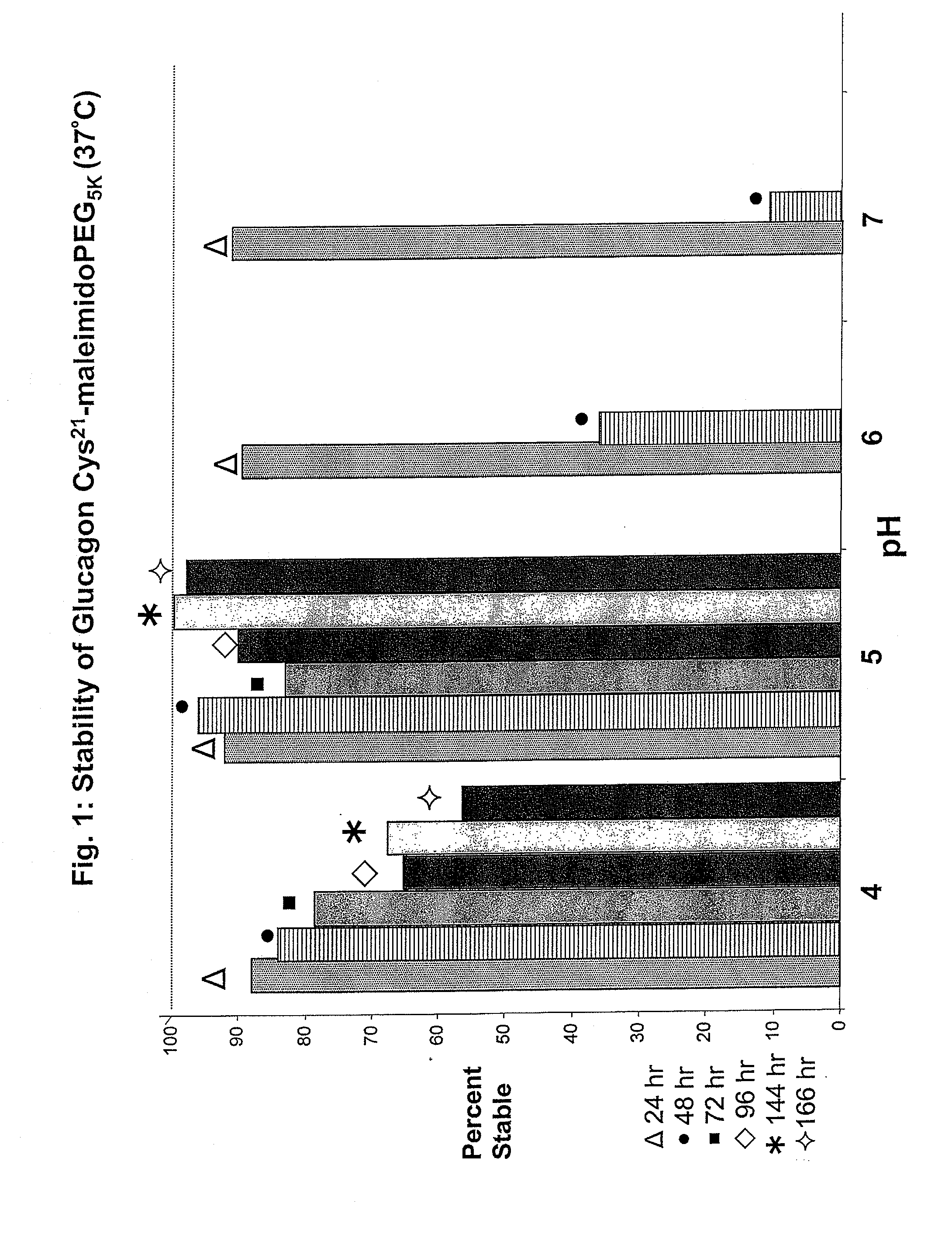
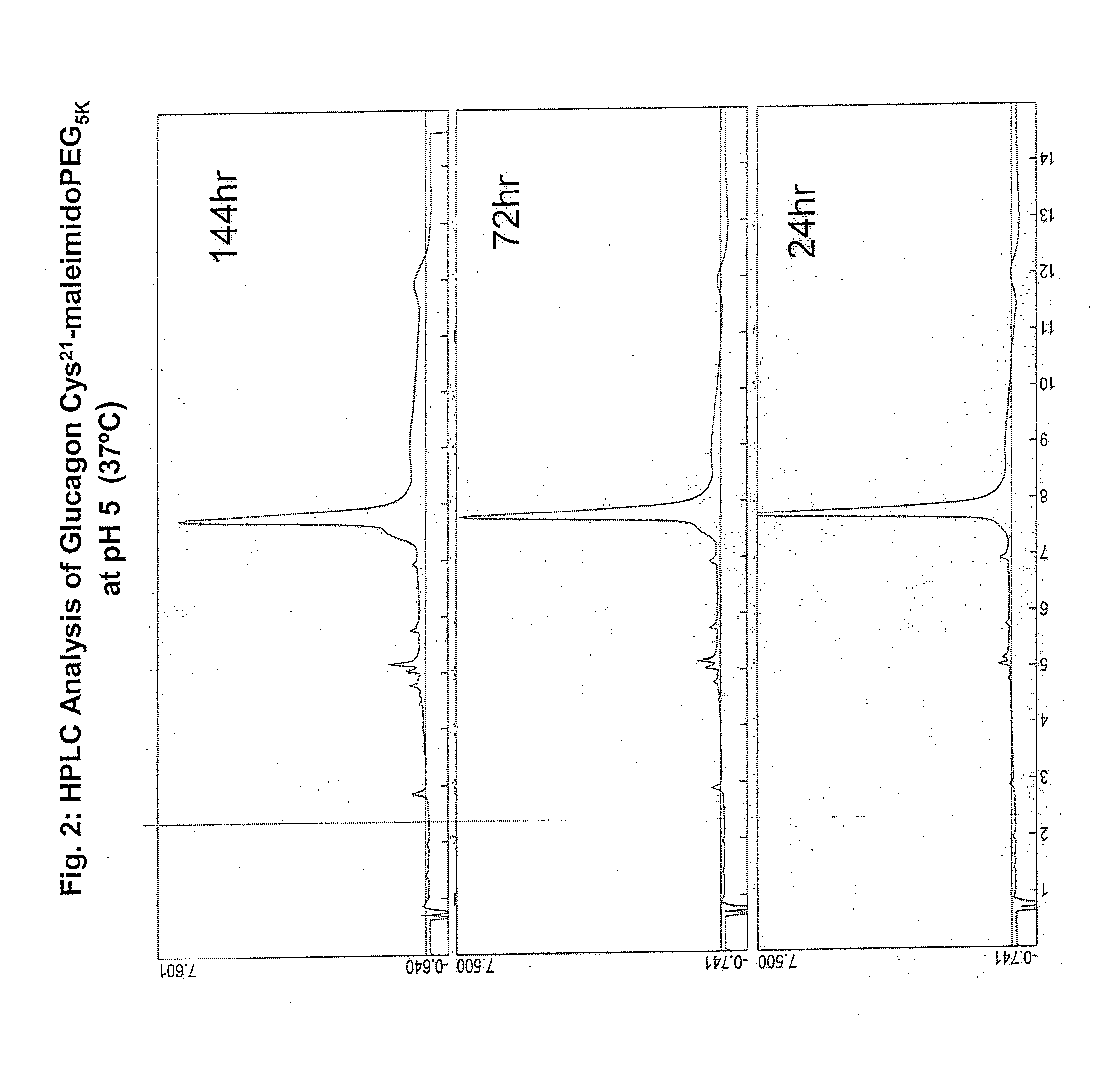
The present invention relates to novel peptide conjugates which have increased stability and are useful in the treatment of excess levels of blood glucose.
Owner:ZP HLDG SPV
Modified exendins and exendin agonists
InactiveUS6924264B1Increase ratingsDecrease amount of potassiumMetabolism disorderSaccharide peptide ingredientsGastric emptyingPolyethylene glycol
Novel modified exendins and exendin agonist analogs having an exendin or exendin agonist analog linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonist analogs, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Glucagon/glp-1 receptor co-agonists
InactiveUS20100190701A1High activityEnhanced biophysical stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityCarboxylic acid
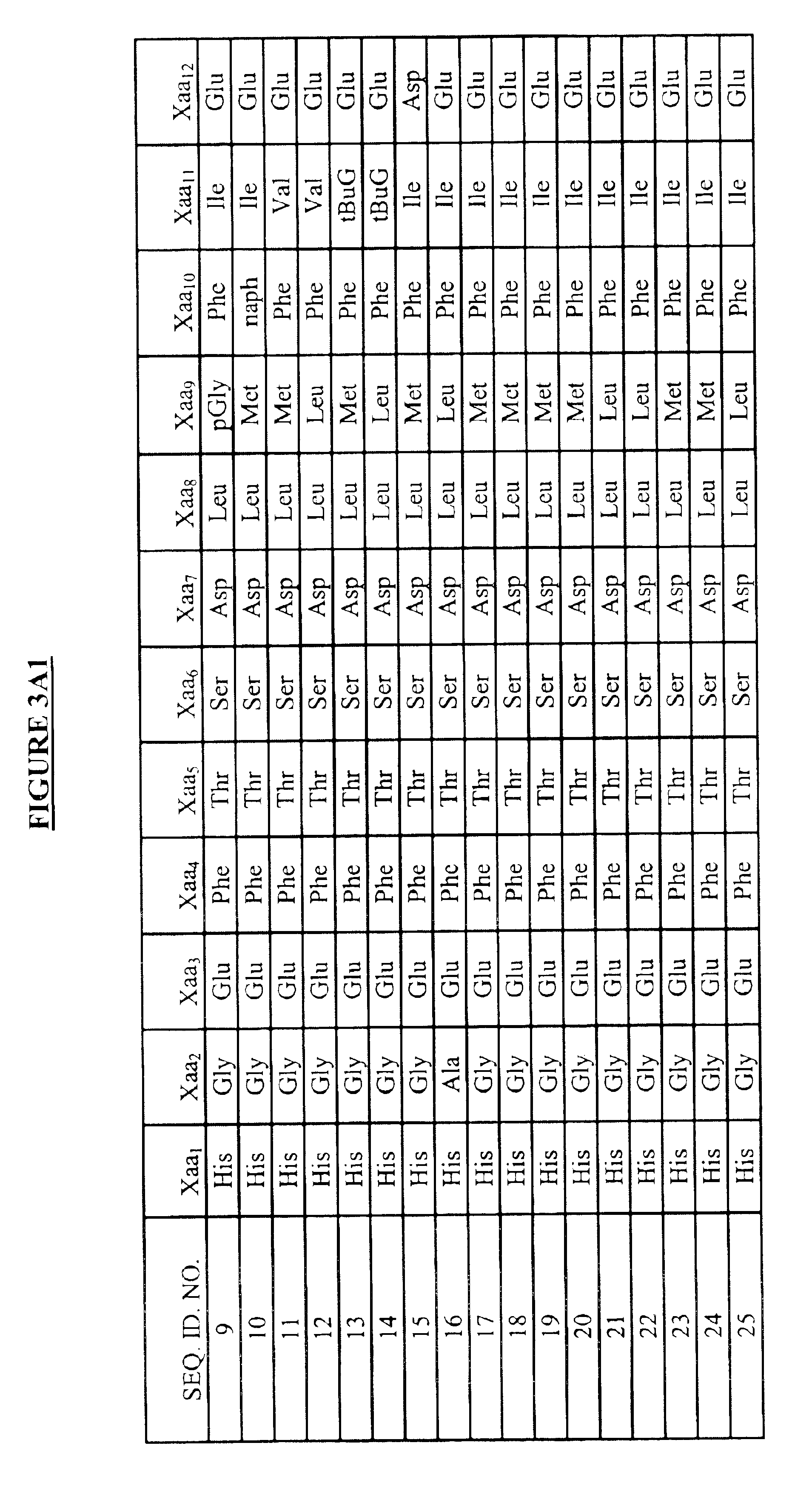
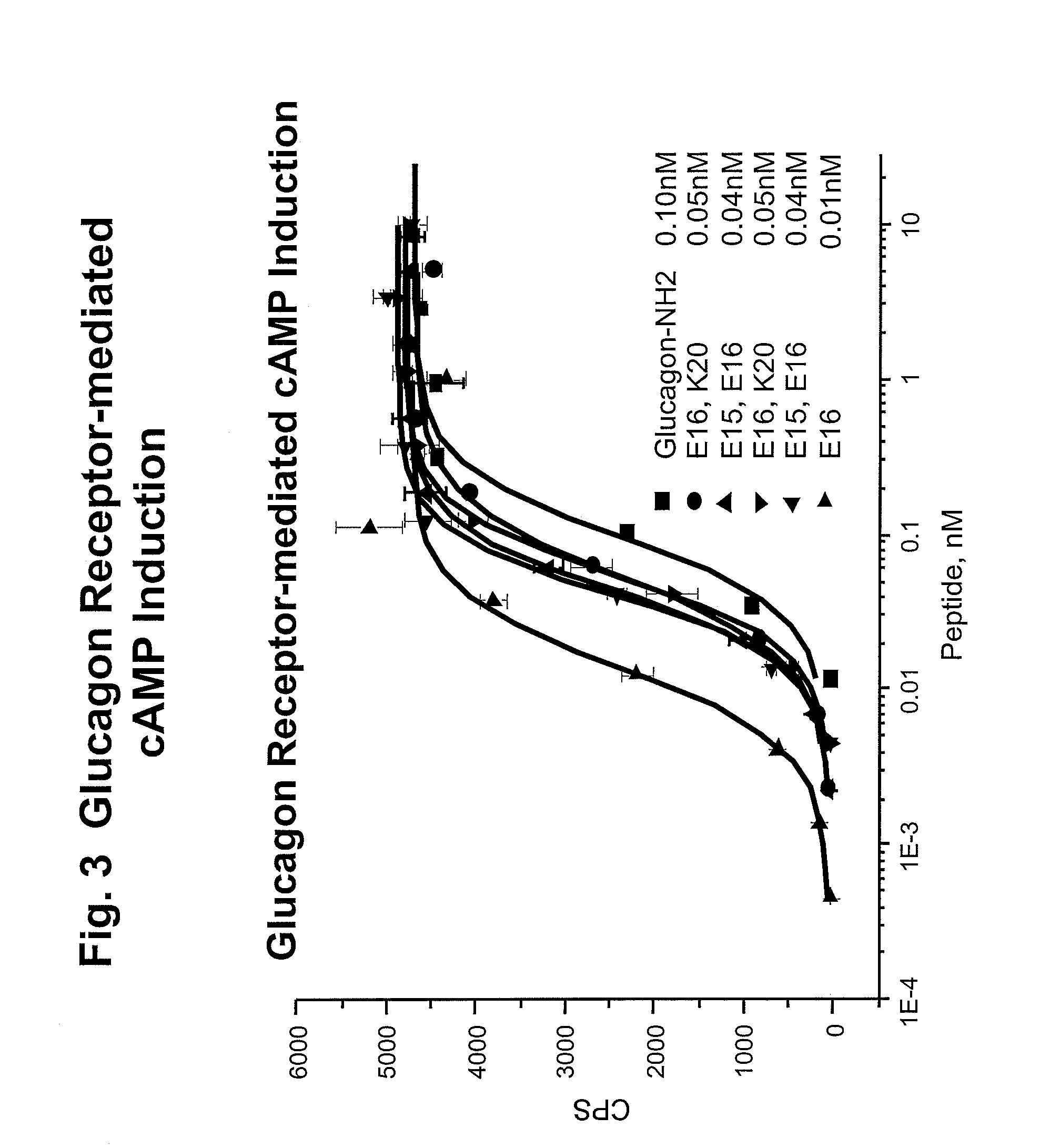
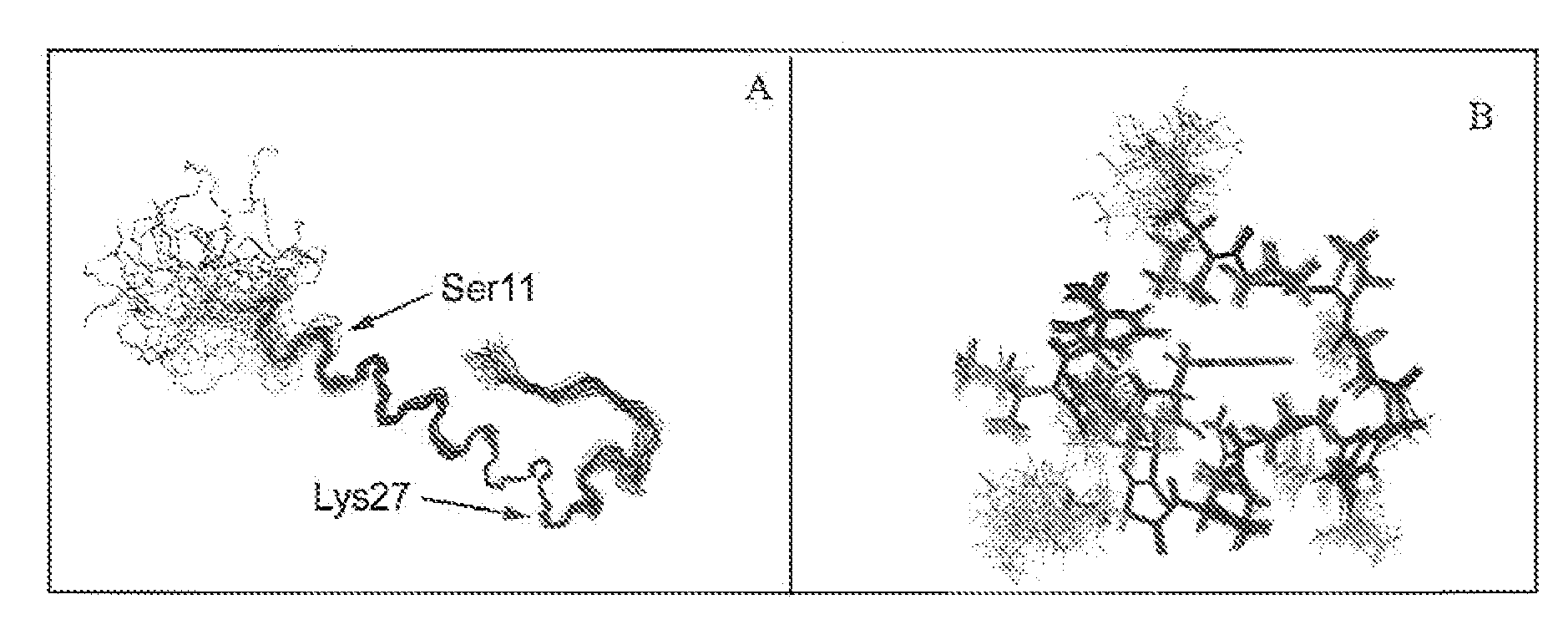
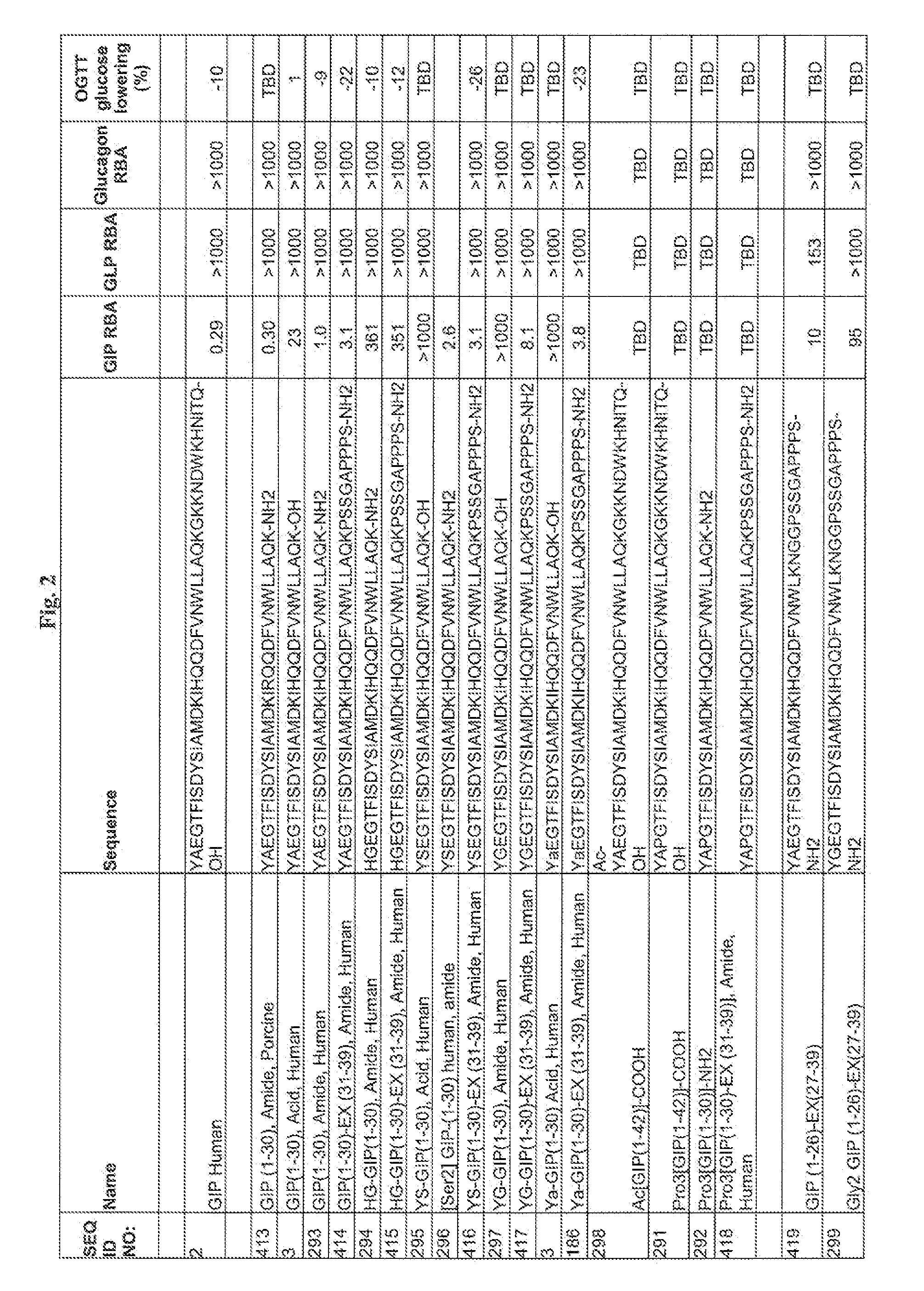
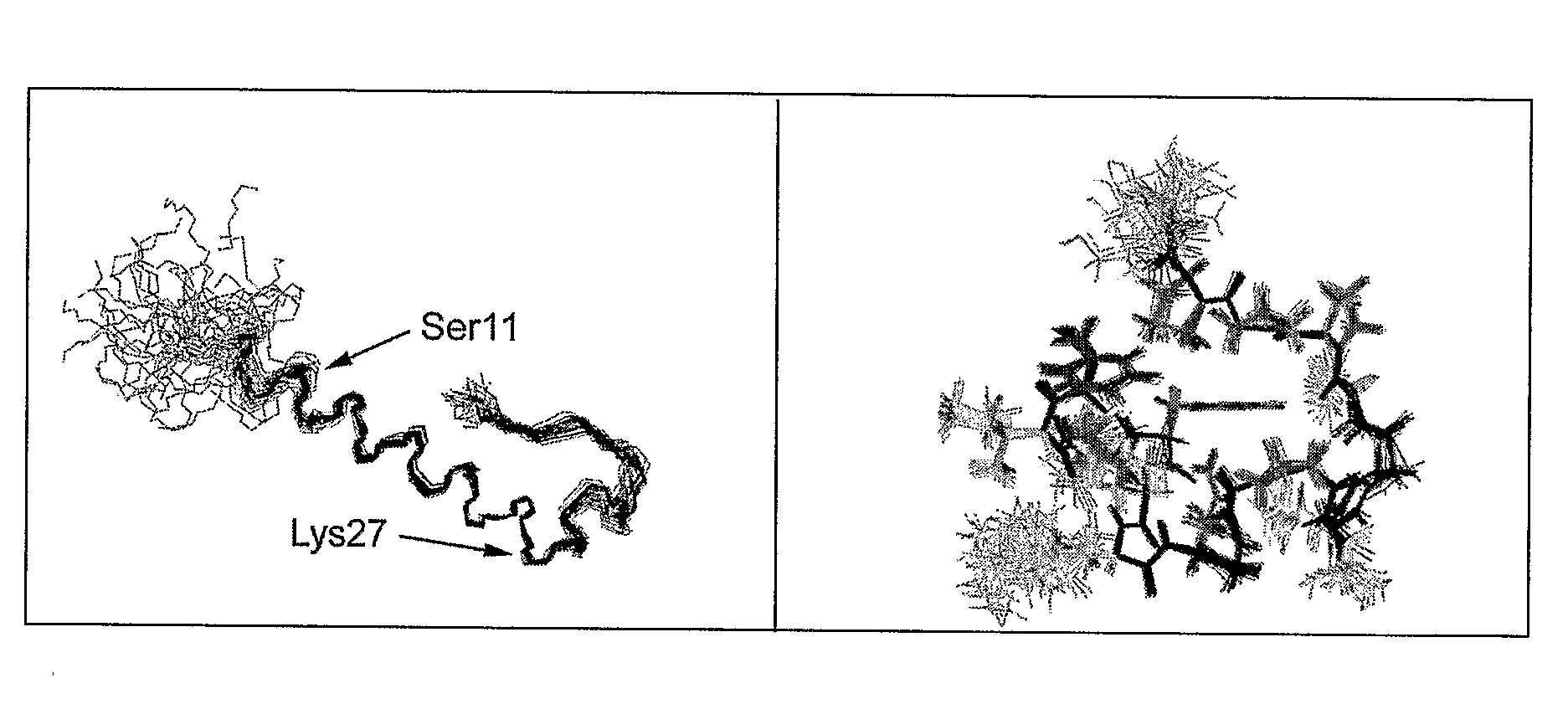
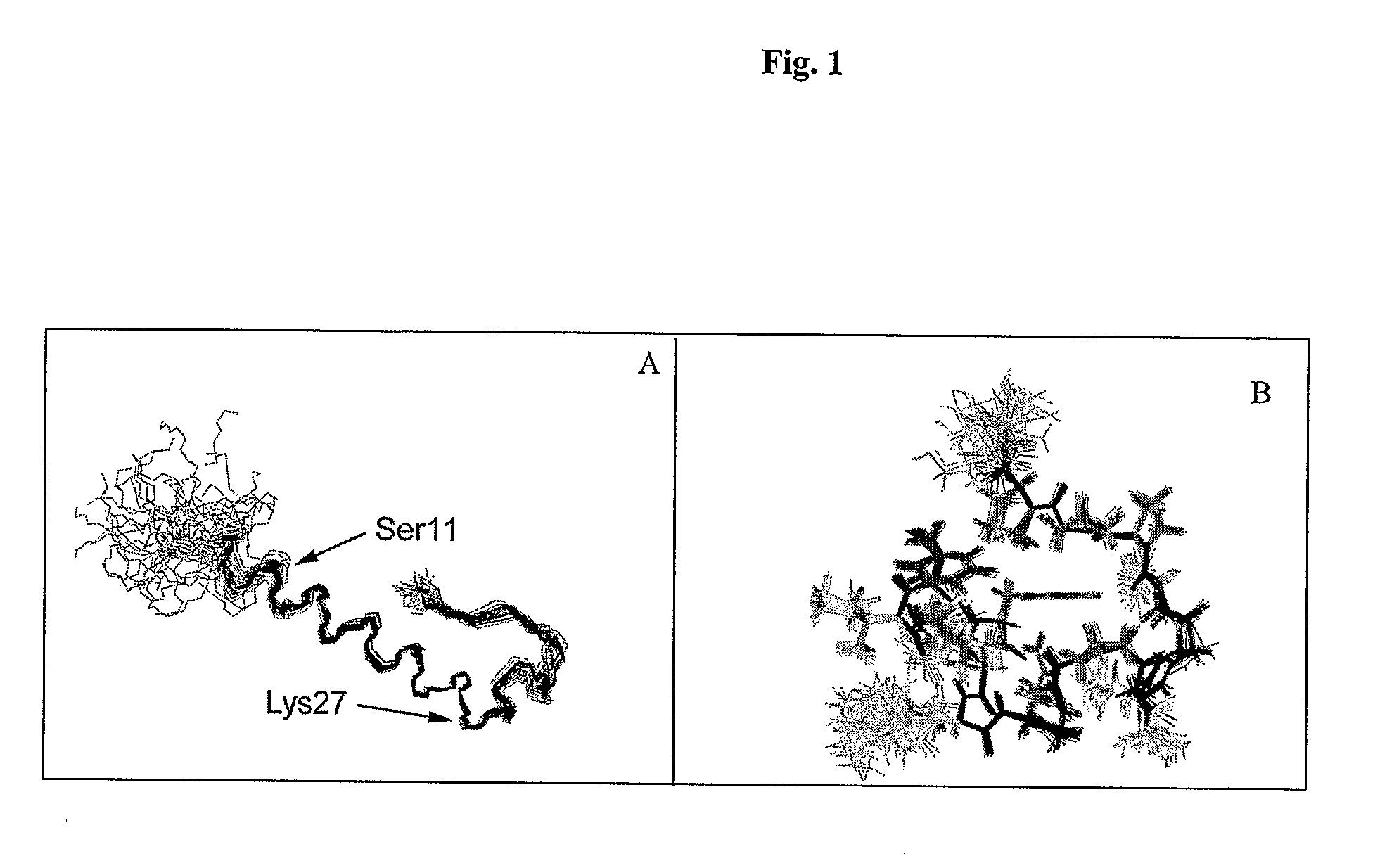
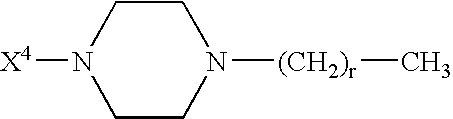
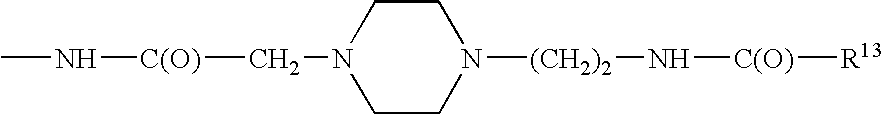
Modified glucagon peptides are disclosed having enhanced potency at the glucagon receptor relative to native glucagon. Further modification of the glucagon peptides by forming lactam bridges or the substitution of the terminal carboxylic acid with an amide group produces peptides exhibiting glucagon / GLP-1 receptor co-agonist activity. The solubility and stability of these high potency glucagon analogs can be further improved by modification of the polypeptides by pegylation, substitution of carboxy terminal amino acids, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID NO: 27 (K-RNRNNIA) and SEQ ID NO: 28 (KRNR).
Owner:INDIANA UNIV RES & TECH CORP
Administration of dipeptidyl peptidase inhibitors
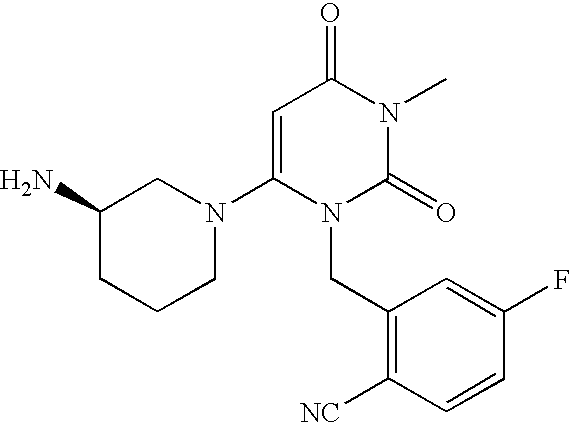
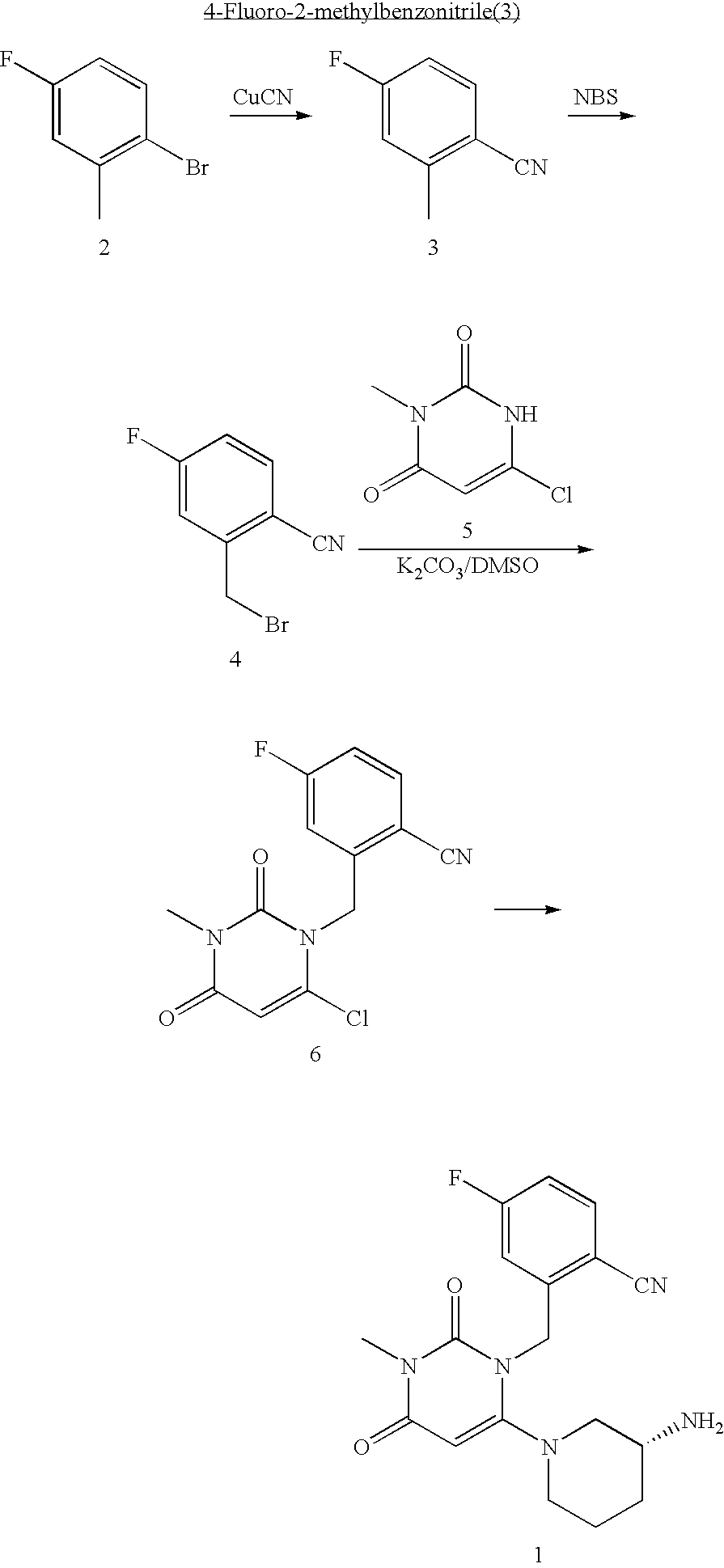
InactiveUS20070060530A1Convenient treatmentEliminate side effectsBiocidePeptide/protein ingredientsDipeptidyl peptidaseBenzonitrile
Pharmaceutical compositions comprising 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile and pharmaceutically acceptable salts thereof are provided as well as kits and articles of manufacture comprising the pharmaceutical compositions as well as methods of using the pharmaceutical compositions.
Owner:TAKEDA PHARMA CO LTD
C-aryl glucoside SGLT2 inhibitors and method
Owner:BRISTOL MYERS SQUIBB CO
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
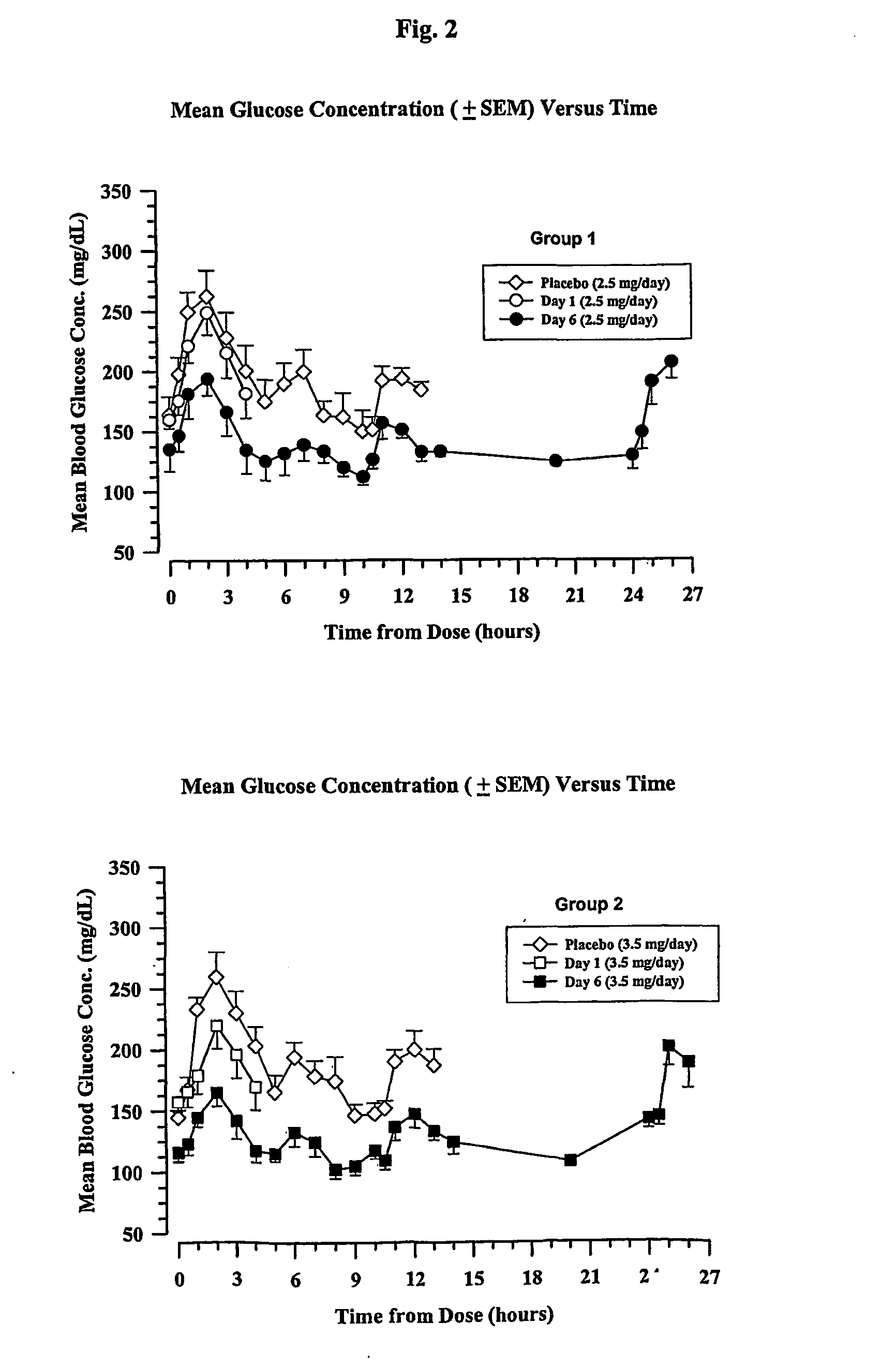
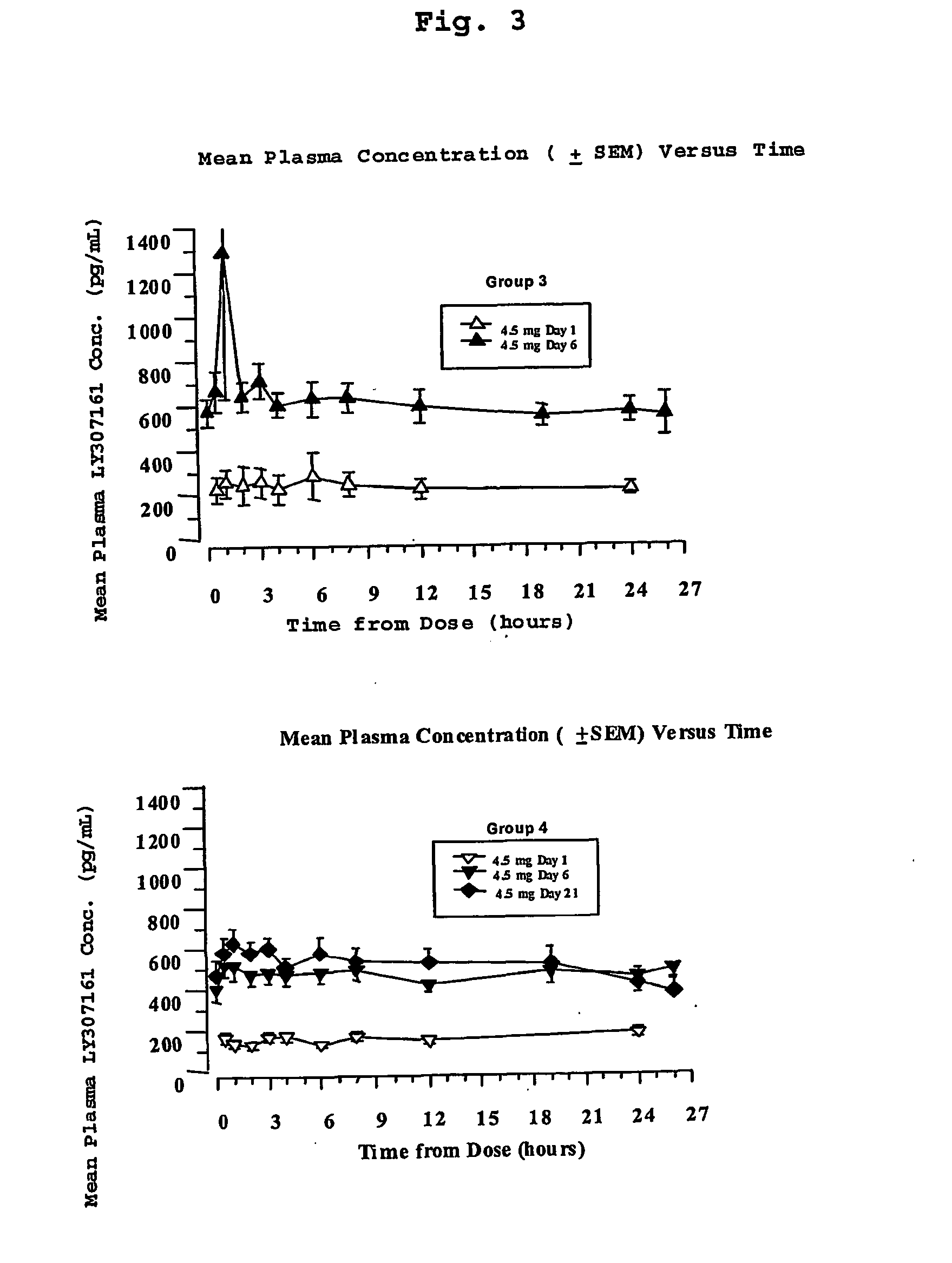
GLP-1 formulations with protracted time action
The present invention encompasses compositions wherein a GLP-1 compound is complexed with a basic polypeptide. The compositions provide a prolonged duration of action and can be administered by the pulmonary route.
Owner:ELI LILLY & CO
Polypeptide compositions with improved stability
InactiveUS7022674B2Good chemical stabilityLower Level RequirementsPeptide/protein ingredientsMetabolism disorderLinear correlationGlycerol
The present invention provides means to improve the chemical stability of aqueous, parenteral pharmaceutical compositions comprising a polypeptide and glycerin. Reactive aldehydes are identified in commercial glycerins, and means for reducing such are provided. Convenient means are provided to assay for reactive aldehydes in glycerin, and a strong linear correlation between the level of reactive aldehydes in glycerin and chemical stability of compositions comprising a polypeptide and glycerin is demonstrated. The invention includes aqueous compositions comprising a polypeptide and glycerin having improved chemical stability compared to compositions previously known.
Owner:ELI LILLY & CO
Chronic treatment regimen using glucagon-like insulinotropic peptides
InactiveUS7259233B2Avoids and minimizes side effectPeptide/protein ingredientsImmunoglobulinsDiseasePeptide
The present invention encompasses a method of treating a disease by maintaining chronic steady state serum levels of a GLP-1 compound within a specified range.
Owner:ELI LILLY & CO
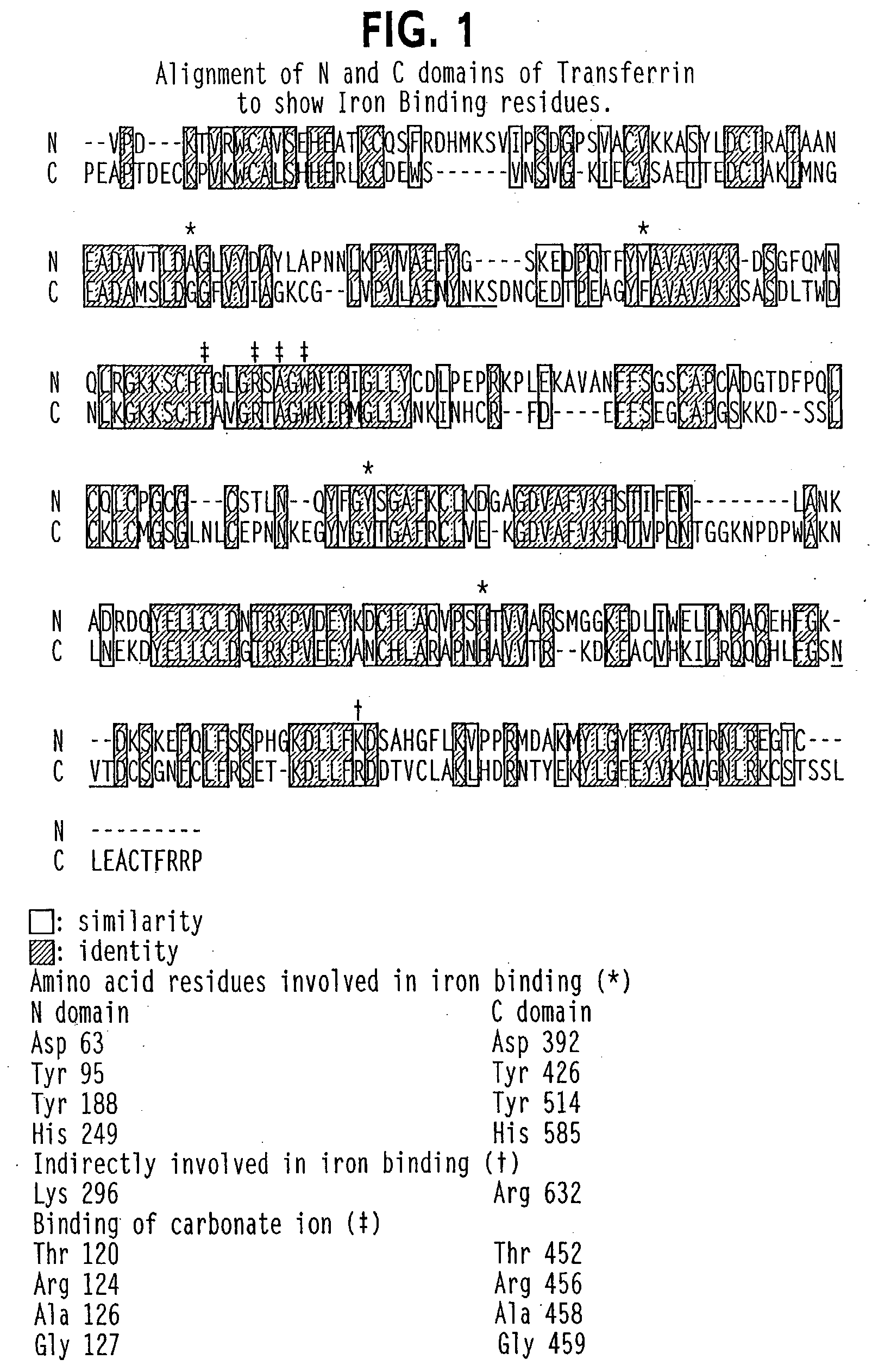
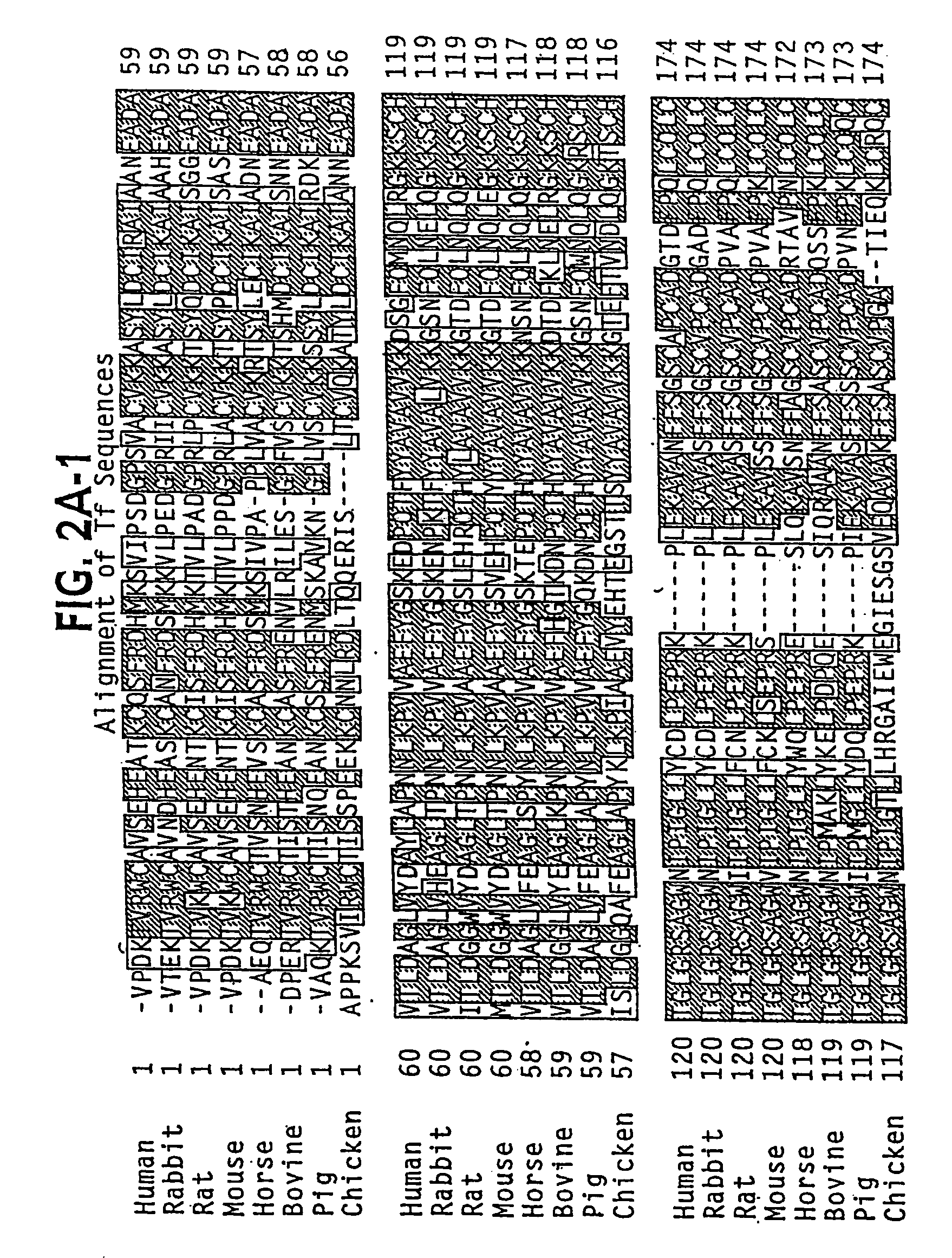
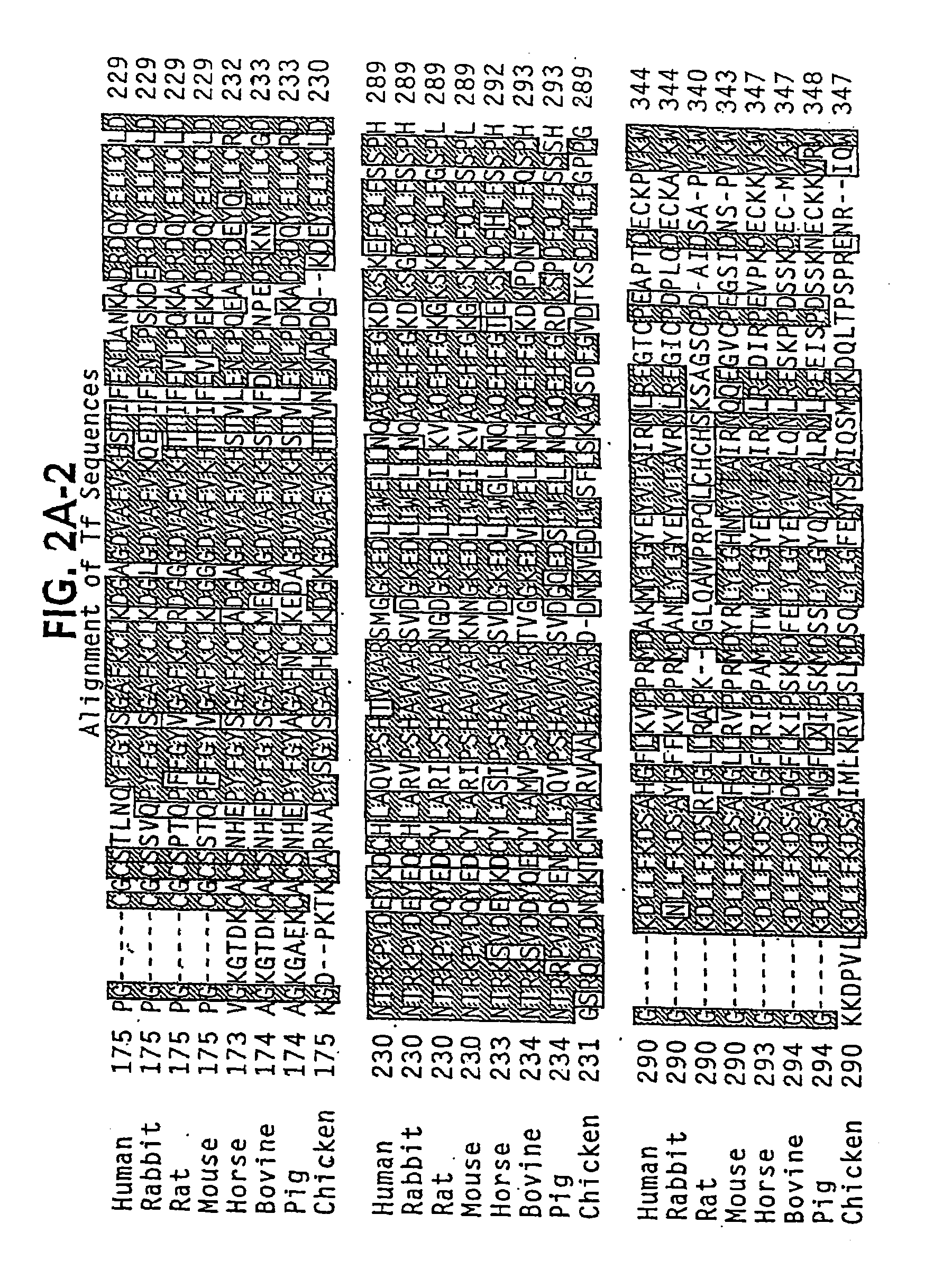
Modified transferrin fusion proteins
InactiveUS7176278B2Increased serum half-lifeImprove bioavailabilityAntibody mimetics/scaffoldsVirus peptidesDiseaseSerum ige
The present invention discloses fusion proteins comprising transferrin, lactoferrin or melanotransferrin fused to glucagon-like peptide 1 (GLP-1). In one embodiment of the invention, the fusion protein displays increased serum half-life as compared to a GLP-1 peptide in an unfused state. The invention includes a pharmaceutical composition comprising the GLP-1 fusion protein of the invention and a carrier. The fusion protein of the invention can be administered to a subject for treatment of diseases or conditions treatable by GLP-1, including, but not limited to, diabetes, obesity, congestive heart failure and inflammatory bowel syndrome.
Owner:BIOREXIS TECH INC
Methods for glucagon suppression using modified exendins
InactiveUS7153825B2Saccharide peptide ingredientsVasoactive intestinal peptideDiseaseBiological half-life
We claim a method of lowering plasma glucagon in a subject in need thereof comprising administering to the subject a composition comprising a modified exendin or modified exendin analog, wherein said modification comprises one or more molecule linked to an exendin or the exendin analog wherein said molecule is selected from the group consisiting of polyethylene glycol, gelatin and / or albumin. The modified exendin or the modified exendin analog has activity of suppressing glucagon secretion and / or lowering glucagon levels in the subject and possesses increased biological half-life compared to unmodified exendin or unmodified exendin analog. The method is useful in treating hyperglucagonemia and other disorders that would be benefited by lowering plasma glucagon or suppressing glucagon secretion.
Owner:AMYLIN PHARMA INC
Activatable clostridial toxins
InactiveUS20080032931A1Minimize security riskEnhanced efficiency and rateHydrolasesPeptide/protein ingredientsClostridial toxinSaxitoxin
Owner:ALLERGAN INC
Treatment of conditions through modulation of the autonomic nervous system during at least one predetermined menstrual cycle phase
ActiveUS20050256028A1Effective treatmentAntibacterial agentsBiocideMenstrual cycle phaseNervous system
Methods are provided for treating a subject for a condition. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is modulated during at least one predetermined phase of the subject's menstrual cycle to alter the parasympathetic activity / sympathetic activity ratio in a manner that is effective to treat the subject for the condition. The subject methods find use in the treatment of a variety of different conditions, including various disease conditions, that increase in severity and / or occurrence during one or more phases of the menstrual cycle. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Gip analog and hybrid polypeptides with selectable properties
ActiveUS20080312157A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsMetabolism disorderDyslipidemiaFeeding disability
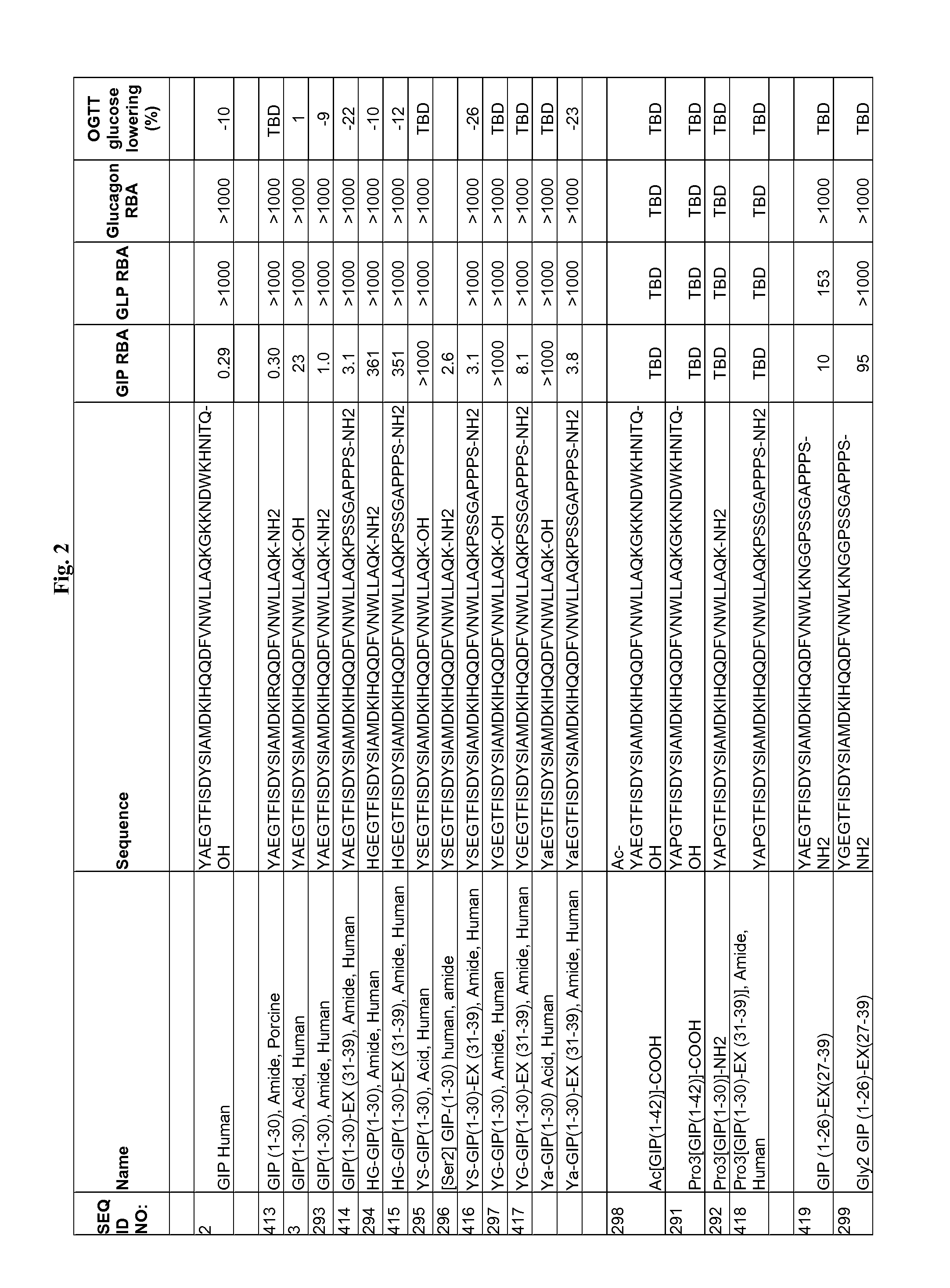
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level
InactiveUS20060154866A1Lower blood sugar levelsImprove the level ofOrganic active ingredientsSenses disorderGpr119 agonistG protein-coupled receptor
The present invention concerns combination of an amount of a GPR119 agonist with an amount of a dipeptidyl peptidase IV (DPP-IV) inhibitor such that the combination provides an effect in lowering a blood glucose level or in increasing a blood GLP-1 level in a subject over that provided by the amount of the GPR119 agonist or the amount of the DPP-IV inhibitor alone and the use of such a combination for treating or preventing diabetes and conditions related thereto or conditions ameliorated by increasing a blood GLP-1 level. The present invention also relates to the use of a G protein-coupled receptor to screen for GLP-1 secretagogues.
Owner:ARENA PHARMA
Use of GLP-1 analogs and derivatives administered peripherally in regulation of obesity
This invention relates the use of glucagon-like peptides such as GLP-1, a GLP-1 analog, or a GLP-1 derivative in methods and compositions for reducing body weight.
Owner:ELI LILLY & CO
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Gip analog and hybrid polypeptides with selectable properties
InactiveUS20090036364A1Increased insulin secretionDecreasing bone loss boneSenses disorderNervous disorderDyslipidemiaPhysiology
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Novel peptide agonists of GLP-1 activity
InactiveUS20040106547A1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsVasoactive intestinal peptideDiseaseGastric emptying
Owner:ZEALAND PHARM AS
Hybrid polypeptides with selectable properties
InactiveUS20060094652A1Reverse glucose intoleranceIncrease beta cell massPeptide/protein ingredientsAntibody mimetics/scaffoldsDyslipidemiaBlood plasma
The present invention relates generally to novel, selectable hybrid polypeptides useful as agents for the treatment and prevention of metabolic diseases and disorders which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, such as diabetes and diabetes-related conditions. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:AMYLIN PHARMA INC
Insulinotropic hormone derivatives and uses thereof
InactiveUS7138486B2Promote insulin secretionIncrease insulin levelsBiocidePeptide/protein ingredientsInsulinotropinPancreatic hormone
Derivatives of glucagon-like peptide I (GLP-1) and especially GLP-1 (7-37) have been found to have insulinotropic activity. The invention pertains to a composition comprising an acid addition salt of GLP-I (7-37) and to a composition comprising a carboxylate salt of GLP-I (7-37). The invention also pertains to method of treating type II diabetes mellitus by providing derivatives of GLP-I (7-37) to the patient.
Owner:THE GENERAL HOSPITAL CORP
Chronic treatment regimen using glucagon-like insulinotropic peptides
InactiveUS20040053819A1Avoids and minimizes side effectPeptide/protein ingredientsImmunoglobulinsDiseasePeptide
The present invention encompasses a method of treating a disease by maintaining chronic steady state serum levels of a GLP-1 compound within a specified range.
Owner:ELI LILLY & CO
GLP-1 gene delivery for the treatment of type 2 diabetes
ActiveUS7374930B2Efficient transfectionEasy to controlSugar derivativesPeptide/protein ingredientsGene deliveryGlucose polymers
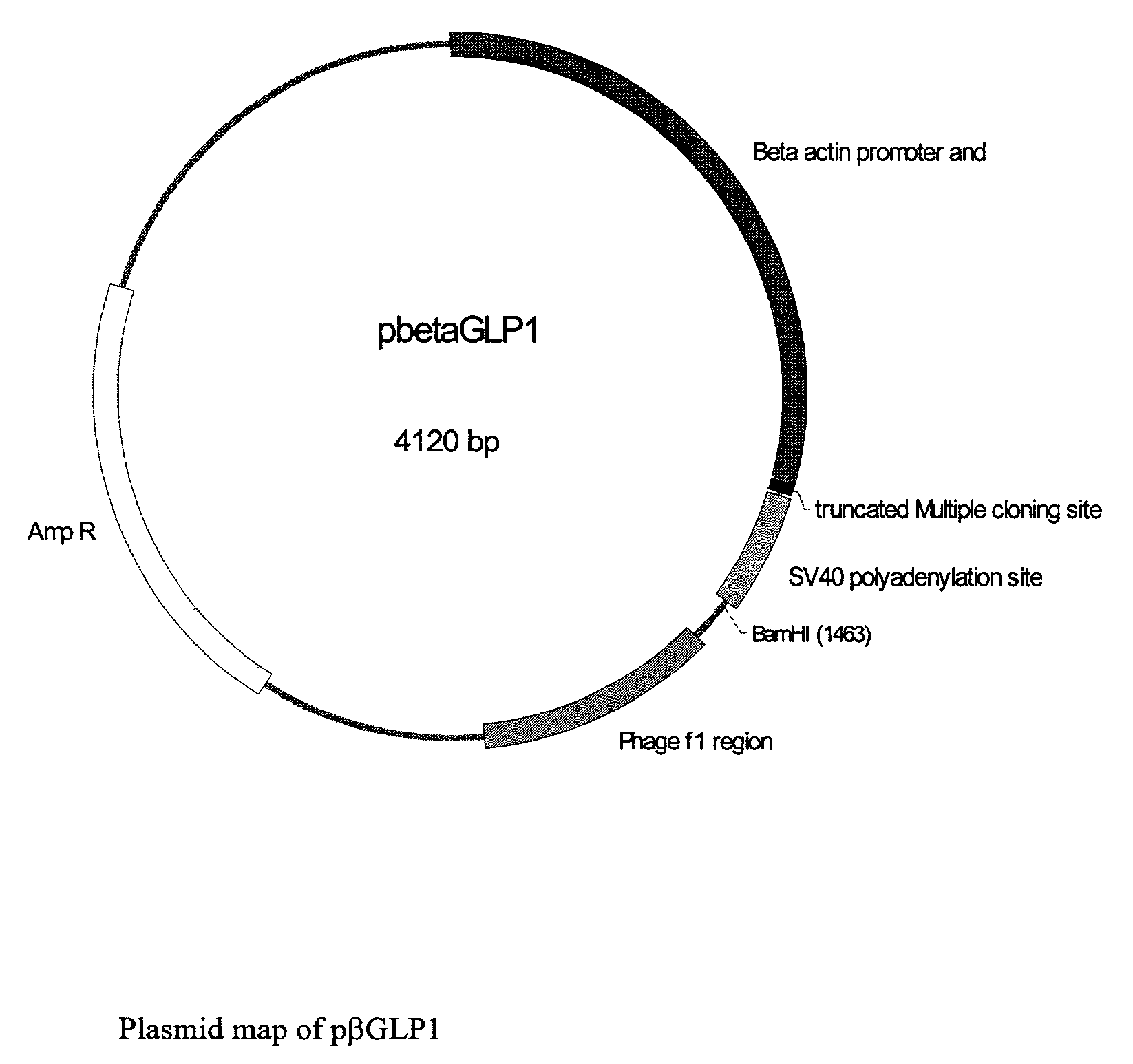
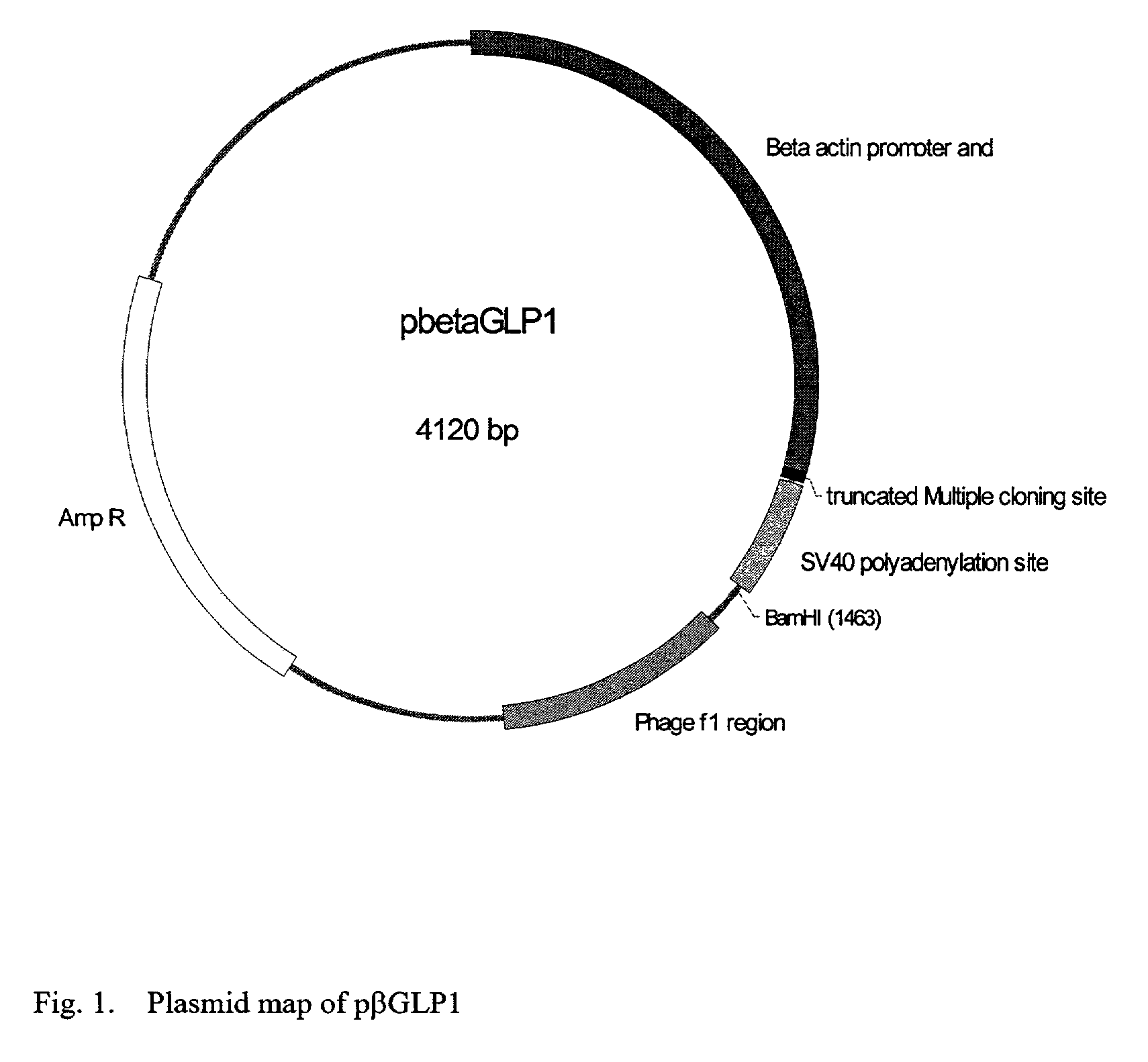
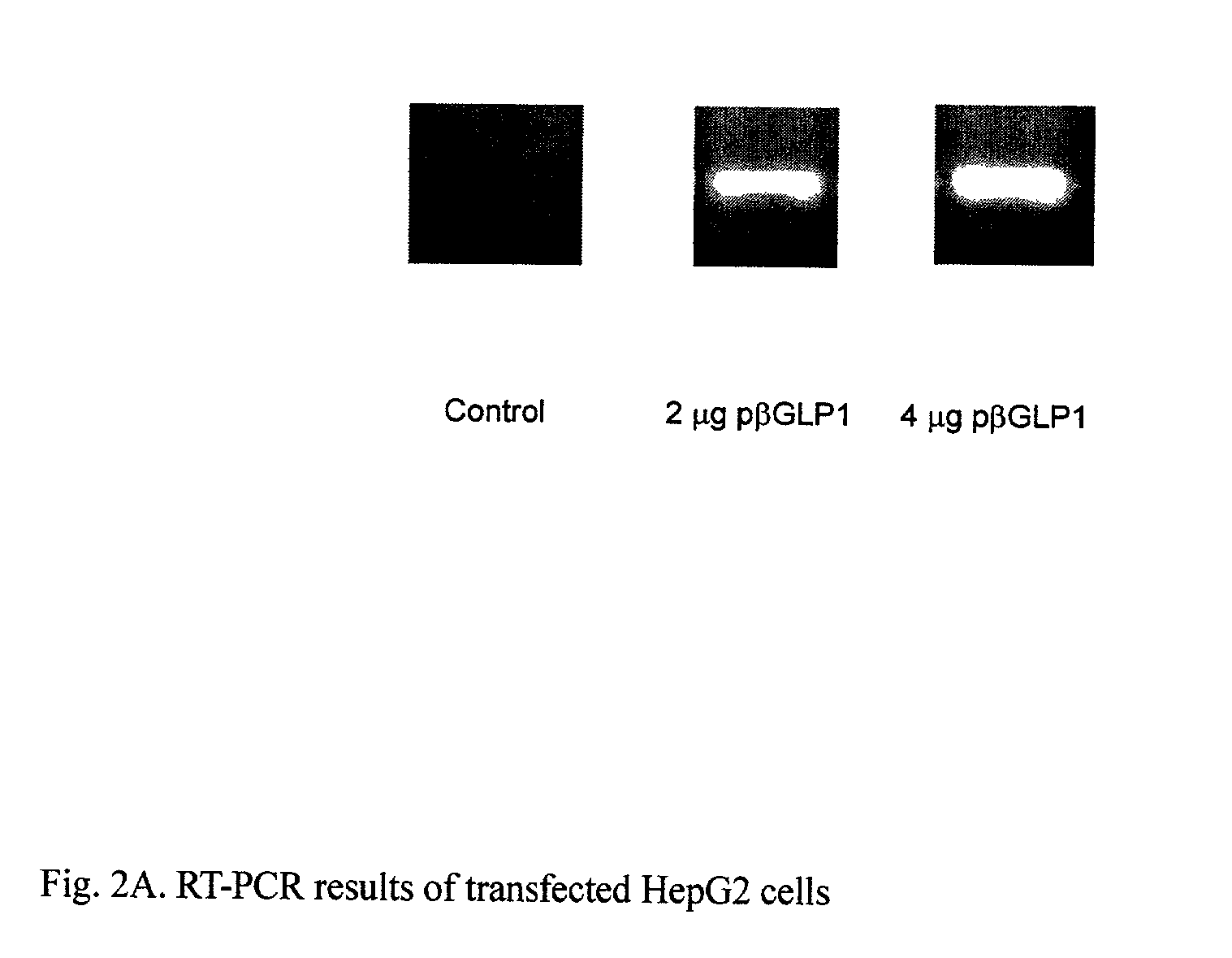
This patent discloses compositions and methods of use thereof to normalize the blood glucose levels of patients with type 2 diabetes. It relates particularly to a plasmid comprising a chicken β actin promoter and enhancer; a modified GLP-1 (7-37) cDNA (pβGLP1), carrying a furin cleavage site, which is constructed and delivered into a cell for the expression of active GLP-1.
Owner:CLSN LAB
Chemotherapy treatment
InactiveUS20030040478A1Improve welfareImprove survivalBiocideOrganic active ingredientsRegimenApoptosis
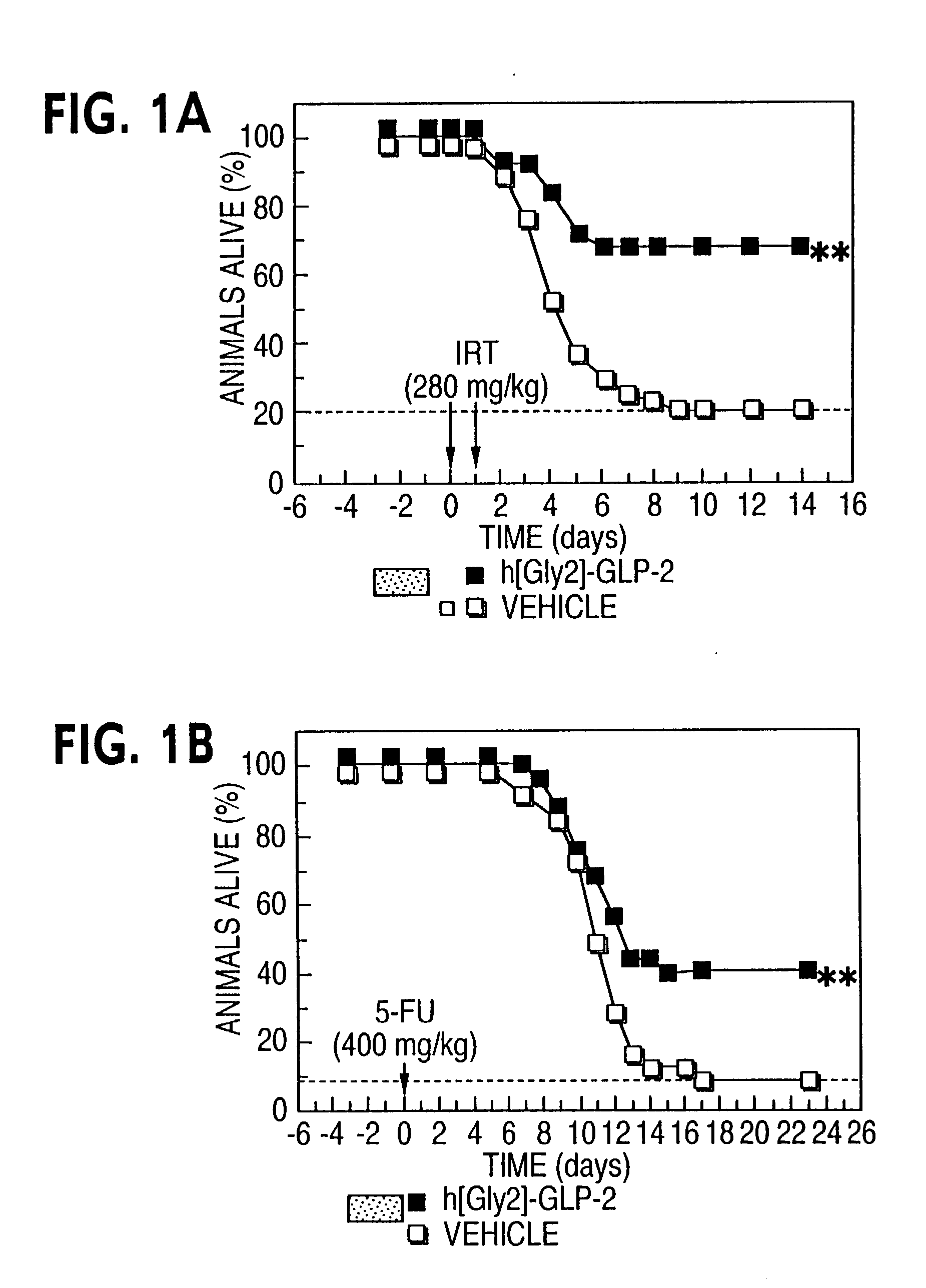
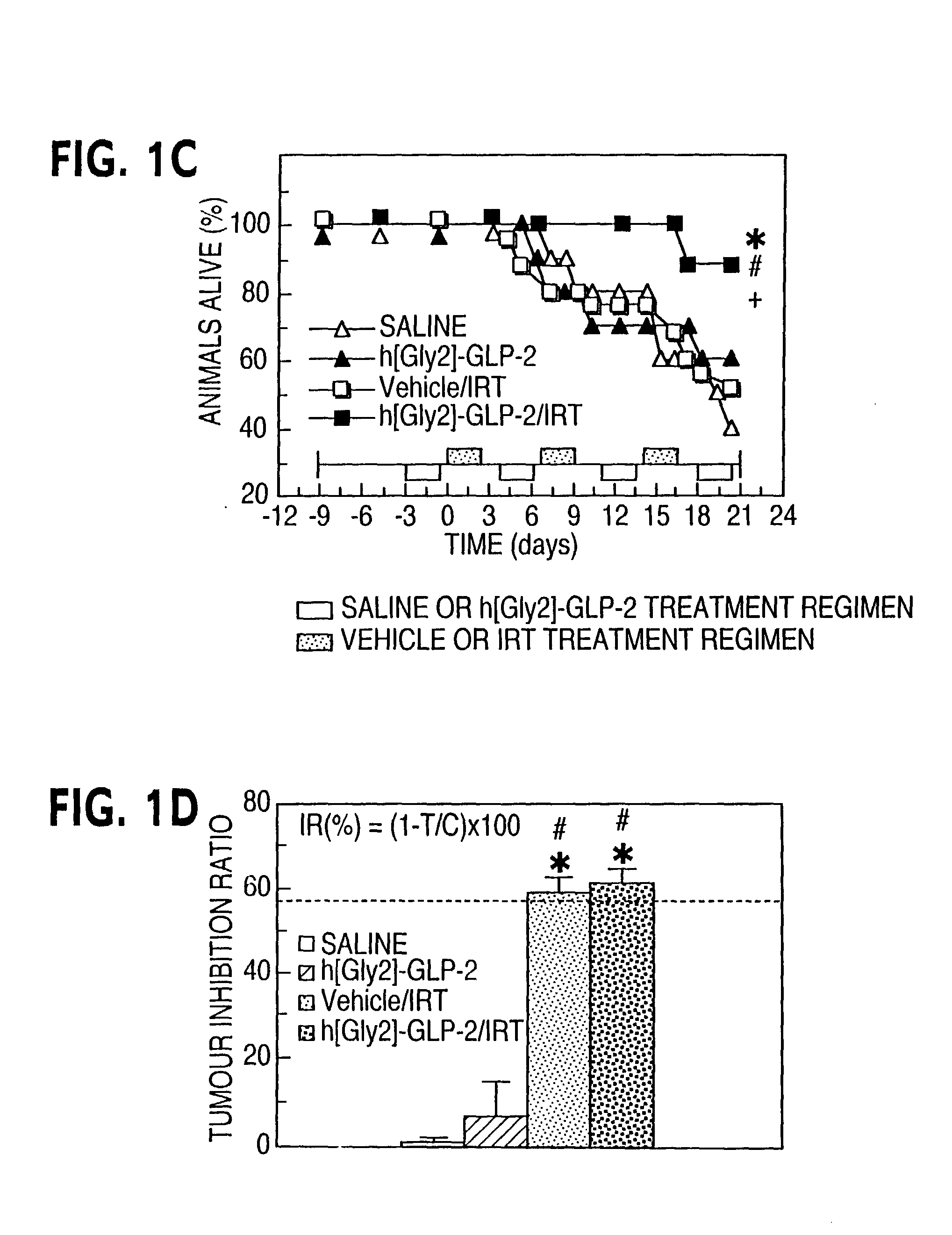
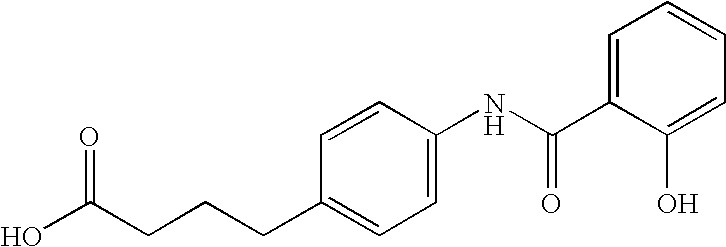
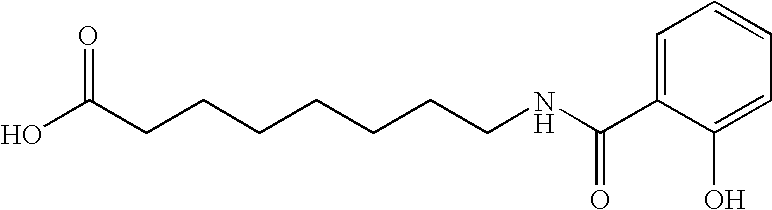
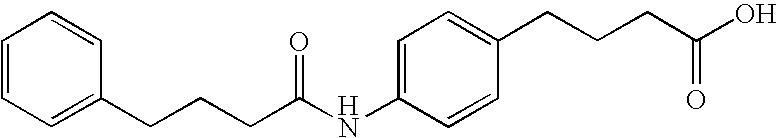
This invention provides a treatment regimen that is effective in inhibiting chemotherapy-induced apoptosis and promoting cell survival. The invention also relates to a treatment regimen that confers resistance to caspase activation, thereby inhibiting caspase-mediated, proteolytic cleavage of functional cellular enzymes. Specifically, subjects undergoing chemotherapy are first exposed to a pretreatment regimen. Under this regimen, a GLP-2 receptor activator, such as h[GLY2]-GLP2, is administered each day for a predetermined beneficial period, e.g., three consecutive days. Approximately about 1 week following pretreatment, the subjects are exposed to an appropriate chemotherapy treatment regimen. Pretreatment with a GLP-2 receptor activator followed by administration of chemotherapeutic agents improves cell survival, reduces bacteremia, attenuates epithelial injury, and inhibits cellular apoptosis. Moreover, it does not impair the effectiveness of chemotherapy nor result in weight loss. The anti-apoptotic effects of GLP-2 may be useful in the reduction of cytoxicity and bacterial infection induced by chemotherapeutic agents.
Owner:1149336 ONTARIO
Oral GLP-1 formulations
InactiveUS20060286129A1Facilitate oral deliveryBioavailabilityPeptide/protein ingredientsMetabolism disorderBioavailabilityDrug
The present invention provides phamaceutical compositions comprising at least one delivery agent and GLP-1. These pharmaceutical compositions facilitate the oral delivery of GLP-1, providing improved (e.g. increased) bioavailability of GLP-1 compared to administration of GLP-1 without a delivery agent.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Derivatives of GLP-1 analogs
InactiveUS7235627B2Improve solubility and stabilityReduce capacityOrganic active ingredientsOrganic detergent compounding agentsActive agentSurface-active agents
The present invention relates to a pharmaceutical composition comprising a GLP-1 derivative having a lipophilic substituent; and a surfactant.
Owner:NOVO NORDISK AS
Biosynthetic polypeptides utilizing non-naturally encoded amino acids
InactiveUS7638299B2Improve stabilityPromote circulationVirus peptidesVasoactive intestinal peptideAmino acidBiosynthesis
Owner:AMBRX
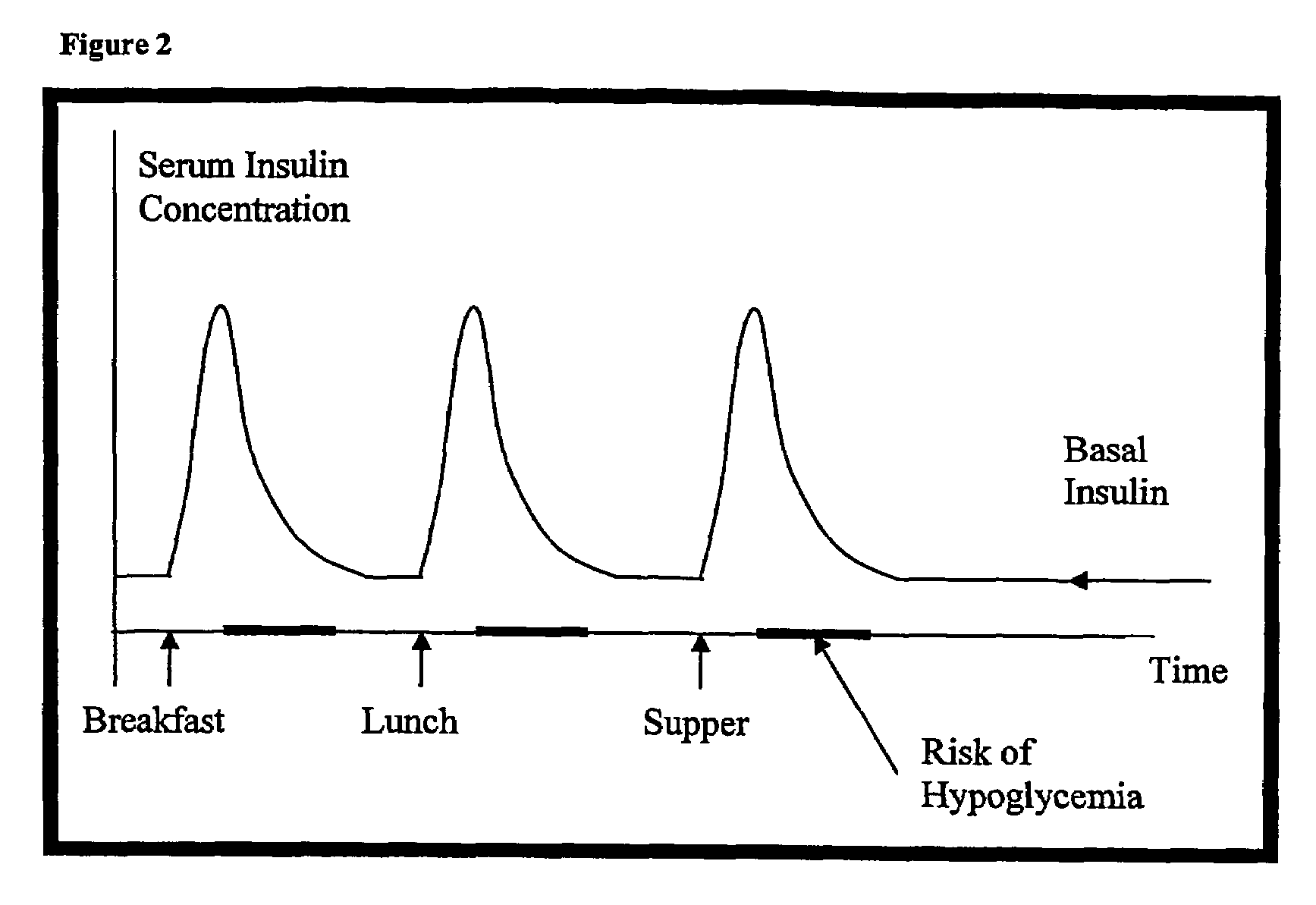
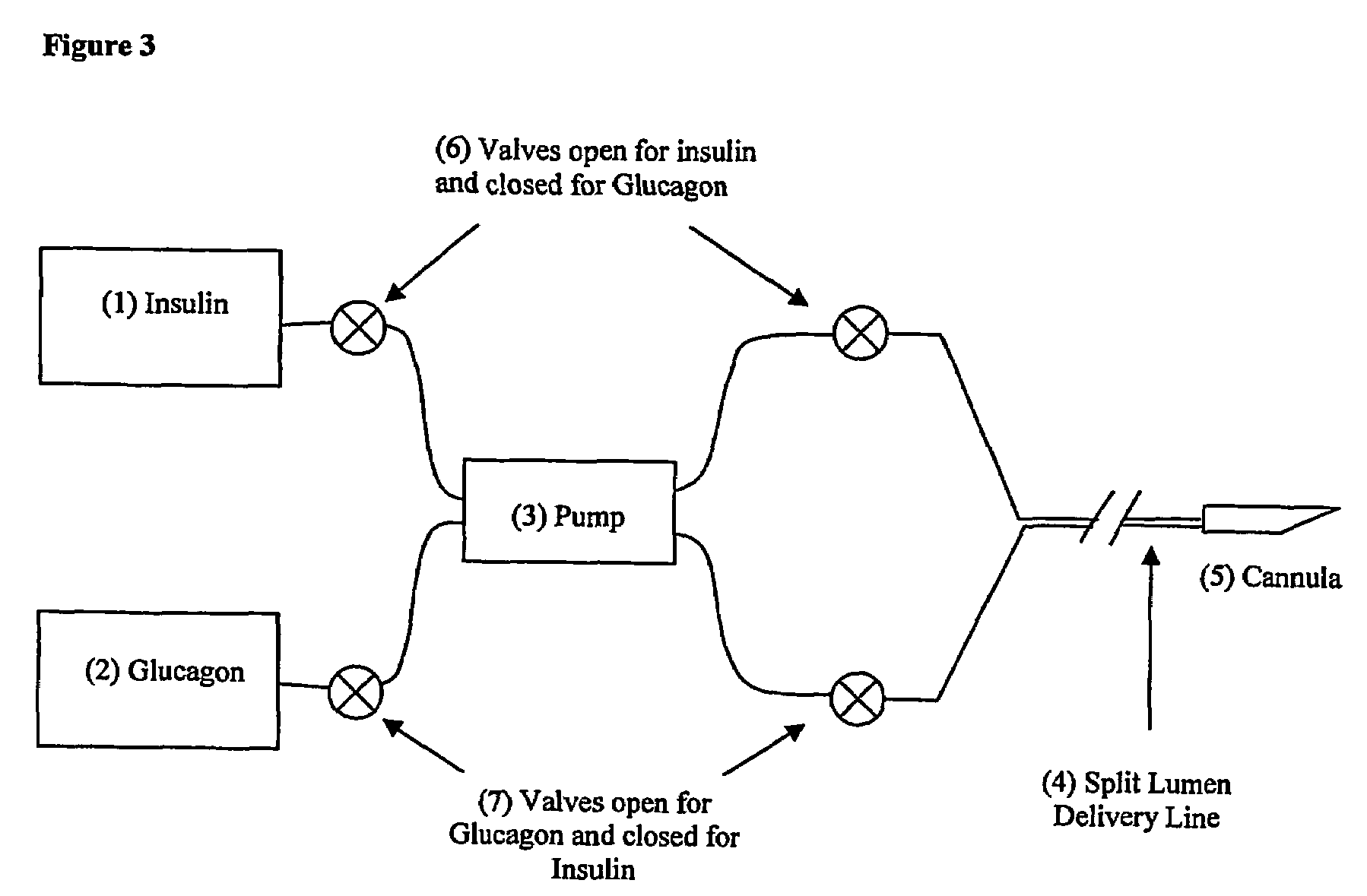
Compositions and methods for the prevention and control of insulin-induced hypoglycemia
InactiveUS7314859B2Extended half-lifePowder deliveryDispersion deliveryInsulin induced hypoglycemiaCvd risk
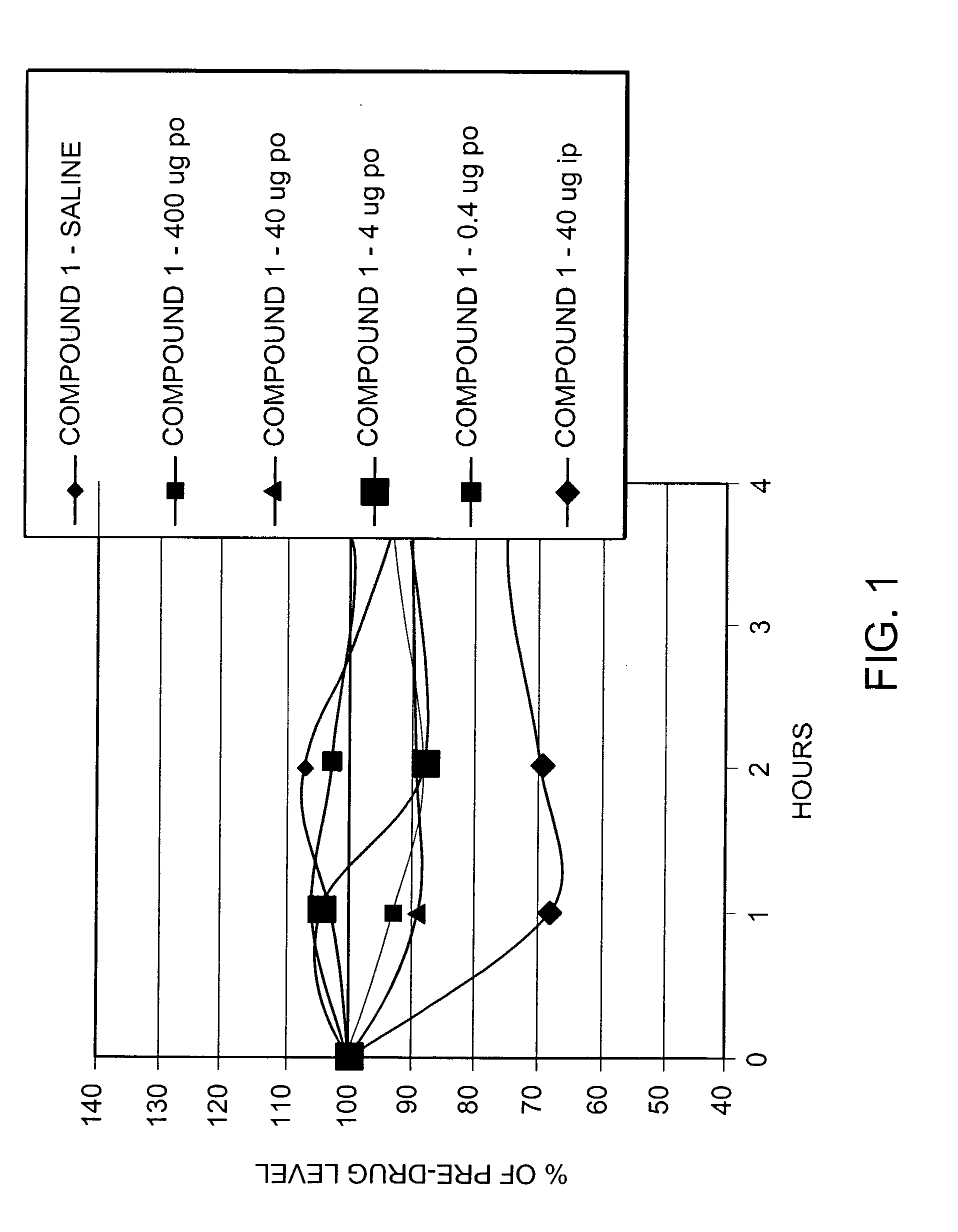
Pharmaceutical composition comprising both insulin and glucagon can be administered to control and treat diabetes while reducing or eliminating the risk of insulin-induced hypoglycemia.
Owner:DIOBEX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com